User login
A 50-year-old male Japanese immigrant with a history of smoking and occasional untreated heartburn presented with the recent onset of flank pain, weight loss, headache, syncope, and blurred vision.
Previously healthy, he began feeling moderate pain in his left flank 1 month ago; it was diagnosed as kidney stones and was treated conservatively. Two weeks later he had an episode of syncope and soon after developed blurred vision, mainly in his left eye, along with severe bifrontal headache. An eye examination and magnetic resonance imaging of the brain indicated optic neuritis, for which he was given glucocorticoids intravenously for 3 days, with moderate improvement.
As his symptoms continued over the next 2 weeks, he lost 20 lb (9.1 kg) due to the pain, loss of appetite, nausea, and occasional vomiting.
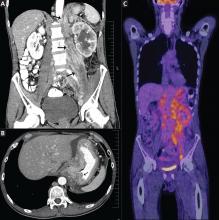
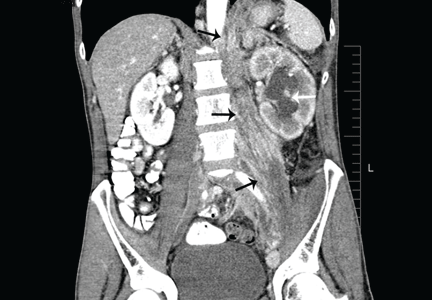
Positron emission tomography showed the retroperitoneal infiltrative process and the thickened gastric cardia to be hypermetabolic (Figure 1C).
The area of retroperitoneal infiltration was biopsied under CT guidance, and pathologic study showed poorly differentiated carcinoma with signet-ring cells, a feature of gastric cancer.
The patient underwent lumbar puncture. His cerebrospinal fluid had 206 white blood cells/μL (reference range 0–5) and large numbers of poorly differentiated malignant cells, most consistent with adenocarcinoma on cytologic study.
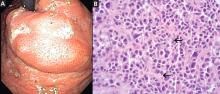
duodenoscopy (EGD) revealed a large, ulcerated, submucosal, nodular mass in the cardia of the stomach extending to the gastroesophageal junction (Figure 2A). Biopsy of the mass again revealed poorly differentiated adenocarcinoma with scattered signet-ring cells undermining the gastric mucosa, favoring a gastric origin (Figure 2B).
THREE SUBTYPES OF GASTRIC CANCER
Worldwide, gastric cancer is the third most common type of cancer and the second most common cause of cancer-related deaths.1 In the United States, blacks and people of Asian ancestry have almost twice the risk of death, with the highest incidence and mortality rates.2,3
Most cases of gastric adenocarcinoma can be categorized as either intestinal or diffuse, but a new proximal subtype is emerging.4
Intestinal-type gastric adenocarcinoma is the most common subtype and accounts for almost all the ethnic and geographic variation in incidence.2 The lesions are often ulcerative and distal; the pathogenesis is stepwise and is initiated by chronic inflammation. Risk factors include old age, Helicobacter pylori infection, tobacco smoking, family history, and high salt intake, with an observed risk-reduction with the use of nonsteroidal anti-inflammatory drugs and with a high intake of fruits and vegetables.3
Diffuse gastric adenocarcinoma, on the other hand, has a uniform distribution worldwide, and its incidence is increasing. It typically carries a poor prognosis. Evidence thus far has shown its pathogenesis to be independent of chronic inflammation, but it has a strong tendency to be hereditary.3
Proximal gastric adenocarcinoma is observed in the gastric cardia and near the gastroesophageal junction. It is often grouped with the distal esophageal adenocarcinomas and has similar risk factors, including reflux disease, obesity, alcohol abuse, and tobacco smoking. Interestingly, however, H pylori infection does not contribute to the pathogenesis of this type, and it may even have a protective role.3
DIFFICULT TO DETECT EARLY
Gastric cancer is difficult to detect early enough in its course to be cured. Understanding its risk factors, recognizing its common symptoms, and regarding its uncommon symptoms with suspicion may lead to earlier diagnosis and more effective treatment.
Our patient’s proximal gastric cancer was diagnosed late even though he had several risk factors for it (he was Japanese, he was a smoker, and he had gastroesophageal reflux disease) because of a late and atypical presentation with misleading paraneoplastic symptoms.
Early diagnosis is difficult because most patients have no symptoms in the early stage; weight loss and abdominal pain are often late signs of tumor progression.
Screening may be justified in high-risk groups in the United States, although the issue is debatable. Diagnostic imaging is the only effective method for screening,5 with EGD considered the first-line targeted evaluation should there be suspicion of gastric cancer either from the clinical presentation or from barium swallow.6 Candidates for screening may include elderly patients with atrophic gastritis or pernicious anemia, immigrants from countries with high rates of gastric carcinoma, and people with a family history of gastrointestinal cancer.7
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55:74–108.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12:354–362.
- Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw 2010; 8:437–447.
- Fine G, Chan K. Alimentary tract. In:Kissane JM, editor. Anderson’s Pathology. 8th ed. Saint Louis, MO: Mosby; 1985:1055–1095.
- Kunisaki C, Ishino J, Nakajima S, et al. Outcomes of mass screening for gastric carcinoma. Ann Surg Oncol 2006; 13:221–228.
- Cappell MS, Friedel D. The role of esophagogastroduodenoscopy in the diagnosis and management of upper gastrointestinal disorders. Med Clin North Am 2002; 86:1165–1216.
- Hisamuchi S, Fukao P, Sugawara N, et al. Evaluation of mass screening programme for stomach cancer in Japan. In:Miller AB, Chamberlain J, Day NE, et al, editors. Cancer Screening. Cambridge, UK: Cambridge University Press; 1991:357–372.
A 50-year-old male Japanese immigrant with a history of smoking and occasional untreated heartburn presented with the recent onset of flank pain, weight loss, headache, syncope, and blurred vision.
Previously healthy, he began feeling moderate pain in his left flank 1 month ago; it was diagnosed as kidney stones and was treated conservatively. Two weeks later he had an episode of syncope and soon after developed blurred vision, mainly in his left eye, along with severe bifrontal headache. An eye examination and magnetic resonance imaging of the brain indicated optic neuritis, for which he was given glucocorticoids intravenously for 3 days, with moderate improvement.
As his symptoms continued over the next 2 weeks, he lost 20 lb (9.1 kg) due to the pain, loss of appetite, nausea, and occasional vomiting.
Positron emission tomography showed the retroperitoneal infiltrative process and the thickened gastric cardia to be hypermetabolic (Figure 1C).
The area of retroperitoneal infiltration was biopsied under CT guidance, and pathologic study showed poorly differentiated carcinoma with signet-ring cells, a feature of gastric cancer.
The patient underwent lumbar puncture. His cerebrospinal fluid had 206 white blood cells/μL (reference range 0–5) and large numbers of poorly differentiated malignant cells, most consistent with adenocarcinoma on cytologic study.
duodenoscopy (EGD) revealed a large, ulcerated, submucosal, nodular mass in the cardia of the stomach extending to the gastroesophageal junction (Figure 2A). Biopsy of the mass again revealed poorly differentiated adenocarcinoma with scattered signet-ring cells undermining the gastric mucosa, favoring a gastric origin (Figure 2B).
THREE SUBTYPES OF GASTRIC CANCER
Worldwide, gastric cancer is the third most common type of cancer and the second most common cause of cancer-related deaths.1 In the United States, blacks and people of Asian ancestry have almost twice the risk of death, with the highest incidence and mortality rates.2,3
Most cases of gastric adenocarcinoma can be categorized as either intestinal or diffuse, but a new proximal subtype is emerging.4
Intestinal-type gastric adenocarcinoma is the most common subtype and accounts for almost all the ethnic and geographic variation in incidence.2 The lesions are often ulcerative and distal; the pathogenesis is stepwise and is initiated by chronic inflammation. Risk factors include old age, Helicobacter pylori infection, tobacco smoking, family history, and high salt intake, with an observed risk-reduction with the use of nonsteroidal anti-inflammatory drugs and with a high intake of fruits and vegetables.3
Diffuse gastric adenocarcinoma, on the other hand, has a uniform distribution worldwide, and its incidence is increasing. It typically carries a poor prognosis. Evidence thus far has shown its pathogenesis to be independent of chronic inflammation, but it has a strong tendency to be hereditary.3
Proximal gastric adenocarcinoma is observed in the gastric cardia and near the gastroesophageal junction. It is often grouped with the distal esophageal adenocarcinomas and has similar risk factors, including reflux disease, obesity, alcohol abuse, and tobacco smoking. Interestingly, however, H pylori infection does not contribute to the pathogenesis of this type, and it may even have a protective role.3
DIFFICULT TO DETECT EARLY
Gastric cancer is difficult to detect early enough in its course to be cured. Understanding its risk factors, recognizing its common symptoms, and regarding its uncommon symptoms with suspicion may lead to earlier diagnosis and more effective treatment.
Our patient’s proximal gastric cancer was diagnosed late even though he had several risk factors for it (he was Japanese, he was a smoker, and he had gastroesophageal reflux disease) because of a late and atypical presentation with misleading paraneoplastic symptoms.
Early diagnosis is difficult because most patients have no symptoms in the early stage; weight loss and abdominal pain are often late signs of tumor progression.
Screening may be justified in high-risk groups in the United States, although the issue is debatable. Diagnostic imaging is the only effective method for screening,5 with EGD considered the first-line targeted evaluation should there be suspicion of gastric cancer either from the clinical presentation or from barium swallow.6 Candidates for screening may include elderly patients with atrophic gastritis or pernicious anemia, immigrants from countries with high rates of gastric carcinoma, and people with a family history of gastrointestinal cancer.7
A 50-year-old male Japanese immigrant with a history of smoking and occasional untreated heartburn presented with the recent onset of flank pain, weight loss, headache, syncope, and blurred vision.
Previously healthy, he began feeling moderate pain in his left flank 1 month ago; it was diagnosed as kidney stones and was treated conservatively. Two weeks later he had an episode of syncope and soon after developed blurred vision, mainly in his left eye, along with severe bifrontal headache. An eye examination and magnetic resonance imaging of the brain indicated optic neuritis, for which he was given glucocorticoids intravenously for 3 days, with moderate improvement.
As his symptoms continued over the next 2 weeks, he lost 20 lb (9.1 kg) due to the pain, loss of appetite, nausea, and occasional vomiting.
Positron emission tomography showed the retroperitoneal infiltrative process and the thickened gastric cardia to be hypermetabolic (Figure 1C).
The area of retroperitoneal infiltration was biopsied under CT guidance, and pathologic study showed poorly differentiated carcinoma with signet-ring cells, a feature of gastric cancer.
The patient underwent lumbar puncture. His cerebrospinal fluid had 206 white blood cells/μL (reference range 0–5) and large numbers of poorly differentiated malignant cells, most consistent with adenocarcinoma on cytologic study.
duodenoscopy (EGD) revealed a large, ulcerated, submucosal, nodular mass in the cardia of the stomach extending to the gastroesophageal junction (Figure 2A). Biopsy of the mass again revealed poorly differentiated adenocarcinoma with scattered signet-ring cells undermining the gastric mucosa, favoring a gastric origin (Figure 2B).
THREE SUBTYPES OF GASTRIC CANCER
Worldwide, gastric cancer is the third most common type of cancer and the second most common cause of cancer-related deaths.1 In the United States, blacks and people of Asian ancestry have almost twice the risk of death, with the highest incidence and mortality rates.2,3
Most cases of gastric adenocarcinoma can be categorized as either intestinal or diffuse, but a new proximal subtype is emerging.4
Intestinal-type gastric adenocarcinoma is the most common subtype and accounts for almost all the ethnic and geographic variation in incidence.2 The lesions are often ulcerative and distal; the pathogenesis is stepwise and is initiated by chronic inflammation. Risk factors include old age, Helicobacter pylori infection, tobacco smoking, family history, and high salt intake, with an observed risk-reduction with the use of nonsteroidal anti-inflammatory drugs and with a high intake of fruits and vegetables.3
Diffuse gastric adenocarcinoma, on the other hand, has a uniform distribution worldwide, and its incidence is increasing. It typically carries a poor prognosis. Evidence thus far has shown its pathogenesis to be independent of chronic inflammation, but it has a strong tendency to be hereditary.3
Proximal gastric adenocarcinoma is observed in the gastric cardia and near the gastroesophageal junction. It is often grouped with the distal esophageal adenocarcinomas and has similar risk factors, including reflux disease, obesity, alcohol abuse, and tobacco smoking. Interestingly, however, H pylori infection does not contribute to the pathogenesis of this type, and it may even have a protective role.3
DIFFICULT TO DETECT EARLY
Gastric cancer is difficult to detect early enough in its course to be cured. Understanding its risk factors, recognizing its common symptoms, and regarding its uncommon symptoms with suspicion may lead to earlier diagnosis and more effective treatment.
Our patient’s proximal gastric cancer was diagnosed late even though he had several risk factors for it (he was Japanese, he was a smoker, and he had gastroesophageal reflux disease) because of a late and atypical presentation with misleading paraneoplastic symptoms.
Early diagnosis is difficult because most patients have no symptoms in the early stage; weight loss and abdominal pain are often late signs of tumor progression.
Screening may be justified in high-risk groups in the United States, although the issue is debatable. Diagnostic imaging is the only effective method for screening,5 with EGD considered the first-line targeted evaluation should there be suspicion of gastric cancer either from the clinical presentation or from barium swallow.6 Candidates for screening may include elderly patients with atrophic gastritis or pernicious anemia, immigrants from countries with high rates of gastric carcinoma, and people with a family history of gastrointestinal cancer.7
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55:74–108.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12:354–362.
- Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw 2010; 8:437–447.
- Fine G, Chan K. Alimentary tract. In:Kissane JM, editor. Anderson’s Pathology. 8th ed. Saint Louis, MO: Mosby; 1985:1055–1095.
- Kunisaki C, Ishino J, Nakajima S, et al. Outcomes of mass screening for gastric carcinoma. Ann Surg Oncol 2006; 13:221–228.
- Cappell MS, Friedel D. The role of esophagogastroduodenoscopy in the diagnosis and management of upper gastrointestinal disorders. Med Clin North Am 2002; 86:1165–1216.
- Hisamuchi S, Fukao P, Sugawara N, et al. Evaluation of mass screening programme for stomach cancer in Japan. In:Miller AB, Chamberlain J, Day NE, et al, editors. Cancer Screening. Cambridge, UK: Cambridge University Press; 1991:357–372.
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55:74–108.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12:354–362.
- Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw 2010; 8:437–447.
- Fine G, Chan K. Alimentary tract. In:Kissane JM, editor. Anderson’s Pathology. 8th ed. Saint Louis, MO: Mosby; 1985:1055–1095.
- Kunisaki C, Ishino J, Nakajima S, et al. Outcomes of mass screening for gastric carcinoma. Ann Surg Oncol 2006; 13:221–228.
- Cappell MS, Friedel D. The role of esophagogastroduodenoscopy in the diagnosis and management of upper gastrointestinal disorders. Med Clin North Am 2002; 86:1165–1216.
- Hisamuchi S, Fukao P, Sugawara N, et al. Evaluation of mass screening programme for stomach cancer in Japan. In:Miller AB, Chamberlain J, Day NE, et al, editors. Cancer Screening. Cambridge, UK: Cambridge University Press; 1991:357–372.