User login
2023 Update on menopause
This year’s menopause Update highlights a highly effective nonhormonal medication that recently received approval by the US Food and Drug Administration (FDA) for the treatment of bothersome menopausal vasomotor symptoms. In addition, the Update provides guidance regarding how ObGyns should respond when an endometrial biopsy for postmenopausal bleeding reveals proliferative changes.
Breakthrough in women’s health: A new nonhormone therapy for vasomotor symptoms
Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a phase 3 RCT. J Clin Endocrinol Metab. 2023;dgad058. doi:10.1210/clinem/dgad058.
Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102. doi:10.1016/S0140-6736(23)00085-5.
A new oral nonestrogen-containing medication for relief of moderate to severe hot flashes, fezolinetant (Veozah) 45 mg daily, has been approved by the FDA and was expected to be available by the end of May 2023. Fezolinetant is a selective neurokinin 3 (NK3) receptor antagonistthat offers a targeted nonhormonal approach to menopausal vasomotor symptoms (VMS), and it is the first in its class to make it to market.
The decline in estrogen at menopause appears to result in increased signaling at kisspeptin/neurokinin B/dynorphin (KNDy) neurons in the thermoregulatory center within the hypothalamus with resultant increases in hot flashes.1,2 Fezolinetant works by binding to and blocking the activities of the NK3 receptor.3-5
Key study findings
Selective NK3 receptor antagonists, including fezolinetant, effectively reduce the frequency and severity of VMS comparable to that of hormone therapy (HT). Two phase 3 clinical trials, Skylight 1 and 2, confirmed the efficacy and safety of fezolinetant 45 mg in treating VMS,6,7 and an additional 52-week placebo-controlled study, Skylight 4, confirmed long-term safety.8 Onset of action occurs within a week. Reported adverse events occurred in 1% to 2% of healthy menopausal women participating in clinical trials; these included headaches, abdominal pain, diarrhea, insomnia, back pain, hot flushes, and reversible elevated hepatic transaminase levels.6-9
The published phase 2 trials9 and the international randomized controlled trial (RCT) 12-week studies, Skylight 1 and 2,6,7 found that once-daily 30-mg and 45-mg doses of fezolinetant significantly reduced VMS frequency and severity at 12 weeks among women aged 40 to 60 years who reported an average of 7 moderate to severe VMS/day; the reduction in reported VMS was sustained at 40 weeks. Phase 3 data from Skylight 1 and 2 demonstrated fezolinetant’s efficacy in reducing the frequency and severity of VMS and provided information on the safety profile of fezolinetant compared with placebo over 12 weeks and a noncontrolled extension for an additional 40 weeks.6,7
Oral fezolinetant was associated with improved quality of life, including reduced VMS-related interference with daily life.10 Johnson and colleagues, reporting for Skylight 2, found VMS frequency and severity improvement by week 1, which achieved statistical significance at weeks 4 and 12, with this improvement maintained through week 52.6 A 64.3% reduction in mean daily VMS from baseline was seen at 12 weeks for fezolinetant 45 mg compared with a 45.4% reduction for placebo. VMS severity significantly decreased compared with placebo at 4 and 12 weeks.6
Serious treatment-emergent adverse events were infrequent, reported by 2%, 1%, and 0% of those receiving fezolinetant 30 mg, fezolinetant 45 mg, and placebo.6 Increases in levels of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) were noted and were described as asymptomatic, isolated, intermittent, or transient, and these levels returned to baseline during treatment or after discontinuation.6
Of the 5 participants taking fezolinetant in Skyline 1 with ALT or AST levels greater than 3 times the upper limit of normal in the 12-week randomized trial, levels returned to normal range while continuing treatment in 2 participants, with treatment interruption in 2, and with discontinuation in 1. No new safety signals were seen in the 40-week extension trial.6
Fezolinetant offers a much-needed effective and safe selective nonhormone NK3 receptor antagonist therapy that reduces the frequency and severity of menopausal VMS and has been shown to be safe through 52 weeks of treatment.
To read more about how fezolinetant specifically targets the hormone receptor that triggers hot flashes as well as on prescribing hormone therapy for women with menopausal symptoms, see “Focus on menopause: Q&A with Jan Shifren, MD, and Genevieve NealPerry, MD, PhD,” in the December 2022 issue of OBG Management at https://www.mdedge.com/obgyn/article/260380/menopause
Continue to: Endometrial and bone safety...
Endometrial and bone safety
Results from Skylight 4, a phase 3, randomized, double-blind, 52-week safety study, provided additional evidence that confirmed the longer-term safety of fezolinetant over a 52-week treatment period.8
Endometrial safety was assessed in postmenopausal women with normal baseline endometrium (n = 599).8 For fezolinetant 45 mg, 1 of 203 participants had endometrial hyperplasia (EH) (0.5%; upper limit of one-sided 95% confidence interval [CI], 2.3%); no cases of EH were noted in the placebo (0 of 186) or fezolinetant 30-mg (0 of 210) groups. The incidence of EH or malignancy in fezolinetant-treated participants was within prespecified limits, as assessed by blinded, centrally read endometrial biopsies. Endometrial malignancy occurred in 1 of 210 in the fezolinetant 30-mg group (0.5%; 95% CI, 2.2%) with no cases in the other groups, thus meeting FDA requirements for endometrial safety.8
In addition, no significant differences were noted in change from baseline endometrial thickness on transvaginal ultrasonography between fezolinetant-treated and placebo groups. Likewise, no loss of bone density was found on dual-energy x-ray absorptiometry (DEXA) scans or trabecular bone scores.8
Liver safety
Although no cases of severe liver injury were noted, elevations in serum transaminase concentrations greater than 3 times the upper limit of normal were observed in the clinical trials. In Skylight 4, liver enzyme elevations more than 3 times the upper limit of normal occurred in 6 of 583 participants taking placebo, 8 of 590 taking fezolinetant 30 mg, and 12 of 589 taking fezolinetant 45 mg.8
The prescribing information for fezolinetant includes a warning for elevated hepatic transaminases: Fezolinetant should not be started if baseline serum transaminase concentration is equal to or exceeds 2 times the upper limit of normal. Liver tests should be obtained at baseline and repeated every 3 months for the first 9 months and then if symptoms suggest liver injury.11,12
Unmet need for nonhormone treatment of VMS
Vasomotor symptoms affect up to 80% of women, with approximately 25% bothersome enough to warrant treatment. Vasomotor symptoms persist for a median of 7 years, with duration and severity differing by race and ethnicity. Black, Hispanic, and possibly Native American women experience the highest burden of VMS.2 Although VMS, including hot flashes, night sweats, and mood and sleep disturbances, often are considered an annoyance to those with mild symptoms, moderate to severe VMS impact women’s lives, including functioning at home or work, affecting relationships, and decreasing perceived quality of life, and they have been associated with workplace absenteeism and increased health care costs, both direct from medical care and testing and indirect costs from lost work.13-15
Women with 7 or more daily moderate to severe VMS (defined as with sweating or affecting function) reported interference with sleep (94%), concentration (84%), mood (85%), energy (77%), and sexual activity (61%).16 Moderately to severely bothersome VMS have been associated with impaired psychological and general well-being, affecting work performance.17 Based on a Mayo Clinic workplace survey, Faubion and colleagues estimated an annual loss of $1.8 billion in the United States for menopause-related missed work and a $28 billion loss when medical expenses were added.15
Menopausal HT has been the primary treatment for VMS and has been shown to reduce the frequency and severity of hot flashes, with additional benefits on sleep, mood, fatigue, bone loss and reduction of fracture, and genitourinary syndrome of menopause (GSM), and with potential improvement in cardiovascular health with decreased type 2 diabetes.18,19 For healthy women with early menopause and no contraindications, HT has been recommended until at least the age of natural menopause, as observational data suggest that HT prevents osteoporosis, cardiovascular disease, neurodegenerative changes, and sexual dysfunction for these women.19,20 Similarly, for healthy women younger than age 60 or within 10 years of menopause, initiating HT has been shown to be safe and effective in treating bothersome VMS and preventing osteoporotic fractures and genitourinary changes.19,21
Most systemic HT formulations are inexpensive (for example, available as generics), with multiple dosing and formulations available for use alone or combined as oral, transdermal, or vaginal therapies. Despite the fear that arose for clinicians and women from the initial 2002 findings of the Women’s Health Initiative regarding increased risk of breast cancer, stroke, venous thrombosis, cardiovascular disease, and dementia, major medical societies agree that when initiated at or soon after menopause, HT is a safe and effective therapy to relieve VMS, protect against bone loss, and treat genitourinary changes.19,21
Many women, however, cannot take HT, including those with estrogen-sensitive cancers, such as breast or uterine cancers; prior cardiovascular disease, stroke, or venous thrombotic events; severe endometriosis; or migraine headaches with visual auras.2 In addition, many symptomatic menopausal women without health contraindications choose not to take HT.2 Until now, the only FDA-approved VMS nonhormone therapy has been a low-dose 7.5-mg paroxetine salt. Unfortunately, this formulation, along with the off-label use of other antidepressants (selective serotonin reuptake inhibitors and serotonin and norepinephrine reuptake inhibitors), gabapentinoids, oxybutynin, and clonidine, are substantially less effective than HT in treating moderate to severe VMS.
Bottom line
A substantial unmet need remains for effective therapy for moderate to severe VMS for women who cannot or choose not to take menopausal HT to relieve VMS.2,16 Effective, safe nonhormone treatment options such as the new NK3 receptor antagonist fezolinetant will address this clinically important need.
One concern is that the cost of developing and bringing to market the first of a new type of medication will be passed on to consumers, which may put it out of the price range for the many women who need it. However, the development and FDA approval of fezolinetant as the first NK3 receptor antagonist to treat menopausal VMS is potentially a practice changer. It provides a novel, effective, and safe FDA-approved nonhormonal treatment for menopausal women with moderate to severe VMS, particularly for women who cannot or will not take hormone therapy.
Continue to: When endometrial biopsy for postmenopausal bleeding reveals proliferative changes, how should we respond?...
When endometrial biopsy for postmenopausal bleeding reveals proliferative changes, how should we respond?
Abraham C. Proliferative endometrium in menopause: to treat or not to treat? Obstet Gynecol. 2023;141:265-267. doi:10.1097/AOG.0000000000005054.
The following case represents a common scenario for ObGyns.
CASE Patient with proliferative endometrial changes
A menopausal patient with a body mass index (BMI) > 30 kg/m2 presents with uterine bleeding. She does not use systemic menopausal hormone therapy. Endometrial biopsy indicates proliferative changes.
When endometrial biopsy performed for bleeding reveals proliferative changes in menopausal women, we traditionally have responded by reassuring the patient that the findings are benign and advising that she should let us know if future spotting or bleeding occurs.
However, a recent review by Abraham published in Obstetrics and Gynecology details the implications of proliferative endometrial changes in menopausal patients, advising that treatment, as well as monitoring, may be appropriate.22
Endometrial changes and what they suggest
In premenopausal women, proliferative endometrial changes are physiologic and result from ovarian estrogen production early in each cycle, during what is called the proliferative (referring to the endometrium) or follicular (referring to the dominant follicle that synthesizes estrogen) phase. In menopausal women who are not using HT, however, proliferative endometrial changes, with orderly uniform glands seen on histologic evaluation, reflect aromatization of androgens by adipose and other tissues into estrogen.
The next step on the continuum to hyperplasia (benign or atypical) after proliferative endometrium is disordered proliferative endometrium. At this stage, histologic evaluation reveals scattered cystic and dilated glands that have a normal gland-to-stroma ratio with a low gland density overall and without any atypia. Randomly distributed glands may have tubal metaplasia or fibrin thrombi associated with microinfarcts, often presenting with irregular bleeding. This is a noncancerous change that occurs with excess estrogen (endogenous or exogenous).23
Progestins reverse endometrial hyperplasia by activating progesterone receptors, which leads to stromal decidualization with thinning of the endometrium. They have a pronounced effect on the histologic appearance of the endometrium. By contrast, endometrial intraepithelial neoplasia (EIN, previously known as endometrial hyperplasiawith atypia) shows underlying molecular mutations and histologic alterations and represents a sharp transition to true neoplasia, which greatly increases the risk of endometrioid endometrial adenocarcinoma.24
For decades, we have been aware that if women diagnosed with endometrial hyperplasia are not treated with progestational therapy, their future risk of endometrial cancer is elevated. More recently, we also recognize that menopausal women found to have proliferative endometrial changes, if not treated, have an increased risk of endometrial cancer.
In a retrospective cohort study of almost 300 menopausal women who were not treated after endometrial biopsy revealed proliferative changes, investigators followed participants for an average of 11 years.25 These women had a mean BMI of 34 kg/m2. During follow-up, almost 12% of these women were diagnosed with endometrial hyperplasia or cancer. This incidence of endometrial neoplasia was some 4 times higher than for women initially found to have atrophic endometrial changes.25
Progestin treatment
Oral progestin therapy with follow-up endometrial biopsy constitutes traditional management for endometrial hyperplasia. Such therapy minimizes the likelihood that hyperplasia will progress to endometrial cancer.
We now recognize that the convenience, as well as the high endometrial progestin levels achieved, with levonorgestrel-releasing intrauterine devices (LNG-IUDs) have advantages over oral progestin therapy in treating endometrial hyperplasia. Indeed, a recent US report found that among women with EIN managed medically, use of progestin-releasing IUDs has grown from 7.7% in 2008 to 35.6% in 2020.26
Although both oral and intrauterine progestin are highly effective in treating simple hyperplasia, progestin IUDs are substantially more effective than oral progestins in treating EIN.27 Progestin concentrations in the endometrium have been shown to be 100-fold higher after LNG-IUD placement compared with oral progestin use.22 In addition, adverse effects, including bloating, unpleasant mood changes, and increased appetite, are more common with oral than intrauterine progestin therapy.28
Unfortunately, data from randomized trials addressing progestational treatment of proliferative endometrium in menopausal women are not available to support the treatment of proliferative endometrium with either oral progestins or the LNG-IUD.22
Role of ultrasonography
Another concern is relying on a finding of thin endometrial thickness on vaginal ultrasonography. In a simulated retrospective cohort study, use of transvaginal ultrasonography to determine the appropriateness of a biopsy was found not to be sufficiently accurate or racially equitable with regard to Black women.29 In simulated data, transvaginal ultrasonography missed almost 5 times more cases of endometrial cancer among Black women compared with White women due to higher fibroid prevalence and nonendometrioid histologic type malignancies in Black women.29
Assessing risk
If proliferative endometrium is found, Abraham suggests assessing risk using22:
- age
- comorbidities (including obesity)
- endometrial echo thickness on vaginal ultrasonography.
Consider the patient’s risk and tolerance of recurrent bleeding as well as her tolerance for progestational adverse effects if medical therapy is chosen. Discussion about next steps should include reviewing the histologic findings with the patient and discussing the difference in risk of progression to endometrial cancer of a finding of proliferative endometrium compared with a histologic finding of endometrial hyperplasia.
Using this patient-centered approach, observation over time with follow-up endometrial biopsies remains a management option. Although some women may tolerate micronized progesterone over synthetic progestins, there is concern that it may be less effective in suppressing the endometrium than synthetic progestins.30 Accordingly, synthetic progestins represent first-line options in this setting.
In her review, Abraham suggests that when endometrial biopsy reveals proliferative changes in a menopausal woman, we should initiate progestin treatment and perform surveillance endometrial sampling every 3 to 6 months. If such sampling reveals benign but not proliferative endometrium, progestin therapy can be stopped and endometrial biopsy repeated if bleeding recurs.22 ●
ObGyns may choose to adopt Abraham’s approach or to hold off on progestin therapy while performing follow-up endometrial sampling. Either way, the take-home message is that the finding of proliferative endometrial changes on biopsy for postmenopausal bleeding requires proactive management.
- Modi M, Dhillo WS. Neurokinin 3 receptor antagonism: a novel treatment for menopausal hot flushes. Neuroendocrinology. 2019;109:242-248. doi:10.1159/000495889
- Pinkerton JV, Redick DL, Homewood LN, et al. Neurokinin receptor antagonist, fezolinetant, for treatment of menopausal vasomotor symptoms. J Clin Endocrinol Metab. 2023;dgad209. doi:10.1210/clinem/dgad209
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211-227. doi:10.1016 /j.yfrne.2013.07.003
- Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci USA. 2012;109:1984619851. doi:10.1073/pnas.1211517109
- Astellas Pharma. Astellas’ Veozah (fezolinetant) approved by US FDA for treatment of vasomotor symptoms due to menopause. May 12, 2023. PR Newswire. Accessed May 15, 2023. https://www.prnewswire.com/news-releases/astellas-veozah-fezolinetant-approved-by-us-fda-for -treatment-of-vasomotor-symptoms-due-to-menopause -301823639.html
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a phase 3 RCT. J Clin Endocrinol Metab. 2023;dgad058. doi:10.1210/clinem/dgad058
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102. doi:10.1016 /S0140-6736(23)00085-5
- Neal-Perry G, Cano A, Lederman S, et al. Safety of fezolinetant for vasomotor symptoms associated with menopause: a randomized controlled trial. Obstet Gynecol. 2023;141:737-747. doi:10.1097/AOG.0000000000005114
- Depypere H, Timmerman D, Donders G, et al. Treatment of menopausal vasomotor symptoms with fezolinetant, a neurokinin 3 receptor antagonist: a phase 2a trial. J Clin Endocrinol Metab. 2019;104:5893-5905. doi: 10.1210/jc .2019-00677
- Santoro N, Waldbaum A, Lederman S, et al. Effect of the neurokinin 3 receptor antagonist fezolinetant on patientreported outcomes in postmenopausal women with vasomotor symptoms: results of a randomized, placebo-controlled, double-blind, dose-ranging study (VESTA). Menopause. 2020;27:1350-1356. doi:10.1097/GME.0000000000001621
- FDA approves novel drug to treat moderate to severe hot flashes caused by menopause. May 12, 2023. US Food and Drug Administration. Accessed May 15, 2023. https://www .fda.gov/news-events/press-announcements/fda-approves -novel-drug-treat-moderate-severe-hot-flashes-caused -menopause
- Veozah. Prescribing information. Astellas; 2023. Accessed May 16, 2023. https://www.astellas.com/us/system/files /veozah_uspi.pdf
- Pinkerton JV. Money talks: untreated hot flashes cost women, the workplace, and society. Menopause. 2015;22:254-255. doi:10.1097/GME.0000000000000427
- Sarrel P, Portman D, Lefebvre P, et al. Incremental direct and indirect costs of untreated vasomotor symptoms. Menopause. 2015;22(3):260-266. doi:10.1097/GME.0000000000000320
- Faubion SS, Enders F, Hedges MS, et al. Impact of menopause symptoms on women in the workplace. Mayo Clin Proc. 2023;98:833-845. doi:10.1016/j.mayocp.2023.02.025
- Williams RE, Levine KB, Kalilani L, et al. Menopause- specific questionnaire assessment in US populationbased study shows negative impact on health-related quality of life. Maturitas. 2009;62:153-159. doi:10.1016 /j.maturitas.2008.12.006
- Gartoulla P, Bell RJ, Worsley R, et al. Moderate-severely bothersome vasomotor symptoms are associated with lowered psychological general wellbeing in women at midlife. Maturitas. 2015;81:487-492. doi:10.1016 /j.maturitas.2015.06.004
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806. doi:10.1056/NEJMp1514242
- 2022 Hormone Therapy Position Statement of the North American Menopause Society Advisory Panel. The 2022 hormone therapy position statement of the North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.0000000000002028
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;12;326:1429-1430. doi:10.1001 /jama.2021.3305
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-455. doi:10.1056 /NEJMcp1714787
- Abraham C. Proliferative endometrium in menopause: to treat or not to treat? Obstet Gynecol. 2023;141:265-267. doi:10.1097/AOG.0000000000005054
- Chandra V, Kim JJ, Benbrook DM, et al. Therapeutic options for management of endometrial hyperplasia. J Gynecol Oncol. 2016;27:e8. doi:10.3802/jgo.2016.27.e8
- Owings RA, Quick CM. Endometrial intraepithelial neoplasia. Arch Pathol Lab Med. 2014;138:484-491. doi:10.5858 /arpa.2012-0709-RA
- Rotenberg O, Doulaveris G, Fridman D, et al. Long-term outcome of postmenopausal women with proliferative endometrium on endometrial sampling. Am J Obstet Gynecol. 2020;223:896.e1-896.e7. doi:10.1016/j.ajog.2020.06.045
- Suzuki Y, Chen L, Hou JY, et al. Systemic progestins and progestin-releasing intrauterine device therapy for premenopausal patients with endometrial intraepithelial neoplasia. Obstet Gynecol. 2023;141:979-987. doi:10.1097 /AOG.0000000000005124
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-103.e13. doi:10.1016/j.ajog.2019.12.273
- Liu S, Kciuk O, Frank M, et al. Progestins of today and tomorrow. Curr Opin Obstet Gynecol. 2022;34:344-350. doi:10.1097 /GCO.0000000000000819
- Doll KM, Romano SS, Marsh EE, et al. Estimated performance of transvaginal ultrasonography for evaluation of postmenopausal bleeding in a simulated cohort of black and white women in the US. JAMA Oncol. 2021;7:1158-1165. doi:10.1001/jamaoncol.2021.1700
- Gompel A. Progesterone and endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2020;69:95-107. doi:10.1016 /j.bpobgyn.2020.05.003
This year’s menopause Update highlights a highly effective nonhormonal medication that recently received approval by the US Food and Drug Administration (FDA) for the treatment of bothersome menopausal vasomotor symptoms. In addition, the Update provides guidance regarding how ObGyns should respond when an endometrial biopsy for postmenopausal bleeding reveals proliferative changes.
Breakthrough in women’s health: A new nonhormone therapy for vasomotor symptoms
Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a phase 3 RCT. J Clin Endocrinol Metab. 2023;dgad058. doi:10.1210/clinem/dgad058.
Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102. doi:10.1016/S0140-6736(23)00085-5.
A new oral nonestrogen-containing medication for relief of moderate to severe hot flashes, fezolinetant (Veozah) 45 mg daily, has been approved by the FDA and was expected to be available by the end of May 2023. Fezolinetant is a selective neurokinin 3 (NK3) receptor antagonistthat offers a targeted nonhormonal approach to menopausal vasomotor symptoms (VMS), and it is the first in its class to make it to market.
The decline in estrogen at menopause appears to result in increased signaling at kisspeptin/neurokinin B/dynorphin (KNDy) neurons in the thermoregulatory center within the hypothalamus with resultant increases in hot flashes.1,2 Fezolinetant works by binding to and blocking the activities of the NK3 receptor.3-5
Key study findings
Selective NK3 receptor antagonists, including fezolinetant, effectively reduce the frequency and severity of VMS comparable to that of hormone therapy (HT). Two phase 3 clinical trials, Skylight 1 and 2, confirmed the efficacy and safety of fezolinetant 45 mg in treating VMS,6,7 and an additional 52-week placebo-controlled study, Skylight 4, confirmed long-term safety.8 Onset of action occurs within a week. Reported adverse events occurred in 1% to 2% of healthy menopausal women participating in clinical trials; these included headaches, abdominal pain, diarrhea, insomnia, back pain, hot flushes, and reversible elevated hepatic transaminase levels.6-9
The published phase 2 trials9 and the international randomized controlled trial (RCT) 12-week studies, Skylight 1 and 2,6,7 found that once-daily 30-mg and 45-mg doses of fezolinetant significantly reduced VMS frequency and severity at 12 weeks among women aged 40 to 60 years who reported an average of 7 moderate to severe VMS/day; the reduction in reported VMS was sustained at 40 weeks. Phase 3 data from Skylight 1 and 2 demonstrated fezolinetant’s efficacy in reducing the frequency and severity of VMS and provided information on the safety profile of fezolinetant compared with placebo over 12 weeks and a noncontrolled extension for an additional 40 weeks.6,7
Oral fezolinetant was associated with improved quality of life, including reduced VMS-related interference with daily life.10 Johnson and colleagues, reporting for Skylight 2, found VMS frequency and severity improvement by week 1, which achieved statistical significance at weeks 4 and 12, with this improvement maintained through week 52.6 A 64.3% reduction in mean daily VMS from baseline was seen at 12 weeks for fezolinetant 45 mg compared with a 45.4% reduction for placebo. VMS severity significantly decreased compared with placebo at 4 and 12 weeks.6
Serious treatment-emergent adverse events were infrequent, reported by 2%, 1%, and 0% of those receiving fezolinetant 30 mg, fezolinetant 45 mg, and placebo.6 Increases in levels of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) were noted and were described as asymptomatic, isolated, intermittent, or transient, and these levels returned to baseline during treatment or after discontinuation.6
Of the 5 participants taking fezolinetant in Skyline 1 with ALT or AST levels greater than 3 times the upper limit of normal in the 12-week randomized trial, levels returned to normal range while continuing treatment in 2 participants, with treatment interruption in 2, and with discontinuation in 1. No new safety signals were seen in the 40-week extension trial.6
Fezolinetant offers a much-needed effective and safe selective nonhormone NK3 receptor antagonist therapy that reduces the frequency and severity of menopausal VMS and has been shown to be safe through 52 weeks of treatment.
To read more about how fezolinetant specifically targets the hormone receptor that triggers hot flashes as well as on prescribing hormone therapy for women with menopausal symptoms, see “Focus on menopause: Q&A with Jan Shifren, MD, and Genevieve NealPerry, MD, PhD,” in the December 2022 issue of OBG Management at https://www.mdedge.com/obgyn/article/260380/menopause
Continue to: Endometrial and bone safety...
Endometrial and bone safety
Results from Skylight 4, a phase 3, randomized, double-blind, 52-week safety study, provided additional evidence that confirmed the longer-term safety of fezolinetant over a 52-week treatment period.8
Endometrial safety was assessed in postmenopausal women with normal baseline endometrium (n = 599).8 For fezolinetant 45 mg, 1 of 203 participants had endometrial hyperplasia (EH) (0.5%; upper limit of one-sided 95% confidence interval [CI], 2.3%); no cases of EH were noted in the placebo (0 of 186) or fezolinetant 30-mg (0 of 210) groups. The incidence of EH or malignancy in fezolinetant-treated participants was within prespecified limits, as assessed by blinded, centrally read endometrial biopsies. Endometrial malignancy occurred in 1 of 210 in the fezolinetant 30-mg group (0.5%; 95% CI, 2.2%) with no cases in the other groups, thus meeting FDA requirements for endometrial safety.8
In addition, no significant differences were noted in change from baseline endometrial thickness on transvaginal ultrasonography between fezolinetant-treated and placebo groups. Likewise, no loss of bone density was found on dual-energy x-ray absorptiometry (DEXA) scans or trabecular bone scores.8
Liver safety
Although no cases of severe liver injury were noted, elevations in serum transaminase concentrations greater than 3 times the upper limit of normal were observed in the clinical trials. In Skylight 4, liver enzyme elevations more than 3 times the upper limit of normal occurred in 6 of 583 participants taking placebo, 8 of 590 taking fezolinetant 30 mg, and 12 of 589 taking fezolinetant 45 mg.8
The prescribing information for fezolinetant includes a warning for elevated hepatic transaminases: Fezolinetant should not be started if baseline serum transaminase concentration is equal to or exceeds 2 times the upper limit of normal. Liver tests should be obtained at baseline and repeated every 3 months for the first 9 months and then if symptoms suggest liver injury.11,12
Unmet need for nonhormone treatment of VMS
Vasomotor symptoms affect up to 80% of women, with approximately 25% bothersome enough to warrant treatment. Vasomotor symptoms persist for a median of 7 years, with duration and severity differing by race and ethnicity. Black, Hispanic, and possibly Native American women experience the highest burden of VMS.2 Although VMS, including hot flashes, night sweats, and mood and sleep disturbances, often are considered an annoyance to those with mild symptoms, moderate to severe VMS impact women’s lives, including functioning at home or work, affecting relationships, and decreasing perceived quality of life, and they have been associated with workplace absenteeism and increased health care costs, both direct from medical care and testing and indirect costs from lost work.13-15
Women with 7 or more daily moderate to severe VMS (defined as with sweating or affecting function) reported interference with sleep (94%), concentration (84%), mood (85%), energy (77%), and sexual activity (61%).16 Moderately to severely bothersome VMS have been associated with impaired psychological and general well-being, affecting work performance.17 Based on a Mayo Clinic workplace survey, Faubion and colleagues estimated an annual loss of $1.8 billion in the United States for menopause-related missed work and a $28 billion loss when medical expenses were added.15
Menopausal HT has been the primary treatment for VMS and has been shown to reduce the frequency and severity of hot flashes, with additional benefits on sleep, mood, fatigue, bone loss and reduction of fracture, and genitourinary syndrome of menopause (GSM), and with potential improvement in cardiovascular health with decreased type 2 diabetes.18,19 For healthy women with early menopause and no contraindications, HT has been recommended until at least the age of natural menopause, as observational data suggest that HT prevents osteoporosis, cardiovascular disease, neurodegenerative changes, and sexual dysfunction for these women.19,20 Similarly, for healthy women younger than age 60 or within 10 years of menopause, initiating HT has been shown to be safe and effective in treating bothersome VMS and preventing osteoporotic fractures and genitourinary changes.19,21
Most systemic HT formulations are inexpensive (for example, available as generics), with multiple dosing and formulations available for use alone or combined as oral, transdermal, or vaginal therapies. Despite the fear that arose for clinicians and women from the initial 2002 findings of the Women’s Health Initiative regarding increased risk of breast cancer, stroke, venous thrombosis, cardiovascular disease, and dementia, major medical societies agree that when initiated at or soon after menopause, HT is a safe and effective therapy to relieve VMS, protect against bone loss, and treat genitourinary changes.19,21
Many women, however, cannot take HT, including those with estrogen-sensitive cancers, such as breast or uterine cancers; prior cardiovascular disease, stroke, or venous thrombotic events; severe endometriosis; or migraine headaches with visual auras.2 In addition, many symptomatic menopausal women without health contraindications choose not to take HT.2 Until now, the only FDA-approved VMS nonhormone therapy has been a low-dose 7.5-mg paroxetine salt. Unfortunately, this formulation, along with the off-label use of other antidepressants (selective serotonin reuptake inhibitors and serotonin and norepinephrine reuptake inhibitors), gabapentinoids, oxybutynin, and clonidine, are substantially less effective than HT in treating moderate to severe VMS.
Bottom line
A substantial unmet need remains for effective therapy for moderate to severe VMS for women who cannot or choose not to take menopausal HT to relieve VMS.2,16 Effective, safe nonhormone treatment options such as the new NK3 receptor antagonist fezolinetant will address this clinically important need.
One concern is that the cost of developing and bringing to market the first of a new type of medication will be passed on to consumers, which may put it out of the price range for the many women who need it. However, the development and FDA approval of fezolinetant as the first NK3 receptor antagonist to treat menopausal VMS is potentially a practice changer. It provides a novel, effective, and safe FDA-approved nonhormonal treatment for menopausal women with moderate to severe VMS, particularly for women who cannot or will not take hormone therapy.
Continue to: When endometrial biopsy for postmenopausal bleeding reveals proliferative changes, how should we respond?...
When endometrial biopsy for postmenopausal bleeding reveals proliferative changes, how should we respond?
Abraham C. Proliferative endometrium in menopause: to treat or not to treat? Obstet Gynecol. 2023;141:265-267. doi:10.1097/AOG.0000000000005054.
The following case represents a common scenario for ObGyns.
CASE Patient with proliferative endometrial changes
A menopausal patient with a body mass index (BMI) > 30 kg/m2 presents with uterine bleeding. She does not use systemic menopausal hormone therapy. Endometrial biopsy indicates proliferative changes.
When endometrial biopsy performed for bleeding reveals proliferative changes in menopausal women, we traditionally have responded by reassuring the patient that the findings are benign and advising that she should let us know if future spotting or bleeding occurs.
However, a recent review by Abraham published in Obstetrics and Gynecology details the implications of proliferative endometrial changes in menopausal patients, advising that treatment, as well as monitoring, may be appropriate.22
Endometrial changes and what they suggest
In premenopausal women, proliferative endometrial changes are physiologic and result from ovarian estrogen production early in each cycle, during what is called the proliferative (referring to the endometrium) or follicular (referring to the dominant follicle that synthesizes estrogen) phase. In menopausal women who are not using HT, however, proliferative endometrial changes, with orderly uniform glands seen on histologic evaluation, reflect aromatization of androgens by adipose and other tissues into estrogen.
The next step on the continuum to hyperplasia (benign or atypical) after proliferative endometrium is disordered proliferative endometrium. At this stage, histologic evaluation reveals scattered cystic and dilated glands that have a normal gland-to-stroma ratio with a low gland density overall and without any atypia. Randomly distributed glands may have tubal metaplasia or fibrin thrombi associated with microinfarcts, often presenting with irregular bleeding. This is a noncancerous change that occurs with excess estrogen (endogenous or exogenous).23
Progestins reverse endometrial hyperplasia by activating progesterone receptors, which leads to stromal decidualization with thinning of the endometrium. They have a pronounced effect on the histologic appearance of the endometrium. By contrast, endometrial intraepithelial neoplasia (EIN, previously known as endometrial hyperplasiawith atypia) shows underlying molecular mutations and histologic alterations and represents a sharp transition to true neoplasia, which greatly increases the risk of endometrioid endometrial adenocarcinoma.24
For decades, we have been aware that if women diagnosed with endometrial hyperplasia are not treated with progestational therapy, their future risk of endometrial cancer is elevated. More recently, we also recognize that menopausal women found to have proliferative endometrial changes, if not treated, have an increased risk of endometrial cancer.
In a retrospective cohort study of almost 300 menopausal women who were not treated after endometrial biopsy revealed proliferative changes, investigators followed participants for an average of 11 years.25 These women had a mean BMI of 34 kg/m2. During follow-up, almost 12% of these women were diagnosed with endometrial hyperplasia or cancer. This incidence of endometrial neoplasia was some 4 times higher than for women initially found to have atrophic endometrial changes.25
Progestin treatment
Oral progestin therapy with follow-up endometrial biopsy constitutes traditional management for endometrial hyperplasia. Such therapy minimizes the likelihood that hyperplasia will progress to endometrial cancer.
We now recognize that the convenience, as well as the high endometrial progestin levels achieved, with levonorgestrel-releasing intrauterine devices (LNG-IUDs) have advantages over oral progestin therapy in treating endometrial hyperplasia. Indeed, a recent US report found that among women with EIN managed medically, use of progestin-releasing IUDs has grown from 7.7% in 2008 to 35.6% in 2020.26
Although both oral and intrauterine progestin are highly effective in treating simple hyperplasia, progestin IUDs are substantially more effective than oral progestins in treating EIN.27 Progestin concentrations in the endometrium have been shown to be 100-fold higher after LNG-IUD placement compared with oral progestin use.22 In addition, adverse effects, including bloating, unpleasant mood changes, and increased appetite, are more common with oral than intrauterine progestin therapy.28
Unfortunately, data from randomized trials addressing progestational treatment of proliferative endometrium in menopausal women are not available to support the treatment of proliferative endometrium with either oral progestins or the LNG-IUD.22
Role of ultrasonography
Another concern is relying on a finding of thin endometrial thickness on vaginal ultrasonography. In a simulated retrospective cohort study, use of transvaginal ultrasonography to determine the appropriateness of a biopsy was found not to be sufficiently accurate or racially equitable with regard to Black women.29 In simulated data, transvaginal ultrasonography missed almost 5 times more cases of endometrial cancer among Black women compared with White women due to higher fibroid prevalence and nonendometrioid histologic type malignancies in Black women.29
Assessing risk
If proliferative endometrium is found, Abraham suggests assessing risk using22:
- age
- comorbidities (including obesity)
- endometrial echo thickness on vaginal ultrasonography.
Consider the patient’s risk and tolerance of recurrent bleeding as well as her tolerance for progestational adverse effects if medical therapy is chosen. Discussion about next steps should include reviewing the histologic findings with the patient and discussing the difference in risk of progression to endometrial cancer of a finding of proliferative endometrium compared with a histologic finding of endometrial hyperplasia.
Using this patient-centered approach, observation over time with follow-up endometrial biopsies remains a management option. Although some women may tolerate micronized progesterone over synthetic progestins, there is concern that it may be less effective in suppressing the endometrium than synthetic progestins.30 Accordingly, synthetic progestins represent first-line options in this setting.
In her review, Abraham suggests that when endometrial biopsy reveals proliferative changes in a menopausal woman, we should initiate progestin treatment and perform surveillance endometrial sampling every 3 to 6 months. If such sampling reveals benign but not proliferative endometrium, progestin therapy can be stopped and endometrial biopsy repeated if bleeding recurs.22 ●
ObGyns may choose to adopt Abraham’s approach or to hold off on progestin therapy while performing follow-up endometrial sampling. Either way, the take-home message is that the finding of proliferative endometrial changes on biopsy for postmenopausal bleeding requires proactive management.
This year’s menopause Update highlights a highly effective nonhormonal medication that recently received approval by the US Food and Drug Administration (FDA) for the treatment of bothersome menopausal vasomotor symptoms. In addition, the Update provides guidance regarding how ObGyns should respond when an endometrial biopsy for postmenopausal bleeding reveals proliferative changes.
Breakthrough in women’s health: A new nonhormone therapy for vasomotor symptoms
Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a phase 3 RCT. J Clin Endocrinol Metab. 2023;dgad058. doi:10.1210/clinem/dgad058.
Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102. doi:10.1016/S0140-6736(23)00085-5.
A new oral nonestrogen-containing medication for relief of moderate to severe hot flashes, fezolinetant (Veozah) 45 mg daily, has been approved by the FDA and was expected to be available by the end of May 2023. Fezolinetant is a selective neurokinin 3 (NK3) receptor antagonistthat offers a targeted nonhormonal approach to menopausal vasomotor symptoms (VMS), and it is the first in its class to make it to market.
The decline in estrogen at menopause appears to result in increased signaling at kisspeptin/neurokinin B/dynorphin (KNDy) neurons in the thermoregulatory center within the hypothalamus with resultant increases in hot flashes.1,2 Fezolinetant works by binding to and blocking the activities of the NK3 receptor.3-5
Key study findings
Selective NK3 receptor antagonists, including fezolinetant, effectively reduce the frequency and severity of VMS comparable to that of hormone therapy (HT). Two phase 3 clinical trials, Skylight 1 and 2, confirmed the efficacy and safety of fezolinetant 45 mg in treating VMS,6,7 and an additional 52-week placebo-controlled study, Skylight 4, confirmed long-term safety.8 Onset of action occurs within a week. Reported adverse events occurred in 1% to 2% of healthy menopausal women participating in clinical trials; these included headaches, abdominal pain, diarrhea, insomnia, back pain, hot flushes, and reversible elevated hepatic transaminase levels.6-9
The published phase 2 trials9 and the international randomized controlled trial (RCT) 12-week studies, Skylight 1 and 2,6,7 found that once-daily 30-mg and 45-mg doses of fezolinetant significantly reduced VMS frequency and severity at 12 weeks among women aged 40 to 60 years who reported an average of 7 moderate to severe VMS/day; the reduction in reported VMS was sustained at 40 weeks. Phase 3 data from Skylight 1 and 2 demonstrated fezolinetant’s efficacy in reducing the frequency and severity of VMS and provided information on the safety profile of fezolinetant compared with placebo over 12 weeks and a noncontrolled extension for an additional 40 weeks.6,7
Oral fezolinetant was associated with improved quality of life, including reduced VMS-related interference with daily life.10 Johnson and colleagues, reporting for Skylight 2, found VMS frequency and severity improvement by week 1, which achieved statistical significance at weeks 4 and 12, with this improvement maintained through week 52.6 A 64.3% reduction in mean daily VMS from baseline was seen at 12 weeks for fezolinetant 45 mg compared with a 45.4% reduction for placebo. VMS severity significantly decreased compared with placebo at 4 and 12 weeks.6
Serious treatment-emergent adverse events were infrequent, reported by 2%, 1%, and 0% of those receiving fezolinetant 30 mg, fezolinetant 45 mg, and placebo.6 Increases in levels of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) were noted and were described as asymptomatic, isolated, intermittent, or transient, and these levels returned to baseline during treatment or after discontinuation.6
Of the 5 participants taking fezolinetant in Skyline 1 with ALT or AST levels greater than 3 times the upper limit of normal in the 12-week randomized trial, levels returned to normal range while continuing treatment in 2 participants, with treatment interruption in 2, and with discontinuation in 1. No new safety signals were seen in the 40-week extension trial.6
Fezolinetant offers a much-needed effective and safe selective nonhormone NK3 receptor antagonist therapy that reduces the frequency and severity of menopausal VMS and has been shown to be safe through 52 weeks of treatment.
To read more about how fezolinetant specifically targets the hormone receptor that triggers hot flashes as well as on prescribing hormone therapy for women with menopausal symptoms, see “Focus on menopause: Q&A with Jan Shifren, MD, and Genevieve NealPerry, MD, PhD,” in the December 2022 issue of OBG Management at https://www.mdedge.com/obgyn/article/260380/menopause
Continue to: Endometrial and bone safety...
Endometrial and bone safety
Results from Skylight 4, a phase 3, randomized, double-blind, 52-week safety study, provided additional evidence that confirmed the longer-term safety of fezolinetant over a 52-week treatment period.8
Endometrial safety was assessed in postmenopausal women with normal baseline endometrium (n = 599).8 For fezolinetant 45 mg, 1 of 203 participants had endometrial hyperplasia (EH) (0.5%; upper limit of one-sided 95% confidence interval [CI], 2.3%); no cases of EH were noted in the placebo (0 of 186) or fezolinetant 30-mg (0 of 210) groups. The incidence of EH or malignancy in fezolinetant-treated participants was within prespecified limits, as assessed by blinded, centrally read endometrial biopsies. Endometrial malignancy occurred in 1 of 210 in the fezolinetant 30-mg group (0.5%; 95% CI, 2.2%) with no cases in the other groups, thus meeting FDA requirements for endometrial safety.8
In addition, no significant differences were noted in change from baseline endometrial thickness on transvaginal ultrasonography between fezolinetant-treated and placebo groups. Likewise, no loss of bone density was found on dual-energy x-ray absorptiometry (DEXA) scans or trabecular bone scores.8
Liver safety
Although no cases of severe liver injury were noted, elevations in serum transaminase concentrations greater than 3 times the upper limit of normal were observed in the clinical trials. In Skylight 4, liver enzyme elevations more than 3 times the upper limit of normal occurred in 6 of 583 participants taking placebo, 8 of 590 taking fezolinetant 30 mg, and 12 of 589 taking fezolinetant 45 mg.8
The prescribing information for fezolinetant includes a warning for elevated hepatic transaminases: Fezolinetant should not be started if baseline serum transaminase concentration is equal to or exceeds 2 times the upper limit of normal. Liver tests should be obtained at baseline and repeated every 3 months for the first 9 months and then if symptoms suggest liver injury.11,12
Unmet need for nonhormone treatment of VMS
Vasomotor symptoms affect up to 80% of women, with approximately 25% bothersome enough to warrant treatment. Vasomotor symptoms persist for a median of 7 years, with duration and severity differing by race and ethnicity. Black, Hispanic, and possibly Native American women experience the highest burden of VMS.2 Although VMS, including hot flashes, night sweats, and mood and sleep disturbances, often are considered an annoyance to those with mild symptoms, moderate to severe VMS impact women’s lives, including functioning at home or work, affecting relationships, and decreasing perceived quality of life, and they have been associated with workplace absenteeism and increased health care costs, both direct from medical care and testing and indirect costs from lost work.13-15
Women with 7 or more daily moderate to severe VMS (defined as with sweating or affecting function) reported interference with sleep (94%), concentration (84%), mood (85%), energy (77%), and sexual activity (61%).16 Moderately to severely bothersome VMS have been associated with impaired psychological and general well-being, affecting work performance.17 Based on a Mayo Clinic workplace survey, Faubion and colleagues estimated an annual loss of $1.8 billion in the United States for menopause-related missed work and a $28 billion loss when medical expenses were added.15
Menopausal HT has been the primary treatment for VMS and has been shown to reduce the frequency and severity of hot flashes, with additional benefits on sleep, mood, fatigue, bone loss and reduction of fracture, and genitourinary syndrome of menopause (GSM), and with potential improvement in cardiovascular health with decreased type 2 diabetes.18,19 For healthy women with early menopause and no contraindications, HT has been recommended until at least the age of natural menopause, as observational data suggest that HT prevents osteoporosis, cardiovascular disease, neurodegenerative changes, and sexual dysfunction for these women.19,20 Similarly, for healthy women younger than age 60 or within 10 years of menopause, initiating HT has been shown to be safe and effective in treating bothersome VMS and preventing osteoporotic fractures and genitourinary changes.19,21
Most systemic HT formulations are inexpensive (for example, available as generics), with multiple dosing and formulations available for use alone or combined as oral, transdermal, or vaginal therapies. Despite the fear that arose for clinicians and women from the initial 2002 findings of the Women’s Health Initiative regarding increased risk of breast cancer, stroke, venous thrombosis, cardiovascular disease, and dementia, major medical societies agree that when initiated at or soon after menopause, HT is a safe and effective therapy to relieve VMS, protect against bone loss, and treat genitourinary changes.19,21
Many women, however, cannot take HT, including those with estrogen-sensitive cancers, such as breast or uterine cancers; prior cardiovascular disease, stroke, or venous thrombotic events; severe endometriosis; or migraine headaches with visual auras.2 In addition, many symptomatic menopausal women without health contraindications choose not to take HT.2 Until now, the only FDA-approved VMS nonhormone therapy has been a low-dose 7.5-mg paroxetine salt. Unfortunately, this formulation, along with the off-label use of other antidepressants (selective serotonin reuptake inhibitors and serotonin and norepinephrine reuptake inhibitors), gabapentinoids, oxybutynin, and clonidine, are substantially less effective than HT in treating moderate to severe VMS.
Bottom line
A substantial unmet need remains for effective therapy for moderate to severe VMS for women who cannot or choose not to take menopausal HT to relieve VMS.2,16 Effective, safe nonhormone treatment options such as the new NK3 receptor antagonist fezolinetant will address this clinically important need.
One concern is that the cost of developing and bringing to market the first of a new type of medication will be passed on to consumers, which may put it out of the price range for the many women who need it. However, the development and FDA approval of fezolinetant as the first NK3 receptor antagonist to treat menopausal VMS is potentially a practice changer. It provides a novel, effective, and safe FDA-approved nonhormonal treatment for menopausal women with moderate to severe VMS, particularly for women who cannot or will not take hormone therapy.
Continue to: When endometrial biopsy for postmenopausal bleeding reveals proliferative changes, how should we respond?...
When endometrial biopsy for postmenopausal bleeding reveals proliferative changes, how should we respond?
Abraham C. Proliferative endometrium in menopause: to treat or not to treat? Obstet Gynecol. 2023;141:265-267. doi:10.1097/AOG.0000000000005054.
The following case represents a common scenario for ObGyns.
CASE Patient with proliferative endometrial changes
A menopausal patient with a body mass index (BMI) > 30 kg/m2 presents with uterine bleeding. She does not use systemic menopausal hormone therapy. Endometrial biopsy indicates proliferative changes.
When endometrial biopsy performed for bleeding reveals proliferative changes in menopausal women, we traditionally have responded by reassuring the patient that the findings are benign and advising that she should let us know if future spotting or bleeding occurs.
However, a recent review by Abraham published in Obstetrics and Gynecology details the implications of proliferative endometrial changes in menopausal patients, advising that treatment, as well as monitoring, may be appropriate.22
Endometrial changes and what they suggest
In premenopausal women, proliferative endometrial changes are physiologic and result from ovarian estrogen production early in each cycle, during what is called the proliferative (referring to the endometrium) or follicular (referring to the dominant follicle that synthesizes estrogen) phase. In menopausal women who are not using HT, however, proliferative endometrial changes, with orderly uniform glands seen on histologic evaluation, reflect aromatization of androgens by adipose and other tissues into estrogen.
The next step on the continuum to hyperplasia (benign or atypical) after proliferative endometrium is disordered proliferative endometrium. At this stage, histologic evaluation reveals scattered cystic and dilated glands that have a normal gland-to-stroma ratio with a low gland density overall and without any atypia. Randomly distributed glands may have tubal metaplasia or fibrin thrombi associated with microinfarcts, often presenting with irregular bleeding. This is a noncancerous change that occurs with excess estrogen (endogenous or exogenous).23
Progestins reverse endometrial hyperplasia by activating progesterone receptors, which leads to stromal decidualization with thinning of the endometrium. They have a pronounced effect on the histologic appearance of the endometrium. By contrast, endometrial intraepithelial neoplasia (EIN, previously known as endometrial hyperplasiawith atypia) shows underlying molecular mutations and histologic alterations and represents a sharp transition to true neoplasia, which greatly increases the risk of endometrioid endometrial adenocarcinoma.24
For decades, we have been aware that if women diagnosed with endometrial hyperplasia are not treated with progestational therapy, their future risk of endometrial cancer is elevated. More recently, we also recognize that menopausal women found to have proliferative endometrial changes, if not treated, have an increased risk of endometrial cancer.
In a retrospective cohort study of almost 300 menopausal women who were not treated after endometrial biopsy revealed proliferative changes, investigators followed participants for an average of 11 years.25 These women had a mean BMI of 34 kg/m2. During follow-up, almost 12% of these women were diagnosed with endometrial hyperplasia or cancer. This incidence of endometrial neoplasia was some 4 times higher than for women initially found to have atrophic endometrial changes.25
Progestin treatment
Oral progestin therapy with follow-up endometrial biopsy constitutes traditional management for endometrial hyperplasia. Such therapy minimizes the likelihood that hyperplasia will progress to endometrial cancer.
We now recognize that the convenience, as well as the high endometrial progestin levels achieved, with levonorgestrel-releasing intrauterine devices (LNG-IUDs) have advantages over oral progestin therapy in treating endometrial hyperplasia. Indeed, a recent US report found that among women with EIN managed medically, use of progestin-releasing IUDs has grown from 7.7% in 2008 to 35.6% in 2020.26
Although both oral and intrauterine progestin are highly effective in treating simple hyperplasia, progestin IUDs are substantially more effective than oral progestins in treating EIN.27 Progestin concentrations in the endometrium have been shown to be 100-fold higher after LNG-IUD placement compared with oral progestin use.22 In addition, adverse effects, including bloating, unpleasant mood changes, and increased appetite, are more common with oral than intrauterine progestin therapy.28
Unfortunately, data from randomized trials addressing progestational treatment of proliferative endometrium in menopausal women are not available to support the treatment of proliferative endometrium with either oral progestins or the LNG-IUD.22
Role of ultrasonography
Another concern is relying on a finding of thin endometrial thickness on vaginal ultrasonography. In a simulated retrospective cohort study, use of transvaginal ultrasonography to determine the appropriateness of a biopsy was found not to be sufficiently accurate or racially equitable with regard to Black women.29 In simulated data, transvaginal ultrasonography missed almost 5 times more cases of endometrial cancer among Black women compared with White women due to higher fibroid prevalence and nonendometrioid histologic type malignancies in Black women.29
Assessing risk
If proliferative endometrium is found, Abraham suggests assessing risk using22:
- age
- comorbidities (including obesity)
- endometrial echo thickness on vaginal ultrasonography.
Consider the patient’s risk and tolerance of recurrent bleeding as well as her tolerance for progestational adverse effects if medical therapy is chosen. Discussion about next steps should include reviewing the histologic findings with the patient and discussing the difference in risk of progression to endometrial cancer of a finding of proliferative endometrium compared with a histologic finding of endometrial hyperplasia.
Using this patient-centered approach, observation over time with follow-up endometrial biopsies remains a management option. Although some women may tolerate micronized progesterone over synthetic progestins, there is concern that it may be less effective in suppressing the endometrium than synthetic progestins.30 Accordingly, synthetic progestins represent first-line options in this setting.
In her review, Abraham suggests that when endometrial biopsy reveals proliferative changes in a menopausal woman, we should initiate progestin treatment and perform surveillance endometrial sampling every 3 to 6 months. If such sampling reveals benign but not proliferative endometrium, progestin therapy can be stopped and endometrial biopsy repeated if bleeding recurs.22 ●
ObGyns may choose to adopt Abraham’s approach or to hold off on progestin therapy while performing follow-up endometrial sampling. Either way, the take-home message is that the finding of proliferative endometrial changes on biopsy for postmenopausal bleeding requires proactive management.
- Modi M, Dhillo WS. Neurokinin 3 receptor antagonism: a novel treatment for menopausal hot flushes. Neuroendocrinology. 2019;109:242-248. doi:10.1159/000495889
- Pinkerton JV, Redick DL, Homewood LN, et al. Neurokinin receptor antagonist, fezolinetant, for treatment of menopausal vasomotor symptoms. J Clin Endocrinol Metab. 2023;dgad209. doi:10.1210/clinem/dgad209
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211-227. doi:10.1016 /j.yfrne.2013.07.003
- Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci USA. 2012;109:1984619851. doi:10.1073/pnas.1211517109
- Astellas Pharma. Astellas’ Veozah (fezolinetant) approved by US FDA for treatment of vasomotor symptoms due to menopause. May 12, 2023. PR Newswire. Accessed May 15, 2023. https://www.prnewswire.com/news-releases/astellas-veozah-fezolinetant-approved-by-us-fda-for -treatment-of-vasomotor-symptoms-due-to-menopause -301823639.html
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a phase 3 RCT. J Clin Endocrinol Metab. 2023;dgad058. doi:10.1210/clinem/dgad058
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102. doi:10.1016 /S0140-6736(23)00085-5
- Neal-Perry G, Cano A, Lederman S, et al. Safety of fezolinetant for vasomotor symptoms associated with menopause: a randomized controlled trial. Obstet Gynecol. 2023;141:737-747. doi:10.1097/AOG.0000000000005114
- Depypere H, Timmerman D, Donders G, et al. Treatment of menopausal vasomotor symptoms with fezolinetant, a neurokinin 3 receptor antagonist: a phase 2a trial. J Clin Endocrinol Metab. 2019;104:5893-5905. doi: 10.1210/jc .2019-00677
- Santoro N, Waldbaum A, Lederman S, et al. Effect of the neurokinin 3 receptor antagonist fezolinetant on patientreported outcomes in postmenopausal women with vasomotor symptoms: results of a randomized, placebo-controlled, double-blind, dose-ranging study (VESTA). Menopause. 2020;27:1350-1356. doi:10.1097/GME.0000000000001621
- FDA approves novel drug to treat moderate to severe hot flashes caused by menopause. May 12, 2023. US Food and Drug Administration. Accessed May 15, 2023. https://www .fda.gov/news-events/press-announcements/fda-approves -novel-drug-treat-moderate-severe-hot-flashes-caused -menopause
- Veozah. Prescribing information. Astellas; 2023. Accessed May 16, 2023. https://www.astellas.com/us/system/files /veozah_uspi.pdf
- Pinkerton JV. Money talks: untreated hot flashes cost women, the workplace, and society. Menopause. 2015;22:254-255. doi:10.1097/GME.0000000000000427
- Sarrel P, Portman D, Lefebvre P, et al. Incremental direct and indirect costs of untreated vasomotor symptoms. Menopause. 2015;22(3):260-266. doi:10.1097/GME.0000000000000320
- Faubion SS, Enders F, Hedges MS, et al. Impact of menopause symptoms on women in the workplace. Mayo Clin Proc. 2023;98:833-845. doi:10.1016/j.mayocp.2023.02.025
- Williams RE, Levine KB, Kalilani L, et al. Menopause- specific questionnaire assessment in US populationbased study shows negative impact on health-related quality of life. Maturitas. 2009;62:153-159. doi:10.1016 /j.maturitas.2008.12.006
- Gartoulla P, Bell RJ, Worsley R, et al. Moderate-severely bothersome vasomotor symptoms are associated with lowered psychological general wellbeing in women at midlife. Maturitas. 2015;81:487-492. doi:10.1016 /j.maturitas.2015.06.004
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806. doi:10.1056/NEJMp1514242
- 2022 Hormone Therapy Position Statement of the North American Menopause Society Advisory Panel. The 2022 hormone therapy position statement of the North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.0000000000002028
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;12;326:1429-1430. doi:10.1001 /jama.2021.3305
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-455. doi:10.1056 /NEJMcp1714787
- Abraham C. Proliferative endometrium in menopause: to treat or not to treat? Obstet Gynecol. 2023;141:265-267. doi:10.1097/AOG.0000000000005054
- Chandra V, Kim JJ, Benbrook DM, et al. Therapeutic options for management of endometrial hyperplasia. J Gynecol Oncol. 2016;27:e8. doi:10.3802/jgo.2016.27.e8
- Owings RA, Quick CM. Endometrial intraepithelial neoplasia. Arch Pathol Lab Med. 2014;138:484-491. doi:10.5858 /arpa.2012-0709-RA
- Rotenberg O, Doulaveris G, Fridman D, et al. Long-term outcome of postmenopausal women with proliferative endometrium on endometrial sampling. Am J Obstet Gynecol. 2020;223:896.e1-896.e7. doi:10.1016/j.ajog.2020.06.045
- Suzuki Y, Chen L, Hou JY, et al. Systemic progestins and progestin-releasing intrauterine device therapy for premenopausal patients with endometrial intraepithelial neoplasia. Obstet Gynecol. 2023;141:979-987. doi:10.1097 /AOG.0000000000005124
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-103.e13. doi:10.1016/j.ajog.2019.12.273
- Liu S, Kciuk O, Frank M, et al. Progestins of today and tomorrow. Curr Opin Obstet Gynecol. 2022;34:344-350. doi:10.1097 /GCO.0000000000000819
- Doll KM, Romano SS, Marsh EE, et al. Estimated performance of transvaginal ultrasonography for evaluation of postmenopausal bleeding in a simulated cohort of black and white women in the US. JAMA Oncol. 2021;7:1158-1165. doi:10.1001/jamaoncol.2021.1700
- Gompel A. Progesterone and endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2020;69:95-107. doi:10.1016 /j.bpobgyn.2020.05.003
- Modi M, Dhillo WS. Neurokinin 3 receptor antagonism: a novel treatment for menopausal hot flushes. Neuroendocrinology. 2019;109:242-248. doi:10.1159/000495889
- Pinkerton JV, Redick DL, Homewood LN, et al. Neurokinin receptor antagonist, fezolinetant, for treatment of menopausal vasomotor symptoms. J Clin Endocrinol Metab. 2023;dgad209. doi:10.1210/clinem/dgad209
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211-227. doi:10.1016 /j.yfrne.2013.07.003
- Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci USA. 2012;109:1984619851. doi:10.1073/pnas.1211517109
- Astellas Pharma. Astellas’ Veozah (fezolinetant) approved by US FDA for treatment of vasomotor symptoms due to menopause. May 12, 2023. PR Newswire. Accessed May 15, 2023. https://www.prnewswire.com/news-releases/astellas-veozah-fezolinetant-approved-by-us-fda-for -treatment-of-vasomotor-symptoms-due-to-menopause -301823639.html
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a phase 3 RCT. J Clin Endocrinol Metab. 2023;dgad058. doi:10.1210/clinem/dgad058
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102. doi:10.1016 /S0140-6736(23)00085-5
- Neal-Perry G, Cano A, Lederman S, et al. Safety of fezolinetant for vasomotor symptoms associated with menopause: a randomized controlled trial. Obstet Gynecol. 2023;141:737-747. doi:10.1097/AOG.0000000000005114
- Depypere H, Timmerman D, Donders G, et al. Treatment of menopausal vasomotor symptoms with fezolinetant, a neurokinin 3 receptor antagonist: a phase 2a trial. J Clin Endocrinol Metab. 2019;104:5893-5905. doi: 10.1210/jc .2019-00677
- Santoro N, Waldbaum A, Lederman S, et al. Effect of the neurokinin 3 receptor antagonist fezolinetant on patientreported outcomes in postmenopausal women with vasomotor symptoms: results of a randomized, placebo-controlled, double-blind, dose-ranging study (VESTA). Menopause. 2020;27:1350-1356. doi:10.1097/GME.0000000000001621
- FDA approves novel drug to treat moderate to severe hot flashes caused by menopause. May 12, 2023. US Food and Drug Administration. Accessed May 15, 2023. https://www .fda.gov/news-events/press-announcements/fda-approves -novel-drug-treat-moderate-severe-hot-flashes-caused -menopause
- Veozah. Prescribing information. Astellas; 2023. Accessed May 16, 2023. https://www.astellas.com/us/system/files /veozah_uspi.pdf
- Pinkerton JV. Money talks: untreated hot flashes cost women, the workplace, and society. Menopause. 2015;22:254-255. doi:10.1097/GME.0000000000000427
- Sarrel P, Portman D, Lefebvre P, et al. Incremental direct and indirect costs of untreated vasomotor symptoms. Menopause. 2015;22(3):260-266. doi:10.1097/GME.0000000000000320
- Faubion SS, Enders F, Hedges MS, et al. Impact of menopause symptoms on women in the workplace. Mayo Clin Proc. 2023;98:833-845. doi:10.1016/j.mayocp.2023.02.025
- Williams RE, Levine KB, Kalilani L, et al. Menopause- specific questionnaire assessment in US populationbased study shows negative impact on health-related quality of life. Maturitas. 2009;62:153-159. doi:10.1016 /j.maturitas.2008.12.006
- Gartoulla P, Bell RJ, Worsley R, et al. Moderate-severely bothersome vasomotor symptoms are associated with lowered psychological general wellbeing in women at midlife. Maturitas. 2015;81:487-492. doi:10.1016 /j.maturitas.2015.06.004
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806. doi:10.1056/NEJMp1514242
- 2022 Hormone Therapy Position Statement of the North American Menopause Society Advisory Panel. The 2022 hormone therapy position statement of the North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.0000000000002028
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;12;326:1429-1430. doi:10.1001 /jama.2021.3305
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-455. doi:10.1056 /NEJMcp1714787
- Abraham C. Proliferative endometrium in menopause: to treat or not to treat? Obstet Gynecol. 2023;141:265-267. doi:10.1097/AOG.0000000000005054
- Chandra V, Kim JJ, Benbrook DM, et al. Therapeutic options for management of endometrial hyperplasia. J Gynecol Oncol. 2016;27:e8. doi:10.3802/jgo.2016.27.e8
- Owings RA, Quick CM. Endometrial intraepithelial neoplasia. Arch Pathol Lab Med. 2014;138:484-491. doi:10.5858 /arpa.2012-0709-RA
- Rotenberg O, Doulaveris G, Fridman D, et al. Long-term outcome of postmenopausal women with proliferative endometrium on endometrial sampling. Am J Obstet Gynecol. 2020;223:896.e1-896.e7. doi:10.1016/j.ajog.2020.06.045
- Suzuki Y, Chen L, Hou JY, et al. Systemic progestins and progestin-releasing intrauterine device therapy for premenopausal patients with endometrial intraepithelial neoplasia. Obstet Gynecol. 2023;141:979-987. doi:10.1097 /AOG.0000000000005124
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-103.e13. doi:10.1016/j.ajog.2019.12.273
- Liu S, Kciuk O, Frank M, et al. Progestins of today and tomorrow. Curr Opin Obstet Gynecol. 2022;34:344-350. doi:10.1097 /GCO.0000000000000819
- Doll KM, Romano SS, Marsh EE, et al. Estimated performance of transvaginal ultrasonography for evaluation of postmenopausal bleeding in a simulated cohort of black and white women in the US. JAMA Oncol. 2021;7:1158-1165. doi:10.1001/jamaoncol.2021.1700
- Gompel A. Progesterone and endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2020;69:95-107. doi:10.1016 /j.bpobgyn.2020.05.003
The perimenopausal period and the benefits of progestin IUDs
Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.
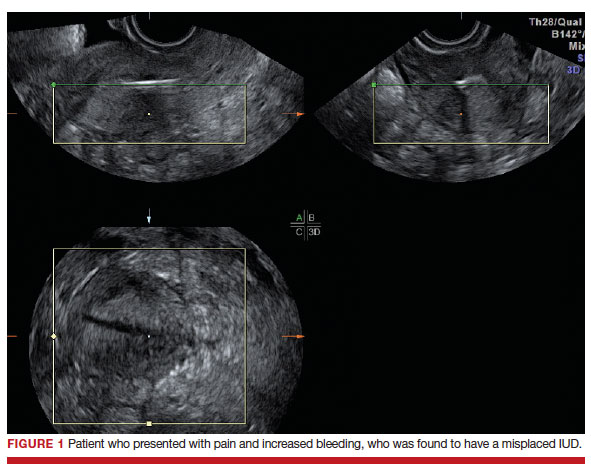
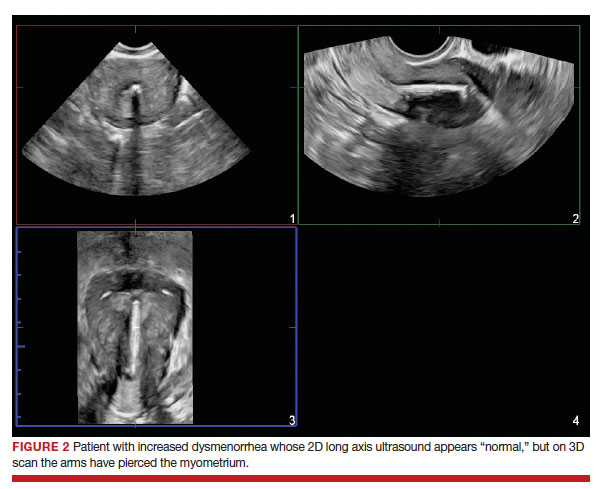
It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.

Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.


It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●

Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.


It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.
2020 Update on Menopause
The term genitourinary syndrome of menopause (GSM) refers to the bothersome symptoms and physical findings associated with estrogen deficiency that involve the labia, vestibular tissue, clitoris, vagina, urethra, and bladder.1 GSM is associated with genital irritation, dryness, and burning; urinary symptoms including urgency, dysuria, and recurrent urinary tract infections; and sexual symptoms including vaginal dryness and pain. Vulvovaginal atrophy (VVA) represents a component of GSM.
GSM is highly prevalent, affecting more than three-quarters of menopausal women. In contrast to menopausal vasomotor symptoms, which often are most severe and frequent in recently menopausal women, GSM commonly presents years following menopause. Unfortunately, VVA symptoms may have a substantial negative impact on women’s quality of life.
In this 2020 Menopause Update, I review a large observational study that provides reassurance to clinicians and patients regarding the safety of the best-studied prescription treatment for GSM—vaginal estrogen. Because some women should not use vaginal estrogen and others choose not to use it, nonhormonal management of GSM is important. Dr. JoAnn Pinkerton provides details on a randomized clinical trial that compared the use of fractionated CO2 laser therapy with vaginal estrogen for the treatment of GSM. In addition, Dr. JoAnn Manson discusses recent studies that found lower health risks with vaginal estrogen use compared with systemic estrogen therapy.
Diagnosing GSM
GSM can be diagnosed presumptively based on a characteristic history in a menopausal patient. Performing a pelvic examination, however, allows clinicians to exclude other conditions that may present with similar symptoms, such as lichen sclerosus, Candida infection, and malignancy.
During inspection of the external genitalia, the clinician may note loss of the fat pad in the labia majora and mons as well as a reduction in labia minora pigmentation and tissue. The urethral meatus often becomes erythematous and prominent. If vaginal or introital narrowing is present, use of a pediatric (ultrathin) speculum reduces patient discomfort. The vaginal mucosa may appear smooth due to loss of rugation; it also may appear shiny and dry. Bleeding (friability) on contact with a spatula or cotton-tipped swab may occur. In addition, the vaginal fornices may become attenuated, leaving the cervix flush with the vaginal apex.
GSM can be diagnosed without laboratory assessment. However, vaginal pH, if measured, is characteristically higher than 5.0; microscopic wet prep often reveals many white blood cells, immature epithelial cells (large nuclei), and reduced or absent lactobacilli.2
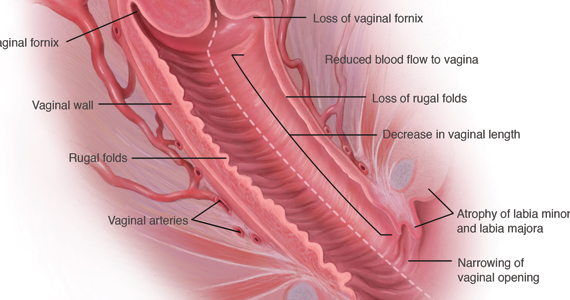
Nonhormonal management of GSM
Water, silicone-based, and oil-based lubricants reduce the friction and discomfort associated with sexual activity. By contrast, vaginal moisturizers act longer than lubricants and can be applied several times weekly or daily. Natural oils, including olive and coconut oil, may be useful both as lubricants and as moisturizers. Aqueous lidocaine 4%, applied to vestibular tissue with cotton balls prior to penetration, reduces dyspareunia in women with GSM.3
Vaginal estrogen therapy
When nonhormonal management does not sufficiently reduce GSM symptoms, use of low-dose vaginal estrogen enhances thickness and elasticity of genital tissue and improves vaginal blood flow. Vaginal estrogen creams, tablets, an insert, and a ring are marketed in the United States. Although clinical improvement may be apparent within several weeks of initiating vaginal estrogen, the full benefit of treatment becomes apparent after 2 to 3 months.3
Despite the availability and effectiveness of low-dose vaginal estrogen, fears regarding the safety of menopausal hormone therapy have resulted in the underutilization of vaginal estrogen.4,5 Unfortunately, the package labeling for low-dose vaginal estrogen can exacerbate these fears.
Continue to: Nurses’ Health Study report...
Nurses’ Health Study report provides reassurance on long-term safety of vaginal estrogen
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610
Bhupathiraju and colleagues published a report from the long-running Nurses’ Health prospective cohort study on the health outcomes associated with the use of vaginal estrogen.
Recap of the study
Starting in 1982, participants in the Nurses’Health Study were asked to report their use of vaginal estrogen via a validated questionnaire. For the years 1982 to 2012, investigators analyzed data from 896 and 52,901 women who had and had not used vaginal estrogen, respectively. The mean duration of vaginal estrogen use was 36 months.
In an analysis adjusted for numerous factors, the investigators observed no statistically significant differences in risk for cardiovascular outcomes (myocardial infarction, stroke, deep vein thrombosis, and pulmonary embolism) or invasive cancers (colorectal, endometrial, ovarian, or breast).
Findings uphold safety of vaginal estrogen
This landmark study provides reassurance that 3 years of use of vaginal estrogen does not increase the risk of cardiovascular events or invasive breast cancer, findings that hopefully will allow clinicians and women to feel comfortable regarding the safety of vaginal estrogen. A study of vaginal estrogen from the Women’s Health Initiative provided similar reassurance. Recent research supports guidance from The North American Menopause Society and the American College of Obstetricians and Gynecologists that vaginal estrogen can be used indefinitely, if indicated, and that use of concomitant progestin is not recommended in women who use vaginal estrogen and have an intact uterus.6,7
I agree with the authors, who point out that since treatment of GSM may need to be continued long term (even indefinitely), it would be helpful to have data that assessed the safety of longer-duration use of vaginal estrogen.
Results from Bhupathiraju and colleagues’ analysis of data from the Nurses’ Health Study on the 3-year safety of vaginal estrogen use encourage clinicians to recommend and women to use this safe and effective treatment for GSM.
How CO2 fractionated vaginal laser therapy compares with vaginal estrogen for relief of GSM symptoms
Paraiso MF, Ferrando CA, Sokol ER, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET trial. Menopause. 2020;27:50-56.
Up to 50% to 60% of postmenopausal women experience GSM symptoms. However, many fewer receive treatment, either because they do not understand that the symptoms are related to menopause or they are not aware that safe and effective treatment is available. Sadly, many women are not asked about their symptoms or are embarrassed to tell providers.
GSM affects relationships and quality of life. Vaginal lubricants or moisturizers may provide relief. US Food and Drug Administration (FDA)–approved therapies include low-dose vaginal estrogen, available as a vaginal tablet, cream, suppository, and ring; intravaginal dehydroepiandrosterone (DHEA); and oral ospemifene, a selective estrogen replacement modulator. If women have an estrogen-sensitive breast or uterine cancer, an oncologist should be involved in decisions about vaginal hormonal therapy.
Energy-based devices such as vaginal lasers appear to induce wound healing; stimulate collagen and elastin fiber formation through increased storage of glycogen; and activate fibroblasts, which leads to increased extracellular matrix and restoration of vaginal pH.
These lasers are FDA approved for use in gynecology but not specifically for the treatment of GSM. In July 2016, the FDA issued a safety alert that energy-based devices, while approved for use in gynecology, have not been approved or adequately tested for menopausal vaginal conditions, and safety concerns include reports of vaginal burns.8 Lacking are publications of adequately powered randomized, sham-con-trolled trials to determine if laser therapy works better for women with GSM than placebos, moisturizers, or vaginal hormone therapies.
Recently, investigators conducted a multicenter, randomized, single-blinded trial of vaginal laser therapy and estrogen cream for treatment of GSM.
Continue to: Details of the study...
Details of the study
Paraiso and colleagues aimed to compare the 6-month efficacy and safety of fractionated CO2 vaginal laser therapy with that of estrogen vaginal cream for the treatment of vaginal dryness/GSM.
Participants randomly assigned to the estrogen therapy arm applied conjugated estrogen cream 0.5 g vaginally daily for 14 days, followed by twice weekly application for 24 weeks (a low-dose vaginal estrogen therapy). Participants randomly assigned to laser therapy underwent 3 vaginal treatments at a minimum of 6 weeks apart.
Sixty-nine women were enrolled in the trial before enrollment was closed because the FDA required that the sponsor obtain and maintain an investigational device exemption. Of 62 women who completed 6 months’ treatment, 30 received 3 laser treatments and 32 received estrogen cream.
The primary outcome compared subjective improvement in vaginal dryness using the visual analog scale (VAS) between the 2 groups at 6 months. Secondary outcomes included comparisons of the vaginal health index (VHI) and vaginal maturation index (VMI), the effect of GSM on quality of life, the effect of treatment on sexual function and urinary symptoms, and patient satisfaction.
Study findings
Efficacy. Laser therapy and estrogen therapy were found to be similarly effective except on the VMI, which favored estrogen. On patient global impression, 85.8% of laser-treated women rated their improvement as ‘‘better or much better’’ and 78.5% reported being either ‘‘satisfied or very satisfied,’’ compared with 70% and 73.3%, respectively, in the estrogen group, a statistically nonsignificant difference.
On linear regression, the investigators found a nonsignificant mean difference in female sexual function index scores. While VMI scores remained higher in the estrogen-treated group (adjusted P = .02), baseline and 6-month follow-up VMI data were available for only 34 participants (16 laser treated, 18 estrogen treated).
Regarding long-term effectiveness, 20% to 25% of the women in the laser-treated group needed further treatment after 1 year while the estrogen cream continued to work as long as it was used as prescribed.
Adverse effects. The incidence of vaginal bleeding was similar in the 2 groups: 6.7% in the laser group and 6.3% in the estrogen group. In the laser therapy group, 3% expe-rienced vaginal pain, discharge, and bladder infections, while in the estrogen cream group, 3% reported breast tenderness, migraine headaches, and abdominal cramping.
Takeaways. This small randomized, open-label (not blinded) trial provides pilot data on the effectiveness of vaginal CO2 laser compared with vaginal estrogen in treating vaginal atrophy, quality-of-life symptoms, sexual function, and urinary symptoms. Adverse events were minimal. Patient global impression of improvement and satisfaction improved for both vaginal laser and vaginal estrogen therapy.
Continue to: Study strengths and limitations...
Study strengths and limitations
To show noninferiority of vaginal laser therapy to vaginal estrogen, 196 study participants were needed. However, after 38% had been enrolled, the FDA sent a warning letter to the Foundation for Female Health Awareness, which required obtainment of an investigational device exemption for the laser and addition of a sham treatment arm.9 Instead of redesigning the trial and reconsenting the participants, the investigators closed the study, and analysis was performed only on the 62 participants who completed the study; vaginal maturation was assessed only in 34 participants.
The study lacked a placebo or sham control, which increases the risk of bias, while small numbers limit the strength of the findings. Longer-term evaluation of the effects of laser therapy beyond 6 months is needed to allow assessment of the effects of scarring on vaginal health, sexual function, and urinary issues.
Discussing therapy with patients
Despite this study’s preliminary findings, and until more robust data are available, providers should discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, FDA-approved vaginal hormone therapies (such as vaginal estrogen and intravaginal dehydroepiandrosterone), and systemic therapies, such as hormone therapy and ospemifene, to determine the best treatment for the individual woman with GSM.
In a healthy postmenopausal woman with bothersome GSM symptoms not responsive to lubricants and moisturizers, I recommend FDA-approved vaginal therapies as first-line treatment if there are no contraindications. For women with breast cancer, I involve their oncologist. If a patient asks about vaginal laser treatment, I share that vaginal energy-based therapies, such as the vaginal laser, have not been approved for menopausal vaginal concerns. In addition to the possibility of adverse events or unsuccessful treatment, there are significant out-of-pocket costs and the potential need for ongoing therapy after the initial 3 laser treatments.
For GSM that does not respond to lubricants and moisturizers, many FDA-approved vaginal and systemic therapies are available to treat vaginal symptoms. Vaginal laser treatment is a promising therapy for vaginal symptoms of GSM that needs further testing to determine its efficacy, safety, and long-term effects. If discussing vaginal energy-based therapies with patients, include the current lack of FDA approval for specific vaginal indications, potential adverse effects, the need for ongoing retreatment, and out-of-pocket costs.
JoAnn E. Manson, MD, DrPH, NCMP
Having more appropriate, evidence-based labeling of low-dose vaginal estrogen continues to be a high priority for The North American Menopause Society (NAMS), the International Society for the Study of Women’s Sexual Health (ISSWSH), and other professional societies.
NAMS and the Working Group on Women’s Health and Well-Being in Menopause had submitted a citizen’s petition to the US Food and Drug Administration (FDA) in 2016 requesting modification of the label—including removal of the “black box warning”—for low-dose vaginal estrogen products. The petition was, disappointingly, denied in 2018.1
Currently, the class labeling, which was based on the results of randomized trials with systemic hormone therapy, is not applicable to low-dose vaginal estrogen, and the inclusion of the black box warning has led to serious underutilization of an effective and safe treatment for a very common and life-altering condition, the genitourinary syndrome of menopause (GSM). This condition affects nearly half of postmenopausal women. It tends to be chronic and progressive and, unlike hot flashes and vasomotor symptoms, it does not remit or decline over time, and it affects women’s health and quality of life.
While removal of the black box warning would be appropriate, labeling should include emphatic reminders for women that if they have any bleeding or spotting they should seek medical attention immediately, and if they have a history of breast cancer or other estrogen-sensitive cancers they should talk with their oncologist prior to starting treatment with low-dose vaginal estrogen. Although the text would still inform women of research results on systemic hormone therapy, it would explain the differences between low-dose vaginal estrogen and systemic therapy.
Studies show vaginal estrogen has good safety profile
In the last several years, large, observational studies of low-dose vaginal estrogen have suggested that this treatment is not associated with an increase in cardiovascular disease, pulmonary embolism, venous thrombosis, cancer, or dementia—conditions listed in the black box warning that were linked to systemic estrogen therapy plus synthetic progestin. Recent data from the Nurses’ Health Study, for example, demonstrated that 3 years of vaginal estrogen use did not increase the risk of cardiovascular events or invasive breast cancer.
Women’s Health Initiative. In a prospective observational cohort study, Crandall and colleagues used data from participants in the Women’s Health Initiative Observational Study to determine the association between use of vaginal estrogen and risk of a global index event (GIE), defined as time to first occurrence of coronary heart disease, invasive breast cancer, stroke, pulmonary embolism, hip fracture, colorectal cancer, endometrial cancer, or death from any cause.2
Women were recruited from multiple clinical centers, were aged 50 to 79 years at baseline, and did not use systemic estrogen therapy during follow-up. The study included 45,663 women and median follow-up was 7.2 years. The investigators collected data on women’s self-reported use of vaginal estrogen as well as the development of the conditions defined above.
In women with a uterus, there was no significant difference between vaginal estrogen users and nonusers in the risk of stroke, invasive breast cancer, colorectal cancer, endometrial cancer, pulmonary embolism, or deep vein thrombosis. The risks of coronary heart disease, fracture, all-cause mortality, and GIE were lower in vaginal estrogen users than in nonusers (GIE adjusted hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.55–0.86).
In women who had undergone hysterectomy, the risks of the individual GIE components and the overall GIE were not significantly different in users of vaginal estrogen compared with nonusers (GIE adjusted HR, 0.94; 95% CI, 0.70–1.26).
The investigators concluded that the risks of cardiovascular disease and cancer were not increased in postmenopausal women who used vaginal estrogen. Thus, this study offers reassurance on the treatment’s safety.2
Meta-analysis on menopausal hormone therapy and breast cancer risk. Further evidence now indicates that low-dose vaginal estrogen is not linked to chronic health conditions. In a large meta-analysis published in 2019, investigators looked at different types of hormone therapies—oral estrogen plus progestin, transdermal estrogen and progestin, estrogen alone, low-dose vaginal estrogen—and their relationship to breast cancer risk.3
Information on individual participants was obtained from 58 studies, 24 prospective and 34 retrospective. Breast cancer relative risks (RR) during years 5 to 14 of current hormone use were assessed according to the main hormonal contituents, doses, and modes of delivery of the last-used menopausal hormone therapy. For all systemic estrogen-only preparations, the RR was 1.33 (95% CI, 1.28–1.38), while for all estrogen-progestogen preparations, the RR was 2.08 (95% CI, 2.02–2.15). For transdermal estrogen, the RR was 1.35 (95% CI, 1.25–1.46). In contrast, for vaginal estrogen, the RR was 1.09 (95% CI, 0.97–1.23).3
Thus, the analysis found that in all the studies that had been done to date, there was no evidence of increased risk of breast cancer with vaginal estrogen therapy.
The evidence is growing that low-dose vaginal estrogen is different from systemic estrogen in terms of its safety profile and benefit-risk pattern. It is important for the FDA to consider these data and revise the vaginal estrogen label.
On the horizon: New estradiol reference ranges
It would be useful if we could accurately compare estradiol levels in women treated with vaginal estrogen against those of women treated with systemic estrogen therapy. In September 2019, NAMS held a workshop with the goal of establishing reference ranges for estradiol in postmenopausal women.4 It is very important to have good, reliable laboratory assays for estradiol and estrone, and to have a clear understanding of what is a reference range, that is, the range of estradiol levels in postmenopausal women who are not treated with estrogen. That way, you can observe what the estradiol blood levels are in women treated with low-dose vaginal estrogen or those treated with systemic estrogen versus the levels observed among postmenopausal women not receiving any estrogen product.
With the reference range information, we could look at data on the blood levels of estradiol with low-dose vaginal estrogen from the various studies available, as well as the increasing evidence from observational studies of the safety of low-dose vaginal estrogen to better understand its relationship with health. If these studies demonstrate that, with certain doses and formulations of low-dose vaginal estrogen, blood estradiol levels stay within the reference range of postmenopausal estradiol levels, it would inform the labeling modifications of these products. We need this information for future discussions with the FDA.
The laboratory assay technology used for such an investigation is primarily liquid chromatography with tandem mass spectrometry, the so-called LC-MS/MS assay. With use of this technology, the reference range for estradiol may be less than 10 picograms per milliliter. Previously, a very wide and inconsistent range—about 5 to 30 picograms per milliliter—was considered a “normal” range.
NAMS is championing the efforts to define a true evidence-based reference range that would represent the range of levels seen in postmenopausal women.5 This effort has been spearheaded by Dr. Richard Santen and colleagues. Using the more sensitive and specific LC-MS/MS assay will enable researchers and clinicians to better understand how levels on low-dose vaginal estrogen relate to the reference range for postmenopausal women. We are hoping to work together with researchers to establish these reference ranges, and to use that information to look at how low-dose vaginal estrogen compares to levels in untreated postmenopausal women, as well as to levels in women on systemic estrogen.
Hopefully, establishing the reference range can be done in an expeditious and timely way, with discussions with the FDA resuming shortly thereafter.
References
1.NAMS Citizen’s Petition and FDA Response, June 7, 2018. http://www.menopause.org/docs/
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. 2018;25:11-20.
3. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394:1159-1168.
4. Santen RJ, Pinkerton JV, Liu JH, et al. Workshop on normal reference ranges for estradiol in postmenopausal women, September 2019, Chicago, Illinois. Menopause. May 4, 2020. doi:10.1097/GME.0000000000001556.
5. Pinkerton JV, Liu JH, Santoro NF, et al. Workshop on normal reference ranges for estradiol in postmenopausal women: commentary from The North American Menopause Society on low-dose vaginal estrogen therapy. Menopause. 2020;27:611-613.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Maturitas. 2014;79:349-354.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126:859-876.
- Shifren JL. Genitourinary syndrome of menopause. Clin Obstet Gynecol. 2018;61:508-516.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806.
- Kingsberg SA, Krychman M, Graham S, et al. The women’s EMPOWER survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 Hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- US Food and Drug Administration website. FDA warns against the use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/medical-devices/safety-communications/fda-warns-against-use-energy-based-devices-perform-vaginal-rejuvenation-or-vaginal-cosmetic. Updated November 20, 2018. Accessed May 21, 2020.
- US Food and Drug Administration website. Letters to industry. https://www.fda.gov/medical-devices/industry-medical-devices/letters-industry. July 24, 2018. Accessed May 21, 2020
The term genitourinary syndrome of menopause (GSM) refers to the bothersome symptoms and physical findings associated with estrogen deficiency that involve the labia, vestibular tissue, clitoris, vagina, urethra, and bladder.1 GSM is associated with genital irritation, dryness, and burning; urinary symptoms including urgency, dysuria, and recurrent urinary tract infections; and sexual symptoms including vaginal dryness and pain. Vulvovaginal atrophy (VVA) represents a component of GSM.
GSM is highly prevalent, affecting more than three-quarters of menopausal women. In contrast to menopausal vasomotor symptoms, which often are most severe and frequent in recently menopausal women, GSM commonly presents years following menopause. Unfortunately, VVA symptoms may have a substantial negative impact on women’s quality of life.
In this 2020 Menopause Update, I review a large observational study that provides reassurance to clinicians and patients regarding the safety of the best-studied prescription treatment for GSM—vaginal estrogen. Because some women should not use vaginal estrogen and others choose not to use it, nonhormonal management of GSM is important. Dr. JoAnn Pinkerton provides details on a randomized clinical trial that compared the use of fractionated CO2 laser therapy with vaginal estrogen for the treatment of GSM. In addition, Dr. JoAnn Manson discusses recent studies that found lower health risks with vaginal estrogen use compared with systemic estrogen therapy.
Diagnosing GSM
GSM can be diagnosed presumptively based on a characteristic history in a menopausal patient. Performing a pelvic examination, however, allows clinicians to exclude other conditions that may present with similar symptoms, such as lichen sclerosus, Candida infection, and malignancy.
During inspection of the external genitalia, the clinician may note loss of the fat pad in the labia majora and mons as well as a reduction in labia minora pigmentation and tissue. The urethral meatus often becomes erythematous and prominent. If vaginal or introital narrowing is present, use of a pediatric (ultrathin) speculum reduces patient discomfort. The vaginal mucosa may appear smooth due to loss of rugation; it also may appear shiny and dry. Bleeding (friability) on contact with a spatula or cotton-tipped swab may occur. In addition, the vaginal fornices may become attenuated, leaving the cervix flush with the vaginal apex.
GSM can be diagnosed without laboratory assessment. However, vaginal pH, if measured, is characteristically higher than 5.0; microscopic wet prep often reveals many white blood cells, immature epithelial cells (large nuclei), and reduced or absent lactobacilli.2

Nonhormonal management of GSM
Water, silicone-based, and oil-based lubricants reduce the friction and discomfort associated with sexual activity. By contrast, vaginal moisturizers act longer than lubricants and can be applied several times weekly or daily. Natural oils, including olive and coconut oil, may be useful both as lubricants and as moisturizers. Aqueous lidocaine 4%, applied to vestibular tissue with cotton balls prior to penetration, reduces dyspareunia in women with GSM.3
Vaginal estrogen therapy
When nonhormonal management does not sufficiently reduce GSM symptoms, use of low-dose vaginal estrogen enhances thickness and elasticity of genital tissue and improves vaginal blood flow. Vaginal estrogen creams, tablets, an insert, and a ring are marketed in the United States. Although clinical improvement may be apparent within several weeks of initiating vaginal estrogen, the full benefit of treatment becomes apparent after 2 to 3 months.3
Despite the availability and effectiveness of low-dose vaginal estrogen, fears regarding the safety of menopausal hormone therapy have resulted in the underutilization of vaginal estrogen.4,5 Unfortunately, the package labeling for low-dose vaginal estrogen can exacerbate these fears.
Continue to: Nurses’ Health Study report...
Nurses’ Health Study report provides reassurance on long-term safety of vaginal estrogen
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610
Bhupathiraju and colleagues published a report from the long-running Nurses’ Health prospective cohort study on the health outcomes associated with the use of vaginal estrogen.
Recap of the study
Starting in 1982, participants in the Nurses’Health Study were asked to report their use of vaginal estrogen via a validated questionnaire. For the years 1982 to 2012, investigators analyzed data from 896 and 52,901 women who had and had not used vaginal estrogen, respectively. The mean duration of vaginal estrogen use was 36 months.
In an analysis adjusted for numerous factors, the investigators observed no statistically significant differences in risk for cardiovascular outcomes (myocardial infarction, stroke, deep vein thrombosis, and pulmonary embolism) or invasive cancers (colorectal, endometrial, ovarian, or breast).
Findings uphold safety of vaginal estrogen
This landmark study provides reassurance that 3 years of use of vaginal estrogen does not increase the risk of cardiovascular events or invasive breast cancer, findings that hopefully will allow clinicians and women to feel comfortable regarding the safety of vaginal estrogen. A study of vaginal estrogen from the Women’s Health Initiative provided similar reassurance. Recent research supports guidance from The North American Menopause Society and the American College of Obstetricians and Gynecologists that vaginal estrogen can be used indefinitely, if indicated, and that use of concomitant progestin is not recommended in women who use vaginal estrogen and have an intact uterus.6,7
I agree with the authors, who point out that since treatment of GSM may need to be continued long term (even indefinitely), it would be helpful to have data that assessed the safety of longer-duration use of vaginal estrogen.
Results from Bhupathiraju and colleagues’ analysis of data from the Nurses’ Health Study on the 3-year safety of vaginal estrogen use encourage clinicians to recommend and women to use this safe and effective treatment for GSM.
How CO2 fractionated vaginal laser therapy compares with vaginal estrogen for relief of GSM symptoms
Paraiso MF, Ferrando CA, Sokol ER, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET trial. Menopause. 2020;27:50-56.
Up to 50% to 60% of postmenopausal women experience GSM symptoms. However, many fewer receive treatment, either because they do not understand that the symptoms are related to menopause or they are not aware that safe and effective treatment is available. Sadly, many women are not asked about their symptoms or are embarrassed to tell providers.
GSM affects relationships and quality of life. Vaginal lubricants or moisturizers may provide relief. US Food and Drug Administration (FDA)–approved therapies include low-dose vaginal estrogen, available as a vaginal tablet, cream, suppository, and ring; intravaginal dehydroepiandrosterone (DHEA); and oral ospemifene, a selective estrogen replacement modulator. If women have an estrogen-sensitive breast or uterine cancer, an oncologist should be involved in decisions about vaginal hormonal therapy.
Energy-based devices such as vaginal lasers appear to induce wound healing; stimulate collagen and elastin fiber formation through increased storage of glycogen; and activate fibroblasts, which leads to increased extracellular matrix and restoration of vaginal pH.
These lasers are FDA approved for use in gynecology but not specifically for the treatment of GSM. In July 2016, the FDA issued a safety alert that energy-based devices, while approved for use in gynecology, have not been approved or adequately tested for menopausal vaginal conditions, and safety concerns include reports of vaginal burns.8 Lacking are publications of adequately powered randomized, sham-con-trolled trials to determine if laser therapy works better for women with GSM than placebos, moisturizers, or vaginal hormone therapies.
Recently, investigators conducted a multicenter, randomized, single-blinded trial of vaginal laser therapy and estrogen cream for treatment of GSM.
Continue to: Details of the study...
Details of the study
Paraiso and colleagues aimed to compare the 6-month efficacy and safety of fractionated CO2 vaginal laser therapy with that of estrogen vaginal cream for the treatment of vaginal dryness/GSM.
Participants randomly assigned to the estrogen therapy arm applied conjugated estrogen cream 0.5 g vaginally daily for 14 days, followed by twice weekly application for 24 weeks (a low-dose vaginal estrogen therapy). Participants randomly assigned to laser therapy underwent 3 vaginal treatments at a minimum of 6 weeks apart.
Sixty-nine women were enrolled in the trial before enrollment was closed because the FDA required that the sponsor obtain and maintain an investigational device exemption. Of 62 women who completed 6 months’ treatment, 30 received 3 laser treatments and 32 received estrogen cream.
The primary outcome compared subjective improvement in vaginal dryness using the visual analog scale (VAS) between the 2 groups at 6 months. Secondary outcomes included comparisons of the vaginal health index (VHI) and vaginal maturation index (VMI), the effect of GSM on quality of life, the effect of treatment on sexual function and urinary symptoms, and patient satisfaction.
Study findings
Efficacy. Laser therapy and estrogen therapy were found to be similarly effective except on the VMI, which favored estrogen. On patient global impression, 85.8% of laser-treated women rated their improvement as ‘‘better or much better’’ and 78.5% reported being either ‘‘satisfied or very satisfied,’’ compared with 70% and 73.3%, respectively, in the estrogen group, a statistically nonsignificant difference.
On linear regression, the investigators found a nonsignificant mean difference in female sexual function index scores. While VMI scores remained higher in the estrogen-treated group (adjusted P = .02), baseline and 6-month follow-up VMI data were available for only 34 participants (16 laser treated, 18 estrogen treated).
Regarding long-term effectiveness, 20% to 25% of the women in the laser-treated group needed further treatment after 1 year while the estrogen cream continued to work as long as it was used as prescribed.
Adverse effects. The incidence of vaginal bleeding was similar in the 2 groups: 6.7% in the laser group and 6.3% in the estrogen group. In the laser therapy group, 3% expe-rienced vaginal pain, discharge, and bladder infections, while in the estrogen cream group, 3% reported breast tenderness, migraine headaches, and abdominal cramping.
Takeaways. This small randomized, open-label (not blinded) trial provides pilot data on the effectiveness of vaginal CO2 laser compared with vaginal estrogen in treating vaginal atrophy, quality-of-life symptoms, sexual function, and urinary symptoms. Adverse events were minimal. Patient global impression of improvement and satisfaction improved for both vaginal laser and vaginal estrogen therapy.
Continue to: Study strengths and limitations...
Study strengths and limitations
To show noninferiority of vaginal laser therapy to vaginal estrogen, 196 study participants were needed. However, after 38% had been enrolled, the FDA sent a warning letter to the Foundation for Female Health Awareness, which required obtainment of an investigational device exemption for the laser and addition of a sham treatment arm.9 Instead of redesigning the trial and reconsenting the participants, the investigators closed the study, and analysis was performed only on the 62 participants who completed the study; vaginal maturation was assessed only in 34 participants.
The study lacked a placebo or sham control, which increases the risk of bias, while small numbers limit the strength of the findings. Longer-term evaluation of the effects of laser therapy beyond 6 months is needed to allow assessment of the effects of scarring on vaginal health, sexual function, and urinary issues.
Discussing therapy with patients
Despite this study’s preliminary findings, and until more robust data are available, providers should discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, FDA-approved vaginal hormone therapies (such as vaginal estrogen and intravaginal dehydroepiandrosterone), and systemic therapies, such as hormone therapy and ospemifene, to determine the best treatment for the individual woman with GSM.
In a healthy postmenopausal woman with bothersome GSM symptoms not responsive to lubricants and moisturizers, I recommend FDA-approved vaginal therapies as first-line treatment if there are no contraindications. For women with breast cancer, I involve their oncologist. If a patient asks about vaginal laser treatment, I share that vaginal energy-based therapies, such as the vaginal laser, have not been approved for menopausal vaginal concerns. In addition to the possibility of adverse events or unsuccessful treatment, there are significant out-of-pocket costs and the potential need for ongoing therapy after the initial 3 laser treatments.
For GSM that does not respond to lubricants and moisturizers, many FDA-approved vaginal and systemic therapies are available to treat vaginal symptoms. Vaginal laser treatment is a promising therapy for vaginal symptoms of GSM that needs further testing to determine its efficacy, safety, and long-term effects. If discussing vaginal energy-based therapies with patients, include the current lack of FDA approval for specific vaginal indications, potential adverse effects, the need for ongoing retreatment, and out-of-pocket costs.
JoAnn E. Manson, MD, DrPH, NCMP
Having more appropriate, evidence-based labeling of low-dose vaginal estrogen continues to be a high priority for The North American Menopause Society (NAMS), the International Society for the Study of Women’s Sexual Health (ISSWSH), and other professional societies.
NAMS and the Working Group on Women’s Health and Well-Being in Menopause had submitted a citizen’s petition to the US Food and Drug Administration (FDA) in 2016 requesting modification of the label—including removal of the “black box warning”—for low-dose vaginal estrogen products. The petition was, disappointingly, denied in 2018.1
Currently, the class labeling, which was based on the results of randomized trials with systemic hormone therapy, is not applicable to low-dose vaginal estrogen, and the inclusion of the black box warning has led to serious underutilization of an effective and safe treatment for a very common and life-altering condition, the genitourinary syndrome of menopause (GSM). This condition affects nearly half of postmenopausal women. It tends to be chronic and progressive and, unlike hot flashes and vasomotor symptoms, it does not remit or decline over time, and it affects women’s health and quality of life.
While removal of the black box warning would be appropriate, labeling should include emphatic reminders for women that if they have any bleeding or spotting they should seek medical attention immediately, and if they have a history of breast cancer or other estrogen-sensitive cancers they should talk with their oncologist prior to starting treatment with low-dose vaginal estrogen. Although the text would still inform women of research results on systemic hormone therapy, it would explain the differences between low-dose vaginal estrogen and systemic therapy.
Studies show vaginal estrogen has good safety profile
In the last several years, large, observational studies of low-dose vaginal estrogen have suggested that this treatment is not associated with an increase in cardiovascular disease, pulmonary embolism, venous thrombosis, cancer, or dementia—conditions listed in the black box warning that were linked to systemic estrogen therapy plus synthetic progestin. Recent data from the Nurses’ Health Study, for example, demonstrated that 3 years of vaginal estrogen use did not increase the risk of cardiovascular events or invasive breast cancer.
Women’s Health Initiative. In a prospective observational cohort study, Crandall and colleagues used data from participants in the Women’s Health Initiative Observational Study to determine the association between use of vaginal estrogen and risk of a global index event (GIE), defined as time to first occurrence of coronary heart disease, invasive breast cancer, stroke, pulmonary embolism, hip fracture, colorectal cancer, endometrial cancer, or death from any cause.2
Women were recruited from multiple clinical centers, were aged 50 to 79 years at baseline, and did not use systemic estrogen therapy during follow-up. The study included 45,663 women and median follow-up was 7.2 years. The investigators collected data on women’s self-reported use of vaginal estrogen as well as the development of the conditions defined above.
In women with a uterus, there was no significant difference between vaginal estrogen users and nonusers in the risk of stroke, invasive breast cancer, colorectal cancer, endometrial cancer, pulmonary embolism, or deep vein thrombosis. The risks of coronary heart disease, fracture, all-cause mortality, and GIE were lower in vaginal estrogen users than in nonusers (GIE adjusted hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.55–0.86).
In women who had undergone hysterectomy, the risks of the individual GIE components and the overall GIE were not significantly different in users of vaginal estrogen compared with nonusers (GIE adjusted HR, 0.94; 95% CI, 0.70–1.26).
The investigators concluded that the risks of cardiovascular disease and cancer were not increased in postmenopausal women who used vaginal estrogen. Thus, this study offers reassurance on the treatment’s safety.2
Meta-analysis on menopausal hormone therapy and breast cancer risk. Further evidence now indicates that low-dose vaginal estrogen is not linked to chronic health conditions. In a large meta-analysis published in 2019, investigators looked at different types of hormone therapies—oral estrogen plus progestin, transdermal estrogen and progestin, estrogen alone, low-dose vaginal estrogen—and their relationship to breast cancer risk.3
Information on individual participants was obtained from 58 studies, 24 prospective and 34 retrospective. Breast cancer relative risks (RR) during years 5 to 14 of current hormone use were assessed according to the main hormonal contituents, doses, and modes of delivery of the last-used menopausal hormone therapy. For all systemic estrogen-only preparations, the RR was 1.33 (95% CI, 1.28–1.38), while for all estrogen-progestogen preparations, the RR was 2.08 (95% CI, 2.02–2.15). For transdermal estrogen, the RR was 1.35 (95% CI, 1.25–1.46). In contrast, for vaginal estrogen, the RR was 1.09 (95% CI, 0.97–1.23).3
Thus, the analysis found that in all the studies that had been done to date, there was no evidence of increased risk of breast cancer with vaginal estrogen therapy.
The evidence is growing that low-dose vaginal estrogen is different from systemic estrogen in terms of its safety profile and benefit-risk pattern. It is important for the FDA to consider these data and revise the vaginal estrogen label.
On the horizon: New estradiol reference ranges
It would be useful if we could accurately compare estradiol levels in women treated with vaginal estrogen against those of women treated with systemic estrogen therapy. In September 2019, NAMS held a workshop with the goal of establishing reference ranges for estradiol in postmenopausal women.4 It is very important to have good, reliable laboratory assays for estradiol and estrone, and to have a clear understanding of what is a reference range, that is, the range of estradiol levels in postmenopausal women who are not treated with estrogen. That way, you can observe what the estradiol blood levels are in women treated with low-dose vaginal estrogen or those treated with systemic estrogen versus the levels observed among postmenopausal women not receiving any estrogen product.
With the reference range information, we could look at data on the blood levels of estradiol with low-dose vaginal estrogen from the various studies available, as well as the increasing evidence from observational studies of the safety of low-dose vaginal estrogen to better understand its relationship with health. If these studies demonstrate that, with certain doses and formulations of low-dose vaginal estrogen, blood estradiol levels stay within the reference range of postmenopausal estradiol levels, it would inform the labeling modifications of these products. We need this information for future discussions with the FDA.
The laboratory assay technology used for such an investigation is primarily liquid chromatography with tandem mass spectrometry, the so-called LC-MS/MS assay. With use of this technology, the reference range for estradiol may be less than 10 picograms per milliliter. Previously, a very wide and inconsistent range—about 5 to 30 picograms per milliliter—was considered a “normal” range.
NAMS is championing the efforts to define a true evidence-based reference range that would represent the range of levels seen in postmenopausal women.5 This effort has been spearheaded by Dr. Richard Santen and colleagues. Using the more sensitive and specific LC-MS/MS assay will enable researchers and clinicians to better understand how levels on low-dose vaginal estrogen relate to the reference range for postmenopausal women. We are hoping to work together with researchers to establish these reference ranges, and to use that information to look at how low-dose vaginal estrogen compares to levels in untreated postmenopausal women, as well as to levels in women on systemic estrogen.
Hopefully, establishing the reference range can be done in an expeditious and timely way, with discussions with the FDA resuming shortly thereafter.
References
1.NAMS Citizen’s Petition and FDA Response, June 7, 2018. http://www.menopause.org/docs/
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. 2018;25:11-20.
3. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394:1159-1168.
4. Santen RJ, Pinkerton JV, Liu JH, et al. Workshop on normal reference ranges for estradiol in postmenopausal women, September 2019, Chicago, Illinois. Menopause. May 4, 2020. doi:10.1097/GME.0000000000001556.
5. Pinkerton JV, Liu JH, Santoro NF, et al. Workshop on normal reference ranges for estradiol in postmenopausal women: commentary from The North American Menopause Society on low-dose vaginal estrogen therapy. Menopause. 2020;27:611-613.
The term genitourinary syndrome of menopause (GSM) refers to the bothersome symptoms and physical findings associated with estrogen deficiency that involve the labia, vestibular tissue, clitoris, vagina, urethra, and bladder.1 GSM is associated with genital irritation, dryness, and burning; urinary symptoms including urgency, dysuria, and recurrent urinary tract infections; and sexual symptoms including vaginal dryness and pain. Vulvovaginal atrophy (VVA) represents a component of GSM.
GSM is highly prevalent, affecting more than three-quarters of menopausal women. In contrast to menopausal vasomotor symptoms, which often are most severe and frequent in recently menopausal women, GSM commonly presents years following menopause. Unfortunately, VVA symptoms may have a substantial negative impact on women’s quality of life.
In this 2020 Menopause Update, I review a large observational study that provides reassurance to clinicians and patients regarding the safety of the best-studied prescription treatment for GSM—vaginal estrogen. Because some women should not use vaginal estrogen and others choose not to use it, nonhormonal management of GSM is important. Dr. JoAnn Pinkerton provides details on a randomized clinical trial that compared the use of fractionated CO2 laser therapy with vaginal estrogen for the treatment of GSM. In addition, Dr. JoAnn Manson discusses recent studies that found lower health risks with vaginal estrogen use compared with systemic estrogen therapy.
Diagnosing GSM
GSM can be diagnosed presumptively based on a characteristic history in a menopausal patient. Performing a pelvic examination, however, allows clinicians to exclude other conditions that may present with similar symptoms, such as lichen sclerosus, Candida infection, and malignancy.
During inspection of the external genitalia, the clinician may note loss of the fat pad in the labia majora and mons as well as a reduction in labia minora pigmentation and tissue. The urethral meatus often becomes erythematous and prominent. If vaginal or introital narrowing is present, use of a pediatric (ultrathin) speculum reduces patient discomfort. The vaginal mucosa may appear smooth due to loss of rugation; it also may appear shiny and dry. Bleeding (friability) on contact with a spatula or cotton-tipped swab may occur. In addition, the vaginal fornices may become attenuated, leaving the cervix flush with the vaginal apex.
GSM can be diagnosed without laboratory assessment. However, vaginal pH, if measured, is characteristically higher than 5.0; microscopic wet prep often reveals many white blood cells, immature epithelial cells (large nuclei), and reduced or absent lactobacilli.2

Nonhormonal management of GSM
Water, silicone-based, and oil-based lubricants reduce the friction and discomfort associated with sexual activity. By contrast, vaginal moisturizers act longer than lubricants and can be applied several times weekly or daily. Natural oils, including olive and coconut oil, may be useful both as lubricants and as moisturizers. Aqueous lidocaine 4%, applied to vestibular tissue with cotton balls prior to penetration, reduces dyspareunia in women with GSM.3
Vaginal estrogen therapy
When nonhormonal management does not sufficiently reduce GSM symptoms, use of low-dose vaginal estrogen enhances thickness and elasticity of genital tissue and improves vaginal blood flow. Vaginal estrogen creams, tablets, an insert, and a ring are marketed in the United States. Although clinical improvement may be apparent within several weeks of initiating vaginal estrogen, the full benefit of treatment becomes apparent after 2 to 3 months.3
Despite the availability and effectiveness of low-dose vaginal estrogen, fears regarding the safety of menopausal hormone therapy have resulted in the underutilization of vaginal estrogen.4,5 Unfortunately, the package labeling for low-dose vaginal estrogen can exacerbate these fears.
Continue to: Nurses’ Health Study report...
Nurses’ Health Study report provides reassurance on long-term safety of vaginal estrogen
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610
Bhupathiraju and colleagues published a report from the long-running Nurses’ Health prospective cohort study on the health outcomes associated with the use of vaginal estrogen.
Recap of the study
Starting in 1982, participants in the Nurses’Health Study were asked to report their use of vaginal estrogen via a validated questionnaire. For the years 1982 to 2012, investigators analyzed data from 896 and 52,901 women who had and had not used vaginal estrogen, respectively. The mean duration of vaginal estrogen use was 36 months.
In an analysis adjusted for numerous factors, the investigators observed no statistically significant differences in risk for cardiovascular outcomes (myocardial infarction, stroke, deep vein thrombosis, and pulmonary embolism) or invasive cancers (colorectal, endometrial, ovarian, or breast).
Findings uphold safety of vaginal estrogen
This landmark study provides reassurance that 3 years of use of vaginal estrogen does not increase the risk of cardiovascular events or invasive breast cancer, findings that hopefully will allow clinicians and women to feel comfortable regarding the safety of vaginal estrogen. A study of vaginal estrogen from the Women’s Health Initiative provided similar reassurance. Recent research supports guidance from The North American Menopause Society and the American College of Obstetricians and Gynecologists that vaginal estrogen can be used indefinitely, if indicated, and that use of concomitant progestin is not recommended in women who use vaginal estrogen and have an intact uterus.6,7
I agree with the authors, who point out that since treatment of GSM may need to be continued long term (even indefinitely), it would be helpful to have data that assessed the safety of longer-duration use of vaginal estrogen.
Results from Bhupathiraju and colleagues’ analysis of data from the Nurses’ Health Study on the 3-year safety of vaginal estrogen use encourage clinicians to recommend and women to use this safe and effective treatment for GSM.
How CO2 fractionated vaginal laser therapy compares with vaginal estrogen for relief of GSM symptoms
Paraiso MF, Ferrando CA, Sokol ER, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET trial. Menopause. 2020;27:50-56.
Up to 50% to 60% of postmenopausal women experience GSM symptoms. However, many fewer receive treatment, either because they do not understand that the symptoms are related to menopause or they are not aware that safe and effective treatment is available. Sadly, many women are not asked about their symptoms or are embarrassed to tell providers.
GSM affects relationships and quality of life. Vaginal lubricants or moisturizers may provide relief. US Food and Drug Administration (FDA)–approved therapies include low-dose vaginal estrogen, available as a vaginal tablet, cream, suppository, and ring; intravaginal dehydroepiandrosterone (DHEA); and oral ospemifene, a selective estrogen replacement modulator. If women have an estrogen-sensitive breast or uterine cancer, an oncologist should be involved in decisions about vaginal hormonal therapy.
Energy-based devices such as vaginal lasers appear to induce wound healing; stimulate collagen and elastin fiber formation through increased storage of glycogen; and activate fibroblasts, which leads to increased extracellular matrix and restoration of vaginal pH.
These lasers are FDA approved for use in gynecology but not specifically for the treatment of GSM. In July 2016, the FDA issued a safety alert that energy-based devices, while approved for use in gynecology, have not been approved or adequately tested for menopausal vaginal conditions, and safety concerns include reports of vaginal burns.8 Lacking are publications of adequately powered randomized, sham-con-trolled trials to determine if laser therapy works better for women with GSM than placebos, moisturizers, or vaginal hormone therapies.
Recently, investigators conducted a multicenter, randomized, single-blinded trial of vaginal laser therapy and estrogen cream for treatment of GSM.
Continue to: Details of the study...
Details of the study
Paraiso and colleagues aimed to compare the 6-month efficacy and safety of fractionated CO2 vaginal laser therapy with that of estrogen vaginal cream for the treatment of vaginal dryness/GSM.
Participants randomly assigned to the estrogen therapy arm applied conjugated estrogen cream 0.5 g vaginally daily for 14 days, followed by twice weekly application for 24 weeks (a low-dose vaginal estrogen therapy). Participants randomly assigned to laser therapy underwent 3 vaginal treatments at a minimum of 6 weeks apart.
Sixty-nine women were enrolled in the trial before enrollment was closed because the FDA required that the sponsor obtain and maintain an investigational device exemption. Of 62 women who completed 6 months’ treatment, 30 received 3 laser treatments and 32 received estrogen cream.
The primary outcome compared subjective improvement in vaginal dryness using the visual analog scale (VAS) between the 2 groups at 6 months. Secondary outcomes included comparisons of the vaginal health index (VHI) and vaginal maturation index (VMI), the effect of GSM on quality of life, the effect of treatment on sexual function and urinary symptoms, and patient satisfaction.
Study findings
Efficacy. Laser therapy and estrogen therapy were found to be similarly effective except on the VMI, which favored estrogen. On patient global impression, 85.8% of laser-treated women rated their improvement as ‘‘better or much better’’ and 78.5% reported being either ‘‘satisfied or very satisfied,’’ compared with 70% and 73.3%, respectively, in the estrogen group, a statistically nonsignificant difference.
On linear regression, the investigators found a nonsignificant mean difference in female sexual function index scores. While VMI scores remained higher in the estrogen-treated group (adjusted P = .02), baseline and 6-month follow-up VMI data were available for only 34 participants (16 laser treated, 18 estrogen treated).
Regarding long-term effectiveness, 20% to 25% of the women in the laser-treated group needed further treatment after 1 year while the estrogen cream continued to work as long as it was used as prescribed.
Adverse effects. The incidence of vaginal bleeding was similar in the 2 groups: 6.7% in the laser group and 6.3% in the estrogen group. In the laser therapy group, 3% expe-rienced vaginal pain, discharge, and bladder infections, while in the estrogen cream group, 3% reported breast tenderness, migraine headaches, and abdominal cramping.
Takeaways. This small randomized, open-label (not blinded) trial provides pilot data on the effectiveness of vaginal CO2 laser compared with vaginal estrogen in treating vaginal atrophy, quality-of-life symptoms, sexual function, and urinary symptoms. Adverse events were minimal. Patient global impression of improvement and satisfaction improved for both vaginal laser and vaginal estrogen therapy.
Continue to: Study strengths and limitations...
Study strengths and limitations
To show noninferiority of vaginal laser therapy to vaginal estrogen, 196 study participants were needed. However, after 38% had been enrolled, the FDA sent a warning letter to the Foundation for Female Health Awareness, which required obtainment of an investigational device exemption for the laser and addition of a sham treatment arm.9 Instead of redesigning the trial and reconsenting the participants, the investigators closed the study, and analysis was performed only on the 62 participants who completed the study; vaginal maturation was assessed only in 34 participants.
The study lacked a placebo or sham control, which increases the risk of bias, while small numbers limit the strength of the findings. Longer-term evaluation of the effects of laser therapy beyond 6 months is needed to allow assessment of the effects of scarring on vaginal health, sexual function, and urinary issues.
Discussing therapy with patients
Despite this study’s preliminary findings, and until more robust data are available, providers should discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, FDA-approved vaginal hormone therapies (such as vaginal estrogen and intravaginal dehydroepiandrosterone), and systemic therapies, such as hormone therapy and ospemifene, to determine the best treatment for the individual woman with GSM.
In a healthy postmenopausal woman with bothersome GSM symptoms not responsive to lubricants and moisturizers, I recommend FDA-approved vaginal therapies as first-line treatment if there are no contraindications. For women with breast cancer, I involve their oncologist. If a patient asks about vaginal laser treatment, I share that vaginal energy-based therapies, such as the vaginal laser, have not been approved for menopausal vaginal concerns. In addition to the possibility of adverse events or unsuccessful treatment, there are significant out-of-pocket costs and the potential need for ongoing therapy after the initial 3 laser treatments.
For GSM that does not respond to lubricants and moisturizers, many FDA-approved vaginal and systemic therapies are available to treat vaginal symptoms. Vaginal laser treatment is a promising therapy for vaginal symptoms of GSM that needs further testing to determine its efficacy, safety, and long-term effects. If discussing vaginal energy-based therapies with patients, include the current lack of FDA approval for specific vaginal indications, potential adverse effects, the need for ongoing retreatment, and out-of-pocket costs.
JoAnn E. Manson, MD, DrPH, NCMP
Having more appropriate, evidence-based labeling of low-dose vaginal estrogen continues to be a high priority for The North American Menopause Society (NAMS), the International Society for the Study of Women’s Sexual Health (ISSWSH), and other professional societies.
NAMS and the Working Group on Women’s Health and Well-Being in Menopause had submitted a citizen’s petition to the US Food and Drug Administration (FDA) in 2016 requesting modification of the label—including removal of the “black box warning”—for low-dose vaginal estrogen products. The petition was, disappointingly, denied in 2018.1
Currently, the class labeling, which was based on the results of randomized trials with systemic hormone therapy, is not applicable to low-dose vaginal estrogen, and the inclusion of the black box warning has led to serious underutilization of an effective and safe treatment for a very common and life-altering condition, the genitourinary syndrome of menopause (GSM). This condition affects nearly half of postmenopausal women. It tends to be chronic and progressive and, unlike hot flashes and vasomotor symptoms, it does not remit or decline over time, and it affects women’s health and quality of life.
While removal of the black box warning would be appropriate, labeling should include emphatic reminders for women that if they have any bleeding or spotting they should seek medical attention immediately, and if they have a history of breast cancer or other estrogen-sensitive cancers they should talk with their oncologist prior to starting treatment with low-dose vaginal estrogen. Although the text would still inform women of research results on systemic hormone therapy, it would explain the differences between low-dose vaginal estrogen and systemic therapy.
Studies show vaginal estrogen has good safety profile
In the last several years, large, observational studies of low-dose vaginal estrogen have suggested that this treatment is not associated with an increase in cardiovascular disease, pulmonary embolism, venous thrombosis, cancer, or dementia—conditions listed in the black box warning that were linked to systemic estrogen therapy plus synthetic progestin. Recent data from the Nurses’ Health Study, for example, demonstrated that 3 years of vaginal estrogen use did not increase the risk of cardiovascular events or invasive breast cancer.
Women’s Health Initiative. In a prospective observational cohort study, Crandall and colleagues used data from participants in the Women’s Health Initiative Observational Study to determine the association between use of vaginal estrogen and risk of a global index event (GIE), defined as time to first occurrence of coronary heart disease, invasive breast cancer, stroke, pulmonary embolism, hip fracture, colorectal cancer, endometrial cancer, or death from any cause.2
Women were recruited from multiple clinical centers, were aged 50 to 79 years at baseline, and did not use systemic estrogen therapy during follow-up. The study included 45,663 women and median follow-up was 7.2 years. The investigators collected data on women’s self-reported use of vaginal estrogen as well as the development of the conditions defined above.
In women with a uterus, there was no significant difference between vaginal estrogen users and nonusers in the risk of stroke, invasive breast cancer, colorectal cancer, endometrial cancer, pulmonary embolism, or deep vein thrombosis. The risks of coronary heart disease, fracture, all-cause mortality, and GIE were lower in vaginal estrogen users than in nonusers (GIE adjusted hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.55–0.86).
In women who had undergone hysterectomy, the risks of the individual GIE components and the overall GIE were not significantly different in users of vaginal estrogen compared with nonusers (GIE adjusted HR, 0.94; 95% CI, 0.70–1.26).
The investigators concluded that the risks of cardiovascular disease and cancer were not increased in postmenopausal women who used vaginal estrogen. Thus, this study offers reassurance on the treatment’s safety.2
Meta-analysis on menopausal hormone therapy and breast cancer risk. Further evidence now indicates that low-dose vaginal estrogen is not linked to chronic health conditions. In a large meta-analysis published in 2019, investigators looked at different types of hormone therapies—oral estrogen plus progestin, transdermal estrogen and progestin, estrogen alone, low-dose vaginal estrogen—and their relationship to breast cancer risk.3
Information on individual participants was obtained from 58 studies, 24 prospective and 34 retrospective. Breast cancer relative risks (RR) during years 5 to 14 of current hormone use were assessed according to the main hormonal contituents, doses, and modes of delivery of the last-used menopausal hormone therapy. For all systemic estrogen-only preparations, the RR was 1.33 (95% CI, 1.28–1.38), while for all estrogen-progestogen preparations, the RR was 2.08 (95% CI, 2.02–2.15). For transdermal estrogen, the RR was 1.35 (95% CI, 1.25–1.46). In contrast, for vaginal estrogen, the RR was 1.09 (95% CI, 0.97–1.23).3
Thus, the analysis found that in all the studies that had been done to date, there was no evidence of increased risk of breast cancer with vaginal estrogen therapy.
The evidence is growing that low-dose vaginal estrogen is different from systemic estrogen in terms of its safety profile and benefit-risk pattern. It is important for the FDA to consider these data and revise the vaginal estrogen label.
On the horizon: New estradiol reference ranges
It would be useful if we could accurately compare estradiol levels in women treated with vaginal estrogen against those of women treated with systemic estrogen therapy. In September 2019, NAMS held a workshop with the goal of establishing reference ranges for estradiol in postmenopausal women.4 It is very important to have good, reliable laboratory assays for estradiol and estrone, and to have a clear understanding of what is a reference range, that is, the range of estradiol levels in postmenopausal women who are not treated with estrogen. That way, you can observe what the estradiol blood levels are in women treated with low-dose vaginal estrogen or those treated with systemic estrogen versus the levels observed among postmenopausal women not receiving any estrogen product.
With the reference range information, we could look at data on the blood levels of estradiol with low-dose vaginal estrogen from the various studies available, as well as the increasing evidence from observational studies of the safety of low-dose vaginal estrogen to better understand its relationship with health. If these studies demonstrate that, with certain doses and formulations of low-dose vaginal estrogen, blood estradiol levels stay within the reference range of postmenopausal estradiol levels, it would inform the labeling modifications of these products. We need this information for future discussions with the FDA.
The laboratory assay technology used for such an investigation is primarily liquid chromatography with tandem mass spectrometry, the so-called LC-MS/MS assay. With use of this technology, the reference range for estradiol may be less than 10 picograms per milliliter. Previously, a very wide and inconsistent range—about 5 to 30 picograms per milliliter—was considered a “normal” range.
NAMS is championing the efforts to define a true evidence-based reference range that would represent the range of levels seen in postmenopausal women.5 This effort has been spearheaded by Dr. Richard Santen and colleagues. Using the more sensitive and specific LC-MS/MS assay will enable researchers and clinicians to better understand how levels on low-dose vaginal estrogen relate to the reference range for postmenopausal women. We are hoping to work together with researchers to establish these reference ranges, and to use that information to look at how low-dose vaginal estrogen compares to levels in untreated postmenopausal women, as well as to levels in women on systemic estrogen.
Hopefully, establishing the reference range can be done in an expeditious and timely way, with discussions with the FDA resuming shortly thereafter.
References
1.NAMS Citizen’s Petition and FDA Response, June 7, 2018. http://www.menopause.org/docs/
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. 2018;25:11-20.
3. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394:1159-1168.
4. Santen RJ, Pinkerton JV, Liu JH, et al. Workshop on normal reference ranges for estradiol in postmenopausal women, September 2019, Chicago, Illinois. Menopause. May 4, 2020. doi:10.1097/GME.0000000000001556.
5. Pinkerton JV, Liu JH, Santoro NF, et al. Workshop on normal reference ranges for estradiol in postmenopausal women: commentary from The North American Menopause Society on low-dose vaginal estrogen therapy. Menopause. 2020;27:611-613.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Maturitas. 2014;79:349-354.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126:859-876.
- Shifren JL. Genitourinary syndrome of menopause. Clin Obstet Gynecol. 2018;61:508-516.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806.
- Kingsberg SA, Krychman M, Graham S, et al. The women’s EMPOWER survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 Hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- US Food and Drug Administration website. FDA warns against the use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/medical-devices/safety-communications/fda-warns-against-use-energy-based-devices-perform-vaginal-rejuvenation-or-vaginal-cosmetic. Updated November 20, 2018. Accessed May 21, 2020.
- US Food and Drug Administration website. Letters to industry. https://www.fda.gov/medical-devices/industry-medical-devices/letters-industry. July 24, 2018. Accessed May 21, 2020
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Maturitas. 2014;79:349-354.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126:859-876.
- Shifren JL. Genitourinary syndrome of menopause. Clin Obstet Gynecol. 2018;61:508-516.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806.
- Kingsberg SA, Krychman M, Graham S, et al. The women’s EMPOWER survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 Hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- US Food and Drug Administration website. FDA warns against the use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/medical-devices/safety-communications/fda-warns-against-use-energy-based-devices-perform-vaginal-rejuvenation-or-vaginal-cosmetic. Updated November 20, 2018. Accessed May 21, 2020.
- US Food and Drug Administration website. Letters to industry. https://www.fda.gov/medical-devices/industry-medical-devices/letters-industry. July 24, 2018. Accessed May 21, 2020
2018 Update on menopause
Our knowledge regarding the benefits and risks of systemic menopausal hormone therapy (HT) has continued to evolve since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI). In late 2017, the US Preventive Services Task Force (USPSTF) issued its recommendation against the use of menopausal HT for the prevention of chronic conditions. In this Menopause Update, Dr. JoAnn Manson, Dr. JoAnn Pinkerton, and I detail why we do not support the Task Force’s recommendation. In a sidebar discussion, Dr. Manson also reviews the results of 2 WHI HT trials, published in September 2017, that analyzed mortality in trial participants over an 18-year follow-up.
In addition, I summarize an observational study that assessed the association of HT and Alzheimer disease (AD) as well as a clinical trial that compared the impact of oral versus transdermal estrogen on sexuality in recently menopausal women.
What's the impact of long-term use of systemic HT on Alzheimer disease risk?
Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062-1068.
Data from the WHI HT randomized trials have clarified that initiation of oral HT among women aged 65 and older increases the risk of cognitive decline. By contrast, an analysis of younger WHI participants found that oral HT had no impact on cognitive function. Recently, Imtiaz and colleagues conducted a prospective cohort study of postmenopausal HT and AD in women residing in a Finnish county, with 25 years of follow-up. A diagnosis of AD was based on administrative health records and use of medications prescribed specifically to treat dementia. Use of systemic HT was identified via self-report. Overall, among more than 8,000 women followed, 227 cases of AD (mean age, 72 years) were identified.
In an analysis that controlled for factors including age, body mass index, alcohol use, smoking, physical activity, occupation status, and parity, up to 5 years of HT use was not associated with a risk of being diagnosed with AD. Five to 10 years of HT use was associated with a hazard ratio (HR) of 0.89, an 11% risk reduction that did not achieve statistical significance. By contrast, more than 10 years' use of systemic HT was associated with an HR of 0.53, a statistically significant 47% reduction in risk of AD.1
Other studies found conflicting results
Three large randomized trials found that HT initiated early in menopause and continued for less than 7 years had no impact on cognitive function.2-4 The Cache County (Utah) long-term prospective cohort study, however, found that HT started early in menopause and continued for 10 years or longer was associated with a significant reduction in risk of AD.5
Of note are results from the 2017 report of 18-year cumulative mortality among WHI participants (see the box on page 30). In that study, mortality from AD and other dementia was lower among participants who were randomly assigned to treatment with estrogen alone versus placebo (HR, 0.74; 95% confidence interval [CI], 0.59-0.94). With estrogen-progestin therapy, the HR was 0.93 (95% CI, 0.77-1.11), and the pooled HR for the 2 trials was 0.85 (95% CI, 0.74-0.98).6
NAMS guidance
The North American Menopause Society (NAMS) HT position statement recommends that prevention of dementia should not be considered an indication for HT use since definitive data are not available.7 The statement indicates also that estrogen therapy may have positive cognitive benefits when initiated immediately after early surgical menopause and taken until the average age of menopause to prevent health risks seen with early loss of hormones.
Definitive data from long-term randomized clinical trials are not likely to become available. Observational trials continue to have methodologic issues, such as "healthy user bias," but the studies are reassuring that initiating HT close to menopause does not increase the risk of dementia. The long-term Finnish study by Imtiaz and colleagues and the Cache County study provide tentative observational data support for a "critical window" hypothesis, leaving open the possibility that initiating systemic HT soon after menopause onset and continuing it long term may reduce the risk of AD. Discussion is needed on individual patient characteristics, potential benefits and risks, and ongoing assessment over time.
Read Dr. Manson’s discussion of 18 years of follow-up data on menopause.
JoAnn E. Manson, MD, DrPH, NCMP
A new analysis from the Women's Health Initiative (WHI) randomized trials examined all-cause and cause-specific mortality during the intervention and postintervention follow-up periods.1 We followed more than 27,000 postmenopausal women aged 50 to 79 (mean age, 63) who were recruited to 2 randomized WHI trials of HT between 1993 and 1998. The trials continued until 2002 for the estrogen-progestin trial and to 2004 for the estrogen-alone trial. The trials ran for 5 to 7 years' duration, with post-stopping follow-up for an additional 10 to 12 years (total cumulative follow-up of 18 years).
The participants were randomly assigned to receive active treatment or placebo. The interventions were conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) versus placebo for women with an intact uterus and CEE alone versus placebo for women who had a hysterectomy.
All-cause mortality did not increase with HT use
The primary outcome measure was all-cause mortality in the 2 pooled trials and in each trial individually. We found that there was no link between HT and all-cause mortality in the overall study population (ages 50-79) in either trial. However, there was a trend toward lower all-cause mortality among the younger women in both trials. In women aged 50 to 59, there was a statistically significant 31% lower risk of mortality in the pooled trials among women taking active HT compared with those taking placebo, but no reduction in mortality with HT among older women (P for trend by age = .01).
Notably, all-cause mortality provides a critically important summary measure for interventions such as HT that have a complex matrix of benefits and risks. We know that HT has a number of benefits in menopausal women. It reduces hot flashes and other menopausal symptoms. It lowers the risk of hip fracture, other types of bone fractures, and type 2 diabetes. However, HT increases the risk of venous thrombosis, stroke, and some forms of cancer.
A summary measure that assesses the net effect of a medication on serious and life-threatening health outcomes is very important. As such, all-cause mortality is the ultimate bottom line for the balance of benefits and risks. This speaks to why we conducted the mortality analysis--WHI is the largest randomized trial of HT with long-term follow-up, allowing detailed analyses by age group. Although there have been previous reports on individual health outcomes in the WHI trials, no previous report had specifically focused on all-cause and cause-specific mortality with HT, stratified by age group, over long-term follow-up.
Hopefully the results of this study will alleviate some of the anxiety associated with HT because, as mentioned, there was no increase in overall total mortality or specific major causes of death. In addition, the younger women had a trend toward benefit for all-cause mortality.
We think that these findings support the recommendations from The North American Menopause Society and other professional societies that endorse the use of HT for managing bothersome menopausal symptoms, especially when started in early menopause. These results should be reassuring that there is no increase in mortality with HT use. Although these findings do not support prescribing HT for the express purpose of trying to prevent cardiovascular disease, dementia, or other chronic diseases (due to some potential risks), they do support an important role of HT for management of bothersome hot flashes, especially in early menopause.
Cause-specific mortality
Regarding cause-specific mortality and HT use, we looked in detail at deaths from cardiovascular causes, cancer, dementia, and other major illness. Overall, we observed no increase or decrease in cardiovascular or cancer deaths. In the estrogen-alone trial, there was a surprising finding of a 26% reduction in dementia deaths. In the estrogen-progestin trial, the results were neutral for dementia deaths.
Overall, the cause-specific mortality results were neutral. This is surprising because even for total cancer deaths there was no increase or decrease, despite a great deal of anxiety about cancer risk with HT. It appears that for cancer, HT has complex effects: it increases some types of cancer, such as breast cancer, and decreases others, such as endometrial cancer (in the estrogen-progestin group), and possibly colorectal cancer. Moreover, CEE alone was associated with a reduction in breast cancer mortality, but it remains unclear if this applies to other formulations. HT's net effect on total cancer mortality was neutral in both trials, that is, no increase or decrease.
Cautions and takeaways
We need to keep in mind that in current clinical practice, lower doses and different formulations and routes of administration of HT are now often used, including transdermal estradiol patches, gels, sprays, and micronized progesterone. These formulations, and the lower doses, may have an even more favorable benefit-risk profile. We need additional research on the long-term benefits and risks of these newer formulations and lower dosages.
Generally, these findings from the WHI trials indicate that for women who have significant hot flashes, night sweats, or other bothersome menopausal symptoms, it's important to discuss their symptoms with their health care provider and understand that hormone therapy may be an option for them. If it's not an option, many other treatments are available, including nonhormonal prescription medications, nonprescription medications, and behavioral approaches.
These findings should alleviate some fear about HT use, especially in younger women who have an overall favorable trend in terms of all-cause mortality with treatment, plus a much lower absolute risk of adverse events than older women. In a woman in early menopause who has bothersome hot flashes or other symptoms that disrupt her sleep or impair her quality of life, it's likely that the benefits of HT will outweigh the risks.
Reference
- Manson JE, Aragaki AK, Rossouw JE, et al; for the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women's Health Initiative randomized trials. JAMA. 2017;318(10):927-938.
Read how the route of HT may affect sexuality outcomes.
Oral vs transdermal estrogen therapy: Is one preferable regarding sexuality?
Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471-1479.
If route of administration of systemic HT influences sexuality outcomes in menopausal women, this would inform how we counsel our patients regarding HT.
Recently, Taylor and colleagues conducted a randomized clinical trial to examine the effects of HT's route of administration on sexual function.8 The 4-year Kronos Early Estrogen Prevention Study (KEEPS) ancillary sexual study randomly assigned 670 recently menopausal women to 0.45 mg of oral conjugated equine estrogens (CEE), an 0.05-mg estradiol transdermal patch, or placebo (with oral micronized progesterone for those on active treatment). The participants were aged 42 to 58 years and were within 36 months from their last menstrual period.
Participants were evaluated using the Female Sexual Function Inventory (FSFI) questionnaire, which assessed desire, arousal, lubrication, orgasm, satisfaction, and pain. The FSFI is scored using a point range of 0 to 36. A higher FSFI score indicates better sexual function. An FSFI score less than 26.55 depicts low sexual function (LSF).
Transdermal estrogen improved sexual function scores
Treatment with oral CEE was associated with no significant change in FSFI score compared with placebo, although benefits were seen for lubrication. By contrast, estrogen patch use improved the FSFI score (mean improvement, 2.6). Although improvement in FSFI score with transdermal estrogen was limited to participants with baseline LSF, most participants in fact had LSF at baseline.
Oral estrogen increases the liver's production of sex hormone-binding globulin, resulting in lower free (bioavailable) testosterone. Transdermal estrogen does not produce this effect. Accordingly, sexuality concerns may represent a reason to prefer the use of transdermal as opposed to oral estrogen.
Read about the authors’ concern over new USPSTF guidance.
The USPSTF recommendation against menopausal HT use for prevention of chronic conditions: Guidance that may confuse--and mislead
US Preventive Services Task Force; Grossman DC, CurrySJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224-2233.
In late 2017, the USPSTF issued its recommendation against the use of menopausal HT for prevention of chronic conditions.9 We are concerned that this recommendation will be misconstrued as suggesting that the use of HT is not appropriate for any indication, including treatment of bothersome menopausal symptoms.
Although the Task Force's report briefly indicated that the guidance does not refer to HT use for treatment of symptoms, this important disclaimer likely will be overlooked or ignored by many readers. The result may be increased uncertainty and anxiety in decision making regarding HT use. Thus, we might see a further decline in the proportion of menopausal women who are prescribed appropriate treatment for symptoms that impair quality of life.
HT use improves menopausal symptoms
According to the 2017 NAMS Position Statement, for symptomatic women in early menopause (that is, younger than age 60 or within 10 years of menopause onset) and free of contraindications to treatment, use of systemic HT is appropriate.7 Currently, clinicians are reluctant to prescribe HT, and women are apprehensive regarding its use.10 Unfortunately, the USPSTF guidance may further discourage appropriate treatment of menopausal symptoms.
Findings from randomized clinical trials, as well as preclinical, clinical, and epidemiologic studies, clarify the favorable benefit-risk profile for HT use by recently menopausal women with bothersome vasomotor and related menopausal symptoms.7,10-12
Notably, the USPSTF guidance does not address women with premature or early menopause, those with persistent (long-duration) vasomotor symptoms, or women at increased risk for osteoporosis and related fractures. Furthermore, the prevalent and undertreated condition, genitourinary syndrome of menopause, deserves but does not receive attention.
In recent decades, our understanding regarding HT's benefits and risks has advanced substantially. Guidance for clinicians and women should reflect this evolution and underscore the individualization and shared decision making that facilitates appropriate decisions regarding the use of HT.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062–1068.
- Espeland MA, Shumaker SA, Leng I, et al; WHIMSY Study Group. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173(15):1429–1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12(6):e1001833;discussion e1001833.
- Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708.
- Shao H, Breitner JC, Whitmer RA, et al; Cache County Investigators. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852.
- Manson JE, Aragaki AK, Rossouw JE, et al; the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318(10):927–938.
- NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471–1479.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224–2233.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015; 126(4):859–876.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
Our knowledge regarding the benefits and risks of systemic menopausal hormone therapy (HT) has continued to evolve since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI). In late 2017, the US Preventive Services Task Force (USPSTF) issued its recommendation against the use of menopausal HT for the prevention of chronic conditions. In this Menopause Update, Dr. JoAnn Manson, Dr. JoAnn Pinkerton, and I detail why we do not support the Task Force’s recommendation. In a sidebar discussion, Dr. Manson also reviews the results of 2 WHI HT trials, published in September 2017, that analyzed mortality in trial participants over an 18-year follow-up.
In addition, I summarize an observational study that assessed the association of HT and Alzheimer disease (AD) as well as a clinical trial that compared the impact of oral versus transdermal estrogen on sexuality in recently menopausal women.
What's the impact of long-term use of systemic HT on Alzheimer disease risk?
Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062-1068.
Data from the WHI HT randomized trials have clarified that initiation of oral HT among women aged 65 and older increases the risk of cognitive decline. By contrast, an analysis of younger WHI participants found that oral HT had no impact on cognitive function. Recently, Imtiaz and colleagues conducted a prospective cohort study of postmenopausal HT and AD in women residing in a Finnish county, with 25 years of follow-up. A diagnosis of AD was based on administrative health records and use of medications prescribed specifically to treat dementia. Use of systemic HT was identified via self-report. Overall, among more than 8,000 women followed, 227 cases of AD (mean age, 72 years) were identified.
In an analysis that controlled for factors including age, body mass index, alcohol use, smoking, physical activity, occupation status, and parity, up to 5 years of HT use was not associated with a risk of being diagnosed with AD. Five to 10 years of HT use was associated with a hazard ratio (HR) of 0.89, an 11% risk reduction that did not achieve statistical significance. By contrast, more than 10 years' use of systemic HT was associated with an HR of 0.53, a statistically significant 47% reduction in risk of AD.1
Other studies found conflicting results
Three large randomized trials found that HT initiated early in menopause and continued for less than 7 years had no impact on cognitive function.2-4 The Cache County (Utah) long-term prospective cohort study, however, found that HT started early in menopause and continued for 10 years or longer was associated with a significant reduction in risk of AD.5
Of note are results from the 2017 report of 18-year cumulative mortality among WHI participants (see the box on page 30). In that study, mortality from AD and other dementia was lower among participants who were randomly assigned to treatment with estrogen alone versus placebo (HR, 0.74; 95% confidence interval [CI], 0.59-0.94). With estrogen-progestin therapy, the HR was 0.93 (95% CI, 0.77-1.11), and the pooled HR for the 2 trials was 0.85 (95% CI, 0.74-0.98).6
NAMS guidance
The North American Menopause Society (NAMS) HT position statement recommends that prevention of dementia should not be considered an indication for HT use since definitive data are not available.7 The statement indicates also that estrogen therapy may have positive cognitive benefits when initiated immediately after early surgical menopause and taken until the average age of menopause to prevent health risks seen with early loss of hormones.
Definitive data from long-term randomized clinical trials are not likely to become available. Observational trials continue to have methodologic issues, such as "healthy user bias," but the studies are reassuring that initiating HT close to menopause does not increase the risk of dementia. The long-term Finnish study by Imtiaz and colleagues and the Cache County study provide tentative observational data support for a "critical window" hypothesis, leaving open the possibility that initiating systemic HT soon after menopause onset and continuing it long term may reduce the risk of AD. Discussion is needed on individual patient characteristics, potential benefits and risks, and ongoing assessment over time.
Read Dr. Manson’s discussion of 18 years of follow-up data on menopause.
JoAnn E. Manson, MD, DrPH, NCMP
A new analysis from the Women's Health Initiative (WHI) randomized trials examined all-cause and cause-specific mortality during the intervention and postintervention follow-up periods.1 We followed more than 27,000 postmenopausal women aged 50 to 79 (mean age, 63) who were recruited to 2 randomized WHI trials of HT between 1993 and 1998. The trials continued until 2002 for the estrogen-progestin trial and to 2004 for the estrogen-alone trial. The trials ran for 5 to 7 years' duration, with post-stopping follow-up for an additional 10 to 12 years (total cumulative follow-up of 18 years).
The participants were randomly assigned to receive active treatment or placebo. The interventions were conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) versus placebo for women with an intact uterus and CEE alone versus placebo for women who had a hysterectomy.
All-cause mortality did not increase with HT use
The primary outcome measure was all-cause mortality in the 2 pooled trials and in each trial individually. We found that there was no link between HT and all-cause mortality in the overall study population (ages 50-79) in either trial. However, there was a trend toward lower all-cause mortality among the younger women in both trials. In women aged 50 to 59, there was a statistically significant 31% lower risk of mortality in the pooled trials among women taking active HT compared with those taking placebo, but no reduction in mortality with HT among older women (P for trend by age = .01).
Notably, all-cause mortality provides a critically important summary measure for interventions such as HT that have a complex matrix of benefits and risks. We know that HT has a number of benefits in menopausal women. It reduces hot flashes and other menopausal symptoms. It lowers the risk of hip fracture, other types of bone fractures, and type 2 diabetes. However, HT increases the risk of venous thrombosis, stroke, and some forms of cancer.
A summary measure that assesses the net effect of a medication on serious and life-threatening health outcomes is very important. As such, all-cause mortality is the ultimate bottom line for the balance of benefits and risks. This speaks to why we conducted the mortality analysis--WHI is the largest randomized trial of HT with long-term follow-up, allowing detailed analyses by age group. Although there have been previous reports on individual health outcomes in the WHI trials, no previous report had specifically focused on all-cause and cause-specific mortality with HT, stratified by age group, over long-term follow-up.
Hopefully the results of this study will alleviate some of the anxiety associated with HT because, as mentioned, there was no increase in overall total mortality or specific major causes of death. In addition, the younger women had a trend toward benefit for all-cause mortality.
We think that these findings support the recommendations from The North American Menopause Society and other professional societies that endorse the use of HT for managing bothersome menopausal symptoms, especially when started in early menopause. These results should be reassuring that there is no increase in mortality with HT use. Although these findings do not support prescribing HT for the express purpose of trying to prevent cardiovascular disease, dementia, or other chronic diseases (due to some potential risks), they do support an important role of HT for management of bothersome hot flashes, especially in early menopause.
Cause-specific mortality
Regarding cause-specific mortality and HT use, we looked in detail at deaths from cardiovascular causes, cancer, dementia, and other major illness. Overall, we observed no increase or decrease in cardiovascular or cancer deaths. In the estrogen-alone trial, there was a surprising finding of a 26% reduction in dementia deaths. In the estrogen-progestin trial, the results were neutral for dementia deaths.
Overall, the cause-specific mortality results were neutral. This is surprising because even for total cancer deaths there was no increase or decrease, despite a great deal of anxiety about cancer risk with HT. It appears that for cancer, HT has complex effects: it increases some types of cancer, such as breast cancer, and decreases others, such as endometrial cancer (in the estrogen-progestin group), and possibly colorectal cancer. Moreover, CEE alone was associated with a reduction in breast cancer mortality, but it remains unclear if this applies to other formulations. HT's net effect on total cancer mortality was neutral in both trials, that is, no increase or decrease.
Cautions and takeaways
We need to keep in mind that in current clinical practice, lower doses and different formulations and routes of administration of HT are now often used, including transdermal estradiol patches, gels, sprays, and micronized progesterone. These formulations, and the lower doses, may have an even more favorable benefit-risk profile. We need additional research on the long-term benefits and risks of these newer formulations and lower dosages.
Generally, these findings from the WHI trials indicate that for women who have significant hot flashes, night sweats, or other bothersome menopausal symptoms, it's important to discuss their symptoms with their health care provider and understand that hormone therapy may be an option for them. If it's not an option, many other treatments are available, including nonhormonal prescription medications, nonprescription medications, and behavioral approaches.
These findings should alleviate some fear about HT use, especially in younger women who have an overall favorable trend in terms of all-cause mortality with treatment, plus a much lower absolute risk of adverse events than older women. In a woman in early menopause who has bothersome hot flashes or other symptoms that disrupt her sleep or impair her quality of life, it's likely that the benefits of HT will outweigh the risks.
Reference
- Manson JE, Aragaki AK, Rossouw JE, et al; for the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women's Health Initiative randomized trials. JAMA. 2017;318(10):927-938.
Read how the route of HT may affect sexuality outcomes.
Oral vs transdermal estrogen therapy: Is one preferable regarding sexuality?
Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471-1479.
If route of administration of systemic HT influences sexuality outcomes in menopausal women, this would inform how we counsel our patients regarding HT.
Recently, Taylor and colleagues conducted a randomized clinical trial to examine the effects of HT's route of administration on sexual function.8 The 4-year Kronos Early Estrogen Prevention Study (KEEPS) ancillary sexual study randomly assigned 670 recently menopausal women to 0.45 mg of oral conjugated equine estrogens (CEE), an 0.05-mg estradiol transdermal patch, or placebo (with oral micronized progesterone for those on active treatment). The participants were aged 42 to 58 years and were within 36 months from their last menstrual period.
Participants were evaluated using the Female Sexual Function Inventory (FSFI) questionnaire, which assessed desire, arousal, lubrication, orgasm, satisfaction, and pain. The FSFI is scored using a point range of 0 to 36. A higher FSFI score indicates better sexual function. An FSFI score less than 26.55 depicts low sexual function (LSF).
Transdermal estrogen improved sexual function scores
Treatment with oral CEE was associated with no significant change in FSFI score compared with placebo, although benefits were seen for lubrication. By contrast, estrogen patch use improved the FSFI score (mean improvement, 2.6). Although improvement in FSFI score with transdermal estrogen was limited to participants with baseline LSF, most participants in fact had LSF at baseline.
Oral estrogen increases the liver's production of sex hormone-binding globulin, resulting in lower free (bioavailable) testosterone. Transdermal estrogen does not produce this effect. Accordingly, sexuality concerns may represent a reason to prefer the use of transdermal as opposed to oral estrogen.
Read about the authors’ concern over new USPSTF guidance.
The USPSTF recommendation against menopausal HT use for prevention of chronic conditions: Guidance that may confuse--and mislead
US Preventive Services Task Force; Grossman DC, CurrySJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224-2233.
In late 2017, the USPSTF issued its recommendation against the use of menopausal HT for prevention of chronic conditions.9 We are concerned that this recommendation will be misconstrued as suggesting that the use of HT is not appropriate for any indication, including treatment of bothersome menopausal symptoms.
Although the Task Force's report briefly indicated that the guidance does not refer to HT use for treatment of symptoms, this important disclaimer likely will be overlooked or ignored by many readers. The result may be increased uncertainty and anxiety in decision making regarding HT use. Thus, we might see a further decline in the proportion of menopausal women who are prescribed appropriate treatment for symptoms that impair quality of life.
HT use improves menopausal symptoms
According to the 2017 NAMS Position Statement, for symptomatic women in early menopause (that is, younger than age 60 or within 10 years of menopause onset) and free of contraindications to treatment, use of systemic HT is appropriate.7 Currently, clinicians are reluctant to prescribe HT, and women are apprehensive regarding its use.10 Unfortunately, the USPSTF guidance may further discourage appropriate treatment of menopausal symptoms.
Findings from randomized clinical trials, as well as preclinical, clinical, and epidemiologic studies, clarify the favorable benefit-risk profile for HT use by recently menopausal women with bothersome vasomotor and related menopausal symptoms.7,10-12
Notably, the USPSTF guidance does not address women with premature or early menopause, those with persistent (long-duration) vasomotor symptoms, or women at increased risk for osteoporosis and related fractures. Furthermore, the prevalent and undertreated condition, genitourinary syndrome of menopause, deserves but does not receive attention.
In recent decades, our understanding regarding HT's benefits and risks has advanced substantially. Guidance for clinicians and women should reflect this evolution and underscore the individualization and shared decision making that facilitates appropriate decisions regarding the use of HT.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Our knowledge regarding the benefits and risks of systemic menopausal hormone therapy (HT) has continued to evolve since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI). In late 2017, the US Preventive Services Task Force (USPSTF) issued its recommendation against the use of menopausal HT for the prevention of chronic conditions. In this Menopause Update, Dr. JoAnn Manson, Dr. JoAnn Pinkerton, and I detail why we do not support the Task Force’s recommendation. In a sidebar discussion, Dr. Manson also reviews the results of 2 WHI HT trials, published in September 2017, that analyzed mortality in trial participants over an 18-year follow-up.
In addition, I summarize an observational study that assessed the association of HT and Alzheimer disease (AD) as well as a clinical trial that compared the impact of oral versus transdermal estrogen on sexuality in recently menopausal women.
What's the impact of long-term use of systemic HT on Alzheimer disease risk?
Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062-1068.
Data from the WHI HT randomized trials have clarified that initiation of oral HT among women aged 65 and older increases the risk of cognitive decline. By contrast, an analysis of younger WHI participants found that oral HT had no impact on cognitive function. Recently, Imtiaz and colleagues conducted a prospective cohort study of postmenopausal HT and AD in women residing in a Finnish county, with 25 years of follow-up. A diagnosis of AD was based on administrative health records and use of medications prescribed specifically to treat dementia. Use of systemic HT was identified via self-report. Overall, among more than 8,000 women followed, 227 cases of AD (mean age, 72 years) were identified.
In an analysis that controlled for factors including age, body mass index, alcohol use, smoking, physical activity, occupation status, and parity, up to 5 years of HT use was not associated with a risk of being diagnosed with AD. Five to 10 years of HT use was associated with a hazard ratio (HR) of 0.89, an 11% risk reduction that did not achieve statistical significance. By contrast, more than 10 years' use of systemic HT was associated with an HR of 0.53, a statistically significant 47% reduction in risk of AD.1
Other studies found conflicting results
Three large randomized trials found that HT initiated early in menopause and continued for less than 7 years had no impact on cognitive function.2-4 The Cache County (Utah) long-term prospective cohort study, however, found that HT started early in menopause and continued for 10 years or longer was associated with a significant reduction in risk of AD.5
Of note are results from the 2017 report of 18-year cumulative mortality among WHI participants (see the box on page 30). In that study, mortality from AD and other dementia was lower among participants who were randomly assigned to treatment with estrogen alone versus placebo (HR, 0.74; 95% confidence interval [CI], 0.59-0.94). With estrogen-progestin therapy, the HR was 0.93 (95% CI, 0.77-1.11), and the pooled HR for the 2 trials was 0.85 (95% CI, 0.74-0.98).6
NAMS guidance
The North American Menopause Society (NAMS) HT position statement recommends that prevention of dementia should not be considered an indication for HT use since definitive data are not available.7 The statement indicates also that estrogen therapy may have positive cognitive benefits when initiated immediately after early surgical menopause and taken until the average age of menopause to prevent health risks seen with early loss of hormones.
Definitive data from long-term randomized clinical trials are not likely to become available. Observational trials continue to have methodologic issues, such as "healthy user bias," but the studies are reassuring that initiating HT close to menopause does not increase the risk of dementia. The long-term Finnish study by Imtiaz and colleagues and the Cache County study provide tentative observational data support for a "critical window" hypothesis, leaving open the possibility that initiating systemic HT soon after menopause onset and continuing it long term may reduce the risk of AD. Discussion is needed on individual patient characteristics, potential benefits and risks, and ongoing assessment over time.
Read Dr. Manson’s discussion of 18 years of follow-up data on menopause.
JoAnn E. Manson, MD, DrPH, NCMP
A new analysis from the Women's Health Initiative (WHI) randomized trials examined all-cause and cause-specific mortality during the intervention and postintervention follow-up periods.1 We followed more than 27,000 postmenopausal women aged 50 to 79 (mean age, 63) who were recruited to 2 randomized WHI trials of HT between 1993 and 1998. The trials continued until 2002 for the estrogen-progestin trial and to 2004 for the estrogen-alone trial. The trials ran for 5 to 7 years' duration, with post-stopping follow-up for an additional 10 to 12 years (total cumulative follow-up of 18 years).
The participants were randomly assigned to receive active treatment or placebo. The interventions were conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) versus placebo for women with an intact uterus and CEE alone versus placebo for women who had a hysterectomy.
All-cause mortality did not increase with HT use
The primary outcome measure was all-cause mortality in the 2 pooled trials and in each trial individually. We found that there was no link between HT and all-cause mortality in the overall study population (ages 50-79) in either trial. However, there was a trend toward lower all-cause mortality among the younger women in both trials. In women aged 50 to 59, there was a statistically significant 31% lower risk of mortality in the pooled trials among women taking active HT compared with those taking placebo, but no reduction in mortality with HT among older women (P for trend by age = .01).
Notably, all-cause mortality provides a critically important summary measure for interventions such as HT that have a complex matrix of benefits and risks. We know that HT has a number of benefits in menopausal women. It reduces hot flashes and other menopausal symptoms. It lowers the risk of hip fracture, other types of bone fractures, and type 2 diabetes. However, HT increases the risk of venous thrombosis, stroke, and some forms of cancer.
A summary measure that assesses the net effect of a medication on serious and life-threatening health outcomes is very important. As such, all-cause mortality is the ultimate bottom line for the balance of benefits and risks. This speaks to why we conducted the mortality analysis--WHI is the largest randomized trial of HT with long-term follow-up, allowing detailed analyses by age group. Although there have been previous reports on individual health outcomes in the WHI trials, no previous report had specifically focused on all-cause and cause-specific mortality with HT, stratified by age group, over long-term follow-up.
Hopefully the results of this study will alleviate some of the anxiety associated with HT because, as mentioned, there was no increase in overall total mortality or specific major causes of death. In addition, the younger women had a trend toward benefit for all-cause mortality.
We think that these findings support the recommendations from The North American Menopause Society and other professional societies that endorse the use of HT for managing bothersome menopausal symptoms, especially when started in early menopause. These results should be reassuring that there is no increase in mortality with HT use. Although these findings do not support prescribing HT for the express purpose of trying to prevent cardiovascular disease, dementia, or other chronic diseases (due to some potential risks), they do support an important role of HT for management of bothersome hot flashes, especially in early menopause.
Cause-specific mortality
Regarding cause-specific mortality and HT use, we looked in detail at deaths from cardiovascular causes, cancer, dementia, and other major illness. Overall, we observed no increase or decrease in cardiovascular or cancer deaths. In the estrogen-alone trial, there was a surprising finding of a 26% reduction in dementia deaths. In the estrogen-progestin trial, the results were neutral for dementia deaths.
Overall, the cause-specific mortality results were neutral. This is surprising because even for total cancer deaths there was no increase or decrease, despite a great deal of anxiety about cancer risk with HT. It appears that for cancer, HT has complex effects: it increases some types of cancer, such as breast cancer, and decreases others, such as endometrial cancer (in the estrogen-progestin group), and possibly colorectal cancer. Moreover, CEE alone was associated with a reduction in breast cancer mortality, but it remains unclear if this applies to other formulations. HT's net effect on total cancer mortality was neutral in both trials, that is, no increase or decrease.
Cautions and takeaways
We need to keep in mind that in current clinical practice, lower doses and different formulations and routes of administration of HT are now often used, including transdermal estradiol patches, gels, sprays, and micronized progesterone. These formulations, and the lower doses, may have an even more favorable benefit-risk profile. We need additional research on the long-term benefits and risks of these newer formulations and lower dosages.
Generally, these findings from the WHI trials indicate that for women who have significant hot flashes, night sweats, or other bothersome menopausal symptoms, it's important to discuss their symptoms with their health care provider and understand that hormone therapy may be an option for them. If it's not an option, many other treatments are available, including nonhormonal prescription medications, nonprescription medications, and behavioral approaches.
These findings should alleviate some fear about HT use, especially in younger women who have an overall favorable trend in terms of all-cause mortality with treatment, plus a much lower absolute risk of adverse events than older women. In a woman in early menopause who has bothersome hot flashes or other symptoms that disrupt her sleep or impair her quality of life, it's likely that the benefits of HT will outweigh the risks.
Reference
- Manson JE, Aragaki AK, Rossouw JE, et al; for the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women's Health Initiative randomized trials. JAMA. 2017;318(10):927-938.
Read how the route of HT may affect sexuality outcomes.
Oral vs transdermal estrogen therapy: Is one preferable regarding sexuality?
Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471-1479.
If route of administration of systemic HT influences sexuality outcomes in menopausal women, this would inform how we counsel our patients regarding HT.
Recently, Taylor and colleagues conducted a randomized clinical trial to examine the effects of HT's route of administration on sexual function.8 The 4-year Kronos Early Estrogen Prevention Study (KEEPS) ancillary sexual study randomly assigned 670 recently menopausal women to 0.45 mg of oral conjugated equine estrogens (CEE), an 0.05-mg estradiol transdermal patch, or placebo (with oral micronized progesterone for those on active treatment). The participants were aged 42 to 58 years and were within 36 months from their last menstrual period.
Participants were evaluated using the Female Sexual Function Inventory (FSFI) questionnaire, which assessed desire, arousal, lubrication, orgasm, satisfaction, and pain. The FSFI is scored using a point range of 0 to 36. A higher FSFI score indicates better sexual function. An FSFI score less than 26.55 depicts low sexual function (LSF).
Transdermal estrogen improved sexual function scores
Treatment with oral CEE was associated with no significant change in FSFI score compared with placebo, although benefits were seen for lubrication. By contrast, estrogen patch use improved the FSFI score (mean improvement, 2.6). Although improvement in FSFI score with transdermal estrogen was limited to participants with baseline LSF, most participants in fact had LSF at baseline.
Oral estrogen increases the liver's production of sex hormone-binding globulin, resulting in lower free (bioavailable) testosterone. Transdermal estrogen does not produce this effect. Accordingly, sexuality concerns may represent a reason to prefer the use of transdermal as opposed to oral estrogen.
Read about the authors’ concern over new USPSTF guidance.
The USPSTF recommendation against menopausal HT use for prevention of chronic conditions: Guidance that may confuse--and mislead
US Preventive Services Task Force; Grossman DC, CurrySJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224-2233.
In late 2017, the USPSTF issued its recommendation against the use of menopausal HT for prevention of chronic conditions.9 We are concerned that this recommendation will be misconstrued as suggesting that the use of HT is not appropriate for any indication, including treatment of bothersome menopausal symptoms.
Although the Task Force's report briefly indicated that the guidance does not refer to HT use for treatment of symptoms, this important disclaimer likely will be overlooked or ignored by many readers. The result may be increased uncertainty and anxiety in decision making regarding HT use. Thus, we might see a further decline in the proportion of menopausal women who are prescribed appropriate treatment for symptoms that impair quality of life.
HT use improves menopausal symptoms
According to the 2017 NAMS Position Statement, for symptomatic women in early menopause (that is, younger than age 60 or within 10 years of menopause onset) and free of contraindications to treatment, use of systemic HT is appropriate.7 Currently, clinicians are reluctant to prescribe HT, and women are apprehensive regarding its use.10 Unfortunately, the USPSTF guidance may further discourage appropriate treatment of menopausal symptoms.
Findings from randomized clinical trials, as well as preclinical, clinical, and epidemiologic studies, clarify the favorable benefit-risk profile for HT use by recently menopausal women with bothersome vasomotor and related menopausal symptoms.7,10-12
Notably, the USPSTF guidance does not address women with premature or early menopause, those with persistent (long-duration) vasomotor symptoms, or women at increased risk for osteoporosis and related fractures. Furthermore, the prevalent and undertreated condition, genitourinary syndrome of menopause, deserves but does not receive attention.
In recent decades, our understanding regarding HT's benefits and risks has advanced substantially. Guidance for clinicians and women should reflect this evolution and underscore the individualization and shared decision making that facilitates appropriate decisions regarding the use of HT.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062–1068.
- Espeland MA, Shumaker SA, Leng I, et al; WHIMSY Study Group. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173(15):1429–1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12(6):e1001833;discussion e1001833.
- Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708.
- Shao H, Breitner JC, Whitmer RA, et al; Cache County Investigators. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852.
- Manson JE, Aragaki AK, Rossouw JE, et al; the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318(10):927–938.
- NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471–1479.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224–2233.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015; 126(4):859–876.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062–1068.
- Espeland MA, Shumaker SA, Leng I, et al; WHIMSY Study Group. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173(15):1429–1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12(6):e1001833;discussion e1001833.
- Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708.
- Shao H, Breitner JC, Whitmer RA, et al; Cache County Investigators. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852.
- Manson JE, Aragaki AK, Rossouw JE, et al; the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318(10):927–938.
- NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471–1479.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224–2233.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015; 126(4):859–876.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
2017 Update on menopause
Since publication of initial findings of the Women’s Health Initiative (WHI) in 2002, use of systemic menopausal hormone therapy (HT) has declined by some 80% among US women.1 Against this backdrop, this year’s Menopause Update highlights the “hot off the press” updated position statement on menopausal HT from The North American Menopause Society (NAMS), summarized by Dr. JoAnn V. Pinkerton. Although this guidance is chock full of practical, evidence-based guidance, the take-home message that Dr. Pinkerton and I would like to leave readers of OBG
Related Article:
Dr. Andrew M. Kaunitz on prescribing systemic HT to older women
Although menopausal vasomotor and related symptoms improve as women age, in untreated women, vulvovaginal atrophy (VVA, also known as genitourinary syndrome of menopause, or GSM) tends to progress, causing vaginal dryness and sexual dysfunction, among other symptoms. When symptomatic GSM represents the only indication for treatment, low-dose local vaginal estrogen, ospemifene, or dehydroepiandrosterone (DHEA; prasterone) is safe and effective. However, as with systemic HT, specific treatments for GSM are substantially underutilized.2 The current package labeling for low-dose vaginal estrogen deters many appropriate candidates from using this safe, effective treatment. In this Update, Dr. JoAnn E. Manson reviews the rationale for updating this labeling as well as recent efforts to accomplish the task.
Read about updated NAMS guidelines on HT
Guidelines on HT have been updated by The North American Menopause Society
The North American Menopause Society Hormone Therapy (HT) Position Statement Advisory Panel, composed of more than 20 experts in menopausal women's HT, including clinicians, researchers, and epidemiologists, reviewed the 2012 HT Position Statement, evaluated prior and new literature and used levels of evidence to identify the quality of the evidence and strength of the recommendations and to find consensus for the guidelines. The following information comes from the NAMS 2017 Hormone Therapy Position Statement.3
What are the major findings?
HT is the most effective treatment for vasomotor symptoms (VMS) and GSM and has been shown to prevent bone loss and fracture. Risks of HT may differ for women depending on type, dose, duration, route of administration, and timing of initiation and whether or not a progestogen is needed. Treatment should be individualized using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation about benefits and risks of continuing or discontinuing HT.
For women who are younger than age 60 or within 10 years of menopause and have no contraindication, the clearest benefit of HT is for the treatment of VMS and prevention of bone loss in those at elevated risk.
The clinical guidelines were presented to NAMS audience at the 2016 annual clinical meeting, where NAMS recommended "determining the most appropriate type, dose, formulation, and duration of HT."4
When to initiate HT and duration of use
In its now-published 2017 guidelines on HT, NAMS affirms the safety and efficacy of HT for symptomatic menopausal women or those at high risk for bone loss who are under age 60 or within 10 years of menopause. NAMS encourages practitioners to employ shared decision making with their patients to find the appropriate type, dose, formulation, and duration of HT, making individualized decisions based on evidence-based information, the unique health risks of women, and with periodic reassessment.
In the clinical guidelines presented in the 2016 NAMS annual meeting,4 key recommendations taken from the 2017 Hormone Therapy Position Statement3 include the following: For women who are aged younger than 60 years or within 10 years of menopause and have no contraindications, the benefit/risk ratio appears favorable for treatment of bothersome VMS and in those at elevated risk for bone loss or fracture.
For women who initiate HT more than 10 years from menopause or after age 60, this benefit/risk ratio appears less favorable because of greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
What about extended use of hormone therapy? There is no evidence to support routine discontinuation of HT after age 65. Decisions about longer durations of HT should be individualized and considered for indications such as persistent VMS or bone loss, with shared decision making, documentation, and periodic reevaluation. Longer duration is more favorable for estrogen therapy than for estrogen-progestin therapy, based on the Women's Health Initiative (WHI) randomized controlled trials.5
What about only vaginal symptoms? For bothersome GSM not relieved with over-the-counter therapies and without indications for use of systemic HT, low-dose vaginal estrogen therapy or other therapies are recommended and can be continued as long as indicated since there is minimal systemic absorption of estrogen, with serum levels remaining within the normal postmenopausal range.6,7 For women with estrogen sensitive cancer, oncologists should be included in decision making, particularly for women on aromatase inhibitors.
Considerations for special populations Early menopause. For women with hypoestrogenism, primary ovarian insufficiency, or premature surgical menopause without contraindications, HT is recommended until at least the median age of menopause (52 years), as studies suggest that benefits outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.8
Family history of breast cancer. Observational evidence suggests that use of HT does not further alter the risk for breast cancer in women with a family history of breast cancer. Family history is one risk, among others, that should be assessed when counseling women regarding HT.
Women who are BRCA-positive without breast cancer. For women who are BRCA-positive (higher genetic risk of breast cancer, primarily estrogen-receptor-negative), and have undergone surgical menopause (bilateral salpingo-oophorectomy), the benefits of estrogen to decrease health risks caused by premature loss of estrogen need to be considered on an individual basis.9 On the basis of limited observational studies, consider offering systemic HT until the median age of menopause (52 years) with longer use individualized.3
Related Article:
Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
Survivors of endometrial and breast cancer with bothersome VMS. For women with prior estrogen-sensitive cancers, non-HTs should be considered first, particularly those agents studied through randomized controlled trials in this population and found to be effective. If systemic estrogen is considered for persistent symptoms after non-HT or complementary options have been unsuccessful, decisions should be made for compelling reasons and after detailed counseling, with shared decision making and in conjunction with their oncologist.3
Bothersome GSM. On the basis of limited observational data, there appears to be minimal to no demonstrated elevation in risk for recurrence of endometrial or breast cancer using low-dose vaginal estrogen,3,10 but decisions should be made in conjunction with an oncologist.
Related Article:
Focus on treating genital atrophy symptoms
The importance of relaying the new guidelines to patients
It is important for clinicians to talk to women about their menopausal symptoms and their options for relief of symptoms or prevention of bone loss. Discussion should take into account age and time from menopause, include evidence-based information11-13 about benefits and risks of different types of therapy, and employ shared decision making to choose the most appropriate therapy to maximize benefits and minimize risks for the individual woman.
Following the WHI initial release in 2002, both women and providers became fearful of HT and believed media hype and celebrities that compounded bioidentical HT was safer than FDA-approved HTs. However, compounded products lack safety and efficacy data, are not monitored or regulated by the FDA, and have unique risks associated with compounding, including concerns about sterility, impurities, and overdosing or underdosing, which could increase cancer risk.3
- Hormone therapy for symptomatic menopausal women is safe and effective for those under age 60 or within 10 years of menopause.
- Identify the most appropriate type, dose, formulation, and duration of hormone therapy for an individual woman based on evidence.
- We want to remove the fear of using hormone therapy for healthy symptomatic women who are under age 60 or within 10 years of menopause.
- Age at initiation of hormone therapy matters.
- NAMS endorses use of FDA-approved hormone therapy over compounded therapies.
Read about modifying low-dose vaginal estrogen’s black box warning
Physicians continue to underwhelmingly prescribe low-dose vaginal estrogen for GSM
Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The Women's EMPOWER survey: identifying women's perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14(3):413-424.
GSM is seriously underrecognized and undertreated.2,8,14 It has a major impact on women's lives--a silent epidemic affecting women's quality of life, sexual health, interpersonal relationships, and even physical health in terms of increased risk of urinary tract infections and urinary symptoms. Unfortunately, patients are reluctant to mention the problem to their clinicians, and they do not clearly recognize it as a medical condition that has available treatment options. Clinicians also rarely receive adequate training in the management of this condition and how to discuss it with their patients. Given busy schedules and time constraints, addressing this topic often falls through the cracks, representing a missed opportunity for helping our patients with safe and effective treatments. In a recent study by Kingsberg and colleagues, an astoundingly low percentageof women with GSM symptoms received treatment.
Details of the study
The study authors evaluated women's perceptions of GSM and available treatment options. US women aged 45 and older who reported GSM symptoms were surveyed. Of 1,858 women with a median age of 58 (range, 45-90), the study authors found that 50% had never used any treatment; 25% used over-the-counter medications; 18% were former users of GSM treatments; and 7% currently used prescribed GSM therapies.
When GSM was discussed, women were more likely than their clinicians to initiate the conversation. The main reason for women not mentioning their symptoms was the perception that GSM symptoms were a natural and inevitable part of aging. Hormonal products were perceived by women as having several downsides, including risk of systemic absorption, messiness of local creams, and the need to reuse an applicator. Overall, clinicians recommended vaginal estrogen therapy to only 23% and oral HTs to 18% of women.
The results of the study are consistent with results of earlier surveys of menopausal women. Although the survey included nearly 2,000 women, it has the potential for selection biases inherent to most Internet-based surveys. In addition, the respondents tended to be white and have higher socieconomic status, with limited representation from other groups.
Calls for the current boxed warning to be revised
GSM is highly prevalent among postmenopausal women; the condition has adverse effects on quality of life and sexual health.2,8,14 Safe and effective treatments are available but are underutilized.1,8,15,16 A current boxed warning appears on low-dose vaginal estrogen--class labeling that appears on all medications in the class of estrogen or HT, regardless of dose or route of administration. These warnings are based on findings from the WHI and other studies of systemic estrogen or estrogen plus progestin, which demonstrated a complex pattern of risks and benefits of HT (including increased risk of venous thrombosis or pulmonary embolism, stroke, and breast cancer [with estrogen plus progestin]).
These findings, however, do not appear to be relevant to low-dose vaginal estrogen, given minimal if any systemic absorption and much lower blood levels of hormones than found with systemic HT. Blood levels of estradiol with low-dose vaginal estrogen remain in the normal postmenopausal range, compared to several-fold elevations in hormone levels with systemic HT.8,15,16 Additionally, observational studies of low-dose vaginal estrogen, as well as short-term randomized clinical trials, show no evidence of an increased risk of venous thromboembolic events, heart disease, stroke, breast cancer, or dementia--the listed possible adverse effects in the boxed warning. The current warning is based on extrapolating findings from systemic HT, which is inappropriate and not evidence-based for low-dose vaginal estrogen.15
The inappropriate boxed warning contributes to the problem of undertreatment of GSM in women by discouraging clinicians from prescribing the medication and dissuading patients from taking it even after purchase. Testimonials from many clinicians caring for these women have underscored that women will fill their prescription, but after seeing the boxed warning will often become alarmed and decide not to take the medication. Clinicians reported that patients often say at their next appointment: "No, I never took it. I got very scared when I saw the boxed warning." As a result, clinicians often have to spend a great deal of time explaining the limitations of, and lack of evidence for, the boxed warning on low-dose vaginal estrogen.
Related Article:
2016 Update on menopause
Recommended label revisions
A modified label, without a boxed warning, would be safer for women because the key messages would not be obscured by the large amount of irrelevant information. Our Working Group recommended that the label explain that the listed risks were found in studies of systemic HT and their relevance to low-dose vaginal estrogen is unknown. The Group also recommended that warning text should be added in bold font to advise patients to seek medical attention if they have vaginal bleeding or spotting while taking the medication. In addition, patients who have a history of breast cancer or other hormone-sensitive cancer should discuss the use of the medication with their oncologist.
Status update on efforts to revise label. A citizen's petition was filed in the Spring of 2016, with signatures from more than 600 clinicians and patients and representatives of medical and professional organizations endorsing a more appropriate evidence-based label for low-dose vaginal estrogen. The FDA is continuing to review and deliberate on these issues but has not yet made a final decision.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Manson JM, Kaunitz AM. Menopause management—Getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- The 2017 hormone therapy position statement of The North American Menopause Society [published online ahead of print June 2017]. Menopause.
- Pinkerton JV. Hormone therapy: 2016 NAMS position statement [abstract]. Menopause. 2016;23:1365.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Sys Rev. 2016;8:CD001500.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Chai X, Domchek S, Kauff N, Rebbeck T, Chen J. RE: Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(9).
- Farrell R; American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice. ACOG Committee Opinion No. 659 summary: The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):618–619.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231.
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;(3):CD002229.
- Parish S, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Manson JE, Goldstein SR, Kagan R, et al; Working Group on Women’s Health and Well-Being in Menopause. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause. 2014;21(9):911–916.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859-876.
Since publication of initial findings of the Women’s Health Initiative (WHI) in 2002, use of systemic menopausal hormone therapy (HT) has declined by some 80% among US women.1 Against this backdrop, this year’s Menopause Update highlights the “hot off the press” updated position statement on menopausal HT from The North American Menopause Society (NAMS), summarized by Dr. JoAnn V. Pinkerton. Although this guidance is chock full of practical, evidence-based guidance, the take-home message that Dr. Pinkerton and I would like to leave readers of OBG
Related Article:
Dr. Andrew M. Kaunitz on prescribing systemic HT to older women
Although menopausal vasomotor and related symptoms improve as women age, in untreated women, vulvovaginal atrophy (VVA, also known as genitourinary syndrome of menopause, or GSM) tends to progress, causing vaginal dryness and sexual dysfunction, among other symptoms. When symptomatic GSM represents the only indication for treatment, low-dose local vaginal estrogen, ospemifene, or dehydroepiandrosterone (DHEA; prasterone) is safe and effective. However, as with systemic HT, specific treatments for GSM are substantially underutilized.2 The current package labeling for low-dose vaginal estrogen deters many appropriate candidates from using this safe, effective treatment. In this Update, Dr. JoAnn E. Manson reviews the rationale for updating this labeling as well as recent efforts to accomplish the task.
Read about updated NAMS guidelines on HT
Guidelines on HT have been updated by The North American Menopause Society
The North American Menopause Society Hormone Therapy (HT) Position Statement Advisory Panel, composed of more than 20 experts in menopausal women's HT, including clinicians, researchers, and epidemiologists, reviewed the 2012 HT Position Statement, evaluated prior and new literature and used levels of evidence to identify the quality of the evidence and strength of the recommendations and to find consensus for the guidelines. The following information comes from the NAMS 2017 Hormone Therapy Position Statement.3
What are the major findings?
HT is the most effective treatment for vasomotor symptoms (VMS) and GSM and has been shown to prevent bone loss and fracture. Risks of HT may differ for women depending on type, dose, duration, route of administration, and timing of initiation and whether or not a progestogen is needed. Treatment should be individualized using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation about benefits and risks of continuing or discontinuing HT.
For women who are younger than age 60 or within 10 years of menopause and have no contraindication, the clearest benefit of HT is for the treatment of VMS and prevention of bone loss in those at elevated risk.
The clinical guidelines were presented to NAMS audience at the 2016 annual clinical meeting, where NAMS recommended "determining the most appropriate type, dose, formulation, and duration of HT."4
When to initiate HT and duration of use
In its now-published 2017 guidelines on HT, NAMS affirms the safety and efficacy of HT for symptomatic menopausal women or those at high risk for bone loss who are under age 60 or within 10 years of menopause. NAMS encourages practitioners to employ shared decision making with their patients to find the appropriate type, dose, formulation, and duration of HT, making individualized decisions based on evidence-based information, the unique health risks of women, and with periodic reassessment.
In the clinical guidelines presented in the 2016 NAMS annual meeting,4 key recommendations taken from the 2017 Hormone Therapy Position Statement3 include the following: For women who are aged younger than 60 years or within 10 years of menopause and have no contraindications, the benefit/risk ratio appears favorable for treatment of bothersome VMS and in those at elevated risk for bone loss or fracture.
For women who initiate HT more than 10 years from menopause or after age 60, this benefit/risk ratio appears less favorable because of greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
What about extended use of hormone therapy? There is no evidence to support routine discontinuation of HT after age 65. Decisions about longer durations of HT should be individualized and considered for indications such as persistent VMS or bone loss, with shared decision making, documentation, and periodic reevaluation. Longer duration is more favorable for estrogen therapy than for estrogen-progestin therapy, based on the Women's Health Initiative (WHI) randomized controlled trials.5
What about only vaginal symptoms? For bothersome GSM not relieved with over-the-counter therapies and without indications for use of systemic HT, low-dose vaginal estrogen therapy or other therapies are recommended and can be continued as long as indicated since there is minimal systemic absorption of estrogen, with serum levels remaining within the normal postmenopausal range.6,7 For women with estrogen sensitive cancer, oncologists should be included in decision making, particularly for women on aromatase inhibitors.
Considerations for special populations Early menopause. For women with hypoestrogenism, primary ovarian insufficiency, or premature surgical menopause without contraindications, HT is recommended until at least the median age of menopause (52 years), as studies suggest that benefits outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.8
Family history of breast cancer. Observational evidence suggests that use of HT does not further alter the risk for breast cancer in women with a family history of breast cancer. Family history is one risk, among others, that should be assessed when counseling women regarding HT.
Women who are BRCA-positive without breast cancer. For women who are BRCA-positive (higher genetic risk of breast cancer, primarily estrogen-receptor-negative), and have undergone surgical menopause (bilateral salpingo-oophorectomy), the benefits of estrogen to decrease health risks caused by premature loss of estrogen need to be considered on an individual basis.9 On the basis of limited observational studies, consider offering systemic HT until the median age of menopause (52 years) with longer use individualized.3
Related Article:
Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
Survivors of endometrial and breast cancer with bothersome VMS. For women with prior estrogen-sensitive cancers, non-HTs should be considered first, particularly those agents studied through randomized controlled trials in this population and found to be effective. If systemic estrogen is considered for persistent symptoms after non-HT or complementary options have been unsuccessful, decisions should be made for compelling reasons and after detailed counseling, with shared decision making and in conjunction with their oncologist.3
Bothersome GSM. On the basis of limited observational data, there appears to be minimal to no demonstrated elevation in risk for recurrence of endometrial or breast cancer using low-dose vaginal estrogen,3,10 but decisions should be made in conjunction with an oncologist.
Related Article:
Focus on treating genital atrophy symptoms
The importance of relaying the new guidelines to patients
It is important for clinicians to talk to women about their menopausal symptoms and their options for relief of symptoms or prevention of bone loss. Discussion should take into account age and time from menopause, include evidence-based information11-13 about benefits and risks of different types of therapy, and employ shared decision making to choose the most appropriate therapy to maximize benefits and minimize risks for the individual woman.
Following the WHI initial release in 2002, both women and providers became fearful of HT and believed media hype and celebrities that compounded bioidentical HT was safer than FDA-approved HTs. However, compounded products lack safety and efficacy data, are not monitored or regulated by the FDA, and have unique risks associated with compounding, including concerns about sterility, impurities, and overdosing or underdosing, which could increase cancer risk.3
- Hormone therapy for symptomatic menopausal women is safe and effective for those under age 60 or within 10 years of menopause.
- Identify the most appropriate type, dose, formulation, and duration of hormone therapy for an individual woman based on evidence.
- We want to remove the fear of using hormone therapy for healthy symptomatic women who are under age 60 or within 10 years of menopause.
- Age at initiation of hormone therapy matters.
- NAMS endorses use of FDA-approved hormone therapy over compounded therapies.
Read about modifying low-dose vaginal estrogen’s black box warning
Physicians continue to underwhelmingly prescribe low-dose vaginal estrogen for GSM
Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The Women's EMPOWER survey: identifying women's perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14(3):413-424.
GSM is seriously underrecognized and undertreated.2,8,14 It has a major impact on women's lives--a silent epidemic affecting women's quality of life, sexual health, interpersonal relationships, and even physical health in terms of increased risk of urinary tract infections and urinary symptoms. Unfortunately, patients are reluctant to mention the problem to their clinicians, and they do not clearly recognize it as a medical condition that has available treatment options. Clinicians also rarely receive adequate training in the management of this condition and how to discuss it with their patients. Given busy schedules and time constraints, addressing this topic often falls through the cracks, representing a missed opportunity for helping our patients with safe and effective treatments. In a recent study by Kingsberg and colleagues, an astoundingly low percentageof women with GSM symptoms received treatment.
Details of the study
The study authors evaluated women's perceptions of GSM and available treatment options. US women aged 45 and older who reported GSM symptoms were surveyed. Of 1,858 women with a median age of 58 (range, 45-90), the study authors found that 50% had never used any treatment; 25% used over-the-counter medications; 18% were former users of GSM treatments; and 7% currently used prescribed GSM therapies.
When GSM was discussed, women were more likely than their clinicians to initiate the conversation. The main reason for women not mentioning their symptoms was the perception that GSM symptoms were a natural and inevitable part of aging. Hormonal products were perceived by women as having several downsides, including risk of systemic absorption, messiness of local creams, and the need to reuse an applicator. Overall, clinicians recommended vaginal estrogen therapy to only 23% and oral HTs to 18% of women.
The results of the study are consistent with results of earlier surveys of menopausal women. Although the survey included nearly 2,000 women, it has the potential for selection biases inherent to most Internet-based surveys. In addition, the respondents tended to be white and have higher socieconomic status, with limited representation from other groups.
Calls for the current boxed warning to be revised
GSM is highly prevalent among postmenopausal women; the condition has adverse effects on quality of life and sexual health.2,8,14 Safe and effective treatments are available but are underutilized.1,8,15,16 A current boxed warning appears on low-dose vaginal estrogen--class labeling that appears on all medications in the class of estrogen or HT, regardless of dose or route of administration. These warnings are based on findings from the WHI and other studies of systemic estrogen or estrogen plus progestin, which demonstrated a complex pattern of risks and benefits of HT (including increased risk of venous thrombosis or pulmonary embolism, stroke, and breast cancer [with estrogen plus progestin]).
These findings, however, do not appear to be relevant to low-dose vaginal estrogen, given minimal if any systemic absorption and much lower blood levels of hormones than found with systemic HT. Blood levels of estradiol with low-dose vaginal estrogen remain in the normal postmenopausal range, compared to several-fold elevations in hormone levels with systemic HT.8,15,16 Additionally, observational studies of low-dose vaginal estrogen, as well as short-term randomized clinical trials, show no evidence of an increased risk of venous thromboembolic events, heart disease, stroke, breast cancer, or dementia--the listed possible adverse effects in the boxed warning. The current warning is based on extrapolating findings from systemic HT, which is inappropriate and not evidence-based for low-dose vaginal estrogen.15
The inappropriate boxed warning contributes to the problem of undertreatment of GSM in women by discouraging clinicians from prescribing the medication and dissuading patients from taking it even after purchase. Testimonials from many clinicians caring for these women have underscored that women will fill their prescription, but after seeing the boxed warning will often become alarmed and decide not to take the medication. Clinicians reported that patients often say at their next appointment: "No, I never took it. I got very scared when I saw the boxed warning." As a result, clinicians often have to spend a great deal of time explaining the limitations of, and lack of evidence for, the boxed warning on low-dose vaginal estrogen.
Related Article:
2016 Update on menopause
Recommended label revisions
A modified label, without a boxed warning, would be safer for women because the key messages would not be obscured by the large amount of irrelevant information. Our Working Group recommended that the label explain that the listed risks were found in studies of systemic HT and their relevance to low-dose vaginal estrogen is unknown. The Group also recommended that warning text should be added in bold font to advise patients to seek medical attention if they have vaginal bleeding or spotting while taking the medication. In addition, patients who have a history of breast cancer or other hormone-sensitive cancer should discuss the use of the medication with their oncologist.
Status update on efforts to revise label. A citizen's petition was filed in the Spring of 2016, with signatures from more than 600 clinicians and patients and representatives of medical and professional organizations endorsing a more appropriate evidence-based label for low-dose vaginal estrogen. The FDA is continuing to review and deliberate on these issues but has not yet made a final decision.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Since publication of initial findings of the Women’s Health Initiative (WHI) in 2002, use of systemic menopausal hormone therapy (HT) has declined by some 80% among US women.1 Against this backdrop, this year’s Menopause Update highlights the “hot off the press” updated position statement on menopausal HT from The North American Menopause Society (NAMS), summarized by Dr. JoAnn V. Pinkerton. Although this guidance is chock full of practical, evidence-based guidance, the take-home message that Dr. Pinkerton and I would like to leave readers of OBG
Related Article:
Dr. Andrew M. Kaunitz on prescribing systemic HT to older women
Although menopausal vasomotor and related symptoms improve as women age, in untreated women, vulvovaginal atrophy (VVA, also known as genitourinary syndrome of menopause, or GSM) tends to progress, causing vaginal dryness and sexual dysfunction, among other symptoms. When symptomatic GSM represents the only indication for treatment, low-dose local vaginal estrogen, ospemifene, or dehydroepiandrosterone (DHEA; prasterone) is safe and effective. However, as with systemic HT, specific treatments for GSM are substantially underutilized.2 The current package labeling for low-dose vaginal estrogen deters many appropriate candidates from using this safe, effective treatment. In this Update, Dr. JoAnn E. Manson reviews the rationale for updating this labeling as well as recent efforts to accomplish the task.
Read about updated NAMS guidelines on HT
Guidelines on HT have been updated by The North American Menopause Society
The North American Menopause Society Hormone Therapy (HT) Position Statement Advisory Panel, composed of more than 20 experts in menopausal women's HT, including clinicians, researchers, and epidemiologists, reviewed the 2012 HT Position Statement, evaluated prior and new literature and used levels of evidence to identify the quality of the evidence and strength of the recommendations and to find consensus for the guidelines. The following information comes from the NAMS 2017 Hormone Therapy Position Statement.3
What are the major findings?
HT is the most effective treatment for vasomotor symptoms (VMS) and GSM and has been shown to prevent bone loss and fracture. Risks of HT may differ for women depending on type, dose, duration, route of administration, and timing of initiation and whether or not a progestogen is needed. Treatment should be individualized using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation about benefits and risks of continuing or discontinuing HT.
For women who are younger than age 60 or within 10 years of menopause and have no contraindication, the clearest benefit of HT is for the treatment of VMS and prevention of bone loss in those at elevated risk.
The clinical guidelines were presented to NAMS audience at the 2016 annual clinical meeting, where NAMS recommended "determining the most appropriate type, dose, formulation, and duration of HT."4
When to initiate HT and duration of use
In its now-published 2017 guidelines on HT, NAMS affirms the safety and efficacy of HT for symptomatic menopausal women or those at high risk for bone loss who are under age 60 or within 10 years of menopause. NAMS encourages practitioners to employ shared decision making with their patients to find the appropriate type, dose, formulation, and duration of HT, making individualized decisions based on evidence-based information, the unique health risks of women, and with periodic reassessment.
In the clinical guidelines presented in the 2016 NAMS annual meeting,4 key recommendations taken from the 2017 Hormone Therapy Position Statement3 include the following: For women who are aged younger than 60 years or within 10 years of menopause and have no contraindications, the benefit/risk ratio appears favorable for treatment of bothersome VMS and in those at elevated risk for bone loss or fracture.
For women who initiate HT more than 10 years from menopause or after age 60, this benefit/risk ratio appears less favorable because of greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
What about extended use of hormone therapy? There is no evidence to support routine discontinuation of HT after age 65. Decisions about longer durations of HT should be individualized and considered for indications such as persistent VMS or bone loss, with shared decision making, documentation, and periodic reevaluation. Longer duration is more favorable for estrogen therapy than for estrogen-progestin therapy, based on the Women's Health Initiative (WHI) randomized controlled trials.5
What about only vaginal symptoms? For bothersome GSM not relieved with over-the-counter therapies and without indications for use of systemic HT, low-dose vaginal estrogen therapy or other therapies are recommended and can be continued as long as indicated since there is minimal systemic absorption of estrogen, with serum levels remaining within the normal postmenopausal range.6,7 For women with estrogen sensitive cancer, oncologists should be included in decision making, particularly for women on aromatase inhibitors.
Considerations for special populations Early menopause. For women with hypoestrogenism, primary ovarian insufficiency, or premature surgical menopause without contraindications, HT is recommended until at least the median age of menopause (52 years), as studies suggest that benefits outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.8
Family history of breast cancer. Observational evidence suggests that use of HT does not further alter the risk for breast cancer in women with a family history of breast cancer. Family history is one risk, among others, that should be assessed when counseling women regarding HT.
Women who are BRCA-positive without breast cancer. For women who are BRCA-positive (higher genetic risk of breast cancer, primarily estrogen-receptor-negative), and have undergone surgical menopause (bilateral salpingo-oophorectomy), the benefits of estrogen to decrease health risks caused by premature loss of estrogen need to be considered on an individual basis.9 On the basis of limited observational studies, consider offering systemic HT until the median age of menopause (52 years) with longer use individualized.3
Related Article:
Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
Survivors of endometrial and breast cancer with bothersome VMS. For women with prior estrogen-sensitive cancers, non-HTs should be considered first, particularly those agents studied through randomized controlled trials in this population and found to be effective. If systemic estrogen is considered for persistent symptoms after non-HT or complementary options have been unsuccessful, decisions should be made for compelling reasons and after detailed counseling, with shared decision making and in conjunction with their oncologist.3
Bothersome GSM. On the basis of limited observational data, there appears to be minimal to no demonstrated elevation in risk for recurrence of endometrial or breast cancer using low-dose vaginal estrogen,3,10 but decisions should be made in conjunction with an oncologist.
Related Article:
Focus on treating genital atrophy symptoms
The importance of relaying the new guidelines to patients
It is important for clinicians to talk to women about their menopausal symptoms and their options for relief of symptoms or prevention of bone loss. Discussion should take into account age and time from menopause, include evidence-based information11-13 about benefits and risks of different types of therapy, and employ shared decision making to choose the most appropriate therapy to maximize benefits and minimize risks for the individual woman.
Following the WHI initial release in 2002, both women and providers became fearful of HT and believed media hype and celebrities that compounded bioidentical HT was safer than FDA-approved HTs. However, compounded products lack safety and efficacy data, are not monitored or regulated by the FDA, and have unique risks associated with compounding, including concerns about sterility, impurities, and overdosing or underdosing, which could increase cancer risk.3
- Hormone therapy for symptomatic menopausal women is safe and effective for those under age 60 or within 10 years of menopause.
- Identify the most appropriate type, dose, formulation, and duration of hormone therapy for an individual woman based on evidence.
- We want to remove the fear of using hormone therapy for healthy symptomatic women who are under age 60 or within 10 years of menopause.
- Age at initiation of hormone therapy matters.
- NAMS endorses use of FDA-approved hormone therapy over compounded therapies.
Read about modifying low-dose vaginal estrogen’s black box warning
Physicians continue to underwhelmingly prescribe low-dose vaginal estrogen for GSM
Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The Women's EMPOWER survey: identifying women's perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14(3):413-424.
GSM is seriously underrecognized and undertreated.2,8,14 It has a major impact on women's lives--a silent epidemic affecting women's quality of life, sexual health, interpersonal relationships, and even physical health in terms of increased risk of urinary tract infections and urinary symptoms. Unfortunately, patients are reluctant to mention the problem to their clinicians, and they do not clearly recognize it as a medical condition that has available treatment options. Clinicians also rarely receive adequate training in the management of this condition and how to discuss it with their patients. Given busy schedules and time constraints, addressing this topic often falls through the cracks, representing a missed opportunity for helping our patients with safe and effective treatments. In a recent study by Kingsberg and colleagues, an astoundingly low percentageof women with GSM symptoms received treatment.
Details of the study
The study authors evaluated women's perceptions of GSM and available treatment options. US women aged 45 and older who reported GSM symptoms were surveyed. Of 1,858 women with a median age of 58 (range, 45-90), the study authors found that 50% had never used any treatment; 25% used over-the-counter medications; 18% were former users of GSM treatments; and 7% currently used prescribed GSM therapies.
When GSM was discussed, women were more likely than their clinicians to initiate the conversation. The main reason for women not mentioning their symptoms was the perception that GSM symptoms were a natural and inevitable part of aging. Hormonal products were perceived by women as having several downsides, including risk of systemic absorption, messiness of local creams, and the need to reuse an applicator. Overall, clinicians recommended vaginal estrogen therapy to only 23% and oral HTs to 18% of women.
The results of the study are consistent with results of earlier surveys of menopausal women. Although the survey included nearly 2,000 women, it has the potential for selection biases inherent to most Internet-based surveys. In addition, the respondents tended to be white and have higher socieconomic status, with limited representation from other groups.
Calls for the current boxed warning to be revised
GSM is highly prevalent among postmenopausal women; the condition has adverse effects on quality of life and sexual health.2,8,14 Safe and effective treatments are available but are underutilized.1,8,15,16 A current boxed warning appears on low-dose vaginal estrogen--class labeling that appears on all medications in the class of estrogen or HT, regardless of dose or route of administration. These warnings are based on findings from the WHI and other studies of systemic estrogen or estrogen plus progestin, which demonstrated a complex pattern of risks and benefits of HT (including increased risk of venous thrombosis or pulmonary embolism, stroke, and breast cancer [with estrogen plus progestin]).
These findings, however, do not appear to be relevant to low-dose vaginal estrogen, given minimal if any systemic absorption and much lower blood levels of hormones than found with systemic HT. Blood levels of estradiol with low-dose vaginal estrogen remain in the normal postmenopausal range, compared to several-fold elevations in hormone levels with systemic HT.8,15,16 Additionally, observational studies of low-dose vaginal estrogen, as well as short-term randomized clinical trials, show no evidence of an increased risk of venous thromboembolic events, heart disease, stroke, breast cancer, or dementia--the listed possible adverse effects in the boxed warning. The current warning is based on extrapolating findings from systemic HT, which is inappropriate and not evidence-based for low-dose vaginal estrogen.15
The inappropriate boxed warning contributes to the problem of undertreatment of GSM in women by discouraging clinicians from prescribing the medication and dissuading patients from taking it even after purchase. Testimonials from many clinicians caring for these women have underscored that women will fill their prescription, but after seeing the boxed warning will often become alarmed and decide not to take the medication. Clinicians reported that patients often say at their next appointment: "No, I never took it. I got very scared when I saw the boxed warning." As a result, clinicians often have to spend a great deal of time explaining the limitations of, and lack of evidence for, the boxed warning on low-dose vaginal estrogen.
Related Article:
2016 Update on menopause
Recommended label revisions
A modified label, without a boxed warning, would be safer for women because the key messages would not be obscured by the large amount of irrelevant information. Our Working Group recommended that the label explain that the listed risks were found in studies of systemic HT and their relevance to low-dose vaginal estrogen is unknown. The Group also recommended that warning text should be added in bold font to advise patients to seek medical attention if they have vaginal bleeding or spotting while taking the medication. In addition, patients who have a history of breast cancer or other hormone-sensitive cancer should discuss the use of the medication with their oncologist.
Status update on efforts to revise label. A citizen's petition was filed in the Spring of 2016, with signatures from more than 600 clinicians and patients and representatives of medical and professional organizations endorsing a more appropriate evidence-based label for low-dose vaginal estrogen. The FDA is continuing to review and deliberate on these issues but has not yet made a final decision.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Manson JM, Kaunitz AM. Menopause management—Getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- The 2017 hormone therapy position statement of The North American Menopause Society [published online ahead of print June 2017]. Menopause.
- Pinkerton JV. Hormone therapy: 2016 NAMS position statement [abstract]. Menopause. 2016;23:1365.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Sys Rev. 2016;8:CD001500.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Chai X, Domchek S, Kauff N, Rebbeck T, Chen J. RE: Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(9).
- Farrell R; American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice. ACOG Committee Opinion No. 659 summary: The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):618–619.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231.
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;(3):CD002229.
- Parish S, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Manson JE, Goldstein SR, Kagan R, et al; Working Group on Women’s Health and Well-Being in Menopause. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause. 2014;21(9):911–916.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859-876.
- Manson JM, Kaunitz AM. Menopause management—Getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- The 2017 hormone therapy position statement of The North American Menopause Society [published online ahead of print June 2017]. Menopause.
- Pinkerton JV. Hormone therapy: 2016 NAMS position statement [abstract]. Menopause. 2016;23:1365.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Sys Rev. 2016;8:CD001500.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Chai X, Domchek S, Kauff N, Rebbeck T, Chen J. RE: Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(9).
- Farrell R; American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice. ACOG Committee Opinion No. 659 summary: The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):618–619.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231.
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;(3):CD002229.
- Parish S, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Manson JE, Goldstein SR, Kagan R, et al; Working Group on Women’s Health and Well-Being in Menopause. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause. 2014;21(9):911–916.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859-876.