User login
Does early repolarization on ECG increase the risk of cardiac death in healthy people?
No. The early repolarization pattern on electrocardiography (ECG) in asymptomatic patients is nearly always a benign incidental finding. However, in a patient with a history of idiopathic ventricular fibrillation or a family history of sudden cardiac death, the finding warrants further evaluation.
DEFINING EARLY REPOLARIZATION
The early repolarization pattern may mimic patterns seen in myocardial infarction, pericarditis, ventricular aneurysm, hyperkalemia, and hypothermia,1,3 and misinterpreting the pattern can lead to unnecessary laboratory testing, imaging, medication use, and hospital admissions. On the other hand, misinterpreting it as benign in the presence of certain features of the history or clinical presentation can delay the diagnosis and treatment of a potentially critical condition.
PREVALENCE AND MECHANISMS
The prevalence of the early repolarization pattern in the general population ranges from 5% to 15%; the wide range reflects differences in the definition, as well as variability in the pattern of early repolarization over time.4
The early repolarization pattern is more commonly seen in African American men and in young, physically active individuals.3 In one study, it was observed in 15% of cases of idiopathic ventricular fibrillation and sudden cardiac death, especially in people ages 35 to 45.4 While there is evidence of a heritable basis in the general population, a family history of early repolarization is not known to increase the risk of sudden cardiac death.
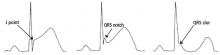
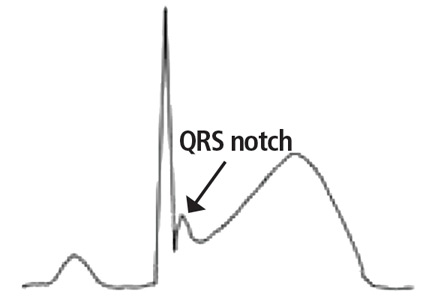
A proposed mechanism for the early repolarization pattern is an imbalance in the ion channel system, resulting in variable refractoriness of multiple myocardial regions and varying excitability in the myocardium. This can produce a voltage gradient between myocardial regions, which is believed to cause the major hallmarks of the early repolarization pattern, ie, ST-segment elevation and QRS notching or slurring.3
MANAGEMENT
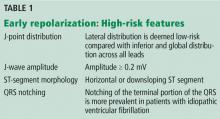
The early repolarization pattern is nearly always a benign incidental finding on ECG, with no specific signs or symptoms attributed to it. High-risk features on ECG are associated with a modest increase in absolute risk of sudden cardiac death and warrant clinical correlation.
In the absence of syncope or family history of sudden cardiac death, early repolarization does not merit further workup.2
In patients with a history of unexplained syncope and a family history of sudden cardiac death, early repolarization should be considered in overall risk stratification.1 Early repolarization in a patient with previous idiopathic ventricular fibrillation warrants referral for electrophysiologic study and, if indicated, insertion of an implantable cardiac defibrillator for secondary prevention.5
- Patton KK, Ellinor PT, Ezekowitz M, et al; American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Functional Genomics and Translational Biology. Electrocardiographic early repolarization: a scientific statement from the American Heart Association. Circulation 2016; 133(15):1520–1529. doi:10.1161/CIR.0000000000000388
- Macfarlane PW, Antzelevitch C, Haissaguerre M, et al. The early repolarization pattern: a consensus paper. J Am Coll Cardiol 2015; 66(4):470–477. doi:10.1016/j.jacc.2015.05.033
- Benito B, Guasch E, Rivard L, Nattel S. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol 2010; 56(15):1177–1186. doi:10.1016/j.jacc.2010.05.037
- Maury P, Rollin A. Prevalence of early repolarisation/J wave patterns in the normal population. J Electrocardiol 2013; 46(5):411–416. doi:10.1016/j.jelectrocard.2013.06.014
- Mahida S, Sacher F, Berte B, et al. Evaluation of patients with early repolarization syndrome. J Atr Fibrillation 2014; 7(3):1083. doi:10.4022/jafib.1083
No. The early repolarization pattern on electrocardiography (ECG) in asymptomatic patients is nearly always a benign incidental finding. However, in a patient with a history of idiopathic ventricular fibrillation or a family history of sudden cardiac death, the finding warrants further evaluation.
DEFINING EARLY REPOLARIZATION
The early repolarization pattern may mimic patterns seen in myocardial infarction, pericarditis, ventricular aneurysm, hyperkalemia, and hypothermia,1,3 and misinterpreting the pattern can lead to unnecessary laboratory testing, imaging, medication use, and hospital admissions. On the other hand, misinterpreting it as benign in the presence of certain features of the history or clinical presentation can delay the diagnosis and treatment of a potentially critical condition.
PREVALENCE AND MECHANISMS
The prevalence of the early repolarization pattern in the general population ranges from 5% to 15%; the wide range reflects differences in the definition, as well as variability in the pattern of early repolarization over time.4
The early repolarization pattern is more commonly seen in African American men and in young, physically active individuals.3 In one study, it was observed in 15% of cases of idiopathic ventricular fibrillation and sudden cardiac death, especially in people ages 35 to 45.4 While there is evidence of a heritable basis in the general population, a family history of early repolarization is not known to increase the risk of sudden cardiac death.
A proposed mechanism for the early repolarization pattern is an imbalance in the ion channel system, resulting in variable refractoriness of multiple myocardial regions and varying excitability in the myocardium. This can produce a voltage gradient between myocardial regions, which is believed to cause the major hallmarks of the early repolarization pattern, ie, ST-segment elevation and QRS notching or slurring.3
MANAGEMENT
The early repolarization pattern is nearly always a benign incidental finding on ECG, with no specific signs or symptoms attributed to it. High-risk features on ECG are associated with a modest increase in absolute risk of sudden cardiac death and warrant clinical correlation.
In the absence of syncope or family history of sudden cardiac death, early repolarization does not merit further workup.2
In patients with a history of unexplained syncope and a family history of sudden cardiac death, early repolarization should be considered in overall risk stratification.1 Early repolarization in a patient with previous idiopathic ventricular fibrillation warrants referral for electrophysiologic study and, if indicated, insertion of an implantable cardiac defibrillator for secondary prevention.5
No. The early repolarization pattern on electrocardiography (ECG) in asymptomatic patients is nearly always a benign incidental finding. However, in a patient with a history of idiopathic ventricular fibrillation or a family history of sudden cardiac death, the finding warrants further evaluation.
DEFINING EARLY REPOLARIZATION
The early repolarization pattern may mimic patterns seen in myocardial infarction, pericarditis, ventricular aneurysm, hyperkalemia, and hypothermia,1,3 and misinterpreting the pattern can lead to unnecessary laboratory testing, imaging, medication use, and hospital admissions. On the other hand, misinterpreting it as benign in the presence of certain features of the history or clinical presentation can delay the diagnosis and treatment of a potentially critical condition.
PREVALENCE AND MECHANISMS
The prevalence of the early repolarization pattern in the general population ranges from 5% to 15%; the wide range reflects differences in the definition, as well as variability in the pattern of early repolarization over time.4
The early repolarization pattern is more commonly seen in African American men and in young, physically active individuals.3 In one study, it was observed in 15% of cases of idiopathic ventricular fibrillation and sudden cardiac death, especially in people ages 35 to 45.4 While there is evidence of a heritable basis in the general population, a family history of early repolarization is not known to increase the risk of sudden cardiac death.
A proposed mechanism for the early repolarization pattern is an imbalance in the ion channel system, resulting in variable refractoriness of multiple myocardial regions and varying excitability in the myocardium. This can produce a voltage gradient between myocardial regions, which is believed to cause the major hallmarks of the early repolarization pattern, ie, ST-segment elevation and QRS notching or slurring.3
MANAGEMENT
The early repolarization pattern is nearly always a benign incidental finding on ECG, with no specific signs or symptoms attributed to it. High-risk features on ECG are associated with a modest increase in absolute risk of sudden cardiac death and warrant clinical correlation.
In the absence of syncope or family history of sudden cardiac death, early repolarization does not merit further workup.2
In patients with a history of unexplained syncope and a family history of sudden cardiac death, early repolarization should be considered in overall risk stratification.1 Early repolarization in a patient with previous idiopathic ventricular fibrillation warrants referral for electrophysiologic study and, if indicated, insertion of an implantable cardiac defibrillator for secondary prevention.5
- Patton KK, Ellinor PT, Ezekowitz M, et al; American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Functional Genomics and Translational Biology. Electrocardiographic early repolarization: a scientific statement from the American Heart Association. Circulation 2016; 133(15):1520–1529. doi:10.1161/CIR.0000000000000388
- Macfarlane PW, Antzelevitch C, Haissaguerre M, et al. The early repolarization pattern: a consensus paper. J Am Coll Cardiol 2015; 66(4):470–477. doi:10.1016/j.jacc.2015.05.033
- Benito B, Guasch E, Rivard L, Nattel S. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol 2010; 56(15):1177–1186. doi:10.1016/j.jacc.2010.05.037
- Maury P, Rollin A. Prevalence of early repolarisation/J wave patterns in the normal population. J Electrocardiol 2013; 46(5):411–416. doi:10.1016/j.jelectrocard.2013.06.014
- Mahida S, Sacher F, Berte B, et al. Evaluation of patients with early repolarization syndrome. J Atr Fibrillation 2014; 7(3):1083. doi:10.4022/jafib.1083
- Patton KK, Ellinor PT, Ezekowitz M, et al; American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Functional Genomics and Translational Biology. Electrocardiographic early repolarization: a scientific statement from the American Heart Association. Circulation 2016; 133(15):1520–1529. doi:10.1161/CIR.0000000000000388
- Macfarlane PW, Antzelevitch C, Haissaguerre M, et al. The early repolarization pattern: a consensus paper. J Am Coll Cardiol 2015; 66(4):470–477. doi:10.1016/j.jacc.2015.05.033
- Benito B, Guasch E, Rivard L, Nattel S. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol 2010; 56(15):1177–1186. doi:10.1016/j.jacc.2010.05.037
- Maury P, Rollin A. Prevalence of early repolarisation/J wave patterns in the normal population. J Electrocardiol 2013; 46(5):411–416. doi:10.1016/j.jelectrocard.2013.06.014
- Mahida S, Sacher F, Berte B, et al. Evaluation of patients with early repolarization syndrome. J Atr Fibrillation 2014; 7(3):1083. doi:10.4022/jafib.1083
Do all hospital inpatients need cardiac telemetry?
No. Continuous monitoring for changes in heart rhythm with cardiac telemetry is recommended for all patients admitted to an intensive care unit (ICU). But routine telemetry monitoring for patients in non-ICU beds is not recommended, as it leads to unnecessary testing and treatment, increasing the cost of care and hospital length of stay.
RISK STRATIFICATION AND INDICATIONS
Telemetry is generally recommended for patients admitted with any type of heart disease, including:
- Acute myocardial infarction with ST-segment elevation or Q waves on 12-lead electrocardiography (ECG)
- Acute ischemia suggested by ST-segment depression or T-wave inversion on ECG
- Systolic blood pressure less than 100 mm Hg
- Acute decompensated heart failure with bilateral rales above the lung bases
- Chest pain that is worse than or the same as that in prior angina or myocardial infarction.1,2
Indications for telemetry are less clear in patients with no history of heart disease. The American Heart Association (AHA)3 has classified admitted patients based on the presence or absence of heart disease3:
- Class I (high risk of arrhythmia): acute coronary syndrome, new arrhythmia (eg, atrial fibrillation or flutter), severe electrolyte imbalance; telemetry is warranted
- Class II (moderate risk): acute decompensated heart failure with stable hemodynamic status, a surgical or medical diagnosis with underlying paced rhythms (ie, with a pacemaker), and chronic arrhythmia (atrial fibrillation or flutter); in these cases, telemetry monitoring may be considered
- Class III (low risk): no history of cardiac disease or arrhythmias, admitted for medical or surgical reasons; in these cases, telemetry is generally not indicated3
Telemetry should also be considered in patients admitted with syncope or stroke, critical illness, or palpitations.
Syncope and stroke
Despite the wide use of telemetry for patients admitted with syncope, current evidence does not support this practice. However, the AHA recommends routine telemetry for patients admitted with idiopathic syncope when there is a high level of suspicion for underlying cardiac arrhythmias as a cause of syncope (risk class II-b).3 In 30% of patients admitted with stroke or transient ischemic attack, the cause is cardioembolic. Therefore, telemetry is indicated to rule out an underlying cardiac cause.4
Critical illness
Patients hospitalized with major trauma, acute respiratory failure, sepsis, shock, or acute pulmonary embolism or for major noncardiac surgery (especially elderly patients with coronary artery disease or at high risk of coronary events) require cardiac telemetry (risk class I-b). Patients admitted with kidney failure, significant electrolyte abnormalities, drug or substance toxicity (especially with known arrhythmogenic drugs) also require cardiac telemetry at the time of admission (risk class I-b).
Recurrent palpitations, arrhythmia
Most patients with palpitations can be evaluated in an outpatient setting.5 However, patients hospitalized for recurrent palpitations or for suspected underlying cardiac disease require telemetric monitoring (risk class II-b).3 Patients with high-degree atrioventricular block admitted after percutaneous temporary pacemaker implantation should be monitored, as should patients with a permanent pacemaker for 12 to 24 hours after implantation (risk class I-c). Also, patients hospitalized after implantable cardioverter-defibrillator (ICD) shock need to be monitored.3,6
Patients with a paced rhythm who do not meet the above criteria do not require routine telemetric monitoring (risk class III-c).7
TELEMETRY IS OVERUSED
Off-site telemetry monitoring can identify significant arrhythmias during hospitalization. It also saves time on nursing staff to focus on bedside patient care. However, its convenience can lower the threshold for ordering it. This can lead to overuse with a major impact on healthcare costs.
Routine use of cardiac telemetry is associated with increased hospitalization costs with little benefit.8 The use of off-site services for continuous monitoring can activate many alarms throughout the day, triggering unnecessary workups and leading to densensitization to alarms (“alarm fatigue”).9
Despite the precise indications outlined in the AHA updated practice standards for inpatient electrocardiographic monitoring,10 telemetry use is expanding to non-ICU units without evidence of benefit,8 and this overuse can result in harmful clinical outcomes and a financial burden. Telemetry monitoring of low-risk patients can cause delays in emergency department and ICU admissions and transfers8,11 of patients who may be sicker and need intensive care.
In a prospective observational study,12 only 11 (6%) of 182 patients admitted to a general medical floor met AHA class I criteria for telemetry; very few patients developed a significant telemetry event such as atrial fibrillation or flutter that necessitated a change in management. Most overprescribers of telemetry monitoring reason that it will catch arrhythmias early.12 In fact, in a study of patients in a cardiac unit, telemetry detected just 50% of in-house cardiac arrest cases, with a potential survival benefit of only 0.02%.13
Another study showed that only 0.01% of all telemetry alarms represented a real emergency. Only 37.2% of emergency alarms were classified as clinically important, and only 48.3% of these led to a change in management within 1 hour.14
Moreover, in a report of trauma patients with abnormal results on ECG at the time of admission, telemetry had negligible clinical benefit.15 And in a study of 414 patients, only 4% of those admitted with chest pain and normal initial ECG had cardiac interventions.16
Another study8 showed that hospital intervention to restrict the use of telemetry guided by AHA recommendations resulted in a 43% reduction in telemetry orders, a 47% reduction in telemetry duration, and a 70% reduction in the mean daily number of patients monitored, with no changes in hospital census or rates of code blue, death, or rapid response team activation.8
The financial cost can be seen in the backup of patients in the emergency department. A study showed that 91% of patients being admitted for chest pain were delayed by more than 3 hours while waiting for monitored beds. This translated into an annual cost of $168,300 to the hospital.17 Adherence to guidelines for appropriate use of telemetry can significantly decrease costs. Applying a simple algorithm for telemetry use was shown8 to decrease daily non-ICU cardiac telemetry costs from $18,971 to $5,772.
CURRENT GUIDELINES ARE LIMITED
The current American College of Cardiology and AHA guidelines are based mostly on expert opinion rather than randomized clinical trials, while most telemetry trials have been performed on patients with a cardiac or possible cardiac diagnosis.3 Current guidelines need to be updated, and more studies are needed to specify the optimal duration of cardiac monitoring in indicated cases. Many noncardiac conditions raise a legitimate concern of dysrhythmia, an indication for cardiac monitoring, but precise recommendations for telemetry for such conditions are lacking.
RECOMMENDATIONS
Raising awareness of the clinical and financial burdens associated with unwise telemetry utilization is critical. We suggest use of a pop-up notification in the electronic medical record to remind the provider of the existing telemetry order and to specify the duration of telemetry monitoring when placing the initial order. The goal is to identify patients in true need of a telemetry bed, to decrease unnecessary testing, and to reduce hospitalization costs.
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol 1991; 18(6):1431–1433. pmid:1939942
- Goldman L, Cook EF, Johnson PA, Brand DA, Rouan GW, Lee TH. Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain. N Engl J Med 1996; 334(23):1498–1504. doi:10.1056/NEJM199606063342303
- Drew BJ, Califf RM, Funk M, et al; American Heart Association; Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110(17):2721–2746. doi:10.1161/01.CIR.0000145144.56673.59
- Ustrell X, Pellise A. Cardiac workup of ischemic stroke. Curr Cardiol Rev 2010; 6(3):175-183. doi:10.2174/157340310791658721
- Olson JA, Fouts AM, Padanilam BJ, Prystowsky EN. Utility of mobile cardiac outpatient telemetry for the diagnosis of palpitations, presyncope, syncope, and the assessment of therapy efficacy. J Cardiovasc Electrophysiol 2007; 18(5):473–477. doi:10.1111/j.1540-8167.2007.00779.x
- Chen EH, Hollander JE. When do patients need admission to a telemetry bed? J Emerg Med 2007; 33(1):53–60. doi:10.1016/j.jemermed.2007.01.017
- Sandau KE, Funk M, Auerbach A, et al; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Cardiovascular Disease in the Young. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med 2014; 174(11):1852–1854. doi:10.1001/jamainternmed.2014.4491
- Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non–critically ill patients. JAMA 2016; 316(5):519–524. doi:10.1001/jama.2016.10258
- Sandau KE, Funk M, Auerbach A, et al. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Atzema C, Schull MJ, Borgundvaag B, Slaughter GR, Lee CK. ALARMED: adverse events in low-risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med 2006; 24(1):62–67. doi:10.1016/j.ajem.2005.05.015
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med 2012; 172(17):1349–1350. doi:10.1001/archinternmed.2012.3163
- Schull MJ, Redelmeier DA. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000; 7(6):647–652. pmid:10905643
- Kansara P, Jackson K, Dressler R, et al. Potential of missing life-threatening arrhythmias after limiting the use of cardiac telemetry. JAMA Intern Med 2015; 175(8):1416–1418. doi:10.1001/jamainternmed.2015.2387
- Nagy KK, Krosner SM, Roberts RR, Joseph KT, Smith RF, Barrett J. Determining which patients require evaluation for blunt cardiac injury following blunt chest trauma. World J Surg 2001; 25(1):108–111. pmid:11213149
- Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002; 122(2):517–523. pmid:12171825
- Bayley MD, Schwartz JS, Shofer FS, et al. The financial burden of emergency department congestion and hospital crowding for chest pain patients awaiting admission. Ann Emerg Med 2005; 45(2):110–117. doi:10.1016/j.annemergmed.2004.09.010
No. Continuous monitoring for changes in heart rhythm with cardiac telemetry is recommended for all patients admitted to an intensive care unit (ICU). But routine telemetry monitoring for patients in non-ICU beds is not recommended, as it leads to unnecessary testing and treatment, increasing the cost of care and hospital length of stay.
RISK STRATIFICATION AND INDICATIONS
Telemetry is generally recommended for patients admitted with any type of heart disease, including:
- Acute myocardial infarction with ST-segment elevation or Q waves on 12-lead electrocardiography (ECG)
- Acute ischemia suggested by ST-segment depression or T-wave inversion on ECG
- Systolic blood pressure less than 100 mm Hg
- Acute decompensated heart failure with bilateral rales above the lung bases
- Chest pain that is worse than or the same as that in prior angina or myocardial infarction.1,2
Indications for telemetry are less clear in patients with no history of heart disease. The American Heart Association (AHA)3 has classified admitted patients based on the presence or absence of heart disease3:
- Class I (high risk of arrhythmia): acute coronary syndrome, new arrhythmia (eg, atrial fibrillation or flutter), severe electrolyte imbalance; telemetry is warranted
- Class II (moderate risk): acute decompensated heart failure with stable hemodynamic status, a surgical or medical diagnosis with underlying paced rhythms (ie, with a pacemaker), and chronic arrhythmia (atrial fibrillation or flutter); in these cases, telemetry monitoring may be considered
- Class III (low risk): no history of cardiac disease or arrhythmias, admitted for medical or surgical reasons; in these cases, telemetry is generally not indicated3
Telemetry should also be considered in patients admitted with syncope or stroke, critical illness, or palpitations.
Syncope and stroke
Despite the wide use of telemetry for patients admitted with syncope, current evidence does not support this practice. However, the AHA recommends routine telemetry for patients admitted with idiopathic syncope when there is a high level of suspicion for underlying cardiac arrhythmias as a cause of syncope (risk class II-b).3 In 30% of patients admitted with stroke or transient ischemic attack, the cause is cardioembolic. Therefore, telemetry is indicated to rule out an underlying cardiac cause.4
Critical illness
Patients hospitalized with major trauma, acute respiratory failure, sepsis, shock, or acute pulmonary embolism or for major noncardiac surgery (especially elderly patients with coronary artery disease or at high risk of coronary events) require cardiac telemetry (risk class I-b). Patients admitted with kidney failure, significant electrolyte abnormalities, drug or substance toxicity (especially with known arrhythmogenic drugs) also require cardiac telemetry at the time of admission (risk class I-b).
Recurrent palpitations, arrhythmia
Most patients with palpitations can be evaluated in an outpatient setting.5 However, patients hospitalized for recurrent palpitations or for suspected underlying cardiac disease require telemetric monitoring (risk class II-b).3 Patients with high-degree atrioventricular block admitted after percutaneous temporary pacemaker implantation should be monitored, as should patients with a permanent pacemaker for 12 to 24 hours after implantation (risk class I-c). Also, patients hospitalized after implantable cardioverter-defibrillator (ICD) shock need to be monitored.3,6
Patients with a paced rhythm who do not meet the above criteria do not require routine telemetric monitoring (risk class III-c).7
TELEMETRY IS OVERUSED
Off-site telemetry monitoring can identify significant arrhythmias during hospitalization. It also saves time on nursing staff to focus on bedside patient care. However, its convenience can lower the threshold for ordering it. This can lead to overuse with a major impact on healthcare costs.
Routine use of cardiac telemetry is associated with increased hospitalization costs with little benefit.8 The use of off-site services for continuous monitoring can activate many alarms throughout the day, triggering unnecessary workups and leading to densensitization to alarms (“alarm fatigue”).9
Despite the precise indications outlined in the AHA updated practice standards for inpatient electrocardiographic monitoring,10 telemetry use is expanding to non-ICU units without evidence of benefit,8 and this overuse can result in harmful clinical outcomes and a financial burden. Telemetry monitoring of low-risk patients can cause delays in emergency department and ICU admissions and transfers8,11 of patients who may be sicker and need intensive care.
In a prospective observational study,12 only 11 (6%) of 182 patients admitted to a general medical floor met AHA class I criteria for telemetry; very few patients developed a significant telemetry event such as atrial fibrillation or flutter that necessitated a change in management. Most overprescribers of telemetry monitoring reason that it will catch arrhythmias early.12 In fact, in a study of patients in a cardiac unit, telemetry detected just 50% of in-house cardiac arrest cases, with a potential survival benefit of only 0.02%.13
Another study showed that only 0.01% of all telemetry alarms represented a real emergency. Only 37.2% of emergency alarms were classified as clinically important, and only 48.3% of these led to a change in management within 1 hour.14
Moreover, in a report of trauma patients with abnormal results on ECG at the time of admission, telemetry had negligible clinical benefit.15 And in a study of 414 patients, only 4% of those admitted with chest pain and normal initial ECG had cardiac interventions.16
Another study8 showed that hospital intervention to restrict the use of telemetry guided by AHA recommendations resulted in a 43% reduction in telemetry orders, a 47% reduction in telemetry duration, and a 70% reduction in the mean daily number of patients monitored, with no changes in hospital census or rates of code blue, death, or rapid response team activation.8
The financial cost can be seen in the backup of patients in the emergency department. A study showed that 91% of patients being admitted for chest pain were delayed by more than 3 hours while waiting for monitored beds. This translated into an annual cost of $168,300 to the hospital.17 Adherence to guidelines for appropriate use of telemetry can significantly decrease costs. Applying a simple algorithm for telemetry use was shown8 to decrease daily non-ICU cardiac telemetry costs from $18,971 to $5,772.
CURRENT GUIDELINES ARE LIMITED
The current American College of Cardiology and AHA guidelines are based mostly on expert opinion rather than randomized clinical trials, while most telemetry trials have been performed on patients with a cardiac or possible cardiac diagnosis.3 Current guidelines need to be updated, and more studies are needed to specify the optimal duration of cardiac monitoring in indicated cases. Many noncardiac conditions raise a legitimate concern of dysrhythmia, an indication for cardiac monitoring, but precise recommendations for telemetry for such conditions are lacking.
RECOMMENDATIONS
Raising awareness of the clinical and financial burdens associated with unwise telemetry utilization is critical. We suggest use of a pop-up notification in the electronic medical record to remind the provider of the existing telemetry order and to specify the duration of telemetry monitoring when placing the initial order. The goal is to identify patients in true need of a telemetry bed, to decrease unnecessary testing, and to reduce hospitalization costs.
No. Continuous monitoring for changes in heart rhythm with cardiac telemetry is recommended for all patients admitted to an intensive care unit (ICU). But routine telemetry monitoring for patients in non-ICU beds is not recommended, as it leads to unnecessary testing and treatment, increasing the cost of care and hospital length of stay.
RISK STRATIFICATION AND INDICATIONS
Telemetry is generally recommended for patients admitted with any type of heart disease, including:
- Acute myocardial infarction with ST-segment elevation or Q waves on 12-lead electrocardiography (ECG)
- Acute ischemia suggested by ST-segment depression or T-wave inversion on ECG
- Systolic blood pressure less than 100 mm Hg
- Acute decompensated heart failure with bilateral rales above the lung bases
- Chest pain that is worse than or the same as that in prior angina or myocardial infarction.1,2
Indications for telemetry are less clear in patients with no history of heart disease. The American Heart Association (AHA)3 has classified admitted patients based on the presence or absence of heart disease3:
- Class I (high risk of arrhythmia): acute coronary syndrome, new arrhythmia (eg, atrial fibrillation or flutter), severe electrolyte imbalance; telemetry is warranted
- Class II (moderate risk): acute decompensated heart failure with stable hemodynamic status, a surgical or medical diagnosis with underlying paced rhythms (ie, with a pacemaker), and chronic arrhythmia (atrial fibrillation or flutter); in these cases, telemetry monitoring may be considered
- Class III (low risk): no history of cardiac disease or arrhythmias, admitted for medical or surgical reasons; in these cases, telemetry is generally not indicated3
Telemetry should also be considered in patients admitted with syncope or stroke, critical illness, or palpitations.
Syncope and stroke
Despite the wide use of telemetry for patients admitted with syncope, current evidence does not support this practice. However, the AHA recommends routine telemetry for patients admitted with idiopathic syncope when there is a high level of suspicion for underlying cardiac arrhythmias as a cause of syncope (risk class II-b).3 In 30% of patients admitted with stroke or transient ischemic attack, the cause is cardioembolic. Therefore, telemetry is indicated to rule out an underlying cardiac cause.4
Critical illness
Patients hospitalized with major trauma, acute respiratory failure, sepsis, shock, or acute pulmonary embolism or for major noncardiac surgery (especially elderly patients with coronary artery disease or at high risk of coronary events) require cardiac telemetry (risk class I-b). Patients admitted with kidney failure, significant electrolyte abnormalities, drug or substance toxicity (especially with known arrhythmogenic drugs) also require cardiac telemetry at the time of admission (risk class I-b).
Recurrent palpitations, arrhythmia
Most patients with palpitations can be evaluated in an outpatient setting.5 However, patients hospitalized for recurrent palpitations or for suspected underlying cardiac disease require telemetric monitoring (risk class II-b).3 Patients with high-degree atrioventricular block admitted after percutaneous temporary pacemaker implantation should be monitored, as should patients with a permanent pacemaker for 12 to 24 hours after implantation (risk class I-c). Also, patients hospitalized after implantable cardioverter-defibrillator (ICD) shock need to be monitored.3,6
Patients with a paced rhythm who do not meet the above criteria do not require routine telemetric monitoring (risk class III-c).7
TELEMETRY IS OVERUSED
Off-site telemetry monitoring can identify significant arrhythmias during hospitalization. It also saves time on nursing staff to focus on bedside patient care. However, its convenience can lower the threshold for ordering it. This can lead to overuse with a major impact on healthcare costs.
Routine use of cardiac telemetry is associated with increased hospitalization costs with little benefit.8 The use of off-site services for continuous monitoring can activate many alarms throughout the day, triggering unnecessary workups and leading to densensitization to alarms (“alarm fatigue”).9
Despite the precise indications outlined in the AHA updated practice standards for inpatient electrocardiographic monitoring,10 telemetry use is expanding to non-ICU units without evidence of benefit,8 and this overuse can result in harmful clinical outcomes and a financial burden. Telemetry monitoring of low-risk patients can cause delays in emergency department and ICU admissions and transfers8,11 of patients who may be sicker and need intensive care.
In a prospective observational study,12 only 11 (6%) of 182 patients admitted to a general medical floor met AHA class I criteria for telemetry; very few patients developed a significant telemetry event such as atrial fibrillation or flutter that necessitated a change in management. Most overprescribers of telemetry monitoring reason that it will catch arrhythmias early.12 In fact, in a study of patients in a cardiac unit, telemetry detected just 50% of in-house cardiac arrest cases, with a potential survival benefit of only 0.02%.13
Another study showed that only 0.01% of all telemetry alarms represented a real emergency. Only 37.2% of emergency alarms were classified as clinically important, and only 48.3% of these led to a change in management within 1 hour.14
Moreover, in a report of trauma patients with abnormal results on ECG at the time of admission, telemetry had negligible clinical benefit.15 And in a study of 414 patients, only 4% of those admitted with chest pain and normal initial ECG had cardiac interventions.16
Another study8 showed that hospital intervention to restrict the use of telemetry guided by AHA recommendations resulted in a 43% reduction in telemetry orders, a 47% reduction in telemetry duration, and a 70% reduction in the mean daily number of patients monitored, with no changes in hospital census or rates of code blue, death, or rapid response team activation.8
The financial cost can be seen in the backup of patients in the emergency department. A study showed that 91% of patients being admitted for chest pain were delayed by more than 3 hours while waiting for monitored beds. This translated into an annual cost of $168,300 to the hospital.17 Adherence to guidelines for appropriate use of telemetry can significantly decrease costs. Applying a simple algorithm for telemetry use was shown8 to decrease daily non-ICU cardiac telemetry costs from $18,971 to $5,772.
CURRENT GUIDELINES ARE LIMITED
The current American College of Cardiology and AHA guidelines are based mostly on expert opinion rather than randomized clinical trials, while most telemetry trials have been performed on patients with a cardiac or possible cardiac diagnosis.3 Current guidelines need to be updated, and more studies are needed to specify the optimal duration of cardiac monitoring in indicated cases. Many noncardiac conditions raise a legitimate concern of dysrhythmia, an indication for cardiac monitoring, but precise recommendations for telemetry for such conditions are lacking.
RECOMMENDATIONS
Raising awareness of the clinical and financial burdens associated with unwise telemetry utilization is critical. We suggest use of a pop-up notification in the electronic medical record to remind the provider of the existing telemetry order and to specify the duration of telemetry monitoring when placing the initial order. The goal is to identify patients in true need of a telemetry bed, to decrease unnecessary testing, and to reduce hospitalization costs.
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol 1991; 18(6):1431–1433. pmid:1939942
- Goldman L, Cook EF, Johnson PA, Brand DA, Rouan GW, Lee TH. Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain. N Engl J Med 1996; 334(23):1498–1504. doi:10.1056/NEJM199606063342303
- Drew BJ, Califf RM, Funk M, et al; American Heart Association; Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110(17):2721–2746. doi:10.1161/01.CIR.0000145144.56673.59
- Ustrell X, Pellise A. Cardiac workup of ischemic stroke. Curr Cardiol Rev 2010; 6(3):175-183. doi:10.2174/157340310791658721
- Olson JA, Fouts AM, Padanilam BJ, Prystowsky EN. Utility of mobile cardiac outpatient telemetry for the diagnosis of palpitations, presyncope, syncope, and the assessment of therapy efficacy. J Cardiovasc Electrophysiol 2007; 18(5):473–477. doi:10.1111/j.1540-8167.2007.00779.x
- Chen EH, Hollander JE. When do patients need admission to a telemetry bed? J Emerg Med 2007; 33(1):53–60. doi:10.1016/j.jemermed.2007.01.017
- Sandau KE, Funk M, Auerbach A, et al; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Cardiovascular Disease in the Young. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med 2014; 174(11):1852–1854. doi:10.1001/jamainternmed.2014.4491
- Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non–critically ill patients. JAMA 2016; 316(5):519–524. doi:10.1001/jama.2016.10258
- Sandau KE, Funk M, Auerbach A, et al. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Atzema C, Schull MJ, Borgundvaag B, Slaughter GR, Lee CK. ALARMED: adverse events in low-risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med 2006; 24(1):62–67. doi:10.1016/j.ajem.2005.05.015
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med 2012; 172(17):1349–1350. doi:10.1001/archinternmed.2012.3163
- Schull MJ, Redelmeier DA. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000; 7(6):647–652. pmid:10905643
- Kansara P, Jackson K, Dressler R, et al. Potential of missing life-threatening arrhythmias after limiting the use of cardiac telemetry. JAMA Intern Med 2015; 175(8):1416–1418. doi:10.1001/jamainternmed.2015.2387
- Nagy KK, Krosner SM, Roberts RR, Joseph KT, Smith RF, Barrett J. Determining which patients require evaluation for blunt cardiac injury following blunt chest trauma. World J Surg 2001; 25(1):108–111. pmid:11213149
- Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002; 122(2):517–523. pmid:12171825
- Bayley MD, Schwartz JS, Shofer FS, et al. The financial burden of emergency department congestion and hospital crowding for chest pain patients awaiting admission. Ann Emerg Med 2005; 45(2):110–117. doi:10.1016/j.annemergmed.2004.09.010
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol 1991; 18(6):1431–1433. pmid:1939942
- Goldman L, Cook EF, Johnson PA, Brand DA, Rouan GW, Lee TH. Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain. N Engl J Med 1996; 334(23):1498–1504. doi:10.1056/NEJM199606063342303
- Drew BJ, Califf RM, Funk M, et al; American Heart Association; Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110(17):2721–2746. doi:10.1161/01.CIR.0000145144.56673.59
- Ustrell X, Pellise A. Cardiac workup of ischemic stroke. Curr Cardiol Rev 2010; 6(3):175-183. doi:10.2174/157340310791658721
- Olson JA, Fouts AM, Padanilam BJ, Prystowsky EN. Utility of mobile cardiac outpatient telemetry for the diagnosis of palpitations, presyncope, syncope, and the assessment of therapy efficacy. J Cardiovasc Electrophysiol 2007; 18(5):473–477. doi:10.1111/j.1540-8167.2007.00779.x
- Chen EH, Hollander JE. When do patients need admission to a telemetry bed? J Emerg Med 2007; 33(1):53–60. doi:10.1016/j.jemermed.2007.01.017
- Sandau KE, Funk M, Auerbach A, et al; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Cardiovascular Disease in the Young. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med 2014; 174(11):1852–1854. doi:10.1001/jamainternmed.2014.4491
- Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non–critically ill patients. JAMA 2016; 316(5):519–524. doi:10.1001/jama.2016.10258
- Sandau KE, Funk M, Auerbach A, et al. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Atzema C, Schull MJ, Borgundvaag B, Slaughter GR, Lee CK. ALARMED: adverse events in low-risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med 2006; 24(1):62–67. doi:10.1016/j.ajem.2005.05.015
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med 2012; 172(17):1349–1350. doi:10.1001/archinternmed.2012.3163
- Schull MJ, Redelmeier DA. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000; 7(6):647–652. pmid:10905643
- Kansara P, Jackson K, Dressler R, et al. Potential of missing life-threatening arrhythmias after limiting the use of cardiac telemetry. JAMA Intern Med 2015; 175(8):1416–1418. doi:10.1001/jamainternmed.2015.2387
- Nagy KK, Krosner SM, Roberts RR, Joseph KT, Smith RF, Barrett J. Determining which patients require evaluation for blunt cardiac injury following blunt chest trauma. World J Surg 2001; 25(1):108–111. pmid:11213149
- Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002; 122(2):517–523. pmid:12171825
- Bayley MD, Schwartz JS, Shofer FS, et al. The financial burden of emergency department congestion and hospital crowding for chest pain patients awaiting admission. Ann Emerg Med 2005; 45(2):110–117. doi:10.1016/j.annemergmed.2004.09.010
Which patients with pulmonary embolism need echocardiography?
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
RISK STRATIFICATION IN PULMONARY EMBOLISM
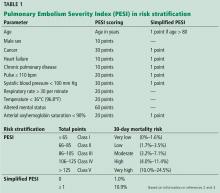
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
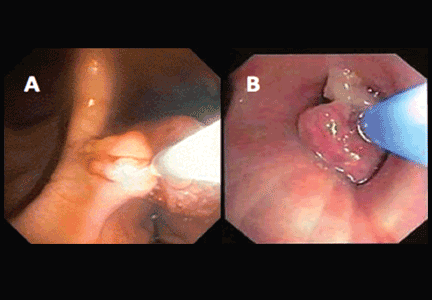
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
RISK STRATIFICATION IN PULMONARY EMBOLISM
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
RISK STRATIFICATION IN PULMONARY EMBOLISM
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011
How soon should patients with infective endocarditis be referred for valve surgery?
WHAT IS ‘EARLY’ SURGERY?
More than 50% of patients with infective endocarditis undergo cardiac surgery during their initial presentation.1
The 2017 guidelines of the American Association for Thoracic Surgery (AATS) recommend surgery once a surgical indication has been established and effective antimicrobial therapy has been started.2
The American Heart Association/American College of Cardiology (ACC/AHA) guidelines recommend surgery during the initial hospitalization before completion of a full course of antibiotics.3
The European Society of Cardiology guidelines define surgery according to the time since the patient received intravenous antibiotic therapy: emergency surgery is performed within 24 hours of therapy, urgent surgery is performed within a few days, and elective surgery is performed after at least 1 to 2 weeks.4
These slight differences are due to the dearth of large randomized trials addressing this question.
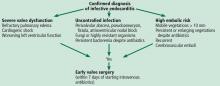
INDICATIONS FOR EARLY SURGERY
Left ventricular dysfunction and heart failure
Of all the complications of infectious endocarditis, concomitant heart failure has the greatest impact on prognosis5 and is one of the most frequent indications for surgery.6
The guidelines recommend emergency surgery during the initial hospitalization for all patients with infective endocarditis who present with refractory pulmonary edema, worsening left ventricular dysfunction, or cardiogenic shock, regardless of whether they have completed a full course of antibiotics. This applies to both native valve endocarditis and prosthetic valve endocarditis.
Uncontrolled persistent infection
Persistent infection is defined as fever and positive cultures persisting after 1 week of appropriate antibiotic treatment.4 However, 1 week is a long time. Persistence of positive blood cultures more than 48 to 72 hours after starting antibiotic therapy is associated with poor outcome and is an independent predictor of in-hospital mortality.7
The ACC/AHA guidelines recommend early surgery in patients with left-sided infective endocarditis caused by fungi or highly resistant organisms such as vancomycin-resistant enterococci or multidrug-resistant gram-negative bacilli.3 Nonetheless, antibiotic resistance is an unusual reason for expediting surgery unless there are additional indications for it.
Extension of the infection beyond the valve annulus, which occurs in about 30% of cases of native valve endocarditis and 50% of cases of prosthetic valve endocarditis,8 is considered a more valid reason to expedite surgery. Similarly, urgent surgery should be considered if there is any evidence of locally uncontrolled infection causing perivalvular abscess, fistula, pseudoaneurysm, or conduction system abnormalities causing atrioventricular nodal block.2–4
Some authors suggest reviewing the surgical pathology and microbial sequencing of excised cardiac valves after surgery to confirm the diagnosis and identify the culprit pathogen.9,10
Right-sided infective endocarditis
Right-sided infective endocarditis has a more favorable prognosis than left-sided infective endocarditis and usually responds well to medical therapy.11
Nevertheless, surgery for right-sided infective endocarditis should be expedited in patients with right heart failure secondary to severe tricuspid regurgitation with poor response to medical therapy or in the case of large tricuspid valve vegetations.12 Likewise, recurrent septic pulmonary emboli can be encountered in the setting of right-sided infective endocarditis and are an indication for early surgery.4,12
Since many patients with right-sided infective endocarditis acquire the infection by intravenous drug use, there is often a reluctance to recommend surgery, given the risk of prosthetic valve infection if they continue to use intravenous drugs.4,12 One study showed that the risk of death or reoperation between 3 and 6 months after surgery for infective endocarditis was 10 times higher in intravenous drug users. Yet their survival after surgery beyond this period was similar to that of patients with endocarditis who did not inject drugs.13 Therefore, the AATS guidelines recommend applying normal indications for surgery to those patients, with emphasis on the need for strict follow-up aimed at addiction treatment.2
Prevention of embolic events
Neurologic embolic events are a frequent complication of infective endocarditis, with the highest risk during the first few days after antibiotics are started. However, this risk decreases significantly after 2 weeks.14
The timing of surgery largely depends on whether the patient has had previous neurologic embolic events and on the size and mobility of the vegetation. The current guidelines recommend early surgery for recurrent emboli and persistent or enlarging vegetations despite appropriate antibiotic therapy, or in case of large vegetations (> 10 mm) on a native valve even in the absence of embolic events.4
A randomized trial by Kang et al15 demonstrated that, compared with conventional care, early surgery (within 48 hours of diagnosis) in patients with native valve endocarditis with large vegetations (> 10 mm) and severe valve dysfunction was associated with a significant reduction in the risk of death and embolic events.
Timing of surgery after a neurologic complication
Determining the right time for surgery is challenging in patients with infective endocarditis who have had neurologic complications, given the risk of hemorrhagic conversion of existing stroke with anticoagulation or exacerbation of cerebral ischemia in case of intraoperative hypotension. The decision should take into account the severity of cardiac decompensation, weighed against the severity of neurologic symptoms.
In general, surgery should be postponed for at least 4 weeks after intracerebral hemorrhage. However, it should be expedited in the event of silent cerebral embolism or transient ischemic attack, or in patients with infective endocarditis with stroke who have other indications for early surgery, as long as cerebral hemorrhage has been excluded by appropriate imaging.4
Early surgery for prosthetic valve endocarditis
The timing of surgery for prosthetic valve endocarditis follows the same general principles as for native valve endocarditis.2–4,12
One study showed that early surgery for prosthetic valve endocarditis was not associated with lower in-hospital and 1-year mortality rates compared with medical therapy.16 On the other hand, a subgroup analysis demonstrated surgery to be significantly beneficial in those with the strongest indications for surgery, including severe valve regurgitation, heart failure, paravalvular abscess, fistula, or prosthetic valve dehiscence.
The decision to proceed with surgery in prosthetic valve endocarditis should be weighed carefully, taking into consideration the patient’s overall clinical condition and estimated surgical risk.16
COLLABORATION IS HELPFUL
Early surgery is indicated for infective endocarditis patients presenting with:
- Refractory heart failure symptoms
- Persistent infection
- Large vegetations with a high risk of embolism.
Expeditious and successful treatment entails multidisciplinary collaboration among experts in cardiology and infectious diseases with access to cardiac surgery input early in the evaluation.
- Lalani T, Cabell CH, Benjamin DK, et al; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010; 121(8):1005–1013. doi:10.1161/CIRCULATIONAHA.109.864488
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson GB, Coselli JS; Writing Committee, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Nishimura RA, Otto CM, Bonow RO, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Prendergast BD, Tornos P. Surgery for infective endocarditis. Who and when? Circulation 2010; 121(9):1141–1152. doi:10.1161/CIRCULATIONAHA.108.773598
- Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005; 91(5):571–575. doi:10.1136/hrt.2003.032128
- López J, Sevilla T, Vilacosta I, et al. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J 2013; 34(23):1749–1754. doi:10.1093/eurheartj/ehs379
- Graupner C, Vilacosta I, SanRoman J, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol 2002; 39(7):1204–1211. doi:10.1016/S0735-1097(02)01747-3
- Shrestha NK, Ledtke CS, Wang H, et al. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg 2015; 99(1):33–37. doi:10.1016/j.athoracsur.2014.07.028
- Shapira N, Merin O, Rosenmann E, et al. Latent infective endocarditis: epidemiology and clinical characteristics of patients with unsuspected endocarditis detected after elective valve replacement. Ann Thorac Surg 2004; 78(5):1623–1629. doi:10.1016/j.athoracsur.2004.05.052
- Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992; 117(7):560–566. doi:10.7326/0003-4819-117-7-560
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015; 100(3):875–882. doi:10.1016/j.athoracsur.2015.03.019
- Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127(23):2272–2284. doi:10.1161/CIRCULATIONAHA.112.000813
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
- Lalani T, Chu VH, Park LP, et al; International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013; 173(16):1495–1504. doi:10.1001/jamainternmed.2013.8203
WHAT IS ‘EARLY’ SURGERY?
More than 50% of patients with infective endocarditis undergo cardiac surgery during their initial presentation.1
The 2017 guidelines of the American Association for Thoracic Surgery (AATS) recommend surgery once a surgical indication has been established and effective antimicrobial therapy has been started.2
The American Heart Association/American College of Cardiology (ACC/AHA) guidelines recommend surgery during the initial hospitalization before completion of a full course of antibiotics.3
The European Society of Cardiology guidelines define surgery according to the time since the patient received intravenous antibiotic therapy: emergency surgery is performed within 24 hours of therapy, urgent surgery is performed within a few days, and elective surgery is performed after at least 1 to 2 weeks.4
These slight differences are due to the dearth of large randomized trials addressing this question.
INDICATIONS FOR EARLY SURGERY
Left ventricular dysfunction and heart failure
Of all the complications of infectious endocarditis, concomitant heart failure has the greatest impact on prognosis5 and is one of the most frequent indications for surgery.6
The guidelines recommend emergency surgery during the initial hospitalization for all patients with infective endocarditis who present with refractory pulmonary edema, worsening left ventricular dysfunction, or cardiogenic shock, regardless of whether they have completed a full course of antibiotics. This applies to both native valve endocarditis and prosthetic valve endocarditis.
Uncontrolled persistent infection
Persistent infection is defined as fever and positive cultures persisting after 1 week of appropriate antibiotic treatment.4 However, 1 week is a long time. Persistence of positive blood cultures more than 48 to 72 hours after starting antibiotic therapy is associated with poor outcome and is an independent predictor of in-hospital mortality.7
The ACC/AHA guidelines recommend early surgery in patients with left-sided infective endocarditis caused by fungi or highly resistant organisms such as vancomycin-resistant enterococci or multidrug-resistant gram-negative bacilli.3 Nonetheless, antibiotic resistance is an unusual reason for expediting surgery unless there are additional indications for it.
Extension of the infection beyond the valve annulus, which occurs in about 30% of cases of native valve endocarditis and 50% of cases of prosthetic valve endocarditis,8 is considered a more valid reason to expedite surgery. Similarly, urgent surgery should be considered if there is any evidence of locally uncontrolled infection causing perivalvular abscess, fistula, pseudoaneurysm, or conduction system abnormalities causing atrioventricular nodal block.2–4
Some authors suggest reviewing the surgical pathology and microbial sequencing of excised cardiac valves after surgery to confirm the diagnosis and identify the culprit pathogen.9,10
Right-sided infective endocarditis
Right-sided infective endocarditis has a more favorable prognosis than left-sided infective endocarditis and usually responds well to medical therapy.11
Nevertheless, surgery for right-sided infective endocarditis should be expedited in patients with right heart failure secondary to severe tricuspid regurgitation with poor response to medical therapy or in the case of large tricuspid valve vegetations.12 Likewise, recurrent septic pulmonary emboli can be encountered in the setting of right-sided infective endocarditis and are an indication for early surgery.4,12
Since many patients with right-sided infective endocarditis acquire the infection by intravenous drug use, there is often a reluctance to recommend surgery, given the risk of prosthetic valve infection if they continue to use intravenous drugs.4,12 One study showed that the risk of death or reoperation between 3 and 6 months after surgery for infective endocarditis was 10 times higher in intravenous drug users. Yet their survival after surgery beyond this period was similar to that of patients with endocarditis who did not inject drugs.13 Therefore, the AATS guidelines recommend applying normal indications for surgery to those patients, with emphasis on the need for strict follow-up aimed at addiction treatment.2
Prevention of embolic events
Neurologic embolic events are a frequent complication of infective endocarditis, with the highest risk during the first few days after antibiotics are started. However, this risk decreases significantly after 2 weeks.14
The timing of surgery largely depends on whether the patient has had previous neurologic embolic events and on the size and mobility of the vegetation. The current guidelines recommend early surgery for recurrent emboli and persistent or enlarging vegetations despite appropriate antibiotic therapy, or in case of large vegetations (> 10 mm) on a native valve even in the absence of embolic events.4
A randomized trial by Kang et al15 demonstrated that, compared with conventional care, early surgery (within 48 hours of diagnosis) in patients with native valve endocarditis with large vegetations (> 10 mm) and severe valve dysfunction was associated with a significant reduction in the risk of death and embolic events.
Timing of surgery after a neurologic complication
Determining the right time for surgery is challenging in patients with infective endocarditis who have had neurologic complications, given the risk of hemorrhagic conversion of existing stroke with anticoagulation or exacerbation of cerebral ischemia in case of intraoperative hypotension. The decision should take into account the severity of cardiac decompensation, weighed against the severity of neurologic symptoms.
In general, surgery should be postponed for at least 4 weeks after intracerebral hemorrhage. However, it should be expedited in the event of silent cerebral embolism or transient ischemic attack, or in patients with infective endocarditis with stroke who have other indications for early surgery, as long as cerebral hemorrhage has been excluded by appropriate imaging.4
Early surgery for prosthetic valve endocarditis
The timing of surgery for prosthetic valve endocarditis follows the same general principles as for native valve endocarditis.2–4,12
One study showed that early surgery for prosthetic valve endocarditis was not associated with lower in-hospital and 1-year mortality rates compared with medical therapy.16 On the other hand, a subgroup analysis demonstrated surgery to be significantly beneficial in those with the strongest indications for surgery, including severe valve regurgitation, heart failure, paravalvular abscess, fistula, or prosthetic valve dehiscence.
The decision to proceed with surgery in prosthetic valve endocarditis should be weighed carefully, taking into consideration the patient’s overall clinical condition and estimated surgical risk.16
COLLABORATION IS HELPFUL
Early surgery is indicated for infective endocarditis patients presenting with:
- Refractory heart failure symptoms
- Persistent infection
- Large vegetations with a high risk of embolism.
Expeditious and successful treatment entails multidisciplinary collaboration among experts in cardiology and infectious diseases with access to cardiac surgery input early in the evaluation.
WHAT IS ‘EARLY’ SURGERY?
More than 50% of patients with infective endocarditis undergo cardiac surgery during their initial presentation.1
The 2017 guidelines of the American Association for Thoracic Surgery (AATS) recommend surgery once a surgical indication has been established and effective antimicrobial therapy has been started.2
The American Heart Association/American College of Cardiology (ACC/AHA) guidelines recommend surgery during the initial hospitalization before completion of a full course of antibiotics.3
The European Society of Cardiology guidelines define surgery according to the time since the patient received intravenous antibiotic therapy: emergency surgery is performed within 24 hours of therapy, urgent surgery is performed within a few days, and elective surgery is performed after at least 1 to 2 weeks.4
These slight differences are due to the dearth of large randomized trials addressing this question.
INDICATIONS FOR EARLY SURGERY
Left ventricular dysfunction and heart failure
Of all the complications of infectious endocarditis, concomitant heart failure has the greatest impact on prognosis5 and is one of the most frequent indications for surgery.6
The guidelines recommend emergency surgery during the initial hospitalization for all patients with infective endocarditis who present with refractory pulmonary edema, worsening left ventricular dysfunction, or cardiogenic shock, regardless of whether they have completed a full course of antibiotics. This applies to both native valve endocarditis and prosthetic valve endocarditis.
Uncontrolled persistent infection
Persistent infection is defined as fever and positive cultures persisting after 1 week of appropriate antibiotic treatment.4 However, 1 week is a long time. Persistence of positive blood cultures more than 48 to 72 hours after starting antibiotic therapy is associated with poor outcome and is an independent predictor of in-hospital mortality.7
The ACC/AHA guidelines recommend early surgery in patients with left-sided infective endocarditis caused by fungi or highly resistant organisms such as vancomycin-resistant enterococci or multidrug-resistant gram-negative bacilli.3 Nonetheless, antibiotic resistance is an unusual reason for expediting surgery unless there are additional indications for it.
Extension of the infection beyond the valve annulus, which occurs in about 30% of cases of native valve endocarditis and 50% of cases of prosthetic valve endocarditis,8 is considered a more valid reason to expedite surgery. Similarly, urgent surgery should be considered if there is any evidence of locally uncontrolled infection causing perivalvular abscess, fistula, pseudoaneurysm, or conduction system abnormalities causing atrioventricular nodal block.2–4
Some authors suggest reviewing the surgical pathology and microbial sequencing of excised cardiac valves after surgery to confirm the diagnosis and identify the culprit pathogen.9,10
Right-sided infective endocarditis
Right-sided infective endocarditis has a more favorable prognosis than left-sided infective endocarditis and usually responds well to medical therapy.11
Nevertheless, surgery for right-sided infective endocarditis should be expedited in patients with right heart failure secondary to severe tricuspid regurgitation with poor response to medical therapy or in the case of large tricuspid valve vegetations.12 Likewise, recurrent septic pulmonary emboli can be encountered in the setting of right-sided infective endocarditis and are an indication for early surgery.4,12
Since many patients with right-sided infective endocarditis acquire the infection by intravenous drug use, there is often a reluctance to recommend surgery, given the risk of prosthetic valve infection if they continue to use intravenous drugs.4,12 One study showed that the risk of death or reoperation between 3 and 6 months after surgery for infective endocarditis was 10 times higher in intravenous drug users. Yet their survival after surgery beyond this period was similar to that of patients with endocarditis who did not inject drugs.13 Therefore, the AATS guidelines recommend applying normal indications for surgery to those patients, with emphasis on the need for strict follow-up aimed at addiction treatment.2
Prevention of embolic events
Neurologic embolic events are a frequent complication of infective endocarditis, with the highest risk during the first few days after antibiotics are started. However, this risk decreases significantly after 2 weeks.14
The timing of surgery largely depends on whether the patient has had previous neurologic embolic events and on the size and mobility of the vegetation. The current guidelines recommend early surgery for recurrent emboli and persistent or enlarging vegetations despite appropriate antibiotic therapy, or in case of large vegetations (> 10 mm) on a native valve even in the absence of embolic events.4
A randomized trial by Kang et al15 demonstrated that, compared with conventional care, early surgery (within 48 hours of diagnosis) in patients with native valve endocarditis with large vegetations (> 10 mm) and severe valve dysfunction was associated with a significant reduction in the risk of death and embolic events.
Timing of surgery after a neurologic complication
Determining the right time for surgery is challenging in patients with infective endocarditis who have had neurologic complications, given the risk of hemorrhagic conversion of existing stroke with anticoagulation or exacerbation of cerebral ischemia in case of intraoperative hypotension. The decision should take into account the severity of cardiac decompensation, weighed against the severity of neurologic symptoms.
In general, surgery should be postponed for at least 4 weeks after intracerebral hemorrhage. However, it should be expedited in the event of silent cerebral embolism or transient ischemic attack, or in patients with infective endocarditis with stroke who have other indications for early surgery, as long as cerebral hemorrhage has been excluded by appropriate imaging.4
Early surgery for prosthetic valve endocarditis
The timing of surgery for prosthetic valve endocarditis follows the same general principles as for native valve endocarditis.2–4,12
One study showed that early surgery for prosthetic valve endocarditis was not associated with lower in-hospital and 1-year mortality rates compared with medical therapy.16 On the other hand, a subgroup analysis demonstrated surgery to be significantly beneficial in those with the strongest indications for surgery, including severe valve regurgitation, heart failure, paravalvular abscess, fistula, or prosthetic valve dehiscence.
The decision to proceed with surgery in prosthetic valve endocarditis should be weighed carefully, taking into consideration the patient’s overall clinical condition and estimated surgical risk.16
COLLABORATION IS HELPFUL
Early surgery is indicated for infective endocarditis patients presenting with:
- Refractory heart failure symptoms
- Persistent infection
- Large vegetations with a high risk of embolism.
Expeditious and successful treatment entails multidisciplinary collaboration among experts in cardiology and infectious diseases with access to cardiac surgery input early in the evaluation.
- Lalani T, Cabell CH, Benjamin DK, et al; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010; 121(8):1005–1013. doi:10.1161/CIRCULATIONAHA.109.864488
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson GB, Coselli JS; Writing Committee, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Nishimura RA, Otto CM, Bonow RO, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Prendergast BD, Tornos P. Surgery for infective endocarditis. Who and when? Circulation 2010; 121(9):1141–1152. doi:10.1161/CIRCULATIONAHA.108.773598
- Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005; 91(5):571–575. doi:10.1136/hrt.2003.032128
- López J, Sevilla T, Vilacosta I, et al. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J 2013; 34(23):1749–1754. doi:10.1093/eurheartj/ehs379
- Graupner C, Vilacosta I, SanRoman J, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol 2002; 39(7):1204–1211. doi:10.1016/S0735-1097(02)01747-3
- Shrestha NK, Ledtke CS, Wang H, et al. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg 2015; 99(1):33–37. doi:10.1016/j.athoracsur.2014.07.028
- Shapira N, Merin O, Rosenmann E, et al. Latent infective endocarditis: epidemiology and clinical characteristics of patients with unsuspected endocarditis detected after elective valve replacement. Ann Thorac Surg 2004; 78(5):1623–1629. doi:10.1016/j.athoracsur.2004.05.052
- Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992; 117(7):560–566. doi:10.7326/0003-4819-117-7-560
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015; 100(3):875–882. doi:10.1016/j.athoracsur.2015.03.019
- Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127(23):2272–2284. doi:10.1161/CIRCULATIONAHA.112.000813
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
- Lalani T, Chu VH, Park LP, et al; International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013; 173(16):1495–1504. doi:10.1001/jamainternmed.2013.8203
- Lalani T, Cabell CH, Benjamin DK, et al; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010; 121(8):1005–1013. doi:10.1161/CIRCULATIONAHA.109.864488
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson GB, Coselli JS; Writing Committee, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Nishimura RA, Otto CM, Bonow RO, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Prendergast BD, Tornos P. Surgery for infective endocarditis. Who and when? Circulation 2010; 121(9):1141–1152. doi:10.1161/CIRCULATIONAHA.108.773598
- Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005; 91(5):571–575. doi:10.1136/hrt.2003.032128
- López J, Sevilla T, Vilacosta I, et al. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J 2013; 34(23):1749–1754. doi:10.1093/eurheartj/ehs379
- Graupner C, Vilacosta I, SanRoman J, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol 2002; 39(7):1204–1211. doi:10.1016/S0735-1097(02)01747-3
- Shrestha NK, Ledtke CS, Wang H, et al. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg 2015; 99(1):33–37. doi:10.1016/j.athoracsur.2014.07.028
- Shapira N, Merin O, Rosenmann E, et al. Latent infective endocarditis: epidemiology and clinical characteristics of patients with unsuspected endocarditis detected after elective valve replacement. Ann Thorac Surg 2004; 78(5):1623–1629. doi:10.1016/j.athoracsur.2004.05.052
- Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992; 117(7):560–566. doi:10.7326/0003-4819-117-7-560
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015; 100(3):875–882. doi:10.1016/j.athoracsur.2015.03.019
- Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127(23):2272–2284. doi:10.1161/CIRCULATIONAHA.112.000813
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
- Lalani T, Chu VH, Park LP, et al; International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013; 173(16):1495–1504. doi:10.1001/jamainternmed.2013.8203
Premature ventricular contractions: Reassure or refer?
Doctor, my heart ______.” Fill in the blank with: skips, flip-flops, hiccups, stops, beats in my throat or chest, or any of the various ways patients describe palpitations. One cannot practice clinical medicine and not see patients with some variation of this chief complaint.1–3 Not every patient who complains of palpitations will be found to have premature ventricular contractions (PVCs), but PVCs are often part of the clinical problem.
This review focuses on the initial evaluation and management of PVCs in the primary care setting (Figure 1). It is not intended to be a comprehensive review of the pathophysiology, electrophysiology, or localization and ablation of PVCs. We will discuss approaches to the initial therapy of symptomatic PVCs. We will not discuss catheter-based therapy in detail except for which patients might benefit from referral to a clinical cardiac electrophysiologist.
Findings that should prompt consideration for referral to a specialist (“red flags”) are summarized at the end of each section. The type of specialist depends to a degree on the cardiology practice available to the referring physician. In our practice, such patients are typically seen by an electrophysiologist. In other practices, a general cardiologist might see such patients initially.
INITIAL EVALUATION
A primary concern of any patient presenting with a new symptom is whether the symptom is a marker of serious risk to health or life. In a patient with palpitations, the answer depends in large part on whether he or she has underlying structural heart disease—and that is the focus of the initial evaluation.
History: Information to ascertain
- When did the patient first notice the palpitations?
- Had there been any significant life events, either illness or emotional stress, at the time the palpitations began?
- Does the patient have a known history of heart disease (myocardial infarction, heart surgery, valvular heart disease, heart failure)?
- What medications is the patient taking?
- Does the patient take any dietary or health supplements? Ask specifically about any supplements taken to help with weight loss or increase energy levels. Almost all of them contain caffeine or other “natural” sympathomimetic agents. Also ask specifically about illicit drug use. If the patient is accompanied by a parent or partner, this question can be challenging.
- When do the palpitations occur? At random? At rest? With exercise? Time of day? In relation to the menstrual cycle? (More about this later.)
- Does anything make the palpitations better? If they occur at rest, does activity make them better or worse?
- Are there symptoms of heart failure, such as dyspnea on exertion, early fatigue, decline in exercise or exertional capacity, orthopnea, or paroxysmal nocturnal dyspnea?
- Are there symptoms suggesting cardiac ischemia, such as substernal chest pain or discomfort, chest pain or discomfort brought on by or made worse by exertion, or chest pain relieved with rest or sublingual nitroglycerin?
- Have the palpitations ever been associated with syncope? Keep in mind that syncope is transient loss of consciousness that spontaneously resolves with no features to suggest seizures.4 Thus, a patient who reports he or she “blacks out” with the palpitations but never falls or slumps has not had loss of consciousness and therefore has not had syncope.5
- Is there any history of unexplained death in the family, especially in younger people? Is there a history of unexplained accidental death in young family members?
Red flags obtained from the history
- Syncope related to palpitations
- Palpitations triggered by activity or exertion
- Known significant heart disease, congenital heart disease, or history of heart surgery
- Family history of premature unexplained sudden death in a first-degree relative.
Physical examination
The physical examination should focus on detecting any signs of underlying heart or vascular disease, eg:
- Significant murmurs
- Abnormal S3 or S4
- Displaced and diffuse point of maximal impulse or precordial heave
- Signs of right or left heart failure, or both, eg, peripheral edema, elevation of jugular venous pulse, rales, S3, S4.
Electrocardiography
We consider 12-lead electrocardiography (ECG) a part of the initial examination and assessment, not an ancillary test. One cannot evaluate a patient’s complaint of palpitations without ECG. Ideally, ECG should include a long 12-lead rhythm strip. The clinician should look for any evidence of underlying structural heart disease, eg:
- Pathologic Q waves
- Long QT interval
- ST-segment elevation in leads V1 and V2 consistent with a Brugada pattern
- Epsilon waves (seen in right ventricular arrhythmogenic cardiomyopathy).
Examples of the above can be found at sites such as ecgpedia.org.
Red flags in the physical examination and ECG
Any of the above findings on physical examination or ECG should prompt consideration of early referral, even though we have yet to establish that the palpitations are due to PVCs. Early consultation is suggested not for treatment of the palpitations but for further evaluation of structural heart disease.
Assuming the history, physical examination, and electrocardiography do not demonstrate any reasons for early cardiology or electrophysiology consultation, what’s next?
FURTHER EVALUATION: EXTENDED MONITORING
With luck, the patient’s typical palpitations will occur during ECG, in which case the palpitations can reasonably be attributed to PVCs. If not, monitoring is required to establish the cause of the patient’s symptoms.
The type of monitoring to order depends on the frequency of the palpitations. If the patient reports several episodes per day, then a 24- or 48-hour Holter monitor should both allow for a diagnosis and document the PVC burden (ie, the percent of the patient’s heartbeats that are PVCs), or the burden of whatever is the cause of the patient’s palpitations.
If the palpitations are less frequent, a 14-to-30-day monitor should be considered. A standard event recorder can confirm that the palpitations are due to PVCs but does not tell you the PVC burden. For that, a system capable of mobile outpatient cardiac telemetry is needed. Several such systems are commercially available.
A Holter monitor or other monitoring system is useful in determining whether the PVCs are unifocal (all look the same) or multifocal (have more than one morphology) and whether, in addition to PVCs, the patient has nonsustained ventricular tachycardia or sustained ventricular tachycardia (by definition lasting longer than 30 seconds or associated with symptoms of hemodynamic compromise such as near-syncope). Even if the patient has nonsustained ventricular tachycardia, if the heart is structurally normal the prognosis remains excellent.
Given the importance of knowing whether the patient has structural heart disease, we have a low threshold for ordering echocardiography, especially if nonsustained ventricular tachycardia has been documented. The finding of significant systolic dysfunction on echocardiography should prompt a cardiology consultation even if the physical examination is normal. In patients who have a high PVC burden, echocardiography is used to monitor for arrhythmia-induced cardiomyopathy.6
If the patient’s symptoms occur with activity, an exercise study can be helpful. It is important to either supervise the study oneself or, at the least, alert the exercise laboratory staff that the study is being performed to evaluate for exercise-induced arrhythmias. If the exercise study induces sustained ventricular tachycardia, the patient is almost invariably admitted to the hospital and inpatient consultation with an electrophysiologist is obtained.
Red flags on extended monitoring
- Multifocal PVCs or nonsustained ventricular tachycardia
- Polymorphic nonsustained ventricular tachycardia
- Sustained ventricular tachycardia; this still may be idiopathic and have a benign prognosis but generally should prompt referral.
If at this point no red flags have been uncovered, monitoring has established the patient’s symptoms are due to PVCs, and our examination and ancillary testing have established the patient has a structurally normal heart, what is the next step?
IDIOPATHIC PVCs
PVCs in a patient with a structurally normal heart are called “idiopathic.” Often, these patients will also be found to have nonsustained ventricular tachycardia, and may also be classified as having “idiopathic ventricular tachycardia.” Regardless of whether the patient has PVCs, nonsustained ventricular tachycardia, or both, the management approach is the same.
Roughly 60% to 80% of idiopathic PVCs originate from the right ventricle, in particular the right ventricular outflow tract.7 Patients with outflow tract PVCs typically present between the ages of 30 and 50 but range from adolescents to elders. More women than men are affected.
Outflow tract PVCs often occur only, or at much greater frequency, within a range of heart rates.8 Individual patients may have different ranges of heart rates at which their PVCs are more frequent. Patients may complain that their palpitations are more frequent at rest, early in exercise, at a peak of exercise, or early in recovery from exercise. It is not unusual for patients with outflow tract PVCs to report that activity reduces the frequency of their palpitations. Women might note an increase in their symptoms during menstruation.9 It is not clear, however, that this perceived increase in palpitations is in fact due to an increase in the number of PVCs.10
If the patient’s PVCs have not been captured on 12-lead ECG (ie, if it is not seen in all 12 leads), 12-lead Holter monitoring, if available, can be helpful. Examination of the morphology of the PVC on 12-lead ECG is extremely helpful. Outflow tract PVCs are the most common cause of idiopathic PVCs and nonsustained ventricular tachycardia and are easily recognizable with 12-lead ECG.
ECG points to the origin of the PVCs
A PVC arising on the right side of the heart will activate the right ventricle first and then the left ventricle. This is analogous to the sequence of ventricular activation in a patient with left bundle-branch block. Not surprisingly, on ECG a right-sided PVC looks similar to the QRS complex seen in left bundle-branch block—similar, but not identical.
When describing PVCs or the morphology of nonsustained ventricular tachycardia, the terms “left bundle-branch block pattern” and “right bundle-branch block pattern” refer to lead V1. If the PVC is negative (or mostly negative) in V1, the PVC has a left bundle-branch block pattern. A PVC that is positive in V1 is said to have a right bundle-branch block pattern and by implication arises from the left side of the heart.
A PVC originating from the top of the heart will move from top to bottom. The electrical axis of the PVC will be directed inferiorly. This means the PVC will be strongly positive in the inferior leads, ie, II, aVF, and III.
The electrocardiogram shown in Figure 2 demonstrates the typical appearance of a right ventricular outflow tract PVC.
If the PVC arises from the left ventricular outflow tract, the axis will still be inferiorly directed. However, the further to the left the origin of the PVC, the earlier the precordial transition will occur (the point at which the PVC is more positive than negative in the precordial leads). A PVC origin far enough to the left will result in a right bundle-branch block pattern PVC.
Not all idiopathic PVCs arise from the outflow tracts. A right bundle branch block pattern PVC does not imply the presence of underlying structural heart disease. PVCs may arise from both the tricuspid and mitral valve annuli, the left ventricular fascicles, or from the epicardium.
Multiple methods have been proposed to locate the origin of the PVC. For example, Park et al reviewed the use of surface ECG in locating the site of origin of ventricular tachycardia.11 All such algorithms should be applied with care, and with awareness of the caveats associated with their use.
Arrhythmogenic right ventricular cardiomyopathy is not benign
Arrhythmogenic right ventricular cardiomyopathy may give rise to PVCs or nonsustained ventricular tachycardia with morphologies similar to those of right ventricular outflow tract PVCs and ventricular tachycardia. The ventricular tachycardia complicating arrhythmogenic cardiomyopathy is, like PVCs arising from the right ventricular outflow tract, commonly associated with exercise or activity.
Unlike right ventricular outflow tract tachycardia, ventricular tachycardia related to arrhythmogenic cardiomyopathy is not benign.12 Distinguishing right ventricular outflow tract tachycardia from tachycardia secondary to arrhythmogenic cardiomyopathy is therefore critical.
Good-quality ECG demonstrating normal right ventricular size and function is reassuring, and if echocardiography is not conclusive, cardiovascular magnetic resonance imaging may provide additional diagnostic and prognostic data, especially when arrhythmogenic cardiomyopathy, cardiac sarcoidosis, or cardiac amyloidosis is suspected.6
Recently, magnetic resonance imaging has been used most for infiltrative diseases as the imaging modality of choice due to its superior tissue characterization and noninvasive morphological and functional evaluation. Magnetic resonance imaging findings in patients with arrhythmogenic cardiomyopathy correlate well with those of endomyocardial biopsy, angiography, and echocardiography and have been associated with incremental arrhythmic risk in the setting of electrical abnormalities. The increasing use of magnetic resonance imaging is leading to the recognition that left ventricular involvement (left-dominant arrhythmogenic right ventricular cardiomyopathy) is more common than previously recognized, with some suggesting that arrhythmogenic right ventricular cardiomyopathy should be simply called “arrhythmogenic cardiomyopathy.”
Although endomyocardial biopsy can establish the diagnosis of arrhythmogenic right ventricular cardiomyopathy, it is rarely performed because it has a high false-negative rate owing to the patchy, epicardial nature of this disorder.13
Red flags for cardiomyopathy
- Multifocal PVCs, or nonsustained ventricular tachycardia of more than one morphology on monitoring
- Syncope associated with active exercise
- Abnormal imaging findings that are consistent with arrhythmogenic right ventricular cardiomyopathy, cardiac sarcoidosis, or amyloidosis.
WHEN TO TREAT IDIOPATHIC PVCs
In our practice we explain to patients that there are two primary indications for treating idiopathic PVCs: (1) to relieve symptoms or (2) in asymptomatic patients with presumed arrhythmia-induced cardiomyopathy, to try to reverse the cardiomyopathy by eliminating the PVCs.
Some patients report severe symptoms due to their PVCs. Other patients appear to have no symptoms whatsoever, while still others are not overly bothered by the PVCs but are concerned that they may indicate they are at increased risk of cardiac events. In this last group, an evaluation such as outlined above that discloses no evidence of structural heart disease and reassurance by the physician may be all the treatment needed.
Even if they have no symptoms or only minimal symptoms, patients with a high PVC burden require follow-up because of the association between frequent PVCs and arrhythmia-induced cardiomyopathy.14,15 What constitutes a “high” PVC burden remains a matter of debate. Left ventricular dysfunction has generally been reported at PVC burdens above 15% to 25% of the total cardiac beats, though this percentage can be as low as 10%.14
Eliminating the high burden of PVCs in patients with left ventricular dysfunction may significantly improve left ventricular systolic function.15 It is likely, however, that more than PVC burden alone contributes to the development of the cardiomyopathy.14
Given these complexities, it is reasonable to request an electrophysiology consultation for patients who have more than rare PVCs. What is rare? There is no defined standard, but a PVC burden less than 1% is reasonable.
Treatment of the PVCs may be indicated in patients with systolic heart failure receiving cardiac resynchronization therapy, ie, a biventricular pacemaker. For cardiac resynchronization therapy to be clinically beneficial, close to 100% of heartbeats need to be paced, and frequent PVCs, even at a burden less than 10%, may undermine its effectiveness.16
HOW TO INTERVENE?
Beta-blockers and nondihydropyridine calcium channel blockers have both been used to treat symptomatic PVCs. If the patient is found to have systolic dysfunction as part of the evaluation, a beta-blocker is indicated, irrespective of any desire to treat the PVCs. Beta-blockers and calcium channel blockers both have low adverse effect profiles. They are available in once-a-day formulations and are inexpensive. Their efficacy is variable. The use of these medications is well within the purview of the primary care physician.
Selective beta-blockers are the first choice in treatment, and metoprolol is commonly used in clinical practice. We start with a low dose and increase it based on symptom relief.
As noted, only nondihydropyridine calcium channel blockers should be used for treatment of PVCs. As with beta-blockers, we start at a low dose and increase as needed based on the response to therapy.
Antiarrhythymic drugs are classified according to the Vaughan-Williams system. The ones most frequently used for PVCs are the class Ic drugs propafenone and flecainide and the class III drugs sotalol, amiodarone, and dofetalide. However, in our experience, if first-line agents (ie, beta-blockers and nondihydropyridine calcium channel blockers) are unsuccessful in controlling the patient’s symptoms, most primary care physicians are uncomfortable prescribing class Ic and class III drugs. Failure of a beta-blocker, a calcium channel blocker, or both often results in referral to a cardiologist or electrophysiologist.
The consultation should include a careful discussion with the patient regarding the risk of treatment with a type I or a type III drug vs catheter ablation. Treatment with class I or class III antiarrhythmic drugs always entails a small risk of proarrhythmia. The choice between drug therapy or ablation therapy is highly individualized. However, if elimination of the PVCs is of paramount importance, such as in cases of arrhythmia-induced cardiomyopathy, ablation therapy is more effective at eliminating the PVCs, although at the cost of an invasive procedure. Fortunately, the risk of complications with ablation therapy is quite low.
No drugs are approved by the US Food and Drug Administration for treating PVCs or nonsustained ventricular tachycardia. The drugs that do have an indication for treatment of ventricular arrhythmias are labeled as being indicated for “sustained” or “life-threatening” ventricular arrhythmias. The use of drugs for the treatment of PVCs or nonsustained ventricular tachycardia represents off-label usage.
Referral to discuss catheter ablation of the PVCs6 should be considered for patients who:
- Have undergone unsuccessful attempts at drug therapy for either symptoms or PVC-related cardiomyopathy
- Refuse drug therapy but have severe symptoms, or
- Do not respond to cardiac resynchronization therapy due to suboptimal pacing due to PVCs.
- Kennedy HL, Underhill SJ. Frequent or complex ventricular ectopy in apparently healthy subjects: a clinical study of 25 cases. Am J Cardiol 1976; 38:141–148.
- Brodsky M, Wu D, Denes P, Kanakis C, Rosen KM. Arrhythmias documented by 24 hour continuous electrocardiographic monitoring in 50 male medical students without apparent heart disease. Am J Cardiol 1977; 39:390–395.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C Jr, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015;12:e41–e63.
- Benditt DG, Adkisson WO. Approach to the patient with syncope: venues, presentations, diagnoses. Cardiol Clin 2013; 31:9–25.
- Pedersen CT, Kay GN, Kalman J, et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Heart Rhythm 2014; 11:e166–e196.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Buxton AE, Waxman HL, Marchlinski FE, Simson MB, Cassidy D, Josephson ME. Right ventricular tachycardia: clinical and electrophysiologic characteristics. Circulation 1983; 68:917–927.
- Marchlinski FE, Deely MP, Zado ES. Sex-specific triggers for right ventricular outflow tract tachycardia. Am Heart J 2000; 139:1009–1013.
- Fuenmayor AJ, Araujo X, Fuenmayor AM. Cardiac arrhythmias during two different stages of the menstrual cycle. Int J Cardiol 1998; 63:267–270.
- Park KM, Kim YH, Marchlinski FE. Using the surface electrocardiogram to localize the origin of idiopathic ventricular tachycardia. Pacing Clin Electrophysiol 2012; 35:1516–1527.
- Te Riele AS, Hauer RN. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: clinical challenges in a changing disease spectrum. Trends Cardiovasc Med 2015; 25:191–198.
- Philips B, Cheng A. 2015 update on the diagnosis and management of arrhythmogenic right ventricular cardiomyopathy. Curr Opin Cardiol 2016; 31:46–56.
- Del Carpio Munoz F, Syed FF, Noheria A, et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J Cardiovasc Electrophysiol 2011; 22:791–798.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Zhang Q, Zhou Y, Yu CM. Incidence, definition, diagnosis, and management of the cardiac resynchronization therapy nonresponder. Curr Opin Cardiol 2015; 30:40–49.
Doctor, my heart ______.” Fill in the blank with: skips, flip-flops, hiccups, stops, beats in my throat or chest, or any of the various ways patients describe palpitations. One cannot practice clinical medicine and not see patients with some variation of this chief complaint.1–3 Not every patient who complains of palpitations will be found to have premature ventricular contractions (PVCs), but PVCs are often part of the clinical problem.
This review focuses on the initial evaluation and management of PVCs in the primary care setting (Figure 1). It is not intended to be a comprehensive review of the pathophysiology, electrophysiology, or localization and ablation of PVCs. We will discuss approaches to the initial therapy of symptomatic PVCs. We will not discuss catheter-based therapy in detail except for which patients might benefit from referral to a clinical cardiac electrophysiologist.
Findings that should prompt consideration for referral to a specialist (“red flags”) are summarized at the end of each section. The type of specialist depends to a degree on the cardiology practice available to the referring physician. In our practice, such patients are typically seen by an electrophysiologist. In other practices, a general cardiologist might see such patients initially.
INITIAL EVALUATION
A primary concern of any patient presenting with a new symptom is whether the symptom is a marker of serious risk to health or life. In a patient with palpitations, the answer depends in large part on whether he or she has underlying structural heart disease—and that is the focus of the initial evaluation.
History: Information to ascertain
- When did the patient first notice the palpitations?
- Had there been any significant life events, either illness or emotional stress, at the time the palpitations began?
- Does the patient have a known history of heart disease (myocardial infarction, heart surgery, valvular heart disease, heart failure)?
- What medications is the patient taking?
- Does the patient take any dietary or health supplements? Ask specifically about any supplements taken to help with weight loss or increase energy levels. Almost all of them contain caffeine or other “natural” sympathomimetic agents. Also ask specifically about illicit drug use. If the patient is accompanied by a parent or partner, this question can be challenging.
- When do the palpitations occur? At random? At rest? With exercise? Time of day? In relation to the menstrual cycle? (More about this later.)
- Does anything make the palpitations better? If they occur at rest, does activity make them better or worse?
- Are there symptoms of heart failure, such as dyspnea on exertion, early fatigue, decline in exercise or exertional capacity, orthopnea, or paroxysmal nocturnal dyspnea?
- Are there symptoms suggesting cardiac ischemia, such as substernal chest pain or discomfort, chest pain or discomfort brought on by or made worse by exertion, or chest pain relieved with rest or sublingual nitroglycerin?
- Have the palpitations ever been associated with syncope? Keep in mind that syncope is transient loss of consciousness that spontaneously resolves with no features to suggest seizures.4 Thus, a patient who reports he or she “blacks out” with the palpitations but never falls or slumps has not had loss of consciousness and therefore has not had syncope.5
- Is there any history of unexplained death in the family, especially in younger people? Is there a history of unexplained accidental death in young family members?
Red flags obtained from the history
- Syncope related to palpitations
- Palpitations triggered by activity or exertion
- Known significant heart disease, congenital heart disease, or history of heart surgery
- Family history of premature unexplained sudden death in a first-degree relative.
Physical examination
The physical examination should focus on detecting any signs of underlying heart or vascular disease, eg:
- Significant murmurs
- Abnormal S3 or S4
- Displaced and diffuse point of maximal impulse or precordial heave
- Signs of right or left heart failure, or both, eg, peripheral edema, elevation of jugular venous pulse, rales, S3, S4.
Electrocardiography
We consider 12-lead electrocardiography (ECG) a part of the initial examination and assessment, not an ancillary test. One cannot evaluate a patient’s complaint of palpitations without ECG. Ideally, ECG should include a long 12-lead rhythm strip. The clinician should look for any evidence of underlying structural heart disease, eg:
- Pathologic Q waves
- Long QT interval
- ST-segment elevation in leads V1 and V2 consistent with a Brugada pattern
- Epsilon waves (seen in right ventricular arrhythmogenic cardiomyopathy).
Examples of the above can be found at sites such as ecgpedia.org.
Red flags in the physical examination and ECG
Any of the above findings on physical examination or ECG should prompt consideration of early referral, even though we have yet to establish that the palpitations are due to PVCs. Early consultation is suggested not for treatment of the palpitations but for further evaluation of structural heart disease.
Assuming the history, physical examination, and electrocardiography do not demonstrate any reasons for early cardiology or electrophysiology consultation, what’s next?
FURTHER EVALUATION: EXTENDED MONITORING
With luck, the patient’s typical palpitations will occur during ECG, in which case the palpitations can reasonably be attributed to PVCs. If not, monitoring is required to establish the cause of the patient’s symptoms.
The type of monitoring to order depends on the frequency of the palpitations. If the patient reports several episodes per day, then a 24- or 48-hour Holter monitor should both allow for a diagnosis and document the PVC burden (ie, the percent of the patient’s heartbeats that are PVCs), or the burden of whatever is the cause of the patient’s palpitations.
If the palpitations are less frequent, a 14-to-30-day monitor should be considered. A standard event recorder can confirm that the palpitations are due to PVCs but does not tell you the PVC burden. For that, a system capable of mobile outpatient cardiac telemetry is needed. Several such systems are commercially available.
A Holter monitor or other monitoring system is useful in determining whether the PVCs are unifocal (all look the same) or multifocal (have more than one morphology) and whether, in addition to PVCs, the patient has nonsustained ventricular tachycardia or sustained ventricular tachycardia (by definition lasting longer than 30 seconds or associated with symptoms of hemodynamic compromise such as near-syncope). Even if the patient has nonsustained ventricular tachycardia, if the heart is structurally normal the prognosis remains excellent.
Given the importance of knowing whether the patient has structural heart disease, we have a low threshold for ordering echocardiography, especially if nonsustained ventricular tachycardia has been documented. The finding of significant systolic dysfunction on echocardiography should prompt a cardiology consultation even if the physical examination is normal. In patients who have a high PVC burden, echocardiography is used to monitor for arrhythmia-induced cardiomyopathy.6
If the patient’s symptoms occur with activity, an exercise study can be helpful. It is important to either supervise the study oneself or, at the least, alert the exercise laboratory staff that the study is being performed to evaluate for exercise-induced arrhythmias. If the exercise study induces sustained ventricular tachycardia, the patient is almost invariably admitted to the hospital and inpatient consultation with an electrophysiologist is obtained.
Red flags on extended monitoring
- Multifocal PVCs or nonsustained ventricular tachycardia
- Polymorphic nonsustained ventricular tachycardia
- Sustained ventricular tachycardia; this still may be idiopathic and have a benign prognosis but generally should prompt referral.
If at this point no red flags have been uncovered, monitoring has established the patient’s symptoms are due to PVCs, and our examination and ancillary testing have established the patient has a structurally normal heart, what is the next step?
IDIOPATHIC PVCs
PVCs in a patient with a structurally normal heart are called “idiopathic.” Often, these patients will also be found to have nonsustained ventricular tachycardia, and may also be classified as having “idiopathic ventricular tachycardia.” Regardless of whether the patient has PVCs, nonsustained ventricular tachycardia, or both, the management approach is the same.
Roughly 60% to 80% of idiopathic PVCs originate from the right ventricle, in particular the right ventricular outflow tract.7 Patients with outflow tract PVCs typically present between the ages of 30 and 50 but range from adolescents to elders. More women than men are affected.
Outflow tract PVCs often occur only, or at much greater frequency, within a range of heart rates.8 Individual patients may have different ranges of heart rates at which their PVCs are more frequent. Patients may complain that their palpitations are more frequent at rest, early in exercise, at a peak of exercise, or early in recovery from exercise. It is not unusual for patients with outflow tract PVCs to report that activity reduces the frequency of their palpitations. Women might note an increase in their symptoms during menstruation.9 It is not clear, however, that this perceived increase in palpitations is in fact due to an increase in the number of PVCs.10
If the patient’s PVCs have not been captured on 12-lead ECG (ie, if it is not seen in all 12 leads), 12-lead Holter monitoring, if available, can be helpful. Examination of the morphology of the PVC on 12-lead ECG is extremely helpful. Outflow tract PVCs are the most common cause of idiopathic PVCs and nonsustained ventricular tachycardia and are easily recognizable with 12-lead ECG.
ECG points to the origin of the PVCs
A PVC arising on the right side of the heart will activate the right ventricle first and then the left ventricle. This is analogous to the sequence of ventricular activation in a patient with left bundle-branch block. Not surprisingly, on ECG a right-sided PVC looks similar to the QRS complex seen in left bundle-branch block—similar, but not identical.
When describing PVCs or the morphology of nonsustained ventricular tachycardia, the terms “left bundle-branch block pattern” and “right bundle-branch block pattern” refer to lead V1. If the PVC is negative (or mostly negative) in V1, the PVC has a left bundle-branch block pattern. A PVC that is positive in V1 is said to have a right bundle-branch block pattern and by implication arises from the left side of the heart.
A PVC originating from the top of the heart will move from top to bottom. The electrical axis of the PVC will be directed inferiorly. This means the PVC will be strongly positive in the inferior leads, ie, II, aVF, and III.
The electrocardiogram shown in Figure 2 demonstrates the typical appearance of a right ventricular outflow tract PVC.
If the PVC arises from the left ventricular outflow tract, the axis will still be inferiorly directed. However, the further to the left the origin of the PVC, the earlier the precordial transition will occur (the point at which the PVC is more positive than negative in the precordial leads). A PVC origin far enough to the left will result in a right bundle-branch block pattern PVC.
Not all idiopathic PVCs arise from the outflow tracts. A right bundle branch block pattern PVC does not imply the presence of underlying structural heart disease. PVCs may arise from both the tricuspid and mitral valve annuli, the left ventricular fascicles, or from the epicardium.
Multiple methods have been proposed to locate the origin of the PVC. For example, Park et al reviewed the use of surface ECG in locating the site of origin of ventricular tachycardia.11 All such algorithms should be applied with care, and with awareness of the caveats associated with their use.
Arrhythmogenic right ventricular cardiomyopathy is not benign
Arrhythmogenic right ventricular cardiomyopathy may give rise to PVCs or nonsustained ventricular tachycardia with morphologies similar to those of right ventricular outflow tract PVCs and ventricular tachycardia. The ventricular tachycardia complicating arrhythmogenic cardiomyopathy is, like PVCs arising from the right ventricular outflow tract, commonly associated with exercise or activity.
Unlike right ventricular outflow tract tachycardia, ventricular tachycardia related to arrhythmogenic cardiomyopathy is not benign.12 Distinguishing right ventricular outflow tract tachycardia from tachycardia secondary to arrhythmogenic cardiomyopathy is therefore critical.
Good-quality ECG demonstrating normal right ventricular size and function is reassuring, and if echocardiography is not conclusive, cardiovascular magnetic resonance imaging may provide additional diagnostic and prognostic data, especially when arrhythmogenic cardiomyopathy, cardiac sarcoidosis, or cardiac amyloidosis is suspected.6
Recently, magnetic resonance imaging has been used most for infiltrative diseases as the imaging modality of choice due to its superior tissue characterization and noninvasive morphological and functional evaluation. Magnetic resonance imaging findings in patients with arrhythmogenic cardiomyopathy correlate well with those of endomyocardial biopsy, angiography, and echocardiography and have been associated with incremental arrhythmic risk in the setting of electrical abnormalities. The increasing use of magnetic resonance imaging is leading to the recognition that left ventricular involvement (left-dominant arrhythmogenic right ventricular cardiomyopathy) is more common than previously recognized, with some suggesting that arrhythmogenic right ventricular cardiomyopathy should be simply called “arrhythmogenic cardiomyopathy.”
Although endomyocardial biopsy can establish the diagnosis of arrhythmogenic right ventricular cardiomyopathy, it is rarely performed because it has a high false-negative rate owing to the patchy, epicardial nature of this disorder.13
Red flags for cardiomyopathy
- Multifocal PVCs, or nonsustained ventricular tachycardia of more than one morphology on monitoring
- Syncope associated with active exercise
- Abnormal imaging findings that are consistent with arrhythmogenic right ventricular cardiomyopathy, cardiac sarcoidosis, or amyloidosis.
WHEN TO TREAT IDIOPATHIC PVCs
In our practice we explain to patients that there are two primary indications for treating idiopathic PVCs: (1) to relieve symptoms or (2) in asymptomatic patients with presumed arrhythmia-induced cardiomyopathy, to try to reverse the cardiomyopathy by eliminating the PVCs.
Some patients report severe symptoms due to their PVCs. Other patients appear to have no symptoms whatsoever, while still others are not overly bothered by the PVCs but are concerned that they may indicate they are at increased risk of cardiac events. In this last group, an evaluation such as outlined above that discloses no evidence of structural heart disease and reassurance by the physician may be all the treatment needed.
Even if they have no symptoms or only minimal symptoms, patients with a high PVC burden require follow-up because of the association between frequent PVCs and arrhythmia-induced cardiomyopathy.14,15 What constitutes a “high” PVC burden remains a matter of debate. Left ventricular dysfunction has generally been reported at PVC burdens above 15% to 25% of the total cardiac beats, though this percentage can be as low as 10%.14
Eliminating the high burden of PVCs in patients with left ventricular dysfunction may significantly improve left ventricular systolic function.15 It is likely, however, that more than PVC burden alone contributes to the development of the cardiomyopathy.14
Given these complexities, it is reasonable to request an electrophysiology consultation for patients who have more than rare PVCs. What is rare? There is no defined standard, but a PVC burden less than 1% is reasonable.
Treatment of the PVCs may be indicated in patients with systolic heart failure receiving cardiac resynchronization therapy, ie, a biventricular pacemaker. For cardiac resynchronization therapy to be clinically beneficial, close to 100% of heartbeats need to be paced, and frequent PVCs, even at a burden less than 10%, may undermine its effectiveness.16
HOW TO INTERVENE?
Beta-blockers and nondihydropyridine calcium channel blockers have both been used to treat symptomatic PVCs. If the patient is found to have systolic dysfunction as part of the evaluation, a beta-blocker is indicated, irrespective of any desire to treat the PVCs. Beta-blockers and calcium channel blockers both have low adverse effect profiles. They are available in once-a-day formulations and are inexpensive. Their efficacy is variable. The use of these medications is well within the purview of the primary care physician.
Selective beta-blockers are the first choice in treatment, and metoprolol is commonly used in clinical practice. We start with a low dose and increase it based on symptom relief.
As noted, only nondihydropyridine calcium channel blockers should be used for treatment of PVCs. As with beta-blockers, we start at a low dose and increase as needed based on the response to therapy.
Antiarrhythymic drugs are classified according to the Vaughan-Williams system. The ones most frequently used for PVCs are the class Ic drugs propafenone and flecainide and the class III drugs sotalol, amiodarone, and dofetalide. However, in our experience, if first-line agents (ie, beta-blockers and nondihydropyridine calcium channel blockers) are unsuccessful in controlling the patient’s symptoms, most primary care physicians are uncomfortable prescribing class Ic and class III drugs. Failure of a beta-blocker, a calcium channel blocker, or both often results in referral to a cardiologist or electrophysiologist.
The consultation should include a careful discussion with the patient regarding the risk of treatment with a type I or a type III drug vs catheter ablation. Treatment with class I or class III antiarrhythmic drugs always entails a small risk of proarrhythmia. The choice between drug therapy or ablation therapy is highly individualized. However, if elimination of the PVCs is of paramount importance, such as in cases of arrhythmia-induced cardiomyopathy, ablation therapy is more effective at eliminating the PVCs, although at the cost of an invasive procedure. Fortunately, the risk of complications with ablation therapy is quite low.
No drugs are approved by the US Food and Drug Administration for treating PVCs or nonsustained ventricular tachycardia. The drugs that do have an indication for treatment of ventricular arrhythmias are labeled as being indicated for “sustained” or “life-threatening” ventricular arrhythmias. The use of drugs for the treatment of PVCs or nonsustained ventricular tachycardia represents off-label usage.
Referral to discuss catheter ablation of the PVCs6 should be considered for patients who:
- Have undergone unsuccessful attempts at drug therapy for either symptoms or PVC-related cardiomyopathy
- Refuse drug therapy but have severe symptoms, or
- Do not respond to cardiac resynchronization therapy due to suboptimal pacing due to PVCs.
Doctor, my heart ______.” Fill in the blank with: skips, flip-flops, hiccups, stops, beats in my throat or chest, or any of the various ways patients describe palpitations. One cannot practice clinical medicine and not see patients with some variation of this chief complaint.1–3 Not every patient who complains of palpitations will be found to have premature ventricular contractions (PVCs), but PVCs are often part of the clinical problem.
This review focuses on the initial evaluation and management of PVCs in the primary care setting (Figure 1). It is not intended to be a comprehensive review of the pathophysiology, electrophysiology, or localization and ablation of PVCs. We will discuss approaches to the initial therapy of symptomatic PVCs. We will not discuss catheter-based therapy in detail except for which patients might benefit from referral to a clinical cardiac electrophysiologist.
Findings that should prompt consideration for referral to a specialist (“red flags”) are summarized at the end of each section. The type of specialist depends to a degree on the cardiology practice available to the referring physician. In our practice, such patients are typically seen by an electrophysiologist. In other practices, a general cardiologist might see such patients initially.
INITIAL EVALUATION
A primary concern of any patient presenting with a new symptom is whether the symptom is a marker of serious risk to health or life. In a patient with palpitations, the answer depends in large part on whether he or she has underlying structural heart disease—and that is the focus of the initial evaluation.
History: Information to ascertain
- When did the patient first notice the palpitations?
- Had there been any significant life events, either illness or emotional stress, at the time the palpitations began?
- Does the patient have a known history of heart disease (myocardial infarction, heart surgery, valvular heart disease, heart failure)?
- What medications is the patient taking?
- Does the patient take any dietary or health supplements? Ask specifically about any supplements taken to help with weight loss or increase energy levels. Almost all of them contain caffeine or other “natural” sympathomimetic agents. Also ask specifically about illicit drug use. If the patient is accompanied by a parent or partner, this question can be challenging.
- When do the palpitations occur? At random? At rest? With exercise? Time of day? In relation to the menstrual cycle? (More about this later.)
- Does anything make the palpitations better? If they occur at rest, does activity make them better or worse?
- Are there symptoms of heart failure, such as dyspnea on exertion, early fatigue, decline in exercise or exertional capacity, orthopnea, or paroxysmal nocturnal dyspnea?
- Are there symptoms suggesting cardiac ischemia, such as substernal chest pain or discomfort, chest pain or discomfort brought on by or made worse by exertion, or chest pain relieved with rest or sublingual nitroglycerin?
- Have the palpitations ever been associated with syncope? Keep in mind that syncope is transient loss of consciousness that spontaneously resolves with no features to suggest seizures.4 Thus, a patient who reports he or she “blacks out” with the palpitations but never falls or slumps has not had loss of consciousness and therefore has not had syncope.5
- Is there any history of unexplained death in the family, especially in younger people? Is there a history of unexplained accidental death in young family members?
Red flags obtained from the history
- Syncope related to palpitations
- Palpitations triggered by activity or exertion
- Known significant heart disease, congenital heart disease, or history of heart surgery
- Family history of premature unexplained sudden death in a first-degree relative.
Physical examination
The physical examination should focus on detecting any signs of underlying heart or vascular disease, eg:
- Significant murmurs
- Abnormal S3 or S4
- Displaced and diffuse point of maximal impulse or precordial heave
- Signs of right or left heart failure, or both, eg, peripheral edema, elevation of jugular venous pulse, rales, S3, S4.
Electrocardiography
We consider 12-lead electrocardiography (ECG) a part of the initial examination and assessment, not an ancillary test. One cannot evaluate a patient’s complaint of palpitations without ECG. Ideally, ECG should include a long 12-lead rhythm strip. The clinician should look for any evidence of underlying structural heart disease, eg:
- Pathologic Q waves
- Long QT interval
- ST-segment elevation in leads V1 and V2 consistent with a Brugada pattern
- Epsilon waves (seen in right ventricular arrhythmogenic cardiomyopathy).
Examples of the above can be found at sites such as ecgpedia.org.
Red flags in the physical examination and ECG
Any of the above findings on physical examination or ECG should prompt consideration of early referral, even though we have yet to establish that the palpitations are due to PVCs. Early consultation is suggested not for treatment of the palpitations but for further evaluation of structural heart disease.
Assuming the history, physical examination, and electrocardiography do not demonstrate any reasons for early cardiology or electrophysiology consultation, what’s next?
FURTHER EVALUATION: EXTENDED MONITORING
With luck, the patient’s typical palpitations will occur during ECG, in which case the palpitations can reasonably be attributed to PVCs. If not, monitoring is required to establish the cause of the patient’s symptoms.
The type of monitoring to order depends on the frequency of the palpitations. If the patient reports several episodes per day, then a 24- or 48-hour Holter monitor should both allow for a diagnosis and document the PVC burden (ie, the percent of the patient’s heartbeats that are PVCs), or the burden of whatever is the cause of the patient’s palpitations.
If the palpitations are less frequent, a 14-to-30-day monitor should be considered. A standard event recorder can confirm that the palpitations are due to PVCs but does not tell you the PVC burden. For that, a system capable of mobile outpatient cardiac telemetry is needed. Several such systems are commercially available.
A Holter monitor or other monitoring system is useful in determining whether the PVCs are unifocal (all look the same) or multifocal (have more than one morphology) and whether, in addition to PVCs, the patient has nonsustained ventricular tachycardia or sustained ventricular tachycardia (by definition lasting longer than 30 seconds or associated with symptoms of hemodynamic compromise such as near-syncope). Even if the patient has nonsustained ventricular tachycardia, if the heart is structurally normal the prognosis remains excellent.
Given the importance of knowing whether the patient has structural heart disease, we have a low threshold for ordering echocardiography, especially if nonsustained ventricular tachycardia has been documented. The finding of significant systolic dysfunction on echocardiography should prompt a cardiology consultation even if the physical examination is normal. In patients who have a high PVC burden, echocardiography is used to monitor for arrhythmia-induced cardiomyopathy.6
If the patient’s symptoms occur with activity, an exercise study can be helpful. It is important to either supervise the study oneself or, at the least, alert the exercise laboratory staff that the study is being performed to evaluate for exercise-induced arrhythmias. If the exercise study induces sustained ventricular tachycardia, the patient is almost invariably admitted to the hospital and inpatient consultation with an electrophysiologist is obtained.
Red flags on extended monitoring
- Multifocal PVCs or nonsustained ventricular tachycardia
- Polymorphic nonsustained ventricular tachycardia
- Sustained ventricular tachycardia; this still may be idiopathic and have a benign prognosis but generally should prompt referral.
If at this point no red flags have been uncovered, monitoring has established the patient’s symptoms are due to PVCs, and our examination and ancillary testing have established the patient has a structurally normal heart, what is the next step?
IDIOPATHIC PVCs
PVCs in a patient with a structurally normal heart are called “idiopathic.” Often, these patients will also be found to have nonsustained ventricular tachycardia, and may also be classified as having “idiopathic ventricular tachycardia.” Regardless of whether the patient has PVCs, nonsustained ventricular tachycardia, or both, the management approach is the same.
Roughly 60% to 80% of idiopathic PVCs originate from the right ventricle, in particular the right ventricular outflow tract.7 Patients with outflow tract PVCs typically present between the ages of 30 and 50 but range from adolescents to elders. More women than men are affected.
Outflow tract PVCs often occur only, or at much greater frequency, within a range of heart rates.8 Individual patients may have different ranges of heart rates at which their PVCs are more frequent. Patients may complain that their palpitations are more frequent at rest, early in exercise, at a peak of exercise, or early in recovery from exercise. It is not unusual for patients with outflow tract PVCs to report that activity reduces the frequency of their palpitations. Women might note an increase in their symptoms during menstruation.9 It is not clear, however, that this perceived increase in palpitations is in fact due to an increase in the number of PVCs.10
If the patient’s PVCs have not been captured on 12-lead ECG (ie, if it is not seen in all 12 leads), 12-lead Holter monitoring, if available, can be helpful. Examination of the morphology of the PVC on 12-lead ECG is extremely helpful. Outflow tract PVCs are the most common cause of idiopathic PVCs and nonsustained ventricular tachycardia and are easily recognizable with 12-lead ECG.
ECG points to the origin of the PVCs
A PVC arising on the right side of the heart will activate the right ventricle first and then the left ventricle. This is analogous to the sequence of ventricular activation in a patient with left bundle-branch block. Not surprisingly, on ECG a right-sided PVC looks similar to the QRS complex seen in left bundle-branch block—similar, but not identical.
When describing PVCs or the morphology of nonsustained ventricular tachycardia, the terms “left bundle-branch block pattern” and “right bundle-branch block pattern” refer to lead V1. If the PVC is negative (or mostly negative) in V1, the PVC has a left bundle-branch block pattern. A PVC that is positive in V1 is said to have a right bundle-branch block pattern and by implication arises from the left side of the heart.
A PVC originating from the top of the heart will move from top to bottom. The electrical axis of the PVC will be directed inferiorly. This means the PVC will be strongly positive in the inferior leads, ie, II, aVF, and III.
The electrocardiogram shown in Figure 2 demonstrates the typical appearance of a right ventricular outflow tract PVC.
If the PVC arises from the left ventricular outflow tract, the axis will still be inferiorly directed. However, the further to the left the origin of the PVC, the earlier the precordial transition will occur (the point at which the PVC is more positive than negative in the precordial leads). A PVC origin far enough to the left will result in a right bundle-branch block pattern PVC.
Not all idiopathic PVCs arise from the outflow tracts. A right bundle branch block pattern PVC does not imply the presence of underlying structural heart disease. PVCs may arise from both the tricuspid and mitral valve annuli, the left ventricular fascicles, or from the epicardium.
Multiple methods have been proposed to locate the origin of the PVC. For example, Park et al reviewed the use of surface ECG in locating the site of origin of ventricular tachycardia.11 All such algorithms should be applied with care, and with awareness of the caveats associated with their use.
Arrhythmogenic right ventricular cardiomyopathy is not benign
Arrhythmogenic right ventricular cardiomyopathy may give rise to PVCs or nonsustained ventricular tachycardia with morphologies similar to those of right ventricular outflow tract PVCs and ventricular tachycardia. The ventricular tachycardia complicating arrhythmogenic cardiomyopathy is, like PVCs arising from the right ventricular outflow tract, commonly associated with exercise or activity.
Unlike right ventricular outflow tract tachycardia, ventricular tachycardia related to arrhythmogenic cardiomyopathy is not benign.12 Distinguishing right ventricular outflow tract tachycardia from tachycardia secondary to arrhythmogenic cardiomyopathy is therefore critical.
Good-quality ECG demonstrating normal right ventricular size and function is reassuring, and if echocardiography is not conclusive, cardiovascular magnetic resonance imaging may provide additional diagnostic and prognostic data, especially when arrhythmogenic cardiomyopathy, cardiac sarcoidosis, or cardiac amyloidosis is suspected.6
Recently, magnetic resonance imaging has been used most for infiltrative diseases as the imaging modality of choice due to its superior tissue characterization and noninvasive morphological and functional evaluation. Magnetic resonance imaging findings in patients with arrhythmogenic cardiomyopathy correlate well with those of endomyocardial biopsy, angiography, and echocardiography and have been associated with incremental arrhythmic risk in the setting of electrical abnormalities. The increasing use of magnetic resonance imaging is leading to the recognition that left ventricular involvement (left-dominant arrhythmogenic right ventricular cardiomyopathy) is more common than previously recognized, with some suggesting that arrhythmogenic right ventricular cardiomyopathy should be simply called “arrhythmogenic cardiomyopathy.”
Although endomyocardial biopsy can establish the diagnosis of arrhythmogenic right ventricular cardiomyopathy, it is rarely performed because it has a high false-negative rate owing to the patchy, epicardial nature of this disorder.13
Red flags for cardiomyopathy
- Multifocal PVCs, or nonsustained ventricular tachycardia of more than one morphology on monitoring
- Syncope associated with active exercise
- Abnormal imaging findings that are consistent with arrhythmogenic right ventricular cardiomyopathy, cardiac sarcoidosis, or amyloidosis.
WHEN TO TREAT IDIOPATHIC PVCs
In our practice we explain to patients that there are two primary indications for treating idiopathic PVCs: (1) to relieve symptoms or (2) in asymptomatic patients with presumed arrhythmia-induced cardiomyopathy, to try to reverse the cardiomyopathy by eliminating the PVCs.
Some patients report severe symptoms due to their PVCs. Other patients appear to have no symptoms whatsoever, while still others are not overly bothered by the PVCs but are concerned that they may indicate they are at increased risk of cardiac events. In this last group, an evaluation such as outlined above that discloses no evidence of structural heart disease and reassurance by the physician may be all the treatment needed.
Even if they have no symptoms or only minimal symptoms, patients with a high PVC burden require follow-up because of the association between frequent PVCs and arrhythmia-induced cardiomyopathy.14,15 What constitutes a “high” PVC burden remains a matter of debate. Left ventricular dysfunction has generally been reported at PVC burdens above 15% to 25% of the total cardiac beats, though this percentage can be as low as 10%.14
Eliminating the high burden of PVCs in patients with left ventricular dysfunction may significantly improve left ventricular systolic function.15 It is likely, however, that more than PVC burden alone contributes to the development of the cardiomyopathy.14
Given these complexities, it is reasonable to request an electrophysiology consultation for patients who have more than rare PVCs. What is rare? There is no defined standard, but a PVC burden less than 1% is reasonable.
Treatment of the PVCs may be indicated in patients with systolic heart failure receiving cardiac resynchronization therapy, ie, a biventricular pacemaker. For cardiac resynchronization therapy to be clinically beneficial, close to 100% of heartbeats need to be paced, and frequent PVCs, even at a burden less than 10%, may undermine its effectiveness.16
HOW TO INTERVENE?
Beta-blockers and nondihydropyridine calcium channel blockers have both been used to treat symptomatic PVCs. If the patient is found to have systolic dysfunction as part of the evaluation, a beta-blocker is indicated, irrespective of any desire to treat the PVCs. Beta-blockers and calcium channel blockers both have low adverse effect profiles. They are available in once-a-day formulations and are inexpensive. Their efficacy is variable. The use of these medications is well within the purview of the primary care physician.
Selective beta-blockers are the first choice in treatment, and metoprolol is commonly used in clinical practice. We start with a low dose and increase it based on symptom relief.
As noted, only nondihydropyridine calcium channel blockers should be used for treatment of PVCs. As with beta-blockers, we start at a low dose and increase as needed based on the response to therapy.
Antiarrhythymic drugs are classified according to the Vaughan-Williams system. The ones most frequently used for PVCs are the class Ic drugs propafenone and flecainide and the class III drugs sotalol, amiodarone, and dofetalide. However, in our experience, if first-line agents (ie, beta-blockers and nondihydropyridine calcium channel blockers) are unsuccessful in controlling the patient’s symptoms, most primary care physicians are uncomfortable prescribing class Ic and class III drugs. Failure of a beta-blocker, a calcium channel blocker, or both often results in referral to a cardiologist or electrophysiologist.
The consultation should include a careful discussion with the patient regarding the risk of treatment with a type I or a type III drug vs catheter ablation. Treatment with class I or class III antiarrhythmic drugs always entails a small risk of proarrhythmia. The choice between drug therapy or ablation therapy is highly individualized. However, if elimination of the PVCs is of paramount importance, such as in cases of arrhythmia-induced cardiomyopathy, ablation therapy is more effective at eliminating the PVCs, although at the cost of an invasive procedure. Fortunately, the risk of complications with ablation therapy is quite low.
No drugs are approved by the US Food and Drug Administration for treating PVCs or nonsustained ventricular tachycardia. The drugs that do have an indication for treatment of ventricular arrhythmias are labeled as being indicated for “sustained” or “life-threatening” ventricular arrhythmias. The use of drugs for the treatment of PVCs or nonsustained ventricular tachycardia represents off-label usage.
Referral to discuss catheter ablation of the PVCs6 should be considered for patients who:
- Have undergone unsuccessful attempts at drug therapy for either symptoms or PVC-related cardiomyopathy
- Refuse drug therapy but have severe symptoms, or
- Do not respond to cardiac resynchronization therapy due to suboptimal pacing due to PVCs.
- Kennedy HL, Underhill SJ. Frequent or complex ventricular ectopy in apparently healthy subjects: a clinical study of 25 cases. Am J Cardiol 1976; 38:141–148.
- Brodsky M, Wu D, Denes P, Kanakis C, Rosen KM. Arrhythmias documented by 24 hour continuous electrocardiographic monitoring in 50 male medical students without apparent heart disease. Am J Cardiol 1977; 39:390–395.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C Jr, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015;12:e41–e63.
- Benditt DG, Adkisson WO. Approach to the patient with syncope: venues, presentations, diagnoses. Cardiol Clin 2013; 31:9–25.
- Pedersen CT, Kay GN, Kalman J, et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Heart Rhythm 2014; 11:e166–e196.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Buxton AE, Waxman HL, Marchlinski FE, Simson MB, Cassidy D, Josephson ME. Right ventricular tachycardia: clinical and electrophysiologic characteristics. Circulation 1983; 68:917–927.
- Marchlinski FE, Deely MP, Zado ES. Sex-specific triggers for right ventricular outflow tract tachycardia. Am Heart J 2000; 139:1009–1013.
- Fuenmayor AJ, Araujo X, Fuenmayor AM. Cardiac arrhythmias during two different stages of the menstrual cycle. Int J Cardiol 1998; 63:267–270.
- Park KM, Kim YH, Marchlinski FE. Using the surface electrocardiogram to localize the origin of idiopathic ventricular tachycardia. Pacing Clin Electrophysiol 2012; 35:1516–1527.
- Te Riele AS, Hauer RN. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: clinical challenges in a changing disease spectrum. Trends Cardiovasc Med 2015; 25:191–198.
- Philips B, Cheng A. 2015 update on the diagnosis and management of arrhythmogenic right ventricular cardiomyopathy. Curr Opin Cardiol 2016; 31:46–56.
- Del Carpio Munoz F, Syed FF, Noheria A, et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J Cardiovasc Electrophysiol 2011; 22:791–798.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Zhang Q, Zhou Y, Yu CM. Incidence, definition, diagnosis, and management of the cardiac resynchronization therapy nonresponder. Curr Opin Cardiol 2015; 30:40–49.
- Kennedy HL, Underhill SJ. Frequent or complex ventricular ectopy in apparently healthy subjects: a clinical study of 25 cases. Am J Cardiol 1976; 38:141–148.
- Brodsky M, Wu D, Denes P, Kanakis C, Rosen KM. Arrhythmias documented by 24 hour continuous electrocardiographic monitoring in 50 male medical students without apparent heart disease. Am J Cardiol 1977; 39:390–395.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C Jr, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015;12:e41–e63.
- Benditt DG, Adkisson WO. Approach to the patient with syncope: venues, presentations, diagnoses. Cardiol Clin 2013; 31:9–25.
- Pedersen CT, Kay GN, Kalman J, et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Heart Rhythm 2014; 11:e166–e196.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Buxton AE, Waxman HL, Marchlinski FE, Simson MB, Cassidy D, Josephson ME. Right ventricular tachycardia: clinical and electrophysiologic characteristics. Circulation 1983; 68:917–927.
- Marchlinski FE, Deely MP, Zado ES. Sex-specific triggers for right ventricular outflow tract tachycardia. Am Heart J 2000; 139:1009–1013.
- Fuenmayor AJ, Araujo X, Fuenmayor AM. Cardiac arrhythmias during two different stages of the menstrual cycle. Int J Cardiol 1998; 63:267–270.
- Park KM, Kim YH, Marchlinski FE. Using the surface electrocardiogram to localize the origin of idiopathic ventricular tachycardia. Pacing Clin Electrophysiol 2012; 35:1516–1527.
- Te Riele AS, Hauer RN. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: clinical challenges in a changing disease spectrum. Trends Cardiovasc Med 2015; 25:191–198.
- Philips B, Cheng A. 2015 update on the diagnosis and management of arrhythmogenic right ventricular cardiomyopathy. Curr Opin Cardiol 2016; 31:46–56.
- Del Carpio Munoz F, Syed FF, Noheria A, et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J Cardiovasc Electrophysiol 2011; 22:791–798.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Zhang Q, Zhou Y, Yu CM. Incidence, definition, diagnosis, and management of the cardiac resynchronization therapy nonresponder. Curr Opin Cardiol 2015; 30:40–49.
KEY POINTS
- The focus of the initial evaluation is to determine whether there is underlying structural heart disease. If there is, early referral to a specialist is probably warranted.
- Idiopathic PVCs (in which there is no structural heart disease) have a benign prognosis.
- Treatment of PVCs is indicated for relief of symptoms if reassurance is not sufficient.
- Patients who have a high PVC burden (> 10% of total heartbeats, though this is a subject of debate) should have an evaluation of their systolic function. If it is normal at baseline, periodic follow-up echocardiograms should be considered.
- Patients with a very high burden (> 20%) are at high risk of arrhythmia-induced cardiomyopathy. In these patients, referral is prudent, as some patients may opt for more aggressive treatment of their PVCs.
- In patients with severe symptoms for whom medical management has failed, referral for consideration of catheter ablation is reasonable.
Can patients opt to turn off implantable cardioverter-defibrillators near the end of life?
Yes. Although implantable cardioverter-defibrillators (ICDs) prevent sudden cardiac death in patients with advanced heart failure, their benefit in terminally ill patients is small.1 Furthermore, the shocks they deliver at the end of life can cause distress. Therefore, it is reasonable to consider ICD deactivation if the patient or family wishes.
A DIFFICULT DECISION
End-of-life decisions place significant emotional burdens on patients, their families, and their healthcare providers and can have social and legal consequences.
Turning off an ICD is an especially difficult decision, considering that these devices protect against sudden cardiac death and fatal arrhythmias. Also, patients and their representatives may find it more difficult to withdraw from active care than to forgo further interventions (more on this below), and they may misunderstand discussions about ICD deactivation, perceiving them as the beginning of abandonment.
ICD DEACTIVATION IS OFTEN DONE HAPHAZARDLY OR NOT AT ALL
Many healthcare providers are not trained in or comfortable with discussing end-of-life issues, and many hospitals and hospice programs lack policies and protocols for managing implanted devices at the end of life. Consequently, ICD management at the end of life varies among providers and tends to be suboptimal.2
In a report of a survey in 414 hospice facilities, 97% of facilities reported that they admitted patients with ICDs, but only 10% had a policy on device deactivation.3
In a survey of 47 European medical centers, only 4% said they addressed ICD deactivation with their patients.4
A study of 125 patients with ICDs who had died found that 52% had do-not-resuscitate orders. Nevertheless, in 100 patients the ICD had remained active in the last 24 hours of their life, and 31 of these patients had received shocks during their last 24 hours.5
In a survey of next of kin of patients with ICDs who had died of any cause,6 in only 27 of 100 cases had the clinician discussed ICD deactivation, and about three-fourths of these discussions had occurred during the last few days of life. Twenty-seven patients had received ICD discharges in the last month of life, and 8% had received a discharge during the final minutes.
TRAINING AND PROTOCOLS ARE NEEDED
Healthcare professionals need education about device deactivation at the end of life so that they are comfortable communicating with patients and families about this critical issue. To this end, several cardiac and palliative care societies have jointly released an expert statement on managing ICDs and other implantable devices in end-of-life situations.7
Many providers harbor a misunderstanding of the difference between withholding a device and withdrawing (or turning off) a device that is already implanted.2 Some mistakenly believe they would be committing a crime by deactivating an implanted life-sustaining device. Legally and ethically, there is no difference between withholding a device and withdrawing a device. Legally, carrying out a request to withdraw life-sustaining treatment is neither physician-assisted suicide nor euthanasia.
DISCUSSION SHOULD BEGIN EARLY AND SHOULD BE ONGOING
The discussion of ICD deactivation should begin before the device is implanted and should continue as the patient’s health status changes. In a survey, 40% of patients said they felt that ICD deactivation should be discussed before the device is implanted, and only 5% felt that this discussion should be undertaken in the last days of life.8
At the least, it is important to identify patients with ICDs on admission to hospice and to have policies in place that ensure adequate patient education to make an informed decision about ICD deactivation at the end of life.
The topic should be discussed when goals of care change and when do-not-resuscitate status is addressed, and also when advanced directives are being acknowledged. If the patient or his or her legal representative wishes to keep the ICD turned on, that wish should be respected. The essence of a discussion is not to impose the providers’ choice on the patient, but to help the patient make the right decision for himself or herself. Of note, patients entering hospice do not have to have do-not-resuscitate status.
We believe that device management in end-of-life circumstances should be part of the discussion of the goals of care. Accordingly, healthcare providers need to be familiar with device management and to have a higher comfort level in addressing such sensitive topics with patients facing the end of life, as well as with their families.
It is also advisable to apply protocols within hospice services to address ICD management options for the patient and the legal representative. An early decision regarding end-of-life deactivation will help patients avoid distressing ICD discharges and the related emotional distress in their last moments.
- Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol 2012; 59:2075–2079.
- Kapa S, Mueller PS, Hayes DL, Asirvatham SJ. Perspectives on withdrawing pacemaker and implantable cardioverter-defibrillator therapies at end of life: results of a survey of medical and legal professionals and patients. Mayo Clin Proc 2010; 85:981–990.
- Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: management of implantable cardioverter-defibrillators in hospice: a nationwide survey. Ann Intern Med 2010; 152:296–299.
- Marinskis G, van Erven L; EHRA Scientific Initiatives Committtee. Deactivation of implanted cardioverter-defibrillators at the end of life: results of the EHRA survey. Europace 2010; 12:1176–1177.
- Kinch Westerdahl A, Sjoblom J, Mattiasson AC, Rosenqvist M, Frykman V. Implantable cardioverter-defibrillator therapy before death: high risk for painful shocks at end of life. Circulation 2014; 129:422–429.
- Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med 2004; 141:835–838.
- Lampert R, Hayes DL, Annas GJ, et al; American College of Cardiology; American Geriatrics Society; American Academy of Hospice and Palliative Medicine; American Heart Association; European Heart Rhythm Association; Hospice and Palliative Nurses Association. HRS expert consensus statement on the management of cardiovascular implantable electronic devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Heart Rhythm 2010; 7:1008–1026.
- Raphael CE, Koa-Wing M, Stain N, Wright I, Francis DP, Kanagaratnam P. Implantable cardioverter-defibrillator recipient attitudes towards device activation: how much do patients want to know? Pacing Clin Electrophysiol 2011; 34:1628–1633.
Yes. Although implantable cardioverter-defibrillators (ICDs) prevent sudden cardiac death in patients with advanced heart failure, their benefit in terminally ill patients is small.1 Furthermore, the shocks they deliver at the end of life can cause distress. Therefore, it is reasonable to consider ICD deactivation if the patient or family wishes.
A DIFFICULT DECISION
End-of-life decisions place significant emotional burdens on patients, their families, and their healthcare providers and can have social and legal consequences.
Turning off an ICD is an especially difficult decision, considering that these devices protect against sudden cardiac death and fatal arrhythmias. Also, patients and their representatives may find it more difficult to withdraw from active care than to forgo further interventions (more on this below), and they may misunderstand discussions about ICD deactivation, perceiving them as the beginning of abandonment.
ICD DEACTIVATION IS OFTEN DONE HAPHAZARDLY OR NOT AT ALL
Many healthcare providers are not trained in or comfortable with discussing end-of-life issues, and many hospitals and hospice programs lack policies and protocols for managing implanted devices at the end of life. Consequently, ICD management at the end of life varies among providers and tends to be suboptimal.2
In a report of a survey in 414 hospice facilities, 97% of facilities reported that they admitted patients with ICDs, but only 10% had a policy on device deactivation.3
In a survey of 47 European medical centers, only 4% said they addressed ICD deactivation with their patients.4
A study of 125 patients with ICDs who had died found that 52% had do-not-resuscitate orders. Nevertheless, in 100 patients the ICD had remained active in the last 24 hours of their life, and 31 of these patients had received shocks during their last 24 hours.5
In a survey of next of kin of patients with ICDs who had died of any cause,6 in only 27 of 100 cases had the clinician discussed ICD deactivation, and about three-fourths of these discussions had occurred during the last few days of life. Twenty-seven patients had received ICD discharges in the last month of life, and 8% had received a discharge during the final minutes.
TRAINING AND PROTOCOLS ARE NEEDED
Healthcare professionals need education about device deactivation at the end of life so that they are comfortable communicating with patients and families about this critical issue. To this end, several cardiac and palliative care societies have jointly released an expert statement on managing ICDs and other implantable devices in end-of-life situations.7
Many providers harbor a misunderstanding of the difference between withholding a device and withdrawing (or turning off) a device that is already implanted.2 Some mistakenly believe they would be committing a crime by deactivating an implanted life-sustaining device. Legally and ethically, there is no difference between withholding a device and withdrawing a device. Legally, carrying out a request to withdraw life-sustaining treatment is neither physician-assisted suicide nor euthanasia.
DISCUSSION SHOULD BEGIN EARLY AND SHOULD BE ONGOING
The discussion of ICD deactivation should begin before the device is implanted and should continue as the patient’s health status changes. In a survey, 40% of patients said they felt that ICD deactivation should be discussed before the device is implanted, and only 5% felt that this discussion should be undertaken in the last days of life.8
At the least, it is important to identify patients with ICDs on admission to hospice and to have policies in place that ensure adequate patient education to make an informed decision about ICD deactivation at the end of life.
The topic should be discussed when goals of care change and when do-not-resuscitate status is addressed, and also when advanced directives are being acknowledged. If the patient or his or her legal representative wishes to keep the ICD turned on, that wish should be respected. The essence of a discussion is not to impose the providers’ choice on the patient, but to help the patient make the right decision for himself or herself. Of note, patients entering hospice do not have to have do-not-resuscitate status.
We believe that device management in end-of-life circumstances should be part of the discussion of the goals of care. Accordingly, healthcare providers need to be familiar with device management and to have a higher comfort level in addressing such sensitive topics with patients facing the end of life, as well as with their families.
It is also advisable to apply protocols within hospice services to address ICD management options for the patient and the legal representative. An early decision regarding end-of-life deactivation will help patients avoid distressing ICD discharges and the related emotional distress in their last moments.
Yes. Although implantable cardioverter-defibrillators (ICDs) prevent sudden cardiac death in patients with advanced heart failure, their benefit in terminally ill patients is small.1 Furthermore, the shocks they deliver at the end of life can cause distress. Therefore, it is reasonable to consider ICD deactivation if the patient or family wishes.
A DIFFICULT DECISION
End-of-life decisions place significant emotional burdens on patients, their families, and their healthcare providers and can have social and legal consequences.
Turning off an ICD is an especially difficult decision, considering that these devices protect against sudden cardiac death and fatal arrhythmias. Also, patients and their representatives may find it more difficult to withdraw from active care than to forgo further interventions (more on this below), and they may misunderstand discussions about ICD deactivation, perceiving them as the beginning of abandonment.
ICD DEACTIVATION IS OFTEN DONE HAPHAZARDLY OR NOT AT ALL
Many healthcare providers are not trained in or comfortable with discussing end-of-life issues, and many hospitals and hospice programs lack policies and protocols for managing implanted devices at the end of life. Consequently, ICD management at the end of life varies among providers and tends to be suboptimal.2
In a report of a survey in 414 hospice facilities, 97% of facilities reported that they admitted patients with ICDs, but only 10% had a policy on device deactivation.3
In a survey of 47 European medical centers, only 4% said they addressed ICD deactivation with their patients.4
A study of 125 patients with ICDs who had died found that 52% had do-not-resuscitate orders. Nevertheless, in 100 patients the ICD had remained active in the last 24 hours of their life, and 31 of these patients had received shocks during their last 24 hours.5
In a survey of next of kin of patients with ICDs who had died of any cause,6 in only 27 of 100 cases had the clinician discussed ICD deactivation, and about three-fourths of these discussions had occurred during the last few days of life. Twenty-seven patients had received ICD discharges in the last month of life, and 8% had received a discharge during the final minutes.
TRAINING AND PROTOCOLS ARE NEEDED
Healthcare professionals need education about device deactivation at the end of life so that they are comfortable communicating with patients and families about this critical issue. To this end, several cardiac and palliative care societies have jointly released an expert statement on managing ICDs and other implantable devices in end-of-life situations.7
Many providers harbor a misunderstanding of the difference between withholding a device and withdrawing (or turning off) a device that is already implanted.2 Some mistakenly believe they would be committing a crime by deactivating an implanted life-sustaining device. Legally and ethically, there is no difference between withholding a device and withdrawing a device. Legally, carrying out a request to withdraw life-sustaining treatment is neither physician-assisted suicide nor euthanasia.
DISCUSSION SHOULD BEGIN EARLY AND SHOULD BE ONGOING
The discussion of ICD deactivation should begin before the device is implanted and should continue as the patient’s health status changes. In a survey, 40% of patients said they felt that ICD deactivation should be discussed before the device is implanted, and only 5% felt that this discussion should be undertaken in the last days of life.8
At the least, it is important to identify patients with ICDs on admission to hospice and to have policies in place that ensure adequate patient education to make an informed decision about ICD deactivation at the end of life.
The topic should be discussed when goals of care change and when do-not-resuscitate status is addressed, and also when advanced directives are being acknowledged. If the patient or his or her legal representative wishes to keep the ICD turned on, that wish should be respected. The essence of a discussion is not to impose the providers’ choice on the patient, but to help the patient make the right decision for himself or herself. Of note, patients entering hospice do not have to have do-not-resuscitate status.
We believe that device management in end-of-life circumstances should be part of the discussion of the goals of care. Accordingly, healthcare providers need to be familiar with device management and to have a higher comfort level in addressing such sensitive topics with patients facing the end of life, as well as with their families.
It is also advisable to apply protocols within hospice services to address ICD management options for the patient and the legal representative. An early decision regarding end-of-life deactivation will help patients avoid distressing ICD discharges and the related emotional distress in their last moments.
- Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol 2012; 59:2075–2079.
- Kapa S, Mueller PS, Hayes DL, Asirvatham SJ. Perspectives on withdrawing pacemaker and implantable cardioverter-defibrillator therapies at end of life: results of a survey of medical and legal professionals and patients. Mayo Clin Proc 2010; 85:981–990.
- Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: management of implantable cardioverter-defibrillators in hospice: a nationwide survey. Ann Intern Med 2010; 152:296–299.
- Marinskis G, van Erven L; EHRA Scientific Initiatives Committtee. Deactivation of implanted cardioverter-defibrillators at the end of life: results of the EHRA survey. Europace 2010; 12:1176–1177.
- Kinch Westerdahl A, Sjoblom J, Mattiasson AC, Rosenqvist M, Frykman V. Implantable cardioverter-defibrillator therapy before death: high risk for painful shocks at end of life. Circulation 2014; 129:422–429.
- Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med 2004; 141:835–838.
- Lampert R, Hayes DL, Annas GJ, et al; American College of Cardiology; American Geriatrics Society; American Academy of Hospice and Palliative Medicine; American Heart Association; European Heart Rhythm Association; Hospice and Palliative Nurses Association. HRS expert consensus statement on the management of cardiovascular implantable electronic devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Heart Rhythm 2010; 7:1008–1026.
- Raphael CE, Koa-Wing M, Stain N, Wright I, Francis DP, Kanagaratnam P. Implantable cardioverter-defibrillator recipient attitudes towards device activation: how much do patients want to know? Pacing Clin Electrophysiol 2011; 34:1628–1633.
- Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol 2012; 59:2075–2079.
- Kapa S, Mueller PS, Hayes DL, Asirvatham SJ. Perspectives on withdrawing pacemaker and implantable cardioverter-defibrillator therapies at end of life: results of a survey of medical and legal professionals and patients. Mayo Clin Proc 2010; 85:981–990.
- Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: management of implantable cardioverter-defibrillators in hospice: a nationwide survey. Ann Intern Med 2010; 152:296–299.
- Marinskis G, van Erven L; EHRA Scientific Initiatives Committtee. Deactivation of implanted cardioverter-defibrillators at the end of life: results of the EHRA survey. Europace 2010; 12:1176–1177.
- Kinch Westerdahl A, Sjoblom J, Mattiasson AC, Rosenqvist M, Frykman V. Implantable cardioverter-defibrillator therapy before death: high risk for painful shocks at end of life. Circulation 2014; 129:422–429.
- Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med 2004; 141:835–838.
- Lampert R, Hayes DL, Annas GJ, et al; American College of Cardiology; American Geriatrics Society; American Academy of Hospice and Palliative Medicine; American Heart Association; European Heart Rhythm Association; Hospice and Palliative Nurses Association. HRS expert consensus statement on the management of cardiovascular implantable electronic devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Heart Rhythm 2010; 7:1008–1026.
- Raphael CE, Koa-Wing M, Stain N, Wright I, Francis DP, Kanagaratnam P. Implantable cardioverter-defibrillator recipient attitudes towards device activation: how much do patients want to know? Pacing Clin Electrophysiol 2011; 34:1628–1633.
Does stenting of severe renal artery stenosis improve outomes compared with medical therapy alone?
No. In patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting offers no additional benefit when added to comprehensive medical therapy.
In these patients, renal artery stenting in addition to antihypertensive drug therapy can improve blood pressure control modestly but has no significant effect on outcomes such as adverse cardiovascular events and death. And because renal artery stenting carries a risk of complications, medical management should continue to be the first-line therapy.
RENAL ARTERY STENOSIS
Renal artery stenosis is a common form of peripheral artery disease. Atherosclerosis is the most common cause, but it can also be caused by fibromuscular dysplasia or vasculitis (eg, Takayasu arteritis). It is most often unilateral, but bilateral disease has also been reported.
The prevalence of atherosclerotic renal vascular disease in the US Medicare population is 0.5%, and 5.5% in those with chronic kidney disease.1 Furthermore, renal artery stenosis is found in 6.8% of adults over age 65.2 The prevalence increases with age and is higher in patients with hyperlipidemia, peripheral arterial disease, and hypertension. The prevalence of renal artery stenosis in patients with atherosclerotic disease and renal dysfunction is as high as 50%.3
Patients with peripheral artery disease may be five times more likely to develop renal artery stenosis than people without peripheral artery disease.4 Significant stenosis can result in resistant arterial hypertension, renal insufficiency, left ventricular hypertrophy, and congestive heart failure.5
Nephropathy due to renal artery stenosis is complex and is caused by hypoperfusion and chronic microatheroembolism. Renal artery stenosis leads to oxidative stress, inflammation, fibrosis in the stenotic kidney, and, over time, loss of kidney function. Hypoperfusion also leads to activation of the renin-angiotensin-aldosterone system, which plays a role in development of left ventricular hypertrophy.5,6
Adequate blood pressure control, goal-directed lipid-lowering therapy, smoking cessation, and other preventive measures are the foundation of management.
RENAL ARTERY STENOSIS AND HYPERTENSION
Renal artery stenosis is a cause of secondary hypertension. The stenosis decreases renal perfusion pressure, activating the release of renin and the production of angiotensin II, which in turn raises the blood pressure by two mechanisms (Figure 1): directly, by causing generalized vasoconstriction, and indirectly, by stimulating the release of aldosterone, which in turn increases the reabsorption of sodium and causes hypervolemia. These two mechanisms play a major role in renal vascular hypertension when renal artery stenosis is bilateral. In unilateral renal artery stenosis, pressure diuresis in the unaffected kidney compensates for the reabsorption of sodium in the affected kidney, keeping the blood pressure down. However, with time, the unaffected kidney will develop hypertensive nephropathy, and pressure diuresis will be lost.7,8 In addition, the activation of the renin-angiotensin-aldosterone system results in structural heart disease, such as left ventricular hypertrophy,5 and may shorten survival.
STENTING PLUS ANTIHYPERTENSIVE DRUG THERAPY
Because observational studies showed improvement in blood pressure control after endovascular stenting of atherosclerotic renal artery stenosis,9,10 this approach became a treatment option for uncontrolled hypertension in these patients. The 2005 joint guidelines of the American College of Cardiology and the American Heart Association11 considered percutaneous revascularization a reasonable option (level of evidence B) for patients who meet one of the following criteria:
- Hemodynamically significant stenosis and accelerated, resistant, or malignant hypertension, hypertension with an unexplained unilateral small kidney, or hypertension with intolerance to medication
- Renal artery stenosis and progressive chronic kidney disease with bilateral stenosis or stenosis in a solitary functioning kidney
- Hemodynamically significant stenosis and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema or unstable angina.11
However, no randomized study has shown a direct benefit of renal artery stenting on rates of cardiovascular events or renal function compared with drug therapy alone.
TRIALS OF STENTING VS MEDICAL THERAPY ALONE
Technical improvements have led to more widespread use of diagnostic and interventional endovascular tools for renal artery revascularization. Studies over the past 10 years examined the impact of stenting in patients with uncontrolled hypertension.
The STAR trial
In the Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial,9 patients with creatinine clearance less than 80 mL/min/1.73 m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy or medical therapy alone. The authors concluded that stenting had no effect on the progression of renal dysfunction but led to a small number of significant, procedure-related complications. The study was criticized for including patients with mild stenosis (< 50% stenosis) and for being underpowered for the primary end point.
The ASTRAL study
The Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) study10 was a similar comparison with similar results, showing no benefit from stenting with respect to renal function, systolic blood pressure control, cardiovascular events, or death.
HERCULES
The Herculink Elite Cobalt Chromium Renal Stent Trial to Demonstrate Efficacy and Safety (HERCULES)12 was a prospective multicenter study of the effects of renal artery stenting in 202 patients with significant renal artery stenosis and uncontrolled hypertension. It showed a reduction in systolic blood pressure from baseline (P < .0001). However, follow-up was only 9 months, which was insufficient to show a significant effect on long-term cardiovascular and cerebrovascular outcomes.
The CORAL trial
The Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial13 used more stringent definitions and longer follow-up. It randomized 947 patients to either stenting plus medical therapy or medical therapy alone. Patients had atherosclerotic renal artery stenosis, defined as stenosis of at least 80% or stenosis of 60% to 80% with a gradient of at least 20 mm Hg in the systolic pressure), and either systolic hypertension while taking two or more antihypertensive drugs or stage 3 or higher chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated by the Modification of Diet in Renal Disease formula).
Participants were followed for 43 months to detect the occurrence of adverse cardiovascular and renal events. There was no significant difference in primary outcome between stenting plus drug therapy and drug therapy alone (35.1% and 35.8%, respectively; P = .58). However, stenting plus drug therapy was associated with modestly lower systolic pressures compared with drug therapy alone (−2.3 mm Hg, 95% confidence interval −4.4 to −0.2 mm Hg, P = .03).13 This study provided strong evidence that renal artery stenting offers no significant benefit to patients with moderately severe atherosclerotic renal artery stenosis, and that stenting may actually pose an unnecessary risk.
COMPLICATIONS OF RENAL ARTERY STENTING
Complications of renal artery stenting are a limiting factor compared with drug therapy alone, especially since the procedure offers no significant benefit in outcome. Procedural complication rates of 10% to 15% have been reported.9,10,12 The CORAL trial reported arterial dissection in 2.2%, branch-vessel occlusion in 1.2%, and distal embolization in 1.2% of patients undergoing stenting.13 Other reported complications have included stent misplacement requiring an additional stent, access-vessel damage, stent embolization, renal artery thrombosis or occlusion, and death.10,12
- Kalra PA, Guo H, Kausz AT, et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 68:293–301.
- Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Uzu T, Takeji M, Yamada N, et al. Prevalence and outcome of renal artery stenosis in atherosclerotic patients with renal dysfunction. Hypertens Res 2002; 25:537–542.
- Benjamin MM, Fazel P, Filardo G, Choi JW, Stoler RC. Prevalence of and risk factors of renal artery stenosis in patients with resistant hypertension. Am J Cardiol 2014; 113:687–690.
- Wu S, Polavarapu N, Stouffer GA. Left ventricular hypertrophy in patients with renal artery stenosis. Am J Med Sci 2006; 332:334–338.
- Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis 2009; 52:196–203.
- Black HR, Glickman MG, Schiff M Jr, Pingoud EG. Renovascular hypertension: pathophysiology, diagnosis, and treatment. Yale J Biol Med 1978; 51:635–654.
- Tobe SW, Burgess E, Lebel M. Atherosclerotic renovascular disease. Can J Cardiol 2006; 22:623–628.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of stent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by atherosclerotic ostial stenosis of the renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
No. In patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting offers no additional benefit when added to comprehensive medical therapy.
In these patients, renal artery stenting in addition to antihypertensive drug therapy can improve blood pressure control modestly but has no significant effect on outcomes such as adverse cardiovascular events and death. And because renal artery stenting carries a risk of complications, medical management should continue to be the first-line therapy.
RENAL ARTERY STENOSIS
Renal artery stenosis is a common form of peripheral artery disease. Atherosclerosis is the most common cause, but it can also be caused by fibromuscular dysplasia or vasculitis (eg, Takayasu arteritis). It is most often unilateral, but bilateral disease has also been reported.
The prevalence of atherosclerotic renal vascular disease in the US Medicare population is 0.5%, and 5.5% in those with chronic kidney disease.1 Furthermore, renal artery stenosis is found in 6.8% of adults over age 65.2 The prevalence increases with age and is higher in patients with hyperlipidemia, peripheral arterial disease, and hypertension. The prevalence of renal artery stenosis in patients with atherosclerotic disease and renal dysfunction is as high as 50%.3
Patients with peripheral artery disease may be five times more likely to develop renal artery stenosis than people without peripheral artery disease.4 Significant stenosis can result in resistant arterial hypertension, renal insufficiency, left ventricular hypertrophy, and congestive heart failure.5
Nephropathy due to renal artery stenosis is complex and is caused by hypoperfusion and chronic microatheroembolism. Renal artery stenosis leads to oxidative stress, inflammation, fibrosis in the stenotic kidney, and, over time, loss of kidney function. Hypoperfusion also leads to activation of the renin-angiotensin-aldosterone system, which plays a role in development of left ventricular hypertrophy.5,6
Adequate blood pressure control, goal-directed lipid-lowering therapy, smoking cessation, and other preventive measures are the foundation of management.
RENAL ARTERY STENOSIS AND HYPERTENSION
Renal artery stenosis is a cause of secondary hypertension. The stenosis decreases renal perfusion pressure, activating the release of renin and the production of angiotensin II, which in turn raises the blood pressure by two mechanisms (Figure 1): directly, by causing generalized vasoconstriction, and indirectly, by stimulating the release of aldosterone, which in turn increases the reabsorption of sodium and causes hypervolemia. These two mechanisms play a major role in renal vascular hypertension when renal artery stenosis is bilateral. In unilateral renal artery stenosis, pressure diuresis in the unaffected kidney compensates for the reabsorption of sodium in the affected kidney, keeping the blood pressure down. However, with time, the unaffected kidney will develop hypertensive nephropathy, and pressure diuresis will be lost.7,8 In addition, the activation of the renin-angiotensin-aldosterone system results in structural heart disease, such as left ventricular hypertrophy,5 and may shorten survival.
STENTING PLUS ANTIHYPERTENSIVE DRUG THERAPY
Because observational studies showed improvement in blood pressure control after endovascular stenting of atherosclerotic renal artery stenosis,9,10 this approach became a treatment option for uncontrolled hypertension in these patients. The 2005 joint guidelines of the American College of Cardiology and the American Heart Association11 considered percutaneous revascularization a reasonable option (level of evidence B) for patients who meet one of the following criteria:
- Hemodynamically significant stenosis and accelerated, resistant, or malignant hypertension, hypertension with an unexplained unilateral small kidney, or hypertension with intolerance to medication
- Renal artery stenosis and progressive chronic kidney disease with bilateral stenosis or stenosis in a solitary functioning kidney
- Hemodynamically significant stenosis and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema or unstable angina.11
However, no randomized study has shown a direct benefit of renal artery stenting on rates of cardiovascular events or renal function compared with drug therapy alone.
TRIALS OF STENTING VS MEDICAL THERAPY ALONE
Technical improvements have led to more widespread use of diagnostic and interventional endovascular tools for renal artery revascularization. Studies over the past 10 years examined the impact of stenting in patients with uncontrolled hypertension.
The STAR trial
In the Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial,9 patients with creatinine clearance less than 80 mL/min/1.73 m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy or medical therapy alone. The authors concluded that stenting had no effect on the progression of renal dysfunction but led to a small number of significant, procedure-related complications. The study was criticized for including patients with mild stenosis (< 50% stenosis) and for being underpowered for the primary end point.
The ASTRAL study
The Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) study10 was a similar comparison with similar results, showing no benefit from stenting with respect to renal function, systolic blood pressure control, cardiovascular events, or death.
HERCULES
The Herculink Elite Cobalt Chromium Renal Stent Trial to Demonstrate Efficacy and Safety (HERCULES)12 was a prospective multicenter study of the effects of renal artery stenting in 202 patients with significant renal artery stenosis and uncontrolled hypertension. It showed a reduction in systolic blood pressure from baseline (P < .0001). However, follow-up was only 9 months, which was insufficient to show a significant effect on long-term cardiovascular and cerebrovascular outcomes.
The CORAL trial
The Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial13 used more stringent definitions and longer follow-up. It randomized 947 patients to either stenting plus medical therapy or medical therapy alone. Patients had atherosclerotic renal artery stenosis, defined as stenosis of at least 80% or stenosis of 60% to 80% with a gradient of at least 20 mm Hg in the systolic pressure), and either systolic hypertension while taking two or more antihypertensive drugs or stage 3 or higher chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated by the Modification of Diet in Renal Disease formula).
Participants were followed for 43 months to detect the occurrence of adverse cardiovascular and renal events. There was no significant difference in primary outcome between stenting plus drug therapy and drug therapy alone (35.1% and 35.8%, respectively; P = .58). However, stenting plus drug therapy was associated with modestly lower systolic pressures compared with drug therapy alone (−2.3 mm Hg, 95% confidence interval −4.4 to −0.2 mm Hg, P = .03).13 This study provided strong evidence that renal artery stenting offers no significant benefit to patients with moderately severe atherosclerotic renal artery stenosis, and that stenting may actually pose an unnecessary risk.
COMPLICATIONS OF RENAL ARTERY STENTING
Complications of renal artery stenting are a limiting factor compared with drug therapy alone, especially since the procedure offers no significant benefit in outcome. Procedural complication rates of 10% to 15% have been reported.9,10,12 The CORAL trial reported arterial dissection in 2.2%, branch-vessel occlusion in 1.2%, and distal embolization in 1.2% of patients undergoing stenting.13 Other reported complications have included stent misplacement requiring an additional stent, access-vessel damage, stent embolization, renal artery thrombosis or occlusion, and death.10,12
No. In patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting offers no additional benefit when added to comprehensive medical therapy.
In these patients, renal artery stenting in addition to antihypertensive drug therapy can improve blood pressure control modestly but has no significant effect on outcomes such as adverse cardiovascular events and death. And because renal artery stenting carries a risk of complications, medical management should continue to be the first-line therapy.
RENAL ARTERY STENOSIS
Renal artery stenosis is a common form of peripheral artery disease. Atherosclerosis is the most common cause, but it can also be caused by fibromuscular dysplasia or vasculitis (eg, Takayasu arteritis). It is most often unilateral, but bilateral disease has also been reported.
The prevalence of atherosclerotic renal vascular disease in the US Medicare population is 0.5%, and 5.5% in those with chronic kidney disease.1 Furthermore, renal artery stenosis is found in 6.8% of adults over age 65.2 The prevalence increases with age and is higher in patients with hyperlipidemia, peripheral arterial disease, and hypertension. The prevalence of renal artery stenosis in patients with atherosclerotic disease and renal dysfunction is as high as 50%.3
Patients with peripheral artery disease may be five times more likely to develop renal artery stenosis than people without peripheral artery disease.4 Significant stenosis can result in resistant arterial hypertension, renal insufficiency, left ventricular hypertrophy, and congestive heart failure.5
Nephropathy due to renal artery stenosis is complex and is caused by hypoperfusion and chronic microatheroembolism. Renal artery stenosis leads to oxidative stress, inflammation, fibrosis in the stenotic kidney, and, over time, loss of kidney function. Hypoperfusion also leads to activation of the renin-angiotensin-aldosterone system, which plays a role in development of left ventricular hypertrophy.5,6
Adequate blood pressure control, goal-directed lipid-lowering therapy, smoking cessation, and other preventive measures are the foundation of management.
RENAL ARTERY STENOSIS AND HYPERTENSION
Renal artery stenosis is a cause of secondary hypertension. The stenosis decreases renal perfusion pressure, activating the release of renin and the production of angiotensin II, which in turn raises the blood pressure by two mechanisms (Figure 1): directly, by causing generalized vasoconstriction, and indirectly, by stimulating the release of aldosterone, which in turn increases the reabsorption of sodium and causes hypervolemia. These two mechanisms play a major role in renal vascular hypertension when renal artery stenosis is bilateral. In unilateral renal artery stenosis, pressure diuresis in the unaffected kidney compensates for the reabsorption of sodium in the affected kidney, keeping the blood pressure down. However, with time, the unaffected kidney will develop hypertensive nephropathy, and pressure diuresis will be lost.7,8 In addition, the activation of the renin-angiotensin-aldosterone system results in structural heart disease, such as left ventricular hypertrophy,5 and may shorten survival.
STENTING PLUS ANTIHYPERTENSIVE DRUG THERAPY
Because observational studies showed improvement in blood pressure control after endovascular stenting of atherosclerotic renal artery stenosis,9,10 this approach became a treatment option for uncontrolled hypertension in these patients. The 2005 joint guidelines of the American College of Cardiology and the American Heart Association11 considered percutaneous revascularization a reasonable option (level of evidence B) for patients who meet one of the following criteria:
- Hemodynamically significant stenosis and accelerated, resistant, or malignant hypertension, hypertension with an unexplained unilateral small kidney, or hypertension with intolerance to medication
- Renal artery stenosis and progressive chronic kidney disease with bilateral stenosis or stenosis in a solitary functioning kidney
- Hemodynamically significant stenosis and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema or unstable angina.11
However, no randomized study has shown a direct benefit of renal artery stenting on rates of cardiovascular events or renal function compared with drug therapy alone.
TRIALS OF STENTING VS MEDICAL THERAPY ALONE
Technical improvements have led to more widespread use of diagnostic and interventional endovascular tools for renal artery revascularization. Studies over the past 10 years examined the impact of stenting in patients with uncontrolled hypertension.
The STAR trial
In the Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial,9 patients with creatinine clearance less than 80 mL/min/1.73 m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy or medical therapy alone. The authors concluded that stenting had no effect on the progression of renal dysfunction but led to a small number of significant, procedure-related complications. The study was criticized for including patients with mild stenosis (< 50% stenosis) and for being underpowered for the primary end point.
The ASTRAL study
The Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) study10 was a similar comparison with similar results, showing no benefit from stenting with respect to renal function, systolic blood pressure control, cardiovascular events, or death.
HERCULES
The Herculink Elite Cobalt Chromium Renal Stent Trial to Demonstrate Efficacy and Safety (HERCULES)12 was a prospective multicenter study of the effects of renal artery stenting in 202 patients with significant renal artery stenosis and uncontrolled hypertension. It showed a reduction in systolic blood pressure from baseline (P < .0001). However, follow-up was only 9 months, which was insufficient to show a significant effect on long-term cardiovascular and cerebrovascular outcomes.
The CORAL trial
The Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial13 used more stringent definitions and longer follow-up. It randomized 947 patients to either stenting plus medical therapy or medical therapy alone. Patients had atherosclerotic renal artery stenosis, defined as stenosis of at least 80% or stenosis of 60% to 80% with a gradient of at least 20 mm Hg in the systolic pressure), and either systolic hypertension while taking two or more antihypertensive drugs or stage 3 or higher chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated by the Modification of Diet in Renal Disease formula).
Participants were followed for 43 months to detect the occurrence of adverse cardiovascular and renal events. There was no significant difference in primary outcome between stenting plus drug therapy and drug therapy alone (35.1% and 35.8%, respectively; P = .58). However, stenting plus drug therapy was associated with modestly lower systolic pressures compared with drug therapy alone (−2.3 mm Hg, 95% confidence interval −4.4 to −0.2 mm Hg, P = .03).13 This study provided strong evidence that renal artery stenting offers no significant benefit to patients with moderately severe atherosclerotic renal artery stenosis, and that stenting may actually pose an unnecessary risk.
COMPLICATIONS OF RENAL ARTERY STENTING
Complications of renal artery stenting are a limiting factor compared with drug therapy alone, especially since the procedure offers no significant benefit in outcome. Procedural complication rates of 10% to 15% have been reported.9,10,12 The CORAL trial reported arterial dissection in 2.2%, branch-vessel occlusion in 1.2%, and distal embolization in 1.2% of patients undergoing stenting.13 Other reported complications have included stent misplacement requiring an additional stent, access-vessel damage, stent embolization, renal artery thrombosis or occlusion, and death.10,12
- Kalra PA, Guo H, Kausz AT, et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 68:293–301.
- Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Uzu T, Takeji M, Yamada N, et al. Prevalence and outcome of renal artery stenosis in atherosclerotic patients with renal dysfunction. Hypertens Res 2002; 25:537–542.
- Benjamin MM, Fazel P, Filardo G, Choi JW, Stoler RC. Prevalence of and risk factors of renal artery stenosis in patients with resistant hypertension. Am J Cardiol 2014; 113:687–690.
- Wu S, Polavarapu N, Stouffer GA. Left ventricular hypertrophy in patients with renal artery stenosis. Am J Med Sci 2006; 332:334–338.
- Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis 2009; 52:196–203.
- Black HR, Glickman MG, Schiff M Jr, Pingoud EG. Renovascular hypertension: pathophysiology, diagnosis, and treatment. Yale J Biol Med 1978; 51:635–654.
- Tobe SW, Burgess E, Lebel M. Atherosclerotic renovascular disease. Can J Cardiol 2006; 22:623–628.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of stent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by atherosclerotic ostial stenosis of the renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
- Kalra PA, Guo H, Kausz AT, et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 68:293–301.
- Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Uzu T, Takeji M, Yamada N, et al. Prevalence and outcome of renal artery stenosis in atherosclerotic patients with renal dysfunction. Hypertens Res 2002; 25:537–542.
- Benjamin MM, Fazel P, Filardo G, Choi JW, Stoler RC. Prevalence of and risk factors of renal artery stenosis in patients with resistant hypertension. Am J Cardiol 2014; 113:687–690.
- Wu S, Polavarapu N, Stouffer GA. Left ventricular hypertrophy in patients with renal artery stenosis. Am J Med Sci 2006; 332:334–338.
- Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis 2009; 52:196–203.
- Black HR, Glickman MG, Schiff M Jr, Pingoud EG. Renovascular hypertension: pathophysiology, diagnosis, and treatment. Yale J Biol Med 1978; 51:635–654.
- Tobe SW, Burgess E, Lebel M. Atherosclerotic renovascular disease. Can J Cardiol 2006; 22:623–628.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of stent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by atherosclerotic ostial stenosis of the renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
When does pericarditis merit a workup for autoimmune or inflammatory disease?
Pericarditis is in most cases a one-time disease simply treated with anti-inflammatory drugs. It requires no extensive workup for systemic inflammatory or autoimmune disease. Further evaluation is required for patients who have recurrent pericarditis resistant to conventional therapy or pericarditis with manifestations of systemic disease.
ACUTE PERICARDITIS
Pericardial disease has different presentations: acute, recurrent, constrictive, effusive-constrictive, and pericardial effusion with or without tamponade. Acute pericarditis is the most common of these and can affect people of all ages. The typical acute manifestations are chest pain (usually pleuritic), a pericardial friction rub, and widespread ST-segment elevation on the electrocardiogram.1,2 The chest pain tends to be sharp and long-lasting; it radiates to the trapezius ridge and increases during respiration or body movements.
Acute pericarditis usually responds to an anti-inflammatory drug such as colchicine 0.6 mg/day for 3 months, a nonsteroidal anti-inflammatory drug such as ibuprofen 600 mg three times a day for 10 days, and in advanced resistant cases, an oral corticosteroid.3,4
Most often, pericarditis is either idiopathic or occurs after a respiratory viral illness. Much less common causes include bacterial infection, postpericardiotomy syndrome, myocardial infarction, primary or metastatic tumors, trauma, radiation, and uremia. However, pericarditis can also be part of the presentation of systemic inflammatory and autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus; hereditary periodic fever syndromes such as familial Mediterranean fever; and systemic-onset juvenile idiopathic arthritis.1,5
In acute pericarditis, a complex workup is usually not justified, since the results will have limited usefulness in the clinical management of the patient. It is most often diagnosed by the presenting symptoms, auscultation, electrocardiography, echocardiography, and chest radiography, and by additional basic tests that include a complete blood cell count, complete metabolic profile, erythrocyte sedimentation rate, and C-reactive protein level. However, if pericarditis does not respond to anti-inflammatory treatment and if an autoimmune or infectious disease is suspected, further evaluation may include antinuclear antibody testing and testing for human immunodeficiency virus and tuberculosis. If the diagnosis of acute pericarditis remains uncertain, cardiac magnetic resonance imaging (MRI) may be useful.
RECURRENT PERICARDITIS
Although acute pericarditis most often has a benign course and responds well to anti-inflammatory drugs, 20% to 30% of patients who have a first attack of acute pericarditis have a recurrence, and up to 50% of patients who have one recurrence will have another.3,4
Disease activity can be followed with serial testing of inflammatory markers—eg, erythrocyte sedimentation rate and C-reactive protein level. Echocardiography, cardiac computed tomography, and cardiac MRI can characterize active inflammation, edema, pericardial thickness, and pericardial effusion.6–8
Recurrent pericarditis is often resistant to standard therapy and requires corticosteroids in high doses, which paradoxically can increase the risk of recurrence. Therefore, further workup for underlying autoimmune disease, systemic inflammatory disease, or infection is necessary. More potent immunosuppressive therapy may be required, not only in pericarditis associated with systemic autoimmune or inflammatory conditions, but even in idiopathic recurrent pericarditis, either to control symptoms or to mitigate the effects of corticosteroids.
SYSTEMIC INFLAMMATION
The true prevalence of pericardial disease in most systemic inflammatory and autoimmune diseases is difficult to determine from current data. But advances in serologic testing and imaging techniques have shown cardiac involvement in a number of inflammatory diseases.9
In one study, a serologic autoimmune workup in patients with acute pericarditis found that 2% had collagen vascular disease.9 Pericardial involvement is likely in systemic lupus erythematosus,10 and a postmortem study of patients with systemic sclerosis found that 72% had pericarditis.11 Mixed connective tissue disease has been associated with pericarditis in 29% of cases and 56% in autopsy studies.12,13 Pericarditis may be the initial manifestation of vasculitis—eg, Takayasu arteritis or granulomatosis with polyangiitis (formerly known as Wegener granulomatosis).
Other diseases with pericardial involvement include Still disease, Sjögren syndrome, sarcoidosis, and inflammatory bowel disease. Symptomatic pericarditis occurs in about 25% of patients with Sjögren syndrome and asymptomatic pericardial involvement in more than half. Autopsy studies reported pericardial involvement in up to 80% of patients with systemic lupus erythematosus. Cardiac tamponade occurs in fewer than 2%, and constrictive pericarditis is extremely rare.5,9–11
RECOMMENDATIONS
Patients with a first episode of pericarditis should be treated with an anti-inflammatory medication, with no comprehensive testing for autoimmune disease. An evaluation for autoimmune and infectious disease should be carried out in patients with fever (temperature > 38°C; 100.4°F), recurrent pericarditis, recurrent large pericardial effusion or tamponade, or night sweats despite conventional medical therapy. Signs of systemic disease such as renal failure, elevated liver enzymes, or skin rash merit further evaluation.
Prospective studies using appropriate serologic testing and imaging are needed to determine the correlation between myopericardial involvement and inflammatory diseases because of increased morbidity and mortality in several of these diseases.
- Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet 2004; 363:717–727.
- Alraies MC, Klein AL. Should we still use electrocardiography to diagnose pericardial disease? Cleve Clin J Med 2013; 80:97–100.
- Imazio M, Brucato A, Cemin R, et al; ICAP Investigators. A randomized trial of colchicine for acute pericarditis. N Engl J Med 2013; 369:1522–1528.
- Imazio M, Cecchi E, Demichelis B, et al. Indicators of poor prognosis of acute pericarditis. Circulation 2007; 115:2739–2744.
- Zayas R, Anguita M, Torres F, et al. Incidence of specific etiology and role of methods for specific etiologic diagnosis of primary acute pericarditis. Am J Cardiol 1995; 75:378–382.
- Verhaert D, Gabriel RS, Johnston D, Lytle BW, Desai MY, Klein AL. The role of multimodality imaging in the management of pericardial disease. Circ Cardiovasc Imaging 2010; 3:333–343.
- Klein AL, Abbara S, Agler DA, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2013; 26:965–1012.e15.
- Yingchoncharoen T, Alraies MC, Kwon DH, Rodriguez ER, Tan CD, Klein AL. Emerging role of multimodality imaging in management of inflammatory pericardial diseases. Expert Rev Cardiovasc Ther 2013; 11:1211–1225.
- Knockaert DC. Cardiac involvement in systemic inflammatory diseases. Eur Heart J 2007; 28:1797–1804.
- Doria A, Iaccarino L, Sarzi-Puttini P, Atzeni F, Turriel M, Petri M. Cardiac involvement in systemic lupus erythematosus. Lupus 2005; 14:683–686.
- Byers RJ, Marshall DA, Freemont AJ. Pericardial involvement in systemic sclerosis. Ann Rheum Dis 1997; 56:393–394.
- Kasukawa R. Mixed connective tissue disease. Intern Med 1999; 38:386–393.
- Bezerra MC, Saraiva F Jr, Carvalho JF, Caleiro MT, Goncalves CR, Borba EF. Cardiac tamponade due to massive pericardial effusion in mixed connective tissue disease: reversal with steroid therapy. Lupus 2004; 13:618–620.
Pericarditis is in most cases a one-time disease simply treated with anti-inflammatory drugs. It requires no extensive workup for systemic inflammatory or autoimmune disease. Further evaluation is required for patients who have recurrent pericarditis resistant to conventional therapy or pericarditis with manifestations of systemic disease.
ACUTE PERICARDITIS
Pericardial disease has different presentations: acute, recurrent, constrictive, effusive-constrictive, and pericardial effusion with or without tamponade. Acute pericarditis is the most common of these and can affect people of all ages. The typical acute manifestations are chest pain (usually pleuritic), a pericardial friction rub, and widespread ST-segment elevation on the electrocardiogram.1,2 The chest pain tends to be sharp and long-lasting; it radiates to the trapezius ridge and increases during respiration or body movements.
Acute pericarditis usually responds to an anti-inflammatory drug such as colchicine 0.6 mg/day for 3 months, a nonsteroidal anti-inflammatory drug such as ibuprofen 600 mg three times a day for 10 days, and in advanced resistant cases, an oral corticosteroid.3,4
Most often, pericarditis is either idiopathic or occurs after a respiratory viral illness. Much less common causes include bacterial infection, postpericardiotomy syndrome, myocardial infarction, primary or metastatic tumors, trauma, radiation, and uremia. However, pericarditis can also be part of the presentation of systemic inflammatory and autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus; hereditary periodic fever syndromes such as familial Mediterranean fever; and systemic-onset juvenile idiopathic arthritis.1,5
In acute pericarditis, a complex workup is usually not justified, since the results will have limited usefulness in the clinical management of the patient. It is most often diagnosed by the presenting symptoms, auscultation, electrocardiography, echocardiography, and chest radiography, and by additional basic tests that include a complete blood cell count, complete metabolic profile, erythrocyte sedimentation rate, and C-reactive protein level. However, if pericarditis does not respond to anti-inflammatory treatment and if an autoimmune or infectious disease is suspected, further evaluation may include antinuclear antibody testing and testing for human immunodeficiency virus and tuberculosis. If the diagnosis of acute pericarditis remains uncertain, cardiac magnetic resonance imaging (MRI) may be useful.
RECURRENT PERICARDITIS
Although acute pericarditis most often has a benign course and responds well to anti-inflammatory drugs, 20% to 30% of patients who have a first attack of acute pericarditis have a recurrence, and up to 50% of patients who have one recurrence will have another.3,4
Disease activity can be followed with serial testing of inflammatory markers—eg, erythrocyte sedimentation rate and C-reactive protein level. Echocardiography, cardiac computed tomography, and cardiac MRI can characterize active inflammation, edema, pericardial thickness, and pericardial effusion.6–8
Recurrent pericarditis is often resistant to standard therapy and requires corticosteroids in high doses, which paradoxically can increase the risk of recurrence. Therefore, further workup for underlying autoimmune disease, systemic inflammatory disease, or infection is necessary. More potent immunosuppressive therapy may be required, not only in pericarditis associated with systemic autoimmune or inflammatory conditions, but even in idiopathic recurrent pericarditis, either to control symptoms or to mitigate the effects of corticosteroids.
SYSTEMIC INFLAMMATION
The true prevalence of pericardial disease in most systemic inflammatory and autoimmune diseases is difficult to determine from current data. But advances in serologic testing and imaging techniques have shown cardiac involvement in a number of inflammatory diseases.9
In one study, a serologic autoimmune workup in patients with acute pericarditis found that 2% had collagen vascular disease.9 Pericardial involvement is likely in systemic lupus erythematosus,10 and a postmortem study of patients with systemic sclerosis found that 72% had pericarditis.11 Mixed connective tissue disease has been associated with pericarditis in 29% of cases and 56% in autopsy studies.12,13 Pericarditis may be the initial manifestation of vasculitis—eg, Takayasu arteritis or granulomatosis with polyangiitis (formerly known as Wegener granulomatosis).
Other diseases with pericardial involvement include Still disease, Sjögren syndrome, sarcoidosis, and inflammatory bowel disease. Symptomatic pericarditis occurs in about 25% of patients with Sjögren syndrome and asymptomatic pericardial involvement in more than half. Autopsy studies reported pericardial involvement in up to 80% of patients with systemic lupus erythematosus. Cardiac tamponade occurs in fewer than 2%, and constrictive pericarditis is extremely rare.5,9–11
RECOMMENDATIONS
Patients with a first episode of pericarditis should be treated with an anti-inflammatory medication, with no comprehensive testing for autoimmune disease. An evaluation for autoimmune and infectious disease should be carried out in patients with fever (temperature > 38°C; 100.4°F), recurrent pericarditis, recurrent large pericardial effusion or tamponade, or night sweats despite conventional medical therapy. Signs of systemic disease such as renal failure, elevated liver enzymes, or skin rash merit further evaluation.
Prospective studies using appropriate serologic testing and imaging are needed to determine the correlation between myopericardial involvement and inflammatory diseases because of increased morbidity and mortality in several of these diseases.
Pericarditis is in most cases a one-time disease simply treated with anti-inflammatory drugs. It requires no extensive workup for systemic inflammatory or autoimmune disease. Further evaluation is required for patients who have recurrent pericarditis resistant to conventional therapy or pericarditis with manifestations of systemic disease.
ACUTE PERICARDITIS
Pericardial disease has different presentations: acute, recurrent, constrictive, effusive-constrictive, and pericardial effusion with or without tamponade. Acute pericarditis is the most common of these and can affect people of all ages. The typical acute manifestations are chest pain (usually pleuritic), a pericardial friction rub, and widespread ST-segment elevation on the electrocardiogram.1,2 The chest pain tends to be sharp and long-lasting; it radiates to the trapezius ridge and increases during respiration or body movements.
Acute pericarditis usually responds to an anti-inflammatory drug such as colchicine 0.6 mg/day for 3 months, a nonsteroidal anti-inflammatory drug such as ibuprofen 600 mg three times a day for 10 days, and in advanced resistant cases, an oral corticosteroid.3,4
Most often, pericarditis is either idiopathic or occurs after a respiratory viral illness. Much less common causes include bacterial infection, postpericardiotomy syndrome, myocardial infarction, primary or metastatic tumors, trauma, radiation, and uremia. However, pericarditis can also be part of the presentation of systemic inflammatory and autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus; hereditary periodic fever syndromes such as familial Mediterranean fever; and systemic-onset juvenile idiopathic arthritis.1,5
In acute pericarditis, a complex workup is usually not justified, since the results will have limited usefulness in the clinical management of the patient. It is most often diagnosed by the presenting symptoms, auscultation, electrocardiography, echocardiography, and chest radiography, and by additional basic tests that include a complete blood cell count, complete metabolic profile, erythrocyte sedimentation rate, and C-reactive protein level. However, if pericarditis does not respond to anti-inflammatory treatment and if an autoimmune or infectious disease is suspected, further evaluation may include antinuclear antibody testing and testing for human immunodeficiency virus and tuberculosis. If the diagnosis of acute pericarditis remains uncertain, cardiac magnetic resonance imaging (MRI) may be useful.
RECURRENT PERICARDITIS
Although acute pericarditis most often has a benign course and responds well to anti-inflammatory drugs, 20% to 30% of patients who have a first attack of acute pericarditis have a recurrence, and up to 50% of patients who have one recurrence will have another.3,4
Disease activity can be followed with serial testing of inflammatory markers—eg, erythrocyte sedimentation rate and C-reactive protein level. Echocardiography, cardiac computed tomography, and cardiac MRI can characterize active inflammation, edema, pericardial thickness, and pericardial effusion.6–8
Recurrent pericarditis is often resistant to standard therapy and requires corticosteroids in high doses, which paradoxically can increase the risk of recurrence. Therefore, further workup for underlying autoimmune disease, systemic inflammatory disease, or infection is necessary. More potent immunosuppressive therapy may be required, not only in pericarditis associated with systemic autoimmune or inflammatory conditions, but even in idiopathic recurrent pericarditis, either to control symptoms or to mitigate the effects of corticosteroids.
SYSTEMIC INFLAMMATION
The true prevalence of pericardial disease in most systemic inflammatory and autoimmune diseases is difficult to determine from current data. But advances in serologic testing and imaging techniques have shown cardiac involvement in a number of inflammatory diseases.9
In one study, a serologic autoimmune workup in patients with acute pericarditis found that 2% had collagen vascular disease.9 Pericardial involvement is likely in systemic lupus erythematosus,10 and a postmortem study of patients with systemic sclerosis found that 72% had pericarditis.11 Mixed connective tissue disease has been associated with pericarditis in 29% of cases and 56% in autopsy studies.12,13 Pericarditis may be the initial manifestation of vasculitis—eg, Takayasu arteritis or granulomatosis with polyangiitis (formerly known as Wegener granulomatosis).
Other diseases with pericardial involvement include Still disease, Sjögren syndrome, sarcoidosis, and inflammatory bowel disease. Symptomatic pericarditis occurs in about 25% of patients with Sjögren syndrome and asymptomatic pericardial involvement in more than half. Autopsy studies reported pericardial involvement in up to 80% of patients with systemic lupus erythematosus. Cardiac tamponade occurs in fewer than 2%, and constrictive pericarditis is extremely rare.5,9–11
RECOMMENDATIONS
Patients with a first episode of pericarditis should be treated with an anti-inflammatory medication, with no comprehensive testing for autoimmune disease. An evaluation for autoimmune and infectious disease should be carried out in patients with fever (temperature > 38°C; 100.4°F), recurrent pericarditis, recurrent large pericardial effusion or tamponade, or night sweats despite conventional medical therapy. Signs of systemic disease such as renal failure, elevated liver enzymes, or skin rash merit further evaluation.
Prospective studies using appropriate serologic testing and imaging are needed to determine the correlation between myopericardial involvement and inflammatory diseases because of increased morbidity and mortality in several of these diseases.
- Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet 2004; 363:717–727.
- Alraies MC, Klein AL. Should we still use electrocardiography to diagnose pericardial disease? Cleve Clin J Med 2013; 80:97–100.
- Imazio M, Brucato A, Cemin R, et al; ICAP Investigators. A randomized trial of colchicine for acute pericarditis. N Engl J Med 2013; 369:1522–1528.
- Imazio M, Cecchi E, Demichelis B, et al. Indicators of poor prognosis of acute pericarditis. Circulation 2007; 115:2739–2744.
- Zayas R, Anguita M, Torres F, et al. Incidence of specific etiology and role of methods for specific etiologic diagnosis of primary acute pericarditis. Am J Cardiol 1995; 75:378–382.
- Verhaert D, Gabriel RS, Johnston D, Lytle BW, Desai MY, Klein AL. The role of multimodality imaging in the management of pericardial disease. Circ Cardiovasc Imaging 2010; 3:333–343.
- Klein AL, Abbara S, Agler DA, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2013; 26:965–1012.e15.
- Yingchoncharoen T, Alraies MC, Kwon DH, Rodriguez ER, Tan CD, Klein AL. Emerging role of multimodality imaging in management of inflammatory pericardial diseases. Expert Rev Cardiovasc Ther 2013; 11:1211–1225.
- Knockaert DC. Cardiac involvement in systemic inflammatory diseases. Eur Heart J 2007; 28:1797–1804.
- Doria A, Iaccarino L, Sarzi-Puttini P, Atzeni F, Turriel M, Petri M. Cardiac involvement in systemic lupus erythematosus. Lupus 2005; 14:683–686.
- Byers RJ, Marshall DA, Freemont AJ. Pericardial involvement in systemic sclerosis. Ann Rheum Dis 1997; 56:393–394.
- Kasukawa R. Mixed connective tissue disease. Intern Med 1999; 38:386–393.
- Bezerra MC, Saraiva F Jr, Carvalho JF, Caleiro MT, Goncalves CR, Borba EF. Cardiac tamponade due to massive pericardial effusion in mixed connective tissue disease: reversal with steroid therapy. Lupus 2004; 13:618–620.
- Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet 2004; 363:717–727.
- Alraies MC, Klein AL. Should we still use electrocardiography to diagnose pericardial disease? Cleve Clin J Med 2013; 80:97–100.
- Imazio M, Brucato A, Cemin R, et al; ICAP Investigators. A randomized trial of colchicine for acute pericarditis. N Engl J Med 2013; 369:1522–1528.
- Imazio M, Cecchi E, Demichelis B, et al. Indicators of poor prognosis of acute pericarditis. Circulation 2007; 115:2739–2744.
- Zayas R, Anguita M, Torres F, et al. Incidence of specific etiology and role of methods for specific etiologic diagnosis of primary acute pericarditis. Am J Cardiol 1995; 75:378–382.
- Verhaert D, Gabriel RS, Johnston D, Lytle BW, Desai MY, Klein AL. The role of multimodality imaging in the management of pericardial disease. Circ Cardiovasc Imaging 2010; 3:333–343.
- Klein AL, Abbara S, Agler DA, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2013; 26:965–1012.e15.
- Yingchoncharoen T, Alraies MC, Kwon DH, Rodriguez ER, Tan CD, Klein AL. Emerging role of multimodality imaging in management of inflammatory pericardial diseases. Expert Rev Cardiovasc Ther 2013; 11:1211–1225.
- Knockaert DC. Cardiac involvement in systemic inflammatory diseases. Eur Heart J 2007; 28:1797–1804.
- Doria A, Iaccarino L, Sarzi-Puttini P, Atzeni F, Turriel M, Petri M. Cardiac involvement in systemic lupus erythematosus. Lupus 2005; 14:683–686.
- Byers RJ, Marshall DA, Freemont AJ. Pericardial involvement in systemic sclerosis. Ann Rheum Dis 1997; 56:393–394.
- Kasukawa R. Mixed connective tissue disease. Intern Med 1999; 38:386–393.
- Bezerra MC, Saraiva F Jr, Carvalho JF, Caleiro MT, Goncalves CR, Borba EF. Cardiac tamponade due to massive pericardial effusion in mixed connective tissue disease: reversal with steroid therapy. Lupus 2004; 13:618–620.
Does massive hemoptysis always merit diagnostic bronchoscopy?
Yes, all patients with massive hemoptysis should undergo diagnostic bronchoscopy. The procedure plays an important role in protecting the airway, maintaining ventilation, finding the site and underlying cause of the bleeding, and in some cases stopping the bleeding, either temporarily or definitively.
Frightening to patients, massive hemoptysis is a medical emergency and demands immediate attention by an experienced pulmonary team.1 Hemoptysis can be the initial presentation of an underlying infectious, autoimmune, or malignant disorder (Table 1).2 Fortunately, most cases of hemoptysis are not massive or life-threatening.1
WHAT IS ‘MASSIVE’ HEMOPTYSIS?
Numerous studies have defined massive hemoptysis on the basis of the volume of blood lost over time, eg, more than 1 L in 24 hours or more than 400 mL in 6 hours.
Ibrahim3 has proposed that we move away from using the word “massive,” which is not useful, and instead think in terms of “life-threatening” hemoptysis, defined as any of the following:
- More than 100 mL of blood lost in 24 hours (a low number, but blood loss is hard to estimate accurately)
- Causing abnormal gas exchange due to airway obstruction
- Causing hemodynamic instability.
In this article, we use the traditional “massive” terminology.
BRONCHOSCOPY IS SUPERIOR TO IMAGING FOR DIAGNOSIS
Radiography can help identify the side or the site of bleeding in 33% to 82% of patients, and computed tomography can in 70% to 88.5%.4 Magnetic resonance imaging may also have a role; one study found it useful in cases of thoracic endometriosis during the quiescent stage.5 However, transferring a patient who is actively bleeding out of the intensive care unit for imaging can be challenging.
Flexible bronchoscopy is superior to radiographic imaging in evaluating massive hemoptysis: it can be performed at the bed-side and can include therapeutic procedures to control the bleeding until the patient can undergo a definitive therapeutic procedure.6 It has been found helpful in identifying the side of bleeding in 73% to 93% of cases of massive hemoptysis.6
However, one should consider starting the procedure with a rigid bronchoscope, which protects the airway better and allows for better ventilation during the procedure than a flexible one. One can use it to isolate the nonbleeding lung and to apply pressure to the bleeding site if it is in the main bronchus.7 Measuring 12 mm in diameter, a rigid scope cannot go as far into the lung as a flexible bronchoscope (measuring 6.4 mm), but a flexible bronchoscope can be introduced through the rigid bronchoscope to go further in.
MANAGEMENT OPTIONS
The management team should include an anesthesiologist, an intensivist, a thoracic surgeon, an interventional radiologist, and an interventional pulmonologist.
In the intensive care unit, the patient should be placed in the lateral decubitus position on the bleeding side. To maintain ventilation, the nonbleeding lung should be intubated with a large-bore endotracheal tube (internal diameter 8.5–9.0 mm) or, ideally, with a rigid bronchoscope.6 Meanwhile, the patient’s circulatory status should be stabilized with adequate fluid resuscitation and transfusion of blood products, with close monitoring.
Once the bleeding site is found, a bronchoscopic treatment is selected based on the cause of the bleeding. Massive hemoptysis usually arises from high-pressure bronchial vessels (90%) or, less commonly, from non-bronchial vessels or capillaries (10%).8 A variety of agents (eg, cold saline lavage, epinephrine 1:20,000) can be instilled through the bronchoscope to slow the bleeding and offer better visualization of the airway.6
If a bleeding intrabronchial lesion is identified, such as a malignant tracheobronchial tumor, local coagulation therapy can be applied through the bronchoscope. Options include laser treatment, argon plasma coagulation, cryotherapy, and electrocautery (Figure 1).9,10
If the bleeding persists or cannot be localized to a particular subsegment, an endobronchial balloon plug can be placed proximally (Figure 2). This can be left in place to isolate the bleeding and apply tamponade until a definitive procedure can be performed, such as bronchial artery embolization, radiation therapy, or surgery.
- Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med 2000; 28:1642–1647.
- Abi Khalil S, Gourdier AL, Aoun N, et al. Cystic and cavitary lesions of the lung: imaging characteristics and differential diagnosis [in French]. J Radiol 2010; 91:465–473.
- Ibrahim WH. Massive haemoptysis: the definition should be revised. Eur Respir J 2008; 32:1131–1132.
- Khalil A, Soussan M, Mangiapan G, Fartoukh M, Parrot A, Carette MF. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol 2007; 80:21–25.
- Cassina PC, Hauser M, Kacl G, Imthurn B, Schröder S, Weder W. Catamenial hemoptysis. Diagnosis with MRI. Chest 1997; 111:1447–1450.
- Sakr L, Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration 2010; 80:38–58.
- Conlan AA, Hurwitz SS. Management of massive haemoptysis with the rigid bronchoscope and cold saline lavage. Thorax 1980; 35:901–904.
- Deffebach ME, Charan NB, Lakshminarayan S, Butler J. The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis 1987; 135:463–481.
- Morice RC, Ece T, Ece F, Keus L. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001; 119:781–787.
- Sheski FD, Mathur PN. Cryotherapy, electrocautery, and brachytherapy. Clin Chest Med 1999; 20:123–138.
Yes, all patients with massive hemoptysis should undergo diagnostic bronchoscopy. The procedure plays an important role in protecting the airway, maintaining ventilation, finding the site and underlying cause of the bleeding, and in some cases stopping the bleeding, either temporarily or definitively.
Frightening to patients, massive hemoptysis is a medical emergency and demands immediate attention by an experienced pulmonary team.1 Hemoptysis can be the initial presentation of an underlying infectious, autoimmune, or malignant disorder (Table 1).2 Fortunately, most cases of hemoptysis are not massive or life-threatening.1
WHAT IS ‘MASSIVE’ HEMOPTYSIS?
Numerous studies have defined massive hemoptysis on the basis of the volume of blood lost over time, eg, more than 1 L in 24 hours or more than 400 mL in 6 hours.
Ibrahim3 has proposed that we move away from using the word “massive,” which is not useful, and instead think in terms of “life-threatening” hemoptysis, defined as any of the following:
- More than 100 mL of blood lost in 24 hours (a low number, but blood loss is hard to estimate accurately)
- Causing abnormal gas exchange due to airway obstruction
- Causing hemodynamic instability.
In this article, we use the traditional “massive” terminology.
BRONCHOSCOPY IS SUPERIOR TO IMAGING FOR DIAGNOSIS
Radiography can help identify the side or the site of bleeding in 33% to 82% of patients, and computed tomography can in 70% to 88.5%.4 Magnetic resonance imaging may also have a role; one study found it useful in cases of thoracic endometriosis during the quiescent stage.5 However, transferring a patient who is actively bleeding out of the intensive care unit for imaging can be challenging.
Flexible bronchoscopy is superior to radiographic imaging in evaluating massive hemoptysis: it can be performed at the bed-side and can include therapeutic procedures to control the bleeding until the patient can undergo a definitive therapeutic procedure.6 It has been found helpful in identifying the side of bleeding in 73% to 93% of cases of massive hemoptysis.6
However, one should consider starting the procedure with a rigid bronchoscope, which protects the airway better and allows for better ventilation during the procedure than a flexible one. One can use it to isolate the nonbleeding lung and to apply pressure to the bleeding site if it is in the main bronchus.7 Measuring 12 mm in diameter, a rigid scope cannot go as far into the lung as a flexible bronchoscope (measuring 6.4 mm), but a flexible bronchoscope can be introduced through the rigid bronchoscope to go further in.
MANAGEMENT OPTIONS
The management team should include an anesthesiologist, an intensivist, a thoracic surgeon, an interventional radiologist, and an interventional pulmonologist.
In the intensive care unit, the patient should be placed in the lateral decubitus position on the bleeding side. To maintain ventilation, the nonbleeding lung should be intubated with a large-bore endotracheal tube (internal diameter 8.5–9.0 mm) or, ideally, with a rigid bronchoscope.6 Meanwhile, the patient’s circulatory status should be stabilized with adequate fluid resuscitation and transfusion of blood products, with close monitoring.
Once the bleeding site is found, a bronchoscopic treatment is selected based on the cause of the bleeding. Massive hemoptysis usually arises from high-pressure bronchial vessels (90%) or, less commonly, from non-bronchial vessels or capillaries (10%).8 A variety of agents (eg, cold saline lavage, epinephrine 1:20,000) can be instilled through the bronchoscope to slow the bleeding and offer better visualization of the airway.6
If a bleeding intrabronchial lesion is identified, such as a malignant tracheobronchial tumor, local coagulation therapy can be applied through the bronchoscope. Options include laser treatment, argon plasma coagulation, cryotherapy, and electrocautery (Figure 1).9,10
If the bleeding persists or cannot be localized to a particular subsegment, an endobronchial balloon plug can be placed proximally (Figure 2). This can be left in place to isolate the bleeding and apply tamponade until a definitive procedure can be performed, such as bronchial artery embolization, radiation therapy, or surgery.
Yes, all patients with massive hemoptysis should undergo diagnostic bronchoscopy. The procedure plays an important role in protecting the airway, maintaining ventilation, finding the site and underlying cause of the bleeding, and in some cases stopping the bleeding, either temporarily or definitively.
Frightening to patients, massive hemoptysis is a medical emergency and demands immediate attention by an experienced pulmonary team.1 Hemoptysis can be the initial presentation of an underlying infectious, autoimmune, or malignant disorder (Table 1).2 Fortunately, most cases of hemoptysis are not massive or life-threatening.1
WHAT IS ‘MASSIVE’ HEMOPTYSIS?
Numerous studies have defined massive hemoptysis on the basis of the volume of blood lost over time, eg, more than 1 L in 24 hours or more than 400 mL in 6 hours.
Ibrahim3 has proposed that we move away from using the word “massive,” which is not useful, and instead think in terms of “life-threatening” hemoptysis, defined as any of the following:
- More than 100 mL of blood lost in 24 hours (a low number, but blood loss is hard to estimate accurately)
- Causing abnormal gas exchange due to airway obstruction
- Causing hemodynamic instability.
In this article, we use the traditional “massive” terminology.
BRONCHOSCOPY IS SUPERIOR TO IMAGING FOR DIAGNOSIS
Radiography can help identify the side or the site of bleeding in 33% to 82% of patients, and computed tomography can in 70% to 88.5%.4 Magnetic resonance imaging may also have a role; one study found it useful in cases of thoracic endometriosis during the quiescent stage.5 However, transferring a patient who is actively bleeding out of the intensive care unit for imaging can be challenging.
Flexible bronchoscopy is superior to radiographic imaging in evaluating massive hemoptysis: it can be performed at the bed-side and can include therapeutic procedures to control the bleeding until the patient can undergo a definitive therapeutic procedure.6 It has been found helpful in identifying the side of bleeding in 73% to 93% of cases of massive hemoptysis.6
However, one should consider starting the procedure with a rigid bronchoscope, which protects the airway better and allows for better ventilation during the procedure than a flexible one. One can use it to isolate the nonbleeding lung and to apply pressure to the bleeding site if it is in the main bronchus.7 Measuring 12 mm in diameter, a rigid scope cannot go as far into the lung as a flexible bronchoscope (measuring 6.4 mm), but a flexible bronchoscope can be introduced through the rigid bronchoscope to go further in.
MANAGEMENT OPTIONS
The management team should include an anesthesiologist, an intensivist, a thoracic surgeon, an interventional radiologist, and an interventional pulmonologist.
In the intensive care unit, the patient should be placed in the lateral decubitus position on the bleeding side. To maintain ventilation, the nonbleeding lung should be intubated with a large-bore endotracheal tube (internal diameter 8.5–9.0 mm) or, ideally, with a rigid bronchoscope.6 Meanwhile, the patient’s circulatory status should be stabilized with adequate fluid resuscitation and transfusion of blood products, with close monitoring.
Once the bleeding site is found, a bronchoscopic treatment is selected based on the cause of the bleeding. Massive hemoptysis usually arises from high-pressure bronchial vessels (90%) or, less commonly, from non-bronchial vessels or capillaries (10%).8 A variety of agents (eg, cold saline lavage, epinephrine 1:20,000) can be instilled through the bronchoscope to slow the bleeding and offer better visualization of the airway.6
If a bleeding intrabronchial lesion is identified, such as a malignant tracheobronchial tumor, local coagulation therapy can be applied through the bronchoscope. Options include laser treatment, argon plasma coagulation, cryotherapy, and electrocautery (Figure 1).9,10
If the bleeding persists or cannot be localized to a particular subsegment, an endobronchial balloon plug can be placed proximally (Figure 2). This can be left in place to isolate the bleeding and apply tamponade until a definitive procedure can be performed, such as bronchial artery embolization, radiation therapy, or surgery.
- Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med 2000; 28:1642–1647.
- Abi Khalil S, Gourdier AL, Aoun N, et al. Cystic and cavitary lesions of the lung: imaging characteristics and differential diagnosis [in French]. J Radiol 2010; 91:465–473.
- Ibrahim WH. Massive haemoptysis: the definition should be revised. Eur Respir J 2008; 32:1131–1132.
- Khalil A, Soussan M, Mangiapan G, Fartoukh M, Parrot A, Carette MF. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol 2007; 80:21–25.
- Cassina PC, Hauser M, Kacl G, Imthurn B, Schröder S, Weder W. Catamenial hemoptysis. Diagnosis with MRI. Chest 1997; 111:1447–1450.
- Sakr L, Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration 2010; 80:38–58.
- Conlan AA, Hurwitz SS. Management of massive haemoptysis with the rigid bronchoscope and cold saline lavage. Thorax 1980; 35:901–904.
- Deffebach ME, Charan NB, Lakshminarayan S, Butler J. The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis 1987; 135:463–481.
- Morice RC, Ece T, Ece F, Keus L. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001; 119:781–787.
- Sheski FD, Mathur PN. Cryotherapy, electrocautery, and brachytherapy. Clin Chest Med 1999; 20:123–138.
- Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med 2000; 28:1642–1647.
- Abi Khalil S, Gourdier AL, Aoun N, et al. Cystic and cavitary lesions of the lung: imaging characteristics and differential diagnosis [in French]. J Radiol 2010; 91:465–473.
- Ibrahim WH. Massive haemoptysis: the definition should be revised. Eur Respir J 2008; 32:1131–1132.
- Khalil A, Soussan M, Mangiapan G, Fartoukh M, Parrot A, Carette MF. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol 2007; 80:21–25.
- Cassina PC, Hauser M, Kacl G, Imthurn B, Schröder S, Weder W. Catamenial hemoptysis. Diagnosis with MRI. Chest 1997; 111:1447–1450.
- Sakr L, Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration 2010; 80:38–58.
- Conlan AA, Hurwitz SS. Management of massive haemoptysis with the rigid bronchoscope and cold saline lavage. Thorax 1980; 35:901–904.
- Deffebach ME, Charan NB, Lakshminarayan S, Butler J. The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis 1987; 135:463–481.
- Morice RC, Ece T, Ece F, Keus L. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001; 119:781–787.
- Sheski FD, Mathur PN. Cryotherapy, electrocautery, and brachytherapy. Clin Chest Med 1999; 20:123–138.
A serious complication of a common stress test
To the Editor: We read with interest the article by Drs. Buitrago et al in the May 2014 issue of Cleveland Clinic Journal of Medicine, “Syncope during a pharmacologic nuclear stress test.”1 It highlights a known, serious interaction between adenosine and dipyridamole (the latter contained in the aspirin-dipyridamole combination Aggrenox) and associated asystole in patients undergoing pharmacologic cardiac stress testing. This interaction is known in the cardiology literature, as it was noted in the current guidelines for pharmacologic stress testing.2 However, I would like to discuss a few points with the authors for a better understanding of the case.
First, the underlying rhythm before the development of complete atrioventricular (AV) dissociation and asystole was significant for second-degree AV block (Mobitz type I, Wenckebach). Second- or third-degree AV block is considered a contraindication to adenosine because of the risk of exacerbating these conditions. This underlying AV nodal disease made dipyridamole not the only culprit. In addition, the patient had been on two agents (labetalol and clonidine) that have AV nodal-blocking properties. Electrolyte imbalances such as hypokalemia, hypomagnesemia, and hypocalcemia are another reason for delayed conduction and PR prolongation, and electrolyte levels should be checked and corrected properly before the stress test or coronary angiography. It would have been helpful if the authors had discussed these points for a better understanding of the drug-drug interaction.
Because of the increasing trend to admit patients with chest pain to observation units to rule out myocardial infarction, the case has a valuable teaching point, especially for hospitalists and emergency physicians in charge of patients admitted with chest pain.3 Since cardiologists rarely get involved in the care of these patients, careful review of medications before scheduling stress testing is of ultimate importance and should be emphasized in the discussion.
Lastly, the number of combined medications that are available commercially is increasing, which puts patients at higher risk of drug interactions. Hospitalists and internists taking care of patients, especially elderly patients, admitted from nursing homes and taking multiple medications should pay extra attention when reviewing medications with brand names.4,5 Furthermore, a 12-lead electrocardiogram should be reviewed, with special attention to the PR interval and QT segment. A pharmacy consultation could be valuable, especially in patients taking multiple drugs.6
- Buitrago I, Wolinsky D, Asher CR. Syncope during a pharmacologic nuclear stress test. Cleve Clin J Med 2014; 81:279–280.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
- Graff LG, Dallara J, Ross MA, et al. Impact on the care of the emergency department chest pain patient from the chest pain evaluation registry (CHEPER) study. Am J Cardiol 1997; 80:563–568.
- Samaras N, Chevalley T, Samaras D, Gold G. Older patients in the emergency department: a review. Ann Emerg Med 2010; 56:261–269.
- Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium.” JAMA 2010; 304:1592–1601.
- Scott IA, Gray LC, Martin JH, Mitchell CA. Minimizing inappropriate medications in older populations: a 10-step conceptual framework. Am J Med 2012; 125:529–537.
To the Editor: We read with interest the article by Drs. Buitrago et al in the May 2014 issue of Cleveland Clinic Journal of Medicine, “Syncope during a pharmacologic nuclear stress test.”1 It highlights a known, serious interaction between adenosine and dipyridamole (the latter contained in the aspirin-dipyridamole combination Aggrenox) and associated asystole in patients undergoing pharmacologic cardiac stress testing. This interaction is known in the cardiology literature, as it was noted in the current guidelines for pharmacologic stress testing.2 However, I would like to discuss a few points with the authors for a better understanding of the case.
First, the underlying rhythm before the development of complete atrioventricular (AV) dissociation and asystole was significant for second-degree AV block (Mobitz type I, Wenckebach). Second- or third-degree AV block is considered a contraindication to adenosine because of the risk of exacerbating these conditions. This underlying AV nodal disease made dipyridamole not the only culprit. In addition, the patient had been on two agents (labetalol and clonidine) that have AV nodal-blocking properties. Electrolyte imbalances such as hypokalemia, hypomagnesemia, and hypocalcemia are another reason for delayed conduction and PR prolongation, and electrolyte levels should be checked and corrected properly before the stress test or coronary angiography. It would have been helpful if the authors had discussed these points for a better understanding of the drug-drug interaction.
Because of the increasing trend to admit patients with chest pain to observation units to rule out myocardial infarction, the case has a valuable teaching point, especially for hospitalists and emergency physicians in charge of patients admitted with chest pain.3 Since cardiologists rarely get involved in the care of these patients, careful review of medications before scheduling stress testing is of ultimate importance and should be emphasized in the discussion.
Lastly, the number of combined medications that are available commercially is increasing, which puts patients at higher risk of drug interactions. Hospitalists and internists taking care of patients, especially elderly patients, admitted from nursing homes and taking multiple medications should pay extra attention when reviewing medications with brand names.4,5 Furthermore, a 12-lead electrocardiogram should be reviewed, with special attention to the PR interval and QT segment. A pharmacy consultation could be valuable, especially in patients taking multiple drugs.6
To the Editor: We read with interest the article by Drs. Buitrago et al in the May 2014 issue of Cleveland Clinic Journal of Medicine, “Syncope during a pharmacologic nuclear stress test.”1 It highlights a known, serious interaction between adenosine and dipyridamole (the latter contained in the aspirin-dipyridamole combination Aggrenox) and associated asystole in patients undergoing pharmacologic cardiac stress testing. This interaction is known in the cardiology literature, as it was noted in the current guidelines for pharmacologic stress testing.2 However, I would like to discuss a few points with the authors for a better understanding of the case.
First, the underlying rhythm before the development of complete atrioventricular (AV) dissociation and asystole was significant for second-degree AV block (Mobitz type I, Wenckebach). Second- or third-degree AV block is considered a contraindication to adenosine because of the risk of exacerbating these conditions. This underlying AV nodal disease made dipyridamole not the only culprit. In addition, the patient had been on two agents (labetalol and clonidine) that have AV nodal-blocking properties. Electrolyte imbalances such as hypokalemia, hypomagnesemia, and hypocalcemia are another reason for delayed conduction and PR prolongation, and electrolyte levels should be checked and corrected properly before the stress test or coronary angiography. It would have been helpful if the authors had discussed these points for a better understanding of the drug-drug interaction.
Because of the increasing trend to admit patients with chest pain to observation units to rule out myocardial infarction, the case has a valuable teaching point, especially for hospitalists and emergency physicians in charge of patients admitted with chest pain.3 Since cardiologists rarely get involved in the care of these patients, careful review of medications before scheduling stress testing is of ultimate importance and should be emphasized in the discussion.
Lastly, the number of combined medications that are available commercially is increasing, which puts patients at higher risk of drug interactions. Hospitalists and internists taking care of patients, especially elderly patients, admitted from nursing homes and taking multiple medications should pay extra attention when reviewing medications with brand names.4,5 Furthermore, a 12-lead electrocardiogram should be reviewed, with special attention to the PR interval and QT segment. A pharmacy consultation could be valuable, especially in patients taking multiple drugs.6
- Buitrago I, Wolinsky D, Asher CR. Syncope during a pharmacologic nuclear stress test. Cleve Clin J Med 2014; 81:279–280.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
- Graff LG, Dallara J, Ross MA, et al. Impact on the care of the emergency department chest pain patient from the chest pain evaluation registry (CHEPER) study. Am J Cardiol 1997; 80:563–568.
- Samaras N, Chevalley T, Samaras D, Gold G. Older patients in the emergency department: a review. Ann Emerg Med 2010; 56:261–269.
- Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium.” JAMA 2010; 304:1592–1601.
- Scott IA, Gray LC, Martin JH, Mitchell CA. Minimizing inappropriate medications in older populations: a 10-step conceptual framework. Am J Med 2012; 125:529–537.
- Buitrago I, Wolinsky D, Asher CR. Syncope during a pharmacologic nuclear stress test. Cleve Clin J Med 2014; 81:279–280.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
- Graff LG, Dallara J, Ross MA, et al. Impact on the care of the emergency department chest pain patient from the chest pain evaluation registry (CHEPER) study. Am J Cardiol 1997; 80:563–568.
- Samaras N, Chevalley T, Samaras D, Gold G. Older patients in the emergency department: a review. Ann Emerg Med 2010; 56:261–269.
- Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium.” JAMA 2010; 304:1592–1601.
- Scott IA, Gray LC, Martin JH, Mitchell CA. Minimizing inappropriate medications in older populations: a 10-step conceptual framework. Am J Med 2012; 125:529–537.