User login
2023 Update on cervical disease
Cervical cancer was the most common cancer killer of persons with a cervix in the early 1900s in the United States. Widespread adoption of the Pap test in the mid-20th century followed by large-scale outreach through programs such as the National Breast and Cervical Cancer Early Detection Program have dramatically reduced deaths from cervical cancer. The development of a highly effective vaccine that targets human papillomavirus (HPV), the virus implicated in all cervical cancers, has made prevention even more accessible and attainable. Primary prevention with HPV vaccination in conjunction with regular screening as recommended by current guidelines is the most effective way we can prevent cervical cancer.
Despite these advances, the incidence and death rates from cervical cancer have plateaued over the last decade.1 Additionally, many fear that due to the poor attendance at screening visits since the beginning of the COVID-19 pandemic, the incidence might further rise in the United States.2 Among those in the United States diagnosed with cervical cancer, more than 50% have not been screened in over 5 years or had their abnormal results not managed as recommended by current guidelines, suggesting that operational and access issues are contributors to incident cervical cancer. In addition, HPV vaccination rates have increased only slightly from year to year. According to the most recent data from the Centers for Disease Control and Prevention (CDC), coverage with 1 or more doses of HPV vaccine in 2021 increased only by 1.8% and has stagnated, with administration to about 75% of those for whom it is recommended.3 The plateauing will limit our ability to eradicate cervical cancer in the United States, permitting death from a largely preventable disease.
Establishing the framework for the eradication of cervical cancer
The World Health Organization (WHO) adopted a global strategy called the Cervical Cancer Elimination Initiative in August 2020. This initiative is a multipronged effort that focuses on vaccination (90% of girls fully vaccinated by age 15), screening (70% of women screened by age 35 with an effective test and again at age 45), and treatment (90% treatment of precancer and 90% management of women with invasive cancer).4
These are the numbers we need to achieve if all countries are to reach a cervical cancer incidence of less than 4 per 100,000 persons with a cervix. The WHO further suggests that each country should meet the “90-70-90” targets by 2030 if we are to achieve the low incidence by the turn of the century.4 To date, few regions of the world have achieved these goals, and sadly the United States is not among them.
In response to this call to action, many medical and policymaking organizations are taking inventory and implementing strategies to achieve the WHO 2030 targets for cervical cancer eradication. In the United States, the Society of Gynecologic Oncology (SGO; www.sgo.org), the American Society for Colposcopy and Cervical Pathology (ASCCP; www.ASCCP.org), the American College of Obstetricians and Gynecologists (ACOG; www.acog.org), the American Cancer Society (ACS; www.cancer.org), and many others have initiated programs in a collaborative esprit de corps with the aim of eradicating this deadly disease.
In this Update, we review several studies with evidence of screening and management strategies that show promise of accelerating the eradication of cervical cancer.
Continue to: Transitioning to primary HPV screening in the United States...
Transitioning to primary HPV screening in the United States
Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
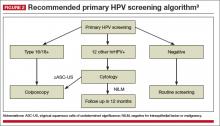
The American Cancer Society released an updated cervical cancer screening guideline in July 2020 that recommended testing for HPV as the preferred strategy. Reasons behind the change, moving away from a Pap test as part of the initial screen, are:
- increased sensitivity of primary HPV testing when compared with conventional cervical cytology (Pap test)
- improved risk stratification to identify who is at risk for cervical cancer now and in the future
- improved efficiency in identifying those who need colposcopy, thus limiting unnecessary procedures without increasing the risk of false-negative tests, thereby missing cervical precancer or invasive cancer.
Some countries with organized screening programs have already made the switch. Self-sampling for HPV is currently being considered for an approved use in the United States, further improving access to screening for cervical cancer when the initial step can be completed by the patient at home or simplified in nontraditional health care settings.2
ACS initiative created to address barriers to primary HPV testing
Challenges to primary HPV testing remain, including laboratory implementation, payment, and operationalizing clinical workflow (for example, HPV testing with reflex cytology instead of cytology with reflex HPV testing).5 There are undoubtedly other unforeseen barriers in the current US health care environment.
In a recent commentary, Downs and colleagues described how the ACS has convened the Primary HPV Screening Initiative (PHSI), nested under the ACS National Roundtable on Cervical Cancer, which is charged with identifying critical barriers to, and opportunities for, transitioning to primary HPV screening.5 The deliverable will be a roadmap with tools and recommendations to support health systems, laboratories, providers, patients, and payers as they make this evolution.
Work groups will develop resources
Patients, particularly those who have had routine cervical cancer screening over their lifetime, also will be curious about the changes in recommendations. The Provider Needs Workgroup within the PHSI structure will develop tools and patient education materials regarding the data, workflow, benefits, and safety of this new paradigm for cervical cancer screening.
Laboratories that process and interpret tests likely will bear the heaviest load of changes. For example, not all commercially available HPV tests in the United States are approved by the US Food and Drug Administration (FDA) for primary HPV testing. Some sites will need to adapt their equipment to ensure adherence to FDA-approved tests. Laboratory workflows will need to be altered for aliquots to be tested for HPV first, and the remainder for cytology. Quality assurance and accreditation requirements for testing will need modifications, and further efforts will be needed to ensure sufficient numbers of trained cytopathologists, whose workforce is rapidly declining, for processing and reading cervical cytology.
In addition, payment for HPV testing alone, without the need for a Pap test, might not be supported by payers that support safety-net providers and sites, who arguably serve the most vulnerable patients and those most at risk for cervical cancer. Collaboration across medical professionals, societies, payers, and policymakers will provide a critical infrastructure to make the change in the most seamless fashion and limit the harm from missed opportunities for screening.
HPV testing as the primary screen for cervical cancer is now recommended in guidelines due to improved sensitivity and improved efficiency when compared with other methods of screening. Implementation of this new workflow for clinicians and labs will require collaboration across multiple stakeholders.
Continue to: The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN...
The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN
Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
One new technology that was recently FDA approved and recommended for management of abnormal cervical cancer screening testing is dual-stain (DS) testing. Dual-stain testing is a cytology-based test that evaluates the concurrent expression of p16, a tumor suppressor protein upregulated in HPV oncogenesis, and Ki-67, a cell proliferation marker.6,7 Two recent studies have showcased the outstanding clinical performance of DS testing and triage strategies that incorporate DS testing.
Higher specificity, fewer colposcopies needed with DS testing
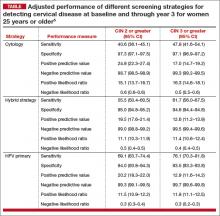
Magkana and colleagues prospectively evaluated patients with atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), or negative for intraepithelial lesion or malignancy (NILM) cytology referred for colposcopy, and they compared p16/Ki-67 DS testing with high-risk HPV (HR-HPV) testing for the detection of cervical intraepithelial neoplasia grade 2 or worse (CIN 2+); comparable sensitivities for CIN 2+ detection were seen (97.3% and 98.7%, respectively).8
Dual-stain testing exhibited higher specificity at 99.3% compared with HR-HPV testing at 52.2%. Incorporating DS testing into triage strategies also led to fewer colposcopies needed to detect CIN 2+ compared with current ASCCP guidelines that use traditional cervical cancer screening algorithms.
DS cytology strategy had the highest sensitivity for CIN 2+ detection
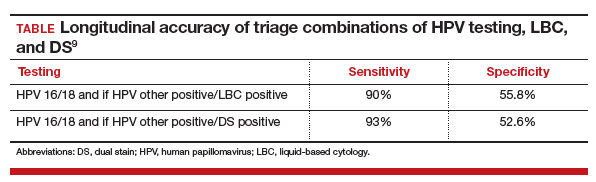
An additional study by Stanczuk and colleagues evaluated triage strategies in a cohort of HR-HPV positive patients who participated in the Scottish Papillomavirus Dumfries and Galloway study with HPV 16/18 genotyping (HPV 16/18), liquid-based cytology (LBC), and p16/Ki-67 DS cytology.9 Of these 3 triage strategies, DS cytology had the highest sensitivity for the detection of CIN 2+, at 77.7% (with a specificity of 74.2%), performance that is arguably better than cytology.
When evaluated in sequence as part of a triage strategy after HPV primary screening, HPV 16/18–positive patients reflexed to DS testing showed a similar sensitivity as those who would be triaged with LBC (TABLE).9
DS testing’s potential
These studies add to the growing body of literature that supports the use of DS testing in cervical cancer screening management guidelines and that are being incorporated into currently existing workflows. Furthermore, with advancements in digital imaging and machine learning, DS testing holds the potential for a high throughput, reproducible, and accurate risk stratification that can replace the current reliance on cytology, furthering the potential for a fully molecular Pap test.10,11
The introduction of p16/Ki-67 dual-stain testing has the potential to allow us to safely move away from a traditional Pap test for cervical cancer screening by allowing for more accurate and reliable identification of high-risk lesions with a molecular test that can be automated and have a high throughput.
Continue to: Cervical cancer screening in women older than age 65: Is there benefit?...
Cervical cancer screening in women older than age 65: Is there benefit?
Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
Current guidelines in the United States recommend that cervical cancer screening for all persons with a cervix end at age 65. These age restrictions were a change in guidelines updated in 2012 and endorsed by the US Preventive Services Task Force.12,13 Evidence suggests that because of high likelihood of regression and slow progression of disease, risks of screening prior to age 21 outweigh its benefits. With primary HPV testing, the age at screening debut is 25 for the same reasons.14 In people with a history of CIN 2+, active surveillance should continue for at least 25 years with HPV-based screening regardless of age. In the absence of a history of CIN 2+, however, the data to support discontinuation of screening after age 65 are less clear.
HPV positivity found to be most substantial risk for CIN 2+
In a study published this year in the Journal of Lower Genital Tract Disease, Firtina Tuncer and colleagues described their experience extending “routine screening” in patients older than 65 years.15 Data including cervical cytology, HPV test results, biopsy findings, and endocervical curettage results were collected, and abnormal findings were managed according to the 2012 and 2019 ASCCP guidelines.
When compared with negative HPV testing and normal cytology, the authors found that HPV positivity and abnormal cytology increased the risk of CIN 2+(odds ratio [OR], 136.1 and 13.1, respectively). Patients whose screening prior to age 65 had been insufficient or demonstrated CIN 2+ in the preceding 10 years were similarly more likely to have findings of CIN 2+ (OR, 9.7 when compared with HPV-negative controls).
The authors concluded that, among persons with a cervix older than age 65, previous screening and abnormal cytology were important in risk stratifications for CIN 2+; however, HPV positivity conferred the most substantial risk.
Study finds cervical dysplasia is prevalent in older populations
It has been suggested that screening for cervical cancer should continue beyond age 65 as cytology-based screening may have decreased sensitivity in older patients, which may contribute to the higher rates of advanced-stage diagnoses and cancer-related death in this population.16,17
Authors of an observational study conducted in Denmark invited persons with a cervix aged 69 and older to have one additional HPV-based screening test, and they referred them for colposcopy if HPV positive or in the presence of ASCUS or greater cytology.18 Among the 191 patients with HPV-positive results, 20% were found to have a diagnosis of CIN 2+, and 24.4% had CIN 2+ detected at another point in the study period. Notably, most patients diagnosed with CIN 2+ had no abnormalities visualized on colposcopy, and the majority of biopsies taken (65.8%) did not contain the transitional zone.
Biopsies underestimated CIN 2+ in 17.9% of cases compared with loop electrosurgical excision procedure (LEEP). These findings suggest both that high-grade cervical dysplasia is prevalent in an older population and that older populations may be susceptible to false-negative results. They also further support the use of HPV-based screening.
There are risk factors overscreening and underscreening that impact decision making regarding restricting screening to persons with a cervix younger than age 65. As more data become available, and as the population ages, it will be essential to closely examine the incidence of and trends in cervical cancer to determine appropriate patterns of screening.
Harnessing the immune system to improve survival rates in recurrent cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Unfortunately, most clinical trials for recurrent or metastatic cervical cancer are negative trials or have results that show limited impact on disease outcomes. Currently, cervical cancer is treated with multiple agents, including platinum-based chemotherapy and bevacizumab, a medication that targets vascular growth. Despite these usually very effective drugs given in combination to cervical cancer patients, long-term survival remains low. Over the past few decades, many trials have been designed to help patients with this terrible disease, but few have shown significant promise.
Immune checkpoint inhibitors, such as pembrolizumab, have revolutionized care for many cancers. Checkpoint inhibitors block the proteins that cause a tumor to remain undetected by the immune system’s army of T cells. By blocking these proteins, the cancer cells can then be recognized by the immune system as foreign. Several studies have concluded that including immune checkpoint inhibitors in the comprehensive regimen for recurrent cervical cancer improves survival.
Addition of pembrolizumab increased survival
Investigators in the phase 3 double-blinded KEYNOTE-826 trial evaluated whether or not the addition of pembrolizumab to standard of care improved progression-free and overall survival in advanced, recurrent, or persistent cervical cancer.19 As part of the evaluation, the investigators measured the protein that turns off the immune system’s ability to recognize tumors, anti-programmed cell death protein-1 (PD-1).
Compared with placebo, the investigators found that, regardless of PD-1 status, the addition of pembrolizumab immunotherapy to the standard regimen increased progression-free survival and overall survival without any significantly increased adverse effects or safety concerns (FIGURE).19 At 1 year after treatment, more patients who received pembrolizumab were still alive regardless of PD-1 status, and their responses lasted longer. The most profound improvements were seen in patients whose tumors exhibited high expression of PD-L1, the target of pembrolizumab and many other immune checkpoint inhibitors.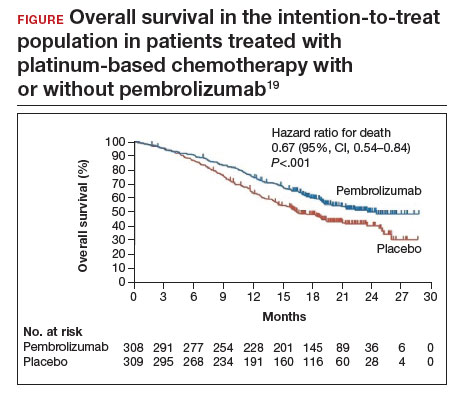
Despite these promising results, more studies are needed to find additional therapeutic targets and treatments. Using the immune system to fight cancer represents a promising step toward the ultimate goal of cervical cancer eradication. ●
Metastatic cervical cancer can be a devastating disease that cannot be treated surgically and therefore has limited treatment options that have curative intent. Immune checkpoint inhibition via pembrolizumab opens new avenues for treatment and is a huge step forward toward the goal of cervical cancer eradication.
- US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. June 2023. Accessed October 9, 2023. https://gis.cdc.gov/Cancer/USCS/#/Trends/
- Einstein MH, Zhou N, Gabor L, et al. Primary human papillomavirus testing and other new technologies for cervical cancer screening. Obstet Gynecol. September 14, 2023. doi:10.1097/AOG.0000000000005393
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2020. MMWR Morbid Mortal Weekly Rep. 2021;70:1183-1190.
- Cervical cancer elimination initiative. World Health Organization. 2023. Accessed October 10, 2023. https ://www.who.int/initiatives/cervical-cancer-eliminationinitiative#cms
- Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
- Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 Dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst. 2015;107:djv257.
- Ikenberg H, Bergeron C, Schmidt D, et al; PALMS Study Group. Screening for cervical cancer precursors with p16 /Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105:1550-1557.
- Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
- Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
- Wright TC Jr, Stoler MH, Behrens CM, et al. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134: 1835-1843.
- Wentzensen N, Lahrmann B, Clarke MA, et al. Accuracy and efficiency of deep-learning-based automation of dual stain cytology in cervical cancer screening. J Natl Cancer Inst. 2021;113:72-79.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-846.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156: 880-891, W312.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
- Hammer A, Hee L, Blaakaer J, et al. Temporal patterns of cervical cancer screening among Danish women 55 years and older diagnosed with cervical cancer. J Low Genit Tract Dis. 2018;22:1-7.
- Hammer A, Soegaard V, Maimburg RD, et al. Cervical cancer screening history prior to a diagnosis of cervical cancer in Danish women aged 60 years and older—A national cohort study. Cancer Med. 2019;8:418-427.
- Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.

Cervical cancer was the most common cancer killer of persons with a cervix in the early 1900s in the United States. Widespread adoption of the Pap test in the mid-20th century followed by large-scale outreach through programs such as the National Breast and Cervical Cancer Early Detection Program have dramatically reduced deaths from cervical cancer. The development of a highly effective vaccine that targets human papillomavirus (HPV), the virus implicated in all cervical cancers, has made prevention even more accessible and attainable. Primary prevention with HPV vaccination in conjunction with regular screening as recommended by current guidelines is the most effective way we can prevent cervical cancer.
Despite these advances, the incidence and death rates from cervical cancer have plateaued over the last decade.1 Additionally, many fear that due to the poor attendance at screening visits since the beginning of the COVID-19 pandemic, the incidence might further rise in the United States.2 Among those in the United States diagnosed with cervical cancer, more than 50% have not been screened in over 5 years or had their abnormal results not managed as recommended by current guidelines, suggesting that operational and access issues are contributors to incident cervical cancer. In addition, HPV vaccination rates have increased only slightly from year to year. According to the most recent data from the Centers for Disease Control and Prevention (CDC), coverage with 1 or more doses of HPV vaccine in 2021 increased only by 1.8% and has stagnated, with administration to about 75% of those for whom it is recommended.3 The plateauing will limit our ability to eradicate cervical cancer in the United States, permitting death from a largely preventable disease.
Establishing the framework for the eradication of cervical cancer
The World Health Organization (WHO) adopted a global strategy called the Cervical Cancer Elimination Initiative in August 2020. This initiative is a multipronged effort that focuses on vaccination (90% of girls fully vaccinated by age 15), screening (70% of women screened by age 35 with an effective test and again at age 45), and treatment (90% treatment of precancer and 90% management of women with invasive cancer).4
These are the numbers we need to achieve if all countries are to reach a cervical cancer incidence of less than 4 per 100,000 persons with a cervix. The WHO further suggests that each country should meet the “90-70-90” targets by 2030 if we are to achieve the low incidence by the turn of the century.4 To date, few regions of the world have achieved these goals, and sadly the United States is not among them.
In response to this call to action, many medical and policymaking organizations are taking inventory and implementing strategies to achieve the WHO 2030 targets for cervical cancer eradication. In the United States, the Society of Gynecologic Oncology (SGO; www.sgo.org), the American Society for Colposcopy and Cervical Pathology (ASCCP; www.ASCCP.org), the American College of Obstetricians and Gynecologists (ACOG; www.acog.org), the American Cancer Society (ACS; www.cancer.org), and many others have initiated programs in a collaborative esprit de corps with the aim of eradicating this deadly disease.
In this Update, we review several studies with evidence of screening and management strategies that show promise of accelerating the eradication of cervical cancer.
Continue to: Transitioning to primary HPV screening in the United States...
Transitioning to primary HPV screening in the United States
Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
The American Cancer Society released an updated cervical cancer screening guideline in July 2020 that recommended testing for HPV as the preferred strategy. Reasons behind the change, moving away from a Pap test as part of the initial screen, are:
- increased sensitivity of primary HPV testing when compared with conventional cervical cytology (Pap test)
- improved risk stratification to identify who is at risk for cervical cancer now and in the future
- improved efficiency in identifying those who need colposcopy, thus limiting unnecessary procedures without increasing the risk of false-negative tests, thereby missing cervical precancer or invasive cancer.
Some countries with organized screening programs have already made the switch. Self-sampling for HPV is currently being considered for an approved use in the United States, further improving access to screening for cervical cancer when the initial step can be completed by the patient at home or simplified in nontraditional health care settings.2
ACS initiative created to address barriers to primary HPV testing
Challenges to primary HPV testing remain, including laboratory implementation, payment, and operationalizing clinical workflow (for example, HPV testing with reflex cytology instead of cytology with reflex HPV testing).5 There are undoubtedly other unforeseen barriers in the current US health care environment.
In a recent commentary, Downs and colleagues described how the ACS has convened the Primary HPV Screening Initiative (PHSI), nested under the ACS National Roundtable on Cervical Cancer, which is charged with identifying critical barriers to, and opportunities for, transitioning to primary HPV screening.5 The deliverable will be a roadmap with tools and recommendations to support health systems, laboratories, providers, patients, and payers as they make this evolution.
Work groups will develop resources
Patients, particularly those who have had routine cervical cancer screening over their lifetime, also will be curious about the changes in recommendations. The Provider Needs Workgroup within the PHSI structure will develop tools and patient education materials regarding the data, workflow, benefits, and safety of this new paradigm for cervical cancer screening.
Laboratories that process and interpret tests likely will bear the heaviest load of changes. For example, not all commercially available HPV tests in the United States are approved by the US Food and Drug Administration (FDA) for primary HPV testing. Some sites will need to adapt their equipment to ensure adherence to FDA-approved tests. Laboratory workflows will need to be altered for aliquots to be tested for HPV first, and the remainder for cytology. Quality assurance and accreditation requirements for testing will need modifications, and further efforts will be needed to ensure sufficient numbers of trained cytopathologists, whose workforce is rapidly declining, for processing and reading cervical cytology.
In addition, payment for HPV testing alone, without the need for a Pap test, might not be supported by payers that support safety-net providers and sites, who arguably serve the most vulnerable patients and those most at risk for cervical cancer. Collaboration across medical professionals, societies, payers, and policymakers will provide a critical infrastructure to make the change in the most seamless fashion and limit the harm from missed opportunities for screening.
HPV testing as the primary screen for cervical cancer is now recommended in guidelines due to improved sensitivity and improved efficiency when compared with other methods of screening. Implementation of this new workflow for clinicians and labs will require collaboration across multiple stakeholders.
Continue to: The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN...
The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN
Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
One new technology that was recently FDA approved and recommended for management of abnormal cervical cancer screening testing is dual-stain (DS) testing. Dual-stain testing is a cytology-based test that evaluates the concurrent expression of p16, a tumor suppressor protein upregulated in HPV oncogenesis, and Ki-67, a cell proliferation marker.6,7 Two recent studies have showcased the outstanding clinical performance of DS testing and triage strategies that incorporate DS testing.
Higher specificity, fewer colposcopies needed with DS testing
Magkana and colleagues prospectively evaluated patients with atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), or negative for intraepithelial lesion or malignancy (NILM) cytology referred for colposcopy, and they compared p16/Ki-67 DS testing with high-risk HPV (HR-HPV) testing for the detection of cervical intraepithelial neoplasia grade 2 or worse (CIN 2+); comparable sensitivities for CIN 2+ detection were seen (97.3% and 98.7%, respectively).8
Dual-stain testing exhibited higher specificity at 99.3% compared with HR-HPV testing at 52.2%. Incorporating DS testing into triage strategies also led to fewer colposcopies needed to detect CIN 2+ compared with current ASCCP guidelines that use traditional cervical cancer screening algorithms.
DS cytology strategy had the highest sensitivity for CIN 2+ detection
An additional study by Stanczuk and colleagues evaluated triage strategies in a cohort of HR-HPV positive patients who participated in the Scottish Papillomavirus Dumfries and Galloway study with HPV 16/18 genotyping (HPV 16/18), liquid-based cytology (LBC), and p16/Ki-67 DS cytology.9 Of these 3 triage strategies, DS cytology had the highest sensitivity for the detection of CIN 2+, at 77.7% (with a specificity of 74.2%), performance that is arguably better than cytology.
When evaluated in sequence as part of a triage strategy after HPV primary screening, HPV 16/18–positive patients reflexed to DS testing showed a similar sensitivity as those who would be triaged with LBC (TABLE).9

DS testing’s potential
These studies add to the growing body of literature that supports the use of DS testing in cervical cancer screening management guidelines and that are being incorporated into currently existing workflows. Furthermore, with advancements in digital imaging and machine learning, DS testing holds the potential for a high throughput, reproducible, and accurate risk stratification that can replace the current reliance on cytology, furthering the potential for a fully molecular Pap test.10,11
The introduction of p16/Ki-67 dual-stain testing has the potential to allow us to safely move away from a traditional Pap test for cervical cancer screening by allowing for more accurate and reliable identification of high-risk lesions with a molecular test that can be automated and have a high throughput.
Continue to: Cervical cancer screening in women older than age 65: Is there benefit?...
Cervical cancer screening in women older than age 65: Is there benefit?
Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
Current guidelines in the United States recommend that cervical cancer screening for all persons with a cervix end at age 65. These age restrictions were a change in guidelines updated in 2012 and endorsed by the US Preventive Services Task Force.12,13 Evidence suggests that because of high likelihood of regression and slow progression of disease, risks of screening prior to age 21 outweigh its benefits. With primary HPV testing, the age at screening debut is 25 for the same reasons.14 In people with a history of CIN 2+, active surveillance should continue for at least 25 years with HPV-based screening regardless of age. In the absence of a history of CIN 2+, however, the data to support discontinuation of screening after age 65 are less clear.
HPV positivity found to be most substantial risk for CIN 2+
In a study published this year in the Journal of Lower Genital Tract Disease, Firtina Tuncer and colleagues described their experience extending “routine screening” in patients older than 65 years.15 Data including cervical cytology, HPV test results, biopsy findings, and endocervical curettage results were collected, and abnormal findings were managed according to the 2012 and 2019 ASCCP guidelines.
When compared with negative HPV testing and normal cytology, the authors found that HPV positivity and abnormal cytology increased the risk of CIN 2+(odds ratio [OR], 136.1 and 13.1, respectively). Patients whose screening prior to age 65 had been insufficient or demonstrated CIN 2+ in the preceding 10 years were similarly more likely to have findings of CIN 2+ (OR, 9.7 when compared with HPV-negative controls).
The authors concluded that, among persons with a cervix older than age 65, previous screening and abnormal cytology were important in risk stratifications for CIN 2+; however, HPV positivity conferred the most substantial risk.
Study finds cervical dysplasia is prevalent in older populations
It has been suggested that screening for cervical cancer should continue beyond age 65 as cytology-based screening may have decreased sensitivity in older patients, which may contribute to the higher rates of advanced-stage diagnoses and cancer-related death in this population.16,17
Authors of an observational study conducted in Denmark invited persons with a cervix aged 69 and older to have one additional HPV-based screening test, and they referred them for colposcopy if HPV positive or in the presence of ASCUS or greater cytology.18 Among the 191 patients with HPV-positive results, 20% were found to have a diagnosis of CIN 2+, and 24.4% had CIN 2+ detected at another point in the study period. Notably, most patients diagnosed with CIN 2+ had no abnormalities visualized on colposcopy, and the majority of biopsies taken (65.8%) did not contain the transitional zone.
Biopsies underestimated CIN 2+ in 17.9% of cases compared with loop electrosurgical excision procedure (LEEP). These findings suggest both that high-grade cervical dysplasia is prevalent in an older population and that older populations may be susceptible to false-negative results. They also further support the use of HPV-based screening.
There are risk factors overscreening and underscreening that impact decision making regarding restricting screening to persons with a cervix younger than age 65. As more data become available, and as the population ages, it will be essential to closely examine the incidence of and trends in cervical cancer to determine appropriate patterns of screening.
Harnessing the immune system to improve survival rates in recurrent cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Unfortunately, most clinical trials for recurrent or metastatic cervical cancer are negative trials or have results that show limited impact on disease outcomes. Currently, cervical cancer is treated with multiple agents, including platinum-based chemotherapy and bevacizumab, a medication that targets vascular growth. Despite these usually very effective drugs given in combination to cervical cancer patients, long-term survival remains low. Over the past few decades, many trials have been designed to help patients with this terrible disease, but few have shown significant promise.
Immune checkpoint inhibitors, such as pembrolizumab, have revolutionized care for many cancers. Checkpoint inhibitors block the proteins that cause a tumor to remain undetected by the immune system’s army of T cells. By blocking these proteins, the cancer cells can then be recognized by the immune system as foreign. Several studies have concluded that including immune checkpoint inhibitors in the comprehensive regimen for recurrent cervical cancer improves survival.
Addition of pembrolizumab increased survival
Investigators in the phase 3 double-blinded KEYNOTE-826 trial evaluated whether or not the addition of pembrolizumab to standard of care improved progression-free and overall survival in advanced, recurrent, or persistent cervical cancer.19 As part of the evaluation, the investigators measured the protein that turns off the immune system’s ability to recognize tumors, anti-programmed cell death protein-1 (PD-1).
Compared with placebo, the investigators found that, regardless of PD-1 status, the addition of pembrolizumab immunotherapy to the standard regimen increased progression-free survival and overall survival without any significantly increased adverse effects or safety concerns (FIGURE).19 At 1 year after treatment, more patients who received pembrolizumab were still alive regardless of PD-1 status, and their responses lasted longer. The most profound improvements were seen in patients whose tumors exhibited high expression of PD-L1, the target of pembrolizumab and many other immune checkpoint inhibitors.
Despite these promising results, more studies are needed to find additional therapeutic targets and treatments. Using the immune system to fight cancer represents a promising step toward the ultimate goal of cervical cancer eradication. ●
Metastatic cervical cancer can be a devastating disease that cannot be treated surgically and therefore has limited treatment options that have curative intent. Immune checkpoint inhibition via pembrolizumab opens new avenues for treatment and is a huge step forward toward the goal of cervical cancer eradication.

Cervical cancer was the most common cancer killer of persons with a cervix in the early 1900s in the United States. Widespread adoption of the Pap test in the mid-20th century followed by large-scale outreach through programs such as the National Breast and Cervical Cancer Early Detection Program have dramatically reduced deaths from cervical cancer. The development of a highly effective vaccine that targets human papillomavirus (HPV), the virus implicated in all cervical cancers, has made prevention even more accessible and attainable. Primary prevention with HPV vaccination in conjunction with regular screening as recommended by current guidelines is the most effective way we can prevent cervical cancer.
Despite these advances, the incidence and death rates from cervical cancer have plateaued over the last decade.1 Additionally, many fear that due to the poor attendance at screening visits since the beginning of the COVID-19 pandemic, the incidence might further rise in the United States.2 Among those in the United States diagnosed with cervical cancer, more than 50% have not been screened in over 5 years or had their abnormal results not managed as recommended by current guidelines, suggesting that operational and access issues are contributors to incident cervical cancer. In addition, HPV vaccination rates have increased only slightly from year to year. According to the most recent data from the Centers for Disease Control and Prevention (CDC), coverage with 1 or more doses of HPV vaccine in 2021 increased only by 1.8% and has stagnated, with administration to about 75% of those for whom it is recommended.3 The plateauing will limit our ability to eradicate cervical cancer in the United States, permitting death from a largely preventable disease.
Establishing the framework for the eradication of cervical cancer
The World Health Organization (WHO) adopted a global strategy called the Cervical Cancer Elimination Initiative in August 2020. This initiative is a multipronged effort that focuses on vaccination (90% of girls fully vaccinated by age 15), screening (70% of women screened by age 35 with an effective test and again at age 45), and treatment (90% treatment of precancer and 90% management of women with invasive cancer).4
These are the numbers we need to achieve if all countries are to reach a cervical cancer incidence of less than 4 per 100,000 persons with a cervix. The WHO further suggests that each country should meet the “90-70-90” targets by 2030 if we are to achieve the low incidence by the turn of the century.4 To date, few regions of the world have achieved these goals, and sadly the United States is not among them.
In response to this call to action, many medical and policymaking organizations are taking inventory and implementing strategies to achieve the WHO 2030 targets for cervical cancer eradication. In the United States, the Society of Gynecologic Oncology (SGO; www.sgo.org), the American Society for Colposcopy and Cervical Pathology (ASCCP; www.ASCCP.org), the American College of Obstetricians and Gynecologists (ACOG; www.acog.org), the American Cancer Society (ACS; www.cancer.org), and many others have initiated programs in a collaborative esprit de corps with the aim of eradicating this deadly disease.
In this Update, we review several studies with evidence of screening and management strategies that show promise of accelerating the eradication of cervical cancer.
Continue to: Transitioning to primary HPV screening in the United States...
Transitioning to primary HPV screening in the United States
Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
The American Cancer Society released an updated cervical cancer screening guideline in July 2020 that recommended testing for HPV as the preferred strategy. Reasons behind the change, moving away from a Pap test as part of the initial screen, are:
- increased sensitivity of primary HPV testing when compared with conventional cervical cytology (Pap test)
- improved risk stratification to identify who is at risk for cervical cancer now and in the future
- improved efficiency in identifying those who need colposcopy, thus limiting unnecessary procedures without increasing the risk of false-negative tests, thereby missing cervical precancer or invasive cancer.
Some countries with organized screening programs have already made the switch. Self-sampling for HPV is currently being considered for an approved use in the United States, further improving access to screening for cervical cancer when the initial step can be completed by the patient at home or simplified in nontraditional health care settings.2
ACS initiative created to address barriers to primary HPV testing
Challenges to primary HPV testing remain, including laboratory implementation, payment, and operationalizing clinical workflow (for example, HPV testing with reflex cytology instead of cytology with reflex HPV testing).5 There are undoubtedly other unforeseen barriers in the current US health care environment.
In a recent commentary, Downs and colleagues described how the ACS has convened the Primary HPV Screening Initiative (PHSI), nested under the ACS National Roundtable on Cervical Cancer, which is charged with identifying critical barriers to, and opportunities for, transitioning to primary HPV screening.5 The deliverable will be a roadmap with tools and recommendations to support health systems, laboratories, providers, patients, and payers as they make this evolution.
Work groups will develop resources
Patients, particularly those who have had routine cervical cancer screening over their lifetime, also will be curious about the changes in recommendations. The Provider Needs Workgroup within the PHSI structure will develop tools and patient education materials regarding the data, workflow, benefits, and safety of this new paradigm for cervical cancer screening.
Laboratories that process and interpret tests likely will bear the heaviest load of changes. For example, not all commercially available HPV tests in the United States are approved by the US Food and Drug Administration (FDA) for primary HPV testing. Some sites will need to adapt their equipment to ensure adherence to FDA-approved tests. Laboratory workflows will need to be altered for aliquots to be tested for HPV first, and the remainder for cytology. Quality assurance and accreditation requirements for testing will need modifications, and further efforts will be needed to ensure sufficient numbers of trained cytopathologists, whose workforce is rapidly declining, for processing and reading cervical cytology.
In addition, payment for HPV testing alone, without the need for a Pap test, might not be supported by payers that support safety-net providers and sites, who arguably serve the most vulnerable patients and those most at risk for cervical cancer. Collaboration across medical professionals, societies, payers, and policymakers will provide a critical infrastructure to make the change in the most seamless fashion and limit the harm from missed opportunities for screening.
HPV testing as the primary screen for cervical cancer is now recommended in guidelines due to improved sensitivity and improved efficiency when compared with other methods of screening. Implementation of this new workflow for clinicians and labs will require collaboration across multiple stakeholders.
Continue to: The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN...
The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN
Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
One new technology that was recently FDA approved and recommended for management of abnormal cervical cancer screening testing is dual-stain (DS) testing. Dual-stain testing is a cytology-based test that evaluates the concurrent expression of p16, a tumor suppressor protein upregulated in HPV oncogenesis, and Ki-67, a cell proliferation marker.6,7 Two recent studies have showcased the outstanding clinical performance of DS testing and triage strategies that incorporate DS testing.
Higher specificity, fewer colposcopies needed with DS testing
Magkana and colleagues prospectively evaluated patients with atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), or negative for intraepithelial lesion or malignancy (NILM) cytology referred for colposcopy, and they compared p16/Ki-67 DS testing with high-risk HPV (HR-HPV) testing for the detection of cervical intraepithelial neoplasia grade 2 or worse (CIN 2+); comparable sensitivities for CIN 2+ detection were seen (97.3% and 98.7%, respectively).8
Dual-stain testing exhibited higher specificity at 99.3% compared with HR-HPV testing at 52.2%. Incorporating DS testing into triage strategies also led to fewer colposcopies needed to detect CIN 2+ compared with current ASCCP guidelines that use traditional cervical cancer screening algorithms.
DS cytology strategy had the highest sensitivity for CIN 2+ detection
An additional study by Stanczuk and colleagues evaluated triage strategies in a cohort of HR-HPV positive patients who participated in the Scottish Papillomavirus Dumfries and Galloway study with HPV 16/18 genotyping (HPV 16/18), liquid-based cytology (LBC), and p16/Ki-67 DS cytology.9 Of these 3 triage strategies, DS cytology had the highest sensitivity for the detection of CIN 2+, at 77.7% (with a specificity of 74.2%), performance that is arguably better than cytology.
When evaluated in sequence as part of a triage strategy after HPV primary screening, HPV 16/18–positive patients reflexed to DS testing showed a similar sensitivity as those who would be triaged with LBC (TABLE).9

DS testing’s potential
These studies add to the growing body of literature that supports the use of DS testing in cervical cancer screening management guidelines and that are being incorporated into currently existing workflows. Furthermore, with advancements in digital imaging and machine learning, DS testing holds the potential for a high throughput, reproducible, and accurate risk stratification that can replace the current reliance on cytology, furthering the potential for a fully molecular Pap test.10,11
The introduction of p16/Ki-67 dual-stain testing has the potential to allow us to safely move away from a traditional Pap test for cervical cancer screening by allowing for more accurate and reliable identification of high-risk lesions with a molecular test that can be automated and have a high throughput.
Continue to: Cervical cancer screening in women older than age 65: Is there benefit?...
Cervical cancer screening in women older than age 65: Is there benefit?
Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
Current guidelines in the United States recommend that cervical cancer screening for all persons with a cervix end at age 65. These age restrictions were a change in guidelines updated in 2012 and endorsed by the US Preventive Services Task Force.12,13 Evidence suggests that because of high likelihood of regression and slow progression of disease, risks of screening prior to age 21 outweigh its benefits. With primary HPV testing, the age at screening debut is 25 for the same reasons.14 In people with a history of CIN 2+, active surveillance should continue for at least 25 years with HPV-based screening regardless of age. In the absence of a history of CIN 2+, however, the data to support discontinuation of screening after age 65 are less clear.
HPV positivity found to be most substantial risk for CIN 2+
In a study published this year in the Journal of Lower Genital Tract Disease, Firtina Tuncer and colleagues described their experience extending “routine screening” in patients older than 65 years.15 Data including cervical cytology, HPV test results, biopsy findings, and endocervical curettage results were collected, and abnormal findings were managed according to the 2012 and 2019 ASCCP guidelines.
When compared with negative HPV testing and normal cytology, the authors found that HPV positivity and abnormal cytology increased the risk of CIN 2+(odds ratio [OR], 136.1 and 13.1, respectively). Patients whose screening prior to age 65 had been insufficient or demonstrated CIN 2+ in the preceding 10 years were similarly more likely to have findings of CIN 2+ (OR, 9.7 when compared with HPV-negative controls).
The authors concluded that, among persons with a cervix older than age 65, previous screening and abnormal cytology were important in risk stratifications for CIN 2+; however, HPV positivity conferred the most substantial risk.
Study finds cervical dysplasia is prevalent in older populations
It has been suggested that screening for cervical cancer should continue beyond age 65 as cytology-based screening may have decreased sensitivity in older patients, which may contribute to the higher rates of advanced-stage diagnoses and cancer-related death in this population.16,17
Authors of an observational study conducted in Denmark invited persons with a cervix aged 69 and older to have one additional HPV-based screening test, and they referred them for colposcopy if HPV positive or in the presence of ASCUS or greater cytology.18 Among the 191 patients with HPV-positive results, 20% were found to have a diagnosis of CIN 2+, and 24.4% had CIN 2+ detected at another point in the study period. Notably, most patients diagnosed with CIN 2+ had no abnormalities visualized on colposcopy, and the majority of biopsies taken (65.8%) did not contain the transitional zone.
Biopsies underestimated CIN 2+ in 17.9% of cases compared with loop electrosurgical excision procedure (LEEP). These findings suggest both that high-grade cervical dysplasia is prevalent in an older population and that older populations may be susceptible to false-negative results. They also further support the use of HPV-based screening.
There are risk factors overscreening and underscreening that impact decision making regarding restricting screening to persons with a cervix younger than age 65. As more data become available, and as the population ages, it will be essential to closely examine the incidence of and trends in cervical cancer to determine appropriate patterns of screening.
Harnessing the immune system to improve survival rates in recurrent cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Unfortunately, most clinical trials for recurrent or metastatic cervical cancer are negative trials or have results that show limited impact on disease outcomes. Currently, cervical cancer is treated with multiple agents, including platinum-based chemotherapy and bevacizumab, a medication that targets vascular growth. Despite these usually very effective drugs given in combination to cervical cancer patients, long-term survival remains low. Over the past few decades, many trials have been designed to help patients with this terrible disease, but few have shown significant promise.
Immune checkpoint inhibitors, such as pembrolizumab, have revolutionized care for many cancers. Checkpoint inhibitors block the proteins that cause a tumor to remain undetected by the immune system’s army of T cells. By blocking these proteins, the cancer cells can then be recognized by the immune system as foreign. Several studies have concluded that including immune checkpoint inhibitors in the comprehensive regimen for recurrent cervical cancer improves survival.
Addition of pembrolizumab increased survival
Investigators in the phase 3 double-blinded KEYNOTE-826 trial evaluated whether or not the addition of pembrolizumab to standard of care improved progression-free and overall survival in advanced, recurrent, or persistent cervical cancer.19 As part of the evaluation, the investigators measured the protein that turns off the immune system’s ability to recognize tumors, anti-programmed cell death protein-1 (PD-1).
Compared with placebo, the investigators found that, regardless of PD-1 status, the addition of pembrolizumab immunotherapy to the standard regimen increased progression-free survival and overall survival without any significantly increased adverse effects or safety concerns (FIGURE).19 At 1 year after treatment, more patients who received pembrolizumab were still alive regardless of PD-1 status, and their responses lasted longer. The most profound improvements were seen in patients whose tumors exhibited high expression of PD-L1, the target of pembrolizumab and many other immune checkpoint inhibitors.
Despite these promising results, more studies are needed to find additional therapeutic targets and treatments. Using the immune system to fight cancer represents a promising step toward the ultimate goal of cervical cancer eradication. ●
Metastatic cervical cancer can be a devastating disease that cannot be treated surgically and therefore has limited treatment options that have curative intent. Immune checkpoint inhibition via pembrolizumab opens new avenues for treatment and is a huge step forward toward the goal of cervical cancer eradication.
- US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. June 2023. Accessed October 9, 2023. https://gis.cdc.gov/Cancer/USCS/#/Trends/
- Einstein MH, Zhou N, Gabor L, et al. Primary human papillomavirus testing and other new technologies for cervical cancer screening. Obstet Gynecol. September 14, 2023. doi:10.1097/AOG.0000000000005393
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2020. MMWR Morbid Mortal Weekly Rep. 2021;70:1183-1190.
- Cervical cancer elimination initiative. World Health Organization. 2023. Accessed October 10, 2023. https ://www.who.int/initiatives/cervical-cancer-eliminationinitiative#cms
- Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
- Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 Dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst. 2015;107:djv257.
- Ikenberg H, Bergeron C, Schmidt D, et al; PALMS Study Group. Screening for cervical cancer precursors with p16 /Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105:1550-1557.
- Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
- Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
- Wright TC Jr, Stoler MH, Behrens CM, et al. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134: 1835-1843.
- Wentzensen N, Lahrmann B, Clarke MA, et al. Accuracy and efficiency of deep-learning-based automation of dual stain cytology in cervical cancer screening. J Natl Cancer Inst. 2021;113:72-79.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-846.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156: 880-891, W312.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
- Hammer A, Hee L, Blaakaer J, et al. Temporal patterns of cervical cancer screening among Danish women 55 years and older diagnosed with cervical cancer. J Low Genit Tract Dis. 2018;22:1-7.
- Hammer A, Soegaard V, Maimburg RD, et al. Cervical cancer screening history prior to a diagnosis of cervical cancer in Danish women aged 60 years and older—A national cohort study. Cancer Med. 2019;8:418-427.
- Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
- US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. June 2023. Accessed October 9, 2023. https://gis.cdc.gov/Cancer/USCS/#/Trends/
- Einstein MH, Zhou N, Gabor L, et al. Primary human papillomavirus testing and other new technologies for cervical cancer screening. Obstet Gynecol. September 14, 2023. doi:10.1097/AOG.0000000000005393
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2020. MMWR Morbid Mortal Weekly Rep. 2021;70:1183-1190.
- Cervical cancer elimination initiative. World Health Organization. 2023. Accessed October 10, 2023. https ://www.who.int/initiatives/cervical-cancer-eliminationinitiative#cms
- Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
- Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 Dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst. 2015;107:djv257.
- Ikenberg H, Bergeron C, Schmidt D, et al; PALMS Study Group. Screening for cervical cancer precursors with p16 /Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105:1550-1557.
- Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
- Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
- Wright TC Jr, Stoler MH, Behrens CM, et al. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134: 1835-1843.
- Wentzensen N, Lahrmann B, Clarke MA, et al. Accuracy and efficiency of deep-learning-based automation of dual stain cytology in cervical cancer screening. J Natl Cancer Inst. 2021;113:72-79.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-846.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156: 880-891, W312.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
- Hammer A, Hee L, Blaakaer J, et al. Temporal patterns of cervical cancer screening among Danish women 55 years and older diagnosed with cervical cancer. J Low Genit Tract Dis. 2018;22:1-7.
- Hammer A, Soegaard V, Maimburg RD, et al. Cervical cancer screening history prior to a diagnosis of cervical cancer in Danish women aged 60 years and older—A national cohort study. Cancer Med. 2019;8:418-427.
- Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
2019 Update on cervical disease
Cervical cancer rates remain low in the United States, with the incidence having plateaued for decades. And yet, in 2019, more than 13,000 US women will be diagnosed with cervical cancer.1 Globally, in 2018 almost 600,000 women were diagnosed with cervical cancer2; it is the fourth most frequent cancer in women. This is despite the fact that we have adequate primary and secondary prevention tools available to minimize—and almost eliminate—cervical cancer. We must continue to raise the bar for preventing, screening for, and managing this disease.
Human papillomavirus (HPV) vaccines provide a highly effective primary prevention strategy, but we need to improve our ability to identify and diagnose dysplastic lesions prior to the development of cervical cancer. Highly sensitive HPV testing and cytology is a powerful secondary prevention approach that enables us to assess a woman’s risk of having precancerous cells both now and in the near future. These modalities have been very successful in decreasing the incidence of cervical cancer in the United States and other areas with organized screening programs. In low- and middle-income countries, however, access to, availability of, and performance with these modalities is not optimal. Innovative strategies and new technologies are being evaluated to overcome these limitations.
Advances in radiation and surgical technology have enabled us to vastly improve cervical cancer treatment. Women with early-stage cervical cancer are candidates for surgical management, which frequently includes a radical hysterectomy and lymph node dissection. While these surgeries traditionally have been performed via an exploratory laparotomy, minimally invasive techniques (laparoscopic and robot-assisted surgical techniques) have decreased the morbidity with these surgeries. Notable new studies have shed light on the comparative effectiveness of minimally invasive technologies and have shown us that new is not always better.
The US Preventive Services Task Force (USPSTF) recently released its updated cervical cancer screening guidelines. The suggested approach to screening differs from previous recommendations. HPV testing as a primary test (that is, HPV testing alone or followed by cytology) takes the spotlight now, according to the analysis by the Task Force.
In this Update, we highlight important studies published in the past year that address these issues.
Continue to: New tech's potential to identify high-grade...
New tech's potential to identify high-grade cervical dysplasia may be a boon to low-resource settings
Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
When cervical screening tests like cytology and HPV testing show abnormal results, colposcopy often is recommended. The goal of colposcopy is to identify the areas that might harbor a high-grade precancerous lesion or worse. The gold standard in this case, however, is histology, not colposcopic impression, as many studies have shown that colposcopy without biopsies is limited and that performance is improved with more biopsies.3,4
Visual inspection with acetic acid (VIA) is an approach used often in low-resource settings where visual impression is the gold standard. However, as with colposcopy, a visual evaluation without histology does not perform well, and often women are overtreated. Many attempts have been made with new technologies to overcome the limitations of time, cost, and workforce required for cytology and histology services. New disruptive technologies may be able to surmount human limitations and improve on not only VIA but also the need for histology.
Novel technology uses images to develop algorithm with predictive ability
In a recent observational study, Hu and colleagues used images that were collected during a large population study in Guanacaste, Costa Rica.5 More than 9,000 women were followed for up to 7 years, and cervical photographs (cervigrams) were obtained. Well-annotated histopathology results were obtained for women with abnormal screening, and 279 women had a high-grade dysplastic lesion or cancer.
Cervigrams from women with high-grade lesions and matched controls were collected, and a deep learning-based algorithm using artificial intelligence technology was developed using 70% of the images. The remaining 30% of images were used as a validation set to test the algorithm's ability to "predict" high-grade dysplasia without knowing the final result.
Findings. Termed automated visual evaluation (AVE), this new technology demonstrated a very accurate ability to identify high-grade dysplasia or worse, with an area under the curve (AUC) of 0.91 from merely a cervicogram (FIGURE). This outperformed conventional Pap smears (AUC, 0.71), liquid-based cytology (AUC, 0.79) and, surprisingly, highly sensitive HPV testing (AUC, 0.82) in women in the prime of their screening ages (>25 years of age).
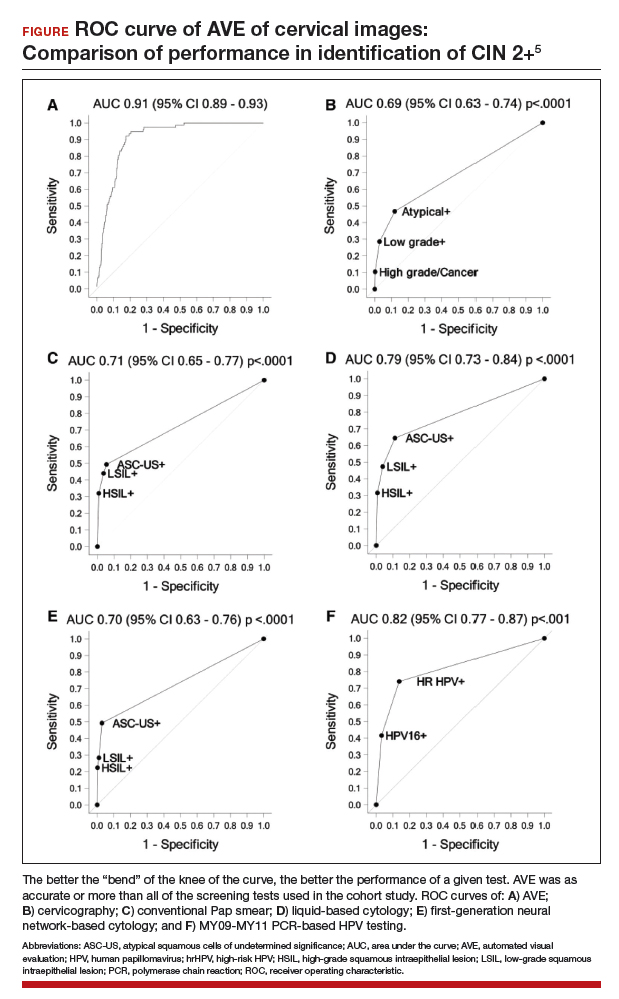
Colposcopy remains the gold standard for evaluating abnormal cervical cancer screening tests in the United States. But can we do better for our patients using new technologies like AVE? If validated in large-scale trials, AVE has the potential to revolutionize cervical cancer screening in low-resource settings where follow-up and adequate histology services are limited or nonexistent. Future large studies are necessary to evaluate the role of AVE alone versus in combination with other diagnostic testing (such as HPV testing) to detect cervical lesions globally.
Continue to: Data offer persuasive evidence...
Data offer persuasive evidence to abandon minimally invasive surgery in management of early-stage cervical cancer
Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
Over the past decade, gynecologic cancer surgery has shifted from what routinely were open procedures to the adoption of minimally invasive techniques. Recently, a large, well-designed prospective study and a large retrospective study both demonstrated worse outcomes with minimally invasive radical hysterectomy (MIRH) as compared with traditional open radical abdominal hysterectomy (RAH). These 2 landmark studies, initially presented at the Society of Gynecologic Oncology's 2018 annual meeting and later published in the New England Journal of Medicine, have really affected the gynecologic oncology community.
Shorter overall survival in women who had MIRH
Melamed and colleagues conducted a large, retrospective US-based study to evaluate all-cause mortality in women with cervical cancer who underwent MIRH compared with those who had RAH.6 The authors also sought to evaluate national trends in 4-year relative survival rates after minimally invasive surgery was adopted.
The study included 2,461 women who met the inclusion criteria; 49.8% (1,225) underwent MIRH procedures and, of those, 79.8% (978) had robot-assisted laparoscopy. Most women had stage IB1 tumors (88%), and most carcinomas were squamous cell (61%); 40.6% of tumors were less than 2 cm in size. There were no differences between the 2 groups with respect to rates of positive parametria, surgical margins, and lymph node involvement. Administration of adjuvant therapy, in those who qualified, was also similar between groups.
Results. At a median follow-up of 45 months, 94 deaths occurred in the minimally invasive group and 70 in the open surgery group. The risk of death at 4 years was 9.1% in the minimally invasive group versus 5.3% in the open surgery group, with a 65% higher risk of death from any cause, which was highly statistically significant.
Prospective trial showed MIRH was associated with lower survival rates
From 2008 to 2017, Ramirez and colleagues conducted a phase 3, multicenter, randomized controlled trial to prospectively establish the noninferiority of MIRH compared with RAH.7 The study included 631 women from 33 centers. The prespecified expected disease-free survival rate was 90% at 4.5 years.
To be included as a site, centers were required to submit details from 10 minimally invasive cases as well as 2 unedited videos for review by the trial management committee. In contrast to Melamed and colleagues' retrospective study, of the 319 procedures that were classified as minimally invasive, only 15.6% were robotically assisted. Similarly, most women had stage IB1 tumors (91.9%), and most were squamous cell carcinomas (67%). There were also no differences in the postoperative pathology findings or the need for adjuvant therapy administered between groups. The median follow-up was 2.5 years.
Results. At that time there were 27 recurrences in the MIRH group and 7 in the RAH group; there were also 19 deaths after MIRH and 3 after RAH. Disease-free survival at 4.5 years was 86% with MIRH versus 96.5% with RAH. Reported 3-year disease-free survival and overall survival were also significantily lower in the minimally invasive subgroup (91.2% vs 97.1%, 93.8% vs 99.0%, respectively).
Study limitations. Criticisms of this trial are that noninferiority could not be declared; in addition, the investigators were unable to complete enrollment secondary to early enrollment termination after the data and safety monitoring board raised survival concerns.
Many argue that subgroup analyses suggest a lower risk of poor outcomes in patients with smaller tumors (<2 cm); however, it is critical to note that this study was not powered to detect these differences.
The evidence is compelling and demonstrates potentially worse disease-related outcomes using MIRH when compared to traditional RAH with respect to cervical cancer recurrence, rates of death, and disease-free and overall survival. Several hypotheses have been proposed, and future research is needed to elucidate the differences in variables responsible for the outcomes demonstrated in these studies. Although there has been no ban on robot-assisted surgical devices or traditional minimally invasive techniques, the National Comprehensive Cancer Network has updated its recommendations to include careful counseling of patients who require a surgical approach for the management of early-stage cervical cancer.
Continue to: USPSTF updated guidance on cervical cancer screening...
USPSTF updated guidance on cervical cancer screening
Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Past guidelines for cervical cancer screening have included testing for high-risk HPV (hrHPV) as a cotest with cytology or for triage of atypical squamous cells of undetermined significance (ASCUS) in women aged 30 to 65 years.8 The American Society for Colposcopy and Cervical Pathology and the Society of Gynecologic Oncology, with other stakeholder organizations, issued interim guidance for primary HPV testing--that is, HPV test first and, in the case of non-16/18 hrHPV types, cytology as a triage. The most recent evidence report and systematic review by Melnikow and colleagues for the USPSTF offers an in-depth analysis of risks, benefits, harms, and value of cotesting and other management strategies.9
Focus on screening effectiveness
Large trials of cotesting were conducted in women aged 25 to 65.10-13 These studies all consistently showed that primary hrHPV screening led to a statistically significant increased detection of cervical intraepithelial neoplasia (CIN) 3+ in the initial round of screening, with a relative risk of detecting CIN 3+ ranging from 1.61 to 7.46 compared with cytology alone.
Four additional studies compared cotesting with conventional cytology for the detection of CIN 3+. None of these trials demonstrated a significantly higher detection rate of CIN 3+ with cotesting compared with conventional cytology testing alone. Notably, the studies reviewed were performed in European countries that had organized screening programs in place and a nationalized health care system. Thus, these data may not be as applicable to women in the United States, particularly to women who have limited health care access.
Risks of screening
In the same studies reviewed for screening effectiveness, the investigators found that overall, screening with hrHPV primary or cotesting was associated with more false-positive results and higher colposcopy rates. Women screened with hrHPV alone had a 7.9% referral rate to colposcopy, while those screened with cytology had a 2.8% referral rate to colposcopy. Similarly, the rate of biopsy was higher in the hrHPV-only group (3.2% vs 1.3%).
Overall, while cotesting might have some improvement in performance compared with hrHPV as a single modality, there might be risks of overreferral to colposcopy and overtreatment with additional cytology over hrHPV testing alone.
This evidence review also included an analysis of more potential harms. Very limited evidence suggests that positive hrHPV test results may be associated with greater psychological harm, including decreased sexual satisfaction, increased anxiety and distress, and worse feelings about sexual partners, than abnormal cytology results. These were assessed, however, 1 to 2 weeks after the test results were provided to the patients, and long-term assessment was not done.
New recommendations from the USPSTF
Based on these data, the USPSTF issued new recommendations regarding screening (TABLE).14 For women aged 21 to 29, cytology alone should be used for screening every 3 years. Women aged 30 to 65 can be screened with cytology alone every 3 years, with hrHPV testing alone every 5 years, or with cotesting every 5 years.
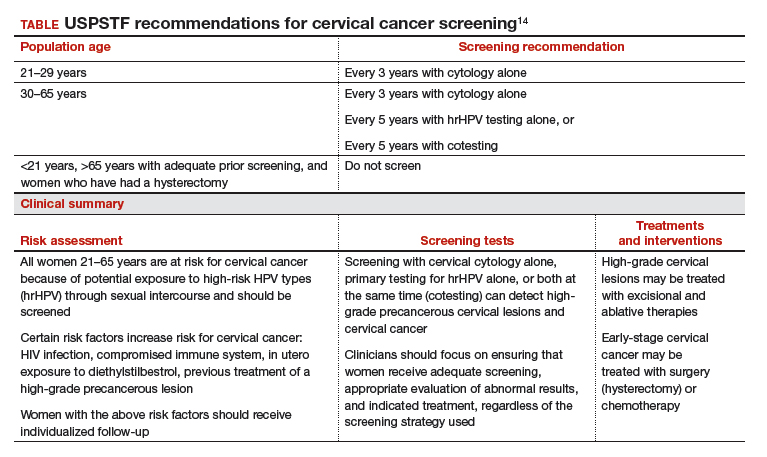
Primary screening with hrHPV is more effective in diagnosing a CIN 3+ than cytology alone. Cotesting with cytology and hrHPV testing appears to have limited performance improvement, with potential harm, compared with hrHPV testing alone in diagnosing CIN 3+. The Task Force recommendation is hrHPV testing alone or cotesting every 5 years.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- World Health Organization website. Cervical cancer. https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/. Accessed April 17, 2019.
- Wentzensen N, Walker JL, Gold MA, et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol. 2015;33:83-89.
- Gage JC, Hanson VW, Abbey K, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108:264-272.
- Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
- Canfell K, Caruana M, Gebski V, et al. Cervical screening with primary HPV testing or cytology in a population of women in which those aged 33 years or younger had previously been offered HPV vaccination: results of the Compass pilot randomised trial. PLoS Med. 2017;14:e1002388. doi:10.1371/journal.pmed.1002388.
- Leinonen MK, Nieminen P, Lonnberg S, et al. Detection rates of precancerous and cancerous cervical lesions within one screening round of primary human papillomavirus DNA testing: prospective randomised trial in Finland. BMJ. 2012;345:e7789.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Ronco G, Fioprgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Cervical cancer rates remain low in the United States, with the incidence having plateaued for decades. And yet, in 2019, more than 13,000 US women will be diagnosed with cervical cancer.1 Globally, in 2018 almost 600,000 women were diagnosed with cervical cancer2; it is the fourth most frequent cancer in women. This is despite the fact that we have adequate primary and secondary prevention tools available to minimize—and almost eliminate—cervical cancer. We must continue to raise the bar for preventing, screening for, and managing this disease.
Human papillomavirus (HPV) vaccines provide a highly effective primary prevention strategy, but we need to improve our ability to identify and diagnose dysplastic lesions prior to the development of cervical cancer. Highly sensitive HPV testing and cytology is a powerful secondary prevention approach that enables us to assess a woman’s risk of having precancerous cells both now and in the near future. These modalities have been very successful in decreasing the incidence of cervical cancer in the United States and other areas with organized screening programs. In low- and middle-income countries, however, access to, availability of, and performance with these modalities is not optimal. Innovative strategies and new technologies are being evaluated to overcome these limitations.
Advances in radiation and surgical technology have enabled us to vastly improve cervical cancer treatment. Women with early-stage cervical cancer are candidates for surgical management, which frequently includes a radical hysterectomy and lymph node dissection. While these surgeries traditionally have been performed via an exploratory laparotomy, minimally invasive techniques (laparoscopic and robot-assisted surgical techniques) have decreased the morbidity with these surgeries. Notable new studies have shed light on the comparative effectiveness of minimally invasive technologies and have shown us that new is not always better.
The US Preventive Services Task Force (USPSTF) recently released its updated cervical cancer screening guidelines. The suggested approach to screening differs from previous recommendations. HPV testing as a primary test (that is, HPV testing alone or followed by cytology) takes the spotlight now, according to the analysis by the Task Force.
In this Update, we highlight important studies published in the past year that address these issues.
Continue to: New tech's potential to identify high-grade...
New tech's potential to identify high-grade cervical dysplasia may be a boon to low-resource settings
Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
When cervical screening tests like cytology and HPV testing show abnormal results, colposcopy often is recommended. The goal of colposcopy is to identify the areas that might harbor a high-grade precancerous lesion or worse. The gold standard in this case, however, is histology, not colposcopic impression, as many studies have shown that colposcopy without biopsies is limited and that performance is improved with more biopsies.3,4
Visual inspection with acetic acid (VIA) is an approach used often in low-resource settings where visual impression is the gold standard. However, as with colposcopy, a visual evaluation without histology does not perform well, and often women are overtreated. Many attempts have been made with new technologies to overcome the limitations of time, cost, and workforce required for cytology and histology services. New disruptive technologies may be able to surmount human limitations and improve on not only VIA but also the need for histology.
Novel technology uses images to develop algorithm with predictive ability
In a recent observational study, Hu and colleagues used images that were collected during a large population study in Guanacaste, Costa Rica.5 More than 9,000 women were followed for up to 7 years, and cervical photographs (cervigrams) were obtained. Well-annotated histopathology results were obtained for women with abnormal screening, and 279 women had a high-grade dysplastic lesion or cancer.
Cervigrams from women with high-grade lesions and matched controls were collected, and a deep learning-based algorithm using artificial intelligence technology was developed using 70% of the images. The remaining 30% of images were used as a validation set to test the algorithm's ability to "predict" high-grade dysplasia without knowing the final result.
Findings. Termed automated visual evaluation (AVE), this new technology demonstrated a very accurate ability to identify high-grade dysplasia or worse, with an area under the curve (AUC) of 0.91 from merely a cervicogram (FIGURE). This outperformed conventional Pap smears (AUC, 0.71), liquid-based cytology (AUC, 0.79) and, surprisingly, highly sensitive HPV testing (AUC, 0.82) in women in the prime of their screening ages (>25 years of age).

Colposcopy remains the gold standard for evaluating abnormal cervical cancer screening tests in the United States. But can we do better for our patients using new technologies like AVE? If validated in large-scale trials, AVE has the potential to revolutionize cervical cancer screening in low-resource settings where follow-up and adequate histology services are limited or nonexistent. Future large studies are necessary to evaluate the role of AVE alone versus in combination with other diagnostic testing (such as HPV testing) to detect cervical lesions globally.
Continue to: Data offer persuasive evidence...
Data offer persuasive evidence to abandon minimally invasive surgery in management of early-stage cervical cancer
Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
Over the past decade, gynecologic cancer surgery has shifted from what routinely were open procedures to the adoption of minimally invasive techniques. Recently, a large, well-designed prospective study and a large retrospective study both demonstrated worse outcomes with minimally invasive radical hysterectomy (MIRH) as compared with traditional open radical abdominal hysterectomy (RAH). These 2 landmark studies, initially presented at the Society of Gynecologic Oncology's 2018 annual meeting and later published in the New England Journal of Medicine, have really affected the gynecologic oncology community.
Shorter overall survival in women who had MIRH
Melamed and colleagues conducted a large, retrospective US-based study to evaluate all-cause mortality in women with cervical cancer who underwent MIRH compared with those who had RAH.6 The authors also sought to evaluate national trends in 4-year relative survival rates after minimally invasive surgery was adopted.
The study included 2,461 women who met the inclusion criteria; 49.8% (1,225) underwent MIRH procedures and, of those, 79.8% (978) had robot-assisted laparoscopy. Most women had stage IB1 tumors (88%), and most carcinomas were squamous cell (61%); 40.6% of tumors were less than 2 cm in size. There were no differences between the 2 groups with respect to rates of positive parametria, surgical margins, and lymph node involvement. Administration of adjuvant therapy, in those who qualified, was also similar between groups.
Results. At a median follow-up of 45 months, 94 deaths occurred in the minimally invasive group and 70 in the open surgery group. The risk of death at 4 years was 9.1% in the minimally invasive group versus 5.3% in the open surgery group, with a 65% higher risk of death from any cause, which was highly statistically significant.
Prospective trial showed MIRH was associated with lower survival rates
From 2008 to 2017, Ramirez and colleagues conducted a phase 3, multicenter, randomized controlled trial to prospectively establish the noninferiority of MIRH compared with RAH.7 The study included 631 women from 33 centers. The prespecified expected disease-free survival rate was 90% at 4.5 years.
To be included as a site, centers were required to submit details from 10 minimally invasive cases as well as 2 unedited videos for review by the trial management committee. In contrast to Melamed and colleagues' retrospective study, of the 319 procedures that were classified as minimally invasive, only 15.6% were robotically assisted. Similarly, most women had stage IB1 tumors (91.9%), and most were squamous cell carcinomas (67%). There were also no differences in the postoperative pathology findings or the need for adjuvant therapy administered between groups. The median follow-up was 2.5 years.
Results. At that time there were 27 recurrences in the MIRH group and 7 in the RAH group; there were also 19 deaths after MIRH and 3 after RAH. Disease-free survival at 4.5 years was 86% with MIRH versus 96.5% with RAH. Reported 3-year disease-free survival and overall survival were also significantily lower in the minimally invasive subgroup (91.2% vs 97.1%, 93.8% vs 99.0%, respectively).
Study limitations. Criticisms of this trial are that noninferiority could not be declared; in addition, the investigators were unable to complete enrollment secondary to early enrollment termination after the data and safety monitoring board raised survival concerns.
Many argue that subgroup analyses suggest a lower risk of poor outcomes in patients with smaller tumors (<2 cm); however, it is critical to note that this study was not powered to detect these differences.
The evidence is compelling and demonstrates potentially worse disease-related outcomes using MIRH when compared to traditional RAH with respect to cervical cancer recurrence, rates of death, and disease-free and overall survival. Several hypotheses have been proposed, and future research is needed to elucidate the differences in variables responsible for the outcomes demonstrated in these studies. Although there has been no ban on robot-assisted surgical devices or traditional minimally invasive techniques, the National Comprehensive Cancer Network has updated its recommendations to include careful counseling of patients who require a surgical approach for the management of early-stage cervical cancer.
Continue to: USPSTF updated guidance on cervical cancer screening...
USPSTF updated guidance on cervical cancer screening
Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Past guidelines for cervical cancer screening have included testing for high-risk HPV (hrHPV) as a cotest with cytology or for triage of atypical squamous cells of undetermined significance (ASCUS) in women aged 30 to 65 years.8 The American Society for Colposcopy and Cervical Pathology and the Society of Gynecologic Oncology, with other stakeholder organizations, issued interim guidance for primary HPV testing--that is, HPV test first and, in the case of non-16/18 hrHPV types, cytology as a triage. The most recent evidence report and systematic review by Melnikow and colleagues for the USPSTF offers an in-depth analysis of risks, benefits, harms, and value of cotesting and other management strategies.9
Focus on screening effectiveness
Large trials of cotesting were conducted in women aged 25 to 65.10-13 These studies all consistently showed that primary hrHPV screening led to a statistically significant increased detection of cervical intraepithelial neoplasia (CIN) 3+ in the initial round of screening, with a relative risk of detecting CIN 3+ ranging from 1.61 to 7.46 compared with cytology alone.
Four additional studies compared cotesting with conventional cytology for the detection of CIN 3+. None of these trials demonstrated a significantly higher detection rate of CIN 3+ with cotesting compared with conventional cytology testing alone. Notably, the studies reviewed were performed in European countries that had organized screening programs in place and a nationalized health care system. Thus, these data may not be as applicable to women in the United States, particularly to women who have limited health care access.
Risks of screening
In the same studies reviewed for screening effectiveness, the investigators found that overall, screening with hrHPV primary or cotesting was associated with more false-positive results and higher colposcopy rates. Women screened with hrHPV alone had a 7.9% referral rate to colposcopy, while those screened with cytology had a 2.8% referral rate to colposcopy. Similarly, the rate of biopsy was higher in the hrHPV-only group (3.2% vs 1.3%).
Overall, while cotesting might have some improvement in performance compared with hrHPV as a single modality, there might be risks of overreferral to colposcopy and overtreatment with additional cytology over hrHPV testing alone.
This evidence review also included an analysis of more potential harms. Very limited evidence suggests that positive hrHPV test results may be associated with greater psychological harm, including decreased sexual satisfaction, increased anxiety and distress, and worse feelings about sexual partners, than abnormal cytology results. These were assessed, however, 1 to 2 weeks after the test results were provided to the patients, and long-term assessment was not done.
New recommendations from the USPSTF
Based on these data, the USPSTF issued new recommendations regarding screening (TABLE).14 For women aged 21 to 29, cytology alone should be used for screening every 3 years. Women aged 30 to 65 can be screened with cytology alone every 3 years, with hrHPV testing alone every 5 years, or with cotesting every 5 years.

Primary screening with hrHPV is more effective in diagnosing a CIN 3+ than cytology alone. Cotesting with cytology and hrHPV testing appears to have limited performance improvement, with potential harm, compared with hrHPV testing alone in diagnosing CIN 3+. The Task Force recommendation is hrHPV testing alone or cotesting every 5 years.
Cervical cancer rates remain low in the United States, with the incidence having plateaued for decades. And yet, in 2019, more than 13,000 US women will be diagnosed with cervical cancer.1 Globally, in 2018 almost 600,000 women were diagnosed with cervical cancer2; it is the fourth most frequent cancer in women. This is despite the fact that we have adequate primary and secondary prevention tools available to minimize—and almost eliminate—cervical cancer. We must continue to raise the bar for preventing, screening for, and managing this disease.
Human papillomavirus (HPV) vaccines provide a highly effective primary prevention strategy, but we need to improve our ability to identify and diagnose dysplastic lesions prior to the development of cervical cancer. Highly sensitive HPV testing and cytology is a powerful secondary prevention approach that enables us to assess a woman’s risk of having precancerous cells both now and in the near future. These modalities have been very successful in decreasing the incidence of cervical cancer in the United States and other areas with organized screening programs. In low- and middle-income countries, however, access to, availability of, and performance with these modalities is not optimal. Innovative strategies and new technologies are being evaluated to overcome these limitations.
Advances in radiation and surgical technology have enabled us to vastly improve cervical cancer treatment. Women with early-stage cervical cancer are candidates for surgical management, which frequently includes a radical hysterectomy and lymph node dissection. While these surgeries traditionally have been performed via an exploratory laparotomy, minimally invasive techniques (laparoscopic and robot-assisted surgical techniques) have decreased the morbidity with these surgeries. Notable new studies have shed light on the comparative effectiveness of minimally invasive technologies and have shown us that new is not always better.
The US Preventive Services Task Force (USPSTF) recently released its updated cervical cancer screening guidelines. The suggested approach to screening differs from previous recommendations. HPV testing as a primary test (that is, HPV testing alone or followed by cytology) takes the spotlight now, according to the analysis by the Task Force.
In this Update, we highlight important studies published in the past year that address these issues.
Continue to: New tech's potential to identify high-grade...
New tech's potential to identify high-grade cervical dysplasia may be a boon to low-resource settings
Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
When cervical screening tests like cytology and HPV testing show abnormal results, colposcopy often is recommended. The goal of colposcopy is to identify the areas that might harbor a high-grade precancerous lesion or worse. The gold standard in this case, however, is histology, not colposcopic impression, as many studies have shown that colposcopy without biopsies is limited and that performance is improved with more biopsies.3,4
Visual inspection with acetic acid (VIA) is an approach used often in low-resource settings where visual impression is the gold standard. However, as with colposcopy, a visual evaluation without histology does not perform well, and often women are overtreated. Many attempts have been made with new technologies to overcome the limitations of time, cost, and workforce required for cytology and histology services. New disruptive technologies may be able to surmount human limitations and improve on not only VIA but also the need for histology.
Novel technology uses images to develop algorithm with predictive ability
In a recent observational study, Hu and colleagues used images that were collected during a large population study in Guanacaste, Costa Rica.5 More than 9,000 women were followed for up to 7 years, and cervical photographs (cervigrams) were obtained. Well-annotated histopathology results were obtained for women with abnormal screening, and 279 women had a high-grade dysplastic lesion or cancer.
Cervigrams from women with high-grade lesions and matched controls were collected, and a deep learning-based algorithm using artificial intelligence technology was developed using 70% of the images. The remaining 30% of images were used as a validation set to test the algorithm's ability to "predict" high-grade dysplasia without knowing the final result.
Findings. Termed automated visual evaluation (AVE), this new technology demonstrated a very accurate ability to identify high-grade dysplasia or worse, with an area under the curve (AUC) of 0.91 from merely a cervicogram (FIGURE). This outperformed conventional Pap smears (AUC, 0.71), liquid-based cytology (AUC, 0.79) and, surprisingly, highly sensitive HPV testing (AUC, 0.82) in women in the prime of their screening ages (>25 years of age).

Colposcopy remains the gold standard for evaluating abnormal cervical cancer screening tests in the United States. But can we do better for our patients using new technologies like AVE? If validated in large-scale trials, AVE has the potential to revolutionize cervical cancer screening in low-resource settings where follow-up and adequate histology services are limited or nonexistent. Future large studies are necessary to evaluate the role of AVE alone versus in combination with other diagnostic testing (such as HPV testing) to detect cervical lesions globally.
Continue to: Data offer persuasive evidence...
Data offer persuasive evidence to abandon minimally invasive surgery in management of early-stage cervical cancer
Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
Over the past decade, gynecologic cancer surgery has shifted from what routinely were open procedures to the adoption of minimally invasive techniques. Recently, a large, well-designed prospective study and a large retrospective study both demonstrated worse outcomes with minimally invasive radical hysterectomy (MIRH) as compared with traditional open radical abdominal hysterectomy (RAH). These 2 landmark studies, initially presented at the Society of Gynecologic Oncology's 2018 annual meeting and later published in the New England Journal of Medicine, have really affected the gynecologic oncology community.
Shorter overall survival in women who had MIRH
Melamed and colleagues conducted a large, retrospective US-based study to evaluate all-cause mortality in women with cervical cancer who underwent MIRH compared with those who had RAH.6 The authors also sought to evaluate national trends in 4-year relative survival rates after minimally invasive surgery was adopted.
The study included 2,461 women who met the inclusion criteria; 49.8% (1,225) underwent MIRH procedures and, of those, 79.8% (978) had robot-assisted laparoscopy. Most women had stage IB1 tumors (88%), and most carcinomas were squamous cell (61%); 40.6% of tumors were less than 2 cm in size. There were no differences between the 2 groups with respect to rates of positive parametria, surgical margins, and lymph node involvement. Administration of adjuvant therapy, in those who qualified, was also similar between groups.
Results. At a median follow-up of 45 months, 94 deaths occurred in the minimally invasive group and 70 in the open surgery group. The risk of death at 4 years was 9.1% in the minimally invasive group versus 5.3% in the open surgery group, with a 65% higher risk of death from any cause, which was highly statistically significant.
Prospective trial showed MIRH was associated with lower survival rates
From 2008 to 2017, Ramirez and colleagues conducted a phase 3, multicenter, randomized controlled trial to prospectively establish the noninferiority of MIRH compared with RAH.7 The study included 631 women from 33 centers. The prespecified expected disease-free survival rate was 90% at 4.5 years.
To be included as a site, centers were required to submit details from 10 minimally invasive cases as well as 2 unedited videos for review by the trial management committee. In contrast to Melamed and colleagues' retrospective study, of the 319 procedures that were classified as minimally invasive, only 15.6% were robotically assisted. Similarly, most women had stage IB1 tumors (91.9%), and most were squamous cell carcinomas (67%). There were also no differences in the postoperative pathology findings or the need for adjuvant therapy administered between groups. The median follow-up was 2.5 years.
Results. At that time there were 27 recurrences in the MIRH group and 7 in the RAH group; there were also 19 deaths after MIRH and 3 after RAH. Disease-free survival at 4.5 years was 86% with MIRH versus 96.5% with RAH. Reported 3-year disease-free survival and overall survival were also significantily lower in the minimally invasive subgroup (91.2% vs 97.1%, 93.8% vs 99.0%, respectively).
Study limitations. Criticisms of this trial are that noninferiority could not be declared; in addition, the investigators were unable to complete enrollment secondary to early enrollment termination after the data and safety monitoring board raised survival concerns.
Many argue that subgroup analyses suggest a lower risk of poor outcomes in patients with smaller tumors (<2 cm); however, it is critical to note that this study was not powered to detect these differences.
The evidence is compelling and demonstrates potentially worse disease-related outcomes using MIRH when compared to traditional RAH with respect to cervical cancer recurrence, rates of death, and disease-free and overall survival. Several hypotheses have been proposed, and future research is needed to elucidate the differences in variables responsible for the outcomes demonstrated in these studies. Although there has been no ban on robot-assisted surgical devices or traditional minimally invasive techniques, the National Comprehensive Cancer Network has updated its recommendations to include careful counseling of patients who require a surgical approach for the management of early-stage cervical cancer.
Continue to: USPSTF updated guidance on cervical cancer screening...
USPSTF updated guidance on cervical cancer screening
Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Past guidelines for cervical cancer screening have included testing for high-risk HPV (hrHPV) as a cotest with cytology or for triage of atypical squamous cells of undetermined significance (ASCUS) in women aged 30 to 65 years.8 The American Society for Colposcopy and Cervical Pathology and the Society of Gynecologic Oncology, with other stakeholder organizations, issued interim guidance for primary HPV testing--that is, HPV test first and, in the case of non-16/18 hrHPV types, cytology as a triage. The most recent evidence report and systematic review by Melnikow and colleagues for the USPSTF offers an in-depth analysis of risks, benefits, harms, and value of cotesting and other management strategies.9
Focus on screening effectiveness
Large trials of cotesting were conducted in women aged 25 to 65.10-13 These studies all consistently showed that primary hrHPV screening led to a statistically significant increased detection of cervical intraepithelial neoplasia (CIN) 3+ in the initial round of screening, with a relative risk of detecting CIN 3+ ranging from 1.61 to 7.46 compared with cytology alone.
Four additional studies compared cotesting with conventional cytology for the detection of CIN 3+. None of these trials demonstrated a significantly higher detection rate of CIN 3+ with cotesting compared with conventional cytology testing alone. Notably, the studies reviewed were performed in European countries that had organized screening programs in place and a nationalized health care system. Thus, these data may not be as applicable to women in the United States, particularly to women who have limited health care access.
Risks of screening
In the same studies reviewed for screening effectiveness, the investigators found that overall, screening with hrHPV primary or cotesting was associated with more false-positive results and higher colposcopy rates. Women screened with hrHPV alone had a 7.9% referral rate to colposcopy, while those screened with cytology had a 2.8% referral rate to colposcopy. Similarly, the rate of biopsy was higher in the hrHPV-only group (3.2% vs 1.3%).
Overall, while cotesting might have some improvement in performance compared with hrHPV as a single modality, there might be risks of overreferral to colposcopy and overtreatment with additional cytology over hrHPV testing alone.
This evidence review also included an analysis of more potential harms. Very limited evidence suggests that positive hrHPV test results may be associated with greater psychological harm, including decreased sexual satisfaction, increased anxiety and distress, and worse feelings about sexual partners, than abnormal cytology results. These were assessed, however, 1 to 2 weeks after the test results were provided to the patients, and long-term assessment was not done.
New recommendations from the USPSTF
Based on these data, the USPSTF issued new recommendations regarding screening (TABLE).14 For women aged 21 to 29, cytology alone should be used for screening every 3 years. Women aged 30 to 65 can be screened with cytology alone every 3 years, with hrHPV testing alone every 5 years, or with cotesting every 5 years.

Primary screening with hrHPV is more effective in diagnosing a CIN 3+ than cytology alone. Cotesting with cytology and hrHPV testing appears to have limited performance improvement, with potential harm, compared with hrHPV testing alone in diagnosing CIN 3+. The Task Force recommendation is hrHPV testing alone or cotesting every 5 years.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- World Health Organization website. Cervical cancer. https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/. Accessed April 17, 2019.
- Wentzensen N, Walker JL, Gold MA, et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol. 2015;33:83-89.
- Gage JC, Hanson VW, Abbey K, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108:264-272.
- Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
- Canfell K, Caruana M, Gebski V, et al. Cervical screening with primary HPV testing or cytology in a population of women in which those aged 33 years or younger had previously been offered HPV vaccination: results of the Compass pilot randomised trial. PLoS Med. 2017;14:e1002388. doi:10.1371/journal.pmed.1002388.
- Leinonen MK, Nieminen P, Lonnberg S, et al. Detection rates of precancerous and cancerous cervical lesions within one screening round of primary human papillomavirus DNA testing: prospective randomised trial in Finland. BMJ. 2012;345:e7789.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Ronco G, Fioprgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- World Health Organization website. Cervical cancer. https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/. Accessed April 17, 2019.
- Wentzensen N, Walker JL, Gold MA, et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol. 2015;33:83-89.
- Gage JC, Hanson VW, Abbey K, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108:264-272.
- Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
- Canfell K, Caruana M, Gebski V, et al. Cervical screening with primary HPV testing or cytology in a population of women in which those aged 33 years or younger had previously been offered HPV vaccination: results of the Compass pilot randomised trial. PLoS Med. 2017;14:e1002388. doi:10.1371/journal.pmed.1002388.
- Leinonen MK, Nieminen P, Lonnberg S, et al. Detection rates of precancerous and cancerous cervical lesions within one screening round of primary human papillomavirus DNA testing: prospective randomised trial in Finland. BMJ. 2012;345:e7789.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Ronco G, Fioprgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
2018 Update on cervical disease
In this Update, I outline important findings from several studies published in the past year. First and foremost, what are best practices for performing colposcopy in the United States? The American Society for Colposcopy and Cervical Pathology (ASCCP) released guidelines addressing such practices. Second, what are the implications of repeated negative screening and patients’ acceptance of extended screening intervals? A recent observational cohort study and a large study of Kaiser Permanente’s practices since 2003 shed light on these questions. Last, where do we stand with HPV vaccination? Two studies shed light on the efficacy of vaccination against human papillomavirus (HPV), and subsequent cervical intraepithelial neoplasia (CIN) and cervical cancer.
ASCCP releases updated quality guidelines for performing colposcopy
Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223-229.
Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235-241.
Wentzensen N, Schiffman M, Silver MI, et al. ASCCP colposcopy standards: Risk-based colposcopy practice. J Low Genit Tract Dis. 2017;21(4):230-234.
In October 2017, the ASCCP released a set of standards on the role and performance of colposcopy that represents best practices in women's health care in the United States. The work of these groups comprised a literature search, a national survey of ASCCP members, public comment, and expert consensus, and addressed:
- establishment of a common understanding of 1) the benefits of colposcopy in health maintenance and risk prevention, 2) risks presented by the procedure, and 3) terminology and criteria for reporting results that reduce subjectivity in reporting
- the rationale for, approach to, and recommendations regarding assessment of cervical precancer at colposcopy
- both minimum and comprehensive guidelines for the colposcopic examination, from preprocedure evaluation to follow-up.
Each Working Group performed the analysis and produced its own report and recommendations, published sequentially in a 2017 issue of the Journal of Lower Urinary Tract Disease. The findings and standards that they produced 1) offer essential insight for high- and low-volume coloposcopists and 2) are intended to improve the quality of colposcopy, reduce subjectivity in reporting findings, and improve the sensitivity of the procedure. Aware of the concerns and objectives of payers and hospital credentialing committees, the ASCCP found it important to establish what would be considered US-based minimum quality standards and to present goals that providers and systems could strive to achieve.
Selected details of the 3 guideline reports
The past 6 years have brought us through a great deal of transition in the prevention of cervical precancer, with regard to screening intervals and types of screening (for example, see "HPV−cytology co-testing every 3 years lowers population rates of cervical precancer and cancer," in the 2017 "Cervical Disease Update," OBG Management, May 2017). The most significant change was in 2012, when American Cancer Society/ASCCP guidelines were revised to abandon screening with annual Pap testing on most patients--an effort to strike a balance between the lifesaving value of identifying precancer and the potential harm of excessive colposcopy.
If, as the US Preventive Services Task Force (USPSTF) has declared, excessive colposcopy is a harm of screening, then we should be adapting our practices, especially in terms of the frequency of screening, to 1) reduce the risk of unnecessarily screening and potentially triaging patients to colposcopy and 2) bring the highest standards of performance and reporting to colposcopic practice (see "Why aren't you doing a Pap on me?"). In other words, "This is the way I've always done it" shouldn't characterize efforts to detect disease, when the data are clear that doing less might be more beneficial for our patients. Adherence to extended screening intervals is not yet good enough to balance benefit and risk of harm, as Rendle and colleagues showed in an article this year in Preventive Medicine (discussed in the next section of this "Update"). We need to do better.
Adherence to extended screening intervals means fewer colposcopies and less exposure to risk of attendant harm. But adherence is not purely mechanical: It can be intertwined with how patients feel about the care we provide and about their safety. When a patient moves from years of annual Pap testing to less frequent screening, she might express her concern by challenging your expertise.
In my practice, I have a simple, 1-minute conversation with the patient that is important to wedge into our discussion of her care. I explain that increasing the frequency of screening is only going to increase the chance that I will perform a colposcopy but not increase the chance that I will identify cancer. I conclude by reassuring her that I do not want to harm her, or to cause her anxiety, pain, cramping, or bleeding--or require her to spend time away from work or show her family that she is suffering. Patients are reassured and happy after that, I find. This is a patient-centered discussion that providers need to have if they hope to establish and maintain adherence to recommended screening intervals.
-- Mark H. Einstein, MD, MS
Here is a limited encapsulation of the 3 wide-ranging reports on the ASCCP colposcopy recommendations:
Role of colposcopy; benefits, potential harms, terminology (Khan et al; Working Group 1). The authors provide reinforcement: The strategic benefit of colposcopy is clear--a "drastic" reduction in excisional procedures by limiting them to patients in whom cervical cancer precursors have been confirmed or who present a high risk of occult invasive cervical cancer. Furthermore, the rate of adverse events for colposcopy−including significant bleeding and infection−is low.
Nevertheless, the potential for harm exists when an unskilled provider performs colposcopy; the Working Group emphasizes that proficiency comes with training and experience. Even in skilled hands, however, anxiety and the discomfort of a speculum examination and from acetic acid, as well as cramping and pain, might dissuade some women from receiving regular cervical screening subsequently. The authors cite research showing that educational interventions can help soothe anxiety about colposcopy and potential findings,1,2 although consensus is lacking on the value of such interventions.
The Working Group 1) developed recommended terminology for reporting findings in colposcopy practice in the United States and 2) defined the comprehensive documentation of the procedure as comprising cervix and squamocolumnar junction visibility; acetowhitening; presence of a lesion; lesion visibility, size and location of lesion(s); vascular changes; other features; and colposcopic impression (TABLE 1).3 Minimum criteria for reporting colposcopy results were also proposed, extracted from the comprehensive standards.
Risk-based colposcopy practice (Wentzensen et al). Women referred to colposcopy present with a range of underlying risk of precancer. Assessing that risk at the colposcopy visit allows the provider to modify and individualize the procedure. Risk can be estimated by referral screening tests (eg, cytology, HPV testing) performed in conjunction with the colposcopic impression. As opposed to a lack of standards for a minimum number of biopsies, the Working Group recommends that, as a standard, multiple targeted biopsies (≥2, as many as 4) are needed to improve detection of prevalent precancers. Colposcopic impression alone is not enough to diagnose precancerous cells. Let's face it: Our eyes with a colposcopic magnification of 15X do not make a microscope.
Implementing the Working Group's recommendations is expected to lead to improved detection of cervical precancers at colposcopy and to provide stronger reassurance of negative colposcopy results. Regarding biopsy of lesions, ASCCP did not find added benefit to taking random (nondirected) biopsies for women at low risk for precancer. The sensitivity of biopsy is increased by taking multiple biopsies of suspicious lesions, based on a risk-based approach detailed in the ASCCP guidelines. So, depending on underlying risk (estimated from screening and triage tests), colposcopy practice can be adapted in a useful manner to account for differences in risk:
- When risk of precancer is very high, for example, immediate treatment might reduce cost and prevent the patient from being lost to follow-up. When risk is very low, consider expectant management (serial cytology and HPV testing) with limited need for biopsy. In a setting of intermediate risk, the Working Group proposes, "multiple biopsies of acetowhite lesions lead to increased detection of precancer."
- Perform multiple biopsies that target all areas characterized by 1) acetowhitening, 2) metaplasia, and 3) higher abnormalities.
- Do not perform nontargeted biopsies on patients at the lowest end of risk who have been referred to colposcopy−ie, those with cytology that is less than HSIL; no evidence of HPV types 16/18; and a normal colposcopic impression (ie, no acetowhitening or metaplasia, or other visible abnormality).
- Immediate excision without biopsy confirmation or colposcopy with multiple targeted biopsies is acceptable in nonpregnant women 25 years and older whose risk of precancer is very high (≥2 of the following: HSIL cytology, HPV 16- or HPV 18-positive(or both), and high-grade colposcopy impression). Endocervical sampling should be conducted according to ASCCP's 2012 management guidelines. If biopsies do not show precancer, manage the patient using ASCCP's 2012 management guidelines, the Working Group recommends.
How do we perform colposcopy? Implications for establishing standards (Waxman et al; Working Group 3). To serve as a guide to standardizing colposcopy across the United States, the authors defined and delineated 6 major components (and their constituent parts) of a comprehensive colposcopy:
- precolposcopy evaluation
- the examination
- use of colposcopy adjuncts
- documentation
- biopsy sampling
- postcolposcopy procedures.
The constituent parts of these components are laid out in TABLE 2.4 A set of components for a minimum colposcopy procedure is drawn mostly from the comprehensive protocol.
The Working Group acknowledges that, in the United States, "the accuracy and reproducibility of colposcopy with biopsy as a diagnostic tool are limited." Why? Three contributing factors, the authors write, might be the absence of practice recommendations for colposcopy-biopsy procedures; of measures of quality assurance; and of standardized terminology.
Standards arrive for practice
Minimum quality standards are becoming part of almost everything US health care providers do−whether it is documentation, billing practices, or good care. Our work in gynecology, including colposcopy, is now being assessed as it is in much of the world, where minimum standards are already in place and guidelines must be followed. (In some countries standards require performing a minimum number of colposcopies per year to be identified as a "certified" colposcopist.)
What should be considered "minimum standards" for colposcopy in the United States? These ASCCP reports ask, and deliver answers to that question, bringing a broad range of concerns about high-quality practice into focus. Physicians and advanced-practice clinicians in this country who perform colposcopies have been trained to do so, but they have never had minimum standards by which to model and assess their performance. A procedure that has the potential to lead to additional testing for either cervical cancer, or to surveillance, should have minimum standards by which it is performed and documented in the United States as it is for much of the world that has widespread cervical cancer screening.
Guidance and recommendations developed by ASCCP offer women's health care providers a set of comprehensive and, alternatively, minimum quality standards that should be incorporated into practice across all aspects of the colposcopic exam, including precolposcopy evaluation, how to perform the procedure, how to document and report findings (TABLE 2), biopsy practice, establish quality control and assurance, as well as postprocedure follow-up. In taking the initiative to draw up these standards, ASCCP encourages providers to exceed the minimum requirements.
Read about adherence to cervical cancer screening.
Cervical screening adherence is relatively low, but safe. Extended intervals are very safe.
Castle PE, Kinney WK, Xue X, et al. Effect of several negative rounds of human papillomavirus and cytology co-testing on safety against cervical cancer: an observational cohort study. Ann Intern Med. 2018;168(1):20-29.
Rendle KA, Schiffman M, Cheung LC, et al. Adherence patterns to extended cervical screening intervals in women undergoing human papillomavirus (HPV) and cytology cotesting. Prev Med. 2018;109:44-50.
Patients who have been screened for cervical cancer for a long time--decades, even--have a diminishing likelihood that cancer will ever be detected. Furthermore, highest-risk patients already have been triaged into further testing or procedures, such as a loop excision electrosurgical procedure or hysterectomy. Two recent studies examined the implications of repeated negative screening and patients' acceptance of extended screening intervals.
Details of the studies
Several negative rounds of cotesting (HPV and cytology) might justify changes to the screening interval. To determine the rate of detection of CIN3, adenocarcinoma in situ, and cervical cancer (≥CIN3) in routine practice after successive negative screening at 3-year intervals, Castle and colleagues looked at records of more than 990,000 women in an integrated health care system who underwent cotesting (HPV and cytology) between 2003 and 2014. They determined that the risk of invasive cervical cancer and ≥CIN3 declined with each round of cotesting; the absolute risk fell more between first and second rounds than between second and third rounds.
At any given round of cotesting, Castle found that the ability to reassure a patient about cancer and cancer risk was similar when looking at an HPV result alone, whatever the cytology or HPV-cytology cotest result was. The investigators concluded that similar patterns of risk would have been seen had stand-alone HPV testing been used, instead of co-testing, (HPV testing alone might have missed a few cases of CIN3 and adenocarcinoma in situ leading to cancer). A single negative cotest was so effective at ruling out ≥CIN3 and cervical cancer that, after a second round of cotesting, they found that no interval cancer cases were detected among women who had a negative HPV result.
Women aged 50 years or older had a 5- to 6-fold lower risk after their third consecutive negative cotest than women aged 30 to 39 years had after their first negative cotest. These data support the ideas, Castle noted, that 1) assigning screening intervals based on both age and number of previous negative screens and 2) extending the screening interval even further than 3 years after 2--perhaps even after 1--negative cotests or HPV tests are worth entertaining. Screening women of this age becomes inefficient and cost-ineffective, even at 5-year intervals.
Is patients' adherence to an extended interval of cotesting reliable enough to change practice? Rendle and colleagues examined the records of more than 491,000 women (in the same integrated health care system that Castle studied) who had undergone routine cervical cancer screening between 2003 and 2015. Their goal was to determine how high adherence had become to the system's recommendation of an every-3-year screening interval--an interval that mirrors long-standing guidelines elsewhere.
In short, researchers observed increasing and relatively rapid clinical adoption of every-3-year cotesting for routine cervical screening over time; between 2003 and 2009, the cohort grew significantly less likely overall to come in early for screening. In this setting, adoption of an extended screeninginterval appears to run counter to earlier understanding that patients are likely to resist such extension.
Women aged 60 to 64 were most likely to screen early across 2 consecutive intervals. What Rendle termed a "modest" decrease in the percentage of late screeners (but still within a 5-year interval) was also noted during adoption of the 3-year interval.
What next?
Molecular-based testing. Research, mostly outside of the United States, is taking us in the direction of molecular-based technologies as at least a component of cervical cancer screening. Today, we rely mostly on Pap tests and colposcopy, but these are subjective screens, with a human operator. With molecular testing (mostly of components of HPV), results are objective--a "Yes" or "No" finding based on clinically validated thresholds. Methods such as genotyping, P16INK4a/Ki-67 gene product dual-stain cytology, and testing for E6 and E7 HPV mRNA transcripts are in development, and hold promise to allow us to screen safely using almost completely molecular testing, thus eliminating human error and subjectivity and enriching the population that needs further management with very sensitive and potentially specific testing.
We are being presented with the possibility that almost all aspects of screening can be done without a provider, until the patient needs treatment.
Access to screening. Research is also looking at improving access, such as self-sampling for primary screening. That includes home cervical and vaginal sampling, with specimens mailed to the laboratory, from where results and follow-up instructions as communicated to patients. The Netherlands and the United Kingdom are moving to self-sampling primary screens; the United States is not--yet. But that is the direction research is taking us.
Modified guidelines. Eyes are on the work of the USPSTF. Last year, the Task Force issued draft recommendations (https://www.uspre ventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2#clinical), followed by a comment period (now closed), for updating 2012 cervical cancer screening guidelines in a way that would trigger a major change in clinical practice. Those draft recommendations and public comments are under review; final recommendations are possible within this calendar year.
Continue to follow current screening guidelines; they are safe and effective for preventing cervical cancer. This assumes adherence to intervals, which is both the provider's and the patient's responsibility: First, less is more; too much screening ("I've always done it this way") can be harmful. Second, screening at intervals set by the guidelines is extremely safe, despite earlier reports or provider concerns that suggest otherwise.
Patients who have undergone several rounds of negative screening have a markedly diminished risk of cervical cancer. Serve them best by performing this underutilized gyn procedure: Sit on your hands.
Be aware that winds of change are blowing: What constitutes appropriate screening intervals is up for discussion this year, and molecular-based testing technologies that are under investigation have the potential to someday be a vast improvement over current good, but subjective, interpretations of results.
Last, promote primary prevention of cervical cancer with HPV vaccination in your practice to increase the percentage of protected patients. Doing so will contribute not only to their long-term health but also, at a societal level, to a herd immunity effect.5 Any positive HPV infection in a future of a well-vaccinated population will be significant, and HPV-targeted technologies to identify the highest risk women will be the most efficient screening.
Read about the safety and efficacy of HPV vaccination.
Primary prevention of cervical cancer with vaccination is critical in any cancer prevention program
Benard VB, Castle PE, Jenison SA, et al; New Mexico HPV Pap Registry Steering Committee. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3(6):833-837.
Luostarinen T, Apter D, Dillner J, et al. Vaccination protects against invasive HPV-associated cancers. Int J Cancer. 2018;142(10):2186-2187.
The success story of HPV vaccination, after more than a decade of use, continued to unfold in important ways over the past year.
Safety. With tens of millions of doses delivered, we know that the vaccine is safe, and we have retreated on some of the precautions that we once took: For example, we no longer perform a routine pregnancy test before vaccination on reproductive-age women.
Efficacy. We have learned, based on what we see in Australia and Western Europe, that vaccination is highly effective. We are also starting to see evidence of efficacy in areas of the United States, even though the vaccine is voluntary and there are no school-based recommendations. And we know that herd vaccination is very effective. The 2 studies described here add to our understanding of how vaccination is having an impact on endpoints.
Findings of the 2 studies
HPV vaccination has a direct impact on the precursor of cancer, CIN. Benard and colleagues examined data from the New Mexico HPV Pap Registry, a mandatory statewide surveillance system of cervical cancer screening that captured estimates of both screening prevalence and CIN since the time HPV vaccination was introduced in 2007 to 2014. The investigators examined registry data to gauge trends in the rate of CIN and to estimate the effect of HPV vaccination on that rate when adjusted for changes in screening for cervical cancer.
The incidence of CIN declined significantly across all grades in 2 groups between 2007 and 2015: females aged 15 to 19 years and females aged 20 to 24 years (but not in females 25 to 29 years of age). During those years, mean uptake of HPV vaccination among females 13 to 17 years of age reached as high as 40% (in 2014).
Although a reduction in CIN2 and CIN3 precancers "are early benchmarks for achieving this aim [of reducing the rate of cancer]," the investigators note, a reduction in CIN1 is "a direct measure of reductions in HPV infections requisite to the development of almost all invasive cervical cancer."
Benard moves on to conclude that a reduction in clinical outcomes of CIN among groups who are partially vaccinated for HPV is going to change clinical practice and reduce the cost-effectiveness of clinical care that supports prevention of cervical cancer. Of greatest importance, modalities and strategies for screening, and management algorithms, are going to need to evolve as HPV vaccination and cervical screening are integrated in a rational manner. Furthermore, it might be feasible to factor in population-level decreases in CIN among cohorts who are partially vaccinated for HPV when reassessing clinical practice guidelines for cervical cancer screening.
What does this mean? As we start to eliminate HPV from the population, any positive screening result will be that much more meaningful because the outcome--cervical cancer--will be much rarer. The onus will be on providers and public health officials to re-strategize how to screen what is going to be a widely-vaccinated population; more and more, we will be looking for needles in a haystack.
How are we going to someday screen women in their 20s who were vaccinated at 11 or 12 years of age? Likely, screening will start at a later age, and screening will be conducted at longer intervals. Any finding of HPV or disease is going to be highly significant, and likely, far less frequent.
HPV vaccination protects against invasive HPV-associated cancer. Luostarinen and colleagues report proof of highly efficacious protection offered by a population-based HPV vaccination program in Finland, in the form of a decrease in the key endpoint: cases of invasive HPV-associated cancer. Examining vaccinated (3,331 females) and unvaccinated (15,665 females) cohorts in the nationwide Finnish Cancer Registry, the investigators identified 10 cases of HPV-caused cancer (8 cervical, 1 oropharyngeal, 1 vulvar) in the unvaccinated females and 0 cases in vaccinated females--a statistically significant difference.
From the evidence gathered in this first intention-to-treat trial, the investigators conclude that vaccination protects against invasive HPV-associated cancer--what they call "an awaited, pivotal corollary" to high vaccine efficacy against HPV infection.
Summing up
This success story continues to unfold, despite well-organized, antivaccine campaigns. The HPV vaccine has been an easy target: It is novel, it involves a sexually transmitted infection, and the endpoint of protecting against invasive HPV-associated cancer is years--decades--away. But antivaccine groups can no longer argue the point that studies have not been designed to yield evidence of the impact of the vaccine on decisive endpoints, including cervical cancer.
The exciting news that the sought-out endpoint of HPV vaccination -- prevention of invasive HPV-associated cervical cancer -- is being realized. This should all the more energize you to:
- urge vaccination for your patients in whom it is indicated
- emphasize vaccine coverage in the young -- especially for the routinely recommended age group of 11 - and 12-year-olds
- strenuously reject and counter arguments made by segments of the public that HPV vaccination is neither safe nor necessary
- prepare for potential changes down the road in practice guidelines regarding screening (eg, raising the age at which screening begins) as the impact of vaccination on the health of women is felt.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Walsh JC, Curtis R, Mylotte M. Anxiety levels in women attending a colposcopy clinic: a randomised trial of an educational intervention using video colposcopy. Patient Educ Couns. 2004;55(2):247–251.
- Tomaino-Brunner C, Freda MC, Damus K, Runowicz CD. Can precolposcopy education increase knowledge and decrease anxiety? J Obstet Gynecol Neonatal Nurs. 1998;27(6):636–645.
- Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223–229.
- Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235–241.
- Wentzensen N, Schiffman M. Accelerating cervical cancer control and prevention. Lancet Public Health. 2018;3(1):e6–e7.
In this Update, I outline important findings from several studies published in the past year. First and foremost, what are best practices for performing colposcopy in the United States? The American Society for Colposcopy and Cervical Pathology (ASCCP) released guidelines addressing such practices. Second, what are the implications of repeated negative screening and patients’ acceptance of extended screening intervals? A recent observational cohort study and a large study of Kaiser Permanente’s practices since 2003 shed light on these questions. Last, where do we stand with HPV vaccination? Two studies shed light on the efficacy of vaccination against human papillomavirus (HPV), and subsequent cervical intraepithelial neoplasia (CIN) and cervical cancer.
ASCCP releases updated quality guidelines for performing colposcopy
Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223-229.
Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235-241.
Wentzensen N, Schiffman M, Silver MI, et al. ASCCP colposcopy standards: Risk-based colposcopy practice. J Low Genit Tract Dis. 2017;21(4):230-234.
In October 2017, the ASCCP released a set of standards on the role and performance of colposcopy that represents best practices in women's health care in the United States. The work of these groups comprised a literature search, a national survey of ASCCP members, public comment, and expert consensus, and addressed:
- establishment of a common understanding of 1) the benefits of colposcopy in health maintenance and risk prevention, 2) risks presented by the procedure, and 3) terminology and criteria for reporting results that reduce subjectivity in reporting
- the rationale for, approach to, and recommendations regarding assessment of cervical precancer at colposcopy
- both minimum and comprehensive guidelines for the colposcopic examination, from preprocedure evaluation to follow-up.
Each Working Group performed the analysis and produced its own report and recommendations, published sequentially in a 2017 issue of the Journal of Lower Urinary Tract Disease. The findings and standards that they produced 1) offer essential insight for high- and low-volume coloposcopists and 2) are intended to improve the quality of colposcopy, reduce subjectivity in reporting findings, and improve the sensitivity of the procedure. Aware of the concerns and objectives of payers and hospital credentialing committees, the ASCCP found it important to establish what would be considered US-based minimum quality standards and to present goals that providers and systems could strive to achieve.
Selected details of the 3 guideline reports
The past 6 years have brought us through a great deal of transition in the prevention of cervical precancer, with regard to screening intervals and types of screening (for example, see "HPV−cytology co-testing every 3 years lowers population rates of cervical precancer and cancer," in the 2017 "Cervical Disease Update," OBG Management, May 2017). The most significant change was in 2012, when American Cancer Society/ASCCP guidelines were revised to abandon screening with annual Pap testing on most patients--an effort to strike a balance between the lifesaving value of identifying precancer and the potential harm of excessive colposcopy.
If, as the US Preventive Services Task Force (USPSTF) has declared, excessive colposcopy is a harm of screening, then we should be adapting our practices, especially in terms of the frequency of screening, to 1) reduce the risk of unnecessarily screening and potentially triaging patients to colposcopy and 2) bring the highest standards of performance and reporting to colposcopic practice (see "Why aren't you doing a Pap on me?"). In other words, "This is the way I've always done it" shouldn't characterize efforts to detect disease, when the data are clear that doing less might be more beneficial for our patients. Adherence to extended screening intervals is not yet good enough to balance benefit and risk of harm, as Rendle and colleagues showed in an article this year in Preventive Medicine (discussed in the next section of this "Update"). We need to do better.
Adherence to extended screening intervals means fewer colposcopies and less exposure to risk of attendant harm. But adherence is not purely mechanical: It can be intertwined with how patients feel about the care we provide and about their safety. When a patient moves from years of annual Pap testing to less frequent screening, she might express her concern by challenging your expertise.
In my practice, I have a simple, 1-minute conversation with the patient that is important to wedge into our discussion of her care. I explain that increasing the frequency of screening is only going to increase the chance that I will perform a colposcopy but not increase the chance that I will identify cancer. I conclude by reassuring her that I do not want to harm her, or to cause her anxiety, pain, cramping, or bleeding--or require her to spend time away from work or show her family that she is suffering. Patients are reassured and happy after that, I find. This is a patient-centered discussion that providers need to have if they hope to establish and maintain adherence to recommended screening intervals.
-- Mark H. Einstein, MD, MS
Here is a limited encapsulation of the 3 wide-ranging reports on the ASCCP colposcopy recommendations:
Role of colposcopy; benefits, potential harms, terminology (Khan et al; Working Group 1). The authors provide reinforcement: The strategic benefit of colposcopy is clear--a "drastic" reduction in excisional procedures by limiting them to patients in whom cervical cancer precursors have been confirmed or who present a high risk of occult invasive cervical cancer. Furthermore, the rate of adverse events for colposcopy−including significant bleeding and infection−is low.
Nevertheless, the potential for harm exists when an unskilled provider performs colposcopy; the Working Group emphasizes that proficiency comes with training and experience. Even in skilled hands, however, anxiety and the discomfort of a speculum examination and from acetic acid, as well as cramping and pain, might dissuade some women from receiving regular cervical screening subsequently. The authors cite research showing that educational interventions can help soothe anxiety about colposcopy and potential findings,1,2 although consensus is lacking on the value of such interventions.
The Working Group 1) developed recommended terminology for reporting findings in colposcopy practice in the United States and 2) defined the comprehensive documentation of the procedure as comprising cervix and squamocolumnar junction visibility; acetowhitening; presence of a lesion; lesion visibility, size and location of lesion(s); vascular changes; other features; and colposcopic impression (TABLE 1).3 Minimum criteria for reporting colposcopy results were also proposed, extracted from the comprehensive standards.
Risk-based colposcopy practice (Wentzensen et al). Women referred to colposcopy present with a range of underlying risk of precancer. Assessing that risk at the colposcopy visit allows the provider to modify and individualize the procedure. Risk can be estimated by referral screening tests (eg, cytology, HPV testing) performed in conjunction with the colposcopic impression. As opposed to a lack of standards for a minimum number of biopsies, the Working Group recommends that, as a standard, multiple targeted biopsies (≥2, as many as 4) are needed to improve detection of prevalent precancers. Colposcopic impression alone is not enough to diagnose precancerous cells. Let's face it: Our eyes with a colposcopic magnification of 15X do not make a microscope.
Implementing the Working Group's recommendations is expected to lead to improved detection of cervical precancers at colposcopy and to provide stronger reassurance of negative colposcopy results. Regarding biopsy of lesions, ASCCP did not find added benefit to taking random (nondirected) biopsies for women at low risk for precancer. The sensitivity of biopsy is increased by taking multiple biopsies of suspicious lesions, based on a risk-based approach detailed in the ASCCP guidelines. So, depending on underlying risk (estimated from screening and triage tests), colposcopy practice can be adapted in a useful manner to account for differences in risk:
- When risk of precancer is very high, for example, immediate treatment might reduce cost and prevent the patient from being lost to follow-up. When risk is very low, consider expectant management (serial cytology and HPV testing) with limited need for biopsy. In a setting of intermediate risk, the Working Group proposes, "multiple biopsies of acetowhite lesions lead to increased detection of precancer."
- Perform multiple biopsies that target all areas characterized by 1) acetowhitening, 2) metaplasia, and 3) higher abnormalities.
- Do not perform nontargeted biopsies on patients at the lowest end of risk who have been referred to colposcopy−ie, those with cytology that is less than HSIL; no evidence of HPV types 16/18; and a normal colposcopic impression (ie, no acetowhitening or metaplasia, or other visible abnormality).
- Immediate excision without biopsy confirmation or colposcopy with multiple targeted biopsies is acceptable in nonpregnant women 25 years and older whose risk of precancer is very high (≥2 of the following: HSIL cytology, HPV 16- or HPV 18-positive(or both), and high-grade colposcopy impression). Endocervical sampling should be conducted according to ASCCP's 2012 management guidelines. If biopsies do not show precancer, manage the patient using ASCCP's 2012 management guidelines, the Working Group recommends.
How do we perform colposcopy? Implications for establishing standards (Waxman et al; Working Group 3). To serve as a guide to standardizing colposcopy across the United States, the authors defined and delineated 6 major components (and their constituent parts) of a comprehensive colposcopy:
- precolposcopy evaluation
- the examination
- use of colposcopy adjuncts
- documentation
- biopsy sampling
- postcolposcopy procedures.
The constituent parts of these components are laid out in TABLE 2.4 A set of components for a minimum colposcopy procedure is drawn mostly from the comprehensive protocol.
The Working Group acknowledges that, in the United States, "the accuracy and reproducibility of colposcopy with biopsy as a diagnostic tool are limited." Why? Three contributing factors, the authors write, might be the absence of practice recommendations for colposcopy-biopsy procedures; of measures of quality assurance; and of standardized terminology.
Standards arrive for practice
Minimum quality standards are becoming part of almost everything US health care providers do−whether it is documentation, billing practices, or good care. Our work in gynecology, including colposcopy, is now being assessed as it is in much of the world, where minimum standards are already in place and guidelines must be followed. (In some countries standards require performing a minimum number of colposcopies per year to be identified as a "certified" colposcopist.)
What should be considered "minimum standards" for colposcopy in the United States? These ASCCP reports ask, and deliver answers to that question, bringing a broad range of concerns about high-quality practice into focus. Physicians and advanced-practice clinicians in this country who perform colposcopies have been trained to do so, but they have never had minimum standards by which to model and assess their performance. A procedure that has the potential to lead to additional testing for either cervical cancer, or to surveillance, should have minimum standards by which it is performed and documented in the United States as it is for much of the world that has widespread cervical cancer screening.
Guidance and recommendations developed by ASCCP offer women's health care providers a set of comprehensive and, alternatively, minimum quality standards that should be incorporated into practice across all aspects of the colposcopic exam, including precolposcopy evaluation, how to perform the procedure, how to document and report findings (TABLE 2), biopsy practice, establish quality control and assurance, as well as postprocedure follow-up. In taking the initiative to draw up these standards, ASCCP encourages providers to exceed the minimum requirements.
Read about adherence to cervical cancer screening.
Cervical screening adherence is relatively low, but safe. Extended intervals are very safe.
Castle PE, Kinney WK, Xue X, et al. Effect of several negative rounds of human papillomavirus and cytology co-testing on safety against cervical cancer: an observational cohort study. Ann Intern Med. 2018;168(1):20-29.
Rendle KA, Schiffman M, Cheung LC, et al. Adherence patterns to extended cervical screening intervals in women undergoing human papillomavirus (HPV) and cytology cotesting. Prev Med. 2018;109:44-50.
Patients who have been screened for cervical cancer for a long time--decades, even--have a diminishing likelihood that cancer will ever be detected. Furthermore, highest-risk patients already have been triaged into further testing or procedures, such as a loop excision electrosurgical procedure or hysterectomy. Two recent studies examined the implications of repeated negative screening and patients' acceptance of extended screening intervals.
Details of the studies
Several negative rounds of cotesting (HPV and cytology) might justify changes to the screening interval. To determine the rate of detection of CIN3, adenocarcinoma in situ, and cervical cancer (≥CIN3) in routine practice after successive negative screening at 3-year intervals, Castle and colleagues looked at records of more than 990,000 women in an integrated health care system who underwent cotesting (HPV and cytology) between 2003 and 2014. They determined that the risk of invasive cervical cancer and ≥CIN3 declined with each round of cotesting; the absolute risk fell more between first and second rounds than between second and third rounds.
At any given round of cotesting, Castle found that the ability to reassure a patient about cancer and cancer risk was similar when looking at an HPV result alone, whatever the cytology or HPV-cytology cotest result was. The investigators concluded that similar patterns of risk would have been seen had stand-alone HPV testing been used, instead of co-testing, (HPV testing alone might have missed a few cases of CIN3 and adenocarcinoma in situ leading to cancer). A single negative cotest was so effective at ruling out ≥CIN3 and cervical cancer that, after a second round of cotesting, they found that no interval cancer cases were detected among women who had a negative HPV result.
Women aged 50 years or older had a 5- to 6-fold lower risk after their third consecutive negative cotest than women aged 30 to 39 years had after their first negative cotest. These data support the ideas, Castle noted, that 1) assigning screening intervals based on both age and number of previous negative screens and 2) extending the screening interval even further than 3 years after 2--perhaps even after 1--negative cotests or HPV tests are worth entertaining. Screening women of this age becomes inefficient and cost-ineffective, even at 5-year intervals.
Is patients' adherence to an extended interval of cotesting reliable enough to change practice? Rendle and colleagues examined the records of more than 491,000 women (in the same integrated health care system that Castle studied) who had undergone routine cervical cancer screening between 2003 and 2015. Their goal was to determine how high adherence had become to the system's recommendation of an every-3-year screening interval--an interval that mirrors long-standing guidelines elsewhere.
In short, researchers observed increasing and relatively rapid clinical adoption of every-3-year cotesting for routine cervical screening over time; between 2003 and 2009, the cohort grew significantly less likely overall to come in early for screening. In this setting, adoption of an extended screeninginterval appears to run counter to earlier understanding that patients are likely to resist such extension.
Women aged 60 to 64 were most likely to screen early across 2 consecutive intervals. What Rendle termed a "modest" decrease in the percentage of late screeners (but still within a 5-year interval) was also noted during adoption of the 3-year interval.
What next?
Molecular-based testing. Research, mostly outside of the United States, is taking us in the direction of molecular-based technologies as at least a component of cervical cancer screening. Today, we rely mostly on Pap tests and colposcopy, but these are subjective screens, with a human operator. With molecular testing (mostly of components of HPV), results are objective--a "Yes" or "No" finding based on clinically validated thresholds. Methods such as genotyping, P16INK4a/Ki-67 gene product dual-stain cytology, and testing for E6 and E7 HPV mRNA transcripts are in development, and hold promise to allow us to screen safely using almost completely molecular testing, thus eliminating human error and subjectivity and enriching the population that needs further management with very sensitive and potentially specific testing.
We are being presented with the possibility that almost all aspects of screening can be done without a provider, until the patient needs treatment.
Access to screening. Research is also looking at improving access, such as self-sampling for primary screening. That includes home cervical and vaginal sampling, with specimens mailed to the laboratory, from where results and follow-up instructions as communicated to patients. The Netherlands and the United Kingdom are moving to self-sampling primary screens; the United States is not--yet. But that is the direction research is taking us.
Modified guidelines. Eyes are on the work of the USPSTF. Last year, the Task Force issued draft recommendations (https://www.uspre ventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2#clinical), followed by a comment period (now closed), for updating 2012 cervical cancer screening guidelines in a way that would trigger a major change in clinical practice. Those draft recommendations and public comments are under review; final recommendations are possible within this calendar year.
Continue to follow current screening guidelines; they are safe and effective for preventing cervical cancer. This assumes adherence to intervals, which is both the provider's and the patient's responsibility: First, less is more; too much screening ("I've always done it this way") can be harmful. Second, screening at intervals set by the guidelines is extremely safe, despite earlier reports or provider concerns that suggest otherwise.
Patients who have undergone several rounds of negative screening have a markedly diminished risk of cervical cancer. Serve them best by performing this underutilized gyn procedure: Sit on your hands.
Be aware that winds of change are blowing: What constitutes appropriate screening intervals is up for discussion this year, and molecular-based testing technologies that are under investigation have the potential to someday be a vast improvement over current good, but subjective, interpretations of results.
Last, promote primary prevention of cervical cancer with HPV vaccination in your practice to increase the percentage of protected patients. Doing so will contribute not only to their long-term health but also, at a societal level, to a herd immunity effect.5 Any positive HPV infection in a future of a well-vaccinated population will be significant, and HPV-targeted technologies to identify the highest risk women will be the most efficient screening.
Read about the safety and efficacy of HPV vaccination.
Primary prevention of cervical cancer with vaccination is critical in any cancer prevention program
Benard VB, Castle PE, Jenison SA, et al; New Mexico HPV Pap Registry Steering Committee. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3(6):833-837.
Luostarinen T, Apter D, Dillner J, et al. Vaccination protects against invasive HPV-associated cancers. Int J Cancer. 2018;142(10):2186-2187.
The success story of HPV vaccination, after more than a decade of use, continued to unfold in important ways over the past year.
Safety. With tens of millions of doses delivered, we know that the vaccine is safe, and we have retreated on some of the precautions that we once took: For example, we no longer perform a routine pregnancy test before vaccination on reproductive-age women.
Efficacy. We have learned, based on what we see in Australia and Western Europe, that vaccination is highly effective. We are also starting to see evidence of efficacy in areas of the United States, even though the vaccine is voluntary and there are no school-based recommendations. And we know that herd vaccination is very effective. The 2 studies described here add to our understanding of how vaccination is having an impact on endpoints.
Findings of the 2 studies
HPV vaccination has a direct impact on the precursor of cancer, CIN. Benard and colleagues examined data from the New Mexico HPV Pap Registry, a mandatory statewide surveillance system of cervical cancer screening that captured estimates of both screening prevalence and CIN since the time HPV vaccination was introduced in 2007 to 2014. The investigators examined registry data to gauge trends in the rate of CIN and to estimate the effect of HPV vaccination on that rate when adjusted for changes in screening for cervical cancer.
The incidence of CIN declined significantly across all grades in 2 groups between 2007 and 2015: females aged 15 to 19 years and females aged 20 to 24 years (but not in females 25 to 29 years of age). During those years, mean uptake of HPV vaccination among females 13 to 17 years of age reached as high as 40% (in 2014).
Although a reduction in CIN2 and CIN3 precancers "are early benchmarks for achieving this aim [of reducing the rate of cancer]," the investigators note, a reduction in CIN1 is "a direct measure of reductions in HPV infections requisite to the development of almost all invasive cervical cancer."
Benard moves on to conclude that a reduction in clinical outcomes of CIN among groups who are partially vaccinated for HPV is going to change clinical practice and reduce the cost-effectiveness of clinical care that supports prevention of cervical cancer. Of greatest importance, modalities and strategies for screening, and management algorithms, are going to need to evolve as HPV vaccination and cervical screening are integrated in a rational manner. Furthermore, it might be feasible to factor in population-level decreases in CIN among cohorts who are partially vaccinated for HPV when reassessing clinical practice guidelines for cervical cancer screening.
What does this mean? As we start to eliminate HPV from the population, any positive screening result will be that much more meaningful because the outcome--cervical cancer--will be much rarer. The onus will be on providers and public health officials to re-strategize how to screen what is going to be a widely-vaccinated population; more and more, we will be looking for needles in a haystack.
How are we going to someday screen women in their 20s who were vaccinated at 11 or 12 years of age? Likely, screening will start at a later age, and screening will be conducted at longer intervals. Any finding of HPV or disease is going to be highly significant, and likely, far less frequent.
HPV vaccination protects against invasive HPV-associated cancer. Luostarinen and colleagues report proof of highly efficacious protection offered by a population-based HPV vaccination program in Finland, in the form of a decrease in the key endpoint: cases of invasive HPV-associated cancer. Examining vaccinated (3,331 females) and unvaccinated (15,665 females) cohorts in the nationwide Finnish Cancer Registry, the investigators identified 10 cases of HPV-caused cancer (8 cervical, 1 oropharyngeal, 1 vulvar) in the unvaccinated females and 0 cases in vaccinated females--a statistically significant difference.
From the evidence gathered in this first intention-to-treat trial, the investigators conclude that vaccination protects against invasive HPV-associated cancer--what they call "an awaited, pivotal corollary" to high vaccine efficacy against HPV infection.
Summing up
This success story continues to unfold, despite well-organized, antivaccine campaigns. The HPV vaccine has been an easy target: It is novel, it involves a sexually transmitted infection, and the endpoint of protecting against invasive HPV-associated cancer is years--decades--away. But antivaccine groups can no longer argue the point that studies have not been designed to yield evidence of the impact of the vaccine on decisive endpoints, including cervical cancer.
The exciting news that the sought-out endpoint of HPV vaccination -- prevention of invasive HPV-associated cervical cancer -- is being realized. This should all the more energize you to:
- urge vaccination for your patients in whom it is indicated
- emphasize vaccine coverage in the young -- especially for the routinely recommended age group of 11 - and 12-year-olds
- strenuously reject and counter arguments made by segments of the public that HPV vaccination is neither safe nor necessary
- prepare for potential changes down the road in practice guidelines regarding screening (eg, raising the age at which screening begins) as the impact of vaccination on the health of women is felt.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
In this Update, I outline important findings from several studies published in the past year. First and foremost, what are best practices for performing colposcopy in the United States? The American Society for Colposcopy and Cervical Pathology (ASCCP) released guidelines addressing such practices. Second, what are the implications of repeated negative screening and patients’ acceptance of extended screening intervals? A recent observational cohort study and a large study of Kaiser Permanente’s practices since 2003 shed light on these questions. Last, where do we stand with HPV vaccination? Two studies shed light on the efficacy of vaccination against human papillomavirus (HPV), and subsequent cervical intraepithelial neoplasia (CIN) and cervical cancer.
ASCCP releases updated quality guidelines for performing colposcopy
Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223-229.
Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235-241.
Wentzensen N, Schiffman M, Silver MI, et al. ASCCP colposcopy standards: Risk-based colposcopy practice. J Low Genit Tract Dis. 2017;21(4):230-234.
In October 2017, the ASCCP released a set of standards on the role and performance of colposcopy that represents best practices in women's health care in the United States. The work of these groups comprised a literature search, a national survey of ASCCP members, public comment, and expert consensus, and addressed:
- establishment of a common understanding of 1) the benefits of colposcopy in health maintenance and risk prevention, 2) risks presented by the procedure, and 3) terminology and criteria for reporting results that reduce subjectivity in reporting
- the rationale for, approach to, and recommendations regarding assessment of cervical precancer at colposcopy
- both minimum and comprehensive guidelines for the colposcopic examination, from preprocedure evaluation to follow-up.
Each Working Group performed the analysis and produced its own report and recommendations, published sequentially in a 2017 issue of the Journal of Lower Urinary Tract Disease. The findings and standards that they produced 1) offer essential insight for high- and low-volume coloposcopists and 2) are intended to improve the quality of colposcopy, reduce subjectivity in reporting findings, and improve the sensitivity of the procedure. Aware of the concerns and objectives of payers and hospital credentialing committees, the ASCCP found it important to establish what would be considered US-based minimum quality standards and to present goals that providers and systems could strive to achieve.
Selected details of the 3 guideline reports
The past 6 years have brought us through a great deal of transition in the prevention of cervical precancer, with regard to screening intervals and types of screening (for example, see "HPV−cytology co-testing every 3 years lowers population rates of cervical precancer and cancer," in the 2017 "Cervical Disease Update," OBG Management, May 2017). The most significant change was in 2012, when American Cancer Society/ASCCP guidelines were revised to abandon screening with annual Pap testing on most patients--an effort to strike a balance between the lifesaving value of identifying precancer and the potential harm of excessive colposcopy.
If, as the US Preventive Services Task Force (USPSTF) has declared, excessive colposcopy is a harm of screening, then we should be adapting our practices, especially in terms of the frequency of screening, to 1) reduce the risk of unnecessarily screening and potentially triaging patients to colposcopy and 2) bring the highest standards of performance and reporting to colposcopic practice (see "Why aren't you doing a Pap on me?"). In other words, "This is the way I've always done it" shouldn't characterize efforts to detect disease, when the data are clear that doing less might be more beneficial for our patients. Adherence to extended screening intervals is not yet good enough to balance benefit and risk of harm, as Rendle and colleagues showed in an article this year in Preventive Medicine (discussed in the next section of this "Update"). We need to do better.
Adherence to extended screening intervals means fewer colposcopies and less exposure to risk of attendant harm. But adherence is not purely mechanical: It can be intertwined with how patients feel about the care we provide and about their safety. When a patient moves from years of annual Pap testing to less frequent screening, she might express her concern by challenging your expertise.
In my practice, I have a simple, 1-minute conversation with the patient that is important to wedge into our discussion of her care. I explain that increasing the frequency of screening is only going to increase the chance that I will perform a colposcopy but not increase the chance that I will identify cancer. I conclude by reassuring her that I do not want to harm her, or to cause her anxiety, pain, cramping, or bleeding--or require her to spend time away from work or show her family that she is suffering. Patients are reassured and happy after that, I find. This is a patient-centered discussion that providers need to have if they hope to establish and maintain adherence to recommended screening intervals.
-- Mark H. Einstein, MD, MS
Here is a limited encapsulation of the 3 wide-ranging reports on the ASCCP colposcopy recommendations:
Role of colposcopy; benefits, potential harms, terminology (Khan et al; Working Group 1). The authors provide reinforcement: The strategic benefit of colposcopy is clear--a "drastic" reduction in excisional procedures by limiting them to patients in whom cervical cancer precursors have been confirmed or who present a high risk of occult invasive cervical cancer. Furthermore, the rate of adverse events for colposcopy−including significant bleeding and infection−is low.
Nevertheless, the potential for harm exists when an unskilled provider performs colposcopy; the Working Group emphasizes that proficiency comes with training and experience. Even in skilled hands, however, anxiety and the discomfort of a speculum examination and from acetic acid, as well as cramping and pain, might dissuade some women from receiving regular cervical screening subsequently. The authors cite research showing that educational interventions can help soothe anxiety about colposcopy and potential findings,1,2 although consensus is lacking on the value of such interventions.
The Working Group 1) developed recommended terminology for reporting findings in colposcopy practice in the United States and 2) defined the comprehensive documentation of the procedure as comprising cervix and squamocolumnar junction visibility; acetowhitening; presence of a lesion; lesion visibility, size and location of lesion(s); vascular changes; other features; and colposcopic impression (TABLE 1).3 Minimum criteria for reporting colposcopy results were also proposed, extracted from the comprehensive standards.
Risk-based colposcopy practice (Wentzensen et al). Women referred to colposcopy present with a range of underlying risk of precancer. Assessing that risk at the colposcopy visit allows the provider to modify and individualize the procedure. Risk can be estimated by referral screening tests (eg, cytology, HPV testing) performed in conjunction with the colposcopic impression. As opposed to a lack of standards for a minimum number of biopsies, the Working Group recommends that, as a standard, multiple targeted biopsies (≥2, as many as 4) are needed to improve detection of prevalent precancers. Colposcopic impression alone is not enough to diagnose precancerous cells. Let's face it: Our eyes with a colposcopic magnification of 15X do not make a microscope.
Implementing the Working Group's recommendations is expected to lead to improved detection of cervical precancers at colposcopy and to provide stronger reassurance of negative colposcopy results. Regarding biopsy of lesions, ASCCP did not find added benefit to taking random (nondirected) biopsies for women at low risk for precancer. The sensitivity of biopsy is increased by taking multiple biopsies of suspicious lesions, based on a risk-based approach detailed in the ASCCP guidelines. So, depending on underlying risk (estimated from screening and triage tests), colposcopy practice can be adapted in a useful manner to account for differences in risk:
- When risk of precancer is very high, for example, immediate treatment might reduce cost and prevent the patient from being lost to follow-up. When risk is very low, consider expectant management (serial cytology and HPV testing) with limited need for biopsy. In a setting of intermediate risk, the Working Group proposes, "multiple biopsies of acetowhite lesions lead to increased detection of precancer."
- Perform multiple biopsies that target all areas characterized by 1) acetowhitening, 2) metaplasia, and 3) higher abnormalities.
- Do not perform nontargeted biopsies on patients at the lowest end of risk who have been referred to colposcopy−ie, those with cytology that is less than HSIL; no evidence of HPV types 16/18; and a normal colposcopic impression (ie, no acetowhitening or metaplasia, or other visible abnormality).
- Immediate excision without biopsy confirmation or colposcopy with multiple targeted biopsies is acceptable in nonpregnant women 25 years and older whose risk of precancer is very high (≥2 of the following: HSIL cytology, HPV 16- or HPV 18-positive(or both), and high-grade colposcopy impression). Endocervical sampling should be conducted according to ASCCP's 2012 management guidelines. If biopsies do not show precancer, manage the patient using ASCCP's 2012 management guidelines, the Working Group recommends.
How do we perform colposcopy? Implications for establishing standards (Waxman et al; Working Group 3). To serve as a guide to standardizing colposcopy across the United States, the authors defined and delineated 6 major components (and their constituent parts) of a comprehensive colposcopy:
- precolposcopy evaluation
- the examination
- use of colposcopy adjuncts
- documentation
- biopsy sampling
- postcolposcopy procedures.
The constituent parts of these components are laid out in TABLE 2.4 A set of components for a minimum colposcopy procedure is drawn mostly from the comprehensive protocol.
The Working Group acknowledges that, in the United States, "the accuracy and reproducibility of colposcopy with biopsy as a diagnostic tool are limited." Why? Three contributing factors, the authors write, might be the absence of practice recommendations for colposcopy-biopsy procedures; of measures of quality assurance; and of standardized terminology.
Standards arrive for practice
Minimum quality standards are becoming part of almost everything US health care providers do−whether it is documentation, billing practices, or good care. Our work in gynecology, including colposcopy, is now being assessed as it is in much of the world, where minimum standards are already in place and guidelines must be followed. (In some countries standards require performing a minimum number of colposcopies per year to be identified as a "certified" colposcopist.)
What should be considered "minimum standards" for colposcopy in the United States? These ASCCP reports ask, and deliver answers to that question, bringing a broad range of concerns about high-quality practice into focus. Physicians and advanced-practice clinicians in this country who perform colposcopies have been trained to do so, but they have never had minimum standards by which to model and assess their performance. A procedure that has the potential to lead to additional testing for either cervical cancer, or to surveillance, should have minimum standards by which it is performed and documented in the United States as it is for much of the world that has widespread cervical cancer screening.
Guidance and recommendations developed by ASCCP offer women's health care providers a set of comprehensive and, alternatively, minimum quality standards that should be incorporated into practice across all aspects of the colposcopic exam, including precolposcopy evaluation, how to perform the procedure, how to document and report findings (TABLE 2), biopsy practice, establish quality control and assurance, as well as postprocedure follow-up. In taking the initiative to draw up these standards, ASCCP encourages providers to exceed the minimum requirements.
Read about adherence to cervical cancer screening.
Cervical screening adherence is relatively low, but safe. Extended intervals are very safe.
Castle PE, Kinney WK, Xue X, et al. Effect of several negative rounds of human papillomavirus and cytology co-testing on safety against cervical cancer: an observational cohort study. Ann Intern Med. 2018;168(1):20-29.
Rendle KA, Schiffman M, Cheung LC, et al. Adherence patterns to extended cervical screening intervals in women undergoing human papillomavirus (HPV) and cytology cotesting. Prev Med. 2018;109:44-50.
Patients who have been screened for cervical cancer for a long time--decades, even--have a diminishing likelihood that cancer will ever be detected. Furthermore, highest-risk patients already have been triaged into further testing or procedures, such as a loop excision electrosurgical procedure or hysterectomy. Two recent studies examined the implications of repeated negative screening and patients' acceptance of extended screening intervals.
Details of the studies
Several negative rounds of cotesting (HPV and cytology) might justify changes to the screening interval. To determine the rate of detection of CIN3, adenocarcinoma in situ, and cervical cancer (≥CIN3) in routine practice after successive negative screening at 3-year intervals, Castle and colleagues looked at records of more than 990,000 women in an integrated health care system who underwent cotesting (HPV and cytology) between 2003 and 2014. They determined that the risk of invasive cervical cancer and ≥CIN3 declined with each round of cotesting; the absolute risk fell more between first and second rounds than between second and third rounds.
At any given round of cotesting, Castle found that the ability to reassure a patient about cancer and cancer risk was similar when looking at an HPV result alone, whatever the cytology or HPV-cytology cotest result was. The investigators concluded that similar patterns of risk would have been seen had stand-alone HPV testing been used, instead of co-testing, (HPV testing alone might have missed a few cases of CIN3 and adenocarcinoma in situ leading to cancer). A single negative cotest was so effective at ruling out ≥CIN3 and cervical cancer that, after a second round of cotesting, they found that no interval cancer cases were detected among women who had a negative HPV result.
Women aged 50 years or older had a 5- to 6-fold lower risk after their third consecutive negative cotest than women aged 30 to 39 years had after their first negative cotest. These data support the ideas, Castle noted, that 1) assigning screening intervals based on both age and number of previous negative screens and 2) extending the screening interval even further than 3 years after 2--perhaps even after 1--negative cotests or HPV tests are worth entertaining. Screening women of this age becomes inefficient and cost-ineffective, even at 5-year intervals.
Is patients' adherence to an extended interval of cotesting reliable enough to change practice? Rendle and colleagues examined the records of more than 491,000 women (in the same integrated health care system that Castle studied) who had undergone routine cervical cancer screening between 2003 and 2015. Their goal was to determine how high adherence had become to the system's recommendation of an every-3-year screening interval--an interval that mirrors long-standing guidelines elsewhere.
In short, researchers observed increasing and relatively rapid clinical adoption of every-3-year cotesting for routine cervical screening over time; between 2003 and 2009, the cohort grew significantly less likely overall to come in early for screening. In this setting, adoption of an extended screeninginterval appears to run counter to earlier understanding that patients are likely to resist such extension.
Women aged 60 to 64 were most likely to screen early across 2 consecutive intervals. What Rendle termed a "modest" decrease in the percentage of late screeners (but still within a 5-year interval) was also noted during adoption of the 3-year interval.
What next?
Molecular-based testing. Research, mostly outside of the United States, is taking us in the direction of molecular-based technologies as at least a component of cervical cancer screening. Today, we rely mostly on Pap tests and colposcopy, but these are subjective screens, with a human operator. With molecular testing (mostly of components of HPV), results are objective--a "Yes" or "No" finding based on clinically validated thresholds. Methods such as genotyping, P16INK4a/Ki-67 gene product dual-stain cytology, and testing for E6 and E7 HPV mRNA transcripts are in development, and hold promise to allow us to screen safely using almost completely molecular testing, thus eliminating human error and subjectivity and enriching the population that needs further management with very sensitive and potentially specific testing.
We are being presented with the possibility that almost all aspects of screening can be done without a provider, until the patient needs treatment.
Access to screening. Research is also looking at improving access, such as self-sampling for primary screening. That includes home cervical and vaginal sampling, with specimens mailed to the laboratory, from where results and follow-up instructions as communicated to patients. The Netherlands and the United Kingdom are moving to self-sampling primary screens; the United States is not--yet. But that is the direction research is taking us.
Modified guidelines. Eyes are on the work of the USPSTF. Last year, the Task Force issued draft recommendations (https://www.uspre ventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2#clinical), followed by a comment period (now closed), for updating 2012 cervical cancer screening guidelines in a way that would trigger a major change in clinical practice. Those draft recommendations and public comments are under review; final recommendations are possible within this calendar year.
Continue to follow current screening guidelines; they are safe and effective for preventing cervical cancer. This assumes adherence to intervals, which is both the provider's and the patient's responsibility: First, less is more; too much screening ("I've always done it this way") can be harmful. Second, screening at intervals set by the guidelines is extremely safe, despite earlier reports or provider concerns that suggest otherwise.
Patients who have undergone several rounds of negative screening have a markedly diminished risk of cervical cancer. Serve them best by performing this underutilized gyn procedure: Sit on your hands.
Be aware that winds of change are blowing: What constitutes appropriate screening intervals is up for discussion this year, and molecular-based testing technologies that are under investigation have the potential to someday be a vast improvement over current good, but subjective, interpretations of results.
Last, promote primary prevention of cervical cancer with HPV vaccination in your practice to increase the percentage of protected patients. Doing so will contribute not only to their long-term health but also, at a societal level, to a herd immunity effect.5 Any positive HPV infection in a future of a well-vaccinated population will be significant, and HPV-targeted technologies to identify the highest risk women will be the most efficient screening.
Read about the safety and efficacy of HPV vaccination.
Primary prevention of cervical cancer with vaccination is critical in any cancer prevention program
Benard VB, Castle PE, Jenison SA, et al; New Mexico HPV Pap Registry Steering Committee. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3(6):833-837.
Luostarinen T, Apter D, Dillner J, et al. Vaccination protects against invasive HPV-associated cancers. Int J Cancer. 2018;142(10):2186-2187.
The success story of HPV vaccination, after more than a decade of use, continued to unfold in important ways over the past year.
Safety. With tens of millions of doses delivered, we know that the vaccine is safe, and we have retreated on some of the precautions that we once took: For example, we no longer perform a routine pregnancy test before vaccination on reproductive-age women.
Efficacy. We have learned, based on what we see in Australia and Western Europe, that vaccination is highly effective. We are also starting to see evidence of efficacy in areas of the United States, even though the vaccine is voluntary and there are no school-based recommendations. And we know that herd vaccination is very effective. The 2 studies described here add to our understanding of how vaccination is having an impact on endpoints.
Findings of the 2 studies
HPV vaccination has a direct impact on the precursor of cancer, CIN. Benard and colleagues examined data from the New Mexico HPV Pap Registry, a mandatory statewide surveillance system of cervical cancer screening that captured estimates of both screening prevalence and CIN since the time HPV vaccination was introduced in 2007 to 2014. The investigators examined registry data to gauge trends in the rate of CIN and to estimate the effect of HPV vaccination on that rate when adjusted for changes in screening for cervical cancer.
The incidence of CIN declined significantly across all grades in 2 groups between 2007 and 2015: females aged 15 to 19 years and females aged 20 to 24 years (but not in females 25 to 29 years of age). During those years, mean uptake of HPV vaccination among females 13 to 17 years of age reached as high as 40% (in 2014).
Although a reduction in CIN2 and CIN3 precancers "are early benchmarks for achieving this aim [of reducing the rate of cancer]," the investigators note, a reduction in CIN1 is "a direct measure of reductions in HPV infections requisite to the development of almost all invasive cervical cancer."
Benard moves on to conclude that a reduction in clinical outcomes of CIN among groups who are partially vaccinated for HPV is going to change clinical practice and reduce the cost-effectiveness of clinical care that supports prevention of cervical cancer. Of greatest importance, modalities and strategies for screening, and management algorithms, are going to need to evolve as HPV vaccination and cervical screening are integrated in a rational manner. Furthermore, it might be feasible to factor in population-level decreases in CIN among cohorts who are partially vaccinated for HPV when reassessing clinical practice guidelines for cervical cancer screening.
What does this mean? As we start to eliminate HPV from the population, any positive screening result will be that much more meaningful because the outcome--cervical cancer--will be much rarer. The onus will be on providers and public health officials to re-strategize how to screen what is going to be a widely-vaccinated population; more and more, we will be looking for needles in a haystack.
How are we going to someday screen women in their 20s who were vaccinated at 11 or 12 years of age? Likely, screening will start at a later age, and screening will be conducted at longer intervals. Any finding of HPV or disease is going to be highly significant, and likely, far less frequent.
HPV vaccination protects against invasive HPV-associated cancer. Luostarinen and colleagues report proof of highly efficacious protection offered by a population-based HPV vaccination program in Finland, in the form of a decrease in the key endpoint: cases of invasive HPV-associated cancer. Examining vaccinated (3,331 females) and unvaccinated (15,665 females) cohorts in the nationwide Finnish Cancer Registry, the investigators identified 10 cases of HPV-caused cancer (8 cervical, 1 oropharyngeal, 1 vulvar) in the unvaccinated females and 0 cases in vaccinated females--a statistically significant difference.
From the evidence gathered in this first intention-to-treat trial, the investigators conclude that vaccination protects against invasive HPV-associated cancer--what they call "an awaited, pivotal corollary" to high vaccine efficacy against HPV infection.
Summing up
This success story continues to unfold, despite well-organized, antivaccine campaigns. The HPV vaccine has been an easy target: It is novel, it involves a sexually transmitted infection, and the endpoint of protecting against invasive HPV-associated cancer is years--decades--away. But antivaccine groups can no longer argue the point that studies have not been designed to yield evidence of the impact of the vaccine on decisive endpoints, including cervical cancer.
The exciting news that the sought-out endpoint of HPV vaccination -- prevention of invasive HPV-associated cervical cancer -- is being realized. This should all the more energize you to:
- urge vaccination for your patients in whom it is indicated
- emphasize vaccine coverage in the young -- especially for the routinely recommended age group of 11 - and 12-year-olds
- strenuously reject and counter arguments made by segments of the public that HPV vaccination is neither safe nor necessary
- prepare for potential changes down the road in practice guidelines regarding screening (eg, raising the age at which screening begins) as the impact of vaccination on the health of women is felt.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Walsh JC, Curtis R, Mylotte M. Anxiety levels in women attending a colposcopy clinic: a randomised trial of an educational intervention using video colposcopy. Patient Educ Couns. 2004;55(2):247–251.
- Tomaino-Brunner C, Freda MC, Damus K, Runowicz CD. Can precolposcopy education increase knowledge and decrease anxiety? J Obstet Gynecol Neonatal Nurs. 1998;27(6):636–645.
- Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223–229.
- Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235–241.
- Wentzensen N, Schiffman M. Accelerating cervical cancer control and prevention. Lancet Public Health. 2018;3(1):e6–e7.
- Walsh JC, Curtis R, Mylotte M. Anxiety levels in women attending a colposcopy clinic: a randomised trial of an educational intervention using video colposcopy. Patient Educ Couns. 2004;55(2):247–251.
- Tomaino-Brunner C, Freda MC, Damus K, Runowicz CD. Can precolposcopy education increase knowledge and decrease anxiety? J Obstet Gynecol Neonatal Nurs. 1998;27(6):636–645.
- Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223–229.
- Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235–241.
- Wentzensen N, Schiffman M. Accelerating cervical cancer control and prevention. Lancet Public Health. 2018;3(1):e6–e7.
The beginning of the end of the Pap?
EXPERT COMMENTARY
Realistic prospective performance data are needed to quantify the additional benefit of the cytology component of cotesting on top of what is already known to be highly sensitive molecular HPV testing. While the addition of cytology to HPV testing can add performance, it also can add further costs and the potential for unnecessary colposcopies for what are merely cytomorphologic manifestations of an active HPV infection. Frequent invasive procedures such as colposcopy, which can be costly and lead to anxiety and distress in generally young women and the potential for overtreatment of likely regressive lesions, has been defined as a harm of screening by the US Preventive Services Task Force (USPSTF).
Details of the study
In a cohort from Kaiser Permanente Northern California, 1,208,710 women aged 30 years or older were screened with cotesting from 2003 to 2015. Those who cotested HPV negative and cytology negative were offered triennial screening. Positive cotest results were managed according to Kaiser protocol. Women with cytologic abnormalities were referred for colposcopy. Those with HPV positive/cytology negative results or HPV negative/cytology equivocal results underwent accelerated testing at 1 year. A total of 623 cervical cancers were identified and included in the analyses.
Using multiple analyses, Schiffman and colleagues demonstrated the sensitivity advantage of HPV testing. They clearly showed that the cytology component to cotesting performance over many years is very limited for detecting precancers and early curable cancers. For example, prediagnostic HPV testing (76.7%) was more likely to be positive than cytology (59.1%; P<.001 for paired comparison); 82.6% of all prediagnostic cotests were positive by HPV and/or cytology; and only 5.9% of the cotests were positive by cytology alone (HPV negative.)
Primary HPV testing is recommended as a potential screening strategy by an interim guidance group led by the Society of Gynecologic Oncology and the American Society for Colposcopy and Cervical Pathology, and it is the primary cervical cancer screening recommendation of USPSTF draft guidelines.1 There have been reports that reliance on primary HPV testing would encourage cervical cancer mortality; Schiffman and colleagues point out, however, that according to their study data, such reports are overstated.
Despite these data, practically speaking, shifting away from standard cotesting poses numerous challenges for clinicians and laboratories alike; however, these data clearly show the limited value of cytology and, due to the overtreatment of likely regressive cervical intraepithelial neoplasia grade 2, the possible increased risk of preterm birth and its subsequent harm as well.
Study strengths and weaknesses
The authors examined the long-term relative history of HPV testing and cytology prior to cancer diagnosis in a large, prospectively followed US cohort where hundreds of women in this cohort developed cancer. There will not be a validation study of this size and scale in the near future. Further, the authors showed that the relative value of cytology to cotesting is minimal. Multiple subsequent rounds of cotesting after negative results also can be questioned.
One weakness of the study is that the data were collected from only one health care system and therefore may not be representative of all populations. Additionally, cotesting was performed on 2 separately collected specimens, which may have reduced HPV testing performance.
Excessive cervical cancer screening, including frequent cotesting, could have minimal cancer prevention benefits while increasing the harms of screening. These data confirm guidance showing HPV testing alone is an effective cervical cancer screening strategy.
-- Mark H. Einstein, MD, MS
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330-337.
EXPERT COMMENTARY
Realistic prospective performance data are needed to quantify the additional benefit of the cytology component of cotesting on top of what is already known to be highly sensitive molecular HPV testing. While the addition of cytology to HPV testing can add performance, it also can add further costs and the potential for unnecessary colposcopies for what are merely cytomorphologic manifestations of an active HPV infection. Frequent invasive procedures such as colposcopy, which can be costly and lead to anxiety and distress in generally young women and the potential for overtreatment of likely regressive lesions, has been defined as a harm of screening by the US Preventive Services Task Force (USPSTF).
Details of the study
In a cohort from Kaiser Permanente Northern California, 1,208,710 women aged 30 years or older were screened with cotesting from 2003 to 2015. Those who cotested HPV negative and cytology negative were offered triennial screening. Positive cotest results were managed according to Kaiser protocol. Women with cytologic abnormalities were referred for colposcopy. Those with HPV positive/cytology negative results or HPV negative/cytology equivocal results underwent accelerated testing at 1 year. A total of 623 cervical cancers were identified and included in the analyses.
Using multiple analyses, Schiffman and colleagues demonstrated the sensitivity advantage of HPV testing. They clearly showed that the cytology component to cotesting performance over many years is very limited for detecting precancers and early curable cancers. For example, prediagnostic HPV testing (76.7%) was more likely to be positive than cytology (59.1%; P<.001 for paired comparison); 82.6% of all prediagnostic cotests were positive by HPV and/or cytology; and only 5.9% of the cotests were positive by cytology alone (HPV negative.)
Primary HPV testing is recommended as a potential screening strategy by an interim guidance group led by the Society of Gynecologic Oncology and the American Society for Colposcopy and Cervical Pathology, and it is the primary cervical cancer screening recommendation of USPSTF draft guidelines.1 There have been reports that reliance on primary HPV testing would encourage cervical cancer mortality; Schiffman and colleagues point out, however, that according to their study data, such reports are overstated.
Despite these data, practically speaking, shifting away from standard cotesting poses numerous challenges for clinicians and laboratories alike; however, these data clearly show the limited value of cytology and, due to the overtreatment of likely regressive cervical intraepithelial neoplasia grade 2, the possible increased risk of preterm birth and its subsequent harm as well.
Study strengths and weaknesses
The authors examined the long-term relative history of HPV testing and cytology prior to cancer diagnosis in a large, prospectively followed US cohort where hundreds of women in this cohort developed cancer. There will not be a validation study of this size and scale in the near future. Further, the authors showed that the relative value of cytology to cotesting is minimal. Multiple subsequent rounds of cotesting after negative results also can be questioned.
One weakness of the study is that the data were collected from only one health care system and therefore may not be representative of all populations. Additionally, cotesting was performed on 2 separately collected specimens, which may have reduced HPV testing performance.
Excessive cervical cancer screening, including frequent cotesting, could have minimal cancer prevention benefits while increasing the harms of screening. These data confirm guidance showing HPV testing alone is an effective cervical cancer screening strategy.
-- Mark H. Einstein, MD, MS
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Realistic prospective performance data are needed to quantify the additional benefit of the cytology component of cotesting on top of what is already known to be highly sensitive molecular HPV testing. While the addition of cytology to HPV testing can add performance, it also can add further costs and the potential for unnecessary colposcopies for what are merely cytomorphologic manifestations of an active HPV infection. Frequent invasive procedures such as colposcopy, which can be costly and lead to anxiety and distress in generally young women and the potential for overtreatment of likely regressive lesions, has been defined as a harm of screening by the US Preventive Services Task Force (USPSTF).
Details of the study
In a cohort from Kaiser Permanente Northern California, 1,208,710 women aged 30 years or older were screened with cotesting from 2003 to 2015. Those who cotested HPV negative and cytology negative were offered triennial screening. Positive cotest results were managed according to Kaiser protocol. Women with cytologic abnormalities were referred for colposcopy. Those with HPV positive/cytology negative results or HPV negative/cytology equivocal results underwent accelerated testing at 1 year. A total of 623 cervical cancers were identified and included in the analyses.
Using multiple analyses, Schiffman and colleagues demonstrated the sensitivity advantage of HPV testing. They clearly showed that the cytology component to cotesting performance over many years is very limited for detecting precancers and early curable cancers. For example, prediagnostic HPV testing (76.7%) was more likely to be positive than cytology (59.1%; P<.001 for paired comparison); 82.6% of all prediagnostic cotests were positive by HPV and/or cytology; and only 5.9% of the cotests were positive by cytology alone (HPV negative.)
Primary HPV testing is recommended as a potential screening strategy by an interim guidance group led by the Society of Gynecologic Oncology and the American Society for Colposcopy and Cervical Pathology, and it is the primary cervical cancer screening recommendation of USPSTF draft guidelines.1 There have been reports that reliance on primary HPV testing would encourage cervical cancer mortality; Schiffman and colleagues point out, however, that according to their study data, such reports are overstated.
Despite these data, practically speaking, shifting away from standard cotesting poses numerous challenges for clinicians and laboratories alike; however, these data clearly show the limited value of cytology and, due to the overtreatment of likely regressive cervical intraepithelial neoplasia grade 2, the possible increased risk of preterm birth and its subsequent harm as well.
Study strengths and weaknesses
The authors examined the long-term relative history of HPV testing and cytology prior to cancer diagnosis in a large, prospectively followed US cohort where hundreds of women in this cohort developed cancer. There will not be a validation study of this size and scale in the near future. Further, the authors showed that the relative value of cytology to cotesting is minimal. Multiple subsequent rounds of cotesting after negative results also can be questioned.
One weakness of the study is that the data were collected from only one health care system and therefore may not be representative of all populations. Additionally, cotesting was performed on 2 separately collected specimens, which may have reduced HPV testing performance.
Excessive cervical cancer screening, including frequent cotesting, could have minimal cancer prevention benefits while increasing the harms of screening. These data confirm guidance showing HPV testing alone is an effective cervical cancer screening strategy.
-- Mark H. Einstein, MD, MS
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330-337.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330-337.
2017 Update on cervical disease
Vaccination against human papillomavirus (HPV) infection and periodic cervical screening have significantly decreased the incidence of invasive cervical cancer. But cancers still exist despite the availability of these useful clinical tools, especially in women of reproductive age in developing regions of the world. In the 2016 update on cervical disease, I reviewed studies on 2 promising and novel immunotherapies for cervical cancer: HPV therapeutic vaccine and adoptive T-cell therapy. This year the focus is on remarkable advances in the field of genomics and related studies that are rapidly expanding our understanding of the molecular characteristics of cervical cancer. Rewards of this research already being explored include novel immunotherapeutic agents as well as the repurposed use of existing drugs.
But first, with regard to cervical screening and follow-up, 2 recent large studies have yielded findings that have important implications for patient management. One pertains to the monitoring of women who have persistent infection with high-risk HPV but cytology results that are negative. Its conclusion was unequivocal and very useful in the management of our patients. The other study tracked HPV screening performed every 3 years and reported on the diagnostic efficiency of this shorter interval screening strategy.
Read about persistent HPV infection and CIN
Persistent HPV infection has a higher risk than most clinicians might think
Elfgren K, Elfström KM, Naucler P, Arnheim-Dahlström L, Dillner J. Management of women with human papillomavirus persistence: long-term follow-up of a randomized clinical trial. Am J Obstet Gynecol. 2017;216(3):264.e1-e7.
It is well known that most cases of cervical cancer arise from persistent HPV infection, with the highest percentage of cancers caused by high-risk types 16 or 18. What has been uncertain, however, is the actual degree of risk that persistent infection confers over time for the development of cervical intraepithelial neoplasia (CIN) or worse when a woman's repeated cytology reports are negative. In an analysis of a long-term double-blind, randomized, controlled screening study, Elfgren and colleagues showed that all women whose HPV infection persisted up to 7 years developed CIN grade 2 (CIN2+), while those whose infection cleared in that period, or changed genotype, had no precancerous lesions out to 13 years of follow-up.
Related Article:
It is time for HPV vaccination to be considered part of routine preventive health care
Details of the study
Between 1997 and 2000, 12,527 Swedish women between the ages of 32 and 38 years who were undergoing organized cervical cancer screening agreed to participate in a 1:1-randomized prospective trial to determine the benefit of screening with HPV and cytology (intervention group) compared with cytology screening alone (control group). However, brush sampling for HPV was performed even on women in the control group, with the samples frozen for later testing. All participants were identified in the Swedish National Cervical Screening Registry.
Women in the intervention group who initially tested positive for HPV but whose cytology test results were negative (n = 341) were invited to return a year later for repeat HPV testing; 270 women returned and 119 had type-specific HPV persistence. Of those with persistent infection, 100 agreed to undergo colposcopy; 111 women from the control group were randomly selected to undergo sham HPV testing and colposcopy, and 95 attended. Women with evident cytologic abnormalities received treatment per protocol. Those with negative cytology results were offered annual HPV testing thereafter, and each follow-up with documented type-specific HPV persistence led to repeat colposcopy. A comparable number of women from the control group had repeat colposcopies.
Although some women were lost to clinical follow-up throughout the trial, all 195 who attended the first colposcopy were followed for at least 5 years in the Swedish registry, and 191 were followed in the registry for 13 years. Of 102 women with known HPV persistence at baseline (100 in the treatment group; 2 in the randomly selected control group), 31 became HPV negative, 4 evidenced a switch in HPV type but cleared the initial infection, 27 had unknown persistence status due to missed HPV tests, and 40 had continuously type-specific persistence. Of note, persistent HPV16 infection seemed to impart a higher risk of CIN development than did persistent HPV18 infection.
All 40 participants with clinically verified continuously persistent HPV infection developed CIN2+ within 7 years of baseline documentation of persistence (FIGURE 1). Among the 27 women with unknown persistence status, risk of CIN2+ occurrence within 7 years was 50%. None of the 35 women who cleared their infection or switched HPV type developed CIN2+.
Read about HPV-cytology cotesting
HPV−cytology cotesting every 3 years lowers population rates of cervical precancer and cancer
Silver MI, Schiffman M, Fetterman B, et al. The population impact of human papillomavirus/cytology cervical cotesting at 3-year intervals: reduced cervical cancer risk and decreased yield of precancer per screen. Cancer. 2016;122(23):3682−3686.
Current guidelines on screening for cervical cancer in women 30 to 65 years of age advise the preferred strategy of using cytology alone every 3 years or combining HPV testing and cytology every 5 years.1 These guidelines, based on data available at the time they were written, were meant to offer a reasonable balance between timely detection of abnormalities and avoidance of potential harms from screening too frequently. However, many patients are reluctant to postpone repeat testing to the extent recommended. Several authorities have in fact asked that screening intervals be revisited, perhaps allowing for a range of strategies, contending that the level of protection once provided by annual screening should be the benchmark by which evolving strategies are judged.2 Today, they point out, the risk of cancer doubles in the 3 years following an initial negative cytology result, and it also increases by lengthening the cotesting interval from 3 to 5 years. They additionally question the validity of using frequency of colposcopies as a surrogate to measure harms of screening, and suggest that many women would willingly accept the procedure's minimal discomfort and inconvenience to gain peace of mind.
The study by Silver and colleagues gives credence to considering a shorter cotesting interval. Since 2003, Kaiser Permanente Northern California (KPNC) has implemented 3-year cotesting. To determine actual clinical outcomes of cotesting at this interval, KPNC analyzed data on more than 1 million women in its care between 2003 and 2012. Although investigators expected that they might see decreasing efficiency in cotesting over time, they instead found an increased detection rate of precancerous lesions per woman screened in the larger of 2 study cohorts.
Related Article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Details of the study
Included were all women 30 years of age or older enrolled in this study at KPNC between 2003 and 2012 who underwent HPV−cytology cotesting every 3 years. The population in its entirety (1,065,273 women) was deemed the "open cohort" and represented KPNC's total annual experience. A subset of this population, the "closed cohort," was designed to gauge the effect of repeated screening on a fixed population and comprised only those women enrolled and initially screened between 2003 and 2004 and then followed longitudinally until 2012.
For each cohort, investigators calculated the ratios of precancer and cancer diagnoses to the total number of cotests performed on the cohort's population. The 3-year testing periods were 2004−2006, 2007−2009, and 2010−2012. Also calculated in these periods were the ratios of colposcopic biopsies to cotests and the rates of precancer diagnoses (TABLE).
In the open cohort, the biopsy rate nearly doubled over the course of the study. Precancer diagnoses per number of cotests rose by 71.5% between the first and second testing periods (P = .001) and then eased off by 10% in the third period (P<.001). These corresponding increases throughout the study yielded a stable number of biopsies (16 to 22) needed to detect precancer.
In the closed long-term cohort, the biopsy rate rose, but not as much as in the open cohort. Precancer diagnoses per number of cotests rose by 47% between the first and second periods (P≤.001), but in the third period fell back by 28% (P<.001) to a level just above the first period results. The number of biopsies needed to detect a precancerous lesion in the closed cohort rose from 19 to 33 over the course of the study, suggesting there may have been some loss of screening efficiency in the fixed group.
Read about molecular profiling of cervical cancer
Molecular profiling of cervical cancer is revolutionizing treatment
The Cancer Genome Atlas Research Network. Integratedgenomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378−384.
Effective treatments for cervical cancer could be close at hand, thanks to a recent explosion of knowledge at the molecular level about how specific cancers arise and what drives them other than HPV. The Cancer Genome Atlas Research Network (TCGA) recently published the results of its genomic and proteomic analyses, which yielded distinct profiles for 178 cervical cancers with important patterns common to other cancers, such as uterine and breast cancer. These recently published findings on cervical cancer highlight areas of gene and protein dysfunction it shares with these other cancers, which could open the doors for new targets for treatments already developed or in the pipeline.
Related Article:
2016 Update on cervical disease
How molecular profiling is paying off for cervical cancer
Cancers develop in any given tissue through the altered function of different genes and signaling pathways in the tissue's cells. The latest extensive investigation conducted by the TCGA network has identified significant mutations in 5 genes previously unrecognized in association with cervical cancer, bringing the total now to 14.
Several highlights are featured in the TCGA's recently published work. One discovery is the amplification of genes CD274 and PDCD1LG2, which are involved with the expression of 2 cytolytic effector genes and are therefore likely targets for immunotherapeutic strategies. Another line of exploration, whole-genome sequencing, has detected an aberration in some cervical cancer tissue with the potential for immediate application. Duplication and copy number gain of BCAR4, a noncoding RNA, facilitates cell proliferation through the HER2/HER3 pathway, a target of the tyrosine-kinase inhibitor, lapatinib, which is currently used to treat breast cancer.
The integration of data from multiple layers of analysis (FIGURE 2) is helping investigators identify variations in cancers. DNA methylation, for instance, is a means by which cells control gene expression. An analysis of this process in cervical tumor tissue has revealed additional cancer subgroups in which messenger RNA increases the transition of epithelial cells to invasive mesenchymal cells. Targeting that process in these subgroups would likely enhance the effectiveness of novel small-molecule inhibitors and some standard cytotoxic chemotherapy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542.
- Kinney W, Wright TC, Dinkelspiel HE, DeFrancesco M, Thomas Cox J, Huh W. Increased cervical cancer risk associated with screening at longer intervals. Obstet Gynecol. 2015;125(2):311–315.
Vaccination against human papillomavirus (HPV) infection and periodic cervical screening have significantly decreased the incidence of invasive cervical cancer. But cancers still exist despite the availability of these useful clinical tools, especially in women of reproductive age in developing regions of the world. In the 2016 update on cervical disease, I reviewed studies on 2 promising and novel immunotherapies for cervical cancer: HPV therapeutic vaccine and adoptive T-cell therapy. This year the focus is on remarkable advances in the field of genomics and related studies that are rapidly expanding our understanding of the molecular characteristics of cervical cancer. Rewards of this research already being explored include novel immunotherapeutic agents as well as the repurposed use of existing drugs.
But first, with regard to cervical screening and follow-up, 2 recent large studies have yielded findings that have important implications for patient management. One pertains to the monitoring of women who have persistent infection with high-risk HPV but cytology results that are negative. Its conclusion was unequivocal and very useful in the management of our patients. The other study tracked HPV screening performed every 3 years and reported on the diagnostic efficiency of this shorter interval screening strategy.
Read about persistent HPV infection and CIN
Persistent HPV infection has a higher risk than most clinicians might think
Elfgren K, Elfström KM, Naucler P, Arnheim-Dahlström L, Dillner J. Management of women with human papillomavirus persistence: long-term follow-up of a randomized clinical trial. Am J Obstet Gynecol. 2017;216(3):264.e1-e7.
It is well known that most cases of cervical cancer arise from persistent HPV infection, with the highest percentage of cancers caused by high-risk types 16 or 18. What has been uncertain, however, is the actual degree of risk that persistent infection confers over time for the development of cervical intraepithelial neoplasia (CIN) or worse when a woman's repeated cytology reports are negative. In an analysis of a long-term double-blind, randomized, controlled screening study, Elfgren and colleagues showed that all women whose HPV infection persisted up to 7 years developed CIN grade 2 (CIN2+), while those whose infection cleared in that period, or changed genotype, had no precancerous lesions out to 13 years of follow-up.
Related Article:
It is time for HPV vaccination to be considered part of routine preventive health care
Details of the study
Between 1997 and 2000, 12,527 Swedish women between the ages of 32 and 38 years who were undergoing organized cervical cancer screening agreed to participate in a 1:1-randomized prospective trial to determine the benefit of screening with HPV and cytology (intervention group) compared with cytology screening alone (control group). However, brush sampling for HPV was performed even on women in the control group, with the samples frozen for later testing. All participants were identified in the Swedish National Cervical Screening Registry.
Women in the intervention group who initially tested positive for HPV but whose cytology test results were negative (n = 341) were invited to return a year later for repeat HPV testing; 270 women returned and 119 had type-specific HPV persistence. Of those with persistent infection, 100 agreed to undergo colposcopy; 111 women from the control group were randomly selected to undergo sham HPV testing and colposcopy, and 95 attended. Women with evident cytologic abnormalities received treatment per protocol. Those with negative cytology results were offered annual HPV testing thereafter, and each follow-up with documented type-specific HPV persistence led to repeat colposcopy. A comparable number of women from the control group had repeat colposcopies.
Although some women were lost to clinical follow-up throughout the trial, all 195 who attended the first colposcopy were followed for at least 5 years in the Swedish registry, and 191 were followed in the registry for 13 years. Of 102 women with known HPV persistence at baseline (100 in the treatment group; 2 in the randomly selected control group), 31 became HPV negative, 4 evidenced a switch in HPV type but cleared the initial infection, 27 had unknown persistence status due to missed HPV tests, and 40 had continuously type-specific persistence. Of note, persistent HPV16 infection seemed to impart a higher risk of CIN development than did persistent HPV18 infection.
All 40 participants with clinically verified continuously persistent HPV infection developed CIN2+ within 7 years of baseline documentation of persistence (FIGURE 1). Among the 27 women with unknown persistence status, risk of CIN2+ occurrence within 7 years was 50%. None of the 35 women who cleared their infection or switched HPV type developed CIN2+.
Read about HPV-cytology cotesting
HPV−cytology cotesting every 3 years lowers population rates of cervical precancer and cancer
Silver MI, Schiffman M, Fetterman B, et al. The population impact of human papillomavirus/cytology cervical cotesting at 3-year intervals: reduced cervical cancer risk and decreased yield of precancer per screen. Cancer. 2016;122(23):3682−3686.
Current guidelines on screening for cervical cancer in women 30 to 65 years of age advise the preferred strategy of using cytology alone every 3 years or combining HPV testing and cytology every 5 years.1 These guidelines, based on data available at the time they were written, were meant to offer a reasonable balance between timely detection of abnormalities and avoidance of potential harms from screening too frequently. However, many patients are reluctant to postpone repeat testing to the extent recommended. Several authorities have in fact asked that screening intervals be revisited, perhaps allowing for a range of strategies, contending that the level of protection once provided by annual screening should be the benchmark by which evolving strategies are judged.2 Today, they point out, the risk of cancer doubles in the 3 years following an initial negative cytology result, and it also increases by lengthening the cotesting interval from 3 to 5 years. They additionally question the validity of using frequency of colposcopies as a surrogate to measure harms of screening, and suggest that many women would willingly accept the procedure's minimal discomfort and inconvenience to gain peace of mind.
The study by Silver and colleagues gives credence to considering a shorter cotesting interval. Since 2003, Kaiser Permanente Northern California (KPNC) has implemented 3-year cotesting. To determine actual clinical outcomes of cotesting at this interval, KPNC analyzed data on more than 1 million women in its care between 2003 and 2012. Although investigators expected that they might see decreasing efficiency in cotesting over time, they instead found an increased detection rate of precancerous lesions per woman screened in the larger of 2 study cohorts.
Related Article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Details of the study
Included were all women 30 years of age or older enrolled in this study at KPNC between 2003 and 2012 who underwent HPV−cytology cotesting every 3 years. The population in its entirety (1,065,273 women) was deemed the "open cohort" and represented KPNC's total annual experience. A subset of this population, the "closed cohort," was designed to gauge the effect of repeated screening on a fixed population and comprised only those women enrolled and initially screened between 2003 and 2004 and then followed longitudinally until 2012.
For each cohort, investigators calculated the ratios of precancer and cancer diagnoses to the total number of cotests performed on the cohort's population. The 3-year testing periods were 2004−2006, 2007−2009, and 2010−2012. Also calculated in these periods were the ratios of colposcopic biopsies to cotests and the rates of precancer diagnoses (TABLE).
In the open cohort, the biopsy rate nearly doubled over the course of the study. Precancer diagnoses per number of cotests rose by 71.5% between the first and second testing periods (P = .001) and then eased off by 10% in the third period (P<.001). These corresponding increases throughout the study yielded a stable number of biopsies (16 to 22) needed to detect precancer.
In the closed long-term cohort, the biopsy rate rose, but not as much as in the open cohort. Precancer diagnoses per number of cotests rose by 47% between the first and second periods (P≤.001), but in the third period fell back by 28% (P<.001) to a level just above the first period results. The number of biopsies needed to detect a precancerous lesion in the closed cohort rose from 19 to 33 over the course of the study, suggesting there may have been some loss of screening efficiency in the fixed group.
Read about molecular profiling of cervical cancer
Molecular profiling of cervical cancer is revolutionizing treatment
The Cancer Genome Atlas Research Network. Integratedgenomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378−384.
Effective treatments for cervical cancer could be close at hand, thanks to a recent explosion of knowledge at the molecular level about how specific cancers arise and what drives them other than HPV. The Cancer Genome Atlas Research Network (TCGA) recently published the results of its genomic and proteomic analyses, which yielded distinct profiles for 178 cervical cancers with important patterns common to other cancers, such as uterine and breast cancer. These recently published findings on cervical cancer highlight areas of gene and protein dysfunction it shares with these other cancers, which could open the doors for new targets for treatments already developed or in the pipeline.
Related Article:
2016 Update on cervical disease
How molecular profiling is paying off for cervical cancer
Cancers develop in any given tissue through the altered function of different genes and signaling pathways in the tissue's cells. The latest extensive investigation conducted by the TCGA network has identified significant mutations in 5 genes previously unrecognized in association with cervical cancer, bringing the total now to 14.
Several highlights are featured in the TCGA's recently published work. One discovery is the amplification of genes CD274 and PDCD1LG2, which are involved with the expression of 2 cytolytic effector genes and are therefore likely targets for immunotherapeutic strategies. Another line of exploration, whole-genome sequencing, has detected an aberration in some cervical cancer tissue with the potential for immediate application. Duplication and copy number gain of BCAR4, a noncoding RNA, facilitates cell proliferation through the HER2/HER3 pathway, a target of the tyrosine-kinase inhibitor, lapatinib, which is currently used to treat breast cancer.
The integration of data from multiple layers of analysis (FIGURE 2) is helping investigators identify variations in cancers. DNA methylation, for instance, is a means by which cells control gene expression. An analysis of this process in cervical tumor tissue has revealed additional cancer subgroups in which messenger RNA increases the transition of epithelial cells to invasive mesenchymal cells. Targeting that process in these subgroups would likely enhance the effectiveness of novel small-molecule inhibitors and some standard cytotoxic chemotherapy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Vaccination against human papillomavirus (HPV) infection and periodic cervical screening have significantly decreased the incidence of invasive cervical cancer. But cancers still exist despite the availability of these useful clinical tools, especially in women of reproductive age in developing regions of the world. In the 2016 update on cervical disease, I reviewed studies on 2 promising and novel immunotherapies for cervical cancer: HPV therapeutic vaccine and adoptive T-cell therapy. This year the focus is on remarkable advances in the field of genomics and related studies that are rapidly expanding our understanding of the molecular characteristics of cervical cancer. Rewards of this research already being explored include novel immunotherapeutic agents as well as the repurposed use of existing drugs.
But first, with regard to cervical screening and follow-up, 2 recent large studies have yielded findings that have important implications for patient management. One pertains to the monitoring of women who have persistent infection with high-risk HPV but cytology results that are negative. Its conclusion was unequivocal and very useful in the management of our patients. The other study tracked HPV screening performed every 3 years and reported on the diagnostic efficiency of this shorter interval screening strategy.
Read about persistent HPV infection and CIN
Persistent HPV infection has a higher risk than most clinicians might think
Elfgren K, Elfström KM, Naucler P, Arnheim-Dahlström L, Dillner J. Management of women with human papillomavirus persistence: long-term follow-up of a randomized clinical trial. Am J Obstet Gynecol. 2017;216(3):264.e1-e7.
It is well known that most cases of cervical cancer arise from persistent HPV infection, with the highest percentage of cancers caused by high-risk types 16 or 18. What has been uncertain, however, is the actual degree of risk that persistent infection confers over time for the development of cervical intraepithelial neoplasia (CIN) or worse when a woman's repeated cytology reports are negative. In an analysis of a long-term double-blind, randomized, controlled screening study, Elfgren and colleagues showed that all women whose HPV infection persisted up to 7 years developed CIN grade 2 (CIN2+), while those whose infection cleared in that period, or changed genotype, had no precancerous lesions out to 13 years of follow-up.
Related Article:
It is time for HPV vaccination to be considered part of routine preventive health care
Details of the study
Between 1997 and 2000, 12,527 Swedish women between the ages of 32 and 38 years who were undergoing organized cervical cancer screening agreed to participate in a 1:1-randomized prospective trial to determine the benefit of screening with HPV and cytology (intervention group) compared with cytology screening alone (control group). However, brush sampling for HPV was performed even on women in the control group, with the samples frozen for later testing. All participants were identified in the Swedish National Cervical Screening Registry.
Women in the intervention group who initially tested positive for HPV but whose cytology test results were negative (n = 341) were invited to return a year later for repeat HPV testing; 270 women returned and 119 had type-specific HPV persistence. Of those with persistent infection, 100 agreed to undergo colposcopy; 111 women from the control group were randomly selected to undergo sham HPV testing and colposcopy, and 95 attended. Women with evident cytologic abnormalities received treatment per protocol. Those with negative cytology results were offered annual HPV testing thereafter, and each follow-up with documented type-specific HPV persistence led to repeat colposcopy. A comparable number of women from the control group had repeat colposcopies.
Although some women were lost to clinical follow-up throughout the trial, all 195 who attended the first colposcopy were followed for at least 5 years in the Swedish registry, and 191 were followed in the registry for 13 years. Of 102 women with known HPV persistence at baseline (100 in the treatment group; 2 in the randomly selected control group), 31 became HPV negative, 4 evidenced a switch in HPV type but cleared the initial infection, 27 had unknown persistence status due to missed HPV tests, and 40 had continuously type-specific persistence. Of note, persistent HPV16 infection seemed to impart a higher risk of CIN development than did persistent HPV18 infection.
All 40 participants with clinically verified continuously persistent HPV infection developed CIN2+ within 7 years of baseline documentation of persistence (FIGURE 1). Among the 27 women with unknown persistence status, risk of CIN2+ occurrence within 7 years was 50%. None of the 35 women who cleared their infection or switched HPV type developed CIN2+.
Read about HPV-cytology cotesting
HPV−cytology cotesting every 3 years lowers population rates of cervical precancer and cancer
Silver MI, Schiffman M, Fetterman B, et al. The population impact of human papillomavirus/cytology cervical cotesting at 3-year intervals: reduced cervical cancer risk and decreased yield of precancer per screen. Cancer. 2016;122(23):3682−3686.
Current guidelines on screening for cervical cancer in women 30 to 65 years of age advise the preferred strategy of using cytology alone every 3 years or combining HPV testing and cytology every 5 years.1 These guidelines, based on data available at the time they were written, were meant to offer a reasonable balance between timely detection of abnormalities and avoidance of potential harms from screening too frequently. However, many patients are reluctant to postpone repeat testing to the extent recommended. Several authorities have in fact asked that screening intervals be revisited, perhaps allowing for a range of strategies, contending that the level of protection once provided by annual screening should be the benchmark by which evolving strategies are judged.2 Today, they point out, the risk of cancer doubles in the 3 years following an initial negative cytology result, and it also increases by lengthening the cotesting interval from 3 to 5 years. They additionally question the validity of using frequency of colposcopies as a surrogate to measure harms of screening, and suggest that many women would willingly accept the procedure's minimal discomfort and inconvenience to gain peace of mind.
The study by Silver and colleagues gives credence to considering a shorter cotesting interval. Since 2003, Kaiser Permanente Northern California (KPNC) has implemented 3-year cotesting. To determine actual clinical outcomes of cotesting at this interval, KPNC analyzed data on more than 1 million women in its care between 2003 and 2012. Although investigators expected that they might see decreasing efficiency in cotesting over time, they instead found an increased detection rate of precancerous lesions per woman screened in the larger of 2 study cohorts.
Related Article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Details of the study
Included were all women 30 years of age or older enrolled in this study at KPNC between 2003 and 2012 who underwent HPV−cytology cotesting every 3 years. The population in its entirety (1,065,273 women) was deemed the "open cohort" and represented KPNC's total annual experience. A subset of this population, the "closed cohort," was designed to gauge the effect of repeated screening on a fixed population and comprised only those women enrolled and initially screened between 2003 and 2004 and then followed longitudinally until 2012.
For each cohort, investigators calculated the ratios of precancer and cancer diagnoses to the total number of cotests performed on the cohort's population. The 3-year testing periods were 2004−2006, 2007−2009, and 2010−2012. Also calculated in these periods were the ratios of colposcopic biopsies to cotests and the rates of precancer diagnoses (TABLE).
In the open cohort, the biopsy rate nearly doubled over the course of the study. Precancer diagnoses per number of cotests rose by 71.5% between the first and second testing periods (P = .001) and then eased off by 10% in the third period (P<.001). These corresponding increases throughout the study yielded a stable number of biopsies (16 to 22) needed to detect precancer.
In the closed long-term cohort, the biopsy rate rose, but not as much as in the open cohort. Precancer diagnoses per number of cotests rose by 47% between the first and second periods (P≤.001), but in the third period fell back by 28% (P<.001) to a level just above the first period results. The number of biopsies needed to detect a precancerous lesion in the closed cohort rose from 19 to 33 over the course of the study, suggesting there may have been some loss of screening efficiency in the fixed group.
Read about molecular profiling of cervical cancer
Molecular profiling of cervical cancer is revolutionizing treatment
The Cancer Genome Atlas Research Network. Integratedgenomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378−384.
Effective treatments for cervical cancer could be close at hand, thanks to a recent explosion of knowledge at the molecular level about how specific cancers arise and what drives them other than HPV. The Cancer Genome Atlas Research Network (TCGA) recently published the results of its genomic and proteomic analyses, which yielded distinct profiles for 178 cervical cancers with important patterns common to other cancers, such as uterine and breast cancer. These recently published findings on cervical cancer highlight areas of gene and protein dysfunction it shares with these other cancers, which could open the doors for new targets for treatments already developed or in the pipeline.
Related Article:
2016 Update on cervical disease
How molecular profiling is paying off for cervical cancer
Cancers develop in any given tissue through the altered function of different genes and signaling pathways in the tissue's cells. The latest extensive investigation conducted by the TCGA network has identified significant mutations in 5 genes previously unrecognized in association with cervical cancer, bringing the total now to 14.
Several highlights are featured in the TCGA's recently published work. One discovery is the amplification of genes CD274 and PDCD1LG2, which are involved with the expression of 2 cytolytic effector genes and are therefore likely targets for immunotherapeutic strategies. Another line of exploration, whole-genome sequencing, has detected an aberration in some cervical cancer tissue with the potential for immediate application. Duplication and copy number gain of BCAR4, a noncoding RNA, facilitates cell proliferation through the HER2/HER3 pathway, a target of the tyrosine-kinase inhibitor, lapatinib, which is currently used to treat breast cancer.
The integration of data from multiple layers of analysis (FIGURE 2) is helping investigators identify variations in cancers. DNA methylation, for instance, is a means by which cells control gene expression. An analysis of this process in cervical tumor tissue has revealed additional cancer subgroups in which messenger RNA increases the transition of epithelial cells to invasive mesenchymal cells. Targeting that process in these subgroups would likely enhance the effectiveness of novel small-molecule inhibitors and some standard cytotoxic chemotherapy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542.
- Kinney W, Wright TC, Dinkelspiel HE, DeFrancesco M, Thomas Cox J, Huh W. Increased cervical cancer risk associated with screening at longer intervals. Obstet Gynecol. 2015;125(2):311–315.
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542.
- Kinney W, Wright TC, Dinkelspiel HE, DeFrancesco M, Thomas Cox J, Huh W. Increased cervical cancer risk associated with screening at longer intervals. Obstet Gynecol. 2015;125(2):311–315.
2016 Update on cervical disease
For the past 40 to 50 years, the first-line treatment for high-grade cervical intraepithelial neoplasia (CIN) has been excisional procedures (including loop electrosurgical excision [LEEP], cone biopsy, cryosurgery, and laser therapy), and these treatments work well. It appears, however, that these procedures potentially can lead to preterm birth.1–3 With results from large, comprehensive meta-analyses that control for such risk factors as smoking and other factors that could contribute to both preterm birth and high-grade CIN, we have learned that excision treatment can result in a 2% to 5% increased risk for preterm birth, depending on the size and the extent of excision performed.1–3 The preterm birth rate in the United States is about 11.4%.4 With about 500,000 excisional treatments for high-grade CIN performed in the United States every year, and about 2% of preterm births caused by excisional procedures, conservatively, about 5,000 to 10,000 US preterm births are directly related to excisional procedures for high-grade CIN annually.
Clearly, excisional treatment for high-grade CIN and its connection to preterm birth adds to health care costs and long-term morbidity because babies that are born preterm potentially have diminished functionality. We need a better treatment approach other than excision to CIN, which is known to be a virally mediated disease. Consider the fact that just because excisional procedures remove potentially cancerous cells does not mean that these treatments remove the underlying reason behind the high-grade CIN—HPV. We cannot cut out a virus. Consequently, many studies have explored better-targeted therapies against high-grade CIN. Immune-based therapies, which can train a patient’s own immune system to attack HPV-infected cells, are exciting possibilities.
In this Update, I focus on 2 studies of immune-based therapies to treat cervical cancer. In addition, I discuss long-term follow-up data that are available regarding efficacy of primary HPV testing.
HPV therapeutic vaccine shows promise in RCT
Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386(10008):2078-2088.
While the promise of immune-based therapies to target a virally mediated disease has good scientific rationale, there have been many generally negative studies published in the past 15 years on immune-based targeted therapies. This study by Trimble and colleagues has interesting results because it is a randomized controlled trial (RCT) using a DNA vaccine delivered with a novel approach called electroporation. Electroporation generates a small electrical shot at the vaccine site that potentially increases a vaccine's DNA uptake and the patient's immune response.
Details of the study
Women aged 18 to 55 years with HPV16- or HPV18-positive high-grade CIN from 36 academic and private gynecology practices in 7 countries were assigned in a 3:1 blinded randomization to receive vaccine (6 mg; VGX-3100) or placebo (1 mL), given intramuscularly at 0, 4, and 12 weeks. Patients were stratified by age 25 or older versus younger than 25 and by CIN2 versus CIN3. The primary efficacy endpoint was regression to CIN1 or normal pathology 36 weeks after the first vaccine dose.
A mandatory interim safety colposcopy was performed 12 weeks after the third vaccine dose. At 36 weeks (the primary endpoint visit), patients with colposcopic evidence of residual disease underwent standard excision (LEEP or cone). In patients with no evidence of disease, investigators could biopsy the site of the original lesions. At 40 weeks, when all patients had completed their first visit after the primary endpoint, the data were unmasked. Long-term follow-up data were collected on all patients with remaining visits. Patients and study site investigators and personnel stayed masked to treatment until study data were final.
Results indicated a significant clinical response as well as an immune response in those patients who were treated with electroporation and the vaccine versus electroporation and placebo. In the per-protocol analysis, 53 (49.5%) of 107 vaccine recipients and 11 (30.6%) of 36 placebo recipients had histopathologic regression (percentage point difference [PPD], 19.0 [95% confidence interval CI, 1.4-36.6]; P = .034) (FIGURE 1). In the modified intention-to-treat analysis, 55 (48.2%) of 114 vaccine recipients and 12 (30.0%) of 40 placebo recipients had histopathologic regression (PPD, 18.2; 95% CI, 1.3-34.4; P = .034).
Injection-site reactions occurred in most patients, but only erythema was significantly more common in the vaccine group than in the placebo group (PPD, 21.3 [95% CI, 5.3-37.8]; P = .007).
What this evidence means for practice
In prior studies of immunotherapies, there have not been good correlations between immune responses and clinical responses, and this is one of the important differences between this study by Trimble and colleagues and prior studies in this space. Unfortunately, immune-based therapies are a "shot in the dark," with researchers not knowing which patients may have an increased immune response but no clinical response or a clinical response but no immune response. The measured immune responses are from peripheral blood, an immune response that might not reflect the milieu of immune responses in the cervical-vaginal tract.
If perfected, technologies like these hold the promise of minimizing the amount of patients who need to undergo excisional procedures because patients' own immune systems have been trained to target HPV-infected cells. The bigger hope is that we will be able to minimize preterm births that are directly related to treatment of dysplasia.
Adoptive T-cell therapy offers targeted treatment for recurrent cervical cancer
Stevanovic S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33(14):1543-1550.
Stevanovic and colleagues have been developing another immune-based therapy that has been tested for other cancers. This uses a method for generating T-cell cultures from HPV-positive cancers and selecting specific HPV oncoprotein- reactive cultures for administration to patients. Termed adoptive T-cell therapy (ACT), this targeted approach to recurrent cervical cancer is what I would consider one of the most intriguing future treatments of cervical disease. In the past, the largest barrier to an effective HPV vaccine to treat cervical cancer has been lack of clinical response to existing cytotoxic regimens. In this, albeit small, trial, investigators found a correlation between HPV reactivity and the infused T cells and objective clinical responses.
What is adoptive T-cell therapy?
ACT allows for more rigorous control over the magnitude of the targeted response than tumor vaccination treatment strategies because the T cells used for therapy are identified and selected in vitro. The cells selected are exposed to cytokines and immunomodulators that influence differentiation during priming and are expanded to large numbers. The resulting number of antigen-specific T cells produced in the peripheral blood is much greater (more than 10-fold) than that possible by current vaccine regimens alone.
Studies conducted by the National Cancer Institute of adoptive transfer of in vitro-selected tumor-infiltrating lymphocytes were the first to demonstrate the potential of T-cell immunotherapy to eradicate solid tumors.5,6 Among 13 patients with melanoma, treatment with adoptive transfer of ex vivo-amplified autologous tumor-infiltrating T cells resulted in treatment response in 10 of the patients—clinical responses in 6 and mixed responses in 4.
Details of the study
This study by Stevanovic and colleagues involved 9 patients with metastatic cervical cancer who previously had received optimal recommended chemotherapy or concomitant chemoradiotherapy regimens. Patients were treated with a single infusion of tumor-infiltrating T cells specifically selected for HPV E6 and E7 reactivity (HPV-TILs). Patients received lymphocyte-depleting chemotherapy before ACT and aldesleukin chemotherapy injection after ACT.
In such a phase I population, one would not expect clinical responses over persistent stable disease. However, in this small trial, 2 patients had complete tumor regression and 1 patient had a partial treatment response, demonstrating that a complete response to metastatic cervical cancer can occur after a single infusion of HPV-TILs. The partial response lasted 3 months. The 2 complete responses were ongoing 22 and 15 months after treatment (FIGURE 2).
Editorialists point out that, only when the infusion product had reactivity against the HPV E6 and E7 peptides did the patients show objective clinical response, suggesting it was the immune response that contributed to the tumor regression.7 In addition, in the 3 patients with objective responses, HPV-specific T cells persisted in peripheral blood for several months.
| FIGURE 2 Patients with complete tumor responses with adoptive T-cell therapy | ||
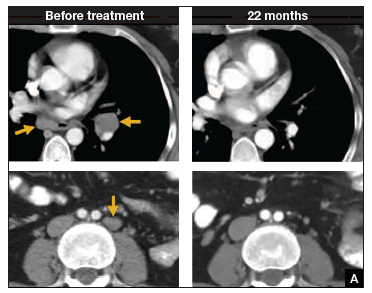 | 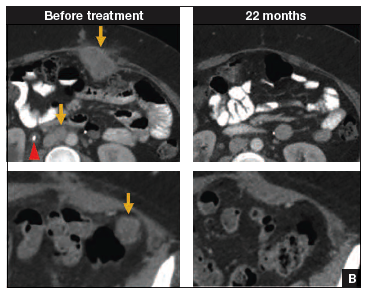 | |
Two patients with metastatic cervical cancer had complete tumor responses with treatment with tumor-infiltrating T cells selected for HPV E6 and E7 reactivity (HPV-TILs). Contrast-enhanced computed tomography scans obtained before treatment and at most recent follow-up for both patients. (A) First patient (patient 3) had disease involving para-aortic, bilateral hilar, subcarinal, and left iliac lymph nodes (gold arrows). Patient had no evidence of disease 22 months after treatment. (B) Second patient (patient 6) had metastatic disease in para-aortic lymph node, abdominal wall, aortocaval lymph node, left pericolic pelvic mass, and right ureteral nodule (gold arrows). Patient had no evidence of disease 15 months after treatment. (Red arrowhead indicates ureteral stent that was removed after right ureteral tumor regressed.) | ||
Stevanovic S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells.J Clin Oncol. 2015;33(14):1543–1550. Used with permission.
What this evidence means for practice
The recent approval of bevacizumab has been a major breakthrough in the treatment of advanced and recurrent cervical cancer. Although ACT is a treatment that is in early clinical development, it is the next major advance in this area. Its promise is currently limited, as the process is cumbersome and complex, involving surgical removal of a patient's lymph nodes, culturing of the T cells from the lymph nodes, and infusing the T cells with the oncoproteins that will train those T cells to infiltrate the cancer tumor. The process is wrought with potential problems in laboratory and translational techniques. However, this group of investigators from the NCI has perfected the process of ACT, creating T cells that will target the HPV that is integrated into each cervical cancer tumor.
The patients who demonstrated good T-cell reactivity against HPV were the ones who had a treatment response, which demonstrates the targeted precision of ACT therapy. There might come a day when we can select patients with recurrent cervical cancer who are going to have T-cell reactivity, and send them for treatment to a center specialized in ACT. Typically in phase 1 trials, we are happy to see a number of patients responding with stable disease. In this trial, 2 patients had a complete response. The results demonstrated by Stevanovic and colleagues are very exciting for the future treatment of patients with cervical cancer.
Primary HPV screening shows up to 70% greater protection against invasive cervical cancer than cytology
Ronco G, Dillner J, Elfstrom KM; International HPV Screening Working Group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):424-532.
In my 2015 "Update on Cervical Disease,"8 I discussed the newly published interim guidance for managing abnormal screening results for cervical cancer from a collective expert panel from the American Society for Colposcopy and Cervical Pathology, Society of Gynecologic Oncology, the American College of Obstetricians and Gynecologists, and 4 more societies.9 The guidelines support use of HPV testing alone or with the Papanicolaou test. In 2016, follow-up data from 4 RCTs provide long-term data on the efficacy of HPV primary testing.
Details of the trial
Incidence of invasive cervical cancer was the endpoint in 4 European trials comparing HPV-based with cytology-based screening. In total, 176,464 women aged 20 to 64 years were randomly assigned to either screening strategy. Median follow-up was 6.5 years (1,214,415 person-years). Using screening, pathology, and cancer registries investigators identified 107 invasive cervical carcinomas, with masked review of histologic specimens and reports.
Investigators calculated the rate ratios (defined as the cancer detection rate in the primary HPV testing-based versus cytology-based arms) for incidence of invasive cervical cancer. During the first 2.5 years of follow-up, detection of invasive cancer was similar between screening methods (0.79, 0.46-1.36). Thereafter, however, cumulative cancer detection was lower in the primary HPV testing-based arm (0.45; 95% CI, 0.25-0.81).
At 3.5 and 5.5 years after a negative cytology test on entry, cumulative cancer incidence was 15.4 per 105 (95% CI, 7.9-27.0) and 36.0 per 105 (23.2-53.5), respectively. At 3.5 and 5.5 years after a negative HPV test on entry, cumulative cancer incidence was 4.6 per 105 (1.1-12.1) and 8.7 per 105 (3.3-18.6), respectively (FIGURE 3).
| FIGURE 3 Cumulative detection of invasive cervical carcinoma | ||
 | ||
*Observations are censored 2.5 years after CIN2 or CIN3 detection, if any.
| ||
What this EVIDENCE means for practice
The 4 studies in this report were completed across Europe (in England, Netherlands, Sweden, and Italy): different regions, different sites, hospitals, and screening systems. The women in Europe are not any different than the women in the United States in terms of rates of HPV and age and incidence of HPV. Therefore, these results are globally generalizable.
The US trial by Wright and colleagues10 that led to US Food and Drug Administration approval of HPV primary testing was different than this European study in that all trial sites had to perform screening in the same way. In addition, the end point was high-grade dysplasia; in this trial by Ronco and colleagues the end point is cancer. These current investigators found no difference with either screening arm in terms of detection of invasive cervical cancer. Even more interesting is that, over time, the cervical cancer rates in the primary HPV testing-based arm were much less than that in the cytology-based arm.
The real strengths of this study are the long-term follow-up and the study size. We are not likely to see validation cohorts this big again. This study demonstrates that, overall, we should be able to continue to reduce the incidence of invasive cervical cancer with a primary HPV testing-based screening strategy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Conner SN, Frey HA, Cahill AG, Macones GA, Colditz GA, Tuuli MG. Loop electrosurgical excision procedure and risk of preterm birth: a systematic review and meta-analysis. Obstet Gynecol. 2014;123(4):752−761.
- Kyrgiou M, Valasoulis G, Stasinou SM, et al. Proportion of cervical excision for cervical intraepithelial neoplasia as a predictor of pregnancy outcomes. Int J Gynaecol Obstet. 2015;128(2):141−147.
- Miller ES, Grobman WA. The association between cervical excisional procedures, midtrimester cervical length, and preterm birth. Am J Obstet Gynecol. 2014;211(3):242.e1−e4.
- Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Mathews TJ. Births: Final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65. http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_01.pdf. Published January 15, 2015. Accessed April 20, 2016.
- Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854.
- Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346−2357.
- Zsiros E, Tsuli T, Odunsi K. Adoptive T-cell therapy is a promising salvage approach for advanced or recurrent metastatic cervical cancer. J Clin Oncol. 2015;33(14):1521−1522.
- Einstein MH. Update on cervical disease: New ammo for HPV prevention and screening. OBG Manag. 2015;27(5):32−39.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
For the past 40 to 50 years, the first-line treatment for high-grade cervical intraepithelial neoplasia (CIN) has been excisional procedures (including loop electrosurgical excision [LEEP], cone biopsy, cryosurgery, and laser therapy), and these treatments work well. It appears, however, that these procedures potentially can lead to preterm birth.1–3 With results from large, comprehensive meta-analyses that control for such risk factors as smoking and other factors that could contribute to both preterm birth and high-grade CIN, we have learned that excision treatment can result in a 2% to 5% increased risk for preterm birth, depending on the size and the extent of excision performed.1–3 The preterm birth rate in the United States is about 11.4%.4 With about 500,000 excisional treatments for high-grade CIN performed in the United States every year, and about 2% of preterm births caused by excisional procedures, conservatively, about 5,000 to 10,000 US preterm births are directly related to excisional procedures for high-grade CIN annually.
Clearly, excisional treatment for high-grade CIN and its connection to preterm birth adds to health care costs and long-term morbidity because babies that are born preterm potentially have diminished functionality. We need a better treatment approach other than excision to CIN, which is known to be a virally mediated disease. Consider the fact that just because excisional procedures remove potentially cancerous cells does not mean that these treatments remove the underlying reason behind the high-grade CIN—HPV. We cannot cut out a virus. Consequently, many studies have explored better-targeted therapies against high-grade CIN. Immune-based therapies, which can train a patient’s own immune system to attack HPV-infected cells, are exciting possibilities.
In this Update, I focus on 2 studies of immune-based therapies to treat cervical cancer. In addition, I discuss long-term follow-up data that are available regarding efficacy of primary HPV testing.
HPV therapeutic vaccine shows promise in RCT
Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386(10008):2078-2088.
While the promise of immune-based therapies to target a virally mediated disease has good scientific rationale, there have been many generally negative studies published in the past 15 years on immune-based targeted therapies. This study by Trimble and colleagues has interesting results because it is a randomized controlled trial (RCT) using a DNA vaccine delivered with a novel approach called electroporation. Electroporation generates a small electrical shot at the vaccine site that potentially increases a vaccine's DNA uptake and the patient's immune response.
Details of the study
Women aged 18 to 55 years with HPV16- or HPV18-positive high-grade CIN from 36 academic and private gynecology practices in 7 countries were assigned in a 3:1 blinded randomization to receive vaccine (6 mg; VGX-3100) or placebo (1 mL), given intramuscularly at 0, 4, and 12 weeks. Patients were stratified by age 25 or older versus younger than 25 and by CIN2 versus CIN3. The primary efficacy endpoint was regression to CIN1 or normal pathology 36 weeks after the first vaccine dose.
A mandatory interim safety colposcopy was performed 12 weeks after the third vaccine dose. At 36 weeks (the primary endpoint visit), patients with colposcopic evidence of residual disease underwent standard excision (LEEP or cone). In patients with no evidence of disease, investigators could biopsy the site of the original lesions. At 40 weeks, when all patients had completed their first visit after the primary endpoint, the data were unmasked. Long-term follow-up data were collected on all patients with remaining visits. Patients and study site investigators and personnel stayed masked to treatment until study data were final.
Results indicated a significant clinical response as well as an immune response in those patients who were treated with electroporation and the vaccine versus electroporation and placebo. In the per-protocol analysis, 53 (49.5%) of 107 vaccine recipients and 11 (30.6%) of 36 placebo recipients had histopathologic regression (percentage point difference [PPD], 19.0 [95% confidence interval CI, 1.4-36.6]; P = .034) (FIGURE 1). In the modified intention-to-treat analysis, 55 (48.2%) of 114 vaccine recipients and 12 (30.0%) of 40 placebo recipients had histopathologic regression (PPD, 18.2; 95% CI, 1.3-34.4; P = .034).
Injection-site reactions occurred in most patients, but only erythema was significantly more common in the vaccine group than in the placebo group (PPD, 21.3 [95% CI, 5.3-37.8]; P = .007).
What this evidence means for practice
In prior studies of immunotherapies, there have not been good correlations between immune responses and clinical responses, and this is one of the important differences between this study by Trimble and colleagues and prior studies in this space. Unfortunately, immune-based therapies are a "shot in the dark," with researchers not knowing which patients may have an increased immune response but no clinical response or a clinical response but no immune response. The measured immune responses are from peripheral blood, an immune response that might not reflect the milieu of immune responses in the cervical-vaginal tract.
If perfected, technologies like these hold the promise of minimizing the amount of patients who need to undergo excisional procedures because patients' own immune systems have been trained to target HPV-infected cells. The bigger hope is that we will be able to minimize preterm births that are directly related to treatment of dysplasia.
Adoptive T-cell therapy offers targeted treatment for recurrent cervical cancer
Stevanovic S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33(14):1543-1550.
Stevanovic and colleagues have been developing another immune-based therapy that has been tested for other cancers. This uses a method for generating T-cell cultures from HPV-positive cancers and selecting specific HPV oncoprotein- reactive cultures for administration to patients. Termed adoptive T-cell therapy (ACT), this targeted approach to recurrent cervical cancer is what I would consider one of the most intriguing future treatments of cervical disease. In the past, the largest barrier to an effective HPV vaccine to treat cervical cancer has been lack of clinical response to existing cytotoxic regimens. In this, albeit small, trial, investigators found a correlation between HPV reactivity and the infused T cells and objective clinical responses.
What is adoptive T-cell therapy?
ACT allows for more rigorous control over the magnitude of the targeted response than tumor vaccination treatment strategies because the T cells used for therapy are identified and selected in vitro. The cells selected are exposed to cytokines and immunomodulators that influence differentiation during priming and are expanded to large numbers. The resulting number of antigen-specific T cells produced in the peripheral blood is much greater (more than 10-fold) than that possible by current vaccine regimens alone.
Studies conducted by the National Cancer Institute of adoptive transfer of in vitro-selected tumor-infiltrating lymphocytes were the first to demonstrate the potential of T-cell immunotherapy to eradicate solid tumors.5,6 Among 13 patients with melanoma, treatment with adoptive transfer of ex vivo-amplified autologous tumor-infiltrating T cells resulted in treatment response in 10 of the patients—clinical responses in 6 and mixed responses in 4.
Details of the study
This study by Stevanovic and colleagues involved 9 patients with metastatic cervical cancer who previously had received optimal recommended chemotherapy or concomitant chemoradiotherapy regimens. Patients were treated with a single infusion of tumor-infiltrating T cells specifically selected for HPV E6 and E7 reactivity (HPV-TILs). Patients received lymphocyte-depleting chemotherapy before ACT and aldesleukin chemotherapy injection after ACT.
In such a phase I population, one would not expect clinical responses over persistent stable disease. However, in this small trial, 2 patients had complete tumor regression and 1 patient had a partial treatment response, demonstrating that a complete response to metastatic cervical cancer can occur after a single infusion of HPV-TILs. The partial response lasted 3 months. The 2 complete responses were ongoing 22 and 15 months after treatment (FIGURE 2).
Editorialists point out that, only when the infusion product had reactivity against the HPV E6 and E7 peptides did the patients show objective clinical response, suggesting it was the immune response that contributed to the tumor regression.7 In addition, in the 3 patients with objective responses, HPV-specific T cells persisted in peripheral blood for several months.
| FIGURE 2 Patients with complete tumor responses with adoptive T-cell therapy | ||
 |  | |
Two patients with metastatic cervical cancer had complete tumor responses with treatment with tumor-infiltrating T cells selected for HPV E6 and E7 reactivity (HPV-TILs). Contrast-enhanced computed tomography scans obtained before treatment and at most recent follow-up for both patients. (A) First patient (patient 3) had disease involving para-aortic, bilateral hilar, subcarinal, and left iliac lymph nodes (gold arrows). Patient had no evidence of disease 22 months after treatment. (B) Second patient (patient 6) had metastatic disease in para-aortic lymph node, abdominal wall, aortocaval lymph node, left pericolic pelvic mass, and right ureteral nodule (gold arrows). Patient had no evidence of disease 15 months after treatment. (Red arrowhead indicates ureteral stent that was removed after right ureteral tumor regressed.) | ||
Stevanovic S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells.J Clin Oncol. 2015;33(14):1543–1550. Used with permission.
What this evidence means for practice
The recent approval of bevacizumab has been a major breakthrough in the treatment of advanced and recurrent cervical cancer. Although ACT is a treatment that is in early clinical development, it is the next major advance in this area. Its promise is currently limited, as the process is cumbersome and complex, involving surgical removal of a patient's lymph nodes, culturing of the T cells from the lymph nodes, and infusing the T cells with the oncoproteins that will train those T cells to infiltrate the cancer tumor. The process is wrought with potential problems in laboratory and translational techniques. However, this group of investigators from the NCI has perfected the process of ACT, creating T cells that will target the HPV that is integrated into each cervical cancer tumor.
The patients who demonstrated good T-cell reactivity against HPV were the ones who had a treatment response, which demonstrates the targeted precision of ACT therapy. There might come a day when we can select patients with recurrent cervical cancer who are going to have T-cell reactivity, and send them for treatment to a center specialized in ACT. Typically in phase 1 trials, we are happy to see a number of patients responding with stable disease. In this trial, 2 patients had a complete response. The results demonstrated by Stevanovic and colleagues are very exciting for the future treatment of patients with cervical cancer.
Primary HPV screening shows up to 70% greater protection against invasive cervical cancer than cytology
Ronco G, Dillner J, Elfstrom KM; International HPV Screening Working Group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):424-532.
In my 2015 "Update on Cervical Disease,"8 I discussed the newly published interim guidance for managing abnormal screening results for cervical cancer from a collective expert panel from the American Society for Colposcopy and Cervical Pathology, Society of Gynecologic Oncology, the American College of Obstetricians and Gynecologists, and 4 more societies.9 The guidelines support use of HPV testing alone or with the Papanicolaou test. In 2016, follow-up data from 4 RCTs provide long-term data on the efficacy of HPV primary testing.
Details of the trial
Incidence of invasive cervical cancer was the endpoint in 4 European trials comparing HPV-based with cytology-based screening. In total, 176,464 women aged 20 to 64 years were randomly assigned to either screening strategy. Median follow-up was 6.5 years (1,214,415 person-years). Using screening, pathology, and cancer registries investigators identified 107 invasive cervical carcinomas, with masked review of histologic specimens and reports.
Investigators calculated the rate ratios (defined as the cancer detection rate in the primary HPV testing-based versus cytology-based arms) for incidence of invasive cervical cancer. During the first 2.5 years of follow-up, detection of invasive cancer was similar between screening methods (0.79, 0.46-1.36). Thereafter, however, cumulative cancer detection was lower in the primary HPV testing-based arm (0.45; 95% CI, 0.25-0.81).
At 3.5 and 5.5 years after a negative cytology test on entry, cumulative cancer incidence was 15.4 per 105 (95% CI, 7.9-27.0) and 36.0 per 105 (23.2-53.5), respectively. At 3.5 and 5.5 years after a negative HPV test on entry, cumulative cancer incidence was 4.6 per 105 (1.1-12.1) and 8.7 per 105 (3.3-18.6), respectively (FIGURE 3).
| FIGURE 3 Cumulative detection of invasive cervical carcinoma | ||
 | ||
*Observations are censored 2.5 years after CIN2 or CIN3 detection, if any.
| ||
What this EVIDENCE means for practice
The 4 studies in this report were completed across Europe (in England, Netherlands, Sweden, and Italy): different regions, different sites, hospitals, and screening systems. The women in Europe are not any different than the women in the United States in terms of rates of HPV and age and incidence of HPV. Therefore, these results are globally generalizable.
The US trial by Wright and colleagues10 that led to US Food and Drug Administration approval of HPV primary testing was different than this European study in that all trial sites had to perform screening in the same way. In addition, the end point was high-grade dysplasia; in this trial by Ronco and colleagues the end point is cancer. These current investigators found no difference with either screening arm in terms of detection of invasive cervical cancer. Even more interesting is that, over time, the cervical cancer rates in the primary HPV testing-based arm were much less than that in the cytology-based arm.
The real strengths of this study are the long-term follow-up and the study size. We are not likely to see validation cohorts this big again. This study demonstrates that, overall, we should be able to continue to reduce the incidence of invasive cervical cancer with a primary HPV testing-based screening strategy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
For the past 40 to 50 years, the first-line treatment for high-grade cervical intraepithelial neoplasia (CIN) has been excisional procedures (including loop electrosurgical excision [LEEP], cone biopsy, cryosurgery, and laser therapy), and these treatments work well. It appears, however, that these procedures potentially can lead to preterm birth.1–3 With results from large, comprehensive meta-analyses that control for such risk factors as smoking and other factors that could contribute to both preterm birth and high-grade CIN, we have learned that excision treatment can result in a 2% to 5% increased risk for preterm birth, depending on the size and the extent of excision performed.1–3 The preterm birth rate in the United States is about 11.4%.4 With about 500,000 excisional treatments for high-grade CIN performed in the United States every year, and about 2% of preterm births caused by excisional procedures, conservatively, about 5,000 to 10,000 US preterm births are directly related to excisional procedures for high-grade CIN annually.
Clearly, excisional treatment for high-grade CIN and its connection to preterm birth adds to health care costs and long-term morbidity because babies that are born preterm potentially have diminished functionality. We need a better treatment approach other than excision to CIN, which is known to be a virally mediated disease. Consider the fact that just because excisional procedures remove potentially cancerous cells does not mean that these treatments remove the underlying reason behind the high-grade CIN—HPV. We cannot cut out a virus. Consequently, many studies have explored better-targeted therapies against high-grade CIN. Immune-based therapies, which can train a patient’s own immune system to attack HPV-infected cells, are exciting possibilities.
In this Update, I focus on 2 studies of immune-based therapies to treat cervical cancer. In addition, I discuss long-term follow-up data that are available regarding efficacy of primary HPV testing.
HPV therapeutic vaccine shows promise in RCT
Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386(10008):2078-2088.
While the promise of immune-based therapies to target a virally mediated disease has good scientific rationale, there have been many generally negative studies published in the past 15 years on immune-based targeted therapies. This study by Trimble and colleagues has interesting results because it is a randomized controlled trial (RCT) using a DNA vaccine delivered with a novel approach called electroporation. Electroporation generates a small electrical shot at the vaccine site that potentially increases a vaccine's DNA uptake and the patient's immune response.
Details of the study
Women aged 18 to 55 years with HPV16- or HPV18-positive high-grade CIN from 36 academic and private gynecology practices in 7 countries were assigned in a 3:1 blinded randomization to receive vaccine (6 mg; VGX-3100) or placebo (1 mL), given intramuscularly at 0, 4, and 12 weeks. Patients were stratified by age 25 or older versus younger than 25 and by CIN2 versus CIN3. The primary efficacy endpoint was regression to CIN1 or normal pathology 36 weeks after the first vaccine dose.
A mandatory interim safety colposcopy was performed 12 weeks after the third vaccine dose. At 36 weeks (the primary endpoint visit), patients with colposcopic evidence of residual disease underwent standard excision (LEEP or cone). In patients with no evidence of disease, investigators could biopsy the site of the original lesions. At 40 weeks, when all patients had completed their first visit after the primary endpoint, the data were unmasked. Long-term follow-up data were collected on all patients with remaining visits. Patients and study site investigators and personnel stayed masked to treatment until study data were final.
Results indicated a significant clinical response as well as an immune response in those patients who were treated with electroporation and the vaccine versus electroporation and placebo. In the per-protocol analysis, 53 (49.5%) of 107 vaccine recipients and 11 (30.6%) of 36 placebo recipients had histopathologic regression (percentage point difference [PPD], 19.0 [95% confidence interval CI, 1.4-36.6]; P = .034) (FIGURE 1). In the modified intention-to-treat analysis, 55 (48.2%) of 114 vaccine recipients and 12 (30.0%) of 40 placebo recipients had histopathologic regression (PPD, 18.2; 95% CI, 1.3-34.4; P = .034).
Injection-site reactions occurred in most patients, but only erythema was significantly more common in the vaccine group than in the placebo group (PPD, 21.3 [95% CI, 5.3-37.8]; P = .007).
What this evidence means for practice
In prior studies of immunotherapies, there have not been good correlations between immune responses and clinical responses, and this is one of the important differences between this study by Trimble and colleagues and prior studies in this space. Unfortunately, immune-based therapies are a "shot in the dark," with researchers not knowing which patients may have an increased immune response but no clinical response or a clinical response but no immune response. The measured immune responses are from peripheral blood, an immune response that might not reflect the milieu of immune responses in the cervical-vaginal tract.
If perfected, technologies like these hold the promise of minimizing the amount of patients who need to undergo excisional procedures because patients' own immune systems have been trained to target HPV-infected cells. The bigger hope is that we will be able to minimize preterm births that are directly related to treatment of dysplasia.
Adoptive T-cell therapy offers targeted treatment for recurrent cervical cancer
Stevanovic S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33(14):1543-1550.
Stevanovic and colleagues have been developing another immune-based therapy that has been tested for other cancers. This uses a method for generating T-cell cultures from HPV-positive cancers and selecting specific HPV oncoprotein- reactive cultures for administration to patients. Termed adoptive T-cell therapy (ACT), this targeted approach to recurrent cervical cancer is what I would consider one of the most intriguing future treatments of cervical disease. In the past, the largest barrier to an effective HPV vaccine to treat cervical cancer has been lack of clinical response to existing cytotoxic regimens. In this, albeit small, trial, investigators found a correlation between HPV reactivity and the infused T cells and objective clinical responses.
What is adoptive T-cell therapy?
ACT allows for more rigorous control over the magnitude of the targeted response than tumor vaccination treatment strategies because the T cells used for therapy are identified and selected in vitro. The cells selected are exposed to cytokines and immunomodulators that influence differentiation during priming and are expanded to large numbers. The resulting number of antigen-specific T cells produced in the peripheral blood is much greater (more than 10-fold) than that possible by current vaccine regimens alone.
Studies conducted by the National Cancer Institute of adoptive transfer of in vitro-selected tumor-infiltrating lymphocytes were the first to demonstrate the potential of T-cell immunotherapy to eradicate solid tumors.5,6 Among 13 patients with melanoma, treatment with adoptive transfer of ex vivo-amplified autologous tumor-infiltrating T cells resulted in treatment response in 10 of the patients—clinical responses in 6 and mixed responses in 4.
Details of the study
This study by Stevanovic and colleagues involved 9 patients with metastatic cervical cancer who previously had received optimal recommended chemotherapy or concomitant chemoradiotherapy regimens. Patients were treated with a single infusion of tumor-infiltrating T cells specifically selected for HPV E6 and E7 reactivity (HPV-TILs). Patients received lymphocyte-depleting chemotherapy before ACT and aldesleukin chemotherapy injection after ACT.
In such a phase I population, one would not expect clinical responses over persistent stable disease. However, in this small trial, 2 patients had complete tumor regression and 1 patient had a partial treatment response, demonstrating that a complete response to metastatic cervical cancer can occur after a single infusion of HPV-TILs. The partial response lasted 3 months. The 2 complete responses were ongoing 22 and 15 months after treatment (FIGURE 2).
Editorialists point out that, only when the infusion product had reactivity against the HPV E6 and E7 peptides did the patients show objective clinical response, suggesting it was the immune response that contributed to the tumor regression.7 In addition, in the 3 patients with objective responses, HPV-specific T cells persisted in peripheral blood for several months.
| FIGURE 2 Patients with complete tumor responses with adoptive T-cell therapy | ||
 |  | |
Two patients with metastatic cervical cancer had complete tumor responses with treatment with tumor-infiltrating T cells selected for HPV E6 and E7 reactivity (HPV-TILs). Contrast-enhanced computed tomography scans obtained before treatment and at most recent follow-up for both patients. (A) First patient (patient 3) had disease involving para-aortic, bilateral hilar, subcarinal, and left iliac lymph nodes (gold arrows). Patient had no evidence of disease 22 months after treatment. (B) Second patient (patient 6) had metastatic disease in para-aortic lymph node, abdominal wall, aortocaval lymph node, left pericolic pelvic mass, and right ureteral nodule (gold arrows). Patient had no evidence of disease 15 months after treatment. (Red arrowhead indicates ureteral stent that was removed after right ureteral tumor regressed.) | ||
Stevanovic S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells.J Clin Oncol. 2015;33(14):1543–1550. Used with permission.
What this evidence means for practice
The recent approval of bevacizumab has been a major breakthrough in the treatment of advanced and recurrent cervical cancer. Although ACT is a treatment that is in early clinical development, it is the next major advance in this area. Its promise is currently limited, as the process is cumbersome and complex, involving surgical removal of a patient's lymph nodes, culturing of the T cells from the lymph nodes, and infusing the T cells with the oncoproteins that will train those T cells to infiltrate the cancer tumor. The process is wrought with potential problems in laboratory and translational techniques. However, this group of investigators from the NCI has perfected the process of ACT, creating T cells that will target the HPV that is integrated into each cervical cancer tumor.
The patients who demonstrated good T-cell reactivity against HPV were the ones who had a treatment response, which demonstrates the targeted precision of ACT therapy. There might come a day when we can select patients with recurrent cervical cancer who are going to have T-cell reactivity, and send them for treatment to a center specialized in ACT. Typically in phase 1 trials, we are happy to see a number of patients responding with stable disease. In this trial, 2 patients had a complete response. The results demonstrated by Stevanovic and colleagues are very exciting for the future treatment of patients with cervical cancer.
Primary HPV screening shows up to 70% greater protection against invasive cervical cancer than cytology
Ronco G, Dillner J, Elfstrom KM; International HPV Screening Working Group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):424-532.
In my 2015 "Update on Cervical Disease,"8 I discussed the newly published interim guidance for managing abnormal screening results for cervical cancer from a collective expert panel from the American Society for Colposcopy and Cervical Pathology, Society of Gynecologic Oncology, the American College of Obstetricians and Gynecologists, and 4 more societies.9 The guidelines support use of HPV testing alone or with the Papanicolaou test. In 2016, follow-up data from 4 RCTs provide long-term data on the efficacy of HPV primary testing.
Details of the trial
Incidence of invasive cervical cancer was the endpoint in 4 European trials comparing HPV-based with cytology-based screening. In total, 176,464 women aged 20 to 64 years were randomly assigned to either screening strategy. Median follow-up was 6.5 years (1,214,415 person-years). Using screening, pathology, and cancer registries investigators identified 107 invasive cervical carcinomas, with masked review of histologic specimens and reports.
Investigators calculated the rate ratios (defined as the cancer detection rate in the primary HPV testing-based versus cytology-based arms) for incidence of invasive cervical cancer. During the first 2.5 years of follow-up, detection of invasive cancer was similar between screening methods (0.79, 0.46-1.36). Thereafter, however, cumulative cancer detection was lower in the primary HPV testing-based arm (0.45; 95% CI, 0.25-0.81).
At 3.5 and 5.5 years after a negative cytology test on entry, cumulative cancer incidence was 15.4 per 105 (95% CI, 7.9-27.0) and 36.0 per 105 (23.2-53.5), respectively. At 3.5 and 5.5 years after a negative HPV test on entry, cumulative cancer incidence was 4.6 per 105 (1.1-12.1) and 8.7 per 105 (3.3-18.6), respectively (FIGURE 3).
| FIGURE 3 Cumulative detection of invasive cervical carcinoma | ||
 | ||
*Observations are censored 2.5 years after CIN2 or CIN3 detection, if any.
| ||
What this EVIDENCE means for practice
The 4 studies in this report were completed across Europe (in England, Netherlands, Sweden, and Italy): different regions, different sites, hospitals, and screening systems. The women in Europe are not any different than the women in the United States in terms of rates of HPV and age and incidence of HPV. Therefore, these results are globally generalizable.
The US trial by Wright and colleagues10 that led to US Food and Drug Administration approval of HPV primary testing was different than this European study in that all trial sites had to perform screening in the same way. In addition, the end point was high-grade dysplasia; in this trial by Ronco and colleagues the end point is cancer. These current investigators found no difference with either screening arm in terms of detection of invasive cervical cancer. Even more interesting is that, over time, the cervical cancer rates in the primary HPV testing-based arm were much less than that in the cytology-based arm.
The real strengths of this study are the long-term follow-up and the study size. We are not likely to see validation cohorts this big again. This study demonstrates that, overall, we should be able to continue to reduce the incidence of invasive cervical cancer with a primary HPV testing-based screening strategy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Conner SN, Frey HA, Cahill AG, Macones GA, Colditz GA, Tuuli MG. Loop electrosurgical excision procedure and risk of preterm birth: a systematic review and meta-analysis. Obstet Gynecol. 2014;123(4):752−761.
- Kyrgiou M, Valasoulis G, Stasinou SM, et al. Proportion of cervical excision for cervical intraepithelial neoplasia as a predictor of pregnancy outcomes. Int J Gynaecol Obstet. 2015;128(2):141−147.
- Miller ES, Grobman WA. The association between cervical excisional procedures, midtrimester cervical length, and preterm birth. Am J Obstet Gynecol. 2014;211(3):242.e1−e4.
- Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Mathews TJ. Births: Final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65. http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_01.pdf. Published January 15, 2015. Accessed April 20, 2016.
- Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854.
- Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346−2357.
- Zsiros E, Tsuli T, Odunsi K. Adoptive T-cell therapy is a promising salvage approach for advanced or recurrent metastatic cervical cancer. J Clin Oncol. 2015;33(14):1521−1522.
- Einstein MH. Update on cervical disease: New ammo for HPV prevention and screening. OBG Manag. 2015;27(5):32−39.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
- Conner SN, Frey HA, Cahill AG, Macones GA, Colditz GA, Tuuli MG. Loop electrosurgical excision procedure and risk of preterm birth: a systematic review and meta-analysis. Obstet Gynecol. 2014;123(4):752−761.
- Kyrgiou M, Valasoulis G, Stasinou SM, et al. Proportion of cervical excision for cervical intraepithelial neoplasia as a predictor of pregnancy outcomes. Int J Gynaecol Obstet. 2015;128(2):141−147.
- Miller ES, Grobman WA. The association between cervical excisional procedures, midtrimester cervical length, and preterm birth. Am J Obstet Gynecol. 2014;211(3):242.e1−e4.
- Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Mathews TJ. Births: Final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65. http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_01.pdf. Published January 15, 2015. Accessed April 20, 2016.
- Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854.
- Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346−2357.
- Zsiros E, Tsuli T, Odunsi K. Adoptive T-cell therapy is a promising salvage approach for advanced or recurrent metastatic cervical cancer. J Clin Oncol. 2015;33(14):1521−1522.
- Einstein MH. Update on cervical disease: New ammo for HPV prevention and screening. OBG Manag. 2015;27(5):32−39.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
In this article
• The success of adoptive T-cell therapy
• Long-term follow-up of primary HPV screening
2015 Update on cervical disease: New ammo for HPV prevention and screening
Two very recent significant advances in cervical disease prevention and screening make this an exciting time for women’s health clinicians. One development, the 9-valent human papillomavirus (HPV) vaccine, offers the potential to increase overall prevention of cervical cancer to over 90%. The other advance offers clinicians a cervical cancer screening alternative, HPV DNA testing, for primary cervical cancer screening. In this article, I underscore the data behind, as well as expert guidance on, these two important developments.
The 9-valent HPV vaccine expands HPV-type coverage and vaccine options for routine use
Joura EA, Giuliano AR, Iversen O, et al. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723.
Two HPV types, 16 and 18, cause the majority—about 70%—of cervical cancers. Vaccination against these types, as well as against types 6 and 11 that cause most condyloma, has been available in the United States since 2006, when the quadrivalent vaccine was approved by the US Food and Drug Administration (FDA).1 Now, based on the results of Joura and colleagues’ randomized, double-blind phase 2b−3 study involving more than 14,000 women, the 9-valent vaccine (Gardasil 9, Merck, Whitehouse Station, New Jersey) has been recommended by the Advisory Committee on Immunization Practices (ACIP) as 1 of 3 HPV vaccines that can be used for routine vaccination.1 (The other 2 vaccines include the bivalent [Cervarix, GlaxoSmithKline, Research Triangle Park, North Carolina] and quadrivalent [Gardasil, Merck]).
Compared with quadrivalent, does the 9-valent vaccine offer compelling additional protection?
The incidence rate of high-grade cervical intraepithelial neoplasia (CIN; ≥CIN 2 or adenocarcinoma in situ) related to the additional HPV types covered with the 9-valent vaccine (31, 33, 45, 52, and 58) was 0.1 per 1,000 person-years in the 9-valent group and 1.6 per 1,000 person-years in the quadrivalent group. This is equivalent to 1 case versus 30 cases of disease and translates to 96.7% efficacy (95% confidence interval [CI], 80.9−99.8) against these 5 additional high-risk HPV types. At 36 months, there was 1 case of high-grade cervical disease in the 9-valent group related to the 5 additional HPV types, compared with 20 cumulative cases in the quadrivalent group. At 48 months, there was 1 case in the 9-valent group and 27 cases in the quadrivalent group (FIGURE 1).
This expanded disease coverage means the vaccine has the potential to prevent an additional 15% to 20% of cervical cancers in addition to the potential to prevent 5% to 20% of other HPV-related cancers.3
The added HPV-type protection resulted in more frequent injection site reactions (90.7% in the 9-valent group vs 84.9% in the quadrivalent group). Pain, erythema, and pruritis were the most common reactions. While rare, events of severe intensity were more common in the 9-valent group. However, less than 0.1% of participants discontinued study vaccination because of a vaccine-related adverse event.
Study strengths and weaknesses
This was a well-designed prospective, randomized controlled trial. Follow-up was limited; however, this is typical for a clinical trial, and extended follow-up analyses have held up in other HPV vaccine trials; I don’t anticipate it will be any different in this case. The control arm in the case of this trial was the quadrivalent vaccine, as that is the routinely recommended vaccine, so it is not ethical to give placebo in this age-range population. The placebo study already was published,4 so Joura and colleagues’ results build on prior findings.
What this EVIDENCE means for practice
In a widely vaccinated population, the 9-valent HPV vaccine has the potential to protect against an additional 20% of cervical cancers, compared with the quadrivalent vaccine. This is an important improvement in HPV infection and cervical disease prevention. Unfortunately, in the United States we still have very low coverage for the first dose of the HPV vaccine, and even lower coverage for the recommended 3-dose series. This is a big problem in the United States. Stakeholders and advocates need to figure out innovative ways to overcome the challenges of full vaccination for the patients in whom it’s routinely recommended—11- and 12-year-old girls and boys. HPV vaccination lags behind coverage for other vaccines recommended in this same age group—by 20% to 25%.3 US HPV vaccination rates are woefully low in comparison with such other countries as Australia, much of western Europe, and the UK. “If teenagers were offered and accepted HPV vaccination every time they received another vaccine, first-dose coverage for HPV would exceed 90%.”3
The ACIP recommends routine vaccination for HPV—with the bivalent, quadrivalent, or 9-valent vaccine—at age 11 or 12 years. They also recommend vaccination for females aged 13 through 26 years and males aged 13 through 21 years who have not been vaccinated previously. Vaccination is also recommended through age 26 years for men who have sex with men and for immunocompromised persons (including those with HIV infection) if not vaccinated previously.1
By the time I retire, I hope that the impact of protection against additional HPV infection types will be felt, with HPV vaccination rates improved and fewer women affected by the morbidity and mortality related to cervical cancer. As ObGyns, we want to do right by our patients; we need to embrace and continue to discuss the message of primary protection with vaccines that protect against HPV in order to overcome the mixed rhetoric patients and parents receive from other groups, including sensational media or political figureheads who might have an alternative agenda that is clearly not in the best interest of our patients.
HPV test alone is as effective as Pap plus HPV test for cervical disease screening
Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
The cobas (Roche Molecular Diagnostics, Pleasanton, California) HPV DNA test received FDA approval as a primary screening test for cervical cancer in women aged 25 and older in April 2015. This is a big paradigm shift from what has long been the way we screen women, starting with cytology. Simplistically, the thinking is that we start with the more sensitive test to enrich the population of women that might need additional testing, which might include cytology.
The FDA considered these end-of-study data by Wright and colleagues, which had not been publically published at the time, in its decision. With the Addressing the Need for Advanced HPV Diagnostics (ATHENA) 3-year prospective study, these investigators sought to address major unresolved issues related to HPV primary screening, such as determining which HPV-positive women should be referred to colposcopy and how HPV primary screening performs in the United States. Such a strategy long has been shown to be effective in large prospective European trials.
Details of the study
Three screening strategies were tested:
- Cytology: HPV testing performed only for atypical cells of undetermined significance (ASC-US).
- Hybrid: Cytology strategy for women aged 25 to 29 and cotesting with both cytology and HPV (pooled 14 genotypes) for women 30 years or older. This strategy mimics current preferred US screening recommendations. With cotesting, HPV-positive women with negative cytology are retested with both tests in 1 year and undergo colposcopy if either test is abnormal.
- HPV primary: HPV-negative women rescreened in 3 years, HPV16/18-positive women receive immediate colposcopy, women positive for the other 12 HPV types receive reflex cytology with colposcopy if the cytology is ASC-US or worse. If cytology results are negative, women are rescreened with HPV and cytology in 1 year.
In all strategies, women who were referred to colposcopy and found not to have CIN 2 or greater were rescreened with both tests in 1 year and referred to colposcopy if the finding was ASC-US or higher-grade or persistently HPV-positive.
Of the 3 screening strategies, HPV primary in women 25 years and older had the highest adjusted sensitivity over 3 years (76.1%; 95% CI, 70.3–81.8) for the detection of CIN 3 or greater, with similar specificity as the cytology and hybrid strategies. In addition, the negative predictive value for not having clinically relevant disease for HPV primary was comparable to or better than the other 2 strategies (TABLE).5
Another important finding was that the number of colposcopies required to detect 1 case of cervical disease, although found to be significantly higher, was comparable for the HPV primary and cytology strategies (7.1 [95% CI, 6.4–8.0] for cytology vs 8.0 for HPV primary for CIN 2 or greater in women 25 years and older). For CIN 3 or greater, the number of colposcopies required to detect 1 case was 12.8 (95% CI, 11.7–14.5) for HPV primary versus 12.9 (95% CI, 11.5–14.8) for hybrid and 10.8 (95% CI, 9.4–12.6) for cytology.
What this EVIDENCE means for practice
These data indicate that HPV primary screening in women aged 25 and older is as effective as a hybrid screening strategy that uses cytology and cotesting in a patient older than 30 years. And HPV primary screening requires fewer overall screening tests to identify women who have clinically significant cervical disease.
Importantly, compared with a cytology-based strategy, the negative predictive value is quite high for HPV primary screening. Therefore, if someone has a negative HPV test result, the likelihood of that person actually having some sort of clinically relevant disease that day or in the next 3 years is incredibly low. And this is really what’s important for our patients who are getting screened for cervical cancer.
Interim guidelines support use of HPV testing alone or with the Pap smear
Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
The most recent set of consensus guidelines for managing abnormal cervical cancer screening tests and cancer precursors is the American Cancer Society/American Society for Colposcopy and Cervical Pathology (ASCCP)/American Society for Clinical Pathology 2012 guidelines,6 which recommend cotesting as the preferred strategy in women aged 30 to 65 years. However, to address increasing evidence that HPV testing alone is an effective primary screening approach and how clinicians should adopt these findings in their practice, an expert panel convened to offer interim guidance. The panel was cosponsored and funded by the Society of Gynecologic Oncology (SGO) and ASCCP and included 13 experts representing 7 societies, including SGO, ASCCP, and the American College of Obstetricians and Gynecologists. This guidance can be adopted as an alternative to the updated 2012 recommendations until the next consensus guidelines panel convenes.
The panel considered a number of questions related to primary HPV testing and overall advantages and disadvantages of this strategy for screening.
Is HPV testing (for high-risk HPV [hrHPV] types) for primary screening as safe and effective as cytology-based screening?
The panel’s answer: Yes. A negative hrHPV test provides greater reassurance of low CIN 3 or greater risk than a negative cytology result. Because of its equivalent, or even superior, effectiveness—which has been demonstrated in the ATHENA study and several European randomized controlled screening trials7,8—primary hrHPV screening can be considered as an alternative to current US cervical cancer screening methods.
A reasonable approach to managing a positive hrHPV result, advises the panel, is to triage hrHPV-positive women using a combination of genotyping for HPV 16 and 18 and reflex cytology for women positive for the 12 other hrHPV genotypes
(FIGURE 2).9
What is the optimal age to begin primary hrHPV screening?
The panel’s clinical guidance is not before age 25. This is a gray area right now, however, as there are concerns regarding the potential harm of screening at age 25 despite the increased detection of disease, particularly with regard to the number of colposcopies that could be performed in this age group due to the high incidence of HPV infection in young women. So the ideal age at which to begin hrHPV screening will need further discussion in future consensus guideline panels.
What is the optimal interval for primary hrHPV screening?
Prospective follow-up in the ATHENA study was restricted to 3 years. The panel advises that rescreening after a negative primary hrHPV screen should occur no sooner than every 3 years.
Outstanding considerations
The changeover from primary cytology to primary HPV testing represents a very different workflow for clinicians and laboratories. It also represents a different mode of screening for our patients, so patient education is essential. Many questions and concerns still need to be considered, for instance:
- There are no real comparative effectiveness data for the number of screening tests that are needed for an HPV primary screening program, including the number of colposcopies.
- There needs to be further discussion about the optimal age to begin primary HPV screening and the appropriate interval for rescreening patients who are HPV-negative.
- There are questions about the sampling from patients, such as specimen adequacy, internal controls, and the impact of other interfering substances in a large screening program.
What this EVIDENCE means for practice
A move to the HPV test for primary screening represents a paradigm shift for clinicians and patients. Such a shift likely will be slow to occur, due to changes in clinical and laboratory workflow, provider and patient education, and systems issues. Also, there are a number of questions that still need to be answered. Primary hrHPV screening at age 25 to 29 years may lead to increased CIN 3 detection, but the impact of the increased number of colposcopies, integration for those women who already have been screened prior to age 25, and actual impact on cancer prevention need further investigation, the panel points out.
However, primary HPV screening can be considered as an alternative to current US cytology-based cervical cancer screening approaches. Over time, use of primary HPV screening appears to make screening more precise and efficient as it will minimize the number of abnormal cytology results that we would consider cytomorphologic manifestations of an active HPV infection that are not clinically relevant.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Petrosky E, Bocchini JA, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR. 2015;62(11):300−304.
2. Joura EA, Giuliano O, Iversen C, et al, for the Broad Spectrum HPV Vaccine Study. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711−723.
3. Schuchat A. HPV “coverage.” N Engl J Med. 2015;372(8): 775−776.
4. Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928−1943.
5. Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
6. Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 suppl 1):S1–S27.
7. Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532.
8. Dillner J, ReboljM, Birembaut P, et al. Long-term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study [published online ahead of print October 13, 2008]. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754.
9. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
Two very recent significant advances in cervical disease prevention and screening make this an exciting time for women’s health clinicians. One development, the 9-valent human papillomavirus (HPV) vaccine, offers the potential to increase overall prevention of cervical cancer to over 90%. The other advance offers clinicians a cervical cancer screening alternative, HPV DNA testing, for primary cervical cancer screening. In this article, I underscore the data behind, as well as expert guidance on, these two important developments.
The 9-valent HPV vaccine expands HPV-type coverage and vaccine options for routine use
Joura EA, Giuliano AR, Iversen O, et al. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723.
Two HPV types, 16 and 18, cause the majority—about 70%—of cervical cancers. Vaccination against these types, as well as against types 6 and 11 that cause most condyloma, has been available in the United States since 2006, when the quadrivalent vaccine was approved by the US Food and Drug Administration (FDA).1 Now, based on the results of Joura and colleagues’ randomized, double-blind phase 2b−3 study involving more than 14,000 women, the 9-valent vaccine (Gardasil 9, Merck, Whitehouse Station, New Jersey) has been recommended by the Advisory Committee on Immunization Practices (ACIP) as 1 of 3 HPV vaccines that can be used for routine vaccination.1 (The other 2 vaccines include the bivalent [Cervarix, GlaxoSmithKline, Research Triangle Park, North Carolina] and quadrivalent [Gardasil, Merck]).
Compared with quadrivalent, does the 9-valent vaccine offer compelling additional protection?
The incidence rate of high-grade cervical intraepithelial neoplasia (CIN; ≥CIN 2 or adenocarcinoma in situ) related to the additional HPV types covered with the 9-valent vaccine (31, 33, 45, 52, and 58) was 0.1 per 1,000 person-years in the 9-valent group and 1.6 per 1,000 person-years in the quadrivalent group. This is equivalent to 1 case versus 30 cases of disease and translates to 96.7% efficacy (95% confidence interval [CI], 80.9−99.8) against these 5 additional high-risk HPV types. At 36 months, there was 1 case of high-grade cervical disease in the 9-valent group related to the 5 additional HPV types, compared with 20 cumulative cases in the quadrivalent group. At 48 months, there was 1 case in the 9-valent group and 27 cases in the quadrivalent group (FIGURE 1).
This expanded disease coverage means the vaccine has the potential to prevent an additional 15% to 20% of cervical cancers in addition to the potential to prevent 5% to 20% of other HPV-related cancers.3
The added HPV-type protection resulted in more frequent injection site reactions (90.7% in the 9-valent group vs 84.9% in the quadrivalent group). Pain, erythema, and pruritis were the most common reactions. While rare, events of severe intensity were more common in the 9-valent group. However, less than 0.1% of participants discontinued study vaccination because of a vaccine-related adverse event.
Study strengths and weaknesses
This was a well-designed prospective, randomized controlled trial. Follow-up was limited; however, this is typical for a clinical trial, and extended follow-up analyses have held up in other HPV vaccine trials; I don’t anticipate it will be any different in this case. The control arm in the case of this trial was the quadrivalent vaccine, as that is the routinely recommended vaccine, so it is not ethical to give placebo in this age-range population. The placebo study already was published,4 so Joura and colleagues’ results build on prior findings.
What this EVIDENCE means for practice
In a widely vaccinated population, the 9-valent HPV vaccine has the potential to protect against an additional 20% of cervical cancers, compared with the quadrivalent vaccine. This is an important improvement in HPV infection and cervical disease prevention. Unfortunately, in the United States we still have very low coverage for the first dose of the HPV vaccine, and even lower coverage for the recommended 3-dose series. This is a big problem in the United States. Stakeholders and advocates need to figure out innovative ways to overcome the challenges of full vaccination for the patients in whom it’s routinely recommended—11- and 12-year-old girls and boys. HPV vaccination lags behind coverage for other vaccines recommended in this same age group—by 20% to 25%.3 US HPV vaccination rates are woefully low in comparison with such other countries as Australia, much of western Europe, and the UK. “If teenagers were offered and accepted HPV vaccination every time they received another vaccine, first-dose coverage for HPV would exceed 90%.”3
The ACIP recommends routine vaccination for HPV—with the bivalent, quadrivalent, or 9-valent vaccine—at age 11 or 12 years. They also recommend vaccination for females aged 13 through 26 years and males aged 13 through 21 years who have not been vaccinated previously. Vaccination is also recommended through age 26 years for men who have sex with men and for immunocompromised persons (including those with HIV infection) if not vaccinated previously.1
By the time I retire, I hope that the impact of protection against additional HPV infection types will be felt, with HPV vaccination rates improved and fewer women affected by the morbidity and mortality related to cervical cancer. As ObGyns, we want to do right by our patients; we need to embrace and continue to discuss the message of primary protection with vaccines that protect against HPV in order to overcome the mixed rhetoric patients and parents receive from other groups, including sensational media or political figureheads who might have an alternative agenda that is clearly not in the best interest of our patients.
HPV test alone is as effective as Pap plus HPV test for cervical disease screening
Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
The cobas (Roche Molecular Diagnostics, Pleasanton, California) HPV DNA test received FDA approval as a primary screening test for cervical cancer in women aged 25 and older in April 2015. This is a big paradigm shift from what has long been the way we screen women, starting with cytology. Simplistically, the thinking is that we start with the more sensitive test to enrich the population of women that might need additional testing, which might include cytology.
The FDA considered these end-of-study data by Wright and colleagues, which had not been publically published at the time, in its decision. With the Addressing the Need for Advanced HPV Diagnostics (ATHENA) 3-year prospective study, these investigators sought to address major unresolved issues related to HPV primary screening, such as determining which HPV-positive women should be referred to colposcopy and how HPV primary screening performs in the United States. Such a strategy long has been shown to be effective in large prospective European trials.
Details of the study
Three screening strategies were tested:
- Cytology: HPV testing performed only for atypical cells of undetermined significance (ASC-US).
- Hybrid: Cytology strategy for women aged 25 to 29 and cotesting with both cytology and HPV (pooled 14 genotypes) for women 30 years or older. This strategy mimics current preferred US screening recommendations. With cotesting, HPV-positive women with negative cytology are retested with both tests in 1 year and undergo colposcopy if either test is abnormal.
- HPV primary: HPV-negative women rescreened in 3 years, HPV16/18-positive women receive immediate colposcopy, women positive for the other 12 HPV types receive reflex cytology with colposcopy if the cytology is ASC-US or worse. If cytology results are negative, women are rescreened with HPV and cytology in 1 year.
In all strategies, women who were referred to colposcopy and found not to have CIN 2 or greater were rescreened with both tests in 1 year and referred to colposcopy if the finding was ASC-US or higher-grade or persistently HPV-positive.
Of the 3 screening strategies, HPV primary in women 25 years and older had the highest adjusted sensitivity over 3 years (76.1%; 95% CI, 70.3–81.8) for the detection of CIN 3 or greater, with similar specificity as the cytology and hybrid strategies. In addition, the negative predictive value for not having clinically relevant disease for HPV primary was comparable to or better than the other 2 strategies (TABLE).5
Another important finding was that the number of colposcopies required to detect 1 case of cervical disease, although found to be significantly higher, was comparable for the HPV primary and cytology strategies (7.1 [95% CI, 6.4–8.0] for cytology vs 8.0 for HPV primary for CIN 2 or greater in women 25 years and older). For CIN 3 or greater, the number of colposcopies required to detect 1 case was 12.8 (95% CI, 11.7–14.5) for HPV primary versus 12.9 (95% CI, 11.5–14.8) for hybrid and 10.8 (95% CI, 9.4–12.6) for cytology.
What this EVIDENCE means for practice
These data indicate that HPV primary screening in women aged 25 and older is as effective as a hybrid screening strategy that uses cytology and cotesting in a patient older than 30 years. And HPV primary screening requires fewer overall screening tests to identify women who have clinically significant cervical disease.
Importantly, compared with a cytology-based strategy, the negative predictive value is quite high for HPV primary screening. Therefore, if someone has a negative HPV test result, the likelihood of that person actually having some sort of clinically relevant disease that day or in the next 3 years is incredibly low. And this is really what’s important for our patients who are getting screened for cervical cancer.
Interim guidelines support use of HPV testing alone or with the Pap smear
Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
The most recent set of consensus guidelines for managing abnormal cervical cancer screening tests and cancer precursors is the American Cancer Society/American Society for Colposcopy and Cervical Pathology (ASCCP)/American Society for Clinical Pathology 2012 guidelines,6 which recommend cotesting as the preferred strategy in women aged 30 to 65 years. However, to address increasing evidence that HPV testing alone is an effective primary screening approach and how clinicians should adopt these findings in their practice, an expert panel convened to offer interim guidance. The panel was cosponsored and funded by the Society of Gynecologic Oncology (SGO) and ASCCP and included 13 experts representing 7 societies, including SGO, ASCCP, and the American College of Obstetricians and Gynecologists. This guidance can be adopted as an alternative to the updated 2012 recommendations until the next consensus guidelines panel convenes.
The panel considered a number of questions related to primary HPV testing and overall advantages and disadvantages of this strategy for screening.
Is HPV testing (for high-risk HPV [hrHPV] types) for primary screening as safe and effective as cytology-based screening?
The panel’s answer: Yes. A negative hrHPV test provides greater reassurance of low CIN 3 or greater risk than a negative cytology result. Because of its equivalent, or even superior, effectiveness—which has been demonstrated in the ATHENA study and several European randomized controlled screening trials7,8—primary hrHPV screening can be considered as an alternative to current US cervical cancer screening methods.
A reasonable approach to managing a positive hrHPV result, advises the panel, is to triage hrHPV-positive women using a combination of genotyping for HPV 16 and 18 and reflex cytology for women positive for the 12 other hrHPV genotypes
(FIGURE 2).9
What is the optimal age to begin primary hrHPV screening?
The panel’s clinical guidance is not before age 25. This is a gray area right now, however, as there are concerns regarding the potential harm of screening at age 25 despite the increased detection of disease, particularly with regard to the number of colposcopies that could be performed in this age group due to the high incidence of HPV infection in young women. So the ideal age at which to begin hrHPV screening will need further discussion in future consensus guideline panels.
What is the optimal interval for primary hrHPV screening?
Prospective follow-up in the ATHENA study was restricted to 3 years. The panel advises that rescreening after a negative primary hrHPV screen should occur no sooner than every 3 years.
Outstanding considerations
The changeover from primary cytology to primary HPV testing represents a very different workflow for clinicians and laboratories. It also represents a different mode of screening for our patients, so patient education is essential. Many questions and concerns still need to be considered, for instance:
- There are no real comparative effectiveness data for the number of screening tests that are needed for an HPV primary screening program, including the number of colposcopies.
- There needs to be further discussion about the optimal age to begin primary HPV screening and the appropriate interval for rescreening patients who are HPV-negative.
- There are questions about the sampling from patients, such as specimen adequacy, internal controls, and the impact of other interfering substances in a large screening program.
What this EVIDENCE means for practice
A move to the HPV test for primary screening represents a paradigm shift for clinicians and patients. Such a shift likely will be slow to occur, due to changes in clinical and laboratory workflow, provider and patient education, and systems issues. Also, there are a number of questions that still need to be answered. Primary hrHPV screening at age 25 to 29 years may lead to increased CIN 3 detection, but the impact of the increased number of colposcopies, integration for those women who already have been screened prior to age 25, and actual impact on cancer prevention need further investigation, the panel points out.
However, primary HPV screening can be considered as an alternative to current US cytology-based cervical cancer screening approaches. Over time, use of primary HPV screening appears to make screening more precise and efficient as it will minimize the number of abnormal cytology results that we would consider cytomorphologic manifestations of an active HPV infection that are not clinically relevant.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Two very recent significant advances in cervical disease prevention and screening make this an exciting time for women’s health clinicians. One development, the 9-valent human papillomavirus (HPV) vaccine, offers the potential to increase overall prevention of cervical cancer to over 90%. The other advance offers clinicians a cervical cancer screening alternative, HPV DNA testing, for primary cervical cancer screening. In this article, I underscore the data behind, as well as expert guidance on, these two important developments.
The 9-valent HPV vaccine expands HPV-type coverage and vaccine options for routine use
Joura EA, Giuliano AR, Iversen O, et al. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723.
Two HPV types, 16 and 18, cause the majority—about 70%—of cervical cancers. Vaccination against these types, as well as against types 6 and 11 that cause most condyloma, has been available in the United States since 2006, when the quadrivalent vaccine was approved by the US Food and Drug Administration (FDA).1 Now, based on the results of Joura and colleagues’ randomized, double-blind phase 2b−3 study involving more than 14,000 women, the 9-valent vaccine (Gardasil 9, Merck, Whitehouse Station, New Jersey) has been recommended by the Advisory Committee on Immunization Practices (ACIP) as 1 of 3 HPV vaccines that can be used for routine vaccination.1 (The other 2 vaccines include the bivalent [Cervarix, GlaxoSmithKline, Research Triangle Park, North Carolina] and quadrivalent [Gardasil, Merck]).
Compared with quadrivalent, does the 9-valent vaccine offer compelling additional protection?
The incidence rate of high-grade cervical intraepithelial neoplasia (CIN; ≥CIN 2 or adenocarcinoma in situ) related to the additional HPV types covered with the 9-valent vaccine (31, 33, 45, 52, and 58) was 0.1 per 1,000 person-years in the 9-valent group and 1.6 per 1,000 person-years in the quadrivalent group. This is equivalent to 1 case versus 30 cases of disease and translates to 96.7% efficacy (95% confidence interval [CI], 80.9−99.8) against these 5 additional high-risk HPV types. At 36 months, there was 1 case of high-grade cervical disease in the 9-valent group related to the 5 additional HPV types, compared with 20 cumulative cases in the quadrivalent group. At 48 months, there was 1 case in the 9-valent group and 27 cases in the quadrivalent group (FIGURE 1).
This expanded disease coverage means the vaccine has the potential to prevent an additional 15% to 20% of cervical cancers in addition to the potential to prevent 5% to 20% of other HPV-related cancers.3
The added HPV-type protection resulted in more frequent injection site reactions (90.7% in the 9-valent group vs 84.9% in the quadrivalent group). Pain, erythema, and pruritis were the most common reactions. While rare, events of severe intensity were more common in the 9-valent group. However, less than 0.1% of participants discontinued study vaccination because of a vaccine-related adverse event.
Study strengths and weaknesses
This was a well-designed prospective, randomized controlled trial. Follow-up was limited; however, this is typical for a clinical trial, and extended follow-up analyses have held up in other HPV vaccine trials; I don’t anticipate it will be any different in this case. The control arm in the case of this trial was the quadrivalent vaccine, as that is the routinely recommended vaccine, so it is not ethical to give placebo in this age-range population. The placebo study already was published,4 so Joura and colleagues’ results build on prior findings.
What this EVIDENCE means for practice
In a widely vaccinated population, the 9-valent HPV vaccine has the potential to protect against an additional 20% of cervical cancers, compared with the quadrivalent vaccine. This is an important improvement in HPV infection and cervical disease prevention. Unfortunately, in the United States we still have very low coverage for the first dose of the HPV vaccine, and even lower coverage for the recommended 3-dose series. This is a big problem in the United States. Stakeholders and advocates need to figure out innovative ways to overcome the challenges of full vaccination for the patients in whom it’s routinely recommended—11- and 12-year-old girls and boys. HPV vaccination lags behind coverage for other vaccines recommended in this same age group—by 20% to 25%.3 US HPV vaccination rates are woefully low in comparison with such other countries as Australia, much of western Europe, and the UK. “If teenagers were offered and accepted HPV vaccination every time they received another vaccine, first-dose coverage for HPV would exceed 90%.”3
The ACIP recommends routine vaccination for HPV—with the bivalent, quadrivalent, or 9-valent vaccine—at age 11 or 12 years. They also recommend vaccination for females aged 13 through 26 years and males aged 13 through 21 years who have not been vaccinated previously. Vaccination is also recommended through age 26 years for men who have sex with men and for immunocompromised persons (including those with HIV infection) if not vaccinated previously.1
By the time I retire, I hope that the impact of protection against additional HPV infection types will be felt, with HPV vaccination rates improved and fewer women affected by the morbidity and mortality related to cervical cancer. As ObGyns, we want to do right by our patients; we need to embrace and continue to discuss the message of primary protection with vaccines that protect against HPV in order to overcome the mixed rhetoric patients and parents receive from other groups, including sensational media or political figureheads who might have an alternative agenda that is clearly not in the best interest of our patients.
HPV test alone is as effective as Pap plus HPV test for cervical disease screening
Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
The cobas (Roche Molecular Diagnostics, Pleasanton, California) HPV DNA test received FDA approval as a primary screening test for cervical cancer in women aged 25 and older in April 2015. This is a big paradigm shift from what has long been the way we screen women, starting with cytology. Simplistically, the thinking is that we start with the more sensitive test to enrich the population of women that might need additional testing, which might include cytology.
The FDA considered these end-of-study data by Wright and colleagues, which had not been publically published at the time, in its decision. With the Addressing the Need for Advanced HPV Diagnostics (ATHENA) 3-year prospective study, these investigators sought to address major unresolved issues related to HPV primary screening, such as determining which HPV-positive women should be referred to colposcopy and how HPV primary screening performs in the United States. Such a strategy long has been shown to be effective in large prospective European trials.
Details of the study
Three screening strategies were tested:
- Cytology: HPV testing performed only for atypical cells of undetermined significance (ASC-US).
- Hybrid: Cytology strategy for women aged 25 to 29 and cotesting with both cytology and HPV (pooled 14 genotypes) for women 30 years or older. This strategy mimics current preferred US screening recommendations. With cotesting, HPV-positive women with negative cytology are retested with both tests in 1 year and undergo colposcopy if either test is abnormal.
- HPV primary: HPV-negative women rescreened in 3 years, HPV16/18-positive women receive immediate colposcopy, women positive for the other 12 HPV types receive reflex cytology with colposcopy if the cytology is ASC-US or worse. If cytology results are negative, women are rescreened with HPV and cytology in 1 year.
In all strategies, women who were referred to colposcopy and found not to have CIN 2 or greater were rescreened with both tests in 1 year and referred to colposcopy if the finding was ASC-US or higher-grade or persistently HPV-positive.
Of the 3 screening strategies, HPV primary in women 25 years and older had the highest adjusted sensitivity over 3 years (76.1%; 95% CI, 70.3–81.8) for the detection of CIN 3 or greater, with similar specificity as the cytology and hybrid strategies. In addition, the negative predictive value for not having clinically relevant disease for HPV primary was comparable to or better than the other 2 strategies (TABLE).5
Another important finding was that the number of colposcopies required to detect 1 case of cervical disease, although found to be significantly higher, was comparable for the HPV primary and cytology strategies (7.1 [95% CI, 6.4–8.0] for cytology vs 8.0 for HPV primary for CIN 2 or greater in women 25 years and older). For CIN 3 or greater, the number of colposcopies required to detect 1 case was 12.8 (95% CI, 11.7–14.5) for HPV primary versus 12.9 (95% CI, 11.5–14.8) for hybrid and 10.8 (95% CI, 9.4–12.6) for cytology.
What this EVIDENCE means for practice
These data indicate that HPV primary screening in women aged 25 and older is as effective as a hybrid screening strategy that uses cytology and cotesting in a patient older than 30 years. And HPV primary screening requires fewer overall screening tests to identify women who have clinically significant cervical disease.
Importantly, compared with a cytology-based strategy, the negative predictive value is quite high for HPV primary screening. Therefore, if someone has a negative HPV test result, the likelihood of that person actually having some sort of clinically relevant disease that day or in the next 3 years is incredibly low. And this is really what’s important for our patients who are getting screened for cervical cancer.
Interim guidelines support use of HPV testing alone or with the Pap smear
Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
The most recent set of consensus guidelines for managing abnormal cervical cancer screening tests and cancer precursors is the American Cancer Society/American Society for Colposcopy and Cervical Pathology (ASCCP)/American Society for Clinical Pathology 2012 guidelines,6 which recommend cotesting as the preferred strategy in women aged 30 to 65 years. However, to address increasing evidence that HPV testing alone is an effective primary screening approach and how clinicians should adopt these findings in their practice, an expert panel convened to offer interim guidance. The panel was cosponsored and funded by the Society of Gynecologic Oncology (SGO) and ASCCP and included 13 experts representing 7 societies, including SGO, ASCCP, and the American College of Obstetricians and Gynecologists. This guidance can be adopted as an alternative to the updated 2012 recommendations until the next consensus guidelines panel convenes.
The panel considered a number of questions related to primary HPV testing and overall advantages and disadvantages of this strategy for screening.
Is HPV testing (for high-risk HPV [hrHPV] types) for primary screening as safe and effective as cytology-based screening?
The panel’s answer: Yes. A negative hrHPV test provides greater reassurance of low CIN 3 or greater risk than a negative cytology result. Because of its equivalent, or even superior, effectiveness—which has been demonstrated in the ATHENA study and several European randomized controlled screening trials7,8—primary hrHPV screening can be considered as an alternative to current US cervical cancer screening methods.
A reasonable approach to managing a positive hrHPV result, advises the panel, is to triage hrHPV-positive women using a combination of genotyping for HPV 16 and 18 and reflex cytology for women positive for the 12 other hrHPV genotypes
(FIGURE 2).9
What is the optimal age to begin primary hrHPV screening?
The panel’s clinical guidance is not before age 25. This is a gray area right now, however, as there are concerns regarding the potential harm of screening at age 25 despite the increased detection of disease, particularly with regard to the number of colposcopies that could be performed in this age group due to the high incidence of HPV infection in young women. So the ideal age at which to begin hrHPV screening will need further discussion in future consensus guideline panels.
What is the optimal interval for primary hrHPV screening?
Prospective follow-up in the ATHENA study was restricted to 3 years. The panel advises that rescreening after a negative primary hrHPV screen should occur no sooner than every 3 years.
Outstanding considerations
The changeover from primary cytology to primary HPV testing represents a very different workflow for clinicians and laboratories. It also represents a different mode of screening for our patients, so patient education is essential. Many questions and concerns still need to be considered, for instance:
- There are no real comparative effectiveness data for the number of screening tests that are needed for an HPV primary screening program, including the number of colposcopies.
- There needs to be further discussion about the optimal age to begin primary HPV screening and the appropriate interval for rescreening patients who are HPV-negative.
- There are questions about the sampling from patients, such as specimen adequacy, internal controls, and the impact of other interfering substances in a large screening program.
What this EVIDENCE means for practice
A move to the HPV test for primary screening represents a paradigm shift for clinicians and patients. Such a shift likely will be slow to occur, due to changes in clinical and laboratory workflow, provider and patient education, and systems issues. Also, there are a number of questions that still need to be answered. Primary hrHPV screening at age 25 to 29 years may lead to increased CIN 3 detection, but the impact of the increased number of colposcopies, integration for those women who already have been screened prior to age 25, and actual impact on cancer prevention need further investigation, the panel points out.
However, primary HPV screening can be considered as an alternative to current US cytology-based cervical cancer screening approaches. Over time, use of primary HPV screening appears to make screening more precise and efficient as it will minimize the number of abnormal cytology results that we would consider cytomorphologic manifestations of an active HPV infection that are not clinically relevant.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Petrosky E, Bocchini JA, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR. 2015;62(11):300−304.
2. Joura EA, Giuliano O, Iversen C, et al, for the Broad Spectrum HPV Vaccine Study. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711−723.
3. Schuchat A. HPV “coverage.” N Engl J Med. 2015;372(8): 775−776.
4. Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928−1943.
5. Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
6. Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 suppl 1):S1–S27.
7. Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532.
8. Dillner J, ReboljM, Birembaut P, et al. Long-term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study [published online ahead of print October 13, 2008]. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754.
9. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
1. Petrosky E, Bocchini JA, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR. 2015;62(11):300−304.
2. Joura EA, Giuliano O, Iversen C, et al, for the Broad Spectrum HPV Vaccine Study. A 9-valent vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711−723.
3. Schuchat A. HPV “coverage.” N Engl J Med. 2015;372(8): 775−776.
4. Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928−1943.
5. Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
6. Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 suppl 1):S1–S27.
7. Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532.
8. Dillner J, ReboljM, Birembaut P, et al. Long-term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study [published online ahead of print October 13, 2008]. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754.
9. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
In this article
- Disease progression with 9-valent versus quadrivalent HPV vaccine
- How well do current screening strategies detect disease?
- Recommended primary HPV screening algorithm
2014 Update on cervical disease
Advances in cervical cancer screening continue apace. We are fortunate that these advances are based on a substantial amount of high-quality prospective evidence. Many of these advances are designed to target the women who have clinically relevant disease while minimizing harm and anxiety caused by unnecessary procedures related to cervical screening test abnormalities that have little clinical relevance.
With clinicians being regularly judged on performance and outcomes, adoption of advances and new guidelines should be considered relatively quickly by women’s health providers.
In this article, I focus on two significant advances of the past (and coming) year:
- recent application and unanimous approval by a Food and Drug Administration (FDA) expert panel for the use of the cobas human papillomavirus (HPV) DNA test as a primary cervical cancer screen
- the latest update of guidelines on the management of abnormal cervical screening tests from the American Society for Colposcopy and Cervical Pathology (ASCCP).
cobas HPV TEST IS POISED FOR FDA APPROVAL AS A PRIMARY SCREEN FOR CERVICAL CANCER
Wright TC Jr, Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012;206(1):46.e1–e11.
An FDA expert panel unanimously approved the cobas (Roche Molecular Diagnostics; Pleasanton, California) HPV DNA test on March 12, 2014. The FDA will decide on potential approval within the coming months. Although the FDA sometimes reaches a different decision from one of its advisory committees when it comes to a final vote on a product or device, most often the FDA concurs with the committee’s judgment. Therefore, approval of the cobas HPV test as a primary screen is likely.
Related article: FDA Advisory Committee recommends HPV test as primary screening tool for cervical cancer Deborah Reale (News for your Practice, March 2014)
The cobas HPV test yields a pooled result for 12 high-risk HPV types (hrHPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68), as well as individual results for types 16 and 18; it also has an internal control for specimen adequacy. HPV 16 and 18 account for roughly 70% of all cases of cervical cancer, and infection with both types are known to place women at high risk for having clinically relevant disease—more so than the other hrHPV types.
COMMITTEE REVIEWED DATA FROM ATHENA IN VOTING FOR APPROVAL
In considering the cobas HPV test, the advisory committee reviewed data from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial, a prospective, multicenter, US-based study of 47,208 women aged 21 and older. These women were recruited at the time of undergoing routine screening for cervical cancer; only 2.6% had been vaccinated against HPV. All were screened by liquid-based cytology and an HPV test. Those who had abnormal cytology or a positive test for a high-risk HPV type underwent colposcopy, as did a randomly selected group of women aged 25 or older who tested negative on both tests.
The prevalence of abnormal findings was:
- 7.1% for liquid-based cytology
- 12.6% for pooled high-risk HPV
- 2.8% for HPV 16
- 1.0% for HPV 18.
As expected, cytologic abnormalities and infection with high-risk HPV types declined with increasing age. The adjusted prevalence of cervical intraepithelial neoplasia (CIN) grade 2 or higher in women aged 25 to 34 years was 2.3%; it declined to 1.5% among women older than age 34. Of note, approximately 500,000 US women are given a diagnosis of CIN 2 or CIN 3 each year in the United States.
WHY ATHENA IS IMPORTANT
This US-based trial was designed to assess the medical utility of pooled high-risk HPV DNA in addition to genotyping for HPV 16 and 18 in three populations:
- women aged 21 and older with a cytologic finding of atypical squamous cells of undetermined significance (ASC-US)
- women aged 30 and older with normal cytology
- women aged 25 and older in the overall screening population with any cytologic finding.
Investigators were particularly interested in the use of the HPV test as:
- a triage for women with abnormal cytologic findings
- an adjunct to guide clinical management of women with negative cytology results
- a potential front-line test in the screening of women aged 25 and older.
Related article: Endometrial cancer update: The move toward personalized cancer care Lindsay M. Kuroki, MD, and David G. Mutch, MD (October 2013)
The participants of the ATHENA trial were representative of women undergoing screening for cervical cancer in the United States—both in terms of demographics and in the distribution of cytologic findings. For example, recent US census data indicate that the female population is 79% white, 13% black, and 16% Hispanic or Latino—figures comparable to the breakdown of race/ethnicity in the ATHENA trial.
The trial was conducted in a baseline phase (published in 2012) and a 3-year follow-up phase (not yet published). The 3-year data were reviewed by the FDA advisory committee during its consideration of the cobas HPV test as a primary screen.
DESPITE PROBABLE APPROVAL, INCREMENTAL CHANGE IS LIKELY
Although a move to the HPV test as the primary screen is a definite paradigm shift for what has been cytology-based screening since the initiation of cervical cancer screening, the changeover from primary cytology to primary HPV testing likely will be slow. It will require education of clinicians as well as patients, and a shift in many internal procedures for pathology laboratories.
The ATHENA trial also leaves some intriguing questions unanswered:
- How do we transition women into the new screening strategy? Many women today still undergo cytology screening with reflex HPV testing, as appropriate, and an increasing number of women aged 30 and older undergo cotesting with both cytology and HPV testing. When should they begin screening in a primary HPV testing setting? And what screening intervals will be recommended? If a woman already has been screened with cytology, how should she transition into and at what interval should she begin primary HPV screening?
- How should we manage women’s care after the first round of primary HPV testing? The ATHENA trial so far only has outcomes data after one round of HPV testing. While some data are available from Europe, we do not know what happens after two or three rounds of screening with primary HPV testing in a large US-based cohort. We clearly will be identifying and treating many women with preinvasive disease from screening after one round of testing, at a rate likely higher than with cytology alone—a good thing. We also likely will be reducing the number of unnecessary colposcopies for cytology that are not related to hrHPV.
What this EVIDENCE means for practice
Screening women using the cobas HPV test as a primary screen will require considerable education of providers and patients to explain how this change will affect how a woman will be managed after being screened for cervical cancer. Though much remains to be determined about this new cervical cancer screening paradigm (eg, logistics, timing, use of secondary tests), it should reduce the number of screening tests and colposcopies necessary to detect clinically relevant disease.
UPDATED ASCCP GUIDELINES EMPHASIZE EQUAL MANAGEMENT FOR EQUAL RISK
Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27.
In formulating this latest set of guidelines for the management of abnormal cervical cancer screening tests and cancer precursors, the ASCCP led a conference consisting of scientific stakeholders to perform a comprehensive review of the literature. Also, with study investigators at Kaiser Permanente Northern California (KPNC) and the National Cancer Institute, the guidelines panel also modeled and assessed data on risk after abnormal tests from almost 1.4 million women followed over 8 years in the KPNC Medical Care Plan—this cohort has provided us with “big data.”
The sheer size of the Kaiser Permanente population made it possible for the ASCCP-led panel to validate its previous guidelines or to modify them, where needed. It also made risk-based stratification possible for even rare abnormalities and clinical outcomes.
Although findings from the KPNC population may not be fully generalizable to the US population as a whole, they enhance our understanding of the optimal management of abnormal cervical cancer screening tests and cancer precursors. More widely dispersed study cohorts on a similar scale in the United States are unlikely in the near future.
Related article: Update on cervical disease Mark H. Einstein, MD, MS, and J. Thomas Cox, MD (May 2013)
SEVERAL SIGNIFICANT MODIFICATIONS
Although the ASCCP reaffirmed most elements of its 2006 consensus management guidelines, it did make a number of changes:
- Women who have ASC-US cytology but test HPV-negative now should be followed with cotesting at 3 years rather than 5 years before they return to routine screening.
- Women near age 65 who have a negative finding on ASC-US cytology and HPV testing should not exit screening.
- Women who have ASC-US cytology and test HPV-positive should go to immediate colposcopy, regardless of hrHPV results, including genotyping.
- Women who test positive for HPV 16 or 18 but have negative cytology should undergo immediate colposcopy.
- Women aged 21 to 24 years should be managed as conservatively and minimally invasively as possible, especially when an abnormality is minor.
- Endocervical curettage reported as CIN 1 should be managed as CIN 1, not as a positive endocervical curettage.
- When a cytologic sample is unsatisfactory, sampling usually should be repeated, even when HPV cotesting results are known. However, negative cytology that lacks sufficient endocervical cells or a transformation zone component usually can be managed without frequent follow-up.
Related article: New cervical Ca screening guidelines recommend less frequent assessment Janelle Yates (News for your Practice; April 2012)
EQUAL MANAGEMENT SHOULD BE PERFORMED FOR ABNORMAL TESTS THAT INDICATE EQUAL RISK
The ASCCP-led management panel unanimously agreed to several basic assumptions in formulating the updated guidelines. For example, they concurred that achieving zero risk for cancer is impossible and that attempts to achieve zero risk (which typically means more frequent testing) may cause harm. They also cited the 2011 American Cancer Society/ASCCP/American Society for Clinical Pathology consensus screening document, which stated: “Optimal prevention strategies should identify those HPV-related abnormalities likely to progress to invasive cancers while avoiding destructive treatment of abnormalities not destined to become cancerous.”1
The panel also agreed that CIN 3+ is a “reasonable proxy for cancer risk.” When calculating risk, the KPNC data were modeled for all combinations of cytology and HPV testing, using CIN 3+ for many of the outcomes, and when outcomes were rare, using CIN 2+. The theme of equal management for equal risk was the rationale behind the management approaches detailed in the TABLE. Risks were deemed to be low and return to normal screening was recommended when the risks were similar to the rate of CIN 3+ 3 years after negative cytology or 5 years after negative cotesting. However, immediate colposcopy was recommended when the 5-year risk of CIN 3+ for the combination of cytology and hrHPV testing, when indicated, exceeded 5%. A 6-month to 12-month return (intermediate risk) is indicated with a risk of CIN3+ of 2% to 5%.
An emphasis on avoiding harms
Abnormal findings at the time of cervical cancer screening can lead to a number of harms for the patient, including anxiety and emotional distress, particularly when colposcopy is necessary, as well as time lost from home and work life. For this reason, the guidelines panel emphasized that colposcopy and other interventions should be avoided when the risk of CIN 3+ is low and when the cervical screening abnormalities are likely to resolve without treatment.
However, women who experience postcoital bleeding, unexplained abnormal vaginal bleeding, pelvic pain, abnormal discharge, or a visible lesion should be managed promptly on an individualized basis.
Long-term effects of HPV vaccination are unknown
Among the areas that remain to be addressed are the unknown effects of widespread prophylactic HPV vaccination over the long term. We also lack full understanding of whether and how HPV vaccination will alter the incidence and management of cytologic and histologic abnormalities. Given the low rates of vaccination against HPV in the United States at present, this will need to be re-evaluated in the future.
What this EVIDENCE means for practice
The updated ASCCP guidelines are inherently complex, but their complexity arises from a large body of high-quality prospective data from a large population of women. Equal risk should result in equal management of cervical screening test abnormalities. Practitioners need not feel obligated to memorize the guidelines, owing to the availability of algorithms for specific findings in specific populations at the ASCCP Web site (www.asccp.org/consensus2012). Apps also are available for the iPhone, iPad, and Android.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: obg@frontlinemedcom.com Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
Reference
- Saslow D, Solomon D, Lawson HW, et al;ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
Advances in cervical cancer screening continue apace. We are fortunate that these advances are based on a substantial amount of high-quality prospective evidence. Many of these advances are designed to target the women who have clinically relevant disease while minimizing harm and anxiety caused by unnecessary procedures related to cervical screening test abnormalities that have little clinical relevance.
With clinicians being regularly judged on performance and outcomes, adoption of advances and new guidelines should be considered relatively quickly by women’s health providers.
In this article, I focus on two significant advances of the past (and coming) year:
- recent application and unanimous approval by a Food and Drug Administration (FDA) expert panel for the use of the cobas human papillomavirus (HPV) DNA test as a primary cervical cancer screen
- the latest update of guidelines on the management of abnormal cervical screening tests from the American Society for Colposcopy and Cervical Pathology (ASCCP).
cobas HPV TEST IS POISED FOR FDA APPROVAL AS A PRIMARY SCREEN FOR CERVICAL CANCER
Wright TC Jr, Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012;206(1):46.e1–e11.
An FDA expert panel unanimously approved the cobas (Roche Molecular Diagnostics; Pleasanton, California) HPV DNA test on March 12, 2014. The FDA will decide on potential approval within the coming months. Although the FDA sometimes reaches a different decision from one of its advisory committees when it comes to a final vote on a product or device, most often the FDA concurs with the committee’s judgment. Therefore, approval of the cobas HPV test as a primary screen is likely.
Related article: FDA Advisory Committee recommends HPV test as primary screening tool for cervical cancer Deborah Reale (News for your Practice, March 2014)
The cobas HPV test yields a pooled result for 12 high-risk HPV types (hrHPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68), as well as individual results for types 16 and 18; it also has an internal control for specimen adequacy. HPV 16 and 18 account for roughly 70% of all cases of cervical cancer, and infection with both types are known to place women at high risk for having clinically relevant disease—more so than the other hrHPV types.
COMMITTEE REVIEWED DATA FROM ATHENA IN VOTING FOR APPROVAL
In considering the cobas HPV test, the advisory committee reviewed data from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial, a prospective, multicenter, US-based study of 47,208 women aged 21 and older. These women were recruited at the time of undergoing routine screening for cervical cancer; only 2.6% had been vaccinated against HPV. All were screened by liquid-based cytology and an HPV test. Those who had abnormal cytology or a positive test for a high-risk HPV type underwent colposcopy, as did a randomly selected group of women aged 25 or older who tested negative on both tests.
The prevalence of abnormal findings was:
- 7.1% for liquid-based cytology
- 12.6% for pooled high-risk HPV
- 2.8% for HPV 16
- 1.0% for HPV 18.
As expected, cytologic abnormalities and infection with high-risk HPV types declined with increasing age. The adjusted prevalence of cervical intraepithelial neoplasia (CIN) grade 2 or higher in women aged 25 to 34 years was 2.3%; it declined to 1.5% among women older than age 34. Of note, approximately 500,000 US women are given a diagnosis of CIN 2 or CIN 3 each year in the United States.
WHY ATHENA IS IMPORTANT
This US-based trial was designed to assess the medical utility of pooled high-risk HPV DNA in addition to genotyping for HPV 16 and 18 in three populations:
- women aged 21 and older with a cytologic finding of atypical squamous cells of undetermined significance (ASC-US)
- women aged 30 and older with normal cytology
- women aged 25 and older in the overall screening population with any cytologic finding.
Investigators were particularly interested in the use of the HPV test as:
- a triage for women with abnormal cytologic findings
- an adjunct to guide clinical management of women with negative cytology results
- a potential front-line test in the screening of women aged 25 and older.
Related article: Endometrial cancer update: The move toward personalized cancer care Lindsay M. Kuroki, MD, and David G. Mutch, MD (October 2013)
The participants of the ATHENA trial were representative of women undergoing screening for cervical cancer in the United States—both in terms of demographics and in the distribution of cytologic findings. For example, recent US census data indicate that the female population is 79% white, 13% black, and 16% Hispanic or Latino—figures comparable to the breakdown of race/ethnicity in the ATHENA trial.
The trial was conducted in a baseline phase (published in 2012) and a 3-year follow-up phase (not yet published). The 3-year data were reviewed by the FDA advisory committee during its consideration of the cobas HPV test as a primary screen.
DESPITE PROBABLE APPROVAL, INCREMENTAL CHANGE IS LIKELY
Although a move to the HPV test as the primary screen is a definite paradigm shift for what has been cytology-based screening since the initiation of cervical cancer screening, the changeover from primary cytology to primary HPV testing likely will be slow. It will require education of clinicians as well as patients, and a shift in many internal procedures for pathology laboratories.
The ATHENA trial also leaves some intriguing questions unanswered:
- How do we transition women into the new screening strategy? Many women today still undergo cytology screening with reflex HPV testing, as appropriate, and an increasing number of women aged 30 and older undergo cotesting with both cytology and HPV testing. When should they begin screening in a primary HPV testing setting? And what screening intervals will be recommended? If a woman already has been screened with cytology, how should she transition into and at what interval should she begin primary HPV screening?
- How should we manage women’s care after the first round of primary HPV testing? The ATHENA trial so far only has outcomes data after one round of HPV testing. While some data are available from Europe, we do not know what happens after two or three rounds of screening with primary HPV testing in a large US-based cohort. We clearly will be identifying and treating many women with preinvasive disease from screening after one round of testing, at a rate likely higher than with cytology alone—a good thing. We also likely will be reducing the number of unnecessary colposcopies for cytology that are not related to hrHPV.
What this EVIDENCE means for practice
Screening women using the cobas HPV test as a primary screen will require considerable education of providers and patients to explain how this change will affect how a woman will be managed after being screened for cervical cancer. Though much remains to be determined about this new cervical cancer screening paradigm (eg, logistics, timing, use of secondary tests), it should reduce the number of screening tests and colposcopies necessary to detect clinically relevant disease.
UPDATED ASCCP GUIDELINES EMPHASIZE EQUAL MANAGEMENT FOR EQUAL RISK
Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27.
In formulating this latest set of guidelines for the management of abnormal cervical cancer screening tests and cancer precursors, the ASCCP led a conference consisting of scientific stakeholders to perform a comprehensive review of the literature. Also, with study investigators at Kaiser Permanente Northern California (KPNC) and the National Cancer Institute, the guidelines panel also modeled and assessed data on risk after abnormal tests from almost 1.4 million women followed over 8 years in the KPNC Medical Care Plan—this cohort has provided us with “big data.”
The sheer size of the Kaiser Permanente population made it possible for the ASCCP-led panel to validate its previous guidelines or to modify them, where needed. It also made risk-based stratification possible for even rare abnormalities and clinical outcomes.
Although findings from the KPNC population may not be fully generalizable to the US population as a whole, they enhance our understanding of the optimal management of abnormal cervical cancer screening tests and cancer precursors. More widely dispersed study cohorts on a similar scale in the United States are unlikely in the near future.
Related article: Update on cervical disease Mark H. Einstein, MD, MS, and J. Thomas Cox, MD (May 2013)
SEVERAL SIGNIFICANT MODIFICATIONS
Although the ASCCP reaffirmed most elements of its 2006 consensus management guidelines, it did make a number of changes:
- Women who have ASC-US cytology but test HPV-negative now should be followed with cotesting at 3 years rather than 5 years before they return to routine screening.
- Women near age 65 who have a negative finding on ASC-US cytology and HPV testing should not exit screening.
- Women who have ASC-US cytology and test HPV-positive should go to immediate colposcopy, regardless of hrHPV results, including genotyping.
- Women who test positive for HPV 16 or 18 but have negative cytology should undergo immediate colposcopy.
- Women aged 21 to 24 years should be managed as conservatively and minimally invasively as possible, especially when an abnormality is minor.
- Endocervical curettage reported as CIN 1 should be managed as CIN 1, not as a positive endocervical curettage.
- When a cytologic sample is unsatisfactory, sampling usually should be repeated, even when HPV cotesting results are known. However, negative cytology that lacks sufficient endocervical cells or a transformation zone component usually can be managed without frequent follow-up.
Related article: New cervical Ca screening guidelines recommend less frequent assessment Janelle Yates (News for your Practice; April 2012)
EQUAL MANAGEMENT SHOULD BE PERFORMED FOR ABNORMAL TESTS THAT INDICATE EQUAL RISK
The ASCCP-led management panel unanimously agreed to several basic assumptions in formulating the updated guidelines. For example, they concurred that achieving zero risk for cancer is impossible and that attempts to achieve zero risk (which typically means more frequent testing) may cause harm. They also cited the 2011 American Cancer Society/ASCCP/American Society for Clinical Pathology consensus screening document, which stated: “Optimal prevention strategies should identify those HPV-related abnormalities likely to progress to invasive cancers while avoiding destructive treatment of abnormalities not destined to become cancerous.”1
The panel also agreed that CIN 3+ is a “reasonable proxy for cancer risk.” When calculating risk, the KPNC data were modeled for all combinations of cytology and HPV testing, using CIN 3+ for many of the outcomes, and when outcomes were rare, using CIN 2+. The theme of equal management for equal risk was the rationale behind the management approaches detailed in the TABLE. Risks were deemed to be low and return to normal screening was recommended when the risks were similar to the rate of CIN 3+ 3 years after negative cytology or 5 years after negative cotesting. However, immediate colposcopy was recommended when the 5-year risk of CIN 3+ for the combination of cytology and hrHPV testing, when indicated, exceeded 5%. A 6-month to 12-month return (intermediate risk) is indicated with a risk of CIN3+ of 2% to 5%.
An emphasis on avoiding harms
Abnormal findings at the time of cervical cancer screening can lead to a number of harms for the patient, including anxiety and emotional distress, particularly when colposcopy is necessary, as well as time lost from home and work life. For this reason, the guidelines panel emphasized that colposcopy and other interventions should be avoided when the risk of CIN 3+ is low and when the cervical screening abnormalities are likely to resolve without treatment.
However, women who experience postcoital bleeding, unexplained abnormal vaginal bleeding, pelvic pain, abnormal discharge, or a visible lesion should be managed promptly on an individualized basis.
Long-term effects of HPV vaccination are unknown
Among the areas that remain to be addressed are the unknown effects of widespread prophylactic HPV vaccination over the long term. We also lack full understanding of whether and how HPV vaccination will alter the incidence and management of cytologic and histologic abnormalities. Given the low rates of vaccination against HPV in the United States at present, this will need to be re-evaluated in the future.
What this EVIDENCE means for practice
The updated ASCCP guidelines are inherently complex, but their complexity arises from a large body of high-quality prospective data from a large population of women. Equal risk should result in equal management of cervical screening test abnormalities. Practitioners need not feel obligated to memorize the guidelines, owing to the availability of algorithms for specific findings in specific populations at the ASCCP Web site (www.asccp.org/consensus2012). Apps also are available for the iPhone, iPad, and Android.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: obg@frontlinemedcom.com Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
Advances in cervical cancer screening continue apace. We are fortunate that these advances are based on a substantial amount of high-quality prospective evidence. Many of these advances are designed to target the women who have clinically relevant disease while minimizing harm and anxiety caused by unnecessary procedures related to cervical screening test abnormalities that have little clinical relevance.
With clinicians being regularly judged on performance and outcomes, adoption of advances and new guidelines should be considered relatively quickly by women’s health providers.
In this article, I focus on two significant advances of the past (and coming) year:
- recent application and unanimous approval by a Food and Drug Administration (FDA) expert panel for the use of the cobas human papillomavirus (HPV) DNA test as a primary cervical cancer screen
- the latest update of guidelines on the management of abnormal cervical screening tests from the American Society for Colposcopy and Cervical Pathology (ASCCP).
cobas HPV TEST IS POISED FOR FDA APPROVAL AS A PRIMARY SCREEN FOR CERVICAL CANCER
Wright TC Jr, Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012;206(1):46.e1–e11.
An FDA expert panel unanimously approved the cobas (Roche Molecular Diagnostics; Pleasanton, California) HPV DNA test on March 12, 2014. The FDA will decide on potential approval within the coming months. Although the FDA sometimes reaches a different decision from one of its advisory committees when it comes to a final vote on a product or device, most often the FDA concurs with the committee’s judgment. Therefore, approval of the cobas HPV test as a primary screen is likely.
Related article: FDA Advisory Committee recommends HPV test as primary screening tool for cervical cancer Deborah Reale (News for your Practice, March 2014)
The cobas HPV test yields a pooled result for 12 high-risk HPV types (hrHPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68), as well as individual results for types 16 and 18; it also has an internal control for specimen adequacy. HPV 16 and 18 account for roughly 70% of all cases of cervical cancer, and infection with both types are known to place women at high risk for having clinically relevant disease—more so than the other hrHPV types.
COMMITTEE REVIEWED DATA FROM ATHENA IN VOTING FOR APPROVAL
In considering the cobas HPV test, the advisory committee reviewed data from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial, a prospective, multicenter, US-based study of 47,208 women aged 21 and older. These women were recruited at the time of undergoing routine screening for cervical cancer; only 2.6% had been vaccinated against HPV. All were screened by liquid-based cytology and an HPV test. Those who had abnormal cytology or a positive test for a high-risk HPV type underwent colposcopy, as did a randomly selected group of women aged 25 or older who tested negative on both tests.
The prevalence of abnormal findings was:
- 7.1% for liquid-based cytology
- 12.6% for pooled high-risk HPV
- 2.8% for HPV 16
- 1.0% for HPV 18.
As expected, cytologic abnormalities and infection with high-risk HPV types declined with increasing age. The adjusted prevalence of cervical intraepithelial neoplasia (CIN) grade 2 or higher in women aged 25 to 34 years was 2.3%; it declined to 1.5% among women older than age 34. Of note, approximately 500,000 US women are given a diagnosis of CIN 2 or CIN 3 each year in the United States.
WHY ATHENA IS IMPORTANT
This US-based trial was designed to assess the medical utility of pooled high-risk HPV DNA in addition to genotyping for HPV 16 and 18 in three populations:
- women aged 21 and older with a cytologic finding of atypical squamous cells of undetermined significance (ASC-US)
- women aged 30 and older with normal cytology
- women aged 25 and older in the overall screening population with any cytologic finding.
Investigators were particularly interested in the use of the HPV test as:
- a triage for women with abnormal cytologic findings
- an adjunct to guide clinical management of women with negative cytology results
- a potential front-line test in the screening of women aged 25 and older.
Related article: Endometrial cancer update: The move toward personalized cancer care Lindsay M. Kuroki, MD, and David G. Mutch, MD (October 2013)
The participants of the ATHENA trial were representative of women undergoing screening for cervical cancer in the United States—both in terms of demographics and in the distribution of cytologic findings. For example, recent US census data indicate that the female population is 79% white, 13% black, and 16% Hispanic or Latino—figures comparable to the breakdown of race/ethnicity in the ATHENA trial.
The trial was conducted in a baseline phase (published in 2012) and a 3-year follow-up phase (not yet published). The 3-year data were reviewed by the FDA advisory committee during its consideration of the cobas HPV test as a primary screen.
DESPITE PROBABLE APPROVAL, INCREMENTAL CHANGE IS LIKELY
Although a move to the HPV test as the primary screen is a definite paradigm shift for what has been cytology-based screening since the initiation of cervical cancer screening, the changeover from primary cytology to primary HPV testing likely will be slow. It will require education of clinicians as well as patients, and a shift in many internal procedures for pathology laboratories.
The ATHENA trial also leaves some intriguing questions unanswered:
- How do we transition women into the new screening strategy? Many women today still undergo cytology screening with reflex HPV testing, as appropriate, and an increasing number of women aged 30 and older undergo cotesting with both cytology and HPV testing. When should they begin screening in a primary HPV testing setting? And what screening intervals will be recommended? If a woman already has been screened with cytology, how should she transition into and at what interval should she begin primary HPV screening?
- How should we manage women’s care after the first round of primary HPV testing? The ATHENA trial so far only has outcomes data after one round of HPV testing. While some data are available from Europe, we do not know what happens after two or three rounds of screening with primary HPV testing in a large US-based cohort. We clearly will be identifying and treating many women with preinvasive disease from screening after one round of testing, at a rate likely higher than with cytology alone—a good thing. We also likely will be reducing the number of unnecessary colposcopies for cytology that are not related to hrHPV.
What this EVIDENCE means for practice
Screening women using the cobas HPV test as a primary screen will require considerable education of providers and patients to explain how this change will affect how a woman will be managed after being screened for cervical cancer. Though much remains to be determined about this new cervical cancer screening paradigm (eg, logistics, timing, use of secondary tests), it should reduce the number of screening tests and colposcopies necessary to detect clinically relevant disease.
UPDATED ASCCP GUIDELINES EMPHASIZE EQUAL MANAGEMENT FOR EQUAL RISK
Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27.
In formulating this latest set of guidelines for the management of abnormal cervical cancer screening tests and cancer precursors, the ASCCP led a conference consisting of scientific stakeholders to perform a comprehensive review of the literature. Also, with study investigators at Kaiser Permanente Northern California (KPNC) and the National Cancer Institute, the guidelines panel also modeled and assessed data on risk after abnormal tests from almost 1.4 million women followed over 8 years in the KPNC Medical Care Plan—this cohort has provided us with “big data.”
The sheer size of the Kaiser Permanente population made it possible for the ASCCP-led panel to validate its previous guidelines or to modify them, where needed. It also made risk-based stratification possible for even rare abnormalities and clinical outcomes.
Although findings from the KPNC population may not be fully generalizable to the US population as a whole, they enhance our understanding of the optimal management of abnormal cervical cancer screening tests and cancer precursors. More widely dispersed study cohorts on a similar scale in the United States are unlikely in the near future.
Related article: Update on cervical disease Mark H. Einstein, MD, MS, and J. Thomas Cox, MD (May 2013)
SEVERAL SIGNIFICANT MODIFICATIONS
Although the ASCCP reaffirmed most elements of its 2006 consensus management guidelines, it did make a number of changes:
- Women who have ASC-US cytology but test HPV-negative now should be followed with cotesting at 3 years rather than 5 years before they return to routine screening.
- Women near age 65 who have a negative finding on ASC-US cytology and HPV testing should not exit screening.
- Women who have ASC-US cytology and test HPV-positive should go to immediate colposcopy, regardless of hrHPV results, including genotyping.
- Women who test positive for HPV 16 or 18 but have negative cytology should undergo immediate colposcopy.
- Women aged 21 to 24 years should be managed as conservatively and minimally invasively as possible, especially when an abnormality is minor.
- Endocervical curettage reported as CIN 1 should be managed as CIN 1, not as a positive endocervical curettage.
- When a cytologic sample is unsatisfactory, sampling usually should be repeated, even when HPV cotesting results are known. However, negative cytology that lacks sufficient endocervical cells or a transformation zone component usually can be managed without frequent follow-up.
Related article: New cervical Ca screening guidelines recommend less frequent assessment Janelle Yates (News for your Practice; April 2012)
EQUAL MANAGEMENT SHOULD BE PERFORMED FOR ABNORMAL TESTS THAT INDICATE EQUAL RISK
The ASCCP-led management panel unanimously agreed to several basic assumptions in formulating the updated guidelines. For example, they concurred that achieving zero risk for cancer is impossible and that attempts to achieve zero risk (which typically means more frequent testing) may cause harm. They also cited the 2011 American Cancer Society/ASCCP/American Society for Clinical Pathology consensus screening document, which stated: “Optimal prevention strategies should identify those HPV-related abnormalities likely to progress to invasive cancers while avoiding destructive treatment of abnormalities not destined to become cancerous.”1
The panel also agreed that CIN 3+ is a “reasonable proxy for cancer risk.” When calculating risk, the KPNC data were modeled for all combinations of cytology and HPV testing, using CIN 3+ for many of the outcomes, and when outcomes were rare, using CIN 2+. The theme of equal management for equal risk was the rationale behind the management approaches detailed in the TABLE. Risks were deemed to be low and return to normal screening was recommended when the risks were similar to the rate of CIN 3+ 3 years after negative cytology or 5 years after negative cotesting. However, immediate colposcopy was recommended when the 5-year risk of CIN 3+ for the combination of cytology and hrHPV testing, when indicated, exceeded 5%. A 6-month to 12-month return (intermediate risk) is indicated with a risk of CIN3+ of 2% to 5%.
An emphasis on avoiding harms
Abnormal findings at the time of cervical cancer screening can lead to a number of harms for the patient, including anxiety and emotional distress, particularly when colposcopy is necessary, as well as time lost from home and work life. For this reason, the guidelines panel emphasized that colposcopy and other interventions should be avoided when the risk of CIN 3+ is low and when the cervical screening abnormalities are likely to resolve without treatment.
However, women who experience postcoital bleeding, unexplained abnormal vaginal bleeding, pelvic pain, abnormal discharge, or a visible lesion should be managed promptly on an individualized basis.
Long-term effects of HPV vaccination are unknown
Among the areas that remain to be addressed are the unknown effects of widespread prophylactic HPV vaccination over the long term. We also lack full understanding of whether and how HPV vaccination will alter the incidence and management of cytologic and histologic abnormalities. Given the low rates of vaccination against HPV in the United States at present, this will need to be re-evaluated in the future.
What this EVIDENCE means for practice
The updated ASCCP guidelines are inherently complex, but their complexity arises from a large body of high-quality prospective data from a large population of women. Equal risk should result in equal management of cervical screening test abnormalities. Practitioners need not feel obligated to memorize the guidelines, owing to the availability of algorithms for specific findings in specific populations at the ASCCP Web site (www.asccp.org/consensus2012). Apps also are available for the iPhone, iPad, and Android.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: obg@frontlinemedcom.com Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
Reference
- Saslow D, Solomon D, Lawson HW, et al;ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
Reference
- Saslow D, Solomon D, Lawson HW, et al;ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
Dr. Mark Einstein anticipated final FDA approval of the first HPV test for primary cervical cancer screening and, in this UPDATE ON CERVICAL DISEASE, expands on the data behind the approval and how your practice could change
UPDATE ON CERVICAL DISEASE
To access 10 recent articles and audiocasts from OBG Management on cervical disease, click here.
Dr. Einstein reports that Montefiore Medical Center has received payment from Roche and Hologic for time he spent as an advisor or educational speaker. In some cases, his travel has been paid for when required for meetings. In addition, Dr. Einstein reports that Montefiore has received grant funding from Roche, Hologic, and Becton-Dickinson for research-related costs of clinical trials that he has been the overall or Montefiore principal investigator.
Dr. Cox reports that he is a consultant to OncoHealth; a member of the Scientific Advisory Boards for Roche and Hologic; a speaker for Roche; and on the Data and Safety Monitoring Board for HPV vaccines for Merck.
Cervical cancer screening is necessarily complex, and guidelines must change fairly frequently as our understanding of the natural history of HPV infection and cervical cancer continues to evolve. Up-to-date guidelines enhance our ability to detect cervical intraepithelial neoplasia and cancer early and manage them appropriately.
In April 2013, the American Society for Colposcopy and Cervical Pathology (ASCCP) updated guidelines for the management of abnormal cervical cytology and cervical cancer precursors for the first time since 2006.1 This update follows new cervical cancer screening guidelines published in 2012 by the ACS/ASCCP/ASCP,2 the USPSTF,3 and the American College of Obstetricians and Gynecologists4 (and reported in OBG Management in June 20125).
For many clinicians, all these modifications amount to a dizzying “sea change” in the way they have been screening and managing patients to prevent cervical cancer. Clinicians often express frustration with the guidelines, both for their complexity and for what seems like all-too-frequent changes. Do they really need to change … again? Do they really need to get even more complex? And what about them is really new?
This article addresses these questions by reviewing the guidelines and their updates in more depth. For a specific answer to the question of “What’s new?” see sidebar below.
The following features of the 2013 ASCCP update to cervical cancer screening guidelines are new:
- The return to “routine” screening is now better defined
- The management of women who have “unsatisfactory” cytology or a specimen lacking endocervical or transformation-zone components now includes the results of HPV testing
- Management guidelines previously used for adolescents (<21 years) now apply to young adult women (<25 years)
- There is now advice on the management of women aged 30 and older who have discordant cotest results, including HPV-positive/cytology-negative findings and HPV-negative/cytology-positive findings of ASC-US or more severe.
Did the guidelines really need to change … again?
Cervical cancer screening tests—be they the Pap test or a human papillomavirus (HPV) test—are not as clear-cut as other tests used to screen for sexually transmitted infections or their effects. We treat a patient whenever her gonorrhea or Chlamydia test is positive, for example. However, other than cytology classified as high-grade (ie, HSIL), which may prompt immediate treatment in women 25 years and older by “see-and-treat” loop electrosurgical excision procedure (LEEP), neither cervical cytology nor HPV testing is sufficiently specific for present disease (cervical intraepithelial neoplasia [CIN] 3 or cancer) to warrant treatment without a diagnostic work-up. That’s because the cause of cervical cancer (infection with HPV) usually does not produce CIN 3 or cancer, and the cell changes that it does produce most often (atypia and koilocytosis) are very common. And other cervical-vaginal changes associated with hormonal fluctuations, tampons, intercourse, and so on, may result in cervical cytologic changes unrelated to HPV and, therefore, do not represent a risk for cervical cancer.
How can we best sort out who needs to be evaluated without under- or overdoing it? When we find CIN, some of which is destined to progress and some not, how do we reduce the risk of overtreatment without increasing the likelihood that some will progress to cancer? If we have treated CIN or adenocarcinoma in situ (AIS), how do we make sure there is no recurrence without risking over-management and potential overtreatment?
The first thing we do is ensure that we use our best clinical judgment and also respect the informed wishes of the patient. Because the guidelines are based on the best available data, and on expert opinion when data are lacking, guidelines developed through a consensus process provide a framework for care that is optimal for most women at each phase of their lives. This knowledge can help the clinician—and often the patient—make the best-informed decisions.
Because only high-risk HPV types cause cervical cancer, testing should be restricted to high-risk (oncogenic) HPV types. Do not test for low-risk HPV types.
The guidelines are intended for use only with HPV tests that have been analytically and clinically validated, as documented by US Food and Drug Administration licensing and approval or by publication in peer-reviewed scientific literature. This distinction is important because management based on results of HPV tests that have not been similarly validated may not result in outcomes intended by these guidelines and may increase the potential for patient harm.
Do guidelines really need to get even more complex?
Consider the myriad management decisions that confront us in the field of cervical cancer screening, and the potential result of each choice. Even when cervical screening involves cytology alone, there are five major categories for abnormal results, each associated with a different level of risk requiring a unique level of management:
- atypical squamous cells – undetermined significance (ASC-US)
- atypical squamous cells – cannot rule out a high-grade lesion (ASC-H)
- atypical glandular cells (AGC)
- low-grade squamous intraepithelial lesion (LSIL)
- high-grade squamous intraepithelial lesion (HSIL).
Add in HPV testing with cervical cytology for women 30 years and older, and there is one more abnormal category—normal Pap/ HPV-positive. And these categories just cover initial management. Also needed are guidelines for appropriate follow-up of women who undergo colposcopy for each abnormal cytologic result when no CIN 2, CIN 3, or AIS is found that requires treatment, as well as guidelines for managing women following treatment when high-grade histology is found.
As our understanding of the natural history of HPV and cervical oncogenesis has increased, it has become clearer that we must further adjust management decisions on the basis of age, essentially creating many parallel sets of guidelines for women aged 21 to 24, 25 to 29, and 30 years and older.
Yes, cervical screening and management are complex. We are fortunate that the Internet and new “apps” for smartphones give us easy access to guidelines for most of the potential combinations of clinical findings and results. The guideline algorithms are available at www.asccp.org, and full explanatory articles are available at www.jlgtd.com and www.greenjournal.org (comprehensive apps are available for download for almost every smartphone device).
Remember, it is impossible to create guidelines for every possible clinical situation, so clinical judgment must always be paramount when applying guidelines to individual patients.1
What are the major changes of the latest set of guidelines and its update?
Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121(4):829–846. [Also published in J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27.]
Let’s start by focusing on how the experts crafted the 2012 guidelines. New evidence to guide decisions about the management of abnormal screening tests, CIN, and AIS emerged in 2012 from a review of the world literature and from analyses of a large 7-year clinical database (1.4 million women) at the Kaiser Permanente Northern California Medical Care Plan, conducted in collaboration with scientists from the National Cancer Institute.1
Most of the 2006 guidelines remain valid, but new evidence has modified some of the guidelines and created others where gaps existed. Guideline developers recognized that cervical cancer prevention is a process that entails both benefits and potential harms, and that the potential risks cannot be reduced to zero with the strategies currently available. Attempts to achieve zero risk could result in unbalanced harms, including overtreatment.
- Anxiety from an abnormal test that the patient might fear to be a sign of cancer
- Stigma from diagnosis of a ubiquitous sexually transmitted infection (HPV)
- Time and patient expense related to screening and management
- Pain and injury from the procedures and treatment
- Increased risk of premature delivery and pregnancy loss.
Defining acceptable risk levels
Applying the concept of “similar management for similar risks,” guideline developers benchmarked risks to the risks associated with accepted screening and management strategies. Because the 5-year risk for CIN 3+ for a woman with an LSIL Pap finding is about 5.2%, and the recommendation for LSIL is colposcopy, 5.2% was set as the lower limit of the level of risk that provides enough benefit (detection of CIN 3+) to balance the potential harms of colposcopy.1 (See the box on harms above.)
When women return to prolonged screening as follow-up to abnormal cytology or a positive HPV test, acceptable risk was considered to be that approximating the risk for CIN3+ three years after negative cytology or 5 years after negative cotesting—as these risks were considered acceptable to guide recent primary cervical screening guidelines.2-4
To be as precise as possible, experts stratified the guidelines by risk, according to the woman’s age, cytologic diagnosis, and HPV status, including HPV genotyping for types 16 and 18, when tested. Of course, guidelines for management apply only to women who are found to have abnormalities during routine screening.1 Women who experience postcoital or unexplained abnormal vaginal bleeding, pelvic pain, abnormal discharge, or a visible lesion need individualized evaluations.1
Only changes or additions to the guidelines are listed here, so be sure to read the published guidelines and supplemental articles and/or visit the Web sites listed earlier for a review of all the guidelines.
What’s new in managing women with unsatisfactory Pap results?
In general, cytology should be repeated in 2 to 4 months.
If the unsatisfactory Pap test is part of a cotest, then the following strategies are appropriate:
- If the HPV test is positive, either repeating the Pap test or moving directly to colposcopy is acceptable
- If HPV genotyping was reported and is positive for type 16 or 18, colposcopy is indicated.
Colposcopy also is recommended when two consecutive Pap tests are unsatisfactory.
What’s new in managing women with normal cytology but no, or insufficient, endocervical cells/transformation-zone component?
The answer varies by age:
- For women 21 to 29 years – routine screening with cytology in 3 years is recommended
- For women 30 years and older:
- When cotesting is done, the HPV result guides management:
- HPV-negative: routine screening with cotesting in 5 years is preferred
- HPV-positive: either cotesting in 1 year or immediate genotyping is recommended
- If HPV testing was not done, then HPV testing is recommended, with management guided by results.
- When cotesting is done, the HPV result guides management:
What’s new in managing women aged 21 to 24 with abnormal cervical cytology or CIN?
Young women of this age are at high risk for HPV infection but very low risk for cancer. Aggressive management usually involves more harm than benefit, promoting observation. Adolescents are no longer screened; management previously reserved for adolescents is now appropriate for women aged 21 to 24 years.
If the Pap result is:
- ASC-US or LSIL:
- No colposcopy is needed. The Pap test should be repeated annually for 2 years, with colposcopy after 1 year only when the finding is HSIL and after 2 years if ASC-US or LSIL findings persist
- HPV triage for ASC-US is not recommended, but if it is done:
- HPV-negative women should continue routine screening with a Pap test in 3 years
- HPV-positive women should have annual cytology for 2 years, with colposcopy after 1 year only if the result is HSIL and after 2 years if ASC-US or LSIL findings persist.
- ASC-H or HSIL:
- Colposcopy is recommended, but immediate treatment (see-and-treat LEEP) is unacceptable
- Women with no CIN 2 or CIN 3 at colposcopy should be followed with colposcopy and cytology every 6 months for as long as 2 years, until two consecutive Pap tests are negative and no high-grade colposcopic abnormality is observed
- Repeat biopsies are indicated if cytology at 1 year is again ASC-H or HSIL
- Diagnostic excision is recommended if HSIL cytology persists for 2 years.
Changes in the management of histologic findings
If CIN 1 is detected, management depends on the antecedent cytology report:
- If the prior Pap finding was ASC-US or LSIL, observation with annual cytology is recommended
- If the prior Pap finding was ASC-H or HSIL, observation for as long as 24 months is recommended, using both colposcopy and cytology at 6-month intervals, provided the colposcopic examination is adequate and endocervical assessment is negative.
If CIN 2 is detected, observation is preferred but treatment is acceptable (see the guidelines for detailed recommendations).
If CIN 2/CIN 3 (not otherwise differentiated) is detected, either observation or treatment is acceptable (see the guidelines for detailed recommendations).
If CIN 3 is detected in a woman of any age, treatment is indicated.
What’s new in managing women 30 years and older who have discordant cotest results?
Use cotesting management recommendations only for women 30 years and older.
If the finding is:
- HPV-positive/Pap-negative (HPV+/ Pap-), the two options are:
- Repeat cotesting in 1 year, with colposcopy if the finding is again HPV+ or the Pap is ASC-US or more severe (including HPV-/ASC-US), and repeat cotesting in 3 years if results for both the HPV test and the Pap are negative (HPV-/Pap-)
- Genotyping, with colposcopy if HPV 16 or 18 is identified and repeat cotesting in 1 year if both HPV 16 and 18 are negative
- HPV-/ASC-US:
- Repeat the cotest in 3 years
- HPV-/LSIL, the options are:
- Cotesting in 1 year (preferred)
- Colposcopy (acceptable)
- HPV+/LSIL or LSIL/no HPV result:
- Colposcopy
- HPV-/HSIL or HPV-/ASC-H:
- Colposcopy
- HPV-/AGC
- Colposcopy, often with endometrial sampling.
New terminology unifies all lower genital tract HPV intraepithelial neoplasia
Darragh TM, Colgan TJ, Cox JT, et al; LAST Project Work Groups. The Lower Anogenital Squamous Terminology Standardization Project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis. 2012;16(3):205–242.
In 2012, the Lower Anogenital Squamous Terminology (LAST) standardization project created new histology terminology for HPV-related lesions of the lower genital tract. The LSIL finding was designated as the all-encompassing term for CIN 1, vaginal intraepithelial neoplasia 1 (VaIN 1), vulvar intraepithelial neoplasia 1 (VIN 1), penile intraepithelial neoplasia 1 (PeIN 1), perianal intraepithelial neoplasia 1 (PAIN 1) and anal intraepithelial neoplasia 1 (AIN 1). Intraepithelial neoplasia (IN) graded 2, 2/3, and 3 from each of these areas is designated HSIL.5
When CIN 2 and CIN 3 can be differentiated, these designations can be reported along with the HSIL diagnosis. However, after thoughtful deliberation, the delegates to the ASCCP consensus conference decided that there is not yet enough outcome data available to determine different management strategies when using the new LAST histopathology terminology. They recommended that, until evidence is available, results reported as histologic (not cytologic) LSIL should be managed as CIN 1, and histologic (not cytologic) HSIL should be managed as CIN 2/CIN 3.
Guidelines for the management of abnormal cervical cytology, CIN, and AIS are necessarily complicated, but they provide the best basis for evidence-based management of these medical challenges. The Web provides easy access to all of the ASCCP guidelines via www.asccp.org, www.jlgtd.com, and www.greenjournal.org.
We want to hear from you! Tell us what you think.
1. Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121(4):829-846.(Also published in J Low Genit Tract Dis. 2013;17[5 Suppl 1]:S1–S27.)
2. Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516-542.
3. Moyer VA. US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880-891, W312.
4. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 131: Screening for cervical cancer. Obstet Gynecol. 2012;120(5):1222-1238.
5. Yates J. New cervical Ca screening guidelines recommend less frequent assessment. OBG Manage. 2012;24(6):40-44.
To access 10 recent articles and audiocasts from OBG Management on cervical disease, click here.
Dr. Einstein reports that Montefiore Medical Center has received payment from Roche and Hologic for time he spent as an advisor or educational speaker. In some cases, his travel has been paid for when required for meetings. In addition, Dr. Einstein reports that Montefiore has received grant funding from Roche, Hologic, and Becton-Dickinson for research-related costs of clinical trials that he has been the overall or Montefiore principal investigator.
Dr. Cox reports that he is a consultant to OncoHealth; a member of the Scientific Advisory Boards for Roche and Hologic; a speaker for Roche; and on the Data and Safety Monitoring Board for HPV vaccines for Merck.

Cervical cancer screening is necessarily complex, and guidelines must change fairly frequently as our understanding of the natural history of HPV infection and cervical cancer continues to evolve. Up-to-date guidelines enhance our ability to detect cervical intraepithelial neoplasia and cancer early and manage them appropriately.
In April 2013, the American Society for Colposcopy and Cervical Pathology (ASCCP) updated guidelines for the management of abnormal cervical cytology and cervical cancer precursors for the first time since 2006.1 This update follows new cervical cancer screening guidelines published in 2012 by the ACS/ASCCP/ASCP,2 the USPSTF,3 and the American College of Obstetricians and Gynecologists4 (and reported in OBG Management in June 20125).
For many clinicians, all these modifications amount to a dizzying “sea change” in the way they have been screening and managing patients to prevent cervical cancer. Clinicians often express frustration with the guidelines, both for their complexity and for what seems like all-too-frequent changes. Do they really need to change … again? Do they really need to get even more complex? And what about them is really new?
This article addresses these questions by reviewing the guidelines and their updates in more depth. For a specific answer to the question of “What’s new?” see sidebar below.
The following features of the 2013 ASCCP update to cervical cancer screening guidelines are new:
- The return to “routine” screening is now better defined
- The management of women who have “unsatisfactory” cytology or a specimen lacking endocervical or transformation-zone components now includes the results of HPV testing
- Management guidelines previously used for adolescents (<21 years) now apply to young adult women (<25 years)
- There is now advice on the management of women aged 30 and older who have discordant cotest results, including HPV-positive/cytology-negative findings and HPV-negative/cytology-positive findings of ASC-US or more severe.
Did the guidelines really need to change … again?
Cervical cancer screening tests—be they the Pap test or a human papillomavirus (HPV) test—are not as clear-cut as other tests used to screen for sexually transmitted infections or their effects. We treat a patient whenever her gonorrhea or Chlamydia test is positive, for example. However, other than cytology classified as high-grade (ie, HSIL), which may prompt immediate treatment in women 25 years and older by “see-and-treat” loop electrosurgical excision procedure (LEEP), neither cervical cytology nor HPV testing is sufficiently specific for present disease (cervical intraepithelial neoplasia [CIN] 3 or cancer) to warrant treatment without a diagnostic work-up. That’s because the cause of cervical cancer (infection with HPV) usually does not produce CIN 3 or cancer, and the cell changes that it does produce most often (atypia and koilocytosis) are very common. And other cervical-vaginal changes associated with hormonal fluctuations, tampons, intercourse, and so on, may result in cervical cytologic changes unrelated to HPV and, therefore, do not represent a risk for cervical cancer.
How can we best sort out who needs to be evaluated without under- or overdoing it? When we find CIN, some of which is destined to progress and some not, how do we reduce the risk of overtreatment without increasing the likelihood that some will progress to cancer? If we have treated CIN or adenocarcinoma in situ (AIS), how do we make sure there is no recurrence without risking over-management and potential overtreatment?
The first thing we do is ensure that we use our best clinical judgment and also respect the informed wishes of the patient. Because the guidelines are based on the best available data, and on expert opinion when data are lacking, guidelines developed through a consensus process provide a framework for care that is optimal for most women at each phase of their lives. This knowledge can help the clinician—and often the patient—make the best-informed decisions.
Because only high-risk HPV types cause cervical cancer, testing should be restricted to high-risk (oncogenic) HPV types. Do not test for low-risk HPV types.
The guidelines are intended for use only with HPV tests that have been analytically and clinically validated, as documented by US Food and Drug Administration licensing and approval or by publication in peer-reviewed scientific literature. This distinction is important because management based on results of HPV tests that have not been similarly validated may not result in outcomes intended by these guidelines and may increase the potential for patient harm.
Do guidelines really need to get even more complex?
Consider the myriad management decisions that confront us in the field of cervical cancer screening, and the potential result of each choice. Even when cervical screening involves cytology alone, there are five major categories for abnormal results, each associated with a different level of risk requiring a unique level of management:
- atypical squamous cells – undetermined significance (ASC-US)
- atypical squamous cells – cannot rule out a high-grade lesion (ASC-H)
- atypical glandular cells (AGC)
- low-grade squamous intraepithelial lesion (LSIL)
- high-grade squamous intraepithelial lesion (HSIL).
Add in HPV testing with cervical cytology for women 30 years and older, and there is one more abnormal category—normal Pap/ HPV-positive. And these categories just cover initial management. Also needed are guidelines for appropriate follow-up of women who undergo colposcopy for each abnormal cytologic result when no CIN 2, CIN 3, or AIS is found that requires treatment, as well as guidelines for managing women following treatment when high-grade histology is found.
As our understanding of the natural history of HPV and cervical oncogenesis has increased, it has become clearer that we must further adjust management decisions on the basis of age, essentially creating many parallel sets of guidelines for women aged 21 to 24, 25 to 29, and 30 years and older.
Yes, cervical screening and management are complex. We are fortunate that the Internet and new “apps” for smartphones give us easy access to guidelines for most of the potential combinations of clinical findings and results. The guideline algorithms are available at www.asccp.org, and full explanatory articles are available at www.jlgtd.com and www.greenjournal.org (comprehensive apps are available for download for almost every smartphone device).
Remember, it is impossible to create guidelines for every possible clinical situation, so clinical judgment must always be paramount when applying guidelines to individual patients.1
What are the major changes of the latest set of guidelines and its update?
Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121(4):829–846. [Also published in J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27.]
Let’s start by focusing on how the experts crafted the 2012 guidelines. New evidence to guide decisions about the management of abnormal screening tests, CIN, and AIS emerged in 2012 from a review of the world literature and from analyses of a large 7-year clinical database (1.4 million women) at the Kaiser Permanente Northern California Medical Care Plan, conducted in collaboration with scientists from the National Cancer Institute.1
Most of the 2006 guidelines remain valid, but new evidence has modified some of the guidelines and created others where gaps existed. Guideline developers recognized that cervical cancer prevention is a process that entails both benefits and potential harms, and that the potential risks cannot be reduced to zero with the strategies currently available. Attempts to achieve zero risk could result in unbalanced harms, including overtreatment.
- Anxiety from an abnormal test that the patient might fear to be a sign of cancer
- Stigma from diagnosis of a ubiquitous sexually transmitted infection (HPV)
- Time and patient expense related to screening and management
- Pain and injury from the procedures and treatment
- Increased risk of premature delivery and pregnancy loss.
Defining acceptable risk levels
Applying the concept of “similar management for similar risks,” guideline developers benchmarked risks to the risks associated with accepted screening and management strategies. Because the 5-year risk for CIN 3+ for a woman with an LSIL Pap finding is about 5.2%, and the recommendation for LSIL is colposcopy, 5.2% was set as the lower limit of the level of risk that provides enough benefit (detection of CIN 3+) to balance the potential harms of colposcopy.1 (See the box on harms above.)
When women return to prolonged screening as follow-up to abnormal cytology or a positive HPV test, acceptable risk was considered to be that approximating the risk for CIN3+ three years after negative cytology or 5 years after negative cotesting—as these risks were considered acceptable to guide recent primary cervical screening guidelines.2-4
To be as precise as possible, experts stratified the guidelines by risk, according to the woman’s age, cytologic diagnosis, and HPV status, including HPV genotyping for types 16 and 18, when tested. Of course, guidelines for management apply only to women who are found to have abnormalities during routine screening.1 Women who experience postcoital or unexplained abnormal vaginal bleeding, pelvic pain, abnormal discharge, or a visible lesion need individualized evaluations.1
Only changes or additions to the guidelines are listed here, so be sure to read the published guidelines and supplemental articles and/or visit the Web sites listed earlier for a review of all the guidelines.
What’s new in managing women with unsatisfactory Pap results?
In general, cytology should be repeated in 2 to 4 months.
If the unsatisfactory Pap test is part of a cotest, then the following strategies are appropriate:
- If the HPV test is positive, either repeating the Pap test or moving directly to colposcopy is acceptable
- If HPV genotyping was reported and is positive for type 16 or 18, colposcopy is indicated.
Colposcopy also is recommended when two consecutive Pap tests are unsatisfactory.
What’s new in managing women with normal cytology but no, or insufficient, endocervical cells/transformation-zone component?
The answer varies by age:
- For women 21 to 29 years – routine screening with cytology in 3 years is recommended
- For women 30 years and older:
- When cotesting is done, the HPV result guides management:
- HPV-negative: routine screening with cotesting in 5 years is preferred
- HPV-positive: either cotesting in 1 year or immediate genotyping is recommended
- If HPV testing was not done, then HPV testing is recommended, with management guided by results.
- When cotesting is done, the HPV result guides management:
What’s new in managing women aged 21 to 24 with abnormal cervical cytology or CIN?
Young women of this age are at high risk for HPV infection but very low risk for cancer. Aggressive management usually involves more harm than benefit, promoting observation. Adolescents are no longer screened; management previously reserved for adolescents is now appropriate for women aged 21 to 24 years.
If the Pap result is:
- ASC-US or LSIL:
- No colposcopy is needed. The Pap test should be repeated annually for 2 years, with colposcopy after 1 year only when the finding is HSIL and after 2 years if ASC-US or LSIL findings persist
- HPV triage for ASC-US is not recommended, but if it is done:
- HPV-negative women should continue routine screening with a Pap test in 3 years
- HPV-positive women should have annual cytology for 2 years, with colposcopy after 1 year only if the result is HSIL and after 2 years if ASC-US or LSIL findings persist.
- ASC-H or HSIL:
- Colposcopy is recommended, but immediate treatment (see-and-treat LEEP) is unacceptable
- Women with no CIN 2 or CIN 3 at colposcopy should be followed with colposcopy and cytology every 6 months for as long as 2 years, until two consecutive Pap tests are negative and no high-grade colposcopic abnormality is observed
- Repeat biopsies are indicated if cytology at 1 year is again ASC-H or HSIL
- Diagnostic excision is recommended if HSIL cytology persists for 2 years.
Changes in the management of histologic findings
If CIN 1 is detected, management depends on the antecedent cytology report:
- If the prior Pap finding was ASC-US or LSIL, observation with annual cytology is recommended
- If the prior Pap finding was ASC-H or HSIL, observation for as long as 24 months is recommended, using both colposcopy and cytology at 6-month intervals, provided the colposcopic examination is adequate and endocervical assessment is negative.
If CIN 2 is detected, observation is preferred but treatment is acceptable (see the guidelines for detailed recommendations).
If CIN 2/CIN 3 (not otherwise differentiated) is detected, either observation or treatment is acceptable (see the guidelines for detailed recommendations).
If CIN 3 is detected in a woman of any age, treatment is indicated.
What’s new in managing women 30 years and older who have discordant cotest results?
Use cotesting management recommendations only for women 30 years and older.
If the finding is:
- HPV-positive/Pap-negative (HPV+/ Pap-), the two options are:
- Repeat cotesting in 1 year, with colposcopy if the finding is again HPV+ or the Pap is ASC-US or more severe (including HPV-/ASC-US), and repeat cotesting in 3 years if results for both the HPV test and the Pap are negative (HPV-/Pap-)
- Genotyping, with colposcopy if HPV 16 or 18 is identified and repeat cotesting in 1 year if both HPV 16 and 18 are negative
- HPV-/ASC-US:
- Repeat the cotest in 3 years
- HPV-/LSIL, the options are:
- Cotesting in 1 year (preferred)
- Colposcopy (acceptable)
- HPV+/LSIL or LSIL/no HPV result:
- Colposcopy
- HPV-/HSIL or HPV-/ASC-H:
- Colposcopy
- HPV-/AGC
- Colposcopy, often with endometrial sampling.
New terminology unifies all lower genital tract HPV intraepithelial neoplasia
Darragh TM, Colgan TJ, Cox JT, et al; LAST Project Work Groups. The Lower Anogenital Squamous Terminology Standardization Project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis. 2012;16(3):205–242.
In 2012, the Lower Anogenital Squamous Terminology (LAST) standardization project created new histology terminology for HPV-related lesions of the lower genital tract. The LSIL finding was designated as the all-encompassing term for CIN 1, vaginal intraepithelial neoplasia 1 (VaIN 1), vulvar intraepithelial neoplasia 1 (VIN 1), penile intraepithelial neoplasia 1 (PeIN 1), perianal intraepithelial neoplasia 1 (PAIN 1) and anal intraepithelial neoplasia 1 (AIN 1). Intraepithelial neoplasia (IN) graded 2, 2/3, and 3 from each of these areas is designated HSIL.5
When CIN 2 and CIN 3 can be differentiated, these designations can be reported along with the HSIL diagnosis. However, after thoughtful deliberation, the delegates to the ASCCP consensus conference decided that there is not yet enough outcome data available to determine different management strategies when using the new LAST histopathology terminology. They recommended that, until evidence is available, results reported as histologic (not cytologic) LSIL should be managed as CIN 1, and histologic (not cytologic) HSIL should be managed as CIN 2/CIN 3.
Guidelines for the management of abnormal cervical cytology, CIN, and AIS are necessarily complicated, but they provide the best basis for evidence-based management of these medical challenges. The Web provides easy access to all of the ASCCP guidelines via www.asccp.org, www.jlgtd.com, and www.greenjournal.org.
We want to hear from you! Tell us what you think.
To access 10 recent articles and audiocasts from OBG Management on cervical disease, click here.
Dr. Einstein reports that Montefiore Medical Center has received payment from Roche and Hologic for time he spent as an advisor or educational speaker. In some cases, his travel has been paid for when required for meetings. In addition, Dr. Einstein reports that Montefiore has received grant funding from Roche, Hologic, and Becton-Dickinson for research-related costs of clinical trials that he has been the overall or Montefiore principal investigator.
Dr. Cox reports that he is a consultant to OncoHealth; a member of the Scientific Advisory Boards for Roche and Hologic; a speaker for Roche; and on the Data and Safety Monitoring Board for HPV vaccines for Merck.

Cervical cancer screening is necessarily complex, and guidelines must change fairly frequently as our understanding of the natural history of HPV infection and cervical cancer continues to evolve. Up-to-date guidelines enhance our ability to detect cervical intraepithelial neoplasia and cancer early and manage them appropriately.
In April 2013, the American Society for Colposcopy and Cervical Pathology (ASCCP) updated guidelines for the management of abnormal cervical cytology and cervical cancer precursors for the first time since 2006.1 This update follows new cervical cancer screening guidelines published in 2012 by the ACS/ASCCP/ASCP,2 the USPSTF,3 and the American College of Obstetricians and Gynecologists4 (and reported in OBG Management in June 20125).
For many clinicians, all these modifications amount to a dizzying “sea change” in the way they have been screening and managing patients to prevent cervical cancer. Clinicians often express frustration with the guidelines, both for their complexity and for what seems like all-too-frequent changes. Do they really need to change … again? Do they really need to get even more complex? And what about them is really new?
This article addresses these questions by reviewing the guidelines and their updates in more depth. For a specific answer to the question of “What’s new?” see sidebar below.
The following features of the 2013 ASCCP update to cervical cancer screening guidelines are new:
- The return to “routine” screening is now better defined
- The management of women who have “unsatisfactory” cytology or a specimen lacking endocervical or transformation-zone components now includes the results of HPV testing
- Management guidelines previously used for adolescents (<21 years) now apply to young adult women (<25 years)
- There is now advice on the management of women aged 30 and older who have discordant cotest results, including HPV-positive/cytology-negative findings and HPV-negative/cytology-positive findings of ASC-US or more severe.
Did the guidelines really need to change … again?
Cervical cancer screening tests—be they the Pap test or a human papillomavirus (HPV) test—are not as clear-cut as other tests used to screen for sexually transmitted infections or their effects. We treat a patient whenever her gonorrhea or Chlamydia test is positive, for example. However, other than cytology classified as high-grade (ie, HSIL), which may prompt immediate treatment in women 25 years and older by “see-and-treat” loop electrosurgical excision procedure (LEEP), neither cervical cytology nor HPV testing is sufficiently specific for present disease (cervical intraepithelial neoplasia [CIN] 3 or cancer) to warrant treatment without a diagnostic work-up. That’s because the cause of cervical cancer (infection with HPV) usually does not produce CIN 3 or cancer, and the cell changes that it does produce most often (atypia and koilocytosis) are very common. And other cervical-vaginal changes associated with hormonal fluctuations, tampons, intercourse, and so on, may result in cervical cytologic changes unrelated to HPV and, therefore, do not represent a risk for cervical cancer.
How can we best sort out who needs to be evaluated without under- or overdoing it? When we find CIN, some of which is destined to progress and some not, how do we reduce the risk of overtreatment without increasing the likelihood that some will progress to cancer? If we have treated CIN or adenocarcinoma in situ (AIS), how do we make sure there is no recurrence without risking over-management and potential overtreatment?
The first thing we do is ensure that we use our best clinical judgment and also respect the informed wishes of the patient. Because the guidelines are based on the best available data, and on expert opinion when data are lacking, guidelines developed through a consensus process provide a framework for care that is optimal for most women at each phase of their lives. This knowledge can help the clinician—and often the patient—make the best-informed decisions.
Because only high-risk HPV types cause cervical cancer, testing should be restricted to high-risk (oncogenic) HPV types. Do not test for low-risk HPV types.
The guidelines are intended for use only with HPV tests that have been analytically and clinically validated, as documented by US Food and Drug Administration licensing and approval or by publication in peer-reviewed scientific literature. This distinction is important because management based on results of HPV tests that have not been similarly validated may not result in outcomes intended by these guidelines and may increase the potential for patient harm.
Do guidelines really need to get even more complex?
Consider the myriad management decisions that confront us in the field of cervical cancer screening, and the potential result of each choice. Even when cervical screening involves cytology alone, there are five major categories for abnormal results, each associated with a different level of risk requiring a unique level of management:
- atypical squamous cells – undetermined significance (ASC-US)
- atypical squamous cells – cannot rule out a high-grade lesion (ASC-H)
- atypical glandular cells (AGC)
- low-grade squamous intraepithelial lesion (LSIL)
- high-grade squamous intraepithelial lesion (HSIL).
Add in HPV testing with cervical cytology for women 30 years and older, and there is one more abnormal category—normal Pap/ HPV-positive. And these categories just cover initial management. Also needed are guidelines for appropriate follow-up of women who undergo colposcopy for each abnormal cytologic result when no CIN 2, CIN 3, or AIS is found that requires treatment, as well as guidelines for managing women following treatment when high-grade histology is found.
As our understanding of the natural history of HPV and cervical oncogenesis has increased, it has become clearer that we must further adjust management decisions on the basis of age, essentially creating many parallel sets of guidelines for women aged 21 to 24, 25 to 29, and 30 years and older.
Yes, cervical screening and management are complex. We are fortunate that the Internet and new “apps” for smartphones give us easy access to guidelines for most of the potential combinations of clinical findings and results. The guideline algorithms are available at www.asccp.org, and full explanatory articles are available at www.jlgtd.com and www.greenjournal.org (comprehensive apps are available for download for almost every smartphone device).
Remember, it is impossible to create guidelines for every possible clinical situation, so clinical judgment must always be paramount when applying guidelines to individual patients.1
What are the major changes of the latest set of guidelines and its update?
Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121(4):829–846. [Also published in J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27.]
Let’s start by focusing on how the experts crafted the 2012 guidelines. New evidence to guide decisions about the management of abnormal screening tests, CIN, and AIS emerged in 2012 from a review of the world literature and from analyses of a large 7-year clinical database (1.4 million women) at the Kaiser Permanente Northern California Medical Care Plan, conducted in collaboration with scientists from the National Cancer Institute.1
Most of the 2006 guidelines remain valid, but new evidence has modified some of the guidelines and created others where gaps existed. Guideline developers recognized that cervical cancer prevention is a process that entails both benefits and potential harms, and that the potential risks cannot be reduced to zero with the strategies currently available. Attempts to achieve zero risk could result in unbalanced harms, including overtreatment.
- Anxiety from an abnormal test that the patient might fear to be a sign of cancer
- Stigma from diagnosis of a ubiquitous sexually transmitted infection (HPV)
- Time and patient expense related to screening and management
- Pain and injury from the procedures and treatment
- Increased risk of premature delivery and pregnancy loss.
Defining acceptable risk levels
Applying the concept of “similar management for similar risks,” guideline developers benchmarked risks to the risks associated with accepted screening and management strategies. Because the 5-year risk for CIN 3+ for a woman with an LSIL Pap finding is about 5.2%, and the recommendation for LSIL is colposcopy, 5.2% was set as the lower limit of the level of risk that provides enough benefit (detection of CIN 3+) to balance the potential harms of colposcopy.1 (See the box on harms above.)
When women return to prolonged screening as follow-up to abnormal cytology or a positive HPV test, acceptable risk was considered to be that approximating the risk for CIN3+ three years after negative cytology or 5 years after negative cotesting—as these risks were considered acceptable to guide recent primary cervical screening guidelines.2-4
To be as precise as possible, experts stratified the guidelines by risk, according to the woman’s age, cytologic diagnosis, and HPV status, including HPV genotyping for types 16 and 18, when tested. Of course, guidelines for management apply only to women who are found to have abnormalities during routine screening.1 Women who experience postcoital or unexplained abnormal vaginal bleeding, pelvic pain, abnormal discharge, or a visible lesion need individualized evaluations.1
Only changes or additions to the guidelines are listed here, so be sure to read the published guidelines and supplemental articles and/or visit the Web sites listed earlier for a review of all the guidelines.
What’s new in managing women with unsatisfactory Pap results?
In general, cytology should be repeated in 2 to 4 months.
If the unsatisfactory Pap test is part of a cotest, then the following strategies are appropriate:
- If the HPV test is positive, either repeating the Pap test or moving directly to colposcopy is acceptable
- If HPV genotyping was reported and is positive for type 16 or 18, colposcopy is indicated.
Colposcopy also is recommended when two consecutive Pap tests are unsatisfactory.
What’s new in managing women with normal cytology but no, or insufficient, endocervical cells/transformation-zone component?
The answer varies by age:
- For women 21 to 29 years – routine screening with cytology in 3 years is recommended
- For women 30 years and older:
- When cotesting is done, the HPV result guides management:
- HPV-negative: routine screening with cotesting in 5 years is preferred
- HPV-positive: either cotesting in 1 year or immediate genotyping is recommended
- If HPV testing was not done, then HPV testing is recommended, with management guided by results.
- When cotesting is done, the HPV result guides management:
What’s new in managing women aged 21 to 24 with abnormal cervical cytology or CIN?
Young women of this age are at high risk for HPV infection but very low risk for cancer. Aggressive management usually involves more harm than benefit, promoting observation. Adolescents are no longer screened; management previously reserved for adolescents is now appropriate for women aged 21 to 24 years.
If the Pap result is:
- ASC-US or LSIL:
- No colposcopy is needed. The Pap test should be repeated annually for 2 years, with colposcopy after 1 year only when the finding is HSIL and after 2 years if ASC-US or LSIL findings persist
- HPV triage for ASC-US is not recommended, but if it is done:
- HPV-negative women should continue routine screening with a Pap test in 3 years
- HPV-positive women should have annual cytology for 2 years, with colposcopy after 1 year only if the result is HSIL and after 2 years if ASC-US or LSIL findings persist.
- ASC-H or HSIL:
- Colposcopy is recommended, but immediate treatment (see-and-treat LEEP) is unacceptable
- Women with no CIN 2 or CIN 3 at colposcopy should be followed with colposcopy and cytology every 6 months for as long as 2 years, until two consecutive Pap tests are negative and no high-grade colposcopic abnormality is observed
- Repeat biopsies are indicated if cytology at 1 year is again ASC-H or HSIL
- Diagnostic excision is recommended if HSIL cytology persists for 2 years.
Changes in the management of histologic findings
If CIN 1 is detected, management depends on the antecedent cytology report:
- If the prior Pap finding was ASC-US or LSIL, observation with annual cytology is recommended
- If the prior Pap finding was ASC-H or HSIL, observation for as long as 24 months is recommended, using both colposcopy and cytology at 6-month intervals, provided the colposcopic examination is adequate and endocervical assessment is negative.
If CIN 2 is detected, observation is preferred but treatment is acceptable (see the guidelines for detailed recommendations).
If CIN 2/CIN 3 (not otherwise differentiated) is detected, either observation or treatment is acceptable (see the guidelines for detailed recommendations).
If CIN 3 is detected in a woman of any age, treatment is indicated.
What’s new in managing women 30 years and older who have discordant cotest results?
Use cotesting management recommendations only for women 30 years and older.
If the finding is:
- HPV-positive/Pap-negative (HPV+/ Pap-), the two options are:
- Repeat cotesting in 1 year, with colposcopy if the finding is again HPV+ or the Pap is ASC-US or more severe (including HPV-/ASC-US), and repeat cotesting in 3 years if results for both the HPV test and the Pap are negative (HPV-/Pap-)
- Genotyping, with colposcopy if HPV 16 or 18 is identified and repeat cotesting in 1 year if both HPV 16 and 18 are negative
- HPV-/ASC-US:
- Repeat the cotest in 3 years
- HPV-/LSIL, the options are:
- Cotesting in 1 year (preferred)
- Colposcopy (acceptable)
- HPV+/LSIL or LSIL/no HPV result:
- Colposcopy
- HPV-/HSIL or HPV-/ASC-H:
- Colposcopy
- HPV-/AGC
- Colposcopy, often with endometrial sampling.
New terminology unifies all lower genital tract HPV intraepithelial neoplasia
Darragh TM, Colgan TJ, Cox JT, et al; LAST Project Work Groups. The Lower Anogenital Squamous Terminology Standardization Project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis. 2012;16(3):205–242.
In 2012, the Lower Anogenital Squamous Terminology (LAST) standardization project created new histology terminology for HPV-related lesions of the lower genital tract. The LSIL finding was designated as the all-encompassing term for CIN 1, vaginal intraepithelial neoplasia 1 (VaIN 1), vulvar intraepithelial neoplasia 1 (VIN 1), penile intraepithelial neoplasia 1 (PeIN 1), perianal intraepithelial neoplasia 1 (PAIN 1) and anal intraepithelial neoplasia 1 (AIN 1). Intraepithelial neoplasia (IN) graded 2, 2/3, and 3 from each of these areas is designated HSIL.5
When CIN 2 and CIN 3 can be differentiated, these designations can be reported along with the HSIL diagnosis. However, after thoughtful deliberation, the delegates to the ASCCP consensus conference decided that there is not yet enough outcome data available to determine different management strategies when using the new LAST histopathology terminology. They recommended that, until evidence is available, results reported as histologic (not cytologic) LSIL should be managed as CIN 1, and histologic (not cytologic) HSIL should be managed as CIN 2/CIN 3.
Guidelines for the management of abnormal cervical cytology, CIN, and AIS are necessarily complicated, but they provide the best basis for evidence-based management of these medical challenges. The Web provides easy access to all of the ASCCP guidelines via www.asccp.org, www.jlgtd.com, and www.greenjournal.org.
We want to hear from you! Tell us what you think.
1. Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121(4):829-846.(Also published in J Low Genit Tract Dis. 2013;17[5 Suppl 1]:S1–S27.)
2. Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516-542.
3. Moyer VA. US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880-891, W312.
4. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 131: Screening for cervical cancer. Obstet Gynecol. 2012;120(5):1222-1238.
5. Yates J. New cervical Ca screening guidelines recommend less frequent assessment. OBG Manage. 2012;24(6):40-44.
1. Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121(4):829-846.(Also published in J Low Genit Tract Dis. 2013;17[5 Suppl 1]:S1–S27.)
2. Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516-542.
3. Moyer VA. US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880-891, W312.
4. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 131: Screening for cervical cancer. Obstet Gynecol. 2012;120(5):1222-1238.
5. Yates J. New cervical Ca screening guidelines recommend less frequent assessment. OBG Manage. 2012;24(6):40-44.