User login
The Translational Revolution in Atopic Dermatitis, and How It Also Translates to Other Inflammatory Skin Diseases
Atopic dermatitis (AD) is the most common inflammatory skin disease in both adults and children.1 Unfortunately, the current treatment armamentarium is largely confined to topical calcineurin inhibitors, topical and systemic steroids, phototherapy, cyclosporine (not approved by the US Food and Drug Administration for AD), and other oral immunosuppressants.2 The availability of partially helpful and highly toxic treatments creates a huge unmet need for more effective and safer treatments, particularly for patients with moderate to severe AD who often require systemic approaches.
Recent extensive translational (bench top to bedside and back) investigations in skin of AD patients has shown that skin phenotype is characterized by increased T-cell infiltration and related inflammatory cytokines as well as epidermal abnormalities (eg, hyperplasia, aberrant differentiation).3 Clinical improvement of AD has been demonstrated with broad T-cell targeted therapeutics, such as cyclosporine and narrowband UVB, coupled with decreases of T-cell infiltrates and inflammatory gene products as well as improvement of the pathologic epidermal phenotype.4,5
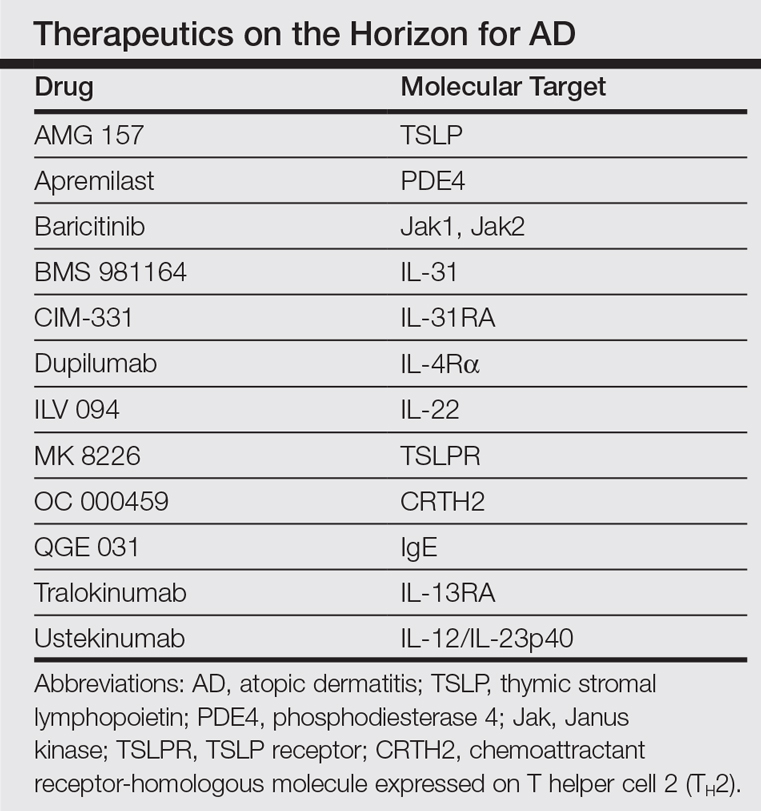
In the past, AD was conceptualized as a T helper cell TH2 (acute disease)/TH1 (chronic disease) bipolar cytokine disorder.6 Acute lesions are characterized by high TH2, TH22, and some TH17 signals, with intensification of these axes and TH1 augmentation orchestrating the chronic phenotype.7 The identification of the inflammatory pathways underlying AD has led to the development and testing of more than 10 broad or targeted therapeutics (Table).8 Phase 1 and phase 2 studies of dupilumab (targeting IL-4Rα) have shown not only tremendous AD improvement (~70%) but also tissue reversal of the immune and barrier abnormalities, including inflammatory cytokines and epidermal hyperplasia.9-11 As a result, other TH2 axis inhibitors (anti–IL-13/tralokinumab, anti–IL-31RA/CIM 331) are now in clinical trials. The identification of IL-22 in AD lesions has prompted trials with an anti–IL-22 (ILV 094) and an IL-12/IL-23p40 (ustekinumab) inhibitor.12 For psoriasis, ustekinumab showed 75% improvement in approximately 70% of patients,13 but for AD, despite clear clinical and molecular effects, differences compared to placebo were not statistically significant,12 probably due to underdosing of the drug in an excessively immune-activated disease14 as well as allowing topical steroids in patients, which may minimize the differences in treatment effect between drug and placebo.
The developments seen in AD are now moving into other inflammatory skin diseases, particularly alopecia areata (AA), a T-cell–mediated disease that shares phenotypic similarities with AD and often is associated with it.15 There is a paucity of effective, remission-sustaining treatments of AA, particularly for patients with severe disease who rarely experience spontaneous hair regrowth and who have a limited response to topical interventions.16,17 Our clinical experience showed that successfully treating patients with concurrent AD and AA has led to hair regrowth. Inspired by these clinical observations and by results obtained in AD,9-12 studying AA skin showed an upregulation of not only the traditionally suspected culprit TH1 but also TH2 and TH9 axes, IL-23 cytokines, and phosphodiesterase 4.18 Subsequently, a pilot study of 3 patients with extensive AA treated with ustekinumab showed that all 3 patients not only experienced hair regrowth but also had a reduction in inflammatory markers in scalp lesions.19 Although these results are promising, AA is an immunologically complex disease and it is yet to be determined if therapeutically targeting 1 (eg, IL-4) rather than a wide array of cytokines can reverse disease phenotype. There are ongoing clinical trials directed at different pathogenic targets (eg, Jak inhibitors, IL-13 antagonist, IL-17 antagonist, phosphodiesterase 4 antagonist); some showed some efficacy in small studies.20,21
The finding of a commonly upregulated TH2 pathway in both AD and AA will pave the way for studies with TH2 antagonists in AA patients. Future targeted therapeutic studies will shed light on the pathogenic pathways of this devastating skin disease and answer the extensive unmet therapeutic need it presents.
- Czarnowicki T, Krueger JG, Guttman-Yassky E. Skin barrier and immune dysregulation in atopic dermatitis: an evolving story with important clinical implications. J Allergy Clin Immunol Pract. 2014;2:371-379; quiz 380-381.
- Roekevisch E, Spuls PI, Kuester D, et al. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133:429-438.
- Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis—part
I: clinical and pathologic concepts. J Allergy Clin Immunol. 2011;127:1110-1118. - Khattri S, Shemer A, Rozenblit M, et al. Cyclosporine in patients with atopic dermatitis modulates activated inflammatory pathways and reverses epidermal pathology. J Allergy Clin Immunol. 2014;133:1626-1634.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response [published online July 16, 2011]. J Allergy Clin Immunol. 2011;128:583-593.
- Eyerich K, Novak N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy. 2013;68:974-982.
- Gittler JK, Shemer A, Suárez-Fariñas M, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis [published online August 27, 2012]. J Allergy Clin Immunol. 2012;130:1344-1354.
- Noda S, Krueger JG, Guttman-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324-336.
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Hamilton JD, Suárez-Fariñas M, Dhingra N, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2014;134:1293-1300.
- Hamilton J, Ren H, Weinstein SP, et al. Dupilumab improved all domains of Eczema Area and Severity Index (EASI) and 5-D pruritus scale in adults with atopic dermatitis in a phase 2 study. J Invest Dermatol. 2014;134:S104.
- Khattri S, Brunner PM, Garcet S, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis [published online June 15, 2016]. Exp Dermatol. doi:10.1111/exd.13112.
- Griffiths CEM, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
- Czarnowicki T, Malajian D, Shemer A, et al. Skin-homing and systemic T-cell subsets show higher activation in atopic dermatitis versus psoriasis. J Allergy Clin Immunol. 2015;136:208-211.
- Barahmani N, Schabath MB, Duvic M. History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol. 2009;61:581-591.
- Price VH, Hordinsky MK, Olsen EA, et al. Subcutaneous efalizumab is not effective in the treatment of alopecia areata. J Am Acad Dermatol. 2008;58:395-402.
- Alkhalifah A, Alsantali A, Wang E, et al. Alopecia areata update part II. treatment. J Am Acad Dermatol. 2010;62:191-202.
- Suárez-Fariñas M, Ungar B, Noda S, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136:1277-1287.
- Guttman-Yassky E, Ungar B, Noda S, et al. Extensive alopecia areata is reversed by IL-12/IL-23p40 cytokine antagonism. J Allergy Clin Immunol. 2016;137:301-304.
- Xing LZ, Dai ZP, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Castela E, Le Duff F, Butori C, et al. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol. 2014;150:748-751.
Atopic dermatitis (AD) is the most common inflammatory skin disease in both adults and children.1 Unfortunately, the current treatment armamentarium is largely confined to topical calcineurin inhibitors, topical and systemic steroids, phototherapy, cyclosporine (not approved by the US Food and Drug Administration for AD), and other oral immunosuppressants.2 The availability of partially helpful and highly toxic treatments creates a huge unmet need for more effective and safer treatments, particularly for patients with moderate to severe AD who often require systemic approaches.
Recent extensive translational (bench top to bedside and back) investigations in skin of AD patients has shown that skin phenotype is characterized by increased T-cell infiltration and related inflammatory cytokines as well as epidermal abnormalities (eg, hyperplasia, aberrant differentiation).3 Clinical improvement of AD has been demonstrated with broad T-cell targeted therapeutics, such as cyclosporine and narrowband UVB, coupled with decreases of T-cell infiltrates and inflammatory gene products as well as improvement of the pathologic epidermal phenotype.4,5
In the past, AD was conceptualized as a T helper cell TH2 (acute disease)/TH1 (chronic disease) bipolar cytokine disorder.6 Acute lesions are characterized by high TH2, TH22, and some TH17 signals, with intensification of these axes and TH1 augmentation orchestrating the chronic phenotype.7 The identification of the inflammatory pathways underlying AD has led to the development and testing of more than 10 broad or targeted therapeutics (Table).8 Phase 1 and phase 2 studies of dupilumab (targeting IL-4Rα) have shown not only tremendous AD improvement (~70%) but also tissue reversal of the immune and barrier abnormalities, including inflammatory cytokines and epidermal hyperplasia.9-11 As a result, other TH2 axis inhibitors (anti–IL-13/tralokinumab, anti–IL-31RA/CIM 331) are now in clinical trials. The identification of IL-22 in AD lesions has prompted trials with an anti–IL-22 (ILV 094) and an IL-12/IL-23p40 (ustekinumab) inhibitor.12 For psoriasis, ustekinumab showed 75% improvement in approximately 70% of patients,13 but for AD, despite clear clinical and molecular effects, differences compared to placebo were not statistically significant,12 probably due to underdosing of the drug in an excessively immune-activated disease14 as well as allowing topical steroids in patients, which may minimize the differences in treatment effect between drug and placebo.
The developments seen in AD are now moving into other inflammatory skin diseases, particularly alopecia areata (AA), a T-cell–mediated disease that shares phenotypic similarities with AD and often is associated with it.15 There is a paucity of effective, remission-sustaining treatments of AA, particularly for patients with severe disease who rarely experience spontaneous hair regrowth and who have a limited response to topical interventions.16,17 Our clinical experience showed that successfully treating patients with concurrent AD and AA has led to hair regrowth. Inspired by these clinical observations and by results obtained in AD,9-12 studying AA skin showed an upregulation of not only the traditionally suspected culprit TH1 but also TH2 and TH9 axes, IL-23 cytokines, and phosphodiesterase 4.18 Subsequently, a pilot study of 3 patients with extensive AA treated with ustekinumab showed that all 3 patients not only experienced hair regrowth but also had a reduction in inflammatory markers in scalp lesions.19 Although these results are promising, AA is an immunologically complex disease and it is yet to be determined if therapeutically targeting 1 (eg, IL-4) rather than a wide array of cytokines can reverse disease phenotype. There are ongoing clinical trials directed at different pathogenic targets (eg, Jak inhibitors, IL-13 antagonist, IL-17 antagonist, phosphodiesterase 4 antagonist); some showed some efficacy in small studies.20,21
The finding of a commonly upregulated TH2 pathway in both AD and AA will pave the way for studies with TH2 antagonists in AA patients. Future targeted therapeutic studies will shed light on the pathogenic pathways of this devastating skin disease and answer the extensive unmet therapeutic need it presents.
Atopic dermatitis (AD) is the most common inflammatory skin disease in both adults and children.1 Unfortunately, the current treatment armamentarium is largely confined to topical calcineurin inhibitors, topical and systemic steroids, phototherapy, cyclosporine (not approved by the US Food and Drug Administration for AD), and other oral immunosuppressants.2 The availability of partially helpful and highly toxic treatments creates a huge unmet need for more effective and safer treatments, particularly for patients with moderate to severe AD who often require systemic approaches.
Recent extensive translational (bench top to bedside and back) investigations in skin of AD patients has shown that skin phenotype is characterized by increased T-cell infiltration and related inflammatory cytokines as well as epidermal abnormalities (eg, hyperplasia, aberrant differentiation).3 Clinical improvement of AD has been demonstrated with broad T-cell targeted therapeutics, such as cyclosporine and narrowband UVB, coupled with decreases of T-cell infiltrates and inflammatory gene products as well as improvement of the pathologic epidermal phenotype.4,5
In the past, AD was conceptualized as a T helper cell TH2 (acute disease)/TH1 (chronic disease) bipolar cytokine disorder.6 Acute lesions are characterized by high TH2, TH22, and some TH17 signals, with intensification of these axes and TH1 augmentation orchestrating the chronic phenotype.7 The identification of the inflammatory pathways underlying AD has led to the development and testing of more than 10 broad or targeted therapeutics (Table).8 Phase 1 and phase 2 studies of dupilumab (targeting IL-4Rα) have shown not only tremendous AD improvement (~70%) but also tissue reversal of the immune and barrier abnormalities, including inflammatory cytokines and epidermal hyperplasia.9-11 As a result, other TH2 axis inhibitors (anti–IL-13/tralokinumab, anti–IL-31RA/CIM 331) are now in clinical trials. The identification of IL-22 in AD lesions has prompted trials with an anti–IL-22 (ILV 094) and an IL-12/IL-23p40 (ustekinumab) inhibitor.12 For psoriasis, ustekinumab showed 75% improvement in approximately 70% of patients,13 but for AD, despite clear clinical and molecular effects, differences compared to placebo were not statistically significant,12 probably due to underdosing of the drug in an excessively immune-activated disease14 as well as allowing topical steroids in patients, which may minimize the differences in treatment effect between drug and placebo.
The developments seen in AD are now moving into other inflammatory skin diseases, particularly alopecia areata (AA), a T-cell–mediated disease that shares phenotypic similarities with AD and often is associated with it.15 There is a paucity of effective, remission-sustaining treatments of AA, particularly for patients with severe disease who rarely experience spontaneous hair regrowth and who have a limited response to topical interventions.16,17 Our clinical experience showed that successfully treating patients with concurrent AD and AA has led to hair regrowth. Inspired by these clinical observations and by results obtained in AD,9-12 studying AA skin showed an upregulation of not only the traditionally suspected culprit TH1 but also TH2 and TH9 axes, IL-23 cytokines, and phosphodiesterase 4.18 Subsequently, a pilot study of 3 patients with extensive AA treated with ustekinumab showed that all 3 patients not only experienced hair regrowth but also had a reduction in inflammatory markers in scalp lesions.19 Although these results are promising, AA is an immunologically complex disease and it is yet to be determined if therapeutically targeting 1 (eg, IL-4) rather than a wide array of cytokines can reverse disease phenotype. There are ongoing clinical trials directed at different pathogenic targets (eg, Jak inhibitors, IL-13 antagonist, IL-17 antagonist, phosphodiesterase 4 antagonist); some showed some efficacy in small studies.20,21
The finding of a commonly upregulated TH2 pathway in both AD and AA will pave the way for studies with TH2 antagonists in AA patients. Future targeted therapeutic studies will shed light on the pathogenic pathways of this devastating skin disease and answer the extensive unmet therapeutic need it presents.
- Czarnowicki T, Krueger JG, Guttman-Yassky E. Skin barrier and immune dysregulation in atopic dermatitis: an evolving story with important clinical implications. J Allergy Clin Immunol Pract. 2014;2:371-379; quiz 380-381.
- Roekevisch E, Spuls PI, Kuester D, et al. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133:429-438.
- Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis—part
I: clinical and pathologic concepts. J Allergy Clin Immunol. 2011;127:1110-1118. - Khattri S, Shemer A, Rozenblit M, et al. Cyclosporine in patients with atopic dermatitis modulates activated inflammatory pathways and reverses epidermal pathology. J Allergy Clin Immunol. 2014;133:1626-1634.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response [published online July 16, 2011]. J Allergy Clin Immunol. 2011;128:583-593.
- Eyerich K, Novak N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy. 2013;68:974-982.
- Gittler JK, Shemer A, Suárez-Fariñas M, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis [published online August 27, 2012]. J Allergy Clin Immunol. 2012;130:1344-1354.
- Noda S, Krueger JG, Guttman-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324-336.
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Hamilton JD, Suárez-Fariñas M, Dhingra N, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2014;134:1293-1300.
- Hamilton J, Ren H, Weinstein SP, et al. Dupilumab improved all domains of Eczema Area and Severity Index (EASI) and 5-D pruritus scale in adults with atopic dermatitis in a phase 2 study. J Invest Dermatol. 2014;134:S104.
- Khattri S, Brunner PM, Garcet S, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis [published online June 15, 2016]. Exp Dermatol. doi:10.1111/exd.13112.
- Griffiths CEM, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
- Czarnowicki T, Malajian D, Shemer A, et al. Skin-homing and systemic T-cell subsets show higher activation in atopic dermatitis versus psoriasis. J Allergy Clin Immunol. 2015;136:208-211.
- Barahmani N, Schabath MB, Duvic M. History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol. 2009;61:581-591.
- Price VH, Hordinsky MK, Olsen EA, et al. Subcutaneous efalizumab is not effective in the treatment of alopecia areata. J Am Acad Dermatol. 2008;58:395-402.
- Alkhalifah A, Alsantali A, Wang E, et al. Alopecia areata update part II. treatment. J Am Acad Dermatol. 2010;62:191-202.
- Suárez-Fariñas M, Ungar B, Noda S, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136:1277-1287.
- Guttman-Yassky E, Ungar B, Noda S, et al. Extensive alopecia areata is reversed by IL-12/IL-23p40 cytokine antagonism. J Allergy Clin Immunol. 2016;137:301-304.
- Xing LZ, Dai ZP, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Castela E, Le Duff F, Butori C, et al. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol. 2014;150:748-751.
- Czarnowicki T, Krueger JG, Guttman-Yassky E. Skin barrier and immune dysregulation in atopic dermatitis: an evolving story with important clinical implications. J Allergy Clin Immunol Pract. 2014;2:371-379; quiz 380-381.
- Roekevisch E, Spuls PI, Kuester D, et al. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133:429-438.
- Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis—part
I: clinical and pathologic concepts. J Allergy Clin Immunol. 2011;127:1110-1118. - Khattri S, Shemer A, Rozenblit M, et al. Cyclosporine in patients with atopic dermatitis modulates activated inflammatory pathways and reverses epidermal pathology. J Allergy Clin Immunol. 2014;133:1626-1634.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response [published online July 16, 2011]. J Allergy Clin Immunol. 2011;128:583-593.
- Eyerich K, Novak N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy. 2013;68:974-982.
- Gittler JK, Shemer A, Suárez-Fariñas M, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis [published online August 27, 2012]. J Allergy Clin Immunol. 2012;130:1344-1354.
- Noda S, Krueger JG, Guttman-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324-336.
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Hamilton JD, Suárez-Fariñas M, Dhingra N, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2014;134:1293-1300.
- Hamilton J, Ren H, Weinstein SP, et al. Dupilumab improved all domains of Eczema Area and Severity Index (EASI) and 5-D pruritus scale in adults with atopic dermatitis in a phase 2 study. J Invest Dermatol. 2014;134:S104.
- Khattri S, Brunner PM, Garcet S, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis [published online June 15, 2016]. Exp Dermatol. doi:10.1111/exd.13112.
- Griffiths CEM, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
- Czarnowicki T, Malajian D, Shemer A, et al. Skin-homing and systemic T-cell subsets show higher activation in atopic dermatitis versus psoriasis. J Allergy Clin Immunol. 2015;136:208-211.
- Barahmani N, Schabath MB, Duvic M. History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol. 2009;61:581-591.
- Price VH, Hordinsky MK, Olsen EA, et al. Subcutaneous efalizumab is not effective in the treatment of alopecia areata. J Am Acad Dermatol. 2008;58:395-402.
- Alkhalifah A, Alsantali A, Wang E, et al. Alopecia areata update part II. treatment. J Am Acad Dermatol. 2010;62:191-202.
- Suárez-Fariñas M, Ungar B, Noda S, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136:1277-1287.
- Guttman-Yassky E, Ungar B, Noda S, et al. Extensive alopecia areata is reversed by IL-12/IL-23p40 cytokine antagonism. J Allergy Clin Immunol. 2016;137:301-304.
- Xing LZ, Dai ZP, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Castela E, Le Duff F, Butori C, et al. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol. 2014;150:748-751.
Atopic Dermatitis Treatments Moving Forward: Report From the AAD Meeting
Although psoriasis was once at the forefront of therapeutic advancements in dermatology, atopic dermatitis (AD) is now taking center stage with several new treatments in the pipeline. Dr. Emma Guttman-Yassky provides an overview of the future of AD treatment, which includes new topical and systemic agents that currently are moving forward in advanced clinical trials or are close to registration. She also discusses strategies for improving disease management in AD patients, noting that prevention and education of both patients and their caregivers are key to effective treatment.
Although psoriasis was once at the forefront of therapeutic advancements in dermatology, atopic dermatitis (AD) is now taking center stage with several new treatments in the pipeline. Dr. Emma Guttman-Yassky provides an overview of the future of AD treatment, which includes new topical and systemic agents that currently are moving forward in advanced clinical trials or are close to registration. She also discusses strategies for improving disease management in AD patients, noting that prevention and education of both patients and their caregivers are key to effective treatment.
Although psoriasis was once at the forefront of therapeutic advancements in dermatology, atopic dermatitis (AD) is now taking center stage with several new treatments in the pipeline. Dr. Emma Guttman-Yassky provides an overview of the future of AD treatment, which includes new topical and systemic agents that currently are moving forward in advanced clinical trials or are close to registration. She also discusses strategies for improving disease management in AD patients, noting that prevention and education of both patients and their caregivers are key to effective treatment.