User login
Laser Safety: The Need for Protocols
The use of lasers in dermatology has evolved and expanded since their first cutaneous use in 1963.1 As the fundamental understanding of the interaction of laser energy with biological tissues increased, the need for laser safety became apparent. Since then, lasers of varying wavelengths have been developed, each with its specific chromophore target and specific safety need. Protocols, such as a checklist, that have been shown to reduce adverse events in surgery and in the intensive care unit can be borrowed to decrease risk from laser injury and optimize laser safety in dermatology.2 The safety of the patient, the laser operator, and the other health care providers involved in the delivery of laser therapy led to the first US Food and Drug Administration (FDA) guidelines for laser use in 1984.3
There are 4 regulatory organizations for laser safety in the United States: the American National Standards Institute (ANSI), the Occupational Health and Safety Administration (OSHA), the FDA’s Center for Devices and Radiological Health, and The Joint Commission.
Laser Principles
The basic principles of lasers include transmission, absorption, scatter, and reflection, all occurring when laser light is applied to biological tissues. The effects of the laser are a function of the target tissue (the chromophore) and the wavelength of light being used.4 In the skin, there are 3 main endogenous chromophores: water, hemoglobin, and melanin. Some experts consider collagen to be a fourth and separate entity as a chromophore. Tattoos are considered exogenous chromophores.3 The basic principles of lasers are important to understand and keep in mind when discussing laser safety, as they are the mechanisms through which unintended consequences can occur.
Laser Safety
Ocular Hazards
Ocular hazards are a notable concern in laser surgery. The eye is uniquely susceptible to laser light, and eye injuries represent a majority of reported injuries, which can occur through direct beam, mirror reflection by surgical instruments, and beam reflection off the skin (4%–7% of light that hits the skin is reflected because of the refractive index between air and the stratum corneum).3 The different wavelengths of lasers affect different parts of the eye. The 3 parts of the eye affected most are the retina, cornea, and lens. Not only is the lens primarily at risk for acute (lenticular burns) and chronic (cataracts) injury from the laser, but secondarily the lens also can concentrate a laser beam onto the retina by a factor of 100,000 (Table 1).3
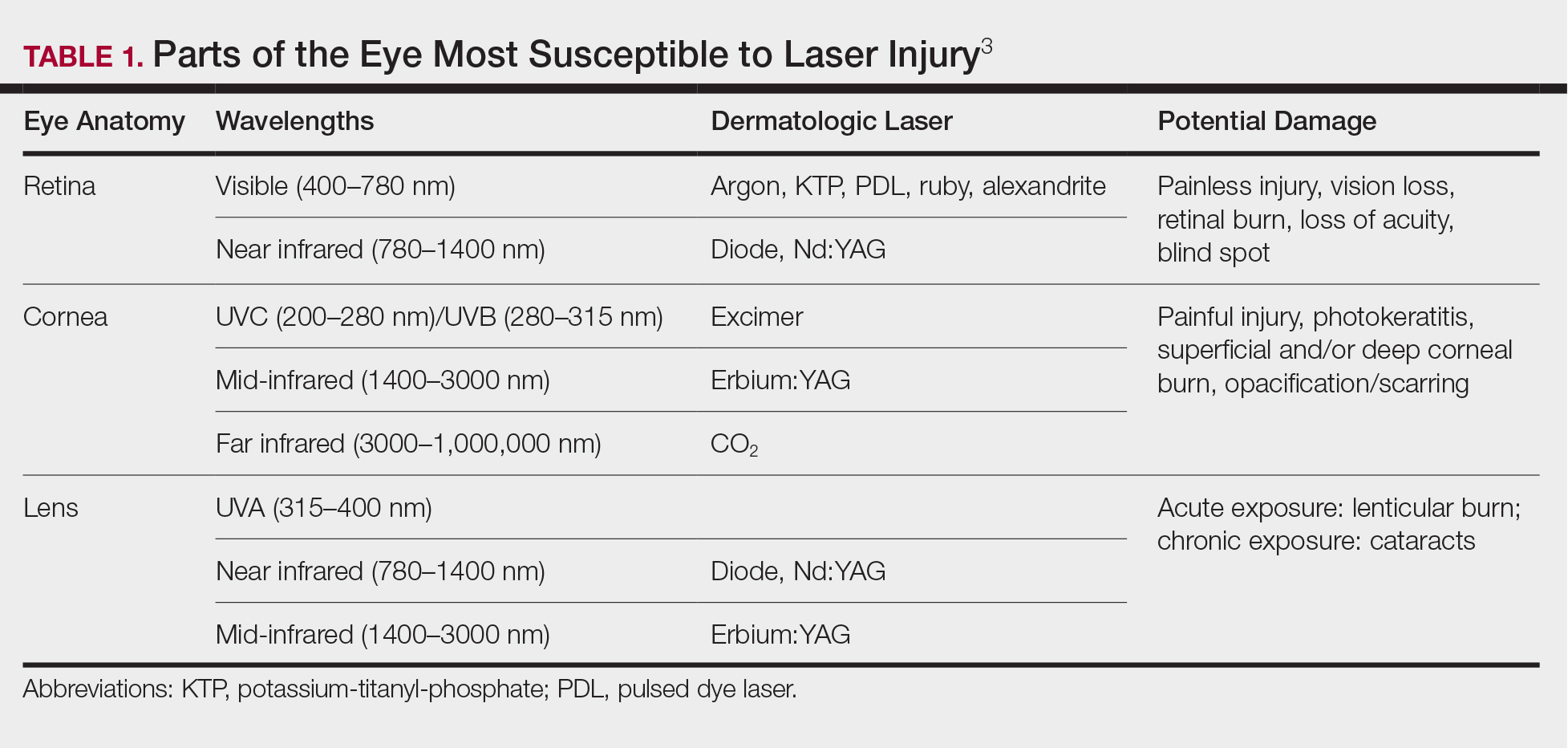
The use of ocular protective equipment, sometimes referred to as personal protective eyewear (PPE), is essential and is mandated by ANSI and OSHA for all class 3 and class 4 lasers. The eyewear must be labeled with the wavelength and the degree of optical protection—termed the optical density (OD) or filter factor—of each lens and should match the laser being used. Laser manufacturers, as required by ANSI, must provide the wavelength and OD of their lasers, and both can be found on each laser as well as in ANSI Z136.1.3
Vendors supplying PPE generally provide the material, usually glass or polycarbonate; color; visible light transmission, which is the actual amount of light that reaches one’s eye through the lens; filter specifications, which contain the OD at certain wavelengths; and the types of lasers for which each specific PPE is used. It is important to match the laser to the correct PPE. The use of multiple types of lasers in the same office or laser treatment area can present challenges regarding eye safety. Matching the PPE to the laser in use is critical, and therefore all steps to prevent error for patients and personnel should be employed. One recommendation is to place each laser in a separate room with the appropriate PPE hung outside on the door of that room.
When the treatment area is in the periocular region, protection of the patient’s cornea is essential. Leaded eye shields with nonreflective surfaces have been shown to offer the best protection.5 Prior to placement, anesthetic eye drops and lubrication are important for patient comfort and protection from corneal injury.
Laser-Generated Airborne Contaminants
Other hazards associated with laser use not directly related to the beam are laser-generated airborne contaminants (LGACs), including chemicals, viruses, bacteria, aerosolized blood products, and nanoparticles (<1 µm) known as ultrafine particles (UFPs). According to ANSI, electrosurgical devices and lasers generate the same smoke. The plume (surgical smoke) is known to contain as many as 60 chemicals, including but not limited to carbon monoxide, acrylonitrite, hydrocyanide, benzene, toluene, naphthalene, and formaldehyde. Several are known carcinogens, and others are environmental toxins.6,7
Smoke management is an important consideration for dermatologists and their patients and generally includes respiratory protection via masks and ventilation techniques. However, the practice is not universal, and oversight agencies such as OSHA and the National Institute for Occupational Safety and Health (NIOSH) provide guidelines only; they do not enforce. As such, smoke management is voluntary and not widely practiced. In a 2014 survey of 997 dermatologic surgeons who were asked if smoke management is used in their practice, 77% of respondents indicated no smoke management was used.6
The Surgical Plume: Composition
A 2014 study from the University of California, San Diego Department of Dermatology analyzed surgical smoke.6 The researchers placed the smoke collection probe 16 to 18 inches above the electrocautery site, which approximates the location of the surgeon’s head during the procedure. Assessing smoke composition, they found high levels of carcinogens and irritants. Two compounds found in their assay—1,3-butadiene and benzene—also are found in secondhand cigarette smoke. However, the concentrations in the plume were 17-fold higher for 1,3-butadiene and 10-fold higher for benzene than those found in secondhand cigarette smoke. The risk from chronic, long-term exposure to these airborne contaminants is notable, as benzene (a known carcinogen as determined by the US Department of Health and Human Services) is known to cause leukemia. For example, a busy Mohs surgeon can reach the equivalent of as many as 50 hours of continuous smoke exposure over the course of a year.6
The Surgical Plume: Particle Concentration
Ultrafine particles can bypass conventional filtering systems (surgical masks and N95 respirators) because of their extremely small size, which allows them to pass further into the lungs and all the way to the alveolar spaces. Geographic regions with high UFPs have been shown to have higher overall mortality rates, as well as higher rates of reactive airway disease, cardiovascular disease, and lung cancer. A 2016 study by Chuang et al7 published in JAMA Dermatology looked at the UFPs in the surgical plume from laser hair removal (LHR) procedures. The plume of LHR has a distinct odor and easily discernible particulates. The investigators measured the UFPs at the level of the laser practitioner and the patient’s face during LHR with a smoke evacuator turned on and again with it turned off for 30 seconds, and then compared them to UFPs measured in the treatment room, the waiting room, and outside the building. There were substantial increases in UFPs from the LHR procedure, especially for the laser practitioner, when the smoke evacuator was off. The ambient baseline particle count, as measured in the clinic waiting area, began at 15,300 particles per cubic centimeter (PPC), and once the LHR procedure began (smoke evacuator on), there was a greater than 8-fold PPC increase above baseline (15,300 PPC to 129,376 PPC) in UFPs measured for the laser practitioner. Importantly, during LHR when the smoke evacuator was turned off for 30 seconds, there was a more than 28-fold increase (15,300 PPC to 435,888 PPC) over baseline to the practitioner (Figure).7
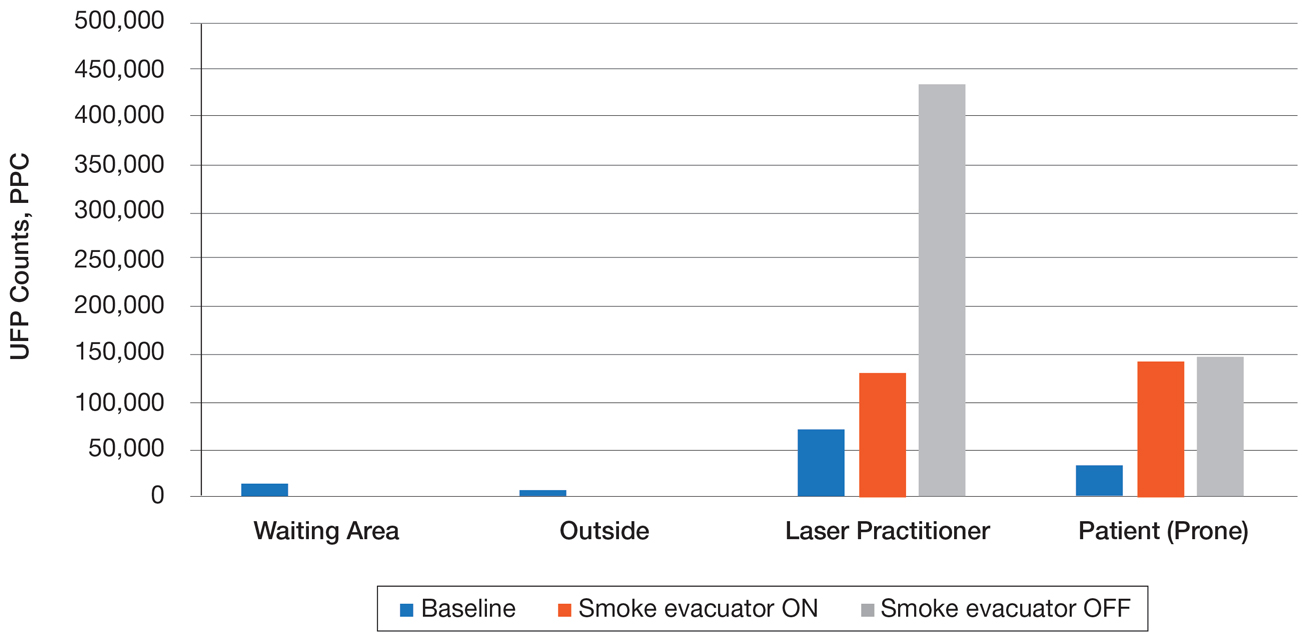
The Surgical Plume: Viruses, Bacteria, and Aerosolized Blood Products
Viruses and bacteria are thought to be transmissible via the plume, and proviral human immunodeficiency virus DNA has been found in the plume as well as evacuator equipment used to reduce plume exposure.8 A study from 1988 found that CO2 laser users treating verrucae had human papillomavirus in the laser plume.9 A comparison study of CO2 laser users treating verrucae had an increased incidence of nasopharyngeal human papillomavirus infection when compared to a control group, and the plume also contained aerosolized blood.10 The American National Standards Institute, OSHA, and NIOSH all agree that LGAC control from lasers is necessary through respiratory protection and ventilation, but none of these organizations provides specific equipment recommendations. The American Society for Laser Medicine and Surgery has published a position statement on laser plume.11
The Surgical Plume: Smoke Management
Many virus particles and UFPs are less than 0.1 µm in size. It is important to note that neither surgical masks nor high-filtration masks, such as the N95 respirator, filter particles smaller than 0.1 µm. The first line of defense in smoke management is the local exhaust ventilation (LEV) system, which includes wall suction and/or a smoke evacuator. The smoke evacuator is considered the more important of the two. General filtration, such as wall suction, is a low-flow system and is really used for liquids. It can be used as a supplement to the smoke evacuator to control small amounts of plume if fitted with an in-line filter. There are 2 types of LEV filters: ultralow particulate air filters filter particles larger than 0.1
Of utmost importance when using a smoke evacuator system is suction tip placement. Placing the suction tip 1 cm from the tissue damage site has been shown to be 98.6% effective at removing laser plume. If moved to 2 cm, effectiveness decreases to less than 50%.11 Proper management recommendations based on current evidence suggest that use of a smoke evacuator and an approved fit-tested N95 respirator might provide maximum protection.6 In addition to plume exposure, tissue splatter can occur, especially during ablative (CO2) and tattoo laser therapy, which should prompt consideration of a face shield.11 There are several vendors and models available online, and a simple Internet search for surgical tissue splatter face shields will provide multiple options.
The standard surgical mask is not NIOSH approved and only effectively (99%) filters particles larger than 5 µm (vs 25% efficacy for 0.3-µm particles). Its main purpose is to protect the patient from the wearer.12
High-filtration masks, which capture particles as small as 0.1 µm, should be used instead. The surgical N95 respirator is a NIOSH-certified respirator and is recommended for use in cases when smoke management is necessary. The FDA does not test or certify these masks; it only clears them after reviewing manufacturer test data. Technically, to be called a surgical mask, it must be cleared by the FDA.12 The 95 of N95 indicates filter efficiency ratings of 95% when testing the filter efficiency using particles of approximately 0.3 µm in diameter (Table 2).13 Because 77% of surgical smoke particles are smaller than 1.1 µm, surgical masks and N95 respirators are never sufficient as stand-alone protection.14 An LEV system is much more important for safe surgical smoke management. However, recommendations call for the use of a smoke evacuator and a high-filtration mask together to obtain the most protection available.14
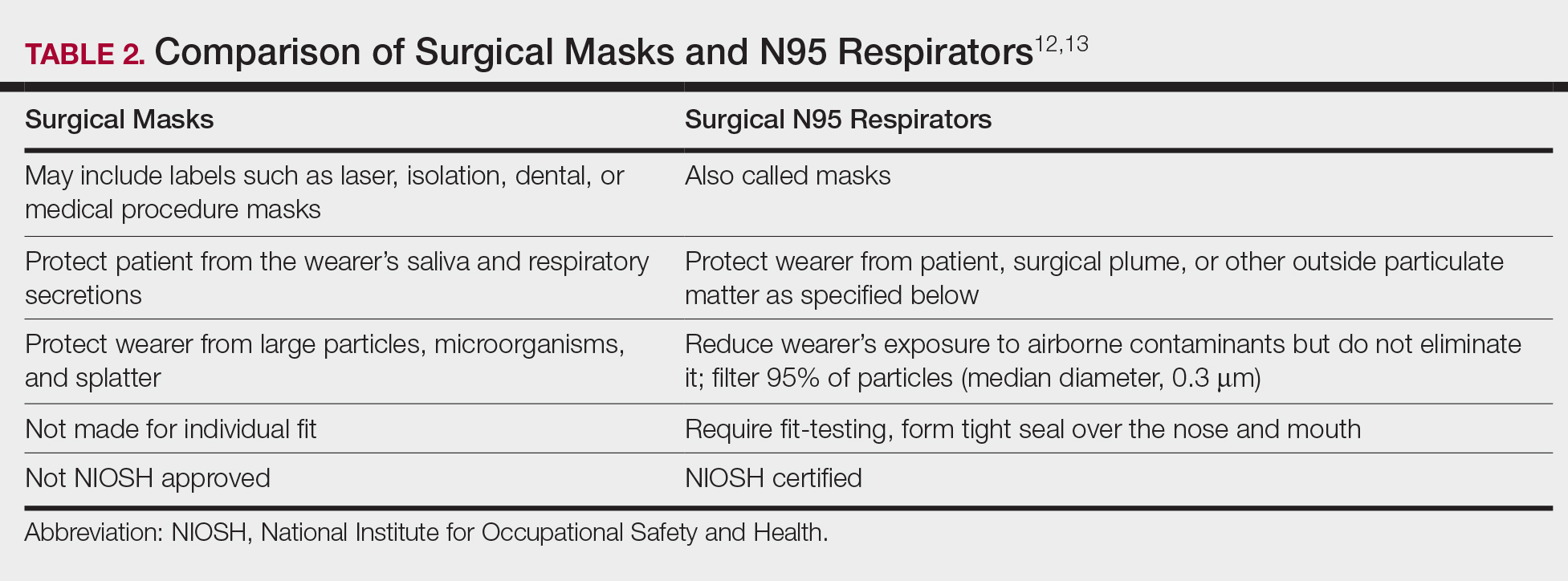
Fire Hazards
Fire hazards constitute another area of concern for the laser user and are seen with class 4 lasers. There usually are 2 types of fire hazards: electrical fires inside the laser (often faulty wiring) and flash fires (laser beam contacts flammable material). Flammable materials (eg, hair, hair products, makeup, fabrics, plastic, alcohol, chlorhexidine, aluminum chloride, elastic strap on safety goggles, gauze, drapes) should be identified and removed prior to laser use. CO2 and erbium:YAG lasers tend to pose the worst risk for flash fires.15
Precautions for fire control in the laser room should include fire extinguishers and/or fire extinguisher blankets, a water basin, and fire-resistant drapes available as needed. Flammable material such as gauze should be kept wet, or a nonflammable version should be used.3
Additional Safety Considerations
Whenever lasers are being used, it is important to cover any windows in the laser treatment area (LTA) to prevent the laser beam from passing through the glass window. Laser-blocking window covers are a requirement and are available from several vendors. Covers that block every laser class are available and come as a shade or a flat cover that is attached with Velcro or magnets. They also come with “Laser in Use” warning signs for additional safety. Access to the LTA when the laser is in use should be controlled and appropriate warning signs placed on the door to prevent inadvertent entry without proper PPE. Locking the door to the LTA while using the laser is an additional safety measure and can be included on a checklist.
For the dermatologist, the skin is a primary focus, and similar to the eye, can be at risk for injury. The most common type of injury resembles a sunburn, such as those seen in the UVB range, that appears as redness and sometimes blistering,15 which is an important consideration, and attention should be given to all those in the laser room.
Checklists
Checklists are ubiquitous throughout many occupations and many medical specialties. Their usefulness in preventing adverse events is well established. Any patient-provider encounter in which a series of sequential actions is required is a perfect situation for a checklist. In dermatologic laser surgery where the eye is uniquely susceptible to injury, a laser safety checklist is essential. Additionally, there are issues with LGACs and fire that are important to consider. Having protocols (ie, a checklist) in place that address these safety issues has been shown to reduce adverse outcomes.2 There are a number of templates available from various sources that can be customized to the laser treatment area. We provide a modifiable example (Table 3).
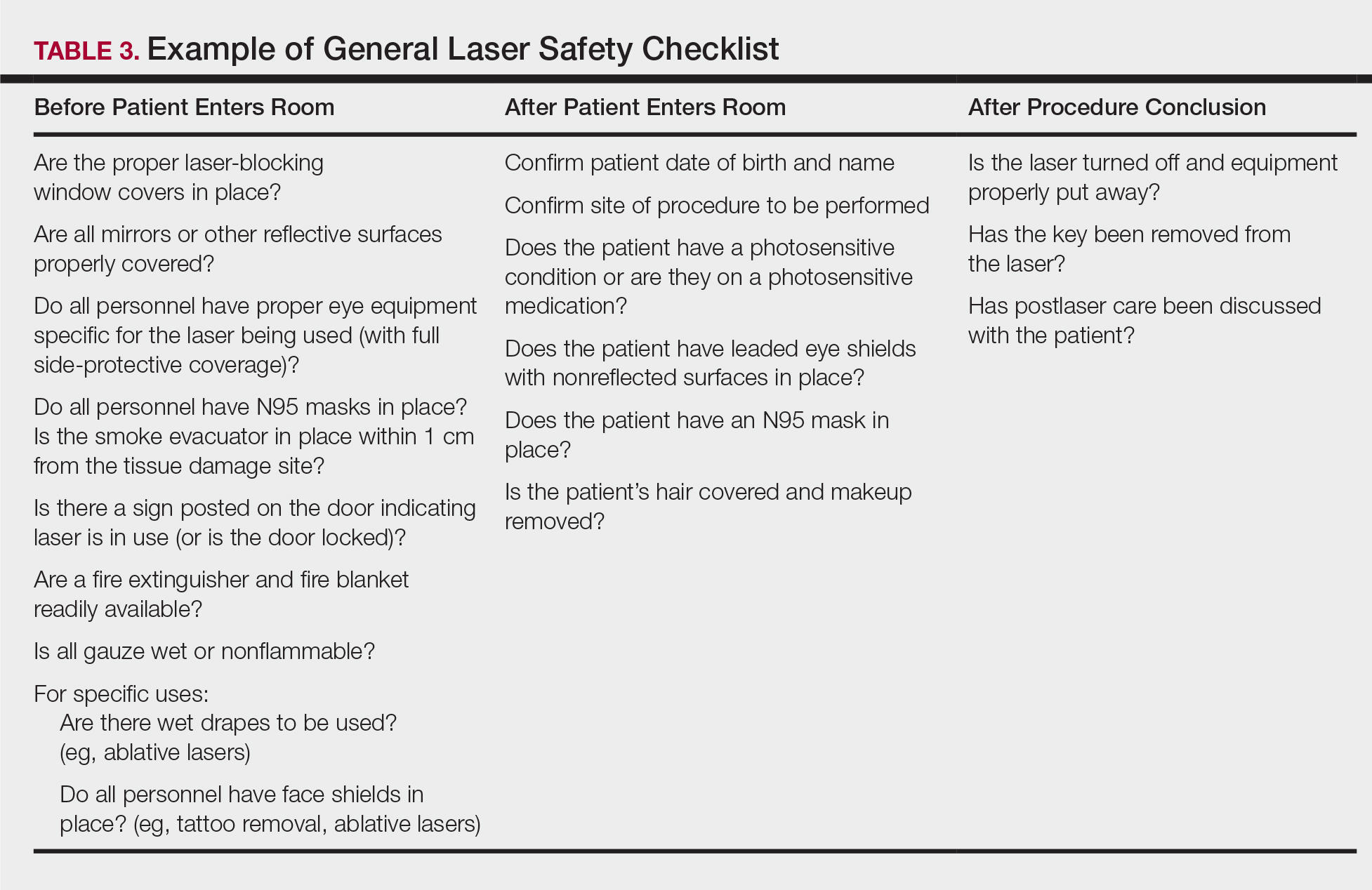
Conclusion
Laser usage in dermatologic surgery has increased. According to surveys from the American Society for Dermatologic Surgery, in 2012 there were approximately 2 million laser/light/energy-based procedures performed. By 2017, there were 3.27 million, up from 2.79 million in 2016, representing an approximate 1-year increase of 17%.16 Lasers have allowed interventions for skin, vascular, and aesthetic conditions that were once untreatable. As their use increases in number and broadens in scope, there also has been an increase in litigation alleging malpractice for misuse of the laser.17 Adverse events, which include photochemical or thermal injuries to the skin, pigmentation issues, scarring, plume-related issues, and fires, do occur. One solution to reduce the chance of an adverse outcome is to implement a checklist. Research using checklists has shown that adverse events are reduced when checklists are created and implemented properly. Improving checklist compliance also improves patient outcomes.17 The American National Standards Institute, in their ANSI Z136 series, and the World Health Organization provide checklist templates. We include our checklist for use in laser surgery (Table 3). Understanding that each laser treatment area is unique, the templates can serve as a starting point and can then be customized to suit the needs of each dermatologist.
- Goldman L, Blaney DJ, Kindel DJ, et al. Effect of the laser beam on the skin. J Invest Dermatol. 1963;40:121-122.
- Daggett C, Daggett A. The surgical check list revisited. Int J Surg Res Pract. 2017;4:051.
- Pritzker RN, Rohrer TE. Laser safety: standards and guidelines. In: Nouri K, ed. Handbook of Lasers in Dermatology. London, England: Springer; 2014:11-28.
- Husain Z, Alster TS. The role of lasers and intense pulsed light technology in dermatology. Clin Cosmet Investig Dermatol. 2016;9:29-40.
- Ries WR, Clymer MA, Reinisch L. Laser safety features of eye shields. Lasers Surg Med. 1996;18:309-315.
- Oganesyan G, Eimputh S, Kim SS, et al. Surgical smoke detection in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chuang GS, Farinelli W, Christiani DC, et al. Gaseous and particulate content of laser hair removal plume. JAMA Dermatol. 2016;152:1320-1326.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papilloma virus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Gloster HM Jr, Roenigk RK. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32:436-441.
- American Society for Laser Medicine and Surgery. ASLMS laser and energy device plume position statement. http://www.aslms.org/for-professionals/professional-resources/safety-and-complications/aslms-laser-and-energy-device-plume-position-statement. Accessed October 4, 2019.
- A comparison of surgical masks, surgical N95 respirators, and industrial N95 respirators. OH&S website. https://ohsonline.com/Articles/2014/05/01/Comparison-Respiratory.aspx?Page=3. Published May 1, 2014. Accessed October 4, 2019.
- 3M Infection Prevention N95 particulate respirators, 1860/1860s and 1870. Frequently Asked Questions. http://multimedia.3m.com/mws/media/323208O/n95-particulate-respirators-1860-1860s-1870-faqs.pdf. Accessed October 4, 2019.
- Lewin JM, Brauer JA, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Arefiev K, Warycha M, Whiting D, et al. Flammability of topical preparations and surgical dressings in cutaneous and laser surgery: a controlled simulation study. J Am Acad Dermatol. 2012;67:700-705.
- ASDS survey on dermatologic procedures. American Society for Dermatologic Surgery website. https://www.asds.net/Medical-Professionals/Practice-Resources/ASDS-Survey-on-Dermatologic-Procedures. Accessed October 4, 2019.
- Jalian HR, Jalian CA, Avram MM. Common causes of injury and legal action in laser surgery. JAMA Dermatol. 2013;149:188-193.
The use of lasers in dermatology has evolved and expanded since their first cutaneous use in 1963.1 As the fundamental understanding of the interaction of laser energy with biological tissues increased, the need for laser safety became apparent. Since then, lasers of varying wavelengths have been developed, each with its specific chromophore target and specific safety need. Protocols, such as a checklist, that have been shown to reduce adverse events in surgery and in the intensive care unit can be borrowed to decrease risk from laser injury and optimize laser safety in dermatology.2 The safety of the patient, the laser operator, and the other health care providers involved in the delivery of laser therapy led to the first US Food and Drug Administration (FDA) guidelines for laser use in 1984.3
There are 4 regulatory organizations for laser safety in the United States: the American National Standards Institute (ANSI), the Occupational Health and Safety Administration (OSHA), the FDA’s Center for Devices and Radiological Health, and The Joint Commission.
Laser Principles
The basic principles of lasers include transmission, absorption, scatter, and reflection, all occurring when laser light is applied to biological tissues. The effects of the laser are a function of the target tissue (the chromophore) and the wavelength of light being used.4 In the skin, there are 3 main endogenous chromophores: water, hemoglobin, and melanin. Some experts consider collagen to be a fourth and separate entity as a chromophore. Tattoos are considered exogenous chromophores.3 The basic principles of lasers are important to understand and keep in mind when discussing laser safety, as they are the mechanisms through which unintended consequences can occur.
Laser Safety
Ocular Hazards
Ocular hazards are a notable concern in laser surgery. The eye is uniquely susceptible to laser light, and eye injuries represent a majority of reported injuries, which can occur through direct beam, mirror reflection by surgical instruments, and beam reflection off the skin (4%–7% of light that hits the skin is reflected because of the refractive index between air and the stratum corneum).3 The different wavelengths of lasers affect different parts of the eye. The 3 parts of the eye affected most are the retina, cornea, and lens. Not only is the lens primarily at risk for acute (lenticular burns) and chronic (cataracts) injury from the laser, but secondarily the lens also can concentrate a laser beam onto the retina by a factor of 100,000 (Table 1).3

The use of ocular protective equipment, sometimes referred to as personal protective eyewear (PPE), is essential and is mandated by ANSI and OSHA for all class 3 and class 4 lasers. The eyewear must be labeled with the wavelength and the degree of optical protection—termed the optical density (OD) or filter factor—of each lens and should match the laser being used. Laser manufacturers, as required by ANSI, must provide the wavelength and OD of their lasers, and both can be found on each laser as well as in ANSI Z136.1.3
Vendors supplying PPE generally provide the material, usually glass or polycarbonate; color; visible light transmission, which is the actual amount of light that reaches one’s eye through the lens; filter specifications, which contain the OD at certain wavelengths; and the types of lasers for which each specific PPE is used. It is important to match the laser to the correct PPE. The use of multiple types of lasers in the same office or laser treatment area can present challenges regarding eye safety. Matching the PPE to the laser in use is critical, and therefore all steps to prevent error for patients and personnel should be employed. One recommendation is to place each laser in a separate room with the appropriate PPE hung outside on the door of that room.
When the treatment area is in the periocular region, protection of the patient’s cornea is essential. Leaded eye shields with nonreflective surfaces have been shown to offer the best protection.5 Prior to placement, anesthetic eye drops and lubrication are important for patient comfort and protection from corneal injury.
Laser-Generated Airborne Contaminants
Other hazards associated with laser use not directly related to the beam are laser-generated airborne contaminants (LGACs), including chemicals, viruses, bacteria, aerosolized blood products, and nanoparticles (<1 µm) known as ultrafine particles (UFPs). According to ANSI, electrosurgical devices and lasers generate the same smoke. The plume (surgical smoke) is known to contain as many as 60 chemicals, including but not limited to carbon monoxide, acrylonitrite, hydrocyanide, benzene, toluene, naphthalene, and formaldehyde. Several are known carcinogens, and others are environmental toxins.6,7
Smoke management is an important consideration for dermatologists and their patients and generally includes respiratory protection via masks and ventilation techniques. However, the practice is not universal, and oversight agencies such as OSHA and the National Institute for Occupational Safety and Health (NIOSH) provide guidelines only; they do not enforce. As such, smoke management is voluntary and not widely practiced. In a 2014 survey of 997 dermatologic surgeons who were asked if smoke management is used in their practice, 77% of respondents indicated no smoke management was used.6
The Surgical Plume: Composition
A 2014 study from the University of California, San Diego Department of Dermatology analyzed surgical smoke.6 The researchers placed the smoke collection probe 16 to 18 inches above the electrocautery site, which approximates the location of the surgeon’s head during the procedure. Assessing smoke composition, they found high levels of carcinogens and irritants. Two compounds found in their assay—1,3-butadiene and benzene—also are found in secondhand cigarette smoke. However, the concentrations in the plume were 17-fold higher for 1,3-butadiene and 10-fold higher for benzene than those found in secondhand cigarette smoke. The risk from chronic, long-term exposure to these airborne contaminants is notable, as benzene (a known carcinogen as determined by the US Department of Health and Human Services) is known to cause leukemia. For example, a busy Mohs surgeon can reach the equivalent of as many as 50 hours of continuous smoke exposure over the course of a year.6
The Surgical Plume: Particle Concentration
Ultrafine particles can bypass conventional filtering systems (surgical masks and N95 respirators) because of their extremely small size, which allows them to pass further into the lungs and all the way to the alveolar spaces. Geographic regions with high UFPs have been shown to have higher overall mortality rates, as well as higher rates of reactive airway disease, cardiovascular disease, and lung cancer. A 2016 study by Chuang et al7 published in JAMA Dermatology looked at the UFPs in the surgical plume from laser hair removal (LHR) procedures. The plume of LHR has a distinct odor and easily discernible particulates. The investigators measured the UFPs at the level of the laser practitioner and the patient’s face during LHR with a smoke evacuator turned on and again with it turned off for 30 seconds, and then compared them to UFPs measured in the treatment room, the waiting room, and outside the building. There were substantial increases in UFPs from the LHR procedure, especially for the laser practitioner, when the smoke evacuator was off. The ambient baseline particle count, as measured in the clinic waiting area, began at 15,300 particles per cubic centimeter (PPC), and once the LHR procedure began (smoke evacuator on), there was a greater than 8-fold PPC increase above baseline (15,300 PPC to 129,376 PPC) in UFPs measured for the laser practitioner. Importantly, during LHR when the smoke evacuator was turned off for 30 seconds, there was a more than 28-fold increase (15,300 PPC to 435,888 PPC) over baseline to the practitioner (Figure).7

The Surgical Plume: Viruses, Bacteria, and Aerosolized Blood Products
Viruses and bacteria are thought to be transmissible via the plume, and proviral human immunodeficiency virus DNA has been found in the plume as well as evacuator equipment used to reduce plume exposure.8 A study from 1988 found that CO2 laser users treating verrucae had human papillomavirus in the laser plume.9 A comparison study of CO2 laser users treating verrucae had an increased incidence of nasopharyngeal human papillomavirus infection when compared to a control group, and the plume also contained aerosolized blood.10 The American National Standards Institute, OSHA, and NIOSH all agree that LGAC control from lasers is necessary through respiratory protection and ventilation, but none of these organizations provides specific equipment recommendations. The American Society for Laser Medicine and Surgery has published a position statement on laser plume.11
The Surgical Plume: Smoke Management
Many virus particles and UFPs are less than 0.1 µm in size. It is important to note that neither surgical masks nor high-filtration masks, such as the N95 respirator, filter particles smaller than 0.1 µm. The first line of defense in smoke management is the local exhaust ventilation (LEV) system, which includes wall suction and/or a smoke evacuator. The smoke evacuator is considered the more important of the two. General filtration, such as wall suction, is a low-flow system and is really used for liquids. It can be used as a supplement to the smoke evacuator to control small amounts of plume if fitted with an in-line filter. There are 2 types of LEV filters: ultralow particulate air filters filter particles larger than 0.1
Of utmost importance when using a smoke evacuator system is suction tip placement. Placing the suction tip 1 cm from the tissue damage site has been shown to be 98.6% effective at removing laser plume. If moved to 2 cm, effectiveness decreases to less than 50%.11 Proper management recommendations based on current evidence suggest that use of a smoke evacuator and an approved fit-tested N95 respirator might provide maximum protection.6 In addition to plume exposure, tissue splatter can occur, especially during ablative (CO2) and tattoo laser therapy, which should prompt consideration of a face shield.11 There are several vendors and models available online, and a simple Internet search for surgical tissue splatter face shields will provide multiple options.
The standard surgical mask is not NIOSH approved and only effectively (99%) filters particles larger than 5 µm (vs 25% efficacy for 0.3-µm particles). Its main purpose is to protect the patient from the wearer.12
High-filtration masks, which capture particles as small as 0.1 µm, should be used instead. The surgical N95 respirator is a NIOSH-certified respirator and is recommended for use in cases when smoke management is necessary. The FDA does not test or certify these masks; it only clears them after reviewing manufacturer test data. Technically, to be called a surgical mask, it must be cleared by the FDA.12 The 95 of N95 indicates filter efficiency ratings of 95% when testing the filter efficiency using particles of approximately 0.3 µm in diameter (Table 2).13 Because 77% of surgical smoke particles are smaller than 1.1 µm, surgical masks and N95 respirators are never sufficient as stand-alone protection.14 An LEV system is much more important for safe surgical smoke management. However, recommendations call for the use of a smoke evacuator and a high-filtration mask together to obtain the most protection available.14

Fire Hazards
Fire hazards constitute another area of concern for the laser user and are seen with class 4 lasers. There usually are 2 types of fire hazards: electrical fires inside the laser (often faulty wiring) and flash fires (laser beam contacts flammable material). Flammable materials (eg, hair, hair products, makeup, fabrics, plastic, alcohol, chlorhexidine, aluminum chloride, elastic strap on safety goggles, gauze, drapes) should be identified and removed prior to laser use. CO2 and erbium:YAG lasers tend to pose the worst risk for flash fires.15
Precautions for fire control in the laser room should include fire extinguishers and/or fire extinguisher blankets, a water basin, and fire-resistant drapes available as needed. Flammable material such as gauze should be kept wet, or a nonflammable version should be used.3
Additional Safety Considerations
Whenever lasers are being used, it is important to cover any windows in the laser treatment area (LTA) to prevent the laser beam from passing through the glass window. Laser-blocking window covers are a requirement and are available from several vendors. Covers that block every laser class are available and come as a shade or a flat cover that is attached with Velcro or magnets. They also come with “Laser in Use” warning signs for additional safety. Access to the LTA when the laser is in use should be controlled and appropriate warning signs placed on the door to prevent inadvertent entry without proper PPE. Locking the door to the LTA while using the laser is an additional safety measure and can be included on a checklist.
For the dermatologist, the skin is a primary focus, and similar to the eye, can be at risk for injury. The most common type of injury resembles a sunburn, such as those seen in the UVB range, that appears as redness and sometimes blistering,15 which is an important consideration, and attention should be given to all those in the laser room.
Checklists
Checklists are ubiquitous throughout many occupations and many medical specialties. Their usefulness in preventing adverse events is well established. Any patient-provider encounter in which a series of sequential actions is required is a perfect situation for a checklist. In dermatologic laser surgery where the eye is uniquely susceptible to injury, a laser safety checklist is essential. Additionally, there are issues with LGACs and fire that are important to consider. Having protocols (ie, a checklist) in place that address these safety issues has been shown to reduce adverse outcomes.2 There are a number of templates available from various sources that can be customized to the laser treatment area. We provide a modifiable example (Table 3).

Conclusion
Laser usage in dermatologic surgery has increased. According to surveys from the American Society for Dermatologic Surgery, in 2012 there were approximately 2 million laser/light/energy-based procedures performed. By 2017, there were 3.27 million, up from 2.79 million in 2016, representing an approximate 1-year increase of 17%.16 Lasers have allowed interventions for skin, vascular, and aesthetic conditions that were once untreatable. As their use increases in number and broadens in scope, there also has been an increase in litigation alleging malpractice for misuse of the laser.17 Adverse events, which include photochemical or thermal injuries to the skin, pigmentation issues, scarring, plume-related issues, and fires, do occur. One solution to reduce the chance of an adverse outcome is to implement a checklist. Research using checklists has shown that adverse events are reduced when checklists are created and implemented properly. Improving checklist compliance also improves patient outcomes.17 The American National Standards Institute, in their ANSI Z136 series, and the World Health Organization provide checklist templates. We include our checklist for use in laser surgery (Table 3). Understanding that each laser treatment area is unique, the templates can serve as a starting point and can then be customized to suit the needs of each dermatologist.
The use of lasers in dermatology has evolved and expanded since their first cutaneous use in 1963.1 As the fundamental understanding of the interaction of laser energy with biological tissues increased, the need for laser safety became apparent. Since then, lasers of varying wavelengths have been developed, each with its specific chromophore target and specific safety need. Protocols, such as a checklist, that have been shown to reduce adverse events in surgery and in the intensive care unit can be borrowed to decrease risk from laser injury and optimize laser safety in dermatology.2 The safety of the patient, the laser operator, and the other health care providers involved in the delivery of laser therapy led to the first US Food and Drug Administration (FDA) guidelines for laser use in 1984.3
There are 4 regulatory organizations for laser safety in the United States: the American National Standards Institute (ANSI), the Occupational Health and Safety Administration (OSHA), the FDA’s Center for Devices and Radiological Health, and The Joint Commission.
Laser Principles
The basic principles of lasers include transmission, absorption, scatter, and reflection, all occurring when laser light is applied to biological tissues. The effects of the laser are a function of the target tissue (the chromophore) and the wavelength of light being used.4 In the skin, there are 3 main endogenous chromophores: water, hemoglobin, and melanin. Some experts consider collagen to be a fourth and separate entity as a chromophore. Tattoos are considered exogenous chromophores.3 The basic principles of lasers are important to understand and keep in mind when discussing laser safety, as they are the mechanisms through which unintended consequences can occur.
Laser Safety
Ocular Hazards
Ocular hazards are a notable concern in laser surgery. The eye is uniquely susceptible to laser light, and eye injuries represent a majority of reported injuries, which can occur through direct beam, mirror reflection by surgical instruments, and beam reflection off the skin (4%–7% of light that hits the skin is reflected because of the refractive index between air and the stratum corneum).3 The different wavelengths of lasers affect different parts of the eye. The 3 parts of the eye affected most are the retina, cornea, and lens. Not only is the lens primarily at risk for acute (lenticular burns) and chronic (cataracts) injury from the laser, but secondarily the lens also can concentrate a laser beam onto the retina by a factor of 100,000 (Table 1).3

The use of ocular protective equipment, sometimes referred to as personal protective eyewear (PPE), is essential and is mandated by ANSI and OSHA for all class 3 and class 4 lasers. The eyewear must be labeled with the wavelength and the degree of optical protection—termed the optical density (OD) or filter factor—of each lens and should match the laser being used. Laser manufacturers, as required by ANSI, must provide the wavelength and OD of their lasers, and both can be found on each laser as well as in ANSI Z136.1.3
Vendors supplying PPE generally provide the material, usually glass or polycarbonate; color; visible light transmission, which is the actual amount of light that reaches one’s eye through the lens; filter specifications, which contain the OD at certain wavelengths; and the types of lasers for which each specific PPE is used. It is important to match the laser to the correct PPE. The use of multiple types of lasers in the same office or laser treatment area can present challenges regarding eye safety. Matching the PPE to the laser in use is critical, and therefore all steps to prevent error for patients and personnel should be employed. One recommendation is to place each laser in a separate room with the appropriate PPE hung outside on the door of that room.
When the treatment area is in the periocular region, protection of the patient’s cornea is essential. Leaded eye shields with nonreflective surfaces have been shown to offer the best protection.5 Prior to placement, anesthetic eye drops and lubrication are important for patient comfort and protection from corneal injury.
Laser-Generated Airborne Contaminants
Other hazards associated with laser use not directly related to the beam are laser-generated airborne contaminants (LGACs), including chemicals, viruses, bacteria, aerosolized blood products, and nanoparticles (<1 µm) known as ultrafine particles (UFPs). According to ANSI, electrosurgical devices and lasers generate the same smoke. The plume (surgical smoke) is known to contain as many as 60 chemicals, including but not limited to carbon monoxide, acrylonitrite, hydrocyanide, benzene, toluene, naphthalene, and formaldehyde. Several are known carcinogens, and others are environmental toxins.6,7
Smoke management is an important consideration for dermatologists and their patients and generally includes respiratory protection via masks and ventilation techniques. However, the practice is not universal, and oversight agencies such as OSHA and the National Institute for Occupational Safety and Health (NIOSH) provide guidelines only; they do not enforce. As such, smoke management is voluntary and not widely practiced. In a 2014 survey of 997 dermatologic surgeons who were asked if smoke management is used in their practice, 77% of respondents indicated no smoke management was used.6
The Surgical Plume: Composition
A 2014 study from the University of California, San Diego Department of Dermatology analyzed surgical smoke.6 The researchers placed the smoke collection probe 16 to 18 inches above the electrocautery site, which approximates the location of the surgeon’s head during the procedure. Assessing smoke composition, they found high levels of carcinogens and irritants. Two compounds found in their assay—1,3-butadiene and benzene—also are found in secondhand cigarette smoke. However, the concentrations in the plume were 17-fold higher for 1,3-butadiene and 10-fold higher for benzene than those found in secondhand cigarette smoke. The risk from chronic, long-term exposure to these airborne contaminants is notable, as benzene (a known carcinogen as determined by the US Department of Health and Human Services) is known to cause leukemia. For example, a busy Mohs surgeon can reach the equivalent of as many as 50 hours of continuous smoke exposure over the course of a year.6
The Surgical Plume: Particle Concentration
Ultrafine particles can bypass conventional filtering systems (surgical masks and N95 respirators) because of their extremely small size, which allows them to pass further into the lungs and all the way to the alveolar spaces. Geographic regions with high UFPs have been shown to have higher overall mortality rates, as well as higher rates of reactive airway disease, cardiovascular disease, and lung cancer. A 2016 study by Chuang et al7 published in JAMA Dermatology looked at the UFPs in the surgical plume from laser hair removal (LHR) procedures. The plume of LHR has a distinct odor and easily discernible particulates. The investigators measured the UFPs at the level of the laser practitioner and the patient’s face during LHR with a smoke evacuator turned on and again with it turned off for 30 seconds, and then compared them to UFPs measured in the treatment room, the waiting room, and outside the building. There were substantial increases in UFPs from the LHR procedure, especially for the laser practitioner, when the smoke evacuator was off. The ambient baseline particle count, as measured in the clinic waiting area, began at 15,300 particles per cubic centimeter (PPC), and once the LHR procedure began (smoke evacuator on), there was a greater than 8-fold PPC increase above baseline (15,300 PPC to 129,376 PPC) in UFPs measured for the laser practitioner. Importantly, during LHR when the smoke evacuator was turned off for 30 seconds, there was a more than 28-fold increase (15,300 PPC to 435,888 PPC) over baseline to the practitioner (Figure).7

The Surgical Plume: Viruses, Bacteria, and Aerosolized Blood Products
Viruses and bacteria are thought to be transmissible via the plume, and proviral human immunodeficiency virus DNA has been found in the plume as well as evacuator equipment used to reduce plume exposure.8 A study from 1988 found that CO2 laser users treating verrucae had human papillomavirus in the laser plume.9 A comparison study of CO2 laser users treating verrucae had an increased incidence of nasopharyngeal human papillomavirus infection when compared to a control group, and the plume also contained aerosolized blood.10 The American National Standards Institute, OSHA, and NIOSH all agree that LGAC control from lasers is necessary through respiratory protection and ventilation, but none of these organizations provides specific equipment recommendations. The American Society for Laser Medicine and Surgery has published a position statement on laser plume.11
The Surgical Plume: Smoke Management
Many virus particles and UFPs are less than 0.1 µm in size. It is important to note that neither surgical masks nor high-filtration masks, such as the N95 respirator, filter particles smaller than 0.1 µm. The first line of defense in smoke management is the local exhaust ventilation (LEV) system, which includes wall suction and/or a smoke evacuator. The smoke evacuator is considered the more important of the two. General filtration, such as wall suction, is a low-flow system and is really used for liquids. It can be used as a supplement to the smoke evacuator to control small amounts of plume if fitted with an in-line filter. There are 2 types of LEV filters: ultralow particulate air filters filter particles larger than 0.1
Of utmost importance when using a smoke evacuator system is suction tip placement. Placing the suction tip 1 cm from the tissue damage site has been shown to be 98.6% effective at removing laser plume. If moved to 2 cm, effectiveness decreases to less than 50%.11 Proper management recommendations based on current evidence suggest that use of a smoke evacuator and an approved fit-tested N95 respirator might provide maximum protection.6 In addition to plume exposure, tissue splatter can occur, especially during ablative (CO2) and tattoo laser therapy, which should prompt consideration of a face shield.11 There are several vendors and models available online, and a simple Internet search for surgical tissue splatter face shields will provide multiple options.
The standard surgical mask is not NIOSH approved and only effectively (99%) filters particles larger than 5 µm (vs 25% efficacy for 0.3-µm particles). Its main purpose is to protect the patient from the wearer.12
High-filtration masks, which capture particles as small as 0.1 µm, should be used instead. The surgical N95 respirator is a NIOSH-certified respirator and is recommended for use in cases when smoke management is necessary. The FDA does not test or certify these masks; it only clears them after reviewing manufacturer test data. Technically, to be called a surgical mask, it must be cleared by the FDA.12 The 95 of N95 indicates filter efficiency ratings of 95% when testing the filter efficiency using particles of approximately 0.3 µm in diameter (Table 2).13 Because 77% of surgical smoke particles are smaller than 1.1 µm, surgical masks and N95 respirators are never sufficient as stand-alone protection.14 An LEV system is much more important for safe surgical smoke management. However, recommendations call for the use of a smoke evacuator and a high-filtration mask together to obtain the most protection available.14

Fire Hazards
Fire hazards constitute another area of concern for the laser user and are seen with class 4 lasers. There usually are 2 types of fire hazards: electrical fires inside the laser (often faulty wiring) and flash fires (laser beam contacts flammable material). Flammable materials (eg, hair, hair products, makeup, fabrics, plastic, alcohol, chlorhexidine, aluminum chloride, elastic strap on safety goggles, gauze, drapes) should be identified and removed prior to laser use. CO2 and erbium:YAG lasers tend to pose the worst risk for flash fires.15
Precautions for fire control in the laser room should include fire extinguishers and/or fire extinguisher blankets, a water basin, and fire-resistant drapes available as needed. Flammable material such as gauze should be kept wet, or a nonflammable version should be used.3
Additional Safety Considerations
Whenever lasers are being used, it is important to cover any windows in the laser treatment area (LTA) to prevent the laser beam from passing through the glass window. Laser-blocking window covers are a requirement and are available from several vendors. Covers that block every laser class are available and come as a shade or a flat cover that is attached with Velcro or magnets. They also come with “Laser in Use” warning signs for additional safety. Access to the LTA when the laser is in use should be controlled and appropriate warning signs placed on the door to prevent inadvertent entry without proper PPE. Locking the door to the LTA while using the laser is an additional safety measure and can be included on a checklist.
For the dermatologist, the skin is a primary focus, and similar to the eye, can be at risk for injury. The most common type of injury resembles a sunburn, such as those seen in the UVB range, that appears as redness and sometimes blistering,15 which is an important consideration, and attention should be given to all those in the laser room.
Checklists
Checklists are ubiquitous throughout many occupations and many medical specialties. Their usefulness in preventing adverse events is well established. Any patient-provider encounter in which a series of sequential actions is required is a perfect situation for a checklist. In dermatologic laser surgery where the eye is uniquely susceptible to injury, a laser safety checklist is essential. Additionally, there are issues with LGACs and fire that are important to consider. Having protocols (ie, a checklist) in place that address these safety issues has been shown to reduce adverse outcomes.2 There are a number of templates available from various sources that can be customized to the laser treatment area. We provide a modifiable example (Table 3).

Conclusion
Laser usage in dermatologic surgery has increased. According to surveys from the American Society for Dermatologic Surgery, in 2012 there were approximately 2 million laser/light/energy-based procedures performed. By 2017, there were 3.27 million, up from 2.79 million in 2016, representing an approximate 1-year increase of 17%.16 Lasers have allowed interventions for skin, vascular, and aesthetic conditions that were once untreatable. As their use increases in number and broadens in scope, there also has been an increase in litigation alleging malpractice for misuse of the laser.17 Adverse events, which include photochemical or thermal injuries to the skin, pigmentation issues, scarring, plume-related issues, and fires, do occur. One solution to reduce the chance of an adverse outcome is to implement a checklist. Research using checklists has shown that adverse events are reduced when checklists are created and implemented properly. Improving checklist compliance also improves patient outcomes.17 The American National Standards Institute, in their ANSI Z136 series, and the World Health Organization provide checklist templates. We include our checklist for use in laser surgery (Table 3). Understanding that each laser treatment area is unique, the templates can serve as a starting point and can then be customized to suit the needs of each dermatologist.
- Goldman L, Blaney DJ, Kindel DJ, et al. Effect of the laser beam on the skin. J Invest Dermatol. 1963;40:121-122.
- Daggett C, Daggett A. The surgical check list revisited. Int J Surg Res Pract. 2017;4:051.
- Pritzker RN, Rohrer TE. Laser safety: standards and guidelines. In: Nouri K, ed. Handbook of Lasers in Dermatology. London, England: Springer; 2014:11-28.
- Husain Z, Alster TS. The role of lasers and intense pulsed light technology in dermatology. Clin Cosmet Investig Dermatol. 2016;9:29-40.
- Ries WR, Clymer MA, Reinisch L. Laser safety features of eye shields. Lasers Surg Med. 1996;18:309-315.
- Oganesyan G, Eimputh S, Kim SS, et al. Surgical smoke detection in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chuang GS, Farinelli W, Christiani DC, et al. Gaseous and particulate content of laser hair removal plume. JAMA Dermatol. 2016;152:1320-1326.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papilloma virus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Gloster HM Jr, Roenigk RK. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32:436-441.
- American Society for Laser Medicine and Surgery. ASLMS laser and energy device plume position statement. http://www.aslms.org/for-professionals/professional-resources/safety-and-complications/aslms-laser-and-energy-device-plume-position-statement. Accessed October 4, 2019.
- A comparison of surgical masks, surgical N95 respirators, and industrial N95 respirators. OH&S website. https://ohsonline.com/Articles/2014/05/01/Comparison-Respiratory.aspx?Page=3. Published May 1, 2014. Accessed October 4, 2019.
- 3M Infection Prevention N95 particulate respirators, 1860/1860s and 1870. Frequently Asked Questions. http://multimedia.3m.com/mws/media/323208O/n95-particulate-respirators-1860-1860s-1870-faqs.pdf. Accessed October 4, 2019.
- Lewin JM, Brauer JA, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Arefiev K, Warycha M, Whiting D, et al. Flammability of topical preparations and surgical dressings in cutaneous and laser surgery: a controlled simulation study. J Am Acad Dermatol. 2012;67:700-705.
- ASDS survey on dermatologic procedures. American Society for Dermatologic Surgery website. https://www.asds.net/Medical-Professionals/Practice-Resources/ASDS-Survey-on-Dermatologic-Procedures. Accessed October 4, 2019.
- Jalian HR, Jalian CA, Avram MM. Common causes of injury and legal action in laser surgery. JAMA Dermatol. 2013;149:188-193.
- Goldman L, Blaney DJ, Kindel DJ, et al. Effect of the laser beam on the skin. J Invest Dermatol. 1963;40:121-122.
- Daggett C, Daggett A. The surgical check list revisited. Int J Surg Res Pract. 2017;4:051.
- Pritzker RN, Rohrer TE. Laser safety: standards and guidelines. In: Nouri K, ed. Handbook of Lasers in Dermatology. London, England: Springer; 2014:11-28.
- Husain Z, Alster TS. The role of lasers and intense pulsed light technology in dermatology. Clin Cosmet Investig Dermatol. 2016;9:29-40.
- Ries WR, Clymer MA, Reinisch L. Laser safety features of eye shields. Lasers Surg Med. 1996;18:309-315.
- Oganesyan G, Eimputh S, Kim SS, et al. Surgical smoke detection in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chuang GS, Farinelli W, Christiani DC, et al. Gaseous and particulate content of laser hair removal plume. JAMA Dermatol. 2016;152:1320-1326.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papilloma virus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Gloster HM Jr, Roenigk RK. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32:436-441.
- American Society for Laser Medicine and Surgery. ASLMS laser and energy device plume position statement. http://www.aslms.org/for-professionals/professional-resources/safety-and-complications/aslms-laser-and-energy-device-plume-position-statement. Accessed October 4, 2019.
- A comparison of surgical masks, surgical N95 respirators, and industrial N95 respirators. OH&S website. https://ohsonline.com/Articles/2014/05/01/Comparison-Respiratory.aspx?Page=3. Published May 1, 2014. Accessed October 4, 2019.
- 3M Infection Prevention N95 particulate respirators, 1860/1860s and 1870. Frequently Asked Questions. http://multimedia.3m.com/mws/media/323208O/n95-particulate-respirators-1860-1860s-1870-faqs.pdf. Accessed October 4, 2019.
- Lewin JM, Brauer JA, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Arefiev K, Warycha M, Whiting D, et al. Flammability of topical preparations and surgical dressings in cutaneous and laser surgery: a controlled simulation study. J Am Acad Dermatol. 2012;67:700-705.
- ASDS survey on dermatologic procedures. American Society for Dermatologic Surgery website. https://www.asds.net/Medical-Professionals/Practice-Resources/ASDS-Survey-on-Dermatologic-Procedures. Accessed October 4, 2019.
- Jalian HR, Jalian CA, Avram MM. Common causes of injury and legal action in laser surgery. JAMA Dermatol. 2013;149:188-193.
Practice Points
- Laser therapy has evolved and expanded since its first cutaneous use in 1963.
- The 4 regulatory agencies for laser safety in the United States establish standards and guidelines, but implementation is voluntary.
- Ocular hazards, laser-generated airborne contaminants, fires, and unintended laser beam injuries constitute the main safety concerns.
- Safety protocols with a laser checklist can reduce adverse outcomes.
