User login
The practice of variolation, or inoculation of the smallpox virus from a pustule into a healthy person, was described as early as 1500
Immunization
Vaccinia is an orthopoxvirus, distinct from the smallpox virus variola, with cross-protective immunity after infection. The smallpox vaccine that is available today is a second-generation vaccinia virus derived from plaque purification cloning from the first-generation version originally licensed in 1932, which was central to eradication.5 Today’s vaccine is administered using a bifurcated needle to puncture the epidermis 15 times. Ideally, a papule forms at the inoculation site 3 to 5 days later, progresses to a vesicle and then a pustule, and finally crusts and reaches maximum size by day 10. The crust separates from the skin at 14 to 21 days, at which time the virus can no longer be isolated from the wound. United States Department of Defense surveillance of the first 450,000 vaccinated personnel noted 1% of recipients developed cutaneous eruptions beyond the vaccination site, 5% developed a localized rash, and 1% experienced a generalized eruption.2 Adverse reactions included generalized vaccinia, erythema multiforme (EM), autoinoculation (including ocular vaccinia), and contact vaccinia. There were no cases of eczema vaccinatum (EV) or progressive vaccinia (PV) reported, and no deaths were attributed to these initial vaccines.2
Immunologic Response
Vaccinia replicates in keratinocytes, spreading from cell to cell, resulting in necrosis and vesicle formation. Components of both cellular and humoral immune responses are in place by 10 days after immunization. Deficiencies in these responses result in vaccine complications secondary to vaccine escape and replication beyond the inoculation site.6 A helper T cell TH2-predominant cytokine response in atopic individuals is the likely pathogenesis required for the rapid viral spread for EV.7 Similarly, patients with cell-mediated immunity deficiencies cannot sufficiently produce enough cytotoxic T cells to eliminate an established infection, which can result in PV. Despite the effectiveness of intravenous vaccinia immunoglobulins (VIGIVs) when administered to patients with certain vaccine complications, observations that children with severe X-linked agammaglobulinemia (Bruton disease) have normal responses to vaccination suggest that antibody production is least important in viral control.8 Simian models also suggest that B-cell depletion has no impact on lesion dissemination, as lesion size is inversely correlated with T-cell count.9
Eczema Vaccinatum
A national survey estimated the prevalence of eczema in the United States at 31.6 million individuals,10 with 2- to 3-fold increases in incidence since the 1970s.11 Due to the risk for developing EV, the Advisory Committee on Immunization Practices considers personal history of eczema or contact with a family member who has eczema (either currently or in the past) contraindications to nonemergency administration of the vaccine.12,13 However, atopic conditions in general are underrecognized, with only approximately one-third of patients carrying an official diagnosis from a physician.10 Despite a large atopic and vaccinated population, EV remains relatively uncommon at 10 to 39 cases per million vaccines.6
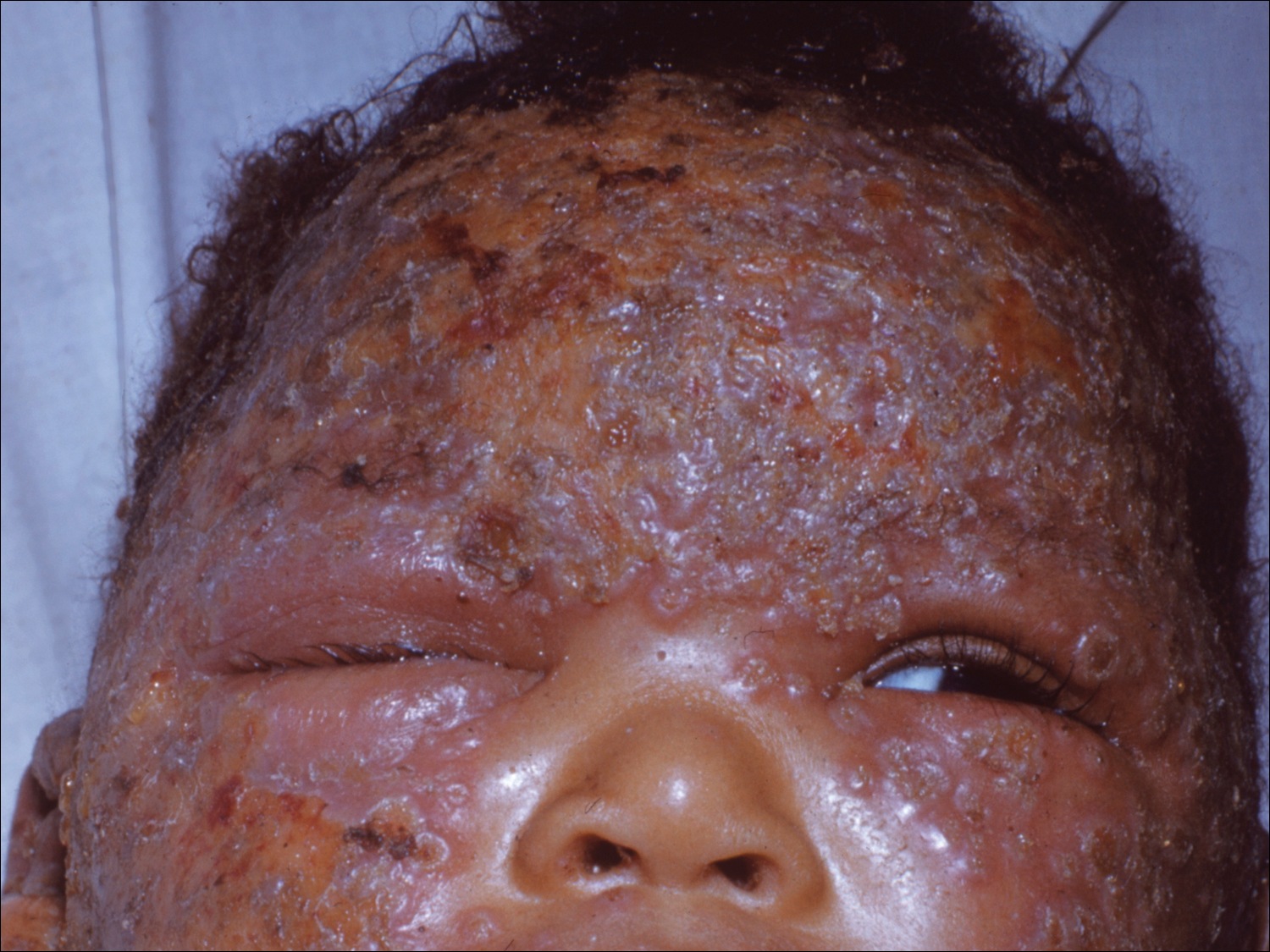
The EV rash classically involves the midface, neck, and antecubital and popliteal fossae but can present in any location. The lesions start as papules that quickly progress to vesicles and pustules with crusting on an erythematous base. Given the extent of denudation of the epidermis, impetiginization can occur. Death rates as high as 30% have been reported14 but have only occurred in instances of secondary contact transmission with no deaths occurring in the primary vaccinees.15 In a case published in 2008, a 2-year-old boy developed the first documented EV case under the new program after exposure to his father’s predeployment vaccine.16 A similar rash is shown in Figure 1 with notable vesicles and pustules. The child required burn patient–type management, VIGIV, and treatment with cidofovir and an investigational antiorthopox agent. He was discharged from the hospital after 48 days without sequelae or considerable scarring.16 If a family member has a contraindication barring secondary contact with the vaccine, the US Department of Defense’s policy defers vaccination in active-duty members until they reach their deployment destination, at which point the inoculation is administered.
Progressive Vaccinia
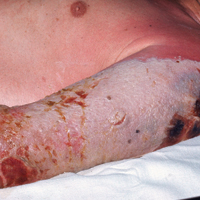
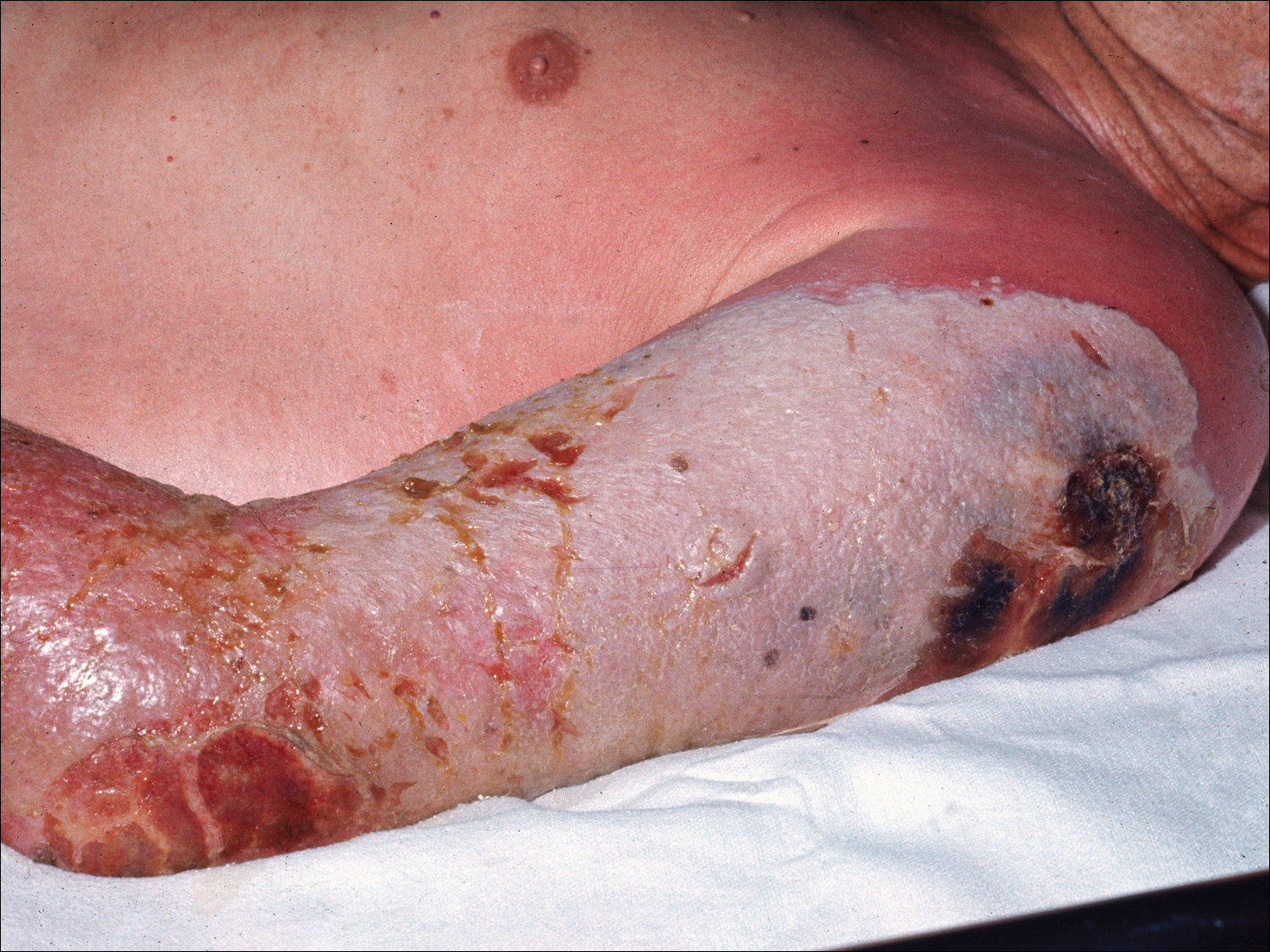
Progressive vaccinia is also known as vaccinia necrosum or vaccinia gangrenosum. It is a dreaded but uncommon complication, occurring once in every 1 million vaccinations. It carries an overall case fatality rate of 15%,17 but it nearly always is fatal in patients with severe T-cell defects.18 Progressive vaccinia occurs exclusively in patients with cell-mediated immunodeficiency, with the severity of the acute illness correlating with the severity of immunodeficiency. In patients with cell-mediated immunodeficiency but intact humoral immunity, progression can be limited to expansion of the lesion, as it is thought that antibody production restricts viremia.18 Progressive vaccinia should be suspected in a patient if the vaccine site shows no signs of improvement by 14 days.19 The PV lesions do not heal and may progress or recur in patients with signs of prior healing. The leading edge has confluent vesicles, and the center of the lesion develops necrosis with thick black eschar formation. Most specifically, there is no surrounding inflammation; however, inflammation can develop later as a response to treatment or secondary infection. Figure 2 shows a PV lesion with black eschar and a transition to intact dermis without inflammation.
The first known case of PV since the 1960s vaccination campaign occurred in an active-duty Marine vaccinated with vaccinia before a diagnosis of acute myelogenous leukemia was recognized 2 weeks later.19 The vaccine site was stable in size and crusted when he received neutropenia-inducing chemotherapy 6.5 weeks after vaccination. The site then progressed in a manner typical for PV with central necrosis and a lack of inflammation at the expanding painless wound edge.19 This classic appearance with progression of satellite lesions prompted the treatment team to obtain wound and serum samples, which yielded the orthopox virus from polymerase chain reaction and viral culture. He required 2 months of care in an intensive care unit and received treatment with topical imiquimod, VIGIV, a topical and intravenous antiorthopox agent, and a second investigational antiorthopox agent; the patient ultimately survived.17,20
Generalized Vaccinia
Generalized vaccinia (GV) typically is a benign vaccine complication resulting from viremic spread from the initial inoculation site and is most commonly seen in healthy patients. Generalized vaccinia is only life threatening in immunocompromised patients. The incidence of GV is 23.4 to 241.5 patients per million vaccines.6 The majority of GV cases occur 5 to 12 days after vaccination when small distant pustules or vesicles appear on any part of the body, including the palms and soles. The lesions usually are smaller than the primary vaccination site and resolve more quickly. Generalized vaccinia can have a few to several hundred pocks, though the rash is rarely as diffuse as EV presentations.3 Given that EV can present diffusely on skin unaffected by atopic dermatitis, GV can be difficult to distinguish from EV. Features more common to EV include more systemically ill patients, increased numbers of lesions, and lesions that become confluent in an atopic distribution. It has been suggested that GV can be differentiated from vesicular or vesiculopapular EM because GV does not develop flaccid bullae and EM typically has targetoid lesions.18 Mild GV disease requires no treatment, but VIGIV can be used in more extensive cases.
Localized Reactions Due to Viral Replication
Accidental autoinoculation can occur when patients touch the vaccination site and then themselves, transferring virus particles to areas of compromised skin integrity, most commonly on the face, eyes, hands, genitalia, anus, or any other broken skin. Autoinoculation happens with some frequency and is of limited clinical concern unless there is ocular involvement. Keratitis develops in 6% of ocular vaccinia cases, and VIGIV is contraindicated, as rabbit models suggest that antigen-antibody precipitates in the cornea can cause scarring.21 Instead, trifluorothymidine is an effective topical treatment available for ocular vaccinia.
A robust response or “take” is defined as a reaction having redness, swelling, and warmth more than 3 inches in diameter at the inoculation site, peaking 6 to 12 days after inoculation with spontaneous regression occurring 1 to 3 days after.22,23 A robust take frequently is of concern to the clinician, as it can be difficult to discern from secondary infection. Secondary infections are uncommon, and a robust take is secondary to viral, not bacterial, cellulitis. Unfortunately, there are no diagnostics that have utility in distinguishing between the two, and the decision to administer empiric antibiotics might be unavoidable in light of the consequences of an untreated, rapidly progressive bacterial cellulitis. Milder cases in the setting of no constitutional symptoms could be safely monitored if close follow-up is assured.
Generalized Skin Reactions Without Viral Replication
Development of erythematous, pruritic, urticarial, and diffuse targetlike lesions of EM is common in first-time vaccinees. Often misdiagnosed as GV, EM is an immunologically mediated, not virally mediated, process. The most common infectious cause prompting EM is herpes simplex virus type 1. In the setting of a live-virus vaccine, it is difficult to determine if the vaccine prompted herpes simplex virus type 1 viral shedding and associated EM or if the vaccinia vaccine is more directly the cause of EM.24 Symptoms typically are mild, but more severe reactions may require treatment with corticosteroids. Stevens-Johnson syndrome with a severe bullous eruption has been linked to vaccinia24 but fortunately is rare. Morbilliform eruptions, urticaria, and angioedema also can occur.
Final Thoughts
Given current world events and ongoing bioterrorism threats, the smallpox vaccine program continues indefinitely. With a brisk military deployment tempo, a larger population of new vaccinees naturally will yield more cutaneous reactions. Military members, civilian health care workers, and members of the National Guard and National Reserves will develop complications and present to dermatologists for care. The historical pool of providers accustomed to seeing these complications from the 1960s eradication campaign is scant. Military and civilian dermatologists alike are uniquely poised to be the experts on protean manifestations of vaccinia reactions.
- Voigt EA, Kennedy RB, Poland GA. Defending against smallpox: a focus on vaccines. Expert Rev Vaccines. 2016;15:1197-1211.
- Grabenstein J, Wikenwerder W Jr. US military smallpox vaccination program experience. JAMA. 2003;289:3278-3282.
- Kelly CD, Egan C, Davis SW, et al. Laboratory confirmation of generalized vaccinia following smallpox vaccination. J Clin Microbiol. 2004;42:1373-1375.
- Slike BM, Creegan M, Marovich M, et al. Humoral immunity to primary smallpox vaccination: impact of childhood versus adult immunization on vaccinia vector vaccine development in military populations. PLoS One. 2017;12:E0169247.
- Notice to readers: newly licensed vaccine to replace old smallpox vaccine. MMWR. 2008;57:207-208.
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101-114.
- Engler R, Kenner J, Leung D. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357-365.
- Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36:766-774.
- Gordon S, Cecchinato V, Andresen V, et al. Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis. 2011;203:1043-1053.
- Hanifin J, Reed M. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;82:82-91.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1-16.
- Petersen BW, Harms TJ, Reynolds MG, et al. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupation exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunizations Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65:257-262.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Eczema Vaccinatum. Eczema vaccinatum as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection analysis, and presentation of immunization safety data. Vaccine. 2007:25;5725-5734.
- Aragón TJ, Ulrich S, Fernyak S, et al. Risks of serious complications and death from smallpox vaccination: a systematic review of the United States experience, 1963-1968. BMC Public Health. 2003;3:26.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555-1561.
- Centers for Disease Control and Prevention (CDC). Progressive vaccinia in a military smallpox vaccinee—United States 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Fulginiti VA, Papier A, Lane M, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Progressive Vaccinia. Progressive vaccinia as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5735-5744.
- Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:E1372-E1385.
- Lane ML, Goldstein J. Adverse events occurring after smallpox vaccination. Semin Ped Infect Dis. 2003;14:189-195.
- Vaccine adverse events. CDC website. http://www.cdc.gov/smallpox/clinicians/vaccine-adverse-events5.html. Accessed January 3, 2018.
- Cono J, Casey CG, Bell DM. Smallpox vaccination and adversereactions, guidance for clinicians. CDC website. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Accessed January 3, 2018.
- Rosenblatt AE, Stein SL. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33:327-332.
The practice of variolation, or inoculation of the smallpox virus from a pustule into a healthy person, was described as early as 1500
Immunization
Vaccinia is an orthopoxvirus, distinct from the smallpox virus variola, with cross-protective immunity after infection. The smallpox vaccine that is available today is a second-generation vaccinia virus derived from plaque purification cloning from the first-generation version originally licensed in 1932, which was central to eradication.5 Today’s vaccine is administered using a bifurcated needle to puncture the epidermis 15 times. Ideally, a papule forms at the inoculation site 3 to 5 days later, progresses to a vesicle and then a pustule, and finally crusts and reaches maximum size by day 10. The crust separates from the skin at 14 to 21 days, at which time the virus can no longer be isolated from the wound. United States Department of Defense surveillance of the first 450,000 vaccinated personnel noted 1% of recipients developed cutaneous eruptions beyond the vaccination site, 5% developed a localized rash, and 1% experienced a generalized eruption.2 Adverse reactions included generalized vaccinia, erythema multiforme (EM), autoinoculation (including ocular vaccinia), and contact vaccinia. There were no cases of eczema vaccinatum (EV) or progressive vaccinia (PV) reported, and no deaths were attributed to these initial vaccines.2
Immunologic Response
Vaccinia replicates in keratinocytes, spreading from cell to cell, resulting in necrosis and vesicle formation. Components of both cellular and humoral immune responses are in place by 10 days after immunization. Deficiencies in these responses result in vaccine complications secondary to vaccine escape and replication beyond the inoculation site.6 A helper T cell TH2-predominant cytokine response in atopic individuals is the likely pathogenesis required for the rapid viral spread for EV.7 Similarly, patients with cell-mediated immunity deficiencies cannot sufficiently produce enough cytotoxic T cells to eliminate an established infection, which can result in PV. Despite the effectiveness of intravenous vaccinia immunoglobulins (VIGIVs) when administered to patients with certain vaccine complications, observations that children with severe X-linked agammaglobulinemia (Bruton disease) have normal responses to vaccination suggest that antibody production is least important in viral control.8 Simian models also suggest that B-cell depletion has no impact on lesion dissemination, as lesion size is inversely correlated with T-cell count.9
Eczema Vaccinatum
A national survey estimated the prevalence of eczema in the United States at 31.6 million individuals,10 with 2- to 3-fold increases in incidence since the 1970s.11 Due to the risk for developing EV, the Advisory Committee on Immunization Practices considers personal history of eczema or contact with a family member who has eczema (either currently or in the past) contraindications to nonemergency administration of the vaccine.12,13 However, atopic conditions in general are underrecognized, with only approximately one-third of patients carrying an official diagnosis from a physician.10 Despite a large atopic and vaccinated population, EV remains relatively uncommon at 10 to 39 cases per million vaccines.6
The EV rash classically involves the midface, neck, and antecubital and popliteal fossae but can present in any location. The lesions start as papules that quickly progress to vesicles and pustules with crusting on an erythematous base. Given the extent of denudation of the epidermis, impetiginization can occur. Death rates as high as 30% have been reported14 but have only occurred in instances of secondary contact transmission with no deaths occurring in the primary vaccinees.15 In a case published in 2008, a 2-year-old boy developed the first documented EV case under the new program after exposure to his father’s predeployment vaccine.16 A similar rash is shown in Figure 1 with notable vesicles and pustules. The child required burn patient–type management, VIGIV, and treatment with cidofovir and an investigational antiorthopox agent. He was discharged from the hospital after 48 days without sequelae or considerable scarring.16 If a family member has a contraindication barring secondary contact with the vaccine, the US Department of Defense’s policy defers vaccination in active-duty members until they reach their deployment destination, at which point the inoculation is administered.

Progressive Vaccinia
Progressive vaccinia is also known as vaccinia necrosum or vaccinia gangrenosum. It is a dreaded but uncommon complication, occurring once in every 1 million vaccinations. It carries an overall case fatality rate of 15%,17 but it nearly always is fatal in patients with severe T-cell defects.18 Progressive vaccinia occurs exclusively in patients with cell-mediated immunodeficiency, with the severity of the acute illness correlating with the severity of immunodeficiency. In patients with cell-mediated immunodeficiency but intact humoral immunity, progression can be limited to expansion of the lesion, as it is thought that antibody production restricts viremia.18 Progressive vaccinia should be suspected in a patient if the vaccine site shows no signs of improvement by 14 days.19 The PV lesions do not heal and may progress or recur in patients with signs of prior healing. The leading edge has confluent vesicles, and the center of the lesion develops necrosis with thick black eschar formation. Most specifically, there is no surrounding inflammation; however, inflammation can develop later as a response to treatment or secondary infection. Figure 2 shows a PV lesion with black eschar and a transition to intact dermis without inflammation.

The first known case of PV since the 1960s vaccination campaign occurred in an active-duty Marine vaccinated with vaccinia before a diagnosis of acute myelogenous leukemia was recognized 2 weeks later.19 The vaccine site was stable in size and crusted when he received neutropenia-inducing chemotherapy 6.5 weeks after vaccination. The site then progressed in a manner typical for PV with central necrosis and a lack of inflammation at the expanding painless wound edge.19 This classic appearance with progression of satellite lesions prompted the treatment team to obtain wound and serum samples, which yielded the orthopox virus from polymerase chain reaction and viral culture. He required 2 months of care in an intensive care unit and received treatment with topical imiquimod, VIGIV, a topical and intravenous antiorthopox agent, and a second investigational antiorthopox agent; the patient ultimately survived.17,20
Generalized Vaccinia
Generalized vaccinia (GV) typically is a benign vaccine complication resulting from viremic spread from the initial inoculation site and is most commonly seen in healthy patients. Generalized vaccinia is only life threatening in immunocompromised patients. The incidence of GV is 23.4 to 241.5 patients per million vaccines.6 The majority of GV cases occur 5 to 12 days after vaccination when small distant pustules or vesicles appear on any part of the body, including the palms and soles. The lesions usually are smaller than the primary vaccination site and resolve more quickly. Generalized vaccinia can have a few to several hundred pocks, though the rash is rarely as diffuse as EV presentations.3 Given that EV can present diffusely on skin unaffected by atopic dermatitis, GV can be difficult to distinguish from EV. Features more common to EV include more systemically ill patients, increased numbers of lesions, and lesions that become confluent in an atopic distribution. It has been suggested that GV can be differentiated from vesicular or vesiculopapular EM because GV does not develop flaccid bullae and EM typically has targetoid lesions.18 Mild GV disease requires no treatment, but VIGIV can be used in more extensive cases.
Localized Reactions Due to Viral Replication
Accidental autoinoculation can occur when patients touch the vaccination site and then themselves, transferring virus particles to areas of compromised skin integrity, most commonly on the face, eyes, hands, genitalia, anus, or any other broken skin. Autoinoculation happens with some frequency and is of limited clinical concern unless there is ocular involvement. Keratitis develops in 6% of ocular vaccinia cases, and VIGIV is contraindicated, as rabbit models suggest that antigen-antibody precipitates in the cornea can cause scarring.21 Instead, trifluorothymidine is an effective topical treatment available for ocular vaccinia.
A robust response or “take” is defined as a reaction having redness, swelling, and warmth more than 3 inches in diameter at the inoculation site, peaking 6 to 12 days after inoculation with spontaneous regression occurring 1 to 3 days after.22,23 A robust take frequently is of concern to the clinician, as it can be difficult to discern from secondary infection. Secondary infections are uncommon, and a robust take is secondary to viral, not bacterial, cellulitis. Unfortunately, there are no diagnostics that have utility in distinguishing between the two, and the decision to administer empiric antibiotics might be unavoidable in light of the consequences of an untreated, rapidly progressive bacterial cellulitis. Milder cases in the setting of no constitutional symptoms could be safely monitored if close follow-up is assured.
Generalized Skin Reactions Without Viral Replication
Development of erythematous, pruritic, urticarial, and diffuse targetlike lesions of EM is common in first-time vaccinees. Often misdiagnosed as GV, EM is an immunologically mediated, not virally mediated, process. The most common infectious cause prompting EM is herpes simplex virus type 1. In the setting of a live-virus vaccine, it is difficult to determine if the vaccine prompted herpes simplex virus type 1 viral shedding and associated EM or if the vaccinia vaccine is more directly the cause of EM.24 Symptoms typically are mild, but more severe reactions may require treatment with corticosteroids. Stevens-Johnson syndrome with a severe bullous eruption has been linked to vaccinia24 but fortunately is rare. Morbilliform eruptions, urticaria, and angioedema also can occur.
Final Thoughts
Given current world events and ongoing bioterrorism threats, the smallpox vaccine program continues indefinitely. With a brisk military deployment tempo, a larger population of new vaccinees naturally will yield more cutaneous reactions. Military members, civilian health care workers, and members of the National Guard and National Reserves will develop complications and present to dermatologists for care. The historical pool of providers accustomed to seeing these complications from the 1960s eradication campaign is scant. Military and civilian dermatologists alike are uniquely poised to be the experts on protean manifestations of vaccinia reactions.
The practice of variolation, or inoculation of the smallpox virus from a pustule into a healthy person, was described as early as 1500
Immunization
Vaccinia is an orthopoxvirus, distinct from the smallpox virus variola, with cross-protective immunity after infection. The smallpox vaccine that is available today is a second-generation vaccinia virus derived from plaque purification cloning from the first-generation version originally licensed in 1932, which was central to eradication.5 Today’s vaccine is administered using a bifurcated needle to puncture the epidermis 15 times. Ideally, a papule forms at the inoculation site 3 to 5 days later, progresses to a vesicle and then a pustule, and finally crusts and reaches maximum size by day 10. The crust separates from the skin at 14 to 21 days, at which time the virus can no longer be isolated from the wound. United States Department of Defense surveillance of the first 450,000 vaccinated personnel noted 1% of recipients developed cutaneous eruptions beyond the vaccination site, 5% developed a localized rash, and 1% experienced a generalized eruption.2 Adverse reactions included generalized vaccinia, erythema multiforme (EM), autoinoculation (including ocular vaccinia), and contact vaccinia. There were no cases of eczema vaccinatum (EV) or progressive vaccinia (PV) reported, and no deaths were attributed to these initial vaccines.2
Immunologic Response
Vaccinia replicates in keratinocytes, spreading from cell to cell, resulting in necrosis and vesicle formation. Components of both cellular and humoral immune responses are in place by 10 days after immunization. Deficiencies in these responses result in vaccine complications secondary to vaccine escape and replication beyond the inoculation site.6 A helper T cell TH2-predominant cytokine response in atopic individuals is the likely pathogenesis required for the rapid viral spread for EV.7 Similarly, patients with cell-mediated immunity deficiencies cannot sufficiently produce enough cytotoxic T cells to eliminate an established infection, which can result in PV. Despite the effectiveness of intravenous vaccinia immunoglobulins (VIGIVs) when administered to patients with certain vaccine complications, observations that children with severe X-linked agammaglobulinemia (Bruton disease) have normal responses to vaccination suggest that antibody production is least important in viral control.8 Simian models also suggest that B-cell depletion has no impact on lesion dissemination, as lesion size is inversely correlated with T-cell count.9
Eczema Vaccinatum
A national survey estimated the prevalence of eczema in the United States at 31.6 million individuals,10 with 2- to 3-fold increases in incidence since the 1970s.11 Due to the risk for developing EV, the Advisory Committee on Immunization Practices considers personal history of eczema or contact with a family member who has eczema (either currently or in the past) contraindications to nonemergency administration of the vaccine.12,13 However, atopic conditions in general are underrecognized, with only approximately one-third of patients carrying an official diagnosis from a physician.10 Despite a large atopic and vaccinated population, EV remains relatively uncommon at 10 to 39 cases per million vaccines.6
The EV rash classically involves the midface, neck, and antecubital and popliteal fossae but can present in any location. The lesions start as papules that quickly progress to vesicles and pustules with crusting on an erythematous base. Given the extent of denudation of the epidermis, impetiginization can occur. Death rates as high as 30% have been reported14 but have only occurred in instances of secondary contact transmission with no deaths occurring in the primary vaccinees.15 In a case published in 2008, a 2-year-old boy developed the first documented EV case under the new program after exposure to his father’s predeployment vaccine.16 A similar rash is shown in Figure 1 with notable vesicles and pustules. The child required burn patient–type management, VIGIV, and treatment with cidofovir and an investigational antiorthopox agent. He was discharged from the hospital after 48 days without sequelae or considerable scarring.16 If a family member has a contraindication barring secondary contact with the vaccine, the US Department of Defense’s policy defers vaccination in active-duty members until they reach their deployment destination, at which point the inoculation is administered.

Progressive Vaccinia
Progressive vaccinia is also known as vaccinia necrosum or vaccinia gangrenosum. It is a dreaded but uncommon complication, occurring once in every 1 million vaccinations. It carries an overall case fatality rate of 15%,17 but it nearly always is fatal in patients with severe T-cell defects.18 Progressive vaccinia occurs exclusively in patients with cell-mediated immunodeficiency, with the severity of the acute illness correlating with the severity of immunodeficiency. In patients with cell-mediated immunodeficiency but intact humoral immunity, progression can be limited to expansion of the lesion, as it is thought that antibody production restricts viremia.18 Progressive vaccinia should be suspected in a patient if the vaccine site shows no signs of improvement by 14 days.19 The PV lesions do not heal and may progress or recur in patients with signs of prior healing. The leading edge has confluent vesicles, and the center of the lesion develops necrosis with thick black eschar formation. Most specifically, there is no surrounding inflammation; however, inflammation can develop later as a response to treatment or secondary infection. Figure 2 shows a PV lesion with black eschar and a transition to intact dermis without inflammation.

The first known case of PV since the 1960s vaccination campaign occurred in an active-duty Marine vaccinated with vaccinia before a diagnosis of acute myelogenous leukemia was recognized 2 weeks later.19 The vaccine site was stable in size and crusted when he received neutropenia-inducing chemotherapy 6.5 weeks after vaccination. The site then progressed in a manner typical for PV with central necrosis and a lack of inflammation at the expanding painless wound edge.19 This classic appearance with progression of satellite lesions prompted the treatment team to obtain wound and serum samples, which yielded the orthopox virus from polymerase chain reaction and viral culture. He required 2 months of care in an intensive care unit and received treatment with topical imiquimod, VIGIV, a topical and intravenous antiorthopox agent, and a second investigational antiorthopox agent; the patient ultimately survived.17,20
Generalized Vaccinia
Generalized vaccinia (GV) typically is a benign vaccine complication resulting from viremic spread from the initial inoculation site and is most commonly seen in healthy patients. Generalized vaccinia is only life threatening in immunocompromised patients. The incidence of GV is 23.4 to 241.5 patients per million vaccines.6 The majority of GV cases occur 5 to 12 days after vaccination when small distant pustules or vesicles appear on any part of the body, including the palms and soles. The lesions usually are smaller than the primary vaccination site and resolve more quickly. Generalized vaccinia can have a few to several hundred pocks, though the rash is rarely as diffuse as EV presentations.3 Given that EV can present diffusely on skin unaffected by atopic dermatitis, GV can be difficult to distinguish from EV. Features more common to EV include more systemically ill patients, increased numbers of lesions, and lesions that become confluent in an atopic distribution. It has been suggested that GV can be differentiated from vesicular or vesiculopapular EM because GV does not develop flaccid bullae and EM typically has targetoid lesions.18 Mild GV disease requires no treatment, but VIGIV can be used in more extensive cases.
Localized Reactions Due to Viral Replication
Accidental autoinoculation can occur when patients touch the vaccination site and then themselves, transferring virus particles to areas of compromised skin integrity, most commonly on the face, eyes, hands, genitalia, anus, or any other broken skin. Autoinoculation happens with some frequency and is of limited clinical concern unless there is ocular involvement. Keratitis develops in 6% of ocular vaccinia cases, and VIGIV is contraindicated, as rabbit models suggest that antigen-antibody precipitates in the cornea can cause scarring.21 Instead, trifluorothymidine is an effective topical treatment available for ocular vaccinia.
A robust response or “take” is defined as a reaction having redness, swelling, and warmth more than 3 inches in diameter at the inoculation site, peaking 6 to 12 days after inoculation with spontaneous regression occurring 1 to 3 days after.22,23 A robust take frequently is of concern to the clinician, as it can be difficult to discern from secondary infection. Secondary infections are uncommon, and a robust take is secondary to viral, not bacterial, cellulitis. Unfortunately, there are no diagnostics that have utility in distinguishing between the two, and the decision to administer empiric antibiotics might be unavoidable in light of the consequences of an untreated, rapidly progressive bacterial cellulitis. Milder cases in the setting of no constitutional symptoms could be safely monitored if close follow-up is assured.
Generalized Skin Reactions Without Viral Replication
Development of erythematous, pruritic, urticarial, and diffuse targetlike lesions of EM is common in first-time vaccinees. Often misdiagnosed as GV, EM is an immunologically mediated, not virally mediated, process. The most common infectious cause prompting EM is herpes simplex virus type 1. In the setting of a live-virus vaccine, it is difficult to determine if the vaccine prompted herpes simplex virus type 1 viral shedding and associated EM or if the vaccinia vaccine is more directly the cause of EM.24 Symptoms typically are mild, but more severe reactions may require treatment with corticosteroids. Stevens-Johnson syndrome with a severe bullous eruption has been linked to vaccinia24 but fortunately is rare. Morbilliform eruptions, urticaria, and angioedema also can occur.
Final Thoughts
Given current world events and ongoing bioterrorism threats, the smallpox vaccine program continues indefinitely. With a brisk military deployment tempo, a larger population of new vaccinees naturally will yield more cutaneous reactions. Military members, civilian health care workers, and members of the National Guard and National Reserves will develop complications and present to dermatologists for care. The historical pool of providers accustomed to seeing these complications from the 1960s eradication campaign is scant. Military and civilian dermatologists alike are uniquely poised to be the experts on protean manifestations of vaccinia reactions.
- Voigt EA, Kennedy RB, Poland GA. Defending against smallpox: a focus on vaccines. Expert Rev Vaccines. 2016;15:1197-1211.
- Grabenstein J, Wikenwerder W Jr. US military smallpox vaccination program experience. JAMA. 2003;289:3278-3282.
- Kelly CD, Egan C, Davis SW, et al. Laboratory confirmation of generalized vaccinia following smallpox vaccination. J Clin Microbiol. 2004;42:1373-1375.
- Slike BM, Creegan M, Marovich M, et al. Humoral immunity to primary smallpox vaccination: impact of childhood versus adult immunization on vaccinia vector vaccine development in military populations. PLoS One. 2017;12:E0169247.
- Notice to readers: newly licensed vaccine to replace old smallpox vaccine. MMWR. 2008;57:207-208.
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101-114.
- Engler R, Kenner J, Leung D. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357-365.
- Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36:766-774.
- Gordon S, Cecchinato V, Andresen V, et al. Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis. 2011;203:1043-1053.
- Hanifin J, Reed M. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;82:82-91.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1-16.
- Petersen BW, Harms TJ, Reynolds MG, et al. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupation exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunizations Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65:257-262.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Eczema Vaccinatum. Eczema vaccinatum as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection analysis, and presentation of immunization safety data. Vaccine. 2007:25;5725-5734.
- Aragón TJ, Ulrich S, Fernyak S, et al. Risks of serious complications and death from smallpox vaccination: a systematic review of the United States experience, 1963-1968. BMC Public Health. 2003;3:26.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555-1561.
- Centers for Disease Control and Prevention (CDC). Progressive vaccinia in a military smallpox vaccinee—United States 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Fulginiti VA, Papier A, Lane M, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Progressive Vaccinia. Progressive vaccinia as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5735-5744.
- Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:E1372-E1385.
- Lane ML, Goldstein J. Adverse events occurring after smallpox vaccination. Semin Ped Infect Dis. 2003;14:189-195.
- Vaccine adverse events. CDC website. http://www.cdc.gov/smallpox/clinicians/vaccine-adverse-events5.html. Accessed January 3, 2018.
- Cono J, Casey CG, Bell DM. Smallpox vaccination and adversereactions, guidance for clinicians. CDC website. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Accessed January 3, 2018.
- Rosenblatt AE, Stein SL. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33:327-332.
- Voigt EA, Kennedy RB, Poland GA. Defending against smallpox: a focus on vaccines. Expert Rev Vaccines. 2016;15:1197-1211.
- Grabenstein J, Wikenwerder W Jr. US military smallpox vaccination program experience. JAMA. 2003;289:3278-3282.
- Kelly CD, Egan C, Davis SW, et al. Laboratory confirmation of generalized vaccinia following smallpox vaccination. J Clin Microbiol. 2004;42:1373-1375.
- Slike BM, Creegan M, Marovich M, et al. Humoral immunity to primary smallpox vaccination: impact of childhood versus adult immunization on vaccinia vector vaccine development in military populations. PLoS One. 2017;12:E0169247.
- Notice to readers: newly licensed vaccine to replace old smallpox vaccine. MMWR. 2008;57:207-208.
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101-114.
- Engler R, Kenner J, Leung D. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357-365.
- Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36:766-774.
- Gordon S, Cecchinato V, Andresen V, et al. Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis. 2011;203:1043-1053.
- Hanifin J, Reed M. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;82:82-91.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1-16.
- Petersen BW, Harms TJ, Reynolds MG, et al. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupation exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunizations Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65:257-262.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Eczema Vaccinatum. Eczema vaccinatum as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection analysis, and presentation of immunization safety data. Vaccine. 2007:25;5725-5734.
- Aragón TJ, Ulrich S, Fernyak S, et al. Risks of serious complications and death from smallpox vaccination: a systematic review of the United States experience, 1963-1968. BMC Public Health. 2003;3:26.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555-1561.
- Centers for Disease Control and Prevention (CDC). Progressive vaccinia in a military smallpox vaccinee—United States 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Fulginiti VA, Papier A, Lane M, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Progressive Vaccinia. Progressive vaccinia as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5735-5744.
- Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:E1372-E1385.
- Lane ML, Goldstein J. Adverse events occurring after smallpox vaccination. Semin Ped Infect Dis. 2003;14:189-195.
- Vaccine adverse events. CDC website. http://www.cdc.gov/smallpox/clinicians/vaccine-adverse-events5.html. Accessed January 3, 2018.
- Cono J, Casey CG, Bell DM. Smallpox vaccination and adversereactions, guidance for clinicians. CDC website. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Accessed January 3, 2018.
- Rosenblatt AE, Stein SL. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33:327-332.
Practice Points
- Dermatologists should be aware that smallpox vaccinations are being administered to patients and may present with a myriad of cutaneous complications.
- Progressive vaccinia should be suspected if a smallpox inoculation has not healed after 14 days and, most specifically, if there is no inflammation surrounding the site.
- Generalized vaccinia generally is a benign condition seen in otherwise healthy patients and usually requires no treatment.
Atopic patients should be educated to avoid receiving routine smallpox vaccinations if they would be considered at risk for requiring the inoculation.