User login
2020 Update on Menopause
The term genitourinary syndrome of menopause (GSM) refers to the bothersome symptoms and physical findings associated with estrogen deficiency that involve the labia, vestibular tissue, clitoris, vagina, urethra, and bladder.1 GSM is associated with genital irritation, dryness, and burning; urinary symptoms including urgency, dysuria, and recurrent urinary tract infections; and sexual symptoms including vaginal dryness and pain. Vulvovaginal atrophy (VVA) represents a component of GSM.
GSM is highly prevalent, affecting more than three-quarters of menopausal women. In contrast to menopausal vasomotor symptoms, which often are most severe and frequent in recently menopausal women, GSM commonly presents years following menopause. Unfortunately, VVA symptoms may have a substantial negative impact on women’s quality of life.
In this 2020 Menopause Update, I review a large observational study that provides reassurance to clinicians and patients regarding the safety of the best-studied prescription treatment for GSM—vaginal estrogen. Because some women should not use vaginal estrogen and others choose not to use it, nonhormonal management of GSM is important. Dr. JoAnn Pinkerton provides details on a randomized clinical trial that compared the use of fractionated CO2 laser therapy with vaginal estrogen for the treatment of GSM. In addition, Dr. JoAnn Manson discusses recent studies that found lower health risks with vaginal estrogen use compared with systemic estrogen therapy.
Diagnosing GSM
GSM can be diagnosed presumptively based on a characteristic history in a menopausal patient. Performing a pelvic examination, however, allows clinicians to exclude other conditions that may present with similar symptoms, such as lichen sclerosus, Candida infection, and malignancy.
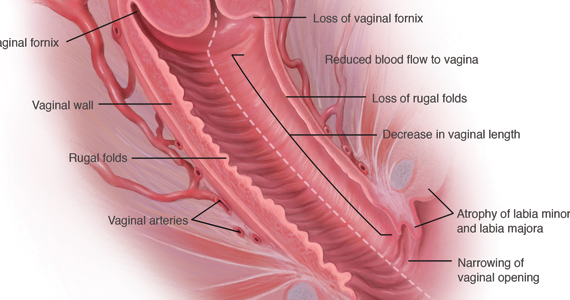
During inspection of the external genitalia, the clinician may note loss of the fat pad in the labia majora and mons as well as a reduction in labia minora pigmentation and tissue. The urethral meatus often becomes erythematous and prominent. If vaginal or introital narrowing is present, use of a pediatric (ultrathin) speculum reduces patient discomfort. The vaginal mucosa may appear smooth due to loss of rugation; it also may appear shiny and dry. Bleeding (friability) on contact with a spatula or cotton-tipped swab may occur. In addition, the vaginal fornices may become attenuated, leaving the cervix flush with the vaginal apex.
GSM can be diagnosed without laboratory assessment. However, vaginal pH, if measured, is characteristically higher than 5.0; microscopic wet prep often reveals many white blood cells, immature epithelial cells (large nuclei), and reduced or absent lactobacilli.2
Nonhormonal management of GSM
Water, silicone-based, and oil-based lubricants reduce the friction and discomfort associated with sexual activity. By contrast, vaginal moisturizers act longer than lubricants and can be applied several times weekly or daily. Natural oils, including olive and coconut oil, may be useful both as lubricants and as moisturizers. Aqueous lidocaine 4%, applied to vestibular tissue with cotton balls prior to penetration, reduces dyspareunia in women with GSM.3
Vaginal estrogen therapy
When nonhormonal management does not sufficiently reduce GSM symptoms, use of low-dose vaginal estrogen enhances thickness and elasticity of genital tissue and improves vaginal blood flow. Vaginal estrogen creams, tablets, an insert, and a ring are marketed in the United States. Although clinical improvement may be apparent within several weeks of initiating vaginal estrogen, the full benefit of treatment becomes apparent after 2 to 3 months.3
Despite the availability and effectiveness of low-dose vaginal estrogen, fears regarding the safety of menopausal hormone therapy have resulted in the underutilization of vaginal estrogen.4,5 Unfortunately, the package labeling for low-dose vaginal estrogen can exacerbate these fears.
Continue to: Nurses’ Health Study report...
Nurses’ Health Study report provides reassurance on long-term safety of vaginal estrogen
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610
Bhupathiraju and colleagues published a report from the long-running Nurses’ Health prospective cohort study on the health outcomes associated with the use of vaginal estrogen.
Recap of the study
Starting in 1982, participants in the Nurses’Health Study were asked to report their use of vaginal estrogen via a validated questionnaire. For the years 1982 to 2012, investigators analyzed data from 896 and 52,901 women who had and had not used vaginal estrogen, respectively. The mean duration of vaginal estrogen use was 36 months.
In an analysis adjusted for numerous factors, the investigators observed no statistically significant differences in risk for cardiovascular outcomes (myocardial infarction, stroke, deep vein thrombosis, and pulmonary embolism) or invasive cancers (colorectal, endometrial, ovarian, or breast).
Findings uphold safety of vaginal estrogen
This landmark study provides reassurance that 3 years of use of vaginal estrogen does not increase the risk of cardiovascular events or invasive breast cancer, findings that hopefully will allow clinicians and women to feel comfortable regarding the safety of vaginal estrogen. A study of vaginal estrogen from the Women’s Health Initiative provided similar reassurance. Recent research supports guidance from The North American Menopause Society and the American College of Obstetricians and Gynecologists that vaginal estrogen can be used indefinitely, if indicated, and that use of concomitant progestin is not recommended in women who use vaginal estrogen and have an intact uterus.6,7
I agree with the authors, who point out that since treatment of GSM may need to be continued long term (even indefinitely), it would be helpful to have data that assessed the safety of longer-duration use of vaginal estrogen.
Results from Bhupathiraju and colleagues’ analysis of data from the Nurses’ Health Study on the 3-year safety of vaginal estrogen use encourage clinicians to recommend and women to use this safe and effective treatment for GSM.
How CO2 fractionated vaginal laser therapy compares with vaginal estrogen for relief of GSM symptoms
Paraiso MF, Ferrando CA, Sokol ER, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET trial. Menopause. 2020;27:50-56.
Up to 50% to 60% of postmenopausal women experience GSM symptoms. However, many fewer receive treatment, either because they do not understand that the symptoms are related to menopause or they are not aware that safe and effective treatment is available. Sadly, many women are not asked about their symptoms or are embarrassed to tell providers.
GSM affects relationships and quality of life. Vaginal lubricants or moisturizers may provide relief. US Food and Drug Administration (FDA)–approved therapies include low-dose vaginal estrogen, available as a vaginal tablet, cream, suppository, and ring; intravaginal dehydroepiandrosterone (DHEA); and oral ospemifene, a selective estrogen replacement modulator. If women have an estrogen-sensitive breast or uterine cancer, an oncologist should be involved in decisions about vaginal hormonal therapy.
Energy-based devices such as vaginal lasers appear to induce wound healing; stimulate collagen and elastin fiber formation through increased storage of glycogen; and activate fibroblasts, which leads to increased extracellular matrix and restoration of vaginal pH.
These lasers are FDA approved for use in gynecology but not specifically for the treatment of GSM. In July 2016, the FDA issued a safety alert that energy-based devices, while approved for use in gynecology, have not been approved or adequately tested for menopausal vaginal conditions, and safety concerns include reports of vaginal burns.8 Lacking are publications of adequately powered randomized, sham-con-trolled trials to determine if laser therapy works better for women with GSM than placebos, moisturizers, or vaginal hormone therapies.
Recently, investigators conducted a multicenter, randomized, single-blinded trial of vaginal laser therapy and estrogen cream for treatment of GSM.
Continue to: Details of the study...
Details of the study
Paraiso and colleagues aimed to compare the 6-month efficacy and safety of fractionated CO2 vaginal laser therapy with that of estrogen vaginal cream for the treatment of vaginal dryness/GSM.
Participants randomly assigned to the estrogen therapy arm applied conjugated estrogen cream 0.5 g vaginally daily for 14 days, followed by twice weekly application for 24 weeks (a low-dose vaginal estrogen therapy). Participants randomly assigned to laser therapy underwent 3 vaginal treatments at a minimum of 6 weeks apart.
Sixty-nine women were enrolled in the trial before enrollment was closed because the FDA required that the sponsor obtain and maintain an investigational device exemption. Of 62 women who completed 6 months’ treatment, 30 received 3 laser treatments and 32 received estrogen cream.
The primary outcome compared subjective improvement in vaginal dryness using the visual analog scale (VAS) between the 2 groups at 6 months. Secondary outcomes included comparisons of the vaginal health index (VHI) and vaginal maturation index (VMI), the effect of GSM on quality of life, the effect of treatment on sexual function and urinary symptoms, and patient satisfaction.
Study findings
Efficacy. Laser therapy and estrogen therapy were found to be similarly effective except on the VMI, which favored estrogen. On patient global impression, 85.8% of laser-treated women rated their improvement as ‘‘better or much better’’ and 78.5% reported being either ‘‘satisfied or very satisfied,’’ compared with 70% and 73.3%, respectively, in the estrogen group, a statistically nonsignificant difference.
On linear regression, the investigators found a nonsignificant mean difference in female sexual function index scores. While VMI scores remained higher in the estrogen-treated group (adjusted P = .02), baseline and 6-month follow-up VMI data were available for only 34 participants (16 laser treated, 18 estrogen treated).
Regarding long-term effectiveness, 20% to 25% of the women in the laser-treated group needed further treatment after 1 year while the estrogen cream continued to work as long as it was used as prescribed.
Adverse effects. The incidence of vaginal bleeding was similar in the 2 groups: 6.7% in the laser group and 6.3% in the estrogen group. In the laser therapy group, 3% expe-rienced vaginal pain, discharge, and bladder infections, while in the estrogen cream group, 3% reported breast tenderness, migraine headaches, and abdominal cramping.
Takeaways. This small randomized, open-label (not blinded) trial provides pilot data on the effectiveness of vaginal CO2 laser compared with vaginal estrogen in treating vaginal atrophy, quality-of-life symptoms, sexual function, and urinary symptoms. Adverse events were minimal. Patient global impression of improvement and satisfaction improved for both vaginal laser and vaginal estrogen therapy.
Continue to: Study strengths and limitations...
Study strengths and limitations
To show noninferiority of vaginal laser therapy to vaginal estrogen, 196 study participants were needed. However, after 38% had been enrolled, the FDA sent a warning letter to the Foundation for Female Health Awareness, which required obtainment of an investigational device exemption for the laser and addition of a sham treatment arm.9 Instead of redesigning the trial and reconsenting the participants, the investigators closed the study, and analysis was performed only on the 62 participants who completed the study; vaginal maturation was assessed only in 34 participants.
The study lacked a placebo or sham control, which increases the risk of bias, while small numbers limit the strength of the findings. Longer-term evaluation of the effects of laser therapy beyond 6 months is needed to allow assessment of the effects of scarring on vaginal health, sexual function, and urinary issues.
Discussing therapy with patients
Despite this study’s preliminary findings, and until more robust data are available, providers should discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, FDA-approved vaginal hormone therapies (such as vaginal estrogen and intravaginal dehydroepiandrosterone), and systemic therapies, such as hormone therapy and ospemifene, to determine the best treatment for the individual woman with GSM.
In a healthy postmenopausal woman with bothersome GSM symptoms not responsive to lubricants and moisturizers, I recommend FDA-approved vaginal therapies as first-line treatment if there are no contraindications. For women with breast cancer, I involve their oncologist. If a patient asks about vaginal laser treatment, I share that vaginal energy-based therapies, such as the vaginal laser, have not been approved for menopausal vaginal concerns. In addition to the possibility of adverse events or unsuccessful treatment, there are significant out-of-pocket costs and the potential need for ongoing therapy after the initial 3 laser treatments.
For GSM that does not respond to lubricants and moisturizers, many FDA-approved vaginal and systemic therapies are available to treat vaginal symptoms. Vaginal laser treatment is a promising therapy for vaginal symptoms of GSM that needs further testing to determine its efficacy, safety, and long-term effects. If discussing vaginal energy-based therapies with patients, include the current lack of FDA approval for specific vaginal indications, potential adverse effects, the need for ongoing retreatment, and out-of-pocket costs.
JoAnn E. Manson, MD, DrPH, NCMP
Having more appropriate, evidence-based labeling of low-dose vaginal estrogen continues to be a high priority for The North American Menopause Society (NAMS), the International Society for the Study of Women’s Sexual Health (ISSWSH), and other professional societies.
NAMS and the Working Group on Women’s Health and Well-Being in Menopause had submitted a citizen’s petition to the US Food and Drug Administration (FDA) in 2016 requesting modification of the label—including removal of the “black box warning”—for low-dose vaginal estrogen products. The petition was, disappointingly, denied in 2018.1
Currently, the class labeling, which was based on the results of randomized trials with systemic hormone therapy, is not applicable to low-dose vaginal estrogen, and the inclusion of the black box warning has led to serious underutilization of an effective and safe treatment for a very common and life-altering condition, the genitourinary syndrome of menopause (GSM). This condition affects nearly half of postmenopausal women. It tends to be chronic and progressive and, unlike hot flashes and vasomotor symptoms, it does not remit or decline over time, and it affects women’s health and quality of life.
While removal of the black box warning would be appropriate, labeling should include emphatic reminders for women that if they have any bleeding or spotting they should seek medical attention immediately, and if they have a history of breast cancer or other estrogen-sensitive cancers they should talk with their oncologist prior to starting treatment with low-dose vaginal estrogen. Although the text would still inform women of research results on systemic hormone therapy, it would explain the differences between low-dose vaginal estrogen and systemic therapy.
Studies show vaginal estrogen has good safety profile
In the last several years, large, observational studies of low-dose vaginal estrogen have suggested that this treatment is not associated with an increase in cardiovascular disease, pulmonary embolism, venous thrombosis, cancer, or dementia—conditions listed in the black box warning that were linked to systemic estrogen therapy plus synthetic progestin. Recent data from the Nurses’ Health Study, for example, demonstrated that 3 years of vaginal estrogen use did not increase the risk of cardiovascular events or invasive breast cancer.
Women’s Health Initiative. In a prospective observational cohort study, Crandall and colleagues used data from participants in the Women’s Health Initiative Observational Study to determine the association between use of vaginal estrogen and risk of a global index event (GIE), defined as time to first occurrence of coronary heart disease, invasive breast cancer, stroke, pulmonary embolism, hip fracture, colorectal cancer, endometrial cancer, or death from any cause.2
Women were recruited from multiple clinical centers, were aged 50 to 79 years at baseline, and did not use systemic estrogen therapy during follow-up. The study included 45,663 women and median follow-up was 7.2 years. The investigators collected data on women’s self-reported use of vaginal estrogen as well as the development of the conditions defined above.
In women with a uterus, there was no significant difference between vaginal estrogen users and nonusers in the risk of stroke, invasive breast cancer, colorectal cancer, endometrial cancer, pulmonary embolism, or deep vein thrombosis. The risks of coronary heart disease, fracture, all-cause mortality, and GIE were lower in vaginal estrogen users than in nonusers (GIE adjusted hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.55–0.86).
In women who had undergone hysterectomy, the risks of the individual GIE components and the overall GIE were not significantly different in users of vaginal estrogen compared with nonusers (GIE adjusted HR, 0.94; 95% CI, 0.70–1.26).
The investigators concluded that the risks of cardiovascular disease and cancer were not increased in postmenopausal women who used vaginal estrogen. Thus, this study offers reassurance on the treatment’s safety.2
Meta-analysis on menopausal hormone therapy and breast cancer risk. Further evidence now indicates that low-dose vaginal estrogen is not linked to chronic health conditions. In a large meta-analysis published in 2019, investigators looked at different types of hormone therapies—oral estrogen plus progestin, transdermal estrogen and progestin, estrogen alone, low-dose vaginal estrogen—and their relationship to breast cancer risk.3
Information on individual participants was obtained from 58 studies, 24 prospective and 34 retrospective. Breast cancer relative risks (RR) during years 5 to 14 of current hormone use were assessed according to the main hormonal contituents, doses, and modes of delivery of the last-used menopausal hormone therapy. For all systemic estrogen-only preparations, the RR was 1.33 (95% CI, 1.28–1.38), while for all estrogen-progestogen preparations, the RR was 2.08 (95% CI, 2.02–2.15). For transdermal estrogen, the RR was 1.35 (95% CI, 1.25–1.46). In contrast, for vaginal estrogen, the RR was 1.09 (95% CI, 0.97–1.23).3
Thus, the analysis found that in all the studies that had been done to date, there was no evidence of increased risk of breast cancer with vaginal estrogen therapy.
The evidence is growing that low-dose vaginal estrogen is different from systemic estrogen in terms of its safety profile and benefit-risk pattern. It is important for the FDA to consider these data and revise the vaginal estrogen label.
On the horizon: New estradiol reference ranges
It would be useful if we could accurately compare estradiol levels in women treated with vaginal estrogen against those of women treated with systemic estrogen therapy. In September 2019, NAMS held a workshop with the goal of establishing reference ranges for estradiol in postmenopausal women.4 It is very important to have good, reliable laboratory assays for estradiol and estrone, and to have a clear understanding of what is a reference range, that is, the range of estradiol levels in postmenopausal women who are not treated with estrogen. That way, you can observe what the estradiol blood levels are in women treated with low-dose vaginal estrogen or those treated with systemic estrogen versus the levels observed among postmenopausal women not receiving any estrogen product.
With the reference range information, we could look at data on the blood levels of estradiol with low-dose vaginal estrogen from the various studies available, as well as the increasing evidence from observational studies of the safety of low-dose vaginal estrogen to better understand its relationship with health. If these studies demonstrate that, with certain doses and formulations of low-dose vaginal estrogen, blood estradiol levels stay within the reference range of postmenopausal estradiol levels, it would inform the labeling modifications of these products. We need this information for future discussions with the FDA.
The laboratory assay technology used for such an investigation is primarily liquid chromatography with tandem mass spectrometry, the so-called LC-MS/MS assay. With use of this technology, the reference range for estradiol may be less than 10 picograms per milliliter. Previously, a very wide and inconsistent range—about 5 to 30 picograms per milliliter—was considered a “normal” range.
NAMS is championing the efforts to define a true evidence-based reference range that would represent the range of levels seen in postmenopausal women.5 This effort has been spearheaded by Dr. Richard Santen and colleagues. Using the more sensitive and specific LC-MS/MS assay will enable researchers and clinicians to better understand how levels on low-dose vaginal estrogen relate to the reference range for postmenopausal women. We are hoping to work together with researchers to establish these reference ranges, and to use that information to look at how low-dose vaginal estrogen compares to levels in untreated postmenopausal women, as well as to levels in women on systemic estrogen.
Hopefully, establishing the reference range can be done in an expeditious and timely way, with discussions with the FDA resuming shortly thereafter.
References
1.NAMS Citizen’s Petition and FDA Response, June 7, 2018. http://www.menopause.org/docs/
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. 2018;25:11-20.
3. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394:1159-1168.
4. Santen RJ, Pinkerton JV, Liu JH, et al. Workshop on normal reference ranges for estradiol in postmenopausal women, September 2019, Chicago, Illinois. Menopause. May 4, 2020. doi:10.1097/GME.0000000000001556.
5. Pinkerton JV, Liu JH, Santoro NF, et al. Workshop on normal reference ranges for estradiol in postmenopausal women: commentary from The North American Menopause Society on low-dose vaginal estrogen therapy. Menopause. 2020;27:611-613.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Maturitas. 2014;79:349-354.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126:859-876.
- Shifren JL. Genitourinary syndrome of menopause. Clin Obstet Gynecol. 2018;61:508-516.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806.
- Kingsberg SA, Krychman M, Graham S, et al. The women’s EMPOWER survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 Hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- US Food and Drug Administration website. FDA warns against the use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/medical-devices/safety-communications/fda-warns-against-use-energy-based-devices-perform-vaginal-rejuvenation-or-vaginal-cosmetic. Updated November 20, 2018. Accessed May 21, 2020.
- US Food and Drug Administration website. Letters to industry. https://www.fda.gov/medical-devices/industry-medical-devices/letters-industry. July 24, 2018. Accessed May 21, 2020
The term genitourinary syndrome of menopause (GSM) refers to the bothersome symptoms and physical findings associated with estrogen deficiency that involve the labia, vestibular tissue, clitoris, vagina, urethra, and bladder.1 GSM is associated with genital irritation, dryness, and burning; urinary symptoms including urgency, dysuria, and recurrent urinary tract infections; and sexual symptoms including vaginal dryness and pain. Vulvovaginal atrophy (VVA) represents a component of GSM.
GSM is highly prevalent, affecting more than three-quarters of menopausal women. In contrast to menopausal vasomotor symptoms, which often are most severe and frequent in recently menopausal women, GSM commonly presents years following menopause. Unfortunately, VVA symptoms may have a substantial negative impact on women’s quality of life.
In this 2020 Menopause Update, I review a large observational study that provides reassurance to clinicians and patients regarding the safety of the best-studied prescription treatment for GSM—vaginal estrogen. Because some women should not use vaginal estrogen and others choose not to use it, nonhormonal management of GSM is important. Dr. JoAnn Pinkerton provides details on a randomized clinical trial that compared the use of fractionated CO2 laser therapy with vaginal estrogen for the treatment of GSM. In addition, Dr. JoAnn Manson discusses recent studies that found lower health risks with vaginal estrogen use compared with systemic estrogen therapy.
Diagnosing GSM
GSM can be diagnosed presumptively based on a characteristic history in a menopausal patient. Performing a pelvic examination, however, allows clinicians to exclude other conditions that may present with similar symptoms, such as lichen sclerosus, Candida infection, and malignancy.
During inspection of the external genitalia, the clinician may note loss of the fat pad in the labia majora and mons as well as a reduction in labia minora pigmentation and tissue. The urethral meatus often becomes erythematous and prominent. If vaginal or introital narrowing is present, use of a pediatric (ultrathin) speculum reduces patient discomfort. The vaginal mucosa may appear smooth due to loss of rugation; it also may appear shiny and dry. Bleeding (friability) on contact with a spatula or cotton-tipped swab may occur. In addition, the vaginal fornices may become attenuated, leaving the cervix flush with the vaginal apex.
GSM can be diagnosed without laboratory assessment. However, vaginal pH, if measured, is characteristically higher than 5.0; microscopic wet prep often reveals many white blood cells, immature epithelial cells (large nuclei), and reduced or absent lactobacilli.2

Nonhormonal management of GSM
Water, silicone-based, and oil-based lubricants reduce the friction and discomfort associated with sexual activity. By contrast, vaginal moisturizers act longer than lubricants and can be applied several times weekly or daily. Natural oils, including olive and coconut oil, may be useful both as lubricants and as moisturizers. Aqueous lidocaine 4%, applied to vestibular tissue with cotton balls prior to penetration, reduces dyspareunia in women with GSM.3
Vaginal estrogen therapy
When nonhormonal management does not sufficiently reduce GSM symptoms, use of low-dose vaginal estrogen enhances thickness and elasticity of genital tissue and improves vaginal blood flow. Vaginal estrogen creams, tablets, an insert, and a ring are marketed in the United States. Although clinical improvement may be apparent within several weeks of initiating vaginal estrogen, the full benefit of treatment becomes apparent after 2 to 3 months.3
Despite the availability and effectiveness of low-dose vaginal estrogen, fears regarding the safety of menopausal hormone therapy have resulted in the underutilization of vaginal estrogen.4,5 Unfortunately, the package labeling for low-dose vaginal estrogen can exacerbate these fears.
Continue to: Nurses’ Health Study report...
Nurses’ Health Study report provides reassurance on long-term safety of vaginal estrogen
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610
Bhupathiraju and colleagues published a report from the long-running Nurses’ Health prospective cohort study on the health outcomes associated with the use of vaginal estrogen.
Recap of the study
Starting in 1982, participants in the Nurses’Health Study were asked to report their use of vaginal estrogen via a validated questionnaire. For the years 1982 to 2012, investigators analyzed data from 896 and 52,901 women who had and had not used vaginal estrogen, respectively. The mean duration of vaginal estrogen use was 36 months.
In an analysis adjusted for numerous factors, the investigators observed no statistically significant differences in risk for cardiovascular outcomes (myocardial infarction, stroke, deep vein thrombosis, and pulmonary embolism) or invasive cancers (colorectal, endometrial, ovarian, or breast).
Findings uphold safety of vaginal estrogen
This landmark study provides reassurance that 3 years of use of vaginal estrogen does not increase the risk of cardiovascular events or invasive breast cancer, findings that hopefully will allow clinicians and women to feel comfortable regarding the safety of vaginal estrogen. A study of vaginal estrogen from the Women’s Health Initiative provided similar reassurance. Recent research supports guidance from The North American Menopause Society and the American College of Obstetricians and Gynecologists that vaginal estrogen can be used indefinitely, if indicated, and that use of concomitant progestin is not recommended in women who use vaginal estrogen and have an intact uterus.6,7
I agree with the authors, who point out that since treatment of GSM may need to be continued long term (even indefinitely), it would be helpful to have data that assessed the safety of longer-duration use of vaginal estrogen.
Results from Bhupathiraju and colleagues’ analysis of data from the Nurses’ Health Study on the 3-year safety of vaginal estrogen use encourage clinicians to recommend and women to use this safe and effective treatment for GSM.
How CO2 fractionated vaginal laser therapy compares with vaginal estrogen for relief of GSM symptoms
Paraiso MF, Ferrando CA, Sokol ER, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET trial. Menopause. 2020;27:50-56.
Up to 50% to 60% of postmenopausal women experience GSM symptoms. However, many fewer receive treatment, either because they do not understand that the symptoms are related to menopause or they are not aware that safe and effective treatment is available. Sadly, many women are not asked about their symptoms or are embarrassed to tell providers.
GSM affects relationships and quality of life. Vaginal lubricants or moisturizers may provide relief. US Food and Drug Administration (FDA)–approved therapies include low-dose vaginal estrogen, available as a vaginal tablet, cream, suppository, and ring; intravaginal dehydroepiandrosterone (DHEA); and oral ospemifene, a selective estrogen replacement modulator. If women have an estrogen-sensitive breast or uterine cancer, an oncologist should be involved in decisions about vaginal hormonal therapy.
Energy-based devices such as vaginal lasers appear to induce wound healing; stimulate collagen and elastin fiber formation through increased storage of glycogen; and activate fibroblasts, which leads to increased extracellular matrix and restoration of vaginal pH.
These lasers are FDA approved for use in gynecology but not specifically for the treatment of GSM. In July 2016, the FDA issued a safety alert that energy-based devices, while approved for use in gynecology, have not been approved or adequately tested for menopausal vaginal conditions, and safety concerns include reports of vaginal burns.8 Lacking are publications of adequately powered randomized, sham-con-trolled trials to determine if laser therapy works better for women with GSM than placebos, moisturizers, or vaginal hormone therapies.
Recently, investigators conducted a multicenter, randomized, single-blinded trial of vaginal laser therapy and estrogen cream for treatment of GSM.
Continue to: Details of the study...
Details of the study
Paraiso and colleagues aimed to compare the 6-month efficacy and safety of fractionated CO2 vaginal laser therapy with that of estrogen vaginal cream for the treatment of vaginal dryness/GSM.
Participants randomly assigned to the estrogen therapy arm applied conjugated estrogen cream 0.5 g vaginally daily for 14 days, followed by twice weekly application for 24 weeks (a low-dose vaginal estrogen therapy). Participants randomly assigned to laser therapy underwent 3 vaginal treatments at a minimum of 6 weeks apart.
Sixty-nine women were enrolled in the trial before enrollment was closed because the FDA required that the sponsor obtain and maintain an investigational device exemption. Of 62 women who completed 6 months’ treatment, 30 received 3 laser treatments and 32 received estrogen cream.
The primary outcome compared subjective improvement in vaginal dryness using the visual analog scale (VAS) between the 2 groups at 6 months. Secondary outcomes included comparisons of the vaginal health index (VHI) and vaginal maturation index (VMI), the effect of GSM on quality of life, the effect of treatment on sexual function and urinary symptoms, and patient satisfaction.
Study findings
Efficacy. Laser therapy and estrogen therapy were found to be similarly effective except on the VMI, which favored estrogen. On patient global impression, 85.8% of laser-treated women rated their improvement as ‘‘better or much better’’ and 78.5% reported being either ‘‘satisfied or very satisfied,’’ compared with 70% and 73.3%, respectively, in the estrogen group, a statistically nonsignificant difference.
On linear regression, the investigators found a nonsignificant mean difference in female sexual function index scores. While VMI scores remained higher in the estrogen-treated group (adjusted P = .02), baseline and 6-month follow-up VMI data were available for only 34 participants (16 laser treated, 18 estrogen treated).
Regarding long-term effectiveness, 20% to 25% of the women in the laser-treated group needed further treatment after 1 year while the estrogen cream continued to work as long as it was used as prescribed.
Adverse effects. The incidence of vaginal bleeding was similar in the 2 groups: 6.7% in the laser group and 6.3% in the estrogen group. In the laser therapy group, 3% expe-rienced vaginal pain, discharge, and bladder infections, while in the estrogen cream group, 3% reported breast tenderness, migraine headaches, and abdominal cramping.
Takeaways. This small randomized, open-label (not blinded) trial provides pilot data on the effectiveness of vaginal CO2 laser compared with vaginal estrogen in treating vaginal atrophy, quality-of-life symptoms, sexual function, and urinary symptoms. Adverse events were minimal. Patient global impression of improvement and satisfaction improved for both vaginal laser and vaginal estrogen therapy.
Continue to: Study strengths and limitations...
Study strengths and limitations
To show noninferiority of vaginal laser therapy to vaginal estrogen, 196 study participants were needed. However, after 38% had been enrolled, the FDA sent a warning letter to the Foundation for Female Health Awareness, which required obtainment of an investigational device exemption for the laser and addition of a sham treatment arm.9 Instead of redesigning the trial and reconsenting the participants, the investigators closed the study, and analysis was performed only on the 62 participants who completed the study; vaginal maturation was assessed only in 34 participants.
The study lacked a placebo or sham control, which increases the risk of bias, while small numbers limit the strength of the findings. Longer-term evaluation of the effects of laser therapy beyond 6 months is needed to allow assessment of the effects of scarring on vaginal health, sexual function, and urinary issues.
Discussing therapy with patients
Despite this study’s preliminary findings, and until more robust data are available, providers should discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, FDA-approved vaginal hormone therapies (such as vaginal estrogen and intravaginal dehydroepiandrosterone), and systemic therapies, such as hormone therapy and ospemifene, to determine the best treatment for the individual woman with GSM.
In a healthy postmenopausal woman with bothersome GSM symptoms not responsive to lubricants and moisturizers, I recommend FDA-approved vaginal therapies as first-line treatment if there are no contraindications. For women with breast cancer, I involve their oncologist. If a patient asks about vaginal laser treatment, I share that vaginal energy-based therapies, such as the vaginal laser, have not been approved for menopausal vaginal concerns. In addition to the possibility of adverse events or unsuccessful treatment, there are significant out-of-pocket costs and the potential need for ongoing therapy after the initial 3 laser treatments.
For GSM that does not respond to lubricants and moisturizers, many FDA-approved vaginal and systemic therapies are available to treat vaginal symptoms. Vaginal laser treatment is a promising therapy for vaginal symptoms of GSM that needs further testing to determine its efficacy, safety, and long-term effects. If discussing vaginal energy-based therapies with patients, include the current lack of FDA approval for specific vaginal indications, potential adverse effects, the need for ongoing retreatment, and out-of-pocket costs.
JoAnn E. Manson, MD, DrPH, NCMP
Having more appropriate, evidence-based labeling of low-dose vaginal estrogen continues to be a high priority for The North American Menopause Society (NAMS), the International Society for the Study of Women’s Sexual Health (ISSWSH), and other professional societies.
NAMS and the Working Group on Women’s Health and Well-Being in Menopause had submitted a citizen’s petition to the US Food and Drug Administration (FDA) in 2016 requesting modification of the label—including removal of the “black box warning”—for low-dose vaginal estrogen products. The petition was, disappointingly, denied in 2018.1
Currently, the class labeling, which was based on the results of randomized trials with systemic hormone therapy, is not applicable to low-dose vaginal estrogen, and the inclusion of the black box warning has led to serious underutilization of an effective and safe treatment for a very common and life-altering condition, the genitourinary syndrome of menopause (GSM). This condition affects nearly half of postmenopausal women. It tends to be chronic and progressive and, unlike hot flashes and vasomotor symptoms, it does not remit or decline over time, and it affects women’s health and quality of life.
While removal of the black box warning would be appropriate, labeling should include emphatic reminders for women that if they have any bleeding or spotting they should seek medical attention immediately, and if they have a history of breast cancer or other estrogen-sensitive cancers they should talk with their oncologist prior to starting treatment with low-dose vaginal estrogen. Although the text would still inform women of research results on systemic hormone therapy, it would explain the differences between low-dose vaginal estrogen and systemic therapy.
Studies show vaginal estrogen has good safety profile
In the last several years, large, observational studies of low-dose vaginal estrogen have suggested that this treatment is not associated with an increase in cardiovascular disease, pulmonary embolism, venous thrombosis, cancer, or dementia—conditions listed in the black box warning that were linked to systemic estrogen therapy plus synthetic progestin. Recent data from the Nurses’ Health Study, for example, demonstrated that 3 years of vaginal estrogen use did not increase the risk of cardiovascular events or invasive breast cancer.
Women’s Health Initiative. In a prospective observational cohort study, Crandall and colleagues used data from participants in the Women’s Health Initiative Observational Study to determine the association between use of vaginal estrogen and risk of a global index event (GIE), defined as time to first occurrence of coronary heart disease, invasive breast cancer, stroke, pulmonary embolism, hip fracture, colorectal cancer, endometrial cancer, or death from any cause.2
Women were recruited from multiple clinical centers, were aged 50 to 79 years at baseline, and did not use systemic estrogen therapy during follow-up. The study included 45,663 women and median follow-up was 7.2 years. The investigators collected data on women’s self-reported use of vaginal estrogen as well as the development of the conditions defined above.
In women with a uterus, there was no significant difference between vaginal estrogen users and nonusers in the risk of stroke, invasive breast cancer, colorectal cancer, endometrial cancer, pulmonary embolism, or deep vein thrombosis. The risks of coronary heart disease, fracture, all-cause mortality, and GIE were lower in vaginal estrogen users than in nonusers (GIE adjusted hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.55–0.86).
In women who had undergone hysterectomy, the risks of the individual GIE components and the overall GIE were not significantly different in users of vaginal estrogen compared with nonusers (GIE adjusted HR, 0.94; 95% CI, 0.70–1.26).
The investigators concluded that the risks of cardiovascular disease and cancer were not increased in postmenopausal women who used vaginal estrogen. Thus, this study offers reassurance on the treatment’s safety.2
Meta-analysis on menopausal hormone therapy and breast cancer risk. Further evidence now indicates that low-dose vaginal estrogen is not linked to chronic health conditions. In a large meta-analysis published in 2019, investigators looked at different types of hormone therapies—oral estrogen plus progestin, transdermal estrogen and progestin, estrogen alone, low-dose vaginal estrogen—and their relationship to breast cancer risk.3
Information on individual participants was obtained from 58 studies, 24 prospective and 34 retrospective. Breast cancer relative risks (RR) during years 5 to 14 of current hormone use were assessed according to the main hormonal contituents, doses, and modes of delivery of the last-used menopausal hormone therapy. For all systemic estrogen-only preparations, the RR was 1.33 (95% CI, 1.28–1.38), while for all estrogen-progestogen preparations, the RR was 2.08 (95% CI, 2.02–2.15). For transdermal estrogen, the RR was 1.35 (95% CI, 1.25–1.46). In contrast, for vaginal estrogen, the RR was 1.09 (95% CI, 0.97–1.23).3
Thus, the analysis found that in all the studies that had been done to date, there was no evidence of increased risk of breast cancer with vaginal estrogen therapy.
The evidence is growing that low-dose vaginal estrogen is different from systemic estrogen in terms of its safety profile and benefit-risk pattern. It is important for the FDA to consider these data and revise the vaginal estrogen label.
On the horizon: New estradiol reference ranges
It would be useful if we could accurately compare estradiol levels in women treated with vaginal estrogen against those of women treated with systemic estrogen therapy. In September 2019, NAMS held a workshop with the goal of establishing reference ranges for estradiol in postmenopausal women.4 It is very important to have good, reliable laboratory assays for estradiol and estrone, and to have a clear understanding of what is a reference range, that is, the range of estradiol levels in postmenopausal women who are not treated with estrogen. That way, you can observe what the estradiol blood levels are in women treated with low-dose vaginal estrogen or those treated with systemic estrogen versus the levels observed among postmenopausal women not receiving any estrogen product.
With the reference range information, we could look at data on the blood levels of estradiol with low-dose vaginal estrogen from the various studies available, as well as the increasing evidence from observational studies of the safety of low-dose vaginal estrogen to better understand its relationship with health. If these studies demonstrate that, with certain doses and formulations of low-dose vaginal estrogen, blood estradiol levels stay within the reference range of postmenopausal estradiol levels, it would inform the labeling modifications of these products. We need this information for future discussions with the FDA.
The laboratory assay technology used for such an investigation is primarily liquid chromatography with tandem mass spectrometry, the so-called LC-MS/MS assay. With use of this technology, the reference range for estradiol may be less than 10 picograms per milliliter. Previously, a very wide and inconsistent range—about 5 to 30 picograms per milliliter—was considered a “normal” range.
NAMS is championing the efforts to define a true evidence-based reference range that would represent the range of levels seen in postmenopausal women.5 This effort has been spearheaded by Dr. Richard Santen and colleagues. Using the more sensitive and specific LC-MS/MS assay will enable researchers and clinicians to better understand how levels on low-dose vaginal estrogen relate to the reference range for postmenopausal women. We are hoping to work together with researchers to establish these reference ranges, and to use that information to look at how low-dose vaginal estrogen compares to levels in untreated postmenopausal women, as well as to levels in women on systemic estrogen.
Hopefully, establishing the reference range can be done in an expeditious and timely way, with discussions with the FDA resuming shortly thereafter.
References
1.NAMS Citizen’s Petition and FDA Response, June 7, 2018. http://www.menopause.org/docs/
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. 2018;25:11-20.
3. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394:1159-1168.
4. Santen RJ, Pinkerton JV, Liu JH, et al. Workshop on normal reference ranges for estradiol in postmenopausal women, September 2019, Chicago, Illinois. Menopause. May 4, 2020. doi:10.1097/GME.0000000000001556.
5. Pinkerton JV, Liu JH, Santoro NF, et al. Workshop on normal reference ranges for estradiol in postmenopausal women: commentary from The North American Menopause Society on low-dose vaginal estrogen therapy. Menopause. 2020;27:611-613.
The term genitourinary syndrome of menopause (GSM) refers to the bothersome symptoms and physical findings associated with estrogen deficiency that involve the labia, vestibular tissue, clitoris, vagina, urethra, and bladder.1 GSM is associated with genital irritation, dryness, and burning; urinary symptoms including urgency, dysuria, and recurrent urinary tract infections; and sexual symptoms including vaginal dryness and pain. Vulvovaginal atrophy (VVA) represents a component of GSM.
GSM is highly prevalent, affecting more than three-quarters of menopausal women. In contrast to menopausal vasomotor symptoms, which often are most severe and frequent in recently menopausal women, GSM commonly presents years following menopause. Unfortunately, VVA symptoms may have a substantial negative impact on women’s quality of life.
In this 2020 Menopause Update, I review a large observational study that provides reassurance to clinicians and patients regarding the safety of the best-studied prescription treatment for GSM—vaginal estrogen. Because some women should not use vaginal estrogen and others choose not to use it, nonhormonal management of GSM is important. Dr. JoAnn Pinkerton provides details on a randomized clinical trial that compared the use of fractionated CO2 laser therapy with vaginal estrogen for the treatment of GSM. In addition, Dr. JoAnn Manson discusses recent studies that found lower health risks with vaginal estrogen use compared with systemic estrogen therapy.
Diagnosing GSM
GSM can be diagnosed presumptively based on a characteristic history in a menopausal patient. Performing a pelvic examination, however, allows clinicians to exclude other conditions that may present with similar symptoms, such as lichen sclerosus, Candida infection, and malignancy.
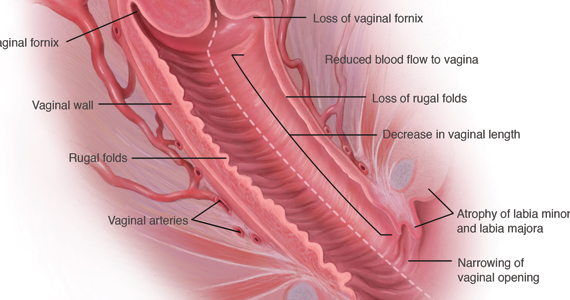
During inspection of the external genitalia, the clinician may note loss of the fat pad in the labia majora and mons as well as a reduction in labia minora pigmentation and tissue. The urethral meatus often becomes erythematous and prominent. If vaginal or introital narrowing is present, use of a pediatric (ultrathin) speculum reduces patient discomfort. The vaginal mucosa may appear smooth due to loss of rugation; it also may appear shiny and dry. Bleeding (friability) on contact with a spatula or cotton-tipped swab may occur. In addition, the vaginal fornices may become attenuated, leaving the cervix flush with the vaginal apex.
GSM can be diagnosed without laboratory assessment. However, vaginal pH, if measured, is characteristically higher than 5.0; microscopic wet prep often reveals many white blood cells, immature epithelial cells (large nuclei), and reduced or absent lactobacilli.2

Nonhormonal management of GSM
Water, silicone-based, and oil-based lubricants reduce the friction and discomfort associated with sexual activity. By contrast, vaginal moisturizers act longer than lubricants and can be applied several times weekly or daily. Natural oils, including olive and coconut oil, may be useful both as lubricants and as moisturizers. Aqueous lidocaine 4%, applied to vestibular tissue with cotton balls prior to penetration, reduces dyspareunia in women with GSM.3
Vaginal estrogen therapy
When nonhormonal management does not sufficiently reduce GSM symptoms, use of low-dose vaginal estrogen enhances thickness and elasticity of genital tissue and improves vaginal blood flow. Vaginal estrogen creams, tablets, an insert, and a ring are marketed in the United States. Although clinical improvement may be apparent within several weeks of initiating vaginal estrogen, the full benefit of treatment becomes apparent after 2 to 3 months.3
Despite the availability and effectiveness of low-dose vaginal estrogen, fears regarding the safety of menopausal hormone therapy have resulted in the underutilization of vaginal estrogen.4,5 Unfortunately, the package labeling for low-dose vaginal estrogen can exacerbate these fears.
Continue to: Nurses’ Health Study report...
Nurses’ Health Study report provides reassurance on long-term safety of vaginal estrogen
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610
Bhupathiraju and colleagues published a report from the long-running Nurses’ Health prospective cohort study on the health outcomes associated with the use of vaginal estrogen.
Recap of the study
Starting in 1982, participants in the Nurses’Health Study were asked to report their use of vaginal estrogen via a validated questionnaire. For the years 1982 to 2012, investigators analyzed data from 896 and 52,901 women who had and had not used vaginal estrogen, respectively. The mean duration of vaginal estrogen use was 36 months.
In an analysis adjusted for numerous factors, the investigators observed no statistically significant differences in risk for cardiovascular outcomes (myocardial infarction, stroke, deep vein thrombosis, and pulmonary embolism) or invasive cancers (colorectal, endometrial, ovarian, or breast).
Findings uphold safety of vaginal estrogen
This landmark study provides reassurance that 3 years of use of vaginal estrogen does not increase the risk of cardiovascular events or invasive breast cancer, findings that hopefully will allow clinicians and women to feel comfortable regarding the safety of vaginal estrogen. A study of vaginal estrogen from the Women’s Health Initiative provided similar reassurance. Recent research supports guidance from The North American Menopause Society and the American College of Obstetricians and Gynecologists that vaginal estrogen can be used indefinitely, if indicated, and that use of concomitant progestin is not recommended in women who use vaginal estrogen and have an intact uterus.6,7
I agree with the authors, who point out that since treatment of GSM may need to be continued long term (even indefinitely), it would be helpful to have data that assessed the safety of longer-duration use of vaginal estrogen.
Results from Bhupathiraju and colleagues’ analysis of data from the Nurses’ Health Study on the 3-year safety of vaginal estrogen use encourage clinicians to recommend and women to use this safe and effective treatment for GSM.
How CO2 fractionated vaginal laser therapy compares with vaginal estrogen for relief of GSM symptoms
Paraiso MF, Ferrando CA, Sokol ER, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET trial. Menopause. 2020;27:50-56.
Up to 50% to 60% of postmenopausal women experience GSM symptoms. However, many fewer receive treatment, either because they do not understand that the symptoms are related to menopause or they are not aware that safe and effective treatment is available. Sadly, many women are not asked about their symptoms or are embarrassed to tell providers.
GSM affects relationships and quality of life. Vaginal lubricants or moisturizers may provide relief. US Food and Drug Administration (FDA)–approved therapies include low-dose vaginal estrogen, available as a vaginal tablet, cream, suppository, and ring; intravaginal dehydroepiandrosterone (DHEA); and oral ospemifene, a selective estrogen replacement modulator. If women have an estrogen-sensitive breast or uterine cancer, an oncologist should be involved in decisions about vaginal hormonal therapy.
Energy-based devices such as vaginal lasers appear to induce wound healing; stimulate collagen and elastin fiber formation through increased storage of glycogen; and activate fibroblasts, which leads to increased extracellular matrix and restoration of vaginal pH.
These lasers are FDA approved for use in gynecology but not specifically for the treatment of GSM. In July 2016, the FDA issued a safety alert that energy-based devices, while approved for use in gynecology, have not been approved or adequately tested for menopausal vaginal conditions, and safety concerns include reports of vaginal burns.8 Lacking are publications of adequately powered randomized, sham-con-trolled trials to determine if laser therapy works better for women with GSM than placebos, moisturizers, or vaginal hormone therapies.
Recently, investigators conducted a multicenter, randomized, single-blinded trial of vaginal laser therapy and estrogen cream for treatment of GSM.
Continue to: Details of the study...
Details of the study
Paraiso and colleagues aimed to compare the 6-month efficacy and safety of fractionated CO2 vaginal laser therapy with that of estrogen vaginal cream for the treatment of vaginal dryness/GSM.
Participants randomly assigned to the estrogen therapy arm applied conjugated estrogen cream 0.5 g vaginally daily for 14 days, followed by twice weekly application for 24 weeks (a low-dose vaginal estrogen therapy). Participants randomly assigned to laser therapy underwent 3 vaginal treatments at a minimum of 6 weeks apart.
Sixty-nine women were enrolled in the trial before enrollment was closed because the FDA required that the sponsor obtain and maintain an investigational device exemption. Of 62 women who completed 6 months’ treatment, 30 received 3 laser treatments and 32 received estrogen cream.
The primary outcome compared subjective improvement in vaginal dryness using the visual analog scale (VAS) between the 2 groups at 6 months. Secondary outcomes included comparisons of the vaginal health index (VHI) and vaginal maturation index (VMI), the effect of GSM on quality of life, the effect of treatment on sexual function and urinary symptoms, and patient satisfaction.
Study findings
Efficacy. Laser therapy and estrogen therapy were found to be similarly effective except on the VMI, which favored estrogen. On patient global impression, 85.8% of laser-treated women rated their improvement as ‘‘better or much better’’ and 78.5% reported being either ‘‘satisfied or very satisfied,’’ compared with 70% and 73.3%, respectively, in the estrogen group, a statistically nonsignificant difference.
On linear regression, the investigators found a nonsignificant mean difference in female sexual function index scores. While VMI scores remained higher in the estrogen-treated group (adjusted P = .02), baseline and 6-month follow-up VMI data were available for only 34 participants (16 laser treated, 18 estrogen treated).
Regarding long-term effectiveness, 20% to 25% of the women in the laser-treated group needed further treatment after 1 year while the estrogen cream continued to work as long as it was used as prescribed.
Adverse effects. The incidence of vaginal bleeding was similar in the 2 groups: 6.7% in the laser group and 6.3% in the estrogen group. In the laser therapy group, 3% expe-rienced vaginal pain, discharge, and bladder infections, while in the estrogen cream group, 3% reported breast tenderness, migraine headaches, and abdominal cramping.
Takeaways. This small randomized, open-label (not blinded) trial provides pilot data on the effectiveness of vaginal CO2 laser compared with vaginal estrogen in treating vaginal atrophy, quality-of-life symptoms, sexual function, and urinary symptoms. Adverse events were minimal. Patient global impression of improvement and satisfaction improved for both vaginal laser and vaginal estrogen therapy.
Continue to: Study strengths and limitations...
Study strengths and limitations
To show noninferiority of vaginal laser therapy to vaginal estrogen, 196 study participants were needed. However, after 38% had been enrolled, the FDA sent a warning letter to the Foundation for Female Health Awareness, which required obtainment of an investigational device exemption for the laser and addition of a sham treatment arm.9 Instead of redesigning the trial and reconsenting the participants, the investigators closed the study, and analysis was performed only on the 62 participants who completed the study; vaginal maturation was assessed only in 34 participants.
The study lacked a placebo or sham control, which increases the risk of bias, while small numbers limit the strength of the findings. Longer-term evaluation of the effects of laser therapy beyond 6 months is needed to allow assessment of the effects of scarring on vaginal health, sexual function, and urinary issues.
Discussing therapy with patients
Despite this study’s preliminary findings, and until more robust data are available, providers should discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, FDA-approved vaginal hormone therapies (such as vaginal estrogen and intravaginal dehydroepiandrosterone), and systemic therapies, such as hormone therapy and ospemifene, to determine the best treatment for the individual woman with GSM.
In a healthy postmenopausal woman with bothersome GSM symptoms not responsive to lubricants and moisturizers, I recommend FDA-approved vaginal therapies as first-line treatment if there are no contraindications. For women with breast cancer, I involve their oncologist. If a patient asks about vaginal laser treatment, I share that vaginal energy-based therapies, such as the vaginal laser, have not been approved for menopausal vaginal concerns. In addition to the possibility of adverse events or unsuccessful treatment, there are significant out-of-pocket costs and the potential need for ongoing therapy after the initial 3 laser treatments.
For GSM that does not respond to lubricants and moisturizers, many FDA-approved vaginal and systemic therapies are available to treat vaginal symptoms. Vaginal laser treatment is a promising therapy for vaginal symptoms of GSM that needs further testing to determine its efficacy, safety, and long-term effects. If discussing vaginal energy-based therapies with patients, include the current lack of FDA approval for specific vaginal indications, potential adverse effects, the need for ongoing retreatment, and out-of-pocket costs.
JoAnn E. Manson, MD, DrPH, NCMP
Having more appropriate, evidence-based labeling of low-dose vaginal estrogen continues to be a high priority for The North American Menopause Society (NAMS), the International Society for the Study of Women’s Sexual Health (ISSWSH), and other professional societies.
NAMS and the Working Group on Women’s Health and Well-Being in Menopause had submitted a citizen’s petition to the US Food and Drug Administration (FDA) in 2016 requesting modification of the label—including removal of the “black box warning”—for low-dose vaginal estrogen products. The petition was, disappointingly, denied in 2018.1
Currently, the class labeling, which was based on the results of randomized trials with systemic hormone therapy, is not applicable to low-dose vaginal estrogen, and the inclusion of the black box warning has led to serious underutilization of an effective and safe treatment for a very common and life-altering condition, the genitourinary syndrome of menopause (GSM). This condition affects nearly half of postmenopausal women. It tends to be chronic and progressive and, unlike hot flashes and vasomotor symptoms, it does not remit or decline over time, and it affects women’s health and quality of life.
While removal of the black box warning would be appropriate, labeling should include emphatic reminders for women that if they have any bleeding or spotting they should seek medical attention immediately, and if they have a history of breast cancer or other estrogen-sensitive cancers they should talk with their oncologist prior to starting treatment with low-dose vaginal estrogen. Although the text would still inform women of research results on systemic hormone therapy, it would explain the differences between low-dose vaginal estrogen and systemic therapy.
Studies show vaginal estrogen has good safety profile
In the last several years, large, observational studies of low-dose vaginal estrogen have suggested that this treatment is not associated with an increase in cardiovascular disease, pulmonary embolism, venous thrombosis, cancer, or dementia—conditions listed in the black box warning that were linked to systemic estrogen therapy plus synthetic progestin. Recent data from the Nurses’ Health Study, for example, demonstrated that 3 years of vaginal estrogen use did not increase the risk of cardiovascular events or invasive breast cancer.
Women’s Health Initiative. In a prospective observational cohort study, Crandall and colleagues used data from participants in the Women’s Health Initiative Observational Study to determine the association between use of vaginal estrogen and risk of a global index event (GIE), defined as time to first occurrence of coronary heart disease, invasive breast cancer, stroke, pulmonary embolism, hip fracture, colorectal cancer, endometrial cancer, or death from any cause.2
Women were recruited from multiple clinical centers, were aged 50 to 79 years at baseline, and did not use systemic estrogen therapy during follow-up. The study included 45,663 women and median follow-up was 7.2 years. The investigators collected data on women’s self-reported use of vaginal estrogen as well as the development of the conditions defined above.
In women with a uterus, there was no significant difference between vaginal estrogen users and nonusers in the risk of stroke, invasive breast cancer, colorectal cancer, endometrial cancer, pulmonary embolism, or deep vein thrombosis. The risks of coronary heart disease, fracture, all-cause mortality, and GIE were lower in vaginal estrogen users than in nonusers (GIE adjusted hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.55–0.86).
In women who had undergone hysterectomy, the risks of the individual GIE components and the overall GIE were not significantly different in users of vaginal estrogen compared with nonusers (GIE adjusted HR, 0.94; 95% CI, 0.70–1.26).
The investigators concluded that the risks of cardiovascular disease and cancer were not increased in postmenopausal women who used vaginal estrogen. Thus, this study offers reassurance on the treatment’s safety.2
Meta-analysis on menopausal hormone therapy and breast cancer risk. Further evidence now indicates that low-dose vaginal estrogen is not linked to chronic health conditions. In a large meta-analysis published in 2019, investigators looked at different types of hormone therapies—oral estrogen plus progestin, transdermal estrogen and progestin, estrogen alone, low-dose vaginal estrogen—and their relationship to breast cancer risk.3
Information on individual participants was obtained from 58 studies, 24 prospective and 34 retrospective. Breast cancer relative risks (RR) during years 5 to 14 of current hormone use were assessed according to the main hormonal contituents, doses, and modes of delivery of the last-used menopausal hormone therapy. For all systemic estrogen-only preparations, the RR was 1.33 (95% CI, 1.28–1.38), while for all estrogen-progestogen preparations, the RR was 2.08 (95% CI, 2.02–2.15). For transdermal estrogen, the RR was 1.35 (95% CI, 1.25–1.46). In contrast, for vaginal estrogen, the RR was 1.09 (95% CI, 0.97–1.23).3
Thus, the analysis found that in all the studies that had been done to date, there was no evidence of increased risk of breast cancer with vaginal estrogen therapy.
The evidence is growing that low-dose vaginal estrogen is different from systemic estrogen in terms of its safety profile and benefit-risk pattern. It is important for the FDA to consider these data and revise the vaginal estrogen label.
On the horizon: New estradiol reference ranges
It would be useful if we could accurately compare estradiol levels in women treated with vaginal estrogen against those of women treated with systemic estrogen therapy. In September 2019, NAMS held a workshop with the goal of establishing reference ranges for estradiol in postmenopausal women.4 It is very important to have good, reliable laboratory assays for estradiol and estrone, and to have a clear understanding of what is a reference range, that is, the range of estradiol levels in postmenopausal women who are not treated with estrogen. That way, you can observe what the estradiol blood levels are in women treated with low-dose vaginal estrogen or those treated with systemic estrogen versus the levels observed among postmenopausal women not receiving any estrogen product.
With the reference range information, we could look at data on the blood levels of estradiol with low-dose vaginal estrogen from the various studies available, as well as the increasing evidence from observational studies of the safety of low-dose vaginal estrogen to better understand its relationship with health. If these studies demonstrate that, with certain doses and formulations of low-dose vaginal estrogen, blood estradiol levels stay within the reference range of postmenopausal estradiol levels, it would inform the labeling modifications of these products. We need this information for future discussions with the FDA.
The laboratory assay technology used for such an investigation is primarily liquid chromatography with tandem mass spectrometry, the so-called LC-MS/MS assay. With use of this technology, the reference range for estradiol may be less than 10 picograms per milliliter. Previously, a very wide and inconsistent range—about 5 to 30 picograms per milliliter—was considered a “normal” range.
NAMS is championing the efforts to define a true evidence-based reference range that would represent the range of levels seen in postmenopausal women.5 This effort has been spearheaded by Dr. Richard Santen and colleagues. Using the more sensitive and specific LC-MS/MS assay will enable researchers and clinicians to better understand how levels on low-dose vaginal estrogen relate to the reference range for postmenopausal women. We are hoping to work together with researchers to establish these reference ranges, and to use that information to look at how low-dose vaginal estrogen compares to levels in untreated postmenopausal women, as well as to levels in women on systemic estrogen.
Hopefully, establishing the reference range can be done in an expeditious and timely way, with discussions with the FDA resuming shortly thereafter.
References
1.NAMS Citizen’s Petition and FDA Response, June 7, 2018. http://www.menopause.org/docs/
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. 2018;25:11-20.
3. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394:1159-1168.
4. Santen RJ, Pinkerton JV, Liu JH, et al. Workshop on normal reference ranges for estradiol in postmenopausal women, September 2019, Chicago, Illinois. Menopause. May 4, 2020. doi:10.1097/GME.0000000000001556.
5. Pinkerton JV, Liu JH, Santoro NF, et al. Workshop on normal reference ranges for estradiol in postmenopausal women: commentary from The North American Menopause Society on low-dose vaginal estrogen therapy. Menopause. 2020;27:611-613.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Maturitas. 2014;79:349-354.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126:859-876.
- Shifren JL. Genitourinary syndrome of menopause. Clin Obstet Gynecol. 2018;61:508-516.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806.
- Kingsberg SA, Krychman M, Graham S, et al. The women’s EMPOWER survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 Hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- US Food and Drug Administration website. FDA warns against the use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/medical-devices/safety-communications/fda-warns-against-use-energy-based-devices-perform-vaginal-rejuvenation-or-vaginal-cosmetic. Updated November 20, 2018. Accessed May 21, 2020.
- US Food and Drug Administration website. Letters to industry. https://www.fda.gov/medical-devices/industry-medical-devices/letters-industry. July 24, 2018. Accessed May 21, 2020
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Maturitas. 2014;79:349-354.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126:859-876.
- Shifren JL. Genitourinary syndrome of menopause. Clin Obstet Gynecol. 2018;61:508-516.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806.
- Kingsberg SA, Krychman M, Graham S, et al. The women’s EMPOWER survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 Hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- US Food and Drug Administration website. FDA warns against the use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/medical-devices/safety-communications/fda-warns-against-use-energy-based-devices-perform-vaginal-rejuvenation-or-vaginal-cosmetic. Updated November 20, 2018. Accessed May 21, 2020.
- US Food and Drug Administration website. Letters to industry. https://www.fda.gov/medical-devices/industry-medical-devices/letters-industry. July 24, 2018. Accessed May 21, 2020
Does BSO status affect health outcomes for women taking estrogen for menopause?
Do health effects of menopausal estrogen therapy differ between women with bilateral oophorectomy versus those with conserved ovaries? To answer this question a group of investigators performed a subanalysis of the Women’s Health Initiative (WHI) Estrogen-Alone Trial,1 which included 40 clinical centers across the United States. They examined estrogen therapy outcomes by bilateral salpingo-oophorectomy (BSO) status, with additional stratification by 10-year age groups in 9,939 women aged 50 to 79 years with prior hysterectomy and known oophorectomy status. In the WHI trial, women were randomly assigned to conjugated equine estrogens (CEE) 0.625 mg/d or placebo for a median of 7.2 years. Investigators assessed the incidence of coronary heart disease and invasive breast cancer (the trial’s 2 primary end points), all-cause mortality, and a “global index”—these end points plus stroke, pulmonary embolism, colorectal cancer, and hip fracture—during the intervention phase and 18-year cumulative follow-up.
OBG Management caught up with lead author JoAnn E. Manson, MD, DrPH, NCMP, to discuss the study’s results.
OBG Management : How many women undergo BSO with their hysterectomy?
Dr. JoAnn E. Manson, MD, DrPH, NCMP: Of the 425,000 women who undergo hysterectomy in the United States for benign reasons each year,2,3 about 40% of them undergo BSO—so between 150,000 and 200,000 women per year undergo BSO with their hysterectomy.4,5
OBG Management : Although BSO is performed with hysterectomy to minimize patients’ future ovarian cancer risk, does BSO have health risks of its own, and how has estrogen been shown to affect these risks?
Dr. Manson: First, yes, BSO has been associated with health risks, especially when it is performed at a young age, such as before age 45. It has been linked to an increased risk of heart disease, osteoporosis, cognitive decline, and all-cause mortality. According to observational studies, estrogen therapy appears to offset many of these risks, particularly those related to heart disease and osteoporosis (the evidence is less clear on cognitive deficits).5
OBG Management : What did you find in your trial when you randomly assigned women in the age groups of 50 to 79 who underwent hysterectomy with and without BSO to estrogen therapy or placebo?
Dr. Manson: The WHI is the first study to be conducted in a randomized trial setting to analyze the health risks and benefits of estrogen therapy according to whether or not women had their ovaries removed. What we found was that the woman’s age had a strong influence on the effects of estrogen therapy among women who had BSO but only a negligible effect among women who had conserved ovaries. Overall, across the full age range, the effects of estrogen therapy did not differ substantially between women who had a BSO and those who had their ovaries conserved.
However, there were major differences by age group among the women who had BSO. A significant 32% reduction in all-cause mortality emerged during the 18-year follow-up period among the younger women (below age 60) who had BSO when they received estrogen therapy as compared with placebo. By contrast, the women who had conserved ovaries did not have this significant reduction in all-cause mortality, or in most of the other outcomes on estrogen compared with placebo. Overall, the effects of estrogen therapy tended to be relatively neutral in the women with conserved ovaries.
Now, the reduction in all-cause mortality with estrogen therapy was particularly pronounced among women who had BSO before age 45. They had a 40% statistically significant reduction in all-cause mortality with estrogen therapy compared with placebo. Also, among the women with BSO, there was a strong association between the timing of estrogen initiation and the magnitude of reduction in mortality. Women who started the estrogen therapy within 10 years of having the BSO had a 34% significant reduction in all-cause mortality, and those who started estrogen more than 20 years after having their ovaries removed had no reduction in mortality.
Continue to:
OBG Management : Do your data give support to the timing hypothesis?
Dr. Manson: Yes, our findings do support a timing hypothesis that was particularly pronounced for women who underwent BSO. It was the women who had early surgical menopause (before age 45) and those who started the estrogen therapy within 10 years of having their ovaries removed who had the greatest reduction in all-cause mortality and the most favorable benefit-risk profile from hormone therapy. So, the results do lend support to the timing hypothesis.
By contrast, women who had BSO at hysterectomy and began hormone therapy at age 70 or older had net adverse effects from hormone therapy. They posted a 40% increase in the global index—which is a summary measure of adverse effects on cardiovascular disease, cancer, and other major health outcomes. So, the women with BSO who were randomized in the trial at age 70 and older, had unfavorable results from estrogen therapy and an increase in the global index, in contrast to the women who were below age 60 or within 10 years of menopause.
OBG Management : Given your study findings, in which women would you recommend estrogen therapy? And are there groups of women in which you would advise avoiding estrogen therapy?
Dr. Manson: Current guidelines6,7 recommend estrogen therapy for women who have early menopause, particularly an early surgical menopause and BSO prior to the average age at natural menopause. Unless the woman has contraindications to estrogen therapy, the recommendations are to treat with estrogen until the average age of menopause—until about age 50 to 51.
Our study findings provide reassurance that, if a woman continues to have indications for estrogen (vasomotor symptoms, or other indications for estrogen therapy), there is relative safety of continuing estrogen-alone therapy through her 50s, until age 60. For example, a woman who, after the average age of menopause continues to have vasomotor symptoms, or if she has bone health problems, our study would suggest that estrogen therapy would continue to have a favorable benefit-risk profile until at least the age of 60. Decisions would have to be individualized, especially after age 60, with shared decision-making particularly important for those decisions. (Some women, depending on their risk profile, may continue to be candidates for estrogen therapy past age 60.)
So, this study provides reassurance regarding use of estrogen therapy for women in their 50s if they have had BSO. Actually, the women who had conserved ovaries also had relative safety with estrogen therapy until age 60. They just didn’t show the significant benefits for all-cause mortality. Overall, their pattern of health-related benefits and risks was neutral. Thus, if vasomotor symptom management, quality of life benefits, or bone health effects are sought, taking hormone therapy is a quite reasonable choice for these women.
By contrast, women who have had a BSO and are age 70 or older should really avoid initiating estrogen therapy because it would follow a prolonged period of estrogen deficiency, or very low estrogen levels, and these women appeared to have a net adverse effect from initiating hormone therapy (with increases in the global index found).
Continue to:
OBG Management : Did taking estrogen therapy prior to trial enrollment make a difference when it came to study outcomes?
Dr. Manson: We found minimal if any effect in our analyses. In fact, even the women who did not have prior (pre-randomization) use of estrogen therapy tended to do well on estrogen-alone therapy if they were younger than age 60. This was particularly true for the women who had BSO. Even if they had not used estrogen previously, and they were many years past the BSO, they still did well on estrogen therapy if they were below age 60.
1. Manson JE, Aragaki AK, Bassuk SS. Menopausal estrogen-alone therapy and health outcomes in women with and without bilateral oophorectomy: a randomized trial. Ann Intern Med. 2019 September 10. doi:10.7326/M19-0274.
2. Einarsson J. Are hysterectomy volumes in the US really falling? Contemporary OB/GYN. 1 September 2017. www.contemporaryobgyn.net/gynecology/are-hysterectomy-volumes-us-really-falling. November 4, 2019.
3. Temkin SM, Minasian L, Noone AM. The end of the hysterectomy epidemic and endometrial cancer incidence: what are the unintended consequences of declining hysterectomy rates? Front Oncol. 2016;6:89.
4. Doll KM, Dusetzina SB, Robinson W. Trends in inpatient and outpatient hysterectomy and oophorectomy rates among commercially insured women in the United States, 2000-2014. JAMA Surg. 2016;151:876-877.
5. Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279.
6. ACOG Practice Bulletin No. 141: management of menopausal symptoms [published corrections appear in: Obstet Gynecol. 2016;127(1):166. and Obstet Gynecol. 2018;131(3):604]. Obstet Gynecol. 2014;123:202-216.
7. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
Do health effects of menopausal estrogen therapy differ between women with bilateral oophorectomy versus those with conserved ovaries? To answer this question a group of investigators performed a subanalysis of the Women’s Health Initiative (WHI) Estrogen-Alone Trial,1 which included 40 clinical centers across the United States. They examined estrogen therapy outcomes by bilateral salpingo-oophorectomy (BSO) status, with additional stratification by 10-year age groups in 9,939 women aged 50 to 79 years with prior hysterectomy and known oophorectomy status. In the WHI trial, women were randomly assigned to conjugated equine estrogens (CEE) 0.625 mg/d or placebo for a median of 7.2 years. Investigators assessed the incidence of coronary heart disease and invasive breast cancer (the trial’s 2 primary end points), all-cause mortality, and a “global index”—these end points plus stroke, pulmonary embolism, colorectal cancer, and hip fracture—during the intervention phase and 18-year cumulative follow-up.
OBG Management caught up with lead author JoAnn E. Manson, MD, DrPH, NCMP, to discuss the study’s results.
OBG Management : How many women undergo BSO with their hysterectomy?
Dr. JoAnn E. Manson, MD, DrPH, NCMP: Of the 425,000 women who undergo hysterectomy in the United States for benign reasons each year,2,3 about 40% of them undergo BSO—so between 150,000 and 200,000 women per year undergo BSO with their hysterectomy.4,5
OBG Management : Although BSO is performed with hysterectomy to minimize patients’ future ovarian cancer risk, does BSO have health risks of its own, and how has estrogen been shown to affect these risks?
Dr. Manson: First, yes, BSO has been associated with health risks, especially when it is performed at a young age, such as before age 45. It has been linked to an increased risk of heart disease, osteoporosis, cognitive decline, and all-cause mortality. According to observational studies, estrogen therapy appears to offset many of these risks, particularly those related to heart disease and osteoporosis (the evidence is less clear on cognitive deficits).5
OBG Management : What did you find in your trial when you randomly assigned women in the age groups of 50 to 79 who underwent hysterectomy with and without BSO to estrogen therapy or placebo?
Dr. Manson: The WHI is the first study to be conducted in a randomized trial setting to analyze the health risks and benefits of estrogen therapy according to whether or not women had their ovaries removed. What we found was that the woman’s age had a strong influence on the effects of estrogen therapy among women who had BSO but only a negligible effect among women who had conserved ovaries. Overall, across the full age range, the effects of estrogen therapy did not differ substantially between women who had a BSO and those who had their ovaries conserved.
However, there were major differences by age group among the women who had BSO. A significant 32% reduction in all-cause mortality emerged during the 18-year follow-up period among the younger women (below age 60) who had BSO when they received estrogen therapy as compared with placebo. By contrast, the women who had conserved ovaries did not have this significant reduction in all-cause mortality, or in most of the other outcomes on estrogen compared with placebo. Overall, the effects of estrogen therapy tended to be relatively neutral in the women with conserved ovaries.
Now, the reduction in all-cause mortality with estrogen therapy was particularly pronounced among women who had BSO before age 45. They had a 40% statistically significant reduction in all-cause mortality with estrogen therapy compared with placebo. Also, among the women with BSO, there was a strong association between the timing of estrogen initiation and the magnitude of reduction in mortality. Women who started the estrogen therapy within 10 years of having the BSO had a 34% significant reduction in all-cause mortality, and those who started estrogen more than 20 years after having their ovaries removed had no reduction in mortality.
Continue to:
OBG Management : Do your data give support to the timing hypothesis?
Dr. Manson: Yes, our findings do support a timing hypothesis that was particularly pronounced for women who underwent BSO. It was the women who had early surgical menopause (before age 45) and those who started the estrogen therapy within 10 years of having their ovaries removed who had the greatest reduction in all-cause mortality and the most favorable benefit-risk profile from hormone therapy. So, the results do lend support to the timing hypothesis.
By contrast, women who had BSO at hysterectomy and began hormone therapy at age 70 or older had net adverse effects from hormone therapy. They posted a 40% increase in the global index—which is a summary measure of adverse effects on cardiovascular disease, cancer, and other major health outcomes. So, the women with BSO who were randomized in the trial at age 70 and older, had unfavorable results from estrogen therapy and an increase in the global index, in contrast to the women who were below age 60 or within 10 years of menopause.
OBG Management : Given your study findings, in which women would you recommend estrogen therapy? And are there groups of women in which you would advise avoiding estrogen therapy?
Dr. Manson: Current guidelines6,7 recommend estrogen therapy for women who have early menopause, particularly an early surgical menopause and BSO prior to the average age at natural menopause. Unless the woman has contraindications to estrogen therapy, the recommendations are to treat with estrogen until the average age of menopause—until about age 50 to 51.
Our study findings provide reassurance that, if a woman continues to have indications for estrogen (vasomotor symptoms, or other indications for estrogen therapy), there is relative safety of continuing estrogen-alone therapy through her 50s, until age 60. For example, a woman who, after the average age of menopause continues to have vasomotor symptoms, or if she has bone health problems, our study would suggest that estrogen therapy would continue to have a favorable benefit-risk profile until at least the age of 60. Decisions would have to be individualized, especially after age 60, with shared decision-making particularly important for those decisions. (Some women, depending on their risk profile, may continue to be candidates for estrogen therapy past age 60.)
So, this study provides reassurance regarding use of estrogen therapy for women in their 50s if they have had BSO. Actually, the women who had conserved ovaries also had relative safety with estrogen therapy until age 60. They just didn’t show the significant benefits for all-cause mortality. Overall, their pattern of health-related benefits and risks was neutral. Thus, if vasomotor symptom management, quality of life benefits, or bone health effects are sought, taking hormone therapy is a quite reasonable choice for these women.
By contrast, women who have had a BSO and are age 70 or older should really avoid initiating estrogen therapy because it would follow a prolonged period of estrogen deficiency, or very low estrogen levels, and these women appeared to have a net adverse effect from initiating hormone therapy (with increases in the global index found).
Continue to:
OBG Management : Did taking estrogen therapy prior to trial enrollment make a difference when it came to study outcomes?
Dr. Manson: We found minimal if any effect in our analyses. In fact, even the women who did not have prior (pre-randomization) use of estrogen therapy tended to do well on estrogen-alone therapy if they were younger than age 60. This was particularly true for the women who had BSO. Even if they had not used estrogen previously, and they were many years past the BSO, they still did well on estrogen therapy if they were below age 60.
Do health effects of menopausal estrogen therapy differ between women with bilateral oophorectomy versus those with conserved ovaries? To answer this question a group of investigators performed a subanalysis of the Women’s Health Initiative (WHI) Estrogen-Alone Trial,1 which included 40 clinical centers across the United States. They examined estrogen therapy outcomes by bilateral salpingo-oophorectomy (BSO) status, with additional stratification by 10-year age groups in 9,939 women aged 50 to 79 years with prior hysterectomy and known oophorectomy status. In the WHI trial, women were randomly assigned to conjugated equine estrogens (CEE) 0.625 mg/d or placebo for a median of 7.2 years. Investigators assessed the incidence of coronary heart disease and invasive breast cancer (the trial’s 2 primary end points), all-cause mortality, and a “global index”—these end points plus stroke, pulmonary embolism, colorectal cancer, and hip fracture—during the intervention phase and 18-year cumulative follow-up.
OBG Management caught up with lead author JoAnn E. Manson, MD, DrPH, NCMP, to discuss the study’s results.
OBG Management : How many women undergo BSO with their hysterectomy?
Dr. JoAnn E. Manson, MD, DrPH, NCMP: Of the 425,000 women who undergo hysterectomy in the United States for benign reasons each year,2,3 about 40% of them undergo BSO—so between 150,000 and 200,000 women per year undergo BSO with their hysterectomy.4,5
OBG Management : Although BSO is performed with hysterectomy to minimize patients’ future ovarian cancer risk, does BSO have health risks of its own, and how has estrogen been shown to affect these risks?
Dr. Manson: First, yes, BSO has been associated with health risks, especially when it is performed at a young age, such as before age 45. It has been linked to an increased risk of heart disease, osteoporosis, cognitive decline, and all-cause mortality. According to observational studies, estrogen therapy appears to offset many of these risks, particularly those related to heart disease and osteoporosis (the evidence is less clear on cognitive deficits).5
OBG Management : What did you find in your trial when you randomly assigned women in the age groups of 50 to 79 who underwent hysterectomy with and without BSO to estrogen therapy or placebo?
Dr. Manson: The WHI is the first study to be conducted in a randomized trial setting to analyze the health risks and benefits of estrogen therapy according to whether or not women had their ovaries removed. What we found was that the woman’s age had a strong influence on the effects of estrogen therapy among women who had BSO but only a negligible effect among women who had conserved ovaries. Overall, across the full age range, the effects of estrogen therapy did not differ substantially between women who had a BSO and those who had their ovaries conserved.
However, there were major differences by age group among the women who had BSO. A significant 32% reduction in all-cause mortality emerged during the 18-year follow-up period among the younger women (below age 60) who had BSO when they received estrogen therapy as compared with placebo. By contrast, the women who had conserved ovaries did not have this significant reduction in all-cause mortality, or in most of the other outcomes on estrogen compared with placebo. Overall, the effects of estrogen therapy tended to be relatively neutral in the women with conserved ovaries.
Now, the reduction in all-cause mortality with estrogen therapy was particularly pronounced among women who had BSO before age 45. They had a 40% statistically significant reduction in all-cause mortality with estrogen therapy compared with placebo. Also, among the women with BSO, there was a strong association between the timing of estrogen initiation and the magnitude of reduction in mortality. Women who started the estrogen therapy within 10 years of having the BSO had a 34% significant reduction in all-cause mortality, and those who started estrogen more than 20 years after having their ovaries removed had no reduction in mortality.
Continue to:
OBG Management : Do your data give support to the timing hypothesis?
Dr. Manson: Yes, our findings do support a timing hypothesis that was particularly pronounced for women who underwent BSO. It was the women who had early surgical menopause (before age 45) and those who started the estrogen therapy within 10 years of having their ovaries removed who had the greatest reduction in all-cause mortality and the most favorable benefit-risk profile from hormone therapy. So, the results do lend support to the timing hypothesis.
By contrast, women who had BSO at hysterectomy and began hormone therapy at age 70 or older had net adverse effects from hormone therapy. They posted a 40% increase in the global index—which is a summary measure of adverse effects on cardiovascular disease, cancer, and other major health outcomes. So, the women with BSO who were randomized in the trial at age 70 and older, had unfavorable results from estrogen therapy and an increase in the global index, in contrast to the women who were below age 60 or within 10 years of menopause.
OBG Management : Given your study findings, in which women would you recommend estrogen therapy? And are there groups of women in which you would advise avoiding estrogen therapy?
Dr. Manson: Current guidelines6,7 recommend estrogen therapy for women who have early menopause, particularly an early surgical menopause and BSO prior to the average age at natural menopause. Unless the woman has contraindications to estrogen therapy, the recommendations are to treat with estrogen until the average age of menopause—until about age 50 to 51.
Our study findings provide reassurance that, if a woman continues to have indications for estrogen (vasomotor symptoms, or other indications for estrogen therapy), there is relative safety of continuing estrogen-alone therapy through her 50s, until age 60. For example, a woman who, after the average age of menopause continues to have vasomotor symptoms, or if she has bone health problems, our study would suggest that estrogen therapy would continue to have a favorable benefit-risk profile until at least the age of 60. Decisions would have to be individualized, especially after age 60, with shared decision-making particularly important for those decisions. (Some women, depending on their risk profile, may continue to be candidates for estrogen therapy past age 60.)
So, this study provides reassurance regarding use of estrogen therapy for women in their 50s if they have had BSO. Actually, the women who had conserved ovaries also had relative safety with estrogen therapy until age 60. They just didn’t show the significant benefits for all-cause mortality. Overall, their pattern of health-related benefits and risks was neutral. Thus, if vasomotor symptom management, quality of life benefits, or bone health effects are sought, taking hormone therapy is a quite reasonable choice for these women.
By contrast, women who have had a BSO and are age 70 or older should really avoid initiating estrogen therapy because it would follow a prolonged period of estrogen deficiency, or very low estrogen levels, and these women appeared to have a net adverse effect from initiating hormone therapy (with increases in the global index found).
Continue to:
OBG Management : Did taking estrogen therapy prior to trial enrollment make a difference when it came to study outcomes?
Dr. Manson: We found minimal if any effect in our analyses. In fact, even the women who did not have prior (pre-randomization) use of estrogen therapy tended to do well on estrogen-alone therapy if they were younger than age 60. This was particularly true for the women who had BSO. Even if they had not used estrogen previously, and they were many years past the BSO, they still did well on estrogen therapy if they were below age 60.
1. Manson JE, Aragaki AK, Bassuk SS. Menopausal estrogen-alone therapy and health outcomes in women with and without bilateral oophorectomy: a randomized trial. Ann Intern Med. 2019 September 10. doi:10.7326/M19-0274.
2. Einarsson J. Are hysterectomy volumes in the US really falling? Contemporary OB/GYN. 1 September 2017. www.contemporaryobgyn.net/gynecology/are-hysterectomy-volumes-us-really-falling. November 4, 2019.
3. Temkin SM, Minasian L, Noone AM. The end of the hysterectomy epidemic and endometrial cancer incidence: what are the unintended consequences of declining hysterectomy rates? Front Oncol. 2016;6:89.
4. Doll KM, Dusetzina SB, Robinson W. Trends in inpatient and outpatient hysterectomy and oophorectomy rates among commercially insured women in the United States, 2000-2014. JAMA Surg. 2016;151:876-877.
5. Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279.
6. ACOG Practice Bulletin No. 141: management of menopausal symptoms [published corrections appear in: Obstet Gynecol. 2016;127(1):166. and Obstet Gynecol. 2018;131(3):604]. Obstet Gynecol. 2014;123:202-216.
7. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
1. Manson JE, Aragaki AK, Bassuk SS. Menopausal estrogen-alone therapy and health outcomes in women with and without bilateral oophorectomy: a randomized trial. Ann Intern Med. 2019 September 10. doi:10.7326/M19-0274.
2. Einarsson J. Are hysterectomy volumes in the US really falling? Contemporary OB/GYN. 1 September 2017. www.contemporaryobgyn.net/gynecology/are-hysterectomy-volumes-us-really-falling. November 4, 2019.
3. Temkin SM, Minasian L, Noone AM. The end of the hysterectomy epidemic and endometrial cancer incidence: what are the unintended consequences of declining hysterectomy rates? Front Oncol. 2016;6:89.
4. Doll KM, Dusetzina SB, Robinson W. Trends in inpatient and outpatient hysterectomy and oophorectomy rates among commercially insured women in the United States, 2000-2014. JAMA Surg. 2016;151:876-877.
5. Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279.
6. ACOG Practice Bulletin No. 141: management of menopausal symptoms [published corrections appear in: Obstet Gynecol. 2016;127(1):166. and Obstet Gynecol. 2018;131(3):604]. Obstet Gynecol. 2014;123:202-216.
7. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2018 Update on menopause
Our knowledge regarding the benefits and risks of systemic menopausal hormone therapy (HT) has continued to evolve since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI). In late 2017, the US Preventive Services Task Force (USPSTF) issued its recommendation against the use of menopausal HT for the prevention of chronic conditions. In this Menopause Update, Dr. JoAnn Manson, Dr. JoAnn Pinkerton, and I detail why we do not support the Task Force’s recommendation. In a sidebar discussion, Dr. Manson also reviews the results of 2 WHI HT trials, published in September 2017, that analyzed mortality in trial participants over an 18-year follow-up.
In addition, I summarize an observational study that assessed the association of HT and Alzheimer disease (AD) as well as a clinical trial that compared the impact of oral versus transdermal estrogen on sexuality in recently menopausal women.
What's the impact of long-term use of systemic HT on Alzheimer disease risk?
Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062-1068.
Data from the WHI HT randomized trials have clarified that initiation of oral HT among women aged 65 and older increases the risk of cognitive decline. By contrast, an analysis of younger WHI participants found that oral HT had no impact on cognitive function. Recently, Imtiaz and colleagues conducted a prospective cohort study of postmenopausal HT and AD in women residing in a Finnish county, with 25 years of follow-up. A diagnosis of AD was based on administrative health records and use of medications prescribed specifically to treat dementia. Use of systemic HT was identified via self-report. Overall, among more than 8,000 women followed, 227 cases of AD (mean age, 72 years) were identified.
In an analysis that controlled for factors including age, body mass index, alcohol use, smoking, physical activity, occupation status, and parity, up to 5 years of HT use was not associated with a risk of being diagnosed with AD. Five to 10 years of HT use was associated with a hazard ratio (HR) of 0.89, an 11% risk reduction that did not achieve statistical significance. By contrast, more than 10 years' use of systemic HT was associated with an HR of 0.53, a statistically significant 47% reduction in risk of AD.1
Other studies found conflicting results
Three large randomized trials found that HT initiated early in menopause and continued for less than 7 years had no impact on cognitive function.2-4 The Cache County (Utah) long-term prospective cohort study, however, found that HT started early in menopause and continued for 10 years or longer was associated with a significant reduction in risk of AD.5
Of note are results from the 2017 report of 18-year cumulative mortality among WHI participants (see the box on page 30). In that study, mortality from AD and other dementia was lower among participants who were randomly assigned to treatment with estrogen alone versus placebo (HR, 0.74; 95% confidence interval [CI], 0.59-0.94). With estrogen-progestin therapy, the HR was 0.93 (95% CI, 0.77-1.11), and the pooled HR for the 2 trials was 0.85 (95% CI, 0.74-0.98).6
NAMS guidance
The North American Menopause Society (NAMS) HT position statement recommends that prevention of dementia should not be considered an indication for HT use since definitive data are not available.7 The statement indicates also that estrogen therapy may have positive cognitive benefits when initiated immediately after early surgical menopause and taken until the average age of menopause to prevent health risks seen with early loss of hormones.
Definitive data from long-term randomized clinical trials are not likely to become available. Observational trials continue to have methodologic issues, such as "healthy user bias," but the studies are reassuring that initiating HT close to menopause does not increase the risk of dementia. The long-term Finnish study by Imtiaz and colleagues and the Cache County study provide tentative observational data support for a "critical window" hypothesis, leaving open the possibility that initiating systemic HT soon after menopause onset and continuing it long term may reduce the risk of AD. Discussion is needed on individual patient characteristics, potential benefits and risks, and ongoing assessment over time.
Read Dr. Manson’s discussion of 18 years of follow-up data on menopause.
JoAnn E. Manson, MD, DrPH, NCMP
A new analysis from the Women's Health Initiative (WHI) randomized trials examined all-cause and cause-specific mortality during the intervention and postintervention follow-up periods.1 We followed more than 27,000 postmenopausal women aged 50 to 79 (mean age, 63) who were recruited to 2 randomized WHI trials of HT between 1993 and 1998. The trials continued until 2002 for the estrogen-progestin trial and to 2004 for the estrogen-alone trial. The trials ran for 5 to 7 years' duration, with post-stopping follow-up for an additional 10 to 12 years (total cumulative follow-up of 18 years).
The participants were randomly assigned to receive active treatment or placebo. The interventions were conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) versus placebo for women with an intact uterus and CEE alone versus placebo for women who had a hysterectomy.
All-cause mortality did not increase with HT use
The primary outcome measure was all-cause mortality in the 2 pooled trials and in each trial individually. We found that there was no link between HT and all-cause mortality in the overall study population (ages 50-79) in either trial. However, there was a trend toward lower all-cause mortality among the younger women in both trials. In women aged 50 to 59, there was a statistically significant 31% lower risk of mortality in the pooled trials among women taking active HT compared with those taking placebo, but no reduction in mortality with HT among older women (P for trend by age = .01).
Notably, all-cause mortality provides a critically important summary measure for interventions such as HT that have a complex matrix of benefits and risks. We know that HT has a number of benefits in menopausal women. It reduces hot flashes and other menopausal symptoms. It lowers the risk of hip fracture, other types of bone fractures, and type 2 diabetes. However, HT increases the risk of venous thrombosis, stroke, and some forms of cancer.
A summary measure that assesses the net effect of a medication on serious and life-threatening health outcomes is very important. As such, all-cause mortality is the ultimate bottom line for the balance of benefits and risks. This speaks to why we conducted the mortality analysis--WHI is the largest randomized trial of HT with long-term follow-up, allowing detailed analyses by age group. Although there have been previous reports on individual health outcomes in the WHI trials, no previous report had specifically focused on all-cause and cause-specific mortality with HT, stratified by age group, over long-term follow-up.
Hopefully the results of this study will alleviate some of the anxiety associated with HT because, as mentioned, there was no increase in overall total mortality or specific major causes of death. In addition, the younger women had a trend toward benefit for all-cause mortality.
We think that these findings support the recommendations from The North American Menopause Society and other professional societies that endorse the use of HT for managing bothersome menopausal symptoms, especially when started in early menopause. These results should be reassuring that there is no increase in mortality with HT use. Although these findings do not support prescribing HT for the express purpose of trying to prevent cardiovascular disease, dementia, or other chronic diseases (due to some potential risks), they do support an important role of HT for management of bothersome hot flashes, especially in early menopause.
Cause-specific mortality
Regarding cause-specific mortality and HT use, we looked in detail at deaths from cardiovascular causes, cancer, dementia, and other major illness. Overall, we observed no increase or decrease in cardiovascular or cancer deaths. In the estrogen-alone trial, there was a surprising finding of a 26% reduction in dementia deaths. In the estrogen-progestin trial, the results were neutral for dementia deaths.
Overall, the cause-specific mortality results were neutral. This is surprising because even for total cancer deaths there was no increase or decrease, despite a great deal of anxiety about cancer risk with HT. It appears that for cancer, HT has complex effects: it increases some types of cancer, such as breast cancer, and decreases others, such as endometrial cancer (in the estrogen-progestin group), and possibly colorectal cancer. Moreover, CEE alone was associated with a reduction in breast cancer mortality, but it remains unclear if this applies to other formulations. HT's net effect on total cancer mortality was neutral in both trials, that is, no increase or decrease.
Cautions and takeaways
We need to keep in mind that in current clinical practice, lower doses and different formulations and routes of administration of HT are now often used, including transdermal estradiol patches, gels, sprays, and micronized progesterone. These formulations, and the lower doses, may have an even more favorable benefit-risk profile. We need additional research on the long-term benefits and risks of these newer formulations and lower dosages.
Generally, these findings from the WHI trials indicate that for women who have significant hot flashes, night sweats, or other bothersome menopausal symptoms, it's important to discuss their symptoms with their health care provider and understand that hormone therapy may be an option for them. If it's not an option, many other treatments are available, including nonhormonal prescription medications, nonprescription medications, and behavioral approaches.
These findings should alleviate some fear about HT use, especially in younger women who have an overall favorable trend in terms of all-cause mortality with treatment, plus a much lower absolute risk of adverse events than older women. In a woman in early menopause who has bothersome hot flashes or other symptoms that disrupt her sleep or impair her quality of life, it's likely that the benefits of HT will outweigh the risks.
Reference
- Manson JE, Aragaki AK, Rossouw JE, et al; for the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women's Health Initiative randomized trials. JAMA. 2017;318(10):927-938.
Read how the route of HT may affect sexuality outcomes.
Oral vs transdermal estrogen therapy: Is one preferable regarding sexuality?
Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471-1479.
If route of administration of systemic HT influences sexuality outcomes in menopausal women, this would inform how we counsel our patients regarding HT.
Recently, Taylor and colleagues conducted a randomized clinical trial to examine the effects of HT's route of administration on sexual function.8 The 4-year Kronos Early Estrogen Prevention Study (KEEPS) ancillary sexual study randomly assigned 670 recently menopausal women to 0.45 mg of oral conjugated equine estrogens (CEE), an 0.05-mg estradiol transdermal patch, or placebo (with oral micronized progesterone for those on active treatment). The participants were aged 42 to 58 years and were within 36 months from their last menstrual period.
Participants were evaluated using the Female Sexual Function Inventory (FSFI) questionnaire, which assessed desire, arousal, lubrication, orgasm, satisfaction, and pain. The FSFI is scored using a point range of 0 to 36. A higher FSFI score indicates better sexual function. An FSFI score less than 26.55 depicts low sexual function (LSF).
Transdermal estrogen improved sexual function scores
Treatment with oral CEE was associated with no significant change in FSFI score compared with placebo, although benefits were seen for lubrication. By contrast, estrogen patch use improved the FSFI score (mean improvement, 2.6). Although improvement in FSFI score with transdermal estrogen was limited to participants with baseline LSF, most participants in fact had LSF at baseline.
Oral estrogen increases the liver's production of sex hormone-binding globulin, resulting in lower free (bioavailable) testosterone. Transdermal estrogen does not produce this effect. Accordingly, sexuality concerns may represent a reason to prefer the use of transdermal as opposed to oral estrogen.
Read about the authors’ concern over new USPSTF guidance.
The USPSTF recommendation against menopausal HT use for prevention of chronic conditions: Guidance that may confuse--and mislead
US Preventive Services Task Force; Grossman DC, CurrySJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224-2233.
In late 2017, the USPSTF issued its recommendation against the use of menopausal HT for prevention of chronic conditions.9 We are concerned that this recommendation will be misconstrued as suggesting that the use of HT is not appropriate for any indication, including treatment of bothersome menopausal symptoms.
Although the Task Force's report briefly indicated that the guidance does not refer to HT use for treatment of symptoms, this important disclaimer likely will be overlooked or ignored by many readers. The result may be increased uncertainty and anxiety in decision making regarding HT use. Thus, we might see a further decline in the proportion of menopausal women who are prescribed appropriate treatment for symptoms that impair quality of life.
HT use improves menopausal symptoms
According to the 2017 NAMS Position Statement, for symptomatic women in early menopause (that is, younger than age 60 or within 10 years of menopause onset) and free of contraindications to treatment, use of systemic HT is appropriate.7 Currently, clinicians are reluctant to prescribe HT, and women are apprehensive regarding its use.10 Unfortunately, the USPSTF guidance may further discourage appropriate treatment of menopausal symptoms.
Findings from randomized clinical trials, as well as preclinical, clinical, and epidemiologic studies, clarify the favorable benefit-risk profile for HT use by recently menopausal women with bothersome vasomotor and related menopausal symptoms.7,10-12
Notably, the USPSTF guidance does not address women with premature or early menopause, those with persistent (long-duration) vasomotor symptoms, or women at increased risk for osteoporosis and related fractures. Furthermore, the prevalent and undertreated condition, genitourinary syndrome of menopause, deserves but does not receive attention.
In recent decades, our understanding regarding HT's benefits and risks has advanced substantially. Guidance for clinicians and women should reflect this evolution and underscore the individualization and shared decision making that facilitates appropriate decisions regarding the use of HT.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062–1068.
- Espeland MA, Shumaker SA, Leng I, et al; WHIMSY Study Group. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173(15):1429–1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12(6):e1001833;discussion e1001833.
- Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708.
- Shao H, Breitner JC, Whitmer RA, et al; Cache County Investigators. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852.
- Manson JE, Aragaki AK, Rossouw JE, et al; the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318(10):927–938.
- NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471–1479.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224–2233.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015; 126(4):859–876.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
Our knowledge regarding the benefits and risks of systemic menopausal hormone therapy (HT) has continued to evolve since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI). In late 2017, the US Preventive Services Task Force (USPSTF) issued its recommendation against the use of menopausal HT for the prevention of chronic conditions. In this Menopause Update, Dr. JoAnn Manson, Dr. JoAnn Pinkerton, and I detail why we do not support the Task Force’s recommendation. In a sidebar discussion, Dr. Manson also reviews the results of 2 WHI HT trials, published in September 2017, that analyzed mortality in trial participants over an 18-year follow-up.
In addition, I summarize an observational study that assessed the association of HT and Alzheimer disease (AD) as well as a clinical trial that compared the impact of oral versus transdermal estrogen on sexuality in recently menopausal women.
What's the impact of long-term use of systemic HT on Alzheimer disease risk?
Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062-1068.
Data from the WHI HT randomized trials have clarified that initiation of oral HT among women aged 65 and older increases the risk of cognitive decline. By contrast, an analysis of younger WHI participants found that oral HT had no impact on cognitive function. Recently, Imtiaz and colleagues conducted a prospective cohort study of postmenopausal HT and AD in women residing in a Finnish county, with 25 years of follow-up. A diagnosis of AD was based on administrative health records and use of medications prescribed specifically to treat dementia. Use of systemic HT was identified via self-report. Overall, among more than 8,000 women followed, 227 cases of AD (mean age, 72 years) were identified.
In an analysis that controlled for factors including age, body mass index, alcohol use, smoking, physical activity, occupation status, and parity, up to 5 years of HT use was not associated with a risk of being diagnosed with AD. Five to 10 years of HT use was associated with a hazard ratio (HR) of 0.89, an 11% risk reduction that did not achieve statistical significance. By contrast, more than 10 years' use of systemic HT was associated with an HR of 0.53, a statistically significant 47% reduction in risk of AD.1
Other studies found conflicting results
Three large randomized trials found that HT initiated early in menopause and continued for less than 7 years had no impact on cognitive function.2-4 The Cache County (Utah) long-term prospective cohort study, however, found that HT started early in menopause and continued for 10 years or longer was associated with a significant reduction in risk of AD.5
Of note are results from the 2017 report of 18-year cumulative mortality among WHI participants (see the box on page 30). In that study, mortality from AD and other dementia was lower among participants who were randomly assigned to treatment with estrogen alone versus placebo (HR, 0.74; 95% confidence interval [CI], 0.59-0.94). With estrogen-progestin therapy, the HR was 0.93 (95% CI, 0.77-1.11), and the pooled HR for the 2 trials was 0.85 (95% CI, 0.74-0.98).6
NAMS guidance
The North American Menopause Society (NAMS) HT position statement recommends that prevention of dementia should not be considered an indication for HT use since definitive data are not available.7 The statement indicates also that estrogen therapy may have positive cognitive benefits when initiated immediately after early surgical menopause and taken until the average age of menopause to prevent health risks seen with early loss of hormones.
Definitive data from long-term randomized clinical trials are not likely to become available. Observational trials continue to have methodologic issues, such as "healthy user bias," but the studies are reassuring that initiating HT close to menopause does not increase the risk of dementia. The long-term Finnish study by Imtiaz and colleagues and the Cache County study provide tentative observational data support for a "critical window" hypothesis, leaving open the possibility that initiating systemic HT soon after menopause onset and continuing it long term may reduce the risk of AD. Discussion is needed on individual patient characteristics, potential benefits and risks, and ongoing assessment over time.
Read Dr. Manson’s discussion of 18 years of follow-up data on menopause.
JoAnn E. Manson, MD, DrPH, NCMP
A new analysis from the Women's Health Initiative (WHI) randomized trials examined all-cause and cause-specific mortality during the intervention and postintervention follow-up periods.1 We followed more than 27,000 postmenopausal women aged 50 to 79 (mean age, 63) who were recruited to 2 randomized WHI trials of HT between 1993 and 1998. The trials continued until 2002 for the estrogen-progestin trial and to 2004 for the estrogen-alone trial. The trials ran for 5 to 7 years' duration, with post-stopping follow-up for an additional 10 to 12 years (total cumulative follow-up of 18 years).
The participants were randomly assigned to receive active treatment or placebo. The interventions were conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) versus placebo for women with an intact uterus and CEE alone versus placebo for women who had a hysterectomy.
All-cause mortality did not increase with HT use
The primary outcome measure was all-cause mortality in the 2 pooled trials and in each trial individually. We found that there was no link between HT and all-cause mortality in the overall study population (ages 50-79) in either trial. However, there was a trend toward lower all-cause mortality among the younger women in both trials. In women aged 50 to 59, there was a statistically significant 31% lower risk of mortality in the pooled trials among women taking active HT compared with those taking placebo, but no reduction in mortality with HT among older women (P for trend by age = .01).
Notably, all-cause mortality provides a critically important summary measure for interventions such as HT that have a complex matrix of benefits and risks. We know that HT has a number of benefits in menopausal women. It reduces hot flashes and other menopausal symptoms. It lowers the risk of hip fracture, other types of bone fractures, and type 2 diabetes. However, HT increases the risk of venous thrombosis, stroke, and some forms of cancer.
A summary measure that assesses the net effect of a medication on serious and life-threatening health outcomes is very important. As such, all-cause mortality is the ultimate bottom line for the balance of benefits and risks. This speaks to why we conducted the mortality analysis--WHI is the largest randomized trial of HT with long-term follow-up, allowing detailed analyses by age group. Although there have been previous reports on individual health outcomes in the WHI trials, no previous report had specifically focused on all-cause and cause-specific mortality with HT, stratified by age group, over long-term follow-up.
Hopefully the results of this study will alleviate some of the anxiety associated with HT because, as mentioned, there was no increase in overall total mortality or specific major causes of death. In addition, the younger women had a trend toward benefit for all-cause mortality.
We think that these findings support the recommendations from The North American Menopause Society and other professional societies that endorse the use of HT for managing bothersome menopausal symptoms, especially when started in early menopause. These results should be reassuring that there is no increase in mortality with HT use. Although these findings do not support prescribing HT for the express purpose of trying to prevent cardiovascular disease, dementia, or other chronic diseases (due to some potential risks), they do support an important role of HT for management of bothersome hot flashes, especially in early menopause.
Cause-specific mortality
Regarding cause-specific mortality and HT use, we looked in detail at deaths from cardiovascular causes, cancer, dementia, and other major illness. Overall, we observed no increase or decrease in cardiovascular or cancer deaths. In the estrogen-alone trial, there was a surprising finding of a 26% reduction in dementia deaths. In the estrogen-progestin trial, the results were neutral for dementia deaths.
Overall, the cause-specific mortality results were neutral. This is surprising because even for total cancer deaths there was no increase or decrease, despite a great deal of anxiety about cancer risk with HT. It appears that for cancer, HT has complex effects: it increases some types of cancer, such as breast cancer, and decreases others, such as endometrial cancer (in the estrogen-progestin group), and possibly colorectal cancer. Moreover, CEE alone was associated with a reduction in breast cancer mortality, but it remains unclear if this applies to other formulations. HT's net effect on total cancer mortality was neutral in both trials, that is, no increase or decrease.
Cautions and takeaways
We need to keep in mind that in current clinical practice, lower doses and different formulations and routes of administration of HT are now often used, including transdermal estradiol patches, gels, sprays, and micronized progesterone. These formulations, and the lower doses, may have an even more favorable benefit-risk profile. We need additional research on the long-term benefits and risks of these newer formulations and lower dosages.
Generally, these findings from the WHI trials indicate that for women who have significant hot flashes, night sweats, or other bothersome menopausal symptoms, it's important to discuss their symptoms with their health care provider and understand that hormone therapy may be an option for them. If it's not an option, many other treatments are available, including nonhormonal prescription medications, nonprescription medications, and behavioral approaches.
These findings should alleviate some fear about HT use, especially in younger women who have an overall favorable trend in terms of all-cause mortality with treatment, plus a much lower absolute risk of adverse events than older women. In a woman in early menopause who has bothersome hot flashes or other symptoms that disrupt her sleep or impair her quality of life, it's likely that the benefits of HT will outweigh the risks.
Reference
- Manson JE, Aragaki AK, Rossouw JE, et al; for the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women's Health Initiative randomized trials. JAMA. 2017;318(10):927-938.
Read how the route of HT may affect sexuality outcomes.
Oral vs transdermal estrogen therapy: Is one preferable regarding sexuality?
Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471-1479.
If route of administration of systemic HT influences sexuality outcomes in menopausal women, this would inform how we counsel our patients regarding HT.
Recently, Taylor and colleagues conducted a randomized clinical trial to examine the effects of HT's route of administration on sexual function.8 The 4-year Kronos Early Estrogen Prevention Study (KEEPS) ancillary sexual study randomly assigned 670 recently menopausal women to 0.45 mg of oral conjugated equine estrogens (CEE), an 0.05-mg estradiol transdermal patch, or placebo (with oral micronized progesterone for those on active treatment). The participants were aged 42 to 58 years and were within 36 months from their last menstrual period.
Participants were evaluated using the Female Sexual Function Inventory (FSFI) questionnaire, which assessed desire, arousal, lubrication, orgasm, satisfaction, and pain. The FSFI is scored using a point range of 0 to 36. A higher FSFI score indicates better sexual function. An FSFI score less than 26.55 depicts low sexual function (LSF).
Transdermal estrogen improved sexual function scores
Treatment with oral CEE was associated with no significant change in FSFI score compared with placebo, although benefits were seen for lubrication. By contrast, estrogen patch use improved the FSFI score (mean improvement, 2.6). Although improvement in FSFI score with transdermal estrogen was limited to participants with baseline LSF, most participants in fact had LSF at baseline.
Oral estrogen increases the liver's production of sex hormone-binding globulin, resulting in lower free (bioavailable) testosterone. Transdermal estrogen does not produce this effect. Accordingly, sexuality concerns may represent a reason to prefer the use of transdermal as opposed to oral estrogen.
Read about the authors’ concern over new USPSTF guidance.
The USPSTF recommendation against menopausal HT use for prevention of chronic conditions: Guidance that may confuse--and mislead
US Preventive Services Task Force; Grossman DC, CurrySJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224-2233.
In late 2017, the USPSTF issued its recommendation against the use of menopausal HT for prevention of chronic conditions.9 We are concerned that this recommendation will be misconstrued as suggesting that the use of HT is not appropriate for any indication, including treatment of bothersome menopausal symptoms.
Although the Task Force's report briefly indicated that the guidance does not refer to HT use for treatment of symptoms, this important disclaimer likely will be overlooked or ignored by many readers. The result may be increased uncertainty and anxiety in decision making regarding HT use. Thus, we might see a further decline in the proportion of menopausal women who are prescribed appropriate treatment for symptoms that impair quality of life.
HT use improves menopausal symptoms
According to the 2017 NAMS Position Statement, for symptomatic women in early menopause (that is, younger than age 60 or within 10 years of menopause onset) and free of contraindications to treatment, use of systemic HT is appropriate.7 Currently, clinicians are reluctant to prescribe HT, and women are apprehensive regarding its use.10 Unfortunately, the USPSTF guidance may further discourage appropriate treatment of menopausal symptoms.
Findings from randomized clinical trials, as well as preclinical, clinical, and epidemiologic studies, clarify the favorable benefit-risk profile for HT use by recently menopausal women with bothersome vasomotor and related menopausal symptoms.7,10-12
Notably, the USPSTF guidance does not address women with premature or early menopause, those with persistent (long-duration) vasomotor symptoms, or women at increased risk for osteoporosis and related fractures. Furthermore, the prevalent and undertreated condition, genitourinary syndrome of menopause, deserves but does not receive attention.
In recent decades, our understanding regarding HT's benefits and risks has advanced substantially. Guidance for clinicians and women should reflect this evolution and underscore the individualization and shared decision making that facilitates appropriate decisions regarding the use of HT.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Our knowledge regarding the benefits and risks of systemic menopausal hormone therapy (HT) has continued to evolve since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI). In late 2017, the US Preventive Services Task Force (USPSTF) issued its recommendation against the use of menopausal HT for the prevention of chronic conditions. In this Menopause Update, Dr. JoAnn Manson, Dr. JoAnn Pinkerton, and I detail why we do not support the Task Force’s recommendation. In a sidebar discussion, Dr. Manson also reviews the results of 2 WHI HT trials, published in September 2017, that analyzed mortality in trial participants over an 18-year follow-up.
In addition, I summarize an observational study that assessed the association of HT and Alzheimer disease (AD) as well as a clinical trial that compared the impact of oral versus transdermal estrogen on sexuality in recently menopausal women.
What's the impact of long-term use of systemic HT on Alzheimer disease risk?
Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062-1068.
Data from the WHI HT randomized trials have clarified that initiation of oral HT among women aged 65 and older increases the risk of cognitive decline. By contrast, an analysis of younger WHI participants found that oral HT had no impact on cognitive function. Recently, Imtiaz and colleagues conducted a prospective cohort study of postmenopausal HT and AD in women residing in a Finnish county, with 25 years of follow-up. A diagnosis of AD was based on administrative health records and use of medications prescribed specifically to treat dementia. Use of systemic HT was identified via self-report. Overall, among more than 8,000 women followed, 227 cases of AD (mean age, 72 years) were identified.
In an analysis that controlled for factors including age, body mass index, alcohol use, smoking, physical activity, occupation status, and parity, up to 5 years of HT use was not associated with a risk of being diagnosed with AD. Five to 10 years of HT use was associated with a hazard ratio (HR) of 0.89, an 11% risk reduction that did not achieve statistical significance. By contrast, more than 10 years' use of systemic HT was associated with an HR of 0.53, a statistically significant 47% reduction in risk of AD.1
Other studies found conflicting results
Three large randomized trials found that HT initiated early in menopause and continued for less than 7 years had no impact on cognitive function.2-4 The Cache County (Utah) long-term prospective cohort study, however, found that HT started early in menopause and continued for 10 years or longer was associated with a significant reduction in risk of AD.5
Of note are results from the 2017 report of 18-year cumulative mortality among WHI participants (see the box on page 30). In that study, mortality from AD and other dementia was lower among participants who were randomly assigned to treatment with estrogen alone versus placebo (HR, 0.74; 95% confidence interval [CI], 0.59-0.94). With estrogen-progestin therapy, the HR was 0.93 (95% CI, 0.77-1.11), and the pooled HR for the 2 trials was 0.85 (95% CI, 0.74-0.98).6
NAMS guidance
The North American Menopause Society (NAMS) HT position statement recommends that prevention of dementia should not be considered an indication for HT use since definitive data are not available.7 The statement indicates also that estrogen therapy may have positive cognitive benefits when initiated immediately after early surgical menopause and taken until the average age of menopause to prevent health risks seen with early loss of hormones.
Definitive data from long-term randomized clinical trials are not likely to become available. Observational trials continue to have methodologic issues, such as "healthy user bias," but the studies are reassuring that initiating HT close to menopause does not increase the risk of dementia. The long-term Finnish study by Imtiaz and colleagues and the Cache County study provide tentative observational data support for a "critical window" hypothesis, leaving open the possibility that initiating systemic HT soon after menopause onset and continuing it long term may reduce the risk of AD. Discussion is needed on individual patient characteristics, potential benefits and risks, and ongoing assessment over time.
Read Dr. Manson’s discussion of 18 years of follow-up data on menopause.
JoAnn E. Manson, MD, DrPH, NCMP
A new analysis from the Women's Health Initiative (WHI) randomized trials examined all-cause and cause-specific mortality during the intervention and postintervention follow-up periods.1 We followed more than 27,000 postmenopausal women aged 50 to 79 (mean age, 63) who were recruited to 2 randomized WHI trials of HT between 1993 and 1998. The trials continued until 2002 for the estrogen-progestin trial and to 2004 for the estrogen-alone trial. The trials ran for 5 to 7 years' duration, with post-stopping follow-up for an additional 10 to 12 years (total cumulative follow-up of 18 years).
The participants were randomly assigned to receive active treatment or placebo. The interventions were conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) versus placebo for women with an intact uterus and CEE alone versus placebo for women who had a hysterectomy.
All-cause mortality did not increase with HT use
The primary outcome measure was all-cause mortality in the 2 pooled trials and in each trial individually. We found that there was no link between HT and all-cause mortality in the overall study population (ages 50-79) in either trial. However, there was a trend toward lower all-cause mortality among the younger women in both trials. In women aged 50 to 59, there was a statistically significant 31% lower risk of mortality in the pooled trials among women taking active HT compared with those taking placebo, but no reduction in mortality with HT among older women (P for trend by age = .01).
Notably, all-cause mortality provides a critically important summary measure for interventions such as HT that have a complex matrix of benefits and risks. We know that HT has a number of benefits in menopausal women. It reduces hot flashes and other menopausal symptoms. It lowers the risk of hip fracture, other types of bone fractures, and type 2 diabetes. However, HT increases the risk of venous thrombosis, stroke, and some forms of cancer.
A summary measure that assesses the net effect of a medication on serious and life-threatening health outcomes is very important. As such, all-cause mortality is the ultimate bottom line for the balance of benefits and risks. This speaks to why we conducted the mortality analysis--WHI is the largest randomized trial of HT with long-term follow-up, allowing detailed analyses by age group. Although there have been previous reports on individual health outcomes in the WHI trials, no previous report had specifically focused on all-cause and cause-specific mortality with HT, stratified by age group, over long-term follow-up.
Hopefully the results of this study will alleviate some of the anxiety associated with HT because, as mentioned, there was no increase in overall total mortality or specific major causes of death. In addition, the younger women had a trend toward benefit for all-cause mortality.
We think that these findings support the recommendations from The North American Menopause Society and other professional societies that endorse the use of HT for managing bothersome menopausal symptoms, especially when started in early menopause. These results should be reassuring that there is no increase in mortality with HT use. Although these findings do not support prescribing HT for the express purpose of trying to prevent cardiovascular disease, dementia, or other chronic diseases (due to some potential risks), they do support an important role of HT for management of bothersome hot flashes, especially in early menopause.
Cause-specific mortality
Regarding cause-specific mortality and HT use, we looked in detail at deaths from cardiovascular causes, cancer, dementia, and other major illness. Overall, we observed no increase or decrease in cardiovascular or cancer deaths. In the estrogen-alone trial, there was a surprising finding of a 26% reduction in dementia deaths. In the estrogen-progestin trial, the results were neutral for dementia deaths.
Overall, the cause-specific mortality results were neutral. This is surprising because even for total cancer deaths there was no increase or decrease, despite a great deal of anxiety about cancer risk with HT. It appears that for cancer, HT has complex effects: it increases some types of cancer, such as breast cancer, and decreases others, such as endometrial cancer (in the estrogen-progestin group), and possibly colorectal cancer. Moreover, CEE alone was associated with a reduction in breast cancer mortality, but it remains unclear if this applies to other formulations. HT's net effect on total cancer mortality was neutral in both trials, that is, no increase or decrease.
Cautions and takeaways
We need to keep in mind that in current clinical practice, lower doses and different formulations and routes of administration of HT are now often used, including transdermal estradiol patches, gels, sprays, and micronized progesterone. These formulations, and the lower doses, may have an even more favorable benefit-risk profile. We need additional research on the long-term benefits and risks of these newer formulations and lower dosages.
Generally, these findings from the WHI trials indicate that for women who have significant hot flashes, night sweats, or other bothersome menopausal symptoms, it's important to discuss their symptoms with their health care provider and understand that hormone therapy may be an option for them. If it's not an option, many other treatments are available, including nonhormonal prescription medications, nonprescription medications, and behavioral approaches.
These findings should alleviate some fear about HT use, especially in younger women who have an overall favorable trend in terms of all-cause mortality with treatment, plus a much lower absolute risk of adverse events than older women. In a woman in early menopause who has bothersome hot flashes or other symptoms that disrupt her sleep or impair her quality of life, it's likely that the benefits of HT will outweigh the risks.
Reference
- Manson JE, Aragaki AK, Rossouw JE, et al; for the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women's Health Initiative randomized trials. JAMA. 2017;318(10):927-938.
Read how the route of HT may affect sexuality outcomes.
Oral vs transdermal estrogen therapy: Is one preferable regarding sexuality?
Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471-1479.
If route of administration of systemic HT influences sexuality outcomes in menopausal women, this would inform how we counsel our patients regarding HT.
Recently, Taylor and colleagues conducted a randomized clinical trial to examine the effects of HT's route of administration on sexual function.8 The 4-year Kronos Early Estrogen Prevention Study (KEEPS) ancillary sexual study randomly assigned 670 recently menopausal women to 0.45 mg of oral conjugated equine estrogens (CEE), an 0.05-mg estradiol transdermal patch, or placebo (with oral micronized progesterone for those on active treatment). The participants were aged 42 to 58 years and were within 36 months from their last menstrual period.
Participants were evaluated using the Female Sexual Function Inventory (FSFI) questionnaire, which assessed desire, arousal, lubrication, orgasm, satisfaction, and pain. The FSFI is scored using a point range of 0 to 36. A higher FSFI score indicates better sexual function. An FSFI score less than 26.55 depicts low sexual function (LSF).
Transdermal estrogen improved sexual function scores
Treatment with oral CEE was associated with no significant change in FSFI score compared with placebo, although benefits were seen for lubrication. By contrast, estrogen patch use improved the FSFI score (mean improvement, 2.6). Although improvement in FSFI score with transdermal estrogen was limited to participants with baseline LSF, most participants in fact had LSF at baseline.
Oral estrogen increases the liver's production of sex hormone-binding globulin, resulting in lower free (bioavailable) testosterone. Transdermal estrogen does not produce this effect. Accordingly, sexuality concerns may represent a reason to prefer the use of transdermal as opposed to oral estrogen.
Read about the authors’ concern over new USPSTF guidance.
The USPSTF recommendation against menopausal HT use for prevention of chronic conditions: Guidance that may confuse--and mislead
US Preventive Services Task Force; Grossman DC, CurrySJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224-2233.
In late 2017, the USPSTF issued its recommendation against the use of menopausal HT for prevention of chronic conditions.9 We are concerned that this recommendation will be misconstrued as suggesting that the use of HT is not appropriate for any indication, including treatment of bothersome menopausal symptoms.
Although the Task Force's report briefly indicated that the guidance does not refer to HT use for treatment of symptoms, this important disclaimer likely will be overlooked or ignored by many readers. The result may be increased uncertainty and anxiety in decision making regarding HT use. Thus, we might see a further decline in the proportion of menopausal women who are prescribed appropriate treatment for symptoms that impair quality of life.
HT use improves menopausal symptoms
According to the 2017 NAMS Position Statement, for symptomatic women in early menopause (that is, younger than age 60 or within 10 years of menopause onset) and free of contraindications to treatment, use of systemic HT is appropriate.7 Currently, clinicians are reluctant to prescribe HT, and women are apprehensive regarding its use.10 Unfortunately, the USPSTF guidance may further discourage appropriate treatment of menopausal symptoms.
Findings from randomized clinical trials, as well as preclinical, clinical, and epidemiologic studies, clarify the favorable benefit-risk profile for HT use by recently menopausal women with bothersome vasomotor and related menopausal symptoms.7,10-12
Notably, the USPSTF guidance does not address women with premature or early menopause, those with persistent (long-duration) vasomotor symptoms, or women at increased risk for osteoporosis and related fractures. Furthermore, the prevalent and undertreated condition, genitourinary syndrome of menopause, deserves but does not receive attention.
In recent decades, our understanding regarding HT's benefits and risks has advanced substantially. Guidance for clinicians and women should reflect this evolution and underscore the individualization and shared decision making that facilitates appropriate decisions regarding the use of HT.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062–1068.
- Espeland MA, Shumaker SA, Leng I, et al; WHIMSY Study Group. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173(15):1429–1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12(6):e1001833;discussion e1001833.
- Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708.
- Shao H, Breitner JC, Whitmer RA, et al; Cache County Investigators. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852.
- Manson JE, Aragaki AK, Rossouw JE, et al; the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318(10):927–938.
- NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471–1479.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224–2233.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015; 126(4):859–876.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062–1068.
- Espeland MA, Shumaker SA, Leng I, et al; WHIMSY Study Group. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173(15):1429–1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12(6):e1001833;discussion e1001833.
- Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708.
- Shao H, Breitner JC, Whitmer RA, et al; Cache County Investigators. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852.
- Manson JE, Aragaki AK, Rossouw JE, et al; the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318(10):927–938.
- NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471–1479.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224–2233.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015; 126(4):859–876.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
2017 Update on menopause
Since publication of initial findings of the Women’s Health Initiative (WHI) in 2002, use of systemic menopausal hormone therapy (HT) has declined by some 80% among US women.1 Against this backdrop, this year’s Menopause Update highlights the “hot off the press” updated position statement on menopausal HT from The North American Menopause Society (NAMS), summarized by Dr. JoAnn V. Pinkerton. Although this guidance is chock full of practical, evidence-based guidance, the take-home message that Dr. Pinkerton and I would like to leave readers of OBG
Related Article:
Dr. Andrew M. Kaunitz on prescribing systemic HT to older women
Although menopausal vasomotor and related symptoms improve as women age, in untreated women, vulvovaginal atrophy (VVA, also known as genitourinary syndrome of menopause, or GSM) tends to progress, causing vaginal dryness and sexual dysfunction, among other symptoms. When symptomatic GSM represents the only indication for treatment, low-dose local vaginal estrogen, ospemifene, or dehydroepiandrosterone (DHEA; prasterone) is safe and effective. However, as with systemic HT, specific treatments for GSM are substantially underutilized.2 The current package labeling for low-dose vaginal estrogen deters many appropriate candidates from using this safe, effective treatment. In this Update, Dr. JoAnn E. Manson reviews the rationale for updating this labeling as well as recent efforts to accomplish the task.
Read about updated NAMS guidelines on HT
Guidelines on HT have been updated by The North American Menopause Society
The North American Menopause Society Hormone Therapy (HT) Position Statement Advisory Panel, composed of more than 20 experts in menopausal women's HT, including clinicians, researchers, and epidemiologists, reviewed the 2012 HT Position Statement, evaluated prior and new literature and used levels of evidence to identify the quality of the evidence and strength of the recommendations and to find consensus for the guidelines. The following information comes from the NAMS 2017 Hormone Therapy Position Statement.3
What are the major findings?
HT is the most effective treatment for vasomotor symptoms (VMS) and GSM and has been shown to prevent bone loss and fracture. Risks of HT may differ for women depending on type, dose, duration, route of administration, and timing of initiation and whether or not a progestogen is needed. Treatment should be individualized using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation about benefits and risks of continuing or discontinuing HT.
For women who are younger than age 60 or within 10 years of menopause and have no contraindication, the clearest benefit of HT is for the treatment of VMS and prevention of bone loss in those at elevated risk.
The clinical guidelines were presented to NAMS audience at the 2016 annual clinical meeting, where NAMS recommended "determining the most appropriate type, dose, formulation, and duration of HT."4
When to initiate HT and duration of use
In its now-published 2017 guidelines on HT, NAMS affirms the safety and efficacy of HT for symptomatic menopausal women or those at high risk for bone loss who are under age 60 or within 10 years of menopause. NAMS encourages practitioners to employ shared decision making with their patients to find the appropriate type, dose, formulation, and duration of HT, making individualized decisions based on evidence-based information, the unique health risks of women, and with periodic reassessment.
In the clinical guidelines presented in the 2016 NAMS annual meeting,4 key recommendations taken from the 2017 Hormone Therapy Position Statement3 include the following: For women who are aged younger than 60 years or within 10 years of menopause and have no contraindications, the benefit/risk ratio appears favorable for treatment of bothersome VMS and in those at elevated risk for bone loss or fracture.
For women who initiate HT more than 10 years from menopause or after age 60, this benefit/risk ratio appears less favorable because of greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
What about extended use of hormone therapy? There is no evidence to support routine discontinuation of HT after age 65. Decisions about longer durations of HT should be individualized and considered for indications such as persistent VMS or bone loss, with shared decision making, documentation, and periodic reevaluation. Longer duration is more favorable for estrogen therapy than for estrogen-progestin therapy, based on the Women's Health Initiative (WHI) randomized controlled trials.5
What about only vaginal symptoms? For bothersome GSM not relieved with over-the-counter therapies and without indications for use of systemic HT, low-dose vaginal estrogen therapy or other therapies are recommended and can be continued as long as indicated since there is minimal systemic absorption of estrogen, with serum levels remaining within the normal postmenopausal range.6,7 For women with estrogen sensitive cancer, oncologists should be included in decision making, particularly for women on aromatase inhibitors.
Considerations for special populations Early menopause. For women with hypoestrogenism, primary ovarian insufficiency, or premature surgical menopause without contraindications, HT is recommended until at least the median age of menopause (52 years), as studies suggest that benefits outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.8
Family history of breast cancer. Observational evidence suggests that use of HT does not further alter the risk for breast cancer in women with a family history of breast cancer. Family history is one risk, among others, that should be assessed when counseling women regarding HT.
Women who are BRCA-positive without breast cancer. For women who are BRCA-positive (higher genetic risk of breast cancer, primarily estrogen-receptor-negative), and have undergone surgical menopause (bilateral salpingo-oophorectomy), the benefits of estrogen to decrease health risks caused by premature loss of estrogen need to be considered on an individual basis.9 On the basis of limited observational studies, consider offering systemic HT until the median age of menopause (52 years) with longer use individualized.3
Related Article:
Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
Survivors of endometrial and breast cancer with bothersome VMS. For women with prior estrogen-sensitive cancers, non-HTs should be considered first, particularly those agents studied through randomized controlled trials in this population and found to be effective. If systemic estrogen is considered for persistent symptoms after non-HT or complementary options have been unsuccessful, decisions should be made for compelling reasons and after detailed counseling, with shared decision making and in conjunction with their oncologist.3
Bothersome GSM. On the basis of limited observational data, there appears to be minimal to no demonstrated elevation in risk for recurrence of endometrial or breast cancer using low-dose vaginal estrogen,3,10 but decisions should be made in conjunction with an oncologist.
Related Article:
Focus on treating genital atrophy symptoms
The importance of relaying the new guidelines to patients
It is important for clinicians to talk to women about their menopausal symptoms and their options for relief of symptoms or prevention of bone loss. Discussion should take into account age and time from menopause, include evidence-based information11-13 about benefits and risks of different types of therapy, and employ shared decision making to choose the most appropriate therapy to maximize benefits and minimize risks for the individual woman.
Following the WHI initial release in 2002, both women and providers became fearful of HT and believed media hype and celebrities that compounded bioidentical HT was safer than FDA-approved HTs. However, compounded products lack safety and efficacy data, are not monitored or regulated by the FDA, and have unique risks associated with compounding, including concerns about sterility, impurities, and overdosing or underdosing, which could increase cancer risk.3
- Hormone therapy for symptomatic menopausal women is safe and effective for those under age 60 or within 10 years of menopause.
- Identify the most appropriate type, dose, formulation, and duration of hormone therapy for an individual woman based on evidence.
- We want to remove the fear of using hormone therapy for healthy symptomatic women who are under age 60 or within 10 years of menopause.
- Age at initiation of hormone therapy matters.
- NAMS endorses use of FDA-approved hormone therapy over compounded therapies.
Read about modifying low-dose vaginal estrogen’s black box warning
Physicians continue to underwhelmingly prescribe low-dose vaginal estrogen for GSM
Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The Women's EMPOWER survey: identifying women's perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14(3):413-424.
GSM is seriously underrecognized and undertreated.2,8,14 It has a major impact on women's lives--a silent epidemic affecting women's quality of life, sexual health, interpersonal relationships, and even physical health in terms of increased risk of urinary tract infections and urinary symptoms. Unfortunately, patients are reluctant to mention the problem to their clinicians, and they do not clearly recognize it as a medical condition that has available treatment options. Clinicians also rarely receive adequate training in the management of this condition and how to discuss it with their patients. Given busy schedules and time constraints, addressing this topic often falls through the cracks, representing a missed opportunity for helping our patients with safe and effective treatments. In a recent study by Kingsberg and colleagues, an astoundingly low percentageof women with GSM symptoms received treatment.
Details of the study
The study authors evaluated women's perceptions of GSM and available treatment options. US women aged 45 and older who reported GSM symptoms were surveyed. Of 1,858 women with a median age of 58 (range, 45-90), the study authors found that 50% had never used any treatment; 25% used over-the-counter medications; 18% were former users of GSM treatments; and 7% currently used prescribed GSM therapies.
When GSM was discussed, women were more likely than their clinicians to initiate the conversation. The main reason for women not mentioning their symptoms was the perception that GSM symptoms were a natural and inevitable part of aging. Hormonal products were perceived by women as having several downsides, including risk of systemic absorption, messiness of local creams, and the need to reuse an applicator. Overall, clinicians recommended vaginal estrogen therapy to only 23% and oral HTs to 18% of women.
The results of the study are consistent with results of earlier surveys of menopausal women. Although the survey included nearly 2,000 women, it has the potential for selection biases inherent to most Internet-based surveys. In addition, the respondents tended to be white and have higher socieconomic status, with limited representation from other groups.
Calls for the current boxed warning to be revised
GSM is highly prevalent among postmenopausal women; the condition has adverse effects on quality of life and sexual health.2,8,14 Safe and effective treatments are available but are underutilized.1,8,15,16 A current boxed warning appears on low-dose vaginal estrogen--class labeling that appears on all medications in the class of estrogen or HT, regardless of dose or route of administration. These warnings are based on findings from the WHI and other studies of systemic estrogen or estrogen plus progestin, which demonstrated a complex pattern of risks and benefits of HT (including increased risk of venous thrombosis or pulmonary embolism, stroke, and breast cancer [with estrogen plus progestin]).
These findings, however, do not appear to be relevant to low-dose vaginal estrogen, given minimal if any systemic absorption and much lower blood levels of hormones than found with systemic HT. Blood levels of estradiol with low-dose vaginal estrogen remain in the normal postmenopausal range, compared to several-fold elevations in hormone levels with systemic HT.8,15,16 Additionally, observational studies of low-dose vaginal estrogen, as well as short-term randomized clinical trials, show no evidence of an increased risk of venous thromboembolic events, heart disease, stroke, breast cancer, or dementia--the listed possible adverse effects in the boxed warning. The current warning is based on extrapolating findings from systemic HT, which is inappropriate and not evidence-based for low-dose vaginal estrogen.15
The inappropriate boxed warning contributes to the problem of undertreatment of GSM in women by discouraging clinicians from prescribing the medication and dissuading patients from taking it even after purchase. Testimonials from many clinicians caring for these women have underscored that women will fill their prescription, but after seeing the boxed warning will often become alarmed and decide not to take the medication. Clinicians reported that patients often say at their next appointment: "No, I never took it. I got very scared when I saw the boxed warning." As a result, clinicians often have to spend a great deal of time explaining the limitations of, and lack of evidence for, the boxed warning on low-dose vaginal estrogen.
Related Article:
2016 Update on menopause
Recommended label revisions
A modified label, without a boxed warning, would be safer for women because the key messages would not be obscured by the large amount of irrelevant information. Our Working Group recommended that the label explain that the listed risks were found in studies of systemic HT and their relevance to low-dose vaginal estrogen is unknown. The Group also recommended that warning text should be added in bold font to advise patients to seek medical attention if they have vaginal bleeding or spotting while taking the medication. In addition, patients who have a history of breast cancer or other hormone-sensitive cancer should discuss the use of the medication with their oncologist.
Status update on efforts to revise label. A citizen's petition was filed in the Spring of 2016, with signatures from more than 600 clinicians and patients and representatives of medical and professional organizations endorsing a more appropriate evidence-based label for low-dose vaginal estrogen. The FDA is continuing to review and deliberate on these issues but has not yet made a final decision.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Manson JM, Kaunitz AM. Menopause management—Getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- The 2017 hormone therapy position statement of The North American Menopause Society [published online ahead of print June 2017]. Menopause.
- Pinkerton JV. Hormone therapy: 2016 NAMS position statement [abstract]. Menopause. 2016;23:1365.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Sys Rev. 2016;8:CD001500.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Chai X, Domchek S, Kauff N, Rebbeck T, Chen J. RE: Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(9).
- Farrell R; American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice. ACOG Committee Opinion No. 659 summary: The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):618–619.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231.
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;(3):CD002229.
- Parish S, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Manson JE, Goldstein SR, Kagan R, et al; Working Group on Women’s Health and Well-Being in Menopause. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause. 2014;21(9):911–916.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859-876.
Since publication of initial findings of the Women’s Health Initiative (WHI) in 2002, use of systemic menopausal hormone therapy (HT) has declined by some 80% among US women.1 Against this backdrop, this year’s Menopause Update highlights the “hot off the press” updated position statement on menopausal HT from The North American Menopause Society (NAMS), summarized by Dr. JoAnn V. Pinkerton. Although this guidance is chock full of practical, evidence-based guidance, the take-home message that Dr. Pinkerton and I would like to leave readers of OBG
Related Article:
Dr. Andrew M. Kaunitz on prescribing systemic HT to older women
Although menopausal vasomotor and related symptoms improve as women age, in untreated women, vulvovaginal atrophy (VVA, also known as genitourinary syndrome of menopause, or GSM) tends to progress, causing vaginal dryness and sexual dysfunction, among other symptoms. When symptomatic GSM represents the only indication for treatment, low-dose local vaginal estrogen, ospemifene, or dehydroepiandrosterone (DHEA; prasterone) is safe and effective. However, as with systemic HT, specific treatments for GSM are substantially underutilized.2 The current package labeling for low-dose vaginal estrogen deters many appropriate candidates from using this safe, effective treatment. In this Update, Dr. JoAnn E. Manson reviews the rationale for updating this labeling as well as recent efforts to accomplish the task.
Read about updated NAMS guidelines on HT
Guidelines on HT have been updated by The North American Menopause Society
The North American Menopause Society Hormone Therapy (HT) Position Statement Advisory Panel, composed of more than 20 experts in menopausal women's HT, including clinicians, researchers, and epidemiologists, reviewed the 2012 HT Position Statement, evaluated prior and new literature and used levels of evidence to identify the quality of the evidence and strength of the recommendations and to find consensus for the guidelines. The following information comes from the NAMS 2017 Hormone Therapy Position Statement.3
What are the major findings?
HT is the most effective treatment for vasomotor symptoms (VMS) and GSM and has been shown to prevent bone loss and fracture. Risks of HT may differ for women depending on type, dose, duration, route of administration, and timing of initiation and whether or not a progestogen is needed. Treatment should be individualized using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation about benefits and risks of continuing or discontinuing HT.
For women who are younger than age 60 or within 10 years of menopause and have no contraindication, the clearest benefit of HT is for the treatment of VMS and prevention of bone loss in those at elevated risk.
The clinical guidelines were presented to NAMS audience at the 2016 annual clinical meeting, where NAMS recommended "determining the most appropriate type, dose, formulation, and duration of HT."4
When to initiate HT and duration of use
In its now-published 2017 guidelines on HT, NAMS affirms the safety and efficacy of HT for symptomatic menopausal women or those at high risk for bone loss who are under age 60 or within 10 years of menopause. NAMS encourages practitioners to employ shared decision making with their patients to find the appropriate type, dose, formulation, and duration of HT, making individualized decisions based on evidence-based information, the unique health risks of women, and with periodic reassessment.
In the clinical guidelines presented in the 2016 NAMS annual meeting,4 key recommendations taken from the 2017 Hormone Therapy Position Statement3 include the following: For women who are aged younger than 60 years or within 10 years of menopause and have no contraindications, the benefit/risk ratio appears favorable for treatment of bothersome VMS and in those at elevated risk for bone loss or fracture.
For women who initiate HT more than 10 years from menopause or after age 60, this benefit/risk ratio appears less favorable because of greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
What about extended use of hormone therapy? There is no evidence to support routine discontinuation of HT after age 65. Decisions about longer durations of HT should be individualized and considered for indications such as persistent VMS or bone loss, with shared decision making, documentation, and periodic reevaluation. Longer duration is more favorable for estrogen therapy than for estrogen-progestin therapy, based on the Women's Health Initiative (WHI) randomized controlled trials.5
What about only vaginal symptoms? For bothersome GSM not relieved with over-the-counter therapies and without indications for use of systemic HT, low-dose vaginal estrogen therapy or other therapies are recommended and can be continued as long as indicated since there is minimal systemic absorption of estrogen, with serum levels remaining within the normal postmenopausal range.6,7 For women with estrogen sensitive cancer, oncologists should be included in decision making, particularly for women on aromatase inhibitors.
Considerations for special populations Early menopause. For women with hypoestrogenism, primary ovarian insufficiency, or premature surgical menopause without contraindications, HT is recommended until at least the median age of menopause (52 years), as studies suggest that benefits outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.8
Family history of breast cancer. Observational evidence suggests that use of HT does not further alter the risk for breast cancer in women with a family history of breast cancer. Family history is one risk, among others, that should be assessed when counseling women regarding HT.
Women who are BRCA-positive without breast cancer. For women who are BRCA-positive (higher genetic risk of breast cancer, primarily estrogen-receptor-negative), and have undergone surgical menopause (bilateral salpingo-oophorectomy), the benefits of estrogen to decrease health risks caused by premature loss of estrogen need to be considered on an individual basis.9 On the basis of limited observational studies, consider offering systemic HT until the median age of menopause (52 years) with longer use individualized.3
Related Article:
Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
Survivors of endometrial and breast cancer with bothersome VMS. For women with prior estrogen-sensitive cancers, non-HTs should be considered first, particularly those agents studied through randomized controlled trials in this population and found to be effective. If systemic estrogen is considered for persistent symptoms after non-HT or complementary options have been unsuccessful, decisions should be made for compelling reasons and after detailed counseling, with shared decision making and in conjunction with their oncologist.3
Bothersome GSM. On the basis of limited observational data, there appears to be minimal to no demonstrated elevation in risk for recurrence of endometrial or breast cancer using low-dose vaginal estrogen,3,10 but decisions should be made in conjunction with an oncologist.
Related Article:
Focus on treating genital atrophy symptoms
The importance of relaying the new guidelines to patients
It is important for clinicians to talk to women about their menopausal symptoms and their options for relief of symptoms or prevention of bone loss. Discussion should take into account age and time from menopause, include evidence-based information11-13 about benefits and risks of different types of therapy, and employ shared decision making to choose the most appropriate therapy to maximize benefits and minimize risks for the individual woman.
Following the WHI initial release in 2002, both women and providers became fearful of HT and believed media hype and celebrities that compounded bioidentical HT was safer than FDA-approved HTs. However, compounded products lack safety and efficacy data, are not monitored or regulated by the FDA, and have unique risks associated with compounding, including concerns about sterility, impurities, and overdosing or underdosing, which could increase cancer risk.3
- Hormone therapy for symptomatic menopausal women is safe and effective for those under age 60 or within 10 years of menopause.
- Identify the most appropriate type, dose, formulation, and duration of hormone therapy for an individual woman based on evidence.
- We want to remove the fear of using hormone therapy for healthy symptomatic women who are under age 60 or within 10 years of menopause.
- Age at initiation of hormone therapy matters.
- NAMS endorses use of FDA-approved hormone therapy over compounded therapies.
Read about modifying low-dose vaginal estrogen’s black box warning
Physicians continue to underwhelmingly prescribe low-dose vaginal estrogen for GSM
Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The Women's EMPOWER survey: identifying women's perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14(3):413-424.
GSM is seriously underrecognized and undertreated.2,8,14 It has a major impact on women's lives--a silent epidemic affecting women's quality of life, sexual health, interpersonal relationships, and even physical health in terms of increased risk of urinary tract infections and urinary symptoms. Unfortunately, patients are reluctant to mention the problem to their clinicians, and they do not clearly recognize it as a medical condition that has available treatment options. Clinicians also rarely receive adequate training in the management of this condition and how to discuss it with their patients. Given busy schedules and time constraints, addressing this topic often falls through the cracks, representing a missed opportunity for helping our patients with safe and effective treatments. In a recent study by Kingsberg and colleagues, an astoundingly low percentageof women with GSM symptoms received treatment.
Details of the study
The study authors evaluated women's perceptions of GSM and available treatment options. US women aged 45 and older who reported GSM symptoms were surveyed. Of 1,858 women with a median age of 58 (range, 45-90), the study authors found that 50% had never used any treatment; 25% used over-the-counter medications; 18% were former users of GSM treatments; and 7% currently used prescribed GSM therapies.
When GSM was discussed, women were more likely than their clinicians to initiate the conversation. The main reason for women not mentioning their symptoms was the perception that GSM symptoms were a natural and inevitable part of aging. Hormonal products were perceived by women as having several downsides, including risk of systemic absorption, messiness of local creams, and the need to reuse an applicator. Overall, clinicians recommended vaginal estrogen therapy to only 23% and oral HTs to 18% of women.
The results of the study are consistent with results of earlier surveys of menopausal women. Although the survey included nearly 2,000 women, it has the potential for selection biases inherent to most Internet-based surveys. In addition, the respondents tended to be white and have higher socieconomic status, with limited representation from other groups.
Calls for the current boxed warning to be revised
GSM is highly prevalent among postmenopausal women; the condition has adverse effects on quality of life and sexual health.2,8,14 Safe and effective treatments are available but are underutilized.1,8,15,16 A current boxed warning appears on low-dose vaginal estrogen--class labeling that appears on all medications in the class of estrogen or HT, regardless of dose or route of administration. These warnings are based on findings from the WHI and other studies of systemic estrogen or estrogen plus progestin, which demonstrated a complex pattern of risks and benefits of HT (including increased risk of venous thrombosis or pulmonary embolism, stroke, and breast cancer [with estrogen plus progestin]).
These findings, however, do not appear to be relevant to low-dose vaginal estrogen, given minimal if any systemic absorption and much lower blood levels of hormones than found with systemic HT. Blood levels of estradiol with low-dose vaginal estrogen remain in the normal postmenopausal range, compared to several-fold elevations in hormone levels with systemic HT.8,15,16 Additionally, observational studies of low-dose vaginal estrogen, as well as short-term randomized clinical trials, show no evidence of an increased risk of venous thromboembolic events, heart disease, stroke, breast cancer, or dementia--the listed possible adverse effects in the boxed warning. The current warning is based on extrapolating findings from systemic HT, which is inappropriate and not evidence-based for low-dose vaginal estrogen.15
The inappropriate boxed warning contributes to the problem of undertreatment of GSM in women by discouraging clinicians from prescribing the medication and dissuading patients from taking it even after purchase. Testimonials from many clinicians caring for these women have underscored that women will fill their prescription, but after seeing the boxed warning will often become alarmed and decide not to take the medication. Clinicians reported that patients often say at their next appointment: "No, I never took it. I got very scared when I saw the boxed warning." As a result, clinicians often have to spend a great deal of time explaining the limitations of, and lack of evidence for, the boxed warning on low-dose vaginal estrogen.
Related Article:
2016 Update on menopause
Recommended label revisions
A modified label, without a boxed warning, would be safer for women because the key messages would not be obscured by the large amount of irrelevant information. Our Working Group recommended that the label explain that the listed risks were found in studies of systemic HT and their relevance to low-dose vaginal estrogen is unknown. The Group also recommended that warning text should be added in bold font to advise patients to seek medical attention if they have vaginal bleeding or spotting while taking the medication. In addition, patients who have a history of breast cancer or other hormone-sensitive cancer should discuss the use of the medication with their oncologist.
Status update on efforts to revise label. A citizen's petition was filed in the Spring of 2016, with signatures from more than 600 clinicians and patients and representatives of medical and professional organizations endorsing a more appropriate evidence-based label for low-dose vaginal estrogen. The FDA is continuing to review and deliberate on these issues but has not yet made a final decision.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Since publication of initial findings of the Women’s Health Initiative (WHI) in 2002, use of systemic menopausal hormone therapy (HT) has declined by some 80% among US women.1 Against this backdrop, this year’s Menopause Update highlights the “hot off the press” updated position statement on menopausal HT from The North American Menopause Society (NAMS), summarized by Dr. JoAnn V. Pinkerton. Although this guidance is chock full of practical, evidence-based guidance, the take-home message that Dr. Pinkerton and I would like to leave readers of OBG
Related Article:
Dr. Andrew M. Kaunitz on prescribing systemic HT to older women
Although menopausal vasomotor and related symptoms improve as women age, in untreated women, vulvovaginal atrophy (VVA, also known as genitourinary syndrome of menopause, or GSM) tends to progress, causing vaginal dryness and sexual dysfunction, among other symptoms. When symptomatic GSM represents the only indication for treatment, low-dose local vaginal estrogen, ospemifene, or dehydroepiandrosterone (DHEA; prasterone) is safe and effective. However, as with systemic HT, specific treatments for GSM are substantially underutilized.2 The current package labeling for low-dose vaginal estrogen deters many appropriate candidates from using this safe, effective treatment. In this Update, Dr. JoAnn E. Manson reviews the rationale for updating this labeling as well as recent efforts to accomplish the task.
Read about updated NAMS guidelines on HT
Guidelines on HT have been updated by The North American Menopause Society
The North American Menopause Society Hormone Therapy (HT) Position Statement Advisory Panel, composed of more than 20 experts in menopausal women's HT, including clinicians, researchers, and epidemiologists, reviewed the 2012 HT Position Statement, evaluated prior and new literature and used levels of evidence to identify the quality of the evidence and strength of the recommendations and to find consensus for the guidelines. The following information comes from the NAMS 2017 Hormone Therapy Position Statement.3
What are the major findings?
HT is the most effective treatment for vasomotor symptoms (VMS) and GSM and has been shown to prevent bone loss and fracture. Risks of HT may differ for women depending on type, dose, duration, route of administration, and timing of initiation and whether or not a progestogen is needed. Treatment should be individualized using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation about benefits and risks of continuing or discontinuing HT.
For women who are younger than age 60 or within 10 years of menopause and have no contraindication, the clearest benefit of HT is for the treatment of VMS and prevention of bone loss in those at elevated risk.
The clinical guidelines were presented to NAMS audience at the 2016 annual clinical meeting, where NAMS recommended "determining the most appropriate type, dose, formulation, and duration of HT."4
When to initiate HT and duration of use
In its now-published 2017 guidelines on HT, NAMS affirms the safety and efficacy of HT for symptomatic menopausal women or those at high risk for bone loss who are under age 60 or within 10 years of menopause. NAMS encourages practitioners to employ shared decision making with their patients to find the appropriate type, dose, formulation, and duration of HT, making individualized decisions based on evidence-based information, the unique health risks of women, and with periodic reassessment.
In the clinical guidelines presented in the 2016 NAMS annual meeting,4 key recommendations taken from the 2017 Hormone Therapy Position Statement3 include the following: For women who are aged younger than 60 years or within 10 years of menopause and have no contraindications, the benefit/risk ratio appears favorable for treatment of bothersome VMS and in those at elevated risk for bone loss or fracture.
For women who initiate HT more than 10 years from menopause or after age 60, this benefit/risk ratio appears less favorable because of greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
What about extended use of hormone therapy? There is no evidence to support routine discontinuation of HT after age 65. Decisions about longer durations of HT should be individualized and considered for indications such as persistent VMS or bone loss, with shared decision making, documentation, and periodic reevaluation. Longer duration is more favorable for estrogen therapy than for estrogen-progestin therapy, based on the Women's Health Initiative (WHI) randomized controlled trials.5
What about only vaginal symptoms? For bothersome GSM not relieved with over-the-counter therapies and without indications for use of systemic HT, low-dose vaginal estrogen therapy or other therapies are recommended and can be continued as long as indicated since there is minimal systemic absorption of estrogen, with serum levels remaining within the normal postmenopausal range.6,7 For women with estrogen sensitive cancer, oncologists should be included in decision making, particularly for women on aromatase inhibitors.
Considerations for special populations Early menopause. For women with hypoestrogenism, primary ovarian insufficiency, or premature surgical menopause without contraindications, HT is recommended until at least the median age of menopause (52 years), as studies suggest that benefits outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.8
Family history of breast cancer. Observational evidence suggests that use of HT does not further alter the risk for breast cancer in women with a family history of breast cancer. Family history is one risk, among others, that should be assessed when counseling women regarding HT.
Women who are BRCA-positive without breast cancer. For women who are BRCA-positive (higher genetic risk of breast cancer, primarily estrogen-receptor-negative), and have undergone surgical menopause (bilateral salpingo-oophorectomy), the benefits of estrogen to decrease health risks caused by premature loss of estrogen need to be considered on an individual basis.9 On the basis of limited observational studies, consider offering systemic HT until the median age of menopause (52 years) with longer use individualized.3
Related Article:
Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
Survivors of endometrial and breast cancer with bothersome VMS. For women with prior estrogen-sensitive cancers, non-HTs should be considered first, particularly those agents studied through randomized controlled trials in this population and found to be effective. If systemic estrogen is considered for persistent symptoms after non-HT or complementary options have been unsuccessful, decisions should be made for compelling reasons and after detailed counseling, with shared decision making and in conjunction with their oncologist.3
Bothersome GSM. On the basis of limited observational data, there appears to be minimal to no demonstrated elevation in risk for recurrence of endometrial or breast cancer using low-dose vaginal estrogen,3,10 but decisions should be made in conjunction with an oncologist.
Related Article:
Focus on treating genital atrophy symptoms
The importance of relaying the new guidelines to patients
It is important for clinicians to talk to women about their menopausal symptoms and their options for relief of symptoms or prevention of bone loss. Discussion should take into account age and time from menopause, include evidence-based information11-13 about benefits and risks of different types of therapy, and employ shared decision making to choose the most appropriate therapy to maximize benefits and minimize risks for the individual woman.
Following the WHI initial release in 2002, both women and providers became fearful of HT and believed media hype and celebrities that compounded bioidentical HT was safer than FDA-approved HTs. However, compounded products lack safety and efficacy data, are not monitored or regulated by the FDA, and have unique risks associated with compounding, including concerns about sterility, impurities, and overdosing or underdosing, which could increase cancer risk.3
- Hormone therapy for symptomatic menopausal women is safe and effective for those under age 60 or within 10 years of menopause.
- Identify the most appropriate type, dose, formulation, and duration of hormone therapy for an individual woman based on evidence.
- We want to remove the fear of using hormone therapy for healthy symptomatic women who are under age 60 or within 10 years of menopause.
- Age at initiation of hormone therapy matters.
- NAMS endorses use of FDA-approved hormone therapy over compounded therapies.
Read about modifying low-dose vaginal estrogen’s black box warning
Physicians continue to underwhelmingly prescribe low-dose vaginal estrogen for GSM
Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The Women's EMPOWER survey: identifying women's perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14(3):413-424.
GSM is seriously underrecognized and undertreated.2,8,14 It has a major impact on women's lives--a silent epidemic affecting women's quality of life, sexual health, interpersonal relationships, and even physical health in terms of increased risk of urinary tract infections and urinary symptoms. Unfortunately, patients are reluctant to mention the problem to their clinicians, and they do not clearly recognize it as a medical condition that has available treatment options. Clinicians also rarely receive adequate training in the management of this condition and how to discuss it with their patients. Given busy schedules and time constraints, addressing this topic often falls through the cracks, representing a missed opportunity for helping our patients with safe and effective treatments. In a recent study by Kingsberg and colleagues, an astoundingly low percentageof women with GSM symptoms received treatment.
Details of the study
The study authors evaluated women's perceptions of GSM and available treatment options. US women aged 45 and older who reported GSM symptoms were surveyed. Of 1,858 women with a median age of 58 (range, 45-90), the study authors found that 50% had never used any treatment; 25% used over-the-counter medications; 18% were former users of GSM treatments; and 7% currently used prescribed GSM therapies.
When GSM was discussed, women were more likely than their clinicians to initiate the conversation. The main reason for women not mentioning their symptoms was the perception that GSM symptoms were a natural and inevitable part of aging. Hormonal products were perceived by women as having several downsides, including risk of systemic absorption, messiness of local creams, and the need to reuse an applicator. Overall, clinicians recommended vaginal estrogen therapy to only 23% and oral HTs to 18% of women.
The results of the study are consistent with results of earlier surveys of menopausal women. Although the survey included nearly 2,000 women, it has the potential for selection biases inherent to most Internet-based surveys. In addition, the respondents tended to be white and have higher socieconomic status, with limited representation from other groups.
Calls for the current boxed warning to be revised
GSM is highly prevalent among postmenopausal women; the condition has adverse effects on quality of life and sexual health.2,8,14 Safe and effective treatments are available but are underutilized.1,8,15,16 A current boxed warning appears on low-dose vaginal estrogen--class labeling that appears on all medications in the class of estrogen or HT, regardless of dose or route of administration. These warnings are based on findings from the WHI and other studies of systemic estrogen or estrogen plus progestin, which demonstrated a complex pattern of risks and benefits of HT (including increased risk of venous thrombosis or pulmonary embolism, stroke, and breast cancer [with estrogen plus progestin]).
These findings, however, do not appear to be relevant to low-dose vaginal estrogen, given minimal if any systemic absorption and much lower blood levels of hormones than found with systemic HT. Blood levels of estradiol with low-dose vaginal estrogen remain in the normal postmenopausal range, compared to several-fold elevations in hormone levels with systemic HT.8,15,16 Additionally, observational studies of low-dose vaginal estrogen, as well as short-term randomized clinical trials, show no evidence of an increased risk of venous thromboembolic events, heart disease, stroke, breast cancer, or dementia--the listed possible adverse effects in the boxed warning. The current warning is based on extrapolating findings from systemic HT, which is inappropriate and not evidence-based for low-dose vaginal estrogen.15
The inappropriate boxed warning contributes to the problem of undertreatment of GSM in women by discouraging clinicians from prescribing the medication and dissuading patients from taking it even after purchase. Testimonials from many clinicians caring for these women have underscored that women will fill their prescription, but after seeing the boxed warning will often become alarmed and decide not to take the medication. Clinicians reported that patients often say at their next appointment: "No, I never took it. I got very scared when I saw the boxed warning." As a result, clinicians often have to spend a great deal of time explaining the limitations of, and lack of evidence for, the boxed warning on low-dose vaginal estrogen.
Related Article:
2016 Update on menopause
Recommended label revisions
A modified label, without a boxed warning, would be safer for women because the key messages would not be obscured by the large amount of irrelevant information. Our Working Group recommended that the label explain that the listed risks were found in studies of systemic HT and their relevance to low-dose vaginal estrogen is unknown. The Group also recommended that warning text should be added in bold font to advise patients to seek medical attention if they have vaginal bleeding or spotting while taking the medication. In addition, patients who have a history of breast cancer or other hormone-sensitive cancer should discuss the use of the medication with their oncologist.
Status update on efforts to revise label. A citizen's petition was filed in the Spring of 2016, with signatures from more than 600 clinicians and patients and representatives of medical and professional organizations endorsing a more appropriate evidence-based label for low-dose vaginal estrogen. The FDA is continuing to review and deliberate on these issues but has not yet made a final decision.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Manson JM, Kaunitz AM. Menopause management—Getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- The 2017 hormone therapy position statement of The North American Menopause Society [published online ahead of print June 2017]. Menopause.
- Pinkerton JV. Hormone therapy: 2016 NAMS position statement [abstract]. Menopause. 2016;23:1365.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Sys Rev. 2016;8:CD001500.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Chai X, Domchek S, Kauff N, Rebbeck T, Chen J. RE: Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(9).
- Farrell R; American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice. ACOG Committee Opinion No. 659 summary: The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):618–619.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231.
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;(3):CD002229.
- Parish S, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Manson JE, Goldstein SR, Kagan R, et al; Working Group on Women’s Health and Well-Being in Menopause. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause. 2014;21(9):911–916.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859-876.
- Manson JM, Kaunitz AM. Menopause management—Getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- The 2017 hormone therapy position statement of The North American Menopause Society [published online ahead of print June 2017]. Menopause.
- Pinkerton JV. Hormone therapy: 2016 NAMS position statement [abstract]. Menopause. 2016;23:1365.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Sys Rev. 2016;8:CD001500.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Chai X, Domchek S, Kauff N, Rebbeck T, Chen J. RE: Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(9).
- Farrell R; American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice. ACOG Committee Opinion No. 659 summary: The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):618–619.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231.
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;(3):CD002229.
- Parish S, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Manson JE, Goldstein SR, Kagan R, et al; Working Group on Women’s Health and Well-Being in Menopause. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause. 2014;21(9):911–916.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859-876.