User login
Diagnosis and Treatment of an Anaplastic Large Cell Primary Central Nervous System Lymphoma
BACKGROUND: Primary central nervous system lymphoma (PCNSL) is a rare and aggressive malignancy of predominantly B-cell origins. Where tolerated, strong sensitivity is seen with induction regimens containing high-dose methotrexate and rituximab. However, little is known regarding ideal therapy for T-cell variants, especially anaplastic large cell lymphoma.
CASE REPORT: A 20-year-old male with no past medical history developed progressive positional headaches, nausea, and dizziness over several months. Between several hospital visits, he was found to have enhancing lesions of his right caudate, left cerebellum, and right frontal lobe. A lumbar puncture demonstrated pleocytosis (152 WBC, 97% lymphocytes) and a small population of atypical CD5- T-cells on flow cytometry. Preliminary biopsy of the right caudate lesion was inconclusive, significant only for demyelination and a subset of LGL-like T-cells expressing CD3 and TIA-1. Neurology was consulted and he was given high-dose methylprednisolone with significant improvement in his symptoms. However, several months later he returned to the emergency department with new headaches, vomiting, and bilateral nystagmus. A repeat brian MRI showed lesion progression and evidence of hydrocephalus. He received hypertonic saline prior to external ventricular drain placement. Once stabilized, he underwent an uncomplicated left retrosigmoid craniotomy with resection of his cerebellar lesion. Histopathology demonstrated strong CD30 and ALK1 expression, with atypical mature T-cells on flow cytometry (CD4+, CD8+, CD5-). PET/CT imaging, bone marrow biopsy, and an ophthalmologic slit lamp exam were without evidence of systemic disease. He was given a diagnosis of PCNSL of T-cell origin (ALK+ anaplastic large cell subtype) and discharged on a dexamethasone taper. After surgical recovery he was started on induction chemotherapy with high-dose methotrexate, procarbazine, and vincristine (MPV). Interval MR imaging demonstrated marked decrease in the size of his intracranial lesions. He was subsequently transitioned to consolidation with HiDAC with the intent to undergo autologous hematopoietic cell transplant.
CONCLUSIONS: Incidence of ALK-positive anaplastic large cell PCNSL is extremely rare, and thus consensus data regarding optimal treatment is lacking. For younger patients with good functional status and renal clearance, induction therapy containing high-dose methotrexate (i.e. MPV) can provide an effective bridge to consolidation and autologous hematopoietic cell transplant.
BACKGROUND: Primary central nervous system lymphoma (PCNSL) is a rare and aggressive malignancy of predominantly B-cell origins. Where tolerated, strong sensitivity is seen with induction regimens containing high-dose methotrexate and rituximab. However, little is known regarding ideal therapy for T-cell variants, especially anaplastic large cell lymphoma.
CASE REPORT: A 20-year-old male with no past medical history developed progressive positional headaches, nausea, and dizziness over several months. Between several hospital visits, he was found to have enhancing lesions of his right caudate, left cerebellum, and right frontal lobe. A lumbar puncture demonstrated pleocytosis (152 WBC, 97% lymphocytes) and a small population of atypical CD5- T-cells on flow cytometry. Preliminary biopsy of the right caudate lesion was inconclusive, significant only for demyelination and a subset of LGL-like T-cells expressing CD3 and TIA-1. Neurology was consulted and he was given high-dose methylprednisolone with significant improvement in his symptoms. However, several months later he returned to the emergency department with new headaches, vomiting, and bilateral nystagmus. A repeat brian MRI showed lesion progression and evidence of hydrocephalus. He received hypertonic saline prior to external ventricular drain placement. Once stabilized, he underwent an uncomplicated left retrosigmoid craniotomy with resection of his cerebellar lesion. Histopathology demonstrated strong CD30 and ALK1 expression, with atypical mature T-cells on flow cytometry (CD4+, CD8+, CD5-). PET/CT imaging, bone marrow biopsy, and an ophthalmologic slit lamp exam were without evidence of systemic disease. He was given a diagnosis of PCNSL of T-cell origin (ALK+ anaplastic large cell subtype) and discharged on a dexamethasone taper. After surgical recovery he was started on induction chemotherapy with high-dose methotrexate, procarbazine, and vincristine (MPV). Interval MR imaging demonstrated marked decrease in the size of his intracranial lesions. He was subsequently transitioned to consolidation with HiDAC with the intent to undergo autologous hematopoietic cell transplant.
CONCLUSIONS: Incidence of ALK-positive anaplastic large cell PCNSL is extremely rare, and thus consensus data regarding optimal treatment is lacking. For younger patients with good functional status and renal clearance, induction therapy containing high-dose methotrexate (i.e. MPV) can provide an effective bridge to consolidation and autologous hematopoietic cell transplant.
BACKGROUND: Primary central nervous system lymphoma (PCNSL) is a rare and aggressive malignancy of predominantly B-cell origins. Where tolerated, strong sensitivity is seen with induction regimens containing high-dose methotrexate and rituximab. However, little is known regarding ideal therapy for T-cell variants, especially anaplastic large cell lymphoma.
CASE REPORT: A 20-year-old male with no past medical history developed progressive positional headaches, nausea, and dizziness over several months. Between several hospital visits, he was found to have enhancing lesions of his right caudate, left cerebellum, and right frontal lobe. A lumbar puncture demonstrated pleocytosis (152 WBC, 97% lymphocytes) and a small population of atypical CD5- T-cells on flow cytometry. Preliminary biopsy of the right caudate lesion was inconclusive, significant only for demyelination and a subset of LGL-like T-cells expressing CD3 and TIA-1. Neurology was consulted and he was given high-dose methylprednisolone with significant improvement in his symptoms. However, several months later he returned to the emergency department with new headaches, vomiting, and bilateral nystagmus. A repeat brian MRI showed lesion progression and evidence of hydrocephalus. He received hypertonic saline prior to external ventricular drain placement. Once stabilized, he underwent an uncomplicated left retrosigmoid craniotomy with resection of his cerebellar lesion. Histopathology demonstrated strong CD30 and ALK1 expression, with atypical mature T-cells on flow cytometry (CD4+, CD8+, CD5-). PET/CT imaging, bone marrow biopsy, and an ophthalmologic slit lamp exam were without evidence of systemic disease. He was given a diagnosis of PCNSL of T-cell origin (ALK+ anaplastic large cell subtype) and discharged on a dexamethasone taper. After surgical recovery he was started on induction chemotherapy with high-dose methotrexate, procarbazine, and vincristine (MPV). Interval MR imaging demonstrated marked decrease in the size of his intracranial lesions. He was subsequently transitioned to consolidation with HiDAC with the intent to undergo autologous hematopoietic cell transplant.
CONCLUSIONS: Incidence of ALK-positive anaplastic large cell PCNSL is extremely rare, and thus consensus data regarding optimal treatment is lacking. For younger patients with good functional status and renal clearance, induction therapy containing high-dose methotrexate (i.e. MPV) can provide an effective bridge to consolidation and autologous hematopoietic cell transplant.
Reducing CV risk in diabetes: An ADA update
More than 29 million Americans have diabetes, and each year another 1.7 million are given the diagnosis.1 Prediabetes is even more common; over one-third of US adults ages 20 years and older, and more than half of those who are ages 65 and older, have attained this precursor status, representing another 86 million Americans.1
Because the evidence base for the management of diabetes is rapidly expanding, the American Diabetes Association’s (ADA) Professional Practice Committee updates its Standards of Medical Care in Diabetes annually to incorporate new evidence into its recommendations. The 2017 Standards of Care are available at: professional.diabetes.org/jfp.2
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality for people with diabetes, and is the largest contributor to the direct and indirect costs of the disease.2 As a result, all patients with diabetes should have cardiovascular (CV) risk factors, including dyslipidemia, hypertension, smoking, a family history of premature coronary disease, and the presence of albuminuria, assessed at least annually.2 Numerous studies have demonstrated the efficacy of controlling individual CV risk factors in preventing or slowing ASCVD in people with diabetes. Even larger benefits, including reduced ASCVD morbidity and mortality, can be achieved when multiple risk factors are addressed simultaneously.3
To hone your management of CV risks in patients with diabetes, we’ve put together this Q&A pointing out the elements of the ADA’s 2017 Standards of Care that are most relevant to the management of patients at risk for, or with established, ASCVD.
Screening
Since ASCVD so commonly co-occurs with diabetes, should I routinely screen asymptomatic patients with diabetes for heart disease?
No. The current evidence suggests that outcomes are NOT improved by screening people before they develop symptoms of ASCVD,4 and widespread ASCVD screening has not been shown to be cost-effective. Cardiac testing should be reserved for those with typical or atypical symptoms or those with an abnormal resting electrocardiogram (EKG).
Lifestyle modification
What are the benefits of lifestyle interventions?
The benefits include not only lost pounds, but improved mobility, physical and sexual functioning, and health-related quality of life. Recommend that all overweight patients with diabetes take advantage of intensive lifestyle interventions focusing on weight loss through decreased caloric intake and increased physical activity as per the Look AHEAD (Action for Health in Diabetes) trial.5 Although the intensive lifestyle intervention in the Look AHEAD trial did not decrease CV outcomes over 10 years of follow-up, it did improve control of CV risk factors and led to people in the intervention group taking fewer glucose-, blood pressure (BP)-, and lipid-lowering medications than those in the standard care group.
There is no one diet that is recommended for all people with diabetes. Weight reduction often requires intensive intervention. In order for weight loss diets to be sustainable, they must include patient preferences.
People with diabetes should be encouraged to receive individualized medical nutrition therapy (MNT), preferably from a registered dietitian who is well versed in nutritional management for diabetes. Such MNT is associated with a 0.5% to 2% decrease in A1c levels for people with type 2 diabetes.6-9 Specific healthy diets include the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and plant-based diets.
A new lifestyle recommendation in this year’s ADA Standards is that periods of prolonged sitting should be interrupted every 30 minutes with a period of physical activity. This appears to have glycemic benefits.2
Hypertension/BP management
When should I initiate hypertension treatment in patients with diabetes?
Nonpharmacologic therapy is reasonable in people with diabetes and mildly elevated BP (>120/80 mm Hg). If systolic blood pressure (SBP) is confirmed to be >140 mm Hg and/or diastolic blood pressure (DBP) is confirmed to be >90 mm Hg, the ADA recommends initiating pharmacologic therapy along with nonpharmacologic strategies. For patients with confirmed office-based BP >160/100 mm Hg, the ADA advises initiating lifestyle modifications as well as 2 pharmacologic medications (or a single pill combination of agents).2
What is the recommended BP target for patients with diabetes and hypertension?
These patients should be treated with a combination of measures, including lifestyle modification and pharmacologic therapy, to a target BP of <140/90 mm Hg. Randomized controlled trials (RCTs) have shown benefits with this target in terms of a reduction in the incidence of coronary heart disease (CHD) events, stroke, and diabetic kidney disease.10,11
A 2012 meta-analysis of randomized trials involving adults with type 2 diabetes mellitus (T2DM) and comparing intensive BP targets (≤130 mm Hg SBP and ≤80 mm Hg DBP) with standard targets (≤140-160 mm Hg SBP and ≤85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MIs associated with more intense BP control. There was a statistically significant 35% relative risk (RR) reduction in stroke with intensive targets, but lower BP was also associated with an increased risk of hypotension and syncope.12
The 2010 Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,13 which randomized 5518 patients with T2DM at high risk for ASCVD to either a target SBP of <120 mm Hg or 130 to 140 mm Hg, found that the patients with the lower SBP target did not benefit in the primary end point (a composite of nonfatal MI, nonfatal stroke, and CV death), but did benefit from nominally significant lower rates of total stroke and nonfatal stroke.
Based on these data, the ADA Standards of Care suggest that, “more intensive BP control may be reasonable in certain motivated, ACCORD-like patients (40-79 years of age with prior evidence of CVD or multiple CV risk factors) who have been educated about the added treatment burden, side effects, and costs of more intensive BP control and for patients who prefer to lower their risk of stroke beyond what can be achieved with usual care.”
Another major study, the 2015 Systolic Blood Pressure Intervention Trial (SPRINT) trial,14 demonstrated that treating patients with hypertension to a target SBP <120 mm Hg compared to the usual target of <140 mm Hg resulted in a 25% lower RR of the primary outcome (a composite of MI, other acute coronary syndromes, stroke, heart failure, or death from CV causes) and about a 25% reduction in all-cause mortality; however, people with diabetes were not included in the trial, so the applicability of the results to decisions about BP management in patients with diabetes is not known.
A 2015 systematic review and meta-analysis of over 100,000 participants looked at SBP lowering in adults with T2DM and found that each 10-mm Hg reduction in SBP was associated with a significantly lower risk of morbidity, CV events, CHD, stroke, albuminuria, and retinopathy.10 When trials were stratified by mean baseline SBP (<140 mm Hg or ≥140 mm Hg), RRs for outcomes other than stroke, retinopathy, and renal failure were lower in studies with greater baseline SBP.
The latest ADA Standards of Care recommend that a lower BP target of 130/80 mm Hg may be appropriate for patients at high risk of CVD if this target can be achieved without undue treatment burden. A DBP of <80 mm Hg may also be appropriate in certain patients including those with a long life expectancy, CKD, elevated urinary albumin excretion, and those with evidence of CVD or associated risk factors.15 Of note, treating older adults with diabetes to an SBP target of <130 mm Hg has not been shown to improve cardiovascular outcomes,16 and treating to a diastolic target of <70 mm Hg has been associated with a greater risk of mortality.17
What are the current recommended treatment options?
Treatment for hypertension in adults with diabetes without albuminuria should include any of the classes of medications demonstrated to reduce CV events in patients with diabetes, such as:
- angiotensin-converting enzyme (ACE) inhibitors,
- angiotensin receptor blockers (ARBs),
- thiazide-like diuretics, and
- dihydropyridine calcium channel blockers.
These recommendations are based on evidence suggesting the lack of superiority of ACE inhibitors and ARBs over other classes of antihypertensive agents for the prevention of CV outcomes in all patients with diabetes.18 However, in people with diabetes at high risk for ASCVD and/or with albuminuria, ACE inhibitors and ARBs do reduce ASCVD outcomes and the progression of kidney disease.19-24 Thus, ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and urine albumin/creatinine ratios ≥30 mg/g, as these medications are associated with a reduction in the rate of kidney disease progression.
The use of both an ACE inhibitor and an ARB in combination is not recommended.25,26 For patients treated with ACE inhibitors, ARBs, or diuretics, serum creatinine/estimated glomerular filtration rate (eGFR) and serum potassium levels should be monitored.
What are the recommended lifestyle modifications for patients with diabetes and hypertension?
Regular exercise and healthy eating are recommended for all people with diabetes to optimize glycemic control and lose weight (if they are overweight or obese). For patients with hypertension, the DASH diet (available at: https://www.nhlbi.nih.gov/health/health-topics/topics/dash/) is effective at lowering BP. The DASH diet emphasizes reducing sodium intake, increasing potassium intake, limiting alcohol intake, and increasing physical activity. Specifically, sodium intake should be restricted to <2300 mg/d and patients should consume approximately 8 to 10 servings of fruits and vegetables per day and 2 to 3 servings of low-fat dairy per day. Alcohol should be limited to 2 drinks per day for men and one drink per day for women.
Most adults with diabetes should perform 150 minutes per week of moderate to vigorous exercise, spread over at least 3 days/week. In addition, it is recommended that resistance exercises be performed at least 2 to 3 days/week. Prolonged inactivity is detrimental to health and should be interrupted with activity every 30 minutes.27
Finally, as a part of lifestyle management for all patients with diabetes, smoking cessation is important, as is attention to stress, depression, and anxiety.
Is there an advantage to nighttime dosing of antihypertensive medications?
Yes. Growing evidence suggests that there is an ASCVD benefit to avoiding nocturnal BP dipping. A 2011 RCT of 448 participants with T2DM and hypertension showed a decrease in CV events and mortality during 5.4 years of follow-up if at least one antihypertensive medication was taken at bedtime.28 As a result of this and other evidence,29 consider administering one or more antihypertensive medications at bedtime, although this is not a formal recommendation in the ADA Standards of Care.
Are there any additional issues to be aware of when treating patients with diabetes and hypertension?
Yes. Sometimes patients who have had diabetes for many years have significant orthostatic hypotension secondary to autonomic neuropathy. Postural changes in BP and pulse may require adjustment of BP targets. Home BP self-monitoring and 24-hour ambulatory BP monitoring may indicate white-coat or masked hypertension.
Lipid management
What is the current evidence for lipid treatment in diabetes?
Lipid abnormalities are common in people with diabetes and contribute to the overall high risk of ASCVD in these patients. Subgroup analyses of patients in large trials with diabetes30 and trials involving patients with diabetes31 have shown significant improvements in primary and secondary prevention of ASCVD with statin use. A 2008 meta-analysis of 18,686 people with diabetes showed a 9% reduction in all-cause mortality and a 13% reduction in vascular mortality for each 39-mg/dL reduction in low-density lipoprotein (LDL) cholesterol.32 Absolute reductions in mortality are greatest in those with highest risk, but the benefits of statin therapy are clear for low- and moderate-risk individuals with diabetes, too.33,34 As a result, statins are the medications of choice for lipid lowering and CV risk reduction and should be used in addition to lifestyle management.
Who should get a statin, and how do I choose the optimum dosage?
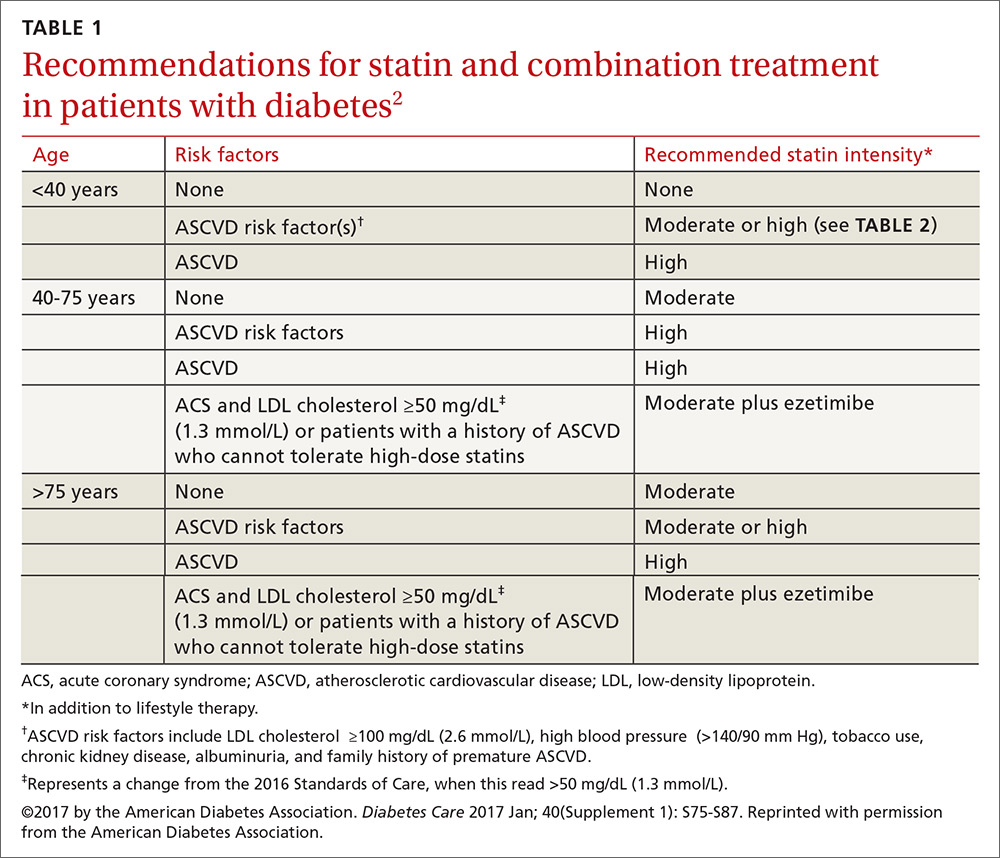
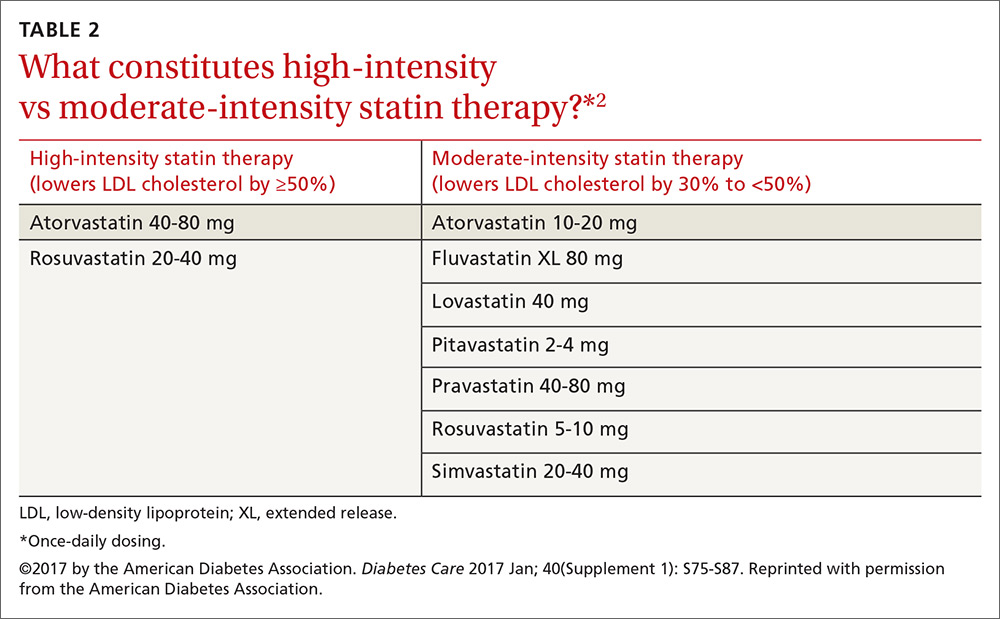
Patients ages 40 to 75 years with diabetes but without additional ASCVD risk factors should receive a moderate-intensity statin, according to the ADA (see TABLES 12 and 22). For those with additional CV risk factors, a high-intensity statin should be considered. The American College of Cardiology/American Heart Association ASCVD risk calculator (available at: http://www.cvriskcalculator.com/) may be useful for some patients, but generally, risk is already known to be high for most patients with diabetes. For patients of all ages with diabetes and established ASCVD, high-intensity statin therapy should be added to lifestyle modifications.35-37
For patients with diabetes who are <40 years with additional ASCVD risk factors, few clinical trial data exist; nevertheless, consider a moderate- or high-intensity statin and lifestyle therapy. Similarly, for patients >75 years who have diabetes and no additional ASCVD risk factors, consider a moderate-intensity statin and lifestyle modifications. For older adults with additional ASCVD risk factors, consider high-intensity statin therapy.35-37
Statins and cognition. It should be noted that published data have not demonstrated an adverse effect of statins on cognition.38 Statins, however, have been linked to an increased risk of developing diabetes,39,40 although the absolute increase in risk is small, and much smaller than the benefit derived from preventing the development of coronary disease.
Should total cholesterol and LDL levels be used as targets with statin treatment?
No. Statin doses have primarily been tested against placebo in clinical trials, rather than testing to specific target LDL levels, suggesting that the initiation and intensification of statin therapy be based on a patient’s risk profile.35 When maximally tolerated doses of statins do not lower LDL cholesterol by more than 30% from the patient’s baseline, there is currently no good evidence that combination therapy would be helpful, so regular monitoring of lipid levels has limited value. A lipid profile that includes levels of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides should be obtained at initial medical evaluation, at diagnosis of diabetes, and every 5 years thereafter or before the initiation of statin therapy. Ongoing testing may be appropriate in individual circumstances and to monitor for adherence to, or efficacy of, therapy.
What should I do for my patients who can’t tolerate statins?
Try a lower dose or a different statin before eliminating the class. Research has shown that even small doses (eg, rosuvastatin 5 mg) have some benefit.41
How do combination treatments figure into the current treatment of lipids in patients with diabetes?
It depends on the agent and the patient’s profile.
Fenofibrate. The ADA does not recommend automatically adding fenofibrate to statin therapy because the combination is associated with increased risks for abnormal transaminase levels, myositis, and rhabdomyolysis. In the ACCORD trial, the combination of fenofibrate and simvastatin did not reduce the rate of fatal CV events, nonfatal MIs, or nonfatal strokes compared with simvastatin alone.42
That said, a subgroup analysis suggested a benefit for men with both a triglyceride level ≥204 mg/dL (2.3 mmol/L) and an HDL cholesterol level ≤34 mg/dL (0.9 mmol/L).42 For this reason, the combination of a statin and fenofibrate may be considered for men who meet these laboratory parameters. In addition, consider medical therapy for triglyceride levels ≥500 mg/dL to reduce the risk of pancreatitis.
Ezetimibe. Recommendations regarding ezetimibe are based on the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), a 2015 RCT including over 18,000 patients that compared treatment with ezetimibe and simvastatin to simvastatin alone.43 Individuals in the trial were ≥50 years of age and had experienced an ACS within the preceding 10 days. In those with diabetes, the combination of moderate-intensity simvastatin (40 mg) and ezetimibe (10 mg) significantly reduced major adverse CV events with an absolute risk reduction of 5% (40% vs 45%) and an RR reduction of 14% over moderate-intensity simvastatin (40 mg) alone.
Based on these results, patients with diabetes and a recent ACS should be considered for combination therapy with ezetimibe and a moderate-intensity statin. The combination should also be considered in patients with diabetes and a history of ASCVD who cannot tolerate high-intensity statins.43
Niacin. The ADA currently does not recommend niacin in combination with a statin because of lack of efficacy on major ASCVD outcomes, possible increased risk of ischemic stroke, and adverse effects.44
What are the recommendations for the use of PCSK-9 inhibitors?
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors (ie, evolucumab and alirocumab) may be considered as adjunctive therapy to statins for patients with diabetes at high risk for ASCVD events who require additional lowering of LDL cholesterol. They may also be considered for those in whom high-intensity statin therapy is indicated, but not tolerated.
Antiplatelet agents
Who should take aspirin for primary prevention of CVD?
Both women and men ages ≥50 years who have diabetes and at least one additional CV risk factor (family history of premature ASCVD, hypertension, tobacco use, dyslipidemia, or albuminuria) should consider taking daily aspirin therapy (75-162 mg/d) if they do not have an excessive bleeding risk.45,46 The most common dose in the United States is 81 mg. This recommendation is supported by a 2010 consensus statement of the American Diabetes Association, American Heart Association, and the American College of Cardiology.47
Should patients with diabetes and heart disease receive antiplatelet therapy?
Yes. The evidence is clear that people with known diabetes and ASCVD benefit from aspirin therapy, according to the 2017 Standards of Care. Clopidogrel 75 mg/d is an appropriate alternative for patients who are allergic to aspirin. Dual antiplatelet therapy (a P2Y12 receptor antagonist and aspirin) should be used for as long as one year after an ACS and may have benefits beyond this period.48
Established heart disease
Are there specific recommendations for patients with diabetes and CHD?
According to the ADA Standards, there is good evidence that both aspirin and statin therapy are beneficial for patients with known ASCVD, and that high-intensity statin therapy should be used. In addition, consider ACE inhibitors to reduce the future risk of CV events. In patients with a prior MI, continue beta-blocker therapy for at least 2 years post event.49
Which medications should I avoid, or approach with caution, in patients with congestive heart failure (CHF)?
Thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, and metformin all require careful attention. This is especially important to know when you consider that almost half of all patients with T2DM will develop heart failure.50
Thiazolidinediones. The 2017 Standards of Care state that patients with diabetes and symptomatic congestive heart failure should not receive thiazolidinediones, as they can worsen heart failure status via fluid retention. As such, they are contraindicated in patients with class III and IV heart failure.51
DPP-4 inhibitors. The studies on DPP-4 inhibitors and heart failure have had mixed results. The 2013 SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) 53 trial52 showed that patients treated with saxagliptin were more likely to be hospitalized for heart failure than those taking placebo (3.5% vs 2.8%, respectively). However, the 2015 EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care)53 trial and the 2015 TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin)54 trial evaluated heart failure and mortality outcomes in patients with alogliptin and sitagliptin, respectively, compared to placebo, and did not show a relationship to heart failure.
Metformin may be used in people who have T2DM and stable CHF if their eGFR remains >30 mL/min; it should be withheld from patients with unstable heart failure and those who are hospitalized with CHF.
Are there antihyperglycemic medications that reduce CV morbidity and mortality in those with established ASCVD?
Yes. This year’s ADA Standards indicate that certain glucose-lowering medications—specifically empagliflozin (a sodium–glucose cotransporter [SGLT]-2 inhibitor) and liraglutide (a glucagon-like peptide [GLP]-1 receptor agonist)—have been shown to be beneficial for those with established CVD. According to the 2017 Standards of Care, “In patients with longstanding suboptimally controlled T2DM and established ASCVD, empagliflozin or liraglutide should be considered, as they have been shown to reduce CV and all-cause mortality when added to standard care.”2 The studies that provide support for their use are summarized below. Ongoing studies are investigating the CV effects of other agents in these drug classes.
Empagliflozin. The 2015 EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study55 was a randomized double-blind study of empagliflozin vs placebo and usual care in patients with diabetes and established CVD. Over a median follow-up of 3.1 years, treatment with empagliflozin reduced the aggregate outcome of MI, stroke, and CV death by 14%, reduced CV deaths by 38%, and decreased deaths from any cause by 32%. In December 2016, the FDA announced a new indication for empagliflozin: to reduce the risk of CV death in adult patients with T2DM and CVD.56
Liraglutide. The LEADER (Liraglutide Effect and Action in Diabetes Evaluation of Cardiovascular Outcome Results: A Long Term Evaluation) trial57 was a double-blind randomized trial of liraglutide vs placebo added to usual care in patients with T2DM at high risk for CVD or with existing CVD. More than 80% of the participants had existing CVD including a history of prior MI, cerebrovascular disease, or peripheral vascular disease. After a median follow-up of 3.8 years, the group taking liraglutide demonstrated a 13% reduction in the composite outcome of MI, stroke, or CV death, a 22% reduction in CV death, and a 15% reduction in death from any cause, compared with placebo.57
CORRESPONDENCE
Neil Skolnik, MD, Abington-Jefferson Health, 500 Old York Rd, Ste 108, Jenkintown, PA 19046; nskolnik@comcast.net.
The authors thank Sarah Bradley, director, professional engagement & collaboration at the American Diabetes Association, for her editorial and organizational assistance in the preparation of this manuscript.
1. Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. Available at: http://templatelab.com/national-diabetes-report-2014/. Accessed April 7, 2017.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Available at: http://professional.diabetes.org/sites/professional.diabetes.org/files/media/dc_40_s1_final.pdf. Accessed April 7, 2017.
3. Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591.
4. Bax JJ, Young LH, Frye RL, et al; American Diabetes Association. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007;30:2729-2736.
5. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154.
6. UK Prospective Diabetes Study (UKDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKDS 34). Lancet. 1998;352:854-865.
7. Ziemer DC, Berkowitz KJ, Panayioto RM, et al. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes. Diabetes Care. 2003;26:1719-1724.
8. Wolf AM, Conaway RM, Crowther JQ, et al; Improving Control with Activity and Nutrition (ICAN) Study. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570-1576.
9. Coppell KJ, Kataoka M, Williams SM, et al. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment-Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. 2010;341:c3337.
10. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603-615.
11. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;10:CD008277.
12. McBrien K, Rabi DM, Campbell N, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2012;172:1296-1303.
13. ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
14. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
15. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
16. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
17. Anderson RJ, Bahn GD, Moritz TE, et al; VADT Study Group. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care. 2011;34:34-38.
18. Bangalore S, Fakheri R, Toklu B, et al. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ. 2016;352:i438.
19. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253-259.
20. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772-776.
21. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
22. Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759-766.
23. Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
24. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047-2056.
25. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
26. Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
27. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079.
28. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
29. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011;10:CD004184.
30. Py̆orälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
31. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care. 2006;29:1478-1485.
32. Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117-125.
33. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816.
34. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610.
35. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
36. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
37. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
38. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688-697.
39. Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924-1929.
40. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
41. Meek C, Wierzbicki AS, Jewkes C, et al. Daily and intermittent rosuvastatin 5 mg therapy in statin intolerant patients: an observational study. Curr Med Res Opin. 2012;28:371-378.
42. ACCORD Study Group, Ginsberg HN, Bam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
43. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
44. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
45. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860.
46. Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635-1701.
47. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes. A position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
48. Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
49. Kezerashvilli A, Marzo K, De Leon J. Beta blocker use after acute myocardial infarction in the patient with normal systolic function: when is it “ok” to discontinue? Curr Cardiol Rev. 2012;8:77-84.
50. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34.
51. Pioglitazone Package Insert. Available at: http://medlibrary.org/lib/rx/meds/pioglitazone-3/. Accessed April 10, 2017.
52. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
53. Zannad F, Cannon CP, Cushman WC, et al; EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
54. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
55. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
56. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. FDA News Release, December 2, 2016. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed February 9, 2017.
57. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
More than 29 million Americans have diabetes, and each year another 1.7 million are given the diagnosis.1 Prediabetes is even more common; over one-third of US adults ages 20 years and older, and more than half of those who are ages 65 and older, have attained this precursor status, representing another 86 million Americans.1
Because the evidence base for the management of diabetes is rapidly expanding, the American Diabetes Association’s (ADA) Professional Practice Committee updates its Standards of Medical Care in Diabetes annually to incorporate new evidence into its recommendations. The 2017 Standards of Care are available at: professional.diabetes.org/jfp.2
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality for people with diabetes, and is the largest contributor to the direct and indirect costs of the disease.2 As a result, all patients with diabetes should have cardiovascular (CV) risk factors, including dyslipidemia, hypertension, smoking, a family history of premature coronary disease, and the presence of albuminuria, assessed at least annually.2 Numerous studies have demonstrated the efficacy of controlling individual CV risk factors in preventing or slowing ASCVD in people with diabetes. Even larger benefits, including reduced ASCVD morbidity and mortality, can be achieved when multiple risk factors are addressed simultaneously.3
To hone your management of CV risks in patients with diabetes, we’ve put together this Q&A pointing out the elements of the ADA’s 2017 Standards of Care that are most relevant to the management of patients at risk for, or with established, ASCVD.
Screening
Since ASCVD so commonly co-occurs with diabetes, should I routinely screen asymptomatic patients with diabetes for heart disease?
No. The current evidence suggests that outcomes are NOT improved by screening people before they develop symptoms of ASCVD,4 and widespread ASCVD screening has not been shown to be cost-effective. Cardiac testing should be reserved for those with typical or atypical symptoms or those with an abnormal resting electrocardiogram (EKG).
Lifestyle modification
What are the benefits of lifestyle interventions?
The benefits include not only lost pounds, but improved mobility, physical and sexual functioning, and health-related quality of life. Recommend that all overweight patients with diabetes take advantage of intensive lifestyle interventions focusing on weight loss through decreased caloric intake and increased physical activity as per the Look AHEAD (Action for Health in Diabetes) trial.5 Although the intensive lifestyle intervention in the Look AHEAD trial did not decrease CV outcomes over 10 years of follow-up, it did improve control of CV risk factors and led to people in the intervention group taking fewer glucose-, blood pressure (BP)-, and lipid-lowering medications than those in the standard care group.
There is no one diet that is recommended for all people with diabetes. Weight reduction often requires intensive intervention. In order for weight loss diets to be sustainable, they must include patient preferences.
People with diabetes should be encouraged to receive individualized medical nutrition therapy (MNT), preferably from a registered dietitian who is well versed in nutritional management for diabetes. Such MNT is associated with a 0.5% to 2% decrease in A1c levels for people with type 2 diabetes.6-9 Specific healthy diets include the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and plant-based diets.
A new lifestyle recommendation in this year’s ADA Standards is that periods of prolonged sitting should be interrupted every 30 minutes with a period of physical activity. This appears to have glycemic benefits.2
Hypertension/BP management
When should I initiate hypertension treatment in patients with diabetes?
Nonpharmacologic therapy is reasonable in people with diabetes and mildly elevated BP (>120/80 mm Hg). If systolic blood pressure (SBP) is confirmed to be >140 mm Hg and/or diastolic blood pressure (DBP) is confirmed to be >90 mm Hg, the ADA recommends initiating pharmacologic therapy along with nonpharmacologic strategies. For patients with confirmed office-based BP >160/100 mm Hg, the ADA advises initiating lifestyle modifications as well as 2 pharmacologic medications (or a single pill combination of agents).2
What is the recommended BP target for patients with diabetes and hypertension?
These patients should be treated with a combination of measures, including lifestyle modification and pharmacologic therapy, to a target BP of <140/90 mm Hg. Randomized controlled trials (RCTs) have shown benefits with this target in terms of a reduction in the incidence of coronary heart disease (CHD) events, stroke, and diabetic kidney disease.10,11
A 2012 meta-analysis of randomized trials involving adults with type 2 diabetes mellitus (T2DM) and comparing intensive BP targets (≤130 mm Hg SBP and ≤80 mm Hg DBP) with standard targets (≤140-160 mm Hg SBP and ≤85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MIs associated with more intense BP control. There was a statistically significant 35% relative risk (RR) reduction in stroke with intensive targets, but lower BP was also associated with an increased risk of hypotension and syncope.12
The 2010 Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,13 which randomized 5518 patients with T2DM at high risk for ASCVD to either a target SBP of <120 mm Hg or 130 to 140 mm Hg, found that the patients with the lower SBP target did not benefit in the primary end point (a composite of nonfatal MI, nonfatal stroke, and CV death), but did benefit from nominally significant lower rates of total stroke and nonfatal stroke.
Based on these data, the ADA Standards of Care suggest that, “more intensive BP control may be reasonable in certain motivated, ACCORD-like patients (40-79 years of age with prior evidence of CVD or multiple CV risk factors) who have been educated about the added treatment burden, side effects, and costs of more intensive BP control and for patients who prefer to lower their risk of stroke beyond what can be achieved with usual care.”
Another major study, the 2015 Systolic Blood Pressure Intervention Trial (SPRINT) trial,14 demonstrated that treating patients with hypertension to a target SBP <120 mm Hg compared to the usual target of <140 mm Hg resulted in a 25% lower RR of the primary outcome (a composite of MI, other acute coronary syndromes, stroke, heart failure, or death from CV causes) and about a 25% reduction in all-cause mortality; however, people with diabetes were not included in the trial, so the applicability of the results to decisions about BP management in patients with diabetes is not known.
A 2015 systematic review and meta-analysis of over 100,000 participants looked at SBP lowering in adults with T2DM and found that each 10-mm Hg reduction in SBP was associated with a significantly lower risk of morbidity, CV events, CHD, stroke, albuminuria, and retinopathy.10 When trials were stratified by mean baseline SBP (<140 mm Hg or ≥140 mm Hg), RRs for outcomes other than stroke, retinopathy, and renal failure were lower in studies with greater baseline SBP.
The latest ADA Standards of Care recommend that a lower BP target of 130/80 mm Hg may be appropriate for patients at high risk of CVD if this target can be achieved without undue treatment burden. A DBP of <80 mm Hg may also be appropriate in certain patients including those with a long life expectancy, CKD, elevated urinary albumin excretion, and those with evidence of CVD or associated risk factors.15 Of note, treating older adults with diabetes to an SBP target of <130 mm Hg has not been shown to improve cardiovascular outcomes,16 and treating to a diastolic target of <70 mm Hg has been associated with a greater risk of mortality.17
What are the current recommended treatment options?
Treatment for hypertension in adults with diabetes without albuminuria should include any of the classes of medications demonstrated to reduce CV events in patients with diabetes, such as:
- angiotensin-converting enzyme (ACE) inhibitors,
- angiotensin receptor blockers (ARBs),
- thiazide-like diuretics, and
- dihydropyridine calcium channel blockers.
These recommendations are based on evidence suggesting the lack of superiority of ACE inhibitors and ARBs over other classes of antihypertensive agents for the prevention of CV outcomes in all patients with diabetes.18 However, in people with diabetes at high risk for ASCVD and/or with albuminuria, ACE inhibitors and ARBs do reduce ASCVD outcomes and the progression of kidney disease.19-24 Thus, ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and urine albumin/creatinine ratios ≥30 mg/g, as these medications are associated with a reduction in the rate of kidney disease progression.
The use of both an ACE inhibitor and an ARB in combination is not recommended.25,26 For patients treated with ACE inhibitors, ARBs, or diuretics, serum creatinine/estimated glomerular filtration rate (eGFR) and serum potassium levels should be monitored.
What are the recommended lifestyle modifications for patients with diabetes and hypertension?
Regular exercise and healthy eating are recommended for all people with diabetes to optimize glycemic control and lose weight (if they are overweight or obese). For patients with hypertension, the DASH diet (available at: https://www.nhlbi.nih.gov/health/health-topics/topics/dash/) is effective at lowering BP. The DASH diet emphasizes reducing sodium intake, increasing potassium intake, limiting alcohol intake, and increasing physical activity. Specifically, sodium intake should be restricted to <2300 mg/d and patients should consume approximately 8 to 10 servings of fruits and vegetables per day and 2 to 3 servings of low-fat dairy per day. Alcohol should be limited to 2 drinks per day for men and one drink per day for women.
Most adults with diabetes should perform 150 minutes per week of moderate to vigorous exercise, spread over at least 3 days/week. In addition, it is recommended that resistance exercises be performed at least 2 to 3 days/week. Prolonged inactivity is detrimental to health and should be interrupted with activity every 30 minutes.27
Finally, as a part of lifestyle management for all patients with diabetes, smoking cessation is important, as is attention to stress, depression, and anxiety.
Is there an advantage to nighttime dosing of antihypertensive medications?
Yes. Growing evidence suggests that there is an ASCVD benefit to avoiding nocturnal BP dipping. A 2011 RCT of 448 participants with T2DM and hypertension showed a decrease in CV events and mortality during 5.4 years of follow-up if at least one antihypertensive medication was taken at bedtime.28 As a result of this and other evidence,29 consider administering one or more antihypertensive medications at bedtime, although this is not a formal recommendation in the ADA Standards of Care.
Are there any additional issues to be aware of when treating patients with diabetes and hypertension?
Yes. Sometimes patients who have had diabetes for many years have significant orthostatic hypotension secondary to autonomic neuropathy. Postural changes in BP and pulse may require adjustment of BP targets. Home BP self-monitoring and 24-hour ambulatory BP monitoring may indicate white-coat or masked hypertension.
Lipid management
What is the current evidence for lipid treatment in diabetes?
Lipid abnormalities are common in people with diabetes and contribute to the overall high risk of ASCVD in these patients. Subgroup analyses of patients in large trials with diabetes30 and trials involving patients with diabetes31 have shown significant improvements in primary and secondary prevention of ASCVD with statin use. A 2008 meta-analysis of 18,686 people with diabetes showed a 9% reduction in all-cause mortality and a 13% reduction in vascular mortality for each 39-mg/dL reduction in low-density lipoprotein (LDL) cholesterol.32 Absolute reductions in mortality are greatest in those with highest risk, but the benefits of statin therapy are clear for low- and moderate-risk individuals with diabetes, too.33,34 As a result, statins are the medications of choice for lipid lowering and CV risk reduction and should be used in addition to lifestyle management.
Who should get a statin, and how do I choose the optimum dosage?
Patients ages 40 to 75 years with diabetes but without additional ASCVD risk factors should receive a moderate-intensity statin, according to the ADA (see TABLES 12 and 22). For those with additional CV risk factors, a high-intensity statin should be considered. The American College of Cardiology/American Heart Association ASCVD risk calculator (available at: http://www.cvriskcalculator.com/) may be useful for some patients, but generally, risk is already known to be high for most patients with diabetes. For patients of all ages with diabetes and established ASCVD, high-intensity statin therapy should be added to lifestyle modifications.35-37
For patients with diabetes who are <40 years with additional ASCVD risk factors, few clinical trial data exist; nevertheless, consider a moderate- or high-intensity statin and lifestyle therapy. Similarly, for patients >75 years who have diabetes and no additional ASCVD risk factors, consider a moderate-intensity statin and lifestyle modifications. For older adults with additional ASCVD risk factors, consider high-intensity statin therapy.35-37
Statins and cognition. It should be noted that published data have not demonstrated an adverse effect of statins on cognition.38 Statins, however, have been linked to an increased risk of developing diabetes,39,40 although the absolute increase in risk is small, and much smaller than the benefit derived from preventing the development of coronary disease.
Should total cholesterol and LDL levels be used as targets with statin treatment?
No. Statin doses have primarily been tested against placebo in clinical trials, rather than testing to specific target LDL levels, suggesting that the initiation and intensification of statin therapy be based on a patient’s risk profile.35 When maximally tolerated doses of statins do not lower LDL cholesterol by more than 30% from the patient’s baseline, there is currently no good evidence that combination therapy would be helpful, so regular monitoring of lipid levels has limited value. A lipid profile that includes levels of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides should be obtained at initial medical evaluation, at diagnosis of diabetes, and every 5 years thereafter or before the initiation of statin therapy. Ongoing testing may be appropriate in individual circumstances and to monitor for adherence to, or efficacy of, therapy.
What should I do for my patients who can’t tolerate statins?
Try a lower dose or a different statin before eliminating the class. Research has shown that even small doses (eg, rosuvastatin 5 mg) have some benefit.41
How do combination treatments figure into the current treatment of lipids in patients with diabetes?
It depends on the agent and the patient’s profile.
Fenofibrate. The ADA does not recommend automatically adding fenofibrate to statin therapy because the combination is associated with increased risks for abnormal transaminase levels, myositis, and rhabdomyolysis. In the ACCORD trial, the combination of fenofibrate and simvastatin did not reduce the rate of fatal CV events, nonfatal MIs, or nonfatal strokes compared with simvastatin alone.42
That said, a subgroup analysis suggested a benefit for men with both a triglyceride level ≥204 mg/dL (2.3 mmol/L) and an HDL cholesterol level ≤34 mg/dL (0.9 mmol/L).42 For this reason, the combination of a statin and fenofibrate may be considered for men who meet these laboratory parameters. In addition, consider medical therapy for triglyceride levels ≥500 mg/dL to reduce the risk of pancreatitis.
Ezetimibe. Recommendations regarding ezetimibe are based on the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), a 2015 RCT including over 18,000 patients that compared treatment with ezetimibe and simvastatin to simvastatin alone.43 Individuals in the trial were ≥50 years of age and had experienced an ACS within the preceding 10 days. In those with diabetes, the combination of moderate-intensity simvastatin (40 mg) and ezetimibe (10 mg) significantly reduced major adverse CV events with an absolute risk reduction of 5% (40% vs 45%) and an RR reduction of 14% over moderate-intensity simvastatin (40 mg) alone.
Based on these results, patients with diabetes and a recent ACS should be considered for combination therapy with ezetimibe and a moderate-intensity statin. The combination should also be considered in patients with diabetes and a history of ASCVD who cannot tolerate high-intensity statins.43
Niacin. The ADA currently does not recommend niacin in combination with a statin because of lack of efficacy on major ASCVD outcomes, possible increased risk of ischemic stroke, and adverse effects.44
What are the recommendations for the use of PCSK-9 inhibitors?
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors (ie, evolucumab and alirocumab) may be considered as adjunctive therapy to statins for patients with diabetes at high risk for ASCVD events who require additional lowering of LDL cholesterol. They may also be considered for those in whom high-intensity statin therapy is indicated, but not tolerated.
Antiplatelet agents
Who should take aspirin for primary prevention of CVD?
Both women and men ages ≥50 years who have diabetes and at least one additional CV risk factor (family history of premature ASCVD, hypertension, tobacco use, dyslipidemia, or albuminuria) should consider taking daily aspirin therapy (75-162 mg/d) if they do not have an excessive bleeding risk.45,46 The most common dose in the United States is 81 mg. This recommendation is supported by a 2010 consensus statement of the American Diabetes Association, American Heart Association, and the American College of Cardiology.47
Should patients with diabetes and heart disease receive antiplatelet therapy?
Yes. The evidence is clear that people with known diabetes and ASCVD benefit from aspirin therapy, according to the 2017 Standards of Care. Clopidogrel 75 mg/d is an appropriate alternative for patients who are allergic to aspirin. Dual antiplatelet therapy (a P2Y12 receptor antagonist and aspirin) should be used for as long as one year after an ACS and may have benefits beyond this period.48
Established heart disease
Are there specific recommendations for patients with diabetes and CHD?
According to the ADA Standards, there is good evidence that both aspirin and statin therapy are beneficial for patients with known ASCVD, and that high-intensity statin therapy should be used. In addition, consider ACE inhibitors to reduce the future risk of CV events. In patients with a prior MI, continue beta-blocker therapy for at least 2 years post event.49
Which medications should I avoid, or approach with caution, in patients with congestive heart failure (CHF)?
Thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, and metformin all require careful attention. This is especially important to know when you consider that almost half of all patients with T2DM will develop heart failure.50
Thiazolidinediones. The 2017 Standards of Care state that patients with diabetes and symptomatic congestive heart failure should not receive thiazolidinediones, as they can worsen heart failure status via fluid retention. As such, they are contraindicated in patients with class III and IV heart failure.51
DPP-4 inhibitors. The studies on DPP-4 inhibitors and heart failure have had mixed results. The 2013 SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) 53 trial52 showed that patients treated with saxagliptin were more likely to be hospitalized for heart failure than those taking placebo (3.5% vs 2.8%, respectively). However, the 2015 EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care)53 trial and the 2015 TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin)54 trial evaluated heart failure and mortality outcomes in patients with alogliptin and sitagliptin, respectively, compared to placebo, and did not show a relationship to heart failure.
Metformin may be used in people who have T2DM and stable CHF if their eGFR remains >30 mL/min; it should be withheld from patients with unstable heart failure and those who are hospitalized with CHF.
Are there antihyperglycemic medications that reduce CV morbidity and mortality in those with established ASCVD?
Yes. This year’s ADA Standards indicate that certain glucose-lowering medications—specifically empagliflozin (a sodium–glucose cotransporter [SGLT]-2 inhibitor) and liraglutide (a glucagon-like peptide [GLP]-1 receptor agonist)—have been shown to be beneficial for those with established CVD. According to the 2017 Standards of Care, “In patients with longstanding suboptimally controlled T2DM and established ASCVD, empagliflozin or liraglutide should be considered, as they have been shown to reduce CV and all-cause mortality when added to standard care.”2 The studies that provide support for their use are summarized below. Ongoing studies are investigating the CV effects of other agents in these drug classes.
Empagliflozin. The 2015 EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study55 was a randomized double-blind study of empagliflozin vs placebo and usual care in patients with diabetes and established CVD. Over a median follow-up of 3.1 years, treatment with empagliflozin reduced the aggregate outcome of MI, stroke, and CV death by 14%, reduced CV deaths by 38%, and decreased deaths from any cause by 32%. In December 2016, the FDA announced a new indication for empagliflozin: to reduce the risk of CV death in adult patients with T2DM and CVD.56
Liraglutide. The LEADER (Liraglutide Effect and Action in Diabetes Evaluation of Cardiovascular Outcome Results: A Long Term Evaluation) trial57 was a double-blind randomized trial of liraglutide vs placebo added to usual care in patients with T2DM at high risk for CVD or with existing CVD. More than 80% of the participants had existing CVD including a history of prior MI, cerebrovascular disease, or peripheral vascular disease. After a median follow-up of 3.8 years, the group taking liraglutide demonstrated a 13% reduction in the composite outcome of MI, stroke, or CV death, a 22% reduction in CV death, and a 15% reduction in death from any cause, compared with placebo.57
CORRESPONDENCE
Neil Skolnik, MD, Abington-Jefferson Health, 500 Old York Rd, Ste 108, Jenkintown, PA 19046; nskolnik@comcast.net.
The authors thank Sarah Bradley, director, professional engagement & collaboration at the American Diabetes Association, for her editorial and organizational assistance in the preparation of this manuscript.
More than 29 million Americans have diabetes, and each year another 1.7 million are given the diagnosis.1 Prediabetes is even more common; over one-third of US adults ages 20 years and older, and more than half of those who are ages 65 and older, have attained this precursor status, representing another 86 million Americans.1
Because the evidence base for the management of diabetes is rapidly expanding, the American Diabetes Association’s (ADA) Professional Practice Committee updates its Standards of Medical Care in Diabetes annually to incorporate new evidence into its recommendations. The 2017 Standards of Care are available at: professional.diabetes.org/jfp.2
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality for people with diabetes, and is the largest contributor to the direct and indirect costs of the disease.2 As a result, all patients with diabetes should have cardiovascular (CV) risk factors, including dyslipidemia, hypertension, smoking, a family history of premature coronary disease, and the presence of albuminuria, assessed at least annually.2 Numerous studies have demonstrated the efficacy of controlling individual CV risk factors in preventing or slowing ASCVD in people with diabetes. Even larger benefits, including reduced ASCVD morbidity and mortality, can be achieved when multiple risk factors are addressed simultaneously.3
To hone your management of CV risks in patients with diabetes, we’ve put together this Q&A pointing out the elements of the ADA’s 2017 Standards of Care that are most relevant to the management of patients at risk for, or with established, ASCVD.
Screening
Since ASCVD so commonly co-occurs with diabetes, should I routinely screen asymptomatic patients with diabetes for heart disease?
No. The current evidence suggests that outcomes are NOT improved by screening people before they develop symptoms of ASCVD,4 and widespread ASCVD screening has not been shown to be cost-effective. Cardiac testing should be reserved for those with typical or atypical symptoms or those with an abnormal resting electrocardiogram (EKG).
Lifestyle modification
What are the benefits of lifestyle interventions?
The benefits include not only lost pounds, but improved mobility, physical and sexual functioning, and health-related quality of life. Recommend that all overweight patients with diabetes take advantage of intensive lifestyle interventions focusing on weight loss through decreased caloric intake and increased physical activity as per the Look AHEAD (Action for Health in Diabetes) trial.5 Although the intensive lifestyle intervention in the Look AHEAD trial did not decrease CV outcomes over 10 years of follow-up, it did improve control of CV risk factors and led to people in the intervention group taking fewer glucose-, blood pressure (BP)-, and lipid-lowering medications than those in the standard care group.
There is no one diet that is recommended for all people with diabetes. Weight reduction often requires intensive intervention. In order for weight loss diets to be sustainable, they must include patient preferences.
People with diabetes should be encouraged to receive individualized medical nutrition therapy (MNT), preferably from a registered dietitian who is well versed in nutritional management for diabetes. Such MNT is associated with a 0.5% to 2% decrease in A1c levels for people with type 2 diabetes.6-9 Specific healthy diets include the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and plant-based diets.
A new lifestyle recommendation in this year’s ADA Standards is that periods of prolonged sitting should be interrupted every 30 minutes with a period of physical activity. This appears to have glycemic benefits.2
Hypertension/BP management
When should I initiate hypertension treatment in patients with diabetes?
Nonpharmacologic therapy is reasonable in people with diabetes and mildly elevated BP (>120/80 mm Hg). If systolic blood pressure (SBP) is confirmed to be >140 mm Hg and/or diastolic blood pressure (DBP) is confirmed to be >90 mm Hg, the ADA recommends initiating pharmacologic therapy along with nonpharmacologic strategies. For patients with confirmed office-based BP >160/100 mm Hg, the ADA advises initiating lifestyle modifications as well as 2 pharmacologic medications (or a single pill combination of agents).2
What is the recommended BP target for patients with diabetes and hypertension?
These patients should be treated with a combination of measures, including lifestyle modification and pharmacologic therapy, to a target BP of <140/90 mm Hg. Randomized controlled trials (RCTs) have shown benefits with this target in terms of a reduction in the incidence of coronary heart disease (CHD) events, stroke, and diabetic kidney disease.10,11
A 2012 meta-analysis of randomized trials involving adults with type 2 diabetes mellitus (T2DM) and comparing intensive BP targets (≤130 mm Hg SBP and ≤80 mm Hg DBP) with standard targets (≤140-160 mm Hg SBP and ≤85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MIs associated with more intense BP control. There was a statistically significant 35% relative risk (RR) reduction in stroke with intensive targets, but lower BP was also associated with an increased risk of hypotension and syncope.12
The 2010 Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,13 which randomized 5518 patients with T2DM at high risk for ASCVD to either a target SBP of <120 mm Hg or 130 to 140 mm Hg, found that the patients with the lower SBP target did not benefit in the primary end point (a composite of nonfatal MI, nonfatal stroke, and CV death), but did benefit from nominally significant lower rates of total stroke and nonfatal stroke.
Based on these data, the ADA Standards of Care suggest that, “more intensive BP control may be reasonable in certain motivated, ACCORD-like patients (40-79 years of age with prior evidence of CVD or multiple CV risk factors) who have been educated about the added treatment burden, side effects, and costs of more intensive BP control and for patients who prefer to lower their risk of stroke beyond what can be achieved with usual care.”
Another major study, the 2015 Systolic Blood Pressure Intervention Trial (SPRINT) trial,14 demonstrated that treating patients with hypertension to a target SBP <120 mm Hg compared to the usual target of <140 mm Hg resulted in a 25% lower RR of the primary outcome (a composite of MI, other acute coronary syndromes, stroke, heart failure, or death from CV causes) and about a 25% reduction in all-cause mortality; however, people with diabetes were not included in the trial, so the applicability of the results to decisions about BP management in patients with diabetes is not known.
A 2015 systematic review and meta-analysis of over 100,000 participants looked at SBP lowering in adults with T2DM and found that each 10-mm Hg reduction in SBP was associated with a significantly lower risk of morbidity, CV events, CHD, stroke, albuminuria, and retinopathy.10 When trials were stratified by mean baseline SBP (<140 mm Hg or ≥140 mm Hg), RRs for outcomes other than stroke, retinopathy, and renal failure were lower in studies with greater baseline SBP.
The latest ADA Standards of Care recommend that a lower BP target of 130/80 mm Hg may be appropriate for patients at high risk of CVD if this target can be achieved without undue treatment burden. A DBP of <80 mm Hg may also be appropriate in certain patients including those with a long life expectancy, CKD, elevated urinary albumin excretion, and those with evidence of CVD or associated risk factors.15 Of note, treating older adults with diabetes to an SBP target of <130 mm Hg has not been shown to improve cardiovascular outcomes,16 and treating to a diastolic target of <70 mm Hg has been associated with a greater risk of mortality.17
What are the current recommended treatment options?
Treatment for hypertension in adults with diabetes without albuminuria should include any of the classes of medications demonstrated to reduce CV events in patients with diabetes, such as:
- angiotensin-converting enzyme (ACE) inhibitors,
- angiotensin receptor blockers (ARBs),
- thiazide-like diuretics, and
- dihydropyridine calcium channel blockers.
These recommendations are based on evidence suggesting the lack of superiority of ACE inhibitors and ARBs over other classes of antihypertensive agents for the prevention of CV outcomes in all patients with diabetes.18 However, in people with diabetes at high risk for ASCVD and/or with albuminuria, ACE inhibitors and ARBs do reduce ASCVD outcomes and the progression of kidney disease.19-24 Thus, ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and urine albumin/creatinine ratios ≥30 mg/g, as these medications are associated with a reduction in the rate of kidney disease progression.
The use of both an ACE inhibitor and an ARB in combination is not recommended.25,26 For patients treated with ACE inhibitors, ARBs, or diuretics, serum creatinine/estimated glomerular filtration rate (eGFR) and serum potassium levels should be monitored.
What are the recommended lifestyle modifications for patients with diabetes and hypertension?
Regular exercise and healthy eating are recommended for all people with diabetes to optimize glycemic control and lose weight (if they are overweight or obese). For patients with hypertension, the DASH diet (available at: https://www.nhlbi.nih.gov/health/health-topics/topics/dash/) is effective at lowering BP. The DASH diet emphasizes reducing sodium intake, increasing potassium intake, limiting alcohol intake, and increasing physical activity. Specifically, sodium intake should be restricted to <2300 mg/d and patients should consume approximately 8 to 10 servings of fruits and vegetables per day and 2 to 3 servings of low-fat dairy per day. Alcohol should be limited to 2 drinks per day for men and one drink per day for women.
Most adults with diabetes should perform 150 minutes per week of moderate to vigorous exercise, spread over at least 3 days/week. In addition, it is recommended that resistance exercises be performed at least 2 to 3 days/week. Prolonged inactivity is detrimental to health and should be interrupted with activity every 30 minutes.27
Finally, as a part of lifestyle management for all patients with diabetes, smoking cessation is important, as is attention to stress, depression, and anxiety.
Is there an advantage to nighttime dosing of antihypertensive medications?
Yes. Growing evidence suggests that there is an ASCVD benefit to avoiding nocturnal BP dipping. A 2011 RCT of 448 participants with T2DM and hypertension showed a decrease in CV events and mortality during 5.4 years of follow-up if at least one antihypertensive medication was taken at bedtime.28 As a result of this and other evidence,29 consider administering one or more antihypertensive medications at bedtime, although this is not a formal recommendation in the ADA Standards of Care.
Are there any additional issues to be aware of when treating patients with diabetes and hypertension?
Yes. Sometimes patients who have had diabetes for many years have significant orthostatic hypotension secondary to autonomic neuropathy. Postural changes in BP and pulse may require adjustment of BP targets. Home BP self-monitoring and 24-hour ambulatory BP monitoring may indicate white-coat or masked hypertension.
Lipid management
What is the current evidence for lipid treatment in diabetes?
Lipid abnormalities are common in people with diabetes and contribute to the overall high risk of ASCVD in these patients. Subgroup analyses of patients in large trials with diabetes30 and trials involving patients with diabetes31 have shown significant improvements in primary and secondary prevention of ASCVD with statin use. A 2008 meta-analysis of 18,686 people with diabetes showed a 9% reduction in all-cause mortality and a 13% reduction in vascular mortality for each 39-mg/dL reduction in low-density lipoprotein (LDL) cholesterol.32 Absolute reductions in mortality are greatest in those with highest risk, but the benefits of statin therapy are clear for low- and moderate-risk individuals with diabetes, too.33,34 As a result, statins are the medications of choice for lipid lowering and CV risk reduction and should be used in addition to lifestyle management.
Who should get a statin, and how do I choose the optimum dosage?
Patients ages 40 to 75 years with diabetes but without additional ASCVD risk factors should receive a moderate-intensity statin, according to the ADA (see TABLES 12 and 22). For those with additional CV risk factors, a high-intensity statin should be considered. The American College of Cardiology/American Heart Association ASCVD risk calculator (available at: http://www.cvriskcalculator.com/) may be useful for some patients, but generally, risk is already known to be high for most patients with diabetes. For patients of all ages with diabetes and established ASCVD, high-intensity statin therapy should be added to lifestyle modifications.35-37
For patients with diabetes who are <40 years with additional ASCVD risk factors, few clinical trial data exist; nevertheless, consider a moderate- or high-intensity statin and lifestyle therapy. Similarly, for patients >75 years who have diabetes and no additional ASCVD risk factors, consider a moderate-intensity statin and lifestyle modifications. For older adults with additional ASCVD risk factors, consider high-intensity statin therapy.35-37
Statins and cognition. It should be noted that published data have not demonstrated an adverse effect of statins on cognition.38 Statins, however, have been linked to an increased risk of developing diabetes,39,40 although the absolute increase in risk is small, and much smaller than the benefit derived from preventing the development of coronary disease.
Should total cholesterol and LDL levels be used as targets with statin treatment?
No. Statin doses have primarily been tested against placebo in clinical trials, rather than testing to specific target LDL levels, suggesting that the initiation and intensification of statin therapy be based on a patient’s risk profile.35 When maximally tolerated doses of statins do not lower LDL cholesterol by more than 30% from the patient’s baseline, there is currently no good evidence that combination therapy would be helpful, so regular monitoring of lipid levels has limited value. A lipid profile that includes levels of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides should be obtained at initial medical evaluation, at diagnosis of diabetes, and every 5 years thereafter or before the initiation of statin therapy. Ongoing testing may be appropriate in individual circumstances and to monitor for adherence to, or efficacy of, therapy.
What should I do for my patients who can’t tolerate statins?
Try a lower dose or a different statin before eliminating the class. Research has shown that even small doses (eg, rosuvastatin 5 mg) have some benefit.41
How do combination treatments figure into the current treatment of lipids in patients with diabetes?
It depends on the agent and the patient’s profile.
Fenofibrate. The ADA does not recommend automatically adding fenofibrate to statin therapy because the combination is associated with increased risks for abnormal transaminase levels, myositis, and rhabdomyolysis. In the ACCORD trial, the combination of fenofibrate and simvastatin did not reduce the rate of fatal CV events, nonfatal MIs, or nonfatal strokes compared with simvastatin alone.42
That said, a subgroup analysis suggested a benefit for men with both a triglyceride level ≥204 mg/dL (2.3 mmol/L) and an HDL cholesterol level ≤34 mg/dL (0.9 mmol/L).42 For this reason, the combination of a statin and fenofibrate may be considered for men who meet these laboratory parameters. In addition, consider medical therapy for triglyceride levels ≥500 mg/dL to reduce the risk of pancreatitis.
Ezetimibe. Recommendations regarding ezetimibe are based on the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), a 2015 RCT including over 18,000 patients that compared treatment with ezetimibe and simvastatin to simvastatin alone.43 Individuals in the trial were ≥50 years of age and had experienced an ACS within the preceding 10 days. In those with diabetes, the combination of moderate-intensity simvastatin (40 mg) and ezetimibe (10 mg) significantly reduced major adverse CV events with an absolute risk reduction of 5% (40% vs 45%) and an RR reduction of 14% over moderate-intensity simvastatin (40 mg) alone.
Based on these results, patients with diabetes and a recent ACS should be considered for combination therapy with ezetimibe and a moderate-intensity statin. The combination should also be considered in patients with diabetes and a history of ASCVD who cannot tolerate high-intensity statins.43
Niacin. The ADA currently does not recommend niacin in combination with a statin because of lack of efficacy on major ASCVD outcomes, possible increased risk of ischemic stroke, and adverse effects.44
What are the recommendations for the use of PCSK-9 inhibitors?
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors (ie, evolucumab and alirocumab) may be considered as adjunctive therapy to statins for patients with diabetes at high risk for ASCVD events who require additional lowering of LDL cholesterol. They may also be considered for those in whom high-intensity statin therapy is indicated, but not tolerated.
Antiplatelet agents
Who should take aspirin for primary prevention of CVD?
Both women and men ages ≥50 years who have diabetes and at least one additional CV risk factor (family history of premature ASCVD, hypertension, tobacco use, dyslipidemia, or albuminuria) should consider taking daily aspirin therapy (75-162 mg/d) if they do not have an excessive bleeding risk.45,46 The most common dose in the United States is 81 mg. This recommendation is supported by a 2010 consensus statement of the American Diabetes Association, American Heart Association, and the American College of Cardiology.47
Should patients with diabetes and heart disease receive antiplatelet therapy?
Yes. The evidence is clear that people with known diabetes and ASCVD benefit from aspirin therapy, according to the 2017 Standards of Care. Clopidogrel 75 mg/d is an appropriate alternative for patients who are allergic to aspirin. Dual antiplatelet therapy (a P2Y12 receptor antagonist and aspirin) should be used for as long as one year after an ACS and may have benefits beyond this period.48
Established heart disease
Are there specific recommendations for patients with diabetes and CHD?
According to the ADA Standards, there is good evidence that both aspirin and statin therapy are beneficial for patients with known ASCVD, and that high-intensity statin therapy should be used. In addition, consider ACE inhibitors to reduce the future risk of CV events. In patients with a prior MI, continue beta-blocker therapy for at least 2 years post event.49
Which medications should I avoid, or approach with caution, in patients with congestive heart failure (CHF)?
Thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, and metformin all require careful attention. This is especially important to know when you consider that almost half of all patients with T2DM will develop heart failure.50
Thiazolidinediones. The 2017 Standards of Care state that patients with diabetes and symptomatic congestive heart failure should not receive thiazolidinediones, as they can worsen heart failure status via fluid retention. As such, they are contraindicated in patients with class III and IV heart failure.51
DPP-4 inhibitors. The studies on DPP-4 inhibitors and heart failure have had mixed results. The 2013 SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) 53 trial52 showed that patients treated with saxagliptin were more likely to be hospitalized for heart failure than those taking placebo (3.5% vs 2.8%, respectively). However, the 2015 EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care)53 trial and the 2015 TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin)54 trial evaluated heart failure and mortality outcomes in patients with alogliptin and sitagliptin, respectively, compared to placebo, and did not show a relationship to heart failure.
Metformin may be used in people who have T2DM and stable CHF if their eGFR remains >30 mL/min; it should be withheld from patients with unstable heart failure and those who are hospitalized with CHF.
Are there antihyperglycemic medications that reduce CV morbidity and mortality in those with established ASCVD?
Yes. This year’s ADA Standards indicate that certain glucose-lowering medications—specifically empagliflozin (a sodium–glucose cotransporter [SGLT]-2 inhibitor) and liraglutide (a glucagon-like peptide [GLP]-1 receptor agonist)—have been shown to be beneficial for those with established CVD. According to the 2017 Standards of Care, “In patients with longstanding suboptimally controlled T2DM and established ASCVD, empagliflozin or liraglutide should be considered, as they have been shown to reduce CV and all-cause mortality when added to standard care.”2 The studies that provide support for their use are summarized below. Ongoing studies are investigating the CV effects of other agents in these drug classes.
Empagliflozin. The 2015 EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study55 was a randomized double-blind study of empagliflozin vs placebo and usual care in patients with diabetes and established CVD. Over a median follow-up of 3.1 years, treatment with empagliflozin reduced the aggregate outcome of MI, stroke, and CV death by 14%, reduced CV deaths by 38%, and decreased deaths from any cause by 32%. In December 2016, the FDA announced a new indication for empagliflozin: to reduce the risk of CV death in adult patients with T2DM and CVD.56
Liraglutide. The LEADER (Liraglutide Effect and Action in Diabetes Evaluation of Cardiovascular Outcome Results: A Long Term Evaluation) trial57 was a double-blind randomized trial of liraglutide vs placebo added to usual care in patients with T2DM at high risk for CVD or with existing CVD. More than 80% of the participants had existing CVD including a history of prior MI, cerebrovascular disease, or peripheral vascular disease. After a median follow-up of 3.8 years, the group taking liraglutide demonstrated a 13% reduction in the composite outcome of MI, stroke, or CV death, a 22% reduction in CV death, and a 15% reduction in death from any cause, compared with placebo.57
CORRESPONDENCE
Neil Skolnik, MD, Abington-Jefferson Health, 500 Old York Rd, Ste 108, Jenkintown, PA 19046; nskolnik@comcast.net.
The authors thank Sarah Bradley, director, professional engagement & collaboration at the American Diabetes Association, for her editorial and organizational assistance in the preparation of this manuscript.
1. Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. Available at: http://templatelab.com/national-diabetes-report-2014/. Accessed April 7, 2017.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Available at: http://professional.diabetes.org/sites/professional.diabetes.org/files/media/dc_40_s1_final.pdf. Accessed April 7, 2017.
3. Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591.
4. Bax JJ, Young LH, Frye RL, et al; American Diabetes Association. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007;30:2729-2736.
5. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154.
6. UK Prospective Diabetes Study (UKDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKDS 34). Lancet. 1998;352:854-865.
7. Ziemer DC, Berkowitz KJ, Panayioto RM, et al. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes. Diabetes Care. 2003;26:1719-1724.
8. Wolf AM, Conaway RM, Crowther JQ, et al; Improving Control with Activity and Nutrition (ICAN) Study. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570-1576.
9. Coppell KJ, Kataoka M, Williams SM, et al. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment-Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. 2010;341:c3337.
10. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603-615.
11. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;10:CD008277.
12. McBrien K, Rabi DM, Campbell N, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2012;172:1296-1303.
13. ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
14. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
15. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
16. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
17. Anderson RJ, Bahn GD, Moritz TE, et al; VADT Study Group. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care. 2011;34:34-38.
18. Bangalore S, Fakheri R, Toklu B, et al. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ. 2016;352:i438.
19. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253-259.
20. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772-776.
21. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
22. Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759-766.
23. Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
24. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047-2056.
25. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
26. Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
27. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079.
28. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
29. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011;10:CD004184.
30. Py̆orälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
31. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care. 2006;29:1478-1485.
32. Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117-125.
33. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816.
34. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610.
35. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
36. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
37. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
38. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688-697.
39. Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924-1929.
40. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
41. Meek C, Wierzbicki AS, Jewkes C, et al. Daily and intermittent rosuvastatin 5 mg therapy in statin intolerant patients: an observational study. Curr Med Res Opin. 2012;28:371-378.
42. ACCORD Study Group, Ginsberg HN, Bam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
43. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
44. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
45. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860.
46. Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635-1701.
47. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes. A position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
48. Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
49. Kezerashvilli A, Marzo K, De Leon J. Beta blocker use after acute myocardial infarction in the patient with normal systolic function: when is it “ok” to discontinue? Curr Cardiol Rev. 2012;8:77-84.
50. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34.
51. Pioglitazone Package Insert. Available at: http://medlibrary.org/lib/rx/meds/pioglitazone-3/. Accessed April 10, 2017.
52. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
53. Zannad F, Cannon CP, Cushman WC, et al; EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
54. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
55. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
56. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. FDA News Release, December 2, 2016. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed February 9, 2017.
57. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
1. Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. Available at: http://templatelab.com/national-diabetes-report-2014/. Accessed April 7, 2017.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Available at: http://professional.diabetes.org/sites/professional.diabetes.org/files/media/dc_40_s1_final.pdf. Accessed April 7, 2017.
3. Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591.
4. Bax JJ, Young LH, Frye RL, et al; American Diabetes Association. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007;30:2729-2736.
5. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154.
6. UK Prospective Diabetes Study (UKDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKDS 34). Lancet. 1998;352:854-865.
7. Ziemer DC, Berkowitz KJ, Panayioto RM, et al. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes. Diabetes Care. 2003;26:1719-1724.
8. Wolf AM, Conaway RM, Crowther JQ, et al; Improving Control with Activity and Nutrition (ICAN) Study. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570-1576.
9. Coppell KJ, Kataoka M, Williams SM, et al. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment-Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. 2010;341:c3337.
10. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603-615.
11. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;10:CD008277.
12. McBrien K, Rabi DM, Campbell N, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2012;172:1296-1303.
13. ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
14. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
15. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
16. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
17. Anderson RJ, Bahn GD, Moritz TE, et al; VADT Study Group. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care. 2011;34:34-38.
18. Bangalore S, Fakheri R, Toklu B, et al. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ. 2016;352:i438.
19. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253-259.
20. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772-776.
21. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
22. Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759-766.
23. Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
24. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047-2056.
25. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
26. Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
27. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079.
28. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
29. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011;10:CD004184.
30. Py̆orälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
31. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care. 2006;29:1478-1485.
32. Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117-125.
33. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816.
34. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610.
35. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
36. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
37. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
38. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688-697.
39. Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924-1929.
40. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
41. Meek C, Wierzbicki AS, Jewkes C, et al. Daily and intermittent rosuvastatin 5 mg therapy in statin intolerant patients: an observational study. Curr Med Res Opin. 2012;28:371-378.
42. ACCORD Study Group, Ginsberg HN, Bam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
43. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
44. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
45. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860.
46. Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635-1701.
47. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes. A position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
48. Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
49. Kezerashvilli A, Marzo K, De Leon J. Beta blocker use after acute myocardial infarction in the patient with normal systolic function: when is it “ok” to discontinue? Curr Cardiol Rev. 2012;8:77-84.
50. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34.
51. Pioglitazone Package Insert. Available at: http://medlibrary.org/lib/rx/meds/pioglitazone-3/. Accessed April 10, 2017.
52. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
53. Zannad F, Cannon CP, Cushman WC, et al; EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
54. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
55. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
56. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. FDA News Release, December 2, 2016. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed February 9, 2017.
57. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
Diabetes update: Your guide to the latest ADA standards
Prevention of diabetes, as well as early detection and treatment of both prediabetes and diabetes, is critical to the health of our country. Because evidence-based guidelines are key to our ability to effectively address the nation’s diabetes epidemic, the American Diabetes Association (ADA) updates its “Standards of Medical Care in Diabetes” annually to incorporate new evidence or clarifications.
The 2016 standards,1 available at professional.diabetes.org/jfp, are a valuable resource. Among the latest revisions: an expansion in screening recommendations, a change in the age at which aspirin therapy for women should be considered, and a change in A1C goals for pregnant women with diabetes.
As members of the ADA’s primary care advisory group, we use a question and answer format in the summary that follows to highlight recent revisions and review other recommendations that are of particular relevance to physicians in primary care. It is important to note, however, that ADA recommendations are not intended to preclude clinical judgment and should be applied in the context of excellent medical care.
Diagnosis and screening
Have the 2016 ADA standards changed the way diabetes is diagnosed?
No. The criteria for a diagnosis of diabetes did not change. Diabetes and prediabetes are still screened for and diagnosed with any of the following: a fasting plasma glucose (FPG); a 2-hour 75-g oral glucose tolerance test (OGTT); a random plasma glucose >200 mg/dL with symptoms of hyperglycemia; or A1C criteria (TABLE 1).1,2 The wording was changed, however, to make it clear that no one test is preferred over another for diagnosis.
Yes. In addition to screening asymptomatic adults of any age who are overweight or obese and have one or more additional risk factors for diabetes, the 2016 standards recommend screening all adults 45 years and older, regardless of weight.
Is an A1C <7% the recommended treatment goal for everyone with diabetes?
No. An A1C <7% is considered reasonable for most, but not all, nonpregnant adults. In the last few years, the ADA has focused more on individualized targets.
Tighter control (<6.5%)—which is associated with lower rates of eye disease, kidney disease, and nerve damage—may be appropriate for patients who have no significant hypoglycemia, no cardiovascular disease (CVD), a shorter duration of diabetes, or a longer expected lifespan.
Conversely, a higher target (<8%) may be appropriate for patients who are older, have longstanding diabetes, advanced macrovascular or microvascular disease, established complications, or a limited life expectancy.3,4
Pregnancy. The 2016 standards have a new target for pregnant women with diabetes: The ADA previously recommended an A1C <6% for this patient population, but now recommends a target A1C between 6% and 6.5%. This may be tightened or relaxed, however, depending on individual risk of hypoglycemia.
In focusing on individualized targets and hypoglycemia avoidance, the ADA notes that attention must be paid to fasting, pre-meal, and post-meal blood glucose levels to achieve treatment goals. The 2016 standards emphasize the importance of patient-centered diabetes care, aligned with a coordinated, team-based chronic care model.
Diabetes self-management education and support is indicated for those who are newly diagnosed, and should be provided periodically based on glucose control and progression of the disease. All patients should receive education on hypoglycemia risk and treatment.
Prediabetes and prevention
What is prediabetes and what can I do to prevent patients with prediabetes from developing diabetes?
Patients with impaired glucose tolerance, impaired fasting glucose, or an A1C between 5.7% and 6.4% are considered to have prediabetes and are at risk for developing type 2 diabetes.
Family physicians should refer patients with prediabetes to intensive diet, physical activity, and behavioral counseling programs like those based on the Diabetes Prevention Program study (www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp/Pages/default.aspx). Goals should include a minimum 7% weight loss and moderate-intensity physical activity, such as brisk walking, for at least 150 minutes per week.
Lifestyle modification programs have been shown to be very effective in preventing diabetes, with about a 58% reduction in the risk of developing type 2 diabetes after 3 years.5 The 2016 standards added a recommendation that physicians encourage the use of new technology, such as text messaging or smart phone apps, to support such efforts.
Should I consider initiating oral antiglycemics in patients with prediabetes?
Yes. Pharmacologic agents, including metformin, acarbose, and pioglitazone, have been shown to decrease progression from prediabetes to type 2 diabetes. Thus, antiglycemics should be considered for certain patients. Metformin is especially appropriate for women with a history of gestational diabetes, patients who are younger than 60 years, and those who have a body mass index (BMI) ≥35 kg/m2.6
How often should I screen patients with prediabetes?
Patients with prediabetes should be screened annually. Such individuals should also be screened and treated for modifiable cardiovascular risk factors. There is strong evidence that the treatment of obesity can be beneficial for those at any stage of the diabetes spectrum.
Obesity management
What do the 2016 ADA standards recommend for obese patients with diabetes?
With more than two-thirds of Americans either overweight or obese, the ADA added a new section on obesity management and calls on health care providers to:
- weigh patients and calculate and document their BMI at every visit, and
- counsel those who are overweight or obese on the benefits of even modest weight loss.
The ADA recommends a sustained weight loss of 5%, which can improve glycemic control and reduce the need for diabetes medications,7-9 although weight loss of ≥7% is optimal. Physicians are also called on to assess each patient’s readiness to engage in therapeutic lifestyle change to maintain a modest weight loss.
Treatment for obesity can include therapeutic lifestyle change (reduction in calories, increase in physical activity) and behavioral therapy. For refractory patients, pharmacologic therapy and bariatric surgery may be considered.
Interventions should be high-intensity (≥16 sessions in 6 months) and focus on diet, physical activity, and behavioral strategies to achieve a 500 to 750 calorie deficit per day.10 Long-term (≥1 year) comprehensive weight maintenance programs should be prescribed for those who achieve short-term weight loss.11,12 Such programs should provide at least monthly contact and encourage ongoing monitoring of body weight (weekly or more frequently), continued consumption of a reduced-calorie diet, and participation in high levels of physical activity (200 to 300 minutes per week).
Glycemic treatment
What are some of the key factors that distinguish the different type 2 diabetes medications from one another?
An increasing understanding of diabetes pathophysiology has led to a wider array of medications, making treatment more complex than ever. It is important for physicians to have a strong working knowledge of the various classes of antidiabetic agents and the subtleties between drugs in the same class to best individualize treatment.
Here are the highlights of each class of medication listed in the ADA/European Association for the Study of Diabetes algorithm for the management of type 2 diabetes,13 which is available at http://care.diabetesjournals.org/content/38/1/140/F2.large.jpg):
Metformin is the preferred initial medication for all patients who can tolerate it and have no contraindications. The drug is cost-effective, weight neutral, and has had positive cardiovascular and mortality outcomes in long-term studies. Adverse gastrointestinal (GI) effects, including nausea, diarrhea, and dyspepsia, are common but can be reduced with a slow titration of the drug. Metformin should be used with caution in those with renal disease. The dose should be reduced if the estimated glomerular filtration rate (eGFR) <45 mL/min/1.73m2 and the drug discontinued if eGFR <30 mL/min/1.73 m2.
Sulfonylureas/meglitinides stimulate insulin secretion in a glucose-independent manner. They are cost-effective and have high efficacy early in the disease and with initial use, but the effect wanes as the disease progresses. This class of drugs is associated with weight gain and hypoglycemia. Second-generation sulfonylureas (glipizide, glimepiride) are recommended; meglitinides are more expensive than sulfonylureas.
Thiazolidinediones work to improve insulin sensitivity in the periphery and have a low risk of hypoglycemia. They have been associated with fluid retention, weight gain, and worsening of pre-existing congestive heart failure, but previous cardiovascular concerns (with rosiglitazone)14 and bladder cancer risks (with pioglitazone)15-17 have been refuted. Thiazolidinediones are contraindicated in those with Class III and IV congestive heart failure, however, and patients taking them require careful monitoring for weight gain, fluid retention, and exacerbation of heart failure.
Dipeptidyl peptidase-4 inhibitors (DPP4Is) work to reduce the breakdown of endogenous incretin hormones. These oral agents increase insulin secretion in a glucose-dependent manner; more insulin is secreted when glucose is higher and less when glucose is closer to normal. This means that there is a much lower risk of hypoglycemia when a DPP4I is used as monotherapy.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs), which are injectable, also work via incretin hormones and stimulate insulin in a glucose-dependent manner. They are associated with weight loss and low rates of hypoglycemia. Adverse GI effects are common with this class of drugs, but can be reduced by titrating the medication and avoiding overeating. GLP-1RAs can be taken twice daily to once weekly, depending on the specific agent.
Sodium glucose transporter 2 inhibitors (SGLT2Is) are oral agents and the newest class of antidiabetes drugs. The drugs help block the reabsorption of glucose, thereby lowering glucose levels, blood pressure, and weight in many patients. The most common adverse effects are urinary tract and genital yeast infections. SGLT2Is should not be given to patients with advanced renal disease (chronic kidney disease Stages 3B-5) because they will not be effectively absorbed.
The US Food and Drug Administration (FDA) recently issued a warning about the risk of ketoacidosis with these agents,18 and patients should be advised to stop taking them and to seek immediate medical attention if they develop symptoms of ketoacidosis, such as excessive thirst, frequent urination, nausea and vomiting, abdominal pain, weakness or fatigue, shortness of breath, fruity-scented breath, or confusion.
Insulin is eventually needed by most patients with type 2 diabetes who live long enough to see the disease progress. The most common adverse effects are weight gain and hypoglycemia. There are many types of insulin, but only one that is delivered via inhalation—human insulin inhaled powder. Inhaled insulin, however, has the potential for adverse pulmonary effects, including cough and reduction of peak expiratory flow. Therefore, pulmonary function testing is recommended prior to its use.
Treatment goal attainment should be evaluated every 3 months, and treatment titrated at 3-month intervals if goals are not achieved. The ADA/European Association for the Study of Diabetes’ algorithm indicates that patients are likely to need insulin a year after diagnosis if their A1C goal has not been achieved or maintained.13
The following medications are not included in the algorithm but are included in the 2016 standards, and may be helpful for certain patients:
Alpha-glucosidase inhibitors delay the absorption of glucose from the proximal to distal GI tract, thereby reducing postprandial hyperglycemia. Flatulence and leakage of stool—the most common adverse effects—have limited their use in the United States.
Bile acid sequestrants (colesevelam) treat both hyperlipidemia and diabetes. The medications work by reducing glucose absorption from the GI tract. They reduce postprandial hyperglycemia, with a low risk of hypoglycemia. Colesevelam’s use is limited, however, because of the number of pills needed (6 daily).
Bromocriptine affects satiety levels via the central nervous system, and is available in a specific formulation for the treatment of diabetes. “First-dose” hypotension, however, is an adverse effect of considerable concern.1
Pramlintide, an injectable amylin mimetic given to patients on prandial insulin, can reduce postprandial glucose levels. The most common adverse effects are upper GI symptoms and hypoglycemia. Due to the adverse effects and the need for an injection with each meal, pramlintide is used infrequently.
Cardiovascular risk reduction
Has the ADA revised its recommendations for cardiovascular disease risk management?
Yes. There have been several changes. The first is in terminology, with atherosclerotic cardiovascular disease (ASCVD) replacing CVD alone. While new recommendations for statin therapy for adults older than 40 years (TABLE 2)1 were also added, the emphasis remains on therapeutic lifestyle change as an effective treatment for hypertension. These modifications should include at least 150 minutes of moderate physical activity per week and, for most patients, a reduction in total calories, saturated fat, and sodium.
It is important to remind patients that to maximize the benefits in terms of treating hyperglycemia, hypertension, and dyslipidemia, such changes must be maintained over the long term.
Aspirin therapy. The ADA also revised its recommendation regarding aspirin therapy. Based on new evidence in the treatment of women with ASCVD risk, the standards now call for considering aspirin therapy (75-162 mg/d) in both women and men ≥50 years as a primary prevention strategy for those with type 1 or type 2 diabetes with a 10-year ASCVD risk of >10%. (The previous standards recommended this only for women older than 60 years.)
Antiplatelet therapy is now recommended for patients younger than 50 years with multiple risk factors, and as secondary prevention in those with a history of ASCVD.19-21
Hypertension. The ADA’s recommendations for treating hypertension in patients with diabetes have not changed; the goal remains <140/<90 mm Hg. Lower targets may be appropriate for younger patients, those with albuminuria, and individuals with additional CVD risk factors; however, systolic pressure <130 mm Hg has not been shown to reduce CVD outcomes, and diastolic pressure <70 mm Hg has been associated with higher mortality.22
Optimal medication and lifestyle therapy are important to achieve goals, with avoidance of undue treatment burden. Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), but not both, should be included as part of treatment. Other agents, such as a thiazide diuretic, may be needed to achieve individual goals. Serum creatinine/eGFR and serum potassium levels should be monitored with the use of diuretics.
Lipids. The 2016 standards include notable changes in lipid management. The ADA sees a role for ezetimibe for select patients, based on studies such as the IMPROVE IT trial23 that included participants with diabetes. The ADA also added a table highlighting statin recommendations and delineating high and moderate-intensity statins (TABLE 3).1 Those younger than 40 years with no other risk factors may not need a statin, but patients ages 40 or older will need moderate- to high-intensity statin therapy to effectively lower ASCVD risk.24-28
These recommendations reflect a comprehensive plan to reduce ASCVD in this at-risk population, which should also include lifestyle modification, including smoking prevention and quit strategies, as needed.
Microvascular complications
DIABETIC KIDNEY DISEASE
How should I diagnose nephropathy?
The ADA changed the terminology, referring to “diabetic kidney disease” (DKD) rather than nephropathy to highlight the fact that the focus is on kidney disease directly linked to diabetes.
Other recommendations include an annual assessment of urinary albumin (eg, spot urine albumin-to-creatinine ratio and eGFR) for patients who have had type 1 diabetes for ≥5 years and all patients who have type 2 diabetes. Two out of 3 abnormal specimens collected within a 3- to 6-month period indicate the presence of albuminuria.
What can be done to prevent or slow the progression of DKD?
Optimal BP and glycemic control are key,29-35 along with diet and medication. For patients with DKD, dietary protein intake should be 0.8 g/kg body weight per day. ACE inhibitors and ARBs have been shown to slow the decline in eGFR in patients with elevated urinary albumin excretion (≥30 mg/day).
However, neither an ACE inhibitor nor an ARB is recommended for the primary prevention of DKD in patients who have normal BP, normal urine albumin-to-creatinine ratio (<30 mg/g), and normal eGFR. In addition, combined use of an ACE inhibitor and an ARB should be avoided, as it provides no additional benefit and increases the risk of adverse effects.29
RETINOPATHY
How should I manage retinopathy in patients with diabetes?
As with the management of DKD, it is important to optimize glycemic and BP control to reduce the risk, or slow the progression, of retinopathy. Intensive diabetes management, with the goal of achieving near-normal glycemic levels, has been shown in large prospective randomized studies to prevent or delay the onset and progression of diabetic retinopathy.33,36 The presence of retinopathy is not a contraindication to aspirin therapy for ASCVD prevention, as aspirin does not increase the risk of retinal hemorrhage.
When should patients with diabetes be screened for retinopathy?
Patients with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years of the onset of diabetes. Those with type 2 diabetes should have such an exam shortly after diagnosis. The exam should be repeated annually; if there is no evidence of retinopathy, however, 2-year intervals may be considered.
PERIPHERAL NEUROPATHY
When and how should I screen patients with diabetes for neuropathy?
All patients should be screened for diabetic peripheral neuropathy (DPN) starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes, and continued at least annually thereafter. Assessment should include a detailed history and 10-g monofilament testing, as well as at least one of the following tests: pinprick, temperature, and vibration sensation.
It is important, too, to screen patients with more advanced diabetes for signs and symptoms of autonomic neuropathy. Signs and symptoms may include resting tachycardia, exercise intolerance, orthostatic hypotension, gastroparesis, constipation, impaired neurovascular function, and autonomic failure in response to hypoglycemia. In men, diabetic autonomic neuropathy may cause erectile dysfunction and/or retrograde ejaculation.
How should I manage patients who have DPN?
Tight glycemic control is the only measure that has been shown to prevent or delay the development of DPN or cardiac autonomic neuropathy in patients with type 1 diabetes,37,38 and to slow the progression of neuropathy in some patients with type 2 diabetes.39
The FDA has approved pregabalin, duloxetine, and tapentadol for the treatment of pain associated with DPN. Tricyclic antidepressants, gabapentin, venlafaxine, carbamazepine, tramadol, and topical capsaicin, although not approved for the treatment of painful DPN, may also be effective in treating neuropathic pain.
For those with autonomic neuropathy, dietary changes and prokinetic agents such as erythromycin may alleviate gastroparesis. Due to extrapyramidal adverse effects, metoclopramide is reserved for the most severe and unresponsive cases. Recurrent urinary tract infections, pyelonephritis, incontinence, or palpable bladder should prompt an evaluation for bladder dysfunction. Controlling lipids and BP, quitting smoking, and making other lifestyle changes can reduce both the development and the progression of autonomic neuropathy.
FOOT CARE/PERIPHERAL ARTERIAL DISEASE
What does the ADA recommend regarding foot care for patients with diabetes?
The ADA’s standards recommend an annual comprehensive foot examination to identify risk factors predictive of ulcers and potential amputations. The exam should start with inspection and assessment of foot pulses and should seek to identify loss of peripheral sensation. The examination should include inspection of the skin, assessment of foot deformities, neurologic assessment including 10-g monofilament testing and pinprick or vibration testing or assessment of ankle reflexes, and vascular assessment, including pulses in the legs and feet.40
It is also important to screen patients for peripheral arterial disease (PAD), with a comprehensive medical history and physical exam of pulses. Ankle-brachial index testing (ABI) should be performed in patients with signs or symptoms of PAD, including claudication or skin and hair changes in the lower extremities. ABI may be considered for all patients with diabetes starting at age 50 and in those younger than 50 years who have risk factors.41
Which patients with diabetes are at higher risk for foot complications?
The following are risk factors for foot complications: previous amputation, prior foot ulcer, peripheral neuropathy, foot deformity, peripheral vascular disease, visual impairment, peripheral neuropathy (especially if on dialysis), poor glycemic control, and smoking. Patients with high-risk foot conditions should be educated about their risk and appropriate management.
A well-fitted walking shoe that cushions the feet and redistributes pressure is one option to help patients. Patients with bony deformities may need extra wide or deep shoes and patients with more advanced disease may need custom-fitted shoes.
When should patients be referred to a foot specialist?
Refer patients to a foot care specialist for ongoing preventive care and lifelong surveillance if they smoke or have a history of lower-extremity complications, a loss of protective sensation, structural abnormalities, or PAD.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/site/misc/2016-Standards-of-Care.pdf. Accessed March 28, 2016.
2. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334.
3. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362.
4. Vijan S, Sussman JB, Yudkin JS, et al. Effect of patients’ risks and p on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–1234.
5. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
6. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737.
7. UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905–912.
8. Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415.
9. Pastors JG, Warshaw H, Daly A, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–613.
10. Selph S, Dana T, Bougatsos C, et al. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 US Preventive Services Task Force Recommendation. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed March 28, 2016.
11. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and metaanalysis. Obesity (Silver Spring). 2006;14:1283–1293.
12. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;99:14–23.
13. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149.
14. Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195.
15. Balaji V, Seshiah V, Ashtalakshmi G, et al. Retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–427.
16. Kuo HW, Tiao MM, Ho SC, et al. Pioglitazone use and the risk of bladder cancer. Kaohsiung J Med Sci. 2014;30:94–97.
17. Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254-259.
18. US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed December 11, 2015.
19. Huxley RR, Peters SAE, Mishra GD, et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206.
20. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551.
21. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973-1980.
22. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
23. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397.
24. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485.
25. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696.
26. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504.
27. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316.
28. Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080.
29. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713.
30. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an American Diabetes Association Consensus Conference. Diabetes Care. 2014;37:2864–2883.
31. The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720.
32. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
33. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
34. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
35. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430.
36. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559.
37. Chew EY, Ambrosius WT, Davis MD, et al; ACCORD Study Group; ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244.
38. Ang L, Jaiswal M, Martin C, et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14:528.
39. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group. Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:31–38.
40. Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765.
41. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341.
42. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Available at: http://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed April 8, 2016.
Prevention of diabetes, as well as early detection and treatment of both prediabetes and diabetes, is critical to the health of our country. Because evidence-based guidelines are key to our ability to effectively address the nation’s diabetes epidemic, the American Diabetes Association (ADA) updates its “Standards of Medical Care in Diabetes” annually to incorporate new evidence or clarifications.
The 2016 standards,1 available at professional.diabetes.org/jfp, are a valuable resource. Among the latest revisions: an expansion in screening recommendations, a change in the age at which aspirin therapy for women should be considered, and a change in A1C goals for pregnant women with diabetes.
As members of the ADA’s primary care advisory group, we use a question and answer format in the summary that follows to highlight recent revisions and review other recommendations that are of particular relevance to physicians in primary care. It is important to note, however, that ADA recommendations are not intended to preclude clinical judgment and should be applied in the context of excellent medical care.
Diagnosis and screening
Have the 2016 ADA standards changed the way diabetes is diagnosed?
No. The criteria for a diagnosis of diabetes did not change. Diabetes and prediabetes are still screened for and diagnosed with any of the following: a fasting plasma glucose (FPG); a 2-hour 75-g oral glucose tolerance test (OGTT); a random plasma glucose >200 mg/dL with symptoms of hyperglycemia; or A1C criteria (TABLE 1).1,2 The wording was changed, however, to make it clear that no one test is preferred over another for diagnosis.
Yes. In addition to screening asymptomatic adults of any age who are overweight or obese and have one or more additional risk factors for diabetes, the 2016 standards recommend screening all adults 45 years and older, regardless of weight.
Is an A1C <7% the recommended treatment goal for everyone with diabetes?
No. An A1C <7% is considered reasonable for most, but not all, nonpregnant adults. In the last few years, the ADA has focused more on individualized targets.
Tighter control (<6.5%)—which is associated with lower rates of eye disease, kidney disease, and nerve damage—may be appropriate for patients who have no significant hypoglycemia, no cardiovascular disease (CVD), a shorter duration of diabetes, or a longer expected lifespan.
Conversely, a higher target (<8%) may be appropriate for patients who are older, have longstanding diabetes, advanced macrovascular or microvascular disease, established complications, or a limited life expectancy.3,4
Pregnancy. The 2016 standards have a new target for pregnant women with diabetes: The ADA previously recommended an A1C <6% for this patient population, but now recommends a target A1C between 6% and 6.5%. This may be tightened or relaxed, however, depending on individual risk of hypoglycemia.
In focusing on individualized targets and hypoglycemia avoidance, the ADA notes that attention must be paid to fasting, pre-meal, and post-meal blood glucose levels to achieve treatment goals. The 2016 standards emphasize the importance of patient-centered diabetes care, aligned with a coordinated, team-based chronic care model.
Diabetes self-management education and support is indicated for those who are newly diagnosed, and should be provided periodically based on glucose control and progression of the disease. All patients should receive education on hypoglycemia risk and treatment.
Prediabetes and prevention
What is prediabetes and what can I do to prevent patients with prediabetes from developing diabetes?
Patients with impaired glucose tolerance, impaired fasting glucose, or an A1C between 5.7% and 6.4% are considered to have prediabetes and are at risk for developing type 2 diabetes.
Family physicians should refer patients with prediabetes to intensive diet, physical activity, and behavioral counseling programs like those based on the Diabetes Prevention Program study (www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp/Pages/default.aspx). Goals should include a minimum 7% weight loss and moderate-intensity physical activity, such as brisk walking, for at least 150 minutes per week.
Lifestyle modification programs have been shown to be very effective in preventing diabetes, with about a 58% reduction in the risk of developing type 2 diabetes after 3 years.5 The 2016 standards added a recommendation that physicians encourage the use of new technology, such as text messaging or smart phone apps, to support such efforts.
Should I consider initiating oral antiglycemics in patients with prediabetes?
Yes. Pharmacologic agents, including metformin, acarbose, and pioglitazone, have been shown to decrease progression from prediabetes to type 2 diabetes. Thus, antiglycemics should be considered for certain patients. Metformin is especially appropriate for women with a history of gestational diabetes, patients who are younger than 60 years, and those who have a body mass index (BMI) ≥35 kg/m2.6
How often should I screen patients with prediabetes?
Patients with prediabetes should be screened annually. Such individuals should also be screened and treated for modifiable cardiovascular risk factors. There is strong evidence that the treatment of obesity can be beneficial for those at any stage of the diabetes spectrum.
Obesity management
What do the 2016 ADA standards recommend for obese patients with diabetes?
With more than two-thirds of Americans either overweight or obese, the ADA added a new section on obesity management and calls on health care providers to:
- weigh patients and calculate and document their BMI at every visit, and
- counsel those who are overweight or obese on the benefits of even modest weight loss.
The ADA recommends a sustained weight loss of 5%, which can improve glycemic control and reduce the need for diabetes medications,7-9 although weight loss of ≥7% is optimal. Physicians are also called on to assess each patient’s readiness to engage in therapeutic lifestyle change to maintain a modest weight loss.
Treatment for obesity can include therapeutic lifestyle change (reduction in calories, increase in physical activity) and behavioral therapy. For refractory patients, pharmacologic therapy and bariatric surgery may be considered.
Interventions should be high-intensity (≥16 sessions in 6 months) and focus on diet, physical activity, and behavioral strategies to achieve a 500 to 750 calorie deficit per day.10 Long-term (≥1 year) comprehensive weight maintenance programs should be prescribed for those who achieve short-term weight loss.11,12 Such programs should provide at least monthly contact and encourage ongoing monitoring of body weight (weekly or more frequently), continued consumption of a reduced-calorie diet, and participation in high levels of physical activity (200 to 300 minutes per week).
Glycemic treatment
What are some of the key factors that distinguish the different type 2 diabetes medications from one another?
An increasing understanding of diabetes pathophysiology has led to a wider array of medications, making treatment more complex than ever. It is important for physicians to have a strong working knowledge of the various classes of antidiabetic agents and the subtleties between drugs in the same class to best individualize treatment.
Here are the highlights of each class of medication listed in the ADA/European Association for the Study of Diabetes algorithm for the management of type 2 diabetes,13 which is available at http://care.diabetesjournals.org/content/38/1/140/F2.large.jpg):
Metformin is the preferred initial medication for all patients who can tolerate it and have no contraindications. The drug is cost-effective, weight neutral, and has had positive cardiovascular and mortality outcomes in long-term studies. Adverse gastrointestinal (GI) effects, including nausea, diarrhea, and dyspepsia, are common but can be reduced with a slow titration of the drug. Metformin should be used with caution in those with renal disease. The dose should be reduced if the estimated glomerular filtration rate (eGFR) <45 mL/min/1.73m2 and the drug discontinued if eGFR <30 mL/min/1.73 m2.
Sulfonylureas/meglitinides stimulate insulin secretion in a glucose-independent manner. They are cost-effective and have high efficacy early in the disease and with initial use, but the effect wanes as the disease progresses. This class of drugs is associated with weight gain and hypoglycemia. Second-generation sulfonylureas (glipizide, glimepiride) are recommended; meglitinides are more expensive than sulfonylureas.
Thiazolidinediones work to improve insulin sensitivity in the periphery and have a low risk of hypoglycemia. They have been associated with fluid retention, weight gain, and worsening of pre-existing congestive heart failure, but previous cardiovascular concerns (with rosiglitazone)14 and bladder cancer risks (with pioglitazone)15-17 have been refuted. Thiazolidinediones are contraindicated in those with Class III and IV congestive heart failure, however, and patients taking them require careful monitoring for weight gain, fluid retention, and exacerbation of heart failure.
Dipeptidyl peptidase-4 inhibitors (DPP4Is) work to reduce the breakdown of endogenous incretin hormones. These oral agents increase insulin secretion in a glucose-dependent manner; more insulin is secreted when glucose is higher and less when glucose is closer to normal. This means that there is a much lower risk of hypoglycemia when a DPP4I is used as monotherapy.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs), which are injectable, also work via incretin hormones and stimulate insulin in a glucose-dependent manner. They are associated with weight loss and low rates of hypoglycemia. Adverse GI effects are common with this class of drugs, but can be reduced by titrating the medication and avoiding overeating. GLP-1RAs can be taken twice daily to once weekly, depending on the specific agent.
Sodium glucose transporter 2 inhibitors (SGLT2Is) are oral agents and the newest class of antidiabetes drugs. The drugs help block the reabsorption of glucose, thereby lowering glucose levels, blood pressure, and weight in many patients. The most common adverse effects are urinary tract and genital yeast infections. SGLT2Is should not be given to patients with advanced renal disease (chronic kidney disease Stages 3B-5) because they will not be effectively absorbed.
The US Food and Drug Administration (FDA) recently issued a warning about the risk of ketoacidosis with these agents,18 and patients should be advised to stop taking them and to seek immediate medical attention if they develop symptoms of ketoacidosis, such as excessive thirst, frequent urination, nausea and vomiting, abdominal pain, weakness or fatigue, shortness of breath, fruity-scented breath, or confusion.
Insulin is eventually needed by most patients with type 2 diabetes who live long enough to see the disease progress. The most common adverse effects are weight gain and hypoglycemia. There are many types of insulin, but only one that is delivered via inhalation—human insulin inhaled powder. Inhaled insulin, however, has the potential for adverse pulmonary effects, including cough and reduction of peak expiratory flow. Therefore, pulmonary function testing is recommended prior to its use.
Treatment goal attainment should be evaluated every 3 months, and treatment titrated at 3-month intervals if goals are not achieved. The ADA/European Association for the Study of Diabetes’ algorithm indicates that patients are likely to need insulin a year after diagnosis if their A1C goal has not been achieved or maintained.13
The following medications are not included in the algorithm but are included in the 2016 standards, and may be helpful for certain patients:
Alpha-glucosidase inhibitors delay the absorption of glucose from the proximal to distal GI tract, thereby reducing postprandial hyperglycemia. Flatulence and leakage of stool—the most common adverse effects—have limited their use in the United States.
Bile acid sequestrants (colesevelam) treat both hyperlipidemia and diabetes. The medications work by reducing glucose absorption from the GI tract. They reduce postprandial hyperglycemia, with a low risk of hypoglycemia. Colesevelam’s use is limited, however, because of the number of pills needed (6 daily).
Bromocriptine affects satiety levels via the central nervous system, and is available in a specific formulation for the treatment of diabetes. “First-dose” hypotension, however, is an adverse effect of considerable concern.1
Pramlintide, an injectable amylin mimetic given to patients on prandial insulin, can reduce postprandial glucose levels. The most common adverse effects are upper GI symptoms and hypoglycemia. Due to the adverse effects and the need for an injection with each meal, pramlintide is used infrequently.
Cardiovascular risk reduction
Has the ADA revised its recommendations for cardiovascular disease risk management?
Yes. There have been several changes. The first is in terminology, with atherosclerotic cardiovascular disease (ASCVD) replacing CVD alone. While new recommendations for statin therapy for adults older than 40 years (TABLE 2)1 were also added, the emphasis remains on therapeutic lifestyle change as an effective treatment for hypertension. These modifications should include at least 150 minutes of moderate physical activity per week and, for most patients, a reduction in total calories, saturated fat, and sodium.
It is important to remind patients that to maximize the benefits in terms of treating hyperglycemia, hypertension, and dyslipidemia, such changes must be maintained over the long term.
Aspirin therapy. The ADA also revised its recommendation regarding aspirin therapy. Based on new evidence in the treatment of women with ASCVD risk, the standards now call for considering aspirin therapy (75-162 mg/d) in both women and men ≥50 years as a primary prevention strategy for those with type 1 or type 2 diabetes with a 10-year ASCVD risk of >10%. (The previous standards recommended this only for women older than 60 years.)
Antiplatelet therapy is now recommended for patients younger than 50 years with multiple risk factors, and as secondary prevention in those with a history of ASCVD.19-21
Hypertension. The ADA’s recommendations for treating hypertension in patients with diabetes have not changed; the goal remains <140/<90 mm Hg. Lower targets may be appropriate for younger patients, those with albuminuria, and individuals with additional CVD risk factors; however, systolic pressure <130 mm Hg has not been shown to reduce CVD outcomes, and diastolic pressure <70 mm Hg has been associated with higher mortality.22
Optimal medication and lifestyle therapy are important to achieve goals, with avoidance of undue treatment burden. Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), but not both, should be included as part of treatment. Other agents, such as a thiazide diuretic, may be needed to achieve individual goals. Serum creatinine/eGFR and serum potassium levels should be monitored with the use of diuretics.
Lipids. The 2016 standards include notable changes in lipid management. The ADA sees a role for ezetimibe for select patients, based on studies such as the IMPROVE IT trial23 that included participants with diabetes. The ADA also added a table highlighting statin recommendations and delineating high and moderate-intensity statins (TABLE 3).1 Those younger than 40 years with no other risk factors may not need a statin, but patients ages 40 or older will need moderate- to high-intensity statin therapy to effectively lower ASCVD risk.24-28
These recommendations reflect a comprehensive plan to reduce ASCVD in this at-risk population, which should also include lifestyle modification, including smoking prevention and quit strategies, as needed.
Microvascular complications
DIABETIC KIDNEY DISEASE
How should I diagnose nephropathy?
The ADA changed the terminology, referring to “diabetic kidney disease” (DKD) rather than nephropathy to highlight the fact that the focus is on kidney disease directly linked to diabetes.
Other recommendations include an annual assessment of urinary albumin (eg, spot urine albumin-to-creatinine ratio and eGFR) for patients who have had type 1 diabetes for ≥5 years and all patients who have type 2 diabetes. Two out of 3 abnormal specimens collected within a 3- to 6-month period indicate the presence of albuminuria.
What can be done to prevent or slow the progression of DKD?
Optimal BP and glycemic control are key,29-35 along with diet and medication. For patients with DKD, dietary protein intake should be 0.8 g/kg body weight per day. ACE inhibitors and ARBs have been shown to slow the decline in eGFR in patients with elevated urinary albumin excretion (≥30 mg/day).
However, neither an ACE inhibitor nor an ARB is recommended for the primary prevention of DKD in patients who have normal BP, normal urine albumin-to-creatinine ratio (<30 mg/g), and normal eGFR. In addition, combined use of an ACE inhibitor and an ARB should be avoided, as it provides no additional benefit and increases the risk of adverse effects.29
RETINOPATHY
How should I manage retinopathy in patients with diabetes?
As with the management of DKD, it is important to optimize glycemic and BP control to reduce the risk, or slow the progression, of retinopathy. Intensive diabetes management, with the goal of achieving near-normal glycemic levels, has been shown in large prospective randomized studies to prevent or delay the onset and progression of diabetic retinopathy.33,36 The presence of retinopathy is not a contraindication to aspirin therapy for ASCVD prevention, as aspirin does not increase the risk of retinal hemorrhage.
When should patients with diabetes be screened for retinopathy?
Patients with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years of the onset of diabetes. Those with type 2 diabetes should have such an exam shortly after diagnosis. The exam should be repeated annually; if there is no evidence of retinopathy, however, 2-year intervals may be considered.
PERIPHERAL NEUROPATHY
When and how should I screen patients with diabetes for neuropathy?
All patients should be screened for diabetic peripheral neuropathy (DPN) starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes, and continued at least annually thereafter. Assessment should include a detailed history and 10-g monofilament testing, as well as at least one of the following tests: pinprick, temperature, and vibration sensation.
It is important, too, to screen patients with more advanced diabetes for signs and symptoms of autonomic neuropathy. Signs and symptoms may include resting tachycardia, exercise intolerance, orthostatic hypotension, gastroparesis, constipation, impaired neurovascular function, and autonomic failure in response to hypoglycemia. In men, diabetic autonomic neuropathy may cause erectile dysfunction and/or retrograde ejaculation.
How should I manage patients who have DPN?
Tight glycemic control is the only measure that has been shown to prevent or delay the development of DPN or cardiac autonomic neuropathy in patients with type 1 diabetes,37,38 and to slow the progression of neuropathy in some patients with type 2 diabetes.39
The FDA has approved pregabalin, duloxetine, and tapentadol for the treatment of pain associated with DPN. Tricyclic antidepressants, gabapentin, venlafaxine, carbamazepine, tramadol, and topical capsaicin, although not approved for the treatment of painful DPN, may also be effective in treating neuropathic pain.
For those with autonomic neuropathy, dietary changes and prokinetic agents such as erythromycin may alleviate gastroparesis. Due to extrapyramidal adverse effects, metoclopramide is reserved for the most severe and unresponsive cases. Recurrent urinary tract infections, pyelonephritis, incontinence, or palpable bladder should prompt an evaluation for bladder dysfunction. Controlling lipids and BP, quitting smoking, and making other lifestyle changes can reduce both the development and the progression of autonomic neuropathy.
FOOT CARE/PERIPHERAL ARTERIAL DISEASE
What does the ADA recommend regarding foot care for patients with diabetes?
The ADA’s standards recommend an annual comprehensive foot examination to identify risk factors predictive of ulcers and potential amputations. The exam should start with inspection and assessment of foot pulses and should seek to identify loss of peripheral sensation. The examination should include inspection of the skin, assessment of foot deformities, neurologic assessment including 10-g monofilament testing and pinprick or vibration testing or assessment of ankle reflexes, and vascular assessment, including pulses in the legs and feet.40
It is also important to screen patients for peripheral arterial disease (PAD), with a comprehensive medical history and physical exam of pulses. Ankle-brachial index testing (ABI) should be performed in patients with signs or symptoms of PAD, including claudication or skin and hair changes in the lower extremities. ABI may be considered for all patients with diabetes starting at age 50 and in those younger than 50 years who have risk factors.41
Which patients with diabetes are at higher risk for foot complications?
The following are risk factors for foot complications: previous amputation, prior foot ulcer, peripheral neuropathy, foot deformity, peripheral vascular disease, visual impairment, peripheral neuropathy (especially if on dialysis), poor glycemic control, and smoking. Patients with high-risk foot conditions should be educated about their risk and appropriate management.
A well-fitted walking shoe that cushions the feet and redistributes pressure is one option to help patients. Patients with bony deformities may need extra wide or deep shoes and patients with more advanced disease may need custom-fitted shoes.
When should patients be referred to a foot specialist?
Refer patients to a foot care specialist for ongoing preventive care and lifelong surveillance if they smoke or have a history of lower-extremity complications, a loss of protective sensation, structural abnormalities, or PAD.
Prevention of diabetes, as well as early detection and treatment of both prediabetes and diabetes, is critical to the health of our country. Because evidence-based guidelines are key to our ability to effectively address the nation’s diabetes epidemic, the American Diabetes Association (ADA) updates its “Standards of Medical Care in Diabetes” annually to incorporate new evidence or clarifications.
The 2016 standards,1 available at professional.diabetes.org/jfp, are a valuable resource. Among the latest revisions: an expansion in screening recommendations, a change in the age at which aspirin therapy for women should be considered, and a change in A1C goals for pregnant women with diabetes.
As members of the ADA’s primary care advisory group, we use a question and answer format in the summary that follows to highlight recent revisions and review other recommendations that are of particular relevance to physicians in primary care. It is important to note, however, that ADA recommendations are not intended to preclude clinical judgment and should be applied in the context of excellent medical care.
Diagnosis and screening
Have the 2016 ADA standards changed the way diabetes is diagnosed?
No. The criteria for a diagnosis of diabetes did not change. Diabetes and prediabetes are still screened for and diagnosed with any of the following: a fasting plasma glucose (FPG); a 2-hour 75-g oral glucose tolerance test (OGTT); a random plasma glucose >200 mg/dL with symptoms of hyperglycemia; or A1C criteria (TABLE 1).1,2 The wording was changed, however, to make it clear that no one test is preferred over another for diagnosis.
Yes. In addition to screening asymptomatic adults of any age who are overweight or obese and have one or more additional risk factors for diabetes, the 2016 standards recommend screening all adults 45 years and older, regardless of weight.
Is an A1C <7% the recommended treatment goal for everyone with diabetes?
No. An A1C <7% is considered reasonable for most, but not all, nonpregnant adults. In the last few years, the ADA has focused more on individualized targets.
Tighter control (<6.5%)—which is associated with lower rates of eye disease, kidney disease, and nerve damage—may be appropriate for patients who have no significant hypoglycemia, no cardiovascular disease (CVD), a shorter duration of diabetes, or a longer expected lifespan.
Conversely, a higher target (<8%) may be appropriate for patients who are older, have longstanding diabetes, advanced macrovascular or microvascular disease, established complications, or a limited life expectancy.3,4
Pregnancy. The 2016 standards have a new target for pregnant women with diabetes: The ADA previously recommended an A1C <6% for this patient population, but now recommends a target A1C between 6% and 6.5%. This may be tightened or relaxed, however, depending on individual risk of hypoglycemia.
In focusing on individualized targets and hypoglycemia avoidance, the ADA notes that attention must be paid to fasting, pre-meal, and post-meal blood glucose levels to achieve treatment goals. The 2016 standards emphasize the importance of patient-centered diabetes care, aligned with a coordinated, team-based chronic care model.
Diabetes self-management education and support is indicated for those who are newly diagnosed, and should be provided periodically based on glucose control and progression of the disease. All patients should receive education on hypoglycemia risk and treatment.
Prediabetes and prevention
What is prediabetes and what can I do to prevent patients with prediabetes from developing diabetes?
Patients with impaired glucose tolerance, impaired fasting glucose, or an A1C between 5.7% and 6.4% are considered to have prediabetes and are at risk for developing type 2 diabetes.
Family physicians should refer patients with prediabetes to intensive diet, physical activity, and behavioral counseling programs like those based on the Diabetes Prevention Program study (www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp/Pages/default.aspx). Goals should include a minimum 7% weight loss and moderate-intensity physical activity, such as brisk walking, for at least 150 minutes per week.
Lifestyle modification programs have been shown to be very effective in preventing diabetes, with about a 58% reduction in the risk of developing type 2 diabetes after 3 years.5 The 2016 standards added a recommendation that physicians encourage the use of new technology, such as text messaging or smart phone apps, to support such efforts.
Should I consider initiating oral antiglycemics in patients with prediabetes?
Yes. Pharmacologic agents, including metformin, acarbose, and pioglitazone, have been shown to decrease progression from prediabetes to type 2 diabetes. Thus, antiglycemics should be considered for certain patients. Metformin is especially appropriate for women with a history of gestational diabetes, patients who are younger than 60 years, and those who have a body mass index (BMI) ≥35 kg/m2.6
How often should I screen patients with prediabetes?
Patients with prediabetes should be screened annually. Such individuals should also be screened and treated for modifiable cardiovascular risk factors. There is strong evidence that the treatment of obesity can be beneficial for those at any stage of the diabetes spectrum.
Obesity management
What do the 2016 ADA standards recommend for obese patients with diabetes?
With more than two-thirds of Americans either overweight or obese, the ADA added a new section on obesity management and calls on health care providers to:
- weigh patients and calculate and document their BMI at every visit, and
- counsel those who are overweight or obese on the benefits of even modest weight loss.
The ADA recommends a sustained weight loss of 5%, which can improve glycemic control and reduce the need for diabetes medications,7-9 although weight loss of ≥7% is optimal. Physicians are also called on to assess each patient’s readiness to engage in therapeutic lifestyle change to maintain a modest weight loss.
Treatment for obesity can include therapeutic lifestyle change (reduction in calories, increase in physical activity) and behavioral therapy. For refractory patients, pharmacologic therapy and bariatric surgery may be considered.
Interventions should be high-intensity (≥16 sessions in 6 months) and focus on diet, physical activity, and behavioral strategies to achieve a 500 to 750 calorie deficit per day.10 Long-term (≥1 year) comprehensive weight maintenance programs should be prescribed for those who achieve short-term weight loss.11,12 Such programs should provide at least monthly contact and encourage ongoing monitoring of body weight (weekly or more frequently), continued consumption of a reduced-calorie diet, and participation in high levels of physical activity (200 to 300 minutes per week).
Glycemic treatment
What are some of the key factors that distinguish the different type 2 diabetes medications from one another?
An increasing understanding of diabetes pathophysiology has led to a wider array of medications, making treatment more complex than ever. It is important for physicians to have a strong working knowledge of the various classes of antidiabetic agents and the subtleties between drugs in the same class to best individualize treatment.
Here are the highlights of each class of medication listed in the ADA/European Association for the Study of Diabetes algorithm for the management of type 2 diabetes,13 which is available at http://care.diabetesjournals.org/content/38/1/140/F2.large.jpg):
Metformin is the preferred initial medication for all patients who can tolerate it and have no contraindications. The drug is cost-effective, weight neutral, and has had positive cardiovascular and mortality outcomes in long-term studies. Adverse gastrointestinal (GI) effects, including nausea, diarrhea, and dyspepsia, are common but can be reduced with a slow titration of the drug. Metformin should be used with caution in those with renal disease. The dose should be reduced if the estimated glomerular filtration rate (eGFR) <45 mL/min/1.73m2 and the drug discontinued if eGFR <30 mL/min/1.73 m2.
Sulfonylureas/meglitinides stimulate insulin secretion in a glucose-independent manner. They are cost-effective and have high efficacy early in the disease and with initial use, but the effect wanes as the disease progresses. This class of drugs is associated with weight gain and hypoglycemia. Second-generation sulfonylureas (glipizide, glimepiride) are recommended; meglitinides are more expensive than sulfonylureas.
Thiazolidinediones work to improve insulin sensitivity in the periphery and have a low risk of hypoglycemia. They have been associated with fluid retention, weight gain, and worsening of pre-existing congestive heart failure, but previous cardiovascular concerns (with rosiglitazone)14 and bladder cancer risks (with pioglitazone)15-17 have been refuted. Thiazolidinediones are contraindicated in those with Class III and IV congestive heart failure, however, and patients taking them require careful monitoring for weight gain, fluid retention, and exacerbation of heart failure.
Dipeptidyl peptidase-4 inhibitors (DPP4Is) work to reduce the breakdown of endogenous incretin hormones. These oral agents increase insulin secretion in a glucose-dependent manner; more insulin is secreted when glucose is higher and less when glucose is closer to normal. This means that there is a much lower risk of hypoglycemia when a DPP4I is used as monotherapy.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs), which are injectable, also work via incretin hormones and stimulate insulin in a glucose-dependent manner. They are associated with weight loss and low rates of hypoglycemia. Adverse GI effects are common with this class of drugs, but can be reduced by titrating the medication and avoiding overeating. GLP-1RAs can be taken twice daily to once weekly, depending on the specific agent.
Sodium glucose transporter 2 inhibitors (SGLT2Is) are oral agents and the newest class of antidiabetes drugs. The drugs help block the reabsorption of glucose, thereby lowering glucose levels, blood pressure, and weight in many patients. The most common adverse effects are urinary tract and genital yeast infections. SGLT2Is should not be given to patients with advanced renal disease (chronic kidney disease Stages 3B-5) because they will not be effectively absorbed.
The US Food and Drug Administration (FDA) recently issued a warning about the risk of ketoacidosis with these agents,18 and patients should be advised to stop taking them and to seek immediate medical attention if they develop symptoms of ketoacidosis, such as excessive thirst, frequent urination, nausea and vomiting, abdominal pain, weakness or fatigue, shortness of breath, fruity-scented breath, or confusion.
Insulin is eventually needed by most patients with type 2 diabetes who live long enough to see the disease progress. The most common adverse effects are weight gain and hypoglycemia. There are many types of insulin, but only one that is delivered via inhalation—human insulin inhaled powder. Inhaled insulin, however, has the potential for adverse pulmonary effects, including cough and reduction of peak expiratory flow. Therefore, pulmonary function testing is recommended prior to its use.
Treatment goal attainment should be evaluated every 3 months, and treatment titrated at 3-month intervals if goals are not achieved. The ADA/European Association for the Study of Diabetes’ algorithm indicates that patients are likely to need insulin a year after diagnosis if their A1C goal has not been achieved or maintained.13
The following medications are not included in the algorithm but are included in the 2016 standards, and may be helpful for certain patients:
Alpha-glucosidase inhibitors delay the absorption of glucose from the proximal to distal GI tract, thereby reducing postprandial hyperglycemia. Flatulence and leakage of stool—the most common adverse effects—have limited their use in the United States.
Bile acid sequestrants (colesevelam) treat both hyperlipidemia and diabetes. The medications work by reducing glucose absorption from the GI tract. They reduce postprandial hyperglycemia, with a low risk of hypoglycemia. Colesevelam’s use is limited, however, because of the number of pills needed (6 daily).
Bromocriptine affects satiety levels via the central nervous system, and is available in a specific formulation for the treatment of diabetes. “First-dose” hypotension, however, is an adverse effect of considerable concern.1
Pramlintide, an injectable amylin mimetic given to patients on prandial insulin, can reduce postprandial glucose levels. The most common adverse effects are upper GI symptoms and hypoglycemia. Due to the adverse effects and the need for an injection with each meal, pramlintide is used infrequently.
Cardiovascular risk reduction
Has the ADA revised its recommendations for cardiovascular disease risk management?
Yes. There have been several changes. The first is in terminology, with atherosclerotic cardiovascular disease (ASCVD) replacing CVD alone. While new recommendations for statin therapy for adults older than 40 years (TABLE 2)1 were also added, the emphasis remains on therapeutic lifestyle change as an effective treatment for hypertension. These modifications should include at least 150 minutes of moderate physical activity per week and, for most patients, a reduction in total calories, saturated fat, and sodium.
It is important to remind patients that to maximize the benefits in terms of treating hyperglycemia, hypertension, and dyslipidemia, such changes must be maintained over the long term.
Aspirin therapy. The ADA also revised its recommendation regarding aspirin therapy. Based on new evidence in the treatment of women with ASCVD risk, the standards now call for considering aspirin therapy (75-162 mg/d) in both women and men ≥50 years as a primary prevention strategy for those with type 1 or type 2 diabetes with a 10-year ASCVD risk of >10%. (The previous standards recommended this only for women older than 60 years.)
Antiplatelet therapy is now recommended for patients younger than 50 years with multiple risk factors, and as secondary prevention in those with a history of ASCVD.19-21
Hypertension. The ADA’s recommendations for treating hypertension in patients with diabetes have not changed; the goal remains <140/<90 mm Hg. Lower targets may be appropriate for younger patients, those with albuminuria, and individuals with additional CVD risk factors; however, systolic pressure <130 mm Hg has not been shown to reduce CVD outcomes, and diastolic pressure <70 mm Hg has been associated with higher mortality.22
Optimal medication and lifestyle therapy are important to achieve goals, with avoidance of undue treatment burden. Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), but not both, should be included as part of treatment. Other agents, such as a thiazide diuretic, may be needed to achieve individual goals. Serum creatinine/eGFR and serum potassium levels should be monitored with the use of diuretics.
Lipids. The 2016 standards include notable changes in lipid management. The ADA sees a role for ezetimibe for select patients, based on studies such as the IMPROVE IT trial23 that included participants with diabetes. The ADA also added a table highlighting statin recommendations and delineating high and moderate-intensity statins (TABLE 3).1 Those younger than 40 years with no other risk factors may not need a statin, but patients ages 40 or older will need moderate- to high-intensity statin therapy to effectively lower ASCVD risk.24-28
These recommendations reflect a comprehensive plan to reduce ASCVD in this at-risk population, which should also include lifestyle modification, including smoking prevention and quit strategies, as needed.
Microvascular complications
DIABETIC KIDNEY DISEASE
How should I diagnose nephropathy?
The ADA changed the terminology, referring to “diabetic kidney disease” (DKD) rather than nephropathy to highlight the fact that the focus is on kidney disease directly linked to diabetes.
Other recommendations include an annual assessment of urinary albumin (eg, spot urine albumin-to-creatinine ratio and eGFR) for patients who have had type 1 diabetes for ≥5 years and all patients who have type 2 diabetes. Two out of 3 abnormal specimens collected within a 3- to 6-month period indicate the presence of albuminuria.
What can be done to prevent or slow the progression of DKD?
Optimal BP and glycemic control are key,29-35 along with diet and medication. For patients with DKD, dietary protein intake should be 0.8 g/kg body weight per day. ACE inhibitors and ARBs have been shown to slow the decline in eGFR in patients with elevated urinary albumin excretion (≥30 mg/day).
However, neither an ACE inhibitor nor an ARB is recommended for the primary prevention of DKD in patients who have normal BP, normal urine albumin-to-creatinine ratio (<30 mg/g), and normal eGFR. In addition, combined use of an ACE inhibitor and an ARB should be avoided, as it provides no additional benefit and increases the risk of adverse effects.29
RETINOPATHY
How should I manage retinopathy in patients with diabetes?
As with the management of DKD, it is important to optimize glycemic and BP control to reduce the risk, or slow the progression, of retinopathy. Intensive diabetes management, with the goal of achieving near-normal glycemic levels, has been shown in large prospective randomized studies to prevent or delay the onset and progression of diabetic retinopathy.33,36 The presence of retinopathy is not a contraindication to aspirin therapy for ASCVD prevention, as aspirin does not increase the risk of retinal hemorrhage.
When should patients with diabetes be screened for retinopathy?
Patients with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years of the onset of diabetes. Those with type 2 diabetes should have such an exam shortly after diagnosis. The exam should be repeated annually; if there is no evidence of retinopathy, however, 2-year intervals may be considered.
PERIPHERAL NEUROPATHY
When and how should I screen patients with diabetes for neuropathy?
All patients should be screened for diabetic peripheral neuropathy (DPN) starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes, and continued at least annually thereafter. Assessment should include a detailed history and 10-g monofilament testing, as well as at least one of the following tests: pinprick, temperature, and vibration sensation.
It is important, too, to screen patients with more advanced diabetes for signs and symptoms of autonomic neuropathy. Signs and symptoms may include resting tachycardia, exercise intolerance, orthostatic hypotension, gastroparesis, constipation, impaired neurovascular function, and autonomic failure in response to hypoglycemia. In men, diabetic autonomic neuropathy may cause erectile dysfunction and/or retrograde ejaculation.
How should I manage patients who have DPN?
Tight glycemic control is the only measure that has been shown to prevent or delay the development of DPN or cardiac autonomic neuropathy in patients with type 1 diabetes,37,38 and to slow the progression of neuropathy in some patients with type 2 diabetes.39
The FDA has approved pregabalin, duloxetine, and tapentadol for the treatment of pain associated with DPN. Tricyclic antidepressants, gabapentin, venlafaxine, carbamazepine, tramadol, and topical capsaicin, although not approved for the treatment of painful DPN, may also be effective in treating neuropathic pain.
For those with autonomic neuropathy, dietary changes and prokinetic agents such as erythromycin may alleviate gastroparesis. Due to extrapyramidal adverse effects, metoclopramide is reserved for the most severe and unresponsive cases. Recurrent urinary tract infections, pyelonephritis, incontinence, or palpable bladder should prompt an evaluation for bladder dysfunction. Controlling lipids and BP, quitting smoking, and making other lifestyle changes can reduce both the development and the progression of autonomic neuropathy.
FOOT CARE/PERIPHERAL ARTERIAL DISEASE
What does the ADA recommend regarding foot care for patients with diabetes?
The ADA’s standards recommend an annual comprehensive foot examination to identify risk factors predictive of ulcers and potential amputations. The exam should start with inspection and assessment of foot pulses and should seek to identify loss of peripheral sensation. The examination should include inspection of the skin, assessment of foot deformities, neurologic assessment including 10-g monofilament testing and pinprick or vibration testing or assessment of ankle reflexes, and vascular assessment, including pulses in the legs and feet.40
It is also important to screen patients for peripheral arterial disease (PAD), with a comprehensive medical history and physical exam of pulses. Ankle-brachial index testing (ABI) should be performed in patients with signs or symptoms of PAD, including claudication or skin and hair changes in the lower extremities. ABI may be considered for all patients with diabetes starting at age 50 and in those younger than 50 years who have risk factors.41
Which patients with diabetes are at higher risk for foot complications?
The following are risk factors for foot complications: previous amputation, prior foot ulcer, peripheral neuropathy, foot deformity, peripheral vascular disease, visual impairment, peripheral neuropathy (especially if on dialysis), poor glycemic control, and smoking. Patients with high-risk foot conditions should be educated about their risk and appropriate management.
A well-fitted walking shoe that cushions the feet and redistributes pressure is one option to help patients. Patients with bony deformities may need extra wide or deep shoes and patients with more advanced disease may need custom-fitted shoes.
When should patients be referred to a foot specialist?
Refer patients to a foot care specialist for ongoing preventive care and lifelong surveillance if they smoke or have a history of lower-extremity complications, a loss of protective sensation, structural abnormalities, or PAD.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/site/misc/2016-Standards-of-Care.pdf. Accessed March 28, 2016.
2. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334.
3. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362.
4. Vijan S, Sussman JB, Yudkin JS, et al. Effect of patients’ risks and p on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–1234.
5. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
6. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737.
7. UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905–912.
8. Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415.
9. Pastors JG, Warshaw H, Daly A, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–613.
10. Selph S, Dana T, Bougatsos C, et al. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 US Preventive Services Task Force Recommendation. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed March 28, 2016.
11. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and metaanalysis. Obesity (Silver Spring). 2006;14:1283–1293.
12. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;99:14–23.
13. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149.
14. Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195.
15. Balaji V, Seshiah V, Ashtalakshmi G, et al. Retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–427.
16. Kuo HW, Tiao MM, Ho SC, et al. Pioglitazone use and the risk of bladder cancer. Kaohsiung J Med Sci. 2014;30:94–97.
17. Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254-259.
18. US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed December 11, 2015.
19. Huxley RR, Peters SAE, Mishra GD, et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206.
20. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551.
21. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973-1980.
22. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
23. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397.
24. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485.
25. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696.
26. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504.
27. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316.
28. Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080.
29. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713.
30. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an American Diabetes Association Consensus Conference. Diabetes Care. 2014;37:2864–2883.
31. The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720.
32. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
33. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
34. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
35. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430.
36. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559.
37. Chew EY, Ambrosius WT, Davis MD, et al; ACCORD Study Group; ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244.
38. Ang L, Jaiswal M, Martin C, et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14:528.
39. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group. Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:31–38.
40. Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765.
41. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341.
42. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Available at: http://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed April 8, 2016.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/site/misc/2016-Standards-of-Care.pdf. Accessed March 28, 2016.
2. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334.
3. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362.
4. Vijan S, Sussman JB, Yudkin JS, et al. Effect of patients’ risks and p on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–1234.
5. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
6. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737.
7. UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905–912.
8. Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415.
9. Pastors JG, Warshaw H, Daly A, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–613.
10. Selph S, Dana T, Bougatsos C, et al. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 US Preventive Services Task Force Recommendation. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed March 28, 2016.
11. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and metaanalysis. Obesity (Silver Spring). 2006;14:1283–1293.
12. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;99:14–23.
13. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149.
14. Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195.
15. Balaji V, Seshiah V, Ashtalakshmi G, et al. Retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–427.
16. Kuo HW, Tiao MM, Ho SC, et al. Pioglitazone use and the risk of bladder cancer. Kaohsiung J Med Sci. 2014;30:94–97.
17. Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254-259.
18. US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed December 11, 2015.
19. Huxley RR, Peters SAE, Mishra GD, et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206.
20. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551.
21. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973-1980.
22. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
23. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397.
24. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485.
25. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696.
26. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504.
27. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316.
28. Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080.
29. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713.
30. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an American Diabetes Association Consensus Conference. Diabetes Care. 2014;37:2864–2883.
31. The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720.
32. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
33. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
34. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
35. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430.
36. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559.
37. Chew EY, Ambrosius WT, Davis MD, et al; ACCORD Study Group; ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244.
38. Ang L, Jaiswal M, Martin C, et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14:528.
39. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group. Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:31–38.
40. Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765.
41. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341.
42. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Available at: http://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed April 8, 2016.