User login
Goodbye, finger sticks; hello, CGMs
Nearly 90% of diabetes management in the United States is provided by primary care clinicians; diabetes is the fifth most common reason for a primary care visit. State-of-the-art technology such as continuous glucose monitors (CGMs) will inevitably transform the management of diabetes in primary care. Clinicians and staff must be ready to educate, counsel, and support primary care patients in the use of CGMs.
CGMs (also called glucose sensors) are small, minimally invasive devices that attach to the skin of the upper arm or trunk. A tiny electrode in the subcutaneous space prompts an enzyme reaction that measures the interstitial (rather than blood) glucose concentration, typically every 5 minutes. The results are displayed on an accompanying reader or transmitted to an app on the user’s mobile phone.
CGMs could eliminate the need for finger-stick blood glucose testing, which until now, has been the much-despised gold standard for self-monitoring of glucose levels in diabetes. Despite being relatively inexpensive and accurate, finger-stick glucose tests are inconvenient and often painful. But of greater significance is this downside: Finger-stick monitoring reveals the patient’s blood glucose concentration at a single point in time, which can be difficult to interpret. Is the blood glucose rising or falling? Multiple finger-stick tests are required to determine the trend of a patient’s glucose levels or the response to food or exercise.
In contrast, the graphic display from a CGM sensor is more like a movie, telling a story as it unfolds. Uninterrupted data provide valuable feedback to patients about the effects of diet, physical activity, stress, or pain on their glucose levels. And for the first time, it’s easy to determine the proportion of time the patient spends in or out of the target glucose range.
Incorporating new technology into your practice may seem like a burden, but the reward is better information that leads to better management of diabetes. If you’re new to glucose sensors, many excellent resources are available to learn how to use them.
I recommend starting with a website called diabeteswise.org, which has both a patient-facing and clinician-facing version. This unbranded site serves as a kind of Consumer Reports for diabetes technology, allowing both patients and professionals to compare and contrast currently available CGM devices.
DiabetesWisePro has information ranging from CGM device fundamentals and best practices to CGM prescribing and reimbursement.
Clinical Diabetes also provides multiple tools to help incorporate these devices into primary care clinical practice, including:
• Continuous Glucose Monitoring: Optimizing Diabetes Care (CME course).
• Diabetes Technology in Primary Care.
The next article in this series will cover two types of CGMs used in primary care: professional and personal devices.
Dr. Shubrook is a professor in the department of primary care, Touro University California College of Osteopathic Medicine, Vallejo, Calif., and director of diabetes services, Solano County Family Health Services, Fairfield, Calif. He disclosed ties with Abbott, Astra Zeneca, Bayer, Nevro, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
Nearly 90% of diabetes management in the United States is provided by primary care clinicians; diabetes is the fifth most common reason for a primary care visit. State-of-the-art technology such as continuous glucose monitors (CGMs) will inevitably transform the management of diabetes in primary care. Clinicians and staff must be ready to educate, counsel, and support primary care patients in the use of CGMs.
CGMs (also called glucose sensors) are small, minimally invasive devices that attach to the skin of the upper arm or trunk. A tiny electrode in the subcutaneous space prompts an enzyme reaction that measures the interstitial (rather than blood) glucose concentration, typically every 5 minutes. The results are displayed on an accompanying reader or transmitted to an app on the user’s mobile phone.
CGMs could eliminate the need for finger-stick blood glucose testing, which until now, has been the much-despised gold standard for self-monitoring of glucose levels in diabetes. Despite being relatively inexpensive and accurate, finger-stick glucose tests are inconvenient and often painful. But of greater significance is this downside: Finger-stick monitoring reveals the patient’s blood glucose concentration at a single point in time, which can be difficult to interpret. Is the blood glucose rising or falling? Multiple finger-stick tests are required to determine the trend of a patient’s glucose levels or the response to food or exercise.
In contrast, the graphic display from a CGM sensor is more like a movie, telling a story as it unfolds. Uninterrupted data provide valuable feedback to patients about the effects of diet, physical activity, stress, or pain on their glucose levels. And for the first time, it’s easy to determine the proportion of time the patient spends in or out of the target glucose range.
Incorporating new technology into your practice may seem like a burden, but the reward is better information that leads to better management of diabetes. If you’re new to glucose sensors, many excellent resources are available to learn how to use them.
I recommend starting with a website called diabeteswise.org, which has both a patient-facing and clinician-facing version. This unbranded site serves as a kind of Consumer Reports for diabetes technology, allowing both patients and professionals to compare and contrast currently available CGM devices.
DiabetesWisePro has information ranging from CGM device fundamentals and best practices to CGM prescribing and reimbursement.
Clinical Diabetes also provides multiple tools to help incorporate these devices into primary care clinical practice, including:
• Continuous Glucose Monitoring: Optimizing Diabetes Care (CME course).
• Diabetes Technology in Primary Care.
The next article in this series will cover two types of CGMs used in primary care: professional and personal devices.
Dr. Shubrook is a professor in the department of primary care, Touro University California College of Osteopathic Medicine, Vallejo, Calif., and director of diabetes services, Solano County Family Health Services, Fairfield, Calif. He disclosed ties with Abbott, Astra Zeneca, Bayer, Nevro, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
Nearly 90% of diabetes management in the United States is provided by primary care clinicians; diabetes is the fifth most common reason for a primary care visit. State-of-the-art technology such as continuous glucose monitors (CGMs) will inevitably transform the management of diabetes in primary care. Clinicians and staff must be ready to educate, counsel, and support primary care patients in the use of CGMs.
CGMs (also called glucose sensors) are small, minimally invasive devices that attach to the skin of the upper arm or trunk. A tiny electrode in the subcutaneous space prompts an enzyme reaction that measures the interstitial (rather than blood) glucose concentration, typically every 5 minutes. The results are displayed on an accompanying reader or transmitted to an app on the user’s mobile phone.
CGMs could eliminate the need for finger-stick blood glucose testing, which until now, has been the much-despised gold standard for self-monitoring of glucose levels in diabetes. Despite being relatively inexpensive and accurate, finger-stick glucose tests are inconvenient and often painful. But of greater significance is this downside: Finger-stick monitoring reveals the patient’s blood glucose concentration at a single point in time, which can be difficult to interpret. Is the blood glucose rising or falling? Multiple finger-stick tests are required to determine the trend of a patient’s glucose levels or the response to food or exercise.
In contrast, the graphic display from a CGM sensor is more like a movie, telling a story as it unfolds. Uninterrupted data provide valuable feedback to patients about the effects of diet, physical activity, stress, or pain on their glucose levels. And for the first time, it’s easy to determine the proportion of time the patient spends in or out of the target glucose range.
Incorporating new technology into your practice may seem like a burden, but the reward is better information that leads to better management of diabetes. If you’re new to glucose sensors, many excellent resources are available to learn how to use them.
I recommend starting with a website called diabeteswise.org, which has both a patient-facing and clinician-facing version. This unbranded site serves as a kind of Consumer Reports for diabetes technology, allowing both patients and professionals to compare and contrast currently available CGM devices.
DiabetesWisePro has information ranging from CGM device fundamentals and best practices to CGM prescribing and reimbursement.
Clinical Diabetes also provides multiple tools to help incorporate these devices into primary care clinical practice, including:
• Continuous Glucose Monitoring: Optimizing Diabetes Care (CME course).
• Diabetes Technology in Primary Care.
The next article in this series will cover two types of CGMs used in primary care: professional and personal devices.
Dr. Shubrook is a professor in the department of primary care, Touro University California College of Osteopathic Medicine, Vallejo, Calif., and director of diabetes services, Solano County Family Health Services, Fairfield, Calif. He disclosed ties with Abbott, Astra Zeneca, Bayer, Nevro, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
Gestational diabetes: Optimizing Dx and management in primary care
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.
If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
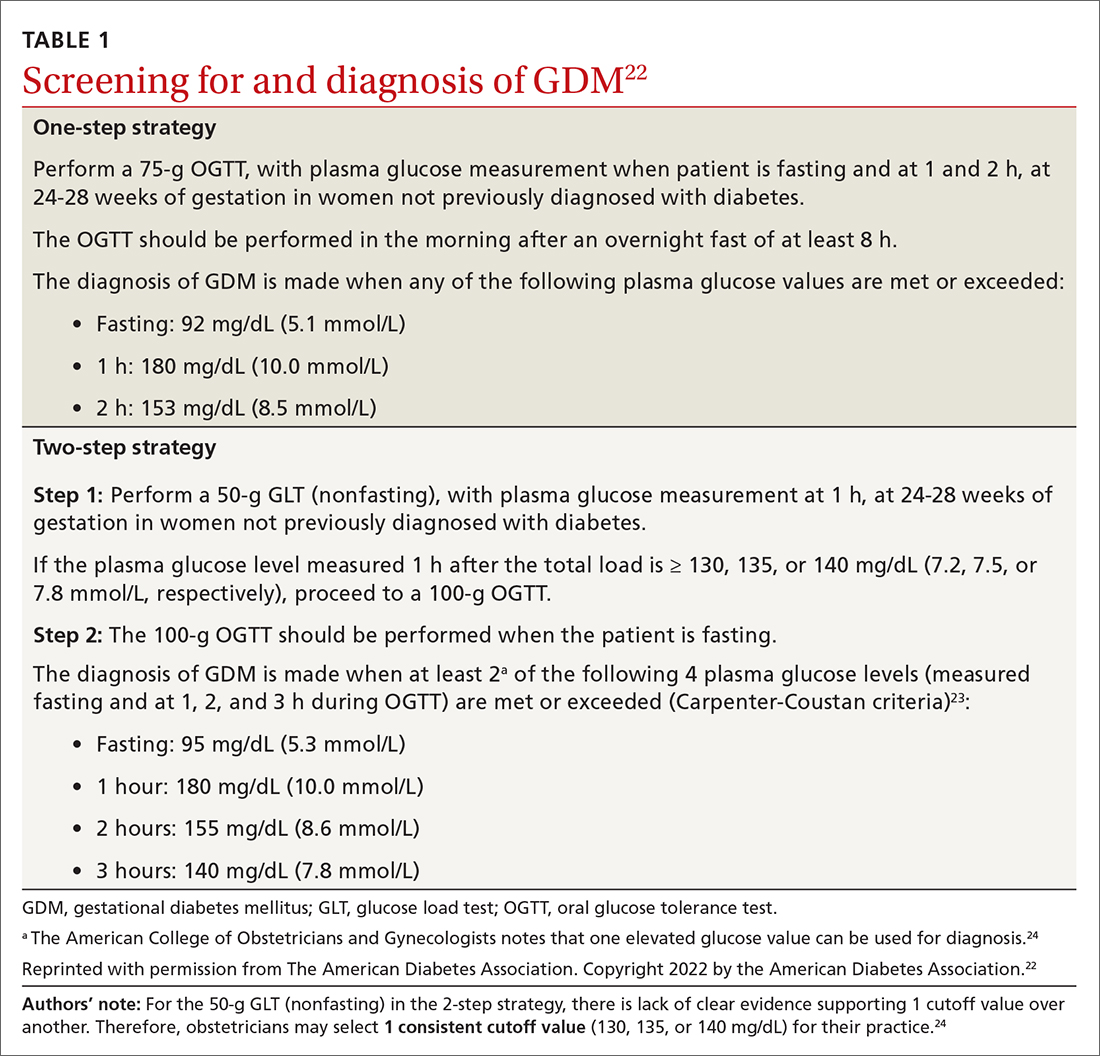
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24
Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49
CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; connie.ha@tu.edu
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.
If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24
Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49
CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; connie.ha@tu.edu
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.
If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24
Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49
CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; connie.ha@tu.edu
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
PRACTICE RECOMMENDATIONS
› Manage gestational diabetes mellitus (GDM) with lifestyle behavior changes first and add insulin as a secondary treatment only if glycemic targets are not being met. A
› Treat hyperglycemia in GDM with insulin, not metformin or glyburide; these agents cross the placenta to the fetus. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Reducing CV risk in diabetes: An ADA update
More than 29 million Americans have diabetes, and each year another 1.7 million are given the diagnosis.1 Prediabetes is even more common; over one-third of US adults ages 20 years and older, and more than half of those who are ages 65 and older, have attained this precursor status, representing another 86 million Americans.1
Because the evidence base for the management of diabetes is rapidly expanding, the American Diabetes Association’s (ADA) Professional Practice Committee updates its Standards of Medical Care in Diabetes annually to incorporate new evidence into its recommendations. The 2017 Standards of Care are available at: professional.diabetes.org/jfp.2
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality for people with diabetes, and is the largest contributor to the direct and indirect costs of the disease.2 As a result, all patients with diabetes should have cardiovascular (CV) risk factors, including dyslipidemia, hypertension, smoking, a family history of premature coronary disease, and the presence of albuminuria, assessed at least annually.2 Numerous studies have demonstrated the efficacy of controlling individual CV risk factors in preventing or slowing ASCVD in people with diabetes. Even larger benefits, including reduced ASCVD morbidity and mortality, can be achieved when multiple risk factors are addressed simultaneously.3
To hone your management of CV risks in patients with diabetes, we’ve put together this Q&A pointing out the elements of the ADA’s 2017 Standards of Care that are most relevant to the management of patients at risk for, or with established, ASCVD.
Screening
Since ASCVD so commonly co-occurs with diabetes, should I routinely screen asymptomatic patients with diabetes for heart disease?
No. The current evidence suggests that outcomes are NOT improved by screening people before they develop symptoms of ASCVD,4 and widespread ASCVD screening has not been shown to be cost-effective. Cardiac testing should be reserved for those with typical or atypical symptoms or those with an abnormal resting electrocardiogram (EKG).
Lifestyle modification
What are the benefits of lifestyle interventions?
The benefits include not only lost pounds, but improved mobility, physical and sexual functioning, and health-related quality of life. Recommend that all overweight patients with diabetes take advantage of intensive lifestyle interventions focusing on weight loss through decreased caloric intake and increased physical activity as per the Look AHEAD (Action for Health in Diabetes) trial.5 Although the intensive lifestyle intervention in the Look AHEAD trial did not decrease CV outcomes over 10 years of follow-up, it did improve control of CV risk factors and led to people in the intervention group taking fewer glucose-, blood pressure (BP)-, and lipid-lowering medications than those in the standard care group.
There is no one diet that is recommended for all people with diabetes. Weight reduction often requires intensive intervention. In order for weight loss diets to be sustainable, they must include patient preferences.
People with diabetes should be encouraged to receive individualized medical nutrition therapy (MNT), preferably from a registered dietitian who is well versed in nutritional management for diabetes. Such MNT is associated with a 0.5% to 2% decrease in A1c levels for people with type 2 diabetes.6-9 Specific healthy diets include the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and plant-based diets.
A new lifestyle recommendation in this year’s ADA Standards is that periods of prolonged sitting should be interrupted every 30 minutes with a period of physical activity. This appears to have glycemic benefits.2
Hypertension/BP management
When should I initiate hypertension treatment in patients with diabetes?
Nonpharmacologic therapy is reasonable in people with diabetes and mildly elevated BP (>120/80 mm Hg). If systolic blood pressure (SBP) is confirmed to be >140 mm Hg and/or diastolic blood pressure (DBP) is confirmed to be >90 mm Hg, the ADA recommends initiating pharmacologic therapy along with nonpharmacologic strategies. For patients with confirmed office-based BP >160/100 mm Hg, the ADA advises initiating lifestyle modifications as well as 2 pharmacologic medications (or a single pill combination of agents).2
What is the recommended BP target for patients with diabetes and hypertension?
These patients should be treated with a combination of measures, including lifestyle modification and pharmacologic therapy, to a target BP of <140/90 mm Hg. Randomized controlled trials (RCTs) have shown benefits with this target in terms of a reduction in the incidence of coronary heart disease (CHD) events, stroke, and diabetic kidney disease.10,11
A 2012 meta-analysis of randomized trials involving adults with type 2 diabetes mellitus (T2DM) and comparing intensive BP targets (≤130 mm Hg SBP and ≤80 mm Hg DBP) with standard targets (≤140-160 mm Hg SBP and ≤85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MIs associated with more intense BP control. There was a statistically significant 35% relative risk (RR) reduction in stroke with intensive targets, but lower BP was also associated with an increased risk of hypotension and syncope.12
The 2010 Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,13 which randomized 5518 patients with T2DM at high risk for ASCVD to either a target SBP of <120 mm Hg or 130 to 140 mm Hg, found that the patients with the lower SBP target did not benefit in the primary end point (a composite of nonfatal MI, nonfatal stroke, and CV death), but did benefit from nominally significant lower rates of total stroke and nonfatal stroke.
Based on these data, the ADA Standards of Care suggest that, “more intensive BP control may be reasonable in certain motivated, ACCORD-like patients (40-79 years of age with prior evidence of CVD or multiple CV risk factors) who have been educated about the added treatment burden, side effects, and costs of more intensive BP control and for patients who prefer to lower their risk of stroke beyond what can be achieved with usual care.”
Another major study, the 2015 Systolic Blood Pressure Intervention Trial (SPRINT) trial,14 demonstrated that treating patients with hypertension to a target SBP <120 mm Hg compared to the usual target of <140 mm Hg resulted in a 25% lower RR of the primary outcome (a composite of MI, other acute coronary syndromes, stroke, heart failure, or death from CV causes) and about a 25% reduction in all-cause mortality; however, people with diabetes were not included in the trial, so the applicability of the results to decisions about BP management in patients with diabetes is not known.
A 2015 systematic review and meta-analysis of over 100,000 participants looked at SBP lowering in adults with T2DM and found that each 10-mm Hg reduction in SBP was associated with a significantly lower risk of morbidity, CV events, CHD, stroke, albuminuria, and retinopathy.10 When trials were stratified by mean baseline SBP (<140 mm Hg or ≥140 mm Hg), RRs for outcomes other than stroke, retinopathy, and renal failure were lower in studies with greater baseline SBP.
The latest ADA Standards of Care recommend that a lower BP target of 130/80 mm Hg may be appropriate for patients at high risk of CVD if this target can be achieved without undue treatment burden. A DBP of <80 mm Hg may also be appropriate in certain patients including those with a long life expectancy, CKD, elevated urinary albumin excretion, and those with evidence of CVD or associated risk factors.15 Of note, treating older adults with diabetes to an SBP target of <130 mm Hg has not been shown to improve cardiovascular outcomes,16 and treating to a diastolic target of <70 mm Hg has been associated with a greater risk of mortality.17
What are the current recommended treatment options?
Treatment for hypertension in adults with diabetes without albuminuria should include any of the classes of medications demonstrated to reduce CV events in patients with diabetes, such as:
- angiotensin-converting enzyme (ACE) inhibitors,
- angiotensin receptor blockers (ARBs),
- thiazide-like diuretics, and
- dihydropyridine calcium channel blockers.
These recommendations are based on evidence suggesting the lack of superiority of ACE inhibitors and ARBs over other classes of antihypertensive agents for the prevention of CV outcomes in all patients with diabetes.18 However, in people with diabetes at high risk for ASCVD and/or with albuminuria, ACE inhibitors and ARBs do reduce ASCVD outcomes and the progression of kidney disease.19-24 Thus, ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and urine albumin/creatinine ratios ≥30 mg/g, as these medications are associated with a reduction in the rate of kidney disease progression.
The use of both an ACE inhibitor and an ARB in combination is not recommended.25,26 For patients treated with ACE inhibitors, ARBs, or diuretics, serum creatinine/estimated glomerular filtration rate (eGFR) and serum potassium levels should be monitored.
What are the recommended lifestyle modifications for patients with diabetes and hypertension?
Regular exercise and healthy eating are recommended for all people with diabetes to optimize glycemic control and lose weight (if they are overweight or obese). For patients with hypertension, the DASH diet (available at: https://www.nhlbi.nih.gov/health/health-topics/topics/dash/) is effective at lowering BP. The DASH diet emphasizes reducing sodium intake, increasing potassium intake, limiting alcohol intake, and increasing physical activity. Specifically, sodium intake should be restricted to <2300 mg/d and patients should consume approximately 8 to 10 servings of fruits and vegetables per day and 2 to 3 servings of low-fat dairy per day. Alcohol should be limited to 2 drinks per day for men and one drink per day for women.
Most adults with diabetes should perform 150 minutes per week of moderate to vigorous exercise, spread over at least 3 days/week. In addition, it is recommended that resistance exercises be performed at least 2 to 3 days/week. Prolonged inactivity is detrimental to health and should be interrupted with activity every 30 minutes.27
Finally, as a part of lifestyle management for all patients with diabetes, smoking cessation is important, as is attention to stress, depression, and anxiety.
Is there an advantage to nighttime dosing of antihypertensive medications?
Yes. Growing evidence suggests that there is an ASCVD benefit to avoiding nocturnal BP dipping. A 2011 RCT of 448 participants with T2DM and hypertension showed a decrease in CV events and mortality during 5.4 years of follow-up if at least one antihypertensive medication was taken at bedtime.28 As a result of this and other evidence,29 consider administering one or more antihypertensive medications at bedtime, although this is not a formal recommendation in the ADA Standards of Care.
Are there any additional issues to be aware of when treating patients with diabetes and hypertension?
Yes. Sometimes patients who have had diabetes for many years have significant orthostatic hypotension secondary to autonomic neuropathy. Postural changes in BP and pulse may require adjustment of BP targets. Home BP self-monitoring and 24-hour ambulatory BP monitoring may indicate white-coat or masked hypertension.
Lipid management
What is the current evidence for lipid treatment in diabetes?
Lipid abnormalities are common in people with diabetes and contribute to the overall high risk of ASCVD in these patients. Subgroup analyses of patients in large trials with diabetes30 and trials involving patients with diabetes31 have shown significant improvements in primary and secondary prevention of ASCVD with statin use. A 2008 meta-analysis of 18,686 people with diabetes showed a 9% reduction in all-cause mortality and a 13% reduction in vascular mortality for each 39-mg/dL reduction in low-density lipoprotein (LDL) cholesterol.32 Absolute reductions in mortality are greatest in those with highest risk, but the benefits of statin therapy are clear for low- and moderate-risk individuals with diabetes, too.33,34 As a result, statins are the medications of choice for lipid lowering and CV risk reduction and should be used in addition to lifestyle management.
Who should get a statin, and how do I choose the optimum dosage?
Patients ages 40 to 75 years with diabetes but without additional ASCVD risk factors should receive a moderate-intensity statin, according to the ADA (see TABLES 12 and 22). For those with additional CV risk factors, a high-intensity statin should be considered. The American College of Cardiology/American Heart Association ASCVD risk calculator (available at: http://www.cvriskcalculator.com/) may be useful for some patients, but generally, risk is already known to be high for most patients with diabetes. For patients of all ages with diabetes and established ASCVD, high-intensity statin therapy should be added to lifestyle modifications.35-37
For patients with diabetes who are <40 years with additional ASCVD risk factors, few clinical trial data exist; nevertheless, consider a moderate- or high-intensity statin and lifestyle therapy. Similarly, for patients >75 years who have diabetes and no additional ASCVD risk factors, consider a moderate-intensity statin and lifestyle modifications. For older adults with additional ASCVD risk factors, consider high-intensity statin therapy.35-37
Statins and cognition. It should be noted that published data have not demonstrated an adverse effect of statins on cognition.38 Statins, however, have been linked to an increased risk of developing diabetes,39,40 although the absolute increase in risk is small, and much smaller than the benefit derived from preventing the development of coronary disease.
Should total cholesterol and LDL levels be used as targets with statin treatment?
No. Statin doses have primarily been tested against placebo in clinical trials, rather than testing to specific target LDL levels, suggesting that the initiation and intensification of statin therapy be based on a patient’s risk profile.35 When maximally tolerated doses of statins do not lower LDL cholesterol by more than 30% from the patient’s baseline, there is currently no good evidence that combination therapy would be helpful, so regular monitoring of lipid levels has limited value. A lipid profile that includes levels of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides should be obtained at initial medical evaluation, at diagnosis of diabetes, and every 5 years thereafter or before the initiation of statin therapy. Ongoing testing may be appropriate in individual circumstances and to monitor for adherence to, or efficacy of, therapy.
What should I do for my patients who can’t tolerate statins?
Try a lower dose or a different statin before eliminating the class. Research has shown that even small doses (eg, rosuvastatin 5 mg) have some benefit.41
How do combination treatments figure into the current treatment of lipids in patients with diabetes?
It depends on the agent and the patient’s profile.
Fenofibrate. The ADA does not recommend automatically adding fenofibrate to statin therapy because the combination is associated with increased risks for abnormal transaminase levels, myositis, and rhabdomyolysis. In the ACCORD trial, the combination of fenofibrate and simvastatin did not reduce the rate of fatal CV events, nonfatal MIs, or nonfatal strokes compared with simvastatin alone.42
That said, a subgroup analysis suggested a benefit for men with both a triglyceride level ≥204 mg/dL (2.3 mmol/L) and an HDL cholesterol level ≤34 mg/dL (0.9 mmol/L).42 For this reason, the combination of a statin and fenofibrate may be considered for men who meet these laboratory parameters. In addition, consider medical therapy for triglyceride levels ≥500 mg/dL to reduce the risk of pancreatitis.
Ezetimibe. Recommendations regarding ezetimibe are based on the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), a 2015 RCT including over 18,000 patients that compared treatment with ezetimibe and simvastatin to simvastatin alone.43 Individuals in the trial were ≥50 years of age and had experienced an ACS within the preceding 10 days. In those with diabetes, the combination of moderate-intensity simvastatin (40 mg) and ezetimibe (10 mg) significantly reduced major adverse CV events with an absolute risk reduction of 5% (40% vs 45%) and an RR reduction of 14% over moderate-intensity simvastatin (40 mg) alone.
Based on these results, patients with diabetes and a recent ACS should be considered for combination therapy with ezetimibe and a moderate-intensity statin. The combination should also be considered in patients with diabetes and a history of ASCVD who cannot tolerate high-intensity statins.43
Niacin. The ADA currently does not recommend niacin in combination with a statin because of lack of efficacy on major ASCVD outcomes, possible increased risk of ischemic stroke, and adverse effects.44
What are the recommendations for the use of PCSK-9 inhibitors?
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors (ie, evolucumab and alirocumab) may be considered as adjunctive therapy to statins for patients with diabetes at high risk for ASCVD events who require additional lowering of LDL cholesterol. They may also be considered for those in whom high-intensity statin therapy is indicated, but not tolerated.
Antiplatelet agents
Who should take aspirin for primary prevention of CVD?
Both women and men ages ≥50 years who have diabetes and at least one additional CV risk factor (family history of premature ASCVD, hypertension, tobacco use, dyslipidemia, or albuminuria) should consider taking daily aspirin therapy (75-162 mg/d) if they do not have an excessive bleeding risk.45,46 The most common dose in the United States is 81 mg. This recommendation is supported by a 2010 consensus statement of the American Diabetes Association, American Heart Association, and the American College of Cardiology.47
Should patients with diabetes and heart disease receive antiplatelet therapy?
Yes. The evidence is clear that people with known diabetes and ASCVD benefit from aspirin therapy, according to the 2017 Standards of Care. Clopidogrel 75 mg/d is an appropriate alternative for patients who are allergic to aspirin. Dual antiplatelet therapy (a P2Y12 receptor antagonist and aspirin) should be used for as long as one year after an ACS and may have benefits beyond this period.48
Established heart disease
Are there specific recommendations for patients with diabetes and CHD?
According to the ADA Standards, there is good evidence that both aspirin and statin therapy are beneficial for patients with known ASCVD, and that high-intensity statin therapy should be used. In addition, consider ACE inhibitors to reduce the future risk of CV events. In patients with a prior MI, continue beta-blocker therapy for at least 2 years post event.49
Which medications should I avoid, or approach with caution, in patients with congestive heart failure (CHF)?
Thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, and metformin all require careful attention. This is especially important to know when you consider that almost half of all patients with T2DM will develop heart failure.50
Thiazolidinediones. The 2017 Standards of Care state that patients with diabetes and symptomatic congestive heart failure should not receive thiazolidinediones, as they can worsen heart failure status via fluid retention. As such, they are contraindicated in patients with class III and IV heart failure.51
DPP-4 inhibitors. The studies on DPP-4 inhibitors and heart failure have had mixed results. The 2013 SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) 53 trial52 showed that patients treated with saxagliptin were more likely to be hospitalized for heart failure than those taking placebo (3.5% vs 2.8%, respectively). However, the 2015 EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care)53 trial and the 2015 TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin)54 trial evaluated heart failure and mortality outcomes in patients with alogliptin and sitagliptin, respectively, compared to placebo, and did not show a relationship to heart failure.
Metformin may be used in people who have T2DM and stable CHF if their eGFR remains >30 mL/min; it should be withheld from patients with unstable heart failure and those who are hospitalized with CHF.
Are there antihyperglycemic medications that reduce CV morbidity and mortality in those with established ASCVD?
Yes. This year’s ADA Standards indicate that certain glucose-lowering medications—specifically empagliflozin (a sodium–glucose cotransporter [SGLT]-2 inhibitor) and liraglutide (a glucagon-like peptide [GLP]-1 receptor agonist)—have been shown to be beneficial for those with established CVD. According to the 2017 Standards of Care, “In patients with longstanding suboptimally controlled T2DM and established ASCVD, empagliflozin or liraglutide should be considered, as they have been shown to reduce CV and all-cause mortality when added to standard care.”2 The studies that provide support for their use are summarized below. Ongoing studies are investigating the CV effects of other agents in these drug classes.
Empagliflozin. The 2015 EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study55 was a randomized double-blind study of empagliflozin vs placebo and usual care in patients with diabetes and established CVD. Over a median follow-up of 3.1 years, treatment with empagliflozin reduced the aggregate outcome of MI, stroke, and CV death by 14%, reduced CV deaths by 38%, and decreased deaths from any cause by 32%. In December 2016, the FDA announced a new indication for empagliflozin: to reduce the risk of CV death in adult patients with T2DM and CVD.56
Liraglutide. The LEADER (Liraglutide Effect and Action in Diabetes Evaluation of Cardiovascular Outcome Results: A Long Term Evaluation) trial57 was a double-blind randomized trial of liraglutide vs placebo added to usual care in patients with T2DM at high risk for CVD or with existing CVD. More than 80% of the participants had existing CVD including a history of prior MI, cerebrovascular disease, or peripheral vascular disease. After a median follow-up of 3.8 years, the group taking liraglutide demonstrated a 13% reduction in the composite outcome of MI, stroke, or CV death, a 22% reduction in CV death, and a 15% reduction in death from any cause, compared with placebo.57
CORRESPONDENCE
Neil Skolnik, MD, Abington-Jefferson Health, 500 Old York Rd, Ste 108, Jenkintown, PA 19046; nskolnik@comcast.net.
The authors thank Sarah Bradley, director, professional engagement & collaboration at the American Diabetes Association, for her editorial and organizational assistance in the preparation of this manuscript.
1. Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. Available at: http://templatelab.com/national-diabetes-report-2014/. Accessed April 7, 2017.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Available at: http://professional.diabetes.org/sites/professional.diabetes.org/files/media/dc_40_s1_final.pdf. Accessed April 7, 2017.
3. Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591.
4. Bax JJ, Young LH, Frye RL, et al; American Diabetes Association. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007;30:2729-2736.
5. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154.
6. UK Prospective Diabetes Study (UKDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKDS 34). Lancet. 1998;352:854-865.
7. Ziemer DC, Berkowitz KJ, Panayioto RM, et al. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes. Diabetes Care. 2003;26:1719-1724.
8. Wolf AM, Conaway RM, Crowther JQ, et al; Improving Control with Activity and Nutrition (ICAN) Study. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570-1576.
9. Coppell KJ, Kataoka M, Williams SM, et al. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment-Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. 2010;341:c3337.
10. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603-615.
11. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;10:CD008277.
12. McBrien K, Rabi DM, Campbell N, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2012;172:1296-1303.
13. ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
14. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
15. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
16. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
17. Anderson RJ, Bahn GD, Moritz TE, et al; VADT Study Group. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care. 2011;34:34-38.
18. Bangalore S, Fakheri R, Toklu B, et al. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ. 2016;352:i438.
19. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253-259.
20. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772-776.
21. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
22. Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759-766.
23. Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
24. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047-2056.
25. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
26. Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
27. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079.
28. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
29. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011;10:CD004184.
30. Py̆orälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
31. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care. 2006;29:1478-1485.
32. Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117-125.
33. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816.
34. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610.
35. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
36. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
37. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
38. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688-697.
39. Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924-1929.
40. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
41. Meek C, Wierzbicki AS, Jewkes C, et al. Daily and intermittent rosuvastatin 5 mg therapy in statin intolerant patients: an observational study. Curr Med Res Opin. 2012;28:371-378.
42. ACCORD Study Group, Ginsberg HN, Bam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
43. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
44. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
45. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860.
46. Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635-1701.
47. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes. A position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
48. Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
49. Kezerashvilli A, Marzo K, De Leon J. Beta blocker use after acute myocardial infarction in the patient with normal systolic function: when is it “ok” to discontinue? Curr Cardiol Rev. 2012;8:77-84.
50. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34.
51. Pioglitazone Package Insert. Available at: http://medlibrary.org/lib/rx/meds/pioglitazone-3/. Accessed April 10, 2017.
52. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
53. Zannad F, Cannon CP, Cushman WC, et al; EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
54. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
55. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
56. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. FDA News Release, December 2, 2016. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed February 9, 2017.
57. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
More than 29 million Americans have diabetes, and each year another 1.7 million are given the diagnosis.1 Prediabetes is even more common; over one-third of US adults ages 20 years and older, and more than half of those who are ages 65 and older, have attained this precursor status, representing another 86 million Americans.1
Because the evidence base for the management of diabetes is rapidly expanding, the American Diabetes Association’s (ADA) Professional Practice Committee updates its Standards of Medical Care in Diabetes annually to incorporate new evidence into its recommendations. The 2017 Standards of Care are available at: professional.diabetes.org/jfp.2
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality for people with diabetes, and is the largest contributor to the direct and indirect costs of the disease.2 As a result, all patients with diabetes should have cardiovascular (CV) risk factors, including dyslipidemia, hypertension, smoking, a family history of premature coronary disease, and the presence of albuminuria, assessed at least annually.2 Numerous studies have demonstrated the efficacy of controlling individual CV risk factors in preventing or slowing ASCVD in people with diabetes. Even larger benefits, including reduced ASCVD morbidity and mortality, can be achieved when multiple risk factors are addressed simultaneously.3
To hone your management of CV risks in patients with diabetes, we’ve put together this Q&A pointing out the elements of the ADA’s 2017 Standards of Care that are most relevant to the management of patients at risk for, or with established, ASCVD.
Screening
Since ASCVD so commonly co-occurs with diabetes, should I routinely screen asymptomatic patients with diabetes for heart disease?
No. The current evidence suggests that outcomes are NOT improved by screening people before they develop symptoms of ASCVD,4 and widespread ASCVD screening has not been shown to be cost-effective. Cardiac testing should be reserved for those with typical or atypical symptoms or those with an abnormal resting electrocardiogram (EKG).
Lifestyle modification
What are the benefits of lifestyle interventions?
The benefits include not only lost pounds, but improved mobility, physical and sexual functioning, and health-related quality of life. Recommend that all overweight patients with diabetes take advantage of intensive lifestyle interventions focusing on weight loss through decreased caloric intake and increased physical activity as per the Look AHEAD (Action for Health in Diabetes) trial.5 Although the intensive lifestyle intervention in the Look AHEAD trial did not decrease CV outcomes over 10 years of follow-up, it did improve control of CV risk factors and led to people in the intervention group taking fewer glucose-, blood pressure (BP)-, and lipid-lowering medications than those in the standard care group.
There is no one diet that is recommended for all people with diabetes. Weight reduction often requires intensive intervention. In order for weight loss diets to be sustainable, they must include patient preferences.
People with diabetes should be encouraged to receive individualized medical nutrition therapy (MNT), preferably from a registered dietitian who is well versed in nutritional management for diabetes. Such MNT is associated with a 0.5% to 2% decrease in A1c levels for people with type 2 diabetes.6-9 Specific healthy diets include the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and plant-based diets.
A new lifestyle recommendation in this year’s ADA Standards is that periods of prolonged sitting should be interrupted every 30 minutes with a period of physical activity. This appears to have glycemic benefits.2
Hypertension/BP management
When should I initiate hypertension treatment in patients with diabetes?
Nonpharmacologic therapy is reasonable in people with diabetes and mildly elevated BP (>120/80 mm Hg). If systolic blood pressure (SBP) is confirmed to be >140 mm Hg and/or diastolic blood pressure (DBP) is confirmed to be >90 mm Hg, the ADA recommends initiating pharmacologic therapy along with nonpharmacologic strategies. For patients with confirmed office-based BP >160/100 mm Hg, the ADA advises initiating lifestyle modifications as well as 2 pharmacologic medications (or a single pill combination of agents).2
What is the recommended BP target for patients with diabetes and hypertension?
These patients should be treated with a combination of measures, including lifestyle modification and pharmacologic therapy, to a target BP of <140/90 mm Hg. Randomized controlled trials (RCTs) have shown benefits with this target in terms of a reduction in the incidence of coronary heart disease (CHD) events, stroke, and diabetic kidney disease.10,11
A 2012 meta-analysis of randomized trials involving adults with type 2 diabetes mellitus (T2DM) and comparing intensive BP targets (≤130 mm Hg SBP and ≤80 mm Hg DBP) with standard targets (≤140-160 mm Hg SBP and ≤85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MIs associated with more intense BP control. There was a statistically significant 35% relative risk (RR) reduction in stroke with intensive targets, but lower BP was also associated with an increased risk of hypotension and syncope.12
The 2010 Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,13 which randomized 5518 patients with T2DM at high risk for ASCVD to either a target SBP of <120 mm Hg or 130 to 140 mm Hg, found that the patients with the lower SBP target did not benefit in the primary end point (a composite of nonfatal MI, nonfatal stroke, and CV death), but did benefit from nominally significant lower rates of total stroke and nonfatal stroke.
Based on these data, the ADA Standards of Care suggest that, “more intensive BP control may be reasonable in certain motivated, ACCORD-like patients (40-79 years of age with prior evidence of CVD or multiple CV risk factors) who have been educated about the added treatment burden, side effects, and costs of more intensive BP control and for patients who prefer to lower their risk of stroke beyond what can be achieved with usual care.”
Another major study, the 2015 Systolic Blood Pressure Intervention Trial (SPRINT) trial,14 demonstrated that treating patients with hypertension to a target SBP <120 mm Hg compared to the usual target of <140 mm Hg resulted in a 25% lower RR of the primary outcome (a composite of MI, other acute coronary syndromes, stroke, heart failure, or death from CV causes) and about a 25% reduction in all-cause mortality; however, people with diabetes were not included in the trial, so the applicability of the results to decisions about BP management in patients with diabetes is not known.
A 2015 systematic review and meta-analysis of over 100,000 participants looked at SBP lowering in adults with T2DM and found that each 10-mm Hg reduction in SBP was associated with a significantly lower risk of morbidity, CV events, CHD, stroke, albuminuria, and retinopathy.10 When trials were stratified by mean baseline SBP (<140 mm Hg or ≥140 mm Hg), RRs for outcomes other than stroke, retinopathy, and renal failure were lower in studies with greater baseline SBP.
The latest ADA Standards of Care recommend that a lower BP target of 130/80 mm Hg may be appropriate for patients at high risk of CVD if this target can be achieved without undue treatment burden. A DBP of <80 mm Hg may also be appropriate in certain patients including those with a long life expectancy, CKD, elevated urinary albumin excretion, and those with evidence of CVD or associated risk factors.15 Of note, treating older adults with diabetes to an SBP target of <130 mm Hg has not been shown to improve cardiovascular outcomes,16 and treating to a diastolic target of <70 mm Hg has been associated with a greater risk of mortality.17
What are the current recommended treatment options?
Treatment for hypertension in adults with diabetes without albuminuria should include any of the classes of medications demonstrated to reduce CV events in patients with diabetes, such as:
- angiotensin-converting enzyme (ACE) inhibitors,
- angiotensin receptor blockers (ARBs),
- thiazide-like diuretics, and
- dihydropyridine calcium channel blockers.
These recommendations are based on evidence suggesting the lack of superiority of ACE inhibitors and ARBs over other classes of antihypertensive agents for the prevention of CV outcomes in all patients with diabetes.18 However, in people with diabetes at high risk for ASCVD and/or with albuminuria, ACE inhibitors and ARBs do reduce ASCVD outcomes and the progression of kidney disease.19-24 Thus, ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and urine albumin/creatinine ratios ≥30 mg/g, as these medications are associated with a reduction in the rate of kidney disease progression.
The use of both an ACE inhibitor and an ARB in combination is not recommended.25,26 For patients treated with ACE inhibitors, ARBs, or diuretics, serum creatinine/estimated glomerular filtration rate (eGFR) and serum potassium levels should be monitored.
What are the recommended lifestyle modifications for patients with diabetes and hypertension?
Regular exercise and healthy eating are recommended for all people with diabetes to optimize glycemic control and lose weight (if they are overweight or obese). For patients with hypertension, the DASH diet (available at: https://www.nhlbi.nih.gov/health/health-topics/topics/dash/) is effective at lowering BP. The DASH diet emphasizes reducing sodium intake, increasing potassium intake, limiting alcohol intake, and increasing physical activity. Specifically, sodium intake should be restricted to <2300 mg/d and patients should consume approximately 8 to 10 servings of fruits and vegetables per day and 2 to 3 servings of low-fat dairy per day. Alcohol should be limited to 2 drinks per day for men and one drink per day for women.
Most adults with diabetes should perform 150 minutes per week of moderate to vigorous exercise, spread over at least 3 days/week. In addition, it is recommended that resistance exercises be performed at least 2 to 3 days/week. Prolonged inactivity is detrimental to health and should be interrupted with activity every 30 minutes.27
Finally, as a part of lifestyle management for all patients with diabetes, smoking cessation is important, as is attention to stress, depression, and anxiety.
Is there an advantage to nighttime dosing of antihypertensive medications?
Yes. Growing evidence suggests that there is an ASCVD benefit to avoiding nocturnal BP dipping. A 2011 RCT of 448 participants with T2DM and hypertension showed a decrease in CV events and mortality during 5.4 years of follow-up if at least one antihypertensive medication was taken at bedtime.28 As a result of this and other evidence,29 consider administering one or more antihypertensive medications at bedtime, although this is not a formal recommendation in the ADA Standards of Care.
Are there any additional issues to be aware of when treating patients with diabetes and hypertension?
Yes. Sometimes patients who have had diabetes for many years have significant orthostatic hypotension secondary to autonomic neuropathy. Postural changes in BP and pulse may require adjustment of BP targets. Home BP self-monitoring and 24-hour ambulatory BP monitoring may indicate white-coat or masked hypertension.
Lipid management
What is the current evidence for lipid treatment in diabetes?
Lipid abnormalities are common in people with diabetes and contribute to the overall high risk of ASCVD in these patients. Subgroup analyses of patients in large trials with diabetes30 and trials involving patients with diabetes31 have shown significant improvements in primary and secondary prevention of ASCVD with statin use. A 2008 meta-analysis of 18,686 people with diabetes showed a 9% reduction in all-cause mortality and a 13% reduction in vascular mortality for each 39-mg/dL reduction in low-density lipoprotein (LDL) cholesterol.32 Absolute reductions in mortality are greatest in those with highest risk, but the benefits of statin therapy are clear for low- and moderate-risk individuals with diabetes, too.33,34 As a result, statins are the medications of choice for lipid lowering and CV risk reduction and should be used in addition to lifestyle management.
Who should get a statin, and how do I choose the optimum dosage?
Patients ages 40 to 75 years with diabetes but without additional ASCVD risk factors should receive a moderate-intensity statin, according to the ADA (see TABLES 12 and 22). For those with additional CV risk factors, a high-intensity statin should be considered. The American College of Cardiology/American Heart Association ASCVD risk calculator (available at: http://www.cvriskcalculator.com/) may be useful for some patients, but generally, risk is already known to be high for most patients with diabetes. For patients of all ages with diabetes and established ASCVD, high-intensity statin therapy should be added to lifestyle modifications.35-37
For patients with diabetes who are <40 years with additional ASCVD risk factors, few clinical trial data exist; nevertheless, consider a moderate- or high-intensity statin and lifestyle therapy. Similarly, for patients >75 years who have diabetes and no additional ASCVD risk factors, consider a moderate-intensity statin and lifestyle modifications. For older adults with additional ASCVD risk factors, consider high-intensity statin therapy.35-37
Statins and cognition. It should be noted that published data have not demonstrated an adverse effect of statins on cognition.38 Statins, however, have been linked to an increased risk of developing diabetes,39,40 although the absolute increase in risk is small, and much smaller than the benefit derived from preventing the development of coronary disease.
Should total cholesterol and LDL levels be used as targets with statin treatment?
No. Statin doses have primarily been tested against placebo in clinical trials, rather than testing to specific target LDL levels, suggesting that the initiation and intensification of statin therapy be based on a patient’s risk profile.35 When maximally tolerated doses of statins do not lower LDL cholesterol by more than 30% from the patient’s baseline, there is currently no good evidence that combination therapy would be helpful, so regular monitoring of lipid levels has limited value. A lipid profile that includes levels of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides should be obtained at initial medical evaluation, at diagnosis of diabetes, and every 5 years thereafter or before the initiation of statin therapy. Ongoing testing may be appropriate in individual circumstances and to monitor for adherence to, or efficacy of, therapy.
What should I do for my patients who can’t tolerate statins?
Try a lower dose or a different statin before eliminating the class. Research has shown that even small doses (eg, rosuvastatin 5 mg) have some benefit.41
How do combination treatments figure into the current treatment of lipids in patients with diabetes?
It depends on the agent and the patient’s profile.
Fenofibrate. The ADA does not recommend automatically adding fenofibrate to statin therapy because the combination is associated with increased risks for abnormal transaminase levels, myositis, and rhabdomyolysis. In the ACCORD trial, the combination of fenofibrate and simvastatin did not reduce the rate of fatal CV events, nonfatal MIs, or nonfatal strokes compared with simvastatin alone.42
That said, a subgroup analysis suggested a benefit for men with both a triglyceride level ≥204 mg/dL (2.3 mmol/L) and an HDL cholesterol level ≤34 mg/dL (0.9 mmol/L).42 For this reason, the combination of a statin and fenofibrate may be considered for men who meet these laboratory parameters. In addition, consider medical therapy for triglyceride levels ≥500 mg/dL to reduce the risk of pancreatitis.
Ezetimibe. Recommendations regarding ezetimibe are based on the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), a 2015 RCT including over 18,000 patients that compared treatment with ezetimibe and simvastatin to simvastatin alone.43 Individuals in the trial were ≥50 years of age and had experienced an ACS within the preceding 10 days. In those with diabetes, the combination of moderate-intensity simvastatin (40 mg) and ezetimibe (10 mg) significantly reduced major adverse CV events with an absolute risk reduction of 5% (40% vs 45%) and an RR reduction of 14% over moderate-intensity simvastatin (40 mg) alone.
Based on these results, patients with diabetes and a recent ACS should be considered for combination therapy with ezetimibe and a moderate-intensity statin. The combination should also be considered in patients with diabetes and a history of ASCVD who cannot tolerate high-intensity statins.43
Niacin. The ADA currently does not recommend niacin in combination with a statin because of lack of efficacy on major ASCVD outcomes, possible increased risk of ischemic stroke, and adverse effects.44
What are the recommendations for the use of PCSK-9 inhibitors?
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors (ie, evolucumab and alirocumab) may be considered as adjunctive therapy to statins for patients with diabetes at high risk for ASCVD events who require additional lowering of LDL cholesterol. They may also be considered for those in whom high-intensity statin therapy is indicated, but not tolerated.
Antiplatelet agents
Who should take aspirin for primary prevention of CVD?
Both women and men ages ≥50 years who have diabetes and at least one additional CV risk factor (family history of premature ASCVD, hypertension, tobacco use, dyslipidemia, or albuminuria) should consider taking daily aspirin therapy (75-162 mg/d) if they do not have an excessive bleeding risk.45,46 The most common dose in the United States is 81 mg. This recommendation is supported by a 2010 consensus statement of the American Diabetes Association, American Heart Association, and the American College of Cardiology.47
Should patients with diabetes and heart disease receive antiplatelet therapy?
Yes. The evidence is clear that people with known diabetes and ASCVD benefit from aspirin therapy, according to the 2017 Standards of Care. Clopidogrel 75 mg/d is an appropriate alternative for patients who are allergic to aspirin. Dual antiplatelet therapy (a P2Y12 receptor antagonist and aspirin) should be used for as long as one year after an ACS and may have benefits beyond this period.48
Established heart disease
Are there specific recommendations for patients with diabetes and CHD?
According to the ADA Standards, there is good evidence that both aspirin and statin therapy are beneficial for patients with known ASCVD, and that high-intensity statin therapy should be used. In addition, consider ACE inhibitors to reduce the future risk of CV events. In patients with a prior MI, continue beta-blocker therapy for at least 2 years post event.49
Which medications should I avoid, or approach with caution, in patients with congestive heart failure (CHF)?
Thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, and metformin all require careful attention. This is especially important to know when you consider that almost half of all patients with T2DM will develop heart failure.50
Thiazolidinediones. The 2017 Standards of Care state that patients with diabetes and symptomatic congestive heart failure should not receive thiazolidinediones, as they can worsen heart failure status via fluid retention. As such, they are contraindicated in patients with class III and IV heart failure.51
DPP-4 inhibitors. The studies on DPP-4 inhibitors and heart failure have had mixed results. The 2013 SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) 53 trial52 showed that patients treated with saxagliptin were more likely to be hospitalized for heart failure than those taking placebo (3.5% vs 2.8%, respectively). However, the 2015 EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care)53 trial and the 2015 TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin)54 trial evaluated heart failure and mortality outcomes in patients with alogliptin and sitagliptin, respectively, compared to placebo, and did not show a relationship to heart failure.
Metformin may be used in people who have T2DM and stable CHF if their eGFR remains >30 mL/min; it should be withheld from patients with unstable heart failure and those who are hospitalized with CHF.
Are there antihyperglycemic medications that reduce CV morbidity and mortality in those with established ASCVD?
Yes. This year’s ADA Standards indicate that certain glucose-lowering medications—specifically empagliflozin (a sodium–glucose cotransporter [SGLT]-2 inhibitor) and liraglutide (a glucagon-like peptide [GLP]-1 receptor agonist)—have been shown to be beneficial for those with established CVD. According to the 2017 Standards of Care, “In patients with longstanding suboptimally controlled T2DM and established ASCVD, empagliflozin or liraglutide should be considered, as they have been shown to reduce CV and all-cause mortality when added to standard care.”2 The studies that provide support for their use are summarized below. Ongoing studies are investigating the CV effects of other agents in these drug classes.
Empagliflozin. The 2015 EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study55 was a randomized double-blind study of empagliflozin vs placebo and usual care in patients with diabetes and established CVD. Over a median follow-up of 3.1 years, treatment with empagliflozin reduced the aggregate outcome of MI, stroke, and CV death by 14%, reduced CV deaths by 38%, and decreased deaths from any cause by 32%. In December 2016, the FDA announced a new indication for empagliflozin: to reduce the risk of CV death in adult patients with T2DM and CVD.56
Liraglutide. The LEADER (Liraglutide Effect and Action in Diabetes Evaluation of Cardiovascular Outcome Results: A Long Term Evaluation) trial57 was a double-blind randomized trial of liraglutide vs placebo added to usual care in patients with T2DM at high risk for CVD or with existing CVD. More than 80% of the participants had existing CVD including a history of prior MI, cerebrovascular disease, or peripheral vascular disease. After a median follow-up of 3.8 years, the group taking liraglutide demonstrated a 13% reduction in the composite outcome of MI, stroke, or CV death, a 22% reduction in CV death, and a 15% reduction in death from any cause, compared with placebo.57
CORRESPONDENCE
Neil Skolnik, MD, Abington-Jefferson Health, 500 Old York Rd, Ste 108, Jenkintown, PA 19046; nskolnik@comcast.net.
The authors thank Sarah Bradley, director, professional engagement & collaboration at the American Diabetes Association, for her editorial and organizational assistance in the preparation of this manuscript.
More than 29 million Americans have diabetes, and each year another 1.7 million are given the diagnosis.1 Prediabetes is even more common; over one-third of US adults ages 20 years and older, and more than half of those who are ages 65 and older, have attained this precursor status, representing another 86 million Americans.1
Because the evidence base for the management of diabetes is rapidly expanding, the American Diabetes Association’s (ADA) Professional Practice Committee updates its Standards of Medical Care in Diabetes annually to incorporate new evidence into its recommendations. The 2017 Standards of Care are available at: professional.diabetes.org/jfp.2
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality for people with diabetes, and is the largest contributor to the direct and indirect costs of the disease.2 As a result, all patients with diabetes should have cardiovascular (CV) risk factors, including dyslipidemia, hypertension, smoking, a family history of premature coronary disease, and the presence of albuminuria, assessed at least annually.2 Numerous studies have demonstrated the efficacy of controlling individual CV risk factors in preventing or slowing ASCVD in people with diabetes. Even larger benefits, including reduced ASCVD morbidity and mortality, can be achieved when multiple risk factors are addressed simultaneously.3
To hone your management of CV risks in patients with diabetes, we’ve put together this Q&A pointing out the elements of the ADA’s 2017 Standards of Care that are most relevant to the management of patients at risk for, or with established, ASCVD.
Screening
Since ASCVD so commonly co-occurs with diabetes, should I routinely screen asymptomatic patients with diabetes for heart disease?
No. The current evidence suggests that outcomes are NOT improved by screening people before they develop symptoms of ASCVD,4 and widespread ASCVD screening has not been shown to be cost-effective. Cardiac testing should be reserved for those with typical or atypical symptoms or those with an abnormal resting electrocardiogram (EKG).
Lifestyle modification
What are the benefits of lifestyle interventions?
The benefits include not only lost pounds, but improved mobility, physical and sexual functioning, and health-related quality of life. Recommend that all overweight patients with diabetes take advantage of intensive lifestyle interventions focusing on weight loss through decreased caloric intake and increased physical activity as per the Look AHEAD (Action for Health in Diabetes) trial.5 Although the intensive lifestyle intervention in the Look AHEAD trial did not decrease CV outcomes over 10 years of follow-up, it did improve control of CV risk factors and led to people in the intervention group taking fewer glucose-, blood pressure (BP)-, and lipid-lowering medications than those in the standard care group.
There is no one diet that is recommended for all people with diabetes. Weight reduction often requires intensive intervention. In order for weight loss diets to be sustainable, they must include patient preferences.
People with diabetes should be encouraged to receive individualized medical nutrition therapy (MNT), preferably from a registered dietitian who is well versed in nutritional management for diabetes. Such MNT is associated with a 0.5% to 2% decrease in A1c levels for people with type 2 diabetes.6-9 Specific healthy diets include the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and plant-based diets.
A new lifestyle recommendation in this year’s ADA Standards is that periods of prolonged sitting should be interrupted every 30 minutes with a period of physical activity. This appears to have glycemic benefits.2
Hypertension/BP management
When should I initiate hypertension treatment in patients with diabetes?
Nonpharmacologic therapy is reasonable in people with diabetes and mildly elevated BP (>120/80 mm Hg). If systolic blood pressure (SBP) is confirmed to be >140 mm Hg and/or diastolic blood pressure (DBP) is confirmed to be >90 mm Hg, the ADA recommends initiating pharmacologic therapy along with nonpharmacologic strategies. For patients with confirmed office-based BP >160/100 mm Hg, the ADA advises initiating lifestyle modifications as well as 2 pharmacologic medications (or a single pill combination of agents).2
What is the recommended BP target for patients with diabetes and hypertension?
These patients should be treated with a combination of measures, including lifestyle modification and pharmacologic therapy, to a target BP of <140/90 mm Hg. Randomized controlled trials (RCTs) have shown benefits with this target in terms of a reduction in the incidence of coronary heart disease (CHD) events, stroke, and diabetic kidney disease.10,11
A 2012 meta-analysis of randomized trials involving adults with type 2 diabetes mellitus (T2DM) and comparing intensive BP targets (≤130 mm Hg SBP and ≤80 mm Hg DBP) with standard targets (≤140-160 mm Hg SBP and ≤85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MIs associated with more intense BP control. There was a statistically significant 35% relative risk (RR) reduction in stroke with intensive targets, but lower BP was also associated with an increased risk of hypotension and syncope.12
The 2010 Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,13 which randomized 5518 patients with T2DM at high risk for ASCVD to either a target SBP of <120 mm Hg or 130 to 140 mm Hg, found that the patients with the lower SBP target did not benefit in the primary end point (a composite of nonfatal MI, nonfatal stroke, and CV death), but did benefit from nominally significant lower rates of total stroke and nonfatal stroke.
Based on these data, the ADA Standards of Care suggest that, “more intensive BP control may be reasonable in certain motivated, ACCORD-like patients (40-79 years of age with prior evidence of CVD or multiple CV risk factors) who have been educated about the added treatment burden, side effects, and costs of more intensive BP control and for patients who prefer to lower their risk of stroke beyond what can be achieved with usual care.”
Another major study, the 2015 Systolic Blood Pressure Intervention Trial (SPRINT) trial,14 demonstrated that treating patients with hypertension to a target SBP <120 mm Hg compared to the usual target of <140 mm Hg resulted in a 25% lower RR of the primary outcome (a composite of MI, other acute coronary syndromes, stroke, heart failure, or death from CV causes) and about a 25% reduction in all-cause mortality; however, people with diabetes were not included in the trial, so the applicability of the results to decisions about BP management in patients with diabetes is not known.
A 2015 systematic review and meta-analysis of over 100,000 participants looked at SBP lowering in adults with T2DM and found that each 10-mm Hg reduction in SBP was associated with a significantly lower risk of morbidity, CV events, CHD, stroke, albuminuria, and retinopathy.10 When trials were stratified by mean baseline SBP (<140 mm Hg or ≥140 mm Hg), RRs for outcomes other than stroke, retinopathy, and renal failure were lower in studies with greater baseline SBP.
The latest ADA Standards of Care recommend that a lower BP target of 130/80 mm Hg may be appropriate for patients at high risk of CVD if this target can be achieved without undue treatment burden. A DBP of <80 mm Hg may also be appropriate in certain patients including those with a long life expectancy, CKD, elevated urinary albumin excretion, and those with evidence of CVD or associated risk factors.15 Of note, treating older adults with diabetes to an SBP target of <130 mm Hg has not been shown to improve cardiovascular outcomes,16 and treating to a diastolic target of <70 mm Hg has been associated with a greater risk of mortality.17
What are the current recommended treatment options?
Treatment for hypertension in adults with diabetes without albuminuria should include any of the classes of medications demonstrated to reduce CV events in patients with diabetes, such as:
- angiotensin-converting enzyme (ACE) inhibitors,
- angiotensin receptor blockers (ARBs),
- thiazide-like diuretics, and
- dihydropyridine calcium channel blockers.
These recommendations are based on evidence suggesting the lack of superiority of ACE inhibitors and ARBs over other classes of antihypertensive agents for the prevention of CV outcomes in all patients with diabetes.18 However, in people with diabetes at high risk for ASCVD and/or with albuminuria, ACE inhibitors and ARBs do reduce ASCVD outcomes and the progression of kidney disease.19-24 Thus, ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and urine albumin/creatinine ratios ≥30 mg/g, as these medications are associated with a reduction in the rate of kidney disease progression.
The use of both an ACE inhibitor and an ARB in combination is not recommended.25,26 For patients treated with ACE inhibitors, ARBs, or diuretics, serum creatinine/estimated glomerular filtration rate (eGFR) and serum potassium levels should be monitored.
What are the recommended lifestyle modifications for patients with diabetes and hypertension?
Regular exercise and healthy eating are recommended for all people with diabetes to optimize glycemic control and lose weight (if they are overweight or obese). For patients with hypertension, the DASH diet (available at: https://www.nhlbi.nih.gov/health/health-topics/topics/dash/) is effective at lowering BP. The DASH diet emphasizes reducing sodium intake, increasing potassium intake, limiting alcohol intake, and increasing physical activity. Specifically, sodium intake should be restricted to <2300 mg/d and patients should consume approximately 8 to 10 servings of fruits and vegetables per day and 2 to 3 servings of low-fat dairy per day. Alcohol should be limited to 2 drinks per day for men and one drink per day for women.
Most adults with diabetes should perform 150 minutes per week of moderate to vigorous exercise, spread over at least 3 days/week. In addition, it is recommended that resistance exercises be performed at least 2 to 3 days/week. Prolonged inactivity is detrimental to health and should be interrupted with activity every 30 minutes.27
Finally, as a part of lifestyle management for all patients with diabetes, smoking cessation is important, as is attention to stress, depression, and anxiety.
Is there an advantage to nighttime dosing of antihypertensive medications?
Yes. Growing evidence suggests that there is an ASCVD benefit to avoiding nocturnal BP dipping. A 2011 RCT of 448 participants with T2DM and hypertension showed a decrease in CV events and mortality during 5.4 years of follow-up if at least one antihypertensive medication was taken at bedtime.28 As a result of this and other evidence,29 consider administering one or more antihypertensive medications at bedtime, although this is not a formal recommendation in the ADA Standards of Care.
Are there any additional issues to be aware of when treating patients with diabetes and hypertension?
Yes. Sometimes patients who have had diabetes for many years have significant orthostatic hypotension secondary to autonomic neuropathy. Postural changes in BP and pulse may require adjustment of BP targets. Home BP self-monitoring and 24-hour ambulatory BP monitoring may indicate white-coat or masked hypertension.
Lipid management
What is the current evidence for lipid treatment in diabetes?
Lipid abnormalities are common in people with diabetes and contribute to the overall high risk of ASCVD in these patients. Subgroup analyses of patients in large trials with diabetes30 and trials involving patients with diabetes31 have shown significant improvements in primary and secondary prevention of ASCVD with statin use. A 2008 meta-analysis of 18,686 people with diabetes showed a 9% reduction in all-cause mortality and a 13% reduction in vascular mortality for each 39-mg/dL reduction in low-density lipoprotein (LDL) cholesterol.32 Absolute reductions in mortality are greatest in those with highest risk, but the benefits of statin therapy are clear for low- and moderate-risk individuals with diabetes, too.33,34 As a result, statins are the medications of choice for lipid lowering and CV risk reduction and should be used in addition to lifestyle management.
Who should get a statin, and how do I choose the optimum dosage?
Patients ages 40 to 75 years with diabetes but without additional ASCVD risk factors should receive a moderate-intensity statin, according to the ADA (see TABLES 12 and 22). For those with additional CV risk factors, a high-intensity statin should be considered. The American College of Cardiology/American Heart Association ASCVD risk calculator (available at: http://www.cvriskcalculator.com/) may be useful for some patients, but generally, risk is already known to be high for most patients with diabetes. For patients of all ages with diabetes and established ASCVD, high-intensity statin therapy should be added to lifestyle modifications.35-37
For patients with diabetes who are <40 years with additional ASCVD risk factors, few clinical trial data exist; nevertheless, consider a moderate- or high-intensity statin and lifestyle therapy. Similarly, for patients >75 years who have diabetes and no additional ASCVD risk factors, consider a moderate-intensity statin and lifestyle modifications. For older adults with additional ASCVD risk factors, consider high-intensity statin therapy.35-37
Statins and cognition. It should be noted that published data have not demonstrated an adverse effect of statins on cognition.38 Statins, however, have been linked to an increased risk of developing diabetes,39,40 although the absolute increase in risk is small, and much smaller than the benefit derived from preventing the development of coronary disease.
Should total cholesterol and LDL levels be used as targets with statin treatment?
No. Statin doses have primarily been tested against placebo in clinical trials, rather than testing to specific target LDL levels, suggesting that the initiation and intensification of statin therapy be based on a patient’s risk profile.35 When maximally tolerated doses of statins do not lower LDL cholesterol by more than 30% from the patient’s baseline, there is currently no good evidence that combination therapy would be helpful, so regular monitoring of lipid levels has limited value. A lipid profile that includes levels of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides should be obtained at initial medical evaluation, at diagnosis of diabetes, and every 5 years thereafter or before the initiation of statin therapy. Ongoing testing may be appropriate in individual circumstances and to monitor for adherence to, or efficacy of, therapy.
What should I do for my patients who can’t tolerate statins?
Try a lower dose or a different statin before eliminating the class. Research has shown that even small doses (eg, rosuvastatin 5 mg) have some benefit.41
How do combination treatments figure into the current treatment of lipids in patients with diabetes?
It depends on the agent and the patient’s profile.
Fenofibrate. The ADA does not recommend automatically adding fenofibrate to statin therapy because the combination is associated with increased risks for abnormal transaminase levels, myositis, and rhabdomyolysis. In the ACCORD trial, the combination of fenofibrate and simvastatin did not reduce the rate of fatal CV events, nonfatal MIs, or nonfatal strokes compared with simvastatin alone.42
That said, a subgroup analysis suggested a benefit for men with both a triglyceride level ≥204 mg/dL (2.3 mmol/L) and an HDL cholesterol level ≤34 mg/dL (0.9 mmol/L).42 For this reason, the combination of a statin and fenofibrate may be considered for men who meet these laboratory parameters. In addition, consider medical therapy for triglyceride levels ≥500 mg/dL to reduce the risk of pancreatitis.
Ezetimibe. Recommendations regarding ezetimibe are based on the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), a 2015 RCT including over 18,000 patients that compared treatment with ezetimibe and simvastatin to simvastatin alone.43 Individuals in the trial were ≥50 years of age and had experienced an ACS within the preceding 10 days. In those with diabetes, the combination of moderate-intensity simvastatin (40 mg) and ezetimibe (10 mg) significantly reduced major adverse CV events with an absolute risk reduction of 5% (40% vs 45%) and an RR reduction of 14% over moderate-intensity simvastatin (40 mg) alone.
Based on these results, patients with diabetes and a recent ACS should be considered for combination therapy with ezetimibe and a moderate-intensity statin. The combination should also be considered in patients with diabetes and a history of ASCVD who cannot tolerate high-intensity statins.43
Niacin. The ADA currently does not recommend niacin in combination with a statin because of lack of efficacy on major ASCVD outcomes, possible increased risk of ischemic stroke, and adverse effects.44
What are the recommendations for the use of PCSK-9 inhibitors?
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors (ie, evolucumab and alirocumab) may be considered as adjunctive therapy to statins for patients with diabetes at high risk for ASCVD events who require additional lowering of LDL cholesterol. They may also be considered for those in whom high-intensity statin therapy is indicated, but not tolerated.
Antiplatelet agents
Who should take aspirin for primary prevention of CVD?
Both women and men ages ≥50 years who have diabetes and at least one additional CV risk factor (family history of premature ASCVD, hypertension, tobacco use, dyslipidemia, or albuminuria) should consider taking daily aspirin therapy (75-162 mg/d) if they do not have an excessive bleeding risk.45,46 The most common dose in the United States is 81 mg. This recommendation is supported by a 2010 consensus statement of the American Diabetes Association, American Heart Association, and the American College of Cardiology.47
Should patients with diabetes and heart disease receive antiplatelet therapy?
Yes. The evidence is clear that people with known diabetes and ASCVD benefit from aspirin therapy, according to the 2017 Standards of Care. Clopidogrel 75 mg/d is an appropriate alternative for patients who are allergic to aspirin. Dual antiplatelet therapy (a P2Y12 receptor antagonist and aspirin) should be used for as long as one year after an ACS and may have benefits beyond this period.48
Established heart disease
Are there specific recommendations for patients with diabetes and CHD?
According to the ADA Standards, there is good evidence that both aspirin and statin therapy are beneficial for patients with known ASCVD, and that high-intensity statin therapy should be used. In addition, consider ACE inhibitors to reduce the future risk of CV events. In patients with a prior MI, continue beta-blocker therapy for at least 2 years post event.49
Which medications should I avoid, or approach with caution, in patients with congestive heart failure (CHF)?
Thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, and metformin all require careful attention. This is especially important to know when you consider that almost half of all patients with T2DM will develop heart failure.50
Thiazolidinediones. The 2017 Standards of Care state that patients with diabetes and symptomatic congestive heart failure should not receive thiazolidinediones, as they can worsen heart failure status via fluid retention. As such, they are contraindicated in patients with class III and IV heart failure.51
DPP-4 inhibitors. The studies on DPP-4 inhibitors and heart failure have had mixed results. The 2013 SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) 53 trial52 showed that patients treated with saxagliptin were more likely to be hospitalized for heart failure than those taking placebo (3.5% vs 2.8%, respectively). However, the 2015 EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care)53 trial and the 2015 TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin)54 trial evaluated heart failure and mortality outcomes in patients with alogliptin and sitagliptin, respectively, compared to placebo, and did not show a relationship to heart failure.
Metformin may be used in people who have T2DM and stable CHF if their eGFR remains >30 mL/min; it should be withheld from patients with unstable heart failure and those who are hospitalized with CHF.
Are there antihyperglycemic medications that reduce CV morbidity and mortality in those with established ASCVD?
Yes. This year’s ADA Standards indicate that certain glucose-lowering medications—specifically empagliflozin (a sodium–glucose cotransporter [SGLT]-2 inhibitor) and liraglutide (a glucagon-like peptide [GLP]-1 receptor agonist)—have been shown to be beneficial for those with established CVD. According to the 2017 Standards of Care, “In patients with longstanding suboptimally controlled T2DM and established ASCVD, empagliflozin or liraglutide should be considered, as they have been shown to reduce CV and all-cause mortality when added to standard care.”2 The studies that provide support for their use are summarized below. Ongoing studies are investigating the CV effects of other agents in these drug classes.
Empagliflozin. The 2015 EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study55 was a randomized double-blind study of empagliflozin vs placebo and usual care in patients with diabetes and established CVD. Over a median follow-up of 3.1 years, treatment with empagliflozin reduced the aggregate outcome of MI, stroke, and CV death by 14%, reduced CV deaths by 38%, and decreased deaths from any cause by 32%. In December 2016, the FDA announced a new indication for empagliflozin: to reduce the risk of CV death in adult patients with T2DM and CVD.56
Liraglutide. The LEADER (Liraglutide Effect and Action in Diabetes Evaluation of Cardiovascular Outcome Results: A Long Term Evaluation) trial57 was a double-blind randomized trial of liraglutide vs placebo added to usual care in patients with T2DM at high risk for CVD or with existing CVD. More than 80% of the participants had existing CVD including a history of prior MI, cerebrovascular disease, or peripheral vascular disease. After a median follow-up of 3.8 years, the group taking liraglutide demonstrated a 13% reduction in the composite outcome of MI, stroke, or CV death, a 22% reduction in CV death, and a 15% reduction in death from any cause, compared with placebo.57
CORRESPONDENCE
Neil Skolnik, MD, Abington-Jefferson Health, 500 Old York Rd, Ste 108, Jenkintown, PA 19046; nskolnik@comcast.net.
The authors thank Sarah Bradley, director, professional engagement & collaboration at the American Diabetes Association, for her editorial and organizational assistance in the preparation of this manuscript.
1. Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. Available at: http://templatelab.com/national-diabetes-report-2014/. Accessed April 7, 2017.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Available at: http://professional.diabetes.org/sites/professional.diabetes.org/files/media/dc_40_s1_final.pdf. Accessed April 7, 2017.
3. Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591.
4. Bax JJ, Young LH, Frye RL, et al; American Diabetes Association. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007;30:2729-2736.
5. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154.
6. UK Prospective Diabetes Study (UKDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKDS 34). Lancet. 1998;352:854-865.
7. Ziemer DC, Berkowitz KJ, Panayioto RM, et al. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes. Diabetes Care. 2003;26:1719-1724.
8. Wolf AM, Conaway RM, Crowther JQ, et al; Improving Control with Activity and Nutrition (ICAN) Study. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570-1576.
9. Coppell KJ, Kataoka M, Williams SM, et al. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment-Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. 2010;341:c3337.
10. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603-615.
11. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;10:CD008277.
12. McBrien K, Rabi DM, Campbell N, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2012;172:1296-1303.
13. ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
14. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
15. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
16. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
17. Anderson RJ, Bahn GD, Moritz TE, et al; VADT Study Group. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care. 2011;34:34-38.
18. Bangalore S, Fakheri R, Toklu B, et al. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ. 2016;352:i438.
19. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253-259.
20. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772-776.
21. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
22. Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759-766.
23. Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
24. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047-2056.
25. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
26. Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
27. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079.
28. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
29. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011;10:CD004184.
30. Py̆orälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
31. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care. 2006;29:1478-1485.
32. Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117-125.
33. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816.
34. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610.
35. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
36. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
37. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
38. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688-697.
39. Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924-1929.
40. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
41. Meek C, Wierzbicki AS, Jewkes C, et al. Daily and intermittent rosuvastatin 5 mg therapy in statin intolerant patients: an observational study. Curr Med Res Opin. 2012;28:371-378.
42. ACCORD Study Group, Ginsberg HN, Bam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
43. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
44. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
45. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860.
46. Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635-1701.
47. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes. A position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
48. Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
49. Kezerashvilli A, Marzo K, De Leon J. Beta blocker use after acute myocardial infarction in the patient with normal systolic function: when is it “ok” to discontinue? Curr Cardiol Rev. 2012;8:77-84.
50. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34.
51. Pioglitazone Package Insert. Available at: http://medlibrary.org/lib/rx/meds/pioglitazone-3/. Accessed April 10, 2017.
52. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
53. Zannad F, Cannon CP, Cushman WC, et al; EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
54. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
55. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
56. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. FDA News Release, December 2, 2016. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed February 9, 2017.
57. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
1. Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. Available at: http://templatelab.com/national-diabetes-report-2014/. Accessed April 7, 2017.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Available at: http://professional.diabetes.org/sites/professional.diabetes.org/files/media/dc_40_s1_final.pdf. Accessed April 7, 2017.
3. Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591.
4. Bax JJ, Young LH, Frye RL, et al; American Diabetes Association. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007;30:2729-2736.
5. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154.
6. UK Prospective Diabetes Study (UKDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKDS 34). Lancet. 1998;352:854-865.
7. Ziemer DC, Berkowitz KJ, Panayioto RM, et al. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes. Diabetes Care. 2003;26:1719-1724.
8. Wolf AM, Conaway RM, Crowther JQ, et al; Improving Control with Activity and Nutrition (ICAN) Study. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570-1576.
9. Coppell KJ, Kataoka M, Williams SM, et al. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment-Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. 2010;341:c3337.
10. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603-615.
11. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;10:CD008277.
12. McBrien K, Rabi DM, Campbell N, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2012;172:1296-1303.
13. ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
14. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
15. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
16. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
17. Anderson RJ, Bahn GD, Moritz TE, et al; VADT Study Group. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care. 2011;34:34-38.
18. Bangalore S, Fakheri R, Toklu B, et al. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ. 2016;352:i438.
19. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253-259.
20. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772-776.
21. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
22. Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759-766.
23. Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
24. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047-2056.
25. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
26. Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
27. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079.
28. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
29. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011;10:CD004184.
30. Py̆orälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
31. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care. 2006;29:1478-1485.
32. Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117-125.
33. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816.
34. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610.
35. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
36. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
37. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
38. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688-697.
39. Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924-1929.
40. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
41. Meek C, Wierzbicki AS, Jewkes C, et al. Daily and intermittent rosuvastatin 5 mg therapy in statin intolerant patients: an observational study. Curr Med Res Opin. 2012;28:371-378.
42. ACCORD Study Group, Ginsberg HN, Bam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
43. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
44. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
45. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860.
46. Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635-1701.
47. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes. A position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
48. Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
49. Kezerashvilli A, Marzo K, De Leon J. Beta blocker use after acute myocardial infarction in the patient with normal systolic function: when is it “ok” to discontinue? Curr Cardiol Rev. 2012;8:77-84.
50. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34.
51. Pioglitazone Package Insert. Available at: http://medlibrary.org/lib/rx/meds/pioglitazone-3/. Accessed April 10, 2017.
52. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
53. Zannad F, Cannon CP, Cushman WC, et al; EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
54. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
55. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
56. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. FDA News Release, December 2, 2016. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed February 9, 2017.
57. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
Insulin pumps: Beyond basal-bolus
The advent of the insulin pump in the late 1970s was a step forward in diabetes treatment,1 and recent improvements make these devices easier to use in intensive insulin management. Today, more than 400,000 people in the United States are thought to be using an insulin pump.2
With a pump, patients can adjust the dosage and discreetly give themselves boluses by simply pushing a button instead of giving themselves multiple daily injections. Also, pump therapy can be tailored to correct for hepatic glucose production in a way that injections cannot.
This article reviews the clinical application of continuous subcutaneous insulin therapy—ie, the insulin pump—and provides recommendations for patient selection and management.
INDICATIONS FOR AN INSULIN PUMP
The American Association of Clinical Endocrinologists3 recommends considering an insulin pump for patients with type 1 or 2 diabetes mellitus who have a clear indication:
- Suboptimal control on basal-bolus injections, ie, not achieving glycemic goals despite maximal adherence to multiple daily injections
- Wide and erratic glycemic excursions
- Frequent severe hypoglycemia, or hypoglycemia unawareness
- A marked “dawn phenomenon” (spike in blood glucose level early in the morning)
- Pregnancy or planning for pregnancy
- Erratic lifestyle
- Personal preference.
WHO IS A GOOD CANDIDATE FOR AN INSULIN PUMP?
Good candidates for a pump are patients with type 1 diabetes (and some with type 2) who are well versed in taking multiple daily injections, are already checking their glucose four or more times daily, “counting carbs” (estimating or, preferably, measuring how much carbohydrate they are eating, and limiting their intake accordingly), and demonstrate the ability to adjust their dosing appropriately (Table 1).
A pump is not a shortcut to checking glucose less frequently or making fewer decisions. However, for those who actively manage their diabetes, it provides more real-time flexibility and some important safety features, as discussed below.
IS A PUMP BETTER THAN INJECTIONS?
Several studies have compared insulin pump therapy and multiple daily injections.4–7 While some found no difference in glucose control in terms of hemoglobin A1c or hypoglycemia, others showed improved glucose control with pumps in patients who had higher baseline hemoglobin A1c levels (> 10%).6 In this subgroup, a pump lowered hemoglobin A1c an additional estimated 0.65% compared with multiple daily injections.6 Fructosamine levels also improved in pump users.6
Using continuous glucose monitoring for 3 days in a study in children with type 1 diabetes, Schreiver et al8 found lower insulin requirements and less-severe glycemic excursions with a pump than with multiple daily injections.
A 2013 study9 of 57 patients ages 13 to 71 with type 2 diabetes who were struggling to control their blood sugar with multiple daily injections found that they achieved better control with less insulin using a pump.
A meta-analysis found pump therapy to be more effective than multiple daily injections for those who used it more than 1 year.10
ADVANTAGES AND DISADVANTAGES OF INSULIN PUMP THERAPY
Intensive glucose control reduces microvascular complications in type 1 diabetes.11–14 The advantages of using a pump include better adherence, more accurate dosing, greater lifestyle flexibility, control of the dawn phenomenon without induction of nocturnal hypoglycemia, and the ability to suspend or temporarily reduce basal insulin to compensate for increased physical activity.15
Disadvantages include the high degree of technical aptitude required, the need for high-level engagement, skin reactions to tape, a higher risk of diabetic ketoacidosis from pump malfunction, infusion-site problems such as “tunneling” of insulin (leakage of insulin along the outside of the cannula and back to the skin surface) and clogging of the infusion set, and a risk of inactivation of insulin from exposure to heat, which can lead to ketoacidosis in a few hours if not addressed promptly.15
IS IT COST-EFFECTIVE?
There is evidence that continuous subcutaneous insulin infusion is cost-effective, both in general and compared with multiple daily injections for children and adults with type 1 diabetes mellitus. Cohen and Shaw16 found that life expectancy and quality-adjusted life-years increased in pump users, although the price per life-year gained varied greatly depending on the model used.
And this therapy is expensive. Most pumps cost more than $6,000, and supplies cost about $300 per month. Most insurance providers cover this therapy for patients with type 1 diabetes (Table 2) but less often for those with type 2. Further, many insurance policies have copayments, and patients may find a 20% co-payment a significant financial burden. Physicians need to obtain preapproval for insulin pumps from the insurance company. Typically, prescriptions for supplies are written annually. Despite these significant costs, most patients with type 1 diabetes who use an insulin pump find that the benefits of improved control and greater independence justify the cost.
An annual review of currently available insulin pumps and other diabetes-related equipment is published in Diabetes Forecast.17
PATIENT PERSPECTIVE ON INSULIN PUMP USE
Many patients who use a pump find that it gives them greater flexibility to adjust to day-to-day changes in schedules and routines. For example, consuming an extra serving at a meal could necessitate another injection for a patient on multiple daily injections, but a pump user would need only to push a few buttons. With cell phone apps available to control some pumps, many people find that an insulin pump is more discreet and easier to manage than carrying around injection supplies. Further, the complex calculations of carbohydrate ratios and correction factors are easier and more accurate with a pump.
In an open-label randomized study,18 29 of 41 patients with type 1 diabetes said they preferred a pump to multiple daily injections.
Conversely, some people do not want a pump because it is attached all the time and identifies them to others as having an illness. Other patients do not trust a machine and want control in their own hands. (Actually, machines typically are much more reliable and less mistake-prone than humans.)
HOW DOES A PUMP WORK COMPARED WITH MULTIPLE DAILY INJECTIONS?
Patients taking multiple daily injections must use two types of insulin: a long-acting one that reaches a steady level in the blood without a peak and lasts from 12 to 24 hours, and a rapid-acting one taken with meals, usually having a peak of action and an effect lasting 3 to 5 hours. The idea is to approximate normal insulin patterns, with a basal level in the background and peaks (boluses) of insulin with carbohydrate intake.
Insulin pumps use only one kind of insulin—a rapid-acting one, ie, lispro, aspart, or glulisine. They preserve the basal-bolus concept, but with many refinements (discussed below).15
Most pumps are attached to the patient by plastic tubing that connects the reservoir to a subcutaneous cannula or steel needle. However, some pumps have a reservoir directly attached to a subcutaneous cannula without the tubing. This type of pump is controlled with a remote device.
The infusion set (cannula or needle and tubing) and the site should be changed every third day to minimize the risk of infection and abnormal delivery due to protein buildup on the cannula os, epithelial healing, and irritation around the site. Failure to do so often results in higher blood glucose concentrations.19
The patient and healthcare team work together to calculate the patient’s daily insulin needs, and the pump is programmed based on the patient’s requirements, lifestyle, and sensitivity to insulin. Once the pump is started, the patient operates it to deliver the insulin dose according to carbohydrate intake and blood glucose level.
PUMP SETTINGS
Basal rate
The basal rate is programmed by the physician and is intended to mimic physiologic insulin release. The pump can be set to a number of basal rates within any 24-hour period. This provides more physiologic matching of insulin delivery to hourly insulin needs based on the patient’s daily schedule.
If the patient has been taking multiple daily injections, the hourly basal rate can be calculated by dividing the daily basal dose by 24. However, lower rates are usually used after midnight, and rates are increased early in the morning to counteract the dawn phenomenon.
The rates can also be adjusted temporarily (for up to 24 hours), with a feature called the temporary basal rate. People tend to have higher blood glucose levels when they have a respiratory illness, are under significant stress, or are menstruating. Thus, a person with influenza could increase the basal rate by 25%, or a student could run a temporary basal rate of 150% for 4 hours before taking a final exam.
Conversely, exercising increases insulin’s effectiveness at the muscle level, and insulin requirements drop. To counteract this, one would temporarily decrease the basal rate in the pump before exercising.
Many factors affect the bolus dose
A bolus of insulin is given for meals and to correct hyperglycemia, as with multiple daily injections. A pump calculates the bolus based on the carbohydrate ratio, correction factor, or both. These ratios are programmed into the pump by the physician. A benefit of the insulin pump is that the patient just has to input the amount of carbohydrates to be eaten or record a blood glucose level and the pump will calculate the bolus dose of insulin to be given.
The carbohydrate ratio is the amount of insulin that should be taken per amount of carbohydrate. A typical ratio is 1:15, meaning that the patient should take 1 unit of insulin for every 15 g of carbohydrates to be eaten. This varies by patient depending on insulin sensitivity.
The correction factor describes how much the glucose level is expected to drop per unit of insulin given. For example, if the target glucose level is 100 mg/dL and the correction factor is 25, then the patient will get 1 unit of correction of insulin if his or her glucose level is 125 mg/dL, 2 units if it is 150 mg/dL, and so on. A pump can dispense fractions of a unit.
The target glucose level or range is set by the physician and patient and is one of the factors the pump uses in calculating a bolus dose. Insulin pumps allow for multiple target glucose levels. Commonly, to minimize the risk of hypoglycemia, a higher (less strict) target is set for bedtime and overnight than for daytime.
Active insulin time defines how soon the patient can take another bolus.
Often, people eat more than they thought they would. They may also find that the glucose level did not increase or decrease as much as expected. Many patients who actively manage their glucose take additional boluses of insulin after a meal if their glucose is higher than they thought it would be. A patient taking injections cannot know how much of the insulin from the before-meal bolus is still working and has to guess.
Insulin pumps use a logarithmic formula to calculate this and prevent the user from “stacking” insulin boluses and lowering the glucose level too much. For example, if the active insulin time is 4 hours and the patient took a bolus for lunch at noon, he or she would be unable to take a full insulin correction dose until 4:00 pm. The patient can override this feature. Although the active insulin time varies from patient to patient, it is rarely more than 4 hours.
Additional safety features
Suspend. When a person who is taking insulin injections starts to experience hypoglycemia, he or she has one option—to eat something to treat the low blood glucose. The insulin injection has already been taken and cannot be reversed. However, with an insulin pump the patient can first suspend the pump so that no additional insulin is infused until it is safe again, and then eat to treat the low sugar level. This allows the patient to eat less, prevent overtreating, and, hopefully, prevent rebound hyperglycemia.
Reverse correction. When patients take insulin for an upcoming meal, they estimate the amount needed for the carbohydrates that they are about to eat as well as how much correction is needed. If their glucose level is below the target range, they may or may not subtract insulin from the dose to achieve the glucose target. The pump does this automatically, resulting in a lower dose of insulin for that bolus. This allows the patient to take a bolus for a meal even if he or she is below the target, and thus prevent hyperglycemia.
CAN INSULIN PUMPS BE USED IN THE HOSPITAL?
Patients can keep using their insulin pump in the hospital under the right conditions.
Inpatient hypoglycemia increases the risk of death, and although not all patients require tight glycemic control, there is still benefit in avoiding extremes in blood sugar levels,20 including at night.20–22 Insulin pump therapy, when used in the hospital, results in fewer episodes of severe hyperglycemia (glucose levels > 300 mg/dL) and hypoglycemia (levels < 40 mg/dL) than multiple daily injections.22 Moreover, most pump users feel more comfortable when they can manage their own therapy. Using the pump in the hospital has the additional benefit that patients can treat themselves before and after meals easily with less staff time and effort.
Bailon et al23 retrospectively studied 35 patients with insulin pumps in 50 hospitalizations. More than half of the patients were allowed to continue using their pump in the hospital. Reasons for discontinuing the pump included lack of access to supplies, unfamiliarity with the pump, attempted suicide, malfunctioning hardware, diabetic ketoacidosis, and altered mental status. Patients using their pump had fewer episodes of hypoglycemia (glucose levels < 70 mg/dL) than patients who removed their pump. In patients who continued using the pump throughout their hospitalization, no adverse events (eg, site infection or mechanical failure) were noted.
Leonhardi et al24 reviewed 25 hospital admissions, and the outcomes were similar to those reported by Bailon et al,23 with no adverse outcomes related to the pumps.
When using an insulin pump in the hospital
When a physician wants a patient to continue using an insulin pump in the hospital, a number of things must happen. The nursing staff must be informed that the patient is wearing a pump and can self-administer insulin. Most facilities will still follow routine protocols for checking blood glucose but will document that the patient is administering his or her own insulin. The patient must be well enough to manage the pump. If the infusion site needs to be changed, the patient would be expected to do so with his or her own supplies.
Imaging and insulin pumps
Advice differs on what to do if a patient with an insulin pump needs to undergo radiographic imaging. For example, the University of Wisconsin radiology department says it is safe to keep an insulin pump in place if the x-ray beam will be on for less than 3 seconds at a time and if the device is covered by a lead apron.25 However, radiation can induce electrical currents in the circuitry, which can alter the function of the pump. For this reason, some manufacturers recommend removing the device before the patient enters any room in which radiation or magnetic resonance imaging will be used.26–31
Insulin pumps and surgery
Insulin pumps have been used in the perioperative and intraoperative periods, with positive outcomes.32 An analysis of 20 patients on pumps undergoing a total of 23 surgeries (mostly orthopedic procedures) found that 13 of the 20 patients wore their pump during surgery. No adverse events were noted in any of these cases, although the sample size was small.33
Corney et al34 retrospectively compared insulin pumps with alternative methods of perioperative glucose management. Multiple surgical specialties were included. No significant difference in mean blood glucose levels was found between those who continued to use their pump and those who used other methods. In those who continued to use their pump, there were no episodes of intraoperative technical difficulties related to the pump.
Any patient who may be undergoing a procedure or surgery must let the surgeon and anesthesiologist know that he or she has a pump. If the infusion site is too close to the site of the surgery or procedure, it must be moved.
Concerns during surgery include catheter or site disconnection or loss, crystallization within the tubing (a potential problem not limited to surgery), and pump malfunction. If the procedure involves imaging, the pump should probably be disconnected or covered by lead shielding as directed in the pump manufacturer’s manual. The surgeon and anesthesiologist must decide whether to continue use of a pump during a surgical procedure. However, the study by Corney et al34 shows it is possible.
Most office-based procedures can be done with the insulin pump in place, as the patient is not under general anesthesia and so can adjust the insulin regimen as needed.
Abdelmalak et al,35 in a comprehensive review of insulin pump use in noncardiac surgery, commented that the type of surgery may play a role in determining the best approach to perioperative glucose management. Major surgery causes a large inflammatory response that makes it difficult to control blood sugar, especially when steroids or beta agonists are given, whereas minor surgery does not affect blood glucose nearly as much. The authors offered recommendations on pump use during various surgical procedures depending on the length of the procedure:
- If surgery is anticipated to last less than 1 hour, then keep the insulin pump on, and have the patient manage corrections preoperatively and postoperatively.
- For surgery of intermediate length (1–3 hours), have the patient take a bolus of 1 hour’s worth of insulin (based on the basal rate for that time period) before the procedure, then remove the insulin pump. Do this only if blood sugar is normal or close to normal. If the patient is severely hyperglycemic, remove the insulin pump and start an intravenous insulin infusion.
- If the procedure will take more than 3 hours, remove the pump and start an insulin infusion regardless of the blood sugar level.35
AIR TRAVEL AND INSULIN PUMPS
Insulin pumps can be easy to manage during airline travel if the user is prepared (Table 3).
First, it is important to have a letter from the treating physician stating that the pump is a necessary medical device. All supplies should be carried on and in a separate bag for easy inspection. The more forthcoming the user is at the security checkpoint, the easier the process.
According to the Transportation Security Administration, insulin pump users can keep their pump on during screening, and the metal detectors and full-body scanners will not harm the device.36
However, manufacturer recommendations differ. Medtronic recommends that patients not expose their insulin pump to x-rays, and that instead of going through a full-body scanner the patient should request a pat-down.37 Animas recommends the same.38 OmniPod states that their system can be worn through airport imaging, making it the only approved continuous insulin delivery system that can be taken through airport imaging.39
Another potential problem is the change in atmospheric pressure during takeoff and landing. Bubbles can form in the insulin reservoir as air pressure decreases with ascent, thereby displacing insulin from the pump to the patient. The opposite happens during descent. King et al40 corroborated this phenomenon with Animas and Medtronic pumps. Asante recommends removing their pump tubing during takeoff and landing.30
If PROBLEMS ARISE
Like any machine, an insulin pump can fail. Most failures result in lack of insulin delivery—the patient does not get excess insulin from insulin pump failure. Excess insulin delivery is most often due to operator error. All insulin is either preprogrammed (basal by provider or patient) or must be confirmed by the patient at the time of delivery (meal or correction boluses).
Pump manufacturers have 24-hour support programs and hotlines, with experts who will either walk the patient through the problem or send a replacement pump—often within 24 hours.
EVOLVING TECHNOLOGY
Pump technology is evolving quickly. On the way are “smart” pumps that interact with other systems, smaller pumps with advanced touch-screen features, and patch pumps that do not have tubing but operate similarly to pumps with tubing (ie, a cannula is still required for insulin delivery).
Some insulin pumps can be linked to an external glucose sensor. These systems provide a great amount of information to the patient and provider. Often, there is increased awareness of fluctuations in glucose, allowing earlier intervention to prevent high and low glucose excursions. Sensor-augmented pumps may further improve safety by suspending infusion during hypoglycemia.41,42
Researchers continue to strive for closed-loop systems that would allow the pump to automatically respond to circulating glucose and thus provide truly physiologic control.43 A recent study showed the effectiveness of the outpatient use of a bihormonal (insulin and glucagon) “bionic pancreas,” which provided improved glucose control and similar or less hypoglycemia in adults and adolescents who had been using a traditional insulin pump.44
- Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care 2002; 25:593–598.
- JDRF and BD collaborate to improve insulin pump delivery. www.bd.com/_Images/BD_JDRF_press_release_2010_tcm49-19552.pdf. Accessed October 14, 2015.
- Grunberger G, Abelseth JM, Bailey TS, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology insulin pump management task force. Endocr Pract 2014; 20:463–489.
- Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001; 24:1722–1727.
- Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care 2005; 28:1568–1573.
- Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care 2004; 27:2590–2596.
- Hirsch IB, Bode BW, Garg S, et al; Insulin Aspart CSII/MDI Comparison Study Group. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005; 28:533–538.
- Schreiver C, Jacoby U, Watzer B, Thomas A, Haffner D, Fischer DC. Glycaemic variability in paediatric patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI): a cross-sectional cohort study. Clin Endocrinol (Oxf) 2013; 79:641–647.
- Leinung MC, Thompson S, Luo M, Leykina L, Nardacci E. Use of insulin pump therapy in patients with type 2 diabetes after failure of multiple daily injections. Endocr Pract 2013; 19:9–13.
- Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003; 26:1079-1087.
- Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care 1995; 18:361–376.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352:837–853.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Skyler JS, Ponder S, Kruger DF, Matheson D, Parkin CG. Is there a place for insulin pump therapy in your practice? Clinical Diabetes 2007; 25:50–56.
- Cohen N, Shaw J. Cost effectiveness of insulin pump therapy. Infusystems Asia 2007; 2:25–28.
- Tucker ME. Insulin pumps: closer to a pancreas. Diabetes Forecast. www.diabetesforecast.org/2015/mar-apr/insulin-pumps-closer-to-pancreas.html. Accessed October 14, 2015.
- Hanaire-Broutin H, Melki V, Bessières-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care 2000; 23:1232–1235.
- Schmid V, Hohberg C, Borchert M, Forst T, Pfützner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol 2010; 4:976–982.
- Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009; 15:353–369.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Cook CB, Beer KA, Seifert KM, Boyle ME, Mackey PA, Castro JC. Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years’ experience with 253 cases. J Diabetes Sci Technol 2012; 6:995–1002.
- Bailon RM, Partlow BJ, Miller-Cage V, et al. Continuous subcutaneous insulin infusion (insulin pump) therapy can be safely used in the hospital in select patients. Endocr Pract 2009; 15:24–29.
- Leonhardi BJ, Boyle ME, Beer KA, et al. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital: a review of one institution’s experience. J Diabetes Sci Technol 2008; 2:948–962.
- Department of Radiology, University of Wisconsin School of Medicine and Public Health. Precautions with implanted devices. www.radiology.wisc.edu/fileShelf/forReferring/PrecautionsWithImplantedDevices_CTandXRAY.php. Accessed October 14, 2015.
- Indications, contraindications, warnings and precautions. Medtronicdiabetes.com/important-safety-information. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- T:slim user guide. www.tandemdiabetes.com/uploadedFiles/Content/_Configuration/Files/Manuals/tslim_User_Guide.pdf. Tandem Diabetes Care. Accessed October 14, 2015.
- OmniPod user guide. www.myomnipodtraining.com/pdf/OmniPod-User-Guide-UST400.pdf. Insulet Corporation. Accessed October 14, 2015.
- Important safety information.Animas Vibe Insulin Pump and CGM System. www.animas.com/safety. Animas Corporation. Accessed October 14, 2015.
- Snap insulin pump safety information. Snappump.com/safety-information. Asante Solutions, Inc. Accessed October 14, 2015.
- ACCU-CHEK Spirit insulin pump system. Pump user guide. www.accu-chekinsulinpumps.com/documents/PumpUserGuide.pdf. Disetronic Medical Systems, Inc. Accessed October 14, 2015.
- White WA Jr, Montalvo H, Monday JM. Continuous subcutaneous insulin infusion during general anesthesia: a case report. AANA J 2004; 72:353–357.
- Boyle ME, Seifert KM, Beer KA, et al. Insulin pump therapy in the perioperative period: a review of care after implementation of institutional guidelines. J Diabetes Sci Technol 2012; 6:1016–1021.
- Corney SM, Dukatz T, Rosenblatt S, et al. Comparison of insulin pump therapy (continuous subcutaneous insulin infusion) to alternative methods for perioperative glycemic management in patients with planned postoperative admissions. J Diabetes Sci Technol 2012; 6:1003–1015.
- Abdelmalak B, Ibrahim M, Yared JP, Modic MB, Nasr C. Perioperative glycemic management in insulin pump patients undergoing noncardiac surgery. Curr Pharm Des 2012; 18:6204–6214.
- US Department of Homeland Security. Travelers with disabilities and medical conditions. www.tsa.gov/travel/special-procedures. Transportation Security Administration. Accessed October 14, 2015.
- Medical emergency card/airport information. www.medtronicdiabetes.com/sites/default/files/library/support/Airport%20Information%20Card.pdf. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- Traveling with an insulin pump. www.animas.com/about-insulin-pump-therapy/traveling-with-diabetes. Animas Corporation. Accessed October 14, 2015.
- Tips for air travel with diabetes supplies. www.myomnipod.com/pdf/14986-AWAirTravelTipsFlyerR2-11-11.pdf. Insulet Corporation. Accessed October 14, 2015.
- King BR, Goss PW, Paterson MA, Crock PA, Anderson DG. Changes in altitude cause unintended insulin delivery from insulin pumps: mechanisms and implications. Diabetes Care 2011; 34:1932–1933.
- Bergenstal RM, Tamborlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010; 363:311–320.
- Bergenstal RM, Klonoff DC, Garg SK, et al; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013; 369:224–232.
- Bequette BW. Challenges and recent progress in the development of a closed-loop artificial pancreas. Annu Rev Control 2012; 36:255–266.
- Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014; 371:313–325.
The advent of the insulin pump in the late 1970s was a step forward in diabetes treatment,1 and recent improvements make these devices easier to use in intensive insulin management. Today, more than 400,000 people in the United States are thought to be using an insulin pump.2
With a pump, patients can adjust the dosage and discreetly give themselves boluses by simply pushing a button instead of giving themselves multiple daily injections. Also, pump therapy can be tailored to correct for hepatic glucose production in a way that injections cannot.
This article reviews the clinical application of continuous subcutaneous insulin therapy—ie, the insulin pump—and provides recommendations for patient selection and management.
INDICATIONS FOR AN INSULIN PUMP
The American Association of Clinical Endocrinologists3 recommends considering an insulin pump for patients with type 1 or 2 diabetes mellitus who have a clear indication:
- Suboptimal control on basal-bolus injections, ie, not achieving glycemic goals despite maximal adherence to multiple daily injections
- Wide and erratic glycemic excursions
- Frequent severe hypoglycemia, or hypoglycemia unawareness
- A marked “dawn phenomenon” (spike in blood glucose level early in the morning)
- Pregnancy or planning for pregnancy
- Erratic lifestyle
- Personal preference.
WHO IS A GOOD CANDIDATE FOR AN INSULIN PUMP?
Good candidates for a pump are patients with type 1 diabetes (and some with type 2) who are well versed in taking multiple daily injections, are already checking their glucose four or more times daily, “counting carbs” (estimating or, preferably, measuring how much carbohydrate they are eating, and limiting their intake accordingly), and demonstrate the ability to adjust their dosing appropriately (Table 1).
A pump is not a shortcut to checking glucose less frequently or making fewer decisions. However, for those who actively manage their diabetes, it provides more real-time flexibility and some important safety features, as discussed below.
IS A PUMP BETTER THAN INJECTIONS?
Several studies have compared insulin pump therapy and multiple daily injections.4–7 While some found no difference in glucose control in terms of hemoglobin A1c or hypoglycemia, others showed improved glucose control with pumps in patients who had higher baseline hemoglobin A1c levels (> 10%).6 In this subgroup, a pump lowered hemoglobin A1c an additional estimated 0.65% compared with multiple daily injections.6 Fructosamine levels also improved in pump users.6
Using continuous glucose monitoring for 3 days in a study in children with type 1 diabetes, Schreiver et al8 found lower insulin requirements and less-severe glycemic excursions with a pump than with multiple daily injections.
A 2013 study9 of 57 patients ages 13 to 71 with type 2 diabetes who were struggling to control their blood sugar with multiple daily injections found that they achieved better control with less insulin using a pump.
A meta-analysis found pump therapy to be more effective than multiple daily injections for those who used it more than 1 year.10
ADVANTAGES AND DISADVANTAGES OF INSULIN PUMP THERAPY
Intensive glucose control reduces microvascular complications in type 1 diabetes.11–14 The advantages of using a pump include better adherence, more accurate dosing, greater lifestyle flexibility, control of the dawn phenomenon without induction of nocturnal hypoglycemia, and the ability to suspend or temporarily reduce basal insulin to compensate for increased physical activity.15
Disadvantages include the high degree of technical aptitude required, the need for high-level engagement, skin reactions to tape, a higher risk of diabetic ketoacidosis from pump malfunction, infusion-site problems such as “tunneling” of insulin (leakage of insulin along the outside of the cannula and back to the skin surface) and clogging of the infusion set, and a risk of inactivation of insulin from exposure to heat, which can lead to ketoacidosis in a few hours if not addressed promptly.15
IS IT COST-EFFECTIVE?
There is evidence that continuous subcutaneous insulin infusion is cost-effective, both in general and compared with multiple daily injections for children and adults with type 1 diabetes mellitus. Cohen and Shaw16 found that life expectancy and quality-adjusted life-years increased in pump users, although the price per life-year gained varied greatly depending on the model used.
And this therapy is expensive. Most pumps cost more than $6,000, and supplies cost about $300 per month. Most insurance providers cover this therapy for patients with type 1 diabetes (Table 2) but less often for those with type 2. Further, many insurance policies have copayments, and patients may find a 20% co-payment a significant financial burden. Physicians need to obtain preapproval for insulin pumps from the insurance company. Typically, prescriptions for supplies are written annually. Despite these significant costs, most patients with type 1 diabetes who use an insulin pump find that the benefits of improved control and greater independence justify the cost.
An annual review of currently available insulin pumps and other diabetes-related equipment is published in Diabetes Forecast.17
PATIENT PERSPECTIVE ON INSULIN PUMP USE
Many patients who use a pump find that it gives them greater flexibility to adjust to day-to-day changes in schedules and routines. For example, consuming an extra serving at a meal could necessitate another injection for a patient on multiple daily injections, but a pump user would need only to push a few buttons. With cell phone apps available to control some pumps, many people find that an insulin pump is more discreet and easier to manage than carrying around injection supplies. Further, the complex calculations of carbohydrate ratios and correction factors are easier and more accurate with a pump.
In an open-label randomized study,18 29 of 41 patients with type 1 diabetes said they preferred a pump to multiple daily injections.
Conversely, some people do not want a pump because it is attached all the time and identifies them to others as having an illness. Other patients do not trust a machine and want control in their own hands. (Actually, machines typically are much more reliable and less mistake-prone than humans.)
HOW DOES A PUMP WORK COMPARED WITH MULTIPLE DAILY INJECTIONS?
Patients taking multiple daily injections must use two types of insulin: a long-acting one that reaches a steady level in the blood without a peak and lasts from 12 to 24 hours, and a rapid-acting one taken with meals, usually having a peak of action and an effect lasting 3 to 5 hours. The idea is to approximate normal insulin patterns, with a basal level in the background and peaks (boluses) of insulin with carbohydrate intake.
Insulin pumps use only one kind of insulin—a rapid-acting one, ie, lispro, aspart, or glulisine. They preserve the basal-bolus concept, but with many refinements (discussed below).15
Most pumps are attached to the patient by plastic tubing that connects the reservoir to a subcutaneous cannula or steel needle. However, some pumps have a reservoir directly attached to a subcutaneous cannula without the tubing. This type of pump is controlled with a remote device.
The infusion set (cannula or needle and tubing) and the site should be changed every third day to minimize the risk of infection and abnormal delivery due to protein buildup on the cannula os, epithelial healing, and irritation around the site. Failure to do so often results in higher blood glucose concentrations.19
The patient and healthcare team work together to calculate the patient’s daily insulin needs, and the pump is programmed based on the patient’s requirements, lifestyle, and sensitivity to insulin. Once the pump is started, the patient operates it to deliver the insulin dose according to carbohydrate intake and blood glucose level.
PUMP SETTINGS
Basal rate
The basal rate is programmed by the physician and is intended to mimic physiologic insulin release. The pump can be set to a number of basal rates within any 24-hour period. This provides more physiologic matching of insulin delivery to hourly insulin needs based on the patient’s daily schedule.
If the patient has been taking multiple daily injections, the hourly basal rate can be calculated by dividing the daily basal dose by 24. However, lower rates are usually used after midnight, and rates are increased early in the morning to counteract the dawn phenomenon.
The rates can also be adjusted temporarily (for up to 24 hours), with a feature called the temporary basal rate. People tend to have higher blood glucose levels when they have a respiratory illness, are under significant stress, or are menstruating. Thus, a person with influenza could increase the basal rate by 25%, or a student could run a temporary basal rate of 150% for 4 hours before taking a final exam.
Conversely, exercising increases insulin’s effectiveness at the muscle level, and insulin requirements drop. To counteract this, one would temporarily decrease the basal rate in the pump before exercising.
Many factors affect the bolus dose
A bolus of insulin is given for meals and to correct hyperglycemia, as with multiple daily injections. A pump calculates the bolus based on the carbohydrate ratio, correction factor, or both. These ratios are programmed into the pump by the physician. A benefit of the insulin pump is that the patient just has to input the amount of carbohydrates to be eaten or record a blood glucose level and the pump will calculate the bolus dose of insulin to be given.
The carbohydrate ratio is the amount of insulin that should be taken per amount of carbohydrate. A typical ratio is 1:15, meaning that the patient should take 1 unit of insulin for every 15 g of carbohydrates to be eaten. This varies by patient depending on insulin sensitivity.
The correction factor describes how much the glucose level is expected to drop per unit of insulin given. For example, if the target glucose level is 100 mg/dL and the correction factor is 25, then the patient will get 1 unit of correction of insulin if his or her glucose level is 125 mg/dL, 2 units if it is 150 mg/dL, and so on. A pump can dispense fractions of a unit.
The target glucose level or range is set by the physician and patient and is one of the factors the pump uses in calculating a bolus dose. Insulin pumps allow for multiple target glucose levels. Commonly, to minimize the risk of hypoglycemia, a higher (less strict) target is set for bedtime and overnight than for daytime.
Active insulin time defines how soon the patient can take another bolus.
Often, people eat more than they thought they would. They may also find that the glucose level did not increase or decrease as much as expected. Many patients who actively manage their glucose take additional boluses of insulin after a meal if their glucose is higher than they thought it would be. A patient taking injections cannot know how much of the insulin from the before-meal bolus is still working and has to guess.
Insulin pumps use a logarithmic formula to calculate this and prevent the user from “stacking” insulin boluses and lowering the glucose level too much. For example, if the active insulin time is 4 hours and the patient took a bolus for lunch at noon, he or she would be unable to take a full insulin correction dose until 4:00 pm. The patient can override this feature. Although the active insulin time varies from patient to patient, it is rarely more than 4 hours.
Additional safety features
Suspend. When a person who is taking insulin injections starts to experience hypoglycemia, he or she has one option—to eat something to treat the low blood glucose. The insulin injection has already been taken and cannot be reversed. However, with an insulin pump the patient can first suspend the pump so that no additional insulin is infused until it is safe again, and then eat to treat the low sugar level. This allows the patient to eat less, prevent overtreating, and, hopefully, prevent rebound hyperglycemia.
Reverse correction. When patients take insulin for an upcoming meal, they estimate the amount needed for the carbohydrates that they are about to eat as well as how much correction is needed. If their glucose level is below the target range, they may or may not subtract insulin from the dose to achieve the glucose target. The pump does this automatically, resulting in a lower dose of insulin for that bolus. This allows the patient to take a bolus for a meal even if he or she is below the target, and thus prevent hyperglycemia.
CAN INSULIN PUMPS BE USED IN THE HOSPITAL?
Patients can keep using their insulin pump in the hospital under the right conditions.
Inpatient hypoglycemia increases the risk of death, and although not all patients require tight glycemic control, there is still benefit in avoiding extremes in blood sugar levels,20 including at night.20–22 Insulin pump therapy, when used in the hospital, results in fewer episodes of severe hyperglycemia (glucose levels > 300 mg/dL) and hypoglycemia (levels < 40 mg/dL) than multiple daily injections.22 Moreover, most pump users feel more comfortable when they can manage their own therapy. Using the pump in the hospital has the additional benefit that patients can treat themselves before and after meals easily with less staff time and effort.
Bailon et al23 retrospectively studied 35 patients with insulin pumps in 50 hospitalizations. More than half of the patients were allowed to continue using their pump in the hospital. Reasons for discontinuing the pump included lack of access to supplies, unfamiliarity with the pump, attempted suicide, malfunctioning hardware, diabetic ketoacidosis, and altered mental status. Patients using their pump had fewer episodes of hypoglycemia (glucose levels < 70 mg/dL) than patients who removed their pump. In patients who continued using the pump throughout their hospitalization, no adverse events (eg, site infection or mechanical failure) were noted.
Leonhardi et al24 reviewed 25 hospital admissions, and the outcomes were similar to those reported by Bailon et al,23 with no adverse outcomes related to the pumps.
When using an insulin pump in the hospital
When a physician wants a patient to continue using an insulin pump in the hospital, a number of things must happen. The nursing staff must be informed that the patient is wearing a pump and can self-administer insulin. Most facilities will still follow routine protocols for checking blood glucose but will document that the patient is administering his or her own insulin. The patient must be well enough to manage the pump. If the infusion site needs to be changed, the patient would be expected to do so with his or her own supplies.
Imaging and insulin pumps
Advice differs on what to do if a patient with an insulin pump needs to undergo radiographic imaging. For example, the University of Wisconsin radiology department says it is safe to keep an insulin pump in place if the x-ray beam will be on for less than 3 seconds at a time and if the device is covered by a lead apron.25 However, radiation can induce electrical currents in the circuitry, which can alter the function of the pump. For this reason, some manufacturers recommend removing the device before the patient enters any room in which radiation or magnetic resonance imaging will be used.26–31
Insulin pumps and surgery
Insulin pumps have been used in the perioperative and intraoperative periods, with positive outcomes.32 An analysis of 20 patients on pumps undergoing a total of 23 surgeries (mostly orthopedic procedures) found that 13 of the 20 patients wore their pump during surgery. No adverse events were noted in any of these cases, although the sample size was small.33
Corney et al34 retrospectively compared insulin pumps with alternative methods of perioperative glucose management. Multiple surgical specialties were included. No significant difference in mean blood glucose levels was found between those who continued to use their pump and those who used other methods. In those who continued to use their pump, there were no episodes of intraoperative technical difficulties related to the pump.
Any patient who may be undergoing a procedure or surgery must let the surgeon and anesthesiologist know that he or she has a pump. If the infusion site is too close to the site of the surgery or procedure, it must be moved.
Concerns during surgery include catheter or site disconnection or loss, crystallization within the tubing (a potential problem not limited to surgery), and pump malfunction. If the procedure involves imaging, the pump should probably be disconnected or covered by lead shielding as directed in the pump manufacturer’s manual. The surgeon and anesthesiologist must decide whether to continue use of a pump during a surgical procedure. However, the study by Corney et al34 shows it is possible.
Most office-based procedures can be done with the insulin pump in place, as the patient is not under general anesthesia and so can adjust the insulin regimen as needed.
Abdelmalak et al,35 in a comprehensive review of insulin pump use in noncardiac surgery, commented that the type of surgery may play a role in determining the best approach to perioperative glucose management. Major surgery causes a large inflammatory response that makes it difficult to control blood sugar, especially when steroids or beta agonists are given, whereas minor surgery does not affect blood glucose nearly as much. The authors offered recommendations on pump use during various surgical procedures depending on the length of the procedure:
- If surgery is anticipated to last less than 1 hour, then keep the insulin pump on, and have the patient manage corrections preoperatively and postoperatively.
- For surgery of intermediate length (1–3 hours), have the patient take a bolus of 1 hour’s worth of insulin (based on the basal rate for that time period) before the procedure, then remove the insulin pump. Do this only if blood sugar is normal or close to normal. If the patient is severely hyperglycemic, remove the insulin pump and start an intravenous insulin infusion.
- If the procedure will take more than 3 hours, remove the pump and start an insulin infusion regardless of the blood sugar level.35
AIR TRAVEL AND INSULIN PUMPS
Insulin pumps can be easy to manage during airline travel if the user is prepared (Table 3).
First, it is important to have a letter from the treating physician stating that the pump is a necessary medical device. All supplies should be carried on and in a separate bag for easy inspection. The more forthcoming the user is at the security checkpoint, the easier the process.
According to the Transportation Security Administration, insulin pump users can keep their pump on during screening, and the metal detectors and full-body scanners will not harm the device.36
However, manufacturer recommendations differ. Medtronic recommends that patients not expose their insulin pump to x-rays, and that instead of going through a full-body scanner the patient should request a pat-down.37 Animas recommends the same.38 OmniPod states that their system can be worn through airport imaging, making it the only approved continuous insulin delivery system that can be taken through airport imaging.39
Another potential problem is the change in atmospheric pressure during takeoff and landing. Bubbles can form in the insulin reservoir as air pressure decreases with ascent, thereby displacing insulin from the pump to the patient. The opposite happens during descent. King et al40 corroborated this phenomenon with Animas and Medtronic pumps. Asante recommends removing their pump tubing during takeoff and landing.30
If PROBLEMS ARISE
Like any machine, an insulin pump can fail. Most failures result in lack of insulin delivery—the patient does not get excess insulin from insulin pump failure. Excess insulin delivery is most often due to operator error. All insulin is either preprogrammed (basal by provider or patient) or must be confirmed by the patient at the time of delivery (meal or correction boluses).
Pump manufacturers have 24-hour support programs and hotlines, with experts who will either walk the patient through the problem or send a replacement pump—often within 24 hours.
EVOLVING TECHNOLOGY
Pump technology is evolving quickly. On the way are “smart” pumps that interact with other systems, smaller pumps with advanced touch-screen features, and patch pumps that do not have tubing but operate similarly to pumps with tubing (ie, a cannula is still required for insulin delivery).
Some insulin pumps can be linked to an external glucose sensor. These systems provide a great amount of information to the patient and provider. Often, there is increased awareness of fluctuations in glucose, allowing earlier intervention to prevent high and low glucose excursions. Sensor-augmented pumps may further improve safety by suspending infusion during hypoglycemia.41,42
Researchers continue to strive for closed-loop systems that would allow the pump to automatically respond to circulating glucose and thus provide truly physiologic control.43 A recent study showed the effectiveness of the outpatient use of a bihormonal (insulin and glucagon) “bionic pancreas,” which provided improved glucose control and similar or less hypoglycemia in adults and adolescents who had been using a traditional insulin pump.44
The advent of the insulin pump in the late 1970s was a step forward in diabetes treatment,1 and recent improvements make these devices easier to use in intensive insulin management. Today, more than 400,000 people in the United States are thought to be using an insulin pump.2
With a pump, patients can adjust the dosage and discreetly give themselves boluses by simply pushing a button instead of giving themselves multiple daily injections. Also, pump therapy can be tailored to correct for hepatic glucose production in a way that injections cannot.
This article reviews the clinical application of continuous subcutaneous insulin therapy—ie, the insulin pump—and provides recommendations for patient selection and management.
INDICATIONS FOR AN INSULIN PUMP
The American Association of Clinical Endocrinologists3 recommends considering an insulin pump for patients with type 1 or 2 diabetes mellitus who have a clear indication:
- Suboptimal control on basal-bolus injections, ie, not achieving glycemic goals despite maximal adherence to multiple daily injections
- Wide and erratic glycemic excursions
- Frequent severe hypoglycemia, or hypoglycemia unawareness
- A marked “dawn phenomenon” (spike in blood glucose level early in the morning)
- Pregnancy or planning for pregnancy
- Erratic lifestyle
- Personal preference.
WHO IS A GOOD CANDIDATE FOR AN INSULIN PUMP?
Good candidates for a pump are patients with type 1 diabetes (and some with type 2) who are well versed in taking multiple daily injections, are already checking their glucose four or more times daily, “counting carbs” (estimating or, preferably, measuring how much carbohydrate they are eating, and limiting their intake accordingly), and demonstrate the ability to adjust their dosing appropriately (Table 1).
A pump is not a shortcut to checking glucose less frequently or making fewer decisions. However, for those who actively manage their diabetes, it provides more real-time flexibility and some important safety features, as discussed below.
IS A PUMP BETTER THAN INJECTIONS?
Several studies have compared insulin pump therapy and multiple daily injections.4–7 While some found no difference in glucose control in terms of hemoglobin A1c or hypoglycemia, others showed improved glucose control with pumps in patients who had higher baseline hemoglobin A1c levels (> 10%).6 In this subgroup, a pump lowered hemoglobin A1c an additional estimated 0.65% compared with multiple daily injections.6 Fructosamine levels also improved in pump users.6
Using continuous glucose monitoring for 3 days in a study in children with type 1 diabetes, Schreiver et al8 found lower insulin requirements and less-severe glycemic excursions with a pump than with multiple daily injections.
A 2013 study9 of 57 patients ages 13 to 71 with type 2 diabetes who were struggling to control their blood sugar with multiple daily injections found that they achieved better control with less insulin using a pump.
A meta-analysis found pump therapy to be more effective than multiple daily injections for those who used it more than 1 year.10
ADVANTAGES AND DISADVANTAGES OF INSULIN PUMP THERAPY
Intensive glucose control reduces microvascular complications in type 1 diabetes.11–14 The advantages of using a pump include better adherence, more accurate dosing, greater lifestyle flexibility, control of the dawn phenomenon without induction of nocturnal hypoglycemia, and the ability to suspend or temporarily reduce basal insulin to compensate for increased physical activity.15
Disadvantages include the high degree of technical aptitude required, the need for high-level engagement, skin reactions to tape, a higher risk of diabetic ketoacidosis from pump malfunction, infusion-site problems such as “tunneling” of insulin (leakage of insulin along the outside of the cannula and back to the skin surface) and clogging of the infusion set, and a risk of inactivation of insulin from exposure to heat, which can lead to ketoacidosis in a few hours if not addressed promptly.15
IS IT COST-EFFECTIVE?
There is evidence that continuous subcutaneous insulin infusion is cost-effective, both in general and compared with multiple daily injections for children and adults with type 1 diabetes mellitus. Cohen and Shaw16 found that life expectancy and quality-adjusted life-years increased in pump users, although the price per life-year gained varied greatly depending on the model used.
And this therapy is expensive. Most pumps cost more than $6,000, and supplies cost about $300 per month. Most insurance providers cover this therapy for patients with type 1 diabetes (Table 2) but less often for those with type 2. Further, many insurance policies have copayments, and patients may find a 20% co-payment a significant financial burden. Physicians need to obtain preapproval for insulin pumps from the insurance company. Typically, prescriptions for supplies are written annually. Despite these significant costs, most patients with type 1 diabetes who use an insulin pump find that the benefits of improved control and greater independence justify the cost.
An annual review of currently available insulin pumps and other diabetes-related equipment is published in Diabetes Forecast.17
PATIENT PERSPECTIVE ON INSULIN PUMP USE
Many patients who use a pump find that it gives them greater flexibility to adjust to day-to-day changes in schedules and routines. For example, consuming an extra serving at a meal could necessitate another injection for a patient on multiple daily injections, but a pump user would need only to push a few buttons. With cell phone apps available to control some pumps, many people find that an insulin pump is more discreet and easier to manage than carrying around injection supplies. Further, the complex calculations of carbohydrate ratios and correction factors are easier and more accurate with a pump.
In an open-label randomized study,18 29 of 41 patients with type 1 diabetes said they preferred a pump to multiple daily injections.
Conversely, some people do not want a pump because it is attached all the time and identifies them to others as having an illness. Other patients do not trust a machine and want control in their own hands. (Actually, machines typically are much more reliable and less mistake-prone than humans.)
HOW DOES A PUMP WORK COMPARED WITH MULTIPLE DAILY INJECTIONS?
Patients taking multiple daily injections must use two types of insulin: a long-acting one that reaches a steady level in the blood without a peak and lasts from 12 to 24 hours, and a rapid-acting one taken with meals, usually having a peak of action and an effect lasting 3 to 5 hours. The idea is to approximate normal insulin patterns, with a basal level in the background and peaks (boluses) of insulin with carbohydrate intake.
Insulin pumps use only one kind of insulin—a rapid-acting one, ie, lispro, aspart, or glulisine. They preserve the basal-bolus concept, but with many refinements (discussed below).15
Most pumps are attached to the patient by plastic tubing that connects the reservoir to a subcutaneous cannula or steel needle. However, some pumps have a reservoir directly attached to a subcutaneous cannula without the tubing. This type of pump is controlled with a remote device.
The infusion set (cannula or needle and tubing) and the site should be changed every third day to minimize the risk of infection and abnormal delivery due to protein buildup on the cannula os, epithelial healing, and irritation around the site. Failure to do so often results in higher blood glucose concentrations.19
The patient and healthcare team work together to calculate the patient’s daily insulin needs, and the pump is programmed based on the patient’s requirements, lifestyle, and sensitivity to insulin. Once the pump is started, the patient operates it to deliver the insulin dose according to carbohydrate intake and blood glucose level.
PUMP SETTINGS
Basal rate
The basal rate is programmed by the physician and is intended to mimic physiologic insulin release. The pump can be set to a number of basal rates within any 24-hour period. This provides more physiologic matching of insulin delivery to hourly insulin needs based on the patient’s daily schedule.
If the patient has been taking multiple daily injections, the hourly basal rate can be calculated by dividing the daily basal dose by 24. However, lower rates are usually used after midnight, and rates are increased early in the morning to counteract the dawn phenomenon.
The rates can also be adjusted temporarily (for up to 24 hours), with a feature called the temporary basal rate. People tend to have higher blood glucose levels when they have a respiratory illness, are under significant stress, or are menstruating. Thus, a person with influenza could increase the basal rate by 25%, or a student could run a temporary basal rate of 150% for 4 hours before taking a final exam.
Conversely, exercising increases insulin’s effectiveness at the muscle level, and insulin requirements drop. To counteract this, one would temporarily decrease the basal rate in the pump before exercising.
Many factors affect the bolus dose
A bolus of insulin is given for meals and to correct hyperglycemia, as with multiple daily injections. A pump calculates the bolus based on the carbohydrate ratio, correction factor, or both. These ratios are programmed into the pump by the physician. A benefit of the insulin pump is that the patient just has to input the amount of carbohydrates to be eaten or record a blood glucose level and the pump will calculate the bolus dose of insulin to be given.
The carbohydrate ratio is the amount of insulin that should be taken per amount of carbohydrate. A typical ratio is 1:15, meaning that the patient should take 1 unit of insulin for every 15 g of carbohydrates to be eaten. This varies by patient depending on insulin sensitivity.
The correction factor describes how much the glucose level is expected to drop per unit of insulin given. For example, if the target glucose level is 100 mg/dL and the correction factor is 25, then the patient will get 1 unit of correction of insulin if his or her glucose level is 125 mg/dL, 2 units if it is 150 mg/dL, and so on. A pump can dispense fractions of a unit.
The target glucose level or range is set by the physician and patient and is one of the factors the pump uses in calculating a bolus dose. Insulin pumps allow for multiple target glucose levels. Commonly, to minimize the risk of hypoglycemia, a higher (less strict) target is set for bedtime and overnight than for daytime.
Active insulin time defines how soon the patient can take another bolus.
Often, people eat more than they thought they would. They may also find that the glucose level did not increase or decrease as much as expected. Many patients who actively manage their glucose take additional boluses of insulin after a meal if their glucose is higher than they thought it would be. A patient taking injections cannot know how much of the insulin from the before-meal bolus is still working and has to guess.
Insulin pumps use a logarithmic formula to calculate this and prevent the user from “stacking” insulin boluses and lowering the glucose level too much. For example, if the active insulin time is 4 hours and the patient took a bolus for lunch at noon, he or she would be unable to take a full insulin correction dose until 4:00 pm. The patient can override this feature. Although the active insulin time varies from patient to patient, it is rarely more than 4 hours.
Additional safety features
Suspend. When a person who is taking insulin injections starts to experience hypoglycemia, he or she has one option—to eat something to treat the low blood glucose. The insulin injection has already been taken and cannot be reversed. However, with an insulin pump the patient can first suspend the pump so that no additional insulin is infused until it is safe again, and then eat to treat the low sugar level. This allows the patient to eat less, prevent overtreating, and, hopefully, prevent rebound hyperglycemia.
Reverse correction. When patients take insulin for an upcoming meal, they estimate the amount needed for the carbohydrates that they are about to eat as well as how much correction is needed. If their glucose level is below the target range, they may or may not subtract insulin from the dose to achieve the glucose target. The pump does this automatically, resulting in a lower dose of insulin for that bolus. This allows the patient to take a bolus for a meal even if he or she is below the target, and thus prevent hyperglycemia.
CAN INSULIN PUMPS BE USED IN THE HOSPITAL?
Patients can keep using their insulin pump in the hospital under the right conditions.
Inpatient hypoglycemia increases the risk of death, and although not all patients require tight glycemic control, there is still benefit in avoiding extremes in blood sugar levels,20 including at night.20–22 Insulin pump therapy, when used in the hospital, results in fewer episodes of severe hyperglycemia (glucose levels > 300 mg/dL) and hypoglycemia (levels < 40 mg/dL) than multiple daily injections.22 Moreover, most pump users feel more comfortable when they can manage their own therapy. Using the pump in the hospital has the additional benefit that patients can treat themselves before and after meals easily with less staff time and effort.
Bailon et al23 retrospectively studied 35 patients with insulin pumps in 50 hospitalizations. More than half of the patients were allowed to continue using their pump in the hospital. Reasons for discontinuing the pump included lack of access to supplies, unfamiliarity with the pump, attempted suicide, malfunctioning hardware, diabetic ketoacidosis, and altered mental status. Patients using their pump had fewer episodes of hypoglycemia (glucose levels < 70 mg/dL) than patients who removed their pump. In patients who continued using the pump throughout their hospitalization, no adverse events (eg, site infection or mechanical failure) were noted.
Leonhardi et al24 reviewed 25 hospital admissions, and the outcomes were similar to those reported by Bailon et al,23 with no adverse outcomes related to the pumps.
When using an insulin pump in the hospital
When a physician wants a patient to continue using an insulin pump in the hospital, a number of things must happen. The nursing staff must be informed that the patient is wearing a pump and can self-administer insulin. Most facilities will still follow routine protocols for checking blood glucose but will document that the patient is administering his or her own insulin. The patient must be well enough to manage the pump. If the infusion site needs to be changed, the patient would be expected to do so with his or her own supplies.
Imaging and insulin pumps
Advice differs on what to do if a patient with an insulin pump needs to undergo radiographic imaging. For example, the University of Wisconsin radiology department says it is safe to keep an insulin pump in place if the x-ray beam will be on for less than 3 seconds at a time and if the device is covered by a lead apron.25 However, radiation can induce electrical currents in the circuitry, which can alter the function of the pump. For this reason, some manufacturers recommend removing the device before the patient enters any room in which radiation or magnetic resonance imaging will be used.26–31
Insulin pumps and surgery
Insulin pumps have been used in the perioperative and intraoperative periods, with positive outcomes.32 An analysis of 20 patients on pumps undergoing a total of 23 surgeries (mostly orthopedic procedures) found that 13 of the 20 patients wore their pump during surgery. No adverse events were noted in any of these cases, although the sample size was small.33
Corney et al34 retrospectively compared insulin pumps with alternative methods of perioperative glucose management. Multiple surgical specialties were included. No significant difference in mean blood glucose levels was found between those who continued to use their pump and those who used other methods. In those who continued to use their pump, there were no episodes of intraoperative technical difficulties related to the pump.
Any patient who may be undergoing a procedure or surgery must let the surgeon and anesthesiologist know that he or she has a pump. If the infusion site is too close to the site of the surgery or procedure, it must be moved.
Concerns during surgery include catheter or site disconnection or loss, crystallization within the tubing (a potential problem not limited to surgery), and pump malfunction. If the procedure involves imaging, the pump should probably be disconnected or covered by lead shielding as directed in the pump manufacturer’s manual. The surgeon and anesthesiologist must decide whether to continue use of a pump during a surgical procedure. However, the study by Corney et al34 shows it is possible.
Most office-based procedures can be done with the insulin pump in place, as the patient is not under general anesthesia and so can adjust the insulin regimen as needed.
Abdelmalak et al,35 in a comprehensive review of insulin pump use in noncardiac surgery, commented that the type of surgery may play a role in determining the best approach to perioperative glucose management. Major surgery causes a large inflammatory response that makes it difficult to control blood sugar, especially when steroids or beta agonists are given, whereas minor surgery does not affect blood glucose nearly as much. The authors offered recommendations on pump use during various surgical procedures depending on the length of the procedure:
- If surgery is anticipated to last less than 1 hour, then keep the insulin pump on, and have the patient manage corrections preoperatively and postoperatively.
- For surgery of intermediate length (1–3 hours), have the patient take a bolus of 1 hour’s worth of insulin (based on the basal rate for that time period) before the procedure, then remove the insulin pump. Do this only if blood sugar is normal or close to normal. If the patient is severely hyperglycemic, remove the insulin pump and start an intravenous insulin infusion.
- If the procedure will take more than 3 hours, remove the pump and start an insulin infusion regardless of the blood sugar level.35
AIR TRAVEL AND INSULIN PUMPS
Insulin pumps can be easy to manage during airline travel if the user is prepared (Table 3).
First, it is important to have a letter from the treating physician stating that the pump is a necessary medical device. All supplies should be carried on and in a separate bag for easy inspection. The more forthcoming the user is at the security checkpoint, the easier the process.
According to the Transportation Security Administration, insulin pump users can keep their pump on during screening, and the metal detectors and full-body scanners will not harm the device.36
However, manufacturer recommendations differ. Medtronic recommends that patients not expose their insulin pump to x-rays, and that instead of going through a full-body scanner the patient should request a pat-down.37 Animas recommends the same.38 OmniPod states that their system can be worn through airport imaging, making it the only approved continuous insulin delivery system that can be taken through airport imaging.39
Another potential problem is the change in atmospheric pressure during takeoff and landing. Bubbles can form in the insulin reservoir as air pressure decreases with ascent, thereby displacing insulin from the pump to the patient. The opposite happens during descent. King et al40 corroborated this phenomenon with Animas and Medtronic pumps. Asante recommends removing their pump tubing during takeoff and landing.30
If PROBLEMS ARISE
Like any machine, an insulin pump can fail. Most failures result in lack of insulin delivery—the patient does not get excess insulin from insulin pump failure. Excess insulin delivery is most often due to operator error. All insulin is either preprogrammed (basal by provider or patient) or must be confirmed by the patient at the time of delivery (meal or correction boluses).
Pump manufacturers have 24-hour support programs and hotlines, with experts who will either walk the patient through the problem or send a replacement pump—often within 24 hours.
EVOLVING TECHNOLOGY
Pump technology is evolving quickly. On the way are “smart” pumps that interact with other systems, smaller pumps with advanced touch-screen features, and patch pumps that do not have tubing but operate similarly to pumps with tubing (ie, a cannula is still required for insulin delivery).
Some insulin pumps can be linked to an external glucose sensor. These systems provide a great amount of information to the patient and provider. Often, there is increased awareness of fluctuations in glucose, allowing earlier intervention to prevent high and low glucose excursions. Sensor-augmented pumps may further improve safety by suspending infusion during hypoglycemia.41,42
Researchers continue to strive for closed-loop systems that would allow the pump to automatically respond to circulating glucose and thus provide truly physiologic control.43 A recent study showed the effectiveness of the outpatient use of a bihormonal (insulin and glucagon) “bionic pancreas,” which provided improved glucose control and similar or less hypoglycemia in adults and adolescents who had been using a traditional insulin pump.44
- Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care 2002; 25:593–598.
- JDRF and BD collaborate to improve insulin pump delivery. www.bd.com/_Images/BD_JDRF_press_release_2010_tcm49-19552.pdf. Accessed October 14, 2015.
- Grunberger G, Abelseth JM, Bailey TS, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology insulin pump management task force. Endocr Pract 2014; 20:463–489.
- Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001; 24:1722–1727.
- Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care 2005; 28:1568–1573.
- Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care 2004; 27:2590–2596.
- Hirsch IB, Bode BW, Garg S, et al; Insulin Aspart CSII/MDI Comparison Study Group. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005; 28:533–538.
- Schreiver C, Jacoby U, Watzer B, Thomas A, Haffner D, Fischer DC. Glycaemic variability in paediatric patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI): a cross-sectional cohort study. Clin Endocrinol (Oxf) 2013; 79:641–647.
- Leinung MC, Thompson S, Luo M, Leykina L, Nardacci E. Use of insulin pump therapy in patients with type 2 diabetes after failure of multiple daily injections. Endocr Pract 2013; 19:9–13.
- Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003; 26:1079-1087.
- Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care 1995; 18:361–376.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352:837–853.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Skyler JS, Ponder S, Kruger DF, Matheson D, Parkin CG. Is there a place for insulin pump therapy in your practice? Clinical Diabetes 2007; 25:50–56.
- Cohen N, Shaw J. Cost effectiveness of insulin pump therapy. Infusystems Asia 2007; 2:25–28.
- Tucker ME. Insulin pumps: closer to a pancreas. Diabetes Forecast. www.diabetesforecast.org/2015/mar-apr/insulin-pumps-closer-to-pancreas.html. Accessed October 14, 2015.
- Hanaire-Broutin H, Melki V, Bessières-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care 2000; 23:1232–1235.
- Schmid V, Hohberg C, Borchert M, Forst T, Pfützner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol 2010; 4:976–982.
- Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009; 15:353–369.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Cook CB, Beer KA, Seifert KM, Boyle ME, Mackey PA, Castro JC. Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years’ experience with 253 cases. J Diabetes Sci Technol 2012; 6:995–1002.
- Bailon RM, Partlow BJ, Miller-Cage V, et al. Continuous subcutaneous insulin infusion (insulin pump) therapy can be safely used in the hospital in select patients. Endocr Pract 2009; 15:24–29.
- Leonhardi BJ, Boyle ME, Beer KA, et al. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital: a review of one institution’s experience. J Diabetes Sci Technol 2008; 2:948–962.
- Department of Radiology, University of Wisconsin School of Medicine and Public Health. Precautions with implanted devices. www.radiology.wisc.edu/fileShelf/forReferring/PrecautionsWithImplantedDevices_CTandXRAY.php. Accessed October 14, 2015.
- Indications, contraindications, warnings and precautions. Medtronicdiabetes.com/important-safety-information. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- T:slim user guide. www.tandemdiabetes.com/uploadedFiles/Content/_Configuration/Files/Manuals/tslim_User_Guide.pdf. Tandem Diabetes Care. Accessed October 14, 2015.
- OmniPod user guide. www.myomnipodtraining.com/pdf/OmniPod-User-Guide-UST400.pdf. Insulet Corporation. Accessed October 14, 2015.
- Important safety information.Animas Vibe Insulin Pump and CGM System. www.animas.com/safety. Animas Corporation. Accessed October 14, 2015.
- Snap insulin pump safety information. Snappump.com/safety-information. Asante Solutions, Inc. Accessed October 14, 2015.
- ACCU-CHEK Spirit insulin pump system. Pump user guide. www.accu-chekinsulinpumps.com/documents/PumpUserGuide.pdf. Disetronic Medical Systems, Inc. Accessed October 14, 2015.
- White WA Jr, Montalvo H, Monday JM. Continuous subcutaneous insulin infusion during general anesthesia: a case report. AANA J 2004; 72:353–357.
- Boyle ME, Seifert KM, Beer KA, et al. Insulin pump therapy in the perioperative period: a review of care after implementation of institutional guidelines. J Diabetes Sci Technol 2012; 6:1016–1021.
- Corney SM, Dukatz T, Rosenblatt S, et al. Comparison of insulin pump therapy (continuous subcutaneous insulin infusion) to alternative methods for perioperative glycemic management in patients with planned postoperative admissions. J Diabetes Sci Technol 2012; 6:1003–1015.
- Abdelmalak B, Ibrahim M, Yared JP, Modic MB, Nasr C. Perioperative glycemic management in insulin pump patients undergoing noncardiac surgery. Curr Pharm Des 2012; 18:6204–6214.
- US Department of Homeland Security. Travelers with disabilities and medical conditions. www.tsa.gov/travel/special-procedures. Transportation Security Administration. Accessed October 14, 2015.
- Medical emergency card/airport information. www.medtronicdiabetes.com/sites/default/files/library/support/Airport%20Information%20Card.pdf. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- Traveling with an insulin pump. www.animas.com/about-insulin-pump-therapy/traveling-with-diabetes. Animas Corporation. Accessed October 14, 2015.
- Tips for air travel with diabetes supplies. www.myomnipod.com/pdf/14986-AWAirTravelTipsFlyerR2-11-11.pdf. Insulet Corporation. Accessed October 14, 2015.
- King BR, Goss PW, Paterson MA, Crock PA, Anderson DG. Changes in altitude cause unintended insulin delivery from insulin pumps: mechanisms and implications. Diabetes Care 2011; 34:1932–1933.
- Bergenstal RM, Tamborlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010; 363:311–320.
- Bergenstal RM, Klonoff DC, Garg SK, et al; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013; 369:224–232.
- Bequette BW. Challenges and recent progress in the development of a closed-loop artificial pancreas. Annu Rev Control 2012; 36:255–266.
- Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014; 371:313–325.
- Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care 2002; 25:593–598.
- JDRF and BD collaborate to improve insulin pump delivery. www.bd.com/_Images/BD_JDRF_press_release_2010_tcm49-19552.pdf. Accessed October 14, 2015.
- Grunberger G, Abelseth JM, Bailey TS, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology insulin pump management task force. Endocr Pract 2014; 20:463–489.
- Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001; 24:1722–1727.
- Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care 2005; 28:1568–1573.
- Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care 2004; 27:2590–2596.
- Hirsch IB, Bode BW, Garg S, et al; Insulin Aspart CSII/MDI Comparison Study Group. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005; 28:533–538.
- Schreiver C, Jacoby U, Watzer B, Thomas A, Haffner D, Fischer DC. Glycaemic variability in paediatric patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI): a cross-sectional cohort study. Clin Endocrinol (Oxf) 2013; 79:641–647.
- Leinung MC, Thompson S, Luo M, Leykina L, Nardacci E. Use of insulin pump therapy in patients with type 2 diabetes after failure of multiple daily injections. Endocr Pract 2013; 19:9–13.
- Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003; 26:1079-1087.
- Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care 1995; 18:361–376.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352:837–853.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Skyler JS, Ponder S, Kruger DF, Matheson D, Parkin CG. Is there a place for insulin pump therapy in your practice? Clinical Diabetes 2007; 25:50–56.
- Cohen N, Shaw J. Cost effectiveness of insulin pump therapy. Infusystems Asia 2007; 2:25–28.
- Tucker ME. Insulin pumps: closer to a pancreas. Diabetes Forecast. www.diabetesforecast.org/2015/mar-apr/insulin-pumps-closer-to-pancreas.html. Accessed October 14, 2015.
- Hanaire-Broutin H, Melki V, Bessières-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care 2000; 23:1232–1235.
- Schmid V, Hohberg C, Borchert M, Forst T, Pfützner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol 2010; 4:976–982.
- Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009; 15:353–369.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Cook CB, Beer KA, Seifert KM, Boyle ME, Mackey PA, Castro JC. Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years’ experience with 253 cases. J Diabetes Sci Technol 2012; 6:995–1002.
- Bailon RM, Partlow BJ, Miller-Cage V, et al. Continuous subcutaneous insulin infusion (insulin pump) therapy can be safely used in the hospital in select patients. Endocr Pract 2009; 15:24–29.
- Leonhardi BJ, Boyle ME, Beer KA, et al. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital: a review of one institution’s experience. J Diabetes Sci Technol 2008; 2:948–962.
- Department of Radiology, University of Wisconsin School of Medicine and Public Health. Precautions with implanted devices. www.radiology.wisc.edu/fileShelf/forReferring/PrecautionsWithImplantedDevices_CTandXRAY.php. Accessed October 14, 2015.
- Indications, contraindications, warnings and precautions. Medtronicdiabetes.com/important-safety-information. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- T:slim user guide. www.tandemdiabetes.com/uploadedFiles/Content/_Configuration/Files/Manuals/tslim_User_Guide.pdf. Tandem Diabetes Care. Accessed October 14, 2015.
- OmniPod user guide. www.myomnipodtraining.com/pdf/OmniPod-User-Guide-UST400.pdf. Insulet Corporation. Accessed October 14, 2015.
- Important safety information.Animas Vibe Insulin Pump and CGM System. www.animas.com/safety. Animas Corporation. Accessed October 14, 2015.
- Snap insulin pump safety information. Snappump.com/safety-information. Asante Solutions, Inc. Accessed October 14, 2015.
- ACCU-CHEK Spirit insulin pump system. Pump user guide. www.accu-chekinsulinpumps.com/documents/PumpUserGuide.pdf. Disetronic Medical Systems, Inc. Accessed October 14, 2015.
- White WA Jr, Montalvo H, Monday JM. Continuous subcutaneous insulin infusion during general anesthesia: a case report. AANA J 2004; 72:353–357.
- Boyle ME, Seifert KM, Beer KA, et al. Insulin pump therapy in the perioperative period: a review of care after implementation of institutional guidelines. J Diabetes Sci Technol 2012; 6:1016–1021.
- Corney SM, Dukatz T, Rosenblatt S, et al. Comparison of insulin pump therapy (continuous subcutaneous insulin infusion) to alternative methods for perioperative glycemic management in patients with planned postoperative admissions. J Diabetes Sci Technol 2012; 6:1003–1015.
- Abdelmalak B, Ibrahim M, Yared JP, Modic MB, Nasr C. Perioperative glycemic management in insulin pump patients undergoing noncardiac surgery. Curr Pharm Des 2012; 18:6204–6214.
- US Department of Homeland Security. Travelers with disabilities and medical conditions. www.tsa.gov/travel/special-procedures. Transportation Security Administration. Accessed October 14, 2015.
- Medical emergency card/airport information. www.medtronicdiabetes.com/sites/default/files/library/support/Airport%20Information%20Card.pdf. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- Traveling with an insulin pump. www.animas.com/about-insulin-pump-therapy/traveling-with-diabetes. Animas Corporation. Accessed October 14, 2015.
- Tips for air travel with diabetes supplies. www.myomnipod.com/pdf/14986-AWAirTravelTipsFlyerR2-11-11.pdf. Insulet Corporation. Accessed October 14, 2015.
- King BR, Goss PW, Paterson MA, Crock PA, Anderson DG. Changes in altitude cause unintended insulin delivery from insulin pumps: mechanisms and implications. Diabetes Care 2011; 34:1932–1933.
- Bergenstal RM, Tamborlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010; 363:311–320.
- Bergenstal RM, Klonoff DC, Garg SK, et al; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013; 369:224–232.
- Bequette BW. Challenges and recent progress in the development of a closed-loop artificial pancreas. Annu Rev Control 2012; 36:255–266.
- Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014; 371:313–325.
KEY POINTS
- Insulin pumps allow for more accurate insulin dosing than multiple daily injections, resulting in less drastic extremes in blood sugar.
- Insulin pumps allow for more individualized basal insulin coverage than long-acting injectable insulin.
- Both the patient and provider need a good understanding of insulin pump therapy for successful pump management.