User login
Type 1 diabetes amputation rates fall in Sweden, rise in U.S.
according to a national registry analysis.
The incidence of any amputation trended downward from 2011 to 2019, Sara Hallström, MD, reported at the annual scientific sessions of the American Diabetes Association.
Levels of hemoglobin A1c have also trended downward over time in Sweden among those with type 1 diabetes, while renal function has remained stable among patients who did not undergo amputations, Dr. Hallström said in a virtual presentation.
“Observing stable renal function and decreasing levels of [hemoglobin] A1c, along with decreasing incidence of amputation, indicates a shift in the prognosis of persons with type 1 diabetes,” she said.
Drilling down on amputation risk in type 1 diabetes
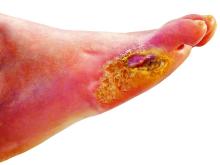
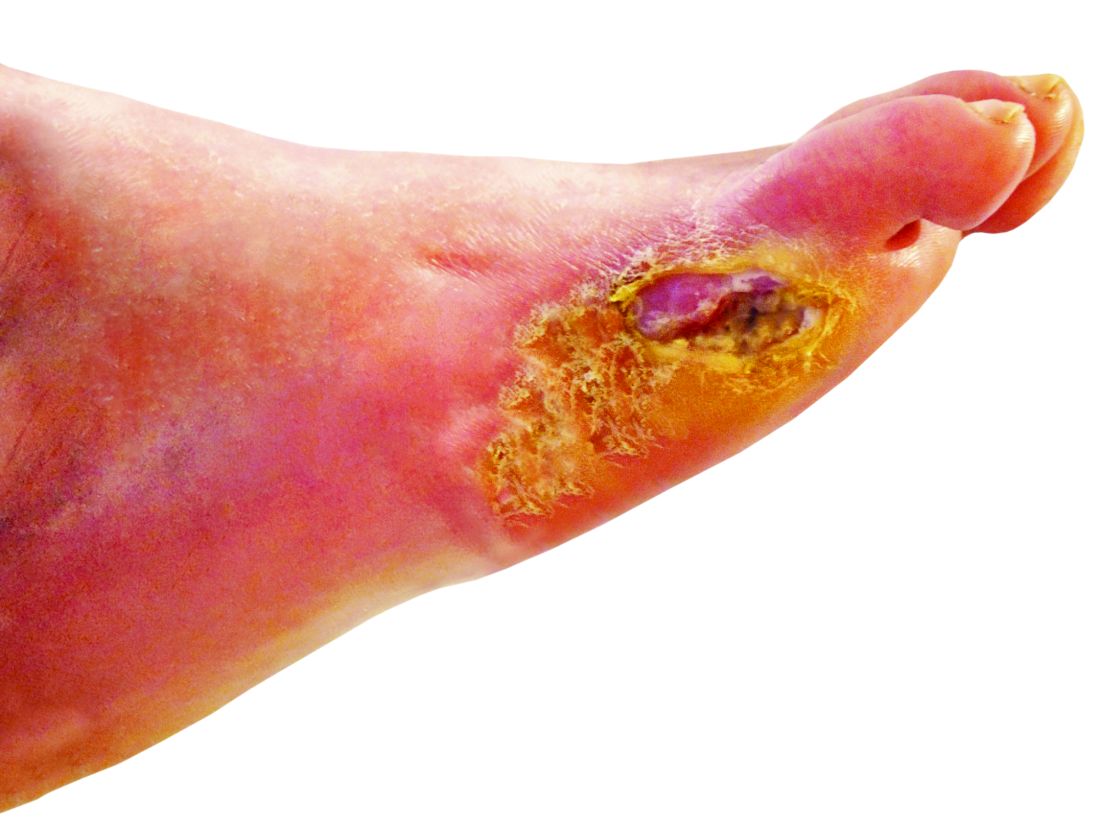
Lower-extremity amputation is a major source of disability and distress in people with diabetes, and also poses a significant financial burden for the health care system, according to Dr. Hallström of Sahlgrenska University Hospital and the University of Gothenburg (Sweden).
“Limb loss due to amputation is not seldom a final outcome of diabetic foot ulcers,” she said in the presentation.
Most studies of amputation incidence and risk factors have grouped patients with different types of diabetes, though a few recent studies have singled out type 1 diabetes.
Among these is a 2019 study indicating a 40-fold higher risk of amputation among individuals with type 1 diabetes, compared with the general population, based on analysis of Swedish National Diabetes Register data from 1998 to 2013.
Trends over time
In the present study, Dr. Hallström and coinvestigators queried that same Swedish registry and identified 46,008 individuals with type 1 diabetes from 1998 to 2019. The mean age was 32.5 years and 55% were male. Overall, 1,519 of these individuals (3.3%) underwent amputation.
The incidence of any amputation fluctuated from 1998 to 2011, followed by a “decreasing trend over time” from 2011 to 2019, Dr. Hallström said.
The incidence of amputation per 1,000 patient-years was 2.84 in the earliest time period of 1998-2001, decreasing to 1.64 in 2017-2019.
Levels of A1c decreased over time, starting at 2012, both in participants with and without amputations, Dr. Hallström said. Renal function over that period remained stable in persons without amputation, and showed a decreasing trend in persons with amputation.
Compared with individuals with no amputations, those undergoing amputation were older (50 years vs. 32 years), had a longer duration of diabetes (34.9 years vs. 16.5 years), and had higher mean A1c, Dr. Hellström said. The amputee group also included a higher proportion of smokers, at 19.4% versus 14.0%, data show.
Risk factors for amputation included renal dysfunction, hyperglycemia, older age, smoking, hypertension, and cardiovascular comorbidities, according to the researcher.
U.S. amputations on the rise overall
While authors say results of this study point to a potentially improved prognosis for individuals with type 1 diabetes in Sweden, Robert A. Gabbay, MD, PhD, chief scientific and medical officer of the ADA, said amputation rates remains “concerning” based on U.S. data focused largely on type 2 diabetes.
“The amputation rate is unfortunately rising,” he said. “Sadly, this continues to be an issue.”
Significant health disparities persist, he added, with Black Americans having two- to threefold higher rates of amputations.
To help reduce amputation rates, clinicians should be asking patient about claudication and using simple screening techniques such as inspecting patient’s feet. “The big deal here is preventing ulcer formation, because once the ulcer forms, it often doesn’t heal, and it’s a downward spiral,” he said.
In addition, recent research suggests seeking a second opinion may help: “Many of those amputations could be avoided, in part because people aren’t aware of some of the treatments that can open up the arteries and reestablish blood flow,” he added.
Dr. Hallström reported no conflicts of interest. One coauthor on the study provided disclosures related to Abbott, AstraZeneca, Boehringer Ingelheim, Lilly Diabetes, and Novo Nordisk.
according to a national registry analysis.
The incidence of any amputation trended downward from 2011 to 2019, Sara Hallström, MD, reported at the annual scientific sessions of the American Diabetes Association.
Levels of hemoglobin A1c have also trended downward over time in Sweden among those with type 1 diabetes, while renal function has remained stable among patients who did not undergo amputations, Dr. Hallström said in a virtual presentation.
“Observing stable renal function and decreasing levels of [hemoglobin] A1c, along with decreasing incidence of amputation, indicates a shift in the prognosis of persons with type 1 diabetes,” she said.
Drilling down on amputation risk in type 1 diabetes
Lower-extremity amputation is a major source of disability and distress in people with diabetes, and also poses a significant financial burden for the health care system, according to Dr. Hallström of Sahlgrenska University Hospital and the University of Gothenburg (Sweden).
“Limb loss due to amputation is not seldom a final outcome of diabetic foot ulcers,” she said in the presentation.
Most studies of amputation incidence and risk factors have grouped patients with different types of diabetes, though a few recent studies have singled out type 1 diabetes.
Among these is a 2019 study indicating a 40-fold higher risk of amputation among individuals with type 1 diabetes, compared with the general population, based on analysis of Swedish National Diabetes Register data from 1998 to 2013.
Trends over time
In the present study, Dr. Hallström and coinvestigators queried that same Swedish registry and identified 46,008 individuals with type 1 diabetes from 1998 to 2019. The mean age was 32.5 years and 55% were male. Overall, 1,519 of these individuals (3.3%) underwent amputation.
The incidence of any amputation fluctuated from 1998 to 2011, followed by a “decreasing trend over time” from 2011 to 2019, Dr. Hallström said.
The incidence of amputation per 1,000 patient-years was 2.84 in the earliest time period of 1998-2001, decreasing to 1.64 in 2017-2019.
Levels of A1c decreased over time, starting at 2012, both in participants with and without amputations, Dr. Hallström said. Renal function over that period remained stable in persons without amputation, and showed a decreasing trend in persons with amputation.
Compared with individuals with no amputations, those undergoing amputation were older (50 years vs. 32 years), had a longer duration of diabetes (34.9 years vs. 16.5 years), and had higher mean A1c, Dr. Hellström said. The amputee group also included a higher proportion of smokers, at 19.4% versus 14.0%, data show.
Risk factors for amputation included renal dysfunction, hyperglycemia, older age, smoking, hypertension, and cardiovascular comorbidities, according to the researcher.
U.S. amputations on the rise overall
While authors say results of this study point to a potentially improved prognosis for individuals with type 1 diabetes in Sweden, Robert A. Gabbay, MD, PhD, chief scientific and medical officer of the ADA, said amputation rates remains “concerning” based on U.S. data focused largely on type 2 diabetes.
“The amputation rate is unfortunately rising,” he said. “Sadly, this continues to be an issue.”
Significant health disparities persist, he added, with Black Americans having two- to threefold higher rates of amputations.
To help reduce amputation rates, clinicians should be asking patient about claudication and using simple screening techniques such as inspecting patient’s feet. “The big deal here is preventing ulcer formation, because once the ulcer forms, it often doesn’t heal, and it’s a downward spiral,” he said.
In addition, recent research suggests seeking a second opinion may help: “Many of those amputations could be avoided, in part because people aren’t aware of some of the treatments that can open up the arteries and reestablish blood flow,” he added.
Dr. Hallström reported no conflicts of interest. One coauthor on the study provided disclosures related to Abbott, AstraZeneca, Boehringer Ingelheim, Lilly Diabetes, and Novo Nordisk.
according to a national registry analysis.
The incidence of any amputation trended downward from 2011 to 2019, Sara Hallström, MD, reported at the annual scientific sessions of the American Diabetes Association.
Levels of hemoglobin A1c have also trended downward over time in Sweden among those with type 1 diabetes, while renal function has remained stable among patients who did not undergo amputations, Dr. Hallström said in a virtual presentation.
“Observing stable renal function and decreasing levels of [hemoglobin] A1c, along with decreasing incidence of amputation, indicates a shift in the prognosis of persons with type 1 diabetes,” she said.
Drilling down on amputation risk in type 1 diabetes
Lower-extremity amputation is a major source of disability and distress in people with diabetes, and also poses a significant financial burden for the health care system, according to Dr. Hallström of Sahlgrenska University Hospital and the University of Gothenburg (Sweden).
“Limb loss due to amputation is not seldom a final outcome of diabetic foot ulcers,” she said in the presentation.
Most studies of amputation incidence and risk factors have grouped patients with different types of diabetes, though a few recent studies have singled out type 1 diabetes.
Among these is a 2019 study indicating a 40-fold higher risk of amputation among individuals with type 1 diabetes, compared with the general population, based on analysis of Swedish National Diabetes Register data from 1998 to 2013.
Trends over time
In the present study, Dr. Hallström and coinvestigators queried that same Swedish registry and identified 46,008 individuals with type 1 diabetes from 1998 to 2019. The mean age was 32.5 years and 55% were male. Overall, 1,519 of these individuals (3.3%) underwent amputation.
The incidence of any amputation fluctuated from 1998 to 2011, followed by a “decreasing trend over time” from 2011 to 2019, Dr. Hallström said.
The incidence of amputation per 1,000 patient-years was 2.84 in the earliest time period of 1998-2001, decreasing to 1.64 in 2017-2019.
Levels of A1c decreased over time, starting at 2012, both in participants with and without amputations, Dr. Hallström said. Renal function over that period remained stable in persons without amputation, and showed a decreasing trend in persons with amputation.
Compared with individuals with no amputations, those undergoing amputation were older (50 years vs. 32 years), had a longer duration of diabetes (34.9 years vs. 16.5 years), and had higher mean A1c, Dr. Hellström said. The amputee group also included a higher proportion of smokers, at 19.4% versus 14.0%, data show.
Risk factors for amputation included renal dysfunction, hyperglycemia, older age, smoking, hypertension, and cardiovascular comorbidities, according to the researcher.
U.S. amputations on the rise overall
While authors say results of this study point to a potentially improved prognosis for individuals with type 1 diabetes in Sweden, Robert A. Gabbay, MD, PhD, chief scientific and medical officer of the ADA, said amputation rates remains “concerning” based on U.S. data focused largely on type 2 diabetes.
“The amputation rate is unfortunately rising,” he said. “Sadly, this continues to be an issue.”
Significant health disparities persist, he added, with Black Americans having two- to threefold higher rates of amputations.
To help reduce amputation rates, clinicians should be asking patient about claudication and using simple screening techniques such as inspecting patient’s feet. “The big deal here is preventing ulcer formation, because once the ulcer forms, it often doesn’t heal, and it’s a downward spiral,” he said.
In addition, recent research suggests seeking a second opinion may help: “Many of those amputations could be avoided, in part because people aren’t aware of some of the treatments that can open up the arteries and reestablish blood flow,” he added.
Dr. Hallström reported no conflicts of interest. One coauthor on the study provided disclosures related to Abbott, AstraZeneca, Boehringer Ingelheim, Lilly Diabetes, and Novo Nordisk.
FROM ADA 2020
Unmanaged diabetes, high blood glucose tied to COVID-19 severity
Unmanaged diabetes and high blood glucose levels are linked to more severe COVID-19 and worse rates of recovery, according to results of a retrospective study.
Patients not managing their diabetes with medication had more severe COVID-19 and length of hospitalization, compared with those who were taking medication, investigator Sudip Bajpeyi, PhD, said at the annual scientific sessions of the American Diabetes Association.
In addition, patients with higher blood glucose levels had more severe COVID-19 and longer hospital stays.
Those findings underscore the need to assess, monitor, and control blood glucose, especially in vulnerable populations, said Dr. Bajpeyi, director of the Metabolic, Nutrition, and Exercise Research Laboratory in the University of Texas, El Paso, who added that nearly 90% of the study subjects were Hispanic.
“As public health decisions are made, we think fasting blood glucose should be considered in the treatment of hospitalized COVID-19 patients,” he said in a press conference.
Links between diabetes and COVID-19
There are now many reports in medical literature that link diabetes to increased risk of COVID-19 severity, according to Ali Mossayebi, a master’s student who worked on the study. However, there are fewer studies that have looked specifically at the implications of poor diabetes management or acute glycemic control, the investigators said.
It’s known that poorly controlled diabetes can have severe health consequences, including higher risks for life-threatening comorbidities, they added.
Their retrospective study focused on medical records from 364 patients with COVID-19 admitted to a medical center in El Paso. Their mean age was 60 years, and their mean body mass index was 30.3 kg/m2; 87% were Hispanic.
Acute glycemic control was assessed by fasting blood glucose at the time of hospitalization, while chronic glycemic control was assessed by hemoglobin A1c, the investigators said. Severity of COVID-19 was measured with the Sequential (Sepsis-Related) Organ Failure Assessment (qSOFA), which is based on the patient’s respiratory rate, blood pressure, and mental status.
Impact of unmanaged diabetes and high blood glucose
Severity of COVID-19 severity and length of hospital stay were significantly greater in patients with unmanaged diabetes, as compared with those who reported that they managed their diabetes with medication, Dr. Bajpeyi and coinvestigators found.
Among patients with unmanaged diabetes, the mean qSOFA score was 0.22, as compared with 0.44 for patients with managed diabetes. The mean length of hospital stay was 10.8 days for patients with unmanaged diabetes and 8.2 days for those with medication-managed diabetes, according to the abstract.
COVID-19 severity and hospital stay length were highest among patients with acute glycemia, the investigators further reported in an electronic poster that was part of the ADA meeting proceedings.
The mean qSOFA score was about 0.6 for patients with blood glucose levels of at least 126 mg/dL and A1c below 6.5%, and roughly 0.2 for those with normal blood glucose and normal A1c. Similarly, duration of hospital stay was significantly higher for patients with high blood glucose and A1c as compared with those with normal blood glucose and A1c.
Aggressive treatment needed
Findings of this study are in line with previous research showing that in-hospital hyperglycemia is a common and important marker of poor clinical outcome and mortality, with or without diabetes, according to Rodolfo J. Galindo, MD, FACE, medical chair of the hospital diabetes task force at Emory Healthcare System, Atlanta.
“These patients need aggressive treatment of hyperglycemia, regardless of the diagnosis of diabetes or A1c value,” said Dr. Galindo, who was not involved in the study. “They also need outpatient follow-up after discharge, because they may develop diabetes soon after.”
Follow-up within is important because roughly 30% of patients with stress hyperglycemia (increases in blood glucose during an acute illness) will develop diabetes within a year, according to Dr. Galindo.
“We do not know in COVID-10 patients if it is only 30%,” he said, “Our thinking in our group is that it’s probably higher.”
Dr. Bajpeyi and coauthors reported no disclosures. Dr. Galindo reported disclosures related to Abbott Diabetes, Boehringer Ingelheim International, Eli Lilly, Novo Nordisk, Sanofi US, Valeritas, and Dexcom.
Unmanaged diabetes and high blood glucose levels are linked to more severe COVID-19 and worse rates of recovery, according to results of a retrospective study.
Patients not managing their diabetes with medication had more severe COVID-19 and length of hospitalization, compared with those who were taking medication, investigator Sudip Bajpeyi, PhD, said at the annual scientific sessions of the American Diabetes Association.
In addition, patients with higher blood glucose levels had more severe COVID-19 and longer hospital stays.
Those findings underscore the need to assess, monitor, and control blood glucose, especially in vulnerable populations, said Dr. Bajpeyi, director of the Metabolic, Nutrition, and Exercise Research Laboratory in the University of Texas, El Paso, who added that nearly 90% of the study subjects were Hispanic.
“As public health decisions are made, we think fasting blood glucose should be considered in the treatment of hospitalized COVID-19 patients,” he said in a press conference.
Links between diabetes and COVID-19
There are now many reports in medical literature that link diabetes to increased risk of COVID-19 severity, according to Ali Mossayebi, a master’s student who worked on the study. However, there are fewer studies that have looked specifically at the implications of poor diabetes management or acute glycemic control, the investigators said.
It’s known that poorly controlled diabetes can have severe health consequences, including higher risks for life-threatening comorbidities, they added.
Their retrospective study focused on medical records from 364 patients with COVID-19 admitted to a medical center in El Paso. Their mean age was 60 years, and their mean body mass index was 30.3 kg/m2; 87% were Hispanic.
Acute glycemic control was assessed by fasting blood glucose at the time of hospitalization, while chronic glycemic control was assessed by hemoglobin A1c, the investigators said. Severity of COVID-19 was measured with the Sequential (Sepsis-Related) Organ Failure Assessment (qSOFA), which is based on the patient’s respiratory rate, blood pressure, and mental status.
Impact of unmanaged diabetes and high blood glucose
Severity of COVID-19 severity and length of hospital stay were significantly greater in patients with unmanaged diabetes, as compared with those who reported that they managed their diabetes with medication, Dr. Bajpeyi and coinvestigators found.
Among patients with unmanaged diabetes, the mean qSOFA score was 0.22, as compared with 0.44 for patients with managed diabetes. The mean length of hospital stay was 10.8 days for patients with unmanaged diabetes and 8.2 days for those with medication-managed diabetes, according to the abstract.
COVID-19 severity and hospital stay length were highest among patients with acute glycemia, the investigators further reported in an electronic poster that was part of the ADA meeting proceedings.
The mean qSOFA score was about 0.6 for patients with blood glucose levels of at least 126 mg/dL and A1c below 6.5%, and roughly 0.2 for those with normal blood glucose and normal A1c. Similarly, duration of hospital stay was significantly higher for patients with high blood glucose and A1c as compared with those with normal blood glucose and A1c.
Aggressive treatment needed
Findings of this study are in line with previous research showing that in-hospital hyperglycemia is a common and important marker of poor clinical outcome and mortality, with or without diabetes, according to Rodolfo J. Galindo, MD, FACE, medical chair of the hospital diabetes task force at Emory Healthcare System, Atlanta.
“These patients need aggressive treatment of hyperglycemia, regardless of the diagnosis of diabetes or A1c value,” said Dr. Galindo, who was not involved in the study. “They also need outpatient follow-up after discharge, because they may develop diabetes soon after.”
Follow-up within is important because roughly 30% of patients with stress hyperglycemia (increases in blood glucose during an acute illness) will develop diabetes within a year, according to Dr. Galindo.
“We do not know in COVID-10 patients if it is only 30%,” he said, “Our thinking in our group is that it’s probably higher.”
Dr. Bajpeyi and coauthors reported no disclosures. Dr. Galindo reported disclosures related to Abbott Diabetes, Boehringer Ingelheim International, Eli Lilly, Novo Nordisk, Sanofi US, Valeritas, and Dexcom.
Unmanaged diabetes and high blood glucose levels are linked to more severe COVID-19 and worse rates of recovery, according to results of a retrospective study.
Patients not managing their diabetes with medication had more severe COVID-19 and length of hospitalization, compared with those who were taking medication, investigator Sudip Bajpeyi, PhD, said at the annual scientific sessions of the American Diabetes Association.
In addition, patients with higher blood glucose levels had more severe COVID-19 and longer hospital stays.
Those findings underscore the need to assess, monitor, and control blood glucose, especially in vulnerable populations, said Dr. Bajpeyi, director of the Metabolic, Nutrition, and Exercise Research Laboratory in the University of Texas, El Paso, who added that nearly 90% of the study subjects were Hispanic.
“As public health decisions are made, we think fasting blood glucose should be considered in the treatment of hospitalized COVID-19 patients,” he said in a press conference.
Links between diabetes and COVID-19
There are now many reports in medical literature that link diabetes to increased risk of COVID-19 severity, according to Ali Mossayebi, a master’s student who worked on the study. However, there are fewer studies that have looked specifically at the implications of poor diabetes management or acute glycemic control, the investigators said.
It’s known that poorly controlled diabetes can have severe health consequences, including higher risks for life-threatening comorbidities, they added.
Their retrospective study focused on medical records from 364 patients with COVID-19 admitted to a medical center in El Paso. Their mean age was 60 years, and their mean body mass index was 30.3 kg/m2; 87% were Hispanic.
Acute glycemic control was assessed by fasting blood glucose at the time of hospitalization, while chronic glycemic control was assessed by hemoglobin A1c, the investigators said. Severity of COVID-19 was measured with the Sequential (Sepsis-Related) Organ Failure Assessment (qSOFA), which is based on the patient’s respiratory rate, blood pressure, and mental status.
Impact of unmanaged diabetes and high blood glucose
Severity of COVID-19 severity and length of hospital stay were significantly greater in patients with unmanaged diabetes, as compared with those who reported that they managed their diabetes with medication, Dr. Bajpeyi and coinvestigators found.
Among patients with unmanaged diabetes, the mean qSOFA score was 0.22, as compared with 0.44 for patients with managed diabetes. The mean length of hospital stay was 10.8 days for patients with unmanaged diabetes and 8.2 days for those with medication-managed diabetes, according to the abstract.
COVID-19 severity and hospital stay length were highest among patients with acute glycemia, the investigators further reported in an electronic poster that was part of the ADA meeting proceedings.
The mean qSOFA score was about 0.6 for patients with blood glucose levels of at least 126 mg/dL and A1c below 6.5%, and roughly 0.2 for those with normal blood glucose and normal A1c. Similarly, duration of hospital stay was significantly higher for patients with high blood glucose and A1c as compared with those with normal blood glucose and A1c.
Aggressive treatment needed
Findings of this study are in line with previous research showing that in-hospital hyperglycemia is a common and important marker of poor clinical outcome and mortality, with or without diabetes, according to Rodolfo J. Galindo, MD, FACE, medical chair of the hospital diabetes task force at Emory Healthcare System, Atlanta.
“These patients need aggressive treatment of hyperglycemia, regardless of the diagnosis of diabetes or A1c value,” said Dr. Galindo, who was not involved in the study. “They also need outpatient follow-up after discharge, because they may develop diabetes soon after.”
Follow-up within is important because roughly 30% of patients with stress hyperglycemia (increases in blood glucose during an acute illness) will develop diabetes within a year, according to Dr. Galindo.
“We do not know in COVID-10 patients if it is only 30%,” he said, “Our thinking in our group is that it’s probably higher.”
Dr. Bajpeyi and coauthors reported no disclosures. Dr. Galindo reported disclosures related to Abbott Diabetes, Boehringer Ingelheim International, Eli Lilly, Novo Nordisk, Sanofi US, Valeritas, and Dexcom.
FROM ADA 2020
FDA approves OTC antihistamine nasal spray
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HER3-targeted treatment demonstrates efficacy and safety in phase 1 lung cancer study
A HER3-targeted therapy has demonstrated clinically meaningful and durable efficacy in heavily pretreated patients with EGFR-mutant non–small cell lung cancer, according to results of a phase 1 study.
Patritumab deruxtecan, an antibody-drug conjugate targeting HER3, had an overall response rate (ORR) of 39% and median progression-free survival (PFS) of 8.2 months in a phase 1 study that included patients previously treated with tyrosine kinase inhibitors (TKIs) and chemotherapy, the results show.
The efficacy was seen across EGFR TKI resistance mechanisms in this very difficult-to-treat patient population, according to investigator Pasi A. Jänne, MD, PhD, of the Dana-Farber Cancer Institute, Boston.
“There is not one category of individuals that are having a response, or not having a response,” Dr. Jänne said in a presentation at the annual meeting of the American Society of Clinical Oncology (Abstract 9007).
“Responses are observed in patients with identifiable resistance mechanisms, and in patients that do not have an identifiable resistance mechanism, but have progressed on prior EGFR TKI therapy,” he added.
More than 80% of non–small cell lung cancer (NSCLC) tumors express HER3, and of note, HER3 alterations do not appear to confer resistance to EGFR TKIs in patients with EGFR-mutant NSCLC, according to Dr. Jänne.
Study details
Also known as HER3-DXd, patritumab deruxtecan consists of a fully human anti-HER3 monoclonal antibody linked to a topoisomerase inhibitor payload by a tetrapeptide-based cleavable linker.
The antibody-drug conjugate is also being evaluated in metastatic breast cancer and colorectal cancer, Dr. Jänne said.
In the present phase 1 dose escalation and dose expansion study, a total of 57 patients were treated with patritumab deruxtecan at 5.6 mg/kg, the recommended dose for the expansion phase.
The median age of these patients was 65 years, and the majority (63%) were women, Dr. Jänne reported. About half had a history of central nervous system metastases.
The median number of prior lines of systemic therapy was four, making this a heavily pretreated patient population, Dr. Jänne said. All patients had received prior EGFR TKI therapy, and 86% specifically had prior osimertinib. Ninety-one percent had prior platinum-based chemotherapy, and 40% had received immunotherapy.
Spectrum of responses
The confirmed ORR of 39% included 1 complete response (2%) and 21 partial responses (37%), Dr. Jänne reported. The disease control rate was 72%, and median duration of response was 6.9 months at a median follow-up of 10.2 months.
The median PFS was 8.2 months in 57 patients overall and in a subset of 44 patients who had received prior osimertinib and platinum-based chemotherapy, according to the report.
Activity of patritumab deruxtecan was seen not only across patients with diverse mechanisms of EGFR TKI resistance, but also regardless of prior number of treatments, and regardless of history of brain metastases, the investigator said.
In addition, clinical responses were seen across a spectrum of baseline HER3 expression by immunohistochemistry, the investigator added.
Safety was assessed in 81 patients treated at a range of doses in the phase 1 trial. The most common grade 3 or greater treatment-emergent adverse events, observed in 5% or more of patients, included thrombocytopenia, neutropenia, and anemia, while other side effects such as fatigue and dyspnea were observed, Dr. Jänne said. About 9% of the adverse events led to treatment discontinuation in the safety cohort.
Interstitial lung disease was observed in four patients, or 5% of the safety cohort. Three of these were grade 1-2 and one was grade 3, according to the report.
Questions to explore
The efficacy of patritumab deruxtecan was “high” in this phase 1 study, based on the reported response rate and median PFS, said discussant Nicolas Girard, MD, PhD, of Institut Curie in Paris.
However, the most striking finding of the study was the efficacy of the antibody-drug conjugate across all reported resistance mechanisms, Dr. Girard said in his remarks.
Questions that remains to be explored, according to Dr. Girard, include the impact of previous treatment sequencing with TKIs and chemotherapy on patient outcomes with patritumab deruxtecan, as well as the assessment of intracranial response and PFS for patients treated with the agent.
In addition, antitumor activity was seen across a wide range of baseline HER3 expression levels in this study, suggesting that a predictive biomarker remains to be identified, according to Dr. Girard.
“HER3 immunohistochemistry does not seem to be the candidate in this setting,” he said.
The study was sponsored by Daiichi Sankyo. Dr. Jänne reported disclosures related to Daiichi Sankyo, as well as Araxes Pharma, Astellas Pharma, AstraZeneca, and multiple other pharmaceutical companies. Dr. Jänne is also coinventor on a Dana Farber Cancer Institute–owned patent on EGFR mutations licensed to Labcorp and receives postmarketing royalties from this invention.
Dr. Girard reported disclosures related to AbbVie, AstraZeneca, Boehringer Ingelheim, and multiple other pharmaceutical companies.
A HER3-targeted therapy has demonstrated clinically meaningful and durable efficacy in heavily pretreated patients with EGFR-mutant non–small cell lung cancer, according to results of a phase 1 study.
Patritumab deruxtecan, an antibody-drug conjugate targeting HER3, had an overall response rate (ORR) of 39% and median progression-free survival (PFS) of 8.2 months in a phase 1 study that included patients previously treated with tyrosine kinase inhibitors (TKIs) and chemotherapy, the results show.
The efficacy was seen across EGFR TKI resistance mechanisms in this very difficult-to-treat patient population, according to investigator Pasi A. Jänne, MD, PhD, of the Dana-Farber Cancer Institute, Boston.
“There is not one category of individuals that are having a response, or not having a response,” Dr. Jänne said in a presentation at the annual meeting of the American Society of Clinical Oncology (Abstract 9007).
“Responses are observed in patients with identifiable resistance mechanisms, and in patients that do not have an identifiable resistance mechanism, but have progressed on prior EGFR TKI therapy,” he added.
More than 80% of non–small cell lung cancer (NSCLC) tumors express HER3, and of note, HER3 alterations do not appear to confer resistance to EGFR TKIs in patients with EGFR-mutant NSCLC, according to Dr. Jänne.
Study details
Also known as HER3-DXd, patritumab deruxtecan consists of a fully human anti-HER3 monoclonal antibody linked to a topoisomerase inhibitor payload by a tetrapeptide-based cleavable linker.
The antibody-drug conjugate is also being evaluated in metastatic breast cancer and colorectal cancer, Dr. Jänne said.
In the present phase 1 dose escalation and dose expansion study, a total of 57 patients were treated with patritumab deruxtecan at 5.6 mg/kg, the recommended dose for the expansion phase.
The median age of these patients was 65 years, and the majority (63%) were women, Dr. Jänne reported. About half had a history of central nervous system metastases.
The median number of prior lines of systemic therapy was four, making this a heavily pretreated patient population, Dr. Jänne said. All patients had received prior EGFR TKI therapy, and 86% specifically had prior osimertinib. Ninety-one percent had prior platinum-based chemotherapy, and 40% had received immunotherapy.
Spectrum of responses
The confirmed ORR of 39% included 1 complete response (2%) and 21 partial responses (37%), Dr. Jänne reported. The disease control rate was 72%, and median duration of response was 6.9 months at a median follow-up of 10.2 months.
The median PFS was 8.2 months in 57 patients overall and in a subset of 44 patients who had received prior osimertinib and platinum-based chemotherapy, according to the report.
Activity of patritumab deruxtecan was seen not only across patients with diverse mechanisms of EGFR TKI resistance, but also regardless of prior number of treatments, and regardless of history of brain metastases, the investigator said.
In addition, clinical responses were seen across a spectrum of baseline HER3 expression by immunohistochemistry, the investigator added.
Safety was assessed in 81 patients treated at a range of doses in the phase 1 trial. The most common grade 3 or greater treatment-emergent adverse events, observed in 5% or more of patients, included thrombocytopenia, neutropenia, and anemia, while other side effects such as fatigue and dyspnea were observed, Dr. Jänne said. About 9% of the adverse events led to treatment discontinuation in the safety cohort.
Interstitial lung disease was observed in four patients, or 5% of the safety cohort. Three of these were grade 1-2 and one was grade 3, according to the report.
Questions to explore
The efficacy of patritumab deruxtecan was “high” in this phase 1 study, based on the reported response rate and median PFS, said discussant Nicolas Girard, MD, PhD, of Institut Curie in Paris.
However, the most striking finding of the study was the efficacy of the antibody-drug conjugate across all reported resistance mechanisms, Dr. Girard said in his remarks.
Questions that remains to be explored, according to Dr. Girard, include the impact of previous treatment sequencing with TKIs and chemotherapy on patient outcomes with patritumab deruxtecan, as well as the assessment of intracranial response and PFS for patients treated with the agent.
In addition, antitumor activity was seen across a wide range of baseline HER3 expression levels in this study, suggesting that a predictive biomarker remains to be identified, according to Dr. Girard.
“HER3 immunohistochemistry does not seem to be the candidate in this setting,” he said.
The study was sponsored by Daiichi Sankyo. Dr. Jänne reported disclosures related to Daiichi Sankyo, as well as Araxes Pharma, Astellas Pharma, AstraZeneca, and multiple other pharmaceutical companies. Dr. Jänne is also coinventor on a Dana Farber Cancer Institute–owned patent on EGFR mutations licensed to Labcorp and receives postmarketing royalties from this invention.
Dr. Girard reported disclosures related to AbbVie, AstraZeneca, Boehringer Ingelheim, and multiple other pharmaceutical companies.
A HER3-targeted therapy has demonstrated clinically meaningful and durable efficacy in heavily pretreated patients with EGFR-mutant non–small cell lung cancer, according to results of a phase 1 study.
Patritumab deruxtecan, an antibody-drug conjugate targeting HER3, had an overall response rate (ORR) of 39% and median progression-free survival (PFS) of 8.2 months in a phase 1 study that included patients previously treated with tyrosine kinase inhibitors (TKIs) and chemotherapy, the results show.
The efficacy was seen across EGFR TKI resistance mechanisms in this very difficult-to-treat patient population, according to investigator Pasi A. Jänne, MD, PhD, of the Dana-Farber Cancer Institute, Boston.
“There is not one category of individuals that are having a response, or not having a response,” Dr. Jänne said in a presentation at the annual meeting of the American Society of Clinical Oncology (Abstract 9007).
“Responses are observed in patients with identifiable resistance mechanisms, and in patients that do not have an identifiable resistance mechanism, but have progressed on prior EGFR TKI therapy,” he added.
More than 80% of non–small cell lung cancer (NSCLC) tumors express HER3, and of note, HER3 alterations do not appear to confer resistance to EGFR TKIs in patients with EGFR-mutant NSCLC, according to Dr. Jänne.
Study details
Also known as HER3-DXd, patritumab deruxtecan consists of a fully human anti-HER3 monoclonal antibody linked to a topoisomerase inhibitor payload by a tetrapeptide-based cleavable linker.
The antibody-drug conjugate is also being evaluated in metastatic breast cancer and colorectal cancer, Dr. Jänne said.
In the present phase 1 dose escalation and dose expansion study, a total of 57 patients were treated with patritumab deruxtecan at 5.6 mg/kg, the recommended dose for the expansion phase.
The median age of these patients was 65 years, and the majority (63%) were women, Dr. Jänne reported. About half had a history of central nervous system metastases.
The median number of prior lines of systemic therapy was four, making this a heavily pretreated patient population, Dr. Jänne said. All patients had received prior EGFR TKI therapy, and 86% specifically had prior osimertinib. Ninety-one percent had prior platinum-based chemotherapy, and 40% had received immunotherapy.
Spectrum of responses
The confirmed ORR of 39% included 1 complete response (2%) and 21 partial responses (37%), Dr. Jänne reported. The disease control rate was 72%, and median duration of response was 6.9 months at a median follow-up of 10.2 months.
The median PFS was 8.2 months in 57 patients overall and in a subset of 44 patients who had received prior osimertinib and platinum-based chemotherapy, according to the report.
Activity of patritumab deruxtecan was seen not only across patients with diverse mechanisms of EGFR TKI resistance, but also regardless of prior number of treatments, and regardless of history of brain metastases, the investigator said.
In addition, clinical responses were seen across a spectrum of baseline HER3 expression by immunohistochemistry, the investigator added.
Safety was assessed in 81 patients treated at a range of doses in the phase 1 trial. The most common grade 3 or greater treatment-emergent adverse events, observed in 5% or more of patients, included thrombocytopenia, neutropenia, and anemia, while other side effects such as fatigue and dyspnea were observed, Dr. Jänne said. About 9% of the adverse events led to treatment discontinuation in the safety cohort.
Interstitial lung disease was observed in four patients, or 5% of the safety cohort. Three of these were grade 1-2 and one was grade 3, according to the report.
Questions to explore
The efficacy of patritumab deruxtecan was “high” in this phase 1 study, based on the reported response rate and median PFS, said discussant Nicolas Girard, MD, PhD, of Institut Curie in Paris.
However, the most striking finding of the study was the efficacy of the antibody-drug conjugate across all reported resistance mechanisms, Dr. Girard said in his remarks.
Questions that remains to be explored, according to Dr. Girard, include the impact of previous treatment sequencing with TKIs and chemotherapy on patient outcomes with patritumab deruxtecan, as well as the assessment of intracranial response and PFS for patients treated with the agent.
In addition, antitumor activity was seen across a wide range of baseline HER3 expression levels in this study, suggesting that a predictive biomarker remains to be identified, according to Dr. Girard.
“HER3 immunohistochemistry does not seem to be the candidate in this setting,” he said.
The study was sponsored by Daiichi Sankyo. Dr. Jänne reported disclosures related to Daiichi Sankyo, as well as Araxes Pharma, Astellas Pharma, AstraZeneca, and multiple other pharmaceutical companies. Dr. Jänne is also coinventor on a Dana Farber Cancer Institute–owned patent on EGFR mutations licensed to Labcorp and receives postmarketing royalties from this invention.
Dr. Girard reported disclosures related to AbbVie, AstraZeneca, Boehringer Ingelheim, and multiple other pharmaceutical companies.
REPORTING FROM ASCO 2021
Surgical outcomes favor addition of nivolumab to neoadjuvant chemo in resectable lung cancers
The addition of nivolumab to neoadjuvant chemotherapy did not impede the feasibility or timing of surgery in patients with resectable lung cancer, according to results from the phase 3 CheckMate 816 trial.
Adding nivolumab to chemotherapy was tolerable and did not increase the rate of surgical complications, investigator Jonathan Spicer, FRCPC, MD, PhD, of McGill University, Montreal, said in his presentation at the annual meeting of the American Society of Clinical Oncology.
His presentation comes about 2 months after the reporting of primary endpoint results of CheckMate 816 (NCT02998528). CheckMate 816 demonstrated that adding nivolumab to neoadjuvant chemotherapy significantly improved pathological complete response (pCR) in patients with resectable non–small cell lung cancer (NSCLC), according to results presented earlier at the American Association for Cancer Research annual meeting.
“The safety and surgical outcome data reported thus far from CheckMate 816, along with significant improvement in pathological complete response, support nivolumab in combination with chemotherapy as an attractive neoadjuvant option for patients with resectable NSCLC,” said Dr. Spicer (Abstract 8503).
Building on previous experience
The CheckMate 816 study builds on extensive experience in advanced NSCLC that has consistently shown better outcomes, including overall survival, with combinations of chemotherapy and immuno-oncology (IO) agents, compared to chemotherapy alone, said discussant Valerie W. Rusch, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Rusch called out “salient and interesting results” regarding surgical management in CheckMate 816, including a lower rate of surgery cancellations and shorter surgical duration in the chemotherapy-plus-IO arm, compared to the chemotherapy-alone arm.
Furthermore, fewer patients required a pneumonectomy and more patients had a complete resection in the chemotherapy-plus-IO arm, compared to chemotherapy alone, she noted.
“These excellent surgical results, along with the data previously presented at AACR regarding the primary endpoint, help to establish a new standard of neoadjuvant care,” Dr. Rusch said in her presentation.
Study details
CheckMate 816 included 358 patients with newly diagnosed, resectable, stage IB-IIIA NSCLC, Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, and no known EGFR mutations or ALK alterations. Patients were randomized to receive nivolumab and platinum-doublet chemotherapy (nivolumab/chemotherapy) or chemotherapy alone every 3 weeks, with surgery to be performed within 6 weeks of the last dose of neoadjuvant treatment.
The median age of patients was 64 years in the nivolumab/chemotherapy arm and 65 years in the chemotherapy-alone arm. About one-third of patients had ECOG performance status of one, and about half had squamous tumor histology, Dr. Spicer said in his report. Almost two-thirds of patients had stage IIIA disease.
In the study results previously presented at the AACR meeting, both pCR and major pathologic response were significantly better following neoadjuvant chemotherapy and IO treatment, compared to chemotherapy alone.
In the intention-to-treat analysis, 24.0% of patients treated with nivolumab/chemotherapy achieved a pCR, compared to 2.2% in the chemotherapy arm, amounting to an approximate 12-fold increase in pCR, Dr. Spicer said. Similarly, the rate of major pathologic response in the intention-to-treat analysis was 36.9% and 8.9% for the nivolumab/chemotherapy and chemotherapy arms, respectively.
Surgical results
In his ASCO presentation, Dr. Spicer reported that definitive surgery was canceled in 16% of patients in the nivolumab/chemotherapy arm, and 21% of the chemotherapy arm. Reasons for surgery cancellation generally included patients declining surgery, unresectable disease, and poor lung function. “Cancellation of surgery due to neoadjuvant therapy toxicity was rare,” Dr. Spicer said in his presentation.
Among patients who did proceed to surgery, the median duration of the procedure was 184 minutes in the nivolumab/chemotherapy arm and 217 minutes in the chemotherapy arm. That half-hour difference in favor of the combination arm suggests that the complexity of surgery was not increased by the addition of nivolumab, Dr. Spicer said.
Median time to surgery was about 5 weeks in both arms, which was “well within accepted standards for a neoadjuvant therapeutic approach,” Dr. Spicer said. Most delays beyond 6 weeks were due to administrative issues, and occurred in similar proportions (21% of the nivolumab/chemotherapy arm and 18% of the chemotherapy arm).
The addition of nivolumab to chemotherapy improved pCR rates regardless of baseline stage of disease, according to Dr. Spicer. Furthermore, the depth of pathological regression in the primary tumor was “dramatically different” across stage groupings, he said. Median residual viable tumor percentage in stage IB/II patients was 28% for nivolumab/chemotherapy and 79% for chemotherapy, and in stage IIIA patients, it was 8% for nivolumab/chemotherapy and 70% for chemotherapy.
Overall, thoracotomy was the most frequent surgical approach in this international phase 3 trial, Dr. Spicer said. However, among patients with stage IIIA disease, minimally invasive approaches were used 30% of the time in the nivolumab/chemotherapy arm and 19% in the chemotherapy arm. Conversely, the rate of conversion from a minimally invasive to open approach in patients with stage IIIA disease was 11% for nivolumab/chemotherapy and 20% for chemotherapy alone.
Lobectomy was more frequent in the nivolumab/chemotherapy arm (77%) compared to the chemotherapy arm (61%), a difference that Dr. Spicer described as clinically important. He said the difference appears to be attributable to a lower rate of pneumonectomy in the nivolumab/chemotherapy arm (17%) than in the chemotherapy arm (25%).
Despite less extensive lung resection being required, the rate of R0 resection was numerically higher in the nivolumab/chemotherapy arm (83%) than in the chemotherapy arm (78%), said Dr. Spicer.
Length of hospital stay was “within expected ranges” from geographic regions represented in the trial, Dr. Spicer said. Median length of stay was 4.0 and 6.0 days, respectively, for nivolumab/chemotherapy and chemotherapy alone in North America, 9.5 and 13.0 days in Europe, and 11.0 and 13.0 days in Asia.
Likewise, 90-day surgical complications were well within expected ranges, according to the investigator, with anemia, pain, and wound complications being the most commonly reported. Rates were generally similar between study arms, other than a twofold higher rate of pain in the chemotherapy arm, possibly due to the lower rate of minimally invasive surgery or higher rate of conversion to an open procedure, compared to the nivolumab/chemotherapy arm, he said.
Awaiting survival
Rates of 30- and 90-day mortality are expected to be evaluated when survival endpoints are available, according to Dr. Spicer. Beyond pCR rate, event-free survival is also a primary endpoint of the study, while overall survival is a secondary endpoint.
The study was supported by Bristol Myers Squibb. Dr. Spicer reported disclosures related to AstraZeneca, Bristol Myers Squibb Foundation, Merck, and Roche. Dr. Rusch reported research funding with Genelux and Genentech, and travel expenses from Intuitive Surgical.
The addition of nivolumab to neoadjuvant chemotherapy did not impede the feasibility or timing of surgery in patients with resectable lung cancer, according to results from the phase 3 CheckMate 816 trial.
Adding nivolumab to chemotherapy was tolerable and did not increase the rate of surgical complications, investigator Jonathan Spicer, FRCPC, MD, PhD, of McGill University, Montreal, said in his presentation at the annual meeting of the American Society of Clinical Oncology.
His presentation comes about 2 months after the reporting of primary endpoint results of CheckMate 816 (NCT02998528). CheckMate 816 demonstrated that adding nivolumab to neoadjuvant chemotherapy significantly improved pathological complete response (pCR) in patients with resectable non–small cell lung cancer (NSCLC), according to results presented earlier at the American Association for Cancer Research annual meeting.
“The safety and surgical outcome data reported thus far from CheckMate 816, along with significant improvement in pathological complete response, support nivolumab in combination with chemotherapy as an attractive neoadjuvant option for patients with resectable NSCLC,” said Dr. Spicer (Abstract 8503).
Building on previous experience
The CheckMate 816 study builds on extensive experience in advanced NSCLC that has consistently shown better outcomes, including overall survival, with combinations of chemotherapy and immuno-oncology (IO) agents, compared to chemotherapy alone, said discussant Valerie W. Rusch, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Rusch called out “salient and interesting results” regarding surgical management in CheckMate 816, including a lower rate of surgery cancellations and shorter surgical duration in the chemotherapy-plus-IO arm, compared to the chemotherapy-alone arm.
Furthermore, fewer patients required a pneumonectomy and more patients had a complete resection in the chemotherapy-plus-IO arm, compared to chemotherapy alone, she noted.
“These excellent surgical results, along with the data previously presented at AACR regarding the primary endpoint, help to establish a new standard of neoadjuvant care,” Dr. Rusch said in her presentation.
Study details
CheckMate 816 included 358 patients with newly diagnosed, resectable, stage IB-IIIA NSCLC, Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, and no known EGFR mutations or ALK alterations. Patients were randomized to receive nivolumab and platinum-doublet chemotherapy (nivolumab/chemotherapy) or chemotherapy alone every 3 weeks, with surgery to be performed within 6 weeks of the last dose of neoadjuvant treatment.
The median age of patients was 64 years in the nivolumab/chemotherapy arm and 65 years in the chemotherapy-alone arm. About one-third of patients had ECOG performance status of one, and about half had squamous tumor histology, Dr. Spicer said in his report. Almost two-thirds of patients had stage IIIA disease.
In the study results previously presented at the AACR meeting, both pCR and major pathologic response were significantly better following neoadjuvant chemotherapy and IO treatment, compared to chemotherapy alone.
In the intention-to-treat analysis, 24.0% of patients treated with nivolumab/chemotherapy achieved a pCR, compared to 2.2% in the chemotherapy arm, amounting to an approximate 12-fold increase in pCR, Dr. Spicer said. Similarly, the rate of major pathologic response in the intention-to-treat analysis was 36.9% and 8.9% for the nivolumab/chemotherapy and chemotherapy arms, respectively.
Surgical results
In his ASCO presentation, Dr. Spicer reported that definitive surgery was canceled in 16% of patients in the nivolumab/chemotherapy arm, and 21% of the chemotherapy arm. Reasons for surgery cancellation generally included patients declining surgery, unresectable disease, and poor lung function. “Cancellation of surgery due to neoadjuvant therapy toxicity was rare,” Dr. Spicer said in his presentation.
Among patients who did proceed to surgery, the median duration of the procedure was 184 minutes in the nivolumab/chemotherapy arm and 217 minutes in the chemotherapy arm. That half-hour difference in favor of the combination arm suggests that the complexity of surgery was not increased by the addition of nivolumab, Dr. Spicer said.
Median time to surgery was about 5 weeks in both arms, which was “well within accepted standards for a neoadjuvant therapeutic approach,” Dr. Spicer said. Most delays beyond 6 weeks were due to administrative issues, and occurred in similar proportions (21% of the nivolumab/chemotherapy arm and 18% of the chemotherapy arm).
The addition of nivolumab to chemotherapy improved pCR rates regardless of baseline stage of disease, according to Dr. Spicer. Furthermore, the depth of pathological regression in the primary tumor was “dramatically different” across stage groupings, he said. Median residual viable tumor percentage in stage IB/II patients was 28% for nivolumab/chemotherapy and 79% for chemotherapy, and in stage IIIA patients, it was 8% for nivolumab/chemotherapy and 70% for chemotherapy.
Overall, thoracotomy was the most frequent surgical approach in this international phase 3 trial, Dr. Spicer said. However, among patients with stage IIIA disease, minimally invasive approaches were used 30% of the time in the nivolumab/chemotherapy arm and 19% in the chemotherapy arm. Conversely, the rate of conversion from a minimally invasive to open approach in patients with stage IIIA disease was 11% for nivolumab/chemotherapy and 20% for chemotherapy alone.
Lobectomy was more frequent in the nivolumab/chemotherapy arm (77%) compared to the chemotherapy arm (61%), a difference that Dr. Spicer described as clinically important. He said the difference appears to be attributable to a lower rate of pneumonectomy in the nivolumab/chemotherapy arm (17%) than in the chemotherapy arm (25%).
Despite less extensive lung resection being required, the rate of R0 resection was numerically higher in the nivolumab/chemotherapy arm (83%) than in the chemotherapy arm (78%), said Dr. Spicer.
Length of hospital stay was “within expected ranges” from geographic regions represented in the trial, Dr. Spicer said. Median length of stay was 4.0 and 6.0 days, respectively, for nivolumab/chemotherapy and chemotherapy alone in North America, 9.5 and 13.0 days in Europe, and 11.0 and 13.0 days in Asia.
Likewise, 90-day surgical complications were well within expected ranges, according to the investigator, with anemia, pain, and wound complications being the most commonly reported. Rates were generally similar between study arms, other than a twofold higher rate of pain in the chemotherapy arm, possibly due to the lower rate of minimally invasive surgery or higher rate of conversion to an open procedure, compared to the nivolumab/chemotherapy arm, he said.
Awaiting survival
Rates of 30- and 90-day mortality are expected to be evaluated when survival endpoints are available, according to Dr. Spicer. Beyond pCR rate, event-free survival is also a primary endpoint of the study, while overall survival is a secondary endpoint.
The study was supported by Bristol Myers Squibb. Dr. Spicer reported disclosures related to AstraZeneca, Bristol Myers Squibb Foundation, Merck, and Roche. Dr. Rusch reported research funding with Genelux and Genentech, and travel expenses from Intuitive Surgical.
The addition of nivolumab to neoadjuvant chemotherapy did not impede the feasibility or timing of surgery in patients with resectable lung cancer, according to results from the phase 3 CheckMate 816 trial.
Adding nivolumab to chemotherapy was tolerable and did not increase the rate of surgical complications, investigator Jonathan Spicer, FRCPC, MD, PhD, of McGill University, Montreal, said in his presentation at the annual meeting of the American Society of Clinical Oncology.
His presentation comes about 2 months after the reporting of primary endpoint results of CheckMate 816 (NCT02998528). CheckMate 816 demonstrated that adding nivolumab to neoadjuvant chemotherapy significantly improved pathological complete response (pCR) in patients with resectable non–small cell lung cancer (NSCLC), according to results presented earlier at the American Association for Cancer Research annual meeting.
“The safety and surgical outcome data reported thus far from CheckMate 816, along with significant improvement in pathological complete response, support nivolumab in combination with chemotherapy as an attractive neoadjuvant option for patients with resectable NSCLC,” said Dr. Spicer (Abstract 8503).
Building on previous experience
The CheckMate 816 study builds on extensive experience in advanced NSCLC that has consistently shown better outcomes, including overall survival, with combinations of chemotherapy and immuno-oncology (IO) agents, compared to chemotherapy alone, said discussant Valerie W. Rusch, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Rusch called out “salient and interesting results” regarding surgical management in CheckMate 816, including a lower rate of surgery cancellations and shorter surgical duration in the chemotherapy-plus-IO arm, compared to the chemotherapy-alone arm.
Furthermore, fewer patients required a pneumonectomy and more patients had a complete resection in the chemotherapy-plus-IO arm, compared to chemotherapy alone, she noted.
“These excellent surgical results, along with the data previously presented at AACR regarding the primary endpoint, help to establish a new standard of neoadjuvant care,” Dr. Rusch said in her presentation.
Study details
CheckMate 816 included 358 patients with newly diagnosed, resectable, stage IB-IIIA NSCLC, Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, and no known EGFR mutations or ALK alterations. Patients were randomized to receive nivolumab and platinum-doublet chemotherapy (nivolumab/chemotherapy) or chemotherapy alone every 3 weeks, with surgery to be performed within 6 weeks of the last dose of neoadjuvant treatment.
The median age of patients was 64 years in the nivolumab/chemotherapy arm and 65 years in the chemotherapy-alone arm. About one-third of patients had ECOG performance status of one, and about half had squamous tumor histology, Dr. Spicer said in his report. Almost two-thirds of patients had stage IIIA disease.
In the study results previously presented at the AACR meeting, both pCR and major pathologic response were significantly better following neoadjuvant chemotherapy and IO treatment, compared to chemotherapy alone.
In the intention-to-treat analysis, 24.0% of patients treated with nivolumab/chemotherapy achieved a pCR, compared to 2.2% in the chemotherapy arm, amounting to an approximate 12-fold increase in pCR, Dr. Spicer said. Similarly, the rate of major pathologic response in the intention-to-treat analysis was 36.9% and 8.9% for the nivolumab/chemotherapy and chemotherapy arms, respectively.
Surgical results
In his ASCO presentation, Dr. Spicer reported that definitive surgery was canceled in 16% of patients in the nivolumab/chemotherapy arm, and 21% of the chemotherapy arm. Reasons for surgery cancellation generally included patients declining surgery, unresectable disease, and poor lung function. “Cancellation of surgery due to neoadjuvant therapy toxicity was rare,” Dr. Spicer said in his presentation.
Among patients who did proceed to surgery, the median duration of the procedure was 184 minutes in the nivolumab/chemotherapy arm and 217 minutes in the chemotherapy arm. That half-hour difference in favor of the combination arm suggests that the complexity of surgery was not increased by the addition of nivolumab, Dr. Spicer said.
Median time to surgery was about 5 weeks in both arms, which was “well within accepted standards for a neoadjuvant therapeutic approach,” Dr. Spicer said. Most delays beyond 6 weeks were due to administrative issues, and occurred in similar proportions (21% of the nivolumab/chemotherapy arm and 18% of the chemotherapy arm).
The addition of nivolumab to chemotherapy improved pCR rates regardless of baseline stage of disease, according to Dr. Spicer. Furthermore, the depth of pathological regression in the primary tumor was “dramatically different” across stage groupings, he said. Median residual viable tumor percentage in stage IB/II patients was 28% for nivolumab/chemotherapy and 79% for chemotherapy, and in stage IIIA patients, it was 8% for nivolumab/chemotherapy and 70% for chemotherapy.
Overall, thoracotomy was the most frequent surgical approach in this international phase 3 trial, Dr. Spicer said. However, among patients with stage IIIA disease, minimally invasive approaches were used 30% of the time in the nivolumab/chemotherapy arm and 19% in the chemotherapy arm. Conversely, the rate of conversion from a minimally invasive to open approach in patients with stage IIIA disease was 11% for nivolumab/chemotherapy and 20% for chemotherapy alone.
Lobectomy was more frequent in the nivolumab/chemotherapy arm (77%) compared to the chemotherapy arm (61%), a difference that Dr. Spicer described as clinically important. He said the difference appears to be attributable to a lower rate of pneumonectomy in the nivolumab/chemotherapy arm (17%) than in the chemotherapy arm (25%).
Despite less extensive lung resection being required, the rate of R0 resection was numerically higher in the nivolumab/chemotherapy arm (83%) than in the chemotherapy arm (78%), said Dr. Spicer.
Length of hospital stay was “within expected ranges” from geographic regions represented in the trial, Dr. Spicer said. Median length of stay was 4.0 and 6.0 days, respectively, for nivolumab/chemotherapy and chemotherapy alone in North America, 9.5 and 13.0 days in Europe, and 11.0 and 13.0 days in Asia.
Likewise, 90-day surgical complications were well within expected ranges, according to the investigator, with anemia, pain, and wound complications being the most commonly reported. Rates were generally similar between study arms, other than a twofold higher rate of pain in the chemotherapy arm, possibly due to the lower rate of minimally invasive surgery or higher rate of conversion to an open procedure, compared to the nivolumab/chemotherapy arm, he said.
Awaiting survival
Rates of 30- and 90-day mortality are expected to be evaluated when survival endpoints are available, according to Dr. Spicer. Beyond pCR rate, event-free survival is also a primary endpoint of the study, while overall survival is a secondary endpoint.
The study was supported by Bristol Myers Squibb. Dr. Spicer reported disclosures related to AstraZeneca, Bristol Myers Squibb Foundation, Merck, and Roche. Dr. Rusch reported research funding with Genelux and Genentech, and travel expenses from Intuitive Surgical.
FROM ASCO 2021
Vinorelbine survival benefit in mesothelioma overshadowed by advances in immuno-oncology
After decades of off-label use, vinorelbine finally has a randomized clinical trial supporting its efficacy as a second-line treatment of mesothelioma, an investigator reported at the annual meeting of the American Society of Clinical Oncology.
However, the development of other treatment regimens, and notably immuno-oncology approaches, are pushing this classic chemotherapy option to later lines of therapy in patients with malignant pleural mesothelioma (MPM), a speaker said at the meeting.
Adding vinorelbine to active symptom control statistically improved progression-free survival (PFS), among patients with MPM who had prior platinum-based therapy, according to results of the randomized Vinorelbine in Mesothelioma (VIM) trial.
Median PFS reached 4.2 months in the vinorelbine arm, versus 2.8 months for active symptom control alone, study results show.
That finding , coupled with safety results, supports the off-label use of vinorelbine as a treatment option for patients with relapsed MPM, according to investigator Dean Anthony Fennell, FRCP, PhD, of the University of Leicester (England).
“Vinorelbine appears to be a safe and effective treatment and could be considered as a treatment option for patients with relapsed mesothelioma,” Prof. Fennell said in his presentation at ASCO (Abstract 8507).
Changing status
While that welcome pronouncement was a long time coming, there are now other promising treatment approaches that relegate vinorelbine to a “lower priority” in the treatment algorithm, said discussant Anna K. Nowak, MBBS, FRACP, PhD, of the University of Western Australia, Nedlands.
In October 2020, the U.S. Food and Drug Administration approved the combination of ipilimumab and nivolumab for the first-line treatment of unresectable malignant pleural mesothelioma, on the basis of results from CHECKMATE-743, a randomized, open-label trial.
“Certainly, we know now that first-line ipilimumab and nivolumab is of very substantial benefit to these patients, and we still have clinical trials open in this space as well,” Prof. Nowak said in her discussion at ASCO.
There is “no doubt” that many patients with mesothelioma should at some point receive an IO agent, particularly now with recently reported randomized clinical trial evidence of an overall survival benefit, she added.
“This really pushes vinorelbine out to be a third- or fourth-line treatment,” she added, “and we know that there are usually diminishing returns from using chemotherapies further down the treatment algorithm.”
Trial details
The VIM trial described at ASCO by Prof. Fennell was a randomized, controlled phase 2 including 154 patients with MPM that had progressed after first-line chemotherapy.
“Vinorelbine has shown useful clinical activity in single-arm phase two studies, however, the specific efficacy of vinorelbine has not been evaluated in an appropriately controlled randomized trial,” Prof. Fennell said in this presentation.
Patients in the trial were randomized 2:1 to either vinorelbine plus active supportive care or active supportive care alone. Vinorelbine was given initially at 60 mg/m2 weekly every 21 days, escalated to 80 mg/m2 from cycle 2.
The median age of patients in VIM was approximately 71 years, and about 80% were male.
More partial responses were seen in the vinorelbine arm, at 3.1% of patients, compared with 1.8% for active supportive care, according to Prof. Fennell. Likewise, the rate of stable disease was higher in the vinorelbine arm, at 62.2%, versus 46.4% in the control arm.
The primary outcome of the study, PFS, was significantly improved in the vinorelbine arm, according to Dr. Fennell. The median PFS was 4.2 months in the vinorelbine arm and 2.8 months in the supportive care arm (P = .002), translating into a hazard ratio of 0.60 (95% confidence interval, 0.41-0.86), Prof. Fennell reported.
The most common grade 3-4 adverse event was neutropenia, occurring in 12.5% of the vinorelbine-treated patients and no patients managed with supportive care alone, according to the report. Other grade 3-4 adverse events occurred in fewer than 10% of patients and included dyspnea, lower respiratory infection, lymphopenia, and fatigue, among others.
Overall survival (OS) was not statistically different between vinorelbine and supportive care arms, with median OS of 9.3 months and 9.1 months, respectively.
However, a number of patients in the control arm went on to receive subsequent therapy, including 15 (or about 27%) who went into CONFIRM, a randomized phase 3 trial that, as recently reported, met its coprimary endpoints of improve OS and PFS with nivolumab vs. placebo in relapsed malignant mesothelioma.
Investigators also sought to test the hypothesis that BRCA1-negative patients might be chemoresistant, based in part on preclinical models demonstrating that BRCA1 predicted sensitivity to vinorelbine. However, there was no difference in PFS by BRCA1 expression in the VIM study, according to Prof. Fennell.
Taken together, findings of the VIM trial suggest vinorelbine is a “modestly active” agent with low cost and acceptable toxicity, according to Prof. Nowak.
“It is incumbent on us to have clear discussions with our patients on the risks and benefits of trying this as a subsequent-line therapy, in the context of this evidence that was generated as a second-line therapy,” she said in her discussant remarks on the study.
“I would say that it is a lower priority in our algorithm than cisplatin and pemetrexed, or of course, immuno-oncology agents,” she added.
Dr. Fennell reported disclosures related to AstraZeneca, Astex Therapeutics, Bayer, and multiple other pharmaceutical companies. Dr. Nowak reported disclosures with AstraZeneca, Atara Biotherapeutics, Boehringer Ingelheim, and multiple other pharmaceutical companies.
After decades of off-label use, vinorelbine finally has a randomized clinical trial supporting its efficacy as a second-line treatment of mesothelioma, an investigator reported at the annual meeting of the American Society of Clinical Oncology.
However, the development of other treatment regimens, and notably immuno-oncology approaches, are pushing this classic chemotherapy option to later lines of therapy in patients with malignant pleural mesothelioma (MPM), a speaker said at the meeting.
Adding vinorelbine to active symptom control statistically improved progression-free survival (PFS), among patients with MPM who had prior platinum-based therapy, according to results of the randomized Vinorelbine in Mesothelioma (VIM) trial.
Median PFS reached 4.2 months in the vinorelbine arm, versus 2.8 months for active symptom control alone, study results show.
That finding , coupled with safety results, supports the off-label use of vinorelbine as a treatment option for patients with relapsed MPM, according to investigator Dean Anthony Fennell, FRCP, PhD, of the University of Leicester (England).
“Vinorelbine appears to be a safe and effective treatment and could be considered as a treatment option for patients with relapsed mesothelioma,” Prof. Fennell said in his presentation at ASCO (Abstract 8507).
Changing status
While that welcome pronouncement was a long time coming, there are now other promising treatment approaches that relegate vinorelbine to a “lower priority” in the treatment algorithm, said discussant Anna K. Nowak, MBBS, FRACP, PhD, of the University of Western Australia, Nedlands.
In October 2020, the U.S. Food and Drug Administration approved the combination of ipilimumab and nivolumab for the first-line treatment of unresectable malignant pleural mesothelioma, on the basis of results from CHECKMATE-743, a randomized, open-label trial.
“Certainly, we know now that first-line ipilimumab and nivolumab is of very substantial benefit to these patients, and we still have clinical trials open in this space as well,” Prof. Nowak said in her discussion at ASCO.
There is “no doubt” that many patients with mesothelioma should at some point receive an IO agent, particularly now with recently reported randomized clinical trial evidence of an overall survival benefit, she added.
“This really pushes vinorelbine out to be a third- or fourth-line treatment,” she added, “and we know that there are usually diminishing returns from using chemotherapies further down the treatment algorithm.”
Trial details
The VIM trial described at ASCO by Prof. Fennell was a randomized, controlled phase 2 including 154 patients with MPM that had progressed after first-line chemotherapy.
“Vinorelbine has shown useful clinical activity in single-arm phase two studies, however, the specific efficacy of vinorelbine has not been evaluated in an appropriately controlled randomized trial,” Prof. Fennell said in this presentation.
Patients in the trial were randomized 2:1 to either vinorelbine plus active supportive care or active supportive care alone. Vinorelbine was given initially at 60 mg/m2 weekly every 21 days, escalated to 80 mg/m2 from cycle 2.
The median age of patients in VIM was approximately 71 years, and about 80% were male.
More partial responses were seen in the vinorelbine arm, at 3.1% of patients, compared with 1.8% for active supportive care, according to Prof. Fennell. Likewise, the rate of stable disease was higher in the vinorelbine arm, at 62.2%, versus 46.4% in the control arm.
The primary outcome of the study, PFS, was significantly improved in the vinorelbine arm, according to Dr. Fennell. The median PFS was 4.2 months in the vinorelbine arm and 2.8 months in the supportive care arm (P = .002), translating into a hazard ratio of 0.60 (95% confidence interval, 0.41-0.86), Prof. Fennell reported.
The most common grade 3-4 adverse event was neutropenia, occurring in 12.5% of the vinorelbine-treated patients and no patients managed with supportive care alone, according to the report. Other grade 3-4 adverse events occurred in fewer than 10% of patients and included dyspnea, lower respiratory infection, lymphopenia, and fatigue, among others.
Overall survival (OS) was not statistically different between vinorelbine and supportive care arms, with median OS of 9.3 months and 9.1 months, respectively.
However, a number of patients in the control arm went on to receive subsequent therapy, including 15 (or about 27%) who went into CONFIRM, a randomized phase 3 trial that, as recently reported, met its coprimary endpoints of improve OS and PFS with nivolumab vs. placebo in relapsed malignant mesothelioma.
Investigators also sought to test the hypothesis that BRCA1-negative patients might be chemoresistant, based in part on preclinical models demonstrating that BRCA1 predicted sensitivity to vinorelbine. However, there was no difference in PFS by BRCA1 expression in the VIM study, according to Prof. Fennell.
Taken together, findings of the VIM trial suggest vinorelbine is a “modestly active” agent with low cost and acceptable toxicity, according to Prof. Nowak.
“It is incumbent on us to have clear discussions with our patients on the risks and benefits of trying this as a subsequent-line therapy, in the context of this evidence that was generated as a second-line therapy,” she said in her discussant remarks on the study.
“I would say that it is a lower priority in our algorithm than cisplatin and pemetrexed, or of course, immuno-oncology agents,” she added.
Dr. Fennell reported disclosures related to AstraZeneca, Astex Therapeutics, Bayer, and multiple other pharmaceutical companies. Dr. Nowak reported disclosures with AstraZeneca, Atara Biotherapeutics, Boehringer Ingelheim, and multiple other pharmaceutical companies.
After decades of off-label use, vinorelbine finally has a randomized clinical trial supporting its efficacy as a second-line treatment of mesothelioma, an investigator reported at the annual meeting of the American Society of Clinical Oncology.
However, the development of other treatment regimens, and notably immuno-oncology approaches, are pushing this classic chemotherapy option to later lines of therapy in patients with malignant pleural mesothelioma (MPM), a speaker said at the meeting.
Adding vinorelbine to active symptom control statistically improved progression-free survival (PFS), among patients with MPM who had prior platinum-based therapy, according to results of the randomized Vinorelbine in Mesothelioma (VIM) trial.
Median PFS reached 4.2 months in the vinorelbine arm, versus 2.8 months for active symptom control alone, study results show.
That finding , coupled with safety results, supports the off-label use of vinorelbine as a treatment option for patients with relapsed MPM, according to investigator Dean Anthony Fennell, FRCP, PhD, of the University of Leicester (England).
“Vinorelbine appears to be a safe and effective treatment and could be considered as a treatment option for patients with relapsed mesothelioma,” Prof. Fennell said in his presentation at ASCO (Abstract 8507).
Changing status
While that welcome pronouncement was a long time coming, there are now other promising treatment approaches that relegate vinorelbine to a “lower priority” in the treatment algorithm, said discussant Anna K. Nowak, MBBS, FRACP, PhD, of the University of Western Australia, Nedlands.
In October 2020, the U.S. Food and Drug Administration approved the combination of ipilimumab and nivolumab for the first-line treatment of unresectable malignant pleural mesothelioma, on the basis of results from CHECKMATE-743, a randomized, open-label trial.
“Certainly, we know now that first-line ipilimumab and nivolumab is of very substantial benefit to these patients, and we still have clinical trials open in this space as well,” Prof. Nowak said in her discussion at ASCO.
There is “no doubt” that many patients with mesothelioma should at some point receive an IO agent, particularly now with recently reported randomized clinical trial evidence of an overall survival benefit, she added.
“This really pushes vinorelbine out to be a third- or fourth-line treatment,” she added, “and we know that there are usually diminishing returns from using chemotherapies further down the treatment algorithm.”
Trial details
The VIM trial described at ASCO by Prof. Fennell was a randomized, controlled phase 2 including 154 patients with MPM that had progressed after first-line chemotherapy.
“Vinorelbine has shown useful clinical activity in single-arm phase two studies, however, the specific efficacy of vinorelbine has not been evaluated in an appropriately controlled randomized trial,” Prof. Fennell said in this presentation.
Patients in the trial were randomized 2:1 to either vinorelbine plus active supportive care or active supportive care alone. Vinorelbine was given initially at 60 mg/m2 weekly every 21 days, escalated to 80 mg/m2 from cycle 2.
The median age of patients in VIM was approximately 71 years, and about 80% were male.
More partial responses were seen in the vinorelbine arm, at 3.1% of patients, compared with 1.8% for active supportive care, according to Prof. Fennell. Likewise, the rate of stable disease was higher in the vinorelbine arm, at 62.2%, versus 46.4% in the control arm.
The primary outcome of the study, PFS, was significantly improved in the vinorelbine arm, according to Dr. Fennell. The median PFS was 4.2 months in the vinorelbine arm and 2.8 months in the supportive care arm (P = .002), translating into a hazard ratio of 0.60 (95% confidence interval, 0.41-0.86), Prof. Fennell reported.
The most common grade 3-4 adverse event was neutropenia, occurring in 12.5% of the vinorelbine-treated patients and no patients managed with supportive care alone, according to the report. Other grade 3-4 adverse events occurred in fewer than 10% of patients and included dyspnea, lower respiratory infection, lymphopenia, and fatigue, among others.
Overall survival (OS) was not statistically different between vinorelbine and supportive care arms, with median OS of 9.3 months and 9.1 months, respectively.
However, a number of patients in the control arm went on to receive subsequent therapy, including 15 (or about 27%) who went into CONFIRM, a randomized phase 3 trial that, as recently reported, met its coprimary endpoints of improve OS and PFS with nivolumab vs. placebo in relapsed malignant mesothelioma.
Investigators also sought to test the hypothesis that BRCA1-negative patients might be chemoresistant, based in part on preclinical models demonstrating that BRCA1 predicted sensitivity to vinorelbine. However, there was no difference in PFS by BRCA1 expression in the VIM study, according to Prof. Fennell.
Taken together, findings of the VIM trial suggest vinorelbine is a “modestly active” agent with low cost and acceptable toxicity, according to Prof. Nowak.
“It is incumbent on us to have clear discussions with our patients on the risks and benefits of trying this as a subsequent-line therapy, in the context of this evidence that was generated as a second-line therapy,” she said in her discussant remarks on the study.
“I would say that it is a lower priority in our algorithm than cisplatin and pemetrexed, or of course, immuno-oncology agents,” she added.
Dr. Fennell reported disclosures related to AstraZeneca, Astex Therapeutics, Bayer, and multiple other pharmaceutical companies. Dr. Nowak reported disclosures with AstraZeneca, Atara Biotherapeutics, Boehringer Ingelheim, and multiple other pharmaceutical companies.
REPORTING FROM ASCO 2021
KRAS inhibitor improved survival in phase 2 lung cancer trial
The first KRAS inhibitor approved for the treatment of lung cancer provided a clinically meaningful overall survival benefit in an updated analysis of a phase 2 study.
Treatment with sotorasib yielded a median overall survival (OS) of 12.5 months in patients with previously treated KRAS p.G12C-mutated non-small cell lung cancer (NSCLC), according to an analysis of the phase 2 CodeBreaK 100 trial data presented at the American Society of Clinical Oncology Annual Meeting.
Median progression-free survival (PFS) was 6.8 months in this update, which included a median follow-up of more than 15 months, according to investigator Ferdinandos Skoulidis, MD, PhD, assistant professor of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center in Houston.
Efficacy responses
The confirmed objective response rate was 37.1%, including a 3.2% complete response rate and a median duration of response of 11.1 months, according to the report by Dr. Skoulidis.
In exploratory analyses, the benefit of sotorasib was consistent across patient subgroups, Dr. Skoulidis said in his presentation (Abstract 9003).
In particular, efficacy was observed in subgroups with co-occurring mutations in TP53, STK11, and KEAP1, which are molecular indicators of suboptimal outcomes on standard systemic treatments, according to Dr. Skoulidis.
This update on the registrational phase 2 CodeBreaK100 trial, published concurrently in the New England Journal of Medicine , came just one week after the U.S. Food and Drug Administration (FDA) granted accelerated approval to sotorasib.
Sotorasib was approved for the treatment of patients with previously treated KRAS G12C‑mutated locally advanced or metastatic NSCLC on the basis of previously reported results from CodeBreaK100.
This sotorasib indication represents a “historic milestone,” Dr. Skoulidis said in an interview.
No previously studied selective KRAS inhibitor has been approved despite scientific research efforts that stretch back nearly four decades, he explained.
“In a way, one can say that we have dealt KRAS-mutant lung cancer a knockdown blow, however, I should point out that the fight is not over,” he added.
“These clinical results will no doubt spearhead and galvanize further efforts to develop even more effective therapeutic combinations in the future, as well as identify and either forestall or overcome the eventual development of acquired resistance,” he said.
Only 1 out of 8 patients
The KRAS p.G12C mutation is present in about 13% of lung adenocarcinomas, or about one in every eight patients with nonsquamous NSCLC, Dr. Skoulidis said in the interview.
“We are estimating that this is in the region of 13,000 patients newly diagnosed every year in the U.S., and approximately 13,000 patients or so that are currently being treated in the second- or third-line setting,” he said.
The CodeBreaK100 trial included 126 patients with locally advanced or metastatic NSCLC and KRAS p.G12C mutation who had progressed on prior systemic therapies. About 43% had one prior line of treatment, while 35% had two lines, and 22% had three lines. A total of 81% had previously received both platinum-based chemotherapy and PD-1/PD-L1 axis inhibitors.
Most treatment-related adverse events in the study were grade 1-2 and generally manageable, according to Dr. Skoulidis. About 20% of patients experienced grade 3 treatment-related adverse events, which were mostly diarrhea or increases in aspartate aminotransferase and alanine aminotransferase levels. A grade 4 treatment-related adverse event, pneumonitis and dyspnea, was reported in one patient or approximately 1%.
Confirmatory trial
Although CodeBreak100 is not a randomized trial, the median OS of 12.5 months compares favorably to median OS times in the range of 7.9-10.3 months reported in randomized phase 3 clinical trials and subgroup analysis of randomized phase 3 trials of docetaxel for patients with KRAS-mutant lung adenocarcinoma, Dr. Skoulidis said in a question-and-answer session.
A confirmatory phase 3 CodeBreaK200 trial of sotorasib versus docetaxel in patients with previously treated KRAS p.G12C-mutated NSCLC is underway. That trial is evaluating PFS as a primary endpoint and OS as a secondary endpoint.
“If the same magnitude of benefit, 12.5 months median overall survival, is confirmed in the larger phase 3 clinical trial, as a clinician I would consider that beneficial for patients, compared to the standard of care,” Dr. Skoulidis said during the session.
Mature data
The updated analysis of the phase 2 CodeBreaK100 study is notable for its mature OS data, updated safety and the first molecular subgroup analyses, according to discussant Christine Marie Lovly, MD, PhD, of the division of hematology-oncology at Vanderbilt University Medical Center in Nashville.
“The objective response rate was 37.1%,” she added. “This is a little bit lower than we’re used to for targeted therapies, but remember, this is a different mutation and a very different class of drugs.”
The KRAS G12C inhibitors, several of which are under clinical development, are not tyrosine kinase inhibitors (TKIs), but rather allele-specific inhibitors that target mutant KRAS, trapping it in an inactive conformation, she explained.
Dr. Lovly referenced the exploratory analyses demonstrating efficacy in molecularly defined subgroups, calling it “interesting” that there was no difference in objective response rate between TP53 wild type and mutant tumors.
“We do have data that mutant TP53 seems to confer inferior outcomes for EGFR TKI-directed therapy in patients with EGFR-mutant lung cancer,” she said.
CodeBreaK100 was supported by Amgen, Inc. and partly by a National Institutes of Health Cancer Center Support Grant at Memorial Sloan Kettering Cancer Center.
Dr. Skoulidis reported honoraria from Bristol-Myers Squibb; research funding from AIMM Therapeutics and Amgen; and travel, accommodations, or expenses from Tango Therapeutics. Dr. Lovly reported disclosures related to Amgen, AstraZeneca, Genentech, Novartis, and Pfizer, among others.
The first KRAS inhibitor approved for the treatment of lung cancer provided a clinically meaningful overall survival benefit in an updated analysis of a phase 2 study.
Treatment with sotorasib yielded a median overall survival (OS) of 12.5 months in patients with previously treated KRAS p.G12C-mutated non-small cell lung cancer (NSCLC), according to an analysis of the phase 2 CodeBreaK 100 trial data presented at the American Society of Clinical Oncology Annual Meeting.
Median progression-free survival (PFS) was 6.8 months in this update, which included a median follow-up of more than 15 months, according to investigator Ferdinandos Skoulidis, MD, PhD, assistant professor of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center in Houston.
Efficacy responses
The confirmed objective response rate was 37.1%, including a 3.2% complete response rate and a median duration of response of 11.1 months, according to the report by Dr. Skoulidis.
In exploratory analyses, the benefit of sotorasib was consistent across patient subgroups, Dr. Skoulidis said in his presentation (Abstract 9003).
In particular, efficacy was observed in subgroups with co-occurring mutations in TP53, STK11, and KEAP1, which are molecular indicators of suboptimal outcomes on standard systemic treatments, according to Dr. Skoulidis.
This update on the registrational phase 2 CodeBreaK100 trial, published concurrently in the New England Journal of Medicine , came just one week after the U.S. Food and Drug Administration (FDA) granted accelerated approval to sotorasib.
Sotorasib was approved for the treatment of patients with previously treated KRAS G12C‑mutated locally advanced or metastatic NSCLC on the basis of previously reported results from CodeBreaK100.
This sotorasib indication represents a “historic milestone,” Dr. Skoulidis said in an interview.
No previously studied selective KRAS inhibitor has been approved despite scientific research efforts that stretch back nearly four decades, he explained.
“In a way, one can say that we have dealt KRAS-mutant lung cancer a knockdown blow, however, I should point out that the fight is not over,” he added.
“These clinical results will no doubt spearhead and galvanize further efforts to develop even more effective therapeutic combinations in the future, as well as identify and either forestall or overcome the eventual development of acquired resistance,” he said.
Only 1 out of 8 patients
The KRAS p.G12C mutation is present in about 13% of lung adenocarcinomas, or about one in every eight patients with nonsquamous NSCLC, Dr. Skoulidis said in the interview.
“We are estimating that this is in the region of 13,000 patients newly diagnosed every year in the U.S., and approximately 13,000 patients or so that are currently being treated in the second- or third-line setting,” he said.
The CodeBreaK100 trial included 126 patients with locally advanced or metastatic NSCLC and KRAS p.G12C mutation who had progressed on prior systemic therapies. About 43% had one prior line of treatment, while 35% had two lines, and 22% had three lines. A total of 81% had previously received both platinum-based chemotherapy and PD-1/PD-L1 axis inhibitors.
Most treatment-related adverse events in the study were grade 1-2 and generally manageable, according to Dr. Skoulidis. About 20% of patients experienced grade 3 treatment-related adverse events, which were mostly diarrhea or increases in aspartate aminotransferase and alanine aminotransferase levels. A grade 4 treatment-related adverse event, pneumonitis and dyspnea, was reported in one patient or approximately 1%.
Confirmatory trial
Although CodeBreak100 is not a randomized trial, the median OS of 12.5 months compares favorably to median OS times in the range of 7.9-10.3 months reported in randomized phase 3 clinical trials and subgroup analysis of randomized phase 3 trials of docetaxel for patients with KRAS-mutant lung adenocarcinoma, Dr. Skoulidis said in a question-and-answer session.
A confirmatory phase 3 CodeBreaK200 trial of sotorasib versus docetaxel in patients with previously treated KRAS p.G12C-mutated NSCLC is underway. That trial is evaluating PFS as a primary endpoint and OS as a secondary endpoint.
“If the same magnitude of benefit, 12.5 months median overall survival, is confirmed in the larger phase 3 clinical trial, as a clinician I would consider that beneficial for patients, compared to the standard of care,” Dr. Skoulidis said during the session.
Mature data
The updated analysis of the phase 2 CodeBreaK100 study is notable for its mature OS data, updated safety and the first molecular subgroup analyses, according to discussant Christine Marie Lovly, MD, PhD, of the division of hematology-oncology at Vanderbilt University Medical Center in Nashville.
“The objective response rate was 37.1%,” she added. “This is a little bit lower than we’re used to for targeted therapies, but remember, this is a different mutation and a very different class of drugs.”
The KRAS G12C inhibitors, several of which are under clinical development, are not tyrosine kinase inhibitors (TKIs), but rather allele-specific inhibitors that target mutant KRAS, trapping it in an inactive conformation, she explained.
Dr. Lovly referenced the exploratory analyses demonstrating efficacy in molecularly defined subgroups, calling it “interesting” that there was no difference in objective response rate between TP53 wild type and mutant tumors.
“We do have data that mutant TP53 seems to confer inferior outcomes for EGFR TKI-directed therapy in patients with EGFR-mutant lung cancer,” she said.
CodeBreaK100 was supported by Amgen, Inc. and partly by a National Institutes of Health Cancer Center Support Grant at Memorial Sloan Kettering Cancer Center.
Dr. Skoulidis reported honoraria from Bristol-Myers Squibb; research funding from AIMM Therapeutics and Amgen; and travel, accommodations, or expenses from Tango Therapeutics. Dr. Lovly reported disclosures related to Amgen, AstraZeneca, Genentech, Novartis, and Pfizer, among others.
The first KRAS inhibitor approved for the treatment of lung cancer provided a clinically meaningful overall survival benefit in an updated analysis of a phase 2 study.
Treatment with sotorasib yielded a median overall survival (OS) of 12.5 months in patients with previously treated KRAS p.G12C-mutated non-small cell lung cancer (NSCLC), according to an analysis of the phase 2 CodeBreaK 100 trial data presented at the American Society of Clinical Oncology Annual Meeting.
Median progression-free survival (PFS) was 6.8 months in this update, which included a median follow-up of more than 15 months, according to investigator Ferdinandos Skoulidis, MD, PhD, assistant professor of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center in Houston.
Efficacy responses
The confirmed objective response rate was 37.1%, including a 3.2% complete response rate and a median duration of response of 11.1 months, according to the report by Dr. Skoulidis.
In exploratory analyses, the benefit of sotorasib was consistent across patient subgroups, Dr. Skoulidis said in his presentation (Abstract 9003).
In particular, efficacy was observed in subgroups with co-occurring mutations in TP53, STK11, and KEAP1, which are molecular indicators of suboptimal outcomes on standard systemic treatments, according to Dr. Skoulidis.
This update on the registrational phase 2 CodeBreaK100 trial, published concurrently in the New England Journal of Medicine , came just one week after the U.S. Food and Drug Administration (FDA) granted accelerated approval to sotorasib.
Sotorasib was approved for the treatment of patients with previously treated KRAS G12C‑mutated locally advanced or metastatic NSCLC on the basis of previously reported results from CodeBreaK100.
This sotorasib indication represents a “historic milestone,” Dr. Skoulidis said in an interview.
No previously studied selective KRAS inhibitor has been approved despite scientific research efforts that stretch back nearly four decades, he explained.
“In a way, one can say that we have dealt KRAS-mutant lung cancer a knockdown blow, however, I should point out that the fight is not over,” he added.
“These clinical results will no doubt spearhead and galvanize further efforts to develop even more effective therapeutic combinations in the future, as well as identify and either forestall or overcome the eventual development of acquired resistance,” he said.
Only 1 out of 8 patients
The KRAS p.G12C mutation is present in about 13% of lung adenocarcinomas, or about one in every eight patients with nonsquamous NSCLC, Dr. Skoulidis said in the interview.
“We are estimating that this is in the region of 13,000 patients newly diagnosed every year in the U.S., and approximately 13,000 patients or so that are currently being treated in the second- or third-line setting,” he said.
The CodeBreaK100 trial included 126 patients with locally advanced or metastatic NSCLC and KRAS p.G12C mutation who had progressed on prior systemic therapies. About 43% had one prior line of treatment, while 35% had two lines, and 22% had three lines. A total of 81% had previously received both platinum-based chemotherapy and PD-1/PD-L1 axis inhibitors.
Most treatment-related adverse events in the study were grade 1-2 and generally manageable, according to Dr. Skoulidis. About 20% of patients experienced grade 3 treatment-related adverse events, which were mostly diarrhea or increases in aspartate aminotransferase and alanine aminotransferase levels. A grade 4 treatment-related adverse event, pneumonitis and dyspnea, was reported in one patient or approximately 1%.
Confirmatory trial
Although CodeBreak100 is not a randomized trial, the median OS of 12.5 months compares favorably to median OS times in the range of 7.9-10.3 months reported in randomized phase 3 clinical trials and subgroup analysis of randomized phase 3 trials of docetaxel for patients with KRAS-mutant lung adenocarcinoma, Dr. Skoulidis said in a question-and-answer session.
A confirmatory phase 3 CodeBreaK200 trial of sotorasib versus docetaxel in patients with previously treated KRAS p.G12C-mutated NSCLC is underway. That trial is evaluating PFS as a primary endpoint and OS as a secondary endpoint.
“If the same magnitude of benefit, 12.5 months median overall survival, is confirmed in the larger phase 3 clinical trial, as a clinician I would consider that beneficial for patients, compared to the standard of care,” Dr. Skoulidis said during the session.
Mature data
The updated analysis of the phase 2 CodeBreaK100 study is notable for its mature OS data, updated safety and the first molecular subgroup analyses, according to discussant Christine Marie Lovly, MD, PhD, of the division of hematology-oncology at Vanderbilt University Medical Center in Nashville.
“The objective response rate was 37.1%,” she added. “This is a little bit lower than we’re used to for targeted therapies, but remember, this is a different mutation and a very different class of drugs.”
The KRAS G12C inhibitors, several of which are under clinical development, are not tyrosine kinase inhibitors (TKIs), but rather allele-specific inhibitors that target mutant KRAS, trapping it in an inactive conformation, she explained.
Dr. Lovly referenced the exploratory analyses demonstrating efficacy in molecularly defined subgroups, calling it “interesting” that there was no difference in objective response rate between TP53 wild type and mutant tumors.
“We do have data that mutant TP53 seems to confer inferior outcomes for EGFR TKI-directed therapy in patients with EGFR-mutant lung cancer,” she said.
CodeBreaK100 was supported by Amgen, Inc. and partly by a National Institutes of Health Cancer Center Support Grant at Memorial Sloan Kettering Cancer Center.
Dr. Skoulidis reported honoraria from Bristol-Myers Squibb; research funding from AIMM Therapeutics and Amgen; and travel, accommodations, or expenses from Tango Therapeutics. Dr. Lovly reported disclosures related to Amgen, AstraZeneca, Genentech, Novartis, and Pfizer, among others.
FROM ASCO 2021
Community practice lung cancer patients insufficiently tested for treatment-related biomarkers
Lung cancer patients treated in community practices are not being comprehensively tested for biomarkers that could guide choice of first-line therapy, a recent retrospective analysis shows.
Less than half of patients with previously untreated non-small cell lung cancer (NSCLC) in a network of community practices underwent testing for all five biomarkers evaluated in the study, which was presented at the annual meeting of the American Society of Clinical Oncology (Abstract 9004).
Almost all of the 3,474 patients in the study (90%) had been tested for at least one biomarker, according to investigator Makenzi Colleen Evangelist, MD, an oncologist with New York Oncology Hematology, a practice in the US Oncology Network.
Only 46% were tested for all five biomarkers—ALK, BRAF, EGFR, ROS1, and PD-L1.
“While the proportion of patients tested for all five biomarkers increased over time, testing rates remain low at approximately 50%,” Dr. Evangelist said in a presentation of the results at the meeting.
This gap in testing illustrates “significant implementation challenges” that exist despite tremendous advances in biomarker-driven drug development and the technology to detect the mutations that can guide therapy, said Christine Marie Lovly, MD, PhD, the invited discussant for the study. “I would strongly argue that we have to apply what we already have to get equity, while still pushing the science forward,” said Dr. Lovly of the division of hematology-oncology at Vanderbilt University Medical Center in Nashville.
“We don’t want to miss the low-hanging fruit,” Dr. Lovly said. “We have to be able to make sure every patient with an EGFR mutation gets an EGFR tyrosine kinase inhibitor, and so forth, for all the other biomarkers that we test for.”
Real-world testing
The retrospective analysis of real-world biomarker testing patterns presented by Dr. Evangelist is the first of three protocols in the MYLUNG Consortium, a collaborative research study being conducted over a five-year period, according to the US Oncology Network.
The review of electronic health records included patients with metastatic NSCLC starting first-line systemic therapy between April 2018 and March 2020 in the US Oncology Network of community practices.
Rates of biomarker testing were highest for PD-L1, which was done for 83% of patients, the data show. EGFR and ALK testing were performed in 70% of patients, while ROS1 was evaluated in 68%. BRAF testing was done in 55% of patients. Testing rates appeared to be numerically higher for lung cancers with nonsquamous histology, according to Dr. Evangelist.
Over time, rates of specific biomarker testing were essentially unchanged, though a significant difference was seen for BRAF testing over time. BRAF was evaluated in 54% of patients starting therapy from April 2018 through September 2018, and 59%-62% in subsequent time periods (P = .005).
The proportion of patients tested for all five biomarkers was 44% in the April-September 2018 time period, and 50%-53% in subsequent time periods, the data show (P = .0056).
The proportion of patients tested with next-generation sequencing rose from 33% to 45% between 2018 and 2020, suggesting that comprehensive testing is increasing, according to Dr. Evangelist.
The turnaround time from testing orders to results was approximately 2 weeks, underscoring a need to get test results to oncologists sooner so they can consider biomarker data as they develop a treatment plan, the US Oncology Network said in a press release that described the study.
Median time from diagnosis to treatment in the study was approximately 5 weeks, which is “a concern for patients anxiously waiting for treatment,” the press release said.
Raising awareness
This study should serve to raise awareness that not all NSCLC patients who should be tested are being tested, study co-author Nicholas Robert, MD, said in an interview.
“There is a great line – ‘right drug, right patient, right time’ – and we’re not meeting that,” said Dr. Robert, vice president of medical affairs for Ontada, an oncology insights and technology company that is part of McKesson, which acquired the US Oncology Network in 2010.
The hope is that general oncologists will begin thinking of biomarker testing in NSCLC as being essential in the same way hormone receptor and HER2 testing are in breast cancer, according to Dr. Robert.
“You would never think about treating anyone with breast cancer without those variables,” he said. “We’d like to think that the general oncologist feels the same way about biomarkers in non-small cell cancer, that it’s something that should be done routinely across the board.”
The next phase of the MYLUNG Consortium study will prospectively evaluate biomarker test-ordering practices, turnaround times, and treatment decision making in approximately 1,000 patients from 11 sites, while the final phase will evaluate interventions to improve biomarker testing and access to therapies in up to 7,500 patients at 20 sites, according to the US Oncology Network.
Dr. Evangelist reported a consulting or advisory role with Takeda and AstraZeneca. Dr. Robert reported employment, leadership, and stock/ownership interest disclosures related to McKesson, along with other disclosures related to Johnson & Johnson, Oncolytics Biotech, Bristol-Myers Squibb, Roche, Advi, Boehringer Ingelheim, and New Century Health. Dr. Lovly reported disclosures related to Amgen, AstraZeneca, Genentech, Novartis, and Pfizer, among others.
Lung cancer patients treated in community practices are not being comprehensively tested for biomarkers that could guide choice of first-line therapy, a recent retrospective analysis shows.
Less than half of patients with previously untreated non-small cell lung cancer (NSCLC) in a network of community practices underwent testing for all five biomarkers evaluated in the study, which was presented at the annual meeting of the American Society of Clinical Oncology (Abstract 9004).
Almost all of the 3,474 patients in the study (90%) had been tested for at least one biomarker, according to investigator Makenzi Colleen Evangelist, MD, an oncologist with New York Oncology Hematology, a practice in the US Oncology Network.
Only 46% were tested for all five biomarkers—ALK, BRAF, EGFR, ROS1, and PD-L1.
“While the proportion of patients tested for all five biomarkers increased over time, testing rates remain low at approximately 50%,” Dr. Evangelist said in a presentation of the results at the meeting.
This gap in testing illustrates “significant implementation challenges” that exist despite tremendous advances in biomarker-driven drug development and the technology to detect the mutations that can guide therapy, said Christine Marie Lovly, MD, PhD, the invited discussant for the study. “I would strongly argue that we have to apply what we already have to get equity, while still pushing the science forward,” said Dr. Lovly of the division of hematology-oncology at Vanderbilt University Medical Center in Nashville.
“We don’t want to miss the low-hanging fruit,” Dr. Lovly said. “We have to be able to make sure every patient with an EGFR mutation gets an EGFR tyrosine kinase inhibitor, and so forth, for all the other biomarkers that we test for.”
Real-world testing
The retrospective analysis of real-world biomarker testing patterns presented by Dr. Evangelist is the first of three protocols in the MYLUNG Consortium, a collaborative research study being conducted over a five-year period, according to the US Oncology Network.
The review of electronic health records included patients with metastatic NSCLC starting first-line systemic therapy between April 2018 and March 2020 in the US Oncology Network of community practices.
Rates of biomarker testing were highest for PD-L1, which was done for 83% of patients, the data show. EGFR and ALK testing were performed in 70% of patients, while ROS1 was evaluated in 68%. BRAF testing was done in 55% of patients. Testing rates appeared to be numerically higher for lung cancers with nonsquamous histology, according to Dr. Evangelist.
Over time, rates of specific biomarker testing were essentially unchanged, though a significant difference was seen for BRAF testing over time. BRAF was evaluated in 54% of patients starting therapy from April 2018 through September 2018, and 59%-62% in subsequent time periods (P = .005).
The proportion of patients tested for all five biomarkers was 44% in the April-September 2018 time period, and 50%-53% in subsequent time periods, the data show (P = .0056).
The proportion of patients tested with next-generation sequencing rose from 33% to 45% between 2018 and 2020, suggesting that comprehensive testing is increasing, according to Dr. Evangelist.
The turnaround time from testing orders to results was approximately 2 weeks, underscoring a need to get test results to oncologists sooner so they can consider biomarker data as they develop a treatment plan, the US Oncology Network said in a press release that described the study.
Median time from diagnosis to treatment in the study was approximately 5 weeks, which is “a concern for patients anxiously waiting for treatment,” the press release said.
Raising awareness
This study should serve to raise awareness that not all NSCLC patients who should be tested are being tested, study co-author Nicholas Robert, MD, said in an interview.
“There is a great line – ‘right drug, right patient, right time’ – and we’re not meeting that,” said Dr. Robert, vice president of medical affairs for Ontada, an oncology insights and technology company that is part of McKesson, which acquired the US Oncology Network in 2010.
The hope is that general oncologists will begin thinking of biomarker testing in NSCLC as being essential in the same way hormone receptor and HER2 testing are in breast cancer, according to Dr. Robert.
“You would never think about treating anyone with breast cancer without those variables,” he said. “We’d like to think that the general oncologist feels the same way about biomarkers in non-small cell cancer, that it’s something that should be done routinely across the board.”
The next phase of the MYLUNG Consortium study will prospectively evaluate biomarker test-ordering practices, turnaround times, and treatment decision making in approximately 1,000 patients from 11 sites, while the final phase will evaluate interventions to improve biomarker testing and access to therapies in up to 7,500 patients at 20 sites, according to the US Oncology Network.
Dr. Evangelist reported a consulting or advisory role with Takeda and AstraZeneca. Dr. Robert reported employment, leadership, and stock/ownership interest disclosures related to McKesson, along with other disclosures related to Johnson & Johnson, Oncolytics Biotech, Bristol-Myers Squibb, Roche, Advi, Boehringer Ingelheim, and New Century Health. Dr. Lovly reported disclosures related to Amgen, AstraZeneca, Genentech, Novartis, and Pfizer, among others.
Lung cancer patients treated in community practices are not being comprehensively tested for biomarkers that could guide choice of first-line therapy, a recent retrospective analysis shows.
Less than half of patients with previously untreated non-small cell lung cancer (NSCLC) in a network of community practices underwent testing for all five biomarkers evaluated in the study, which was presented at the annual meeting of the American Society of Clinical Oncology (Abstract 9004).
Almost all of the 3,474 patients in the study (90%) had been tested for at least one biomarker, according to investigator Makenzi Colleen Evangelist, MD, an oncologist with New York Oncology Hematology, a practice in the US Oncology Network.
Only 46% were tested for all five biomarkers—ALK, BRAF, EGFR, ROS1, and PD-L1.
“While the proportion of patients tested for all five biomarkers increased over time, testing rates remain low at approximately 50%,” Dr. Evangelist said in a presentation of the results at the meeting.
This gap in testing illustrates “significant implementation challenges” that exist despite tremendous advances in biomarker-driven drug development and the technology to detect the mutations that can guide therapy, said Christine Marie Lovly, MD, PhD, the invited discussant for the study. “I would strongly argue that we have to apply what we already have to get equity, while still pushing the science forward,” said Dr. Lovly of the division of hematology-oncology at Vanderbilt University Medical Center in Nashville.
“We don’t want to miss the low-hanging fruit,” Dr. Lovly said. “We have to be able to make sure every patient with an EGFR mutation gets an EGFR tyrosine kinase inhibitor, and so forth, for all the other biomarkers that we test for.”
Real-world testing
The retrospective analysis of real-world biomarker testing patterns presented by Dr. Evangelist is the first of three protocols in the MYLUNG Consortium, a collaborative research study being conducted over a five-year period, according to the US Oncology Network.
The review of electronic health records included patients with metastatic NSCLC starting first-line systemic therapy between April 2018 and March 2020 in the US Oncology Network of community practices.
Rates of biomarker testing were highest for PD-L1, which was done for 83% of patients, the data show. EGFR and ALK testing were performed in 70% of patients, while ROS1 was evaluated in 68%. BRAF testing was done in 55% of patients. Testing rates appeared to be numerically higher for lung cancers with nonsquamous histology, according to Dr. Evangelist.
Over time, rates of specific biomarker testing were essentially unchanged, though a significant difference was seen for BRAF testing over time. BRAF was evaluated in 54% of patients starting therapy from April 2018 through September 2018, and 59%-62% in subsequent time periods (P = .005).
The proportion of patients tested for all five biomarkers was 44% in the April-September 2018 time period, and 50%-53% in subsequent time periods, the data show (P = .0056).
The proportion of patients tested with next-generation sequencing rose from 33% to 45% between 2018 and 2020, suggesting that comprehensive testing is increasing, according to Dr. Evangelist.
The turnaround time from testing orders to results was approximately 2 weeks, underscoring a need to get test results to oncologists sooner so they can consider biomarker data as they develop a treatment plan, the US Oncology Network said in a press release that described the study.
Median time from diagnosis to treatment in the study was approximately 5 weeks, which is “a concern for patients anxiously waiting for treatment,” the press release said.
Raising awareness
This study should serve to raise awareness that not all NSCLC patients who should be tested are being tested, study co-author Nicholas Robert, MD, said in an interview.
“There is a great line – ‘right drug, right patient, right time’ – and we’re not meeting that,” said Dr. Robert, vice president of medical affairs for Ontada, an oncology insights and technology company that is part of McKesson, which acquired the US Oncology Network in 2010.
The hope is that general oncologists will begin thinking of biomarker testing in NSCLC as being essential in the same way hormone receptor and HER2 testing are in breast cancer, according to Dr. Robert.
“You would never think about treating anyone with breast cancer without those variables,” he said. “We’d like to think that the general oncologist feels the same way about biomarkers in non-small cell cancer, that it’s something that should be done routinely across the board.”
The next phase of the MYLUNG Consortium study will prospectively evaluate biomarker test-ordering practices, turnaround times, and treatment decision making in approximately 1,000 patients from 11 sites, while the final phase will evaluate interventions to improve biomarker testing and access to therapies in up to 7,500 patients at 20 sites, according to the US Oncology Network.
Dr. Evangelist reported a consulting or advisory role with Takeda and AstraZeneca. Dr. Robert reported employment, leadership, and stock/ownership interest disclosures related to McKesson, along with other disclosures related to Johnson & Johnson, Oncolytics Biotech, Bristol-Myers Squibb, Roche, Advi, Boehringer Ingelheim, and New Century Health. Dr. Lovly reported disclosures related to Amgen, AstraZeneca, Genentech, Novartis, and Pfizer, among others.
FROM ASCO 2021
NSCLC: Immune-related AEs during checkpoint inhibitor therapy may predict outcomes
Experiencing an immune-related adverse event during checkpoint inhibitor treatment may predict outcomes in patients with non-small cell lung cancer, exploratory analyses of phase 3 trials suggest.
Immune-related adverse events (irAEs) were tied to longer overall survival (OS) in exploratory pooled analyses of three phase 3 clinical trials evaluating atezolizumab-based regimens, according to investigator Mark A. Socinski, MD, of AdventHealth Cancer Institute, Orlando, Fla.
Median OS approached 26 months for patients who received first-line atezolizumab and experienced an irAE, compared with just 13 months for those who did not experience an irAE, according to results reported at the American Society of Clinical Oncology Annual Meeting (Abstract 9002).
Atezolizumab-treated patients with grade 3 or greater irAEs had the shortest OS, shorter than those atezolizumab-treated patients who experienced grade 1-2 irAEs or no irAEs at all. That short OS may be due to treatment interruptions or discontinuations, said Dr. Socinski.
“Data from these analyses suggest an association between irAEs and efficacy in patients with [non-small cell cancer] NSCLC,” he stated in his presentation of the results.
A lot more to learn about irAEs
Similar linkages between irAEs and outcomes were observed in pooled analyses of patients enrolled in the control arms of the phase 3 trials, with a median OS of about 20 months for control patients experiencing an irAE, versus about 13 months for those who did not.
That linkage in the control arm prompted a question from an ASCO attendee about why an effect of irAEs, commonly associated with immune checkpoint inhibitor therapy, would be evident in analyses of patients who did not receive those agents.
In his response, Dr. Socinski characterized the finding as “a surprise” and said the finding may either reflect how adverse events are characterized or how chemotherapy impacts the immune system.
“I don’t know that our definition of irAEs is perfect,” he said, “and maybe we don’t understand what impact chemotherapy may have on the immune system, and may actually engender what historically we’ve always seen as an adverse event, but didn’t necessarily classify as an immune-related adverse event.”
More work is needed to better understand the connection between irAES and outcomes, and whether anything can be done as a result of that improved understanding, said discussant Mary Weber Redman, PhD.
“The question is, ‘what is actionable?’” added Dr. Redman, a biostatistician at the Fred Hutchinson Cancer Research Center, Seattle.
A firmer understanding of the relationship between irAEs and outcomes could change how clinicians monitor patients for irAEs, lead to better prediction of which patients may experience higher grade irAEs, and ultimately impact treatment selection potentially to avoid those higher grade events, Dr. Redman said in her remarks.
“Doing these types of analyses are quite important, because we have to look at the breadth of information that we have to be able to interpret that and think about what are future questions,” she said in the question-and-answer session accompanying Dr. Socinski’s presentation.
“I think the key is that we shouldn’t use these analyses to be definitive, but we should use them as to be hypothesis generating,” she added.
More evidence to link irAEs and outcomes
Immune-related AEs caused by off-target immune and inflammatory activity have been reported in up to 80% of patients receiving immune checkpoint inhibitors as monotherapy and up to 95% in combination regimens, Dr. Socinski said in his presentation.
“Increasing evidence suggests that the occurrence of immune-related adverse events with PD-L1 or PD-1 inhibitor therapy may be predictive of improved outcomes in cancers such as NSCLC, “ he added.
In their exploratory pooled analyses, Dr. Socinski and co-investigators looked at data from the phase 3 IMpower130 and IMpower132 trials, which evaluated first-line atezolizumab and chemotherapy for NSCLC, and the phase 3 IMpower150 trial, which evaluated atezolizumab plus chemotherapy with or without bevacizumab.
In all, they analyzed data for 1,557 atezolizumab-treated patients, and 900 patients who had been in the control arms of the studies.
Forty-eight percent of atezolizumab-treated patients experienced irAEs of any grade, while 11% experienced irAEs of grade 3-5, according to the presented data. In the control arm, 32% experienced irAEs of any grade and 5% experienced grade 3-5 irAEs.
The most common irAEs of any grade were rash, hepatitis, and hypothyroidism, occurring in 28%, 15%, and 12% of atezolizumab-treated patients, respectively.
Median OS in the atezolizumab arm was 25.7 months for patients with irAEs and 13.0 for patients with no irAEs, with a hazard ratio (HR) of 0.69 using a time-dependent Cox model.
Median OS in the control arm was 20.2 months for patients with irAEs and 12.8 months for patients with no irAEs, with an HR of 0.82.
The overall response rate (ORR) in the atezolizumab arm was 61.1% for patients with irAEs and 37.2% for those without irAEs; in the control arm, ORR was 42.2% for patients with irAEs and 34.0% for those with no irAEs.
Atezolizumab-treated patients who experienced grade 3-5 irAEs had the shortest OS, according to Dr. Socinski. The HRs for OS at 1, 3, 6, and 12 months in atezolizumab-treated patients with grade 3-5 irAEs (compared with those without irAEs) ranged from 1.25 to 0.87. By contrast, HRs at those time points for patients with grade 1-2 irAEs ranged from 0.78 to 0.72, Dr. Socinski said.
Dr. Socinski reported disclosures related to AstraZeneca/MedImmune, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Genentech, Guardant Health, Janssen, Lilly, Merck, Novartis, Roche/Genentech, and Spectrum Pharmaceuticals. Dr. Redman reported a consulting or advisory role with AstraZeneca.
Experiencing an immune-related adverse event during checkpoint inhibitor treatment may predict outcomes in patients with non-small cell lung cancer, exploratory analyses of phase 3 trials suggest.
Immune-related adverse events (irAEs) were tied to longer overall survival (OS) in exploratory pooled analyses of three phase 3 clinical trials evaluating atezolizumab-based regimens, according to investigator Mark A. Socinski, MD, of AdventHealth Cancer Institute, Orlando, Fla.
Median OS approached 26 months for patients who received first-line atezolizumab and experienced an irAE, compared with just 13 months for those who did not experience an irAE, according to results reported at the American Society of Clinical Oncology Annual Meeting (Abstract 9002).
Atezolizumab-treated patients with grade 3 or greater irAEs had the shortest OS, shorter than those atezolizumab-treated patients who experienced grade 1-2 irAEs or no irAEs at all. That short OS may be due to treatment interruptions or discontinuations, said Dr. Socinski.
“Data from these analyses suggest an association between irAEs and efficacy in patients with [non-small cell cancer] NSCLC,” he stated in his presentation of the results.
A lot more to learn about irAEs
Similar linkages between irAEs and outcomes were observed in pooled analyses of patients enrolled in the control arms of the phase 3 trials, with a median OS of about 20 months for control patients experiencing an irAE, versus about 13 months for those who did not.
That linkage in the control arm prompted a question from an ASCO attendee about why an effect of irAEs, commonly associated with immune checkpoint inhibitor therapy, would be evident in analyses of patients who did not receive those agents.
In his response, Dr. Socinski characterized the finding as “a surprise” and said the finding may either reflect how adverse events are characterized or how chemotherapy impacts the immune system.
“I don’t know that our definition of irAEs is perfect,” he said, “and maybe we don’t understand what impact chemotherapy may have on the immune system, and may actually engender what historically we’ve always seen as an adverse event, but didn’t necessarily classify as an immune-related adverse event.”
More work is needed to better understand the connection between irAES and outcomes, and whether anything can be done as a result of that improved understanding, said discussant Mary Weber Redman, PhD.
“The question is, ‘what is actionable?’” added Dr. Redman, a biostatistician at the Fred Hutchinson Cancer Research Center, Seattle.
A firmer understanding of the relationship between irAEs and outcomes could change how clinicians monitor patients for irAEs, lead to better prediction of which patients may experience higher grade irAEs, and ultimately impact treatment selection potentially to avoid those higher grade events, Dr. Redman said in her remarks.
“Doing these types of analyses are quite important, because we have to look at the breadth of information that we have to be able to interpret that and think about what are future questions,” she said in the question-and-answer session accompanying Dr. Socinski’s presentation.
“I think the key is that we shouldn’t use these analyses to be definitive, but we should use them as to be hypothesis generating,” she added.
More evidence to link irAEs and outcomes
Immune-related AEs caused by off-target immune and inflammatory activity have been reported in up to 80% of patients receiving immune checkpoint inhibitors as monotherapy and up to 95% in combination regimens, Dr. Socinski said in his presentation.
“Increasing evidence suggests that the occurrence of immune-related adverse events with PD-L1 or PD-1 inhibitor therapy may be predictive of improved outcomes in cancers such as NSCLC, “ he added.
In their exploratory pooled analyses, Dr. Socinski and co-investigators looked at data from the phase 3 IMpower130 and IMpower132 trials, which evaluated first-line atezolizumab and chemotherapy for NSCLC, and the phase 3 IMpower150 trial, which evaluated atezolizumab plus chemotherapy with or without bevacizumab.
In all, they analyzed data for 1,557 atezolizumab-treated patients, and 900 patients who had been in the control arms of the studies.
Forty-eight percent of atezolizumab-treated patients experienced irAEs of any grade, while 11% experienced irAEs of grade 3-5, according to the presented data. In the control arm, 32% experienced irAEs of any grade and 5% experienced grade 3-5 irAEs.
The most common irAEs of any grade were rash, hepatitis, and hypothyroidism, occurring in 28%, 15%, and 12% of atezolizumab-treated patients, respectively.
Median OS in the atezolizumab arm was 25.7 months for patients with irAEs and 13.0 for patients with no irAEs, with a hazard ratio (HR) of 0.69 using a time-dependent Cox model.
Median OS in the control arm was 20.2 months for patients with irAEs and 12.8 months for patients with no irAEs, with an HR of 0.82.
The overall response rate (ORR) in the atezolizumab arm was 61.1% for patients with irAEs and 37.2% for those without irAEs; in the control arm, ORR was 42.2% for patients with irAEs and 34.0% for those with no irAEs.
Atezolizumab-treated patients who experienced grade 3-5 irAEs had the shortest OS, according to Dr. Socinski. The HRs for OS at 1, 3, 6, and 12 months in atezolizumab-treated patients with grade 3-5 irAEs (compared with those without irAEs) ranged from 1.25 to 0.87. By contrast, HRs at those time points for patients with grade 1-2 irAEs ranged from 0.78 to 0.72, Dr. Socinski said.
Dr. Socinski reported disclosures related to AstraZeneca/MedImmune, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Genentech, Guardant Health, Janssen, Lilly, Merck, Novartis, Roche/Genentech, and Spectrum Pharmaceuticals. Dr. Redman reported a consulting or advisory role with AstraZeneca.
Experiencing an immune-related adverse event during checkpoint inhibitor treatment may predict outcomes in patients with non-small cell lung cancer, exploratory analyses of phase 3 trials suggest.
Immune-related adverse events (irAEs) were tied to longer overall survival (OS) in exploratory pooled analyses of three phase 3 clinical trials evaluating atezolizumab-based regimens, according to investigator Mark A. Socinski, MD, of AdventHealth Cancer Institute, Orlando, Fla.
Median OS approached 26 months for patients who received first-line atezolizumab and experienced an irAE, compared with just 13 months for those who did not experience an irAE, according to results reported at the American Society of Clinical Oncology Annual Meeting (Abstract 9002).
Atezolizumab-treated patients with grade 3 or greater irAEs had the shortest OS, shorter than those atezolizumab-treated patients who experienced grade 1-2 irAEs or no irAEs at all. That short OS may be due to treatment interruptions or discontinuations, said Dr. Socinski.
“Data from these analyses suggest an association between irAEs and efficacy in patients with [non-small cell cancer] NSCLC,” he stated in his presentation of the results.
A lot more to learn about irAEs
Similar linkages between irAEs and outcomes were observed in pooled analyses of patients enrolled in the control arms of the phase 3 trials, with a median OS of about 20 months for control patients experiencing an irAE, versus about 13 months for those who did not.
That linkage in the control arm prompted a question from an ASCO attendee about why an effect of irAEs, commonly associated with immune checkpoint inhibitor therapy, would be evident in analyses of patients who did not receive those agents.
In his response, Dr. Socinski characterized the finding as “a surprise” and said the finding may either reflect how adverse events are characterized or how chemotherapy impacts the immune system.
“I don’t know that our definition of irAEs is perfect,” he said, “and maybe we don’t understand what impact chemotherapy may have on the immune system, and may actually engender what historically we’ve always seen as an adverse event, but didn’t necessarily classify as an immune-related adverse event.”
More work is needed to better understand the connection between irAES and outcomes, and whether anything can be done as a result of that improved understanding, said discussant Mary Weber Redman, PhD.
“The question is, ‘what is actionable?’” added Dr. Redman, a biostatistician at the Fred Hutchinson Cancer Research Center, Seattle.
A firmer understanding of the relationship between irAEs and outcomes could change how clinicians monitor patients for irAEs, lead to better prediction of which patients may experience higher grade irAEs, and ultimately impact treatment selection potentially to avoid those higher grade events, Dr. Redman said in her remarks.
“Doing these types of analyses are quite important, because we have to look at the breadth of information that we have to be able to interpret that and think about what are future questions,” she said in the question-and-answer session accompanying Dr. Socinski’s presentation.
“I think the key is that we shouldn’t use these analyses to be definitive, but we should use them as to be hypothesis generating,” she added.
More evidence to link irAEs and outcomes
Immune-related AEs caused by off-target immune and inflammatory activity have been reported in up to 80% of patients receiving immune checkpoint inhibitors as monotherapy and up to 95% in combination regimens, Dr. Socinski said in his presentation.
“Increasing evidence suggests that the occurrence of immune-related adverse events with PD-L1 or PD-1 inhibitor therapy may be predictive of improved outcomes in cancers such as NSCLC, “ he added.
In their exploratory pooled analyses, Dr. Socinski and co-investigators looked at data from the phase 3 IMpower130 and IMpower132 trials, which evaluated first-line atezolizumab and chemotherapy for NSCLC, and the phase 3 IMpower150 trial, which evaluated atezolizumab plus chemotherapy with or without bevacizumab.
In all, they analyzed data for 1,557 atezolizumab-treated patients, and 900 patients who had been in the control arms of the studies.
Forty-eight percent of atezolizumab-treated patients experienced irAEs of any grade, while 11% experienced irAEs of grade 3-5, according to the presented data. In the control arm, 32% experienced irAEs of any grade and 5% experienced grade 3-5 irAEs.
The most common irAEs of any grade were rash, hepatitis, and hypothyroidism, occurring in 28%, 15%, and 12% of atezolizumab-treated patients, respectively.
Median OS in the atezolizumab arm was 25.7 months for patients with irAEs and 13.0 for patients with no irAEs, with a hazard ratio (HR) of 0.69 using a time-dependent Cox model.
Median OS in the control arm was 20.2 months for patients with irAEs and 12.8 months for patients with no irAEs, with an HR of 0.82.
The overall response rate (ORR) in the atezolizumab arm was 61.1% for patients with irAEs and 37.2% for those without irAEs; in the control arm, ORR was 42.2% for patients with irAEs and 34.0% for those with no irAEs.
Atezolizumab-treated patients who experienced grade 3-5 irAEs had the shortest OS, according to Dr. Socinski. The HRs for OS at 1, 3, 6, and 12 months in atezolizumab-treated patients with grade 3-5 irAEs (compared with those without irAEs) ranged from 1.25 to 0.87. By contrast, HRs at those time points for patients with grade 1-2 irAEs ranged from 0.78 to 0.72, Dr. Socinski said.
Dr. Socinski reported disclosures related to AstraZeneca/MedImmune, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Genentech, Guardant Health, Janssen, Lilly, Merck, Novartis, Roche/Genentech, and Spectrum Pharmaceuticals. Dr. Redman reported a consulting or advisory role with AstraZeneca.
REPORTING FROM ASCO 2021
GI symptoms and chronic fatigue may persist months after COVID-19
Gastrointestinal symptoms and chronic fatigue may persist months after the COVID-19 virus infection resolves, results of a recent cohort-controlled study suggest.
About 5 months after SARS-CoV-2 infection, relative risks of loose stools, somatization, and chronic fatigue were increased by approximately two- to three fold, compared to individuals who had not been infected, according to study results presented at the annual Digestive Disease Week® (DDW).
These longer-term consequences of SARS-CoV-2 appeared to be more severe in patients who had experienced diarrhea during the acute infection, according to investigator Daniele Noviello, MD, a second-year resident in gastroenterology and hepatology at the University of Milan.
This is the first cohort-controlled study that specifically investigates gastrointestinal symptoms and somatoform disorders, Dr. Noviello said in a virtual presentation of the results.
“Based on our data, chronic fatigue, gastrointestinal, and somatoform symptoms may have a common postinfectious origin, and they should be investigated in the follow-up of SARS-CoV-2 patients,” he said.
Links between SARS-CoV-2 and gastrointestinal symptoms
Gastrointestinal symptoms are known to be relatively common during acute infection. According to Dr. Noviello, the most frequent gastrointestinal symptom associated with SARS-CoV-2 is diarrhea, occurring in 4% to nearly 40% of patients in case series to date.
However, data on the longer-term gastrointestinal impacts of SARS-CoV-2 remain scarce.
In one noncontrolled cohort study in China, loss of appetite, nausea, acid reflux, and diarrhea were seen in 15%-24% of patients 3 months after the infection, Dr. Noviello said. In another cohort study in China, diarrhea and vomiting were reported in 5% of patients 6 months after infection.
In any case, it is known that viral, bacterial, and protozoal infections of the gastrointestinal tract are a risk factor for development of functional disorders including irritable bowel syndrome (IBS), functional dyspepsia, and chronic fatigue, according to Dr. Noviello.
Accordingly, the results of the present study suggest that SARS-CoV-2 also “may affect the brain-gut axis in the long term,” Dr. Noviello and coauthors wrote in an abstract of the study.
It is plausible that SARS-CoV-2 infection could be a trigger for longer-term gastrointestinal symptoms, especially given the previous evidence linking infections and IBS symptoms, or postinfectious IBS, said Juan Pablo Stefanolo, MD, a physician with the neurogastroenterology and motility section, Hospital de Clínicas José de San Martín, Buenos Aires University.
“If it is demonstrated [that SARS-CoV-2 infection is a trigger], the microbiota-gut-brain axis concept in IBS pathophysiology is reinforced,” Dr. Stefanolo said in an interview.
In the meantime, practitioners may want to take into account COVID-19 infection history in the evaluation of a patient with IBS-like symptoms and, in case of a known positive COVID-19 result in an IBS patient, be aware of the possibility of symptom exacerbation, Dr. Stefanolo said.
Pandemic in Italy: Unique study opportunity
The severe outbreak in the Milan region early in the COVID-19 pandemic provided a “unique opportunity” to assess the long-term impact of infection on gastrointestinal and extraintestinal somatoform symptoms, said Dr. Noviello.
The investigators sent an online questionnaire to patients who had a molecular diagnosis of SARS-CoV-2 infection by nasal swab between February and April of 2020. To form a control group, they also sent questionnaires to hospital employees and health care providers who had tested negative over that same time period.
In all, 378 questionnaires were completed by 177 SARS-CoV-2–positive individuals and 201 controls. The SARS-CoV-2–positive patients were somewhat older (about 44 years vs. 40 years for controls), were less often female (40% vs. 61%), had a lower education level, and smoked less than did controls, according to the investigators.
A mean of 4.8 months had elapsed between the time of SARS-CoV-2 infection and when the questionnaires were compiled, said Dr. Noviello.
In the acute phase, diarrhea was the most common gastrointestinal symptom among virus-positive individuals, occurring in about 50% compared to 20% of controls (P < .001), data show. Other symptoms reported by 40% of SARS-CoV-2–infected individuals included fever, dyspnea, loss of smell or taste, weight loss, myalgia, arthralgia, and asthenia in the acute phase controls in the acute phase, Dr. Noviello said.
Persistent gastrointestinal symptoms after SARS-CoV-2
Persistent symptoms included loose stools, as measured by the Bristol Stool scale, occurring in 17.8% of SARS-CoV-2–positive individuals, but only 9.3% of the SARS-CoV-2–negative controls, according to Dr. Noviello, with an adjusted risk ratio of 1.88 (95% confidence interval, 0.99-3.54).
Chronic fatigue symptoms, as measured by the Structured Assessment of Gastrointestinal Symptoms questionnaire, were reported by about 30% of SARS-CoV-2–positive patients and about 15% of controls, for an adjusted risk ratio of 2.24 (95% CI, 1.48-3.37), according to Dr. Noviello’s presentation.
The mean t-score on the Symptom Checklist–12 for somatoform disorders was higher for the virus-positive patients compared to controls, according to Dr. Noviello. The scores were 54.6 and 50.5, respectively, with an adjusted score difference of 3.6 (95% CI, 1.0-6.2).
The longer-term sequelae of SARS-CoV-2 infection might be more severe in individuals who experienced diarrhea during acute infection, according to Dr. Noviello. In a post hoc analysis, reports of irritable bowel syndrome and loose stools were significantly higher in SARS-CoV-2–infected individuals who had diarrhea in the acute phase compared to those who did not experience diarrhea, he said.
Somatoform disorder scores were significantly higher, and reports of headache, back pain, and chronic fatigue were significantly more common, in individuals who had diarrhea at the time of SARS-CoV-2 infection, he added.
Dr. Noviello and coauthors reported no competing interests related to the study. Dr. Stefanolo had no disclosures to report.
Gastrointestinal symptoms and chronic fatigue may persist months after the COVID-19 virus infection resolves, results of a recent cohort-controlled study suggest.
About 5 months after SARS-CoV-2 infection, relative risks of loose stools, somatization, and chronic fatigue were increased by approximately two- to three fold, compared to individuals who had not been infected, according to study results presented at the annual Digestive Disease Week® (DDW).
These longer-term consequences of SARS-CoV-2 appeared to be more severe in patients who had experienced diarrhea during the acute infection, according to investigator Daniele Noviello, MD, a second-year resident in gastroenterology and hepatology at the University of Milan.
This is the first cohort-controlled study that specifically investigates gastrointestinal symptoms and somatoform disorders, Dr. Noviello said in a virtual presentation of the results.
“Based on our data, chronic fatigue, gastrointestinal, and somatoform symptoms may have a common postinfectious origin, and they should be investigated in the follow-up of SARS-CoV-2 patients,” he said.
Links between SARS-CoV-2 and gastrointestinal symptoms
Gastrointestinal symptoms are known to be relatively common during acute infection. According to Dr. Noviello, the most frequent gastrointestinal symptom associated with SARS-CoV-2 is diarrhea, occurring in 4% to nearly 40% of patients in case series to date.
However, data on the longer-term gastrointestinal impacts of SARS-CoV-2 remain scarce.
In one noncontrolled cohort study in China, loss of appetite, nausea, acid reflux, and diarrhea were seen in 15%-24% of patients 3 months after the infection, Dr. Noviello said. In another cohort study in China, diarrhea and vomiting were reported in 5% of patients 6 months after infection.
In any case, it is known that viral, bacterial, and protozoal infections of the gastrointestinal tract are a risk factor for development of functional disorders including irritable bowel syndrome (IBS), functional dyspepsia, and chronic fatigue, according to Dr. Noviello.
Accordingly, the results of the present study suggest that SARS-CoV-2 also “may affect the brain-gut axis in the long term,” Dr. Noviello and coauthors wrote in an abstract of the study.
It is plausible that SARS-CoV-2 infection could be a trigger for longer-term gastrointestinal symptoms, especially given the previous evidence linking infections and IBS symptoms, or postinfectious IBS, said Juan Pablo Stefanolo, MD, a physician with the neurogastroenterology and motility section, Hospital de Clínicas José de San Martín, Buenos Aires University.
“If it is demonstrated [that SARS-CoV-2 infection is a trigger], the microbiota-gut-brain axis concept in IBS pathophysiology is reinforced,” Dr. Stefanolo said in an interview.
In the meantime, practitioners may want to take into account COVID-19 infection history in the evaluation of a patient with IBS-like symptoms and, in case of a known positive COVID-19 result in an IBS patient, be aware of the possibility of symptom exacerbation, Dr. Stefanolo said.
Pandemic in Italy: Unique study opportunity
The severe outbreak in the Milan region early in the COVID-19 pandemic provided a “unique opportunity” to assess the long-term impact of infection on gastrointestinal and extraintestinal somatoform symptoms, said Dr. Noviello.
The investigators sent an online questionnaire to patients who had a molecular diagnosis of SARS-CoV-2 infection by nasal swab between February and April of 2020. To form a control group, they also sent questionnaires to hospital employees and health care providers who had tested negative over that same time period.
In all, 378 questionnaires were completed by 177 SARS-CoV-2–positive individuals and 201 controls. The SARS-CoV-2–positive patients were somewhat older (about 44 years vs. 40 years for controls), were less often female (40% vs. 61%), had a lower education level, and smoked less than did controls, according to the investigators.
A mean of 4.8 months had elapsed between the time of SARS-CoV-2 infection and when the questionnaires were compiled, said Dr. Noviello.
In the acute phase, diarrhea was the most common gastrointestinal symptom among virus-positive individuals, occurring in about 50% compared to 20% of controls (P < .001), data show. Other symptoms reported by 40% of SARS-CoV-2–infected individuals included fever, dyspnea, loss of smell or taste, weight loss, myalgia, arthralgia, and asthenia in the acute phase controls in the acute phase, Dr. Noviello said.
Persistent gastrointestinal symptoms after SARS-CoV-2
Persistent symptoms included loose stools, as measured by the Bristol Stool scale, occurring in 17.8% of SARS-CoV-2–positive individuals, but only 9.3% of the SARS-CoV-2–negative controls, according to Dr. Noviello, with an adjusted risk ratio of 1.88 (95% confidence interval, 0.99-3.54).
Chronic fatigue symptoms, as measured by the Structured Assessment of Gastrointestinal Symptoms questionnaire, were reported by about 30% of SARS-CoV-2–positive patients and about 15% of controls, for an adjusted risk ratio of 2.24 (95% CI, 1.48-3.37), according to Dr. Noviello’s presentation.
The mean t-score on the Symptom Checklist–12 for somatoform disorders was higher for the virus-positive patients compared to controls, according to Dr. Noviello. The scores were 54.6 and 50.5, respectively, with an adjusted score difference of 3.6 (95% CI, 1.0-6.2).
The longer-term sequelae of SARS-CoV-2 infection might be more severe in individuals who experienced diarrhea during acute infection, according to Dr. Noviello. In a post hoc analysis, reports of irritable bowel syndrome and loose stools were significantly higher in SARS-CoV-2–infected individuals who had diarrhea in the acute phase compared to those who did not experience diarrhea, he said.
Somatoform disorder scores were significantly higher, and reports of headache, back pain, and chronic fatigue were significantly more common, in individuals who had diarrhea at the time of SARS-CoV-2 infection, he added.
Dr. Noviello and coauthors reported no competing interests related to the study. Dr. Stefanolo had no disclosures to report.
Gastrointestinal symptoms and chronic fatigue may persist months after the COVID-19 virus infection resolves, results of a recent cohort-controlled study suggest.
About 5 months after SARS-CoV-2 infection, relative risks of loose stools, somatization, and chronic fatigue were increased by approximately two- to three fold, compared to individuals who had not been infected, according to study results presented at the annual Digestive Disease Week® (DDW).
These longer-term consequences of SARS-CoV-2 appeared to be more severe in patients who had experienced diarrhea during the acute infection, according to investigator Daniele Noviello, MD, a second-year resident in gastroenterology and hepatology at the University of Milan.
This is the first cohort-controlled study that specifically investigates gastrointestinal symptoms and somatoform disorders, Dr. Noviello said in a virtual presentation of the results.
“Based on our data, chronic fatigue, gastrointestinal, and somatoform symptoms may have a common postinfectious origin, and they should be investigated in the follow-up of SARS-CoV-2 patients,” he said.
Links between SARS-CoV-2 and gastrointestinal symptoms
Gastrointestinal symptoms are known to be relatively common during acute infection. According to Dr. Noviello, the most frequent gastrointestinal symptom associated with SARS-CoV-2 is diarrhea, occurring in 4% to nearly 40% of patients in case series to date.
However, data on the longer-term gastrointestinal impacts of SARS-CoV-2 remain scarce.
In one noncontrolled cohort study in China, loss of appetite, nausea, acid reflux, and diarrhea were seen in 15%-24% of patients 3 months after the infection, Dr. Noviello said. In another cohort study in China, diarrhea and vomiting were reported in 5% of patients 6 months after infection.
In any case, it is known that viral, bacterial, and protozoal infections of the gastrointestinal tract are a risk factor for development of functional disorders including irritable bowel syndrome (IBS), functional dyspepsia, and chronic fatigue, according to Dr. Noviello.
Accordingly, the results of the present study suggest that SARS-CoV-2 also “may affect the brain-gut axis in the long term,” Dr. Noviello and coauthors wrote in an abstract of the study.
It is plausible that SARS-CoV-2 infection could be a trigger for longer-term gastrointestinal symptoms, especially given the previous evidence linking infections and IBS symptoms, or postinfectious IBS, said Juan Pablo Stefanolo, MD, a physician with the neurogastroenterology and motility section, Hospital de Clínicas José de San Martín, Buenos Aires University.
“If it is demonstrated [that SARS-CoV-2 infection is a trigger], the microbiota-gut-brain axis concept in IBS pathophysiology is reinforced,” Dr. Stefanolo said in an interview.
In the meantime, practitioners may want to take into account COVID-19 infection history in the evaluation of a patient with IBS-like symptoms and, in case of a known positive COVID-19 result in an IBS patient, be aware of the possibility of symptom exacerbation, Dr. Stefanolo said.
Pandemic in Italy: Unique study opportunity
The severe outbreak in the Milan region early in the COVID-19 pandemic provided a “unique opportunity” to assess the long-term impact of infection on gastrointestinal and extraintestinal somatoform symptoms, said Dr. Noviello.
The investigators sent an online questionnaire to patients who had a molecular diagnosis of SARS-CoV-2 infection by nasal swab between February and April of 2020. To form a control group, they also sent questionnaires to hospital employees and health care providers who had tested negative over that same time period.
In all, 378 questionnaires were completed by 177 SARS-CoV-2–positive individuals and 201 controls. The SARS-CoV-2–positive patients were somewhat older (about 44 years vs. 40 years for controls), were less often female (40% vs. 61%), had a lower education level, and smoked less than did controls, according to the investigators.
A mean of 4.8 months had elapsed between the time of SARS-CoV-2 infection and when the questionnaires were compiled, said Dr. Noviello.
In the acute phase, diarrhea was the most common gastrointestinal symptom among virus-positive individuals, occurring in about 50% compared to 20% of controls (P < .001), data show. Other symptoms reported by 40% of SARS-CoV-2–infected individuals included fever, dyspnea, loss of smell or taste, weight loss, myalgia, arthralgia, and asthenia in the acute phase controls in the acute phase, Dr. Noviello said.
Persistent gastrointestinal symptoms after SARS-CoV-2
Persistent symptoms included loose stools, as measured by the Bristol Stool scale, occurring in 17.8% of SARS-CoV-2–positive individuals, but only 9.3% of the SARS-CoV-2–negative controls, according to Dr. Noviello, with an adjusted risk ratio of 1.88 (95% confidence interval, 0.99-3.54).
Chronic fatigue symptoms, as measured by the Structured Assessment of Gastrointestinal Symptoms questionnaire, were reported by about 30% of SARS-CoV-2–positive patients and about 15% of controls, for an adjusted risk ratio of 2.24 (95% CI, 1.48-3.37), according to Dr. Noviello’s presentation.
The mean t-score on the Symptom Checklist–12 for somatoform disorders was higher for the virus-positive patients compared to controls, according to Dr. Noviello. The scores were 54.6 and 50.5, respectively, with an adjusted score difference of 3.6 (95% CI, 1.0-6.2).
The longer-term sequelae of SARS-CoV-2 infection might be more severe in individuals who experienced diarrhea during acute infection, according to Dr. Noviello. In a post hoc analysis, reports of irritable bowel syndrome and loose stools were significantly higher in SARS-CoV-2–infected individuals who had diarrhea in the acute phase compared to those who did not experience diarrhea, he said.
Somatoform disorder scores were significantly higher, and reports of headache, back pain, and chronic fatigue were significantly more common, in individuals who had diarrhea at the time of SARS-CoV-2 infection, he added.
Dr. Noviello and coauthors reported no competing interests related to the study. Dr. Stefanolo had no disclosures to report.
FROM DDW 2021