User login
The Impact of Sequencing of Abiraterone and Enzalutamide in Veterans With Metastatic Castration- Resistant Prostate Cancer
PURPOSE: To evaluate outcomes of disease progression based on the sequence of abiraterone and enzalutamide in veterans diagnosed with metastatic castration-resistant prostate cancer (mCRPC). BACKGROUND: Two of the current options for mCRPC treatment are the novel oral hormonal agents abiraterone and enzalutamide. After progression on one of these agents, one option is to switch to the other agent not previously used. Previously published retrospective studies and one prospective study have shown a difference in outcomes favoring abiraterone followed by enzalutamide, while others have shown no difference based on sequence. The optimal sequence of abiraterone and enzalutamide is still unclear.
METHODS: This was a retrospective chart review of patients who received abiraterone and enzalutamide in sequence for the treatment of mCRPC within our healthcare system from April 28, 2011 through October 31, 2019. Baseline demographic information such as age, race, Gleason score, and prior treatments were collected. The primary outcome was combined prostate-specific antigen progression-free survival (cPSA-PFS). Secondary outcomes included radiographic PFS (rPFS), overall survival (OS), adverse events causing treatment discontinuation, and medication adherence. Between-group survival differences were estimated by the Kaplan-Meier method and an unadjusted Cox regression model.
RESULTS: A total of 77 patients met criteria for study inclusion, with 51 in the abiraterone-to-enzalutamide group (ABI-ENZ) and 26 in the enzalutamide-to-abiraterone group (ENZ-ABI). For the primary outcome of cPSA-PFS, the median survival of the ABI-ENZ and ENZ-ABI groups was 17.3 months (95% CI, 10.3-24.3 months) and 10.2 months (95% CI, 8.5-11.8 months), respectively, which was significantly different (log-rank P=0.009) in favor of the ABI-ENZ sequence (HR 0.46; 95% CI, 0.26-0.83). Secondary outcomes of rPFS and OS were not significantly different between groups.
CONCLUSION: This study adds to the evidence supporting the sequence of abiraterone before enzalutamide for improving PSA-PFS. It is thought this might be related to differences in mechanisms of resistance between the two drugs. This benefit has not yet translated to an improvement in rPFS and OS. Based on the results of this study in conjunction with previously published studies, use of abiraterone before enzalutamide should be considered over the alternate sequence.
PURPOSE: To evaluate outcomes of disease progression based on the sequence of abiraterone and enzalutamide in veterans diagnosed with metastatic castration-resistant prostate cancer (mCRPC). BACKGROUND: Two of the current options for mCRPC treatment are the novel oral hormonal agents abiraterone and enzalutamide. After progression on one of these agents, one option is to switch to the other agent not previously used. Previously published retrospective studies and one prospective study have shown a difference in outcomes favoring abiraterone followed by enzalutamide, while others have shown no difference based on sequence. The optimal sequence of abiraterone and enzalutamide is still unclear.
METHODS: This was a retrospective chart review of patients who received abiraterone and enzalutamide in sequence for the treatment of mCRPC within our healthcare system from April 28, 2011 through October 31, 2019. Baseline demographic information such as age, race, Gleason score, and prior treatments were collected. The primary outcome was combined prostate-specific antigen progression-free survival (cPSA-PFS). Secondary outcomes included radiographic PFS (rPFS), overall survival (OS), adverse events causing treatment discontinuation, and medication adherence. Between-group survival differences were estimated by the Kaplan-Meier method and an unadjusted Cox regression model.
RESULTS: A total of 77 patients met criteria for study inclusion, with 51 in the abiraterone-to-enzalutamide group (ABI-ENZ) and 26 in the enzalutamide-to-abiraterone group (ENZ-ABI). For the primary outcome of cPSA-PFS, the median survival of the ABI-ENZ and ENZ-ABI groups was 17.3 months (95% CI, 10.3-24.3 months) and 10.2 months (95% CI, 8.5-11.8 months), respectively, which was significantly different (log-rank P=0.009) in favor of the ABI-ENZ sequence (HR 0.46; 95% CI, 0.26-0.83). Secondary outcomes of rPFS and OS were not significantly different between groups.
CONCLUSION: This study adds to the evidence supporting the sequence of abiraterone before enzalutamide for improving PSA-PFS. It is thought this might be related to differences in mechanisms of resistance between the two drugs. This benefit has not yet translated to an improvement in rPFS and OS. Based on the results of this study in conjunction with previously published studies, use of abiraterone before enzalutamide should be considered over the alternate sequence.
PURPOSE: To evaluate outcomes of disease progression based on the sequence of abiraterone and enzalutamide in veterans diagnosed with metastatic castration-resistant prostate cancer (mCRPC). BACKGROUND: Two of the current options for mCRPC treatment are the novel oral hormonal agents abiraterone and enzalutamide. After progression on one of these agents, one option is to switch to the other agent not previously used. Previously published retrospective studies and one prospective study have shown a difference in outcomes favoring abiraterone followed by enzalutamide, while others have shown no difference based on sequence. The optimal sequence of abiraterone and enzalutamide is still unclear.
METHODS: This was a retrospective chart review of patients who received abiraterone and enzalutamide in sequence for the treatment of mCRPC within our healthcare system from April 28, 2011 through October 31, 2019. Baseline demographic information such as age, race, Gleason score, and prior treatments were collected. The primary outcome was combined prostate-specific antigen progression-free survival (cPSA-PFS). Secondary outcomes included radiographic PFS (rPFS), overall survival (OS), adverse events causing treatment discontinuation, and medication adherence. Between-group survival differences were estimated by the Kaplan-Meier method and an unadjusted Cox regression model.
RESULTS: A total of 77 patients met criteria for study inclusion, with 51 in the abiraterone-to-enzalutamide group (ABI-ENZ) and 26 in the enzalutamide-to-abiraterone group (ENZ-ABI). For the primary outcome of cPSA-PFS, the median survival of the ABI-ENZ and ENZ-ABI groups was 17.3 months (95% CI, 10.3-24.3 months) and 10.2 months (95% CI, 8.5-11.8 months), respectively, which was significantly different (log-rank P=0.009) in favor of the ABI-ENZ sequence (HR 0.46; 95% CI, 0.26-0.83). Secondary outcomes of rPFS and OS were not significantly different between groups.
CONCLUSION: This study adds to the evidence supporting the sequence of abiraterone before enzalutamide for improving PSA-PFS. It is thought this might be related to differences in mechanisms of resistance between the two drugs. This benefit has not yet translated to an improvement in rPFS and OS. Based on the results of this study in conjunction with previously published studies, use of abiraterone before enzalutamide should be considered over the alternate sequence.
The Impact of Sequencing of Abiraterone and Enzalutamide in Veterans With Metastatic Castration- Resistant Prostate Cancer
PURPOSE: To evaluate outcomes of disease progression based on the sequence of abiraterone and enzalutamide in veterans diagnosed with metastatic castration-resistant prostate cancer (mCRPC).
BACKGROUND: Two of the current options for mCRPC treatment are the novel oral hormonal agents abiraterone and enzalutamide. After progression on one of these agents, one option is to switch to the other agent not previously used. Previously published retrospective studies and one prospective study have shown a difference in outcomes favoring abiraterone followed by enzalutamide, while others have shown no difference based on sequence. The optimal sequence of abiraterone and enzalutamide is still unclear.
METHODS: This was a retrospective chart review of patients who received abiraterone and enzalutamide in sequence for the treatment of mCRPC within our healthcare system from April 28, 2011 through October 31, 2019. Baseline demographic information such as age, race, Gleason score, and prior treatments were collected. The primary outcome was combined prostate-specific antigen progression-free survival (cPSA-PFS). Secondary outcomes included radiographic PFS (rPFS), overall survival (OS), adverse events causing treatment discontinuation, and medication adherence. Between-group survival differences were estimated by the Kaplan-Meier method and an unadjusted Cox regression model.
RESULTS: A total of 77 patients met criteria for study inclusion, with 51 in the abiraterone-to-enzalutamide group (ABI-ENZ) and 26 in the enzalutamide-to-abiraterone group (ENZ-ABI). For the primary outcome of cPSA-PFS, the median survival of the ABI-ENZ and ENZ-ABI groups was 17.3 months (95% CI, 10.3-24.3 months) and 10.2 months (95% CI, 8.5-11.8 months), respectively, which was significantly different (log-rank P=0.009) in favor of the ABI-ENZ sequence (HR 0.46; 95% CI, 0.26-0.83). Secondary outcomes of rPFS and OS were not significantly different between groups.
CONCLUSION: This study adds to the evidence supporting the sequence of abiraterone before enzalutamide for improving PSA-PFS. It is thought this might be related to differences in mechanisms of resistance between the two drugs. This benefit has not yet translated to an improvement in rPFS and OS. Based on the results of this study in conjunction with previously published studies, use of abiraterone before enzalutamide should be considered over the alternate sequence.
PURPOSE: To evaluate outcomes of disease progression based on the sequence of abiraterone and enzalutamide in veterans diagnosed with metastatic castration-resistant prostate cancer (mCRPC).
BACKGROUND: Two of the current options for mCRPC treatment are the novel oral hormonal agents abiraterone and enzalutamide. After progression on one of these agents, one option is to switch to the other agent not previously used. Previously published retrospective studies and one prospective study have shown a difference in outcomes favoring abiraterone followed by enzalutamide, while others have shown no difference based on sequence. The optimal sequence of abiraterone and enzalutamide is still unclear.
METHODS: This was a retrospective chart review of patients who received abiraterone and enzalutamide in sequence for the treatment of mCRPC within our healthcare system from April 28, 2011 through October 31, 2019. Baseline demographic information such as age, race, Gleason score, and prior treatments were collected. The primary outcome was combined prostate-specific antigen progression-free survival (cPSA-PFS). Secondary outcomes included radiographic PFS (rPFS), overall survival (OS), adverse events causing treatment discontinuation, and medication adherence. Between-group survival differences were estimated by the Kaplan-Meier method and an unadjusted Cox regression model.
RESULTS: A total of 77 patients met criteria for study inclusion, with 51 in the abiraterone-to-enzalutamide group (ABI-ENZ) and 26 in the enzalutamide-to-abiraterone group (ENZ-ABI). For the primary outcome of cPSA-PFS, the median survival of the ABI-ENZ and ENZ-ABI groups was 17.3 months (95% CI, 10.3-24.3 months) and 10.2 months (95% CI, 8.5-11.8 months), respectively, which was significantly different (log-rank P=0.009) in favor of the ABI-ENZ sequence (HR 0.46; 95% CI, 0.26-0.83). Secondary outcomes of rPFS and OS were not significantly different between groups.
CONCLUSION: This study adds to the evidence supporting the sequence of abiraterone before enzalutamide for improving PSA-PFS. It is thought this might be related to differences in mechanisms of resistance between the two drugs. This benefit has not yet translated to an improvement in rPFS and OS. Based on the results of this study in conjunction with previously published studies, use of abiraterone before enzalutamide should be considered over the alternate sequence.
PURPOSE: To evaluate outcomes of disease progression based on the sequence of abiraterone and enzalutamide in veterans diagnosed with metastatic castration-resistant prostate cancer (mCRPC).
BACKGROUND: Two of the current options for mCRPC treatment are the novel oral hormonal agents abiraterone and enzalutamide. After progression on one of these agents, one option is to switch to the other agent not previously used. Previously published retrospective studies and one prospective study have shown a difference in outcomes favoring abiraterone followed by enzalutamide, while others have shown no difference based on sequence. The optimal sequence of abiraterone and enzalutamide is still unclear.
METHODS: This was a retrospective chart review of patients who received abiraterone and enzalutamide in sequence for the treatment of mCRPC within our healthcare system from April 28, 2011 through October 31, 2019. Baseline demographic information such as age, race, Gleason score, and prior treatments were collected. The primary outcome was combined prostate-specific antigen progression-free survival (cPSA-PFS). Secondary outcomes included radiographic PFS (rPFS), overall survival (OS), adverse events causing treatment discontinuation, and medication adherence. Between-group survival differences were estimated by the Kaplan-Meier method and an unadjusted Cox regression model.
RESULTS: A total of 77 patients met criteria for study inclusion, with 51 in the abiraterone-to-enzalutamide group (ABI-ENZ) and 26 in the enzalutamide-to-abiraterone group (ENZ-ABI). For the primary outcome of cPSA-PFS, the median survival of the ABI-ENZ and ENZ-ABI groups was 17.3 months (95% CI, 10.3-24.3 months) and 10.2 months (95% CI, 8.5-11.8 months), respectively, which was significantly different (log-rank P=0.009) in favor of the ABI-ENZ sequence (HR 0.46; 95% CI, 0.26-0.83). Secondary outcomes of rPFS and OS were not significantly different between groups.
CONCLUSION: This study adds to the evidence supporting the sequence of abiraterone before enzalutamide for improving PSA-PFS. It is thought this might be related to differences in mechanisms of resistance between the two drugs. This benefit has not yet translated to an improvement in rPFS and OS. Based on the results of this study in conjunction with previously published studies, use of abiraterone before enzalutamide should be considered over the alternate sequence.
Malnutrition as a Fall Risk Factor
Falls often result in injuries with devastating outcomes. In the elderly, falls are the largest cause of injury, mortality, and functional decline, leading to 40% of nursing home admissions.1 Nationally, falls with injury are estimated to cost $19 billion in direct medical costs.2 According to the National Quality Forum (NQF), hospital falls resulting in injury are reportable events. Beginning in fiscal year 2015, reportable events labeled as hospital acquired conditions (HAC) are subject to nonpayment, creating increased regulatory and reimbursement pressure on hospitals.3
Due to the major impact of a fall, The Joint Commission (TJC) requires hospitals to assess a patient’s fall risk on admission and whenever the patient’s condition changes.4 Despite decades of research evaluating various predictive strategies to identify individuals at fall risk, nutritional issues as interactive risk factors have received little attention.
A comparative study on the validity of fall risk assessment scales revealed that tools claiming to predict risk factors do not work well.5 Falls are the result of multiple interactive, synergistic pathologies and risk factors. In a multivariate regression study conducted by Lichtenstein and colleagues in Canada, lower body weight was found to be a statistically significant risk factor for falling.6 In 2004, Oliver and colleagues conducted a systematic review of fall risk assessment tools that included validation testing with sufficient data to allow for calculation of sensitivity, specificity, negative and positive predictive values, odds ratios, and confidence intervals.5
Fourteen studies identified common fall risk factors. The majority of these studies identified impaired cognition as a risk factor.5 Of the 14 studies included in the systematic analysis of Oliver and colleagues,6-19 6 identified medications,6,8,11,12,17,18, and 8 noted weakness and unsteady gait as risk factors.6,9-11,14,16,18,19 Only 1 study noted anemia as a risk factor for falls among patients who were post cerebral vascular accident.11 Additionally, only 1 study noted an association between falls resulting in hip fracture and lower body weight.6
One in 4 adults admitted to a hospital is malnourished.20,21 Components of malnutrition, including but not limited to anemia, clinically significant weight loss, and vitamin D deficiency, may be unrecognized interactive risk factors that increase the risk of hospital falls. Malnutrition and dehydration symptoms include fatigue, dizziness, irritability, loss of muscle mass, impulsivity, and the potential for poor judgment. Therefore, it is likely that the severity of specific malnutrition parameters is associated with recurrent falls and possibly injurious falls.
Hunger and inadequate food intake due to chronic disease or chronic food insecurity are real issues for the elderly. Insufficient caloric intake often leads to slow, progressive, and often unnoticed weight loss. The Academy of Nutrition and Dietetics (AND) and the American Association of Parenteral and Enteral Nutrition (ASPEN) have defined clinically significant weight loss categories to aid in the diagnosis of malnutrition. To aid in the diagnosis of various degrees of malnutrition, clinically significant weight loss is classified as ≥ 5% weight loss in 30 days; ≥ 7.5% weight loss in 90 days; > 10% in 180 days.21 The World Health Organization (WHO) identifies iron deficiency measured by hemoglobin (Hgb) value (for men < 13 g/dL) as the most common and widespread nutritional disorder in the world (Tables 1a, 1b, and 1c).22
Eat Well, Fall Less is a retrospective chart review approved by the Louis Stokes Cleveland VAMC (LSCVAMC) Internal Review Board. The review seeks to determine whether degree of weight loss and decline in Hgb and vitamin D deficiency, factors of malnutrition, are present in recurrent fallers vs single-event fallers. Researchers hypothesized that individuals who experienced recurrent falls during hospitalization would demonstrate a greater degree of clinically significant weight loss compared with those who had experienced a single fall. The second hypothesis was that recurrent fallers also would have lower Hgb values than that of single-event fallers. The tertiary hypothesis was that individuals with a greater degree of vitamin D deficiency were more likely to be recurrent fallers compared with single-event fallers.
In addition, dementia has been previously identified as an independent risk factor for falls and recurrent falls.23 During phase 2 of the study, the researchers hypothesized that in individuals with dementia, the concurrent diagnosis of malnutrition would be greatest in the recurrent fall population. A total of 30 subjects were included in the analysis.
Methods
Patient record search of note titles for falls was compiled daily. A random sample of 170 veterans who had experienced a fall was screened for study inclusion. Of the 170 charts, data from a total of 120 veterans who experienced a documented fall during a hospitalization between October 1, 2010 and October 31, 2012, were included in this analysis.
Eligibility Criteria and Baseline Characteristics
Chart reviews of veterans aged > 18 years experiencing a fall while hospitalized at the LSCVAMC between October 1, 2010 and October 31, 2012, were eligible to be included in this study. Each veteran needed to have 1 documented weight in a maximum of 24 months before the first fall and a minimum of 1 documented Hgb value prior to the first fall.
Patients were excluded if they had experienced a cerebrovascular accident or transient ischemic attack; a documented orthopedic fracture in the 12 months before the first fall; a documented amputation of a lower limb in the past 24 months; diagnosis of blindness; a lack of outpatient weight for greater than 24 months before the first fall; a history of volume overload, renal, cardiovascular, or other in nature during hospitalization; and alcoholism. Additionally, if any of the study investigators felt as though a patient had commorientes that made weight history inaccurate, those patients were excluded. Data were reviewed on 170 randomly selected subjects. A total of 50 subjects were excluded for not meeting the inclusion criteria; 120 individuals met eligible criteria. The patients who had experienced falls were divided into 2 groups: single-event fallers (1 fall documented during the study period) and recurrent fallers (2 or more falls documented during the study period). Fifty subjects were excluded from the final analysis because they met the following exclusion criteria: volume overload anytime in the 12 months before the documented fall (42); amputation of a lower limb before the fall (6); and prosthetic device alteration within the 12 months before the fall (3). One of the subjects was eliminated for both volume overload and amputation.
Data Collection
Data obtained from the Computerized Patient Record System review included age, gender, diagnosis, and body weight at the time of the first fall and 1 month, 3 months, 6 months, and 1 year before the first fall. Hemoglobin values at time of the first fall, 1 month, 3 months, 6 months, and 1 year before first documented fall also were collected. Vitamin D values and date of value also were collected. In year 2 of this multiphase retrospective review, charts again were reviewed and additional data collected, which included absence or presence of dementia along with type of dementia and the presence or absence of cancer. Year 2 data collection also included diagnosis of malnutrition by provider and registered dietitian assessment of degree of malnutrition.
Analysis
Data analysis comparing single-event fallers and frequent fallers was performed using IBM SPSS Statistic V22.0s. This pilot study has recognized weight loss and anemia as being associated with repeat falls. A 2-tailed t test was performed to evaluate differences in weight history, 25-hydroxyvitamin D, and Hgb characteristics between single fallers and frequent fallers. A repeated analysis of variance was performed to evaluate changes in weight and Hgb values over time for single and multiple falls. During phase 2 of this trial, a subanalysis comparing individuals with and without the diagnosis of dementia was conducted.
Results
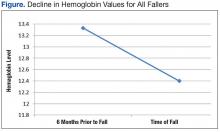
The average age of the patients was 68 years. There was a significant decline in Hgb levels in both single-event fallers and frequent fallers at 12 and 6 months before the first fall event (95% confidence interval; P = .001). One year before the first fall, 28% of eventual fallers met WHO criteria for the diagnosis of mild anemia. One year before the first fall, none of the eventual fallers met WHO criteria for moderate or severe anemia. Using the lab data just before the first fall, 10% of fallers met WHO criteria for mild anemia, 48% met WHO criteria for moderate anemia, and 31% met WHO criteria for severe anemia. The degree of anemia in single-event fallers when compared with multiple-event fallers was insignificant. Interestingly, the degree of decline of Hgb value during the 6 months before the first fall event was notable for all fallers (Figure).
Only 60 of the 120 included patients had a documented vitamin D level. At the time of the fall, 46 patients had moderate vitamin D deficiency, and 23 patients had severe vitamin D deficiency, defined as < 32 mg/dL. The authors speculate that vitamin D status declines with malnutrition and increases fall risk.
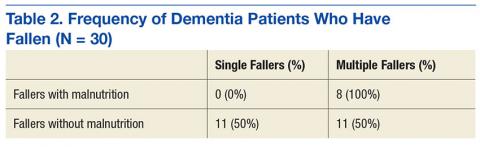
Thirty participants were included in the dementia arm; 36.7% were single-event fallers, and 63.3% were multiple-event fallers (Table 2). The average age of the groups was 76.7 years and 73.9 years, respectively. Physician diagnosis of malnutrition was collected for both single- and multiple-event fallers. Of the single-event fallers with dementia, none had a diagnosis of malnutrition before the first hospital fall; and of the multiple-event fallers with dementia, all had a diagnosis of malnutrition before their first hospital fall. Individuals with a diagnosis of dementia and malnutrition fall frequently (P = .0028).
Discussion
In this study, falls occurred in a variety of patient populations. Both single-event fallers and recurrent fallers had a significant drop in Hgb values at 12, 6, and 3 months before the first fall. There was not a strong difference of the Hgb value between single-event falls and multiple fallers in the total population. Anemia was a significant risk factor for all fallers. The decline in the Hgb level before a fall is highly predictive of fall risk.
In individuals with dementia, those with the diagnosis of malnutrition are frequent fallers. A tool to assist in identification of this patient population along with a focused intervention strategy for this population is an area of needed research.
Further research is under way to determine which components of malnutrition diagnosis contribute to fall risk. If so, development of a fall assessment tool, including various components of malnutrition is warranted. Intervention strategies to reduce fall risk may soon include new nutrition and education techniques based on the faller constellation. Falls instruments that explore nutritional risk factors and falls should be investigated (ie, weight loss, vitamin D status, and anemia).
Falls occur in patients with a variety of risk factors (eg, mobility and cognition). The current screening instruments to assess fall risk factors do not sufficiently account for nutritional risk factors. In the Eat Well, Fall Less Study of hospitalized veterans, nutritional risk factors of anemia and weight loss also were associated with single- and multiple-event fallers. The AUTUMN falls instrument that includes critical elements of malnutrition, such as a decline in Hgb and weight loss, is currently being created and is in the process of being validated at LSCVAMC; this tool will incorporate components of malnutrition.
Acknowledgments
The authors acknowledge Michelle Pearson, Laura Guidotti, Adam Weier, Elizabeth Gable, and Shannon Corlett for their research contributions. In memory of Anne Raguso, RD, PhD, for her lifelong focus on nutrition research.
1. Centers for Disease Control and Prevention. Older adult falls: important facts about falls. http://www.cdc.gov/homeandrecreationalsafety/falls/adultfalls.html. Updated September 20, 2016. Accessed December 2, 2016.
2. Centers for Disease Control and Prevention. Older adult falls: cost of falls among older adults. http://www.cdc.gov/HomeandRecreationalSafety/Falls/fallcost.html. Updated August 19, 2016. Accessed December 2, 2016.
3. National Quality Forum. Serious reportable events in healthcare—2011 update: a consensus report https://www.qualityforum.org/Publications/2011/12/SRE_2011_Final_Report.aspx. Published 2011. Accessed December 2, 2016.
4. DuPree E. Taking a stand against falls. https://www.jointcommission.org/jc_physician_blog/taking_a_stand_against_falls. Published May1, 2014. Accessed December 2, 2016.
5. Oliver D, Daly F, Martin FC, McMurdo ME. Risk factors and risk assessment tools for falls in hospital in-patients: a systematic review. Age Ageing. 2004;33(2):122-130.
6. Lichtenstein MJ, Griffin MR, Cornell JE, Malcolm E, Ray WA. Risk factors for hip fractures occurring in the hospital. Am J Epidemiol. 1994;140(9):830-838.
7. Ballinger BR, Ramsay AC. Accidents and drug treatment in a psychiatric hospital. Br J Psychiatry. 1975;126:462-463.
8. Bates D, Pruess K, Souney P, Platt R. Serious falls in hospitalized patients correlates and resource utilization. Am J Med. 1995;99(2):137-143.
9. Byers V, Arrington ME, Finstuen K. Predictive risk factors associated with stroke patient falls in acute care settings. J Neurosci Nurs. 1990;22(3):147-154.
10. Chu LW, Pei CK, Chiu A, et al. Risk factors for falls in hospitalized older medical patients. J Gerontol A Biol Sci Med Sci. 1999;54(1):M38-M48.
11. Gales BJ, Menard SM. Relationship between administration of selected medications and falls in hospitalized elderly patients. Ann Pharmacother. 1995;29(4):354-358.
12. Gluck T, Wientjes HJ, Rai GS. An evaluation of risk factors for inpatient falls in acute care and rehabilitation elderly care wards. Gerontology. 1996:42(2):104-107.
13. Janken J, Reynolds B. Patient falls in the acute care setting: identifying risk factors. Nurs Res.1986;35(4):215-219.
14. Morse JM, Tylko SJ, Dixon HA. Characteristics of the fall-prone patient. Gerontologist. 1987;27(4):516-522.
15. Oliver D, Britton M, Seed P, Martin FC, Hopper AH. Development and evaluation of an evidenced based risk assessment tool (STRATIFY) to predict which elderly outpatients will fall: case-control and cohort studies. BMJ. 1997;315(7115):1049-1053.
16. Passaro A, Volpato S. Benzodiazepenes with different half-life and falling in a hospitalized population: the GIFA study. Gruppo Italiano di Farmacovigilanza nell’Anziano. J Clin Epidemol. 2000;53(12):1222-1229.
17. Salgado R, Lord SR, Packer J, Ehrlich F. Factors associated with falling in elderly hospitalized inpatients. Gerentology. 1994;40(6):325-331.
18. Schmidt NA. 1989 federal nursing service award winner. reducing patient falls: a research-based comprehensive fall prevention program. Mil Med. 1990;155(5):202-207.
19. Sutton JC, Standon PJ, Wallace WA. Patient accidents in hospital: incidence, documentation and significance. Br J Clin Pract. 1994;48(2):63-66.
20. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parental and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738.
21. Russell C, Elia M. Nutrition screening survey in the UK in 2008: hospitals, care homes and mental health units. http://www.bapen.org.uk/pdfs/nsw/nsw_report2008-09.pdf. Published 2009. Accessed December 2, 2016.
22. Cansado P, Ravasco P, Camilo M. A longitudinal study of hospital undernutrition in the elderly: comparison of four validated methods. J Nutr Health Aging. 2009;13(2):159-164.
23. World Health Organization. Nutrition: micronutrient deficiencies. http://www.who.int/nutrition/topics/ida/en. Accessed December 2, 2016.
Falls often result in injuries with devastating outcomes. In the elderly, falls are the largest cause of injury, mortality, and functional decline, leading to 40% of nursing home admissions.1 Nationally, falls with injury are estimated to cost $19 billion in direct medical costs.2 According to the National Quality Forum (NQF), hospital falls resulting in injury are reportable events. Beginning in fiscal year 2015, reportable events labeled as hospital acquired conditions (HAC) are subject to nonpayment, creating increased regulatory and reimbursement pressure on hospitals.3
Due to the major impact of a fall, The Joint Commission (TJC) requires hospitals to assess a patient’s fall risk on admission and whenever the patient’s condition changes.4 Despite decades of research evaluating various predictive strategies to identify individuals at fall risk, nutritional issues as interactive risk factors have received little attention.
A comparative study on the validity of fall risk assessment scales revealed that tools claiming to predict risk factors do not work well.5 Falls are the result of multiple interactive, synergistic pathologies and risk factors. In a multivariate regression study conducted by Lichtenstein and colleagues in Canada, lower body weight was found to be a statistically significant risk factor for falling.6 In 2004, Oliver and colleagues conducted a systematic review of fall risk assessment tools that included validation testing with sufficient data to allow for calculation of sensitivity, specificity, negative and positive predictive values, odds ratios, and confidence intervals.5
Fourteen studies identified common fall risk factors. The majority of these studies identified impaired cognition as a risk factor.5 Of the 14 studies included in the systematic analysis of Oliver and colleagues,6-19 6 identified medications,6,8,11,12,17,18, and 8 noted weakness and unsteady gait as risk factors.6,9-11,14,16,18,19 Only 1 study noted anemia as a risk factor for falls among patients who were post cerebral vascular accident.11 Additionally, only 1 study noted an association between falls resulting in hip fracture and lower body weight.6
One in 4 adults admitted to a hospital is malnourished.20,21 Components of malnutrition, including but not limited to anemia, clinically significant weight loss, and vitamin D deficiency, may be unrecognized interactive risk factors that increase the risk of hospital falls. Malnutrition and dehydration symptoms include fatigue, dizziness, irritability, loss of muscle mass, impulsivity, and the potential for poor judgment. Therefore, it is likely that the severity of specific malnutrition parameters is associated with recurrent falls and possibly injurious falls.
Hunger and inadequate food intake due to chronic disease or chronic food insecurity are real issues for the elderly. Insufficient caloric intake often leads to slow, progressive, and often unnoticed weight loss. The Academy of Nutrition and Dietetics (AND) and the American Association of Parenteral and Enteral Nutrition (ASPEN) have defined clinically significant weight loss categories to aid in the diagnosis of malnutrition. To aid in the diagnosis of various degrees of malnutrition, clinically significant weight loss is classified as ≥ 5% weight loss in 30 days; ≥ 7.5% weight loss in 90 days; > 10% in 180 days.21 The World Health Organization (WHO) identifies iron deficiency measured by hemoglobin (Hgb) value (for men < 13 g/dL) as the most common and widespread nutritional disorder in the world (Tables 1a, 1b, and 1c).22
Eat Well, Fall Less is a retrospective chart review approved by the Louis Stokes Cleveland VAMC (LSCVAMC) Internal Review Board. The review seeks to determine whether degree of weight loss and decline in Hgb and vitamin D deficiency, factors of malnutrition, are present in recurrent fallers vs single-event fallers. Researchers hypothesized that individuals who experienced recurrent falls during hospitalization would demonstrate a greater degree of clinically significant weight loss compared with those who had experienced a single fall. The second hypothesis was that recurrent fallers also would have lower Hgb values than that of single-event fallers. The tertiary hypothesis was that individuals with a greater degree of vitamin D deficiency were more likely to be recurrent fallers compared with single-event fallers.
In addition, dementia has been previously identified as an independent risk factor for falls and recurrent falls.23 During phase 2 of the study, the researchers hypothesized that in individuals with dementia, the concurrent diagnosis of malnutrition would be greatest in the recurrent fall population. A total of 30 subjects were included in the analysis.
Methods
Patient record search of note titles for falls was compiled daily. A random sample of 170 veterans who had experienced a fall was screened for study inclusion. Of the 170 charts, data from a total of 120 veterans who experienced a documented fall during a hospitalization between October 1, 2010 and October 31, 2012, were included in this analysis.
Eligibility Criteria and Baseline Characteristics
Chart reviews of veterans aged > 18 years experiencing a fall while hospitalized at the LSCVAMC between October 1, 2010 and October 31, 2012, were eligible to be included in this study. Each veteran needed to have 1 documented weight in a maximum of 24 months before the first fall and a minimum of 1 documented Hgb value prior to the first fall.
Patients were excluded if they had experienced a cerebrovascular accident or transient ischemic attack; a documented orthopedic fracture in the 12 months before the first fall; a documented amputation of a lower limb in the past 24 months; diagnosis of blindness; a lack of outpatient weight for greater than 24 months before the first fall; a history of volume overload, renal, cardiovascular, or other in nature during hospitalization; and alcoholism. Additionally, if any of the study investigators felt as though a patient had commorientes that made weight history inaccurate, those patients were excluded. Data were reviewed on 170 randomly selected subjects. A total of 50 subjects were excluded for not meeting the inclusion criteria; 120 individuals met eligible criteria. The patients who had experienced falls were divided into 2 groups: single-event fallers (1 fall documented during the study period) and recurrent fallers (2 or more falls documented during the study period). Fifty subjects were excluded from the final analysis because they met the following exclusion criteria: volume overload anytime in the 12 months before the documented fall (42); amputation of a lower limb before the fall (6); and prosthetic device alteration within the 12 months before the fall (3). One of the subjects was eliminated for both volume overload and amputation.
Data Collection
Data obtained from the Computerized Patient Record System review included age, gender, diagnosis, and body weight at the time of the first fall and 1 month, 3 months, 6 months, and 1 year before the first fall. Hemoglobin values at time of the first fall, 1 month, 3 months, 6 months, and 1 year before first documented fall also were collected. Vitamin D values and date of value also were collected. In year 2 of this multiphase retrospective review, charts again were reviewed and additional data collected, which included absence or presence of dementia along with type of dementia and the presence or absence of cancer. Year 2 data collection also included diagnosis of malnutrition by provider and registered dietitian assessment of degree of malnutrition.
Analysis
Data analysis comparing single-event fallers and frequent fallers was performed using IBM SPSS Statistic V22.0s. This pilot study has recognized weight loss and anemia as being associated with repeat falls. A 2-tailed t test was performed to evaluate differences in weight history, 25-hydroxyvitamin D, and Hgb characteristics between single fallers and frequent fallers. A repeated analysis of variance was performed to evaluate changes in weight and Hgb values over time for single and multiple falls. During phase 2 of this trial, a subanalysis comparing individuals with and without the diagnosis of dementia was conducted.
Results
The average age of the patients was 68 years. There was a significant decline in Hgb levels in both single-event fallers and frequent fallers at 12 and 6 months before the first fall event (95% confidence interval; P = .001). One year before the first fall, 28% of eventual fallers met WHO criteria for the diagnosis of mild anemia. One year before the first fall, none of the eventual fallers met WHO criteria for moderate or severe anemia. Using the lab data just before the first fall, 10% of fallers met WHO criteria for mild anemia, 48% met WHO criteria for moderate anemia, and 31% met WHO criteria for severe anemia. The degree of anemia in single-event fallers when compared with multiple-event fallers was insignificant. Interestingly, the degree of decline of Hgb value during the 6 months before the first fall event was notable for all fallers (Figure).
Only 60 of the 120 included patients had a documented vitamin D level. At the time of the fall, 46 patients had moderate vitamin D deficiency, and 23 patients had severe vitamin D deficiency, defined as < 32 mg/dL. The authors speculate that vitamin D status declines with malnutrition and increases fall risk.
Thirty participants were included in the dementia arm; 36.7% were single-event fallers, and 63.3% were multiple-event fallers (Table 2). The average age of the groups was 76.7 years and 73.9 years, respectively. Physician diagnosis of malnutrition was collected for both single- and multiple-event fallers. Of the single-event fallers with dementia, none had a diagnosis of malnutrition before the first hospital fall; and of the multiple-event fallers with dementia, all had a diagnosis of malnutrition before their first hospital fall. Individuals with a diagnosis of dementia and malnutrition fall frequently (P = .0028).
Discussion
In this study, falls occurred in a variety of patient populations. Both single-event fallers and recurrent fallers had a significant drop in Hgb values at 12, 6, and 3 months before the first fall. There was not a strong difference of the Hgb value between single-event falls and multiple fallers in the total population. Anemia was a significant risk factor for all fallers. The decline in the Hgb level before a fall is highly predictive of fall risk.
In individuals with dementia, those with the diagnosis of malnutrition are frequent fallers. A tool to assist in identification of this patient population along with a focused intervention strategy for this population is an area of needed research.
Further research is under way to determine which components of malnutrition diagnosis contribute to fall risk. If so, development of a fall assessment tool, including various components of malnutrition is warranted. Intervention strategies to reduce fall risk may soon include new nutrition and education techniques based on the faller constellation. Falls instruments that explore nutritional risk factors and falls should be investigated (ie, weight loss, vitamin D status, and anemia).
Falls occur in patients with a variety of risk factors (eg, mobility and cognition). The current screening instruments to assess fall risk factors do not sufficiently account for nutritional risk factors. In the Eat Well, Fall Less Study of hospitalized veterans, nutritional risk factors of anemia and weight loss also were associated with single- and multiple-event fallers. The AUTUMN falls instrument that includes critical elements of malnutrition, such as a decline in Hgb and weight loss, is currently being created and is in the process of being validated at LSCVAMC; this tool will incorporate components of malnutrition.
Acknowledgments
The authors acknowledge Michelle Pearson, Laura Guidotti, Adam Weier, Elizabeth Gable, and Shannon Corlett for their research contributions. In memory of Anne Raguso, RD, PhD, for her lifelong focus on nutrition research.
Falls often result in injuries with devastating outcomes. In the elderly, falls are the largest cause of injury, mortality, and functional decline, leading to 40% of nursing home admissions.1 Nationally, falls with injury are estimated to cost $19 billion in direct medical costs.2 According to the National Quality Forum (NQF), hospital falls resulting in injury are reportable events. Beginning in fiscal year 2015, reportable events labeled as hospital acquired conditions (HAC) are subject to nonpayment, creating increased regulatory and reimbursement pressure on hospitals.3
Due to the major impact of a fall, The Joint Commission (TJC) requires hospitals to assess a patient’s fall risk on admission and whenever the patient’s condition changes.4 Despite decades of research evaluating various predictive strategies to identify individuals at fall risk, nutritional issues as interactive risk factors have received little attention.
A comparative study on the validity of fall risk assessment scales revealed that tools claiming to predict risk factors do not work well.5 Falls are the result of multiple interactive, synergistic pathologies and risk factors. In a multivariate regression study conducted by Lichtenstein and colleagues in Canada, lower body weight was found to be a statistically significant risk factor for falling.6 In 2004, Oliver and colleagues conducted a systematic review of fall risk assessment tools that included validation testing with sufficient data to allow for calculation of sensitivity, specificity, negative and positive predictive values, odds ratios, and confidence intervals.5
Fourteen studies identified common fall risk factors. The majority of these studies identified impaired cognition as a risk factor.5 Of the 14 studies included in the systematic analysis of Oliver and colleagues,6-19 6 identified medications,6,8,11,12,17,18, and 8 noted weakness and unsteady gait as risk factors.6,9-11,14,16,18,19 Only 1 study noted anemia as a risk factor for falls among patients who were post cerebral vascular accident.11 Additionally, only 1 study noted an association between falls resulting in hip fracture and lower body weight.6
One in 4 adults admitted to a hospital is malnourished.20,21 Components of malnutrition, including but not limited to anemia, clinically significant weight loss, and vitamin D deficiency, may be unrecognized interactive risk factors that increase the risk of hospital falls. Malnutrition and dehydration symptoms include fatigue, dizziness, irritability, loss of muscle mass, impulsivity, and the potential for poor judgment. Therefore, it is likely that the severity of specific malnutrition parameters is associated with recurrent falls and possibly injurious falls.
Hunger and inadequate food intake due to chronic disease or chronic food insecurity are real issues for the elderly. Insufficient caloric intake often leads to slow, progressive, and often unnoticed weight loss. The Academy of Nutrition and Dietetics (AND) and the American Association of Parenteral and Enteral Nutrition (ASPEN) have defined clinically significant weight loss categories to aid in the diagnosis of malnutrition. To aid in the diagnosis of various degrees of malnutrition, clinically significant weight loss is classified as ≥ 5% weight loss in 30 days; ≥ 7.5% weight loss in 90 days; > 10% in 180 days.21 The World Health Organization (WHO) identifies iron deficiency measured by hemoglobin (Hgb) value (for men < 13 g/dL) as the most common and widespread nutritional disorder in the world (Tables 1a, 1b, and 1c).22
Eat Well, Fall Less is a retrospective chart review approved by the Louis Stokes Cleveland VAMC (LSCVAMC) Internal Review Board. The review seeks to determine whether degree of weight loss and decline in Hgb and vitamin D deficiency, factors of malnutrition, are present in recurrent fallers vs single-event fallers. Researchers hypothesized that individuals who experienced recurrent falls during hospitalization would demonstrate a greater degree of clinically significant weight loss compared with those who had experienced a single fall. The second hypothesis was that recurrent fallers also would have lower Hgb values than that of single-event fallers. The tertiary hypothesis was that individuals with a greater degree of vitamin D deficiency were more likely to be recurrent fallers compared with single-event fallers.
In addition, dementia has been previously identified as an independent risk factor for falls and recurrent falls.23 During phase 2 of the study, the researchers hypothesized that in individuals with dementia, the concurrent diagnosis of malnutrition would be greatest in the recurrent fall population. A total of 30 subjects were included in the analysis.
Methods
Patient record search of note titles for falls was compiled daily. A random sample of 170 veterans who had experienced a fall was screened for study inclusion. Of the 170 charts, data from a total of 120 veterans who experienced a documented fall during a hospitalization between October 1, 2010 and October 31, 2012, were included in this analysis.
Eligibility Criteria and Baseline Characteristics
Chart reviews of veterans aged > 18 years experiencing a fall while hospitalized at the LSCVAMC between October 1, 2010 and October 31, 2012, were eligible to be included in this study. Each veteran needed to have 1 documented weight in a maximum of 24 months before the first fall and a minimum of 1 documented Hgb value prior to the first fall.
Patients were excluded if they had experienced a cerebrovascular accident or transient ischemic attack; a documented orthopedic fracture in the 12 months before the first fall; a documented amputation of a lower limb in the past 24 months; diagnosis of blindness; a lack of outpatient weight for greater than 24 months before the first fall; a history of volume overload, renal, cardiovascular, or other in nature during hospitalization; and alcoholism. Additionally, if any of the study investigators felt as though a patient had commorientes that made weight history inaccurate, those patients were excluded. Data were reviewed on 170 randomly selected subjects. A total of 50 subjects were excluded for not meeting the inclusion criteria; 120 individuals met eligible criteria. The patients who had experienced falls were divided into 2 groups: single-event fallers (1 fall documented during the study period) and recurrent fallers (2 or more falls documented during the study period). Fifty subjects were excluded from the final analysis because they met the following exclusion criteria: volume overload anytime in the 12 months before the documented fall (42); amputation of a lower limb before the fall (6); and prosthetic device alteration within the 12 months before the fall (3). One of the subjects was eliminated for both volume overload and amputation.
Data Collection
Data obtained from the Computerized Patient Record System review included age, gender, diagnosis, and body weight at the time of the first fall and 1 month, 3 months, 6 months, and 1 year before the first fall. Hemoglobin values at time of the first fall, 1 month, 3 months, 6 months, and 1 year before first documented fall also were collected. Vitamin D values and date of value also were collected. In year 2 of this multiphase retrospective review, charts again were reviewed and additional data collected, which included absence or presence of dementia along with type of dementia and the presence or absence of cancer. Year 2 data collection also included diagnosis of malnutrition by provider and registered dietitian assessment of degree of malnutrition.
Analysis
Data analysis comparing single-event fallers and frequent fallers was performed using IBM SPSS Statistic V22.0s. This pilot study has recognized weight loss and anemia as being associated with repeat falls. A 2-tailed t test was performed to evaluate differences in weight history, 25-hydroxyvitamin D, and Hgb characteristics between single fallers and frequent fallers. A repeated analysis of variance was performed to evaluate changes in weight and Hgb values over time for single and multiple falls. During phase 2 of this trial, a subanalysis comparing individuals with and without the diagnosis of dementia was conducted.
Results
The average age of the patients was 68 years. There was a significant decline in Hgb levels in both single-event fallers and frequent fallers at 12 and 6 months before the first fall event (95% confidence interval; P = .001). One year before the first fall, 28% of eventual fallers met WHO criteria for the diagnosis of mild anemia. One year before the first fall, none of the eventual fallers met WHO criteria for moderate or severe anemia. Using the lab data just before the first fall, 10% of fallers met WHO criteria for mild anemia, 48% met WHO criteria for moderate anemia, and 31% met WHO criteria for severe anemia. The degree of anemia in single-event fallers when compared with multiple-event fallers was insignificant. Interestingly, the degree of decline of Hgb value during the 6 months before the first fall event was notable for all fallers (Figure).
Only 60 of the 120 included patients had a documented vitamin D level. At the time of the fall, 46 patients had moderate vitamin D deficiency, and 23 patients had severe vitamin D deficiency, defined as < 32 mg/dL. The authors speculate that vitamin D status declines with malnutrition and increases fall risk.
Thirty participants were included in the dementia arm; 36.7% were single-event fallers, and 63.3% were multiple-event fallers (Table 2). The average age of the groups was 76.7 years and 73.9 years, respectively. Physician diagnosis of malnutrition was collected for both single- and multiple-event fallers. Of the single-event fallers with dementia, none had a diagnosis of malnutrition before the first hospital fall; and of the multiple-event fallers with dementia, all had a diagnosis of malnutrition before their first hospital fall. Individuals with a diagnosis of dementia and malnutrition fall frequently (P = .0028).
Discussion
In this study, falls occurred in a variety of patient populations. Both single-event fallers and recurrent fallers had a significant drop in Hgb values at 12, 6, and 3 months before the first fall. There was not a strong difference of the Hgb value between single-event falls and multiple fallers in the total population. Anemia was a significant risk factor for all fallers. The decline in the Hgb level before a fall is highly predictive of fall risk.
In individuals with dementia, those with the diagnosis of malnutrition are frequent fallers. A tool to assist in identification of this patient population along with a focused intervention strategy for this population is an area of needed research.
Further research is under way to determine which components of malnutrition diagnosis contribute to fall risk. If so, development of a fall assessment tool, including various components of malnutrition is warranted. Intervention strategies to reduce fall risk may soon include new nutrition and education techniques based on the faller constellation. Falls instruments that explore nutritional risk factors and falls should be investigated (ie, weight loss, vitamin D status, and anemia).
Falls occur in patients with a variety of risk factors (eg, mobility and cognition). The current screening instruments to assess fall risk factors do not sufficiently account for nutritional risk factors. In the Eat Well, Fall Less Study of hospitalized veterans, nutritional risk factors of anemia and weight loss also were associated with single- and multiple-event fallers. The AUTUMN falls instrument that includes critical elements of malnutrition, such as a decline in Hgb and weight loss, is currently being created and is in the process of being validated at LSCVAMC; this tool will incorporate components of malnutrition.
Acknowledgments
The authors acknowledge Michelle Pearson, Laura Guidotti, Adam Weier, Elizabeth Gable, and Shannon Corlett for their research contributions. In memory of Anne Raguso, RD, PhD, for her lifelong focus on nutrition research.
1. Centers for Disease Control and Prevention. Older adult falls: important facts about falls. http://www.cdc.gov/homeandrecreationalsafety/falls/adultfalls.html. Updated September 20, 2016. Accessed December 2, 2016.
2. Centers for Disease Control and Prevention. Older adult falls: cost of falls among older adults. http://www.cdc.gov/HomeandRecreationalSafety/Falls/fallcost.html. Updated August 19, 2016. Accessed December 2, 2016.
3. National Quality Forum. Serious reportable events in healthcare—2011 update: a consensus report https://www.qualityforum.org/Publications/2011/12/SRE_2011_Final_Report.aspx. Published 2011. Accessed December 2, 2016.
4. DuPree E. Taking a stand against falls. https://www.jointcommission.org/jc_physician_blog/taking_a_stand_against_falls. Published May1, 2014. Accessed December 2, 2016.
5. Oliver D, Daly F, Martin FC, McMurdo ME. Risk factors and risk assessment tools for falls in hospital in-patients: a systematic review. Age Ageing. 2004;33(2):122-130.
6. Lichtenstein MJ, Griffin MR, Cornell JE, Malcolm E, Ray WA. Risk factors for hip fractures occurring in the hospital. Am J Epidemiol. 1994;140(9):830-838.
7. Ballinger BR, Ramsay AC. Accidents and drug treatment in a psychiatric hospital. Br J Psychiatry. 1975;126:462-463.
8. Bates D, Pruess K, Souney P, Platt R. Serious falls in hospitalized patients correlates and resource utilization. Am J Med. 1995;99(2):137-143.
9. Byers V, Arrington ME, Finstuen K. Predictive risk factors associated with stroke patient falls in acute care settings. J Neurosci Nurs. 1990;22(3):147-154.
10. Chu LW, Pei CK, Chiu A, et al. Risk factors for falls in hospitalized older medical patients. J Gerontol A Biol Sci Med Sci. 1999;54(1):M38-M48.
11. Gales BJ, Menard SM. Relationship between administration of selected medications and falls in hospitalized elderly patients. Ann Pharmacother. 1995;29(4):354-358.
12. Gluck T, Wientjes HJ, Rai GS. An evaluation of risk factors for inpatient falls in acute care and rehabilitation elderly care wards. Gerontology. 1996:42(2):104-107.
13. Janken J, Reynolds B. Patient falls in the acute care setting: identifying risk factors. Nurs Res.1986;35(4):215-219.
14. Morse JM, Tylko SJ, Dixon HA. Characteristics of the fall-prone patient. Gerontologist. 1987;27(4):516-522.
15. Oliver D, Britton M, Seed P, Martin FC, Hopper AH. Development and evaluation of an evidenced based risk assessment tool (STRATIFY) to predict which elderly outpatients will fall: case-control and cohort studies. BMJ. 1997;315(7115):1049-1053.
16. Passaro A, Volpato S. Benzodiazepenes with different half-life and falling in a hospitalized population: the GIFA study. Gruppo Italiano di Farmacovigilanza nell’Anziano. J Clin Epidemol. 2000;53(12):1222-1229.
17. Salgado R, Lord SR, Packer J, Ehrlich F. Factors associated with falling in elderly hospitalized inpatients. Gerentology. 1994;40(6):325-331.
18. Schmidt NA. 1989 federal nursing service award winner. reducing patient falls: a research-based comprehensive fall prevention program. Mil Med. 1990;155(5):202-207.
19. Sutton JC, Standon PJ, Wallace WA. Patient accidents in hospital: incidence, documentation and significance. Br J Clin Pract. 1994;48(2):63-66.
20. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parental and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738.
21. Russell C, Elia M. Nutrition screening survey in the UK in 2008: hospitals, care homes and mental health units. http://www.bapen.org.uk/pdfs/nsw/nsw_report2008-09.pdf. Published 2009. Accessed December 2, 2016.
22. Cansado P, Ravasco P, Camilo M. A longitudinal study of hospital undernutrition in the elderly: comparison of four validated methods. J Nutr Health Aging. 2009;13(2):159-164.
23. World Health Organization. Nutrition: micronutrient deficiencies. http://www.who.int/nutrition/topics/ida/en. Accessed December 2, 2016.
1. Centers for Disease Control and Prevention. Older adult falls: important facts about falls. http://www.cdc.gov/homeandrecreationalsafety/falls/adultfalls.html. Updated September 20, 2016. Accessed December 2, 2016.
2. Centers for Disease Control and Prevention. Older adult falls: cost of falls among older adults. http://www.cdc.gov/HomeandRecreationalSafety/Falls/fallcost.html. Updated August 19, 2016. Accessed December 2, 2016.
3. National Quality Forum. Serious reportable events in healthcare—2011 update: a consensus report https://www.qualityforum.org/Publications/2011/12/SRE_2011_Final_Report.aspx. Published 2011. Accessed December 2, 2016.
4. DuPree E. Taking a stand against falls. https://www.jointcommission.org/jc_physician_blog/taking_a_stand_against_falls. Published May1, 2014. Accessed December 2, 2016.
5. Oliver D, Daly F, Martin FC, McMurdo ME. Risk factors and risk assessment tools for falls in hospital in-patients: a systematic review. Age Ageing. 2004;33(2):122-130.
6. Lichtenstein MJ, Griffin MR, Cornell JE, Malcolm E, Ray WA. Risk factors for hip fractures occurring in the hospital. Am J Epidemiol. 1994;140(9):830-838.
7. Ballinger BR, Ramsay AC. Accidents and drug treatment in a psychiatric hospital. Br J Psychiatry. 1975;126:462-463.
8. Bates D, Pruess K, Souney P, Platt R. Serious falls in hospitalized patients correlates and resource utilization. Am J Med. 1995;99(2):137-143.
9. Byers V, Arrington ME, Finstuen K. Predictive risk factors associated with stroke patient falls in acute care settings. J Neurosci Nurs. 1990;22(3):147-154.
10. Chu LW, Pei CK, Chiu A, et al. Risk factors for falls in hospitalized older medical patients. J Gerontol A Biol Sci Med Sci. 1999;54(1):M38-M48.
11. Gales BJ, Menard SM. Relationship between administration of selected medications and falls in hospitalized elderly patients. Ann Pharmacother. 1995;29(4):354-358.
12. Gluck T, Wientjes HJ, Rai GS. An evaluation of risk factors for inpatient falls in acute care and rehabilitation elderly care wards. Gerontology. 1996:42(2):104-107.
13. Janken J, Reynolds B. Patient falls in the acute care setting: identifying risk factors. Nurs Res.1986;35(4):215-219.
14. Morse JM, Tylko SJ, Dixon HA. Characteristics of the fall-prone patient. Gerontologist. 1987;27(4):516-522.
15. Oliver D, Britton M, Seed P, Martin FC, Hopper AH. Development and evaluation of an evidenced based risk assessment tool (STRATIFY) to predict which elderly outpatients will fall: case-control and cohort studies. BMJ. 1997;315(7115):1049-1053.
16. Passaro A, Volpato S. Benzodiazepenes with different half-life and falling in a hospitalized population: the GIFA study. Gruppo Italiano di Farmacovigilanza nell’Anziano. J Clin Epidemol. 2000;53(12):1222-1229.
17. Salgado R, Lord SR, Packer J, Ehrlich F. Factors associated with falling in elderly hospitalized inpatients. Gerentology. 1994;40(6):325-331.
18. Schmidt NA. 1989 federal nursing service award winner. reducing patient falls: a research-based comprehensive fall prevention program. Mil Med. 1990;155(5):202-207.
19. Sutton JC, Standon PJ, Wallace WA. Patient accidents in hospital: incidence, documentation and significance. Br J Clin Pract. 1994;48(2):63-66.
20. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parental and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738.
21. Russell C, Elia M. Nutrition screening survey in the UK in 2008: hospitals, care homes and mental health units. http://www.bapen.org.uk/pdfs/nsw/nsw_report2008-09.pdf. Published 2009. Accessed December 2, 2016.
22. Cansado P, Ravasco P, Camilo M. A longitudinal study of hospital undernutrition in the elderly: comparison of four validated methods. J Nutr Health Aging. 2009;13(2):159-164.
23. World Health Organization. Nutrition: micronutrient deficiencies. http://www.who.int/nutrition/topics/ida/en. Accessed December 2, 2016.
Simulation Training, Coaching, and Cue Cards Improve Delirium Care
Many clinicians continue to think that delirium or acute confusion is inevitable, untreatable, and harmless. However, nothing could be further from the truth.1 Delirium is a common and costly clinical syndrome that plagues a large percentage of older adults in a variety of settings. Numerous clinical studies conducted over the past 20 years have validated a high incidence of delirium in acute care hospitals.2-5 Reported rates of delirium among veteran and nonveteran populations vary widely, from 20% to 80%, but even these rates may reflect underrecognition and underreporting.6
Veterans with delirium pose a unique challenge to clinicians and health care systems because they often concurrently experience dementia, depression, posttraumatic stress disorder, and delirium. This complex syndrome caused by a myriad of environmental, physiologic, and psychological factors has been associated with profoundly poor clinical outcomes, including increased institutionalization, hospital length of stay, medication use, restraint use, falls, and mortality.3,7,8
Financial costs associated with delirium have been estimated at between $38 billion and $152 billion per year.9 In addition, this syndrome is costly in human resource expenditures, including increased burden on family members and the need for additional care providers, such as “sitters.” Families and clinicians report increased burden and stress in their interactions with these patients.10-12 Mounting evidence exists that some people with delirium never return to baseline cognitive function after even a single episode of delirium.1,8 Unfortunately, many clinicians do not recognize the seriousness of acute confusion.
Clinical practices related to routine screening for delirium vary widely. Although the Confusion Assessment Method (CAM) screening tool has high sensitivity and specificity, only 17% of hospitals consistently use this tool in clinical practice.2,6,13,14 According to a survey by Ely and colleagues, physicians reported being aware of delirium but inconsistently applying treatment protocols in clinical practice.2 Nurses noted similar difficulties in consistently screening patients and using delirium management protocols.15 Given the high incidence of delirium and its associated morbidity, including long-term cognitive impairment and human and financial costs, there is an urgent need to implement programs that enhance delirium prevention, timely recognition, and effective management to improve patient outcomes and address caregiver burden.
Over the past decade, educational strategies for improving delirium prevention, recognition, and management have included didactic education, consultation, and use of protocols.2,3,5,16 Bedside mentoring, implementation of protocols, and other interventions have been proposed as well.16,17 Several program models, including consultation by psychiatrists or psychiatric advanced practice nurses, have been implemented to increase detection and treatment of delirium.2,3,5,15 These pilot programs have been successful to varying degrees but in general have not shown independent effects beyond intervention or significantly increased recognition and management of adverse consequences for most patients. The cause of these outcomes seem to be multifactorial, but the complexity of the syndrome is part of the problem.18-20
Other possible barriers to change regarding delirium-related issues in clinical practice are lack of knowledge and skills and individual attitudes.20-22 Continuing evidence exists that clinicians feel ill-prepared to help delirious patients and frustrated enough to resort to using restraints and medication as first-line treatment.17 Yanamadala and colleagues reviewed 26 studies that identified strategies for delirium education.23 Most of the studies reported on didactic teaching methods that included information on resources. Only 1 study with nursing students reported using actors for simulation training. The programs most successful in improving knowledge, skills, attitudes, practice changes, and patient outcomes seemed to be those that used multiple educational methods, including information dissemination, use of guidelines and protocols, and peer and expert feedback.
This finding is consistent with the report that didactic learning alone, though improving competency, is less likely to change behavior or improve outcomes.24 A constellation of didactic education, mentoring, use of protocols to target high-risk patients, and a therapeutic environment has helped to reduce delirium incidence.4,25,26 Rudolph and colleagues found an association between multimodal education (risk assessment, sensory improvement, sleep promotion) and shorter hospital stays and less use of restraints.27 In clinical practice,however, implementation of evidence-based nonpharmacologic interventions, such as enhanced communication, mobility, nutrition, and meaningful activities, continues to lag despite education.28,29
Multimodal Education
To address these gaps in knowledge and skills, a multidisciplinary delirium resource team at the Louis Stokes Cleveland VAMC in Ohio developed a multimodal educational program incorporating simulation. The team of physicians, nurses, care coordinators, and social workers met regularly and developed interventions, educational materials, cue cards (eFigure 1), sense-enhancing aids (hearing amplifiers, puzzles, books, music CDs, prism glasses), clinical protocols, and delirium resources, such as CARES (Confusion Assessment Resource Enhancement Supplies) activity carts.
The CARES carts are small, rolling wooden carts stocked with various resources that focus on comfort and entertainment. The carts hold guided imagery CDs and Playaways (small audio players that come with ear buds for individual use and preloaded with a specific guided imagery session). The carts also hold books, books on tape, magazines, portable CD players, music CDs, games, exercise bands, healthful snacks, DVDs, and a portable DVD player.
Bedside mentoring continued throughout this quality improvement (QI) project, and a CARES teaching tool kit was developed. This kit, which continues to be used, includes videos and webinars for professionals and family caregivers; delirium pocket cue cards for physicians, nurses, aides, and sitters; a list of patient diversion supplies; and a family brochure. Delirium resource team members continue to provide the health care team with education and support. Given the emphasis on clinicians and patient outcomes in intensive care units (ICUs), the teaching tool kit is a valuable guide for assessing and treating patients with delirium during rounds and consultations.
Simulation
Using a simulation center and standardized patients (SPs), teams of interdisciplinary care providers practiced communication techniques and recommended treatment strategies with the help of a delirium coach. Sessions were videotaped. This intervention, which used simulation training, was supported by VA grant T-21, to reduce institutionalization and promote patient-centered care.
In a clinical context, simulation involves activities that mimick the reality of the clinical environment, including physical symptoms, communication patterns, and critical decision making. Trained SPs have the unique advantage of providing interactive practice and immediate feedback in a safe, controlled setting.30
Standardized patient programs provide learners with real-life interactions for the development and practice of interpersonal communication and clinical skills. In a laboratory setting, SP programs use role-play scenarios that allow learners to practice complex assessment and communication skills. Standardized patients are effective in teaching clinical, interviewing, and communication skills to learners from a variety of disciplines, including medicine, nursing, dental, and law.31 Standardized patients also provide a safe, supportive environment conducive to learning and standardized assessment.
Standardized patients can serve as practice models and participate in sophisticated assessment and feedback of learners’ abilities and services. Interacting with SPs gives learners a chance to practice clinical and interpersonal skills with an emphasis on communication before meeting actual patients. After interacting with an SP, a learner receives feedback from a preceptor and/or the learner’s peers. The SP also may be asked to provide brief feedback—a component of the SP training process. Allowing time for feedback is an integral part of student learning.
For this QI project, the delirium team used the Mt. Sinai Skills and Simulation Center at Case Western Reserve University School of Medicine. The facility focuses on creative, innovative continuing learning for health care providers at all levels and is certified by the American College of Surgeons as a level 1 Comprehensive Accredited Education Institute.
After a literature review and several brainstorming sessions, the delirium team tailored case studies to veterans to simulate the intervention and train SPs for the delirium program. During training, SPs reviewed scenarios, engaged in practice sessions, and answered questions. Several SPs were familiar with the behavior of delirious patients from personal experience.
The goals of the program were to increase knowledge of delirium signs and symptoms as a medical condition that requires immediate attention; increase competency in administering CAM and in documenting its results; increase interdisciplinary communication; and increase knowledge using nonpharmacologic interventions for sensory enhancement and agitation. Enhanced interdisciplinary communication was accomplished during the simulation by assigning individuals from different disciplines to work in teams. To maximize the use of resources and limit participants’ time away from the clinical area, the administration planned and supported a daylong program that included didactic education, videos, group work sessions, and the simulation sessions with resource team members as coaches.
Methods
All participants attended an hour-long introductory didactic lecture together. Then, they were randomly assigned to 1 of 4 remaining 45-minute training sessions. Each participant attended a session that combined a video and a case study; a session of role-playing with group discussion; and 2 simulation scenario sessions. Concurrent training sessions were needed to facilitate having all 100 attendees participate within 6 hours. Attendees were multidisciplinary providers from various non-ICU medical/surgical units and outpatient geriatric clinics. They rotated among sessions to accommodate all participants.
For the simulation scenarios, 8 simulation rooms were used over 2 periods—for 16 simulation sessions total. Participants were randomly assigned to multidisciplinary groups that worked in teams to assess and recommend care and treatments for SPs who were stimulating delirium. During the simulation, delirium coaches used cue cards and verbal hints to direct teams (Simulation Exercise). After the session, participants received verbal and
Outcomes
The impact of this multimodal intervention was measured in a variety of ways—with preintervention and postintervention knowledge tests, postsimulation surveys, program surveys, and patient chart reviews. Simulation sessions had 100 attendees, including mentors (interdisciplinary resource team), champions, and nursing staff from various hospital units. Champions represented multiple disciplines and had varying levels of experience. Most of the participants were nurses (62%), followed by social workers (12%), nursing assistants (12%), psychologists (6%), and others (11%). Participants’ years of experience were < 1 year (6%), 1 to 5 years (21%), 6 to 10 years (21%), and > 10 years (52%).
Mean knowledge survey score was 84% before training and 92% after training. Recognition of delirium as a medical emergency requiring immediate follow-up was increased (P = .02), as was knowledge about delirium management, as in increasing daytime activities (P < .001) and using distraction techniques (P = .03) (Table).
More than 94% of participants said the simulation training fulfilled their education needs. More than 80% reported using the information from the delirium workshop in their practice. In reviewing the techniques presented during the workshop, participants reported that they would approach situations differently from before the workshop by using more nonpharmacologic interventions (40%), enhanced communication (24%), and more in-depth assessment for medical causes of delirium (19%). Thirteen percent said they would not change their approach.
Thirty-five percent of respondents had positive feelings after the simulation exercise, 40% had cared for patients in similar situations, and 35% knew delirium care should start with assessment for medical causes.
The team reviewed patient charts for documentation of confusion assessment (signs and symptoms of confusion), including the standardized CAM method and nonpharmacologic interventions. Random monthly audits, 1 month before training and 5 months after, indicated an increase in confusion assessment and documentation. For veterans with delirium, nonpharmacologic interventions increased from 9% at baseline to 53% at the 5-month audit. Hospital length of stay, however, trended toward a slight increase in number of days. These findings are consistent with those reported by Rudolph and colleagues, who also piloted multimodal education and sensory enhancement.27
Discussion
Delirium assessment and management are complex skills that require well-coordinated interdisciplinary care and significant administrative support. Clinicians are becoming increasingly aware of the mounting evidence that patients with delirium feel immediate and often long-term negative effects. Strategies that support clinicians and enhance clinical care must include multimodal education and support.
In this QI project, participants supported use of simulation education, bedside coaching, and pocket cue cards to enhance delirium caregiving knowledge and skills. The majority of participants indicated that, though the simulation sessions were challenging, they also were realistic and helpful. Standardized patients provided feedback and often advised teams of needed improvement, such as spending more time in helping patients feel safe and comfortable. Coaches noted that many team members collaborated with one another but often neglected to use the pocket cue cards, family brochures, and other resources in the room. The reason is not clear. Perhaps the novelty of the resources and potential participant anxiety during the simulation were contributing factors. In future sessions, coaches must address making use of available resources.
Chart reviews indicated that nonpharmacologic management of delirium increased to 53% from < 10%. The increased use of resources in caring for patients with delirium was confirmed by the need to restock the CARES activity carts with patient diversion supplies. Given the success of this first program, another ICU education and simulation program was initiated. Findings from this QI project support using multimodal education that incorporates simulation training, bedside coaching, and pocket cue cards to enhance clinical practices for care of patients with delirium.33
Methods facilitated team collaboration, patient family communication, and synthesis of much information over a relatively short period. Didactic education alone may be insufficient to adequately enhance clinical care for delirium. The impact of a multimodal strategy—including a delirium resource consultation team that provided bedside mentoring, encouraged use of pocket cue cards, and supported evidence-based nonpharmacologic interventions—cannot be underestimated. In addition, simulation education also provided a unique opportunity for the health care teams to “practice” assessment, communication, and collaboration skills in a supportive setting with real-time feedback. These resources are being disseminated throughout the Louis Stokes Cleveland VAMC. Plans to disseminate this information to a broader national audience are under way.
Although not all facilities can access the simulation laboratory, many may be able to implement use of videos, case studies, and role-playing to enhance didactic education, to improve outcomes for patients with delirium. Enhanced clinical management of complex syndromes such as delirium may be influenced most by a combination of education, practice, and mentoring methods. Use of simulation as an adjunct teaching method is a promising strategy that may enhance care of patients with delirium. This QI project demonstrated positive educational and clinical trends in a VA setting. More studies, including randomized clinical trials, are needed in a variety of settings to further test these strategies.
Acknowledgments
The authors give special thanks to Brigid Wilson, PhD, Heather O’Leary, RN, and Patrick Kilroy, SN, for their assistance in this project. The project was developed by the Cleveland VAMC interdisciplinary delirium resource team with support of a VA T-21 grant and the VISN 10 Geriatric Research Education and Clinical Centers.
1. ICU Delirium and Cognitive Impairment Study Group, Center for Health Services Research, Vanderbilt University Medical Center. Delirium prevention and safety: starting with the ABCDEF’s. http://www.icudelirium.org/medicalprofessionals .html. Accessed November 9, 2016.
2. Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: an under-recognized syndrome of organ dysfunction. Semin Respir Crit Care Med. 2001;22(2):115-126.
3. Inouye SK, Bogardus ST Jr, Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70-83.
4. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522.
5. Foreman MD, Wakefield B, Culp K, Milisen K. Delirium in elderly patients: an overview of the state of the science. J Gerontol Nurs. 2001;27(4):12-20.
6. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948.
7. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856.
8. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451.
9. Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32.
10. Bruera E, Bush SH, Willey J, et al. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer. 2009;115(9):2004-2012.
11. Cohen MZ, Pace EA, Kaur G, Bruera E. Delirium in advanced cancer leading to distress in patients and family caregivers. J Palliat Care. 2009;25(3):164-171.
12. Patel RP, Gambrell M, Speroff T, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009;37(3):825-832.
13. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
14. Neuman MD, Speck RM, Karlawish JH, Schwartz JS, Shea JA. Hospital protocols for the inpatient care of older adults: results from a statewide survey. J Am Geriatr Soc. 2010;58(10):1959-1964.
15. Idemoto BK. The assessment of delirium and depression in the intensive care unit [doctoral dissertation]. Cleveland, OH: Case Western Reserve University; 2005. No. 137.
16. Balas MC, Vasilevskis EE, Burke WJ, et al. Critical care nurses’ role in implementing the “ABCDE bundle” into practice. Crit Care Nurse. 2012;32(2):35-38, 40-47.
17. Barr J, Fraser GL, Puntillo K, et al; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit: executive summary. Am J Health Syst Pharm. 2013;70(1):53-58.
18. Pun BT, Ely EW. The importance of diagnosing and managing ICU delirium. Chest. 2007;132(2):624-636.
19. Pisani MA, Araujo KL, Van Ness PH, Zhang Y, Ely EW, Inouye SK. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10(4):R121.
20. Voyer P, Cole MG, McCusker J, St-Jacques S, Laplante J. Accuracy of nurse documentation of delirium symptoms in medical charts. Int J Nurs Pract. 2008;14(2):165-167.
21. Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34-41.
22. Steis MR, Fick DM. Are nurses recognizing delirium? A systematic review. J Gerontol Nurs. 2008;34(9):40-48.
23. Yanamadala M, Wieland D, Heflin MT. Educational interventions to improve recognition of delirium: a systematic review. J Am Geriatr Soc. 2013;61(11):1983-1993.
24. Ramaswamy R, Dix EF, Drew JE, Diamond JJ, Inouye SK, Roehl BJ. Beyond grand rounds: a comprehensive and sequential intervention to improve identification of delirium. Gerontologist. 2011;51(1):122-131.
25. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007;19(3):178-186.
26. Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord. 1999;10(5):393-400.
27. Rudolph JL, Archambault E, Kelly B; VA Boston Delirium Task Force. A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e7-e11.
28. Hartford Institute for Geriatric Nursing. http://hartfordign.org. Accessed November 9, 2016.
29. Inouye SK. A practical program for preventing delirium in hospitalized elderly patients. Cleve Clin J Med. 2004;71(11):890-896.
30. Raurell-Torredà M, Olivet-Pujol J, Romero-Collado À, Malagon-Aguilera MC, Patiño-Masó J, Baltasar-Bagué A. Case-based learning and simulation: useful tools to enhance nurses’ education? Nonrandomized controlled trial. J Nurs Scholarsh. 2015;47(1):34-42.
31. Mast TA, Coulson LR, Meyer TC, et al. Self directed learning: wisdom from independent study programs. Res Med Educ. 1985;24:305-311.
32. Massachusetts Department of Higher Education Nursing Initiative—Simulation Scenario Library. http://www.mass.edu/nahi/Sim/Welcome.asp. Accessed November 1, 2016.
33. Vancouver Island Health Authority. Delirium in the older person: a medical emergency. Pre-Post Test. http://www.viha.ca/NR/rdonlyres/03D50FE6-AF90 -4F98-8B3B-7AE87A4B481D/0/preposttest.pdf. Reviewed August 2014. Accessed November 9, 2016.
Many clinicians continue to think that delirium or acute confusion is inevitable, untreatable, and harmless. However, nothing could be further from the truth.1 Delirium is a common and costly clinical syndrome that plagues a large percentage of older adults in a variety of settings. Numerous clinical studies conducted over the past 20 years have validated a high incidence of delirium in acute care hospitals.2-5 Reported rates of delirium among veteran and nonveteran populations vary widely, from 20% to 80%, but even these rates may reflect underrecognition and underreporting.6
Veterans with delirium pose a unique challenge to clinicians and health care systems because they often concurrently experience dementia, depression, posttraumatic stress disorder, and delirium. This complex syndrome caused by a myriad of environmental, physiologic, and psychological factors has been associated with profoundly poor clinical outcomes, including increased institutionalization, hospital length of stay, medication use, restraint use, falls, and mortality.3,7,8
Financial costs associated with delirium have been estimated at between $38 billion and $152 billion per year.9 In addition, this syndrome is costly in human resource expenditures, including increased burden on family members and the need for additional care providers, such as “sitters.” Families and clinicians report increased burden and stress in their interactions with these patients.10-12 Mounting evidence exists that some people with delirium never return to baseline cognitive function after even a single episode of delirium.1,8 Unfortunately, many clinicians do not recognize the seriousness of acute confusion.
Clinical practices related to routine screening for delirium vary widely. Although the Confusion Assessment Method (CAM) screening tool has high sensitivity and specificity, only 17% of hospitals consistently use this tool in clinical practice.2,6,13,14 According to a survey by Ely and colleagues, physicians reported being aware of delirium but inconsistently applying treatment protocols in clinical practice.2 Nurses noted similar difficulties in consistently screening patients and using delirium management protocols.15 Given the high incidence of delirium and its associated morbidity, including long-term cognitive impairment and human and financial costs, there is an urgent need to implement programs that enhance delirium prevention, timely recognition, and effective management to improve patient outcomes and address caregiver burden.
Over the past decade, educational strategies for improving delirium prevention, recognition, and management have included didactic education, consultation, and use of protocols.2,3,5,16 Bedside mentoring, implementation of protocols, and other interventions have been proposed as well.16,17 Several program models, including consultation by psychiatrists or psychiatric advanced practice nurses, have been implemented to increase detection and treatment of delirium.2,3,5,15 These pilot programs have been successful to varying degrees but in general have not shown independent effects beyond intervention or significantly increased recognition and management of adverse consequences for most patients. The cause of these outcomes seem to be multifactorial, but the complexity of the syndrome is part of the problem.18-20
Other possible barriers to change regarding delirium-related issues in clinical practice are lack of knowledge and skills and individual attitudes.20-22 Continuing evidence exists that clinicians feel ill-prepared to help delirious patients and frustrated enough to resort to using restraints and medication as first-line treatment.17 Yanamadala and colleagues reviewed 26 studies that identified strategies for delirium education.23 Most of the studies reported on didactic teaching methods that included information on resources. Only 1 study with nursing students reported using actors for simulation training. The programs most successful in improving knowledge, skills, attitudes, practice changes, and patient outcomes seemed to be those that used multiple educational methods, including information dissemination, use of guidelines and protocols, and peer and expert feedback.
This finding is consistent with the report that didactic learning alone, though improving competency, is less likely to change behavior or improve outcomes.24 A constellation of didactic education, mentoring, use of protocols to target high-risk patients, and a therapeutic environment has helped to reduce delirium incidence.4,25,26 Rudolph and colleagues found an association between multimodal education (risk assessment, sensory improvement, sleep promotion) and shorter hospital stays and less use of restraints.27 In clinical practice,however, implementation of evidence-based nonpharmacologic interventions, such as enhanced communication, mobility, nutrition, and meaningful activities, continues to lag despite education.28,29
Multimodal Education
To address these gaps in knowledge and skills, a multidisciplinary delirium resource team at the Louis Stokes Cleveland VAMC in Ohio developed a multimodal educational program incorporating simulation. The team of physicians, nurses, care coordinators, and social workers met regularly and developed interventions, educational materials, cue cards (eFigure 1), sense-enhancing aids (hearing amplifiers, puzzles, books, music CDs, prism glasses), clinical protocols, and delirium resources, such as CARES (Confusion Assessment Resource Enhancement Supplies) activity carts.
The CARES carts are small, rolling wooden carts stocked with various resources that focus on comfort and entertainment. The carts hold guided imagery CDs and Playaways (small audio players that come with ear buds for individual use and preloaded with a specific guided imagery session). The carts also hold books, books on tape, magazines, portable CD players, music CDs, games, exercise bands, healthful snacks, DVDs, and a portable DVD player.
Bedside mentoring continued throughout this quality improvement (QI) project, and a CARES teaching tool kit was developed. This kit, which continues to be used, includes videos and webinars for professionals and family caregivers; delirium pocket cue cards for physicians, nurses, aides, and sitters; a list of patient diversion supplies; and a family brochure. Delirium resource team members continue to provide the health care team with education and support. Given the emphasis on clinicians and patient outcomes in intensive care units (ICUs), the teaching tool kit is a valuable guide for assessing and treating patients with delirium during rounds and consultations.
Simulation
Using a simulation center and standardized patients (SPs), teams of interdisciplinary care providers practiced communication techniques and recommended treatment strategies with the help of a delirium coach. Sessions were videotaped. This intervention, which used simulation training, was supported by VA grant T-21, to reduce institutionalization and promote patient-centered care.
In a clinical context, simulation involves activities that mimick the reality of the clinical environment, including physical symptoms, communication patterns, and critical decision making. Trained SPs have the unique advantage of providing interactive practice and immediate feedback in a safe, controlled setting.30
Standardized patient programs provide learners with real-life interactions for the development and practice of interpersonal communication and clinical skills. In a laboratory setting, SP programs use role-play scenarios that allow learners to practice complex assessment and communication skills. Standardized patients are effective in teaching clinical, interviewing, and communication skills to learners from a variety of disciplines, including medicine, nursing, dental, and law.31 Standardized patients also provide a safe, supportive environment conducive to learning and standardized assessment.
Standardized patients can serve as practice models and participate in sophisticated assessment and feedback of learners’ abilities and services. Interacting with SPs gives learners a chance to practice clinical and interpersonal skills with an emphasis on communication before meeting actual patients. After interacting with an SP, a learner receives feedback from a preceptor and/or the learner’s peers. The SP also may be asked to provide brief feedback—a component of the SP training process. Allowing time for feedback is an integral part of student learning.
For this QI project, the delirium team used the Mt. Sinai Skills and Simulation Center at Case Western Reserve University School of Medicine. The facility focuses on creative, innovative continuing learning for health care providers at all levels and is certified by the American College of Surgeons as a level 1 Comprehensive Accredited Education Institute.
After a literature review and several brainstorming sessions, the delirium team tailored case studies to veterans to simulate the intervention and train SPs for the delirium program. During training, SPs reviewed scenarios, engaged in practice sessions, and answered questions. Several SPs were familiar with the behavior of delirious patients from personal experience.
The goals of the program were to increase knowledge of delirium signs and symptoms as a medical condition that requires immediate attention; increase competency in administering CAM and in documenting its results; increase interdisciplinary communication; and increase knowledge using nonpharmacologic interventions for sensory enhancement and agitation. Enhanced interdisciplinary communication was accomplished during the simulation by assigning individuals from different disciplines to work in teams. To maximize the use of resources and limit participants’ time away from the clinical area, the administration planned and supported a daylong program that included didactic education, videos, group work sessions, and the simulation sessions with resource team members as coaches.
Methods
All participants attended an hour-long introductory didactic lecture together. Then, they were randomly assigned to 1 of 4 remaining 45-minute training sessions. Each participant attended a session that combined a video and a case study; a session of role-playing with group discussion; and 2 simulation scenario sessions. Concurrent training sessions were needed to facilitate having all 100 attendees participate within 6 hours. Attendees were multidisciplinary providers from various non-ICU medical/surgical units and outpatient geriatric clinics. They rotated among sessions to accommodate all participants.
For the simulation scenarios, 8 simulation rooms were used over 2 periods—for 16 simulation sessions total. Participants were randomly assigned to multidisciplinary groups that worked in teams to assess and recommend care and treatments for SPs who were stimulating delirium. During the simulation, delirium coaches used cue cards and verbal hints to direct teams (Simulation Exercise). After the session, participants received verbal and
Outcomes
The impact of this multimodal intervention was measured in a variety of ways—with preintervention and postintervention knowledge tests, postsimulation surveys, program surveys, and patient chart reviews. Simulation sessions had 100 attendees, including mentors (interdisciplinary resource team), champions, and nursing staff from various hospital units. Champions represented multiple disciplines and had varying levels of experience. Most of the participants were nurses (62%), followed by social workers (12%), nursing assistants (12%), psychologists (6%), and others (11%). Participants’ years of experience were < 1 year (6%), 1 to 5 years (21%), 6 to 10 years (21%), and > 10 years (52%).
Mean knowledge survey score was 84% before training and 92% after training. Recognition of delirium as a medical emergency requiring immediate follow-up was increased (P = .02), as was knowledge about delirium management, as in increasing daytime activities (P < .001) and using distraction techniques (P = .03) (Table).
More than 94% of participants said the simulation training fulfilled their education needs. More than 80% reported using the information from the delirium workshop in their practice. In reviewing the techniques presented during the workshop, participants reported that they would approach situations differently from before the workshop by using more nonpharmacologic interventions (40%), enhanced communication (24%), and more in-depth assessment for medical causes of delirium (19%). Thirteen percent said they would not change their approach.
Thirty-five percent of respondents had positive feelings after the simulation exercise, 40% had cared for patients in similar situations, and 35% knew delirium care should start with assessment for medical causes.
The team reviewed patient charts for documentation of confusion assessment (signs and symptoms of confusion), including the standardized CAM method and nonpharmacologic interventions. Random monthly audits, 1 month before training and 5 months after, indicated an increase in confusion assessment and documentation. For veterans with delirium, nonpharmacologic interventions increased from 9% at baseline to 53% at the 5-month audit. Hospital length of stay, however, trended toward a slight increase in number of days. These findings are consistent with those reported by Rudolph and colleagues, who also piloted multimodal education and sensory enhancement.27
Discussion
Delirium assessment and management are complex skills that require well-coordinated interdisciplinary care and significant administrative support. Clinicians are becoming increasingly aware of the mounting evidence that patients with delirium feel immediate and often long-term negative effects. Strategies that support clinicians and enhance clinical care must include multimodal education and support.
In this QI project, participants supported use of simulation education, bedside coaching, and pocket cue cards to enhance delirium caregiving knowledge and skills. The majority of participants indicated that, though the simulation sessions were challenging, they also were realistic and helpful. Standardized patients provided feedback and often advised teams of needed improvement, such as spending more time in helping patients feel safe and comfortable. Coaches noted that many team members collaborated with one another but often neglected to use the pocket cue cards, family brochures, and other resources in the room. The reason is not clear. Perhaps the novelty of the resources and potential participant anxiety during the simulation were contributing factors. In future sessions, coaches must address making use of available resources.
Chart reviews indicated that nonpharmacologic management of delirium increased to 53% from < 10%. The increased use of resources in caring for patients with delirium was confirmed by the need to restock the CARES activity carts with patient diversion supplies. Given the success of this first program, another ICU education and simulation program was initiated. Findings from this QI project support using multimodal education that incorporates simulation training, bedside coaching, and pocket cue cards to enhance clinical practices for care of patients with delirium.33
Methods facilitated team collaboration, patient family communication, and synthesis of much information over a relatively short period. Didactic education alone may be insufficient to adequately enhance clinical care for delirium. The impact of a multimodal strategy—including a delirium resource consultation team that provided bedside mentoring, encouraged use of pocket cue cards, and supported evidence-based nonpharmacologic interventions—cannot be underestimated. In addition, simulation education also provided a unique opportunity for the health care teams to “practice” assessment, communication, and collaboration skills in a supportive setting with real-time feedback. These resources are being disseminated throughout the Louis Stokes Cleveland VAMC. Plans to disseminate this information to a broader national audience are under way.
Although not all facilities can access the simulation laboratory, many may be able to implement use of videos, case studies, and role-playing to enhance didactic education, to improve outcomes for patients with delirium. Enhanced clinical management of complex syndromes such as delirium may be influenced most by a combination of education, practice, and mentoring methods. Use of simulation as an adjunct teaching method is a promising strategy that may enhance care of patients with delirium. This QI project demonstrated positive educational and clinical trends in a VA setting. More studies, including randomized clinical trials, are needed in a variety of settings to further test these strategies.
Acknowledgments
The authors give special thanks to Brigid Wilson, PhD, Heather O’Leary, RN, and Patrick Kilroy, SN, for their assistance in this project. The project was developed by the Cleveland VAMC interdisciplinary delirium resource team with support of a VA T-21 grant and the VISN 10 Geriatric Research Education and Clinical Centers.
Many clinicians continue to think that delirium or acute confusion is inevitable, untreatable, and harmless. However, nothing could be further from the truth.1 Delirium is a common and costly clinical syndrome that plagues a large percentage of older adults in a variety of settings. Numerous clinical studies conducted over the past 20 years have validated a high incidence of delirium in acute care hospitals.2-5 Reported rates of delirium among veteran and nonveteran populations vary widely, from 20% to 80%, but even these rates may reflect underrecognition and underreporting.6
Veterans with delirium pose a unique challenge to clinicians and health care systems because they often concurrently experience dementia, depression, posttraumatic stress disorder, and delirium. This complex syndrome caused by a myriad of environmental, physiologic, and psychological factors has been associated with profoundly poor clinical outcomes, including increased institutionalization, hospital length of stay, medication use, restraint use, falls, and mortality.3,7,8
Financial costs associated with delirium have been estimated at between $38 billion and $152 billion per year.9 In addition, this syndrome is costly in human resource expenditures, including increased burden on family members and the need for additional care providers, such as “sitters.” Families and clinicians report increased burden and stress in their interactions with these patients.10-12 Mounting evidence exists that some people with delirium never return to baseline cognitive function after even a single episode of delirium.1,8 Unfortunately, many clinicians do not recognize the seriousness of acute confusion.
Clinical practices related to routine screening for delirium vary widely. Although the Confusion Assessment Method (CAM) screening tool has high sensitivity and specificity, only 17% of hospitals consistently use this tool in clinical practice.2,6,13,14 According to a survey by Ely and colleagues, physicians reported being aware of delirium but inconsistently applying treatment protocols in clinical practice.2 Nurses noted similar difficulties in consistently screening patients and using delirium management protocols.15 Given the high incidence of delirium and its associated morbidity, including long-term cognitive impairment and human and financial costs, there is an urgent need to implement programs that enhance delirium prevention, timely recognition, and effective management to improve patient outcomes and address caregiver burden.
Over the past decade, educational strategies for improving delirium prevention, recognition, and management have included didactic education, consultation, and use of protocols.2,3,5,16 Bedside mentoring, implementation of protocols, and other interventions have been proposed as well.16,17 Several program models, including consultation by psychiatrists or psychiatric advanced practice nurses, have been implemented to increase detection and treatment of delirium.2,3,5,15 These pilot programs have been successful to varying degrees but in general have not shown independent effects beyond intervention or significantly increased recognition and management of adverse consequences for most patients. The cause of these outcomes seem to be multifactorial, but the complexity of the syndrome is part of the problem.18-20
Other possible barriers to change regarding delirium-related issues in clinical practice are lack of knowledge and skills and individual attitudes.20-22 Continuing evidence exists that clinicians feel ill-prepared to help delirious patients and frustrated enough to resort to using restraints and medication as first-line treatment.17 Yanamadala and colleagues reviewed 26 studies that identified strategies for delirium education.23 Most of the studies reported on didactic teaching methods that included information on resources. Only 1 study with nursing students reported using actors for simulation training. The programs most successful in improving knowledge, skills, attitudes, practice changes, and patient outcomes seemed to be those that used multiple educational methods, including information dissemination, use of guidelines and protocols, and peer and expert feedback.
This finding is consistent with the report that didactic learning alone, though improving competency, is less likely to change behavior or improve outcomes.24 A constellation of didactic education, mentoring, use of protocols to target high-risk patients, and a therapeutic environment has helped to reduce delirium incidence.4,25,26 Rudolph and colleagues found an association between multimodal education (risk assessment, sensory improvement, sleep promotion) and shorter hospital stays and less use of restraints.27 In clinical practice,however, implementation of evidence-based nonpharmacologic interventions, such as enhanced communication, mobility, nutrition, and meaningful activities, continues to lag despite education.28,29
Multimodal Education
To address these gaps in knowledge and skills, a multidisciplinary delirium resource team at the Louis Stokes Cleveland VAMC in Ohio developed a multimodal educational program incorporating simulation. The team of physicians, nurses, care coordinators, and social workers met regularly and developed interventions, educational materials, cue cards (eFigure 1), sense-enhancing aids (hearing amplifiers, puzzles, books, music CDs, prism glasses), clinical protocols, and delirium resources, such as CARES (Confusion Assessment Resource Enhancement Supplies) activity carts.
The CARES carts are small, rolling wooden carts stocked with various resources that focus on comfort and entertainment. The carts hold guided imagery CDs and Playaways (small audio players that come with ear buds for individual use and preloaded with a specific guided imagery session). The carts also hold books, books on tape, magazines, portable CD players, music CDs, games, exercise bands, healthful snacks, DVDs, and a portable DVD player.
Bedside mentoring continued throughout this quality improvement (QI) project, and a CARES teaching tool kit was developed. This kit, which continues to be used, includes videos and webinars for professionals and family caregivers; delirium pocket cue cards for physicians, nurses, aides, and sitters; a list of patient diversion supplies; and a family brochure. Delirium resource team members continue to provide the health care team with education and support. Given the emphasis on clinicians and patient outcomes in intensive care units (ICUs), the teaching tool kit is a valuable guide for assessing and treating patients with delirium during rounds and consultations.
Simulation
Using a simulation center and standardized patients (SPs), teams of interdisciplinary care providers practiced communication techniques and recommended treatment strategies with the help of a delirium coach. Sessions were videotaped. This intervention, which used simulation training, was supported by VA grant T-21, to reduce institutionalization and promote patient-centered care.
In a clinical context, simulation involves activities that mimick the reality of the clinical environment, including physical symptoms, communication patterns, and critical decision making. Trained SPs have the unique advantage of providing interactive practice and immediate feedback in a safe, controlled setting.30
Standardized patient programs provide learners with real-life interactions for the development and practice of interpersonal communication and clinical skills. In a laboratory setting, SP programs use role-play scenarios that allow learners to practice complex assessment and communication skills. Standardized patients are effective in teaching clinical, interviewing, and communication skills to learners from a variety of disciplines, including medicine, nursing, dental, and law.31 Standardized patients also provide a safe, supportive environment conducive to learning and standardized assessment.
Standardized patients can serve as practice models and participate in sophisticated assessment and feedback of learners’ abilities and services. Interacting with SPs gives learners a chance to practice clinical and interpersonal skills with an emphasis on communication before meeting actual patients. After interacting with an SP, a learner receives feedback from a preceptor and/or the learner’s peers. The SP also may be asked to provide brief feedback—a component of the SP training process. Allowing time for feedback is an integral part of student learning.
For this QI project, the delirium team used the Mt. Sinai Skills and Simulation Center at Case Western Reserve University School of Medicine. The facility focuses on creative, innovative continuing learning for health care providers at all levels and is certified by the American College of Surgeons as a level 1 Comprehensive Accredited Education Institute.
After a literature review and several brainstorming sessions, the delirium team tailored case studies to veterans to simulate the intervention and train SPs for the delirium program. During training, SPs reviewed scenarios, engaged in practice sessions, and answered questions. Several SPs were familiar with the behavior of delirious patients from personal experience.
The goals of the program were to increase knowledge of delirium signs and symptoms as a medical condition that requires immediate attention; increase competency in administering CAM and in documenting its results; increase interdisciplinary communication; and increase knowledge using nonpharmacologic interventions for sensory enhancement and agitation. Enhanced interdisciplinary communication was accomplished during the simulation by assigning individuals from different disciplines to work in teams. To maximize the use of resources and limit participants’ time away from the clinical area, the administration planned and supported a daylong program that included didactic education, videos, group work sessions, and the simulation sessions with resource team members as coaches.
Methods
All participants attended an hour-long introductory didactic lecture together. Then, they were randomly assigned to 1 of 4 remaining 45-minute training sessions. Each participant attended a session that combined a video and a case study; a session of role-playing with group discussion; and 2 simulation scenario sessions. Concurrent training sessions were needed to facilitate having all 100 attendees participate within 6 hours. Attendees were multidisciplinary providers from various non-ICU medical/surgical units and outpatient geriatric clinics. They rotated among sessions to accommodate all participants.
For the simulation scenarios, 8 simulation rooms were used over 2 periods—for 16 simulation sessions total. Participants were randomly assigned to multidisciplinary groups that worked in teams to assess and recommend care and treatments for SPs who were stimulating delirium. During the simulation, delirium coaches used cue cards and verbal hints to direct teams (Simulation Exercise). After the session, participants received verbal and
Outcomes
The impact of this multimodal intervention was measured in a variety of ways—with preintervention and postintervention knowledge tests, postsimulation surveys, program surveys, and patient chart reviews. Simulation sessions had 100 attendees, including mentors (interdisciplinary resource team), champions, and nursing staff from various hospital units. Champions represented multiple disciplines and had varying levels of experience. Most of the participants were nurses (62%), followed by social workers (12%), nursing assistants (12%), psychologists (6%), and others (11%). Participants’ years of experience were < 1 year (6%), 1 to 5 years (21%), 6 to 10 years (21%), and > 10 years (52%).
Mean knowledge survey score was 84% before training and 92% after training. Recognition of delirium as a medical emergency requiring immediate follow-up was increased (P = .02), as was knowledge about delirium management, as in increasing daytime activities (P < .001) and using distraction techniques (P = .03) (Table).
More than 94% of participants said the simulation training fulfilled their education needs. More than 80% reported using the information from the delirium workshop in their practice. In reviewing the techniques presented during the workshop, participants reported that they would approach situations differently from before the workshop by using more nonpharmacologic interventions (40%), enhanced communication (24%), and more in-depth assessment for medical causes of delirium (19%). Thirteen percent said they would not change their approach.
Thirty-five percent of respondents had positive feelings after the simulation exercise, 40% had cared for patients in similar situations, and 35% knew delirium care should start with assessment for medical causes.
The team reviewed patient charts for documentation of confusion assessment (signs and symptoms of confusion), including the standardized CAM method and nonpharmacologic interventions. Random monthly audits, 1 month before training and 5 months after, indicated an increase in confusion assessment and documentation. For veterans with delirium, nonpharmacologic interventions increased from 9% at baseline to 53% at the 5-month audit. Hospital length of stay, however, trended toward a slight increase in number of days. These findings are consistent with those reported by Rudolph and colleagues, who also piloted multimodal education and sensory enhancement.27
Discussion
Delirium assessment and management are complex skills that require well-coordinated interdisciplinary care and significant administrative support. Clinicians are becoming increasingly aware of the mounting evidence that patients with delirium feel immediate and often long-term negative effects. Strategies that support clinicians and enhance clinical care must include multimodal education and support.
In this QI project, participants supported use of simulation education, bedside coaching, and pocket cue cards to enhance delirium caregiving knowledge and skills. The majority of participants indicated that, though the simulation sessions were challenging, they also were realistic and helpful. Standardized patients provided feedback and often advised teams of needed improvement, such as spending more time in helping patients feel safe and comfortable. Coaches noted that many team members collaborated with one another but often neglected to use the pocket cue cards, family brochures, and other resources in the room. The reason is not clear. Perhaps the novelty of the resources and potential participant anxiety during the simulation were contributing factors. In future sessions, coaches must address making use of available resources.
Chart reviews indicated that nonpharmacologic management of delirium increased to 53% from < 10%. The increased use of resources in caring for patients with delirium was confirmed by the need to restock the CARES activity carts with patient diversion supplies. Given the success of this first program, another ICU education and simulation program was initiated. Findings from this QI project support using multimodal education that incorporates simulation training, bedside coaching, and pocket cue cards to enhance clinical practices for care of patients with delirium.33
Methods facilitated team collaboration, patient family communication, and synthesis of much information over a relatively short period. Didactic education alone may be insufficient to adequately enhance clinical care for delirium. The impact of a multimodal strategy—including a delirium resource consultation team that provided bedside mentoring, encouraged use of pocket cue cards, and supported evidence-based nonpharmacologic interventions—cannot be underestimated. In addition, simulation education also provided a unique opportunity for the health care teams to “practice” assessment, communication, and collaboration skills in a supportive setting with real-time feedback. These resources are being disseminated throughout the Louis Stokes Cleveland VAMC. Plans to disseminate this information to a broader national audience are under way.
Although not all facilities can access the simulation laboratory, many may be able to implement use of videos, case studies, and role-playing to enhance didactic education, to improve outcomes for patients with delirium. Enhanced clinical management of complex syndromes such as delirium may be influenced most by a combination of education, practice, and mentoring methods. Use of simulation as an adjunct teaching method is a promising strategy that may enhance care of patients with delirium. This QI project demonstrated positive educational and clinical trends in a VA setting. More studies, including randomized clinical trials, are needed in a variety of settings to further test these strategies.
Acknowledgments
The authors give special thanks to Brigid Wilson, PhD, Heather O’Leary, RN, and Patrick Kilroy, SN, for their assistance in this project. The project was developed by the Cleveland VAMC interdisciplinary delirium resource team with support of a VA T-21 grant and the VISN 10 Geriatric Research Education and Clinical Centers.
1. ICU Delirium and Cognitive Impairment Study Group, Center for Health Services Research, Vanderbilt University Medical Center. Delirium prevention and safety: starting with the ABCDEF’s. http://www.icudelirium.org/medicalprofessionals .html. Accessed November 9, 2016.
2. Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: an under-recognized syndrome of organ dysfunction. Semin Respir Crit Care Med. 2001;22(2):115-126.
3. Inouye SK, Bogardus ST Jr, Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70-83.
4. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522.
5. Foreman MD, Wakefield B, Culp K, Milisen K. Delirium in elderly patients: an overview of the state of the science. J Gerontol Nurs. 2001;27(4):12-20.
6. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948.
7. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856.
8. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451.
9. Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32.
10. Bruera E, Bush SH, Willey J, et al. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer. 2009;115(9):2004-2012.
11. Cohen MZ, Pace EA, Kaur G, Bruera E. Delirium in advanced cancer leading to distress in patients and family caregivers. J Palliat Care. 2009;25(3):164-171.
12. Patel RP, Gambrell M, Speroff T, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009;37(3):825-832.
13. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
14. Neuman MD, Speck RM, Karlawish JH, Schwartz JS, Shea JA. Hospital protocols for the inpatient care of older adults: results from a statewide survey. J Am Geriatr Soc. 2010;58(10):1959-1964.
15. Idemoto BK. The assessment of delirium and depression in the intensive care unit [doctoral dissertation]. Cleveland, OH: Case Western Reserve University; 2005. No. 137.
16. Balas MC, Vasilevskis EE, Burke WJ, et al. Critical care nurses’ role in implementing the “ABCDE bundle” into practice. Crit Care Nurse. 2012;32(2):35-38, 40-47.
17. Barr J, Fraser GL, Puntillo K, et al; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit: executive summary. Am J Health Syst Pharm. 2013;70(1):53-58.
18. Pun BT, Ely EW. The importance of diagnosing and managing ICU delirium. Chest. 2007;132(2):624-636.
19. Pisani MA, Araujo KL, Van Ness PH, Zhang Y, Ely EW, Inouye SK. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10(4):R121.
20. Voyer P, Cole MG, McCusker J, St-Jacques S, Laplante J. Accuracy of nurse documentation of delirium symptoms in medical charts. Int J Nurs Pract. 2008;14(2):165-167.
21. Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34-41.
22. Steis MR, Fick DM. Are nurses recognizing delirium? A systematic review. J Gerontol Nurs. 2008;34(9):40-48.
23. Yanamadala M, Wieland D, Heflin MT. Educational interventions to improve recognition of delirium: a systematic review. J Am Geriatr Soc. 2013;61(11):1983-1993.
24. Ramaswamy R, Dix EF, Drew JE, Diamond JJ, Inouye SK, Roehl BJ. Beyond grand rounds: a comprehensive and sequential intervention to improve identification of delirium. Gerontologist. 2011;51(1):122-131.
25. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007;19(3):178-186.
26. Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord. 1999;10(5):393-400.
27. Rudolph JL, Archambault E, Kelly B; VA Boston Delirium Task Force. A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e7-e11.
28. Hartford Institute for Geriatric Nursing. http://hartfordign.org. Accessed November 9, 2016.
29. Inouye SK. A practical program for preventing delirium in hospitalized elderly patients. Cleve Clin J Med. 2004;71(11):890-896.
30. Raurell-Torredà M, Olivet-Pujol J, Romero-Collado À, Malagon-Aguilera MC, Patiño-Masó J, Baltasar-Bagué A. Case-based learning and simulation: useful tools to enhance nurses’ education? Nonrandomized controlled trial. J Nurs Scholarsh. 2015;47(1):34-42.
31. Mast TA, Coulson LR, Meyer TC, et al. Self directed learning: wisdom from independent study programs. Res Med Educ. 1985;24:305-311.
32. Massachusetts Department of Higher Education Nursing Initiative—Simulation Scenario Library. http://www.mass.edu/nahi/Sim/Welcome.asp. Accessed November 1, 2016.
33. Vancouver Island Health Authority. Delirium in the older person: a medical emergency. Pre-Post Test. http://www.viha.ca/NR/rdonlyres/03D50FE6-AF90 -4F98-8B3B-7AE87A4B481D/0/preposttest.pdf. Reviewed August 2014. Accessed November 9, 2016.
1. ICU Delirium and Cognitive Impairment Study Group, Center for Health Services Research, Vanderbilt University Medical Center. Delirium prevention and safety: starting with the ABCDEF’s. http://www.icudelirium.org/medicalprofessionals .html. Accessed November 9, 2016.
2. Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: an under-recognized syndrome of organ dysfunction. Semin Respir Crit Care Med. 2001;22(2):115-126.
3. Inouye SK, Bogardus ST Jr, Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70-83.
4. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522.
5. Foreman MD, Wakefield B, Culp K, Milisen K. Delirium in elderly patients: an overview of the state of the science. J Gerontol Nurs. 2001;27(4):12-20.
6. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948.
7. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856.
8. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451.
9. Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32.
10. Bruera E, Bush SH, Willey J, et al. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer. 2009;115(9):2004-2012.
11. Cohen MZ, Pace EA, Kaur G, Bruera E. Delirium in advanced cancer leading to distress in patients and family caregivers. J Palliat Care. 2009;25(3):164-171.
12. Patel RP, Gambrell M, Speroff T, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009;37(3):825-832.
13. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
14. Neuman MD, Speck RM, Karlawish JH, Schwartz JS, Shea JA. Hospital protocols for the inpatient care of older adults: results from a statewide survey. J Am Geriatr Soc. 2010;58(10):1959-1964.
15. Idemoto BK. The assessment of delirium and depression in the intensive care unit [doctoral dissertation]. Cleveland, OH: Case Western Reserve University; 2005. No. 137.
16. Balas MC, Vasilevskis EE, Burke WJ, et al. Critical care nurses’ role in implementing the “ABCDE bundle” into practice. Crit Care Nurse. 2012;32(2):35-38, 40-47.
17. Barr J, Fraser GL, Puntillo K, et al; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit: executive summary. Am J Health Syst Pharm. 2013;70(1):53-58.
18. Pun BT, Ely EW. The importance of diagnosing and managing ICU delirium. Chest. 2007;132(2):624-636.
19. Pisani MA, Araujo KL, Van Ness PH, Zhang Y, Ely EW, Inouye SK. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10(4):R121.
20. Voyer P, Cole MG, McCusker J, St-Jacques S, Laplante J. Accuracy of nurse documentation of delirium symptoms in medical charts. Int J Nurs Pract. 2008;14(2):165-167.
21. Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34-41.
22. Steis MR, Fick DM. Are nurses recognizing delirium? A systematic review. J Gerontol Nurs. 2008;34(9):40-48.
23. Yanamadala M, Wieland D, Heflin MT. Educational interventions to improve recognition of delirium: a systematic review. J Am Geriatr Soc. 2013;61(11):1983-1993.
24. Ramaswamy R, Dix EF, Drew JE, Diamond JJ, Inouye SK, Roehl BJ. Beyond grand rounds: a comprehensive and sequential intervention to improve identification of delirium. Gerontologist. 2011;51(1):122-131.
25. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007;19(3):178-186.
26. Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord. 1999;10(5):393-400.
27. Rudolph JL, Archambault E, Kelly B; VA Boston Delirium Task Force. A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e7-e11.
28. Hartford Institute for Geriatric Nursing. http://hartfordign.org. Accessed November 9, 2016.
29. Inouye SK. A practical program for preventing delirium in hospitalized elderly patients. Cleve Clin J Med. 2004;71(11):890-896.
30. Raurell-Torredà M, Olivet-Pujol J, Romero-Collado À, Malagon-Aguilera MC, Patiño-Masó J, Baltasar-Bagué A. Case-based learning and simulation: useful tools to enhance nurses’ education? Nonrandomized controlled trial. J Nurs Scholarsh. 2015;47(1):34-42.
31. Mast TA, Coulson LR, Meyer TC, et al. Self directed learning: wisdom from independent study programs. Res Med Educ. 1985;24:305-311.
32. Massachusetts Department of Higher Education Nursing Initiative—Simulation Scenario Library. http://www.mass.edu/nahi/Sim/Welcome.asp. Accessed November 1, 2016.
33. Vancouver Island Health Authority. Delirium in the older person: a medical emergency. Pre-Post Test. http://www.viha.ca/NR/rdonlyres/03D50FE6-AF90 -4F98-8B3B-7AE87A4B481D/0/preposttest.pdf. Reviewed August 2014. Accessed November 9, 2016.