User login
Ticking Time: Spreading Awareness About African Tick-Bite Fever
To the Editor:
One of the more common tick-borne infections seen in travelers returning from sub-Saharan Africa is caused by Rickettsia africae, which is the etiologic agent of African tick-bite fever (ATBF), the most common tick-borne bacterial zoonosis.1 There are 2 known tick vectors of disease: Amblyomma variegatum, found in sub-Saharan Africa and the West Indies, and Amblyomma hebraeum, found specifically in southern Africa.2,3
Unlike other disease-carrying ticks that passively wait on vegetation to be picked up by a host, A hebraeum uniquely attract other nearby ticks to the host. Studies have shown that male ticks feeding on a nonhuman host (usually cattle) can emit an aggression-attachment pheromone that attracts other ticks to the host. The presence of the pheromone allows unfed ticks to actively discriminate between hosts on which these parasites have fed successfully (ie, suitable hosts) and those on which they have not.4
The aggressive hunting nature of A hebraeum explains the clinical presentation of multiple eschars and why large groups of exposed travelers—such as soldiers, leisure safari tourists, game hunters, and foreign-aid workers—are affected.2
Another southern African spotted fever group, Rickettsia conorii is the causative agent of Mediterranean spotted fever (MSF). Ticks carrying R conorii exhibit a much less aggressive hunting stylethan A hebraeum; consequently, infected patients present with a single eschar site.5
Rickettsia africae is estimated to have very high prevalence (95.2%) in Amblyomma ticks and a fairly high prevalence (approximately 4.0% to 8.6%) in travelers coming from rural southern Africa,6,7 with an incubation period of 5 to 10 days after inoculation by an infected tick.8 Signs include fever, a generalized maculopapular or papulovesicular rash, and regional lymphadenopathy; symptoms include fatigue, headache, and myalgia.
The inoculation eschar—single or multiple—commonly presents on the legs and is accompanied by tender lymphadenopathy of draining nodes1,8 More severe findings, such as myocarditis and subacute neuropathy, have been reported in elderly patients.9
A 77-year-old man presented with a pruritic maculopapular and papulovesicular rash distributed over the upper and lower extremities of 3 weeks’ duration. The patient reported having been on a 12-day mission trip to Limpopo, South Africa, where he was constructing and installing safe toilets to replace dangerous toilet pits. He believed he had been bitten by 2 ticks, after which he noted a dark purple and black patch on the left lower leg by the third day of the trip. He developed a sudden persistent pruritic rash, first on the lower extremities and then spreading to the upper extremities. The patient was seen by an American physician in South Africa who gave him a 7-day course of oral doxycycline monohydrate 100 mg twice daily. He then returned to the United States.
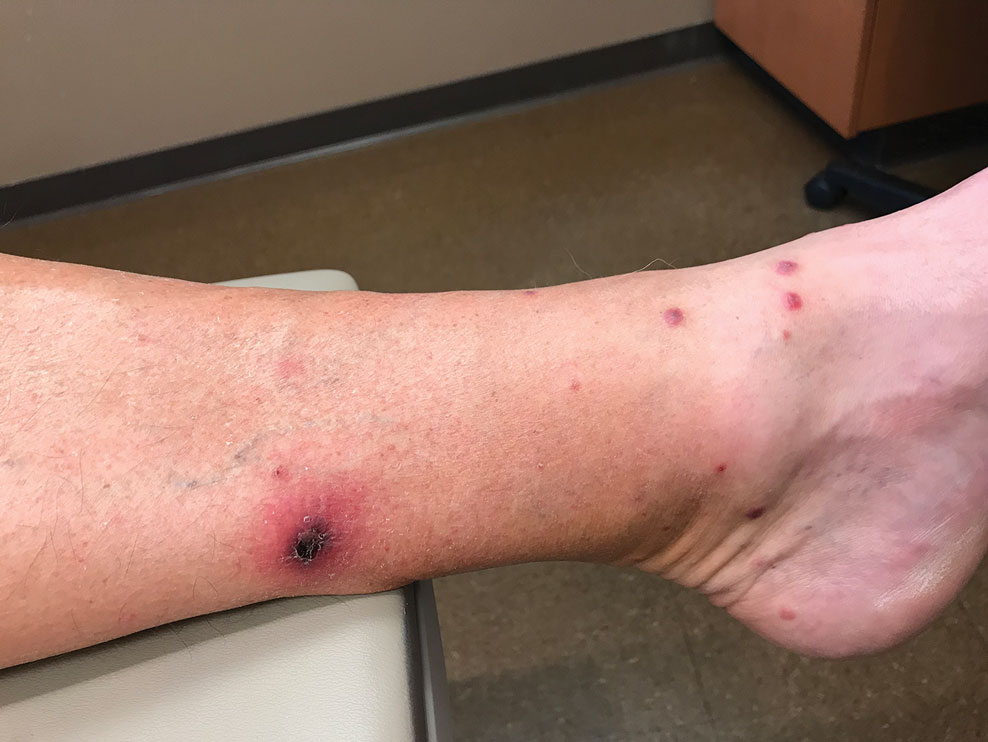
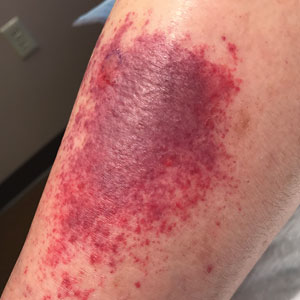
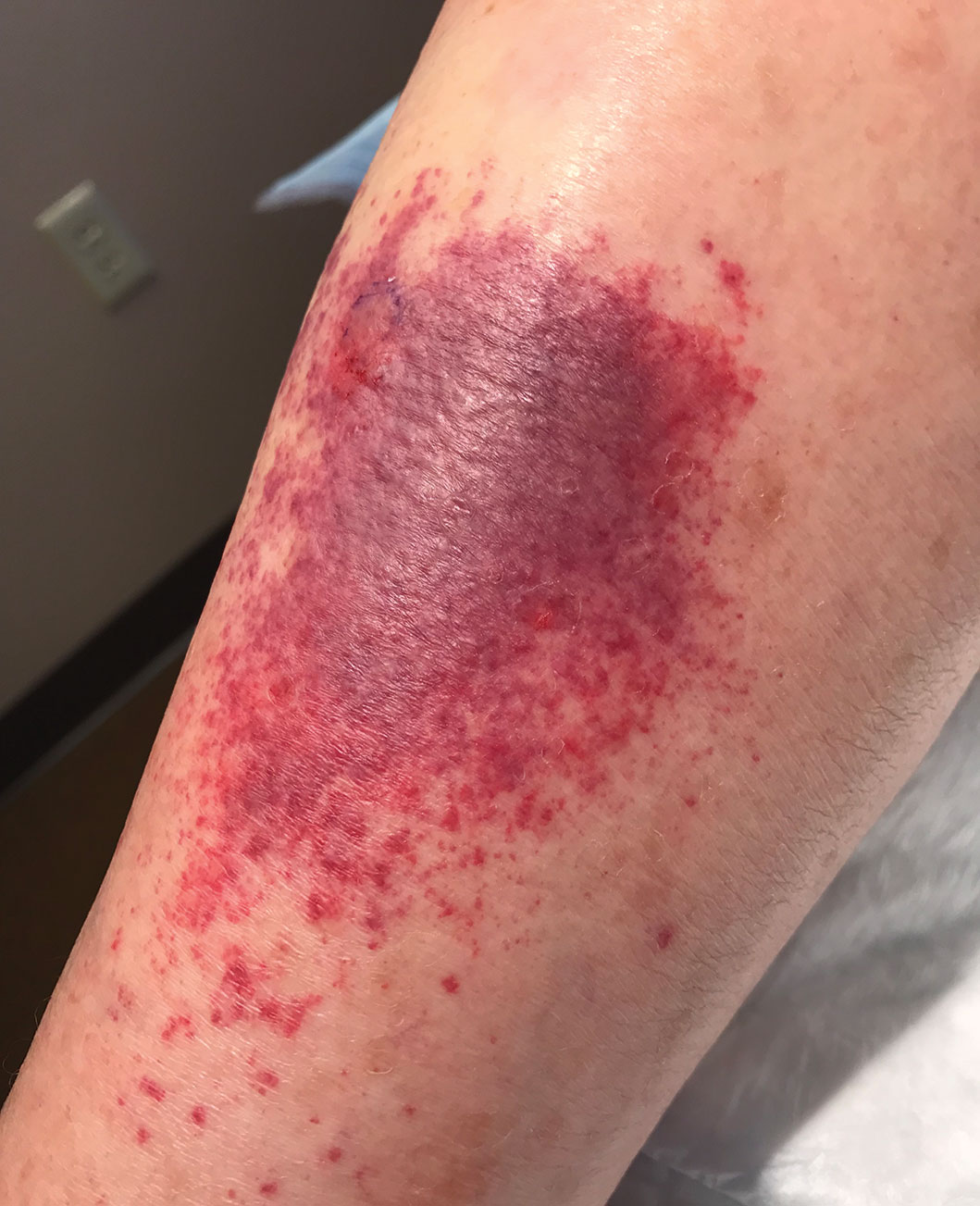
Sixteen days after being bitten by the ticks, the patient was examined in our dermatology office. Physical examination revealed an erythematous plaque with a central eschar over the medial aspect of the left leg (Figure 1) and multiple 3- to 6-mm, erythematous, dome-shaped papules scattered over the dorsal aspects of the feet and ankles (Figure 2). The examination was otherwise normal. Blood was drawn the same day for laboratory analysis; no abnormalities of platelets, red blood cells, or white blood cells were found. Results of a chemistry panel and liver enzyme tests were within reference range.
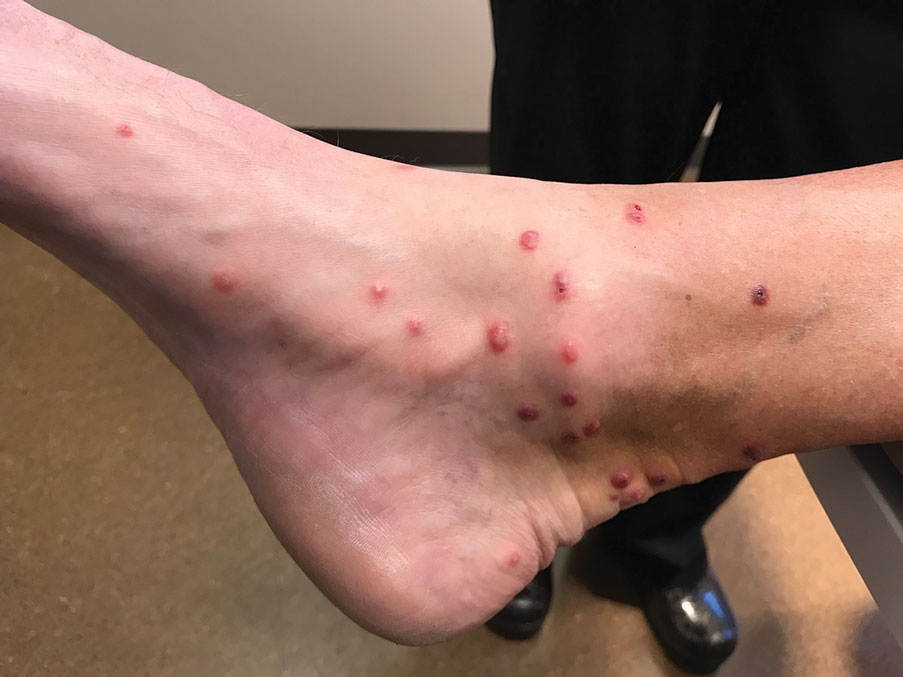
Skin biopsies were taken to elucidate the underlying pathology. Although an arthropod assault was suspected, there also was concern for deep vessel vasculitis because of the presence of considerable petechiae and purpura (Figure 3). Histologically, leukocytoclasia was seen in deep dermal blood vessels. A mild eosinophilic spongiosis with a mixed dermal infiltrate was identified—strengthening our suspicion of an arthropod assault. Bacterial cultures for aerobes and anaerobes using material taken from the right shin showed no growth.
Ten days after the initial biopsies, serum specimens were drawn and swabs of eschar were collected and sent to the Centers for Disease Control and Prevention for further testing. Serum was tested by immunofluorescence assay (IFA) for spotted fever group IgG to detect Rocky Mountain spotted fever and ATBF antibodies; both tests were negative. Swab material from eschar was tested again by IFA for spotted fever group IgG (Rocky Mountain spotted fever) and antibodies to ATBF and with bacterial culture isolation and nucleic acid amplification; the culture and amplification came back positive for R africae.
Because the specialized tests confirmed infection with R africae, the patient was given triamcinolone cream 0.1% to apply twice daily to the pruritic lesions for as long as 4 weeks; an additional 14-day course of oral doxycycline monohydrate (100 mg twice daily) was given. At follow-up, the lesions had fully resolved without evident scarring.
Various diagnostic techniques can detect R africae. Bacterial culture and the polymerase chain reaction are specific and therefore diagnostic. In addition, the diagnosis of rickettsiosis can be made with serology testing, in which disease-specific antibodies are detected by indirect IFA using disease-specific antigens.
Antigens from R rickettsii (the agent of Rocky Mountain spotted fever), R conorii (Mediterranean spotted fever), and R africae (ATBF) are commercially available for making the diagnosis of rickettsiosis. However, antigens from R conorii exhibit cross-reactivity with R africae, which can confound the diagnosis.1,10 Serologic IFA tests have been shown to be less sensitive, especially when performed after antibacterial treatment has started.
In a study, 17 of 65 (26%) ATBF-confirmed patients were seronegative (acute and convalescent-phase sera) against R africae; 14 had received doxycycline during the first week of clinical signs. The current hypothesis is that R africae is highly sensitive to doxycycline and that early exposure to the drug prevented development of detectable titers of reactive antibodies, thus producing a negative serology test.11
Furthermore, it has been shown that seroconversion of IgG and IgM antibodies in R africae–infected sera is delayed compared to what is observed with R conorii–infected sera. Typically, seroconversion of R conorii–infected sera can be detected within 2 weeks; seroconversion in R africae–infected sera can take 4 weeks or longer.11
Our patient had a confirmed case of ATBF secondary to R africae infection, which was evident from tissue culture isolation and polymerase chain reaction analysis of swab material obtained from eschar sites, both of which yielded a positive result for R africae. The traveler’s negative serologic status might be due to his early exposure to doxycycline or to the 4-week delay in R africae seroconversion; his serum was collected only 3 weeks after the tick bites.
Clinical signs also aid in making the diagnosis of ATBF and distinguishing R conorii from R africae infection. Because of the aggressive hunting nature of the tick carrying R africae, they are associated with multiple eschars and tend to affect groups of multiple people, especially in rural areas.4,5 In contrast, ticks carrying R conorii yield a single eschar due to their passive style of infecting a host and because they favor a single host within urban areas.5 Both infections exhibit a maculopapular or papulovesicular rash and are accompanied by fatigue, headache, and myalgia, though ATBF tends to present with a milder rash than MSF.
Infection with either R conorii or R africae responds to tetracyclines, quinolones, and macrolides.10,12
African tick-bite fever is becoming more common, which should encourage clinicians to become familiar with the disease. Less than 2 decades ago, ATBF virtually was unknown outside of Zimbabwe, Botswana, Tanzania, Zambia, and Kenya, where it is endemic. However, after the abolition of apartheid in the 1990s, international tourism in southern Africa increased 6-fold.13 African tick-bite fever is now one of the most common rickettsial infections in Africa.7 In addition to diagnosing ATBF and managing infected patients, clinicians can help prevent ATBF in individuals who travel to endemic areas by recommending commercial topical insect repellents containing at least 19.5% N,N-diethyl-meta-toluamide (DEET).14
- Parola P, Paddock CD, Socolovschi C, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev. 2013;26:657-702. doi:10.1128/CMR.00032-13
- Jensenius M, Fournier P-E, Vene S, et al; Norwegian African Tick Bite Fever Study Group. African tick bite fever in travelers to rural sub-equatorial Africa. Clin Infect Dis. 2003;36:1411-1417. doi:10.1086/375083
- Parola P, Jourdan J, Raoult D. Tick-borne infection caused by Rickettsia africae in the West Indies. N Engl J Med. 1998;338:1391-1392. doi:10.1056/NEJM199805073381918
- Norval RA, Andrew HR, Yunker CE. Pheromone-mediation of host-selection in bont ticks (Amblyomma hebraeum Koch). Science. 1989;243:364-365. doi:10.1126/science.2911745
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928. doi:10.1086/319347
- Kelly PJ, Mason PR. Transmission of a spotted fever group rickettsia by Amblyomma hebraeum (Acari: Ixodidae). J Med Entomol. 1991;28:598-600. doi:10.1093/jmedent/28.5.598
- Maina AN, Jiang J, Omulo SA, et al. High prevalence of Rickettsia africae variants in Amblyomma variegatum ticks from domestic mammals in rural Western Kenya: implications for human health. Vector Borne Zoonotic Dis. 2014;14:693-702. doi:10.1089/vbz.2014.1578
- Jensenius M, Fournier P-E, Kelly P, et al. African tick bite fever. Lancet Infect Dis. 2003;3:557-564. doi:10.1016/s1473-3099(03)00739-4
- Roch N, Epaulard O, Pelloux I, et al. African tick bite fever in elderly patients: 8 cases in French tourists returning from South Africa. Clin Infect Dis. 2008;47:E28-E35. doi:10.1086/589868
- Palau L, Pankey GA. Mediterranean spotted fever in travelers from the United States. J Travel Med. 1997;4:179-182. doi:10.1111/j.1708-8305.1997.tb00816.x
- Fournier P-E, Jensenius M, Laferl H, et al. Kinetics of antibody responses in Rickettsia africae and Rickettsia conorii infections. Clin Diagn Lab Immunol. 2002;9:324-328. doi:10.1128/cdli.9.2.324-328.2002
- Brouqui P, Bacellar F, Baranton G, et al; ; . Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin Microbiol Infect. 2004;10:1108-1132. doi:10.1111/j.1469-0691.2004.01019.x
- Rolain JM, Jensenius M, Raoult D. Rickettsial infections—a threat to travelers? Curr Opin Infect Dis. 2004;17:433-437. doi:10.1097/00001432-200410000-00008
- Jensenius M, Pretorius AM, Clarke F, et al. Repellent efficacy of four commercial DEET lotions against Amblyomma hebraeum (Acari: Ixodidae), the principal vector of Rickettsia africae in southern Africa. Trans R Soc Trop Med Hyg. 2005;99:708-711. doi:10.1016/j.trstmh.2005.01.006
To the Editor:
One of the more common tick-borne infections seen in travelers returning from sub-Saharan Africa is caused by Rickettsia africae, which is the etiologic agent of African tick-bite fever (ATBF), the most common tick-borne bacterial zoonosis.1 There are 2 known tick vectors of disease: Amblyomma variegatum, found in sub-Saharan Africa and the West Indies, and Amblyomma hebraeum, found specifically in southern Africa.2,3
Unlike other disease-carrying ticks that passively wait on vegetation to be picked up by a host, A hebraeum uniquely attract other nearby ticks to the host. Studies have shown that male ticks feeding on a nonhuman host (usually cattle) can emit an aggression-attachment pheromone that attracts other ticks to the host. The presence of the pheromone allows unfed ticks to actively discriminate between hosts on which these parasites have fed successfully (ie, suitable hosts) and those on which they have not.4
The aggressive hunting nature of A hebraeum explains the clinical presentation of multiple eschars and why large groups of exposed travelers—such as soldiers, leisure safari tourists, game hunters, and foreign-aid workers—are affected.2
Another southern African spotted fever group, Rickettsia conorii is the causative agent of Mediterranean spotted fever (MSF). Ticks carrying R conorii exhibit a much less aggressive hunting stylethan A hebraeum; consequently, infected patients present with a single eschar site.5
Rickettsia africae is estimated to have very high prevalence (95.2%) in Amblyomma ticks and a fairly high prevalence (approximately 4.0% to 8.6%) in travelers coming from rural southern Africa,6,7 with an incubation period of 5 to 10 days after inoculation by an infected tick.8 Signs include fever, a generalized maculopapular or papulovesicular rash, and regional lymphadenopathy; symptoms include fatigue, headache, and myalgia.
The inoculation eschar—single or multiple—commonly presents on the legs and is accompanied by tender lymphadenopathy of draining nodes1,8 More severe findings, such as myocarditis and subacute neuropathy, have been reported in elderly patients.9
A 77-year-old man presented with a pruritic maculopapular and papulovesicular rash distributed over the upper and lower extremities of 3 weeks’ duration. The patient reported having been on a 12-day mission trip to Limpopo, South Africa, where he was constructing and installing safe toilets to replace dangerous toilet pits. He believed he had been bitten by 2 ticks, after which he noted a dark purple and black patch on the left lower leg by the third day of the trip. He developed a sudden persistent pruritic rash, first on the lower extremities and then spreading to the upper extremities. The patient was seen by an American physician in South Africa who gave him a 7-day course of oral doxycycline monohydrate 100 mg twice daily. He then returned to the United States.

Sixteen days after being bitten by the ticks, the patient was examined in our dermatology office. Physical examination revealed an erythematous plaque with a central eschar over the medial aspect of the left leg (Figure 1) and multiple 3- to 6-mm, erythematous, dome-shaped papules scattered over the dorsal aspects of the feet and ankles (Figure 2). The examination was otherwise normal. Blood was drawn the same day for laboratory analysis; no abnormalities of platelets, red blood cells, or white blood cells were found. Results of a chemistry panel and liver enzyme tests were within reference range.

Skin biopsies were taken to elucidate the underlying pathology. Although an arthropod assault was suspected, there also was concern for deep vessel vasculitis because of the presence of considerable petechiae and purpura (Figure 3). Histologically, leukocytoclasia was seen in deep dermal blood vessels. A mild eosinophilic spongiosis with a mixed dermal infiltrate was identified—strengthening our suspicion of an arthropod assault. Bacterial cultures for aerobes and anaerobes using material taken from the right shin showed no growth.

Ten days after the initial biopsies, serum specimens were drawn and swabs of eschar were collected and sent to the Centers for Disease Control and Prevention for further testing. Serum was tested by immunofluorescence assay (IFA) for spotted fever group IgG to detect Rocky Mountain spotted fever and ATBF antibodies; both tests were negative. Swab material from eschar was tested again by IFA for spotted fever group IgG (Rocky Mountain spotted fever) and antibodies to ATBF and with bacterial culture isolation and nucleic acid amplification; the culture and amplification came back positive for R africae.
Because the specialized tests confirmed infection with R africae, the patient was given triamcinolone cream 0.1% to apply twice daily to the pruritic lesions for as long as 4 weeks; an additional 14-day course of oral doxycycline monohydrate (100 mg twice daily) was given. At follow-up, the lesions had fully resolved without evident scarring.
Various diagnostic techniques can detect R africae. Bacterial culture and the polymerase chain reaction are specific and therefore diagnostic. In addition, the diagnosis of rickettsiosis can be made with serology testing, in which disease-specific antibodies are detected by indirect IFA using disease-specific antigens.
Antigens from R rickettsii (the agent of Rocky Mountain spotted fever), R conorii (Mediterranean spotted fever), and R africae (ATBF) are commercially available for making the diagnosis of rickettsiosis. However, antigens from R conorii exhibit cross-reactivity with R africae, which can confound the diagnosis.1,10 Serologic IFA tests have been shown to be less sensitive, especially when performed after antibacterial treatment has started.
In a study, 17 of 65 (26%) ATBF-confirmed patients were seronegative (acute and convalescent-phase sera) against R africae; 14 had received doxycycline during the first week of clinical signs. The current hypothesis is that R africae is highly sensitive to doxycycline and that early exposure to the drug prevented development of detectable titers of reactive antibodies, thus producing a negative serology test.11
Furthermore, it has been shown that seroconversion of IgG and IgM antibodies in R africae–infected sera is delayed compared to what is observed with R conorii–infected sera. Typically, seroconversion of R conorii–infected sera can be detected within 2 weeks; seroconversion in R africae–infected sera can take 4 weeks or longer.11
Our patient had a confirmed case of ATBF secondary to R africae infection, which was evident from tissue culture isolation and polymerase chain reaction analysis of swab material obtained from eschar sites, both of which yielded a positive result for R africae. The traveler’s negative serologic status might be due to his early exposure to doxycycline or to the 4-week delay in R africae seroconversion; his serum was collected only 3 weeks after the tick bites.
Clinical signs also aid in making the diagnosis of ATBF and distinguishing R conorii from R africae infection. Because of the aggressive hunting nature of the tick carrying R africae, they are associated with multiple eschars and tend to affect groups of multiple people, especially in rural areas.4,5 In contrast, ticks carrying R conorii yield a single eschar due to their passive style of infecting a host and because they favor a single host within urban areas.5 Both infections exhibit a maculopapular or papulovesicular rash and are accompanied by fatigue, headache, and myalgia, though ATBF tends to present with a milder rash than MSF.
Infection with either R conorii or R africae responds to tetracyclines, quinolones, and macrolides.10,12
African tick-bite fever is becoming more common, which should encourage clinicians to become familiar with the disease. Less than 2 decades ago, ATBF virtually was unknown outside of Zimbabwe, Botswana, Tanzania, Zambia, and Kenya, where it is endemic. However, after the abolition of apartheid in the 1990s, international tourism in southern Africa increased 6-fold.13 African tick-bite fever is now one of the most common rickettsial infections in Africa.7 In addition to diagnosing ATBF and managing infected patients, clinicians can help prevent ATBF in individuals who travel to endemic areas by recommending commercial topical insect repellents containing at least 19.5% N,N-diethyl-meta-toluamide (DEET).14
To the Editor:
One of the more common tick-borne infections seen in travelers returning from sub-Saharan Africa is caused by Rickettsia africae, which is the etiologic agent of African tick-bite fever (ATBF), the most common tick-borne bacterial zoonosis.1 There are 2 known tick vectors of disease: Amblyomma variegatum, found in sub-Saharan Africa and the West Indies, and Amblyomma hebraeum, found specifically in southern Africa.2,3
Unlike other disease-carrying ticks that passively wait on vegetation to be picked up by a host, A hebraeum uniquely attract other nearby ticks to the host. Studies have shown that male ticks feeding on a nonhuman host (usually cattle) can emit an aggression-attachment pheromone that attracts other ticks to the host. The presence of the pheromone allows unfed ticks to actively discriminate between hosts on which these parasites have fed successfully (ie, suitable hosts) and those on which they have not.4
The aggressive hunting nature of A hebraeum explains the clinical presentation of multiple eschars and why large groups of exposed travelers—such as soldiers, leisure safari tourists, game hunters, and foreign-aid workers—are affected.2
Another southern African spotted fever group, Rickettsia conorii is the causative agent of Mediterranean spotted fever (MSF). Ticks carrying R conorii exhibit a much less aggressive hunting stylethan A hebraeum; consequently, infected patients present with a single eschar site.5
Rickettsia africae is estimated to have very high prevalence (95.2%) in Amblyomma ticks and a fairly high prevalence (approximately 4.0% to 8.6%) in travelers coming from rural southern Africa,6,7 with an incubation period of 5 to 10 days after inoculation by an infected tick.8 Signs include fever, a generalized maculopapular or papulovesicular rash, and regional lymphadenopathy; symptoms include fatigue, headache, and myalgia.
The inoculation eschar—single or multiple—commonly presents on the legs and is accompanied by tender lymphadenopathy of draining nodes1,8 More severe findings, such as myocarditis and subacute neuropathy, have been reported in elderly patients.9
A 77-year-old man presented with a pruritic maculopapular and papulovesicular rash distributed over the upper and lower extremities of 3 weeks’ duration. The patient reported having been on a 12-day mission trip to Limpopo, South Africa, where he was constructing and installing safe toilets to replace dangerous toilet pits. He believed he had been bitten by 2 ticks, after which he noted a dark purple and black patch on the left lower leg by the third day of the trip. He developed a sudden persistent pruritic rash, first on the lower extremities and then spreading to the upper extremities. The patient was seen by an American physician in South Africa who gave him a 7-day course of oral doxycycline monohydrate 100 mg twice daily. He then returned to the United States.

Sixteen days after being bitten by the ticks, the patient was examined in our dermatology office. Physical examination revealed an erythematous plaque with a central eschar over the medial aspect of the left leg (Figure 1) and multiple 3- to 6-mm, erythematous, dome-shaped papules scattered over the dorsal aspects of the feet and ankles (Figure 2). The examination was otherwise normal. Blood was drawn the same day for laboratory analysis; no abnormalities of platelets, red blood cells, or white blood cells were found. Results of a chemistry panel and liver enzyme tests were within reference range.

Skin biopsies were taken to elucidate the underlying pathology. Although an arthropod assault was suspected, there also was concern for deep vessel vasculitis because of the presence of considerable petechiae and purpura (Figure 3). Histologically, leukocytoclasia was seen in deep dermal blood vessels. A mild eosinophilic spongiosis with a mixed dermal infiltrate was identified—strengthening our suspicion of an arthropod assault. Bacterial cultures for aerobes and anaerobes using material taken from the right shin showed no growth.

Ten days after the initial biopsies, serum specimens were drawn and swabs of eschar were collected and sent to the Centers for Disease Control and Prevention for further testing. Serum was tested by immunofluorescence assay (IFA) for spotted fever group IgG to detect Rocky Mountain spotted fever and ATBF antibodies; both tests were negative. Swab material from eschar was tested again by IFA for spotted fever group IgG (Rocky Mountain spotted fever) and antibodies to ATBF and with bacterial culture isolation and nucleic acid amplification; the culture and amplification came back positive for R africae.
Because the specialized tests confirmed infection with R africae, the patient was given triamcinolone cream 0.1% to apply twice daily to the pruritic lesions for as long as 4 weeks; an additional 14-day course of oral doxycycline monohydrate (100 mg twice daily) was given. At follow-up, the lesions had fully resolved without evident scarring.
Various diagnostic techniques can detect R africae. Bacterial culture and the polymerase chain reaction are specific and therefore diagnostic. In addition, the diagnosis of rickettsiosis can be made with serology testing, in which disease-specific antibodies are detected by indirect IFA using disease-specific antigens.
Antigens from R rickettsii (the agent of Rocky Mountain spotted fever), R conorii (Mediterranean spotted fever), and R africae (ATBF) are commercially available for making the diagnosis of rickettsiosis. However, antigens from R conorii exhibit cross-reactivity with R africae, which can confound the diagnosis.1,10 Serologic IFA tests have been shown to be less sensitive, especially when performed after antibacterial treatment has started.
In a study, 17 of 65 (26%) ATBF-confirmed patients were seronegative (acute and convalescent-phase sera) against R africae; 14 had received doxycycline during the first week of clinical signs. The current hypothesis is that R africae is highly sensitive to doxycycline and that early exposure to the drug prevented development of detectable titers of reactive antibodies, thus producing a negative serology test.11
Furthermore, it has been shown that seroconversion of IgG and IgM antibodies in R africae–infected sera is delayed compared to what is observed with R conorii–infected sera. Typically, seroconversion of R conorii–infected sera can be detected within 2 weeks; seroconversion in R africae–infected sera can take 4 weeks or longer.11
Our patient had a confirmed case of ATBF secondary to R africae infection, which was evident from tissue culture isolation and polymerase chain reaction analysis of swab material obtained from eschar sites, both of which yielded a positive result for R africae. The traveler’s negative serologic status might be due to his early exposure to doxycycline or to the 4-week delay in R africae seroconversion; his serum was collected only 3 weeks after the tick bites.
Clinical signs also aid in making the diagnosis of ATBF and distinguishing R conorii from R africae infection. Because of the aggressive hunting nature of the tick carrying R africae, they are associated with multiple eschars and tend to affect groups of multiple people, especially in rural areas.4,5 In contrast, ticks carrying R conorii yield a single eschar due to their passive style of infecting a host and because they favor a single host within urban areas.5 Both infections exhibit a maculopapular or papulovesicular rash and are accompanied by fatigue, headache, and myalgia, though ATBF tends to present with a milder rash than MSF.
Infection with either R conorii or R africae responds to tetracyclines, quinolones, and macrolides.10,12
African tick-bite fever is becoming more common, which should encourage clinicians to become familiar with the disease. Less than 2 decades ago, ATBF virtually was unknown outside of Zimbabwe, Botswana, Tanzania, Zambia, and Kenya, where it is endemic. However, after the abolition of apartheid in the 1990s, international tourism in southern Africa increased 6-fold.13 African tick-bite fever is now one of the most common rickettsial infections in Africa.7 In addition to diagnosing ATBF and managing infected patients, clinicians can help prevent ATBF in individuals who travel to endemic areas by recommending commercial topical insect repellents containing at least 19.5% N,N-diethyl-meta-toluamide (DEET).14
- Parola P, Paddock CD, Socolovschi C, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev. 2013;26:657-702. doi:10.1128/CMR.00032-13
- Jensenius M, Fournier P-E, Vene S, et al; Norwegian African Tick Bite Fever Study Group. African tick bite fever in travelers to rural sub-equatorial Africa. Clin Infect Dis. 2003;36:1411-1417. doi:10.1086/375083
- Parola P, Jourdan J, Raoult D. Tick-borne infection caused by Rickettsia africae in the West Indies. N Engl J Med. 1998;338:1391-1392. doi:10.1056/NEJM199805073381918
- Norval RA, Andrew HR, Yunker CE. Pheromone-mediation of host-selection in bont ticks (Amblyomma hebraeum Koch). Science. 1989;243:364-365. doi:10.1126/science.2911745
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928. doi:10.1086/319347
- Kelly PJ, Mason PR. Transmission of a spotted fever group rickettsia by Amblyomma hebraeum (Acari: Ixodidae). J Med Entomol. 1991;28:598-600. doi:10.1093/jmedent/28.5.598
- Maina AN, Jiang J, Omulo SA, et al. High prevalence of Rickettsia africae variants in Amblyomma variegatum ticks from domestic mammals in rural Western Kenya: implications for human health. Vector Borne Zoonotic Dis. 2014;14:693-702. doi:10.1089/vbz.2014.1578
- Jensenius M, Fournier P-E, Kelly P, et al. African tick bite fever. Lancet Infect Dis. 2003;3:557-564. doi:10.1016/s1473-3099(03)00739-4
- Roch N, Epaulard O, Pelloux I, et al. African tick bite fever in elderly patients: 8 cases in French tourists returning from South Africa. Clin Infect Dis. 2008;47:E28-E35. doi:10.1086/589868
- Palau L, Pankey GA. Mediterranean spotted fever in travelers from the United States. J Travel Med. 1997;4:179-182. doi:10.1111/j.1708-8305.1997.tb00816.x
- Fournier P-E, Jensenius M, Laferl H, et al. Kinetics of antibody responses in Rickettsia africae and Rickettsia conorii infections. Clin Diagn Lab Immunol. 2002;9:324-328. doi:10.1128/cdli.9.2.324-328.2002
- Brouqui P, Bacellar F, Baranton G, et al; ; . Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin Microbiol Infect. 2004;10:1108-1132. doi:10.1111/j.1469-0691.2004.01019.x
- Rolain JM, Jensenius M, Raoult D. Rickettsial infections—a threat to travelers? Curr Opin Infect Dis. 2004;17:433-437. doi:10.1097/00001432-200410000-00008
- Jensenius M, Pretorius AM, Clarke F, et al. Repellent efficacy of four commercial DEET lotions against Amblyomma hebraeum (Acari: Ixodidae), the principal vector of Rickettsia africae in southern Africa. Trans R Soc Trop Med Hyg. 2005;99:708-711. doi:10.1016/j.trstmh.2005.01.006
- Parola P, Paddock CD, Socolovschi C, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev. 2013;26:657-702. doi:10.1128/CMR.00032-13
- Jensenius M, Fournier P-E, Vene S, et al; Norwegian African Tick Bite Fever Study Group. African tick bite fever in travelers to rural sub-equatorial Africa. Clin Infect Dis. 2003;36:1411-1417. doi:10.1086/375083
- Parola P, Jourdan J, Raoult D. Tick-borne infection caused by Rickettsia africae in the West Indies. N Engl J Med. 1998;338:1391-1392. doi:10.1056/NEJM199805073381918
- Norval RA, Andrew HR, Yunker CE. Pheromone-mediation of host-selection in bont ticks (Amblyomma hebraeum Koch). Science. 1989;243:364-365. doi:10.1126/science.2911745
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928. doi:10.1086/319347
- Kelly PJ, Mason PR. Transmission of a spotted fever group rickettsia by Amblyomma hebraeum (Acari: Ixodidae). J Med Entomol. 1991;28:598-600. doi:10.1093/jmedent/28.5.598
- Maina AN, Jiang J, Omulo SA, et al. High prevalence of Rickettsia africae variants in Amblyomma variegatum ticks from domestic mammals in rural Western Kenya: implications for human health. Vector Borne Zoonotic Dis. 2014;14:693-702. doi:10.1089/vbz.2014.1578
- Jensenius M, Fournier P-E, Kelly P, et al. African tick bite fever. Lancet Infect Dis. 2003;3:557-564. doi:10.1016/s1473-3099(03)00739-4
- Roch N, Epaulard O, Pelloux I, et al. African tick bite fever in elderly patients: 8 cases in French tourists returning from South Africa. Clin Infect Dis. 2008;47:E28-E35. doi:10.1086/589868
- Palau L, Pankey GA. Mediterranean spotted fever in travelers from the United States. J Travel Med. 1997;4:179-182. doi:10.1111/j.1708-8305.1997.tb00816.x
- Fournier P-E, Jensenius M, Laferl H, et al. Kinetics of antibody responses in Rickettsia africae and Rickettsia conorii infections. Clin Diagn Lab Immunol. 2002;9:324-328. doi:10.1128/cdli.9.2.324-328.2002
- Brouqui P, Bacellar F, Baranton G, et al; ; . Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin Microbiol Infect. 2004;10:1108-1132. doi:10.1111/j.1469-0691.2004.01019.x
- Rolain JM, Jensenius M, Raoult D. Rickettsial infections—a threat to travelers? Curr Opin Infect Dis. 2004;17:433-437. doi:10.1097/00001432-200410000-00008
- Jensenius M, Pretorius AM, Clarke F, et al. Repellent efficacy of four commercial DEET lotions against Amblyomma hebraeum (Acari: Ixodidae), the principal vector of Rickettsia africae in southern Africa. Trans R Soc Trop Med Hyg. 2005;99:708-711. doi:10.1016/j.trstmh.2005.01.006
PRACTICE POINTS
- African tick-bite fever (ATBF) is one of the more common tick-borne bacterial zoonoses and should be considered in patients presenting with multiple eschar sites who have had exposure to rural areas of southern Africa in the preceding 2 weeks.
- Ticks carrying Rickettsia africae are unique, given their ability to actively be attracted to and hunt their nonhuman hosts via an aggression-attachment pheromone.
- Laboratory diagnosis of ATBF can be challenging due to the high cross-reactivity of Helvetica Neue LT StdR africae with Helvetica Neue LT StdRickettsia conorii in serologic testing and the delay in seroconversion in Helvetica Neue LT StdR africae infection.