User login
Graft-vs-host Disease and Toxic Epidermal Necrolysis Following Hematopoietic Stem Cell Transplantation
To the Editor:
Acute graft-vs-host disease (GVHD) remains a limitation to hematopoietic stem cell transplantation (HSCT) in 20% to 50% of patients after transplant. Furthermore, failed treatment with corticosteroids is frequent and portends a poor prognosis.1 Toxic epidermal necrolysis (TEN) is an epidermolytic skin disorder thought to represent an adverse drug reaction, though its pathogenesis remains unclear. Severe forms of acute GVHD can mimic TEN clinically and histologically. Both can present with widespread cutaneous and mucosal bullae, erosions, and desquamation. Toxic epidermal necrolysis in the context of allogeneic hematopoietic stem cell transplantation is extremely rare, with almost 100% mortality in adult patients. Features that favor acute GVHD over TEN include diarrhea, elevation in bilirubin level, and chimerism.2 However, these features might be absent, posing a therapeutic dilemma, as current treatment preferences for each of these entities differ.
Growing evidence supports the use of anti–tumor necrosis factor (TNF) α drugs for the treatment of TEN. Success has been reported with both anti–TNF-α monoclonal antibodies as well as the soluble fusion protein etanercept.3,4 The use of TNF-α inhibitors in acute GVHD remains anecdotal.
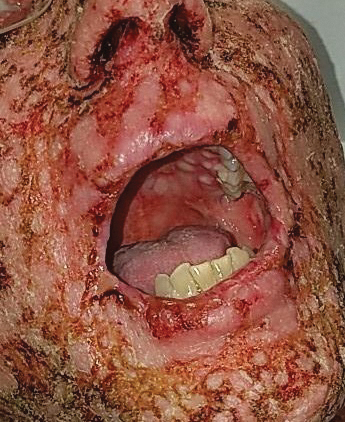
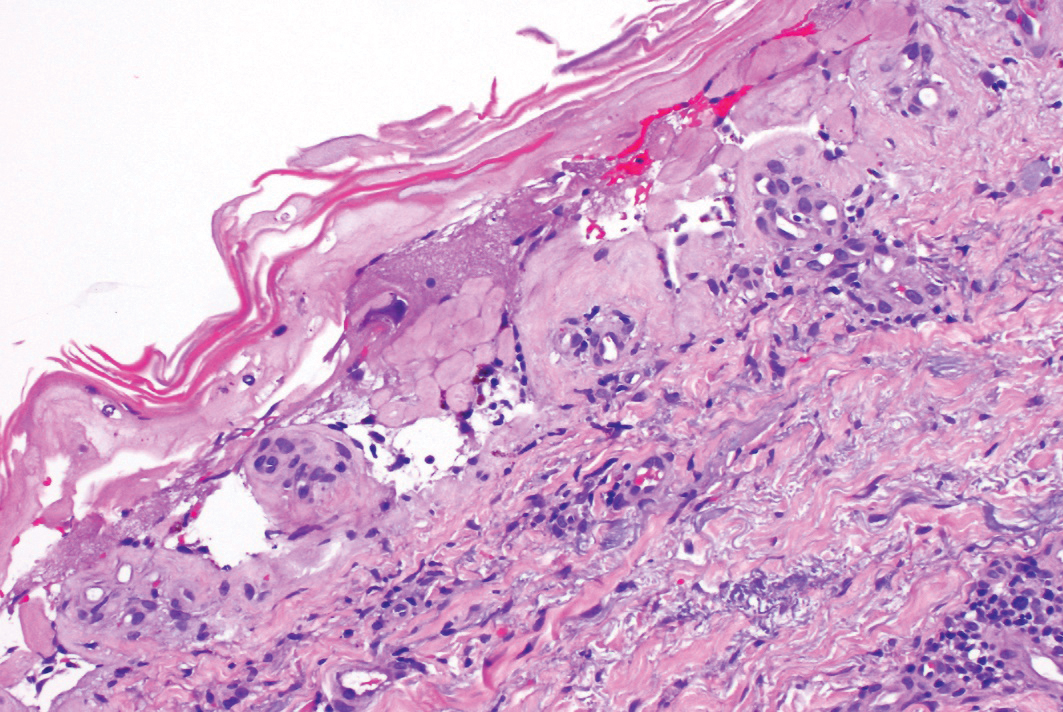
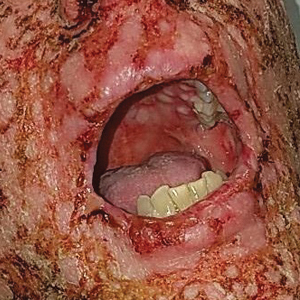
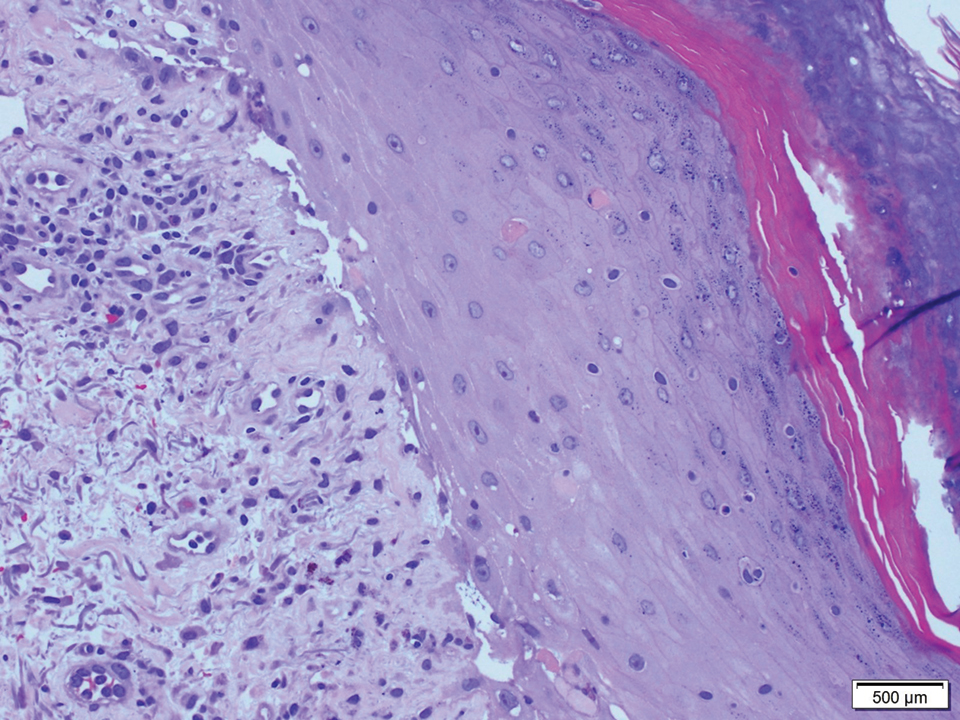
A 58-year-old man (patient 1) with a history of acute myelogenous leukemia presented with a pruritic morbilliform eruption 28 days after HSCT. There was no desquamation or mucosal involvement and the biopsy obtained was histologically suggestive of grade 2 acute GVHD. His immunosuppressive regimen included sirolimus and cyclophosphamide. He was receiving trimethoprim-sulfamethoxazole (TMP-SMX), voriconazole, and acyclovir for infectious prophylaxis. At the time of presentation, he was treated with high-dose systemic steroids (prednisone 2 mg/kg/d) for acute GVHD with partial improvement. Upon tapering of the steroids 3 weeks after initiating TMP-SMX and 1 week after initiating voriconazole, he developed painful desquamation and erosions involving 95% of the body surface area (BSA), necessitating admission to the local burn unit (Figure 1). Biopsies demonstrated full-thickness epidermal necrosis with subepidermal blistering and interface dermatitis (Figure 2). No gastrointestinal tract involvement of acute GVHD was noted. The patient was a 100% donor chimera, supporting the diagnosis of acute GVHD; however, the patient and donor carried the HLA-C*06:02 allele, which previously has been described in association with TMP-SMX–related Stevens-Johnson syndrome/TEN.5 In addition, causality assessment using the algorithm of drug causality for epidermal necrolysis indicated TMP-SMX as a probable cause and voriconazole as a possible cause. The diagnosis of TEN with a SCORe of Toxic Epidermal Necrosis (SCORTEN) of 4 in the setting of acute GVHD was favored, though grade 4 acute GVHD could not be excluded. Trimethoprim-sulfamethoxazole was discontinued, and voriconazole was changed to posaconazole. He received supportive care along with 1 dose of 25-mg subcutaneous etanercept and 3 days of intravenous immunoglobulin (IVIG). Skin re-epithelialization was complete by 3 weeks. At 4 weeks, the patient developed a new asymptomatic erythematous eruption. Biopsies demonstrated changes of acute and chronic GVHD (Figure 3) that resolved with up-titration of sirolimus. The patient remained hospitalized for 96 days and continued to follow up with his transplant team as well as ophthalmology and dermatology. He died 2 years after HSCT.
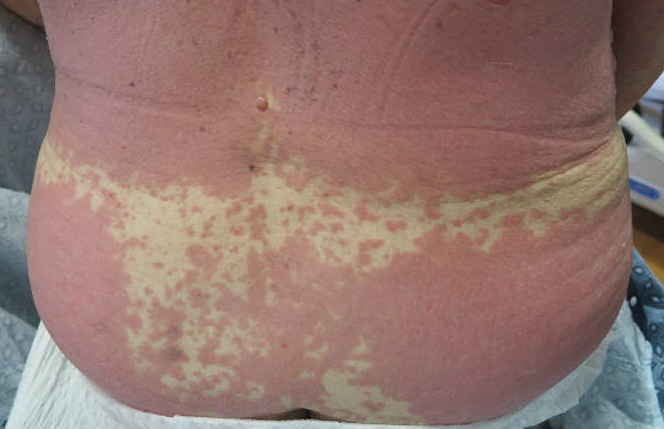
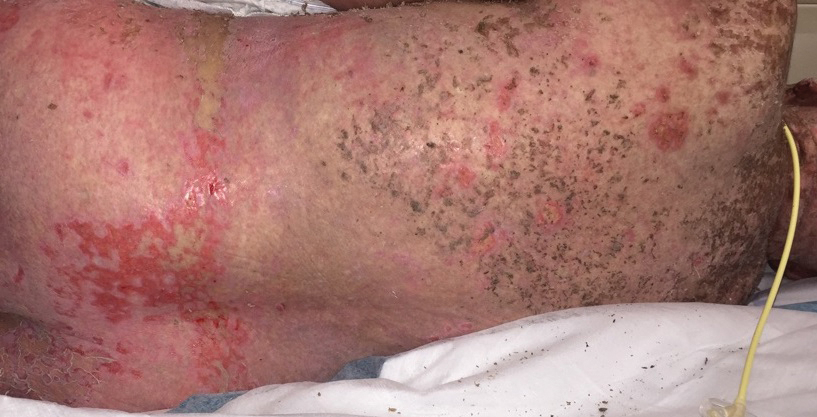
A 67-year-old woman (patient 2) with high-grade myelodysplastic syndrome presented with an erythematous morbilliform eruption on the torso on day 20 after a matched unrelated HSCT that histologically was consistent with grade 2 GVHD (Figure 4). She had been receiving sirolimus and tacrolimus for GVHD prophylaxis. Infectious prophylaxis included acyclovir, pentamidine, micafungin, and TMP-SMX. Despite high-dose systemic steroids, the rash progressed and ultimately involved 80% BSA. A positive Nikolsky sign was noted involving 21% BSA (Figure 5), in addition to oral and genital mucosal ulcers. She denied nausea, vomiting, fever, or diarrhea. Chimerism studies were negative. Trimethoprim-sulfamethoxazole was discontinued, and she was transferred to a burn unit. Biopsies showed full-thickness epidermal necrosis. A diagnosis of TEN with a SCORTEN of 4 in the setting of acute GVHD was favored; grade 4 acute GVHD could not be excluded. Steroids were discontinued. Because laboratory studies indicated IgA deficiency, IVIG was not considered as a systemic option for therapy. The patient received 1 dose of infliximab (5 mg/kg). Cyclophosphamide 1600 mg weekly was added for GVHD therapy. The wounds progressively healed, and 2 weeks into her admission she was noted to have only 3% BSA with denuded skin. The patient was transferred to the cancer treatment center for further management of the malignancy. Unfortunately, after 2 months she died due to ischemic colitis that was confirmed on autopsy.
Graft-vs-host disease and TEN are rare, life-threatening complications seen in patients with allogeneic HSCT.2 Graft-vs-host disease and TEN share clinicopathologic characteristics and effector immune mechanisms, largely the substantial role of T-cell activation and tissue destruction, which occur through mediators such as TNF-α.6-8
Given the sparse lymphocytic infiltrate, keratinocyte death in TEN is thought to result from soluble molecules, including TNF-α and TNF-related apoptosis-inducing ligand.9 Tumor necrosis factor α has been identified in blister fluid, biopsy specimens, and serum of patients with TEN. Tumor necrosis factor α increases the expression of keratinocyte-inducible nitric oxide synthase, which upregulates keratinocyte Fas ligand expression and subsequent Fas- and caspase-8–mediated keratinocyte cell death.10
Acute GVHD results from donor lymphocyte activation after infusion into damaged recipient tissues that previously have been radiated or chemoablated. Mismatches in histocompatibility complexes between donor cells and recipient tissue antigens serve as the initial trigger for immune activation. Activation of antigen-presenting cells followed by activation, proliferation, differentiation, and migration of donor T cells ultimately results in destruction of the target tissue.11 Immune mediators, such as TNF-α and lymphotoxin α (another member of the TNF superfamily), play a nonredundant role in the pathogenesis of GVHD.12
Current treatment strategies for severe acute GVHD and TEN differ. In North America, high-dose IVIG frequently is used as first-line systemic therapy, while high-dose systemic corticosteroids rarely are used.13 Studies have demonstrated successful use of anti–TNF-α drugs for the treatment of TEN.3,4 Moreover, etanercept has shown to effectively inhibit lymphotoxin α.14 Similarly, TNF inhibition in the management of steroid-refractory acute GVHD has been successful.1 These studies coupled with the underlying immune mechanisms that both diseases share encouraged initiating a trial of anti–TNF-α therapy in our patients.
Patient 1 merits further discussion because he was both a 100% donor chimera as well as a carrier of an human leukocyte antigen susceptibility candidate allele to TMP-SMX. Historical features of his presentation are consistent with either steroid-refractory GVHD or TEN superimposed on acute GVHD. His initial presentation of the more typical macular exanthem of cutaneous acute GVHD was both biopsy proven and supported by clinical improvement with steroid therapy, which was later followed by a robust blistering mucocutaneous presentation approximately 3 weeks after the administration of TMP-SMX and 1 week after initiating voriconazole that improved with IVIG and etanercept.
It is difficult to determine if TEN represents a continuum or result of the underlying drivers of acute GVHD vs a drug reaction. Although there is insufficient evidence to establish a clear-cut diagnosis of TEN, these cases illustrate the need for better diagnostic techniques to allow differentiation between TEN and grade 4 acute GVHD, and in the context of uncertainty, TNF-α inhibition poses a viable therapeutic strategy for these 2 often lethal conditions. Our cases do unequivocally indicate the benefit of this therapeutic modality, add to the current body of literature supporting the use of TNF-α inhibitors in patients such as ours without an official TEN diagnosis, and may guide future investigative efforts.
- Couriel DR, Saliba R, de Lima M, et al. A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:1555-1562.
- Jeanmonod P, Hubbuch M, Grünhage F, et al. Graft-versus-host disease or toxic epidermal necrolysis: diagnostic dilemma after liver transplantation. Transpl Infect Dis. 2012;14:422-426.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Scott-Lang V, Tidman M, McKay D. Toxic epidermal necrolysis in a child successfully treated with infliximab. Pediatr Dermatol. 2014;31:532-534.
- Kingpin T, Mahasirimongkol S, Konyoung P, et al. Candidate HLA genes for prediction of co-trimoxazole-induced severe cutaneous reactions. Pharmacogenet Genomics. 2015;25:402-411.
- Correia O, Delgado L, Barbosa IL, et al. Increased interleukin 10, tumor necrosis factor alpha, and interleukin 6 levels in blister fluid of toxic epidermal necrolysis. J Am Acad Dermatol. 2002;47:58-62.
- French LE, Tschopp J. Fas-mediated cell death in toxic epidermal necrolysis and graft-versus-host disease: potential for therapeutic inhibition. Schweiz Med Wochenschr. 2000;130:1656-1661.
- Downey A, Jackson C, Harun N, et al. Toxic epidermal necrolysis: review of pathogenesis and management. J Am Acad Dermatol. 2012;66:995-1003.
- de Araujo E, Dessirier V, Laprée G, et al. Death ligand TRAIL, secreted by CD1a+ and CD14+ cells in blister fluids, is involved in killing keratinocytes in toxic epidermal necrolysis. Exp Dermatol. 2011;20:107-112.
- Viard-Leveugle I, Gaide O, Jankovic D, et al. TNF-α and IFN-γ are potential inducers of Fas-mediated keratinocyte apoptosis through activation of inducible nitric oxide synthase in toxic epidermal necrolysis. J Invest Dermatol. 2013;133:489-498.
- Choi SW, Levine JE, Ferrara JL. Pathogenesis and management of graft-versus-host disease. Immunol Allergy Clin North Am. 2010;30:75-101.
- Markey KA, Burman AC, Banovic T, et al. Soluble lymphotoxin is an important effector molecule in GVHD and GVL. Blood. 2010;115:122-132.
- Dodiuk-Gad RP, Olteanu C, Jeschke MG, et al. Treatment of toxic epidermal necrolysis in North America. J Am Acad Dermatol. 2015;73:876-877.
- Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244-279.
To the Editor:
Acute graft-vs-host disease (GVHD) remains a limitation to hematopoietic stem cell transplantation (HSCT) in 20% to 50% of patients after transplant. Furthermore, failed treatment with corticosteroids is frequent and portends a poor prognosis.1 Toxic epidermal necrolysis (TEN) is an epidermolytic skin disorder thought to represent an adverse drug reaction, though its pathogenesis remains unclear. Severe forms of acute GVHD can mimic TEN clinically and histologically. Both can present with widespread cutaneous and mucosal bullae, erosions, and desquamation. Toxic epidermal necrolysis in the context of allogeneic hematopoietic stem cell transplantation is extremely rare, with almost 100% mortality in adult patients. Features that favor acute GVHD over TEN include diarrhea, elevation in bilirubin level, and chimerism.2 However, these features might be absent, posing a therapeutic dilemma, as current treatment preferences for each of these entities differ.
Growing evidence supports the use of anti–tumor necrosis factor (TNF) α drugs for the treatment of TEN. Success has been reported with both anti–TNF-α monoclonal antibodies as well as the soluble fusion protein etanercept.3,4 The use of TNF-α inhibitors in acute GVHD remains anecdotal.
A 58-year-old man (patient 1) with a history of acute myelogenous leukemia presented with a pruritic morbilliform eruption 28 days after HSCT. There was no desquamation or mucosal involvement and the biopsy obtained was histologically suggestive of grade 2 acute GVHD. His immunosuppressive regimen included sirolimus and cyclophosphamide. He was receiving trimethoprim-sulfamethoxazole (TMP-SMX), voriconazole, and acyclovir for infectious prophylaxis. At the time of presentation, he was treated with high-dose systemic steroids (prednisone 2 mg/kg/d) for acute GVHD with partial improvement. Upon tapering of the steroids 3 weeks after initiating TMP-SMX and 1 week after initiating voriconazole, he developed painful desquamation and erosions involving 95% of the body surface area (BSA), necessitating admission to the local burn unit (Figure 1). Biopsies demonstrated full-thickness epidermal necrosis with subepidermal blistering and interface dermatitis (Figure 2). No gastrointestinal tract involvement of acute GVHD was noted. The patient was a 100% donor chimera, supporting the diagnosis of acute GVHD; however, the patient and donor carried the HLA-C*06:02 allele, which previously has been described in association with TMP-SMX–related Stevens-Johnson syndrome/TEN.5 In addition, causality assessment using the algorithm of drug causality for epidermal necrolysis indicated TMP-SMX as a probable cause and voriconazole as a possible cause. The diagnosis of TEN with a SCORe of Toxic Epidermal Necrosis (SCORTEN) of 4 in the setting of acute GVHD was favored, though grade 4 acute GVHD could not be excluded. Trimethoprim-sulfamethoxazole was discontinued, and voriconazole was changed to posaconazole. He received supportive care along with 1 dose of 25-mg subcutaneous etanercept and 3 days of intravenous immunoglobulin (IVIG). Skin re-epithelialization was complete by 3 weeks. At 4 weeks, the patient developed a new asymptomatic erythematous eruption. Biopsies demonstrated changes of acute and chronic GVHD (Figure 3) that resolved with up-titration of sirolimus. The patient remained hospitalized for 96 days and continued to follow up with his transplant team as well as ophthalmology and dermatology. He died 2 years after HSCT.
A 67-year-old woman (patient 2) with high-grade myelodysplastic syndrome presented with an erythematous morbilliform eruption on the torso on day 20 after a matched unrelated HSCT that histologically was consistent with grade 2 GVHD (Figure 4). She had been receiving sirolimus and tacrolimus for GVHD prophylaxis. Infectious prophylaxis included acyclovir, pentamidine, micafungin, and TMP-SMX. Despite high-dose systemic steroids, the rash progressed and ultimately involved 80% BSA. A positive Nikolsky sign was noted involving 21% BSA (Figure 5), in addition to oral and genital mucosal ulcers. She denied nausea, vomiting, fever, or diarrhea. Chimerism studies were negative. Trimethoprim-sulfamethoxazole was discontinued, and she was transferred to a burn unit. Biopsies showed full-thickness epidermal necrosis. A diagnosis of TEN with a SCORTEN of 4 in the setting of acute GVHD was favored; grade 4 acute GVHD could not be excluded. Steroids were discontinued. Because laboratory studies indicated IgA deficiency, IVIG was not considered as a systemic option for therapy. The patient received 1 dose of infliximab (5 mg/kg). Cyclophosphamide 1600 mg weekly was added for GVHD therapy. The wounds progressively healed, and 2 weeks into her admission she was noted to have only 3% BSA with denuded skin. The patient was transferred to the cancer treatment center for further management of the malignancy. Unfortunately, after 2 months she died due to ischemic colitis that was confirmed on autopsy.

Graft-vs-host disease and TEN are rare, life-threatening complications seen in patients with allogeneic HSCT.2 Graft-vs-host disease and TEN share clinicopathologic characteristics and effector immune mechanisms, largely the substantial role of T-cell activation and tissue destruction, which occur through mediators such as TNF-α.6-8
Given the sparse lymphocytic infiltrate, keratinocyte death in TEN is thought to result from soluble molecules, including TNF-α and TNF-related apoptosis-inducing ligand.9 Tumor necrosis factor α has been identified in blister fluid, biopsy specimens, and serum of patients with TEN. Tumor necrosis factor α increases the expression of keratinocyte-inducible nitric oxide synthase, which upregulates keratinocyte Fas ligand expression and subsequent Fas- and caspase-8–mediated keratinocyte cell death.10
Acute GVHD results from donor lymphocyte activation after infusion into damaged recipient tissues that previously have been radiated or chemoablated. Mismatches in histocompatibility complexes between donor cells and recipient tissue antigens serve as the initial trigger for immune activation. Activation of antigen-presenting cells followed by activation, proliferation, differentiation, and migration of donor T cells ultimately results in destruction of the target tissue.11 Immune mediators, such as TNF-α and lymphotoxin α (another member of the TNF superfamily), play a nonredundant role in the pathogenesis of GVHD.12
Current treatment strategies for severe acute GVHD and TEN differ. In North America, high-dose IVIG frequently is used as first-line systemic therapy, while high-dose systemic corticosteroids rarely are used.13 Studies have demonstrated successful use of anti–TNF-α drugs for the treatment of TEN.3,4 Moreover, etanercept has shown to effectively inhibit lymphotoxin α.14 Similarly, TNF inhibition in the management of steroid-refractory acute GVHD has been successful.1 These studies coupled with the underlying immune mechanisms that both diseases share encouraged initiating a trial of anti–TNF-α therapy in our patients.
Patient 1 merits further discussion because he was both a 100% donor chimera as well as a carrier of an human leukocyte antigen susceptibility candidate allele to TMP-SMX. Historical features of his presentation are consistent with either steroid-refractory GVHD or TEN superimposed on acute GVHD. His initial presentation of the more typical macular exanthem of cutaneous acute GVHD was both biopsy proven and supported by clinical improvement with steroid therapy, which was later followed by a robust blistering mucocutaneous presentation approximately 3 weeks after the administration of TMP-SMX and 1 week after initiating voriconazole that improved with IVIG and etanercept.
It is difficult to determine if TEN represents a continuum or result of the underlying drivers of acute GVHD vs a drug reaction. Although there is insufficient evidence to establish a clear-cut diagnosis of TEN, these cases illustrate the need for better diagnostic techniques to allow differentiation between TEN and grade 4 acute GVHD, and in the context of uncertainty, TNF-α inhibition poses a viable therapeutic strategy for these 2 often lethal conditions. Our cases do unequivocally indicate the benefit of this therapeutic modality, add to the current body of literature supporting the use of TNF-α inhibitors in patients such as ours without an official TEN diagnosis, and may guide future investigative efforts.
To the Editor:
Acute graft-vs-host disease (GVHD) remains a limitation to hematopoietic stem cell transplantation (HSCT) in 20% to 50% of patients after transplant. Furthermore, failed treatment with corticosteroids is frequent and portends a poor prognosis.1 Toxic epidermal necrolysis (TEN) is an epidermolytic skin disorder thought to represent an adverse drug reaction, though its pathogenesis remains unclear. Severe forms of acute GVHD can mimic TEN clinically and histologically. Both can present with widespread cutaneous and mucosal bullae, erosions, and desquamation. Toxic epidermal necrolysis in the context of allogeneic hematopoietic stem cell transplantation is extremely rare, with almost 100% mortality in adult patients. Features that favor acute GVHD over TEN include diarrhea, elevation in bilirubin level, and chimerism.2 However, these features might be absent, posing a therapeutic dilemma, as current treatment preferences for each of these entities differ.
Growing evidence supports the use of anti–tumor necrosis factor (TNF) α drugs for the treatment of TEN. Success has been reported with both anti–TNF-α monoclonal antibodies as well as the soluble fusion protein etanercept.3,4 The use of TNF-α inhibitors in acute GVHD remains anecdotal.
A 58-year-old man (patient 1) with a history of acute myelogenous leukemia presented with a pruritic morbilliform eruption 28 days after HSCT. There was no desquamation or mucosal involvement and the biopsy obtained was histologically suggestive of grade 2 acute GVHD. His immunosuppressive regimen included sirolimus and cyclophosphamide. He was receiving trimethoprim-sulfamethoxazole (TMP-SMX), voriconazole, and acyclovir for infectious prophylaxis. At the time of presentation, he was treated with high-dose systemic steroids (prednisone 2 mg/kg/d) for acute GVHD with partial improvement. Upon tapering of the steroids 3 weeks after initiating TMP-SMX and 1 week after initiating voriconazole, he developed painful desquamation and erosions involving 95% of the body surface area (BSA), necessitating admission to the local burn unit (Figure 1). Biopsies demonstrated full-thickness epidermal necrosis with subepidermal blistering and interface dermatitis (Figure 2). No gastrointestinal tract involvement of acute GVHD was noted. The patient was a 100% donor chimera, supporting the diagnosis of acute GVHD; however, the patient and donor carried the HLA-C*06:02 allele, which previously has been described in association with TMP-SMX–related Stevens-Johnson syndrome/TEN.5 In addition, causality assessment using the algorithm of drug causality for epidermal necrolysis indicated TMP-SMX as a probable cause and voriconazole as a possible cause. The diagnosis of TEN with a SCORe of Toxic Epidermal Necrosis (SCORTEN) of 4 in the setting of acute GVHD was favored, though grade 4 acute GVHD could not be excluded. Trimethoprim-sulfamethoxazole was discontinued, and voriconazole was changed to posaconazole. He received supportive care along with 1 dose of 25-mg subcutaneous etanercept and 3 days of intravenous immunoglobulin (IVIG). Skin re-epithelialization was complete by 3 weeks. At 4 weeks, the patient developed a new asymptomatic erythematous eruption. Biopsies demonstrated changes of acute and chronic GVHD (Figure 3) that resolved with up-titration of sirolimus. The patient remained hospitalized for 96 days and continued to follow up with his transplant team as well as ophthalmology and dermatology. He died 2 years after HSCT.
A 67-year-old woman (patient 2) with high-grade myelodysplastic syndrome presented with an erythematous morbilliform eruption on the torso on day 20 after a matched unrelated HSCT that histologically was consistent with grade 2 GVHD (Figure 4). She had been receiving sirolimus and tacrolimus for GVHD prophylaxis. Infectious prophylaxis included acyclovir, pentamidine, micafungin, and TMP-SMX. Despite high-dose systemic steroids, the rash progressed and ultimately involved 80% BSA. A positive Nikolsky sign was noted involving 21% BSA (Figure 5), in addition to oral and genital mucosal ulcers. She denied nausea, vomiting, fever, or diarrhea. Chimerism studies were negative. Trimethoprim-sulfamethoxazole was discontinued, and she was transferred to a burn unit. Biopsies showed full-thickness epidermal necrosis. A diagnosis of TEN with a SCORTEN of 4 in the setting of acute GVHD was favored; grade 4 acute GVHD could not be excluded. Steroids were discontinued. Because laboratory studies indicated IgA deficiency, IVIG was not considered as a systemic option for therapy. The patient received 1 dose of infliximab (5 mg/kg). Cyclophosphamide 1600 mg weekly was added for GVHD therapy. The wounds progressively healed, and 2 weeks into her admission she was noted to have only 3% BSA with denuded skin. The patient was transferred to the cancer treatment center for further management of the malignancy. Unfortunately, after 2 months she died due to ischemic colitis that was confirmed on autopsy.

Graft-vs-host disease and TEN are rare, life-threatening complications seen in patients with allogeneic HSCT.2 Graft-vs-host disease and TEN share clinicopathologic characteristics and effector immune mechanisms, largely the substantial role of T-cell activation and tissue destruction, which occur through mediators such as TNF-α.6-8
Given the sparse lymphocytic infiltrate, keratinocyte death in TEN is thought to result from soluble molecules, including TNF-α and TNF-related apoptosis-inducing ligand.9 Tumor necrosis factor α has been identified in blister fluid, biopsy specimens, and serum of patients with TEN. Tumor necrosis factor α increases the expression of keratinocyte-inducible nitric oxide synthase, which upregulates keratinocyte Fas ligand expression and subsequent Fas- and caspase-8–mediated keratinocyte cell death.10
Acute GVHD results from donor lymphocyte activation after infusion into damaged recipient tissues that previously have been radiated or chemoablated. Mismatches in histocompatibility complexes between donor cells and recipient tissue antigens serve as the initial trigger for immune activation. Activation of antigen-presenting cells followed by activation, proliferation, differentiation, and migration of donor T cells ultimately results in destruction of the target tissue.11 Immune mediators, such as TNF-α and lymphotoxin α (another member of the TNF superfamily), play a nonredundant role in the pathogenesis of GVHD.12
Current treatment strategies for severe acute GVHD and TEN differ. In North America, high-dose IVIG frequently is used as first-line systemic therapy, while high-dose systemic corticosteroids rarely are used.13 Studies have demonstrated successful use of anti–TNF-α drugs for the treatment of TEN.3,4 Moreover, etanercept has shown to effectively inhibit lymphotoxin α.14 Similarly, TNF inhibition in the management of steroid-refractory acute GVHD has been successful.1 These studies coupled with the underlying immune mechanisms that both diseases share encouraged initiating a trial of anti–TNF-α therapy in our patients.
Patient 1 merits further discussion because he was both a 100% donor chimera as well as a carrier of an human leukocyte antigen susceptibility candidate allele to TMP-SMX. Historical features of his presentation are consistent with either steroid-refractory GVHD or TEN superimposed on acute GVHD. His initial presentation of the more typical macular exanthem of cutaneous acute GVHD was both biopsy proven and supported by clinical improvement with steroid therapy, which was later followed by a robust blistering mucocutaneous presentation approximately 3 weeks after the administration of TMP-SMX and 1 week after initiating voriconazole that improved with IVIG and etanercept.
It is difficult to determine if TEN represents a continuum or result of the underlying drivers of acute GVHD vs a drug reaction. Although there is insufficient evidence to establish a clear-cut diagnosis of TEN, these cases illustrate the need for better diagnostic techniques to allow differentiation between TEN and grade 4 acute GVHD, and in the context of uncertainty, TNF-α inhibition poses a viable therapeutic strategy for these 2 often lethal conditions. Our cases do unequivocally indicate the benefit of this therapeutic modality, add to the current body of literature supporting the use of TNF-α inhibitors in patients such as ours without an official TEN diagnosis, and may guide future investigative efforts.
- Couriel DR, Saliba R, de Lima M, et al. A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:1555-1562.
- Jeanmonod P, Hubbuch M, Grünhage F, et al. Graft-versus-host disease or toxic epidermal necrolysis: diagnostic dilemma after liver transplantation. Transpl Infect Dis. 2012;14:422-426.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Scott-Lang V, Tidman M, McKay D. Toxic epidermal necrolysis in a child successfully treated with infliximab. Pediatr Dermatol. 2014;31:532-534.
- Kingpin T, Mahasirimongkol S, Konyoung P, et al. Candidate HLA genes for prediction of co-trimoxazole-induced severe cutaneous reactions. Pharmacogenet Genomics. 2015;25:402-411.
- Correia O, Delgado L, Barbosa IL, et al. Increased interleukin 10, tumor necrosis factor alpha, and interleukin 6 levels in blister fluid of toxic epidermal necrolysis. J Am Acad Dermatol. 2002;47:58-62.
- French LE, Tschopp J. Fas-mediated cell death in toxic epidermal necrolysis and graft-versus-host disease: potential for therapeutic inhibition. Schweiz Med Wochenschr. 2000;130:1656-1661.
- Downey A, Jackson C, Harun N, et al. Toxic epidermal necrolysis: review of pathogenesis and management. J Am Acad Dermatol. 2012;66:995-1003.
- de Araujo E, Dessirier V, Laprée G, et al. Death ligand TRAIL, secreted by CD1a+ and CD14+ cells in blister fluids, is involved in killing keratinocytes in toxic epidermal necrolysis. Exp Dermatol. 2011;20:107-112.
- Viard-Leveugle I, Gaide O, Jankovic D, et al. TNF-α and IFN-γ are potential inducers of Fas-mediated keratinocyte apoptosis through activation of inducible nitric oxide synthase in toxic epidermal necrolysis. J Invest Dermatol. 2013;133:489-498.
- Choi SW, Levine JE, Ferrara JL. Pathogenesis and management of graft-versus-host disease. Immunol Allergy Clin North Am. 2010;30:75-101.
- Markey KA, Burman AC, Banovic T, et al. Soluble lymphotoxin is an important effector molecule in GVHD and GVL. Blood. 2010;115:122-132.
- Dodiuk-Gad RP, Olteanu C, Jeschke MG, et al. Treatment of toxic epidermal necrolysis in North America. J Am Acad Dermatol. 2015;73:876-877.
- Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244-279.
- Couriel DR, Saliba R, de Lima M, et al. A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:1555-1562.
- Jeanmonod P, Hubbuch M, Grünhage F, et al. Graft-versus-host disease or toxic epidermal necrolysis: diagnostic dilemma after liver transplantation. Transpl Infect Dis. 2012;14:422-426.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Scott-Lang V, Tidman M, McKay D. Toxic epidermal necrolysis in a child successfully treated with infliximab. Pediatr Dermatol. 2014;31:532-534.
- Kingpin T, Mahasirimongkol S, Konyoung P, et al. Candidate HLA genes for prediction of co-trimoxazole-induced severe cutaneous reactions. Pharmacogenet Genomics. 2015;25:402-411.
- Correia O, Delgado L, Barbosa IL, et al. Increased interleukin 10, tumor necrosis factor alpha, and interleukin 6 levels in blister fluid of toxic epidermal necrolysis. J Am Acad Dermatol. 2002;47:58-62.
- French LE, Tschopp J. Fas-mediated cell death in toxic epidermal necrolysis and graft-versus-host disease: potential for therapeutic inhibition. Schweiz Med Wochenschr. 2000;130:1656-1661.
- Downey A, Jackson C, Harun N, et al. Toxic epidermal necrolysis: review of pathogenesis and management. J Am Acad Dermatol. 2012;66:995-1003.
- de Araujo E, Dessirier V, Laprée G, et al. Death ligand TRAIL, secreted by CD1a+ and CD14+ cells in blister fluids, is involved in killing keratinocytes in toxic epidermal necrolysis. Exp Dermatol. 2011;20:107-112.
- Viard-Leveugle I, Gaide O, Jankovic D, et al. TNF-α and IFN-γ are potential inducers of Fas-mediated keratinocyte apoptosis through activation of inducible nitric oxide synthase in toxic epidermal necrolysis. J Invest Dermatol. 2013;133:489-498.
- Choi SW, Levine JE, Ferrara JL. Pathogenesis and management of graft-versus-host disease. Immunol Allergy Clin North Am. 2010;30:75-101.
- Markey KA, Burman AC, Banovic T, et al. Soluble lymphotoxin is an important effector molecule in GVHD and GVL. Blood. 2010;115:122-132.
- Dodiuk-Gad RP, Olteanu C, Jeschke MG, et al. Treatment of toxic epidermal necrolysis in North America. J Am Acad Dermatol. 2015;73:876-877.
- Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244-279.
Practice Points
- Graft-vs-host disease (GVHD) and toxic epidermal necrolysis (TEN) are rare life-threatening complications seen in patients with allogeneic hematopoietic stem cell transplantation.
- Although mild acute GVHD easily is distinguished from TEN, severe acute GVHD and TEN share overlapping features and present a diagnostic challenge.
- Therapeutic decisions and associated outcomes hinge on accurate diagnosis, as high-dose systemic corticosteroids have been associated with higher mortality rates in TEN.
An erythematous facial rash
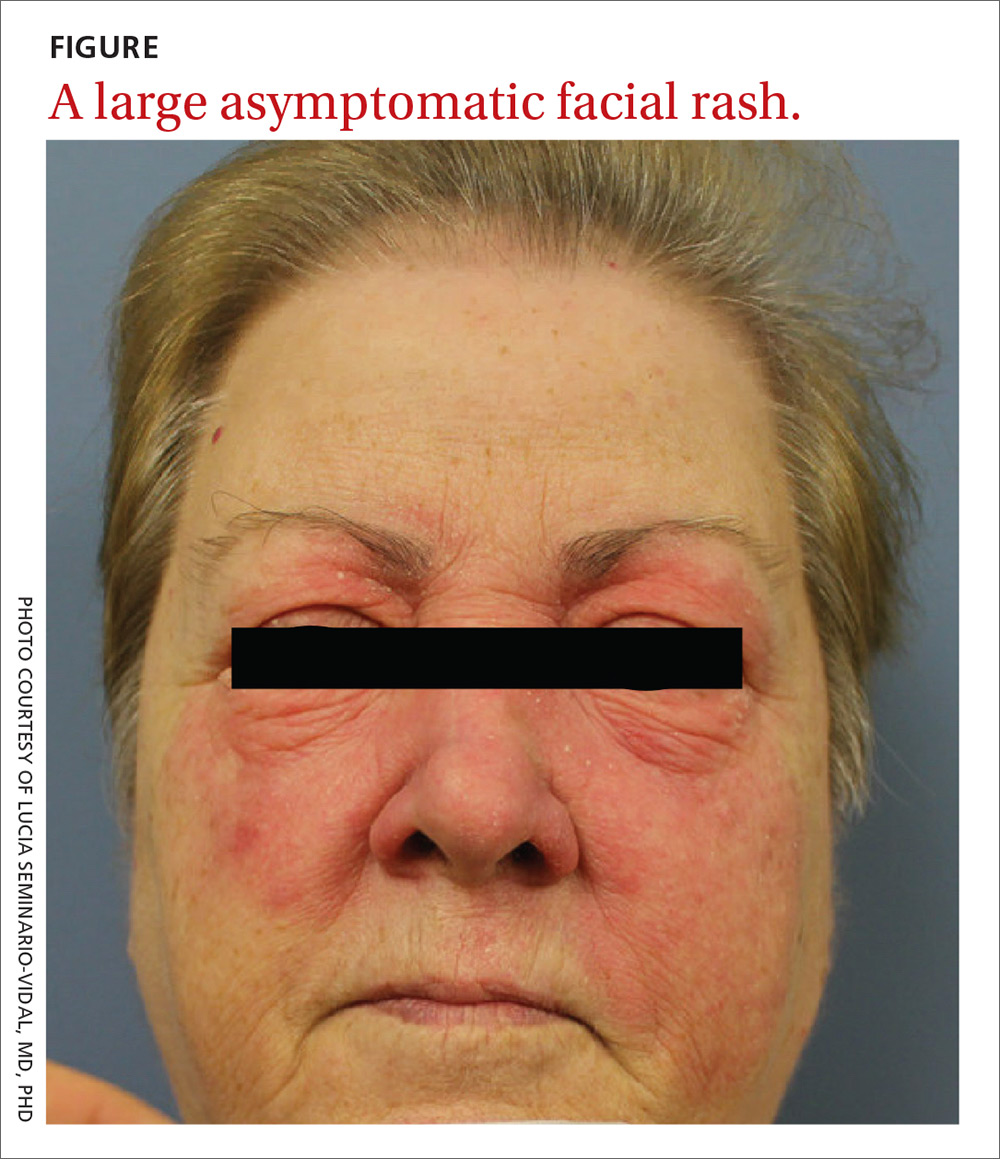
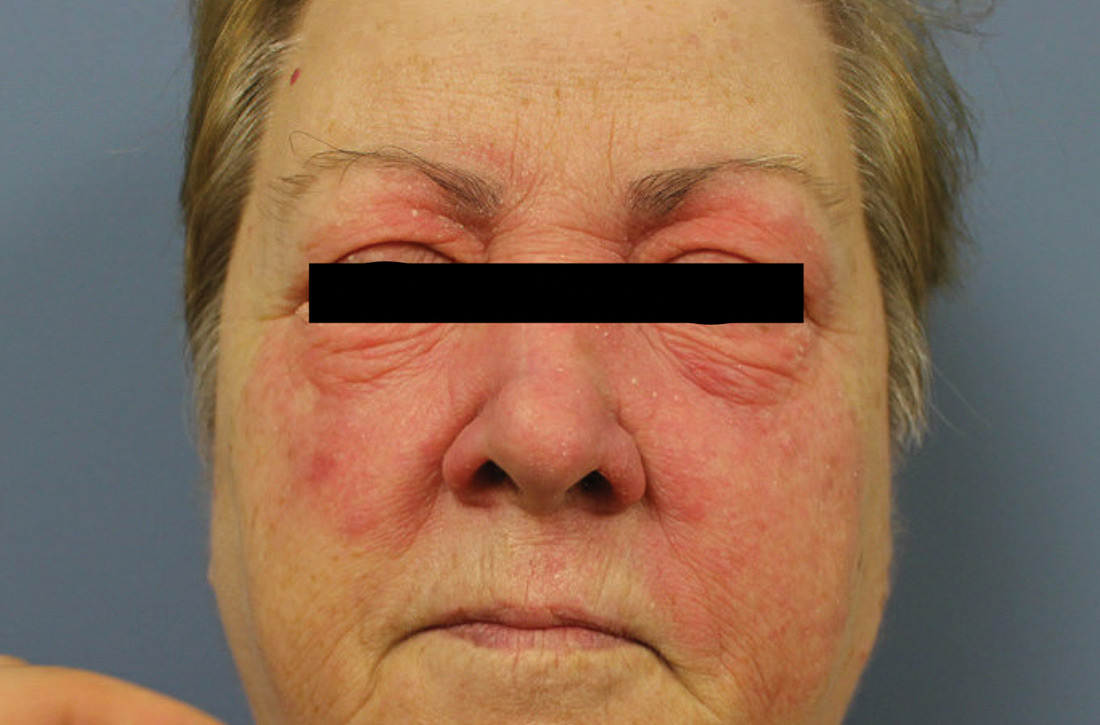
A 59-year-old woman presented to our clinic with a large asymptomatic facial rash that had developed several months earlier. The rash had been slowly growing but did not change day to day. Her past medical history was significant for hypertension, hyperlipidemia, and cutaneous lymphoma, which was localized to her arms. She denied the use of any new products, including hair or facial products, nail polish, or any new medications.
Initially, she was presumed (by an outside provider) to have rosacea, and she received treatment with doxycycline 100 mg/d for 2 months. However, the rash did not improve.
Physical examination revealed a large erythematous rash involving her cheeks, nose, and periocular area with no other significant findings (FIGURE).
A biopsy of her right cheek was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Mycosis fungoides
Following the biopsy of her right cheek, a histopathologic analysis demonstrated an atypical lymphocytic infiltrate positive for CD3 and CD4. These histopathologic features led to a diagnosis of recurrent mycosis fungoides (MF), a type of cutaneous lymphoma. (Our patient’s cutaneous lymphoma had been in remission for a year following local radiotherapy.)
MF is the most common type of cutaneous lymphoma, with an incidence of 6.4 to 9.6 cases per million people in the United States.1 There are also 2 rare subtypes of MF: the psoriasiform and palmoplantar forms. Psoriasiform MF presents with psoriasis-like plaques, while palmoplantar MF initially presents on the palms and soles.
Patients with classic MF typically present with patches and plaques—with the late evolution of tumors—on non–sun-exposed areas.1 Our patient’s clinical presentation was atypical because the rash manifested on a sun-exposed area of her body.
MF and other cutaneous lymphomas should always be part of the differential diagnosis for an unexplained persistent rash, especially in a patient with a history of MF. The development of lymphomas is thought to be a stepwise process through which chronic antigenic stimulation results in an accumulation of genetic mutations that then cause cells to undergo clonal expansion and, ultimately, malignant transformation. Genetic, environmental, and immunologic factors that contribute to the disease pathogenesis have been identified.2
Once clinical features point toward MF, the diagnosis can be further differentiated from other benign inflammatory mimics with a biopsy demonstrating cerebriform lymphocytes homing toward the epidermis, monoclonal expansion of T cells, and defective apoptosis.3
Continue to: Differential includes rosacea and seborrheic dermatitis
Differential includes rosacea and seborrheic dermatitis
The diagnosis of MF can be difficult as it often imitates other benign inflammatory conditions.
Rosacea manifests as an erythematous facial rash but usually spares the nasolabial folds and eyelids. There are several forms, including ocular (featuring swollen and irritated conjunctiva), erythematotelangiectatic (with visible blood vessels), and papulopustular (with acneic lesions). Over time, the skin may develop a thickened, bumpy texture, referred to as phymatous rosacea.4 A history of acute worsening with exposure to certain hot or spicy foods, alcohol, or ultraviolet light suggests a diagnosis of rosacea.
Seborrheic dermatitis classically presents as yellow scaling on a mildly erythematous base and often involves nasolabial folds and eyebrows. Seborrheic dermatitis can be associated with human immunodeficiency virus, Parkinson’s disease, and other chronic medical conditions.
Allergic contact dermatitis can look identical to MF, but in our case, there was no new allergen in the history. A thorough history regarding new medications, creams, and household supplies is integral to differentiating this diagnosis.
Misdiagnosis can lead to advanced-stage disease
This case of persistent facial erythema, originally treated as rosacea, highlights the importance of having a low threshold of suspicion of MF, especially in a patient with a prior history of MF. A recent study by Kelati et al3 indicated that certain subtypes of MF are easily misdiagnosed and treated as psoriasis or eczema respectively for an average of 10.5 years.3 These years of misdiagnosis are significantly correlated with the development of advanced-stage MF, which is more difficult to treat.3
Continue to: Treatment with topical desonide and mechlorethamine
Treatment with topical desonide and mechlorethamine
There are multiple treatment options for MF, depending on the stage, starting with topical therapies and advancing to systemic therapies in more advanced stages. Topical treatments include steroids, nitrogen mustard, and retinoids.5 Our patient was referred to a multidisciplinary lymphoma clinic, where topical treatment was initiated with desonide cream .05% and mechlorethamine gel .016%. Our patient experienced a 50% improvement in skin involvement at 3 months.
As MF progresses to more advanced stages, treatment often combines skin-directed therapies with systemic immunomodulators, biologics, radiation, and total skin electron beam therapy.6 TSEBT is a low-dose full-body radiation treatment that targets the skin surface and therefore effectively treats cutaneous lymphoma. Although TSEBT is usually well tolerated, there have been documented acute and chronic adverse effects, including dermatitis, alopecia, peripheral edema, cutaneous malignancies, and infertility in men.7
While the use of topical desonide and mechlorethamine was initially favored over radiation due to eyelid involvement, our patient developed new patches on her legs 11 months after her initial visit. When biopsies indicated MF with large cell transformation, she received 1 course of low-dose TSEBT (12 Gy), with complete response noted at the 2 month follow-up.
CORRESPONDENCE
Lucia Seminario-Vidal, MD, PhD, Department of Dermatology and Cutaneous Surgery, 13330 USF Laurel Drive, Tampa, FL 33612; luciasem@usf.edu
1. Jawed S, Myskowski P, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome). Part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-e16.
2. Wohl Y, Tur E. Environmental risk factors for mycosis fungoides. Curr Probl Dermatol. 2007;35:52-64.
3. Kelati A, Gallouj S, Tahiri L, et al. Defining the mimics and clinico-histological diagnosis criteria for mycosis fungoides to minimize misdiagnosis. Int J Womens Dermatol. 2017;3:100-106.
4. Two AM, Wu W, Gallo RL, et al. Rosacea. part I. Introduction, categorization, histology, pathogenesis, and risk factors. J AM Acad Dermatol. 2015;72:749-758.
5. Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
6. Jawed S, Myskowski P, Horwitz S, et al. Continuing medical education: Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-e17.
7. De Moraes FY, Carvalho Hde A, Hanna SA, et al. Literature review of clinical results of total skin electron irradiation (TSEBT) of mycosis fungoides in adults. Rep Pract Oncol Radiother. 2014;19:92-98.
A 59-year-old woman presented to our clinic with a large asymptomatic facial rash that had developed several months earlier. The rash had been slowly growing but did not change day to day. Her past medical history was significant for hypertension, hyperlipidemia, and cutaneous lymphoma, which was localized to her arms. She denied the use of any new products, including hair or facial products, nail polish, or any new medications.
Initially, she was presumed (by an outside provider) to have rosacea, and she received treatment with doxycycline 100 mg/d for 2 months. However, the rash did not improve.
Physical examination revealed a large erythematous rash involving her cheeks, nose, and periocular area with no other significant findings (FIGURE).
A biopsy of her right cheek was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Mycosis fungoides
Following the biopsy of her right cheek, a histopathologic analysis demonstrated an atypical lymphocytic infiltrate positive for CD3 and CD4. These histopathologic features led to a diagnosis of recurrent mycosis fungoides (MF), a type of cutaneous lymphoma. (Our patient’s cutaneous lymphoma had been in remission for a year following local radiotherapy.)
MF is the most common type of cutaneous lymphoma, with an incidence of 6.4 to 9.6 cases per million people in the United States.1 There are also 2 rare subtypes of MF: the psoriasiform and palmoplantar forms. Psoriasiform MF presents with psoriasis-like plaques, while palmoplantar MF initially presents on the palms and soles.
Patients with classic MF typically present with patches and plaques—with the late evolution of tumors—on non–sun-exposed areas.1 Our patient’s clinical presentation was atypical because the rash manifested on a sun-exposed area of her body.
MF and other cutaneous lymphomas should always be part of the differential diagnosis for an unexplained persistent rash, especially in a patient with a history of MF. The development of lymphomas is thought to be a stepwise process through which chronic antigenic stimulation results in an accumulation of genetic mutations that then cause cells to undergo clonal expansion and, ultimately, malignant transformation. Genetic, environmental, and immunologic factors that contribute to the disease pathogenesis have been identified.2
Once clinical features point toward MF, the diagnosis can be further differentiated from other benign inflammatory mimics with a biopsy demonstrating cerebriform lymphocytes homing toward the epidermis, monoclonal expansion of T cells, and defective apoptosis.3
Continue to: Differential includes rosacea and seborrheic dermatitis
Differential includes rosacea and seborrheic dermatitis
The diagnosis of MF can be difficult as it often imitates other benign inflammatory conditions.
Rosacea manifests as an erythematous facial rash but usually spares the nasolabial folds and eyelids. There are several forms, including ocular (featuring swollen and irritated conjunctiva), erythematotelangiectatic (with visible blood vessels), and papulopustular (with acneic lesions). Over time, the skin may develop a thickened, bumpy texture, referred to as phymatous rosacea.4 A history of acute worsening with exposure to certain hot or spicy foods, alcohol, or ultraviolet light suggests a diagnosis of rosacea.
Seborrheic dermatitis classically presents as yellow scaling on a mildly erythematous base and often involves nasolabial folds and eyebrows. Seborrheic dermatitis can be associated with human immunodeficiency virus, Parkinson’s disease, and other chronic medical conditions.
Allergic contact dermatitis can look identical to MF, but in our case, there was no new allergen in the history. A thorough history regarding new medications, creams, and household supplies is integral to differentiating this diagnosis.
Misdiagnosis can lead to advanced-stage disease
This case of persistent facial erythema, originally treated as rosacea, highlights the importance of having a low threshold of suspicion of MF, especially in a patient with a prior history of MF. A recent study by Kelati et al3 indicated that certain subtypes of MF are easily misdiagnosed and treated as psoriasis or eczema respectively for an average of 10.5 years.3 These years of misdiagnosis are significantly correlated with the development of advanced-stage MF, which is more difficult to treat.3
Continue to: Treatment with topical desonide and mechlorethamine
Treatment with topical desonide and mechlorethamine
There are multiple treatment options for MF, depending on the stage, starting with topical therapies and advancing to systemic therapies in more advanced stages. Topical treatments include steroids, nitrogen mustard, and retinoids.5 Our patient was referred to a multidisciplinary lymphoma clinic, where topical treatment was initiated with desonide cream .05% and mechlorethamine gel .016%. Our patient experienced a 50% improvement in skin involvement at 3 months.
As MF progresses to more advanced stages, treatment often combines skin-directed therapies with systemic immunomodulators, biologics, radiation, and total skin electron beam therapy.6 TSEBT is a low-dose full-body radiation treatment that targets the skin surface and therefore effectively treats cutaneous lymphoma. Although TSEBT is usually well tolerated, there have been documented acute and chronic adverse effects, including dermatitis, alopecia, peripheral edema, cutaneous malignancies, and infertility in men.7
While the use of topical desonide and mechlorethamine was initially favored over radiation due to eyelid involvement, our patient developed new patches on her legs 11 months after her initial visit. When biopsies indicated MF with large cell transformation, she received 1 course of low-dose TSEBT (12 Gy), with complete response noted at the 2 month follow-up.
CORRESPONDENCE
Lucia Seminario-Vidal, MD, PhD, Department of Dermatology and Cutaneous Surgery, 13330 USF Laurel Drive, Tampa, FL 33612; luciasem@usf.edu
A 59-year-old woman presented to our clinic with a large asymptomatic facial rash that had developed several months earlier. The rash had been slowly growing but did not change day to day. Her past medical history was significant for hypertension, hyperlipidemia, and cutaneous lymphoma, which was localized to her arms. She denied the use of any new products, including hair or facial products, nail polish, or any new medications.
Initially, she was presumed (by an outside provider) to have rosacea, and she received treatment with doxycycline 100 mg/d for 2 months. However, the rash did not improve.
Physical examination revealed a large erythematous rash involving her cheeks, nose, and periocular area with no other significant findings (FIGURE).
A biopsy of her right cheek was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Mycosis fungoides
Following the biopsy of her right cheek, a histopathologic analysis demonstrated an atypical lymphocytic infiltrate positive for CD3 and CD4. These histopathologic features led to a diagnosis of recurrent mycosis fungoides (MF), a type of cutaneous lymphoma. (Our patient’s cutaneous lymphoma had been in remission for a year following local radiotherapy.)
MF is the most common type of cutaneous lymphoma, with an incidence of 6.4 to 9.6 cases per million people in the United States.1 There are also 2 rare subtypes of MF: the psoriasiform and palmoplantar forms. Psoriasiform MF presents with psoriasis-like plaques, while palmoplantar MF initially presents on the palms and soles.
Patients with classic MF typically present with patches and plaques—with the late evolution of tumors—on non–sun-exposed areas.1 Our patient’s clinical presentation was atypical because the rash manifested on a sun-exposed area of her body.
MF and other cutaneous lymphomas should always be part of the differential diagnosis for an unexplained persistent rash, especially in a patient with a history of MF. The development of lymphomas is thought to be a stepwise process through which chronic antigenic stimulation results in an accumulation of genetic mutations that then cause cells to undergo clonal expansion and, ultimately, malignant transformation. Genetic, environmental, and immunologic factors that contribute to the disease pathogenesis have been identified.2
Once clinical features point toward MF, the diagnosis can be further differentiated from other benign inflammatory mimics with a biopsy demonstrating cerebriform lymphocytes homing toward the epidermis, monoclonal expansion of T cells, and defective apoptosis.3
Continue to: Differential includes rosacea and seborrheic dermatitis
Differential includes rosacea and seborrheic dermatitis
The diagnosis of MF can be difficult as it often imitates other benign inflammatory conditions.
Rosacea manifests as an erythematous facial rash but usually spares the nasolabial folds and eyelids. There are several forms, including ocular (featuring swollen and irritated conjunctiva), erythematotelangiectatic (with visible blood vessels), and papulopustular (with acneic lesions). Over time, the skin may develop a thickened, bumpy texture, referred to as phymatous rosacea.4 A history of acute worsening with exposure to certain hot or spicy foods, alcohol, or ultraviolet light suggests a diagnosis of rosacea.
Seborrheic dermatitis classically presents as yellow scaling on a mildly erythematous base and often involves nasolabial folds and eyebrows. Seborrheic dermatitis can be associated with human immunodeficiency virus, Parkinson’s disease, and other chronic medical conditions.
Allergic contact dermatitis can look identical to MF, but in our case, there was no new allergen in the history. A thorough history regarding new medications, creams, and household supplies is integral to differentiating this diagnosis.
Misdiagnosis can lead to advanced-stage disease
This case of persistent facial erythema, originally treated as rosacea, highlights the importance of having a low threshold of suspicion of MF, especially in a patient with a prior history of MF. A recent study by Kelati et al3 indicated that certain subtypes of MF are easily misdiagnosed and treated as psoriasis or eczema respectively for an average of 10.5 years.3 These years of misdiagnosis are significantly correlated with the development of advanced-stage MF, which is more difficult to treat.3
Continue to: Treatment with topical desonide and mechlorethamine
Treatment with topical desonide and mechlorethamine
There are multiple treatment options for MF, depending on the stage, starting with topical therapies and advancing to systemic therapies in more advanced stages. Topical treatments include steroids, nitrogen mustard, and retinoids.5 Our patient was referred to a multidisciplinary lymphoma clinic, where topical treatment was initiated with desonide cream .05% and mechlorethamine gel .016%. Our patient experienced a 50% improvement in skin involvement at 3 months.
As MF progresses to more advanced stages, treatment often combines skin-directed therapies with systemic immunomodulators, biologics, radiation, and total skin electron beam therapy.6 TSEBT is a low-dose full-body radiation treatment that targets the skin surface and therefore effectively treats cutaneous lymphoma. Although TSEBT is usually well tolerated, there have been documented acute and chronic adverse effects, including dermatitis, alopecia, peripheral edema, cutaneous malignancies, and infertility in men.7
While the use of topical desonide and mechlorethamine was initially favored over radiation due to eyelid involvement, our patient developed new patches on her legs 11 months after her initial visit. When biopsies indicated MF with large cell transformation, she received 1 course of low-dose TSEBT (12 Gy), with complete response noted at the 2 month follow-up.
CORRESPONDENCE
Lucia Seminario-Vidal, MD, PhD, Department of Dermatology and Cutaneous Surgery, 13330 USF Laurel Drive, Tampa, FL 33612; luciasem@usf.edu
1. Jawed S, Myskowski P, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome). Part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-e16.
2. Wohl Y, Tur E. Environmental risk factors for mycosis fungoides. Curr Probl Dermatol. 2007;35:52-64.
3. Kelati A, Gallouj S, Tahiri L, et al. Defining the mimics and clinico-histological diagnosis criteria for mycosis fungoides to minimize misdiagnosis. Int J Womens Dermatol. 2017;3:100-106.
4. Two AM, Wu W, Gallo RL, et al. Rosacea. part I. Introduction, categorization, histology, pathogenesis, and risk factors. J AM Acad Dermatol. 2015;72:749-758.
5. Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
6. Jawed S, Myskowski P, Horwitz S, et al. Continuing medical education: Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-e17.
7. De Moraes FY, Carvalho Hde A, Hanna SA, et al. Literature review of clinical results of total skin electron irradiation (TSEBT) of mycosis fungoides in adults. Rep Pract Oncol Radiother. 2014;19:92-98.
1. Jawed S, Myskowski P, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome). Part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-e16.
2. Wohl Y, Tur E. Environmental risk factors for mycosis fungoides. Curr Probl Dermatol. 2007;35:52-64.
3. Kelati A, Gallouj S, Tahiri L, et al. Defining the mimics and clinico-histological diagnosis criteria for mycosis fungoides to minimize misdiagnosis. Int J Womens Dermatol. 2017;3:100-106.
4. Two AM, Wu W, Gallo RL, et al. Rosacea. part I. Introduction, categorization, histology, pathogenesis, and risk factors. J AM Acad Dermatol. 2015;72:749-758.
5. Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
6. Jawed S, Myskowski P, Horwitz S, et al. Continuing medical education: Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-e17.
7. De Moraes FY, Carvalho Hde A, Hanna SA, et al. Literature review of clinical results of total skin electron irradiation (TSEBT) of mycosis fungoides in adults. Rep Pract Oncol Radiother. 2014;19:92-98.