User login
Erythematous Papules and Pustules on the Nose
The Diagnosis: Granulosis Rubra Nasi
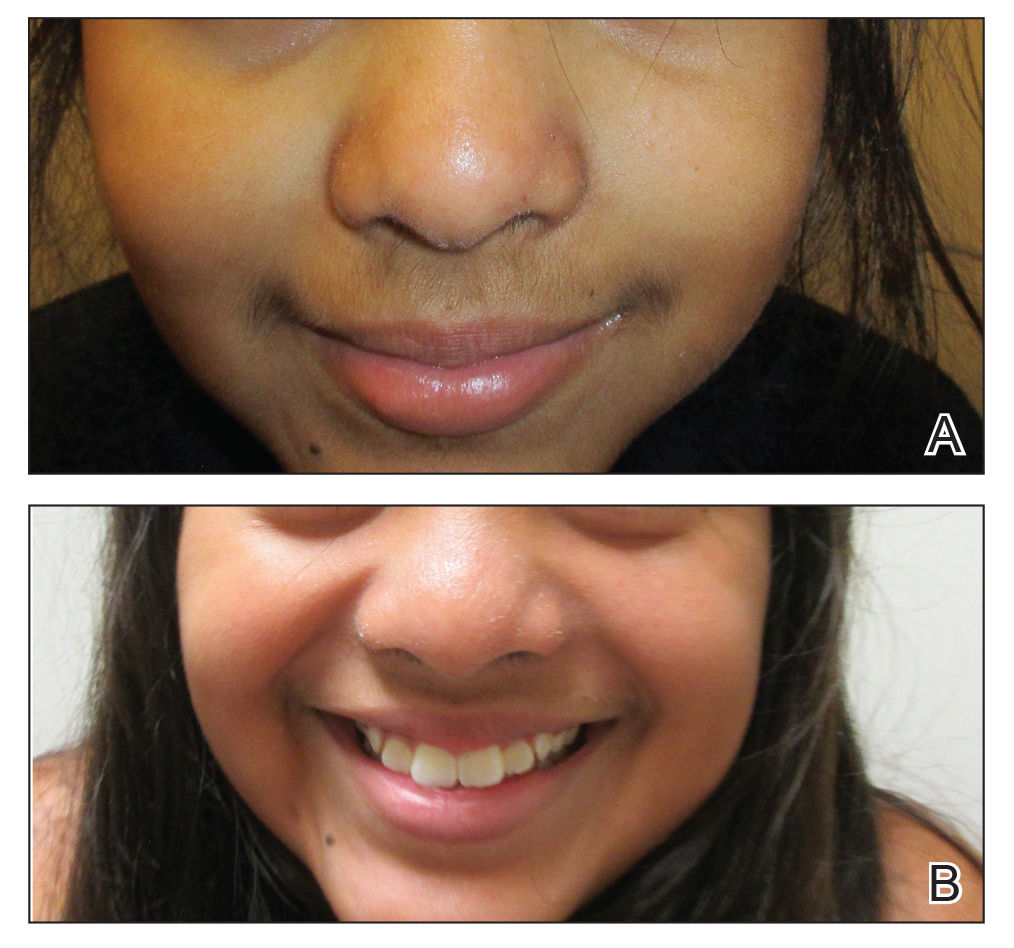
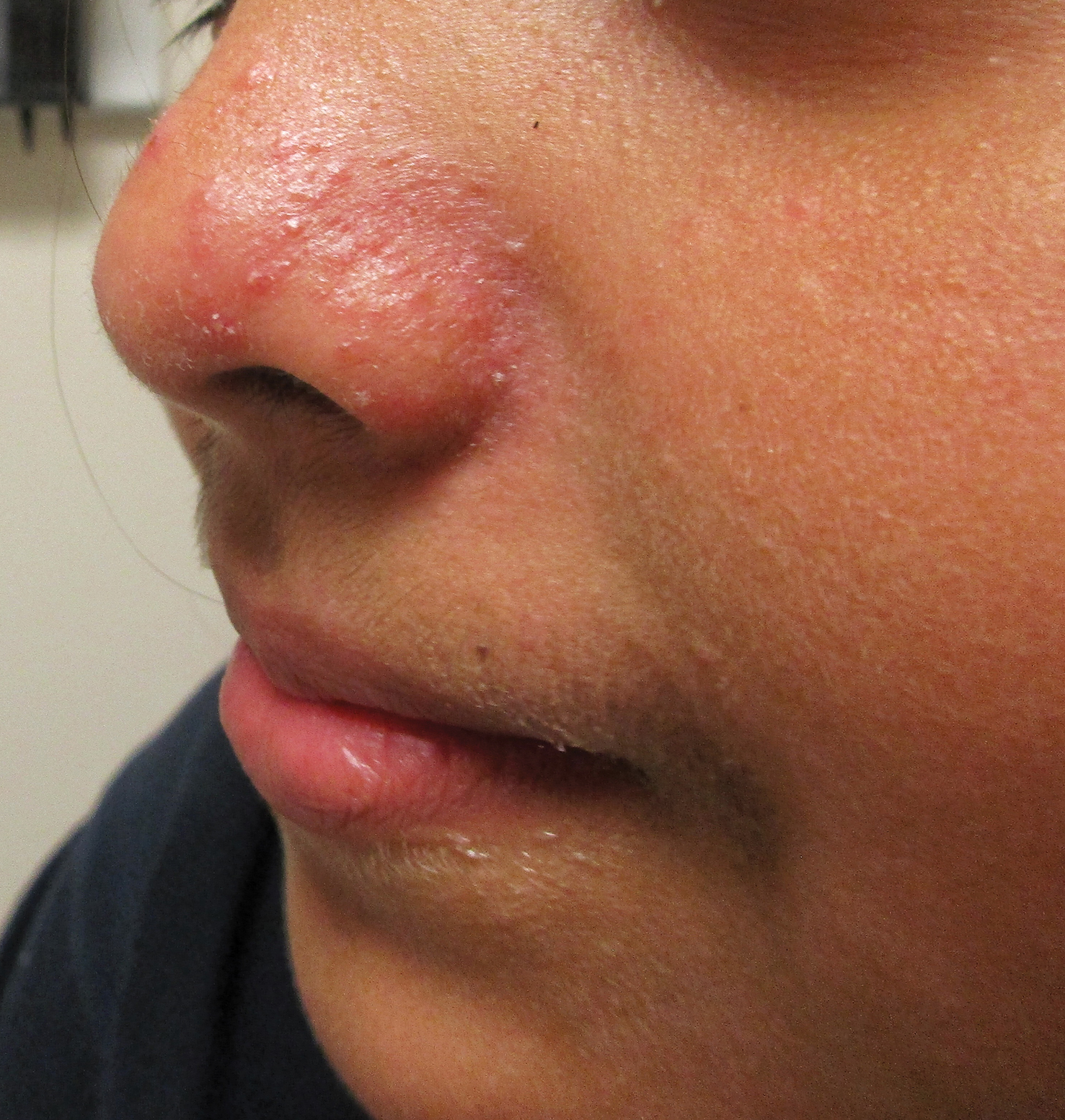
A history of prominent nasal sweating was later elicited and the patient was subsequently diagnosed with granulosis rubra nasi. She was instructed to continue daily use of topical pimecrolimus with the addition of topical atropine, resulting in complete resolution of the eruption at 6-week follow-up (Figure, A). She was then maintained on topical atropine monotherapy, only noting recurrence with cessation of the atropine (Figure, B).
Other successful treatment regimens of granulosis rubra nasi include injection of botulinum toxin into the nose,1 monotherapy with topical tacrolimus,2 topical indomethacin, steroids, and cryotherapy, among other modalities.1 Topical atropine and pimecrolimus were selected as first-line agents for treating our pediatric patient due to tolerability and their anti-inflammatory and anticholinergic properties.
Granulosis rubra nasi is a form of focal hyperhidrosis that presents as erythematous papules, pustules, and vesicles of the midface, especially the nose.3 It is a fairly rare condition that can mimic many other common clinical entities, including comedonal acne, nevus comedonicus, periorificial dermatitis, and tinea faciei, but is resistant to treatments aimed at these disorders. It was first described as a "peculiar disease of the skin of the nose in children" in a case report by Jadassohn4 in 1901. It is most common in children aged 7 to 12 years and typically resolves at puberty; adults rarely are affected. Although the etiology has not yet been elucidated, autosomal-dominant transmission has been described, and the cutaneous changes are hypothesized to be secondary to hyperhidrosis.5 This postulation is further corroborated by a case report of a pheochromocytoma-associated granulosis rubra nasi that resolved with surgical excision of the pheochromocytoma.6 It is not uncommon for patients to have concomitant palmoplantar hyperhidrosis and acrocyanosis.5 Histopathologic examination is not necessary for diagnosis, but when performed, it discloses a mononuclear cellular infiltrate surrounding eccrine sweat ducts, blood vessels, and lymphatics without other abnormalities of the epidermis or pilosebaceous unit.1-3,7
- Grazziotin TC, Buffon RB, Da Silva Manzoni AP, et al. Treatment of granulosis rubra nasi with botulinum toxin. Dermatol Surg. 2009;35:1298-1299.
- Kumar P, Gosai A, Mondal AK, et al. Granulosis rubra nasi: a rare condition treated successfully with topical tacrolimus. Dermatol Reports. 2012;4:E5.
- Sargunam C, Thomas J, Ahmed NA. Granulosis rubra nasi. Indian Dermatol Online J. 2013;4:208-209.
- Jadassohn J. Ueber eine eigenartige erkrankung der nasenhaut bei kindern. Arch Derm Syph. 1901;58:145-158.
- Hellier FF. Granulosis rubra nasi in a mother and daughter. Br Med J. 1937;2:1068.
- Heid E, Samain F, Jelen G, et al. Granulosis rubra nasi and pheochromocytoma. Ann Dermatol Venereol. 1996;123:106-108.
- Akhdari N. Granulosis rubra nasi. Int J Dermatol. 2007;46:396.
The Diagnosis: Granulosis Rubra Nasi
A history of prominent nasal sweating was later elicited and the patient was subsequently diagnosed with granulosis rubra nasi. She was instructed to continue daily use of topical pimecrolimus with the addition of topical atropine, resulting in complete resolution of the eruption at 6-week follow-up (Figure, A). She was then maintained on topical atropine monotherapy, only noting recurrence with cessation of the atropine (Figure, B).
Other successful treatment regimens of granulosis rubra nasi include injection of botulinum toxin into the nose,1 monotherapy with topical tacrolimus,2 topical indomethacin, steroids, and cryotherapy, among other modalities.1 Topical atropine and pimecrolimus were selected as first-line agents for treating our pediatric patient due to tolerability and their anti-inflammatory and anticholinergic properties.
Granulosis rubra nasi is a form of focal hyperhidrosis that presents as erythematous papules, pustules, and vesicles of the midface, especially the nose.3 It is a fairly rare condition that can mimic many other common clinical entities, including comedonal acne, nevus comedonicus, periorificial dermatitis, and tinea faciei, but is resistant to treatments aimed at these disorders. It was first described as a "peculiar disease of the skin of the nose in children" in a case report by Jadassohn4 in 1901. It is most common in children aged 7 to 12 years and typically resolves at puberty; adults rarely are affected. Although the etiology has not yet been elucidated, autosomal-dominant transmission has been described, and the cutaneous changes are hypothesized to be secondary to hyperhidrosis.5 This postulation is further corroborated by a case report of a pheochromocytoma-associated granulosis rubra nasi that resolved with surgical excision of the pheochromocytoma.6 It is not uncommon for patients to have concomitant palmoplantar hyperhidrosis and acrocyanosis.5 Histopathologic examination is not necessary for diagnosis, but when performed, it discloses a mononuclear cellular infiltrate surrounding eccrine sweat ducts, blood vessels, and lymphatics without other abnormalities of the epidermis or pilosebaceous unit.1-3,7
The Diagnosis: Granulosis Rubra Nasi
A history of prominent nasal sweating was later elicited and the patient was subsequently diagnosed with granulosis rubra nasi. She was instructed to continue daily use of topical pimecrolimus with the addition of topical atropine, resulting in complete resolution of the eruption at 6-week follow-up (Figure, A). She was then maintained on topical atropine monotherapy, only noting recurrence with cessation of the atropine (Figure, B).
Other successful treatment regimens of granulosis rubra nasi include injection of botulinum toxin into the nose,1 monotherapy with topical tacrolimus,2 topical indomethacin, steroids, and cryotherapy, among other modalities.1 Topical atropine and pimecrolimus were selected as first-line agents for treating our pediatric patient due to tolerability and their anti-inflammatory and anticholinergic properties.
Granulosis rubra nasi is a form of focal hyperhidrosis that presents as erythematous papules, pustules, and vesicles of the midface, especially the nose.3 It is a fairly rare condition that can mimic many other common clinical entities, including comedonal acne, nevus comedonicus, periorificial dermatitis, and tinea faciei, but is resistant to treatments aimed at these disorders. It was first described as a "peculiar disease of the skin of the nose in children" in a case report by Jadassohn4 in 1901. It is most common in children aged 7 to 12 years and typically resolves at puberty; adults rarely are affected. Although the etiology has not yet been elucidated, autosomal-dominant transmission has been described, and the cutaneous changes are hypothesized to be secondary to hyperhidrosis.5 This postulation is further corroborated by a case report of a pheochromocytoma-associated granulosis rubra nasi that resolved with surgical excision of the pheochromocytoma.6 It is not uncommon for patients to have concomitant palmoplantar hyperhidrosis and acrocyanosis.5 Histopathologic examination is not necessary for diagnosis, but when performed, it discloses a mononuclear cellular infiltrate surrounding eccrine sweat ducts, blood vessels, and lymphatics without other abnormalities of the epidermis or pilosebaceous unit.1-3,7
- Grazziotin TC, Buffon RB, Da Silva Manzoni AP, et al. Treatment of granulosis rubra nasi with botulinum toxin. Dermatol Surg. 2009;35:1298-1299.
- Kumar P, Gosai A, Mondal AK, et al. Granulosis rubra nasi: a rare condition treated successfully with topical tacrolimus. Dermatol Reports. 2012;4:E5.
- Sargunam C, Thomas J, Ahmed NA. Granulosis rubra nasi. Indian Dermatol Online J. 2013;4:208-209.
- Jadassohn J. Ueber eine eigenartige erkrankung der nasenhaut bei kindern. Arch Derm Syph. 1901;58:145-158.
- Hellier FF. Granulosis rubra nasi in a mother and daughter. Br Med J. 1937;2:1068.
- Heid E, Samain F, Jelen G, et al. Granulosis rubra nasi and pheochromocytoma. Ann Dermatol Venereol. 1996;123:106-108.
- Akhdari N. Granulosis rubra nasi. Int J Dermatol. 2007;46:396.
- Grazziotin TC, Buffon RB, Da Silva Manzoni AP, et al. Treatment of granulosis rubra nasi with botulinum toxin. Dermatol Surg. 2009;35:1298-1299.
- Kumar P, Gosai A, Mondal AK, et al. Granulosis rubra nasi: a rare condition treated successfully with topical tacrolimus. Dermatol Reports. 2012;4:E5.
- Sargunam C, Thomas J, Ahmed NA. Granulosis rubra nasi. Indian Dermatol Online J. 2013;4:208-209.
- Jadassohn J. Ueber eine eigenartige erkrankung der nasenhaut bei kindern. Arch Derm Syph. 1901;58:145-158.
- Hellier FF. Granulosis rubra nasi in a mother and daughter. Br Med J. 1937;2:1068.
- Heid E, Samain F, Jelen G, et al. Granulosis rubra nasi and pheochromocytoma. Ann Dermatol Venereol. 1996;123:106-108.
- Akhdari N. Granulosis rubra nasi. Int J Dermatol. 2007;46:396.
A healthy 9-year-old girl presented with a 2-year history of erythematous papules and pustules on the nose. There was no involvement of the rest of the face or body. At the time of presentation, she had been treated with several topical therapies including steroids, calcineurin inhibitors, antibiotics, and retinoids without improvement. A potassium hydroxide preparation from a pustule was performed and revealed only normal keratinocytes.
Allergy Testing in Dermatology and Beyond
Allergy testing typically refers to evaluation of a patient for suspected type I or type IV hypersensitivity.1,2 The possibility of type I hypersensitivity is raised in patients presenting with food allergies, allergic rhinitis, asthma, and immediate adverse reactions to medications, whereas type IV hypersensitivity is suspected in patients with eczematous eruptions, delayed adverse cutaneous reactions to medications, and failure of metallic implants (eg, metal joint replacements, cardiac stents) in conjunction with overlying skin rashes (Table 1).1-5 Type II (eg, pemphigus vulgaris) and type III (eg, IgA vasculitis) hypersensitivities are not evaluated with screening allergy tests.
Type I Sensitization
Type I hypersensitivity is an immediate hypersensitivity mediated predominantly by IgE activation of mast cells in the skin as well as the respiratory and gastric mucosa.1 Sensitization of an individual patient occurs when antigen-presenting cells induce a helper T cell (TH2) cytokine response leading to B-cell class switching and allergen-specific IgE production. Upon repeat exposure to the allergen, circulating antibodies then bind to high-affinity receptors on mast cells and basophils and initiate an allergic inflammatory response, leading to a clinical presentation of allergic rhinitis, urticaria, or immediate drug reactions. Confirming type I sensitization may be performed via serologic (in vitro) or skin testing (in vivo).5,6
Serologic Testing (In Vitro)
Serologic testing is a blood test that detects circulating IgE levels against specific allergens.5 The first such test, the radioallergosorbent test, was introduced in the 1970s but is not quantitative and is no longer used. Although common, it is inaccurate to describe current serum IgE (s-IgE) testing as radioallergosorbent testing. There are several US Food and Drug Administration-approved s-IgE assays in common use, and these tests may be helpful in elucidating relevant allergens and for tailoring therapy appropriately, which may consist of avoidance of certain foods or environmental agents and/or allergen immunotherapy.
Skin Testing (In Vivo)
Skin testing can be performed percutaneously (eg, percutaneous skin testing) or intradermally (eg, intradermal testing).6 Percutaneous skin testing is performed by placing a drop of allergen extract on the skin, after which a lancet is used to lightly scratch the skin; intradermal testing is performed by injecting a small amount of allergen extract into the dermis. In both cases, the skin is evaluated after 15 to 20 minutes for the presence and size of a cutaneous wheal. Medications with antihistaminergic activity must be discontinued prior to testing. Both s-IgE and skin testing assess for type I hypersensitivity, and factors such as extensive rash, concern for anaphylaxis, or inability to discontinue antihistamines may favor s-IgE testing versus skin testing. False-positive results can occur with both tests, and for this reason, test results should always be interpreted in conjunction with clinical examination and patient history to determine relevant allergies.
Type IV Sensitization
Type IV hypersensitivity is a delayed hypersensitivity mediated primarily by lymphocytes.2 Sensitization occurs when haptens bind to host proteins and are presented by epidermal and dermal dendritic cells to T lymphocytes in the skin. These lymphocytes then migrate to regional lymph nodes where antigen-specific T lymphocytes are produced and home back to the skin. Upon reexposure to the allergen, these memory T lymphocytes become activated and incite a delayed allergic response. Confirming type IV hypersensitivity primarily is accomplished via patch testing, though other testing modalities exist.
Skin Biopsy
Biopsy is sometimes performed in the workup of an individual presenting with allergic contact dermatitis (ACD) and typically will show spongiosis with normal stratum corneum and epidermal thickness in the setting of acute ACD and mild to marked acanthosis and parakeratosis in chronic ACD.7 The findings, however, are nonspecific and the differential of these histopathologic findings encompasses nummular dermatitis, atopic dermatitis, irritant contact dermatitis, and dyshidrotic eczema, among others. The presence of eosinophils and Langerhans cell microabscesses may provide supportive evidence for ACD over the other spongiotic dermatitides.7,8
Patch Testing
Patch testing is the gold standard in diagnosing type IV hypersensitivities resulting in a clinical presentation of ACD. Hundreds of allergens are commercially available for patch testing, and more commonly tested allergens fall into one of several categories, such as cosmetic preservatives, rubbers, metals, textiles, fragrances, adhesives, antibiotics, plants, and even corticosteroids. Of note, a common misconception is that ACD must result from new exposures; however, patients may develop ACD secondary to an exposure or product they have been using for many years without a problem.
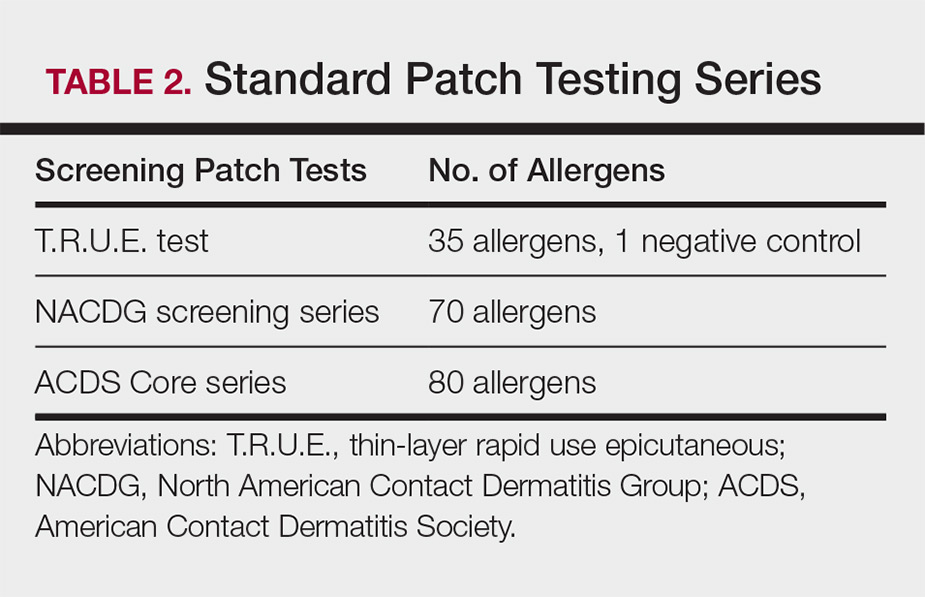
Three commonly used screening series are the thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice), North American Contact Dermatitis Group screening series, and American Contact Dermatitis Society Core 80 allergen series, which have some variation in the type and number of allergens included (Table 2). The T.R.U.E. test will miss a notable number of clinically relevant allergens in comparison to the North American Contact Dermatitis Group and American Contact Dermatitis Society Core series, and it may be of particularly low utility in identifying fragrance or preservative ACD.9
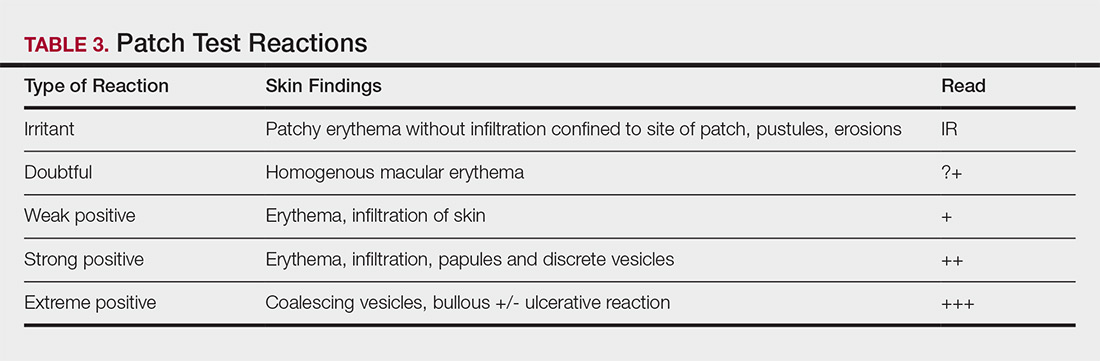
Allergens are placed on the back in chambers in a petrolatum or aqueous medium. The patches remain affixed for 48 hours, during which time the patient is asked to refrain from showering or exercising to prevent loss of patches. The patient's skin is then evaluated for reactions to allergens on 2 separate occasions: at the time of patch removal 48 hours after initial placement, then the areas of patches are marked for delayed readings at day 4 to day 7 after initial patch placement. Results are scored based on the degree of the inflammatory reaction (Table 3). Delayed readings beyond day 7 may be necessary for metals, specific preservatives (eg, dodecyl gallate, propolis), and neomycin.10
There is a wide spectrum of cutaneous disease that should prompt consideration of patch testing, including well-circumscribed eczematous dermatitis (eg, recurrent lip, hand, and foot dermatitis); patchy or diffuse eczema, especially if recently worsened and/or unresponsive to topical steroids; lichenoid eruptions, particularly of mucosal surfaces; mucous membrane eruptions (eg, stomatitis, vulvitis); and eczematous presentations that raise concern for airborne (photodistributed) or systemic contact dermatitis.11-13 Although further studies of efficacy and safety are ongoing, patch testing also may be useful in the diagnosis of nonimmediate cutaneous adverse drug reactions, especially fixed drug eruptions, acute generalized exanthematous pustulosis, systemic contact dermatitis from medications, and drug-induced hypersensitivity syndrome.3 Lastly, patients with type IV hypersensitivity to metals, adhesives, or antibiotics used in metallic orthopedic or cardiac implants may experience implant failure, regional contact dermatitis, or both, and benefit from patch testing prior to implant replacement to assess for potential allergens. Of the joints that fail, it is estimated that up to 5% are due to metal hypersensitivity.4
Throughout patch testing, patients may continue to manage their skin condition with oral antihistamines and topical steroids, though application to the site at which the patches are applied should be avoided throughout patch testing and during the week prior. According to expert consensus, immunosuppressive medications that are less likely to impact patch testing and therefore may be continued include low-dose methotrexate, oral prednisone less than 10 mg daily, biologic therapy, and low-dose cyclosporine (<2 mg/kg daily). Therapeutic interventions that are more likely to impact patch testing and should be avoided include phototherapy or extensive sun exposure within a week prior to testing, oral prednisone more than 10 mg daily, intramuscular triamcinolone within the preceding month, and high-dose cyclosporine (>2 mg/kg daily).14
An important component to successful patch testing is posttest patient counseling. Providers can create a safe list of products for patients by logging onto the American Contact Dermatitis Society website and accessing the Contact Allergen Management Program (CAMP).15 All relevant allergens found on patch testing may be selected and patient-specific identification codes generated. Once these codes are entered into the CAMP app on the patient's cellular device, a personalized, regularly updated list of safe products appears for many categories of products, including shampoos, sunscreens, moisturizers, cosmetic products, and laundry or dish detergents, among others. Of note, this app is not helpful for avoidance in patients with textile allergies. Patients should be counseled that improvement occurs with avoidance, which usually occurs within weeks but may slowly occur over time in some cases.
Lymphocyte Transformation Test (In Vitro)
The lymphocyte transformation test is an experimental in vitro test for type IV hypersensitivity. This serologic test utilizes allergens to stimulate memory T lymphocytes in vitro and measures the degree of response to the allergen. Although this test has generated excitement, particularly for the potential to safely evaluate for severe adverse cutaneous drug reactions, it currently is not the standard of care and is not utilized in the United States.16
Conclusion
Dermatologists play a vital role in the workup of suspected type IV hypersensitivities. Patch testing is an important but underutilized tool in the arsenal of allergy testing and may be indicated in a wide variety of cutaneous presentations, adverse reactions to medications, and implanted device failures. Identification and avoidance of a culprit allergen has the potential to lead to complete resolution of disease and notable improvement in quality of life for patients.
Acknowledgments
The author thanks Nina Botto, MD (San Francisco, California), for her mentorship in the arena of ACD as well as the Women's Dermatologic Society for the support they provided through the mentorship program.
- Oettgen H, Broide DH. Introduction to the mechanisms of allergic disease. In: Holgate ST, Church MK, Broide DH, et al, eds. Allergy. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1-32.
- Werfel T, Kapp A. Atopic dermatitis and allergic contact dermatitis. In: Holgate ST, Church MK, Broide DH, et al, eds. Allergy. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:263-286.
- Zinn A, Gayam S, Chelliah MP, et al. Patch testing for nonimmediate cutaneous adverse drug reactions. J Am Acad Dermatol. 2018;78:421-423.
- Thyssen JP, Menne T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Cox L. Overview of serological-specific IgE antibody testing in children. Curr Allergy Asthma Rep. 2011;11:447-453.
- Dolen WK. Skin testing and immunoassays for allergen-specific IgE. Clin Rev Allergy Immunol. 2001;21:229-239.
- Keeling BH, Gavino AC, Gavino AC. Skin biopsy, the allergists' tool: how to interpret a report. Curr Allergy Asthma Rep. 2015;15:62.
- Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504.
- DeKoven JG, Warshaw EM, Belsito DV. North American Contact Dermatitis Group patch test results 2013-2014. Dermatitis. 2017;28:33-46.
- Davis MD, Bhate K, Rohlinger AL, et al. Delayed patch test reading after 5 days: the Mayo Clinic experience. J Am Acad Dermatol. 2008;59:225-233.
- Rajagopalan R, Anderson RT. The profile of a patient with contact dermatitis and a suspicion of contact allergy (history, physical characteristics, and dermatology-specific quality of life). Am J Contact Dermat. 1997;8:26-31.
- Huygens S, Goossens A. An update on airborne contact dermatitis. Contact Dermatitis. 2001;44:1-6.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- ACDS CAMP. American Contact Dermatitis Society website. https://www.contactderm.org/i4a/pages/index.cfm?pageid=3489. Accessed November 14, 2018.
- Popple A, Williams J, Maxwell G, et al. The lymphocyte transformation test in allergic contact dermatitis: new opportunities. J Immunotoxicol. 2016;13:84-91.
Allergy testing typically refers to evaluation of a patient for suspected type I or type IV hypersensitivity.1,2 The possibility of type I hypersensitivity is raised in patients presenting with food allergies, allergic rhinitis, asthma, and immediate adverse reactions to medications, whereas type IV hypersensitivity is suspected in patients with eczematous eruptions, delayed adverse cutaneous reactions to medications, and failure of metallic implants (eg, metal joint replacements, cardiac stents) in conjunction with overlying skin rashes (Table 1).1-5 Type II (eg, pemphigus vulgaris) and type III (eg, IgA vasculitis) hypersensitivities are not evaluated with screening allergy tests.
Type I Sensitization
Type I hypersensitivity is an immediate hypersensitivity mediated predominantly by IgE activation of mast cells in the skin as well as the respiratory and gastric mucosa.1 Sensitization of an individual patient occurs when antigen-presenting cells induce a helper T cell (TH2) cytokine response leading to B-cell class switching and allergen-specific IgE production. Upon repeat exposure to the allergen, circulating antibodies then bind to high-affinity receptors on mast cells and basophils and initiate an allergic inflammatory response, leading to a clinical presentation of allergic rhinitis, urticaria, or immediate drug reactions. Confirming type I sensitization may be performed via serologic (in vitro) or skin testing (in vivo).5,6
Serologic Testing (In Vitro)
Serologic testing is a blood test that detects circulating IgE levels against specific allergens.5 The first such test, the radioallergosorbent test, was introduced in the 1970s but is not quantitative and is no longer used. Although common, it is inaccurate to describe current serum IgE (s-IgE) testing as radioallergosorbent testing. There are several US Food and Drug Administration-approved s-IgE assays in common use, and these tests may be helpful in elucidating relevant allergens and for tailoring therapy appropriately, which may consist of avoidance of certain foods or environmental agents and/or allergen immunotherapy.
Skin Testing (In Vivo)
Skin testing can be performed percutaneously (eg, percutaneous skin testing) or intradermally (eg, intradermal testing).6 Percutaneous skin testing is performed by placing a drop of allergen extract on the skin, after which a lancet is used to lightly scratch the skin; intradermal testing is performed by injecting a small amount of allergen extract into the dermis. In both cases, the skin is evaluated after 15 to 20 minutes for the presence and size of a cutaneous wheal. Medications with antihistaminergic activity must be discontinued prior to testing. Both s-IgE and skin testing assess for type I hypersensitivity, and factors such as extensive rash, concern for anaphylaxis, or inability to discontinue antihistamines may favor s-IgE testing versus skin testing. False-positive results can occur with both tests, and for this reason, test results should always be interpreted in conjunction with clinical examination and patient history to determine relevant allergies.
Type IV Sensitization
Type IV hypersensitivity is a delayed hypersensitivity mediated primarily by lymphocytes.2 Sensitization occurs when haptens bind to host proteins and are presented by epidermal and dermal dendritic cells to T lymphocytes in the skin. These lymphocytes then migrate to regional lymph nodes where antigen-specific T lymphocytes are produced and home back to the skin. Upon reexposure to the allergen, these memory T lymphocytes become activated and incite a delayed allergic response. Confirming type IV hypersensitivity primarily is accomplished via patch testing, though other testing modalities exist.
Skin Biopsy
Biopsy is sometimes performed in the workup of an individual presenting with allergic contact dermatitis (ACD) and typically will show spongiosis with normal stratum corneum and epidermal thickness in the setting of acute ACD and mild to marked acanthosis and parakeratosis in chronic ACD.7 The findings, however, are nonspecific and the differential of these histopathologic findings encompasses nummular dermatitis, atopic dermatitis, irritant contact dermatitis, and dyshidrotic eczema, among others. The presence of eosinophils and Langerhans cell microabscesses may provide supportive evidence for ACD over the other spongiotic dermatitides.7,8
Patch Testing
Patch testing is the gold standard in diagnosing type IV hypersensitivities resulting in a clinical presentation of ACD. Hundreds of allergens are commercially available for patch testing, and more commonly tested allergens fall into one of several categories, such as cosmetic preservatives, rubbers, metals, textiles, fragrances, adhesives, antibiotics, plants, and even corticosteroids. Of note, a common misconception is that ACD must result from new exposures; however, patients may develop ACD secondary to an exposure or product they have been using for many years without a problem.
Three commonly used screening series are the thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice), North American Contact Dermatitis Group screening series, and American Contact Dermatitis Society Core 80 allergen series, which have some variation in the type and number of allergens included (Table 2). The T.R.U.E. test will miss a notable number of clinically relevant allergens in comparison to the North American Contact Dermatitis Group and American Contact Dermatitis Society Core series, and it may be of particularly low utility in identifying fragrance or preservative ACD.9
Allergens are placed on the back in chambers in a petrolatum or aqueous medium. The patches remain affixed for 48 hours, during which time the patient is asked to refrain from showering or exercising to prevent loss of patches. The patient's skin is then evaluated for reactions to allergens on 2 separate occasions: at the time of patch removal 48 hours after initial placement, then the areas of patches are marked for delayed readings at day 4 to day 7 after initial patch placement. Results are scored based on the degree of the inflammatory reaction (Table 3). Delayed readings beyond day 7 may be necessary for metals, specific preservatives (eg, dodecyl gallate, propolis), and neomycin.10
There is a wide spectrum of cutaneous disease that should prompt consideration of patch testing, including well-circumscribed eczematous dermatitis (eg, recurrent lip, hand, and foot dermatitis); patchy or diffuse eczema, especially if recently worsened and/or unresponsive to topical steroids; lichenoid eruptions, particularly of mucosal surfaces; mucous membrane eruptions (eg, stomatitis, vulvitis); and eczematous presentations that raise concern for airborne (photodistributed) or systemic contact dermatitis.11-13 Although further studies of efficacy and safety are ongoing, patch testing also may be useful in the diagnosis of nonimmediate cutaneous adverse drug reactions, especially fixed drug eruptions, acute generalized exanthematous pustulosis, systemic contact dermatitis from medications, and drug-induced hypersensitivity syndrome.3 Lastly, patients with type IV hypersensitivity to metals, adhesives, or antibiotics used in metallic orthopedic or cardiac implants may experience implant failure, regional contact dermatitis, or both, and benefit from patch testing prior to implant replacement to assess for potential allergens. Of the joints that fail, it is estimated that up to 5% are due to metal hypersensitivity.4
Throughout patch testing, patients may continue to manage their skin condition with oral antihistamines and topical steroids, though application to the site at which the patches are applied should be avoided throughout patch testing and during the week prior. According to expert consensus, immunosuppressive medications that are less likely to impact patch testing and therefore may be continued include low-dose methotrexate, oral prednisone less than 10 mg daily, biologic therapy, and low-dose cyclosporine (<2 mg/kg daily). Therapeutic interventions that are more likely to impact patch testing and should be avoided include phototherapy or extensive sun exposure within a week prior to testing, oral prednisone more than 10 mg daily, intramuscular triamcinolone within the preceding month, and high-dose cyclosporine (>2 mg/kg daily).14
An important component to successful patch testing is posttest patient counseling. Providers can create a safe list of products for patients by logging onto the American Contact Dermatitis Society website and accessing the Contact Allergen Management Program (CAMP).15 All relevant allergens found on patch testing may be selected and patient-specific identification codes generated. Once these codes are entered into the CAMP app on the patient's cellular device, a personalized, regularly updated list of safe products appears for many categories of products, including shampoos, sunscreens, moisturizers, cosmetic products, and laundry or dish detergents, among others. Of note, this app is not helpful for avoidance in patients with textile allergies. Patients should be counseled that improvement occurs with avoidance, which usually occurs within weeks but may slowly occur over time in some cases.
Lymphocyte Transformation Test (In Vitro)
The lymphocyte transformation test is an experimental in vitro test for type IV hypersensitivity. This serologic test utilizes allergens to stimulate memory T lymphocytes in vitro and measures the degree of response to the allergen. Although this test has generated excitement, particularly for the potential to safely evaluate for severe adverse cutaneous drug reactions, it currently is not the standard of care and is not utilized in the United States.16
Conclusion
Dermatologists play a vital role in the workup of suspected type IV hypersensitivities. Patch testing is an important but underutilized tool in the arsenal of allergy testing and may be indicated in a wide variety of cutaneous presentations, adverse reactions to medications, and implanted device failures. Identification and avoidance of a culprit allergen has the potential to lead to complete resolution of disease and notable improvement in quality of life for patients.
Acknowledgments
The author thanks Nina Botto, MD (San Francisco, California), for her mentorship in the arena of ACD as well as the Women's Dermatologic Society for the support they provided through the mentorship program.
Allergy testing typically refers to evaluation of a patient for suspected type I or type IV hypersensitivity.1,2 The possibility of type I hypersensitivity is raised in patients presenting with food allergies, allergic rhinitis, asthma, and immediate adverse reactions to medications, whereas type IV hypersensitivity is suspected in patients with eczematous eruptions, delayed adverse cutaneous reactions to medications, and failure of metallic implants (eg, metal joint replacements, cardiac stents) in conjunction with overlying skin rashes (Table 1).1-5 Type II (eg, pemphigus vulgaris) and type III (eg, IgA vasculitis) hypersensitivities are not evaluated with screening allergy tests.
Type I Sensitization
Type I hypersensitivity is an immediate hypersensitivity mediated predominantly by IgE activation of mast cells in the skin as well as the respiratory and gastric mucosa.1 Sensitization of an individual patient occurs when antigen-presenting cells induce a helper T cell (TH2) cytokine response leading to B-cell class switching and allergen-specific IgE production. Upon repeat exposure to the allergen, circulating antibodies then bind to high-affinity receptors on mast cells and basophils and initiate an allergic inflammatory response, leading to a clinical presentation of allergic rhinitis, urticaria, or immediate drug reactions. Confirming type I sensitization may be performed via serologic (in vitro) or skin testing (in vivo).5,6
Serologic Testing (In Vitro)
Serologic testing is a blood test that detects circulating IgE levels against specific allergens.5 The first such test, the radioallergosorbent test, was introduced in the 1970s but is not quantitative and is no longer used. Although common, it is inaccurate to describe current serum IgE (s-IgE) testing as radioallergosorbent testing. There are several US Food and Drug Administration-approved s-IgE assays in common use, and these tests may be helpful in elucidating relevant allergens and for tailoring therapy appropriately, which may consist of avoidance of certain foods or environmental agents and/or allergen immunotherapy.
Skin Testing (In Vivo)
Skin testing can be performed percutaneously (eg, percutaneous skin testing) or intradermally (eg, intradermal testing).6 Percutaneous skin testing is performed by placing a drop of allergen extract on the skin, after which a lancet is used to lightly scratch the skin; intradermal testing is performed by injecting a small amount of allergen extract into the dermis. In both cases, the skin is evaluated after 15 to 20 minutes for the presence and size of a cutaneous wheal. Medications with antihistaminergic activity must be discontinued prior to testing. Both s-IgE and skin testing assess for type I hypersensitivity, and factors such as extensive rash, concern for anaphylaxis, or inability to discontinue antihistamines may favor s-IgE testing versus skin testing. False-positive results can occur with both tests, and for this reason, test results should always be interpreted in conjunction with clinical examination and patient history to determine relevant allergies.
Type IV Sensitization
Type IV hypersensitivity is a delayed hypersensitivity mediated primarily by lymphocytes.2 Sensitization occurs when haptens bind to host proteins and are presented by epidermal and dermal dendritic cells to T lymphocytes in the skin. These lymphocytes then migrate to regional lymph nodes where antigen-specific T lymphocytes are produced and home back to the skin. Upon reexposure to the allergen, these memory T lymphocytes become activated and incite a delayed allergic response. Confirming type IV hypersensitivity primarily is accomplished via patch testing, though other testing modalities exist.
Skin Biopsy
Biopsy is sometimes performed in the workup of an individual presenting with allergic contact dermatitis (ACD) and typically will show spongiosis with normal stratum corneum and epidermal thickness in the setting of acute ACD and mild to marked acanthosis and parakeratosis in chronic ACD.7 The findings, however, are nonspecific and the differential of these histopathologic findings encompasses nummular dermatitis, atopic dermatitis, irritant contact dermatitis, and dyshidrotic eczema, among others. The presence of eosinophils and Langerhans cell microabscesses may provide supportive evidence for ACD over the other spongiotic dermatitides.7,8
Patch Testing
Patch testing is the gold standard in diagnosing type IV hypersensitivities resulting in a clinical presentation of ACD. Hundreds of allergens are commercially available for patch testing, and more commonly tested allergens fall into one of several categories, such as cosmetic preservatives, rubbers, metals, textiles, fragrances, adhesives, antibiotics, plants, and even corticosteroids. Of note, a common misconception is that ACD must result from new exposures; however, patients may develop ACD secondary to an exposure or product they have been using for many years without a problem.
Three commonly used screening series are the thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice), North American Contact Dermatitis Group screening series, and American Contact Dermatitis Society Core 80 allergen series, which have some variation in the type and number of allergens included (Table 2). The T.R.U.E. test will miss a notable number of clinically relevant allergens in comparison to the North American Contact Dermatitis Group and American Contact Dermatitis Society Core series, and it may be of particularly low utility in identifying fragrance or preservative ACD.9
Allergens are placed on the back in chambers in a petrolatum or aqueous medium. The patches remain affixed for 48 hours, during which time the patient is asked to refrain from showering or exercising to prevent loss of patches. The patient's skin is then evaluated for reactions to allergens on 2 separate occasions: at the time of patch removal 48 hours after initial placement, then the areas of patches are marked for delayed readings at day 4 to day 7 after initial patch placement. Results are scored based on the degree of the inflammatory reaction (Table 3). Delayed readings beyond day 7 may be necessary for metals, specific preservatives (eg, dodecyl gallate, propolis), and neomycin.10
There is a wide spectrum of cutaneous disease that should prompt consideration of patch testing, including well-circumscribed eczematous dermatitis (eg, recurrent lip, hand, and foot dermatitis); patchy or diffuse eczema, especially if recently worsened and/or unresponsive to topical steroids; lichenoid eruptions, particularly of mucosal surfaces; mucous membrane eruptions (eg, stomatitis, vulvitis); and eczematous presentations that raise concern for airborne (photodistributed) or systemic contact dermatitis.11-13 Although further studies of efficacy and safety are ongoing, patch testing also may be useful in the diagnosis of nonimmediate cutaneous adverse drug reactions, especially fixed drug eruptions, acute generalized exanthematous pustulosis, systemic contact dermatitis from medications, and drug-induced hypersensitivity syndrome.3 Lastly, patients with type IV hypersensitivity to metals, adhesives, or antibiotics used in metallic orthopedic or cardiac implants may experience implant failure, regional contact dermatitis, or both, and benefit from patch testing prior to implant replacement to assess for potential allergens. Of the joints that fail, it is estimated that up to 5% are due to metal hypersensitivity.4
Throughout patch testing, patients may continue to manage their skin condition with oral antihistamines and topical steroids, though application to the site at which the patches are applied should be avoided throughout patch testing and during the week prior. According to expert consensus, immunosuppressive medications that are less likely to impact patch testing and therefore may be continued include low-dose methotrexate, oral prednisone less than 10 mg daily, biologic therapy, and low-dose cyclosporine (<2 mg/kg daily). Therapeutic interventions that are more likely to impact patch testing and should be avoided include phototherapy or extensive sun exposure within a week prior to testing, oral prednisone more than 10 mg daily, intramuscular triamcinolone within the preceding month, and high-dose cyclosporine (>2 mg/kg daily).14
An important component to successful patch testing is posttest patient counseling. Providers can create a safe list of products for patients by logging onto the American Contact Dermatitis Society website and accessing the Contact Allergen Management Program (CAMP).15 All relevant allergens found on patch testing may be selected and patient-specific identification codes generated. Once these codes are entered into the CAMP app on the patient's cellular device, a personalized, regularly updated list of safe products appears for many categories of products, including shampoos, sunscreens, moisturizers, cosmetic products, and laundry or dish detergents, among others. Of note, this app is not helpful for avoidance in patients with textile allergies. Patients should be counseled that improvement occurs with avoidance, which usually occurs within weeks but may slowly occur over time in some cases.
Lymphocyte Transformation Test (In Vitro)
The lymphocyte transformation test is an experimental in vitro test for type IV hypersensitivity. This serologic test utilizes allergens to stimulate memory T lymphocytes in vitro and measures the degree of response to the allergen. Although this test has generated excitement, particularly for the potential to safely evaluate for severe adverse cutaneous drug reactions, it currently is not the standard of care and is not utilized in the United States.16
Conclusion
Dermatologists play a vital role in the workup of suspected type IV hypersensitivities. Patch testing is an important but underutilized tool in the arsenal of allergy testing and may be indicated in a wide variety of cutaneous presentations, adverse reactions to medications, and implanted device failures. Identification and avoidance of a culprit allergen has the potential to lead to complete resolution of disease and notable improvement in quality of life for patients.
Acknowledgments
The author thanks Nina Botto, MD (San Francisco, California), for her mentorship in the arena of ACD as well as the Women's Dermatologic Society for the support they provided through the mentorship program.
- Oettgen H, Broide DH. Introduction to the mechanisms of allergic disease. In: Holgate ST, Church MK, Broide DH, et al, eds. Allergy. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1-32.
- Werfel T, Kapp A. Atopic dermatitis and allergic contact dermatitis. In: Holgate ST, Church MK, Broide DH, et al, eds. Allergy. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:263-286.
- Zinn A, Gayam S, Chelliah MP, et al. Patch testing for nonimmediate cutaneous adverse drug reactions. J Am Acad Dermatol. 2018;78:421-423.
- Thyssen JP, Menne T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Cox L. Overview of serological-specific IgE antibody testing in children. Curr Allergy Asthma Rep. 2011;11:447-453.
- Dolen WK. Skin testing and immunoassays for allergen-specific IgE. Clin Rev Allergy Immunol. 2001;21:229-239.
- Keeling BH, Gavino AC, Gavino AC. Skin biopsy, the allergists' tool: how to interpret a report. Curr Allergy Asthma Rep. 2015;15:62.
- Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504.
- DeKoven JG, Warshaw EM, Belsito DV. North American Contact Dermatitis Group patch test results 2013-2014. Dermatitis. 2017;28:33-46.
- Davis MD, Bhate K, Rohlinger AL, et al. Delayed patch test reading after 5 days: the Mayo Clinic experience. J Am Acad Dermatol. 2008;59:225-233.
- Rajagopalan R, Anderson RT. The profile of a patient with contact dermatitis and a suspicion of contact allergy (history, physical characteristics, and dermatology-specific quality of life). Am J Contact Dermat. 1997;8:26-31.
- Huygens S, Goossens A. An update on airborne contact dermatitis. Contact Dermatitis. 2001;44:1-6.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- ACDS CAMP. American Contact Dermatitis Society website. https://www.contactderm.org/i4a/pages/index.cfm?pageid=3489. Accessed November 14, 2018.
- Popple A, Williams J, Maxwell G, et al. The lymphocyte transformation test in allergic contact dermatitis: new opportunities. J Immunotoxicol. 2016;13:84-91.
- Oettgen H, Broide DH. Introduction to the mechanisms of allergic disease. In: Holgate ST, Church MK, Broide DH, et al, eds. Allergy. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1-32.
- Werfel T, Kapp A. Atopic dermatitis and allergic contact dermatitis. In: Holgate ST, Church MK, Broide DH, et al, eds. Allergy. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:263-286.
- Zinn A, Gayam S, Chelliah MP, et al. Patch testing for nonimmediate cutaneous adverse drug reactions. J Am Acad Dermatol. 2018;78:421-423.
- Thyssen JP, Menne T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Cox L. Overview of serological-specific IgE antibody testing in children. Curr Allergy Asthma Rep. 2011;11:447-453.
- Dolen WK. Skin testing and immunoassays for allergen-specific IgE. Clin Rev Allergy Immunol. 2001;21:229-239.
- Keeling BH, Gavino AC, Gavino AC. Skin biopsy, the allergists' tool: how to interpret a report. Curr Allergy Asthma Rep. 2015;15:62.
- Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504.
- DeKoven JG, Warshaw EM, Belsito DV. North American Contact Dermatitis Group patch test results 2013-2014. Dermatitis. 2017;28:33-46.
- Davis MD, Bhate K, Rohlinger AL, et al. Delayed patch test reading after 5 days: the Mayo Clinic experience. J Am Acad Dermatol. 2008;59:225-233.
- Rajagopalan R, Anderson RT. The profile of a patient with contact dermatitis and a suspicion of contact allergy (history, physical characteristics, and dermatology-specific quality of life). Am J Contact Dermat. 1997;8:26-31.
- Huygens S, Goossens A. An update on airborne contact dermatitis. Contact Dermatitis. 2001;44:1-6.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- ACDS CAMP. American Contact Dermatitis Society website. https://www.contactderm.org/i4a/pages/index.cfm?pageid=3489. Accessed November 14, 2018.
- Popple A, Williams J, Maxwell G, et al. The lymphocyte transformation test in allergic contact dermatitis: new opportunities. J Immunotoxicol. 2016;13:84-91.
Bedside Microscopy for the Beginner
Dermatologists are uniquely equipped amongst clinicians to make bedside diagnoses because of the focus on histopathology and microscopy inherent in our training. This skill is highly valuable in both an inpatient and outpatient setting because it may lead to a rapid diagnosis or be a useful adjunct in the initial clinical decision-making process. Although expert microscopists may be able to garner relevant information from scraping almost any type of lesion, bedside microscopy primarily is used by dermatologists in the United States for consideration of infectious etiologies of a variety of cutaneous manifestations.1,2
Basic Principles
Lesions that should be considered for bedside microscopic analysis in outpatient settings are scaly lesions, vesiculobullous lesions, inflammatory papules, and pustules1; microscopic evaluation also can be useful for myriad trichoscopic considerations.3,4 In some instances, direct visualization of the pathogen is possible (eg, cutaneous fungal infections, demodicidosis, scabetic infections), and in other circumstances reactive changes of keratinocytes or the presence of specific cell types can aid in diagnosis (eg, ballooning degeneration and multinucleation of keratinocytes in herpetic lesions, an abundance of eosinophils in erythema toxicum neonatorum). Different types of media are used to best prepare tissue based on the suspected etiology of the condition.
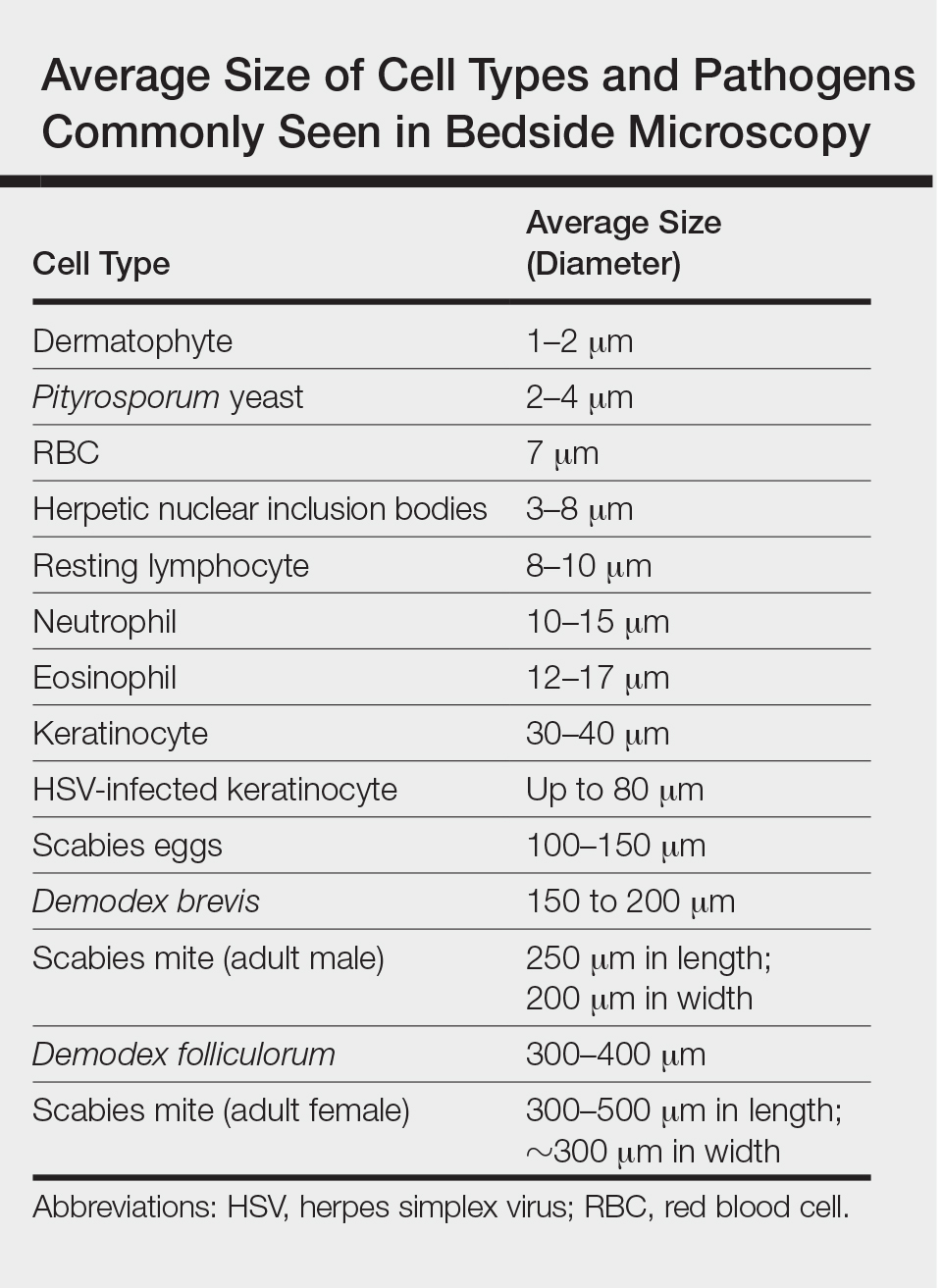
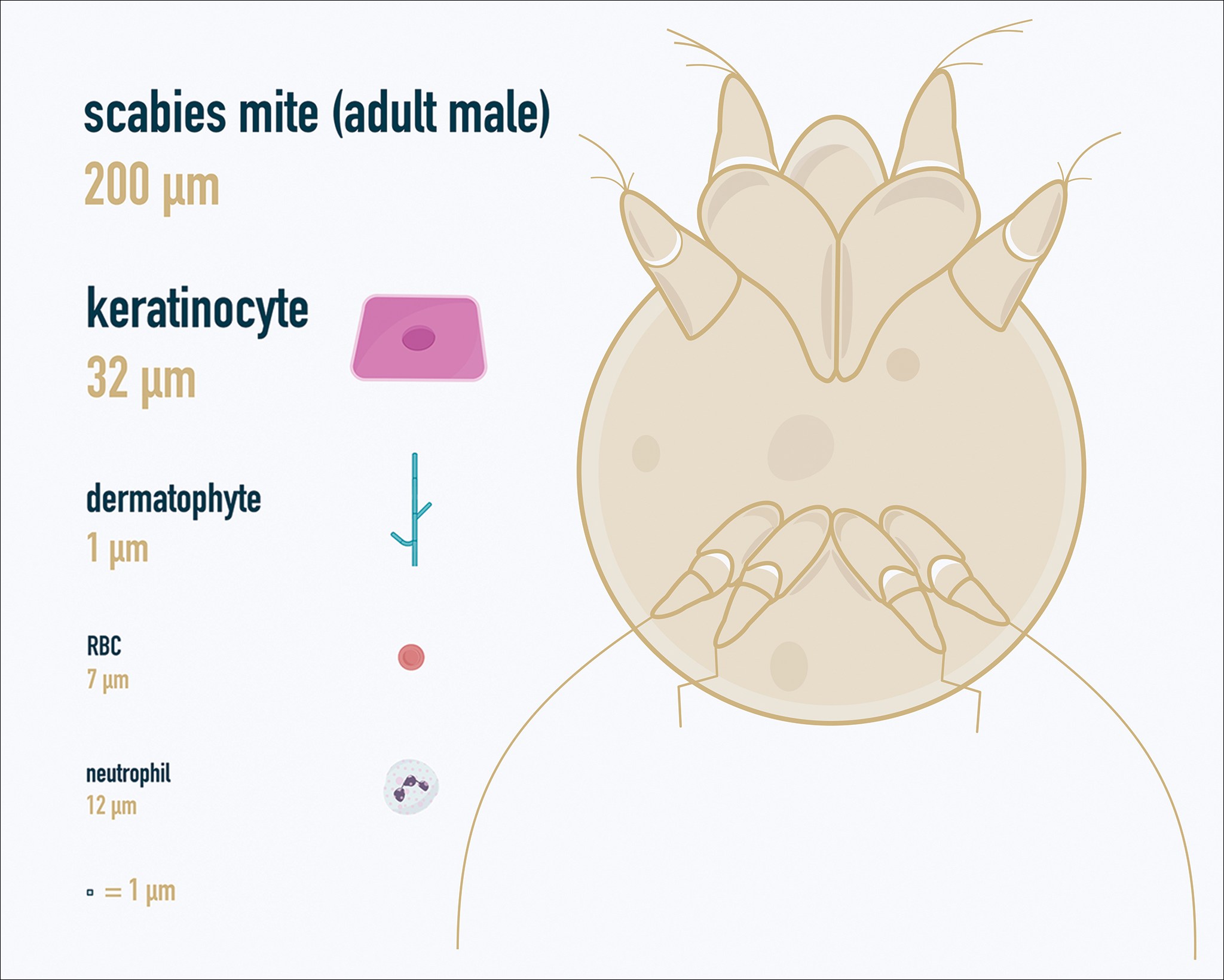
One major stumbling block for residents when beginning to perform bedside testing is the lack of dimensional understanding of the structures they are searching for; for example, medical students and residents often may mistake fibers for dermatophytes, which typically are much larger than fungal hyphae. Familiarizing oneself with the basic dimensions of different cell types or pathogens in relation to each other (Table) will help further refine the beginner’s ability to effectively search for and identify pathogenic features. This concept is further schematized in Figure 1 to help visualize scale differences.
Examination of the Specimen
Slide preparation depends on the primary lesion in consideration and will be discussed in greater detail in the following sections. Once the slide is prepared, place it on the microscope stage and adjust the condenser and light source for optimal visualization. Scan the specimen in a gridlike fashion on low power (usually ×10) and then inspect suspicious findings on higher power (×40 or higher).
Dermatomycoses
Fungal infections of the skin can present as annular papulosquamous lesions, follicular pustules or papules, bullous lesions, hypopigmented patches, and mucosal exudate or erosions, among other manifestations.5 Potassium hydroxide (KOH) is the classic medium used in preparation of lesions being assessed for evidence of fungus because it leads to lysis of keratinocytes for better visualization of fungal hyphae and spores. Other media that contain KOH and additional substrates such as dimethyl sulfoxide or chlorazol black E can be used to better highlight fungal elements.6
Dermatophytosis
Dermatophytes lead to superficial infection of the epidermis and epidermal appendages and present in a variety of ways, including site-specific infections manifesting typically as erythematous, annular or arcuate scaling (eg, tinea faciei, tinea corporis, tinea cruris, tinea manus, tinea pedis), alopecia with broken hair shafts, black dots, boggy nodules and/or scaling of the scalp (eg, tinea capitis, favus, kerion), and dystrophic nails (eg, onychomycosis).5,7 For examination of lesional skin scrapings, one can either use clear cellophane tape against the skin to remove scale, which is especially useful in the case of pediatric patients, and then press the tape against a slide prepared with several drops of a KOH-based medium to directly visualize without a coverslip, or scrape the lesion with a No. 15 blade and place the scales onto the glass slide, with further preparation as described below.8 For assessment of alopecia or dystrophic nails, scrape lesional skin with a No. 15 blade to obtain affected hair follicles and proximal subungual debris, respectively.6,9
Once the cellular debris has been obtained and placed on the slide, a coverslip can be overlaid and KOH applied laterally to be taken up across the slide by capillary action. Allow the slide to sit for at least 5 minutes before analyzing to better visualize fungal elements. Both tinea and onychomycosis will show branching septate hyphae extending across keratinocytes; a common false-positive is identifying overlapping keratinocyte edges, which are a similar size, but they can be distinguished from fungi because they do not cross multiple keratinocytes.1,8 Tinea capitis may demonstrate similar findings or may reveal hair shafts with spores contained within or surrounding it, corresponding to endothrix or ectothrix infection, respectively.5
Pityriasis Versicolor and Malassezia Folliculitis
Pityriasis versicolor presents with hypopigmented to pink, finely scaling ovoid papules, usually on the upper back, shoulders, and neck, and is caused by Malassezia furfur and other Malassezia species.5 Malassezia folliculitis also is caused by this fungus and presents with monomorphic follicular papules and pustules. Scrapings from the scaly papules will demonstrate keratinocytes with the classic “spaghetti and meatballs” fungal elements, whereas Malassezia folliculitis demonstrates only spores.5,7
Candidiasis
One possible outpatient presentation of candidiasis is oral thrush, which can exhibit white mucosal exudate or erythematous patches. A tongue blade can be used to scrape the tongue or cheek wall, with subsequent preparatory steps with application of KOH as described for dermatophytes. Cutaneous candidiasis most often develops in intertriginous regions and will exhibit erosive painful lesions with satellite pustules. In both cases, analysis of the specimen will show shorter fatter hyphal elements than seen in dermatophytosis, with pseudohyphae, blunted ends, and potentially yeast forms.5
Vesiculobullous Lesions
The Tzanck smear has been used since the 1940s to differentiate between etiologies of blistering disorders and is now most commonly used for the quick identification of herpetic lesions.1 The test is performed by scraping the base of a deroofed vesicle, pustule, or bulla, and smearing the cellular materials onto a glass slide. The most commonly utilized media for staining in the outpatient setting at my institution (University of Texas Dell Medical School, Austin) is Giemsa, which is composed of azure II–eosin, glycerin, and methanol. It stains nuclei a reddish blue to pink and the cytoplasm blue.10 After being applied to the slide, the cells are allowed to air-dry for 5 to 10 minutes, and Giemsa stain is subsequently applied and allowed to incubate for 15 minutes, then rinsed carefully with water and directly examined.
Other stains that can be used to perform the Tzanck smear include commercial preparations that may be more accessible in the inpatient settings such as the Wright-Giemsa, Quik-Dip, and Diff-Quick.1,10
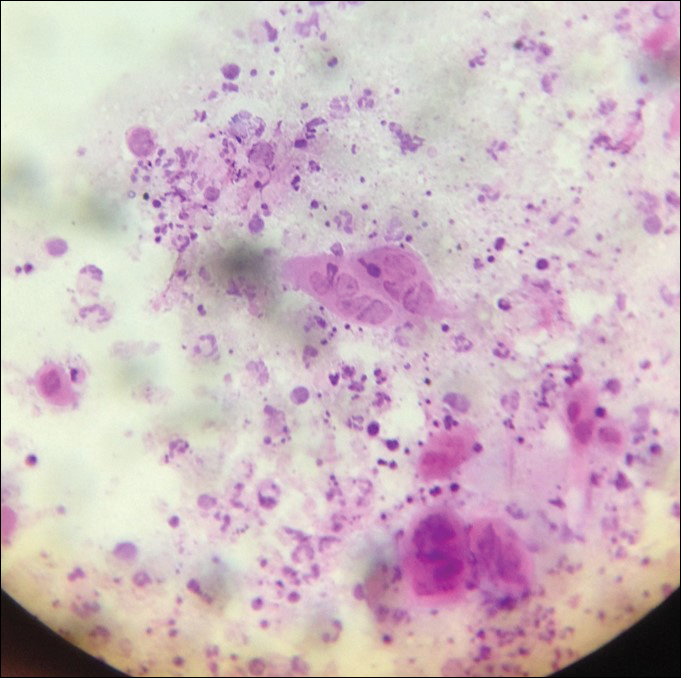
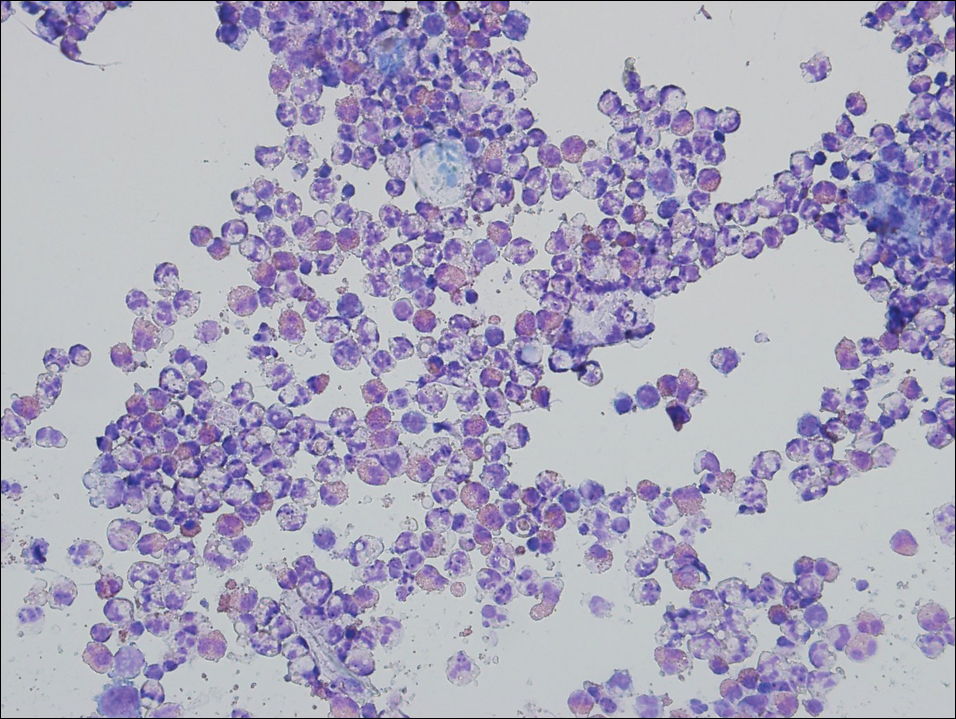
Examination of a Tzanck smear from a herpetic lesion will yield acantholytic, enlarged keratinocytes up to twice their usual size (referred to as ballooning degeneration), and multinucleation. In addition, molding of the nuclei to each other within the multinucleated cells and margination of the nuclear chromatin may be appreciated (Figure 2). Intranuclear inclusion bodies, also known as Cowdry type A bodies, can be seen that are nearly the size of red blood cells but are rare to find, with only 10% of specimens exhibiting this finding in a prospective review of 299 patients with herpetic vesiculobullous lesions.11 Evaluation of the contents of blisters caused by bullous pemphigoid and erythema toxicum neonatorum may yield high densities of eosinophils with normal keratinocyte morphology (Figure 3). Other blistering eruptions such as pemphigus vulgaris and bullous drug eruptions also have characteristic findings.1,2
Gout Preparation
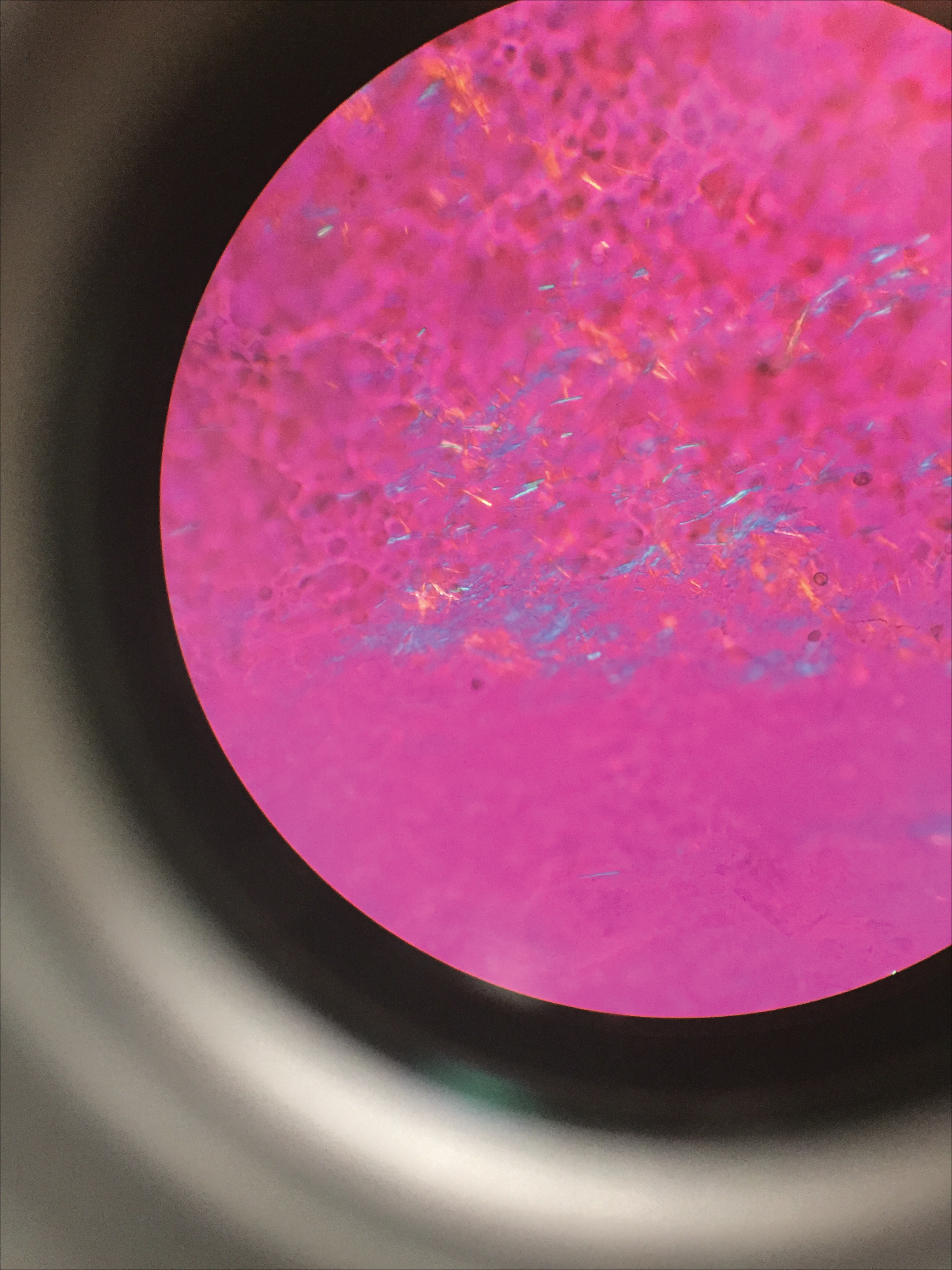
Gout is a systemic disease caused by uric acid accumulation that can present with joint pain and white to red nodules on digits, joints, and ears (known as tophi). Material may be expressed from tophi and examined immediately by polarized light microscopy to confirm the diagnosis.5 Specimens will demonstrate needle-shaped, negatively birefringent monosodium urate crystals on polarized light microscopy (Figure 4). An ordinary light microscope can be converted for such use with the lenses of inexpensive polarized sunglasses, placing one lens between the light source and specimen and the other lens between the examiner’s eye and the specimen.12
Parasitic Infections
Two common parasitic infections identified in outpatient dermatology clinics are scabies mites and Demodex mites. Human scabies is extremely pruritic and caused by infestation with Sarcoptes scabiei var hominis; the typical presentation in an adult is erythematous and crusted papules, linear burrows, and vesiculopustules, especially of the interdigital spaces, wrists, axillae, umbilicus, and genital region.1,13 Demodicidosis presents with papules and pustules on the face, usually in a patient with background rosacea and diffuse erythema.1,5,14
If either of these conditions are suspected, mineral oil should be used to prepare the slide because it will maintain viability of the organisms, which are visualized better in motion. Adult scabies mites are roughly 10 times larger than keratinocytes, measuring approximately 250 to 450 µm in length with 8 legs.13 Eggs also may be visualized within the cellular debris and typically are 100 to 150 µm in size and ovoid in shape. Of note, polariscopic examination may be a useful adjunct for evaluation of scabies because scabetic spines and scybala (or fecal material) are polarizable.15
Two types of Demodex mites typically are found in the skin: Demodex folliculorum, which are similarly sized to scabies mites with a more oblong body and occur most commonly in mature hair follicles (eg, eyelashes), and Demodex brevis, which are about half the size (150–200 µm) and live in the sebaceous glands of vellus hairs (Figure 5).14 Both of these mites have 8 legs, similar to the scabies mite.
Hair Preparations
Hair preparations for bulbar examination (eg, trichogram) may prove useful in the evaluation of many types of alopecia, and elaboration on this topic is beyond the scope of this article. Microscopic evaluation of the hair shaft may be an underutilized technique in the outpatient setting and is capable of yielding a variety of diagnoses, including monilethrix, pili torti, and pili trianguli et canaliculi, among others.3 One particularly useful scenario for hair shaft examination (usually of the eyebrow) is in the setting of a patient with severe atopic dermatitis or a baby with ichthyosiform erythroderma, as discovery of trichorrhexis invaginata is pathognomonic for the diagnosis of Netherton syndrome.16 Lastly, evaluation of the hair shaft in patients with patchy and diffuse hair loss whose clinical impression is reminiscent of alopecia areata, or those with concerns of inability to grow hair beyond a short length, may lead to diagnosis of loose anagen syndrome, especially if more than 70% of hair fibers examined exhibit the classic findings of a ruffled proximal cuticle and lack of root sheath.4
Final Thoughts
Bedside microscopy is a rapid and cost-sensitive way to confirm diagnoses that are clinically suspected and remains a valuable tool to acquire during residency training.
- Wanat KA, Dominguez AR, Carter Z, et al. Bedside diagnostics in dermatology: viral, bacterial, and fungal infections. J Am Acad Dermatol. 2017;77:197-218.
- Micheletti RG, Dominguez AR, Wanat KA. Bedside diagnostics in dermatology: parasitic and noninfectious diseases. J Am Acad Dermatol. 2017;77:221-230.
- Whiting DA, Dy LC. Office diagnosis of hair shaft defects. Semin Cutan Med Surg. 2006;25:24-34.
- Tosti A. Loose anagen hair syndrome and loose anagen hair. Arch Dermatol. 2002;138:521-522.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia PA: Elsevier; 2017.
- Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;55:620-626.
- Elder DE, ed. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009.
- Raghukumar S, Ravikumar BC. Potassium hydroxide mount with cellophane adhesive: a method for direct diagnosis of dermatophyte skin infections [published online May 29, 2018]. Clin Exp Dermatol. doi:10.1111/ced.13573.
- Bhat YJ, Zeerak S, Kanth F, et al. Clinicoepidemiological and mycological study of tinea capitis in the pediatric population of Kashmir Valley: a study from a tertiary care centre. Indian Dermatol Online J. 2017;8:100-103.
- Gupta LK, Singhi MK. Tzanck smear: a useful diagnostic tool. Indian J Dermatol Venereol Leprol. 2005;71:295-299.
- Durdu M, Baba M, Seçkin D. The value of Tzanck smear test in diagnosis of erosive, vesicular, bullous, and pustular skin lesions. J Am Acad Dermatol. 2008;59:958-964.
- Fagan TJ, Lidsky MD. Compensated polarized light microscopy using cellophane adhesive tape. Arthritis Rheum. 1974;17:256-262.
- Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007;20:268-279.
- Desch C, Nutting WB. Demodex folliculorum (Simon) and D. brevis akbulatova of man: redescription and reevaluation. J Parasitol. 1972;58:169-177.
- Foo CW, Florell SR, Bowen AR. Polarizable elements in scabies infestation: a clue to diagnosis. J Cutan Pathol. 2013;40:6-10.
- Akkurt ZM, Tuncel T, Ayhan E, et al. Rapid and easy diagnosis of Netherton syndrome with dermoscopy. J Cutan Med Surg. 2014;18:280-282.
Dermatologists are uniquely equipped amongst clinicians to make bedside diagnoses because of the focus on histopathology and microscopy inherent in our training. This skill is highly valuable in both an inpatient and outpatient setting because it may lead to a rapid diagnosis or be a useful adjunct in the initial clinical decision-making process. Although expert microscopists may be able to garner relevant information from scraping almost any type of lesion, bedside microscopy primarily is used by dermatologists in the United States for consideration of infectious etiologies of a variety of cutaneous manifestations.1,2
Basic Principles
Lesions that should be considered for bedside microscopic analysis in outpatient settings are scaly lesions, vesiculobullous lesions, inflammatory papules, and pustules1; microscopic evaluation also can be useful for myriad trichoscopic considerations.3,4 In some instances, direct visualization of the pathogen is possible (eg, cutaneous fungal infections, demodicidosis, scabetic infections), and in other circumstances reactive changes of keratinocytes or the presence of specific cell types can aid in diagnosis (eg, ballooning degeneration and multinucleation of keratinocytes in herpetic lesions, an abundance of eosinophils in erythema toxicum neonatorum). Different types of media are used to best prepare tissue based on the suspected etiology of the condition.
One major stumbling block for residents when beginning to perform bedside testing is the lack of dimensional understanding of the structures they are searching for; for example, medical students and residents often may mistake fibers for dermatophytes, which typically are much larger than fungal hyphae. Familiarizing oneself with the basic dimensions of different cell types or pathogens in relation to each other (Table) will help further refine the beginner’s ability to effectively search for and identify pathogenic features. This concept is further schematized in Figure 1 to help visualize scale differences.

Examination of the Specimen
Slide preparation depends on the primary lesion in consideration and will be discussed in greater detail in the following sections. Once the slide is prepared, place it on the microscope stage and adjust the condenser and light source for optimal visualization. Scan the specimen in a gridlike fashion on low power (usually ×10) and then inspect suspicious findings on higher power (×40 or higher).
Dermatomycoses
Fungal infections of the skin can present as annular papulosquamous lesions, follicular pustules or papules, bullous lesions, hypopigmented patches, and mucosal exudate or erosions, among other manifestations.5 Potassium hydroxide (KOH) is the classic medium used in preparation of lesions being assessed for evidence of fungus because it leads to lysis of keratinocytes for better visualization of fungal hyphae and spores. Other media that contain KOH and additional substrates such as dimethyl sulfoxide or chlorazol black E can be used to better highlight fungal elements.6
Dermatophytosis
Dermatophytes lead to superficial infection of the epidermis and epidermal appendages and present in a variety of ways, including site-specific infections manifesting typically as erythematous, annular or arcuate scaling (eg, tinea faciei, tinea corporis, tinea cruris, tinea manus, tinea pedis), alopecia with broken hair shafts, black dots, boggy nodules and/or scaling of the scalp (eg, tinea capitis, favus, kerion), and dystrophic nails (eg, onychomycosis).5,7 For examination of lesional skin scrapings, one can either use clear cellophane tape against the skin to remove scale, which is especially useful in the case of pediatric patients, and then press the tape against a slide prepared with several drops of a KOH-based medium to directly visualize without a coverslip, or scrape the lesion with a No. 15 blade and place the scales onto the glass slide, with further preparation as described below.8 For assessment of alopecia or dystrophic nails, scrape lesional skin with a No. 15 blade to obtain affected hair follicles and proximal subungual debris, respectively.6,9
Once the cellular debris has been obtained and placed on the slide, a coverslip can be overlaid and KOH applied laterally to be taken up across the slide by capillary action. Allow the slide to sit for at least 5 minutes before analyzing to better visualize fungal elements. Both tinea and onychomycosis will show branching septate hyphae extending across keratinocytes; a common false-positive is identifying overlapping keratinocyte edges, which are a similar size, but they can be distinguished from fungi because they do not cross multiple keratinocytes.1,8 Tinea capitis may demonstrate similar findings or may reveal hair shafts with spores contained within or surrounding it, corresponding to endothrix or ectothrix infection, respectively.5
Pityriasis Versicolor and Malassezia Folliculitis
Pityriasis versicolor presents with hypopigmented to pink, finely scaling ovoid papules, usually on the upper back, shoulders, and neck, and is caused by Malassezia furfur and other Malassezia species.5 Malassezia folliculitis also is caused by this fungus and presents with monomorphic follicular papules and pustules. Scrapings from the scaly papules will demonstrate keratinocytes with the classic “spaghetti and meatballs” fungal elements, whereas Malassezia folliculitis demonstrates only spores.5,7
Candidiasis
One possible outpatient presentation of candidiasis is oral thrush, which can exhibit white mucosal exudate or erythematous patches. A tongue blade can be used to scrape the tongue or cheek wall, with subsequent preparatory steps with application of KOH as described for dermatophytes. Cutaneous candidiasis most often develops in intertriginous regions and will exhibit erosive painful lesions with satellite pustules. In both cases, analysis of the specimen will show shorter fatter hyphal elements than seen in dermatophytosis, with pseudohyphae, blunted ends, and potentially yeast forms.5
Vesiculobullous Lesions
The Tzanck smear has been used since the 1940s to differentiate between etiologies of blistering disorders and is now most commonly used for the quick identification of herpetic lesions.1 The test is performed by scraping the base of a deroofed vesicle, pustule, or bulla, and smearing the cellular materials onto a glass slide. The most commonly utilized media for staining in the outpatient setting at my institution (University of Texas Dell Medical School, Austin) is Giemsa, which is composed of azure II–eosin, glycerin, and methanol. It stains nuclei a reddish blue to pink and the cytoplasm blue.10 After being applied to the slide, the cells are allowed to air-dry for 5 to 10 minutes, and Giemsa stain is subsequently applied and allowed to incubate for 15 minutes, then rinsed carefully with water and directly examined.
Other stains that can be used to perform the Tzanck smear include commercial preparations that may be more accessible in the inpatient settings such as the Wright-Giemsa, Quik-Dip, and Diff-Quick.1,10
Examination of a Tzanck smear from a herpetic lesion will yield acantholytic, enlarged keratinocytes up to twice their usual size (referred to as ballooning degeneration), and multinucleation. In addition, molding of the nuclei to each other within the multinucleated cells and margination of the nuclear chromatin may be appreciated (Figure 2). Intranuclear inclusion bodies, also known as Cowdry type A bodies, can be seen that are nearly the size of red blood cells but are rare to find, with only 10% of specimens exhibiting this finding in a prospective review of 299 patients with herpetic vesiculobullous lesions.11 Evaluation of the contents of blisters caused by bullous pemphigoid and erythema toxicum neonatorum may yield high densities of eosinophils with normal keratinocyte morphology (Figure 3). Other blistering eruptions such as pemphigus vulgaris and bullous drug eruptions also have characteristic findings.1,2


Gout Preparation
Gout is a systemic disease caused by uric acid accumulation that can present with joint pain and white to red nodules on digits, joints, and ears (known as tophi). Material may be expressed from tophi and examined immediately by polarized light microscopy to confirm the diagnosis.5 Specimens will demonstrate needle-shaped, negatively birefringent monosodium urate crystals on polarized light microscopy (Figure 4). An ordinary light microscope can be converted for such use with the lenses of inexpensive polarized sunglasses, placing one lens between the light source and specimen and the other lens between the examiner’s eye and the specimen.12

Parasitic Infections
Two common parasitic infections identified in outpatient dermatology clinics are scabies mites and Demodex mites. Human scabies is extremely pruritic and caused by infestation with Sarcoptes scabiei var hominis; the typical presentation in an adult is erythematous and crusted papules, linear burrows, and vesiculopustules, especially of the interdigital spaces, wrists, axillae, umbilicus, and genital region.1,13 Demodicidosis presents with papules and pustules on the face, usually in a patient with background rosacea and diffuse erythema.1,5,14
If either of these conditions are suspected, mineral oil should be used to prepare the slide because it will maintain viability of the organisms, which are visualized better in motion. Adult scabies mites are roughly 10 times larger than keratinocytes, measuring approximately 250 to 450 µm in length with 8 legs.13 Eggs also may be visualized within the cellular debris and typically are 100 to 150 µm in size and ovoid in shape. Of note, polariscopic examination may be a useful adjunct for evaluation of scabies because scabetic spines and scybala (or fecal material) are polarizable.15
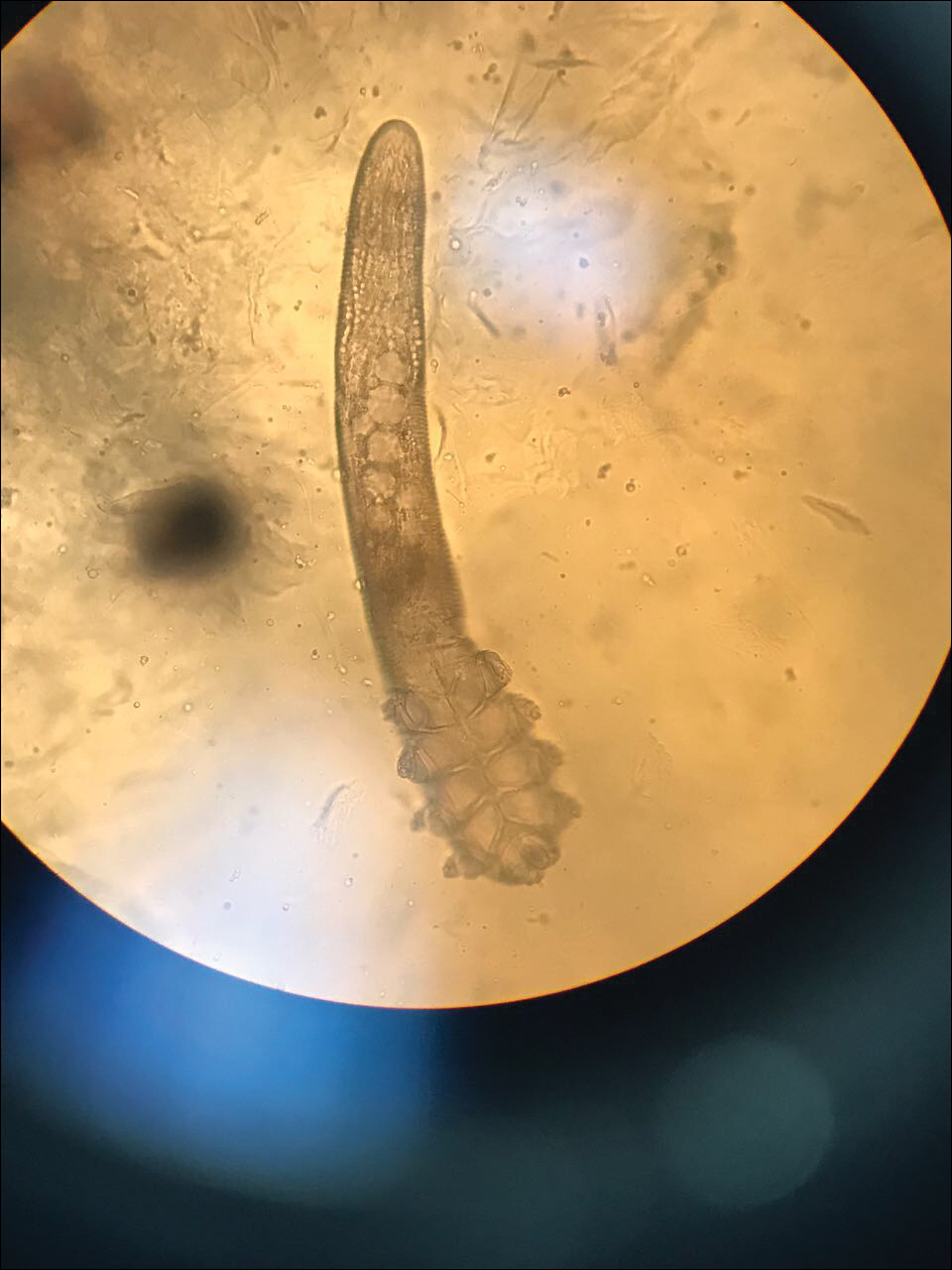
Two types of Demodex mites typically are found in the skin: Demodex folliculorum, which are similarly sized to scabies mites with a more oblong body and occur most commonly in mature hair follicles (eg, eyelashes), and Demodex brevis, which are about half the size (150–200 µm) and live in the sebaceous glands of vellus hairs (Figure 5).14 Both of these mites have 8 legs, similar to the scabies mite.

Hair Preparations
Hair preparations for bulbar examination (eg, trichogram) may prove useful in the evaluation of many types of alopecia, and elaboration on this topic is beyond the scope of this article. Microscopic evaluation of the hair shaft may be an underutilized technique in the outpatient setting and is capable of yielding a variety of diagnoses, including monilethrix, pili torti, and pili trianguli et canaliculi, among others.3 One particularly useful scenario for hair shaft examination (usually of the eyebrow) is in the setting of a patient with severe atopic dermatitis or a baby with ichthyosiform erythroderma, as discovery of trichorrhexis invaginata is pathognomonic for the diagnosis of Netherton syndrome.16 Lastly, evaluation of the hair shaft in patients with patchy and diffuse hair loss whose clinical impression is reminiscent of alopecia areata, or those with concerns of inability to grow hair beyond a short length, may lead to diagnosis of loose anagen syndrome, especially if more than 70% of hair fibers examined exhibit the classic findings of a ruffled proximal cuticle and lack of root sheath.4
Final Thoughts
Bedside microscopy is a rapid and cost-sensitive way to confirm diagnoses that are clinically suspected and remains a valuable tool to acquire during residency training.
Dermatologists are uniquely equipped amongst clinicians to make bedside diagnoses because of the focus on histopathology and microscopy inherent in our training. This skill is highly valuable in both an inpatient and outpatient setting because it may lead to a rapid diagnosis or be a useful adjunct in the initial clinical decision-making process. Although expert microscopists may be able to garner relevant information from scraping almost any type of lesion, bedside microscopy primarily is used by dermatologists in the United States for consideration of infectious etiologies of a variety of cutaneous manifestations.1,2
Basic Principles
Lesions that should be considered for bedside microscopic analysis in outpatient settings are scaly lesions, vesiculobullous lesions, inflammatory papules, and pustules1; microscopic evaluation also can be useful for myriad trichoscopic considerations.3,4 In some instances, direct visualization of the pathogen is possible (eg, cutaneous fungal infections, demodicidosis, scabetic infections), and in other circumstances reactive changes of keratinocytes or the presence of specific cell types can aid in diagnosis (eg, ballooning degeneration and multinucleation of keratinocytes in herpetic lesions, an abundance of eosinophils in erythema toxicum neonatorum). Different types of media are used to best prepare tissue based on the suspected etiology of the condition.
One major stumbling block for residents when beginning to perform bedside testing is the lack of dimensional understanding of the structures they are searching for; for example, medical students and residents often may mistake fibers for dermatophytes, which typically are much larger than fungal hyphae. Familiarizing oneself with the basic dimensions of different cell types or pathogens in relation to each other (Table) will help further refine the beginner’s ability to effectively search for and identify pathogenic features. This concept is further schematized in Figure 1 to help visualize scale differences.

Examination of the Specimen
Slide preparation depends on the primary lesion in consideration and will be discussed in greater detail in the following sections. Once the slide is prepared, place it on the microscope stage and adjust the condenser and light source for optimal visualization. Scan the specimen in a gridlike fashion on low power (usually ×10) and then inspect suspicious findings on higher power (×40 or higher).
Dermatomycoses
Fungal infections of the skin can present as annular papulosquamous lesions, follicular pustules or papules, bullous lesions, hypopigmented patches, and mucosal exudate or erosions, among other manifestations.5 Potassium hydroxide (KOH) is the classic medium used in preparation of lesions being assessed for evidence of fungus because it leads to lysis of keratinocytes for better visualization of fungal hyphae and spores. Other media that contain KOH and additional substrates such as dimethyl sulfoxide or chlorazol black E can be used to better highlight fungal elements.6
Dermatophytosis
Dermatophytes lead to superficial infection of the epidermis and epidermal appendages and present in a variety of ways, including site-specific infections manifesting typically as erythematous, annular or arcuate scaling (eg, tinea faciei, tinea corporis, tinea cruris, tinea manus, tinea pedis), alopecia with broken hair shafts, black dots, boggy nodules and/or scaling of the scalp (eg, tinea capitis, favus, kerion), and dystrophic nails (eg, onychomycosis).5,7 For examination of lesional skin scrapings, one can either use clear cellophane tape against the skin to remove scale, which is especially useful in the case of pediatric patients, and then press the tape against a slide prepared with several drops of a KOH-based medium to directly visualize without a coverslip, or scrape the lesion with a No. 15 blade and place the scales onto the glass slide, with further preparation as described below.8 For assessment of alopecia or dystrophic nails, scrape lesional skin with a No. 15 blade to obtain affected hair follicles and proximal subungual debris, respectively.6,9
Once the cellular debris has been obtained and placed on the slide, a coverslip can be overlaid and KOH applied laterally to be taken up across the slide by capillary action. Allow the slide to sit for at least 5 minutes before analyzing to better visualize fungal elements. Both tinea and onychomycosis will show branching septate hyphae extending across keratinocytes; a common false-positive is identifying overlapping keratinocyte edges, which are a similar size, but they can be distinguished from fungi because they do not cross multiple keratinocytes.1,8 Tinea capitis may demonstrate similar findings or may reveal hair shafts with spores contained within or surrounding it, corresponding to endothrix or ectothrix infection, respectively.5
Pityriasis Versicolor and Malassezia Folliculitis
Pityriasis versicolor presents with hypopigmented to pink, finely scaling ovoid papules, usually on the upper back, shoulders, and neck, and is caused by Malassezia furfur and other Malassezia species.5 Malassezia folliculitis also is caused by this fungus and presents with monomorphic follicular papules and pustules. Scrapings from the scaly papules will demonstrate keratinocytes with the classic “spaghetti and meatballs” fungal elements, whereas Malassezia folliculitis demonstrates only spores.5,7
Candidiasis
One possible outpatient presentation of candidiasis is oral thrush, which can exhibit white mucosal exudate or erythematous patches. A tongue blade can be used to scrape the tongue or cheek wall, with subsequent preparatory steps with application of KOH as described for dermatophytes. Cutaneous candidiasis most often develops in intertriginous regions and will exhibit erosive painful lesions with satellite pustules. In both cases, analysis of the specimen will show shorter fatter hyphal elements than seen in dermatophytosis, with pseudohyphae, blunted ends, and potentially yeast forms.5
Vesiculobullous Lesions
The Tzanck smear has been used since the 1940s to differentiate between etiologies of blistering disorders and is now most commonly used for the quick identification of herpetic lesions.1 The test is performed by scraping the base of a deroofed vesicle, pustule, or bulla, and smearing the cellular materials onto a glass slide. The most commonly utilized media for staining in the outpatient setting at my institution (University of Texas Dell Medical School, Austin) is Giemsa, which is composed of azure II–eosin, glycerin, and methanol. It stains nuclei a reddish blue to pink and the cytoplasm blue.10 After being applied to the slide, the cells are allowed to air-dry for 5 to 10 minutes, and Giemsa stain is subsequently applied and allowed to incubate for 15 minutes, then rinsed carefully with water and directly examined.
Other stains that can be used to perform the Tzanck smear include commercial preparations that may be more accessible in the inpatient settings such as the Wright-Giemsa, Quik-Dip, and Diff-Quick.1,10
Examination of a Tzanck smear from a herpetic lesion will yield acantholytic, enlarged keratinocytes up to twice their usual size (referred to as ballooning degeneration), and multinucleation. In addition, molding of the nuclei to each other within the multinucleated cells and margination of the nuclear chromatin may be appreciated (Figure 2). Intranuclear inclusion bodies, also known as Cowdry type A bodies, can be seen that are nearly the size of red blood cells but are rare to find, with only 10% of specimens exhibiting this finding in a prospective review of 299 patients with herpetic vesiculobullous lesions.11 Evaluation of the contents of blisters caused by bullous pemphigoid and erythema toxicum neonatorum may yield high densities of eosinophils with normal keratinocyte morphology (Figure 3). Other blistering eruptions such as pemphigus vulgaris and bullous drug eruptions also have characteristic findings.1,2


Gout Preparation
Gout is a systemic disease caused by uric acid accumulation that can present with joint pain and white to red nodules on digits, joints, and ears (known as tophi). Material may be expressed from tophi and examined immediately by polarized light microscopy to confirm the diagnosis.5 Specimens will demonstrate needle-shaped, negatively birefringent monosodium urate crystals on polarized light microscopy (Figure 4). An ordinary light microscope can be converted for such use with the lenses of inexpensive polarized sunglasses, placing one lens between the light source and specimen and the other lens between the examiner’s eye and the specimen.12

Parasitic Infections
Two common parasitic infections identified in outpatient dermatology clinics are scabies mites and Demodex mites. Human scabies is extremely pruritic and caused by infestation with Sarcoptes scabiei var hominis; the typical presentation in an adult is erythematous and crusted papules, linear burrows, and vesiculopustules, especially of the interdigital spaces, wrists, axillae, umbilicus, and genital region.1,13 Demodicidosis presents with papules and pustules on the face, usually in a patient with background rosacea and diffuse erythema.1,5,14
If either of these conditions are suspected, mineral oil should be used to prepare the slide because it will maintain viability of the organisms, which are visualized better in motion. Adult scabies mites are roughly 10 times larger than keratinocytes, measuring approximately 250 to 450 µm in length with 8 legs.13 Eggs also may be visualized within the cellular debris and typically are 100 to 150 µm in size and ovoid in shape. Of note, polariscopic examination may be a useful adjunct for evaluation of scabies because scabetic spines and scybala (or fecal material) are polarizable.15
Two types of Demodex mites typically are found in the skin: Demodex folliculorum, which are similarly sized to scabies mites with a more oblong body and occur most commonly in mature hair follicles (eg, eyelashes), and Demodex brevis, which are about half the size (150–200 µm) and live in the sebaceous glands of vellus hairs (Figure 5).14 Both of these mites have 8 legs, similar to the scabies mite.

Hair Preparations
Hair preparations for bulbar examination (eg, trichogram) may prove useful in the evaluation of many types of alopecia, and elaboration on this topic is beyond the scope of this article. Microscopic evaluation of the hair shaft may be an underutilized technique in the outpatient setting and is capable of yielding a variety of diagnoses, including monilethrix, pili torti, and pili trianguli et canaliculi, among others.3 One particularly useful scenario for hair shaft examination (usually of the eyebrow) is in the setting of a patient with severe atopic dermatitis or a baby with ichthyosiform erythroderma, as discovery of trichorrhexis invaginata is pathognomonic for the diagnosis of Netherton syndrome.16 Lastly, evaluation of the hair shaft in patients with patchy and diffuse hair loss whose clinical impression is reminiscent of alopecia areata, or those with concerns of inability to grow hair beyond a short length, may lead to diagnosis of loose anagen syndrome, especially if more than 70% of hair fibers examined exhibit the classic findings of a ruffled proximal cuticle and lack of root sheath.4
Final Thoughts
Bedside microscopy is a rapid and cost-sensitive way to confirm diagnoses that are clinically suspected and remains a valuable tool to acquire during residency training.
- Wanat KA, Dominguez AR, Carter Z, et al. Bedside diagnostics in dermatology: viral, bacterial, and fungal infections. J Am Acad Dermatol. 2017;77:197-218.
- Micheletti RG, Dominguez AR, Wanat KA. Bedside diagnostics in dermatology: parasitic and noninfectious diseases. J Am Acad Dermatol. 2017;77:221-230.
- Whiting DA, Dy LC. Office diagnosis of hair shaft defects. Semin Cutan Med Surg. 2006;25:24-34.
- Tosti A. Loose anagen hair syndrome and loose anagen hair. Arch Dermatol. 2002;138:521-522.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia PA: Elsevier; 2017.
- Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;55:620-626.
- Elder DE, ed. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009.
- Raghukumar S, Ravikumar BC. Potassium hydroxide mount with cellophane adhesive: a method for direct diagnosis of dermatophyte skin infections [published online May 29, 2018]. Clin Exp Dermatol. doi:10.1111/ced.13573.
- Bhat YJ, Zeerak S, Kanth F, et al. Clinicoepidemiological and mycological study of tinea capitis in the pediatric population of Kashmir Valley: a study from a tertiary care centre. Indian Dermatol Online J. 2017;8:100-103.
- Gupta LK, Singhi MK. Tzanck smear: a useful diagnostic tool. Indian J Dermatol Venereol Leprol. 2005;71:295-299.
- Durdu M, Baba M, Seçkin D. The value of Tzanck smear test in diagnosis of erosive, vesicular, bullous, and pustular skin lesions. J Am Acad Dermatol. 2008;59:958-964.
- Fagan TJ, Lidsky MD. Compensated polarized light microscopy using cellophane adhesive tape. Arthritis Rheum. 1974;17:256-262.
- Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007;20:268-279.
- Desch C, Nutting WB. Demodex folliculorum (Simon) and D. brevis akbulatova of man: redescription and reevaluation. J Parasitol. 1972;58:169-177.
- Foo CW, Florell SR, Bowen AR. Polarizable elements in scabies infestation: a clue to diagnosis. J Cutan Pathol. 2013;40:6-10.
- Akkurt ZM, Tuncel T, Ayhan E, et al. Rapid and easy diagnosis of Netherton syndrome with dermoscopy. J Cutan Med Surg. 2014;18:280-282.
- Wanat KA, Dominguez AR, Carter Z, et al. Bedside diagnostics in dermatology: viral, bacterial, and fungal infections. J Am Acad Dermatol. 2017;77:197-218.
- Micheletti RG, Dominguez AR, Wanat KA. Bedside diagnostics in dermatology: parasitic and noninfectious diseases. J Am Acad Dermatol. 2017;77:221-230.
- Whiting DA, Dy LC. Office diagnosis of hair shaft defects. Semin Cutan Med Surg. 2006;25:24-34.
- Tosti A. Loose anagen hair syndrome and loose anagen hair. Arch Dermatol. 2002;138:521-522.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia PA: Elsevier; 2017.
- Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;55:620-626.
- Elder DE, ed. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009.
- Raghukumar S, Ravikumar BC. Potassium hydroxide mount with cellophane adhesive: a method for direct diagnosis of dermatophyte skin infections [published online May 29, 2018]. Clin Exp Dermatol. doi:10.1111/ced.13573.
- Bhat YJ, Zeerak S, Kanth F, et al. Clinicoepidemiological and mycological study of tinea capitis in the pediatric population of Kashmir Valley: a study from a tertiary care centre. Indian Dermatol Online J. 2017;8:100-103.
- Gupta LK, Singhi MK. Tzanck smear: a useful diagnostic tool. Indian J Dermatol Venereol Leprol. 2005;71:295-299.
- Durdu M, Baba M, Seçkin D. The value of Tzanck smear test in diagnosis of erosive, vesicular, bullous, and pustular skin lesions. J Am Acad Dermatol. 2008;59:958-964.
- Fagan TJ, Lidsky MD. Compensated polarized light microscopy using cellophane adhesive tape. Arthritis Rheum. 1974;17:256-262.
- Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007;20:268-279.
- Desch C, Nutting WB. Demodex folliculorum (Simon) and D. brevis akbulatova of man: redescription and reevaluation. J Parasitol. 1972;58:169-177.
- Foo CW, Florell SR, Bowen AR. Polarizable elements in scabies infestation: a clue to diagnosis. J Cutan Pathol. 2013;40:6-10.
- Akkurt ZM, Tuncel T, Ayhan E, et al. Rapid and easy diagnosis of Netherton syndrome with dermoscopy. J Cutan Med Surg. 2014;18:280-282.
Establishing Financial Literacy: What Every Resident Needs to Know
The average debt of graduating medical students today is $190,000, which has increased from $32,000 since 1986 (or the equivalent of $70,000 in 2017 dollars when adjusted for inflation).1 This fact is especially disconcerting given that medical trainees and professionals are not known for being financially sophisticated, and rising levels of high-interest educational debt, increasing years of training, and stagnant or decreasing physician salaries make this status quo untenable.2 Building foundational financial literacy and establishing good financial practices should start during medical school and residency; these basics are a crucial component of long-term job satisfaction and professional resilience.
One prominent physician finance writer advocates that residents should consider the following 5 big-ticket financial steps: acquire life and disability insurance, open a Roth IRA, engage yearly in some type of financial education, and learn about billing and coding in your specialty.3 These exercises, except life insurance for a resident without dependents, are all nonnegotiable, yet alone are insufficient actions to build a solid financial foundation. The purpose of this article is to address additional steps every resident should take, including establishing a workable budget, learning how and why to calculate net worth yearly, determining what percentage of income to save for retirement and basic investing strategies, and managing student loans.
Establish a Workable Budget
Living on a budget is a form of reality acceptance. It may feel impossible to save or budget on a resident salary, but residents earn approximately the median US household income of $59,039, according to the US Census Bureau from September 2017.4,5 There are many tools that can be used to create a budget and to track monthly expenses. However, the simplest way to budget is to pay yourself first with automatic deductions to retirement and savings accounts as well as automated bill payments. Making a habit of reviewing all expenses at the end of every month allows you to see if expenditures remain aligned to your personal values and to reallocate funds for the upcoming month if they are not.
Calculate Net Worth Yearly
Calculating personal net worth may appear to be a discouraging activity to advocate for residents, as many will have a negative 6-figure net worth. The purpose is two-fold: Firstly, to compel you to become well acquainted with your varying types of debt and their respective interest rates. Secondly, similar to taking serial photographs of vitiligo patients to monitor for improvement, it may be the only thing in a long slow slog that indicates beneficial change is occurring because small daily efforts over time yield surprisingly impressive results and the calculation factors in both debt repayment and contributions to all savings vehicles. An example of a simplified method to calculate net worth is demonstrated in the Table.
Understand Your Retirement Account and Asset Distribution
Contributing to a retirement account should start day 1 of intern year. A simple rule of thumb to estimate how much money you need to save for retirement is to divide how much you expect to spend on a yearly basis by 4%. For example, if you anticipate spending $80,000 per year during retirement, you will need $2 million in savings (0.04×$2,000,000=$80,000). The amount saved depends on the aggressiveness of your financial goals, but it should be a minimum of 10% to 15% of income during residency and at least 20% afterwards. This strategy allows even a resident to save $25,000 to $50,000 over a 4-year period (depending on employer match), which can accrue additional value in the stock market. One advantage of contributing to an employer-based retirement account, which usually is a 403(b) plan for residents, is that it lowers your tax burden for the year because the savings are tax deferred, in contrast to a Roth IRA, which is funded with posttax dollars. Roth accounts often are recommended for residents because contributions are made during a period in which the physician is presumably in the lowest tax bracket, as account earnings and withdrawals from a Roth IRA after 59.5 years of age, when most physicians expect to be in a higher tax bracket, are tax free. Another advantage of contributing to a 403(b) account is that many residency programs offer a match, which provides for an immediate and substantial return on invested money. Because most residents do not have the cash flow to fully fund both a Roth IRA and 403(b) account (2018 contribution limits are $5500 and $18,500, respectively),6,7 one strategy to utilize both is to save enough to the 403(b) to capture the employer match and place whatever additional savings you can afford into the Roth IRA.
Many different investment strategies exist, and a thorough discussion of them is beyond the scope of this article. Simply speaking, there are 4 major asset classes in which to invest: US stocks, foreign stocks, real estate, and bonds. The variation of recommended contributions to each asset is limitless, and every resident should spend time considering the best strategy for his/her goals. One example of a simple effective investing strategy is to utilize index funds, which track the market and therefore rise with the market, as they tend to go up (at least historically, though temporary setbacks occur).8 If you are investing in funds available through your employer-sponsored retirement account, examine the funds you are automatically assigned and their associated fee and expense ratio (ER) disclosures, which are typically available through the online portal. A general rule of thumb is that good funds have ERs of less than 0.5% and bad funds have ERs greater than 1% and additional associated fees. The funds available to you also can be researched on the Morningstar, Inc, website (www.morningstar.com). My institution (University of Texas Dell Medical School, Austin) offers a variety of options with ERs varying from 0.02% to 1.02%. The difference in the costs associated with these funds over decades is notable, and it pays (literally) to understand the nuances. Reallocation of funds usually can be done easily online and are effective within 24 hours.
Student Loans
Although many residents agonize most over management of student loans, the simple solution is do not defer them. Refinancing federal loans with a private company versus enrolling in an income-based repayment program depends on many factors, including whether you have a high-earning spouse, how many dependents you have, and whether you expect to stay in academia and will be eligible for Public Service Loan Forgiveness, among others. Look critically at your situation and likely future employment to decide what is most appropriate for you; doing so can save you thousands of dollars in interest over the course of your residency.
Final Thoughts
To the detriment of residents and the attending physicians they will become, discussing financial matters in medicine remains rare, perhaps because it seems to shift what should be the singular focus of our profession, namely to help the sick, to thoughts of personal gain, which is a false dichotomy. Unquestionably, the physician’s role that supersedes all others is to care for the patient and to honor the oath we all took: “Into whatsoever houses I enter, I will enter to help the sick.” But this commitment should not preclude the mastery of financial concepts that promote personal and professional health and well-being. After all, the joy in work is maximized when you are not enslaved to it.
Your reading assignment, paper revision, or presentation can wait. Making time to understand your current financial health, to build your own financial literacy, and to plan for your future is an important component of a long satisfying career. Start now.
- Grischkan J, George BP, Chaiyachati K, et al. Distribution of medical education debt by specialty, 2010-2016. JAMA Intern Med. 2017;177:1532-1535.
- Ahmad FA, White AJ, Hiller KM, et al. An assessment of residents’ and fellows’ personal finance literacy: an unmet medical education need. Int J Med Educ. 2017;8:192-204.
- The five big money items you should do as a resident. The White Coat Investor website. https://www.whitecoatinvestor.com/the-five-big-money-items-you-should-do-as-a-resident. Published July 7, 2011. Accessed May 14, 2018.
- Income, poverty and health insurance coverage in the United States: 2016. United States Census Bureau website. https://www.census.gov/newsroom/press-releases/2017/income-povery.html. Published September 12, 2017. Accessed May 14, 2018.
- Levy S. Residents salary and debt report 2017. Medscape website. https://www.medscape.com/slideshow/residents-salary-and-debt-report-2017-6008931. Published July 26, 2017. Accessed May 22, 2018.
- Retirement topics - IRA contribution limits. Internal Revenue Service website. https://www.irs.gov/retirement-plans/plan-participant-employee/retirement-topics-ira-contribution-limits. Updated October 20, 2017. Accessed May 22, 2018.
- Retirement plan FAQs regarding 403(b) tax-sheltered annuity plans. Internal Revenue Service website. https://www.irs.gov/retirement-plans/retirement-plans-faqs-regarding-403b-tax-sheltered-annuity-plans#conts. Updated November 14, 2017. Accessed May 22, 2018.
- Collins JL. Stock series. JLCollins website. http://jlcollinsnh.com/stock-series/. Accessed May 14, 2018.
The average debt of graduating medical students today is $190,000, which has increased from $32,000 since 1986 (or the equivalent of $70,000 in 2017 dollars when adjusted for inflation).1 This fact is especially disconcerting given that medical trainees and professionals are not known for being financially sophisticated, and rising levels of high-interest educational debt, increasing years of training, and stagnant or decreasing physician salaries make this status quo untenable.2 Building foundational financial literacy and establishing good financial practices should start during medical school and residency; these basics are a crucial component of long-term job satisfaction and professional resilience.
One prominent physician finance writer advocates that residents should consider the following 5 big-ticket financial steps: acquire life and disability insurance, open a Roth IRA, engage yearly in some type of financial education, and learn about billing and coding in your specialty.3 These exercises, except life insurance for a resident without dependents, are all nonnegotiable, yet alone are insufficient actions to build a solid financial foundation. The purpose of this article is to address additional steps every resident should take, including establishing a workable budget, learning how and why to calculate net worth yearly, determining what percentage of income to save for retirement and basic investing strategies, and managing student loans.
Establish a Workable Budget
Living on a budget is a form of reality acceptance. It may feel impossible to save or budget on a resident salary, but residents earn approximately the median US household income of $59,039, according to the US Census Bureau from September 2017.4,5 There are many tools that can be used to create a budget and to track monthly expenses. However, the simplest way to budget is to pay yourself first with automatic deductions to retirement and savings accounts as well as automated bill payments. Making a habit of reviewing all expenses at the end of every month allows you to see if expenditures remain aligned to your personal values and to reallocate funds for the upcoming month if they are not.
Calculate Net Worth Yearly
Calculating personal net worth may appear to be a discouraging activity to advocate for residents, as many will have a negative 6-figure net worth. The purpose is two-fold: Firstly, to compel you to become well acquainted with your varying types of debt and their respective interest rates. Secondly, similar to taking serial photographs of vitiligo patients to monitor for improvement, it may be the only thing in a long slow slog that indicates beneficial change is occurring because small daily efforts over time yield surprisingly impressive results and the calculation factors in both debt repayment and contributions to all savings vehicles. An example of a simplified method to calculate net worth is demonstrated in the Table.
Understand Your Retirement Account and Asset Distribution
Contributing to a retirement account should start day 1 of intern year. A simple rule of thumb to estimate how much money you need to save for retirement is to divide how much you expect to spend on a yearly basis by 4%. For example, if you anticipate spending $80,000 per year during retirement, you will need $2 million in savings (0.04×$2,000,000=$80,000). The amount saved depends on the aggressiveness of your financial goals, but it should be a minimum of 10% to 15% of income during residency and at least 20% afterwards. This strategy allows even a resident to save $25,000 to $50,000 over a 4-year period (depending on employer match), which can accrue additional value in the stock market. One advantage of contributing to an employer-based retirement account, which usually is a 403(b) plan for residents, is that it lowers your tax burden for the year because the savings are tax deferred, in contrast to a Roth IRA, which is funded with posttax dollars. Roth accounts often are recommended for residents because contributions are made during a period in which the physician is presumably in the lowest tax bracket, as account earnings and withdrawals from a Roth IRA after 59.5 years of age, when most physicians expect to be in a higher tax bracket, are tax free. Another advantage of contributing to a 403(b) account is that many residency programs offer a match, which provides for an immediate and substantial return on invested money. Because most residents do not have the cash flow to fully fund both a Roth IRA and 403(b) account (2018 contribution limits are $5500 and $18,500, respectively),6,7 one strategy to utilize both is to save enough to the 403(b) to capture the employer match and place whatever additional savings you can afford into the Roth IRA.
Many different investment strategies exist, and a thorough discussion of them is beyond the scope of this article. Simply speaking, there are 4 major asset classes in which to invest: US stocks, foreign stocks, real estate, and bonds. The variation of recommended contributions to each asset is limitless, and every resident should spend time considering the best strategy for his/her goals. One example of a simple effective investing strategy is to utilize index funds, which track the market and therefore rise with the market, as they tend to go up (at least historically, though temporary setbacks occur).8 If you are investing in funds available through your employer-sponsored retirement account, examine the funds you are automatically assigned and their associated fee and expense ratio (ER) disclosures, which are typically available through the online portal. A general rule of thumb is that good funds have ERs of less than 0.5% and bad funds have ERs greater than 1% and additional associated fees. The funds available to you also can be researched on the Morningstar, Inc, website (www.morningstar.com). My institution (University of Texas Dell Medical School, Austin) offers a variety of options with ERs varying from 0.02% to 1.02%. The difference in the costs associated with these funds over decades is notable, and it pays (literally) to understand the nuances. Reallocation of funds usually can be done easily online and are effective within 24 hours.
Student Loans
Although many residents agonize most over management of student loans, the simple solution is do not defer them. Refinancing federal loans with a private company versus enrolling in an income-based repayment program depends on many factors, including whether you have a high-earning spouse, how many dependents you have, and whether you expect to stay in academia and will be eligible for Public Service Loan Forgiveness, among others. Look critically at your situation and likely future employment to decide what is most appropriate for you; doing so can save you thousands of dollars in interest over the course of your residency.
Final Thoughts
To the detriment of residents and the attending physicians they will become, discussing financial matters in medicine remains rare, perhaps because it seems to shift what should be the singular focus of our profession, namely to help the sick, to thoughts of personal gain, which is a false dichotomy. Unquestionably, the physician’s role that supersedes all others is to care for the patient and to honor the oath we all took: “Into whatsoever houses I enter, I will enter to help the sick.” But this commitment should not preclude the mastery of financial concepts that promote personal and professional health and well-being. After all, the joy in work is maximized when you are not enslaved to it.
Your reading assignment, paper revision, or presentation can wait. Making time to understand your current financial health, to build your own financial literacy, and to plan for your future is an important component of a long satisfying career. Start now.
The average debt of graduating medical students today is $190,000, which has increased from $32,000 since 1986 (or the equivalent of $70,000 in 2017 dollars when adjusted for inflation).1 This fact is especially disconcerting given that medical trainees and professionals are not known for being financially sophisticated, and rising levels of high-interest educational debt, increasing years of training, and stagnant or decreasing physician salaries make this status quo untenable.2 Building foundational financial literacy and establishing good financial practices should start during medical school and residency; these basics are a crucial component of long-term job satisfaction and professional resilience.
One prominent physician finance writer advocates that residents should consider the following 5 big-ticket financial steps: acquire life and disability insurance, open a Roth IRA, engage yearly in some type of financial education, and learn about billing and coding in your specialty.3 These exercises, except life insurance for a resident without dependents, are all nonnegotiable, yet alone are insufficient actions to build a solid financial foundation. The purpose of this article is to address additional steps every resident should take, including establishing a workable budget, learning how and why to calculate net worth yearly, determining what percentage of income to save for retirement and basic investing strategies, and managing student loans.
Establish a Workable Budget
Living on a budget is a form of reality acceptance. It may feel impossible to save or budget on a resident salary, but residents earn approximately the median US household income of $59,039, according to the US Census Bureau from September 2017.4,5 There are many tools that can be used to create a budget and to track monthly expenses. However, the simplest way to budget is to pay yourself first with automatic deductions to retirement and savings accounts as well as automated bill payments. Making a habit of reviewing all expenses at the end of every month allows you to see if expenditures remain aligned to your personal values and to reallocate funds for the upcoming month if they are not.
Calculate Net Worth Yearly
Calculating personal net worth may appear to be a discouraging activity to advocate for residents, as many will have a negative 6-figure net worth. The purpose is two-fold: Firstly, to compel you to become well acquainted with your varying types of debt and their respective interest rates. Secondly, similar to taking serial photographs of vitiligo patients to monitor for improvement, it may be the only thing in a long slow slog that indicates beneficial change is occurring because small daily efforts over time yield surprisingly impressive results and the calculation factors in both debt repayment and contributions to all savings vehicles. An example of a simplified method to calculate net worth is demonstrated in the Table.
Understand Your Retirement Account and Asset Distribution
Contributing to a retirement account should start day 1 of intern year. A simple rule of thumb to estimate how much money you need to save for retirement is to divide how much you expect to spend on a yearly basis by 4%. For example, if you anticipate spending $80,000 per year during retirement, you will need $2 million in savings (0.04×$2,000,000=$80,000). The amount saved depends on the aggressiveness of your financial goals, but it should be a minimum of 10% to 15% of income during residency and at least 20% afterwards. This strategy allows even a resident to save $25,000 to $50,000 over a 4-year period (depending on employer match), which can accrue additional value in the stock market. One advantage of contributing to an employer-based retirement account, which usually is a 403(b) plan for residents, is that it lowers your tax burden for the year because the savings are tax deferred, in contrast to a Roth IRA, which is funded with posttax dollars. Roth accounts often are recommended for residents because contributions are made during a period in which the physician is presumably in the lowest tax bracket, as account earnings and withdrawals from a Roth IRA after 59.5 years of age, when most physicians expect to be in a higher tax bracket, are tax free. Another advantage of contributing to a 403(b) account is that many residency programs offer a match, which provides for an immediate and substantial return on invested money. Because most residents do not have the cash flow to fully fund both a Roth IRA and 403(b) account (2018 contribution limits are $5500 and $18,500, respectively),6,7 one strategy to utilize both is to save enough to the 403(b) to capture the employer match and place whatever additional savings you can afford into the Roth IRA.
Many different investment strategies exist, and a thorough discussion of them is beyond the scope of this article. Simply speaking, there are 4 major asset classes in which to invest: US stocks, foreign stocks, real estate, and bonds. The variation of recommended contributions to each asset is limitless, and every resident should spend time considering the best strategy for his/her goals. One example of a simple effective investing strategy is to utilize index funds, which track the market and therefore rise with the market, as they tend to go up (at least historically, though temporary setbacks occur).8 If you are investing in funds available through your employer-sponsored retirement account, examine the funds you are automatically assigned and their associated fee and expense ratio (ER) disclosures, which are typically available through the online portal. A general rule of thumb is that good funds have ERs of less than 0.5% and bad funds have ERs greater than 1% and additional associated fees. The funds available to you also can be researched on the Morningstar, Inc, website (www.morningstar.com). My institution (University of Texas Dell Medical School, Austin) offers a variety of options with ERs varying from 0.02% to 1.02%. The difference in the costs associated with these funds over decades is notable, and it pays (literally) to understand the nuances. Reallocation of funds usually can be done easily online and are effective within 24 hours.
Student Loans
Although many residents agonize most over management of student loans, the simple solution is do not defer them. Refinancing federal loans with a private company versus enrolling in an income-based repayment program depends on many factors, including whether you have a high-earning spouse, how many dependents you have, and whether you expect to stay in academia and will be eligible for Public Service Loan Forgiveness, among others. Look critically at your situation and likely future employment to decide what is most appropriate for you; doing so can save you thousands of dollars in interest over the course of your residency.
Final Thoughts
To the detriment of residents and the attending physicians they will become, discussing financial matters in medicine remains rare, perhaps because it seems to shift what should be the singular focus of our profession, namely to help the sick, to thoughts of personal gain, which is a false dichotomy. Unquestionably, the physician’s role that supersedes all others is to care for the patient and to honor the oath we all took: “Into whatsoever houses I enter, I will enter to help the sick.” But this commitment should not preclude the mastery of financial concepts that promote personal and professional health and well-being. After all, the joy in work is maximized when you are not enslaved to it.
Your reading assignment, paper revision, or presentation can wait. Making time to understand your current financial health, to build your own financial literacy, and to plan for your future is an important component of a long satisfying career. Start now.
- Grischkan J, George BP, Chaiyachati K, et al. Distribution of medical education debt by specialty, 2010-2016. JAMA Intern Med. 2017;177:1532-1535.
- Ahmad FA, White AJ, Hiller KM, et al. An assessment of residents’ and fellows’ personal finance literacy: an unmet medical education need. Int J Med Educ. 2017;8:192-204.
- The five big money items you should do as a resident. The White Coat Investor website. https://www.whitecoatinvestor.com/the-five-big-money-items-you-should-do-as-a-resident. Published July 7, 2011. Accessed May 14, 2018.
- Income, poverty and health insurance coverage in the United States: 2016. United States Census Bureau website. https://www.census.gov/newsroom/press-releases/2017/income-povery.html. Published September 12, 2017. Accessed May 14, 2018.
- Levy S. Residents salary and debt report 2017. Medscape website. https://www.medscape.com/slideshow/residents-salary-and-debt-report-2017-6008931. Published July 26, 2017. Accessed May 22, 2018.
- Retirement topics - IRA contribution limits. Internal Revenue Service website. https://www.irs.gov/retirement-plans/plan-participant-employee/retirement-topics-ira-contribution-limits. Updated October 20, 2017. Accessed May 22, 2018.
- Retirement plan FAQs regarding 403(b) tax-sheltered annuity plans. Internal Revenue Service website. https://www.irs.gov/retirement-plans/retirement-plans-faqs-regarding-403b-tax-sheltered-annuity-plans#conts. Updated November 14, 2017. Accessed May 22, 2018.
- Collins JL. Stock series. JLCollins website. http://jlcollinsnh.com/stock-series/. Accessed May 14, 2018.
- Grischkan J, George BP, Chaiyachati K, et al. Distribution of medical education debt by specialty, 2010-2016. JAMA Intern Med. 2017;177:1532-1535.
- Ahmad FA, White AJ, Hiller KM, et al. An assessment of residents’ and fellows’ personal finance literacy: an unmet medical education need. Int J Med Educ. 2017;8:192-204.
- The five big money items you should do as a resident. The White Coat Investor website. https://www.whitecoatinvestor.com/the-five-big-money-items-you-should-do-as-a-resident. Published July 7, 2011. Accessed May 14, 2018.
- Income, poverty and health insurance coverage in the United States: 2016. United States Census Bureau website. https://www.census.gov/newsroom/press-releases/2017/income-povery.html. Published September 12, 2017. Accessed May 14, 2018.
- Levy S. Residents salary and debt report 2017. Medscape website. https://www.medscape.com/slideshow/residents-salary-and-debt-report-2017-6008931. Published July 26, 2017. Accessed May 22, 2018.
- Retirement topics - IRA contribution limits. Internal Revenue Service website. https://www.irs.gov/retirement-plans/plan-participant-employee/retirement-topics-ira-contribution-limits. Updated October 20, 2017. Accessed May 22, 2018.
- Retirement plan FAQs regarding 403(b) tax-sheltered annuity plans. Internal Revenue Service website. https://www.irs.gov/retirement-plans/retirement-plans-faqs-regarding-403b-tax-sheltered-annuity-plans#conts. Updated November 14, 2017. Accessed May 22, 2018.
- Collins JL. Stock series. JLCollins website. http://jlcollinsnh.com/stock-series/. Accessed May 14, 2018.
Climate Change and Skin Disease
The term climate refers to the average weather conditions of a specific geographic location measured over several decades.1 While a certain degree of variation in the Earth’s climate is expected, a persistent warming or cooling trend is not. The factors driving the Earth’s warming remain difficult to prove.2 We know the Earth previously has undergone dramatic climate changes and that natural factors driving these changes are varied (eg, the relationship between the Earth and the Sun, volcanic eruptions, solar irradiance).1,3 These factors ideally change over protracted periods of time in a way that allows organisms to adapt to new environments.
Anthropogenic climate change refers to human-caused climate change. This is thought to be a major driving factor in the Earth’s recent warming trend, partly due to the rapidity of warming in recent years.3 According to climate scientists, the Earth’s temperature has risen 4°C to 7°C over the past 5000 years, but it has risen 0.7°C in just the past 100 years alone.4 Greenhouse gases such as carbon dioxide are emitted by various natural processes and human activities and play a central role in current warming because they trap solar heat and increase ambient temperature.3
In a recent edition of the commonly cited textbook Dermatology, Bolognia et al5 referenced climate change only once in a figure legend regarding the expansion of dengue fever in the Americas. However, climate change may have the potential to cause outright skin disease epidemics worldwide, and the Climate Change Committee of the International Society of Dermatology has called upon dermatologists across the globe to help raise awareness of this issue.6
Much of the literature regarding the effects of climate change on human health focuses on insect-borne diseases, but over the past decade other areas of impact also have been investigated, such as increases in airborne diseases, zoonoses, newly endemic saprophytic and dimorphic fungal infections, fecal-oral diseases, and severe allergic disease.7,8 It is postulated that climate change leads to region-specific increases in human disease because it creates newly favorable habitats for infectious agents, their vectors. and their reservoirs, allowing expansion of their ranges and access to immunologically naïve populations.9 Furthermore, extreme weather events such as heat waves, hurricanes, and flooding, which are expected to increase in frequency as a result of climate change, have all been linked to infectious disease outbreaks.10
Lyme Disease
In the past 20 years, Lyme disease incidence has tripled in the United States.11 It has been hypothesized that the increase may be occurring as a result of the expanding geographic distribution of the Ixodes tick and its mammalian hosts (eg, white-tailed deer) under the influence of climate change.12 Lyme disease is a multisystem disease affecting the skin, joints, heart, and nervous system. Its most characteristic manifestation is cutaneous in the form of erythema migrans. Dermatologists may be called upon to play an increasingly important role in early detection and treatment of this potentially chronic and debilitating condition.
Arboviruses
Arboviruses are transmitted by arthropods and are an important category of climate change–related diseases due to the expansion of the mosquito habitat worldwide. The vectorial capacity for the transmission of dengue fever has increased worldwide by 9.4% via Aedes aegypti and 11.1% via Aedes albopictus since 1950.13 Dengue fever, also known as breakbone fever, presents with intense joint pain, fevers, headaches, and a transient morbilliform rash that desquamates with defervescence and in some cases will incite hemorrhagic skin lesions.14 Dengue fever previously was considered to be a tropical disease but locally acquired cases have been reported in the United States, including Texas, Hawaii, and Florida.15,16
Reports of local transmission of chikungunya, another arbovirus transmitted by A albopictus and A aegypti mosquitoes in Florida, the US Virgin Islands, and Puerto Rico, began in 2014.17 A higher prevalence of these diseases within the United States also may be related to increased globalization, with US travelers returning from endemic regions with infections. Prior to 2014, transmission occurred in traditional endemic regions, primarily in Asia, Africa, or island nations in the Indian Ocean. Like dengue fever, chikungunya causes high fevers, cutaneous manifestations (eg, urticarial papules, morbilliform eruption, hypermelanosis, intertriginous lesions, lymphedema),17 and intense joint pain. Unlike dengue fever, however, joint involvement can be chronic, erosive, and debilitating.
Lastly, New World leishmaniasis, an arboviral disease characterized by mucocutaneous ulcers and transmitted by phlebotomine sand flies, has been acquired locally in Oklahoma and Texas when it was previously considered to be endemic to Mexico and Central and South America.14,18 The habitats of New World Leishmania species are expected to expand northward, with an ecological niche model predicting that they reach southern Canada by the year 2080 due to the expanding habitats of sand fly and rodent vectors.19
Fungal Infections
In the Pacific Northwest, there have been reports of newly endemic Cryptococcus gattii and Coccidioides immitis, both of which previously had been confined to the southwestern United States.8 Endemic ranges of these mycotic pathogens may be expanding for a variety of reasons, with climate change creating new regions conducive to the colonization of these species.8,20,21Coccidioides immitis is a soil-dwelling fungus that usually presents with primary pulmonary disease that can disseminate acutely or even months later. Prompt recognition of disseminated disease may allow life-saving therapy to be initiated. Cryptococcus gattii is a fungus with multiple niches, including oil, trees, and birds.20 This fungus also is acquired via inhalation, with dissemination occurring most commonly in immunosuppressed patients to the central nervous system, bone, and skin. Primary or secondary infection with both of these fungi may present with cutaneous manifestations presenting as polymorphous lesions, including umbilicated or ulcerated papules, indurated nodules, and acneiform pustules.
Final Thoughts
Awareness of the shifting habitats of microorganisms and vectors locally is important in order for clinicians to make correct diagnoses in a timely fashion. Regional or endemic diseases are presenting outside their traditional boundaries due to changing habitats of microbes and vectors and may be easily overlooked, resulting in a delayed diagnosis. Being prepared to diagnose diseases with increasing incidence secondary to climate change and discussing this with patients is an important physician obligation, but it is not the only one. We cannot effectively advocate for the health of patients and the community while ignoring the destruction of the environment. Our additional responsibility is straightforward—being advocates for good stewardship of the Earth’s resources now on both a personal and a policy level.22
- Climate Central. Global Weirdness: Severe Storms, Deadly Heat Waves, Relentless Drought, Rising Seas, and the Weather of the Future. New York, NY: Pantheon Books; 2012.
- Cook J, Nuccitelli D, Green SA, et al. Quantifying the consensus on anthropogenic global warming in the scientific literature. Environ Res Lett. 2013;8:024024.
- A blanket around the Earth. NASA Climate website. https://climate.nasa.gov/causes/. Accessed February 5, 2018.
- How is today’s warming different from the past? NASA Earth Observatory website. https://earthobservatory.nasa.gov/Features/GlobalWarming/page3.php. Accessed February 5, 2018.
- Mancini AJ, Shani-Adir A, Sidbury R. Other viral diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2017:1425-1446.
- Andersen LK, Davis MDP. A wake-up call to dermatologists—climate change affects the skin. Int J Dermatol. 2017;56:E198-E199.
- Liang L, Gong P. Climate change and human infectious diseases: a synthesis of research findings from global and spatio-temporal perspectives [published online March 23, 2017]. Environ Int. 2017;103:99-108.
- Lockhart SR, McCotter OZ, Chiller TM. Emerging fungal infections in the Pacific Northwest: the unrecognized burden and geographic range of Cryptococcus gattii and Coccidioides immitis. Microbiol Spectr. 2016;4. doi:10.1128/microbiolspec.EI10-0016-2016.
- Kilpatrick AM, Randolph SE. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. 2012;380:1946-1955.
- McMichael AJ. Extreme weather events and infectious disease outbreaks. Virulence. 2015;6:543-547.
- Lyme disease graphs. CDC website. https://www.cdc.gov/lyme/stats/graphs.html. Updated November 1, 2017. Accessed April 12, 2018.
- Stone BL, Tourand Y, Brissette CA. Brave new worlds: the expanding universe of Lyme disease. Vector Borne Zoonotic Dis. 2017;17:619-629.
- Watts N, Amann M, Ayeb-Karlsson S, et al. The Lancet Countdown on health and climate change: from 25 years of inaction to a global transformation for public health [published online October 30, 2017]. Lancet. doi:10.1016/S0140-6736(17)32464-9.
- Nawas ZY, Tong Y, Kollipara R, et al. Emerging infectious diseases with cutaneous manifestations: viral and bacterial infections. J Am Acad Dermatol. 2016;75:1-16.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Dengue. CDC website. https://www.cdc.gov/dengue/epidemiology/index.html. Updated June 9, 2014. Accessed April 3, 2018.
- Chikungunya virus in the United States. CDC website. https://www.cdc.gov/chikungunya/geo/united-states.html. Updated October 30, 2017. Accessed April 4, 2018.
- Clarke CF, Bradley KK, Wright JH, et al. Emergence of autochthonous cutaneous leishmaniasis in northeastern Texas and southeastern Oklahoma. Am J Trop Med Hyg. 2013;88:157-61.
- González C, Wang O, Strutz SE, et al. Climate change and risk of leishmaniasis in North America: predictions from ecological niche models of vector and reservoir species. PLoS Negl Trop Dis. 2010;4:E585.
- Chang CC, Chen SC. Colliding epidemics and the rise of cryptococcosis. J Fungi (Basel). 2015;2. doi: 10.3390/jof2010001.
- Marsden-Haug N, Goldoft M, Ralston C, et al. Coccidioidomycosis acquired in Washington state. Clin Infect Dis. 2013;56:847-850.
- Rosenbach M. Climate change & dermatology: what can you do? Paper presented at: American Academy of Dermatology Annual Meeting; March 3-7, 2017; Orlando, FL.
The term climate refers to the average weather conditions of a specific geographic location measured over several decades.1 While a certain degree of variation in the Earth’s climate is expected, a persistent warming or cooling trend is not. The factors driving the Earth’s warming remain difficult to prove.2 We know the Earth previously has undergone dramatic climate changes and that natural factors driving these changes are varied (eg, the relationship between the Earth and the Sun, volcanic eruptions, solar irradiance).1,3 These factors ideally change over protracted periods of time in a way that allows organisms to adapt to new environments.
Anthropogenic climate change refers to human-caused climate change. This is thought to be a major driving factor in the Earth’s recent warming trend, partly due to the rapidity of warming in recent years.3 According to climate scientists, the Earth’s temperature has risen 4°C to 7°C over the past 5000 years, but it has risen 0.7°C in just the past 100 years alone.4 Greenhouse gases such as carbon dioxide are emitted by various natural processes and human activities and play a central role in current warming because they trap solar heat and increase ambient temperature.3
In a recent edition of the commonly cited textbook Dermatology, Bolognia et al5 referenced climate change only once in a figure legend regarding the expansion of dengue fever in the Americas. However, climate change may have the potential to cause outright skin disease epidemics worldwide, and the Climate Change Committee of the International Society of Dermatology has called upon dermatologists across the globe to help raise awareness of this issue.6
Much of the literature regarding the effects of climate change on human health focuses on insect-borne diseases, but over the past decade other areas of impact also have been investigated, such as increases in airborne diseases, zoonoses, newly endemic saprophytic and dimorphic fungal infections, fecal-oral diseases, and severe allergic disease.7,8 It is postulated that climate change leads to region-specific increases in human disease because it creates newly favorable habitats for infectious agents, their vectors. and their reservoirs, allowing expansion of their ranges and access to immunologically naïve populations.9 Furthermore, extreme weather events such as heat waves, hurricanes, and flooding, which are expected to increase in frequency as a result of climate change, have all been linked to infectious disease outbreaks.10
Lyme Disease
In the past 20 years, Lyme disease incidence has tripled in the United States.11 It has been hypothesized that the increase may be occurring as a result of the expanding geographic distribution of the Ixodes tick and its mammalian hosts (eg, white-tailed deer) under the influence of climate change.12 Lyme disease is a multisystem disease affecting the skin, joints, heart, and nervous system. Its most characteristic manifestation is cutaneous in the form of erythema migrans. Dermatologists may be called upon to play an increasingly important role in early detection and treatment of this potentially chronic and debilitating condition.
Arboviruses
Arboviruses are transmitted by arthropods and are an important category of climate change–related diseases due to the expansion of the mosquito habitat worldwide. The vectorial capacity for the transmission of dengue fever has increased worldwide by 9.4% via Aedes aegypti and 11.1% via Aedes albopictus since 1950.13 Dengue fever, also known as breakbone fever, presents with intense joint pain, fevers, headaches, and a transient morbilliform rash that desquamates with defervescence and in some cases will incite hemorrhagic skin lesions.14 Dengue fever previously was considered to be a tropical disease but locally acquired cases have been reported in the United States, including Texas, Hawaii, and Florida.15,16
Reports of local transmission of chikungunya, another arbovirus transmitted by A albopictus and A aegypti mosquitoes in Florida, the US Virgin Islands, and Puerto Rico, began in 2014.17 A higher prevalence of these diseases within the United States also may be related to increased globalization, with US travelers returning from endemic regions with infections. Prior to 2014, transmission occurred in traditional endemic regions, primarily in Asia, Africa, or island nations in the Indian Ocean. Like dengue fever, chikungunya causes high fevers, cutaneous manifestations (eg, urticarial papules, morbilliform eruption, hypermelanosis, intertriginous lesions, lymphedema),17 and intense joint pain. Unlike dengue fever, however, joint involvement can be chronic, erosive, and debilitating.
Lastly, New World leishmaniasis, an arboviral disease characterized by mucocutaneous ulcers and transmitted by phlebotomine sand flies, has been acquired locally in Oklahoma and Texas when it was previously considered to be endemic to Mexico and Central and South America.14,18 The habitats of New World Leishmania species are expected to expand northward, with an ecological niche model predicting that they reach southern Canada by the year 2080 due to the expanding habitats of sand fly and rodent vectors.19
Fungal Infections
In the Pacific Northwest, there have been reports of newly endemic Cryptococcus gattii and Coccidioides immitis, both of which previously had been confined to the southwestern United States.8 Endemic ranges of these mycotic pathogens may be expanding for a variety of reasons, with climate change creating new regions conducive to the colonization of these species.8,20,21Coccidioides immitis is a soil-dwelling fungus that usually presents with primary pulmonary disease that can disseminate acutely or even months later. Prompt recognition of disseminated disease may allow life-saving therapy to be initiated. Cryptococcus gattii is a fungus with multiple niches, including oil, trees, and birds.20 This fungus also is acquired via inhalation, with dissemination occurring most commonly in immunosuppressed patients to the central nervous system, bone, and skin. Primary or secondary infection with both of these fungi may present with cutaneous manifestations presenting as polymorphous lesions, including umbilicated or ulcerated papules, indurated nodules, and acneiform pustules.
Final Thoughts
Awareness of the shifting habitats of microorganisms and vectors locally is important in order for clinicians to make correct diagnoses in a timely fashion. Regional or endemic diseases are presenting outside their traditional boundaries due to changing habitats of microbes and vectors and may be easily overlooked, resulting in a delayed diagnosis. Being prepared to diagnose diseases with increasing incidence secondary to climate change and discussing this with patients is an important physician obligation, but it is not the only one. We cannot effectively advocate for the health of patients and the community while ignoring the destruction of the environment. Our additional responsibility is straightforward—being advocates for good stewardship of the Earth’s resources now on both a personal and a policy level.22
The term climate refers to the average weather conditions of a specific geographic location measured over several decades.1 While a certain degree of variation in the Earth’s climate is expected, a persistent warming or cooling trend is not. The factors driving the Earth’s warming remain difficult to prove.2 We know the Earth previously has undergone dramatic climate changes and that natural factors driving these changes are varied (eg, the relationship between the Earth and the Sun, volcanic eruptions, solar irradiance).1,3 These factors ideally change over protracted periods of time in a way that allows organisms to adapt to new environments.
Anthropogenic climate change refers to human-caused climate change. This is thought to be a major driving factor in the Earth’s recent warming trend, partly due to the rapidity of warming in recent years.3 According to climate scientists, the Earth’s temperature has risen 4°C to 7°C over the past 5000 years, but it has risen 0.7°C in just the past 100 years alone.4 Greenhouse gases such as carbon dioxide are emitted by various natural processes and human activities and play a central role in current warming because they trap solar heat and increase ambient temperature.3
In a recent edition of the commonly cited textbook Dermatology, Bolognia et al5 referenced climate change only once in a figure legend regarding the expansion of dengue fever in the Americas. However, climate change may have the potential to cause outright skin disease epidemics worldwide, and the Climate Change Committee of the International Society of Dermatology has called upon dermatologists across the globe to help raise awareness of this issue.6
Much of the literature regarding the effects of climate change on human health focuses on insect-borne diseases, but over the past decade other areas of impact also have been investigated, such as increases in airborne diseases, zoonoses, newly endemic saprophytic and dimorphic fungal infections, fecal-oral diseases, and severe allergic disease.7,8 It is postulated that climate change leads to region-specific increases in human disease because it creates newly favorable habitats for infectious agents, their vectors. and their reservoirs, allowing expansion of their ranges and access to immunologically naïve populations.9 Furthermore, extreme weather events such as heat waves, hurricanes, and flooding, which are expected to increase in frequency as a result of climate change, have all been linked to infectious disease outbreaks.10
Lyme Disease
In the past 20 years, Lyme disease incidence has tripled in the United States.11 It has been hypothesized that the increase may be occurring as a result of the expanding geographic distribution of the Ixodes tick and its mammalian hosts (eg, white-tailed deer) under the influence of climate change.12 Lyme disease is a multisystem disease affecting the skin, joints, heart, and nervous system. Its most characteristic manifestation is cutaneous in the form of erythema migrans. Dermatologists may be called upon to play an increasingly important role in early detection and treatment of this potentially chronic and debilitating condition.
Arboviruses
Arboviruses are transmitted by arthropods and are an important category of climate change–related diseases due to the expansion of the mosquito habitat worldwide. The vectorial capacity for the transmission of dengue fever has increased worldwide by 9.4% via Aedes aegypti and 11.1% via Aedes albopictus since 1950.13 Dengue fever, also known as breakbone fever, presents with intense joint pain, fevers, headaches, and a transient morbilliform rash that desquamates with defervescence and in some cases will incite hemorrhagic skin lesions.14 Dengue fever previously was considered to be a tropical disease but locally acquired cases have been reported in the United States, including Texas, Hawaii, and Florida.15,16
Reports of local transmission of chikungunya, another arbovirus transmitted by A albopictus and A aegypti mosquitoes in Florida, the US Virgin Islands, and Puerto Rico, began in 2014.17 A higher prevalence of these diseases within the United States also may be related to increased globalization, with US travelers returning from endemic regions with infections. Prior to 2014, transmission occurred in traditional endemic regions, primarily in Asia, Africa, or island nations in the Indian Ocean. Like dengue fever, chikungunya causes high fevers, cutaneous manifestations (eg, urticarial papules, morbilliform eruption, hypermelanosis, intertriginous lesions, lymphedema),17 and intense joint pain. Unlike dengue fever, however, joint involvement can be chronic, erosive, and debilitating.
Lastly, New World leishmaniasis, an arboviral disease characterized by mucocutaneous ulcers and transmitted by phlebotomine sand flies, has been acquired locally in Oklahoma and Texas when it was previously considered to be endemic to Mexico and Central and South America.14,18 The habitats of New World Leishmania species are expected to expand northward, with an ecological niche model predicting that they reach southern Canada by the year 2080 due to the expanding habitats of sand fly and rodent vectors.19
Fungal Infections
In the Pacific Northwest, there have been reports of newly endemic Cryptococcus gattii and Coccidioides immitis, both of which previously had been confined to the southwestern United States.8 Endemic ranges of these mycotic pathogens may be expanding for a variety of reasons, with climate change creating new regions conducive to the colonization of these species.8,20,21Coccidioides immitis is a soil-dwelling fungus that usually presents with primary pulmonary disease that can disseminate acutely or even months later. Prompt recognition of disseminated disease may allow life-saving therapy to be initiated. Cryptococcus gattii is a fungus with multiple niches, including oil, trees, and birds.20 This fungus also is acquired via inhalation, with dissemination occurring most commonly in immunosuppressed patients to the central nervous system, bone, and skin. Primary or secondary infection with both of these fungi may present with cutaneous manifestations presenting as polymorphous lesions, including umbilicated or ulcerated papules, indurated nodules, and acneiform pustules.
Final Thoughts
Awareness of the shifting habitats of microorganisms and vectors locally is important in order for clinicians to make correct diagnoses in a timely fashion. Regional or endemic diseases are presenting outside their traditional boundaries due to changing habitats of microbes and vectors and may be easily overlooked, resulting in a delayed diagnosis. Being prepared to diagnose diseases with increasing incidence secondary to climate change and discussing this with patients is an important physician obligation, but it is not the only one. We cannot effectively advocate for the health of patients and the community while ignoring the destruction of the environment. Our additional responsibility is straightforward—being advocates for good stewardship of the Earth’s resources now on both a personal and a policy level.22
- Climate Central. Global Weirdness: Severe Storms, Deadly Heat Waves, Relentless Drought, Rising Seas, and the Weather of the Future. New York, NY: Pantheon Books; 2012.
- Cook J, Nuccitelli D, Green SA, et al. Quantifying the consensus on anthropogenic global warming in the scientific literature. Environ Res Lett. 2013;8:024024.
- A blanket around the Earth. NASA Climate website. https://climate.nasa.gov/causes/. Accessed February 5, 2018.
- How is today’s warming different from the past? NASA Earth Observatory website. https://earthobservatory.nasa.gov/Features/GlobalWarming/page3.php. Accessed February 5, 2018.
- Mancini AJ, Shani-Adir A, Sidbury R. Other viral diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2017:1425-1446.
- Andersen LK, Davis MDP. A wake-up call to dermatologists—climate change affects the skin. Int J Dermatol. 2017;56:E198-E199.
- Liang L, Gong P. Climate change and human infectious diseases: a synthesis of research findings from global and spatio-temporal perspectives [published online March 23, 2017]. Environ Int. 2017;103:99-108.
- Lockhart SR, McCotter OZ, Chiller TM. Emerging fungal infections in the Pacific Northwest: the unrecognized burden and geographic range of Cryptococcus gattii and Coccidioides immitis. Microbiol Spectr. 2016;4. doi:10.1128/microbiolspec.EI10-0016-2016.
- Kilpatrick AM, Randolph SE. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. 2012;380:1946-1955.
- McMichael AJ. Extreme weather events and infectious disease outbreaks. Virulence. 2015;6:543-547.
- Lyme disease graphs. CDC website. https://www.cdc.gov/lyme/stats/graphs.html. Updated November 1, 2017. Accessed April 12, 2018.
- Stone BL, Tourand Y, Brissette CA. Brave new worlds: the expanding universe of Lyme disease. Vector Borne Zoonotic Dis. 2017;17:619-629.
- Watts N, Amann M, Ayeb-Karlsson S, et al. The Lancet Countdown on health and climate change: from 25 years of inaction to a global transformation for public health [published online October 30, 2017]. Lancet. doi:10.1016/S0140-6736(17)32464-9.
- Nawas ZY, Tong Y, Kollipara R, et al. Emerging infectious diseases with cutaneous manifestations: viral and bacterial infections. J Am Acad Dermatol. 2016;75:1-16.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Dengue. CDC website. https://www.cdc.gov/dengue/epidemiology/index.html. Updated June 9, 2014. Accessed April 3, 2018.
- Chikungunya virus in the United States. CDC website. https://www.cdc.gov/chikungunya/geo/united-states.html. Updated October 30, 2017. Accessed April 4, 2018.
- Clarke CF, Bradley KK, Wright JH, et al. Emergence of autochthonous cutaneous leishmaniasis in northeastern Texas and southeastern Oklahoma. Am J Trop Med Hyg. 2013;88:157-61.
- González C, Wang O, Strutz SE, et al. Climate change and risk of leishmaniasis in North America: predictions from ecological niche models of vector and reservoir species. PLoS Negl Trop Dis. 2010;4:E585.
- Chang CC, Chen SC. Colliding epidemics and the rise of cryptococcosis. J Fungi (Basel). 2015;2. doi: 10.3390/jof2010001.
- Marsden-Haug N, Goldoft M, Ralston C, et al. Coccidioidomycosis acquired in Washington state. Clin Infect Dis. 2013;56:847-850.
- Rosenbach M. Climate change & dermatology: what can you do? Paper presented at: American Academy of Dermatology Annual Meeting; March 3-7, 2017; Orlando, FL.
- Climate Central. Global Weirdness: Severe Storms, Deadly Heat Waves, Relentless Drought, Rising Seas, and the Weather of the Future. New York, NY: Pantheon Books; 2012.
- Cook J, Nuccitelli D, Green SA, et al. Quantifying the consensus on anthropogenic global warming in the scientific literature. Environ Res Lett. 2013;8:024024.
- A blanket around the Earth. NASA Climate website. https://climate.nasa.gov/causes/. Accessed February 5, 2018.
- How is today’s warming different from the past? NASA Earth Observatory website. https://earthobservatory.nasa.gov/Features/GlobalWarming/page3.php. Accessed February 5, 2018.
- Mancini AJ, Shani-Adir A, Sidbury R. Other viral diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2017:1425-1446.
- Andersen LK, Davis MDP. A wake-up call to dermatologists—climate change affects the skin. Int J Dermatol. 2017;56:E198-E199.
- Liang L, Gong P. Climate change and human infectious diseases: a synthesis of research findings from global and spatio-temporal perspectives [published online March 23, 2017]. Environ Int. 2017;103:99-108.
- Lockhart SR, McCotter OZ, Chiller TM. Emerging fungal infections in the Pacific Northwest: the unrecognized burden and geographic range of Cryptococcus gattii and Coccidioides immitis. Microbiol Spectr. 2016;4. doi:10.1128/microbiolspec.EI10-0016-2016.
- Kilpatrick AM, Randolph SE. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. 2012;380:1946-1955.
- McMichael AJ. Extreme weather events and infectious disease outbreaks. Virulence. 2015;6:543-547.
- Lyme disease graphs. CDC website. https://www.cdc.gov/lyme/stats/graphs.html. Updated November 1, 2017. Accessed April 12, 2018.
- Stone BL, Tourand Y, Brissette CA. Brave new worlds: the expanding universe of Lyme disease. Vector Borne Zoonotic Dis. 2017;17:619-629.
- Watts N, Amann M, Ayeb-Karlsson S, et al. The Lancet Countdown on health and climate change: from 25 years of inaction to a global transformation for public health [published online October 30, 2017]. Lancet. doi:10.1016/S0140-6736(17)32464-9.
- Nawas ZY, Tong Y, Kollipara R, et al. Emerging infectious diseases with cutaneous manifestations: viral and bacterial infections. J Am Acad Dermatol. 2016;75:1-16.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Dengue. CDC website. https://www.cdc.gov/dengue/epidemiology/index.html. Updated June 9, 2014. Accessed April 3, 2018.
- Chikungunya virus in the United States. CDC website. https://www.cdc.gov/chikungunya/geo/united-states.html. Updated October 30, 2017. Accessed April 4, 2018.
- Clarke CF, Bradley KK, Wright JH, et al. Emergence of autochthonous cutaneous leishmaniasis in northeastern Texas and southeastern Oklahoma. Am J Trop Med Hyg. 2013;88:157-61.
- González C, Wang O, Strutz SE, et al. Climate change and risk of leishmaniasis in North America: predictions from ecological niche models of vector and reservoir species. PLoS Negl Trop Dis. 2010;4:E585.
- Chang CC, Chen SC. Colliding epidemics and the rise of cryptococcosis. J Fungi (Basel). 2015;2. doi: 10.3390/jof2010001.
- Marsden-Haug N, Goldoft M, Ralston C, et al. Coccidioidomycosis acquired in Washington state. Clin Infect Dis. 2013;56:847-850.
- Rosenbach M. Climate change & dermatology: what can you do? Paper presented at: American Academy of Dermatology Annual Meeting; March 3-7, 2017; Orlando, FL.