User login
Prevention of diabetes, as well as early detection and treatment of both prediabetes and diabetes, is critical to the health of our country. Because evidence-based guidelines are key to our ability to effectively address the nation’s diabetes epidemic, the American Diabetes Association (ADA) updates its “Standards of Medical Care in Diabetes” annually to incorporate new evidence or clarifications.
The 2016 standards,1 available at professional.diabetes.org/jfp, are a valuable resource. Among the latest revisions: an expansion in screening recommendations, a change in the age at which aspirin therapy for women should be considered, and a change in A1C goals for pregnant women with diabetes.
As members of the ADA’s primary care advisory group, we use a question and answer format in the summary that follows to highlight recent revisions and review other recommendations that are of particular relevance to physicians in primary care. It is important to note, however, that ADA recommendations are not intended to preclude clinical judgment and should be applied in the context of excellent medical care.
Diagnosis and screening
Have the 2016 ADA standards changed the way diabetes is diagnosed?
No. The criteria for a diagnosis of diabetes did not change. Diabetes and prediabetes are still screened for and diagnosed with any of the following: a fasting plasma glucose (FPG); a 2-hour 75-g oral glucose tolerance test (OGTT); a random plasma glucose >200 mg/dL with symptoms of hyperglycemia; or A1C criteria (TABLE 1).1,2 The wording was changed, however, to make it clear that no one test is preferred over another for diagnosis.
Yes. In addition to screening asymptomatic adults of any age who are overweight or obese and have one or more additional risk factors for diabetes, the 2016 standards recommend screening all adults 45 years and older, regardless of weight.
Is an A1C <7% the recommended treatment goal for everyone with diabetes?
No. An A1C <7% is considered reasonable for most, but not all, nonpregnant adults. In the last few years, the ADA has focused more on individualized targets.
Tighter control (<6.5%)—which is associated with lower rates of eye disease, kidney disease, and nerve damage—may be appropriate for patients who have no significant hypoglycemia, no cardiovascular disease (CVD), a shorter duration of diabetes, or a longer expected lifespan.
Conversely, a higher target (<8%) may be appropriate for patients who are older, have longstanding diabetes, advanced macrovascular or microvascular disease, established complications, or a limited life expectancy.3,4
Pregnancy. The 2016 standards have a new target for pregnant women with diabetes: The ADA previously recommended an A1C <6% for this patient population, but now recommends a target A1C between 6% and 6.5%. This may be tightened or relaxed, however, depending on individual risk of hypoglycemia.
In focusing on individualized targets and hypoglycemia avoidance, the ADA notes that attention must be paid to fasting, pre-meal, and post-meal blood glucose levels to achieve treatment goals. The 2016 standards emphasize the importance of patient-centered diabetes care, aligned with a coordinated, team-based chronic care model.
Diabetes self-management education and support is indicated for those who are newly diagnosed, and should be provided periodically based on glucose control and progression of the disease. All patients should receive education on hypoglycemia risk and treatment.
Prediabetes and prevention
What is prediabetes and what can I do to prevent patients with prediabetes from developing diabetes?
Patients with impaired glucose tolerance, impaired fasting glucose, or an A1C between 5.7% and 6.4% are considered to have prediabetes and are at risk for developing type 2 diabetes.
Family physicians should refer patients with prediabetes to intensive diet, physical activity, and behavioral counseling programs like those based on the Diabetes Prevention Program study (www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp/Pages/default.aspx). Goals should include a minimum 7% weight loss and moderate-intensity physical activity, such as brisk walking, for at least 150 minutes per week.
Lifestyle modification programs have been shown to be very effective in preventing diabetes, with about a 58% reduction in the risk of developing type 2 diabetes after 3 years.5 The 2016 standards added a recommendation that physicians encourage the use of new technology, such as text messaging or smart phone apps, to support such efforts.
Should I consider initiating oral antiglycemics in patients with prediabetes?
Yes. Pharmacologic agents, including metformin, acarbose, and pioglitazone, have been shown to decrease progression from prediabetes to type 2 diabetes. Thus, antiglycemics should be considered for certain patients. Metformin is especially appropriate for women with a history of gestational diabetes, patients who are younger than 60 years, and those who have a body mass index (BMI) ≥35 kg/m2.6
How often should I screen patients with prediabetes?
Patients with prediabetes should be screened annually. Such individuals should also be screened and treated for modifiable cardiovascular risk factors. There is strong evidence that the treatment of obesity can be beneficial for those at any stage of the diabetes spectrum.
Obesity management
What do the 2016 ADA standards recommend for obese patients with diabetes?
With more than two-thirds of Americans either overweight or obese, the ADA added a new section on obesity management and calls on health care providers to:
- weigh patients and calculate and document their BMI at every visit, and
- counsel those who are overweight or obese on the benefits of even modest weight loss.
The ADA recommends a sustained weight loss of 5%, which can improve glycemic control and reduce the need for diabetes medications,7-9 although weight loss of ≥7% is optimal. Physicians are also called on to assess each patient’s readiness to engage in therapeutic lifestyle change to maintain a modest weight loss.
Treatment for obesity can include therapeutic lifestyle change (reduction in calories, increase in physical activity) and behavioral therapy. For refractory patients, pharmacologic therapy and bariatric surgery may be considered.
Interventions should be high-intensity (≥16 sessions in 6 months) and focus on diet, physical activity, and behavioral strategies to achieve a 500 to 750 calorie deficit per day.10 Long-term (≥1 year) comprehensive weight maintenance programs should be prescribed for those who achieve short-term weight loss.11,12 Such programs should provide at least monthly contact and encourage ongoing monitoring of body weight (weekly or more frequently), continued consumption of a reduced-calorie diet, and participation in high levels of physical activity (200 to 300 minutes per week).
Glycemic treatment
What are some of the key factors that distinguish the different type 2 diabetes medications from one another?
An increasing understanding of diabetes pathophysiology has led to a wider array of medications, making treatment more complex than ever. It is important for physicians to have a strong working knowledge of the various classes of antidiabetic agents and the subtleties between drugs in the same class to best individualize treatment.
Here are the highlights of each class of medication listed in the ADA/European Association for the Study of Diabetes algorithm for the management of type 2 diabetes,13 which is available at http://care.diabetesjournals.org/content/38/1/140/F2.large.jpg):
Metformin is the preferred initial medication for all patients who can tolerate it and have no contraindications. The drug is cost-effective, weight neutral, and has had positive cardiovascular and mortality outcomes in long-term studies. Adverse gastrointestinal (GI) effects, including nausea, diarrhea, and dyspepsia, are common but can be reduced with a slow titration of the drug. Metformin should be used with caution in those with renal disease. The dose should be reduced if the estimated glomerular filtration rate (eGFR) <45 mL/min/1.73m2 and the drug discontinued if eGFR <30 mL/min/1.73 m2.
Sulfonylureas/meglitinides stimulate insulin secretion in a glucose-independent manner. They are cost-effective and have high efficacy early in the disease and with initial use, but the effect wanes as the disease progresses. This class of drugs is associated with weight gain and hypoglycemia. Second-generation sulfonylureas (glipizide, glimepiride) are recommended; meglitinides are more expensive than sulfonylureas.
Thiazolidinediones work to improve insulin sensitivity in the periphery and have a low risk of hypoglycemia. They have been associated with fluid retention, weight gain, and worsening of pre-existing congestive heart failure, but previous cardiovascular concerns (with rosiglitazone)14 and bladder cancer risks (with pioglitazone)15-17 have been refuted. Thiazolidinediones are contraindicated in those with Class III and IV congestive heart failure, however, and patients taking them require careful monitoring for weight gain, fluid retention, and exacerbation of heart failure.
Dipeptidyl peptidase-4 inhibitors (DPP4Is) work to reduce the breakdown of endogenous incretin hormones. These oral agents increase insulin secretion in a glucose-dependent manner; more insulin is secreted when glucose is higher and less when glucose is closer to normal. This means that there is a much lower risk of hypoglycemia when a DPP4I is used as monotherapy.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs), which are injectable, also work via incretin hormones and stimulate insulin in a glucose-dependent manner. They are associated with weight loss and low rates of hypoglycemia. Adverse GI effects are common with this class of drugs, but can be reduced by titrating the medication and avoiding overeating. GLP-1RAs can be taken twice daily to once weekly, depending on the specific agent.
Sodium glucose transporter 2 inhibitors (SGLT2Is) are oral agents and the newest class of antidiabetes drugs. The drugs help block the reabsorption of glucose, thereby lowering glucose levels, blood pressure, and weight in many patients. The most common adverse effects are urinary tract and genital yeast infections. SGLT2Is should not be given to patients with advanced renal disease (chronic kidney disease Stages 3B-5) because they will not be effectively absorbed.
The US Food and Drug Administration (FDA) recently issued a warning about the risk of ketoacidosis with these agents,18 and patients should be advised to stop taking them and to seek immediate medical attention if they develop symptoms of ketoacidosis, such as excessive thirst, frequent urination, nausea and vomiting, abdominal pain, weakness or fatigue, shortness of breath, fruity-scented breath, or confusion.
Insulin is eventually needed by most patients with type 2 diabetes who live long enough to see the disease progress. The most common adverse effects are weight gain and hypoglycemia. There are many types of insulin, but only one that is delivered via inhalation—human insulin inhaled powder. Inhaled insulin, however, has the potential for adverse pulmonary effects, including cough and reduction of peak expiratory flow. Therefore, pulmonary function testing is recommended prior to its use.
Treatment goal attainment should be evaluated every 3 months, and treatment titrated at 3-month intervals if goals are not achieved. The ADA/European Association for the Study of Diabetes’ algorithm indicates that patients are likely to need insulin a year after diagnosis if their A1C goal has not been achieved or maintained.13
The following medications are not included in the algorithm but are included in the 2016 standards, and may be helpful for certain patients:
Alpha-glucosidase inhibitors delay the absorption of glucose from the proximal to distal GI tract, thereby reducing postprandial hyperglycemia. Flatulence and leakage of stool—the most common adverse effects—have limited their use in the United States.
Bile acid sequestrants (colesevelam) treat both hyperlipidemia and diabetes. The medications work by reducing glucose absorption from the GI tract. They reduce postprandial hyperglycemia, with a low risk of hypoglycemia. Colesevelam’s use is limited, however, because of the number of pills needed (6 daily).
Bromocriptine affects satiety levels via the central nervous system, and is available in a specific formulation for the treatment of diabetes. “First-dose” hypotension, however, is an adverse effect of considerable concern.1
Pramlintide, an injectable amylin mimetic given to patients on prandial insulin, can reduce postprandial glucose levels. The most common adverse effects are upper GI symptoms and hypoglycemia. Due to the adverse effects and the need for an injection with each meal, pramlintide is used infrequently.
Cardiovascular risk reduction
Has the ADA revised its recommendations for cardiovascular disease risk management?
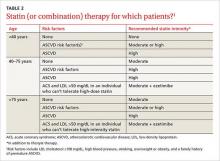
Yes. There have been several changes. The first is in terminology, with atherosclerotic cardiovascular disease (ASCVD) replacing CVD alone. While new recommendations for statin therapy for adults older than 40 years (TABLE 2)1 were also added, the emphasis remains on therapeutic lifestyle change as an effective treatment for hypertension. These modifications should include at least 150 minutes of moderate physical activity per week and, for most patients, a reduction in total calories, saturated fat, and sodium.
It is important to remind patients that to maximize the benefits in terms of treating hyperglycemia, hypertension, and dyslipidemia, such changes must be maintained over the long term.
Aspirin therapy. The ADA also revised its recommendation regarding aspirin therapy. Based on new evidence in the treatment of women with ASCVD risk, the standards now call for considering aspirin therapy (75-162 mg/d) in both women and men ≥50 years as a primary prevention strategy for those with type 1 or type 2 diabetes with a 10-year ASCVD risk of >10%. (The previous standards recommended this only for women older than 60 years.)
Antiplatelet therapy is now recommended for patients younger than 50 years with multiple risk factors, and as secondary prevention in those with a history of ASCVD.19-21
Hypertension. The ADA’s recommendations for treating hypertension in patients with diabetes have not changed; the goal remains <140/<90 mm Hg. Lower targets may be appropriate for younger patients, those with albuminuria, and individuals with additional CVD risk factors; however, systolic pressure <130 mm Hg has not been shown to reduce CVD outcomes, and diastolic pressure <70 mm Hg has been associated with higher mortality.22
Optimal medication and lifestyle therapy are important to achieve goals, with avoidance of undue treatment burden. Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), but not both, should be included as part of treatment. Other agents, such as a thiazide diuretic, may be needed to achieve individual goals. Serum creatinine/eGFR and serum potassium levels should be monitored with the use of diuretics.
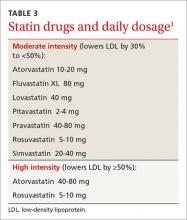
Lipids. The 2016 standards include notable changes in lipid management. The ADA sees a role for ezetimibe for select patients, based on studies such as the IMPROVE IT trial23 that included participants with diabetes. The ADA also added a table highlighting statin recommendations and delineating high and moderate-intensity statins (TABLE 3).1 Those younger than 40 years with no other risk factors may not need a statin, but patients ages 40 or older will need moderate- to high-intensity statin therapy to effectively lower ASCVD risk.24-28
These recommendations reflect a comprehensive plan to reduce ASCVD in this at-risk population, which should also include lifestyle modification, including smoking prevention and quit strategies, as needed.
Microvascular complications
DIABETIC KIDNEY DISEASE
How should I diagnose nephropathy?
The ADA changed the terminology, referring to “diabetic kidney disease” (DKD) rather than nephropathy to highlight the fact that the focus is on kidney disease directly linked to diabetes.
Other recommendations include an annual assessment of urinary albumin (eg, spot urine albumin-to-creatinine ratio and eGFR) for patients who have had type 1 diabetes for ≥5 years and all patients who have type 2 diabetes. Two out of 3 abnormal specimens collected within a 3- to 6-month period indicate the presence of albuminuria.
What can be done to prevent or slow the progression of DKD?
Optimal BP and glycemic control are key,29-35 along with diet and medication. For patients with DKD, dietary protein intake should be 0.8 g/kg body weight per day. ACE inhibitors and ARBs have been shown to slow the decline in eGFR in patients with elevated urinary albumin excretion (≥30 mg/day).
However, neither an ACE inhibitor nor an ARB is recommended for the primary prevention of DKD in patients who have normal BP, normal urine albumin-to-creatinine ratio (<30 mg/g), and normal eGFR. In addition, combined use of an ACE inhibitor and an ARB should be avoided, as it provides no additional benefit and increases the risk of adverse effects.29
RETINOPATHY
How should I manage retinopathy in patients with diabetes?
As with the management of DKD, it is important to optimize glycemic and BP control to reduce the risk, or slow the progression, of retinopathy. Intensive diabetes management, with the goal of achieving near-normal glycemic levels, has been shown in large prospective randomized studies to prevent or delay the onset and progression of diabetic retinopathy.33,36 The presence of retinopathy is not a contraindication to aspirin therapy for ASCVD prevention, as aspirin does not increase the risk of retinal hemorrhage.
When should patients with diabetes be screened for retinopathy?
Patients with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years of the onset of diabetes. Those with type 2 diabetes should have such an exam shortly after diagnosis. The exam should be repeated annually; if there is no evidence of retinopathy, however, 2-year intervals may be considered.
PERIPHERAL NEUROPATHY
When and how should I screen patients with diabetes for neuropathy?
All patients should be screened for diabetic peripheral neuropathy (DPN) starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes, and continued at least annually thereafter. Assessment should include a detailed history and 10-g monofilament testing, as well as at least one of the following tests: pinprick, temperature, and vibration sensation.
It is important, too, to screen patients with more advanced diabetes for signs and symptoms of autonomic neuropathy. Signs and symptoms may include resting tachycardia, exercise intolerance, orthostatic hypotension, gastroparesis, constipation, impaired neurovascular function, and autonomic failure in response to hypoglycemia. In men, diabetic autonomic neuropathy may cause erectile dysfunction and/or retrograde ejaculation.
How should I manage patients who have DPN?
Tight glycemic control is the only measure that has been shown to prevent or delay the development of DPN or cardiac autonomic neuropathy in patients with type 1 diabetes,37,38 and to slow the progression of neuropathy in some patients with type 2 diabetes.39
The FDA has approved pregabalin, duloxetine, and tapentadol for the treatment of pain associated with DPN. Tricyclic antidepressants, gabapentin, venlafaxine, carbamazepine, tramadol, and topical capsaicin, although not approved for the treatment of painful DPN, may also be effective in treating neuropathic pain.
For those with autonomic neuropathy, dietary changes and prokinetic agents such as erythromycin may alleviate gastroparesis. Due to extrapyramidal adverse effects, metoclopramide is reserved for the most severe and unresponsive cases. Recurrent urinary tract infections, pyelonephritis, incontinence, or palpable bladder should prompt an evaluation for bladder dysfunction. Controlling lipids and BP, quitting smoking, and making other lifestyle changes can reduce both the development and the progression of autonomic neuropathy.
FOOT CARE/PERIPHERAL ARTERIAL DISEASE
What does the ADA recommend regarding foot care for patients with diabetes?
The ADA’s standards recommend an annual comprehensive foot examination to identify risk factors predictive of ulcers and potential amputations. The exam should start with inspection and assessment of foot pulses and should seek to identify loss of peripheral sensation. The examination should include inspection of the skin, assessment of foot deformities, neurologic assessment including 10-g monofilament testing and pinprick or vibration testing or assessment of ankle reflexes, and vascular assessment, including pulses in the legs and feet.40
It is also important to screen patients for peripheral arterial disease (PAD), with a comprehensive medical history and physical exam of pulses. Ankle-brachial index testing (ABI) should be performed in patients with signs or symptoms of PAD, including claudication or skin and hair changes in the lower extremities. ABI may be considered for all patients with diabetes starting at age 50 and in those younger than 50 years who have risk factors.41
Which patients with diabetes are at higher risk for foot complications?
The following are risk factors for foot complications: previous amputation, prior foot ulcer, peripheral neuropathy, foot deformity, peripheral vascular disease, visual impairment, peripheral neuropathy (especially if on dialysis), poor glycemic control, and smoking. Patients with high-risk foot conditions should be educated about their risk and appropriate management.
A well-fitted walking shoe that cushions the feet and redistributes pressure is one option to help patients. Patients with bony deformities may need extra wide or deep shoes and patients with more advanced disease may need custom-fitted shoes.
When should patients be referred to a foot specialist?
Refer patients to a foot care specialist for ongoing preventive care and lifelong surveillance if they smoke or have a history of lower-extremity complications, a loss of protective sensation, structural abnormalities, or PAD.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/site/misc/2016-Standards-of-Care.pdf. Accessed March 28, 2016.
2. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334.
3. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362.
4. Vijan S, Sussman JB, Yudkin JS, et al. Effect of patients’ risks and p on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–1234.
5. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
6. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737.
7. UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905–912.
8. Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415.
9. Pastors JG, Warshaw H, Daly A, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–613.
10. Selph S, Dana T, Bougatsos C, et al. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 US Preventive Services Task Force Recommendation. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed March 28, 2016.
11. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and metaanalysis. Obesity (Silver Spring). 2006;14:1283–1293.
12. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;99:14–23.
13. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149.
14. Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195.
15. Balaji V, Seshiah V, Ashtalakshmi G, et al. Retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–427.
16. Kuo HW, Tiao MM, Ho SC, et al. Pioglitazone use and the risk of bladder cancer. Kaohsiung J Med Sci. 2014;30:94–97.
17. Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254-259.
18. US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed December 11, 2015.
19. Huxley RR, Peters SAE, Mishra GD, et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206.
20. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551.
21. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973-1980.
22. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
23. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397.
24. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485.
25. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696.
26. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504.
27. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316.
28. Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080.
29. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713.
30. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an American Diabetes Association Consensus Conference. Diabetes Care. 2014;37:2864–2883.
31. The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720.
32. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
33. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
34. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
35. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430.
36. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559.
37. Chew EY, Ambrosius WT, Davis MD, et al; ACCORD Study Group; ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244.
38. Ang L, Jaiswal M, Martin C, et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14:528.
39. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group. Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:31–38.
40. Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765.
41. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341.
42. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Available at: http://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed April 8, 2016.
Prevention of diabetes, as well as early detection and treatment of both prediabetes and diabetes, is critical to the health of our country. Because evidence-based guidelines are key to our ability to effectively address the nation’s diabetes epidemic, the American Diabetes Association (ADA) updates its “Standards of Medical Care in Diabetes” annually to incorporate new evidence or clarifications.
The 2016 standards,1 available at professional.diabetes.org/jfp, are a valuable resource. Among the latest revisions: an expansion in screening recommendations, a change in the age at which aspirin therapy for women should be considered, and a change in A1C goals for pregnant women with diabetes.
As members of the ADA’s primary care advisory group, we use a question and answer format in the summary that follows to highlight recent revisions and review other recommendations that are of particular relevance to physicians in primary care. It is important to note, however, that ADA recommendations are not intended to preclude clinical judgment and should be applied in the context of excellent medical care.
Diagnosis and screening
Have the 2016 ADA standards changed the way diabetes is diagnosed?
No. The criteria for a diagnosis of diabetes did not change. Diabetes and prediabetes are still screened for and diagnosed with any of the following: a fasting plasma glucose (FPG); a 2-hour 75-g oral glucose tolerance test (OGTT); a random plasma glucose >200 mg/dL with symptoms of hyperglycemia; or A1C criteria (TABLE 1).1,2 The wording was changed, however, to make it clear that no one test is preferred over another for diagnosis.
Yes. In addition to screening asymptomatic adults of any age who are overweight or obese and have one or more additional risk factors for diabetes, the 2016 standards recommend screening all adults 45 years and older, regardless of weight.
Is an A1C <7% the recommended treatment goal for everyone with diabetes?
No. An A1C <7% is considered reasonable for most, but not all, nonpregnant adults. In the last few years, the ADA has focused more on individualized targets.
Tighter control (<6.5%)—which is associated with lower rates of eye disease, kidney disease, and nerve damage—may be appropriate for patients who have no significant hypoglycemia, no cardiovascular disease (CVD), a shorter duration of diabetes, or a longer expected lifespan.
Conversely, a higher target (<8%) may be appropriate for patients who are older, have longstanding diabetes, advanced macrovascular or microvascular disease, established complications, or a limited life expectancy.3,4
Pregnancy. The 2016 standards have a new target for pregnant women with diabetes: The ADA previously recommended an A1C <6% for this patient population, but now recommends a target A1C between 6% and 6.5%. This may be tightened or relaxed, however, depending on individual risk of hypoglycemia.
In focusing on individualized targets and hypoglycemia avoidance, the ADA notes that attention must be paid to fasting, pre-meal, and post-meal blood glucose levels to achieve treatment goals. The 2016 standards emphasize the importance of patient-centered diabetes care, aligned with a coordinated, team-based chronic care model.
Diabetes self-management education and support is indicated for those who are newly diagnosed, and should be provided periodically based on glucose control and progression of the disease. All patients should receive education on hypoglycemia risk and treatment.
Prediabetes and prevention
What is prediabetes and what can I do to prevent patients with prediabetes from developing diabetes?
Patients with impaired glucose tolerance, impaired fasting glucose, or an A1C between 5.7% and 6.4% are considered to have prediabetes and are at risk for developing type 2 diabetes.
Family physicians should refer patients with prediabetes to intensive diet, physical activity, and behavioral counseling programs like those based on the Diabetes Prevention Program study (www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp/Pages/default.aspx). Goals should include a minimum 7% weight loss and moderate-intensity physical activity, such as brisk walking, for at least 150 minutes per week.
Lifestyle modification programs have been shown to be very effective in preventing diabetes, with about a 58% reduction in the risk of developing type 2 diabetes after 3 years.5 The 2016 standards added a recommendation that physicians encourage the use of new technology, such as text messaging or smart phone apps, to support such efforts.
Should I consider initiating oral antiglycemics in patients with prediabetes?
Yes. Pharmacologic agents, including metformin, acarbose, and pioglitazone, have been shown to decrease progression from prediabetes to type 2 diabetes. Thus, antiglycemics should be considered for certain patients. Metformin is especially appropriate for women with a history of gestational diabetes, patients who are younger than 60 years, and those who have a body mass index (BMI) ≥35 kg/m2.6
How often should I screen patients with prediabetes?
Patients with prediabetes should be screened annually. Such individuals should also be screened and treated for modifiable cardiovascular risk factors. There is strong evidence that the treatment of obesity can be beneficial for those at any stage of the diabetes spectrum.
Obesity management
What do the 2016 ADA standards recommend for obese patients with diabetes?
With more than two-thirds of Americans either overweight or obese, the ADA added a new section on obesity management and calls on health care providers to:
- weigh patients and calculate and document their BMI at every visit, and
- counsel those who are overweight or obese on the benefits of even modest weight loss.
The ADA recommends a sustained weight loss of 5%, which can improve glycemic control and reduce the need for diabetes medications,7-9 although weight loss of ≥7% is optimal. Physicians are also called on to assess each patient’s readiness to engage in therapeutic lifestyle change to maintain a modest weight loss.
Treatment for obesity can include therapeutic lifestyle change (reduction in calories, increase in physical activity) and behavioral therapy. For refractory patients, pharmacologic therapy and bariatric surgery may be considered.
Interventions should be high-intensity (≥16 sessions in 6 months) and focus on diet, physical activity, and behavioral strategies to achieve a 500 to 750 calorie deficit per day.10 Long-term (≥1 year) comprehensive weight maintenance programs should be prescribed for those who achieve short-term weight loss.11,12 Such programs should provide at least monthly contact and encourage ongoing monitoring of body weight (weekly or more frequently), continued consumption of a reduced-calorie diet, and participation in high levels of physical activity (200 to 300 minutes per week).
Glycemic treatment
What are some of the key factors that distinguish the different type 2 diabetes medications from one another?
An increasing understanding of diabetes pathophysiology has led to a wider array of medications, making treatment more complex than ever. It is important for physicians to have a strong working knowledge of the various classes of antidiabetic agents and the subtleties between drugs in the same class to best individualize treatment.
Here are the highlights of each class of medication listed in the ADA/European Association for the Study of Diabetes algorithm for the management of type 2 diabetes,13 which is available at http://care.diabetesjournals.org/content/38/1/140/F2.large.jpg):
Metformin is the preferred initial medication for all patients who can tolerate it and have no contraindications. The drug is cost-effective, weight neutral, and has had positive cardiovascular and mortality outcomes in long-term studies. Adverse gastrointestinal (GI) effects, including nausea, diarrhea, and dyspepsia, are common but can be reduced with a slow titration of the drug. Metformin should be used with caution in those with renal disease. The dose should be reduced if the estimated glomerular filtration rate (eGFR) <45 mL/min/1.73m2 and the drug discontinued if eGFR <30 mL/min/1.73 m2.
Sulfonylureas/meglitinides stimulate insulin secretion in a glucose-independent manner. They are cost-effective and have high efficacy early in the disease and with initial use, but the effect wanes as the disease progresses. This class of drugs is associated with weight gain and hypoglycemia. Second-generation sulfonylureas (glipizide, glimepiride) are recommended; meglitinides are more expensive than sulfonylureas.
Thiazolidinediones work to improve insulin sensitivity in the periphery and have a low risk of hypoglycemia. They have been associated with fluid retention, weight gain, and worsening of pre-existing congestive heart failure, but previous cardiovascular concerns (with rosiglitazone)14 and bladder cancer risks (with pioglitazone)15-17 have been refuted. Thiazolidinediones are contraindicated in those with Class III and IV congestive heart failure, however, and patients taking them require careful monitoring for weight gain, fluid retention, and exacerbation of heart failure.
Dipeptidyl peptidase-4 inhibitors (DPP4Is) work to reduce the breakdown of endogenous incretin hormones. These oral agents increase insulin secretion in a glucose-dependent manner; more insulin is secreted when glucose is higher and less when glucose is closer to normal. This means that there is a much lower risk of hypoglycemia when a DPP4I is used as monotherapy.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs), which are injectable, also work via incretin hormones and stimulate insulin in a glucose-dependent manner. They are associated with weight loss and low rates of hypoglycemia. Adverse GI effects are common with this class of drugs, but can be reduced by titrating the medication and avoiding overeating. GLP-1RAs can be taken twice daily to once weekly, depending on the specific agent.
Sodium glucose transporter 2 inhibitors (SGLT2Is) are oral agents and the newest class of antidiabetes drugs. The drugs help block the reabsorption of glucose, thereby lowering glucose levels, blood pressure, and weight in many patients. The most common adverse effects are urinary tract and genital yeast infections. SGLT2Is should not be given to patients with advanced renal disease (chronic kidney disease Stages 3B-5) because they will not be effectively absorbed.
The US Food and Drug Administration (FDA) recently issued a warning about the risk of ketoacidosis with these agents,18 and patients should be advised to stop taking them and to seek immediate medical attention if they develop symptoms of ketoacidosis, such as excessive thirst, frequent urination, nausea and vomiting, abdominal pain, weakness or fatigue, shortness of breath, fruity-scented breath, or confusion.
Insulin is eventually needed by most patients with type 2 diabetes who live long enough to see the disease progress. The most common adverse effects are weight gain and hypoglycemia. There are many types of insulin, but only one that is delivered via inhalation—human insulin inhaled powder. Inhaled insulin, however, has the potential for adverse pulmonary effects, including cough and reduction of peak expiratory flow. Therefore, pulmonary function testing is recommended prior to its use.
Treatment goal attainment should be evaluated every 3 months, and treatment titrated at 3-month intervals if goals are not achieved. The ADA/European Association for the Study of Diabetes’ algorithm indicates that patients are likely to need insulin a year after diagnosis if their A1C goal has not been achieved or maintained.13
The following medications are not included in the algorithm but are included in the 2016 standards, and may be helpful for certain patients:
Alpha-glucosidase inhibitors delay the absorption of glucose from the proximal to distal GI tract, thereby reducing postprandial hyperglycemia. Flatulence and leakage of stool—the most common adverse effects—have limited their use in the United States.
Bile acid sequestrants (colesevelam) treat both hyperlipidemia and diabetes. The medications work by reducing glucose absorption from the GI tract. They reduce postprandial hyperglycemia, with a low risk of hypoglycemia. Colesevelam’s use is limited, however, because of the number of pills needed (6 daily).
Bromocriptine affects satiety levels via the central nervous system, and is available in a specific formulation for the treatment of diabetes. “First-dose” hypotension, however, is an adverse effect of considerable concern.1
Pramlintide, an injectable amylin mimetic given to patients on prandial insulin, can reduce postprandial glucose levels. The most common adverse effects are upper GI symptoms and hypoglycemia. Due to the adverse effects and the need for an injection with each meal, pramlintide is used infrequently.
Cardiovascular risk reduction
Has the ADA revised its recommendations for cardiovascular disease risk management?
Yes. There have been several changes. The first is in terminology, with atherosclerotic cardiovascular disease (ASCVD) replacing CVD alone. While new recommendations for statin therapy for adults older than 40 years (TABLE 2)1 were also added, the emphasis remains on therapeutic lifestyle change as an effective treatment for hypertension. These modifications should include at least 150 minutes of moderate physical activity per week and, for most patients, a reduction in total calories, saturated fat, and sodium.
It is important to remind patients that to maximize the benefits in terms of treating hyperglycemia, hypertension, and dyslipidemia, such changes must be maintained over the long term.
Aspirin therapy. The ADA also revised its recommendation regarding aspirin therapy. Based on new evidence in the treatment of women with ASCVD risk, the standards now call for considering aspirin therapy (75-162 mg/d) in both women and men ≥50 years as a primary prevention strategy for those with type 1 or type 2 diabetes with a 10-year ASCVD risk of >10%. (The previous standards recommended this only for women older than 60 years.)
Antiplatelet therapy is now recommended for patients younger than 50 years with multiple risk factors, and as secondary prevention in those with a history of ASCVD.19-21
Hypertension. The ADA’s recommendations for treating hypertension in patients with diabetes have not changed; the goal remains <140/<90 mm Hg. Lower targets may be appropriate for younger patients, those with albuminuria, and individuals with additional CVD risk factors; however, systolic pressure <130 mm Hg has not been shown to reduce CVD outcomes, and diastolic pressure <70 mm Hg has been associated with higher mortality.22
Optimal medication and lifestyle therapy are important to achieve goals, with avoidance of undue treatment burden. Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), but not both, should be included as part of treatment. Other agents, such as a thiazide diuretic, may be needed to achieve individual goals. Serum creatinine/eGFR and serum potassium levels should be monitored with the use of diuretics.
Lipids. The 2016 standards include notable changes in lipid management. The ADA sees a role for ezetimibe for select patients, based on studies such as the IMPROVE IT trial23 that included participants with diabetes. The ADA also added a table highlighting statin recommendations and delineating high and moderate-intensity statins (TABLE 3).1 Those younger than 40 years with no other risk factors may not need a statin, but patients ages 40 or older will need moderate- to high-intensity statin therapy to effectively lower ASCVD risk.24-28
These recommendations reflect a comprehensive plan to reduce ASCVD in this at-risk population, which should also include lifestyle modification, including smoking prevention and quit strategies, as needed.
Microvascular complications
DIABETIC KIDNEY DISEASE
How should I diagnose nephropathy?
The ADA changed the terminology, referring to “diabetic kidney disease” (DKD) rather than nephropathy to highlight the fact that the focus is on kidney disease directly linked to diabetes.
Other recommendations include an annual assessment of urinary albumin (eg, spot urine albumin-to-creatinine ratio and eGFR) for patients who have had type 1 diabetes for ≥5 years and all patients who have type 2 diabetes. Two out of 3 abnormal specimens collected within a 3- to 6-month period indicate the presence of albuminuria.
What can be done to prevent or slow the progression of DKD?
Optimal BP and glycemic control are key,29-35 along with diet and medication. For patients with DKD, dietary protein intake should be 0.8 g/kg body weight per day. ACE inhibitors and ARBs have been shown to slow the decline in eGFR in patients with elevated urinary albumin excretion (≥30 mg/day).
However, neither an ACE inhibitor nor an ARB is recommended for the primary prevention of DKD in patients who have normal BP, normal urine albumin-to-creatinine ratio (<30 mg/g), and normal eGFR. In addition, combined use of an ACE inhibitor and an ARB should be avoided, as it provides no additional benefit and increases the risk of adverse effects.29
RETINOPATHY
How should I manage retinopathy in patients with diabetes?
As with the management of DKD, it is important to optimize glycemic and BP control to reduce the risk, or slow the progression, of retinopathy. Intensive diabetes management, with the goal of achieving near-normal glycemic levels, has been shown in large prospective randomized studies to prevent or delay the onset and progression of diabetic retinopathy.33,36 The presence of retinopathy is not a contraindication to aspirin therapy for ASCVD prevention, as aspirin does not increase the risk of retinal hemorrhage.
When should patients with diabetes be screened for retinopathy?
Patients with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years of the onset of diabetes. Those with type 2 diabetes should have such an exam shortly after diagnosis. The exam should be repeated annually; if there is no evidence of retinopathy, however, 2-year intervals may be considered.
PERIPHERAL NEUROPATHY
When and how should I screen patients with diabetes for neuropathy?
All patients should be screened for diabetic peripheral neuropathy (DPN) starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes, and continued at least annually thereafter. Assessment should include a detailed history and 10-g monofilament testing, as well as at least one of the following tests: pinprick, temperature, and vibration sensation.
It is important, too, to screen patients with more advanced diabetes for signs and symptoms of autonomic neuropathy. Signs and symptoms may include resting tachycardia, exercise intolerance, orthostatic hypotension, gastroparesis, constipation, impaired neurovascular function, and autonomic failure in response to hypoglycemia. In men, diabetic autonomic neuropathy may cause erectile dysfunction and/or retrograde ejaculation.
How should I manage patients who have DPN?
Tight glycemic control is the only measure that has been shown to prevent or delay the development of DPN or cardiac autonomic neuropathy in patients with type 1 diabetes,37,38 and to slow the progression of neuropathy in some patients with type 2 diabetes.39
The FDA has approved pregabalin, duloxetine, and tapentadol for the treatment of pain associated with DPN. Tricyclic antidepressants, gabapentin, venlafaxine, carbamazepine, tramadol, and topical capsaicin, although not approved for the treatment of painful DPN, may also be effective in treating neuropathic pain.
For those with autonomic neuropathy, dietary changes and prokinetic agents such as erythromycin may alleviate gastroparesis. Due to extrapyramidal adverse effects, metoclopramide is reserved for the most severe and unresponsive cases. Recurrent urinary tract infections, pyelonephritis, incontinence, or palpable bladder should prompt an evaluation for bladder dysfunction. Controlling lipids and BP, quitting smoking, and making other lifestyle changes can reduce both the development and the progression of autonomic neuropathy.
FOOT CARE/PERIPHERAL ARTERIAL DISEASE
What does the ADA recommend regarding foot care for patients with diabetes?
The ADA’s standards recommend an annual comprehensive foot examination to identify risk factors predictive of ulcers and potential amputations. The exam should start with inspection and assessment of foot pulses and should seek to identify loss of peripheral sensation. The examination should include inspection of the skin, assessment of foot deformities, neurologic assessment including 10-g monofilament testing and pinprick or vibration testing or assessment of ankle reflexes, and vascular assessment, including pulses in the legs and feet.40
It is also important to screen patients for peripheral arterial disease (PAD), with a comprehensive medical history and physical exam of pulses. Ankle-brachial index testing (ABI) should be performed in patients with signs or symptoms of PAD, including claudication or skin and hair changes in the lower extremities. ABI may be considered for all patients with diabetes starting at age 50 and in those younger than 50 years who have risk factors.41
Which patients with diabetes are at higher risk for foot complications?
The following are risk factors for foot complications: previous amputation, prior foot ulcer, peripheral neuropathy, foot deformity, peripheral vascular disease, visual impairment, peripheral neuropathy (especially if on dialysis), poor glycemic control, and smoking. Patients with high-risk foot conditions should be educated about their risk and appropriate management.
A well-fitted walking shoe that cushions the feet and redistributes pressure is one option to help patients. Patients with bony deformities may need extra wide or deep shoes and patients with more advanced disease may need custom-fitted shoes.
When should patients be referred to a foot specialist?
Refer patients to a foot care specialist for ongoing preventive care and lifelong surveillance if they smoke or have a history of lower-extremity complications, a loss of protective sensation, structural abnormalities, or PAD.
Prevention of diabetes, as well as early detection and treatment of both prediabetes and diabetes, is critical to the health of our country. Because evidence-based guidelines are key to our ability to effectively address the nation’s diabetes epidemic, the American Diabetes Association (ADA) updates its “Standards of Medical Care in Diabetes” annually to incorporate new evidence or clarifications.
The 2016 standards,1 available at professional.diabetes.org/jfp, are a valuable resource. Among the latest revisions: an expansion in screening recommendations, a change in the age at which aspirin therapy for women should be considered, and a change in A1C goals for pregnant women with diabetes.
As members of the ADA’s primary care advisory group, we use a question and answer format in the summary that follows to highlight recent revisions and review other recommendations that are of particular relevance to physicians in primary care. It is important to note, however, that ADA recommendations are not intended to preclude clinical judgment and should be applied in the context of excellent medical care.
Diagnosis and screening
Have the 2016 ADA standards changed the way diabetes is diagnosed?
No. The criteria for a diagnosis of diabetes did not change. Diabetes and prediabetes are still screened for and diagnosed with any of the following: a fasting plasma glucose (FPG); a 2-hour 75-g oral glucose tolerance test (OGTT); a random plasma glucose >200 mg/dL with symptoms of hyperglycemia; or A1C criteria (TABLE 1).1,2 The wording was changed, however, to make it clear that no one test is preferred over another for diagnosis.
Yes. In addition to screening asymptomatic adults of any age who are overweight or obese and have one or more additional risk factors for diabetes, the 2016 standards recommend screening all adults 45 years and older, regardless of weight.
Is an A1C <7% the recommended treatment goal for everyone with diabetes?
No. An A1C <7% is considered reasonable for most, but not all, nonpregnant adults. In the last few years, the ADA has focused more on individualized targets.
Tighter control (<6.5%)—which is associated with lower rates of eye disease, kidney disease, and nerve damage—may be appropriate for patients who have no significant hypoglycemia, no cardiovascular disease (CVD), a shorter duration of diabetes, or a longer expected lifespan.
Conversely, a higher target (<8%) may be appropriate for patients who are older, have longstanding diabetes, advanced macrovascular or microvascular disease, established complications, or a limited life expectancy.3,4
Pregnancy. The 2016 standards have a new target for pregnant women with diabetes: The ADA previously recommended an A1C <6% for this patient population, but now recommends a target A1C between 6% and 6.5%. This may be tightened or relaxed, however, depending on individual risk of hypoglycemia.
In focusing on individualized targets and hypoglycemia avoidance, the ADA notes that attention must be paid to fasting, pre-meal, and post-meal blood glucose levels to achieve treatment goals. The 2016 standards emphasize the importance of patient-centered diabetes care, aligned with a coordinated, team-based chronic care model.
Diabetes self-management education and support is indicated for those who are newly diagnosed, and should be provided periodically based on glucose control and progression of the disease. All patients should receive education on hypoglycemia risk and treatment.
Prediabetes and prevention
What is prediabetes and what can I do to prevent patients with prediabetes from developing diabetes?
Patients with impaired glucose tolerance, impaired fasting glucose, or an A1C between 5.7% and 6.4% are considered to have prediabetes and are at risk for developing type 2 diabetes.
Family physicians should refer patients with prediabetes to intensive diet, physical activity, and behavioral counseling programs like those based on the Diabetes Prevention Program study (www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp/Pages/default.aspx). Goals should include a minimum 7% weight loss and moderate-intensity physical activity, such as brisk walking, for at least 150 minutes per week.
Lifestyle modification programs have been shown to be very effective in preventing diabetes, with about a 58% reduction in the risk of developing type 2 diabetes after 3 years.5 The 2016 standards added a recommendation that physicians encourage the use of new technology, such as text messaging or smart phone apps, to support such efforts.
Should I consider initiating oral antiglycemics in patients with prediabetes?
Yes. Pharmacologic agents, including metformin, acarbose, and pioglitazone, have been shown to decrease progression from prediabetes to type 2 diabetes. Thus, antiglycemics should be considered for certain patients. Metformin is especially appropriate for women with a history of gestational diabetes, patients who are younger than 60 years, and those who have a body mass index (BMI) ≥35 kg/m2.6
How often should I screen patients with prediabetes?
Patients with prediabetes should be screened annually. Such individuals should also be screened and treated for modifiable cardiovascular risk factors. There is strong evidence that the treatment of obesity can be beneficial for those at any stage of the diabetes spectrum.
Obesity management
What do the 2016 ADA standards recommend for obese patients with diabetes?
With more than two-thirds of Americans either overweight or obese, the ADA added a new section on obesity management and calls on health care providers to:
- weigh patients and calculate and document their BMI at every visit, and
- counsel those who are overweight or obese on the benefits of even modest weight loss.
The ADA recommends a sustained weight loss of 5%, which can improve glycemic control and reduce the need for diabetes medications,7-9 although weight loss of ≥7% is optimal. Physicians are also called on to assess each patient’s readiness to engage in therapeutic lifestyle change to maintain a modest weight loss.
Treatment for obesity can include therapeutic lifestyle change (reduction in calories, increase in physical activity) and behavioral therapy. For refractory patients, pharmacologic therapy and bariatric surgery may be considered.
Interventions should be high-intensity (≥16 sessions in 6 months) and focus on diet, physical activity, and behavioral strategies to achieve a 500 to 750 calorie deficit per day.10 Long-term (≥1 year) comprehensive weight maintenance programs should be prescribed for those who achieve short-term weight loss.11,12 Such programs should provide at least monthly contact and encourage ongoing monitoring of body weight (weekly or more frequently), continued consumption of a reduced-calorie diet, and participation in high levels of physical activity (200 to 300 minutes per week).
Glycemic treatment
What are some of the key factors that distinguish the different type 2 diabetes medications from one another?
An increasing understanding of diabetes pathophysiology has led to a wider array of medications, making treatment more complex than ever. It is important for physicians to have a strong working knowledge of the various classes of antidiabetic agents and the subtleties between drugs in the same class to best individualize treatment.
Here are the highlights of each class of medication listed in the ADA/European Association for the Study of Diabetes algorithm for the management of type 2 diabetes,13 which is available at http://care.diabetesjournals.org/content/38/1/140/F2.large.jpg):
Metformin is the preferred initial medication for all patients who can tolerate it and have no contraindications. The drug is cost-effective, weight neutral, and has had positive cardiovascular and mortality outcomes in long-term studies. Adverse gastrointestinal (GI) effects, including nausea, diarrhea, and dyspepsia, are common but can be reduced with a slow titration of the drug. Metformin should be used with caution in those with renal disease. The dose should be reduced if the estimated glomerular filtration rate (eGFR) <45 mL/min/1.73m2 and the drug discontinued if eGFR <30 mL/min/1.73 m2.
Sulfonylureas/meglitinides stimulate insulin secretion in a glucose-independent manner. They are cost-effective and have high efficacy early in the disease and with initial use, but the effect wanes as the disease progresses. This class of drugs is associated with weight gain and hypoglycemia. Second-generation sulfonylureas (glipizide, glimepiride) are recommended; meglitinides are more expensive than sulfonylureas.
Thiazolidinediones work to improve insulin sensitivity in the periphery and have a low risk of hypoglycemia. They have been associated with fluid retention, weight gain, and worsening of pre-existing congestive heart failure, but previous cardiovascular concerns (with rosiglitazone)14 and bladder cancer risks (with pioglitazone)15-17 have been refuted. Thiazolidinediones are contraindicated in those with Class III and IV congestive heart failure, however, and patients taking them require careful monitoring for weight gain, fluid retention, and exacerbation of heart failure.
Dipeptidyl peptidase-4 inhibitors (DPP4Is) work to reduce the breakdown of endogenous incretin hormones. These oral agents increase insulin secretion in a glucose-dependent manner; more insulin is secreted when glucose is higher and less when glucose is closer to normal. This means that there is a much lower risk of hypoglycemia when a DPP4I is used as monotherapy.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs), which are injectable, also work via incretin hormones and stimulate insulin in a glucose-dependent manner. They are associated with weight loss and low rates of hypoglycemia. Adverse GI effects are common with this class of drugs, but can be reduced by titrating the medication and avoiding overeating. GLP-1RAs can be taken twice daily to once weekly, depending on the specific agent.
Sodium glucose transporter 2 inhibitors (SGLT2Is) are oral agents and the newest class of antidiabetes drugs. The drugs help block the reabsorption of glucose, thereby lowering glucose levels, blood pressure, and weight in many patients. The most common adverse effects are urinary tract and genital yeast infections. SGLT2Is should not be given to patients with advanced renal disease (chronic kidney disease Stages 3B-5) because they will not be effectively absorbed.
The US Food and Drug Administration (FDA) recently issued a warning about the risk of ketoacidosis with these agents,18 and patients should be advised to stop taking them and to seek immediate medical attention if they develop symptoms of ketoacidosis, such as excessive thirst, frequent urination, nausea and vomiting, abdominal pain, weakness or fatigue, shortness of breath, fruity-scented breath, or confusion.
Insulin is eventually needed by most patients with type 2 diabetes who live long enough to see the disease progress. The most common adverse effects are weight gain and hypoglycemia. There are many types of insulin, but only one that is delivered via inhalation—human insulin inhaled powder. Inhaled insulin, however, has the potential for adverse pulmonary effects, including cough and reduction of peak expiratory flow. Therefore, pulmonary function testing is recommended prior to its use.
Treatment goal attainment should be evaluated every 3 months, and treatment titrated at 3-month intervals if goals are not achieved. The ADA/European Association for the Study of Diabetes’ algorithm indicates that patients are likely to need insulin a year after diagnosis if their A1C goal has not been achieved or maintained.13
The following medications are not included in the algorithm but are included in the 2016 standards, and may be helpful for certain patients:
Alpha-glucosidase inhibitors delay the absorption of glucose from the proximal to distal GI tract, thereby reducing postprandial hyperglycemia. Flatulence and leakage of stool—the most common adverse effects—have limited their use in the United States.
Bile acid sequestrants (colesevelam) treat both hyperlipidemia and diabetes. The medications work by reducing glucose absorption from the GI tract. They reduce postprandial hyperglycemia, with a low risk of hypoglycemia. Colesevelam’s use is limited, however, because of the number of pills needed (6 daily).
Bromocriptine affects satiety levels via the central nervous system, and is available in a specific formulation for the treatment of diabetes. “First-dose” hypotension, however, is an adverse effect of considerable concern.1
Pramlintide, an injectable amylin mimetic given to patients on prandial insulin, can reduce postprandial glucose levels. The most common adverse effects are upper GI symptoms and hypoglycemia. Due to the adverse effects and the need for an injection with each meal, pramlintide is used infrequently.
Cardiovascular risk reduction
Has the ADA revised its recommendations for cardiovascular disease risk management?
Yes. There have been several changes. The first is in terminology, with atherosclerotic cardiovascular disease (ASCVD) replacing CVD alone. While new recommendations for statin therapy for adults older than 40 years (TABLE 2)1 were also added, the emphasis remains on therapeutic lifestyle change as an effective treatment for hypertension. These modifications should include at least 150 minutes of moderate physical activity per week and, for most patients, a reduction in total calories, saturated fat, and sodium.
It is important to remind patients that to maximize the benefits in terms of treating hyperglycemia, hypertension, and dyslipidemia, such changes must be maintained over the long term.
Aspirin therapy. The ADA also revised its recommendation regarding aspirin therapy. Based on new evidence in the treatment of women with ASCVD risk, the standards now call for considering aspirin therapy (75-162 mg/d) in both women and men ≥50 years as a primary prevention strategy for those with type 1 or type 2 diabetes with a 10-year ASCVD risk of >10%. (The previous standards recommended this only for women older than 60 years.)
Antiplatelet therapy is now recommended for patients younger than 50 years with multiple risk factors, and as secondary prevention in those with a history of ASCVD.19-21
Hypertension. The ADA’s recommendations for treating hypertension in patients with diabetes have not changed; the goal remains <140/<90 mm Hg. Lower targets may be appropriate for younger patients, those with albuminuria, and individuals with additional CVD risk factors; however, systolic pressure <130 mm Hg has not been shown to reduce CVD outcomes, and diastolic pressure <70 mm Hg has been associated with higher mortality.22
Optimal medication and lifestyle therapy are important to achieve goals, with avoidance of undue treatment burden. Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), but not both, should be included as part of treatment. Other agents, such as a thiazide diuretic, may be needed to achieve individual goals. Serum creatinine/eGFR and serum potassium levels should be monitored with the use of diuretics.
Lipids. The 2016 standards include notable changes in lipid management. The ADA sees a role for ezetimibe for select patients, based on studies such as the IMPROVE IT trial23 that included participants with diabetes. The ADA also added a table highlighting statin recommendations and delineating high and moderate-intensity statins (TABLE 3).1 Those younger than 40 years with no other risk factors may not need a statin, but patients ages 40 or older will need moderate- to high-intensity statin therapy to effectively lower ASCVD risk.24-28
These recommendations reflect a comprehensive plan to reduce ASCVD in this at-risk population, which should also include lifestyle modification, including smoking prevention and quit strategies, as needed.
Microvascular complications
DIABETIC KIDNEY DISEASE
How should I diagnose nephropathy?
The ADA changed the terminology, referring to “diabetic kidney disease” (DKD) rather than nephropathy to highlight the fact that the focus is on kidney disease directly linked to diabetes.
Other recommendations include an annual assessment of urinary albumin (eg, spot urine albumin-to-creatinine ratio and eGFR) for patients who have had type 1 diabetes for ≥5 years and all patients who have type 2 diabetes. Two out of 3 abnormal specimens collected within a 3- to 6-month period indicate the presence of albuminuria.
What can be done to prevent or slow the progression of DKD?
Optimal BP and glycemic control are key,29-35 along with diet and medication. For patients with DKD, dietary protein intake should be 0.8 g/kg body weight per day. ACE inhibitors and ARBs have been shown to slow the decline in eGFR in patients with elevated urinary albumin excretion (≥30 mg/day).
However, neither an ACE inhibitor nor an ARB is recommended for the primary prevention of DKD in patients who have normal BP, normal urine albumin-to-creatinine ratio (<30 mg/g), and normal eGFR. In addition, combined use of an ACE inhibitor and an ARB should be avoided, as it provides no additional benefit and increases the risk of adverse effects.29
RETINOPATHY
How should I manage retinopathy in patients with diabetes?
As with the management of DKD, it is important to optimize glycemic and BP control to reduce the risk, or slow the progression, of retinopathy. Intensive diabetes management, with the goal of achieving near-normal glycemic levels, has been shown in large prospective randomized studies to prevent or delay the onset and progression of diabetic retinopathy.33,36 The presence of retinopathy is not a contraindication to aspirin therapy for ASCVD prevention, as aspirin does not increase the risk of retinal hemorrhage.
When should patients with diabetes be screened for retinopathy?
Patients with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years of the onset of diabetes. Those with type 2 diabetes should have such an exam shortly after diagnosis. The exam should be repeated annually; if there is no evidence of retinopathy, however, 2-year intervals may be considered.
PERIPHERAL NEUROPATHY
When and how should I screen patients with diabetes for neuropathy?
All patients should be screened for diabetic peripheral neuropathy (DPN) starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes, and continued at least annually thereafter. Assessment should include a detailed history and 10-g monofilament testing, as well as at least one of the following tests: pinprick, temperature, and vibration sensation.
It is important, too, to screen patients with more advanced diabetes for signs and symptoms of autonomic neuropathy. Signs and symptoms may include resting tachycardia, exercise intolerance, orthostatic hypotension, gastroparesis, constipation, impaired neurovascular function, and autonomic failure in response to hypoglycemia. In men, diabetic autonomic neuropathy may cause erectile dysfunction and/or retrograde ejaculation.
How should I manage patients who have DPN?
Tight glycemic control is the only measure that has been shown to prevent or delay the development of DPN or cardiac autonomic neuropathy in patients with type 1 diabetes,37,38 and to slow the progression of neuropathy in some patients with type 2 diabetes.39
The FDA has approved pregabalin, duloxetine, and tapentadol for the treatment of pain associated with DPN. Tricyclic antidepressants, gabapentin, venlafaxine, carbamazepine, tramadol, and topical capsaicin, although not approved for the treatment of painful DPN, may also be effective in treating neuropathic pain.
For those with autonomic neuropathy, dietary changes and prokinetic agents such as erythromycin may alleviate gastroparesis. Due to extrapyramidal adverse effects, metoclopramide is reserved for the most severe and unresponsive cases. Recurrent urinary tract infections, pyelonephritis, incontinence, or palpable bladder should prompt an evaluation for bladder dysfunction. Controlling lipids and BP, quitting smoking, and making other lifestyle changes can reduce both the development and the progression of autonomic neuropathy.
FOOT CARE/PERIPHERAL ARTERIAL DISEASE
What does the ADA recommend regarding foot care for patients with diabetes?
The ADA’s standards recommend an annual comprehensive foot examination to identify risk factors predictive of ulcers and potential amputations. The exam should start with inspection and assessment of foot pulses and should seek to identify loss of peripheral sensation. The examination should include inspection of the skin, assessment of foot deformities, neurologic assessment including 10-g monofilament testing and pinprick or vibration testing or assessment of ankle reflexes, and vascular assessment, including pulses in the legs and feet.40
It is also important to screen patients for peripheral arterial disease (PAD), with a comprehensive medical history and physical exam of pulses. Ankle-brachial index testing (ABI) should be performed in patients with signs or symptoms of PAD, including claudication or skin and hair changes in the lower extremities. ABI may be considered for all patients with diabetes starting at age 50 and in those younger than 50 years who have risk factors.41
Which patients with diabetes are at higher risk for foot complications?
The following are risk factors for foot complications: previous amputation, prior foot ulcer, peripheral neuropathy, foot deformity, peripheral vascular disease, visual impairment, peripheral neuropathy (especially if on dialysis), poor glycemic control, and smoking. Patients with high-risk foot conditions should be educated about their risk and appropriate management.
A well-fitted walking shoe that cushions the feet and redistributes pressure is one option to help patients. Patients with bony deformities may need extra wide or deep shoes and patients with more advanced disease may need custom-fitted shoes.
When should patients be referred to a foot specialist?
Refer patients to a foot care specialist for ongoing preventive care and lifelong surveillance if they smoke or have a history of lower-extremity complications, a loss of protective sensation, structural abnormalities, or PAD.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/site/misc/2016-Standards-of-Care.pdf. Accessed March 28, 2016.
2. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334.
3. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362.
4. Vijan S, Sussman JB, Yudkin JS, et al. Effect of patients’ risks and p on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–1234.
5. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
6. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737.
7. UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905–912.
8. Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415.
9. Pastors JG, Warshaw H, Daly A, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–613.
10. Selph S, Dana T, Bougatsos C, et al. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 US Preventive Services Task Force Recommendation. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed March 28, 2016.
11. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and metaanalysis. Obesity (Silver Spring). 2006;14:1283–1293.
12. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;99:14–23.
13. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149.
14. Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195.
15. Balaji V, Seshiah V, Ashtalakshmi G, et al. Retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–427.
16. Kuo HW, Tiao MM, Ho SC, et al. Pioglitazone use and the risk of bladder cancer. Kaohsiung J Med Sci. 2014;30:94–97.
17. Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254-259.
18. US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed December 11, 2015.
19. Huxley RR, Peters SAE, Mishra GD, et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206.
20. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551.
21. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973-1980.
22. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
23. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397.
24. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485.
25. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696.
26. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504.
27. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316.
28. Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080.
29. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713.
30. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an American Diabetes Association Consensus Conference. Diabetes Care. 2014;37:2864–2883.
31. The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720.
32. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
33. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
34. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
35. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430.
36. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559.
37. Chew EY, Ambrosius WT, Davis MD, et al; ACCORD Study Group; ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244.
38. Ang L, Jaiswal M, Martin C, et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14:528.
39. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group. Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:31–38.
40. Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765.
41. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341.
42. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Available at: http://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed April 8, 2016.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/site/misc/2016-Standards-of-Care.pdf. Accessed March 28, 2016.
2. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334.
3. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362.
4. Vijan S, Sussman JB, Yudkin JS, et al. Effect of patients’ risks and p on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–1234.
5. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
6. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737.
7. UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905–912.
8. Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415.
9. Pastors JG, Warshaw H, Daly A, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–613.
10. Selph S, Dana T, Bougatsos C, et al. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 US Preventive Services Task Force Recommendation. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed March 28, 2016.
11. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and metaanalysis. Obesity (Silver Spring). 2006;14:1283–1293.
12. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;99:14–23.
13. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149.
14. Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195.
15. Balaji V, Seshiah V, Ashtalakshmi G, et al. Retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–427.
16. Kuo HW, Tiao MM, Ho SC, et al. Pioglitazone use and the risk of bladder cancer. Kaohsiung J Med Sci. 2014;30:94–97.
17. Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254-259.
18. US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed December 11, 2015.
19. Huxley RR, Peters SAE, Mishra GD, et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206.
20. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551.
21. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973-1980.
22. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
23. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397.
24. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485.
25. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696.
26. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504.
27. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316.
28. Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080.
29. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713.
30. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an American Diabetes Association Consensus Conference. Diabetes Care. 2014;37:2864–2883.
31. The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720.
32. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
33. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
34. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
35. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430.
36. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559.
37. Chew EY, Ambrosius WT, Davis MD, et al; ACCORD Study Group; ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244.
38. Ang L, Jaiswal M, Martin C, et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14:528.
39. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group. Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:31–38.
40. Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765.
41. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341.
42. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Available at: http://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed April 8, 2016.