User login
› Intensify diabetes treatment for patients who have a normal fasting glucose, but an HbA1c >7% and daytime hyperglycemia, and for those who are not at goal despite basal insulin doses >0.5 units/kg/d. B
› Consider intensifying diabetes management beyond basal insulin therapy by adding a glucagon-like peptide 1 receptor agonist, insulin prior to one meal each day, or insulin prior to all meals. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Diabetes mellitus is a complex, progressive disease that affects every family physician’s practice. Major diabetes organizations recommend that treatment be ongoing and progressive in order to control the disease. The American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists recommend that patients be assessed every 2 to 3 months after diagnosis and that treatment should be intensified if the patient is not meeting treatment goals.1,2 Using this approach, all people with type 2 diabetes could be on insulin one year after diagnosis.1,2
While many family physicians have become comfortable with using once-daily basal insulin such as glargine or detemir, what to do after basal insulin is much more complex. This review builds upon an earlier article in this journal, “Insulin for type 2 diabetes: How and when to get started,”3 by explaining 3 strategies to consider when basal insulin alone isn't enough.
3 main strategies for intensifying treatment
Basal insulin is indicated for patients who have glucose toxicity and persistently elevated hemoglobin A1c (HbA1c) despite using 2 or more oral agents, or for those who have not achieved glucose goals one year into treatment.3,4 ADA/EASD recommends initiating a weight-based approach for basal insulin therapy based on initial HbA1c levels >7% or >8%.4 Instructing and encouraging patients to titrate their own insulin dose based on fasting glucose readings provides greater and faster glucose control.1,2
Despite these attempts, some patients will not reach their glucose goals with basal insulin. When intensifying treatment beyond basal insulin therapy, patient preference, cost-effectiveness, safety, tolerability, glycemic efficacy, risk of hypoglycemia, effects on cardiovascular risk factors, and other non-glycemic effects should be considered in the shared decision-making process. There are 3 main strategies for intensifying treatment:
1. Basal plus incretin therapy. Add a newer injectable agent such as a glucagon-like peptide 1 receptor agonist (GLP-1RA).
2. Basal plus one strategy. Add prandial insulin prior to the largest meal of the day.
3. Basal-bolus combination. Add insulin prior to all meals.
TABLE 15-8 provides details of several studies that have documented the efficacy of these 3 strategies.
CLICK IMAGE TO ENLARGE
Monitoring blood glucose to guide the way
Blood glucose monitoring using either a 7-point glucose monitoring technique or staggered glucose checks should guide insulin intensification. A 7-point glucose profile includes pre-meal and post-meal readings for 3 meals a day and an additional bedtime reading.9 This is typically performed for 3 to 7 days prior to an appointment and provides an estimate of a typical full day’s glucose pattern.
Staggered monitoring includes a pair of glucose checks taken immediately before and typically 90 minutes after a meal. This is assigned to a different meal each day in order to obtain the same information as is achieved with 7-point monitoring, but with fewer checks on any given day. It may take up to 2 to 3 weeks to gather the necessary information using the staggered monitoring technique.
In order to optimize insulin strategies for tighter glycemic control, it is important to review blood glucose logs at each office visit with either of the above techniques.
Basal plus incretin therapy
GLP-1RAs are subcutaneously administered injectable incretin agents. They mimic the action of endogenous GLP-1 hormones, which are normally secreted in response to meals by the cells of the small intestine.10 GLP-1 stimulates glucose-dependent insulin secretion, suppresses postprandial glucagon release from pancreatic alpha cells, signals satiety, and slows gastric emptying.10 In other words, GLP-1 appears to be a physiologic regulator of appetite and food intake. GLP-1 is rapidly metabolized and inactivated by dipeptidyl peptidase-4 (DPP-4) enzymes.10 The amplification of insulin secretion elicited by hormones secreted from the gastrointestinal (GI) tract is called the “incretin effect.”10 Obesity, insulin resistance, and type 2 diabetes greatly reduce the incretin effect.10
GLP-1RAs mimic the incretin effect and are not degraded by endogenous DPP-4 enzymes.10 They provide a pharmacologic level of GLP-1 activity, including beneficial glucose effects (via insulin secretion and glucagon suppression), but they also increase GI adverse effects, such as nausea and vomiting.11-15 Further, they can suppress appetite and contribute to weight loss.11-15
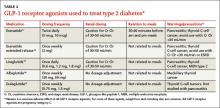
GLP-1RAs can be considered as an add-on therapy for patients whose HbA1c exceeds 7% and whose fasting blood glucose ranges from 80 to 130 mg/dL, or for patients with a basal insulin dose >0.5 unit/kg/d. The 5 currently available GLP-1RAs (exenatide, exenatide extended-release, liraglutide, albiglutide, and dulaglutide) are compared in TABLE 2.11-15
Dosing varies with each agent and includes twice daily before meals for exenatide, once daily (independent of meals) for liraglutide, and once weekly for exenatide extended-release, albiglutide, and dulaglutide. These agents should not be used for patients with a history of pancreatitis or a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia type 2. Because exenatide is cleared through the kidneys, its use is contraindicated in patients with a creatinine clearance <30 mL/min or end-stage renal disease. Caution is advised for its use in patients with a creatinine clearance of 30 to 50 mL/min.11
Basal plus one strategy
To best utilize prandial insulin, it is important to know what the patient’s glucose readings are before and after meals as assessed by the 7-point or staggered blood glucose monitoring techniques described earlier. Once you have clarified which meal(s) are raising the patient’s glucose levels, selecting appropriate treatment becomes easier. To reduce the glucose-monitoring burden for the patient, it may be acceptable to allow the patient to omit the fasting glucose measurement (if stable).
The first major decision is whether to treat one meal per day (basal plus one) or all meals (basal-bolus). Adding a rapid-acting insulin prior to one meal a day (usually the largest meal) is a reasonable starting point.16
The meal that produces the highest postprandial glucose readings can be considered the meal of greatest glycemic impact. The “delta” value—the difference between pre-meal glucose and 2-hour postprandial glucose readings—also helps to determine the largest meal of the day.17 The average physiologic delta is ≤50 mg/dL.17 If the delta for a meal is >75 mg/dL, consider initiating prandial insulin prior to that meal and titrating the dose to achieve a target glucose level of <130 mg/dL before the next meal.
Using 4 to 6 units of a rapid-acting insulin per meal is a good initial regimen for a basal plus one (as well as for a basal-bolus) approach.16 If the patient experiences significantly increased insulin demands as indicated by glucose patterns where the post-meal glucose is still consistently above 180 mg/dL, the initial regimen may be modified to 0.1 unit per kg per meal,17-19 and then titrated up to a maximum of 50% of the total daily insulin dose (TDD) for basal plus one16 (or 10%-20% of TDD per meal for basal-bolus).
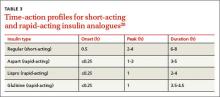
Consider the timing of administration. Rapid-acting insulin analogs exhibit peak pharmacodynamic activity 60 minutes after injection (TABLE 3).20
Peak carbohydrate absorption following a meal occurs approximately 75 to 90 minutes after eating begins.17,21 Thus, to synchronize the action of insulin with carbohydrate digestion, the analog should be injected 15 minutes before meals. This can be increased by titrating prandial insulin by 1 unit/d to a goal of either a 90-minute to 2-hour postprandial glucose of <140 to 180 mg/dL or the next preprandial glucose of <130 mg/dL.16 The goal is to obtain a near-normal physiologic delta of <50 mg/dL. The drop in delta noted with every unit of insulin added to the current dose can provide a rough approximation of how many additional insulin titrations will be needed to achieve a delta of <50 mg/dL.
Basal-bolus combination
A gradual increase from one injection before a single meal each day to as-needed multiple daily injections (MDIs) is the next step in hyperglycemia management. Starting slow and building up to insulin therapy prior to each meal offers structure, simplicity, and physician-patient confidence in diabetes management. The slow progression from basal plus one to basal-bolus combination allows the patient ease into a complex, labor-intensive regimen of MDIs. Additionally, the stepwise reduction of postprandial hyperglycemia with this slow approach often reduces the incidence of hypoglycemia (more on this in a moment).8
Advanced insulin users can calculate an “insulin-to-carbohydrate ratio” (ICR) to estimate the amount of insulin they need to accommodate the amount of carbohydrates they ingest per meal. An ICR of 1:10 implies that the patient administers 1 unit of insulin for every 10 grams of carbohydrates ingested. For example, if a patient with an ICR of 1:10 concludes that his meal contains a total of 60 grams of carbohydrates, then he would administer 6 units of insulin prior to this meal to address the anticipated post-meal hyperglycemia.
In order to use the ICR regimen, a patient would need to be able to accurately determine the nutritional content of his meals (starch, protein, carbohydrates, and fat) and calculate the appropriate insulin dosage. For successful diabetes management, it is essential to evaluate the patient’s skills in these areas before starting an ICR regimen, and to routinely assess hypoglycemic episodes at follow-up visits.
An ICR approach is usually reserved for patients who require tighter glucose control than that obtained from fixed prandial insulin doses, such as patients with type 1 diabetes, those with variable meal schedules and content, those with a malabsorption syndrome that requires consuming meals with a specific amount of carbohydrates, athletes on a structured diet with specific carbohydrate content, and patients who want flexibility with carbohydrate intake with meals.
The risk of hypoglycemia is a major barrier to initiating basal-bolus insulin therapy. Hypoglycemia is classified as a blood glucose level of <70 mg/dL, and severe hypoglycemia as <50 mg/dL, regardless of whether the patient develops symptoms.22 Symptoms of hypoglycemia include dizziness, difficulty speaking, anxiety, confusion, and lethargy. Hypoglycemia can result in loss of consciousness or even death.22
A patient who has frequent hypoglycemic episodes may lose the protective physiologic response and may not recognize that he is experiencing a hypoglycemic episode (“hypoglycemia unawareness”). This is why it is crucial to ask patients if they have had symptoms of hypoglycemia, and to correlate the timing of these symptoms with blood glucose logs. For example, it is possible for a patient to experience hypoglycemic symptoms for blood glucose readings in the 100 to 200 mg/dL range if his or her average blood glucose has been in the 250 to 300 mg/dL range. Such patient may not realize he is experiencing hypoglycemia until he develops severe symptoms, such as loss of consciousness.
Hypoglycemia unawareness must be addressed immediately by reducing insulin dosing to prevent all hypoglycemic episodes for 2 to 3 weeks. This has been shown to “reset” the normal physiologic response to hypoglycemia, regardless of how long the patient has had diabetes.23,24 Even if your patient is aware of the warning signs of a hypoglycemic episode, it is important to routinely ask about hypoglycemia at all diabetes visits because patients may reduce insulin doses, skip doses, or eat defensively to prevent hypoglycemia.
Other than the risk of hypoglycemia, insulin typically has fewer adverse effects than oral medications used to treat diabetes. Most common concerns include weight gain, hypoglycemia, injection site reactions and, rarely, allergy to insulin or its vehicle.16
CORRESPONDENCE
Jay Shubrook, DO, FAAF P, FACOF P, BC-ADM, Touro University College of Osteopathic Medicine, 1310 Club Drive, Vallejo, CA 94592; jay.shubrook@tu.edu
1. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE Comprehensive Diabetes Management Algorithm 2013. Endocr Pract. 2013;19:327-336.
2. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient centered approach. A position statement of the ADA and the EASD. Diabetes Care. 2012;35:1364-1379.
3. Shubrook, J. Insulin for type 2 diabetes: How and when to get started. J Fam Pract. 2014; 63:76-81.
4. Nathan D, Buse J, Davidson M, et al; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
5. Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice daily prandial insulin lispro. Diabetes Care. 2014;37:2317-2325.
6. Owens DR, Luzio SD, Sert-Langeron C, et al. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab. 2011;13:1020-1027.
7. Lankisch MR, Ferlinz KC, Leahy JL, et al; Orals Plus Apidra and LANTUS (OPAL) study group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two singledose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab. 2008;10:1178-1185.
8. Davidson MB, Raskin P, Tanenberg RJ, et al. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract. 2011;17:395-403.
9. Owens DR. Stepwise intensification of insulin therapy in type 2 diabetes management--exploring the concept of basal-plus approach in clinical practice. Diabet Med. 2013;30:276-288.
10. Holst J. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409-1439.
11. Byetta [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2015.
12. Bydureon [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2014.
13. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2015.
14. Tanzeum [package insert]. Wilmington, DE: GlaxoSmithKline; 2014.
15. Trulicity [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
16. Vaidya A, McMahon GT. Initiating insulin for type 2 diabetes: Strategies for success. J Clin Outcomes Manag. 2009;16:127-136.
17. Unger J. Insulin initiation and intensification in patients with T2DM for the primary care physician. Diabetes Metab Syndr Obes. 2011;4:253-261.
18. Sharma MD, Garber AJ. Progression from basal to pre-mixed or rapid-acting insulin – Options for intensification and the use of pumps. US Endocrinology. 2009;5:40-44.
19. Mooradian AD, Bernbaum M, Albert SG. Narrative review: A rational approach to starting insulin therapy. Ann Intern Med. 2006;145:125-134.
20. Monthly Prescribing Reference (MPR). Insulin. Monthly Prescribing Reference Web site. Available at: http://www.empr.com/insulins/article/123739/. Accessed January 10, 2014.
21. Guyton AC, Hall JE. Insulin, glucagon, and diabetes mellitus. In: Guyton AC, Hall JE, eds. Textbook of Medical Physiology. 11th ed. Philadelphia, PA: Elsevier Saunders; 2006:961-977.
22. Kitabchi AE, Gosmanov AR. Safety of rapid-acting insulin analogs versus regular human insulin. Am J Med Sci. 2012;344:136-141.
23. Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Eng J Med. 2004;350:2272-2279.
24. Gehlaut RR, Shubrook JH. Revisiting hypoglycemia in diabetes. Osteopathic Family Physician. 2014;1:19-25.
› Intensify diabetes treatment for patients who have a normal fasting glucose, but an HbA1c >7% and daytime hyperglycemia, and for those who are not at goal despite basal insulin doses >0.5 units/kg/d. B
› Consider intensifying diabetes management beyond basal insulin therapy by adding a glucagon-like peptide 1 receptor agonist, insulin prior to one meal each day, or insulin prior to all meals. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Diabetes mellitus is a complex, progressive disease that affects every family physician’s practice. Major diabetes organizations recommend that treatment be ongoing and progressive in order to control the disease. The American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists recommend that patients be assessed every 2 to 3 months after diagnosis and that treatment should be intensified if the patient is not meeting treatment goals.1,2 Using this approach, all people with type 2 diabetes could be on insulin one year after diagnosis.1,2
While many family physicians have become comfortable with using once-daily basal insulin such as glargine or detemir, what to do after basal insulin is much more complex. This review builds upon an earlier article in this journal, “Insulin for type 2 diabetes: How and when to get started,”3 by explaining 3 strategies to consider when basal insulin alone isn't enough.
3 main strategies for intensifying treatment
Basal insulin is indicated for patients who have glucose toxicity and persistently elevated hemoglobin A1c (HbA1c) despite using 2 or more oral agents, or for those who have not achieved glucose goals one year into treatment.3,4 ADA/EASD recommends initiating a weight-based approach for basal insulin therapy based on initial HbA1c levels >7% or >8%.4 Instructing and encouraging patients to titrate their own insulin dose based on fasting glucose readings provides greater and faster glucose control.1,2
Despite these attempts, some patients will not reach their glucose goals with basal insulin. When intensifying treatment beyond basal insulin therapy, patient preference, cost-effectiveness, safety, tolerability, glycemic efficacy, risk of hypoglycemia, effects on cardiovascular risk factors, and other non-glycemic effects should be considered in the shared decision-making process. There are 3 main strategies for intensifying treatment:
1. Basal plus incretin therapy. Add a newer injectable agent such as a glucagon-like peptide 1 receptor agonist (GLP-1RA).
2. Basal plus one strategy. Add prandial insulin prior to the largest meal of the day.
3. Basal-bolus combination. Add insulin prior to all meals.
TABLE 15-8 provides details of several studies that have documented the efficacy of these 3 strategies.
CLICK IMAGE TO ENLARGE
Monitoring blood glucose to guide the way
Blood glucose monitoring using either a 7-point glucose monitoring technique or staggered glucose checks should guide insulin intensification. A 7-point glucose profile includes pre-meal and post-meal readings for 3 meals a day and an additional bedtime reading.9 This is typically performed for 3 to 7 days prior to an appointment and provides an estimate of a typical full day’s glucose pattern.
Staggered monitoring includes a pair of glucose checks taken immediately before and typically 90 minutes after a meal. This is assigned to a different meal each day in order to obtain the same information as is achieved with 7-point monitoring, but with fewer checks on any given day. It may take up to 2 to 3 weeks to gather the necessary information using the staggered monitoring technique.
In order to optimize insulin strategies for tighter glycemic control, it is important to review blood glucose logs at each office visit with either of the above techniques.
Basal plus incretin therapy
GLP-1RAs are subcutaneously administered injectable incretin agents. They mimic the action of endogenous GLP-1 hormones, which are normally secreted in response to meals by the cells of the small intestine.10 GLP-1 stimulates glucose-dependent insulin secretion, suppresses postprandial glucagon release from pancreatic alpha cells, signals satiety, and slows gastric emptying.10 In other words, GLP-1 appears to be a physiologic regulator of appetite and food intake. GLP-1 is rapidly metabolized and inactivated by dipeptidyl peptidase-4 (DPP-4) enzymes.10 The amplification of insulin secretion elicited by hormones secreted from the gastrointestinal (GI) tract is called the “incretin effect.”10 Obesity, insulin resistance, and type 2 diabetes greatly reduce the incretin effect.10
GLP-1RAs mimic the incretin effect and are not degraded by endogenous DPP-4 enzymes.10 They provide a pharmacologic level of GLP-1 activity, including beneficial glucose effects (via insulin secretion and glucagon suppression), but they also increase GI adverse effects, such as nausea and vomiting.11-15 Further, they can suppress appetite and contribute to weight loss.11-15
GLP-1RAs can be considered as an add-on therapy for patients whose HbA1c exceeds 7% and whose fasting blood glucose ranges from 80 to 130 mg/dL, or for patients with a basal insulin dose >0.5 unit/kg/d. The 5 currently available GLP-1RAs (exenatide, exenatide extended-release, liraglutide, albiglutide, and dulaglutide) are compared in TABLE 2.11-15
Dosing varies with each agent and includes twice daily before meals for exenatide, once daily (independent of meals) for liraglutide, and once weekly for exenatide extended-release, albiglutide, and dulaglutide. These agents should not be used for patients with a history of pancreatitis or a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia type 2. Because exenatide is cleared through the kidneys, its use is contraindicated in patients with a creatinine clearance <30 mL/min or end-stage renal disease. Caution is advised for its use in patients with a creatinine clearance of 30 to 50 mL/min.11
Basal plus one strategy
To best utilize prandial insulin, it is important to know what the patient’s glucose readings are before and after meals as assessed by the 7-point or staggered blood glucose monitoring techniques described earlier. Once you have clarified which meal(s) are raising the patient’s glucose levels, selecting appropriate treatment becomes easier. To reduce the glucose-monitoring burden for the patient, it may be acceptable to allow the patient to omit the fasting glucose measurement (if stable).
The first major decision is whether to treat one meal per day (basal plus one) or all meals (basal-bolus). Adding a rapid-acting insulin prior to one meal a day (usually the largest meal) is a reasonable starting point.16
The meal that produces the highest postprandial glucose readings can be considered the meal of greatest glycemic impact. The “delta” value—the difference between pre-meal glucose and 2-hour postprandial glucose readings—also helps to determine the largest meal of the day.17 The average physiologic delta is ≤50 mg/dL.17 If the delta for a meal is >75 mg/dL, consider initiating prandial insulin prior to that meal and titrating the dose to achieve a target glucose level of <130 mg/dL before the next meal.
Using 4 to 6 units of a rapid-acting insulin per meal is a good initial regimen for a basal plus one (as well as for a basal-bolus) approach.16 If the patient experiences significantly increased insulin demands as indicated by glucose patterns where the post-meal glucose is still consistently above 180 mg/dL, the initial regimen may be modified to 0.1 unit per kg per meal,17-19 and then titrated up to a maximum of 50% of the total daily insulin dose (TDD) for basal plus one16 (or 10%-20% of TDD per meal for basal-bolus).
Consider the timing of administration. Rapid-acting insulin analogs exhibit peak pharmacodynamic activity 60 minutes after injection (TABLE 3).20
Peak carbohydrate absorption following a meal occurs approximately 75 to 90 minutes after eating begins.17,21 Thus, to synchronize the action of insulin with carbohydrate digestion, the analog should be injected 15 minutes before meals. This can be increased by titrating prandial insulin by 1 unit/d to a goal of either a 90-minute to 2-hour postprandial glucose of <140 to 180 mg/dL or the next preprandial glucose of <130 mg/dL.16 The goal is to obtain a near-normal physiologic delta of <50 mg/dL. The drop in delta noted with every unit of insulin added to the current dose can provide a rough approximation of how many additional insulin titrations will be needed to achieve a delta of <50 mg/dL.
Basal-bolus combination
A gradual increase from one injection before a single meal each day to as-needed multiple daily injections (MDIs) is the next step in hyperglycemia management. Starting slow and building up to insulin therapy prior to each meal offers structure, simplicity, and physician-patient confidence in diabetes management. The slow progression from basal plus one to basal-bolus combination allows the patient ease into a complex, labor-intensive regimen of MDIs. Additionally, the stepwise reduction of postprandial hyperglycemia with this slow approach often reduces the incidence of hypoglycemia (more on this in a moment).8
Advanced insulin users can calculate an “insulin-to-carbohydrate ratio” (ICR) to estimate the amount of insulin they need to accommodate the amount of carbohydrates they ingest per meal. An ICR of 1:10 implies that the patient administers 1 unit of insulin for every 10 grams of carbohydrates ingested. For example, if a patient with an ICR of 1:10 concludes that his meal contains a total of 60 grams of carbohydrates, then he would administer 6 units of insulin prior to this meal to address the anticipated post-meal hyperglycemia.
In order to use the ICR regimen, a patient would need to be able to accurately determine the nutritional content of his meals (starch, protein, carbohydrates, and fat) and calculate the appropriate insulin dosage. For successful diabetes management, it is essential to evaluate the patient’s skills in these areas before starting an ICR regimen, and to routinely assess hypoglycemic episodes at follow-up visits.
An ICR approach is usually reserved for patients who require tighter glucose control than that obtained from fixed prandial insulin doses, such as patients with type 1 diabetes, those with variable meal schedules and content, those with a malabsorption syndrome that requires consuming meals with a specific amount of carbohydrates, athletes on a structured diet with specific carbohydrate content, and patients who want flexibility with carbohydrate intake with meals.
The risk of hypoglycemia is a major barrier to initiating basal-bolus insulin therapy. Hypoglycemia is classified as a blood glucose level of <70 mg/dL, and severe hypoglycemia as <50 mg/dL, regardless of whether the patient develops symptoms.22 Symptoms of hypoglycemia include dizziness, difficulty speaking, anxiety, confusion, and lethargy. Hypoglycemia can result in loss of consciousness or even death.22
A patient who has frequent hypoglycemic episodes may lose the protective physiologic response and may not recognize that he is experiencing a hypoglycemic episode (“hypoglycemia unawareness”). This is why it is crucial to ask patients if they have had symptoms of hypoglycemia, and to correlate the timing of these symptoms with blood glucose logs. For example, it is possible for a patient to experience hypoglycemic symptoms for blood glucose readings in the 100 to 200 mg/dL range if his or her average blood glucose has been in the 250 to 300 mg/dL range. Such patient may not realize he is experiencing hypoglycemia until he develops severe symptoms, such as loss of consciousness.
Hypoglycemia unawareness must be addressed immediately by reducing insulin dosing to prevent all hypoglycemic episodes for 2 to 3 weeks. This has been shown to “reset” the normal physiologic response to hypoglycemia, regardless of how long the patient has had diabetes.23,24 Even if your patient is aware of the warning signs of a hypoglycemic episode, it is important to routinely ask about hypoglycemia at all diabetes visits because patients may reduce insulin doses, skip doses, or eat defensively to prevent hypoglycemia.
Other than the risk of hypoglycemia, insulin typically has fewer adverse effects than oral medications used to treat diabetes. Most common concerns include weight gain, hypoglycemia, injection site reactions and, rarely, allergy to insulin or its vehicle.16
CORRESPONDENCE
Jay Shubrook, DO, FAAF P, FACOF P, BC-ADM, Touro University College of Osteopathic Medicine, 1310 Club Drive, Vallejo, CA 94592; jay.shubrook@tu.edu
› Intensify diabetes treatment for patients who have a normal fasting glucose, but an HbA1c >7% and daytime hyperglycemia, and for those who are not at goal despite basal insulin doses >0.5 units/kg/d. B
› Consider intensifying diabetes management beyond basal insulin therapy by adding a glucagon-like peptide 1 receptor agonist, insulin prior to one meal each day, or insulin prior to all meals. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Diabetes mellitus is a complex, progressive disease that affects every family physician’s practice. Major diabetes organizations recommend that treatment be ongoing and progressive in order to control the disease. The American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists recommend that patients be assessed every 2 to 3 months after diagnosis and that treatment should be intensified if the patient is not meeting treatment goals.1,2 Using this approach, all people with type 2 diabetes could be on insulin one year after diagnosis.1,2
While many family physicians have become comfortable with using once-daily basal insulin such as glargine or detemir, what to do after basal insulin is much more complex. This review builds upon an earlier article in this journal, “Insulin for type 2 diabetes: How and when to get started,”3 by explaining 3 strategies to consider when basal insulin alone isn't enough.
3 main strategies for intensifying treatment
Basal insulin is indicated for patients who have glucose toxicity and persistently elevated hemoglobin A1c (HbA1c) despite using 2 or more oral agents, or for those who have not achieved glucose goals one year into treatment.3,4 ADA/EASD recommends initiating a weight-based approach for basal insulin therapy based on initial HbA1c levels >7% or >8%.4 Instructing and encouraging patients to titrate their own insulin dose based on fasting glucose readings provides greater and faster glucose control.1,2
Despite these attempts, some patients will not reach their glucose goals with basal insulin. When intensifying treatment beyond basal insulin therapy, patient preference, cost-effectiveness, safety, tolerability, glycemic efficacy, risk of hypoglycemia, effects on cardiovascular risk factors, and other non-glycemic effects should be considered in the shared decision-making process. There are 3 main strategies for intensifying treatment:
1. Basal plus incretin therapy. Add a newer injectable agent such as a glucagon-like peptide 1 receptor agonist (GLP-1RA).
2. Basal plus one strategy. Add prandial insulin prior to the largest meal of the day.
3. Basal-bolus combination. Add insulin prior to all meals.
TABLE 15-8 provides details of several studies that have documented the efficacy of these 3 strategies.
CLICK IMAGE TO ENLARGE
Monitoring blood glucose to guide the way
Blood glucose monitoring using either a 7-point glucose monitoring technique or staggered glucose checks should guide insulin intensification. A 7-point glucose profile includes pre-meal and post-meal readings for 3 meals a day and an additional bedtime reading.9 This is typically performed for 3 to 7 days prior to an appointment and provides an estimate of a typical full day’s glucose pattern.
Staggered monitoring includes a pair of glucose checks taken immediately before and typically 90 minutes after a meal. This is assigned to a different meal each day in order to obtain the same information as is achieved with 7-point monitoring, but with fewer checks on any given day. It may take up to 2 to 3 weeks to gather the necessary information using the staggered monitoring technique.
In order to optimize insulin strategies for tighter glycemic control, it is important to review blood glucose logs at each office visit with either of the above techniques.
Basal plus incretin therapy
GLP-1RAs are subcutaneously administered injectable incretin agents. They mimic the action of endogenous GLP-1 hormones, which are normally secreted in response to meals by the cells of the small intestine.10 GLP-1 stimulates glucose-dependent insulin secretion, suppresses postprandial glucagon release from pancreatic alpha cells, signals satiety, and slows gastric emptying.10 In other words, GLP-1 appears to be a physiologic regulator of appetite and food intake. GLP-1 is rapidly metabolized and inactivated by dipeptidyl peptidase-4 (DPP-4) enzymes.10 The amplification of insulin secretion elicited by hormones secreted from the gastrointestinal (GI) tract is called the “incretin effect.”10 Obesity, insulin resistance, and type 2 diabetes greatly reduce the incretin effect.10
GLP-1RAs mimic the incretin effect and are not degraded by endogenous DPP-4 enzymes.10 They provide a pharmacologic level of GLP-1 activity, including beneficial glucose effects (via insulin secretion and glucagon suppression), but they also increase GI adverse effects, such as nausea and vomiting.11-15 Further, they can suppress appetite and contribute to weight loss.11-15
GLP-1RAs can be considered as an add-on therapy for patients whose HbA1c exceeds 7% and whose fasting blood glucose ranges from 80 to 130 mg/dL, or for patients with a basal insulin dose >0.5 unit/kg/d. The 5 currently available GLP-1RAs (exenatide, exenatide extended-release, liraglutide, albiglutide, and dulaglutide) are compared in TABLE 2.11-15
Dosing varies with each agent and includes twice daily before meals for exenatide, once daily (independent of meals) for liraglutide, and once weekly for exenatide extended-release, albiglutide, and dulaglutide. These agents should not be used for patients with a history of pancreatitis or a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia type 2. Because exenatide is cleared through the kidneys, its use is contraindicated in patients with a creatinine clearance <30 mL/min or end-stage renal disease. Caution is advised for its use in patients with a creatinine clearance of 30 to 50 mL/min.11
Basal plus one strategy
To best utilize prandial insulin, it is important to know what the patient’s glucose readings are before and after meals as assessed by the 7-point or staggered blood glucose monitoring techniques described earlier. Once you have clarified which meal(s) are raising the patient’s glucose levels, selecting appropriate treatment becomes easier. To reduce the glucose-monitoring burden for the patient, it may be acceptable to allow the patient to omit the fasting glucose measurement (if stable).
The first major decision is whether to treat one meal per day (basal plus one) or all meals (basal-bolus). Adding a rapid-acting insulin prior to one meal a day (usually the largest meal) is a reasonable starting point.16
The meal that produces the highest postprandial glucose readings can be considered the meal of greatest glycemic impact. The “delta” value—the difference between pre-meal glucose and 2-hour postprandial glucose readings—also helps to determine the largest meal of the day.17 The average physiologic delta is ≤50 mg/dL.17 If the delta for a meal is >75 mg/dL, consider initiating prandial insulin prior to that meal and titrating the dose to achieve a target glucose level of <130 mg/dL before the next meal.
Using 4 to 6 units of a rapid-acting insulin per meal is a good initial regimen for a basal plus one (as well as for a basal-bolus) approach.16 If the patient experiences significantly increased insulin demands as indicated by glucose patterns where the post-meal glucose is still consistently above 180 mg/dL, the initial regimen may be modified to 0.1 unit per kg per meal,17-19 and then titrated up to a maximum of 50% of the total daily insulin dose (TDD) for basal plus one16 (or 10%-20% of TDD per meal for basal-bolus).
Consider the timing of administration. Rapid-acting insulin analogs exhibit peak pharmacodynamic activity 60 minutes after injection (TABLE 3).20
Peak carbohydrate absorption following a meal occurs approximately 75 to 90 minutes after eating begins.17,21 Thus, to synchronize the action of insulin with carbohydrate digestion, the analog should be injected 15 minutes before meals. This can be increased by titrating prandial insulin by 1 unit/d to a goal of either a 90-minute to 2-hour postprandial glucose of <140 to 180 mg/dL or the next preprandial glucose of <130 mg/dL.16 The goal is to obtain a near-normal physiologic delta of <50 mg/dL. The drop in delta noted with every unit of insulin added to the current dose can provide a rough approximation of how many additional insulin titrations will be needed to achieve a delta of <50 mg/dL.
Basal-bolus combination
A gradual increase from one injection before a single meal each day to as-needed multiple daily injections (MDIs) is the next step in hyperglycemia management. Starting slow and building up to insulin therapy prior to each meal offers structure, simplicity, and physician-patient confidence in diabetes management. The slow progression from basal plus one to basal-bolus combination allows the patient ease into a complex, labor-intensive regimen of MDIs. Additionally, the stepwise reduction of postprandial hyperglycemia with this slow approach often reduces the incidence of hypoglycemia (more on this in a moment).8
Advanced insulin users can calculate an “insulin-to-carbohydrate ratio” (ICR) to estimate the amount of insulin they need to accommodate the amount of carbohydrates they ingest per meal. An ICR of 1:10 implies that the patient administers 1 unit of insulin for every 10 grams of carbohydrates ingested. For example, if a patient with an ICR of 1:10 concludes that his meal contains a total of 60 grams of carbohydrates, then he would administer 6 units of insulin prior to this meal to address the anticipated post-meal hyperglycemia.
In order to use the ICR regimen, a patient would need to be able to accurately determine the nutritional content of his meals (starch, protein, carbohydrates, and fat) and calculate the appropriate insulin dosage. For successful diabetes management, it is essential to evaluate the patient’s skills in these areas before starting an ICR regimen, and to routinely assess hypoglycemic episodes at follow-up visits.
An ICR approach is usually reserved for patients who require tighter glucose control than that obtained from fixed prandial insulin doses, such as patients with type 1 diabetes, those with variable meal schedules and content, those with a malabsorption syndrome that requires consuming meals with a specific amount of carbohydrates, athletes on a structured diet with specific carbohydrate content, and patients who want flexibility with carbohydrate intake with meals.
The risk of hypoglycemia is a major barrier to initiating basal-bolus insulin therapy. Hypoglycemia is classified as a blood glucose level of <70 mg/dL, and severe hypoglycemia as <50 mg/dL, regardless of whether the patient develops symptoms.22 Symptoms of hypoglycemia include dizziness, difficulty speaking, anxiety, confusion, and lethargy. Hypoglycemia can result in loss of consciousness or even death.22
A patient who has frequent hypoglycemic episodes may lose the protective physiologic response and may not recognize that he is experiencing a hypoglycemic episode (“hypoglycemia unawareness”). This is why it is crucial to ask patients if they have had symptoms of hypoglycemia, and to correlate the timing of these symptoms with blood glucose logs. For example, it is possible for a patient to experience hypoglycemic symptoms for blood glucose readings in the 100 to 200 mg/dL range if his or her average blood glucose has been in the 250 to 300 mg/dL range. Such patient may not realize he is experiencing hypoglycemia until he develops severe symptoms, such as loss of consciousness.
Hypoglycemia unawareness must be addressed immediately by reducing insulin dosing to prevent all hypoglycemic episodes for 2 to 3 weeks. This has been shown to “reset” the normal physiologic response to hypoglycemia, regardless of how long the patient has had diabetes.23,24 Even if your patient is aware of the warning signs of a hypoglycemic episode, it is important to routinely ask about hypoglycemia at all diabetes visits because patients may reduce insulin doses, skip doses, or eat defensively to prevent hypoglycemia.
Other than the risk of hypoglycemia, insulin typically has fewer adverse effects than oral medications used to treat diabetes. Most common concerns include weight gain, hypoglycemia, injection site reactions and, rarely, allergy to insulin or its vehicle.16
CORRESPONDENCE
Jay Shubrook, DO, FAAF P, FACOF P, BC-ADM, Touro University College of Osteopathic Medicine, 1310 Club Drive, Vallejo, CA 94592; jay.shubrook@tu.edu
1. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE Comprehensive Diabetes Management Algorithm 2013. Endocr Pract. 2013;19:327-336.
2. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient centered approach. A position statement of the ADA and the EASD. Diabetes Care. 2012;35:1364-1379.
3. Shubrook, J. Insulin for type 2 diabetes: How and when to get started. J Fam Pract. 2014; 63:76-81.
4. Nathan D, Buse J, Davidson M, et al; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
5. Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice daily prandial insulin lispro. Diabetes Care. 2014;37:2317-2325.
6. Owens DR, Luzio SD, Sert-Langeron C, et al. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab. 2011;13:1020-1027.
7. Lankisch MR, Ferlinz KC, Leahy JL, et al; Orals Plus Apidra and LANTUS (OPAL) study group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two singledose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab. 2008;10:1178-1185.
8. Davidson MB, Raskin P, Tanenberg RJ, et al. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract. 2011;17:395-403.
9. Owens DR. Stepwise intensification of insulin therapy in type 2 diabetes management--exploring the concept of basal-plus approach in clinical practice. Diabet Med. 2013;30:276-288.
10. Holst J. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409-1439.
11. Byetta [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2015.
12. Bydureon [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2014.
13. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2015.
14. Tanzeum [package insert]. Wilmington, DE: GlaxoSmithKline; 2014.
15. Trulicity [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
16. Vaidya A, McMahon GT. Initiating insulin for type 2 diabetes: Strategies for success. J Clin Outcomes Manag. 2009;16:127-136.
17. Unger J. Insulin initiation and intensification in patients with T2DM for the primary care physician. Diabetes Metab Syndr Obes. 2011;4:253-261.
18. Sharma MD, Garber AJ. Progression from basal to pre-mixed or rapid-acting insulin – Options for intensification and the use of pumps. US Endocrinology. 2009;5:40-44.
19. Mooradian AD, Bernbaum M, Albert SG. Narrative review: A rational approach to starting insulin therapy. Ann Intern Med. 2006;145:125-134.
20. Monthly Prescribing Reference (MPR). Insulin. Monthly Prescribing Reference Web site. Available at: http://www.empr.com/insulins/article/123739/. Accessed January 10, 2014.
21. Guyton AC, Hall JE. Insulin, glucagon, and diabetes mellitus. In: Guyton AC, Hall JE, eds. Textbook of Medical Physiology. 11th ed. Philadelphia, PA: Elsevier Saunders; 2006:961-977.
22. Kitabchi AE, Gosmanov AR. Safety of rapid-acting insulin analogs versus regular human insulin. Am J Med Sci. 2012;344:136-141.
23. Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Eng J Med. 2004;350:2272-2279.
24. Gehlaut RR, Shubrook JH. Revisiting hypoglycemia in diabetes. Osteopathic Family Physician. 2014;1:19-25.
1. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE Comprehensive Diabetes Management Algorithm 2013. Endocr Pract. 2013;19:327-336.
2. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient centered approach. A position statement of the ADA and the EASD. Diabetes Care. 2012;35:1364-1379.
3. Shubrook, J. Insulin for type 2 diabetes: How and when to get started. J Fam Pract. 2014; 63:76-81.
4. Nathan D, Buse J, Davidson M, et al; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
5. Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice daily prandial insulin lispro. Diabetes Care. 2014;37:2317-2325.
6. Owens DR, Luzio SD, Sert-Langeron C, et al. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab. 2011;13:1020-1027.
7. Lankisch MR, Ferlinz KC, Leahy JL, et al; Orals Plus Apidra and LANTUS (OPAL) study group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two singledose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab. 2008;10:1178-1185.
8. Davidson MB, Raskin P, Tanenberg RJ, et al. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract. 2011;17:395-403.
9. Owens DR. Stepwise intensification of insulin therapy in type 2 diabetes management--exploring the concept of basal-plus approach in clinical practice. Diabet Med. 2013;30:276-288.
10. Holst J. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409-1439.
11. Byetta [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2015.
12. Bydureon [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2014.
13. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2015.
14. Tanzeum [package insert]. Wilmington, DE: GlaxoSmithKline; 2014.
15. Trulicity [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
16. Vaidya A, McMahon GT. Initiating insulin for type 2 diabetes: Strategies for success. J Clin Outcomes Manag. 2009;16:127-136.
17. Unger J. Insulin initiation and intensification in patients with T2DM for the primary care physician. Diabetes Metab Syndr Obes. 2011;4:253-261.
18. Sharma MD, Garber AJ. Progression from basal to pre-mixed or rapid-acting insulin – Options for intensification and the use of pumps. US Endocrinology. 2009;5:40-44.
19. Mooradian AD, Bernbaum M, Albert SG. Narrative review: A rational approach to starting insulin therapy. Ann Intern Med. 2006;145:125-134.
20. Monthly Prescribing Reference (MPR). Insulin. Monthly Prescribing Reference Web site. Available at: http://www.empr.com/insulins/article/123739/. Accessed January 10, 2014.
21. Guyton AC, Hall JE. Insulin, glucagon, and diabetes mellitus. In: Guyton AC, Hall JE, eds. Textbook of Medical Physiology. 11th ed. Philadelphia, PA: Elsevier Saunders; 2006:961-977.
22. Kitabchi AE, Gosmanov AR. Safety of rapid-acting insulin analogs versus regular human insulin. Am J Med Sci. 2012;344:136-141.
23. Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Eng J Med. 2004;350:2272-2279.
24. Gehlaut RR, Shubrook JH. Revisiting hypoglycemia in diabetes. Osteopathic Family Physician. 2014;1:19-25.