User login
Hair oiling: Practices, benefits, and caveats
including in India and among people of the African diaspora. Hair oiling typically entails combing the hair, followed by applying oil from the roots to the ends about once a week prior to shampooing. Hair oiling daily or every other day, often with a hair braid, is also practiced, using coconut oil or other oils. Oil is found in various hair products and has been popular in the United States, particularly as hot oil treatments in the 1980s.
Oils are used as part of Abhyanga massage, either by an Ayurvedic practitioner or as self-massage, as part of ancient Indian Ayurvedic medicine practice. The type of oil used is determined by the practitioner depending on the individual’s needs; cold-pressed sesame oil or coconut oil is often used as the base oil. Abhyanga is often performed with oil on the entire body as an act of self-care, which includes massage of the hair and scalp. The oil and herbs that are often added to the oil, as well as the scalp massage itself, are explained by practitioners as having therapeutic effects on the hair, including exfoliation of a dry scalp and on overall well-being. Outside of Ayurvedic medicine, hair oiling is also sometimes performed in India as part of routine hair care and can be a bonding experience among parents, grandparents, and children.
Amla oil, which is derived from Indian gooseberry (Phyllanthus emblica L.) and is often used in India on the hair, contains Vitamin C and has been shown to have activity against dermatophytes. In a study conducted in India, amla oil was found to have the most activity against Microsporum canis, M. gypseum, and Trichophyton rubrum, followed by cantharidine and coconut oil, while T. mentagrophytes was most susceptible to coconut oil, followed by amla and cantharidine oil.1 A study conducted in mice in Thailand found that 5-alpha-reductase was reduced with the dried form of the herb Phyllanthus emblica L, as well as with some other traditional Thai herbs used as hair treatments.2
Castor oil has been utilized in many cultures to promote hair growth. Ricinoleic acid, an unsaturated omega-9 fatty acid and hydroxy acid, is the major component of seed oil obtained from mature castor plants. It has anti-inflammatory and antimicrobial effects, and in one study,3 was found to inhibit prostaglandin D2, which has been implicated in the pathogenesis of androgenetic alopecia.4 Jamaican black castor oil is darker in color and has a more alkaline pH compared with traditional castor oil (both contain ricinoleic acid). To produce Jamaican black castor oil, the seed is roasted, ground to a thick paste, boiled in a pot of hot water, then the oil is skimmed off of the top into individual bottles, whereas regular castor oil is cold pressed. The alkalinity of Jamaican black castor oil may play a role in opening up the hair cuticle, which may be beneficial, but application also needs to be followed by a routine that includes closing the cuticle to avoid increasing hair fragility; such a routine could include, for example, leave-in conditioner or rinsing conditioner with colder water.
Castor oil is sometimes used on its own or in combination with other oils, and it is also an ingredient in some hair care products. However, publicly available peer-reviewed research articles demonstrating the effects of castor oil when applied directly to the hair and scalp for hair growth are needed.
Different types of hair oils are used in African American hair care products and, worldwide, by people of the African diaspora as part of regular hair care and hair styling. Common oils include jojoba, coconut, castor, argan, olive, sunflower, and almond oils. Very curly hair often dries out more easily, especially in drier climates; and sebum build-up on the scalp does not occur as quickly, so hair typically does not need to be shampooed frequently. As such, over-shampooing also often dries the hair out faster, especially if hair has been chemically processed. Thus, oils help to coat and protect the hair, and smooth the cuticle. Oils themselves do not moisturize, but can seal moisture into the hair or act as humectants and draw moisture in. Rather than applying on dry hair, oils, when used as daily care as opposed to before shampooing, are often applied on clean hair after shampooing and after a leave-in conditioner has been applied to help seal in moisture for the most benefit. For treatment of scalp conditions, depending on the type of hair care practice performed at home, oils may be preferred over potentially drying solutions and foams if available, for optimum hair care.
Hair oiling is a long-standing practice which, when used correctly, can be beneficial for hair management and health. There are, however, caveats to hair oiling, which include being careful not to excessively brush or comb hair that has a lot of oil in it because the hair is slick, which can cause hair to fall out during combing. Some oils have elevated levels of lauric acid (coconut oil has 50%), which has a high affinity for hair proteins.5 While this can support hair strength, care should be taken not to overuse some oils or other products known as “protein packs,” which should be used as directed. Since hair is protein, excess protein build-up coating the hair can block needed moisture, making the hair more knotted and brittle, potentially resulting in more breakage with brushing or other hair care practices.
Some oil-containing hair products also contain artificial fragrances and/or dyes, which in some individuals, may promote an immunogenic effect, such as contact dermatitis. Certain oils, particularly olive oil, can promote an environment favorable to yeast growth, especially Malassezia species, implicated in seborrheic dermatitis. Practices that involve excessive application of oil to the scalp can also result in build up if not shampooed regularly. Sulfonated castor oil (also known as Turkey Red oil) has been reported to cause contact dermatitis.6
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
including in India and among people of the African diaspora. Hair oiling typically entails combing the hair, followed by applying oil from the roots to the ends about once a week prior to shampooing. Hair oiling daily or every other day, often with a hair braid, is also practiced, using coconut oil or other oils. Oil is found in various hair products and has been popular in the United States, particularly as hot oil treatments in the 1980s.
Oils are used as part of Abhyanga massage, either by an Ayurvedic practitioner or as self-massage, as part of ancient Indian Ayurvedic medicine practice. The type of oil used is determined by the practitioner depending on the individual’s needs; cold-pressed sesame oil or coconut oil is often used as the base oil. Abhyanga is often performed with oil on the entire body as an act of self-care, which includes massage of the hair and scalp. The oil and herbs that are often added to the oil, as well as the scalp massage itself, are explained by practitioners as having therapeutic effects on the hair, including exfoliation of a dry scalp and on overall well-being. Outside of Ayurvedic medicine, hair oiling is also sometimes performed in India as part of routine hair care and can be a bonding experience among parents, grandparents, and children.
Amla oil, which is derived from Indian gooseberry (Phyllanthus emblica L.) and is often used in India on the hair, contains Vitamin C and has been shown to have activity against dermatophytes. In a study conducted in India, amla oil was found to have the most activity against Microsporum canis, M. gypseum, and Trichophyton rubrum, followed by cantharidine and coconut oil, while T. mentagrophytes was most susceptible to coconut oil, followed by amla and cantharidine oil.1 A study conducted in mice in Thailand found that 5-alpha-reductase was reduced with the dried form of the herb Phyllanthus emblica L, as well as with some other traditional Thai herbs used as hair treatments.2
Castor oil has been utilized in many cultures to promote hair growth. Ricinoleic acid, an unsaturated omega-9 fatty acid and hydroxy acid, is the major component of seed oil obtained from mature castor plants. It has anti-inflammatory and antimicrobial effects, and in one study,3 was found to inhibit prostaglandin D2, which has been implicated in the pathogenesis of androgenetic alopecia.4 Jamaican black castor oil is darker in color and has a more alkaline pH compared with traditional castor oil (both contain ricinoleic acid). To produce Jamaican black castor oil, the seed is roasted, ground to a thick paste, boiled in a pot of hot water, then the oil is skimmed off of the top into individual bottles, whereas regular castor oil is cold pressed. The alkalinity of Jamaican black castor oil may play a role in opening up the hair cuticle, which may be beneficial, but application also needs to be followed by a routine that includes closing the cuticle to avoid increasing hair fragility; such a routine could include, for example, leave-in conditioner or rinsing conditioner with colder water.
Castor oil is sometimes used on its own or in combination with other oils, and it is also an ingredient in some hair care products. However, publicly available peer-reviewed research articles demonstrating the effects of castor oil when applied directly to the hair and scalp for hair growth are needed.
Different types of hair oils are used in African American hair care products and, worldwide, by people of the African diaspora as part of regular hair care and hair styling. Common oils include jojoba, coconut, castor, argan, olive, sunflower, and almond oils. Very curly hair often dries out more easily, especially in drier climates; and sebum build-up on the scalp does not occur as quickly, so hair typically does not need to be shampooed frequently. As such, over-shampooing also often dries the hair out faster, especially if hair has been chemically processed. Thus, oils help to coat and protect the hair, and smooth the cuticle. Oils themselves do not moisturize, but can seal moisture into the hair or act as humectants and draw moisture in. Rather than applying on dry hair, oils, when used as daily care as opposed to before shampooing, are often applied on clean hair after shampooing and after a leave-in conditioner has been applied to help seal in moisture for the most benefit. For treatment of scalp conditions, depending on the type of hair care practice performed at home, oils may be preferred over potentially drying solutions and foams if available, for optimum hair care.
Hair oiling is a long-standing practice which, when used correctly, can be beneficial for hair management and health. There are, however, caveats to hair oiling, which include being careful not to excessively brush or comb hair that has a lot of oil in it because the hair is slick, which can cause hair to fall out during combing. Some oils have elevated levels of lauric acid (coconut oil has 50%), which has a high affinity for hair proteins.5 While this can support hair strength, care should be taken not to overuse some oils or other products known as “protein packs,” which should be used as directed. Since hair is protein, excess protein build-up coating the hair can block needed moisture, making the hair more knotted and brittle, potentially resulting in more breakage with brushing or other hair care practices.
Some oil-containing hair products also contain artificial fragrances and/or dyes, which in some individuals, may promote an immunogenic effect, such as contact dermatitis. Certain oils, particularly olive oil, can promote an environment favorable to yeast growth, especially Malassezia species, implicated in seborrheic dermatitis. Practices that involve excessive application of oil to the scalp can also result in build up if not shampooed regularly. Sulfonated castor oil (also known as Turkey Red oil) has been reported to cause contact dermatitis.6
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
including in India and among people of the African diaspora. Hair oiling typically entails combing the hair, followed by applying oil from the roots to the ends about once a week prior to shampooing. Hair oiling daily or every other day, often with a hair braid, is also practiced, using coconut oil or other oils. Oil is found in various hair products and has been popular in the United States, particularly as hot oil treatments in the 1980s.
Oils are used as part of Abhyanga massage, either by an Ayurvedic practitioner or as self-massage, as part of ancient Indian Ayurvedic medicine practice. The type of oil used is determined by the practitioner depending on the individual’s needs; cold-pressed sesame oil or coconut oil is often used as the base oil. Abhyanga is often performed with oil on the entire body as an act of self-care, which includes massage of the hair and scalp. The oil and herbs that are often added to the oil, as well as the scalp massage itself, are explained by practitioners as having therapeutic effects on the hair, including exfoliation of a dry scalp and on overall well-being. Outside of Ayurvedic medicine, hair oiling is also sometimes performed in India as part of routine hair care and can be a bonding experience among parents, grandparents, and children.
Amla oil, which is derived from Indian gooseberry (Phyllanthus emblica L.) and is often used in India on the hair, contains Vitamin C and has been shown to have activity against dermatophytes. In a study conducted in India, amla oil was found to have the most activity against Microsporum canis, M. gypseum, and Trichophyton rubrum, followed by cantharidine and coconut oil, while T. mentagrophytes was most susceptible to coconut oil, followed by amla and cantharidine oil.1 A study conducted in mice in Thailand found that 5-alpha-reductase was reduced with the dried form of the herb Phyllanthus emblica L, as well as with some other traditional Thai herbs used as hair treatments.2
Castor oil has been utilized in many cultures to promote hair growth. Ricinoleic acid, an unsaturated omega-9 fatty acid and hydroxy acid, is the major component of seed oil obtained from mature castor plants. It has anti-inflammatory and antimicrobial effects, and in one study,3 was found to inhibit prostaglandin D2, which has been implicated in the pathogenesis of androgenetic alopecia.4 Jamaican black castor oil is darker in color and has a more alkaline pH compared with traditional castor oil (both contain ricinoleic acid). To produce Jamaican black castor oil, the seed is roasted, ground to a thick paste, boiled in a pot of hot water, then the oil is skimmed off of the top into individual bottles, whereas regular castor oil is cold pressed. The alkalinity of Jamaican black castor oil may play a role in opening up the hair cuticle, which may be beneficial, but application also needs to be followed by a routine that includes closing the cuticle to avoid increasing hair fragility; such a routine could include, for example, leave-in conditioner or rinsing conditioner with colder water.
Castor oil is sometimes used on its own or in combination with other oils, and it is also an ingredient in some hair care products. However, publicly available peer-reviewed research articles demonstrating the effects of castor oil when applied directly to the hair and scalp for hair growth are needed.
Different types of hair oils are used in African American hair care products and, worldwide, by people of the African diaspora as part of regular hair care and hair styling. Common oils include jojoba, coconut, castor, argan, olive, sunflower, and almond oils. Very curly hair often dries out more easily, especially in drier climates; and sebum build-up on the scalp does not occur as quickly, so hair typically does not need to be shampooed frequently. As such, over-shampooing also often dries the hair out faster, especially if hair has been chemically processed. Thus, oils help to coat and protect the hair, and smooth the cuticle. Oils themselves do not moisturize, but can seal moisture into the hair or act as humectants and draw moisture in. Rather than applying on dry hair, oils, when used as daily care as opposed to before shampooing, are often applied on clean hair after shampooing and after a leave-in conditioner has been applied to help seal in moisture for the most benefit. For treatment of scalp conditions, depending on the type of hair care practice performed at home, oils may be preferred over potentially drying solutions and foams if available, for optimum hair care.
Hair oiling is a long-standing practice which, when used correctly, can be beneficial for hair management and health. There are, however, caveats to hair oiling, which include being careful not to excessively brush or comb hair that has a lot of oil in it because the hair is slick, which can cause hair to fall out during combing. Some oils have elevated levels of lauric acid (coconut oil has 50%), which has a high affinity for hair proteins.5 While this can support hair strength, care should be taken not to overuse some oils or other products known as “protein packs,” which should be used as directed. Since hair is protein, excess protein build-up coating the hair can block needed moisture, making the hair more knotted and brittle, potentially resulting in more breakage with brushing or other hair care practices.
Some oil-containing hair products also contain artificial fragrances and/or dyes, which in some individuals, may promote an immunogenic effect, such as contact dermatitis. Certain oils, particularly olive oil, can promote an environment favorable to yeast growth, especially Malassezia species, implicated in seborrheic dermatitis. Practices that involve excessive application of oil to the scalp can also result in build up if not shampooed regularly. Sulfonated castor oil (also known as Turkey Red oil) has been reported to cause contact dermatitis.6
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Biologic responses to metal implants: Dermatologic implications
Hypersensitivity to implantable devices, albeit rare, is a growing problem. according to a report on biological responses to metal implants released by the Food and Drug Administration in September 2019. Large controlled studies are lacking, and the FDA has initiated extensive postmarketing reviews of certain metal implants in response to safety concerns. Further research is needed on the composition of these implants, the diverse spectrum of metals used, the physical environment in which they are implanted, and the immune response associated with implants.
Local and systemic type IV hypersensitivity reactions can result from exposure to metal ions, which are thought to act as haptens and bind to proteins. The hapten-protein complex acts as the antigen for the T cell. Additionally, both acute and chronic inflammatory responses secondary to wound healing and foreign body reactions can occur. Neutrophils and macrophages elicit a tissue response, which can cause aseptic infection, loosening of joints, and tissue damage. Furthermore, corrosion of metal implants can lead to release of metal ions, which can have genotoxic and carcinogenic effects.
Clinical and subclinical effects of implantable devices depend on the device itself, the composition of the device, the tissue type, and an individual’s immune characteristics. Metal debris released from implants can activate innate and adaptive immune responses through a variety of different mechanisms, depending on the implant type and in what tissues the implant is placed. In the case of orthopedic implants, the most common implants, osteoclasts can sense metal and induce proinflammatory cytokines, which can result in corrosion and uptake of metal particles. Metal devices used in the central nervous system, such as intracerebral electrodes, can cause inflammatory responses leading to tissue encapsulation of electrodes. Corrosion of electrodes and release of metal ions can also impede ion channels in the CNS, blocking critical neuron-signaling pathways. Inflammatory reactions surrounding cardiac and vascular implants containing metal activate coagulation cascades, resulting in endothelial injury and activation of thrombi.
Despite the commonly used term “metal allergy” that delineates a type IV hypersensitivity reaction, reports in the literature supports the existence of both innate and adaptive immune responses to metal implanted in tissues. The recommended terminology is “adverse reactions to metal debris.” The clinical presentation may not be straightforward or easily attributed to the implant. Diagnostic tools are limited and may not detect a causal relationship.
Clinical symptoms can range from local rashes and pruritus to cardiac damage, depression, vertigo, and neurologic symptoms; autoimmune/autoinflammatory reactions including chronic fatigue and autoimmune-like systemic symptoms, such as joint pain, headaches, and hair loss, have also been reported in association with implants containing metal. In addition to pruritus, dermatologic manifestations can include erythema, edema, papules, vesicles, as well as systemic hypersensitivity reactions. Typically, cutaneous reactions usually present within 2 days to 24 months of implantation and may be considered surgical-site infections. Although these reactions can be treated with topical or oral corticosteroids, removal of the device is frequently needed for complete clearance.
In clinical practice, it has been frustrating that potential adverse reactions to metal implants are often overlooked because they are thought to be so rare. There are case series documenting metal implant hypersensitivity, but the actual prevalence of hypersensitivity or autoinflammatory reactions is not known. Testing methods are often inaccurate; therefore, identification of at-risk individuals and management of symptomatic patients with implants is important.
The 2016 American Contact Dermatitis Society guidelines do not recommend preimplantation patch testing unless there is a suspected metal allergy. However, patch testing cannot identify the extent of corrosion, autoinflammatory reactions, and foreign body reactions that can occur.
We must keep an open mind in patients who have implanted devices and have unusual or otherwise undefined symptoms. Often, the symptoms do not directly correspond to the site of implantation and the only way to discern whether the implant is the cause and to treat symptoms is removal of the implanted device.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
Food and Drug Administration. Biological Responses to Metal Implants. 2019 Sep. https://www.fda.gov/media/131150/download.
Atwater AR, Reeder M. Cutis. 2020 Feb;105(2):68-70.
Schalock PC et al. Dermatitis. Sep-Oct 2016;27(5):241-7.
Hypersensitivity to implantable devices, albeit rare, is a growing problem. according to a report on biological responses to metal implants released by the Food and Drug Administration in September 2019. Large controlled studies are lacking, and the FDA has initiated extensive postmarketing reviews of certain metal implants in response to safety concerns. Further research is needed on the composition of these implants, the diverse spectrum of metals used, the physical environment in which they are implanted, and the immune response associated with implants.
Local and systemic type IV hypersensitivity reactions can result from exposure to metal ions, which are thought to act as haptens and bind to proteins. The hapten-protein complex acts as the antigen for the T cell. Additionally, both acute and chronic inflammatory responses secondary to wound healing and foreign body reactions can occur. Neutrophils and macrophages elicit a tissue response, which can cause aseptic infection, loosening of joints, and tissue damage. Furthermore, corrosion of metal implants can lead to release of metal ions, which can have genotoxic and carcinogenic effects.
Clinical and subclinical effects of implantable devices depend on the device itself, the composition of the device, the tissue type, and an individual’s immune characteristics. Metal debris released from implants can activate innate and adaptive immune responses through a variety of different mechanisms, depending on the implant type and in what tissues the implant is placed. In the case of orthopedic implants, the most common implants, osteoclasts can sense metal and induce proinflammatory cytokines, which can result in corrosion and uptake of metal particles. Metal devices used in the central nervous system, such as intracerebral electrodes, can cause inflammatory responses leading to tissue encapsulation of electrodes. Corrosion of electrodes and release of metal ions can also impede ion channels in the CNS, blocking critical neuron-signaling pathways. Inflammatory reactions surrounding cardiac and vascular implants containing metal activate coagulation cascades, resulting in endothelial injury and activation of thrombi.
Despite the commonly used term “metal allergy” that delineates a type IV hypersensitivity reaction, reports in the literature supports the existence of both innate and adaptive immune responses to metal implanted in tissues. The recommended terminology is “adverse reactions to metal debris.” The clinical presentation may not be straightforward or easily attributed to the implant. Diagnostic tools are limited and may not detect a causal relationship.
Clinical symptoms can range from local rashes and pruritus to cardiac damage, depression, vertigo, and neurologic symptoms; autoimmune/autoinflammatory reactions including chronic fatigue and autoimmune-like systemic symptoms, such as joint pain, headaches, and hair loss, have also been reported in association with implants containing metal. In addition to pruritus, dermatologic manifestations can include erythema, edema, papules, vesicles, as well as systemic hypersensitivity reactions. Typically, cutaneous reactions usually present within 2 days to 24 months of implantation and may be considered surgical-site infections. Although these reactions can be treated with topical or oral corticosteroids, removal of the device is frequently needed for complete clearance.
In clinical practice, it has been frustrating that potential adverse reactions to metal implants are often overlooked because they are thought to be so rare. There are case series documenting metal implant hypersensitivity, but the actual prevalence of hypersensitivity or autoinflammatory reactions is not known. Testing methods are often inaccurate; therefore, identification of at-risk individuals and management of symptomatic patients with implants is important.
The 2016 American Contact Dermatitis Society guidelines do not recommend preimplantation patch testing unless there is a suspected metal allergy. However, patch testing cannot identify the extent of corrosion, autoinflammatory reactions, and foreign body reactions that can occur.
We must keep an open mind in patients who have implanted devices and have unusual or otherwise undefined symptoms. Often, the symptoms do not directly correspond to the site of implantation and the only way to discern whether the implant is the cause and to treat symptoms is removal of the implanted device.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
Food and Drug Administration. Biological Responses to Metal Implants. 2019 Sep. https://www.fda.gov/media/131150/download.
Atwater AR, Reeder M. Cutis. 2020 Feb;105(2):68-70.
Schalock PC et al. Dermatitis. Sep-Oct 2016;27(5):241-7.
Hypersensitivity to implantable devices, albeit rare, is a growing problem. according to a report on biological responses to metal implants released by the Food and Drug Administration in September 2019. Large controlled studies are lacking, and the FDA has initiated extensive postmarketing reviews of certain metal implants in response to safety concerns. Further research is needed on the composition of these implants, the diverse spectrum of metals used, the physical environment in which they are implanted, and the immune response associated with implants.
Local and systemic type IV hypersensitivity reactions can result from exposure to metal ions, which are thought to act as haptens and bind to proteins. The hapten-protein complex acts as the antigen for the T cell. Additionally, both acute and chronic inflammatory responses secondary to wound healing and foreign body reactions can occur. Neutrophils and macrophages elicit a tissue response, which can cause aseptic infection, loosening of joints, and tissue damage. Furthermore, corrosion of metal implants can lead to release of metal ions, which can have genotoxic and carcinogenic effects.
Clinical and subclinical effects of implantable devices depend on the device itself, the composition of the device, the tissue type, and an individual’s immune characteristics. Metal debris released from implants can activate innate and adaptive immune responses through a variety of different mechanisms, depending on the implant type and in what tissues the implant is placed. In the case of orthopedic implants, the most common implants, osteoclasts can sense metal and induce proinflammatory cytokines, which can result in corrosion and uptake of metal particles. Metal devices used in the central nervous system, such as intracerebral electrodes, can cause inflammatory responses leading to tissue encapsulation of electrodes. Corrosion of electrodes and release of metal ions can also impede ion channels in the CNS, blocking critical neuron-signaling pathways. Inflammatory reactions surrounding cardiac and vascular implants containing metal activate coagulation cascades, resulting in endothelial injury and activation of thrombi.
Despite the commonly used term “metal allergy” that delineates a type IV hypersensitivity reaction, reports in the literature supports the existence of both innate and adaptive immune responses to metal implanted in tissues. The recommended terminology is “adverse reactions to metal debris.” The clinical presentation may not be straightforward or easily attributed to the implant. Diagnostic tools are limited and may not detect a causal relationship.
Clinical symptoms can range from local rashes and pruritus to cardiac damage, depression, vertigo, and neurologic symptoms; autoimmune/autoinflammatory reactions including chronic fatigue and autoimmune-like systemic symptoms, such as joint pain, headaches, and hair loss, have also been reported in association with implants containing metal. In addition to pruritus, dermatologic manifestations can include erythema, edema, papules, vesicles, as well as systemic hypersensitivity reactions. Typically, cutaneous reactions usually present within 2 days to 24 months of implantation and may be considered surgical-site infections. Although these reactions can be treated with topical or oral corticosteroids, removal of the device is frequently needed for complete clearance.
In clinical practice, it has been frustrating that potential adverse reactions to metal implants are often overlooked because they are thought to be so rare. There are case series documenting metal implant hypersensitivity, but the actual prevalence of hypersensitivity or autoinflammatory reactions is not known. Testing methods are often inaccurate; therefore, identification of at-risk individuals and management of symptomatic patients with implants is important.
The 2016 American Contact Dermatitis Society guidelines do not recommend preimplantation patch testing unless there is a suspected metal allergy. However, patch testing cannot identify the extent of corrosion, autoinflammatory reactions, and foreign body reactions that can occur.
We must keep an open mind in patients who have implanted devices and have unusual or otherwise undefined symptoms. Often, the symptoms do not directly correspond to the site of implantation and the only way to discern whether the implant is the cause and to treat symptoms is removal of the implanted device.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
Food and Drug Administration. Biological Responses to Metal Implants. 2019 Sep. https://www.fda.gov/media/131150/download.
Atwater AR, Reeder M. Cutis. 2020 Feb;105(2):68-70.
Schalock PC et al. Dermatitis. Sep-Oct 2016;27(5):241-7.
Colorism and dermatology
With the world currently really listening and engaged (hopefully) on making positive changes with regards to racism and systemic racial injustices, skin color has come to the forefront. Racism because of skin color has been an unfortunate part of our history and foundation of the United States with a capitalist society built and thriving on the profits of slavery, and a democracy founded on equality – unless you had black skin. These issues are at the forefront in the United States, but have also significantly impacted other parts of the world, including the Caribbean and South America having a significant African slave trade history and impacts, with Brazil currently facing the same systemic racial injustices and police brutality among black men, and King Leopold II of Belgium slaughtering an estimated 10-15 million Congolese people in the name of colonialism, slavery, and robbing resources (natural resources as well as servitude) in the Congo as late as the early 1900s.
These are just a few of the many historical examples of racial injustice, which remains ingrained in many parts of our society today. With this worldwide history, it has been advantageous for people to have lighter skin with regards to money, politics, jobs, education, the justice system, modeling/acting opportunities and contracts, home ownership, and opportunities for generational wealth for years to come. It has ingrained some unfortunate beliefs among some that having lighter skin is better, advantageous, and will make them more desirable or more beautiful.
and across the African continent. It is estimated that 77% of women in Nigeria and 55% of women in China use bleaching creams to achieve overall skin lightening. Unilever’s Fair & Lovely skin-whitening cream has long been a popular over-the-counter product in India, with an estimated market worth of 270 billion rupees ($4 billion USD). On June 25, 2020, Unilever vowed to rename and rebrand Fair & Lovely. With such an offensive name for a product that further promotes colorism, this is an effort in the right direction and has been a long time coming since its debut in 1975. Unilever’s Fair and Lovely Foundation for women’s causes still exists, and has not been renamed at the time of this writing.
Controversy remains on whether this product and other products such as these should exist for the purposes they are used for. Johnson & Johnson has decided that it will no longer produce and sell the Neutrogena Fine Fairness line, sold only in Asia and the Middle East, and the Clean & Clear Fairness line, sold in India. There are arguments to the contrary that halting production of skin-lightening products altogether may result in an influx of unsafe alternatives.
As dermatologists, we use skin-lightening products appropriately for the purposes of treating skin conditions such as postinflammatory hyperpigmentation, melasma, and photoaging. This is where the use of such products should largely end. While it is up to individuals about what they do with their skin and their bodies, we, as health care skin professionals, should be furthering the notion that all skin colors and types are beautiful. Moreover, we should not be encouraging the use of these products for overall skin whitening. Part of the issue is that these products are available often at high concentrations over the counter or in the illegal market, especially in parts of Asia and Africa where colorism is more common and skin whitening is more commonly practiced. The dangers are not only the risk of ochronosis with high concentrations or long term use of hydroquinone, but also what the Centre for Science and Environment found in a 2014 study, that 44% of the skin “fairness” creams in India contained mercury, which is illegal and a health concern.
In my practice, I have also had patients (several originally from Nigeria) who have admitted to long term use of skin-bleaching products for the purposes of all over face- and body-skin lightening who now suffer from very sensitive skin and experience bouts of eczematous dermatitis from time to time, despite having stopped using lightening cream. While there are adverse physical effects resulting from the use of these topicals for this purpose, the effects on the psyche are what concern me the most.
The beauty industry has also been an unfortunate part of furthering thoughts and attitudes concerning colorism over the years with lighter skin and Caucasian ideals being set as standards of beauty. One of many examples is a deodorant ad in the Middle East with the tagline “White is Purity” on a woman, which was pulled by Nivea in 2017 after it was slammed as racist. Another is the 2017 Dove ad for body wash that showed a smiling black woman peel off her brown shirt to reveal a white woman in a lighter-color shirt.
A shift has occurred in recent years with more ethnic images of beauty appearing in magazines and film. However, such opportunities are still less plentiful, pay discrepancies still occur, and sexual objectification of women of color as opposed to beautification is still rampant. As such, it is also up to us to do our part in studying and utilizing ethnic and racial differences in skin and beauty to maximize our efforts in promoting what is inherently beautiful as opposed to one standard of beauty. The education begins with the images we see, what we teach our children, loving ourselves, and as doctors, being knowledgeable about the right aesthetic choices for patients with different skin colors and types.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
With the world currently really listening and engaged (hopefully) on making positive changes with regards to racism and systemic racial injustices, skin color has come to the forefront. Racism because of skin color has been an unfortunate part of our history and foundation of the United States with a capitalist society built and thriving on the profits of slavery, and a democracy founded on equality – unless you had black skin. These issues are at the forefront in the United States, but have also significantly impacted other parts of the world, including the Caribbean and South America having a significant African slave trade history and impacts, with Brazil currently facing the same systemic racial injustices and police brutality among black men, and King Leopold II of Belgium slaughtering an estimated 10-15 million Congolese people in the name of colonialism, slavery, and robbing resources (natural resources as well as servitude) in the Congo as late as the early 1900s.
These are just a few of the many historical examples of racial injustice, which remains ingrained in many parts of our society today. With this worldwide history, it has been advantageous for people to have lighter skin with regards to money, politics, jobs, education, the justice system, modeling/acting opportunities and contracts, home ownership, and opportunities for generational wealth for years to come. It has ingrained some unfortunate beliefs among some that having lighter skin is better, advantageous, and will make them more desirable or more beautiful.
and across the African continent. It is estimated that 77% of women in Nigeria and 55% of women in China use bleaching creams to achieve overall skin lightening. Unilever’s Fair & Lovely skin-whitening cream has long been a popular over-the-counter product in India, with an estimated market worth of 270 billion rupees ($4 billion USD). On June 25, 2020, Unilever vowed to rename and rebrand Fair & Lovely. With such an offensive name for a product that further promotes colorism, this is an effort in the right direction and has been a long time coming since its debut in 1975. Unilever’s Fair and Lovely Foundation for women’s causes still exists, and has not been renamed at the time of this writing.
Controversy remains on whether this product and other products such as these should exist for the purposes they are used for. Johnson & Johnson has decided that it will no longer produce and sell the Neutrogena Fine Fairness line, sold only in Asia and the Middle East, and the Clean & Clear Fairness line, sold in India. There are arguments to the contrary that halting production of skin-lightening products altogether may result in an influx of unsafe alternatives.
As dermatologists, we use skin-lightening products appropriately for the purposes of treating skin conditions such as postinflammatory hyperpigmentation, melasma, and photoaging. This is where the use of such products should largely end. While it is up to individuals about what they do with their skin and their bodies, we, as health care skin professionals, should be furthering the notion that all skin colors and types are beautiful. Moreover, we should not be encouraging the use of these products for overall skin whitening. Part of the issue is that these products are available often at high concentrations over the counter or in the illegal market, especially in parts of Asia and Africa where colorism is more common and skin whitening is more commonly practiced. The dangers are not only the risk of ochronosis with high concentrations or long term use of hydroquinone, but also what the Centre for Science and Environment found in a 2014 study, that 44% of the skin “fairness” creams in India contained mercury, which is illegal and a health concern.
In my practice, I have also had patients (several originally from Nigeria) who have admitted to long term use of skin-bleaching products for the purposes of all over face- and body-skin lightening who now suffer from very sensitive skin and experience bouts of eczematous dermatitis from time to time, despite having stopped using lightening cream. While there are adverse physical effects resulting from the use of these topicals for this purpose, the effects on the psyche are what concern me the most.
The beauty industry has also been an unfortunate part of furthering thoughts and attitudes concerning colorism over the years with lighter skin and Caucasian ideals being set as standards of beauty. One of many examples is a deodorant ad in the Middle East with the tagline “White is Purity” on a woman, which was pulled by Nivea in 2017 after it was slammed as racist. Another is the 2017 Dove ad for body wash that showed a smiling black woman peel off her brown shirt to reveal a white woman in a lighter-color shirt.
A shift has occurred in recent years with more ethnic images of beauty appearing in magazines and film. However, such opportunities are still less plentiful, pay discrepancies still occur, and sexual objectification of women of color as opposed to beautification is still rampant. As such, it is also up to us to do our part in studying and utilizing ethnic and racial differences in skin and beauty to maximize our efforts in promoting what is inherently beautiful as opposed to one standard of beauty. The education begins with the images we see, what we teach our children, loving ourselves, and as doctors, being knowledgeable about the right aesthetic choices for patients with different skin colors and types.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
With the world currently really listening and engaged (hopefully) on making positive changes with regards to racism and systemic racial injustices, skin color has come to the forefront. Racism because of skin color has been an unfortunate part of our history and foundation of the United States with a capitalist society built and thriving on the profits of slavery, and a democracy founded on equality – unless you had black skin. These issues are at the forefront in the United States, but have also significantly impacted other parts of the world, including the Caribbean and South America having a significant African slave trade history and impacts, with Brazil currently facing the same systemic racial injustices and police brutality among black men, and King Leopold II of Belgium slaughtering an estimated 10-15 million Congolese people in the name of colonialism, slavery, and robbing resources (natural resources as well as servitude) in the Congo as late as the early 1900s.
These are just a few of the many historical examples of racial injustice, which remains ingrained in many parts of our society today. With this worldwide history, it has been advantageous for people to have lighter skin with regards to money, politics, jobs, education, the justice system, modeling/acting opportunities and contracts, home ownership, and opportunities for generational wealth for years to come. It has ingrained some unfortunate beliefs among some that having lighter skin is better, advantageous, and will make them more desirable or more beautiful.
and across the African continent. It is estimated that 77% of women in Nigeria and 55% of women in China use bleaching creams to achieve overall skin lightening. Unilever’s Fair & Lovely skin-whitening cream has long been a popular over-the-counter product in India, with an estimated market worth of 270 billion rupees ($4 billion USD). On June 25, 2020, Unilever vowed to rename and rebrand Fair & Lovely. With such an offensive name for a product that further promotes colorism, this is an effort in the right direction and has been a long time coming since its debut in 1975. Unilever’s Fair and Lovely Foundation for women’s causes still exists, and has not been renamed at the time of this writing.
Controversy remains on whether this product and other products such as these should exist for the purposes they are used for. Johnson & Johnson has decided that it will no longer produce and sell the Neutrogena Fine Fairness line, sold only in Asia and the Middle East, and the Clean & Clear Fairness line, sold in India. There are arguments to the contrary that halting production of skin-lightening products altogether may result in an influx of unsafe alternatives.
As dermatologists, we use skin-lightening products appropriately for the purposes of treating skin conditions such as postinflammatory hyperpigmentation, melasma, and photoaging. This is where the use of such products should largely end. While it is up to individuals about what they do with their skin and their bodies, we, as health care skin professionals, should be furthering the notion that all skin colors and types are beautiful. Moreover, we should not be encouraging the use of these products for overall skin whitening. Part of the issue is that these products are available often at high concentrations over the counter or in the illegal market, especially in parts of Asia and Africa where colorism is more common and skin whitening is more commonly practiced. The dangers are not only the risk of ochronosis with high concentrations or long term use of hydroquinone, but also what the Centre for Science and Environment found in a 2014 study, that 44% of the skin “fairness” creams in India contained mercury, which is illegal and a health concern.
In my practice, I have also had patients (several originally from Nigeria) who have admitted to long term use of skin-bleaching products for the purposes of all over face- and body-skin lightening who now suffer from very sensitive skin and experience bouts of eczematous dermatitis from time to time, despite having stopped using lightening cream. While there are adverse physical effects resulting from the use of these topicals for this purpose, the effects on the psyche are what concern me the most.
The beauty industry has also been an unfortunate part of furthering thoughts and attitudes concerning colorism over the years with lighter skin and Caucasian ideals being set as standards of beauty. One of many examples is a deodorant ad in the Middle East with the tagline “White is Purity” on a woman, which was pulled by Nivea in 2017 after it was slammed as racist. Another is the 2017 Dove ad for body wash that showed a smiling black woman peel off her brown shirt to reveal a white woman in a lighter-color shirt.
A shift has occurred in recent years with more ethnic images of beauty appearing in magazines and film. However, such opportunities are still less plentiful, pay discrepancies still occur, and sexual objectification of women of color as opposed to beautification is still rampant. As such, it is also up to us to do our part in studying and utilizing ethnic and racial differences in skin and beauty to maximize our efforts in promoting what is inherently beautiful as opposed to one standard of beauty. The education begins with the images we see, what we teach our children, loving ourselves, and as doctors, being knowledgeable about the right aesthetic choices for patients with different skin colors and types.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Your diet may be aging you
Recent studies have shown a correlation between many dietary elements and skin diseases including acne, rosacea, and perioral dermatitis. and there is now evidence that the aging process can also be slowed with a healthy diet. Previous studies have shown that intake of vegetables, fish, and foods high in vitamin C, carotenoids, olive oil, and linoleic acid are associated with decreased wrinkles.
In a Dutch population-based cohort study published in the Journal of the American Academy of Dermatology in 2019, Mekić et al. investigated the association between diet and facial wrinkles in an elderly population. Facial photographs were used to evaluate wrinkle severity and diet of the participants was assessed with the Food Frequency Questionnaire and adherence to the Dutch Healthy Diet Index (DHDI).
The DHDI is a measure of the ability to adhere to the Dutch Guidelines for a Healthy Diet. The guidelines recommend a daily intake in the diet of at least 200 g of vegetables daily; at least 200 g of fruit; 90 g of brown bread, wholemeal bread, or other whole-grain products; and at least 15 g of unsalted nuts. One serving of fish (preferably oily fish) per week and little to no dairy, alcohol, red meat, cooking fats, and sugar is also recommended.
The study revealed that better adherence to the DHDI was significantly associated with fewer wrinkles among women but not men. Women who ate more animal meat and fats and carbohydrates had more wrinkles than did those with a fruit-dominant diet.
Although other healthy behaviors such as exercise and alcohol are likely to play a role in confounding these data, UV exposure as a cause of wrinkling was accounted for, and in the study, increased outdoor exercise was associated with more wrinkles. Unhealthy food can induce oxidative stress, increased skin and gut inflammation, and glycation, which are some of the physiologic mechanisms suggested to increase wrinkle formation. In contrast, nutrients in fruits and vegetables stimulate collagen production and DNA repair and reduce oxidative stress on the skin.
Nutritional advice is largely rare in internal medicine, cardiology, and even endocrinology. We are developing better ways to assess and understand the way foods interact and cause inflammation of the gut and the body and skin. I highly recommend nutritional education be a part of our residency training programs and to make better guidelines on the prevention of skin disease and aging available for both practitioners and patients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
Mekić S et al. J Am Acad Dermatol. 2019 May;80(5):1358-1363.e2.
Purba MB et al. J Am Coll Nutr. 2001;20(1):71‐80.
van Lee L et al. Nutr J. 2012 Jul 20;11:49.
Kromhout D et al. Eur J Clin Nutr. 2016 Aug;70(8):869‐78.
Recent studies have shown a correlation between many dietary elements and skin diseases including acne, rosacea, and perioral dermatitis. and there is now evidence that the aging process can also be slowed with a healthy diet. Previous studies have shown that intake of vegetables, fish, and foods high in vitamin C, carotenoids, olive oil, and linoleic acid are associated with decreased wrinkles.
In a Dutch population-based cohort study published in the Journal of the American Academy of Dermatology in 2019, Mekić et al. investigated the association between diet and facial wrinkles in an elderly population. Facial photographs were used to evaluate wrinkle severity and diet of the participants was assessed with the Food Frequency Questionnaire and adherence to the Dutch Healthy Diet Index (DHDI).
The DHDI is a measure of the ability to adhere to the Dutch Guidelines for a Healthy Diet. The guidelines recommend a daily intake in the diet of at least 200 g of vegetables daily; at least 200 g of fruit; 90 g of brown bread, wholemeal bread, or other whole-grain products; and at least 15 g of unsalted nuts. One serving of fish (preferably oily fish) per week and little to no dairy, alcohol, red meat, cooking fats, and sugar is also recommended.
The study revealed that better adherence to the DHDI was significantly associated with fewer wrinkles among women but not men. Women who ate more animal meat and fats and carbohydrates had more wrinkles than did those with a fruit-dominant diet.
Although other healthy behaviors such as exercise and alcohol are likely to play a role in confounding these data, UV exposure as a cause of wrinkling was accounted for, and in the study, increased outdoor exercise was associated with more wrinkles. Unhealthy food can induce oxidative stress, increased skin and gut inflammation, and glycation, which are some of the physiologic mechanisms suggested to increase wrinkle formation. In contrast, nutrients in fruits and vegetables stimulate collagen production and DNA repair and reduce oxidative stress on the skin.
Nutritional advice is largely rare in internal medicine, cardiology, and even endocrinology. We are developing better ways to assess and understand the way foods interact and cause inflammation of the gut and the body and skin. I highly recommend nutritional education be a part of our residency training programs and to make better guidelines on the prevention of skin disease and aging available for both practitioners and patients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
Mekić S et al. J Am Acad Dermatol. 2019 May;80(5):1358-1363.e2.
Purba MB et al. J Am Coll Nutr. 2001;20(1):71‐80.
van Lee L et al. Nutr J. 2012 Jul 20;11:49.
Kromhout D et al. Eur J Clin Nutr. 2016 Aug;70(8):869‐78.
Recent studies have shown a correlation between many dietary elements and skin diseases including acne, rosacea, and perioral dermatitis. and there is now evidence that the aging process can also be slowed with a healthy diet. Previous studies have shown that intake of vegetables, fish, and foods high in vitamin C, carotenoids, olive oil, and linoleic acid are associated with decreased wrinkles.
In a Dutch population-based cohort study published in the Journal of the American Academy of Dermatology in 2019, Mekić et al. investigated the association between diet and facial wrinkles in an elderly population. Facial photographs were used to evaluate wrinkle severity and diet of the participants was assessed with the Food Frequency Questionnaire and adherence to the Dutch Healthy Diet Index (DHDI).
The DHDI is a measure of the ability to adhere to the Dutch Guidelines for a Healthy Diet. The guidelines recommend a daily intake in the diet of at least 200 g of vegetables daily; at least 200 g of fruit; 90 g of brown bread, wholemeal bread, or other whole-grain products; and at least 15 g of unsalted nuts. One serving of fish (preferably oily fish) per week and little to no dairy, alcohol, red meat, cooking fats, and sugar is also recommended.
The study revealed that better adherence to the DHDI was significantly associated with fewer wrinkles among women but not men. Women who ate more animal meat and fats and carbohydrates had more wrinkles than did those with a fruit-dominant diet.
Although other healthy behaviors such as exercise and alcohol are likely to play a role in confounding these data, UV exposure as a cause of wrinkling was accounted for, and in the study, increased outdoor exercise was associated with more wrinkles. Unhealthy food can induce oxidative stress, increased skin and gut inflammation, and glycation, which are some of the physiologic mechanisms suggested to increase wrinkle formation. In contrast, nutrients in fruits and vegetables stimulate collagen production and DNA repair and reduce oxidative stress on the skin.
Nutritional advice is largely rare in internal medicine, cardiology, and even endocrinology. We are developing better ways to assess and understand the way foods interact and cause inflammation of the gut and the body and skin. I highly recommend nutritional education be a part of our residency training programs and to make better guidelines on the prevention of skin disease and aging available for both practitioners and patients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
Mekić S et al. J Am Acad Dermatol. 2019 May;80(5):1358-1363.e2.
Purba MB et al. J Am Coll Nutr. 2001;20(1):71‐80.
van Lee L et al. Nutr J. 2012 Jul 20;11:49.
Kromhout D et al. Eur J Clin Nutr. 2016 Aug;70(8):869‐78.
Laser surgery precautions as clinics begin to reopen amid COVID-19
Protective measures recommended for cosmetic procedures have recently been published by Dover et al. in Facial Plastic Surgery & Aesthetic Medicine. The manuscript, titled “A path to resume aesthetic care Project AesCert Guidance Supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” provides thorough, detailed recommendations on all aspects of protection and preparedness for aesthetic clinical practices.
in this uncharted territory. During the last pandemic, the 1918 Spanish flu, caused by an H1N1 virus, laser procedures didn’t exist. Discussion among dermatologists and laser surgeons, including the aforementioned publication, have led to the following initial office recommendations (subject to change).
Office preparation and safety including:
- Prescreening patients for symptoms.
- Social distancing in the office, including waiting room areas (or eliminating waiting areas and bringing patients into exam rooms upon arrival).
- Decreasing patient load and increasing length of appointment times.
- Having no additional visitors during patient appointments, unless necessary (minor, caregiver).
- Patients wearing masks to appointments and hand washing/sanitizing upon arrival/departure.
- Providers wearing appropriate personal protective equipment (PPE) during visits.
- Instituting office disinfectant checklists.
For nonablative laser surgery specifically, especially for therapy of the face and neck, recommendations include the following:
- Lasers and office areas are thoroughly sanitized between each procedure.
- Providers wear appropriate PPE, including N95 masks if possible, wraparound safety glasses, gloves, as well as strong consideration of face shields).
- The duration and number of procedures should be limited, as should intraprocedure conversations and close face-to-face proximity with patient’s airways.
- Lasers with increased plume, including laser tattoo removal and laser hair removal, are the procedures with the most concern with regards to viral particle or infection transmission.
PPE is recommended (including masks – N95 if available – gloves, and face shield), as well as evacuator suction systems of the two-stage filtration type, and/or negative room pressure if available. For air-filtration evacuator suction systems, the device vacuum must be held within 2 inches of the treatment area for the best efficacy. Some have suggested performing laser tattoo removal through a hydrogel patch to help eliminate plume, which may also increase the cost of the procedure and may depend on the availability of the patches themselves. Nothing has been published on the use of the hydrogel patch in laser hair removal. Shaving or trimming of hairs prior to the procedure is critical.
While pulse dye and intense pulsed light (IPL) lasers have generally been deemed safer to use during the COVID-19 pandemic – with appropriate protective gear and general office precautions – I would recommend being mindful of potential plume created when using these lasers in hair-bearing areas. IPL is generally avoided in these regions, unless specific filters are used for hair removal treatment. But if use an IPL in a hair-bearing region, shaving or trimming of the hairs with the above precautions should be done first to reduce plume. As with all face-to-face procedures, the above PPE, contact, and intraprocedure conversation precautions should be taken.
Nonablative fractional resurfacing lasers are areas in which more questions lie. Some providers are comfortable performing nonablative fractional lasers with protective gear and air filtration systems, while others are recommending delaying these procedures until more information is available. The question essentially involves whether infection risk is higher with these procedures because of plume and if depth of penetration of the laser can release viral particles.
In addition to the other precautions above, with the high transmissibility of COVID-19, I would recommend considering precleansing the treatment area with soap and water or a sterile prep that won’t irritate the skin, which has activity against coronaviruses. A study by Kampf et al. demonstrated that coronaviruses can persist on surfaces such as metal, glass, or plastic for up to 9 days (human skin surface unknown) but can be effectively inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents that may be more tolerable on the skin surface, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate were less effective. Washing the face with soap and water may be the most tolerated and easiest cleansing method. Face-to-face respiratory transmission should be mitigated by the aforementioned methods.
Ablative laser surgery
Most laser surgeons agree that ablative laser surgery procedures should likely be delayed until the virus has waned more, because of the increased invasiveness of and recovery of wound healing from the procedure. There is increased evidence of SARS-CoV-2 infecting endothelial cells, raising concern about transmission via blood. A study of the cardiovascular manifestations seen in COVID-19 infection, published in The Lancet, showed the virus directly targets the endothelial cells that line blood vessels. Ablative laser surgery (fractional and fully ablative) is associated with blood or serous fluid on the skin surface immediately after the procedure and for up to 5-7 days post procedure, particularly with Er:Yag than with the CO2 laser. Antibacterial and antiviral prophylaxis often is used with these procedures. While the aforementioned protocols for other nonablative lasers may help with ablative laser treatment, there is currently no known effective and available antiviral prophylactic medication against SARS-CoV-2, if needed.
PPE
Personal protective equipment shortages are still a concern. Many hospitals are sterilizing and reusing traditionally disposable N95 masks in the inpatient setting, which is unprecedented. Resterilization will likely be necessary in outpatient medical offices as well, if the supply of masks does not increase. The supply chain will be a factor in considering PPE use in outpatient offices affecting the availability of PPE for emergency medicine, inpatient hospital, and ICU providers in direct contact with known COVID-19 patients.
With asymptomatic spread and the lack of adequate testing for COVID-19, as practices reopen, all practitioners will be on the front lines and should treat their practice and protect their patients, staff and themselves as such.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They have no relevant disclosures.
References:
Dover JS et al. Facial Plast Surg Aesthet Med. 2020 May 5. doi: 10.1089/fpsam.2020.0239.
Kampf G et al. J Hosp Infect. 2020 Mar;104(3):246-51.
Varga Z et al. Lancet. 2020 May 2;395(10234):1417-8.
Protective measures recommended for cosmetic procedures have recently been published by Dover et al. in Facial Plastic Surgery & Aesthetic Medicine. The manuscript, titled “A path to resume aesthetic care Project AesCert Guidance Supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” provides thorough, detailed recommendations on all aspects of protection and preparedness for aesthetic clinical practices.
in this uncharted territory. During the last pandemic, the 1918 Spanish flu, caused by an H1N1 virus, laser procedures didn’t exist. Discussion among dermatologists and laser surgeons, including the aforementioned publication, have led to the following initial office recommendations (subject to change).
Office preparation and safety including:
- Prescreening patients for symptoms.
- Social distancing in the office, including waiting room areas (or eliminating waiting areas and bringing patients into exam rooms upon arrival).
- Decreasing patient load and increasing length of appointment times.
- Having no additional visitors during patient appointments, unless necessary (minor, caregiver).
- Patients wearing masks to appointments and hand washing/sanitizing upon arrival/departure.
- Providers wearing appropriate personal protective equipment (PPE) during visits.
- Instituting office disinfectant checklists.
For nonablative laser surgery specifically, especially for therapy of the face and neck, recommendations include the following:
- Lasers and office areas are thoroughly sanitized between each procedure.
- Providers wear appropriate PPE, including N95 masks if possible, wraparound safety glasses, gloves, as well as strong consideration of face shields).
- The duration and number of procedures should be limited, as should intraprocedure conversations and close face-to-face proximity with patient’s airways.
- Lasers with increased plume, including laser tattoo removal and laser hair removal, are the procedures with the most concern with regards to viral particle or infection transmission.
PPE is recommended (including masks – N95 if available – gloves, and face shield), as well as evacuator suction systems of the two-stage filtration type, and/or negative room pressure if available. For air-filtration evacuator suction systems, the device vacuum must be held within 2 inches of the treatment area for the best efficacy. Some have suggested performing laser tattoo removal through a hydrogel patch to help eliminate plume, which may also increase the cost of the procedure and may depend on the availability of the patches themselves. Nothing has been published on the use of the hydrogel patch in laser hair removal. Shaving or trimming of hairs prior to the procedure is critical.
While pulse dye and intense pulsed light (IPL) lasers have generally been deemed safer to use during the COVID-19 pandemic – with appropriate protective gear and general office precautions – I would recommend being mindful of potential plume created when using these lasers in hair-bearing areas. IPL is generally avoided in these regions, unless specific filters are used for hair removal treatment. But if use an IPL in a hair-bearing region, shaving or trimming of the hairs with the above precautions should be done first to reduce plume. As with all face-to-face procedures, the above PPE, contact, and intraprocedure conversation precautions should be taken.
Nonablative fractional resurfacing lasers are areas in which more questions lie. Some providers are comfortable performing nonablative fractional lasers with protective gear and air filtration systems, while others are recommending delaying these procedures until more information is available. The question essentially involves whether infection risk is higher with these procedures because of plume and if depth of penetration of the laser can release viral particles.
In addition to the other precautions above, with the high transmissibility of COVID-19, I would recommend considering precleansing the treatment area with soap and water or a sterile prep that won’t irritate the skin, which has activity against coronaviruses. A study by Kampf et al. demonstrated that coronaviruses can persist on surfaces such as metal, glass, or plastic for up to 9 days (human skin surface unknown) but can be effectively inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents that may be more tolerable on the skin surface, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate were less effective. Washing the face with soap and water may be the most tolerated and easiest cleansing method. Face-to-face respiratory transmission should be mitigated by the aforementioned methods.
Ablative laser surgery
Most laser surgeons agree that ablative laser surgery procedures should likely be delayed until the virus has waned more, because of the increased invasiveness of and recovery of wound healing from the procedure. There is increased evidence of SARS-CoV-2 infecting endothelial cells, raising concern about transmission via blood. A study of the cardiovascular manifestations seen in COVID-19 infection, published in The Lancet, showed the virus directly targets the endothelial cells that line blood vessels. Ablative laser surgery (fractional and fully ablative) is associated with blood or serous fluid on the skin surface immediately after the procedure and for up to 5-7 days post procedure, particularly with Er:Yag than with the CO2 laser. Antibacterial and antiviral prophylaxis often is used with these procedures. While the aforementioned protocols for other nonablative lasers may help with ablative laser treatment, there is currently no known effective and available antiviral prophylactic medication against SARS-CoV-2, if needed.
PPE
Personal protective equipment shortages are still a concern. Many hospitals are sterilizing and reusing traditionally disposable N95 masks in the inpatient setting, which is unprecedented. Resterilization will likely be necessary in outpatient medical offices as well, if the supply of masks does not increase. The supply chain will be a factor in considering PPE use in outpatient offices affecting the availability of PPE for emergency medicine, inpatient hospital, and ICU providers in direct contact with known COVID-19 patients.
With asymptomatic spread and the lack of adequate testing for COVID-19, as practices reopen, all practitioners will be on the front lines and should treat their practice and protect their patients, staff and themselves as such.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They have no relevant disclosures.
References:
Dover JS et al. Facial Plast Surg Aesthet Med. 2020 May 5. doi: 10.1089/fpsam.2020.0239.
Kampf G et al. J Hosp Infect. 2020 Mar;104(3):246-51.
Varga Z et al. Lancet. 2020 May 2;395(10234):1417-8.
Protective measures recommended for cosmetic procedures have recently been published by Dover et al. in Facial Plastic Surgery & Aesthetic Medicine. The manuscript, titled “A path to resume aesthetic care Project AesCert Guidance Supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” provides thorough, detailed recommendations on all aspects of protection and preparedness for aesthetic clinical practices.
in this uncharted territory. During the last pandemic, the 1918 Spanish flu, caused by an H1N1 virus, laser procedures didn’t exist. Discussion among dermatologists and laser surgeons, including the aforementioned publication, have led to the following initial office recommendations (subject to change).
Office preparation and safety including:
- Prescreening patients for symptoms.
- Social distancing in the office, including waiting room areas (or eliminating waiting areas and bringing patients into exam rooms upon arrival).
- Decreasing patient load and increasing length of appointment times.
- Having no additional visitors during patient appointments, unless necessary (minor, caregiver).
- Patients wearing masks to appointments and hand washing/sanitizing upon arrival/departure.
- Providers wearing appropriate personal protective equipment (PPE) during visits.
- Instituting office disinfectant checklists.
For nonablative laser surgery specifically, especially for therapy of the face and neck, recommendations include the following:
- Lasers and office areas are thoroughly sanitized between each procedure.
- Providers wear appropriate PPE, including N95 masks if possible, wraparound safety glasses, gloves, as well as strong consideration of face shields).
- The duration and number of procedures should be limited, as should intraprocedure conversations and close face-to-face proximity with patient’s airways.
- Lasers with increased plume, including laser tattoo removal and laser hair removal, are the procedures with the most concern with regards to viral particle or infection transmission.
PPE is recommended (including masks – N95 if available – gloves, and face shield), as well as evacuator suction systems of the two-stage filtration type, and/or negative room pressure if available. For air-filtration evacuator suction systems, the device vacuum must be held within 2 inches of the treatment area for the best efficacy. Some have suggested performing laser tattoo removal through a hydrogel patch to help eliminate plume, which may also increase the cost of the procedure and may depend on the availability of the patches themselves. Nothing has been published on the use of the hydrogel patch in laser hair removal. Shaving or trimming of hairs prior to the procedure is critical.
While pulse dye and intense pulsed light (IPL) lasers have generally been deemed safer to use during the COVID-19 pandemic – with appropriate protective gear and general office precautions – I would recommend being mindful of potential plume created when using these lasers in hair-bearing areas. IPL is generally avoided in these regions, unless specific filters are used for hair removal treatment. But if use an IPL in a hair-bearing region, shaving or trimming of the hairs with the above precautions should be done first to reduce plume. As with all face-to-face procedures, the above PPE, contact, and intraprocedure conversation precautions should be taken.
Nonablative fractional resurfacing lasers are areas in which more questions lie. Some providers are comfortable performing nonablative fractional lasers with protective gear and air filtration systems, while others are recommending delaying these procedures until more information is available. The question essentially involves whether infection risk is higher with these procedures because of plume and if depth of penetration of the laser can release viral particles.
In addition to the other precautions above, with the high transmissibility of COVID-19, I would recommend considering precleansing the treatment area with soap and water or a sterile prep that won’t irritate the skin, which has activity against coronaviruses. A study by Kampf et al. demonstrated that coronaviruses can persist on surfaces such as metal, glass, or plastic for up to 9 days (human skin surface unknown) but can be effectively inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents that may be more tolerable on the skin surface, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate were less effective. Washing the face with soap and water may be the most tolerated and easiest cleansing method. Face-to-face respiratory transmission should be mitigated by the aforementioned methods.
Ablative laser surgery
Most laser surgeons agree that ablative laser surgery procedures should likely be delayed until the virus has waned more, because of the increased invasiveness of and recovery of wound healing from the procedure. There is increased evidence of SARS-CoV-2 infecting endothelial cells, raising concern about transmission via blood. A study of the cardiovascular manifestations seen in COVID-19 infection, published in The Lancet, showed the virus directly targets the endothelial cells that line blood vessels. Ablative laser surgery (fractional and fully ablative) is associated with blood or serous fluid on the skin surface immediately after the procedure and for up to 5-7 days post procedure, particularly with Er:Yag than with the CO2 laser. Antibacterial and antiviral prophylaxis often is used with these procedures. While the aforementioned protocols for other nonablative lasers may help with ablative laser treatment, there is currently no known effective and available antiviral prophylactic medication against SARS-CoV-2, if needed.
PPE
Personal protective equipment shortages are still a concern. Many hospitals are sterilizing and reusing traditionally disposable N95 masks in the inpatient setting, which is unprecedented. Resterilization will likely be necessary in outpatient medical offices as well, if the supply of masks does not increase. The supply chain will be a factor in considering PPE use in outpatient offices affecting the availability of PPE for emergency medicine, inpatient hospital, and ICU providers in direct contact with known COVID-19 patients.
With asymptomatic spread and the lack of adequate testing for COVID-19, as practices reopen, all practitioners will be on the front lines and should treat their practice and protect their patients, staff and themselves as such.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They have no relevant disclosures.
References:
Dover JS et al. Facial Plast Surg Aesthet Med. 2020 May 5. doi: 10.1089/fpsam.2020.0239.
Kampf G et al. J Hosp Infect. 2020 Mar;104(3):246-51.
Varga Z et al. Lancet. 2020 May 2;395(10234):1417-8.
The resurgence of Plaquenil (hydroxychloroquine)
Two of the most unusual dermatologic drugs have resurged as possible first-line therapy for rescue treatment of hospitalized patients with SARS-CoV-2, despite extremely limited clinical data supporting their efficacy, optimal dose, treatment duration, and potential adverse effects.
Chloroquine and hydroxychloroquine were introduced as treatment and prophylaxis of malaria and approved by the Food and Drug Administration in 1949 and 1955, respectively. They belong to a class of drugs called 4-aminoquinolones and have a flat aromatic core and a basic side chain. The basic property of these drugs contribute to their ability to accumulate in lysosomes. They have a large volume of distribution in the blood and a half-life of 40-60 days. Important interactions include use with tamoxifen, proton pump inhibitors, and with smoking. Although both drugs cross the placenta, they don’t have any notable effects on the fetus.
Chloroquine and hydroxychloroquine enter the cell and accumulate in the lysosomes along a pH gradient. Within the lysosome, they increase the pH, thereby stabilizing lysosomes and inhibiting eosinophil and neutrophil chemotaxis and phagocytic activity. They also inhibit complement-mediated hemolysis, reduce acute phase reactants, and prevent MHC class II–mediated auto antigen presentation. Additionally, they decrease cell-mediated immunity by decreasing the production of interleukin-1 and plasma cell synthesis. Hydroxychloroquine can also accumulate in endosomes and inhibit toll-like receptor signaling, thereby reducing the production of proinflammatory cytokines.
One of the ways SARS-CoV-2 enters cells is by up-regulating and binding to ACE2. Chloroquine/hydroxychloroquine reduce glycosylation of ACE2 and thus inhibit viral entry. Additionally, by increasing the endosomal pH, they potentially inactivate enzymes that viruses require for replication. Their lifesaving benefits, however, are thought to involve blocking the proinflammatory cytokine IL-6 and suppressing the cytokine storm thought to induce acute respiratory distress syndrome. Interestingly, chloroquine has also been shown to allow zinc ions into the cell, and zinc is a potent inhibitor of coronavirus RNA polymerase.
Side effects of chloroquine and hydroxychloroquine include GI upset, retinal toxicity with long-term use, hypoglycemia, cardiomyopathy, QT prolongation, ventricular arrhythmias, and renal and liver toxicity. Adverse effects have been observed with long-term daily doses of more than 3.5 mg/kg of chloroquine or more than 6.5 mg/kg of hydroxychloroquine. Cutaneous effects include pruritus, morbilliform rashes (in an estimated 10% of those treated) and psoriasis flares, and blue-black hyperpigmentation (in about 25%) of the shins, face, oral palate, and nails.
Initial In February 2020, the first clinical results of 100 patients treated with chloroquine were reported in a news briefing by the Chinese government. On March 20, the first clinical trial was published offering guidelines for the treatment of COVID-19 using hydroxychloroquine and azithromycin combination therapy – albeit with many limitations and reported biases in the study. Despite the poorly designed studies and inconclusive evidence, on March 28, the FDA issued an Emergency Use Authorization that allows providers to request a supply of hydroxychloroquine or chloroquine for hospitalized patients with COVID-19 who are unable to join a clinical trial.
On April 2, the first clinical trial to evaluate the safety and efficacy of hydroxychloroquine in adults hospitalized with COVID-19 began at Vanderbilt University Medical Center, Nashville, Tenn. The ORCHID trial (Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease), funded by the National Heart, Lung, and Blood Institute. This blinded, placebo-controlled study is evaluating hydroxychloroquine treatment of hospitalized patients with COVID-19 in hopes of treating the severe complications of acute respiratory distress syndrome. Participants are randomly assigned to receive 400 mg hydroxychloroquine twice daily as a loading dose and then 200 mg twice daily thereafter on days 2-5. As of this writing, this study is currently underway and outcomes are expected in the upcoming weeks.
There is now a shortage of chloroquine and hydroxychloroquine in patients who have severe dermatologic and rheumatologic diseases, which include some who been in remission for years because of these medications and are in grave danger of recurrence. During this crisis, we desperately need well-controlled, randomized studies to test the efficacy and prolonged safety profile of these drugs in COVID-19 patients, as well as appropriate funding to source these medications for hospitalized and nonhospitalized patients in need.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at dermnews@mdedge.com.
Sources
Liu J et al. Cell Discov. 2020 Mar 18. doi: 10.1038/s41421-020-0156-0.
Vincent MJ et al. Virol J. 2005 Aug 22;2:69.
Gautret P et al. Int J Antimicrob Agents. 2020 Mar 20. doi: 10.1016/j.ijantimicag.2020.105949.
Devaux CA et al. Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Aronson J et al. COVID-19 trials registered up to 8 March 2020 – an analysis of 382 studies. 2020. Centre for Evidence-Based Medicine. https://www.cebm.net/oxford-covid-19/covid-19-registered-trials-and-analysis/
Savarino A et al. Lancet Infect Dis. 2003 Nov;3(11):722-7.
Yazdany J, Kim AHJ. Ann Intern Med. 2020 Mar 31. doi: 10.7326/M20-1334.
Xue J et al. PLoS One. 2014 Oct 1;9(10):e109180.
te Velthuis AJ et al. PLoS Pathog. 2010 Nov 4;6(11):e1001176.
Two of the most unusual dermatologic drugs have resurged as possible first-line therapy for rescue treatment of hospitalized patients with SARS-CoV-2, despite extremely limited clinical data supporting their efficacy, optimal dose, treatment duration, and potential adverse effects.
Chloroquine and hydroxychloroquine were introduced as treatment and prophylaxis of malaria and approved by the Food and Drug Administration in 1949 and 1955, respectively. They belong to a class of drugs called 4-aminoquinolones and have a flat aromatic core and a basic side chain. The basic property of these drugs contribute to their ability to accumulate in lysosomes. They have a large volume of distribution in the blood and a half-life of 40-60 days. Important interactions include use with tamoxifen, proton pump inhibitors, and with smoking. Although both drugs cross the placenta, they don’t have any notable effects on the fetus.
Chloroquine and hydroxychloroquine enter the cell and accumulate in the lysosomes along a pH gradient. Within the lysosome, they increase the pH, thereby stabilizing lysosomes and inhibiting eosinophil and neutrophil chemotaxis and phagocytic activity. They also inhibit complement-mediated hemolysis, reduce acute phase reactants, and prevent MHC class II–mediated auto antigen presentation. Additionally, they decrease cell-mediated immunity by decreasing the production of interleukin-1 and plasma cell synthesis. Hydroxychloroquine can also accumulate in endosomes and inhibit toll-like receptor signaling, thereby reducing the production of proinflammatory cytokines.
One of the ways SARS-CoV-2 enters cells is by up-regulating and binding to ACE2. Chloroquine/hydroxychloroquine reduce glycosylation of ACE2 and thus inhibit viral entry. Additionally, by increasing the endosomal pH, they potentially inactivate enzymes that viruses require for replication. Their lifesaving benefits, however, are thought to involve blocking the proinflammatory cytokine IL-6 and suppressing the cytokine storm thought to induce acute respiratory distress syndrome. Interestingly, chloroquine has also been shown to allow zinc ions into the cell, and zinc is a potent inhibitor of coronavirus RNA polymerase.
Side effects of chloroquine and hydroxychloroquine include GI upset, retinal toxicity with long-term use, hypoglycemia, cardiomyopathy, QT prolongation, ventricular arrhythmias, and renal and liver toxicity. Adverse effects have been observed with long-term daily doses of more than 3.5 mg/kg of chloroquine or more than 6.5 mg/kg of hydroxychloroquine. Cutaneous effects include pruritus, morbilliform rashes (in an estimated 10% of those treated) and psoriasis flares, and blue-black hyperpigmentation (in about 25%) of the shins, face, oral palate, and nails.
Initial In February 2020, the first clinical results of 100 patients treated with chloroquine were reported in a news briefing by the Chinese government. On March 20, the first clinical trial was published offering guidelines for the treatment of COVID-19 using hydroxychloroquine and azithromycin combination therapy – albeit with many limitations and reported biases in the study. Despite the poorly designed studies and inconclusive evidence, on March 28, the FDA issued an Emergency Use Authorization that allows providers to request a supply of hydroxychloroquine or chloroquine for hospitalized patients with COVID-19 who are unable to join a clinical trial.
On April 2, the first clinical trial to evaluate the safety and efficacy of hydroxychloroquine in adults hospitalized with COVID-19 began at Vanderbilt University Medical Center, Nashville, Tenn. The ORCHID trial (Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease), funded by the National Heart, Lung, and Blood Institute. This blinded, placebo-controlled study is evaluating hydroxychloroquine treatment of hospitalized patients with COVID-19 in hopes of treating the severe complications of acute respiratory distress syndrome. Participants are randomly assigned to receive 400 mg hydroxychloroquine twice daily as a loading dose and then 200 mg twice daily thereafter on days 2-5. As of this writing, this study is currently underway and outcomes are expected in the upcoming weeks.
There is now a shortage of chloroquine and hydroxychloroquine in patients who have severe dermatologic and rheumatologic diseases, which include some who been in remission for years because of these medications and are in grave danger of recurrence. During this crisis, we desperately need well-controlled, randomized studies to test the efficacy and prolonged safety profile of these drugs in COVID-19 patients, as well as appropriate funding to source these medications for hospitalized and nonhospitalized patients in need.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at dermnews@mdedge.com.
Sources
Liu J et al. Cell Discov. 2020 Mar 18. doi: 10.1038/s41421-020-0156-0.
Vincent MJ et al. Virol J. 2005 Aug 22;2:69.
Gautret P et al. Int J Antimicrob Agents. 2020 Mar 20. doi: 10.1016/j.ijantimicag.2020.105949.
Devaux CA et al. Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Aronson J et al. COVID-19 trials registered up to 8 March 2020 – an analysis of 382 studies. 2020. Centre for Evidence-Based Medicine. https://www.cebm.net/oxford-covid-19/covid-19-registered-trials-and-analysis/
Savarino A et al. Lancet Infect Dis. 2003 Nov;3(11):722-7.
Yazdany J, Kim AHJ. Ann Intern Med. 2020 Mar 31. doi: 10.7326/M20-1334.
Xue J et al. PLoS One. 2014 Oct 1;9(10):e109180.
te Velthuis AJ et al. PLoS Pathog. 2010 Nov 4;6(11):e1001176.
Two of the most unusual dermatologic drugs have resurged as possible first-line therapy for rescue treatment of hospitalized patients with SARS-CoV-2, despite extremely limited clinical data supporting their efficacy, optimal dose, treatment duration, and potential adverse effects.
Chloroquine and hydroxychloroquine were introduced as treatment and prophylaxis of malaria and approved by the Food and Drug Administration in 1949 and 1955, respectively. They belong to a class of drugs called 4-aminoquinolones and have a flat aromatic core and a basic side chain. The basic property of these drugs contribute to their ability to accumulate in lysosomes. They have a large volume of distribution in the blood and a half-life of 40-60 days. Important interactions include use with tamoxifen, proton pump inhibitors, and with smoking. Although both drugs cross the placenta, they don’t have any notable effects on the fetus.
Chloroquine and hydroxychloroquine enter the cell and accumulate in the lysosomes along a pH gradient. Within the lysosome, they increase the pH, thereby stabilizing lysosomes and inhibiting eosinophil and neutrophil chemotaxis and phagocytic activity. They also inhibit complement-mediated hemolysis, reduce acute phase reactants, and prevent MHC class II–mediated auto antigen presentation. Additionally, they decrease cell-mediated immunity by decreasing the production of interleukin-1 and plasma cell synthesis. Hydroxychloroquine can also accumulate in endosomes and inhibit toll-like receptor signaling, thereby reducing the production of proinflammatory cytokines.
One of the ways SARS-CoV-2 enters cells is by up-regulating and binding to ACE2. Chloroquine/hydroxychloroquine reduce glycosylation of ACE2 and thus inhibit viral entry. Additionally, by increasing the endosomal pH, they potentially inactivate enzymes that viruses require for replication. Their lifesaving benefits, however, are thought to involve blocking the proinflammatory cytokine IL-6 and suppressing the cytokine storm thought to induce acute respiratory distress syndrome. Interestingly, chloroquine has also been shown to allow zinc ions into the cell, and zinc is a potent inhibitor of coronavirus RNA polymerase.
Side effects of chloroquine and hydroxychloroquine include GI upset, retinal toxicity with long-term use, hypoglycemia, cardiomyopathy, QT prolongation, ventricular arrhythmias, and renal and liver toxicity. Adverse effects have been observed with long-term daily doses of more than 3.5 mg/kg of chloroquine or more than 6.5 mg/kg of hydroxychloroquine. Cutaneous effects include pruritus, morbilliform rashes (in an estimated 10% of those treated) and psoriasis flares, and blue-black hyperpigmentation (in about 25%) of the shins, face, oral palate, and nails.
Initial In February 2020, the first clinical results of 100 patients treated with chloroquine were reported in a news briefing by the Chinese government. On March 20, the first clinical trial was published offering guidelines for the treatment of COVID-19 using hydroxychloroquine and azithromycin combination therapy – albeit with many limitations and reported biases in the study. Despite the poorly designed studies and inconclusive evidence, on March 28, the FDA issued an Emergency Use Authorization that allows providers to request a supply of hydroxychloroquine or chloroquine for hospitalized patients with COVID-19 who are unable to join a clinical trial.
On April 2, the first clinical trial to evaluate the safety and efficacy of hydroxychloroquine in adults hospitalized with COVID-19 began at Vanderbilt University Medical Center, Nashville, Tenn. The ORCHID trial (Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease), funded by the National Heart, Lung, and Blood Institute. This blinded, placebo-controlled study is evaluating hydroxychloroquine treatment of hospitalized patients with COVID-19 in hopes of treating the severe complications of acute respiratory distress syndrome. Participants are randomly assigned to receive 400 mg hydroxychloroquine twice daily as a loading dose and then 200 mg twice daily thereafter on days 2-5. As of this writing, this study is currently underway and outcomes are expected in the upcoming weeks.
There is now a shortage of chloroquine and hydroxychloroquine in patients who have severe dermatologic and rheumatologic diseases, which include some who been in remission for years because of these medications and are in grave danger of recurrence. During this crisis, we desperately need well-controlled, randomized studies to test the efficacy and prolonged safety profile of these drugs in COVID-19 patients, as well as appropriate funding to source these medications for hospitalized and nonhospitalized patients in need.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at dermnews@mdedge.com.
Sources
Liu J et al. Cell Discov. 2020 Mar 18. doi: 10.1038/s41421-020-0156-0.
Vincent MJ et al. Virol J. 2005 Aug 22;2:69.
Gautret P et al. Int J Antimicrob Agents. 2020 Mar 20. doi: 10.1016/j.ijantimicag.2020.105949.
Devaux CA et al. Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Aronson J et al. COVID-19 trials registered up to 8 March 2020 – an analysis of 382 studies. 2020. Centre for Evidence-Based Medicine. https://www.cebm.net/oxford-covid-19/covid-19-registered-trials-and-analysis/
Savarino A et al. Lancet Infect Dis. 2003 Nov;3(11):722-7.
Yazdany J, Kim AHJ. Ann Intern Med. 2020 Mar 31. doi: 10.7326/M20-1334.
Xue J et al. PLoS One. 2014 Oct 1;9(10):e109180.
te Velthuis AJ et al. PLoS Pathog. 2010 Nov 4;6(11):e1001176.
Hand washing and hand sanitizer on the skin and COVID-19 infection risk
As we deal with the effects of the COVID-19 pandemic, hand washing and the use of hand sanitizers have been key for infection prevention. With drier, colder weather in many of the communities initially affected by COVID-19, skin was already prone to dryness and a skin barrier compromised, and hand eczema was more prevalent because of these factors alone. This article explores the while maintaining the maximum possible degree of infection prevention.
With many viruses, including coronavirus, the virus is a self-assembled nanoparticle in which the most vulnerable structure is the outer lipid bilayer. Soaps dissolve the lipid membrane and the virus breaks apart, inactivating it; they are also alkaline surfactants that pick up particles – including dirt, bacteria, and viruses – which are removed from the surface of the skin when the soaps are rinsed off. In the process of washing, the alkalinity of the soap (pH approximately 9-10), compared with the normal outer skin pH of approximately 5.5 or lower, also can affect the skin barrier as well as the resident skin microflora. In a study by Lambers et al., it was found that an acid skin pH (4-4.5) keeps the resident bacterial flora attached to the skin, whereas an alkaline pH (8-9) promotes the dispersal from the skin in assessments of the volar forearm.
With regard to the effectiveness of hand washing against viruses, the length of time spent hand washing has been shown to have an impact on influenza-like illness. In a recent study of 2,082 participants by Bin Abdulrahman et al., those who spent only 5-10 seconds hand washing with soap and hand rubbing were at a higher risk of more frequent influenza-like illness (odds ratio, 1.37; 95% confidence interval, 1.08-1.75), compared with those who washed their hands for 15 seconds or longer. Moreover, hand washing with soap and rubbing after shaking hands was found to be an independent protective factor against frequent influenza-like illness (adjusted OR, 0.59; 95% confidence interval, 0.37-0.94). Previous studies on the impact of hand washing on bacterial and parasitic illnesses also found similar results: Hand washing for 15-20 seconds or longer reduces infection.
Alcohol, long known as a disinfectant, has been recommended for disinfecting the hands since the late 1800s. Most alcohol-based hand antiseptics contain isopropanol, ethanol, N-propanol, or a combination of two of these products. The antimicrobial activity of alcohols can be attributed to their ability to denature and coagulate proteins, thereby lysing microorganisms’ cells, and disrupting their cellular metabolism. Alcohol solutions containing 60%-95% alcohol are the most effective. Notably, very high concentrations of alcohol are less potent because less water is found in higher concentrations of alcohol and proteins are not denatured easily in the absence of water. Alcohol-based hand sanitizers also often contain humectants, such as glycerin and/or aloe vera, to help prevent skin dryness and replace water content that is stripped by the use of alcohol on the skin surface.
Other topical disinfectants can also be used to inactivate coronaviruses from surfaces, including the skin. A recently published analysis of 22 studies found that human coronaviruses – such as severe acute respiratory syndrome (SARS) coronavirus, Middle East respiratory syndrome (MERS) coronavirus, or endemic human coronaviruses (HCoV) – can persist on inanimate surfaces such as metal, glass, or plastic for up to 9 days (COVID-19 was found in a study to persist on metal for up to 2-3 days), but can be efficiently inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate, are less effective.
In the case of SARS, treatment of SARS-CoV with povidone-iodine products for 2 minutes reduced virus infectivity to below the detectable level, equivalent to the effect of ethanol, in one study. Formalin fixation of the infected cells and heating the virus to 56° C, as used in routine tissue processing, were found to inactivate several coronaviruses as well. Based on this information, ethanol-based hand sanitizers, typically containing ethanol content of 60% or higher, can be used to inactivate coronaviruses on the skin, including COVID-19.
In patients with influenza-virus infections, whether pathogens were in wet or dried mucus played a role in whether hand washing or rubbing with hand sanitizer was more effective. In a study that examined the effects of hand washing versus antiseptic hand rubbing with an ethanol-based hand disinfectant on inactivation of influenza A virus adhered to the hands, the investigators showed that the effectiveness of the ethanol-based disinfectant against influenza A virus in mucus was reduced, compared with influenza A virus in saline. Influenza A in mucus remained active, despite 120 seconds of hand rubbing with hand sanitizer; however, influenza A in saline was completely inactivated within 30 seconds. Interestingly, rubbing hands with an ethanol-based disinfectant inactivated influenza A virus in mucus within 30 seconds with mucus that had dried completely because the hydrogel characteristics had been eliminated. Hand washing rapidly inactivated influenza A virus whether in mucus form, saline, or dried mucous.
It is important to note that in COVID-19 infections, a productive cough or rhinorrhea are not as common compared with dry cough. Regardless, the findings of the study described above should be considered if mucous symptoms develop during a COVID-19 infection when determining infection control. Luckily, with COVID-19, both hand washing and use of an ethanol-based hand sanitizer are seemingly effective in inactivating the virus or removing it from the skin surface.
After frequent hand washing, we all can experience dryness and potentially cracked skin as well. With hand sanitizer, the alcohol content can also cause burning of skin, especially compromised skin.
Vanilloid receptor-1 (VR1), a heat-gated ion channel, is responsible for the burning sensation caused by capsaicin. Ethanol lowers the amount of heat needed to turn on VR1 nocioceptive pain receptors by almost ten degrees, resulting in a potential burning sensation when applied.
Nails are affected as well with frequent hand washing and/or application of hand sanitizer and can become cracked or brittle. Contact dermatitis, both irritant and allergic, can occur with increased use of disinfectants, particularly household cleaners without proper barrier protection.
We’ve previously mentioned the effect of hand washing disrupting the resident skin microflora. Maintaining the skin microflora and barrier is an important component of skin health for preventing both dermatitis and infection. Hand washing or use of hand sanitizer is of paramount importance and effective in infection control for COVID-19. To maintain skin health and the skin barrier, applying lotion or cream after hand washing is recommended. It is recommended to avoid scrubbing hands while washing, since this causes breaks in the skin. Using water that is too hot is not recommended as it can inflame the skin further and disrupt the skin barrier.
Wearing gloves, if possible, is recommended when using household disinfectant products to further decrease skin irritation, barrier disruption, and risk of contact dermatitis. I have found hand emollients that contain ceramides or ingredients higher in omega 6 fatty acids, such as borage seed oil or other oils high in linoleic acid content, to be helpful. In addition to improving the skin barrier, emollients and perhaps those with topical pre- or probiotics, may help restore the skin microflora, potentially improving infection control further. Application of hand moisturizer each time after hand washing to maintain better infection control and barrier protection was also recommended by the recent consensus statement of Chinese experts on protection of skin and mucous membrane barrier for health care workers fighting against COVID-19.
We and our patients have remarked how it seems like our hands have aged 20-50 years in the previous 2 weeks. No one is complaining, everyone understands that protecting themselves and others against a potentially lethal virus is paramount. Maintaining skin health is of secondary concern, but maintaining healthy skin may also protect the skin barrier, another important component of potential infection control.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. They had no relevant disclosures. Write to them at dermnews@mdedge.com.
Resources
Lambers H et al. Int J Cosmet Sci. 2006 Oct;28(5):359-70.
Bin Abdulrahman AK et al. BMC Public Health. 2019 Oct 22;19(1):1324. doi: 10.1186/s12889-019-77.
Kariwa H et al. Dermatology. 2006;212 Suppl 1:119-23.
HIrose R et al. mSphere. 2019 Sep 18;4(5). pii: e00474-19. doi: 10.1128/mSphere.00474-19.
Trevisani M et al. Nat Neurosci. 2002 Jun;5(6):546-51.
Yan Y et al. Dermatol Ther. 2020 Mar 13:e13310. doi: 10.1111/dth.13310.
As we deal with the effects of the COVID-19 pandemic, hand washing and the use of hand sanitizers have been key for infection prevention. With drier, colder weather in many of the communities initially affected by COVID-19, skin was already prone to dryness and a skin barrier compromised, and hand eczema was more prevalent because of these factors alone. This article explores the while maintaining the maximum possible degree of infection prevention.
With many viruses, including coronavirus, the virus is a self-assembled nanoparticle in which the most vulnerable structure is the outer lipid bilayer. Soaps dissolve the lipid membrane and the virus breaks apart, inactivating it; they are also alkaline surfactants that pick up particles – including dirt, bacteria, and viruses – which are removed from the surface of the skin when the soaps are rinsed off. In the process of washing, the alkalinity of the soap (pH approximately 9-10), compared with the normal outer skin pH of approximately 5.5 or lower, also can affect the skin barrier as well as the resident skin microflora. In a study by Lambers et al., it was found that an acid skin pH (4-4.5) keeps the resident bacterial flora attached to the skin, whereas an alkaline pH (8-9) promotes the dispersal from the skin in assessments of the volar forearm.
With regard to the effectiveness of hand washing against viruses, the length of time spent hand washing has been shown to have an impact on influenza-like illness. In a recent study of 2,082 participants by Bin Abdulrahman et al., those who spent only 5-10 seconds hand washing with soap and hand rubbing were at a higher risk of more frequent influenza-like illness (odds ratio, 1.37; 95% confidence interval, 1.08-1.75), compared with those who washed their hands for 15 seconds or longer. Moreover, hand washing with soap and rubbing after shaking hands was found to be an independent protective factor against frequent influenza-like illness (adjusted OR, 0.59; 95% confidence interval, 0.37-0.94). Previous studies on the impact of hand washing on bacterial and parasitic illnesses also found similar results: Hand washing for 15-20 seconds or longer reduces infection.
Alcohol, long known as a disinfectant, has been recommended for disinfecting the hands since the late 1800s. Most alcohol-based hand antiseptics contain isopropanol, ethanol, N-propanol, or a combination of two of these products. The antimicrobial activity of alcohols can be attributed to their ability to denature and coagulate proteins, thereby lysing microorganisms’ cells, and disrupting their cellular metabolism. Alcohol solutions containing 60%-95% alcohol are the most effective. Notably, very high concentrations of alcohol are less potent because less water is found in higher concentrations of alcohol and proteins are not denatured easily in the absence of water. Alcohol-based hand sanitizers also often contain humectants, such as glycerin and/or aloe vera, to help prevent skin dryness and replace water content that is stripped by the use of alcohol on the skin surface.
Other topical disinfectants can also be used to inactivate coronaviruses from surfaces, including the skin. A recently published analysis of 22 studies found that human coronaviruses – such as severe acute respiratory syndrome (SARS) coronavirus, Middle East respiratory syndrome (MERS) coronavirus, or endemic human coronaviruses (HCoV) – can persist on inanimate surfaces such as metal, glass, or plastic for up to 9 days (COVID-19 was found in a study to persist on metal for up to 2-3 days), but can be efficiently inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate, are less effective.
In the case of SARS, treatment of SARS-CoV with povidone-iodine products for 2 minutes reduced virus infectivity to below the detectable level, equivalent to the effect of ethanol, in one study. Formalin fixation of the infected cells and heating the virus to 56° C, as used in routine tissue processing, were found to inactivate several coronaviruses as well. Based on this information, ethanol-based hand sanitizers, typically containing ethanol content of 60% or higher, can be used to inactivate coronaviruses on the skin, including COVID-19.
In patients with influenza-virus infections, whether pathogens were in wet or dried mucus played a role in whether hand washing or rubbing with hand sanitizer was more effective. In a study that examined the effects of hand washing versus antiseptic hand rubbing with an ethanol-based hand disinfectant on inactivation of influenza A virus adhered to the hands, the investigators showed that the effectiveness of the ethanol-based disinfectant against influenza A virus in mucus was reduced, compared with influenza A virus in saline. Influenza A in mucus remained active, despite 120 seconds of hand rubbing with hand sanitizer; however, influenza A in saline was completely inactivated within 30 seconds. Interestingly, rubbing hands with an ethanol-based disinfectant inactivated influenza A virus in mucus within 30 seconds with mucus that had dried completely because the hydrogel characteristics had been eliminated. Hand washing rapidly inactivated influenza A virus whether in mucus form, saline, or dried mucous.
It is important to note that in COVID-19 infections, a productive cough or rhinorrhea are not as common compared with dry cough. Regardless, the findings of the study described above should be considered if mucous symptoms develop during a COVID-19 infection when determining infection control. Luckily, with COVID-19, both hand washing and use of an ethanol-based hand sanitizer are seemingly effective in inactivating the virus or removing it from the skin surface.
After frequent hand washing, we all can experience dryness and potentially cracked skin as well. With hand sanitizer, the alcohol content can also cause burning of skin, especially compromised skin.
Vanilloid receptor-1 (VR1), a heat-gated ion channel, is responsible for the burning sensation caused by capsaicin. Ethanol lowers the amount of heat needed to turn on VR1 nocioceptive pain receptors by almost ten degrees, resulting in a potential burning sensation when applied.
Nails are affected as well with frequent hand washing and/or application of hand sanitizer and can become cracked or brittle. Contact dermatitis, both irritant and allergic, can occur with increased use of disinfectants, particularly household cleaners without proper barrier protection.
We’ve previously mentioned the effect of hand washing disrupting the resident skin microflora. Maintaining the skin microflora and barrier is an important component of skin health for preventing both dermatitis and infection. Hand washing or use of hand sanitizer is of paramount importance and effective in infection control for COVID-19. To maintain skin health and the skin barrier, applying lotion or cream after hand washing is recommended. It is recommended to avoid scrubbing hands while washing, since this causes breaks in the skin. Using water that is too hot is not recommended as it can inflame the skin further and disrupt the skin barrier.
Wearing gloves, if possible, is recommended when using household disinfectant products to further decrease skin irritation, barrier disruption, and risk of contact dermatitis. I have found hand emollients that contain ceramides or ingredients higher in omega 6 fatty acids, such as borage seed oil or other oils high in linoleic acid content, to be helpful. In addition to improving the skin barrier, emollients and perhaps those with topical pre- or probiotics, may help restore the skin microflora, potentially improving infection control further. Application of hand moisturizer each time after hand washing to maintain better infection control and barrier protection was also recommended by the recent consensus statement of Chinese experts on protection of skin and mucous membrane barrier for health care workers fighting against COVID-19.
We and our patients have remarked how it seems like our hands have aged 20-50 years in the previous 2 weeks. No one is complaining, everyone understands that protecting themselves and others against a potentially lethal virus is paramount. Maintaining skin health is of secondary concern, but maintaining healthy skin may also protect the skin barrier, another important component of potential infection control.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. They had no relevant disclosures. Write to them at dermnews@mdedge.com.
Resources
Lambers H et al. Int J Cosmet Sci. 2006 Oct;28(5):359-70.
Bin Abdulrahman AK et al. BMC Public Health. 2019 Oct 22;19(1):1324. doi: 10.1186/s12889-019-77.
Kariwa H et al. Dermatology. 2006;212 Suppl 1:119-23.
HIrose R et al. mSphere. 2019 Sep 18;4(5). pii: e00474-19. doi: 10.1128/mSphere.00474-19.
Trevisani M et al. Nat Neurosci. 2002 Jun;5(6):546-51.
Yan Y et al. Dermatol Ther. 2020 Mar 13:e13310. doi: 10.1111/dth.13310.
As we deal with the effects of the COVID-19 pandemic, hand washing and the use of hand sanitizers have been key for infection prevention. With drier, colder weather in many of the communities initially affected by COVID-19, skin was already prone to dryness and a skin barrier compromised, and hand eczema was more prevalent because of these factors alone. This article explores the while maintaining the maximum possible degree of infection prevention.
With many viruses, including coronavirus, the virus is a self-assembled nanoparticle in which the most vulnerable structure is the outer lipid bilayer. Soaps dissolve the lipid membrane and the virus breaks apart, inactivating it; they are also alkaline surfactants that pick up particles – including dirt, bacteria, and viruses – which are removed from the surface of the skin when the soaps are rinsed off. In the process of washing, the alkalinity of the soap (pH approximately 9-10), compared with the normal outer skin pH of approximately 5.5 or lower, also can affect the skin barrier as well as the resident skin microflora. In a study by Lambers et al., it was found that an acid skin pH (4-4.5) keeps the resident bacterial flora attached to the skin, whereas an alkaline pH (8-9) promotes the dispersal from the skin in assessments of the volar forearm.
With regard to the effectiveness of hand washing against viruses, the length of time spent hand washing has been shown to have an impact on influenza-like illness. In a recent study of 2,082 participants by Bin Abdulrahman et al., those who spent only 5-10 seconds hand washing with soap and hand rubbing were at a higher risk of more frequent influenza-like illness (odds ratio, 1.37; 95% confidence interval, 1.08-1.75), compared with those who washed their hands for 15 seconds or longer. Moreover, hand washing with soap and rubbing after shaking hands was found to be an independent protective factor against frequent influenza-like illness (adjusted OR, 0.59; 95% confidence interval, 0.37-0.94). Previous studies on the impact of hand washing on bacterial and parasitic illnesses also found similar results: Hand washing for 15-20 seconds or longer reduces infection.
Alcohol, long known as a disinfectant, has been recommended for disinfecting the hands since the late 1800s. Most alcohol-based hand antiseptics contain isopropanol, ethanol, N-propanol, or a combination of two of these products. The antimicrobial activity of alcohols can be attributed to their ability to denature and coagulate proteins, thereby lysing microorganisms’ cells, and disrupting their cellular metabolism. Alcohol solutions containing 60%-95% alcohol are the most effective. Notably, very high concentrations of alcohol are less potent because less water is found in higher concentrations of alcohol and proteins are not denatured easily in the absence of water. Alcohol-based hand sanitizers also often contain humectants, such as glycerin and/or aloe vera, to help prevent skin dryness and replace water content that is stripped by the use of alcohol on the skin surface.
Other topical disinfectants can also be used to inactivate coronaviruses from surfaces, including the skin. A recently published analysis of 22 studies found that human coronaviruses – such as severe acute respiratory syndrome (SARS) coronavirus, Middle East respiratory syndrome (MERS) coronavirus, or endemic human coronaviruses (HCoV) – can persist on inanimate surfaces such as metal, glass, or plastic for up to 9 days (COVID-19 was found in a study to persist on metal for up to 2-3 days), but can be efficiently inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate, are less effective.
In the case of SARS, treatment of SARS-CoV with povidone-iodine products for 2 minutes reduced virus infectivity to below the detectable level, equivalent to the effect of ethanol, in one study. Formalin fixation of the infected cells and heating the virus to 56° C, as used in routine tissue processing, were found to inactivate several coronaviruses as well. Based on this information, ethanol-based hand sanitizers, typically containing ethanol content of 60% or higher, can be used to inactivate coronaviruses on the skin, including COVID-19.
In patients with influenza-virus infections, whether pathogens were in wet or dried mucus played a role in whether hand washing or rubbing with hand sanitizer was more effective. In a study that examined the effects of hand washing versus antiseptic hand rubbing with an ethanol-based hand disinfectant on inactivation of influenza A virus adhered to the hands, the investigators showed that the effectiveness of the ethanol-based disinfectant against influenza A virus in mucus was reduced, compared with influenza A virus in saline. Influenza A in mucus remained active, despite 120 seconds of hand rubbing with hand sanitizer; however, influenza A in saline was completely inactivated within 30 seconds. Interestingly, rubbing hands with an ethanol-based disinfectant inactivated influenza A virus in mucus within 30 seconds with mucus that had dried completely because the hydrogel characteristics had been eliminated. Hand washing rapidly inactivated influenza A virus whether in mucus form, saline, or dried mucous.
It is important to note that in COVID-19 infections, a productive cough or rhinorrhea are not as common compared with dry cough. Regardless, the findings of the study described above should be considered if mucous symptoms develop during a COVID-19 infection when determining infection control. Luckily, with COVID-19, both hand washing and use of an ethanol-based hand sanitizer are seemingly effective in inactivating the virus or removing it from the skin surface.
After frequent hand washing, we all can experience dryness and potentially cracked skin as well. With hand sanitizer, the alcohol content can also cause burning of skin, especially compromised skin.
Vanilloid receptor-1 (VR1), a heat-gated ion channel, is responsible for the burning sensation caused by capsaicin. Ethanol lowers the amount of heat needed to turn on VR1 nocioceptive pain receptors by almost ten degrees, resulting in a potential burning sensation when applied.
Nails are affected as well with frequent hand washing and/or application of hand sanitizer and can become cracked or brittle. Contact dermatitis, both irritant and allergic, can occur with increased use of disinfectants, particularly household cleaners without proper barrier protection.
We’ve previously mentioned the effect of hand washing disrupting the resident skin microflora. Maintaining the skin microflora and barrier is an important component of skin health for preventing both dermatitis and infection. Hand washing or use of hand sanitizer is of paramount importance and effective in infection control for COVID-19. To maintain skin health and the skin barrier, applying lotion or cream after hand washing is recommended. It is recommended to avoid scrubbing hands while washing, since this causes breaks in the skin. Using water that is too hot is not recommended as it can inflame the skin further and disrupt the skin barrier.
Wearing gloves, if possible, is recommended when using household disinfectant products to further decrease skin irritation, barrier disruption, and risk of contact dermatitis. I have found hand emollients that contain ceramides or ingredients higher in omega 6 fatty acids, such as borage seed oil or other oils high in linoleic acid content, to be helpful. In addition to improving the skin barrier, emollients and perhaps those with topical pre- or probiotics, may help restore the skin microflora, potentially improving infection control further. Application of hand moisturizer each time after hand washing to maintain better infection control and barrier protection was also recommended by the recent consensus statement of Chinese experts on protection of skin and mucous membrane barrier for health care workers fighting against COVID-19.
We and our patients have remarked how it seems like our hands have aged 20-50 years in the previous 2 weeks. No one is complaining, everyone understands that protecting themselves and others against a potentially lethal virus is paramount. Maintaining skin health is of secondary concern, but maintaining healthy skin may also protect the skin barrier, another important component of potential infection control.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. They had no relevant disclosures. Write to them at dermnews@mdedge.com.
Resources
Lambers H et al. Int J Cosmet Sci. 2006 Oct;28(5):359-70.
Bin Abdulrahman AK et al. BMC Public Health. 2019 Oct 22;19(1):1324. doi: 10.1186/s12889-019-77.
Kariwa H et al. Dermatology. 2006;212 Suppl 1:119-23.
HIrose R et al. mSphere. 2019 Sep 18;4(5). pii: e00474-19. doi: 10.1128/mSphere.00474-19.
Trevisani M et al. Nat Neurosci. 2002 Jun;5(6):546-51.
Yan Y et al. Dermatol Ther. 2020 Mar 13:e13310. doi: 10.1111/dth.13310.
Vascular occlusion management
The time course and proper management of vascular occlusion attributable to interarterial hyaluronic acid fillers is critical. Albeit a rare complication, off-label uses of HA fillers, lack of proper training of injectors, and lack of clear appropriate guidelines in the management of these complications are some of the causes of delayed treatment and necrotic complications.
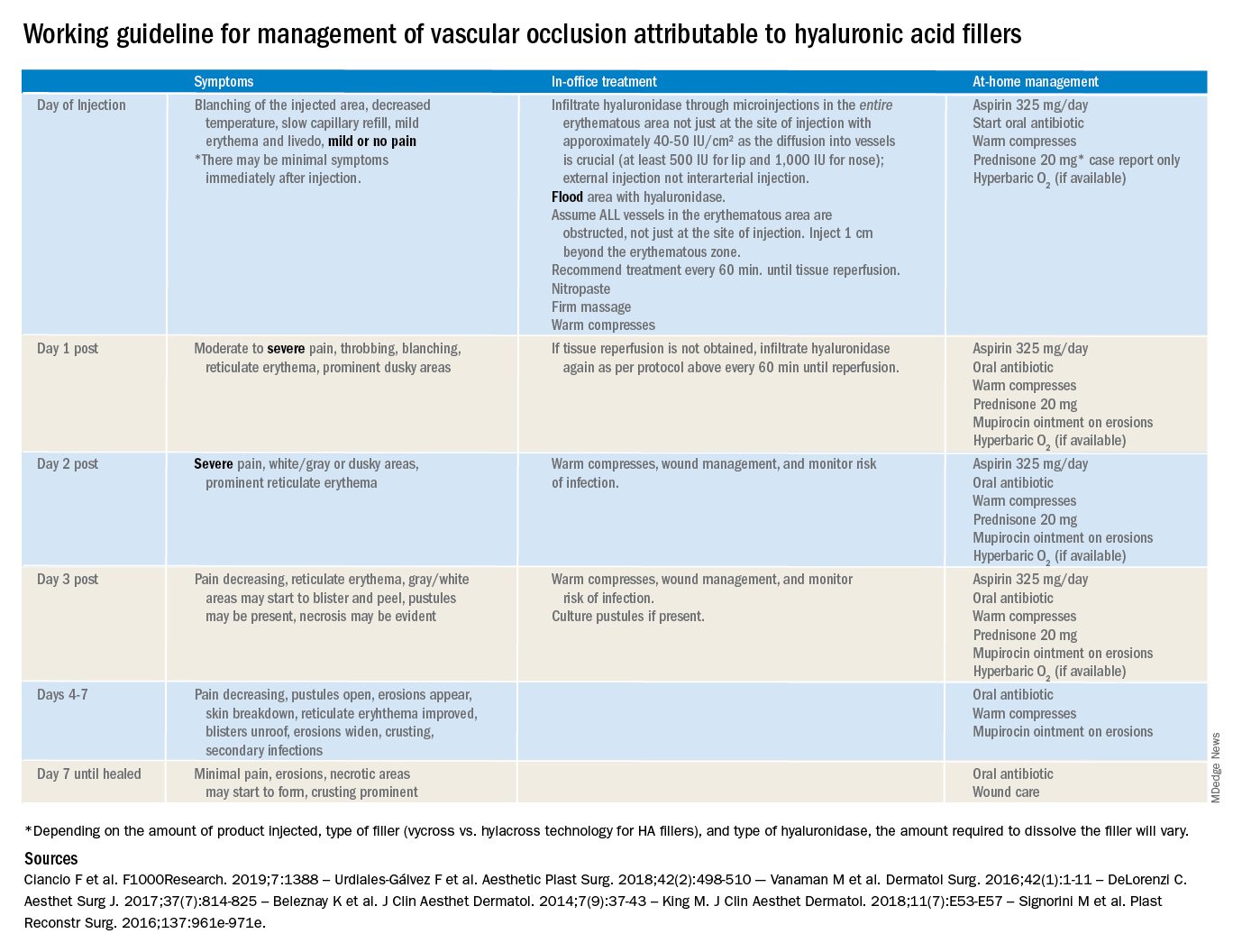
There are currently no definitive guidelines for the management of filler-associated cutaneous necrosis as experience with its treatment continues to evolve and be reported. In an attempt to consolidate the published data, as well as to give somewhat of a clear guideline of expectations, a time course and treatment guide has been outlined. The following is a working guideline for management of vascular occlusion attributable to HA fillers based on reports in the literature. This is not a consensus statement, rather it is a consolidation of the anecdotal reports and case studies outlined to help practitioners. It is also not inclusive of all the presentations of vascular occlusion. There are delayed cases of vascular occlusion beginning several days after injection, as well as alternative treatment options that may be considered.
These guidelines also are not for the devastating complication of blindness because of vascular occlusion secondary to fillers. Blindness is beyond the scope of the current article; however, we believe all experienced injectors should have emergency preparations in place and a relationship with an ophthalmologist or other trained surgeons experienced in performing retrobulbar hyaluronidase injections who can be reached in the event of a suspected occlusion. Any symptoms of eye pain, headache, or visual changes need to be immediately treated. Vascular occlusion is an emergency and timing is critical to prevent permanent blindness and facial deformities.
As with all filler injections, risks and complications can happen, and we cannot stress enough the appropriate level of training, as well as expert understanding of anatomy and injection technique, in minimizing potential risks. We encourage regulations and a required level of training to perform these procedures.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
The time course and proper management of vascular occlusion attributable to interarterial hyaluronic acid fillers is critical. Albeit a rare complication, off-label uses of HA fillers, lack of proper training of injectors, and lack of clear appropriate guidelines in the management of these complications are some of the causes of delayed treatment and necrotic complications.

There are currently no definitive guidelines for the management of filler-associated cutaneous necrosis as experience with its treatment continues to evolve and be reported. In an attempt to consolidate the published data, as well as to give somewhat of a clear guideline of expectations, a time course and treatment guide has been outlined. The following is a working guideline for management of vascular occlusion attributable to HA fillers based on reports in the literature. This is not a consensus statement, rather it is a consolidation of the anecdotal reports and case studies outlined to help practitioners. It is also not inclusive of all the presentations of vascular occlusion. There are delayed cases of vascular occlusion beginning several days after injection, as well as alternative treatment options that may be considered.
These guidelines also are not for the devastating complication of blindness because of vascular occlusion secondary to fillers. Blindness is beyond the scope of the current article; however, we believe all experienced injectors should have emergency preparations in place and a relationship with an ophthalmologist or other trained surgeons experienced in performing retrobulbar hyaluronidase injections who can be reached in the event of a suspected occlusion. Any symptoms of eye pain, headache, or visual changes need to be immediately treated. Vascular occlusion is an emergency and timing is critical to prevent permanent blindness and facial deformities.
As with all filler injections, risks and complications can happen, and we cannot stress enough the appropriate level of training, as well as expert understanding of anatomy and injection technique, in minimizing potential risks. We encourage regulations and a required level of training to perform these procedures.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
The time course and proper management of vascular occlusion attributable to interarterial hyaluronic acid fillers is critical. Albeit a rare complication, off-label uses of HA fillers, lack of proper training of injectors, and lack of clear appropriate guidelines in the management of these complications are some of the causes of delayed treatment and necrotic complications.

There are currently no definitive guidelines for the management of filler-associated cutaneous necrosis as experience with its treatment continues to evolve and be reported. In an attempt to consolidate the published data, as well as to give somewhat of a clear guideline of expectations, a time course and treatment guide has been outlined. The following is a working guideline for management of vascular occlusion attributable to HA fillers based on reports in the literature. This is not a consensus statement, rather it is a consolidation of the anecdotal reports and case studies outlined to help practitioners. It is also not inclusive of all the presentations of vascular occlusion. There are delayed cases of vascular occlusion beginning several days after injection, as well as alternative treatment options that may be considered.
These guidelines also are not for the devastating complication of blindness because of vascular occlusion secondary to fillers. Blindness is beyond the scope of the current article; however, we believe all experienced injectors should have emergency preparations in place and a relationship with an ophthalmologist or other trained surgeons experienced in performing retrobulbar hyaluronidase injections who can be reached in the event of a suspected occlusion. Any symptoms of eye pain, headache, or visual changes need to be immediately treated. Vascular occlusion is an emergency and timing is critical to prevent permanent blindness and facial deformities.
As with all filler injections, risks and complications can happen, and we cannot stress enough the appropriate level of training, as well as expert understanding of anatomy and injection technique, in minimizing potential risks. We encourage regulations and a required level of training to perform these procedures.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
CD1a and cosmetic-related contact dermatitis
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
Makeup is contaminated with pathogenic bacteria
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.








