User login
Physician Burnout Meta‐analysis
Hospital medicine is a rapidly growing field of US clinical practice.[1] Almost since its advent, concerns have been expressed about the potential for hospitalists to burn out.[2] Hospitalists are not unique in this; similar concerns heralded the arrival of other location‐defined specialties, including emergency medicine[3] and the full‐time intensivist model,[4] a fact that has not gone unnoted in the literature about hospitalists.[5]
The existing international literature on physician burnout provides good reason for this concern. Inpatient‐based physicians tend to work unpredictable schedules, with substantial impact on home life.[6] They tend to be young, and much burnout literature suggests a higher risk among younger, less‐experienced physicians.[7] When surveyed, hospitalists have expressed more concerns about their potential for burnout than their outpatient‐based colleagues.[8]
In fact, data suggesting a correlation between inpatient practice and burnout predate the advent of the US hospitalist movement. Increased hospital time was reported to correlate with higher rates of burnout in internists,[9] family practitioners,[10] palliative physicians,[11] junior doctors,[12] radiologists,[13] and cystic fibrosis caregivers.[14] In 1987, Keinan and Melamed[15] noted, Hospital work by its very nature, as compared to the work of a general practitioner, deals with the more severe and complicated illnesses, coupled with continuous daily contacts with patients and their anxious families. In addition, these physicians may find themselves embroiled in the power struggles and competition so common in their work environment.
There are other features, however, that may protect inpatient physicians from burnout. Hospital practice can facilitate favorable social relations involving colleagues, co‐workers, and patients,[16] a factor that may be protective.[17] A hospitalist schedule also can allow more focused time for continuing medical education, research, and teaching,[18] which have all been associated with reduced risk of burnout in some studies.[17] Studies of psychiatrists[19] and pediatricians[20] have shown a lower rate of burnout among physicians with more inpatient duties. Finally, a practice model involving a seemingly stable cadre of inpatient physicians has existed in Europe for decades,[2] indicating at least a degree of sustainability.
Information suggesting a higher rate of burnout among inpatient physicians could be used to target therapeutic interventions and to adjust schedules, whereas the opposite outcome could refute a pervasive myth. We therefore endeavored to summarize the literature on burnout among inpatient versus outpatient physicians in a systematic fashion, and to include data not only from the US hospitalist experience but also from other countries that have used a similar model for decades. Our primary hypothesis was that inpatient physicians experience more burnout than outpatient physicians.
It is important to distinguish burnout from depression, job dissatisfaction, and occupational stress, all of which have been studied extensively in physicians. Burnout, as introduced by Freudenberger[21] and further characterized by Maslach,[22] is a condition in which emotional exhaustion, depersonalization, and a low sense of personal accomplishment combine to negatively affect work life (as opposed to clinical depression, which affects all aspects of life). Job satisfaction can correlate inversely with burnout, but it is a separate process[23] and the subject of a recent systematic review.[24] The importance of distinguishing burnout from job dissatisfaction is illustrated by a survey of head and neck surgeons, in which 97% of those surveyed indicated satisfaction with their jobs and 34% of the same group answered in the affirmative when asked if they felt burned out.[25]
One obstacle to the meaningful comparison of burnout prevalence across time, geography, and specialty is the myriad ways in which burnout is measured and reported. The oldest and most commonly used instrument to measure burnout is the Maslach Burnout Inventory (MBI), which contains 22 items assessing 3 components of burnout (emotional exhaustion, depersonalization, and low personal accomplishment).[26] Other measures include the Copenhagen Burnout Inventory[27] (19 items with the components personal burnout, work‐related burnout, and client‐related burnout), Utrecht Burnout Inventory[28] (20‐item modification of the MBI), Boudreau Burnout Questionnaire[29] (30 items), Arbeitsbezogenes Verhaltens und Erlebensmuster[30] (66‐item questionnaire assessing professional commitment, resistance to stress, and emotional well‐being), Shirom‐Melamed Burnout Measure[31] (22 items with subscales for physical fatigue, cognitive weariness, tension, and listlessness), and a validated single‐item questionnaire.[32]
METHODS
Electronic searches of MEDLINE, EMBASE, PsycINFO, SCOPUS, and PubMed were undertaken for articles published from January 1, 1974 (the year in which burnout was first described by Freudenberger[21]) to 2012 (last accessed, September 12, 2012) using the Medical Subject Headings (MeSH) terms stress, psychological; burnout, professional; adaptation, psychological; and the keyword burnout. The same sources were searched to create another set for the MeSH terms hospitalists, physician's practice patterns, physicians/px, professional practice location, and the keyword hospitalist#. Where exact subject headings did not exist in databases, comparable subject headings or keywords were used. The 2 sets were then combined using the operator and. Abstracts from the Society of Hospital Medicine annual conferences were hand‐searched, as were reference lists from identified articles. To ensure that pertinent international literature was captured, there was no language restriction in the search.
A 2‐stage screening process was used. The titles and abstracts of all articles identified in the search were independently reviewed by 2 investigators (D.L.R. and K.J.C.) who had no knowledge of each other's results. An article was obtained when either reviewer deemed it worthy of full‐text review.
All full‐text articles were independently reviewed by the same 2 investigators. The inclusion criterion was the measurement of burnout in physicians who are stated to or can be reasonably assumed to spend the substantial majority of their clinical practice exclusively in either the inpatient or the outpatient setting. Studies of emergency department physicians or specialists who invariably spend substantial amounts of time in both settings (eg, surgeons, anesthesiologists) were excluded. Studies limited to trainees or nonphysicians were also excluded. For both stages of review, agreement between the 2 investigators was assessed by calculating the statistic. Disagreements about inclusion were adjudicated by a third investigator (A.I.B.).
Because our goal was to establish and compare the rate of burnout among US hospitalists and other inpatient physicians around the world, we included studies of hospitalists according to the definition in use at the time of the individual study, noting that the formal definition of a hospitalist has changed over the years.[33] Because practice patterns for physicians described as primary care physicians, family doctors, hospital doctors, and others differ substantially from country to country, we otherwise included only the studies where the practice location was stated explicitly or where the authors confirmed that their study participants either are known or can be reasonably assumed to spend more than 75% of their time caring for hospital inpatients, or are known or can be reasonably assumed to spend the vast majority of their time caring for outpatients.
Data were abstracted using a standardized form and included the measure of burnout used in the study, results, practice location of study subjects, and total number of study subjects. When data were not clear (eg, burnout measured but not reported by the authors, practice location of study subjects not clear), authors were contacted by email, or when no current email address could be located or no response was received, by telephone or letter. In instances where burnout was measured repeatedly over time or before and after a specific intervention, only the baseline measurement was recorded. Because all studies were expected to be nonrandomized, methodological quality was assessed using a version of the tool of Downs and Black,[34] adapted where necessary by omitting questions not applicable to the specific study type (eg randomization for survey studies)[35] and giving a maximum of 1 point for the inclusion of a power calculation.
Two a priori analyses were planned: (1) a statistical comparison of articles directly comparing burnout among inpatient and outpatient physicians, and (2) a statistical comparison of articles measuring burnout among inpatient physicians with articles measuring burnout among outpatient physicians by the most frequently reported measuremean subset scores for emotional exhaustion, depersonalization, and personal accomplishment on the MBI.
The primary outcome measures were the differences between mean subset scores for emotional exhaustion, depersonalization, and personal accomplishment on the MBI. All differences are expressed as (outpatient meaninpatient mean). The variance of each outcome was calculated with standard formulas.[36] To calculate the overall estimate, each study was weighted by the reciprocal of its variance. Studies with fewer than 10 subjects were excluded from statistical analysis but retained in the systematic review.
For studies that reported data for both inpatient and outpatient physicians (double‐armed studies), Cochran Q test and the I2 value were used to assess heterogeneity.[37, 38] Substantial heterogeneity was expected because these individual studies were conducted for different populations in different settings with different study designs, and this expectation was confirmed statistically. Therefore, we used a random effects model to estimate the overall effect, providing a conservative approach that accounted for heterogeneity among studies.[39]
To assess the durability of our findings, we performed separate multivariate meta‐regression analyses by including single‐armed studies only and including both single‐armed and double‐armed studies. For these meta‐regressions, means were again weighted by the reciprocal of their variances, and the arms of 2‐armed studies were considered separately. This approach allowed us to generate an estimate of the differences between MBI subset scores from studies that did not include such an estimate when analyzed separately.[40]
We examined the potential for publication bias in double‐armed studies by constructing a funnel plot, in which mean scores were plotted against their standard errors.[41] The trim‐and‐fill method was used to determine whether adjustment for publication bias was necessary. In addition, Begg's rank correlation test[42] was completed to test for statistically significant publication bias.
Stata 10.0 statistical software (StataCorp, College Station, TX) was used for data analyses. A P value of 0.05 or less was deemed statistically significant. The Preferred Reporting Items for Systematic Reviews and Meta‐analysis checklist was used for the design and execution of the systematic review and meta‐analysis.[43]
Subgroup analyses based on location were undertaken a posteriori. All data (double‐armed meta‐analysis, meta‐regression of single‐armed studies, and meta‐regression of single‐ and double‐armed studies) were analyzed by location (United States vs other; United States vs Europe vs other).
RESULTS
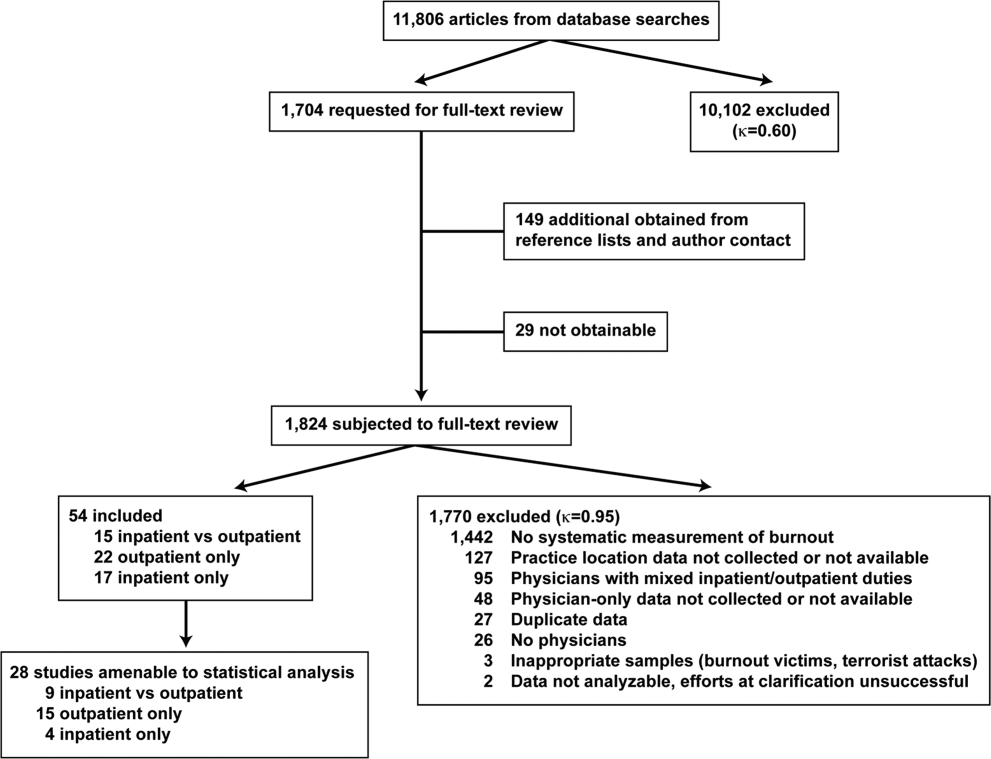
The search results are outlined in Figure 1. In total, 1704 articles met the criteria for full‐text review. A review of pertinent reference lists and author contacts led to the addition of 149 articles. Twenty‐nine references could not be located by any means, despite repeated attempts. Therefore, 1824 articles were subjected to full‐text review by the 2 investigators.
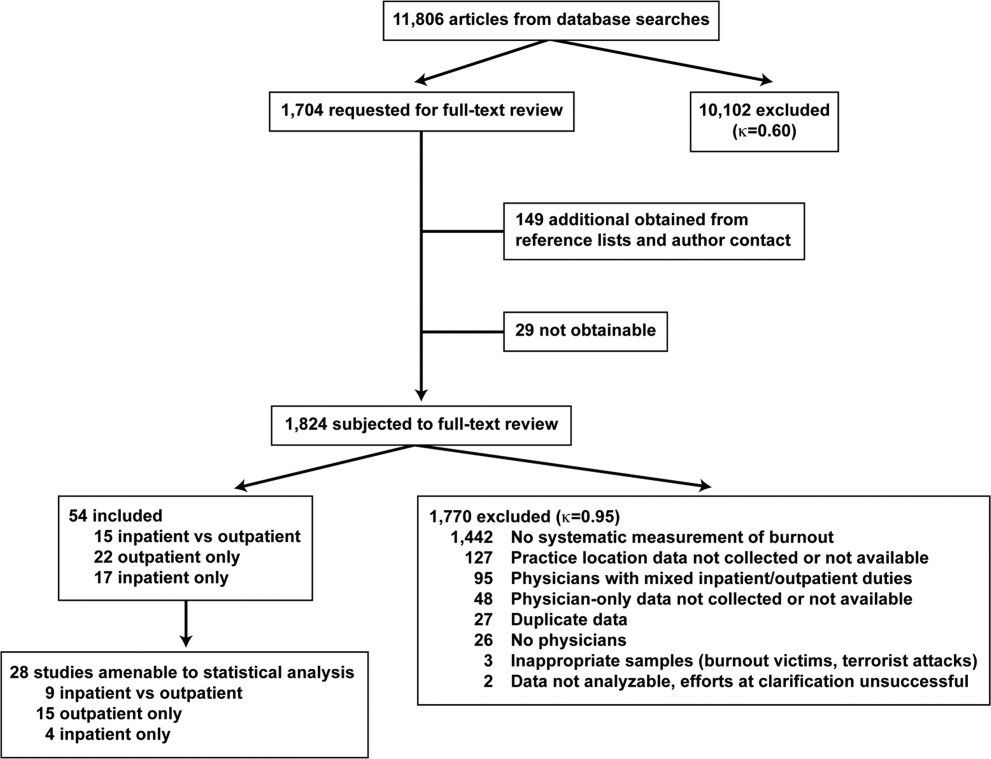
Initially, 57 articles were found that met criteria for inclusion. Of these, 2 articles reported data in formats that could not be interpreted.[44, 45] When efforts to clarify the data with the authors were unsuccessful, these studies were excluded. A study specifically designed to assess the response of physicians to a recent series of terrorist attacks[46] was excluded a posteriori because of lack of generalizability. Of the other 54 studies, 15 reported burnout data on both outpatient physicians and inpatient physicians, 22 reported data on outpatient physicians only, and 17 reported data on inpatient physicians only. Table 1 summarizes the results of the 37 studies involving outpatient physicians; Table 2 summarizes the 32 studies involving inpatient physicians.
| Lead Author, Publication Year | Study Population and Location | Instrument | No. of Participants | EE Score (SD)a | DP Score (SD) | PA Score (SD) | Other Results |
|---|---|---|---|---|---|---|---|
| |||||||
| Schweitzer, 1994[12] | Young physicians of various specialties in South Africa | Single‐item survey | 7 | 6 (83%) endorsed burnout | |||
| Aasland, 1997 [54]b | General practitioners in Norway | Modified MBI (22 items; scale, 15) | 298 | 2.65 (0.80) | 1.90 (0.59) | 3.45 (0.40) | |
| Grassi, 2000 [58] | General practitioners in Italy | MBI | 182 | 18.49 (11.49) | 6.11 (5.86) | 38.52 (7.60) | |
| McManus, 2000 [59]b | General practitioners in United Kingdom | Modified MBI (9 items; scale, 06) | 800 | 8.34 (4.39) | 3.18 (3.40) | 14.16 (2.95) | |
| Yaman, 2002 [60] | General practitioners in 8 European nations | MBI | 98 | 25.1 (8.50) | 7.3 (4.92) | 34.5 (7.67) | |
| Cathbras, 2004 [61] | General practitioners in France | MBI | 306 | 21.85 (12.4) | 9.13 (6.7) | 38.7 (7.1) | |
| Goehring, 2005 [63] | General practitioners, general internists, pediatricians in Switzerland | MBI | 1755 | 17.9 (9.8) | 6.5 (4.7) | 39.6 (6.5) | |
| Esteva, 2006 [64] | General practitioners, pediatricians in Spain | MBI | 261 | 27.4 (11.8) | 10.07 (6.4) | 35.9 (7.06) | |
| Gandini, 2006 [65]b | Physicians of various specialties in Argentina | MBI | 67 | 31.0 (13.8) | 10.2 (6.6) | 38.4 (6.8) | |
| Ozyurt, 2006 [66] | General practitioners in Turkey | Modified MBI (22 items; scale, 04) | 55 | 15.23 (5.80) | 4.47 (3.31) | 23.38 (4.29) | |
| Deighton, 2007 [67]b | Psychiatrists in several German‐speaking nations | MBI | 19 | 30.68 (9.92) | 13.42 (4.23) | 37.16 (3.39) | |
| Dunwoodie, 2007 [68]b | Palliative care physicians in Australia | MBI | 21 | 14.95 (9.14) | 3.95 (3.40) | 38.90 (2.88) | |
| Srgaard, 2007 [69]b | Psychiatrists in 5 European nations | MBI | 22 | 19.41 (8.08) | 6.68 (4.93) | 39.00 (4.40) | |
| Sosa Oberlin, 2007 [56]b | Physicians of various specialties in Argentina | Author‐designed instrument | 33 | 26 (78.8%) had 4 burnout symptoms, 6.15 symptoms per physician | |||
| Voltmer, 2007 [57]b | Physicians of various specialties in Germany | AVEM | 46 | 11 (23.9%) exhibited burnout (type B) pattern | |||
| dm, 2008 [70]b | Physicians of various specialties in Hungary | MBI | 163 | 17.45 (11.12) | 4.86 (4.91) | 36.56 (7.03) | |
| Di Iorio, 2008 [71]b | Dialysis physicians in Italy | Author‐designed instrument | 54 | Work: 2.6 (1.5), Material: 3.1 (2.1), Climate: 3.0 (1.1), Objectives: 3.4 (1.6), Quality: 2.2 (1.5), Justification: 3.2 (2.0) | |||
| Lee, 2008 [49]b | Family physicians in Canada | MBI | 123 | 26.26 (9.53) | 10.20 (5.22) | 38.43 (7.34) | |
| Truchot, 2008 [72] | General practitioners in France | MBI | 259 | 25.4 (11.7) | 7.5 (5.5) | 36.5 (7.1) | |
| Twellaar, 2008 [73]b | General practitioners in the Netherlands | Utrecht Burnout Inventory | 349 | 2.06 (1.11) | 1.71 (1.05) | 5.08 (0.77) | |
| Arigoni, 2009 [17] | General practitioners, pediatricians in Switzerland | MBI | 258 | 22.8 (12.0) | 6.9 (6.1) | 39.0 (7.2) | |
| Bernhardt, 2009 [75] | Clinical geneticists in United States | MBI | 72 | 25.8 (10.01)c | 10.9 (4.16)c | 34.8 (5.43)c | |
| Bressi, 2009 [76]b | Psychiatrists in Italy | MBI | 53 | 23.15 (11.99) | 7.02 (6.29) | 36.41 (7.54) | |
| Krasner, 2009 [77] | General practitioners in United States | MBI | 60 | 26.8 (10.9)d | 8.4 (5.1)d | 40.2 (5.3)d | |
| Lasalvia, 2009 [55]b | Psychiatrists in Italy | Modified MBI (16 items; scale, 06) | 38 | 2.37 (1.27) | 1.51 (1.15) | 4.46 (0.87) | |
| Peisah, 2009 [79]b | Physicians of various specialties in Australia | MBI | 28 | 13.92 (9.24) | 3.66 (3.95) | 39.34 (8.55) | |
| Shanafelt, 2009 [80]b | Physicians of various specialties in United States | MBI | 408 | 20.5 (11.10) | 4.3 (4.74) | 40.8 (6.26) | |
| Zantinge, 2009 [81] | General practitioners in the Netherlands | Utrecht Burnout Inventory | 126 | 1.58 (0.79) | 1.32 (0.72) | 4.27 (0.77) | |
| Voltmer, 2010 [83]b | Psychiatrists in Germany | AVEM | 526 | 114 (21.7%) exhibited burnout (type B) pattern | |||
| Maccacaro, 2011 [85]b | Physicians of various specialties in Italy | MBI | 42 | 14.31 (11.98) | 3.62 (4.95) | 38.24 (6.22) | |
| Lucas, 2011 [84]b | Outpatient physicians periodically staffing an academic hospital teaching service in United States | MBI (EE only) | 30 | 24.37 (14.95) | |||
| Shanafelt, 2012 [87]b | General internists in United States | MBI | 447 | 25.4 (14.0) | 7.5 (6.3) | 41.4 (6.0) | |
| Kushnir, 2004 [62] | General practitioners and pediatricians in Israel | MBI (DP only) and SMBM | 309 | 9.15 (3.95) | SMBM mean (SD), 2.73 per item (0.86) | ||
| Vela‐Bueno, 2008 [74]b | General practitioners in Spain | MBI | 240 | 26.91 (11.61) | 9.20 (6.35) | 35.92 (7.92) | |
| Lesic, 2009 [78]b | General practitioners in Serbia | MBI | 38 | 24.71 (10.81) | 7.47 (5.51) | 37.21 (7.44) | |
| Demirci, 2010 [82]b | Medical specialists related to oncology practice in Hungary | MBI | 26 | 23.31 (11.2) | 6.46 (5.7) | 37.7 (8.14) | |
| Putnik, 2011 [86]b | General practitioners in Hungary | MBI | 370 | 22.22 (11.75) | 3.66 (4.40) | 41.40 (6.85) | |
| Lead Author, Publication Year | Study Population and Location | Instrument | No. of Participants | EE Score (SD)a | DP Score (SD) | PA Score (SD) | Other Results |
|---|---|---|---|---|---|---|---|
| |||||||
| Varga, 1996 [88] | Hospital doctors in Spain | MBI | 179 | 21.61b | 7.33b | 35.28b | |
| Aasland, 1997 [54] | Hospital doctors in Norway | Modified MBI (22 items; scale, 15) | 582 | 2.39 (0.80) | 1.81 (0.65) | 3.51 (0.46) | |
| Bargellini, 2000 [89] | Hospital doctors in Italy | MBI | 51 | 17.45 (9.87) | 7.06 (5.54) | 35.33 (7.90) | |
| Grassi, 2000 [58] | Hospital doctors in Italy | MBI | 146 | 16.17 (9.64) | 5.32 (4.76) | 38.71 (7.28) | |
| Hoff, 2001 [33] | Hospitalists in United States | Single‐item surveyc | 393 | 12.9% burned out (>4/5), 24.9% at risk for burnout (34/5), 62.2% at no current risk (mean, 2.86 on 15 scale) | |||
| Trichard, 2005 [90] | Hospital doctors in France | MBI | 199 | 16 (10.7) | 6.6 (5.7) | 38.5 (6.5) | |
| Gandini, 2006 [65]d | Hospital doctors in Argentina | MBI | 290 | 25.0 (12.7) | 7.9 (6.2) | 40.1 (7.0) | |
| Dunwoodie, 2007 [68]d | Palliative care doctors in Australia | MBI | 14 | 18.29 (14.24) | 5.29 (5.89) | 38.86 (3.42) | |
| Srgaard, 2007 [69]d | Psychiatrists in 5 European nations | MBI | 18 | 18.56 (9.32) | 5.50 (3.79) | 39.08 (5.39) | |
| Sosa Oberlin, 2007 [56]d | Hospital doctors in Argentina | Author‐designed instrument | 3 | 3 (100%) had 4 burnout symptoms, 8.67 symptoms per physician | |||
| Voltmer, 2007 [57]d | Hospital doctors in Germany | AVEM | 271 | 77 (28.4%) exhibited burnout (type B) pattern | |||
| dm, 2008 [70]b | Physicians of various specialties in Hungary | MBI | 194 | 19.23 (10.79) | 4.88 (4.61) | 35.26 (8.42) | |
| Di Iorio, 2008 [71]d | Dialysis physicians in Italy | Author‐designed instrument | 62 | Work, mean (SD), 3.1 (1.4); Material, mean (SD), 3.3 (1.5); Climate, mean (SD), 2.9 (1.1); Objectives, mean (SD), 2.5 (1.5); Quality, mean (SD), 3.0 (1.1); Justification, mean (SD), 3.1 (2.1) | |||
| Fuss, 2008 [91]d | Hospital doctors in Germany | Copenhagen Burnout Inventory | 292 | Mean Copenhagen Burnout Inventory, mean (SD), 46.90 (18.45) | |||
| Marner, 2008 [92]d | Psychiatrists and 1 generalist in United States | MBI | 9 | 20.67 (9.75) | 7.78 (5.14) | 35.33 (6.44) | |
| Shehabi, 2008 [93]d | Intensivists in Australia | Modified MBI (6 items; scale, 15) | 86 | 2.85 (0.93) | 2.64 (0.85) | 2.58 (0.83) | |
| Bressi, 2009 [76]d | Psychiatrists in Italy | MBI | 28 | 17.89 (14.46) | 5.32 (7.01) | 34.57 (11.27) | |
| Brown, 2009 [94] | Hospital doctors in Australia | MBI | 12 | 22.25 (8.59) | 6.33 (2.71) | 39.83 (7.31) | |
| Lasalvia, 2009 [55]d | Psychiatrists in Italy | Modified MBI (16 items; scale, 06) | 21 | 1.95 (1.04) | 1.35 (0.85) | 4.46 (1.04) | |
| Peisah, 2009 [79]d | Hospital doctors in Australia | MBI | 62 | 20.09 (9.91) | 6.34 (4.90) | 35.06 (7.33) | |
| Shanafelt, 2009 [80]d | Hospitalists and intensivists in United States | MBI | 19 | 25.2 (11.59) | 4.4 (3.79) | 38.5 (8.04) | |
| Tunc, 2009 [95] | Hospital doctors in Turkey | Modified MBI (22 items; scale, 04) | 62 | 1.18 (0.78) | 0.81 (0.73) | 3.10 (0.59)e | |
| Cocco, 2010 [96]d | Hospital geriatricians in Italy | MBI | 38 | 16.21 (11.56) | 4.53 (4.63) | 39.13 (7.09) | |
| Doppia, 2011 [97]d | Hospital doctors in France | Copenhagen Burnout Inventory | 1,684 | Mean work‐related burnout score, 2.72 (0.75) | |||
| Glasheen, 2011 [98] | Hospitalists in United States | Single‐item survey | 265 | Mean, 2.08 on 15 scale 62 (23.4%) burned out | |||
| Lucas, 2011 [84]d | Academic hospitalists in United States | MBI (EE only) | 26 | 19.54 (12.85) | |||
| Thorsen, 2011 [99] | Hospital doctors in Malawi | MBI | 2 | 25.5 (4.95) | 8.5 (6.36) | 25.0 (5.66) | |
| Hinami, 2012 [50]d | Hospital doctors in United States | Single‐item survey | 793 | Mean, 2.24 on 15 scale 261 (27.2%) burned out | |||
| Quenot, 2012 [100]d | Intensivists in France | MBI | 4 | 33.25 (4.57) | 13.50 (5.45) | 35.25 (4.86) | |
| Ruitenburg, 2012 [101] | Hospital doctors in the Netherlands | MBI (EE and DP only) | 214 | 13.3 (8.0) | 4.5 (4.1) | ||
| Seibt, 2012 [102]d | Hospital doctors in Germany | Modified MBI (16 items; scale, 06, reported per item rather than totals) | 2,154 | 2.2 (1.4) | 1.4 (1.2) | 5.1 (0.9) | |
| Shanafelt, 2012 [87]d | Hospitalists in United States | MBI | 130 | 24.7 (12.5) | 9.1 (6.9) | 39.0 (7.6) | |
Table 3 summarizes the results of the 15 studies that reported burnout data for both inpatient and outpatient physicians, allowing direct comparisons to be made. Nine studies reported MBI subset totals with standard deviations, 2 used different modifications of the MBI, 2 used different author‐derived measures, 1 used only the emotional exhaustion subscale of the MBI, and 1 used the Arbeitsbezogenes Verhaltens und Erlebensmuster. Therefore, statistical comparison was attempted only for the 9 studies reporting comparable MBI data, comprising burnout data on 1390 outpatient physicians and 899 inpatient physicians.
| Lead Author, Publication Year | Location | Instrument | Inpatient‐Based Physicians | Outpatient‐Based Physicians | ||
|---|---|---|---|---|---|---|
| No. | Results, Score (SD)a | No. | Results, Score (SD)a | |||
| ||||||
| Aasland, 1997 [54]b | Norway | Modified MBI (22 items; scale, 15) | 582 | EE, 2.39 (0.80); DP, 1.81 (0.65); PA, 3.51 (0.46) | 298 | EE, 2.65 (0.80); DP, 1.90 (0.59); PA, 3.45 (0.40) |
| Grassi, 2000 [58] | Italy | MBI | 146 | EE, 16.17 (9.64); DP, 5.32 (4.76); PA, 38.71 (7.28) | 182 | EE, 18.49 (11.49); DP, 6.11 (5.86); PA, 38.52 (7.60) |
| Gandini, 2006 [65]b | Argentina | MBI | 290 | EE, 25.0 (12.7);DP, 7.9 (6.2); PA, 40.1 (7.0) | 67 | EE, 31.0 (13.8); DP, 10.2 (6.6); PA, 38.4 (6.8) |
| Dunwoodie, 2007 [68]b | Australia | MBI | 14 | EE, 18.29 (14.24); DP, 5.29 (5.89); PA, 38.86 (3.42) | 21 | EE, 14.95 (9.14); DP, 3.95 (3.40); PA, 38.90 (2.88) |
| Srgaard, 2007 [69]b | 5 European nations | MBI | 18 | EE, 18.56 (9.32); DP, 5.50 (3.79); PA, 39.08 (5.39) | 22 | EE, 19.41 (8.08); DP, 6.68 (4.93); PA, 39.00 (4.40) |
| Sosa Oberlin, 2007 [56]b | Argentina | Author‐designed instrument | 3 | 3 (100%) had 4 burnout symptoms, 8.67 symptoms per physician | 33 | 26 (78.8%) had 4 burnout symptoms, 6.15 symptoms per physician |
| Voltmer, 2007 [57]b | Germany | AVEM | 271 | 77 (28.4%) exhibited burnout (type B) pattern | 46 | 11 (23.9%) exhibited burnout (type B) pattern |
| dm, 2008 [70]b | Hungary | MBI | 194 | EE, 19.23 (10.79); DP, 4.88 (4.61); PA, 35.26 (8.42) | 163 | EE, 17.45 (11.12); DP, 4.86 (4.91); PA, 36.56 (7.03) |
| Di Iorio, 2008 [71]b | Italy | Author‐designed instrument | 62 | Work: 3.1 (1.4); material: 3.3 (1.5); climate: 2.9 (1.1); objectives: 2.5 (1.5); quality: 3.0 (1.1); justification: 3.1 (2.1) | 54 | Work: 2.6 (1.5); material: 3.1 (2.1); climate: 3.0 (1.1); objectives: 3.4 (1.6); quality: 2.2 (1.5); justification: 3.2 (2.0) |
| Bressi, 2009 [76]b | Italy | MBI | 28 | EE, 17.89 (14.46); DP, 5.32 (7.01); PA, 34.57 (11.27) | 53 | EE, 23.15 (11.99); DP, 7.02 (6.29); PA, 36.41 (7.54) |
| Lasalvia, 2009[55]b | Italy | Modified MBI (16 items; scale, 06) | 21 | EE, 1.95 (1.04); DP, 1.35 (0.85); PA, 4.46 (1.04) | 38 | EE, 2.37 (1.27); DP, 1.51 (1.15); PA, 4.46 (0.87) |
| Peisah, 2009 [79]b | Australia | MBI | 62 | EE, 20.09 (9.91); DP, 6.34 (4.90); PA, 35.06 (7.33) | 28 | EE, 13.92 (9.24); DP, 3.66 (3.95); PA, 39.34 (8.55) |
| Shanafelt, 2009 [80]b | United States | MBI | 19 | EE, 25.2 (11.59); DP, 4.4 (3.79); PA, 38.5 (8.04) | 408 | EE, 20.5 (11.10); DP, 4.3 (4.74); PA, 40.8 (6.26) |
| Lucas, 2011 [84]b | United States | MBI (EE only) | 26 | EE, 19.54 (12.85) | 30 | EE, 24.37 (14.95) |
| Shanafelt, 2012 [87]b | United States | MBI | 130 | EE, 24.7 (12.5); DP, 9.1 (6.9); PA, 39.0 (7.6) | 447 | EE, 25.4 (14.0); DP, 7.5 (6.3); PA, 41.4 (6.0) |
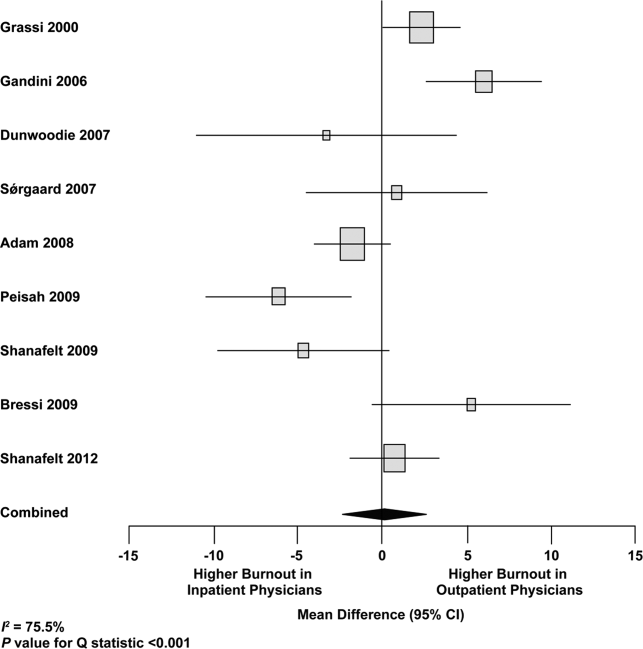
Figure 2 shows that no significant difference existed between the groups regarding emotional exhaustion (mean difference, 0.11 points on a 54‐point scale; 95% confidence interval [CI], 2.40 to 2.61; P=0.94). In addition, there was no significant difference between the groups regarding depersonalization (Figure 3; mean difference, 0.00 points on a 30‐point scale; 95% CI, 1.03 to 1.02; P=0.99) and personal accomplishment (Figure 4; mean difference, 0.93 points on a 48‐point scale; 95% CI, 0.23 to 2.09; P=0.11).
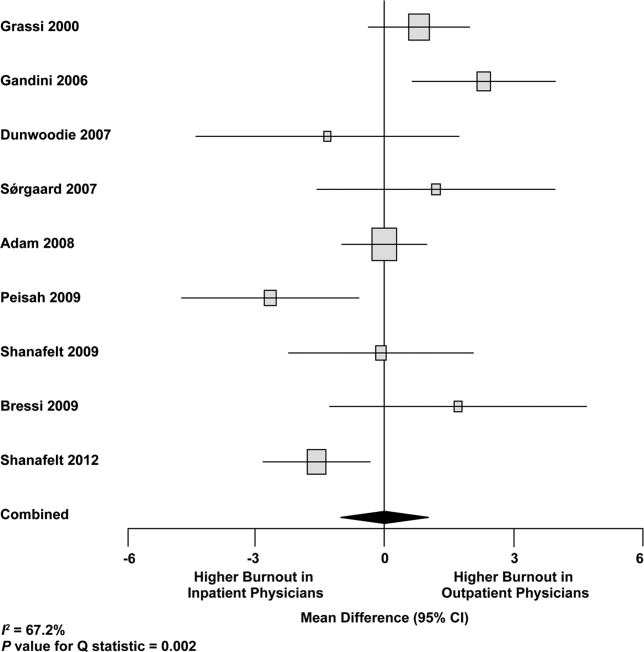
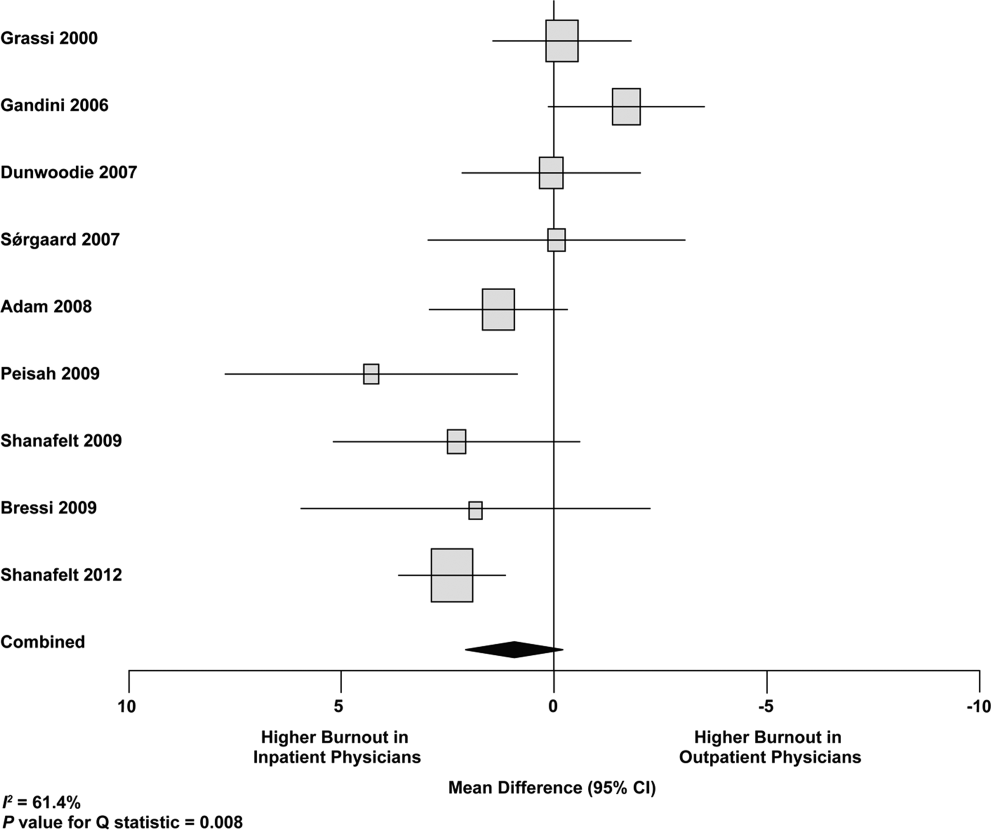
We used meta‐regression to allow the incorporation of single‐armed MBI studies. Whether single‐armed studies were analyzed separately (15 outpatient studies comprising 3927 physicians, 4 inpatient studies comprising 300 physicians) or analyzed with double‐armed studies (24 outpatient arms comprising 5318 physicians, 13 inpatient arms comprising 1301 physicians), the lack of a significant difference between the groups persisted for the depersonalization and personal accomplishment scales (Figure 5). Emotional exhaustion was significantly higher in outpatient physicians when single‐armed studies were considered separately (mean difference, 6.36 points; 95% CI, 2.24 to 10.48; P=0.002), and this difference persisted when all studies were combined (mean difference, 3.00 points; 95% CI, 0.05 to 5.94, P=0.046).
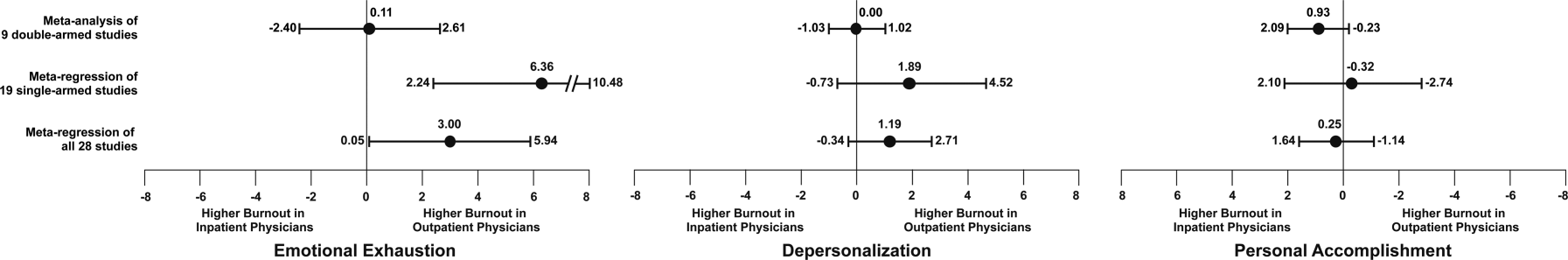
Subgroup analysis by geographic location showed US outpatient physicians had a significantly higher personal accomplishment score than US inpatient physicians (mean difference, 2.38 points; 95% CI, 1.22 to 3.55; P<0.001) in double‐armed studies. This difference did not persist when single‐armed studies were included through meta‐regression (mean difference, 0.55 points, 95% CI, 4.30 to 5.40, P=0.83).
Table 4 demonstrates that methodological quality was generally good from the standpoint of the reporting and bias subsections of the Downs and Black tool. External validity was scored lower for many studies due to the use of convenience samples and lack of information about physicians who declined to participate.
| Lead Author, Publication Year | Reporting | External Validity | Internal Validity: Bias | Internal Validity: Confounding | Power |
|---|---|---|---|---|---|
| Schweitzer, 1994 [12] | 5 of 6 points | 1 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Varga, 1996 [88] | 5 of 6 points | 1 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Aasland, 1997 [54] | 3 of 6 points | 2 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Bargellini, 2000 [89] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Grassi, 2000 [58] | 6 of 6 points | 0 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| McManus, 2000 [59] | 5 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Hoff, 2001 [33] | 6 of 6 points | 2 of 2 points | 2 of 4 points | 1 of 1 point | 0 of 1 point |
| Yaman, 2002 [60] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Cathbras, 2004 [61] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Kushnir, 2004 [62] | 5 of 6 points | 0 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Goehring, 2005 [63] | 6 of 6 points | 1 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Trichard, 2005 [90] | 3 of 6 points | 1 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Esteva, 2006 [64] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Gandini, 2006 [65] | 6 of 6 points | 1 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Ozyurt, 2006 [66] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Deighton, 2007 [67] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Dunwoodie, 2007 [68] | 5 of 6 points | 2 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Srgaard, 2007 [69] | 6 of 6 points | 0 of 2 points | 3 of 4 points | 1 of 1 point | 1 of 1 point |
| Sosa Oberlin, 2007 [56] | 4 of 6 points | 0 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Voltmer, 2007 [57] | 4 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| dm, 2008 [70] | 5 of 6 points | 2 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Di Iorio, 2008 [71] | 6 of 6 points | 0 of 2 points | 2 of 4 points | 0 of 1 point | 0 of 1 point |
| Fuss, 2008 [91] | 6 of 6 points | 0 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Lee, 2008 [49] | 4 of 6 points | 2 of 2 points | 4 of 4 points | 0 of 1 point | 1 of 1 point |
| Marner, 2008 [92] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Shehabi, 2008 [93] | 3 of 6 points | 1 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Truchot, 2008 [72] | 5 of 6 points | 1 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Twellaar, 2008 [73] | 6 of 6 points | 2 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Vela‐Bueno, 2008 [74] | 5 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Arigoni, 2009 [17] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Bernhardt, 2009 [75] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Bressi, 2009 [76] | 6 of 6 points | 0 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Brown, 2009 [94] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Krasner, 2009 [77] | 9 of 11 points | 0 of 3 points | 6 of 7 points | 1 of 2 points | 1 of 1 point |
| Lasalvia, 2009 [55] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Lesic, 2009 [78] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Peisah, 2009 [79] | 6 of 6 points | 2 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Shanafelt, 2009 [80] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Tunc, 2009 [95] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Zantinge, 2009 [81] | 5 of 6 points | 0 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Cocco, 2010 [96] | 4 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Demirci, 2010 [82] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Voltmer, 2010 [83] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Doppia, 2011 [97] | 5 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Glasheen, 2011 [98] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Lucas, 2011 [84] | 10 of 11 points | 2 of 3 points | 7 of 7 points | 5 of 6 points | 1 of 1 point |
| Maccacaro, 2011 [85] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Putnik, 2011 [86] | 6 of 6 points | 1 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Thorsen, 2011 [99] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Hinami, 2012 [50] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 1 of 1 point |
| Quenot, 2012 [100] | 8 of 11 points | 1 of 3 points | 6 of 7 points | 1 of 2 points | 0 of 1 point |
| Ruitenburg, 2012 [101] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Seibt, 2012 [102] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Shanafelt, 2012 [87] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
Funnel plots were used to evaluate for publication bias in the meta‐analysis of the 8 double‐armed studies (Figure 6). We found no significant evidence of bias, which was supported by Begg's test P values of 0.90 for emotional exhaustion, >0.99 for depersonalization, and 0.54 for personal accomplishment. A trim‐and‐fill analysis determined that no adjustment was necessary.
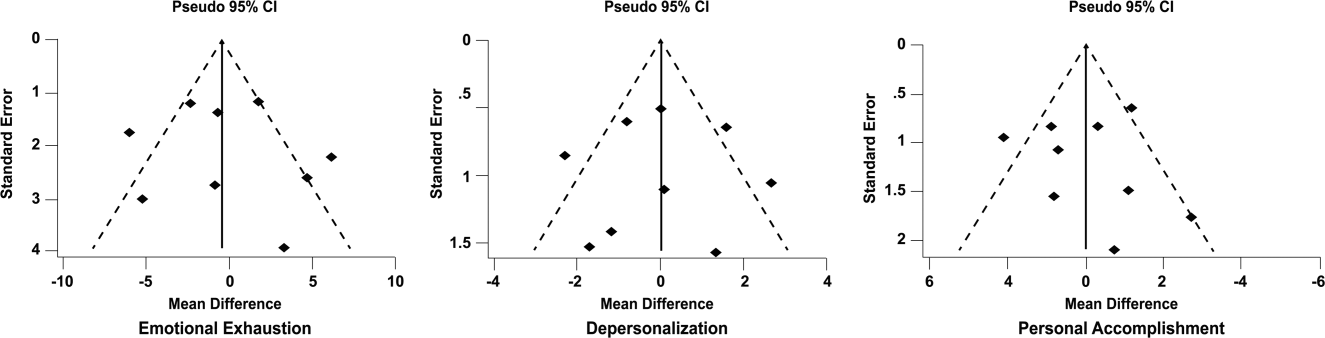
DISCUSSION
There appears to be no support for the long‐held belief that inpatient physicians are particularly prone to burnout. Among studies for which practice location was stated explicitly or could be obtained from the authors, and who used the MBI, no differences were found among inpatient and outpatient physicians with regard to depersonalization or personal accomplishment. This finding persisted whether double‐armed studies were compared directly, single‐armed studies were incorporated into this analysis, or single‐armed studies were analyzed separately. Outpatient physicians had a higher degree of emotional exhaustion when all studies were considered.
There are several reasons why outpatient physicians may be more prone to emotional exhaustion than their inpatient colleagues. Although it is by no means true that all inpatient physicians work in shifts, the increased availability of shift work may allow some inpatient physicians to better balance their professional and personal lives, a factor of work with which some outpatient physicians have struggled.[47] Inpatient practice may also afford more opportunity for teamwork, a factor that has been shown to correlate with reduced burnout.[48] When surveyed about burnout, outpatient physicians have cited patient volumes, paperwork, medicolegal concerns, and lack of community support as factors.[49] Inpatient physicians are not immune to these forces, but they arguably experience them to different degrees.
The absence of a higher rate of depersonalization among inpatient physicians is particularly reassuring in light of concerns expressed with the advent of US hospital medicinethat some hospitalists would be prone to viewing patients as an impediment to the efficient running of the hospital,[2] the very definition of depersonalization.
Although the difference in the whole sample was not statistically significant, the consistent tendency toward a greater sense of personal accomplishment among outpatient physicians is also noteworthy, particularly because post hoc subgroup analysis of US physicians did show statistical significance in both 2‐armed studies. Without detailed age data for the physicians in each study, we could not separate the possible impact of age on personal accomplishment; hospital medicine is a newer specialty staffed by generally younger physicians, and hospitalists may not have had time to develop a sense of accomplishment. When surveyed about job satisfaction, hospitalists have also reported the feeling that they were treated as glorified residents,[50] a factor that, if shared by other inpatient physicians, must surely affect their sense of personal accomplishment. The lack of longitudinal care for patients and the substantial provision of end‐of‐life care also may diminish the sense of personal accomplishment among inpatient physicians.
Another important finding from this systematic review is the marked heterogeneity of the instruments used to measure physician burnout. Many of the identified studies could not be subjected to meta‐analysis because of their use of differing burnout measures. Drawing more substantial conclusions about burnout and practice location is limited by the fact that, although the majority of studies used the full MBI, the largest study of European hospital doctors used the Copenhagen Burnout Inventory, and the studies thus far of US hospitalists have used single‐item surveys or portions of the MBI. Not reflected in this review is the fact that a large study of US burnout and job satisfaction[51] did not formally address practice location (M. Linzer, personal communication, August 2012). Similarly, a large study of British hospital doctors[52] is not included herein because many of the physicians involved had substantial outpatient duties (C. Taylor, personal communication, July 2012). Varying burnout measures have complicated a previous systematic review of burnout in oncologists.[53] Two studies that directly compared inpatient and outpatient physicians but that were excluded from our statistical analysis because of their modified versions of the MBI,[54, 55] showed higher burnout scores in outpatient physicians. Two other studies that provided direct inpatient versus outpatient comparisons but that used alternative burnout measures[56, 57] showed a greater frequency of burnout in inpatient physicians, but of these, 1 study[56] involved only 3 inpatient physicians.
Several limitations of our study should be considered. Although we endeavored to obtain information from authors (with some success) about specific local practice patterns and eliminated many studies because of incomplete data or mixed practice patterns (eg, general practitioners who take frequent hospital calls, hospital physicians with extensive outpatient duties in a clinic attached to their hospital), it remains likely that many physicians identified as outpatient provided some inpatient care (attending a few weeks per year on a teaching service, for example) and that some physicians identified as inpatient have minimal outpatient duties.
More importantly, the dataset analyzed is heterogeneous. Studies of the incidence of burnout are naturally observational and therefore not randomized. Inclusion of international studies is necessary to answer the research question (because published data on US hospitalists are sparse) but naturally introduces differences in practice settings, local factors, and other factors for which we cannot possibly account fully.
Our meta‐analysis therefore addressed a broad question about burnout among inpatient and outpatient physicians in various diverse settings. Applying it to any 1 population (including US hospitalists) is, by necessity, imprecise.
Post hoc analysis should be viewed with caution. For example, the finding of a statistical difference between US inpatient and outpatient physicians with regard to personal accomplishment score is compelling from the standpoint of hypothesis generation. However, it is worth bearing in mind that this analysis contained only 2 studies, both by the same primary author, and compared 855 outpatient physicians to only 149 hospitalists. This difference was no longer significant when 2 outpatient studies were added through meta‐regression.
Finally, the specific focus of this study on practice location precluded comparison with emergency physicians and anesthesiologists, 2 specialist types that have been the subject of particularly robust burnout literature. As the literature on hospitalist burnout becomes more extensive, comparative studies with these groups and with intensivists might prove instructive.
In summary, analysis of 24 studies comprising data on 5318 outpatient physicians and 1301 inpatient physicians provides no support for the commonly held belief that hospital‐based physicians are particularly prone to burnout. Outpatient physicians reported higher emotional exhaustion. Further studies of the incidence and severity of burnout according to practice location are indicated. We propose that in future studies, to avoid the difficulties with statistical analysis summarized herein, investigators ask about and explicitly report practice location (inpatient vs outpatient vs both) and report mean MBI subset data and standard deviations. Such information about US hospitalists would allow comparison with a robust (if heterogeneous) international literature on burnout.
Acknowledgments
The authors gratefully acknowledge all of the study authors who contributed clarification and guidance for this project, particularly the following authors who provided unpublished data for further analysis: Olaf Aasland, MD; Szilvia dm, PhD; Annalisa Bargellini, PhD; Cinzia Bressi, MD, PhD; Darrell Campbell Jr, MD; Ennio Cocco, MD; Russell Deighton, PhD; Senem Demirci Alanyali, MD; Biagio Di Iorio, MD, PhD; David Dunwoodie, MBBS; Sharon Einav, MD; Madeleine Estryn‐Behar, PhD; Bernardo Gandini, MD; Keiki Hinami, MD; Antonio Lasalvia, MD, PhD; Joseph Lee, MD; Guido Maccacaro, MD; Swati Marner, EdD; Chris McManus, MD, PhD; Carmelle Peisah, MBBS, MD; Katarina Putnik, MSc; Alfredo Rodrguez‐Muoz, PhD; Yahya Shehabi, MD; Evelyn Sosa Oberlin, MD; Jean Karl Soler, MD, MSc; Knut Srgaard, PhD; Cath Taylor; Viva Thorsen, MPH; Mascha Twellaar, MD; Edgar Voltmer, MD; Colin West, MD, PhD; and Deborah Whippen. The authors also thank the following colleagues for their help with translation: Dusanka Anastasijevic (Norwegian); Joyce Cheung‐Flynn, PhD (simplified Chinese); Ales Hlubocky, MD (Czech); Lena Jungheim, RN (Swedish); Erez Kessler (Hebrew); Kanae Mukai, MD (Japanese); Eliane Purchase (French); Aaron Shmookler, MD (Russian); Jan Stepanek, MD (German); Fernando Tondato, MD (Portuguese); Laszlo Vaszar, MD (Hungarian); and Joseph Verheidje, PhD (Dutch). Finally, the authors thank Cynthia Heltne and Diana Rogers for their expert and tireless library assistance, Bonnie Schimek for her help with figures, and Cindy Laureano and Elizabeth Jones for their help with author contact.
- . Just because you can, doesn't mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398–402.
- . Hospitalists. Hospitalist. 1998;1(4):5–6.
- , . The occupational hazards of emergency physicians. Am J Emerg Med. 2000;18(3):300–311.
- , , , et al. Physician burnout in pediatric critical care medicine. Crit Care Med. 1995;23(8):1425–1429.
- , . The hospitalist: new boon for internal medicine or retreat from primary care? Ann Intern Med. 1999;130(4 pt 2): 382–387.
- , , , et al. Enquete sur les horaires et la charge de travail des medecins dans un establissement de l'Assitance Publique‐Hopitaux de Paris. Arch Mal Prof. 1996;57:438–444.
- , , , , . Burn‐out of urologists in the county of Schleswig‐Holstein, Germany: a comparison of hospital and private practice urologists. J Urol. 2001;165(4): 1158–1161.
- , , , et al. Satisfaction and worklife of academic hospitalist and non‐hospitalist attendings on general medical inpatient rotations. J Gen Internal Med. 2006;21(S4):128.
- , , , , , ; SGIM Career Satisfaction Group. What effect does increasing inpatient time have on outpatient‐oriented internist satisfaction? J Gen Intern Med. 2003;18(9):725–729.
- , , . Burnout and career‐choice regret among family practice physicians in early practice. Fam Pract Res J. 1994;14(3):213–222.
- , , , , , . Job stress and satisfaction among palliative physicians. Palliat Med. 1996; 10(3):185–194.
- . Stress and burnout in junior doctors. S Afr Med J. 1994; 84(6):352–354.
- , . Work stress, satisfaction and burnout in New Zealand radiologists: comparison of public hospital and private practice in New Zealand. J Med Imaging Radiat Oncol. 2009;53(2):194–199.
- , , . Measurement of hypothetical burnout in cystic fibrosis caregivers. Acta Paediatr Scand. 1981; 70(6):935–939.
- , . Personality characteristics and proneness to burnout: a study among internists. Stress Med. 1987;3(4):307–315.
- , , . Thriving and surviving in a new medical career: the case of hospitalist physicians. J Health Soc Behav. 2002;43(1):72–91.
- , , , , . Prevalence of burnout among Swiss cancer clinicians, paediatricians and general practitioners: who are most at risk? Support Care Cancer. 2009;17(1): 75–81.
- , , , . Preparing for “diastole”: advanced training opportunities for academic hospitalists. J Hosp Med. 2006;1(6):368–377.
- , , , , , . Mental health, “burnout” and job satisfaction among hospital and community‐based mental health staff. Br J Psychiatry. 1996;169(3):334–337.
- , , , . Role of a pediatric department chair: factors leading to satisfaction and burnout. J Pediatr. 2007;151(4):425–430.
- . Staff burn‐out. J Soc Iss. 1974;30(1):159–165.
- . Burned‐out. Hum Behav. 1976;5(9):16–22.
- . Underpaid women, stressed out men, satisfied emergency physicians. Ann Emerg Med. 2008;51(6):729–731.
- , , , . U.S. physician satisfaction: a systematic review. J Hosp Med. 2009;4(9):560–568.
- , , , . Professional burnout among head and neck surgeons: results of a survey. Head Neck. 1993;15(6):557–560.
- , . The measurement of experienced burnout. J Occup Behav. 1981;2(2):99–113.
- , , , . The Copenhagen Burnout Inventory: a new tool for the assessment of burnout. Work Stress. 2005;19(3):192–207.
- , . Handleiding van de Utrechtse Burnout Schaal (UBOS). Lisse, the Netherlands: Swets Test Services; 2000.
- . Alberta Physician Burnout [master's thesis]. Alberta, Canada: The University of Lethbridge; 2003.
- , . Arbeitsbezogenes Verhaltensund Erlebensmuster AVEM. Frankfurt, Germany: Swets Test Services; 2003.
- , , , . Internal construct validity of the Shirom‐Melamed Burnout Questionnaire (SMBQ). BMC Public Health. 2012;12:1.
- , , . Validation of a single‐item measure of burnout against the Maslach Burnout Inventory among physicians. Stress Health. 2004;20(2):75–79.
- , , , , . Characteristics and work experiences of hospitalists in the United States. Arch Intern Med. 2001;161(6):851–858.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , . Including nonrandomized studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1. The Cochrane Collaboration; 2008. Available at: www.cochrane‐handbook. org. Accessed July 24, 2013.
- , . Statistical Methods in Epidemiology. New York, NY: Oxford University Press; 1989.
- , . Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539–1558.
- , , , . Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557–560.
- , , . Can we individualize the “number needed to treat”? An empirical study of summary effect measures in meta‐analyses. Int J Epidemiol. 2002;31(1):72–76.
- , . Applications of estimating treatment effects in metaanalyses with missing data. Technical Report No. 2000‐25, 2000. Available at: http://statistics.stanford.edu/_ckirby/techreports/GEN/2000/2000‐25.pdf. Accessed July 22, 2013.
- , , . Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119–1129.
- , . Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101.
- , , , et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009; 339:b2700.
- , , , , , . Job satisfaction and burnout in general practitioners [in Spanish]. Aten Primaria. 2003;31(4):227–233.
- , , . Burnout syndrome among health staff [in Spanish]. Rev Med Inst Mex Seguro Soc. 2006;44(3):221–226.
- , , , , , . Differences in psychological effects in hospital doctors with and without post‐traumatic stress disorder. Br J Psychiatry. 2008;193(2):165–166.
- , , , , . Crossing boundaries: family physicians' struggles to protect their private lives. Can Fam Physician. 2009;55(3):286–287.e5.
- , , , , , , et al. Emergency physicians accumulate more stress factors than other physicians: results from the French SESMAT study. Emerg Med J. 2011 May;28(5):397–410. Epub 2010 Dec 1.
- , , . Stress, burnout, and strategies for reducing them: what's the situation among Canadian family physicians? Can Fam Physician. 2008;54(2):234–235.
- , , , , . Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27(1):28–36.
- , , , , , , et al; MEMO (Minimizing Error, Maximizing Outcome) Investigators. Working conditions in primary care: physician reactions and care quality. Ann Intern Med. 2009 Jul 7;151(1):28–36.
- , , , , . Mental health of hospital consultants: the effects of stress and satisfaction at work. Lancet. 1996 Mar 16;347(9003):724–8.
- , , , . Burnout, psychiatric morbidity, and work‐related sources of stress in paediatric oncology staff: a review of the literature. Psycho‐Oncology. 2009 Oct;18(10):1019–28.
- , , , , . Health complaints and job stress in Norwegian physicians: the use of an overlapping questionnaire design. Soc Sci Med. 1997;45(11):1615–1629.
- , , , et al. Influence of perceived organisational factors on job burnout: survey of community mental health staff. Br J Psychiatry. 2009;195(6):537–544.
- . Frecuencia de los sintomas del syndrome de burnout en profesionales medicos. Rev Med Rosario. 2007;73:12–20.
- , , . Work‐related behaviour and experience patterns of physicians compared to other professions. Swiss Med Wkly. 2007;137(31‐32):448–453.
- , . Psychiatric morbidity and burnout in the medical profession: an Italian study of general practitioners and hospital physicians. Psychother Psychosom. 2000;69(6):329–334.
- , , . Duties of a doctor: UK doctors and good medical practice. Qual Health Care. 2000;9(1):14–22.
- , . The job related burnout questionnaire: a multinational pilot study. Aust Fam Physician. 2002;31(11):1055–1056.
- , , , , . Burn out among French general practitioners [in French]. Presse Med. 2004;33(22): 1569–1574.
- , , . Are burnout levels increasing? The experience of Israeli primary care physicians. Isr Med Assoc J. 2004; 6(8):451–455.
- , , , . Psychosocial and professional characteristics of burnout in Swiss primary care practitioners: a cross‐sectional survey. Swiss Med Wkly. 2005;135(7‐8):101–108.
- , , . Mental health in family doctors: effects of satisfaction and stress at work [in Spanish]. Rev Clin Esp. 2006;206(2):77–83.
- , , , , . The professional wearing down or syndrome of welfare labor stress (“burnout”) among health professionals in the city of Cordoba [in Spanish]. Rev Fac Cien Med Univ Nac Cordoba. 2006;63(1):18–25.
- , , . Predictors of burnout and job satisfaction among Turkish physicians. QJM. 2006;99(3):161–169.
- , , . Factors affecting burnout and compassion fatigue in psychotherapists treating torture survivors: is the therapist's attitude to working through trauma relevant? J Trauma Stress. 2007;20(1):63–75.
- , . Psychological morbidity and burnout in palliative care doctors in Western Australia. Intern Med J. 2007;37(10): 693–698.
- , , , ; OSCAR Group. Sources of stress and burnout in acute psychiatric care: inpatient vs. community staff. Soc Psychiatry Psychiatr Epidemiol. 2007;42(10):794–802.
- , , . Physician burnout in Hungary: a potential role for work‐family conflict. J Health Psychol. 2008;13:847–856.
- , , , . Burn‐out in the dialysis unit. J Nephrol. 2008;21(suppl 13):S158–S162.
- . Career orientation and burnout in French general practitioners. Psychol Rep. 2008;103(3):875–881.
- , , . How healthy are Dutch general practitioners? Self‐reported (mental) health among Dutch general practitioners. Eur J Gen Pract. 2008;14(1):4–9.
- , , , et al. Insomnia and sleep quality among primary care physicians with low and high burnout levels. J Psychosom Res. 2008;64(4):435–442.
- , , , , , . Distress and burnout among genetic service providers. Genet Med. 2009;11(7):527–535.
- , , , et al. Burnout among psychiatrists in Milan: a multicenter survey. Psychiatr Serv. 2009;60(7):985–988.
- , , , et al. Association of an educational program in mindful communication with burnout, empathy, and attitudes among primary care physicians. JAMA. 2009;302(12): 1284–1293.
- , , , , , . Burnout in Belgrade orthopaedic surgeons and general practitioners, a preliminary report. Acta Chir Iugosl. 2009; 56(2):53–59.
- , , , . Secrets to psychological success: why older doctors might have lower psychological distress and burnout than younger doctors. Aging Ment Health. 2009;13(2):300–307.
- , , , et al. Career fit and burnout among academic faculty. Arch Intern Med. 2009;169(10):990–995.
- , , , , . Does burnout among doctors affect their involvement in patients' mental health problems? A study of videotaped consultations. BMC Fam Pract. 2009;10:60.
- , , , , , . Evaluation of burnout syndrome in oncology employees. Med Oncol. 2010;27(3):968–974.
- , , , , . Workrelated behavior and experience patterns and predictors of mental health in German physicians in medical practice. Fam Med. 2010; 42(6):433–439.
- , , , et al. Emotional exhaustion, life stress, and perceived control among medicine ward attending physicians: a randomized trial of 2‐ versus 4‐week ward rotations [abstract]. J Hosp Med. 2011; 6(4 suppl 2):S43–S44.
- , , , , , . The effort of being male: a survey on gender and burnout [in Italian]. Med Lav. 2011;102(3):286–296.
- , . Word related characteristics, work‐home and home‐work interference and burnout among primary healthcare physicians: a gender perspective in a Serbian context. BMC Public Health. 2011;11:716.
- , , , et al. Burnout and satisfaction with work‐life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172(18):1377–1385.
- , , . Burnout syndrome in general hospital doctors. Eur J Psychiat. 1996;10:207–213.
- , , , , , . Relation between immune variables and burnout in a sample of physicians. Occup Environ Med. 2000;57(7):453–457.
- , , . Epuisement professionnel et consummation de psychotropes chez les medecins hospitaliers. Alcoologie et Addictologie. 2005;27(4):303–308.
- , , , , . Working conditions and Work‐Family Conflict in German hospital physicians: psychosocial and organisational predictors and consequences. BMC Public Health. 2008;8:353.
- . The Role of Empathy and Witnessed Aggression in Stress Reactions Among Staff Working in a Psychiatric Hospital [dissertation]. New Brunswick, NJ: Rutgers University; 2008.
- , , , , , . Burnout syndrome among Australian intensivists: a survey. Crit Care Resusc. 2008;10(4):312–315.
- , , , , , . Doctors' stress responses and poor communication performance in simulated bad‐news consultations. Acad Med. 2009;84(11):1595–1602.
- , . Role conflict, role ambiguity, and burnout in nurses and physicians at a university hospital in Turkey. Nurs Health Sci. 2009;11(4):410–416.
- . How much is geriatric caregivers burnout caring‐specific? Questions from a questionnaire survey. Clin Pract Epidemiol Ment Health. 2010;6:66–71.
- , , , , ; comite de pilotage de l'enquete SESMAT. Burnout in French doctors: a comparative study among anaesthesiologists and other specialists in French hospitals (SESMAT study) [in French]. Ann Fr Anesth Reanim. 2011;30(11):782–794.
- , , , , , . Career satisfaction and burnout in academic hospital medicine. Arch Intern Med. 2011;171(8):782–785.
- , , . High rates of burnout among maternal health staff at a referral hospital in Malawi: a cross‐sectional study. BMC Nurs. 2011;10:9.
- , , , et al. Suffering among careers working in critical care can be reduced by an intensive communication strategy on end‐of‐life practices. Intensive Care Med. 2012;38:55–61.
- , , . The prevalence of common mental disorders among hospital physicians and their association with self‐reported work ability: a cross‐sectional study. BMC Health Serv Res. 2012;12:292–298.
- , , , . Effort‐reward‐ratio and burnout risk among female teachers and hospital‐employed female physicians: a comparison between professions [in German]. Arbeitsmed Sozialmed Umweltmed. 2012;47:396–406.
Hospital medicine is a rapidly growing field of US clinical practice.[1] Almost since its advent, concerns have been expressed about the potential for hospitalists to burn out.[2] Hospitalists are not unique in this; similar concerns heralded the arrival of other location‐defined specialties, including emergency medicine[3] and the full‐time intensivist model,[4] a fact that has not gone unnoted in the literature about hospitalists.[5]
The existing international literature on physician burnout provides good reason for this concern. Inpatient‐based physicians tend to work unpredictable schedules, with substantial impact on home life.[6] They tend to be young, and much burnout literature suggests a higher risk among younger, less‐experienced physicians.[7] When surveyed, hospitalists have expressed more concerns about their potential for burnout than their outpatient‐based colleagues.[8]
In fact, data suggesting a correlation between inpatient practice and burnout predate the advent of the US hospitalist movement. Increased hospital time was reported to correlate with higher rates of burnout in internists,[9] family practitioners,[10] palliative physicians,[11] junior doctors,[12] radiologists,[13] and cystic fibrosis caregivers.[14] In 1987, Keinan and Melamed[15] noted, Hospital work by its very nature, as compared to the work of a general practitioner, deals with the more severe and complicated illnesses, coupled with continuous daily contacts with patients and their anxious families. In addition, these physicians may find themselves embroiled in the power struggles and competition so common in their work environment.
There are other features, however, that may protect inpatient physicians from burnout. Hospital practice can facilitate favorable social relations involving colleagues, co‐workers, and patients,[16] a factor that may be protective.[17] A hospitalist schedule also can allow more focused time for continuing medical education, research, and teaching,[18] which have all been associated with reduced risk of burnout in some studies.[17] Studies of psychiatrists[19] and pediatricians[20] have shown a lower rate of burnout among physicians with more inpatient duties. Finally, a practice model involving a seemingly stable cadre of inpatient physicians has existed in Europe for decades,[2] indicating at least a degree of sustainability.
Information suggesting a higher rate of burnout among inpatient physicians could be used to target therapeutic interventions and to adjust schedules, whereas the opposite outcome could refute a pervasive myth. We therefore endeavored to summarize the literature on burnout among inpatient versus outpatient physicians in a systematic fashion, and to include data not only from the US hospitalist experience but also from other countries that have used a similar model for decades. Our primary hypothesis was that inpatient physicians experience more burnout than outpatient physicians.
It is important to distinguish burnout from depression, job dissatisfaction, and occupational stress, all of which have been studied extensively in physicians. Burnout, as introduced by Freudenberger[21] and further characterized by Maslach,[22] is a condition in which emotional exhaustion, depersonalization, and a low sense of personal accomplishment combine to negatively affect work life (as opposed to clinical depression, which affects all aspects of life). Job satisfaction can correlate inversely with burnout, but it is a separate process[23] and the subject of a recent systematic review.[24] The importance of distinguishing burnout from job dissatisfaction is illustrated by a survey of head and neck surgeons, in which 97% of those surveyed indicated satisfaction with their jobs and 34% of the same group answered in the affirmative when asked if they felt burned out.[25]
One obstacle to the meaningful comparison of burnout prevalence across time, geography, and specialty is the myriad ways in which burnout is measured and reported. The oldest and most commonly used instrument to measure burnout is the Maslach Burnout Inventory (MBI), which contains 22 items assessing 3 components of burnout (emotional exhaustion, depersonalization, and low personal accomplishment).[26] Other measures include the Copenhagen Burnout Inventory[27] (19 items with the components personal burnout, work‐related burnout, and client‐related burnout), Utrecht Burnout Inventory[28] (20‐item modification of the MBI), Boudreau Burnout Questionnaire[29] (30 items), Arbeitsbezogenes Verhaltens und Erlebensmuster[30] (66‐item questionnaire assessing professional commitment, resistance to stress, and emotional well‐being), Shirom‐Melamed Burnout Measure[31] (22 items with subscales for physical fatigue, cognitive weariness, tension, and listlessness), and a validated single‐item questionnaire.[32]
METHODS
Electronic searches of MEDLINE, EMBASE, PsycINFO, SCOPUS, and PubMed were undertaken for articles published from January 1, 1974 (the year in which burnout was first described by Freudenberger[21]) to 2012 (last accessed, September 12, 2012) using the Medical Subject Headings (MeSH) terms stress, psychological; burnout, professional; adaptation, psychological; and the keyword burnout. The same sources were searched to create another set for the MeSH terms hospitalists, physician's practice patterns, physicians/px, professional practice location, and the keyword hospitalist#. Where exact subject headings did not exist in databases, comparable subject headings or keywords were used. The 2 sets were then combined using the operator and. Abstracts from the Society of Hospital Medicine annual conferences were hand‐searched, as were reference lists from identified articles. To ensure that pertinent international literature was captured, there was no language restriction in the search.
A 2‐stage screening process was used. The titles and abstracts of all articles identified in the search were independently reviewed by 2 investigators (D.L.R. and K.J.C.) who had no knowledge of each other's results. An article was obtained when either reviewer deemed it worthy of full‐text review.
All full‐text articles were independently reviewed by the same 2 investigators. The inclusion criterion was the measurement of burnout in physicians who are stated to or can be reasonably assumed to spend the substantial majority of their clinical practice exclusively in either the inpatient or the outpatient setting. Studies of emergency department physicians or specialists who invariably spend substantial amounts of time in both settings (eg, surgeons, anesthesiologists) were excluded. Studies limited to trainees or nonphysicians were also excluded. For both stages of review, agreement between the 2 investigators was assessed by calculating the statistic. Disagreements about inclusion were adjudicated by a third investigator (A.I.B.).
Because our goal was to establish and compare the rate of burnout among US hospitalists and other inpatient physicians around the world, we included studies of hospitalists according to the definition in use at the time of the individual study, noting that the formal definition of a hospitalist has changed over the years.[33] Because practice patterns for physicians described as primary care physicians, family doctors, hospital doctors, and others differ substantially from country to country, we otherwise included only the studies where the practice location was stated explicitly or where the authors confirmed that their study participants either are known or can be reasonably assumed to spend more than 75% of their time caring for hospital inpatients, or are known or can be reasonably assumed to spend the vast majority of their time caring for outpatients.
Data were abstracted using a standardized form and included the measure of burnout used in the study, results, practice location of study subjects, and total number of study subjects. When data were not clear (eg, burnout measured but not reported by the authors, practice location of study subjects not clear), authors were contacted by email, or when no current email address could be located or no response was received, by telephone or letter. In instances where burnout was measured repeatedly over time or before and after a specific intervention, only the baseline measurement was recorded. Because all studies were expected to be nonrandomized, methodological quality was assessed using a version of the tool of Downs and Black,[34] adapted where necessary by omitting questions not applicable to the specific study type (eg randomization for survey studies)[35] and giving a maximum of 1 point for the inclusion of a power calculation.
Two a priori analyses were planned: (1) a statistical comparison of articles directly comparing burnout among inpatient and outpatient physicians, and (2) a statistical comparison of articles measuring burnout among inpatient physicians with articles measuring burnout among outpatient physicians by the most frequently reported measuremean subset scores for emotional exhaustion, depersonalization, and personal accomplishment on the MBI.
The primary outcome measures were the differences between mean subset scores for emotional exhaustion, depersonalization, and personal accomplishment on the MBI. All differences are expressed as (outpatient meaninpatient mean). The variance of each outcome was calculated with standard formulas.[36] To calculate the overall estimate, each study was weighted by the reciprocal of its variance. Studies with fewer than 10 subjects were excluded from statistical analysis but retained in the systematic review.
For studies that reported data for both inpatient and outpatient physicians (double‐armed studies), Cochran Q test and the I2 value were used to assess heterogeneity.[37, 38] Substantial heterogeneity was expected because these individual studies were conducted for different populations in different settings with different study designs, and this expectation was confirmed statistically. Therefore, we used a random effects model to estimate the overall effect, providing a conservative approach that accounted for heterogeneity among studies.[39]
To assess the durability of our findings, we performed separate multivariate meta‐regression analyses by including single‐armed studies only and including both single‐armed and double‐armed studies. For these meta‐regressions, means were again weighted by the reciprocal of their variances, and the arms of 2‐armed studies were considered separately. This approach allowed us to generate an estimate of the differences between MBI subset scores from studies that did not include such an estimate when analyzed separately.[40]
We examined the potential for publication bias in double‐armed studies by constructing a funnel plot, in which mean scores were plotted against their standard errors.[41] The trim‐and‐fill method was used to determine whether adjustment for publication bias was necessary. In addition, Begg's rank correlation test[42] was completed to test for statistically significant publication bias.
Stata 10.0 statistical software (StataCorp, College Station, TX) was used for data analyses. A P value of 0.05 or less was deemed statistically significant. The Preferred Reporting Items for Systematic Reviews and Meta‐analysis checklist was used for the design and execution of the systematic review and meta‐analysis.[43]
Subgroup analyses based on location were undertaken a posteriori. All data (double‐armed meta‐analysis, meta‐regression of single‐armed studies, and meta‐regression of single‐ and double‐armed studies) were analyzed by location (United States vs other; United States vs Europe vs other).
RESULTS
The search results are outlined in Figure 1. In total, 1704 articles met the criteria for full‐text review. A review of pertinent reference lists and author contacts led to the addition of 149 articles. Twenty‐nine references could not be located by any means, despite repeated attempts. Therefore, 1824 articles were subjected to full‐text review by the 2 investigators.

Initially, 57 articles were found that met criteria for inclusion. Of these, 2 articles reported data in formats that could not be interpreted.[44, 45] When efforts to clarify the data with the authors were unsuccessful, these studies were excluded. A study specifically designed to assess the response of physicians to a recent series of terrorist attacks[46] was excluded a posteriori because of lack of generalizability. Of the other 54 studies, 15 reported burnout data on both outpatient physicians and inpatient physicians, 22 reported data on outpatient physicians only, and 17 reported data on inpatient physicians only. Table 1 summarizes the results of the 37 studies involving outpatient physicians; Table 2 summarizes the 32 studies involving inpatient physicians.
| Lead Author, Publication Year | Study Population and Location | Instrument | No. of Participants | EE Score (SD)a | DP Score (SD) | PA Score (SD) | Other Results |
|---|---|---|---|---|---|---|---|
| |||||||
| Schweitzer, 1994[12] | Young physicians of various specialties in South Africa | Single‐item survey | 7 | 6 (83%) endorsed burnout | |||
| Aasland, 1997 [54]b | General practitioners in Norway | Modified MBI (22 items; scale, 15) | 298 | 2.65 (0.80) | 1.90 (0.59) | 3.45 (0.40) | |
| Grassi, 2000 [58] | General practitioners in Italy | MBI | 182 | 18.49 (11.49) | 6.11 (5.86) | 38.52 (7.60) | |
| McManus, 2000 [59]b | General practitioners in United Kingdom | Modified MBI (9 items; scale, 06) | 800 | 8.34 (4.39) | 3.18 (3.40) | 14.16 (2.95) | |
| Yaman, 2002 [60] | General practitioners in 8 European nations | MBI | 98 | 25.1 (8.50) | 7.3 (4.92) | 34.5 (7.67) | |
| Cathbras, 2004 [61] | General practitioners in France | MBI | 306 | 21.85 (12.4) | 9.13 (6.7) | 38.7 (7.1) | |
| Goehring, 2005 [63] | General practitioners, general internists, pediatricians in Switzerland | MBI | 1755 | 17.9 (9.8) | 6.5 (4.7) | 39.6 (6.5) | |
| Esteva, 2006 [64] | General practitioners, pediatricians in Spain | MBI | 261 | 27.4 (11.8) | 10.07 (6.4) | 35.9 (7.06) | |
| Gandini, 2006 [65]b | Physicians of various specialties in Argentina | MBI | 67 | 31.0 (13.8) | 10.2 (6.6) | 38.4 (6.8) | |
| Ozyurt, 2006 [66] | General practitioners in Turkey | Modified MBI (22 items; scale, 04) | 55 | 15.23 (5.80) | 4.47 (3.31) | 23.38 (4.29) | |
| Deighton, 2007 [67]b | Psychiatrists in several German‐speaking nations | MBI | 19 | 30.68 (9.92) | 13.42 (4.23) | 37.16 (3.39) | |
| Dunwoodie, 2007 [68]b | Palliative care physicians in Australia | MBI | 21 | 14.95 (9.14) | 3.95 (3.40) | 38.90 (2.88) | |
| Srgaard, 2007 [69]b | Psychiatrists in 5 European nations | MBI | 22 | 19.41 (8.08) | 6.68 (4.93) | 39.00 (4.40) | |
| Sosa Oberlin, 2007 [56]b | Physicians of various specialties in Argentina | Author‐designed instrument | 33 | 26 (78.8%) had 4 burnout symptoms, 6.15 symptoms per physician | |||
| Voltmer, 2007 [57]b | Physicians of various specialties in Germany | AVEM | 46 | 11 (23.9%) exhibited burnout (type B) pattern | |||
| dm, 2008 [70]b | Physicians of various specialties in Hungary | MBI | 163 | 17.45 (11.12) | 4.86 (4.91) | 36.56 (7.03) | |
| Di Iorio, 2008 [71]b | Dialysis physicians in Italy | Author‐designed instrument | 54 | Work: 2.6 (1.5), Material: 3.1 (2.1), Climate: 3.0 (1.1), Objectives: 3.4 (1.6), Quality: 2.2 (1.5), Justification: 3.2 (2.0) | |||
| Lee, 2008 [49]b | Family physicians in Canada | MBI | 123 | 26.26 (9.53) | 10.20 (5.22) | 38.43 (7.34) | |
| Truchot, 2008 [72] | General practitioners in France | MBI | 259 | 25.4 (11.7) | 7.5 (5.5) | 36.5 (7.1) | |
| Twellaar, 2008 [73]b | General practitioners in the Netherlands | Utrecht Burnout Inventory | 349 | 2.06 (1.11) | 1.71 (1.05) | 5.08 (0.77) | |
| Arigoni, 2009 [17] | General practitioners, pediatricians in Switzerland | MBI | 258 | 22.8 (12.0) | 6.9 (6.1) | 39.0 (7.2) | |
| Bernhardt, 2009 [75] | Clinical geneticists in United States | MBI | 72 | 25.8 (10.01)c | 10.9 (4.16)c | 34.8 (5.43)c | |
| Bressi, 2009 [76]b | Psychiatrists in Italy | MBI | 53 | 23.15 (11.99) | 7.02 (6.29) | 36.41 (7.54) | |
| Krasner, 2009 [77] | General practitioners in United States | MBI | 60 | 26.8 (10.9)d | 8.4 (5.1)d | 40.2 (5.3)d | |
| Lasalvia, 2009 [55]b | Psychiatrists in Italy | Modified MBI (16 items; scale, 06) | 38 | 2.37 (1.27) | 1.51 (1.15) | 4.46 (0.87) | |
| Peisah, 2009 [79]b | Physicians of various specialties in Australia | MBI | 28 | 13.92 (9.24) | 3.66 (3.95) | 39.34 (8.55) | |
| Shanafelt, 2009 [80]b | Physicians of various specialties in United States | MBI | 408 | 20.5 (11.10) | 4.3 (4.74) | 40.8 (6.26) | |
| Zantinge, 2009 [81] | General practitioners in the Netherlands | Utrecht Burnout Inventory | 126 | 1.58 (0.79) | 1.32 (0.72) | 4.27 (0.77) | |
| Voltmer, 2010 [83]b | Psychiatrists in Germany | AVEM | 526 | 114 (21.7%) exhibited burnout (type B) pattern | |||
| Maccacaro, 2011 [85]b | Physicians of various specialties in Italy | MBI | 42 | 14.31 (11.98) | 3.62 (4.95) | 38.24 (6.22) | |
| Lucas, 2011 [84]b | Outpatient physicians periodically staffing an academic hospital teaching service in United States | MBI (EE only) | 30 | 24.37 (14.95) | |||
| Shanafelt, 2012 [87]b | General internists in United States | MBI | 447 | 25.4 (14.0) | 7.5 (6.3) | 41.4 (6.0) | |
| Kushnir, 2004 [62] | General practitioners and pediatricians in Israel | MBI (DP only) and SMBM | 309 | 9.15 (3.95) | SMBM mean (SD), 2.73 per item (0.86) | ||
| Vela‐Bueno, 2008 [74]b | General practitioners in Spain | MBI | 240 | 26.91 (11.61) | 9.20 (6.35) | 35.92 (7.92) | |
| Lesic, 2009 [78]b | General practitioners in Serbia | MBI | 38 | 24.71 (10.81) | 7.47 (5.51) | 37.21 (7.44) | |
| Demirci, 2010 [82]b | Medical specialists related to oncology practice in Hungary | MBI | 26 | 23.31 (11.2) | 6.46 (5.7) | 37.7 (8.14) | |
| Putnik, 2011 [86]b | General practitioners in Hungary | MBI | 370 | 22.22 (11.75) | 3.66 (4.40) | 41.40 (6.85) | |
| Lead Author, Publication Year | Study Population and Location | Instrument | No. of Participants | EE Score (SD)a | DP Score (SD) | PA Score (SD) | Other Results |
|---|---|---|---|---|---|---|---|
| |||||||
| Varga, 1996 [88] | Hospital doctors in Spain | MBI | 179 | 21.61b | 7.33b | 35.28b | |
| Aasland, 1997 [54] | Hospital doctors in Norway | Modified MBI (22 items; scale, 15) | 582 | 2.39 (0.80) | 1.81 (0.65) | 3.51 (0.46) | |
| Bargellini, 2000 [89] | Hospital doctors in Italy | MBI | 51 | 17.45 (9.87) | 7.06 (5.54) | 35.33 (7.90) | |
| Grassi, 2000 [58] | Hospital doctors in Italy | MBI | 146 | 16.17 (9.64) | 5.32 (4.76) | 38.71 (7.28) | |
| Hoff, 2001 [33] | Hospitalists in United States | Single‐item surveyc | 393 | 12.9% burned out (>4/5), 24.9% at risk for burnout (34/5), 62.2% at no current risk (mean, 2.86 on 15 scale) | |||
| Trichard, 2005 [90] | Hospital doctors in France | MBI | 199 | 16 (10.7) | 6.6 (5.7) | 38.5 (6.5) | |
| Gandini, 2006 [65]d | Hospital doctors in Argentina | MBI | 290 | 25.0 (12.7) | 7.9 (6.2) | 40.1 (7.0) | |
| Dunwoodie, 2007 [68]d | Palliative care doctors in Australia | MBI | 14 | 18.29 (14.24) | 5.29 (5.89) | 38.86 (3.42) | |
| Srgaard, 2007 [69]d | Psychiatrists in 5 European nations | MBI | 18 | 18.56 (9.32) | 5.50 (3.79) | 39.08 (5.39) | |
| Sosa Oberlin, 2007 [56]d | Hospital doctors in Argentina | Author‐designed instrument | 3 | 3 (100%) had 4 burnout symptoms, 8.67 symptoms per physician | |||
| Voltmer, 2007 [57]d | Hospital doctors in Germany | AVEM | 271 | 77 (28.4%) exhibited burnout (type B) pattern | |||
| dm, 2008 [70]b | Physicians of various specialties in Hungary | MBI | 194 | 19.23 (10.79) | 4.88 (4.61) | 35.26 (8.42) | |
| Di Iorio, 2008 [71]d | Dialysis physicians in Italy | Author‐designed instrument | 62 | Work, mean (SD), 3.1 (1.4); Material, mean (SD), 3.3 (1.5); Climate, mean (SD), 2.9 (1.1); Objectives, mean (SD), 2.5 (1.5); Quality, mean (SD), 3.0 (1.1); Justification, mean (SD), 3.1 (2.1) | |||
| Fuss, 2008 [91]d | Hospital doctors in Germany | Copenhagen Burnout Inventory | 292 | Mean Copenhagen Burnout Inventory, mean (SD), 46.90 (18.45) | |||
| Marner, 2008 [92]d | Psychiatrists and 1 generalist in United States | MBI | 9 | 20.67 (9.75) | 7.78 (5.14) | 35.33 (6.44) | |
| Shehabi, 2008 [93]d | Intensivists in Australia | Modified MBI (6 items; scale, 15) | 86 | 2.85 (0.93) | 2.64 (0.85) | 2.58 (0.83) | |
| Bressi, 2009 [76]d | Psychiatrists in Italy | MBI | 28 | 17.89 (14.46) | 5.32 (7.01) | 34.57 (11.27) | |
| Brown, 2009 [94] | Hospital doctors in Australia | MBI | 12 | 22.25 (8.59) | 6.33 (2.71) | 39.83 (7.31) | |
| Lasalvia, 2009 [55]d | Psychiatrists in Italy | Modified MBI (16 items; scale, 06) | 21 | 1.95 (1.04) | 1.35 (0.85) | 4.46 (1.04) | |
| Peisah, 2009 [79]d | Hospital doctors in Australia | MBI | 62 | 20.09 (9.91) | 6.34 (4.90) | 35.06 (7.33) | |
| Shanafelt, 2009 [80]d | Hospitalists and intensivists in United States | MBI | 19 | 25.2 (11.59) | 4.4 (3.79) | 38.5 (8.04) | |
| Tunc, 2009 [95] | Hospital doctors in Turkey | Modified MBI (22 items; scale, 04) | 62 | 1.18 (0.78) | 0.81 (0.73) | 3.10 (0.59)e | |
| Cocco, 2010 [96]d | Hospital geriatricians in Italy | MBI | 38 | 16.21 (11.56) | 4.53 (4.63) | 39.13 (7.09) | |
| Doppia, 2011 [97]d | Hospital doctors in France | Copenhagen Burnout Inventory | 1,684 | Mean work‐related burnout score, 2.72 (0.75) | |||
| Glasheen, 2011 [98] | Hospitalists in United States | Single‐item survey | 265 | Mean, 2.08 on 15 scale 62 (23.4%) burned out | |||
| Lucas, 2011 [84]d | Academic hospitalists in United States | MBI (EE only) | 26 | 19.54 (12.85) | |||
| Thorsen, 2011 [99] | Hospital doctors in Malawi | MBI | 2 | 25.5 (4.95) | 8.5 (6.36) | 25.0 (5.66) | |
| Hinami, 2012 [50]d | Hospital doctors in United States | Single‐item survey | 793 | Mean, 2.24 on 15 scale 261 (27.2%) burned out | |||
| Quenot, 2012 [100]d | Intensivists in France | MBI | 4 | 33.25 (4.57) | 13.50 (5.45) | 35.25 (4.86) | |
| Ruitenburg, 2012 [101] | Hospital doctors in the Netherlands | MBI (EE and DP only) | 214 | 13.3 (8.0) | 4.5 (4.1) | ||
| Seibt, 2012 [102]d | Hospital doctors in Germany | Modified MBI (16 items; scale, 06, reported per item rather than totals) | 2,154 | 2.2 (1.4) | 1.4 (1.2) | 5.1 (0.9) | |
| Shanafelt, 2012 [87]d | Hospitalists in United States | MBI | 130 | 24.7 (12.5) | 9.1 (6.9) | 39.0 (7.6) | |
Table 3 summarizes the results of the 15 studies that reported burnout data for both inpatient and outpatient physicians, allowing direct comparisons to be made. Nine studies reported MBI subset totals with standard deviations, 2 used different modifications of the MBI, 2 used different author‐derived measures, 1 used only the emotional exhaustion subscale of the MBI, and 1 used the Arbeitsbezogenes Verhaltens und Erlebensmuster. Therefore, statistical comparison was attempted only for the 9 studies reporting comparable MBI data, comprising burnout data on 1390 outpatient physicians and 899 inpatient physicians.
| Lead Author, Publication Year | Location | Instrument | Inpatient‐Based Physicians | Outpatient‐Based Physicians | ||
|---|---|---|---|---|---|---|
| No. | Results, Score (SD)a | No. | Results, Score (SD)a | |||
| ||||||
| Aasland, 1997 [54]b | Norway | Modified MBI (22 items; scale, 15) | 582 | EE, 2.39 (0.80); DP, 1.81 (0.65); PA, 3.51 (0.46) | 298 | EE, 2.65 (0.80); DP, 1.90 (0.59); PA, 3.45 (0.40) |
| Grassi, 2000 [58] | Italy | MBI | 146 | EE, 16.17 (9.64); DP, 5.32 (4.76); PA, 38.71 (7.28) | 182 | EE, 18.49 (11.49); DP, 6.11 (5.86); PA, 38.52 (7.60) |
| Gandini, 2006 [65]b | Argentina | MBI | 290 | EE, 25.0 (12.7);DP, 7.9 (6.2); PA, 40.1 (7.0) | 67 | EE, 31.0 (13.8); DP, 10.2 (6.6); PA, 38.4 (6.8) |
| Dunwoodie, 2007 [68]b | Australia | MBI | 14 | EE, 18.29 (14.24); DP, 5.29 (5.89); PA, 38.86 (3.42) | 21 | EE, 14.95 (9.14); DP, 3.95 (3.40); PA, 38.90 (2.88) |
| Srgaard, 2007 [69]b | 5 European nations | MBI | 18 | EE, 18.56 (9.32); DP, 5.50 (3.79); PA, 39.08 (5.39) | 22 | EE, 19.41 (8.08); DP, 6.68 (4.93); PA, 39.00 (4.40) |
| Sosa Oberlin, 2007 [56]b | Argentina | Author‐designed instrument | 3 | 3 (100%) had 4 burnout symptoms, 8.67 symptoms per physician | 33 | 26 (78.8%) had 4 burnout symptoms, 6.15 symptoms per physician |
| Voltmer, 2007 [57]b | Germany | AVEM | 271 | 77 (28.4%) exhibited burnout (type B) pattern | 46 | 11 (23.9%) exhibited burnout (type B) pattern |
| dm, 2008 [70]b | Hungary | MBI | 194 | EE, 19.23 (10.79); DP, 4.88 (4.61); PA, 35.26 (8.42) | 163 | EE, 17.45 (11.12); DP, 4.86 (4.91); PA, 36.56 (7.03) |
| Di Iorio, 2008 [71]b | Italy | Author‐designed instrument | 62 | Work: 3.1 (1.4); material: 3.3 (1.5); climate: 2.9 (1.1); objectives: 2.5 (1.5); quality: 3.0 (1.1); justification: 3.1 (2.1) | 54 | Work: 2.6 (1.5); material: 3.1 (2.1); climate: 3.0 (1.1); objectives: 3.4 (1.6); quality: 2.2 (1.5); justification: 3.2 (2.0) |
| Bressi, 2009 [76]b | Italy | MBI | 28 | EE, 17.89 (14.46); DP, 5.32 (7.01); PA, 34.57 (11.27) | 53 | EE, 23.15 (11.99); DP, 7.02 (6.29); PA, 36.41 (7.54) |
| Lasalvia, 2009[55]b | Italy | Modified MBI (16 items; scale, 06) | 21 | EE, 1.95 (1.04); DP, 1.35 (0.85); PA, 4.46 (1.04) | 38 | EE, 2.37 (1.27); DP, 1.51 (1.15); PA, 4.46 (0.87) |
| Peisah, 2009 [79]b | Australia | MBI | 62 | EE, 20.09 (9.91); DP, 6.34 (4.90); PA, 35.06 (7.33) | 28 | EE, 13.92 (9.24); DP, 3.66 (3.95); PA, 39.34 (8.55) |
| Shanafelt, 2009 [80]b | United States | MBI | 19 | EE, 25.2 (11.59); DP, 4.4 (3.79); PA, 38.5 (8.04) | 408 | EE, 20.5 (11.10); DP, 4.3 (4.74); PA, 40.8 (6.26) |
| Lucas, 2011 [84]b | United States | MBI (EE only) | 26 | EE, 19.54 (12.85) | 30 | EE, 24.37 (14.95) |
| Shanafelt, 2012 [87]b | United States | MBI | 130 | EE, 24.7 (12.5); DP, 9.1 (6.9); PA, 39.0 (7.6) | 447 | EE, 25.4 (14.0); DP, 7.5 (6.3); PA, 41.4 (6.0) |
Figure 2 shows that no significant difference existed between the groups regarding emotional exhaustion (mean difference, 0.11 points on a 54‐point scale; 95% confidence interval [CI], 2.40 to 2.61; P=0.94). In addition, there was no significant difference between the groups regarding depersonalization (Figure 3; mean difference, 0.00 points on a 30‐point scale; 95% CI, 1.03 to 1.02; P=0.99) and personal accomplishment (Figure 4; mean difference, 0.93 points on a 48‐point scale; 95% CI, 0.23 to 2.09; P=0.11).



We used meta‐regression to allow the incorporation of single‐armed MBI studies. Whether single‐armed studies were analyzed separately (15 outpatient studies comprising 3927 physicians, 4 inpatient studies comprising 300 physicians) or analyzed with double‐armed studies (24 outpatient arms comprising 5318 physicians, 13 inpatient arms comprising 1301 physicians), the lack of a significant difference between the groups persisted for the depersonalization and personal accomplishment scales (Figure 5). Emotional exhaustion was significantly higher in outpatient physicians when single‐armed studies were considered separately (mean difference, 6.36 points; 95% CI, 2.24 to 10.48; P=0.002), and this difference persisted when all studies were combined (mean difference, 3.00 points; 95% CI, 0.05 to 5.94, P=0.046).

Subgroup analysis by geographic location showed US outpatient physicians had a significantly higher personal accomplishment score than US inpatient physicians (mean difference, 2.38 points; 95% CI, 1.22 to 3.55; P<0.001) in double‐armed studies. This difference did not persist when single‐armed studies were included through meta‐regression (mean difference, 0.55 points, 95% CI, 4.30 to 5.40, P=0.83).
Table 4 demonstrates that methodological quality was generally good from the standpoint of the reporting and bias subsections of the Downs and Black tool. External validity was scored lower for many studies due to the use of convenience samples and lack of information about physicians who declined to participate.
| Lead Author, Publication Year | Reporting | External Validity | Internal Validity: Bias | Internal Validity: Confounding | Power |
|---|---|---|---|---|---|
| Schweitzer, 1994 [12] | 5 of 6 points | 1 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Varga, 1996 [88] | 5 of 6 points | 1 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Aasland, 1997 [54] | 3 of 6 points | 2 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Bargellini, 2000 [89] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Grassi, 2000 [58] | 6 of 6 points | 0 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| McManus, 2000 [59] | 5 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Hoff, 2001 [33] | 6 of 6 points | 2 of 2 points | 2 of 4 points | 1 of 1 point | 0 of 1 point |
| Yaman, 2002 [60] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Cathbras, 2004 [61] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Kushnir, 2004 [62] | 5 of 6 points | 0 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Goehring, 2005 [63] | 6 of 6 points | 1 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Trichard, 2005 [90] | 3 of 6 points | 1 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Esteva, 2006 [64] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Gandini, 2006 [65] | 6 of 6 points | 1 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Ozyurt, 2006 [66] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Deighton, 2007 [67] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Dunwoodie, 2007 [68] | 5 of 6 points | 2 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Srgaard, 2007 [69] | 6 of 6 points | 0 of 2 points | 3 of 4 points | 1 of 1 point | 1 of 1 point |
| Sosa Oberlin, 2007 [56] | 4 of 6 points | 0 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Voltmer, 2007 [57] | 4 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| dm, 2008 [70] | 5 of 6 points | 2 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Di Iorio, 2008 [71] | 6 of 6 points | 0 of 2 points | 2 of 4 points | 0 of 1 point | 0 of 1 point |
| Fuss, 2008 [91] | 6 of 6 points | 0 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Lee, 2008 [49] | 4 of 6 points | 2 of 2 points | 4 of 4 points | 0 of 1 point | 1 of 1 point |
| Marner, 2008 [92] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Shehabi, 2008 [93] | 3 of 6 points | 1 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Truchot, 2008 [72] | 5 of 6 points | 1 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Twellaar, 2008 [73] | 6 of 6 points | 2 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Vela‐Bueno, 2008 [74] | 5 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Arigoni, 2009 [17] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Bernhardt, 2009 [75] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Bressi, 2009 [76] | 6 of 6 points | 0 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Brown, 2009 [94] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Krasner, 2009 [77] | 9 of 11 points | 0 of 3 points | 6 of 7 points | 1 of 2 points | 1 of 1 point |
| Lasalvia, 2009 [55] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Lesic, 2009 [78] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Peisah, 2009 [79] | 6 of 6 points | 2 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Shanafelt, 2009 [80] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Tunc, 2009 [95] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Zantinge, 2009 [81] | 5 of 6 points | 0 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Cocco, 2010 [96] | 4 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Demirci, 2010 [82] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Voltmer, 2010 [83] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Doppia, 2011 [97] | 5 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Glasheen, 2011 [98] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Lucas, 2011 [84] | 10 of 11 points | 2 of 3 points | 7 of 7 points | 5 of 6 points | 1 of 1 point |
| Maccacaro, 2011 [85] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Putnik, 2011 [86] | 6 of 6 points | 1 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Thorsen, 2011 [99] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Hinami, 2012 [50] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 1 of 1 point |
| Quenot, 2012 [100] | 8 of 11 points | 1 of 3 points | 6 of 7 points | 1 of 2 points | 0 of 1 point |
| Ruitenburg, 2012 [101] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Seibt, 2012 [102] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Shanafelt, 2012 [87] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
Funnel plots were used to evaluate for publication bias in the meta‐analysis of the 8 double‐armed studies (Figure 6). We found no significant evidence of bias, which was supported by Begg's test P values of 0.90 for emotional exhaustion, >0.99 for depersonalization, and 0.54 for personal accomplishment. A trim‐and‐fill analysis determined that no adjustment was necessary.

DISCUSSION
There appears to be no support for the long‐held belief that inpatient physicians are particularly prone to burnout. Among studies for which practice location was stated explicitly or could be obtained from the authors, and who used the MBI, no differences were found among inpatient and outpatient physicians with regard to depersonalization or personal accomplishment. This finding persisted whether double‐armed studies were compared directly, single‐armed studies were incorporated into this analysis, or single‐armed studies were analyzed separately. Outpatient physicians had a higher degree of emotional exhaustion when all studies were considered.
There are several reasons why outpatient physicians may be more prone to emotional exhaustion than their inpatient colleagues. Although it is by no means true that all inpatient physicians work in shifts, the increased availability of shift work may allow some inpatient physicians to better balance their professional and personal lives, a factor of work with which some outpatient physicians have struggled.[47] Inpatient practice may also afford more opportunity for teamwork, a factor that has been shown to correlate with reduced burnout.[48] When surveyed about burnout, outpatient physicians have cited patient volumes, paperwork, medicolegal concerns, and lack of community support as factors.[49] Inpatient physicians are not immune to these forces, but they arguably experience them to different degrees.
The absence of a higher rate of depersonalization among inpatient physicians is particularly reassuring in light of concerns expressed with the advent of US hospital medicinethat some hospitalists would be prone to viewing patients as an impediment to the efficient running of the hospital,[2] the very definition of depersonalization.
Although the difference in the whole sample was not statistically significant, the consistent tendency toward a greater sense of personal accomplishment among outpatient physicians is also noteworthy, particularly because post hoc subgroup analysis of US physicians did show statistical significance in both 2‐armed studies. Without detailed age data for the physicians in each study, we could not separate the possible impact of age on personal accomplishment; hospital medicine is a newer specialty staffed by generally younger physicians, and hospitalists may not have had time to develop a sense of accomplishment. When surveyed about job satisfaction, hospitalists have also reported the feeling that they were treated as glorified residents,[50] a factor that, if shared by other inpatient physicians, must surely affect their sense of personal accomplishment. The lack of longitudinal care for patients and the substantial provision of end‐of‐life care also may diminish the sense of personal accomplishment among inpatient physicians.
Another important finding from this systematic review is the marked heterogeneity of the instruments used to measure physician burnout. Many of the identified studies could not be subjected to meta‐analysis because of their use of differing burnout measures. Drawing more substantial conclusions about burnout and practice location is limited by the fact that, although the majority of studies used the full MBI, the largest study of European hospital doctors used the Copenhagen Burnout Inventory, and the studies thus far of US hospitalists have used single‐item surveys or portions of the MBI. Not reflected in this review is the fact that a large study of US burnout and job satisfaction[51] did not formally address practice location (M. Linzer, personal communication, August 2012). Similarly, a large study of British hospital doctors[52] is not included herein because many of the physicians involved had substantial outpatient duties (C. Taylor, personal communication, July 2012). Varying burnout measures have complicated a previous systematic review of burnout in oncologists.[53] Two studies that directly compared inpatient and outpatient physicians but that were excluded from our statistical analysis because of their modified versions of the MBI,[54, 55] showed higher burnout scores in outpatient physicians. Two other studies that provided direct inpatient versus outpatient comparisons but that used alternative burnout measures[56, 57] showed a greater frequency of burnout in inpatient physicians, but of these, 1 study[56] involved only 3 inpatient physicians.
Several limitations of our study should be considered. Although we endeavored to obtain information from authors (with some success) about specific local practice patterns and eliminated many studies because of incomplete data or mixed practice patterns (eg, general practitioners who take frequent hospital calls, hospital physicians with extensive outpatient duties in a clinic attached to their hospital), it remains likely that many physicians identified as outpatient provided some inpatient care (attending a few weeks per year on a teaching service, for example) and that some physicians identified as inpatient have minimal outpatient duties.
More importantly, the dataset analyzed is heterogeneous. Studies of the incidence of burnout are naturally observational and therefore not randomized. Inclusion of international studies is necessary to answer the research question (because published data on US hospitalists are sparse) but naturally introduces differences in practice settings, local factors, and other factors for which we cannot possibly account fully.
Our meta‐analysis therefore addressed a broad question about burnout among inpatient and outpatient physicians in various diverse settings. Applying it to any 1 population (including US hospitalists) is, by necessity, imprecise.
Post hoc analysis should be viewed with caution. For example, the finding of a statistical difference between US inpatient and outpatient physicians with regard to personal accomplishment score is compelling from the standpoint of hypothesis generation. However, it is worth bearing in mind that this analysis contained only 2 studies, both by the same primary author, and compared 855 outpatient physicians to only 149 hospitalists. This difference was no longer significant when 2 outpatient studies were added through meta‐regression.
Finally, the specific focus of this study on practice location precluded comparison with emergency physicians and anesthesiologists, 2 specialist types that have been the subject of particularly robust burnout literature. As the literature on hospitalist burnout becomes more extensive, comparative studies with these groups and with intensivists might prove instructive.
In summary, analysis of 24 studies comprising data on 5318 outpatient physicians and 1301 inpatient physicians provides no support for the commonly held belief that hospital‐based physicians are particularly prone to burnout. Outpatient physicians reported higher emotional exhaustion. Further studies of the incidence and severity of burnout according to practice location are indicated. We propose that in future studies, to avoid the difficulties with statistical analysis summarized herein, investigators ask about and explicitly report practice location (inpatient vs outpatient vs both) and report mean MBI subset data and standard deviations. Such information about US hospitalists would allow comparison with a robust (if heterogeneous) international literature on burnout.
Acknowledgments
The authors gratefully acknowledge all of the study authors who contributed clarification and guidance for this project, particularly the following authors who provided unpublished data for further analysis: Olaf Aasland, MD; Szilvia dm, PhD; Annalisa Bargellini, PhD; Cinzia Bressi, MD, PhD; Darrell Campbell Jr, MD; Ennio Cocco, MD; Russell Deighton, PhD; Senem Demirci Alanyali, MD; Biagio Di Iorio, MD, PhD; David Dunwoodie, MBBS; Sharon Einav, MD; Madeleine Estryn‐Behar, PhD; Bernardo Gandini, MD; Keiki Hinami, MD; Antonio Lasalvia, MD, PhD; Joseph Lee, MD; Guido Maccacaro, MD; Swati Marner, EdD; Chris McManus, MD, PhD; Carmelle Peisah, MBBS, MD; Katarina Putnik, MSc; Alfredo Rodrguez‐Muoz, PhD; Yahya Shehabi, MD; Evelyn Sosa Oberlin, MD; Jean Karl Soler, MD, MSc; Knut Srgaard, PhD; Cath Taylor; Viva Thorsen, MPH; Mascha Twellaar, MD; Edgar Voltmer, MD; Colin West, MD, PhD; and Deborah Whippen. The authors also thank the following colleagues for their help with translation: Dusanka Anastasijevic (Norwegian); Joyce Cheung‐Flynn, PhD (simplified Chinese); Ales Hlubocky, MD (Czech); Lena Jungheim, RN (Swedish); Erez Kessler (Hebrew); Kanae Mukai, MD (Japanese); Eliane Purchase (French); Aaron Shmookler, MD (Russian); Jan Stepanek, MD (German); Fernando Tondato, MD (Portuguese); Laszlo Vaszar, MD (Hungarian); and Joseph Verheidje, PhD (Dutch). Finally, the authors thank Cynthia Heltne and Diana Rogers for their expert and tireless library assistance, Bonnie Schimek for her help with figures, and Cindy Laureano and Elizabeth Jones for their help with author contact.
Hospital medicine is a rapidly growing field of US clinical practice.[1] Almost since its advent, concerns have been expressed about the potential for hospitalists to burn out.[2] Hospitalists are not unique in this; similar concerns heralded the arrival of other location‐defined specialties, including emergency medicine[3] and the full‐time intensivist model,[4] a fact that has not gone unnoted in the literature about hospitalists.[5]
The existing international literature on physician burnout provides good reason for this concern. Inpatient‐based physicians tend to work unpredictable schedules, with substantial impact on home life.[6] They tend to be young, and much burnout literature suggests a higher risk among younger, less‐experienced physicians.[7] When surveyed, hospitalists have expressed more concerns about their potential for burnout than their outpatient‐based colleagues.[8]
In fact, data suggesting a correlation between inpatient practice and burnout predate the advent of the US hospitalist movement. Increased hospital time was reported to correlate with higher rates of burnout in internists,[9] family practitioners,[10] palliative physicians,[11] junior doctors,[12] radiologists,[13] and cystic fibrosis caregivers.[14] In 1987, Keinan and Melamed[15] noted, Hospital work by its very nature, as compared to the work of a general practitioner, deals with the more severe and complicated illnesses, coupled with continuous daily contacts with patients and their anxious families. In addition, these physicians may find themselves embroiled in the power struggles and competition so common in their work environment.
There are other features, however, that may protect inpatient physicians from burnout. Hospital practice can facilitate favorable social relations involving colleagues, co‐workers, and patients,[16] a factor that may be protective.[17] A hospitalist schedule also can allow more focused time for continuing medical education, research, and teaching,[18] which have all been associated with reduced risk of burnout in some studies.[17] Studies of psychiatrists[19] and pediatricians[20] have shown a lower rate of burnout among physicians with more inpatient duties. Finally, a practice model involving a seemingly stable cadre of inpatient physicians has existed in Europe for decades,[2] indicating at least a degree of sustainability.
Information suggesting a higher rate of burnout among inpatient physicians could be used to target therapeutic interventions and to adjust schedules, whereas the opposite outcome could refute a pervasive myth. We therefore endeavored to summarize the literature on burnout among inpatient versus outpatient physicians in a systematic fashion, and to include data not only from the US hospitalist experience but also from other countries that have used a similar model for decades. Our primary hypothesis was that inpatient physicians experience more burnout than outpatient physicians.
It is important to distinguish burnout from depression, job dissatisfaction, and occupational stress, all of which have been studied extensively in physicians. Burnout, as introduced by Freudenberger[21] and further characterized by Maslach,[22] is a condition in which emotional exhaustion, depersonalization, and a low sense of personal accomplishment combine to negatively affect work life (as opposed to clinical depression, which affects all aspects of life). Job satisfaction can correlate inversely with burnout, but it is a separate process[23] and the subject of a recent systematic review.[24] The importance of distinguishing burnout from job dissatisfaction is illustrated by a survey of head and neck surgeons, in which 97% of those surveyed indicated satisfaction with their jobs and 34% of the same group answered in the affirmative when asked if they felt burned out.[25]
One obstacle to the meaningful comparison of burnout prevalence across time, geography, and specialty is the myriad ways in which burnout is measured and reported. The oldest and most commonly used instrument to measure burnout is the Maslach Burnout Inventory (MBI), which contains 22 items assessing 3 components of burnout (emotional exhaustion, depersonalization, and low personal accomplishment).[26] Other measures include the Copenhagen Burnout Inventory[27] (19 items with the components personal burnout, work‐related burnout, and client‐related burnout), Utrecht Burnout Inventory[28] (20‐item modification of the MBI), Boudreau Burnout Questionnaire[29] (30 items), Arbeitsbezogenes Verhaltens und Erlebensmuster[30] (66‐item questionnaire assessing professional commitment, resistance to stress, and emotional well‐being), Shirom‐Melamed Burnout Measure[31] (22 items with subscales for physical fatigue, cognitive weariness, tension, and listlessness), and a validated single‐item questionnaire.[32]
METHODS
Electronic searches of MEDLINE, EMBASE, PsycINFO, SCOPUS, and PubMed were undertaken for articles published from January 1, 1974 (the year in which burnout was first described by Freudenberger[21]) to 2012 (last accessed, September 12, 2012) using the Medical Subject Headings (MeSH) terms stress, psychological; burnout, professional; adaptation, psychological; and the keyword burnout. The same sources were searched to create another set for the MeSH terms hospitalists, physician's practice patterns, physicians/px, professional practice location, and the keyword hospitalist#. Where exact subject headings did not exist in databases, comparable subject headings or keywords were used. The 2 sets were then combined using the operator and. Abstracts from the Society of Hospital Medicine annual conferences were hand‐searched, as were reference lists from identified articles. To ensure that pertinent international literature was captured, there was no language restriction in the search.
A 2‐stage screening process was used. The titles and abstracts of all articles identified in the search were independently reviewed by 2 investigators (D.L.R. and K.J.C.) who had no knowledge of each other's results. An article was obtained when either reviewer deemed it worthy of full‐text review.
All full‐text articles were independently reviewed by the same 2 investigators. The inclusion criterion was the measurement of burnout in physicians who are stated to or can be reasonably assumed to spend the substantial majority of their clinical practice exclusively in either the inpatient or the outpatient setting. Studies of emergency department physicians or specialists who invariably spend substantial amounts of time in both settings (eg, surgeons, anesthesiologists) were excluded. Studies limited to trainees or nonphysicians were also excluded. For both stages of review, agreement between the 2 investigators was assessed by calculating the statistic. Disagreements about inclusion were adjudicated by a third investigator (A.I.B.).
Because our goal was to establish and compare the rate of burnout among US hospitalists and other inpatient physicians around the world, we included studies of hospitalists according to the definition in use at the time of the individual study, noting that the formal definition of a hospitalist has changed over the years.[33] Because practice patterns for physicians described as primary care physicians, family doctors, hospital doctors, and others differ substantially from country to country, we otherwise included only the studies where the practice location was stated explicitly or where the authors confirmed that their study participants either are known or can be reasonably assumed to spend more than 75% of their time caring for hospital inpatients, or are known or can be reasonably assumed to spend the vast majority of their time caring for outpatients.
Data were abstracted using a standardized form and included the measure of burnout used in the study, results, practice location of study subjects, and total number of study subjects. When data were not clear (eg, burnout measured but not reported by the authors, practice location of study subjects not clear), authors were contacted by email, or when no current email address could be located or no response was received, by telephone or letter. In instances where burnout was measured repeatedly over time or before and after a specific intervention, only the baseline measurement was recorded. Because all studies were expected to be nonrandomized, methodological quality was assessed using a version of the tool of Downs and Black,[34] adapted where necessary by omitting questions not applicable to the specific study type (eg randomization for survey studies)[35] and giving a maximum of 1 point for the inclusion of a power calculation.
Two a priori analyses were planned: (1) a statistical comparison of articles directly comparing burnout among inpatient and outpatient physicians, and (2) a statistical comparison of articles measuring burnout among inpatient physicians with articles measuring burnout among outpatient physicians by the most frequently reported measuremean subset scores for emotional exhaustion, depersonalization, and personal accomplishment on the MBI.
The primary outcome measures were the differences between mean subset scores for emotional exhaustion, depersonalization, and personal accomplishment on the MBI. All differences are expressed as (outpatient meaninpatient mean). The variance of each outcome was calculated with standard formulas.[36] To calculate the overall estimate, each study was weighted by the reciprocal of its variance. Studies with fewer than 10 subjects were excluded from statistical analysis but retained in the systematic review.
For studies that reported data for both inpatient and outpatient physicians (double‐armed studies), Cochran Q test and the I2 value were used to assess heterogeneity.[37, 38] Substantial heterogeneity was expected because these individual studies were conducted for different populations in different settings with different study designs, and this expectation was confirmed statistically. Therefore, we used a random effects model to estimate the overall effect, providing a conservative approach that accounted for heterogeneity among studies.[39]
To assess the durability of our findings, we performed separate multivariate meta‐regression analyses by including single‐armed studies only and including both single‐armed and double‐armed studies. For these meta‐regressions, means were again weighted by the reciprocal of their variances, and the arms of 2‐armed studies were considered separately. This approach allowed us to generate an estimate of the differences between MBI subset scores from studies that did not include such an estimate when analyzed separately.[40]
We examined the potential for publication bias in double‐armed studies by constructing a funnel plot, in which mean scores were plotted against their standard errors.[41] The trim‐and‐fill method was used to determine whether adjustment for publication bias was necessary. In addition, Begg's rank correlation test[42] was completed to test for statistically significant publication bias.
Stata 10.0 statistical software (StataCorp, College Station, TX) was used for data analyses. A P value of 0.05 or less was deemed statistically significant. The Preferred Reporting Items for Systematic Reviews and Meta‐analysis checklist was used for the design and execution of the systematic review and meta‐analysis.[43]
Subgroup analyses based on location were undertaken a posteriori. All data (double‐armed meta‐analysis, meta‐regression of single‐armed studies, and meta‐regression of single‐ and double‐armed studies) were analyzed by location (United States vs other; United States vs Europe vs other).
RESULTS
The search results are outlined in Figure 1. In total, 1704 articles met the criteria for full‐text review. A review of pertinent reference lists and author contacts led to the addition of 149 articles. Twenty‐nine references could not be located by any means, despite repeated attempts. Therefore, 1824 articles were subjected to full‐text review by the 2 investigators.

Initially, 57 articles were found that met criteria for inclusion. Of these, 2 articles reported data in formats that could not be interpreted.[44, 45] When efforts to clarify the data with the authors were unsuccessful, these studies were excluded. A study specifically designed to assess the response of physicians to a recent series of terrorist attacks[46] was excluded a posteriori because of lack of generalizability. Of the other 54 studies, 15 reported burnout data on both outpatient physicians and inpatient physicians, 22 reported data on outpatient physicians only, and 17 reported data on inpatient physicians only. Table 1 summarizes the results of the 37 studies involving outpatient physicians; Table 2 summarizes the 32 studies involving inpatient physicians.
| Lead Author, Publication Year | Study Population and Location | Instrument | No. of Participants | EE Score (SD)a | DP Score (SD) | PA Score (SD) | Other Results |
|---|---|---|---|---|---|---|---|
| |||||||
| Schweitzer, 1994[12] | Young physicians of various specialties in South Africa | Single‐item survey | 7 | 6 (83%) endorsed burnout | |||
| Aasland, 1997 [54]b | General practitioners in Norway | Modified MBI (22 items; scale, 15) | 298 | 2.65 (0.80) | 1.90 (0.59) | 3.45 (0.40) | |
| Grassi, 2000 [58] | General practitioners in Italy | MBI | 182 | 18.49 (11.49) | 6.11 (5.86) | 38.52 (7.60) | |
| McManus, 2000 [59]b | General practitioners in United Kingdom | Modified MBI (9 items; scale, 06) | 800 | 8.34 (4.39) | 3.18 (3.40) | 14.16 (2.95) | |
| Yaman, 2002 [60] | General practitioners in 8 European nations | MBI | 98 | 25.1 (8.50) | 7.3 (4.92) | 34.5 (7.67) | |
| Cathbras, 2004 [61] | General practitioners in France | MBI | 306 | 21.85 (12.4) | 9.13 (6.7) | 38.7 (7.1) | |
| Goehring, 2005 [63] | General practitioners, general internists, pediatricians in Switzerland | MBI | 1755 | 17.9 (9.8) | 6.5 (4.7) | 39.6 (6.5) | |
| Esteva, 2006 [64] | General practitioners, pediatricians in Spain | MBI | 261 | 27.4 (11.8) | 10.07 (6.4) | 35.9 (7.06) | |
| Gandini, 2006 [65]b | Physicians of various specialties in Argentina | MBI | 67 | 31.0 (13.8) | 10.2 (6.6) | 38.4 (6.8) | |
| Ozyurt, 2006 [66] | General practitioners in Turkey | Modified MBI (22 items; scale, 04) | 55 | 15.23 (5.80) | 4.47 (3.31) | 23.38 (4.29) | |
| Deighton, 2007 [67]b | Psychiatrists in several German‐speaking nations | MBI | 19 | 30.68 (9.92) | 13.42 (4.23) | 37.16 (3.39) | |
| Dunwoodie, 2007 [68]b | Palliative care physicians in Australia | MBI | 21 | 14.95 (9.14) | 3.95 (3.40) | 38.90 (2.88) | |
| Srgaard, 2007 [69]b | Psychiatrists in 5 European nations | MBI | 22 | 19.41 (8.08) | 6.68 (4.93) | 39.00 (4.40) | |
| Sosa Oberlin, 2007 [56]b | Physicians of various specialties in Argentina | Author‐designed instrument | 33 | 26 (78.8%) had 4 burnout symptoms, 6.15 symptoms per physician | |||
| Voltmer, 2007 [57]b | Physicians of various specialties in Germany | AVEM | 46 | 11 (23.9%) exhibited burnout (type B) pattern | |||
| dm, 2008 [70]b | Physicians of various specialties in Hungary | MBI | 163 | 17.45 (11.12) | 4.86 (4.91) | 36.56 (7.03) | |
| Di Iorio, 2008 [71]b | Dialysis physicians in Italy | Author‐designed instrument | 54 | Work: 2.6 (1.5), Material: 3.1 (2.1), Climate: 3.0 (1.1), Objectives: 3.4 (1.6), Quality: 2.2 (1.5), Justification: 3.2 (2.0) | |||
| Lee, 2008 [49]b | Family physicians in Canada | MBI | 123 | 26.26 (9.53) | 10.20 (5.22) | 38.43 (7.34) | |
| Truchot, 2008 [72] | General practitioners in France | MBI | 259 | 25.4 (11.7) | 7.5 (5.5) | 36.5 (7.1) | |
| Twellaar, 2008 [73]b | General practitioners in the Netherlands | Utrecht Burnout Inventory | 349 | 2.06 (1.11) | 1.71 (1.05) | 5.08 (0.77) | |
| Arigoni, 2009 [17] | General practitioners, pediatricians in Switzerland | MBI | 258 | 22.8 (12.0) | 6.9 (6.1) | 39.0 (7.2) | |
| Bernhardt, 2009 [75] | Clinical geneticists in United States | MBI | 72 | 25.8 (10.01)c | 10.9 (4.16)c | 34.8 (5.43)c | |
| Bressi, 2009 [76]b | Psychiatrists in Italy | MBI | 53 | 23.15 (11.99) | 7.02 (6.29) | 36.41 (7.54) | |
| Krasner, 2009 [77] | General practitioners in United States | MBI | 60 | 26.8 (10.9)d | 8.4 (5.1)d | 40.2 (5.3)d | |
| Lasalvia, 2009 [55]b | Psychiatrists in Italy | Modified MBI (16 items; scale, 06) | 38 | 2.37 (1.27) | 1.51 (1.15) | 4.46 (0.87) | |
| Peisah, 2009 [79]b | Physicians of various specialties in Australia | MBI | 28 | 13.92 (9.24) | 3.66 (3.95) | 39.34 (8.55) | |
| Shanafelt, 2009 [80]b | Physicians of various specialties in United States | MBI | 408 | 20.5 (11.10) | 4.3 (4.74) | 40.8 (6.26) | |
| Zantinge, 2009 [81] | General practitioners in the Netherlands | Utrecht Burnout Inventory | 126 | 1.58 (0.79) | 1.32 (0.72) | 4.27 (0.77) | |
| Voltmer, 2010 [83]b | Psychiatrists in Germany | AVEM | 526 | 114 (21.7%) exhibited burnout (type B) pattern | |||
| Maccacaro, 2011 [85]b | Physicians of various specialties in Italy | MBI | 42 | 14.31 (11.98) | 3.62 (4.95) | 38.24 (6.22) | |
| Lucas, 2011 [84]b | Outpatient physicians periodically staffing an academic hospital teaching service in United States | MBI (EE only) | 30 | 24.37 (14.95) | |||
| Shanafelt, 2012 [87]b | General internists in United States | MBI | 447 | 25.4 (14.0) | 7.5 (6.3) | 41.4 (6.0) | |
| Kushnir, 2004 [62] | General practitioners and pediatricians in Israel | MBI (DP only) and SMBM | 309 | 9.15 (3.95) | SMBM mean (SD), 2.73 per item (0.86) | ||
| Vela‐Bueno, 2008 [74]b | General practitioners in Spain | MBI | 240 | 26.91 (11.61) | 9.20 (6.35) | 35.92 (7.92) | |
| Lesic, 2009 [78]b | General practitioners in Serbia | MBI | 38 | 24.71 (10.81) | 7.47 (5.51) | 37.21 (7.44) | |
| Demirci, 2010 [82]b | Medical specialists related to oncology practice in Hungary | MBI | 26 | 23.31 (11.2) | 6.46 (5.7) | 37.7 (8.14) | |
| Putnik, 2011 [86]b | General practitioners in Hungary | MBI | 370 | 22.22 (11.75) | 3.66 (4.40) | 41.40 (6.85) | |
| Lead Author, Publication Year | Study Population and Location | Instrument | No. of Participants | EE Score (SD)a | DP Score (SD) | PA Score (SD) | Other Results |
|---|---|---|---|---|---|---|---|
| |||||||
| Varga, 1996 [88] | Hospital doctors in Spain | MBI | 179 | 21.61b | 7.33b | 35.28b | |
| Aasland, 1997 [54] | Hospital doctors in Norway | Modified MBI (22 items; scale, 15) | 582 | 2.39 (0.80) | 1.81 (0.65) | 3.51 (0.46) | |
| Bargellini, 2000 [89] | Hospital doctors in Italy | MBI | 51 | 17.45 (9.87) | 7.06 (5.54) | 35.33 (7.90) | |
| Grassi, 2000 [58] | Hospital doctors in Italy | MBI | 146 | 16.17 (9.64) | 5.32 (4.76) | 38.71 (7.28) | |
| Hoff, 2001 [33] | Hospitalists in United States | Single‐item surveyc | 393 | 12.9% burned out (>4/5), 24.9% at risk for burnout (34/5), 62.2% at no current risk (mean, 2.86 on 15 scale) | |||
| Trichard, 2005 [90] | Hospital doctors in France | MBI | 199 | 16 (10.7) | 6.6 (5.7) | 38.5 (6.5) | |
| Gandini, 2006 [65]d | Hospital doctors in Argentina | MBI | 290 | 25.0 (12.7) | 7.9 (6.2) | 40.1 (7.0) | |
| Dunwoodie, 2007 [68]d | Palliative care doctors in Australia | MBI | 14 | 18.29 (14.24) | 5.29 (5.89) | 38.86 (3.42) | |
| Srgaard, 2007 [69]d | Psychiatrists in 5 European nations | MBI | 18 | 18.56 (9.32) | 5.50 (3.79) | 39.08 (5.39) | |
| Sosa Oberlin, 2007 [56]d | Hospital doctors in Argentina | Author‐designed instrument | 3 | 3 (100%) had 4 burnout symptoms, 8.67 symptoms per physician | |||
| Voltmer, 2007 [57]d | Hospital doctors in Germany | AVEM | 271 | 77 (28.4%) exhibited burnout (type B) pattern | |||
| dm, 2008 [70]b | Physicians of various specialties in Hungary | MBI | 194 | 19.23 (10.79) | 4.88 (4.61) | 35.26 (8.42) | |
| Di Iorio, 2008 [71]d | Dialysis physicians in Italy | Author‐designed instrument | 62 | Work, mean (SD), 3.1 (1.4); Material, mean (SD), 3.3 (1.5); Climate, mean (SD), 2.9 (1.1); Objectives, mean (SD), 2.5 (1.5); Quality, mean (SD), 3.0 (1.1); Justification, mean (SD), 3.1 (2.1) | |||
| Fuss, 2008 [91]d | Hospital doctors in Germany | Copenhagen Burnout Inventory | 292 | Mean Copenhagen Burnout Inventory, mean (SD), 46.90 (18.45) | |||
| Marner, 2008 [92]d | Psychiatrists and 1 generalist in United States | MBI | 9 | 20.67 (9.75) | 7.78 (5.14) | 35.33 (6.44) | |
| Shehabi, 2008 [93]d | Intensivists in Australia | Modified MBI (6 items; scale, 15) | 86 | 2.85 (0.93) | 2.64 (0.85) | 2.58 (0.83) | |
| Bressi, 2009 [76]d | Psychiatrists in Italy | MBI | 28 | 17.89 (14.46) | 5.32 (7.01) | 34.57 (11.27) | |
| Brown, 2009 [94] | Hospital doctors in Australia | MBI | 12 | 22.25 (8.59) | 6.33 (2.71) | 39.83 (7.31) | |
| Lasalvia, 2009 [55]d | Psychiatrists in Italy | Modified MBI (16 items; scale, 06) | 21 | 1.95 (1.04) | 1.35 (0.85) | 4.46 (1.04) | |
| Peisah, 2009 [79]d | Hospital doctors in Australia | MBI | 62 | 20.09 (9.91) | 6.34 (4.90) | 35.06 (7.33) | |
| Shanafelt, 2009 [80]d | Hospitalists and intensivists in United States | MBI | 19 | 25.2 (11.59) | 4.4 (3.79) | 38.5 (8.04) | |
| Tunc, 2009 [95] | Hospital doctors in Turkey | Modified MBI (22 items; scale, 04) | 62 | 1.18 (0.78) | 0.81 (0.73) | 3.10 (0.59)e | |
| Cocco, 2010 [96]d | Hospital geriatricians in Italy | MBI | 38 | 16.21 (11.56) | 4.53 (4.63) | 39.13 (7.09) | |
| Doppia, 2011 [97]d | Hospital doctors in France | Copenhagen Burnout Inventory | 1,684 | Mean work‐related burnout score, 2.72 (0.75) | |||
| Glasheen, 2011 [98] | Hospitalists in United States | Single‐item survey | 265 | Mean, 2.08 on 15 scale 62 (23.4%) burned out | |||
| Lucas, 2011 [84]d | Academic hospitalists in United States | MBI (EE only) | 26 | 19.54 (12.85) | |||
| Thorsen, 2011 [99] | Hospital doctors in Malawi | MBI | 2 | 25.5 (4.95) | 8.5 (6.36) | 25.0 (5.66) | |
| Hinami, 2012 [50]d | Hospital doctors in United States | Single‐item survey | 793 | Mean, 2.24 on 15 scale 261 (27.2%) burned out | |||
| Quenot, 2012 [100]d | Intensivists in France | MBI | 4 | 33.25 (4.57) | 13.50 (5.45) | 35.25 (4.86) | |
| Ruitenburg, 2012 [101] | Hospital doctors in the Netherlands | MBI (EE and DP only) | 214 | 13.3 (8.0) | 4.5 (4.1) | ||
| Seibt, 2012 [102]d | Hospital doctors in Germany | Modified MBI (16 items; scale, 06, reported per item rather than totals) | 2,154 | 2.2 (1.4) | 1.4 (1.2) | 5.1 (0.9) | |
| Shanafelt, 2012 [87]d | Hospitalists in United States | MBI | 130 | 24.7 (12.5) | 9.1 (6.9) | 39.0 (7.6) | |
Table 3 summarizes the results of the 15 studies that reported burnout data for both inpatient and outpatient physicians, allowing direct comparisons to be made. Nine studies reported MBI subset totals with standard deviations, 2 used different modifications of the MBI, 2 used different author‐derived measures, 1 used only the emotional exhaustion subscale of the MBI, and 1 used the Arbeitsbezogenes Verhaltens und Erlebensmuster. Therefore, statistical comparison was attempted only for the 9 studies reporting comparable MBI data, comprising burnout data on 1390 outpatient physicians and 899 inpatient physicians.
| Lead Author, Publication Year | Location | Instrument | Inpatient‐Based Physicians | Outpatient‐Based Physicians | ||
|---|---|---|---|---|---|---|
| No. | Results, Score (SD)a | No. | Results, Score (SD)a | |||
| ||||||
| Aasland, 1997 [54]b | Norway | Modified MBI (22 items; scale, 15) | 582 | EE, 2.39 (0.80); DP, 1.81 (0.65); PA, 3.51 (0.46) | 298 | EE, 2.65 (0.80); DP, 1.90 (0.59); PA, 3.45 (0.40) |
| Grassi, 2000 [58] | Italy | MBI | 146 | EE, 16.17 (9.64); DP, 5.32 (4.76); PA, 38.71 (7.28) | 182 | EE, 18.49 (11.49); DP, 6.11 (5.86); PA, 38.52 (7.60) |
| Gandini, 2006 [65]b | Argentina | MBI | 290 | EE, 25.0 (12.7);DP, 7.9 (6.2); PA, 40.1 (7.0) | 67 | EE, 31.0 (13.8); DP, 10.2 (6.6); PA, 38.4 (6.8) |
| Dunwoodie, 2007 [68]b | Australia | MBI | 14 | EE, 18.29 (14.24); DP, 5.29 (5.89); PA, 38.86 (3.42) | 21 | EE, 14.95 (9.14); DP, 3.95 (3.40); PA, 38.90 (2.88) |
| Srgaard, 2007 [69]b | 5 European nations | MBI | 18 | EE, 18.56 (9.32); DP, 5.50 (3.79); PA, 39.08 (5.39) | 22 | EE, 19.41 (8.08); DP, 6.68 (4.93); PA, 39.00 (4.40) |
| Sosa Oberlin, 2007 [56]b | Argentina | Author‐designed instrument | 3 | 3 (100%) had 4 burnout symptoms, 8.67 symptoms per physician | 33 | 26 (78.8%) had 4 burnout symptoms, 6.15 symptoms per physician |
| Voltmer, 2007 [57]b | Germany | AVEM | 271 | 77 (28.4%) exhibited burnout (type B) pattern | 46 | 11 (23.9%) exhibited burnout (type B) pattern |
| dm, 2008 [70]b | Hungary | MBI | 194 | EE, 19.23 (10.79); DP, 4.88 (4.61); PA, 35.26 (8.42) | 163 | EE, 17.45 (11.12); DP, 4.86 (4.91); PA, 36.56 (7.03) |
| Di Iorio, 2008 [71]b | Italy | Author‐designed instrument | 62 | Work: 3.1 (1.4); material: 3.3 (1.5); climate: 2.9 (1.1); objectives: 2.5 (1.5); quality: 3.0 (1.1); justification: 3.1 (2.1) | 54 | Work: 2.6 (1.5); material: 3.1 (2.1); climate: 3.0 (1.1); objectives: 3.4 (1.6); quality: 2.2 (1.5); justification: 3.2 (2.0) |
| Bressi, 2009 [76]b | Italy | MBI | 28 | EE, 17.89 (14.46); DP, 5.32 (7.01); PA, 34.57 (11.27) | 53 | EE, 23.15 (11.99); DP, 7.02 (6.29); PA, 36.41 (7.54) |
| Lasalvia, 2009[55]b | Italy | Modified MBI (16 items; scale, 06) | 21 | EE, 1.95 (1.04); DP, 1.35 (0.85); PA, 4.46 (1.04) | 38 | EE, 2.37 (1.27); DP, 1.51 (1.15); PA, 4.46 (0.87) |
| Peisah, 2009 [79]b | Australia | MBI | 62 | EE, 20.09 (9.91); DP, 6.34 (4.90); PA, 35.06 (7.33) | 28 | EE, 13.92 (9.24); DP, 3.66 (3.95); PA, 39.34 (8.55) |
| Shanafelt, 2009 [80]b | United States | MBI | 19 | EE, 25.2 (11.59); DP, 4.4 (3.79); PA, 38.5 (8.04) | 408 | EE, 20.5 (11.10); DP, 4.3 (4.74); PA, 40.8 (6.26) |
| Lucas, 2011 [84]b | United States | MBI (EE only) | 26 | EE, 19.54 (12.85) | 30 | EE, 24.37 (14.95) |
| Shanafelt, 2012 [87]b | United States | MBI | 130 | EE, 24.7 (12.5); DP, 9.1 (6.9); PA, 39.0 (7.6) | 447 | EE, 25.4 (14.0); DP, 7.5 (6.3); PA, 41.4 (6.0) |
Figure 2 shows that no significant difference existed between the groups regarding emotional exhaustion (mean difference, 0.11 points on a 54‐point scale; 95% confidence interval [CI], 2.40 to 2.61; P=0.94). In addition, there was no significant difference between the groups regarding depersonalization (Figure 3; mean difference, 0.00 points on a 30‐point scale; 95% CI, 1.03 to 1.02; P=0.99) and personal accomplishment (Figure 4; mean difference, 0.93 points on a 48‐point scale; 95% CI, 0.23 to 2.09; P=0.11).



We used meta‐regression to allow the incorporation of single‐armed MBI studies. Whether single‐armed studies were analyzed separately (15 outpatient studies comprising 3927 physicians, 4 inpatient studies comprising 300 physicians) or analyzed with double‐armed studies (24 outpatient arms comprising 5318 physicians, 13 inpatient arms comprising 1301 physicians), the lack of a significant difference between the groups persisted for the depersonalization and personal accomplishment scales (Figure 5). Emotional exhaustion was significantly higher in outpatient physicians when single‐armed studies were considered separately (mean difference, 6.36 points; 95% CI, 2.24 to 10.48; P=0.002), and this difference persisted when all studies were combined (mean difference, 3.00 points; 95% CI, 0.05 to 5.94, P=0.046).

Subgroup analysis by geographic location showed US outpatient physicians had a significantly higher personal accomplishment score than US inpatient physicians (mean difference, 2.38 points; 95% CI, 1.22 to 3.55; P<0.001) in double‐armed studies. This difference did not persist when single‐armed studies were included through meta‐regression (mean difference, 0.55 points, 95% CI, 4.30 to 5.40, P=0.83).
Table 4 demonstrates that methodological quality was generally good from the standpoint of the reporting and bias subsections of the Downs and Black tool. External validity was scored lower for many studies due to the use of convenience samples and lack of information about physicians who declined to participate.
| Lead Author, Publication Year | Reporting | External Validity | Internal Validity: Bias | Internal Validity: Confounding | Power |
|---|---|---|---|---|---|
| Schweitzer, 1994 [12] | 5 of 6 points | 1 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Varga, 1996 [88] | 5 of 6 points | 1 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Aasland, 1997 [54] | 3 of 6 points | 2 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Bargellini, 2000 [89] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Grassi, 2000 [58] | 6 of 6 points | 0 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| McManus, 2000 [59] | 5 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Hoff, 2001 [33] | 6 of 6 points | 2 of 2 points | 2 of 4 points | 1 of 1 point | 0 of 1 point |
| Yaman, 2002 [60] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Cathbras, 2004 [61] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Kushnir, 2004 [62] | 5 of 6 points | 0 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Goehring, 2005 [63] | 6 of 6 points | 1 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Trichard, 2005 [90] | 3 of 6 points | 1 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Esteva, 2006 [64] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Gandini, 2006 [65] | 6 of 6 points | 1 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Ozyurt, 2006 [66] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Deighton, 2007 [67] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Dunwoodie, 2007 [68] | 5 of 6 points | 2 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Srgaard, 2007 [69] | 6 of 6 points | 0 of 2 points | 3 of 4 points | 1 of 1 point | 1 of 1 point |
| Sosa Oberlin, 2007 [56] | 4 of 6 points | 0 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Voltmer, 2007 [57] | 4 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| dm, 2008 [70] | 5 of 6 points | 2 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Di Iorio, 2008 [71] | 6 of 6 points | 0 of 2 points | 2 of 4 points | 0 of 1 point | 0 of 1 point |
| Fuss, 2008 [91] | 6 of 6 points | 0 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Lee, 2008 [49] | 4 of 6 points | 2 of 2 points | 4 of 4 points | 0 of 1 point | 1 of 1 point |
| Marner, 2008 [92] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Shehabi, 2008 [93] | 3 of 6 points | 1 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Truchot, 2008 [72] | 5 of 6 points | 1 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Twellaar, 2008 [73] | 6 of 6 points | 2 of 2 points | 3 of 4 points | 0 of 1 point | 0 of 1 point |
| Vela‐Bueno, 2008 [74] | 5 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Arigoni, 2009 [17] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Bernhardt, 2009 [75] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Bressi, 2009 [76] | 6 of 6 points | 0 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Brown, 2009 [94] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Krasner, 2009 [77] | 9 of 11 points | 0 of 3 points | 6 of 7 points | 1 of 2 points | 1 of 1 point |
| Lasalvia, 2009 [55] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Lesic, 2009 [78] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Peisah, 2009 [79] | 6 of 6 points | 2 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Shanafelt, 2009 [80] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Tunc, 2009 [95] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Zantinge, 2009 [81] | 5 of 6 points | 0 of 2 points | 3 of 4 points | 1 of 1 point | 0 of 1 point |
| Cocco, 2010 [96] | 4 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Demirci, 2010 [82] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Voltmer, 2010 [83] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Doppia, 2011 [97] | 5 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Glasheen, 2011 [98] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Lucas, 2011 [84] | 10 of 11 points | 2 of 3 points | 7 of 7 points | 5 of 6 points | 1 of 1 point |
| Maccacaro, 2011 [85] | 5 of 6 points | 0 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Putnik, 2011 [86] | 6 of 6 points | 1 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Thorsen, 2011 [99] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Hinami, 2012 [50] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 1 of 1 point |
| Quenot, 2012 [100] | 8 of 11 points | 1 of 3 points | 6 of 7 points | 1 of 2 points | 0 of 1 point |
| Ruitenburg, 2012 [101] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 0 of 1 point | 0 of 1 point |
| Seibt, 2012 [102] | 6 of 6 points | 0 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
| Shanafelt, 2012 [87] | 6 of 6 points | 2 of 2 points | 4 of 4 points | 1 of 1 point | 0 of 1 point |
Funnel plots were used to evaluate for publication bias in the meta‐analysis of the 8 double‐armed studies (Figure 6). We found no significant evidence of bias, which was supported by Begg's test P values of 0.90 for emotional exhaustion, >0.99 for depersonalization, and 0.54 for personal accomplishment. A trim‐and‐fill analysis determined that no adjustment was necessary.

DISCUSSION
There appears to be no support for the long‐held belief that inpatient physicians are particularly prone to burnout. Among studies for which practice location was stated explicitly or could be obtained from the authors, and who used the MBI, no differences were found among inpatient and outpatient physicians with regard to depersonalization or personal accomplishment. This finding persisted whether double‐armed studies were compared directly, single‐armed studies were incorporated into this analysis, or single‐armed studies were analyzed separately. Outpatient physicians had a higher degree of emotional exhaustion when all studies were considered.
There are several reasons why outpatient physicians may be more prone to emotional exhaustion than their inpatient colleagues. Although it is by no means true that all inpatient physicians work in shifts, the increased availability of shift work may allow some inpatient physicians to better balance their professional and personal lives, a factor of work with which some outpatient physicians have struggled.[47] Inpatient practice may also afford more opportunity for teamwork, a factor that has been shown to correlate with reduced burnout.[48] When surveyed about burnout, outpatient physicians have cited patient volumes, paperwork, medicolegal concerns, and lack of community support as factors.[49] Inpatient physicians are not immune to these forces, but they arguably experience them to different degrees.
The absence of a higher rate of depersonalization among inpatient physicians is particularly reassuring in light of concerns expressed with the advent of US hospital medicinethat some hospitalists would be prone to viewing patients as an impediment to the efficient running of the hospital,[2] the very definition of depersonalization.
Although the difference in the whole sample was not statistically significant, the consistent tendency toward a greater sense of personal accomplishment among outpatient physicians is also noteworthy, particularly because post hoc subgroup analysis of US physicians did show statistical significance in both 2‐armed studies. Without detailed age data for the physicians in each study, we could not separate the possible impact of age on personal accomplishment; hospital medicine is a newer specialty staffed by generally younger physicians, and hospitalists may not have had time to develop a sense of accomplishment. When surveyed about job satisfaction, hospitalists have also reported the feeling that they were treated as glorified residents,[50] a factor that, if shared by other inpatient physicians, must surely affect their sense of personal accomplishment. The lack of longitudinal care for patients and the substantial provision of end‐of‐life care also may diminish the sense of personal accomplishment among inpatient physicians.
Another important finding from this systematic review is the marked heterogeneity of the instruments used to measure physician burnout. Many of the identified studies could not be subjected to meta‐analysis because of their use of differing burnout measures. Drawing more substantial conclusions about burnout and practice location is limited by the fact that, although the majority of studies used the full MBI, the largest study of European hospital doctors used the Copenhagen Burnout Inventory, and the studies thus far of US hospitalists have used single‐item surveys or portions of the MBI. Not reflected in this review is the fact that a large study of US burnout and job satisfaction[51] did not formally address practice location (M. Linzer, personal communication, August 2012). Similarly, a large study of British hospital doctors[52] is not included herein because many of the physicians involved had substantial outpatient duties (C. Taylor, personal communication, July 2012). Varying burnout measures have complicated a previous systematic review of burnout in oncologists.[53] Two studies that directly compared inpatient and outpatient physicians but that were excluded from our statistical analysis because of their modified versions of the MBI,[54, 55] showed higher burnout scores in outpatient physicians. Two other studies that provided direct inpatient versus outpatient comparisons but that used alternative burnout measures[56, 57] showed a greater frequency of burnout in inpatient physicians, but of these, 1 study[56] involved only 3 inpatient physicians.
Several limitations of our study should be considered. Although we endeavored to obtain information from authors (with some success) about specific local practice patterns and eliminated many studies because of incomplete data or mixed practice patterns (eg, general practitioners who take frequent hospital calls, hospital physicians with extensive outpatient duties in a clinic attached to their hospital), it remains likely that many physicians identified as outpatient provided some inpatient care (attending a few weeks per year on a teaching service, for example) and that some physicians identified as inpatient have minimal outpatient duties.
More importantly, the dataset analyzed is heterogeneous. Studies of the incidence of burnout are naturally observational and therefore not randomized. Inclusion of international studies is necessary to answer the research question (because published data on US hospitalists are sparse) but naturally introduces differences in practice settings, local factors, and other factors for which we cannot possibly account fully.
Our meta‐analysis therefore addressed a broad question about burnout among inpatient and outpatient physicians in various diverse settings. Applying it to any 1 population (including US hospitalists) is, by necessity, imprecise.
Post hoc analysis should be viewed with caution. For example, the finding of a statistical difference between US inpatient and outpatient physicians with regard to personal accomplishment score is compelling from the standpoint of hypothesis generation. However, it is worth bearing in mind that this analysis contained only 2 studies, both by the same primary author, and compared 855 outpatient physicians to only 149 hospitalists. This difference was no longer significant when 2 outpatient studies were added through meta‐regression.
Finally, the specific focus of this study on practice location precluded comparison with emergency physicians and anesthesiologists, 2 specialist types that have been the subject of particularly robust burnout literature. As the literature on hospitalist burnout becomes more extensive, comparative studies with these groups and with intensivists might prove instructive.
In summary, analysis of 24 studies comprising data on 5318 outpatient physicians and 1301 inpatient physicians provides no support for the commonly held belief that hospital‐based physicians are particularly prone to burnout. Outpatient physicians reported higher emotional exhaustion. Further studies of the incidence and severity of burnout according to practice location are indicated. We propose that in future studies, to avoid the difficulties with statistical analysis summarized herein, investigators ask about and explicitly report practice location (inpatient vs outpatient vs both) and report mean MBI subset data and standard deviations. Such information about US hospitalists would allow comparison with a robust (if heterogeneous) international literature on burnout.
Acknowledgments
The authors gratefully acknowledge all of the study authors who contributed clarification and guidance for this project, particularly the following authors who provided unpublished data for further analysis: Olaf Aasland, MD; Szilvia dm, PhD; Annalisa Bargellini, PhD; Cinzia Bressi, MD, PhD; Darrell Campbell Jr, MD; Ennio Cocco, MD; Russell Deighton, PhD; Senem Demirci Alanyali, MD; Biagio Di Iorio, MD, PhD; David Dunwoodie, MBBS; Sharon Einav, MD; Madeleine Estryn‐Behar, PhD; Bernardo Gandini, MD; Keiki Hinami, MD; Antonio Lasalvia, MD, PhD; Joseph Lee, MD; Guido Maccacaro, MD; Swati Marner, EdD; Chris McManus, MD, PhD; Carmelle Peisah, MBBS, MD; Katarina Putnik, MSc; Alfredo Rodrguez‐Muoz, PhD; Yahya Shehabi, MD; Evelyn Sosa Oberlin, MD; Jean Karl Soler, MD, MSc; Knut Srgaard, PhD; Cath Taylor; Viva Thorsen, MPH; Mascha Twellaar, MD; Edgar Voltmer, MD; Colin West, MD, PhD; and Deborah Whippen. The authors also thank the following colleagues for their help with translation: Dusanka Anastasijevic (Norwegian); Joyce Cheung‐Flynn, PhD (simplified Chinese); Ales Hlubocky, MD (Czech); Lena Jungheim, RN (Swedish); Erez Kessler (Hebrew); Kanae Mukai, MD (Japanese); Eliane Purchase (French); Aaron Shmookler, MD (Russian); Jan Stepanek, MD (German); Fernando Tondato, MD (Portuguese); Laszlo Vaszar, MD (Hungarian); and Joseph Verheidje, PhD (Dutch). Finally, the authors thank Cynthia Heltne and Diana Rogers for their expert and tireless library assistance, Bonnie Schimek for her help with figures, and Cindy Laureano and Elizabeth Jones for their help with author contact.
- . Just because you can, doesn't mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398–402.
- . Hospitalists. Hospitalist. 1998;1(4):5–6.
- , . The occupational hazards of emergency physicians. Am J Emerg Med. 2000;18(3):300–311.
- , , , et al. Physician burnout in pediatric critical care medicine. Crit Care Med. 1995;23(8):1425–1429.
- , . The hospitalist: new boon for internal medicine or retreat from primary care? Ann Intern Med. 1999;130(4 pt 2): 382–387.
- , , , et al. Enquete sur les horaires et la charge de travail des medecins dans un establissement de l'Assitance Publique‐Hopitaux de Paris. Arch Mal Prof. 1996;57:438–444.
- , , , , . Burn‐out of urologists in the county of Schleswig‐Holstein, Germany: a comparison of hospital and private practice urologists. J Urol. 2001;165(4): 1158–1161.
- , , , et al. Satisfaction and worklife of academic hospitalist and non‐hospitalist attendings on general medical inpatient rotations. J Gen Internal Med. 2006;21(S4):128.
- , , , , , ; SGIM Career Satisfaction Group. What effect does increasing inpatient time have on outpatient‐oriented internist satisfaction? J Gen Intern Med. 2003;18(9):725–729.
- , , . Burnout and career‐choice regret among family practice physicians in early practice. Fam Pract Res J. 1994;14(3):213–222.
- , , , , , . Job stress and satisfaction among palliative physicians. Palliat Med. 1996; 10(3):185–194.
- . Stress and burnout in junior doctors. S Afr Med J. 1994; 84(6):352–354.
- , . Work stress, satisfaction and burnout in New Zealand radiologists: comparison of public hospital and private practice in New Zealand. J Med Imaging Radiat Oncol. 2009;53(2):194–199.
- , , . Measurement of hypothetical burnout in cystic fibrosis caregivers. Acta Paediatr Scand. 1981; 70(6):935–939.
- , . Personality characteristics and proneness to burnout: a study among internists. Stress Med. 1987;3(4):307–315.
- , , . Thriving and surviving in a new medical career: the case of hospitalist physicians. J Health Soc Behav. 2002;43(1):72–91.
- , , , , . Prevalence of burnout among Swiss cancer clinicians, paediatricians and general practitioners: who are most at risk? Support Care Cancer. 2009;17(1): 75–81.
- , , , . Preparing for “diastole”: advanced training opportunities for academic hospitalists. J Hosp Med. 2006;1(6):368–377.
- , , , , , . Mental health, “burnout” and job satisfaction among hospital and community‐based mental health staff. Br J Psychiatry. 1996;169(3):334–337.
- , , , . Role of a pediatric department chair: factors leading to satisfaction and burnout. J Pediatr. 2007;151(4):425–430.
- . Staff burn‐out. J Soc Iss. 1974;30(1):159–165.
- . Burned‐out. Hum Behav. 1976;5(9):16–22.
- . Underpaid women, stressed out men, satisfied emergency physicians. Ann Emerg Med. 2008;51(6):729–731.
- , , , . U.S. physician satisfaction: a systematic review. J Hosp Med. 2009;4(9):560–568.
- , , , . Professional burnout among head and neck surgeons: results of a survey. Head Neck. 1993;15(6):557–560.
- , . The measurement of experienced burnout. J Occup Behav. 1981;2(2):99–113.
- , , , . The Copenhagen Burnout Inventory: a new tool for the assessment of burnout. Work Stress. 2005;19(3):192–207.
- , . Handleiding van de Utrechtse Burnout Schaal (UBOS). Lisse, the Netherlands: Swets Test Services; 2000.
- . Alberta Physician Burnout [master's thesis]. Alberta, Canada: The University of Lethbridge; 2003.
- , . Arbeitsbezogenes Verhaltensund Erlebensmuster AVEM. Frankfurt, Germany: Swets Test Services; 2003.
- , , , . Internal construct validity of the Shirom‐Melamed Burnout Questionnaire (SMBQ). BMC Public Health. 2012;12:1.
- , , . Validation of a single‐item measure of burnout against the Maslach Burnout Inventory among physicians. Stress Health. 2004;20(2):75–79.
- , , , , . Characteristics and work experiences of hospitalists in the United States. Arch Intern Med. 2001;161(6):851–858.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , . Including nonrandomized studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1. The Cochrane Collaboration; 2008. Available at: www.cochrane‐handbook. org. Accessed July 24, 2013.
- , . Statistical Methods in Epidemiology. New York, NY: Oxford University Press; 1989.
- , . Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539–1558.
- , , , . Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557–560.
- , , . Can we individualize the “number needed to treat”? An empirical study of summary effect measures in meta‐analyses. Int J Epidemiol. 2002;31(1):72–76.
- , . Applications of estimating treatment effects in metaanalyses with missing data. Technical Report No. 2000‐25, 2000. Available at: http://statistics.stanford.edu/_ckirby/techreports/GEN/2000/2000‐25.pdf. Accessed July 22, 2013.
- , , . Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119–1129.
- , . Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101.
- , , , et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009; 339:b2700.
- , , , , , . Job satisfaction and burnout in general practitioners [in Spanish]. Aten Primaria. 2003;31(4):227–233.
- , , . Burnout syndrome among health staff [in Spanish]. Rev Med Inst Mex Seguro Soc. 2006;44(3):221–226.
- , , , , , . Differences in psychological effects in hospital doctors with and without post‐traumatic stress disorder. Br J Psychiatry. 2008;193(2):165–166.
- , , , , . Crossing boundaries: family physicians' struggles to protect their private lives. Can Fam Physician. 2009;55(3):286–287.e5.
- , , , , , , et al. Emergency physicians accumulate more stress factors than other physicians: results from the French SESMAT study. Emerg Med J. 2011 May;28(5):397–410. Epub 2010 Dec 1.
- , , . Stress, burnout, and strategies for reducing them: what's the situation among Canadian family physicians? Can Fam Physician. 2008;54(2):234–235.
- , , , , . Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27(1):28–36.
- , , , , , , et al; MEMO (Minimizing Error, Maximizing Outcome) Investigators. Working conditions in primary care: physician reactions and care quality. Ann Intern Med. 2009 Jul 7;151(1):28–36.
- , , , , . Mental health of hospital consultants: the effects of stress and satisfaction at work. Lancet. 1996 Mar 16;347(9003):724–8.
- , , , . Burnout, psychiatric morbidity, and work‐related sources of stress in paediatric oncology staff: a review of the literature. Psycho‐Oncology. 2009 Oct;18(10):1019–28.
- , , , , . Health complaints and job stress in Norwegian physicians: the use of an overlapping questionnaire design. Soc Sci Med. 1997;45(11):1615–1629.
- , , , et al. Influence of perceived organisational factors on job burnout: survey of community mental health staff. Br J Psychiatry. 2009;195(6):537–544.
- . Frecuencia de los sintomas del syndrome de burnout en profesionales medicos. Rev Med Rosario. 2007;73:12–20.
- , , . Work‐related behaviour and experience patterns of physicians compared to other professions. Swiss Med Wkly. 2007;137(31‐32):448–453.
- , . Psychiatric morbidity and burnout in the medical profession: an Italian study of general practitioners and hospital physicians. Psychother Psychosom. 2000;69(6):329–334.
- , , . Duties of a doctor: UK doctors and good medical practice. Qual Health Care. 2000;9(1):14–22.
- , . The job related burnout questionnaire: a multinational pilot study. Aust Fam Physician. 2002;31(11):1055–1056.
- , , , , . Burn out among French general practitioners [in French]. Presse Med. 2004;33(22): 1569–1574.
- , , . Are burnout levels increasing? The experience of Israeli primary care physicians. Isr Med Assoc J. 2004; 6(8):451–455.
- , , , . Psychosocial and professional characteristics of burnout in Swiss primary care practitioners: a cross‐sectional survey. Swiss Med Wkly. 2005;135(7‐8):101–108.
- , , . Mental health in family doctors: effects of satisfaction and stress at work [in Spanish]. Rev Clin Esp. 2006;206(2):77–83.
- , , , , . The professional wearing down or syndrome of welfare labor stress (“burnout”) among health professionals in the city of Cordoba [in Spanish]. Rev Fac Cien Med Univ Nac Cordoba. 2006;63(1):18–25.
- , , . Predictors of burnout and job satisfaction among Turkish physicians. QJM. 2006;99(3):161–169.
- , , . Factors affecting burnout and compassion fatigue in psychotherapists treating torture survivors: is the therapist's attitude to working through trauma relevant? J Trauma Stress. 2007;20(1):63–75.
- , . Psychological morbidity and burnout in palliative care doctors in Western Australia. Intern Med J. 2007;37(10): 693–698.
- , , , ; OSCAR Group. Sources of stress and burnout in acute psychiatric care: inpatient vs. community staff. Soc Psychiatry Psychiatr Epidemiol. 2007;42(10):794–802.
- , , . Physician burnout in Hungary: a potential role for work‐family conflict. J Health Psychol. 2008;13:847–856.
- , , , . Burn‐out in the dialysis unit. J Nephrol. 2008;21(suppl 13):S158–S162.
- . Career orientation and burnout in French general practitioners. Psychol Rep. 2008;103(3):875–881.
- , , . How healthy are Dutch general practitioners? Self‐reported (mental) health among Dutch general practitioners. Eur J Gen Pract. 2008;14(1):4–9.
- , , , et al. Insomnia and sleep quality among primary care physicians with low and high burnout levels. J Psychosom Res. 2008;64(4):435–442.
- , , , , , . Distress and burnout among genetic service providers. Genet Med. 2009;11(7):527–535.
- , , , et al. Burnout among psychiatrists in Milan: a multicenter survey. Psychiatr Serv. 2009;60(7):985–988.
- , , , et al. Association of an educational program in mindful communication with burnout, empathy, and attitudes among primary care physicians. JAMA. 2009;302(12): 1284–1293.
- , , , , , . Burnout in Belgrade orthopaedic surgeons and general practitioners, a preliminary report. Acta Chir Iugosl. 2009; 56(2):53–59.
- , , , . Secrets to psychological success: why older doctors might have lower psychological distress and burnout than younger doctors. Aging Ment Health. 2009;13(2):300–307.
- , , , et al. Career fit and burnout among academic faculty. Arch Intern Med. 2009;169(10):990–995.
- , , , , . Does burnout among doctors affect their involvement in patients' mental health problems? A study of videotaped consultations. BMC Fam Pract. 2009;10:60.
- , , , , , . Evaluation of burnout syndrome in oncology employees. Med Oncol. 2010;27(3):968–974.
- , , , , . Workrelated behavior and experience patterns and predictors of mental health in German physicians in medical practice. Fam Med. 2010; 42(6):433–439.
- , , , et al. Emotional exhaustion, life stress, and perceived control among medicine ward attending physicians: a randomized trial of 2‐ versus 4‐week ward rotations [abstract]. J Hosp Med. 2011; 6(4 suppl 2):S43–S44.
- , , , , , . The effort of being male: a survey on gender and burnout [in Italian]. Med Lav. 2011;102(3):286–296.
- , . Word related characteristics, work‐home and home‐work interference and burnout among primary healthcare physicians: a gender perspective in a Serbian context. BMC Public Health. 2011;11:716.
- , , , et al. Burnout and satisfaction with work‐life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172(18):1377–1385.
- , , . Burnout syndrome in general hospital doctors. Eur J Psychiat. 1996;10:207–213.
- , , , , , . Relation between immune variables and burnout in a sample of physicians. Occup Environ Med. 2000;57(7):453–457.
- , , . Epuisement professionnel et consummation de psychotropes chez les medecins hospitaliers. Alcoologie et Addictologie. 2005;27(4):303–308.
- , , , , . Working conditions and Work‐Family Conflict in German hospital physicians: psychosocial and organisational predictors and consequences. BMC Public Health. 2008;8:353.
- . The Role of Empathy and Witnessed Aggression in Stress Reactions Among Staff Working in a Psychiatric Hospital [dissertation]. New Brunswick, NJ: Rutgers University; 2008.
- , , , , , . Burnout syndrome among Australian intensivists: a survey. Crit Care Resusc. 2008;10(4):312–315.
- , , , , , . Doctors' stress responses and poor communication performance in simulated bad‐news consultations. Acad Med. 2009;84(11):1595–1602.
- , . Role conflict, role ambiguity, and burnout in nurses and physicians at a university hospital in Turkey. Nurs Health Sci. 2009;11(4):410–416.
- . How much is geriatric caregivers burnout caring‐specific? Questions from a questionnaire survey. Clin Pract Epidemiol Ment Health. 2010;6:66–71.
- , , , , ; comite de pilotage de l'enquete SESMAT. Burnout in French doctors: a comparative study among anaesthesiologists and other specialists in French hospitals (SESMAT study) [in French]. Ann Fr Anesth Reanim. 2011;30(11):782–794.
- , , , , , . Career satisfaction and burnout in academic hospital medicine. Arch Intern Med. 2011;171(8):782–785.
- , , . High rates of burnout among maternal health staff at a referral hospital in Malawi: a cross‐sectional study. BMC Nurs. 2011;10:9.
- , , , et al. Suffering among careers working in critical care can be reduced by an intensive communication strategy on end‐of‐life practices. Intensive Care Med. 2012;38:55–61.
- , , . The prevalence of common mental disorders among hospital physicians and their association with self‐reported work ability: a cross‐sectional study. BMC Health Serv Res. 2012;12:292–298.
- , , , . Effort‐reward‐ratio and burnout risk among female teachers and hospital‐employed female physicians: a comparison between professions [in German]. Arbeitsmed Sozialmed Umweltmed. 2012;47:396–406.
- . Just because you can, doesn't mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398–402.
- . Hospitalists. Hospitalist. 1998;1(4):5–6.
- , . The occupational hazards of emergency physicians. Am J Emerg Med. 2000;18(3):300–311.
- , , , et al. Physician burnout in pediatric critical care medicine. Crit Care Med. 1995;23(8):1425–1429.
- , . The hospitalist: new boon for internal medicine or retreat from primary care? Ann Intern Med. 1999;130(4 pt 2): 382–387.
- , , , et al. Enquete sur les horaires et la charge de travail des medecins dans un establissement de l'Assitance Publique‐Hopitaux de Paris. Arch Mal Prof. 1996;57:438–444.
- , , , , . Burn‐out of urologists in the county of Schleswig‐Holstein, Germany: a comparison of hospital and private practice urologists. J Urol. 2001;165(4): 1158–1161.
- , , , et al. Satisfaction and worklife of academic hospitalist and non‐hospitalist attendings on general medical inpatient rotations. J Gen Internal Med. 2006;21(S4):128.
- , , , , , ; SGIM Career Satisfaction Group. What effect does increasing inpatient time have on outpatient‐oriented internist satisfaction? J Gen Intern Med. 2003;18(9):725–729.
- , , . Burnout and career‐choice regret among family practice physicians in early practice. Fam Pract Res J. 1994;14(3):213–222.
- , , , , , . Job stress and satisfaction among palliative physicians. Palliat Med. 1996; 10(3):185–194.
- . Stress and burnout in junior doctors. S Afr Med J. 1994; 84(6):352–354.
- , . Work stress, satisfaction and burnout in New Zealand radiologists: comparison of public hospital and private practice in New Zealand. J Med Imaging Radiat Oncol. 2009;53(2):194–199.
- , , . Measurement of hypothetical burnout in cystic fibrosis caregivers. Acta Paediatr Scand. 1981; 70(6):935–939.
- , . Personality characteristics and proneness to burnout: a study among internists. Stress Med. 1987;3(4):307–315.
- , , . Thriving and surviving in a new medical career: the case of hospitalist physicians. J Health Soc Behav. 2002;43(1):72–91.
- , , , , . Prevalence of burnout among Swiss cancer clinicians, paediatricians and general practitioners: who are most at risk? Support Care Cancer. 2009;17(1): 75–81.
- , , , . Preparing for “diastole”: advanced training opportunities for academic hospitalists. J Hosp Med. 2006;1(6):368–377.
- , , , , , . Mental health, “burnout” and job satisfaction among hospital and community‐based mental health staff. Br J Psychiatry. 1996;169(3):334–337.
- , , , . Role of a pediatric department chair: factors leading to satisfaction and burnout. J Pediatr. 2007;151(4):425–430.
- . Staff burn‐out. J Soc Iss. 1974;30(1):159–165.
- . Burned‐out. Hum Behav. 1976;5(9):16–22.
- . Underpaid women, stressed out men, satisfied emergency physicians. Ann Emerg Med. 2008;51(6):729–731.
- , , , . U.S. physician satisfaction: a systematic review. J Hosp Med. 2009;4(9):560–568.
- , , , . Professional burnout among head and neck surgeons: results of a survey. Head Neck. 1993;15(6):557–560.
- , . The measurement of experienced burnout. J Occup Behav. 1981;2(2):99–113.
- , , , . The Copenhagen Burnout Inventory: a new tool for the assessment of burnout. Work Stress. 2005;19(3):192–207.
- , . Handleiding van de Utrechtse Burnout Schaal (UBOS). Lisse, the Netherlands: Swets Test Services; 2000.
- . Alberta Physician Burnout [master's thesis]. Alberta, Canada: The University of Lethbridge; 2003.
- , . Arbeitsbezogenes Verhaltensund Erlebensmuster AVEM. Frankfurt, Germany: Swets Test Services; 2003.
- , , , . Internal construct validity of the Shirom‐Melamed Burnout Questionnaire (SMBQ). BMC Public Health. 2012;12:1.
- , , . Validation of a single‐item measure of burnout against the Maslach Burnout Inventory among physicians. Stress Health. 2004;20(2):75–79.
- , , , , . Characteristics and work experiences of hospitalists in the United States. Arch Intern Med. 2001;161(6):851–858.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , . Including nonrandomized studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1. The Cochrane Collaboration; 2008. Available at: www.cochrane‐handbook. org. Accessed July 24, 2013.
- , . Statistical Methods in Epidemiology. New York, NY: Oxford University Press; 1989.
- , . Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539–1558.
- , , , . Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557–560.
- , , . Can we individualize the “number needed to treat”? An empirical study of summary effect measures in meta‐analyses. Int J Epidemiol. 2002;31(1):72–76.
- , . Applications of estimating treatment effects in metaanalyses with missing data. Technical Report No. 2000‐25, 2000. Available at: http://statistics.stanford.edu/_ckirby/techreports/GEN/2000/2000‐25.pdf. Accessed July 22, 2013.
- , , . Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119–1129.
- , . Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101.
- , , , et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009; 339:b2700.
- , , , , , . Job satisfaction and burnout in general practitioners [in Spanish]. Aten Primaria. 2003;31(4):227–233.
- , , . Burnout syndrome among health staff [in Spanish]. Rev Med Inst Mex Seguro Soc. 2006;44(3):221–226.
- , , , , , . Differences in psychological effects in hospital doctors with and without post‐traumatic stress disorder. Br J Psychiatry. 2008;193(2):165–166.
- , , , , . Crossing boundaries: family physicians' struggles to protect their private lives. Can Fam Physician. 2009;55(3):286–287.e5.
- , , , , , , et al. Emergency physicians accumulate more stress factors than other physicians: results from the French SESMAT study. Emerg Med J. 2011 May;28(5):397–410. Epub 2010 Dec 1.
- , , . Stress, burnout, and strategies for reducing them: what's the situation among Canadian family physicians? Can Fam Physician. 2008;54(2):234–235.
- , , , , . Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27(1):28–36.
- , , , , , , et al; MEMO (Minimizing Error, Maximizing Outcome) Investigators. Working conditions in primary care: physician reactions and care quality. Ann Intern Med. 2009 Jul 7;151(1):28–36.
- , , , , . Mental health of hospital consultants: the effects of stress and satisfaction at work. Lancet. 1996 Mar 16;347(9003):724–8.
- , , , . Burnout, psychiatric morbidity, and work‐related sources of stress in paediatric oncology staff: a review of the literature. Psycho‐Oncology. 2009 Oct;18(10):1019–28.
- , , , , . Health complaints and job stress in Norwegian physicians: the use of an overlapping questionnaire design. Soc Sci Med. 1997;45(11):1615–1629.
- , , , et al. Influence of perceived organisational factors on job burnout: survey of community mental health staff. Br J Psychiatry. 2009;195(6):537–544.
- . Frecuencia de los sintomas del syndrome de burnout en profesionales medicos. Rev Med Rosario. 2007;73:12–20.
- , , . Work‐related behaviour and experience patterns of physicians compared to other professions. Swiss Med Wkly. 2007;137(31‐32):448–453.
- , . Psychiatric morbidity and burnout in the medical profession: an Italian study of general practitioners and hospital physicians. Psychother Psychosom. 2000;69(6):329–334.
- , , . Duties of a doctor: UK doctors and good medical practice. Qual Health Care. 2000;9(1):14–22.
- , . The job related burnout questionnaire: a multinational pilot study. Aust Fam Physician. 2002;31(11):1055–1056.
- , , , , . Burn out among French general practitioners [in French]. Presse Med. 2004;33(22): 1569–1574.
- , , . Are burnout levels increasing? The experience of Israeli primary care physicians. Isr Med Assoc J. 2004; 6(8):451–455.
- , , , . Psychosocial and professional characteristics of burnout in Swiss primary care practitioners: a cross‐sectional survey. Swiss Med Wkly. 2005;135(7‐8):101–108.
- , , . Mental health in family doctors: effects of satisfaction and stress at work [in Spanish]. Rev Clin Esp. 2006;206(2):77–83.
- , , , , . The professional wearing down or syndrome of welfare labor stress (“burnout”) among health professionals in the city of Cordoba [in Spanish]. Rev Fac Cien Med Univ Nac Cordoba. 2006;63(1):18–25.
- , , . Predictors of burnout and job satisfaction among Turkish physicians. QJM. 2006;99(3):161–169.
- , , . Factors affecting burnout and compassion fatigue in psychotherapists treating torture survivors: is the therapist's attitude to working through trauma relevant? J Trauma Stress. 2007;20(1):63–75.
- , . Psychological morbidity and burnout in palliative care doctors in Western Australia. Intern Med J. 2007;37(10): 693–698.
- , , , ; OSCAR Group. Sources of stress and burnout in acute psychiatric care: inpatient vs. community staff. Soc Psychiatry Psychiatr Epidemiol. 2007;42(10):794–802.
- , , . Physician burnout in Hungary: a potential role for work‐family conflict. J Health Psychol. 2008;13:847–856.
- , , , . Burn‐out in the dialysis unit. J Nephrol. 2008;21(suppl 13):S158–S162.
- . Career orientation and burnout in French general practitioners. Psychol Rep. 2008;103(3):875–881.
- , , . How healthy are Dutch general practitioners? Self‐reported (mental) health among Dutch general practitioners. Eur J Gen Pract. 2008;14(1):4–9.
- , , , et al. Insomnia and sleep quality among primary care physicians with low and high burnout levels. J Psychosom Res. 2008;64(4):435–442.
- , , , , , . Distress and burnout among genetic service providers. Genet Med. 2009;11(7):527–535.
- , , , et al. Burnout among psychiatrists in Milan: a multicenter survey. Psychiatr Serv. 2009;60(7):985–988.
- , , , et al. Association of an educational program in mindful communication with burnout, empathy, and attitudes among primary care physicians. JAMA. 2009;302(12): 1284–1293.
- , , , , , . Burnout in Belgrade orthopaedic surgeons and general practitioners, a preliminary report. Acta Chir Iugosl. 2009; 56(2):53–59.
- , , , . Secrets to psychological success: why older doctors might have lower psychological distress and burnout than younger doctors. Aging Ment Health. 2009;13(2):300–307.
- , , , et al. Career fit and burnout among academic faculty. Arch Intern Med. 2009;169(10):990–995.
- , , , , . Does burnout among doctors affect their involvement in patients' mental health problems? A study of videotaped consultations. BMC Fam Pract. 2009;10:60.
- , , , , , . Evaluation of burnout syndrome in oncology employees. Med Oncol. 2010;27(3):968–974.
- , , , , . Workrelated behavior and experience patterns and predictors of mental health in German physicians in medical practice. Fam Med. 2010; 42(6):433–439.
- , , , et al. Emotional exhaustion, life stress, and perceived control among medicine ward attending physicians: a randomized trial of 2‐ versus 4‐week ward rotations [abstract]. J Hosp Med. 2011; 6(4 suppl 2):S43–S44.
- , , , , , . The effort of being male: a survey on gender and burnout [in Italian]. Med Lav. 2011;102(3):286–296.
- , . Word related characteristics, work‐home and home‐work interference and burnout among primary healthcare physicians: a gender perspective in a Serbian context. BMC Public Health. 2011;11:716.
- , , , et al. Burnout and satisfaction with work‐life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172(18):1377–1385.
- , , . Burnout syndrome in general hospital doctors. Eur J Psychiat. 1996;10:207–213.
- , , , , , . Relation between immune variables and burnout in a sample of physicians. Occup Environ Med. 2000;57(7):453–457.
- , , . Epuisement professionnel et consummation de psychotropes chez les medecins hospitaliers. Alcoologie et Addictologie. 2005;27(4):303–308.
- , , , , . Working conditions and Work‐Family Conflict in German hospital physicians: psychosocial and organisational predictors and consequences. BMC Public Health. 2008;8:353.
- . The Role of Empathy and Witnessed Aggression in Stress Reactions Among Staff Working in a Psychiatric Hospital [dissertation]. New Brunswick, NJ: Rutgers University; 2008.
- , , , , , . Burnout syndrome among Australian intensivists: a survey. Crit Care Resusc. 2008;10(4):312–315.
- , , , , , . Doctors' stress responses and poor communication performance in simulated bad‐news consultations. Acad Med. 2009;84(11):1595–1602.
- , . Role conflict, role ambiguity, and burnout in nurses and physicians at a university hospital in Turkey. Nurs Health Sci. 2009;11(4):410–416.
- . How much is geriatric caregivers burnout caring‐specific? Questions from a questionnaire survey. Clin Pract Epidemiol Ment Health. 2010;6:66–71.
- , , , , ; comite de pilotage de l'enquete SESMAT. Burnout in French doctors: a comparative study among anaesthesiologists and other specialists in French hospitals (SESMAT study) [in French]. Ann Fr Anesth Reanim. 2011;30(11):782–794.
- , , , , , . Career satisfaction and burnout in academic hospital medicine. Arch Intern Med. 2011;171(8):782–785.
- , , . High rates of burnout among maternal health staff at a referral hospital in Malawi: a cross‐sectional study. BMC Nurs. 2011;10:9.
- , , , et al. Suffering among careers working in critical care can be reduced by an intensive communication strategy on end‐of‐life practices. Intensive Care Med. 2012;38:55–61.
- , , . The prevalence of common mental disorders among hospital physicians and their association with self‐reported work ability: a cross‐sectional study. BMC Health Serv Res. 2012;12:292–298.
- , , , . Effort‐reward‐ratio and burnout risk among female teachers and hospital‐employed female physicians: a comparison between professions [in German]. Arbeitsmed Sozialmed Umweltmed. 2012;47:396–406.
Hospitalist Postgraduate PA Training Program
In recent years, the demand for hospitalists has outstripped the supply, creating a national shortage.1, 2 A recent Society of Hospital Medicine (SHM) survey found that in the last 2 years there has been a 31% mean growth increase in the number of hospitalist groups.3 As hospitalists are becoming more difficult to recruit, many practices are utilizing physician assistants (PAs) and nurse practitioners (NPs), collectively referred to as nonphysician providers (NPPs) to help offset the workload.4 The SHM survey also noted that the number of hospitalist groups utilizing NPPs increased from 29% to 38%.3 The exact number of NPPs working for hospitalist groups is unknown.
Hospitalist NPPs are in demand for reasons other than just physician shortages. NPPs have been utilized to fill the gap in many institutions where the workforce was impacted by the 2002 Accreditation Council for Graduate Medical Education (ACGME) ruling to restrict resident work hours. Several studies have documented NPPs' ability to assist with the compliance of ACCGME resident work‐hour restrictions while maintaining patient continuity of care, improving length of stays, and reducing health care costs on various hospital services.59 Dresselhaus et al.10 found that 56% of medical resident's time on service was delegated to tasks not related to direct patient care. They proposed that these tasks can be delegated to the NPPs, leaving more time for the residents to focus on direct patient care. In a recent study performed at a Pennsylvania hospital, patients presenting to the emergency department with low‐risk chest pain (based upon thrombolysis in myocardial infarction [TIMI] risk score) were admitted to a nonteaching service staffed with NPPs and attending physicians. Simultaneously, a similar group of low‐risk chest pain patients were admitted to a traditional internal medicine resident service. The results demonstrated lower median length of stay and hospital charges on the nonteaching service. This study suggested that NPPs can offset the workload volume for medical residents, allowing them to focus on patients with higher acuity and greater learning value.11
Barriers to Finding Experienced NPPs in Hospital Medicine
Although many hospitalist groups are interested in hiring NPPs, there can be significant obstacles to recruitment. For example, most experienced PAs and NPs have clinical backgrounds in either surgical or medical subspecialties and therefore typically need extensive on‐the‐job training in hospital medicine, which can often take at least 6 to 12 months to acquire the basic skill set.12 Hiring new graduates may require even longer training periods.
The inexperience of new graduates has become an even more pertinent issue due to recent changes in PA education. Traditionally, PA programs attracted older students with prior healthcare experience, who wished to return to school for additional training. However, in 2005 a major shift occurred in PA education: programs began transitioning from graduating trainees with a bachelor's degree to now requiring a master's level degree for completion of the PA program.13 The acquisition of more advanced degrees has changed the demographics of the students matriculating into PA programs, attracting younger students, straight from undergraduate institutions, with less prior healthcare experience.14 As a result, not only are new PA graduates less experienced overall, but they are particularly lacking in exposure to hospital medicine. After PA students complete their first 12 months of PA school in the basic sciences and didactic coursework, they embark on 12 to 15 months of clinical rotations, which are largely rooted in primary care. In fact, many PA programs find it difficult to offer hospital‐based rotations while fulfilling the required rotations in primary care. These factors have resulted in the need for more extensive on‐the‐job training particularly for those new graduates interested in hospital medicine. In light of these challenges, our institution created a 12‐month postgraduate PA fellowship program in Hospital Medicine.
Postgraduate PA Training Programs
Postgraduate PA fellowships, interchangeably called residencies, are voluntary 1‐year training programs that provide both didactic instruction and clinical experience in a medical or surgical subspecialty, thereby lessening the need for on‐the‐job training. These programs are recognized by the Association of Postgraduate Physician Assistant Programs.15 Currently, there are 44 postgraduate training programs in the United States, in a wide range of medical and surgical specialties. At the end of these 1‐year postgraduate PA programs, most graduates receive a certificate of completion. Until now, the only postgraduate education option for PAs interested in Hospital Medicine was a master's completion program only available to PAs who were already employed by a hospitalist group.15 This work reviews the first reported postgraduate hospitalist training program for PAs. Specifically, the program's background, curriculum, anticipated program outcomes, and future plans are discussed.
Background for A Hospitalist Postgraduate PA Fellowship
Mayo Clinic Arizona is a multispecialty private group comprised of both outpatient services and a tertiary care hospital medical center, located in the metropolitan Phoenix, AZ, area. The Mayo Clinic Hospital is a 7‐story facility with 244 licensed beds, 18 operating rooms, and a Level II emergency department. The Mayo Hospitalist group is composed of 15 full time hospitalists and 6 part‐time hospitalists, all of whom are salaried Mayo employees. The group provides 24‐hour in‐house staffing, covering both resident services (teams composed of interns and residents supervised by a staff hospitalist) and nonresident services (staff hospitalists). Over the years there has been steady growth in the number of nonresident services, in part due to resident work‐hour restrictions. To support the physicians working on these nonresident services, the first PA was hired in 2001. Since then, the number of NPPs in our Hospitalist group has increased to 9.35 full‐time equivalents (FTEs), including 1 nurse practitioner. However, one of the greatest challenges in expanding the NPP service was the difficulty finding candidates with experience in hospital internal medicine. This need inspired the creation of a PA fellowship in Hospital Medicine. At the time, there were 2 other postgraduate PA training programs at the Mayo Clinic Arizona in Hepatology and Otolaryngology/Ear, Nose, and Throat (ENT) Surgery.
Program Description
The Mayo Clinic Arizona PA fellowship in Hospital Medicine began in October 2007 and currently accepts 1 fellow per year. Applicants must be graduates of an Accreditation Review Commission in Education for the Physician Assistant (ARC‐PA)‐accredited PA program and be certified through the National Commission on Certification of Physician Assistants (NCCPA). Furthermore, they must be licensed to work as a PA in the state of Arizona. The program is 12 months in duration, and is comprised of both didactic and clinical components. Upon graduation, the fellow earns a certificate of completion from the Mayo Clinic College of Medicine. The program has received recognition with the Association of Postgraduate Physician Assistant Programs (APPAP).
Two physician assistants act as co‐program directors of the PA fellowship in hospital medicine. They are given 0.10 full‐time equivalent (FTE) for management of the program, which includes day‐to‐day operations, curriculum development, and candidate selection. The program also has 2 volunteer physician medical directors, both of whom have previous medical residency experience. The physicians and NPPs in our hospitalist group volunteer their time to serve as faculty for the program, assisting with much of the didactic and clinical education. The program receives a budget of $99,500 per year, which is funded by the organization's foundation through the department of education. This includes the fellow stipend of $44,000 per 12 months and institutional malpractice insurance coverage. The fellow also receives health and dental insurance, 2 weeks of paid vacation, and $500 stipend toward attendance of a continuing medical education (CME) conference.
CURRICULUM
The PA fellowship curriculum is designed in a diverse unique format that strives to accommodate all types of learners. It includes clinical rotations in various medicine/surgical subspecialties, didactic instruction, and teaching modules (Figure 1). The curriculum is based upon the SHM Core Competencies.15
Clinical Rotations
The PA fellow completes 12 to 14 general hospital medicine and medical specialty rotations, each 2 to 4 weeks in duration. The rotation calendar for the current fellow is given in Figure 2. These rotations are all inpatient‐based and are supervised by either the hospitalist or the respective inpatient subspecialists. The PA fellow's specific clinical responsibilities vary from rotation to rotation, and are designed to maximize the fellow's exposure to that particular specialty. Each rotation has specific written objectives created by the program directors and reviewed by the rotation's preceptor(s) (Figure 2). During the clinical rotations, complementary didactic lectures, coursework, and readings are provided to ensure the PA fellow receives a strong foundation. Didactic instruction is designed by the program directors, physician preceptors and staff NPPs, and is coordinated with the clinical rotation specialty. At the end of each rotation the fellow is evaluated by the preceptor and given direct feedback on their performance.

Didactic Instruction
The didactic instruction is organized in a system‐based manner and occurs on a weekly basis during the Hospital Internal Medicine service and Medicine Consults rotations. Hospitalist NPPs and physician faculty are responsible for most of the teaching. This formal didactic instruction is supplemented by journal club presentations given by the PA fellow to faculty in the division of hospital internal medicine. The fellow is also required to attend daily medical resident lunchtime educational lectures, weekly medical grand rounds, and any lectures provided by the medicine subspecialties while the PA is on that particular rotation.
Teaching Modules
One component of the Hospital Medicine PA fellowship curriculum that may be unique is the concept of teaching modules. While receiving regular didactic instruction and completing their clinical rotations, the PA is also expected to complete self‐directed teaching module assignments. These modules serve to educate the PA fellow on the hospital as a systemthe true essence of hospital medicine. The modules cover a variety of topics not directly addressed during their rotations. These topics are outlined in Figure 3. Each teaching module consists of a didactic component, clinical application, and assessment (Figure 4) and has its own specific objectives and goals. Teaching modules are often taught by the local expert in the hospital in that particular area. For example, for the infectious control teaching module, the PA fellow will rotate with the infection control nursing staff learning about the isolation and infection control policies of the institution.

Assessment Tools
There are several tools utilized to assess both the PA fellow and the fellowship program itself (Figure 5). The assessment tools used include both ongoing and summative assessments. To fulfill the ongoing assessment, each rotation and teaching module contains assessment tools provided by the preceptor, which are reviewed by the program directors. Additionally, during the clinical rotations, skills are assessed using competency checklists that require the preceptor to directly observe the PA fellow perform a specific task or skill‐set and sign off on its successful completion (Supplementary Figures 6, 7).

There are 2 forms of summative assessment for the PA fellow. First, to assess the PA fellow's knowledge, comprehensive mid‐year and end‐year examinations are utilized. These multiple‐choice examinations are comprised of questions which align with the didactic lectures/objectives provided by the Hospital Medicine faculty throughout the year. The second form of summative evaluation of the fellow is project‐based and divided into 2 parts. First, the fellow is expected to write a publication‐quality manuscript on a hospital medicine topic by the end of the year. Second, the PA fellow is expected to create a professional portfolio, which is comprised of a collection of all of the rotation/module assessments, the formal program assessments, and documentation of all of the skills obtained by the fellow throughout year (competency checklists). This portfolio can be used by the graduate to demonstrate to future employers what skills they possess and provide documentation of knowledge gained during the fellowship.
The program itself is evaluated by several measures. First, the fellow provides formal feedback during the mid‐year and end‐of‐the‐year assessments, which are used to enhance the experience of future fellows. Second, there is ongoing review by both the division of Hospital Medicine and the institution's Allied Health Education Committee, which ensures that the program maintains the appropriate standards and goals.
Future Goals for the PA Fellowship
The program graduated its first fellow at the end of October 2008 and has enjoyed early success. Integrating the PA fellow onto the hospitalist services augmented the present mid‐level and physician teams. There has been excellent institutional support for the program with extremely positive feedback from the rotation preceptors. There are several futures plans for the program. Our first goal is to seek accreditation from the Accreditation Review Commission for Physician Assistants (ARC‐PA), the organization that accredits entry level PA programs and which began formal, voluntary accreditation of postgraduate programs in early 2008. We plan to begin this process within the next academic year.
Our second long‐term goal for the program is to include NPs in the training program. Because of the desire to seek accreditation, the program directors felt temporarily limiting the fellowship to PAs would aide in the rigorous accreditation process, which can take approximately 1 year to complete. There is an NP on our faculty and the program has received interest from NPs. Once we obtain accreditation, expand the program enrollment, and develop an NP curriculum, we plan to open the fellowship to either PA or NP applicants.
Our third goal is to substantiate our PA Fellowship validity with outcome measures and ultimately publishable data. Thus far, the success of the PA fellowship is qualitative, and with small numbers of graduates it is difficult to quantify. After graduation of many subsequent PA fellows, our goal is to obtain quantifiable data that can be used to improve the quality of the PA fellowship and demonstrate the value of postgraduate training for physician assistants.
Perhaps the most important goal of the program is to eventually accept additional PA/NP fellows per year. While 1 program does not meet the demands of a national shortage of hospitalist providers, it may serve as a model that other institutions can adapt to their own needs. Since the program is based upon the SHM Core Competencies, the curriculum can be applied to a variety of hospitalist programs, and its relatively low operating cost makes it feasible for both academic‐based and community‐based institutions. Importantly, since recruitment and retention of employees is such a challenge for most hospitalist groups, this PA fellowship program may serve as a vehicle for recruitment and long‐term retention of well‐trained employees. This precedent has been set, as our division has hired our first PA fellow, whose transition from PA fellow to PA staff was seamless.
In conclusion, our PA fellowship in Hospital Medicine represents the first reported postgraduate PA program of this kind in the United States offering a certificate of completion. As the need for hospitalists increase so will the need for NPPs, particularly those with additional training in hospital medicine. This program serves as an example of 1 type of training tool for physician assistants looking to work in hospital medicine.
- ,,,.Health care market trends and the evolution of hospitalist use and roles.J Gen Intern Med.2004;20:101–107.
- .Innovations in the management of hospitalized patients. Nurse Pract Spring2006 (suppl):2–3.
- .Hospitalist pay up, productivity steady in SHM's latest survey.Hospitalist.2008;12(5):7,16.
- .Physician assistants: filling the gap in patient care in academic hospitals.Perspect Physician Assist Educ.2003;14(3):158–167.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,, et al.Physician extenders impact trauma systems.J Trauma.2005;58(5):917–920.
- ,,.The role of physician assistants in critical care units.Chest.1991;99:89–91.
- .Alliances: invaluable assistants.Hospitalist.2006;April:32–33.
- ,,.Resource use by physician assistant services versus teaching services.JAAPA.2002;15:33–42.
- ,,,,,.Analyzing the time and value of house staff inpatient work.J Intern Med.1998;13:534–540.
- ,,, et al.Improving resource utilization in a teaching hospital: Development of a nonteaching service for chest pain admissions.Acad Med.2006;81(5):432–435.
- .Midlevels make a rocky entrance into hospital medicine.Todays Hospitalist.2007;5(1):28–32.
- Accreditation Review Commission for Physician Assistant Education.3rd ed. 2005. Available at: http://www.arc‐pa.org/Standards/standards.html. Accessed September2009.
- 22nd Annual Report on Physician Assistant Education in the U.S., 2005–2006. Available at: http://www.paeaonline.org. Accessed September2009.
- Association of Postgraduate Physician Assistant Programs. Available at: http://www.appap.org. Accessed September2009.
- ,,,,.The core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
In recent years, the demand for hospitalists has outstripped the supply, creating a national shortage.1, 2 A recent Society of Hospital Medicine (SHM) survey found that in the last 2 years there has been a 31% mean growth increase in the number of hospitalist groups.3 As hospitalists are becoming more difficult to recruit, many practices are utilizing physician assistants (PAs) and nurse practitioners (NPs), collectively referred to as nonphysician providers (NPPs) to help offset the workload.4 The SHM survey also noted that the number of hospitalist groups utilizing NPPs increased from 29% to 38%.3 The exact number of NPPs working for hospitalist groups is unknown.
Hospitalist NPPs are in demand for reasons other than just physician shortages. NPPs have been utilized to fill the gap in many institutions where the workforce was impacted by the 2002 Accreditation Council for Graduate Medical Education (ACGME) ruling to restrict resident work hours. Several studies have documented NPPs' ability to assist with the compliance of ACCGME resident work‐hour restrictions while maintaining patient continuity of care, improving length of stays, and reducing health care costs on various hospital services.59 Dresselhaus et al.10 found that 56% of medical resident's time on service was delegated to tasks not related to direct patient care. They proposed that these tasks can be delegated to the NPPs, leaving more time for the residents to focus on direct patient care. In a recent study performed at a Pennsylvania hospital, patients presenting to the emergency department with low‐risk chest pain (based upon thrombolysis in myocardial infarction [TIMI] risk score) were admitted to a nonteaching service staffed with NPPs and attending physicians. Simultaneously, a similar group of low‐risk chest pain patients were admitted to a traditional internal medicine resident service. The results demonstrated lower median length of stay and hospital charges on the nonteaching service. This study suggested that NPPs can offset the workload volume for medical residents, allowing them to focus on patients with higher acuity and greater learning value.11
Barriers to Finding Experienced NPPs in Hospital Medicine
Although many hospitalist groups are interested in hiring NPPs, there can be significant obstacles to recruitment. For example, most experienced PAs and NPs have clinical backgrounds in either surgical or medical subspecialties and therefore typically need extensive on‐the‐job training in hospital medicine, which can often take at least 6 to 12 months to acquire the basic skill set.12 Hiring new graduates may require even longer training periods.
The inexperience of new graduates has become an even more pertinent issue due to recent changes in PA education. Traditionally, PA programs attracted older students with prior healthcare experience, who wished to return to school for additional training. However, in 2005 a major shift occurred in PA education: programs began transitioning from graduating trainees with a bachelor's degree to now requiring a master's level degree for completion of the PA program.13 The acquisition of more advanced degrees has changed the demographics of the students matriculating into PA programs, attracting younger students, straight from undergraduate institutions, with less prior healthcare experience.14 As a result, not only are new PA graduates less experienced overall, but they are particularly lacking in exposure to hospital medicine. After PA students complete their first 12 months of PA school in the basic sciences and didactic coursework, they embark on 12 to 15 months of clinical rotations, which are largely rooted in primary care. In fact, many PA programs find it difficult to offer hospital‐based rotations while fulfilling the required rotations in primary care. These factors have resulted in the need for more extensive on‐the‐job training particularly for those new graduates interested in hospital medicine. In light of these challenges, our institution created a 12‐month postgraduate PA fellowship program in Hospital Medicine.
Postgraduate PA Training Programs
Postgraduate PA fellowships, interchangeably called residencies, are voluntary 1‐year training programs that provide both didactic instruction and clinical experience in a medical or surgical subspecialty, thereby lessening the need for on‐the‐job training. These programs are recognized by the Association of Postgraduate Physician Assistant Programs.15 Currently, there are 44 postgraduate training programs in the United States, in a wide range of medical and surgical specialties. At the end of these 1‐year postgraduate PA programs, most graduates receive a certificate of completion. Until now, the only postgraduate education option for PAs interested in Hospital Medicine was a master's completion program only available to PAs who were already employed by a hospitalist group.15 This work reviews the first reported postgraduate hospitalist training program for PAs. Specifically, the program's background, curriculum, anticipated program outcomes, and future plans are discussed.
Background for A Hospitalist Postgraduate PA Fellowship
Mayo Clinic Arizona is a multispecialty private group comprised of both outpatient services and a tertiary care hospital medical center, located in the metropolitan Phoenix, AZ, area. The Mayo Clinic Hospital is a 7‐story facility with 244 licensed beds, 18 operating rooms, and a Level II emergency department. The Mayo Hospitalist group is composed of 15 full time hospitalists and 6 part‐time hospitalists, all of whom are salaried Mayo employees. The group provides 24‐hour in‐house staffing, covering both resident services (teams composed of interns and residents supervised by a staff hospitalist) and nonresident services (staff hospitalists). Over the years there has been steady growth in the number of nonresident services, in part due to resident work‐hour restrictions. To support the physicians working on these nonresident services, the first PA was hired in 2001. Since then, the number of NPPs in our Hospitalist group has increased to 9.35 full‐time equivalents (FTEs), including 1 nurse practitioner. However, one of the greatest challenges in expanding the NPP service was the difficulty finding candidates with experience in hospital internal medicine. This need inspired the creation of a PA fellowship in Hospital Medicine. At the time, there were 2 other postgraduate PA training programs at the Mayo Clinic Arizona in Hepatology and Otolaryngology/Ear, Nose, and Throat (ENT) Surgery.
Program Description
The Mayo Clinic Arizona PA fellowship in Hospital Medicine began in October 2007 and currently accepts 1 fellow per year. Applicants must be graduates of an Accreditation Review Commission in Education for the Physician Assistant (ARC‐PA)‐accredited PA program and be certified through the National Commission on Certification of Physician Assistants (NCCPA). Furthermore, they must be licensed to work as a PA in the state of Arizona. The program is 12 months in duration, and is comprised of both didactic and clinical components. Upon graduation, the fellow earns a certificate of completion from the Mayo Clinic College of Medicine. The program has received recognition with the Association of Postgraduate Physician Assistant Programs (APPAP).
Two physician assistants act as co‐program directors of the PA fellowship in hospital medicine. They are given 0.10 full‐time equivalent (FTE) for management of the program, which includes day‐to‐day operations, curriculum development, and candidate selection. The program also has 2 volunteer physician medical directors, both of whom have previous medical residency experience. The physicians and NPPs in our hospitalist group volunteer their time to serve as faculty for the program, assisting with much of the didactic and clinical education. The program receives a budget of $99,500 per year, which is funded by the organization's foundation through the department of education. This includes the fellow stipend of $44,000 per 12 months and institutional malpractice insurance coverage. The fellow also receives health and dental insurance, 2 weeks of paid vacation, and $500 stipend toward attendance of a continuing medical education (CME) conference.
CURRICULUM
The PA fellowship curriculum is designed in a diverse unique format that strives to accommodate all types of learners. It includes clinical rotations in various medicine/surgical subspecialties, didactic instruction, and teaching modules (Figure 1). The curriculum is based upon the SHM Core Competencies.15

Clinical Rotations
The PA fellow completes 12 to 14 general hospital medicine and medical specialty rotations, each 2 to 4 weeks in duration. The rotation calendar for the current fellow is given in Figure 2. These rotations are all inpatient‐based and are supervised by either the hospitalist or the respective inpatient subspecialists. The PA fellow's specific clinical responsibilities vary from rotation to rotation, and are designed to maximize the fellow's exposure to that particular specialty. Each rotation has specific written objectives created by the program directors and reviewed by the rotation's preceptor(s) (Figure 2). During the clinical rotations, complementary didactic lectures, coursework, and readings are provided to ensure the PA fellow receives a strong foundation. Didactic instruction is designed by the program directors, physician preceptors and staff NPPs, and is coordinated with the clinical rotation specialty. At the end of each rotation the fellow is evaluated by the preceptor and given direct feedback on their performance.

Didactic Instruction
The didactic instruction is organized in a system‐based manner and occurs on a weekly basis during the Hospital Internal Medicine service and Medicine Consults rotations. Hospitalist NPPs and physician faculty are responsible for most of the teaching. This formal didactic instruction is supplemented by journal club presentations given by the PA fellow to faculty in the division of hospital internal medicine. The fellow is also required to attend daily medical resident lunchtime educational lectures, weekly medical grand rounds, and any lectures provided by the medicine subspecialties while the PA is on that particular rotation.
Teaching Modules
One component of the Hospital Medicine PA fellowship curriculum that may be unique is the concept of teaching modules. While receiving regular didactic instruction and completing their clinical rotations, the PA is also expected to complete self‐directed teaching module assignments. These modules serve to educate the PA fellow on the hospital as a systemthe true essence of hospital medicine. The modules cover a variety of topics not directly addressed during their rotations. These topics are outlined in Figure 3. Each teaching module consists of a didactic component, clinical application, and assessment (Figure 4) and has its own specific objectives and goals. Teaching modules are often taught by the local expert in the hospital in that particular area. For example, for the infectious control teaching module, the PA fellow will rotate with the infection control nursing staff learning about the isolation and infection control policies of the institution.


Assessment Tools
There are several tools utilized to assess both the PA fellow and the fellowship program itself (Figure 5). The assessment tools used include both ongoing and summative assessments. To fulfill the ongoing assessment, each rotation and teaching module contains assessment tools provided by the preceptor, which are reviewed by the program directors. Additionally, during the clinical rotations, skills are assessed using competency checklists that require the preceptor to directly observe the PA fellow perform a specific task or skill‐set and sign off on its successful completion (Supplementary Figures 6, 7).

There are 2 forms of summative assessment for the PA fellow. First, to assess the PA fellow's knowledge, comprehensive mid‐year and end‐year examinations are utilized. These multiple‐choice examinations are comprised of questions which align with the didactic lectures/objectives provided by the Hospital Medicine faculty throughout the year. The second form of summative evaluation of the fellow is project‐based and divided into 2 parts. First, the fellow is expected to write a publication‐quality manuscript on a hospital medicine topic by the end of the year. Second, the PA fellow is expected to create a professional portfolio, which is comprised of a collection of all of the rotation/module assessments, the formal program assessments, and documentation of all of the skills obtained by the fellow throughout year (competency checklists). This portfolio can be used by the graduate to demonstrate to future employers what skills they possess and provide documentation of knowledge gained during the fellowship.
The program itself is evaluated by several measures. First, the fellow provides formal feedback during the mid‐year and end‐of‐the‐year assessments, which are used to enhance the experience of future fellows. Second, there is ongoing review by both the division of Hospital Medicine and the institution's Allied Health Education Committee, which ensures that the program maintains the appropriate standards and goals.
Future Goals for the PA Fellowship
The program graduated its first fellow at the end of October 2008 and has enjoyed early success. Integrating the PA fellow onto the hospitalist services augmented the present mid‐level and physician teams. There has been excellent institutional support for the program with extremely positive feedback from the rotation preceptors. There are several futures plans for the program. Our first goal is to seek accreditation from the Accreditation Review Commission for Physician Assistants (ARC‐PA), the organization that accredits entry level PA programs and which began formal, voluntary accreditation of postgraduate programs in early 2008. We plan to begin this process within the next academic year.
Our second long‐term goal for the program is to include NPs in the training program. Because of the desire to seek accreditation, the program directors felt temporarily limiting the fellowship to PAs would aide in the rigorous accreditation process, which can take approximately 1 year to complete. There is an NP on our faculty and the program has received interest from NPs. Once we obtain accreditation, expand the program enrollment, and develop an NP curriculum, we plan to open the fellowship to either PA or NP applicants.
Our third goal is to substantiate our PA Fellowship validity with outcome measures and ultimately publishable data. Thus far, the success of the PA fellowship is qualitative, and with small numbers of graduates it is difficult to quantify. After graduation of many subsequent PA fellows, our goal is to obtain quantifiable data that can be used to improve the quality of the PA fellowship and demonstrate the value of postgraduate training for physician assistants.
Perhaps the most important goal of the program is to eventually accept additional PA/NP fellows per year. While 1 program does not meet the demands of a national shortage of hospitalist providers, it may serve as a model that other institutions can adapt to their own needs. Since the program is based upon the SHM Core Competencies, the curriculum can be applied to a variety of hospitalist programs, and its relatively low operating cost makes it feasible for both academic‐based and community‐based institutions. Importantly, since recruitment and retention of employees is such a challenge for most hospitalist groups, this PA fellowship program may serve as a vehicle for recruitment and long‐term retention of well‐trained employees. This precedent has been set, as our division has hired our first PA fellow, whose transition from PA fellow to PA staff was seamless.
In conclusion, our PA fellowship in Hospital Medicine represents the first reported postgraduate PA program of this kind in the United States offering a certificate of completion. As the need for hospitalists increase so will the need for NPPs, particularly those with additional training in hospital medicine. This program serves as an example of 1 type of training tool for physician assistants looking to work in hospital medicine.
In recent years, the demand for hospitalists has outstripped the supply, creating a national shortage.1, 2 A recent Society of Hospital Medicine (SHM) survey found that in the last 2 years there has been a 31% mean growth increase in the number of hospitalist groups.3 As hospitalists are becoming more difficult to recruit, many practices are utilizing physician assistants (PAs) and nurse practitioners (NPs), collectively referred to as nonphysician providers (NPPs) to help offset the workload.4 The SHM survey also noted that the number of hospitalist groups utilizing NPPs increased from 29% to 38%.3 The exact number of NPPs working for hospitalist groups is unknown.
Hospitalist NPPs are in demand for reasons other than just physician shortages. NPPs have been utilized to fill the gap in many institutions where the workforce was impacted by the 2002 Accreditation Council for Graduate Medical Education (ACGME) ruling to restrict resident work hours. Several studies have documented NPPs' ability to assist with the compliance of ACCGME resident work‐hour restrictions while maintaining patient continuity of care, improving length of stays, and reducing health care costs on various hospital services.59 Dresselhaus et al.10 found that 56% of medical resident's time on service was delegated to tasks not related to direct patient care. They proposed that these tasks can be delegated to the NPPs, leaving more time for the residents to focus on direct patient care. In a recent study performed at a Pennsylvania hospital, patients presenting to the emergency department with low‐risk chest pain (based upon thrombolysis in myocardial infarction [TIMI] risk score) were admitted to a nonteaching service staffed with NPPs and attending physicians. Simultaneously, a similar group of low‐risk chest pain patients were admitted to a traditional internal medicine resident service. The results demonstrated lower median length of stay and hospital charges on the nonteaching service. This study suggested that NPPs can offset the workload volume for medical residents, allowing them to focus on patients with higher acuity and greater learning value.11
Barriers to Finding Experienced NPPs in Hospital Medicine
Although many hospitalist groups are interested in hiring NPPs, there can be significant obstacles to recruitment. For example, most experienced PAs and NPs have clinical backgrounds in either surgical or medical subspecialties and therefore typically need extensive on‐the‐job training in hospital medicine, which can often take at least 6 to 12 months to acquire the basic skill set.12 Hiring new graduates may require even longer training periods.
The inexperience of new graduates has become an even more pertinent issue due to recent changes in PA education. Traditionally, PA programs attracted older students with prior healthcare experience, who wished to return to school for additional training. However, in 2005 a major shift occurred in PA education: programs began transitioning from graduating trainees with a bachelor's degree to now requiring a master's level degree for completion of the PA program.13 The acquisition of more advanced degrees has changed the demographics of the students matriculating into PA programs, attracting younger students, straight from undergraduate institutions, with less prior healthcare experience.14 As a result, not only are new PA graduates less experienced overall, but they are particularly lacking in exposure to hospital medicine. After PA students complete their first 12 months of PA school in the basic sciences and didactic coursework, they embark on 12 to 15 months of clinical rotations, which are largely rooted in primary care. In fact, many PA programs find it difficult to offer hospital‐based rotations while fulfilling the required rotations in primary care. These factors have resulted in the need for more extensive on‐the‐job training particularly for those new graduates interested in hospital medicine. In light of these challenges, our institution created a 12‐month postgraduate PA fellowship program in Hospital Medicine.
Postgraduate PA Training Programs
Postgraduate PA fellowships, interchangeably called residencies, are voluntary 1‐year training programs that provide both didactic instruction and clinical experience in a medical or surgical subspecialty, thereby lessening the need for on‐the‐job training. These programs are recognized by the Association of Postgraduate Physician Assistant Programs.15 Currently, there are 44 postgraduate training programs in the United States, in a wide range of medical and surgical specialties. At the end of these 1‐year postgraduate PA programs, most graduates receive a certificate of completion. Until now, the only postgraduate education option for PAs interested in Hospital Medicine was a master's completion program only available to PAs who were already employed by a hospitalist group.15 This work reviews the first reported postgraduate hospitalist training program for PAs. Specifically, the program's background, curriculum, anticipated program outcomes, and future plans are discussed.
Background for A Hospitalist Postgraduate PA Fellowship
Mayo Clinic Arizona is a multispecialty private group comprised of both outpatient services and a tertiary care hospital medical center, located in the metropolitan Phoenix, AZ, area. The Mayo Clinic Hospital is a 7‐story facility with 244 licensed beds, 18 operating rooms, and a Level II emergency department. The Mayo Hospitalist group is composed of 15 full time hospitalists and 6 part‐time hospitalists, all of whom are salaried Mayo employees. The group provides 24‐hour in‐house staffing, covering both resident services (teams composed of interns and residents supervised by a staff hospitalist) and nonresident services (staff hospitalists). Over the years there has been steady growth in the number of nonresident services, in part due to resident work‐hour restrictions. To support the physicians working on these nonresident services, the first PA was hired in 2001. Since then, the number of NPPs in our Hospitalist group has increased to 9.35 full‐time equivalents (FTEs), including 1 nurse practitioner. However, one of the greatest challenges in expanding the NPP service was the difficulty finding candidates with experience in hospital internal medicine. This need inspired the creation of a PA fellowship in Hospital Medicine. At the time, there were 2 other postgraduate PA training programs at the Mayo Clinic Arizona in Hepatology and Otolaryngology/Ear, Nose, and Throat (ENT) Surgery.
Program Description
The Mayo Clinic Arizona PA fellowship in Hospital Medicine began in October 2007 and currently accepts 1 fellow per year. Applicants must be graduates of an Accreditation Review Commission in Education for the Physician Assistant (ARC‐PA)‐accredited PA program and be certified through the National Commission on Certification of Physician Assistants (NCCPA). Furthermore, they must be licensed to work as a PA in the state of Arizona. The program is 12 months in duration, and is comprised of both didactic and clinical components. Upon graduation, the fellow earns a certificate of completion from the Mayo Clinic College of Medicine. The program has received recognition with the Association of Postgraduate Physician Assistant Programs (APPAP).
Two physician assistants act as co‐program directors of the PA fellowship in hospital medicine. They are given 0.10 full‐time equivalent (FTE) for management of the program, which includes day‐to‐day operations, curriculum development, and candidate selection. The program also has 2 volunteer physician medical directors, both of whom have previous medical residency experience. The physicians and NPPs in our hospitalist group volunteer their time to serve as faculty for the program, assisting with much of the didactic and clinical education. The program receives a budget of $99,500 per year, which is funded by the organization's foundation through the department of education. This includes the fellow stipend of $44,000 per 12 months and institutional malpractice insurance coverage. The fellow also receives health and dental insurance, 2 weeks of paid vacation, and $500 stipend toward attendance of a continuing medical education (CME) conference.
CURRICULUM
The PA fellowship curriculum is designed in a diverse unique format that strives to accommodate all types of learners. It includes clinical rotations in various medicine/surgical subspecialties, didactic instruction, and teaching modules (Figure 1). The curriculum is based upon the SHM Core Competencies.15

Clinical Rotations
The PA fellow completes 12 to 14 general hospital medicine and medical specialty rotations, each 2 to 4 weeks in duration. The rotation calendar for the current fellow is given in Figure 2. These rotations are all inpatient‐based and are supervised by either the hospitalist or the respective inpatient subspecialists. The PA fellow's specific clinical responsibilities vary from rotation to rotation, and are designed to maximize the fellow's exposure to that particular specialty. Each rotation has specific written objectives created by the program directors and reviewed by the rotation's preceptor(s) (Figure 2). During the clinical rotations, complementary didactic lectures, coursework, and readings are provided to ensure the PA fellow receives a strong foundation. Didactic instruction is designed by the program directors, physician preceptors and staff NPPs, and is coordinated with the clinical rotation specialty. At the end of each rotation the fellow is evaluated by the preceptor and given direct feedback on their performance.

Didactic Instruction
The didactic instruction is organized in a system‐based manner and occurs on a weekly basis during the Hospital Internal Medicine service and Medicine Consults rotations. Hospitalist NPPs and physician faculty are responsible for most of the teaching. This formal didactic instruction is supplemented by journal club presentations given by the PA fellow to faculty in the division of hospital internal medicine. The fellow is also required to attend daily medical resident lunchtime educational lectures, weekly medical grand rounds, and any lectures provided by the medicine subspecialties while the PA is on that particular rotation.
Teaching Modules
One component of the Hospital Medicine PA fellowship curriculum that may be unique is the concept of teaching modules. While receiving regular didactic instruction and completing their clinical rotations, the PA is also expected to complete self‐directed teaching module assignments. These modules serve to educate the PA fellow on the hospital as a systemthe true essence of hospital medicine. The modules cover a variety of topics not directly addressed during their rotations. These topics are outlined in Figure 3. Each teaching module consists of a didactic component, clinical application, and assessment (Figure 4) and has its own specific objectives and goals. Teaching modules are often taught by the local expert in the hospital in that particular area. For example, for the infectious control teaching module, the PA fellow will rotate with the infection control nursing staff learning about the isolation and infection control policies of the institution.


Assessment Tools
There are several tools utilized to assess both the PA fellow and the fellowship program itself (Figure 5). The assessment tools used include both ongoing and summative assessments. To fulfill the ongoing assessment, each rotation and teaching module contains assessment tools provided by the preceptor, which are reviewed by the program directors. Additionally, during the clinical rotations, skills are assessed using competency checklists that require the preceptor to directly observe the PA fellow perform a specific task or skill‐set and sign off on its successful completion (Supplementary Figures 6, 7).

There are 2 forms of summative assessment for the PA fellow. First, to assess the PA fellow's knowledge, comprehensive mid‐year and end‐year examinations are utilized. These multiple‐choice examinations are comprised of questions which align with the didactic lectures/objectives provided by the Hospital Medicine faculty throughout the year. The second form of summative evaluation of the fellow is project‐based and divided into 2 parts. First, the fellow is expected to write a publication‐quality manuscript on a hospital medicine topic by the end of the year. Second, the PA fellow is expected to create a professional portfolio, which is comprised of a collection of all of the rotation/module assessments, the formal program assessments, and documentation of all of the skills obtained by the fellow throughout year (competency checklists). This portfolio can be used by the graduate to demonstrate to future employers what skills they possess and provide documentation of knowledge gained during the fellowship.
The program itself is evaluated by several measures. First, the fellow provides formal feedback during the mid‐year and end‐of‐the‐year assessments, which are used to enhance the experience of future fellows. Second, there is ongoing review by both the division of Hospital Medicine and the institution's Allied Health Education Committee, which ensures that the program maintains the appropriate standards and goals.
Future Goals for the PA Fellowship
The program graduated its first fellow at the end of October 2008 and has enjoyed early success. Integrating the PA fellow onto the hospitalist services augmented the present mid‐level and physician teams. There has been excellent institutional support for the program with extremely positive feedback from the rotation preceptors. There are several futures plans for the program. Our first goal is to seek accreditation from the Accreditation Review Commission for Physician Assistants (ARC‐PA), the organization that accredits entry level PA programs and which began formal, voluntary accreditation of postgraduate programs in early 2008. We plan to begin this process within the next academic year.
Our second long‐term goal for the program is to include NPs in the training program. Because of the desire to seek accreditation, the program directors felt temporarily limiting the fellowship to PAs would aide in the rigorous accreditation process, which can take approximately 1 year to complete. There is an NP on our faculty and the program has received interest from NPs. Once we obtain accreditation, expand the program enrollment, and develop an NP curriculum, we plan to open the fellowship to either PA or NP applicants.
Our third goal is to substantiate our PA Fellowship validity with outcome measures and ultimately publishable data. Thus far, the success of the PA fellowship is qualitative, and with small numbers of graduates it is difficult to quantify. After graduation of many subsequent PA fellows, our goal is to obtain quantifiable data that can be used to improve the quality of the PA fellowship and demonstrate the value of postgraduate training for physician assistants.
Perhaps the most important goal of the program is to eventually accept additional PA/NP fellows per year. While 1 program does not meet the demands of a national shortage of hospitalist providers, it may serve as a model that other institutions can adapt to their own needs. Since the program is based upon the SHM Core Competencies, the curriculum can be applied to a variety of hospitalist programs, and its relatively low operating cost makes it feasible for both academic‐based and community‐based institutions. Importantly, since recruitment and retention of employees is such a challenge for most hospitalist groups, this PA fellowship program may serve as a vehicle for recruitment and long‐term retention of well‐trained employees. This precedent has been set, as our division has hired our first PA fellow, whose transition from PA fellow to PA staff was seamless.
In conclusion, our PA fellowship in Hospital Medicine represents the first reported postgraduate PA program of this kind in the United States offering a certificate of completion. As the need for hospitalists increase so will the need for NPPs, particularly those with additional training in hospital medicine. This program serves as an example of 1 type of training tool for physician assistants looking to work in hospital medicine.
- ,,,.Health care market trends and the evolution of hospitalist use and roles.J Gen Intern Med.2004;20:101–107.
- .Innovations in the management of hospitalized patients. Nurse Pract Spring2006 (suppl):2–3.
- .Hospitalist pay up, productivity steady in SHM's latest survey.Hospitalist.2008;12(5):7,16.
- .Physician assistants: filling the gap in patient care in academic hospitals.Perspect Physician Assist Educ.2003;14(3):158–167.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,, et al.Physician extenders impact trauma systems.J Trauma.2005;58(5):917–920.
- ,,.The role of physician assistants in critical care units.Chest.1991;99:89–91.
- .Alliances: invaluable assistants.Hospitalist.2006;April:32–33.
- ,,.Resource use by physician assistant services versus teaching services.JAAPA.2002;15:33–42.
- ,,,,,.Analyzing the time and value of house staff inpatient work.J Intern Med.1998;13:534–540.
- ,,, et al.Improving resource utilization in a teaching hospital: Development of a nonteaching service for chest pain admissions.Acad Med.2006;81(5):432–435.
- .Midlevels make a rocky entrance into hospital medicine.Todays Hospitalist.2007;5(1):28–32.
- Accreditation Review Commission for Physician Assistant Education.3rd ed. 2005. Available at: http://www.arc‐pa.org/Standards/standards.html. Accessed September2009.
- 22nd Annual Report on Physician Assistant Education in the U.S., 2005–2006. Available at: http://www.paeaonline.org. Accessed September2009.
- Association of Postgraduate Physician Assistant Programs. Available at: http://www.appap.org. Accessed September2009.
- ,,,,.The core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
- ,,,.Health care market trends and the evolution of hospitalist use and roles.J Gen Intern Med.2004;20:101–107.
- .Innovations in the management of hospitalized patients. Nurse Pract Spring2006 (suppl):2–3.
- .Hospitalist pay up, productivity steady in SHM's latest survey.Hospitalist.2008;12(5):7,16.
- .Physician assistants: filling the gap in patient care in academic hospitals.Perspect Physician Assist Educ.2003;14(3):158–167.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,, et al.Physician extenders impact trauma systems.J Trauma.2005;58(5):917–920.
- ,,.The role of physician assistants in critical care units.Chest.1991;99:89–91.
- .Alliances: invaluable assistants.Hospitalist.2006;April:32–33.
- ,,.Resource use by physician assistant services versus teaching services.JAAPA.2002;15:33–42.
- ,,,,,.Analyzing the time and value of house staff inpatient work.J Intern Med.1998;13:534–540.
- ,,, et al.Improving resource utilization in a teaching hospital: Development of a nonteaching service for chest pain admissions.Acad Med.2006;81(5):432–435.
- .Midlevels make a rocky entrance into hospital medicine.Todays Hospitalist.2007;5(1):28–32.
- Accreditation Review Commission for Physician Assistant Education.3rd ed. 2005. Available at: http://www.arc‐pa.org/Standards/standards.html. Accessed September2009.
- 22nd Annual Report on Physician Assistant Education in the U.S., 2005–2006. Available at: http://www.paeaonline.org. Accessed September2009.
- Association of Postgraduate Physician Assistant Programs. Available at: http://www.appap.org. Accessed September2009.
- ,,,,.The core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
Copyright © 2010 Society of Hospital Medicine

PE and AMI in Undiagnosed PFO
Acute pulmonary embolism (PE) and acute myocardial infarction (AMI) are common inpatient diagnoses, and are frequently in the differential diagnosis of patients evaluated for chest pain and dyspnea. We present a case with 1 unifying explanation for these entities to coexist. Acute PE with subsequent embolism to the coronary arteries via a patent foramen ovale (PFO) is rare, but the underlying disorder and anatomical variant are common. Of practical significance, hospitalized patients with acute PE and PFO may have up to a 5‐fold increase in morbidity compared to patients with isolated PE.
CASE REPORT
A 79‐year‐old male smoker underwent resection of a recurrent high‐grade liposarcoma of the right upper extremity. He had no antecedent history of coronary artery disease (CAD) or atrial fibrillation, and had no additional vascular risk factors. On postoperative day 2, he developed acute chest pain, dyspnea, and hypoxia. He appeared alert but was diaphoretic and in moderate distress. Pulse was 84 beats per minute, blood pressure 230/120 mm Hg, and oxygen saturation 59% on room air (93% on supplemental oxygen). Heart and lung exam were unremarkable. Neck veins were not distended. Extremity exam was negative for edema, asymmetry, or calf tenderness, and pedal pulses were palpable bilaterally.
The patient's initial complete blood count, metabolic panel, and cardiac enzymes were within normal limits. Arterial blood gas (on 4‐L nasal cannula) revealed pH of 7.35, partial pressure of arterial oxygen (PaO2) of 65.5 mm Hg, partial pressure of arterial CO2 (PaCO2) of 45.4 mm Hg, and an alveolar‐arterial gradient of 131.3 mm Hg. Electrocardiogram (ECG) (Figure 1) showed an unchanged right bundle branch block, but new 2.5‐mm ST segment elevation in leads V4‐6, III, and aVF, and ST depression in aVL. At this point, the available data suggested either PE with secondary ECG changes or acute ST‐elevation MI with hypoxia. Given the ST elevation in 2 coronary distributions and concern for multivessel CAD, the patient was referred for emergent coronary angiography.
The patient was given aspirin, intravenous unfractionated heparin, and morphine. Left heart catheterization showed an abrupt cutoff in the distal left anterior descending artery (LAD), suggestive of thrombosis secondary to coronary embolism (Figure 2A); angioplasty was not attempted due to the distal location of the occlusion. The posterior descending artery from the right coronary artery was relatively short and the inferior apex was supplied by the distal LAD. The left ventriculogram demonstrated preserved ejection fraction but severe apical hypokinesis, correlating with the occluded vascular territory. The remaining coronary arteries were without significant stenosis. Based on the angiogram findings, paradoxical embolism was suspected. Right heart catheterization identified a previously undiagnosed PFO (Figure 2B); no thrombus was visualized. Right to left shunt was not identified; right ventricular systolic pressure was 46 mm Hg (normal, 15‐25 mm Hg). Subsequent spiral computed tomography (CT) revealed bilateral PEs. It was concluded that the patient had suffered an acute PE, from which the thrombus was able to traverse the PFO and left heart, ultimately entering the LAD, causing the acute embolic MI (Figure 3). ST elevation was present in the inferior leads secondary to the wraparound LAD that supplied the inferior apex, as demonstrated by the wall motion abnormality present on ventriculography.

The patient was felt to be in a hypercoagulable state due to his malignancy and recent surgery; given the diagnosis of an acute PE, no ultrasound was performed to search for a deep vein thrombosis. The patient was transferred to the intensive care unit, continued on intravenous heparin and oxygen and started on oral warfarin. Subsequent hospital course was complicated by aspiration pneumonia and worsening hypoxia, but after a 17‐day hospital stay he was weaned off supplemental oxygen and transferred to an extended care facility with a therapeutic international normalized ratio (INR).
DISCUSSION
PFOs are congenital cardiac lesions that may persist through adulthood1 and are found incidentally in 19% to 36% of the normal population.2 They may range from 1 to 19 mm in size.2, 3 Contrast echocardiography has enabled a simple, accurate, and safe procedure for diagnosis (>5 microbubbles in the left heart cavities within three cardiac cycles after their appearance in the right atrium is considered diagnostic), though transesophageal echocardiography (TEE) is the gold standard.4
First described by Cohnheim in 1877,5 paradoxical embolism refers to the passage of embolic material from the venous to arterial circulation through a cardiac defect such as a PFO. However, definite confirmation of paradoxical embolism essentially requires catching the thrombus in the act of crossing the foramen ovale. Direct observation of this during life is rarely possible, and remains confined to isolated echocardiographic reports.6‐13
In clinical practice, the diagnosis of paradoxical embolism is almost always presumptive and relies on: (1) the occurrence of an arterial thromboembolic event in the absence of atrial fibrillation, left‐sided heart disease, or severe atherosclerosis; (2) the detection of right‐to‐left shunt, usually through a PFO or an atrial septal defect (ASD); and (3) the presence of venous thrombosis or PE.14
Although most patients are asymptomatic, during the past 20 years an association of PFO with stroke, migraine headache, peripheral arterial occlusion, and decompression induced neurologic dysfunction has been suggested.1 The neurological symptoms are proposed to be secondary to passage of small thrombi from the venous system through the PFO into arterial circulation during a transient right‐to‐left shunt. The source of clots cannot be established in most patients; fewer than 10% will have detectable deep vein thrombosis.15 Even less common are paradoxical emboli to the coronary arteries,1 estimated at 5% to 10% of all paradoxical emboli.16
In order for a thrombus to paradoxically embolize across a PFO, an atrial right‐to‐left pressure gradient must be present. Such a gradient occurs in normal individuals during early ventricular systole and with a Valsalva maneuver.17‐19 In a community‐based cohort study conducted to evaluate potential stroke risk, 148 (out of 581) subjects were found to have a PFO by TEE; 84 (57%) had right‐to‐left shunting at rest, and 136 (92%) had right‐to‐left shunting with Valsalva.2 Pathologic instances of pulmonary hypertension such as PE further elevate right‐heart pressures, further promoting intracardiac shunt.
Acute PE in the setting of PFO carries important prognostic implications. Konstantinides et al.20 prospectively evaluated 139 patients with large acute PE, all of whom had pulmonary hypertension and 96% of whom had right ventricular dilatation. They found a high prevalence of PFOs (35%) in this population. Furthermore, the subgroup with both PE and PFO had a high mortality (33% death rate compared with 14% in those without PFO; P = 0.015). When logistic regression analysis was performed, only arterial hypotension (odds ratio [OR] 26.3; P < 0.001) and the presence of PFO (OR 11.4; P < 0.001) remained significantly correlated with mortality. The authors reported that the presence of a PFO was associated with more than a 5‐fold increase in the adjusted risk of major in‐hospital complications (P < 0.001); no specific etiologic factors were proposed for this association.
In general, MI in the absence of CAD is uncommon, comprising <1% to 6% of all cases.21 No cause is found for the majority, but reported etiologies include coronary spasm in 15%, hypercoagulable states in 13%, collagen vascular diseases in 2%, and paradoxical embolism in 2%.22 AMI due to coronary embolism is uncommon, and when it does occur, left‐to‐left emboli in the setting of atrial fibrillation or prosthetic valves are far more common than paradoxical emboli. In an autopsy series of 1,050 patients with MIs, Prizel et al.23 identified only 55 patients with coronary embolism, none of which was right‐sided in origin.
A handful of published case reports documented paradoxical embolism as a cause for AMI.24‐26 Reported cases more often involved an ASD rather than PFO.27, 28 In most cases, diagnosis was made postmortem, though in a comprehensive review of the literature, Meier‐Ewert et al.13 identified 8 cases of AMI due to paradoxical embolism being diagnosed antemortem. Paradoxical emboli have been identified in all major divisions of the epicardial circulation, though involvement of the left coronary circulation is more common than the right.16
It is well‐established that the prevalence of PFO in patients with cryptogenic stroke is significantly higher than in the general population,1 and Crump et al.21 examined a case‐matched series of 18 patients with AMI who had little to no CAD (<30% stenosis) to see if the frequency of PFO was similarly higher in this group. Each group had identical frequency of PFO (28%; P = NS). The authors concluded that PFO is unlikely to contribute significantly to AMI. However, this study was limited by the small number of patients and the fact that transthoracic echocardiography (TTE) was used instead of TEE for the diagnosis of PFO.
The definitive diagnosis of AMI due to paradoxical embolism requires angiographic findings consistent with embolic occlusion (such as the cutoff sign in a distal coronary artery we observed in Figure 2A), cardiac defects predisposing to paradoxical emboli (such as PFO), and evidence of a venous source for thromboembolism. Alternatively, diagnosis can be made via direct visualization of emboli in the coronary arteries by TEE, or by autopsy.
Although electrocardiography is essential in the diagnosis and treatment of MI, it has the potential to be deceptive. Acute pulmonary hypertension caused by PE may be accompanied by ST elevation in inferior leads II, III, and aVF in a pseudoinfarction pattern mimicking AMI.29 This ECG abnormality probably reflects reciprocal changes of inferoposterior ischemia from right ventricular pressure overloading. However, clearly distinguishing between pseudoinfarction and true inferior infarction in the setting of PE requires coronary angiography.
Regarding therapy, acute treatment of PE is well‐established and consists of at least 5 days of therapeutically‐dosed heparin product that overlaps with therapeutic warfarin anticoagulation. Management of the PFO and coronary embolism is less clear; there are no guidelines for treatment of coronary embolism. Management strategies should focus on treatment of acute ischemia as well as prevention of future emboli, principally anticoagulation. Because the pathogenesis of AMI in this setting is drastically different from MI secondary to atherosclerosis, there is neither a biological basis nor clinical data to suggest benefit from initiation of beta‐blockers, aspirin, angiotensin converting enzyme inhibitors, or statins.
While studies have been done, and are underway to address optimal management of PFO in the setting of both stroke and migraine headache, to our knowledge, no such trials have addressed PFO and MI. Mehan et al.30 reported 2 cases of AMI caused by suspected paradoxical embolism, and in both cases, instant percutaneous closure of PFO was undertaken. However, there are no data to support or refute such an intervention in this particular setting.
- ,,, et al.Patent foramen ovale: current pathology, pathophysiology, and clinical status.J Am Coll Cardiol.2005;46:1768–1776.
- ,,, et al.Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC Study.Mayo Clin Proc.1999;74:862–869.
- ,,.Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts.Mayo Clin Proc.1984;59:17–20.
- ,,.Diagnosis of an anatomically and physiologically significant patent foramen ovale.Echocardiography.2006;23:810–815.
- .Thrombose und Embolie: Vorlesung über allgemeine Pathologie. Vol.1.Berlin:Hirschwald;1877;134.
- ,,,,,.Impending paradoxical embolism from atrial thrombus: correct diagnosis by transesophageal echocardiography and prevention by surgery.J Am Coll Cardiol.1985;5:1002–1004.
- ,,.Diagnosis of paradoxic embolism by transesophageal echocardiography.Am Heart J.1991;121:1552–1554.
- ,,,,,.Impending paradoxical embolism: echocardiographic diagnosis of an intracardiac thrombus crossing a patent foramen ovale.Am Heart J.1991;122(3 Pt 1):859–862.
- ,,,,,.Cryptogenic ischemic stroke and paradoxical embolism: should a patent foramen ovale be closed? Case report and literature review.Angiology.2001;52:793–799.
- ,,,,.Thrombus‐in‐transit and paradoxical embolism.J Am Soc Echocardiogr.2002;15:1021–1022.
- ,,,.Caught in the act: impending paradoxical embolism.Asian Cardiovasc Thorac Ann.2002;10:342–343.
- .Paradoxical embolism to the left main coronary artery: visualization by transesophageal echocardiography.J Am Soc Echocardiogr.2002;15:1417–1418.
- ,,,,,.Paradoxical embolism in the left main coronary artery: diagnosis by transesophageal echocardiography.Mayo Clin Proc.2003;78:103–106.
- .Specifics of patent foramen ovale.Adv Neurol.2003;92:197–202.
- ,,, et al.Frequency of deep vein thrombosis in patients with patent foramen ovale and ischemic stroke or transient ischemic attack.Am J Cardiol.1997;80:1066–1069.
- ,.Paradoxical coronary embolism: a rare cause of acute myocardial infarction.Rev Cardiovasc Med.2003;4:107–111.
- ,.Positive contrast echocardiography in patients with patent foramen ovale and normal right heart hemodynamics.Am J Cardiol.1982;49:1806–1809.
- ,,, et al.Contrast echocardiographic visualization of cough‐induced right to left shunt through a patent foramen ovale.J Am Coll Cardiol.1984;4:587–594.
- ,,,,.Transesophageal echocardiographic demonstration of distinct mechanisms for right to left shunting across a patent foramen ovale in the absence of pulmonary hypertension.J Am Coll Cardiol.1991;18:1112–1117.
- ,,,,,.Patent foramen ovale is an important predictor of adverse outcome in patients with major acute pulmonary embolism.Circulation.1998;97:1946–1951.
- ,,,.Prevalence of patent foramen ovale in patients with acute myocardial infarction and angiographically normal coronary arteries.Am J Cardiol.2000;85:1368–1370.
- ,,,,,.Clinical characteristics, aetiological factors and long‐term prognosis of myocardial infarction with an absolutely normal coronary angiogram; a 3‐year follow‐up study of 91 patients.Eur Heart J.2001;22:1459–1465.
- ,,.Coronary artery embolism and myocardial infarction.Ann Intern Med.1978;88:155–161.
- ,..Myocardial infarction due to a paradoxical embolism.Am J Med.1969;47:995–998.
- ,,,,,.[Myocardial infarction caused by probable paradoxical embolism and aneurysm of the interatrial septum].Presse Med.1996;25:907 [French].
- ,,.Acute myocardial infarction probably caused by paradoxical embolus in a pregnant woman.Heart.2004;90:e12.
- ,,,,.A case of acute pulmonary embolism and acute myocardial infarction with suspected paradoxical embolism after laparoscopic surgery.Heart Vessels.1999;4:197–200.
- ,,,,,.Acute myocardial infarction caused by paradoxical coronary embolization in a patient with a patent foramen ovale.J Am Soc Echocardiogr.2001;14:1227–1229.
- .ECG manifestations of selected extracardiac diseases.Emerg Med Clin N Am.2006;24:133–143.
- ,,,.Instant percutaneous closure of patent foramen ovale in patients with acute myocardial infarction and normal coronary arteries.Catheter Cardiovasc Interv.2006;67:279–282.
Acute pulmonary embolism (PE) and acute myocardial infarction (AMI) are common inpatient diagnoses, and are frequently in the differential diagnosis of patients evaluated for chest pain and dyspnea. We present a case with 1 unifying explanation for these entities to coexist. Acute PE with subsequent embolism to the coronary arteries via a patent foramen ovale (PFO) is rare, but the underlying disorder and anatomical variant are common. Of practical significance, hospitalized patients with acute PE and PFO may have up to a 5‐fold increase in morbidity compared to patients with isolated PE.
CASE REPORT
A 79‐year‐old male smoker underwent resection of a recurrent high‐grade liposarcoma of the right upper extremity. He had no antecedent history of coronary artery disease (CAD) or atrial fibrillation, and had no additional vascular risk factors. On postoperative day 2, he developed acute chest pain, dyspnea, and hypoxia. He appeared alert but was diaphoretic and in moderate distress. Pulse was 84 beats per minute, blood pressure 230/120 mm Hg, and oxygen saturation 59% on room air (93% on supplemental oxygen). Heart and lung exam were unremarkable. Neck veins were not distended. Extremity exam was negative for edema, asymmetry, or calf tenderness, and pedal pulses were palpable bilaterally.
The patient's initial complete blood count, metabolic panel, and cardiac enzymes were within normal limits. Arterial blood gas (on 4‐L nasal cannula) revealed pH of 7.35, partial pressure of arterial oxygen (PaO2) of 65.5 mm Hg, partial pressure of arterial CO2 (PaCO2) of 45.4 mm Hg, and an alveolar‐arterial gradient of 131.3 mm Hg. Electrocardiogram (ECG) (Figure 1) showed an unchanged right bundle branch block, but new 2.5‐mm ST segment elevation in leads V4‐6, III, and aVF, and ST depression in aVL. At this point, the available data suggested either PE with secondary ECG changes or acute ST‐elevation MI with hypoxia. Given the ST elevation in 2 coronary distributions and concern for multivessel CAD, the patient was referred for emergent coronary angiography.

The patient was given aspirin, intravenous unfractionated heparin, and morphine. Left heart catheterization showed an abrupt cutoff in the distal left anterior descending artery (LAD), suggestive of thrombosis secondary to coronary embolism (Figure 2A); angioplasty was not attempted due to the distal location of the occlusion. The posterior descending artery from the right coronary artery was relatively short and the inferior apex was supplied by the distal LAD. The left ventriculogram demonstrated preserved ejection fraction but severe apical hypokinesis, correlating with the occluded vascular territory. The remaining coronary arteries were without significant stenosis. Based on the angiogram findings, paradoxical embolism was suspected. Right heart catheterization identified a previously undiagnosed PFO (Figure 2B); no thrombus was visualized. Right to left shunt was not identified; right ventricular systolic pressure was 46 mm Hg (normal, 15‐25 mm Hg). Subsequent spiral computed tomography (CT) revealed bilateral PEs. It was concluded that the patient had suffered an acute PE, from which the thrombus was able to traverse the PFO and left heart, ultimately entering the LAD, causing the acute embolic MI (Figure 3). ST elevation was present in the inferior leads secondary to the wraparound LAD that supplied the inferior apex, as demonstrated by the wall motion abnormality present on ventriculography.


The patient was felt to be in a hypercoagulable state due to his malignancy and recent surgery; given the diagnosis of an acute PE, no ultrasound was performed to search for a deep vein thrombosis. The patient was transferred to the intensive care unit, continued on intravenous heparin and oxygen and started on oral warfarin. Subsequent hospital course was complicated by aspiration pneumonia and worsening hypoxia, but after a 17‐day hospital stay he was weaned off supplemental oxygen and transferred to an extended care facility with a therapeutic international normalized ratio (INR).
DISCUSSION
PFOs are congenital cardiac lesions that may persist through adulthood1 and are found incidentally in 19% to 36% of the normal population.2 They may range from 1 to 19 mm in size.2, 3 Contrast echocardiography has enabled a simple, accurate, and safe procedure for diagnosis (>5 microbubbles in the left heart cavities within three cardiac cycles after their appearance in the right atrium is considered diagnostic), though transesophageal echocardiography (TEE) is the gold standard.4
First described by Cohnheim in 1877,5 paradoxical embolism refers to the passage of embolic material from the venous to arterial circulation through a cardiac defect such as a PFO. However, definite confirmation of paradoxical embolism essentially requires catching the thrombus in the act of crossing the foramen ovale. Direct observation of this during life is rarely possible, and remains confined to isolated echocardiographic reports.6‐13
In clinical practice, the diagnosis of paradoxical embolism is almost always presumptive and relies on: (1) the occurrence of an arterial thromboembolic event in the absence of atrial fibrillation, left‐sided heart disease, or severe atherosclerosis; (2) the detection of right‐to‐left shunt, usually through a PFO or an atrial septal defect (ASD); and (3) the presence of venous thrombosis or PE.14
Although most patients are asymptomatic, during the past 20 years an association of PFO with stroke, migraine headache, peripheral arterial occlusion, and decompression induced neurologic dysfunction has been suggested.1 The neurological symptoms are proposed to be secondary to passage of small thrombi from the venous system through the PFO into arterial circulation during a transient right‐to‐left shunt. The source of clots cannot be established in most patients; fewer than 10% will have detectable deep vein thrombosis.15 Even less common are paradoxical emboli to the coronary arteries,1 estimated at 5% to 10% of all paradoxical emboli.16
In order for a thrombus to paradoxically embolize across a PFO, an atrial right‐to‐left pressure gradient must be present. Such a gradient occurs in normal individuals during early ventricular systole and with a Valsalva maneuver.17‐19 In a community‐based cohort study conducted to evaluate potential stroke risk, 148 (out of 581) subjects were found to have a PFO by TEE; 84 (57%) had right‐to‐left shunting at rest, and 136 (92%) had right‐to‐left shunting with Valsalva.2 Pathologic instances of pulmonary hypertension such as PE further elevate right‐heart pressures, further promoting intracardiac shunt.
Acute PE in the setting of PFO carries important prognostic implications. Konstantinides et al.20 prospectively evaluated 139 patients with large acute PE, all of whom had pulmonary hypertension and 96% of whom had right ventricular dilatation. They found a high prevalence of PFOs (35%) in this population. Furthermore, the subgroup with both PE and PFO had a high mortality (33% death rate compared with 14% in those without PFO; P = 0.015). When logistic regression analysis was performed, only arterial hypotension (odds ratio [OR] 26.3; P < 0.001) and the presence of PFO (OR 11.4; P < 0.001) remained significantly correlated with mortality. The authors reported that the presence of a PFO was associated with more than a 5‐fold increase in the adjusted risk of major in‐hospital complications (P < 0.001); no specific etiologic factors were proposed for this association.
In general, MI in the absence of CAD is uncommon, comprising <1% to 6% of all cases.21 No cause is found for the majority, but reported etiologies include coronary spasm in 15%, hypercoagulable states in 13%, collagen vascular diseases in 2%, and paradoxical embolism in 2%.22 AMI due to coronary embolism is uncommon, and when it does occur, left‐to‐left emboli in the setting of atrial fibrillation or prosthetic valves are far more common than paradoxical emboli. In an autopsy series of 1,050 patients with MIs, Prizel et al.23 identified only 55 patients with coronary embolism, none of which was right‐sided in origin.
A handful of published case reports documented paradoxical embolism as a cause for AMI.24‐26 Reported cases more often involved an ASD rather than PFO.27, 28 In most cases, diagnosis was made postmortem, though in a comprehensive review of the literature, Meier‐Ewert et al.13 identified 8 cases of AMI due to paradoxical embolism being diagnosed antemortem. Paradoxical emboli have been identified in all major divisions of the epicardial circulation, though involvement of the left coronary circulation is more common than the right.16
It is well‐established that the prevalence of PFO in patients with cryptogenic stroke is significantly higher than in the general population,1 and Crump et al.21 examined a case‐matched series of 18 patients with AMI who had little to no CAD (<30% stenosis) to see if the frequency of PFO was similarly higher in this group. Each group had identical frequency of PFO (28%; P = NS). The authors concluded that PFO is unlikely to contribute significantly to AMI. However, this study was limited by the small number of patients and the fact that transthoracic echocardiography (TTE) was used instead of TEE for the diagnosis of PFO.
The definitive diagnosis of AMI due to paradoxical embolism requires angiographic findings consistent with embolic occlusion (such as the cutoff sign in a distal coronary artery we observed in Figure 2A), cardiac defects predisposing to paradoxical emboli (such as PFO), and evidence of a venous source for thromboembolism. Alternatively, diagnosis can be made via direct visualization of emboli in the coronary arteries by TEE, or by autopsy.
Although electrocardiography is essential in the diagnosis and treatment of MI, it has the potential to be deceptive. Acute pulmonary hypertension caused by PE may be accompanied by ST elevation in inferior leads II, III, and aVF in a pseudoinfarction pattern mimicking AMI.29 This ECG abnormality probably reflects reciprocal changes of inferoposterior ischemia from right ventricular pressure overloading. However, clearly distinguishing between pseudoinfarction and true inferior infarction in the setting of PE requires coronary angiography.
Regarding therapy, acute treatment of PE is well‐established and consists of at least 5 days of therapeutically‐dosed heparin product that overlaps with therapeutic warfarin anticoagulation. Management of the PFO and coronary embolism is less clear; there are no guidelines for treatment of coronary embolism. Management strategies should focus on treatment of acute ischemia as well as prevention of future emboli, principally anticoagulation. Because the pathogenesis of AMI in this setting is drastically different from MI secondary to atherosclerosis, there is neither a biological basis nor clinical data to suggest benefit from initiation of beta‐blockers, aspirin, angiotensin converting enzyme inhibitors, or statins.
While studies have been done, and are underway to address optimal management of PFO in the setting of both stroke and migraine headache, to our knowledge, no such trials have addressed PFO and MI. Mehan et al.30 reported 2 cases of AMI caused by suspected paradoxical embolism, and in both cases, instant percutaneous closure of PFO was undertaken. However, there are no data to support or refute such an intervention in this particular setting.
Acute pulmonary embolism (PE) and acute myocardial infarction (AMI) are common inpatient diagnoses, and are frequently in the differential diagnosis of patients evaluated for chest pain and dyspnea. We present a case with 1 unifying explanation for these entities to coexist. Acute PE with subsequent embolism to the coronary arteries via a patent foramen ovale (PFO) is rare, but the underlying disorder and anatomical variant are common. Of practical significance, hospitalized patients with acute PE and PFO may have up to a 5‐fold increase in morbidity compared to patients with isolated PE.
CASE REPORT
A 79‐year‐old male smoker underwent resection of a recurrent high‐grade liposarcoma of the right upper extremity. He had no antecedent history of coronary artery disease (CAD) or atrial fibrillation, and had no additional vascular risk factors. On postoperative day 2, he developed acute chest pain, dyspnea, and hypoxia. He appeared alert but was diaphoretic and in moderate distress. Pulse was 84 beats per minute, blood pressure 230/120 mm Hg, and oxygen saturation 59% on room air (93% on supplemental oxygen). Heart and lung exam were unremarkable. Neck veins were not distended. Extremity exam was negative for edema, asymmetry, or calf tenderness, and pedal pulses were palpable bilaterally.
The patient's initial complete blood count, metabolic panel, and cardiac enzymes were within normal limits. Arterial blood gas (on 4‐L nasal cannula) revealed pH of 7.35, partial pressure of arterial oxygen (PaO2) of 65.5 mm Hg, partial pressure of arterial CO2 (PaCO2) of 45.4 mm Hg, and an alveolar‐arterial gradient of 131.3 mm Hg. Electrocardiogram (ECG) (Figure 1) showed an unchanged right bundle branch block, but new 2.5‐mm ST segment elevation in leads V4‐6, III, and aVF, and ST depression in aVL. At this point, the available data suggested either PE with secondary ECG changes or acute ST‐elevation MI with hypoxia. Given the ST elevation in 2 coronary distributions and concern for multivessel CAD, the patient was referred for emergent coronary angiography.

The patient was given aspirin, intravenous unfractionated heparin, and morphine. Left heart catheterization showed an abrupt cutoff in the distal left anterior descending artery (LAD), suggestive of thrombosis secondary to coronary embolism (Figure 2A); angioplasty was not attempted due to the distal location of the occlusion. The posterior descending artery from the right coronary artery was relatively short and the inferior apex was supplied by the distal LAD. The left ventriculogram demonstrated preserved ejection fraction but severe apical hypokinesis, correlating with the occluded vascular territory. The remaining coronary arteries were without significant stenosis. Based on the angiogram findings, paradoxical embolism was suspected. Right heart catheterization identified a previously undiagnosed PFO (Figure 2B); no thrombus was visualized. Right to left shunt was not identified; right ventricular systolic pressure was 46 mm Hg (normal, 15‐25 mm Hg). Subsequent spiral computed tomography (CT) revealed bilateral PEs. It was concluded that the patient had suffered an acute PE, from which the thrombus was able to traverse the PFO and left heart, ultimately entering the LAD, causing the acute embolic MI (Figure 3). ST elevation was present in the inferior leads secondary to the wraparound LAD that supplied the inferior apex, as demonstrated by the wall motion abnormality present on ventriculography.


The patient was felt to be in a hypercoagulable state due to his malignancy and recent surgery; given the diagnosis of an acute PE, no ultrasound was performed to search for a deep vein thrombosis. The patient was transferred to the intensive care unit, continued on intravenous heparin and oxygen and started on oral warfarin. Subsequent hospital course was complicated by aspiration pneumonia and worsening hypoxia, but after a 17‐day hospital stay he was weaned off supplemental oxygen and transferred to an extended care facility with a therapeutic international normalized ratio (INR).
DISCUSSION
PFOs are congenital cardiac lesions that may persist through adulthood1 and are found incidentally in 19% to 36% of the normal population.2 They may range from 1 to 19 mm in size.2, 3 Contrast echocardiography has enabled a simple, accurate, and safe procedure for diagnosis (>5 microbubbles in the left heart cavities within three cardiac cycles after their appearance in the right atrium is considered diagnostic), though transesophageal echocardiography (TEE) is the gold standard.4
First described by Cohnheim in 1877,5 paradoxical embolism refers to the passage of embolic material from the venous to arterial circulation through a cardiac defect such as a PFO. However, definite confirmation of paradoxical embolism essentially requires catching the thrombus in the act of crossing the foramen ovale. Direct observation of this during life is rarely possible, and remains confined to isolated echocardiographic reports.6‐13
In clinical practice, the diagnosis of paradoxical embolism is almost always presumptive and relies on: (1) the occurrence of an arterial thromboembolic event in the absence of atrial fibrillation, left‐sided heart disease, or severe atherosclerosis; (2) the detection of right‐to‐left shunt, usually through a PFO or an atrial septal defect (ASD); and (3) the presence of venous thrombosis or PE.14
Although most patients are asymptomatic, during the past 20 years an association of PFO with stroke, migraine headache, peripheral arterial occlusion, and decompression induced neurologic dysfunction has been suggested.1 The neurological symptoms are proposed to be secondary to passage of small thrombi from the venous system through the PFO into arterial circulation during a transient right‐to‐left shunt. The source of clots cannot be established in most patients; fewer than 10% will have detectable deep vein thrombosis.15 Even less common are paradoxical emboli to the coronary arteries,1 estimated at 5% to 10% of all paradoxical emboli.16
In order for a thrombus to paradoxically embolize across a PFO, an atrial right‐to‐left pressure gradient must be present. Such a gradient occurs in normal individuals during early ventricular systole and with a Valsalva maneuver.17‐19 In a community‐based cohort study conducted to evaluate potential stroke risk, 148 (out of 581) subjects were found to have a PFO by TEE; 84 (57%) had right‐to‐left shunting at rest, and 136 (92%) had right‐to‐left shunting with Valsalva.2 Pathologic instances of pulmonary hypertension such as PE further elevate right‐heart pressures, further promoting intracardiac shunt.
Acute PE in the setting of PFO carries important prognostic implications. Konstantinides et al.20 prospectively evaluated 139 patients with large acute PE, all of whom had pulmonary hypertension and 96% of whom had right ventricular dilatation. They found a high prevalence of PFOs (35%) in this population. Furthermore, the subgroup with both PE and PFO had a high mortality (33% death rate compared with 14% in those without PFO; P = 0.015). When logistic regression analysis was performed, only arterial hypotension (odds ratio [OR] 26.3; P < 0.001) and the presence of PFO (OR 11.4; P < 0.001) remained significantly correlated with mortality. The authors reported that the presence of a PFO was associated with more than a 5‐fold increase in the adjusted risk of major in‐hospital complications (P < 0.001); no specific etiologic factors were proposed for this association.
In general, MI in the absence of CAD is uncommon, comprising <1% to 6% of all cases.21 No cause is found for the majority, but reported etiologies include coronary spasm in 15%, hypercoagulable states in 13%, collagen vascular diseases in 2%, and paradoxical embolism in 2%.22 AMI due to coronary embolism is uncommon, and when it does occur, left‐to‐left emboli in the setting of atrial fibrillation or prosthetic valves are far more common than paradoxical emboli. In an autopsy series of 1,050 patients with MIs, Prizel et al.23 identified only 55 patients with coronary embolism, none of which was right‐sided in origin.
A handful of published case reports documented paradoxical embolism as a cause for AMI.24‐26 Reported cases more often involved an ASD rather than PFO.27, 28 In most cases, diagnosis was made postmortem, though in a comprehensive review of the literature, Meier‐Ewert et al.13 identified 8 cases of AMI due to paradoxical embolism being diagnosed antemortem. Paradoxical emboli have been identified in all major divisions of the epicardial circulation, though involvement of the left coronary circulation is more common than the right.16
It is well‐established that the prevalence of PFO in patients with cryptogenic stroke is significantly higher than in the general population,1 and Crump et al.21 examined a case‐matched series of 18 patients with AMI who had little to no CAD (<30% stenosis) to see if the frequency of PFO was similarly higher in this group. Each group had identical frequency of PFO (28%; P = NS). The authors concluded that PFO is unlikely to contribute significantly to AMI. However, this study was limited by the small number of patients and the fact that transthoracic echocardiography (TTE) was used instead of TEE for the diagnosis of PFO.
The definitive diagnosis of AMI due to paradoxical embolism requires angiographic findings consistent with embolic occlusion (such as the cutoff sign in a distal coronary artery we observed in Figure 2A), cardiac defects predisposing to paradoxical emboli (such as PFO), and evidence of a venous source for thromboembolism. Alternatively, diagnosis can be made via direct visualization of emboli in the coronary arteries by TEE, or by autopsy.
Although electrocardiography is essential in the diagnosis and treatment of MI, it has the potential to be deceptive. Acute pulmonary hypertension caused by PE may be accompanied by ST elevation in inferior leads II, III, and aVF in a pseudoinfarction pattern mimicking AMI.29 This ECG abnormality probably reflects reciprocal changes of inferoposterior ischemia from right ventricular pressure overloading. However, clearly distinguishing between pseudoinfarction and true inferior infarction in the setting of PE requires coronary angiography.
Regarding therapy, acute treatment of PE is well‐established and consists of at least 5 days of therapeutically‐dosed heparin product that overlaps with therapeutic warfarin anticoagulation. Management of the PFO and coronary embolism is less clear; there are no guidelines for treatment of coronary embolism. Management strategies should focus on treatment of acute ischemia as well as prevention of future emboli, principally anticoagulation. Because the pathogenesis of AMI in this setting is drastically different from MI secondary to atherosclerosis, there is neither a biological basis nor clinical data to suggest benefit from initiation of beta‐blockers, aspirin, angiotensin converting enzyme inhibitors, or statins.
While studies have been done, and are underway to address optimal management of PFO in the setting of both stroke and migraine headache, to our knowledge, no such trials have addressed PFO and MI. Mehan et al.30 reported 2 cases of AMI caused by suspected paradoxical embolism, and in both cases, instant percutaneous closure of PFO was undertaken. However, there are no data to support or refute such an intervention in this particular setting.
- ,,, et al.Patent foramen ovale: current pathology, pathophysiology, and clinical status.J Am Coll Cardiol.2005;46:1768–1776.
- ,,, et al.Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC Study.Mayo Clin Proc.1999;74:862–869.
- ,,.Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts.Mayo Clin Proc.1984;59:17–20.
- ,,.Diagnosis of an anatomically and physiologically significant patent foramen ovale.Echocardiography.2006;23:810–815.
- .Thrombose und Embolie: Vorlesung über allgemeine Pathologie. Vol.1.Berlin:Hirschwald;1877;134.
- ,,,,,.Impending paradoxical embolism from atrial thrombus: correct diagnosis by transesophageal echocardiography and prevention by surgery.J Am Coll Cardiol.1985;5:1002–1004.
- ,,.Diagnosis of paradoxic embolism by transesophageal echocardiography.Am Heart J.1991;121:1552–1554.
- ,,,,,.Impending paradoxical embolism: echocardiographic diagnosis of an intracardiac thrombus crossing a patent foramen ovale.Am Heart J.1991;122(3 Pt 1):859–862.
- ,,,,,.Cryptogenic ischemic stroke and paradoxical embolism: should a patent foramen ovale be closed? Case report and literature review.Angiology.2001;52:793–799.
- ,,,,.Thrombus‐in‐transit and paradoxical embolism.J Am Soc Echocardiogr.2002;15:1021–1022.
- ,,,.Caught in the act: impending paradoxical embolism.Asian Cardiovasc Thorac Ann.2002;10:342–343.
- .Paradoxical embolism to the left main coronary artery: visualization by transesophageal echocardiography.J Am Soc Echocardiogr.2002;15:1417–1418.
- ,,,,,.Paradoxical embolism in the left main coronary artery: diagnosis by transesophageal echocardiography.Mayo Clin Proc.2003;78:103–106.
- .Specifics of patent foramen ovale.Adv Neurol.2003;92:197–202.
- ,,, et al.Frequency of deep vein thrombosis in patients with patent foramen ovale and ischemic stroke or transient ischemic attack.Am J Cardiol.1997;80:1066–1069.
- ,.Paradoxical coronary embolism: a rare cause of acute myocardial infarction.Rev Cardiovasc Med.2003;4:107–111.
- ,.Positive contrast echocardiography in patients with patent foramen ovale and normal right heart hemodynamics.Am J Cardiol.1982;49:1806–1809.
- ,,, et al.Contrast echocardiographic visualization of cough‐induced right to left shunt through a patent foramen ovale.J Am Coll Cardiol.1984;4:587–594.
- ,,,,.Transesophageal echocardiographic demonstration of distinct mechanisms for right to left shunting across a patent foramen ovale in the absence of pulmonary hypertension.J Am Coll Cardiol.1991;18:1112–1117.
- ,,,,,.Patent foramen ovale is an important predictor of adverse outcome in patients with major acute pulmonary embolism.Circulation.1998;97:1946–1951.
- ,,,.Prevalence of patent foramen ovale in patients with acute myocardial infarction and angiographically normal coronary arteries.Am J Cardiol.2000;85:1368–1370.
- ,,,,,.Clinical characteristics, aetiological factors and long‐term prognosis of myocardial infarction with an absolutely normal coronary angiogram; a 3‐year follow‐up study of 91 patients.Eur Heart J.2001;22:1459–1465.
- ,,.Coronary artery embolism and myocardial infarction.Ann Intern Med.1978;88:155–161.
- ,..Myocardial infarction due to a paradoxical embolism.Am J Med.1969;47:995–998.
- ,,,,,.[Myocardial infarction caused by probable paradoxical embolism and aneurysm of the interatrial septum].Presse Med.1996;25:907 [French].
- ,,.Acute myocardial infarction probably caused by paradoxical embolus in a pregnant woman.Heart.2004;90:e12.
- ,,,,.A case of acute pulmonary embolism and acute myocardial infarction with suspected paradoxical embolism after laparoscopic surgery.Heart Vessels.1999;4:197–200.
- ,,,,,.Acute myocardial infarction caused by paradoxical coronary embolization in a patient with a patent foramen ovale.J Am Soc Echocardiogr.2001;14:1227–1229.
- .ECG manifestations of selected extracardiac diseases.Emerg Med Clin N Am.2006;24:133–143.
- ,,,.Instant percutaneous closure of patent foramen ovale in patients with acute myocardial infarction and normal coronary arteries.Catheter Cardiovasc Interv.2006;67:279–282.
- ,,, et al.Patent foramen ovale: current pathology, pathophysiology, and clinical status.J Am Coll Cardiol.2005;46:1768–1776.
- ,,, et al.Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC Study.Mayo Clin Proc.1999;74:862–869.
- ,,.Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts.Mayo Clin Proc.1984;59:17–20.
- ,,.Diagnosis of an anatomically and physiologically significant patent foramen ovale.Echocardiography.2006;23:810–815.
- .Thrombose und Embolie: Vorlesung über allgemeine Pathologie. Vol.1.Berlin:Hirschwald;1877;134.
- ,,,,,.Impending paradoxical embolism from atrial thrombus: correct diagnosis by transesophageal echocardiography and prevention by surgery.J Am Coll Cardiol.1985;5:1002–1004.
- ,,.Diagnosis of paradoxic embolism by transesophageal echocardiography.Am Heart J.1991;121:1552–1554.
- ,,,,,.Impending paradoxical embolism: echocardiographic diagnosis of an intracardiac thrombus crossing a patent foramen ovale.Am Heart J.1991;122(3 Pt 1):859–862.
- ,,,,,.Cryptogenic ischemic stroke and paradoxical embolism: should a patent foramen ovale be closed? Case report and literature review.Angiology.2001;52:793–799.
- ,,,,.Thrombus‐in‐transit and paradoxical embolism.J Am Soc Echocardiogr.2002;15:1021–1022.
- ,,,.Caught in the act: impending paradoxical embolism.Asian Cardiovasc Thorac Ann.2002;10:342–343.
- .Paradoxical embolism to the left main coronary artery: visualization by transesophageal echocardiography.J Am Soc Echocardiogr.2002;15:1417–1418.
- ,,,,,.Paradoxical embolism in the left main coronary artery: diagnosis by transesophageal echocardiography.Mayo Clin Proc.2003;78:103–106.
- .Specifics of patent foramen ovale.Adv Neurol.2003;92:197–202.
- ,,, et al.Frequency of deep vein thrombosis in patients with patent foramen ovale and ischemic stroke or transient ischemic attack.Am J Cardiol.1997;80:1066–1069.
- ,.Paradoxical coronary embolism: a rare cause of acute myocardial infarction.Rev Cardiovasc Med.2003;4:107–111.
- ,.Positive contrast echocardiography in patients with patent foramen ovale and normal right heart hemodynamics.Am J Cardiol.1982;49:1806–1809.
- ,,, et al.Contrast echocardiographic visualization of cough‐induced right to left shunt through a patent foramen ovale.J Am Coll Cardiol.1984;4:587–594.
- ,,,,.Transesophageal echocardiographic demonstration of distinct mechanisms for right to left shunting across a patent foramen ovale in the absence of pulmonary hypertension.J Am Coll Cardiol.1991;18:1112–1117.
- ,,,,,.Patent foramen ovale is an important predictor of adverse outcome in patients with major acute pulmonary embolism.Circulation.1998;97:1946–1951.
- ,,,.Prevalence of patent foramen ovale in patients with acute myocardial infarction and angiographically normal coronary arteries.Am J Cardiol.2000;85:1368–1370.
- ,,,,,.Clinical characteristics, aetiological factors and long‐term prognosis of myocardial infarction with an absolutely normal coronary angiogram; a 3‐year follow‐up study of 91 patients.Eur Heart J.2001;22:1459–1465.
- ,,.Coronary artery embolism and myocardial infarction.Ann Intern Med.1978;88:155–161.
- ,..Myocardial infarction due to a paradoxical embolism.Am J Med.1969;47:995–998.
- ,,,,,.[Myocardial infarction caused by probable paradoxical embolism and aneurysm of the interatrial septum].Presse Med.1996;25:907 [French].
- ,,.Acute myocardial infarction probably caused by paradoxical embolus in a pregnant woman.Heart.2004;90:e12.
- ,,,,.A case of acute pulmonary embolism and acute myocardial infarction with suspected paradoxical embolism after laparoscopic surgery.Heart Vessels.1999;4:197–200.
- ,,,,,.Acute myocardial infarction caused by paradoxical coronary embolization in a patient with a patent foramen ovale.J Am Soc Echocardiogr.2001;14:1227–1229.
- .ECG manifestations of selected extracardiac diseases.Emerg Med Clin N Am.2006;24:133–143.
- ,,,.Instant percutaneous closure of patent foramen ovale in patients with acute myocardial infarction and normal coronary arteries.Catheter Cardiovasc Interv.2006;67:279–282.