User login
From Buns to Braids and Ponytails: Entering a New Era of Female Military Hair-Grooming Standards
Professional appearance of servicemembers has been a long-standing custom in the US Military. Specific standards are determined by each branch. Initially, men dominated the military.1,2 As the number of women as well as racial diversity increased in the military, modifications to grooming standards were slow to change and resulted in female hair standards requiring a uniform tight and sleek style or short haircut. Clinicians can be attuned to these occupational standards and their implications on the diagnosis and management of common diseases of the hair and scalp.
History of Hairstyle Standards for Female Servicemembers
For half a century, female servicemembers had limited hairstyle choices. They were not authorized to have hair shorter than one-quarter inch in length. They could choose either short hair worn down or long hair with neatly secured loose ends in the form of a bun or a tucked braid—both of which could not extend past the bottom edge of the uniform collar.3-5 Female navy sailors and air force airmen with long hair were only allowed to wear ponytails during physical training; however, army soldiers previously were limited to wearing a bun.3,6,7 Cornrows and microbraids were authorized in the mid-1990s for the US Air Force, but policy stated that locs were prohibited due to their “unkempt” and “matted” nature. Furthermore, the size of hair bulk in the air force was restricted to no more than 3 inches and could not obstruct wear of the uniform cap.5 Based on these regulations, female servicemembers with longer hair had to utilize tight hairstyles that caused prolonged traction and pressure along the scalp, which contributed to headaches, a sore scalp, and alopecia over time. Normalization of these symptoms led to underreporting, as women lived with the consequences or turned to shorter hairstyles.
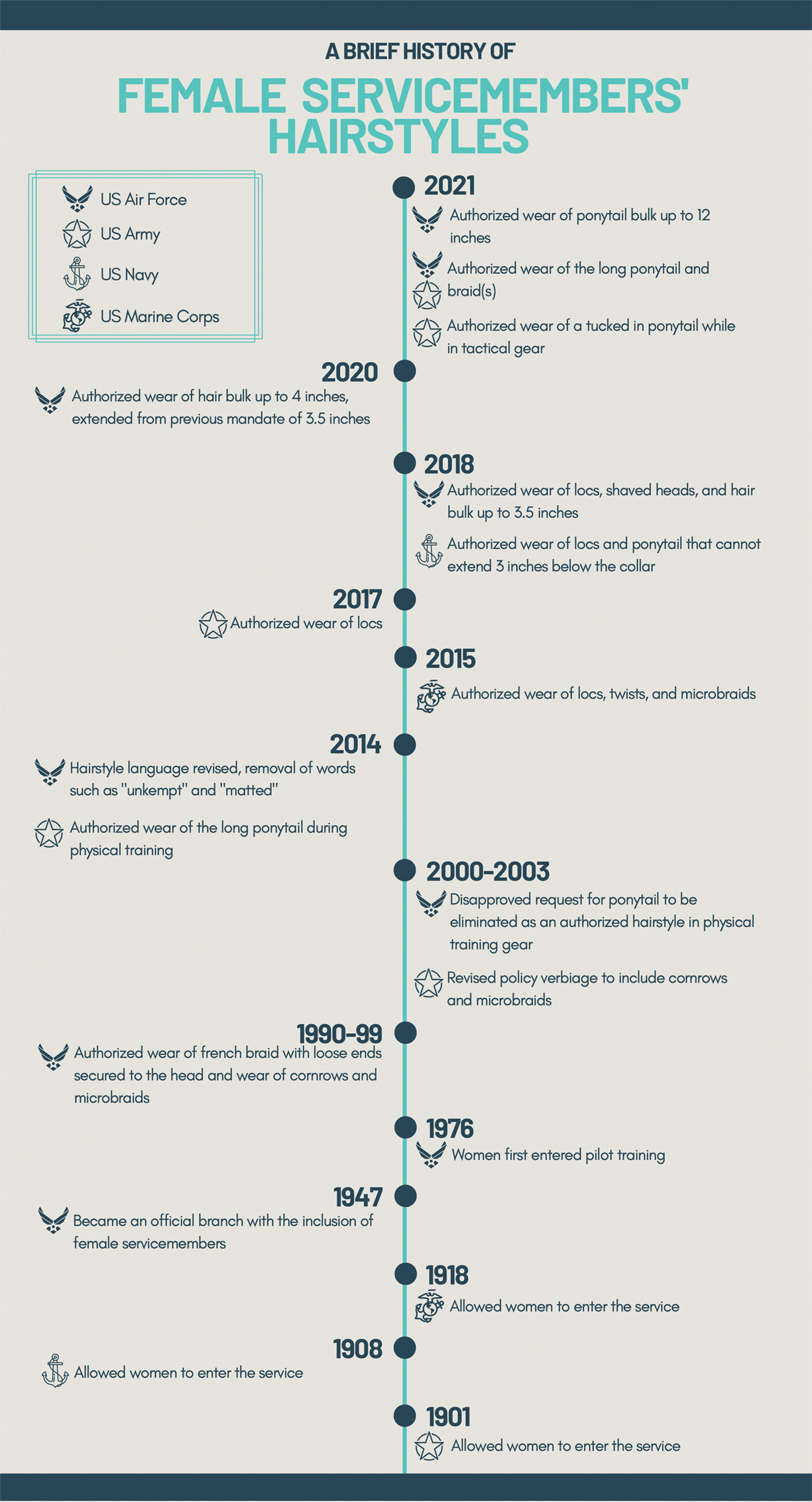
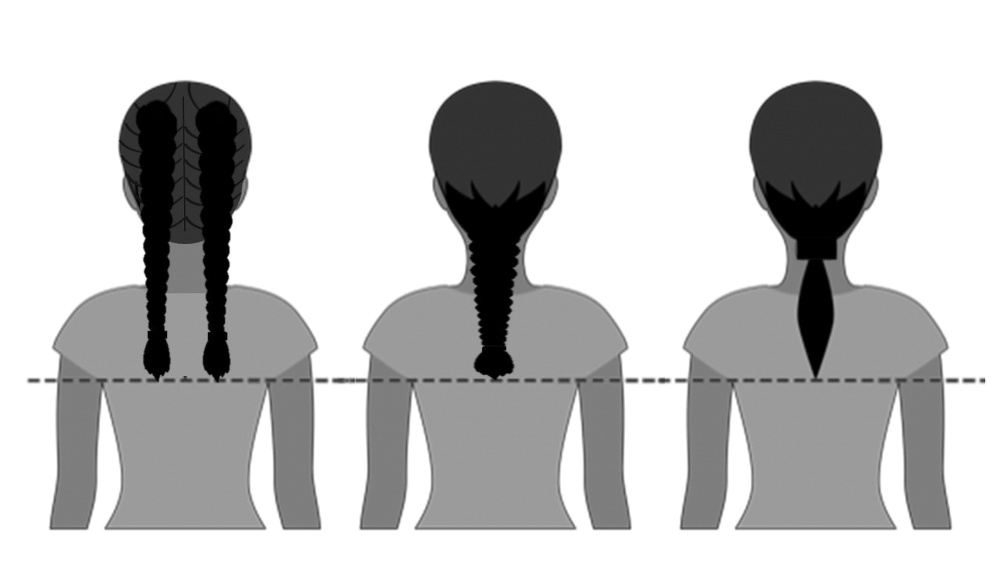
In the last decade alone, female servicemembers have witnessed the greatest number of changes in authorized hairstyles despite being part of the military for more than 50 years (Figure 1).1-11 In 2014, the language used in the air force instructions to describe locs was revised to remove ethnically offensive terms.4,5 This same year, the army allowed female soldiers to wear ponytails during physical training, a privilege that had been authorized by other services years prior.3,6,7 By the end of 2018, locs were authorized by all services, and female sailors could wear a ponytail in all navy uniforms as long as it did not extend 3 inches below the collar.3,4,6-8 In 2018, the air force increased authorized hair bulk up to 3.5 inches from the previous mandate of 3 inches and approved female buzz cuts6,9; in 2020, it allowed hair bulk up to 4 inches. As of 2021, female airmen can wear a ponytail and/or braid(s) as long as it starts below the crown of the head and the length does not extend below a horizontal line running between the top of each sleeve inseam at the underarm (Figures 2–4).6 In an ongoing effort to be more inclusive of hair density differences, female airmen will be authorized to wear a ponytail not exceeding a maximum width bulk of 1 ft starting June 25, 2021, so long as they can comply with the above regulations.11 The army now allows ponytails and braids across all uniforms, as long they do not extend past the bottom of the shoulder blades. This change came just months after authorizing the wearing of ponytails tucked under the uniform blouse with tactical headgear.10 These changes allow for a variety of hairstyles for members to practice while avoiding the physical consequences that develop from repetitive traction and pressure along the same areas of the hair and scalp.
Common Hair Disorders in Female Servicemembers
Herein, we discuss 3 of the most common hair and scalp disorders linked to grooming practices utilized by women to meet prior military regulations: trichorrhexis nodosa (TN), extracranial headaches, and traction alopecia (TA). It is essential that health care providers are able to promptly recognize these conditions, understand their risk factors, and be familiar with first-line treatment options. With these new standards, the hope is that the incidence of the following conditions decreases, thus improving servicemembers’ medical readiness and overall quality of life.
Trichorrhexis Nodosa
Acquired TN is a defect in the hair shaft that causes the hair to break easily secondary to chemical, thermal, or mechanical trauma. This can include but is not limited to chemical relaxers, blow-dryers, excessive brushing or styling, flat irons, and tightly packed hairstyles. The condition is characterized by a thickened hair diameter and splitting at the tip. Clinically, it may present as brittle, lusterless, broken hair with split ends, as well as a positive tug test.14 Management includes gentle hair care and avoidance of harsh hair care practices and treatments.
Extracranial Headaches
Headaches are a common concern among military servicemembers15 and generally are classified as primary or secondary. A less commonly discussed primary headache disorder includes external-pressure headaches, which result from either sustained compression or traction of the soft tissues of the scalp, usually from wearing headbands, helmets, or tight hairstyles.16 Additional at-risk groups include those who chronically wear surgical scrub caps or flight caps, especially if clipped or pinned to the hair. In our 38 years of combined military clinical experience, we can attest that these types of headaches are common among female servicemembers. The diagnostic criteria for an external-pressure headache, commonly referred to by patients as a “ponytail headache,” includes at least 2 headache episodes triggered within 1 hour of sustained traction on the scalp, maximal at the site of traction and resolving within 1 hour after relieving the traction.16 Management includes removal of the pressure-causing source, usually a tight ponytail or bun.
Traction Alopecia
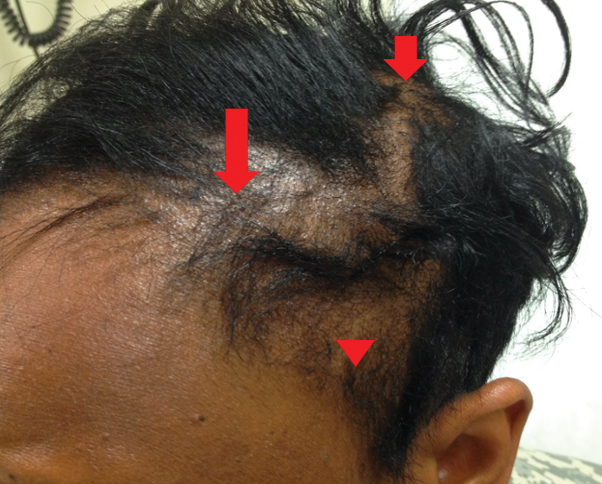
Traction alopecia is hair loss caused by repetitive or prolonged tension on the hair secondary to tight hairstyles. It can be clinically classified into 2 types: marginal and nonmarginal patchy alopecia (Figure 5).13,17,18 Traction alopecia most commonly is found in individuals with ethnic hair, predominantly Black women. Hairstyles with the highest risk for causing TA include tight buns, ponytails, cornrows, weaves, and locs—all of which are utilized by female servicemembers to maintain a professional appearance and adhere to grooming regulations.13,18 Other groups at risk include athletes (eg, ballerinas, gymnasts) and those with chronic headwear use (eg, turbans, helmets, nurse caps, wigs).18 Early TA typically presents with perifollicular erythema followed by follicular-based papules or pustules.13,18 Marginal TA classically includes frontotemporal hair loss or thinning with or without a fringe sign.17,18 Nonmarginal TA includes patchy alopecia most commonly involving the parietal or occipital scalp, seen with chignons, buns, ponytails, or the use of clips, extensions, or bobby pins.18 The first line in management is avoidance of traction-causing hairstyles or headgear. Medical therapy may be warranted and consists of a single agent or combination regimen to include oral or topical antibiotics, topical or intralesional steroids, and topical minoxidil.13,18
Final Thoughts
Military hair-grooming standards have evolved over time. Recent changes show that the US Department of Defense is seriously evaluating policies that may be inherently exclusive. Prior grooming standards resulted in the widespread use of tight hairstyles and harsh hair treatments among female servicemembers with long hair. These practices resulted in TN, extracranial headaches, and TA, among other hair and scalp disorders. These occupational-related hair conditions impact female servicemembers’ mental and physical well-being and thus impact military readiness. Physicians should recognize that these conditions can be related to occupational grooming standards that may impact hair care practices.
The challenge that remains is a lack of standardized documentation for hair and scalp symptoms in the medical record. Due to a paucity in reporting and documentation, limited objective data exist to guide future recommendations for military grooming standards. Another obstacle is the lack of knowledge of hair diseases among primary care providers and patients, especially due to the underrepresentation of ethnic hair in medical textbooks.19 As a result, women frequently accept their hair symptoms as normal and either suffer through them, cut their hair short, or wear wigs before considering a visit to the doctor. Furthermore, hair-grooming standards can expose racial disparities, which are the driving force behind the current policy changes. Clinicians can strive to ask about hair and scalp symptoms and document the following in relation to hair and scalp disorders: occupational grooming requirements; skin and hair type; location, number, and size of scalp lesion(s); onset; duration; current and prior hair care practices; history of treatment; and clinical course accompanied with photographic documentation. Ultimately, improved awareness in patients, collaboration between physicians, and consistent clinical documentation can help create positive change and continued improvement in hair-grooming standards within the military. Improved reporting and documentation will facilitate further study into the effectiveness of the updated hair-grooming standards in female servicemembers.
- United States Air Force Statistical Digest FY 1999. United States Air Force; 2000. Accessed June 8, 2021. https://media.defense.gov/2011/Apr/14/2001330240/-1/-1/0/AFD-110414-048.pdf
- Air Force demographics. Air Force Personnel Center website. Accessed June 8, 2021. https://www.afpc.af.mil/About/Air-Force-Demographics/
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Department of the Army; 2021. Accessed June 8, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Losey S. Loc hairstyles, off-duty earrings for men ok’d in new dress regs. Air Force Times. Published July 16, 2018. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-air-force/2018/07/16/loc-hairstyles-off-duty-earrings-for-men-okd-in-new-dress-regs/
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2011. Accessed June 8, 2021. https://www.uc.edu/content/dam/uc/afrotc/docs/Documents/AFI36-2903.pdf
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2021. Accessed June 8, 2021. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf
- U.S. Navy uniform regulations: summary of changes (26 February 2020). Navy Personnel Command website. Accessed June 8, 2021. https://www.mynavyhr.navy.mil/Portals/55/Navy%20Uniforms/Uniform%20Regulations/Documents/SOC_2020_02_26.pdf?ver=y8Wd0ykVXgISfFpOy8qHkg%3d%3d
- US Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. United States Marine Corps, 2018. Accessed June 8, 2021. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Secretary of the Air Force Public Affairs. Air Force to allow longer braids, ponytails, bangs for women. United States Air Force website. Published January 21, 2021. Accessed June 8, 2021. https://www.af.mil/News/Article-Display/Article/2478173/air-force-to-allow-longer-braids-ponytails-bangs-for-women/
- Britzky H. The Army will now allow women to wear ponytails in all uniforms. Task & Purpose. Published May 6, 2021. Accessed June 8, 2021. https://taskandpurpose.com/news/army-women-ponytails-all-uniforms/
- Secretary of the Air Force Public Affairs. Air Force readdresses women’s hair standard after feedback. US Air Force website. Published June 11, 2021. Accessed June 27, 2021. https://www.af.mil/News/Article-Display/Article/2654774/air-force-readdresses-womens-hair-standard-after-feedback/
- Myers M. Esper direct services to review racial bias in grooming standards, training and more. Air Force Times. Published July 15, 2020. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-military/2020/07/15/esper-directs-services-to-review-racial-bias-in-grooming-standards-training-and-more/
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Quaresma M, Martinez Velasco M, Tosti A. Hair breakage in patients of African descent: role of dermoscopy. Skin Appendage Disord. 2015;1:99-104.
- Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77.
- Sperling L, Cowper S, Knopp E. An Atlas of Hair Pathology with Clinical Correlations. CRC Press; 2012:67-68.
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
Professional appearance of servicemembers has been a long-standing custom in the US Military. Specific standards are determined by each branch. Initially, men dominated the military.1,2 As the number of women as well as racial diversity increased in the military, modifications to grooming standards were slow to change and resulted in female hair standards requiring a uniform tight and sleek style or short haircut. Clinicians can be attuned to these occupational standards and their implications on the diagnosis and management of common diseases of the hair and scalp.
History of Hairstyle Standards for Female Servicemembers
For half a century, female servicemembers had limited hairstyle choices. They were not authorized to have hair shorter than one-quarter inch in length. They could choose either short hair worn down or long hair with neatly secured loose ends in the form of a bun or a tucked braid—both of which could not extend past the bottom edge of the uniform collar.3-5 Female navy sailors and air force airmen with long hair were only allowed to wear ponytails during physical training; however, army soldiers previously were limited to wearing a bun.3,6,7 Cornrows and microbraids were authorized in the mid-1990s for the US Air Force, but policy stated that locs were prohibited due to their “unkempt” and “matted” nature. Furthermore, the size of hair bulk in the air force was restricted to no more than 3 inches and could not obstruct wear of the uniform cap.5 Based on these regulations, female servicemembers with longer hair had to utilize tight hairstyles that caused prolonged traction and pressure along the scalp, which contributed to headaches, a sore scalp, and alopecia over time. Normalization of these symptoms led to underreporting, as women lived with the consequences or turned to shorter hairstyles.
In the last decade alone, female servicemembers have witnessed the greatest number of changes in authorized hairstyles despite being part of the military for more than 50 years (Figure 1).1-11 In 2014, the language used in the air force instructions to describe locs was revised to remove ethnically offensive terms.4,5 This same year, the army allowed female soldiers to wear ponytails during physical training, a privilege that had been authorized by other services years prior.3,6,7 By the end of 2018, locs were authorized by all services, and female sailors could wear a ponytail in all navy uniforms as long as it did not extend 3 inches below the collar.3,4,6-8 In 2018, the air force increased authorized hair bulk up to 3.5 inches from the previous mandate of 3 inches and approved female buzz cuts6,9; in 2020, it allowed hair bulk up to 4 inches. As of 2021, female airmen can wear a ponytail and/or braid(s) as long as it starts below the crown of the head and the length does not extend below a horizontal line running between the top of each sleeve inseam at the underarm (Figures 2–4).6 In an ongoing effort to be more inclusive of hair density differences, female airmen will be authorized to wear a ponytail not exceeding a maximum width bulk of 1 ft starting June 25, 2021, so long as they can comply with the above regulations.11 The army now allows ponytails and braids across all uniforms, as long they do not extend past the bottom of the shoulder blades. This change came just months after authorizing the wearing of ponytails tucked under the uniform blouse with tactical headgear.10 These changes allow for a variety of hairstyles for members to practice while avoiding the physical consequences that develop from repetitive traction and pressure along the same areas of the hair and scalp.
Common Hair Disorders in Female Servicemembers
Herein, we discuss 3 of the most common hair and scalp disorders linked to grooming practices utilized by women to meet prior military regulations: trichorrhexis nodosa (TN), extracranial headaches, and traction alopecia (TA). It is essential that health care providers are able to promptly recognize these conditions, understand their risk factors, and be familiar with first-line treatment options. With these new standards, the hope is that the incidence of the following conditions decreases, thus improving servicemembers’ medical readiness and overall quality of life.
Trichorrhexis Nodosa
Acquired TN is a defect in the hair shaft that causes the hair to break easily secondary to chemical, thermal, or mechanical trauma. This can include but is not limited to chemical relaxers, blow-dryers, excessive brushing or styling, flat irons, and tightly packed hairstyles. The condition is characterized by a thickened hair diameter and splitting at the tip. Clinically, it may present as brittle, lusterless, broken hair with split ends, as well as a positive tug test.14 Management includes gentle hair care and avoidance of harsh hair care practices and treatments.
Extracranial Headaches
Headaches are a common concern among military servicemembers15 and generally are classified as primary or secondary. A less commonly discussed primary headache disorder includes external-pressure headaches, which result from either sustained compression or traction of the soft tissues of the scalp, usually from wearing headbands, helmets, or tight hairstyles.16 Additional at-risk groups include those who chronically wear surgical scrub caps or flight caps, especially if clipped or pinned to the hair. In our 38 years of combined military clinical experience, we can attest that these types of headaches are common among female servicemembers. The diagnostic criteria for an external-pressure headache, commonly referred to by patients as a “ponytail headache,” includes at least 2 headache episodes triggered within 1 hour of sustained traction on the scalp, maximal at the site of traction and resolving within 1 hour after relieving the traction.16 Management includes removal of the pressure-causing source, usually a tight ponytail or bun.
Traction Alopecia
Traction alopecia is hair loss caused by repetitive or prolonged tension on the hair secondary to tight hairstyles. It can be clinically classified into 2 types: marginal and nonmarginal patchy alopecia (Figure 5).13,17,18 Traction alopecia most commonly is found in individuals with ethnic hair, predominantly Black women. Hairstyles with the highest risk for causing TA include tight buns, ponytails, cornrows, weaves, and locs—all of which are utilized by female servicemembers to maintain a professional appearance and adhere to grooming regulations.13,18 Other groups at risk include athletes (eg, ballerinas, gymnasts) and those with chronic headwear use (eg, turbans, helmets, nurse caps, wigs).18 Early TA typically presents with perifollicular erythema followed by follicular-based papules or pustules.13,18 Marginal TA classically includes frontotemporal hair loss or thinning with or without a fringe sign.17,18 Nonmarginal TA includes patchy alopecia most commonly involving the parietal or occipital scalp, seen with chignons, buns, ponytails, or the use of clips, extensions, or bobby pins.18 The first line in management is avoidance of traction-causing hairstyles or headgear. Medical therapy may be warranted and consists of a single agent or combination regimen to include oral or topical antibiotics, topical or intralesional steroids, and topical minoxidil.13,18
Final Thoughts
Military hair-grooming standards have evolved over time. Recent changes show that the US Department of Defense is seriously evaluating policies that may be inherently exclusive. Prior grooming standards resulted in the widespread use of tight hairstyles and harsh hair treatments among female servicemembers with long hair. These practices resulted in TN, extracranial headaches, and TA, among other hair and scalp disorders. These occupational-related hair conditions impact female servicemembers’ mental and physical well-being and thus impact military readiness. Physicians should recognize that these conditions can be related to occupational grooming standards that may impact hair care practices.
The challenge that remains is a lack of standardized documentation for hair and scalp symptoms in the medical record. Due to a paucity in reporting and documentation, limited objective data exist to guide future recommendations for military grooming standards. Another obstacle is the lack of knowledge of hair diseases among primary care providers and patients, especially due to the underrepresentation of ethnic hair in medical textbooks.19 As a result, women frequently accept their hair symptoms as normal and either suffer through them, cut their hair short, or wear wigs before considering a visit to the doctor. Furthermore, hair-grooming standards can expose racial disparities, which are the driving force behind the current policy changes. Clinicians can strive to ask about hair and scalp symptoms and document the following in relation to hair and scalp disorders: occupational grooming requirements; skin and hair type; location, number, and size of scalp lesion(s); onset; duration; current and prior hair care practices; history of treatment; and clinical course accompanied with photographic documentation. Ultimately, improved awareness in patients, collaboration between physicians, and consistent clinical documentation can help create positive change and continued improvement in hair-grooming standards within the military. Improved reporting and documentation will facilitate further study into the effectiveness of the updated hair-grooming standards in female servicemembers.
Professional appearance of servicemembers has been a long-standing custom in the US Military. Specific standards are determined by each branch. Initially, men dominated the military.1,2 As the number of women as well as racial diversity increased in the military, modifications to grooming standards were slow to change and resulted in female hair standards requiring a uniform tight and sleek style or short haircut. Clinicians can be attuned to these occupational standards and their implications on the diagnosis and management of common diseases of the hair and scalp.
History of Hairstyle Standards for Female Servicemembers
For half a century, female servicemembers had limited hairstyle choices. They were not authorized to have hair shorter than one-quarter inch in length. They could choose either short hair worn down or long hair with neatly secured loose ends in the form of a bun or a tucked braid—both of which could not extend past the bottom edge of the uniform collar.3-5 Female navy sailors and air force airmen with long hair were only allowed to wear ponytails during physical training; however, army soldiers previously were limited to wearing a bun.3,6,7 Cornrows and microbraids were authorized in the mid-1990s for the US Air Force, but policy stated that locs were prohibited due to their “unkempt” and “matted” nature. Furthermore, the size of hair bulk in the air force was restricted to no more than 3 inches and could not obstruct wear of the uniform cap.5 Based on these regulations, female servicemembers with longer hair had to utilize tight hairstyles that caused prolonged traction and pressure along the scalp, which contributed to headaches, a sore scalp, and alopecia over time. Normalization of these symptoms led to underreporting, as women lived with the consequences or turned to shorter hairstyles.
In the last decade alone, female servicemembers have witnessed the greatest number of changes in authorized hairstyles despite being part of the military for more than 50 years (Figure 1).1-11 In 2014, the language used in the air force instructions to describe locs was revised to remove ethnically offensive terms.4,5 This same year, the army allowed female soldiers to wear ponytails during physical training, a privilege that had been authorized by other services years prior.3,6,7 By the end of 2018, locs were authorized by all services, and female sailors could wear a ponytail in all navy uniforms as long as it did not extend 3 inches below the collar.3,4,6-8 In 2018, the air force increased authorized hair bulk up to 3.5 inches from the previous mandate of 3 inches and approved female buzz cuts6,9; in 2020, it allowed hair bulk up to 4 inches. As of 2021, female airmen can wear a ponytail and/or braid(s) as long as it starts below the crown of the head and the length does not extend below a horizontal line running between the top of each sleeve inseam at the underarm (Figures 2–4).6 In an ongoing effort to be more inclusive of hair density differences, female airmen will be authorized to wear a ponytail not exceeding a maximum width bulk of 1 ft starting June 25, 2021, so long as they can comply with the above regulations.11 The army now allows ponytails and braids across all uniforms, as long they do not extend past the bottom of the shoulder blades. This change came just months after authorizing the wearing of ponytails tucked under the uniform blouse with tactical headgear.10 These changes allow for a variety of hairstyles for members to practice while avoiding the physical consequences that develop from repetitive traction and pressure along the same areas of the hair and scalp.
Common Hair Disorders in Female Servicemembers
Herein, we discuss 3 of the most common hair and scalp disorders linked to grooming practices utilized by women to meet prior military regulations: trichorrhexis nodosa (TN), extracranial headaches, and traction alopecia (TA). It is essential that health care providers are able to promptly recognize these conditions, understand their risk factors, and be familiar with first-line treatment options. With these new standards, the hope is that the incidence of the following conditions decreases, thus improving servicemembers’ medical readiness and overall quality of life.
Trichorrhexis Nodosa
Acquired TN is a defect in the hair shaft that causes the hair to break easily secondary to chemical, thermal, or mechanical trauma. This can include but is not limited to chemical relaxers, blow-dryers, excessive brushing or styling, flat irons, and tightly packed hairstyles. The condition is characterized by a thickened hair diameter and splitting at the tip. Clinically, it may present as brittle, lusterless, broken hair with split ends, as well as a positive tug test.14 Management includes gentle hair care and avoidance of harsh hair care practices and treatments.
Extracranial Headaches
Headaches are a common concern among military servicemembers15 and generally are classified as primary or secondary. A less commonly discussed primary headache disorder includes external-pressure headaches, which result from either sustained compression or traction of the soft tissues of the scalp, usually from wearing headbands, helmets, or tight hairstyles.16 Additional at-risk groups include those who chronically wear surgical scrub caps or flight caps, especially if clipped or pinned to the hair. In our 38 years of combined military clinical experience, we can attest that these types of headaches are common among female servicemembers. The diagnostic criteria for an external-pressure headache, commonly referred to by patients as a “ponytail headache,” includes at least 2 headache episodes triggered within 1 hour of sustained traction on the scalp, maximal at the site of traction and resolving within 1 hour after relieving the traction.16 Management includes removal of the pressure-causing source, usually a tight ponytail or bun.
Traction Alopecia
Traction alopecia is hair loss caused by repetitive or prolonged tension on the hair secondary to tight hairstyles. It can be clinically classified into 2 types: marginal and nonmarginal patchy alopecia (Figure 5).13,17,18 Traction alopecia most commonly is found in individuals with ethnic hair, predominantly Black women. Hairstyles with the highest risk for causing TA include tight buns, ponytails, cornrows, weaves, and locs—all of which are utilized by female servicemembers to maintain a professional appearance and adhere to grooming regulations.13,18 Other groups at risk include athletes (eg, ballerinas, gymnasts) and those with chronic headwear use (eg, turbans, helmets, nurse caps, wigs).18 Early TA typically presents with perifollicular erythema followed by follicular-based papules or pustules.13,18 Marginal TA classically includes frontotemporal hair loss or thinning with or without a fringe sign.17,18 Nonmarginal TA includes patchy alopecia most commonly involving the parietal or occipital scalp, seen with chignons, buns, ponytails, or the use of clips, extensions, or bobby pins.18 The first line in management is avoidance of traction-causing hairstyles or headgear. Medical therapy may be warranted and consists of a single agent or combination regimen to include oral or topical antibiotics, topical or intralesional steroids, and topical minoxidil.13,18
Final Thoughts
Military hair-grooming standards have evolved over time. Recent changes show that the US Department of Defense is seriously evaluating policies that may be inherently exclusive. Prior grooming standards resulted in the widespread use of tight hairstyles and harsh hair treatments among female servicemembers with long hair. These practices resulted in TN, extracranial headaches, and TA, among other hair and scalp disorders. These occupational-related hair conditions impact female servicemembers’ mental and physical well-being and thus impact military readiness. Physicians should recognize that these conditions can be related to occupational grooming standards that may impact hair care practices.
The challenge that remains is a lack of standardized documentation for hair and scalp symptoms in the medical record. Due to a paucity in reporting and documentation, limited objective data exist to guide future recommendations for military grooming standards. Another obstacle is the lack of knowledge of hair diseases among primary care providers and patients, especially due to the underrepresentation of ethnic hair in medical textbooks.19 As a result, women frequently accept their hair symptoms as normal and either suffer through them, cut their hair short, or wear wigs before considering a visit to the doctor. Furthermore, hair-grooming standards can expose racial disparities, which are the driving force behind the current policy changes. Clinicians can strive to ask about hair and scalp symptoms and document the following in relation to hair and scalp disorders: occupational grooming requirements; skin and hair type; location, number, and size of scalp lesion(s); onset; duration; current and prior hair care practices; history of treatment; and clinical course accompanied with photographic documentation. Ultimately, improved awareness in patients, collaboration between physicians, and consistent clinical documentation can help create positive change and continued improvement in hair-grooming standards within the military. Improved reporting and documentation will facilitate further study into the effectiveness of the updated hair-grooming standards in female servicemembers.
- United States Air Force Statistical Digest FY 1999. United States Air Force; 2000. Accessed June 8, 2021. https://media.defense.gov/2011/Apr/14/2001330240/-1/-1/0/AFD-110414-048.pdf
- Air Force demographics. Air Force Personnel Center website. Accessed June 8, 2021. https://www.afpc.af.mil/About/Air-Force-Demographics/
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Department of the Army; 2021. Accessed June 8, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Losey S. Loc hairstyles, off-duty earrings for men ok’d in new dress regs. Air Force Times. Published July 16, 2018. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-air-force/2018/07/16/loc-hairstyles-off-duty-earrings-for-men-okd-in-new-dress-regs/
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2011. Accessed June 8, 2021. https://www.uc.edu/content/dam/uc/afrotc/docs/Documents/AFI36-2903.pdf
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2021. Accessed June 8, 2021. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf
- U.S. Navy uniform regulations: summary of changes (26 February 2020). Navy Personnel Command website. Accessed June 8, 2021. https://www.mynavyhr.navy.mil/Portals/55/Navy%20Uniforms/Uniform%20Regulations/Documents/SOC_2020_02_26.pdf?ver=y8Wd0ykVXgISfFpOy8qHkg%3d%3d
- US Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. United States Marine Corps, 2018. Accessed June 8, 2021. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Secretary of the Air Force Public Affairs. Air Force to allow longer braids, ponytails, bangs for women. United States Air Force website. Published January 21, 2021. Accessed June 8, 2021. https://www.af.mil/News/Article-Display/Article/2478173/air-force-to-allow-longer-braids-ponytails-bangs-for-women/
- Britzky H. The Army will now allow women to wear ponytails in all uniforms. Task & Purpose. Published May 6, 2021. Accessed June 8, 2021. https://taskandpurpose.com/news/army-women-ponytails-all-uniforms/
- Secretary of the Air Force Public Affairs. Air Force readdresses women’s hair standard after feedback. US Air Force website. Published June 11, 2021. Accessed June 27, 2021. https://www.af.mil/News/Article-Display/Article/2654774/air-force-readdresses-womens-hair-standard-after-feedback/
- Myers M. Esper direct services to review racial bias in grooming standards, training and more. Air Force Times. Published July 15, 2020. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-military/2020/07/15/esper-directs-services-to-review-racial-bias-in-grooming-standards-training-and-more/
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Quaresma M, Martinez Velasco M, Tosti A. Hair breakage in patients of African descent: role of dermoscopy. Skin Appendage Disord. 2015;1:99-104.
- Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77.
- Sperling L, Cowper S, Knopp E. An Atlas of Hair Pathology with Clinical Correlations. CRC Press; 2012:67-68.
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
- United States Air Force Statistical Digest FY 1999. United States Air Force; 2000. Accessed June 8, 2021. https://media.defense.gov/2011/Apr/14/2001330240/-1/-1/0/AFD-110414-048.pdf
- Air Force demographics. Air Force Personnel Center website. Accessed June 8, 2021. https://www.afpc.af.mil/About/Air-Force-Demographics/
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Department of the Army; 2021. Accessed June 8, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Losey S. Loc hairstyles, off-duty earrings for men ok’d in new dress regs. Air Force Times. Published July 16, 2018. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-air-force/2018/07/16/loc-hairstyles-off-duty-earrings-for-men-okd-in-new-dress-regs/
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2011. Accessed June 8, 2021. https://www.uc.edu/content/dam/uc/afrotc/docs/Documents/AFI36-2903.pdf
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2021. Accessed June 8, 2021. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf
- U.S. Navy uniform regulations: summary of changes (26 February 2020). Navy Personnel Command website. Accessed June 8, 2021. https://www.mynavyhr.navy.mil/Portals/55/Navy%20Uniforms/Uniform%20Regulations/Documents/SOC_2020_02_26.pdf?ver=y8Wd0ykVXgISfFpOy8qHkg%3d%3d
- US Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. United States Marine Corps, 2018. Accessed June 8, 2021. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Secretary of the Air Force Public Affairs. Air Force to allow longer braids, ponytails, bangs for women. United States Air Force website. Published January 21, 2021. Accessed June 8, 2021. https://www.af.mil/News/Article-Display/Article/2478173/air-force-to-allow-longer-braids-ponytails-bangs-for-women/
- Britzky H. The Army will now allow women to wear ponytails in all uniforms. Task & Purpose. Published May 6, 2021. Accessed June 8, 2021. https://taskandpurpose.com/news/army-women-ponytails-all-uniforms/
- Secretary of the Air Force Public Affairs. Air Force readdresses women’s hair standard after feedback. US Air Force website. Published June 11, 2021. Accessed June 27, 2021. https://www.af.mil/News/Article-Display/Article/2654774/air-force-readdresses-womens-hair-standard-after-feedback/
- Myers M. Esper direct services to review racial bias in grooming standards, training and more. Air Force Times. Published July 15, 2020. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-military/2020/07/15/esper-directs-services-to-review-racial-bias-in-grooming-standards-training-and-more/
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Quaresma M, Martinez Velasco M, Tosti A. Hair breakage in patients of African descent: role of dermoscopy. Skin Appendage Disord. 2015;1:99-104.
- Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77.
- Sperling L, Cowper S, Knopp E. An Atlas of Hair Pathology with Clinical Correlations. CRC Press; 2012:67-68.
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
Practice Points
- Military hair-grooming standards have undergone considerable changes to foster inclusivity and acknowledge racial diversity in hair and skin types.
- The chronic wearing of tight hairstyles can lead to hair breakage, headaches, and traction alopecia.
- A deliberate focus on diversity and inclusivity has started to drive policy change that eliminates racial and gender bias.
Hyperbaric Oxygen Therapy in Dermatology
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4
Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
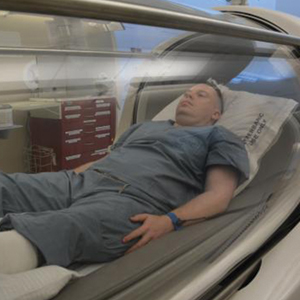
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8
Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
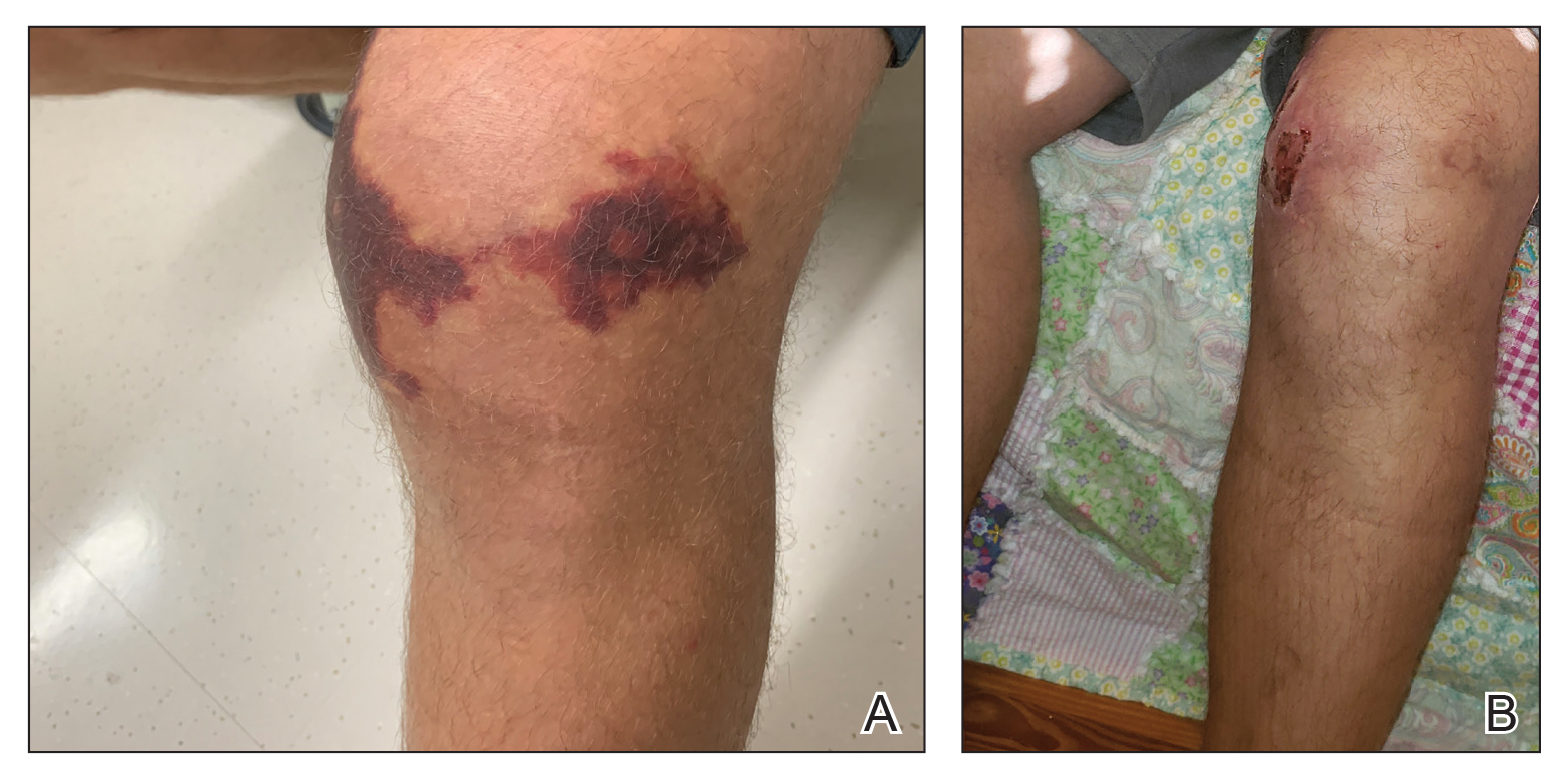
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.
Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4
Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8
Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.
Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4
Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8
Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.
Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
Practice Points
- Hyperbaric oxygen therapy can be considered for the treatment of failing cutaneous grafts and flaps, chronic ulcerations caused by vasculitis or autoimmune disorders, and vascular compromise, including cutaneous ischemia caused by fillers.
- Hyperbaric oxygen therapy involves 1- to 2-hour treatments, 5 days a week, for as long as 1 month.
- Hyperbaric oxygen therapy is safe and well-tolerated, with few contraindications. The sooner therapy is started, the greater the potential for benefit.
Atopic Dermatitis in the US Military
Dermatologic conditions historically have affected military members’ ability to serve during times of peace and conflict. These conditions range from chronic dermatologic diseases to environment- or occupation-related dermatologic diseases. Mild to moderate atopic dermatitis (AD) typically is a manageable skin condition. However, in a deployed setting, a flare of AD can result in the inability of a member to perform their military duty, which directly compromises mission safety and effectiveness. The military developed and updates medical standards for entry and retention of service members. These standards are designed to ensure the greatest potential for a military member to successfully serve at home station and during combat operations.
Impact of Injuries in Military
Historically, disease and nonbattle injuries have resulted in notably more hospitalizations and time lost than injuries sustained on the battlefield.1 A review of major conflicts dating from World War II shows approximately 10% of all dermatologic concerns were related to eczematous dermatitis, with 2% specifically related to AD. These numbers varied remarkably depending on the location and environment of the conflict, with eczema accounting for 25% of dermatologic concerns during the Gulf War.2 During the initial phases of Operation Iraqi Freedom, approximately 75% of hospitalizations were from disease and nonbattle injuries, of which dermatologic disease accounted for 3%.1 From 2003 to 2006 in Iraq, 35 service members were evacuated from combat zones specifically for uncontrolled AD.3 In a deployed environment, each member is critical to the unit’s success in completing their mission. A single member of a unit often is the only person qualified to perform a function for that team. There are rarely extra people with similar skills to replace a member unable to complete his/her duties. The loss of a single member compromises the effectiveness and safety of the team and can lead to mission failure. Therefore, AD can have a profound impact on military operations in a deployed environment.
Military Medical Standards for Accession and Retention
There are 2 main goals of the military medical standards. First, the individual health of the applicant or military member is of utmost importance. Applicants with medical conditions that will be exacerbated by military service or that limit the ability for successful military operations are not accepted for military service. Once an active-duty member is diagnosed with a medical condition, the military determines if limitations are needed for military assignments and deployments based on available medical care in those locations. Second, mission accomplishment in combat operations requires that healthy military members are able to complete their jobs in extreme environments and under notable stress. If an applicant has a medical condition unsuitable for military service, it is in the best interest of the applicant and the military to deny entry.
The Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03) lists conditions that are disqualifying for military service.4 Section 5.21 lists the following as disqualifying for military service in relation to eczematous dermatitis:
d. History of AD or eczema after the 12th birthday. History of residual or recurrent lesions in characteristic areas (face, neck, antecubital or popliteal fossae, occasionally wrists and hands).
e. History of recurrent or chronic nonspecific dermatitis within the past 2 years to include contact (irritant or allergic) or dyshidrotic dermatitis requiring more than treatment with topical corticosteroid.4
Although cases of incorrect diagnosis or very mild AD can be considered for a waiver, the process can be laborious and consideration or approval is not guaranteed. For current military members with new chronic eczematous dermatitis, each service has a process for evaluation and treatment. Some special operational jobs, such as aircrew, missile operators, and divers, have more restrictive medical requirements that are monitored by physicians with special training in these populations.
Atopic dermatitis affects 25% of children and 2% to 3% of adults.5 Approximately 60% of patients with AD will develop their first eruption by 1 year of age, and 90% by 5 years of age. Although the majority of patients will have resolution of their disease during childhood, 10% to 30% will have persistent disease into adulthood.5 Because the majority of AD resolves in childhood, it is understandable that asymptomatic individuals with a history of AD before 12 years of age meet military entrance medical standards.
Provoking Factors
The US Military maintains stringent medical standards because of the nature of the dynamic, rapidly changing military environment and its demands. Whether training for readiness in an austere location, deploying to extreme climates, or being stationed overseas, service members must be prepared to encounter a myriad of environmental extremes, physical stress, and psychological stressors. Environmental factors commonly experienced in the military can provoke or exacerbate symptoms of AD (Figures 1 and 2). Ideally, an individual with AD lives in a stable climate, has access to moisturizers and topical steroids, bathes regularly to remove dust and debris, wears 100% cotton garments to avoid irritation, and avoids using gear that would cause exacerbations. Service members rarely have such accommodations in deployed settings. A recent article in Military Medicine explained quite well, “If someone wanted to design an experience with the explicit goal to flare a person with otherwise well controlled atopic dermatitis it would probably look like a military deployment.”3
The United States has a military presence in countries with extreme temperature and humidity variations all over the world. Uniforms are standardized, and members are required to wear prescribed clothing with no alternatives. Uniforms are made of durable sturdy material. If uniforms can be laundered, they often are grouped together, and sensitive detergent cannot be specified. Bathing is challenging in deployed locations, with troops often going weeks using baby wipes for self-hygiene. These conditions increase risk for development of contact allergens, and little access to proper hygiene practices also increases risk for secondary infections in members with AD.
In addition to environmental challenges, the military gear and equipment used can flare AD. Service members must wear protective gear such as body armor. These heavy hard pieces of material are bulky; difficult to wash; and cause friction, sweating, and irritation. The military prepares for operations in chemical, biological, radiological, or nuclear environments, which requires wearing a rubber mask, multiple layers of boots and gloves, and thick charcoal impregnated over garments for many hours. Such conditions may flare AD or make it intolerable.
Although stress is a part of any deployment experience, excessive or prolonged stress can lead to combat operational stress reactions that inhibit a service member’s ability to function.6 Stressors during deployment can accumulate and may be caused by the operational environment, loss of fellow service members to injury or death, illness, leadership demands, personal choices, issues on the home front, interpersonal conflicts, and sleep loss.7 Atopic dermatitis can be exacerbated by such stress, leading to increased pruritus and scratching.7-9 Symptomatic AD also can play a role in worsening combat stress. Although severe pruritus may affect attentiveness to job duties during the day, these symptoms, if uncontrolled, also can negatively affect sleep. As many as 60% of patients with AD at baseline and 83% of patients with exacerbations experience sleep disturbance due to their disease.5 These stressors experienced by deployed military personnel can contribute to combat stress reactions, which may vary from simple inattentiveness to more serious behaviors such as suicidal ideation.6 Combat stress reactions inhibit a military member’s ability to function properly in the deployed environment and can lead to notable safety concerns and potential mission failure.
Vaccinations
Military members deploying overseas are required to receive specific vaccinations, including the smallpox vaccine. Although the virus was eradicated in 1980, the concern for smallpox to be used as a biological weapon in certain areas of the world necessitates continued vaccination of military populations. According to the Centers for Disease Control and Prevention, the only known reservoir for the virus is humans, and the disease has a mortality rate of 30%.10 A history of or present AD is a contraindication for primary smallpox vaccination and revaccination for nonemergency use because of the risk for eczema vaccinatum.11 The risk also applies to close contacts of vaccinated members. For 30 days after vaccination, service members must avoid skin-to-skin contact with anyone who has active AD.12 Eczema vaccinatum in vaccinated individuals is typically self-limited; however, eczema vaccinatum in nonvaccinated contacts can be severe. One case report described a 28-month-old child with refractory AD who developed severe eczema vaccinatum after contact with her recently vaccinated military parent. The child required a 48-day admission to the intensive care unit and multiple skin grafts; fortunately, the child did not develop any apparent long-term sequelae.13 This case highlights the importance of understanding the risks associated with smallpox vaccination in military members with AD and the responsibility of health care providers to properly screen and counsel individuals prior to administering smallpox vaccines.
Treatment
Treatment of mild to moderate AD is relatively straightforward in developed countries with good access to medical care. The most recent American Academy of Dermatology clinical guidelines for AD focus on minimizing irritants and triggers, regularly using moisturizers soon after bathing, and using topical steroids as needed.5 Military members face specific challenges regarding treatment of AD, particularly when deployed to remote locations without access to treatment facilities or medications. Military members are required to carry all necessary personal medications with them for at least 6 months and preferably the duration of the deployment, sometimes up to 1 year. Military members carry a large amount of gear for deployments, and it is not feasible to pack an additional 10 to 20 lb worth of emollients and topical steroids to last the entire deployment. Routine laboratory monitoring is limited or completely unavailable. Refrigeration typically is not available, making use of systemic medications nearly impossible during deployments. In the event of complications such as eczema herpeticum or secondary bacterial infection, service members could require evacuation from the deployed location to a larger field hospital or to the United States, which is costly and also removes a valuable team member from the deployed unit. These limitations in access to care, medications, and treatment options make AD a difficult condition to treat in the deployed setting.
Nonmilitary Medical Providers
Civilian providers play an important role in diagnosing and treating AD. It is vital to completely and accurately document treatment of all skin diseases; however, it is especially important for those who desire to or currently serve in the military. Military primary care providers or military dermatologists must review the information from civilian providers to aid in determining suitability for entry or retention in the military. Clearly documenting the morphology, extent of disease involvement (eg, body surface area), treatment plan, response to treatment, and exacerbating factors will aid in ensuring the patient’s medical record accurately reflects their skin disease. Ultimately, this record often is the only information available to make health determinations regarding military service.
Conclusion
A career in the military is challenging and rewarding for those who volunteer to serve. Because of the demanding and unpredictable lifestyle inherent with military service, the Department of Defense maintains strict medical standards for entrance and retention. These standards ensure members are capable of safely completing training and deploying anywhere in the world. Although AD is a relatively common and treatable skin disease in locations with well-established medical care, it can pose a notable problem for service members while deployed to austere locations with variable environments around the world. Environmental factors and gear requirements, coupled with limited access to treatment facilities and medications, render AD a potentially serious issue. Atopic dermatitis in military members can affect individual medical readiness and unit success. It is important that all providers understand the myriad effects that AD can have on an individual who wishes to join or continue service in the military.
- Zouris JM, Wade AL, Magno CP. Injury and illness casualty distributions among U.S. Army and Marine Corps personnel during Operation Iraqi Freedom. Mil Med. 2008;173:247-252.
- Gelman, AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:177-182.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 8, 2019.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Force Health Protection (Army Techniques Publication No. 4-02.8). Washington, DC: Department of the Army; March 2016. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/atp4_02x8.pdf. Accessed August 19, 2019.
- Judkins JL, Bradley DL. A review of the effectiveness of a combat and operational stress control restoration center in Afghanistan. Mil Med. 2017;182:1755-1762.
- Suarez AL, Feramisco JD, Koo J, et al. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Dermatol Venereol. 2012;92:7-15.
- Mochizuki H, Lavery MJ, Nattkemper LA, et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br J Dermatol. 2019;180:821-827.
- Centers for Disease Control and Prevention. Smallpox: contraindications to vaccination. https://www.cdc.gov/smallpox/clinicians/vaccination-contraindications1.html. Updated December 5, 2016. Accessed August 19, 2019.
- Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5:84-90.
- Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis. 2012;54:832-840.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccine. Clin Infect Dis. 2008;46:1555-1561.
Dermatologic conditions historically have affected military members’ ability to serve during times of peace and conflict. These conditions range from chronic dermatologic diseases to environment- or occupation-related dermatologic diseases. Mild to moderate atopic dermatitis (AD) typically is a manageable skin condition. However, in a deployed setting, a flare of AD can result in the inability of a member to perform their military duty, which directly compromises mission safety and effectiveness. The military developed and updates medical standards for entry and retention of service members. These standards are designed to ensure the greatest potential for a military member to successfully serve at home station and during combat operations.
Impact of Injuries in Military
Historically, disease and nonbattle injuries have resulted in notably more hospitalizations and time lost than injuries sustained on the battlefield.1 A review of major conflicts dating from World War II shows approximately 10% of all dermatologic concerns were related to eczematous dermatitis, with 2% specifically related to AD. These numbers varied remarkably depending on the location and environment of the conflict, with eczema accounting for 25% of dermatologic concerns during the Gulf War.2 During the initial phases of Operation Iraqi Freedom, approximately 75% of hospitalizations were from disease and nonbattle injuries, of which dermatologic disease accounted for 3%.1 From 2003 to 2006 in Iraq, 35 service members were evacuated from combat zones specifically for uncontrolled AD.3 In a deployed environment, each member is critical to the unit’s success in completing their mission. A single member of a unit often is the only person qualified to perform a function for that team. There are rarely extra people with similar skills to replace a member unable to complete his/her duties. The loss of a single member compromises the effectiveness and safety of the team and can lead to mission failure. Therefore, AD can have a profound impact on military operations in a deployed environment.
Military Medical Standards for Accession and Retention
There are 2 main goals of the military medical standards. First, the individual health of the applicant or military member is of utmost importance. Applicants with medical conditions that will be exacerbated by military service or that limit the ability for successful military operations are not accepted for military service. Once an active-duty member is diagnosed with a medical condition, the military determines if limitations are needed for military assignments and deployments based on available medical care in those locations. Second, mission accomplishment in combat operations requires that healthy military members are able to complete their jobs in extreme environments and under notable stress. If an applicant has a medical condition unsuitable for military service, it is in the best interest of the applicant and the military to deny entry.
The Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03) lists conditions that are disqualifying for military service.4 Section 5.21 lists the following as disqualifying for military service in relation to eczematous dermatitis:
d. History of AD or eczema after the 12th birthday. History of residual or recurrent lesions in characteristic areas (face, neck, antecubital or popliteal fossae, occasionally wrists and hands).
e. History of recurrent or chronic nonspecific dermatitis within the past 2 years to include contact (irritant or allergic) or dyshidrotic dermatitis requiring more than treatment with topical corticosteroid.4
Although cases of incorrect diagnosis or very mild AD can be considered for a waiver, the process can be laborious and consideration or approval is not guaranteed. For current military members with new chronic eczematous dermatitis, each service has a process for evaluation and treatment. Some special operational jobs, such as aircrew, missile operators, and divers, have more restrictive medical requirements that are monitored by physicians with special training in these populations.
Atopic dermatitis affects 25% of children and 2% to 3% of adults.5 Approximately 60% of patients with AD will develop their first eruption by 1 year of age, and 90% by 5 years of age. Although the majority of patients will have resolution of their disease during childhood, 10% to 30% will have persistent disease into adulthood.5 Because the majority of AD resolves in childhood, it is understandable that asymptomatic individuals with a history of AD before 12 years of age meet military entrance medical standards.
Provoking Factors
The US Military maintains stringent medical standards because of the nature of the dynamic, rapidly changing military environment and its demands. Whether training for readiness in an austere location, deploying to extreme climates, or being stationed overseas, service members must be prepared to encounter a myriad of environmental extremes, physical stress, and psychological stressors. Environmental factors commonly experienced in the military can provoke or exacerbate symptoms of AD (Figures 1 and 2). Ideally, an individual with AD lives in a stable climate, has access to moisturizers and topical steroids, bathes regularly to remove dust and debris, wears 100% cotton garments to avoid irritation, and avoids using gear that would cause exacerbations. Service members rarely have such accommodations in deployed settings. A recent article in Military Medicine explained quite well, “If someone wanted to design an experience with the explicit goal to flare a person with otherwise well controlled atopic dermatitis it would probably look like a military deployment.”3
The United States has a military presence in countries with extreme temperature and humidity variations all over the world. Uniforms are standardized, and members are required to wear prescribed clothing with no alternatives. Uniforms are made of durable sturdy material. If uniforms can be laundered, they often are grouped together, and sensitive detergent cannot be specified. Bathing is challenging in deployed locations, with troops often going weeks using baby wipes for self-hygiene. These conditions increase risk for development of contact allergens, and little access to proper hygiene practices also increases risk for secondary infections in members with AD.
In addition to environmental challenges, the military gear and equipment used can flare AD. Service members must wear protective gear such as body armor. These heavy hard pieces of material are bulky; difficult to wash; and cause friction, sweating, and irritation. The military prepares for operations in chemical, biological, radiological, or nuclear environments, which requires wearing a rubber mask, multiple layers of boots and gloves, and thick charcoal impregnated over garments for many hours. Such conditions may flare AD or make it intolerable.
Although stress is a part of any deployment experience, excessive or prolonged stress can lead to combat operational stress reactions that inhibit a service member’s ability to function.6 Stressors during deployment can accumulate and may be caused by the operational environment, loss of fellow service members to injury or death, illness, leadership demands, personal choices, issues on the home front, interpersonal conflicts, and sleep loss.7 Atopic dermatitis can be exacerbated by such stress, leading to increased pruritus and scratching.7-9 Symptomatic AD also can play a role in worsening combat stress. Although severe pruritus may affect attentiveness to job duties during the day, these symptoms, if uncontrolled, also can negatively affect sleep. As many as 60% of patients with AD at baseline and 83% of patients with exacerbations experience sleep disturbance due to their disease.5 These stressors experienced by deployed military personnel can contribute to combat stress reactions, which may vary from simple inattentiveness to more serious behaviors such as suicidal ideation.6 Combat stress reactions inhibit a military member’s ability to function properly in the deployed environment and can lead to notable safety concerns and potential mission failure.
Vaccinations
Military members deploying overseas are required to receive specific vaccinations, including the smallpox vaccine. Although the virus was eradicated in 1980, the concern for smallpox to be used as a biological weapon in certain areas of the world necessitates continued vaccination of military populations. According to the Centers for Disease Control and Prevention, the only known reservoir for the virus is humans, and the disease has a mortality rate of 30%.10 A history of or present AD is a contraindication for primary smallpox vaccination and revaccination for nonemergency use because of the risk for eczema vaccinatum.11 The risk also applies to close contacts of vaccinated members. For 30 days after vaccination, service members must avoid skin-to-skin contact with anyone who has active AD.12 Eczema vaccinatum in vaccinated individuals is typically self-limited; however, eczema vaccinatum in nonvaccinated contacts can be severe. One case report described a 28-month-old child with refractory AD who developed severe eczema vaccinatum after contact with her recently vaccinated military parent. The child required a 48-day admission to the intensive care unit and multiple skin grafts; fortunately, the child did not develop any apparent long-term sequelae.13 This case highlights the importance of understanding the risks associated with smallpox vaccination in military members with AD and the responsibility of health care providers to properly screen and counsel individuals prior to administering smallpox vaccines.
Treatment
Treatment of mild to moderate AD is relatively straightforward in developed countries with good access to medical care. The most recent American Academy of Dermatology clinical guidelines for AD focus on minimizing irritants and triggers, regularly using moisturizers soon after bathing, and using topical steroids as needed.5 Military members face specific challenges regarding treatment of AD, particularly when deployed to remote locations without access to treatment facilities or medications. Military members are required to carry all necessary personal medications with them for at least 6 months and preferably the duration of the deployment, sometimes up to 1 year. Military members carry a large amount of gear for deployments, and it is not feasible to pack an additional 10 to 20 lb worth of emollients and topical steroids to last the entire deployment. Routine laboratory monitoring is limited or completely unavailable. Refrigeration typically is not available, making use of systemic medications nearly impossible during deployments. In the event of complications such as eczema herpeticum or secondary bacterial infection, service members could require evacuation from the deployed location to a larger field hospital or to the United States, which is costly and also removes a valuable team member from the deployed unit. These limitations in access to care, medications, and treatment options make AD a difficult condition to treat in the deployed setting.
Nonmilitary Medical Providers
Civilian providers play an important role in diagnosing and treating AD. It is vital to completely and accurately document treatment of all skin diseases; however, it is especially important for those who desire to or currently serve in the military. Military primary care providers or military dermatologists must review the information from civilian providers to aid in determining suitability for entry or retention in the military. Clearly documenting the morphology, extent of disease involvement (eg, body surface area), treatment plan, response to treatment, and exacerbating factors will aid in ensuring the patient’s medical record accurately reflects their skin disease. Ultimately, this record often is the only information available to make health determinations regarding military service.
Conclusion
A career in the military is challenging and rewarding for those who volunteer to serve. Because of the demanding and unpredictable lifestyle inherent with military service, the Department of Defense maintains strict medical standards for entrance and retention. These standards ensure members are capable of safely completing training and deploying anywhere in the world. Although AD is a relatively common and treatable skin disease in locations with well-established medical care, it can pose a notable problem for service members while deployed to austere locations with variable environments around the world. Environmental factors and gear requirements, coupled with limited access to treatment facilities and medications, render AD a potentially serious issue. Atopic dermatitis in military members can affect individual medical readiness and unit success. It is important that all providers understand the myriad effects that AD can have on an individual who wishes to join or continue service in the military.
Dermatologic conditions historically have affected military members’ ability to serve during times of peace and conflict. These conditions range from chronic dermatologic diseases to environment- or occupation-related dermatologic diseases. Mild to moderate atopic dermatitis (AD) typically is a manageable skin condition. However, in a deployed setting, a flare of AD can result in the inability of a member to perform their military duty, which directly compromises mission safety and effectiveness. The military developed and updates medical standards for entry and retention of service members. These standards are designed to ensure the greatest potential for a military member to successfully serve at home station and during combat operations.
Impact of Injuries in Military
Historically, disease and nonbattle injuries have resulted in notably more hospitalizations and time lost than injuries sustained on the battlefield.1 A review of major conflicts dating from World War II shows approximately 10% of all dermatologic concerns were related to eczematous dermatitis, with 2% specifically related to AD. These numbers varied remarkably depending on the location and environment of the conflict, with eczema accounting for 25% of dermatologic concerns during the Gulf War.2 During the initial phases of Operation Iraqi Freedom, approximately 75% of hospitalizations were from disease and nonbattle injuries, of which dermatologic disease accounted for 3%.1 From 2003 to 2006 in Iraq, 35 service members were evacuated from combat zones specifically for uncontrolled AD.3 In a deployed environment, each member is critical to the unit’s success in completing their mission. A single member of a unit often is the only person qualified to perform a function for that team. There are rarely extra people with similar skills to replace a member unable to complete his/her duties. The loss of a single member compromises the effectiveness and safety of the team and can lead to mission failure. Therefore, AD can have a profound impact on military operations in a deployed environment.
Military Medical Standards for Accession and Retention
There are 2 main goals of the military medical standards. First, the individual health of the applicant or military member is of utmost importance. Applicants with medical conditions that will be exacerbated by military service or that limit the ability for successful military operations are not accepted for military service. Once an active-duty member is diagnosed with a medical condition, the military determines if limitations are needed for military assignments and deployments based on available medical care in those locations. Second, mission accomplishment in combat operations requires that healthy military members are able to complete their jobs in extreme environments and under notable stress. If an applicant has a medical condition unsuitable for military service, it is in the best interest of the applicant and the military to deny entry.
The Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03) lists conditions that are disqualifying for military service.4 Section 5.21 lists the following as disqualifying for military service in relation to eczematous dermatitis:
d. History of AD or eczema after the 12th birthday. History of residual or recurrent lesions in characteristic areas (face, neck, antecubital or popliteal fossae, occasionally wrists and hands).
e. History of recurrent or chronic nonspecific dermatitis within the past 2 years to include contact (irritant or allergic) or dyshidrotic dermatitis requiring more than treatment with topical corticosteroid.4
Although cases of incorrect diagnosis or very mild AD can be considered for a waiver, the process can be laborious and consideration or approval is not guaranteed. For current military members with new chronic eczematous dermatitis, each service has a process for evaluation and treatment. Some special operational jobs, such as aircrew, missile operators, and divers, have more restrictive medical requirements that are monitored by physicians with special training in these populations.
Atopic dermatitis affects 25% of children and 2% to 3% of adults.5 Approximately 60% of patients with AD will develop their first eruption by 1 year of age, and 90% by 5 years of age. Although the majority of patients will have resolution of their disease during childhood, 10% to 30% will have persistent disease into adulthood.5 Because the majority of AD resolves in childhood, it is understandable that asymptomatic individuals with a history of AD before 12 years of age meet military entrance medical standards.
Provoking Factors
The US Military maintains stringent medical standards because of the nature of the dynamic, rapidly changing military environment and its demands. Whether training for readiness in an austere location, deploying to extreme climates, or being stationed overseas, service members must be prepared to encounter a myriad of environmental extremes, physical stress, and psychological stressors. Environmental factors commonly experienced in the military can provoke or exacerbate symptoms of AD (Figures 1 and 2). Ideally, an individual with AD lives in a stable climate, has access to moisturizers and topical steroids, bathes regularly to remove dust and debris, wears 100% cotton garments to avoid irritation, and avoids using gear that would cause exacerbations. Service members rarely have such accommodations in deployed settings. A recent article in Military Medicine explained quite well, “If someone wanted to design an experience with the explicit goal to flare a person with otherwise well controlled atopic dermatitis it would probably look like a military deployment.”3
The United States has a military presence in countries with extreme temperature and humidity variations all over the world. Uniforms are standardized, and members are required to wear prescribed clothing with no alternatives. Uniforms are made of durable sturdy material. If uniforms can be laundered, they often are grouped together, and sensitive detergent cannot be specified. Bathing is challenging in deployed locations, with troops often going weeks using baby wipes for self-hygiene. These conditions increase risk for development of contact allergens, and little access to proper hygiene practices also increases risk for secondary infections in members with AD.
In addition to environmental challenges, the military gear and equipment used can flare AD. Service members must wear protective gear such as body armor. These heavy hard pieces of material are bulky; difficult to wash; and cause friction, sweating, and irritation. The military prepares for operations in chemical, biological, radiological, or nuclear environments, which requires wearing a rubber mask, multiple layers of boots and gloves, and thick charcoal impregnated over garments for many hours. Such conditions may flare AD or make it intolerable.
Although stress is a part of any deployment experience, excessive or prolonged stress can lead to combat operational stress reactions that inhibit a service member’s ability to function.6 Stressors during deployment can accumulate and may be caused by the operational environment, loss of fellow service members to injury or death, illness, leadership demands, personal choices, issues on the home front, interpersonal conflicts, and sleep loss.7 Atopic dermatitis can be exacerbated by such stress, leading to increased pruritus and scratching.7-9 Symptomatic AD also can play a role in worsening combat stress. Although severe pruritus may affect attentiveness to job duties during the day, these symptoms, if uncontrolled, also can negatively affect sleep. As many as 60% of patients with AD at baseline and 83% of patients with exacerbations experience sleep disturbance due to their disease.5 These stressors experienced by deployed military personnel can contribute to combat stress reactions, which may vary from simple inattentiveness to more serious behaviors such as suicidal ideation.6 Combat stress reactions inhibit a military member’s ability to function properly in the deployed environment and can lead to notable safety concerns and potential mission failure.
Vaccinations
Military members deploying overseas are required to receive specific vaccinations, including the smallpox vaccine. Although the virus was eradicated in 1980, the concern for smallpox to be used as a biological weapon in certain areas of the world necessitates continued vaccination of military populations. According to the Centers for Disease Control and Prevention, the only known reservoir for the virus is humans, and the disease has a mortality rate of 30%.10 A history of or present AD is a contraindication for primary smallpox vaccination and revaccination for nonemergency use because of the risk for eczema vaccinatum.11 The risk also applies to close contacts of vaccinated members. For 30 days after vaccination, service members must avoid skin-to-skin contact with anyone who has active AD.12 Eczema vaccinatum in vaccinated individuals is typically self-limited; however, eczema vaccinatum in nonvaccinated contacts can be severe. One case report described a 28-month-old child with refractory AD who developed severe eczema vaccinatum after contact with her recently vaccinated military parent. The child required a 48-day admission to the intensive care unit and multiple skin grafts; fortunately, the child did not develop any apparent long-term sequelae.13 This case highlights the importance of understanding the risks associated with smallpox vaccination in military members with AD and the responsibility of health care providers to properly screen and counsel individuals prior to administering smallpox vaccines.
Treatment
Treatment of mild to moderate AD is relatively straightforward in developed countries with good access to medical care. The most recent American Academy of Dermatology clinical guidelines for AD focus on minimizing irritants and triggers, regularly using moisturizers soon after bathing, and using topical steroids as needed.5 Military members face specific challenges regarding treatment of AD, particularly when deployed to remote locations without access to treatment facilities or medications. Military members are required to carry all necessary personal medications with them for at least 6 months and preferably the duration of the deployment, sometimes up to 1 year. Military members carry a large amount of gear for deployments, and it is not feasible to pack an additional 10 to 20 lb worth of emollients and topical steroids to last the entire deployment. Routine laboratory monitoring is limited or completely unavailable. Refrigeration typically is not available, making use of systemic medications nearly impossible during deployments. In the event of complications such as eczema herpeticum or secondary bacterial infection, service members could require evacuation from the deployed location to a larger field hospital or to the United States, which is costly and also removes a valuable team member from the deployed unit. These limitations in access to care, medications, and treatment options make AD a difficult condition to treat in the deployed setting.
Nonmilitary Medical Providers
Civilian providers play an important role in diagnosing and treating AD. It is vital to completely and accurately document treatment of all skin diseases; however, it is especially important for those who desire to or currently serve in the military. Military primary care providers or military dermatologists must review the information from civilian providers to aid in determining suitability for entry or retention in the military. Clearly documenting the morphology, extent of disease involvement (eg, body surface area), treatment plan, response to treatment, and exacerbating factors will aid in ensuring the patient’s medical record accurately reflects their skin disease. Ultimately, this record often is the only information available to make health determinations regarding military service.
Conclusion
A career in the military is challenging and rewarding for those who volunteer to serve. Because of the demanding and unpredictable lifestyle inherent with military service, the Department of Defense maintains strict medical standards for entrance and retention. These standards ensure members are capable of safely completing training and deploying anywhere in the world. Although AD is a relatively common and treatable skin disease in locations with well-established medical care, it can pose a notable problem for service members while deployed to austere locations with variable environments around the world. Environmental factors and gear requirements, coupled with limited access to treatment facilities and medications, render AD a potentially serious issue. Atopic dermatitis in military members can affect individual medical readiness and unit success. It is important that all providers understand the myriad effects that AD can have on an individual who wishes to join or continue service in the military.
- Zouris JM, Wade AL, Magno CP. Injury and illness casualty distributions among U.S. Army and Marine Corps personnel during Operation Iraqi Freedom. Mil Med. 2008;173:247-252.
- Gelman, AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:177-182.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 8, 2019.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Force Health Protection (Army Techniques Publication No. 4-02.8). Washington, DC: Department of the Army; March 2016. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/atp4_02x8.pdf. Accessed August 19, 2019.
- Judkins JL, Bradley DL. A review of the effectiveness of a combat and operational stress control restoration center in Afghanistan. Mil Med. 2017;182:1755-1762.
- Suarez AL, Feramisco JD, Koo J, et al. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Dermatol Venereol. 2012;92:7-15.
- Mochizuki H, Lavery MJ, Nattkemper LA, et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br J Dermatol. 2019;180:821-827.
- Centers for Disease Control and Prevention. Smallpox: contraindications to vaccination. https://www.cdc.gov/smallpox/clinicians/vaccination-contraindications1.html. Updated December 5, 2016. Accessed August 19, 2019.
- Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5:84-90.
- Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis. 2012;54:832-840.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccine. Clin Infect Dis. 2008;46:1555-1561.
- Zouris JM, Wade AL, Magno CP. Injury and illness casualty distributions among U.S. Army and Marine Corps personnel during Operation Iraqi Freedom. Mil Med. 2008;173:247-252.
- Gelman, AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:177-182.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 8, 2019.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Force Health Protection (Army Techniques Publication No. 4-02.8). Washington, DC: Department of the Army; March 2016. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/atp4_02x8.pdf. Accessed August 19, 2019.
- Judkins JL, Bradley DL. A review of the effectiveness of a combat and operational stress control restoration center in Afghanistan. Mil Med. 2017;182:1755-1762.
- Suarez AL, Feramisco JD, Koo J, et al. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Dermatol Venereol. 2012;92:7-15.
- Mochizuki H, Lavery MJ, Nattkemper LA, et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br J Dermatol. 2019;180:821-827.
- Centers for Disease Control and Prevention. Smallpox: contraindications to vaccination. https://www.cdc.gov/smallpox/clinicians/vaccination-contraindications1.html. Updated December 5, 2016. Accessed August 19, 2019.
- Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5:84-90.
- Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis. 2012;54:832-840.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccine. Clin Infect Dis. 2008;46:1555-1561.
Practice Points
- The US Military follows strict medical eligibility requirements for enlistment and retention. Atopic dermatitis (AD) and chronic eczematous conditions after 12 years of age is disqualifying for military service, but waivers may be possible for mild cases.
- Unpredictable and rigorous environmental and occupational stressors associated with military service as well as limited access to medical care make AD a challenging condition to manage for service members, particularly during military deployment.
- Accurate diagnosis and documentation of AD in childhood and adolescence by nonmilitary providers are essential, as they will aid in appropriately determining an applicant’s potential to successfully serve in the military.
- For current service members, nonmilitary providers play a vital role in diagnosis and management where military dermatologists are not readily available.
Smallpox Vaccine Complications: The Dermatologist’s Role in Diagnosis and Management
The practice of variolation, or inoculation of the smallpox virus from a pustule into a healthy person, was described as early as 1500
Immunization
Vaccinia is an orthopoxvirus, distinct from the smallpox virus variola, with cross-protective immunity after infection. The smallpox vaccine that is available today is a second-generation vaccinia virus derived from plaque purification cloning from the first-generation version originally licensed in 1932, which was central to eradication.5 Today’s vaccine is administered using a bifurcated needle to puncture the epidermis 15 times. Ideally, a papule forms at the inoculation site 3 to 5 days later, progresses to a vesicle and then a pustule, and finally crusts and reaches maximum size by day 10. The crust separates from the skin at 14 to 21 days, at which time the virus can no longer be isolated from the wound. United States Department of Defense surveillance of the first 450,000 vaccinated personnel noted 1% of recipients developed cutaneous eruptions beyond the vaccination site, 5% developed a localized rash, and 1% experienced a generalized eruption.2 Adverse reactions included generalized vaccinia, erythema multiforme (EM), autoinoculation (including ocular vaccinia), and contact vaccinia. There were no cases of eczema vaccinatum (EV) or progressive vaccinia (PV) reported, and no deaths were attributed to these initial vaccines.2
Immunologic Response
Vaccinia replicates in keratinocytes, spreading from cell to cell, resulting in necrosis and vesicle formation. Components of both cellular and humoral immune responses are in place by 10 days after immunization. Deficiencies in these responses result in vaccine complications secondary to vaccine escape and replication beyond the inoculation site.6 A helper T cell TH2-predominant cytokine response in atopic individuals is the likely pathogenesis required for the rapid viral spread for EV.7 Similarly, patients with cell-mediated immunity deficiencies cannot sufficiently produce enough cytotoxic T cells to eliminate an established infection, which can result in PV. Despite the effectiveness of intravenous vaccinia immunoglobulins (VIGIVs) when administered to patients with certain vaccine complications, observations that children with severe X-linked agammaglobulinemia (Bruton disease) have normal responses to vaccination suggest that antibody production is least important in viral control.8 Simian models also suggest that B-cell depletion has no impact on lesion dissemination, as lesion size is inversely correlated with T-cell count.9
Eczema Vaccinatum
A national survey estimated the prevalence of eczema in the United States at 31.6 million individuals,10 with 2- to 3-fold increases in incidence since the 1970s.11 Due to the risk for developing EV, the Advisory Committee on Immunization Practices considers personal history of eczema or contact with a family member who has eczema (either currently or in the past) contraindications to nonemergency administration of the vaccine.12,13 However, atopic conditions in general are underrecognized, with only approximately one-third of patients carrying an official diagnosis from a physician.10 Despite a large atopic and vaccinated population, EV remains relatively uncommon at 10 to 39 cases per million vaccines.6
The EV rash classically involves the midface, neck, and antecubital and popliteal fossae but can present in any location. The lesions start as papules that quickly progress to vesicles and pustules with crusting on an erythematous base. Given the extent of denudation of the epidermis, impetiginization can occur. Death rates as high as 30% have been reported14 but have only occurred in instances of secondary contact transmission with no deaths occurring in the primary vaccinees.15 In a case published in 2008, a 2-year-old boy developed the first documented EV case under the new program after exposure to his father’s predeployment vaccine.16 A similar rash is shown in Figure 1 with notable vesicles and pustules. The child required burn patient–type management, VIGIV, and treatment with cidofovir and an investigational antiorthopox agent. He was discharged from the hospital after 48 days without sequelae or considerable scarring.16 If a family member has a contraindication barring secondary contact with the vaccine, the US Department of Defense’s policy defers vaccination in active-duty members until they reach their deployment destination, at which point the inoculation is administered.
Progressive Vaccinia
Progressive vaccinia is also known as vaccinia necrosum or vaccinia gangrenosum. It is a dreaded but uncommon complication, occurring once in every 1 million vaccinations. It carries an overall case fatality rate of 15%,17 but it nearly always is fatal in patients with severe T-cell defects.18 Progressive vaccinia occurs exclusively in patients with cell-mediated immunodeficiency, with the severity of the acute illness correlating with the severity of immunodeficiency. In patients with cell-mediated immunodeficiency but intact humoral immunity, progression can be limited to expansion of the lesion, as it is thought that antibody production restricts viremia.18 Progressive vaccinia should be suspected in a patient if the vaccine site shows no signs of improvement by 14 days.19 The PV lesions do not heal and may progress or recur in patients with signs of prior healing. The leading edge has confluent vesicles, and the center of the lesion develops necrosis with thick black eschar formation. Most specifically, there is no surrounding inflammation; however, inflammation can develop later as a response to treatment or secondary infection. Figure 2 shows a PV lesion with black eschar and a transition to intact dermis without inflammation.
The first known case of PV since the 1960s vaccination campaign occurred in an active-duty Marine vaccinated with vaccinia before a diagnosis of acute myelogenous leukemia was recognized 2 weeks later.19 The vaccine site was stable in size and crusted when he received neutropenia-inducing chemotherapy 6.5 weeks after vaccination. The site then progressed in a manner typical for PV with central necrosis and a lack of inflammation at the expanding painless wound edge.19 This classic appearance with progression of satellite lesions prompted the treatment team to obtain wound and serum samples, which yielded the orthopox virus from polymerase chain reaction and viral culture. He required 2 months of care in an intensive care unit and received treatment with topical imiquimod, VIGIV, a topical and intravenous antiorthopox agent, and a second investigational antiorthopox agent; the patient ultimately survived.17,20
Generalized Vaccinia
Generalized vaccinia (GV) typically is a benign vaccine complication resulting from viremic spread from the initial inoculation site and is most commonly seen in healthy patients. Generalized vaccinia is only life threatening in immunocompromised patients. The incidence of GV is 23.4 to 241.5 patients per million vaccines.6 The majority of GV cases occur 5 to 12 days after vaccination when small distant pustules or vesicles appear on any part of the body, including the palms and soles. The lesions usually are smaller than the primary vaccination site and resolve more quickly. Generalized vaccinia can have a few to several hundred pocks, though the rash is rarely as diffuse as EV presentations.3 Given that EV can present diffusely on skin unaffected by atopic dermatitis, GV can be difficult to distinguish from EV. Features more common to EV include more systemically ill patients, increased numbers of lesions, and lesions that become confluent in an atopic distribution. It has been suggested that GV can be differentiated from vesicular or vesiculopapular EM because GV does not develop flaccid bullae and EM typically has targetoid lesions.18 Mild GV disease requires no treatment, but VIGIV can be used in more extensive cases.
Localized Reactions Due to Viral Replication
Accidental autoinoculation can occur when patients touch the vaccination site and then themselves, transferring virus particles to areas of compromised skin integrity, most commonly on the face, eyes, hands, genitalia, anus, or any other broken skin. Autoinoculation happens with some frequency and is of limited clinical concern unless there is ocular involvement. Keratitis develops in 6% of ocular vaccinia cases, and VIGIV is contraindicated, as rabbit models suggest that antigen-antibody precipitates in the cornea can cause scarring.21 Instead, trifluorothymidine is an effective topical treatment available for ocular vaccinia.
A robust response or “take” is defined as a reaction having redness, swelling, and warmth more than 3 inches in diameter at the inoculation site, peaking 6 to 12 days after inoculation with spontaneous regression occurring 1 to 3 days after.22,23 A robust take frequently is of concern to the clinician, as it can be difficult to discern from secondary infection. Secondary infections are uncommon, and a robust take is secondary to viral, not bacterial, cellulitis. Unfortunately, there are no diagnostics that have utility in distinguishing between the two, and the decision to administer empiric antibiotics might be unavoidable in light of the consequences of an untreated, rapidly progressive bacterial cellulitis. Milder cases in the setting of no constitutional symptoms could be safely monitored if close follow-up is assured.
Generalized Skin Reactions Without Viral Replication
Development of erythematous, pruritic, urticarial, and diffuse targetlike lesions of EM is common in first-time vaccinees. Often misdiagnosed as GV, EM is an immunologically mediated, not virally mediated, process. The most common infectious cause prompting EM is herpes simplex virus type 1. In the setting of a live-virus vaccine, it is difficult to determine if the vaccine prompted herpes simplex virus type 1 viral shedding and associated EM or if the vaccinia vaccine is more directly the cause of EM.24 Symptoms typically are mild, but more severe reactions may require treatment with corticosteroids. Stevens-Johnson syndrome with a severe bullous eruption has been linked to vaccinia24 but fortunately is rare. Morbilliform eruptions, urticaria, and angioedema also can occur.
Final Thoughts
Given current world events and ongoing bioterrorism threats, the smallpox vaccine program continues indefinitely. With a brisk military deployment tempo, a larger population of new vaccinees naturally will yield more cutaneous reactions. Military members, civilian health care workers, and members of the National Guard and National Reserves will develop complications and present to dermatologists for care. The historical pool of providers accustomed to seeing these complications from the 1960s eradication campaign is scant. Military and civilian dermatologists alike are uniquely poised to be the experts on protean manifestations of vaccinia reactions.
- Voigt EA, Kennedy RB, Poland GA. Defending against smallpox: a focus on vaccines. Expert Rev Vaccines. 2016;15:1197-1211.
- Grabenstein J, Wikenwerder W Jr. US military smallpox vaccination program experience. JAMA. 2003;289:3278-3282.
- Kelly CD, Egan C, Davis SW, et al. Laboratory confirmation of generalized vaccinia following smallpox vaccination. J Clin Microbiol. 2004;42:1373-1375.
- Slike BM, Creegan M, Marovich M, et al. Humoral immunity to primary smallpox vaccination: impact of childhood versus adult immunization on vaccinia vector vaccine development in military populations. PLoS One. 2017;12:E0169247.
- Notice to readers: newly licensed vaccine to replace old smallpox vaccine. MMWR. 2008;57:207-208.
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101-114.
- Engler R, Kenner J, Leung D. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357-365.
- Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36:766-774.
- Gordon S, Cecchinato V, Andresen V, et al. Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis. 2011;203:1043-1053.
- Hanifin J, Reed M. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;82:82-91.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1-16.
- Petersen BW, Harms TJ, Reynolds MG, et al. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupation exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunizations Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65:257-262.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Eczema Vaccinatum. Eczema vaccinatum as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection analysis, and presentation of immunization safety data. Vaccine. 2007:25;5725-5734.
- Aragón TJ, Ulrich S, Fernyak S, et al. Risks of serious complications and death from smallpox vaccination: a systematic review of the United States experience, 1963-1968. BMC Public Health. 2003;3:26.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555-1561.
- Centers for Disease Control and Prevention (CDC). Progressive vaccinia in a military smallpox vaccinee—United States 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Fulginiti VA, Papier A, Lane M, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Progressive Vaccinia. Progressive vaccinia as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5735-5744.
- Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:E1372-E1385.
- Lane ML, Goldstein J. Adverse events occurring after smallpox vaccination. Semin Ped Infect Dis. 2003;14:189-195.
- Vaccine adverse events. CDC website. http://www.cdc.gov/smallpox/clinicians/vaccine-adverse-events5.html. Accessed January 3, 2018.
- Cono J, Casey CG, Bell DM. Smallpox vaccination and adversereactions, guidance for clinicians. CDC website. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Accessed January 3, 2018.
- Rosenblatt AE, Stein SL. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33:327-332.
The practice of variolation, or inoculation of the smallpox virus from a pustule into a healthy person, was described as early as 1500
Immunization
Vaccinia is an orthopoxvirus, distinct from the smallpox virus variola, with cross-protective immunity after infection. The smallpox vaccine that is available today is a second-generation vaccinia virus derived from plaque purification cloning from the first-generation version originally licensed in 1932, which was central to eradication.5 Today’s vaccine is administered using a bifurcated needle to puncture the epidermis 15 times. Ideally, a papule forms at the inoculation site 3 to 5 days later, progresses to a vesicle and then a pustule, and finally crusts and reaches maximum size by day 10. The crust separates from the skin at 14 to 21 days, at which time the virus can no longer be isolated from the wound. United States Department of Defense surveillance of the first 450,000 vaccinated personnel noted 1% of recipients developed cutaneous eruptions beyond the vaccination site, 5% developed a localized rash, and 1% experienced a generalized eruption.2 Adverse reactions included generalized vaccinia, erythema multiforme (EM), autoinoculation (including ocular vaccinia), and contact vaccinia. There were no cases of eczema vaccinatum (EV) or progressive vaccinia (PV) reported, and no deaths were attributed to these initial vaccines.2
Immunologic Response
Vaccinia replicates in keratinocytes, spreading from cell to cell, resulting in necrosis and vesicle formation. Components of both cellular and humoral immune responses are in place by 10 days after immunization. Deficiencies in these responses result in vaccine complications secondary to vaccine escape and replication beyond the inoculation site.6 A helper T cell TH2-predominant cytokine response in atopic individuals is the likely pathogenesis required for the rapid viral spread for EV.7 Similarly, patients with cell-mediated immunity deficiencies cannot sufficiently produce enough cytotoxic T cells to eliminate an established infection, which can result in PV. Despite the effectiveness of intravenous vaccinia immunoglobulins (VIGIVs) when administered to patients with certain vaccine complications, observations that children with severe X-linked agammaglobulinemia (Bruton disease) have normal responses to vaccination suggest that antibody production is least important in viral control.8 Simian models also suggest that B-cell depletion has no impact on lesion dissemination, as lesion size is inversely correlated with T-cell count.9
Eczema Vaccinatum
A national survey estimated the prevalence of eczema in the United States at 31.6 million individuals,10 with 2- to 3-fold increases in incidence since the 1970s.11 Due to the risk for developing EV, the Advisory Committee on Immunization Practices considers personal history of eczema or contact with a family member who has eczema (either currently or in the past) contraindications to nonemergency administration of the vaccine.12,13 However, atopic conditions in general are underrecognized, with only approximately one-third of patients carrying an official diagnosis from a physician.10 Despite a large atopic and vaccinated population, EV remains relatively uncommon at 10 to 39 cases per million vaccines.6
The EV rash classically involves the midface, neck, and antecubital and popliteal fossae but can present in any location. The lesions start as papules that quickly progress to vesicles and pustules with crusting on an erythematous base. Given the extent of denudation of the epidermis, impetiginization can occur. Death rates as high as 30% have been reported14 but have only occurred in instances of secondary contact transmission with no deaths occurring in the primary vaccinees.15 In a case published in 2008, a 2-year-old boy developed the first documented EV case under the new program after exposure to his father’s predeployment vaccine.16 A similar rash is shown in Figure 1 with notable vesicles and pustules. The child required burn patient–type management, VIGIV, and treatment with cidofovir and an investigational antiorthopox agent. He was discharged from the hospital after 48 days without sequelae or considerable scarring.16 If a family member has a contraindication barring secondary contact with the vaccine, the US Department of Defense’s policy defers vaccination in active-duty members until they reach their deployment destination, at which point the inoculation is administered.

Progressive Vaccinia
Progressive vaccinia is also known as vaccinia necrosum or vaccinia gangrenosum. It is a dreaded but uncommon complication, occurring once in every 1 million vaccinations. It carries an overall case fatality rate of 15%,17 but it nearly always is fatal in patients with severe T-cell defects.18 Progressive vaccinia occurs exclusively in patients with cell-mediated immunodeficiency, with the severity of the acute illness correlating with the severity of immunodeficiency. In patients with cell-mediated immunodeficiency but intact humoral immunity, progression can be limited to expansion of the lesion, as it is thought that antibody production restricts viremia.18 Progressive vaccinia should be suspected in a patient if the vaccine site shows no signs of improvement by 14 days.19 The PV lesions do not heal and may progress or recur in patients with signs of prior healing. The leading edge has confluent vesicles, and the center of the lesion develops necrosis with thick black eschar formation. Most specifically, there is no surrounding inflammation; however, inflammation can develop later as a response to treatment or secondary infection. Figure 2 shows a PV lesion with black eschar and a transition to intact dermis without inflammation.

The first known case of PV since the 1960s vaccination campaign occurred in an active-duty Marine vaccinated with vaccinia before a diagnosis of acute myelogenous leukemia was recognized 2 weeks later.19 The vaccine site was stable in size and crusted when he received neutropenia-inducing chemotherapy 6.5 weeks after vaccination. The site then progressed in a manner typical for PV with central necrosis and a lack of inflammation at the expanding painless wound edge.19 This classic appearance with progression of satellite lesions prompted the treatment team to obtain wound and serum samples, which yielded the orthopox virus from polymerase chain reaction and viral culture. He required 2 months of care in an intensive care unit and received treatment with topical imiquimod, VIGIV, a topical and intravenous antiorthopox agent, and a second investigational antiorthopox agent; the patient ultimately survived.17,20
Generalized Vaccinia
Generalized vaccinia (GV) typically is a benign vaccine complication resulting from viremic spread from the initial inoculation site and is most commonly seen in healthy patients. Generalized vaccinia is only life threatening in immunocompromised patients. The incidence of GV is 23.4 to 241.5 patients per million vaccines.6 The majority of GV cases occur 5 to 12 days after vaccination when small distant pustules or vesicles appear on any part of the body, including the palms and soles. The lesions usually are smaller than the primary vaccination site and resolve more quickly. Generalized vaccinia can have a few to several hundred pocks, though the rash is rarely as diffuse as EV presentations.3 Given that EV can present diffusely on skin unaffected by atopic dermatitis, GV can be difficult to distinguish from EV. Features more common to EV include more systemically ill patients, increased numbers of lesions, and lesions that become confluent in an atopic distribution. It has been suggested that GV can be differentiated from vesicular or vesiculopapular EM because GV does not develop flaccid bullae and EM typically has targetoid lesions.18 Mild GV disease requires no treatment, but VIGIV can be used in more extensive cases.
Localized Reactions Due to Viral Replication
Accidental autoinoculation can occur when patients touch the vaccination site and then themselves, transferring virus particles to areas of compromised skin integrity, most commonly on the face, eyes, hands, genitalia, anus, or any other broken skin. Autoinoculation happens with some frequency and is of limited clinical concern unless there is ocular involvement. Keratitis develops in 6% of ocular vaccinia cases, and VIGIV is contraindicated, as rabbit models suggest that antigen-antibody precipitates in the cornea can cause scarring.21 Instead, trifluorothymidine is an effective topical treatment available for ocular vaccinia.
A robust response or “take” is defined as a reaction having redness, swelling, and warmth more than 3 inches in diameter at the inoculation site, peaking 6 to 12 days after inoculation with spontaneous regression occurring 1 to 3 days after.22,23 A robust take frequently is of concern to the clinician, as it can be difficult to discern from secondary infection. Secondary infections are uncommon, and a robust take is secondary to viral, not bacterial, cellulitis. Unfortunately, there are no diagnostics that have utility in distinguishing between the two, and the decision to administer empiric antibiotics might be unavoidable in light of the consequences of an untreated, rapidly progressive bacterial cellulitis. Milder cases in the setting of no constitutional symptoms could be safely monitored if close follow-up is assured.
Generalized Skin Reactions Without Viral Replication
Development of erythematous, pruritic, urticarial, and diffuse targetlike lesions of EM is common in first-time vaccinees. Often misdiagnosed as GV, EM is an immunologically mediated, not virally mediated, process. The most common infectious cause prompting EM is herpes simplex virus type 1. In the setting of a live-virus vaccine, it is difficult to determine if the vaccine prompted herpes simplex virus type 1 viral shedding and associated EM or if the vaccinia vaccine is more directly the cause of EM.24 Symptoms typically are mild, but more severe reactions may require treatment with corticosteroids. Stevens-Johnson syndrome with a severe bullous eruption has been linked to vaccinia24 but fortunately is rare. Morbilliform eruptions, urticaria, and angioedema also can occur.
Final Thoughts
Given current world events and ongoing bioterrorism threats, the smallpox vaccine program continues indefinitely. With a brisk military deployment tempo, a larger population of new vaccinees naturally will yield more cutaneous reactions. Military members, civilian health care workers, and members of the National Guard and National Reserves will develop complications and present to dermatologists for care. The historical pool of providers accustomed to seeing these complications from the 1960s eradication campaign is scant. Military and civilian dermatologists alike are uniquely poised to be the experts on protean manifestations of vaccinia reactions.
The practice of variolation, or inoculation of the smallpox virus from a pustule into a healthy person, was described as early as 1500
Immunization
Vaccinia is an orthopoxvirus, distinct from the smallpox virus variola, with cross-protective immunity after infection. The smallpox vaccine that is available today is a second-generation vaccinia virus derived from plaque purification cloning from the first-generation version originally licensed in 1932, which was central to eradication.5 Today’s vaccine is administered using a bifurcated needle to puncture the epidermis 15 times. Ideally, a papule forms at the inoculation site 3 to 5 days later, progresses to a vesicle and then a pustule, and finally crusts and reaches maximum size by day 10. The crust separates from the skin at 14 to 21 days, at which time the virus can no longer be isolated from the wound. United States Department of Defense surveillance of the first 450,000 vaccinated personnel noted 1% of recipients developed cutaneous eruptions beyond the vaccination site, 5% developed a localized rash, and 1% experienced a generalized eruption.2 Adverse reactions included generalized vaccinia, erythema multiforme (EM), autoinoculation (including ocular vaccinia), and contact vaccinia. There were no cases of eczema vaccinatum (EV) or progressive vaccinia (PV) reported, and no deaths were attributed to these initial vaccines.2
Immunologic Response
Vaccinia replicates in keratinocytes, spreading from cell to cell, resulting in necrosis and vesicle formation. Components of both cellular and humoral immune responses are in place by 10 days after immunization. Deficiencies in these responses result in vaccine complications secondary to vaccine escape and replication beyond the inoculation site.6 A helper T cell TH2-predominant cytokine response in atopic individuals is the likely pathogenesis required for the rapid viral spread for EV.7 Similarly, patients with cell-mediated immunity deficiencies cannot sufficiently produce enough cytotoxic T cells to eliminate an established infection, which can result in PV. Despite the effectiveness of intravenous vaccinia immunoglobulins (VIGIVs) when administered to patients with certain vaccine complications, observations that children with severe X-linked agammaglobulinemia (Bruton disease) have normal responses to vaccination suggest that antibody production is least important in viral control.8 Simian models also suggest that B-cell depletion has no impact on lesion dissemination, as lesion size is inversely correlated with T-cell count.9
Eczema Vaccinatum
A national survey estimated the prevalence of eczema in the United States at 31.6 million individuals,10 with 2- to 3-fold increases in incidence since the 1970s.11 Due to the risk for developing EV, the Advisory Committee on Immunization Practices considers personal history of eczema or contact with a family member who has eczema (either currently or in the past) contraindications to nonemergency administration of the vaccine.12,13 However, atopic conditions in general are underrecognized, with only approximately one-third of patients carrying an official diagnosis from a physician.10 Despite a large atopic and vaccinated population, EV remains relatively uncommon at 10 to 39 cases per million vaccines.6
The EV rash classically involves the midface, neck, and antecubital and popliteal fossae but can present in any location. The lesions start as papules that quickly progress to vesicles and pustules with crusting on an erythematous base. Given the extent of denudation of the epidermis, impetiginization can occur. Death rates as high as 30% have been reported14 but have only occurred in instances of secondary contact transmission with no deaths occurring in the primary vaccinees.15 In a case published in 2008, a 2-year-old boy developed the first documented EV case under the new program after exposure to his father’s predeployment vaccine.16 A similar rash is shown in Figure 1 with notable vesicles and pustules. The child required burn patient–type management, VIGIV, and treatment with cidofovir and an investigational antiorthopox agent. He was discharged from the hospital after 48 days without sequelae or considerable scarring.16 If a family member has a contraindication barring secondary contact with the vaccine, the US Department of Defense’s policy defers vaccination in active-duty members until they reach their deployment destination, at which point the inoculation is administered.

Progressive Vaccinia
Progressive vaccinia is also known as vaccinia necrosum or vaccinia gangrenosum. It is a dreaded but uncommon complication, occurring once in every 1 million vaccinations. It carries an overall case fatality rate of 15%,17 but it nearly always is fatal in patients with severe T-cell defects.18 Progressive vaccinia occurs exclusively in patients with cell-mediated immunodeficiency, with the severity of the acute illness correlating with the severity of immunodeficiency. In patients with cell-mediated immunodeficiency but intact humoral immunity, progression can be limited to expansion of the lesion, as it is thought that antibody production restricts viremia.18 Progressive vaccinia should be suspected in a patient if the vaccine site shows no signs of improvement by 14 days.19 The PV lesions do not heal and may progress or recur in patients with signs of prior healing. The leading edge has confluent vesicles, and the center of the lesion develops necrosis with thick black eschar formation. Most specifically, there is no surrounding inflammation; however, inflammation can develop later as a response to treatment or secondary infection. Figure 2 shows a PV lesion with black eschar and a transition to intact dermis without inflammation.

The first known case of PV since the 1960s vaccination campaign occurred in an active-duty Marine vaccinated with vaccinia before a diagnosis of acute myelogenous leukemia was recognized 2 weeks later.19 The vaccine site was stable in size and crusted when he received neutropenia-inducing chemotherapy 6.5 weeks after vaccination. The site then progressed in a manner typical for PV with central necrosis and a lack of inflammation at the expanding painless wound edge.19 This classic appearance with progression of satellite lesions prompted the treatment team to obtain wound and serum samples, which yielded the orthopox virus from polymerase chain reaction and viral culture. He required 2 months of care in an intensive care unit and received treatment with topical imiquimod, VIGIV, a topical and intravenous antiorthopox agent, and a second investigational antiorthopox agent; the patient ultimately survived.17,20
Generalized Vaccinia
Generalized vaccinia (GV) typically is a benign vaccine complication resulting from viremic spread from the initial inoculation site and is most commonly seen in healthy patients. Generalized vaccinia is only life threatening in immunocompromised patients. The incidence of GV is 23.4 to 241.5 patients per million vaccines.6 The majority of GV cases occur 5 to 12 days after vaccination when small distant pustules or vesicles appear on any part of the body, including the palms and soles. The lesions usually are smaller than the primary vaccination site and resolve more quickly. Generalized vaccinia can have a few to several hundred pocks, though the rash is rarely as diffuse as EV presentations.3 Given that EV can present diffusely on skin unaffected by atopic dermatitis, GV can be difficult to distinguish from EV. Features more common to EV include more systemically ill patients, increased numbers of lesions, and lesions that become confluent in an atopic distribution. It has been suggested that GV can be differentiated from vesicular or vesiculopapular EM because GV does not develop flaccid bullae and EM typically has targetoid lesions.18 Mild GV disease requires no treatment, but VIGIV can be used in more extensive cases.
Localized Reactions Due to Viral Replication
Accidental autoinoculation can occur when patients touch the vaccination site and then themselves, transferring virus particles to areas of compromised skin integrity, most commonly on the face, eyes, hands, genitalia, anus, or any other broken skin. Autoinoculation happens with some frequency and is of limited clinical concern unless there is ocular involvement. Keratitis develops in 6% of ocular vaccinia cases, and VIGIV is contraindicated, as rabbit models suggest that antigen-antibody precipitates in the cornea can cause scarring.21 Instead, trifluorothymidine is an effective topical treatment available for ocular vaccinia.
A robust response or “take” is defined as a reaction having redness, swelling, and warmth more than 3 inches in diameter at the inoculation site, peaking 6 to 12 days after inoculation with spontaneous regression occurring 1 to 3 days after.22,23 A robust take frequently is of concern to the clinician, as it can be difficult to discern from secondary infection. Secondary infections are uncommon, and a robust take is secondary to viral, not bacterial, cellulitis. Unfortunately, there are no diagnostics that have utility in distinguishing between the two, and the decision to administer empiric antibiotics might be unavoidable in light of the consequences of an untreated, rapidly progressive bacterial cellulitis. Milder cases in the setting of no constitutional symptoms could be safely monitored if close follow-up is assured.
Generalized Skin Reactions Without Viral Replication
Development of erythematous, pruritic, urticarial, and diffuse targetlike lesions of EM is common in first-time vaccinees. Often misdiagnosed as GV, EM is an immunologically mediated, not virally mediated, process. The most common infectious cause prompting EM is herpes simplex virus type 1. In the setting of a live-virus vaccine, it is difficult to determine if the vaccine prompted herpes simplex virus type 1 viral shedding and associated EM or if the vaccinia vaccine is more directly the cause of EM.24 Symptoms typically are mild, but more severe reactions may require treatment with corticosteroids. Stevens-Johnson syndrome with a severe bullous eruption has been linked to vaccinia24 but fortunately is rare. Morbilliform eruptions, urticaria, and angioedema also can occur.
Final Thoughts
Given current world events and ongoing bioterrorism threats, the smallpox vaccine program continues indefinitely. With a brisk military deployment tempo, a larger population of new vaccinees naturally will yield more cutaneous reactions. Military members, civilian health care workers, and members of the National Guard and National Reserves will develop complications and present to dermatologists for care. The historical pool of providers accustomed to seeing these complications from the 1960s eradication campaign is scant. Military and civilian dermatologists alike are uniquely poised to be the experts on protean manifestations of vaccinia reactions.
- Voigt EA, Kennedy RB, Poland GA. Defending against smallpox: a focus on vaccines. Expert Rev Vaccines. 2016;15:1197-1211.
- Grabenstein J, Wikenwerder W Jr. US military smallpox vaccination program experience. JAMA. 2003;289:3278-3282.
- Kelly CD, Egan C, Davis SW, et al. Laboratory confirmation of generalized vaccinia following smallpox vaccination. J Clin Microbiol. 2004;42:1373-1375.
- Slike BM, Creegan M, Marovich M, et al. Humoral immunity to primary smallpox vaccination: impact of childhood versus adult immunization on vaccinia vector vaccine development in military populations. PLoS One. 2017;12:E0169247.
- Notice to readers: newly licensed vaccine to replace old smallpox vaccine. MMWR. 2008;57:207-208.
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101-114.
- Engler R, Kenner J, Leung D. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357-365.
- Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36:766-774.
- Gordon S, Cecchinato V, Andresen V, et al. Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis. 2011;203:1043-1053.
- Hanifin J, Reed M. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;82:82-91.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1-16.
- Petersen BW, Harms TJ, Reynolds MG, et al. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupation exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunizations Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65:257-262.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Eczema Vaccinatum. Eczema vaccinatum as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection analysis, and presentation of immunization safety data. Vaccine. 2007:25;5725-5734.
- Aragón TJ, Ulrich S, Fernyak S, et al. Risks of serious complications and death from smallpox vaccination: a systematic review of the United States experience, 1963-1968. BMC Public Health. 2003;3:26.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555-1561.
- Centers for Disease Control and Prevention (CDC). Progressive vaccinia in a military smallpox vaccinee—United States 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Fulginiti VA, Papier A, Lane M, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Progressive Vaccinia. Progressive vaccinia as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5735-5744.
- Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:E1372-E1385.
- Lane ML, Goldstein J. Adverse events occurring after smallpox vaccination. Semin Ped Infect Dis. 2003;14:189-195.
- Vaccine adverse events. CDC website. http://www.cdc.gov/smallpox/clinicians/vaccine-adverse-events5.html. Accessed January 3, 2018.
- Cono J, Casey CG, Bell DM. Smallpox vaccination and adversereactions, guidance for clinicians. CDC website. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Accessed January 3, 2018.
- Rosenblatt AE, Stein SL. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33:327-332.
- Voigt EA, Kennedy RB, Poland GA. Defending against smallpox: a focus on vaccines. Expert Rev Vaccines. 2016;15:1197-1211.
- Grabenstein J, Wikenwerder W Jr. US military smallpox vaccination program experience. JAMA. 2003;289:3278-3282.
- Kelly CD, Egan C, Davis SW, et al. Laboratory confirmation of generalized vaccinia following smallpox vaccination. J Clin Microbiol. 2004;42:1373-1375.
- Slike BM, Creegan M, Marovich M, et al. Humoral immunity to primary smallpox vaccination: impact of childhood versus adult immunization on vaccinia vector vaccine development in military populations. PLoS One. 2017;12:E0169247.
- Notice to readers: newly licensed vaccine to replace old smallpox vaccine. MMWR. 2008;57:207-208.
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101-114.
- Engler R, Kenner J, Leung D. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357-365.
- Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36:766-774.
- Gordon S, Cecchinato V, Andresen V, et al. Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis. 2011;203:1043-1053.
- Hanifin J, Reed M. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;82:82-91.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1-16.
- Petersen BW, Harms TJ, Reynolds MG, et al. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupation exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunizations Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65:257-262.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Eczema Vaccinatum. Eczema vaccinatum as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection analysis, and presentation of immunization safety data. Vaccine. 2007:25;5725-5734.
- Aragón TJ, Ulrich S, Fernyak S, et al. Risks of serious complications and death from smallpox vaccination: a systematic review of the United States experience, 1963-1968. BMC Public Health. 2003;3:26.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555-1561.
- Centers for Disease Control and Prevention (CDC). Progressive vaccinia in a military smallpox vaccinee—United States 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Fulginiti VA, Papier A, Lane M, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Progressive Vaccinia. Progressive vaccinia as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5735-5744.
- Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:E1372-E1385.
- Lane ML, Goldstein J. Adverse events occurring after smallpox vaccination. Semin Ped Infect Dis. 2003;14:189-195.
- Vaccine adverse events. CDC website. http://www.cdc.gov/smallpox/clinicians/vaccine-adverse-events5.html. Accessed January 3, 2018.
- Cono J, Casey CG, Bell DM. Smallpox vaccination and adversereactions, guidance for clinicians. CDC website. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Accessed January 3, 2018.
- Rosenblatt AE, Stein SL. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33:327-332.
Practice Points
- Dermatologists should be aware that smallpox vaccinations are being administered to patients and may present with a myriad of cutaneous complications.
- Progressive vaccinia should be suspected if a smallpox inoculation has not healed after 14 days and, most specifically, if there is no inflammation surrounding the site.
- Generalized vaccinia generally is a benign condition seen in otherwise healthy patients and usually requires no treatment.
Atopic patients should be educated to avoid receiving routine smallpox vaccinations if they would be considered at risk for requiring the inoculation.
Skin Cancer in Military Pilots: A Special Population With Special Risk Factors
Military dermatologists are charged with caring for a diverse population of active-duty members, civilian dependents, and military retirees. Although certain risk factors for cutaneous malignancies are common in all of these groups, the active-duty population experiences unique exposures to be considered when determining their risk for skin cancer. One subset that may be at a higher risk is military pilots who fly at high altitudes on irregular schedules in austere environments. Through the unparalleled comradeship inherent in many military units, pilots “hear” from their fellow pilots that they are at increased risk for skin cancer. Do their occupational exposures translate into increased risk for cutaneous malignancy? This article will survey the literature pertaining to pilots and skin cancer so that all dermatologists may better care for this unique population.
Epidemiology
Anecdotally, we have observed basal cell carcinoma in pilots in their 20s and early 30s, earlier than would be expected in an otherwise healthy prescreened military population.1 Woolley and Hughes2 published a case report of skin cancer in a young military aviator. The patient was a 32-year-old male helicopter pilot with Fitzpatrick skin type II and no personal or family history of skin cancer who was diagnosed with a periocular nodular basal cell carcinoma. He deployed to locations with high UV radiation (UVR) indices, and his vacation time also was spent in such areas.2 UV radiation exposure and Fitzpatrick skin type are known risk factors across occupations, but are there special exposures that come with military aviation service?
To better understand the risk for malignancy in this special population, the US Air Force examined the rates of all cancer types among a cohort of flying versus nonflying officers.3 Aviation personnel showed increased incidence of testicular, bladder, and all-site cancers combined. Noticeably absent was a statistically significant increased risk for malignant melanoma (MM) and nonmelanoma skin cancer (NMSC). Other epidemiological studies examined the incidence rates of MM in the US Armed Forces compared with age- and race-matched civilian populations and showed mixed results: 2 studies showed increased risk,4,5 while a third showed decreased risk.6 Despite finding opposite results of MM rates in military members versus the civilian population, 2 of these studies showed US Air Force members to have higher rates of MM than those in the US Army or Navy.4,6 Interestingly, the air force has the highest number of pilots among all the services, with 4000 more pilots than the army and navy.7 Further studies are needed to determine if the higher air force MM rates occur in pilots.
Although there are mixed and limited data pertaining to military flight crews, there is more robust literature concerning civilian flight personnel. One meta-analysis pooled studies related to cancer risk in cabin crews and civil and military pilots.8 In military pilots, they found a standardized incidence ratio (SIR) of 1.43 (95% confidence interval [CI], 1.09-1.87) for MM and 1.80 (95% CI, 1.25-2.80) for NMSC. The SIRs were higher for male cabin attendants (3.42 and 7.46, respectively) and civil pilots (2.18 and 1.88, respectively). They also found the most common cause of mortality in civilian cabin crews was AIDS, possibly explaining the higher SIRs for all types of malignancy in that population.8 In the United States, many civilian pilots previously were military pilots9 who likely served in the military for at least 10 years.10 A 2015 meta-analysis of 19 studies of more than 266,000 civil pilots and aircrew members found an SIR for MM of 2.22 (95% CI, 1.67-2.93) for civil pilots and 2.09 (95% CI, 1.67-2.62) for aircrews, stating the risk for MM is at least twice that of the general population.11
Risk Factors
UV Radiation
These studies suggest flight duties increase the risk for cutaneous malignancy. UV radiation is a known risk factor for skin cancer.12 The main body of the aircraft may protect the cabin’s crew and passengers from UVR, but pilots are exposed to more UVR, especially in aircraft with larger windshields. A government study in 2007 examined the transmittance of UVR through windscreens of 8 aircraft: 3 commercial jets, 2 commercial propeller planes, 1 private jet, and 2 small propeller planes.13 UVB was attenuated by all the windscreens (<1% transmittance), but 43% to 54% of UVA was transmitted, with plastic windshields attenuating more than glass. Sanlorenzo et al14 measured UVA irradiance at the pilot’s seat of a turboprop aircraft at 30,000-ft altitude. They compared this exposure to a UVA tanning bed and estimated that 57 minutes of flight at 30,000-ft altitude was equivalent to 20 minutes inside a UVA tanning booth, a startling finding.14
Cosmic Radiation
Cosmic radiation consists of neutrons and gamma rays that originate outside Earth’s atmosphere. Pilots are exposed to higher doses of cosmic radiation than nonpilots, but the health effects are difficult to study. Boice et al15 described how factors such as altitude, latitude, and flight time determine pilots’ cumulative exposure. With longer flight times at higher altitudes, a pilot’s exposure to cosmic radiation is increasing over the years.15 A 2012 review found that aircrews have low-level cosmic radiation exposure. Despite increases in MM and NMSC in pilots and increased rates of breast cancer in female aircrew, overall cancer-related mortality was lower in flying versus nonflying controls.16 Thus, cosmic radiation may not be as onerous of an occupational hazard for pilots as has been postulated.
Altered Circadian Rhythms
Aviation duties, especially in the military, require irregular work schedules that repeatedly interfere with normal sleep-wake cycles, disrupt circadian rhythms, and lead to reduced melatonin levels.8 Evidence suggests that low levels of melatonin could increase the risk for breast and prostate cancer—both cancers that occur more frequently in female aircrew and male pilots, respectively—by reducing melatonin’s natural protective role in such malignancies.17,18 A World Health Organization working group categorized shift work as “probably carcinogenic” and cited alterations of melatonin levels, changes in other circadian rhythm–related gene pathways, and relative immunosuppression as likely causative factors.19 In a 2011 study, exposing mice to UVR during times when nucleotide excision repair mechanisms were at their lowest activity caused an increased rate of skin cancers.20 A 2014 review discussed how epidemiological studies of shift workers such as nurses, firefighters, pilots, and flight crews found contradictory data, but molecular studies show that circadian rhythm–linked repair and tumorigenesis mechanisms are altered by aberrations in the normal sleep-wake cycle.21
Cockpit Instrumentation
Electromagnetic energy from the flight instruments in the cockpit also could influence malignancy risk. Nicholas et al22 found magnetic field measurements within the cockpit to be 2 to 10 times that experienced within the home or office. However, no studies examining the health effects of cockpit flight instruments and magnetic fields were found.
Final Thoughts
It is important to counsel pilots on the generally recognized, nonaviation-specific risk factors of family history, skin type, and UVR exposure in the development of skin cancer. Additionally, it is important to explain the possible role of exposure to UVR at higher altitudes, cosmic radiation, and electromagnetic energy from cockpit instruments, as well as altered sleep-wake cycles. A pilot’s risk for MM may be twice that of matched controls, and the risk for NMSC could be higher.8,11 Although the literature lacks specific recommendations for pilots, it is reasonable to screen pilots once per year to better assess their individual risk and encourage diligent use of sunscreen and sun-protective measures when flying. It also may be important to advocate for the development of engineering controls that decrease UVR transmittance through windscreens, particularly for aircraft flying at higher altitudes for longer flights. More research is needed to determine if changes in circadian rhythm and decreases in melatonin increase skin cancer risk, which could impact how pilots’ schedules are managed. Together, we can ensure adequate surveillance, diagnosis, and treatment in this at-risk population.
- Roewert‐Huber J, Lange-Asschenfeldt B, Stockfleth E, et al. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2007;157(suppl 2):47-51.
- Woolley SD, Hughes C. A young military pilot presents with a periocular basal cell carcinoma: a case report. Travel Med Infect Dis. 2013;11:435-437.
- Grayson JK, Lyons TJ. Cancer incidence in United States Air Force aircrew, 1975-89. Aviat Space Environ Med. 1996;67:101-104.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Garland FC, White MR, Garland CF, et al. Occupational sunlight exposure and melanoma in the US Navy. Arc Environ Health. 1990;45:261-267.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the US military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Active Duty Master Personnel File: Active Duty Tactical Operations Officers. Seaside, CA: Defense Manpower Data Center; August 31, 2017. Accessed September 22, 2017.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Jansen HS, Oster CV, eds. Taking Flight: Education and Training for Aviation Careers. Washington, DC: National Academy Press; 1997.
- About AFROTC Service Commitment. US Air Force ROTC website. https://www.afrotc.com/about/service. Accessed September 20, 2017.
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58.
- Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990;52:1119-1136.
- Nakagawara VB, Montgomery RW, Marshall WJ. Optical Radiation Transmittance of Aircraft Windscreens and Pilot Vision. Oklahoma City, OK: Federal Aviation Administration; 2007.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Boice JD, Blettner M, Auvinen A. Epidemiologic studies of pilots and aircrew. Health Phys. 2000;79:576-584.
- Zeeb H, Hammer GP, Blettner M. Epidemiological investigations of aircrew: an occupational group with low-level cosmic radiation exposure [published online March 6, 2012]. J Radiol Prot. 2012;32:N15-N19.
- Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16:254-258.
- Siu SW, Lau KW, Tam PC, et al. Melatonin and prostate cancer cell proliferation: interplay with castration, epidermal growth factor, and androgen sensitivity. Prostate. 2002;52:106-122.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. Lyon, France: World Health Organization International Agency for Research on Cancer; 2010.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci. 2011;108:18790-18795.
- Markova-Car EP, Jurišic´ D, Ilic´ N, et al. Running for time: circadian rhythms and melanoma. Tumour Biol. 2014;35:8359-8368.
- Nicholas JS, Lackland DT, Butler GC, et al. Cosmic radiation and magnetic field exposure to airline flight crews. Am J Ind Med. 1998;34:574-580.
Military dermatologists are charged with caring for a diverse population of active-duty members, civilian dependents, and military retirees. Although certain risk factors for cutaneous malignancies are common in all of these groups, the active-duty population experiences unique exposures to be considered when determining their risk for skin cancer. One subset that may be at a higher risk is military pilots who fly at high altitudes on irregular schedules in austere environments. Through the unparalleled comradeship inherent in many military units, pilots “hear” from their fellow pilots that they are at increased risk for skin cancer. Do their occupational exposures translate into increased risk for cutaneous malignancy? This article will survey the literature pertaining to pilots and skin cancer so that all dermatologists may better care for this unique population.
Epidemiology
Anecdotally, we have observed basal cell carcinoma in pilots in their 20s and early 30s, earlier than would be expected in an otherwise healthy prescreened military population.1 Woolley and Hughes2 published a case report of skin cancer in a young military aviator. The patient was a 32-year-old male helicopter pilot with Fitzpatrick skin type II and no personal or family history of skin cancer who was diagnosed with a periocular nodular basal cell carcinoma. He deployed to locations with high UV radiation (UVR) indices, and his vacation time also was spent in such areas.2 UV radiation exposure and Fitzpatrick skin type are known risk factors across occupations, but are there special exposures that come with military aviation service?
To better understand the risk for malignancy in this special population, the US Air Force examined the rates of all cancer types among a cohort of flying versus nonflying officers.3 Aviation personnel showed increased incidence of testicular, bladder, and all-site cancers combined. Noticeably absent was a statistically significant increased risk for malignant melanoma (MM) and nonmelanoma skin cancer (NMSC). Other epidemiological studies examined the incidence rates of MM in the US Armed Forces compared with age- and race-matched civilian populations and showed mixed results: 2 studies showed increased risk,4,5 while a third showed decreased risk.6 Despite finding opposite results of MM rates in military members versus the civilian population, 2 of these studies showed US Air Force members to have higher rates of MM than those in the US Army or Navy.4,6 Interestingly, the air force has the highest number of pilots among all the services, with 4000 more pilots than the army and navy.7 Further studies are needed to determine if the higher air force MM rates occur in pilots.
Although there are mixed and limited data pertaining to military flight crews, there is more robust literature concerning civilian flight personnel. One meta-analysis pooled studies related to cancer risk in cabin crews and civil and military pilots.8 In military pilots, they found a standardized incidence ratio (SIR) of 1.43 (95% confidence interval [CI], 1.09-1.87) for MM and 1.80 (95% CI, 1.25-2.80) for NMSC. The SIRs were higher for male cabin attendants (3.42 and 7.46, respectively) and civil pilots (2.18 and 1.88, respectively). They also found the most common cause of mortality in civilian cabin crews was AIDS, possibly explaining the higher SIRs for all types of malignancy in that population.8 In the United States, many civilian pilots previously were military pilots9 who likely served in the military for at least 10 years.10 A 2015 meta-analysis of 19 studies of more than 266,000 civil pilots and aircrew members found an SIR for MM of 2.22 (95% CI, 1.67-2.93) for civil pilots and 2.09 (95% CI, 1.67-2.62) for aircrews, stating the risk for MM is at least twice that of the general population.11
Risk Factors
UV Radiation
These studies suggest flight duties increase the risk for cutaneous malignancy. UV radiation is a known risk factor for skin cancer.12 The main body of the aircraft may protect the cabin’s crew and passengers from UVR, but pilots are exposed to more UVR, especially in aircraft with larger windshields. A government study in 2007 examined the transmittance of UVR through windscreens of 8 aircraft: 3 commercial jets, 2 commercial propeller planes, 1 private jet, and 2 small propeller planes.13 UVB was attenuated by all the windscreens (<1% transmittance), but 43% to 54% of UVA was transmitted, with plastic windshields attenuating more than glass. Sanlorenzo et al14 measured UVA irradiance at the pilot’s seat of a turboprop aircraft at 30,000-ft altitude. They compared this exposure to a UVA tanning bed and estimated that 57 minutes of flight at 30,000-ft altitude was equivalent to 20 minutes inside a UVA tanning booth, a startling finding.14
Cosmic Radiation
Cosmic radiation consists of neutrons and gamma rays that originate outside Earth’s atmosphere. Pilots are exposed to higher doses of cosmic radiation than nonpilots, but the health effects are difficult to study. Boice et al15 described how factors such as altitude, latitude, and flight time determine pilots’ cumulative exposure. With longer flight times at higher altitudes, a pilot’s exposure to cosmic radiation is increasing over the years.15 A 2012 review found that aircrews have low-level cosmic radiation exposure. Despite increases in MM and NMSC in pilots and increased rates of breast cancer in female aircrew, overall cancer-related mortality was lower in flying versus nonflying controls.16 Thus, cosmic radiation may not be as onerous of an occupational hazard for pilots as has been postulated.
Altered Circadian Rhythms
Aviation duties, especially in the military, require irregular work schedules that repeatedly interfere with normal sleep-wake cycles, disrupt circadian rhythms, and lead to reduced melatonin levels.8 Evidence suggests that low levels of melatonin could increase the risk for breast and prostate cancer—both cancers that occur more frequently in female aircrew and male pilots, respectively—by reducing melatonin’s natural protective role in such malignancies.17,18 A World Health Organization working group categorized shift work as “probably carcinogenic” and cited alterations of melatonin levels, changes in other circadian rhythm–related gene pathways, and relative immunosuppression as likely causative factors.19 In a 2011 study, exposing mice to UVR during times when nucleotide excision repair mechanisms were at their lowest activity caused an increased rate of skin cancers.20 A 2014 review discussed how epidemiological studies of shift workers such as nurses, firefighters, pilots, and flight crews found contradictory data, but molecular studies show that circadian rhythm–linked repair and tumorigenesis mechanisms are altered by aberrations in the normal sleep-wake cycle.21
Cockpit Instrumentation
Electromagnetic energy from the flight instruments in the cockpit also could influence malignancy risk. Nicholas et al22 found magnetic field measurements within the cockpit to be 2 to 10 times that experienced within the home or office. However, no studies examining the health effects of cockpit flight instruments and magnetic fields were found.
Final Thoughts
It is important to counsel pilots on the generally recognized, nonaviation-specific risk factors of family history, skin type, and UVR exposure in the development of skin cancer. Additionally, it is important to explain the possible role of exposure to UVR at higher altitudes, cosmic radiation, and electromagnetic energy from cockpit instruments, as well as altered sleep-wake cycles. A pilot’s risk for MM may be twice that of matched controls, and the risk for NMSC could be higher.8,11 Although the literature lacks specific recommendations for pilots, it is reasonable to screen pilots once per year to better assess their individual risk and encourage diligent use of sunscreen and sun-protective measures when flying. It also may be important to advocate for the development of engineering controls that decrease UVR transmittance through windscreens, particularly for aircraft flying at higher altitudes for longer flights. More research is needed to determine if changes in circadian rhythm and decreases in melatonin increase skin cancer risk, which could impact how pilots’ schedules are managed. Together, we can ensure adequate surveillance, diagnosis, and treatment in this at-risk population.
Military dermatologists are charged with caring for a diverse population of active-duty members, civilian dependents, and military retirees. Although certain risk factors for cutaneous malignancies are common in all of these groups, the active-duty population experiences unique exposures to be considered when determining their risk for skin cancer. One subset that may be at a higher risk is military pilots who fly at high altitudes on irregular schedules in austere environments. Through the unparalleled comradeship inherent in many military units, pilots “hear” from their fellow pilots that they are at increased risk for skin cancer. Do their occupational exposures translate into increased risk for cutaneous malignancy? This article will survey the literature pertaining to pilots and skin cancer so that all dermatologists may better care for this unique population.
Epidemiology
Anecdotally, we have observed basal cell carcinoma in pilots in their 20s and early 30s, earlier than would be expected in an otherwise healthy prescreened military population.1 Woolley and Hughes2 published a case report of skin cancer in a young military aviator. The patient was a 32-year-old male helicopter pilot with Fitzpatrick skin type II and no personal or family history of skin cancer who was diagnosed with a periocular nodular basal cell carcinoma. He deployed to locations with high UV radiation (UVR) indices, and his vacation time also was spent in such areas.2 UV radiation exposure and Fitzpatrick skin type are known risk factors across occupations, but are there special exposures that come with military aviation service?
To better understand the risk for malignancy in this special population, the US Air Force examined the rates of all cancer types among a cohort of flying versus nonflying officers.3 Aviation personnel showed increased incidence of testicular, bladder, and all-site cancers combined. Noticeably absent was a statistically significant increased risk for malignant melanoma (MM) and nonmelanoma skin cancer (NMSC). Other epidemiological studies examined the incidence rates of MM in the US Armed Forces compared with age- and race-matched civilian populations and showed mixed results: 2 studies showed increased risk,4,5 while a third showed decreased risk.6 Despite finding opposite results of MM rates in military members versus the civilian population, 2 of these studies showed US Air Force members to have higher rates of MM than those in the US Army or Navy.4,6 Interestingly, the air force has the highest number of pilots among all the services, with 4000 more pilots than the army and navy.7 Further studies are needed to determine if the higher air force MM rates occur in pilots.
Although there are mixed and limited data pertaining to military flight crews, there is more robust literature concerning civilian flight personnel. One meta-analysis pooled studies related to cancer risk in cabin crews and civil and military pilots.8 In military pilots, they found a standardized incidence ratio (SIR) of 1.43 (95% confidence interval [CI], 1.09-1.87) for MM and 1.80 (95% CI, 1.25-2.80) for NMSC. The SIRs were higher for male cabin attendants (3.42 and 7.46, respectively) and civil pilots (2.18 and 1.88, respectively). They also found the most common cause of mortality in civilian cabin crews was AIDS, possibly explaining the higher SIRs for all types of malignancy in that population.8 In the United States, many civilian pilots previously were military pilots9 who likely served in the military for at least 10 years.10 A 2015 meta-analysis of 19 studies of more than 266,000 civil pilots and aircrew members found an SIR for MM of 2.22 (95% CI, 1.67-2.93) for civil pilots and 2.09 (95% CI, 1.67-2.62) for aircrews, stating the risk for MM is at least twice that of the general population.11
Risk Factors
UV Radiation
These studies suggest flight duties increase the risk for cutaneous malignancy. UV radiation is a known risk factor for skin cancer.12 The main body of the aircraft may protect the cabin’s crew and passengers from UVR, but pilots are exposed to more UVR, especially in aircraft with larger windshields. A government study in 2007 examined the transmittance of UVR through windscreens of 8 aircraft: 3 commercial jets, 2 commercial propeller planes, 1 private jet, and 2 small propeller planes.13 UVB was attenuated by all the windscreens (<1% transmittance), but 43% to 54% of UVA was transmitted, with plastic windshields attenuating more than glass. Sanlorenzo et al14 measured UVA irradiance at the pilot’s seat of a turboprop aircraft at 30,000-ft altitude. They compared this exposure to a UVA tanning bed and estimated that 57 minutes of flight at 30,000-ft altitude was equivalent to 20 minutes inside a UVA tanning booth, a startling finding.14
Cosmic Radiation
Cosmic radiation consists of neutrons and gamma rays that originate outside Earth’s atmosphere. Pilots are exposed to higher doses of cosmic radiation than nonpilots, but the health effects are difficult to study. Boice et al15 described how factors such as altitude, latitude, and flight time determine pilots’ cumulative exposure. With longer flight times at higher altitudes, a pilot’s exposure to cosmic radiation is increasing over the years.15 A 2012 review found that aircrews have low-level cosmic radiation exposure. Despite increases in MM and NMSC in pilots and increased rates of breast cancer in female aircrew, overall cancer-related mortality was lower in flying versus nonflying controls.16 Thus, cosmic radiation may not be as onerous of an occupational hazard for pilots as has been postulated.
Altered Circadian Rhythms
Aviation duties, especially in the military, require irregular work schedules that repeatedly interfere with normal sleep-wake cycles, disrupt circadian rhythms, and lead to reduced melatonin levels.8 Evidence suggests that low levels of melatonin could increase the risk for breast and prostate cancer—both cancers that occur more frequently in female aircrew and male pilots, respectively—by reducing melatonin’s natural protective role in such malignancies.17,18 A World Health Organization working group categorized shift work as “probably carcinogenic” and cited alterations of melatonin levels, changes in other circadian rhythm–related gene pathways, and relative immunosuppression as likely causative factors.19 In a 2011 study, exposing mice to UVR during times when nucleotide excision repair mechanisms were at their lowest activity caused an increased rate of skin cancers.20 A 2014 review discussed how epidemiological studies of shift workers such as nurses, firefighters, pilots, and flight crews found contradictory data, but molecular studies show that circadian rhythm–linked repair and tumorigenesis mechanisms are altered by aberrations in the normal sleep-wake cycle.21
Cockpit Instrumentation
Electromagnetic energy from the flight instruments in the cockpit also could influence malignancy risk. Nicholas et al22 found magnetic field measurements within the cockpit to be 2 to 10 times that experienced within the home or office. However, no studies examining the health effects of cockpit flight instruments and magnetic fields were found.
Final Thoughts
It is important to counsel pilots on the generally recognized, nonaviation-specific risk factors of family history, skin type, and UVR exposure in the development of skin cancer. Additionally, it is important to explain the possible role of exposure to UVR at higher altitudes, cosmic radiation, and electromagnetic energy from cockpit instruments, as well as altered sleep-wake cycles. A pilot’s risk for MM may be twice that of matched controls, and the risk for NMSC could be higher.8,11 Although the literature lacks specific recommendations for pilots, it is reasonable to screen pilots once per year to better assess their individual risk and encourage diligent use of sunscreen and sun-protective measures when flying. It also may be important to advocate for the development of engineering controls that decrease UVR transmittance through windscreens, particularly for aircraft flying at higher altitudes for longer flights. More research is needed to determine if changes in circadian rhythm and decreases in melatonin increase skin cancer risk, which could impact how pilots’ schedules are managed. Together, we can ensure adequate surveillance, diagnosis, and treatment in this at-risk population.
- Roewert‐Huber J, Lange-Asschenfeldt B, Stockfleth E, et al. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2007;157(suppl 2):47-51.
- Woolley SD, Hughes C. A young military pilot presents with a periocular basal cell carcinoma: a case report. Travel Med Infect Dis. 2013;11:435-437.
- Grayson JK, Lyons TJ. Cancer incidence in United States Air Force aircrew, 1975-89. Aviat Space Environ Med. 1996;67:101-104.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Garland FC, White MR, Garland CF, et al. Occupational sunlight exposure and melanoma in the US Navy. Arc Environ Health. 1990;45:261-267.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the US military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Active Duty Master Personnel File: Active Duty Tactical Operations Officers. Seaside, CA: Defense Manpower Data Center; August 31, 2017. Accessed September 22, 2017.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Jansen HS, Oster CV, eds. Taking Flight: Education and Training for Aviation Careers. Washington, DC: National Academy Press; 1997.
- About AFROTC Service Commitment. US Air Force ROTC website. https://www.afrotc.com/about/service. Accessed September 20, 2017.
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58.
- Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990;52:1119-1136.
- Nakagawara VB, Montgomery RW, Marshall WJ. Optical Radiation Transmittance of Aircraft Windscreens and Pilot Vision. Oklahoma City, OK: Federal Aviation Administration; 2007.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Boice JD, Blettner M, Auvinen A. Epidemiologic studies of pilots and aircrew. Health Phys. 2000;79:576-584.
- Zeeb H, Hammer GP, Blettner M. Epidemiological investigations of aircrew: an occupational group with low-level cosmic radiation exposure [published online March 6, 2012]. J Radiol Prot. 2012;32:N15-N19.
- Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16:254-258.
- Siu SW, Lau KW, Tam PC, et al. Melatonin and prostate cancer cell proliferation: interplay with castration, epidermal growth factor, and androgen sensitivity. Prostate. 2002;52:106-122.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. Lyon, France: World Health Organization International Agency for Research on Cancer; 2010.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci. 2011;108:18790-18795.
- Markova-Car EP, Jurišic´ D, Ilic´ N, et al. Running for time: circadian rhythms and melanoma. Tumour Biol. 2014;35:8359-8368.
- Nicholas JS, Lackland DT, Butler GC, et al. Cosmic radiation and magnetic field exposure to airline flight crews. Am J Ind Med. 1998;34:574-580.
- Roewert‐Huber J, Lange-Asschenfeldt B, Stockfleth E, et al. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2007;157(suppl 2):47-51.
- Woolley SD, Hughes C. A young military pilot presents with a periocular basal cell carcinoma: a case report. Travel Med Infect Dis. 2013;11:435-437.
- Grayson JK, Lyons TJ. Cancer incidence in United States Air Force aircrew, 1975-89. Aviat Space Environ Med. 1996;67:101-104.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Garland FC, White MR, Garland CF, et al. Occupational sunlight exposure and melanoma in the US Navy. Arc Environ Health. 1990;45:261-267.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the US military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Active Duty Master Personnel File: Active Duty Tactical Operations Officers. Seaside, CA: Defense Manpower Data Center; August 31, 2017. Accessed September 22, 2017.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Jansen HS, Oster CV, eds. Taking Flight: Education and Training for Aviation Careers. Washington, DC: National Academy Press; 1997.
- About AFROTC Service Commitment. US Air Force ROTC website. https://www.afrotc.com/about/service. Accessed September 20, 2017.
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58.
- Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990;52:1119-1136.
- Nakagawara VB, Montgomery RW, Marshall WJ. Optical Radiation Transmittance of Aircraft Windscreens and Pilot Vision. Oklahoma City, OK: Federal Aviation Administration; 2007.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Boice JD, Blettner M, Auvinen A. Epidemiologic studies of pilots and aircrew. Health Phys. 2000;79:576-584.
- Zeeb H, Hammer GP, Blettner M. Epidemiological investigations of aircrew: an occupational group with low-level cosmic radiation exposure [published online March 6, 2012]. J Radiol Prot. 2012;32:N15-N19.
- Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16:254-258.
- Siu SW, Lau KW, Tam PC, et al. Melatonin and prostate cancer cell proliferation: interplay with castration, epidermal growth factor, and androgen sensitivity. Prostate. 2002;52:106-122.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. Lyon, France: World Health Organization International Agency for Research on Cancer; 2010.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci. 2011;108:18790-18795.
- Markova-Car EP, Jurišic´ D, Ilic´ N, et al. Running for time: circadian rhythms and melanoma. Tumour Biol. 2014;35:8359-8368.
- Nicholas JS, Lackland DT, Butler GC, et al. Cosmic radiation and magnetic field exposure to airline flight crews. Am J Ind Med. 1998;34:574-580.
Practice Points
- Military and civilian pilots have an increased risk for melanoma and nonmelanoma skin cancer, likely due to unique occupational exposures.
- We recommend annual skin cancer screening for all pilots to help assess their individual risk.
- Pilots should be educated on their increased risk for skin cancer and encouraged to use sun-protective measures during their flying duties and leisure activities.