User login
Impact of the COVID-19 Pandemic on Characteristics of Cutaneous Tumors Treated by Mohs Micrographic Surgery
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
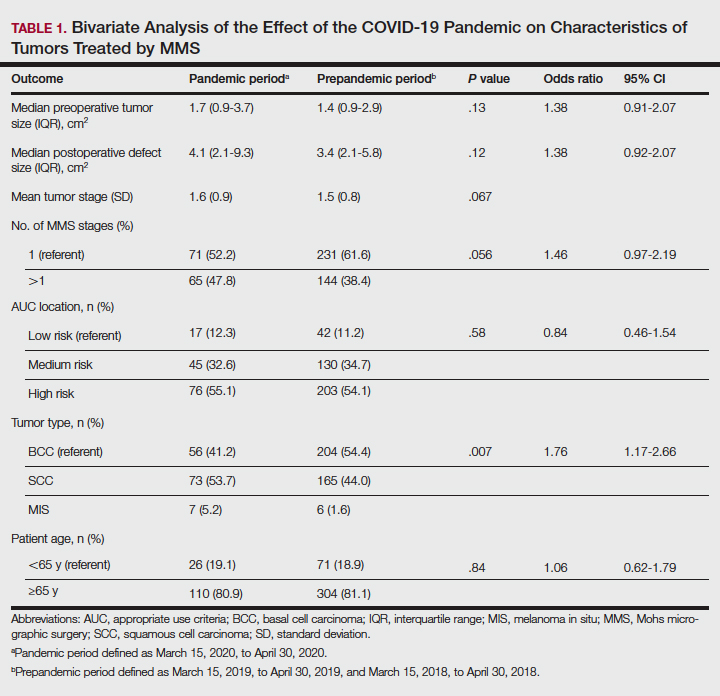
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.
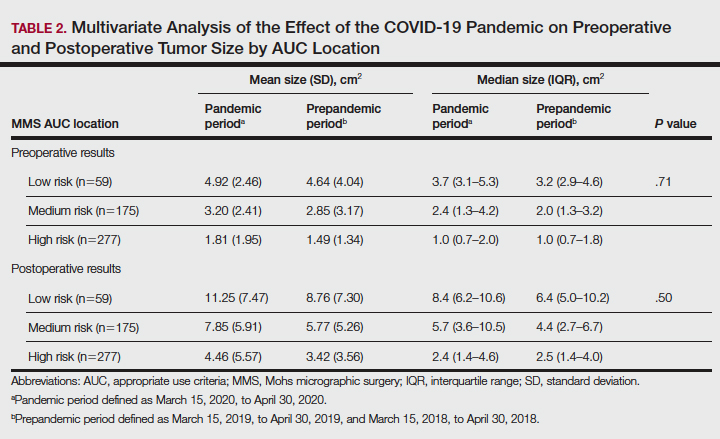
Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).
Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.
Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).
Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.
Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).
Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
Practice Points
- Mohs surgeons should follow best practice guidelines dictated by our governing professional societies in selecting skin cancers for treatment by Mohs micrographic surgery (MMS) during the COVID-19 pandemic and beyond.
- The COVID-19 pandemic has impacted the characteristics of skin cancers treated by MMS, largely driven by new guidelines.
- Changing characteristics of skin cancers treated by MMS are of clinical significance, potentially affecting the extent of reconstructive surgery, cost, operating time, and future tumor characteristics.
Magnification for the Dermatologic Surgeon
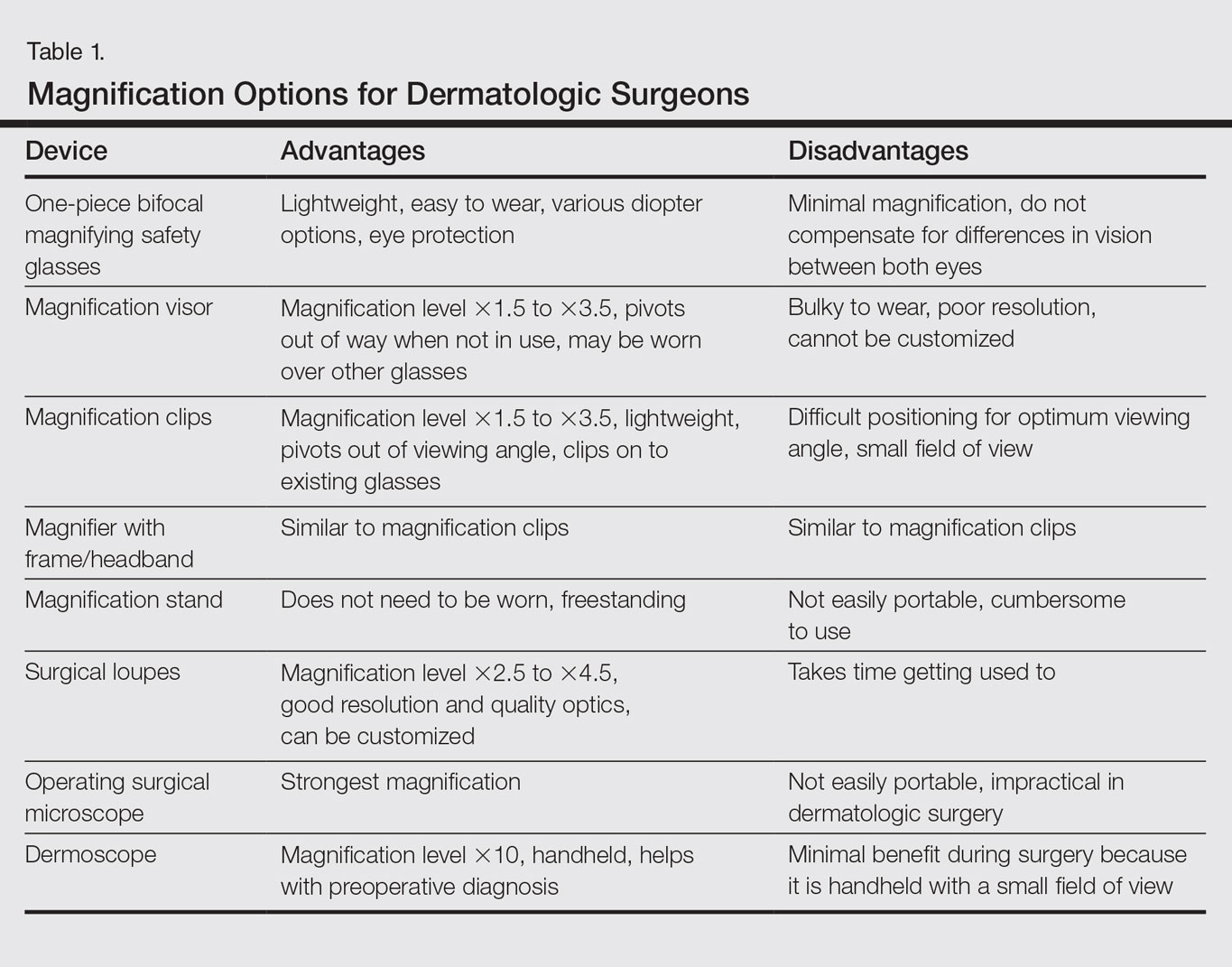
Dermatologic surgeons are susceptible to work-related ailments given the nature of their working posture, the most common of which are pain and stiffness in the neck, shoulders, and lower back, as well as headaches.1,2 Awkward posture and positioning, for the sake of getting a better view of the task at hand, puts the surgeon in ergonomically disagreeable positions. Because the prime working years for a dermatologic surgeon tend to coincide with the age of presbyopia onset, magnification may help reduce and thwart musculoskeletal problems and eye strain. Indeed, a multitude of surgical specialties and dentists use intraoperative magnification.3 Knowledge and use of available magnification options can be a key addition to the dermatologic surgeon’s armamentarium. We discuss the need for magnification and review magnification devices that are readily available to the dermatologic surgeon. Table 1 presents a summary of all magnification options discussed.
Need for Magnification
Presbyopia is a condition of aging in which one loses the ability to accommodate and focus at near distances. The estimated prevalence of presbyopia in North America is 83%, typically with onset by 45 years of age.4 Individuals with presbyopia often hold objects farther away from their eyes to bring them into focus, causing eye strain, headaches, and musculoskeletal injury.
Use of intraoperative magnification allows for enhanced visualization of fine anatomic details and precise suture placement for the surgeon with or without presbyopia. Higher magnification produces a larger image; however, it also reduces field of view and depth of field (ie, the amount of depth that stays in focus without repositioning). The resolution and quality of the image are dependent on the optical properties of the lens system. The ideal optic system is surgeon dependent and involves a combination of magnification level that will not result in dramatic loss of view and depth of field, while maintaining crispness and quality of image.
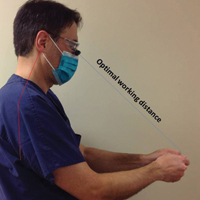
Intraoperative magnification yields ergonomic benefits by promoting a safer neck flexion angle by increasing the working distance to a more ideal position (Figure). In doing so, it improves posture and minimizes eye and musculoskeletal strain secondary to awkward positioning and presbyopia.1,5 Stationary working position and neck flexion and rotation with precise and repetitive tasks are risk factors for strain and injuries that dermatologic surgeons often encounter.1 Magnification devices are tools that the dermatologic surgeon can utilize for a more ergonomically sound practice. Indeed, magnification has been shown to improve posture in the dental literature, a specialty with similar occupational risk factors to dermatologic surgery.6-8 Ergonomic practice reduces occupational injuries and improves work quality and productivity, thereby having a favorable effect on both the patient and the physician.
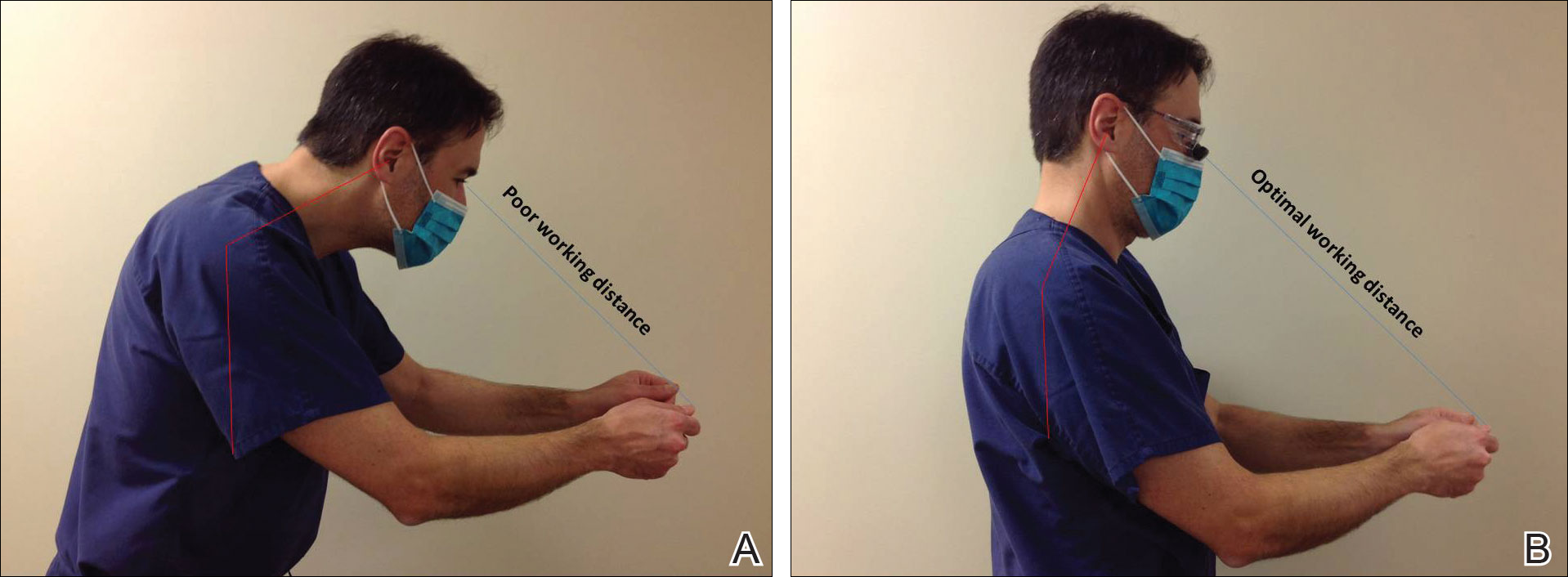
Improved Outcomes With Magnification
There are many examples of improved surgical quality and outcomes with magnification in other specialties. Hart and Hall5 illustrated the advantage of magnification in laceration repairs in the emergency department. In one study, increased magnification resulted in a substantial decrease in positive surgical margin rates in open radical retropubic prostatectomy.9 Schoeffl et al10 demonstrated that the microsurgical success of fine surgical procedures was directly related to optical magnification strength when comparing the unaided eye, surgical loupes, and the operating microscope. The dental literature also has numerous examples of magnification producing improved quality dentistry.11-13 Although magnification is not a novel concept to dermatologic surgery, little has been written about its use in the dermatologic surgery literature.
Magnification Options
One-Piece Bifocal Magnifying Safety Glasses
Bifocal magnifying safety glasses are polycarbonate safety glasses made with lenses in which the lower half is a magnifying lens. They are available in +1.5, +2.0, +2.5, and +3.0 diopter strengths. The total magnification power is calculated as follows: (diopter/4) + 1. The glasses are lightweight, easy to wear, inexpensive, and protect the eyes; however, they provide minimal magnification and do not compensate for differences in vision between both eyes.
Magnification Visor
The magnification visor is a headband visor with magnification lenses. It comes in various levels of magnification ranging from ×1.5 to ×3.5. It can be worn over prescription or safety glasses, may be pivoted out of the way when not in use, and is inexpensive. Conversely, it may be bulky to wear, cannot be customized, and does not offer the best resolution.
Magnification Clips
Magnification clips are hard-coated magnifying lens plates that fasten to eyeglass frames and range in level of magnification from ×1.5 to ×3.5. They can be pivoted out of the viewing angle, are lightweight, and are inexpensive; however, positioning may be difficult for ideal working distance and viewing angle.
Magnifier With Frame/Headband
The magnifier with frame is similar to magnification clips, but the magnification lens plate comes with a frame. It can be used with or without glasses and comes in magnification levels of ×1.5 to ×3.5. It is light, inexpensive, and may be pivoted out of sight, but similar to magnification clips, positioning for the right viewing angle and working distance may be difficult.
The magnifier with headband is essentially the same as the magnifier with frame. The only difference is the magnification plate is attached to a headband as opposed to a frame. It has similar benefits and limitations as the magnifier with frame.
Magnification Stand
The magnification stand comes as a large magnification lens with a flexible arm attached to a stand. It is a basic magnification tool and does not need to be worn; however, the stand is not easily portable and may be cumbersome to use.
Surgical Loupes
Surgical loupes are a robust magnification choice and the mainstay in magnification for the dermatologic surgeon. Loupes have proven to have comparable results in some procedures to the powerful operating surgical microscope.14-17 Factors to consider with loupes include brand, design, lens, magnification, resolution, optimal working distance, field depth, and declination angle.18
The 2 surgical loupe designs—flip-up loupes and through-the-lens loupes—differ in the mounting of the optic lenses on safety glasses. Flip-up loupes have the optics mounted to the bridge of the frame, whereas through-the-lens loupes are fixed in the lenses.
There are 3 different optical systems for surgical loupe magnification: simple, compound, and prismatic. Simple lenses consist of one pair of positive meniscus lenses similar to reading glasses. Compound lenses are made of 2 magnification lenses. Prismatic lenses magnify using a prism that folds and lengthens the light path.19,20
Loupes range in magnification level from ×2.5 to ×4.5. Compared to other magnification modalities, they can be customized and offer better resolution with quality magnification. Additionally, loupes can be fitted with a light source; however, they are expensive and surgeons need time to get used to the increased magnification as well as wearing the loupes.
There are advantages and disadvantages to the different loupe designs (Table 2). Flip-up loupes are more versatile, allowing for use on various safety glasses. They can be flipped out of view, and the declination angle may be altered; however, flip-up loupes have a narrower field of view and are heavier and bulkier than through-the-lens loupes. Through-the-lens loupes are lighter and have a larger field of view, as the optics are closer to the eye. They are customized to the declination angle and working distance of the surgeon. Conversely, through-the-lens loupes are more expensive and cannot be adjusted or moved from the line of vision.
Operating Surgical Microscope
The operating surgical microscope is not practical in the dermatologic surgeon’s practice. It is expensive and provides unnecessarily powerful magnification for dermatologic surgery. This tool usually is used in the operating room for suturing nerves and vessels with sutures sized 8-0 and smaller. Most skin procedures require size 6-0 and larger sutures.
Dermoscope
Dermoscopy, also known as epiluminescence microscopy, is a technique utilizing a handheld device made up of polarized light and a ×10 magnifying lens to evaluate skin lesions. In skilled hands, dermoscopy allows for the examination of characteristic patterns and morphologic features of skin lesions to enhance the clinician’s diagnostic accuracy.21 It may aid the dermatologic surgeon in identifying the surgical margins of difficult-to-define skin cancers. It is small and mobile; however, it has minimal benefit to the dermatologic surgeon during surgery because it is handheld and has a small field of view.
Conclusion
Good ergonomic practices facilitate a healthier and prolonged career for the dermatologic surgeon. When used properly, magnification devices can be a beneficial adjunct to the dermatologic surgeon by promoting better posture, preventing eyestrain, and providing enhanced visualization of the operating field and instruments. Use of magnification devices has been demonstrated to improve patient outcomes in other specialties. There are opportunities for further research specific to magnification improving dermatologic surgery outcomes given the high level of precision and accuracy needed for Mohs micrographic surgery, wound reconstruction, nail surgery, and hair transplantation.
- Liang CA, Levine VJ, Dusza SW, et al. Musculoskeletal disorders and ergonomics in dermatologic surgery: a survey of Mohs surgeons in 2010. Dermatol Surg. 2012;38:240-248.
- Esser AC, Koshy JG, Randle HW. Ergonomics in office-based surgery: a survey-guided observational study. Dermatol Surg. 2007;33:1304-1313; discussion, 1313-1314.
- Jarrett PM. Intraoperative magnification: who uses it? Microsurgery. 2004;24:420-422.
- Holden BA, Fricke TR, Ho SM, et al. Global vision impairment due to uncorrected presbyopia. Arch Ophthalmol. 2008;126:1731-1739.
- Hart RG, Hall J. The value of loupe magnification: an underused tool in emergency medicine. Am J Emerg Med. 2007;25:704-707.
- Branson BG, Bray KK, Gadbury-Amyot C, et al. Effect of magnification lenses on student operator posture. J Dent Educ. 2004;68:384-389.
- Maillet JP, Millar AM, Burke JM, et al. Effect of magnification loupes on dental hygiene student posture. J Dent Educ. 2008;72:33-44.
- Branson BG, Black MA, Simmer-Beck M. Changes in posture: a case study of a dental hygienist’s use of magnification loupes. Work. 2010;35:467-476.
- Magera JS Jr, Inman BA, Slezak JM, et al. Increased optical magnification from 2.5× to 4.3× with technical modification lowers the positive margin rate in open radical retropubic prostatectomy [published online November 13, 2007].J Urol. 2008;179:130-135.
- Schoeffl H, Lazzeri D, Schnelzer R, et al. Optical magnification should be mandatory for microsurgery: scientific basis and clinical data contributing to quality assurance. Arch Plast Surg. 2013;40:104-108.
- Taschieri S, Del Fabbro M, Testori T, et al. Endodontic surgery using 2 different magnification devices: preliminary results of a randomized controlled study. J Oral Maxillofac Surg. 2006;64:235-242.
- Christensen GJ. Magnification in dentistry: useful tool or another gimmick? J Am Dent Assoc. 2003;134:1647-1650.
- Syme SE, Fried JL, Strassler HE. Enhanced visualization using magnification systems. J Dent Hyg. 1997;71:202-206.
- Pieptu D, Luchian S. Loupes-only microsurgery. Microsurgery. 2003;23:181-188.
- Shenaq SM, Klebuc MJ, Vargo D. Free-tissue transfer with the aid of loupe magnification: experience with 251 procedures. Plast Reconstr Surg. 1995;95:261-269.
- Serletti JM, Deuber MA, Guidera PM, et al. Comparison of the operating microscope and loupes for free microvascular tissue transfer. Plast Reconstr Surg. 1995;95:270-276.
- Ross DA, Ariyan S, Restifo R, et al. Use of the operating microscope and loupes for head and neck free microvascular tissue transfer: a retrospective comparison. Arch Otolaryngol Head Neck Surg. 2003;129:189-193.
- Mungadi IA. Refinement on surgical technique: role of magnification. J Surg Tech Case Rep. 2010;2:1-2.
- Stanbury SJ, Elfar J. The use of surgical loupes in microsurgery. J Hand Surg Am. 2011;36:154-156.
- Baker JM, Meals RA. A practical guide to surgical loupes. J Hand Surg Am. 1997;22:967-974.
- Campos-do-Carmo G, Ramos-e-Silva M. Dermoscopy: basic concepts. Int J Dermatol. 2008;47:712-719.
Dermatologic surgeons are susceptible to work-related ailments given the nature of their working posture, the most common of which are pain and stiffness in the neck, shoulders, and lower back, as well as headaches.1,2 Awkward posture and positioning, for the sake of getting a better view of the task at hand, puts the surgeon in ergonomically disagreeable positions. Because the prime working years for a dermatologic surgeon tend to coincide with the age of presbyopia onset, magnification may help reduce and thwart musculoskeletal problems and eye strain. Indeed, a multitude of surgical specialties and dentists use intraoperative magnification.3 Knowledge and use of available magnification options can be a key addition to the dermatologic surgeon’s armamentarium. We discuss the need for magnification and review magnification devices that are readily available to the dermatologic surgeon. Table 1 presents a summary of all magnification options discussed.
Need for Magnification
Presbyopia is a condition of aging in which one loses the ability to accommodate and focus at near distances. The estimated prevalence of presbyopia in North America is 83%, typically with onset by 45 years of age.4 Individuals with presbyopia often hold objects farther away from their eyes to bring them into focus, causing eye strain, headaches, and musculoskeletal injury.
Use of intraoperative magnification allows for enhanced visualization of fine anatomic details and precise suture placement for the surgeon with or without presbyopia. Higher magnification produces a larger image; however, it also reduces field of view and depth of field (ie, the amount of depth that stays in focus without repositioning). The resolution and quality of the image are dependent on the optical properties of the lens system. The ideal optic system is surgeon dependent and involves a combination of magnification level that will not result in dramatic loss of view and depth of field, while maintaining crispness and quality of image.
Intraoperative magnification yields ergonomic benefits by promoting a safer neck flexion angle by increasing the working distance to a more ideal position (Figure). In doing so, it improves posture and minimizes eye and musculoskeletal strain secondary to awkward positioning and presbyopia.1,5 Stationary working position and neck flexion and rotation with precise and repetitive tasks are risk factors for strain and injuries that dermatologic surgeons often encounter.1 Magnification devices are tools that the dermatologic surgeon can utilize for a more ergonomically sound practice. Indeed, magnification has been shown to improve posture in the dental literature, a specialty with similar occupational risk factors to dermatologic surgery.6-8 Ergonomic practice reduces occupational injuries and improves work quality and productivity, thereby having a favorable effect on both the patient and the physician.

Improved Outcomes With Magnification
There are many examples of improved surgical quality and outcomes with magnification in other specialties. Hart and Hall5 illustrated the advantage of magnification in laceration repairs in the emergency department. In one study, increased magnification resulted in a substantial decrease in positive surgical margin rates in open radical retropubic prostatectomy.9 Schoeffl et al10 demonstrated that the microsurgical success of fine surgical procedures was directly related to optical magnification strength when comparing the unaided eye, surgical loupes, and the operating microscope. The dental literature also has numerous examples of magnification producing improved quality dentistry.11-13 Although magnification is not a novel concept to dermatologic surgery, little has been written about its use in the dermatologic surgery literature.
Magnification Options
One-Piece Bifocal Magnifying Safety Glasses
Bifocal magnifying safety glasses are polycarbonate safety glasses made with lenses in which the lower half is a magnifying lens. They are available in +1.5, +2.0, +2.5, and +3.0 diopter strengths. The total magnification power is calculated as follows: (diopter/4) + 1. The glasses are lightweight, easy to wear, inexpensive, and protect the eyes; however, they provide minimal magnification and do not compensate for differences in vision between both eyes.
Magnification Visor
The magnification visor is a headband visor with magnification lenses. It comes in various levels of magnification ranging from ×1.5 to ×3.5. It can be worn over prescription or safety glasses, may be pivoted out of the way when not in use, and is inexpensive. Conversely, it may be bulky to wear, cannot be customized, and does not offer the best resolution.
Magnification Clips
Magnification clips are hard-coated magnifying lens plates that fasten to eyeglass frames and range in level of magnification from ×1.5 to ×3.5. They can be pivoted out of the viewing angle, are lightweight, and are inexpensive; however, positioning may be difficult for ideal working distance and viewing angle.
Magnifier With Frame/Headband
The magnifier with frame is similar to magnification clips, but the magnification lens plate comes with a frame. It can be used with or without glasses and comes in magnification levels of ×1.5 to ×3.5. It is light, inexpensive, and may be pivoted out of sight, but similar to magnification clips, positioning for the right viewing angle and working distance may be difficult.
The magnifier with headband is essentially the same as the magnifier with frame. The only difference is the magnification plate is attached to a headband as opposed to a frame. It has similar benefits and limitations as the magnifier with frame.
Magnification Stand
The magnification stand comes as a large magnification lens with a flexible arm attached to a stand. It is a basic magnification tool and does not need to be worn; however, the stand is not easily portable and may be cumbersome to use.
Surgical Loupes
Surgical loupes are a robust magnification choice and the mainstay in magnification for the dermatologic surgeon. Loupes have proven to have comparable results in some procedures to the powerful operating surgical microscope.14-17 Factors to consider with loupes include brand, design, lens, magnification, resolution, optimal working distance, field depth, and declination angle.18
The 2 surgical loupe designs—flip-up loupes and through-the-lens loupes—differ in the mounting of the optic lenses on safety glasses. Flip-up loupes have the optics mounted to the bridge of the frame, whereas through-the-lens loupes are fixed in the lenses.
There are 3 different optical systems for surgical loupe magnification: simple, compound, and prismatic. Simple lenses consist of one pair of positive meniscus lenses similar to reading glasses. Compound lenses are made of 2 magnification lenses. Prismatic lenses magnify using a prism that folds and lengthens the light path.19,20
Loupes range in magnification level from ×2.5 to ×4.5. Compared to other magnification modalities, they can be customized and offer better resolution with quality magnification. Additionally, loupes can be fitted with a light source; however, they are expensive and surgeons need time to get used to the increased magnification as well as wearing the loupes.
There are advantages and disadvantages to the different loupe designs (Table 2). Flip-up loupes are more versatile, allowing for use on various safety glasses. They can be flipped out of view, and the declination angle may be altered; however, flip-up loupes have a narrower field of view and are heavier and bulkier than through-the-lens loupes. Through-the-lens loupes are lighter and have a larger field of view, as the optics are closer to the eye. They are customized to the declination angle and working distance of the surgeon. Conversely, through-the-lens loupes are more expensive and cannot be adjusted or moved from the line of vision.
Operating Surgical Microscope
The operating surgical microscope is not practical in the dermatologic surgeon’s practice. It is expensive and provides unnecessarily powerful magnification for dermatologic surgery. This tool usually is used in the operating room for suturing nerves and vessels with sutures sized 8-0 and smaller. Most skin procedures require size 6-0 and larger sutures.
Dermoscope
Dermoscopy, also known as epiluminescence microscopy, is a technique utilizing a handheld device made up of polarized light and a ×10 magnifying lens to evaluate skin lesions. In skilled hands, dermoscopy allows for the examination of characteristic patterns and morphologic features of skin lesions to enhance the clinician’s diagnostic accuracy.21 It may aid the dermatologic surgeon in identifying the surgical margins of difficult-to-define skin cancers. It is small and mobile; however, it has minimal benefit to the dermatologic surgeon during surgery because it is handheld and has a small field of view.
Conclusion
Good ergonomic practices facilitate a healthier and prolonged career for the dermatologic surgeon. When used properly, magnification devices can be a beneficial adjunct to the dermatologic surgeon by promoting better posture, preventing eyestrain, and providing enhanced visualization of the operating field and instruments. Use of magnification devices has been demonstrated to improve patient outcomes in other specialties. There are opportunities for further research specific to magnification improving dermatologic surgery outcomes given the high level of precision and accuracy needed for Mohs micrographic surgery, wound reconstruction, nail surgery, and hair transplantation.
Dermatologic surgeons are susceptible to work-related ailments given the nature of their working posture, the most common of which are pain and stiffness in the neck, shoulders, and lower back, as well as headaches.1,2 Awkward posture and positioning, for the sake of getting a better view of the task at hand, puts the surgeon in ergonomically disagreeable positions. Because the prime working years for a dermatologic surgeon tend to coincide with the age of presbyopia onset, magnification may help reduce and thwart musculoskeletal problems and eye strain. Indeed, a multitude of surgical specialties and dentists use intraoperative magnification.3 Knowledge and use of available magnification options can be a key addition to the dermatologic surgeon’s armamentarium. We discuss the need for magnification and review magnification devices that are readily available to the dermatologic surgeon. Table 1 presents a summary of all magnification options discussed.
Need for Magnification
Presbyopia is a condition of aging in which one loses the ability to accommodate and focus at near distances. The estimated prevalence of presbyopia in North America is 83%, typically with onset by 45 years of age.4 Individuals with presbyopia often hold objects farther away from their eyes to bring them into focus, causing eye strain, headaches, and musculoskeletal injury.
Use of intraoperative magnification allows for enhanced visualization of fine anatomic details and precise suture placement for the surgeon with or without presbyopia. Higher magnification produces a larger image; however, it also reduces field of view and depth of field (ie, the amount of depth that stays in focus without repositioning). The resolution and quality of the image are dependent on the optical properties of the lens system. The ideal optic system is surgeon dependent and involves a combination of magnification level that will not result in dramatic loss of view and depth of field, while maintaining crispness and quality of image.
Intraoperative magnification yields ergonomic benefits by promoting a safer neck flexion angle by increasing the working distance to a more ideal position (Figure). In doing so, it improves posture and minimizes eye and musculoskeletal strain secondary to awkward positioning and presbyopia.1,5 Stationary working position and neck flexion and rotation with precise and repetitive tasks are risk factors for strain and injuries that dermatologic surgeons often encounter.1 Magnification devices are tools that the dermatologic surgeon can utilize for a more ergonomically sound practice. Indeed, magnification has been shown to improve posture in the dental literature, a specialty with similar occupational risk factors to dermatologic surgery.6-8 Ergonomic practice reduces occupational injuries and improves work quality and productivity, thereby having a favorable effect on both the patient and the physician.

Improved Outcomes With Magnification
There are many examples of improved surgical quality and outcomes with magnification in other specialties. Hart and Hall5 illustrated the advantage of magnification in laceration repairs in the emergency department. In one study, increased magnification resulted in a substantial decrease in positive surgical margin rates in open radical retropubic prostatectomy.9 Schoeffl et al10 demonstrated that the microsurgical success of fine surgical procedures was directly related to optical magnification strength when comparing the unaided eye, surgical loupes, and the operating microscope. The dental literature also has numerous examples of magnification producing improved quality dentistry.11-13 Although magnification is not a novel concept to dermatologic surgery, little has been written about its use in the dermatologic surgery literature.
Magnification Options
One-Piece Bifocal Magnifying Safety Glasses
Bifocal magnifying safety glasses are polycarbonate safety glasses made with lenses in which the lower half is a magnifying lens. They are available in +1.5, +2.0, +2.5, and +3.0 diopter strengths. The total magnification power is calculated as follows: (diopter/4) + 1. The glasses are lightweight, easy to wear, inexpensive, and protect the eyes; however, they provide minimal magnification and do not compensate for differences in vision between both eyes.
Magnification Visor
The magnification visor is a headband visor with magnification lenses. It comes in various levels of magnification ranging from ×1.5 to ×3.5. It can be worn over prescription or safety glasses, may be pivoted out of the way when not in use, and is inexpensive. Conversely, it may be bulky to wear, cannot be customized, and does not offer the best resolution.
Magnification Clips
Magnification clips are hard-coated magnifying lens plates that fasten to eyeglass frames and range in level of magnification from ×1.5 to ×3.5. They can be pivoted out of the viewing angle, are lightweight, and are inexpensive; however, positioning may be difficult for ideal working distance and viewing angle.
Magnifier With Frame/Headband
The magnifier with frame is similar to magnification clips, but the magnification lens plate comes with a frame. It can be used with or without glasses and comes in magnification levels of ×1.5 to ×3.5. It is light, inexpensive, and may be pivoted out of sight, but similar to magnification clips, positioning for the right viewing angle and working distance may be difficult.
The magnifier with headband is essentially the same as the magnifier with frame. The only difference is the magnification plate is attached to a headband as opposed to a frame. It has similar benefits and limitations as the magnifier with frame.
Magnification Stand
The magnification stand comes as a large magnification lens with a flexible arm attached to a stand. It is a basic magnification tool and does not need to be worn; however, the stand is not easily portable and may be cumbersome to use.
Surgical Loupes
Surgical loupes are a robust magnification choice and the mainstay in magnification for the dermatologic surgeon. Loupes have proven to have comparable results in some procedures to the powerful operating surgical microscope.14-17 Factors to consider with loupes include brand, design, lens, magnification, resolution, optimal working distance, field depth, and declination angle.18
The 2 surgical loupe designs—flip-up loupes and through-the-lens loupes—differ in the mounting of the optic lenses on safety glasses. Flip-up loupes have the optics mounted to the bridge of the frame, whereas through-the-lens loupes are fixed in the lenses.
There are 3 different optical systems for surgical loupe magnification: simple, compound, and prismatic. Simple lenses consist of one pair of positive meniscus lenses similar to reading glasses. Compound lenses are made of 2 magnification lenses. Prismatic lenses magnify using a prism that folds and lengthens the light path.19,20
Loupes range in magnification level from ×2.5 to ×4.5. Compared to other magnification modalities, they can be customized and offer better resolution with quality magnification. Additionally, loupes can be fitted with a light source; however, they are expensive and surgeons need time to get used to the increased magnification as well as wearing the loupes.
There are advantages and disadvantages to the different loupe designs (Table 2). Flip-up loupes are more versatile, allowing for use on various safety glasses. They can be flipped out of view, and the declination angle may be altered; however, flip-up loupes have a narrower field of view and are heavier and bulkier than through-the-lens loupes. Through-the-lens loupes are lighter and have a larger field of view, as the optics are closer to the eye. They are customized to the declination angle and working distance of the surgeon. Conversely, through-the-lens loupes are more expensive and cannot be adjusted or moved from the line of vision.
Operating Surgical Microscope
The operating surgical microscope is not practical in the dermatologic surgeon’s practice. It is expensive and provides unnecessarily powerful magnification for dermatologic surgery. This tool usually is used in the operating room for suturing nerves and vessels with sutures sized 8-0 and smaller. Most skin procedures require size 6-0 and larger sutures.
Dermoscope
Dermoscopy, also known as epiluminescence microscopy, is a technique utilizing a handheld device made up of polarized light and a ×10 magnifying lens to evaluate skin lesions. In skilled hands, dermoscopy allows for the examination of characteristic patterns and morphologic features of skin lesions to enhance the clinician’s diagnostic accuracy.21 It may aid the dermatologic surgeon in identifying the surgical margins of difficult-to-define skin cancers. It is small and mobile; however, it has minimal benefit to the dermatologic surgeon during surgery because it is handheld and has a small field of view.
Conclusion
Good ergonomic practices facilitate a healthier and prolonged career for the dermatologic surgeon. When used properly, magnification devices can be a beneficial adjunct to the dermatologic surgeon by promoting better posture, preventing eyestrain, and providing enhanced visualization of the operating field and instruments. Use of magnification devices has been demonstrated to improve patient outcomes in other specialties. There are opportunities for further research specific to magnification improving dermatologic surgery outcomes given the high level of precision and accuracy needed for Mohs micrographic surgery, wound reconstruction, nail surgery, and hair transplantation.
- Liang CA, Levine VJ, Dusza SW, et al. Musculoskeletal disorders and ergonomics in dermatologic surgery: a survey of Mohs surgeons in 2010. Dermatol Surg. 2012;38:240-248.
- Esser AC, Koshy JG, Randle HW. Ergonomics in office-based surgery: a survey-guided observational study. Dermatol Surg. 2007;33:1304-1313; discussion, 1313-1314.
- Jarrett PM. Intraoperative magnification: who uses it? Microsurgery. 2004;24:420-422.
- Holden BA, Fricke TR, Ho SM, et al. Global vision impairment due to uncorrected presbyopia. Arch Ophthalmol. 2008;126:1731-1739.
- Hart RG, Hall J. The value of loupe magnification: an underused tool in emergency medicine. Am J Emerg Med. 2007;25:704-707.
- Branson BG, Bray KK, Gadbury-Amyot C, et al. Effect of magnification lenses on student operator posture. J Dent Educ. 2004;68:384-389.
- Maillet JP, Millar AM, Burke JM, et al. Effect of magnification loupes on dental hygiene student posture. J Dent Educ. 2008;72:33-44.
- Branson BG, Black MA, Simmer-Beck M. Changes in posture: a case study of a dental hygienist’s use of magnification loupes. Work. 2010;35:467-476.
- Magera JS Jr, Inman BA, Slezak JM, et al. Increased optical magnification from 2.5× to 4.3× with technical modification lowers the positive margin rate in open radical retropubic prostatectomy [published online November 13, 2007].J Urol. 2008;179:130-135.
- Schoeffl H, Lazzeri D, Schnelzer R, et al. Optical magnification should be mandatory for microsurgery: scientific basis and clinical data contributing to quality assurance. Arch Plast Surg. 2013;40:104-108.
- Taschieri S, Del Fabbro M, Testori T, et al. Endodontic surgery using 2 different magnification devices: preliminary results of a randomized controlled study. J Oral Maxillofac Surg. 2006;64:235-242.
- Christensen GJ. Magnification in dentistry: useful tool or another gimmick? J Am Dent Assoc. 2003;134:1647-1650.
- Syme SE, Fried JL, Strassler HE. Enhanced visualization using magnification systems. J Dent Hyg. 1997;71:202-206.
- Pieptu D, Luchian S. Loupes-only microsurgery. Microsurgery. 2003;23:181-188.
- Shenaq SM, Klebuc MJ, Vargo D. Free-tissue transfer with the aid of loupe magnification: experience with 251 procedures. Plast Reconstr Surg. 1995;95:261-269.
- Serletti JM, Deuber MA, Guidera PM, et al. Comparison of the operating microscope and loupes for free microvascular tissue transfer. Plast Reconstr Surg. 1995;95:270-276.
- Ross DA, Ariyan S, Restifo R, et al. Use of the operating microscope and loupes for head and neck free microvascular tissue transfer: a retrospective comparison. Arch Otolaryngol Head Neck Surg. 2003;129:189-193.
- Mungadi IA. Refinement on surgical technique: role of magnification. J Surg Tech Case Rep. 2010;2:1-2.
- Stanbury SJ, Elfar J. The use of surgical loupes in microsurgery. J Hand Surg Am. 2011;36:154-156.
- Baker JM, Meals RA. A practical guide to surgical loupes. J Hand Surg Am. 1997;22:967-974.
- Campos-do-Carmo G, Ramos-e-Silva M. Dermoscopy: basic concepts. Int J Dermatol. 2008;47:712-719.
- Liang CA, Levine VJ, Dusza SW, et al. Musculoskeletal disorders and ergonomics in dermatologic surgery: a survey of Mohs surgeons in 2010. Dermatol Surg. 2012;38:240-248.
- Esser AC, Koshy JG, Randle HW. Ergonomics in office-based surgery: a survey-guided observational study. Dermatol Surg. 2007;33:1304-1313; discussion, 1313-1314.
- Jarrett PM. Intraoperative magnification: who uses it? Microsurgery. 2004;24:420-422.
- Holden BA, Fricke TR, Ho SM, et al. Global vision impairment due to uncorrected presbyopia. Arch Ophthalmol. 2008;126:1731-1739.
- Hart RG, Hall J. The value of loupe magnification: an underused tool in emergency medicine. Am J Emerg Med. 2007;25:704-707.
- Branson BG, Bray KK, Gadbury-Amyot C, et al. Effect of magnification lenses on student operator posture. J Dent Educ. 2004;68:384-389.
- Maillet JP, Millar AM, Burke JM, et al. Effect of magnification loupes on dental hygiene student posture. J Dent Educ. 2008;72:33-44.
- Branson BG, Black MA, Simmer-Beck M. Changes in posture: a case study of a dental hygienist’s use of magnification loupes. Work. 2010;35:467-476.
- Magera JS Jr, Inman BA, Slezak JM, et al. Increased optical magnification from 2.5× to 4.3× with technical modification lowers the positive margin rate in open radical retropubic prostatectomy [published online November 13, 2007].J Urol. 2008;179:130-135.
- Schoeffl H, Lazzeri D, Schnelzer R, et al. Optical magnification should be mandatory for microsurgery: scientific basis and clinical data contributing to quality assurance. Arch Plast Surg. 2013;40:104-108.
- Taschieri S, Del Fabbro M, Testori T, et al. Endodontic surgery using 2 different magnification devices: preliminary results of a randomized controlled study. J Oral Maxillofac Surg. 2006;64:235-242.
- Christensen GJ. Magnification in dentistry: useful tool or another gimmick? J Am Dent Assoc. 2003;134:1647-1650.
- Syme SE, Fried JL, Strassler HE. Enhanced visualization using magnification systems. J Dent Hyg. 1997;71:202-206.
- Pieptu D, Luchian S. Loupes-only microsurgery. Microsurgery. 2003;23:181-188.
- Shenaq SM, Klebuc MJ, Vargo D. Free-tissue transfer with the aid of loupe magnification: experience with 251 procedures. Plast Reconstr Surg. 1995;95:261-269.
- Serletti JM, Deuber MA, Guidera PM, et al. Comparison of the operating microscope and loupes for free microvascular tissue transfer. Plast Reconstr Surg. 1995;95:270-276.
- Ross DA, Ariyan S, Restifo R, et al. Use of the operating microscope and loupes for head and neck free microvascular tissue transfer: a retrospective comparison. Arch Otolaryngol Head Neck Surg. 2003;129:189-193.
- Mungadi IA. Refinement on surgical technique: role of magnification. J Surg Tech Case Rep. 2010;2:1-2.
- Stanbury SJ, Elfar J. The use of surgical loupes in microsurgery. J Hand Surg Am. 2011;36:154-156.
- Baker JM, Meals RA. A practical guide to surgical loupes. J Hand Surg Am. 1997;22:967-974.
- Campos-do-Carmo G, Ramos-e-Silva M. Dermoscopy: basic concepts. Int J Dermatol. 2008;47:712-719.
Practice Points
- Ergonomic practice is paramount in preserving the longevity and productivity of the dermatologic surgeon.
- A magnification device may be a helpful addition for a dermatologic surgeon to achieve a healthier and more productive practice.