User login
Hospitalists and ACC in Pandemic Flu
Major natural disasters, such as Hurricane Rita and Hurricane Katrina in 2005, have reinforced the reality that health care workers may be asked to treat patients outside the traditional hospital setting.1 The emergence of H5N1 avian influenza in Southeast Asia has also raised concerns about a potential worldwide pandemic influenza.2 Since 2003, the number of avian influenza cases in humans has totaled 387, with 245 deaths.3 While H5N1 influenza has thus far been largely confined to avian populations, the virulence of this strain has raised concern regarding the possible emergence of enhanced human transmission.4 While impossible to accurately forecast the devastation of the next pandemic on the health system, anything similar to the pandemics of the past century will require a large coordinated response by the health system. The most severe pandemic in the past century occurred in 1918 to 1919. The estimated deaths attributed to this worldwide ranges from 20 to 100 million persons,57 with >500,000 of these deaths in the United States.6, 7 In comparison, the annual rate of deaths related to influenza in the United States ranges from 30,000 to 50,000.2, 5 It has been estimated that the next pandemic influenza could cause 75 to 100 million people to become ill, and lead to as many as 1.9 million deaths in the United States.8 In response, the Department of Health and Human Services (HHS) has stressed the importance of advanced planning,9 and the most recent Homeland Security Presidential Directive (HSPD‐21) directs health care organizations and the federal government to develop preparedness plans to provide surge capacity care in times of a catastrophic health event.10 A previous report by one of the authors emphasized the need for hospitalists to play a major role in institutional planning for a pandemic influenza.11
The Alternate Care Center
The concept of offsite care in an influenza pandemic has previously been described, and we will refer to these as Alternate Care Centers (ACCs). Although the literature describes different models of care at an ACC (Table 1),12 we believe an ACC should be activated as an extension of the supporting hospital, once the hospital becomes over capacity despite measures to grow its inpatient service volume.
| Overflow hospital providing full range of care |
| Patient isolation and alternative to home care for infectious patients |
| Expanded ambulatory care |
| Care for recovering, noninfectious patients |
| Limited supportive care for noncritical patients |
| Primary triage and rapid patient screening |
| Quarantine |
Our health system is a large academic medical center, and we have been working with our state to develop a plan to establish and operate an ACC for the next pandemic influenza. Our plans call for an ACC to be activated as an overflow hospital once our hospitals are beyond 120% capacity. We have gone through several functional and tabletop exercises to help identify critical issues that are likely to arise during a real pandemic. Subsequent to these exercises, we have convened an ACC Planning Work Group, reviewed the available literature on surge hospitals, and have focused our recent efforts on several key areas.13 First, it will be important to clearly outline the general services that will be available at this offsite location (Table 2), and this information should be disseminated to the local medical community and the general public. An informed public, with a clear understanding that the ACC is an extension of the hospital with hospitalists in charge of medical care, is more likely to accept getting healthcare in this setting.
|
| IVF administration |
| Parenteral medication administration (eg, antibiotics, steroids, narcotic analgesics, antiemetics) |
| Oxygen support |
| Palliative care services |
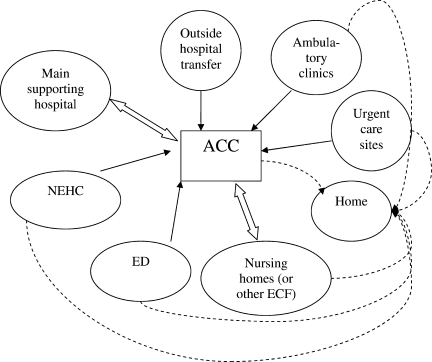
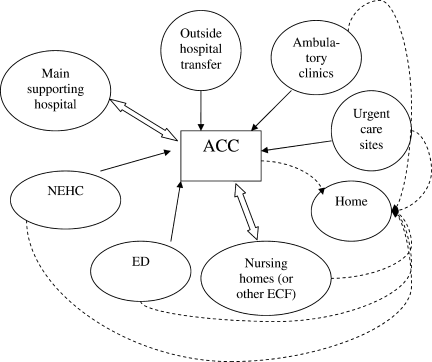
Second, hospitals and the ACCas an extension to the main hospitalwill be asked to provide care to patients referred from several external facilities. Thus, the relationship between the ACC and the main hospital is critical. In a situation where local and even national health care assets will be overwhelmed, having a traditional hospital take full ownership of the ACC and facilitate the transport of patients in and out of the center will be vital to the maintenance of operations. Figure 1 illustrates an example of how patients may be transitioned from 1 site of care to another.
Third, the logistics of establishing an ACC should include details regarding: (1) securing a location that is able to accommodate the needs of the ACC; (2) predetermining the scope of care that can be provided; (3) procuring the necessary equipment and supplies; (4) planning for an adequate number of workforce and staff members; and (5) ensuring a reliable communication plan within the local health system and with state and federal public health officials.14 Staffing shortages and communication barriers are worthy of further emphasis. Given conservative estimates that up to 35% of staff may become ill, refuse to work, or remain home to care for ill family members,15 it is essential that hospitals and regional emergency planners develop a staffing model for the ACC, well in advance of a pandemic. These may include scenarios in which the recommended provider‐to‐patient ratio can not be met. Among the essential lessons learned from the severe acute respiratory syndrome (SARS) outbreak in Toronto (Ontario, Canada) was the importance of developing redundant and reliable communication plans among the healthcare providers.16, 17
Last, healthcare workers' concerns about occupational health and safety must be addressed, and strict measures to protect providers in the ACC need to be implemented.16 This includes providing all exposed staff with adequate personal protective equipment (eg, N‐95 masks), ensuring that all staff are vaccinated against the influenza virus, and implementing strict infection control (eg, hand washing) practices.
For more information, we refer the reader to references that contain further details on our ACC exercises13 and documents that outline concepts of operations in an ACC, developed by the Joint Commission and a multiagency working group.1, 14
The Hospitalist Physician and the ACC
During an influenza pandemic, physicians from all specialties will be vital to the success of the health systems' response. General internists,18 family practitioners, and pediatricians will be overextended in the ambulatory setting to provide intravenous (IV) fluids, antibiotics, and vaccines. Emergency physicians will be called upon to provide care for a burgeoning number of patient arrivals to the Emergency Department (ED), whose acuity is higher than in nonpandemic times. These physicians' clinical expertise at their sites of practice may be severely tested. Hospitalists, given their inpatient focus will be ideally suited to provide medical care to patients admitted to the ACC.
Previous physician leadership at surge hospitals has come from multiple specialties. Case studies describing the heroic physician leadership after Hurricane Katrina and Hurricane Rita represented pediatricians, family physicians, emergency department physicians, and internists.1 In an influenza pandemic, patients in the ACC will require medical care that would, under nonsurge situations, warrant inpatient care. Hospitalists are well poised to lead the response in the ACC for pandemic flu. Hospitalists have expanded their presence into many clinical and administrative responsibilities in their local health systems,19 and the specialty of hospital medicine has evolved to incorporate many of the skills and expertise that would be required of physician leaders who manage an ACC during an influenza pandemic.
While the actual morbidity and mortality associated with the next pandemic are uncertain, it is likely that the number of patients who seek out medical care will exceed current capacity. With constrained space and resources, patients will require appropriate and safe transition to and from the hospital and the ACC. Hospitalists have become leaders in developing and promoting quality transition of care out of acute care settings.20, 21 Their expertise in optimizing this vulnerable time period in patients' healthcare experience should help hospitalists make efficient and appropriate transition care decisions even during busy times and in an alternate care location. Many hospitalists have also developed local and national expertise in quality improvement (QI) and patient safety (PS) initiatives in acute care settings.22 Hospitalists can lead the efforts to apply QI and PS practices in the ACC. These interventions should focus on the potential to be effective in improving patient care, but also consider issues such as ease of implementation, cost, and potential for harm.23
An influenza pandemic will require all levels of the healthcare system to work together to develop a coordinated approach to patient care. Previously, Kisuule et al.24 described how hospitalists can expand their role to include public health. The hospitalists' leadership in the ACC fits well with their descriptions, and hospitalists should work with local, state, and national public health officials in pandemic flu planning. Their scope of practice and clinical expertise will call on them to play key roles in recognition of the development of a pandemic; help lead the response efforts; provide education to staff, patients, and family members; develop clinical care guidelines and pathways for patients; utilize best practices in the use of antimicrobial therapy; and provide appropriate palliative care. Depending on the severity of the influenza pandemic, mortality could be considerable. Many hospitalists have expertise in palliative care at their hospitals,2527 and this skill set will be invaluable in providing compassionate end‐of‐life care to patients in the ACC.
In a pandemic, the most vulnerable patient populations will likely be disproportionately affected, including the elderly, children, and the immune‐compromised. Hospitalists who care regularly for these diverse groups of patients through the spectrum of illness and recovery will be able to address the variety of clinical and nonclinical issues that arise. If the ACC will provide care for children, hospitalists with training in pediatrics, medicine‐pediatrics, or family medicine should be available.
Additional Considerations
While many unanswered questions remain about how to best utilize the ACC, hospitalists are ideally suited to help lead planning efforts for an ACC for pandemic flu. Other issues that may require additional considerations include: (1) whether to strictly care for patients with influenza symptoms and influenza‐related illnesses or to provide care for all patients at the ACC; (2) what to do when patients refuse transfer to and from the ACC; (3) determining the optimal staffing model for patient care providers and to provide care for a wide range of age groups; (4) how the ACC will be funded; (5) how and where to store stockpiles; (6) developing redundant and coordinated communication plans; and (7) planning for reliable access to information and technology from the ACC.
Conclusions
We have introduced the concept of the ACC for the hospitalist community, and emphasized the benefits of engaging hospitalists to lead the ACC initiative at their own health organizations during pandemic flu. As hospitalists currently serve in many of these roles and possess the skills to provide care and lead these initiatives, we encourage hospitalists to contact their hospital administrators to volunteer to assist with preparation efforts.
- Joint Commission on Accreditation of Healthcare Organizations. Surge Hospitals: Providing Safe Care in Emergencies;2006. Available at: http://www.jointcommission.org/NR/rdonlyres/802E9DA4‐AE80‐4584‐A205‐48989C5BD684/0/surge_hospital.pdf. Accessed May 2009.
- .Pandemic influenza: are we ready?Disaster Manag Response.2005;3(3):61–67.
- Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO.2008. Available at: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2008_09_10/en/index.html. Accessed May 2009.
- ,,, et al.Human infection with highly pathogenic H5N1 influenza virus.Lancet.2008;371(9622):1464–1475.
- .Preparing for the next pandemic.N Engl J Med.2005;352(18):1839–1842.
- ,,.Influenza pandemic preparedness action plan for the United States: 2002 update.Clin Infect Dis.2002;35(5):590–596.
- ,,, et al.Nonpharmaceutical interventions implemented by US cities during the 1918‐1919 influenza pandemic.JAMA.2007;298(6):644–654.
- The Health Care Response to Pandemic Influenza: Position Paper.Philadelphia, PA:American College of Physicians;2006.
- U.S. Department of Health and Human Services (HHS). HHS Pandemic Influenza Plan. November2005. Available at: http://www.hhs.gov/pandemicflu/plan. Accessed May 2009.
- Homeland Security Presidential Directive/HSPD‐21.2007. Available at: http://www.whitehouse.gov/news/releases/2007/10/20071018‐10.html. Accessed May 2009.
- ,.Pandemic influenza and the hospitalist: apocalypse when?J Hosp Med.2006;1(2):118–123.
- ,,,,.The prospect of using alternative medical care facilities in an influenza pandemic.Biosecur Bioterror.2006;4(4):384–390.
- ,,, et al.Pandemic influenza and acute care centers (ACCs): taking care of sick patients in a non‐hospital setting.Biosecur Bioterror.2008;6(4):335–348.
- ,,.Acute Care Center. Modular Emergency Medical System: Concept of Operations for the Acute Care Center (ACC).Mass Casualty Care Strategy for A Biological Terrorism Incident. May2003. Available at: http://dms.dartmouth.edu/nnemmrs/resources/surge_capacity_guidance/documents/acute_care_center__concept_ of_operations. pdf. Accessed May 2009.
- Illinois Department of Public Health. Influenza.2007. Available at: http://www.idph.state.il.us/flu/pandemicfs.htm. Accessed May 2009.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291(20):2483–2487.
- .Planning for epidemics—the lessons of SARS.N Engl J Med.2004;350(23):2332–2334.
- .The role of internists during epidemics, outbreaks, and bioterrorist attacks.J Gen Intern Med.2007;22(1):131–136.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136(37‐38):591–596.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,.Executing high‐quality care transitions: a call to do it right.J Hosp Med.2007;2(5):287–290.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1(4):248–252.
- ,.Implementing patient safety interventions in your hospital: what to try and what to avoid.Med Clin North Am.2008;92(2):275–293, vii‐viii.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2(,2):93–101.
- ,,,,,.Evaluating the California hospital initiative in palliative services.Arch Intern Med.2006;166(2):227–230.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1(1):5–6.
- .Palliative care in hospitals.J Hosp Med.2006;1(1):21–28.
Major natural disasters, such as Hurricane Rita and Hurricane Katrina in 2005, have reinforced the reality that health care workers may be asked to treat patients outside the traditional hospital setting.1 The emergence of H5N1 avian influenza in Southeast Asia has also raised concerns about a potential worldwide pandemic influenza.2 Since 2003, the number of avian influenza cases in humans has totaled 387, with 245 deaths.3 While H5N1 influenza has thus far been largely confined to avian populations, the virulence of this strain has raised concern regarding the possible emergence of enhanced human transmission.4 While impossible to accurately forecast the devastation of the next pandemic on the health system, anything similar to the pandemics of the past century will require a large coordinated response by the health system. The most severe pandemic in the past century occurred in 1918 to 1919. The estimated deaths attributed to this worldwide ranges from 20 to 100 million persons,57 with >500,000 of these deaths in the United States.6, 7 In comparison, the annual rate of deaths related to influenza in the United States ranges from 30,000 to 50,000.2, 5 It has been estimated that the next pandemic influenza could cause 75 to 100 million people to become ill, and lead to as many as 1.9 million deaths in the United States.8 In response, the Department of Health and Human Services (HHS) has stressed the importance of advanced planning,9 and the most recent Homeland Security Presidential Directive (HSPD‐21) directs health care organizations and the federal government to develop preparedness plans to provide surge capacity care in times of a catastrophic health event.10 A previous report by one of the authors emphasized the need for hospitalists to play a major role in institutional planning for a pandemic influenza.11
The Alternate Care Center
The concept of offsite care in an influenza pandemic has previously been described, and we will refer to these as Alternate Care Centers (ACCs). Although the literature describes different models of care at an ACC (Table 1),12 we believe an ACC should be activated as an extension of the supporting hospital, once the hospital becomes over capacity despite measures to grow its inpatient service volume.
| Overflow hospital providing full range of care |
| Patient isolation and alternative to home care for infectious patients |
| Expanded ambulatory care |
| Care for recovering, noninfectious patients |
| Limited supportive care for noncritical patients |
| Primary triage and rapid patient screening |
| Quarantine |
Our health system is a large academic medical center, and we have been working with our state to develop a plan to establish and operate an ACC for the next pandemic influenza. Our plans call for an ACC to be activated as an overflow hospital once our hospitals are beyond 120% capacity. We have gone through several functional and tabletop exercises to help identify critical issues that are likely to arise during a real pandemic. Subsequent to these exercises, we have convened an ACC Planning Work Group, reviewed the available literature on surge hospitals, and have focused our recent efforts on several key areas.13 First, it will be important to clearly outline the general services that will be available at this offsite location (Table 2), and this information should be disseminated to the local medical community and the general public. An informed public, with a clear understanding that the ACC is an extension of the hospital with hospitalists in charge of medical care, is more likely to accept getting healthcare in this setting.
|
| IVF administration |
| Parenteral medication administration (eg, antibiotics, steroids, narcotic analgesics, antiemetics) |
| Oxygen support |
| Palliative care services |
Second, hospitals and the ACCas an extension to the main hospitalwill be asked to provide care to patients referred from several external facilities. Thus, the relationship between the ACC and the main hospital is critical. In a situation where local and even national health care assets will be overwhelmed, having a traditional hospital take full ownership of the ACC and facilitate the transport of patients in and out of the center will be vital to the maintenance of operations. Figure 1 illustrates an example of how patients may be transitioned from 1 site of care to another.

Third, the logistics of establishing an ACC should include details regarding: (1) securing a location that is able to accommodate the needs of the ACC; (2) predetermining the scope of care that can be provided; (3) procuring the necessary equipment and supplies; (4) planning for an adequate number of workforce and staff members; and (5) ensuring a reliable communication plan within the local health system and with state and federal public health officials.14 Staffing shortages and communication barriers are worthy of further emphasis. Given conservative estimates that up to 35% of staff may become ill, refuse to work, or remain home to care for ill family members,15 it is essential that hospitals and regional emergency planners develop a staffing model for the ACC, well in advance of a pandemic. These may include scenarios in which the recommended provider‐to‐patient ratio can not be met. Among the essential lessons learned from the severe acute respiratory syndrome (SARS) outbreak in Toronto (Ontario, Canada) was the importance of developing redundant and reliable communication plans among the healthcare providers.16, 17
Last, healthcare workers' concerns about occupational health and safety must be addressed, and strict measures to protect providers in the ACC need to be implemented.16 This includes providing all exposed staff with adequate personal protective equipment (eg, N‐95 masks), ensuring that all staff are vaccinated against the influenza virus, and implementing strict infection control (eg, hand washing) practices.
For more information, we refer the reader to references that contain further details on our ACC exercises13 and documents that outline concepts of operations in an ACC, developed by the Joint Commission and a multiagency working group.1, 14
The Hospitalist Physician and the ACC
During an influenza pandemic, physicians from all specialties will be vital to the success of the health systems' response. General internists,18 family practitioners, and pediatricians will be overextended in the ambulatory setting to provide intravenous (IV) fluids, antibiotics, and vaccines. Emergency physicians will be called upon to provide care for a burgeoning number of patient arrivals to the Emergency Department (ED), whose acuity is higher than in nonpandemic times. These physicians' clinical expertise at their sites of practice may be severely tested. Hospitalists, given their inpatient focus will be ideally suited to provide medical care to patients admitted to the ACC.
Previous physician leadership at surge hospitals has come from multiple specialties. Case studies describing the heroic physician leadership after Hurricane Katrina and Hurricane Rita represented pediatricians, family physicians, emergency department physicians, and internists.1 In an influenza pandemic, patients in the ACC will require medical care that would, under nonsurge situations, warrant inpatient care. Hospitalists are well poised to lead the response in the ACC for pandemic flu. Hospitalists have expanded their presence into many clinical and administrative responsibilities in their local health systems,19 and the specialty of hospital medicine has evolved to incorporate many of the skills and expertise that would be required of physician leaders who manage an ACC during an influenza pandemic.
While the actual morbidity and mortality associated with the next pandemic are uncertain, it is likely that the number of patients who seek out medical care will exceed current capacity. With constrained space and resources, patients will require appropriate and safe transition to and from the hospital and the ACC. Hospitalists have become leaders in developing and promoting quality transition of care out of acute care settings.20, 21 Their expertise in optimizing this vulnerable time period in patients' healthcare experience should help hospitalists make efficient and appropriate transition care decisions even during busy times and in an alternate care location. Many hospitalists have also developed local and national expertise in quality improvement (QI) and patient safety (PS) initiatives in acute care settings.22 Hospitalists can lead the efforts to apply QI and PS practices in the ACC. These interventions should focus on the potential to be effective in improving patient care, but also consider issues such as ease of implementation, cost, and potential for harm.23
An influenza pandemic will require all levels of the healthcare system to work together to develop a coordinated approach to patient care. Previously, Kisuule et al.24 described how hospitalists can expand their role to include public health. The hospitalists' leadership in the ACC fits well with their descriptions, and hospitalists should work with local, state, and national public health officials in pandemic flu planning. Their scope of practice and clinical expertise will call on them to play key roles in recognition of the development of a pandemic; help lead the response efforts; provide education to staff, patients, and family members; develop clinical care guidelines and pathways for patients; utilize best practices in the use of antimicrobial therapy; and provide appropriate palliative care. Depending on the severity of the influenza pandemic, mortality could be considerable. Many hospitalists have expertise in palliative care at their hospitals,2527 and this skill set will be invaluable in providing compassionate end‐of‐life care to patients in the ACC.
In a pandemic, the most vulnerable patient populations will likely be disproportionately affected, including the elderly, children, and the immune‐compromised. Hospitalists who care regularly for these diverse groups of patients through the spectrum of illness and recovery will be able to address the variety of clinical and nonclinical issues that arise. If the ACC will provide care for children, hospitalists with training in pediatrics, medicine‐pediatrics, or family medicine should be available.
Additional Considerations
While many unanswered questions remain about how to best utilize the ACC, hospitalists are ideally suited to help lead planning efforts for an ACC for pandemic flu. Other issues that may require additional considerations include: (1) whether to strictly care for patients with influenza symptoms and influenza‐related illnesses or to provide care for all patients at the ACC; (2) what to do when patients refuse transfer to and from the ACC; (3) determining the optimal staffing model for patient care providers and to provide care for a wide range of age groups; (4) how the ACC will be funded; (5) how and where to store stockpiles; (6) developing redundant and coordinated communication plans; and (7) planning for reliable access to information and technology from the ACC.
Conclusions
We have introduced the concept of the ACC for the hospitalist community, and emphasized the benefits of engaging hospitalists to lead the ACC initiative at their own health organizations during pandemic flu. As hospitalists currently serve in many of these roles and possess the skills to provide care and lead these initiatives, we encourage hospitalists to contact their hospital administrators to volunteer to assist with preparation efforts.
Major natural disasters, such as Hurricane Rita and Hurricane Katrina in 2005, have reinforced the reality that health care workers may be asked to treat patients outside the traditional hospital setting.1 The emergence of H5N1 avian influenza in Southeast Asia has also raised concerns about a potential worldwide pandemic influenza.2 Since 2003, the number of avian influenza cases in humans has totaled 387, with 245 deaths.3 While H5N1 influenza has thus far been largely confined to avian populations, the virulence of this strain has raised concern regarding the possible emergence of enhanced human transmission.4 While impossible to accurately forecast the devastation of the next pandemic on the health system, anything similar to the pandemics of the past century will require a large coordinated response by the health system. The most severe pandemic in the past century occurred in 1918 to 1919. The estimated deaths attributed to this worldwide ranges from 20 to 100 million persons,57 with >500,000 of these deaths in the United States.6, 7 In comparison, the annual rate of deaths related to influenza in the United States ranges from 30,000 to 50,000.2, 5 It has been estimated that the next pandemic influenza could cause 75 to 100 million people to become ill, and lead to as many as 1.9 million deaths in the United States.8 In response, the Department of Health and Human Services (HHS) has stressed the importance of advanced planning,9 and the most recent Homeland Security Presidential Directive (HSPD‐21) directs health care organizations and the federal government to develop preparedness plans to provide surge capacity care in times of a catastrophic health event.10 A previous report by one of the authors emphasized the need for hospitalists to play a major role in institutional planning for a pandemic influenza.11
The Alternate Care Center
The concept of offsite care in an influenza pandemic has previously been described, and we will refer to these as Alternate Care Centers (ACCs). Although the literature describes different models of care at an ACC (Table 1),12 we believe an ACC should be activated as an extension of the supporting hospital, once the hospital becomes over capacity despite measures to grow its inpatient service volume.
| Overflow hospital providing full range of care |
| Patient isolation and alternative to home care for infectious patients |
| Expanded ambulatory care |
| Care for recovering, noninfectious patients |
| Limited supportive care for noncritical patients |
| Primary triage and rapid patient screening |
| Quarantine |
Our health system is a large academic medical center, and we have been working with our state to develop a plan to establish and operate an ACC for the next pandemic influenza. Our plans call for an ACC to be activated as an overflow hospital once our hospitals are beyond 120% capacity. We have gone through several functional and tabletop exercises to help identify critical issues that are likely to arise during a real pandemic. Subsequent to these exercises, we have convened an ACC Planning Work Group, reviewed the available literature on surge hospitals, and have focused our recent efforts on several key areas.13 First, it will be important to clearly outline the general services that will be available at this offsite location (Table 2), and this information should be disseminated to the local medical community and the general public. An informed public, with a clear understanding that the ACC is an extension of the hospital with hospitalists in charge of medical care, is more likely to accept getting healthcare in this setting.
|
| IVF administration |
| Parenteral medication administration (eg, antibiotics, steroids, narcotic analgesics, antiemetics) |
| Oxygen support |
| Palliative care services |
Second, hospitals and the ACCas an extension to the main hospitalwill be asked to provide care to patients referred from several external facilities. Thus, the relationship between the ACC and the main hospital is critical. In a situation where local and even national health care assets will be overwhelmed, having a traditional hospital take full ownership of the ACC and facilitate the transport of patients in and out of the center will be vital to the maintenance of operations. Figure 1 illustrates an example of how patients may be transitioned from 1 site of care to another.

Third, the logistics of establishing an ACC should include details regarding: (1) securing a location that is able to accommodate the needs of the ACC; (2) predetermining the scope of care that can be provided; (3) procuring the necessary equipment and supplies; (4) planning for an adequate number of workforce and staff members; and (5) ensuring a reliable communication plan within the local health system and with state and federal public health officials.14 Staffing shortages and communication barriers are worthy of further emphasis. Given conservative estimates that up to 35% of staff may become ill, refuse to work, or remain home to care for ill family members,15 it is essential that hospitals and regional emergency planners develop a staffing model for the ACC, well in advance of a pandemic. These may include scenarios in which the recommended provider‐to‐patient ratio can not be met. Among the essential lessons learned from the severe acute respiratory syndrome (SARS) outbreak in Toronto (Ontario, Canada) was the importance of developing redundant and reliable communication plans among the healthcare providers.16, 17
Last, healthcare workers' concerns about occupational health and safety must be addressed, and strict measures to protect providers in the ACC need to be implemented.16 This includes providing all exposed staff with adequate personal protective equipment (eg, N‐95 masks), ensuring that all staff are vaccinated against the influenza virus, and implementing strict infection control (eg, hand washing) practices.
For more information, we refer the reader to references that contain further details on our ACC exercises13 and documents that outline concepts of operations in an ACC, developed by the Joint Commission and a multiagency working group.1, 14
The Hospitalist Physician and the ACC
During an influenza pandemic, physicians from all specialties will be vital to the success of the health systems' response. General internists,18 family practitioners, and pediatricians will be overextended in the ambulatory setting to provide intravenous (IV) fluids, antibiotics, and vaccines. Emergency physicians will be called upon to provide care for a burgeoning number of patient arrivals to the Emergency Department (ED), whose acuity is higher than in nonpandemic times. These physicians' clinical expertise at their sites of practice may be severely tested. Hospitalists, given their inpatient focus will be ideally suited to provide medical care to patients admitted to the ACC.
Previous physician leadership at surge hospitals has come from multiple specialties. Case studies describing the heroic physician leadership after Hurricane Katrina and Hurricane Rita represented pediatricians, family physicians, emergency department physicians, and internists.1 In an influenza pandemic, patients in the ACC will require medical care that would, under nonsurge situations, warrant inpatient care. Hospitalists are well poised to lead the response in the ACC for pandemic flu. Hospitalists have expanded their presence into many clinical and administrative responsibilities in their local health systems,19 and the specialty of hospital medicine has evolved to incorporate many of the skills and expertise that would be required of physician leaders who manage an ACC during an influenza pandemic.
While the actual morbidity and mortality associated with the next pandemic are uncertain, it is likely that the number of patients who seek out medical care will exceed current capacity. With constrained space and resources, patients will require appropriate and safe transition to and from the hospital and the ACC. Hospitalists have become leaders in developing and promoting quality transition of care out of acute care settings.20, 21 Their expertise in optimizing this vulnerable time period in patients' healthcare experience should help hospitalists make efficient and appropriate transition care decisions even during busy times and in an alternate care location. Many hospitalists have also developed local and national expertise in quality improvement (QI) and patient safety (PS) initiatives in acute care settings.22 Hospitalists can lead the efforts to apply QI and PS practices in the ACC. These interventions should focus on the potential to be effective in improving patient care, but also consider issues such as ease of implementation, cost, and potential for harm.23
An influenza pandemic will require all levels of the healthcare system to work together to develop a coordinated approach to patient care. Previously, Kisuule et al.24 described how hospitalists can expand their role to include public health. The hospitalists' leadership in the ACC fits well with their descriptions, and hospitalists should work with local, state, and national public health officials in pandemic flu planning. Their scope of practice and clinical expertise will call on them to play key roles in recognition of the development of a pandemic; help lead the response efforts; provide education to staff, patients, and family members; develop clinical care guidelines and pathways for patients; utilize best practices in the use of antimicrobial therapy; and provide appropriate palliative care. Depending on the severity of the influenza pandemic, mortality could be considerable. Many hospitalists have expertise in palliative care at their hospitals,2527 and this skill set will be invaluable in providing compassionate end‐of‐life care to patients in the ACC.
In a pandemic, the most vulnerable patient populations will likely be disproportionately affected, including the elderly, children, and the immune‐compromised. Hospitalists who care regularly for these diverse groups of patients through the spectrum of illness and recovery will be able to address the variety of clinical and nonclinical issues that arise. If the ACC will provide care for children, hospitalists with training in pediatrics, medicine‐pediatrics, or family medicine should be available.
Additional Considerations
While many unanswered questions remain about how to best utilize the ACC, hospitalists are ideally suited to help lead planning efforts for an ACC for pandemic flu. Other issues that may require additional considerations include: (1) whether to strictly care for patients with influenza symptoms and influenza‐related illnesses or to provide care for all patients at the ACC; (2) what to do when patients refuse transfer to and from the ACC; (3) determining the optimal staffing model for patient care providers and to provide care for a wide range of age groups; (4) how the ACC will be funded; (5) how and where to store stockpiles; (6) developing redundant and coordinated communication plans; and (7) planning for reliable access to information and technology from the ACC.
Conclusions
We have introduced the concept of the ACC for the hospitalist community, and emphasized the benefits of engaging hospitalists to lead the ACC initiative at their own health organizations during pandemic flu. As hospitalists currently serve in many of these roles and possess the skills to provide care and lead these initiatives, we encourage hospitalists to contact their hospital administrators to volunteer to assist with preparation efforts.
- Joint Commission on Accreditation of Healthcare Organizations. Surge Hospitals: Providing Safe Care in Emergencies;2006. Available at: http://www.jointcommission.org/NR/rdonlyres/802E9DA4‐AE80‐4584‐A205‐48989C5BD684/0/surge_hospital.pdf. Accessed May 2009.
- .Pandemic influenza: are we ready?Disaster Manag Response.2005;3(3):61–67.
- Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO.2008. Available at: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2008_09_10/en/index.html. Accessed May 2009.
- ,,, et al.Human infection with highly pathogenic H5N1 influenza virus.Lancet.2008;371(9622):1464–1475.
- .Preparing for the next pandemic.N Engl J Med.2005;352(18):1839–1842.
- ,,.Influenza pandemic preparedness action plan for the United States: 2002 update.Clin Infect Dis.2002;35(5):590–596.
- ,,, et al.Nonpharmaceutical interventions implemented by US cities during the 1918‐1919 influenza pandemic.JAMA.2007;298(6):644–654.
- The Health Care Response to Pandemic Influenza: Position Paper.Philadelphia, PA:American College of Physicians;2006.
- U.S. Department of Health and Human Services (HHS). HHS Pandemic Influenza Plan. November2005. Available at: http://www.hhs.gov/pandemicflu/plan. Accessed May 2009.
- Homeland Security Presidential Directive/HSPD‐21.2007. Available at: http://www.whitehouse.gov/news/releases/2007/10/20071018‐10.html. Accessed May 2009.
- ,.Pandemic influenza and the hospitalist: apocalypse when?J Hosp Med.2006;1(2):118–123.
- ,,,,.The prospect of using alternative medical care facilities in an influenza pandemic.Biosecur Bioterror.2006;4(4):384–390.
- ,,, et al.Pandemic influenza and acute care centers (ACCs): taking care of sick patients in a non‐hospital setting.Biosecur Bioterror.2008;6(4):335–348.
- ,,.Acute Care Center. Modular Emergency Medical System: Concept of Operations for the Acute Care Center (ACC).Mass Casualty Care Strategy for A Biological Terrorism Incident. May2003. Available at: http://dms.dartmouth.edu/nnemmrs/resources/surge_capacity_guidance/documents/acute_care_center__concept_ of_operations. pdf. Accessed May 2009.
- Illinois Department of Public Health. Influenza.2007. Available at: http://www.idph.state.il.us/flu/pandemicfs.htm. Accessed May 2009.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291(20):2483–2487.
- .Planning for epidemics—the lessons of SARS.N Engl J Med.2004;350(23):2332–2334.
- .The role of internists during epidemics, outbreaks, and bioterrorist attacks.J Gen Intern Med.2007;22(1):131–136.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136(37‐38):591–596.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,.Executing high‐quality care transitions: a call to do it right.J Hosp Med.2007;2(5):287–290.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1(4):248–252.
- ,.Implementing patient safety interventions in your hospital: what to try and what to avoid.Med Clin North Am.2008;92(2):275–293, vii‐viii.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2(,2):93–101.
- ,,,,,.Evaluating the California hospital initiative in palliative services.Arch Intern Med.2006;166(2):227–230.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1(1):5–6.
- .Palliative care in hospitals.J Hosp Med.2006;1(1):21–28.
- Joint Commission on Accreditation of Healthcare Organizations. Surge Hospitals: Providing Safe Care in Emergencies;2006. Available at: http://www.jointcommission.org/NR/rdonlyres/802E9DA4‐AE80‐4584‐A205‐48989C5BD684/0/surge_hospital.pdf. Accessed May 2009.
- .Pandemic influenza: are we ready?Disaster Manag Response.2005;3(3):61–67.
- Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO.2008. Available at: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2008_09_10/en/index.html. Accessed May 2009.
- ,,, et al.Human infection with highly pathogenic H5N1 influenza virus.Lancet.2008;371(9622):1464–1475.
- .Preparing for the next pandemic.N Engl J Med.2005;352(18):1839–1842.
- ,,.Influenza pandemic preparedness action plan for the United States: 2002 update.Clin Infect Dis.2002;35(5):590–596.
- ,,, et al.Nonpharmaceutical interventions implemented by US cities during the 1918‐1919 influenza pandemic.JAMA.2007;298(6):644–654.
- The Health Care Response to Pandemic Influenza: Position Paper.Philadelphia, PA:American College of Physicians;2006.
- U.S. Department of Health and Human Services (HHS). HHS Pandemic Influenza Plan. November2005. Available at: http://www.hhs.gov/pandemicflu/plan. Accessed May 2009.
- Homeland Security Presidential Directive/HSPD‐21.2007. Available at: http://www.whitehouse.gov/news/releases/2007/10/20071018‐10.html. Accessed May 2009.
- ,.Pandemic influenza and the hospitalist: apocalypse when?J Hosp Med.2006;1(2):118–123.
- ,,,,.The prospect of using alternative medical care facilities in an influenza pandemic.Biosecur Bioterror.2006;4(4):384–390.
- ,,, et al.Pandemic influenza and acute care centers (ACCs): taking care of sick patients in a non‐hospital setting.Biosecur Bioterror.2008;6(4):335–348.
- ,,.Acute Care Center. Modular Emergency Medical System: Concept of Operations for the Acute Care Center (ACC).Mass Casualty Care Strategy for A Biological Terrorism Incident. May2003. Available at: http://dms.dartmouth.edu/nnemmrs/resources/surge_capacity_guidance/documents/acute_care_center__concept_ of_operations. pdf. Accessed May 2009.
- Illinois Department of Public Health. Influenza.2007. Available at: http://www.idph.state.il.us/flu/pandemicfs.htm. Accessed May 2009.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291(20):2483–2487.
- .Planning for epidemics—the lessons of SARS.N Engl J Med.2004;350(23):2332–2334.
- .The role of internists during epidemics, outbreaks, and bioterrorist attacks.J Gen Intern Med.2007;22(1):131–136.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136(37‐38):591–596.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,.Executing high‐quality care transitions: a call to do it right.J Hosp Med.2007;2(5):287–290.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1(4):248–252.
- ,.Implementing patient safety interventions in your hospital: what to try and what to avoid.Med Clin North Am.2008;92(2):275–293, vii‐viii.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2(,2):93–101.
- ,,,,,.Evaluating the California hospital initiative in palliative services.Arch Intern Med.2006;166(2):227–230.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1(1):5–6.
- .Palliative care in hospitals.J Hosp Med.2006;1(1):21–28.
Newer Antifungal Agents
Therapy of serious fungal infections, for decades largely limited to the deoxycholate (regular) preparation of amphotericin B (D‐AmB), expanded significantly with the introduction of fluconazole, followed by lipid‐based formulations of amphotericin B (L‐AmB) and itraconazole. More recently the antifungal armamentarium has broadened further with the approval of voriconazole and posaconazole, as well as the echinocandins caspofungin, micafungin, and anidulafungin. Clinicians, including hospitalists, primary care, emergency medicine, and critical care physicians, may find it challenging to remain abreast of indications for these novel agents, and we review these below, with a focus on adult patients. Manuscripts used in the review were identified by a search of English‐language articles in the PubMed MEDLINE database from 1994 to the present, using the keywords triazoles, echinocandins, voriconazole, posaconazole, caspofungin, micafungin, anidulafungin, candidemia, candidiasis, aspergillosis, invasive Aspergillus, zygomycosis, febrile neutropenia, endemic mycosis, histoplasmosis, and coccidioidomycosis. In addition, reference lists for the majority of the identified manuscripts were hand‐searched for additional pertinent citations.
Table 1 summarizes the newer systemic antifungal therapies and Table 2 summarizes the significant drug‐drug interactions with the newer antifungals.
| Antifungals | Trade Name | FDA‐Approved Indications | Usual Adult Dosing | Adverse Effects |
|---|---|---|---|---|
| ||||
| Azoles | ||||
| Voriconazole | Vfend | Invasive aspergillosis. | Intravenous: 6 mg/kg IV every 12 hours, then 4 mg/kg IV every 12 hours. | Transient visual disturbances (up to 30% in trials), rash, increases in hepatic enzymes, severe hepatotoxicity, and hallucinations. |
| Candidemia in nonneutropenic patients and the following Candida infections: disseminated infections in skin and infections in abdomen, kidney, bladder wall, and wounds. | Oral: 200 mg PO every 12 hours if 40 kg, 100 mg PO every 12 hours if <40 kg. | Accumulation of sulfobutyl ester ‐cyclodextrin, a solubilizing excipient, may occur in patients with creatinine clearance <50 mL/minute receiving the intravenous formulation. | ||
| Esophageal candidiasis. | ||||
| Fungal infections due to Scedosporium apiospermum (asexual form of Pseudallescheria boydii) and Fusarium spp. including Fusarium solani, in patients intolerant of, or refractory to, other therapy. | ||||
| Posaconazole | Noxafil | Prophylaxis of invasive Aspergillus and Candida infections in patients, 13 years of age and older, who are at high risk of developing these infections due to being severely immunocompromised, such as HSCT recipients with graft‐versus‐host disease or those with hematologic malignancies with prolonged neutropenia from chemotherapy. | Prophylaxis of invasive fungal infections: 200 mg (5 mL) PO TID. | Fever, headache, dry mouth, dizziness, fatigue, nausea, vomiting, diarrhea, rash, QT interval prolongation, and elevation of hepatic enzymes. |
| Oropharyngeal candidiasis, including oropharyngeal candidiasis refractory to itraconazole and/or fluconazole. | Oropharyngeal candidiasis: loading dose of 100 mg (2.5 mL) PO BID on day 1, then 100 mg (2.5 mL) PO once daily. | |||
| Oropharyngeal candidiasis refractory to itraconazole and/or fluconazole: 400 mg (10 mL) PO BID. | ||||
| To enhance oral absorption, administer with a full meal or liquid nutritional supplement. | ||||
| Echinocandins | ||||
| Caspofungin | Cancidas | Empirical therapy for presumed fungal infections in febrile, neutropenic patients. | All indications: 70 mg IV loading dose 1, followed by 50 mg IV daily. | Phlebitis, elevation of hepatic enzymes, headache, fever, nausea, vomiting, leukopenia, and histamine mediated symptoms including rash, pruritus, facial swelling, and vasodilatation. |
| Candidemia and the following Candida infections: intraabdominal abscesses, peritonitis, and pleural space infections. | No loading dose required for esophageal candidiasis. | |||
| Esophageal candidiasis. | ||||
| Invasive aspergillosis in patients who are refractory to or intolerant of other therapies (ie, amphotericin B, lipid formulations of amphotericin B, and/or itraconazole). | ||||
| Micafungin | Mycamine | Candidemia, acute disseminated candidiasis, Candida peritonitis and abscesses. | Candidemia, acute disseminated candidiasis, Candida peritonitis and abscesses: 100 mg IV daily. | Similar to caspofungin. |
| Esophageal candidiasis. | Esophageal candidiasis: 150 mg IV daily. | |||
| Prophylaxis of Candida infections in patients undergoing HSCT. | Prophylaxis of Candida infections in HSCT recipients: 50 mg IV daily. | |||
| Anidulafungin | Eraxis | Candidemia and other forms of Candida infections (intraabdominal abscess, peritonitis). | Candidemia/other Candida infections: 200 mg IV loading dose 1, followed by 100 mg IV daily. | Similar to caspofungin. |
| Esophageal candidiasis. | Esophageal candidiasis: 100 mg IV loading dose 1, followed by 50 mg IV Q daily thereafter. | |||
| Antifungal | Effect | Interacting Drugs |
|---|---|---|
| ||
| Voriconazole | Decreased azole serum concentration | Rifampin, rifabutin, carbamazepine, long‐acting barbiturates, efavirenz, high‐dose ritonavir (400 mg twice daily), phenytoin |
| Increased azole serum concentration | Oral contraceptives containing ethinyl estradiol and norethindrone, HIV protease inhibitors other than ritonavir, and nonnucleoside reverse transcriptase inhibitors other than efavirenz | |
| Increased serum concentration of coadministered drug | Sirolimus, rifabutin, efavirenz, terfenadine, astemizole, cisapride, pimozide, quinine, cyclosporine, methadone, tacrolimus, oral contraceptives containing ethinyl estradiol and norethindrone, HIV protease inhibitors other than ritonavir, nonnucleoside reverse transcriptase inhibitors other than efavirenz, benzodiazepines, HMG‐CoA reductase inhibitors, dihydropyridine calcium channel blockers, vinca alkaloids, omeprazole, phenytoin, warfarin, sulfonylurea oral hypoglycemics, and ergot alkaloids | |
| Posaconazole | Decreased azole serum concentration | Cimetidine, rifabutin, phenytoin |
| Increased serum concentration of coadministered drug | Cyclosporine, tacrolimus, rifabutin, midazolam, pheytoin, terfenidine, astemizole, pimozide, cisapride, quinidine, ergot alkaloids, vinca alkaloids, sirolimus, HMG Co‐A reductase inhibitors, and calcium channel blockers | |
| Caspofungin | Decreased serum concentration of caspofungin | Efavirenz, nevirapine, phenytoin, dexamethasone, and carbamazepine |
| Increased serum concentration of caspofungin | Cyclosporine | |
| Decreased serum concentration of coadministered drug | Tacrolimus | |
| Micafungin | Increased serum concentration of coadministered drug | Sirolimus, nifedipine, and itraconazole |
| Anidulafungin | No clinically relevant drug‐drug interactions | |
INVASIVE CANDIDIASIS
Candida has become a leading cause of nosocomial bloodstream infections, and is associated with an attributable mortality of 15% to 25%.1 Candidemia results in an estimated 10‐day increase in hospital length of stay, as well as an average $40,000 (US) increase in costs.2 Invasive candidiasis may be defined as catheter‐related candidemia, other hematogenously disseminated disease, or visceral involvement.3 Risk factors are present in most patients with invasive candidiasis, and include broad‐spectrum antibiotics; parenteral nutrition; central catheters; hospitalization in the intensive care unit setting; renal failure; burns; gastrointestinal and cardiac surgery; and colonization with Candida, particularly at multiple sites.1, 2
Historically, treatment of invasive candidiasis consisted of D‐AmB, with fluconazole largely but not completely replacing amphotericin after prospective trials demonstrated comparable efficacy with markedly improved tolerability. Fluconazole has poor or uncertain activity against C. krusei and C. glabrata, however, leading to reluctance on the part of many clinicians to use it for non‐C. albicans infection (or empirically in the unstable patient). Others have raised concerns regarding the use of fluconazole even for C. albicans in the setting of an unstable or neutropenic patient, given its fungistatic rather than fungicidal activity, although this is a theoretical rather than proven shortcoming.1 Current Infectious Diseases Society of America (IDSA) guidelines for the treatment of candidemia recommend the use of caspofungin, fluconazole, D‐AmB, or the combination of D‐AmB and fluconazole.4 The IDSA recommendations are under revision, however, and we summarize newer evidence below.
Mora‐Duarte et al.,5 in a 2002 trial, randomized patients with invasive candidiasis to caspofungin or D‐AmB, and found a favorable response in 73% and 62%, respectively, which fell just short of statistical significance. Caspofungin was better tolerated than D‐AmB, and the authors concluded that caspofungin was at least as effective as D‐AmB, with fewer adverse effects.5 A 2007 study randomized invasive candidiasis patients to micafungin or L‐AmB, and reported similar efficacy in both arms, with less drug‐related adverse events in the echinocandin‐treated group.6 Reboli et al.7 conducted a noninferiority trial comparing anidulafungin to fluconazole, and found a significantly superior outcome in the anidulafungin arm. Perhaps surprisingly, the outcome difference between the 2 groups was greater for C. albicans than for any other species.7 Although the large majority of patients in the preceding trials had candidemia, one study demonstrated a favorable response to caspofungin in 81% of patients with invasive candidal infections other than candidemia.8
Fewer data exist regarding the use of newer azoles for the treatment of invasive candidiasis. Ostrosky‐Zeichner et al.3 utilized voriconazole as salvage therapy in 52 patients with invasive candidiasis either refractory to or intolerant of other antifungals (almost all of whom had failed therapy with D‐AmB and/or other azoles), and found a 56% favorable response rate in this challenging population. More recently, Kullberg et al.9 studied voriconazole versus D‐AmB followed by fluconazole in candidemic patients, with a similar outcome but somewhat better tolerability in the voriconazole arm. We are unaware of comparative studies involving posaconazole for invasive candidiasis.
In summary, although fluconazole is the drug of choice for most invasive candidal infections, the initial use of an echinocandin should be considered when infection with a non‐C. albicans species is likely, particularly if the patient is unstable. Provided the organism later proves likely to be sensitive, switching to fluconazole is reasonable, particularly given the absence of an oral echinocandin formulation. The 3 currently available echinocandins appear to be interchangeable for the treatment of serious Candida infections.
NEUTROPENIC FEVER
Neutropenia is the most critical factor leading to infection in patients with cancer. Empiric treatment with broad‐spectrum antimicrobials should be initiated at the first sign of infection, since delay can lead to increased mortality.10 There are numerous causes for fever in the neutropenic host, although bacterial infection is most common. Fungal infections can cause unexplained fever and should be considered in neutropenic patients who remain febrile despite broad‐spectrum antibiotics.
Fungal infections in the neutropenic host can have severe consequences. Given their high morbidity and mortality and a lack of effective diagnostic techniques for early detection, empiric antifungal therapy is mandatory in the appropriate setting. Antifungal therapy should be considered in patients who remain febrile and neutropenic for 5 days despite broad‐spectrum antibiotics. The most common fungal pathogens include Candida and Aspergillus spp.11 Other considerations include the emergence of non‐albicans Candida infections and other opportunistic pathogens such as Zygomycetes (Mucor and related pathogens), Fusarium spp, and Scedosporium spp.
Empiric antifungal coverage in the neutropenic host has evolved over the past 2 decades, with the first trials demonstrating the utility of empiric antifungal treatment in the neutropenic host published in the 1980s. These trials demonstrated that addition of D‐AmB to broad spectrum antibiotics decreased development of fungal infections, and led to better outcomes.12, 13 While these studies established D‐AmB as standard empiric antifungal therapy in neutropenic fever, nephrotoxicity and infusion‐related reactions limited its subsequent use as less toxic alternatives were developed. The lipid formulations of amphotericin B, in particular liposomal AmB and amphotericin B lipid complex, have been shown to be as effective as D‐AmB for empiric treatment of febrile neutropenia, with less toxicities but significantly higher expense.14, 15 The older generation azoles itraconazole and fluconazole have also been studied. Itraconazole has been proven to be as effective as D‐AmB in febrile neutropenia with less toxicity; however, the oral capsule has erratic absorption and should be used cautiously.16
Newer agents studied for use in febrile neutropenia include caspofungin and voriconazole. Caspofungin is active against azole‐resistant Candida spp and Aspergillus spp with a favorable toxicity profile, making it an attractive candidate for use in febrile neutropenia. Caspofungin was compared to L‐AmB as empiric antifungal therapy in a randomized double‐blind trial of 1,095 patients with febrile neutropenia.17 The overall success rate was essentially identical for both agents, demonstrating noninferiority of caspofungin therapy. Among patients with baseline fungal infections, significantly more patients receiving caspofungin than L‐AmB had successful outcomes (52% versus 26%, P = 0.04). Overall, caspofungin was better tolerated and associated with fewer complications than L‐AmB.17 The other available echinocandins, micafungin and anidulafungin, have not yet been studied for febrile neutropenia in randomized, controlled fashion.
Voriconazole is a second‐generation azole with activity against fluconazole‐resistant Candida strains; however, the minimum inhibitory concentrations (MICs) are proportionally higher, suggesting a possible cross‐resistance mechanism among highly azole‐resistant strains.18 Voriconazole is active against most Aspergillus spp, Fusarium spp, and Scedosporium apiospermum.19 Voriconazole was compared to L‐AmB in an open‐label, randomized trial of 837 patients with febrile neutropenia.20 Patients were stratified according to risk of fungal infection and previous antifungal prophylaxis. Toxic side effects were similar in both groups. Less breakthrough fungal infections were seen in the voriconazole group; however, there were more discontinuations due to lack of efficacy in patients receiving voriconazole compared to L‐AmB. The overall success rate was 26% with voriconazole and 31% with L‐AmB (95% confidence interval [CI] for absolute difference in success rates: 10.6% to 1.6%), with the low figures reflective not only of infection severity, but also gravity of underlying disease, persistent fever presumably not of fungal origin, and adverse drug effects. Because the predetermined definition of noninferiority for the confidence interval difference between the groups was not met, the U.S. Food and Drug Administration (FDA) voted against approval of voriconazole for febrile neutropenia.
Overall, the role of newer antifungals in the treatment of febrile neutropenia is evolving. Based on current evidence, we prefer caspofungin as the treatment of choice for patients with febrile neutropenia because of its low toxicity profile and good clinical spectrum against most likely pathogens. D‐AmB has long been the gold standard; however, due to toxicity concerns, lipid‐based formulations have largely replaced it, with a notable increase in cost. Voriconazole cannot be recommended at this time based on failure to meet the noninferiority endpoint when compared to L‐AmB. However, for cases in which there is a high suspicion of invasive aspergillosis infection, voriconazole should be considered.
INVASIVE ASPERGILLOSIS
Invasive aspergillosis infection has become an increasing threat in immunocompromised patients, including those treated for cancer, undergoing organ transplantation, or with advanced human immunodeficiency virus (HIV) infection. In particular, patients being treated for hematologic malignancies and those undergoing hematopoietic stem cell transplant (HSCT) are at highest risk, due to prolonged, severe neutropenia. Infection with invasive aspergillosis also occurs when steroids are used for treatment of graft‐versus‐host disease in the HSCT population.
Aspergillus species are saprobic molds found ubiquitously in nature. Most diseases are caused by Aspergillus fumigatus, followed by A. flavus, A. niger, and A. terreus. Infection with Aspergillus can cause a wide spectrum of illnesses, ranging from allergic reactions to fulminant, lethal infections. The lungs are the most common site of primary invasive disease and are associated with high mortality, especially in severely immunocompromised patients.21 Infection is rapidly progressive and can be refractory to treatment, due to the organism's ability to grow quickly and invade blood vessels. Susceptible patients are unable to control infection and thus at high risk for dissemination and death. Prompt administration of an effective antifungal agent is necessary upon suspicion of invasive disease.
The choice of antifungals for invasive Aspergillus infection has grown significantly over the past decade. Current FDA‐approved agents with activity and indications for Aspergillus infection include D‐AmB and its lipid formulations, itraconazole, voriconazole, posaconazole, and caspofungin. D‐AmB and voriconazole are the only agents licensed in the US for the primary treatment of invasive aspergillosis, with D‐AmB the sole therapeutic option until recently. The lipid formulations of amphotericin B, itraconazole, and caspofungin are approved for salvage therapy. Posaconazole is licensed for prophylaxis of invasive aspergillosis in patients who are severely immunocompromised, including those with HSCT and graft‐versus‐host disease as well as those with hematologic malignancies and prolonged neutropenia. Besides caspofungin, the other available echinocandins, micafungin and anidulafungin, are active against Aspergillus species, but not yet FDA‐approved for this indication.
Voriconazole has replaced D‐AmB as the primary treatment of invasive pulmonary aspergillosis.21 Voriconazole was compared to D‐AmB in a randomized, multicenter, open‐label trial of 277 immunocompromised patients with definite or probable disease. The underlying condition in most patients was acute leukemia or allogeneic HSCT, and the majority of patients had invasive pulmonary disease. A successful outcome at week 12 was seen in 53% in the voriconazole group and 32% in the D‐AmB group, with survival rates of 71% and 58%, respectively; both differences were statistically significant. There were more adverse events in the D‐AmB group. Overall, the authors concluded that initial therapy with voriconazole led to better responses, improved survival and fewer side effects than D‐AmB.22
Caspofungin and micafungin have been studied for use as salvage therapy in invasive Aspergillus infection. Caspofungin was studied in 83 patients with invasive aspergillosis refractory to or intolerant of D‐AmB, lipid formulations of amphotericin B, or triazoles, most of whom had hematologic malignancy and allogeneic HSCT. The majority of patients had invasive pulmonary aspergillosis, and a favorable response was seen in 45% of this extremely high‐risk population.23 Micafungin was evaluated in a phase II study as primary or salvage therapy for invasive aspergillosis in adults and children. Of the patients receiving micafungin alone, those receiving the drug as primary therapy had a 50% (n = 6/12) response rate, compared to 41% (9/22) in the salvage therapy group.24 Optimal dosing of micafungin for the treatment of Aspergillus has not yet been established.
Posaconazole, the newest triazole antifungal, has been shown to be effective for the prevention of invasive aspergillosis in immunocompromised patients25, 26 and has also been studied for the treatment of invasive disease. In an open‐label trial, patients with invasive aspergillosis refractory or intolerant to conventional therapy were administered posaconazole, with historical controls as a comparator group.27 The majority of patients had underlying hematologic malignancies with approximately half undergoing HSCT, and most patients had pulmonary infection. The overall success rate was 42% for posaconazole and 26% for the control group. Posaconazole appeared to confer a survival benefit over control at 30 days and end of therapy (P = 0.0003).
Based on current data, we recommend voriconazole for primary treatment of invasive pulmonary aspergillosis. Alternatives include L‐AmB, caspofungin, micafungin, or posaconazole; of these agents, only L‐AmB has been studied as primary (as opposed to salvage) therapy for invasive aspergillosis in a reasonably‐powered trial.28 We agree with current IDSA guidelines, which suggest L‐AmB as a possible alternative to voriconazole for primary therapy of invasive aspergillosis in some patients, particularly where drug‐drug interactions make the use of voriconazole problematic.21
MUCOCUTANEOUS CANDIDIASIS
Oropharyngeal candidiasis, or thrush, is a common infection in infants; those receiving antibiotics, chemotherapy or inhaled corticosteroids; and those with underlying immunodeficiency states. Esophageal candidiasis is most common in patients infected with HIV. Oral candidiasis usually does not cause symptoms, while esophageal disease is associated with odynophagia and dysphagia.
Candida albicans is the most common cause of mucocutaneous candidiasis. Treatment of thrush usually entails topical antifungal agents such as clotrimazole troches or nystatin, or oral azoles such as fluconazole or itraconazole. Topical therapy is ineffective for esophageal candidiasis, and oral or intravenous azoles are required as first‐line therapy with fluconazole being preferred. The treatment of oral and esophageal candidiasis is often complicated by recurrence, especially in immunodeficient patients, and resistance to standard treatments occurs frequently. Identification of Candida to the species level should be performed in the setting of refractory mucocutaneous disease, as this may play a role in the choice of therapy. The 2004 IDSA Guidelines, currently under revision, contain recommendations for treatment of refractory mucocutaneous candidiasis.4 The guidelines recommend a trial of oral itraconazole for fluconazole‐refractory thrush. Intravenous caspofungin and D‐AmB are usually effective alternatives. For treatment of fluconazole‐refractory esophageal disease, the guidelines recommend itraconazole solution, voriconazole, or caspofungin, with D‐AmB recommended as second line therapy, though it is now seldom used in this setting due to significant adverse affects. Experience using newer antifungals is increasing, and these data are summarized below.
Voriconazole has been shown at least as effective as fluconazole in the treatment of esophageal candidiasis in immunocompromised patients.29 A study involving 256 patients revealed success rates of 98% for voriconazole and 95% for fluconazole. C. albicans was the most common pathogen isolated. Perfect et al.30 demonstrated the utility of voriconazole for refractory esophageal candidiasis in 38 patients. A successful outcome was seen in 61% of patients treated with intravenous followed by oral voriconazole. The most common pathogen was C. albicans, although the series included several cases of infection with C. krusei.
Caspofungin was compared to D‐AmB for the treatment of esophageal candidiasis in a multicenter, double‐blind, randomized trial of 128 patients.31 Caspofungin appeared to be at least as effective as D‐AmB, with a significantly higher incidence of drug‐related adverse effects seen in the D‐AmB arm. Caspofungin was also compared to fluconazole in a double‐blind, randomized trial of 177 patients with Candida esophagitis. Favorable responses were seen in 81% and 85% of caspofungin and fluconazole treated patients, respectively. A trend toward higher relapse rate 4 weeks after stopping therapy was seen with caspofungin compared to fluconazole, as was a trend toward superior eradication rates for C. glabrata in the caspofungin arm compared to the fluconazole arm, although neither reached statistical significance.32
Micafungin was used for the treatment of esophageal candidiasis in a dose‐ranging trial of 245 HIV‐infected patients.33 Endoscopic combined cure rate for the 100 mg and 150 mg doses of micafungin (84%) was comparable to that of intravenous fluconazole 200 mg/day (87%). In the posttreatment period, 9 patients in the micafungin arm had a worsening of severity score or received nonprophylactic antifungal therapy. No patients in the fluconazole group experienced a relapse.
Anidulafungin has been compared with fluconazole for the treatment of Candida esophagitis in a randomized, double‐blind trial of 601 patients, with an initial endoscopic success rate approaching 100% in both groups.34 The 2‐week follow‐up examination revealed that 64% and 90% of patients treated with anidulafungin and fluconazole, respectively, sustained endoscopic success (P < 0.001).
Posaconazole was compared with fluconazole for treatment of thrush in 350 patients with HIV/acquired immunodeficiency syndrome (AIDS) in a randomized, blinded study.35 Both posaconazole and fluconazole were administered at a dose of 200 mg on day 1, followed by 100 mg/day. Clinical success occurred in 92% of patients receiving posaconazole and 93% receiving fluconazole. Mycological success was equivalent on day 14 in both arms; however, by day 42, significantly more posaconazole recipients continued to demonstrate mycological success. Posaconazole was recently evaluated for the treatment of azole‐refractory thrush and esophageal candidiasis in patients with advanced HIV infection, demonstrating a success rate of 75% in this population failing fluconazole or itraconazole therapy.36
Multiple new agents are available for the treatment of mucocutaneous candidiasis. Aside from topical antifungals for the initial treatment of thrush, fluconazole remains first line systemic therapy for both oral and esophageal candidiasis due to safety, tolerability, and cost. For fluconazole‐refractory disease, newer choices include voriconazole, the echinocandins, and posaconazole. Voriconazole and posaconazole are attractive options given their oral availability. The relapse rates seen in trials with the echinocandins are concerning; however, these are useful options when azole resistance is suspected.
ZYGOMYCOSIS
Zygomycosis (often referred to less correctly as mucormycosis) is a devastating opportunistic fungal infection that appears to be increasing in frequency. Historically, zygomycosis has commonly occurred in poorly controlled diabetic patients, particularly in the setting of diabetic ketoacidosis, and classically results in rhinocerebral disease with a relatively poor outcome. In recent years, a striking increase has been seen in patients with more profound immunosuppression, particularly those with hematologic malignancies or undergoing HSCT. Sinopulmonary rather than rhinocerebral disease is the most common manifestation in this population.3739 Other well‐described risk factors include iron chelation therapy with deferoxamine, intravenous drug use, solid organ transplantation, metabolic acidosis, trauma, and burns. Disease is also occasionally seen in the seemingly immunocompetent, with 176 of 929 (19%) patients in a comprehensive review lacking an obvious risk factor.37, 40
Invasive mold infections caused by the Zygomycetes are associated with a poor outcome, with Roden et al.37 reporting mortality in excess of 50% in their series. Mortality in patients with hematological malignancies has been reported to be particularly high.37, 38 The cornerstones of successful therapy include early detection of infection, correction or improvement of immunosuppression when possible, prompt surgical debridement of infected tissue, and appropriate antifungal therapy.40 D‐AmB has constituted standard zygomycosis therapy for decades, although it has recently been largely replaced by L‐AmB. Overall survival rates have been reported to be 61% and 69% with the use of D‐AmB and lipid preparations, respectively.37
Given the relatively poor outcomes and substantial infusion‐related toxicity and nephrotoxicity associated with even liposomal preparations of AmB, considerable interest exists in the identification of alternative therapeutic agents. Unfortunately, echinocandins and most triazoles appear to have modest to no activity against Zygomycetes, with a recent case‐control study indicating that widespread use of voriconazole in high‐risk populations may be helping to drive the emergence of breakthrough zygomycosis.39 Posaconazole appears to be an exception, however; with in vitro and murine studies suggesting it compares favorably to D‐AmB in this setting.4143 Numerous case reports describe favorable outcomes with the use of posaconazole as salvage therapy for zygomycosis, and 2 recent retrospective studies support its role in this setting.44, 45 Currently, use of posaconazole for the treatment of zygomycosis is limited by the absence of an intravenous preparation, although this is reportedly under development.46 At present, the role of posaconazole in this setting appears limited to step‐down therapy in those patients who have responded appropriately to L‐AmB, and for salvage therapy. Although an intravenous preparation of posaconazole appears attractive as a first‐line agent for zygomycosis, currently studied patients (ie, those unresponsive to or intolerant of D‐AmB) may not be fully representative of a broader population, and clinical trials will be necessary before more definitive conclusions may be drawn.47
ENDEMIC MYCOSES
Coccidioidomycosis
Coccidioidomycosis results from environmental exposure to either Coccidioides immitis or C. posadii. At least 50% of infections are asymptomatic, with the majority of the remaining individuals exhibiting acute, self‐limited pulmonary symptoms. A small percentage of patients develop chronic illness, either pulmonary or disseminated disease, including involvement of skin, bone/joint, and central nervous system (CNS).48, 49 Current therapy consists of either fluconazole or itraconazole for CNS disease and non‐life‐threatening disease elsewhere, with D‐AmB reserved for pregnancy and more fulminant illness.49 Unfortunately, response failures and relapses are seen commonly with all of these agents, with a resultant need for alternative antifungals.
The echinocandins have no clear role in the treatment of coccidioidomycosis.49 More interest surrounds the use of the newer azoles, with multiple studies demonstrating excellent in vitro activity of both voriconazole and posaconazole against Coccidioides species.5052 Several recently reported open‐label studies have reported good results with the use of posaconazole for chronic coccidioidomycosis, 2 of which enrolled patients intolerant of or refractory to usual agents.5355 Based on these data, posaconazole appears to be highly active against Coccidioides, and should perhaps be the drug of choice in the majority of patients who fail to respond to or tolerate older triazoles.
Histoplasmosis
Histoplasmosis is particularly endemic in the Ohio and Mississippi valleys, although it occurs less commonly in many other areas as well. Inhaled Histoplasma capsulatum conidia result in subclinical infection in the majority of exposed individuals, with self‐limited pneumonia the rule in most others. A minority of patients will experience chronic pulmonary disease or dissemination.56 Not all disease requires treatment, with most pulmonary disease resolving spontaneously; but definite indications for treatment include moderate or severe pneumonia, chronic cavitary lung disease, CNS involvement, and progressive disseminated disease.56 Standard therapy consists of itraconazole or lipid formulations of amphotericin B, based on severity. Multiple studies have demonstrated excellent in vitro activity of voriconazole and particularly posaconazole against H. capsulatum.52, 5759 Recently, in 2 small series of patients, patients failing either to improve with or tolerate conventional agents demonstrated favorable outcomes when they were treated with voriconazole or posaconazole.60, 61 Both drugs appear to be appropriate second‐line agents, with posaconazole arguably preferable based on current evidence.
CONCLUSIONS
The spectrum of available antifungal agents has expanded considerably in recent years, and the advent of additional drugs is expected shortly. Well‐tolerated and effective drugs are now available for most fungal infections, although the precise role for newer agents in some of these diseases has yet to be defined. Future clinical trials should help resolve these uncertainties.
- ,,.Current treatment strategies for disseminated candidiasis.Clin Infect Dis.2006;42:244–251.
- .Echinocandins for candidemia in adults without neutropenia.N Engl J Med.2006;355:1154–1159.
- ,,,.Voriconazole salvage treatment of invasive candidiasis.Eur J Clin Microbiol Infect Dis.2003;22:651–655.
- ,,, et al.Guidelines for treatment of candidiasis.Clin Infect Dis.2004;38:161–189.
- ,,, et al.Comparison of caspofugin and amphotericin B for invasive candidiasis.N Engl J Med.2002;347:2020–2029.
- ,,, et al.Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomized double‐blind trial.Lancet.2007;369:1519–1527.
- ,,, et al.Anidulafungin versus fluconazole for invasive candidiasis.N Engl J Med.2007;356:2472–2482.
- ,,, et al.Caspofungin for the treatment of less common forms of invasive candidiasis.J Antimicrob Chemother.2007;60:363–369.
- ,,, et al.Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non‐neutropenic patients: a randomized non‐inferiority trial.Lancet.2005;366:1435–1442.
- ,.Fever of unknown origin in febrile leucopenia.Infect Dis Clin North Am.2007;21:1055–1090.
- ,,, et al.2002 Guidelines for the use of antimicrobial agents in neutropenic patients with cancer.Clin Infect Dis.2002;34:730–751.
- ,,, et al.Empiric antibiotic and antifungal therapy for cancer patients with prolonged fever and granulocytopenia.Am J Med.1982;72:101–111.
- EORTC International Antimicrobial Therapy Cooperative Group.Empiric antifungal therapy in febrile granulocytopenic patients.Am J Med.1989;86:668–672.
- ,,, et al.Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia.N Engl J Med.1999;340:7644–7671.
- ,,, et al.A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patients.Br J Haematol.1997;98:711–718.
- ,,, et al.Pharmacokinetics and safety of a 7 day administration of intravenous itraconazole followed by a 14‐day administration of itraconazole oral solution in patients with hematologic malignancy.Antimicrob Agents Chemother.2001;45:981–985.
- ,,, et al.Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia.N Engl J Med.2004;351:1392–1402.
- ,,, et al.In vitro activities of voriconazole and four other antifungal agents against 394 clinical isolates of Candida spp.Antimicrob Agents Chemother.1998;42:161–163.
- ,,, et al.Antifungal activity of a new triazole, voriconazole (UK‐109,496) compared with three other antifungal agents tested against clinical isolates of filamentous fungi.Med Mycol.1998;36:433–436.
- ,,, et al.Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever.N Engl J Med.2002;346:225–234.
- ,,, et al.Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America.Clin Infect Dis.2008;46:327–360.
- ,,, et al.Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis.N Engl J Med.2002;347:408–415.
- ,,, et al.Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy.Clin Infect Dis.2004;39:1563–1571.
- ,,, et al.Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis.J Infect2006;53:337–349.
- ,,, et al.Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia.N Engl J Med.2007;356:348–359.
- ,,, et al.Posaconazole or fluconazole for prophylaxis in severe graft versus host disease.N Engl J Med.2007;356:335–347.
- ,,, et al.Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial.Clin Infect Dis.2007;44:2–12.
- ,,, et al.Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high‐loading dose regimen with standard dosing (AmBiLoad trial).Clin Infect Dis.2007;44:1289–1297.
- ,,, et al.A randomized, double‐blind, double‐dummy, multicenter trial of voriconazole and fluconazole in the treatment of esophageal candidiasis in immunocompromised patients.Clin Infect Dis.2001;33:1447–1454.
- ,,, et al.Voriconazole treatment for less‐common, emerging, or refractory fungal infections.Clin Infect Dis.2003;36:1122–1131.
- ,,, et al.A randomized double‐blind study of caspofungin versus amphotericin for the treatment of candidal esophagitis.Clin Infect Dis.2001;33:1529–1535.
- ,,, et al.A randomized double‐blind study of caspofungin versus fluconazole for the treatment of esophageal candidiasis.Am J Med.2002;113:294–299.
- ,,, et al.A randomized, double‐blind, parallel‐group, dose‐response study of micafungin compared with fluconazole for the treatment of esophageal candidiasis in HIV‐positive patients.Clin Infect Dis.2004;39:842–849.
- ,,, et al.A randomized, double‐blind trial of anidulafungin versus fluconazole for the treatment of esophageal candidiasis.Clin Infect Dis.2004;39:770–775.
- ,,, et al.A multicenter randomized trial evaluating posaconazole versus fluconazole for the treatment of oropharyngeal candidiasis in subjects with HIV/AIDS.Clin Infect Dis.2006;42:1179–1186.
- ,,, et al.Posaconazole for the treatment of azole‐refractory oropharyngeal and esophageal candidiasis in subjects with HIV infection.Clin Infect Dis.2007;44:607–614.
- ,,, et al.Epidemiology and outcome of zygomycosis: a review of 929 reported cases.Clin Infect Dis.2005;41:634–653.
- ,,,.Zygomycosis in the 1990s in a tertiary‐care cancer center.Clin Infect Dis.2000;30:851–856.
- ,,, et al.Zygomycosis in a tertiary‐care center in the era of Aspergillus‐active antifungal therapy: a case‐control observational study of 27 recent cases.J Infect Dis.2005;191:1350–1360.
- ,.Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management.Infect Dis Clin North Am.2006;20:581–607.
- ,,,,.In vitro activities of posaconazole itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes.Antimicrob Agents Chemother.2002;46:1581–1582.
- ,,,,.In vivo activity of posaconazole against mucor spp. in an immunosuppressed‐mouse model.Antimicrob Agents Chemother.2002;46:2310–2312.
- ,,,,.In vitro susceptibilities of 217 clinical isolates of zygomycetes to conventional and new antifungal agents.Antimicrob Agents Chemother.2007;51:2587–2590.
- ,,, et al.Posaconazole as salvage therapy for zygomycosis.Antimicrob Agents Chemother.2006;50:126–133.
- ,,,,.Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases.Clin Infect Dis.2006;42:e61–e65.
- ,.Changing epidemiology of rare mould infections.Drugs.2007;67:1803–1812.
- .Posaconazole.Drugs.2005;65:1568–1569.
- ,,, et al.Coccidioidomycosis.Clin Infect Dis.2005;41:1217–1223.
- ,,.Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis.J Clin Microbiol.2007;4:26–30.
- ,,,.Therapeutic efficacy of caspofungin alone and in combination with amphotericin B deoxycholate for coccidioidomycosis in a mouse model.J Antimicrob Chemother.2007;60:1341–1346.
- ,.Antifungal susceptibility profiles of Coccidioides immitis and Coccidioides posadasii from endemic and non‐endemic areas.Mycopathologia.2007;163:31–19.
- ,,,,,.In vitro activities of voriconazole, itraconazole, and amphotericin B against Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum.Antimicrob Agents Chemother.2000;44:1734–1736.
- ,,,,.Refractory coccidioidomycosis treated with posaconazole.Clin Infect Dis.2005;40:1770–1776.
- ,,, et al.Safety, tolerance, and efficacy of posaconazole therapy in patients with nonmeningeal disseminated or chronic pulmonary coccidioidomycosis.Clin Infect Dis.2007;45:562–568.
- ,,, et al.Posaconazole therapy for chronic refractory coccidioidomycosis.Chest.2007;132:952–958.
- ,,, et al.Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America.Clin Infect Dis.2007;45:807–825.
- ,,,,.In vitro activities of new and established triazoles against opportunistic filamentous and dimorphic fungi.Med Mycol.2005;43:281–284.
- ,,, et al.Comparison of a new triazole antifungal agent, Schering 56592, with itraconazole and amphotericin B for treatment of histoplasmosis in immunocompetent mice.Antimicrob Agents Chemother.1999;439:322–328.
- ,,, et al.Activity of newer triazoles against Histoplasma capsulatum from patients with AIDS who failed fluconazole.J Antimicrob Chemother.2006;57:1235–1239.
- ,,,,,.Histoplasmosis in solid organ transplant recipients at a large midwestern university transplant center.Transpl Infect Dis.2005;7:109–115.
- ,,, et al.Salvage treatment of histoplasmosis with posaconazole.J Infect.2007;54:319–327.
Therapy of serious fungal infections, for decades largely limited to the deoxycholate (regular) preparation of amphotericin B (D‐AmB), expanded significantly with the introduction of fluconazole, followed by lipid‐based formulations of amphotericin B (L‐AmB) and itraconazole. More recently the antifungal armamentarium has broadened further with the approval of voriconazole and posaconazole, as well as the echinocandins caspofungin, micafungin, and anidulafungin. Clinicians, including hospitalists, primary care, emergency medicine, and critical care physicians, may find it challenging to remain abreast of indications for these novel agents, and we review these below, with a focus on adult patients. Manuscripts used in the review were identified by a search of English‐language articles in the PubMed MEDLINE database from 1994 to the present, using the keywords triazoles, echinocandins, voriconazole, posaconazole, caspofungin, micafungin, anidulafungin, candidemia, candidiasis, aspergillosis, invasive Aspergillus, zygomycosis, febrile neutropenia, endemic mycosis, histoplasmosis, and coccidioidomycosis. In addition, reference lists for the majority of the identified manuscripts were hand‐searched for additional pertinent citations.
Table 1 summarizes the newer systemic antifungal therapies and Table 2 summarizes the significant drug‐drug interactions with the newer antifungals.
| Antifungals | Trade Name | FDA‐Approved Indications | Usual Adult Dosing | Adverse Effects |
|---|---|---|---|---|
| ||||
| Azoles | ||||
| Voriconazole | Vfend | Invasive aspergillosis. | Intravenous: 6 mg/kg IV every 12 hours, then 4 mg/kg IV every 12 hours. | Transient visual disturbances (up to 30% in trials), rash, increases in hepatic enzymes, severe hepatotoxicity, and hallucinations. |
| Candidemia in nonneutropenic patients and the following Candida infections: disseminated infections in skin and infections in abdomen, kidney, bladder wall, and wounds. | Oral: 200 mg PO every 12 hours if 40 kg, 100 mg PO every 12 hours if <40 kg. | Accumulation of sulfobutyl ester ‐cyclodextrin, a solubilizing excipient, may occur in patients with creatinine clearance <50 mL/minute receiving the intravenous formulation. | ||
| Esophageal candidiasis. | ||||
| Fungal infections due to Scedosporium apiospermum (asexual form of Pseudallescheria boydii) and Fusarium spp. including Fusarium solani, in patients intolerant of, or refractory to, other therapy. | ||||
| Posaconazole | Noxafil | Prophylaxis of invasive Aspergillus and Candida infections in patients, 13 years of age and older, who are at high risk of developing these infections due to being severely immunocompromised, such as HSCT recipients with graft‐versus‐host disease or those with hematologic malignancies with prolonged neutropenia from chemotherapy. | Prophylaxis of invasive fungal infections: 200 mg (5 mL) PO TID. | Fever, headache, dry mouth, dizziness, fatigue, nausea, vomiting, diarrhea, rash, QT interval prolongation, and elevation of hepatic enzymes. |
| Oropharyngeal candidiasis, including oropharyngeal candidiasis refractory to itraconazole and/or fluconazole. | Oropharyngeal candidiasis: loading dose of 100 mg (2.5 mL) PO BID on day 1, then 100 mg (2.5 mL) PO once daily. | |||
| Oropharyngeal candidiasis refractory to itraconazole and/or fluconazole: 400 mg (10 mL) PO BID. | ||||
| To enhance oral absorption, administer with a full meal or liquid nutritional supplement. | ||||
| Echinocandins | ||||
| Caspofungin | Cancidas | Empirical therapy for presumed fungal infections in febrile, neutropenic patients. | All indications: 70 mg IV loading dose 1, followed by 50 mg IV daily. | Phlebitis, elevation of hepatic enzymes, headache, fever, nausea, vomiting, leukopenia, and histamine mediated symptoms including rash, pruritus, facial swelling, and vasodilatation. |
| Candidemia and the following Candida infections: intraabdominal abscesses, peritonitis, and pleural space infections. | No loading dose required for esophageal candidiasis. | |||
| Esophageal candidiasis. | ||||
| Invasive aspergillosis in patients who are refractory to or intolerant of other therapies (ie, amphotericin B, lipid formulations of amphotericin B, and/or itraconazole). | ||||
| Micafungin | Mycamine | Candidemia, acute disseminated candidiasis, Candida peritonitis and abscesses. | Candidemia, acute disseminated candidiasis, Candida peritonitis and abscesses: 100 mg IV daily. | Similar to caspofungin. |
| Esophageal candidiasis. | Esophageal candidiasis: 150 mg IV daily. | |||
| Prophylaxis of Candida infections in patients undergoing HSCT. | Prophylaxis of Candida infections in HSCT recipients: 50 mg IV daily. | |||
| Anidulafungin | Eraxis | Candidemia and other forms of Candida infections (intraabdominal abscess, peritonitis). | Candidemia/other Candida infections: 200 mg IV loading dose 1, followed by 100 mg IV daily. | Similar to caspofungin. |
| Esophageal candidiasis. | Esophageal candidiasis: 100 mg IV loading dose 1, followed by 50 mg IV Q daily thereafter. | |||
| Antifungal | Effect | Interacting Drugs |
|---|---|---|
| ||
| Voriconazole | Decreased azole serum concentration | Rifampin, rifabutin, carbamazepine, long‐acting barbiturates, efavirenz, high‐dose ritonavir (400 mg twice daily), phenytoin |
| Increased azole serum concentration | Oral contraceptives containing ethinyl estradiol and norethindrone, HIV protease inhibitors other than ritonavir, and nonnucleoside reverse transcriptase inhibitors other than efavirenz | |
| Increased serum concentration of coadministered drug | Sirolimus, rifabutin, efavirenz, terfenadine, astemizole, cisapride, pimozide, quinine, cyclosporine, methadone, tacrolimus, oral contraceptives containing ethinyl estradiol and norethindrone, HIV protease inhibitors other than ritonavir, nonnucleoside reverse transcriptase inhibitors other than efavirenz, benzodiazepines, HMG‐CoA reductase inhibitors, dihydropyridine calcium channel blockers, vinca alkaloids, omeprazole, phenytoin, warfarin, sulfonylurea oral hypoglycemics, and ergot alkaloids | |
| Posaconazole | Decreased azole serum concentration | Cimetidine, rifabutin, phenytoin |
| Increased serum concentration of coadministered drug | Cyclosporine, tacrolimus, rifabutin, midazolam, pheytoin, terfenidine, astemizole, pimozide, cisapride, quinidine, ergot alkaloids, vinca alkaloids, sirolimus, HMG Co‐A reductase inhibitors, and calcium channel blockers | |
| Caspofungin | Decreased serum concentration of caspofungin | Efavirenz, nevirapine, phenytoin, dexamethasone, and carbamazepine |
| Increased serum concentration of caspofungin | Cyclosporine | |
| Decreased serum concentration of coadministered drug | Tacrolimus | |
| Micafungin | Increased serum concentration of coadministered drug | Sirolimus, nifedipine, and itraconazole |
| Anidulafungin | No clinically relevant drug‐drug interactions | |
INVASIVE CANDIDIASIS
Candida has become a leading cause of nosocomial bloodstream infections, and is associated with an attributable mortality of 15% to 25%.1 Candidemia results in an estimated 10‐day increase in hospital length of stay, as well as an average $40,000 (US) increase in costs.2 Invasive candidiasis may be defined as catheter‐related candidemia, other hematogenously disseminated disease, or visceral involvement.3 Risk factors are present in most patients with invasive candidiasis, and include broad‐spectrum antibiotics; parenteral nutrition; central catheters; hospitalization in the intensive care unit setting; renal failure; burns; gastrointestinal and cardiac surgery; and colonization with Candida, particularly at multiple sites.1, 2
Historically, treatment of invasive candidiasis consisted of D‐AmB, with fluconazole largely but not completely replacing amphotericin after prospective trials demonstrated comparable efficacy with markedly improved tolerability. Fluconazole has poor or uncertain activity against C. krusei and C. glabrata, however, leading to reluctance on the part of many clinicians to use it for non‐C. albicans infection (or empirically in the unstable patient). Others have raised concerns regarding the use of fluconazole even for C. albicans in the setting of an unstable or neutropenic patient, given its fungistatic rather than fungicidal activity, although this is a theoretical rather than proven shortcoming.1 Current Infectious Diseases Society of America (IDSA) guidelines for the treatment of candidemia recommend the use of caspofungin, fluconazole, D‐AmB, or the combination of D‐AmB and fluconazole.4 The IDSA recommendations are under revision, however, and we summarize newer evidence below.
Mora‐Duarte et al.,5 in a 2002 trial, randomized patients with invasive candidiasis to caspofungin or D‐AmB, and found a favorable response in 73% and 62%, respectively, which fell just short of statistical significance. Caspofungin was better tolerated than D‐AmB, and the authors concluded that caspofungin was at least as effective as D‐AmB, with fewer adverse effects.5 A 2007 study randomized invasive candidiasis patients to micafungin or L‐AmB, and reported similar efficacy in both arms, with less drug‐related adverse events in the echinocandin‐treated group.6 Reboli et al.7 conducted a noninferiority trial comparing anidulafungin to fluconazole, and found a significantly superior outcome in the anidulafungin arm. Perhaps surprisingly, the outcome difference between the 2 groups was greater for C. albicans than for any other species.7 Although the large majority of patients in the preceding trials had candidemia, one study demonstrated a favorable response to caspofungin in 81% of patients with invasive candidal infections other than candidemia.8
Fewer data exist regarding the use of newer azoles for the treatment of invasive candidiasis. Ostrosky‐Zeichner et al.3 utilized voriconazole as salvage therapy in 52 patients with invasive candidiasis either refractory to or intolerant of other antifungals (almost all of whom had failed therapy with D‐AmB and/or other azoles), and found a 56% favorable response rate in this challenging population. More recently, Kullberg et al.9 studied voriconazole versus D‐AmB followed by fluconazole in candidemic patients, with a similar outcome but somewhat better tolerability in the voriconazole arm. We are unaware of comparative studies involving posaconazole for invasive candidiasis.
In summary, although fluconazole is the drug of choice for most invasive candidal infections, the initial use of an echinocandin should be considered when infection with a non‐C. albicans species is likely, particularly if the patient is unstable. Provided the organism later proves likely to be sensitive, switching to fluconazole is reasonable, particularly given the absence of an oral echinocandin formulation. The 3 currently available echinocandins appear to be interchangeable for the treatment of serious Candida infections.
NEUTROPENIC FEVER
Neutropenia is the most critical factor leading to infection in patients with cancer. Empiric treatment with broad‐spectrum antimicrobials should be initiated at the first sign of infection, since delay can lead to increased mortality.10 There are numerous causes for fever in the neutropenic host, although bacterial infection is most common. Fungal infections can cause unexplained fever and should be considered in neutropenic patients who remain febrile despite broad‐spectrum antibiotics.
Fungal infections in the neutropenic host can have severe consequences. Given their high morbidity and mortality and a lack of effective diagnostic techniques for early detection, empiric antifungal therapy is mandatory in the appropriate setting. Antifungal therapy should be considered in patients who remain febrile and neutropenic for 5 days despite broad‐spectrum antibiotics. The most common fungal pathogens include Candida and Aspergillus spp.11 Other considerations include the emergence of non‐albicans Candida infections and other opportunistic pathogens such as Zygomycetes (Mucor and related pathogens), Fusarium spp, and Scedosporium spp.
Empiric antifungal coverage in the neutropenic host has evolved over the past 2 decades, with the first trials demonstrating the utility of empiric antifungal treatment in the neutropenic host published in the 1980s. These trials demonstrated that addition of D‐AmB to broad spectrum antibiotics decreased development of fungal infections, and led to better outcomes.12, 13 While these studies established D‐AmB as standard empiric antifungal therapy in neutropenic fever, nephrotoxicity and infusion‐related reactions limited its subsequent use as less toxic alternatives were developed. The lipid formulations of amphotericin B, in particular liposomal AmB and amphotericin B lipid complex, have been shown to be as effective as D‐AmB for empiric treatment of febrile neutropenia, with less toxicities but significantly higher expense.14, 15 The older generation azoles itraconazole and fluconazole have also been studied. Itraconazole has been proven to be as effective as D‐AmB in febrile neutropenia with less toxicity; however, the oral capsule has erratic absorption and should be used cautiously.16
Newer agents studied for use in febrile neutropenia include caspofungin and voriconazole. Caspofungin is active against azole‐resistant Candida spp and Aspergillus spp with a favorable toxicity profile, making it an attractive candidate for use in febrile neutropenia. Caspofungin was compared to L‐AmB as empiric antifungal therapy in a randomized double‐blind trial of 1,095 patients with febrile neutropenia.17 The overall success rate was essentially identical for both agents, demonstrating noninferiority of caspofungin therapy. Among patients with baseline fungal infections, significantly more patients receiving caspofungin than L‐AmB had successful outcomes (52% versus 26%, P = 0.04). Overall, caspofungin was better tolerated and associated with fewer complications than L‐AmB.17 The other available echinocandins, micafungin and anidulafungin, have not yet been studied for febrile neutropenia in randomized, controlled fashion.
Voriconazole is a second‐generation azole with activity against fluconazole‐resistant Candida strains; however, the minimum inhibitory concentrations (MICs) are proportionally higher, suggesting a possible cross‐resistance mechanism among highly azole‐resistant strains.18 Voriconazole is active against most Aspergillus spp, Fusarium spp, and Scedosporium apiospermum.19 Voriconazole was compared to L‐AmB in an open‐label, randomized trial of 837 patients with febrile neutropenia.20 Patients were stratified according to risk of fungal infection and previous antifungal prophylaxis. Toxic side effects were similar in both groups. Less breakthrough fungal infections were seen in the voriconazole group; however, there were more discontinuations due to lack of efficacy in patients receiving voriconazole compared to L‐AmB. The overall success rate was 26% with voriconazole and 31% with L‐AmB (95% confidence interval [CI] for absolute difference in success rates: 10.6% to 1.6%), with the low figures reflective not only of infection severity, but also gravity of underlying disease, persistent fever presumably not of fungal origin, and adverse drug effects. Because the predetermined definition of noninferiority for the confidence interval difference between the groups was not met, the U.S. Food and Drug Administration (FDA) voted against approval of voriconazole for febrile neutropenia.
Overall, the role of newer antifungals in the treatment of febrile neutropenia is evolving. Based on current evidence, we prefer caspofungin as the treatment of choice for patients with febrile neutropenia because of its low toxicity profile and good clinical spectrum against most likely pathogens. D‐AmB has long been the gold standard; however, due to toxicity concerns, lipid‐based formulations have largely replaced it, with a notable increase in cost. Voriconazole cannot be recommended at this time based on failure to meet the noninferiority endpoint when compared to L‐AmB. However, for cases in which there is a high suspicion of invasive aspergillosis infection, voriconazole should be considered.
INVASIVE ASPERGILLOSIS
Invasive aspergillosis infection has become an increasing threat in immunocompromised patients, including those treated for cancer, undergoing organ transplantation, or with advanced human immunodeficiency virus (HIV) infection. In particular, patients being treated for hematologic malignancies and those undergoing hematopoietic stem cell transplant (HSCT) are at highest risk, due to prolonged, severe neutropenia. Infection with invasive aspergillosis also occurs when steroids are used for treatment of graft‐versus‐host disease in the HSCT population.
Aspergillus species are saprobic molds found ubiquitously in nature. Most diseases are caused by Aspergillus fumigatus, followed by A. flavus, A. niger, and A. terreus. Infection with Aspergillus can cause a wide spectrum of illnesses, ranging from allergic reactions to fulminant, lethal infections. The lungs are the most common site of primary invasive disease and are associated with high mortality, especially in severely immunocompromised patients.21 Infection is rapidly progressive and can be refractory to treatment, due to the organism's ability to grow quickly and invade blood vessels. Susceptible patients are unable to control infection and thus at high risk for dissemination and death. Prompt administration of an effective antifungal agent is necessary upon suspicion of invasive disease.
The choice of antifungals for invasive Aspergillus infection has grown significantly over the past decade. Current FDA‐approved agents with activity and indications for Aspergillus infection include D‐AmB and its lipid formulations, itraconazole, voriconazole, posaconazole, and caspofungin. D‐AmB and voriconazole are the only agents licensed in the US for the primary treatment of invasive aspergillosis, with D‐AmB the sole therapeutic option until recently. The lipid formulations of amphotericin B, itraconazole, and caspofungin are approved for salvage therapy. Posaconazole is licensed for prophylaxis of invasive aspergillosis in patients who are severely immunocompromised, including those with HSCT and graft‐versus‐host disease as well as those with hematologic malignancies and prolonged neutropenia. Besides caspofungin, the other available echinocandins, micafungin and anidulafungin, are active against Aspergillus species, but not yet FDA‐approved for this indication.
Voriconazole has replaced D‐AmB as the primary treatment of invasive pulmonary aspergillosis.21 Voriconazole was compared to D‐AmB in a randomized, multicenter, open‐label trial of 277 immunocompromised patients with definite or probable disease. The underlying condition in most patients was acute leukemia or allogeneic HSCT, and the majority of patients had invasive pulmonary disease. A successful outcome at week 12 was seen in 53% in the voriconazole group and 32% in the D‐AmB group, with survival rates of 71% and 58%, respectively; both differences were statistically significant. There were more adverse events in the D‐AmB group. Overall, the authors concluded that initial therapy with voriconazole led to better responses, improved survival and fewer side effects than D‐AmB.22
Caspofungin and micafungin have been studied for use as salvage therapy in invasive Aspergillus infection. Caspofungin was studied in 83 patients with invasive aspergillosis refractory to or intolerant of D‐AmB, lipid formulations of amphotericin B, or triazoles, most of whom had hematologic malignancy and allogeneic HSCT. The majority of patients had invasive pulmonary aspergillosis, and a favorable response was seen in 45% of this extremely high‐risk population.23 Micafungin was evaluated in a phase II study as primary or salvage therapy for invasive aspergillosis in adults and children. Of the patients receiving micafungin alone, those receiving the drug as primary therapy had a 50% (n = 6/12) response rate, compared to 41% (9/22) in the salvage therapy group.24 Optimal dosing of micafungin for the treatment of Aspergillus has not yet been established.
Posaconazole, the newest triazole antifungal, has been shown to be effective for the prevention of invasive aspergillosis in immunocompromised patients25, 26 and has also been studied for the treatment of invasive disease. In an open‐label trial, patients with invasive aspergillosis refractory or intolerant to conventional therapy were administered posaconazole, with historical controls as a comparator group.27 The majority of patients had underlying hematologic malignancies with approximately half undergoing HSCT, and most patients had pulmonary infection. The overall success rate was 42% for posaconazole and 26% for the control group. Posaconazole appeared to confer a survival benefit over control at 30 days and end of therapy (P = 0.0003).
Based on current data, we recommend voriconazole for primary treatment of invasive pulmonary aspergillosis. Alternatives include L‐AmB, caspofungin, micafungin, or posaconazole; of these agents, only L‐AmB has been studied as primary (as opposed to salvage) therapy for invasive aspergillosis in a reasonably‐powered trial.28 We agree with current IDSA guidelines, which suggest L‐AmB as a possible alternative to voriconazole for primary therapy of invasive aspergillosis in some patients, particularly where drug‐drug interactions make the use of voriconazole problematic.21
MUCOCUTANEOUS CANDIDIASIS
Oropharyngeal candidiasis, or thrush, is a common infection in infants; those receiving antibiotics, chemotherapy or inhaled corticosteroids; and those with underlying immunodeficiency states. Esophageal candidiasis is most common in patients infected with HIV. Oral candidiasis usually does not cause symptoms, while esophageal disease is associated with odynophagia and dysphagia.
Candida albicans is the most common cause of mucocutaneous candidiasis. Treatment of thrush usually entails topical antifungal agents such as clotrimazole troches or nystatin, or oral azoles such as fluconazole or itraconazole. Topical therapy is ineffective for esophageal candidiasis, and oral or intravenous azoles are required as first‐line therapy with fluconazole being preferred. The treatment of oral and esophageal candidiasis is often complicated by recurrence, especially in immunodeficient patients, and resistance to standard treatments occurs frequently. Identification of Candida to the species level should be performed in the setting of refractory mucocutaneous disease, as this may play a role in the choice of therapy. The 2004 IDSA Guidelines, currently under revision, contain recommendations for treatment of refractory mucocutaneous candidiasis.4 The guidelines recommend a trial of oral itraconazole for fluconazole‐refractory thrush. Intravenous caspofungin and D‐AmB are usually effective alternatives. For treatment of fluconazole‐refractory esophageal disease, the guidelines recommend itraconazole solution, voriconazole, or caspofungin, with D‐AmB recommended as second line therapy, though it is now seldom used in this setting due to significant adverse affects. Experience using newer antifungals is increasing, and these data are summarized below.
Voriconazole has been shown at least as effective as fluconazole in the treatment of esophageal candidiasis in immunocompromised patients.29 A study involving 256 patients revealed success rates of 98% for voriconazole and 95% for fluconazole. C. albicans was the most common pathogen isolated. Perfect et al.30 demonstrated the utility of voriconazole for refractory esophageal candidiasis in 38 patients. A successful outcome was seen in 61% of patients treated with intravenous followed by oral voriconazole. The most common pathogen was C. albicans, although the series included several cases of infection with C. krusei.
Caspofungin was compared to D‐AmB for the treatment of esophageal candidiasis in a multicenter, double‐blind, randomized trial of 128 patients.31 Caspofungin appeared to be at least as effective as D‐AmB, with a significantly higher incidence of drug‐related adverse effects seen in the D‐AmB arm. Caspofungin was also compared to fluconazole in a double‐blind, randomized trial of 177 patients with Candida esophagitis. Favorable responses were seen in 81% and 85% of caspofungin and fluconazole treated patients, respectively. A trend toward higher relapse rate 4 weeks after stopping therapy was seen with caspofungin compared to fluconazole, as was a trend toward superior eradication rates for C. glabrata in the caspofungin arm compared to the fluconazole arm, although neither reached statistical significance.32
Micafungin was used for the treatment of esophageal candidiasis in a dose‐ranging trial of 245 HIV‐infected patients.33 Endoscopic combined cure rate for the 100 mg and 150 mg doses of micafungin (84%) was comparable to that of intravenous fluconazole 200 mg/day (87%). In the posttreatment period, 9 patients in the micafungin arm had a worsening of severity score or received nonprophylactic antifungal therapy. No patients in the fluconazole group experienced a relapse.
Anidulafungin has been compared with fluconazole for the treatment of Candida esophagitis in a randomized, double‐blind trial of 601 patients, with an initial endoscopic success rate approaching 100% in both groups.34 The 2‐week follow‐up examination revealed that 64% and 90% of patients treated with anidulafungin and fluconazole, respectively, sustained endoscopic success (P < 0.001).
Posaconazole was compared with fluconazole for treatment of thrush in 350 patients with HIV/acquired immunodeficiency syndrome (AIDS) in a randomized, blinded study.35 Both posaconazole and fluconazole were administered at a dose of 200 mg on day 1, followed by 100 mg/day. Clinical success occurred in 92% of patients receiving posaconazole and 93% receiving fluconazole. Mycological success was equivalent on day 14 in both arms; however, by day 42, significantly more posaconazole recipients continued to demonstrate mycological success. Posaconazole was recently evaluated for the treatment of azole‐refractory thrush and esophageal candidiasis in patients with advanced HIV infection, demonstrating a success rate of 75% in this population failing fluconazole or itraconazole therapy.36
Multiple new agents are available for the treatment of mucocutaneous candidiasis. Aside from topical antifungals for the initial treatment of thrush, fluconazole remains first line systemic therapy for both oral and esophageal candidiasis due to safety, tolerability, and cost. For fluconazole‐refractory disease, newer choices include voriconazole, the echinocandins, and posaconazole. Voriconazole and posaconazole are attractive options given their oral availability. The relapse rates seen in trials with the echinocandins are concerning; however, these are useful options when azole resistance is suspected.
ZYGOMYCOSIS
Zygomycosis (often referred to less correctly as mucormycosis) is a devastating opportunistic fungal infection that appears to be increasing in frequency. Historically, zygomycosis has commonly occurred in poorly controlled diabetic patients, particularly in the setting of diabetic ketoacidosis, and classically results in rhinocerebral disease with a relatively poor outcome. In recent years, a striking increase has been seen in patients with more profound immunosuppression, particularly those with hematologic malignancies or undergoing HSCT. Sinopulmonary rather than rhinocerebral disease is the most common manifestation in this population.3739 Other well‐described risk factors include iron chelation therapy with deferoxamine, intravenous drug use, solid organ transplantation, metabolic acidosis, trauma, and burns. Disease is also occasionally seen in the seemingly immunocompetent, with 176 of 929 (19%) patients in a comprehensive review lacking an obvious risk factor.37, 40
Invasive mold infections caused by the Zygomycetes are associated with a poor outcome, with Roden et al.37 reporting mortality in excess of 50% in their series. Mortality in patients with hematological malignancies has been reported to be particularly high.37, 38 The cornerstones of successful therapy include early detection of infection, correction or improvement of immunosuppression when possible, prompt surgical debridement of infected tissue, and appropriate antifungal therapy.40 D‐AmB has constituted standard zygomycosis therapy for decades, although it has recently been largely replaced by L‐AmB. Overall survival rates have been reported to be 61% and 69% with the use of D‐AmB and lipid preparations, respectively.37
Given the relatively poor outcomes and substantial infusion‐related toxicity and nephrotoxicity associated with even liposomal preparations of AmB, considerable interest exists in the identification of alternative therapeutic agents. Unfortunately, echinocandins and most triazoles appear to have modest to no activity against Zygomycetes, with a recent case‐control study indicating that widespread use of voriconazole in high‐risk populations may be helping to drive the emergence of breakthrough zygomycosis.39 Posaconazole appears to be an exception, however; with in vitro and murine studies suggesting it compares favorably to D‐AmB in this setting.4143 Numerous case reports describe favorable outcomes with the use of posaconazole as salvage therapy for zygomycosis, and 2 recent retrospective studies support its role in this setting.44, 45 Currently, use of posaconazole for the treatment of zygomycosis is limited by the absence of an intravenous preparation, although this is reportedly under development.46 At present, the role of posaconazole in this setting appears limited to step‐down therapy in those patients who have responded appropriately to L‐AmB, and for salvage therapy. Although an intravenous preparation of posaconazole appears attractive as a first‐line agent for zygomycosis, currently studied patients (ie, those unresponsive to or intolerant of D‐AmB) may not be fully representative of a broader population, and clinical trials will be necessary before more definitive conclusions may be drawn.47
ENDEMIC MYCOSES
Coccidioidomycosis
Coccidioidomycosis results from environmental exposure to either Coccidioides immitis or C. posadii. At least 50% of infections are asymptomatic, with the majority of the remaining individuals exhibiting acute, self‐limited pulmonary symptoms. A small percentage of patients develop chronic illness, either pulmonary or disseminated disease, including involvement of skin, bone/joint, and central nervous system (CNS).48, 49 Current therapy consists of either fluconazole or itraconazole for CNS disease and non‐life‐threatening disease elsewhere, with D‐AmB reserved for pregnancy and more fulminant illness.49 Unfortunately, response failures and relapses are seen commonly with all of these agents, with a resultant need for alternative antifungals.
The echinocandins have no clear role in the treatment of coccidioidomycosis.49 More interest surrounds the use of the newer azoles, with multiple studies demonstrating excellent in vitro activity of both voriconazole and posaconazole against Coccidioides species.5052 Several recently reported open‐label studies have reported good results with the use of posaconazole for chronic coccidioidomycosis, 2 of which enrolled patients intolerant of or refractory to usual agents.5355 Based on these data, posaconazole appears to be highly active against Coccidioides, and should perhaps be the drug of choice in the majority of patients who fail to respond to or tolerate older triazoles.
Histoplasmosis
Histoplasmosis is particularly endemic in the Ohio and Mississippi valleys, although it occurs less commonly in many other areas as well. Inhaled Histoplasma capsulatum conidia result in subclinical infection in the majority of exposed individuals, with self‐limited pneumonia the rule in most others. A minority of patients will experience chronic pulmonary disease or dissemination.56 Not all disease requires treatment, with most pulmonary disease resolving spontaneously; but definite indications for treatment include moderate or severe pneumonia, chronic cavitary lung disease, CNS involvement, and progressive disseminated disease.56 Standard therapy consists of itraconazole or lipid formulations of amphotericin B, based on severity. Multiple studies have demonstrated excellent in vitro activity of voriconazole and particularly posaconazole against H. capsulatum.52, 5759 Recently, in 2 small series of patients, patients failing either to improve with or tolerate conventional agents demonstrated favorable outcomes when they were treated with voriconazole or posaconazole.60, 61 Both drugs appear to be appropriate second‐line agents, with posaconazole arguably preferable based on current evidence.
CONCLUSIONS
The spectrum of available antifungal agents has expanded considerably in recent years, and the advent of additional drugs is expected shortly. Well‐tolerated and effective drugs are now available for most fungal infections, although the precise role for newer agents in some of these diseases has yet to be defined. Future clinical trials should help resolve these uncertainties.
Therapy of serious fungal infections, for decades largely limited to the deoxycholate (regular) preparation of amphotericin B (D‐AmB), expanded significantly with the introduction of fluconazole, followed by lipid‐based formulations of amphotericin B (L‐AmB) and itraconazole. More recently the antifungal armamentarium has broadened further with the approval of voriconazole and posaconazole, as well as the echinocandins caspofungin, micafungin, and anidulafungin. Clinicians, including hospitalists, primary care, emergency medicine, and critical care physicians, may find it challenging to remain abreast of indications for these novel agents, and we review these below, with a focus on adult patients. Manuscripts used in the review were identified by a search of English‐language articles in the PubMed MEDLINE database from 1994 to the present, using the keywords triazoles, echinocandins, voriconazole, posaconazole, caspofungin, micafungin, anidulafungin, candidemia, candidiasis, aspergillosis, invasive Aspergillus, zygomycosis, febrile neutropenia, endemic mycosis, histoplasmosis, and coccidioidomycosis. In addition, reference lists for the majority of the identified manuscripts were hand‐searched for additional pertinent citations.
Table 1 summarizes the newer systemic antifungal therapies and Table 2 summarizes the significant drug‐drug interactions with the newer antifungals.
| Antifungals | Trade Name | FDA‐Approved Indications | Usual Adult Dosing | Adverse Effects |
|---|---|---|---|---|
| ||||
| Azoles | ||||
| Voriconazole | Vfend | Invasive aspergillosis. | Intravenous: 6 mg/kg IV every 12 hours, then 4 mg/kg IV every 12 hours. | Transient visual disturbances (up to 30% in trials), rash, increases in hepatic enzymes, severe hepatotoxicity, and hallucinations. |
| Candidemia in nonneutropenic patients and the following Candida infections: disseminated infections in skin and infections in abdomen, kidney, bladder wall, and wounds. | Oral: 200 mg PO every 12 hours if 40 kg, 100 mg PO every 12 hours if <40 kg. | Accumulation of sulfobutyl ester ‐cyclodextrin, a solubilizing excipient, may occur in patients with creatinine clearance <50 mL/minute receiving the intravenous formulation. | ||
| Esophageal candidiasis. | ||||
| Fungal infections due to Scedosporium apiospermum (asexual form of Pseudallescheria boydii) and Fusarium spp. including Fusarium solani, in patients intolerant of, or refractory to, other therapy. | ||||
| Posaconazole | Noxafil | Prophylaxis of invasive Aspergillus and Candida infections in patients, 13 years of age and older, who are at high risk of developing these infections due to being severely immunocompromised, such as HSCT recipients with graft‐versus‐host disease or those with hematologic malignancies with prolonged neutropenia from chemotherapy. | Prophylaxis of invasive fungal infections: 200 mg (5 mL) PO TID. | Fever, headache, dry mouth, dizziness, fatigue, nausea, vomiting, diarrhea, rash, QT interval prolongation, and elevation of hepatic enzymes. |
| Oropharyngeal candidiasis, including oropharyngeal candidiasis refractory to itraconazole and/or fluconazole. | Oropharyngeal candidiasis: loading dose of 100 mg (2.5 mL) PO BID on day 1, then 100 mg (2.5 mL) PO once daily. | |||
| Oropharyngeal candidiasis refractory to itraconazole and/or fluconazole: 400 mg (10 mL) PO BID. | ||||
| To enhance oral absorption, administer with a full meal or liquid nutritional supplement. | ||||
| Echinocandins | ||||
| Caspofungin | Cancidas | Empirical therapy for presumed fungal infections in febrile, neutropenic patients. | All indications: 70 mg IV loading dose 1, followed by 50 mg IV daily. | Phlebitis, elevation of hepatic enzymes, headache, fever, nausea, vomiting, leukopenia, and histamine mediated symptoms including rash, pruritus, facial swelling, and vasodilatation. |
| Candidemia and the following Candida infections: intraabdominal abscesses, peritonitis, and pleural space infections. | No loading dose required for esophageal candidiasis. | |||
| Esophageal candidiasis. | ||||
| Invasive aspergillosis in patients who are refractory to or intolerant of other therapies (ie, amphotericin B, lipid formulations of amphotericin B, and/or itraconazole). | ||||
| Micafungin | Mycamine | Candidemia, acute disseminated candidiasis, Candida peritonitis and abscesses. | Candidemia, acute disseminated candidiasis, Candida peritonitis and abscesses: 100 mg IV daily. | Similar to caspofungin. |
| Esophageal candidiasis. | Esophageal candidiasis: 150 mg IV daily. | |||
| Prophylaxis of Candida infections in patients undergoing HSCT. | Prophylaxis of Candida infections in HSCT recipients: 50 mg IV daily. | |||
| Anidulafungin | Eraxis | Candidemia and other forms of Candida infections (intraabdominal abscess, peritonitis). | Candidemia/other Candida infections: 200 mg IV loading dose 1, followed by 100 mg IV daily. | Similar to caspofungin. |
| Esophageal candidiasis. | Esophageal candidiasis: 100 mg IV loading dose 1, followed by 50 mg IV Q daily thereafter. | |||
| Antifungal | Effect | Interacting Drugs |
|---|---|---|
| ||
| Voriconazole | Decreased azole serum concentration | Rifampin, rifabutin, carbamazepine, long‐acting barbiturates, efavirenz, high‐dose ritonavir (400 mg twice daily), phenytoin |
| Increased azole serum concentration | Oral contraceptives containing ethinyl estradiol and norethindrone, HIV protease inhibitors other than ritonavir, and nonnucleoside reverse transcriptase inhibitors other than efavirenz | |
| Increased serum concentration of coadministered drug | Sirolimus, rifabutin, efavirenz, terfenadine, astemizole, cisapride, pimozide, quinine, cyclosporine, methadone, tacrolimus, oral contraceptives containing ethinyl estradiol and norethindrone, HIV protease inhibitors other than ritonavir, nonnucleoside reverse transcriptase inhibitors other than efavirenz, benzodiazepines, HMG‐CoA reductase inhibitors, dihydropyridine calcium channel blockers, vinca alkaloids, omeprazole, phenytoin, warfarin, sulfonylurea oral hypoglycemics, and ergot alkaloids | |
| Posaconazole | Decreased azole serum concentration | Cimetidine, rifabutin, phenytoin |
| Increased serum concentration of coadministered drug | Cyclosporine, tacrolimus, rifabutin, midazolam, pheytoin, terfenidine, astemizole, pimozide, cisapride, quinidine, ergot alkaloids, vinca alkaloids, sirolimus, HMG Co‐A reductase inhibitors, and calcium channel blockers | |
| Caspofungin | Decreased serum concentration of caspofungin | Efavirenz, nevirapine, phenytoin, dexamethasone, and carbamazepine |
| Increased serum concentration of caspofungin | Cyclosporine | |
| Decreased serum concentration of coadministered drug | Tacrolimus | |
| Micafungin | Increased serum concentration of coadministered drug | Sirolimus, nifedipine, and itraconazole |
| Anidulafungin | No clinically relevant drug‐drug interactions | |
INVASIVE CANDIDIASIS
Candida has become a leading cause of nosocomial bloodstream infections, and is associated with an attributable mortality of 15% to 25%.1 Candidemia results in an estimated 10‐day increase in hospital length of stay, as well as an average $40,000 (US) increase in costs.2 Invasive candidiasis may be defined as catheter‐related candidemia, other hematogenously disseminated disease, or visceral involvement.3 Risk factors are present in most patients with invasive candidiasis, and include broad‐spectrum antibiotics; parenteral nutrition; central catheters; hospitalization in the intensive care unit setting; renal failure; burns; gastrointestinal and cardiac surgery; and colonization with Candida, particularly at multiple sites.1, 2
Historically, treatment of invasive candidiasis consisted of D‐AmB, with fluconazole largely but not completely replacing amphotericin after prospective trials demonstrated comparable efficacy with markedly improved tolerability. Fluconazole has poor or uncertain activity against C. krusei and C. glabrata, however, leading to reluctance on the part of many clinicians to use it for non‐C. albicans infection (or empirically in the unstable patient). Others have raised concerns regarding the use of fluconazole even for C. albicans in the setting of an unstable or neutropenic patient, given its fungistatic rather than fungicidal activity, although this is a theoretical rather than proven shortcoming.1 Current Infectious Diseases Society of America (IDSA) guidelines for the treatment of candidemia recommend the use of caspofungin, fluconazole, D‐AmB, or the combination of D‐AmB and fluconazole.4 The IDSA recommendations are under revision, however, and we summarize newer evidence below.
Mora‐Duarte et al.,5 in a 2002 trial, randomized patients with invasive candidiasis to caspofungin or D‐AmB, and found a favorable response in 73% and 62%, respectively, which fell just short of statistical significance. Caspofungin was better tolerated than D‐AmB, and the authors concluded that caspofungin was at least as effective as D‐AmB, with fewer adverse effects.5 A 2007 study randomized invasive candidiasis patients to micafungin or L‐AmB, and reported similar efficacy in both arms, with less drug‐related adverse events in the echinocandin‐treated group.6 Reboli et al.7 conducted a noninferiority trial comparing anidulafungin to fluconazole, and found a significantly superior outcome in the anidulafungin arm. Perhaps surprisingly, the outcome difference between the 2 groups was greater for C. albicans than for any other species.7 Although the large majority of patients in the preceding trials had candidemia, one study demonstrated a favorable response to caspofungin in 81% of patients with invasive candidal infections other than candidemia.8
Fewer data exist regarding the use of newer azoles for the treatment of invasive candidiasis. Ostrosky‐Zeichner et al.3 utilized voriconazole as salvage therapy in 52 patients with invasive candidiasis either refractory to or intolerant of other antifungals (almost all of whom had failed therapy with D‐AmB and/or other azoles), and found a 56% favorable response rate in this challenging population. More recently, Kullberg et al.9 studied voriconazole versus D‐AmB followed by fluconazole in candidemic patients, with a similar outcome but somewhat better tolerability in the voriconazole arm. We are unaware of comparative studies involving posaconazole for invasive candidiasis.
In summary, although fluconazole is the drug of choice for most invasive candidal infections, the initial use of an echinocandin should be considered when infection with a non‐C. albicans species is likely, particularly if the patient is unstable. Provided the organism later proves likely to be sensitive, switching to fluconazole is reasonable, particularly given the absence of an oral echinocandin formulation. The 3 currently available echinocandins appear to be interchangeable for the treatment of serious Candida infections.
NEUTROPENIC FEVER
Neutropenia is the most critical factor leading to infection in patients with cancer. Empiric treatment with broad‐spectrum antimicrobials should be initiated at the first sign of infection, since delay can lead to increased mortality.10 There are numerous causes for fever in the neutropenic host, although bacterial infection is most common. Fungal infections can cause unexplained fever and should be considered in neutropenic patients who remain febrile despite broad‐spectrum antibiotics.
Fungal infections in the neutropenic host can have severe consequences. Given their high morbidity and mortality and a lack of effective diagnostic techniques for early detection, empiric antifungal therapy is mandatory in the appropriate setting. Antifungal therapy should be considered in patients who remain febrile and neutropenic for 5 days despite broad‐spectrum antibiotics. The most common fungal pathogens include Candida and Aspergillus spp.11 Other considerations include the emergence of non‐albicans Candida infections and other opportunistic pathogens such as Zygomycetes (Mucor and related pathogens), Fusarium spp, and Scedosporium spp.
Empiric antifungal coverage in the neutropenic host has evolved over the past 2 decades, with the first trials demonstrating the utility of empiric antifungal treatment in the neutropenic host published in the 1980s. These trials demonstrated that addition of D‐AmB to broad spectrum antibiotics decreased development of fungal infections, and led to better outcomes.12, 13 While these studies established D‐AmB as standard empiric antifungal therapy in neutropenic fever, nephrotoxicity and infusion‐related reactions limited its subsequent use as less toxic alternatives were developed. The lipid formulations of amphotericin B, in particular liposomal AmB and amphotericin B lipid complex, have been shown to be as effective as D‐AmB for empiric treatment of febrile neutropenia, with less toxicities but significantly higher expense.14, 15 The older generation azoles itraconazole and fluconazole have also been studied. Itraconazole has been proven to be as effective as D‐AmB in febrile neutropenia with less toxicity; however, the oral capsule has erratic absorption and should be used cautiously.16
Newer agents studied for use in febrile neutropenia include caspofungin and voriconazole. Caspofungin is active against azole‐resistant Candida spp and Aspergillus spp with a favorable toxicity profile, making it an attractive candidate for use in febrile neutropenia. Caspofungin was compared to L‐AmB as empiric antifungal therapy in a randomized double‐blind trial of 1,095 patients with febrile neutropenia.17 The overall success rate was essentially identical for both agents, demonstrating noninferiority of caspofungin therapy. Among patients with baseline fungal infections, significantly more patients receiving caspofungin than L‐AmB had successful outcomes (52% versus 26%, P = 0.04). Overall, caspofungin was better tolerated and associated with fewer complications than L‐AmB.17 The other available echinocandins, micafungin and anidulafungin, have not yet been studied for febrile neutropenia in randomized, controlled fashion.
Voriconazole is a second‐generation azole with activity against fluconazole‐resistant Candida strains; however, the minimum inhibitory concentrations (MICs) are proportionally higher, suggesting a possible cross‐resistance mechanism among highly azole‐resistant strains.18 Voriconazole is active against most Aspergillus spp, Fusarium spp, and Scedosporium apiospermum.19 Voriconazole was compared to L‐AmB in an open‐label, randomized trial of 837 patients with febrile neutropenia.20 Patients were stratified according to risk of fungal infection and previous antifungal prophylaxis. Toxic side effects were similar in both groups. Less breakthrough fungal infections were seen in the voriconazole group; however, there were more discontinuations due to lack of efficacy in patients receiving voriconazole compared to L‐AmB. The overall success rate was 26% with voriconazole and 31% with L‐AmB (95% confidence interval [CI] for absolute difference in success rates: 10.6% to 1.6%), with the low figures reflective not only of infection severity, but also gravity of underlying disease, persistent fever presumably not of fungal origin, and adverse drug effects. Because the predetermined definition of noninferiority for the confidence interval difference between the groups was not met, the U.S. Food and Drug Administration (FDA) voted against approval of voriconazole for febrile neutropenia.
Overall, the role of newer antifungals in the treatment of febrile neutropenia is evolving. Based on current evidence, we prefer caspofungin as the treatment of choice for patients with febrile neutropenia because of its low toxicity profile and good clinical spectrum against most likely pathogens. D‐AmB has long been the gold standard; however, due to toxicity concerns, lipid‐based formulations have largely replaced it, with a notable increase in cost. Voriconazole cannot be recommended at this time based on failure to meet the noninferiority endpoint when compared to L‐AmB. However, for cases in which there is a high suspicion of invasive aspergillosis infection, voriconazole should be considered.
INVASIVE ASPERGILLOSIS
Invasive aspergillosis infection has become an increasing threat in immunocompromised patients, including those treated for cancer, undergoing organ transplantation, or with advanced human immunodeficiency virus (HIV) infection. In particular, patients being treated for hematologic malignancies and those undergoing hematopoietic stem cell transplant (HSCT) are at highest risk, due to prolonged, severe neutropenia. Infection with invasive aspergillosis also occurs when steroids are used for treatment of graft‐versus‐host disease in the HSCT population.
Aspergillus species are saprobic molds found ubiquitously in nature. Most diseases are caused by Aspergillus fumigatus, followed by A. flavus, A. niger, and A. terreus. Infection with Aspergillus can cause a wide spectrum of illnesses, ranging from allergic reactions to fulminant, lethal infections. The lungs are the most common site of primary invasive disease and are associated with high mortality, especially in severely immunocompromised patients.21 Infection is rapidly progressive and can be refractory to treatment, due to the organism's ability to grow quickly and invade blood vessels. Susceptible patients are unable to control infection and thus at high risk for dissemination and death. Prompt administration of an effective antifungal agent is necessary upon suspicion of invasive disease.
The choice of antifungals for invasive Aspergillus infection has grown significantly over the past decade. Current FDA‐approved agents with activity and indications for Aspergillus infection include D‐AmB and its lipid formulations, itraconazole, voriconazole, posaconazole, and caspofungin. D‐AmB and voriconazole are the only agents licensed in the US for the primary treatment of invasive aspergillosis, with D‐AmB the sole therapeutic option until recently. The lipid formulations of amphotericin B, itraconazole, and caspofungin are approved for salvage therapy. Posaconazole is licensed for prophylaxis of invasive aspergillosis in patients who are severely immunocompromised, including those with HSCT and graft‐versus‐host disease as well as those with hematologic malignancies and prolonged neutropenia. Besides caspofungin, the other available echinocandins, micafungin and anidulafungin, are active against Aspergillus species, but not yet FDA‐approved for this indication.
Voriconazole has replaced D‐AmB as the primary treatment of invasive pulmonary aspergillosis.21 Voriconazole was compared to D‐AmB in a randomized, multicenter, open‐label trial of 277 immunocompromised patients with definite or probable disease. The underlying condition in most patients was acute leukemia or allogeneic HSCT, and the majority of patients had invasive pulmonary disease. A successful outcome at week 12 was seen in 53% in the voriconazole group and 32% in the D‐AmB group, with survival rates of 71% and 58%, respectively; both differences were statistically significant. There were more adverse events in the D‐AmB group. Overall, the authors concluded that initial therapy with voriconazole led to better responses, improved survival and fewer side effects than D‐AmB.22
Caspofungin and micafungin have been studied for use as salvage therapy in invasive Aspergillus infection. Caspofungin was studied in 83 patients with invasive aspergillosis refractory to or intolerant of D‐AmB, lipid formulations of amphotericin B, or triazoles, most of whom had hematologic malignancy and allogeneic HSCT. The majority of patients had invasive pulmonary aspergillosis, and a favorable response was seen in 45% of this extremely high‐risk population.23 Micafungin was evaluated in a phase II study as primary or salvage therapy for invasive aspergillosis in adults and children. Of the patients receiving micafungin alone, those receiving the drug as primary therapy had a 50% (n = 6/12) response rate, compared to 41% (9/22) in the salvage therapy group.24 Optimal dosing of micafungin for the treatment of Aspergillus has not yet been established.
Posaconazole, the newest triazole antifungal, has been shown to be effective for the prevention of invasive aspergillosis in immunocompromised patients25, 26 and has also been studied for the treatment of invasive disease. In an open‐label trial, patients with invasive aspergillosis refractory or intolerant to conventional therapy were administered posaconazole, with historical controls as a comparator group.27 The majority of patients had underlying hematologic malignancies with approximately half undergoing HSCT, and most patients had pulmonary infection. The overall success rate was 42% for posaconazole and 26% for the control group. Posaconazole appeared to confer a survival benefit over control at 30 days and end of therapy (P = 0.0003).
Based on current data, we recommend voriconazole for primary treatment of invasive pulmonary aspergillosis. Alternatives include L‐AmB, caspofungin, micafungin, or posaconazole; of these agents, only L‐AmB has been studied as primary (as opposed to salvage) therapy for invasive aspergillosis in a reasonably‐powered trial.28 We agree with current IDSA guidelines, which suggest L‐AmB as a possible alternative to voriconazole for primary therapy of invasive aspergillosis in some patients, particularly where drug‐drug interactions make the use of voriconazole problematic.21
MUCOCUTANEOUS CANDIDIASIS
Oropharyngeal candidiasis, or thrush, is a common infection in infants; those receiving antibiotics, chemotherapy or inhaled corticosteroids; and those with underlying immunodeficiency states. Esophageal candidiasis is most common in patients infected with HIV. Oral candidiasis usually does not cause symptoms, while esophageal disease is associated with odynophagia and dysphagia.
Candida albicans is the most common cause of mucocutaneous candidiasis. Treatment of thrush usually entails topical antifungal agents such as clotrimazole troches or nystatin, or oral azoles such as fluconazole or itraconazole. Topical therapy is ineffective for esophageal candidiasis, and oral or intravenous azoles are required as first‐line therapy with fluconazole being preferred. The treatment of oral and esophageal candidiasis is often complicated by recurrence, especially in immunodeficient patients, and resistance to standard treatments occurs frequently. Identification of Candida to the species level should be performed in the setting of refractory mucocutaneous disease, as this may play a role in the choice of therapy. The 2004 IDSA Guidelines, currently under revision, contain recommendations for treatment of refractory mucocutaneous candidiasis.4 The guidelines recommend a trial of oral itraconazole for fluconazole‐refractory thrush. Intravenous caspofungin and D‐AmB are usually effective alternatives. For treatment of fluconazole‐refractory esophageal disease, the guidelines recommend itraconazole solution, voriconazole, or caspofungin, with D‐AmB recommended as second line therapy, though it is now seldom used in this setting due to significant adverse affects. Experience using newer antifungals is increasing, and these data are summarized below.
Voriconazole has been shown at least as effective as fluconazole in the treatment of esophageal candidiasis in immunocompromised patients.29 A study involving 256 patients revealed success rates of 98% for voriconazole and 95% for fluconazole. C. albicans was the most common pathogen isolated. Perfect et al.30 demonstrated the utility of voriconazole for refractory esophageal candidiasis in 38 patients. A successful outcome was seen in 61% of patients treated with intravenous followed by oral voriconazole. The most common pathogen was C. albicans, although the series included several cases of infection with C. krusei.
Caspofungin was compared to D‐AmB for the treatment of esophageal candidiasis in a multicenter, double‐blind, randomized trial of 128 patients.31 Caspofungin appeared to be at least as effective as D‐AmB, with a significantly higher incidence of drug‐related adverse effects seen in the D‐AmB arm. Caspofungin was also compared to fluconazole in a double‐blind, randomized trial of 177 patients with Candida esophagitis. Favorable responses were seen in 81% and 85% of caspofungin and fluconazole treated patients, respectively. A trend toward higher relapse rate 4 weeks after stopping therapy was seen with caspofungin compared to fluconazole, as was a trend toward superior eradication rates for C. glabrata in the caspofungin arm compared to the fluconazole arm, although neither reached statistical significance.32
Micafungin was used for the treatment of esophageal candidiasis in a dose‐ranging trial of 245 HIV‐infected patients.33 Endoscopic combined cure rate for the 100 mg and 150 mg doses of micafungin (84%) was comparable to that of intravenous fluconazole 200 mg/day (87%). In the posttreatment period, 9 patients in the micafungin arm had a worsening of severity score or received nonprophylactic antifungal therapy. No patients in the fluconazole group experienced a relapse.
Anidulafungin has been compared with fluconazole for the treatment of Candida esophagitis in a randomized, double‐blind trial of 601 patients, with an initial endoscopic success rate approaching 100% in both groups.34 The 2‐week follow‐up examination revealed that 64% and 90% of patients treated with anidulafungin and fluconazole, respectively, sustained endoscopic success (P < 0.001).
Posaconazole was compared with fluconazole for treatment of thrush in 350 patients with HIV/acquired immunodeficiency syndrome (AIDS) in a randomized, blinded study.35 Both posaconazole and fluconazole were administered at a dose of 200 mg on day 1, followed by 100 mg/day. Clinical success occurred in 92% of patients receiving posaconazole and 93% receiving fluconazole. Mycological success was equivalent on day 14 in both arms; however, by day 42, significantly more posaconazole recipients continued to demonstrate mycological success. Posaconazole was recently evaluated for the treatment of azole‐refractory thrush and esophageal candidiasis in patients with advanced HIV infection, demonstrating a success rate of 75% in this population failing fluconazole or itraconazole therapy.36
Multiple new agents are available for the treatment of mucocutaneous candidiasis. Aside from topical antifungals for the initial treatment of thrush, fluconazole remains first line systemic therapy for both oral and esophageal candidiasis due to safety, tolerability, and cost. For fluconazole‐refractory disease, newer choices include voriconazole, the echinocandins, and posaconazole. Voriconazole and posaconazole are attractive options given their oral availability. The relapse rates seen in trials with the echinocandins are concerning; however, these are useful options when azole resistance is suspected.
ZYGOMYCOSIS
Zygomycosis (often referred to less correctly as mucormycosis) is a devastating opportunistic fungal infection that appears to be increasing in frequency. Historically, zygomycosis has commonly occurred in poorly controlled diabetic patients, particularly in the setting of diabetic ketoacidosis, and classically results in rhinocerebral disease with a relatively poor outcome. In recent years, a striking increase has been seen in patients with more profound immunosuppression, particularly those with hematologic malignancies or undergoing HSCT. Sinopulmonary rather than rhinocerebral disease is the most common manifestation in this population.3739 Other well‐described risk factors include iron chelation therapy with deferoxamine, intravenous drug use, solid organ transplantation, metabolic acidosis, trauma, and burns. Disease is also occasionally seen in the seemingly immunocompetent, with 176 of 929 (19%) patients in a comprehensive review lacking an obvious risk factor.37, 40
Invasive mold infections caused by the Zygomycetes are associated with a poor outcome, with Roden et al.37 reporting mortality in excess of 50% in their series. Mortality in patients with hematological malignancies has been reported to be particularly high.37, 38 The cornerstones of successful therapy include early detection of infection, correction or improvement of immunosuppression when possible, prompt surgical debridement of infected tissue, and appropriate antifungal therapy.40 D‐AmB has constituted standard zygomycosis therapy for decades, although it has recently been largely replaced by L‐AmB. Overall survival rates have been reported to be 61% and 69% with the use of D‐AmB and lipid preparations, respectively.37
Given the relatively poor outcomes and substantial infusion‐related toxicity and nephrotoxicity associated with even liposomal preparations of AmB, considerable interest exists in the identification of alternative therapeutic agents. Unfortunately, echinocandins and most triazoles appear to have modest to no activity against Zygomycetes, with a recent case‐control study indicating that widespread use of voriconazole in high‐risk populations may be helping to drive the emergence of breakthrough zygomycosis.39 Posaconazole appears to be an exception, however; with in vitro and murine studies suggesting it compares favorably to D‐AmB in this setting.4143 Numerous case reports describe favorable outcomes with the use of posaconazole as salvage therapy for zygomycosis, and 2 recent retrospective studies support its role in this setting.44, 45 Currently, use of posaconazole for the treatment of zygomycosis is limited by the absence of an intravenous preparation, although this is reportedly under development.46 At present, the role of posaconazole in this setting appears limited to step‐down therapy in those patients who have responded appropriately to L‐AmB, and for salvage therapy. Although an intravenous preparation of posaconazole appears attractive as a first‐line agent for zygomycosis, currently studied patients (ie, those unresponsive to or intolerant of D‐AmB) may not be fully representative of a broader population, and clinical trials will be necessary before more definitive conclusions may be drawn.47
ENDEMIC MYCOSES
Coccidioidomycosis
Coccidioidomycosis results from environmental exposure to either Coccidioides immitis or C. posadii. At least 50% of infections are asymptomatic, with the majority of the remaining individuals exhibiting acute, self‐limited pulmonary symptoms. A small percentage of patients develop chronic illness, either pulmonary or disseminated disease, including involvement of skin, bone/joint, and central nervous system (CNS).48, 49 Current therapy consists of either fluconazole or itraconazole for CNS disease and non‐life‐threatening disease elsewhere, with D‐AmB reserved for pregnancy and more fulminant illness.49 Unfortunately, response failures and relapses are seen commonly with all of these agents, with a resultant need for alternative antifungals.
The echinocandins have no clear role in the treatment of coccidioidomycosis.49 More interest surrounds the use of the newer azoles, with multiple studies demonstrating excellent in vitro activity of both voriconazole and posaconazole against Coccidioides species.5052 Several recently reported open‐label studies have reported good results with the use of posaconazole for chronic coccidioidomycosis, 2 of which enrolled patients intolerant of or refractory to usual agents.5355 Based on these data, posaconazole appears to be highly active against Coccidioides, and should perhaps be the drug of choice in the majority of patients who fail to respond to or tolerate older triazoles.
Histoplasmosis
Histoplasmosis is particularly endemic in the Ohio and Mississippi valleys, although it occurs less commonly in many other areas as well. Inhaled Histoplasma capsulatum conidia result in subclinical infection in the majority of exposed individuals, with self‐limited pneumonia the rule in most others. A minority of patients will experience chronic pulmonary disease or dissemination.56 Not all disease requires treatment, with most pulmonary disease resolving spontaneously; but definite indications for treatment include moderate or severe pneumonia, chronic cavitary lung disease, CNS involvement, and progressive disseminated disease.56 Standard therapy consists of itraconazole or lipid formulations of amphotericin B, based on severity. Multiple studies have demonstrated excellent in vitro activity of voriconazole and particularly posaconazole against H. capsulatum.52, 5759 Recently, in 2 small series of patients, patients failing either to improve with or tolerate conventional agents demonstrated favorable outcomes when they were treated with voriconazole or posaconazole.60, 61 Both drugs appear to be appropriate second‐line agents, with posaconazole arguably preferable based on current evidence.
CONCLUSIONS
The spectrum of available antifungal agents has expanded considerably in recent years, and the advent of additional drugs is expected shortly. Well‐tolerated and effective drugs are now available for most fungal infections, although the precise role for newer agents in some of these diseases has yet to be defined. Future clinical trials should help resolve these uncertainties.
- ,,.Current treatment strategies for disseminated candidiasis.Clin Infect Dis.2006;42:244–251.
- .Echinocandins for candidemia in adults without neutropenia.N Engl J Med.2006;355:1154–1159.
- ,,,.Voriconazole salvage treatment of invasive candidiasis.Eur J Clin Microbiol Infect Dis.2003;22:651–655.
- ,,, et al.Guidelines for treatment of candidiasis.Clin Infect Dis.2004;38:161–189.
- ,,, et al.Comparison of caspofugin and amphotericin B for invasive candidiasis.N Engl J Med.2002;347:2020–2029.
- ,,, et al.Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomized double‐blind trial.Lancet.2007;369:1519–1527.
- ,,, et al.Anidulafungin versus fluconazole for invasive candidiasis.N Engl J Med.2007;356:2472–2482.
- ,,, et al.Caspofungin for the treatment of less common forms of invasive candidiasis.J Antimicrob Chemother.2007;60:363–369.
- ,,, et al.Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non‐neutropenic patients: a randomized non‐inferiority trial.Lancet.2005;366:1435–1442.
- ,.Fever of unknown origin in febrile leucopenia.Infect Dis Clin North Am.2007;21:1055–1090.
- ,,, et al.2002 Guidelines for the use of antimicrobial agents in neutropenic patients with cancer.Clin Infect Dis.2002;34:730–751.
- ,,, et al.Empiric antibiotic and antifungal therapy for cancer patients with prolonged fever and granulocytopenia.Am J Med.1982;72:101–111.
- EORTC International Antimicrobial Therapy Cooperative Group.Empiric antifungal therapy in febrile granulocytopenic patients.Am J Med.1989;86:668–672.
- ,,, et al.Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia.N Engl J Med.1999;340:7644–7671.
- ,,, et al.A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patients.Br J Haematol.1997;98:711–718.
- ,,, et al.Pharmacokinetics and safety of a 7 day administration of intravenous itraconazole followed by a 14‐day administration of itraconazole oral solution in patients with hematologic malignancy.Antimicrob Agents Chemother.2001;45:981–985.
- ,,, et al.Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia.N Engl J Med.2004;351:1392–1402.
- ,,, et al.In vitro activities of voriconazole and four other antifungal agents against 394 clinical isolates of Candida spp.Antimicrob Agents Chemother.1998;42:161–163.
- ,,, et al.Antifungal activity of a new triazole, voriconazole (UK‐109,496) compared with three other antifungal agents tested against clinical isolates of filamentous fungi.Med Mycol.1998;36:433–436.
- ,,, et al.Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever.N Engl J Med.2002;346:225–234.
- ,,, et al.Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America.Clin Infect Dis.2008;46:327–360.
- ,,, et al.Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis.N Engl J Med.2002;347:408–415.
- ,,, et al.Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy.Clin Infect Dis.2004;39:1563–1571.
- ,,, et al.Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis.J Infect2006;53:337–349.
- ,,, et al.Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia.N Engl J Med.2007;356:348–359.
- ,,, et al.Posaconazole or fluconazole for prophylaxis in severe graft versus host disease.N Engl J Med.2007;356:335–347.
- ,,, et al.Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial.Clin Infect Dis.2007;44:2–12.
- ,,, et al.Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high‐loading dose regimen with standard dosing (AmBiLoad trial).Clin Infect Dis.2007;44:1289–1297.
- ,,, et al.A randomized, double‐blind, double‐dummy, multicenter trial of voriconazole and fluconazole in the treatment of esophageal candidiasis in immunocompromised patients.Clin Infect Dis.2001;33:1447–1454.
- ,,, et al.Voriconazole treatment for less‐common, emerging, or refractory fungal infections.Clin Infect Dis.2003;36:1122–1131.
- ,,, et al.A randomized double‐blind study of caspofungin versus amphotericin for the treatment of candidal esophagitis.Clin Infect Dis.2001;33:1529–1535.
- ,,, et al.A randomized double‐blind study of caspofungin versus fluconazole for the treatment of esophageal candidiasis.Am J Med.2002;113:294–299.
- ,,, et al.A randomized, double‐blind, parallel‐group, dose‐response study of micafungin compared with fluconazole for the treatment of esophageal candidiasis in HIV‐positive patients.Clin Infect Dis.2004;39:842–849.
- ,,, et al.A randomized, double‐blind trial of anidulafungin versus fluconazole for the treatment of esophageal candidiasis.Clin Infect Dis.2004;39:770–775.
- ,,, et al.A multicenter randomized trial evaluating posaconazole versus fluconazole for the treatment of oropharyngeal candidiasis in subjects with HIV/AIDS.Clin Infect Dis.2006;42:1179–1186.
- ,,, et al.Posaconazole for the treatment of azole‐refractory oropharyngeal and esophageal candidiasis in subjects with HIV infection.Clin Infect Dis.2007;44:607–614.
- ,,, et al.Epidemiology and outcome of zygomycosis: a review of 929 reported cases.Clin Infect Dis.2005;41:634–653.
- ,,,.Zygomycosis in the 1990s in a tertiary‐care cancer center.Clin Infect Dis.2000;30:851–856.
- ,,, et al.Zygomycosis in a tertiary‐care center in the era of Aspergillus‐active antifungal therapy: a case‐control observational study of 27 recent cases.J Infect Dis.2005;191:1350–1360.
- ,.Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management.Infect Dis Clin North Am.2006;20:581–607.
- ,,,,.In vitro activities of posaconazole itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes.Antimicrob Agents Chemother.2002;46:1581–1582.
- ,,,,.In vivo activity of posaconazole against mucor spp. in an immunosuppressed‐mouse model.Antimicrob Agents Chemother.2002;46:2310–2312.
- ,,,,.In vitro susceptibilities of 217 clinical isolates of zygomycetes to conventional and new antifungal agents.Antimicrob Agents Chemother.2007;51:2587–2590.
- ,,, et al.Posaconazole as salvage therapy for zygomycosis.Antimicrob Agents Chemother.2006;50:126–133.
- ,,,,.Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases.Clin Infect Dis.2006;42:e61–e65.
- ,.Changing epidemiology of rare mould infections.Drugs.2007;67:1803–1812.
- .Posaconazole.Drugs.2005;65:1568–1569.
- ,,, et al.Coccidioidomycosis.Clin Infect Dis.2005;41:1217–1223.
- ,,.Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis.J Clin Microbiol.2007;4:26–30.
- ,,,.Therapeutic efficacy of caspofungin alone and in combination with amphotericin B deoxycholate for coccidioidomycosis in a mouse model.J Antimicrob Chemother.2007;60:1341–1346.
- ,.Antifungal susceptibility profiles of Coccidioides immitis and Coccidioides posadasii from endemic and non‐endemic areas.Mycopathologia.2007;163:31–19.
- ,,,,,.In vitro activities of voriconazole, itraconazole, and amphotericin B against Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum.Antimicrob Agents Chemother.2000;44:1734–1736.
- ,,,,.Refractory coccidioidomycosis treated with posaconazole.Clin Infect Dis.2005;40:1770–1776.
- ,,, et al.Safety, tolerance, and efficacy of posaconazole therapy in patients with nonmeningeal disseminated or chronic pulmonary coccidioidomycosis.Clin Infect Dis.2007;45:562–568.
- ,,, et al.Posaconazole therapy for chronic refractory coccidioidomycosis.Chest.2007;132:952–958.
- ,,, et al.Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America.Clin Infect Dis.2007;45:807–825.
- ,,,,.In vitro activities of new and established triazoles against opportunistic filamentous and dimorphic fungi.Med Mycol.2005;43:281–284.
- ,,, et al.Comparison of a new triazole antifungal agent, Schering 56592, with itraconazole and amphotericin B for treatment of histoplasmosis in immunocompetent mice.Antimicrob Agents Chemother.1999;439:322–328.
- ,,, et al.Activity of newer triazoles against Histoplasma capsulatum from patients with AIDS who failed fluconazole.J Antimicrob Chemother.2006;57:1235–1239.
- ,,,,,.Histoplasmosis in solid organ transplant recipients at a large midwestern university transplant center.Transpl Infect Dis.2005;7:109–115.
- ,,, et al.Salvage treatment of histoplasmosis with posaconazole.J Infect.2007;54:319–327.
- ,,.Current treatment strategies for disseminated candidiasis.Clin Infect Dis.2006;42:244–251.
- .Echinocandins for candidemia in adults without neutropenia.N Engl J Med.2006;355:1154–1159.
- ,,,.Voriconazole salvage treatment of invasive candidiasis.Eur J Clin Microbiol Infect Dis.2003;22:651–655.
- ,,, et al.Guidelines for treatment of candidiasis.Clin Infect Dis.2004;38:161–189.
- ,,, et al.Comparison of caspofugin and amphotericin B for invasive candidiasis.N Engl J Med.2002;347:2020–2029.
- ,,, et al.Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomized double‐blind trial.Lancet.2007;369:1519–1527.
- ,,, et al.Anidulafungin versus fluconazole for invasive candidiasis.N Engl J Med.2007;356:2472–2482.
- ,,, et al.Caspofungin for the treatment of less common forms of invasive candidiasis.J Antimicrob Chemother.2007;60:363–369.
- ,,, et al.Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non‐neutropenic patients: a randomized non‐inferiority trial.Lancet.2005;366:1435–1442.
- ,.Fever of unknown origin in febrile leucopenia.Infect Dis Clin North Am.2007;21:1055–1090.
- ,,, et al.2002 Guidelines for the use of antimicrobial agents in neutropenic patients with cancer.Clin Infect Dis.2002;34:730–751.
- ,,, et al.Empiric antibiotic and antifungal therapy for cancer patients with prolonged fever and granulocytopenia.Am J Med.1982;72:101–111.
- EORTC International Antimicrobial Therapy Cooperative Group.Empiric antifungal therapy in febrile granulocytopenic patients.Am J Med.1989;86:668–672.
- ,,, et al.Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia.N Engl J Med.1999;340:7644–7671.
- ,,, et al.A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patients.Br J Haematol.1997;98:711–718.
- ,,, et al.Pharmacokinetics and safety of a 7 day administration of intravenous itraconazole followed by a 14‐day administration of itraconazole oral solution in patients with hematologic malignancy.Antimicrob Agents Chemother.2001;45:981–985.
- ,,, et al.Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia.N Engl J Med.2004;351:1392–1402.
- ,,, et al.In vitro activities of voriconazole and four other antifungal agents against 394 clinical isolates of Candida spp.Antimicrob Agents Chemother.1998;42:161–163.
- ,,, et al.Antifungal activity of a new triazole, voriconazole (UK‐109,496) compared with three other antifungal agents tested against clinical isolates of filamentous fungi.Med Mycol.1998;36:433–436.
- ,,, et al.Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever.N Engl J Med.2002;346:225–234.
- ,,, et al.Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America.Clin Infect Dis.2008;46:327–360.
- ,,, et al.Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis.N Engl J Med.2002;347:408–415.
- ,,, et al.Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy.Clin Infect Dis.2004;39:1563–1571.
- ,,, et al.Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis.J Infect2006;53:337–349.
- ,,, et al.Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia.N Engl J Med.2007;356:348–359.
- ,,, et al.Posaconazole or fluconazole for prophylaxis in severe graft versus host disease.N Engl J Med.2007;356:335–347.
- ,,, et al.Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial.Clin Infect Dis.2007;44:2–12.
- ,,, et al.Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high‐loading dose regimen with standard dosing (AmBiLoad trial).Clin Infect Dis.2007;44:1289–1297.
- ,,, et al.A randomized, double‐blind, double‐dummy, multicenter trial of voriconazole and fluconazole in the treatment of esophageal candidiasis in immunocompromised patients.Clin Infect Dis.2001;33:1447–1454.
- ,,, et al.Voriconazole treatment for less‐common, emerging, or refractory fungal infections.Clin Infect Dis.2003;36:1122–1131.
- ,,, et al.A randomized double‐blind study of caspofungin versus amphotericin for the treatment of candidal esophagitis.Clin Infect Dis.2001;33:1529–1535.
- ,,, et al.A randomized double‐blind study of caspofungin versus fluconazole for the treatment of esophageal candidiasis.Am J Med.2002;113:294–299.
- ,,, et al.A randomized, double‐blind, parallel‐group, dose‐response study of micafungin compared with fluconazole for the treatment of esophageal candidiasis in HIV‐positive patients.Clin Infect Dis.2004;39:842–849.
- ,,, et al.A randomized, double‐blind trial of anidulafungin versus fluconazole for the treatment of esophageal candidiasis.Clin Infect Dis.2004;39:770–775.
- ,,, et al.A multicenter randomized trial evaluating posaconazole versus fluconazole for the treatment of oropharyngeal candidiasis in subjects with HIV/AIDS.Clin Infect Dis.2006;42:1179–1186.
- ,,, et al.Posaconazole for the treatment of azole‐refractory oropharyngeal and esophageal candidiasis in subjects with HIV infection.Clin Infect Dis.2007;44:607–614.
- ,,, et al.Epidemiology and outcome of zygomycosis: a review of 929 reported cases.Clin Infect Dis.2005;41:634–653.
- ,,,.Zygomycosis in the 1990s in a tertiary‐care cancer center.Clin Infect Dis.2000;30:851–856.
- ,,, et al.Zygomycosis in a tertiary‐care center in the era of Aspergillus‐active antifungal therapy: a case‐control observational study of 27 recent cases.J Infect Dis.2005;191:1350–1360.
- ,.Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management.Infect Dis Clin North Am.2006;20:581–607.
- ,,,,.In vitro activities of posaconazole itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes.Antimicrob Agents Chemother.2002;46:1581–1582.
- ,,,,.In vivo activity of posaconazole against mucor spp. in an immunosuppressed‐mouse model.Antimicrob Agents Chemother.2002;46:2310–2312.
- ,,,,.In vitro susceptibilities of 217 clinical isolates of zygomycetes to conventional and new antifungal agents.Antimicrob Agents Chemother.2007;51:2587–2590.
- ,,, et al.Posaconazole as salvage therapy for zygomycosis.Antimicrob Agents Chemother.2006;50:126–133.
- ,,,,.Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases.Clin Infect Dis.2006;42:e61–e65.
- ,.Changing epidemiology of rare mould infections.Drugs.2007;67:1803–1812.
- .Posaconazole.Drugs.2005;65:1568–1569.
- ,,, et al.Coccidioidomycosis.Clin Infect Dis.2005;41:1217–1223.
- ,,.Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis.J Clin Microbiol.2007;4:26–30.
- ,,,.Therapeutic efficacy of caspofungin alone and in combination with amphotericin B deoxycholate for coccidioidomycosis in a mouse model.J Antimicrob Chemother.2007;60:1341–1346.
- ,.Antifungal susceptibility profiles of Coccidioides immitis and Coccidioides posadasii from endemic and non‐endemic areas.Mycopathologia.2007;163:31–19.
- ,,,,,.In vitro activities of voriconazole, itraconazole, and amphotericin B against Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum.Antimicrob Agents Chemother.2000;44:1734–1736.
- ,,,,.Refractory coccidioidomycosis treated with posaconazole.Clin Infect Dis.2005;40:1770–1776.
- ,,, et al.Safety, tolerance, and efficacy of posaconazole therapy in patients with nonmeningeal disseminated or chronic pulmonary coccidioidomycosis.Clin Infect Dis.2007;45:562–568.
- ,,, et al.Posaconazole therapy for chronic refractory coccidioidomycosis.Chest.2007;132:952–958.
- ,,, et al.Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America.Clin Infect Dis.2007;45:807–825.
- ,,,,.In vitro activities of new and established triazoles against opportunistic filamentous and dimorphic fungi.Med Mycol.2005;43:281–284.
- ,,, et al.Comparison of a new triazole antifungal agent, Schering 56592, with itraconazole and amphotericin B for treatment of histoplasmosis in immunocompetent mice.Antimicrob Agents Chemother.1999;439:322–328.
- ,,, et al.Activity of newer triazoles against Histoplasma capsulatum from patients with AIDS who failed fluconazole.J Antimicrob Chemother.2006;57:1235–1239.
- ,,,,,.Histoplasmosis in solid organ transplant recipients at a large midwestern university transplant center.Transpl Infect Dis.2005;7:109–115.
- ,,, et al.Salvage treatment of histoplasmosis with posaconazole.J Infect.2007;54:319–327.
A Rash Decision
A 38‐year‐old HIV+ Ohio man with a recent CD4+ count of 534 cells/mL presented to his physician with 3 weeks of fever as high as 102F. He noted mild myalgias, pruritus, and an occasional cough but no headache, sore throat, dyspnea, rash, or gastrointestinal or genitourinary complaints. He had been seen elsewhere 2 weeks previously, when he had reported a single episode of receptive oral sex with a male partner several weeks earlier. He had been prescribed ciprofloxacin and azithromycin, but a throat swab came back negative for Chlamydia and Neisseria gonorrhoeae, and he reported no change in his symptoms after the course of antibiotics. He denied smoking or using street drugs. His only medications were citalopram and trazodone for depression.
This is a HIV+ man with a mild degree of immunosuppression with a fever of unknown origin (FUO). It is not yet known if the requisite basic infectious evaluation has been completed to meet this definition, but the duration certainly qualifies, and regardless of semantics, the FUO framework is a helpful starting point. The primary considerations in FUO are infections, neoplasms, and autoimmune illnesses. Autoimmune diseases are relatively less common in HIV patients. Although pruritis is quite common in HIV alone, it may also herald renal failure, cholestasis, or a malignancy (usually hematologic). Drugs must also be considered as a cause of unexplained fever; the pruritis might suggest an allergic reaction, although I do not think of citalopram or trazodone as having this effect. The failure to respond to broad‐spectrum antimicrobials (along with the duration) lowers my suspicion for common infections such as pneumonia, urinary tract infection, or cellulitis. Among sexually transmitted diseases, syphilis can be protean and merits consideration.
On examination he appeared well. His temperature was 102.4F, pulse 111 beats/min, blood pressure 138/78 mm Hg. The head, neck, cardiovascular system, and lungs appeared normal on examination. The abdomen was soft and nontender without organomegaly; skin, extremities, and neurological system were unremarkable. Rectal examination showed small anal condylomata. Hemoglobin was 14.3 g/dL, white blood cell count 6200/cm3, and platelet count 230,000/cm3. Serum electrolytes and lactate dehydrogenase were normal. The results of his liver function tests (LFTs) demonstrated a serum aspartate transaminase of 60 U/L (normal, 7‐40 U/L), alanine transaminase of 125 U/L (normal, 5‐50 U/L), alkaline phosphatase 218 U/L (normal, 40‐150 U/L), and total bilirubin 2.1 mg/dL (normal, 0.0‐1.5 mg/dL). Urinalysis demonstrated 2+ bilirubin and was otherwise normal. His erythrocyte sedimentation rate was 32 mm/hr (normal, 0‐15 mm/hr).
After 3 weeks of illness, his CBC demonstrates no signs of chronic illness (such as anemia of a chronic disease or a reactive leukocytosis or thrombocytosis). The results of his liver function tests showed moderate elevation, slightly more cholestatic than hepatocellular. This finding may reflect a disease process involving the liver, but such abnormal findings are often nonspecific in acute and chronic illnesses. With an unremitting fever, infectious complications in the liver merit early consideration. The time course rules out common biliary disorders such as cholangitis or cholecystitis. Pyogenic or amoebic liver abscesses are possible (homosexual men are at increased risk for the latter), but the absence of pain or abdominal tenderness is atypical. This biochemical profile can also be seen in chronic (but not acute) viral infections of the liver. Chronic hepatitis B and C predispose to hepatocellular carcinoma (HCC), which can be associated with fever. Cancers that infiltrate the liver, such as lymphoma or carcinoma, could also account for this picture. Indolent infections such as tuberculosis (TB) and syphilis are also possible, so associated signs of these systemic diseases should be sought. I do not believe either of his antibiotics is commonly associated with LFT abnormalities, and his CD4 count is too high for HIV cholangiopathy. In sum, a host of liver diseases are possible, but an extrahepatic systemic disease deserves equal attention.
His CD4+ count was 537 cells/mL, and his HIV RNA viral load was 44,300 copies/mL. Radiographs of the chest were normal. Two sets of blood cultures were negative. The rapid plasma reagin (RPR) was nonreactive. The results of serologies for acute hepatitis A, B, C, and E, chronic hepatitis B and C, and toxoplasmosis were negative. Testing for both Epstein‐Barr virus and cytomegalovirus showed evidence of remote infection. Results of serologies for bartonella species, human herpesviruses 6 and 7, and parvovirus B19 were negative.
The negative RPR makes disseminated (secondary) syphilis improbable, provided the prozone phenomenon has been excluded. An extensive serological workup is common in the evaluation of fever of unknown origin, although the threat of false‐positive results always looms when many studies are sent simultaneously. This must be considered in advance here, as his relatively preserved CD4 count affords him significant protection against many opportunistic infections. His HIV infection, however, regardless of CD4 count, increases his risk for TB and lymphoma, which remain high on my list. Both may be residing primarily in the liver. In FUO, the abdominal CT is frequently a high‐yield test (primarily by demonstrating unsuspected tumors and abscesses), even in the absence of symptoms, and would certainly be of interest here given the liver function test results. Imaging could diagnose febrile tumors such as lymphoma, HCC, or renal cell carcinoma. In the event that imaging is unrevealing, causes of granulomatous hepatitis should be entertained. The constellation of cough, LFT abnormalities, and fever is compatible with Q fever. As with any FUO case, I would also carefully revisit this patient's history to discern where he was born, where he has been, and what activities or exposures he is engaged in.
He was seen 2 days later with fever of 104F and new papules over his sternal area. Over the next week, he had intermittent fevers and severe fatigue. The rash progressed, predominantly involving his chest and back, but also his legs, arms, and face (see Fig. 1). The lesions spared his palms and soles. The exanthem was intensely pruritic and maculopapular, consisting of lesions with a diameter of 0.5 cm or less, with some scaling. There were no vesicles or pustular lesions. There were no other new findings on examination. His transaminase and bilirubin had normalized, and his CBC and electrolytes were unchanged. Repeat blood cultures held for extended incubation were negative. Computerized tomography of the chest, abdomen, and pelvis demonstrated mild lymphadenopathy at the porta hepatis with increased portocaval and periaortic lymphadenopathy.
The only LFT abnormality that persists is the elevated alkaline phosphatase, which suggests (1) that liver involvement was not specific and that there is a disease process involving the bone, (2) that there is a persistent infiltrative disorder of the liver such as infection or malignancy or, less likely, amyloidosis or sarcoidosis, or (3) that the porta hepatis lymphadenopathy is causing biliary obstruction. The underlying diagnosis must explain the rash, intraabdominal lymphadenopathy, and fever. The time course does somewhat limit the extensive differential of fever and rash. After 3 weeks of illness, some of the most life‐threatening entities such as meningococcal disease, Rocky Mountain spotted fever, and toxic shock syndrome are unlikely. Concern remains for infections that are more indolent, such as mycobacteria, fungi, or spirochetes. The most striking elements of the rash are the extensive distribution, rapid progression, large number, and discreteness of the lesions, which collectively point more toward disseminated fungal (eg, histoplasmosis, as he lives in Ohio), spirochetal, rickettsial, or viral etiologies, rather than bacterial or mycobacterial entities. The absence of vesicles detracts from the diagnosis of a disseminated herpes virus such as herpes simplex or varicella. I believe that this rash is too disseminated to be caused by a common mycobacterial illness. This extent of cutaneous metastases would usually accompany a far more ill patient with an obvious primary cancer (none is seen on imaging, including the liver), and it appears too extensive to be caused by a paraneoplastic phenomenon such as Sweet's syndrome. A systemic vasculitis or another autoimmune disease remains possible, but there is minimal evidence of visceral organ involvement. All the aforementioned diseases could explain the intraabdominal lymphadenopathy, but my suspicion is highest for infection. I would biopsy and culture the skin lesions, repeat the RPR and/or send a treponemal‐specific test, place a PPD skin test, and send fungal studies (serum serologies and urine antigens) for evaluation. If the results of these noninvasive studies are unrevealing, I would consider a liver biopsy.
The patient's medications were discontinued, and a skin biopsy of the rash from his chest showed atypical lymphohistiocytic infiltrates without acute inflammatory cells and with negative Gomori methenamine silver (GMS), acid‐fast bacilli (AFB), and Fite (for Nocardia) stains. The infiltrates were predominantly T cells with a 1:1 CD4:CD8 ratio. This was read as suspicious for cytotoxic (CD8) mycosis fungoides.
I do not have reason to doubt the pathologist's impression of mycosis fungoides on histopathologic grounds, but from a clinical standpoint, I do not think mycosis fungoides is a disease that has a prolonged febrile prodrome or an explosive cutaneous onset. Rather, it is frequently preceded by nonspecific skin findings over a long period. Thinking broadly and pathophysiologically and noting that T cells are the predominant lymphocytes in skin, I wonder if they could represent a nonmalignant, immunological reaction in the skin. The stains, although not perfectly sensitive, make mycobacterial and fungal diseases less likely, although incubation of cultures is necessary.
Over the next 10 days (bringing the total duration of the patient's illness to 6 weeks), the skin lesions increased in number. In the physician's office at his next follow‐up, the patient had a temperature of 104.1F, was uncomfortable, shivering, and ill‐appearing. His blood pressure was 108/66 mm Hg, and his pulse 114 beats/min. He complained of severe shooting pains, predominantly in his pretibial regions and arms. Examination showed no other new findings, including no focal neurological findings. The results of the T‐cell rearrangement study from the skin biopsy showed evidence of a monoclonal T‐cell population. He was admitted to the hospital for further evaluation and treatment.
The extremity dysesthesias could represent a lesion of the spinal cord (including the CSF/meninges), a polyradiculopathy, or a polyneuropathy. Unfortunately, this does not add a tremendous amount of diagnostic resolution, as infection, malignancy, and autoimmune syndromes, such as vasculitis, may all involve the nervous system in these ways. In general, I associate monoclonal lymphocyte responses with hematological malignancies and polyclonal responses with the less specific inflammation that could accompany infection, autoimmunity, or solid malignancies. His age, fever, and rapid progression seem atypical for mycosis fungoides, but given the monoclonal T cells, this must now be considered. Adult T‐cell leukemia/lymphoma, with its prominent skin manifestations and its association with HLTV‐1, is an alternative T‐cell malignancy that could explain the fever, neurological symptoms, and possible visceral involvement (elevated alkaline phosphatase, which could reflect liver or bone). In cases that are diagnostic challenges, one of the highest‐yield maneuvers is to repeat the preceding evaluation, starting with the history, exam, and basic labs, and if necessary, to review or repeat the imaging or skin biopsy. Given the elevated alkaline phosphatase, disseminated rash, new neurological symptoms, and his HIV status, I remain particularly concerned about syphilis and would do further testing (accounting for the prozone phenomenon) before proceeding with the malignancy evaluation.
A lumbar puncture demonstrated clear cerebrospinal fluid, with 2 leukocytes and 195 erythrocytes/cm3, protein of 26 mg/dL, and glucose of 52 mg/dL. Bacterial and fungal cultures of the fluid were negative. The results of colonoscopy were normal. A bone marrow biopsy demonstrated ring granulomas. GMS, AFB, Fite, and Steiner (for spirochetes) stains were negative, cultures of the aspirate were negative for bacteria, and smears were negative for fungi and mycobacteria. Antibody tests for human T‐cell lymphotropic virus types I and II, Coxiella burnetii, and Bartonella henselae were negative. The dermatology consultant believed the absence of lymphadenopathy and the pruritic nature of the lesions was atypical for cytotoxic T‐cell lymphoma (CTCL). Before initiating therapy for CTCL, she suggested repeating the skin biopsy and RPR.
The repeat RPR was positive at 1:64 dilutions, and a confirmatory fluorescent treponemal antibody absorption test showed a positive result. He was prescribed intramuscular benzathine pencillin 2.4 million units weekly for 3 weeks, with almost immediate defervescence and slower resolution of his rash and shooting pains in his limbs. The repeat skin biopsy done during the hospitalization demonstrated lichenoid‐type dermatitis with interstitial and perivascular lymphohistiocytic infiltrates and granulomas. Steiner stains for spirochetes were positive. Immunohistochemical stains ruled out a lymphoproliferative process. One year later his RPR was nonreactive.
COMMENTARY
Fever of unknown origin (FUO) was first defined by Petersdorf and Beeson in 1961 as a temperature higher than 38.3C on several occasions lasting longer than 3 weeks and defying diagnosis despite 1 week of inpatient investigation.1 Dramatic changes in medical practice have rendered this definition outdated, with more recent proposals allowing thoughtful outpatient investigation to serve as a surrogate for hospitalization. Some have proposed that HIV‐associated FUO be considered a distinct entity, with the most complete North American series finding the etiology of the HIV‐associated FUO in 56 of 70 patients.2 The mean CD4+ count in this series was 58/cm3. Disseminated M. avium was the most frequently diagnosed cause, followed by P. jirovecii pneumonia, cytomegalovirus infection, disseminated histoplasmosis, and lymphoma. Of 14 patients with fever of no definable etiology, 12 eventually proved to have self‐limiting illness.
Despite numerous attempts to reduce the investigation of the patient with FUO to an algorithm, the approach must be individualized. A thorough history and careful, serial physical examinations are frequently and appropriately stressed as the foundation, followed by thoughtful selection of laboratory and imaging studies. Although FUO has a lengthy differential diagnosis, it often proves to be, as Mackowiak and Durack stress, an unusual manifestation of a common disease, rather than a typical presentation of a rare disease.3 A relatively uncommon disease in conjunction with an initially negative diagnostic test result, as was the case with this patient, may lead to a protracted diagnostic puzzle.
Syphilis is a rare cause of FUO. In 6 large studies of a total of 947 patients published over a 40‐year period, only 2 cases of syphilis (1 secondary and 1 neurosyphilis) were reported.1, 48 Syphilis as a cause of prolonged cryptic fever appears to have been seen with greater frequency in the preantibiotic era.9 In the first half of the 20th century, syphilis was known as the great imitator, with its unusual manifestations recognized and indeed expected. As a result of the dramatically lower incidence of syphilis in recent decades, these lessons have largely been forgotten, however, which may lead to diagnostic confusion when syphilis presents atypically. The manifestations of secondary syphilis are protean, including a variety of rashes, aphthous ulcers, arthralgias, pharyngitis, weight loss, fever, meningitis, ocular symptoms, cranial nerve palsies, glomerulonephritis, hepatitis, and periostitis (which afflicted this patient, who complained of severe shooting pains in his arms and shins).
After declining in the last decade of the 20th century, the rates of primary and secondary syphilis are rising in the United States.10 Oral sex is a clear risk factor for syphilis transmission, particularly for men who have sex with men.11 Because of the patient's exposure history and clinical picture, his outpatient physician considered the diagnosis of secondary syphilis early in the course of his illness. The diagnosis was not entertained further when an RPR test, highly sensitive at this stage of the disease, returned nonreactive. Likewise, when a rash subsequently appeared, the lack of palm and sole involvement dissuaded multiple clinicians from reconsidering the diagnosis of syphilis. A skin biopsy that appeared to lead in a distinctly different direction understandably confused the picture still further. Even at the time of the lumbar puncture, VDRL of the CSF was not ordered.
In retrospect, the chief confounder in the case was the false‐negative RPR test, as the discussant suspected early on. Although nontreponemal tests are generally accurate in individuals with HIV, delayed seropositivity and false‐negatives have been reported in this population.12 The false‐negative could have also been a result of the prozone phenomenon, an unusual event, occurring in fewer than 2% of cases of secondary syphilis and attributed to a mismatch between antibody and very high antigen level. The prozone reaction can be corrected for by requesting dilution of the serum prior to repeating the test. Simple lab error must be considered as well, but without access to this patient's serum from his original testing, the cause of his initial false‐negative test cannot be known with certainty.
An unusual presentation in conjunction with failure to recognize the causes of rare false‐negative testing for secondary syphilis led to a delayed diagnosis in this patient. Although syphilis and mycosis fungoides have previously been reported to mimic one another both clinically and histopathologically, the potential for secondary syphilis to be misdiagnosed in this fashion is not generally appreciated.1315 Recognition of the possibility of secondary syphilis occurred just in time to spare this patient the rash decision of treating him with cytotoxic therapy directed against CTCL.
Teaching Points
-
HIV‐associated FUO can be a diagnostic challenge, but an etiology can be found in most cases.
-
Syphilis continues to be an unusual cause of FUO and can have protean manifestations affecting nearly every organ system
-
The sensitivity of RPR is extremely high in secondary syphilis, but false‐negative tests can be seen in HIV because of both the prozone phenomenon and a delayed rise in antibodies.
- ,.Fever of unexplained origin: Report on 100 cases.Medicine.1961;40:1–30.
- ,,.Human immunodeficiency virus‐associated fever of unknown origin: A study of 70 patients in the United States and review.Clin Infect Dis.1999;28:341–345.
- ,.Fever of unknown origin. In:Mandell GL,Bennett JE,Dolin R, eds.Principles and Practice of Infectious Diseases.6th ed.Philadelphia:Elsevier Churchill Livingstone;2005:718–729.
- ,,.Fever of unknown origin: Diagnosis and follow‐up of 105 cases, 1970‐1980.Medicine.1982;61:269–292.
- ,,,.Fever of unknown origin in the 1980s: An update of the diagnostic spectrum.Arch Intern Med.1992;152:51–55.
- .Fever of unknown origin: Review of 86 patients treated in community hospitals.Clin Infect Dis.1992;15:968–973.
- ,,.Fever of unknown origin (FUO). I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO study group.Medicine.1997;76:392–400.
- ,,, et al.From prolonged febrile illness to fever of unknown origin: The challenge continues.Arch Intern Med.2003;163:1033–1041.
- ,.The diagnosis of obscure fever. II. The diagnosis of unexplained high fever.Bull Johns Hopkins Hosp.1936;58:307–331.
- Centers for Disease Control and Prevention.Primary and secondary syphilis—United States, 2003–2004.MMWR.2006;55:269–273.
- Transmission of primary and secondary syphilis by oral sex—Chicago, Illinois, 1998‐2202.MMWR.2004;53:966–968.
- ,,, et al.Seronegative secondary syphilis in 2 patients coinfected with human immunodeficiency virus.Arch Dermatol.2005;141:431–433.
- ,,,.Secondary syphilis mimicking mycosis fungoides.J Am Acad Dermatol.1980;3:92–94
- ,.A case of lues maligna in a patient with acquired immunodeficiency syndrome (AIDS).Scand J Infect Dis.2005;37:697–700.
- ,,,,.Unusual presentation of secondary syphilis in 2 HIV‐1 positive patients.Cutis.2000;66:383–389.
A 38‐year‐old HIV+ Ohio man with a recent CD4+ count of 534 cells/mL presented to his physician with 3 weeks of fever as high as 102F. He noted mild myalgias, pruritus, and an occasional cough but no headache, sore throat, dyspnea, rash, or gastrointestinal or genitourinary complaints. He had been seen elsewhere 2 weeks previously, when he had reported a single episode of receptive oral sex with a male partner several weeks earlier. He had been prescribed ciprofloxacin and azithromycin, but a throat swab came back negative for Chlamydia and Neisseria gonorrhoeae, and he reported no change in his symptoms after the course of antibiotics. He denied smoking or using street drugs. His only medications were citalopram and trazodone for depression.
This is a HIV+ man with a mild degree of immunosuppression with a fever of unknown origin (FUO). It is not yet known if the requisite basic infectious evaluation has been completed to meet this definition, but the duration certainly qualifies, and regardless of semantics, the FUO framework is a helpful starting point. The primary considerations in FUO are infections, neoplasms, and autoimmune illnesses. Autoimmune diseases are relatively less common in HIV patients. Although pruritis is quite common in HIV alone, it may also herald renal failure, cholestasis, or a malignancy (usually hematologic). Drugs must also be considered as a cause of unexplained fever; the pruritis might suggest an allergic reaction, although I do not think of citalopram or trazodone as having this effect. The failure to respond to broad‐spectrum antimicrobials (along with the duration) lowers my suspicion for common infections such as pneumonia, urinary tract infection, or cellulitis. Among sexually transmitted diseases, syphilis can be protean and merits consideration.
On examination he appeared well. His temperature was 102.4F, pulse 111 beats/min, blood pressure 138/78 mm Hg. The head, neck, cardiovascular system, and lungs appeared normal on examination. The abdomen was soft and nontender without organomegaly; skin, extremities, and neurological system were unremarkable. Rectal examination showed small anal condylomata. Hemoglobin was 14.3 g/dL, white blood cell count 6200/cm3, and platelet count 230,000/cm3. Serum electrolytes and lactate dehydrogenase were normal. The results of his liver function tests (LFTs) demonstrated a serum aspartate transaminase of 60 U/L (normal, 7‐40 U/L), alanine transaminase of 125 U/L (normal, 5‐50 U/L), alkaline phosphatase 218 U/L (normal, 40‐150 U/L), and total bilirubin 2.1 mg/dL (normal, 0.0‐1.5 mg/dL). Urinalysis demonstrated 2+ bilirubin and was otherwise normal. His erythrocyte sedimentation rate was 32 mm/hr (normal, 0‐15 mm/hr).
After 3 weeks of illness, his CBC demonstrates no signs of chronic illness (such as anemia of a chronic disease or a reactive leukocytosis or thrombocytosis). The results of his liver function tests showed moderate elevation, slightly more cholestatic than hepatocellular. This finding may reflect a disease process involving the liver, but such abnormal findings are often nonspecific in acute and chronic illnesses. With an unremitting fever, infectious complications in the liver merit early consideration. The time course rules out common biliary disorders such as cholangitis or cholecystitis. Pyogenic or amoebic liver abscesses are possible (homosexual men are at increased risk for the latter), but the absence of pain or abdominal tenderness is atypical. This biochemical profile can also be seen in chronic (but not acute) viral infections of the liver. Chronic hepatitis B and C predispose to hepatocellular carcinoma (HCC), which can be associated with fever. Cancers that infiltrate the liver, such as lymphoma or carcinoma, could also account for this picture. Indolent infections such as tuberculosis (TB) and syphilis are also possible, so associated signs of these systemic diseases should be sought. I do not believe either of his antibiotics is commonly associated with LFT abnormalities, and his CD4 count is too high for HIV cholangiopathy. In sum, a host of liver diseases are possible, but an extrahepatic systemic disease deserves equal attention.
His CD4+ count was 537 cells/mL, and his HIV RNA viral load was 44,300 copies/mL. Radiographs of the chest were normal. Two sets of blood cultures were negative. The rapid plasma reagin (RPR) was nonreactive. The results of serologies for acute hepatitis A, B, C, and E, chronic hepatitis B and C, and toxoplasmosis were negative. Testing for both Epstein‐Barr virus and cytomegalovirus showed evidence of remote infection. Results of serologies for bartonella species, human herpesviruses 6 and 7, and parvovirus B19 were negative.
The negative RPR makes disseminated (secondary) syphilis improbable, provided the prozone phenomenon has been excluded. An extensive serological workup is common in the evaluation of fever of unknown origin, although the threat of false‐positive results always looms when many studies are sent simultaneously. This must be considered in advance here, as his relatively preserved CD4 count affords him significant protection against many opportunistic infections. His HIV infection, however, regardless of CD4 count, increases his risk for TB and lymphoma, which remain high on my list. Both may be residing primarily in the liver. In FUO, the abdominal CT is frequently a high‐yield test (primarily by demonstrating unsuspected tumors and abscesses), even in the absence of symptoms, and would certainly be of interest here given the liver function test results. Imaging could diagnose febrile tumors such as lymphoma, HCC, or renal cell carcinoma. In the event that imaging is unrevealing, causes of granulomatous hepatitis should be entertained. The constellation of cough, LFT abnormalities, and fever is compatible with Q fever. As with any FUO case, I would also carefully revisit this patient's history to discern where he was born, where he has been, and what activities or exposures he is engaged in.
He was seen 2 days later with fever of 104F and new papules over his sternal area. Over the next week, he had intermittent fevers and severe fatigue. The rash progressed, predominantly involving his chest and back, but also his legs, arms, and face (see Fig. 1). The lesions spared his palms and soles. The exanthem was intensely pruritic and maculopapular, consisting of lesions with a diameter of 0.5 cm or less, with some scaling. There were no vesicles or pustular lesions. There were no other new findings on examination. His transaminase and bilirubin had normalized, and his CBC and electrolytes were unchanged. Repeat blood cultures held for extended incubation were negative. Computerized tomography of the chest, abdomen, and pelvis demonstrated mild lymphadenopathy at the porta hepatis with increased portocaval and periaortic lymphadenopathy.

The only LFT abnormality that persists is the elevated alkaline phosphatase, which suggests (1) that liver involvement was not specific and that there is a disease process involving the bone, (2) that there is a persistent infiltrative disorder of the liver such as infection or malignancy or, less likely, amyloidosis or sarcoidosis, or (3) that the porta hepatis lymphadenopathy is causing biliary obstruction. The underlying diagnosis must explain the rash, intraabdominal lymphadenopathy, and fever. The time course does somewhat limit the extensive differential of fever and rash. After 3 weeks of illness, some of the most life‐threatening entities such as meningococcal disease, Rocky Mountain spotted fever, and toxic shock syndrome are unlikely. Concern remains for infections that are more indolent, such as mycobacteria, fungi, or spirochetes. The most striking elements of the rash are the extensive distribution, rapid progression, large number, and discreteness of the lesions, which collectively point more toward disseminated fungal (eg, histoplasmosis, as he lives in Ohio), spirochetal, rickettsial, or viral etiologies, rather than bacterial or mycobacterial entities. The absence of vesicles detracts from the diagnosis of a disseminated herpes virus such as herpes simplex or varicella. I believe that this rash is too disseminated to be caused by a common mycobacterial illness. This extent of cutaneous metastases would usually accompany a far more ill patient with an obvious primary cancer (none is seen on imaging, including the liver), and it appears too extensive to be caused by a paraneoplastic phenomenon such as Sweet's syndrome. A systemic vasculitis or another autoimmune disease remains possible, but there is minimal evidence of visceral organ involvement. All the aforementioned diseases could explain the intraabdominal lymphadenopathy, but my suspicion is highest for infection. I would biopsy and culture the skin lesions, repeat the RPR and/or send a treponemal‐specific test, place a PPD skin test, and send fungal studies (serum serologies and urine antigens) for evaluation. If the results of these noninvasive studies are unrevealing, I would consider a liver biopsy.
The patient's medications were discontinued, and a skin biopsy of the rash from his chest showed atypical lymphohistiocytic infiltrates without acute inflammatory cells and with negative Gomori methenamine silver (GMS), acid‐fast bacilli (AFB), and Fite (for Nocardia) stains. The infiltrates were predominantly T cells with a 1:1 CD4:CD8 ratio. This was read as suspicious for cytotoxic (CD8) mycosis fungoides.
I do not have reason to doubt the pathologist's impression of mycosis fungoides on histopathologic grounds, but from a clinical standpoint, I do not think mycosis fungoides is a disease that has a prolonged febrile prodrome or an explosive cutaneous onset. Rather, it is frequently preceded by nonspecific skin findings over a long period. Thinking broadly and pathophysiologically and noting that T cells are the predominant lymphocytes in skin, I wonder if they could represent a nonmalignant, immunological reaction in the skin. The stains, although not perfectly sensitive, make mycobacterial and fungal diseases less likely, although incubation of cultures is necessary.
Over the next 10 days (bringing the total duration of the patient's illness to 6 weeks), the skin lesions increased in number. In the physician's office at his next follow‐up, the patient had a temperature of 104.1F, was uncomfortable, shivering, and ill‐appearing. His blood pressure was 108/66 mm Hg, and his pulse 114 beats/min. He complained of severe shooting pains, predominantly in his pretibial regions and arms. Examination showed no other new findings, including no focal neurological findings. The results of the T‐cell rearrangement study from the skin biopsy showed evidence of a monoclonal T‐cell population. He was admitted to the hospital for further evaluation and treatment.
The extremity dysesthesias could represent a lesion of the spinal cord (including the CSF/meninges), a polyradiculopathy, or a polyneuropathy. Unfortunately, this does not add a tremendous amount of diagnostic resolution, as infection, malignancy, and autoimmune syndromes, such as vasculitis, may all involve the nervous system in these ways. In general, I associate monoclonal lymphocyte responses with hematological malignancies and polyclonal responses with the less specific inflammation that could accompany infection, autoimmunity, or solid malignancies. His age, fever, and rapid progression seem atypical for mycosis fungoides, but given the monoclonal T cells, this must now be considered. Adult T‐cell leukemia/lymphoma, with its prominent skin manifestations and its association with HLTV‐1, is an alternative T‐cell malignancy that could explain the fever, neurological symptoms, and possible visceral involvement (elevated alkaline phosphatase, which could reflect liver or bone). In cases that are diagnostic challenges, one of the highest‐yield maneuvers is to repeat the preceding evaluation, starting with the history, exam, and basic labs, and if necessary, to review or repeat the imaging or skin biopsy. Given the elevated alkaline phosphatase, disseminated rash, new neurological symptoms, and his HIV status, I remain particularly concerned about syphilis and would do further testing (accounting for the prozone phenomenon) before proceeding with the malignancy evaluation.
A lumbar puncture demonstrated clear cerebrospinal fluid, with 2 leukocytes and 195 erythrocytes/cm3, protein of 26 mg/dL, and glucose of 52 mg/dL. Bacterial and fungal cultures of the fluid were negative. The results of colonoscopy were normal. A bone marrow biopsy demonstrated ring granulomas. GMS, AFB, Fite, and Steiner (for spirochetes) stains were negative, cultures of the aspirate were negative for bacteria, and smears were negative for fungi and mycobacteria. Antibody tests for human T‐cell lymphotropic virus types I and II, Coxiella burnetii, and Bartonella henselae were negative. The dermatology consultant believed the absence of lymphadenopathy and the pruritic nature of the lesions was atypical for cytotoxic T‐cell lymphoma (CTCL). Before initiating therapy for CTCL, she suggested repeating the skin biopsy and RPR.
The repeat RPR was positive at 1:64 dilutions, and a confirmatory fluorescent treponemal antibody absorption test showed a positive result. He was prescribed intramuscular benzathine pencillin 2.4 million units weekly for 3 weeks, with almost immediate defervescence and slower resolution of his rash and shooting pains in his limbs. The repeat skin biopsy done during the hospitalization demonstrated lichenoid‐type dermatitis with interstitial and perivascular lymphohistiocytic infiltrates and granulomas. Steiner stains for spirochetes were positive. Immunohistochemical stains ruled out a lymphoproliferative process. One year later his RPR was nonreactive.
COMMENTARY
Fever of unknown origin (FUO) was first defined by Petersdorf and Beeson in 1961 as a temperature higher than 38.3C on several occasions lasting longer than 3 weeks and defying diagnosis despite 1 week of inpatient investigation.1 Dramatic changes in medical practice have rendered this definition outdated, with more recent proposals allowing thoughtful outpatient investigation to serve as a surrogate for hospitalization. Some have proposed that HIV‐associated FUO be considered a distinct entity, with the most complete North American series finding the etiology of the HIV‐associated FUO in 56 of 70 patients.2 The mean CD4+ count in this series was 58/cm3. Disseminated M. avium was the most frequently diagnosed cause, followed by P. jirovecii pneumonia, cytomegalovirus infection, disseminated histoplasmosis, and lymphoma. Of 14 patients with fever of no definable etiology, 12 eventually proved to have self‐limiting illness.
Despite numerous attempts to reduce the investigation of the patient with FUO to an algorithm, the approach must be individualized. A thorough history and careful, serial physical examinations are frequently and appropriately stressed as the foundation, followed by thoughtful selection of laboratory and imaging studies. Although FUO has a lengthy differential diagnosis, it often proves to be, as Mackowiak and Durack stress, an unusual manifestation of a common disease, rather than a typical presentation of a rare disease.3 A relatively uncommon disease in conjunction with an initially negative diagnostic test result, as was the case with this patient, may lead to a protracted diagnostic puzzle.
Syphilis is a rare cause of FUO. In 6 large studies of a total of 947 patients published over a 40‐year period, only 2 cases of syphilis (1 secondary and 1 neurosyphilis) were reported.1, 48 Syphilis as a cause of prolonged cryptic fever appears to have been seen with greater frequency in the preantibiotic era.9 In the first half of the 20th century, syphilis was known as the great imitator, with its unusual manifestations recognized and indeed expected. As a result of the dramatically lower incidence of syphilis in recent decades, these lessons have largely been forgotten, however, which may lead to diagnostic confusion when syphilis presents atypically. The manifestations of secondary syphilis are protean, including a variety of rashes, aphthous ulcers, arthralgias, pharyngitis, weight loss, fever, meningitis, ocular symptoms, cranial nerve palsies, glomerulonephritis, hepatitis, and periostitis (which afflicted this patient, who complained of severe shooting pains in his arms and shins).
After declining in the last decade of the 20th century, the rates of primary and secondary syphilis are rising in the United States.10 Oral sex is a clear risk factor for syphilis transmission, particularly for men who have sex with men.11 Because of the patient's exposure history and clinical picture, his outpatient physician considered the diagnosis of secondary syphilis early in the course of his illness. The diagnosis was not entertained further when an RPR test, highly sensitive at this stage of the disease, returned nonreactive. Likewise, when a rash subsequently appeared, the lack of palm and sole involvement dissuaded multiple clinicians from reconsidering the diagnosis of syphilis. A skin biopsy that appeared to lead in a distinctly different direction understandably confused the picture still further. Even at the time of the lumbar puncture, VDRL of the CSF was not ordered.
In retrospect, the chief confounder in the case was the false‐negative RPR test, as the discussant suspected early on. Although nontreponemal tests are generally accurate in individuals with HIV, delayed seropositivity and false‐negatives have been reported in this population.12 The false‐negative could have also been a result of the prozone phenomenon, an unusual event, occurring in fewer than 2% of cases of secondary syphilis and attributed to a mismatch between antibody and very high antigen level. The prozone reaction can be corrected for by requesting dilution of the serum prior to repeating the test. Simple lab error must be considered as well, but without access to this patient's serum from his original testing, the cause of his initial false‐negative test cannot be known with certainty.
An unusual presentation in conjunction with failure to recognize the causes of rare false‐negative testing for secondary syphilis led to a delayed diagnosis in this patient. Although syphilis and mycosis fungoides have previously been reported to mimic one another both clinically and histopathologically, the potential for secondary syphilis to be misdiagnosed in this fashion is not generally appreciated.1315 Recognition of the possibility of secondary syphilis occurred just in time to spare this patient the rash decision of treating him with cytotoxic therapy directed against CTCL.
Teaching Points
-
HIV‐associated FUO can be a diagnostic challenge, but an etiology can be found in most cases.
-
Syphilis continues to be an unusual cause of FUO and can have protean manifestations affecting nearly every organ system
-
The sensitivity of RPR is extremely high in secondary syphilis, but false‐negative tests can be seen in HIV because of both the prozone phenomenon and a delayed rise in antibodies.
A 38‐year‐old HIV+ Ohio man with a recent CD4+ count of 534 cells/mL presented to his physician with 3 weeks of fever as high as 102F. He noted mild myalgias, pruritus, and an occasional cough but no headache, sore throat, dyspnea, rash, or gastrointestinal or genitourinary complaints. He had been seen elsewhere 2 weeks previously, when he had reported a single episode of receptive oral sex with a male partner several weeks earlier. He had been prescribed ciprofloxacin and azithromycin, but a throat swab came back negative for Chlamydia and Neisseria gonorrhoeae, and he reported no change in his symptoms after the course of antibiotics. He denied smoking or using street drugs. His only medications were citalopram and trazodone for depression.
This is a HIV+ man with a mild degree of immunosuppression with a fever of unknown origin (FUO). It is not yet known if the requisite basic infectious evaluation has been completed to meet this definition, but the duration certainly qualifies, and regardless of semantics, the FUO framework is a helpful starting point. The primary considerations in FUO are infections, neoplasms, and autoimmune illnesses. Autoimmune diseases are relatively less common in HIV patients. Although pruritis is quite common in HIV alone, it may also herald renal failure, cholestasis, or a malignancy (usually hematologic). Drugs must also be considered as a cause of unexplained fever; the pruritis might suggest an allergic reaction, although I do not think of citalopram or trazodone as having this effect. The failure to respond to broad‐spectrum antimicrobials (along with the duration) lowers my suspicion for common infections such as pneumonia, urinary tract infection, or cellulitis. Among sexually transmitted diseases, syphilis can be protean and merits consideration.
On examination he appeared well. His temperature was 102.4F, pulse 111 beats/min, blood pressure 138/78 mm Hg. The head, neck, cardiovascular system, and lungs appeared normal on examination. The abdomen was soft and nontender without organomegaly; skin, extremities, and neurological system were unremarkable. Rectal examination showed small anal condylomata. Hemoglobin was 14.3 g/dL, white blood cell count 6200/cm3, and platelet count 230,000/cm3. Serum electrolytes and lactate dehydrogenase were normal. The results of his liver function tests (LFTs) demonstrated a serum aspartate transaminase of 60 U/L (normal, 7‐40 U/L), alanine transaminase of 125 U/L (normal, 5‐50 U/L), alkaline phosphatase 218 U/L (normal, 40‐150 U/L), and total bilirubin 2.1 mg/dL (normal, 0.0‐1.5 mg/dL). Urinalysis demonstrated 2+ bilirubin and was otherwise normal. His erythrocyte sedimentation rate was 32 mm/hr (normal, 0‐15 mm/hr).
After 3 weeks of illness, his CBC demonstrates no signs of chronic illness (such as anemia of a chronic disease or a reactive leukocytosis or thrombocytosis). The results of his liver function tests showed moderate elevation, slightly more cholestatic than hepatocellular. This finding may reflect a disease process involving the liver, but such abnormal findings are often nonspecific in acute and chronic illnesses. With an unremitting fever, infectious complications in the liver merit early consideration. The time course rules out common biliary disorders such as cholangitis or cholecystitis. Pyogenic or amoebic liver abscesses are possible (homosexual men are at increased risk for the latter), but the absence of pain or abdominal tenderness is atypical. This biochemical profile can also be seen in chronic (but not acute) viral infections of the liver. Chronic hepatitis B and C predispose to hepatocellular carcinoma (HCC), which can be associated with fever. Cancers that infiltrate the liver, such as lymphoma or carcinoma, could also account for this picture. Indolent infections such as tuberculosis (TB) and syphilis are also possible, so associated signs of these systemic diseases should be sought. I do not believe either of his antibiotics is commonly associated with LFT abnormalities, and his CD4 count is too high for HIV cholangiopathy. In sum, a host of liver diseases are possible, but an extrahepatic systemic disease deserves equal attention.
His CD4+ count was 537 cells/mL, and his HIV RNA viral load was 44,300 copies/mL. Radiographs of the chest were normal. Two sets of blood cultures were negative. The rapid plasma reagin (RPR) was nonreactive. The results of serologies for acute hepatitis A, B, C, and E, chronic hepatitis B and C, and toxoplasmosis were negative. Testing for both Epstein‐Barr virus and cytomegalovirus showed evidence of remote infection. Results of serologies for bartonella species, human herpesviruses 6 and 7, and parvovirus B19 were negative.
The negative RPR makes disseminated (secondary) syphilis improbable, provided the prozone phenomenon has been excluded. An extensive serological workup is common in the evaluation of fever of unknown origin, although the threat of false‐positive results always looms when many studies are sent simultaneously. This must be considered in advance here, as his relatively preserved CD4 count affords him significant protection against many opportunistic infections. His HIV infection, however, regardless of CD4 count, increases his risk for TB and lymphoma, which remain high on my list. Both may be residing primarily in the liver. In FUO, the abdominal CT is frequently a high‐yield test (primarily by demonstrating unsuspected tumors and abscesses), even in the absence of symptoms, and would certainly be of interest here given the liver function test results. Imaging could diagnose febrile tumors such as lymphoma, HCC, or renal cell carcinoma. In the event that imaging is unrevealing, causes of granulomatous hepatitis should be entertained. The constellation of cough, LFT abnormalities, and fever is compatible with Q fever. As with any FUO case, I would also carefully revisit this patient's history to discern where he was born, where he has been, and what activities or exposures he is engaged in.
He was seen 2 days later with fever of 104F and new papules over his sternal area. Over the next week, he had intermittent fevers and severe fatigue. The rash progressed, predominantly involving his chest and back, but also his legs, arms, and face (see Fig. 1). The lesions spared his palms and soles. The exanthem was intensely pruritic and maculopapular, consisting of lesions with a diameter of 0.5 cm or less, with some scaling. There were no vesicles or pustular lesions. There were no other new findings on examination. His transaminase and bilirubin had normalized, and his CBC and electrolytes were unchanged. Repeat blood cultures held for extended incubation were negative. Computerized tomography of the chest, abdomen, and pelvis demonstrated mild lymphadenopathy at the porta hepatis with increased portocaval and periaortic lymphadenopathy.

The only LFT abnormality that persists is the elevated alkaline phosphatase, which suggests (1) that liver involvement was not specific and that there is a disease process involving the bone, (2) that there is a persistent infiltrative disorder of the liver such as infection or malignancy or, less likely, amyloidosis or sarcoidosis, or (3) that the porta hepatis lymphadenopathy is causing biliary obstruction. The underlying diagnosis must explain the rash, intraabdominal lymphadenopathy, and fever. The time course does somewhat limit the extensive differential of fever and rash. After 3 weeks of illness, some of the most life‐threatening entities such as meningococcal disease, Rocky Mountain spotted fever, and toxic shock syndrome are unlikely. Concern remains for infections that are more indolent, such as mycobacteria, fungi, or spirochetes. The most striking elements of the rash are the extensive distribution, rapid progression, large number, and discreteness of the lesions, which collectively point more toward disseminated fungal (eg, histoplasmosis, as he lives in Ohio), spirochetal, rickettsial, or viral etiologies, rather than bacterial or mycobacterial entities. The absence of vesicles detracts from the diagnosis of a disseminated herpes virus such as herpes simplex or varicella. I believe that this rash is too disseminated to be caused by a common mycobacterial illness. This extent of cutaneous metastases would usually accompany a far more ill patient with an obvious primary cancer (none is seen on imaging, including the liver), and it appears too extensive to be caused by a paraneoplastic phenomenon such as Sweet's syndrome. A systemic vasculitis or another autoimmune disease remains possible, but there is minimal evidence of visceral organ involvement. All the aforementioned diseases could explain the intraabdominal lymphadenopathy, but my suspicion is highest for infection. I would biopsy and culture the skin lesions, repeat the RPR and/or send a treponemal‐specific test, place a PPD skin test, and send fungal studies (serum serologies and urine antigens) for evaluation. If the results of these noninvasive studies are unrevealing, I would consider a liver biopsy.
The patient's medications were discontinued, and a skin biopsy of the rash from his chest showed atypical lymphohistiocytic infiltrates without acute inflammatory cells and with negative Gomori methenamine silver (GMS), acid‐fast bacilli (AFB), and Fite (for Nocardia) stains. The infiltrates were predominantly T cells with a 1:1 CD4:CD8 ratio. This was read as suspicious for cytotoxic (CD8) mycosis fungoides.
I do not have reason to doubt the pathologist's impression of mycosis fungoides on histopathologic grounds, but from a clinical standpoint, I do not think mycosis fungoides is a disease that has a prolonged febrile prodrome or an explosive cutaneous onset. Rather, it is frequently preceded by nonspecific skin findings over a long period. Thinking broadly and pathophysiologically and noting that T cells are the predominant lymphocytes in skin, I wonder if they could represent a nonmalignant, immunological reaction in the skin. The stains, although not perfectly sensitive, make mycobacterial and fungal diseases less likely, although incubation of cultures is necessary.
Over the next 10 days (bringing the total duration of the patient's illness to 6 weeks), the skin lesions increased in number. In the physician's office at his next follow‐up, the patient had a temperature of 104.1F, was uncomfortable, shivering, and ill‐appearing. His blood pressure was 108/66 mm Hg, and his pulse 114 beats/min. He complained of severe shooting pains, predominantly in his pretibial regions and arms. Examination showed no other new findings, including no focal neurological findings. The results of the T‐cell rearrangement study from the skin biopsy showed evidence of a monoclonal T‐cell population. He was admitted to the hospital for further evaluation and treatment.
The extremity dysesthesias could represent a lesion of the spinal cord (including the CSF/meninges), a polyradiculopathy, or a polyneuropathy. Unfortunately, this does not add a tremendous amount of diagnostic resolution, as infection, malignancy, and autoimmune syndromes, such as vasculitis, may all involve the nervous system in these ways. In general, I associate monoclonal lymphocyte responses with hematological malignancies and polyclonal responses with the less specific inflammation that could accompany infection, autoimmunity, or solid malignancies. His age, fever, and rapid progression seem atypical for mycosis fungoides, but given the monoclonal T cells, this must now be considered. Adult T‐cell leukemia/lymphoma, with its prominent skin manifestations and its association with HLTV‐1, is an alternative T‐cell malignancy that could explain the fever, neurological symptoms, and possible visceral involvement (elevated alkaline phosphatase, which could reflect liver or bone). In cases that are diagnostic challenges, one of the highest‐yield maneuvers is to repeat the preceding evaluation, starting with the history, exam, and basic labs, and if necessary, to review or repeat the imaging or skin biopsy. Given the elevated alkaline phosphatase, disseminated rash, new neurological symptoms, and his HIV status, I remain particularly concerned about syphilis and would do further testing (accounting for the prozone phenomenon) before proceeding with the malignancy evaluation.
A lumbar puncture demonstrated clear cerebrospinal fluid, with 2 leukocytes and 195 erythrocytes/cm3, protein of 26 mg/dL, and glucose of 52 mg/dL. Bacterial and fungal cultures of the fluid were negative. The results of colonoscopy were normal. A bone marrow biopsy demonstrated ring granulomas. GMS, AFB, Fite, and Steiner (for spirochetes) stains were negative, cultures of the aspirate were negative for bacteria, and smears were negative for fungi and mycobacteria. Antibody tests for human T‐cell lymphotropic virus types I and II, Coxiella burnetii, and Bartonella henselae were negative. The dermatology consultant believed the absence of lymphadenopathy and the pruritic nature of the lesions was atypical for cytotoxic T‐cell lymphoma (CTCL). Before initiating therapy for CTCL, she suggested repeating the skin biopsy and RPR.
The repeat RPR was positive at 1:64 dilutions, and a confirmatory fluorescent treponemal antibody absorption test showed a positive result. He was prescribed intramuscular benzathine pencillin 2.4 million units weekly for 3 weeks, with almost immediate defervescence and slower resolution of his rash and shooting pains in his limbs. The repeat skin biopsy done during the hospitalization demonstrated lichenoid‐type dermatitis with interstitial and perivascular lymphohistiocytic infiltrates and granulomas. Steiner stains for spirochetes were positive. Immunohistochemical stains ruled out a lymphoproliferative process. One year later his RPR was nonreactive.
COMMENTARY
Fever of unknown origin (FUO) was first defined by Petersdorf and Beeson in 1961 as a temperature higher than 38.3C on several occasions lasting longer than 3 weeks and defying diagnosis despite 1 week of inpatient investigation.1 Dramatic changes in medical practice have rendered this definition outdated, with more recent proposals allowing thoughtful outpatient investigation to serve as a surrogate for hospitalization. Some have proposed that HIV‐associated FUO be considered a distinct entity, with the most complete North American series finding the etiology of the HIV‐associated FUO in 56 of 70 patients.2 The mean CD4+ count in this series was 58/cm3. Disseminated M. avium was the most frequently diagnosed cause, followed by P. jirovecii pneumonia, cytomegalovirus infection, disseminated histoplasmosis, and lymphoma. Of 14 patients with fever of no definable etiology, 12 eventually proved to have self‐limiting illness.
Despite numerous attempts to reduce the investigation of the patient with FUO to an algorithm, the approach must be individualized. A thorough history and careful, serial physical examinations are frequently and appropriately stressed as the foundation, followed by thoughtful selection of laboratory and imaging studies. Although FUO has a lengthy differential diagnosis, it often proves to be, as Mackowiak and Durack stress, an unusual manifestation of a common disease, rather than a typical presentation of a rare disease.3 A relatively uncommon disease in conjunction with an initially negative diagnostic test result, as was the case with this patient, may lead to a protracted diagnostic puzzle.
Syphilis is a rare cause of FUO. In 6 large studies of a total of 947 patients published over a 40‐year period, only 2 cases of syphilis (1 secondary and 1 neurosyphilis) were reported.1, 48 Syphilis as a cause of prolonged cryptic fever appears to have been seen with greater frequency in the preantibiotic era.9 In the first half of the 20th century, syphilis was known as the great imitator, with its unusual manifestations recognized and indeed expected. As a result of the dramatically lower incidence of syphilis in recent decades, these lessons have largely been forgotten, however, which may lead to diagnostic confusion when syphilis presents atypically. The manifestations of secondary syphilis are protean, including a variety of rashes, aphthous ulcers, arthralgias, pharyngitis, weight loss, fever, meningitis, ocular symptoms, cranial nerve palsies, glomerulonephritis, hepatitis, and periostitis (which afflicted this patient, who complained of severe shooting pains in his arms and shins).
After declining in the last decade of the 20th century, the rates of primary and secondary syphilis are rising in the United States.10 Oral sex is a clear risk factor for syphilis transmission, particularly for men who have sex with men.11 Because of the patient's exposure history and clinical picture, his outpatient physician considered the diagnosis of secondary syphilis early in the course of his illness. The diagnosis was not entertained further when an RPR test, highly sensitive at this stage of the disease, returned nonreactive. Likewise, when a rash subsequently appeared, the lack of palm and sole involvement dissuaded multiple clinicians from reconsidering the diagnosis of syphilis. A skin biopsy that appeared to lead in a distinctly different direction understandably confused the picture still further. Even at the time of the lumbar puncture, VDRL of the CSF was not ordered.
In retrospect, the chief confounder in the case was the false‐negative RPR test, as the discussant suspected early on. Although nontreponemal tests are generally accurate in individuals with HIV, delayed seropositivity and false‐negatives have been reported in this population.12 The false‐negative could have also been a result of the prozone phenomenon, an unusual event, occurring in fewer than 2% of cases of secondary syphilis and attributed to a mismatch between antibody and very high antigen level. The prozone reaction can be corrected for by requesting dilution of the serum prior to repeating the test. Simple lab error must be considered as well, but without access to this patient's serum from his original testing, the cause of his initial false‐negative test cannot be known with certainty.
An unusual presentation in conjunction with failure to recognize the causes of rare false‐negative testing for secondary syphilis led to a delayed diagnosis in this patient. Although syphilis and mycosis fungoides have previously been reported to mimic one another both clinically and histopathologically, the potential for secondary syphilis to be misdiagnosed in this fashion is not generally appreciated.1315 Recognition of the possibility of secondary syphilis occurred just in time to spare this patient the rash decision of treating him with cytotoxic therapy directed against CTCL.
Teaching Points
-
HIV‐associated FUO can be a diagnostic challenge, but an etiology can be found in most cases.
-
Syphilis continues to be an unusual cause of FUO and can have protean manifestations affecting nearly every organ system
-
The sensitivity of RPR is extremely high in secondary syphilis, but false‐negative tests can be seen in HIV because of both the prozone phenomenon and a delayed rise in antibodies.
- ,.Fever of unexplained origin: Report on 100 cases.Medicine.1961;40:1–30.
- ,,.Human immunodeficiency virus‐associated fever of unknown origin: A study of 70 patients in the United States and review.Clin Infect Dis.1999;28:341–345.
- ,.Fever of unknown origin. In:Mandell GL,Bennett JE,Dolin R, eds.Principles and Practice of Infectious Diseases.6th ed.Philadelphia:Elsevier Churchill Livingstone;2005:718–729.
- ,,.Fever of unknown origin: Diagnosis and follow‐up of 105 cases, 1970‐1980.Medicine.1982;61:269–292.
- ,,,.Fever of unknown origin in the 1980s: An update of the diagnostic spectrum.Arch Intern Med.1992;152:51–55.
- .Fever of unknown origin: Review of 86 patients treated in community hospitals.Clin Infect Dis.1992;15:968–973.
- ,,.Fever of unknown origin (FUO). I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO study group.Medicine.1997;76:392–400.
- ,,, et al.From prolonged febrile illness to fever of unknown origin: The challenge continues.Arch Intern Med.2003;163:1033–1041.
- ,.The diagnosis of obscure fever. II. The diagnosis of unexplained high fever.Bull Johns Hopkins Hosp.1936;58:307–331.
- Centers for Disease Control and Prevention.Primary and secondary syphilis—United States, 2003–2004.MMWR.2006;55:269–273.
- Transmission of primary and secondary syphilis by oral sex—Chicago, Illinois, 1998‐2202.MMWR.2004;53:966–968.
- ,,, et al.Seronegative secondary syphilis in 2 patients coinfected with human immunodeficiency virus.Arch Dermatol.2005;141:431–433.
- ,,,.Secondary syphilis mimicking mycosis fungoides.J Am Acad Dermatol.1980;3:92–94
- ,.A case of lues maligna in a patient with acquired immunodeficiency syndrome (AIDS).Scand J Infect Dis.2005;37:697–700.
- ,,,,.Unusual presentation of secondary syphilis in 2 HIV‐1 positive patients.Cutis.2000;66:383–389.
- ,.Fever of unexplained origin: Report on 100 cases.Medicine.1961;40:1–30.
- ,,.Human immunodeficiency virus‐associated fever of unknown origin: A study of 70 patients in the United States and review.Clin Infect Dis.1999;28:341–345.
- ,.Fever of unknown origin. In:Mandell GL,Bennett JE,Dolin R, eds.Principles and Practice of Infectious Diseases.6th ed.Philadelphia:Elsevier Churchill Livingstone;2005:718–729.
- ,,.Fever of unknown origin: Diagnosis and follow‐up of 105 cases, 1970‐1980.Medicine.1982;61:269–292.
- ,,,.Fever of unknown origin in the 1980s: An update of the diagnostic spectrum.Arch Intern Med.1992;152:51–55.
- .Fever of unknown origin: Review of 86 patients treated in community hospitals.Clin Infect Dis.1992;15:968–973.
- ,,.Fever of unknown origin (FUO). I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO study group.Medicine.1997;76:392–400.
- ,,, et al.From prolonged febrile illness to fever of unknown origin: The challenge continues.Arch Intern Med.2003;163:1033–1041.
- ,.The diagnosis of obscure fever. II. The diagnosis of unexplained high fever.Bull Johns Hopkins Hosp.1936;58:307–331.
- Centers for Disease Control and Prevention.Primary and secondary syphilis—United States, 2003–2004.MMWR.2006;55:269–273.
- Transmission of primary and secondary syphilis by oral sex—Chicago, Illinois, 1998‐2202.MMWR.2004;53:966–968.
- ,,, et al.Seronegative secondary syphilis in 2 patients coinfected with human immunodeficiency virus.Arch Dermatol.2005;141:431–433.
- ,,,.Secondary syphilis mimicking mycosis fungoides.J Am Acad Dermatol.1980;3:92–94
- ,.A case of lues maligna in a patient with acquired immunodeficiency syndrome (AIDS).Scand J Infect Dis.2005;37:697–700.
- ,,,,.Unusual presentation of secondary syphilis in 2 HIV‐1 positive patients.Cutis.2000;66:383–389.
Pandemic Influenza and the Hospitalist
Background
Influenza viruses are among the most common respiratory viral infections in humans. There are two major types of human influenza viruses, A and B, with influenza A strains responsible for seasonal or pandemic influenza. Influenza illness is characterized by fever, lower respiratory and often upper respiratory symptoms, myalgia, and malaise and occurs seasonally in temperate climates between late fall and early spring. The average flu season in the United States is marked by 30,000‐40,000 deaths, primarily in elderly patients with significant comorbidity and in the very young. Many of these deaths are caused by secondary bacterial pneumonias. Long interpandemic periods, including the current one of almost 40 years, involve minor mutations of the predominant influenza strain from year to year. Typically, adequate time exists to predict the prevailing strain with reasonable accuracy and to tailor a vaccine accordingly. Periodically an influenza pandemic involving a novel influenza strain emerges, attended by greater‐than‐expected morbidity and mortality.
All influenza viruses are subtyped on the basis of two surface glycoproteins. One of these, hemagglutinin (H), is responsible for viral cell entry; whereas the other, neuraminidase (N), facilitates release of the virus from infected cells, thus allowing perpetuation and amplification of infection. Antigenic drift is the ongoing process of genetic mutations that lead to new strains demonstrating variable change in antigenicity and is the basis for the annual updating of vaccine strains. Antigenic shift is the emergence of a novel influenza A subtype among humans, usually as the result of a recombination event. This radical change is necessary but not sufficient to initiate pandemic influenza, with efficient transmission from person to person also a critical feature. Pandemic influenza strains arise in 1 of 2 fashions. Genetic reassortment may occur when a mammalian host (human or porcine) is infected with both an avian and a human influenza virus, with subsequent dramatic movement into human populations, the source of the 1957 and 1968 pandemics. Alternatively, a novel virus may, after sufficient mutation, move directly from the avian population to humans, as appears to have occurred in 1918.
The 1918‐19 Pandemic
Abruptly in 1918, an influenza pandemic of seemingly unprecedented severity swept the world. Although disagreement remains regarding the source of the outbreak (China, the front lines of World War I, and even the United States have all been suggested), within 6‐9 months essentially the entire globe had been affected. Unlike more typical influenza seasons, the virus preferentially infected previously healthy young individuals, with those aged 15‐40 bearing the brunt of the illness. US military training installations, overcrowded with troops staging for service on the European front, played a particularly ill‐fated role in the pandemic as it swept through the United States.
Estimates of the pandemic's worldwide impact on mortality are sketchy at best, but many authorities believe that at least 50 million deaths resulted, with some suggesting a figure as high as 100 million. In the United States the virus was responsible for an estimated 700,000 deaths, with an untold burden of morbidity. Economic and social disruption was the norm in many areas, with widespread closure of businesses and schools and suspension of public gatherings of any kind. Many communities were simply overwhelmed by the sheer numbers of dying individuals. In Philadelphia, steam shovels were used to dig mass graves for influenza victims.1 The pandemic's effect on the health care system was likewise profound. Most hospitals counted their own physicians and nurses among those who died during the pandemic, and many of the health care workers who succumbed were infected in the course of caring for influenza patients. Overall, an estimated 2%‐3% of those infected with the virus died, a far higher percentage than is seen during interpandemic seasons. Strikingly, the vast majority of deaths do not appear to have resulted from secondary bacterial pneumonias, but rather to have been directly virally mediated through ARDS, a necrotizing viral pneumonia, or both.
The mystery of the 1918 pandemic has recently been partially unlocked, with the successful sequencing of the entire RNA genome of strains recovered from pathology tissue of two soldiers, as well as from lung tissue of a victim frozen in Alaskan permafrost since 1918.2, 3 The data suggest that the 1918 virus was derived from an avian source. Notably, some of the same changes in the polymerase proteins have been found in the highly pathogenic H5N1 viruses.
Avian Influenza Viruses
Influenza viruses that primarily infect birds are characterized as avian influenza viruses. These are always type A and are classified as either of low or high pathogenicity on the basis of the severity of the illness they cause in birds. The currently circulating H5N1 avian viruses are highly pathogenic.
Avian influenza viruses do not usually infect humans; however, several instances of human infections have been reported since 1997. The 1997 Hong Kong outbreak of avian (H5N1) influenza in 18 humans resulted in 6 deaths and was a seminal event that provided evidence that avian influenza viruses can infect people. It also provided the epidemiologic link between avian influenza infection in poultry with disease in humans and was proclaimed as a pandemic warning. These sentinel human infections led to the culling of the entire Hong Kong poultry population, with no subsequent human infection reported at that time. In 2003, more than 80 cases of avian influenza A (H7N7) illness occurred in the Netherlands among persons who handled infected poultry. Sustained human‐to‐human transmission did not occur in this or other outbreaks of avian influenza to date.
Since 2003, sporadic human cases of H5N1 have occurred, most recently reported from Turkey and Iraq. Human cases have also occurred in Vietnam, China, Cambodia, Thailand, and Indonesia, with a total of 173 reported cases and a case fatality rate exceeding 50% as of this writing.4 This mortality rate may be artificially inflated, as less severe cases have certainly gone unreported. All countries reporting human avian influenza diseases since 2003 have had concurrent epizoonotics in birds (both poultry and migratory birds).
Human cases of H5N1 influenza illness have been characterized by high fever and symptoms in the lower respiratory tract, as would be expected. Less predictable has been the presence of watery diarrhea in many patients and of abdominal and pleuritic pain and bleeding from the nose and gums in some. Sputum production has been variably present, and hemoptysis has been seen in some individuals. Most patients have had clinical and radiological evidence of pneumonia at the time they sought medical care, and progression to ARDS and multiorgan failure has been common. The majority of patients to date have required the initiation of mechanical ventilation early in their hospital course. Laboratory studies have typically shown lymphopenia, thrombocytopenia, and, in many cases, modestly elevated transaminase levels.5 Notably, the currently predominant strain of H5N1 (Z strain) is resistant to the M2 ion channel inhibitors amantadine and rimantadine but is susceptible to the newer class of neuraminidase inhibitors, zanamivir (Relenza) and oseltamivir (Tamiflu). Neuraminidase inhibitors and corticosteroids have been used to treat patients, although their efficacy in this setting is unclear. To date, virtually all cases appear to have been transmitted directly from poultry, although person‐to‐person transmission appears likely to have occurred in at least one family in Thailand.6 A recent study of the 14 clusters of avian influenza among humans emphasized the lack of sustained person‐to‐person transmission of H5N1 to date.7
Three factors are necessary for the emergence of a pandemic influenza strain: the ability to infect humans, a novel genetic makeup, and the ability for sustained transmission between people. A virus that in addition proves highly virulent, as did the 1918‐19 H1N1 strain, essentially creates the perfect storm. H5N1 influenza has currently fulfilled 2 of these 3 criteria. The virus is highly pathogenic, although how much of this fitness would be sacrificed with mutation to a more transmissible strain is uncertain. As many have observed, whether there will be another influenza pandemic does not seem in doubt; rather, it is when such a pandemic will occur and whether the pandemic will be caused by H5N1 or another influenza virus, that are the questions.
Potential Effects of the Next Pandemic
The global and national effects of an influenza pandemic will vary in direct proportion to the virulence of the circulating viral strain, but if such a virus is highly virulent, significant and perhaps severe economic and social disruption are likely.
The global economic impact has been estimated to be $800 billion with anticipated quarantines and interruption in global trade. On a national level, it has been estimated that in the United States a pandemic virus whose severity is comparable to that of the 1968 Hong Kong influenza pandemic would lead to approximately 200,000 deaths and 700,000 hospitalizations, of which roughly 100,000 would require treatment in intensive care unit settings. A more virulent strain, similar to that of the 1918‐19 pandemic, might easily result in 1 million deaths; with the number of patients hospitalized approaching 10 million, well over 1 million of which would require ICU‐level care. As an estimated 75% of the 105,000 ventilators in this country are in use at any given time under normal circumstances, the potential for demand to greatly outstrip supply is evident.8 Depending on the severity of a pandemic, suspension or curtailment of international trade and travel could be reasonably likely. Although the World Health Organization has recommended against closing borders or quarantining countries even in the throes of a pandemic, the prospect of this occurring does not seem implausible. In a worst‐case scenario, even the type of national and international chaos envisioned in the Dark Winter smallpox planning exercise might occur.9
Fortress America Versus Containment Strategies
Although the pandemic influenza plan calls for stockpiling antiviral drugs and increasing vaccine production capabilities, the most effective plan for pandemic preparedness may involve a surveillance and containment strategy. No country has enough medicines or vaccines to control a widespread outbreak of pandemic avian influenza. The best solution to prevention of a pandemic is stopping any virus from spreading in the first place. Increased surveillance for avian influenza among poultry and migratory birds in key Asian countries, along with provision of funds to compensate farmers for culling of potentially infected flocks, would align incentives for early detection and eradication. Containing an initial outbreak wherever it occurs is the best defense against a pandemic. Notably, China is thought to be a potential hot zone for emergence of pandemic avian influenza. China is not only the most populous nation in the world but has one quarter of the world's chickens, two thirds of the world's domesticated ducks, and 90% of the world's domesticated geese.
The challenges of biosecurity (protecting humans against animal‐borne diseases such as bird flu) in developing countries include the reality that populations living in close proximity to poultry are also the most illiterate and impoverished, with the most limited access to health care. The recent introduction of H5N1 into Europe has heightened surveillance efforts in the United States. The introduction of H5N1 into the United States may occur through movement of migratory birds and/or importation of exotic birds. The surveillance system has been expanded to include sampling for the influenza virus not only in poultry but also in bodies of water, as the virus is shed in bird feces.
Pandemic Planning
In the setting of a severe pandemic, hospitals will face an enormous burden of patients, with a huge influx of individuals requiring both intensive care unit as well as regular nursing floor care. At the local height of such a pandemic, the ability to successfully discharge every patient whose condition will permit this to the community or elsewhere will be critical, and almost certainly hospitals will need to expand to accept more patients than they are normally configured to hold. Hospitals staffs, particularly nurses and physicians, will be required to handle very large patient censuses. Among medical staffs, emergency physicians, hospitalists, critical care specialists, and infectious disease specialists will certainly be called on to play leading roles, much as they were during and in the aftermath of Hurricane Katrina recently. Despite all of the above, the ability of existing hospitals to accommodate all gravely ill patients may be outstripped, and auxiliary hospitals in schools and other public edifices may need to be established. Hospitalists are likely to be called on to play a major role in such temporary hospitals. The frustration and anguish of not being able to provide a standard level of care to patients (for example, being forced to triage which patients are most deserving of mechanical ventilation) should not be underestimated.
Although characterized by a relatively limited number of patients, the 2003 severe acute respiratory syndrome (SARS) outbreak in Toronto, Ontario, Canada, presented some of the same challenges that will be encountered in a virulent influenza pandemic. These include the need to quickly and drastically modify the usual emergency department and inpatient procedures, as hospitals initially serve to amplify the epidemic, as well as the additional stressor of health care workers becoming ill as a result of work‐related exposure. That fewer than 400 cases of SARS pushed the medical system of one of North America's largest cities nearly to its breaking point is both sobering and instructive.10, 11 Interested readers are directed to an excellent summary of lessons learned from the SARS outbreak, most of which are widely applicable to preparations for future infectious epidemics.12
Infection Control
Although the CDC and other Web sites currently recommend airborne isolation (respiratory personal protection) for avian influenza in humans, there is not strong epidemiologic evidence of transmission other than via droplets (the transmission mode of human influenza). The emergence of a limited number of cases of avian influenza in the United States would allow employment of airborne isolation measures; but in the event of a larger outbreak, the use of surgical masks and the practice of good hand hygiene would be sufficient by health care workers caring for persons with suspected or proven disease.
The CDC recently released proposed changes to help prevent disease outbreaks from contacts of those exposed to ill persons on airplanes. Proposed guidelines would require airlines to maintain computerized lists of passengers taken at point of departure in order to facilitate tracking of contacts and implementation of quarantine if necessary. These measures are part of pandemic planning and result from problems in tracking passengers on planes with SARS cases. By executive order, imposition of quarantine is limited to 9 diseases: cholera, diphtheria, smallpox, yellow fever, viral hemorrhagic fevers (eg, Ebola), plague, infectious tuberculosis, SARS and influenza caused by new strains with pandemic potential.
What Can Be Done?
Although valuable time has elapsed to prepare for the possibility of an H5N1 influenza pandemic, the US and global communities are presently taking the threat seriously and are engaging in a variety of activities to prepare for such an eventuality. Although currently available influenza vaccines do not provide any appreciable protection against H5N1, significant work is under way to develop an effective vaccine; with Chiron and sanofi pasteur preparing vaccine trials in association with the National Institute of Allergy and Infectious Diseases. Current influenza vaccine production is hampered by use of obsolete egg‐based manufacturing processes requiring 6 months, along with a limited capacity to manufacture adequate vaccine supplies even in many usual influenza seasons. The herculean task of providing hundreds of millions of doses of vaccine as soon as possible after the emergence of a pandemic strain, as daunting as it is, is further complicated by the fact that a successful H5N1 vaccine would not necessarily be effective against a strain that mutated sufficiently to move efficiently from person to person. Nonetheless, even partially solving these problems will pay dividends, whether or not H5N1 proves to be responsible for the next pandemic.
Given these difficulties with vaccine development and production, the backbone of any successful early response to a pandemic in the near future will be development of an adequate stockpile of antiviral medication, accompanied by a successful plan to distribute the drug when and where disease erupts. Despite uncertainties regarding their effectiveness as well as questions regarding optimal dose and duration in the setting of avian influenza, the neuraminidase inhibitors are the current drugs of choice. Of the 2 currently available agents, oseltamivir is the preferred drug for pandemic use, given its oral administration,. Unfortunately, the ability to manufacture the drug in sufficient quantities to stockpile has thus far proved problematic. Roche, the manufacturer of Tamiflu, has recently opened a new manufacturing plant and has stated that it can increase its current production of 55 million doses per year to 300 million doses by 2007. We do not recommend a role for personal stockpiling of neuraminidase inhibitors. Concerns include a shortage of the drug for seasonal influenza, absence of a pandemic at present, ignorance regarding the efficacy and optimal dose for H5N1, inappropriate use by individuals, and inequitable distribution. Recent case reports of oseltamivir resistance emerging during prophylaxis13 and treatment14 are of potential concern but do not alter current recommendations.
What can be done locally and specifically, and what can hospitalists do to prepare? First, although we are not sure that Dr Michael Osterholm's goal that planning for a pandemic must be on the agenda of every public health agency, school board, manufacturing plant, investment firm, mortuary, state legislature, and food distributor8 is entirely realistic, every hospital clearly needs to include pandemic influenza as a significant part of its disaster preparedness plan. Such planning will have broad overlap with planning for other potential disasters, including bioterrorist attacks, SARS outbreaks, and others. Hospitals must develop a plan for surge capacity, and such a plan should include not only coordination with other local hospitals, but also planning with local communities to identify sites where temporary flu hospitals can be established. Within hospital medicine groups, emergency staffing plans should be established before pandemic influenza (or another disaster) strikes. Such staffing plans need to include the ability to care for a much higher than normal number of patients for an extended period. Conceivably, a large number of patients will need to be manually ventilated for prolonged periods, which of course will tax the resources of any institution. Prompt discharge of all patients stable enough to leave the hospital will be critical, and given the investment of most hospital medicine groups in hospital throughput issues under normal conditions, much of the responsibility for helping to create beds during a crisis will inevitably fall on the shoulders of hospitalists.
Experiences during and shortly after Hurricane Katrina served to underscore that issues such as physical and mental fatigue, concern for the safety of family members, lack of supplies, communication difficulties, and absenteeism all add additional layers of complexity to the task of providing hospital care under extraordinary conditions such as during a natural disaster. These lessons can and should be extended to a major epidemic. This disaster also showed the importance of military involvement in the response to disasters that exceed local and state capabilities. The primary objective of the federal government in responding to disaster is to maintain security and essential services while preventing chaos. A pandemic of virulent influenza will raise the stakes still further, as physicians and nurses become casualties themselves. Despite these challenges, we are confident that the vast majority of hospitalists and other health care workers will rise to the occasion, and just as during the peri‐Katrina period, stories of selflessness and heroism will be de rigueur. Appropriate advance planning on all levels will serve to reduce the morbidity and mortality associated with the next pandemic and will help to ensure that health care workers do not sacrifice needlessly.0
|
1. World Health Organization (WHO) Website: |
|
2. Centers for Disease Control and Prevention (CDC): |
|
3. U.S. Government Avian Influenza Website: |
|
4. U.S. Department of Health and Human Services Pandemic Influenza Plan: |
|
5. Infectious Diseases Society of America (IDSA) Website: |
- .The Great Influenza.New York, NY:Viking Penguin,2004.
- ,,,,,.Characterization of the 1918 influenza virus polymerase genes.Nature.2005;437:889–893.
- ,,, et al.Characterization of the reconstructed 1918 Spanish influenza pandemic virus.Science.2005;310:77–80.
- WHO Epidemic and Pandemic Alert and Response. Confirmed cases of avian influenza A (H5N1). Available at http://www.who.int/csr/disease/avian_influenza/country/en/index.html. Accessed on February 28,2006.
- Writing Committee of the WHO Consultation on Human Influenza A/H5.Avian influenza A (H5N1) infection in humans.N Engl J Med.2005;353:1374–1385.
- ,,, et al.Probable person‐to‐person transmission of avian influenza A (H5N1).N Engl J Med.2005;352:333–40.
- ,,, et al.Family clustering of avian influenza A (H5N1).EID.2005;11:1799–1801.
- .Preparing for the next pandemic.N Engl J Med.2005;352:1839–1842.
- Center for Biosecurity. Dark Winter overview. Available at http://www.upmc‐biosecurity.org/pages/events/dark_winter/dark_winter.html. Accessed November 28,2005.
- ,,, et al.SARS outbreak in the Greater Toronto Area: the emergency department experience.CMAJ.2004;171:1342–1344.
- ,.Severe acute respiratory syndrome and critical care medicine: The Toronto experience.Crit Care Med.2005;33(suppl):S53–S60.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291:2483–2487.
- ,,, et al.Avian flu: Isolation of drug‐resistant H5N1 virus.Nature.2005;438:754.
- ,,, et al.Oseltamivir resistance during treatment of influenza A (H5N1) infection.N Engl J Med.2005;353:2667–2672.
Background
Influenza viruses are among the most common respiratory viral infections in humans. There are two major types of human influenza viruses, A and B, with influenza A strains responsible for seasonal or pandemic influenza. Influenza illness is characterized by fever, lower respiratory and often upper respiratory symptoms, myalgia, and malaise and occurs seasonally in temperate climates between late fall and early spring. The average flu season in the United States is marked by 30,000‐40,000 deaths, primarily in elderly patients with significant comorbidity and in the very young. Many of these deaths are caused by secondary bacterial pneumonias. Long interpandemic periods, including the current one of almost 40 years, involve minor mutations of the predominant influenza strain from year to year. Typically, adequate time exists to predict the prevailing strain with reasonable accuracy and to tailor a vaccine accordingly. Periodically an influenza pandemic involving a novel influenza strain emerges, attended by greater‐than‐expected morbidity and mortality.
All influenza viruses are subtyped on the basis of two surface glycoproteins. One of these, hemagglutinin (H), is responsible for viral cell entry; whereas the other, neuraminidase (N), facilitates release of the virus from infected cells, thus allowing perpetuation and amplification of infection. Antigenic drift is the ongoing process of genetic mutations that lead to new strains demonstrating variable change in antigenicity and is the basis for the annual updating of vaccine strains. Antigenic shift is the emergence of a novel influenza A subtype among humans, usually as the result of a recombination event. This radical change is necessary but not sufficient to initiate pandemic influenza, with efficient transmission from person to person also a critical feature. Pandemic influenza strains arise in 1 of 2 fashions. Genetic reassortment may occur when a mammalian host (human or porcine) is infected with both an avian and a human influenza virus, with subsequent dramatic movement into human populations, the source of the 1957 and 1968 pandemics. Alternatively, a novel virus may, after sufficient mutation, move directly from the avian population to humans, as appears to have occurred in 1918.
The 1918‐19 Pandemic
Abruptly in 1918, an influenza pandemic of seemingly unprecedented severity swept the world. Although disagreement remains regarding the source of the outbreak (China, the front lines of World War I, and even the United States have all been suggested), within 6‐9 months essentially the entire globe had been affected. Unlike more typical influenza seasons, the virus preferentially infected previously healthy young individuals, with those aged 15‐40 bearing the brunt of the illness. US military training installations, overcrowded with troops staging for service on the European front, played a particularly ill‐fated role in the pandemic as it swept through the United States.
Estimates of the pandemic's worldwide impact on mortality are sketchy at best, but many authorities believe that at least 50 million deaths resulted, with some suggesting a figure as high as 100 million. In the United States the virus was responsible for an estimated 700,000 deaths, with an untold burden of morbidity. Economic and social disruption was the norm in many areas, with widespread closure of businesses and schools and suspension of public gatherings of any kind. Many communities were simply overwhelmed by the sheer numbers of dying individuals. In Philadelphia, steam shovels were used to dig mass graves for influenza victims.1 The pandemic's effect on the health care system was likewise profound. Most hospitals counted their own physicians and nurses among those who died during the pandemic, and many of the health care workers who succumbed were infected in the course of caring for influenza patients. Overall, an estimated 2%‐3% of those infected with the virus died, a far higher percentage than is seen during interpandemic seasons. Strikingly, the vast majority of deaths do not appear to have resulted from secondary bacterial pneumonias, but rather to have been directly virally mediated through ARDS, a necrotizing viral pneumonia, or both.
The mystery of the 1918 pandemic has recently been partially unlocked, with the successful sequencing of the entire RNA genome of strains recovered from pathology tissue of two soldiers, as well as from lung tissue of a victim frozen in Alaskan permafrost since 1918.2, 3 The data suggest that the 1918 virus was derived from an avian source. Notably, some of the same changes in the polymerase proteins have been found in the highly pathogenic H5N1 viruses.
Avian Influenza Viruses
Influenza viruses that primarily infect birds are characterized as avian influenza viruses. These are always type A and are classified as either of low or high pathogenicity on the basis of the severity of the illness they cause in birds. The currently circulating H5N1 avian viruses are highly pathogenic.
Avian influenza viruses do not usually infect humans; however, several instances of human infections have been reported since 1997. The 1997 Hong Kong outbreak of avian (H5N1) influenza in 18 humans resulted in 6 deaths and was a seminal event that provided evidence that avian influenza viruses can infect people. It also provided the epidemiologic link between avian influenza infection in poultry with disease in humans and was proclaimed as a pandemic warning. These sentinel human infections led to the culling of the entire Hong Kong poultry population, with no subsequent human infection reported at that time. In 2003, more than 80 cases of avian influenza A (H7N7) illness occurred in the Netherlands among persons who handled infected poultry. Sustained human‐to‐human transmission did not occur in this or other outbreaks of avian influenza to date.
Since 2003, sporadic human cases of H5N1 have occurred, most recently reported from Turkey and Iraq. Human cases have also occurred in Vietnam, China, Cambodia, Thailand, and Indonesia, with a total of 173 reported cases and a case fatality rate exceeding 50% as of this writing.4 This mortality rate may be artificially inflated, as less severe cases have certainly gone unreported. All countries reporting human avian influenza diseases since 2003 have had concurrent epizoonotics in birds (both poultry and migratory birds).
Human cases of H5N1 influenza illness have been characterized by high fever and symptoms in the lower respiratory tract, as would be expected. Less predictable has been the presence of watery diarrhea in many patients and of abdominal and pleuritic pain and bleeding from the nose and gums in some. Sputum production has been variably present, and hemoptysis has been seen in some individuals. Most patients have had clinical and radiological evidence of pneumonia at the time they sought medical care, and progression to ARDS and multiorgan failure has been common. The majority of patients to date have required the initiation of mechanical ventilation early in their hospital course. Laboratory studies have typically shown lymphopenia, thrombocytopenia, and, in many cases, modestly elevated transaminase levels.5 Notably, the currently predominant strain of H5N1 (Z strain) is resistant to the M2 ion channel inhibitors amantadine and rimantadine but is susceptible to the newer class of neuraminidase inhibitors, zanamivir (Relenza) and oseltamivir (Tamiflu). Neuraminidase inhibitors and corticosteroids have been used to treat patients, although their efficacy in this setting is unclear. To date, virtually all cases appear to have been transmitted directly from poultry, although person‐to‐person transmission appears likely to have occurred in at least one family in Thailand.6 A recent study of the 14 clusters of avian influenza among humans emphasized the lack of sustained person‐to‐person transmission of H5N1 to date.7
Three factors are necessary for the emergence of a pandemic influenza strain: the ability to infect humans, a novel genetic makeup, and the ability for sustained transmission between people. A virus that in addition proves highly virulent, as did the 1918‐19 H1N1 strain, essentially creates the perfect storm. H5N1 influenza has currently fulfilled 2 of these 3 criteria. The virus is highly pathogenic, although how much of this fitness would be sacrificed with mutation to a more transmissible strain is uncertain. As many have observed, whether there will be another influenza pandemic does not seem in doubt; rather, it is when such a pandemic will occur and whether the pandemic will be caused by H5N1 or another influenza virus, that are the questions.
Potential Effects of the Next Pandemic
The global and national effects of an influenza pandemic will vary in direct proportion to the virulence of the circulating viral strain, but if such a virus is highly virulent, significant and perhaps severe economic and social disruption are likely.
The global economic impact has been estimated to be $800 billion with anticipated quarantines and interruption in global trade. On a national level, it has been estimated that in the United States a pandemic virus whose severity is comparable to that of the 1968 Hong Kong influenza pandemic would lead to approximately 200,000 deaths and 700,000 hospitalizations, of which roughly 100,000 would require treatment in intensive care unit settings. A more virulent strain, similar to that of the 1918‐19 pandemic, might easily result in 1 million deaths; with the number of patients hospitalized approaching 10 million, well over 1 million of which would require ICU‐level care. As an estimated 75% of the 105,000 ventilators in this country are in use at any given time under normal circumstances, the potential for demand to greatly outstrip supply is evident.8 Depending on the severity of a pandemic, suspension or curtailment of international trade and travel could be reasonably likely. Although the World Health Organization has recommended against closing borders or quarantining countries even in the throes of a pandemic, the prospect of this occurring does not seem implausible. In a worst‐case scenario, even the type of national and international chaos envisioned in the Dark Winter smallpox planning exercise might occur.9
Fortress America Versus Containment Strategies
Although the pandemic influenza plan calls for stockpiling antiviral drugs and increasing vaccine production capabilities, the most effective plan for pandemic preparedness may involve a surveillance and containment strategy. No country has enough medicines or vaccines to control a widespread outbreak of pandemic avian influenza. The best solution to prevention of a pandemic is stopping any virus from spreading in the first place. Increased surveillance for avian influenza among poultry and migratory birds in key Asian countries, along with provision of funds to compensate farmers for culling of potentially infected flocks, would align incentives for early detection and eradication. Containing an initial outbreak wherever it occurs is the best defense against a pandemic. Notably, China is thought to be a potential hot zone for emergence of pandemic avian influenza. China is not only the most populous nation in the world but has one quarter of the world's chickens, two thirds of the world's domesticated ducks, and 90% of the world's domesticated geese.
The challenges of biosecurity (protecting humans against animal‐borne diseases such as bird flu) in developing countries include the reality that populations living in close proximity to poultry are also the most illiterate and impoverished, with the most limited access to health care. The recent introduction of H5N1 into Europe has heightened surveillance efforts in the United States. The introduction of H5N1 into the United States may occur through movement of migratory birds and/or importation of exotic birds. The surveillance system has been expanded to include sampling for the influenza virus not only in poultry but also in bodies of water, as the virus is shed in bird feces.
Pandemic Planning
In the setting of a severe pandemic, hospitals will face an enormous burden of patients, with a huge influx of individuals requiring both intensive care unit as well as regular nursing floor care. At the local height of such a pandemic, the ability to successfully discharge every patient whose condition will permit this to the community or elsewhere will be critical, and almost certainly hospitals will need to expand to accept more patients than they are normally configured to hold. Hospitals staffs, particularly nurses and physicians, will be required to handle very large patient censuses. Among medical staffs, emergency physicians, hospitalists, critical care specialists, and infectious disease specialists will certainly be called on to play leading roles, much as they were during and in the aftermath of Hurricane Katrina recently. Despite all of the above, the ability of existing hospitals to accommodate all gravely ill patients may be outstripped, and auxiliary hospitals in schools and other public edifices may need to be established. Hospitalists are likely to be called on to play a major role in such temporary hospitals. The frustration and anguish of not being able to provide a standard level of care to patients (for example, being forced to triage which patients are most deserving of mechanical ventilation) should not be underestimated.
Although characterized by a relatively limited number of patients, the 2003 severe acute respiratory syndrome (SARS) outbreak in Toronto, Ontario, Canada, presented some of the same challenges that will be encountered in a virulent influenza pandemic. These include the need to quickly and drastically modify the usual emergency department and inpatient procedures, as hospitals initially serve to amplify the epidemic, as well as the additional stressor of health care workers becoming ill as a result of work‐related exposure. That fewer than 400 cases of SARS pushed the medical system of one of North America's largest cities nearly to its breaking point is both sobering and instructive.10, 11 Interested readers are directed to an excellent summary of lessons learned from the SARS outbreak, most of which are widely applicable to preparations for future infectious epidemics.12
Infection Control
Although the CDC and other Web sites currently recommend airborne isolation (respiratory personal protection) for avian influenza in humans, there is not strong epidemiologic evidence of transmission other than via droplets (the transmission mode of human influenza). The emergence of a limited number of cases of avian influenza in the United States would allow employment of airborne isolation measures; but in the event of a larger outbreak, the use of surgical masks and the practice of good hand hygiene would be sufficient by health care workers caring for persons with suspected or proven disease.
The CDC recently released proposed changes to help prevent disease outbreaks from contacts of those exposed to ill persons on airplanes. Proposed guidelines would require airlines to maintain computerized lists of passengers taken at point of departure in order to facilitate tracking of contacts and implementation of quarantine if necessary. These measures are part of pandemic planning and result from problems in tracking passengers on planes with SARS cases. By executive order, imposition of quarantine is limited to 9 diseases: cholera, diphtheria, smallpox, yellow fever, viral hemorrhagic fevers (eg, Ebola), plague, infectious tuberculosis, SARS and influenza caused by new strains with pandemic potential.
What Can Be Done?
Although valuable time has elapsed to prepare for the possibility of an H5N1 influenza pandemic, the US and global communities are presently taking the threat seriously and are engaging in a variety of activities to prepare for such an eventuality. Although currently available influenza vaccines do not provide any appreciable protection against H5N1, significant work is under way to develop an effective vaccine; with Chiron and sanofi pasteur preparing vaccine trials in association with the National Institute of Allergy and Infectious Diseases. Current influenza vaccine production is hampered by use of obsolete egg‐based manufacturing processes requiring 6 months, along with a limited capacity to manufacture adequate vaccine supplies even in many usual influenza seasons. The herculean task of providing hundreds of millions of doses of vaccine as soon as possible after the emergence of a pandemic strain, as daunting as it is, is further complicated by the fact that a successful H5N1 vaccine would not necessarily be effective against a strain that mutated sufficiently to move efficiently from person to person. Nonetheless, even partially solving these problems will pay dividends, whether or not H5N1 proves to be responsible for the next pandemic.
Given these difficulties with vaccine development and production, the backbone of any successful early response to a pandemic in the near future will be development of an adequate stockpile of antiviral medication, accompanied by a successful plan to distribute the drug when and where disease erupts. Despite uncertainties regarding their effectiveness as well as questions regarding optimal dose and duration in the setting of avian influenza, the neuraminidase inhibitors are the current drugs of choice. Of the 2 currently available agents, oseltamivir is the preferred drug for pandemic use, given its oral administration,. Unfortunately, the ability to manufacture the drug in sufficient quantities to stockpile has thus far proved problematic. Roche, the manufacturer of Tamiflu, has recently opened a new manufacturing plant and has stated that it can increase its current production of 55 million doses per year to 300 million doses by 2007. We do not recommend a role for personal stockpiling of neuraminidase inhibitors. Concerns include a shortage of the drug for seasonal influenza, absence of a pandemic at present, ignorance regarding the efficacy and optimal dose for H5N1, inappropriate use by individuals, and inequitable distribution. Recent case reports of oseltamivir resistance emerging during prophylaxis13 and treatment14 are of potential concern but do not alter current recommendations.
What can be done locally and specifically, and what can hospitalists do to prepare? First, although we are not sure that Dr Michael Osterholm's goal that planning for a pandemic must be on the agenda of every public health agency, school board, manufacturing plant, investment firm, mortuary, state legislature, and food distributor8 is entirely realistic, every hospital clearly needs to include pandemic influenza as a significant part of its disaster preparedness plan. Such planning will have broad overlap with planning for other potential disasters, including bioterrorist attacks, SARS outbreaks, and others. Hospitals must develop a plan for surge capacity, and such a plan should include not only coordination with other local hospitals, but also planning with local communities to identify sites where temporary flu hospitals can be established. Within hospital medicine groups, emergency staffing plans should be established before pandemic influenza (or another disaster) strikes. Such staffing plans need to include the ability to care for a much higher than normal number of patients for an extended period. Conceivably, a large number of patients will need to be manually ventilated for prolonged periods, which of course will tax the resources of any institution. Prompt discharge of all patients stable enough to leave the hospital will be critical, and given the investment of most hospital medicine groups in hospital throughput issues under normal conditions, much of the responsibility for helping to create beds during a crisis will inevitably fall on the shoulders of hospitalists.
Experiences during and shortly after Hurricane Katrina served to underscore that issues such as physical and mental fatigue, concern for the safety of family members, lack of supplies, communication difficulties, and absenteeism all add additional layers of complexity to the task of providing hospital care under extraordinary conditions such as during a natural disaster. These lessons can and should be extended to a major epidemic. This disaster also showed the importance of military involvement in the response to disasters that exceed local and state capabilities. The primary objective of the federal government in responding to disaster is to maintain security and essential services while preventing chaos. A pandemic of virulent influenza will raise the stakes still further, as physicians and nurses become casualties themselves. Despite these challenges, we are confident that the vast majority of hospitalists and other health care workers will rise to the occasion, and just as during the peri‐Katrina period, stories of selflessness and heroism will be de rigueur. Appropriate advance planning on all levels will serve to reduce the morbidity and mortality associated with the next pandemic and will help to ensure that health care workers do not sacrifice needlessly.0
|
1. World Health Organization (WHO) Website: |
|
2. Centers for Disease Control and Prevention (CDC): |
|
3. U.S. Government Avian Influenza Website: |
|
4. U.S. Department of Health and Human Services Pandemic Influenza Plan: |
|
5. Infectious Diseases Society of America (IDSA) Website: |
Background
Influenza viruses are among the most common respiratory viral infections in humans. There are two major types of human influenza viruses, A and B, with influenza A strains responsible for seasonal or pandemic influenza. Influenza illness is characterized by fever, lower respiratory and often upper respiratory symptoms, myalgia, and malaise and occurs seasonally in temperate climates between late fall and early spring. The average flu season in the United States is marked by 30,000‐40,000 deaths, primarily in elderly patients with significant comorbidity and in the very young. Many of these deaths are caused by secondary bacterial pneumonias. Long interpandemic periods, including the current one of almost 40 years, involve minor mutations of the predominant influenza strain from year to year. Typically, adequate time exists to predict the prevailing strain with reasonable accuracy and to tailor a vaccine accordingly. Periodically an influenza pandemic involving a novel influenza strain emerges, attended by greater‐than‐expected morbidity and mortality.
All influenza viruses are subtyped on the basis of two surface glycoproteins. One of these, hemagglutinin (H), is responsible for viral cell entry; whereas the other, neuraminidase (N), facilitates release of the virus from infected cells, thus allowing perpetuation and amplification of infection. Antigenic drift is the ongoing process of genetic mutations that lead to new strains demonstrating variable change in antigenicity and is the basis for the annual updating of vaccine strains. Antigenic shift is the emergence of a novel influenza A subtype among humans, usually as the result of a recombination event. This radical change is necessary but not sufficient to initiate pandemic influenza, with efficient transmission from person to person also a critical feature. Pandemic influenza strains arise in 1 of 2 fashions. Genetic reassortment may occur when a mammalian host (human or porcine) is infected with both an avian and a human influenza virus, with subsequent dramatic movement into human populations, the source of the 1957 and 1968 pandemics. Alternatively, a novel virus may, after sufficient mutation, move directly from the avian population to humans, as appears to have occurred in 1918.
The 1918‐19 Pandemic
Abruptly in 1918, an influenza pandemic of seemingly unprecedented severity swept the world. Although disagreement remains regarding the source of the outbreak (China, the front lines of World War I, and even the United States have all been suggested), within 6‐9 months essentially the entire globe had been affected. Unlike more typical influenza seasons, the virus preferentially infected previously healthy young individuals, with those aged 15‐40 bearing the brunt of the illness. US military training installations, overcrowded with troops staging for service on the European front, played a particularly ill‐fated role in the pandemic as it swept through the United States.
Estimates of the pandemic's worldwide impact on mortality are sketchy at best, but many authorities believe that at least 50 million deaths resulted, with some suggesting a figure as high as 100 million. In the United States the virus was responsible for an estimated 700,000 deaths, with an untold burden of morbidity. Economic and social disruption was the norm in many areas, with widespread closure of businesses and schools and suspension of public gatherings of any kind. Many communities were simply overwhelmed by the sheer numbers of dying individuals. In Philadelphia, steam shovels were used to dig mass graves for influenza victims.1 The pandemic's effect on the health care system was likewise profound. Most hospitals counted their own physicians and nurses among those who died during the pandemic, and many of the health care workers who succumbed were infected in the course of caring for influenza patients. Overall, an estimated 2%‐3% of those infected with the virus died, a far higher percentage than is seen during interpandemic seasons. Strikingly, the vast majority of deaths do not appear to have resulted from secondary bacterial pneumonias, but rather to have been directly virally mediated through ARDS, a necrotizing viral pneumonia, or both.
The mystery of the 1918 pandemic has recently been partially unlocked, with the successful sequencing of the entire RNA genome of strains recovered from pathology tissue of two soldiers, as well as from lung tissue of a victim frozen in Alaskan permafrost since 1918.2, 3 The data suggest that the 1918 virus was derived from an avian source. Notably, some of the same changes in the polymerase proteins have been found in the highly pathogenic H5N1 viruses.
Avian Influenza Viruses
Influenza viruses that primarily infect birds are characterized as avian influenza viruses. These are always type A and are classified as either of low or high pathogenicity on the basis of the severity of the illness they cause in birds. The currently circulating H5N1 avian viruses are highly pathogenic.
Avian influenza viruses do not usually infect humans; however, several instances of human infections have been reported since 1997. The 1997 Hong Kong outbreak of avian (H5N1) influenza in 18 humans resulted in 6 deaths and was a seminal event that provided evidence that avian influenza viruses can infect people. It also provided the epidemiologic link between avian influenza infection in poultry with disease in humans and was proclaimed as a pandemic warning. These sentinel human infections led to the culling of the entire Hong Kong poultry population, with no subsequent human infection reported at that time. In 2003, more than 80 cases of avian influenza A (H7N7) illness occurred in the Netherlands among persons who handled infected poultry. Sustained human‐to‐human transmission did not occur in this or other outbreaks of avian influenza to date.
Since 2003, sporadic human cases of H5N1 have occurred, most recently reported from Turkey and Iraq. Human cases have also occurred in Vietnam, China, Cambodia, Thailand, and Indonesia, with a total of 173 reported cases and a case fatality rate exceeding 50% as of this writing.4 This mortality rate may be artificially inflated, as less severe cases have certainly gone unreported. All countries reporting human avian influenza diseases since 2003 have had concurrent epizoonotics in birds (both poultry and migratory birds).
Human cases of H5N1 influenza illness have been characterized by high fever and symptoms in the lower respiratory tract, as would be expected. Less predictable has been the presence of watery diarrhea in many patients and of abdominal and pleuritic pain and bleeding from the nose and gums in some. Sputum production has been variably present, and hemoptysis has been seen in some individuals. Most patients have had clinical and radiological evidence of pneumonia at the time they sought medical care, and progression to ARDS and multiorgan failure has been common. The majority of patients to date have required the initiation of mechanical ventilation early in their hospital course. Laboratory studies have typically shown lymphopenia, thrombocytopenia, and, in many cases, modestly elevated transaminase levels.5 Notably, the currently predominant strain of H5N1 (Z strain) is resistant to the M2 ion channel inhibitors amantadine and rimantadine but is susceptible to the newer class of neuraminidase inhibitors, zanamivir (Relenza) and oseltamivir (Tamiflu). Neuraminidase inhibitors and corticosteroids have been used to treat patients, although their efficacy in this setting is unclear. To date, virtually all cases appear to have been transmitted directly from poultry, although person‐to‐person transmission appears likely to have occurred in at least one family in Thailand.6 A recent study of the 14 clusters of avian influenza among humans emphasized the lack of sustained person‐to‐person transmission of H5N1 to date.7
Three factors are necessary for the emergence of a pandemic influenza strain: the ability to infect humans, a novel genetic makeup, and the ability for sustained transmission between people. A virus that in addition proves highly virulent, as did the 1918‐19 H1N1 strain, essentially creates the perfect storm. H5N1 influenza has currently fulfilled 2 of these 3 criteria. The virus is highly pathogenic, although how much of this fitness would be sacrificed with mutation to a more transmissible strain is uncertain. As many have observed, whether there will be another influenza pandemic does not seem in doubt; rather, it is when such a pandemic will occur and whether the pandemic will be caused by H5N1 or another influenza virus, that are the questions.
Potential Effects of the Next Pandemic
The global and national effects of an influenza pandemic will vary in direct proportion to the virulence of the circulating viral strain, but if such a virus is highly virulent, significant and perhaps severe economic and social disruption are likely.
The global economic impact has been estimated to be $800 billion with anticipated quarantines and interruption in global trade. On a national level, it has been estimated that in the United States a pandemic virus whose severity is comparable to that of the 1968 Hong Kong influenza pandemic would lead to approximately 200,000 deaths and 700,000 hospitalizations, of which roughly 100,000 would require treatment in intensive care unit settings. A more virulent strain, similar to that of the 1918‐19 pandemic, might easily result in 1 million deaths; with the number of patients hospitalized approaching 10 million, well over 1 million of which would require ICU‐level care. As an estimated 75% of the 105,000 ventilators in this country are in use at any given time under normal circumstances, the potential for demand to greatly outstrip supply is evident.8 Depending on the severity of a pandemic, suspension or curtailment of international trade and travel could be reasonably likely. Although the World Health Organization has recommended against closing borders or quarantining countries even in the throes of a pandemic, the prospect of this occurring does not seem implausible. In a worst‐case scenario, even the type of national and international chaos envisioned in the Dark Winter smallpox planning exercise might occur.9
Fortress America Versus Containment Strategies
Although the pandemic influenza plan calls for stockpiling antiviral drugs and increasing vaccine production capabilities, the most effective plan for pandemic preparedness may involve a surveillance and containment strategy. No country has enough medicines or vaccines to control a widespread outbreak of pandemic avian influenza. The best solution to prevention of a pandemic is stopping any virus from spreading in the first place. Increased surveillance for avian influenza among poultry and migratory birds in key Asian countries, along with provision of funds to compensate farmers for culling of potentially infected flocks, would align incentives for early detection and eradication. Containing an initial outbreak wherever it occurs is the best defense against a pandemic. Notably, China is thought to be a potential hot zone for emergence of pandemic avian influenza. China is not only the most populous nation in the world but has one quarter of the world's chickens, two thirds of the world's domesticated ducks, and 90% of the world's domesticated geese.
The challenges of biosecurity (protecting humans against animal‐borne diseases such as bird flu) in developing countries include the reality that populations living in close proximity to poultry are also the most illiterate and impoverished, with the most limited access to health care. The recent introduction of H5N1 into Europe has heightened surveillance efforts in the United States. The introduction of H5N1 into the United States may occur through movement of migratory birds and/or importation of exotic birds. The surveillance system has been expanded to include sampling for the influenza virus not only in poultry but also in bodies of water, as the virus is shed in bird feces.
Pandemic Planning
In the setting of a severe pandemic, hospitals will face an enormous burden of patients, with a huge influx of individuals requiring both intensive care unit as well as regular nursing floor care. At the local height of such a pandemic, the ability to successfully discharge every patient whose condition will permit this to the community or elsewhere will be critical, and almost certainly hospitals will need to expand to accept more patients than they are normally configured to hold. Hospitals staffs, particularly nurses and physicians, will be required to handle very large patient censuses. Among medical staffs, emergency physicians, hospitalists, critical care specialists, and infectious disease specialists will certainly be called on to play leading roles, much as they were during and in the aftermath of Hurricane Katrina recently. Despite all of the above, the ability of existing hospitals to accommodate all gravely ill patients may be outstripped, and auxiliary hospitals in schools and other public edifices may need to be established. Hospitalists are likely to be called on to play a major role in such temporary hospitals. The frustration and anguish of not being able to provide a standard level of care to patients (for example, being forced to triage which patients are most deserving of mechanical ventilation) should not be underestimated.
Although characterized by a relatively limited number of patients, the 2003 severe acute respiratory syndrome (SARS) outbreak in Toronto, Ontario, Canada, presented some of the same challenges that will be encountered in a virulent influenza pandemic. These include the need to quickly and drastically modify the usual emergency department and inpatient procedures, as hospitals initially serve to amplify the epidemic, as well as the additional stressor of health care workers becoming ill as a result of work‐related exposure. That fewer than 400 cases of SARS pushed the medical system of one of North America's largest cities nearly to its breaking point is both sobering and instructive.10, 11 Interested readers are directed to an excellent summary of lessons learned from the SARS outbreak, most of which are widely applicable to preparations for future infectious epidemics.12
Infection Control
Although the CDC and other Web sites currently recommend airborne isolation (respiratory personal protection) for avian influenza in humans, there is not strong epidemiologic evidence of transmission other than via droplets (the transmission mode of human influenza). The emergence of a limited number of cases of avian influenza in the United States would allow employment of airborne isolation measures; but in the event of a larger outbreak, the use of surgical masks and the practice of good hand hygiene would be sufficient by health care workers caring for persons with suspected or proven disease.
The CDC recently released proposed changes to help prevent disease outbreaks from contacts of those exposed to ill persons on airplanes. Proposed guidelines would require airlines to maintain computerized lists of passengers taken at point of departure in order to facilitate tracking of contacts and implementation of quarantine if necessary. These measures are part of pandemic planning and result from problems in tracking passengers on planes with SARS cases. By executive order, imposition of quarantine is limited to 9 diseases: cholera, diphtheria, smallpox, yellow fever, viral hemorrhagic fevers (eg, Ebola), plague, infectious tuberculosis, SARS and influenza caused by new strains with pandemic potential.
What Can Be Done?
Although valuable time has elapsed to prepare for the possibility of an H5N1 influenza pandemic, the US and global communities are presently taking the threat seriously and are engaging in a variety of activities to prepare for such an eventuality. Although currently available influenza vaccines do not provide any appreciable protection against H5N1, significant work is under way to develop an effective vaccine; with Chiron and sanofi pasteur preparing vaccine trials in association with the National Institute of Allergy and Infectious Diseases. Current influenza vaccine production is hampered by use of obsolete egg‐based manufacturing processes requiring 6 months, along with a limited capacity to manufacture adequate vaccine supplies even in many usual influenza seasons. The herculean task of providing hundreds of millions of doses of vaccine as soon as possible after the emergence of a pandemic strain, as daunting as it is, is further complicated by the fact that a successful H5N1 vaccine would not necessarily be effective against a strain that mutated sufficiently to move efficiently from person to person. Nonetheless, even partially solving these problems will pay dividends, whether or not H5N1 proves to be responsible for the next pandemic.
Given these difficulties with vaccine development and production, the backbone of any successful early response to a pandemic in the near future will be development of an adequate stockpile of antiviral medication, accompanied by a successful plan to distribute the drug when and where disease erupts. Despite uncertainties regarding their effectiveness as well as questions regarding optimal dose and duration in the setting of avian influenza, the neuraminidase inhibitors are the current drugs of choice. Of the 2 currently available agents, oseltamivir is the preferred drug for pandemic use, given its oral administration,. Unfortunately, the ability to manufacture the drug in sufficient quantities to stockpile has thus far proved problematic. Roche, the manufacturer of Tamiflu, has recently opened a new manufacturing plant and has stated that it can increase its current production of 55 million doses per year to 300 million doses by 2007. We do not recommend a role for personal stockpiling of neuraminidase inhibitors. Concerns include a shortage of the drug for seasonal influenza, absence of a pandemic at present, ignorance regarding the efficacy and optimal dose for H5N1, inappropriate use by individuals, and inequitable distribution. Recent case reports of oseltamivir resistance emerging during prophylaxis13 and treatment14 are of potential concern but do not alter current recommendations.
What can be done locally and specifically, and what can hospitalists do to prepare? First, although we are not sure that Dr Michael Osterholm's goal that planning for a pandemic must be on the agenda of every public health agency, school board, manufacturing plant, investment firm, mortuary, state legislature, and food distributor8 is entirely realistic, every hospital clearly needs to include pandemic influenza as a significant part of its disaster preparedness plan. Such planning will have broad overlap with planning for other potential disasters, including bioterrorist attacks, SARS outbreaks, and others. Hospitals must develop a plan for surge capacity, and such a plan should include not only coordination with other local hospitals, but also planning with local communities to identify sites where temporary flu hospitals can be established. Within hospital medicine groups, emergency staffing plans should be established before pandemic influenza (or another disaster) strikes. Such staffing plans need to include the ability to care for a much higher than normal number of patients for an extended period. Conceivably, a large number of patients will need to be manually ventilated for prolonged periods, which of course will tax the resources of any institution. Prompt discharge of all patients stable enough to leave the hospital will be critical, and given the investment of most hospital medicine groups in hospital throughput issues under normal conditions, much of the responsibility for helping to create beds during a crisis will inevitably fall on the shoulders of hospitalists.
Experiences during and shortly after Hurricane Katrina served to underscore that issues such as physical and mental fatigue, concern for the safety of family members, lack of supplies, communication difficulties, and absenteeism all add additional layers of complexity to the task of providing hospital care under extraordinary conditions such as during a natural disaster. These lessons can and should be extended to a major epidemic. This disaster also showed the importance of military involvement in the response to disasters that exceed local and state capabilities. The primary objective of the federal government in responding to disaster is to maintain security and essential services while preventing chaos. A pandemic of virulent influenza will raise the stakes still further, as physicians and nurses become casualties themselves. Despite these challenges, we are confident that the vast majority of hospitalists and other health care workers will rise to the occasion, and just as during the peri‐Katrina period, stories of selflessness and heroism will be de rigueur. Appropriate advance planning on all levels will serve to reduce the morbidity and mortality associated with the next pandemic and will help to ensure that health care workers do not sacrifice needlessly.0
|
1. World Health Organization (WHO) Website: |
|
2. Centers for Disease Control and Prevention (CDC): |
|
3. U.S. Government Avian Influenza Website: |
|
4. U.S. Department of Health and Human Services Pandemic Influenza Plan: |
|
5. Infectious Diseases Society of America (IDSA) Website: |
- .The Great Influenza.New York, NY:Viking Penguin,2004.
- ,,,,,.Characterization of the 1918 influenza virus polymerase genes.Nature.2005;437:889–893.
- ,,, et al.Characterization of the reconstructed 1918 Spanish influenza pandemic virus.Science.2005;310:77–80.
- WHO Epidemic and Pandemic Alert and Response. Confirmed cases of avian influenza A (H5N1). Available at http://www.who.int/csr/disease/avian_influenza/country/en/index.html. Accessed on February 28,2006.
- Writing Committee of the WHO Consultation on Human Influenza A/H5.Avian influenza A (H5N1) infection in humans.N Engl J Med.2005;353:1374–1385.
- ,,, et al.Probable person‐to‐person transmission of avian influenza A (H5N1).N Engl J Med.2005;352:333–40.
- ,,, et al.Family clustering of avian influenza A (H5N1).EID.2005;11:1799–1801.
- .Preparing for the next pandemic.N Engl J Med.2005;352:1839–1842.
- Center for Biosecurity. Dark Winter overview. Available at http://www.upmc‐biosecurity.org/pages/events/dark_winter/dark_winter.html. Accessed November 28,2005.
- ,,, et al.SARS outbreak in the Greater Toronto Area: the emergency department experience.CMAJ.2004;171:1342–1344.
- ,.Severe acute respiratory syndrome and critical care medicine: The Toronto experience.Crit Care Med.2005;33(suppl):S53–S60.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291:2483–2487.
- ,,, et al.Avian flu: Isolation of drug‐resistant H5N1 virus.Nature.2005;438:754.
- ,,, et al.Oseltamivir resistance during treatment of influenza A (H5N1) infection.N Engl J Med.2005;353:2667–2672.
- .The Great Influenza.New York, NY:Viking Penguin,2004.
- ,,,,,.Characterization of the 1918 influenza virus polymerase genes.Nature.2005;437:889–893.
- ,,, et al.Characterization of the reconstructed 1918 Spanish influenza pandemic virus.Science.2005;310:77–80.
- WHO Epidemic and Pandemic Alert and Response. Confirmed cases of avian influenza A (H5N1). Available at http://www.who.int/csr/disease/avian_influenza/country/en/index.html. Accessed on February 28,2006.
- Writing Committee of the WHO Consultation on Human Influenza A/H5.Avian influenza A (H5N1) infection in humans.N Engl J Med.2005;353:1374–1385.
- ,,, et al.Probable person‐to‐person transmission of avian influenza A (H5N1).N Engl J Med.2005;352:333–40.
- ,,, et al.Family clustering of avian influenza A (H5N1).EID.2005;11:1799–1801.
- .Preparing for the next pandemic.N Engl J Med.2005;352:1839–1842.
- Center for Biosecurity. Dark Winter overview. Available at http://www.upmc‐biosecurity.org/pages/events/dark_winter/dark_winter.html. Accessed November 28,2005.
- ,,, et al.SARS outbreak in the Greater Toronto Area: the emergency department experience.CMAJ.2004;171:1342–1344.
- ,.Severe acute respiratory syndrome and critical care medicine: The Toronto experience.Crit Care Med.2005;33(suppl):S53–S60.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291:2483–2487.
- ,,, et al.Avian flu: Isolation of drug‐resistant H5N1 virus.Nature.2005;438:754.
- ,,, et al.Oseltamivir resistance during treatment of influenza A (H5N1) infection.N Engl J Med.2005;353:2667–2672.