User login
Premature ventricular contractions: Reassure or refer?
Doctor, my heart ______.” Fill in the blank with: skips, flip-flops, hiccups, stops, beats in my throat or chest, or any of the various ways patients describe palpitations. One cannot practice clinical medicine and not see patients with some variation of this chief complaint.1–3 Not every patient who complains of palpitations will be found to have premature ventricular contractions (PVCs), but PVCs are often part of the clinical problem.
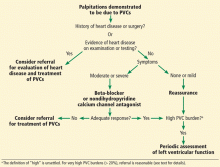
This review focuses on the initial evaluation and management of PVCs in the primary care setting (Figure 1). It is not intended to be a comprehensive review of the pathophysiology, electrophysiology, or localization and ablation of PVCs. We will discuss approaches to the initial therapy of symptomatic PVCs. We will not discuss catheter-based therapy in detail except for which patients might benefit from referral to a clinical cardiac electrophysiologist.
Findings that should prompt consideration for referral to a specialist (“red flags”) are summarized at the end of each section. The type of specialist depends to a degree on the cardiology practice available to the referring physician. In our practice, such patients are typically seen by an electrophysiologist. In other practices, a general cardiologist might see such patients initially.
INITIAL EVALUATION
A primary concern of any patient presenting with a new symptom is whether the symptom is a marker of serious risk to health or life. In a patient with palpitations, the answer depends in large part on whether he or she has underlying structural heart disease—and that is the focus of the initial evaluation.
History: Information to ascertain
- When did the patient first notice the palpitations?
- Had there been any significant life events, either illness or emotional stress, at the time the palpitations began?
- Does the patient have a known history of heart disease (myocardial infarction, heart surgery, valvular heart disease, heart failure)?
- What medications is the patient taking?
- Does the patient take any dietary or health supplements? Ask specifically about any supplements taken to help with weight loss or increase energy levels. Almost all of them contain caffeine or other “natural” sympathomimetic agents. Also ask specifically about illicit drug use. If the patient is accompanied by a parent or partner, this question can be challenging.
- When do the palpitations occur? At random? At rest? With exercise? Time of day? In relation to the menstrual cycle? (More about this later.)
- Does anything make the palpitations better? If they occur at rest, does activity make them better or worse?
- Are there symptoms of heart failure, such as dyspnea on exertion, early fatigue, decline in exercise or exertional capacity, orthopnea, or paroxysmal nocturnal dyspnea?
- Are there symptoms suggesting cardiac ischemia, such as substernal chest pain or discomfort, chest pain or discomfort brought on by or made worse by exertion, or chest pain relieved with rest or sublingual nitroglycerin?
- Have the palpitations ever been associated with syncope? Keep in mind that syncope is transient loss of consciousness that spontaneously resolves with no features to suggest seizures.4 Thus, a patient who reports he or she “blacks out” with the palpitations but never falls or slumps has not had loss of consciousness and therefore has not had syncope.5
- Is there any history of unexplained death in the family, especially in younger people? Is there a history of unexplained accidental death in young family members?
Red flags obtained from the history
- Syncope related to palpitations
- Palpitations triggered by activity or exertion
- Known significant heart disease, congenital heart disease, or history of heart surgery
- Family history of premature unexplained sudden death in a first-degree relative.
Physical examination
The physical examination should focus on detecting any signs of underlying heart or vascular disease, eg:
- Significant murmurs
- Abnormal S3 or S4
- Displaced and diffuse point of maximal impulse or precordial heave
- Signs of right or left heart failure, or both, eg, peripheral edema, elevation of jugular venous pulse, rales, S3, S4.
Electrocardiography
We consider 12-lead electrocardiography (ECG) a part of the initial examination and assessment, not an ancillary test. One cannot evaluate a patient’s complaint of palpitations without ECG. Ideally, ECG should include a long 12-lead rhythm strip. The clinician should look for any evidence of underlying structural heart disease, eg:
- Pathologic Q waves
- Long QT interval
- ST-segment elevation in leads V1 and V2 consistent with a Brugada pattern
- Epsilon waves (seen in right ventricular arrhythmogenic cardiomyopathy).
Examples of the above can be found at sites such as ecgpedia.org.
Red flags in the physical examination and ECG
Any of the above findings on physical examination or ECG should prompt consideration of early referral, even though we have yet to establish that the palpitations are due to PVCs. Early consultation is suggested not for treatment of the palpitations but for further evaluation of structural heart disease.
Assuming the history, physical examination, and electrocardiography do not demonstrate any reasons for early cardiology or electrophysiology consultation, what’s next?
FURTHER EVALUATION: EXTENDED MONITORING
With luck, the patient’s typical palpitations will occur during ECG, in which case the palpitations can reasonably be attributed to PVCs. If not, monitoring is required to establish the cause of the patient’s symptoms.
The type of monitoring to order depends on the frequency of the palpitations. If the patient reports several episodes per day, then a 24- or 48-hour Holter monitor should both allow for a diagnosis and document the PVC burden (ie, the percent of the patient’s heartbeats that are PVCs), or the burden of whatever is the cause of the patient’s palpitations.
If the palpitations are less frequent, a 14-to-30-day monitor should be considered. A standard event recorder can confirm that the palpitations are due to PVCs but does not tell you the PVC burden. For that, a system capable of mobile outpatient cardiac telemetry is needed. Several such systems are commercially available.
A Holter monitor or other monitoring system is useful in determining whether the PVCs are unifocal (all look the same) or multifocal (have more than one morphology) and whether, in addition to PVCs, the patient has nonsustained ventricular tachycardia or sustained ventricular tachycardia (by definition lasting longer than 30 seconds or associated with symptoms of hemodynamic compromise such as near-syncope). Even if the patient has nonsustained ventricular tachycardia, if the heart is structurally normal the prognosis remains excellent.
Given the importance of knowing whether the patient has structural heart disease, we have a low threshold for ordering echocardiography, especially if nonsustained ventricular tachycardia has been documented. The finding of significant systolic dysfunction on echocardiography should prompt a cardiology consultation even if the physical examination is normal. In patients who have a high PVC burden, echocardiography is used to monitor for arrhythmia-induced cardiomyopathy.6
If the patient’s symptoms occur with activity, an exercise study can be helpful. It is important to either supervise the study oneself or, at the least, alert the exercise laboratory staff that the study is being performed to evaluate for exercise-induced arrhythmias. If the exercise study induces sustained ventricular tachycardia, the patient is almost invariably admitted to the hospital and inpatient consultation with an electrophysiologist is obtained.
Red flags on extended monitoring
- Multifocal PVCs or nonsustained ventricular tachycardia
- Polymorphic nonsustained ventricular tachycardia
- Sustained ventricular tachycardia; this still may be idiopathic and have a benign prognosis but generally should prompt referral.
If at this point no red flags have been uncovered, monitoring has established the patient’s symptoms are due to PVCs, and our examination and ancillary testing have established the patient has a structurally normal heart, what is the next step?
IDIOPATHIC PVCs
PVCs in a patient with a structurally normal heart are called “idiopathic.” Often, these patients will also be found to have nonsustained ventricular tachycardia, and may also be classified as having “idiopathic ventricular tachycardia.” Regardless of whether the patient has PVCs, nonsustained ventricular tachycardia, or both, the management approach is the same.
Roughly 60% to 80% of idiopathic PVCs originate from the right ventricle, in particular the right ventricular outflow tract.7 Patients with outflow tract PVCs typically present between the ages of 30 and 50 but range from adolescents to elders. More women than men are affected.
Outflow tract PVCs often occur only, or at much greater frequency, within a range of heart rates.8 Individual patients may have different ranges of heart rates at which their PVCs are more frequent. Patients may complain that their palpitations are more frequent at rest, early in exercise, at a peak of exercise, or early in recovery from exercise. It is not unusual for patients with outflow tract PVCs to report that activity reduces the frequency of their palpitations. Women might note an increase in their symptoms during menstruation.9 It is not clear, however, that this perceived increase in palpitations is in fact due to an increase in the number of PVCs.10
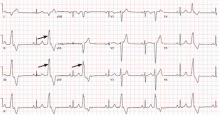
If the patient’s PVCs have not been captured on 12-lead ECG (ie, if it is not seen in all 12 leads), 12-lead Holter monitoring, if available, can be helpful. Examination of the morphology of the PVC on 12-lead ECG is extremely helpful. Outflow tract PVCs are the most common cause of idiopathic PVCs and nonsustained ventricular tachycardia and are easily recognizable with 12-lead ECG.
ECG points to the origin of the PVCs
A PVC arising on the right side of the heart will activate the right ventricle first and then the left ventricle. This is analogous to the sequence of ventricular activation in a patient with left bundle-branch block. Not surprisingly, on ECG a right-sided PVC looks similar to the QRS complex seen in left bundle-branch block—similar, but not identical.
When describing PVCs or the morphology of nonsustained ventricular tachycardia, the terms “left bundle-branch block pattern” and “right bundle-branch block pattern” refer to lead V1. If the PVC is negative (or mostly negative) in V1, the PVC has a left bundle-branch block pattern. A PVC that is positive in V1 is said to have a right bundle-branch block pattern and by implication arises from the left side of the heart.
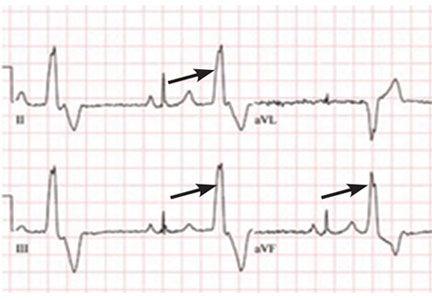
A PVC originating from the top of the heart will move from top to bottom. The electrical axis of the PVC will be directed inferiorly. This means the PVC will be strongly positive in the inferior leads, ie, II, aVF, and III.
The electrocardiogram shown in Figure 2 demonstrates the typical appearance of a right ventricular outflow tract PVC.
If the PVC arises from the left ventricular outflow tract, the axis will still be inferiorly directed. However, the further to the left the origin of the PVC, the earlier the precordial transition will occur (the point at which the PVC is more positive than negative in the precordial leads). A PVC origin far enough to the left will result in a right bundle-branch block pattern PVC.
Not all idiopathic PVCs arise from the outflow tracts. A right bundle branch block pattern PVC does not imply the presence of underlying structural heart disease. PVCs may arise from both the tricuspid and mitral valve annuli, the left ventricular fascicles, or from the epicardium.
Multiple methods have been proposed to locate the origin of the PVC. For example, Park et al reviewed the use of surface ECG in locating the site of origin of ventricular tachycardia.11 All such algorithms should be applied with care, and with awareness of the caveats associated with their use.
Arrhythmogenic right ventricular cardiomyopathy is not benign
Arrhythmogenic right ventricular cardiomyopathy may give rise to PVCs or nonsustained ventricular tachycardia with morphologies similar to those of right ventricular outflow tract PVCs and ventricular tachycardia. The ventricular tachycardia complicating arrhythmogenic cardiomyopathy is, like PVCs arising from the right ventricular outflow tract, commonly associated with exercise or activity.
Unlike right ventricular outflow tract tachycardia, ventricular tachycardia related to arrhythmogenic cardiomyopathy is not benign.12 Distinguishing right ventricular outflow tract tachycardia from tachycardia secondary to arrhythmogenic cardiomyopathy is therefore critical.
Good-quality ECG demonstrating normal right ventricular size and function is reassuring, and if echocardiography is not conclusive, cardiovascular magnetic resonance imaging may provide additional diagnostic and prognostic data, especially when arrhythmogenic cardiomyopathy, cardiac sarcoidosis, or cardiac amyloidosis is suspected.6
Recently, magnetic resonance imaging has been used most for infiltrative diseases as the imaging modality of choice due to its superior tissue characterization and noninvasive morphological and functional evaluation. Magnetic resonance imaging findings in patients with arrhythmogenic cardiomyopathy correlate well with those of endomyocardial biopsy, angiography, and echocardiography and have been associated with incremental arrhythmic risk in the setting of electrical abnormalities. The increasing use of magnetic resonance imaging is leading to the recognition that left ventricular involvement (left-dominant arrhythmogenic right ventricular cardiomyopathy) is more common than previously recognized, with some suggesting that arrhythmogenic right ventricular cardiomyopathy should be simply called “arrhythmogenic cardiomyopathy.”
Although endomyocardial biopsy can establish the diagnosis of arrhythmogenic right ventricular cardiomyopathy, it is rarely performed because it has a high false-negative rate owing to the patchy, epicardial nature of this disorder.13
Red flags for cardiomyopathy
- Multifocal PVCs, or nonsustained ventricular tachycardia of more than one morphology on monitoring
- Syncope associated with active exercise
- Abnormal imaging findings that are consistent with arrhythmogenic right ventricular cardiomyopathy, cardiac sarcoidosis, or amyloidosis.
WHEN TO TREAT IDIOPATHIC PVCs
In our practice we explain to patients that there are two primary indications for treating idiopathic PVCs: (1) to relieve symptoms or (2) in asymptomatic patients with presumed arrhythmia-induced cardiomyopathy, to try to reverse the cardiomyopathy by eliminating the PVCs.
Some patients report severe symptoms due to their PVCs. Other patients appear to have no symptoms whatsoever, while still others are not overly bothered by the PVCs but are concerned that they may indicate they are at increased risk of cardiac events. In this last group, an evaluation such as outlined above that discloses no evidence of structural heart disease and reassurance by the physician may be all the treatment needed.
Even if they have no symptoms or only minimal symptoms, patients with a high PVC burden require follow-up because of the association between frequent PVCs and arrhythmia-induced cardiomyopathy.14,15 What constitutes a “high” PVC burden remains a matter of debate. Left ventricular dysfunction has generally been reported at PVC burdens above 15% to 25% of the total cardiac beats, though this percentage can be as low as 10%.14
Eliminating the high burden of PVCs in patients with left ventricular dysfunction may significantly improve left ventricular systolic function.15 It is likely, however, that more than PVC burden alone contributes to the development of the cardiomyopathy.14
Given these complexities, it is reasonable to request an electrophysiology consultation for patients who have more than rare PVCs. What is rare? There is no defined standard, but a PVC burden less than 1% is reasonable.
Treatment of the PVCs may be indicated in patients with systolic heart failure receiving cardiac resynchronization therapy, ie, a biventricular pacemaker. For cardiac resynchronization therapy to be clinically beneficial, close to 100% of heartbeats need to be paced, and frequent PVCs, even at a burden less than 10%, may undermine its effectiveness.16
HOW TO INTERVENE?
Beta-blockers and nondihydropyridine calcium channel blockers have both been used to treat symptomatic PVCs. If the patient is found to have systolic dysfunction as part of the evaluation, a beta-blocker is indicated, irrespective of any desire to treat the PVCs. Beta-blockers and calcium channel blockers both have low adverse effect profiles. They are available in once-a-day formulations and are inexpensive. Their efficacy is variable. The use of these medications is well within the purview of the primary care physician.
Selective beta-blockers are the first choice in treatment, and metoprolol is commonly used in clinical practice. We start with a low dose and increase it based on symptom relief.
As noted, only nondihydropyridine calcium channel blockers should be used for treatment of PVCs. As with beta-blockers, we start at a low dose and increase as needed based on the response to therapy.
Antiarrhythymic drugs are classified according to the Vaughan-Williams system. The ones most frequently used for PVCs are the class Ic drugs propafenone and flecainide and the class III drugs sotalol, amiodarone, and dofetalide. However, in our experience, if first-line agents (ie, beta-blockers and nondihydropyridine calcium channel blockers) are unsuccessful in controlling the patient’s symptoms, most primary care physicians are uncomfortable prescribing class Ic and class III drugs. Failure of a beta-blocker, a calcium channel blocker, or both often results in referral to a cardiologist or electrophysiologist.
The consultation should include a careful discussion with the patient regarding the risk of treatment with a type I or a type III drug vs catheter ablation. Treatment with class I or class III antiarrhythmic drugs always entails a small risk of proarrhythmia. The choice between drug therapy or ablation therapy is highly individualized. However, if elimination of the PVCs is of paramount importance, such as in cases of arrhythmia-induced cardiomyopathy, ablation therapy is more effective at eliminating the PVCs, although at the cost of an invasive procedure. Fortunately, the risk of complications with ablation therapy is quite low.
No drugs are approved by the US Food and Drug Administration for treating PVCs or nonsustained ventricular tachycardia. The drugs that do have an indication for treatment of ventricular arrhythmias are labeled as being indicated for “sustained” or “life-threatening” ventricular arrhythmias. The use of drugs for the treatment of PVCs or nonsustained ventricular tachycardia represents off-label usage.
Referral to discuss catheter ablation of the PVCs6 should be considered for patients who:
- Have undergone unsuccessful attempts at drug therapy for either symptoms or PVC-related cardiomyopathy
- Refuse drug therapy but have severe symptoms, or
- Do not respond to cardiac resynchronization therapy due to suboptimal pacing due to PVCs.
- Kennedy HL, Underhill SJ. Frequent or complex ventricular ectopy in apparently healthy subjects: a clinical study of 25 cases. Am J Cardiol 1976; 38:141–148.
- Brodsky M, Wu D, Denes P, Kanakis C, Rosen KM. Arrhythmias documented by 24 hour continuous electrocardiographic monitoring in 50 male medical students without apparent heart disease. Am J Cardiol 1977; 39:390–395.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C Jr, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015;12:e41–e63.
- Benditt DG, Adkisson WO. Approach to the patient with syncope: venues, presentations, diagnoses. Cardiol Clin 2013; 31:9–25.
- Pedersen CT, Kay GN, Kalman J, et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Heart Rhythm 2014; 11:e166–e196.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Buxton AE, Waxman HL, Marchlinski FE, Simson MB, Cassidy D, Josephson ME. Right ventricular tachycardia: clinical and electrophysiologic characteristics. Circulation 1983; 68:917–927.
- Marchlinski FE, Deely MP, Zado ES. Sex-specific triggers for right ventricular outflow tract tachycardia. Am Heart J 2000; 139:1009–1013.
- Fuenmayor AJ, Araujo X, Fuenmayor AM. Cardiac arrhythmias during two different stages of the menstrual cycle. Int J Cardiol 1998; 63:267–270.
- Park KM, Kim YH, Marchlinski FE. Using the surface electrocardiogram to localize the origin of idiopathic ventricular tachycardia. Pacing Clin Electrophysiol 2012; 35:1516–1527.
- Te Riele AS, Hauer RN. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: clinical challenges in a changing disease spectrum. Trends Cardiovasc Med 2015; 25:191–198.
- Philips B, Cheng A. 2015 update on the diagnosis and management of arrhythmogenic right ventricular cardiomyopathy. Curr Opin Cardiol 2016; 31:46–56.
- Del Carpio Munoz F, Syed FF, Noheria A, et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J Cardiovasc Electrophysiol 2011; 22:791–798.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Zhang Q, Zhou Y, Yu CM. Incidence, definition, diagnosis, and management of the cardiac resynchronization therapy nonresponder. Curr Opin Cardiol 2015; 30:40–49.
Doctor, my heart ______.” Fill in the blank with: skips, flip-flops, hiccups, stops, beats in my throat or chest, or any of the various ways patients describe palpitations. One cannot practice clinical medicine and not see patients with some variation of this chief complaint.1–3 Not every patient who complains of palpitations will be found to have premature ventricular contractions (PVCs), but PVCs are often part of the clinical problem.
This review focuses on the initial evaluation and management of PVCs in the primary care setting (Figure 1). It is not intended to be a comprehensive review of the pathophysiology, electrophysiology, or localization and ablation of PVCs. We will discuss approaches to the initial therapy of symptomatic PVCs. We will not discuss catheter-based therapy in detail except for which patients might benefit from referral to a clinical cardiac electrophysiologist.
Findings that should prompt consideration for referral to a specialist (“red flags”) are summarized at the end of each section. The type of specialist depends to a degree on the cardiology practice available to the referring physician. In our practice, such patients are typically seen by an electrophysiologist. In other practices, a general cardiologist might see such patients initially.
INITIAL EVALUATION
A primary concern of any patient presenting with a new symptom is whether the symptom is a marker of serious risk to health or life. In a patient with palpitations, the answer depends in large part on whether he or she has underlying structural heart disease—and that is the focus of the initial evaluation.
History: Information to ascertain
- When did the patient first notice the palpitations?
- Had there been any significant life events, either illness or emotional stress, at the time the palpitations began?
- Does the patient have a known history of heart disease (myocardial infarction, heart surgery, valvular heart disease, heart failure)?
- What medications is the patient taking?
- Does the patient take any dietary or health supplements? Ask specifically about any supplements taken to help with weight loss or increase energy levels. Almost all of them contain caffeine or other “natural” sympathomimetic agents. Also ask specifically about illicit drug use. If the patient is accompanied by a parent or partner, this question can be challenging.
- When do the palpitations occur? At random? At rest? With exercise? Time of day? In relation to the menstrual cycle? (More about this later.)
- Does anything make the palpitations better? If they occur at rest, does activity make them better or worse?
- Are there symptoms of heart failure, such as dyspnea on exertion, early fatigue, decline in exercise or exertional capacity, orthopnea, or paroxysmal nocturnal dyspnea?
- Are there symptoms suggesting cardiac ischemia, such as substernal chest pain or discomfort, chest pain or discomfort brought on by or made worse by exertion, or chest pain relieved with rest or sublingual nitroglycerin?
- Have the palpitations ever been associated with syncope? Keep in mind that syncope is transient loss of consciousness that spontaneously resolves with no features to suggest seizures.4 Thus, a patient who reports he or she “blacks out” with the palpitations but never falls or slumps has not had loss of consciousness and therefore has not had syncope.5
- Is there any history of unexplained death in the family, especially in younger people? Is there a history of unexplained accidental death in young family members?
Red flags obtained from the history
- Syncope related to palpitations
- Palpitations triggered by activity or exertion
- Known significant heart disease, congenital heart disease, or history of heart surgery
- Family history of premature unexplained sudden death in a first-degree relative.
Physical examination
The physical examination should focus on detecting any signs of underlying heart or vascular disease, eg:
- Significant murmurs
- Abnormal S3 or S4
- Displaced and diffuse point of maximal impulse or precordial heave
- Signs of right or left heart failure, or both, eg, peripheral edema, elevation of jugular venous pulse, rales, S3, S4.
Electrocardiography
We consider 12-lead electrocardiography (ECG) a part of the initial examination and assessment, not an ancillary test. One cannot evaluate a patient’s complaint of palpitations without ECG. Ideally, ECG should include a long 12-lead rhythm strip. The clinician should look for any evidence of underlying structural heart disease, eg:
- Pathologic Q waves
- Long QT interval
- ST-segment elevation in leads V1 and V2 consistent with a Brugada pattern
- Epsilon waves (seen in right ventricular arrhythmogenic cardiomyopathy).
Examples of the above can be found at sites such as ecgpedia.org.
Red flags in the physical examination and ECG
Any of the above findings on physical examination or ECG should prompt consideration of early referral, even though we have yet to establish that the palpitations are due to PVCs. Early consultation is suggested not for treatment of the palpitations but for further evaluation of structural heart disease.
Assuming the history, physical examination, and electrocardiography do not demonstrate any reasons for early cardiology or electrophysiology consultation, what’s next?
FURTHER EVALUATION: EXTENDED MONITORING
With luck, the patient’s typical palpitations will occur during ECG, in which case the palpitations can reasonably be attributed to PVCs. If not, monitoring is required to establish the cause of the patient’s symptoms.
The type of monitoring to order depends on the frequency of the palpitations. If the patient reports several episodes per day, then a 24- or 48-hour Holter monitor should both allow for a diagnosis and document the PVC burden (ie, the percent of the patient’s heartbeats that are PVCs), or the burden of whatever is the cause of the patient’s palpitations.
If the palpitations are less frequent, a 14-to-30-day monitor should be considered. A standard event recorder can confirm that the palpitations are due to PVCs but does not tell you the PVC burden. For that, a system capable of mobile outpatient cardiac telemetry is needed. Several such systems are commercially available.
A Holter monitor or other monitoring system is useful in determining whether the PVCs are unifocal (all look the same) or multifocal (have more than one morphology) and whether, in addition to PVCs, the patient has nonsustained ventricular tachycardia or sustained ventricular tachycardia (by definition lasting longer than 30 seconds or associated with symptoms of hemodynamic compromise such as near-syncope). Even if the patient has nonsustained ventricular tachycardia, if the heart is structurally normal the prognosis remains excellent.
Given the importance of knowing whether the patient has structural heart disease, we have a low threshold for ordering echocardiography, especially if nonsustained ventricular tachycardia has been documented. The finding of significant systolic dysfunction on echocardiography should prompt a cardiology consultation even if the physical examination is normal. In patients who have a high PVC burden, echocardiography is used to monitor for arrhythmia-induced cardiomyopathy.6
If the patient’s symptoms occur with activity, an exercise study can be helpful. It is important to either supervise the study oneself or, at the least, alert the exercise laboratory staff that the study is being performed to evaluate for exercise-induced arrhythmias. If the exercise study induces sustained ventricular tachycardia, the patient is almost invariably admitted to the hospital and inpatient consultation with an electrophysiologist is obtained.
Red flags on extended monitoring
- Multifocal PVCs or nonsustained ventricular tachycardia
- Polymorphic nonsustained ventricular tachycardia
- Sustained ventricular tachycardia; this still may be idiopathic and have a benign prognosis but generally should prompt referral.
If at this point no red flags have been uncovered, monitoring has established the patient’s symptoms are due to PVCs, and our examination and ancillary testing have established the patient has a structurally normal heart, what is the next step?
IDIOPATHIC PVCs
PVCs in a patient with a structurally normal heart are called “idiopathic.” Often, these patients will also be found to have nonsustained ventricular tachycardia, and may also be classified as having “idiopathic ventricular tachycardia.” Regardless of whether the patient has PVCs, nonsustained ventricular tachycardia, or both, the management approach is the same.
Roughly 60% to 80% of idiopathic PVCs originate from the right ventricle, in particular the right ventricular outflow tract.7 Patients with outflow tract PVCs typically present between the ages of 30 and 50 but range from adolescents to elders. More women than men are affected.
Outflow tract PVCs often occur only, or at much greater frequency, within a range of heart rates.8 Individual patients may have different ranges of heart rates at which their PVCs are more frequent. Patients may complain that their palpitations are more frequent at rest, early in exercise, at a peak of exercise, or early in recovery from exercise. It is not unusual for patients with outflow tract PVCs to report that activity reduces the frequency of their palpitations. Women might note an increase in their symptoms during menstruation.9 It is not clear, however, that this perceived increase in palpitations is in fact due to an increase in the number of PVCs.10
If the patient’s PVCs have not been captured on 12-lead ECG (ie, if it is not seen in all 12 leads), 12-lead Holter monitoring, if available, can be helpful. Examination of the morphology of the PVC on 12-lead ECG is extremely helpful. Outflow tract PVCs are the most common cause of idiopathic PVCs and nonsustained ventricular tachycardia and are easily recognizable with 12-lead ECG.
ECG points to the origin of the PVCs
A PVC arising on the right side of the heart will activate the right ventricle first and then the left ventricle. This is analogous to the sequence of ventricular activation in a patient with left bundle-branch block. Not surprisingly, on ECG a right-sided PVC looks similar to the QRS complex seen in left bundle-branch block—similar, but not identical.
When describing PVCs or the morphology of nonsustained ventricular tachycardia, the terms “left bundle-branch block pattern” and “right bundle-branch block pattern” refer to lead V1. If the PVC is negative (or mostly negative) in V1, the PVC has a left bundle-branch block pattern. A PVC that is positive in V1 is said to have a right bundle-branch block pattern and by implication arises from the left side of the heart.
A PVC originating from the top of the heart will move from top to bottom. The electrical axis of the PVC will be directed inferiorly. This means the PVC will be strongly positive in the inferior leads, ie, II, aVF, and III.
The electrocardiogram shown in Figure 2 demonstrates the typical appearance of a right ventricular outflow tract PVC.
If the PVC arises from the left ventricular outflow tract, the axis will still be inferiorly directed. However, the further to the left the origin of the PVC, the earlier the precordial transition will occur (the point at which the PVC is more positive than negative in the precordial leads). A PVC origin far enough to the left will result in a right bundle-branch block pattern PVC.
Not all idiopathic PVCs arise from the outflow tracts. A right bundle branch block pattern PVC does not imply the presence of underlying structural heart disease. PVCs may arise from both the tricuspid and mitral valve annuli, the left ventricular fascicles, or from the epicardium.
Multiple methods have been proposed to locate the origin of the PVC. For example, Park et al reviewed the use of surface ECG in locating the site of origin of ventricular tachycardia.11 All such algorithms should be applied with care, and with awareness of the caveats associated with their use.
Arrhythmogenic right ventricular cardiomyopathy is not benign
Arrhythmogenic right ventricular cardiomyopathy may give rise to PVCs or nonsustained ventricular tachycardia with morphologies similar to those of right ventricular outflow tract PVCs and ventricular tachycardia. The ventricular tachycardia complicating arrhythmogenic cardiomyopathy is, like PVCs arising from the right ventricular outflow tract, commonly associated with exercise or activity.
Unlike right ventricular outflow tract tachycardia, ventricular tachycardia related to arrhythmogenic cardiomyopathy is not benign.12 Distinguishing right ventricular outflow tract tachycardia from tachycardia secondary to arrhythmogenic cardiomyopathy is therefore critical.
Good-quality ECG demonstrating normal right ventricular size and function is reassuring, and if echocardiography is not conclusive, cardiovascular magnetic resonance imaging may provide additional diagnostic and prognostic data, especially when arrhythmogenic cardiomyopathy, cardiac sarcoidosis, or cardiac amyloidosis is suspected.6
Recently, magnetic resonance imaging has been used most for infiltrative diseases as the imaging modality of choice due to its superior tissue characterization and noninvasive morphological and functional evaluation. Magnetic resonance imaging findings in patients with arrhythmogenic cardiomyopathy correlate well with those of endomyocardial biopsy, angiography, and echocardiography and have been associated with incremental arrhythmic risk in the setting of electrical abnormalities. The increasing use of magnetic resonance imaging is leading to the recognition that left ventricular involvement (left-dominant arrhythmogenic right ventricular cardiomyopathy) is more common than previously recognized, with some suggesting that arrhythmogenic right ventricular cardiomyopathy should be simply called “arrhythmogenic cardiomyopathy.”
Although endomyocardial biopsy can establish the diagnosis of arrhythmogenic right ventricular cardiomyopathy, it is rarely performed because it has a high false-negative rate owing to the patchy, epicardial nature of this disorder.13
Red flags for cardiomyopathy
- Multifocal PVCs, or nonsustained ventricular tachycardia of more than one morphology on monitoring
- Syncope associated with active exercise
- Abnormal imaging findings that are consistent with arrhythmogenic right ventricular cardiomyopathy, cardiac sarcoidosis, or amyloidosis.
WHEN TO TREAT IDIOPATHIC PVCs
In our practice we explain to patients that there are two primary indications for treating idiopathic PVCs: (1) to relieve symptoms or (2) in asymptomatic patients with presumed arrhythmia-induced cardiomyopathy, to try to reverse the cardiomyopathy by eliminating the PVCs.
Some patients report severe symptoms due to their PVCs. Other patients appear to have no symptoms whatsoever, while still others are not overly bothered by the PVCs but are concerned that they may indicate they are at increased risk of cardiac events. In this last group, an evaluation such as outlined above that discloses no evidence of structural heart disease and reassurance by the physician may be all the treatment needed.
Even if they have no symptoms or only minimal symptoms, patients with a high PVC burden require follow-up because of the association between frequent PVCs and arrhythmia-induced cardiomyopathy.14,15 What constitutes a “high” PVC burden remains a matter of debate. Left ventricular dysfunction has generally been reported at PVC burdens above 15% to 25% of the total cardiac beats, though this percentage can be as low as 10%.14
Eliminating the high burden of PVCs in patients with left ventricular dysfunction may significantly improve left ventricular systolic function.15 It is likely, however, that more than PVC burden alone contributes to the development of the cardiomyopathy.14
Given these complexities, it is reasonable to request an electrophysiology consultation for patients who have more than rare PVCs. What is rare? There is no defined standard, but a PVC burden less than 1% is reasonable.
Treatment of the PVCs may be indicated in patients with systolic heart failure receiving cardiac resynchronization therapy, ie, a biventricular pacemaker. For cardiac resynchronization therapy to be clinically beneficial, close to 100% of heartbeats need to be paced, and frequent PVCs, even at a burden less than 10%, may undermine its effectiveness.16
HOW TO INTERVENE?
Beta-blockers and nondihydropyridine calcium channel blockers have both been used to treat symptomatic PVCs. If the patient is found to have systolic dysfunction as part of the evaluation, a beta-blocker is indicated, irrespective of any desire to treat the PVCs. Beta-blockers and calcium channel blockers both have low adverse effect profiles. They are available in once-a-day formulations and are inexpensive. Their efficacy is variable. The use of these medications is well within the purview of the primary care physician.
Selective beta-blockers are the first choice in treatment, and metoprolol is commonly used in clinical practice. We start with a low dose and increase it based on symptom relief.
As noted, only nondihydropyridine calcium channel blockers should be used for treatment of PVCs. As with beta-blockers, we start at a low dose and increase as needed based on the response to therapy.
Antiarrhythymic drugs are classified according to the Vaughan-Williams system. The ones most frequently used for PVCs are the class Ic drugs propafenone and flecainide and the class III drugs sotalol, amiodarone, and dofetalide. However, in our experience, if first-line agents (ie, beta-blockers and nondihydropyridine calcium channel blockers) are unsuccessful in controlling the patient’s symptoms, most primary care physicians are uncomfortable prescribing class Ic and class III drugs. Failure of a beta-blocker, a calcium channel blocker, or both often results in referral to a cardiologist or electrophysiologist.
The consultation should include a careful discussion with the patient regarding the risk of treatment with a type I or a type III drug vs catheter ablation. Treatment with class I or class III antiarrhythmic drugs always entails a small risk of proarrhythmia. The choice between drug therapy or ablation therapy is highly individualized. However, if elimination of the PVCs is of paramount importance, such as in cases of arrhythmia-induced cardiomyopathy, ablation therapy is more effective at eliminating the PVCs, although at the cost of an invasive procedure. Fortunately, the risk of complications with ablation therapy is quite low.
No drugs are approved by the US Food and Drug Administration for treating PVCs or nonsustained ventricular tachycardia. The drugs that do have an indication for treatment of ventricular arrhythmias are labeled as being indicated for “sustained” or “life-threatening” ventricular arrhythmias. The use of drugs for the treatment of PVCs or nonsustained ventricular tachycardia represents off-label usage.
Referral to discuss catheter ablation of the PVCs6 should be considered for patients who:
- Have undergone unsuccessful attempts at drug therapy for either symptoms or PVC-related cardiomyopathy
- Refuse drug therapy but have severe symptoms, or
- Do not respond to cardiac resynchronization therapy due to suboptimal pacing due to PVCs.
Doctor, my heart ______.” Fill in the blank with: skips, flip-flops, hiccups, stops, beats in my throat or chest, or any of the various ways patients describe palpitations. One cannot practice clinical medicine and not see patients with some variation of this chief complaint.1–3 Not every patient who complains of palpitations will be found to have premature ventricular contractions (PVCs), but PVCs are often part of the clinical problem.
This review focuses on the initial evaluation and management of PVCs in the primary care setting (Figure 1). It is not intended to be a comprehensive review of the pathophysiology, electrophysiology, or localization and ablation of PVCs. We will discuss approaches to the initial therapy of symptomatic PVCs. We will not discuss catheter-based therapy in detail except for which patients might benefit from referral to a clinical cardiac electrophysiologist.
Findings that should prompt consideration for referral to a specialist (“red flags”) are summarized at the end of each section. The type of specialist depends to a degree on the cardiology practice available to the referring physician. In our practice, such patients are typically seen by an electrophysiologist. In other practices, a general cardiologist might see such patients initially.
INITIAL EVALUATION
A primary concern of any patient presenting with a new symptom is whether the symptom is a marker of serious risk to health or life. In a patient with palpitations, the answer depends in large part on whether he or she has underlying structural heart disease—and that is the focus of the initial evaluation.
History: Information to ascertain
- When did the patient first notice the palpitations?
- Had there been any significant life events, either illness or emotional stress, at the time the palpitations began?
- Does the patient have a known history of heart disease (myocardial infarction, heart surgery, valvular heart disease, heart failure)?
- What medications is the patient taking?
- Does the patient take any dietary or health supplements? Ask specifically about any supplements taken to help with weight loss or increase energy levels. Almost all of them contain caffeine or other “natural” sympathomimetic agents. Also ask specifically about illicit drug use. If the patient is accompanied by a parent or partner, this question can be challenging.
- When do the palpitations occur? At random? At rest? With exercise? Time of day? In relation to the menstrual cycle? (More about this later.)
- Does anything make the palpitations better? If they occur at rest, does activity make them better or worse?
- Are there symptoms of heart failure, such as dyspnea on exertion, early fatigue, decline in exercise or exertional capacity, orthopnea, or paroxysmal nocturnal dyspnea?
- Are there symptoms suggesting cardiac ischemia, such as substernal chest pain or discomfort, chest pain or discomfort brought on by or made worse by exertion, or chest pain relieved with rest or sublingual nitroglycerin?
- Have the palpitations ever been associated with syncope? Keep in mind that syncope is transient loss of consciousness that spontaneously resolves with no features to suggest seizures.4 Thus, a patient who reports he or she “blacks out” with the palpitations but never falls or slumps has not had loss of consciousness and therefore has not had syncope.5
- Is there any history of unexplained death in the family, especially in younger people? Is there a history of unexplained accidental death in young family members?
Red flags obtained from the history
- Syncope related to palpitations
- Palpitations triggered by activity or exertion
- Known significant heart disease, congenital heart disease, or history of heart surgery
- Family history of premature unexplained sudden death in a first-degree relative.
Physical examination
The physical examination should focus on detecting any signs of underlying heart or vascular disease, eg:
- Significant murmurs
- Abnormal S3 or S4
- Displaced and diffuse point of maximal impulse or precordial heave
- Signs of right or left heart failure, or both, eg, peripheral edema, elevation of jugular venous pulse, rales, S3, S4.
Electrocardiography
We consider 12-lead electrocardiography (ECG) a part of the initial examination and assessment, not an ancillary test. One cannot evaluate a patient’s complaint of palpitations without ECG. Ideally, ECG should include a long 12-lead rhythm strip. The clinician should look for any evidence of underlying structural heart disease, eg:
- Pathologic Q waves
- Long QT interval
- ST-segment elevation in leads V1 and V2 consistent with a Brugada pattern
- Epsilon waves (seen in right ventricular arrhythmogenic cardiomyopathy).
Examples of the above can be found at sites such as ecgpedia.org.
Red flags in the physical examination and ECG
Any of the above findings on physical examination or ECG should prompt consideration of early referral, even though we have yet to establish that the palpitations are due to PVCs. Early consultation is suggested not for treatment of the palpitations but for further evaluation of structural heart disease.
Assuming the history, physical examination, and electrocardiography do not demonstrate any reasons for early cardiology or electrophysiology consultation, what’s next?
FURTHER EVALUATION: EXTENDED MONITORING
With luck, the patient’s typical palpitations will occur during ECG, in which case the palpitations can reasonably be attributed to PVCs. If not, monitoring is required to establish the cause of the patient’s symptoms.
The type of monitoring to order depends on the frequency of the palpitations. If the patient reports several episodes per day, then a 24- or 48-hour Holter monitor should both allow for a diagnosis and document the PVC burden (ie, the percent of the patient’s heartbeats that are PVCs), or the burden of whatever is the cause of the patient’s palpitations.
If the palpitations are less frequent, a 14-to-30-day monitor should be considered. A standard event recorder can confirm that the palpitations are due to PVCs but does not tell you the PVC burden. For that, a system capable of mobile outpatient cardiac telemetry is needed. Several such systems are commercially available.
A Holter monitor or other monitoring system is useful in determining whether the PVCs are unifocal (all look the same) or multifocal (have more than one morphology) and whether, in addition to PVCs, the patient has nonsustained ventricular tachycardia or sustained ventricular tachycardia (by definition lasting longer than 30 seconds or associated with symptoms of hemodynamic compromise such as near-syncope). Even if the patient has nonsustained ventricular tachycardia, if the heart is structurally normal the prognosis remains excellent.
Given the importance of knowing whether the patient has structural heart disease, we have a low threshold for ordering echocardiography, especially if nonsustained ventricular tachycardia has been documented. The finding of significant systolic dysfunction on echocardiography should prompt a cardiology consultation even if the physical examination is normal. In patients who have a high PVC burden, echocardiography is used to monitor for arrhythmia-induced cardiomyopathy.6
If the patient’s symptoms occur with activity, an exercise study can be helpful. It is important to either supervise the study oneself or, at the least, alert the exercise laboratory staff that the study is being performed to evaluate for exercise-induced arrhythmias. If the exercise study induces sustained ventricular tachycardia, the patient is almost invariably admitted to the hospital and inpatient consultation with an electrophysiologist is obtained.
Red flags on extended monitoring
- Multifocal PVCs or nonsustained ventricular tachycardia
- Polymorphic nonsustained ventricular tachycardia
- Sustained ventricular tachycardia; this still may be idiopathic and have a benign prognosis but generally should prompt referral.
If at this point no red flags have been uncovered, monitoring has established the patient’s symptoms are due to PVCs, and our examination and ancillary testing have established the patient has a structurally normal heart, what is the next step?
IDIOPATHIC PVCs
PVCs in a patient with a structurally normal heart are called “idiopathic.” Often, these patients will also be found to have nonsustained ventricular tachycardia, and may also be classified as having “idiopathic ventricular tachycardia.” Regardless of whether the patient has PVCs, nonsustained ventricular tachycardia, or both, the management approach is the same.
Roughly 60% to 80% of idiopathic PVCs originate from the right ventricle, in particular the right ventricular outflow tract.7 Patients with outflow tract PVCs typically present between the ages of 30 and 50 but range from adolescents to elders. More women than men are affected.
Outflow tract PVCs often occur only, or at much greater frequency, within a range of heart rates.8 Individual patients may have different ranges of heart rates at which their PVCs are more frequent. Patients may complain that their palpitations are more frequent at rest, early in exercise, at a peak of exercise, or early in recovery from exercise. It is not unusual for patients with outflow tract PVCs to report that activity reduces the frequency of their palpitations. Women might note an increase in their symptoms during menstruation.9 It is not clear, however, that this perceived increase in palpitations is in fact due to an increase in the number of PVCs.10
If the patient’s PVCs have not been captured on 12-lead ECG (ie, if it is not seen in all 12 leads), 12-lead Holter monitoring, if available, can be helpful. Examination of the morphology of the PVC on 12-lead ECG is extremely helpful. Outflow tract PVCs are the most common cause of idiopathic PVCs and nonsustained ventricular tachycardia and are easily recognizable with 12-lead ECG.
ECG points to the origin of the PVCs
A PVC arising on the right side of the heart will activate the right ventricle first and then the left ventricle. This is analogous to the sequence of ventricular activation in a patient with left bundle-branch block. Not surprisingly, on ECG a right-sided PVC looks similar to the QRS complex seen in left bundle-branch block—similar, but not identical.
When describing PVCs or the morphology of nonsustained ventricular tachycardia, the terms “left bundle-branch block pattern” and “right bundle-branch block pattern” refer to lead V1. If the PVC is negative (or mostly negative) in V1, the PVC has a left bundle-branch block pattern. A PVC that is positive in V1 is said to have a right bundle-branch block pattern and by implication arises from the left side of the heart.
A PVC originating from the top of the heart will move from top to bottom. The electrical axis of the PVC will be directed inferiorly. This means the PVC will be strongly positive in the inferior leads, ie, II, aVF, and III.
The electrocardiogram shown in Figure 2 demonstrates the typical appearance of a right ventricular outflow tract PVC.
If the PVC arises from the left ventricular outflow tract, the axis will still be inferiorly directed. However, the further to the left the origin of the PVC, the earlier the precordial transition will occur (the point at which the PVC is more positive than negative in the precordial leads). A PVC origin far enough to the left will result in a right bundle-branch block pattern PVC.
Not all idiopathic PVCs arise from the outflow tracts. A right bundle branch block pattern PVC does not imply the presence of underlying structural heart disease. PVCs may arise from both the tricuspid and mitral valve annuli, the left ventricular fascicles, or from the epicardium.
Multiple methods have been proposed to locate the origin of the PVC. For example, Park et al reviewed the use of surface ECG in locating the site of origin of ventricular tachycardia.11 All such algorithms should be applied with care, and with awareness of the caveats associated with their use.
Arrhythmogenic right ventricular cardiomyopathy is not benign
Arrhythmogenic right ventricular cardiomyopathy may give rise to PVCs or nonsustained ventricular tachycardia with morphologies similar to those of right ventricular outflow tract PVCs and ventricular tachycardia. The ventricular tachycardia complicating arrhythmogenic cardiomyopathy is, like PVCs arising from the right ventricular outflow tract, commonly associated with exercise or activity.
Unlike right ventricular outflow tract tachycardia, ventricular tachycardia related to arrhythmogenic cardiomyopathy is not benign.12 Distinguishing right ventricular outflow tract tachycardia from tachycardia secondary to arrhythmogenic cardiomyopathy is therefore critical.
Good-quality ECG demonstrating normal right ventricular size and function is reassuring, and if echocardiography is not conclusive, cardiovascular magnetic resonance imaging may provide additional diagnostic and prognostic data, especially when arrhythmogenic cardiomyopathy, cardiac sarcoidosis, or cardiac amyloidosis is suspected.6
Recently, magnetic resonance imaging has been used most for infiltrative diseases as the imaging modality of choice due to its superior tissue characterization and noninvasive morphological and functional evaluation. Magnetic resonance imaging findings in patients with arrhythmogenic cardiomyopathy correlate well with those of endomyocardial biopsy, angiography, and echocardiography and have been associated with incremental arrhythmic risk in the setting of electrical abnormalities. The increasing use of magnetic resonance imaging is leading to the recognition that left ventricular involvement (left-dominant arrhythmogenic right ventricular cardiomyopathy) is more common than previously recognized, with some suggesting that arrhythmogenic right ventricular cardiomyopathy should be simply called “arrhythmogenic cardiomyopathy.”
Although endomyocardial biopsy can establish the diagnosis of arrhythmogenic right ventricular cardiomyopathy, it is rarely performed because it has a high false-negative rate owing to the patchy, epicardial nature of this disorder.13
Red flags for cardiomyopathy
- Multifocal PVCs, or nonsustained ventricular tachycardia of more than one morphology on monitoring
- Syncope associated with active exercise
- Abnormal imaging findings that are consistent with arrhythmogenic right ventricular cardiomyopathy, cardiac sarcoidosis, or amyloidosis.
WHEN TO TREAT IDIOPATHIC PVCs
In our practice we explain to patients that there are two primary indications for treating idiopathic PVCs: (1) to relieve symptoms or (2) in asymptomatic patients with presumed arrhythmia-induced cardiomyopathy, to try to reverse the cardiomyopathy by eliminating the PVCs.
Some patients report severe symptoms due to their PVCs. Other patients appear to have no symptoms whatsoever, while still others are not overly bothered by the PVCs but are concerned that they may indicate they are at increased risk of cardiac events. In this last group, an evaluation such as outlined above that discloses no evidence of structural heart disease and reassurance by the physician may be all the treatment needed.
Even if they have no symptoms or only minimal symptoms, patients with a high PVC burden require follow-up because of the association between frequent PVCs and arrhythmia-induced cardiomyopathy.14,15 What constitutes a “high” PVC burden remains a matter of debate. Left ventricular dysfunction has generally been reported at PVC burdens above 15% to 25% of the total cardiac beats, though this percentage can be as low as 10%.14
Eliminating the high burden of PVCs in patients with left ventricular dysfunction may significantly improve left ventricular systolic function.15 It is likely, however, that more than PVC burden alone contributes to the development of the cardiomyopathy.14
Given these complexities, it is reasonable to request an electrophysiology consultation for patients who have more than rare PVCs. What is rare? There is no defined standard, but a PVC burden less than 1% is reasonable.
Treatment of the PVCs may be indicated in patients with systolic heart failure receiving cardiac resynchronization therapy, ie, a biventricular pacemaker. For cardiac resynchronization therapy to be clinically beneficial, close to 100% of heartbeats need to be paced, and frequent PVCs, even at a burden less than 10%, may undermine its effectiveness.16
HOW TO INTERVENE?
Beta-blockers and nondihydropyridine calcium channel blockers have both been used to treat symptomatic PVCs. If the patient is found to have systolic dysfunction as part of the evaluation, a beta-blocker is indicated, irrespective of any desire to treat the PVCs. Beta-blockers and calcium channel blockers both have low adverse effect profiles. They are available in once-a-day formulations and are inexpensive. Their efficacy is variable. The use of these medications is well within the purview of the primary care physician.
Selective beta-blockers are the first choice in treatment, and metoprolol is commonly used in clinical practice. We start with a low dose and increase it based on symptom relief.
As noted, only nondihydropyridine calcium channel blockers should be used for treatment of PVCs. As with beta-blockers, we start at a low dose and increase as needed based on the response to therapy.
Antiarrhythymic drugs are classified according to the Vaughan-Williams system. The ones most frequently used for PVCs are the class Ic drugs propafenone and flecainide and the class III drugs sotalol, amiodarone, and dofetalide. However, in our experience, if first-line agents (ie, beta-blockers and nondihydropyridine calcium channel blockers) are unsuccessful in controlling the patient’s symptoms, most primary care physicians are uncomfortable prescribing class Ic and class III drugs. Failure of a beta-blocker, a calcium channel blocker, or both often results in referral to a cardiologist or electrophysiologist.
The consultation should include a careful discussion with the patient regarding the risk of treatment with a type I or a type III drug vs catheter ablation. Treatment with class I or class III antiarrhythmic drugs always entails a small risk of proarrhythmia. The choice between drug therapy or ablation therapy is highly individualized. However, if elimination of the PVCs is of paramount importance, such as in cases of arrhythmia-induced cardiomyopathy, ablation therapy is more effective at eliminating the PVCs, although at the cost of an invasive procedure. Fortunately, the risk of complications with ablation therapy is quite low.
No drugs are approved by the US Food and Drug Administration for treating PVCs or nonsustained ventricular tachycardia. The drugs that do have an indication for treatment of ventricular arrhythmias are labeled as being indicated for “sustained” or “life-threatening” ventricular arrhythmias. The use of drugs for the treatment of PVCs or nonsustained ventricular tachycardia represents off-label usage.
Referral to discuss catheter ablation of the PVCs6 should be considered for patients who:
- Have undergone unsuccessful attempts at drug therapy for either symptoms or PVC-related cardiomyopathy
- Refuse drug therapy but have severe symptoms, or
- Do not respond to cardiac resynchronization therapy due to suboptimal pacing due to PVCs.
- Kennedy HL, Underhill SJ. Frequent or complex ventricular ectopy in apparently healthy subjects: a clinical study of 25 cases. Am J Cardiol 1976; 38:141–148.
- Brodsky M, Wu D, Denes P, Kanakis C, Rosen KM. Arrhythmias documented by 24 hour continuous electrocardiographic monitoring in 50 male medical students without apparent heart disease. Am J Cardiol 1977; 39:390–395.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C Jr, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015;12:e41–e63.
- Benditt DG, Adkisson WO. Approach to the patient with syncope: venues, presentations, diagnoses. Cardiol Clin 2013; 31:9–25.
- Pedersen CT, Kay GN, Kalman J, et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Heart Rhythm 2014; 11:e166–e196.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Buxton AE, Waxman HL, Marchlinski FE, Simson MB, Cassidy D, Josephson ME. Right ventricular tachycardia: clinical and electrophysiologic characteristics. Circulation 1983; 68:917–927.
- Marchlinski FE, Deely MP, Zado ES. Sex-specific triggers for right ventricular outflow tract tachycardia. Am Heart J 2000; 139:1009–1013.
- Fuenmayor AJ, Araujo X, Fuenmayor AM. Cardiac arrhythmias during two different stages of the menstrual cycle. Int J Cardiol 1998; 63:267–270.
- Park KM, Kim YH, Marchlinski FE. Using the surface electrocardiogram to localize the origin of idiopathic ventricular tachycardia. Pacing Clin Electrophysiol 2012; 35:1516–1527.
- Te Riele AS, Hauer RN. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: clinical challenges in a changing disease spectrum. Trends Cardiovasc Med 2015; 25:191–198.
- Philips B, Cheng A. 2015 update on the diagnosis and management of arrhythmogenic right ventricular cardiomyopathy. Curr Opin Cardiol 2016; 31:46–56.
- Del Carpio Munoz F, Syed FF, Noheria A, et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J Cardiovasc Electrophysiol 2011; 22:791–798.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Zhang Q, Zhou Y, Yu CM. Incidence, definition, diagnosis, and management of the cardiac resynchronization therapy nonresponder. Curr Opin Cardiol 2015; 30:40–49.
- Kennedy HL, Underhill SJ. Frequent or complex ventricular ectopy in apparently healthy subjects: a clinical study of 25 cases. Am J Cardiol 1976; 38:141–148.
- Brodsky M, Wu D, Denes P, Kanakis C, Rosen KM. Arrhythmias documented by 24 hour continuous electrocardiographic monitoring in 50 male medical students without apparent heart disease. Am J Cardiol 1977; 39:390–395.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C Jr, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015;12:e41–e63.
- Benditt DG, Adkisson WO. Approach to the patient with syncope: venues, presentations, diagnoses. Cardiol Clin 2013; 31:9–25.
- Pedersen CT, Kay GN, Kalman J, et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Heart Rhythm 2014; 11:e166–e196.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Buxton AE, Waxman HL, Marchlinski FE, Simson MB, Cassidy D, Josephson ME. Right ventricular tachycardia: clinical and electrophysiologic characteristics. Circulation 1983; 68:917–927.
- Marchlinski FE, Deely MP, Zado ES. Sex-specific triggers for right ventricular outflow tract tachycardia. Am Heart J 2000; 139:1009–1013.
- Fuenmayor AJ, Araujo X, Fuenmayor AM. Cardiac arrhythmias during two different stages of the menstrual cycle. Int J Cardiol 1998; 63:267–270.
- Park KM, Kim YH, Marchlinski FE. Using the surface electrocardiogram to localize the origin of idiopathic ventricular tachycardia. Pacing Clin Electrophysiol 2012; 35:1516–1527.
- Te Riele AS, Hauer RN. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: clinical challenges in a changing disease spectrum. Trends Cardiovasc Med 2015; 25:191–198.
- Philips B, Cheng A. 2015 update on the diagnosis and management of arrhythmogenic right ventricular cardiomyopathy. Curr Opin Cardiol 2016; 31:46–56.
- Del Carpio Munoz F, Syed FF, Noheria A, et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J Cardiovasc Electrophysiol 2011; 22:791–798.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Zhang Q, Zhou Y, Yu CM. Incidence, definition, diagnosis, and management of the cardiac resynchronization therapy nonresponder. Curr Opin Cardiol 2015; 30:40–49.
KEY POINTS
- The focus of the initial evaluation is to determine whether there is underlying structural heart disease. If there is, early referral to a specialist is probably warranted.
- Idiopathic PVCs (in which there is no structural heart disease) have a benign prognosis.
- Treatment of PVCs is indicated for relief of symptoms if reassurance is not sufficient.
- Patients who have a high PVC burden (> 10% of total heartbeats, though this is a subject of debate) should have an evaluation of their systolic function. If it is normal at baseline, periodic follow-up echocardiograms should be considered.
- Patients with a very high burden (> 20%) are at high risk of arrhythmia-induced cardiomyopathy. In these patients, referral is prudent, as some patients may opt for more aggressive treatment of their PVCs.
- In patients with severe symptoms for whom medical management has failed, referral for consideration of catheter ablation is reasonable.