User login
A minimally invasive treatment for early GI cancers
The treatment of early esophageal, gastric, and colorectal cancer is changing.1 For many years, surgery was the mainstay of treatment for early-stage gastrointestinal cancer. Unfortunately, surgery leads to significant loss of function of the organ, resulting in increased morbidity and decreased quality of life.2
Endoscopic techniques, particularly endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), have been developed and are widely used in Japan, where gastrointestinal cancer is more common than in the West. This article reviews the indications, complications, and outcomes of ESD for early gastrointestinal neoplasms, so that readers will recognize the subset of patients who would benefit from ESD in a Western setting.
ENDOSCOPIC MUCOSAL RESECTION AND SUBMUCOSAL DISSECTION
Since the first therapeutic polypectomy was performed in Japan in 1974, several endoscopic techniques for tumor resection have been developed.3
EMR, one of the most successful and widely used techniques, involves elevating the lesion either with submucosal injection of a solution or with cap suction, and then removing it with a snare.4 Most lesions smaller than 20 mm can be removed in one piece (en bloc).5 Larger lesions are removed in multiple pieces (ie, piecemeal). Unfortunately, some fibrotic lesions, which are usually difficult to lift, cannot be completely removed by EMR.
ESD was first performed in the late 1990s with the aim of overcoming the limitations of EMR in resecting large or fibrotic tumors en bloc.6,7 Since then, ESD technique has been standardized and training centers have been created, especially in Asia, where it is widely used for treatment of early gastric cancer.3,8–10 Since 2012 it has been covered by the Japanese National Health Insurance for treatment of early gastric cancer, and since 2014 for treatment of colorectal malignant tumors measuring 2 to 5 cm.11
Adoption of ESD has been slow in Western countries, where many patients are still referred for surgery or undergo EMR for removal of superficial neoplasms. Reasons for this slow adoption are that gastric cancer is much less common in Western countries, and also that ESD demands a high level of technical skill, is difficult to learn, and is expensive.3,12,13 However, small groups of Western endoscopists have become interested and are advocating it, first studying it on their own and then training in a Japanese center and learning from experts performing the procedure.
Therefore, in a Western setting, ESD should be performed in specialized endoscopy centers and offered to selected patients.1
CANDIDATES SHOULD HAVE EARLY-STAGE, SUPERFICIAL TUMORS
Ideal candidates for endoscopic resection are patients who have early cancer with a negligible risk of lymph node metastasis, such as cancer limited to the mucosa (stage T1a).7 Therefore, to determine the best treatment for a patient with a newly diagnosed gastrointestinal neoplasm, it is mandatory to estimate the depth of invasion.
The depth of invasion is directly correlated with lymph node involvement, which is ultimately the main predictive factor for long-term adverse outcomes of gastrointestinal tumors.4,14–17 Accurate multidisciplinary preprocedure estimations are mandatory, as incorrect evaluations may result in inappropriate therapy and residual cancer.18
Other factors that have been used to predict lymph node involvement include tumor size, macroscopic appearance, histologic differentiation, and lymphatic and vascular involvement.19 Some of these factors can be assessed by special endoscopic techniques (chromoendoscopy and narrow-band imaging with magnifying endoscopy) that allow accurate real-time estimation of the depth of invasion of the lesion.5,17,20–27 Evaluation of microsurface and microvascular arrangements is especially useful for determining the feasibility of ESD in gastric tumors, evaluation of intracapillary loops is useful in esophageal lesions, and assessment of mucosal pit patterns is useful for colorectal lesions.21–29
Endoscopic ultrasonography is another tool that has been used to estimate the depth of the tumor. Although it can differentiate between definite intramucosal and definite submucosal invasive cancers, its ability to confirm minute submucosal invasion is limited. Its use as the sole tumor staging modality is not encouraged, and it should always be used in conjunction with endoscopic evaluation.18
Though the aforementioned factors help stratify patients, pathologic staging is the best predictor of lymph node metastasis. ESD provides adequate specimens for accurate pathologic evaluation, as it removes lesions en bloc.30
All patients found to have risk factors for lymph node metastasis on endoscopic, ultrasonographic, or pathologic analysis should be referred for surgical evaluation.9,19,31,32
ENDOSCOPIC SUBMUCOSAL DISSECTION
Before the procedure, the patient’s physicians need to do the following:
Determine the best type of intervention (EMR, ESD, ablation, surgery) for the specific lesion.3 A multidisciplinary approach is encouraged, with involvement of the internist, gastroenterologist, and surgeon.
Plan for anesthesia, additional consultations, pre- and postprocedural hospital admission, and need for special equipment.33
During the procedure
Define the lateral extent of the lesion using magnification chromoendoscopy or narrow-band imaging. In the stomach, a biopsy sample should be taken from the worst-looking segment and from normal-looking mucosa. Multiple biopsies should be avoided to prevent subsequent fibrosis.33 In the colon, biopsy should be avoided.34
Identify and circumferentially mark the target lesion. Cautery or argon plasma coagulation can be used for making markings at a distance of 5 to 10 mm from the edges.33 This is done to recognize the borders of the lesion, because they can become distorted after submucosal injection.14 This step is unnecessary in colorectal cases, as tumor margins can be adequately visualized after chromoendoscopy.16,35
Lift the lesion by injecting saline, 0.5% hyaluronate, or glycerin to create a submucosal fluid cushion.19,33
Perform a circumferential incision lateral to the mucosal margins to allow for a normal tissue margin.33 Partial incision is performed for esophageal and colorectal ESD to avoid fluid leakage from the submucosal layer, achieving a sustained submucosal lift and safer dissection.16
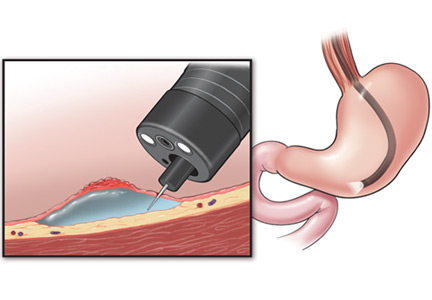
Submucosal dissection. The submucosal layer is dissected with an electrocautery knife until the lesion is completely removed. Dissection should be done carefully to keep the submucosal plane.33 Hemoclips or hemostat forceps can be used to control visible bleeding. The resected specimen is then stretched and fixed to a board using small pins for further histopathologic evaluation.35
Postprocedural monitoring. All patients should be admitted for overnight observation. Those who undergo gastric ESD should receive high-dose acid suppression, and the next day they can be started on a liquid diet.19
STOMACH CANCER
Indications for ESD for stomach cancer in the East
The incidence of gastric cancer is higher in Japan and Korea, where widespread screening programs have led to early identification and early treatment of this disease.36
Pathology studies37 of samples from patients with gastric cancer identified the following as risk factors for lymph node metastasis, which would make ESD unsuitable:
- Undifferentiated type
- Tumors larger than 2 cm
- Lymphatic or venous involvement
- Submucosal invasion
- Ulcerative change.
Based on these findings, the situations in which there was no risk of lymph node involvement (ie, when none of the above factors are present) were accepted as absolute indications for endoscopic resection of early gastric cancer.38 Further histologic studies identified a subset of patients with lesions with very low risk of lymph node metastasis, which outweighed the risk of surgery. Based on these findings, expanded criteria for gastric ESD were proposed,39,40 and the Japanese gastric cancer treatment guidelines now include these expanded preoperative indications9,17 (Table 1).
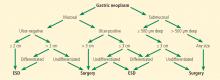
The Japanese Gastric Cancer Association has proposed a treatment algorithm based on the histopathologic evaluation after resection (Figure 2).9
Outcomes
In the largest series of patients who underwent curative ESD for early gastric cancer, the 5-year survival rate was 92.6%, the 5-year disease-specific survival rate was 99.9%, and the 5-year relative survival rate was 105%.41
Similarly, in a Japanese population-based survival analysis, the relative 5-year survival rate for localized gastric cancer was 94.4%.42 Rates of en bloc resection and complete resection with ESD are higher than those with EMR, resulting in a lower risk of local recurrence in selected patients who undergo ESD.8,43,44
Although rare, local recurrence after curative gastric ESD has been reported.45 The annual incidence of local recurrence has been estimated to be 0.84%.46
ESD entails a shorter hospital stay and requires fewer resources than surgery, resulting in lower medical costs (Table 2).44 Additionally, as endoscopic resection is associated with less morbidity, fewer procedure-related adverse events, and fewer complications, ESD could be used as the standard treatment for early gastric cancer.47,48
The Western perspective on endoscopic submucosal dissection for gastric cancer
Since the prevalence of gastric cancer in Western countries is significantly lower than in Japan and Korea, local data and experience are scarce. However, experts performing ESD in the West have adopted the indications of the Japan Gastroenterological Endoscopy Society. The European Society of Gastrointestinal Endoscopy recommends ESD for excision of most superficial gastric neoplasms, with EMR being preferred only in lesions smaller than 15 mm, Paris classification 0 or IIA.5,32
Patients with gastric lesions measuring 15 mm or larger should undergo high-quality endoscopy, preferably chromoendoscopy, to evaluate the mucosal patterns and determine the depth of invasion. If superficial involvement is confirmed, other imaging techniques are not routinely recommended.5 A surgery consult is also recommended.
ESOPHAGEAL CANCER
Indications for ESD for esophageal cancer in the East
Due to the success of ESD for early gastric cancer, this technique is now also used for superficial esophageal neoplasms.19,49 It should be done in a specialized center, as it is more technically difficult than gastric ESD: the esophageal lumen is narrow, the wall is thin, and the esophagus moves with respiration and heartbeat.50 A multidisciplinary approach including an endoscopist, a surgeon, and a pathologist is highly recommended for evaluation and treatment.
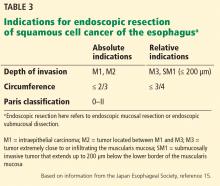
EMR is preferred for removal of mucosal cancer, in view of its safety profile and success rates. ESD can be considered in cases of lesions larger than 15 mm, poorly lifting tumors, and those with the possibility of submucosal invasion (Table 3).5,45,49,51
Circumference involvement is critical when determining eligible candidates, as a defect involving more than three-fourths of the esophageal circumference can lead to esophageal strictures.52 Controlled prospective studies have shown promising results from giving intralesional and oral steroids to prevent stricture after ESD, which could potentially overcome this size limitation.53,54
Outcomes for esophageal cancer
ESD has been shown to be safe and effective, achieving en bloc resection in 85% to 100% of patients.19,51 Its advantages over EMR include en bloc resection, complete resection, and high curative rates, resulting in higher recurrence-free survival.2,55,56 Although the incidence of complications such as bleeding, perforation, and stricture formation are higher with ESD, patients usually recover uneventfully.2,19,20
ESD in the esophagus: The Western perspective
As data on the efficacy of EMR vs ESD for the treatment of Barrett esophagus with adenocarcinoma are limited, EMR is the gold standard endoscopic technique for removal of visible esophageal dysplastic lesions.5,51,57 ESD can be considered for tumors larger than 15 mm, for poorly lifting lesions, and if there is suspicion of submucosal invasion.5
Patients should be evaluated by an experienced endoscopist, using an advanced imaging technique such as narrow-band imaging or chromoendoscopy. If suspicious features are found, endoscopic ultrasonography should be considered to confirm submucosal invasion or lymph node involvement.5
COLORECTAL CANCER
Indications for ESD for colorectal cancer in the East
Colon cancer is one of the leading causes of cancer-related deaths worldwide.58 Since ESD has been found to be effective and safe in treating gastric cancer, it has also been used to remove large colorectal tumors.59 However, ESD is not universally accepted in the treatment of colorectal neoplasms due to its greater technical difficulty, longer procedural time, and higher risk of perforating the thinner colonic wall compared with EMR.21,60
Outcomes for colorectal cancer
Tumor size of 50 mm or larger is a risk factor for complications, while a high procedure volume at the center is a protective factor.60
Endoscopic treatment of colorectal cancer: The Western perspective
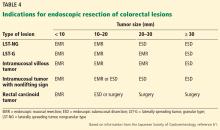
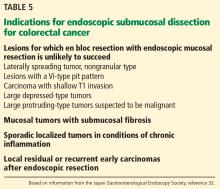
EMR is the gold standard for removal of superficial colorectal lesions. However, ESD can be considered if there is suspicion of superficial submucosal invasion, especially for lesions larger than 20 mm that cannot be resected en bloc by EMR.32 ESD can also be used for fibrotic lesions not amenable to complete EMR removal, or as a salvage procedure after recurrence after EMR.67 Proper selection of cases is critical.1
Patients who have a superficial colonic lesion should be evaluated by means of high-definition endoscopy and chromoendoscopy to assess the mucosal pattern and establish feasibility of endoscopic resection. If submucosal invasion is suspected, staging with endoscopic ultrasonography or magnetic resonance imaging should be considered.5
FOLLOW-UP AFTER ESD
Endoscopic surveillance after the procedure is recommended, given the persistent risk of metachronous cancer after curative ESD due to its organ-sparing quality.68 Surveillance endoscopy aims to achieve early detection and subsequent endoscopic resection of metachronous lesions.
Histopathologic evaluation assessing the presence of malignant cells in the margins of a resected sample is mandatory for determining the next step in treatment. If margins are negative, follow-up endoscopy can be done every 6 to 12 months. If margins are positive, the approach includes surgery, reattempting ESD or endoscopic surveillance in 3 or 6 months.3,32 Although the surveillance strategy varies according to individual risk of metachronous cancer, it should be continued indefinitely.68
COMPLICATIONS OF ESD
The most common procedure-related complications of ESD are bleeding, perforation, and stricture. Most intraprocedural adverse events can be managed endoscopically.69
Bleeding
Most bleeding occurs during the procedure or early after it and can be controlled with electrocautery.49,69 No episodes of massive bleeding, defined as causing clinical symptoms and requiring transfusion or surgery, have been reported.20,43,55
In gastric ESD, delayed bleeding rates have ranged from 0 to 15.6%.69 Bleeding may be prevented with endoscopic coagulation of visible vessels after dissection has been completed and by proton pump inhibitor therapy.70,71 Excessive coagulation should be avoided to lower the risk of perforation.33
In colorectal ESD the bleeding rate has been reported to be 2.2%; applying coagulation to an area where a blood vessel is suspected before cutting (precoagulation) may prevent subsequent bleeding.21
Perforation
For gastric ESD, perforation rates range from 1.2% to 5.2%.69 Esophageal perforation rates can be up to 4%.49 In colorectal ESD, perforation rates have been reported to be 1.6% to 6.6%.60,72
Although most of the cases were successfully managed with conservative treatment, some required emergency surgery.60,73
Strictures
In a case series of 532 patients undergoing gastric ESD, stricture was reported in 5 patients, all of whom presented with obstructive symptoms.74 Risk factors for post-ESD gastric stenosis are a mucosal defect with a circumferential extent of more than three-fourths or a longitudinal extent of more than 5 cm.75
Strictures are common after esophageal ESD, with rates ranging from 2% to 26%. The risk is higher when longer segments are removed or circumferential resection is performed. As previously mentioned, this complication may be reduced with ingestion or injection of steroids after the procedure.53,54
Surprisingly, ESD of large colorectal lesions involving more than three-fourths of the circumference of the rectum is rarely complicated by stenosis.76
LIMITATIONS OF ESD
ESD requires a high level of technical skill, is time-consuming, and has a higher rate of complications than conventional endoscopic resection. A standardized ESD training system is needed, as the procedure is more difficult than EMR. Training in porcine models has been shown to confer competency in ESD in a Western setting.13,16,33
Colorectal ESD is an even more challenging procedure, given the potential for complications related to its anatomy. Training centers in Japan usually have their trainees first master gastric ESD, then assist in more than 20 colorectal ESDs conducted by experienced endoscopists, and accomplish 30 cases before performing the procedure safely and independently.
As the incidence of gastric cancer is low in Western countries, trainees may also begin with lower rectal lesions, which are easier to remove.77 Incorporation of ESD in the West would require a clear treatment algorithm. It is a complex procedure, with higher rates of complications, a prolonged learning curve, and prolonged procedure time. Therefore, it should be performed in specialized centers and under the special situations discussed here to ensure that the benefits for the patients outweigh the risks.
VALUE OF ENDOSCOPIC SUBMUCOSAL DISSECTION
The optimal method for resecting gastrointestinal neoplasms should be safe, cost-effective, and quick and should also completely remove the lesion. The best treatment strategy takes into account the characteristics of the lesion and the comorbidities and wishes of the patient. Internists should be aware of the multiple options available to achieve the best outcome for the patient.1
Endoscopic resection of superficial gastrointestinal neoplasms, including EMR and ESD, has been a subject of increasing interest due to its minimally invasive and potentially curative character. However, cancer can recur after endoscopic resection because the procedure is organ-sparing.
ESD allows resection of early gastrointestinal tumors with a minimally invasive technique. It can achieve higher curative resection rates and lower recurrence rates compared with EMR. Compared with surgery, ESD leads to less morbidity, fewer procedure-related complications, and lower medical costs. Indications should be rigorously followed to achieve successful treatments in selected patients.
Multiple variables have to be taken into account when deciding which treatment is best, such as tumor characteristics, the patient’s baseline condition, physician expertise, and hospital resources.48 Less-invasive treatments may improve the prognosis of patients. No matter the approach, patients should be treated in specialized treatment centers.
Internal medicine physicians should be aware of the advances in treatments for early gastrointestinal cancer so appropriate options can be considered.
- Burgess NG, Bourke MJ. Endoscopic resection of colorectal lesions: the narrowing divide between East and West. Dig Endosc 2016; 28:296–305.
- Kim DH, Jung HY, Gong EJ, et al. Endoscopic and oncologic outcomes of endoscopic resection for superficial esophageal neoplasm. Gut Liver 2015; 9:470–477.
- Draganov PV, Gotoda T, Chavalitdhamrong D, Wallace MB. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013; 78:677–688.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47:829–854.
- Farhat S, Chaussade S, Ponchon T, et al; SFED ESD Study Group. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011; 43:664–670.
- Gotoda T, Jung H. Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig Endosc 2013; 25(suppl 1):55–63.
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009; 69:1228–1235.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123.
- Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005; 6:119–121.
- Watanabe T, Itabashi M, Shimada Y, et al; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20:207–239.
- Oyama T, Yahagi N, Ponchon T, Kiesslich T, Berr F. How to establish endoscopic submucosal dissection in Western countries. World J Gastroenterol 2015; 21:11209–11220.
- Bhatt A, Abe S, Kumaravel A, et al. SU1575 Western skill training in endoscopic submucosal dissection (ESD)—an international remote video based study—the WEST ESD Study. Gastrointest Endosc 2015; 81(suppl):AB335–AB336.
- Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer follow-up of 1475 patients and review of the Japanese literature. Cancer 1993; 72:3174–3178.
- Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus 2009; 6:1–25.
- Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol 2015; 110:784–791.
- Eleftheriadis N, Inoue H, Ikeda H, et al. Definition and staging of early esophageal, gastric and colorectal cancer. J Tumor 2014; 2:161–178.
- Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc 2012; 4:218–226.
- Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi51–vi56.
- Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc 2013; 78:704–710.
- Sakamoto T, Mori G, Yamada M, et al. Endoscopic submucosal dissection for colorectal neoplasms: a review. World J Gastroenterol 2014; 20:16153–16158.
- Ohta A, Tominaga K, Sakai Y. Efficacy of magnifying colonoscopy for the diagnosis of colorectal neoplasia: comparison with histopathological findings. Dig Endosc 2004; 16:308–314.
- Katagiri A, Fu K, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther 2008; 27:1269–1274.
- Fu KI, Kato S, Sano Y, et al. Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci 2008; 53:1886–1892.
- Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc 2011; 23(suppl 1):112–115.
- Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol 2010;10:33.
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008; 103:2700–2706.
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015; 8:122–128.
- Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc 2016; 28:379–393.
- Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi57–vi63.
- Kuwano H, Nishimura Y, Ohtsu A, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus. April 2007 edition: part I - Edited by the Japan Esophageal Society. Esophagus 2008; 5:61–73.
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015; 27:417–434.
- Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin North Am 2014; 24:213–233.
- Saito Y, Sakamoto T, Nakajima T, Matsuda T. Colorectal ESD: current indications and latest technical advances. Gastrointest Endosc Clin N Am 2014; 24:245–255.
- Saito Y, Otake Y, Sakamoto T, et al. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver 2013; 7:263–269.
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 2015; 12:355–361.
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999; 125:148–154.
- Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003; 57:567–579.
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12:148–152.
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225.
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016; 19:198–205.
- Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T; Research Group of Population-Based Cancer Registries of Japan. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011; 41:40–51.
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011; 74:485–493.
- Kim Y, Kim YW, Choi IJ, et al. Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea. Gut Liver 2015; 9:174–180.
- Abe S, Oda I, Nakajima T, et al. A case of local recurrence and distant metastasis following curative endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2015; 18:188–192.
- Hahn KY, Park JC, Kim EH, et al. Incidence and impact of scheduled endoscopic surveillance on recurrence after curative endoscopic resection for early gastric cancer. Gastrointest Endosc 2016; 84:628–638.e1.
- Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One 2015; 10:e0144774.
- Kondo A, de Moura EG, Bernardo WM, et al. Endoscopy vs surgery in the treatment of early gastric cancer: systematic review. World J Gastroenterol 2015; 21:13177–13187.
- Kothari S, Kaul V. Endoscopic mucosal resection and endoscopic submucosal dissection for endoscopic therapy of Barrett’s esophagus-related neoplasia. Gastroenterol Clin North Am 2015; 44:317–335.
- Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011; 25:2541–2546.
- Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett’s adenocarcinoma. Gastrointest Endosc 2014; 80:239–245.
- Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003; 57:165–169.
- Hanaoka N, Ishihara R, Takeuchi Y, et al. 1139: A single session of intralesional steroid injection to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Gastrointest Endosc 2012; 75(suppl):AB175.
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011; 73:1115–1121.
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70:860–866.
- Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc 2005; 61:219–225.
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010; 72:960–966.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013; 27:3262–3770.
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010; 72:1217–1225.
- Tanaka S, Saitoh Y, Matsuda T, et al; Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol 2015; 50:252–260.
- Oka S, Tanaka S, Saito Y, et al; Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015; 110:697–707.
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010; 24:343–352.
- Makazu M, Sakamoto T, So E, et al. Relationship between indeterminate or positive lateral margin and local recurrence after endoscopic resection of colorectal polyps. Endosc Int Open 2015; 3:E252–E257.
- Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014; 46:388–402.
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015; 81:583–595.
- Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015; 16:14–21.
- Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015; 47:1113–1118.
- Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013; 25(suppl 1):71–78.
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection—an analysis of risk factors. Endoscopy 2008; 40:179–183.
- Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007; 102:1610–1616.
- Hayashi N, Tanaka S, Nishiyama S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2014; 79:427–435.
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015; 21:12635–12643.
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008; 67:979–983.
- Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009; 41:421–426.
- Abe S, Sakamoto T, Takamaru H, et al. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc 2016; 30:5459–5464.
- Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 2011; 54:1307–1312.
The treatment of early esophageal, gastric, and colorectal cancer is changing.1 For many years, surgery was the mainstay of treatment for early-stage gastrointestinal cancer. Unfortunately, surgery leads to significant loss of function of the organ, resulting in increased morbidity and decreased quality of life.2
Endoscopic techniques, particularly endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), have been developed and are widely used in Japan, where gastrointestinal cancer is more common than in the West. This article reviews the indications, complications, and outcomes of ESD for early gastrointestinal neoplasms, so that readers will recognize the subset of patients who would benefit from ESD in a Western setting.
ENDOSCOPIC MUCOSAL RESECTION AND SUBMUCOSAL DISSECTION
Since the first therapeutic polypectomy was performed in Japan in 1974, several endoscopic techniques for tumor resection have been developed.3
EMR, one of the most successful and widely used techniques, involves elevating the lesion either with submucosal injection of a solution or with cap suction, and then removing it with a snare.4 Most lesions smaller than 20 mm can be removed in one piece (en bloc).5 Larger lesions are removed in multiple pieces (ie, piecemeal). Unfortunately, some fibrotic lesions, which are usually difficult to lift, cannot be completely removed by EMR.
ESD was first performed in the late 1990s with the aim of overcoming the limitations of EMR in resecting large or fibrotic tumors en bloc.6,7 Since then, ESD technique has been standardized and training centers have been created, especially in Asia, where it is widely used for treatment of early gastric cancer.3,8–10 Since 2012 it has been covered by the Japanese National Health Insurance for treatment of early gastric cancer, and since 2014 for treatment of colorectal malignant tumors measuring 2 to 5 cm.11
Adoption of ESD has been slow in Western countries, where many patients are still referred for surgery or undergo EMR for removal of superficial neoplasms. Reasons for this slow adoption are that gastric cancer is much less common in Western countries, and also that ESD demands a high level of technical skill, is difficult to learn, and is expensive.3,12,13 However, small groups of Western endoscopists have become interested and are advocating it, first studying it on their own and then training in a Japanese center and learning from experts performing the procedure.
Therefore, in a Western setting, ESD should be performed in specialized endoscopy centers and offered to selected patients.1
CANDIDATES SHOULD HAVE EARLY-STAGE, SUPERFICIAL TUMORS
Ideal candidates for endoscopic resection are patients who have early cancer with a negligible risk of lymph node metastasis, such as cancer limited to the mucosa (stage T1a).7 Therefore, to determine the best treatment for a patient with a newly diagnosed gastrointestinal neoplasm, it is mandatory to estimate the depth of invasion.
The depth of invasion is directly correlated with lymph node involvement, which is ultimately the main predictive factor for long-term adverse outcomes of gastrointestinal tumors.4,14–17 Accurate multidisciplinary preprocedure estimations are mandatory, as incorrect evaluations may result in inappropriate therapy and residual cancer.18
Other factors that have been used to predict lymph node involvement include tumor size, macroscopic appearance, histologic differentiation, and lymphatic and vascular involvement.19 Some of these factors can be assessed by special endoscopic techniques (chromoendoscopy and narrow-band imaging with magnifying endoscopy) that allow accurate real-time estimation of the depth of invasion of the lesion.5,17,20–27 Evaluation of microsurface and microvascular arrangements is especially useful for determining the feasibility of ESD in gastric tumors, evaluation of intracapillary loops is useful in esophageal lesions, and assessment of mucosal pit patterns is useful for colorectal lesions.21–29
Endoscopic ultrasonography is another tool that has been used to estimate the depth of the tumor. Although it can differentiate between definite intramucosal and definite submucosal invasive cancers, its ability to confirm minute submucosal invasion is limited. Its use as the sole tumor staging modality is not encouraged, and it should always be used in conjunction with endoscopic evaluation.18
Though the aforementioned factors help stratify patients, pathologic staging is the best predictor of lymph node metastasis. ESD provides adequate specimens for accurate pathologic evaluation, as it removes lesions en bloc.30
All patients found to have risk factors for lymph node metastasis on endoscopic, ultrasonographic, or pathologic analysis should be referred for surgical evaluation.9,19,31,32
ENDOSCOPIC SUBMUCOSAL DISSECTION
Before the procedure, the patient’s physicians need to do the following:
Determine the best type of intervention (EMR, ESD, ablation, surgery) for the specific lesion.3 A multidisciplinary approach is encouraged, with involvement of the internist, gastroenterologist, and surgeon.
Plan for anesthesia, additional consultations, pre- and postprocedural hospital admission, and need for special equipment.33
During the procedure
Define the lateral extent of the lesion using magnification chromoendoscopy or narrow-band imaging. In the stomach, a biopsy sample should be taken from the worst-looking segment and from normal-looking mucosa. Multiple biopsies should be avoided to prevent subsequent fibrosis.33 In the colon, biopsy should be avoided.34
Identify and circumferentially mark the target lesion. Cautery or argon plasma coagulation can be used for making markings at a distance of 5 to 10 mm from the edges.33 This is done to recognize the borders of the lesion, because they can become distorted after submucosal injection.14 This step is unnecessary in colorectal cases, as tumor margins can be adequately visualized after chromoendoscopy.16,35
Lift the lesion by injecting saline, 0.5% hyaluronate, or glycerin to create a submucosal fluid cushion.19,33
Perform a circumferential incision lateral to the mucosal margins to allow for a normal tissue margin.33 Partial incision is performed for esophageal and colorectal ESD to avoid fluid leakage from the submucosal layer, achieving a sustained submucosal lift and safer dissection.16
Submucosal dissection. The submucosal layer is dissected with an electrocautery knife until the lesion is completely removed. Dissection should be done carefully to keep the submucosal plane.33 Hemoclips or hemostat forceps can be used to control visible bleeding. The resected specimen is then stretched and fixed to a board using small pins for further histopathologic evaluation.35
Postprocedural monitoring. All patients should be admitted for overnight observation. Those who undergo gastric ESD should receive high-dose acid suppression, and the next day they can be started on a liquid diet.19
STOMACH CANCER
Indications for ESD for stomach cancer in the East
The incidence of gastric cancer is higher in Japan and Korea, where widespread screening programs have led to early identification and early treatment of this disease.36
Pathology studies37 of samples from patients with gastric cancer identified the following as risk factors for lymph node metastasis, which would make ESD unsuitable:
- Undifferentiated type
- Tumors larger than 2 cm
- Lymphatic or venous involvement
- Submucosal invasion
- Ulcerative change.
Based on these findings, the situations in which there was no risk of lymph node involvement (ie, when none of the above factors are present) were accepted as absolute indications for endoscopic resection of early gastric cancer.38 Further histologic studies identified a subset of patients with lesions with very low risk of lymph node metastasis, which outweighed the risk of surgery. Based on these findings, expanded criteria for gastric ESD were proposed,39,40 and the Japanese gastric cancer treatment guidelines now include these expanded preoperative indications9,17 (Table 1).
The Japanese Gastric Cancer Association has proposed a treatment algorithm based on the histopathologic evaluation after resection (Figure 2).9
Outcomes
In the largest series of patients who underwent curative ESD for early gastric cancer, the 5-year survival rate was 92.6%, the 5-year disease-specific survival rate was 99.9%, and the 5-year relative survival rate was 105%.41
Similarly, in a Japanese population-based survival analysis, the relative 5-year survival rate for localized gastric cancer was 94.4%.42 Rates of en bloc resection and complete resection with ESD are higher than those with EMR, resulting in a lower risk of local recurrence in selected patients who undergo ESD.8,43,44
Although rare, local recurrence after curative gastric ESD has been reported.45 The annual incidence of local recurrence has been estimated to be 0.84%.46
ESD entails a shorter hospital stay and requires fewer resources than surgery, resulting in lower medical costs (Table 2).44 Additionally, as endoscopic resection is associated with less morbidity, fewer procedure-related adverse events, and fewer complications, ESD could be used as the standard treatment for early gastric cancer.47,48
The Western perspective on endoscopic submucosal dissection for gastric cancer
Since the prevalence of gastric cancer in Western countries is significantly lower than in Japan and Korea, local data and experience are scarce. However, experts performing ESD in the West have adopted the indications of the Japan Gastroenterological Endoscopy Society. The European Society of Gastrointestinal Endoscopy recommends ESD for excision of most superficial gastric neoplasms, with EMR being preferred only in lesions smaller than 15 mm, Paris classification 0 or IIA.5,32
Patients with gastric lesions measuring 15 mm or larger should undergo high-quality endoscopy, preferably chromoendoscopy, to evaluate the mucosal patterns and determine the depth of invasion. If superficial involvement is confirmed, other imaging techniques are not routinely recommended.5 A surgery consult is also recommended.
ESOPHAGEAL CANCER
Indications for ESD for esophageal cancer in the East
Due to the success of ESD for early gastric cancer, this technique is now also used for superficial esophageal neoplasms.19,49 It should be done in a specialized center, as it is more technically difficult than gastric ESD: the esophageal lumen is narrow, the wall is thin, and the esophagus moves with respiration and heartbeat.50 A multidisciplinary approach including an endoscopist, a surgeon, and a pathologist is highly recommended for evaluation and treatment.
EMR is preferred for removal of mucosal cancer, in view of its safety profile and success rates. ESD can be considered in cases of lesions larger than 15 mm, poorly lifting tumors, and those with the possibility of submucosal invasion (Table 3).5,45,49,51
Circumference involvement is critical when determining eligible candidates, as a defect involving more than three-fourths of the esophageal circumference can lead to esophageal strictures.52 Controlled prospective studies have shown promising results from giving intralesional and oral steroids to prevent stricture after ESD, which could potentially overcome this size limitation.53,54
Outcomes for esophageal cancer
ESD has been shown to be safe and effective, achieving en bloc resection in 85% to 100% of patients.19,51 Its advantages over EMR include en bloc resection, complete resection, and high curative rates, resulting in higher recurrence-free survival.2,55,56 Although the incidence of complications such as bleeding, perforation, and stricture formation are higher with ESD, patients usually recover uneventfully.2,19,20
ESD in the esophagus: The Western perspective
As data on the efficacy of EMR vs ESD for the treatment of Barrett esophagus with adenocarcinoma are limited, EMR is the gold standard endoscopic technique for removal of visible esophageal dysplastic lesions.5,51,57 ESD can be considered for tumors larger than 15 mm, for poorly lifting lesions, and if there is suspicion of submucosal invasion.5
Patients should be evaluated by an experienced endoscopist, using an advanced imaging technique such as narrow-band imaging or chromoendoscopy. If suspicious features are found, endoscopic ultrasonography should be considered to confirm submucosal invasion or lymph node involvement.5
COLORECTAL CANCER
Indications for ESD for colorectal cancer in the East
Colon cancer is one of the leading causes of cancer-related deaths worldwide.58 Since ESD has been found to be effective and safe in treating gastric cancer, it has also been used to remove large colorectal tumors.59 However, ESD is not universally accepted in the treatment of colorectal neoplasms due to its greater technical difficulty, longer procedural time, and higher risk of perforating the thinner colonic wall compared with EMR.21,60
Outcomes for colorectal cancer
Tumor size of 50 mm or larger is a risk factor for complications, while a high procedure volume at the center is a protective factor.60
Endoscopic treatment of colorectal cancer: The Western perspective
EMR is the gold standard for removal of superficial colorectal lesions. However, ESD can be considered if there is suspicion of superficial submucosal invasion, especially for lesions larger than 20 mm that cannot be resected en bloc by EMR.32 ESD can also be used for fibrotic lesions not amenable to complete EMR removal, or as a salvage procedure after recurrence after EMR.67 Proper selection of cases is critical.1
Patients who have a superficial colonic lesion should be evaluated by means of high-definition endoscopy and chromoendoscopy to assess the mucosal pattern and establish feasibility of endoscopic resection. If submucosal invasion is suspected, staging with endoscopic ultrasonography or magnetic resonance imaging should be considered.5
FOLLOW-UP AFTER ESD
Endoscopic surveillance after the procedure is recommended, given the persistent risk of metachronous cancer after curative ESD due to its organ-sparing quality.68 Surveillance endoscopy aims to achieve early detection and subsequent endoscopic resection of metachronous lesions.
Histopathologic evaluation assessing the presence of malignant cells in the margins of a resected sample is mandatory for determining the next step in treatment. If margins are negative, follow-up endoscopy can be done every 6 to 12 months. If margins are positive, the approach includes surgery, reattempting ESD or endoscopic surveillance in 3 or 6 months.3,32 Although the surveillance strategy varies according to individual risk of metachronous cancer, it should be continued indefinitely.68
COMPLICATIONS OF ESD
The most common procedure-related complications of ESD are bleeding, perforation, and stricture. Most intraprocedural adverse events can be managed endoscopically.69
Bleeding
Most bleeding occurs during the procedure or early after it and can be controlled with electrocautery.49,69 No episodes of massive bleeding, defined as causing clinical symptoms and requiring transfusion or surgery, have been reported.20,43,55
In gastric ESD, delayed bleeding rates have ranged from 0 to 15.6%.69 Bleeding may be prevented with endoscopic coagulation of visible vessels after dissection has been completed and by proton pump inhibitor therapy.70,71 Excessive coagulation should be avoided to lower the risk of perforation.33
In colorectal ESD the bleeding rate has been reported to be 2.2%; applying coagulation to an area where a blood vessel is suspected before cutting (precoagulation) may prevent subsequent bleeding.21
Perforation
For gastric ESD, perforation rates range from 1.2% to 5.2%.69 Esophageal perforation rates can be up to 4%.49 In colorectal ESD, perforation rates have been reported to be 1.6% to 6.6%.60,72
Although most of the cases were successfully managed with conservative treatment, some required emergency surgery.60,73
Strictures
In a case series of 532 patients undergoing gastric ESD, stricture was reported in 5 patients, all of whom presented with obstructive symptoms.74 Risk factors for post-ESD gastric stenosis are a mucosal defect with a circumferential extent of more than three-fourths or a longitudinal extent of more than 5 cm.75
Strictures are common after esophageal ESD, with rates ranging from 2% to 26%. The risk is higher when longer segments are removed or circumferential resection is performed. As previously mentioned, this complication may be reduced with ingestion or injection of steroids after the procedure.53,54
Surprisingly, ESD of large colorectal lesions involving more than three-fourths of the circumference of the rectum is rarely complicated by stenosis.76
LIMITATIONS OF ESD
ESD requires a high level of technical skill, is time-consuming, and has a higher rate of complications than conventional endoscopic resection. A standardized ESD training system is needed, as the procedure is more difficult than EMR. Training in porcine models has been shown to confer competency in ESD in a Western setting.13,16,33
Colorectal ESD is an even more challenging procedure, given the potential for complications related to its anatomy. Training centers in Japan usually have their trainees first master gastric ESD, then assist in more than 20 colorectal ESDs conducted by experienced endoscopists, and accomplish 30 cases before performing the procedure safely and independently.
As the incidence of gastric cancer is low in Western countries, trainees may also begin with lower rectal lesions, which are easier to remove.77 Incorporation of ESD in the West would require a clear treatment algorithm. It is a complex procedure, with higher rates of complications, a prolonged learning curve, and prolonged procedure time. Therefore, it should be performed in specialized centers and under the special situations discussed here to ensure that the benefits for the patients outweigh the risks.
VALUE OF ENDOSCOPIC SUBMUCOSAL DISSECTION
The optimal method for resecting gastrointestinal neoplasms should be safe, cost-effective, and quick and should also completely remove the lesion. The best treatment strategy takes into account the characteristics of the lesion and the comorbidities and wishes of the patient. Internists should be aware of the multiple options available to achieve the best outcome for the patient.1
Endoscopic resection of superficial gastrointestinal neoplasms, including EMR and ESD, has been a subject of increasing interest due to its minimally invasive and potentially curative character. However, cancer can recur after endoscopic resection because the procedure is organ-sparing.
ESD allows resection of early gastrointestinal tumors with a minimally invasive technique. It can achieve higher curative resection rates and lower recurrence rates compared with EMR. Compared with surgery, ESD leads to less morbidity, fewer procedure-related complications, and lower medical costs. Indications should be rigorously followed to achieve successful treatments in selected patients.
Multiple variables have to be taken into account when deciding which treatment is best, such as tumor characteristics, the patient’s baseline condition, physician expertise, and hospital resources.48 Less-invasive treatments may improve the prognosis of patients. No matter the approach, patients should be treated in specialized treatment centers.
Internal medicine physicians should be aware of the advances in treatments for early gastrointestinal cancer so appropriate options can be considered.
The treatment of early esophageal, gastric, and colorectal cancer is changing.1 For many years, surgery was the mainstay of treatment for early-stage gastrointestinal cancer. Unfortunately, surgery leads to significant loss of function of the organ, resulting in increased morbidity and decreased quality of life.2
Endoscopic techniques, particularly endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), have been developed and are widely used in Japan, where gastrointestinal cancer is more common than in the West. This article reviews the indications, complications, and outcomes of ESD for early gastrointestinal neoplasms, so that readers will recognize the subset of patients who would benefit from ESD in a Western setting.
ENDOSCOPIC MUCOSAL RESECTION AND SUBMUCOSAL DISSECTION
Since the first therapeutic polypectomy was performed in Japan in 1974, several endoscopic techniques for tumor resection have been developed.3
EMR, one of the most successful and widely used techniques, involves elevating the lesion either with submucosal injection of a solution or with cap suction, and then removing it with a snare.4 Most lesions smaller than 20 mm can be removed in one piece (en bloc).5 Larger lesions are removed in multiple pieces (ie, piecemeal). Unfortunately, some fibrotic lesions, which are usually difficult to lift, cannot be completely removed by EMR.
ESD was first performed in the late 1990s with the aim of overcoming the limitations of EMR in resecting large or fibrotic tumors en bloc.6,7 Since then, ESD technique has been standardized and training centers have been created, especially in Asia, where it is widely used for treatment of early gastric cancer.3,8–10 Since 2012 it has been covered by the Japanese National Health Insurance for treatment of early gastric cancer, and since 2014 for treatment of colorectal malignant tumors measuring 2 to 5 cm.11
Adoption of ESD has been slow in Western countries, where many patients are still referred for surgery or undergo EMR for removal of superficial neoplasms. Reasons for this slow adoption are that gastric cancer is much less common in Western countries, and also that ESD demands a high level of technical skill, is difficult to learn, and is expensive.3,12,13 However, small groups of Western endoscopists have become interested and are advocating it, first studying it on their own and then training in a Japanese center and learning from experts performing the procedure.
Therefore, in a Western setting, ESD should be performed in specialized endoscopy centers and offered to selected patients.1
CANDIDATES SHOULD HAVE EARLY-STAGE, SUPERFICIAL TUMORS
Ideal candidates for endoscopic resection are patients who have early cancer with a negligible risk of lymph node metastasis, such as cancer limited to the mucosa (stage T1a).7 Therefore, to determine the best treatment for a patient with a newly diagnosed gastrointestinal neoplasm, it is mandatory to estimate the depth of invasion.
The depth of invasion is directly correlated with lymph node involvement, which is ultimately the main predictive factor for long-term adverse outcomes of gastrointestinal tumors.4,14–17 Accurate multidisciplinary preprocedure estimations are mandatory, as incorrect evaluations may result in inappropriate therapy and residual cancer.18
Other factors that have been used to predict lymph node involvement include tumor size, macroscopic appearance, histologic differentiation, and lymphatic and vascular involvement.19 Some of these factors can be assessed by special endoscopic techniques (chromoendoscopy and narrow-band imaging with magnifying endoscopy) that allow accurate real-time estimation of the depth of invasion of the lesion.5,17,20–27 Evaluation of microsurface and microvascular arrangements is especially useful for determining the feasibility of ESD in gastric tumors, evaluation of intracapillary loops is useful in esophageal lesions, and assessment of mucosal pit patterns is useful for colorectal lesions.21–29
Endoscopic ultrasonography is another tool that has been used to estimate the depth of the tumor. Although it can differentiate between definite intramucosal and definite submucosal invasive cancers, its ability to confirm minute submucosal invasion is limited. Its use as the sole tumor staging modality is not encouraged, and it should always be used in conjunction with endoscopic evaluation.18
Though the aforementioned factors help stratify patients, pathologic staging is the best predictor of lymph node metastasis. ESD provides adequate specimens for accurate pathologic evaluation, as it removes lesions en bloc.30
All patients found to have risk factors for lymph node metastasis on endoscopic, ultrasonographic, or pathologic analysis should be referred for surgical evaluation.9,19,31,32
ENDOSCOPIC SUBMUCOSAL DISSECTION
Before the procedure, the patient’s physicians need to do the following:
Determine the best type of intervention (EMR, ESD, ablation, surgery) for the specific lesion.3 A multidisciplinary approach is encouraged, with involvement of the internist, gastroenterologist, and surgeon.
Plan for anesthesia, additional consultations, pre- and postprocedural hospital admission, and need for special equipment.33
During the procedure
Define the lateral extent of the lesion using magnification chromoendoscopy or narrow-band imaging. In the stomach, a biopsy sample should be taken from the worst-looking segment and from normal-looking mucosa. Multiple biopsies should be avoided to prevent subsequent fibrosis.33 In the colon, biopsy should be avoided.34
Identify and circumferentially mark the target lesion. Cautery or argon plasma coagulation can be used for making markings at a distance of 5 to 10 mm from the edges.33 This is done to recognize the borders of the lesion, because they can become distorted after submucosal injection.14 This step is unnecessary in colorectal cases, as tumor margins can be adequately visualized after chromoendoscopy.16,35
Lift the lesion by injecting saline, 0.5% hyaluronate, or glycerin to create a submucosal fluid cushion.19,33
Perform a circumferential incision lateral to the mucosal margins to allow for a normal tissue margin.33 Partial incision is performed for esophageal and colorectal ESD to avoid fluid leakage from the submucosal layer, achieving a sustained submucosal lift and safer dissection.16
Submucosal dissection. The submucosal layer is dissected with an electrocautery knife until the lesion is completely removed. Dissection should be done carefully to keep the submucosal plane.33 Hemoclips or hemostat forceps can be used to control visible bleeding. The resected specimen is then stretched and fixed to a board using small pins for further histopathologic evaluation.35
Postprocedural monitoring. All patients should be admitted for overnight observation. Those who undergo gastric ESD should receive high-dose acid suppression, and the next day they can be started on a liquid diet.19
STOMACH CANCER
Indications for ESD for stomach cancer in the East
The incidence of gastric cancer is higher in Japan and Korea, where widespread screening programs have led to early identification and early treatment of this disease.36
Pathology studies37 of samples from patients with gastric cancer identified the following as risk factors for lymph node metastasis, which would make ESD unsuitable:
- Undifferentiated type
- Tumors larger than 2 cm
- Lymphatic or venous involvement
- Submucosal invasion
- Ulcerative change.
Based on these findings, the situations in which there was no risk of lymph node involvement (ie, when none of the above factors are present) were accepted as absolute indications for endoscopic resection of early gastric cancer.38 Further histologic studies identified a subset of patients with lesions with very low risk of lymph node metastasis, which outweighed the risk of surgery. Based on these findings, expanded criteria for gastric ESD were proposed,39,40 and the Japanese gastric cancer treatment guidelines now include these expanded preoperative indications9,17 (Table 1).
The Japanese Gastric Cancer Association has proposed a treatment algorithm based on the histopathologic evaluation after resection (Figure 2).9
Outcomes
In the largest series of patients who underwent curative ESD for early gastric cancer, the 5-year survival rate was 92.6%, the 5-year disease-specific survival rate was 99.9%, and the 5-year relative survival rate was 105%.41
Similarly, in a Japanese population-based survival analysis, the relative 5-year survival rate for localized gastric cancer was 94.4%.42 Rates of en bloc resection and complete resection with ESD are higher than those with EMR, resulting in a lower risk of local recurrence in selected patients who undergo ESD.8,43,44
Although rare, local recurrence after curative gastric ESD has been reported.45 The annual incidence of local recurrence has been estimated to be 0.84%.46
ESD entails a shorter hospital stay and requires fewer resources than surgery, resulting in lower medical costs (Table 2).44 Additionally, as endoscopic resection is associated with less morbidity, fewer procedure-related adverse events, and fewer complications, ESD could be used as the standard treatment for early gastric cancer.47,48
The Western perspective on endoscopic submucosal dissection for gastric cancer
Since the prevalence of gastric cancer in Western countries is significantly lower than in Japan and Korea, local data and experience are scarce. However, experts performing ESD in the West have adopted the indications of the Japan Gastroenterological Endoscopy Society. The European Society of Gastrointestinal Endoscopy recommends ESD for excision of most superficial gastric neoplasms, with EMR being preferred only in lesions smaller than 15 mm, Paris classification 0 or IIA.5,32
Patients with gastric lesions measuring 15 mm or larger should undergo high-quality endoscopy, preferably chromoendoscopy, to evaluate the mucosal patterns and determine the depth of invasion. If superficial involvement is confirmed, other imaging techniques are not routinely recommended.5 A surgery consult is also recommended.
ESOPHAGEAL CANCER
Indications for ESD for esophageal cancer in the East
Due to the success of ESD for early gastric cancer, this technique is now also used for superficial esophageal neoplasms.19,49 It should be done in a specialized center, as it is more technically difficult than gastric ESD: the esophageal lumen is narrow, the wall is thin, and the esophagus moves with respiration and heartbeat.50 A multidisciplinary approach including an endoscopist, a surgeon, and a pathologist is highly recommended for evaluation and treatment.
EMR is preferred for removal of mucosal cancer, in view of its safety profile and success rates. ESD can be considered in cases of lesions larger than 15 mm, poorly lifting tumors, and those with the possibility of submucosal invasion (Table 3).5,45,49,51
Circumference involvement is critical when determining eligible candidates, as a defect involving more than three-fourths of the esophageal circumference can lead to esophageal strictures.52 Controlled prospective studies have shown promising results from giving intralesional and oral steroids to prevent stricture after ESD, which could potentially overcome this size limitation.53,54
Outcomes for esophageal cancer
ESD has been shown to be safe and effective, achieving en bloc resection in 85% to 100% of patients.19,51 Its advantages over EMR include en bloc resection, complete resection, and high curative rates, resulting in higher recurrence-free survival.2,55,56 Although the incidence of complications such as bleeding, perforation, and stricture formation are higher with ESD, patients usually recover uneventfully.2,19,20
ESD in the esophagus: The Western perspective
As data on the efficacy of EMR vs ESD for the treatment of Barrett esophagus with adenocarcinoma are limited, EMR is the gold standard endoscopic technique for removal of visible esophageal dysplastic lesions.5,51,57 ESD can be considered for tumors larger than 15 mm, for poorly lifting lesions, and if there is suspicion of submucosal invasion.5
Patients should be evaluated by an experienced endoscopist, using an advanced imaging technique such as narrow-band imaging or chromoendoscopy. If suspicious features are found, endoscopic ultrasonography should be considered to confirm submucosal invasion or lymph node involvement.5
COLORECTAL CANCER
Indications for ESD for colorectal cancer in the East
Colon cancer is one of the leading causes of cancer-related deaths worldwide.58 Since ESD has been found to be effective and safe in treating gastric cancer, it has also been used to remove large colorectal tumors.59 However, ESD is not universally accepted in the treatment of colorectal neoplasms due to its greater technical difficulty, longer procedural time, and higher risk of perforating the thinner colonic wall compared with EMR.21,60
Outcomes for colorectal cancer
Tumor size of 50 mm or larger is a risk factor for complications, while a high procedure volume at the center is a protective factor.60
Endoscopic treatment of colorectal cancer: The Western perspective
EMR is the gold standard for removal of superficial colorectal lesions. However, ESD can be considered if there is suspicion of superficial submucosal invasion, especially for lesions larger than 20 mm that cannot be resected en bloc by EMR.32 ESD can also be used for fibrotic lesions not amenable to complete EMR removal, or as a salvage procedure after recurrence after EMR.67 Proper selection of cases is critical.1
Patients who have a superficial colonic lesion should be evaluated by means of high-definition endoscopy and chromoendoscopy to assess the mucosal pattern and establish feasibility of endoscopic resection. If submucosal invasion is suspected, staging with endoscopic ultrasonography or magnetic resonance imaging should be considered.5
FOLLOW-UP AFTER ESD
Endoscopic surveillance after the procedure is recommended, given the persistent risk of metachronous cancer after curative ESD due to its organ-sparing quality.68 Surveillance endoscopy aims to achieve early detection and subsequent endoscopic resection of metachronous lesions.
Histopathologic evaluation assessing the presence of malignant cells in the margins of a resected sample is mandatory for determining the next step in treatment. If margins are negative, follow-up endoscopy can be done every 6 to 12 months. If margins are positive, the approach includes surgery, reattempting ESD or endoscopic surveillance in 3 or 6 months.3,32 Although the surveillance strategy varies according to individual risk of metachronous cancer, it should be continued indefinitely.68
COMPLICATIONS OF ESD
The most common procedure-related complications of ESD are bleeding, perforation, and stricture. Most intraprocedural adverse events can be managed endoscopically.69
Bleeding
Most bleeding occurs during the procedure or early after it and can be controlled with electrocautery.49,69 No episodes of massive bleeding, defined as causing clinical symptoms and requiring transfusion or surgery, have been reported.20,43,55
In gastric ESD, delayed bleeding rates have ranged from 0 to 15.6%.69 Bleeding may be prevented with endoscopic coagulation of visible vessels after dissection has been completed and by proton pump inhibitor therapy.70,71 Excessive coagulation should be avoided to lower the risk of perforation.33
In colorectal ESD the bleeding rate has been reported to be 2.2%; applying coagulation to an area where a blood vessel is suspected before cutting (precoagulation) may prevent subsequent bleeding.21
Perforation
For gastric ESD, perforation rates range from 1.2% to 5.2%.69 Esophageal perforation rates can be up to 4%.49 In colorectal ESD, perforation rates have been reported to be 1.6% to 6.6%.60,72
Although most of the cases were successfully managed with conservative treatment, some required emergency surgery.60,73
Strictures
In a case series of 532 patients undergoing gastric ESD, stricture was reported in 5 patients, all of whom presented with obstructive symptoms.74 Risk factors for post-ESD gastric stenosis are a mucosal defect with a circumferential extent of more than three-fourths or a longitudinal extent of more than 5 cm.75
Strictures are common after esophageal ESD, with rates ranging from 2% to 26%. The risk is higher when longer segments are removed or circumferential resection is performed. As previously mentioned, this complication may be reduced with ingestion or injection of steroids after the procedure.53,54
Surprisingly, ESD of large colorectal lesions involving more than three-fourths of the circumference of the rectum is rarely complicated by stenosis.76
LIMITATIONS OF ESD
ESD requires a high level of technical skill, is time-consuming, and has a higher rate of complications than conventional endoscopic resection. A standardized ESD training system is needed, as the procedure is more difficult than EMR. Training in porcine models has been shown to confer competency in ESD in a Western setting.13,16,33
Colorectal ESD is an even more challenging procedure, given the potential for complications related to its anatomy. Training centers in Japan usually have their trainees first master gastric ESD, then assist in more than 20 colorectal ESDs conducted by experienced endoscopists, and accomplish 30 cases before performing the procedure safely and independently.
As the incidence of gastric cancer is low in Western countries, trainees may also begin with lower rectal lesions, which are easier to remove.77 Incorporation of ESD in the West would require a clear treatment algorithm. It is a complex procedure, with higher rates of complications, a prolonged learning curve, and prolonged procedure time. Therefore, it should be performed in specialized centers and under the special situations discussed here to ensure that the benefits for the patients outweigh the risks.
VALUE OF ENDOSCOPIC SUBMUCOSAL DISSECTION
The optimal method for resecting gastrointestinal neoplasms should be safe, cost-effective, and quick and should also completely remove the lesion. The best treatment strategy takes into account the characteristics of the lesion and the comorbidities and wishes of the patient. Internists should be aware of the multiple options available to achieve the best outcome for the patient.1
Endoscopic resection of superficial gastrointestinal neoplasms, including EMR and ESD, has been a subject of increasing interest due to its minimally invasive and potentially curative character. However, cancer can recur after endoscopic resection because the procedure is organ-sparing.
ESD allows resection of early gastrointestinal tumors with a minimally invasive technique. It can achieve higher curative resection rates and lower recurrence rates compared with EMR. Compared with surgery, ESD leads to less morbidity, fewer procedure-related complications, and lower medical costs. Indications should be rigorously followed to achieve successful treatments in selected patients.
Multiple variables have to be taken into account when deciding which treatment is best, such as tumor characteristics, the patient’s baseline condition, physician expertise, and hospital resources.48 Less-invasive treatments may improve the prognosis of patients. No matter the approach, patients should be treated in specialized treatment centers.
Internal medicine physicians should be aware of the advances in treatments for early gastrointestinal cancer so appropriate options can be considered.
- Burgess NG, Bourke MJ. Endoscopic resection of colorectal lesions: the narrowing divide between East and West. Dig Endosc 2016; 28:296–305.
- Kim DH, Jung HY, Gong EJ, et al. Endoscopic and oncologic outcomes of endoscopic resection for superficial esophageal neoplasm. Gut Liver 2015; 9:470–477.
- Draganov PV, Gotoda T, Chavalitdhamrong D, Wallace MB. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013; 78:677–688.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47:829–854.
- Farhat S, Chaussade S, Ponchon T, et al; SFED ESD Study Group. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011; 43:664–670.
- Gotoda T, Jung H. Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig Endosc 2013; 25(suppl 1):55–63.
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009; 69:1228–1235.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123.
- Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005; 6:119–121.
- Watanabe T, Itabashi M, Shimada Y, et al; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20:207–239.
- Oyama T, Yahagi N, Ponchon T, Kiesslich T, Berr F. How to establish endoscopic submucosal dissection in Western countries. World J Gastroenterol 2015; 21:11209–11220.
- Bhatt A, Abe S, Kumaravel A, et al. SU1575 Western skill training in endoscopic submucosal dissection (ESD)—an international remote video based study—the WEST ESD Study. Gastrointest Endosc 2015; 81(suppl):AB335–AB336.
- Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer follow-up of 1475 patients and review of the Japanese literature. Cancer 1993; 72:3174–3178.
- Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus 2009; 6:1–25.
- Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol 2015; 110:784–791.
- Eleftheriadis N, Inoue H, Ikeda H, et al. Definition and staging of early esophageal, gastric and colorectal cancer. J Tumor 2014; 2:161–178.
- Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc 2012; 4:218–226.
- Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi51–vi56.
- Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc 2013; 78:704–710.
- Sakamoto T, Mori G, Yamada M, et al. Endoscopic submucosal dissection for colorectal neoplasms: a review. World J Gastroenterol 2014; 20:16153–16158.
- Ohta A, Tominaga K, Sakai Y. Efficacy of magnifying colonoscopy for the diagnosis of colorectal neoplasia: comparison with histopathological findings. Dig Endosc 2004; 16:308–314.
- Katagiri A, Fu K, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther 2008; 27:1269–1274.
- Fu KI, Kato S, Sano Y, et al. Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci 2008; 53:1886–1892.
- Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc 2011; 23(suppl 1):112–115.
- Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol 2010;10:33.
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008; 103:2700–2706.
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015; 8:122–128.
- Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc 2016; 28:379–393.
- Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi57–vi63.
- Kuwano H, Nishimura Y, Ohtsu A, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus. April 2007 edition: part I - Edited by the Japan Esophageal Society. Esophagus 2008; 5:61–73.
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015; 27:417–434.
- Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin North Am 2014; 24:213–233.
- Saito Y, Sakamoto T, Nakajima T, Matsuda T. Colorectal ESD: current indications and latest technical advances. Gastrointest Endosc Clin N Am 2014; 24:245–255.
- Saito Y, Otake Y, Sakamoto T, et al. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver 2013; 7:263–269.
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 2015; 12:355–361.
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999; 125:148–154.
- Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003; 57:567–579.
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12:148–152.
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225.
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016; 19:198–205.
- Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T; Research Group of Population-Based Cancer Registries of Japan. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011; 41:40–51.
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011; 74:485–493.
- Kim Y, Kim YW, Choi IJ, et al. Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea. Gut Liver 2015; 9:174–180.
- Abe S, Oda I, Nakajima T, et al. A case of local recurrence and distant metastasis following curative endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2015; 18:188–192.
- Hahn KY, Park JC, Kim EH, et al. Incidence and impact of scheduled endoscopic surveillance on recurrence after curative endoscopic resection for early gastric cancer. Gastrointest Endosc 2016; 84:628–638.e1.
- Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One 2015; 10:e0144774.
- Kondo A, de Moura EG, Bernardo WM, et al. Endoscopy vs surgery in the treatment of early gastric cancer: systematic review. World J Gastroenterol 2015; 21:13177–13187.
- Kothari S, Kaul V. Endoscopic mucosal resection and endoscopic submucosal dissection for endoscopic therapy of Barrett’s esophagus-related neoplasia. Gastroenterol Clin North Am 2015; 44:317–335.
- Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011; 25:2541–2546.
- Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett’s adenocarcinoma. Gastrointest Endosc 2014; 80:239–245.
- Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003; 57:165–169.
- Hanaoka N, Ishihara R, Takeuchi Y, et al. 1139: A single session of intralesional steroid injection to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Gastrointest Endosc 2012; 75(suppl):AB175.
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011; 73:1115–1121.
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70:860–866.
- Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc 2005; 61:219–225.
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010; 72:960–966.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013; 27:3262–3770.
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010; 72:1217–1225.
- Tanaka S, Saitoh Y, Matsuda T, et al; Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol 2015; 50:252–260.
- Oka S, Tanaka S, Saito Y, et al; Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015; 110:697–707.
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010; 24:343–352.
- Makazu M, Sakamoto T, So E, et al. Relationship between indeterminate or positive lateral margin and local recurrence after endoscopic resection of colorectal polyps. Endosc Int Open 2015; 3:E252–E257.
- Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014; 46:388–402.
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015; 81:583–595.
- Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015; 16:14–21.
- Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015; 47:1113–1118.
- Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013; 25(suppl 1):71–78.
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection—an analysis of risk factors. Endoscopy 2008; 40:179–183.
- Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007; 102:1610–1616.
- Hayashi N, Tanaka S, Nishiyama S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2014; 79:427–435.
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015; 21:12635–12643.
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008; 67:979–983.
- Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009; 41:421–426.
- Abe S, Sakamoto T, Takamaru H, et al. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc 2016; 30:5459–5464.
- Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 2011; 54:1307–1312.
- Burgess NG, Bourke MJ. Endoscopic resection of colorectal lesions: the narrowing divide between East and West. Dig Endosc 2016; 28:296–305.
- Kim DH, Jung HY, Gong EJ, et al. Endoscopic and oncologic outcomes of endoscopic resection for superficial esophageal neoplasm. Gut Liver 2015; 9:470–477.
- Draganov PV, Gotoda T, Chavalitdhamrong D, Wallace MB. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013; 78:677–688.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47:829–854.
- Farhat S, Chaussade S, Ponchon T, et al; SFED ESD Study Group. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011; 43:664–670.
- Gotoda T, Jung H. Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig Endosc 2013; 25(suppl 1):55–63.
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009; 69:1228–1235.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123.
- Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005; 6:119–121.
- Watanabe T, Itabashi M, Shimada Y, et al; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20:207–239.
- Oyama T, Yahagi N, Ponchon T, Kiesslich T, Berr F. How to establish endoscopic submucosal dissection in Western countries. World J Gastroenterol 2015; 21:11209–11220.
- Bhatt A, Abe S, Kumaravel A, et al. SU1575 Western skill training in endoscopic submucosal dissection (ESD)—an international remote video based study—the WEST ESD Study. Gastrointest Endosc 2015; 81(suppl):AB335–AB336.
- Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer follow-up of 1475 patients and review of the Japanese literature. Cancer 1993; 72:3174–3178.
- Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus 2009; 6:1–25.
- Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol 2015; 110:784–791.
- Eleftheriadis N, Inoue H, Ikeda H, et al. Definition and staging of early esophageal, gastric and colorectal cancer. J Tumor 2014; 2:161–178.
- Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc 2012; 4:218–226.
- Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi51–vi56.
- Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc 2013; 78:704–710.
- Sakamoto T, Mori G, Yamada M, et al. Endoscopic submucosal dissection for colorectal neoplasms: a review. World J Gastroenterol 2014; 20:16153–16158.
- Ohta A, Tominaga K, Sakai Y. Efficacy of magnifying colonoscopy for the diagnosis of colorectal neoplasia: comparison with histopathological findings. Dig Endosc 2004; 16:308–314.
- Katagiri A, Fu K, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther 2008; 27:1269–1274.
- Fu KI, Kato S, Sano Y, et al. Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci 2008; 53:1886–1892.
- Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc 2011; 23(suppl 1):112–115.
- Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol 2010;10:33.
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008; 103:2700–2706.
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015; 8:122–128.
- Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc 2016; 28:379–393.
- Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi57–vi63.
- Kuwano H, Nishimura Y, Ohtsu A, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus. April 2007 edition: part I - Edited by the Japan Esophageal Society. Esophagus 2008; 5:61–73.
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015; 27:417–434.
- Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin North Am 2014; 24:213–233.
- Saito Y, Sakamoto T, Nakajima T, Matsuda T. Colorectal ESD: current indications and latest technical advances. Gastrointest Endosc Clin N Am 2014; 24:245–255.
- Saito Y, Otake Y, Sakamoto T, et al. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver 2013; 7:263–269.
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 2015; 12:355–361.
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999; 125:148–154.
- Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003; 57:567–579.
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12:148–152.
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225.
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016; 19:198–205.
- Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T; Research Group of Population-Based Cancer Registries of Japan. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011; 41:40–51.
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011; 74:485–493.
- Kim Y, Kim YW, Choi IJ, et al. Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea. Gut Liver 2015; 9:174–180.
- Abe S, Oda I, Nakajima T, et al. A case of local recurrence and distant metastasis following curative endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2015; 18:188–192.
- Hahn KY, Park JC, Kim EH, et al. Incidence and impact of scheduled endoscopic surveillance on recurrence after curative endoscopic resection for early gastric cancer. Gastrointest Endosc 2016; 84:628–638.e1.
- Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One 2015; 10:e0144774.
- Kondo A, de Moura EG, Bernardo WM, et al. Endoscopy vs surgery in the treatment of early gastric cancer: systematic review. World J Gastroenterol 2015; 21:13177–13187.
- Kothari S, Kaul V. Endoscopic mucosal resection and endoscopic submucosal dissection for endoscopic therapy of Barrett’s esophagus-related neoplasia. Gastroenterol Clin North Am 2015; 44:317–335.
- Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011; 25:2541–2546.
- Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett’s adenocarcinoma. Gastrointest Endosc 2014; 80:239–245.
- Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003; 57:165–169.
- Hanaoka N, Ishihara R, Takeuchi Y, et al. 1139: A single session of intralesional steroid injection to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Gastrointest Endosc 2012; 75(suppl):AB175.
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011; 73:1115–1121.
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70:860–866.
- Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc 2005; 61:219–225.
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010; 72:960–966.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013; 27:3262–3770.
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010; 72:1217–1225.
- Tanaka S, Saitoh Y, Matsuda T, et al; Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol 2015; 50:252–260.
- Oka S, Tanaka S, Saito Y, et al; Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015; 110:697–707.
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010; 24:343–352.
- Makazu M, Sakamoto T, So E, et al. Relationship between indeterminate or positive lateral margin and local recurrence after endoscopic resection of colorectal polyps. Endosc Int Open 2015; 3:E252–E257.
- Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014; 46:388–402.
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015; 81:583–595.
- Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015; 16:14–21.
- Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015; 47:1113–1118.
- Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013; 25(suppl 1):71–78.
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection—an analysis of risk factors. Endoscopy 2008; 40:179–183.
- Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007; 102:1610–1616.
- Hayashi N, Tanaka S, Nishiyama S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2014; 79:427–435.
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015; 21:12635–12643.
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008; 67:979–983.
- Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009; 41:421–426.
- Abe S, Sakamoto T, Takamaru H, et al. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc 2016; 30:5459–5464.
- Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 2011; 54:1307–1312.
KEY POINTS
- ESD is a minimally invasive endoscopic technique with curative potential for patients with superficial GI neoplasia.
- ESD preserves the integrity of the organ while achieving curative resection of large neoplasms.
- ESD is indicated rather than surgery in patients with early GI lesions with a negligible risk of lymph node metastasis.
- Complications of the procedure include bleeding, perforation, and stenosis. Most of these respond to endoscopic treatment.
- Successful ESD requires supportive teamwork among internists, gastroenterologists, pathologists, and surgeons.
The Courvoisier sign
A 60-year-old woman has had jaundice, dark-colored urine, and light-colored stools for the past several days. She has no history of jaundice or gallstone disease.
Over a century ago, Courvoisier observed that a palpable gallbladder in a patient with obstructive jaundice is often caused by a non-calculus abnormality of the biliary system, such as pancreatic cancer or cholangiocarcinoma, distal to the insertion of the cystic duct.1–4 He attributed his findings to a higher likelihood of fibrosis of the gallbladder, with stone disease rendering it less distensible.4
Although often associated with malignancy, the Courvoisier sign can also be seen in benign processes causing obstruction of the common bile duct.5
For decades after its initial description, the Courvoisier sign was used as an important sign for the differential diagnosis of jaundice, but advances in diagnostic imaging have led to a more accurate and earlier diagnosis with less reliance on this sign. In this patient, tissue diagnosis confirmed a clinical suspicion of pancreatic adenocarcinoma.
- Anonymous. Ludwig Courvoisier (1843–1918): Courvoisier’s sign. JAMA 1968; 204:627.
- Chung RS. Pathogenesis of the “Courvoisier gallbladder.” Dig Dis Sci 1983; 28:33–38.
- Watts GT. Courvoisier’s law. Lancet 1985; 2:1293–1294.
- Fitzgerald JE, White MJ, Lobo DN. Courvoisier’s gallbladder: law or sign? World J Surg 2009; 33:886–891.
- Parmar MS. Courvoisier’s law. CMAJ 2003; 168:876–877.
A 60-year-old woman has had jaundice, dark-colored urine, and light-colored stools for the past several days. She has no history of jaundice or gallstone disease.
Over a century ago, Courvoisier observed that a palpable gallbladder in a patient with obstructive jaundice is often caused by a non-calculus abnormality of the biliary system, such as pancreatic cancer or cholangiocarcinoma, distal to the insertion of the cystic duct.1–4 He attributed his findings to a higher likelihood of fibrosis of the gallbladder, with stone disease rendering it less distensible.4
Although often associated with malignancy, the Courvoisier sign can also be seen in benign processes causing obstruction of the common bile duct.5
For decades after its initial description, the Courvoisier sign was used as an important sign for the differential diagnosis of jaundice, but advances in diagnostic imaging have led to a more accurate and earlier diagnosis with less reliance on this sign. In this patient, tissue diagnosis confirmed a clinical suspicion of pancreatic adenocarcinoma.
A 60-year-old woman has had jaundice, dark-colored urine, and light-colored stools for the past several days. She has no history of jaundice or gallstone disease.
Over a century ago, Courvoisier observed that a palpable gallbladder in a patient with obstructive jaundice is often caused by a non-calculus abnormality of the biliary system, such as pancreatic cancer or cholangiocarcinoma, distal to the insertion of the cystic duct.1–4 He attributed his findings to a higher likelihood of fibrosis of the gallbladder, with stone disease rendering it less distensible.4
Although often associated with malignancy, the Courvoisier sign can also be seen in benign processes causing obstruction of the common bile duct.5
For decades after its initial description, the Courvoisier sign was used as an important sign for the differential diagnosis of jaundice, but advances in diagnostic imaging have led to a more accurate and earlier diagnosis with less reliance on this sign. In this patient, tissue diagnosis confirmed a clinical suspicion of pancreatic adenocarcinoma.
- Anonymous. Ludwig Courvoisier (1843–1918): Courvoisier’s sign. JAMA 1968; 204:627.
- Chung RS. Pathogenesis of the “Courvoisier gallbladder.” Dig Dis Sci 1983; 28:33–38.
- Watts GT. Courvoisier’s law. Lancet 1985; 2:1293–1294.
- Fitzgerald JE, White MJ, Lobo DN. Courvoisier’s gallbladder: law or sign? World J Surg 2009; 33:886–891.
- Parmar MS. Courvoisier’s law. CMAJ 2003; 168:876–877.
- Anonymous. Ludwig Courvoisier (1843–1918): Courvoisier’s sign. JAMA 1968; 204:627.
- Chung RS. Pathogenesis of the “Courvoisier gallbladder.” Dig Dis Sci 1983; 28:33–38.
- Watts GT. Courvoisier’s law. Lancet 1985; 2:1293–1294.
- Fitzgerald JE, White MJ, Lobo DN. Courvoisier’s gallbladder: law or sign? World J Surg 2009; 33:886–891.
- Parmar MS. Courvoisier’s law. CMAJ 2003; 168:876–877.
Acute pancreatitis: Problems in adherence to guidelines
Several major gastroenterological and surgical societies have issued guidelines on how to manage acute pancreatitis, based on evidence from high-quality randomized trials and nonrandomized studies as well as on expert opinion.1–3 Information is limited on how well physicians in the United States comply with these guidelines, but compliance is suboptimal in other developed countries, according to several studies,4–8 and we suspect that many US physicians are not following the guidelines either.
Acute pancreatitis is a frequent inpatient diagnosis that internists, gastroenterologists, and surgeons all confront. The most common causes are gallstones and heavy alcohol intake. Its management is typically straightforward: intravenous fluids, analgesia, and nothing by mouth. However, treatment of severe cases can be quite complex, particularly if multiple organ systems are involved or if there are local complications.
The primary aim of this article is to raise awareness of recognized deviations from established recommendations that may lead to adverse patient outcomes.
MEASURING ENZYME LEVELS DAILY ADDS COST BUT LITTLE BENEFIT
Problem: Serum amylase and lipase levels are often needlessly measured every day.
Measuring the serum amylase and lipase levels is useful in diagnosing acute pancreatitis, which requires two of the following three features1:
- Characteristic abdominal pain
- Levels of serum amylase or serum lipase, or both, that are three or more times the upper limit of normal
- Findings of acute pancreatitis on computed tomography (CT).
However, the magnitude or duration of the serum enzyme elevation does not correlate with the severity of the attack. Further, we have noticed that physicians at our hospital often order daily serum amylase and lipase levels in patients admitted with acute pancreatitis.
The American College of Gastroenterology (ACG) guidelines1 state that daily monitoring of amylase and lipase has limited value in managing acute pancreatitis. Rechecking these concentrations may be reasonable if pain fails to resolve or worsens during a prolonged hospitalization, as this may suggest a recurrent attack of acute pancreatitis or a developing pseudocyst. But in most cases of acute pancreatitis, daily serum enzyme measurements add cost but little benefit.
REGULAR ASSESSMENT IS IMPORTANT
Problem: Often, severity assessments are not performed regularly or acted on.
Most cases of acute pancreatitis are mild, with rapid recovery and excellent prognosis. However, 15% to 20% are severe and may result in a prolonged hospitalization, systemic inflammatory response syndrome (SIRS), multiorgan system failure, and death.
In severe acute pancreatitis, as pancreatic enzymes and inflammatory cytokines damage the blood vessels, a vast amount of fluid leaks out into the interstitial (“third”) space. This fluid extravasation leads to decreased effective circulating volume, local pancreatic necrosis, hemodynamic instability, and end-organ failure.
It is important to recognize severe acute pancreatitis early because the patient needs to be transferred to a step-down unit or intensive care unit to receive optimal fluid resuscitation and supportive care for organ dysfunction. After 48 to 72 hours, a prediction of severe acute pancreatitis should also prompt the physician to order CT to detect pancreatic necrosis, and also to initiate nutritional support.
Assessment of severity begins in the emergency room or on admission to the hospital. Older age, obesity, organ failure, and pulmonary infiltrates or pleural effusions are initial indicators of poor prognosis. Signs of SIRS (high or low core body temperature, tachycardia, tachypnea, low or high peripheral white blood cell count) or organ failure (eg, elevated serum creatinine) are present on admission in 21% of patients with acute pancreatitis.9
Hemoconcentration is a marker of decreased effective circulating volume in severe acute pancreatitis. A hematocrit higher than 44% at admission or that rises in the first 24 to 48 hours of admission predicts necrosis.10,11 However, a more robust marker of organ failure may be the blood urea nitrogen level.12
Clinical scoring systems
Several clinical scoring systems have been studied for assessing severity.
Limitations of the Ranson score are that it can only be completed after 48 hours, all the data points are not always obtained, and it cannot be repeated on a daily basis. Owing to these limitations and its less-than-optimal predictive value, the Ranson score has fallen into disuse.
The APACHE II (Acute Physiology and Chronic Health Evaluation II) score is more versatile. It is based on multiple clinical and laboratory values, and it correlates very well with the risk of death in acute pancreatitis. Death rates are less than 4% when the APACHE II score is less than 8, and 11% to 18% when it is 8 or higher.1 The trajectory of the APACHE II score in the first 48 hours is also an accurate prognostic indicator.
Previous limitations of the APACHE II score were that it was complicated and timeconsuming to calculate and required arterial blood gas measurements. Easy-to-use online calculators are now available (eg, www.globalrph.com/apacheii.htm), and the venous bicarbonate level and the oxygen saturation can be substituted for the arterial pH and oxygen partial pressure.
BISAP, a new five-point scoring system,15 was recently prospectively validated.12 “BISAP” is an acronym for the five markers it is based on, each of which has been shown to predict severe illness in acute pancreatitis:
- Blood urea nitrogen level > 25 mg/dL
- Impaired mental status
- SIRS
- Age > 60 years
- Pleural effusion.
The presence of three or more of these factors correlates with higher risk of death, organ failure, and pancreatic necrosis.12
Compared with APACHE II, BISAP has similar accuracy and is easier to calculate. Also, BISAP was specifically developed for acute pancreatitis, whereas APACHE II is a generic score for all critically ill patients.
The Atlanta criteria16 define severe acute pancreatitis as one or more of the following:
- A Ranson score of 3 or higher during the first 48 hours
- An APACHE II score of 8 or higher at any time
- Failure of one or more organs
- One or more local complications (eg, necrosis, pseudocysts, abscesses).
Recommendation: Assess severity at least daily
A severity assessment should be performed at admission and at least every day thereafter. Clinical guidelines recognize the importance of severity assessment but vary in their specific recommendations.
The ACG advises calculating the APACHE II score within 3 days of admission and measuring the hematocrit at admission, at 12 hours, and at 24 hours. The level of evidence is III, ie, “from published well-designed trials without randomization, single group prepost, cohort, time series, or matched case controlled studies”.1
The American Gastroenterological Association (AGA) provides a more generalized recommendation, that “clinical judgment” should take into account the presence of risk factors (eg, age, obesity), presence or absence of SIRS, routine laboratory values (eg, hematocrit, serum creatinine), and APACHE II score when assessing severity and making decisions.2
In a German survey, only 32% of gastroenterologists used the APACHE II score for assessing risk in acute pancreatitis, in spite of national guidelines emphasizing its importance.7 Also, not all patients with severe acute pancreatitis are transferred to a step-down unit or intensive care unit as recommended. In a British study,4 only 8 (17%) of 46 patients with predicted severe acute pancreatitis were transferred, and 8 of the 38 patients who were not transferred died.
FLUID MUST BE AGGRESSIVELY REPLACED AND MONITORED
Problem: Often, not enough fluid is replaced, or fluid status is not adequately monitored.
Fluid must be aggressively replaced to balance the massive third-space fluid losses that occur in the early inflammatory phase of acute pancreatitis. Intravascular volume depletion can develop rapidly and result in tachycardia, hypotension, and renal failure. It may also impair the blood flow to the pancreas and worsen necrosis.
Animal studies show that aggressive fluid replacement supports the pancreatic microcirculation and prevents necrosis.17 It may also support the intestinal microcirculation and gut barrier, preventing bacterial translocation.
In humans, no controlled trials have been done to test the efficacy of aggressive fluid resuscitation in acute pancreatitis. However, the notion that intravascular fluid loss contributes to poor outcomes is inferred from human studies showing more necrosis and deaths in patients with hemoconcentration. In one study, patients who received inadequate fluid replacement (evidenced by a rise in hematocrit at 24 hours) were more likely to develop necrotizing pancreatitis.18
Recommendation: Early, aggressive fluid replacement
Experts have suggested initially infusing 500 to 1,000 mL of fluid per hour in those who are volume-depleted, initially infusing 250 to 350 mL per hour in those who are not volumedepleted, and adjusting the fluid rate every 1 to 4 hours on the basis of clinical variables.19 The sufficiency of fluid replacement should be carefully monitored by vital signs, urine output, and serum hematocrit.
On the other hand, overly aggressive fluid resuscitation can be detrimental in patients at risk of volume overload or pulmonary edema. Fluid replacement should be tempered in elderly patients and those with cardiac or renal comorbidities, and may require monitoring of central venous pressure.
The ACG and AGA guidelines both recognize the need for early aggressive volume replacement in acute pancreatitis (level of evidence III), but they do not specify the exact amounts and rates. Young and healthy patients should receive a rapid bolus of isotonic saline or Ringer’s lactate solution followed by an infusion at a high initial maintenance rate.
Few studies have been done to assess physicians’ compliance with recommendations for aggressive volume replacement. In an Italian multicenter study, patients with mild or severe acute pancreatitis received an average of only 2.5 L of fluid per day (about 100 mL/hour).20 Gardner et al21 recently summarized the available evidence for fluid support in acute pancreatitis.
NUTRITIONAL SUPPORT
Problem: In many severe cases, enteral or parenteral feeding is not started soon enough.
Nutritional support entails enteral or parenteral feeding when an oral diet is contraindicated. Enteral feeding is usually via a nasojejunal tube, which may need to be placed under endoscopic or radiographic guidance. Neither parenteral nor nasojejunal feeding stimulates pancreatic secretion, and both are safe in acute pancreatitis.
Severe acute pancreatitis is an intensely catabolic state characterized by increased energy expenditure, protein breakdown, and substrate utilization. Patients may not be able to resume an oral diet for weeks or even months, particularly if local complications develop. Early nutritional support has been shown to improve outcomes in severe acute pancreatitis.22 Therefore, nutritional support should be started as soon as possible in severe acute pancreatitis based on initial clinical and radiographic indicators of severity, optimally within the first 2 or 3 days.
Enteral nutrition is preferred to parenteral nutrition in pancreatitis: it is less expensive and does not pose a risk of catheter-related infection or thrombosis or hepatic complications. Also, there is experimental evidence that enteral nutrition may preserve the gut barrier, decreasing mucosal permeability and bacterial translocation.
A number of small randomized trials compared enteral and parenteral nutrition in acute pancreatitis, but they yielded mixed results. A meta-analysis of six trials showed a lower rate of infectious complications with enteral than with parenteral nutrition. 23 However, no significant difference was found in the rates of death or noninfectious complications.
Recommendation: Enteral feeding, when possible
Nutritional support is unnecessary in most cases of mild acute pancreatitis. Pancreatic inflammation typically resolves within a few days, allowing patients to resume eating. Occasionally, patients in whom pain resolves slowly and who fast for more than 5 to 7 days need nutritional support to prevent proteincalorie malnutrition.
The ACG guidelines1 and most others suggest that, whenever possible, enteral rather than parenteral feeding should be given to those who require nutritional support. The level of evidence is II (“strong evidence from at least one published properly designed randomized controlled trial of appropriate size and in an appropriate clinical setting”).
However, not all physicians recognize the benefit of enteral feeding. In a cohort of German gastroenterologists, only 73% favored enteral over parenteral feeding in acute pancreatitis.7
COMPUTED TOMOGRAPHY
Problem: CT is not done in many patients with severe acute pancreatitis, or it is done too soon during the admission.
Dual-phase, contrast-enhanced, pancreatic-protocol CT provides a sensitive structural evaluation of the pancreas and is useful to diagnose necrotizing pancreatitis. Pancreatic necrosis is correlated with a severe clinical course, the development of single or multiorgan dysfunction, and death.
Recommendation: CT in severe cases
Not every patient with acute pancreatitis needs to undergo CT. Most mild cases do not require routine CT, since necrosis and other local complications are infrequent in this group.
Also, CT is often ordered too soon during the hospitalization. Indicators of severity on CT are not usually evident until 2 to 3 days after admission.25 CT should be considered about 3 days after the onset of symptoms rather than immediately upon admission.
On the other hand, CT at the time of admission may be warranted to rule out other life-threatening causes of abdominal pain and hyperamylasemia (eg, bowel obstruction, viscus perforation). CT may also be useful in the late phase of acute pancreatitis (weeks after admission) to diagnose or monitor complications (eg, pseudocysts, abscesses, splenic vein thrombosis, splenic artery pseudoaneurysms). Magnetic resonance imaging with gadolinium contrast is a reasonable alternative to CT for detecting pancreatic necrosis and other local complications.
In patients who have severe acute pancreatitis and compromised renal function (serum creatinine > 1.5 mg/dL), CT can be performed without contrast to assess severity based on a limited Balthazar score (ie, without a necrosis score). Studies in rats suggest that iodinated contrast may decrease pancreatic microcirculation and worsen or precipitate necrosis,26 although published human studies do not support this contention.27,28
Guidelines uniformly recommend CT for patients with severe acute pancreatitis (the ACG guideline gives it a level of evidence of III), but this recommendation is not always followed. A study from Australia showed that CT was done in only 27% to 67% of patients with severe acute pancreatitis.5 In a British study, only 8 of 46 patients with clinically predicted severe pancreatitis underwent CT within the first 10 days of admission.4
SUSPECTED INFECTED NECROSIS
Problem: Fine-needle aspiration is not done in many cases of suspected infected necrosis.
Approximately one-third of patients with necrotizing pancreatitis develop infected necrosis. The death rate for patients with infected pancreatic necrosis is high—30%, compared with 12% in those with sterile necrosis.1 Differentiating sterile and infected necrosis is therefore essential.
Clinical signs such as fever are poor predictors of infection. Signs of SIRS can be present in both sterile and infected necrotizing pancreatitis.
Recommendation: Fine-needle aspiration of necrosis
For the reasons given above, the findings of necrosis on CT and persistent SIRS should prompt consideration of fine-needle aspiration with Gram stain and culture to differentiate sterile and infected necrosis (ACG guideline, level of evidence III).1 If infection is confirmed, surgical debridement should be strongly considered. Other less-invasive approaches such as endoscopic debridement can be used in selected cases.
Fine-needle aspiration of necrosis is too often neglected. In a cohort of German surgeons, only 55% complied with International Association of Pancreatology recommendations to perform biopsy to differentiate sterile from infected necrosis in patients with signs of sepsis.29
BROAD-SPECTRUM ANTIBIOTICS
Problem: Broad-spectrum antibiotics are often used inappropriately in patients with mild acute pancreatitis and in patients with sterile necrotizing pancreatitis who are clinically stable and have no signs of sepsis.
Antibiotics are not indicated in mild acute pancreatitis. A limited course of antibiotics is typically indicated in severe cases with suspected or proven infected necrosis (in conjunction with surgical necrosectomy). However, the use of antibiotics in sterile necrosis has been very controversial.
At least six small, nonblinded, randomized trials have evaluated the benefit of giving antibiotics prophylactically for presumed sterile necrosis. A recent Cochrane analysis of five of these trials (294 patients) suggested that patients who got antibiotics had a lower risk of death (odds ratio 0.37, 95% confidence interval [CI] 0.17–0.83) but no difference in the rates of pancreatic infection or surgery.30 These paradoxical results suggest that antibiotics may prevent death by preventing nonpancreatic infections (eg, pneumonia, line infections) rather than by preventing infection of necrotic pancreatic tissue. The five trials in the meta-analysis are limited by significant methodologic heterogeneity and by lack of double-blinding.
In spite of the overall lower death rate observed in the meta-analysis, the prophylactic use of antibiotics in sterile necrosis remains controversial. One concern is that patients given long prophylactic courses of antibiotics may develop resistant bacterial or fungal infections. However, the Cochrane and other meta-analyses have not shown a higher rate of fungal infections in those given antibiotics.31
Recommendation: No routine antibiotics for mild cases
The AGA guidelines recommend against routinely giving antibiotics in mild acute pancreatitis and do not provide strict recommendations for prophylactic antibiotic use in necrotizing acute pancreatitis.2 The guidelines state that antibiotics can be used “on demand” based on clinical signs of infection (eg, high fevers, rising leukocytosis, hypotension) or worsening organ failure.
If a purely prophylactic strategy is used, only patients at high risk of developing infection (eg, those with necrosis in more than 30% of the pancreas) should receive antibiotics. Antibiotics with high tissue-penetration should be used, such as imipenem-cilastin (Primaxin IV) or ciprofloxacin (Cipro) plus metronidazole (Flagyl).
Adherence to these guidelines is not optimal. For example, in an Italian multicenter study, 9% of patients with mild acute pancreatitis were treated with antibiotics.19 Moreover, many patients with proven infected necrosis received antibiotics that do not penetrate the pancreatic tissue very well.
ERCP IN SEVERE BILIARY ACUTE PANCREATITIS
Problem: Endoscopic retrograde cholangiopancreatography (ERCP) often is performed inappropriately in mild biliary acute pancreatitis or is not performed urgently in severe cases.
In most cases of mild biliary pancreatitis, the stones pass spontaneously, as verified by cholangiography done during laparoscopic cholecystectomy. Ongoing ampullary obstruction by impacted biliary stones can perpetuate pancreatic inflammation and delay recovery.
Two early randomized trials showed a benefit from early ERCP (within 72 hours) with sphincterotomy and stone extraction, primarily in those with severe biliary acute pancreatitis or ascending cholangitis,32,33 but a third trial failed to reveal a benefit.34 A Cochrane metaanalysis of these three trials failed to show a lower death rate with ERCP in mild or severe biliary pancreatitis.35 However, early ERCP did prevent complications in severe biliary pancreatitis (odds ratio 0.27, 95% CI 0.14–0.53).
Later, a fourth randomized trial was restricted to patients with suspected biliary pancreatitis, evidence of biliary obstruction, and no signs of cholangitis36: 103 patients were randomized to undergo either ERCP within 72 hours or conservative management. No difference was observed in rates of death or organ failure or in the CT severity index.
Recommendation: ER CP for suspected retained stones
ERCP has a limited role in patients with biliary pancreatitis, being used to clear retained bile duct stones or to relieve ongoing biliary obstruction.
The decision to perform ERCP before surgery should be based on how strongly one suspects retained stones. ERCP is most appropriate if the suspicion of retained stones and the likelihood of therapeutic intervention are high (eg, if the serum bilirubin and alkaline phosphatase levels are rising and ultrasonography shows a dilated bile duct). If there is moderate suspicion, a safer and less-invasive imaging study such as magnetic resonance cholangiopancreatography (MRCP) or endoscopic ultrasonography can be done to screen for bile duct stones before proceeding to ERCP.
The ACG guidelines suggest urgent ERCP (preferably within 24 hours) for those with severe biliary pancreatitis complicated by organ failure or those with suspicion of cholangitis. The level of evidence is I, ie, “strong evidence from at least one published systematic review of multiple well-designed randomized controlled trials.”1
Elective ERCP is recommended for those who are poor surgical candidates. ERCP is also recommended for those with rising liver enzyme values or imaging findings suggesting a retained common bile duct stone (including intraoperative cholangiography). Endoscopic ultrasonography or MRCP is recommended for those with slow clinical resolution, who are pregnant, or in whom uncertainty exists regarding the biliary etiology of pancreatitis.
Compliance rates with these and similar guidelines are not adequate. In an audit of adherence to the British Society of Gastroenterology guidelines, early ERCP was performed in only 25% of patients with severe biliary acute pancreatitis.6
LAPAROSCOPIC CHOLECYSTECTOMY FOR MILD BILIARY PANCREATITIS
Problem: Laparoscopic cholecystectomy is not done at admission or within 2 weeks in many patients with mild biliary pancreatitis.
If the gallbladder is not removed, biliary pancreatitis may recur in up to 61% of patients within 6 weeks of hospital discharge.37 This is the basis for guideline recommendations for surgery (or a confirmation of a surgery date) prior to hospital discharge.
The International Association of Pancreatology recommends early cholecystectomy (preferably during the same hospitalization) for patients with mild gallstone-associated acute pancreatitis.38 In severe gallstone-associated acute pancreatitis, cholecystectomy should be delayed until there is sufficient resolution of the inflammatory response and clinical recovery. The AGA guidelines advocate cholecystectomy as soon as possible and in no case later than 4 weeks after discharge to prevent relapse. ERCP with biliary sphinc-terotomy may also protect against relapse in those who are not fit to undergo surgery.
Recommendations for definitive management of gallstones (laparoscopic cholecystectomy or ERCP, or both) are not always followed. For example, a British study showed 70% compliance with this recommendation.4 A similar compliance audit in Germany revealed that cholecystectomy was performed during the initial hospital stay in only 23% of cases.7 In a New Zealand study, a regular compliance audit with feedback to surgeons resulted in an increase in the early cholecystectomy rate from 54% to 80%.8
- Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006; 101:2379–2400.
- Forsmark CE, Baillie J; AGA Institute Clinical Practice and Economics Committee. AGA Institute technical review on acute pancreatitis. Gastroenterology 2007; 132:2022–2044.
- United Kingdom guidelines for the management of acute pancreatitis. British Society of Gastroenterology. Gut 1998; 42(suppl 2):S1–S13.
- Norton SA, Cheruvu CV, Collins J, Dix FP, Eyre-Brook IA. An assessment of clinical guidelines for the management of acute pancreatitis. Ann R Coll Surg Engl 2001; 83:399–405.
- Chiang DT, Anozie A, Fleming WR, Kiroff GK. Comparative study on acute pancreatitis management. ANZ J Surg 2004; 74:218–221.
- Barnard J, Siriwardena AK. Variations in implementation of current national guidelines for the treatment of acute pancreatitis: implications for acute surgical service provision. Ann R Coll Surg Engl 2002; 84:79–81.
- Lankisch PG, Weber-Dany B, Lerch MM. Clinical perspectives in pancreatology: compliance with acute pancreatitis in Germany [letter]. Pancreatology 2005; 5:591–593.
- Connor SJ, Lienert AR, Brown LA, Bagshaw PF. Closing the audit loop is necessary to achieve compliance with evidence-based guidelines in the management of acute pancreatitis. N Z Med J 2008; 121:19–25.
- Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction, and death in acute pancreatitis. Br J Surg 2006; 93:738–744.
- Brown A, Orav J, Banks PA. Hemoconcentration is an early marker for organ failure and necrotizing pancreatitis. Pancreas 2000; 20:367–372.
- Lankisch PG, Mahlke R, Blum T, et al. Hemoconcentration: an early marker of severe and/or necrotizing pancreatitis? A critical appraisal. Am J Gastroenterol 2001; 96:2081–2085.
- Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009; 104:966–971.
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974; 139:69–81.
- Larvin M. Assessment of clinical severity and prognosis. In:Beger HG, Warshaw AL, Buchler MW, et al, editors. The Pancreas. Blackwell Science: New York, 1998:489–502.
- Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008; 57:1698–1703.
- Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993, 128:586–590.
- Forgacs B, Eible G, Faulhaber J, Kahrau S, Buhr H, Foitzik T. Effect of fluid resuscitation with and without endothelin A receptor blockade on hemoconcentration and organ function in experimental pancreatitis. Eur Surg Res 2000; 32:162–168.
- Brown A, Baillargeon JD, Hughes MD, Banks PA. Can fluid resuscitation prevent pancreatic necrosis in severe acute pancreatitis? Pancreatology 2002; 2:104–107.
- Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology 2007; 132:1127–1151.
- Pezzilli R, Uomo G, Gabbrielli A, et al; ProInf-AISP Study Group. A prospective multicenter survey on the treatment of acute pancreatitis in Italy. Dig Liver Dis 2007; 39:838–846.
- Gardner TB, Vege SS, Pearson RK, Chari ST. Fluid resuscitation in acute pancreatitis. Clin Gastroenterol Hepatol 2008; 6:1070–1076.
- Petrov MS, Pylypchuk RD, Emelyanov NV. Systematic review: nutritional support in acute pancreatitis. Aliment Pharmacol Ther 2008; 28:704–712.
- Marik PE, Zaloga GP. Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ 2004; 328:1407.
- Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990; 174:331–336.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Foitzik T, Bassi DG, Schmidt J, et al. Intravenous contrast medium accentuates the severity of acute necrotizing pancreatitis in the rat. Gastroenterology 1994; 106:207–214.
- Carmona-Sanchez R, Uscanga L, Bezaury-Rivas P, Robles-Díaz G, Suazo-Barahona J, Vargas-Vorácková F. Potential harmful effect of iodinated intravenous contrast medium on the clinical course of mild acute pancreatitis. Arch Surg 2000; 135:1280–1284.
- Uhl W, Roggo A, Kirschstein T, et al. Influence of contrast-enhanced computed tomography on couse and outcome in patients with acute pancreatitis. Pancreas 2002; 24:191–197.
- Foitzik T, Klar E. Non-compliance with guidelines for the management of severe acute pancreatitis among German surgeons. Pancreatology 2007; 7:80–85.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006;CD002941.
- Heinrich S, Schafer M, Rousson V, Clavien PA. Evidence-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg 2006; 243:154–168.
- Neoptolemos JP, Carr-Locke DL, London NJ, Bailey IA, James D, Fossard DP. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet 1988; 2:979–983.
- Fan ST, Lai EC, Mok FP, Lo CM, Zheng SS, Wong J. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med 1993; 328:228–232.
- Folsch UR, Nitsche R, Ludtke R, Hilgers RA, Creutzfeldt W. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. The German Study Group on Acute Biliary Pancreatitis. N Engl J Med 1997; 336:237–242.
- Ayub K, Imada R, Slavin J. Endoscopic retrograde cholangiopancreatography in gallstone associated pancreatitis. Cochrane Database Syst Rev 2004;CD003630
- Oria A, Cimmino D, Ocampo C, et al. Early endoscopic intervention versus early conservative management in patients with acute gallstone pancreatitis and biliopancreatic obstruction. A randomized clinical trial. Ann Surg 2007; 245:10–17.
- Frei GJ, Frei VT, Thirlby RC, McClelland RN. Biliary pancreatitis: clinical presentation and surgical management. Am J Surg 1986; 151:170–175.
- Uhl W, Warshaw A, Imrie C, et al; International Association of Pancreatology. IAP guidelines on the surgical management of acute pancreatitis. Pancreatology 2002; 2:565–573.
Several major gastroenterological and surgical societies have issued guidelines on how to manage acute pancreatitis, based on evidence from high-quality randomized trials and nonrandomized studies as well as on expert opinion.1–3 Information is limited on how well physicians in the United States comply with these guidelines, but compliance is suboptimal in other developed countries, according to several studies,4–8 and we suspect that many US physicians are not following the guidelines either.
Acute pancreatitis is a frequent inpatient diagnosis that internists, gastroenterologists, and surgeons all confront. The most common causes are gallstones and heavy alcohol intake. Its management is typically straightforward: intravenous fluids, analgesia, and nothing by mouth. However, treatment of severe cases can be quite complex, particularly if multiple organ systems are involved or if there are local complications.
The primary aim of this article is to raise awareness of recognized deviations from established recommendations that may lead to adverse patient outcomes.
MEASURING ENZYME LEVELS DAILY ADDS COST BUT LITTLE BENEFIT
Problem: Serum amylase and lipase levels are often needlessly measured every day.
Measuring the serum amylase and lipase levels is useful in diagnosing acute pancreatitis, which requires two of the following three features1:
- Characteristic abdominal pain
- Levels of serum amylase or serum lipase, or both, that are three or more times the upper limit of normal
- Findings of acute pancreatitis on computed tomography (CT).
However, the magnitude or duration of the serum enzyme elevation does not correlate with the severity of the attack. Further, we have noticed that physicians at our hospital often order daily serum amylase and lipase levels in patients admitted with acute pancreatitis.
The American College of Gastroenterology (ACG) guidelines1 state that daily monitoring of amylase and lipase has limited value in managing acute pancreatitis. Rechecking these concentrations may be reasonable if pain fails to resolve or worsens during a prolonged hospitalization, as this may suggest a recurrent attack of acute pancreatitis or a developing pseudocyst. But in most cases of acute pancreatitis, daily serum enzyme measurements add cost but little benefit.
REGULAR ASSESSMENT IS IMPORTANT
Problem: Often, severity assessments are not performed regularly or acted on.
Most cases of acute pancreatitis are mild, with rapid recovery and excellent prognosis. However, 15% to 20% are severe and may result in a prolonged hospitalization, systemic inflammatory response syndrome (SIRS), multiorgan system failure, and death.
In severe acute pancreatitis, as pancreatic enzymes and inflammatory cytokines damage the blood vessels, a vast amount of fluid leaks out into the interstitial (“third”) space. This fluid extravasation leads to decreased effective circulating volume, local pancreatic necrosis, hemodynamic instability, and end-organ failure.
It is important to recognize severe acute pancreatitis early because the patient needs to be transferred to a step-down unit or intensive care unit to receive optimal fluid resuscitation and supportive care for organ dysfunction. After 48 to 72 hours, a prediction of severe acute pancreatitis should also prompt the physician to order CT to detect pancreatic necrosis, and also to initiate nutritional support.
Assessment of severity begins in the emergency room or on admission to the hospital. Older age, obesity, organ failure, and pulmonary infiltrates or pleural effusions are initial indicators of poor prognosis. Signs of SIRS (high or low core body temperature, tachycardia, tachypnea, low or high peripheral white blood cell count) or organ failure (eg, elevated serum creatinine) are present on admission in 21% of patients with acute pancreatitis.9
Hemoconcentration is a marker of decreased effective circulating volume in severe acute pancreatitis. A hematocrit higher than 44% at admission or that rises in the first 24 to 48 hours of admission predicts necrosis.10,11 However, a more robust marker of organ failure may be the blood urea nitrogen level.12
Clinical scoring systems
Several clinical scoring systems have been studied for assessing severity.
Limitations of the Ranson score are that it can only be completed after 48 hours, all the data points are not always obtained, and it cannot be repeated on a daily basis. Owing to these limitations and its less-than-optimal predictive value, the Ranson score has fallen into disuse.
The APACHE II (Acute Physiology and Chronic Health Evaluation II) score is more versatile. It is based on multiple clinical and laboratory values, and it correlates very well with the risk of death in acute pancreatitis. Death rates are less than 4% when the APACHE II score is less than 8, and 11% to 18% when it is 8 or higher.1 The trajectory of the APACHE II score in the first 48 hours is also an accurate prognostic indicator.
Previous limitations of the APACHE II score were that it was complicated and timeconsuming to calculate and required arterial blood gas measurements. Easy-to-use online calculators are now available (eg, www.globalrph.com/apacheii.htm), and the venous bicarbonate level and the oxygen saturation can be substituted for the arterial pH and oxygen partial pressure.
BISAP, a new five-point scoring system,15 was recently prospectively validated.12 “BISAP” is an acronym for the five markers it is based on, each of which has been shown to predict severe illness in acute pancreatitis:
- Blood urea nitrogen level > 25 mg/dL
- Impaired mental status
- SIRS
- Age > 60 years
- Pleural effusion.
The presence of three or more of these factors correlates with higher risk of death, organ failure, and pancreatic necrosis.12
Compared with APACHE II, BISAP has similar accuracy and is easier to calculate. Also, BISAP was specifically developed for acute pancreatitis, whereas APACHE II is a generic score for all critically ill patients.
The Atlanta criteria16 define severe acute pancreatitis as one or more of the following:
- A Ranson score of 3 or higher during the first 48 hours
- An APACHE II score of 8 or higher at any time
- Failure of one or more organs
- One or more local complications (eg, necrosis, pseudocysts, abscesses).
Recommendation: Assess severity at least daily
A severity assessment should be performed at admission and at least every day thereafter. Clinical guidelines recognize the importance of severity assessment but vary in their specific recommendations.
The ACG advises calculating the APACHE II score within 3 days of admission and measuring the hematocrit at admission, at 12 hours, and at 24 hours. The level of evidence is III, ie, “from published well-designed trials without randomization, single group prepost, cohort, time series, or matched case controlled studies”.1
The American Gastroenterological Association (AGA) provides a more generalized recommendation, that “clinical judgment” should take into account the presence of risk factors (eg, age, obesity), presence or absence of SIRS, routine laboratory values (eg, hematocrit, serum creatinine), and APACHE II score when assessing severity and making decisions.2
In a German survey, only 32% of gastroenterologists used the APACHE II score for assessing risk in acute pancreatitis, in spite of national guidelines emphasizing its importance.7 Also, not all patients with severe acute pancreatitis are transferred to a step-down unit or intensive care unit as recommended. In a British study,4 only 8 (17%) of 46 patients with predicted severe acute pancreatitis were transferred, and 8 of the 38 patients who were not transferred died.
FLUID MUST BE AGGRESSIVELY REPLACED AND MONITORED
Problem: Often, not enough fluid is replaced, or fluid status is not adequately monitored.
Fluid must be aggressively replaced to balance the massive third-space fluid losses that occur in the early inflammatory phase of acute pancreatitis. Intravascular volume depletion can develop rapidly and result in tachycardia, hypotension, and renal failure. It may also impair the blood flow to the pancreas and worsen necrosis.
Animal studies show that aggressive fluid replacement supports the pancreatic microcirculation and prevents necrosis.17 It may also support the intestinal microcirculation and gut barrier, preventing bacterial translocation.
In humans, no controlled trials have been done to test the efficacy of aggressive fluid resuscitation in acute pancreatitis. However, the notion that intravascular fluid loss contributes to poor outcomes is inferred from human studies showing more necrosis and deaths in patients with hemoconcentration. In one study, patients who received inadequate fluid replacement (evidenced by a rise in hematocrit at 24 hours) were more likely to develop necrotizing pancreatitis.18
Recommendation: Early, aggressive fluid replacement
Experts have suggested initially infusing 500 to 1,000 mL of fluid per hour in those who are volume-depleted, initially infusing 250 to 350 mL per hour in those who are not volumedepleted, and adjusting the fluid rate every 1 to 4 hours on the basis of clinical variables.19 The sufficiency of fluid replacement should be carefully monitored by vital signs, urine output, and serum hematocrit.
On the other hand, overly aggressive fluid resuscitation can be detrimental in patients at risk of volume overload or pulmonary edema. Fluid replacement should be tempered in elderly patients and those with cardiac or renal comorbidities, and may require monitoring of central venous pressure.
The ACG and AGA guidelines both recognize the need for early aggressive volume replacement in acute pancreatitis (level of evidence III), but they do not specify the exact amounts and rates. Young and healthy patients should receive a rapid bolus of isotonic saline or Ringer’s lactate solution followed by an infusion at a high initial maintenance rate.
Few studies have been done to assess physicians’ compliance with recommendations for aggressive volume replacement. In an Italian multicenter study, patients with mild or severe acute pancreatitis received an average of only 2.5 L of fluid per day (about 100 mL/hour).20 Gardner et al21 recently summarized the available evidence for fluid support in acute pancreatitis.
NUTRITIONAL SUPPORT
Problem: In many severe cases, enteral or parenteral feeding is not started soon enough.
Nutritional support entails enteral or parenteral feeding when an oral diet is contraindicated. Enteral feeding is usually via a nasojejunal tube, which may need to be placed under endoscopic or radiographic guidance. Neither parenteral nor nasojejunal feeding stimulates pancreatic secretion, and both are safe in acute pancreatitis.
Severe acute pancreatitis is an intensely catabolic state characterized by increased energy expenditure, protein breakdown, and substrate utilization. Patients may not be able to resume an oral diet for weeks or even months, particularly if local complications develop. Early nutritional support has been shown to improve outcomes in severe acute pancreatitis.22 Therefore, nutritional support should be started as soon as possible in severe acute pancreatitis based on initial clinical and radiographic indicators of severity, optimally within the first 2 or 3 days.
Enteral nutrition is preferred to parenteral nutrition in pancreatitis: it is less expensive and does not pose a risk of catheter-related infection or thrombosis or hepatic complications. Also, there is experimental evidence that enteral nutrition may preserve the gut barrier, decreasing mucosal permeability and bacterial translocation.
A number of small randomized trials compared enteral and parenteral nutrition in acute pancreatitis, but they yielded mixed results. A meta-analysis of six trials showed a lower rate of infectious complications with enteral than with parenteral nutrition. 23 However, no significant difference was found in the rates of death or noninfectious complications.
Recommendation: Enteral feeding, when possible
Nutritional support is unnecessary in most cases of mild acute pancreatitis. Pancreatic inflammation typically resolves within a few days, allowing patients to resume eating. Occasionally, patients in whom pain resolves slowly and who fast for more than 5 to 7 days need nutritional support to prevent proteincalorie malnutrition.
The ACG guidelines1 and most others suggest that, whenever possible, enteral rather than parenteral feeding should be given to those who require nutritional support. The level of evidence is II (“strong evidence from at least one published properly designed randomized controlled trial of appropriate size and in an appropriate clinical setting”).
However, not all physicians recognize the benefit of enteral feeding. In a cohort of German gastroenterologists, only 73% favored enteral over parenteral feeding in acute pancreatitis.7
COMPUTED TOMOGRAPHY
Problem: CT is not done in many patients with severe acute pancreatitis, or it is done too soon during the admission.
Dual-phase, contrast-enhanced, pancreatic-protocol CT provides a sensitive structural evaluation of the pancreas and is useful to diagnose necrotizing pancreatitis. Pancreatic necrosis is correlated with a severe clinical course, the development of single or multiorgan dysfunction, and death.
Recommendation: CT in severe cases
Not every patient with acute pancreatitis needs to undergo CT. Most mild cases do not require routine CT, since necrosis and other local complications are infrequent in this group.
Also, CT is often ordered too soon during the hospitalization. Indicators of severity on CT are not usually evident until 2 to 3 days after admission.25 CT should be considered about 3 days after the onset of symptoms rather than immediately upon admission.
On the other hand, CT at the time of admission may be warranted to rule out other life-threatening causes of abdominal pain and hyperamylasemia (eg, bowel obstruction, viscus perforation). CT may also be useful in the late phase of acute pancreatitis (weeks after admission) to diagnose or monitor complications (eg, pseudocysts, abscesses, splenic vein thrombosis, splenic artery pseudoaneurysms). Magnetic resonance imaging with gadolinium contrast is a reasonable alternative to CT for detecting pancreatic necrosis and other local complications.
In patients who have severe acute pancreatitis and compromised renal function (serum creatinine > 1.5 mg/dL), CT can be performed without contrast to assess severity based on a limited Balthazar score (ie, without a necrosis score). Studies in rats suggest that iodinated contrast may decrease pancreatic microcirculation and worsen or precipitate necrosis,26 although published human studies do not support this contention.27,28
Guidelines uniformly recommend CT for patients with severe acute pancreatitis (the ACG guideline gives it a level of evidence of III), but this recommendation is not always followed. A study from Australia showed that CT was done in only 27% to 67% of patients with severe acute pancreatitis.5 In a British study, only 8 of 46 patients with clinically predicted severe pancreatitis underwent CT within the first 10 days of admission.4
SUSPECTED INFECTED NECROSIS
Problem: Fine-needle aspiration is not done in many cases of suspected infected necrosis.
Approximately one-third of patients with necrotizing pancreatitis develop infected necrosis. The death rate for patients with infected pancreatic necrosis is high—30%, compared with 12% in those with sterile necrosis.1 Differentiating sterile and infected necrosis is therefore essential.
Clinical signs such as fever are poor predictors of infection. Signs of SIRS can be present in both sterile and infected necrotizing pancreatitis.
Recommendation: Fine-needle aspiration of necrosis
For the reasons given above, the findings of necrosis on CT and persistent SIRS should prompt consideration of fine-needle aspiration with Gram stain and culture to differentiate sterile and infected necrosis (ACG guideline, level of evidence III).1 If infection is confirmed, surgical debridement should be strongly considered. Other less-invasive approaches such as endoscopic debridement can be used in selected cases.
Fine-needle aspiration of necrosis is too often neglected. In a cohort of German surgeons, only 55% complied with International Association of Pancreatology recommendations to perform biopsy to differentiate sterile from infected necrosis in patients with signs of sepsis.29
BROAD-SPECTRUM ANTIBIOTICS
Problem: Broad-spectrum antibiotics are often used inappropriately in patients with mild acute pancreatitis and in patients with sterile necrotizing pancreatitis who are clinically stable and have no signs of sepsis.
Antibiotics are not indicated in mild acute pancreatitis. A limited course of antibiotics is typically indicated in severe cases with suspected or proven infected necrosis (in conjunction with surgical necrosectomy). However, the use of antibiotics in sterile necrosis has been very controversial.
At least six small, nonblinded, randomized trials have evaluated the benefit of giving antibiotics prophylactically for presumed sterile necrosis. A recent Cochrane analysis of five of these trials (294 patients) suggested that patients who got antibiotics had a lower risk of death (odds ratio 0.37, 95% confidence interval [CI] 0.17–0.83) but no difference in the rates of pancreatic infection or surgery.30 These paradoxical results suggest that antibiotics may prevent death by preventing nonpancreatic infections (eg, pneumonia, line infections) rather than by preventing infection of necrotic pancreatic tissue. The five trials in the meta-analysis are limited by significant methodologic heterogeneity and by lack of double-blinding.
In spite of the overall lower death rate observed in the meta-analysis, the prophylactic use of antibiotics in sterile necrosis remains controversial. One concern is that patients given long prophylactic courses of antibiotics may develop resistant bacterial or fungal infections. However, the Cochrane and other meta-analyses have not shown a higher rate of fungal infections in those given antibiotics.31
Recommendation: No routine antibiotics for mild cases
The AGA guidelines recommend against routinely giving antibiotics in mild acute pancreatitis and do not provide strict recommendations for prophylactic antibiotic use in necrotizing acute pancreatitis.2 The guidelines state that antibiotics can be used “on demand” based on clinical signs of infection (eg, high fevers, rising leukocytosis, hypotension) or worsening organ failure.
If a purely prophylactic strategy is used, only patients at high risk of developing infection (eg, those with necrosis in more than 30% of the pancreas) should receive antibiotics. Antibiotics with high tissue-penetration should be used, such as imipenem-cilastin (Primaxin IV) or ciprofloxacin (Cipro) plus metronidazole (Flagyl).
Adherence to these guidelines is not optimal. For example, in an Italian multicenter study, 9% of patients with mild acute pancreatitis were treated with antibiotics.19 Moreover, many patients with proven infected necrosis received antibiotics that do not penetrate the pancreatic tissue very well.
ERCP IN SEVERE BILIARY ACUTE PANCREATITIS
Problem: Endoscopic retrograde cholangiopancreatography (ERCP) often is performed inappropriately in mild biliary acute pancreatitis or is not performed urgently in severe cases.
In most cases of mild biliary pancreatitis, the stones pass spontaneously, as verified by cholangiography done during laparoscopic cholecystectomy. Ongoing ampullary obstruction by impacted biliary stones can perpetuate pancreatic inflammation and delay recovery.
Two early randomized trials showed a benefit from early ERCP (within 72 hours) with sphincterotomy and stone extraction, primarily in those with severe biliary acute pancreatitis or ascending cholangitis,32,33 but a third trial failed to reveal a benefit.34 A Cochrane metaanalysis of these three trials failed to show a lower death rate with ERCP in mild or severe biliary pancreatitis.35 However, early ERCP did prevent complications in severe biliary pancreatitis (odds ratio 0.27, 95% CI 0.14–0.53).
Later, a fourth randomized trial was restricted to patients with suspected biliary pancreatitis, evidence of biliary obstruction, and no signs of cholangitis36: 103 patients were randomized to undergo either ERCP within 72 hours or conservative management. No difference was observed in rates of death or organ failure or in the CT severity index.
Recommendation: ER CP for suspected retained stones
ERCP has a limited role in patients with biliary pancreatitis, being used to clear retained bile duct stones or to relieve ongoing biliary obstruction.
The decision to perform ERCP before surgery should be based on how strongly one suspects retained stones. ERCP is most appropriate if the suspicion of retained stones and the likelihood of therapeutic intervention are high (eg, if the serum bilirubin and alkaline phosphatase levels are rising and ultrasonography shows a dilated bile duct). If there is moderate suspicion, a safer and less-invasive imaging study such as magnetic resonance cholangiopancreatography (MRCP) or endoscopic ultrasonography can be done to screen for bile duct stones before proceeding to ERCP.
The ACG guidelines suggest urgent ERCP (preferably within 24 hours) for those with severe biliary pancreatitis complicated by organ failure or those with suspicion of cholangitis. The level of evidence is I, ie, “strong evidence from at least one published systematic review of multiple well-designed randomized controlled trials.”1
Elective ERCP is recommended for those who are poor surgical candidates. ERCP is also recommended for those with rising liver enzyme values or imaging findings suggesting a retained common bile duct stone (including intraoperative cholangiography). Endoscopic ultrasonography or MRCP is recommended for those with slow clinical resolution, who are pregnant, or in whom uncertainty exists regarding the biliary etiology of pancreatitis.
Compliance rates with these and similar guidelines are not adequate. In an audit of adherence to the British Society of Gastroenterology guidelines, early ERCP was performed in only 25% of patients with severe biliary acute pancreatitis.6
LAPAROSCOPIC CHOLECYSTECTOMY FOR MILD BILIARY PANCREATITIS
Problem: Laparoscopic cholecystectomy is not done at admission or within 2 weeks in many patients with mild biliary pancreatitis.
If the gallbladder is not removed, biliary pancreatitis may recur in up to 61% of patients within 6 weeks of hospital discharge.37 This is the basis for guideline recommendations for surgery (or a confirmation of a surgery date) prior to hospital discharge.
The International Association of Pancreatology recommends early cholecystectomy (preferably during the same hospitalization) for patients with mild gallstone-associated acute pancreatitis.38 In severe gallstone-associated acute pancreatitis, cholecystectomy should be delayed until there is sufficient resolution of the inflammatory response and clinical recovery. The AGA guidelines advocate cholecystectomy as soon as possible and in no case later than 4 weeks after discharge to prevent relapse. ERCP with biliary sphinc-terotomy may also protect against relapse in those who are not fit to undergo surgery.
Recommendations for definitive management of gallstones (laparoscopic cholecystectomy or ERCP, or both) are not always followed. For example, a British study showed 70% compliance with this recommendation.4 A similar compliance audit in Germany revealed that cholecystectomy was performed during the initial hospital stay in only 23% of cases.7 In a New Zealand study, a regular compliance audit with feedback to surgeons resulted in an increase in the early cholecystectomy rate from 54% to 80%.8
Several major gastroenterological and surgical societies have issued guidelines on how to manage acute pancreatitis, based on evidence from high-quality randomized trials and nonrandomized studies as well as on expert opinion.1–3 Information is limited on how well physicians in the United States comply with these guidelines, but compliance is suboptimal in other developed countries, according to several studies,4–8 and we suspect that many US physicians are not following the guidelines either.
Acute pancreatitis is a frequent inpatient diagnosis that internists, gastroenterologists, and surgeons all confront. The most common causes are gallstones and heavy alcohol intake. Its management is typically straightforward: intravenous fluids, analgesia, and nothing by mouth. However, treatment of severe cases can be quite complex, particularly if multiple organ systems are involved or if there are local complications.
The primary aim of this article is to raise awareness of recognized deviations from established recommendations that may lead to adverse patient outcomes.
MEASURING ENZYME LEVELS DAILY ADDS COST BUT LITTLE BENEFIT
Problem: Serum amylase and lipase levels are often needlessly measured every day.
Measuring the serum amylase and lipase levels is useful in diagnosing acute pancreatitis, which requires two of the following three features1:
- Characteristic abdominal pain
- Levels of serum amylase or serum lipase, or both, that are three or more times the upper limit of normal
- Findings of acute pancreatitis on computed tomography (CT).
However, the magnitude or duration of the serum enzyme elevation does not correlate with the severity of the attack. Further, we have noticed that physicians at our hospital often order daily serum amylase and lipase levels in patients admitted with acute pancreatitis.
The American College of Gastroenterology (ACG) guidelines1 state that daily monitoring of amylase and lipase has limited value in managing acute pancreatitis. Rechecking these concentrations may be reasonable if pain fails to resolve or worsens during a prolonged hospitalization, as this may suggest a recurrent attack of acute pancreatitis or a developing pseudocyst. But in most cases of acute pancreatitis, daily serum enzyme measurements add cost but little benefit.
REGULAR ASSESSMENT IS IMPORTANT
Problem: Often, severity assessments are not performed regularly or acted on.
Most cases of acute pancreatitis are mild, with rapid recovery and excellent prognosis. However, 15% to 20% are severe and may result in a prolonged hospitalization, systemic inflammatory response syndrome (SIRS), multiorgan system failure, and death.
In severe acute pancreatitis, as pancreatic enzymes and inflammatory cytokines damage the blood vessels, a vast amount of fluid leaks out into the interstitial (“third”) space. This fluid extravasation leads to decreased effective circulating volume, local pancreatic necrosis, hemodynamic instability, and end-organ failure.
It is important to recognize severe acute pancreatitis early because the patient needs to be transferred to a step-down unit or intensive care unit to receive optimal fluid resuscitation and supportive care for organ dysfunction. After 48 to 72 hours, a prediction of severe acute pancreatitis should also prompt the physician to order CT to detect pancreatic necrosis, and also to initiate nutritional support.
Assessment of severity begins in the emergency room or on admission to the hospital. Older age, obesity, organ failure, and pulmonary infiltrates or pleural effusions are initial indicators of poor prognosis. Signs of SIRS (high or low core body temperature, tachycardia, tachypnea, low or high peripheral white blood cell count) or organ failure (eg, elevated serum creatinine) are present on admission in 21% of patients with acute pancreatitis.9
Hemoconcentration is a marker of decreased effective circulating volume in severe acute pancreatitis. A hematocrit higher than 44% at admission or that rises in the first 24 to 48 hours of admission predicts necrosis.10,11 However, a more robust marker of organ failure may be the blood urea nitrogen level.12
Clinical scoring systems
Several clinical scoring systems have been studied for assessing severity.
Limitations of the Ranson score are that it can only be completed after 48 hours, all the data points are not always obtained, and it cannot be repeated on a daily basis. Owing to these limitations and its less-than-optimal predictive value, the Ranson score has fallen into disuse.
The APACHE II (Acute Physiology and Chronic Health Evaluation II) score is more versatile. It is based on multiple clinical and laboratory values, and it correlates very well with the risk of death in acute pancreatitis. Death rates are less than 4% when the APACHE II score is less than 8, and 11% to 18% when it is 8 or higher.1 The trajectory of the APACHE II score in the first 48 hours is also an accurate prognostic indicator.
Previous limitations of the APACHE II score were that it was complicated and timeconsuming to calculate and required arterial blood gas measurements. Easy-to-use online calculators are now available (eg, www.globalrph.com/apacheii.htm), and the venous bicarbonate level and the oxygen saturation can be substituted for the arterial pH and oxygen partial pressure.
BISAP, a new five-point scoring system,15 was recently prospectively validated.12 “BISAP” is an acronym for the five markers it is based on, each of which has been shown to predict severe illness in acute pancreatitis:
- Blood urea nitrogen level > 25 mg/dL
- Impaired mental status
- SIRS
- Age > 60 years
- Pleural effusion.
The presence of three or more of these factors correlates with higher risk of death, organ failure, and pancreatic necrosis.12
Compared with APACHE II, BISAP has similar accuracy and is easier to calculate. Also, BISAP was specifically developed for acute pancreatitis, whereas APACHE II is a generic score for all critically ill patients.
The Atlanta criteria16 define severe acute pancreatitis as one or more of the following:
- A Ranson score of 3 or higher during the first 48 hours
- An APACHE II score of 8 or higher at any time
- Failure of one or more organs
- One or more local complications (eg, necrosis, pseudocysts, abscesses).
Recommendation: Assess severity at least daily
A severity assessment should be performed at admission and at least every day thereafter. Clinical guidelines recognize the importance of severity assessment but vary in their specific recommendations.
The ACG advises calculating the APACHE II score within 3 days of admission and measuring the hematocrit at admission, at 12 hours, and at 24 hours. The level of evidence is III, ie, “from published well-designed trials without randomization, single group prepost, cohort, time series, or matched case controlled studies”.1
The American Gastroenterological Association (AGA) provides a more generalized recommendation, that “clinical judgment” should take into account the presence of risk factors (eg, age, obesity), presence or absence of SIRS, routine laboratory values (eg, hematocrit, serum creatinine), and APACHE II score when assessing severity and making decisions.2
In a German survey, only 32% of gastroenterologists used the APACHE II score for assessing risk in acute pancreatitis, in spite of national guidelines emphasizing its importance.7 Also, not all patients with severe acute pancreatitis are transferred to a step-down unit or intensive care unit as recommended. In a British study,4 only 8 (17%) of 46 patients with predicted severe acute pancreatitis were transferred, and 8 of the 38 patients who were not transferred died.
FLUID MUST BE AGGRESSIVELY REPLACED AND MONITORED
Problem: Often, not enough fluid is replaced, or fluid status is not adequately monitored.
Fluid must be aggressively replaced to balance the massive third-space fluid losses that occur in the early inflammatory phase of acute pancreatitis. Intravascular volume depletion can develop rapidly and result in tachycardia, hypotension, and renal failure. It may also impair the blood flow to the pancreas and worsen necrosis.
Animal studies show that aggressive fluid replacement supports the pancreatic microcirculation and prevents necrosis.17 It may also support the intestinal microcirculation and gut barrier, preventing bacterial translocation.
In humans, no controlled trials have been done to test the efficacy of aggressive fluid resuscitation in acute pancreatitis. However, the notion that intravascular fluid loss contributes to poor outcomes is inferred from human studies showing more necrosis and deaths in patients with hemoconcentration. In one study, patients who received inadequate fluid replacement (evidenced by a rise in hematocrit at 24 hours) were more likely to develop necrotizing pancreatitis.18
Recommendation: Early, aggressive fluid replacement
Experts have suggested initially infusing 500 to 1,000 mL of fluid per hour in those who are volume-depleted, initially infusing 250 to 350 mL per hour in those who are not volumedepleted, and adjusting the fluid rate every 1 to 4 hours on the basis of clinical variables.19 The sufficiency of fluid replacement should be carefully monitored by vital signs, urine output, and serum hematocrit.
On the other hand, overly aggressive fluid resuscitation can be detrimental in patients at risk of volume overload or pulmonary edema. Fluid replacement should be tempered in elderly patients and those with cardiac or renal comorbidities, and may require monitoring of central venous pressure.
The ACG and AGA guidelines both recognize the need for early aggressive volume replacement in acute pancreatitis (level of evidence III), but they do not specify the exact amounts and rates. Young and healthy patients should receive a rapid bolus of isotonic saline or Ringer’s lactate solution followed by an infusion at a high initial maintenance rate.
Few studies have been done to assess physicians’ compliance with recommendations for aggressive volume replacement. In an Italian multicenter study, patients with mild or severe acute pancreatitis received an average of only 2.5 L of fluid per day (about 100 mL/hour).20 Gardner et al21 recently summarized the available evidence for fluid support in acute pancreatitis.
NUTRITIONAL SUPPORT
Problem: In many severe cases, enteral or parenteral feeding is not started soon enough.
Nutritional support entails enteral or parenteral feeding when an oral diet is contraindicated. Enteral feeding is usually via a nasojejunal tube, which may need to be placed under endoscopic or radiographic guidance. Neither parenteral nor nasojejunal feeding stimulates pancreatic secretion, and both are safe in acute pancreatitis.
Severe acute pancreatitis is an intensely catabolic state characterized by increased energy expenditure, protein breakdown, and substrate utilization. Patients may not be able to resume an oral diet for weeks or even months, particularly if local complications develop. Early nutritional support has been shown to improve outcomes in severe acute pancreatitis.22 Therefore, nutritional support should be started as soon as possible in severe acute pancreatitis based on initial clinical and radiographic indicators of severity, optimally within the first 2 or 3 days.
Enteral nutrition is preferred to parenteral nutrition in pancreatitis: it is less expensive and does not pose a risk of catheter-related infection or thrombosis or hepatic complications. Also, there is experimental evidence that enteral nutrition may preserve the gut barrier, decreasing mucosal permeability and bacterial translocation.
A number of small randomized trials compared enteral and parenteral nutrition in acute pancreatitis, but they yielded mixed results. A meta-analysis of six trials showed a lower rate of infectious complications with enteral than with parenteral nutrition. 23 However, no significant difference was found in the rates of death or noninfectious complications.
Recommendation: Enteral feeding, when possible
Nutritional support is unnecessary in most cases of mild acute pancreatitis. Pancreatic inflammation typically resolves within a few days, allowing patients to resume eating. Occasionally, patients in whom pain resolves slowly and who fast for more than 5 to 7 days need nutritional support to prevent proteincalorie malnutrition.
The ACG guidelines1 and most others suggest that, whenever possible, enteral rather than parenteral feeding should be given to those who require nutritional support. The level of evidence is II (“strong evidence from at least one published properly designed randomized controlled trial of appropriate size and in an appropriate clinical setting”).
However, not all physicians recognize the benefit of enteral feeding. In a cohort of German gastroenterologists, only 73% favored enteral over parenteral feeding in acute pancreatitis.7
COMPUTED TOMOGRAPHY
Problem: CT is not done in many patients with severe acute pancreatitis, or it is done too soon during the admission.
Dual-phase, contrast-enhanced, pancreatic-protocol CT provides a sensitive structural evaluation of the pancreas and is useful to diagnose necrotizing pancreatitis. Pancreatic necrosis is correlated with a severe clinical course, the development of single or multiorgan dysfunction, and death.
Recommendation: CT in severe cases
Not every patient with acute pancreatitis needs to undergo CT. Most mild cases do not require routine CT, since necrosis and other local complications are infrequent in this group.
Also, CT is often ordered too soon during the hospitalization. Indicators of severity on CT are not usually evident until 2 to 3 days after admission.25 CT should be considered about 3 days after the onset of symptoms rather than immediately upon admission.
On the other hand, CT at the time of admission may be warranted to rule out other life-threatening causes of abdominal pain and hyperamylasemia (eg, bowel obstruction, viscus perforation). CT may also be useful in the late phase of acute pancreatitis (weeks after admission) to diagnose or monitor complications (eg, pseudocysts, abscesses, splenic vein thrombosis, splenic artery pseudoaneurysms). Magnetic resonance imaging with gadolinium contrast is a reasonable alternative to CT for detecting pancreatic necrosis and other local complications.
In patients who have severe acute pancreatitis and compromised renal function (serum creatinine > 1.5 mg/dL), CT can be performed without contrast to assess severity based on a limited Balthazar score (ie, without a necrosis score). Studies in rats suggest that iodinated contrast may decrease pancreatic microcirculation and worsen or precipitate necrosis,26 although published human studies do not support this contention.27,28
Guidelines uniformly recommend CT for patients with severe acute pancreatitis (the ACG guideline gives it a level of evidence of III), but this recommendation is not always followed. A study from Australia showed that CT was done in only 27% to 67% of patients with severe acute pancreatitis.5 In a British study, only 8 of 46 patients with clinically predicted severe pancreatitis underwent CT within the first 10 days of admission.4
SUSPECTED INFECTED NECROSIS
Problem: Fine-needle aspiration is not done in many cases of suspected infected necrosis.
Approximately one-third of patients with necrotizing pancreatitis develop infected necrosis. The death rate for patients with infected pancreatic necrosis is high—30%, compared with 12% in those with sterile necrosis.1 Differentiating sterile and infected necrosis is therefore essential.
Clinical signs such as fever are poor predictors of infection. Signs of SIRS can be present in both sterile and infected necrotizing pancreatitis.
Recommendation: Fine-needle aspiration of necrosis
For the reasons given above, the findings of necrosis on CT and persistent SIRS should prompt consideration of fine-needle aspiration with Gram stain and culture to differentiate sterile and infected necrosis (ACG guideline, level of evidence III).1 If infection is confirmed, surgical debridement should be strongly considered. Other less-invasive approaches such as endoscopic debridement can be used in selected cases.
Fine-needle aspiration of necrosis is too often neglected. In a cohort of German surgeons, only 55% complied with International Association of Pancreatology recommendations to perform biopsy to differentiate sterile from infected necrosis in patients with signs of sepsis.29
BROAD-SPECTRUM ANTIBIOTICS
Problem: Broad-spectrum antibiotics are often used inappropriately in patients with mild acute pancreatitis and in patients with sterile necrotizing pancreatitis who are clinically stable and have no signs of sepsis.
Antibiotics are not indicated in mild acute pancreatitis. A limited course of antibiotics is typically indicated in severe cases with suspected or proven infected necrosis (in conjunction with surgical necrosectomy). However, the use of antibiotics in sterile necrosis has been very controversial.
At least six small, nonblinded, randomized trials have evaluated the benefit of giving antibiotics prophylactically for presumed sterile necrosis. A recent Cochrane analysis of five of these trials (294 patients) suggested that patients who got antibiotics had a lower risk of death (odds ratio 0.37, 95% confidence interval [CI] 0.17–0.83) but no difference in the rates of pancreatic infection or surgery.30 These paradoxical results suggest that antibiotics may prevent death by preventing nonpancreatic infections (eg, pneumonia, line infections) rather than by preventing infection of necrotic pancreatic tissue. The five trials in the meta-analysis are limited by significant methodologic heterogeneity and by lack of double-blinding.
In spite of the overall lower death rate observed in the meta-analysis, the prophylactic use of antibiotics in sterile necrosis remains controversial. One concern is that patients given long prophylactic courses of antibiotics may develop resistant bacterial or fungal infections. However, the Cochrane and other meta-analyses have not shown a higher rate of fungal infections in those given antibiotics.31
Recommendation: No routine antibiotics for mild cases
The AGA guidelines recommend against routinely giving antibiotics in mild acute pancreatitis and do not provide strict recommendations for prophylactic antibiotic use in necrotizing acute pancreatitis.2 The guidelines state that antibiotics can be used “on demand” based on clinical signs of infection (eg, high fevers, rising leukocytosis, hypotension) or worsening organ failure.
If a purely prophylactic strategy is used, only patients at high risk of developing infection (eg, those with necrosis in more than 30% of the pancreas) should receive antibiotics. Antibiotics with high tissue-penetration should be used, such as imipenem-cilastin (Primaxin IV) or ciprofloxacin (Cipro) plus metronidazole (Flagyl).
Adherence to these guidelines is not optimal. For example, in an Italian multicenter study, 9% of patients with mild acute pancreatitis were treated with antibiotics.19 Moreover, many patients with proven infected necrosis received antibiotics that do not penetrate the pancreatic tissue very well.
ERCP IN SEVERE BILIARY ACUTE PANCREATITIS
Problem: Endoscopic retrograde cholangiopancreatography (ERCP) often is performed inappropriately in mild biliary acute pancreatitis or is not performed urgently in severe cases.
In most cases of mild biliary pancreatitis, the stones pass spontaneously, as verified by cholangiography done during laparoscopic cholecystectomy. Ongoing ampullary obstruction by impacted biliary stones can perpetuate pancreatic inflammation and delay recovery.
Two early randomized trials showed a benefit from early ERCP (within 72 hours) with sphincterotomy and stone extraction, primarily in those with severe biliary acute pancreatitis or ascending cholangitis,32,33 but a third trial failed to reveal a benefit.34 A Cochrane metaanalysis of these three trials failed to show a lower death rate with ERCP in mild or severe biliary pancreatitis.35 However, early ERCP did prevent complications in severe biliary pancreatitis (odds ratio 0.27, 95% CI 0.14–0.53).
Later, a fourth randomized trial was restricted to patients with suspected biliary pancreatitis, evidence of biliary obstruction, and no signs of cholangitis36: 103 patients were randomized to undergo either ERCP within 72 hours or conservative management. No difference was observed in rates of death or organ failure or in the CT severity index.
Recommendation: ER CP for suspected retained stones
ERCP has a limited role in patients with biliary pancreatitis, being used to clear retained bile duct stones or to relieve ongoing biliary obstruction.
The decision to perform ERCP before surgery should be based on how strongly one suspects retained stones. ERCP is most appropriate if the suspicion of retained stones and the likelihood of therapeutic intervention are high (eg, if the serum bilirubin and alkaline phosphatase levels are rising and ultrasonography shows a dilated bile duct). If there is moderate suspicion, a safer and less-invasive imaging study such as magnetic resonance cholangiopancreatography (MRCP) or endoscopic ultrasonography can be done to screen for bile duct stones before proceeding to ERCP.
The ACG guidelines suggest urgent ERCP (preferably within 24 hours) for those with severe biliary pancreatitis complicated by organ failure or those with suspicion of cholangitis. The level of evidence is I, ie, “strong evidence from at least one published systematic review of multiple well-designed randomized controlled trials.”1
Elective ERCP is recommended for those who are poor surgical candidates. ERCP is also recommended for those with rising liver enzyme values or imaging findings suggesting a retained common bile duct stone (including intraoperative cholangiography). Endoscopic ultrasonography or MRCP is recommended for those with slow clinical resolution, who are pregnant, or in whom uncertainty exists regarding the biliary etiology of pancreatitis.
Compliance rates with these and similar guidelines are not adequate. In an audit of adherence to the British Society of Gastroenterology guidelines, early ERCP was performed in only 25% of patients with severe biliary acute pancreatitis.6
LAPAROSCOPIC CHOLECYSTECTOMY FOR MILD BILIARY PANCREATITIS
Problem: Laparoscopic cholecystectomy is not done at admission or within 2 weeks in many patients with mild biliary pancreatitis.
If the gallbladder is not removed, biliary pancreatitis may recur in up to 61% of patients within 6 weeks of hospital discharge.37 This is the basis for guideline recommendations for surgery (or a confirmation of a surgery date) prior to hospital discharge.
The International Association of Pancreatology recommends early cholecystectomy (preferably during the same hospitalization) for patients with mild gallstone-associated acute pancreatitis.38 In severe gallstone-associated acute pancreatitis, cholecystectomy should be delayed until there is sufficient resolution of the inflammatory response and clinical recovery. The AGA guidelines advocate cholecystectomy as soon as possible and in no case later than 4 weeks after discharge to prevent relapse. ERCP with biliary sphinc-terotomy may also protect against relapse in those who are not fit to undergo surgery.
Recommendations for definitive management of gallstones (laparoscopic cholecystectomy or ERCP, or both) are not always followed. For example, a British study showed 70% compliance with this recommendation.4 A similar compliance audit in Germany revealed that cholecystectomy was performed during the initial hospital stay in only 23% of cases.7 In a New Zealand study, a regular compliance audit with feedback to surgeons resulted in an increase in the early cholecystectomy rate from 54% to 80%.8
- Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006; 101:2379–2400.
- Forsmark CE, Baillie J; AGA Institute Clinical Practice and Economics Committee. AGA Institute technical review on acute pancreatitis. Gastroenterology 2007; 132:2022–2044.
- United Kingdom guidelines for the management of acute pancreatitis. British Society of Gastroenterology. Gut 1998; 42(suppl 2):S1–S13.
- Norton SA, Cheruvu CV, Collins J, Dix FP, Eyre-Brook IA. An assessment of clinical guidelines for the management of acute pancreatitis. Ann R Coll Surg Engl 2001; 83:399–405.
- Chiang DT, Anozie A, Fleming WR, Kiroff GK. Comparative study on acute pancreatitis management. ANZ J Surg 2004; 74:218–221.
- Barnard J, Siriwardena AK. Variations in implementation of current national guidelines for the treatment of acute pancreatitis: implications for acute surgical service provision. Ann R Coll Surg Engl 2002; 84:79–81.
- Lankisch PG, Weber-Dany B, Lerch MM. Clinical perspectives in pancreatology: compliance with acute pancreatitis in Germany [letter]. Pancreatology 2005; 5:591–593.
- Connor SJ, Lienert AR, Brown LA, Bagshaw PF. Closing the audit loop is necessary to achieve compliance with evidence-based guidelines in the management of acute pancreatitis. N Z Med J 2008; 121:19–25.
- Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction, and death in acute pancreatitis. Br J Surg 2006; 93:738–744.
- Brown A, Orav J, Banks PA. Hemoconcentration is an early marker for organ failure and necrotizing pancreatitis. Pancreas 2000; 20:367–372.
- Lankisch PG, Mahlke R, Blum T, et al. Hemoconcentration: an early marker of severe and/or necrotizing pancreatitis? A critical appraisal. Am J Gastroenterol 2001; 96:2081–2085.
- Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009; 104:966–971.
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974; 139:69–81.
- Larvin M. Assessment of clinical severity and prognosis. In:Beger HG, Warshaw AL, Buchler MW, et al, editors. The Pancreas. Blackwell Science: New York, 1998:489–502.
- Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008; 57:1698–1703.
- Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993, 128:586–590.
- Forgacs B, Eible G, Faulhaber J, Kahrau S, Buhr H, Foitzik T. Effect of fluid resuscitation with and without endothelin A receptor blockade on hemoconcentration and organ function in experimental pancreatitis. Eur Surg Res 2000; 32:162–168.
- Brown A, Baillargeon JD, Hughes MD, Banks PA. Can fluid resuscitation prevent pancreatic necrosis in severe acute pancreatitis? Pancreatology 2002; 2:104–107.
- Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology 2007; 132:1127–1151.
- Pezzilli R, Uomo G, Gabbrielli A, et al; ProInf-AISP Study Group. A prospective multicenter survey on the treatment of acute pancreatitis in Italy. Dig Liver Dis 2007; 39:838–846.
- Gardner TB, Vege SS, Pearson RK, Chari ST. Fluid resuscitation in acute pancreatitis. Clin Gastroenterol Hepatol 2008; 6:1070–1076.
- Petrov MS, Pylypchuk RD, Emelyanov NV. Systematic review: nutritional support in acute pancreatitis. Aliment Pharmacol Ther 2008; 28:704–712.
- Marik PE, Zaloga GP. Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ 2004; 328:1407.
- Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990; 174:331–336.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Foitzik T, Bassi DG, Schmidt J, et al. Intravenous contrast medium accentuates the severity of acute necrotizing pancreatitis in the rat. Gastroenterology 1994; 106:207–214.
- Carmona-Sanchez R, Uscanga L, Bezaury-Rivas P, Robles-Díaz G, Suazo-Barahona J, Vargas-Vorácková F. Potential harmful effect of iodinated intravenous contrast medium on the clinical course of mild acute pancreatitis. Arch Surg 2000; 135:1280–1284.
- Uhl W, Roggo A, Kirschstein T, et al. Influence of contrast-enhanced computed tomography on couse and outcome in patients with acute pancreatitis. Pancreas 2002; 24:191–197.
- Foitzik T, Klar E. Non-compliance with guidelines for the management of severe acute pancreatitis among German surgeons. Pancreatology 2007; 7:80–85.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006;CD002941.
- Heinrich S, Schafer M, Rousson V, Clavien PA. Evidence-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg 2006; 243:154–168.
- Neoptolemos JP, Carr-Locke DL, London NJ, Bailey IA, James D, Fossard DP. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet 1988; 2:979–983.
- Fan ST, Lai EC, Mok FP, Lo CM, Zheng SS, Wong J. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med 1993; 328:228–232.
- Folsch UR, Nitsche R, Ludtke R, Hilgers RA, Creutzfeldt W. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. The German Study Group on Acute Biliary Pancreatitis. N Engl J Med 1997; 336:237–242.
- Ayub K, Imada R, Slavin J. Endoscopic retrograde cholangiopancreatography in gallstone associated pancreatitis. Cochrane Database Syst Rev 2004;CD003630
- Oria A, Cimmino D, Ocampo C, et al. Early endoscopic intervention versus early conservative management in patients with acute gallstone pancreatitis and biliopancreatic obstruction. A randomized clinical trial. Ann Surg 2007; 245:10–17.
- Frei GJ, Frei VT, Thirlby RC, McClelland RN. Biliary pancreatitis: clinical presentation and surgical management. Am J Surg 1986; 151:170–175.
- Uhl W, Warshaw A, Imrie C, et al; International Association of Pancreatology. IAP guidelines on the surgical management of acute pancreatitis. Pancreatology 2002; 2:565–573.
- Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006; 101:2379–2400.
- Forsmark CE, Baillie J; AGA Institute Clinical Practice and Economics Committee. AGA Institute technical review on acute pancreatitis. Gastroenterology 2007; 132:2022–2044.
- United Kingdom guidelines for the management of acute pancreatitis. British Society of Gastroenterology. Gut 1998; 42(suppl 2):S1–S13.
- Norton SA, Cheruvu CV, Collins J, Dix FP, Eyre-Brook IA. An assessment of clinical guidelines for the management of acute pancreatitis. Ann R Coll Surg Engl 2001; 83:399–405.
- Chiang DT, Anozie A, Fleming WR, Kiroff GK. Comparative study on acute pancreatitis management. ANZ J Surg 2004; 74:218–221.
- Barnard J, Siriwardena AK. Variations in implementation of current national guidelines for the treatment of acute pancreatitis: implications for acute surgical service provision. Ann R Coll Surg Engl 2002; 84:79–81.
- Lankisch PG, Weber-Dany B, Lerch MM. Clinical perspectives in pancreatology: compliance with acute pancreatitis in Germany [letter]. Pancreatology 2005; 5:591–593.
- Connor SJ, Lienert AR, Brown LA, Bagshaw PF. Closing the audit loop is necessary to achieve compliance with evidence-based guidelines in the management of acute pancreatitis. N Z Med J 2008; 121:19–25.
- Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction, and death in acute pancreatitis. Br J Surg 2006; 93:738–744.
- Brown A, Orav J, Banks PA. Hemoconcentration is an early marker for organ failure and necrotizing pancreatitis. Pancreas 2000; 20:367–372.
- Lankisch PG, Mahlke R, Blum T, et al. Hemoconcentration: an early marker of severe and/or necrotizing pancreatitis? A critical appraisal. Am J Gastroenterol 2001; 96:2081–2085.
- Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009; 104:966–971.
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974; 139:69–81.
- Larvin M. Assessment of clinical severity and prognosis. In:Beger HG, Warshaw AL, Buchler MW, et al, editors. The Pancreas. Blackwell Science: New York, 1998:489–502.
- Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008; 57:1698–1703.
- Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993, 128:586–590.
- Forgacs B, Eible G, Faulhaber J, Kahrau S, Buhr H, Foitzik T. Effect of fluid resuscitation with and without endothelin A receptor blockade on hemoconcentration and organ function in experimental pancreatitis. Eur Surg Res 2000; 32:162–168.
- Brown A, Baillargeon JD, Hughes MD, Banks PA. Can fluid resuscitation prevent pancreatic necrosis in severe acute pancreatitis? Pancreatology 2002; 2:104–107.
- Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology 2007; 132:1127–1151.
- Pezzilli R, Uomo G, Gabbrielli A, et al; ProInf-AISP Study Group. A prospective multicenter survey on the treatment of acute pancreatitis in Italy. Dig Liver Dis 2007; 39:838–846.
- Gardner TB, Vege SS, Pearson RK, Chari ST. Fluid resuscitation in acute pancreatitis. Clin Gastroenterol Hepatol 2008; 6:1070–1076.
- Petrov MS, Pylypchuk RD, Emelyanov NV. Systematic review: nutritional support in acute pancreatitis. Aliment Pharmacol Ther 2008; 28:704–712.
- Marik PE, Zaloga GP. Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ 2004; 328:1407.
- Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990; 174:331–336.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Foitzik T, Bassi DG, Schmidt J, et al. Intravenous contrast medium accentuates the severity of acute necrotizing pancreatitis in the rat. Gastroenterology 1994; 106:207–214.
- Carmona-Sanchez R, Uscanga L, Bezaury-Rivas P, Robles-Díaz G, Suazo-Barahona J, Vargas-Vorácková F. Potential harmful effect of iodinated intravenous contrast medium on the clinical course of mild acute pancreatitis. Arch Surg 2000; 135:1280–1284.
- Uhl W, Roggo A, Kirschstein T, et al. Influence of contrast-enhanced computed tomography on couse and outcome in patients with acute pancreatitis. Pancreas 2002; 24:191–197.
- Foitzik T, Klar E. Non-compliance with guidelines for the management of severe acute pancreatitis among German surgeons. Pancreatology 2007; 7:80–85.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006;CD002941.
- Heinrich S, Schafer M, Rousson V, Clavien PA. Evidence-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg 2006; 243:154–168.
- Neoptolemos JP, Carr-Locke DL, London NJ, Bailey IA, James D, Fossard DP. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet 1988; 2:979–983.
- Fan ST, Lai EC, Mok FP, Lo CM, Zheng SS, Wong J. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med 1993; 328:228–232.
- Folsch UR, Nitsche R, Ludtke R, Hilgers RA, Creutzfeldt W. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. The German Study Group on Acute Biliary Pancreatitis. N Engl J Med 1997; 336:237–242.
- Ayub K, Imada R, Slavin J. Endoscopic retrograde cholangiopancreatography in gallstone associated pancreatitis. Cochrane Database Syst Rev 2004;CD003630
- Oria A, Cimmino D, Ocampo C, et al. Early endoscopic intervention versus early conservative management in patients with acute gallstone pancreatitis and biliopancreatic obstruction. A randomized clinical trial. Ann Surg 2007; 245:10–17.
- Frei GJ, Frei VT, Thirlby RC, McClelland RN. Biliary pancreatitis: clinical presentation and surgical management. Am J Surg 1986; 151:170–175.
- Uhl W, Warshaw A, Imrie C, et al; International Association of Pancreatology. IAP guidelines on the surgical management of acute pancreatitis. Pancreatology 2002; 2:565–573.
KEY POINTS
- Serum amylase and lipase levels are often needlessly measured every day.
- Often, severity assessments are not performed regularly or acted on.
- Often, not enough fluid is replaced, or fluid status is not adequately monitored.
- In many severe cases, enteral or parenteral feeding is not started soon enough.
- Computed tomography is not done in many patients with severe acute pancreatitis, or it is performed too soon.
- In many cases of suspected infected necrosis, fine-needle aspiration is not done.
- Broad-spectrum antibiotics are often used inappropriately in patients with mild acute pancreatitis and in patients with sterile necrotizing pancreatitis who are clinically stable and have no signs of sepsis.
Endoscopic therapy of recurrent acute pancreatitis
Endoscopic therapy has become an alternative to surgery for some patients with acute recurrent pancreatitis, ie, those whose disease is caused by gallstones or other mechanical processes that can obstruct the outflow from the pancreas.
In this paper, we review the specific situations in which endoscopic therapy might be useful in patients with acute recurrent pancreatitis.
ACUTE PANCREATITIS IS MANAGED DIFFERENTLY IF IT RECURS
Recurrent acute pancreatitis is defined as more than one episode of acute pancreatitis.1 In clinical practice, it is important to distinguish between the first and recurrent episodes of acute pancreatitis.
Most patients who have one episode of acute pancreatitis never have another one.2,3 Therefore, for patients having an initial attack, we recommend a limited workup that includes a detailed history, laboratory evaluation, and a noninvasive imaging study such as transcutaneous ultrasonography or computed tomography.
On the other hand, people who have a second attack are at higher risk of more recurrences. Therefore, patients having recurrent attacks need a more extensive workup to determine the underlying cause. We recommend referring them to a gastroenterologist for further evaluation.
WHICH CAUSES CAN BE MANAGED ENDOSCOPICALLY?
In the Western world, 70% to 80% of cases of recurrent pancreatitis are due to either alcohol abuse or gallstone disease.2,4 The rest are related to:
- Autoimmune disorders
- Cancer, including occult malignancies and premalignant conditions such as intraductal papillary mucinous neoplasm
- Chronic pancreatitis
- Drugs
- Heredity
- Metabolic abnormalities (hypertriglyceridemia, hypercalcemia)
- Sphincter of Oddi dysfunction
- Structural or congenital abnormalities (pancreas divisum)
- Trauma.
- Gallstone disease, including biliary microlithiasis and sludge (in patients with or without a gallbladder)
- Sphincter of Oddi dysfunction
- Pancreas divisum
- Obstruction to flow of pancreatic juice.
Endoscopy is not completely benign
Although endoscopic procedures are less invasive than surgery, they are not completely benign. They can cause anxiety and are associated with risks such as bleeding, perforation, and pancreatitis.5 The risks, benefits, and alternatives to these procedures should be discussed with the patient, and informed consent should be obtained before any endoscopic procedure.6
STONES (LARGE OR SMALL) OR SLUDGE IN PATIENTS WITH A GALLBLADDER
Gallstones can be large, but small stones (microlithiasis) and sludge are more common and therefore account for more cases of pancreatitis.
Strictly defined, microlithiasis refers to stones smaller than 2 mm in diameter in the biliary tract, whereas sludge is a suspension of biliary crystals, mucin, and cellular debris in the gallbladder or bile ducts.7 The terms are often used interchangeably, since the conditions often coexist and their treatment is similar.
Theories differ as to how microlithiasis or sludge can cause recurrent pancreatitis. According to one theory, the debris blocks the common channel, increasing the pancreatic intraductal pressure and leading to pancreatitis.8 A second theory is that small stones or biliary crystals passing through the sphincter of Oddi cause inflammation, and that repeated inflammation eventually leads to stenosis or dyskinesia of the sphincter, both of which have been associated with pancreatitis.9
Studies suggest that microlithiasis and sludge are common causes of recurrent pancreatitis, accounting for about two-thirds of cases according to estimates by Ros et al10 and Lee et al.11
Detecting small stones and sludge
The diagnosis of microlithiasis and biliary sludge in patients with a gallbladder is based on imaging studies and bile microscopy.12
Transabdominal ultrasonography is the imaging study most often used for diagnosing microlithiasis. The technology and expertise for this test are widely available, and it is relatively inexpensive.
Endoscopic ultrasonography is more sensitive for detecting microlithiasis and can examine the distal common bile duct.
Bile microscopy involves obtaining bile from the second portion of the duodenum (via an endoscope or a duodenal tube) or from the bile ducts (by cannulating the common bile duct and stimulating the gallbladder with cholecystokinin). The bile sample is centrifuged and inspected microscopically under plain light and polarized light (which aids the visualization of biliary crystals). The crystals can be cholesterol monohydrate, calcium bilirubinate, or calcium carbonate.7,13,14
Removing the gallbladder is the treatment of choice for small stones and sludge
Treatments to prevent recurrent attacks of acute pancreatitis due to microlithiasis and sludge include cholecystectomy, biliary sphincterotomy, and ursodioxycholic acid.10,11,15
In prospective observational studies by Ros et al10 and Lee et al,11 about half of the patients with recurrent pancreatitis were treated with cholecystectomy, endoscopic sphincterotomy, or ursodioxycholic acid in a nonrandomized fashion. The choice of therapy was based on the patient’s medical status and the preferences of the patient and the physician. Half the patients received no treatment. In both studies the median follow-up was 4 years. Treated patients had a significantly lower rate of recurrent attacks of pancreatitis during follow-up: less than 20% with therapy compared with more than 60% without therapy. Unfortunately, no published study has compared these three treatments head to head.
Cholecystectomy, however, is the most definitive therapy and is generally considered the treatment of choice.
Biliary sphincterotomy is an endoscopic procedure that involves cutting the sphincter of Oddi to allow the stones and sludge to pass more freely. It is as effective as cholecystectomy in preventing recurrent attacks but does not eliminate the risk of cholecystitis and cholangitis (Figure 1). For this reason, it is usually reserved for patients who cannot tolerate surgery due to comorbidities, those who refuse surgery, or those who are pregnant.16
Ursodeoxycholic acid is a reasonable alternative in patients who cannot tolerate surgical or endoscopic biliary sphincterotomy.1,17–20 The dosage is 10 mg/kg/day, which can be in two or three divided doses. The optimal duration of treatment is not known; however, since this drug works slowly, it may need to be taken for 2 years or more. Ursodeoxycholic acid is more effective in patients with cholesterol-based stones and crystals. It is not effective for large stones (> 1 cm in diameter) or calcified stones.
STONES AFTER CHOLECYSTECTOMY
Bile duct stones can be classified as primary or secondary. A primary stone is one that remains where it was formed, whereas a secondary stone is one that has migrated from its site of formation.21
Some suggest that bile duct stones that are detected within 2 years of cholecystectomy originated in the gallbladder and were missed when the gallbladder was removed (and therefore are considered secondary stones), and that stones that present more than 2 years after cholecystectomy are de novo (ie, primary) stones.22,23
In any event, stones have been found in the common bile duct in 4% to 24% of patients up to 15 years after cholecystectomy.24–26 A fair number of these patients have no symptoms.27 Risk factors for stone recurrence are lithogenic bile (ie, high concentration of cholesterol, low concentration of bile salts), biliary stasis, strictures, dilated bile ducts, and advanced age.28–30
No role for crystal analysis after cholecystectomy
Biliary crystal analysis does not seem to have diagnostic value in patients with recurrent acute pancreatitis after cholecystectomy,31 because removing the gallbladder eliminates the crystals and sludge. Imaging studies are therefore the cornerstone of diagnosis.
Transabdominal ultrasonography is the most commonly used initial imaging test. However, abdominal fat and gas in the duodenum can obscure the distal common bile duct and decrease the sensitivity of this test.32
Endoscopic ultrasonography involves positioning the transducer in the second part of the duodenum, where it can show the adjacent biliary tree without interference from digestive gas or abdominal fat.
Magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasonography are both highly sensitive for detecting common bile duct stones and are recommended if they can be done without delay.
Endoscopic retrograde cholangiopancreatography (ERCP). As a rule, patients who are very likely to have gallstones are best served by proceeding directly to ERCP, a procedure that enables both imaging and treatment. However, ERCP exposes the patient to radiation and the risk of pancreatitis, so in some patients (eg, pregnant women, people who recently had acute pancreatitis), one may want to do ultrasonography first.
ERCP is the treatment of choice after cholecystectomy
The treatment of choice in patients with choledocholithiasis is ERCP with biliary sphincterotomy and stone extraction. Success at clearing the biliary tree of all stones depends on the size, number, and location of the stones, the anatomy of the digestive tract and the bile duct, and the experience of the endoscopist. At specialized centers, the rate of successful clearance with subsequent procedures is close to 100%. Large stones may require fragmentation inside the bile duct to aid their removal.33
SPHINCTER OF ODDI DYSFUNCTION
The sphincter of Oddi, located where the bile and pancreatic ducts penetrate the wall of the duodenum, actually consists of three sphincters: the common, the biliary, and the pancreatic. Its physiologic role is to regulate the flow of bile and pancreatic juice into the duodenum and to prevent reflux into the ducts from the duodenum.34 Its basal pressure is the main regulating mechanism for pancreatic and biliary secretions into the intestine, and its phasic contractile activity is closely associated with duodenal motility.
Sphincter dysfunction: Stenosis, dyskinesia
The sphincter of Oddi can obstruct the flow of bile and pancreatic juice owing either to stenosis or to dyskinesia.35,36 Stenosis refers to structural alteration of the sphincter, probably from inflammation and subsequent fibrosis. In contrast, dyskinesia refers to a motor abnormality of the sphincter that makes it hypertonic.
Stenosis or dyskinesia can occur in the biliary sphincter, the pancreatic sphincter, the common sphincter, or any combination of the three. For example, dysfunction of the biliary sphincter can cause abnormalities in liver-associated enzyme levels and biliary-type pain, whereas pancreatic sphincter dysfunction can cause recurrent attacks of pancreatitis and pancreatic-type pain.37 Elevated pancreatic sphincter pressure has been shown to correlate with increased pancreatic ductal pressure, suggesting that the sphincter plays a role in the pathogenesis of acute pancreatitis.23,38
Sphincter pressure can be measured during ERCP, but ERCP is risky
The gold standard for the diagnosis of sphincter of Oddi dysfunction is manometry,23,35 ie, direct measurement of sphincter pressure via a thin catheter placed inside the pancreatic or biliary sphincter during ERCP (Figure 1).
However, in patients with suspected sphincter of Oddi dysfunction, ERCP with or without manometry is associated with a high rate of complications, with pancreatitis occurring in up to 25% of cases.39–41 Therefore, several noninvasive and provocative tests have been designed in an attempt to identify patients with this disorder. Unfortunately, none of them seems to be as sensitive and specific as manometry for diagnosing sphincter of Oddi dysfunction, and so they have not gained widespread use.
Opening the sphincter of Oddi with drugs, endoscopy, or surgery
Drug treatment of sphincter of Oddi dysfunction is based on drugs that relax smooth muscle, such as calcium channel blockers and nitrates. The treatment must be lifelong. Also, it does not improve sphincter stenosis, and only half of patients with sphincter dyskinesia respond to it. For these reasons, drug treatment of sphincter of Oddi dysfunction has not gained widespread acceptance.36,42
Endoscopic sphincterotomy is the current standard endoscopic therapy for sphincter of Oddi dysfunction. This procedure is performed during ERCP and involves cutting the sphincter with electrocautery.
Endoscopic pancreatic sphincterotomy prevents recurrent attacks of pancreatitis in patients with pancreatic sphincter dysfunction in more than 60% of cases.23,43–46 A potential complication is pancreatitis, which occurs more often in patients with pancreatic sphincter dyskinesia. Placing a stent in the pancreatic duct after pancreatic sphincterotomy reduces the risk of pancreatitis after ERCP.37,47,48
Surgery. Pancreatic sphincterotomy can also be done surgically, most commonly via transduodenal pancreatic sphincteroplasty. Surgical sphincteroplasty is as effective as endoscopic sphincterotomy for preventing recurrent attacks of pancreatitis in patients with pancreatic sphincter dysfunction.49 However, endoscopic therapy is much less invasive and remains the preferred treatment for sphincter of Oddi dysfunction in most centers with experience in this technique.50
PANCREAS DIVISUM
Pancreas divisum is the most common congenital anomaly of the pancreatic duct. Autopsy studies show it occurs in 5% to 10% of the population.51–53
At approximately the 5th week of gestation, there are two pancreatic buds: a ventral and a dorsal bud. The ventral bud eventually gives rise to part of the pancreatic head and uncinate process of the pancreas in the adult. The dorsal bud eventually gives rise to the rest of the pancreatic head, the pancreatic body, and the pancreatic tail. At 6 to 7 weeks of gestation, the ventral bud rotates clockwise and lies posterior to the dorsal bud. At this stage, both the dorsal and ventral pancreata have their own ducts, which do not communicate with each other. Normally, the ventral and dorsal pancreas and their ducts fuse together at 8 weeks of gestation; in people with pancreas divisum, this ductal fusion does not occur.51
The pancreas secretes 1.5 L of fluid per day. Normally, 90% to 95% of this volume drains through the major papilla. In people with pancreas divisum, 90% to 95% of the fluid drains through the minor papilla.
People with pancreas divisum are a heterogeneous group. Most have no symptoms, and their ductal anatomy is diagnosed only incidentally. However, a subgroup is prone to develop acute pancreatitis. The cause is thought to be the small diameter of the minor papilla, which poses a relative obstruction to the flow of pancreatic juice.54 Direct support for this theory comes from a study in which investigators measured pancreatic ductal pressures in eight people with normal anatomy and six people with pancreas divisum. The pressure in the main pancreatic duct in those with pancreas divisum was significantly higher than in those with normal anatomy.55 Additional evidence in favor of this theory is the effectiveness of treatment, which involves widening the minor papillary opening (minor papillary sphincterotomy).
Diagnosis of pancreas divisum
The diagnosis of pancreas divisum is based on imaging studies, and ERCP remains the gold standard for patients with equivocal results on noninvasive imaging. However, MRCP, especially secretin-enhanced MRCP, is as accurate as ERCP. In most cases, MRCP has replaced ERCP for the diagnosis of this condition, although a recent study suggests that MRCP is inferior to ERCP in the diagnosis of pancreas divisum.56 We recommend secretin-enhanced MRCP for this purpose.
Computed tomography and endoscopic ultrasonography can also diagnose pancreas divisum, but their diagnostic accuracy is lower than that of ERCP and MRCP.
Minor papillary sphincterotomy
Treating recurrent pancreatitis due to pancreas divisum involves relieving the relative obstruction of the minor papilla by minor papillary sphincterotomy. This can be done surgically or endoscopically (Figure 1).
Surgery. No randomized, controlled study has yet assessed the efficacy of surgical sphincteroplasty for recurrent pancreatitis in patients with pancreas divisum. However, retrospective studies and one prospective study have been published.57,58
In the retrospective study with the largest number of patients, Warshaw et al57 reported their experience in 49 patients who had recurrent pancreatitis due to pancreas divisum. After surgical sphincteroplasty, the patients were followed for a mean of 53 months; 40 (82%) of the 49 patients had no further episodes of acute pancreatitis during this time.
Bradley and Stephan58 studied 37 patients with pancreas divisum and recurrent pancreatitis.58 After surgical sphincteroplasty, the patients were followed for a mean of 60 months; 31 of the 37 patients had no further attacks, a success rate of 84%.
Endoscopic therapy. As with surgical therapy trials, most trials of endoscopic therapy of recurrent pancreatitis in patients with pancreas divisum are small case series. In a retrospective study with one of the largest number of patients, Heyries et al59 reported their experience with 24 patients with pancreas divisum and recurrent pancreatitis. After undergoing endoscopic minor papillary sphincterotomy, all patients were followed for a mean of 39 months, during which 22 (92%) did not have further episodes of acute pancreatitis.
In the only randomized controlled trial available, 19 patients with recurrent pancreatitis and pancreas divisum underwent either no treatment or endoscopic minor papillary sphincterotomy.60 In the treatment group, 9 of 10 patients had no further episodes of acute pancreatitis during the 3 years of follow-up, while 6 of 9 patients who were randomized to no treatment had at least one episode.60
Although surgical and endoscopic minor papillary sphincterotomy are equally effective, endoscopic therapy is preferred since it is less invasive, is associated with less morbidity, and costs less. It is also more convenient for patients, since it is an outpatient procedure. Surgical treatment is usually reserved for those in whom endoscopic treatment has failed or is not technically possible.
OTHER PROCESSES OBSTRUCTING THE FLOW OF PANCREATIC JUICE
Any process preventing free flow of pancreatic juice can lead to acute pancreatitis. The cause of the blockage can be around the ampulla, in the ampulla, or in the duct.61
Periampullary lesions, tumors, or polyps can press on the ampulla and cause complete or relative obstruction of the pancreatic duct with a subsequent increase in intraductal pressure and, thus, acute pancreatitis.62 Tumors or polyps of the ampulla, such as ampullary adenoma or carcinoma, can cause pancreatitis by directly obstructing the pancreatic duct where it opens into the duodenum.63–66 Intraductal processes such as ductal adenocarcinoma, intraductal papillary mucinous tumor, pancreatic duct stone, and intraductal stricture due to cancer, chronic pancreatitis, or trauma can also cause pancreatitis by preventing free flow of pancreatic juice.67–71
Although it is well known that sequelae of severe chronic pancreatitis such as ductal strictures or intraductal stones can lead to recurrent attacks of acute pancreatitis by preventing the free flow of pancreatic juice, a relationship also seems to exist between early chronic pancreatitis and recurrent acute pancreatitis.72 Several studies have shown that up to 50% of patients with idiopathic recurrent pancreatitis have evidence of chronic pancreatitis.72–74 However, it is still unclear whether early chronic pancreatitis is the underlying cause of the recurrent attacks of acute pancreatitis or whether recurrent attacks of acute pancreatitis might have led to the development of chronic pancreatitis.
Diagnosis
Ampullary and periampullary neoplasms can be diagnosed endoscopically. Intraductal lesions such as strictures can be diagnosed by MRCP, especially secretin-enhanced MRCP, or by ERCP. ERCP has the additional advantage of being able to deliver treatment, ie, balloon dilation and stenting. In the case of ductal strictures, upsizing of the stents or placement of multiple stents during subsequent procedures is usually needed. Pancreatic ductal calcifications associated with chronic pancreatitis are usually radiopaque and are easily visible on plain films or computed tomography of the abdomen. Parenchymal and ductal changes of chronic pancreatitis can be diagnosed by endoscopic ultrasonography.
Treatment
The treatment is to relieve the obstruction and re-establish the free flow of pancreatic juice.
Periampullary tumors or polyps can be resected surgically or, if they involve only the mucosa, by endoscopic mucosal resection. Ampullary adenomas can be resected endoscopically. Ampullary carcinomas usually require surgical resection.
Small, nonobstructive stones in the pancreatic duct can be removed during ERCP.75 Larger stones may need to be fragmented by extracorporeal shock wave lithotripsy to facilitate removal by ERCP.75
Intraductal strictures should raise the suspicion of pancreatic adenocarcinoma, especially in older patients.61 In these cases, relief of the obstruction by placement of a pancreatic stent can prevent further attacks of pancreatitis until a diagnosis can be established and a more definitive treatment can be offered.
- Levy MJ, Geenen JE. Idiopathic acute recurrent pancreatitis. Am J Gastroenterol 2001; 96:2540–2555.
- Gullo L, Migliori M, Pezzilli R, et al. An update on recurrent acute pancreatitis: data from five European countries. Am J Gastroenterol 2002; 97:1959–1962.
- Gao YJ, Li YQ, Wang Q, et al. Analysis of the clinical features of recurrent acute pancreatitis in China. J Gastroenterol 2006; 41:681–685.
- Somogyi L, Martin SP, Venkatesan T, Ulrich CD. Recurrent acute pancreatitis: an algorithmic approach to identification and elimination of inciting factors. Gastroenterology 2001; 120:708–717.
- Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 2007; 102:1781–1788.
- Standards of Practice Committee,Zuckerman MJ, Shen B, Harrison ME, et al. Informed consent for GI endoscopy. Gastrointest Endosc 2007; 66:213–218.
- Lee SP, Hayashi A, Kim YS. Biliary sludge: curiosity or culprit? Hepatology 1994; 20:523–525.
- Opie E. The etiology of acute hemorrhagic pancreatitis. Bull Johns Hopkins Hosp 1901; 12:182–188.
- Hernandez CA, Lerch MM. Sphincter stenosis and gallstone migration through the biliary tract. Lancet 1993; 341:1371–1373.
- Ros E, Navarro S, Bru C, Garcia-Puges A, Valderrama R. Occult microlithiasis in 'idiopathic' acute pancreatitis: prevention of relapses by cholecystectomy or ursodeoxycholic acid therapy. Gastroenterology 1991; 101:1701–1709.
- Lee SP, Nicholls JF, Park HZ. Biliary sludge as a cause of acute pancreatitis. N Engl J Med 1992; 326:589–593.
- Levy MJ. The hunt for microlithiasis in idiopathic acute recurrent pancreatitis: should we abandon the search or intensify our efforts? Gastrointest Endosc 2002; 55:286–293.
- Delchier JC, Benfredj P, Preaux AM, Metreau JM, Dhumeaux D. The usefulness of microscopic bile examination in patients with suspected microlithiasis: a prospective evaluation. Hepatology 1986; 6:118–122.
- Lee SP, Nicholls JF. Nature and composition of biliary sludge. Gastroenterology 1986; 90:677–686.
- Testoni PA, Caporuscio S, Bagnolo F, Lella F. Idiopathic recurrent pancreatitis: long-term results after ERCP, endoscopic sphincterotomy, or ursodeoxycholic acid treatment. Am J Gastroenterol 2000; 95:1702–1707.
- Siddiqui AA, Mitroo P, Kowalski T, Loren D. Endoscopic sphincterotomy with or without cholecystectomy for choledocholithiasis in high-risk surgical patients: a decision analysis. Aliment Pharmacol Ther 2006; 24:1059–1066.
- Steinberg WM, Chari ST, Forsmark CE, et al. Controversies in clinical pancreatology: management of acute idiopathic recurrent pancreatitis. Pancreas 2003; 27:103–117.
- Khalid A, Slivka A. Approach to idiopathic recurrent pancreatitis. Gastrointest Endosc Clin North Am 2003; 13:695–716.
- Adler DG, Baron TH, Davila RE, et al; Standards of Practice Committee of American Society for Gastrointestinal Endoscopy. ASGE guideline: the role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest Endosc 2005; 62:1–8.
- Draganov P, Forsmark CE. “Idiopathic” pancreatitis. Gastroenterology 2005; 128:756–763.
- Chung EJ, Kim MH, Lee SS, Lee SK. Primary vs. secondary common bile duct stones: apples and oranges. Endoscopy 2003; 35:92.
- Saharia PC, Zuidema GD, Cameron JL. Primary common duct stones. Ann Surg 1977; 185:598–604.
- Elta GH. Sphincter of Oddi dysfunction and bile duct microlithiasis in acute idiopathic pancreatitis. World J Gastroenterol 2008; 14:1023–1026.
- Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med 1996; 335:909–918.
- Prat F, Malak NA, Pelletier G, et al. Biliary symptoms and complications more than 8 years after endoscopic sphincterotomy for choledocholithiasis. Gastroenterology 1996; 110:894–899.
- Hawes RH, Cotton PB, Vallon AG. Follow-up 6 to 11 years after duo-denoscopic sphincterotomy for stones in patients with prior cholecystectomy. Gastroenterology 1990; 98:1008–1012.
- Lai KH, Lo GH, Lin CK, et al. Do patients with recurrent choledocholithiasis after endoscopic sphincterotomy benefit from regular follow-up? Gastrointest Endosc 2002; 55:523–526.
- Kim DI, Kim MH, Lee SK, et al. Risk factors for recurrence of primary bile duct stones after endoscopic biliary sphincterotomy. Gastrointest Endosc 2001; 54:42–48.
- Costamagna G, Tringali A, Shah SK, Mutignani M, Zuccala G, Perri V. Long-term follow-up of patients after endoscopic sphincterotomy for choledocholithiasis, and risk factors for recurrence. Endoscopy 2002; 34:273–279.
- Keizman D, Ish Shalom M, Konikoff FM. Recurrent symptomatic common bile duct stones after endoscopic stone extraction in elderly patients. Gastrointest Endosc 2006; 64:60–65.
- Kaw M, Brodmerkel GJ. ERCP, biliary crystal analysis, and sphincter of Oddi manometry in idiopathic recurrent pancreatitis. Gastrointest Endosc 2002; 55:157–162.
- Chak A, Hawes RH, Cooper GS, et al. Prospective assessment of the utility of EUS in the evaluation of gallstone pancreatitis. Gastrointest Endosc 1999; 49:599–604.
- Parsi MA, Neuhaus H, Pleskow D, et al. Peroral cholangioscopy guided stone therapy—report of an international multicenter registry [abstract]. Gastrointest Endosc 2008; 67:AB102.
- Woods CM, Mawe GM, Toouli J, Saccone GT. The sphincter of Oddi: understanding its control and function. Neurogastroenterol Motil 2005; 17 suppl 1:31–40.
- McLoughlin MT, Mitchell RM. Sphincter of Oddi dysfunction and pancreatitis. World J Gastroenterol 2007; 13:6333–6343.
- Bosch A, Pena LR. The sphincter of Oddi. Dig Dis Sci 2007; 52:1211–1218.
- Devereaux BM, Sherman S, Lehman GA. Sphincter of Oddi (pancreatic) hypertension and recurrent pancreatitis. Curr Gastroenterol Rep 2002; 4:153–159.
- Fazel A, Geenen JE, MoezArdalan K, Catalano MF. Intrapancreatic ductal pressure in sphincter of Oddi dysfunction. Pancreas 2005; 30:359–362.
- Freeman ML. Role of pancreatic stents in prevention of post-ERCP pancreatitis. JOP 2004; 5:322–327.
- Singh P, Gurudu SR, Davidoff S, et al. Sphincter of Oddi manometry does not predispose to post-ERCP acute pancreatitis. Gastrointest Endosc 2004; 59:499–505.
- Guda NM, Freeman ML. True culprit or guilt by association? Is sphincter of Oddi manometry the cause of post-ERCP pancreatitis in patients with suspected sphincter of Oddi dysfunction, or is it the patients' susceptibility? Rev Gastroenterol Disord 2004; 4:211–213.
- Craig A, Toouli J. Sphincter of Oddi dysfunction: is there a role for medical therapy? Curr Gastroenterol Rep 2002; 4:172–176.
- Freeman ML, Gill M, Overby C, Cen YY. Predictors of outcomes after biliary and pancreatic sphincterotomy for sphincter of Oddi dysfunction. J Clin Gastroenterol 2007; 41:94–102.
- Sgouros SN, Pereira SP. Systematic review: sphincter of Oddi dysfunction—non-invasive diagnostic methods and long-term outcome after endoscopic sphincterotomy. Aliment Pharmacol Ther 2006; 24:237–246.
- Venu RP, Geenen JE, Hogan W, Stone J, Johnson GK, Soergel K. Idiopathic recurrent pancreatitis. An approach to diagnosis and treatment. Dig Dis Sci 1989; 34:56–60.
- Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Engl J Med 1989; 320:82–87.
- Fogel EL, Eversman D, Jamidar P, Sherman S, Lehman GA. Sphincter of Oddi dysfunction: pancreaticobiliary sphincterotomy with pancreatic stent placement has a lower rate of pancreatitis than biliary sphincterotomy alone. Endoscopy 2002; 34:280–285.
- Freeman ML. Pancreatic stents for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol 2007; 5:1354–1365.
- Toouli J. The sphincter of Oddi and acute pancreatitis - revisited. HPB (Oxford) 2003; 5:142–145.
- Sherman S, Lehman GA. Sphincter of Oddi dysfunction: diagnosis and treatment. JOP 2001; 2:382–400.
- Klein SD, Affronti JP. Pancreas divisum, an evidence-based review: part I, pathophysiology. Gastrointest Endosc 2004; 60:419–425.
- Fogel EL, Toth TG, Lehman GA, DiMagno MJ, DiMagno EP. Does endoscopic therapy favorably affect the outcome of patients who have recurrent acute pancreatitis and pancreas divisum? Pancreas 2007; 34:21–45.
- Lehman GA. Acute recurrent pancreatitis. Can J Gastroenterol 2003; 17:381–383.
- Lehman GA, Sherman S. Pancreas divisum. Diagnosis, clinical significance, and management alternatives. Gastrointest Endosc Clin N Am 1995; 5:145–170.
- Staritz M, Meyer zum Buschenfelde KH. Elevated pressure in the dorsal part of pancreas divisum: the cause of chronic pancreatitis? Pancreas 1988; 3:108–110.
- Carnes M, Romagnuolo J, Cotton P. Miss rate of pancreas divisum by magnetic resonance cholangiopancreatography in clinical practice. Pancreas 2008; 37:151–153.
- Warshaw AL, Simeone JF, Schapiro RH, Flavin-Warshaw B. Evaluation and treatment of the dominant dorsal duct syndrome (pancreas divisum redefined). Am J Surg 1990; 159:59–64.
- Bradley EL, Stephan RN. Accessory duct sphincteroplasty is preferred for long-term prevention of recurrent acute pancreatitis in patients with pancreas divisum. J Am Coll Surg 1996; 183:65–70.
- Heyries L, Barthet M, Delvasto C, Zamora C, Bernard JP, Sahel J. Long-term results of endoscopic management of pancreas divisum with recurrent acute pancreatitis. Gastrointest Endosc 2002; 55:376–381.
- Lans JI, Geenen JE, Johanson JF, Hogan WJ. Endoscopic therapy in patients with pancreas divisum and acute pancreatitis: a prospective, randomized, controlled clinical trial. Gastrointest Endosc 1992; 38:430–434.
- Delhaye M, Matos C, Arvanitakis M, Deviere J. Pancreatic ductal system obstruction and acute recurrent pancreatitis. World J Gastroenterol 2008; 14:1027–1033.
- Finnie IA, Ghosh P, Garvey C, Poston GJ, Rhodes JM. Intraluminal duodenal diverticulum causing recurrent pancreatitis: treatment by endoscopic incision. Gut 1994; 35:557–559.
- Guzzardo G, Kleinman MS, Krackov JH, Schwartz SI. Recurrent acute pancreatitis caused by ampullary villous adenoma. J Clin Gastroenterol 1990; 12:200–202.
- Wright BE, Kozarek RA, Traverso LW, Wechter D, Thirlby R, Raltz SL. Recurrent pancreatitis in Gardner variant familial polyposis: etiology, diagnostic approach, and interventional results. Arch Surg 1999; 134:311–315.
- Tanasijtchouk T, Vaisbein E, Lachter J, Nassar F. Carcinoma of Papilla Vateri presenting as recurrent acute pancreatitis. Acta Gastroenterol Belg 2004; 67:309–310.
- Kwon TH, Park do H, Shim KY, et al. Ampullary adenomyoma presenting as acute recurrent pancreatitis. World J Gastroenterol 2007; 13:2892–2894.
- Lorente JA, Ruiz del Arbol L, Moreira VF, Garcia-Plaza A. Recurrent pancreatitis in a young patient associated with a solitary nonopaque concretion in the main pancreatic duct. Gastrointest Endosc 1990; 36:63–65.
- Chung JP, Chi SW, Park YN, et al. A case of minute intraductal papillary mucinous tumor of the pancreas presenting with recurrent acute pancreatitis. Yonsei Med J 2000; 41:528–532.
- Tikhomirov V, Tikhomirova S, Sieber S, Schiffman MK. A pancreatic intraductal papillary mucinous tumor causing recurrent acute pancreatitis at the onset of menstrual periods. J Clin Gastroenterol 2000; 31:172–174.
- Mosca S, Bottino V, Molino C. Hepatobiliary and pancreatic: a woman with recurrent idiopathic acute pancreatitis. Intraductal papillary mucinous tumor of the pancreas. J Gastroenterol Hepatol 2001; 16:1070,1075.
- Howard TJ, Moore SA, Saxena R, Matthews DE, Schmidt CM, Wiebke EA. Pancreatic duct strictures are a common cause of recurrent pancreatitis after successful management of pancreatic necrosis. Surgery 2004; 136:909–916.
- Garg PK, Tandon RK, Madan K. Is biliary microlithiasis a significant cause of idiopathic recurrent acute pancreatitis? A long-term follow-up study. Clin Gastroenterol Hepatol 2007; 5:75–79.
- Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol 2001; 96:705–709.
- Yusoff IF, Raymond G, Sahai AV. A prospective comparison of the yield of EUS in primary vs. recurrent idiopathic acute pancreatitis. Gastrointest Endosc 2004; 60:673–678.
- Cahen DL, Gouma DJ, Nio Y, et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med 2007; 356:676–684.
Endoscopic therapy has become an alternative to surgery for some patients with acute recurrent pancreatitis, ie, those whose disease is caused by gallstones or other mechanical processes that can obstruct the outflow from the pancreas.
In this paper, we review the specific situations in which endoscopic therapy might be useful in patients with acute recurrent pancreatitis.
ACUTE PANCREATITIS IS MANAGED DIFFERENTLY IF IT RECURS
Recurrent acute pancreatitis is defined as more than one episode of acute pancreatitis.1 In clinical practice, it is important to distinguish between the first and recurrent episodes of acute pancreatitis.
Most patients who have one episode of acute pancreatitis never have another one.2,3 Therefore, for patients having an initial attack, we recommend a limited workup that includes a detailed history, laboratory evaluation, and a noninvasive imaging study such as transcutaneous ultrasonography or computed tomography.
On the other hand, people who have a second attack are at higher risk of more recurrences. Therefore, patients having recurrent attacks need a more extensive workup to determine the underlying cause. We recommend referring them to a gastroenterologist for further evaluation.
WHICH CAUSES CAN BE MANAGED ENDOSCOPICALLY?
In the Western world, 70% to 80% of cases of recurrent pancreatitis are due to either alcohol abuse or gallstone disease.2,4 The rest are related to:
- Autoimmune disorders
- Cancer, including occult malignancies and premalignant conditions such as intraductal papillary mucinous neoplasm
- Chronic pancreatitis
- Drugs
- Heredity
- Metabolic abnormalities (hypertriglyceridemia, hypercalcemia)
- Sphincter of Oddi dysfunction
- Structural or congenital abnormalities (pancreas divisum)
- Trauma.
- Gallstone disease, including biliary microlithiasis and sludge (in patients with or without a gallbladder)
- Sphincter of Oddi dysfunction
- Pancreas divisum
- Obstruction to flow of pancreatic juice.
Endoscopy is not completely benign
Although endoscopic procedures are less invasive than surgery, they are not completely benign. They can cause anxiety and are associated with risks such as bleeding, perforation, and pancreatitis.5 The risks, benefits, and alternatives to these procedures should be discussed with the patient, and informed consent should be obtained before any endoscopic procedure.6
STONES (LARGE OR SMALL) OR SLUDGE IN PATIENTS WITH A GALLBLADDER
Gallstones can be large, but small stones (microlithiasis) and sludge are more common and therefore account for more cases of pancreatitis.
Strictly defined, microlithiasis refers to stones smaller than 2 mm in diameter in the biliary tract, whereas sludge is a suspension of biliary crystals, mucin, and cellular debris in the gallbladder or bile ducts.7 The terms are often used interchangeably, since the conditions often coexist and their treatment is similar.
Theories differ as to how microlithiasis or sludge can cause recurrent pancreatitis. According to one theory, the debris blocks the common channel, increasing the pancreatic intraductal pressure and leading to pancreatitis.8 A second theory is that small stones or biliary crystals passing through the sphincter of Oddi cause inflammation, and that repeated inflammation eventually leads to stenosis or dyskinesia of the sphincter, both of which have been associated with pancreatitis.9
Studies suggest that microlithiasis and sludge are common causes of recurrent pancreatitis, accounting for about two-thirds of cases according to estimates by Ros et al10 and Lee et al.11
Detecting small stones and sludge
The diagnosis of microlithiasis and biliary sludge in patients with a gallbladder is based on imaging studies and bile microscopy.12
Transabdominal ultrasonography is the imaging study most often used for diagnosing microlithiasis. The technology and expertise for this test are widely available, and it is relatively inexpensive.
Endoscopic ultrasonography is more sensitive for detecting microlithiasis and can examine the distal common bile duct.
Bile microscopy involves obtaining bile from the second portion of the duodenum (via an endoscope or a duodenal tube) or from the bile ducts (by cannulating the common bile duct and stimulating the gallbladder with cholecystokinin). The bile sample is centrifuged and inspected microscopically under plain light and polarized light (which aids the visualization of biliary crystals). The crystals can be cholesterol monohydrate, calcium bilirubinate, or calcium carbonate.7,13,14
Removing the gallbladder is the treatment of choice for small stones and sludge
Treatments to prevent recurrent attacks of acute pancreatitis due to microlithiasis and sludge include cholecystectomy, biliary sphincterotomy, and ursodioxycholic acid.10,11,15
In prospective observational studies by Ros et al10 and Lee et al,11 about half of the patients with recurrent pancreatitis were treated with cholecystectomy, endoscopic sphincterotomy, or ursodioxycholic acid in a nonrandomized fashion. The choice of therapy was based on the patient’s medical status and the preferences of the patient and the physician. Half the patients received no treatment. In both studies the median follow-up was 4 years. Treated patients had a significantly lower rate of recurrent attacks of pancreatitis during follow-up: less than 20% with therapy compared with more than 60% without therapy. Unfortunately, no published study has compared these three treatments head to head.
Cholecystectomy, however, is the most definitive therapy and is generally considered the treatment of choice.
Biliary sphincterotomy is an endoscopic procedure that involves cutting the sphincter of Oddi to allow the stones and sludge to pass more freely. It is as effective as cholecystectomy in preventing recurrent attacks but does not eliminate the risk of cholecystitis and cholangitis (Figure 1). For this reason, it is usually reserved for patients who cannot tolerate surgery due to comorbidities, those who refuse surgery, or those who are pregnant.16
Ursodeoxycholic acid is a reasonable alternative in patients who cannot tolerate surgical or endoscopic biliary sphincterotomy.1,17–20 The dosage is 10 mg/kg/day, which can be in two or three divided doses. The optimal duration of treatment is not known; however, since this drug works slowly, it may need to be taken for 2 years or more. Ursodeoxycholic acid is more effective in patients with cholesterol-based stones and crystals. It is not effective for large stones (> 1 cm in diameter) or calcified stones.
STONES AFTER CHOLECYSTECTOMY
Bile duct stones can be classified as primary or secondary. A primary stone is one that remains where it was formed, whereas a secondary stone is one that has migrated from its site of formation.21
Some suggest that bile duct stones that are detected within 2 years of cholecystectomy originated in the gallbladder and were missed when the gallbladder was removed (and therefore are considered secondary stones), and that stones that present more than 2 years after cholecystectomy are de novo (ie, primary) stones.22,23
In any event, stones have been found in the common bile duct in 4% to 24% of patients up to 15 years after cholecystectomy.24–26 A fair number of these patients have no symptoms.27 Risk factors for stone recurrence are lithogenic bile (ie, high concentration of cholesterol, low concentration of bile salts), biliary stasis, strictures, dilated bile ducts, and advanced age.28–30
No role for crystal analysis after cholecystectomy
Biliary crystal analysis does not seem to have diagnostic value in patients with recurrent acute pancreatitis after cholecystectomy,31 because removing the gallbladder eliminates the crystals and sludge. Imaging studies are therefore the cornerstone of diagnosis.
Transabdominal ultrasonography is the most commonly used initial imaging test. However, abdominal fat and gas in the duodenum can obscure the distal common bile duct and decrease the sensitivity of this test.32
Endoscopic ultrasonography involves positioning the transducer in the second part of the duodenum, where it can show the adjacent biliary tree without interference from digestive gas or abdominal fat.
Magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasonography are both highly sensitive for detecting common bile duct stones and are recommended if they can be done without delay.
Endoscopic retrograde cholangiopancreatography (ERCP). As a rule, patients who are very likely to have gallstones are best served by proceeding directly to ERCP, a procedure that enables both imaging and treatment. However, ERCP exposes the patient to radiation and the risk of pancreatitis, so in some patients (eg, pregnant women, people who recently had acute pancreatitis), one may want to do ultrasonography first.
ERCP is the treatment of choice after cholecystectomy
The treatment of choice in patients with choledocholithiasis is ERCP with biliary sphincterotomy and stone extraction. Success at clearing the biliary tree of all stones depends on the size, number, and location of the stones, the anatomy of the digestive tract and the bile duct, and the experience of the endoscopist. At specialized centers, the rate of successful clearance with subsequent procedures is close to 100%. Large stones may require fragmentation inside the bile duct to aid their removal.33
SPHINCTER OF ODDI DYSFUNCTION
The sphincter of Oddi, located where the bile and pancreatic ducts penetrate the wall of the duodenum, actually consists of three sphincters: the common, the biliary, and the pancreatic. Its physiologic role is to regulate the flow of bile and pancreatic juice into the duodenum and to prevent reflux into the ducts from the duodenum.34 Its basal pressure is the main regulating mechanism for pancreatic and biliary secretions into the intestine, and its phasic contractile activity is closely associated with duodenal motility.
Sphincter dysfunction: Stenosis, dyskinesia
The sphincter of Oddi can obstruct the flow of bile and pancreatic juice owing either to stenosis or to dyskinesia.35,36 Stenosis refers to structural alteration of the sphincter, probably from inflammation and subsequent fibrosis. In contrast, dyskinesia refers to a motor abnormality of the sphincter that makes it hypertonic.
Stenosis or dyskinesia can occur in the biliary sphincter, the pancreatic sphincter, the common sphincter, or any combination of the three. For example, dysfunction of the biliary sphincter can cause abnormalities in liver-associated enzyme levels and biliary-type pain, whereas pancreatic sphincter dysfunction can cause recurrent attacks of pancreatitis and pancreatic-type pain.37 Elevated pancreatic sphincter pressure has been shown to correlate with increased pancreatic ductal pressure, suggesting that the sphincter plays a role in the pathogenesis of acute pancreatitis.23,38
Sphincter pressure can be measured during ERCP, but ERCP is risky
The gold standard for the diagnosis of sphincter of Oddi dysfunction is manometry,23,35 ie, direct measurement of sphincter pressure via a thin catheter placed inside the pancreatic or biliary sphincter during ERCP (Figure 1).
However, in patients with suspected sphincter of Oddi dysfunction, ERCP with or without manometry is associated with a high rate of complications, with pancreatitis occurring in up to 25% of cases.39–41 Therefore, several noninvasive and provocative tests have been designed in an attempt to identify patients with this disorder. Unfortunately, none of them seems to be as sensitive and specific as manometry for diagnosing sphincter of Oddi dysfunction, and so they have not gained widespread use.
Opening the sphincter of Oddi with drugs, endoscopy, or surgery
Drug treatment of sphincter of Oddi dysfunction is based on drugs that relax smooth muscle, such as calcium channel blockers and nitrates. The treatment must be lifelong. Also, it does not improve sphincter stenosis, and only half of patients with sphincter dyskinesia respond to it. For these reasons, drug treatment of sphincter of Oddi dysfunction has not gained widespread acceptance.36,42
Endoscopic sphincterotomy is the current standard endoscopic therapy for sphincter of Oddi dysfunction. This procedure is performed during ERCP and involves cutting the sphincter with electrocautery.
Endoscopic pancreatic sphincterotomy prevents recurrent attacks of pancreatitis in patients with pancreatic sphincter dysfunction in more than 60% of cases.23,43–46 A potential complication is pancreatitis, which occurs more often in patients with pancreatic sphincter dyskinesia. Placing a stent in the pancreatic duct after pancreatic sphincterotomy reduces the risk of pancreatitis after ERCP.37,47,48
Surgery. Pancreatic sphincterotomy can also be done surgically, most commonly via transduodenal pancreatic sphincteroplasty. Surgical sphincteroplasty is as effective as endoscopic sphincterotomy for preventing recurrent attacks of pancreatitis in patients with pancreatic sphincter dysfunction.49 However, endoscopic therapy is much less invasive and remains the preferred treatment for sphincter of Oddi dysfunction in most centers with experience in this technique.50
PANCREAS DIVISUM
Pancreas divisum is the most common congenital anomaly of the pancreatic duct. Autopsy studies show it occurs in 5% to 10% of the population.51–53
At approximately the 5th week of gestation, there are two pancreatic buds: a ventral and a dorsal bud. The ventral bud eventually gives rise to part of the pancreatic head and uncinate process of the pancreas in the adult. The dorsal bud eventually gives rise to the rest of the pancreatic head, the pancreatic body, and the pancreatic tail. At 6 to 7 weeks of gestation, the ventral bud rotates clockwise and lies posterior to the dorsal bud. At this stage, both the dorsal and ventral pancreata have their own ducts, which do not communicate with each other. Normally, the ventral and dorsal pancreas and their ducts fuse together at 8 weeks of gestation; in people with pancreas divisum, this ductal fusion does not occur.51
The pancreas secretes 1.5 L of fluid per day. Normally, 90% to 95% of this volume drains through the major papilla. In people with pancreas divisum, 90% to 95% of the fluid drains through the minor papilla.
People with pancreas divisum are a heterogeneous group. Most have no symptoms, and their ductal anatomy is diagnosed only incidentally. However, a subgroup is prone to develop acute pancreatitis. The cause is thought to be the small diameter of the minor papilla, which poses a relative obstruction to the flow of pancreatic juice.54 Direct support for this theory comes from a study in which investigators measured pancreatic ductal pressures in eight people with normal anatomy and six people with pancreas divisum. The pressure in the main pancreatic duct in those with pancreas divisum was significantly higher than in those with normal anatomy.55 Additional evidence in favor of this theory is the effectiveness of treatment, which involves widening the minor papillary opening (minor papillary sphincterotomy).
Diagnosis of pancreas divisum
The diagnosis of pancreas divisum is based on imaging studies, and ERCP remains the gold standard for patients with equivocal results on noninvasive imaging. However, MRCP, especially secretin-enhanced MRCP, is as accurate as ERCP. In most cases, MRCP has replaced ERCP for the diagnosis of this condition, although a recent study suggests that MRCP is inferior to ERCP in the diagnosis of pancreas divisum.56 We recommend secretin-enhanced MRCP for this purpose.
Computed tomography and endoscopic ultrasonography can also diagnose pancreas divisum, but their diagnostic accuracy is lower than that of ERCP and MRCP.
Minor papillary sphincterotomy
Treating recurrent pancreatitis due to pancreas divisum involves relieving the relative obstruction of the minor papilla by minor papillary sphincterotomy. This can be done surgically or endoscopically (Figure 1).
Surgery. No randomized, controlled study has yet assessed the efficacy of surgical sphincteroplasty for recurrent pancreatitis in patients with pancreas divisum. However, retrospective studies and one prospective study have been published.57,58
In the retrospective study with the largest number of patients, Warshaw et al57 reported their experience in 49 patients who had recurrent pancreatitis due to pancreas divisum. After surgical sphincteroplasty, the patients were followed for a mean of 53 months; 40 (82%) of the 49 patients had no further episodes of acute pancreatitis during this time.
Bradley and Stephan58 studied 37 patients with pancreas divisum and recurrent pancreatitis.58 After surgical sphincteroplasty, the patients were followed for a mean of 60 months; 31 of the 37 patients had no further attacks, a success rate of 84%.
Endoscopic therapy. As with surgical therapy trials, most trials of endoscopic therapy of recurrent pancreatitis in patients with pancreas divisum are small case series. In a retrospective study with one of the largest number of patients, Heyries et al59 reported their experience with 24 patients with pancreas divisum and recurrent pancreatitis. After undergoing endoscopic minor papillary sphincterotomy, all patients were followed for a mean of 39 months, during which 22 (92%) did not have further episodes of acute pancreatitis.
In the only randomized controlled trial available, 19 patients with recurrent pancreatitis and pancreas divisum underwent either no treatment or endoscopic minor papillary sphincterotomy.60 In the treatment group, 9 of 10 patients had no further episodes of acute pancreatitis during the 3 years of follow-up, while 6 of 9 patients who were randomized to no treatment had at least one episode.60
Although surgical and endoscopic minor papillary sphincterotomy are equally effective, endoscopic therapy is preferred since it is less invasive, is associated with less morbidity, and costs less. It is also more convenient for patients, since it is an outpatient procedure. Surgical treatment is usually reserved for those in whom endoscopic treatment has failed or is not technically possible.
OTHER PROCESSES OBSTRUCTING THE FLOW OF PANCREATIC JUICE
Any process preventing free flow of pancreatic juice can lead to acute pancreatitis. The cause of the blockage can be around the ampulla, in the ampulla, or in the duct.61
Periampullary lesions, tumors, or polyps can press on the ampulla and cause complete or relative obstruction of the pancreatic duct with a subsequent increase in intraductal pressure and, thus, acute pancreatitis.62 Tumors or polyps of the ampulla, such as ampullary adenoma or carcinoma, can cause pancreatitis by directly obstructing the pancreatic duct where it opens into the duodenum.63–66 Intraductal processes such as ductal adenocarcinoma, intraductal papillary mucinous tumor, pancreatic duct stone, and intraductal stricture due to cancer, chronic pancreatitis, or trauma can also cause pancreatitis by preventing free flow of pancreatic juice.67–71
Although it is well known that sequelae of severe chronic pancreatitis such as ductal strictures or intraductal stones can lead to recurrent attacks of acute pancreatitis by preventing the free flow of pancreatic juice, a relationship also seems to exist between early chronic pancreatitis and recurrent acute pancreatitis.72 Several studies have shown that up to 50% of patients with idiopathic recurrent pancreatitis have evidence of chronic pancreatitis.72–74 However, it is still unclear whether early chronic pancreatitis is the underlying cause of the recurrent attacks of acute pancreatitis or whether recurrent attacks of acute pancreatitis might have led to the development of chronic pancreatitis.
Diagnosis
Ampullary and periampullary neoplasms can be diagnosed endoscopically. Intraductal lesions such as strictures can be diagnosed by MRCP, especially secretin-enhanced MRCP, or by ERCP. ERCP has the additional advantage of being able to deliver treatment, ie, balloon dilation and stenting. In the case of ductal strictures, upsizing of the stents or placement of multiple stents during subsequent procedures is usually needed. Pancreatic ductal calcifications associated with chronic pancreatitis are usually radiopaque and are easily visible on plain films or computed tomography of the abdomen. Parenchymal and ductal changes of chronic pancreatitis can be diagnosed by endoscopic ultrasonography.
Treatment
The treatment is to relieve the obstruction and re-establish the free flow of pancreatic juice.
Periampullary tumors or polyps can be resected surgically or, if they involve only the mucosa, by endoscopic mucosal resection. Ampullary adenomas can be resected endoscopically. Ampullary carcinomas usually require surgical resection.
Small, nonobstructive stones in the pancreatic duct can be removed during ERCP.75 Larger stones may need to be fragmented by extracorporeal shock wave lithotripsy to facilitate removal by ERCP.75
Intraductal strictures should raise the suspicion of pancreatic adenocarcinoma, especially in older patients.61 In these cases, relief of the obstruction by placement of a pancreatic stent can prevent further attacks of pancreatitis until a diagnosis can be established and a more definitive treatment can be offered.
Endoscopic therapy has become an alternative to surgery for some patients with acute recurrent pancreatitis, ie, those whose disease is caused by gallstones or other mechanical processes that can obstruct the outflow from the pancreas.
In this paper, we review the specific situations in which endoscopic therapy might be useful in patients with acute recurrent pancreatitis.
ACUTE PANCREATITIS IS MANAGED DIFFERENTLY IF IT RECURS
Recurrent acute pancreatitis is defined as more than one episode of acute pancreatitis.1 In clinical practice, it is important to distinguish between the first and recurrent episodes of acute pancreatitis.
Most patients who have one episode of acute pancreatitis never have another one.2,3 Therefore, for patients having an initial attack, we recommend a limited workup that includes a detailed history, laboratory evaluation, and a noninvasive imaging study such as transcutaneous ultrasonography or computed tomography.
On the other hand, people who have a second attack are at higher risk of more recurrences. Therefore, patients having recurrent attacks need a more extensive workup to determine the underlying cause. We recommend referring them to a gastroenterologist for further evaluation.
WHICH CAUSES CAN BE MANAGED ENDOSCOPICALLY?
In the Western world, 70% to 80% of cases of recurrent pancreatitis are due to either alcohol abuse or gallstone disease.2,4 The rest are related to:
- Autoimmune disorders
- Cancer, including occult malignancies and premalignant conditions such as intraductal papillary mucinous neoplasm
- Chronic pancreatitis
- Drugs
- Heredity
- Metabolic abnormalities (hypertriglyceridemia, hypercalcemia)
- Sphincter of Oddi dysfunction
- Structural or congenital abnormalities (pancreas divisum)
- Trauma.
- Gallstone disease, including biliary microlithiasis and sludge (in patients with or without a gallbladder)
- Sphincter of Oddi dysfunction
- Pancreas divisum
- Obstruction to flow of pancreatic juice.
Endoscopy is not completely benign
Although endoscopic procedures are less invasive than surgery, they are not completely benign. They can cause anxiety and are associated with risks such as bleeding, perforation, and pancreatitis.5 The risks, benefits, and alternatives to these procedures should be discussed with the patient, and informed consent should be obtained before any endoscopic procedure.6
STONES (LARGE OR SMALL) OR SLUDGE IN PATIENTS WITH A GALLBLADDER
Gallstones can be large, but small stones (microlithiasis) and sludge are more common and therefore account for more cases of pancreatitis.
Strictly defined, microlithiasis refers to stones smaller than 2 mm in diameter in the biliary tract, whereas sludge is a suspension of biliary crystals, mucin, and cellular debris in the gallbladder or bile ducts.7 The terms are often used interchangeably, since the conditions often coexist and their treatment is similar.
Theories differ as to how microlithiasis or sludge can cause recurrent pancreatitis. According to one theory, the debris blocks the common channel, increasing the pancreatic intraductal pressure and leading to pancreatitis.8 A second theory is that small stones or biliary crystals passing through the sphincter of Oddi cause inflammation, and that repeated inflammation eventually leads to stenosis or dyskinesia of the sphincter, both of which have been associated with pancreatitis.9
Studies suggest that microlithiasis and sludge are common causes of recurrent pancreatitis, accounting for about two-thirds of cases according to estimates by Ros et al10 and Lee et al.11
Detecting small stones and sludge
The diagnosis of microlithiasis and biliary sludge in patients with a gallbladder is based on imaging studies and bile microscopy.12
Transabdominal ultrasonography is the imaging study most often used for diagnosing microlithiasis. The technology and expertise for this test are widely available, and it is relatively inexpensive.
Endoscopic ultrasonography is more sensitive for detecting microlithiasis and can examine the distal common bile duct.
Bile microscopy involves obtaining bile from the second portion of the duodenum (via an endoscope or a duodenal tube) or from the bile ducts (by cannulating the common bile duct and stimulating the gallbladder with cholecystokinin). The bile sample is centrifuged and inspected microscopically under plain light and polarized light (which aids the visualization of biliary crystals). The crystals can be cholesterol monohydrate, calcium bilirubinate, or calcium carbonate.7,13,14
Removing the gallbladder is the treatment of choice for small stones and sludge
Treatments to prevent recurrent attacks of acute pancreatitis due to microlithiasis and sludge include cholecystectomy, biliary sphincterotomy, and ursodioxycholic acid.10,11,15
In prospective observational studies by Ros et al10 and Lee et al,11 about half of the patients with recurrent pancreatitis were treated with cholecystectomy, endoscopic sphincterotomy, or ursodioxycholic acid in a nonrandomized fashion. The choice of therapy was based on the patient’s medical status and the preferences of the patient and the physician. Half the patients received no treatment. In both studies the median follow-up was 4 years. Treated patients had a significantly lower rate of recurrent attacks of pancreatitis during follow-up: less than 20% with therapy compared with more than 60% without therapy. Unfortunately, no published study has compared these three treatments head to head.
Cholecystectomy, however, is the most definitive therapy and is generally considered the treatment of choice.
Biliary sphincterotomy is an endoscopic procedure that involves cutting the sphincter of Oddi to allow the stones and sludge to pass more freely. It is as effective as cholecystectomy in preventing recurrent attacks but does not eliminate the risk of cholecystitis and cholangitis (Figure 1). For this reason, it is usually reserved for patients who cannot tolerate surgery due to comorbidities, those who refuse surgery, or those who are pregnant.16
Ursodeoxycholic acid is a reasonable alternative in patients who cannot tolerate surgical or endoscopic biliary sphincterotomy.1,17–20 The dosage is 10 mg/kg/day, which can be in two or three divided doses. The optimal duration of treatment is not known; however, since this drug works slowly, it may need to be taken for 2 years or more. Ursodeoxycholic acid is more effective in patients with cholesterol-based stones and crystals. It is not effective for large stones (> 1 cm in diameter) or calcified stones.
STONES AFTER CHOLECYSTECTOMY
Bile duct stones can be classified as primary or secondary. A primary stone is one that remains where it was formed, whereas a secondary stone is one that has migrated from its site of formation.21
Some suggest that bile duct stones that are detected within 2 years of cholecystectomy originated in the gallbladder and were missed when the gallbladder was removed (and therefore are considered secondary stones), and that stones that present more than 2 years after cholecystectomy are de novo (ie, primary) stones.22,23
In any event, stones have been found in the common bile duct in 4% to 24% of patients up to 15 years after cholecystectomy.24–26 A fair number of these patients have no symptoms.27 Risk factors for stone recurrence are lithogenic bile (ie, high concentration of cholesterol, low concentration of bile salts), biliary stasis, strictures, dilated bile ducts, and advanced age.28–30
No role for crystal analysis after cholecystectomy
Biliary crystal analysis does not seem to have diagnostic value in patients with recurrent acute pancreatitis after cholecystectomy,31 because removing the gallbladder eliminates the crystals and sludge. Imaging studies are therefore the cornerstone of diagnosis.
Transabdominal ultrasonography is the most commonly used initial imaging test. However, abdominal fat and gas in the duodenum can obscure the distal common bile duct and decrease the sensitivity of this test.32
Endoscopic ultrasonography involves positioning the transducer in the second part of the duodenum, where it can show the adjacent biliary tree without interference from digestive gas or abdominal fat.
Magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasonography are both highly sensitive for detecting common bile duct stones and are recommended if they can be done without delay.
Endoscopic retrograde cholangiopancreatography (ERCP). As a rule, patients who are very likely to have gallstones are best served by proceeding directly to ERCP, a procedure that enables both imaging and treatment. However, ERCP exposes the patient to radiation and the risk of pancreatitis, so in some patients (eg, pregnant women, people who recently had acute pancreatitis), one may want to do ultrasonography first.
ERCP is the treatment of choice after cholecystectomy
The treatment of choice in patients with choledocholithiasis is ERCP with biliary sphincterotomy and stone extraction. Success at clearing the biliary tree of all stones depends on the size, number, and location of the stones, the anatomy of the digestive tract and the bile duct, and the experience of the endoscopist. At specialized centers, the rate of successful clearance with subsequent procedures is close to 100%. Large stones may require fragmentation inside the bile duct to aid their removal.33
SPHINCTER OF ODDI DYSFUNCTION
The sphincter of Oddi, located where the bile and pancreatic ducts penetrate the wall of the duodenum, actually consists of three sphincters: the common, the biliary, and the pancreatic. Its physiologic role is to regulate the flow of bile and pancreatic juice into the duodenum and to prevent reflux into the ducts from the duodenum.34 Its basal pressure is the main regulating mechanism for pancreatic and biliary secretions into the intestine, and its phasic contractile activity is closely associated with duodenal motility.
Sphincter dysfunction: Stenosis, dyskinesia
The sphincter of Oddi can obstruct the flow of bile and pancreatic juice owing either to stenosis or to dyskinesia.35,36 Stenosis refers to structural alteration of the sphincter, probably from inflammation and subsequent fibrosis. In contrast, dyskinesia refers to a motor abnormality of the sphincter that makes it hypertonic.
Stenosis or dyskinesia can occur in the biliary sphincter, the pancreatic sphincter, the common sphincter, or any combination of the three. For example, dysfunction of the biliary sphincter can cause abnormalities in liver-associated enzyme levels and biliary-type pain, whereas pancreatic sphincter dysfunction can cause recurrent attacks of pancreatitis and pancreatic-type pain.37 Elevated pancreatic sphincter pressure has been shown to correlate with increased pancreatic ductal pressure, suggesting that the sphincter plays a role in the pathogenesis of acute pancreatitis.23,38
Sphincter pressure can be measured during ERCP, but ERCP is risky
The gold standard for the diagnosis of sphincter of Oddi dysfunction is manometry,23,35 ie, direct measurement of sphincter pressure via a thin catheter placed inside the pancreatic or biliary sphincter during ERCP (Figure 1).
However, in patients with suspected sphincter of Oddi dysfunction, ERCP with or without manometry is associated with a high rate of complications, with pancreatitis occurring in up to 25% of cases.39–41 Therefore, several noninvasive and provocative tests have been designed in an attempt to identify patients with this disorder. Unfortunately, none of them seems to be as sensitive and specific as manometry for diagnosing sphincter of Oddi dysfunction, and so they have not gained widespread use.
Opening the sphincter of Oddi with drugs, endoscopy, or surgery
Drug treatment of sphincter of Oddi dysfunction is based on drugs that relax smooth muscle, such as calcium channel blockers and nitrates. The treatment must be lifelong. Also, it does not improve sphincter stenosis, and only half of patients with sphincter dyskinesia respond to it. For these reasons, drug treatment of sphincter of Oddi dysfunction has not gained widespread acceptance.36,42
Endoscopic sphincterotomy is the current standard endoscopic therapy for sphincter of Oddi dysfunction. This procedure is performed during ERCP and involves cutting the sphincter with electrocautery.
Endoscopic pancreatic sphincterotomy prevents recurrent attacks of pancreatitis in patients with pancreatic sphincter dysfunction in more than 60% of cases.23,43–46 A potential complication is pancreatitis, which occurs more often in patients with pancreatic sphincter dyskinesia. Placing a stent in the pancreatic duct after pancreatic sphincterotomy reduces the risk of pancreatitis after ERCP.37,47,48
Surgery. Pancreatic sphincterotomy can also be done surgically, most commonly via transduodenal pancreatic sphincteroplasty. Surgical sphincteroplasty is as effective as endoscopic sphincterotomy for preventing recurrent attacks of pancreatitis in patients with pancreatic sphincter dysfunction.49 However, endoscopic therapy is much less invasive and remains the preferred treatment for sphincter of Oddi dysfunction in most centers with experience in this technique.50
PANCREAS DIVISUM
Pancreas divisum is the most common congenital anomaly of the pancreatic duct. Autopsy studies show it occurs in 5% to 10% of the population.51–53
At approximately the 5th week of gestation, there are two pancreatic buds: a ventral and a dorsal bud. The ventral bud eventually gives rise to part of the pancreatic head and uncinate process of the pancreas in the adult. The dorsal bud eventually gives rise to the rest of the pancreatic head, the pancreatic body, and the pancreatic tail. At 6 to 7 weeks of gestation, the ventral bud rotates clockwise and lies posterior to the dorsal bud. At this stage, both the dorsal and ventral pancreata have their own ducts, which do not communicate with each other. Normally, the ventral and dorsal pancreas and their ducts fuse together at 8 weeks of gestation; in people with pancreas divisum, this ductal fusion does not occur.51
The pancreas secretes 1.5 L of fluid per day. Normally, 90% to 95% of this volume drains through the major papilla. In people with pancreas divisum, 90% to 95% of the fluid drains through the minor papilla.
People with pancreas divisum are a heterogeneous group. Most have no symptoms, and their ductal anatomy is diagnosed only incidentally. However, a subgroup is prone to develop acute pancreatitis. The cause is thought to be the small diameter of the minor papilla, which poses a relative obstruction to the flow of pancreatic juice.54 Direct support for this theory comes from a study in which investigators measured pancreatic ductal pressures in eight people with normal anatomy and six people with pancreas divisum. The pressure in the main pancreatic duct in those with pancreas divisum was significantly higher than in those with normal anatomy.55 Additional evidence in favor of this theory is the effectiveness of treatment, which involves widening the minor papillary opening (minor papillary sphincterotomy).
Diagnosis of pancreas divisum
The diagnosis of pancreas divisum is based on imaging studies, and ERCP remains the gold standard for patients with equivocal results on noninvasive imaging. However, MRCP, especially secretin-enhanced MRCP, is as accurate as ERCP. In most cases, MRCP has replaced ERCP for the diagnosis of this condition, although a recent study suggests that MRCP is inferior to ERCP in the diagnosis of pancreas divisum.56 We recommend secretin-enhanced MRCP for this purpose.
Computed tomography and endoscopic ultrasonography can also diagnose pancreas divisum, but their diagnostic accuracy is lower than that of ERCP and MRCP.
Minor papillary sphincterotomy
Treating recurrent pancreatitis due to pancreas divisum involves relieving the relative obstruction of the minor papilla by minor papillary sphincterotomy. This can be done surgically or endoscopically (Figure 1).
Surgery. No randomized, controlled study has yet assessed the efficacy of surgical sphincteroplasty for recurrent pancreatitis in patients with pancreas divisum. However, retrospective studies and one prospective study have been published.57,58
In the retrospective study with the largest number of patients, Warshaw et al57 reported their experience in 49 patients who had recurrent pancreatitis due to pancreas divisum. After surgical sphincteroplasty, the patients were followed for a mean of 53 months; 40 (82%) of the 49 patients had no further episodes of acute pancreatitis during this time.
Bradley and Stephan58 studied 37 patients with pancreas divisum and recurrent pancreatitis.58 After surgical sphincteroplasty, the patients were followed for a mean of 60 months; 31 of the 37 patients had no further attacks, a success rate of 84%.
Endoscopic therapy. As with surgical therapy trials, most trials of endoscopic therapy of recurrent pancreatitis in patients with pancreas divisum are small case series. In a retrospective study with one of the largest number of patients, Heyries et al59 reported their experience with 24 patients with pancreas divisum and recurrent pancreatitis. After undergoing endoscopic minor papillary sphincterotomy, all patients were followed for a mean of 39 months, during which 22 (92%) did not have further episodes of acute pancreatitis.
In the only randomized controlled trial available, 19 patients with recurrent pancreatitis and pancreas divisum underwent either no treatment or endoscopic minor papillary sphincterotomy.60 In the treatment group, 9 of 10 patients had no further episodes of acute pancreatitis during the 3 years of follow-up, while 6 of 9 patients who were randomized to no treatment had at least one episode.60
Although surgical and endoscopic minor papillary sphincterotomy are equally effective, endoscopic therapy is preferred since it is less invasive, is associated with less morbidity, and costs less. It is also more convenient for patients, since it is an outpatient procedure. Surgical treatment is usually reserved for those in whom endoscopic treatment has failed or is not technically possible.
OTHER PROCESSES OBSTRUCTING THE FLOW OF PANCREATIC JUICE
Any process preventing free flow of pancreatic juice can lead to acute pancreatitis. The cause of the blockage can be around the ampulla, in the ampulla, or in the duct.61
Periampullary lesions, tumors, or polyps can press on the ampulla and cause complete or relative obstruction of the pancreatic duct with a subsequent increase in intraductal pressure and, thus, acute pancreatitis.62 Tumors or polyps of the ampulla, such as ampullary adenoma or carcinoma, can cause pancreatitis by directly obstructing the pancreatic duct where it opens into the duodenum.63–66 Intraductal processes such as ductal adenocarcinoma, intraductal papillary mucinous tumor, pancreatic duct stone, and intraductal stricture due to cancer, chronic pancreatitis, or trauma can also cause pancreatitis by preventing free flow of pancreatic juice.67–71
Although it is well known that sequelae of severe chronic pancreatitis such as ductal strictures or intraductal stones can lead to recurrent attacks of acute pancreatitis by preventing the free flow of pancreatic juice, a relationship also seems to exist between early chronic pancreatitis and recurrent acute pancreatitis.72 Several studies have shown that up to 50% of patients with idiopathic recurrent pancreatitis have evidence of chronic pancreatitis.72–74 However, it is still unclear whether early chronic pancreatitis is the underlying cause of the recurrent attacks of acute pancreatitis or whether recurrent attacks of acute pancreatitis might have led to the development of chronic pancreatitis.
Diagnosis
Ampullary and periampullary neoplasms can be diagnosed endoscopically. Intraductal lesions such as strictures can be diagnosed by MRCP, especially secretin-enhanced MRCP, or by ERCP. ERCP has the additional advantage of being able to deliver treatment, ie, balloon dilation and stenting. In the case of ductal strictures, upsizing of the stents or placement of multiple stents during subsequent procedures is usually needed. Pancreatic ductal calcifications associated with chronic pancreatitis are usually radiopaque and are easily visible on plain films or computed tomography of the abdomen. Parenchymal and ductal changes of chronic pancreatitis can be diagnosed by endoscopic ultrasonography.
Treatment
The treatment is to relieve the obstruction and re-establish the free flow of pancreatic juice.
Periampullary tumors or polyps can be resected surgically or, if they involve only the mucosa, by endoscopic mucosal resection. Ampullary adenomas can be resected endoscopically. Ampullary carcinomas usually require surgical resection.
Small, nonobstructive stones in the pancreatic duct can be removed during ERCP.75 Larger stones may need to be fragmented by extracorporeal shock wave lithotripsy to facilitate removal by ERCP.75
Intraductal strictures should raise the suspicion of pancreatic adenocarcinoma, especially in older patients.61 In these cases, relief of the obstruction by placement of a pancreatic stent can prevent further attacks of pancreatitis until a diagnosis can be established and a more definitive treatment can be offered.
- Levy MJ, Geenen JE. Idiopathic acute recurrent pancreatitis. Am J Gastroenterol 2001; 96:2540–2555.
- Gullo L, Migliori M, Pezzilli R, et al. An update on recurrent acute pancreatitis: data from five European countries. Am J Gastroenterol 2002; 97:1959–1962.
- Gao YJ, Li YQ, Wang Q, et al. Analysis of the clinical features of recurrent acute pancreatitis in China. J Gastroenterol 2006; 41:681–685.
- Somogyi L, Martin SP, Venkatesan T, Ulrich CD. Recurrent acute pancreatitis: an algorithmic approach to identification and elimination of inciting factors. Gastroenterology 2001; 120:708–717.
- Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 2007; 102:1781–1788.
- Standards of Practice Committee,Zuckerman MJ, Shen B, Harrison ME, et al. Informed consent for GI endoscopy. Gastrointest Endosc 2007; 66:213–218.
- Lee SP, Hayashi A, Kim YS. Biliary sludge: curiosity or culprit? Hepatology 1994; 20:523–525.
- Opie E. The etiology of acute hemorrhagic pancreatitis. Bull Johns Hopkins Hosp 1901; 12:182–188.
- Hernandez CA, Lerch MM. Sphincter stenosis and gallstone migration through the biliary tract. Lancet 1993; 341:1371–1373.
- Ros E, Navarro S, Bru C, Garcia-Puges A, Valderrama R. Occult microlithiasis in 'idiopathic' acute pancreatitis: prevention of relapses by cholecystectomy or ursodeoxycholic acid therapy. Gastroenterology 1991; 101:1701–1709.
- Lee SP, Nicholls JF, Park HZ. Biliary sludge as a cause of acute pancreatitis. N Engl J Med 1992; 326:589–593.
- Levy MJ. The hunt for microlithiasis in idiopathic acute recurrent pancreatitis: should we abandon the search or intensify our efforts? Gastrointest Endosc 2002; 55:286–293.
- Delchier JC, Benfredj P, Preaux AM, Metreau JM, Dhumeaux D. The usefulness of microscopic bile examination in patients with suspected microlithiasis: a prospective evaluation. Hepatology 1986; 6:118–122.
- Lee SP, Nicholls JF. Nature and composition of biliary sludge. Gastroenterology 1986; 90:677–686.
- Testoni PA, Caporuscio S, Bagnolo F, Lella F. Idiopathic recurrent pancreatitis: long-term results after ERCP, endoscopic sphincterotomy, or ursodeoxycholic acid treatment. Am J Gastroenterol 2000; 95:1702–1707.
- Siddiqui AA, Mitroo P, Kowalski T, Loren D. Endoscopic sphincterotomy with or without cholecystectomy for choledocholithiasis in high-risk surgical patients: a decision analysis. Aliment Pharmacol Ther 2006; 24:1059–1066.
- Steinberg WM, Chari ST, Forsmark CE, et al. Controversies in clinical pancreatology: management of acute idiopathic recurrent pancreatitis. Pancreas 2003; 27:103–117.
- Khalid A, Slivka A. Approach to idiopathic recurrent pancreatitis. Gastrointest Endosc Clin North Am 2003; 13:695–716.
- Adler DG, Baron TH, Davila RE, et al; Standards of Practice Committee of American Society for Gastrointestinal Endoscopy. ASGE guideline: the role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest Endosc 2005; 62:1–8.
- Draganov P, Forsmark CE. “Idiopathic” pancreatitis. Gastroenterology 2005; 128:756–763.
- Chung EJ, Kim MH, Lee SS, Lee SK. Primary vs. secondary common bile duct stones: apples and oranges. Endoscopy 2003; 35:92.
- Saharia PC, Zuidema GD, Cameron JL. Primary common duct stones. Ann Surg 1977; 185:598–604.
- Elta GH. Sphincter of Oddi dysfunction and bile duct microlithiasis in acute idiopathic pancreatitis. World J Gastroenterol 2008; 14:1023–1026.
- Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med 1996; 335:909–918.
- Prat F, Malak NA, Pelletier G, et al. Biliary symptoms and complications more than 8 years after endoscopic sphincterotomy for choledocholithiasis. Gastroenterology 1996; 110:894–899.
- Hawes RH, Cotton PB, Vallon AG. Follow-up 6 to 11 years after duo-denoscopic sphincterotomy for stones in patients with prior cholecystectomy. Gastroenterology 1990; 98:1008–1012.
- Lai KH, Lo GH, Lin CK, et al. Do patients with recurrent choledocholithiasis after endoscopic sphincterotomy benefit from regular follow-up? Gastrointest Endosc 2002; 55:523–526.
- Kim DI, Kim MH, Lee SK, et al. Risk factors for recurrence of primary bile duct stones after endoscopic biliary sphincterotomy. Gastrointest Endosc 2001; 54:42–48.
- Costamagna G, Tringali A, Shah SK, Mutignani M, Zuccala G, Perri V. Long-term follow-up of patients after endoscopic sphincterotomy for choledocholithiasis, and risk factors for recurrence. Endoscopy 2002; 34:273–279.
- Keizman D, Ish Shalom M, Konikoff FM. Recurrent symptomatic common bile duct stones after endoscopic stone extraction in elderly patients. Gastrointest Endosc 2006; 64:60–65.
- Kaw M, Brodmerkel GJ. ERCP, biliary crystal analysis, and sphincter of Oddi manometry in idiopathic recurrent pancreatitis. Gastrointest Endosc 2002; 55:157–162.
- Chak A, Hawes RH, Cooper GS, et al. Prospective assessment of the utility of EUS in the evaluation of gallstone pancreatitis. Gastrointest Endosc 1999; 49:599–604.
- Parsi MA, Neuhaus H, Pleskow D, et al. Peroral cholangioscopy guided stone therapy—report of an international multicenter registry [abstract]. Gastrointest Endosc 2008; 67:AB102.
- Woods CM, Mawe GM, Toouli J, Saccone GT. The sphincter of Oddi: understanding its control and function. Neurogastroenterol Motil 2005; 17 suppl 1:31–40.
- McLoughlin MT, Mitchell RM. Sphincter of Oddi dysfunction and pancreatitis. World J Gastroenterol 2007; 13:6333–6343.
- Bosch A, Pena LR. The sphincter of Oddi. Dig Dis Sci 2007; 52:1211–1218.
- Devereaux BM, Sherman S, Lehman GA. Sphincter of Oddi (pancreatic) hypertension and recurrent pancreatitis. Curr Gastroenterol Rep 2002; 4:153–159.
- Fazel A, Geenen JE, MoezArdalan K, Catalano MF. Intrapancreatic ductal pressure in sphincter of Oddi dysfunction. Pancreas 2005; 30:359–362.
- Freeman ML. Role of pancreatic stents in prevention of post-ERCP pancreatitis. JOP 2004; 5:322–327.
- Singh P, Gurudu SR, Davidoff S, et al. Sphincter of Oddi manometry does not predispose to post-ERCP acute pancreatitis. Gastrointest Endosc 2004; 59:499–505.
- Guda NM, Freeman ML. True culprit or guilt by association? Is sphincter of Oddi manometry the cause of post-ERCP pancreatitis in patients with suspected sphincter of Oddi dysfunction, or is it the patients' susceptibility? Rev Gastroenterol Disord 2004; 4:211–213.
- Craig A, Toouli J. Sphincter of Oddi dysfunction: is there a role for medical therapy? Curr Gastroenterol Rep 2002; 4:172–176.
- Freeman ML, Gill M, Overby C, Cen YY. Predictors of outcomes after biliary and pancreatic sphincterotomy for sphincter of Oddi dysfunction. J Clin Gastroenterol 2007; 41:94–102.
- Sgouros SN, Pereira SP. Systematic review: sphincter of Oddi dysfunction—non-invasive diagnostic methods and long-term outcome after endoscopic sphincterotomy. Aliment Pharmacol Ther 2006; 24:237–246.
- Venu RP, Geenen JE, Hogan W, Stone J, Johnson GK, Soergel K. Idiopathic recurrent pancreatitis. An approach to diagnosis and treatment. Dig Dis Sci 1989; 34:56–60.
- Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Engl J Med 1989; 320:82–87.
- Fogel EL, Eversman D, Jamidar P, Sherman S, Lehman GA. Sphincter of Oddi dysfunction: pancreaticobiliary sphincterotomy with pancreatic stent placement has a lower rate of pancreatitis than biliary sphincterotomy alone. Endoscopy 2002; 34:280–285.
- Freeman ML. Pancreatic stents for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol 2007; 5:1354–1365.
- Toouli J. The sphincter of Oddi and acute pancreatitis - revisited. HPB (Oxford) 2003; 5:142–145.
- Sherman S, Lehman GA. Sphincter of Oddi dysfunction: diagnosis and treatment. JOP 2001; 2:382–400.
- Klein SD, Affronti JP. Pancreas divisum, an evidence-based review: part I, pathophysiology. Gastrointest Endosc 2004; 60:419–425.
- Fogel EL, Toth TG, Lehman GA, DiMagno MJ, DiMagno EP. Does endoscopic therapy favorably affect the outcome of patients who have recurrent acute pancreatitis and pancreas divisum? Pancreas 2007; 34:21–45.
- Lehman GA. Acute recurrent pancreatitis. Can J Gastroenterol 2003; 17:381–383.
- Lehman GA, Sherman S. Pancreas divisum. Diagnosis, clinical significance, and management alternatives. Gastrointest Endosc Clin N Am 1995; 5:145–170.
- Staritz M, Meyer zum Buschenfelde KH. Elevated pressure in the dorsal part of pancreas divisum: the cause of chronic pancreatitis? Pancreas 1988; 3:108–110.
- Carnes M, Romagnuolo J, Cotton P. Miss rate of pancreas divisum by magnetic resonance cholangiopancreatography in clinical practice. Pancreas 2008; 37:151–153.
- Warshaw AL, Simeone JF, Schapiro RH, Flavin-Warshaw B. Evaluation and treatment of the dominant dorsal duct syndrome (pancreas divisum redefined). Am J Surg 1990; 159:59–64.
- Bradley EL, Stephan RN. Accessory duct sphincteroplasty is preferred for long-term prevention of recurrent acute pancreatitis in patients with pancreas divisum. J Am Coll Surg 1996; 183:65–70.
- Heyries L, Barthet M, Delvasto C, Zamora C, Bernard JP, Sahel J. Long-term results of endoscopic management of pancreas divisum with recurrent acute pancreatitis. Gastrointest Endosc 2002; 55:376–381.
- Lans JI, Geenen JE, Johanson JF, Hogan WJ. Endoscopic therapy in patients with pancreas divisum and acute pancreatitis: a prospective, randomized, controlled clinical trial. Gastrointest Endosc 1992; 38:430–434.
- Delhaye M, Matos C, Arvanitakis M, Deviere J. Pancreatic ductal system obstruction and acute recurrent pancreatitis. World J Gastroenterol 2008; 14:1027–1033.
- Finnie IA, Ghosh P, Garvey C, Poston GJ, Rhodes JM. Intraluminal duodenal diverticulum causing recurrent pancreatitis: treatment by endoscopic incision. Gut 1994; 35:557–559.
- Guzzardo G, Kleinman MS, Krackov JH, Schwartz SI. Recurrent acute pancreatitis caused by ampullary villous adenoma. J Clin Gastroenterol 1990; 12:200–202.
- Wright BE, Kozarek RA, Traverso LW, Wechter D, Thirlby R, Raltz SL. Recurrent pancreatitis in Gardner variant familial polyposis: etiology, diagnostic approach, and interventional results. Arch Surg 1999; 134:311–315.
- Tanasijtchouk T, Vaisbein E, Lachter J, Nassar F. Carcinoma of Papilla Vateri presenting as recurrent acute pancreatitis. Acta Gastroenterol Belg 2004; 67:309–310.
- Kwon TH, Park do H, Shim KY, et al. Ampullary adenomyoma presenting as acute recurrent pancreatitis. World J Gastroenterol 2007; 13:2892–2894.
- Lorente JA, Ruiz del Arbol L, Moreira VF, Garcia-Plaza A. Recurrent pancreatitis in a young patient associated with a solitary nonopaque concretion in the main pancreatic duct. Gastrointest Endosc 1990; 36:63–65.
- Chung JP, Chi SW, Park YN, et al. A case of minute intraductal papillary mucinous tumor of the pancreas presenting with recurrent acute pancreatitis. Yonsei Med J 2000; 41:528–532.
- Tikhomirov V, Tikhomirova S, Sieber S, Schiffman MK. A pancreatic intraductal papillary mucinous tumor causing recurrent acute pancreatitis at the onset of menstrual periods. J Clin Gastroenterol 2000; 31:172–174.
- Mosca S, Bottino V, Molino C. Hepatobiliary and pancreatic: a woman with recurrent idiopathic acute pancreatitis. Intraductal papillary mucinous tumor of the pancreas. J Gastroenterol Hepatol 2001; 16:1070,1075.
- Howard TJ, Moore SA, Saxena R, Matthews DE, Schmidt CM, Wiebke EA. Pancreatic duct strictures are a common cause of recurrent pancreatitis after successful management of pancreatic necrosis. Surgery 2004; 136:909–916.
- Garg PK, Tandon RK, Madan K. Is biliary microlithiasis a significant cause of idiopathic recurrent acute pancreatitis? A long-term follow-up study. Clin Gastroenterol Hepatol 2007; 5:75–79.
- Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol 2001; 96:705–709.
- Yusoff IF, Raymond G, Sahai AV. A prospective comparison of the yield of EUS in primary vs. recurrent idiopathic acute pancreatitis. Gastrointest Endosc 2004; 60:673–678.
- Cahen DL, Gouma DJ, Nio Y, et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med 2007; 356:676–684.
- Levy MJ, Geenen JE. Idiopathic acute recurrent pancreatitis. Am J Gastroenterol 2001; 96:2540–2555.
- Gullo L, Migliori M, Pezzilli R, et al. An update on recurrent acute pancreatitis: data from five European countries. Am J Gastroenterol 2002; 97:1959–1962.
- Gao YJ, Li YQ, Wang Q, et al. Analysis of the clinical features of recurrent acute pancreatitis in China. J Gastroenterol 2006; 41:681–685.
- Somogyi L, Martin SP, Venkatesan T, Ulrich CD. Recurrent acute pancreatitis: an algorithmic approach to identification and elimination of inciting factors. Gastroenterology 2001; 120:708–717.
- Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 2007; 102:1781–1788.
- Standards of Practice Committee,Zuckerman MJ, Shen B, Harrison ME, et al. Informed consent for GI endoscopy. Gastrointest Endosc 2007; 66:213–218.
- Lee SP, Hayashi A, Kim YS. Biliary sludge: curiosity or culprit? Hepatology 1994; 20:523–525.
- Opie E. The etiology of acute hemorrhagic pancreatitis. Bull Johns Hopkins Hosp 1901; 12:182–188.
- Hernandez CA, Lerch MM. Sphincter stenosis and gallstone migration through the biliary tract. Lancet 1993; 341:1371–1373.
- Ros E, Navarro S, Bru C, Garcia-Puges A, Valderrama R. Occult microlithiasis in 'idiopathic' acute pancreatitis: prevention of relapses by cholecystectomy or ursodeoxycholic acid therapy. Gastroenterology 1991; 101:1701–1709.
- Lee SP, Nicholls JF, Park HZ. Biliary sludge as a cause of acute pancreatitis. N Engl J Med 1992; 326:589–593.
- Levy MJ. The hunt for microlithiasis in idiopathic acute recurrent pancreatitis: should we abandon the search or intensify our efforts? Gastrointest Endosc 2002; 55:286–293.
- Delchier JC, Benfredj P, Preaux AM, Metreau JM, Dhumeaux D. The usefulness of microscopic bile examination in patients with suspected microlithiasis: a prospective evaluation. Hepatology 1986; 6:118–122.
- Lee SP, Nicholls JF. Nature and composition of biliary sludge. Gastroenterology 1986; 90:677–686.
- Testoni PA, Caporuscio S, Bagnolo F, Lella F. Idiopathic recurrent pancreatitis: long-term results after ERCP, endoscopic sphincterotomy, or ursodeoxycholic acid treatment. Am J Gastroenterol 2000; 95:1702–1707.
- Siddiqui AA, Mitroo P, Kowalski T, Loren D. Endoscopic sphincterotomy with or without cholecystectomy for choledocholithiasis in high-risk surgical patients: a decision analysis. Aliment Pharmacol Ther 2006; 24:1059–1066.
- Steinberg WM, Chari ST, Forsmark CE, et al. Controversies in clinical pancreatology: management of acute idiopathic recurrent pancreatitis. Pancreas 2003; 27:103–117.
- Khalid A, Slivka A. Approach to idiopathic recurrent pancreatitis. Gastrointest Endosc Clin North Am 2003; 13:695–716.
- Adler DG, Baron TH, Davila RE, et al; Standards of Practice Committee of American Society for Gastrointestinal Endoscopy. ASGE guideline: the role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest Endosc 2005; 62:1–8.
- Draganov P, Forsmark CE. “Idiopathic” pancreatitis. Gastroenterology 2005; 128:756–763.
- Chung EJ, Kim MH, Lee SS, Lee SK. Primary vs. secondary common bile duct stones: apples and oranges. Endoscopy 2003; 35:92.
- Saharia PC, Zuidema GD, Cameron JL. Primary common duct stones. Ann Surg 1977; 185:598–604.
- Elta GH. Sphincter of Oddi dysfunction and bile duct microlithiasis in acute idiopathic pancreatitis. World J Gastroenterol 2008; 14:1023–1026.
- Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med 1996; 335:909–918.
- Prat F, Malak NA, Pelletier G, et al. Biliary symptoms and complications more than 8 years after endoscopic sphincterotomy for choledocholithiasis. Gastroenterology 1996; 110:894–899.
- Hawes RH, Cotton PB, Vallon AG. Follow-up 6 to 11 years after duo-denoscopic sphincterotomy for stones in patients with prior cholecystectomy. Gastroenterology 1990; 98:1008–1012.
- Lai KH, Lo GH, Lin CK, et al. Do patients with recurrent choledocholithiasis after endoscopic sphincterotomy benefit from regular follow-up? Gastrointest Endosc 2002; 55:523–526.
- Kim DI, Kim MH, Lee SK, et al. Risk factors for recurrence of primary bile duct stones after endoscopic biliary sphincterotomy. Gastrointest Endosc 2001; 54:42–48.
- Costamagna G, Tringali A, Shah SK, Mutignani M, Zuccala G, Perri V. Long-term follow-up of patients after endoscopic sphincterotomy for choledocholithiasis, and risk factors for recurrence. Endoscopy 2002; 34:273–279.
- Keizman D, Ish Shalom M, Konikoff FM. Recurrent symptomatic common bile duct stones after endoscopic stone extraction in elderly patients. Gastrointest Endosc 2006; 64:60–65.
- Kaw M, Brodmerkel GJ. ERCP, biliary crystal analysis, and sphincter of Oddi manometry in idiopathic recurrent pancreatitis. Gastrointest Endosc 2002; 55:157–162.
- Chak A, Hawes RH, Cooper GS, et al. Prospective assessment of the utility of EUS in the evaluation of gallstone pancreatitis. Gastrointest Endosc 1999; 49:599–604.
- Parsi MA, Neuhaus H, Pleskow D, et al. Peroral cholangioscopy guided stone therapy—report of an international multicenter registry [abstract]. Gastrointest Endosc 2008; 67:AB102.
- Woods CM, Mawe GM, Toouli J, Saccone GT. The sphincter of Oddi: understanding its control and function. Neurogastroenterol Motil 2005; 17 suppl 1:31–40.
- McLoughlin MT, Mitchell RM. Sphincter of Oddi dysfunction and pancreatitis. World J Gastroenterol 2007; 13:6333–6343.
- Bosch A, Pena LR. The sphincter of Oddi. Dig Dis Sci 2007; 52:1211–1218.
- Devereaux BM, Sherman S, Lehman GA. Sphincter of Oddi (pancreatic) hypertension and recurrent pancreatitis. Curr Gastroenterol Rep 2002; 4:153–159.
- Fazel A, Geenen JE, MoezArdalan K, Catalano MF. Intrapancreatic ductal pressure in sphincter of Oddi dysfunction. Pancreas 2005; 30:359–362.
- Freeman ML. Role of pancreatic stents in prevention of post-ERCP pancreatitis. JOP 2004; 5:322–327.
- Singh P, Gurudu SR, Davidoff S, et al. Sphincter of Oddi manometry does not predispose to post-ERCP acute pancreatitis. Gastrointest Endosc 2004; 59:499–505.
- Guda NM, Freeman ML. True culprit or guilt by association? Is sphincter of Oddi manometry the cause of post-ERCP pancreatitis in patients with suspected sphincter of Oddi dysfunction, or is it the patients' susceptibility? Rev Gastroenterol Disord 2004; 4:211–213.
- Craig A, Toouli J. Sphincter of Oddi dysfunction: is there a role for medical therapy? Curr Gastroenterol Rep 2002; 4:172–176.
- Freeman ML, Gill M, Overby C, Cen YY. Predictors of outcomes after biliary and pancreatic sphincterotomy for sphincter of Oddi dysfunction. J Clin Gastroenterol 2007; 41:94–102.
- Sgouros SN, Pereira SP. Systematic review: sphincter of Oddi dysfunction—non-invasive diagnostic methods and long-term outcome after endoscopic sphincterotomy. Aliment Pharmacol Ther 2006; 24:237–246.
- Venu RP, Geenen JE, Hogan W, Stone J, Johnson GK, Soergel K. Idiopathic recurrent pancreatitis. An approach to diagnosis and treatment. Dig Dis Sci 1989; 34:56–60.
- Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Engl J Med 1989; 320:82–87.
- Fogel EL, Eversman D, Jamidar P, Sherman S, Lehman GA. Sphincter of Oddi dysfunction: pancreaticobiliary sphincterotomy with pancreatic stent placement has a lower rate of pancreatitis than biliary sphincterotomy alone. Endoscopy 2002; 34:280–285.
- Freeman ML. Pancreatic stents for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol 2007; 5:1354–1365.
- Toouli J. The sphincter of Oddi and acute pancreatitis - revisited. HPB (Oxford) 2003; 5:142–145.
- Sherman S, Lehman GA. Sphincter of Oddi dysfunction: diagnosis and treatment. JOP 2001; 2:382–400.
- Klein SD, Affronti JP. Pancreas divisum, an evidence-based review: part I, pathophysiology. Gastrointest Endosc 2004; 60:419–425.
- Fogel EL, Toth TG, Lehman GA, DiMagno MJ, DiMagno EP. Does endoscopic therapy favorably affect the outcome of patients who have recurrent acute pancreatitis and pancreas divisum? Pancreas 2007; 34:21–45.
- Lehman GA. Acute recurrent pancreatitis. Can J Gastroenterol 2003; 17:381–383.
- Lehman GA, Sherman S. Pancreas divisum. Diagnosis, clinical significance, and management alternatives. Gastrointest Endosc Clin N Am 1995; 5:145–170.
- Staritz M, Meyer zum Buschenfelde KH. Elevated pressure in the dorsal part of pancreas divisum: the cause of chronic pancreatitis? Pancreas 1988; 3:108–110.
- Carnes M, Romagnuolo J, Cotton P. Miss rate of pancreas divisum by magnetic resonance cholangiopancreatography in clinical practice. Pancreas 2008; 37:151–153.
- Warshaw AL, Simeone JF, Schapiro RH, Flavin-Warshaw B. Evaluation and treatment of the dominant dorsal duct syndrome (pancreas divisum redefined). Am J Surg 1990; 159:59–64.
- Bradley EL, Stephan RN. Accessory duct sphincteroplasty is preferred for long-term prevention of recurrent acute pancreatitis in patients with pancreas divisum. J Am Coll Surg 1996; 183:65–70.
- Heyries L, Barthet M, Delvasto C, Zamora C, Bernard JP, Sahel J. Long-term results of endoscopic management of pancreas divisum with recurrent acute pancreatitis. Gastrointest Endosc 2002; 55:376–381.
- Lans JI, Geenen JE, Johanson JF, Hogan WJ. Endoscopic therapy in patients with pancreas divisum and acute pancreatitis: a prospective, randomized, controlled clinical trial. Gastrointest Endosc 1992; 38:430–434.
- Delhaye M, Matos C, Arvanitakis M, Deviere J. Pancreatic ductal system obstruction and acute recurrent pancreatitis. World J Gastroenterol 2008; 14:1027–1033.
- Finnie IA, Ghosh P, Garvey C, Poston GJ, Rhodes JM. Intraluminal duodenal diverticulum causing recurrent pancreatitis: treatment by endoscopic incision. Gut 1994; 35:557–559.
- Guzzardo G, Kleinman MS, Krackov JH, Schwartz SI. Recurrent acute pancreatitis caused by ampullary villous adenoma. J Clin Gastroenterol 1990; 12:200–202.
- Wright BE, Kozarek RA, Traverso LW, Wechter D, Thirlby R, Raltz SL. Recurrent pancreatitis in Gardner variant familial polyposis: etiology, diagnostic approach, and interventional results. Arch Surg 1999; 134:311–315.
- Tanasijtchouk T, Vaisbein E, Lachter J, Nassar F. Carcinoma of Papilla Vateri presenting as recurrent acute pancreatitis. Acta Gastroenterol Belg 2004; 67:309–310.
- Kwon TH, Park do H, Shim KY, et al. Ampullary adenomyoma presenting as acute recurrent pancreatitis. World J Gastroenterol 2007; 13:2892–2894.
- Lorente JA, Ruiz del Arbol L, Moreira VF, Garcia-Plaza A. Recurrent pancreatitis in a young patient associated with a solitary nonopaque concretion in the main pancreatic duct. Gastrointest Endosc 1990; 36:63–65.
- Chung JP, Chi SW, Park YN, et al. A case of minute intraductal papillary mucinous tumor of the pancreas presenting with recurrent acute pancreatitis. Yonsei Med J 2000; 41:528–532.
- Tikhomirov V, Tikhomirova S, Sieber S, Schiffman MK. A pancreatic intraductal papillary mucinous tumor causing recurrent acute pancreatitis at the onset of menstrual periods. J Clin Gastroenterol 2000; 31:172–174.
- Mosca S, Bottino V, Molino C. Hepatobiliary and pancreatic: a woman with recurrent idiopathic acute pancreatitis. Intraductal papillary mucinous tumor of the pancreas. J Gastroenterol Hepatol 2001; 16:1070,1075.
- Howard TJ, Moore SA, Saxena R, Matthews DE, Schmidt CM, Wiebke EA. Pancreatic duct strictures are a common cause of recurrent pancreatitis after successful management of pancreatic necrosis. Surgery 2004; 136:909–916.
- Garg PK, Tandon RK, Madan K. Is biliary microlithiasis a significant cause of idiopathic recurrent acute pancreatitis? A long-term follow-up study. Clin Gastroenterol Hepatol 2007; 5:75–79.
- Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol 2001; 96:705–709.
- Yusoff IF, Raymond G, Sahai AV. A prospective comparison of the yield of EUS in primary vs. recurrent idiopathic acute pancreatitis. Gastrointest Endosc 2004; 60:673–678.
- Cahen DL, Gouma DJ, Nio Y, et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med 2007; 356:676–684.
KEY POINTS
- Recurrent attacks of acute pancreatitis can be prevented only by determining and treating the underlying cause.
- Endoscopic procedures can cause anxiety and carry a risk of bleeding, perforation, and pancreatitis. The risks, benefits, and other treatment options should be discussed with the patient.
- Endoscopic therapy is now the preferred treatment of sphincter of Oddi dysfunction at centers that have experience with this technique.
- In patients with pancreas divisum and recurrent acute pancreatitis, surgical and endoscopic minor sphincterotomy are equally effective.