User login
Preventing herpes zoster through vaccination: New developments
Herpes zoster (HZ), or shingles, represents a reactivation of the varicella-zoster virus (VZV). Following primary infection, usually in childhood, the virus typically lies dormant in the dorsal root and sensory nerve ganglia for decades. The precise mechanism of reactivation is not well understood, but it is associated with a decline in cell-mediated immunity that occurs with advancing age, immune-compromising conditions such as HIV infection and cancer, or immunosuppressive therapies, including corticosteroids.1 HZ is usually a self-limited disease characterized by unilateral dermatomal rash and pain, but can cause disseminated infection in immunocompromised individuals.2
Treatment with antiviral medications within 72 hours of rash onset can reduce acute HZ symptoms.1 However, antiviral agents are only minimally effective in preventing postherpetic neuralgia, the most common complication of HZ.3 Therefore, efforts to reduce the burden of HZ morbidity have focused on prevention through vaccination.
Currently, the only shingles vaccine approved by the US Food and Drug Administration (FDA) is Zostavax (Merck), which contains the live-attenuated Oka strain of VZV at a concentration 14 times greater than that of the varicella vaccine (Varivax, Merck). The live-attenuated vaccine boosts VZV-specific cell-mediated immunity, preventing reactivation of the latent virus.
In this article, we describe the burden of disease and review recent developments in the literature on HZ vaccine, including duration of efficacy, uptake and barriers to vaccination, cost-effectiveness, and the outlook for future vaccines.
INCIDENCE INCREASES WITH AGE
The incidence of herpes zoster in the general population is between 3 and 5 per 1,000 person-years4 and increases with age, especially after age 60 when the incidence can approach 13 to 15 per 1,000 person-years.5,6 An estimated 1 million new cases occur each year in the United States, and about 6% of patients experience a second episode of HZ within 8 years.7,8 In immunocompromised patients, the incidence of HZ is 2 to 10 times higher than in the general population.9
The incidence of HZ has been increasing for reasons that are unclear. After varicella vaccine was introduced into the routine childhood immunization schedule in 1995, it was hypothesized that the resultant decrease in primary varicella infections would remove a natural source of immune boosting and cause an increase in HZ incidence for up to 20 years.10 However, recent studies demonstrate that the observed increase in HZ incidence actually predates the introduction of varicella vaccine,11–13 and the widespread use of varicella vaccine has not resulted in an increase in the incidence of HZ.14
Other potential explanations for the rise in reported incidence include increasing awareness among patients, who might previously not have sought care and among physicians, who may be more likely to make the diagnosis. Advertisement of new treatments for HZ, including gabapentin and capsaicin, probably began to increase awareness in the 1990s, as did promotion of the HZ vaccine after its licensure in 2006.
HZ can occur in people who have been vaccinated against varicella due to reactivation of the vaccine-strain virus, but the risk is lower than after infection with wild-type varicella.15 Given that the varicella vaccine has been routinely used in children for only 20 years, the long-term effect of varicella vaccination on the incidence of HZ in elderly people is unknown.
Serious complications
HZ can cause rare but serious complications including encephalitis, herpes ophthalmicus, herpes oticus, myelitis, and retinitis.1 These can lead to long-term disability including unilateral blindness and deafness.
The most common and debilitating complication is postherpetic neuralgia, a persistent pain lasting at least 3 months, with a mean duration of 3.3 years and sometimes as long as 10 years.16 Postherpetic neuralgia occurs in 8% to 32% of patients after acute HZ,4 and the incidence increases with age, being most common after age 70. The chronic pain of postherpetic neuralgia has a significant adverse impact on patients’ quality of life, including physical disability and emotional distress.17 Some pain is intense, and anecdotal reports of patients committing suicide were included in the Advisory Committee on Immunization Practices (ACIP) recommendations regarding herpes zoster vaccine.18
HZ and its complications also impose a substantial economic burden on society.19 In a population-based study, the mean direct medical costs of HZ ranged from $620 to $1,160 (2015 dollars) depending on age,20 and the mean costs of postherpetic neuralgia were 2 to 5 times higher than that.20–22 Immunocompromised patients had costs 2 to 3 times higher than those of immunocompetent adults.23 In addition, for employed patients, HZ resulted in an average loss of 32 hours of work due to absenteeism and 84 hours due to presenteeism (ie, working while sick and therefore suboptimally).24
Assuming there are 1 million cases of HZ each year, if 8% to 32% of patients go on to develop postherpetic neuralgia, that would translate into approximately $1 to $2 billion in direct medical costs. With 60% of adult patients working,25 at an average wage of $23.23 per hour,26 HZ illness could be responsible for another $1.6 billion in lost productivity.
EFFICACY AND SAFETY OF HZ VACCINE
In 2006, the FDA approved the live-attenuated Oka strain VZV vaccine for prevention of HZ and postherpetic neuralgia in adults age 60 and older based on findings from the Shingles Prevention Study (SPS).27
The Shingles Prevention Study
This multicenter randomized placebo-controlled trial27 enrolled 38,546 immunocompetent persons age 60 and older. Subjects in the intervention group received a single dose of live-attenuated vaccine, and all participants were followed for up to 4.9 years after vaccination.
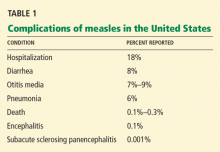
HZ occurred in 315 (1.636%) of the 19,254 participants in the vaccine group and in 642 (3.336%) of the 19,247 participants in the placebo group, an absolute risk reduction of 1.7%, number needed to treat 59, relative risk reduction 51%, P < .001. Similarly, postherpetic neuralgia occurred in 27 (0.140%) of the 19,254 vaccine recipients and in 80 (0.416%) of the placebo recipients (an absolute risk reduction of 0.276%, number needed to treat 362, relative risk reduction 66%, P < .001). The investigators calculated that vaccination reduced the overall burden of illness by 61% (Table 1).
The efficacy against HZ incidence decreased with age,28 but the efficacy against postherpetic neuralgia did not. In addition, vaccine recipients who developed HZ generally had less severe manifestations.
The safety of the vaccine was assessed for all participants in the SPS. In addition, one-sixth of SPS participants were enrolled in a safety substudy. These participants completed a detailed report card regarding all medically important events within the first 42 days. Forty-eight percent of the vaccine group and 17% of the placebo group (P < .05) experienced adverse events, primarily at the injection site. Less than 1% of all local reactions were severe.29 Serious adverse events were rare (< 2%), but occurred significantly more often in the vaccinated group.
Short-Term Persistence Substudy
Short-term efficacy of the live-attenuated vaccine (up to 7 years) was assessed in the Short-Term Persistence Substudy (STPS), which involved 14,270 of the initial participants and reported yearly and overall vaccine efficacy.30 After 5 years, the yearly efficacy against postherpetic neuralgia incidence declined to 32% and was no longer statistically significant. Efficacy against HZ incidence and burden of illness displayed the same pattern. After the end of the STPS, all subjects in the placebo group received vaccination.
Long-Term Persistence Substudy
Those in the intervention group were followed for an additional 4 years in the Long-Term Persistence Substudy (LTPS).31 Due to the lack of concurrent controls in the LTPS, the authors used regression models based on historical controls to estimate contemporary population incidence of HZ and postherpetic neuralgia for comparison.
Efficacy continued to decline over time, and by 10 years after vaccination there was no difference between vaccinated patients and historical controls in the rate of any end point (ie, efficacy declined to zero).
A trial of booster vaccination
Because many patients are vaccinated at age 60, waning immunity could leave them vulnerable to HZ and postherpetic neuralgia by age 70. A potential solution would be to give a booster dose after 10 years.
A recent phase 3 clinical trial of adults age 70 years and older found that a booster dose of live-attenuated vaccine was as safe and immunogenic as an initial dose.32 While antibody responses were similar in the boosted group and the newly vaccinated group, cell-mediated immunity was higher in the boosted group.
Because prevention of HZ is generally via cell-mediated immunity, the booster might be more effective than the initial vaccination, but clinical trials measuring actual cases prevented will be required to prove it. A booster dose is not currently recommended.
A trial of vaccination in adults 50 to 59
In 2011, the FDA extended its approval of HZ vaccine for use in adults ages 50 to 59.33
In a randomized, double-blind, placebo-controlled trial in this age group,33 the vaccine reduced HZ incidence by almost 70% (absolute risk reduction 0.614%, number needed to treat 156; Table 1), but the severity of HZ cases was not affected. There were too few cases of postherpetic neuralgia to assess the efficacy for this end point. The study followed patients for only 1.5 years after vaccination, so the duration of efficacy is unknown.
As in the older recipients, the vaccine was well tolerated; injection-site reactions and headache were the major adverse effects reported among vaccine recipients.33
INDICATIONS AND CONTRAINDICATIONS
Although HZ vaccine is licensed for use in adults age 50 and older, the ACIP recommends it only for immunocompetent adults age 60 and older. At this time, the ACIP does not recommend HZ vaccine in those younger than 60 because of the low risk of HZ in this age group.34
Any person age 60 or older should receive a single dose of the live-attenuated HZ vaccine subcutaneously, regardless of past history of HZ.
The vaccine is contraindicated in patients who have a history of allergic reaction to any vaccine component, immunosuppression or immunodeficiency conditions, and pregnancy. Specifically, people who will receive immunosuppressive therapies should have the vaccine at least 14 days before beginning treatment. Antiviral medications such as acyclovir, famciclovir, and valacyclovir should be discontinued at least 24 hours before vaccination and not resumed until 14 days later. Patients taking high-dose corticosteroids for more than 2 weeks should not be vaccinated until at least 1 month after therapy is completed.
In contrast, HZ vaccine is not contraindicated for leukemia patients who are in remission and who have not received chemotherapy or radiation for at least 3 months, or for patients receiving short-term, low-to-moderate dose, topical, intra-articular, bursal, or tendon injections of corticosteroids. Patients on low-dose methotrexate, azathioprine, or 6-mercaptopurine can also receive the vaccine.18
VACCINATION RATES ARE LOW
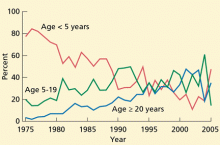
Although the vaccine has been recommended since 2008, uptake has been slow. Figure 1 shows the rate of HZ vaccination in adults age 60 and older surveyed in the National Health Interview Survey from 2007 to 2013.35 Eight years after the vaccine was licensed, only 28% of eligible patients had been vaccinated. Assuming the current rate of increase remains constant, it will take 7 more years to reach a 60% coverage rate—the same as for pneumococcal vaccine36—and 18 years to reach universal coverage.
Barriers to vaccination
Several barriers to HZ vaccination might account for the slow uptake.
For the first few years the vaccine was available, the requirement to store it frozen presented an obstacle for some physicians.37 Physicians may also have been discouraged by the cumbersome Medicare reimbursement process because while the administration fee is covered through Medicare Part B, the live-attenuated vaccine is reimbursed only through Medicare Part D, a benefit that varies by plans. Other barriers to physicians are supply shortages, high up-front costs, and uncertainties regarding the duration of vaccine protection, its safety, and side effects.38–40
Patient barriers include lack of physician recommendation, lack of familiarity with the vaccine, high out-of-pocket costs, the perception that they are at low risk for HZ, underestimation of the pain associated with HZ and postherpetic neuralgia, and fear of vaccine adverse effects.39,41,42
Interventions to increase vaccination rates
Certain interventions have been shown to increase vaccination adherence in general and HZ vaccination in particular. In randomized trials involving other vaccines, electronic medical record reminders supporting panel management or nurse-initiated protocols have been proven to increase vaccination rates, but these methods have not been tested for HZ vaccine specifically.43,44
In an observational study, Chaudhry et al found that the number of HZ vaccinations administered at the Mayo Clinic increased 43% in one practice and 54% in another after the implementation of an electronic alert.45 A randomized controlled trial showed that an informational package discussing HZ and the vaccine sent to patients via either their electronic personal health record or traditional mail increased HZ vaccination by almost 3 times.46
Pharmacists can also influence vaccination rates. States that provide full immunization privileges to pharmacists have vaccination rates significantly higher than states with restricted or no authorization.47
COST-EFFECTIVENESS CONSIDERATIONS
Unlike the Centers for Medicare and Medicaid Services, the ACIP does consider cost-effectiveness in their vaccine recommendations. Because of the morbidity associated with HZ and postherpetic neuralgia as well as the economic impact, vaccination is generally considered cost-effective for adults age 60 and older.48,49
Analyses have demonstrated that cost-effectiveness hinges on 4 factors: initial vaccine efficacy, the duration of efficacy, the age-specific incidence of HZ, and the cost of the vaccine.
For patients ages 50 to 59, the incidence of HZ is low, and because the duration of vaccine efficacy is short even though initial vaccine efficacy is high, vaccination in this age group offers poor value.50 At older ages, the incidence of HZ and postherpetic neuralgia rises, making vaccination more cost-effective. After age 60, the vaccine is cost-effective at all ages, although age 70 appears to offer the optimal trade-off between increasing incidence and declining vaccine efficacy.48,49
For patients who plan to be vaccinated only once, waiting until age 70 would appear to offer the best value.51 For those who are willing to receive a booster dose, the optimal age for vaccination is unknown, but will likely depend on the effectiveness, cost, and duration of the booster.
A NEW HZ VACCINE
In 2015, GlaxoSmithKline tested a new HZ vaccine containing a single VZV glycoprotein in an AS01B adjuvant system (HZ/su vaccine).52 In a phase 3 randomized trial involving 15,411 immunocompetent persons age 50 and older, a 2-dose schedule of HZ/su vaccine was 97% effective in preventing HZ (Table 1).53 Importantly, the vaccine was equally effective in older patients.
This vaccine also had a high rate of adverse reactions, with 17% of vaccine recipients vs 3% of placebo recipients reporting events that prevented normal everyday activities for at least 1 day. However, the rate of serious adverse reactions was the same in both groups (9%). The company announced that they intended to submit a regulatory application for HZ/su vaccine in the second half of 2016.54
Because of its high efficacy, HZ/su vaccine has the potential to change practice, but several issues must be resolved before it can supplant the current vaccine.
First, the AS01B adjuvant is not currently licensed in the United States, so it is unclear if the HZ/su vaccine can get FDA approval.52,55
Second, there are several questions about the efficacy of the vaccine, including long-term efficacy, efficacy in the elderly, and efficacy in the case of a patient receiving only 1 of the 2 required doses.
Third, the impact of HZ/su vaccine on complications such as postherpetic neuralgia has not been established. The clinical trial (NCT01165229) examining vaccine efficacy against postherpetic neuralgia incidence and other complications in adults age 70 and older has recently been completed and data should be available soon. Given the extremely high efficacy against HZ, it is likely that it will be close to 100% effective against this complication.
Fourth, there is uncertainty as to how the HZ/su vaccine should be used in patients who have already received the live-attenuated vaccine, if it is determined that a booster is necessary.
Finally, the vaccine is not yet priced. Given its superior effectiveness, particularly in older individuals, competitive pricing could dramatically affect the market. How Medicare or other insurers cover the new vaccine will likely influence its acceptance.
HZ VACCINATION OF IMMUNOCOMPROMISED PATIENTS
Immunocompromised patients are at highest risk for developing HZ. Unfortunately, there are currently no HZ vaccines approved for use in this population. The current live-attenuated vaccine has been demonstrated to be safe, well tolerated, and immunogenic in patients age 60 and older who are receiving chronic or maintenance low to moderate doses of corticosteroids.56
A clinical trial is being conducted to assess the immunogenicity, clinical effectiveness, and safety of the vaccine in rheumatoid arthritis patients receiving antitumor necrosis factor therapy (NCT01967316). Other trials are examining vaccine efficacy and safety in patients with solid organ tumors prior to chemotherapy (NCT02444936) and in patients who will be undergoing living donor kidney transplantation (NCT00940940). Researchers are also investigating the possibility of vaccinating allogeneic stem cell donors before donation in order to protect transplant recipients against HZ (NCT01573182).
ZVHT and HZ/su vaccination in immunocompromised patients
Heat-treated varicella-zoster vaccine (ZVHT) is a potential alternative for immunocompromised patients. A 4-dose regimen has been proven to reduce the risk of HZ in patients receiving autologous hematopoietic-cell transplants for non-Hodgkin or Hodgkin lymphoma.57
In another trial, the 4-dose ZVHT was safe and elicited significant VZV-specific T-cell response through 28 days in immunosuppressed patients with solid tumor malignancy, hematologic malignancy, human immunodeficiency virus infection with CD4 counts of 200 cells/mm3 or less, and autologous hematopoietic-cell transplants. The T-cell response was poor in allogeneic hematopoietic-cell transplant recipients, however.58
Because the HZ/su vaccine does not contain live virus, it seems particularly promising for immunocompromised patients. In phase 1 and 2 studies, a 3-dose regimen has been shown to be safe and immunogenic in hematopoietic-cell transplant recipients and HIV-infected adults with CD4 count higher than 200 cells/mm3.59,60 A phase 3 trial assessing the efficacy of HZ/su vaccine in autologous hematopoietic-cell transplant recipients is under way (NCT01610414). Changes in recommendations for HZ vaccine in these most vulnerable populations await the results of these studies.
- Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis 2007; 44(suppl 1):S1–S26.
- Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines 2010; 9(suppl):21–26.
- Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev 2014; 2:CD006866.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4:e004833.
- Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA 2011; 305:160–166.
- Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med 2013; 10:e1001420.
- Yawn BP, Saddier P, Wollan PC, St. Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007; 82:1341–1349.
- Yawn BP, Wollan PC, Kurland MJ, St. Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc 2011; 86:88–93.
- Chen SY, Suaya JA, Li Q, et al. Incidence of herpes zoster in patients with altered immune function. Infection 2014; 42:325–334.
- Edmunds WJ, Brisson M. The effect of vaccination on the epidemiology of varicella zoster virus. J Infect 2002; 44:211–219.
- Hales CM, Harpaz R, Joesoef MR, Bialek SR. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann Intern Med 2013; 159:739–745.
- Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis 2011; 52:332–340.
- Rimland D, Moanna A. Increasing incidence of herpes zoster among veterans. Clin Infect Dis 2010; 50:1000–1005.
- Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992-2002. J Infect Dis 2005; 191:2002–2007.
- Plotkin SA, Starr SE, Connor K, Morton D. Zoster in normal children after varicella vaccine. J Infect Dis 1989; 159:1000–1001.
- Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain 2005; 6:356–363.
- Johnson RW, Bouhassira D, Kassianos G, Leplege A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med 2010; 8:37.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1–30.
- Panatto D, Bragazzi NL, Rizzitelli E, et al. Evaluation of the economic burden of herpes zoster (HZ) infection. Hum Vaccin Immunother 2015; 11:245–262.
- Yawn BP, Itzler RF, Wollan PC, Pellissier JM, Sy LS, Saddier P. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc 2009; 84:787–794.
- Dworkin RH, White R, O’Connor AB, Hawkins K. Health care expenditure burden of persisting herpes zoster pain. Pain Med 2008; 9:348–353.
- White RR, Lenhart G, Singhal PK, et al. Incremental 1-year medical resource utilization and costs for patients with herpes zoster from a set of US health plans. Pharmacoeconomics 2009; 27:781–792.
- Insinga RP, Itzler RF, Pellissier JM. Acute/subacute herpes zoster: healthcare resource utilisation and costs in a group of US health plans. Pharmacoeconomics 2007; 25:155–169.
- Singhal PK, Makin C, Pellissier J, Sy L, White R, Saddier P. Work and productivity loss related to herpes zoster. J Med Econ 2011; 14:639–645.
- US Bureau of Labor Statistics. Labor force statistics from the current population survey. www.bls.gov/web/empsit/cpseea13.htm. Accessed April 6, 2017.
- US Bureau of Labor Statistic. Occupational employment statistics. www.bls.gov/oes/current/oes_nat.htm. Accessed April 6, 2017.
- Oxman MN, Levin MJ, Johnson GR, et al; Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–2284.
- Food and Drug Administration (FDA). FDA clinical briefing document for Merck & Co., Inc. Zoster vaccine live (Oka/Merck) Zostavax. www.fda.gov/ohrms/dockets/ac/05/briefing/5-4198b2_1.pdf. Accessed April 6, 2017.
- Simberkoff MS, Arbeit RD, Johnson GR, et al; Shingles Prevention Study Group. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Ann Intern Med 2010; 152:545–554.
- Schmader KE, Oxman MN, Levin MJ, et al; Shingles Prevention Study Group. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis 2012; 55:1320–1328.
- Morrison VA, Johnson GR, Schmader KE, et al; Shingles Prevention Study Group. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 2015; 60:900–909.
- Levin MJ, Schmader KE, Pang L, et al. Cellular and humoral responses to a second dose of herpes zoster vaccine administered 10 years after the first dose among older adults. J Infect Dis 2016; 213:14–22.
- Schmader KE, Levin MJ, Gnann JW Jr, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis 2012; 54:922–928.
- Hales CM, Harpaz R, Ortega-Sanchez I, Bialek SR; Centers for Disease Control and Prevention (CDC). Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep 2014; 63:729–731.
- Centers for Disease Control and Prevention (CDC). Surveillance of vaccination coverage among adult populations—United States, 2014. MMWR Morb Mortal Wkly Rep 2016; 65(1):1–36. Accessed April 12, 2017.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination—United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64:95–102.
- Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis 2010; 51:197–213.
- Hurley LP, Lindley MC, Harpaz R, et al. Barriers to the use of herpes zoster vaccine. Ann Intern Med 2010; 152:555–560.
- Lu PJ, Euler GL, Jumaan AO, Harpaz R. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine 2009; 27:882–887.
- Hurley LP, Harpaz R, Daley MF, et al. National survey of primary care physicians regarding herpes zoster and the herpes zoster vaccine. J Infect Dis 2008; 197(suppl 2):S216–S223.
- Joon Lee T, Hayes S, Cummings DM, et al. Herpes zoster knowledge, prevalence, and vaccination rate by race. J Am Board Fam Med 2013; 26:45–51.
- Opstelten W, van Essen GA, Hak E. Determinants of non-compliance with herpes zoster vaccination in the community-dwelling elderly. Vaccine 2009; 27:192–196.
- Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med 2011; 171:1552–1558.
- Rhew DC, Glassman PA, Goetz MB. Improving pneumococcal vaccine rates. Nurse protocols versus clinical reminders. J Gen Intern Med 1999; 14:351–356.
- Chaudhry R, Schietel SM, North F, Dejesus R, Kesman RL, Stroebel RJ. Improving rates of herpes zoster vaccination with a clinical decision support system in a primary care practice. J Eval Clin Pract 2013; 19:263–266.
- Otsuka SH, Tayal NH, Porter K, Embi PJ, Beatty SJ. Improving herpes zoster vaccination rates through use of a clinical pharmacist and a personal health record. Am J Med 2013; 126:832.e1–832.e6.
- Taitel MS, Fensterheim LE, Cannon AE, Cohen ES. Improving pneumococcal and herpes zoster vaccination uptake: expanding pharmacist privileges. Am J Manag Care 2013; 19:e309–e313.
- Kawai K, Preaud E, Baron-Papillon F, Largeron N, Acosta CJ. Cost-effectiveness of vaccination against herpes zoster and postherpetic neuralgia: a critical review. Vaccine 2014; 32:1645–1653.
- Szucs TD, Pfeil AM. A systematic review of the cost effectiveness of herpes zoster vaccination. Pharmacoeconomics 2013; 31:125–136.
- Le P, Rothberg MB. Cost-effectiveness of herpes zoster vaccine for persons aged 50 years. Ann Intern Med 2015; 163:489–497.
- Le P, Rothberg MB. Determining the optimal age to vaccinate against herpes zoster: a cost-effectiveness analysis. Society for Medical Decision Making 37th Annual North American Meeting. St. Louis, MO; October 18-21, 2015.
- Cohen JI. Clinical practice: herpes zoster. N Engl J Med 2013; 369:255–263.
- Lal H, Cunningham AL, Godeaux O, et al; ZOE-50 Study Group. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–2096.
- GlaxoSmithKline plc. GSK’s candidate shingles vaccine demonstrates 90% efficacy against shingles in people 70 years of age and over. www.gsk.com/en-gb/media/press-releases/gsk-s-candidate-shingles-vaccine-demonstrates-90-efficacy-against-shingles-in-people-70-years-of-age-and-over/. Accessed April 6, 2017.
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med 2013; 19:1597–1608.
- Russell AF, Parrino J, Fisher CL Jr, et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects on chronic/maintenance corticosteroids. Vaccine 2015; 33:3129–3134.
- Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med 2002; 347:26–34.
- Mullane KM, Winston DJ, Wertheim MS, et al. Safety and immunogenicity of heat-treated zoster vaccine (ZVHT) in immunocompromised adults. J Infect Dis 2013; 208:1375–1385.
- Stadtmauer EA, Sullivan KM, Marty FM, et al. A phase 1/2 study of an adjuvanted varicella-zoster virus subunit vaccine in autologous hematopoietic cell transplant recipients. Blood 2014; 124:2921–2929.
- Berkowitz EM, Moyle G, Stellbrink HJ, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis 2015; 211:1279–1287.
Herpes zoster (HZ), or shingles, represents a reactivation of the varicella-zoster virus (VZV). Following primary infection, usually in childhood, the virus typically lies dormant in the dorsal root and sensory nerve ganglia for decades. The precise mechanism of reactivation is not well understood, but it is associated with a decline in cell-mediated immunity that occurs with advancing age, immune-compromising conditions such as HIV infection and cancer, or immunosuppressive therapies, including corticosteroids.1 HZ is usually a self-limited disease characterized by unilateral dermatomal rash and pain, but can cause disseminated infection in immunocompromised individuals.2
Treatment with antiviral medications within 72 hours of rash onset can reduce acute HZ symptoms.1 However, antiviral agents are only minimally effective in preventing postherpetic neuralgia, the most common complication of HZ.3 Therefore, efforts to reduce the burden of HZ morbidity have focused on prevention through vaccination.
Currently, the only shingles vaccine approved by the US Food and Drug Administration (FDA) is Zostavax (Merck), which contains the live-attenuated Oka strain of VZV at a concentration 14 times greater than that of the varicella vaccine (Varivax, Merck). The live-attenuated vaccine boosts VZV-specific cell-mediated immunity, preventing reactivation of the latent virus.
In this article, we describe the burden of disease and review recent developments in the literature on HZ vaccine, including duration of efficacy, uptake and barriers to vaccination, cost-effectiveness, and the outlook for future vaccines.
INCIDENCE INCREASES WITH AGE
The incidence of herpes zoster in the general population is between 3 and 5 per 1,000 person-years4 and increases with age, especially after age 60 when the incidence can approach 13 to 15 per 1,000 person-years.5,6 An estimated 1 million new cases occur each year in the United States, and about 6% of patients experience a second episode of HZ within 8 years.7,8 In immunocompromised patients, the incidence of HZ is 2 to 10 times higher than in the general population.9
The incidence of HZ has been increasing for reasons that are unclear. After varicella vaccine was introduced into the routine childhood immunization schedule in 1995, it was hypothesized that the resultant decrease in primary varicella infections would remove a natural source of immune boosting and cause an increase in HZ incidence for up to 20 years.10 However, recent studies demonstrate that the observed increase in HZ incidence actually predates the introduction of varicella vaccine,11–13 and the widespread use of varicella vaccine has not resulted in an increase in the incidence of HZ.14
Other potential explanations for the rise in reported incidence include increasing awareness among patients, who might previously not have sought care and among physicians, who may be more likely to make the diagnosis. Advertisement of new treatments for HZ, including gabapentin and capsaicin, probably began to increase awareness in the 1990s, as did promotion of the HZ vaccine after its licensure in 2006.
HZ can occur in people who have been vaccinated against varicella due to reactivation of the vaccine-strain virus, but the risk is lower than after infection with wild-type varicella.15 Given that the varicella vaccine has been routinely used in children for only 20 years, the long-term effect of varicella vaccination on the incidence of HZ in elderly people is unknown.
Serious complications
HZ can cause rare but serious complications including encephalitis, herpes ophthalmicus, herpes oticus, myelitis, and retinitis.1 These can lead to long-term disability including unilateral blindness and deafness.
The most common and debilitating complication is postherpetic neuralgia, a persistent pain lasting at least 3 months, with a mean duration of 3.3 years and sometimes as long as 10 years.16 Postherpetic neuralgia occurs in 8% to 32% of patients after acute HZ,4 and the incidence increases with age, being most common after age 70. The chronic pain of postherpetic neuralgia has a significant adverse impact on patients’ quality of life, including physical disability and emotional distress.17 Some pain is intense, and anecdotal reports of patients committing suicide were included in the Advisory Committee on Immunization Practices (ACIP) recommendations regarding herpes zoster vaccine.18
HZ and its complications also impose a substantial economic burden on society.19 In a population-based study, the mean direct medical costs of HZ ranged from $620 to $1,160 (2015 dollars) depending on age,20 and the mean costs of postherpetic neuralgia were 2 to 5 times higher than that.20–22 Immunocompromised patients had costs 2 to 3 times higher than those of immunocompetent adults.23 In addition, for employed patients, HZ resulted in an average loss of 32 hours of work due to absenteeism and 84 hours due to presenteeism (ie, working while sick and therefore suboptimally).24
Assuming there are 1 million cases of HZ each year, if 8% to 32% of patients go on to develop postherpetic neuralgia, that would translate into approximately $1 to $2 billion in direct medical costs. With 60% of adult patients working,25 at an average wage of $23.23 per hour,26 HZ illness could be responsible for another $1.6 billion in lost productivity.
EFFICACY AND SAFETY OF HZ VACCINE
In 2006, the FDA approved the live-attenuated Oka strain VZV vaccine for prevention of HZ and postherpetic neuralgia in adults age 60 and older based on findings from the Shingles Prevention Study (SPS).27
The Shingles Prevention Study
This multicenter randomized placebo-controlled trial27 enrolled 38,546 immunocompetent persons age 60 and older. Subjects in the intervention group received a single dose of live-attenuated vaccine, and all participants were followed for up to 4.9 years after vaccination.
HZ occurred in 315 (1.636%) of the 19,254 participants in the vaccine group and in 642 (3.336%) of the 19,247 participants in the placebo group, an absolute risk reduction of 1.7%, number needed to treat 59, relative risk reduction 51%, P < .001. Similarly, postherpetic neuralgia occurred in 27 (0.140%) of the 19,254 vaccine recipients and in 80 (0.416%) of the placebo recipients (an absolute risk reduction of 0.276%, number needed to treat 362, relative risk reduction 66%, P < .001). The investigators calculated that vaccination reduced the overall burden of illness by 61% (Table 1).
The efficacy against HZ incidence decreased with age,28 but the efficacy against postherpetic neuralgia did not. In addition, vaccine recipients who developed HZ generally had less severe manifestations.
The safety of the vaccine was assessed for all participants in the SPS. In addition, one-sixth of SPS participants were enrolled in a safety substudy. These participants completed a detailed report card regarding all medically important events within the first 42 days. Forty-eight percent of the vaccine group and 17% of the placebo group (P < .05) experienced adverse events, primarily at the injection site. Less than 1% of all local reactions were severe.29 Serious adverse events were rare (< 2%), but occurred significantly more often in the vaccinated group.
Short-Term Persistence Substudy
Short-term efficacy of the live-attenuated vaccine (up to 7 years) was assessed in the Short-Term Persistence Substudy (STPS), which involved 14,270 of the initial participants and reported yearly and overall vaccine efficacy.30 After 5 years, the yearly efficacy against postherpetic neuralgia incidence declined to 32% and was no longer statistically significant. Efficacy against HZ incidence and burden of illness displayed the same pattern. After the end of the STPS, all subjects in the placebo group received vaccination.
Long-Term Persistence Substudy
Those in the intervention group were followed for an additional 4 years in the Long-Term Persistence Substudy (LTPS).31 Due to the lack of concurrent controls in the LTPS, the authors used regression models based on historical controls to estimate contemporary population incidence of HZ and postherpetic neuralgia for comparison.
Efficacy continued to decline over time, and by 10 years after vaccination there was no difference between vaccinated patients and historical controls in the rate of any end point (ie, efficacy declined to zero).
A trial of booster vaccination
Because many patients are vaccinated at age 60, waning immunity could leave them vulnerable to HZ and postherpetic neuralgia by age 70. A potential solution would be to give a booster dose after 10 years.
A recent phase 3 clinical trial of adults age 70 years and older found that a booster dose of live-attenuated vaccine was as safe and immunogenic as an initial dose.32 While antibody responses were similar in the boosted group and the newly vaccinated group, cell-mediated immunity was higher in the boosted group.
Because prevention of HZ is generally via cell-mediated immunity, the booster might be more effective than the initial vaccination, but clinical trials measuring actual cases prevented will be required to prove it. A booster dose is not currently recommended.
A trial of vaccination in adults 50 to 59
In 2011, the FDA extended its approval of HZ vaccine for use in adults ages 50 to 59.33
In a randomized, double-blind, placebo-controlled trial in this age group,33 the vaccine reduced HZ incidence by almost 70% (absolute risk reduction 0.614%, number needed to treat 156; Table 1), but the severity of HZ cases was not affected. There were too few cases of postherpetic neuralgia to assess the efficacy for this end point. The study followed patients for only 1.5 years after vaccination, so the duration of efficacy is unknown.
As in the older recipients, the vaccine was well tolerated; injection-site reactions and headache were the major adverse effects reported among vaccine recipients.33
INDICATIONS AND CONTRAINDICATIONS
Although HZ vaccine is licensed for use in adults age 50 and older, the ACIP recommends it only for immunocompetent adults age 60 and older. At this time, the ACIP does not recommend HZ vaccine in those younger than 60 because of the low risk of HZ in this age group.34
Any person age 60 or older should receive a single dose of the live-attenuated HZ vaccine subcutaneously, regardless of past history of HZ.
The vaccine is contraindicated in patients who have a history of allergic reaction to any vaccine component, immunosuppression or immunodeficiency conditions, and pregnancy. Specifically, people who will receive immunosuppressive therapies should have the vaccine at least 14 days before beginning treatment. Antiviral medications such as acyclovir, famciclovir, and valacyclovir should be discontinued at least 24 hours before vaccination and not resumed until 14 days later. Patients taking high-dose corticosteroids for more than 2 weeks should not be vaccinated until at least 1 month after therapy is completed.
In contrast, HZ vaccine is not contraindicated for leukemia patients who are in remission and who have not received chemotherapy or radiation for at least 3 months, or for patients receiving short-term, low-to-moderate dose, topical, intra-articular, bursal, or tendon injections of corticosteroids. Patients on low-dose methotrexate, azathioprine, or 6-mercaptopurine can also receive the vaccine.18
VACCINATION RATES ARE LOW
Although the vaccine has been recommended since 2008, uptake has been slow. Figure 1 shows the rate of HZ vaccination in adults age 60 and older surveyed in the National Health Interview Survey from 2007 to 2013.35 Eight years after the vaccine was licensed, only 28% of eligible patients had been vaccinated. Assuming the current rate of increase remains constant, it will take 7 more years to reach a 60% coverage rate—the same as for pneumococcal vaccine36—and 18 years to reach universal coverage.
Barriers to vaccination
Several barriers to HZ vaccination might account for the slow uptake.
For the first few years the vaccine was available, the requirement to store it frozen presented an obstacle for some physicians.37 Physicians may also have been discouraged by the cumbersome Medicare reimbursement process because while the administration fee is covered through Medicare Part B, the live-attenuated vaccine is reimbursed only through Medicare Part D, a benefit that varies by plans. Other barriers to physicians are supply shortages, high up-front costs, and uncertainties regarding the duration of vaccine protection, its safety, and side effects.38–40
Patient barriers include lack of physician recommendation, lack of familiarity with the vaccine, high out-of-pocket costs, the perception that they are at low risk for HZ, underestimation of the pain associated with HZ and postherpetic neuralgia, and fear of vaccine adverse effects.39,41,42
Interventions to increase vaccination rates
Certain interventions have been shown to increase vaccination adherence in general and HZ vaccination in particular. In randomized trials involving other vaccines, electronic medical record reminders supporting panel management or nurse-initiated protocols have been proven to increase vaccination rates, but these methods have not been tested for HZ vaccine specifically.43,44
In an observational study, Chaudhry et al found that the number of HZ vaccinations administered at the Mayo Clinic increased 43% in one practice and 54% in another after the implementation of an electronic alert.45 A randomized controlled trial showed that an informational package discussing HZ and the vaccine sent to patients via either their electronic personal health record or traditional mail increased HZ vaccination by almost 3 times.46
Pharmacists can also influence vaccination rates. States that provide full immunization privileges to pharmacists have vaccination rates significantly higher than states with restricted or no authorization.47
COST-EFFECTIVENESS CONSIDERATIONS
Unlike the Centers for Medicare and Medicaid Services, the ACIP does consider cost-effectiveness in their vaccine recommendations. Because of the morbidity associated with HZ and postherpetic neuralgia as well as the economic impact, vaccination is generally considered cost-effective for adults age 60 and older.48,49
Analyses have demonstrated that cost-effectiveness hinges on 4 factors: initial vaccine efficacy, the duration of efficacy, the age-specific incidence of HZ, and the cost of the vaccine.
For patients ages 50 to 59, the incidence of HZ is low, and because the duration of vaccine efficacy is short even though initial vaccine efficacy is high, vaccination in this age group offers poor value.50 At older ages, the incidence of HZ and postherpetic neuralgia rises, making vaccination more cost-effective. After age 60, the vaccine is cost-effective at all ages, although age 70 appears to offer the optimal trade-off between increasing incidence and declining vaccine efficacy.48,49
For patients who plan to be vaccinated only once, waiting until age 70 would appear to offer the best value.51 For those who are willing to receive a booster dose, the optimal age for vaccination is unknown, but will likely depend on the effectiveness, cost, and duration of the booster.
A NEW HZ VACCINE
In 2015, GlaxoSmithKline tested a new HZ vaccine containing a single VZV glycoprotein in an AS01B adjuvant system (HZ/su vaccine).52 In a phase 3 randomized trial involving 15,411 immunocompetent persons age 50 and older, a 2-dose schedule of HZ/su vaccine was 97% effective in preventing HZ (Table 1).53 Importantly, the vaccine was equally effective in older patients.
This vaccine also had a high rate of adverse reactions, with 17% of vaccine recipients vs 3% of placebo recipients reporting events that prevented normal everyday activities for at least 1 day. However, the rate of serious adverse reactions was the same in both groups (9%). The company announced that they intended to submit a regulatory application for HZ/su vaccine in the second half of 2016.54
Because of its high efficacy, HZ/su vaccine has the potential to change practice, but several issues must be resolved before it can supplant the current vaccine.
First, the AS01B adjuvant is not currently licensed in the United States, so it is unclear if the HZ/su vaccine can get FDA approval.52,55
Second, there are several questions about the efficacy of the vaccine, including long-term efficacy, efficacy in the elderly, and efficacy in the case of a patient receiving only 1 of the 2 required doses.
Third, the impact of HZ/su vaccine on complications such as postherpetic neuralgia has not been established. The clinical trial (NCT01165229) examining vaccine efficacy against postherpetic neuralgia incidence and other complications in adults age 70 and older has recently been completed and data should be available soon. Given the extremely high efficacy against HZ, it is likely that it will be close to 100% effective against this complication.
Fourth, there is uncertainty as to how the HZ/su vaccine should be used in patients who have already received the live-attenuated vaccine, if it is determined that a booster is necessary.
Finally, the vaccine is not yet priced. Given its superior effectiveness, particularly in older individuals, competitive pricing could dramatically affect the market. How Medicare or other insurers cover the new vaccine will likely influence its acceptance.
HZ VACCINATION OF IMMUNOCOMPROMISED PATIENTS
Immunocompromised patients are at highest risk for developing HZ. Unfortunately, there are currently no HZ vaccines approved for use in this population. The current live-attenuated vaccine has been demonstrated to be safe, well tolerated, and immunogenic in patients age 60 and older who are receiving chronic or maintenance low to moderate doses of corticosteroids.56
A clinical trial is being conducted to assess the immunogenicity, clinical effectiveness, and safety of the vaccine in rheumatoid arthritis patients receiving antitumor necrosis factor therapy (NCT01967316). Other trials are examining vaccine efficacy and safety in patients with solid organ tumors prior to chemotherapy (NCT02444936) and in patients who will be undergoing living donor kidney transplantation (NCT00940940). Researchers are also investigating the possibility of vaccinating allogeneic stem cell donors before donation in order to protect transplant recipients against HZ (NCT01573182).
ZVHT and HZ/su vaccination in immunocompromised patients
Heat-treated varicella-zoster vaccine (ZVHT) is a potential alternative for immunocompromised patients. A 4-dose regimen has been proven to reduce the risk of HZ in patients receiving autologous hematopoietic-cell transplants for non-Hodgkin or Hodgkin lymphoma.57
In another trial, the 4-dose ZVHT was safe and elicited significant VZV-specific T-cell response through 28 days in immunosuppressed patients with solid tumor malignancy, hematologic malignancy, human immunodeficiency virus infection with CD4 counts of 200 cells/mm3 or less, and autologous hematopoietic-cell transplants. The T-cell response was poor in allogeneic hematopoietic-cell transplant recipients, however.58
Because the HZ/su vaccine does not contain live virus, it seems particularly promising for immunocompromised patients. In phase 1 and 2 studies, a 3-dose regimen has been shown to be safe and immunogenic in hematopoietic-cell transplant recipients and HIV-infected adults with CD4 count higher than 200 cells/mm3.59,60 A phase 3 trial assessing the efficacy of HZ/su vaccine in autologous hematopoietic-cell transplant recipients is under way (NCT01610414). Changes in recommendations for HZ vaccine in these most vulnerable populations await the results of these studies.
Herpes zoster (HZ), or shingles, represents a reactivation of the varicella-zoster virus (VZV). Following primary infection, usually in childhood, the virus typically lies dormant in the dorsal root and sensory nerve ganglia for decades. The precise mechanism of reactivation is not well understood, but it is associated with a decline in cell-mediated immunity that occurs with advancing age, immune-compromising conditions such as HIV infection and cancer, or immunosuppressive therapies, including corticosteroids.1 HZ is usually a self-limited disease characterized by unilateral dermatomal rash and pain, but can cause disseminated infection in immunocompromised individuals.2
Treatment with antiviral medications within 72 hours of rash onset can reduce acute HZ symptoms.1 However, antiviral agents are only minimally effective in preventing postherpetic neuralgia, the most common complication of HZ.3 Therefore, efforts to reduce the burden of HZ morbidity have focused on prevention through vaccination.
Currently, the only shingles vaccine approved by the US Food and Drug Administration (FDA) is Zostavax (Merck), which contains the live-attenuated Oka strain of VZV at a concentration 14 times greater than that of the varicella vaccine (Varivax, Merck). The live-attenuated vaccine boosts VZV-specific cell-mediated immunity, preventing reactivation of the latent virus.
In this article, we describe the burden of disease and review recent developments in the literature on HZ vaccine, including duration of efficacy, uptake and barriers to vaccination, cost-effectiveness, and the outlook for future vaccines.
INCIDENCE INCREASES WITH AGE
The incidence of herpes zoster in the general population is between 3 and 5 per 1,000 person-years4 and increases with age, especially after age 60 when the incidence can approach 13 to 15 per 1,000 person-years.5,6 An estimated 1 million new cases occur each year in the United States, and about 6% of patients experience a second episode of HZ within 8 years.7,8 In immunocompromised patients, the incidence of HZ is 2 to 10 times higher than in the general population.9
The incidence of HZ has been increasing for reasons that are unclear. After varicella vaccine was introduced into the routine childhood immunization schedule in 1995, it was hypothesized that the resultant decrease in primary varicella infections would remove a natural source of immune boosting and cause an increase in HZ incidence for up to 20 years.10 However, recent studies demonstrate that the observed increase in HZ incidence actually predates the introduction of varicella vaccine,11–13 and the widespread use of varicella vaccine has not resulted in an increase in the incidence of HZ.14
Other potential explanations for the rise in reported incidence include increasing awareness among patients, who might previously not have sought care and among physicians, who may be more likely to make the diagnosis. Advertisement of new treatments for HZ, including gabapentin and capsaicin, probably began to increase awareness in the 1990s, as did promotion of the HZ vaccine after its licensure in 2006.
HZ can occur in people who have been vaccinated against varicella due to reactivation of the vaccine-strain virus, but the risk is lower than after infection with wild-type varicella.15 Given that the varicella vaccine has been routinely used in children for only 20 years, the long-term effect of varicella vaccination on the incidence of HZ in elderly people is unknown.
Serious complications
HZ can cause rare but serious complications including encephalitis, herpes ophthalmicus, herpes oticus, myelitis, and retinitis.1 These can lead to long-term disability including unilateral blindness and deafness.
The most common and debilitating complication is postherpetic neuralgia, a persistent pain lasting at least 3 months, with a mean duration of 3.3 years and sometimes as long as 10 years.16 Postherpetic neuralgia occurs in 8% to 32% of patients after acute HZ,4 and the incidence increases with age, being most common after age 70. The chronic pain of postherpetic neuralgia has a significant adverse impact on patients’ quality of life, including physical disability and emotional distress.17 Some pain is intense, and anecdotal reports of patients committing suicide were included in the Advisory Committee on Immunization Practices (ACIP) recommendations regarding herpes zoster vaccine.18
HZ and its complications also impose a substantial economic burden on society.19 In a population-based study, the mean direct medical costs of HZ ranged from $620 to $1,160 (2015 dollars) depending on age,20 and the mean costs of postherpetic neuralgia were 2 to 5 times higher than that.20–22 Immunocompromised patients had costs 2 to 3 times higher than those of immunocompetent adults.23 In addition, for employed patients, HZ resulted in an average loss of 32 hours of work due to absenteeism and 84 hours due to presenteeism (ie, working while sick and therefore suboptimally).24
Assuming there are 1 million cases of HZ each year, if 8% to 32% of patients go on to develop postherpetic neuralgia, that would translate into approximately $1 to $2 billion in direct medical costs. With 60% of adult patients working,25 at an average wage of $23.23 per hour,26 HZ illness could be responsible for another $1.6 billion in lost productivity.
EFFICACY AND SAFETY OF HZ VACCINE
In 2006, the FDA approved the live-attenuated Oka strain VZV vaccine for prevention of HZ and postherpetic neuralgia in adults age 60 and older based on findings from the Shingles Prevention Study (SPS).27
The Shingles Prevention Study
This multicenter randomized placebo-controlled trial27 enrolled 38,546 immunocompetent persons age 60 and older. Subjects in the intervention group received a single dose of live-attenuated vaccine, and all participants were followed for up to 4.9 years after vaccination.
HZ occurred in 315 (1.636%) of the 19,254 participants in the vaccine group and in 642 (3.336%) of the 19,247 participants in the placebo group, an absolute risk reduction of 1.7%, number needed to treat 59, relative risk reduction 51%, P < .001. Similarly, postherpetic neuralgia occurred in 27 (0.140%) of the 19,254 vaccine recipients and in 80 (0.416%) of the placebo recipients (an absolute risk reduction of 0.276%, number needed to treat 362, relative risk reduction 66%, P < .001). The investigators calculated that vaccination reduced the overall burden of illness by 61% (Table 1).
The efficacy against HZ incidence decreased with age,28 but the efficacy against postherpetic neuralgia did not. In addition, vaccine recipients who developed HZ generally had less severe manifestations.
The safety of the vaccine was assessed for all participants in the SPS. In addition, one-sixth of SPS participants were enrolled in a safety substudy. These participants completed a detailed report card regarding all medically important events within the first 42 days. Forty-eight percent of the vaccine group and 17% of the placebo group (P < .05) experienced adverse events, primarily at the injection site. Less than 1% of all local reactions were severe.29 Serious adverse events were rare (< 2%), but occurred significantly more often in the vaccinated group.
Short-Term Persistence Substudy
Short-term efficacy of the live-attenuated vaccine (up to 7 years) was assessed in the Short-Term Persistence Substudy (STPS), which involved 14,270 of the initial participants and reported yearly and overall vaccine efficacy.30 After 5 years, the yearly efficacy against postherpetic neuralgia incidence declined to 32% and was no longer statistically significant. Efficacy against HZ incidence and burden of illness displayed the same pattern. After the end of the STPS, all subjects in the placebo group received vaccination.
Long-Term Persistence Substudy
Those in the intervention group were followed for an additional 4 years in the Long-Term Persistence Substudy (LTPS).31 Due to the lack of concurrent controls in the LTPS, the authors used regression models based on historical controls to estimate contemporary population incidence of HZ and postherpetic neuralgia for comparison.
Efficacy continued to decline over time, and by 10 years after vaccination there was no difference between vaccinated patients and historical controls in the rate of any end point (ie, efficacy declined to zero).
A trial of booster vaccination
Because many patients are vaccinated at age 60, waning immunity could leave them vulnerable to HZ and postherpetic neuralgia by age 70. A potential solution would be to give a booster dose after 10 years.
A recent phase 3 clinical trial of adults age 70 years and older found that a booster dose of live-attenuated vaccine was as safe and immunogenic as an initial dose.32 While antibody responses were similar in the boosted group and the newly vaccinated group, cell-mediated immunity was higher in the boosted group.
Because prevention of HZ is generally via cell-mediated immunity, the booster might be more effective than the initial vaccination, but clinical trials measuring actual cases prevented will be required to prove it. A booster dose is not currently recommended.
A trial of vaccination in adults 50 to 59
In 2011, the FDA extended its approval of HZ vaccine for use in adults ages 50 to 59.33
In a randomized, double-blind, placebo-controlled trial in this age group,33 the vaccine reduced HZ incidence by almost 70% (absolute risk reduction 0.614%, number needed to treat 156; Table 1), but the severity of HZ cases was not affected. There were too few cases of postherpetic neuralgia to assess the efficacy for this end point. The study followed patients for only 1.5 years after vaccination, so the duration of efficacy is unknown.
As in the older recipients, the vaccine was well tolerated; injection-site reactions and headache were the major adverse effects reported among vaccine recipients.33
INDICATIONS AND CONTRAINDICATIONS
Although HZ vaccine is licensed for use in adults age 50 and older, the ACIP recommends it only for immunocompetent adults age 60 and older. At this time, the ACIP does not recommend HZ vaccine in those younger than 60 because of the low risk of HZ in this age group.34
Any person age 60 or older should receive a single dose of the live-attenuated HZ vaccine subcutaneously, regardless of past history of HZ.
The vaccine is contraindicated in patients who have a history of allergic reaction to any vaccine component, immunosuppression or immunodeficiency conditions, and pregnancy. Specifically, people who will receive immunosuppressive therapies should have the vaccine at least 14 days before beginning treatment. Antiviral medications such as acyclovir, famciclovir, and valacyclovir should be discontinued at least 24 hours before vaccination and not resumed until 14 days later. Patients taking high-dose corticosteroids for more than 2 weeks should not be vaccinated until at least 1 month after therapy is completed.
In contrast, HZ vaccine is not contraindicated for leukemia patients who are in remission and who have not received chemotherapy or radiation for at least 3 months, or for patients receiving short-term, low-to-moderate dose, topical, intra-articular, bursal, or tendon injections of corticosteroids. Patients on low-dose methotrexate, azathioprine, or 6-mercaptopurine can also receive the vaccine.18
VACCINATION RATES ARE LOW
Although the vaccine has been recommended since 2008, uptake has been slow. Figure 1 shows the rate of HZ vaccination in adults age 60 and older surveyed in the National Health Interview Survey from 2007 to 2013.35 Eight years after the vaccine was licensed, only 28% of eligible patients had been vaccinated. Assuming the current rate of increase remains constant, it will take 7 more years to reach a 60% coverage rate—the same as for pneumococcal vaccine36—and 18 years to reach universal coverage.
Barriers to vaccination
Several barriers to HZ vaccination might account for the slow uptake.
For the first few years the vaccine was available, the requirement to store it frozen presented an obstacle for some physicians.37 Physicians may also have been discouraged by the cumbersome Medicare reimbursement process because while the administration fee is covered through Medicare Part B, the live-attenuated vaccine is reimbursed only through Medicare Part D, a benefit that varies by plans. Other barriers to physicians are supply shortages, high up-front costs, and uncertainties regarding the duration of vaccine protection, its safety, and side effects.38–40
Patient barriers include lack of physician recommendation, lack of familiarity with the vaccine, high out-of-pocket costs, the perception that they are at low risk for HZ, underestimation of the pain associated with HZ and postherpetic neuralgia, and fear of vaccine adverse effects.39,41,42
Interventions to increase vaccination rates
Certain interventions have been shown to increase vaccination adherence in general and HZ vaccination in particular. In randomized trials involving other vaccines, electronic medical record reminders supporting panel management or nurse-initiated protocols have been proven to increase vaccination rates, but these methods have not been tested for HZ vaccine specifically.43,44
In an observational study, Chaudhry et al found that the number of HZ vaccinations administered at the Mayo Clinic increased 43% in one practice and 54% in another after the implementation of an electronic alert.45 A randomized controlled trial showed that an informational package discussing HZ and the vaccine sent to patients via either their electronic personal health record or traditional mail increased HZ vaccination by almost 3 times.46
Pharmacists can also influence vaccination rates. States that provide full immunization privileges to pharmacists have vaccination rates significantly higher than states with restricted or no authorization.47
COST-EFFECTIVENESS CONSIDERATIONS
Unlike the Centers for Medicare and Medicaid Services, the ACIP does consider cost-effectiveness in their vaccine recommendations. Because of the morbidity associated with HZ and postherpetic neuralgia as well as the economic impact, vaccination is generally considered cost-effective for adults age 60 and older.48,49
Analyses have demonstrated that cost-effectiveness hinges on 4 factors: initial vaccine efficacy, the duration of efficacy, the age-specific incidence of HZ, and the cost of the vaccine.
For patients ages 50 to 59, the incidence of HZ is low, and because the duration of vaccine efficacy is short even though initial vaccine efficacy is high, vaccination in this age group offers poor value.50 At older ages, the incidence of HZ and postherpetic neuralgia rises, making vaccination more cost-effective. After age 60, the vaccine is cost-effective at all ages, although age 70 appears to offer the optimal trade-off between increasing incidence and declining vaccine efficacy.48,49
For patients who plan to be vaccinated only once, waiting until age 70 would appear to offer the best value.51 For those who are willing to receive a booster dose, the optimal age for vaccination is unknown, but will likely depend on the effectiveness, cost, and duration of the booster.
A NEW HZ VACCINE
In 2015, GlaxoSmithKline tested a new HZ vaccine containing a single VZV glycoprotein in an AS01B adjuvant system (HZ/su vaccine).52 In a phase 3 randomized trial involving 15,411 immunocompetent persons age 50 and older, a 2-dose schedule of HZ/su vaccine was 97% effective in preventing HZ (Table 1).53 Importantly, the vaccine was equally effective in older patients.
This vaccine also had a high rate of adverse reactions, with 17% of vaccine recipients vs 3% of placebo recipients reporting events that prevented normal everyday activities for at least 1 day. However, the rate of serious adverse reactions was the same in both groups (9%). The company announced that they intended to submit a regulatory application for HZ/su vaccine in the second half of 2016.54
Because of its high efficacy, HZ/su vaccine has the potential to change practice, but several issues must be resolved before it can supplant the current vaccine.
First, the AS01B adjuvant is not currently licensed in the United States, so it is unclear if the HZ/su vaccine can get FDA approval.52,55
Second, there are several questions about the efficacy of the vaccine, including long-term efficacy, efficacy in the elderly, and efficacy in the case of a patient receiving only 1 of the 2 required doses.
Third, the impact of HZ/su vaccine on complications such as postherpetic neuralgia has not been established. The clinical trial (NCT01165229) examining vaccine efficacy against postherpetic neuralgia incidence and other complications in adults age 70 and older has recently been completed and data should be available soon. Given the extremely high efficacy against HZ, it is likely that it will be close to 100% effective against this complication.
Fourth, there is uncertainty as to how the HZ/su vaccine should be used in patients who have already received the live-attenuated vaccine, if it is determined that a booster is necessary.
Finally, the vaccine is not yet priced. Given its superior effectiveness, particularly in older individuals, competitive pricing could dramatically affect the market. How Medicare or other insurers cover the new vaccine will likely influence its acceptance.
HZ VACCINATION OF IMMUNOCOMPROMISED PATIENTS
Immunocompromised patients are at highest risk for developing HZ. Unfortunately, there are currently no HZ vaccines approved for use in this population. The current live-attenuated vaccine has been demonstrated to be safe, well tolerated, and immunogenic in patients age 60 and older who are receiving chronic or maintenance low to moderate doses of corticosteroids.56
A clinical trial is being conducted to assess the immunogenicity, clinical effectiveness, and safety of the vaccine in rheumatoid arthritis patients receiving antitumor necrosis factor therapy (NCT01967316). Other trials are examining vaccine efficacy and safety in patients with solid organ tumors prior to chemotherapy (NCT02444936) and in patients who will be undergoing living donor kidney transplantation (NCT00940940). Researchers are also investigating the possibility of vaccinating allogeneic stem cell donors before donation in order to protect transplant recipients against HZ (NCT01573182).
ZVHT and HZ/su vaccination in immunocompromised patients
Heat-treated varicella-zoster vaccine (ZVHT) is a potential alternative for immunocompromised patients. A 4-dose regimen has been proven to reduce the risk of HZ in patients receiving autologous hematopoietic-cell transplants for non-Hodgkin or Hodgkin lymphoma.57
In another trial, the 4-dose ZVHT was safe and elicited significant VZV-specific T-cell response through 28 days in immunosuppressed patients with solid tumor malignancy, hematologic malignancy, human immunodeficiency virus infection with CD4 counts of 200 cells/mm3 or less, and autologous hematopoietic-cell transplants. The T-cell response was poor in allogeneic hematopoietic-cell transplant recipients, however.58
Because the HZ/su vaccine does not contain live virus, it seems particularly promising for immunocompromised patients. In phase 1 and 2 studies, a 3-dose regimen has been shown to be safe and immunogenic in hematopoietic-cell transplant recipients and HIV-infected adults with CD4 count higher than 200 cells/mm3.59,60 A phase 3 trial assessing the efficacy of HZ/su vaccine in autologous hematopoietic-cell transplant recipients is under way (NCT01610414). Changes in recommendations for HZ vaccine in these most vulnerable populations await the results of these studies.
- Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis 2007; 44(suppl 1):S1–S26.
- Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines 2010; 9(suppl):21–26.
- Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev 2014; 2:CD006866.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4:e004833.
- Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA 2011; 305:160–166.
- Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med 2013; 10:e1001420.
- Yawn BP, Saddier P, Wollan PC, St. Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007; 82:1341–1349.
- Yawn BP, Wollan PC, Kurland MJ, St. Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc 2011; 86:88–93.
- Chen SY, Suaya JA, Li Q, et al. Incidence of herpes zoster in patients with altered immune function. Infection 2014; 42:325–334.
- Edmunds WJ, Brisson M. The effect of vaccination on the epidemiology of varicella zoster virus. J Infect 2002; 44:211–219.
- Hales CM, Harpaz R, Joesoef MR, Bialek SR. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann Intern Med 2013; 159:739–745.
- Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis 2011; 52:332–340.
- Rimland D, Moanna A. Increasing incidence of herpes zoster among veterans. Clin Infect Dis 2010; 50:1000–1005.
- Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992-2002. J Infect Dis 2005; 191:2002–2007.
- Plotkin SA, Starr SE, Connor K, Morton D. Zoster in normal children after varicella vaccine. J Infect Dis 1989; 159:1000–1001.
- Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain 2005; 6:356–363.
- Johnson RW, Bouhassira D, Kassianos G, Leplege A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med 2010; 8:37.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1–30.
- Panatto D, Bragazzi NL, Rizzitelli E, et al. Evaluation of the economic burden of herpes zoster (HZ) infection. Hum Vaccin Immunother 2015; 11:245–262.
- Yawn BP, Itzler RF, Wollan PC, Pellissier JM, Sy LS, Saddier P. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc 2009; 84:787–794.
- Dworkin RH, White R, O’Connor AB, Hawkins K. Health care expenditure burden of persisting herpes zoster pain. Pain Med 2008; 9:348–353.
- White RR, Lenhart G, Singhal PK, et al. Incremental 1-year medical resource utilization and costs for patients with herpes zoster from a set of US health plans. Pharmacoeconomics 2009; 27:781–792.
- Insinga RP, Itzler RF, Pellissier JM. Acute/subacute herpes zoster: healthcare resource utilisation and costs in a group of US health plans. Pharmacoeconomics 2007; 25:155–169.
- Singhal PK, Makin C, Pellissier J, Sy L, White R, Saddier P. Work and productivity loss related to herpes zoster. J Med Econ 2011; 14:639–645.
- US Bureau of Labor Statistics. Labor force statistics from the current population survey. www.bls.gov/web/empsit/cpseea13.htm. Accessed April 6, 2017.
- US Bureau of Labor Statistic. Occupational employment statistics. www.bls.gov/oes/current/oes_nat.htm. Accessed April 6, 2017.
- Oxman MN, Levin MJ, Johnson GR, et al; Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–2284.
- Food and Drug Administration (FDA). FDA clinical briefing document for Merck & Co., Inc. Zoster vaccine live (Oka/Merck) Zostavax. www.fda.gov/ohrms/dockets/ac/05/briefing/5-4198b2_1.pdf. Accessed April 6, 2017.
- Simberkoff MS, Arbeit RD, Johnson GR, et al; Shingles Prevention Study Group. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Ann Intern Med 2010; 152:545–554.
- Schmader KE, Oxman MN, Levin MJ, et al; Shingles Prevention Study Group. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis 2012; 55:1320–1328.
- Morrison VA, Johnson GR, Schmader KE, et al; Shingles Prevention Study Group. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 2015; 60:900–909.
- Levin MJ, Schmader KE, Pang L, et al. Cellular and humoral responses to a second dose of herpes zoster vaccine administered 10 years after the first dose among older adults. J Infect Dis 2016; 213:14–22.
- Schmader KE, Levin MJ, Gnann JW Jr, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis 2012; 54:922–928.
- Hales CM, Harpaz R, Ortega-Sanchez I, Bialek SR; Centers for Disease Control and Prevention (CDC). Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep 2014; 63:729–731.
- Centers for Disease Control and Prevention (CDC). Surveillance of vaccination coverage among adult populations—United States, 2014. MMWR Morb Mortal Wkly Rep 2016; 65(1):1–36. Accessed April 12, 2017.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination—United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64:95–102.
- Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis 2010; 51:197–213.
- Hurley LP, Lindley MC, Harpaz R, et al. Barriers to the use of herpes zoster vaccine. Ann Intern Med 2010; 152:555–560.
- Lu PJ, Euler GL, Jumaan AO, Harpaz R. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine 2009; 27:882–887.
- Hurley LP, Harpaz R, Daley MF, et al. National survey of primary care physicians regarding herpes zoster and the herpes zoster vaccine. J Infect Dis 2008; 197(suppl 2):S216–S223.
- Joon Lee T, Hayes S, Cummings DM, et al. Herpes zoster knowledge, prevalence, and vaccination rate by race. J Am Board Fam Med 2013; 26:45–51.
- Opstelten W, van Essen GA, Hak E. Determinants of non-compliance with herpes zoster vaccination in the community-dwelling elderly. Vaccine 2009; 27:192–196.
- Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med 2011; 171:1552–1558.
- Rhew DC, Glassman PA, Goetz MB. Improving pneumococcal vaccine rates. Nurse protocols versus clinical reminders. J Gen Intern Med 1999; 14:351–356.
- Chaudhry R, Schietel SM, North F, Dejesus R, Kesman RL, Stroebel RJ. Improving rates of herpes zoster vaccination with a clinical decision support system in a primary care practice. J Eval Clin Pract 2013; 19:263–266.
- Otsuka SH, Tayal NH, Porter K, Embi PJ, Beatty SJ. Improving herpes zoster vaccination rates through use of a clinical pharmacist and a personal health record. Am J Med 2013; 126:832.e1–832.e6.
- Taitel MS, Fensterheim LE, Cannon AE, Cohen ES. Improving pneumococcal and herpes zoster vaccination uptake: expanding pharmacist privileges. Am J Manag Care 2013; 19:e309–e313.
- Kawai K, Preaud E, Baron-Papillon F, Largeron N, Acosta CJ. Cost-effectiveness of vaccination against herpes zoster and postherpetic neuralgia: a critical review. Vaccine 2014; 32:1645–1653.
- Szucs TD, Pfeil AM. A systematic review of the cost effectiveness of herpes zoster vaccination. Pharmacoeconomics 2013; 31:125–136.
- Le P, Rothberg MB. Cost-effectiveness of herpes zoster vaccine for persons aged 50 years. Ann Intern Med 2015; 163:489–497.
- Le P, Rothberg MB. Determining the optimal age to vaccinate against herpes zoster: a cost-effectiveness analysis. Society for Medical Decision Making 37th Annual North American Meeting. St. Louis, MO; October 18-21, 2015.
- Cohen JI. Clinical practice: herpes zoster. N Engl J Med 2013; 369:255–263.
- Lal H, Cunningham AL, Godeaux O, et al; ZOE-50 Study Group. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–2096.
- GlaxoSmithKline plc. GSK’s candidate shingles vaccine demonstrates 90% efficacy against shingles in people 70 years of age and over. www.gsk.com/en-gb/media/press-releases/gsk-s-candidate-shingles-vaccine-demonstrates-90-efficacy-against-shingles-in-people-70-years-of-age-and-over/. Accessed April 6, 2017.
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med 2013; 19:1597–1608.
- Russell AF, Parrino J, Fisher CL Jr, et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects on chronic/maintenance corticosteroids. Vaccine 2015; 33:3129–3134.
- Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med 2002; 347:26–34.
- Mullane KM, Winston DJ, Wertheim MS, et al. Safety and immunogenicity of heat-treated zoster vaccine (ZVHT) in immunocompromised adults. J Infect Dis 2013; 208:1375–1385.
- Stadtmauer EA, Sullivan KM, Marty FM, et al. A phase 1/2 study of an adjuvanted varicella-zoster virus subunit vaccine in autologous hematopoietic cell transplant recipients. Blood 2014; 124:2921–2929.
- Berkowitz EM, Moyle G, Stellbrink HJ, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis 2015; 211:1279–1287.
- Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis 2007; 44(suppl 1):S1–S26.
- Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines 2010; 9(suppl):21–26.
- Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev 2014; 2:CD006866.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4:e004833.
- Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA 2011; 305:160–166.
- Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med 2013; 10:e1001420.
- Yawn BP, Saddier P, Wollan PC, St. Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007; 82:1341–1349.
- Yawn BP, Wollan PC, Kurland MJ, St. Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc 2011; 86:88–93.
- Chen SY, Suaya JA, Li Q, et al. Incidence of herpes zoster in patients with altered immune function. Infection 2014; 42:325–334.
- Edmunds WJ, Brisson M. The effect of vaccination on the epidemiology of varicella zoster virus. J Infect 2002; 44:211–219.
- Hales CM, Harpaz R, Joesoef MR, Bialek SR. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann Intern Med 2013; 159:739–745.
- Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis 2011; 52:332–340.
- Rimland D, Moanna A. Increasing incidence of herpes zoster among veterans. Clin Infect Dis 2010; 50:1000–1005.
- Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992-2002. J Infect Dis 2005; 191:2002–2007.
- Plotkin SA, Starr SE, Connor K, Morton D. Zoster in normal children after varicella vaccine. J Infect Dis 1989; 159:1000–1001.
- Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain 2005; 6:356–363.
- Johnson RW, Bouhassira D, Kassianos G, Leplege A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med 2010; 8:37.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1–30.
- Panatto D, Bragazzi NL, Rizzitelli E, et al. Evaluation of the economic burden of herpes zoster (HZ) infection. Hum Vaccin Immunother 2015; 11:245–262.
- Yawn BP, Itzler RF, Wollan PC, Pellissier JM, Sy LS, Saddier P. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc 2009; 84:787–794.
- Dworkin RH, White R, O’Connor AB, Hawkins K. Health care expenditure burden of persisting herpes zoster pain. Pain Med 2008; 9:348–353.
- White RR, Lenhart G, Singhal PK, et al. Incremental 1-year medical resource utilization and costs for patients with herpes zoster from a set of US health plans. Pharmacoeconomics 2009; 27:781–792.
- Insinga RP, Itzler RF, Pellissier JM. Acute/subacute herpes zoster: healthcare resource utilisation and costs in a group of US health plans. Pharmacoeconomics 2007; 25:155–169.
- Singhal PK, Makin C, Pellissier J, Sy L, White R, Saddier P. Work and productivity loss related to herpes zoster. J Med Econ 2011; 14:639–645.
- US Bureau of Labor Statistics. Labor force statistics from the current population survey. www.bls.gov/web/empsit/cpseea13.htm. Accessed April 6, 2017.
- US Bureau of Labor Statistic. Occupational employment statistics. www.bls.gov/oes/current/oes_nat.htm. Accessed April 6, 2017.
- Oxman MN, Levin MJ, Johnson GR, et al; Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–2284.
- Food and Drug Administration (FDA). FDA clinical briefing document for Merck & Co., Inc. Zoster vaccine live (Oka/Merck) Zostavax. www.fda.gov/ohrms/dockets/ac/05/briefing/5-4198b2_1.pdf. Accessed April 6, 2017.
- Simberkoff MS, Arbeit RD, Johnson GR, et al; Shingles Prevention Study Group. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Ann Intern Med 2010; 152:545–554.
- Schmader KE, Oxman MN, Levin MJ, et al; Shingles Prevention Study Group. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis 2012; 55:1320–1328.
- Morrison VA, Johnson GR, Schmader KE, et al; Shingles Prevention Study Group. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 2015; 60:900–909.
- Levin MJ, Schmader KE, Pang L, et al. Cellular and humoral responses to a second dose of herpes zoster vaccine administered 10 years after the first dose among older adults. J Infect Dis 2016; 213:14–22.
- Schmader KE, Levin MJ, Gnann JW Jr, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis 2012; 54:922–928.
- Hales CM, Harpaz R, Ortega-Sanchez I, Bialek SR; Centers for Disease Control and Prevention (CDC). Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep 2014; 63:729–731.
- Centers for Disease Control and Prevention (CDC). Surveillance of vaccination coverage among adult populations—United States, 2014. MMWR Morb Mortal Wkly Rep 2016; 65(1):1–36. Accessed April 12, 2017.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination—United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64:95–102.
- Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis 2010; 51:197–213.
- Hurley LP, Lindley MC, Harpaz R, et al. Barriers to the use of herpes zoster vaccine. Ann Intern Med 2010; 152:555–560.
- Lu PJ, Euler GL, Jumaan AO, Harpaz R. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine 2009; 27:882–887.
- Hurley LP, Harpaz R, Daley MF, et al. National survey of primary care physicians regarding herpes zoster and the herpes zoster vaccine. J Infect Dis 2008; 197(suppl 2):S216–S223.
- Joon Lee T, Hayes S, Cummings DM, et al. Herpes zoster knowledge, prevalence, and vaccination rate by race. J Am Board Fam Med 2013; 26:45–51.
- Opstelten W, van Essen GA, Hak E. Determinants of non-compliance with herpes zoster vaccination in the community-dwelling elderly. Vaccine 2009; 27:192–196.
- Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med 2011; 171:1552–1558.
- Rhew DC, Glassman PA, Goetz MB. Improving pneumococcal vaccine rates. Nurse protocols versus clinical reminders. J Gen Intern Med 1999; 14:351–356.
- Chaudhry R, Schietel SM, North F, Dejesus R, Kesman RL, Stroebel RJ. Improving rates of herpes zoster vaccination with a clinical decision support system in a primary care practice. J Eval Clin Pract 2013; 19:263–266.
- Otsuka SH, Tayal NH, Porter K, Embi PJ, Beatty SJ. Improving herpes zoster vaccination rates through use of a clinical pharmacist and a personal health record. Am J Med 2013; 126:832.e1–832.e6.
- Taitel MS, Fensterheim LE, Cannon AE, Cohen ES. Improving pneumococcal and herpes zoster vaccination uptake: expanding pharmacist privileges. Am J Manag Care 2013; 19:e309–e313.
- Kawai K, Preaud E, Baron-Papillon F, Largeron N, Acosta CJ. Cost-effectiveness of vaccination against herpes zoster and postherpetic neuralgia: a critical review. Vaccine 2014; 32:1645–1653.
- Szucs TD, Pfeil AM. A systematic review of the cost effectiveness of herpes zoster vaccination. Pharmacoeconomics 2013; 31:125–136.
- Le P, Rothberg MB. Cost-effectiveness of herpes zoster vaccine for persons aged 50 years. Ann Intern Med 2015; 163:489–497.
- Le P, Rothberg MB. Determining the optimal age to vaccinate against herpes zoster: a cost-effectiveness analysis. Society for Medical Decision Making 37th Annual North American Meeting. St. Louis, MO; October 18-21, 2015.
- Cohen JI. Clinical practice: herpes zoster. N Engl J Med 2013; 369:255–263.
- Lal H, Cunningham AL, Godeaux O, et al; ZOE-50 Study Group. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–2096.
- GlaxoSmithKline plc. GSK’s candidate shingles vaccine demonstrates 90% efficacy against shingles in people 70 years of age and over. www.gsk.com/en-gb/media/press-releases/gsk-s-candidate-shingles-vaccine-demonstrates-90-efficacy-against-shingles-in-people-70-years-of-age-and-over/. Accessed April 6, 2017.
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med 2013; 19:1597–1608.
- Russell AF, Parrino J, Fisher CL Jr, et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects on chronic/maintenance corticosteroids. Vaccine 2015; 33:3129–3134.
- Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med 2002; 347:26–34.
- Mullane KM, Winston DJ, Wertheim MS, et al. Safety and immunogenicity of heat-treated zoster vaccine (ZVHT) in immunocompromised adults. J Infect Dis 2013; 208:1375–1385.
- Stadtmauer EA, Sullivan KM, Marty FM, et al. A phase 1/2 study of an adjuvanted varicella-zoster virus subunit vaccine in autologous hematopoietic cell transplant recipients. Blood 2014; 124:2921–2929.
- Berkowitz EM, Moyle G, Stellbrink HJ, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis 2015; 211:1279–1287.
KEY POINTS
- HZ continues to be an important public health problem, with substantial morbidity and economic impact. Because of the lack of effective treatment, vaccination provides the best strategy for disease mitigation.
- Physicians can reduce the impact of HZ by educating patients about its complications and recommending immunization for all patients age 60 and older. Patients can protect themselves by seeking vaccination.
- Vaccine protection wanes completely after 10 years, and physicians should be prepared to offer a booster dose should the Advisory Committee on Immunization Practices issue such recommendations.
- Newer vaccines offer promise for greater efficacy, especially for the elderly. For immunocompromised patients, a safe and effective vaccine may be available in the near future.
In reply: Measles: More than the rash
In Reply: We thank Dr. Cunha for his comments and appreciate the opportunity to emphasize important points that he highlights.
We agree that measles is a systemic illness with important extradermatologic manifestations that are critical to the diagnosis, and that the nondermatologic manifestations often precede the rash and serve to distinguish measles from other systemic illnesses. As discussed in our review, the respiratory prodrome of cough, coryza, and conjunctivitis is very distinctive and serves as an important clue to the diagnosis. Likewise, we acknowledge the importance of gastrointestinal findings in measles and note appendicitis as an important complication that is well described. Although Koplik spots are pathognomonic, we do stress that these often are not present at the time of presentation.
Finally, we agree that measles is a clinical diagnosis, and that the clinical manifestations beyond the dermatologic manifestations noted in our review and highlighted by Dr. Cunha are extremely helpful to the clinician in considering the diagnosis.
In Reply: We thank Dr. Cunha for his comments and appreciate the opportunity to emphasize important points that he highlights.
We agree that measles is a systemic illness with important extradermatologic manifestations that are critical to the diagnosis, and that the nondermatologic manifestations often precede the rash and serve to distinguish measles from other systemic illnesses. As discussed in our review, the respiratory prodrome of cough, coryza, and conjunctivitis is very distinctive and serves as an important clue to the diagnosis. Likewise, we acknowledge the importance of gastrointestinal findings in measles and note appendicitis as an important complication that is well described. Although Koplik spots are pathognomonic, we do stress that these often are not present at the time of presentation.
Finally, we agree that measles is a clinical diagnosis, and that the clinical manifestations beyond the dermatologic manifestations noted in our review and highlighted by Dr. Cunha are extremely helpful to the clinician in considering the diagnosis.
In Reply: We thank Dr. Cunha for his comments and appreciate the opportunity to emphasize important points that he highlights.
We agree that measles is a systemic illness with important extradermatologic manifestations that are critical to the diagnosis, and that the nondermatologic manifestations often precede the rash and serve to distinguish measles from other systemic illnesses. As discussed in our review, the respiratory prodrome of cough, coryza, and conjunctivitis is very distinctive and serves as an important clue to the diagnosis. Likewise, we acknowledge the importance of gastrointestinal findings in measles and note appendicitis as an important complication that is well described. Although Koplik spots are pathognomonic, we do stress that these often are not present at the time of presentation.
Finally, we agree that measles is a clinical diagnosis, and that the clinical manifestations beyond the dermatologic manifestations noted in our review and highlighted by Dr. Cunha are extremely helpful to the clinician in considering the diagnosis.
Measles: Back again
Measles continues to rear its head in the United States. Because it is so contagious, even the few cases introduced by travelers quickly spread to susceptible contacts. Life-threatening and severely disabling complications can occur, although this is rare. Widespread immunization and prompt recognition and isolation of contacts are key to controlling outbreaks.
This article reviews the epidemiology of measles, describes its distinctive clinical picture, and provides recommendations for infection control and prevention, including in immunosuppressed populations.
MEASLES IS SERIOUS AND HIGHLY CONTAGIOUS
Up to 90% of susceptible people develop measles after exposure, making it one of the most contagious of infections. The virus is transmitted by airborne spread when an infected person coughs or sneezes, or by direct contact with infectious droplets. The virus can remain infectious in the air or on a surface for up to 2 hours.1
Worldwide, an estimated 20 million people are infected with measles each year, and 146,000 die of complications. In 1980, before widespread vaccination, 2.6 million deaths were attributable to measles annually. In the United States before the introduction of measles vaccine in 1963, measles was a significant cause of disease and death: an estimated 3 to 4 million people were infected annually, although only about 549,000 were reported. There were 48,000 hospitalizations, 1,000 cases of permanent brain damage from measles encephalitis, and 495 deaths annually.2
Outbreaks still occur regularly
In 2000, measles was declared eliminated from the United States,3 but annual outbreaks have occurred since then as a result of cases imported from other countries and their subsequent transmission to unvaccinated people. From 2001 to 2012, a median of four outbreaks and 60 cases were reported annually to the US Centers for Disease Control and Prevention.4
In January 2015, a multistate measles outbreak originating in Disneyland in California was recognized. As of April 17, when the outbreak was declared over, 111 measles cases from seven states had been linked to this outbreak.5 Of the evaluable cases, 44% were in unvaccinated people and 38% were in those whose vaccination status was unknown or undocumented. The median age of patients was 21, and 20% required hospitalization.
This outbreak, as well as four other smaller US outbreaks the same year, underscores the transmissibility of the virus in populations containing only a small percentage of unvaccinated people.6
DISTINCTIVE CLINICAL PICTURE
The incubation period for measles infection is 7 to 21 days, with most cases becoming apparent 10 to 12 days after exposure. Measles should be suspected in a patient with the following clinical features whose history indicates susceptibility and exposure (ie, an unimmunized person with a history of exposure or travel):
Severe acute respiratory illness. Measles usually presents as an acute respiratory viral illness, which typically lasts 2 to 4 days. The illness involves high fevers, malaise, anorexia, and the “three Cs”: cough, coryza (rhinitis), and conjunctivitis. Patients usually appear sicker than those with more common viral illnesses.
Koplik spots, which are pathognomonic for measles, are seen in the first few days of illness. They are bluish-white, slightly raised lesions on an erythematous base on the buccal mucosa, usually opposite the first molar (Figure 1). Spots can also be seen on the soft palate, conjunctiva, and vaginal mucosa. Koplik spots usually disappear after a few days and often are not appreciable at the time of evaluation.
Discrete erythematous patches develop on the face and neck a day after the appearance of Koplik spots. This rash becomes more confluent as it spreads to involve the entire body (Figure 2). It typically lasts for 3 to 7 days, then fades in a similar pattern. The confluent nature of this rash and its spread from the face and neck to the entire body are characteristic of measles. Patients are highly contagious from 4 days before the onset of the rash to 4 days after.
COMPLICATIONS CAN BE SEVERE
Those at highest risk for measles complications are infants, children under age 5, adults over age 20, pregnant women, and immunosuppressed individuals.7
Pneumonia—either a primary measles pneumonia or a secondary viral or bacterial pneumonia—is the most common cause of death.8,9 Viruses complicating measles are typically adenovirus and herpes simplex virus. Bacteria causing secondary infection are usually Staphylococcus aureus and Streptococcus pneumoniae and, less commonly, gram-negative bacteria.
Laryngotracheobronchitis (croup) is the second most common cause of death, with bacteria and viruses similar to those causing measles-related pneumonia.
Otitis media is the most common complication of measles. Other respiratory complications include mastoiditis, pneumothorax, and mediastinal emphysema.
Acute measles encephalitis occurs in 1 measles case per 1,000 and often results in permanent brain damage. During the convalescent phase of the illness, fever again emerges, with the development of headaches, seizures, and altered consciousness.10
Subacute sclerosing panencephalitis is a rare fatal degenerative disease of the central nervous system caused by a persistent infection with a defective measles virus. The precise pathophysiology is unclear, but it is thought that mutations of the viral genome lead to altered cellular immunity.11 The condition typically occurs 7 to 10 years after the initial measles infection, particularly in those who developed measles before age 2. Clinical manifestations include behavioral disturbances, intellectual deterioration, and myoclonic seizures, slowly progressing to a vegetative state and death.12
Other complications of measles include diarrhea and stomatitis, which are associated with malnutrition in developing countries, and subclinical hepatitis, thrombocytopenia, appendicitis, ileocolitis, hypokalemia, and myocarditis.
During pregnancy, measles infection can be complicated by primary measles pneumonia and is associated with an increased risk of miscarriage and premature birth.13
Patients with a cell-mediated immunodeficiency who develop measles are particularly susceptible to fatal measles pneumonia and acute progressive encephalitis.14
ATYPICAL MEASLES IN THOSE WHO RECEIVED KILLED VACCINE
From 1963 to 1967, a killed measles vaccine was available in the United States. Those who received this vaccine are susceptible to an atypical form of measles when exposed to the virus,15 characterized by a 1- to 2-day prodrome, followed by the appearance of a maculopapular or petechial rash on the distal extremities that spreads centripetally. Patients develop high fever and edema of the hands and feet, and have a more prolonged course than with classic measles. It is believed not to be contagious.16
LABORATORY CONFIRMATION
Laboratory confirmation of measles is recommended for suspected cases. Because viral isolation is technically difficult and is not readily available in most laboratories, measles-specific immunoglobulin M antibody serologic testing is most commonly used. It is almost 100% sensitive when done 2 to 3 days after the onset of the rash.17
Measles RNA testing by real-time polymerase chain reaction to detect measles virus in the blood, throat, or urine is more specific and if available may be preferred over serologic testing.18
SUPPORTIVE MANAGEMENT AND VITAMIN A SUPPLEMENTATION
No specific antiviral therapy for measles is available. Management involves supportive measures and monitoring for secondary bacterial complications.
The World Health Organization and the American Academy of Pediatrics recommend vitamin A supplementation for all children with acute measles.19 In developing countries, it has been shown to reduce rates of morbidity and death in measles-infected children.20 In the United States, children with measles have been found to have low serum levels of vitamin A, with lower levels associated with more severe disease.
VACCINATION RECOMMENDATIONS
The only measles vaccine available in the United States is a live further-attenuated strain prepared in chick embryo cell culture and combined with mumps and rubella vaccine (MMR) or with measles, mumps, rubella, and varicella vaccine (MMRV).
Healthy children. Two doses of measles vaccine are recommended, as a single dose is associated with a 5% failure rate. The recommended schedule is:
- First dose at age 12 to 15 months
- Second dose at the time of school entry (ages 4 to 6), or at any time at least 28 days after the first dose.19
More than 99% of children who receive two doses of vaccine according to this schedule develop serologic evidence of measles immunity. Vaccination provides long-term immunity, and many epidemiologic studies have documented that waning immunity after vaccination occurs only very rarely.21
All school-age children, including elementary, middle, and high school students, who received only one dose of measles vaccine should receive the second dose.
Adults born in 1957 or later should receive at least one dose of measles vaccine unless they have other acceptable evidence of immunity, such as:4
- Documentation of age-appropriate live measles vaccine, ie, one dose of vaccine for adults not at high risk, or two doses for those at high risk (see below)
- Laboratory evidence of immunity (ie, measles immunoglobulin G in serum)
- Laboratory confirmation of disease.
Adults born before 1957 can be considered to be immune to measles, although MMR vaccine can be administered in those without contraindications.
Adults at increased risk of exposure or transmission of measles and who do not have evidence of immunity should receive two doses of MMR vaccine, given at least 28 days apart. This high-risk group includes:
- Students attending college or other post-high school educational institution
- Healthcare personnel
- International travelers.
During measles outbreaks, every effort should be made to ensure that those at high risk are vaccinated with two doses of MMR or have other acceptable evidence of immunity.
LIVE VACCINE IS SAFE FOR MOST PEOPLE
Mild side effects. A transient fever, which may be accompanied by a discrete or confluent rash, occurs in 5% to 15% of recipients 5 to 12 days after vaccination.
Transmission does not occur. People who have been newly vaccinated do not transmit the virus to susceptible contacts, even if they develop a vaccine-associated rash. The vaccine can safely be given to close contacts of immunocompromised and other susceptible people.
Egg allergy not a concern. Measles vaccine is produced in chick embryo cell culture but has been shown to be safe for people with egg allergy and is recommended without the need for egg allergy testing.19
Autism link debunked. No scientific evidence shows that the risk of autism is higher in children who receive MMR vaccine than in those who do not. In 2001, an Institute of Medicine report rejected a causal relationship between MMR vaccine and autism spectrum disorders.22
CONTRAINDICATIONS
Measles vaccine is contraindicated for:
- Patients who have cell-mediated immune deficiencies, except human immunodeficiency virus (HIV) infection
- Pregnant women
- Those who have had a severe allergic reaction to a vaccine component in the past
- Those with moderate or severe acute illness
- Those who have recently received immunoglobulin products.
People with HIV infection who are severely immunosuppressed should not receive live measles vaccine. However, because of the risk of severe measles in HIV-infected patients and because the vaccine has been shown to be safe for patients with HIV without severe immunosuppression, the vaccine is recommended for those with asymptomatic or mildly symptomatic HIV infection who do not have evidence of severe immunosuppression (ie, CD4 lymphocytes < 15% or < 200 cells/µL).3,4
INFECTION CONTROL AND PREVENTION
Healthcare workers should maintain a high index of suspicion for measles and implement isolation procedures promptly in patients with a febrile illness, rash, and a history of travel abroad or contact with travelers from abroad.23 Suspected cases should be reported promptly to local health agencies to help limit spread.
Patients with measles should be placed in airborne isolation (eg, use of an N95 or higher level respirator and an airborne infection isolation room) for 4 days after the onset of the rash in a normal host and for the duration of the illness in an immunocompromised patient. Healthcare staff, regardless of their immunity status, should adhere to these precautions when entering the room of infected patients.
Immunization programs should be established to ensure that everyone who works or volunteers in healthcare facilities is protected against measles.4
Postexposure prophylaxis. Measles vaccination given to susceptible contacts within 72 hours of exposure may provide protection against infection and induces protection against subsequent measles exposures.24,25
Vaccination is the best intervention for susceptible contacts older than 12 months who do not have a contraindication to measles vaccination, and for those who have received only one dose of measles vaccine.
Passive immunization. Active immunization is the best strategy for controlling measles outbreaks. Passive immunization with intramuscularly or intravenously administered immunoglobulin given within 6 days of exposure can be used to prevent transmission or modify the clinical course of infection for susceptible contacts at high risk of developing severe or fatal measles. This includes people who are being treated with immunosuppressive agents, HIV-infected, pregnant, or younger than 1 year of age.
- Stokes J Jr, Reilly CM, Buynak EB, Hilleman MR. Immunologic studies of measles. Am J Hyg 1961; 74:293–303.
- Bloch AB, Orenstein WA, Stetler HC, et al. Health impact of measles vaccination in the United States. Pediatrics 1985; 76:524–532.
- Katz SL, Hinman AR. Summary and conclusions: measles elimination meeting, 16-17 March 2000. J Infect Dis 2004; 189(suppl 1):S43–S47.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention (CDC). Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013; 62:1–34.
- Clemmons NS, Gastanaduy PA, Fiebelkorn AP, Redd SB, Wallace GS; Centers for Disease Control and Prevention (CDC). Measles—United States, January 4 - April 2, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:373–376.
- Gay NJ. The theory of measles elimination: implications for the design of elimination strategies. J Infect Dis 2004; 189(suppl 1):S27–S35.
- Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189(suppl 1):S4–S16).
- Gremillion DH, Crawford GE. Measles pneumonia in young adults. An analysis of 106 cases. Am J Med 1981; 71:539–542.
- Quiambao BP, Gatchalian SR, Halonen P, et al. Coinfection is common in measles-associated pneumonia. Pediatr Infect Dis J 1998; 17:89–93.
- Johnson RT, Griffin D, Hirsch R, et al. Measles encephalomyelitis—clinical and immunologic studies. N Engl J Med 1984; 310:137–141.
- Garg RK. Subacute sclerosing panencephalitis. Postgrad Med J 2002; 78:63–70.
- Sever JL. Persistent measles infection of the central nervous system: subacute sclerosing panencephalitis. Rev Infect Dis 1983; 5:467–473.
- Atmar RL, Englund JA, Hammill H. Complications of measles during pregnancy. Clin Infect Dis 1992; 14:217–226.
- Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients. JAMA 1992; 267:1237–1241.
- Frey HM, Krugman S. Atypical measles syndrome: unusual hepatic, pulmonary, and immunologic aspects. Am J Med Sci 1981; 281:51–55.
- Fulginiti VA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA 1967; 202:1075–1080.
- Bellini WJ, Helfand RF. The challenges and strategies for laboratory diagnosis of measles in an international setting. J Infect Dis 2003; 187(suppl 1):S283–S290.
- Riddell MA, Chibo D, Kelly HA, Catton MG, Birch CJ. Investigation of optimal specimen type and sampling time for detection of measles virus RNA during a measles epidemic. J Clin Microbiol 2001; 39:375–376.
- Measles. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book: 2012 Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:489-499.
- Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev 2005; 4:CD001479.
- Markowitz LE, Preblud SR, Fine PE, Orenstein WA. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J 1990; 9:101–110.
- Stratton K, Gable A, Shetty P, McCormick M. Immunization safety review: measles-mumps-rubella vaccine and autism. Washington, DC: National Academy Press; 2001.
- Centers for Disease Control and Prevention (CDC). 2007 guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. www.cdc.gov/hicpac/2007ip/2007isolationprecautions.html. Accessed April 8, 2016.
- Berkovich S, Starr S. Use of live-measles-virus vaccine to abort an expected outbreak of measles within a closed population. N Engl J Med 1963; 269:75–77.
- Ruuskanen O, Salmi TT, Halonen P. Measles vaccination after exposure to natural measles. J Pediatr 1978; 93:43–46.
Measles continues to rear its head in the United States. Because it is so contagious, even the few cases introduced by travelers quickly spread to susceptible contacts. Life-threatening and severely disabling complications can occur, although this is rare. Widespread immunization and prompt recognition and isolation of contacts are key to controlling outbreaks.
This article reviews the epidemiology of measles, describes its distinctive clinical picture, and provides recommendations for infection control and prevention, including in immunosuppressed populations.
MEASLES IS SERIOUS AND HIGHLY CONTAGIOUS
Up to 90% of susceptible people develop measles after exposure, making it one of the most contagious of infections. The virus is transmitted by airborne spread when an infected person coughs or sneezes, or by direct contact with infectious droplets. The virus can remain infectious in the air or on a surface for up to 2 hours.1
Worldwide, an estimated 20 million people are infected with measles each year, and 146,000 die of complications. In 1980, before widespread vaccination, 2.6 million deaths were attributable to measles annually. In the United States before the introduction of measles vaccine in 1963, measles was a significant cause of disease and death: an estimated 3 to 4 million people were infected annually, although only about 549,000 were reported. There were 48,000 hospitalizations, 1,000 cases of permanent brain damage from measles encephalitis, and 495 deaths annually.2
Outbreaks still occur regularly
In 2000, measles was declared eliminated from the United States,3 but annual outbreaks have occurred since then as a result of cases imported from other countries and their subsequent transmission to unvaccinated people. From 2001 to 2012, a median of four outbreaks and 60 cases were reported annually to the US Centers for Disease Control and Prevention.4
In January 2015, a multistate measles outbreak originating in Disneyland in California was recognized. As of April 17, when the outbreak was declared over, 111 measles cases from seven states had been linked to this outbreak.5 Of the evaluable cases, 44% were in unvaccinated people and 38% were in those whose vaccination status was unknown or undocumented. The median age of patients was 21, and 20% required hospitalization.
This outbreak, as well as four other smaller US outbreaks the same year, underscores the transmissibility of the virus in populations containing only a small percentage of unvaccinated people.6
DISTINCTIVE CLINICAL PICTURE
The incubation period for measles infection is 7 to 21 days, with most cases becoming apparent 10 to 12 days after exposure. Measles should be suspected in a patient with the following clinical features whose history indicates susceptibility and exposure (ie, an unimmunized person with a history of exposure or travel):
Severe acute respiratory illness. Measles usually presents as an acute respiratory viral illness, which typically lasts 2 to 4 days. The illness involves high fevers, malaise, anorexia, and the “three Cs”: cough, coryza (rhinitis), and conjunctivitis. Patients usually appear sicker than those with more common viral illnesses.
Koplik spots, which are pathognomonic for measles, are seen in the first few days of illness. They are bluish-white, slightly raised lesions on an erythematous base on the buccal mucosa, usually opposite the first molar (Figure 1). Spots can also be seen on the soft palate, conjunctiva, and vaginal mucosa. Koplik spots usually disappear after a few days and often are not appreciable at the time of evaluation.
Discrete erythematous patches develop on the face and neck a day after the appearance of Koplik spots. This rash becomes more confluent as it spreads to involve the entire body (Figure 2). It typically lasts for 3 to 7 days, then fades in a similar pattern. The confluent nature of this rash and its spread from the face and neck to the entire body are characteristic of measles. Patients are highly contagious from 4 days before the onset of the rash to 4 days after.
COMPLICATIONS CAN BE SEVERE
Those at highest risk for measles complications are infants, children under age 5, adults over age 20, pregnant women, and immunosuppressed individuals.7
Pneumonia—either a primary measles pneumonia or a secondary viral or bacterial pneumonia—is the most common cause of death.8,9 Viruses complicating measles are typically adenovirus and herpes simplex virus. Bacteria causing secondary infection are usually Staphylococcus aureus and Streptococcus pneumoniae and, less commonly, gram-negative bacteria.
Laryngotracheobronchitis (croup) is the second most common cause of death, with bacteria and viruses similar to those causing measles-related pneumonia.
Otitis media is the most common complication of measles. Other respiratory complications include mastoiditis, pneumothorax, and mediastinal emphysema.
Acute measles encephalitis occurs in 1 measles case per 1,000 and often results in permanent brain damage. During the convalescent phase of the illness, fever again emerges, with the development of headaches, seizures, and altered consciousness.10
Subacute sclerosing panencephalitis is a rare fatal degenerative disease of the central nervous system caused by a persistent infection with a defective measles virus. The precise pathophysiology is unclear, but it is thought that mutations of the viral genome lead to altered cellular immunity.11 The condition typically occurs 7 to 10 years after the initial measles infection, particularly in those who developed measles before age 2. Clinical manifestations include behavioral disturbances, intellectual deterioration, and myoclonic seizures, slowly progressing to a vegetative state and death.12
Other complications of measles include diarrhea and stomatitis, which are associated with malnutrition in developing countries, and subclinical hepatitis, thrombocytopenia, appendicitis, ileocolitis, hypokalemia, and myocarditis.
During pregnancy, measles infection can be complicated by primary measles pneumonia and is associated with an increased risk of miscarriage and premature birth.13
Patients with a cell-mediated immunodeficiency who develop measles are particularly susceptible to fatal measles pneumonia and acute progressive encephalitis.14
ATYPICAL MEASLES IN THOSE WHO RECEIVED KILLED VACCINE
From 1963 to 1967, a killed measles vaccine was available in the United States. Those who received this vaccine are susceptible to an atypical form of measles when exposed to the virus,15 characterized by a 1- to 2-day prodrome, followed by the appearance of a maculopapular or petechial rash on the distal extremities that spreads centripetally. Patients develop high fever and edema of the hands and feet, and have a more prolonged course than with classic measles. It is believed not to be contagious.16
LABORATORY CONFIRMATION
Laboratory confirmation of measles is recommended for suspected cases. Because viral isolation is technically difficult and is not readily available in most laboratories, measles-specific immunoglobulin M antibody serologic testing is most commonly used. It is almost 100% sensitive when done 2 to 3 days after the onset of the rash.17
Measles RNA testing by real-time polymerase chain reaction to detect measles virus in the blood, throat, or urine is more specific and if available may be preferred over serologic testing.18
SUPPORTIVE MANAGEMENT AND VITAMIN A SUPPLEMENTATION
No specific antiviral therapy for measles is available. Management involves supportive measures and monitoring for secondary bacterial complications.
The World Health Organization and the American Academy of Pediatrics recommend vitamin A supplementation for all children with acute measles.19 In developing countries, it has been shown to reduce rates of morbidity and death in measles-infected children.20 In the United States, children with measles have been found to have low serum levels of vitamin A, with lower levels associated with more severe disease.
VACCINATION RECOMMENDATIONS
The only measles vaccine available in the United States is a live further-attenuated strain prepared in chick embryo cell culture and combined with mumps and rubella vaccine (MMR) or with measles, mumps, rubella, and varicella vaccine (MMRV).
Healthy children. Two doses of measles vaccine are recommended, as a single dose is associated with a 5% failure rate. The recommended schedule is:
- First dose at age 12 to 15 months
- Second dose at the time of school entry (ages 4 to 6), or at any time at least 28 days after the first dose.19
More than 99% of children who receive two doses of vaccine according to this schedule develop serologic evidence of measles immunity. Vaccination provides long-term immunity, and many epidemiologic studies have documented that waning immunity after vaccination occurs only very rarely.21
All school-age children, including elementary, middle, and high school students, who received only one dose of measles vaccine should receive the second dose.
Adults born in 1957 or later should receive at least one dose of measles vaccine unless they have other acceptable evidence of immunity, such as:4
- Documentation of age-appropriate live measles vaccine, ie, one dose of vaccine for adults not at high risk, or two doses for those at high risk (see below)
- Laboratory evidence of immunity (ie, measles immunoglobulin G in serum)
- Laboratory confirmation of disease.
Adults born before 1957 can be considered to be immune to measles, although MMR vaccine can be administered in those without contraindications.
Adults at increased risk of exposure or transmission of measles and who do not have evidence of immunity should receive two doses of MMR vaccine, given at least 28 days apart. This high-risk group includes:
- Students attending college or other post-high school educational institution
- Healthcare personnel
- International travelers.
During measles outbreaks, every effort should be made to ensure that those at high risk are vaccinated with two doses of MMR or have other acceptable evidence of immunity.
LIVE VACCINE IS SAFE FOR MOST PEOPLE
Mild side effects. A transient fever, which may be accompanied by a discrete or confluent rash, occurs in 5% to 15% of recipients 5 to 12 days after vaccination.
Transmission does not occur. People who have been newly vaccinated do not transmit the virus to susceptible contacts, even if they develop a vaccine-associated rash. The vaccine can safely be given to close contacts of immunocompromised and other susceptible people.
Egg allergy not a concern. Measles vaccine is produced in chick embryo cell culture but has been shown to be safe for people with egg allergy and is recommended without the need for egg allergy testing.19
Autism link debunked. No scientific evidence shows that the risk of autism is higher in children who receive MMR vaccine than in those who do not. In 2001, an Institute of Medicine report rejected a causal relationship between MMR vaccine and autism spectrum disorders.22
CONTRAINDICATIONS
Measles vaccine is contraindicated for:
- Patients who have cell-mediated immune deficiencies, except human immunodeficiency virus (HIV) infection
- Pregnant women
- Those who have had a severe allergic reaction to a vaccine component in the past
- Those with moderate or severe acute illness
- Those who have recently received immunoglobulin products.
People with HIV infection who are severely immunosuppressed should not receive live measles vaccine. However, because of the risk of severe measles in HIV-infected patients and because the vaccine has been shown to be safe for patients with HIV without severe immunosuppression, the vaccine is recommended for those with asymptomatic or mildly symptomatic HIV infection who do not have evidence of severe immunosuppression (ie, CD4 lymphocytes < 15% or < 200 cells/µL).3,4
INFECTION CONTROL AND PREVENTION
Healthcare workers should maintain a high index of suspicion for measles and implement isolation procedures promptly in patients with a febrile illness, rash, and a history of travel abroad or contact with travelers from abroad.23 Suspected cases should be reported promptly to local health agencies to help limit spread.
Patients with measles should be placed in airborne isolation (eg, use of an N95 or higher level respirator and an airborne infection isolation room) for 4 days after the onset of the rash in a normal host and for the duration of the illness in an immunocompromised patient. Healthcare staff, regardless of their immunity status, should adhere to these precautions when entering the room of infected patients.
Immunization programs should be established to ensure that everyone who works or volunteers in healthcare facilities is protected against measles.4
Postexposure prophylaxis. Measles vaccination given to susceptible contacts within 72 hours of exposure may provide protection against infection and induces protection against subsequent measles exposures.24,25
Vaccination is the best intervention for susceptible contacts older than 12 months who do not have a contraindication to measles vaccination, and for those who have received only one dose of measles vaccine.
Passive immunization. Active immunization is the best strategy for controlling measles outbreaks. Passive immunization with intramuscularly or intravenously administered immunoglobulin given within 6 days of exposure can be used to prevent transmission or modify the clinical course of infection for susceptible contacts at high risk of developing severe or fatal measles. This includes people who are being treated with immunosuppressive agents, HIV-infected, pregnant, or younger than 1 year of age.
Measles continues to rear its head in the United States. Because it is so contagious, even the few cases introduced by travelers quickly spread to susceptible contacts. Life-threatening and severely disabling complications can occur, although this is rare. Widespread immunization and prompt recognition and isolation of contacts are key to controlling outbreaks.
This article reviews the epidemiology of measles, describes its distinctive clinical picture, and provides recommendations for infection control and prevention, including in immunosuppressed populations.
MEASLES IS SERIOUS AND HIGHLY CONTAGIOUS
Up to 90% of susceptible people develop measles after exposure, making it one of the most contagious of infections. The virus is transmitted by airborne spread when an infected person coughs or sneezes, or by direct contact with infectious droplets. The virus can remain infectious in the air or on a surface for up to 2 hours.1
Worldwide, an estimated 20 million people are infected with measles each year, and 146,000 die of complications. In 1980, before widespread vaccination, 2.6 million deaths were attributable to measles annually. In the United States before the introduction of measles vaccine in 1963, measles was a significant cause of disease and death: an estimated 3 to 4 million people were infected annually, although only about 549,000 were reported. There were 48,000 hospitalizations, 1,000 cases of permanent brain damage from measles encephalitis, and 495 deaths annually.2
Outbreaks still occur regularly
In 2000, measles was declared eliminated from the United States,3 but annual outbreaks have occurred since then as a result of cases imported from other countries and their subsequent transmission to unvaccinated people. From 2001 to 2012, a median of four outbreaks and 60 cases were reported annually to the US Centers for Disease Control and Prevention.4
In January 2015, a multistate measles outbreak originating in Disneyland in California was recognized. As of April 17, when the outbreak was declared over, 111 measles cases from seven states had been linked to this outbreak.5 Of the evaluable cases, 44% were in unvaccinated people and 38% were in those whose vaccination status was unknown or undocumented. The median age of patients was 21, and 20% required hospitalization.
This outbreak, as well as four other smaller US outbreaks the same year, underscores the transmissibility of the virus in populations containing only a small percentage of unvaccinated people.6
DISTINCTIVE CLINICAL PICTURE
The incubation period for measles infection is 7 to 21 days, with most cases becoming apparent 10 to 12 days after exposure. Measles should be suspected in a patient with the following clinical features whose history indicates susceptibility and exposure (ie, an unimmunized person with a history of exposure or travel):
Severe acute respiratory illness. Measles usually presents as an acute respiratory viral illness, which typically lasts 2 to 4 days. The illness involves high fevers, malaise, anorexia, and the “three Cs”: cough, coryza (rhinitis), and conjunctivitis. Patients usually appear sicker than those with more common viral illnesses.
Koplik spots, which are pathognomonic for measles, are seen in the first few days of illness. They are bluish-white, slightly raised lesions on an erythematous base on the buccal mucosa, usually opposite the first molar (Figure 1). Spots can also be seen on the soft palate, conjunctiva, and vaginal mucosa. Koplik spots usually disappear after a few days and often are not appreciable at the time of evaluation.
Discrete erythematous patches develop on the face and neck a day after the appearance of Koplik spots. This rash becomes more confluent as it spreads to involve the entire body (Figure 2). It typically lasts for 3 to 7 days, then fades in a similar pattern. The confluent nature of this rash and its spread from the face and neck to the entire body are characteristic of measles. Patients are highly contagious from 4 days before the onset of the rash to 4 days after.
COMPLICATIONS CAN BE SEVERE
Those at highest risk for measles complications are infants, children under age 5, adults over age 20, pregnant women, and immunosuppressed individuals.7
Pneumonia—either a primary measles pneumonia or a secondary viral or bacterial pneumonia—is the most common cause of death.8,9 Viruses complicating measles are typically adenovirus and herpes simplex virus. Bacteria causing secondary infection are usually Staphylococcus aureus and Streptococcus pneumoniae and, less commonly, gram-negative bacteria.
Laryngotracheobronchitis (croup) is the second most common cause of death, with bacteria and viruses similar to those causing measles-related pneumonia.
Otitis media is the most common complication of measles. Other respiratory complications include mastoiditis, pneumothorax, and mediastinal emphysema.
Acute measles encephalitis occurs in 1 measles case per 1,000 and often results in permanent brain damage. During the convalescent phase of the illness, fever again emerges, with the development of headaches, seizures, and altered consciousness.10
Subacute sclerosing panencephalitis is a rare fatal degenerative disease of the central nervous system caused by a persistent infection with a defective measles virus. The precise pathophysiology is unclear, but it is thought that mutations of the viral genome lead to altered cellular immunity.11 The condition typically occurs 7 to 10 years after the initial measles infection, particularly in those who developed measles before age 2. Clinical manifestations include behavioral disturbances, intellectual deterioration, and myoclonic seizures, slowly progressing to a vegetative state and death.12
Other complications of measles include diarrhea and stomatitis, which are associated with malnutrition in developing countries, and subclinical hepatitis, thrombocytopenia, appendicitis, ileocolitis, hypokalemia, and myocarditis.
During pregnancy, measles infection can be complicated by primary measles pneumonia and is associated with an increased risk of miscarriage and premature birth.13
Patients with a cell-mediated immunodeficiency who develop measles are particularly susceptible to fatal measles pneumonia and acute progressive encephalitis.14
ATYPICAL MEASLES IN THOSE WHO RECEIVED KILLED VACCINE
From 1963 to 1967, a killed measles vaccine was available in the United States. Those who received this vaccine are susceptible to an atypical form of measles when exposed to the virus,15 characterized by a 1- to 2-day prodrome, followed by the appearance of a maculopapular or petechial rash on the distal extremities that spreads centripetally. Patients develop high fever and edema of the hands and feet, and have a more prolonged course than with classic measles. It is believed not to be contagious.16
LABORATORY CONFIRMATION
Laboratory confirmation of measles is recommended for suspected cases. Because viral isolation is technically difficult and is not readily available in most laboratories, measles-specific immunoglobulin M antibody serologic testing is most commonly used. It is almost 100% sensitive when done 2 to 3 days after the onset of the rash.17
Measles RNA testing by real-time polymerase chain reaction to detect measles virus in the blood, throat, or urine is more specific and if available may be preferred over serologic testing.18
SUPPORTIVE MANAGEMENT AND VITAMIN A SUPPLEMENTATION
No specific antiviral therapy for measles is available. Management involves supportive measures and monitoring for secondary bacterial complications.
The World Health Organization and the American Academy of Pediatrics recommend vitamin A supplementation for all children with acute measles.19 In developing countries, it has been shown to reduce rates of morbidity and death in measles-infected children.20 In the United States, children with measles have been found to have low serum levels of vitamin A, with lower levels associated with more severe disease.
VACCINATION RECOMMENDATIONS
The only measles vaccine available in the United States is a live further-attenuated strain prepared in chick embryo cell culture and combined with mumps and rubella vaccine (MMR) or with measles, mumps, rubella, and varicella vaccine (MMRV).
Healthy children. Two doses of measles vaccine are recommended, as a single dose is associated with a 5% failure rate. The recommended schedule is:
- First dose at age 12 to 15 months
- Second dose at the time of school entry (ages 4 to 6), or at any time at least 28 days after the first dose.19
More than 99% of children who receive two doses of vaccine according to this schedule develop serologic evidence of measles immunity. Vaccination provides long-term immunity, and many epidemiologic studies have documented that waning immunity after vaccination occurs only very rarely.21
All school-age children, including elementary, middle, and high school students, who received only one dose of measles vaccine should receive the second dose.
Adults born in 1957 or later should receive at least one dose of measles vaccine unless they have other acceptable evidence of immunity, such as:4
- Documentation of age-appropriate live measles vaccine, ie, one dose of vaccine for adults not at high risk, or two doses for those at high risk (see below)
- Laboratory evidence of immunity (ie, measles immunoglobulin G in serum)
- Laboratory confirmation of disease.
Adults born before 1957 can be considered to be immune to measles, although MMR vaccine can be administered in those without contraindications.
Adults at increased risk of exposure or transmission of measles and who do not have evidence of immunity should receive two doses of MMR vaccine, given at least 28 days apart. This high-risk group includes:
- Students attending college or other post-high school educational institution
- Healthcare personnel
- International travelers.
During measles outbreaks, every effort should be made to ensure that those at high risk are vaccinated with two doses of MMR or have other acceptable evidence of immunity.
LIVE VACCINE IS SAFE FOR MOST PEOPLE
Mild side effects. A transient fever, which may be accompanied by a discrete or confluent rash, occurs in 5% to 15% of recipients 5 to 12 days after vaccination.
Transmission does not occur. People who have been newly vaccinated do not transmit the virus to susceptible contacts, even if they develop a vaccine-associated rash. The vaccine can safely be given to close contacts of immunocompromised and other susceptible people.
Egg allergy not a concern. Measles vaccine is produced in chick embryo cell culture but has been shown to be safe for people with egg allergy and is recommended without the need for egg allergy testing.19
Autism link debunked. No scientific evidence shows that the risk of autism is higher in children who receive MMR vaccine than in those who do not. In 2001, an Institute of Medicine report rejected a causal relationship between MMR vaccine and autism spectrum disorders.22
CONTRAINDICATIONS
Measles vaccine is contraindicated for:
- Patients who have cell-mediated immune deficiencies, except human immunodeficiency virus (HIV) infection
- Pregnant women
- Those who have had a severe allergic reaction to a vaccine component in the past
- Those with moderate or severe acute illness
- Those who have recently received immunoglobulin products.
People with HIV infection who are severely immunosuppressed should not receive live measles vaccine. However, because of the risk of severe measles in HIV-infected patients and because the vaccine has been shown to be safe for patients with HIV without severe immunosuppression, the vaccine is recommended for those with asymptomatic or mildly symptomatic HIV infection who do not have evidence of severe immunosuppression (ie, CD4 lymphocytes < 15% or < 200 cells/µL).3,4
INFECTION CONTROL AND PREVENTION
Healthcare workers should maintain a high index of suspicion for measles and implement isolation procedures promptly in patients with a febrile illness, rash, and a history of travel abroad or contact with travelers from abroad.23 Suspected cases should be reported promptly to local health agencies to help limit spread.
Patients with measles should be placed in airborne isolation (eg, use of an N95 or higher level respirator and an airborne infection isolation room) for 4 days after the onset of the rash in a normal host and for the duration of the illness in an immunocompromised patient. Healthcare staff, regardless of their immunity status, should adhere to these precautions when entering the room of infected patients.
Immunization programs should be established to ensure that everyone who works or volunteers in healthcare facilities is protected against measles.4
Postexposure prophylaxis. Measles vaccination given to susceptible contacts within 72 hours of exposure may provide protection against infection and induces protection against subsequent measles exposures.24,25
Vaccination is the best intervention for susceptible contacts older than 12 months who do not have a contraindication to measles vaccination, and for those who have received only one dose of measles vaccine.
Passive immunization. Active immunization is the best strategy for controlling measles outbreaks. Passive immunization with intramuscularly or intravenously administered immunoglobulin given within 6 days of exposure can be used to prevent transmission or modify the clinical course of infection for susceptible contacts at high risk of developing severe or fatal measles. This includes people who are being treated with immunosuppressive agents, HIV-infected, pregnant, or younger than 1 year of age.
- Stokes J Jr, Reilly CM, Buynak EB, Hilleman MR. Immunologic studies of measles. Am J Hyg 1961; 74:293–303.
- Bloch AB, Orenstein WA, Stetler HC, et al. Health impact of measles vaccination in the United States. Pediatrics 1985; 76:524–532.
- Katz SL, Hinman AR. Summary and conclusions: measles elimination meeting, 16-17 March 2000. J Infect Dis 2004; 189(suppl 1):S43–S47.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention (CDC). Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013; 62:1–34.
- Clemmons NS, Gastanaduy PA, Fiebelkorn AP, Redd SB, Wallace GS; Centers for Disease Control and Prevention (CDC). Measles—United States, January 4 - April 2, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:373–376.
- Gay NJ. The theory of measles elimination: implications for the design of elimination strategies. J Infect Dis 2004; 189(suppl 1):S27–S35.
- Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189(suppl 1):S4–S16).
- Gremillion DH, Crawford GE. Measles pneumonia in young adults. An analysis of 106 cases. Am J Med 1981; 71:539–542.
- Quiambao BP, Gatchalian SR, Halonen P, et al. Coinfection is common in measles-associated pneumonia. Pediatr Infect Dis J 1998; 17:89–93.
- Johnson RT, Griffin D, Hirsch R, et al. Measles encephalomyelitis—clinical and immunologic studies. N Engl J Med 1984; 310:137–141.
- Garg RK. Subacute sclerosing panencephalitis. Postgrad Med J 2002; 78:63–70.
- Sever JL. Persistent measles infection of the central nervous system: subacute sclerosing panencephalitis. Rev Infect Dis 1983; 5:467–473.
- Atmar RL, Englund JA, Hammill H. Complications of measles during pregnancy. Clin Infect Dis 1992; 14:217–226.
- Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients. JAMA 1992; 267:1237–1241.
- Frey HM, Krugman S. Atypical measles syndrome: unusual hepatic, pulmonary, and immunologic aspects. Am J Med Sci 1981; 281:51–55.
- Fulginiti VA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA 1967; 202:1075–1080.
- Bellini WJ, Helfand RF. The challenges and strategies for laboratory diagnosis of measles in an international setting. J Infect Dis 2003; 187(suppl 1):S283–S290.
- Riddell MA, Chibo D, Kelly HA, Catton MG, Birch CJ. Investigation of optimal specimen type and sampling time for detection of measles virus RNA during a measles epidemic. J Clin Microbiol 2001; 39:375–376.
- Measles. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book: 2012 Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:489-499.
- Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev 2005; 4:CD001479.
- Markowitz LE, Preblud SR, Fine PE, Orenstein WA. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J 1990; 9:101–110.
- Stratton K, Gable A, Shetty P, McCormick M. Immunization safety review: measles-mumps-rubella vaccine and autism. Washington, DC: National Academy Press; 2001.
- Centers for Disease Control and Prevention (CDC). 2007 guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. www.cdc.gov/hicpac/2007ip/2007isolationprecautions.html. Accessed April 8, 2016.
- Berkovich S, Starr S. Use of live-measles-virus vaccine to abort an expected outbreak of measles within a closed population. N Engl J Med 1963; 269:75–77.
- Ruuskanen O, Salmi TT, Halonen P. Measles vaccination after exposure to natural measles. J Pediatr 1978; 93:43–46.
- Stokes J Jr, Reilly CM, Buynak EB, Hilleman MR. Immunologic studies of measles. Am J Hyg 1961; 74:293–303.
- Bloch AB, Orenstein WA, Stetler HC, et al. Health impact of measles vaccination in the United States. Pediatrics 1985; 76:524–532.
- Katz SL, Hinman AR. Summary and conclusions: measles elimination meeting, 16-17 March 2000. J Infect Dis 2004; 189(suppl 1):S43–S47.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention (CDC). Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013; 62:1–34.
- Clemmons NS, Gastanaduy PA, Fiebelkorn AP, Redd SB, Wallace GS; Centers for Disease Control and Prevention (CDC). Measles—United States, January 4 - April 2, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:373–376.
- Gay NJ. The theory of measles elimination: implications for the design of elimination strategies. J Infect Dis 2004; 189(suppl 1):S27–S35.
- Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189(suppl 1):S4–S16).
- Gremillion DH, Crawford GE. Measles pneumonia in young adults. An analysis of 106 cases. Am J Med 1981; 71:539–542.
- Quiambao BP, Gatchalian SR, Halonen P, et al. Coinfection is common in measles-associated pneumonia. Pediatr Infect Dis J 1998; 17:89–93.
- Johnson RT, Griffin D, Hirsch R, et al. Measles encephalomyelitis—clinical and immunologic studies. N Engl J Med 1984; 310:137–141.
- Garg RK. Subacute sclerosing panencephalitis. Postgrad Med J 2002; 78:63–70.
- Sever JL. Persistent measles infection of the central nervous system: subacute sclerosing panencephalitis. Rev Infect Dis 1983; 5:467–473.
- Atmar RL, Englund JA, Hammill H. Complications of measles during pregnancy. Clin Infect Dis 1992; 14:217–226.
- Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients. JAMA 1992; 267:1237–1241.
- Frey HM, Krugman S. Atypical measles syndrome: unusual hepatic, pulmonary, and immunologic aspects. Am J Med Sci 1981; 281:51–55.
- Fulginiti VA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA 1967; 202:1075–1080.
- Bellini WJ, Helfand RF. The challenges and strategies for laboratory diagnosis of measles in an international setting. J Infect Dis 2003; 187(suppl 1):S283–S290.
- Riddell MA, Chibo D, Kelly HA, Catton MG, Birch CJ. Investigation of optimal specimen type and sampling time for detection of measles virus RNA during a measles epidemic. J Clin Microbiol 2001; 39:375–376.
- Measles. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book: 2012 Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:489-499.
- Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev 2005; 4:CD001479.
- Markowitz LE, Preblud SR, Fine PE, Orenstein WA. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J 1990; 9:101–110.
- Stratton K, Gable A, Shetty P, McCormick M. Immunization safety review: measles-mumps-rubella vaccine and autism. Washington, DC: National Academy Press; 2001.
- Centers for Disease Control and Prevention (CDC). 2007 guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. www.cdc.gov/hicpac/2007ip/2007isolationprecautions.html. Accessed April 8, 2016.
- Berkovich S, Starr S. Use of live-measles-virus vaccine to abort an expected outbreak of measles within a closed population. N Engl J Med 1963; 269:75–77.
- Ruuskanen O, Salmi TT, Halonen P. Measles vaccination after exposure to natural measles. J Pediatr 1978; 93:43–46.
KEY POINTS
- Patients with measles are usually very sick with high fever, cough, rhinitis, and conjunctivitis.
- Koplik spots—small bluish-white lesions on the buccal mucosa—are usually evident only in the first few days of illness. Soon after, a patchy red rash develops, starting with the face and neck, then spreading to the entire body.
- Measles can lead to pneumonia, encephalitis, brain damage, and death.
- Suspected cases should be isolated and susceptible contacts vaccinated or given immunoglobulin if at high risk of developing severe disease.
- The diagnosis should be confirmed by serologic testing with measles-specific immunoglobulin M antibody.
- Vaccination confers lifelong immunity and is recommended for all healthy children in two doses: the first at 12 to 15 months of age and the second at the time of school entry.
Measles: Not just a childhood rash
Although measles is generally considered a disease of children, it affects people of all ages. While the incidence of measles in the United States is significantly lower than in 1963, when an effective measles vaccine was first introduced, recent increases in the number of sporadic cases and community outbreaks in adults show that measles is still a significant health problem.
PATHOGENESIS OF MEASLES
Measles is a highly contagious viral infection, whose manifestations have been recognized since the 7th century. The measles virus is an RNA virus of the Paramyxoviridae family. It is very difficult to isolate from clinical specimens, requiring special cell lines for in vitro propagation.
After acquisition, the measles virus establishes localized infection of the respiratory epithelium and then spreads to the regional lymphatics. A primary viremia then occurs, in which the virus replicates at the site of inoculation and in the reticuloendothelial tissues. A secondary viremia follows, in which the virus infects and replicates in the skin, conjunctiva, respiratory tract, and other distant organs.
The measles rash is thought to be due to a hypersensitivity reaction.1 Cell-mediated responses are the main line of defense against measles, as evidenced by the fact that people with cell-mediated deficiencies develop severe measles infection.2 Immunity to wild-type measles is believed to be lifelong.3,4
MEASLES IS HIGHLY CONTAGIOUS
Measles is one of the most contagious infectious diseases, with a secondary attack rate of at least 90% in susceptible household contacts. 4 The fact that emergency departments and physicians’ offices have become sites of measles transmission in recent years underscores the transmissibility of the virus.5–7
Although the virus is very labile, it can remain infective in respiratory droplets from the air for many hours. Thus, measles virus spreads from person to person by direct contact with droplets from respiratory secretions of infected persons.
The period of maximal contagion is the late prodrome, ie, 2 to 4 days before the onset of the rash. People who are generally in good health are contagious through 4 days after the onset of the rash, whereas people with compromised immunity can continue to shed the virus for the entire duration of the illness.
Airborne transmission precautions are required for 4 days after the onset of the rash in hospitalized, non-immunocompromised patients with measles, and for the duration of the illness for immunocompromised patients.
In the absence of widespread measles vaccination, measles infection peaks in late winter and early spring.
EPIDEMIOLOGIC TRENDS: CAUSE FOR CONCERN
Since an effective vaccine became available in 1963, the annual incidence of measles cases in the United States has decreased by more than 99%. A significant resurgence from 1989 to 1991 affected mainly unvaccinated preschoolers and resulted in more than 55,000 cases and 130 deaths.8 This resurgence prompted widespread, intensive immunization efforts and the recommendation that a second dose of measles vaccine be given to school-aged children. This led to the effective elimination of endemic transmission of measles in the United States.9
Although 90% of these cases either were directly imported or were associated with importation from other countries,10 the reason for the large number of cases was clearly the greater transmission after importation of the virus into the United States. This transmission was the direct result of the fact that 91% of the cases occurred in unvaccinated people or people whose vaccination status was not known or was not documented. A high proportion— at least 61 (47%)—of the 131 measles cases in 2008 were in school-aged children whose parents chose not to have them vaccinated. Although no deaths were reported in these 131 patients, 15 required hospitalization.
Although most reported measles cases are still in young and school-aged children, recent cases and outbreaks have also occurred in isolated communities of adults. Approximately 25% of the cases reported in 2008 were in people age 20 and older. Most adults who contracted measles had unknown or undocumented vaccination status. Similarly, a small measles outbreak occurred in Indiana in 2005, when an adolescent US citizen traveling in Europe became infected in Romania and exposed 500 people at a church gathering upon her return. Thirty-four cases of measles were reported from this exposure, and many were in adults.11
The recent increase in the number of cases reported and the continued reports of outbreaks highlight the fact that measles outbreaks can occur in communities with a high number of unvaccinated people, and underscore the need for high overall measles vaccination coverage to limit the spread of this infection.12
CLINICAL FEATURES OF MEASLES
The first sign of measles is a distinct prodrome, which occurs after an incubation period of 10 to 12 days. The prodrome is characterized by fever, malaise, anorexia, conjunctivitis, coryza, and cough and may resemble an upper respiratory tract infection; it lasts 2 to 4 days.
Towards the end of the prodrome, the body temperature can rise to as high as 40°C, and Koplik spots, pathognomonic for measles, appear. Koplik spots, bluish-gray specks on an erythematous base, usually appear on the buccal mucosa opposite the second molars 1 to 2 days before the onset of the rash, and last for 1 to 2 days after the onset of the rash. Thus, it is not unusual for Koplik spots to have disappeared at the time the diagnosis of measles is entertained.
The classic measles rash is an erythematous maculopapular eruption that begins on the head and face and spreads to involve the entire body. It usually persists for 4 to 5 days and is most confluent on the face and upper body. The rash fades in order of appearance, and may desquamate. People with measles appear ill, especially 1 to 2 days after the rash appears.
The entire course of measles usually lasts 7 to 10 days in patients with a healthy immune system. The cough, a manifestation of tracheobronchitis, is usually the last symptom to resolve. Patients are contagious 2 to 4 days before the onset of the rash, and remain so through 4 days after the onset of the rash.
COMPLICATIONS
Respiratory complications
Pneumonia is responsible for 60% of deaths associated with measles.13 Although radiographic evidence of pneumonia is found in measles patients with no complications, symptomatic pneumonia occurs in 1% to 6% of patients. It is the result of either direct invasion by the virus or secondary bacterial infection,17 most often with Staphylococcus aureus and Streptococcus pneumoniae. Other respiratory complications include otitis media, sinusitis, and laryngotracheobronchitis.
Neurologic complications
Acute measles encephalitis is more common in adults than in children. Occuring in 1 in 1,000 to 2,000 patients,18 it is characterized by the resurgence of fever during the convalescent phase of the illness, along with headaches, seizures, and altered consciousness. These manifestations may be mild or severe, but they lead to permanent neurologic sequelae in a substantial proportion of affected patients. It is not clear whether acute measles encephalitis represents direct invasion of the virus or a postinfectious process from a hypersensitivity to the virus.19
Subacute sclerosing panencephalitis is a rare, chronic, degenerative central nervous system disease that occurs secondary to persistent infection with a defective measles virus.20 The prevalence is estimated at 1 per 100,000 cases. Signs and symptoms appear an average of 7 years after the initial infection and include personality changes, myoclonic seizures, and motor disturbances. Often, coma and death follow.
This condition occurs particularly in those who had measles at a very young age, ie, before the age of 2 years, and it occurs despite a vigorous host-immune response to the virus. Patients have high titers of measles-specific antibody in the sera and cerebrospinal fluid.
Other complications
Diarrhea and stomatitis account for much of the sickness and death from measles in developing countries.
Subclinical hepatitis occurs in at least 30% of adult measles patients.
Less common complications include thrombocytopenia, appendicitis, ileocolitis, pericarditis, myocarditis, and hypocalcemia.
MEASLES DURING PREGNANCY
Measles during pregnancy may be severe, mainly due to primary measles pneumonia.21 Measles is associated with a risk of miscarriage and prematurity, but congenital anomalies of the fetus have not been described, as they have for rubella infection.22
MEASLES IN COMPROMISED IMMUNITY
Measles patients with deficiencies of cellmediated immunity have a prolonged, severe, and often fatal course.2,23,24 This includes patients with:
- Human immunodeficiency virus (HIV) infection
- Congenital immunodeficiencies
- Disorders requiring chemotherapeutic and immunosuppressive therapy.
These patients are particularly susceptible to acute progressive encephalitis and measles pneumonitis. Case-fatality rates of 70% in cancer patients and 40% in HIV-infected patients have been reported.24
The diagnosis of measles may be difficult in patients without cell-mediated immunity, as 25% to 40% of them do not develop the characteristic rash.2,23 The absence of rash supports the theory that the rash is a hypersensitivity reaction to the virus.
MODIFIED AND ATYPICAL MEASLES
Modified measles
A modified form of measles can occur in people with some degree of passive immunity to the virus, including those previously vaccinated. It occurs mostly in patients who recently received immunoglobulin products, or in young infants who have residual maternal antibody. A modified measles illness can also follow vaccination with live-virus vaccine (see later discussion).
The clinical manifestations vary, and the illness may not have the classic features of prodrome, rash, and Koplik spots.
Atypical measles
Atypical measles is an unusual form that can occur when a person previously vaccinated with a killed-virus measles vaccine (used from 1963 to 1967) is exposed to wild-type measles.25 Features include a shorter prodrome (1 to 2 days), followed by appearance of a rash that begins on the distal extremities and spreads centripetally, usually sparing the neck, face, and head. The rash may be petechial, maculopapular, urticarial, vesicular, or a combination. The rash is accompanied by high fever and edema of the extremities. Complications such as pneumonia and hepatitis may occur.
The course of atypical measles is more prolonged than with classic measles, but because these patients are thought to have partial protection against the virus, they do not transmit it and are not considered contagious.26
DIAGNOSIS OF MEASLES
The classic clinical features are usually enough to distinguish measles from other febrile illnesses with similar clinical manifestions, such as rubella, dengue, parvovirus B19 infection, erythema multiforme, Stevens-Johnson syndrome, and streptococcal scarlet fever. The distinctive measles prodrome, Koplik spots, the progression of the rash from the head and neck to the trunk and the extremities, and the severity of disease are distinctive features of measles.
Laboratory tests to confirm the diagnosis are often used in areas where measles is rare, and laboratory confirmation is currently recommended in the United States. Because viral isolation is technically difficult and is not widely available, serologic testing is the method most commonly used. The measles-specific immunoglobulin M (IgM) antibody assay, the test used most often, is almost 100% sensitive when done 2 to 3 days after the onset of the rash.27,28 Measles IgM antibody peaks at 4 weeks after the infection and disappears by 6 to 8 weeks.
It is important to remember that false-positive measles IgM antibody may occur with other viral infections, such as parvovirus B19 and rubella. Because measles-specific IgG antibody is produced with the onset of infection and peaks at 4 weeks, a fourfold rise in the IgG titer is useful in confirming the diagnosis. Measles IgG antibody after infection is sustained for life.
Reverse transcription-polymerase chain reaction testing can also detect measles virus in the blood and urine when direct evidence of the virus is necessary, such as in immunocompromised patients.29
TREATMENT IS SUPPORTIVE
Treatment of measles mainly involves supportive measures, such as fluids and antipyretics. Antiviral agents such as ribavirin and interferon have in vitro activity against the measles virus and have been used to treat severe measles infection in immunocompromised patients. However, their clinical efficacy is unproven.30
Routine use of antibacterial agents to prevent secondary bacterial infection is not recommended.
CURRENT RECOMMENDATIONS FOR ACTIVE IMMUNIZATION
Active immunization for measles has been available since 1963. Between 1963 and 1967, both killed-virus and live-virus vaccines were available. As atypical measles cases became recognized, the killed-virus vaccine was withdrawn.
The vaccine currently available in the United States is a live-attenuated strain prepared in chicken embryo cell culture and combined with mumps and rubella vaccine (MMR) or mumps, rubella, and varicella vaccine (MMRV).
Two doses of live-virus measles vaccine are recommended for all healthy children before they begin school, with the first dose given at 12 to 15 months of age. A second dose is needed because the failure rate with one dose is 5%. More than 99% of people who receive two doses separated by 4 weeks develop serologic evidence of measles.
Waning immunity after vaccination occurs very rarely, with approximately 5% of children developing secondary vaccine failure 10 to 15 years after vaccination.3,31
Although rates of vaccination in the United States are high, cases of measles continue to occur in unvaccinated infants and in children who are either too young to be vaccinated or whose parents claimed exemption because of religious or personal beliefs.
Because of the occurrence of measles cases in adolescents, young adults, and adults, potentially susceptible people should be identified and vaccinated according to current guidelines. People should be considered susceptible unless they have documentation of at least two doses of measles vaccine given at least 28 days apart, physician-diagnosed measles, laboratory evidence of immunity to measles, or were born before 1957. All adults who are susceptible should receive at least one dose of measles vaccine.10 Adults at higher risk of contracting measles include:
- Students in high school and college
- International travelers
- Health care personnel.
For these adults, two doses of measles vaccine, at least 28 days apart, are recommended.32
Postexposure prophylaxis
Measles vaccination given to susceptible contacts within 72 hours of exposure as postexposure prophylaxis may protect against infection and induces protection against subsequent exposures to measles.33,34 Vaccination is the intervention of choice for susceptible individuals older than 12 months of age who are exposed to measles and who do not have a contraindication to measles vaccination.35 Active rather than passive immunization is also the strategy of choice for controlling measles outbreaks.
Passive immunization with intramuscular immune globulin within 6 days of exposure can be used in selected circumstances to prevent transmission or to modify the clinical course of the infection.36 Immune globulin therapy is recommended for susceptible individuals who are exposed to measles and who are at high risk of developing severe or fatal measles. This includes individuals who are being treated with immunosuppressive agents, those with HIV infection, pregnant women, and infants less than 1 year of age. Immune globulin should not be used to control measles outbreaks.
ADVERSE EFFECTS OF MEASLES VACCINE
Live-virus measles vaccine has an excellent safety record. A transient fever, which may be accompanied by a measles-like rash, occurs in 5% to 15% of people 5 to 12 days after vaccination. The rash may be discrete or confluent and is self-limited.
Although measles vaccine is a live-attenuated vaccine, vaccinated people do not transmit the virus to susceptible contacts and are not considered contagious, even if they develop a vaccine-associated rash. Thus, the vaccine can be safely given to close contacts of immunocompromised and other susceptible people. Encephalitis is exceedingly rare following vaccination.
There is no scientific evidence that the risk of autism is higher in children who receive measles or MMR vaccine than in unvaccinated children.37 An Institute of Medicine report in 2001 rejected a causal relationship between MMR vaccine and autism spectrum disorders.38
CONTRAINDICATIONS TO MEASLES VACCINATION
Measles vaccine is contraindicated for:
- People who have cell-mediated immune deficiencies (except patients wtih HIV infection—see discussion just below)
- Pregnant women
- Those who had a severe allergic reaction to a vaccine component after a previous dose
- Those with moderate or severe acute illness
- Those who have recently received immune globulin products.
HIV-infected patients with severe immunosuppression should not receive the liveattenuated measles vaccine. However, because patients with HIV are at risk of severe measles, and because the vaccine has been shown to be safe in HIV patients who do not have severe immunosuppression, the vaccine is recommended for those with asymptomatic or mildly symptomatic HIV infection who do not have evidence of severe immunosuppression. 39
After receiving immune globulin
Anyone who has recently received immune globulin should not receive measles vaccine until sufficient time has passed, since passively acquired antibodies interfere with the immune response to live-virus vaccines. How long to wait depends on the type of immune globulin, the indication, the amount, and the route of administration. In general, the waiting period is:
- At least 3 months after intramuscular immune globulin or tetanus, hepatitis A, or hepatitis B prophylaxis
- At least 4 months after intramuscular immune globulin for rabies, or 6 months after intravenous immune globulin for cytomegalovirus (dose, 150 mg/kg)
- At least 8 months after intravenous immune globulin as replacement or therapy for immune deficiencies (dose, 400 mg/kg), or after intravenous immune globulin for immune thrombocytopenic purpura (400 mg/kg)
- At least 10 months after intravenous immune globulin for immune thrombocytopenic pupura at a dose of 1 g/kg.39
Egg allergy is not a contraindication
Although measles vaccine is produced in chick embryo cell culture, the vaccine has been shown to be safe in people with egg allergy, so they may be vaccinated without first being tested for egg allergy.39,40
- Lachmann PJ. Immunopathology of measles. Proc R Soc Med 1974; 67:1120–1122.
- Enders JF, McCarthy K, Mitus A, Cheatham WJ. Isolation of measles virus at autopsy in case of giant cell pneumonia without rash. N Engl J Med 1959; 261:875–881.
- Markowitz LE, Preblud SR, Fine PE, Orenstein WA. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J 1990; 9:101–110.
- Stokes J, Reilly CM, Buynak EB, Hilleman MR. Immunologic studies of measles. Am J Hyg 1961; 74:293–303.
- Farizo KM, Stehr-Green PA, Simpson DM, Markowitz LE. Pediatric emergency room visits: a risk factor for acquiring measles. Pediatrics 1991; 87:74–79.
- Bloch AB, Orenstein W, Ewing WM, et al. Measles outbreak in a pediatric practice: airborne transmission in an office setting. Pediatrics 1985; 75:676–683.
- Remington PL, Hall WN, Davis IH, et al. Airborne transmission of measles in a physician’s office. JAMA 1985; 253:1574–1577.
- US Centers for Disease Control and Prevention. Reported vaccine-preventable diseases—United States, 1993, and the Childhood Immunization Initiative. MMWR 1994; 43:57–60.
- Orenstein WA, Papania MJ, Wharton ME. Measles elimination in the United States. J Infect Dis 2004; 189 (suppl 1):S1–S3.
- US Centers for Disease Control and Prevention. Update: Measles—United States, January–July 2008. MMWR 2008; 57:893–896.
- Parker AA, Staggs W, Dayan GH, et al. Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med 2006; 355:447–455.
- Mulholland EK. Measles in the United States, 2006. N Engl J Med 2006; 355:440–443.
- Barkin RM. Measles mortality. Analysis of the primary cause of death. Am J Dis Child 1975; 129:307–309.
- Barkin RM. Measles mortality: a retrospective look at the vaccine era. Am J Epidemiol 1975; 102:341–349.
- US Centers for Disease Control and Prevention. Public-sector vaccination efforts in response to the resurgence of measles among preschool-aged children—United States, 1989–1991. MMWR 1992; 41:522–525.
- Gremillion DH, Crawford GE. Measles pneumonia in young adults. an analysis of 106 cases. Am J Med 1981; 71:539–542.
- Quiambao BP, Gatchalian SR, Halonen P, et al. Coinfection is common in measles-associated pneumonia. Pediatr Infect Dis J 1998; 17:89–93.
- Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189( suppl 1):S4–S16.
- Johnson RT, Griffin DE, Hirsch RL, et al. Measles encephalomyelitis—clinical and immunologic studies. N Engl J Med 1984; 310:137–141.
- Sever JL. Persistent measles infection of the central nervous system: subacute sclerosing panencephalitis. Rev Infect Dis 1983; 5:467–473.
- Atmar RL, Englund JA, Hammill H. Complications of measles during pregnancy. Clin Infect Dis 1992; 14:217–226.
- Gershon AA. Chickenpox, measles, and mumps. In:Remington J, Klein J, eds. Infectious Diseases of the Fetus and Newborn Infant. Elsevier Saunders: Philadelphia, 2006:693–738.
- Mitus A, Enders JF, Craig JM, Holloway A. Persistence of measles virus and depression of antibody formation in patients with giant-cell pneumonia after measles. N Engl J Med 1959; 261:882–889.
- Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients JAMA 1992; 267:1237–1241.
- Frey HM, Krugman S. Atypical measles syndrome: unusual hepatic, pulmonary, and immunologic aspects. Am J Med Sci 1981; 281:51–55.
- Fulginiti VA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA 1967; 202:1075–1080.
- Mayo DR, Brennan T, Cormier DP, Hadler J, Lamb P. Evaluation of a commercial measles virus immunoglobulin M enzyme immunoassay. J Clin Microbiol 1991; 29:2865–2867.
- Bellini WJ, Helfand RF. The challenges and strategies for laboratory diagnosis of measles in an international setting. J Infect Dis 2003; 187( suppl 1):S283–S290.
- Riddell MA, Chibo D, Kelly HA, Catton MG, Birch CJ. Investigation of optimal specimen type and sampling time for detection of measles virus RNA during a measles epidemic. J Clin Microbiol 2001; 39:375–376.
- Forni Al, Schluger NW, Roberts RB. Severe measles pneumonitis in adults: evaluation of clinical characteristics and therapy with intravenous ribavirin. Clin Infect Dis 1994; 19:454–462.
- Anders JF, Jacobsen RM, Poland GA, Jacobsen SJ, Wollan PC. Secondary failure rates of measles vaccines: a metaanalysis of published studes. Pediar Infect Dis J 1996; 15:62–66.
- US Centers for Disease Control and Prevention. Measles—United States, January 1–April 25, 2008. MMWR 2008; 57:494–498.
- Berkovitz S, Starr S. Use of live-measles-virus vaccine to abort an expected outbreak of measles within a closed population. N Engl J Med 1963; 269:75–77.
- Ruuskanen O, Salmi TT, Halonen P. Measles vaccination after exposure to natural measles. J Pediatr 1978; 93:43–46.
- Strebel PM, Papania MJ, Halsey NA. Measles vaccine. In:Plotkin SA, Orenstein WA, eds. Vaccines. Saunders: New York, 2004:389–440.
- US Centers for Disease Control and Prevention. Measles, mumps and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1998; 47( RR-8):1–57. http://www.cdc.gov/mmwr/PDF/rr/rr4708.pdf. Accessed December 30, 2009.
- Madsen KM, Hviid A, Vestergaard M, et al. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med 2002; 347:1477–1482.
- Straton K, Gable A, Shetty P, McCormick M; for the Institute of Medicine. Immunization Safety Review: Measles-Mumps-Rubella Vaccine and Autism. National Academy Press: Washington, DC, 2001.
- Pickerington LK, Baker CJ, Long SS, McMillan JA, editors. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2009:444–455.
- James JM, Burks AW, Roberson PK, Sampson HA. Safe administration of the measles vaccine to children allergic to eggs. N Engl J Med 1995; 332:1262–1266.
Although measles is generally considered a disease of children, it affects people of all ages. While the incidence of measles in the United States is significantly lower than in 1963, when an effective measles vaccine was first introduced, recent increases in the number of sporadic cases and community outbreaks in adults show that measles is still a significant health problem.
PATHOGENESIS OF MEASLES
Measles is a highly contagious viral infection, whose manifestations have been recognized since the 7th century. The measles virus is an RNA virus of the Paramyxoviridae family. It is very difficult to isolate from clinical specimens, requiring special cell lines for in vitro propagation.
After acquisition, the measles virus establishes localized infection of the respiratory epithelium and then spreads to the regional lymphatics. A primary viremia then occurs, in which the virus replicates at the site of inoculation and in the reticuloendothelial tissues. A secondary viremia follows, in which the virus infects and replicates in the skin, conjunctiva, respiratory tract, and other distant organs.
The measles rash is thought to be due to a hypersensitivity reaction.1 Cell-mediated responses are the main line of defense against measles, as evidenced by the fact that people with cell-mediated deficiencies develop severe measles infection.2 Immunity to wild-type measles is believed to be lifelong.3,4
MEASLES IS HIGHLY CONTAGIOUS
Measles is one of the most contagious infectious diseases, with a secondary attack rate of at least 90% in susceptible household contacts. 4 The fact that emergency departments and physicians’ offices have become sites of measles transmission in recent years underscores the transmissibility of the virus.5–7
Although the virus is very labile, it can remain infective in respiratory droplets from the air for many hours. Thus, measles virus spreads from person to person by direct contact with droplets from respiratory secretions of infected persons.
The period of maximal contagion is the late prodrome, ie, 2 to 4 days before the onset of the rash. People who are generally in good health are contagious through 4 days after the onset of the rash, whereas people with compromised immunity can continue to shed the virus for the entire duration of the illness.
Airborne transmission precautions are required for 4 days after the onset of the rash in hospitalized, non-immunocompromised patients with measles, and for the duration of the illness for immunocompromised patients.
In the absence of widespread measles vaccination, measles infection peaks in late winter and early spring.
EPIDEMIOLOGIC TRENDS: CAUSE FOR CONCERN
Since an effective vaccine became available in 1963, the annual incidence of measles cases in the United States has decreased by more than 99%. A significant resurgence from 1989 to 1991 affected mainly unvaccinated preschoolers and resulted in more than 55,000 cases and 130 deaths.8 This resurgence prompted widespread, intensive immunization efforts and the recommendation that a second dose of measles vaccine be given to school-aged children. This led to the effective elimination of endemic transmission of measles in the United States.9
Although 90% of these cases either were directly imported or were associated with importation from other countries,10 the reason for the large number of cases was clearly the greater transmission after importation of the virus into the United States. This transmission was the direct result of the fact that 91% of the cases occurred in unvaccinated people or people whose vaccination status was not known or was not documented. A high proportion— at least 61 (47%)—of the 131 measles cases in 2008 were in school-aged children whose parents chose not to have them vaccinated. Although no deaths were reported in these 131 patients, 15 required hospitalization.
Although most reported measles cases are still in young and school-aged children, recent cases and outbreaks have also occurred in isolated communities of adults. Approximately 25% of the cases reported in 2008 were in people age 20 and older. Most adults who contracted measles had unknown or undocumented vaccination status. Similarly, a small measles outbreak occurred in Indiana in 2005, when an adolescent US citizen traveling in Europe became infected in Romania and exposed 500 people at a church gathering upon her return. Thirty-four cases of measles were reported from this exposure, and many were in adults.11
The recent increase in the number of cases reported and the continued reports of outbreaks highlight the fact that measles outbreaks can occur in communities with a high number of unvaccinated people, and underscore the need for high overall measles vaccination coverage to limit the spread of this infection.12
CLINICAL FEATURES OF MEASLES
The first sign of measles is a distinct prodrome, which occurs after an incubation period of 10 to 12 days. The prodrome is characterized by fever, malaise, anorexia, conjunctivitis, coryza, and cough and may resemble an upper respiratory tract infection; it lasts 2 to 4 days.
Towards the end of the prodrome, the body temperature can rise to as high as 40°C, and Koplik spots, pathognomonic for measles, appear. Koplik spots, bluish-gray specks on an erythematous base, usually appear on the buccal mucosa opposite the second molars 1 to 2 days before the onset of the rash, and last for 1 to 2 days after the onset of the rash. Thus, it is not unusual for Koplik spots to have disappeared at the time the diagnosis of measles is entertained.
The classic measles rash is an erythematous maculopapular eruption that begins on the head and face and spreads to involve the entire body. It usually persists for 4 to 5 days and is most confluent on the face and upper body. The rash fades in order of appearance, and may desquamate. People with measles appear ill, especially 1 to 2 days after the rash appears.
The entire course of measles usually lasts 7 to 10 days in patients with a healthy immune system. The cough, a manifestation of tracheobronchitis, is usually the last symptom to resolve. Patients are contagious 2 to 4 days before the onset of the rash, and remain so through 4 days after the onset of the rash.
COMPLICATIONS
Respiratory complications
Pneumonia is responsible for 60% of deaths associated with measles.13 Although radiographic evidence of pneumonia is found in measles patients with no complications, symptomatic pneumonia occurs in 1% to 6% of patients. It is the result of either direct invasion by the virus or secondary bacterial infection,17 most often with Staphylococcus aureus and Streptococcus pneumoniae. Other respiratory complications include otitis media, sinusitis, and laryngotracheobronchitis.
Neurologic complications
Acute measles encephalitis is more common in adults than in children. Occuring in 1 in 1,000 to 2,000 patients,18 it is characterized by the resurgence of fever during the convalescent phase of the illness, along with headaches, seizures, and altered consciousness. These manifestations may be mild or severe, but they lead to permanent neurologic sequelae in a substantial proportion of affected patients. It is not clear whether acute measles encephalitis represents direct invasion of the virus or a postinfectious process from a hypersensitivity to the virus.19
Subacute sclerosing panencephalitis is a rare, chronic, degenerative central nervous system disease that occurs secondary to persistent infection with a defective measles virus.20 The prevalence is estimated at 1 per 100,000 cases. Signs and symptoms appear an average of 7 years after the initial infection and include personality changes, myoclonic seizures, and motor disturbances. Often, coma and death follow.
This condition occurs particularly in those who had measles at a very young age, ie, before the age of 2 years, and it occurs despite a vigorous host-immune response to the virus. Patients have high titers of measles-specific antibody in the sera and cerebrospinal fluid.
Other complications
Diarrhea and stomatitis account for much of the sickness and death from measles in developing countries.
Subclinical hepatitis occurs in at least 30% of adult measles patients.
Less common complications include thrombocytopenia, appendicitis, ileocolitis, pericarditis, myocarditis, and hypocalcemia.
MEASLES DURING PREGNANCY
Measles during pregnancy may be severe, mainly due to primary measles pneumonia.21 Measles is associated with a risk of miscarriage and prematurity, but congenital anomalies of the fetus have not been described, as they have for rubella infection.22
MEASLES IN COMPROMISED IMMUNITY
Measles patients with deficiencies of cellmediated immunity have a prolonged, severe, and often fatal course.2,23,24 This includes patients with:
- Human immunodeficiency virus (HIV) infection
- Congenital immunodeficiencies
- Disorders requiring chemotherapeutic and immunosuppressive therapy.
These patients are particularly susceptible to acute progressive encephalitis and measles pneumonitis. Case-fatality rates of 70% in cancer patients and 40% in HIV-infected patients have been reported.24
The diagnosis of measles may be difficult in patients without cell-mediated immunity, as 25% to 40% of them do not develop the characteristic rash.2,23 The absence of rash supports the theory that the rash is a hypersensitivity reaction to the virus.
MODIFIED AND ATYPICAL MEASLES
Modified measles
A modified form of measles can occur in people with some degree of passive immunity to the virus, including those previously vaccinated. It occurs mostly in patients who recently received immunoglobulin products, or in young infants who have residual maternal antibody. A modified measles illness can also follow vaccination with live-virus vaccine (see later discussion).
The clinical manifestations vary, and the illness may not have the classic features of prodrome, rash, and Koplik spots.
Atypical measles
Atypical measles is an unusual form that can occur when a person previously vaccinated with a killed-virus measles vaccine (used from 1963 to 1967) is exposed to wild-type measles.25 Features include a shorter prodrome (1 to 2 days), followed by appearance of a rash that begins on the distal extremities and spreads centripetally, usually sparing the neck, face, and head. The rash may be petechial, maculopapular, urticarial, vesicular, or a combination. The rash is accompanied by high fever and edema of the extremities. Complications such as pneumonia and hepatitis may occur.
The course of atypical measles is more prolonged than with classic measles, but because these patients are thought to have partial protection against the virus, they do not transmit it and are not considered contagious.26
DIAGNOSIS OF MEASLES
The classic clinical features are usually enough to distinguish measles from other febrile illnesses with similar clinical manifestions, such as rubella, dengue, parvovirus B19 infection, erythema multiforme, Stevens-Johnson syndrome, and streptococcal scarlet fever. The distinctive measles prodrome, Koplik spots, the progression of the rash from the head and neck to the trunk and the extremities, and the severity of disease are distinctive features of measles.
Laboratory tests to confirm the diagnosis are often used in areas where measles is rare, and laboratory confirmation is currently recommended in the United States. Because viral isolation is technically difficult and is not widely available, serologic testing is the method most commonly used. The measles-specific immunoglobulin M (IgM) antibody assay, the test used most often, is almost 100% sensitive when done 2 to 3 days after the onset of the rash.27,28 Measles IgM antibody peaks at 4 weeks after the infection and disappears by 6 to 8 weeks.
It is important to remember that false-positive measles IgM antibody may occur with other viral infections, such as parvovirus B19 and rubella. Because measles-specific IgG antibody is produced with the onset of infection and peaks at 4 weeks, a fourfold rise in the IgG titer is useful in confirming the diagnosis. Measles IgG antibody after infection is sustained for life.
Reverse transcription-polymerase chain reaction testing can also detect measles virus in the blood and urine when direct evidence of the virus is necessary, such as in immunocompromised patients.29
TREATMENT IS SUPPORTIVE
Treatment of measles mainly involves supportive measures, such as fluids and antipyretics. Antiviral agents such as ribavirin and interferon have in vitro activity against the measles virus and have been used to treat severe measles infection in immunocompromised patients. However, their clinical efficacy is unproven.30
Routine use of antibacterial agents to prevent secondary bacterial infection is not recommended.
CURRENT RECOMMENDATIONS FOR ACTIVE IMMUNIZATION
Active immunization for measles has been available since 1963. Between 1963 and 1967, both killed-virus and live-virus vaccines were available. As atypical measles cases became recognized, the killed-virus vaccine was withdrawn.
The vaccine currently available in the United States is a live-attenuated strain prepared in chicken embryo cell culture and combined with mumps and rubella vaccine (MMR) or mumps, rubella, and varicella vaccine (MMRV).
Two doses of live-virus measles vaccine are recommended for all healthy children before they begin school, with the first dose given at 12 to 15 months of age. A second dose is needed because the failure rate with one dose is 5%. More than 99% of people who receive two doses separated by 4 weeks develop serologic evidence of measles.
Waning immunity after vaccination occurs very rarely, with approximately 5% of children developing secondary vaccine failure 10 to 15 years after vaccination.3,31
Although rates of vaccination in the United States are high, cases of measles continue to occur in unvaccinated infants and in children who are either too young to be vaccinated or whose parents claimed exemption because of religious or personal beliefs.
Because of the occurrence of measles cases in adolescents, young adults, and adults, potentially susceptible people should be identified and vaccinated according to current guidelines. People should be considered susceptible unless they have documentation of at least two doses of measles vaccine given at least 28 days apart, physician-diagnosed measles, laboratory evidence of immunity to measles, or were born before 1957. All adults who are susceptible should receive at least one dose of measles vaccine.10 Adults at higher risk of contracting measles include:
- Students in high school and college
- International travelers
- Health care personnel.
For these adults, two doses of measles vaccine, at least 28 days apart, are recommended.32
Postexposure prophylaxis
Measles vaccination given to susceptible contacts within 72 hours of exposure as postexposure prophylaxis may protect against infection and induces protection against subsequent exposures to measles.33,34 Vaccination is the intervention of choice for susceptible individuals older than 12 months of age who are exposed to measles and who do not have a contraindication to measles vaccination.35 Active rather than passive immunization is also the strategy of choice for controlling measles outbreaks.
Passive immunization with intramuscular immune globulin within 6 days of exposure can be used in selected circumstances to prevent transmission or to modify the clinical course of the infection.36 Immune globulin therapy is recommended for susceptible individuals who are exposed to measles and who are at high risk of developing severe or fatal measles. This includes individuals who are being treated with immunosuppressive agents, those with HIV infection, pregnant women, and infants less than 1 year of age. Immune globulin should not be used to control measles outbreaks.
ADVERSE EFFECTS OF MEASLES VACCINE
Live-virus measles vaccine has an excellent safety record. A transient fever, which may be accompanied by a measles-like rash, occurs in 5% to 15% of people 5 to 12 days after vaccination. The rash may be discrete or confluent and is self-limited.
Although measles vaccine is a live-attenuated vaccine, vaccinated people do not transmit the virus to susceptible contacts and are not considered contagious, even if they develop a vaccine-associated rash. Thus, the vaccine can be safely given to close contacts of immunocompromised and other susceptible people. Encephalitis is exceedingly rare following vaccination.
There is no scientific evidence that the risk of autism is higher in children who receive measles or MMR vaccine than in unvaccinated children.37 An Institute of Medicine report in 2001 rejected a causal relationship between MMR vaccine and autism spectrum disorders.38
CONTRAINDICATIONS TO MEASLES VACCINATION
Measles vaccine is contraindicated for:
- People who have cell-mediated immune deficiencies (except patients wtih HIV infection—see discussion just below)
- Pregnant women
- Those who had a severe allergic reaction to a vaccine component after a previous dose
- Those with moderate or severe acute illness
- Those who have recently received immune globulin products.
HIV-infected patients with severe immunosuppression should not receive the liveattenuated measles vaccine. However, because patients with HIV are at risk of severe measles, and because the vaccine has been shown to be safe in HIV patients who do not have severe immunosuppression, the vaccine is recommended for those with asymptomatic or mildly symptomatic HIV infection who do not have evidence of severe immunosuppression. 39
After receiving immune globulin
Anyone who has recently received immune globulin should not receive measles vaccine until sufficient time has passed, since passively acquired antibodies interfere with the immune response to live-virus vaccines. How long to wait depends on the type of immune globulin, the indication, the amount, and the route of administration. In general, the waiting period is:
- At least 3 months after intramuscular immune globulin or tetanus, hepatitis A, or hepatitis B prophylaxis
- At least 4 months after intramuscular immune globulin for rabies, or 6 months after intravenous immune globulin for cytomegalovirus (dose, 150 mg/kg)
- At least 8 months after intravenous immune globulin as replacement or therapy for immune deficiencies (dose, 400 mg/kg), or after intravenous immune globulin for immune thrombocytopenic purpura (400 mg/kg)
- At least 10 months after intravenous immune globulin for immune thrombocytopenic pupura at a dose of 1 g/kg.39
Egg allergy is not a contraindication
Although measles vaccine is produced in chick embryo cell culture, the vaccine has been shown to be safe in people with egg allergy, so they may be vaccinated without first being tested for egg allergy.39,40
Although measles is generally considered a disease of children, it affects people of all ages. While the incidence of measles in the United States is significantly lower than in 1963, when an effective measles vaccine was first introduced, recent increases in the number of sporadic cases and community outbreaks in adults show that measles is still a significant health problem.
PATHOGENESIS OF MEASLES
Measles is a highly contagious viral infection, whose manifestations have been recognized since the 7th century. The measles virus is an RNA virus of the Paramyxoviridae family. It is very difficult to isolate from clinical specimens, requiring special cell lines for in vitro propagation.
After acquisition, the measles virus establishes localized infection of the respiratory epithelium and then spreads to the regional lymphatics. A primary viremia then occurs, in which the virus replicates at the site of inoculation and in the reticuloendothelial tissues. A secondary viremia follows, in which the virus infects and replicates in the skin, conjunctiva, respiratory tract, and other distant organs.
The measles rash is thought to be due to a hypersensitivity reaction.1 Cell-mediated responses are the main line of defense against measles, as evidenced by the fact that people with cell-mediated deficiencies develop severe measles infection.2 Immunity to wild-type measles is believed to be lifelong.3,4
MEASLES IS HIGHLY CONTAGIOUS
Measles is one of the most contagious infectious diseases, with a secondary attack rate of at least 90% in susceptible household contacts. 4 The fact that emergency departments and physicians’ offices have become sites of measles transmission in recent years underscores the transmissibility of the virus.5–7
Although the virus is very labile, it can remain infective in respiratory droplets from the air for many hours. Thus, measles virus spreads from person to person by direct contact with droplets from respiratory secretions of infected persons.
The period of maximal contagion is the late prodrome, ie, 2 to 4 days before the onset of the rash. People who are generally in good health are contagious through 4 days after the onset of the rash, whereas people with compromised immunity can continue to shed the virus for the entire duration of the illness.
Airborne transmission precautions are required for 4 days after the onset of the rash in hospitalized, non-immunocompromised patients with measles, and for the duration of the illness for immunocompromised patients.
In the absence of widespread measles vaccination, measles infection peaks in late winter and early spring.
EPIDEMIOLOGIC TRENDS: CAUSE FOR CONCERN
Since an effective vaccine became available in 1963, the annual incidence of measles cases in the United States has decreased by more than 99%. A significant resurgence from 1989 to 1991 affected mainly unvaccinated preschoolers and resulted in more than 55,000 cases and 130 deaths.8 This resurgence prompted widespread, intensive immunization efforts and the recommendation that a second dose of measles vaccine be given to school-aged children. This led to the effective elimination of endemic transmission of measles in the United States.9
Although 90% of these cases either were directly imported or were associated with importation from other countries,10 the reason for the large number of cases was clearly the greater transmission after importation of the virus into the United States. This transmission was the direct result of the fact that 91% of the cases occurred in unvaccinated people or people whose vaccination status was not known or was not documented. A high proportion— at least 61 (47%)—of the 131 measles cases in 2008 were in school-aged children whose parents chose not to have them vaccinated. Although no deaths were reported in these 131 patients, 15 required hospitalization.
Although most reported measles cases are still in young and school-aged children, recent cases and outbreaks have also occurred in isolated communities of adults. Approximately 25% of the cases reported in 2008 were in people age 20 and older. Most adults who contracted measles had unknown or undocumented vaccination status. Similarly, a small measles outbreak occurred in Indiana in 2005, when an adolescent US citizen traveling in Europe became infected in Romania and exposed 500 people at a church gathering upon her return. Thirty-four cases of measles were reported from this exposure, and many were in adults.11
The recent increase in the number of cases reported and the continued reports of outbreaks highlight the fact that measles outbreaks can occur in communities with a high number of unvaccinated people, and underscore the need for high overall measles vaccination coverage to limit the spread of this infection.12
CLINICAL FEATURES OF MEASLES
The first sign of measles is a distinct prodrome, which occurs after an incubation period of 10 to 12 days. The prodrome is characterized by fever, malaise, anorexia, conjunctivitis, coryza, and cough and may resemble an upper respiratory tract infection; it lasts 2 to 4 days.
Towards the end of the prodrome, the body temperature can rise to as high as 40°C, and Koplik spots, pathognomonic for measles, appear. Koplik spots, bluish-gray specks on an erythematous base, usually appear on the buccal mucosa opposite the second molars 1 to 2 days before the onset of the rash, and last for 1 to 2 days after the onset of the rash. Thus, it is not unusual for Koplik spots to have disappeared at the time the diagnosis of measles is entertained.
The classic measles rash is an erythematous maculopapular eruption that begins on the head and face and spreads to involve the entire body. It usually persists for 4 to 5 days and is most confluent on the face and upper body. The rash fades in order of appearance, and may desquamate. People with measles appear ill, especially 1 to 2 days after the rash appears.
The entire course of measles usually lasts 7 to 10 days in patients with a healthy immune system. The cough, a manifestation of tracheobronchitis, is usually the last symptom to resolve. Patients are contagious 2 to 4 days before the onset of the rash, and remain so through 4 days after the onset of the rash.
COMPLICATIONS
Respiratory complications
Pneumonia is responsible for 60% of deaths associated with measles.13 Although radiographic evidence of pneumonia is found in measles patients with no complications, symptomatic pneumonia occurs in 1% to 6% of patients. It is the result of either direct invasion by the virus or secondary bacterial infection,17 most often with Staphylococcus aureus and Streptococcus pneumoniae. Other respiratory complications include otitis media, sinusitis, and laryngotracheobronchitis.
Neurologic complications
Acute measles encephalitis is more common in adults than in children. Occuring in 1 in 1,000 to 2,000 patients,18 it is characterized by the resurgence of fever during the convalescent phase of the illness, along with headaches, seizures, and altered consciousness. These manifestations may be mild or severe, but they lead to permanent neurologic sequelae in a substantial proportion of affected patients. It is not clear whether acute measles encephalitis represents direct invasion of the virus or a postinfectious process from a hypersensitivity to the virus.19
Subacute sclerosing panencephalitis is a rare, chronic, degenerative central nervous system disease that occurs secondary to persistent infection with a defective measles virus.20 The prevalence is estimated at 1 per 100,000 cases. Signs and symptoms appear an average of 7 years after the initial infection and include personality changes, myoclonic seizures, and motor disturbances. Often, coma and death follow.
This condition occurs particularly in those who had measles at a very young age, ie, before the age of 2 years, and it occurs despite a vigorous host-immune response to the virus. Patients have high titers of measles-specific antibody in the sera and cerebrospinal fluid.
Other complications
Diarrhea and stomatitis account for much of the sickness and death from measles in developing countries.
Subclinical hepatitis occurs in at least 30% of adult measles patients.
Less common complications include thrombocytopenia, appendicitis, ileocolitis, pericarditis, myocarditis, and hypocalcemia.
MEASLES DURING PREGNANCY
Measles during pregnancy may be severe, mainly due to primary measles pneumonia.21 Measles is associated with a risk of miscarriage and prematurity, but congenital anomalies of the fetus have not been described, as they have for rubella infection.22
MEASLES IN COMPROMISED IMMUNITY
Measles patients with deficiencies of cellmediated immunity have a prolonged, severe, and often fatal course.2,23,24 This includes patients with:
- Human immunodeficiency virus (HIV) infection
- Congenital immunodeficiencies
- Disorders requiring chemotherapeutic and immunosuppressive therapy.
These patients are particularly susceptible to acute progressive encephalitis and measles pneumonitis. Case-fatality rates of 70% in cancer patients and 40% in HIV-infected patients have been reported.24
The diagnosis of measles may be difficult in patients without cell-mediated immunity, as 25% to 40% of them do not develop the characteristic rash.2,23 The absence of rash supports the theory that the rash is a hypersensitivity reaction to the virus.
MODIFIED AND ATYPICAL MEASLES
Modified measles
A modified form of measles can occur in people with some degree of passive immunity to the virus, including those previously vaccinated. It occurs mostly in patients who recently received immunoglobulin products, or in young infants who have residual maternal antibody. A modified measles illness can also follow vaccination with live-virus vaccine (see later discussion).
The clinical manifestations vary, and the illness may not have the classic features of prodrome, rash, and Koplik spots.
Atypical measles
Atypical measles is an unusual form that can occur when a person previously vaccinated with a killed-virus measles vaccine (used from 1963 to 1967) is exposed to wild-type measles.25 Features include a shorter prodrome (1 to 2 days), followed by appearance of a rash that begins on the distal extremities and spreads centripetally, usually sparing the neck, face, and head. The rash may be petechial, maculopapular, urticarial, vesicular, or a combination. The rash is accompanied by high fever and edema of the extremities. Complications such as pneumonia and hepatitis may occur.
The course of atypical measles is more prolonged than with classic measles, but because these patients are thought to have partial protection against the virus, they do not transmit it and are not considered contagious.26
DIAGNOSIS OF MEASLES
The classic clinical features are usually enough to distinguish measles from other febrile illnesses with similar clinical manifestions, such as rubella, dengue, parvovirus B19 infection, erythema multiforme, Stevens-Johnson syndrome, and streptococcal scarlet fever. The distinctive measles prodrome, Koplik spots, the progression of the rash from the head and neck to the trunk and the extremities, and the severity of disease are distinctive features of measles.
Laboratory tests to confirm the diagnosis are often used in areas where measles is rare, and laboratory confirmation is currently recommended in the United States. Because viral isolation is technically difficult and is not widely available, serologic testing is the method most commonly used. The measles-specific immunoglobulin M (IgM) antibody assay, the test used most often, is almost 100% sensitive when done 2 to 3 days after the onset of the rash.27,28 Measles IgM antibody peaks at 4 weeks after the infection and disappears by 6 to 8 weeks.
It is important to remember that false-positive measles IgM antibody may occur with other viral infections, such as parvovirus B19 and rubella. Because measles-specific IgG antibody is produced with the onset of infection and peaks at 4 weeks, a fourfold rise in the IgG titer is useful in confirming the diagnosis. Measles IgG antibody after infection is sustained for life.
Reverse transcription-polymerase chain reaction testing can also detect measles virus in the blood and urine when direct evidence of the virus is necessary, such as in immunocompromised patients.29
TREATMENT IS SUPPORTIVE
Treatment of measles mainly involves supportive measures, such as fluids and antipyretics. Antiviral agents such as ribavirin and interferon have in vitro activity against the measles virus and have been used to treat severe measles infection in immunocompromised patients. However, their clinical efficacy is unproven.30
Routine use of antibacterial agents to prevent secondary bacterial infection is not recommended.
CURRENT RECOMMENDATIONS FOR ACTIVE IMMUNIZATION
Active immunization for measles has been available since 1963. Between 1963 and 1967, both killed-virus and live-virus vaccines were available. As atypical measles cases became recognized, the killed-virus vaccine was withdrawn.
The vaccine currently available in the United States is a live-attenuated strain prepared in chicken embryo cell culture and combined with mumps and rubella vaccine (MMR) or mumps, rubella, and varicella vaccine (MMRV).
Two doses of live-virus measles vaccine are recommended for all healthy children before they begin school, with the first dose given at 12 to 15 months of age. A second dose is needed because the failure rate with one dose is 5%. More than 99% of people who receive two doses separated by 4 weeks develop serologic evidence of measles.
Waning immunity after vaccination occurs very rarely, with approximately 5% of children developing secondary vaccine failure 10 to 15 years after vaccination.3,31
Although rates of vaccination in the United States are high, cases of measles continue to occur in unvaccinated infants and in children who are either too young to be vaccinated or whose parents claimed exemption because of religious or personal beliefs.
Because of the occurrence of measles cases in adolescents, young adults, and adults, potentially susceptible people should be identified and vaccinated according to current guidelines. People should be considered susceptible unless they have documentation of at least two doses of measles vaccine given at least 28 days apart, physician-diagnosed measles, laboratory evidence of immunity to measles, or were born before 1957. All adults who are susceptible should receive at least one dose of measles vaccine.10 Adults at higher risk of contracting measles include:
- Students in high school and college
- International travelers
- Health care personnel.
For these adults, two doses of measles vaccine, at least 28 days apart, are recommended.32
Postexposure prophylaxis
Measles vaccination given to susceptible contacts within 72 hours of exposure as postexposure prophylaxis may protect against infection and induces protection against subsequent exposures to measles.33,34 Vaccination is the intervention of choice for susceptible individuals older than 12 months of age who are exposed to measles and who do not have a contraindication to measles vaccination.35 Active rather than passive immunization is also the strategy of choice for controlling measles outbreaks.
Passive immunization with intramuscular immune globulin within 6 days of exposure can be used in selected circumstances to prevent transmission or to modify the clinical course of the infection.36 Immune globulin therapy is recommended for susceptible individuals who are exposed to measles and who are at high risk of developing severe or fatal measles. This includes individuals who are being treated with immunosuppressive agents, those with HIV infection, pregnant women, and infants less than 1 year of age. Immune globulin should not be used to control measles outbreaks.
ADVERSE EFFECTS OF MEASLES VACCINE
Live-virus measles vaccine has an excellent safety record. A transient fever, which may be accompanied by a measles-like rash, occurs in 5% to 15% of people 5 to 12 days after vaccination. The rash may be discrete or confluent and is self-limited.
Although measles vaccine is a live-attenuated vaccine, vaccinated people do not transmit the virus to susceptible contacts and are not considered contagious, even if they develop a vaccine-associated rash. Thus, the vaccine can be safely given to close contacts of immunocompromised and other susceptible people. Encephalitis is exceedingly rare following vaccination.
There is no scientific evidence that the risk of autism is higher in children who receive measles or MMR vaccine than in unvaccinated children.37 An Institute of Medicine report in 2001 rejected a causal relationship between MMR vaccine and autism spectrum disorders.38
CONTRAINDICATIONS TO MEASLES VACCINATION
Measles vaccine is contraindicated for:
- People who have cell-mediated immune deficiencies (except patients wtih HIV infection—see discussion just below)
- Pregnant women
- Those who had a severe allergic reaction to a vaccine component after a previous dose
- Those with moderate or severe acute illness
- Those who have recently received immune globulin products.
HIV-infected patients with severe immunosuppression should not receive the liveattenuated measles vaccine. However, because patients with HIV are at risk of severe measles, and because the vaccine has been shown to be safe in HIV patients who do not have severe immunosuppression, the vaccine is recommended for those with asymptomatic or mildly symptomatic HIV infection who do not have evidence of severe immunosuppression. 39
After receiving immune globulin
Anyone who has recently received immune globulin should not receive measles vaccine until sufficient time has passed, since passively acquired antibodies interfere with the immune response to live-virus vaccines. How long to wait depends on the type of immune globulin, the indication, the amount, and the route of administration. In general, the waiting period is:
- At least 3 months after intramuscular immune globulin or tetanus, hepatitis A, or hepatitis B prophylaxis
- At least 4 months after intramuscular immune globulin for rabies, or 6 months after intravenous immune globulin for cytomegalovirus (dose, 150 mg/kg)
- At least 8 months after intravenous immune globulin as replacement or therapy for immune deficiencies (dose, 400 mg/kg), or after intravenous immune globulin for immune thrombocytopenic purpura (400 mg/kg)
- At least 10 months after intravenous immune globulin for immune thrombocytopenic pupura at a dose of 1 g/kg.39
Egg allergy is not a contraindication
Although measles vaccine is produced in chick embryo cell culture, the vaccine has been shown to be safe in people with egg allergy, so they may be vaccinated without first being tested for egg allergy.39,40
- Lachmann PJ. Immunopathology of measles. Proc R Soc Med 1974; 67:1120–1122.
- Enders JF, McCarthy K, Mitus A, Cheatham WJ. Isolation of measles virus at autopsy in case of giant cell pneumonia without rash. N Engl J Med 1959; 261:875–881.
- Markowitz LE, Preblud SR, Fine PE, Orenstein WA. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J 1990; 9:101–110.
- Stokes J, Reilly CM, Buynak EB, Hilleman MR. Immunologic studies of measles. Am J Hyg 1961; 74:293–303.
- Farizo KM, Stehr-Green PA, Simpson DM, Markowitz LE. Pediatric emergency room visits: a risk factor for acquiring measles. Pediatrics 1991; 87:74–79.
- Bloch AB, Orenstein W, Ewing WM, et al. Measles outbreak in a pediatric practice: airborne transmission in an office setting. Pediatrics 1985; 75:676–683.
- Remington PL, Hall WN, Davis IH, et al. Airborne transmission of measles in a physician’s office. JAMA 1985; 253:1574–1577.
- US Centers for Disease Control and Prevention. Reported vaccine-preventable diseases—United States, 1993, and the Childhood Immunization Initiative. MMWR 1994; 43:57–60.
- Orenstein WA, Papania MJ, Wharton ME. Measles elimination in the United States. J Infect Dis 2004; 189 (suppl 1):S1–S3.
- US Centers for Disease Control and Prevention. Update: Measles—United States, January–July 2008. MMWR 2008; 57:893–896.
- Parker AA, Staggs W, Dayan GH, et al. Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med 2006; 355:447–455.
- Mulholland EK. Measles in the United States, 2006. N Engl J Med 2006; 355:440–443.
- Barkin RM. Measles mortality. Analysis of the primary cause of death. Am J Dis Child 1975; 129:307–309.
- Barkin RM. Measles mortality: a retrospective look at the vaccine era. Am J Epidemiol 1975; 102:341–349.
- US Centers for Disease Control and Prevention. Public-sector vaccination efforts in response to the resurgence of measles among preschool-aged children—United States, 1989–1991. MMWR 1992; 41:522–525.
- Gremillion DH, Crawford GE. Measles pneumonia in young adults. an analysis of 106 cases. Am J Med 1981; 71:539–542.
- Quiambao BP, Gatchalian SR, Halonen P, et al. Coinfection is common in measles-associated pneumonia. Pediatr Infect Dis J 1998; 17:89–93.
- Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189( suppl 1):S4–S16.
- Johnson RT, Griffin DE, Hirsch RL, et al. Measles encephalomyelitis—clinical and immunologic studies. N Engl J Med 1984; 310:137–141.
- Sever JL. Persistent measles infection of the central nervous system: subacute sclerosing panencephalitis. Rev Infect Dis 1983; 5:467–473.
- Atmar RL, Englund JA, Hammill H. Complications of measles during pregnancy. Clin Infect Dis 1992; 14:217–226.
- Gershon AA. Chickenpox, measles, and mumps. In:Remington J, Klein J, eds. Infectious Diseases of the Fetus and Newborn Infant. Elsevier Saunders: Philadelphia, 2006:693–738.
- Mitus A, Enders JF, Craig JM, Holloway A. Persistence of measles virus and depression of antibody formation in patients with giant-cell pneumonia after measles. N Engl J Med 1959; 261:882–889.
- Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients JAMA 1992; 267:1237–1241.
- Frey HM, Krugman S. Atypical measles syndrome: unusual hepatic, pulmonary, and immunologic aspects. Am J Med Sci 1981; 281:51–55.
- Fulginiti VA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA 1967; 202:1075–1080.
- Mayo DR, Brennan T, Cormier DP, Hadler J, Lamb P. Evaluation of a commercial measles virus immunoglobulin M enzyme immunoassay. J Clin Microbiol 1991; 29:2865–2867.
- Bellini WJ, Helfand RF. The challenges and strategies for laboratory diagnosis of measles in an international setting. J Infect Dis 2003; 187( suppl 1):S283–S290.
- Riddell MA, Chibo D, Kelly HA, Catton MG, Birch CJ. Investigation of optimal specimen type and sampling time for detection of measles virus RNA during a measles epidemic. J Clin Microbiol 2001; 39:375–376.
- Forni Al, Schluger NW, Roberts RB. Severe measles pneumonitis in adults: evaluation of clinical characteristics and therapy with intravenous ribavirin. Clin Infect Dis 1994; 19:454–462.
- Anders JF, Jacobsen RM, Poland GA, Jacobsen SJ, Wollan PC. Secondary failure rates of measles vaccines: a metaanalysis of published studes. Pediar Infect Dis J 1996; 15:62–66.
- US Centers for Disease Control and Prevention. Measles—United States, January 1–April 25, 2008. MMWR 2008; 57:494–498.
- Berkovitz S, Starr S. Use of live-measles-virus vaccine to abort an expected outbreak of measles within a closed population. N Engl J Med 1963; 269:75–77.
- Ruuskanen O, Salmi TT, Halonen P. Measles vaccination after exposure to natural measles. J Pediatr 1978; 93:43–46.
- Strebel PM, Papania MJ, Halsey NA. Measles vaccine. In:Plotkin SA, Orenstein WA, eds. Vaccines. Saunders: New York, 2004:389–440.
- US Centers for Disease Control and Prevention. Measles, mumps and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1998; 47( RR-8):1–57. http://www.cdc.gov/mmwr/PDF/rr/rr4708.pdf. Accessed December 30, 2009.
- Madsen KM, Hviid A, Vestergaard M, et al. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med 2002; 347:1477–1482.
- Straton K, Gable A, Shetty P, McCormick M; for the Institute of Medicine. Immunization Safety Review: Measles-Mumps-Rubella Vaccine and Autism. National Academy Press: Washington, DC, 2001.
- Pickerington LK, Baker CJ, Long SS, McMillan JA, editors. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2009:444–455.
- James JM, Burks AW, Roberson PK, Sampson HA. Safe administration of the measles vaccine to children allergic to eggs. N Engl J Med 1995; 332:1262–1266.
- Lachmann PJ. Immunopathology of measles. Proc R Soc Med 1974; 67:1120–1122.
- Enders JF, McCarthy K, Mitus A, Cheatham WJ. Isolation of measles virus at autopsy in case of giant cell pneumonia without rash. N Engl J Med 1959; 261:875–881.
- Markowitz LE, Preblud SR, Fine PE, Orenstein WA. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J 1990; 9:101–110.
- Stokes J, Reilly CM, Buynak EB, Hilleman MR. Immunologic studies of measles. Am J Hyg 1961; 74:293–303.
- Farizo KM, Stehr-Green PA, Simpson DM, Markowitz LE. Pediatric emergency room visits: a risk factor for acquiring measles. Pediatrics 1991; 87:74–79.
- Bloch AB, Orenstein W, Ewing WM, et al. Measles outbreak in a pediatric practice: airborne transmission in an office setting. Pediatrics 1985; 75:676–683.
- Remington PL, Hall WN, Davis IH, et al. Airborne transmission of measles in a physician’s office. JAMA 1985; 253:1574–1577.
- US Centers for Disease Control and Prevention. Reported vaccine-preventable diseases—United States, 1993, and the Childhood Immunization Initiative. MMWR 1994; 43:57–60.
- Orenstein WA, Papania MJ, Wharton ME. Measles elimination in the United States. J Infect Dis 2004; 189 (suppl 1):S1–S3.
- US Centers for Disease Control and Prevention. Update: Measles—United States, January–July 2008. MMWR 2008; 57:893–896.
- Parker AA, Staggs W, Dayan GH, et al. Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med 2006; 355:447–455.
- Mulholland EK. Measles in the United States, 2006. N Engl J Med 2006; 355:440–443.
- Barkin RM. Measles mortality. Analysis of the primary cause of death. Am J Dis Child 1975; 129:307–309.
- Barkin RM. Measles mortality: a retrospective look at the vaccine era. Am J Epidemiol 1975; 102:341–349.
- US Centers for Disease Control and Prevention. Public-sector vaccination efforts in response to the resurgence of measles among preschool-aged children—United States, 1989–1991. MMWR 1992; 41:522–525.
- Gremillion DH, Crawford GE. Measles pneumonia in young adults. an analysis of 106 cases. Am J Med 1981; 71:539–542.
- Quiambao BP, Gatchalian SR, Halonen P, et al. Coinfection is common in measles-associated pneumonia. Pediatr Infect Dis J 1998; 17:89–93.
- Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189( suppl 1):S4–S16.
- Johnson RT, Griffin DE, Hirsch RL, et al. Measles encephalomyelitis—clinical and immunologic studies. N Engl J Med 1984; 310:137–141.
- Sever JL. Persistent measles infection of the central nervous system: subacute sclerosing panencephalitis. Rev Infect Dis 1983; 5:467–473.
- Atmar RL, Englund JA, Hammill H. Complications of measles during pregnancy. Clin Infect Dis 1992; 14:217–226.
- Gershon AA. Chickenpox, measles, and mumps. In:Remington J, Klein J, eds. Infectious Diseases of the Fetus and Newborn Infant. Elsevier Saunders: Philadelphia, 2006:693–738.
- Mitus A, Enders JF, Craig JM, Holloway A. Persistence of measles virus and depression of antibody formation in patients with giant-cell pneumonia after measles. N Engl J Med 1959; 261:882–889.
- Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients JAMA 1992; 267:1237–1241.
- Frey HM, Krugman S. Atypical measles syndrome: unusual hepatic, pulmonary, and immunologic aspects. Am J Med Sci 1981; 281:51–55.
- Fulginiti VA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA 1967; 202:1075–1080.
- Mayo DR, Brennan T, Cormier DP, Hadler J, Lamb P. Evaluation of a commercial measles virus immunoglobulin M enzyme immunoassay. J Clin Microbiol 1991; 29:2865–2867.
- Bellini WJ, Helfand RF. The challenges and strategies for laboratory diagnosis of measles in an international setting. J Infect Dis 2003; 187( suppl 1):S283–S290.
- Riddell MA, Chibo D, Kelly HA, Catton MG, Birch CJ. Investigation of optimal specimen type and sampling time for detection of measles virus RNA during a measles epidemic. J Clin Microbiol 2001; 39:375–376.
- Forni Al, Schluger NW, Roberts RB. Severe measles pneumonitis in adults: evaluation of clinical characteristics and therapy with intravenous ribavirin. Clin Infect Dis 1994; 19:454–462.
- Anders JF, Jacobsen RM, Poland GA, Jacobsen SJ, Wollan PC. Secondary failure rates of measles vaccines: a metaanalysis of published studes. Pediar Infect Dis J 1996; 15:62–66.
- US Centers for Disease Control and Prevention. Measles—United States, January 1–April 25, 2008. MMWR 2008; 57:494–498.
- Berkovitz S, Starr S. Use of live-measles-virus vaccine to abort an expected outbreak of measles within a closed population. N Engl J Med 1963; 269:75–77.
- Ruuskanen O, Salmi TT, Halonen P. Measles vaccination after exposure to natural measles. J Pediatr 1978; 93:43–46.
- Strebel PM, Papania MJ, Halsey NA. Measles vaccine. In:Plotkin SA, Orenstein WA, eds. Vaccines. Saunders: New York, 2004:389–440.
- US Centers for Disease Control and Prevention. Measles, mumps and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1998; 47( RR-8):1–57. http://www.cdc.gov/mmwr/PDF/rr/rr4708.pdf. Accessed December 30, 2009.
- Madsen KM, Hviid A, Vestergaard M, et al. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med 2002; 347:1477–1482.
- Straton K, Gable A, Shetty P, McCormick M; for the Institute of Medicine. Immunization Safety Review: Measles-Mumps-Rubella Vaccine and Autism. National Academy Press: Washington, DC, 2001.
- Pickerington LK, Baker CJ, Long SS, McMillan JA, editors. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2009:444–455.
- James JM, Burks AW, Roberson PK, Sampson HA. Safe administration of the measles vaccine to children allergic to eggs. N Engl J Med 1995; 332:1262–1266.
KEY POINTS
- Measles is one of the most contagious infectious diseases, with a secondary attack rate of at least 90% in susceptible household contacts.
- Since 1993, most reported cases of measles have been directly or indirectly linked to international travel, and many have occurred in adults.
- Acute measles encephalitis, a neurologic complication of measles, is more common in adults than in children and is characterized by the resurgence of fever during the convalescent phase, along with headaches, seizures, and altered consciousness.