User login
A Facility-Wide Plan to Increase Access to Medication for Opioid Use Disorder in Primary Care and General Mental Health Settings
In the United States, opioid use disorder (OUD) is a major public health challenge. In 2018 drug overdose deaths were 4 times higher than they were in 1999.1 This increase highlights a critical need to expand treatment access. Medication for opioid use disorder (MOUD), including methadone, naltrexone, and buprenorphine, improves outcomes for patients retained in care.2 Compared with the general population, veterans, particularly those with co-occurring posttraumatic stress disorder (PTSD) or depression, are more likely to receive higher dosages of opioid medications and experience opioid-related adverse outcomes (eg, overdose, OUD).3,4 As a risk reduction strategy, patients receiving potentially dangerous full-dose agonist opioid medication who are unable to taper to safer dosages may be eligible to transition to buprenorphine.5
Buprenorphine and naltrexone can be prescribed in office-based settings or in addiction, primary care, mental health, and pain clinics. Office-based opioid treatment with buprenorphine (OBOT-B) expands access to patients who are not reached by addiction treatment programs.6,7 This is particularly true in rural settings, where addiction care services are typically scarce.8 OBOT-B prevents relapse and maintains opioid-free days and may increase patient engagement by reducing stigma and providing treatment within an existing clinical care team.9 For many patients, OBOT-B results in good retention with just medical monitoring and minimal or no ancillary addiction counseling.10,11
Successful implementation of OBOT-B has occurred through a variety of care models in selected community health care settings.8,12,13 Historically in the Veterans Health Administration (VHA), MOUD has been prescribed in substance use disorder clinics by mental health practitioners. Currently, more than 44% of veterans with OUD are on MOUD.14
The VHA has invested significant resources to improve access to MOUD. In 2018, the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative launched, with the aim to improve access within primary care, mental health, and pain clinics.15 SCOUTT emphasizes stepped-care treatment, with patients engaging in the step of care most appropriate to their needs. Step 0 is self-directed care/self-management, including mutual support groups; step-1 environments include office-based primary care, mental health, and pain clinics; and step-2 environments are specialty care settings. Through a series of remote webinars, an in-person national 2-day conference, and external facilitation, SCOUTT engaged 18 teams representing each Veterans Integrated Service Network (VISN) across the country to assist in implementing MOUD within 2 step-1 clinics. These teams have developed several models of providing step-1 care, including an interdisciplinary team-based primary care delivery model as well as a pharmacist care manager model.16, 17
US Department of Veterans Affairs (VA) Connecticut Health Care System (VACHS), which delivers care to approximately 58,000 veterans, was chosen to be a phase 1 SCOUTT site. Though all patients in VACHS have access to specialty care step-2 clinics, including methadone and buprenorphine programs, there remained many patients not yet on MOUD who could benefit from it. Baseline data (fiscal year [FY] 2018 4th quarter), obtained through electronic health record (EHR) database dashboards indicated that 710 (56%) patients with an OUD diagnosis were not receiving MOUD. International Classification of Disease, 10th Revision codes are the foundation for VA population management dashboards, and based their data on codes for opioid abuse and opioid dependence. These tools are limited by the accuracy of coding in EHRs. Additionally, 366 patients receiving long-term opioid prescriptions were identified as moderate, high, or very high risk for overdose or death based on an algorithm that considered prescribed medications, sociodemographics, and comorbid conditions, as characterized in the VA EHR (Stratification Tool for Opioid Risk Mitigation [STORM] report).18
This article describes the VACHSquality-improvement effort to extend OBOT-B into step-1 primary care and general mental health clinics. Our objectives are to (1) outline the process for initiating SCOUTT within VACHS; (2) examine barriers to implementation and the SCOUTT team response; (3) review VACHS patient and prescriber data at baseline and 1 year after implementation; and (4) explore future implementation strategies.
SCOUTT Team
A VACHS interdisciplinary team was formed and attended the national SCOUTT kickoff conference in 2018.15 Similar to other SCOUTT teams, the team consisted of VISN leadership (in primary care, mental health, and addiction care), pharmacists, and a team of health care practitioners (HCPs) from step-2 clinics (including 2 addiction psychiatrists, and an advanced practice registered nurse, a registered nurse specializing in addiction care), and a team of HCPs from prospective step-1 clinics (including a clinical psychologist and 2 primary care physicians). An external facilitator was provided from outside the VISN who met remotely with the team to assist in facilitation. Our team met monthly, with the goal to identify local barriers and facilitators to OBOT-B and implement interventions to enhance prescribing in step-1 primary care and general mental health clinics.
Implementation Steps
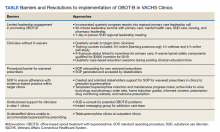
The team identified multiple barriers to dissemination of OBOT-B in target clinics (Table). The 3 main barriers were limited leadership engagement in promoting OBOT-B in target clinics, inadequate number of HCPs with active X-waivered prescribing status in the targeted clinics, and the need for standardized processes and tools to facilitate prescribing and follow-up.
To address leadership engagement, the SCOUTT team held quarterly presentations of SCOUTT goals and progress on target clinic leadership calls (usually 15 minutes) and arranged a 90-minute multidisciplinary leadership summit with key leadership representation from primary care, general mental health, specialty addiction care, nursing, and pharmacy. To enhance X-waivered prescribers in target clinics, the SCOUTT team sent quarterly emails with brief education points on MOUD and links to waiver trainings. At the time of implementation, in order to prescribe buprenorphine and meet qualifications to treat OUD, prescribers were required to complete specialized training as necessitated by the Drug Addiction Treatment Act of 2000. X-waivered status can now be obtained without requiring training
The SCOUTT team advocated for X-waivered status to be incentivized by performance pay for primary care practitioners and held quarterly case-based education sessions during preexisting allotted time. The onboarding process for new waivered prescribers to navigate from waiver training to active prescribing within the EHR was standardized via development of a standard operating procedure (SOP).
The SCOUTT team also assisted in the development of standardized processes and tools for prescribing in target clinics, including implementation of a standard operating procedure regarding prescribing (both initiation of buprenorphine, and maintenance) in target clinics. This procedure specifies that target clinic HCPs prescribe for patients requiring less intensive management, and who are appropriate for office-based treatment based on specific criteria (eAppendix
Templated progress notes were created for buprenorphine initiation and buprenorphine maintenance with links to recommended laboratory tests and urine toxicology test ordering, home induction guides, prescription drug monitoring database, naloxone prescribing, and pharmacy order sets. Communication with specialty HCPs was facilitated by development of e-consultation within the EHR and instant messaging options within the local intranet. In the SCOUTT team model, the prescriber independently completed assessment/follow-up without nursing or clinical pharmacy support.
Analysis
We examined changes in MOUD receipt and prescriber characteristics at baseline (FY 2018 4th quarter) and 1 year after implementation (FY 2019 4th quarter). Patient data were extracted from the VHA Corporate Data Warehouse (CDW), which contains data from all VHA EHRs. The VA STORM, is a CDW tool that automatically flags patients prescribed opioids who are at risk for overdose and suicide. Prescriber data were obtained from the Buprenorphine/X-Waivered Provider Report, a VA Academic Detailing Service database that provides details on HCP type, X-waivered status, and prescribing by location. χ2 analyses were conducted on before and after measures when total values were available.
Results
There was a 4% increase in patients with an OUD diagnosis receiving MOUD, from 552 (44%) to 582 (48%) (P = .04), over this time. The number of waivered prescribers increased from 67 to 131, the number of prescribers of buprenorphine in a 6-month span increased from 35 to 52, and the percentage of HCPs capable of prescribing within the EHR increased from 75% to 89% (P =.01).
Initially, addiction HCPs prescribed to about 68% of patients on buprenorphine, with target clinic HCPs prescribing to 24% (with the remaining coming from other specialty HCPs). On follow-up, addiction professionals prescribed to 63%, with target clinic clincians prescribing to 32%.
Interpretation
SCOUTT team interventions succeeded in increasing the number of patients receiving MOUD, a substantial increase in waivered HCPs, an increase in the number of waivered HCPs prescribing MOUD, and an increase in the proportion of patients receiving MOUD in step-1 target clinics. It is important to note that within the quality-improvement framework and goals of our SCOUTT team that the data were not collected as part of a research study but to assess impact of our interventions. Within this framework, it is not possible to directly attribute the increase in eligible patients receiving MOUD solely to SCOUTT team interventions, as other factors may have contributed, including improved awareness of HCPs.
Summary and Future Directions
Since implementation of SCOUTT in August 2018, VACHS has identified several barriers to buprenorphine prescribing in step-1 clinics and implemented strategies to overcome them. Describing our approach will hopefully inform other large health care systems (VA or non-VA) on changes required in order to scale up implementation of OBOT-B. The VACHS SCOUTT team was successful at enhancing a ready workforce in step-1 clinics, though noted a delay in changing prescribing practice and culture.
We recommend utilizing academic detailing to work with clinics and individual HCPs to identify and overcome barriers to prescribing. Also, we recommend implementation of a nursing or clinical pharmacy collaborative care model in target step-1 clinics (rather than the HCP-driven model). A collaborative care model reflects the patient aligned care team (PACT) principle of team-based efficient care, and PACT nurses or clinical pharmacists should be able to provide the minimal quarterly follow-up of clinically stable patients on MOUD within the step-1 clinics. Templated notes for assessment, initiation, and follow-up of patients on MOUD are now available from the SCOUTT national program and should be broadly implemented to facilitate adoption of the collaborative model in target clinics. In order to accomplish a full collaborative model, the VHA would need to enhance appropriate staffing to support this model, broaden access to telehealth, and expand incentives to teams/clinicians who prescribe in these settings.
Acknowledgments/Funding
This material is based upon work supported by the US Department of Veterans Affairs (VA), Office of Mental Health and Suicide Prevention, Veterans Health Administration; the VA Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (PEC) grants #19-001. Supporting organizations had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
1. Centers for Disease Control and Prevention. Understanding the epidemic. Updated March 17, 2021. Accessed September 17, 2021. https://www.cdc.gov/drugoverdose/epidemic/index.html
2. Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. 2019;393(10182):1760-1772. doi:10.1016/S0140-6736(18)33078-2
3. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan [published correction appears in JAMA. 2012 Jun 20;307(23):2489]. JAMA. 2012;307(9):940-947. doi:10.1001/jama.2012.234
4. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612. doi:10.1097/AJP.0000000000000011
5. US Department of Health and Human Services, Working Group on Patient-Centered Reduction or Discontinuation of Long-term Opioid Analgesics. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of Long-term opioid analgesics. Published October 2019. Accessed September 17, 2021. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf
6. Sullivan LE, Chawarski M, O’Connor PG, Schottenfeld RS, Fiellin DA. The practice of office-based buprenorphine treatment of opioid dependence: is it associated with new patients entering into treatment?. Drug Alcohol Depend. 2005;79(1):113-116. doi:10.1016/j.drugalcdep.2004.12.008
7. LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts collaborative care model in community health centers. J Subst Abuse Treat. 2016;60:6-13. doi:10.1016/j.jsat.2015.06.010
8. Rubin R. Rural veterans less likely to get medication for opioid use disorder. JAMA. 2020;323(4):300. doi:10.1001/jama.2019.21856
9. Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2011;57(3):281-289.
10. Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2-5 years. Am J Addict. 2008;17(2):116-120. doi:10.1080/10550490701860971
11. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365-374. doi:10.1056/NEJMoa055255
12. Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1-2):127-135. doi:10.1016/j.drugalcdep.2012.12.008
13. Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22(2):171-176. doi:10.1007/s11606-006-0023-1
14. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
15. Gordon AJ, Drexler K, Hawkins EJ, et al. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: Expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abus. 2020;41(3):275-282. doi:10.1080/08897077.2020.1787299
16. Codell N, Kelley AT, Jones AL, et al. Aims, development, and early results of an interdisciplinary primary care initiative to address patient vulnerabilities. Am J Drug Alcohol Abuse. 2021;47(2):160-169. doi:10.1080/00952990.2020.1832507
17. DeRonne BM, Wong KR, Schultz E, Jones E, Krebs EE. Implementation of a pharmacist care manager model to expand availability of medications for opioid use disorder. Am J Health Syst Pharm. 2021;78(4):354-359. doi:10.1093/ajhp/zxaa405
18. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
19. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
In the United States, opioid use disorder (OUD) is a major public health challenge. In 2018 drug overdose deaths were 4 times higher than they were in 1999.1 This increase highlights a critical need to expand treatment access. Medication for opioid use disorder (MOUD), including methadone, naltrexone, and buprenorphine, improves outcomes for patients retained in care.2 Compared with the general population, veterans, particularly those with co-occurring posttraumatic stress disorder (PTSD) or depression, are more likely to receive higher dosages of opioid medications and experience opioid-related adverse outcomes (eg, overdose, OUD).3,4 As a risk reduction strategy, patients receiving potentially dangerous full-dose agonist opioid medication who are unable to taper to safer dosages may be eligible to transition to buprenorphine.5
Buprenorphine and naltrexone can be prescribed in office-based settings or in addiction, primary care, mental health, and pain clinics. Office-based opioid treatment with buprenorphine (OBOT-B) expands access to patients who are not reached by addiction treatment programs.6,7 This is particularly true in rural settings, where addiction care services are typically scarce.8 OBOT-B prevents relapse and maintains opioid-free days and may increase patient engagement by reducing stigma and providing treatment within an existing clinical care team.9 For many patients, OBOT-B results in good retention with just medical monitoring and minimal or no ancillary addiction counseling.10,11
Successful implementation of OBOT-B has occurred through a variety of care models in selected community health care settings.8,12,13 Historically in the Veterans Health Administration (VHA), MOUD has been prescribed in substance use disorder clinics by mental health practitioners. Currently, more than 44% of veterans with OUD are on MOUD.14
The VHA has invested significant resources to improve access to MOUD. In 2018, the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative launched, with the aim to improve access within primary care, mental health, and pain clinics.15 SCOUTT emphasizes stepped-care treatment, with patients engaging in the step of care most appropriate to their needs. Step 0 is self-directed care/self-management, including mutual support groups; step-1 environments include office-based primary care, mental health, and pain clinics; and step-2 environments are specialty care settings. Through a series of remote webinars, an in-person national 2-day conference, and external facilitation, SCOUTT engaged 18 teams representing each Veterans Integrated Service Network (VISN) across the country to assist in implementing MOUD within 2 step-1 clinics. These teams have developed several models of providing step-1 care, including an interdisciplinary team-based primary care delivery model as well as a pharmacist care manager model.16, 17
US Department of Veterans Affairs (VA) Connecticut Health Care System (VACHS), which delivers care to approximately 58,000 veterans, was chosen to be a phase 1 SCOUTT site. Though all patients in VACHS have access to specialty care step-2 clinics, including methadone and buprenorphine programs, there remained many patients not yet on MOUD who could benefit from it. Baseline data (fiscal year [FY] 2018 4th quarter), obtained through electronic health record (EHR) database dashboards indicated that 710 (56%) patients with an OUD diagnosis were not receiving MOUD. International Classification of Disease, 10th Revision codes are the foundation for VA population management dashboards, and based their data on codes for opioid abuse and opioid dependence. These tools are limited by the accuracy of coding in EHRs. Additionally, 366 patients receiving long-term opioid prescriptions were identified as moderate, high, or very high risk for overdose or death based on an algorithm that considered prescribed medications, sociodemographics, and comorbid conditions, as characterized in the VA EHR (Stratification Tool for Opioid Risk Mitigation [STORM] report).18
This article describes the VACHSquality-improvement effort to extend OBOT-B into step-1 primary care and general mental health clinics. Our objectives are to (1) outline the process for initiating SCOUTT within VACHS; (2) examine barriers to implementation and the SCOUTT team response; (3) review VACHS patient and prescriber data at baseline and 1 year after implementation; and (4) explore future implementation strategies.
SCOUTT Team
A VACHS interdisciplinary team was formed and attended the national SCOUTT kickoff conference in 2018.15 Similar to other SCOUTT teams, the team consisted of VISN leadership (in primary care, mental health, and addiction care), pharmacists, and a team of health care practitioners (HCPs) from step-2 clinics (including 2 addiction psychiatrists, and an advanced practice registered nurse, a registered nurse specializing in addiction care), and a team of HCPs from prospective step-1 clinics (including a clinical psychologist and 2 primary care physicians). An external facilitator was provided from outside the VISN who met remotely with the team to assist in facilitation. Our team met monthly, with the goal to identify local barriers and facilitators to OBOT-B and implement interventions to enhance prescribing in step-1 primary care and general mental health clinics.
Implementation Steps
The team identified multiple barriers to dissemination of OBOT-B in target clinics (Table). The 3 main barriers were limited leadership engagement in promoting OBOT-B in target clinics, inadequate number of HCPs with active X-waivered prescribing status in the targeted clinics, and the need for standardized processes and tools to facilitate prescribing and follow-up.
To address leadership engagement, the SCOUTT team held quarterly presentations of SCOUTT goals and progress on target clinic leadership calls (usually 15 minutes) and arranged a 90-minute multidisciplinary leadership summit with key leadership representation from primary care, general mental health, specialty addiction care, nursing, and pharmacy. To enhance X-waivered prescribers in target clinics, the SCOUTT team sent quarterly emails with brief education points on MOUD and links to waiver trainings. At the time of implementation, in order to prescribe buprenorphine and meet qualifications to treat OUD, prescribers were required to complete specialized training as necessitated by the Drug Addiction Treatment Act of 2000. X-waivered status can now be obtained without requiring training
The SCOUTT team advocated for X-waivered status to be incentivized by performance pay for primary care practitioners and held quarterly case-based education sessions during preexisting allotted time. The onboarding process for new waivered prescribers to navigate from waiver training to active prescribing within the EHR was standardized via development of a standard operating procedure (SOP).
The SCOUTT team also assisted in the development of standardized processes and tools for prescribing in target clinics, including implementation of a standard operating procedure regarding prescribing (both initiation of buprenorphine, and maintenance) in target clinics. This procedure specifies that target clinic HCPs prescribe for patients requiring less intensive management, and who are appropriate for office-based treatment based on specific criteria (eAppendix
Templated progress notes were created for buprenorphine initiation and buprenorphine maintenance with links to recommended laboratory tests and urine toxicology test ordering, home induction guides, prescription drug monitoring database, naloxone prescribing, and pharmacy order sets. Communication with specialty HCPs was facilitated by development of e-consultation within the EHR and instant messaging options within the local intranet. In the SCOUTT team model, the prescriber independently completed assessment/follow-up without nursing or clinical pharmacy support.
Analysis
We examined changes in MOUD receipt and prescriber characteristics at baseline (FY 2018 4th quarter) and 1 year after implementation (FY 2019 4th quarter). Patient data were extracted from the VHA Corporate Data Warehouse (CDW), which contains data from all VHA EHRs. The VA STORM, is a CDW tool that automatically flags patients prescribed opioids who are at risk for overdose and suicide. Prescriber data were obtained from the Buprenorphine/X-Waivered Provider Report, a VA Academic Detailing Service database that provides details on HCP type, X-waivered status, and prescribing by location. χ2 analyses were conducted on before and after measures when total values were available.
Results
There was a 4% increase in patients with an OUD diagnosis receiving MOUD, from 552 (44%) to 582 (48%) (P = .04), over this time. The number of waivered prescribers increased from 67 to 131, the number of prescribers of buprenorphine in a 6-month span increased from 35 to 52, and the percentage of HCPs capable of prescribing within the EHR increased from 75% to 89% (P =.01).
Initially, addiction HCPs prescribed to about 68% of patients on buprenorphine, with target clinic HCPs prescribing to 24% (with the remaining coming from other specialty HCPs). On follow-up, addiction professionals prescribed to 63%, with target clinic clincians prescribing to 32%.
Interpretation
SCOUTT team interventions succeeded in increasing the number of patients receiving MOUD, a substantial increase in waivered HCPs, an increase in the number of waivered HCPs prescribing MOUD, and an increase in the proportion of patients receiving MOUD in step-1 target clinics. It is important to note that within the quality-improvement framework and goals of our SCOUTT team that the data were not collected as part of a research study but to assess impact of our interventions. Within this framework, it is not possible to directly attribute the increase in eligible patients receiving MOUD solely to SCOUTT team interventions, as other factors may have contributed, including improved awareness of HCPs.
Summary and Future Directions
Since implementation of SCOUTT in August 2018, VACHS has identified several barriers to buprenorphine prescribing in step-1 clinics and implemented strategies to overcome them. Describing our approach will hopefully inform other large health care systems (VA or non-VA) on changes required in order to scale up implementation of OBOT-B. The VACHS SCOUTT team was successful at enhancing a ready workforce in step-1 clinics, though noted a delay in changing prescribing practice and culture.
We recommend utilizing academic detailing to work with clinics and individual HCPs to identify and overcome barriers to prescribing. Also, we recommend implementation of a nursing or clinical pharmacy collaborative care model in target step-1 clinics (rather than the HCP-driven model). A collaborative care model reflects the patient aligned care team (PACT) principle of team-based efficient care, and PACT nurses or clinical pharmacists should be able to provide the minimal quarterly follow-up of clinically stable patients on MOUD within the step-1 clinics. Templated notes for assessment, initiation, and follow-up of patients on MOUD are now available from the SCOUTT national program and should be broadly implemented to facilitate adoption of the collaborative model in target clinics. In order to accomplish a full collaborative model, the VHA would need to enhance appropriate staffing to support this model, broaden access to telehealth, and expand incentives to teams/clinicians who prescribe in these settings.
Acknowledgments/Funding
This material is based upon work supported by the US Department of Veterans Affairs (VA), Office of Mental Health and Suicide Prevention, Veterans Health Administration; the VA Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (PEC) grants #19-001. Supporting organizations had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
In the United States, opioid use disorder (OUD) is a major public health challenge. In 2018 drug overdose deaths were 4 times higher than they were in 1999.1 This increase highlights a critical need to expand treatment access. Medication for opioid use disorder (MOUD), including methadone, naltrexone, and buprenorphine, improves outcomes for patients retained in care.2 Compared with the general population, veterans, particularly those with co-occurring posttraumatic stress disorder (PTSD) or depression, are more likely to receive higher dosages of opioid medications and experience opioid-related adverse outcomes (eg, overdose, OUD).3,4 As a risk reduction strategy, patients receiving potentially dangerous full-dose agonist opioid medication who are unable to taper to safer dosages may be eligible to transition to buprenorphine.5
Buprenorphine and naltrexone can be prescribed in office-based settings or in addiction, primary care, mental health, and pain clinics. Office-based opioid treatment with buprenorphine (OBOT-B) expands access to patients who are not reached by addiction treatment programs.6,7 This is particularly true in rural settings, where addiction care services are typically scarce.8 OBOT-B prevents relapse and maintains opioid-free days and may increase patient engagement by reducing stigma and providing treatment within an existing clinical care team.9 For many patients, OBOT-B results in good retention with just medical monitoring and minimal or no ancillary addiction counseling.10,11
Successful implementation of OBOT-B has occurred through a variety of care models in selected community health care settings.8,12,13 Historically in the Veterans Health Administration (VHA), MOUD has been prescribed in substance use disorder clinics by mental health practitioners. Currently, more than 44% of veterans with OUD are on MOUD.14
The VHA has invested significant resources to improve access to MOUD. In 2018, the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative launched, with the aim to improve access within primary care, mental health, and pain clinics.15 SCOUTT emphasizes stepped-care treatment, with patients engaging in the step of care most appropriate to their needs. Step 0 is self-directed care/self-management, including mutual support groups; step-1 environments include office-based primary care, mental health, and pain clinics; and step-2 environments are specialty care settings. Through a series of remote webinars, an in-person national 2-day conference, and external facilitation, SCOUTT engaged 18 teams representing each Veterans Integrated Service Network (VISN) across the country to assist in implementing MOUD within 2 step-1 clinics. These teams have developed several models of providing step-1 care, including an interdisciplinary team-based primary care delivery model as well as a pharmacist care manager model.16, 17
US Department of Veterans Affairs (VA) Connecticut Health Care System (VACHS), which delivers care to approximately 58,000 veterans, was chosen to be a phase 1 SCOUTT site. Though all patients in VACHS have access to specialty care step-2 clinics, including methadone and buprenorphine programs, there remained many patients not yet on MOUD who could benefit from it. Baseline data (fiscal year [FY] 2018 4th quarter), obtained through electronic health record (EHR) database dashboards indicated that 710 (56%) patients with an OUD diagnosis were not receiving MOUD. International Classification of Disease, 10th Revision codes are the foundation for VA population management dashboards, and based their data on codes for opioid abuse and opioid dependence. These tools are limited by the accuracy of coding in EHRs. Additionally, 366 patients receiving long-term opioid prescriptions were identified as moderate, high, or very high risk for overdose or death based on an algorithm that considered prescribed medications, sociodemographics, and comorbid conditions, as characterized in the VA EHR (Stratification Tool for Opioid Risk Mitigation [STORM] report).18
This article describes the VACHSquality-improvement effort to extend OBOT-B into step-1 primary care and general mental health clinics. Our objectives are to (1) outline the process for initiating SCOUTT within VACHS; (2) examine barriers to implementation and the SCOUTT team response; (3) review VACHS patient and prescriber data at baseline and 1 year after implementation; and (4) explore future implementation strategies.
SCOUTT Team
A VACHS interdisciplinary team was formed and attended the national SCOUTT kickoff conference in 2018.15 Similar to other SCOUTT teams, the team consisted of VISN leadership (in primary care, mental health, and addiction care), pharmacists, and a team of health care practitioners (HCPs) from step-2 clinics (including 2 addiction psychiatrists, and an advanced practice registered nurse, a registered nurse specializing in addiction care), and a team of HCPs from prospective step-1 clinics (including a clinical psychologist and 2 primary care physicians). An external facilitator was provided from outside the VISN who met remotely with the team to assist in facilitation. Our team met monthly, with the goal to identify local barriers and facilitators to OBOT-B and implement interventions to enhance prescribing in step-1 primary care and general mental health clinics.
Implementation Steps
The team identified multiple barriers to dissemination of OBOT-B in target clinics (Table). The 3 main barriers were limited leadership engagement in promoting OBOT-B in target clinics, inadequate number of HCPs with active X-waivered prescribing status in the targeted clinics, and the need for standardized processes and tools to facilitate prescribing and follow-up.
To address leadership engagement, the SCOUTT team held quarterly presentations of SCOUTT goals and progress on target clinic leadership calls (usually 15 minutes) and arranged a 90-minute multidisciplinary leadership summit with key leadership representation from primary care, general mental health, specialty addiction care, nursing, and pharmacy. To enhance X-waivered prescribers in target clinics, the SCOUTT team sent quarterly emails with brief education points on MOUD and links to waiver trainings. At the time of implementation, in order to prescribe buprenorphine and meet qualifications to treat OUD, prescribers were required to complete specialized training as necessitated by the Drug Addiction Treatment Act of 2000. X-waivered status can now be obtained without requiring training
The SCOUTT team advocated for X-waivered status to be incentivized by performance pay for primary care practitioners and held quarterly case-based education sessions during preexisting allotted time. The onboarding process for new waivered prescribers to navigate from waiver training to active prescribing within the EHR was standardized via development of a standard operating procedure (SOP).
The SCOUTT team also assisted in the development of standardized processes and tools for prescribing in target clinics, including implementation of a standard operating procedure regarding prescribing (both initiation of buprenorphine, and maintenance) in target clinics. This procedure specifies that target clinic HCPs prescribe for patients requiring less intensive management, and who are appropriate for office-based treatment based on specific criteria (eAppendix
Templated progress notes were created for buprenorphine initiation and buprenorphine maintenance with links to recommended laboratory tests and urine toxicology test ordering, home induction guides, prescription drug monitoring database, naloxone prescribing, and pharmacy order sets. Communication with specialty HCPs was facilitated by development of e-consultation within the EHR and instant messaging options within the local intranet. In the SCOUTT team model, the prescriber independently completed assessment/follow-up without nursing or clinical pharmacy support.
Analysis
We examined changes in MOUD receipt and prescriber characteristics at baseline (FY 2018 4th quarter) and 1 year after implementation (FY 2019 4th quarter). Patient data were extracted from the VHA Corporate Data Warehouse (CDW), which contains data from all VHA EHRs. The VA STORM, is a CDW tool that automatically flags patients prescribed opioids who are at risk for overdose and suicide. Prescriber data were obtained from the Buprenorphine/X-Waivered Provider Report, a VA Academic Detailing Service database that provides details on HCP type, X-waivered status, and prescribing by location. χ2 analyses were conducted on before and after measures when total values were available.
Results
There was a 4% increase in patients with an OUD diagnosis receiving MOUD, from 552 (44%) to 582 (48%) (P = .04), over this time. The number of waivered prescribers increased from 67 to 131, the number of prescribers of buprenorphine in a 6-month span increased from 35 to 52, and the percentage of HCPs capable of prescribing within the EHR increased from 75% to 89% (P =.01).
Initially, addiction HCPs prescribed to about 68% of patients on buprenorphine, with target clinic HCPs prescribing to 24% (with the remaining coming from other specialty HCPs). On follow-up, addiction professionals prescribed to 63%, with target clinic clincians prescribing to 32%.
Interpretation
SCOUTT team interventions succeeded in increasing the number of patients receiving MOUD, a substantial increase in waivered HCPs, an increase in the number of waivered HCPs prescribing MOUD, and an increase in the proportion of patients receiving MOUD in step-1 target clinics. It is important to note that within the quality-improvement framework and goals of our SCOUTT team that the data were not collected as part of a research study but to assess impact of our interventions. Within this framework, it is not possible to directly attribute the increase in eligible patients receiving MOUD solely to SCOUTT team interventions, as other factors may have contributed, including improved awareness of HCPs.
Summary and Future Directions
Since implementation of SCOUTT in August 2018, VACHS has identified several barriers to buprenorphine prescribing in step-1 clinics and implemented strategies to overcome them. Describing our approach will hopefully inform other large health care systems (VA or non-VA) on changes required in order to scale up implementation of OBOT-B. The VACHS SCOUTT team was successful at enhancing a ready workforce in step-1 clinics, though noted a delay in changing prescribing practice and culture.
We recommend utilizing academic detailing to work with clinics and individual HCPs to identify and overcome barriers to prescribing. Also, we recommend implementation of a nursing or clinical pharmacy collaborative care model in target step-1 clinics (rather than the HCP-driven model). A collaborative care model reflects the patient aligned care team (PACT) principle of team-based efficient care, and PACT nurses or clinical pharmacists should be able to provide the minimal quarterly follow-up of clinically stable patients on MOUD within the step-1 clinics. Templated notes for assessment, initiation, and follow-up of patients on MOUD are now available from the SCOUTT national program and should be broadly implemented to facilitate adoption of the collaborative model in target clinics. In order to accomplish a full collaborative model, the VHA would need to enhance appropriate staffing to support this model, broaden access to telehealth, and expand incentives to teams/clinicians who prescribe in these settings.
Acknowledgments/Funding
This material is based upon work supported by the US Department of Veterans Affairs (VA), Office of Mental Health and Suicide Prevention, Veterans Health Administration; the VA Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (PEC) grants #19-001. Supporting organizations had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
1. Centers for Disease Control and Prevention. Understanding the epidemic. Updated March 17, 2021. Accessed September 17, 2021. https://www.cdc.gov/drugoverdose/epidemic/index.html
2. Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. 2019;393(10182):1760-1772. doi:10.1016/S0140-6736(18)33078-2
3. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan [published correction appears in JAMA. 2012 Jun 20;307(23):2489]. JAMA. 2012;307(9):940-947. doi:10.1001/jama.2012.234
4. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612. doi:10.1097/AJP.0000000000000011
5. US Department of Health and Human Services, Working Group on Patient-Centered Reduction or Discontinuation of Long-term Opioid Analgesics. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of Long-term opioid analgesics. Published October 2019. Accessed September 17, 2021. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf
6. Sullivan LE, Chawarski M, O’Connor PG, Schottenfeld RS, Fiellin DA. The practice of office-based buprenorphine treatment of opioid dependence: is it associated with new patients entering into treatment?. Drug Alcohol Depend. 2005;79(1):113-116. doi:10.1016/j.drugalcdep.2004.12.008
7. LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts collaborative care model in community health centers. J Subst Abuse Treat. 2016;60:6-13. doi:10.1016/j.jsat.2015.06.010
8. Rubin R. Rural veterans less likely to get medication for opioid use disorder. JAMA. 2020;323(4):300. doi:10.1001/jama.2019.21856
9. Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2011;57(3):281-289.
10. Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2-5 years. Am J Addict. 2008;17(2):116-120. doi:10.1080/10550490701860971
11. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365-374. doi:10.1056/NEJMoa055255
12. Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1-2):127-135. doi:10.1016/j.drugalcdep.2012.12.008
13. Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22(2):171-176. doi:10.1007/s11606-006-0023-1
14. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
15. Gordon AJ, Drexler K, Hawkins EJ, et al. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: Expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abus. 2020;41(3):275-282. doi:10.1080/08897077.2020.1787299
16. Codell N, Kelley AT, Jones AL, et al. Aims, development, and early results of an interdisciplinary primary care initiative to address patient vulnerabilities. Am J Drug Alcohol Abuse. 2021;47(2):160-169. doi:10.1080/00952990.2020.1832507
17. DeRonne BM, Wong KR, Schultz E, Jones E, Krebs EE. Implementation of a pharmacist care manager model to expand availability of medications for opioid use disorder. Am J Health Syst Pharm. 2021;78(4):354-359. doi:10.1093/ajhp/zxaa405
18. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
19. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
1. Centers for Disease Control and Prevention. Understanding the epidemic. Updated March 17, 2021. Accessed September 17, 2021. https://www.cdc.gov/drugoverdose/epidemic/index.html
2. Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. 2019;393(10182):1760-1772. doi:10.1016/S0140-6736(18)33078-2
3. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan [published correction appears in JAMA. 2012 Jun 20;307(23):2489]. JAMA. 2012;307(9):940-947. doi:10.1001/jama.2012.234
4. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612. doi:10.1097/AJP.0000000000000011
5. US Department of Health and Human Services, Working Group on Patient-Centered Reduction or Discontinuation of Long-term Opioid Analgesics. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of Long-term opioid analgesics. Published October 2019. Accessed September 17, 2021. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf
6. Sullivan LE, Chawarski M, O’Connor PG, Schottenfeld RS, Fiellin DA. The practice of office-based buprenorphine treatment of opioid dependence: is it associated with new patients entering into treatment?. Drug Alcohol Depend. 2005;79(1):113-116. doi:10.1016/j.drugalcdep.2004.12.008
7. LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts collaborative care model in community health centers. J Subst Abuse Treat. 2016;60:6-13. doi:10.1016/j.jsat.2015.06.010
8. Rubin R. Rural veterans less likely to get medication for opioid use disorder. JAMA. 2020;323(4):300. doi:10.1001/jama.2019.21856
9. Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2011;57(3):281-289.
10. Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2-5 years. Am J Addict. 2008;17(2):116-120. doi:10.1080/10550490701860971
11. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365-374. doi:10.1056/NEJMoa055255
12. Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1-2):127-135. doi:10.1016/j.drugalcdep.2012.12.008
13. Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22(2):171-176. doi:10.1007/s11606-006-0023-1
14. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
15. Gordon AJ, Drexler K, Hawkins EJ, et al. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: Expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abus. 2020;41(3):275-282. doi:10.1080/08897077.2020.1787299
16. Codell N, Kelley AT, Jones AL, et al. Aims, development, and early results of an interdisciplinary primary care initiative to address patient vulnerabilities. Am J Drug Alcohol Abuse. 2021;47(2):160-169. doi:10.1080/00952990.2020.1832507
17. DeRonne BM, Wong KR, Schultz E, Jones E, Krebs EE. Implementation of a pharmacist care manager model to expand availability of medications for opioid use disorder. Am J Health Syst Pharm. 2021;78(4):354-359. doi:10.1093/ajhp/zxaa405
18. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
19. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
Models for Implementing Buprenorphine Treatment in the VHA
Primary Care Medical Services for Homeless Veterans
On a single night in 2012, 62,619 veterans experienced homelessness.1 With high rates of illness, as well as alcohol, tobacco, and other drug (ATOD) use among homeless adults, ending veteran homelessness is a signature initiative of the VA.2-4 However, despite increasing efforts to improve health care for homeless individuals, little is known about best practices in homeless-focused primary care.2,5
With an age-adjusted mortality that is 2 to 10 times higher than that of their housed counterparts, homeless veterans pose a formidable challenge for primary care providers (PCPs).6,7 The complexity of homeless persons’ primary care needs is compounded by poor social support and the need to navigate priorities (eg, shelter) that compete with medical care.6,8,9 Moreover, veterans may face unique vulnerabilities conferred by military-specific experiences.2,10
As of 2012, only 2 VA facilities had primary care clinics tailored to the needs of homeless veterans. McGuire and colleagues built a system of colocated primary care, mental health, and homeless services for mentally ill veterans in Los Angeles, California.11 Over 18 months, this clinic facilitated greater primary and preventive care delivery, though the population’s physical health status did not improve.11 More recently, O’Toole and colleagues implemented a homeless-focused primary care clinic in Providence, Rhode Island.6 Compared with a historical sample of homeless veterans in traditional VA primary care, veterans in this homeless-tailored clinic had greater improvements in some chronic disease outcomes.6 This clinic also decreased nonacute emergency department (ED) use and hospitalizations for general medical conditions.6
Despite these promising outcomes, the VA lacked a nationwide homeless-focused primary care initiative. In 2012 the VA Office of Homeless Programs and the Office of Primary Care Operations funded a national demonstration project to create Homeless Patient-Aligned Care Teams (HPACTs)—primary care medical clinics for homeless veterans—at 32 facilities. This demonstration project guided HPACTs to tailor clinical and social services to homeless veterans’ needs, establish processes to identify and refer appropriate veterans, and integrate distinct services.
There were no explicit instructions that detailed HPACT structure. Because new VA programs must fit local contextual factors, including infrastructure, space, personnel, and institutional/community resources, different models of homeless-focused primary care have evolved.
This article is a case study of HPACTs at 3 of the 32 participating VA facilities, each reflecting a distinct community and organizational context. In light of projected HPACT expansion and concerns that current services are better tailored to sheltered homeless veterans than to their unsheltered peers, there is particular importance to detailed clinic descriptions that vividly portray the intricate relationships between service design and populations served.1
METHODS
VA HPACTs established in May 2012 at 3 facilities were examined: Birmingham VAMC in Alabama (BIR), West Los Angeles VAMC in California (WLA), and VA Pittsburgh Healthcare System in Pennsylvania (PIT). Prior to this demonstration project, each facility offered a range of housing/social services and traditional primary care for veterans. These sites are a geographically diverse convenience sample that emerged from existing homeless-focused collaborations among the authors and represent geographically diverse HPACTs.
The national director of VA Homeless Programs formally determined that this comparison constitutes a VA operations activity that is not research.12 This activity was exempt from Institutional Review Board review.
Study Design
Timed at an early stage of HPACT implementation, this project had 3 aims: (1) To identify noteworthy similarities and/or differences among the initial HPACT clinic structures; (2) To compare and contrast the patient characteristics of veterans enrolled in each of these clinics; and (3) To use these data to inform ongoing HPACT service design.
HPACT program evaluation data are not presented. Rather, a nascent system of care is illustrated that contributes to the limited literature concerning the design and implementation of homeless-focused primary care. Such organizational profiles inform novel program delivery and hold particular utility for heterogeneous populations who are difficult to engage in care.13,14
Authors at each site independently developed lists of variables that fell within the 3 guiding principles of this demonstration project. These variables were compiled and iteratively reduced to a consolidated table that assessed each clinic, including location, operating hours, methods of patient identification and referral, and linkages to distinct services (eg, primary care, mental health, addiction, and social services).
This table also became a guide for HPACT directors to generate narrative clinic descriptions. Characteristics of VA medical homes that are embraced regardless of patients’ housing status (eg, patient-centered, team-based care) were also incorporated into these descriptions.15
Patient Characteristics
The VA electronic health record (EHR) was used to identify all patients enrolled in HPACTs at BIR, WLA, and PIT from May 1, 2012 (clinic inception), through September 30, 2012. Authors developed a standardized template for EHR review and coined the first HPACT record as the patient’s index visit. This record was used to code initial housing status, demographics, and acute medical conditions diagnosed/treated. If housing status was not recorded at the index visit, the first preceding informative record was used.
Records for 6 months preceding the index visit were used to identify the presence or absence of common medical conditions, psychiatric diagnoses, and ATOD abuse or dependence. Medical conditions were identified from a list of common outpatient diagnoses from the National Ambulatory Medical Care Survey, supplemented by common conditions among homeless men.16-18 The EHR problem list (a list of diagnoses, by patient) was used to obtain diagnoses, supplemented by notes from inpatient admissions/discharges, as well as ED, primary care, and subspecialty consultations within 6 months preceding the index visit. Prior VA health care use was ascertained from the EHR review of the same 6-month window, reflecting ED visits, inpatient admissions, primary care visits, subspecialty visits, and individual/group therapy for ATOD or other mental health problems. Data were used to generate site-specific descriptive statistics.
RESULTS
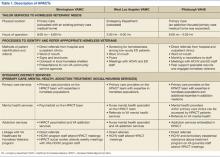
Like all VA hospital-based clinics, a universal EHR captured all medical and social service notes, orders, medications, and administrative records. All facilities had on-site EDs, medical/mental health specialty care, and pharmacies. Social services, including benefits counseling and housing services, were available on site. Table 1 summarizes the HPACT structures at BIR, WLA, and PIT.
Birmingham
The BIR HPACT was devised as a new homeless-focused team located within a VA primary care clinic where other providers continued to see primary care patients. Staff and space were reallocated for this HPACT, which recruited patients in 4 ways: (1) the facility’s primary homeless program, Healthcare for Homeless Veterans, referred patients who previously sought VA housing but who required primary care; (2) an outreach specialist sought homeless veterans in shelters and on the streets; (3) HPACT staff marketed the clinic with presentations and flyers to other VA services and non-VA community agencies; and (4) a referral mechanism within the EHR.
A nurse practitioner was the PCP at this site, supervised by an academic internist experienced in the care of underserved populations, and the HPACT director, a physician certified in internal and addiction medicine. Patients at the BIR HPACT also received care from a social worker, registered nurse, licensed practical nurse, and psychiatrist who received all or a portion of their salaries from this demonstration project. This clinic accommodated walk-in appointments during business hours. Clinicians discussed clinical cases daily, and the full clinical and administrative team met weekly. To promote service integration, HPACT staff attended meetings of the BIR general primary care and Healthcare for Homeless Veterans programs, and vice versa.
West Los Angeles
At WLA, a previously established Homeless Screening Clinic that offered integrated social and medical services for homeless veterans with mental illness was already available during business hours and located within the site’s mental health program.11 However, because ED use for homeless veterans peaked after hours, the new WLA HPACT was established within the WLA ED.
During WLA HPACT hours (3 weekday evenings/week), routine nursing triage occurred for all patients who presented to the ED. However, distinct from other times of day, veterans who were triaged with low-acuity and who were appropriate for outpatient care were given a self-administered, 4-item questionnaire to identify patients who were homeless or at risk for becoming homeless. Veterans who were identified with this screening tool were offered the choice of an ED or HPACT visit. Veterans who chose the latter were assigned to the queue for an HPACT primary care visit instead of the ED.
A physician led the clinical team, with additional services from a mental health clinical nurse specialist and clerks who worked with homeless and/or mental health patients during business hours and provided part-time HPACT coverage. When needed, additional services were provided from colocated ED nurses and social workers. Providers were chosen for their aptitude in culturally responsive communication.
Patients who chose to be seen in HPACT received a primary care visit that also addressed the reason for ED presentation. HPACT staff worked collaboratively, with interdisciplinary team huddles that preceded each clinic session and ended each patient visit. Referrals and social service needs were tracked and monitored by the PCP. Specialty care referrals were also tracked and facilitated when possible with direct communication between HPACT providers and specialty services. Meetings with daytime Homeless Screening Clinic and ED staff facilitated cross-departmental collaborations that helped veterans prepare for and retain housing.
Pittsburgh
The HPACT at PIT evolved from an existing PACT that provided primary care-based addiction services.2 That team included an internist credentialed in addiction medicine and experienced in homeless health care, nurse practitioner, nurse care manager, nursing assistant, and clerks. At this site, HPACT providers had subspecialty expertise in the assessment and treatment of ATOD use, certification to prescribe buprenorphine for outpatient opioid detoxification and/or maintenance, and experience engaging homeless and other vulnerable veterans. The existing colocated clinic included 2 additional addiction medicine clinicians and a physician assistant experienced in ATOD use. The PIT HPACT was not restricted to patients with ATOD use.
At PIT, HPACT care was provided during business hours in a flexible model that allowed for walk-in visits. Providers from the existing addiction-focused PACT identified and referred homeless veterans, as well as patients deemed at-risk for becoming homeless. In addition, other homeless veterans who did not have a PCP could be referred through e-consults placed by any VA staff member. If a referred homeless veteran was already enrolled on a PCP’s panel, HPACT facilitated reengagement with the existing provider. Near the end of this data collection period, a peer support specialist also began community outreach to engage both enrolled and potential HPACT patients.
Patient Characteristics
Table 2 presents the demographic and housing characteristics of enrolled HPACT patients at BIR, WLA, and PIT from May 1, 2012, to September 30, 2012. Each site had a similar number of patients (n = 35/47/43 at BIR/WLA/PIT, respectively). Across sites, most patients were male and African American or white. The majority of patients at each site were housed in VA transitional housing/residential rehabilitation programs (33%/32%/40% at BIR/WLA/PIT, respectively). Fewer patients were unsheltered (on the streets or other places not meant for sleeping), though WLA had the most unsheltered patients (26%) compared with BIR (6%) and PIT (2%). BIR had more patients from emergency shelters (14%) than did the other 2 sites (4% at WLA and 0% at PIT). PIT had the most domiciled patients in houses/apartments (26%, compared with 6% each at BIR and WLA), but who were at risk for homelessness.
Table 3 summarizes the diagnoses and VA health care use patterns of these cohorts. In the first week of HPACT care, WLA addressed the most acute medical conditions (81% of patients had≥ 1 common condition), followed by BIR (54%) and PIT (23%). Among chronic conditions, chronic pain was diagnosed in 51% of patients at BIR, 30% of patients at WLA, and 12% of patients at PIT. Hepatitis C diagnoses were most common at WLA (36%), similar at PIT (33%), and lower at BIR (17%). Overall, chronic medical diagnoses were most common among patients at BIR (91%), followed by WLA (85%), and PIT (60%).
Among mental health diagnoses, mood disorders were the most prevalent across sites (49%/55%/42% at BIR/WLA/PIT, respectively), followed by posttraumatic stress disorder (17%/21%/9% at BIR/WLA/PIT, respectively). WLA had more patients with psychosis (19%) than did the other 2 sites (7% at PIT and 0% at BIR). Overall, mental illness was common among HPACT patients across sites (60%/72%/72% at BIR/WLA/PIT, respectively).
Health care use in the 6 months before HPACT enrollment differed between sites. BIR and PIT had similar rates of ED/urgent care use (46% and 47% sought care over the prior 6 months, respectively), but WLA had the highest rate (62%). However, inpatient admission rates were lowest at WLA (13%) and again similar at BIR (23%) and PIT (26%). BIR patients had the most VA primary care exposure before HPACT entry (71% obtained primary care in the past 6 months), followed by WLA (38%) and PIT (18%). Mental health specialty care was also highest at BIR (60%), followed by PIT (56%), then WLA (39%)
Table 4 presents the prevalence of ATOD abuse or dependence among HPACT patients in the6 months before clinic enrollment. The most commonly misused substances were alcohol (60%/35%/44% at BIR/WLA/PIT, respectively), tobacco (71%/47%/40% at BIR/WLA/PIT, respectively), and cocaine (37%/19%/23% at BIR/WLA/PIT, respectively). PIT had the highest percentages of patients with opioid misuse (21%), followed by BIR (9%) and WLA (6%). Overall, these disorders were prevalent across sites, highest at BIR (94%) and similar at WLA (72%) and PIT (67%).
DISCUSSION
In this case report of early HPACT implementation, strikingly different models of homeless-focused primary care at the geographically distinct facilities were found. The lack of a gold standard primary care medical home for homeless persons—compounded by contrasting local contextual features—led to distinct clinic designs. BIR capitalized on primary care needs among veterans who previously sought housing and relied on the available space within an operating primary care clinic. As WLA already offered primary care for homeless veterans that was colocated with mental health services, the HPACT at this site was devised as an after-hours clinic colocated with the ED.11 At PIT, an existing primary care team with addiction expertise expanded its role to include a focus on homelessness, without needing new space or staff.
The initial HPACT patient cohorts likely reflected these contrasting clinic structures. That is, at WLA, the higher rates of unsheltered patients, prevalence of acute medical conditions and psychotic disorders, and greater ED use was likely driven by ED colocation. The physical location of this site’s HPACT may also speak to greater future decreases in ED use. At BIR, the use of a dedicated HPACT community outreach worker likely led to greater recruitment from emergency shelters. However, the clinic mainly recruited veterans who had previously engaged in VA mainstream primary care services and individuals with ATOD use. At PIT, higher rates of psychotherapy were likely facilitated by the HPACT’s placement within an addiction treatment setting, which may favor psychosocial rehabilitation. The distinctly higher rate of patients with opioid misuse at PIT likely paralleled the ATOD expertise of its providers and/or buprenorphine availability.
The more challenging questions surround the implementation of the current clinic models to address the needs of these patient cohorts and possible avenues to improve each clinic. High rates of chronic medical illness, mental illness, and ATOD use are well known in the homeless veteran and general populations.2,9 Within these 3 HPACTs, the high rates of medical/mental illness and ATOD use speak favorably about the clinics’ respective recruitment strategies; ie, normative homeless populations with high rates of illness are enrolling in these clinics. However, current service integration practices may be enhanced with the specific knowledge gained from this examination. For example, the very high rates of mood and anxiety disorders at each site suggest a role for an embedded mental health provider with prescribing privileges (the model adopted by BIR) as opposed to mental health referrals used at WLA and PIT. There may also be a role for cognitive behavioral therapy services within these clinics. Similarly, the high rates of ATOD (especially alcohol, tobacco, and cocaine) misuse suggest a role for addiction medicine training among the PCPs (the PIT model) as well as psychosocial rehabilitation for ATOD use within the HPACTs. High rates of chronic medical conditions, such as diabetes, hepatitis C, and hypertension elucidate possible roles for specialty care integration and/or chronic disease management programs tailored to the homeless.
Comparing the housing status of these cohorts can help in the design of future homeless-tailored primary care operations and improve these HPACTs. Most patients across sites lived in VA transitional housing/residential rehabilitation programs. As such, current referral practices at these 3 HPACTs proved sufficient in recruiting this subpopulation of homeless veterans. However, in light of national data showing that the count of unsheltered homeless veterans has not declined as rapidly as the count of homeless veterans overall, the higher numbers of veterans recruited from interim sheltering arrangements suggest a need for enhanced outreach to unsheltered individuals.1 WLA data suggest that linkages to EDs can advance this objective. BIR data show that targeted outreach in shelters can engage this high-risk, transiently sheltered subpopulation in primary care. At the time of this project, PIT just began using a peer support specialist for outreach to unsheltered veterans. It will be important to evaluate the outcomes of this new referral strategy.
Limitations
These exploratory findings—though from a small convenience sample within a nascent, growing program—generated critical and detailed information to guide ongoing policies and service design. However, these findings have limitations. First, though this research contributes to limited existing literature about the operational design of homeless-focused primary care, no outcome data were included. Although a comprehensive evaluation of all HPACT sites is a distinct and useful endeavor, this project instead offers a rapid, detailed illustration of 3 early-stage clinics. Though smaller in scope, this effort informs other facilities developing homeless-focused primary care initiatives and the larger demonstration project.
Second, a convenience sample of 3 urban facilities with strong academic ties and community commitment to providing services for homeless persons was presented. It may be difficult to translate these findings to communities with fewer resources.
Third, EHR review was used to determine patient demographics, diagnoses, and patterns of health care use. Though EHR review offers detailed information that is unavailable from administrative data, EHR is subject to variations in documentation patterns.
Last, differing characteristics of the homeless veteran population in each city may interact with contrasting HPACT structures to influence the characteristics of patients served. For example, though the data suggest that linkages with the ED may facilitate greater recruitment of unsheltered veterans in WLA, Los Angeles is known to have particularly high rates of unsheltered individuals.1
CONCLUSIONS
Clinicians, administrators, and researchers in the safety net may benefit from the experience implementing new clinics to recruit and engage homeless veterans in primary care. In a relatively nascent field with few accepted models of care, this paper offers detailed descriptions of newly developed homeless-focused primary care clinics at 3 VA facilities, which can inform other sites undertaking similar initiatives. This study highlights the wide range of approaches to building such clinics, with important variations in structural characteristics such as clinic location and operating hours, as well as within the intricacies of service integration patterns.
Within primary care clinics for homeless adults, this study suggests a role for embedded mental health (medication management and psychotherapy) and substance abuse services, chronic disease management programs tailored to this vulnerable population, and the role of linkages to the ED or community-based outreach to recruit unsheltered homeless patients. To pave a path toward identifying an evidence-based model of homeless-focused primary care, future studies are needed to study nationwide HPACT outcomes, including health status, patient satisfaction, quality of life, housing, and cost-effectiveness.
Acknowledgements
This work was undertaken in part by the VA’s PACT Demonstration Laboratory initiative, supporting and evaluating VA’s transition to a patient-centered medical home. Funding for the PACT Demonstration Laboratory initiative was provided by the VA Office of Patient Care Services. This project received support from the VISN 22 VA Assessment and Improvement Lab for Patient Centered Care (VAIL-PCC) (XVA 65-018; PI: Rubenstein).
Dr. Gabrielian was supported in part by the VA Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment. Drs. Gelberg and Andersen were supported in part by NIDA DA 022445. Dr. Andersen received additional support from the UCLA/DREW Project EXPORT, National Center on Minority Health and Health Disparities, P20MD000148/P20MD000182. Dr. Broyles was supported by a Career Development Award (CDA 10–014) from the VA Health Services Research & Development service. Dr. Kertesz was supported through funding of VISN 7.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cortes A, Henry M, de la Cruz RJ, Brown S. The 2012 Point-in-Time Estimates of Homelessness: Volume I of the 2012 Annual Homeless Assessment Report. Washington, DC: The U.S. Department of Housing and Urban Development, Office of Community Planning and Development; 2012.
2. Balshem H, Christensen V, Tuepker A, Kansagara D. A Critical Review of the Literature Regarding Homelessness Among Veterans. U.S. Department of Veterans Affairs, Veterans Health Administration, Health Services Research & Development Service; 2011. VA-ESP Project #05-225.
3. O’Toole TP, Pirraglia PA, Dosa D, et al; Primary Care-Special Populations Treatment Team. Building care systems to improve access for high-risk and vulnerable veteran populations. J Gen Intern Med. 2011;26(suppl 2):683-688.
4. Opening Doors: Federal Strategic Plan to Prevent and End Homelessness. Washington, DC: The United States Interagency Council on Homelessness; 2010.
5. Nakashima J, McGuire J, Berman S, Daniels W. Developing programs for homeless veterans: Understanding driving forces in implementation. Soc Work Health Care. 2004;40(2):1-12.
6. O’Toole TP, Buckel L, Bourgault C, et al. Applying the chronic care model to homeless veterans: Effect of a population approach to primary care on utilization and clinical outcomes. Am J Public Health. 2010;100(12):2493-2499.
7. Desai MM, Rosenheck RA, Kasprow WJ. Determinants of receipt of ambulatory medical care in a national sample of mentally ill homeless veterans. Med Care. 2003;41(2):275-287.
8. Irene Wong YL, Stanhope V. Conceptualizing community: A comparison of neighborhood characteristics of supportive housing for persons with psychiatric and developmental disabilities. Soc Sci Med. 2009;68(8):1376-1387.
9. Gelberg L, Gallagher TC, Andersen RM, Koegel P. Competing priorities as a barrier to medical care among homeless adults in Los Angeles. Am J Public Health. 1997;87(2):217-220.
10. Hamilton AB, Poza I, Washington DL. “Homelessness and trauma go hand-in-hand”: Pathways to homelessness among women veterans. Womens Health Issues. 2011;21(suppl 4):S203-S209.
11. McGuire J, Gelberg L, Blue-Howells J, Rosenheck RA. Access to primary care for homeless veterans with serious mental illness or substance abuse: A follow-up evaluation of co-located primary care and homeless social services. Adm Policy Ment Health. 2009;36(4):255-264.
12. VHA Operations Activities That May Constitute Research. Washington, DC: U.S. Department of Veterans Affairs, Veterans Health Administration; 2011. VHA Handbook 1058.05.
13. Resnick SG, Armstrong M, Sperrazza M, Harkness L, Rosenheck RA. A model of consumer-provider partnership: Vet-to-Vet. Psychiatr Rehabil J. 2004;28(2):185-187.
14. Hogan TP, Wakefield B, Nazi KM, Houston TK, Weaver FM. Promoting access through complementary eHealth technologies: Recommendations for VA’s Home Telehealth and personal health record programs. J Gen Intern Med. 2011;26(suppl 2):628-635.
15. Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
16. Hsiao CJ, Cherry DK, Beatty PC, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2007 summary. Natl Health Stat Report. 2010;3(27):1-32.
17. Hwang SW, Orav EJ, O’Connell JJ, Lebow JM, Brennan TA. Causes of death in homeless adults in Boston. Ann Intern Med. 1997;126(8):625-628.
18. Hwang SW, Tolomiczenko G, Kouyoumdjian FG, Garner RE. Interventions to improve the health of the homeless: A systematic review. Am J Prev Med. 2005;29(4):311-319.
On a single night in 2012, 62,619 veterans experienced homelessness.1 With high rates of illness, as well as alcohol, tobacco, and other drug (ATOD) use among homeless adults, ending veteran homelessness is a signature initiative of the VA.2-4 However, despite increasing efforts to improve health care for homeless individuals, little is known about best practices in homeless-focused primary care.2,5
With an age-adjusted mortality that is 2 to 10 times higher than that of their housed counterparts, homeless veterans pose a formidable challenge for primary care providers (PCPs).6,7 The complexity of homeless persons’ primary care needs is compounded by poor social support and the need to navigate priorities (eg, shelter) that compete with medical care.6,8,9 Moreover, veterans may face unique vulnerabilities conferred by military-specific experiences.2,10
As of 2012, only 2 VA facilities had primary care clinics tailored to the needs of homeless veterans. McGuire and colleagues built a system of colocated primary care, mental health, and homeless services for mentally ill veterans in Los Angeles, California.11 Over 18 months, this clinic facilitated greater primary and preventive care delivery, though the population’s physical health status did not improve.11 More recently, O’Toole and colleagues implemented a homeless-focused primary care clinic in Providence, Rhode Island.6 Compared with a historical sample of homeless veterans in traditional VA primary care, veterans in this homeless-tailored clinic had greater improvements in some chronic disease outcomes.6 This clinic also decreased nonacute emergency department (ED) use and hospitalizations for general medical conditions.6
Despite these promising outcomes, the VA lacked a nationwide homeless-focused primary care initiative. In 2012 the VA Office of Homeless Programs and the Office of Primary Care Operations funded a national demonstration project to create Homeless Patient-Aligned Care Teams (HPACTs)—primary care medical clinics for homeless veterans—at 32 facilities. This demonstration project guided HPACTs to tailor clinical and social services to homeless veterans’ needs, establish processes to identify and refer appropriate veterans, and integrate distinct services.
There were no explicit instructions that detailed HPACT structure. Because new VA programs must fit local contextual factors, including infrastructure, space, personnel, and institutional/community resources, different models of homeless-focused primary care have evolved.
This article is a case study of HPACTs at 3 of the 32 participating VA facilities, each reflecting a distinct community and organizational context. In light of projected HPACT expansion and concerns that current services are better tailored to sheltered homeless veterans than to their unsheltered peers, there is particular importance to detailed clinic descriptions that vividly portray the intricate relationships between service design and populations served.1
METHODS
VA HPACTs established in May 2012 at 3 facilities were examined: Birmingham VAMC in Alabama (BIR), West Los Angeles VAMC in California (WLA), and VA Pittsburgh Healthcare System in Pennsylvania (PIT). Prior to this demonstration project, each facility offered a range of housing/social services and traditional primary care for veterans. These sites are a geographically diverse convenience sample that emerged from existing homeless-focused collaborations among the authors and represent geographically diverse HPACTs.
The national director of VA Homeless Programs formally determined that this comparison constitutes a VA operations activity that is not research.12 This activity was exempt from Institutional Review Board review.
Study Design
Timed at an early stage of HPACT implementation, this project had 3 aims: (1) To identify noteworthy similarities and/or differences among the initial HPACT clinic structures; (2) To compare and contrast the patient characteristics of veterans enrolled in each of these clinics; and (3) To use these data to inform ongoing HPACT service design.
HPACT program evaluation data are not presented. Rather, a nascent system of care is illustrated that contributes to the limited literature concerning the design and implementation of homeless-focused primary care. Such organizational profiles inform novel program delivery and hold particular utility for heterogeneous populations who are difficult to engage in care.13,14
Authors at each site independently developed lists of variables that fell within the 3 guiding principles of this demonstration project. These variables were compiled and iteratively reduced to a consolidated table that assessed each clinic, including location, operating hours, methods of patient identification and referral, and linkages to distinct services (eg, primary care, mental health, addiction, and social services).
This table also became a guide for HPACT directors to generate narrative clinic descriptions. Characteristics of VA medical homes that are embraced regardless of patients’ housing status (eg, patient-centered, team-based care) were also incorporated into these descriptions.15
Patient Characteristics
The VA electronic health record (EHR) was used to identify all patients enrolled in HPACTs at BIR, WLA, and PIT from May 1, 2012 (clinic inception), through September 30, 2012. Authors developed a standardized template for EHR review and coined the first HPACT record as the patient’s index visit. This record was used to code initial housing status, demographics, and acute medical conditions diagnosed/treated. If housing status was not recorded at the index visit, the first preceding informative record was used.
Records for 6 months preceding the index visit were used to identify the presence or absence of common medical conditions, psychiatric diagnoses, and ATOD abuse or dependence. Medical conditions were identified from a list of common outpatient diagnoses from the National Ambulatory Medical Care Survey, supplemented by common conditions among homeless men.16-18 The EHR problem list (a list of diagnoses, by patient) was used to obtain diagnoses, supplemented by notes from inpatient admissions/discharges, as well as ED, primary care, and subspecialty consultations within 6 months preceding the index visit. Prior VA health care use was ascertained from the EHR review of the same 6-month window, reflecting ED visits, inpatient admissions, primary care visits, subspecialty visits, and individual/group therapy for ATOD or other mental health problems. Data were used to generate site-specific descriptive statistics.
RESULTS
Like all VA hospital-based clinics, a universal EHR captured all medical and social service notes, orders, medications, and administrative records. All facilities had on-site EDs, medical/mental health specialty care, and pharmacies. Social services, including benefits counseling and housing services, were available on site. Table 1 summarizes the HPACT structures at BIR, WLA, and PIT.
Birmingham
The BIR HPACT was devised as a new homeless-focused team located within a VA primary care clinic where other providers continued to see primary care patients. Staff and space were reallocated for this HPACT, which recruited patients in 4 ways: (1) the facility’s primary homeless program, Healthcare for Homeless Veterans, referred patients who previously sought VA housing but who required primary care; (2) an outreach specialist sought homeless veterans in shelters and on the streets; (3) HPACT staff marketed the clinic with presentations and flyers to other VA services and non-VA community agencies; and (4) a referral mechanism within the EHR.
A nurse practitioner was the PCP at this site, supervised by an academic internist experienced in the care of underserved populations, and the HPACT director, a physician certified in internal and addiction medicine. Patients at the BIR HPACT also received care from a social worker, registered nurse, licensed practical nurse, and psychiatrist who received all or a portion of their salaries from this demonstration project. This clinic accommodated walk-in appointments during business hours. Clinicians discussed clinical cases daily, and the full clinical and administrative team met weekly. To promote service integration, HPACT staff attended meetings of the BIR general primary care and Healthcare for Homeless Veterans programs, and vice versa.
West Los Angeles
At WLA, a previously established Homeless Screening Clinic that offered integrated social and medical services for homeless veterans with mental illness was already available during business hours and located within the site’s mental health program.11 However, because ED use for homeless veterans peaked after hours, the new WLA HPACT was established within the WLA ED.
During WLA HPACT hours (3 weekday evenings/week), routine nursing triage occurred for all patients who presented to the ED. However, distinct from other times of day, veterans who were triaged with low-acuity and who were appropriate for outpatient care were given a self-administered, 4-item questionnaire to identify patients who were homeless or at risk for becoming homeless. Veterans who were identified with this screening tool were offered the choice of an ED or HPACT visit. Veterans who chose the latter were assigned to the queue for an HPACT primary care visit instead of the ED.
A physician led the clinical team, with additional services from a mental health clinical nurse specialist and clerks who worked with homeless and/or mental health patients during business hours and provided part-time HPACT coverage. When needed, additional services were provided from colocated ED nurses and social workers. Providers were chosen for their aptitude in culturally responsive communication.
Patients who chose to be seen in HPACT received a primary care visit that also addressed the reason for ED presentation. HPACT staff worked collaboratively, with interdisciplinary team huddles that preceded each clinic session and ended each patient visit. Referrals and social service needs were tracked and monitored by the PCP. Specialty care referrals were also tracked and facilitated when possible with direct communication between HPACT providers and specialty services. Meetings with daytime Homeless Screening Clinic and ED staff facilitated cross-departmental collaborations that helped veterans prepare for and retain housing.
Pittsburgh
The HPACT at PIT evolved from an existing PACT that provided primary care-based addiction services.2 That team included an internist credentialed in addiction medicine and experienced in homeless health care, nurse practitioner, nurse care manager, nursing assistant, and clerks. At this site, HPACT providers had subspecialty expertise in the assessment and treatment of ATOD use, certification to prescribe buprenorphine for outpatient opioid detoxification and/or maintenance, and experience engaging homeless and other vulnerable veterans. The existing colocated clinic included 2 additional addiction medicine clinicians and a physician assistant experienced in ATOD use. The PIT HPACT was not restricted to patients with ATOD use.
At PIT, HPACT care was provided during business hours in a flexible model that allowed for walk-in visits. Providers from the existing addiction-focused PACT identified and referred homeless veterans, as well as patients deemed at-risk for becoming homeless. In addition, other homeless veterans who did not have a PCP could be referred through e-consults placed by any VA staff member. If a referred homeless veteran was already enrolled on a PCP’s panel, HPACT facilitated reengagement with the existing provider. Near the end of this data collection period, a peer support specialist also began community outreach to engage both enrolled and potential HPACT patients.
Patient Characteristics
Table 2 presents the demographic and housing characteristics of enrolled HPACT patients at BIR, WLA, and PIT from May 1, 2012, to September 30, 2012. Each site had a similar number of patients (n = 35/47/43 at BIR/WLA/PIT, respectively). Across sites, most patients were male and African American or white. The majority of patients at each site were housed in VA transitional housing/residential rehabilitation programs (33%/32%/40% at BIR/WLA/PIT, respectively). Fewer patients were unsheltered (on the streets or other places not meant for sleeping), though WLA had the most unsheltered patients (26%) compared with BIR (6%) and PIT (2%). BIR had more patients from emergency shelters (14%) than did the other 2 sites (4% at WLA and 0% at PIT). PIT had the most domiciled patients in houses/apartments (26%, compared with 6% each at BIR and WLA), but who were at risk for homelessness.
Table 3 summarizes the diagnoses and VA health care use patterns of these cohorts. In the first week of HPACT care, WLA addressed the most acute medical conditions (81% of patients had≥ 1 common condition), followed by BIR (54%) and PIT (23%). Among chronic conditions, chronic pain was diagnosed in 51% of patients at BIR, 30% of patients at WLA, and 12% of patients at PIT. Hepatitis C diagnoses were most common at WLA (36%), similar at PIT (33%), and lower at BIR (17%). Overall, chronic medical diagnoses were most common among patients at BIR (91%), followed by WLA (85%), and PIT (60%).
Among mental health diagnoses, mood disorders were the most prevalent across sites (49%/55%/42% at BIR/WLA/PIT, respectively), followed by posttraumatic stress disorder (17%/21%/9% at BIR/WLA/PIT, respectively). WLA had more patients with psychosis (19%) than did the other 2 sites (7% at PIT and 0% at BIR). Overall, mental illness was common among HPACT patients across sites (60%/72%/72% at BIR/WLA/PIT, respectively).
Health care use in the 6 months before HPACT enrollment differed between sites. BIR and PIT had similar rates of ED/urgent care use (46% and 47% sought care over the prior 6 months, respectively), but WLA had the highest rate (62%). However, inpatient admission rates were lowest at WLA (13%) and again similar at BIR (23%) and PIT (26%). BIR patients had the most VA primary care exposure before HPACT entry (71% obtained primary care in the past 6 months), followed by WLA (38%) and PIT (18%). Mental health specialty care was also highest at BIR (60%), followed by PIT (56%), then WLA (39%)
Table 4 presents the prevalence of ATOD abuse or dependence among HPACT patients in the6 months before clinic enrollment. The most commonly misused substances were alcohol (60%/35%/44% at BIR/WLA/PIT, respectively), tobacco (71%/47%/40% at BIR/WLA/PIT, respectively), and cocaine (37%/19%/23% at BIR/WLA/PIT, respectively). PIT had the highest percentages of patients with opioid misuse (21%), followed by BIR (9%) and WLA (6%). Overall, these disorders were prevalent across sites, highest at BIR (94%) and similar at WLA (72%) and PIT (67%).
DISCUSSION
In this case report of early HPACT implementation, strikingly different models of homeless-focused primary care at the geographically distinct facilities were found. The lack of a gold standard primary care medical home for homeless persons—compounded by contrasting local contextual features—led to distinct clinic designs. BIR capitalized on primary care needs among veterans who previously sought housing and relied on the available space within an operating primary care clinic. As WLA already offered primary care for homeless veterans that was colocated with mental health services, the HPACT at this site was devised as an after-hours clinic colocated with the ED.11 At PIT, an existing primary care team with addiction expertise expanded its role to include a focus on homelessness, without needing new space or staff.
The initial HPACT patient cohorts likely reflected these contrasting clinic structures. That is, at WLA, the higher rates of unsheltered patients, prevalence of acute medical conditions and psychotic disorders, and greater ED use was likely driven by ED colocation. The physical location of this site’s HPACT may also speak to greater future decreases in ED use. At BIR, the use of a dedicated HPACT community outreach worker likely led to greater recruitment from emergency shelters. However, the clinic mainly recruited veterans who had previously engaged in VA mainstream primary care services and individuals with ATOD use. At PIT, higher rates of psychotherapy were likely facilitated by the HPACT’s placement within an addiction treatment setting, which may favor psychosocial rehabilitation. The distinctly higher rate of patients with opioid misuse at PIT likely paralleled the ATOD expertise of its providers and/or buprenorphine availability.
The more challenging questions surround the implementation of the current clinic models to address the needs of these patient cohorts and possible avenues to improve each clinic. High rates of chronic medical illness, mental illness, and ATOD use are well known in the homeless veteran and general populations.2,9 Within these 3 HPACTs, the high rates of medical/mental illness and ATOD use speak favorably about the clinics’ respective recruitment strategies; ie, normative homeless populations with high rates of illness are enrolling in these clinics. However, current service integration practices may be enhanced with the specific knowledge gained from this examination. For example, the very high rates of mood and anxiety disorders at each site suggest a role for an embedded mental health provider with prescribing privileges (the model adopted by BIR) as opposed to mental health referrals used at WLA and PIT. There may also be a role for cognitive behavioral therapy services within these clinics. Similarly, the high rates of ATOD (especially alcohol, tobacco, and cocaine) misuse suggest a role for addiction medicine training among the PCPs (the PIT model) as well as psychosocial rehabilitation for ATOD use within the HPACTs. High rates of chronic medical conditions, such as diabetes, hepatitis C, and hypertension elucidate possible roles for specialty care integration and/or chronic disease management programs tailored to the homeless.
Comparing the housing status of these cohorts can help in the design of future homeless-tailored primary care operations and improve these HPACTs. Most patients across sites lived in VA transitional housing/residential rehabilitation programs. As such, current referral practices at these 3 HPACTs proved sufficient in recruiting this subpopulation of homeless veterans. However, in light of national data showing that the count of unsheltered homeless veterans has not declined as rapidly as the count of homeless veterans overall, the higher numbers of veterans recruited from interim sheltering arrangements suggest a need for enhanced outreach to unsheltered individuals.1 WLA data suggest that linkages to EDs can advance this objective. BIR data show that targeted outreach in shelters can engage this high-risk, transiently sheltered subpopulation in primary care. At the time of this project, PIT just began using a peer support specialist for outreach to unsheltered veterans. It will be important to evaluate the outcomes of this new referral strategy.
Limitations
These exploratory findings—though from a small convenience sample within a nascent, growing program—generated critical and detailed information to guide ongoing policies and service design. However, these findings have limitations. First, though this research contributes to limited existing literature about the operational design of homeless-focused primary care, no outcome data were included. Although a comprehensive evaluation of all HPACT sites is a distinct and useful endeavor, this project instead offers a rapid, detailed illustration of 3 early-stage clinics. Though smaller in scope, this effort informs other facilities developing homeless-focused primary care initiatives and the larger demonstration project.
Second, a convenience sample of 3 urban facilities with strong academic ties and community commitment to providing services for homeless persons was presented. It may be difficult to translate these findings to communities with fewer resources.
Third, EHR review was used to determine patient demographics, diagnoses, and patterns of health care use. Though EHR review offers detailed information that is unavailable from administrative data, EHR is subject to variations in documentation patterns.
Last, differing characteristics of the homeless veteran population in each city may interact with contrasting HPACT structures to influence the characteristics of patients served. For example, though the data suggest that linkages with the ED may facilitate greater recruitment of unsheltered veterans in WLA, Los Angeles is known to have particularly high rates of unsheltered individuals.1
CONCLUSIONS
Clinicians, administrators, and researchers in the safety net may benefit from the experience implementing new clinics to recruit and engage homeless veterans in primary care. In a relatively nascent field with few accepted models of care, this paper offers detailed descriptions of newly developed homeless-focused primary care clinics at 3 VA facilities, which can inform other sites undertaking similar initiatives. This study highlights the wide range of approaches to building such clinics, with important variations in structural characteristics such as clinic location and operating hours, as well as within the intricacies of service integration patterns.
Within primary care clinics for homeless adults, this study suggests a role for embedded mental health (medication management and psychotherapy) and substance abuse services, chronic disease management programs tailored to this vulnerable population, and the role of linkages to the ED or community-based outreach to recruit unsheltered homeless patients. To pave a path toward identifying an evidence-based model of homeless-focused primary care, future studies are needed to study nationwide HPACT outcomes, including health status, patient satisfaction, quality of life, housing, and cost-effectiveness.
Acknowledgements
This work was undertaken in part by the VA’s PACT Demonstration Laboratory initiative, supporting and evaluating VA’s transition to a patient-centered medical home. Funding for the PACT Demonstration Laboratory initiative was provided by the VA Office of Patient Care Services. This project received support from the VISN 22 VA Assessment and Improvement Lab for Patient Centered Care (VAIL-PCC) (XVA 65-018; PI: Rubenstein).
Dr. Gabrielian was supported in part by the VA Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment. Drs. Gelberg and Andersen were supported in part by NIDA DA 022445. Dr. Andersen received additional support from the UCLA/DREW Project EXPORT, National Center on Minority Health and Health Disparities, P20MD000148/P20MD000182. Dr. Broyles was supported by a Career Development Award (CDA 10–014) from the VA Health Services Research & Development service. Dr. Kertesz was supported through funding of VISN 7.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
On a single night in 2012, 62,619 veterans experienced homelessness.1 With high rates of illness, as well as alcohol, tobacco, and other drug (ATOD) use among homeless adults, ending veteran homelessness is a signature initiative of the VA.2-4 However, despite increasing efforts to improve health care for homeless individuals, little is known about best practices in homeless-focused primary care.2,5
With an age-adjusted mortality that is 2 to 10 times higher than that of their housed counterparts, homeless veterans pose a formidable challenge for primary care providers (PCPs).6,7 The complexity of homeless persons’ primary care needs is compounded by poor social support and the need to navigate priorities (eg, shelter) that compete with medical care.6,8,9 Moreover, veterans may face unique vulnerabilities conferred by military-specific experiences.2,10
As of 2012, only 2 VA facilities had primary care clinics tailored to the needs of homeless veterans. McGuire and colleagues built a system of colocated primary care, mental health, and homeless services for mentally ill veterans in Los Angeles, California.11 Over 18 months, this clinic facilitated greater primary and preventive care delivery, though the population’s physical health status did not improve.11 More recently, O’Toole and colleagues implemented a homeless-focused primary care clinic in Providence, Rhode Island.6 Compared with a historical sample of homeless veterans in traditional VA primary care, veterans in this homeless-tailored clinic had greater improvements in some chronic disease outcomes.6 This clinic also decreased nonacute emergency department (ED) use and hospitalizations for general medical conditions.6
Despite these promising outcomes, the VA lacked a nationwide homeless-focused primary care initiative. In 2012 the VA Office of Homeless Programs and the Office of Primary Care Operations funded a national demonstration project to create Homeless Patient-Aligned Care Teams (HPACTs)—primary care medical clinics for homeless veterans—at 32 facilities. This demonstration project guided HPACTs to tailor clinical and social services to homeless veterans’ needs, establish processes to identify and refer appropriate veterans, and integrate distinct services.
There were no explicit instructions that detailed HPACT structure. Because new VA programs must fit local contextual factors, including infrastructure, space, personnel, and institutional/community resources, different models of homeless-focused primary care have evolved.
This article is a case study of HPACTs at 3 of the 32 participating VA facilities, each reflecting a distinct community and organizational context. In light of projected HPACT expansion and concerns that current services are better tailored to sheltered homeless veterans than to their unsheltered peers, there is particular importance to detailed clinic descriptions that vividly portray the intricate relationships between service design and populations served.1
METHODS
VA HPACTs established in May 2012 at 3 facilities were examined: Birmingham VAMC in Alabama (BIR), West Los Angeles VAMC in California (WLA), and VA Pittsburgh Healthcare System in Pennsylvania (PIT). Prior to this demonstration project, each facility offered a range of housing/social services and traditional primary care for veterans. These sites are a geographically diverse convenience sample that emerged from existing homeless-focused collaborations among the authors and represent geographically diverse HPACTs.
The national director of VA Homeless Programs formally determined that this comparison constitutes a VA operations activity that is not research.12 This activity was exempt from Institutional Review Board review.
Study Design
Timed at an early stage of HPACT implementation, this project had 3 aims: (1) To identify noteworthy similarities and/or differences among the initial HPACT clinic structures; (2) To compare and contrast the patient characteristics of veterans enrolled in each of these clinics; and (3) To use these data to inform ongoing HPACT service design.
HPACT program evaluation data are not presented. Rather, a nascent system of care is illustrated that contributes to the limited literature concerning the design and implementation of homeless-focused primary care. Such organizational profiles inform novel program delivery and hold particular utility for heterogeneous populations who are difficult to engage in care.13,14
Authors at each site independently developed lists of variables that fell within the 3 guiding principles of this demonstration project. These variables were compiled and iteratively reduced to a consolidated table that assessed each clinic, including location, operating hours, methods of patient identification and referral, and linkages to distinct services (eg, primary care, mental health, addiction, and social services).
This table also became a guide for HPACT directors to generate narrative clinic descriptions. Characteristics of VA medical homes that are embraced regardless of patients’ housing status (eg, patient-centered, team-based care) were also incorporated into these descriptions.15
Patient Characteristics
The VA electronic health record (EHR) was used to identify all patients enrolled in HPACTs at BIR, WLA, and PIT from May 1, 2012 (clinic inception), through September 30, 2012. Authors developed a standardized template for EHR review and coined the first HPACT record as the patient’s index visit. This record was used to code initial housing status, demographics, and acute medical conditions diagnosed/treated. If housing status was not recorded at the index visit, the first preceding informative record was used.
Records for 6 months preceding the index visit were used to identify the presence or absence of common medical conditions, psychiatric diagnoses, and ATOD abuse or dependence. Medical conditions were identified from a list of common outpatient diagnoses from the National Ambulatory Medical Care Survey, supplemented by common conditions among homeless men.16-18 The EHR problem list (a list of diagnoses, by patient) was used to obtain diagnoses, supplemented by notes from inpatient admissions/discharges, as well as ED, primary care, and subspecialty consultations within 6 months preceding the index visit. Prior VA health care use was ascertained from the EHR review of the same 6-month window, reflecting ED visits, inpatient admissions, primary care visits, subspecialty visits, and individual/group therapy for ATOD or other mental health problems. Data were used to generate site-specific descriptive statistics.
RESULTS
Like all VA hospital-based clinics, a universal EHR captured all medical and social service notes, orders, medications, and administrative records. All facilities had on-site EDs, medical/mental health specialty care, and pharmacies. Social services, including benefits counseling and housing services, were available on site. Table 1 summarizes the HPACT structures at BIR, WLA, and PIT.
Birmingham
The BIR HPACT was devised as a new homeless-focused team located within a VA primary care clinic where other providers continued to see primary care patients. Staff and space were reallocated for this HPACT, which recruited patients in 4 ways: (1) the facility’s primary homeless program, Healthcare for Homeless Veterans, referred patients who previously sought VA housing but who required primary care; (2) an outreach specialist sought homeless veterans in shelters and on the streets; (3) HPACT staff marketed the clinic with presentations and flyers to other VA services and non-VA community agencies; and (4) a referral mechanism within the EHR.
A nurse practitioner was the PCP at this site, supervised by an academic internist experienced in the care of underserved populations, and the HPACT director, a physician certified in internal and addiction medicine. Patients at the BIR HPACT also received care from a social worker, registered nurse, licensed practical nurse, and psychiatrist who received all or a portion of their salaries from this demonstration project. This clinic accommodated walk-in appointments during business hours. Clinicians discussed clinical cases daily, and the full clinical and administrative team met weekly. To promote service integration, HPACT staff attended meetings of the BIR general primary care and Healthcare for Homeless Veterans programs, and vice versa.
West Los Angeles
At WLA, a previously established Homeless Screening Clinic that offered integrated social and medical services for homeless veterans with mental illness was already available during business hours and located within the site’s mental health program.11 However, because ED use for homeless veterans peaked after hours, the new WLA HPACT was established within the WLA ED.
During WLA HPACT hours (3 weekday evenings/week), routine nursing triage occurred for all patients who presented to the ED. However, distinct from other times of day, veterans who were triaged with low-acuity and who were appropriate for outpatient care were given a self-administered, 4-item questionnaire to identify patients who were homeless or at risk for becoming homeless. Veterans who were identified with this screening tool were offered the choice of an ED or HPACT visit. Veterans who chose the latter were assigned to the queue for an HPACT primary care visit instead of the ED.
A physician led the clinical team, with additional services from a mental health clinical nurse specialist and clerks who worked with homeless and/or mental health patients during business hours and provided part-time HPACT coverage. When needed, additional services were provided from colocated ED nurses and social workers. Providers were chosen for their aptitude in culturally responsive communication.
Patients who chose to be seen in HPACT received a primary care visit that also addressed the reason for ED presentation. HPACT staff worked collaboratively, with interdisciplinary team huddles that preceded each clinic session and ended each patient visit. Referrals and social service needs were tracked and monitored by the PCP. Specialty care referrals were also tracked and facilitated when possible with direct communication between HPACT providers and specialty services. Meetings with daytime Homeless Screening Clinic and ED staff facilitated cross-departmental collaborations that helped veterans prepare for and retain housing.
Pittsburgh
The HPACT at PIT evolved from an existing PACT that provided primary care-based addiction services.2 That team included an internist credentialed in addiction medicine and experienced in homeless health care, nurse practitioner, nurse care manager, nursing assistant, and clerks. At this site, HPACT providers had subspecialty expertise in the assessment and treatment of ATOD use, certification to prescribe buprenorphine for outpatient opioid detoxification and/or maintenance, and experience engaging homeless and other vulnerable veterans. The existing colocated clinic included 2 additional addiction medicine clinicians and a physician assistant experienced in ATOD use. The PIT HPACT was not restricted to patients with ATOD use.
At PIT, HPACT care was provided during business hours in a flexible model that allowed for walk-in visits. Providers from the existing addiction-focused PACT identified and referred homeless veterans, as well as patients deemed at-risk for becoming homeless. In addition, other homeless veterans who did not have a PCP could be referred through e-consults placed by any VA staff member. If a referred homeless veteran was already enrolled on a PCP’s panel, HPACT facilitated reengagement with the existing provider. Near the end of this data collection period, a peer support specialist also began community outreach to engage both enrolled and potential HPACT patients.
Patient Characteristics
Table 2 presents the demographic and housing characteristics of enrolled HPACT patients at BIR, WLA, and PIT from May 1, 2012, to September 30, 2012. Each site had a similar number of patients (n = 35/47/43 at BIR/WLA/PIT, respectively). Across sites, most patients were male and African American or white. The majority of patients at each site were housed in VA transitional housing/residential rehabilitation programs (33%/32%/40% at BIR/WLA/PIT, respectively). Fewer patients were unsheltered (on the streets or other places not meant for sleeping), though WLA had the most unsheltered patients (26%) compared with BIR (6%) and PIT (2%). BIR had more patients from emergency shelters (14%) than did the other 2 sites (4% at WLA and 0% at PIT). PIT had the most domiciled patients in houses/apartments (26%, compared with 6% each at BIR and WLA), but who were at risk for homelessness.
Table 3 summarizes the diagnoses and VA health care use patterns of these cohorts. In the first week of HPACT care, WLA addressed the most acute medical conditions (81% of patients had≥ 1 common condition), followed by BIR (54%) and PIT (23%). Among chronic conditions, chronic pain was diagnosed in 51% of patients at BIR, 30% of patients at WLA, and 12% of patients at PIT. Hepatitis C diagnoses were most common at WLA (36%), similar at PIT (33%), and lower at BIR (17%). Overall, chronic medical diagnoses were most common among patients at BIR (91%), followed by WLA (85%), and PIT (60%).
Among mental health diagnoses, mood disorders were the most prevalent across sites (49%/55%/42% at BIR/WLA/PIT, respectively), followed by posttraumatic stress disorder (17%/21%/9% at BIR/WLA/PIT, respectively). WLA had more patients with psychosis (19%) than did the other 2 sites (7% at PIT and 0% at BIR). Overall, mental illness was common among HPACT patients across sites (60%/72%/72% at BIR/WLA/PIT, respectively).
Health care use in the 6 months before HPACT enrollment differed between sites. BIR and PIT had similar rates of ED/urgent care use (46% and 47% sought care over the prior 6 months, respectively), but WLA had the highest rate (62%). However, inpatient admission rates were lowest at WLA (13%) and again similar at BIR (23%) and PIT (26%). BIR patients had the most VA primary care exposure before HPACT entry (71% obtained primary care in the past 6 months), followed by WLA (38%) and PIT (18%). Mental health specialty care was also highest at BIR (60%), followed by PIT (56%), then WLA (39%)
Table 4 presents the prevalence of ATOD abuse or dependence among HPACT patients in the6 months before clinic enrollment. The most commonly misused substances were alcohol (60%/35%/44% at BIR/WLA/PIT, respectively), tobacco (71%/47%/40% at BIR/WLA/PIT, respectively), and cocaine (37%/19%/23% at BIR/WLA/PIT, respectively). PIT had the highest percentages of patients with opioid misuse (21%), followed by BIR (9%) and WLA (6%). Overall, these disorders were prevalent across sites, highest at BIR (94%) and similar at WLA (72%) and PIT (67%).
DISCUSSION
In this case report of early HPACT implementation, strikingly different models of homeless-focused primary care at the geographically distinct facilities were found. The lack of a gold standard primary care medical home for homeless persons—compounded by contrasting local contextual features—led to distinct clinic designs. BIR capitalized on primary care needs among veterans who previously sought housing and relied on the available space within an operating primary care clinic. As WLA already offered primary care for homeless veterans that was colocated with mental health services, the HPACT at this site was devised as an after-hours clinic colocated with the ED.11 At PIT, an existing primary care team with addiction expertise expanded its role to include a focus on homelessness, without needing new space or staff.
The initial HPACT patient cohorts likely reflected these contrasting clinic structures. That is, at WLA, the higher rates of unsheltered patients, prevalence of acute medical conditions and psychotic disorders, and greater ED use was likely driven by ED colocation. The physical location of this site’s HPACT may also speak to greater future decreases in ED use. At BIR, the use of a dedicated HPACT community outreach worker likely led to greater recruitment from emergency shelters. However, the clinic mainly recruited veterans who had previously engaged in VA mainstream primary care services and individuals with ATOD use. At PIT, higher rates of psychotherapy were likely facilitated by the HPACT’s placement within an addiction treatment setting, which may favor psychosocial rehabilitation. The distinctly higher rate of patients with opioid misuse at PIT likely paralleled the ATOD expertise of its providers and/or buprenorphine availability.
The more challenging questions surround the implementation of the current clinic models to address the needs of these patient cohorts and possible avenues to improve each clinic. High rates of chronic medical illness, mental illness, and ATOD use are well known in the homeless veteran and general populations.2,9 Within these 3 HPACTs, the high rates of medical/mental illness and ATOD use speak favorably about the clinics’ respective recruitment strategies; ie, normative homeless populations with high rates of illness are enrolling in these clinics. However, current service integration practices may be enhanced with the specific knowledge gained from this examination. For example, the very high rates of mood and anxiety disorders at each site suggest a role for an embedded mental health provider with prescribing privileges (the model adopted by BIR) as opposed to mental health referrals used at WLA and PIT. There may also be a role for cognitive behavioral therapy services within these clinics. Similarly, the high rates of ATOD (especially alcohol, tobacco, and cocaine) misuse suggest a role for addiction medicine training among the PCPs (the PIT model) as well as psychosocial rehabilitation for ATOD use within the HPACTs. High rates of chronic medical conditions, such as diabetes, hepatitis C, and hypertension elucidate possible roles for specialty care integration and/or chronic disease management programs tailored to the homeless.
Comparing the housing status of these cohorts can help in the design of future homeless-tailored primary care operations and improve these HPACTs. Most patients across sites lived in VA transitional housing/residential rehabilitation programs. As such, current referral practices at these 3 HPACTs proved sufficient in recruiting this subpopulation of homeless veterans. However, in light of national data showing that the count of unsheltered homeless veterans has not declined as rapidly as the count of homeless veterans overall, the higher numbers of veterans recruited from interim sheltering arrangements suggest a need for enhanced outreach to unsheltered individuals.1 WLA data suggest that linkages to EDs can advance this objective. BIR data show that targeted outreach in shelters can engage this high-risk, transiently sheltered subpopulation in primary care. At the time of this project, PIT just began using a peer support specialist for outreach to unsheltered veterans. It will be important to evaluate the outcomes of this new referral strategy.
Limitations
These exploratory findings—though from a small convenience sample within a nascent, growing program—generated critical and detailed information to guide ongoing policies and service design. However, these findings have limitations. First, though this research contributes to limited existing literature about the operational design of homeless-focused primary care, no outcome data were included. Although a comprehensive evaluation of all HPACT sites is a distinct and useful endeavor, this project instead offers a rapid, detailed illustration of 3 early-stage clinics. Though smaller in scope, this effort informs other facilities developing homeless-focused primary care initiatives and the larger demonstration project.
Second, a convenience sample of 3 urban facilities with strong academic ties and community commitment to providing services for homeless persons was presented. It may be difficult to translate these findings to communities with fewer resources.
Third, EHR review was used to determine patient demographics, diagnoses, and patterns of health care use. Though EHR review offers detailed information that is unavailable from administrative data, EHR is subject to variations in documentation patterns.
Last, differing characteristics of the homeless veteran population in each city may interact with contrasting HPACT structures to influence the characteristics of patients served. For example, though the data suggest that linkages with the ED may facilitate greater recruitment of unsheltered veterans in WLA, Los Angeles is known to have particularly high rates of unsheltered individuals.1
CONCLUSIONS
Clinicians, administrators, and researchers in the safety net may benefit from the experience implementing new clinics to recruit and engage homeless veterans in primary care. In a relatively nascent field with few accepted models of care, this paper offers detailed descriptions of newly developed homeless-focused primary care clinics at 3 VA facilities, which can inform other sites undertaking similar initiatives. This study highlights the wide range of approaches to building such clinics, with important variations in structural characteristics such as clinic location and operating hours, as well as within the intricacies of service integration patterns.
Within primary care clinics for homeless adults, this study suggests a role for embedded mental health (medication management and psychotherapy) and substance abuse services, chronic disease management programs tailored to this vulnerable population, and the role of linkages to the ED or community-based outreach to recruit unsheltered homeless patients. To pave a path toward identifying an evidence-based model of homeless-focused primary care, future studies are needed to study nationwide HPACT outcomes, including health status, patient satisfaction, quality of life, housing, and cost-effectiveness.
Acknowledgements
This work was undertaken in part by the VA’s PACT Demonstration Laboratory initiative, supporting and evaluating VA’s transition to a patient-centered medical home. Funding for the PACT Demonstration Laboratory initiative was provided by the VA Office of Patient Care Services. This project received support from the VISN 22 VA Assessment and Improvement Lab for Patient Centered Care (VAIL-PCC) (XVA 65-018; PI: Rubenstein).
Dr. Gabrielian was supported in part by the VA Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment. Drs. Gelberg and Andersen were supported in part by NIDA DA 022445. Dr. Andersen received additional support from the UCLA/DREW Project EXPORT, National Center on Minority Health and Health Disparities, P20MD000148/P20MD000182. Dr. Broyles was supported by a Career Development Award (CDA 10–014) from the VA Health Services Research & Development service. Dr. Kertesz was supported through funding of VISN 7.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cortes A, Henry M, de la Cruz RJ, Brown S. The 2012 Point-in-Time Estimates of Homelessness: Volume I of the 2012 Annual Homeless Assessment Report. Washington, DC: The U.S. Department of Housing and Urban Development, Office of Community Planning and Development; 2012.
2. Balshem H, Christensen V, Tuepker A, Kansagara D. A Critical Review of the Literature Regarding Homelessness Among Veterans. U.S. Department of Veterans Affairs, Veterans Health Administration, Health Services Research & Development Service; 2011. VA-ESP Project #05-225.
3. O’Toole TP, Pirraglia PA, Dosa D, et al; Primary Care-Special Populations Treatment Team. Building care systems to improve access for high-risk and vulnerable veteran populations. J Gen Intern Med. 2011;26(suppl 2):683-688.
4. Opening Doors: Federal Strategic Plan to Prevent and End Homelessness. Washington, DC: The United States Interagency Council on Homelessness; 2010.
5. Nakashima J, McGuire J, Berman S, Daniels W. Developing programs for homeless veterans: Understanding driving forces in implementation. Soc Work Health Care. 2004;40(2):1-12.
6. O’Toole TP, Buckel L, Bourgault C, et al. Applying the chronic care model to homeless veterans: Effect of a population approach to primary care on utilization and clinical outcomes. Am J Public Health. 2010;100(12):2493-2499.
7. Desai MM, Rosenheck RA, Kasprow WJ. Determinants of receipt of ambulatory medical care in a national sample of mentally ill homeless veterans. Med Care. 2003;41(2):275-287.
8. Irene Wong YL, Stanhope V. Conceptualizing community: A comparison of neighborhood characteristics of supportive housing for persons with psychiatric and developmental disabilities. Soc Sci Med. 2009;68(8):1376-1387.
9. Gelberg L, Gallagher TC, Andersen RM, Koegel P. Competing priorities as a barrier to medical care among homeless adults in Los Angeles. Am J Public Health. 1997;87(2):217-220.
10. Hamilton AB, Poza I, Washington DL. “Homelessness and trauma go hand-in-hand”: Pathways to homelessness among women veterans. Womens Health Issues. 2011;21(suppl 4):S203-S209.
11. McGuire J, Gelberg L, Blue-Howells J, Rosenheck RA. Access to primary care for homeless veterans with serious mental illness or substance abuse: A follow-up evaluation of co-located primary care and homeless social services. Adm Policy Ment Health. 2009;36(4):255-264.
12. VHA Operations Activities That May Constitute Research. Washington, DC: U.S. Department of Veterans Affairs, Veterans Health Administration; 2011. VHA Handbook 1058.05.
13. Resnick SG, Armstrong M, Sperrazza M, Harkness L, Rosenheck RA. A model of consumer-provider partnership: Vet-to-Vet. Psychiatr Rehabil J. 2004;28(2):185-187.
14. Hogan TP, Wakefield B, Nazi KM, Houston TK, Weaver FM. Promoting access through complementary eHealth technologies: Recommendations for VA’s Home Telehealth and personal health record programs. J Gen Intern Med. 2011;26(suppl 2):628-635.
15. Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
16. Hsiao CJ, Cherry DK, Beatty PC, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2007 summary. Natl Health Stat Report. 2010;3(27):1-32.
17. Hwang SW, Orav EJ, O’Connell JJ, Lebow JM, Brennan TA. Causes of death in homeless adults in Boston. Ann Intern Med. 1997;126(8):625-628.
18. Hwang SW, Tolomiczenko G, Kouyoumdjian FG, Garner RE. Interventions to improve the health of the homeless: A systematic review. Am J Prev Med. 2005;29(4):311-319.
1. Cortes A, Henry M, de la Cruz RJ, Brown S. The 2012 Point-in-Time Estimates of Homelessness: Volume I of the 2012 Annual Homeless Assessment Report. Washington, DC: The U.S. Department of Housing and Urban Development, Office of Community Planning and Development; 2012.
2. Balshem H, Christensen V, Tuepker A, Kansagara D. A Critical Review of the Literature Regarding Homelessness Among Veterans. U.S. Department of Veterans Affairs, Veterans Health Administration, Health Services Research & Development Service; 2011. VA-ESP Project #05-225.
3. O’Toole TP, Pirraglia PA, Dosa D, et al; Primary Care-Special Populations Treatment Team. Building care systems to improve access for high-risk and vulnerable veteran populations. J Gen Intern Med. 2011;26(suppl 2):683-688.
4. Opening Doors: Federal Strategic Plan to Prevent and End Homelessness. Washington, DC: The United States Interagency Council on Homelessness; 2010.
5. Nakashima J, McGuire J, Berman S, Daniels W. Developing programs for homeless veterans: Understanding driving forces in implementation. Soc Work Health Care. 2004;40(2):1-12.
6. O’Toole TP, Buckel L, Bourgault C, et al. Applying the chronic care model to homeless veterans: Effect of a population approach to primary care on utilization and clinical outcomes. Am J Public Health. 2010;100(12):2493-2499.
7. Desai MM, Rosenheck RA, Kasprow WJ. Determinants of receipt of ambulatory medical care in a national sample of mentally ill homeless veterans. Med Care. 2003;41(2):275-287.
8. Irene Wong YL, Stanhope V. Conceptualizing community: A comparison of neighborhood characteristics of supportive housing for persons with psychiatric and developmental disabilities. Soc Sci Med. 2009;68(8):1376-1387.
9. Gelberg L, Gallagher TC, Andersen RM, Koegel P. Competing priorities as a barrier to medical care among homeless adults in Los Angeles. Am J Public Health. 1997;87(2):217-220.
10. Hamilton AB, Poza I, Washington DL. “Homelessness and trauma go hand-in-hand”: Pathways to homelessness among women veterans. Womens Health Issues. 2011;21(suppl 4):S203-S209.
11. McGuire J, Gelberg L, Blue-Howells J, Rosenheck RA. Access to primary care for homeless veterans with serious mental illness or substance abuse: A follow-up evaluation of co-located primary care and homeless social services. Adm Policy Ment Health. 2009;36(4):255-264.
12. VHA Operations Activities That May Constitute Research. Washington, DC: U.S. Department of Veterans Affairs, Veterans Health Administration; 2011. VHA Handbook 1058.05.
13. Resnick SG, Armstrong M, Sperrazza M, Harkness L, Rosenheck RA. A model of consumer-provider partnership: Vet-to-Vet. Psychiatr Rehabil J. 2004;28(2):185-187.
14. Hogan TP, Wakefield B, Nazi KM, Houston TK, Weaver FM. Promoting access through complementary eHealth technologies: Recommendations for VA’s Home Telehealth and personal health record programs. J Gen Intern Med. 2011;26(suppl 2):628-635.
15. Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
16. Hsiao CJ, Cherry DK, Beatty PC, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2007 summary. Natl Health Stat Report. 2010;3(27):1-32.
17. Hwang SW, Orav EJ, O’Connell JJ, Lebow JM, Brennan TA. Causes of death in homeless adults in Boston. Ann Intern Med. 1997;126(8):625-628.
18. Hwang SW, Tolomiczenko G, Kouyoumdjian FG, Garner RE. Interventions to improve the health of the homeless: A systematic review. Am J Prev Med. 2005;29(4):311-319.
Three Questions Can Detect Hazardous Drinkers
STUDY DESIGN: Cross-sectional survey.
POPULATION: Patients waiting for care at 12 primary care sites in western Pennsylvania from October 1995 to December 1997.
OUTCOMES MEASURED: Sensitivity, specificity, likelihood ratios, and predictive values for the AUDIT, AUDIT-C, and AUDIT-3.
RESULTS: A total of 13,438 patients were surveyed. Compared with a quantity-frequency definition of hazardous drinking (Ž16 drinks/week for men and Ž12 drinks/week for women), the AUDIT, AUDIT-C, and AUDIT-3 had areas under the receiver-operating characteristic curves (AUROC) of 0.940, 0.949, and 0.871, respectively. The AUROCs of the AUDIT and AUDIT-C were significantly different (P=.004). The AUROCs of the AUDIT-C (P <.001) and AUDIT (P <.001) were significantly larger than the AUDIT-3. When compared with a positive AUDIT score of 8 or higher, the AUDIT-C (score Ž3) and the AUDIT-3 (score Ž1) were 94.9% and 99.6% sensitive and 68.8% and 51.1% specific in detecting individuals as hazardous drinkers.
CONCLUSIONS: In a large primary care sample, a 3-question version of the AUDIT identified hazardous drinkers as well as the full AUDIT when such drinkers were defined by quantity-frequency criterion. This version of the AUDIT may be useful as an initial screen for assessing hazardous drinking behavior.
Hazardous drinkers consume enough alcohol to be at risk for adverse consequences but do not meet criteria for alcohol abuse or dependence. They are, however, are at risk for more harmful alcohol abuse.1-5 Such drinking behavior has been defined by quantity and frequency criteria.6 It is estimated that up to 20% of primary care patients are at least hazardous drinkers.7-9 Effective interventions to reduce alcohol consumption exist in primary care settings, so it is important for care providers to reliably and efficiently identify patients who are hazardous drinkers.1,10,11 Traditionally,12-14 care providers are poor at identifying such drinkers, and as many as 72% escape their detection.15-17 This ineffectiveness may be because of a lack of brief and simple questions that aid in patient identification.18-20
Formal screening instruments have been promoted to aid in identification of patients with alcohol problems. The Alcohol Use Disorders Identification Test (AUDIT) was developed by the World Health Organization (WHO) and consists of 2 distinct instruments: a 10-item AUDIT core questionnaire and a clinical screening procedure.1,9,21 The AUDIT core questions can detect hazardous drinkers and have been used alone as a screening instrument.22 The AUDIT questions address intake, dependence, and adverse consequences of drinking,23 emphasize drinking in the past year,5,24 and are indifferent to sex or ethnicity.4,25 It is most useful at detecting drinkers who do not meet criteria for alcohol abuse or dependence.26 Because of its ability to detect less severe alcohol drinkers, the AUDIT seems to have practical value in primary care settings.4,15,21,26
Because of trends toward shorter patient visits, the 10-question AUDIT may be too lengthy to be clinically useful in primary care settings.5,27,28 The shorter CAGE questionnaire, therefore, is often recommended for use in limited time situations.18,20 However, although the CAGE is a valuable tool for identifying alcohol abuse and dependence, it is not as useful for identifying less serious behaviors, such as hazardous drinking.5,28-32
A shorter version of the AUDIT may prove beneficial for use by the busy physician for identifying hazardous drinking behavior. The AUDIT-C (consisting of the first 3 questions of the AUDIT) was shown to be as effective as the full AUDIT in detecting hazardous drinking in a population of veterans.33 Also, the AUDIT-3 (the third question of the AUDIT) may be effective for identifying hazardous drinkers.5,34
We investigated the performance of the AUDIT, AUDIT-C, and AUDIT-3 in detecting such drinkers in a large primary care sample. We also compared the AUDIT-C and the AUDIT-3 to the full AUDIT. We hypothesized that the abbreviated instruments would be comparable with the AUDIT for detecting hazardous drinkers as defined by a quantity-frequency standard.
Methods
Design
We based our study on screening data obtained as part of a large randomized clinical trial of brief interventions for hazardous drinkers (the Early Lifestyle Modification [ELM] Study). Screening forms were administered at 12 primary care sites in the western Pennsylvania area from October 1995 to December 1997. The institutional review board at the University of Pittsburgh and equivalent review groups from each primary care setting approved the ELM study and screening protocol.
Setting
The 12 primary care sites included a Veterans Affairs Medical Center internal medicine clinic, a university-based internal medicine clinic, 2 university-affiliated community care clinics, 3 health maintenance organization clinics, 3 university-affiliated family medicine clinics, and 2 private practice family medicine clinics. All clinics were staffed by physicians. The Veterans Administration and university-based clinics had internal medicine residents participating in patient care. Also, physician assistants and nurse practitioners were involved with primary care at some sites.
Patients
Patients were eligible for the study if they were in the waiting rooms of one of the clinic sites during the screening period, were approached by a research assistant in the waiting room, and agreed to answer the screening questionnaire. Screening of eligible patients occurred from October 1995 to December 1997.
Screening Instruments
In the primary care clinics, patients self-administered an 8-page survey consisting of questions about lifestyle habits. Research assistants approached as many patients in the waiting rooms of the primary care sites as possible. The survey included questions about stress management (8 questions), smoking habits (8), the AUDIT (10), and quantity-frequency questions (3). In initial surveys, just before the alcohol-related questions patients were instructed not to answer alcohol questions if they responded “never” to the following question: “How often do you have a drink containing alcohol (for example: beer, wine, wine coolers, sherry, gin, vodka, or other hard liquor)?” This question was removed in later surveys, because it limited the number of people responding to the alcohol instruments under comparison. We also asked for categorical responses to questions about age, sex, education background, race, marital status, and occupation. We did not compensate patients for completing this questionnaire, and their responses were anonymous.
Alcohol Screening Instruments
The AUDIT consists of 10 questions Figure 1. The AUDIT-C includes the following first 3 questions of the AUDIT: How often do you have a drink containing alcohol? How many drinks containing alcohol do you have on a typical day when you are drinking? How often do you have 6 or more drinks on one occasion? The AUDIT-3 is the third question alone. Each individual question is scored from 0 to 4 points on a Likert scale, with higher numbers indicating more severe drinking behavior. For AUDIT questions with only 3 possible answers (questions 9 and 10), the scores were 0, 2, and 4. Thus, the range of possible scores on the AUDIT are from 0 to 40, the AUDIT-C from 0 to 12, and the AUDIT-3 from 0 to 4.
Our quantity-frequency questionnaire consisted of the questions: “If you drink, how many days per week do you have a drink?” (answers: 1 through 7); “If you drink less than once a week, how many days per month do you drink?” (answers: 1 or less, 2, 3, or 4 or more); and “How many drinks containing alcohol do you have on a typical day when you are drinking?” (answers: 1 through 10 or more). The number of average drinks per week was computed for analysis, and if the answer was “or more” we used the maximum number indicated in the question (4 or 10).
Outcome Measures and Analysis
The main outcome measures concerned the accuracy of the full AUDIT, AUDITC, and AUDIT-3. For comparative purposes, patients with missing responses were not used in subsequent statistical analyses.
The AUDIT, AUDIT-C, and AUDIT-3 were compared with a hazardous drinking criterion defined by the quantity-frequency questions. This criterion was 16 or more drinks per week for men and 12 or more drinks per week for women.6 Clinically, quantity-frequency assessment is often used to determine hazardous drinking behavior.16 The area under the receiver-operating characteristic curve (AUROC) was used to compare each instrument’s diagnostic ability.35 AUROC is an indication of the ability of a test to discriminate between false positives and false negatives. A score approaching 1 will be more sensitive and specific over a range of cutoff points than a score of 0.5, which is a nondiscriminating test. To calculate and compare the AUROCs we used published standards developed by Hanley and McNeil36,37 for curves derived by same cases. We performed chi-square analysis on comparisons of categorical data.
We then compared the AUDIT-C and AUDIT-3 with a score of the full AUDIT as a criterion for hazardous drinking. An AUDIT result of 8 or higher was accepted as a criterion for hazardous drinking. We measured the sensitivity and specificity of the AUDIT-C, using a cutoff score of 3 or higher and AUDIT-3 cutoff score of 1 or higher compared with the criterion of hazardous drinking defined by the full AUDIT.38
Results
At least 1 question on the screening survey was answered by 13,438 patients. Overall, 13,198 (98%) either answered that they currently drink or never drank alcohol. Not all questions were answered by each individual. Of patients who indicated that they currently drink alcohol, the full AUDIT was completed by 7035 (52%). The AUDIT-C was completed by 7190 (54%) patients, and the AUDIT-3 was answered by 7303 (54%) patients. Both the entire AUDIT and quantity-frequency questions were answered by 6954 (52%) patients. Of the 13,438 patients, 36% indicated no alcohol consumption. The majority of the surveyed sample were men, white, married, employed, high school graduates, and younger than 60 years Table 1.
AUDITs Compared with Quantity-Frequency Criterion
Table 2 compares the likelihood of hazardous alcohol use, defined by a quantity-frequency criterion, associated with each score of the AUDIT, AUDIT-C, and AUDIT-3. It is important to note that at each score the true positives of screening are only persons identified at that score. For example, at an AUDIT score of 7, 53 of 754 hazardous drinkers were identified with the resulting likelihood ratio (3.0) and predictive value (26.9%).
For comparisons of the AUDIT, AUDIT-C, and AUDIT-3 at identifying hazardous drinkers who scored at or greater than a minimal cutoff, the sensitivity and specificity compared with a quantity-frequency criterion is shown in Table 3. For cut-point values of an AUDIT score of 8 or higher, the sensitivity of the AUDIT was 76%. Similarly, the sensitivities of the AUDIT-C (with score Ž3) and AUDIT-3 (with score Ž1) were 99.6% and 89.1%, as sensitive as the quantity-frequency questions in detecting these patients. Specificity of the AUDIT, AUDIT-C, and AUDIT-3 at these cutoff values was 92%, 48%, and 65%, respectively.
AUROCs were constructed from all cut-point values Figure 2. Computation of the AUROC indicates the effectiveness of the instrument to discriminate hazardous drinkers over a range of AUDIT scores. The AUROCs for the AUDIT, AUDIT-C, and AUDIT-3 were significantly more discriminating than the line of identity (AUROC=0.5). The AUROC of the AUDIT was significantly different from the AUDIT-C (z=2.69; P=.004). The AUDIT-3 AUROC was significantly different than the AUDIT (z=10.03; P <.001) and AUDIT-C (z=12.69; P <.001).
Abbreviated AUDITs Compared with Full AUDIT Criterion
The full AUDIT is often used as a standard to assess hazardous drinking. We compared the abbreviated instruments to the full AUDIT, with a positive score of 8 or higher as a criterion for such drinking. The AUDIT-C (score (3) and AUDIT-3 (score Ž1) were 94.9% and 99.9% as sensitive and 68.8% and 51.1% as specific as the full AUDIT in obtaining a positive score. We also determined the performance of the AUDIT-3 when compared with a reference standard of a positive AUDIT-C. The AUDIT-3 (score Ž1) was 69% sensitive and 95% specific as the AUDIT-C (score Ž3) at identifying hazardous drinkers (data not shown).
Discussion
We evaluated the performance of the AUDIT and abbreviated AUDIT instruments to detect hazardous drinking in a large multisite primary care sample. The abbreviated forms of the AUDIT were as effective as the AUDIT at identifying hazardous drinkers. Compared with quantity-frequency questions, the AUDIT and AUDIT-C were superior at identifying hazardous drinkers than the AUDIT-3. The abbreviated forms of the AUDIT were as sensitive as the full AUDIT at detecting hazardous drinkers when using standard cutoff values for hazardous drinking.
As with the 4-item CAGE questionnaire for alcohol dependence, a 1- or 3-item AUDIT instrument may increase care providers’ recognition of hazardous drinkers. Providers do not routinely ask standard alcohol questions, are particularly poor at identifying hazardous drinkers, and do not enter patients into alcohol treatment.16 Therefore, providing clinicians with a few easily remembered questions to determine hazardous drinking behavior would be beneficial.39 A short questionnaire would be simple to administer and applicable in a wide variety of practice settings. A positive response would increase suspicion regarding hazardous or abusive drinking behavior and prompt additional questions about patients’ alcohol use.18,40 For example, care providers could use the AUDIT-3 or AUDIT-C (to detect at least hazardous drinking), then administer the questionnaire (to detect abuse and dependence), if the patient’s response was positive.
It is important to realize that the AUDIT and its abbreviated forms are only sensitive to detect hazardous drinking, not to specifically assign patients’ drinking habits as hazardous only. The AUDIT was originally designed to distinguish a person with hazardous drinking from one with nonhazardous drinking.26 As such, this instrument may not be specific enough to distinguish hazardous drinkers from others with severe alcohol behaviors; people who score positive may qualify for alcohol abuse and dependence. For less risky alcohol behaviors, it is more important for a health care provider to identify all hazardous drinkers (true positives), at the risk of falsely identifying a person who may not have this behavior (false positives).18 Therefore, when screening to establish a threshold level of treatment intervention, screening instruments should maximize sensitivity, even at the expense of low specificity.
In our sample, the AUDIT (score Ž8), AUDIT-C (score Ž3), and AUDIT-3 (score Ž1) were as sensitive as quantity-frequency questions in detecting hazardous drinking. This increase in sensitivity of the AUDIT-C and AUDIT-3 is likely related to the consumption focus of these questions. The AUDIT-C consists of quantity and frequency type questions, and the third question is specific for quantity of drinking at one session. However, the performance of AUDIT instruments in our study is comparable and confirms results found in a study by Bush and colleagues,34 even though the studies used different criteria for assessment of abbreviated instruments.
If the AUDIT is used as a standard to detect hazardous drinkers, would the AUDIT-C or AUDIT-3 identify the same patients as the full AUDIT? Using cutoff points for the AUDIT-C of 3 or higher and an AUDIT-3 score of 1 or higher, these instruments were 99.7% and 98.3% as sensitive as the full AUDIT. As expected, specificity is much less for both abbreviated instruments. However, the high sensitivity suggests a clinical utility for these abbreviated instruments. It is unlikely that by asking the 3 AUDIT-C questions or the single AUDIT-3 question, a primary care provider will miss identification of a person who is at least a hazardous drinker.
Limitations
There is no gold standard to identify hazardous drinkers.41 The definition of what level of drinking constitutes that label is controversial, and providers do not routinely ask standard questions about drinking behavior.16 Our criterion, the quantity-frequency questions, may be considered a poor standard to compare survey instruments.7,42 In research, quantity-frequency consumption questions are helpful in specific identification of hazardous drinkers when a cutoff value is defined.5 However, patients may prefer not to answer questions about quantity or frequency of alcohol use and may not respond consistently to heterogeneous provider questions. Therefore, quantity-frequency questions may be useful as a standard to compare similar instruments such as the AUDIT and its abbreviations, although they may not be particularly effective in clinical practice.
Not all surveyed individuals completed the full AUDIT instrument. This was primarily because patients who answered “never” to our initial question regarding any alcohol use did not proceed to the AUDIT. Before completion of the study, we eliminated this question from the survey. However, it is not known whether patients who answered “never” to the initial question, were, in fact, drinkers. Consistency response bias may also have occurred as patients may have wished to answer similar items similarly. In addition, the similarity of the AUDIT-C and AUDIT-3 to quantity-frequency questions suggests that our sensitivity analysis is perhaps only the upper bounds of the briefer instruments.
We derived the abbreviated tests directly from the AUDIT, thus no assessment of the instruments out of the context of the full AUDIT was performed. Independent testing of abbreviated AUDIT instruments is needed. Recruitment was conducted by research assistants who solicited and provided forms to patients in the waiting rooms of primary care clinics. This convenience sample may have led to a selection bias in obtaining survey data. Patient recall bias may also have affected survey answers as patients may have had difficulty answering quantity and frequency questions accurately (an advantage of the AUDIT over quantity-frequency type questions). Also, our study investigates identification of individuals who are at least hazardous drinkers, but may also be abusive or dependent. We did not study the instruments’ ability to distinguish between hazardous drinking and abuse or dependence.
Conclusions
Our results confirm that the AUDIT-C and AUDIT-3 are useful screening tests for hazardous drinking. Because treatment of such drinkers can be effective, identifying people with less severe alcohol problems is crucial and an important public health initiative.21 Abbreviated instruments identify hazardous drinkers quickly, efficiently, and effectively, and may encourage early treatment to prevent the occurrence of alcohol-related consequences, abuse, or dependence. We recommend using the AUDIT-C and CAGE as brief screening instruments for hazardous drinking and alcohol abuse and dependence. This approach warrants further investigation.
Acknowledgments
Our work was supported by a grant to Dr Maisto from the National Institute of Alcohol Abuse and Alcoholism (AA10291). Dr Gordon is supported by a faculty development grant in general internal medicine from the VA Pittsburgh Healthcare System and the VISN 4 Mental Illness Research, Education, and Clinical Center. Dr Kraemer is supported by a Mentored Clinical Scientist Development Award from the National Institute of Alcohol Abuse and Alcoholism (AA00235). Dr J. Conigliaro is supported by a Career Development Award from the HSR & D Service, Department of Veterans Affairs (CD-97324-A) and is a generalist physician faculty scholar of the Robert Wood Johnson Foundation (#031500). We thank the ELM research study staff, Monica O’Connor (the ELM project coordinator), and all the patients who participated in the ELM study.
1. A cross-national trial of brief interventions with heavy drinkers: WHO Brief Intervention Study Group. Am J Public Health 1996;86:948-55.
2. Babor TF, de la Fuente JR, Saunders J, Grant M. The Alcohol Use Disorders Identification Test: guidelines for use in primary health care. Geneva, Switzerland: World Health Organization; 1989.
3. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1995.
4. Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcoholism 1997;21:613-19.
5. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Studies Alcohol 1995;56:423-32.
6. Sanchez-Craig M, Wilkinson DA, Davila R. Empirically based guidelines for moderate drinking: 1-year results from three studies with problem drinkers. Am J Public Health 1995;85:823-28.
7. Bradley KA. Screening and diagnosis of alcoholism in the primary care setting. West J Med 1992;156:166-71.
8. Schorling JB, Klas PT, Willems JP, Everett AS. Addressing alcohol use among primary care patients: differences between family medicine and internal medicine residents. J Gen Intern Med 1994;9:248-54.
9. Saunders JB, Aasland OG, Amundsen A, Grant M. Alcohol consumption and related problems among primary health care patients: WHO collaborative project on early detection of persons with harmful alcohol consumption—I. Addiction 1993;88:349-62.
10. Wallace P, Cutler S, Haines A. Randomised controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ 1988;297:663-68.
11. Fleming MF, Barry KL, Manwell LB, Johnson K, London R. Brief physician advice for problem alcohol drinkers: a randomized controlled trial in community-based primary care practices. JAMA 1997;277:1039-45.
12. Kristenson H, Ohlin H, Hulten-Nosslin MB, Trell E, Hood B. Identification and intervention of heavy drinking in middle-aged men: results and follow-up of 24-60 months of long-term study with randomized controls. Alcoholism 1983;7:203-09.
13. Bien TH, Miller WR, Tonigan JS. Brief interventions for alcohol problems: a review. Addiction 1993;88:315-35.
14. Wilk AI, Jensen NM, Havighurst TC. Meta-analysis of randomized control trials addressing brief interventions in heavy alcohol drinkers. J Gen Intern Med 1997;12:274-83.
15. Conigrave KM, Saunders JB, Reznik RB. Predictive capacity of the AUDIT questionnaire for alcohol-related harm. Addiction 1995;90:1479-85.
16. Friedmann PD, McCullough D, Chin MH, Saitz R. Screening and intervention for alcohol problems: a national survey of primary care physicians and psychiatrists. J Gen Intern Med 2000;15:84-91.
17. Bowen OR, Sammons JH. The alcohol-abusing patient: a challenge to the profession. JAMA 1988;260:2267-70.
18. Allen JP, Maisto SA, Connors GJ. Self-report screening tests for alcohol problems in primary care. Arch Intern Med 1995;155:1726-30.
19. Saunders JB, Conigrave KM. Early identification of alcohol problems. CMAJ 1990;143:1060-69.
20. Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry 1974;131:1121-23.
21. Barry KL, Fleming MF. The Alcohol Use Disorders Identification Test (AUDIT) and the SMAST-13: predictive validity in a rural primary care sample. Alcohol Alcoholism 1993;28:33-42.
22. Schmidt A, Barry KL, Fleming MF. Detection of problem drinkers: the Alcohol Use Disorders Identification Test (AUDIT). South Med J 1995;88:52-59.
23. Volk RJ, Steinbauer JR, Cantor SB, Holzer CE, III. The Alcohol Use Disorders Identification Test (AUDIT) as a screen for at-risk drinking in primary care patients of different racial/ethnic backgrounds. Addiction 1997;92:197-206.
24. Kettl PA. Detecting problem drinkers in your practice. Patient Care 1997;30:27-41.
25. Steinbauer JR, Cantor SB, Holzer CE, III, Volk RJ. Ethnic and sex bias in primary care screening tests for alcohol use disorders. Ann Intern Med 1998;129:353-62.
26. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction 1993;88:791-804.
27. Foster AI, Blondell RD, Looney SW. The practicality of using the SMAST and AUDIT to screen for alcoholism among adolescents in an urban private family practice. J Kentucky Med Assoc 1997;95:105-07.
28. Bradley KA, Bush KR, McDonell MB, Malone T, Fihn SD. Screening for problem drinking: comparison of CAGE and AUDIT: Ambulatory Care Quality Improvement Project (ACQUIP). J Gen Intern Med 1998;13:379-88.
29. Morton JL, Jones TV, Manganaro MA. Performance of alcoholism screening questionnaires in elderly veterans. Am J Med 1996;101:153-59.
30. MacKenzie D, Langa A, Brown TM. Identifying hazardous or harmful alcohol use in medical admissions: a comparison of audit, cage and brief mast. Alcohol Alcoholism 1996;31:591-99.
31. Buchsbaum DG, Buchanan RG, Centor RM, Schnoll SH, Lawton MJ. Screening for alcohol abuse using CAGE scores and likelihood ratios. Ann Intern Med 1991;115:774-77.
32. Seppa K, Makela R, Sillanaukee P. Effectiveness of the Alcohol Use Disorders Identification Test in occupational health screenings. Alcoholism 1995;19:999-1003.
33. Piccinelli M, Tessari E, Bortolomasi M, et al. Efficacy of the alcohol use disorders identification test as a screening tool for hazardous alcohol intake and related disorders in primary care: a validity study. BMJ 1997;314:420-24.
34. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Arch Intern Med 1998;158:1789-95.
35. van Kammen DP, Kelley ME, Gurklis JA, et al. Behavioral vs biochemical prediction of clinical stability following haloperidol withdrawal in schizophrenia. Arch Gen Psychiatry 1995;52:673-78.
36. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver-operating characteristic (ROC) curve. Radiology 1982;143:29-36.
37. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983;148:839-43.
38. Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Alcohol Use Disorder Identification Test. Addiction 1995;90:1349-56.
39. Cherpitel CJ, Clark WB. Ethnic differences in performance of screening instruments for identifying harmful drinking and alcohol dependence in the emergency room. Alcoholism 1995;19:628-34.
40. Reid MC, Fiellin DA, O’Connor PG. Hazardous and harmful alcohol consumption in primary care. Arch Intern Med 1999;159:1681-89.
41. Fink A, Hays RD, Moore AA, Beck JC. Alcohol-related problems in older persons. Determinants, consequences, and screening. Arch Intern Med 1996;156:1150-56.
42. Bradley KA, Boyd-Wickizer J, Powell SH, Burman ML. Alcohol screening questionnaires in women: a critical review. JAMA 1998;280:166-71.
STUDY DESIGN: Cross-sectional survey.
POPULATION: Patients waiting for care at 12 primary care sites in western Pennsylvania from October 1995 to December 1997.
OUTCOMES MEASURED: Sensitivity, specificity, likelihood ratios, and predictive values for the AUDIT, AUDIT-C, and AUDIT-3.
RESULTS: A total of 13,438 patients were surveyed. Compared with a quantity-frequency definition of hazardous drinking (Ž16 drinks/week for men and Ž12 drinks/week for women), the AUDIT, AUDIT-C, and AUDIT-3 had areas under the receiver-operating characteristic curves (AUROC) of 0.940, 0.949, and 0.871, respectively. The AUROCs of the AUDIT and AUDIT-C were significantly different (P=.004). The AUROCs of the AUDIT-C (P <.001) and AUDIT (P <.001) were significantly larger than the AUDIT-3. When compared with a positive AUDIT score of 8 or higher, the AUDIT-C (score Ž3) and the AUDIT-3 (score Ž1) were 94.9% and 99.6% sensitive and 68.8% and 51.1% specific in detecting individuals as hazardous drinkers.
CONCLUSIONS: In a large primary care sample, a 3-question version of the AUDIT identified hazardous drinkers as well as the full AUDIT when such drinkers were defined by quantity-frequency criterion. This version of the AUDIT may be useful as an initial screen for assessing hazardous drinking behavior.
Hazardous drinkers consume enough alcohol to be at risk for adverse consequences but do not meet criteria for alcohol abuse or dependence. They are, however, are at risk for more harmful alcohol abuse.1-5 Such drinking behavior has been defined by quantity and frequency criteria.6 It is estimated that up to 20% of primary care patients are at least hazardous drinkers.7-9 Effective interventions to reduce alcohol consumption exist in primary care settings, so it is important for care providers to reliably and efficiently identify patients who are hazardous drinkers.1,10,11 Traditionally,12-14 care providers are poor at identifying such drinkers, and as many as 72% escape their detection.15-17 This ineffectiveness may be because of a lack of brief and simple questions that aid in patient identification.18-20
Formal screening instruments have been promoted to aid in identification of patients with alcohol problems. The Alcohol Use Disorders Identification Test (AUDIT) was developed by the World Health Organization (WHO) and consists of 2 distinct instruments: a 10-item AUDIT core questionnaire and a clinical screening procedure.1,9,21 The AUDIT core questions can detect hazardous drinkers and have been used alone as a screening instrument.22 The AUDIT questions address intake, dependence, and adverse consequences of drinking,23 emphasize drinking in the past year,5,24 and are indifferent to sex or ethnicity.4,25 It is most useful at detecting drinkers who do not meet criteria for alcohol abuse or dependence.26 Because of its ability to detect less severe alcohol drinkers, the AUDIT seems to have practical value in primary care settings.4,15,21,26
Because of trends toward shorter patient visits, the 10-question AUDIT may be too lengthy to be clinically useful in primary care settings.5,27,28 The shorter CAGE questionnaire, therefore, is often recommended for use in limited time situations.18,20 However, although the CAGE is a valuable tool for identifying alcohol abuse and dependence, it is not as useful for identifying less serious behaviors, such as hazardous drinking.5,28-32
A shorter version of the AUDIT may prove beneficial for use by the busy physician for identifying hazardous drinking behavior. The AUDIT-C (consisting of the first 3 questions of the AUDIT) was shown to be as effective as the full AUDIT in detecting hazardous drinking in a population of veterans.33 Also, the AUDIT-3 (the third question of the AUDIT) may be effective for identifying hazardous drinkers.5,34
We investigated the performance of the AUDIT, AUDIT-C, and AUDIT-3 in detecting such drinkers in a large primary care sample. We also compared the AUDIT-C and the AUDIT-3 to the full AUDIT. We hypothesized that the abbreviated instruments would be comparable with the AUDIT for detecting hazardous drinkers as defined by a quantity-frequency standard.
Methods
Design
We based our study on screening data obtained as part of a large randomized clinical trial of brief interventions for hazardous drinkers (the Early Lifestyle Modification [ELM] Study). Screening forms were administered at 12 primary care sites in the western Pennsylvania area from October 1995 to December 1997. The institutional review board at the University of Pittsburgh and equivalent review groups from each primary care setting approved the ELM study and screening protocol.
Setting
The 12 primary care sites included a Veterans Affairs Medical Center internal medicine clinic, a university-based internal medicine clinic, 2 university-affiliated community care clinics, 3 health maintenance organization clinics, 3 university-affiliated family medicine clinics, and 2 private practice family medicine clinics. All clinics were staffed by physicians. The Veterans Administration and university-based clinics had internal medicine residents participating in patient care. Also, physician assistants and nurse practitioners were involved with primary care at some sites.
Patients
Patients were eligible for the study if they were in the waiting rooms of one of the clinic sites during the screening period, were approached by a research assistant in the waiting room, and agreed to answer the screening questionnaire. Screening of eligible patients occurred from October 1995 to December 1997.
Screening Instruments
In the primary care clinics, patients self-administered an 8-page survey consisting of questions about lifestyle habits. Research assistants approached as many patients in the waiting rooms of the primary care sites as possible. The survey included questions about stress management (8 questions), smoking habits (8), the AUDIT (10), and quantity-frequency questions (3). In initial surveys, just before the alcohol-related questions patients were instructed not to answer alcohol questions if they responded “never” to the following question: “How often do you have a drink containing alcohol (for example: beer, wine, wine coolers, sherry, gin, vodka, or other hard liquor)?” This question was removed in later surveys, because it limited the number of people responding to the alcohol instruments under comparison. We also asked for categorical responses to questions about age, sex, education background, race, marital status, and occupation. We did not compensate patients for completing this questionnaire, and their responses were anonymous.
Alcohol Screening Instruments
The AUDIT consists of 10 questions Figure 1. The AUDIT-C includes the following first 3 questions of the AUDIT: How often do you have a drink containing alcohol? How many drinks containing alcohol do you have on a typical day when you are drinking? How often do you have 6 or more drinks on one occasion? The AUDIT-3 is the third question alone. Each individual question is scored from 0 to 4 points on a Likert scale, with higher numbers indicating more severe drinking behavior. For AUDIT questions with only 3 possible answers (questions 9 and 10), the scores were 0, 2, and 4. Thus, the range of possible scores on the AUDIT are from 0 to 40, the AUDIT-C from 0 to 12, and the AUDIT-3 from 0 to 4.
Our quantity-frequency questionnaire consisted of the questions: “If you drink, how many days per week do you have a drink?” (answers: 1 through 7); “If you drink less than once a week, how many days per month do you drink?” (answers: 1 or less, 2, 3, or 4 or more); and “How many drinks containing alcohol do you have on a typical day when you are drinking?” (answers: 1 through 10 or more). The number of average drinks per week was computed for analysis, and if the answer was “or more” we used the maximum number indicated in the question (4 or 10).
Outcome Measures and Analysis
The main outcome measures concerned the accuracy of the full AUDIT, AUDITC, and AUDIT-3. For comparative purposes, patients with missing responses were not used in subsequent statistical analyses.
The AUDIT, AUDIT-C, and AUDIT-3 were compared with a hazardous drinking criterion defined by the quantity-frequency questions. This criterion was 16 or more drinks per week for men and 12 or more drinks per week for women.6 Clinically, quantity-frequency assessment is often used to determine hazardous drinking behavior.16 The area under the receiver-operating characteristic curve (AUROC) was used to compare each instrument’s diagnostic ability.35 AUROC is an indication of the ability of a test to discriminate between false positives and false negatives. A score approaching 1 will be more sensitive and specific over a range of cutoff points than a score of 0.5, which is a nondiscriminating test. To calculate and compare the AUROCs we used published standards developed by Hanley and McNeil36,37 for curves derived by same cases. We performed chi-square analysis on comparisons of categorical data.
We then compared the AUDIT-C and AUDIT-3 with a score of the full AUDIT as a criterion for hazardous drinking. An AUDIT result of 8 or higher was accepted as a criterion for hazardous drinking. We measured the sensitivity and specificity of the AUDIT-C, using a cutoff score of 3 or higher and AUDIT-3 cutoff score of 1 or higher compared with the criterion of hazardous drinking defined by the full AUDIT.38
Results
At least 1 question on the screening survey was answered by 13,438 patients. Overall, 13,198 (98%) either answered that they currently drink or never drank alcohol. Not all questions were answered by each individual. Of patients who indicated that they currently drink alcohol, the full AUDIT was completed by 7035 (52%). The AUDIT-C was completed by 7190 (54%) patients, and the AUDIT-3 was answered by 7303 (54%) patients. Both the entire AUDIT and quantity-frequency questions were answered by 6954 (52%) patients. Of the 13,438 patients, 36% indicated no alcohol consumption. The majority of the surveyed sample were men, white, married, employed, high school graduates, and younger than 60 years Table 1.
AUDITs Compared with Quantity-Frequency Criterion
Table 2 compares the likelihood of hazardous alcohol use, defined by a quantity-frequency criterion, associated with each score of the AUDIT, AUDIT-C, and AUDIT-3. It is important to note that at each score the true positives of screening are only persons identified at that score. For example, at an AUDIT score of 7, 53 of 754 hazardous drinkers were identified with the resulting likelihood ratio (3.0) and predictive value (26.9%).
For comparisons of the AUDIT, AUDIT-C, and AUDIT-3 at identifying hazardous drinkers who scored at or greater than a minimal cutoff, the sensitivity and specificity compared with a quantity-frequency criterion is shown in Table 3. For cut-point values of an AUDIT score of 8 or higher, the sensitivity of the AUDIT was 76%. Similarly, the sensitivities of the AUDIT-C (with score Ž3) and AUDIT-3 (with score Ž1) were 99.6% and 89.1%, as sensitive as the quantity-frequency questions in detecting these patients. Specificity of the AUDIT, AUDIT-C, and AUDIT-3 at these cutoff values was 92%, 48%, and 65%, respectively.
AUROCs were constructed from all cut-point values Figure 2. Computation of the AUROC indicates the effectiveness of the instrument to discriminate hazardous drinkers over a range of AUDIT scores. The AUROCs for the AUDIT, AUDIT-C, and AUDIT-3 were significantly more discriminating than the line of identity (AUROC=0.5). The AUROC of the AUDIT was significantly different from the AUDIT-C (z=2.69; P=.004). The AUDIT-3 AUROC was significantly different than the AUDIT (z=10.03; P <.001) and AUDIT-C (z=12.69; P <.001).
Abbreviated AUDITs Compared with Full AUDIT Criterion
The full AUDIT is often used as a standard to assess hazardous drinking. We compared the abbreviated instruments to the full AUDIT, with a positive score of 8 or higher as a criterion for such drinking. The AUDIT-C (score (3) and AUDIT-3 (score Ž1) were 94.9% and 99.9% as sensitive and 68.8% and 51.1% as specific as the full AUDIT in obtaining a positive score. We also determined the performance of the AUDIT-3 when compared with a reference standard of a positive AUDIT-C. The AUDIT-3 (score Ž1) was 69% sensitive and 95% specific as the AUDIT-C (score Ž3) at identifying hazardous drinkers (data not shown).
Discussion
We evaluated the performance of the AUDIT and abbreviated AUDIT instruments to detect hazardous drinking in a large multisite primary care sample. The abbreviated forms of the AUDIT were as effective as the AUDIT at identifying hazardous drinkers. Compared with quantity-frequency questions, the AUDIT and AUDIT-C were superior at identifying hazardous drinkers than the AUDIT-3. The abbreviated forms of the AUDIT were as sensitive as the full AUDIT at detecting hazardous drinkers when using standard cutoff values for hazardous drinking.
As with the 4-item CAGE questionnaire for alcohol dependence, a 1- or 3-item AUDIT instrument may increase care providers’ recognition of hazardous drinkers. Providers do not routinely ask standard alcohol questions, are particularly poor at identifying hazardous drinkers, and do not enter patients into alcohol treatment.16 Therefore, providing clinicians with a few easily remembered questions to determine hazardous drinking behavior would be beneficial.39 A short questionnaire would be simple to administer and applicable in a wide variety of practice settings. A positive response would increase suspicion regarding hazardous or abusive drinking behavior and prompt additional questions about patients’ alcohol use.18,40 For example, care providers could use the AUDIT-3 or AUDIT-C (to detect at least hazardous drinking), then administer the questionnaire (to detect abuse and dependence), if the patient’s response was positive.
It is important to realize that the AUDIT and its abbreviated forms are only sensitive to detect hazardous drinking, not to specifically assign patients’ drinking habits as hazardous only. The AUDIT was originally designed to distinguish a person with hazardous drinking from one with nonhazardous drinking.26 As such, this instrument may not be specific enough to distinguish hazardous drinkers from others with severe alcohol behaviors; people who score positive may qualify for alcohol abuse and dependence. For less risky alcohol behaviors, it is more important for a health care provider to identify all hazardous drinkers (true positives), at the risk of falsely identifying a person who may not have this behavior (false positives).18 Therefore, when screening to establish a threshold level of treatment intervention, screening instruments should maximize sensitivity, even at the expense of low specificity.
In our sample, the AUDIT (score Ž8), AUDIT-C (score Ž3), and AUDIT-3 (score Ž1) were as sensitive as quantity-frequency questions in detecting hazardous drinking. This increase in sensitivity of the AUDIT-C and AUDIT-3 is likely related to the consumption focus of these questions. The AUDIT-C consists of quantity and frequency type questions, and the third question is specific for quantity of drinking at one session. However, the performance of AUDIT instruments in our study is comparable and confirms results found in a study by Bush and colleagues,34 even though the studies used different criteria for assessment of abbreviated instruments.
If the AUDIT is used as a standard to detect hazardous drinkers, would the AUDIT-C or AUDIT-3 identify the same patients as the full AUDIT? Using cutoff points for the AUDIT-C of 3 or higher and an AUDIT-3 score of 1 or higher, these instruments were 99.7% and 98.3% as sensitive as the full AUDIT. As expected, specificity is much less for both abbreviated instruments. However, the high sensitivity suggests a clinical utility for these abbreviated instruments. It is unlikely that by asking the 3 AUDIT-C questions or the single AUDIT-3 question, a primary care provider will miss identification of a person who is at least a hazardous drinker.
Limitations
There is no gold standard to identify hazardous drinkers.41 The definition of what level of drinking constitutes that label is controversial, and providers do not routinely ask standard questions about drinking behavior.16 Our criterion, the quantity-frequency questions, may be considered a poor standard to compare survey instruments.7,42 In research, quantity-frequency consumption questions are helpful in specific identification of hazardous drinkers when a cutoff value is defined.5 However, patients may prefer not to answer questions about quantity or frequency of alcohol use and may not respond consistently to heterogeneous provider questions. Therefore, quantity-frequency questions may be useful as a standard to compare similar instruments such as the AUDIT and its abbreviations, although they may not be particularly effective in clinical practice.
Not all surveyed individuals completed the full AUDIT instrument. This was primarily because patients who answered “never” to our initial question regarding any alcohol use did not proceed to the AUDIT. Before completion of the study, we eliminated this question from the survey. However, it is not known whether patients who answered “never” to the initial question, were, in fact, drinkers. Consistency response bias may also have occurred as patients may have wished to answer similar items similarly. In addition, the similarity of the AUDIT-C and AUDIT-3 to quantity-frequency questions suggests that our sensitivity analysis is perhaps only the upper bounds of the briefer instruments.
We derived the abbreviated tests directly from the AUDIT, thus no assessment of the instruments out of the context of the full AUDIT was performed. Independent testing of abbreviated AUDIT instruments is needed. Recruitment was conducted by research assistants who solicited and provided forms to patients in the waiting rooms of primary care clinics. This convenience sample may have led to a selection bias in obtaining survey data. Patient recall bias may also have affected survey answers as patients may have had difficulty answering quantity and frequency questions accurately (an advantage of the AUDIT over quantity-frequency type questions). Also, our study investigates identification of individuals who are at least hazardous drinkers, but may also be abusive or dependent. We did not study the instruments’ ability to distinguish between hazardous drinking and abuse or dependence.
Conclusions
Our results confirm that the AUDIT-C and AUDIT-3 are useful screening tests for hazardous drinking. Because treatment of such drinkers can be effective, identifying people with less severe alcohol problems is crucial and an important public health initiative.21 Abbreviated instruments identify hazardous drinkers quickly, efficiently, and effectively, and may encourage early treatment to prevent the occurrence of alcohol-related consequences, abuse, or dependence. We recommend using the AUDIT-C and CAGE as brief screening instruments for hazardous drinking and alcohol abuse and dependence. This approach warrants further investigation.
Acknowledgments
Our work was supported by a grant to Dr Maisto from the National Institute of Alcohol Abuse and Alcoholism (AA10291). Dr Gordon is supported by a faculty development grant in general internal medicine from the VA Pittsburgh Healthcare System and the VISN 4 Mental Illness Research, Education, and Clinical Center. Dr Kraemer is supported by a Mentored Clinical Scientist Development Award from the National Institute of Alcohol Abuse and Alcoholism (AA00235). Dr J. Conigliaro is supported by a Career Development Award from the HSR & D Service, Department of Veterans Affairs (CD-97324-A) and is a generalist physician faculty scholar of the Robert Wood Johnson Foundation (#031500). We thank the ELM research study staff, Monica O’Connor (the ELM project coordinator), and all the patients who participated in the ELM study.
STUDY DESIGN: Cross-sectional survey.
POPULATION: Patients waiting for care at 12 primary care sites in western Pennsylvania from October 1995 to December 1997.
OUTCOMES MEASURED: Sensitivity, specificity, likelihood ratios, and predictive values for the AUDIT, AUDIT-C, and AUDIT-3.
RESULTS: A total of 13,438 patients were surveyed. Compared with a quantity-frequency definition of hazardous drinking (Ž16 drinks/week for men and Ž12 drinks/week for women), the AUDIT, AUDIT-C, and AUDIT-3 had areas under the receiver-operating characteristic curves (AUROC) of 0.940, 0.949, and 0.871, respectively. The AUROCs of the AUDIT and AUDIT-C were significantly different (P=.004). The AUROCs of the AUDIT-C (P <.001) and AUDIT (P <.001) were significantly larger than the AUDIT-3. When compared with a positive AUDIT score of 8 or higher, the AUDIT-C (score Ž3) and the AUDIT-3 (score Ž1) were 94.9% and 99.6% sensitive and 68.8% and 51.1% specific in detecting individuals as hazardous drinkers.
CONCLUSIONS: In a large primary care sample, a 3-question version of the AUDIT identified hazardous drinkers as well as the full AUDIT when such drinkers were defined by quantity-frequency criterion. This version of the AUDIT may be useful as an initial screen for assessing hazardous drinking behavior.
Hazardous drinkers consume enough alcohol to be at risk for adverse consequences but do not meet criteria for alcohol abuse or dependence. They are, however, are at risk for more harmful alcohol abuse.1-5 Such drinking behavior has been defined by quantity and frequency criteria.6 It is estimated that up to 20% of primary care patients are at least hazardous drinkers.7-9 Effective interventions to reduce alcohol consumption exist in primary care settings, so it is important for care providers to reliably and efficiently identify patients who are hazardous drinkers.1,10,11 Traditionally,12-14 care providers are poor at identifying such drinkers, and as many as 72% escape their detection.15-17 This ineffectiveness may be because of a lack of brief and simple questions that aid in patient identification.18-20
Formal screening instruments have been promoted to aid in identification of patients with alcohol problems. The Alcohol Use Disorders Identification Test (AUDIT) was developed by the World Health Organization (WHO) and consists of 2 distinct instruments: a 10-item AUDIT core questionnaire and a clinical screening procedure.1,9,21 The AUDIT core questions can detect hazardous drinkers and have been used alone as a screening instrument.22 The AUDIT questions address intake, dependence, and adverse consequences of drinking,23 emphasize drinking in the past year,5,24 and are indifferent to sex or ethnicity.4,25 It is most useful at detecting drinkers who do not meet criteria for alcohol abuse or dependence.26 Because of its ability to detect less severe alcohol drinkers, the AUDIT seems to have practical value in primary care settings.4,15,21,26
Because of trends toward shorter patient visits, the 10-question AUDIT may be too lengthy to be clinically useful in primary care settings.5,27,28 The shorter CAGE questionnaire, therefore, is often recommended for use in limited time situations.18,20 However, although the CAGE is a valuable tool for identifying alcohol abuse and dependence, it is not as useful for identifying less serious behaviors, such as hazardous drinking.5,28-32
A shorter version of the AUDIT may prove beneficial for use by the busy physician for identifying hazardous drinking behavior. The AUDIT-C (consisting of the first 3 questions of the AUDIT) was shown to be as effective as the full AUDIT in detecting hazardous drinking in a population of veterans.33 Also, the AUDIT-3 (the third question of the AUDIT) may be effective for identifying hazardous drinkers.5,34
We investigated the performance of the AUDIT, AUDIT-C, and AUDIT-3 in detecting such drinkers in a large primary care sample. We also compared the AUDIT-C and the AUDIT-3 to the full AUDIT. We hypothesized that the abbreviated instruments would be comparable with the AUDIT for detecting hazardous drinkers as defined by a quantity-frequency standard.
Methods
Design
We based our study on screening data obtained as part of a large randomized clinical trial of brief interventions for hazardous drinkers (the Early Lifestyle Modification [ELM] Study). Screening forms were administered at 12 primary care sites in the western Pennsylvania area from October 1995 to December 1997. The institutional review board at the University of Pittsburgh and equivalent review groups from each primary care setting approved the ELM study and screening protocol.
Setting
The 12 primary care sites included a Veterans Affairs Medical Center internal medicine clinic, a university-based internal medicine clinic, 2 university-affiliated community care clinics, 3 health maintenance organization clinics, 3 university-affiliated family medicine clinics, and 2 private practice family medicine clinics. All clinics were staffed by physicians. The Veterans Administration and university-based clinics had internal medicine residents participating in patient care. Also, physician assistants and nurse practitioners were involved with primary care at some sites.
Patients
Patients were eligible for the study if they were in the waiting rooms of one of the clinic sites during the screening period, were approached by a research assistant in the waiting room, and agreed to answer the screening questionnaire. Screening of eligible patients occurred from October 1995 to December 1997.
Screening Instruments
In the primary care clinics, patients self-administered an 8-page survey consisting of questions about lifestyle habits. Research assistants approached as many patients in the waiting rooms of the primary care sites as possible. The survey included questions about stress management (8 questions), smoking habits (8), the AUDIT (10), and quantity-frequency questions (3). In initial surveys, just before the alcohol-related questions patients were instructed not to answer alcohol questions if they responded “never” to the following question: “How often do you have a drink containing alcohol (for example: beer, wine, wine coolers, sherry, gin, vodka, or other hard liquor)?” This question was removed in later surveys, because it limited the number of people responding to the alcohol instruments under comparison. We also asked for categorical responses to questions about age, sex, education background, race, marital status, and occupation. We did not compensate patients for completing this questionnaire, and their responses were anonymous.
Alcohol Screening Instruments
The AUDIT consists of 10 questions Figure 1. The AUDIT-C includes the following first 3 questions of the AUDIT: How often do you have a drink containing alcohol? How many drinks containing alcohol do you have on a typical day when you are drinking? How often do you have 6 or more drinks on one occasion? The AUDIT-3 is the third question alone. Each individual question is scored from 0 to 4 points on a Likert scale, with higher numbers indicating more severe drinking behavior. For AUDIT questions with only 3 possible answers (questions 9 and 10), the scores were 0, 2, and 4. Thus, the range of possible scores on the AUDIT are from 0 to 40, the AUDIT-C from 0 to 12, and the AUDIT-3 from 0 to 4.
Our quantity-frequency questionnaire consisted of the questions: “If you drink, how many days per week do you have a drink?” (answers: 1 through 7); “If you drink less than once a week, how many days per month do you drink?” (answers: 1 or less, 2, 3, or 4 or more); and “How many drinks containing alcohol do you have on a typical day when you are drinking?” (answers: 1 through 10 or more). The number of average drinks per week was computed for analysis, and if the answer was “or more” we used the maximum number indicated in the question (4 or 10).
Outcome Measures and Analysis
The main outcome measures concerned the accuracy of the full AUDIT, AUDITC, and AUDIT-3. For comparative purposes, patients with missing responses were not used in subsequent statistical analyses.
The AUDIT, AUDIT-C, and AUDIT-3 were compared with a hazardous drinking criterion defined by the quantity-frequency questions. This criterion was 16 or more drinks per week for men and 12 or more drinks per week for women.6 Clinically, quantity-frequency assessment is often used to determine hazardous drinking behavior.16 The area under the receiver-operating characteristic curve (AUROC) was used to compare each instrument’s diagnostic ability.35 AUROC is an indication of the ability of a test to discriminate between false positives and false negatives. A score approaching 1 will be more sensitive and specific over a range of cutoff points than a score of 0.5, which is a nondiscriminating test. To calculate and compare the AUROCs we used published standards developed by Hanley and McNeil36,37 for curves derived by same cases. We performed chi-square analysis on comparisons of categorical data.
We then compared the AUDIT-C and AUDIT-3 with a score of the full AUDIT as a criterion for hazardous drinking. An AUDIT result of 8 or higher was accepted as a criterion for hazardous drinking. We measured the sensitivity and specificity of the AUDIT-C, using a cutoff score of 3 or higher and AUDIT-3 cutoff score of 1 or higher compared with the criterion of hazardous drinking defined by the full AUDIT.38
Results
At least 1 question on the screening survey was answered by 13,438 patients. Overall, 13,198 (98%) either answered that they currently drink or never drank alcohol. Not all questions were answered by each individual. Of patients who indicated that they currently drink alcohol, the full AUDIT was completed by 7035 (52%). The AUDIT-C was completed by 7190 (54%) patients, and the AUDIT-3 was answered by 7303 (54%) patients. Both the entire AUDIT and quantity-frequency questions were answered by 6954 (52%) patients. Of the 13,438 patients, 36% indicated no alcohol consumption. The majority of the surveyed sample were men, white, married, employed, high school graduates, and younger than 60 years Table 1.
AUDITs Compared with Quantity-Frequency Criterion
Table 2 compares the likelihood of hazardous alcohol use, defined by a quantity-frequency criterion, associated with each score of the AUDIT, AUDIT-C, and AUDIT-3. It is important to note that at each score the true positives of screening are only persons identified at that score. For example, at an AUDIT score of 7, 53 of 754 hazardous drinkers were identified with the resulting likelihood ratio (3.0) and predictive value (26.9%).
For comparisons of the AUDIT, AUDIT-C, and AUDIT-3 at identifying hazardous drinkers who scored at or greater than a minimal cutoff, the sensitivity and specificity compared with a quantity-frequency criterion is shown in Table 3. For cut-point values of an AUDIT score of 8 or higher, the sensitivity of the AUDIT was 76%. Similarly, the sensitivities of the AUDIT-C (with score Ž3) and AUDIT-3 (with score Ž1) were 99.6% and 89.1%, as sensitive as the quantity-frequency questions in detecting these patients. Specificity of the AUDIT, AUDIT-C, and AUDIT-3 at these cutoff values was 92%, 48%, and 65%, respectively.
AUROCs were constructed from all cut-point values Figure 2. Computation of the AUROC indicates the effectiveness of the instrument to discriminate hazardous drinkers over a range of AUDIT scores. The AUROCs for the AUDIT, AUDIT-C, and AUDIT-3 were significantly more discriminating than the line of identity (AUROC=0.5). The AUROC of the AUDIT was significantly different from the AUDIT-C (z=2.69; P=.004). The AUDIT-3 AUROC was significantly different than the AUDIT (z=10.03; P <.001) and AUDIT-C (z=12.69; P <.001).
Abbreviated AUDITs Compared with Full AUDIT Criterion
The full AUDIT is often used as a standard to assess hazardous drinking. We compared the abbreviated instruments to the full AUDIT, with a positive score of 8 or higher as a criterion for such drinking. The AUDIT-C (score (3) and AUDIT-3 (score Ž1) were 94.9% and 99.9% as sensitive and 68.8% and 51.1% as specific as the full AUDIT in obtaining a positive score. We also determined the performance of the AUDIT-3 when compared with a reference standard of a positive AUDIT-C. The AUDIT-3 (score Ž1) was 69% sensitive and 95% specific as the AUDIT-C (score Ž3) at identifying hazardous drinkers (data not shown).
Discussion
We evaluated the performance of the AUDIT and abbreviated AUDIT instruments to detect hazardous drinking in a large multisite primary care sample. The abbreviated forms of the AUDIT were as effective as the AUDIT at identifying hazardous drinkers. Compared with quantity-frequency questions, the AUDIT and AUDIT-C were superior at identifying hazardous drinkers than the AUDIT-3. The abbreviated forms of the AUDIT were as sensitive as the full AUDIT at detecting hazardous drinkers when using standard cutoff values for hazardous drinking.
As with the 4-item CAGE questionnaire for alcohol dependence, a 1- or 3-item AUDIT instrument may increase care providers’ recognition of hazardous drinkers. Providers do not routinely ask standard alcohol questions, are particularly poor at identifying hazardous drinkers, and do not enter patients into alcohol treatment.16 Therefore, providing clinicians with a few easily remembered questions to determine hazardous drinking behavior would be beneficial.39 A short questionnaire would be simple to administer and applicable in a wide variety of practice settings. A positive response would increase suspicion regarding hazardous or abusive drinking behavior and prompt additional questions about patients’ alcohol use.18,40 For example, care providers could use the AUDIT-3 or AUDIT-C (to detect at least hazardous drinking), then administer the questionnaire (to detect abuse and dependence), if the patient’s response was positive.
It is important to realize that the AUDIT and its abbreviated forms are only sensitive to detect hazardous drinking, not to specifically assign patients’ drinking habits as hazardous only. The AUDIT was originally designed to distinguish a person with hazardous drinking from one with nonhazardous drinking.26 As such, this instrument may not be specific enough to distinguish hazardous drinkers from others with severe alcohol behaviors; people who score positive may qualify for alcohol abuse and dependence. For less risky alcohol behaviors, it is more important for a health care provider to identify all hazardous drinkers (true positives), at the risk of falsely identifying a person who may not have this behavior (false positives).18 Therefore, when screening to establish a threshold level of treatment intervention, screening instruments should maximize sensitivity, even at the expense of low specificity.
In our sample, the AUDIT (score Ž8), AUDIT-C (score Ž3), and AUDIT-3 (score Ž1) were as sensitive as quantity-frequency questions in detecting hazardous drinking. This increase in sensitivity of the AUDIT-C and AUDIT-3 is likely related to the consumption focus of these questions. The AUDIT-C consists of quantity and frequency type questions, and the third question is specific for quantity of drinking at one session. However, the performance of AUDIT instruments in our study is comparable and confirms results found in a study by Bush and colleagues,34 even though the studies used different criteria for assessment of abbreviated instruments.
If the AUDIT is used as a standard to detect hazardous drinkers, would the AUDIT-C or AUDIT-3 identify the same patients as the full AUDIT? Using cutoff points for the AUDIT-C of 3 or higher and an AUDIT-3 score of 1 or higher, these instruments were 99.7% and 98.3% as sensitive as the full AUDIT. As expected, specificity is much less for both abbreviated instruments. However, the high sensitivity suggests a clinical utility for these abbreviated instruments. It is unlikely that by asking the 3 AUDIT-C questions or the single AUDIT-3 question, a primary care provider will miss identification of a person who is at least a hazardous drinker.
Limitations
There is no gold standard to identify hazardous drinkers.41 The definition of what level of drinking constitutes that label is controversial, and providers do not routinely ask standard questions about drinking behavior.16 Our criterion, the quantity-frequency questions, may be considered a poor standard to compare survey instruments.7,42 In research, quantity-frequency consumption questions are helpful in specific identification of hazardous drinkers when a cutoff value is defined.5 However, patients may prefer not to answer questions about quantity or frequency of alcohol use and may not respond consistently to heterogeneous provider questions. Therefore, quantity-frequency questions may be useful as a standard to compare similar instruments such as the AUDIT and its abbreviations, although they may not be particularly effective in clinical practice.
Not all surveyed individuals completed the full AUDIT instrument. This was primarily because patients who answered “never” to our initial question regarding any alcohol use did not proceed to the AUDIT. Before completion of the study, we eliminated this question from the survey. However, it is not known whether patients who answered “never” to the initial question, were, in fact, drinkers. Consistency response bias may also have occurred as patients may have wished to answer similar items similarly. In addition, the similarity of the AUDIT-C and AUDIT-3 to quantity-frequency questions suggests that our sensitivity analysis is perhaps only the upper bounds of the briefer instruments.
We derived the abbreviated tests directly from the AUDIT, thus no assessment of the instruments out of the context of the full AUDIT was performed. Independent testing of abbreviated AUDIT instruments is needed. Recruitment was conducted by research assistants who solicited and provided forms to patients in the waiting rooms of primary care clinics. This convenience sample may have led to a selection bias in obtaining survey data. Patient recall bias may also have affected survey answers as patients may have had difficulty answering quantity and frequency questions accurately (an advantage of the AUDIT over quantity-frequency type questions). Also, our study investigates identification of individuals who are at least hazardous drinkers, but may also be abusive or dependent. We did not study the instruments’ ability to distinguish between hazardous drinking and abuse or dependence.
Conclusions
Our results confirm that the AUDIT-C and AUDIT-3 are useful screening tests for hazardous drinking. Because treatment of such drinkers can be effective, identifying people with less severe alcohol problems is crucial and an important public health initiative.21 Abbreviated instruments identify hazardous drinkers quickly, efficiently, and effectively, and may encourage early treatment to prevent the occurrence of alcohol-related consequences, abuse, or dependence. We recommend using the AUDIT-C and CAGE as brief screening instruments for hazardous drinking and alcohol abuse and dependence. This approach warrants further investigation.
Acknowledgments
Our work was supported by a grant to Dr Maisto from the National Institute of Alcohol Abuse and Alcoholism (AA10291). Dr Gordon is supported by a faculty development grant in general internal medicine from the VA Pittsburgh Healthcare System and the VISN 4 Mental Illness Research, Education, and Clinical Center. Dr Kraemer is supported by a Mentored Clinical Scientist Development Award from the National Institute of Alcohol Abuse and Alcoholism (AA00235). Dr J. Conigliaro is supported by a Career Development Award from the HSR & D Service, Department of Veterans Affairs (CD-97324-A) and is a generalist physician faculty scholar of the Robert Wood Johnson Foundation (#031500). We thank the ELM research study staff, Monica O’Connor (the ELM project coordinator), and all the patients who participated in the ELM study.
1. A cross-national trial of brief interventions with heavy drinkers: WHO Brief Intervention Study Group. Am J Public Health 1996;86:948-55.
2. Babor TF, de la Fuente JR, Saunders J, Grant M. The Alcohol Use Disorders Identification Test: guidelines for use in primary health care. Geneva, Switzerland: World Health Organization; 1989.
3. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1995.
4. Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcoholism 1997;21:613-19.
5. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Studies Alcohol 1995;56:423-32.
6. Sanchez-Craig M, Wilkinson DA, Davila R. Empirically based guidelines for moderate drinking: 1-year results from three studies with problem drinkers. Am J Public Health 1995;85:823-28.
7. Bradley KA. Screening and diagnosis of alcoholism in the primary care setting. West J Med 1992;156:166-71.
8. Schorling JB, Klas PT, Willems JP, Everett AS. Addressing alcohol use among primary care patients: differences between family medicine and internal medicine residents. J Gen Intern Med 1994;9:248-54.
9. Saunders JB, Aasland OG, Amundsen A, Grant M. Alcohol consumption and related problems among primary health care patients: WHO collaborative project on early detection of persons with harmful alcohol consumption—I. Addiction 1993;88:349-62.
10. Wallace P, Cutler S, Haines A. Randomised controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ 1988;297:663-68.
11. Fleming MF, Barry KL, Manwell LB, Johnson K, London R. Brief physician advice for problem alcohol drinkers: a randomized controlled trial in community-based primary care practices. JAMA 1997;277:1039-45.
12. Kristenson H, Ohlin H, Hulten-Nosslin MB, Trell E, Hood B. Identification and intervention of heavy drinking in middle-aged men: results and follow-up of 24-60 months of long-term study with randomized controls. Alcoholism 1983;7:203-09.
13. Bien TH, Miller WR, Tonigan JS. Brief interventions for alcohol problems: a review. Addiction 1993;88:315-35.
14. Wilk AI, Jensen NM, Havighurst TC. Meta-analysis of randomized control trials addressing brief interventions in heavy alcohol drinkers. J Gen Intern Med 1997;12:274-83.
15. Conigrave KM, Saunders JB, Reznik RB. Predictive capacity of the AUDIT questionnaire for alcohol-related harm. Addiction 1995;90:1479-85.
16. Friedmann PD, McCullough D, Chin MH, Saitz R. Screening and intervention for alcohol problems: a national survey of primary care physicians and psychiatrists. J Gen Intern Med 2000;15:84-91.
17. Bowen OR, Sammons JH. The alcohol-abusing patient: a challenge to the profession. JAMA 1988;260:2267-70.
18. Allen JP, Maisto SA, Connors GJ. Self-report screening tests for alcohol problems in primary care. Arch Intern Med 1995;155:1726-30.
19. Saunders JB, Conigrave KM. Early identification of alcohol problems. CMAJ 1990;143:1060-69.
20. Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry 1974;131:1121-23.
21. Barry KL, Fleming MF. The Alcohol Use Disorders Identification Test (AUDIT) and the SMAST-13: predictive validity in a rural primary care sample. Alcohol Alcoholism 1993;28:33-42.
22. Schmidt A, Barry KL, Fleming MF. Detection of problem drinkers: the Alcohol Use Disorders Identification Test (AUDIT). South Med J 1995;88:52-59.
23. Volk RJ, Steinbauer JR, Cantor SB, Holzer CE, III. The Alcohol Use Disorders Identification Test (AUDIT) as a screen for at-risk drinking in primary care patients of different racial/ethnic backgrounds. Addiction 1997;92:197-206.
24. Kettl PA. Detecting problem drinkers in your practice. Patient Care 1997;30:27-41.
25. Steinbauer JR, Cantor SB, Holzer CE, III, Volk RJ. Ethnic and sex bias in primary care screening tests for alcohol use disorders. Ann Intern Med 1998;129:353-62.
26. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction 1993;88:791-804.
27. Foster AI, Blondell RD, Looney SW. The practicality of using the SMAST and AUDIT to screen for alcoholism among adolescents in an urban private family practice. J Kentucky Med Assoc 1997;95:105-07.
28. Bradley KA, Bush KR, McDonell MB, Malone T, Fihn SD. Screening for problem drinking: comparison of CAGE and AUDIT: Ambulatory Care Quality Improvement Project (ACQUIP). J Gen Intern Med 1998;13:379-88.
29. Morton JL, Jones TV, Manganaro MA. Performance of alcoholism screening questionnaires in elderly veterans. Am J Med 1996;101:153-59.
30. MacKenzie D, Langa A, Brown TM. Identifying hazardous or harmful alcohol use in medical admissions: a comparison of audit, cage and brief mast. Alcohol Alcoholism 1996;31:591-99.
31. Buchsbaum DG, Buchanan RG, Centor RM, Schnoll SH, Lawton MJ. Screening for alcohol abuse using CAGE scores and likelihood ratios. Ann Intern Med 1991;115:774-77.
32. Seppa K, Makela R, Sillanaukee P. Effectiveness of the Alcohol Use Disorders Identification Test in occupational health screenings. Alcoholism 1995;19:999-1003.
33. Piccinelli M, Tessari E, Bortolomasi M, et al. Efficacy of the alcohol use disorders identification test as a screening tool for hazardous alcohol intake and related disorders in primary care: a validity study. BMJ 1997;314:420-24.
34. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Arch Intern Med 1998;158:1789-95.
35. van Kammen DP, Kelley ME, Gurklis JA, et al. Behavioral vs biochemical prediction of clinical stability following haloperidol withdrawal in schizophrenia. Arch Gen Psychiatry 1995;52:673-78.
36. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver-operating characteristic (ROC) curve. Radiology 1982;143:29-36.
37. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983;148:839-43.
38. Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Alcohol Use Disorder Identification Test. Addiction 1995;90:1349-56.
39. Cherpitel CJ, Clark WB. Ethnic differences in performance of screening instruments for identifying harmful drinking and alcohol dependence in the emergency room. Alcoholism 1995;19:628-34.
40. Reid MC, Fiellin DA, O’Connor PG. Hazardous and harmful alcohol consumption in primary care. Arch Intern Med 1999;159:1681-89.
41. Fink A, Hays RD, Moore AA, Beck JC. Alcohol-related problems in older persons. Determinants, consequences, and screening. Arch Intern Med 1996;156:1150-56.
42. Bradley KA, Boyd-Wickizer J, Powell SH, Burman ML. Alcohol screening questionnaires in women: a critical review. JAMA 1998;280:166-71.
1. A cross-national trial of brief interventions with heavy drinkers: WHO Brief Intervention Study Group. Am J Public Health 1996;86:948-55.
2. Babor TF, de la Fuente JR, Saunders J, Grant M. The Alcohol Use Disorders Identification Test: guidelines for use in primary health care. Geneva, Switzerland: World Health Organization; 1989.
3. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1995.
4. Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcoholism 1997;21:613-19.
5. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Studies Alcohol 1995;56:423-32.
6. Sanchez-Craig M, Wilkinson DA, Davila R. Empirically based guidelines for moderate drinking: 1-year results from three studies with problem drinkers. Am J Public Health 1995;85:823-28.
7. Bradley KA. Screening and diagnosis of alcoholism in the primary care setting. West J Med 1992;156:166-71.
8. Schorling JB, Klas PT, Willems JP, Everett AS. Addressing alcohol use among primary care patients: differences between family medicine and internal medicine residents. J Gen Intern Med 1994;9:248-54.
9. Saunders JB, Aasland OG, Amundsen A, Grant M. Alcohol consumption and related problems among primary health care patients: WHO collaborative project on early detection of persons with harmful alcohol consumption—I. Addiction 1993;88:349-62.
10. Wallace P, Cutler S, Haines A. Randomised controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ 1988;297:663-68.
11. Fleming MF, Barry KL, Manwell LB, Johnson K, London R. Brief physician advice for problem alcohol drinkers: a randomized controlled trial in community-based primary care practices. JAMA 1997;277:1039-45.
12. Kristenson H, Ohlin H, Hulten-Nosslin MB, Trell E, Hood B. Identification and intervention of heavy drinking in middle-aged men: results and follow-up of 24-60 months of long-term study with randomized controls. Alcoholism 1983;7:203-09.
13. Bien TH, Miller WR, Tonigan JS. Brief interventions for alcohol problems: a review. Addiction 1993;88:315-35.
14. Wilk AI, Jensen NM, Havighurst TC. Meta-analysis of randomized control trials addressing brief interventions in heavy alcohol drinkers. J Gen Intern Med 1997;12:274-83.
15. Conigrave KM, Saunders JB, Reznik RB. Predictive capacity of the AUDIT questionnaire for alcohol-related harm. Addiction 1995;90:1479-85.
16. Friedmann PD, McCullough D, Chin MH, Saitz R. Screening and intervention for alcohol problems: a national survey of primary care physicians and psychiatrists. J Gen Intern Med 2000;15:84-91.
17. Bowen OR, Sammons JH. The alcohol-abusing patient: a challenge to the profession. JAMA 1988;260:2267-70.
18. Allen JP, Maisto SA, Connors GJ. Self-report screening tests for alcohol problems in primary care. Arch Intern Med 1995;155:1726-30.
19. Saunders JB, Conigrave KM. Early identification of alcohol problems. CMAJ 1990;143:1060-69.
20. Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry 1974;131:1121-23.
21. Barry KL, Fleming MF. The Alcohol Use Disorders Identification Test (AUDIT) and the SMAST-13: predictive validity in a rural primary care sample. Alcohol Alcoholism 1993;28:33-42.
22. Schmidt A, Barry KL, Fleming MF. Detection of problem drinkers: the Alcohol Use Disorders Identification Test (AUDIT). South Med J 1995;88:52-59.
23. Volk RJ, Steinbauer JR, Cantor SB, Holzer CE, III. The Alcohol Use Disorders Identification Test (AUDIT) as a screen for at-risk drinking in primary care patients of different racial/ethnic backgrounds. Addiction 1997;92:197-206.
24. Kettl PA. Detecting problem drinkers in your practice. Patient Care 1997;30:27-41.
25. Steinbauer JR, Cantor SB, Holzer CE, III, Volk RJ. Ethnic and sex bias in primary care screening tests for alcohol use disorders. Ann Intern Med 1998;129:353-62.
26. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction 1993;88:791-804.
27. Foster AI, Blondell RD, Looney SW. The practicality of using the SMAST and AUDIT to screen for alcoholism among adolescents in an urban private family practice. J Kentucky Med Assoc 1997;95:105-07.
28. Bradley KA, Bush KR, McDonell MB, Malone T, Fihn SD. Screening for problem drinking: comparison of CAGE and AUDIT: Ambulatory Care Quality Improvement Project (ACQUIP). J Gen Intern Med 1998;13:379-88.
29. Morton JL, Jones TV, Manganaro MA. Performance of alcoholism screening questionnaires in elderly veterans. Am J Med 1996;101:153-59.
30. MacKenzie D, Langa A, Brown TM. Identifying hazardous or harmful alcohol use in medical admissions: a comparison of audit, cage and brief mast. Alcohol Alcoholism 1996;31:591-99.
31. Buchsbaum DG, Buchanan RG, Centor RM, Schnoll SH, Lawton MJ. Screening for alcohol abuse using CAGE scores and likelihood ratios. Ann Intern Med 1991;115:774-77.
32. Seppa K, Makela R, Sillanaukee P. Effectiveness of the Alcohol Use Disorders Identification Test in occupational health screenings. Alcoholism 1995;19:999-1003.
33. Piccinelli M, Tessari E, Bortolomasi M, et al. Efficacy of the alcohol use disorders identification test as a screening tool for hazardous alcohol intake and related disorders in primary care: a validity study. BMJ 1997;314:420-24.
34. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Arch Intern Med 1998;158:1789-95.
35. van Kammen DP, Kelley ME, Gurklis JA, et al. Behavioral vs biochemical prediction of clinical stability following haloperidol withdrawal in schizophrenia. Arch Gen Psychiatry 1995;52:673-78.
36. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver-operating characteristic (ROC) curve. Radiology 1982;143:29-36.
37. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983;148:839-43.
38. Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Alcohol Use Disorder Identification Test. Addiction 1995;90:1349-56.
39. Cherpitel CJ, Clark WB. Ethnic differences in performance of screening instruments for identifying harmful drinking and alcohol dependence in the emergency room. Alcoholism 1995;19:628-34.
40. Reid MC, Fiellin DA, O’Connor PG. Hazardous and harmful alcohol consumption in primary care. Arch Intern Med 1999;159:1681-89.
41. Fink A, Hays RD, Moore AA, Beck JC. Alcohol-related problems in older persons. Determinants, consequences, and screening. Arch Intern Med 1996;156:1150-56.
42. Bradley KA, Boyd-Wickizer J, Powell SH, Burman ML. Alcohol screening questionnaires in women: a critical review. JAMA 1998;280:166-71.