User login
Management of Acute Opioid Toxicity in the Outpatient Setting
Dermatologists’ offices are not immune from potentially fatal medical events. As a result, it is imperative that dermatologists are well versed in how to manage emergency situations in an outpatient setting. We discuss signs, symptoms, and management of opioid toxicity with an instructive case from our outpatient, hospital-based dermatology clinic.
A 55-year-old woman presented for Mohs micrographic surgery for a large recurrent basal cell carcinoma on the right medial cheek. After informed consent was obtained and the procedure was discussed with the patient, she took one 0.5-mg tablet of clonazepam for perioperative anxiety, which was part of her standard home medication regimen and preoperative administration of clonazepam had been discussed with the treating physician prior to her appointment. During tissue processing, the patient waited alone in the procedure room, with nursing checks every 10 to 15 minutes. Roughly 30 minutes after the initial stage was taken and clear margins were confirmed, the patient was found to be somnolent and unresponsive to voice, light, or touch. Physical examination revealed pupillary constriction, labored breathing, and absent blink reflex. Subsequent examination of the arms, which initially were covered by sleeves, revealed track marks. She was only aroused by a deep sternal rub, which caused her to moan and open her eyes. Her vital signs remained stable, with oxygen saturation greater than 90% and respiratory rate greater than 12 breaths per minute, and a registered nurse remained at her bedside to monitor her clinical status and vitals. Because this event took place in a hospital setting and the patient adequately maintained her airway, respiratory rate, and oxygenation status, the decision was made to closely observe the patient in our clinic. Without additional intervention, the patient gradually regained full awareness, orientation, and mental capacity over the course of 90 minutes. She was ambulatory and conversant at the completion of the procedure, and she declined additional screening for drug abuse or transfer to an acute care facility. She elected for discharge and was accompanied by a family member to drive her home. Later, a search of the state’s prescription monitoring service revealed she had multiple prescriptions from numerous providers for benzodiazepines and opioids. We suspect that her intoxication was the result of ingestion or injection of an opioid medication when she left to visit the restroom unaccompanied, which occurred on at least one known occasion while awaiting tissue processing.
Patients may experience several side effects when using opioid analgesics, most commonly nausea and constipation. When opioids are used long-term, patients are at increased risk for developing fractures, as opioids may decrease bone mineral density by impairing the production of exogenous sex steroid hormones.1 Respiratory depression also can occur, especially when combined with alcohol and other medications such as benzodiazepines. Lastly, opioid dependence can develop in 1 week of regular use.1,2
If opioid overdose is suspected in the office setting, early intervention is critical. Rapid serum glucose should be obtained if a glucometer is available, as hypoglycemia can be confused with opioid toxicity and is easily correctable. If serum glucose is normal, the provider should notify emergency services. In a hospital setting, a rapid response or code can be initiated. In the office setting, dial 911. If not already in place, noninvasive continuous monitoring of the patient’s pulse, oxygen saturation, and blood pressure is needed.1
The provider’s primary concern should be ensuring the patient is adequately ventilated and oxygenated. If the patient’s respiratory rate is greater than 12 breaths per minute and oxygen saturation is greater than 90% on room air, as was the case with our patient, observe and reassess the patient frequently. If the oxygen saturation drops to less than 90% but the patient is breathing spontaneously, administer supplemental oxygen followed by naloxone. If the patient is breathing fewer than 12 breaths per minute, the airway can be maintained with the head tilt–chin lift technique while ventilating using a bag valve mask with supplemental oxygen, followed by administration of naloxone.1
Naloxone is a short-acting opioid antagonist used to treat potentially fatal respiratory depression associated with opioid overdose. It is available in intramuscular (IM), intravenous (IV), and intranasal forms. Intramuscular and IV administration are preferred due to a more rapid onset compared to intranasal. The dosage is 0.04 to 2 mg for IM or IV formulations and 4 mg for the intranasal formulation.1,3 The anterolateral thigh is the preferred IM injection site. Lower initial doses for the IM and IV forms generally are advisable because of the possibility of naloxone precipitating opioid withdrawal in opioid-dependent patients. Naloxone may be administered every 2 to 3 minutes until emergency personnel arrive. Repeat dosing of naloxone should be given until ventilation is greater than 12 breaths per minute while ensuring oxygen saturation is greater than 90%. If there is an inadequate response after 5 to 10 mg of naloxone administration, reconsider the diagnosis. If there is no response after naloxone administration, continue to provide respiratory support with the bag valve mask and supplemental oxygen. After the administration of naloxone, the patient should be transported to the nearest emergency department regardless of the clinical appearance, as naloxone’s half-life may be shorter than the ingested opioid, requiring further observation in a monitored setting.1,3
We recommend that dermatologists consider keeping naloxone in their offices. The medication is easily administered and has a relatively long shelf-life of 1 to 2 years, with a 10-mL vial of 0.4 mg/mL solution costing less than $200 in most cases.3 Increasing cases of opioid abuse could lead to more clinical scenarios similar to what we experienced. Proper identification and management of opioid overdose is within the purview of the dermatologist and can be lifesaving.
- Stolbach A, Hoffman RS. Acute opioid intoxication in adults. UpToDate website. https://www.uptodate.com/contents/acute-opioid-intoxication-in-adults?search=acute%20opioid%20intoxication%20in%20adults&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Updated October 1, 2019. Accessed July 23, 2020.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560.
- Pruyn S, Frey J, Baker B, et al. Quality assessment of expired naloxone products from first-responders’ supplies. Prehosp Emerg Care. 2018;23:647-653.
Dermatologists’ offices are not immune from potentially fatal medical events. As a result, it is imperative that dermatologists are well versed in how to manage emergency situations in an outpatient setting. We discuss signs, symptoms, and management of opioid toxicity with an instructive case from our outpatient, hospital-based dermatology clinic.
A 55-year-old woman presented for Mohs micrographic surgery for a large recurrent basal cell carcinoma on the right medial cheek. After informed consent was obtained and the procedure was discussed with the patient, she took one 0.5-mg tablet of clonazepam for perioperative anxiety, which was part of her standard home medication regimen and preoperative administration of clonazepam had been discussed with the treating physician prior to her appointment. During tissue processing, the patient waited alone in the procedure room, with nursing checks every 10 to 15 minutes. Roughly 30 minutes after the initial stage was taken and clear margins were confirmed, the patient was found to be somnolent and unresponsive to voice, light, or touch. Physical examination revealed pupillary constriction, labored breathing, and absent blink reflex. Subsequent examination of the arms, which initially were covered by sleeves, revealed track marks. She was only aroused by a deep sternal rub, which caused her to moan and open her eyes. Her vital signs remained stable, with oxygen saturation greater than 90% and respiratory rate greater than 12 breaths per minute, and a registered nurse remained at her bedside to monitor her clinical status and vitals. Because this event took place in a hospital setting and the patient adequately maintained her airway, respiratory rate, and oxygenation status, the decision was made to closely observe the patient in our clinic. Without additional intervention, the patient gradually regained full awareness, orientation, and mental capacity over the course of 90 minutes. She was ambulatory and conversant at the completion of the procedure, and she declined additional screening for drug abuse or transfer to an acute care facility. She elected for discharge and was accompanied by a family member to drive her home. Later, a search of the state’s prescription monitoring service revealed she had multiple prescriptions from numerous providers for benzodiazepines and opioids. We suspect that her intoxication was the result of ingestion or injection of an opioid medication when she left to visit the restroom unaccompanied, which occurred on at least one known occasion while awaiting tissue processing.
Patients may experience several side effects when using opioid analgesics, most commonly nausea and constipation. When opioids are used long-term, patients are at increased risk for developing fractures, as opioids may decrease bone mineral density by impairing the production of exogenous sex steroid hormones.1 Respiratory depression also can occur, especially when combined with alcohol and other medications such as benzodiazepines. Lastly, opioid dependence can develop in 1 week of regular use.1,2
If opioid overdose is suspected in the office setting, early intervention is critical. Rapid serum glucose should be obtained if a glucometer is available, as hypoglycemia can be confused with opioid toxicity and is easily correctable. If serum glucose is normal, the provider should notify emergency services. In a hospital setting, a rapid response or code can be initiated. In the office setting, dial 911. If not already in place, noninvasive continuous monitoring of the patient’s pulse, oxygen saturation, and blood pressure is needed.1
The provider’s primary concern should be ensuring the patient is adequately ventilated and oxygenated. If the patient’s respiratory rate is greater than 12 breaths per minute and oxygen saturation is greater than 90% on room air, as was the case with our patient, observe and reassess the patient frequently. If the oxygen saturation drops to less than 90% but the patient is breathing spontaneously, administer supplemental oxygen followed by naloxone. If the patient is breathing fewer than 12 breaths per minute, the airway can be maintained with the head tilt–chin lift technique while ventilating using a bag valve mask with supplemental oxygen, followed by administration of naloxone.1
Naloxone is a short-acting opioid antagonist used to treat potentially fatal respiratory depression associated with opioid overdose. It is available in intramuscular (IM), intravenous (IV), and intranasal forms. Intramuscular and IV administration are preferred due to a more rapid onset compared to intranasal. The dosage is 0.04 to 2 mg for IM or IV formulations and 4 mg for the intranasal formulation.1,3 The anterolateral thigh is the preferred IM injection site. Lower initial doses for the IM and IV forms generally are advisable because of the possibility of naloxone precipitating opioid withdrawal in opioid-dependent patients. Naloxone may be administered every 2 to 3 minutes until emergency personnel arrive. Repeat dosing of naloxone should be given until ventilation is greater than 12 breaths per minute while ensuring oxygen saturation is greater than 90%. If there is an inadequate response after 5 to 10 mg of naloxone administration, reconsider the diagnosis. If there is no response after naloxone administration, continue to provide respiratory support with the bag valve mask and supplemental oxygen. After the administration of naloxone, the patient should be transported to the nearest emergency department regardless of the clinical appearance, as naloxone’s half-life may be shorter than the ingested opioid, requiring further observation in a monitored setting.1,3
We recommend that dermatologists consider keeping naloxone in their offices. The medication is easily administered and has a relatively long shelf-life of 1 to 2 years, with a 10-mL vial of 0.4 mg/mL solution costing less than $200 in most cases.3 Increasing cases of opioid abuse could lead to more clinical scenarios similar to what we experienced. Proper identification and management of opioid overdose is within the purview of the dermatologist and can be lifesaving.
Dermatologists’ offices are not immune from potentially fatal medical events. As a result, it is imperative that dermatologists are well versed in how to manage emergency situations in an outpatient setting. We discuss signs, symptoms, and management of opioid toxicity with an instructive case from our outpatient, hospital-based dermatology clinic.
A 55-year-old woman presented for Mohs micrographic surgery for a large recurrent basal cell carcinoma on the right medial cheek. After informed consent was obtained and the procedure was discussed with the patient, she took one 0.5-mg tablet of clonazepam for perioperative anxiety, which was part of her standard home medication regimen and preoperative administration of clonazepam had been discussed with the treating physician prior to her appointment. During tissue processing, the patient waited alone in the procedure room, with nursing checks every 10 to 15 minutes. Roughly 30 minutes after the initial stage was taken and clear margins were confirmed, the patient was found to be somnolent and unresponsive to voice, light, or touch. Physical examination revealed pupillary constriction, labored breathing, and absent blink reflex. Subsequent examination of the arms, which initially were covered by sleeves, revealed track marks. She was only aroused by a deep sternal rub, which caused her to moan and open her eyes. Her vital signs remained stable, with oxygen saturation greater than 90% and respiratory rate greater than 12 breaths per minute, and a registered nurse remained at her bedside to monitor her clinical status and vitals. Because this event took place in a hospital setting and the patient adequately maintained her airway, respiratory rate, and oxygenation status, the decision was made to closely observe the patient in our clinic. Without additional intervention, the patient gradually regained full awareness, orientation, and mental capacity over the course of 90 minutes. She was ambulatory and conversant at the completion of the procedure, and she declined additional screening for drug abuse or transfer to an acute care facility. She elected for discharge and was accompanied by a family member to drive her home. Later, a search of the state’s prescription monitoring service revealed she had multiple prescriptions from numerous providers for benzodiazepines and opioids. We suspect that her intoxication was the result of ingestion or injection of an opioid medication when she left to visit the restroom unaccompanied, which occurred on at least one known occasion while awaiting tissue processing.
Patients may experience several side effects when using opioid analgesics, most commonly nausea and constipation. When opioids are used long-term, patients are at increased risk for developing fractures, as opioids may decrease bone mineral density by impairing the production of exogenous sex steroid hormones.1 Respiratory depression also can occur, especially when combined with alcohol and other medications such as benzodiazepines. Lastly, opioid dependence can develop in 1 week of regular use.1,2
If opioid overdose is suspected in the office setting, early intervention is critical. Rapid serum glucose should be obtained if a glucometer is available, as hypoglycemia can be confused with opioid toxicity and is easily correctable. If serum glucose is normal, the provider should notify emergency services. In a hospital setting, a rapid response or code can be initiated. In the office setting, dial 911. If not already in place, noninvasive continuous monitoring of the patient’s pulse, oxygen saturation, and blood pressure is needed.1
The provider’s primary concern should be ensuring the patient is adequately ventilated and oxygenated. If the patient’s respiratory rate is greater than 12 breaths per minute and oxygen saturation is greater than 90% on room air, as was the case with our patient, observe and reassess the patient frequently. If the oxygen saturation drops to less than 90% but the patient is breathing spontaneously, administer supplemental oxygen followed by naloxone. If the patient is breathing fewer than 12 breaths per minute, the airway can be maintained with the head tilt–chin lift technique while ventilating using a bag valve mask with supplemental oxygen, followed by administration of naloxone.1
Naloxone is a short-acting opioid antagonist used to treat potentially fatal respiratory depression associated with opioid overdose. It is available in intramuscular (IM), intravenous (IV), and intranasal forms. Intramuscular and IV administration are preferred due to a more rapid onset compared to intranasal. The dosage is 0.04 to 2 mg for IM or IV formulations and 4 mg for the intranasal formulation.1,3 The anterolateral thigh is the preferred IM injection site. Lower initial doses for the IM and IV forms generally are advisable because of the possibility of naloxone precipitating opioid withdrawal in opioid-dependent patients. Naloxone may be administered every 2 to 3 minutes until emergency personnel arrive. Repeat dosing of naloxone should be given until ventilation is greater than 12 breaths per minute while ensuring oxygen saturation is greater than 90%. If there is an inadequate response after 5 to 10 mg of naloxone administration, reconsider the diagnosis. If there is no response after naloxone administration, continue to provide respiratory support with the bag valve mask and supplemental oxygen. After the administration of naloxone, the patient should be transported to the nearest emergency department regardless of the clinical appearance, as naloxone’s half-life may be shorter than the ingested opioid, requiring further observation in a monitored setting.1,3
We recommend that dermatologists consider keeping naloxone in their offices. The medication is easily administered and has a relatively long shelf-life of 1 to 2 years, with a 10-mL vial of 0.4 mg/mL solution costing less than $200 in most cases.3 Increasing cases of opioid abuse could lead to more clinical scenarios similar to what we experienced. Proper identification and management of opioid overdose is within the purview of the dermatologist and can be lifesaving.
- Stolbach A, Hoffman RS. Acute opioid intoxication in adults. UpToDate website. https://www.uptodate.com/contents/acute-opioid-intoxication-in-adults?search=acute%20opioid%20intoxication%20in%20adults&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Updated October 1, 2019. Accessed July 23, 2020.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560.
- Pruyn S, Frey J, Baker B, et al. Quality assessment of expired naloxone products from first-responders’ supplies. Prehosp Emerg Care. 2018;23:647-653.
- Stolbach A, Hoffman RS. Acute opioid intoxication in adults. UpToDate website. https://www.uptodate.com/contents/acute-opioid-intoxication-in-adults?search=acute%20opioid%20intoxication%20in%20adults&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Updated October 1, 2019. Accessed July 23, 2020.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560.
- Pruyn S, Frey J, Baker B, et al. Quality assessment of expired naloxone products from first-responders’ supplies. Prehosp Emerg Care. 2018;23:647-653.
Practice Points
- Opioid overdose continues to be a major public health concern. Dermatologists may encounter opioid toxicity in their practice, and prompt recognition and treatment are crucial.
- Naloxone is a quick-acting, easy-to-use, and relatively inexpensive medication that can easily be stored and administered in dermatologists’ offices
What’s Eating You? Cheyletiella Mites
Identifying Characteristics and Disease Transmission
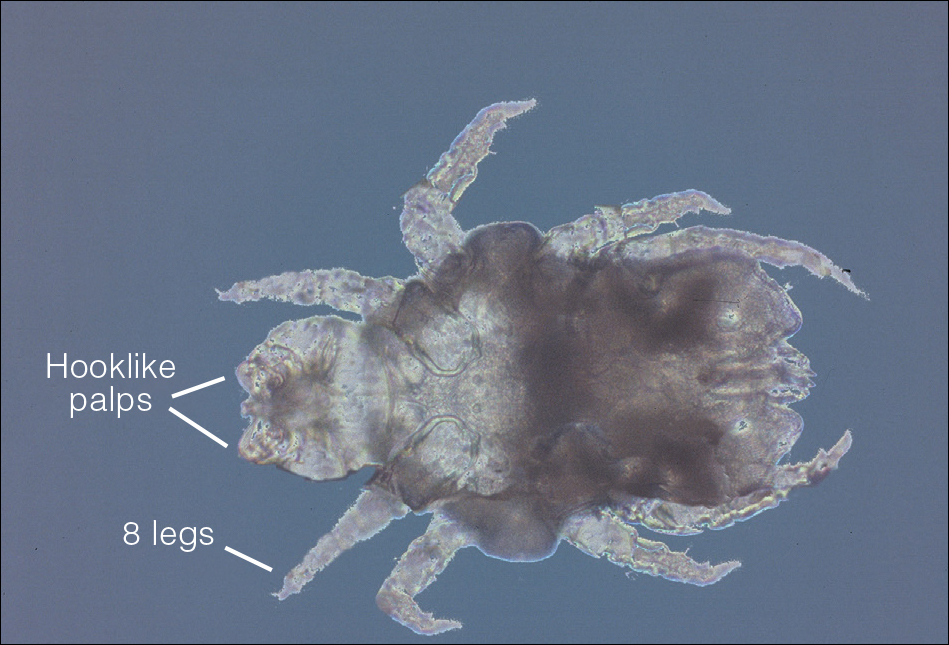
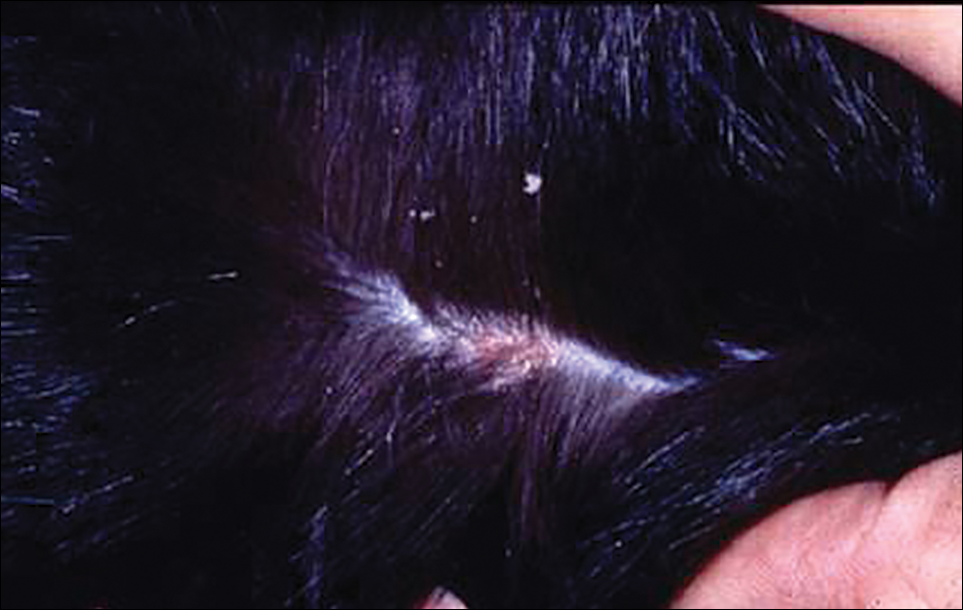
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4
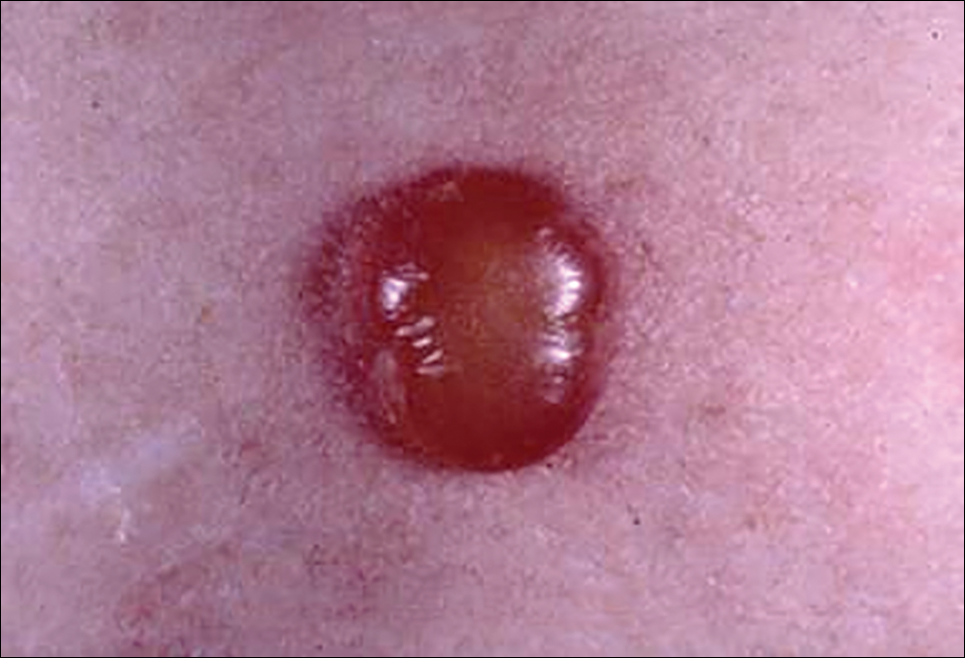
The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11
Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
Identifying Characteristics and Disease Transmission
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4


The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11

Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
Identifying Characteristics and Disease Transmission
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4


The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11

Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
Practice Points
- Cheyletiella mites can cause a range of cutaneous and systemic symptoms in affected individuals.
- Diagnosis can be difficult and requires a high level of suspicion, with inquiries directed at animal exposures.
- Identification of the animal vector and treatment by a knowledgeable veterinarian is necessary to prevent recurrence in humans.
What’s Eating You? Lone Star Tick (Amblyomma americanum)
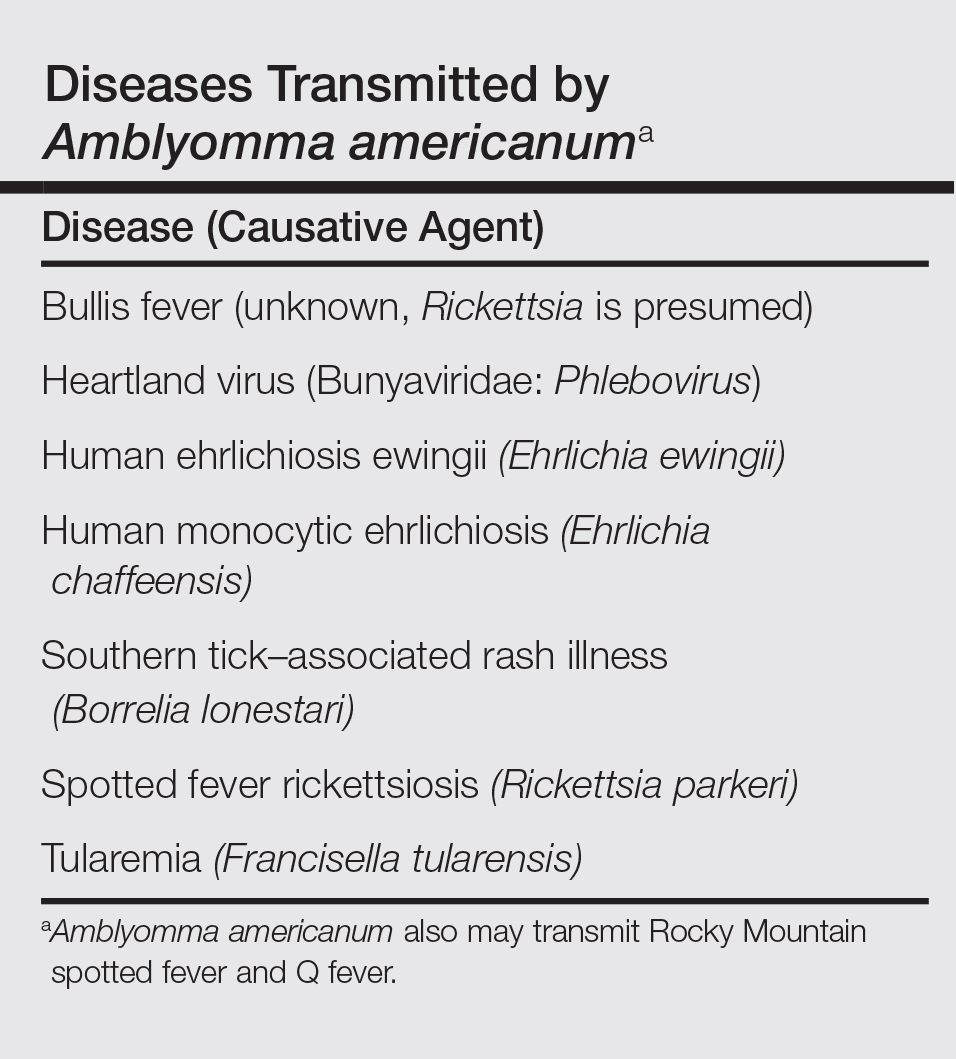
The lone star tick (Amblyomma americanum) is distributed throughout much of the eastern United States. It serves as a vector for species of Rickettsia, Ehrlichia, and Borrelia that are an important cause of tick-borne illness (Table). In addition, the bite of the lone star tick can cause impressive local and systemic reactions. Delayed anaphylaxis to ingestion of red meat has been attributed to the bite of A americanum.1 Herein, we discuss human disease associated with the lone star tick as well as potential tick-control measures.
Tick Characteristics
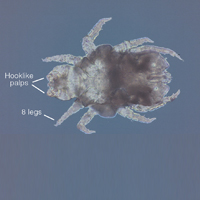
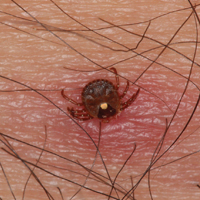
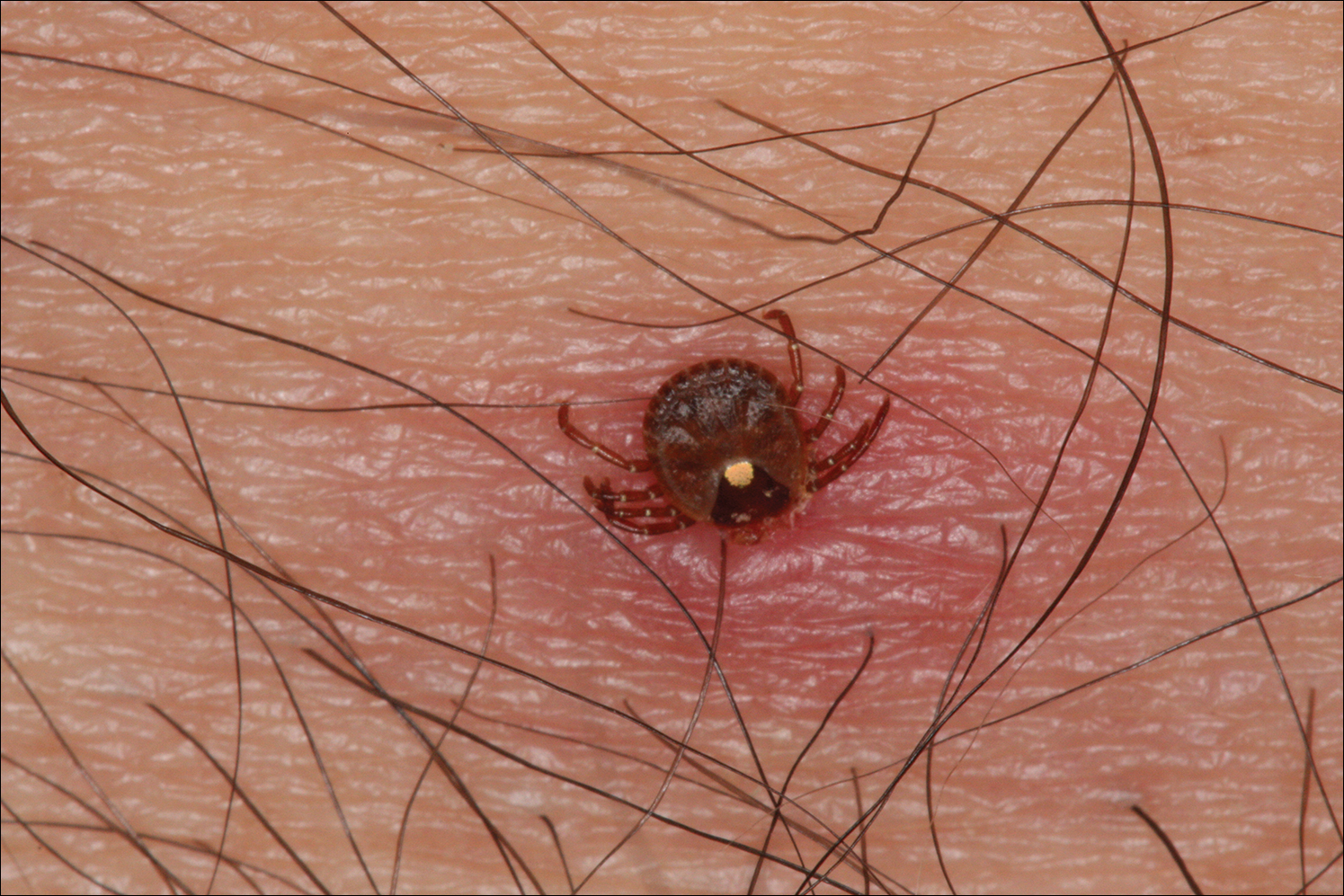
Lone star ticks are characterized by long anterior mouthparts and an ornate scutum (hard dorsal plate). Widely spaced eyes and posterior festoons also are present. In contrast to some other ticks, adanal plates are absent on the ventral surface in male lone star ticks. Amblyomma americanum demonstrates a single white spot on the female’s scutum (Figure 1). The male has inverted horseshoe markings on the posterior scutum. The female’s scutum often covers only a portion of the body to allow room for engorgement.
Patients usually become aware of tick bites while the tick is still attached to the skin, which provides the physician with an opportunity to identify the tick and discuss tick-control measures as well as symptoms of tick-borne disease. Once the tick has been removed, delayed-type hypersensitivity to the tick antigens continues at the attachment site. Erythema and pruritus can be dramatic. Nodules with a pseudolymphomatous histology can occur. Milder reactions respond to application of topical corticosteroids. More intense reactions may require intralesional corticosteroid injection or even surgical excision.
Most hard ticks have a 3-host life cycle, meaning they attach for one long blood meal during each phase of the life cycle. Because they search for a new host for each blood meal, they are efficient disease vectors. The larval ticks, so-called seed ticks, have 6 legs and feed on small animals. Nymphs and adults feed on larger animals. Nymphs resemble small adult ticks with 8 legs but are sexually immature.
Distribution
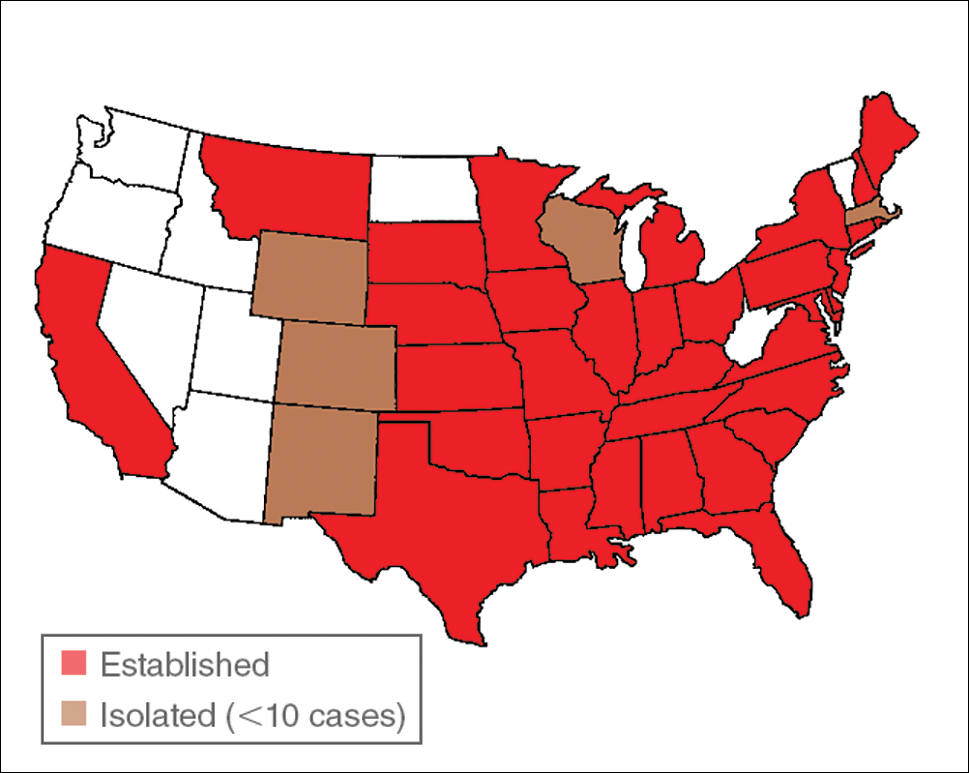
Amblyomma americanum has a wide distribution in the United States from Texas to Iowa and as far north as Maine (Figure 2).2 Tick attachments often are seen in individuals who work outdoors, especially in areas where new commercial or residential development disrupts the environment and the tick’s usual hosts move out of the area. Hungry ticks are left behind in search of a host.
Disease Transmission
Lone star ticks have been implicated as vectors of Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME),3 which has been documented from the mid-Atlantic to south-central United States. It may present as a somewhat milder Rocky Mountain spotted fever–like illness with fever and headache or as a life-threatening systemic illness with organ failure. Prompt diagnosis and treatment with a tetracycline has been correlated with a better prognosis.4 Immunofluorescent antibody testing and polymerase chain reaction can be used to establish the diagnosis.5 Two tick species—A americanum and Dermacentor variabilis—have been implicated as vectors, but A americanum appears to be the major vector.6,7
The lone star tick also is a vector for Erlichia ewingii, the cause of human ehrlichiosis ewingii. Human ehrlichiosis ewingii is a rare disease that presents similar to HME, with most reported cases occurring in immunocompromised hosts.8
A novel member of the Phlebovirus genus, the Heartland virus, was first described in 2 Missouri farmers who presented with symptoms similar to HME but did not respond to doxycycline treatment.9 The virus has since been isolated from A americanum adult ticks, implicating them as the major vectors of the disease.10
Rickettsia parkeri, a cause of spotted fever rickettsiosis, is responsible for an eschar-associated illness in affected individuals.11 The organism has been detected in A americanum ticks collected from the wild. Experiments show the tick is capable of transmitting R parkeri to animals in the laboratory. It is unclear, however, what role A americanum plays in the natural transmission of the disease.12
In Missouri, strains of Borrelia have been isolated from A americanum ticks that feed on cottontail rabbits, but it seems unlikely that the tick plays any role in transmission of true Lyme disease13,14; Borrelia has been shown to have poor survival in the saliva of A americanum beyond 24 hours.15 Southern tick–associated rash illness is a Lyme disease–like illness with several reported cases due to A americanum.16 Patients generally present with an erythema migrans–like rash and may have headache, fever, arthralgia, or myalgia.16 The causative organism remains unclear, though Borrelia lonestari has been implicated.17 Lone star ticks also transmit tularemia and may transmit Rocky Mountain spotted fever and Q fever.13
Bullis fever (first reported at Camp Bullis near San Antonio, Texas) affected huge numbers of military personnel from 1942 to 1943.18 The causative organism appears to be rickettsial. During one outbreak of Bullis fever, it was noted that A americanum was so numerous that more than 4000 adult ticks were collected under a single juniper tree and more than 1000 ticks were removed from a single soldier who sat in a thicket for 2 hours.12 No cases of Bullis fever have been reported in recent years,12 which probably relates to the introduction of fire ants.
Disease Hosts
At Little Rock Air Force Base in Arkansas, A americanum has been a source of Ehrlichia infection. During one outbreak, deer in the area were found to have as many as 2550 ticks per ear,19 which demonstrates the magnitude of tick infestation in some areas of the United States. Tick infestation is not merely of concern to the US military. Ticks are ubiquitous and can be found on neatly trimmed suburban lawns as well as in rough thickets.
More recently, bites from A americanum have been found to induce allergies to red meat in some patients.1 IgE antibodies directed against galactose-alpha-1,3-galactose (alpha gal) have been implicated as the cause of this reaction. These antibodies cause delayed-onset anaphylaxis occurring 3 to 6 hours after ingestion of red meat. Tick bites appear to be the most important and perhaps the only cause of IgE antibodies to alpha gal in the United States.1
Wild white-tailed deer serve as reservoir hosts for several diseases transmitted by A americanum, including HME, human ehrlichiosis ewingii, and Southern tick–associated rash illness.12,20 Communities located close to wildlife reserves may have higher rates of infection.21 Application of acaricides to corn contained in deer feeders has been shown to be an effective method of decreasing local tick populations, which is a potential method for disease control in at-risk areas, though it is costly and time consuming.22
Tick-Control Measures
Hard ticks produce little urine. Instead, excess water is eliminated via salivation back into the host. Loss of water also occurs through spiracles. Absorption of water from the atmosphere is important for the tick to maintain hydration. The tick produces intensely hygroscopic saliva that absorbs water from surrounding moist air. The humidified saliva is then reingested by the tick. In hot climates, ticks are prone to dehydration unless they can find a source of moist air, usually within a layer of leaf debris.23 When the leaf debris is stirred by a human walking through the area, the tick can make contact with the human. Therefore, removal of leaf debris is a critical part of tick-control efforts, as it reduces tick numbers by means of dehydration. Tick eggs also require sufficient humidity to hatch. Leaf removal increases the effectiveness of insecticide applications, which would otherwise do little harm to the ticks below if sprayed on top of leaf debris.
Some lone star ticks attach to birds and disseminate widely. Attachments to animal hosts with long-range migration patterns complicate tick-control efforts.24 Animal migration may contribute to the spread of disease from one geographic region to another.
Imported fire ants are voracious eaters that gather and consume ticks eggs. Fire ants provide an excellent natural means of tick control. Tick numbers in places such as Camp Bullis have declined dramatically since the introduction of imported fire ants.25
- Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13:354-359.
- Springer YP, Eisen L, Beati L, et al. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol. 2014;51:342-351.
- Yu X, Piesman JF, Olson JG, et al. Geographic distribution of different genetic types of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1997;56:679-680.
- Dumler JS, Bakken JS. Human ehrlichiosis: newly recognized infections transmitted by ticks. An Rev Med. 1998;49:201-213.
- Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(suppl 1):S45-S51.
- Lockhart JM, Davidson WR, Stallknecht DE, et al. Natural history of Ehrlichia chaffeensis (Ricketsiales: Ehrlichiea) in the piedmont physiographic province of Georgia. J Parasitol. 1997;83:887-894.
- Centers for Disease Control and Prevention (CDC). Human ehrlichiosis—Maryland, 1994. MMWR Morb Mortal Wkly Rep. 1996;45:798-802.
- Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:261-292.
- McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834-841.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae) [published online March 30, 2016]. J Med Entomol. pii:tjw028.
- Cragun WC, Bartlett BL, Ellis MW, et al. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch Dermatol. 2010;146:641-648.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805-811.
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.) in human and animal diseases. Vet Parasitol. 2009;160:1-12.
- Oliver JH, Kollars TM, Chandler FW, et al. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J Clin Microbiol. 1998;36:1-5.
- Ledin KE, Zeidner NS, Ribeiro JM, et al. Borreliacidal activity of saliva of the tick Amblyomma americanum. Med Vet Entomol. 2005;19:90-95.
- Feder HM Jr, Hoss DM, Zemel L, et al. Southern tick-associated rash illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis. 2011;53:e142-e146.
- Varela AS, Luttrell MP, Howerth EW, et al. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol. 2004;42:1163-1169.
- Livesay HR, Pollard M. Laboratory report on a clinical syndrome referred to as “Bullis Fever.” Am J Trop Med. 1943;23:475-479.
- Goddard J. Ticks and tickborne diseases affecting military personnel. US Air Force School of Aerospace Medicine USAFSAM-SR-89-2. http://www.dtic.mil/dtic/tr/fulltext/u2/a221956.pdf. Published September 1989. Accessed January 19, 2017.
- Lockhart JM, Davidson WR, Stallkneeckt DE, et al. Isolation of Ehrlichia chaffeensis from wild white tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681-1686.
- Standaert SM, Dawson JE, Schaffner W, et al. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420-425.
- Schulze TL, Jordan RA, Hung RW, et al. Effectiveness of the 4-Poster passive topical treatment device in the control of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in New Jersey. Vector Borne Zoonotic Dis. 2009;9:389-400.
- Strey OF, Teel PD, Longnecker MT, et al. Survival and water-balance characteristics of unfed Amblyomma cajennense (Acari: Ixodidae). J Med Entomol. 1996;33:63-73.
- Popham TW, Garris GI, Barre N. Development of a computer model of the population dynamics of Amblyomma variegatum and simulations of eradication strategies for use in the Caribbean. Ann New York Acad Sci. 1996;791:452-465.
- Burns EC, Melancon DG. Effect of important fire ant (Hymenoptera: Formicidae) invasion on lone star tick (Acarina: Ixodidae) populations. J Med Entomol. 1977;14:247-249.
The lone star tick (Amblyomma americanum) is distributed throughout much of the eastern United States. It serves as a vector for species of Rickettsia, Ehrlichia, and Borrelia that are an important cause of tick-borne illness (Table). In addition, the bite of the lone star tick can cause impressive local and systemic reactions. Delayed anaphylaxis to ingestion of red meat has been attributed to the bite of A americanum.1 Herein, we discuss human disease associated with the lone star tick as well as potential tick-control measures.
Tick Characteristics
Lone star ticks are characterized by long anterior mouthparts and an ornate scutum (hard dorsal plate). Widely spaced eyes and posterior festoons also are present. In contrast to some other ticks, adanal plates are absent on the ventral surface in male lone star ticks. Amblyomma americanum demonstrates a single white spot on the female’s scutum (Figure 1). The male has inverted horseshoe markings on the posterior scutum. The female’s scutum often covers only a portion of the body to allow room for engorgement.

Patients usually become aware of tick bites while the tick is still attached to the skin, which provides the physician with an opportunity to identify the tick and discuss tick-control measures as well as symptoms of tick-borne disease. Once the tick has been removed, delayed-type hypersensitivity to the tick antigens continues at the attachment site. Erythema and pruritus can be dramatic. Nodules with a pseudolymphomatous histology can occur. Milder reactions respond to application of topical corticosteroids. More intense reactions may require intralesional corticosteroid injection or even surgical excision.
Most hard ticks have a 3-host life cycle, meaning they attach for one long blood meal during each phase of the life cycle. Because they search for a new host for each blood meal, they are efficient disease vectors. The larval ticks, so-called seed ticks, have 6 legs and feed on small animals. Nymphs and adults feed on larger animals. Nymphs resemble small adult ticks with 8 legs but are sexually immature.
Distribution
Amblyomma americanum has a wide distribution in the United States from Texas to Iowa and as far north as Maine (Figure 2).2 Tick attachments often are seen in individuals who work outdoors, especially in areas where new commercial or residential development disrupts the environment and the tick’s usual hosts move out of the area. Hungry ticks are left behind in search of a host.

Disease Transmission
Lone star ticks have been implicated as vectors of Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME),3 which has been documented from the mid-Atlantic to south-central United States. It may present as a somewhat milder Rocky Mountain spotted fever–like illness with fever and headache or as a life-threatening systemic illness with organ failure. Prompt diagnosis and treatment with a tetracycline has been correlated with a better prognosis.4 Immunofluorescent antibody testing and polymerase chain reaction can be used to establish the diagnosis.5 Two tick species—A americanum and Dermacentor variabilis—have been implicated as vectors, but A americanum appears to be the major vector.6,7
The lone star tick also is a vector for Erlichia ewingii, the cause of human ehrlichiosis ewingii. Human ehrlichiosis ewingii is a rare disease that presents similar to HME, with most reported cases occurring in immunocompromised hosts.8
A novel member of the Phlebovirus genus, the Heartland virus, was first described in 2 Missouri farmers who presented with symptoms similar to HME but did not respond to doxycycline treatment.9 The virus has since been isolated from A americanum adult ticks, implicating them as the major vectors of the disease.10
Rickettsia parkeri, a cause of spotted fever rickettsiosis, is responsible for an eschar-associated illness in affected individuals.11 The organism has been detected in A americanum ticks collected from the wild. Experiments show the tick is capable of transmitting R parkeri to animals in the laboratory. It is unclear, however, what role A americanum plays in the natural transmission of the disease.12
In Missouri, strains of Borrelia have been isolated from A americanum ticks that feed on cottontail rabbits, but it seems unlikely that the tick plays any role in transmission of true Lyme disease13,14; Borrelia has been shown to have poor survival in the saliva of A americanum beyond 24 hours.15 Southern tick–associated rash illness is a Lyme disease–like illness with several reported cases due to A americanum.16 Patients generally present with an erythema migrans–like rash and may have headache, fever, arthralgia, or myalgia.16 The causative organism remains unclear, though Borrelia lonestari has been implicated.17 Lone star ticks also transmit tularemia and may transmit Rocky Mountain spotted fever and Q fever.13
Bullis fever (first reported at Camp Bullis near San Antonio, Texas) affected huge numbers of military personnel from 1942 to 1943.18 The causative organism appears to be rickettsial. During one outbreak of Bullis fever, it was noted that A americanum was so numerous that more than 4000 adult ticks were collected under a single juniper tree and more than 1000 ticks were removed from a single soldier who sat in a thicket for 2 hours.12 No cases of Bullis fever have been reported in recent years,12 which probably relates to the introduction of fire ants.
Disease Hosts
At Little Rock Air Force Base in Arkansas, A americanum has been a source of Ehrlichia infection. During one outbreak, deer in the area were found to have as many as 2550 ticks per ear,19 which demonstrates the magnitude of tick infestation in some areas of the United States. Tick infestation is not merely of concern to the US military. Ticks are ubiquitous and can be found on neatly trimmed suburban lawns as well as in rough thickets.
More recently, bites from A americanum have been found to induce allergies to red meat in some patients.1 IgE antibodies directed against galactose-alpha-1,3-galactose (alpha gal) have been implicated as the cause of this reaction. These antibodies cause delayed-onset anaphylaxis occurring 3 to 6 hours after ingestion of red meat. Tick bites appear to be the most important and perhaps the only cause of IgE antibodies to alpha gal in the United States.1
Wild white-tailed deer serve as reservoir hosts for several diseases transmitted by A americanum, including HME, human ehrlichiosis ewingii, and Southern tick–associated rash illness.12,20 Communities located close to wildlife reserves may have higher rates of infection.21 Application of acaricides to corn contained in deer feeders has been shown to be an effective method of decreasing local tick populations, which is a potential method for disease control in at-risk areas, though it is costly and time consuming.22
Tick-Control Measures
Hard ticks produce little urine. Instead, excess water is eliminated via salivation back into the host. Loss of water also occurs through spiracles. Absorption of water from the atmosphere is important for the tick to maintain hydration. The tick produces intensely hygroscopic saliva that absorbs water from surrounding moist air. The humidified saliva is then reingested by the tick. In hot climates, ticks are prone to dehydration unless they can find a source of moist air, usually within a layer of leaf debris.23 When the leaf debris is stirred by a human walking through the area, the tick can make contact with the human. Therefore, removal of leaf debris is a critical part of tick-control efforts, as it reduces tick numbers by means of dehydration. Tick eggs also require sufficient humidity to hatch. Leaf removal increases the effectiveness of insecticide applications, which would otherwise do little harm to the ticks below if sprayed on top of leaf debris.
Some lone star ticks attach to birds and disseminate widely. Attachments to animal hosts with long-range migration patterns complicate tick-control efforts.24 Animal migration may contribute to the spread of disease from one geographic region to another.
Imported fire ants are voracious eaters that gather and consume ticks eggs. Fire ants provide an excellent natural means of tick control. Tick numbers in places such as Camp Bullis have declined dramatically since the introduction of imported fire ants.25
The lone star tick (Amblyomma americanum) is distributed throughout much of the eastern United States. It serves as a vector for species of Rickettsia, Ehrlichia, and Borrelia that are an important cause of tick-borne illness (Table). In addition, the bite of the lone star tick can cause impressive local and systemic reactions. Delayed anaphylaxis to ingestion of red meat has been attributed to the bite of A americanum.1 Herein, we discuss human disease associated with the lone star tick as well as potential tick-control measures.
Tick Characteristics
Lone star ticks are characterized by long anterior mouthparts and an ornate scutum (hard dorsal plate). Widely spaced eyes and posterior festoons also are present. In contrast to some other ticks, adanal plates are absent on the ventral surface in male lone star ticks. Amblyomma americanum demonstrates a single white spot on the female’s scutum (Figure 1). The male has inverted horseshoe markings on the posterior scutum. The female’s scutum often covers only a portion of the body to allow room for engorgement.

Patients usually become aware of tick bites while the tick is still attached to the skin, which provides the physician with an opportunity to identify the tick and discuss tick-control measures as well as symptoms of tick-borne disease. Once the tick has been removed, delayed-type hypersensitivity to the tick antigens continues at the attachment site. Erythema and pruritus can be dramatic. Nodules with a pseudolymphomatous histology can occur. Milder reactions respond to application of topical corticosteroids. More intense reactions may require intralesional corticosteroid injection or even surgical excision.
Most hard ticks have a 3-host life cycle, meaning they attach for one long blood meal during each phase of the life cycle. Because they search for a new host for each blood meal, they are efficient disease vectors. The larval ticks, so-called seed ticks, have 6 legs and feed on small animals. Nymphs and adults feed on larger animals. Nymphs resemble small adult ticks with 8 legs but are sexually immature.
Distribution
Amblyomma americanum has a wide distribution in the United States from Texas to Iowa and as far north as Maine (Figure 2).2 Tick attachments often are seen in individuals who work outdoors, especially in areas where new commercial or residential development disrupts the environment and the tick’s usual hosts move out of the area. Hungry ticks are left behind in search of a host.

Disease Transmission
Lone star ticks have been implicated as vectors of Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME),3 which has been documented from the mid-Atlantic to south-central United States. It may present as a somewhat milder Rocky Mountain spotted fever–like illness with fever and headache or as a life-threatening systemic illness with organ failure. Prompt diagnosis and treatment with a tetracycline has been correlated with a better prognosis.4 Immunofluorescent antibody testing and polymerase chain reaction can be used to establish the diagnosis.5 Two tick species—A americanum and Dermacentor variabilis—have been implicated as vectors, but A americanum appears to be the major vector.6,7
The lone star tick also is a vector for Erlichia ewingii, the cause of human ehrlichiosis ewingii. Human ehrlichiosis ewingii is a rare disease that presents similar to HME, with most reported cases occurring in immunocompromised hosts.8
A novel member of the Phlebovirus genus, the Heartland virus, was first described in 2 Missouri farmers who presented with symptoms similar to HME but did not respond to doxycycline treatment.9 The virus has since been isolated from A americanum adult ticks, implicating them as the major vectors of the disease.10
Rickettsia parkeri, a cause of spotted fever rickettsiosis, is responsible for an eschar-associated illness in affected individuals.11 The organism has been detected in A americanum ticks collected from the wild. Experiments show the tick is capable of transmitting R parkeri to animals in the laboratory. It is unclear, however, what role A americanum plays in the natural transmission of the disease.12
In Missouri, strains of Borrelia have been isolated from A americanum ticks that feed on cottontail rabbits, but it seems unlikely that the tick plays any role in transmission of true Lyme disease13,14; Borrelia has been shown to have poor survival in the saliva of A americanum beyond 24 hours.15 Southern tick–associated rash illness is a Lyme disease–like illness with several reported cases due to A americanum.16 Patients generally present with an erythema migrans–like rash and may have headache, fever, arthralgia, or myalgia.16 The causative organism remains unclear, though Borrelia lonestari has been implicated.17 Lone star ticks also transmit tularemia and may transmit Rocky Mountain spotted fever and Q fever.13
Bullis fever (first reported at Camp Bullis near San Antonio, Texas) affected huge numbers of military personnel from 1942 to 1943.18 The causative organism appears to be rickettsial. During one outbreak of Bullis fever, it was noted that A americanum was so numerous that more than 4000 adult ticks were collected under a single juniper tree and more than 1000 ticks were removed from a single soldier who sat in a thicket for 2 hours.12 No cases of Bullis fever have been reported in recent years,12 which probably relates to the introduction of fire ants.
Disease Hosts
At Little Rock Air Force Base in Arkansas, A americanum has been a source of Ehrlichia infection. During one outbreak, deer in the area were found to have as many as 2550 ticks per ear,19 which demonstrates the magnitude of tick infestation in some areas of the United States. Tick infestation is not merely of concern to the US military. Ticks are ubiquitous and can be found on neatly trimmed suburban lawns as well as in rough thickets.
More recently, bites from A americanum have been found to induce allergies to red meat in some patients.1 IgE antibodies directed against galactose-alpha-1,3-galactose (alpha gal) have been implicated as the cause of this reaction. These antibodies cause delayed-onset anaphylaxis occurring 3 to 6 hours after ingestion of red meat. Tick bites appear to be the most important and perhaps the only cause of IgE antibodies to alpha gal in the United States.1
Wild white-tailed deer serve as reservoir hosts for several diseases transmitted by A americanum, including HME, human ehrlichiosis ewingii, and Southern tick–associated rash illness.12,20 Communities located close to wildlife reserves may have higher rates of infection.21 Application of acaricides to corn contained in deer feeders has been shown to be an effective method of decreasing local tick populations, which is a potential method for disease control in at-risk areas, though it is costly and time consuming.22
Tick-Control Measures
Hard ticks produce little urine. Instead, excess water is eliminated via salivation back into the host. Loss of water also occurs through spiracles. Absorption of water from the atmosphere is important for the tick to maintain hydration. The tick produces intensely hygroscopic saliva that absorbs water from surrounding moist air. The humidified saliva is then reingested by the tick. In hot climates, ticks are prone to dehydration unless they can find a source of moist air, usually within a layer of leaf debris.23 When the leaf debris is stirred by a human walking through the area, the tick can make contact with the human. Therefore, removal of leaf debris is a critical part of tick-control efforts, as it reduces tick numbers by means of dehydration. Tick eggs also require sufficient humidity to hatch. Leaf removal increases the effectiveness of insecticide applications, which would otherwise do little harm to the ticks below if sprayed on top of leaf debris.
Some lone star ticks attach to birds and disseminate widely. Attachments to animal hosts with long-range migration patterns complicate tick-control efforts.24 Animal migration may contribute to the spread of disease from one geographic region to another.
Imported fire ants are voracious eaters that gather and consume ticks eggs. Fire ants provide an excellent natural means of tick control. Tick numbers in places such as Camp Bullis have declined dramatically since the introduction of imported fire ants.25
- Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13:354-359.
- Springer YP, Eisen L, Beati L, et al. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol. 2014;51:342-351.
- Yu X, Piesman JF, Olson JG, et al. Geographic distribution of different genetic types of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1997;56:679-680.
- Dumler JS, Bakken JS. Human ehrlichiosis: newly recognized infections transmitted by ticks. An Rev Med. 1998;49:201-213.
- Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(suppl 1):S45-S51.
- Lockhart JM, Davidson WR, Stallknecht DE, et al. Natural history of Ehrlichia chaffeensis (Ricketsiales: Ehrlichiea) in the piedmont physiographic province of Georgia. J Parasitol. 1997;83:887-894.
- Centers for Disease Control and Prevention (CDC). Human ehrlichiosis—Maryland, 1994. MMWR Morb Mortal Wkly Rep. 1996;45:798-802.
- Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:261-292.
- McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834-841.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae) [published online March 30, 2016]. J Med Entomol. pii:tjw028.
- Cragun WC, Bartlett BL, Ellis MW, et al. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch Dermatol. 2010;146:641-648.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805-811.
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.) in human and animal diseases. Vet Parasitol. 2009;160:1-12.
- Oliver JH, Kollars TM, Chandler FW, et al. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J Clin Microbiol. 1998;36:1-5.
- Ledin KE, Zeidner NS, Ribeiro JM, et al. Borreliacidal activity of saliva of the tick Amblyomma americanum. Med Vet Entomol. 2005;19:90-95.
- Feder HM Jr, Hoss DM, Zemel L, et al. Southern tick-associated rash illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis. 2011;53:e142-e146.
- Varela AS, Luttrell MP, Howerth EW, et al. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol. 2004;42:1163-1169.
- Livesay HR, Pollard M. Laboratory report on a clinical syndrome referred to as “Bullis Fever.” Am J Trop Med. 1943;23:475-479.
- Goddard J. Ticks and tickborne diseases affecting military personnel. US Air Force School of Aerospace Medicine USAFSAM-SR-89-2. http://www.dtic.mil/dtic/tr/fulltext/u2/a221956.pdf. Published September 1989. Accessed January 19, 2017.
- Lockhart JM, Davidson WR, Stallkneeckt DE, et al. Isolation of Ehrlichia chaffeensis from wild white tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681-1686.
- Standaert SM, Dawson JE, Schaffner W, et al. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420-425.
- Schulze TL, Jordan RA, Hung RW, et al. Effectiveness of the 4-Poster passive topical treatment device in the control of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in New Jersey. Vector Borne Zoonotic Dis. 2009;9:389-400.
- Strey OF, Teel PD, Longnecker MT, et al. Survival and water-balance characteristics of unfed Amblyomma cajennense (Acari: Ixodidae). J Med Entomol. 1996;33:63-73.
- Popham TW, Garris GI, Barre N. Development of a computer model of the population dynamics of Amblyomma variegatum and simulations of eradication strategies for use in the Caribbean. Ann New York Acad Sci. 1996;791:452-465.
- Burns EC, Melancon DG. Effect of important fire ant (Hymenoptera: Formicidae) invasion on lone star tick (Acarina: Ixodidae) populations. J Med Entomol. 1977;14:247-249.
- Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13:354-359.
- Springer YP, Eisen L, Beati L, et al. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol. 2014;51:342-351.
- Yu X, Piesman JF, Olson JG, et al. Geographic distribution of different genetic types of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1997;56:679-680.
- Dumler JS, Bakken JS. Human ehrlichiosis: newly recognized infections transmitted by ticks. An Rev Med. 1998;49:201-213.
- Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(suppl 1):S45-S51.
- Lockhart JM, Davidson WR, Stallknecht DE, et al. Natural history of Ehrlichia chaffeensis (Ricketsiales: Ehrlichiea) in the piedmont physiographic province of Georgia. J Parasitol. 1997;83:887-894.
- Centers for Disease Control and Prevention (CDC). Human ehrlichiosis—Maryland, 1994. MMWR Morb Mortal Wkly Rep. 1996;45:798-802.
- Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:261-292.
- McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834-841.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae) [published online March 30, 2016]. J Med Entomol. pii:tjw028.
- Cragun WC, Bartlett BL, Ellis MW, et al. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch Dermatol. 2010;146:641-648.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805-811.
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.) in human and animal diseases. Vet Parasitol. 2009;160:1-12.
- Oliver JH, Kollars TM, Chandler FW, et al. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J Clin Microbiol. 1998;36:1-5.
- Ledin KE, Zeidner NS, Ribeiro JM, et al. Borreliacidal activity of saliva of the tick Amblyomma americanum. Med Vet Entomol. 2005;19:90-95.
- Feder HM Jr, Hoss DM, Zemel L, et al. Southern tick-associated rash illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis. 2011;53:e142-e146.
- Varela AS, Luttrell MP, Howerth EW, et al. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol. 2004;42:1163-1169.
- Livesay HR, Pollard M. Laboratory report on a clinical syndrome referred to as “Bullis Fever.” Am J Trop Med. 1943;23:475-479.
- Goddard J. Ticks and tickborne diseases affecting military personnel. US Air Force School of Aerospace Medicine USAFSAM-SR-89-2. http://www.dtic.mil/dtic/tr/fulltext/u2/a221956.pdf. Published September 1989. Accessed January 19, 2017.
- Lockhart JM, Davidson WR, Stallkneeckt DE, et al. Isolation of Ehrlichia chaffeensis from wild white tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681-1686.
- Standaert SM, Dawson JE, Schaffner W, et al. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420-425.
- Schulze TL, Jordan RA, Hung RW, et al. Effectiveness of the 4-Poster passive topical treatment device in the control of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in New Jersey. Vector Borne Zoonotic Dis. 2009;9:389-400.
- Strey OF, Teel PD, Longnecker MT, et al. Survival and water-balance characteristics of unfed Amblyomma cajennense (Acari: Ixodidae). J Med Entomol. 1996;33:63-73.
- Popham TW, Garris GI, Barre N. Development of a computer model of the population dynamics of Amblyomma variegatum and simulations of eradication strategies for use in the Caribbean. Ann New York Acad Sci. 1996;791:452-465.
- Burns EC, Melancon DG. Effect of important fire ant (Hymenoptera: Formicidae) invasion on lone star tick (Acarina: Ixodidae) populations. J Med Entomol. 1977;14:247-249.
Practice Points
- Amblyomma americanum (lone star tick) is widely distributed throughout the United States and is an important cause of several tick-borne illnesses.
- Prompt diagnosis and treatment of tick-borne disease improves patient outcomes.
- In some cases, tick bites may cause the human host to develop certain IgE antibodies that result in a delayed-onset anaphylaxis after ingestion of red meat.