User login
Small-volume blood sample tubes may reduce anemia and transfusions in intensive care
according to a new study. The change does not appear to impair biospecimen sufficiency for lab analysis.
In addition, by reducing blood transfusion during ICU admission by about 10 units per 100 patients, the change may enable hospitals and health systems to sustain blood product supply during ongoing worldwide shortages.
“It doesn’t take long working in a hospital or being a patient or family member to realize how much blood we take to do lab work. As a result, patients may develop anemia and low RBC counts, which can be associated with worse health outcomes,” lead author Deborah Siegal, MD, a hematologist at the Ottawa Hospital and associate professor of medicine at the University of Ottawa, said in an interview.
“Unfortunately, the majority of the blood we take is discarded as waste,” she said. “Here’s an opportunity to move the needle on reducing anemia in hospitalized patients, where the benefit also doesn’t come at a cost.”
The study was published online in JAMA.
Reducing Blood Loss
Among ICU patients with critical illness, there is a high prevalence of anemia, Siegal noted. More than 90% of these patients have some degree of anemia after a 3-day stay. Typically, RBC transfusions are given to correct the low blood counts, and as many as 40% of ICU patients receive at least one RBC transfusion. Anemia and RBC transfusion are each associated with adverse outcomes, including higher mortality and longer ICU and hospital stays.
Although anemia in critically ill ICU patients can have several causes, blood sampling can be substantial because of the need to draw multiple tubes several times per day. During 8 days in an ICU, the amount of blood drawn equals about 1 unit of whole blood, the authors noted, and ICU patients often struggle to increase RBC production and compensate for blood loss.
Even then, only 10% of the blood collected is required for lab testing; the remaining 90% is often discarded as waste, the authors noted. Small-volume tubes (1.8 to 3.5 mL), which are designed to draw about 50% less than standard-volume tubes (4 to 6 mL) by using less vacuum strength, are of the same size and cost as standard-volume tubes, and the collection technique is the same. They are produced by the same manufacturers and are compatible with existing lab equipment.
Siegal and colleagues conducted a stepped-wedge cluster randomized trial to test the switch to small-volume tubes in 25 adult medical-surgical ICUs in Canada between February 2019 and January 2021. They analyzed data from more than 27,000 patients admitted to the ICU for 48 hours or longer. ICUs were randomly assigned to switch from standard-volume tubes to small-volume tubes for lab testing. The research team primarily assessed RBC transfusion in units per patient per ICU stay, as well as hemoglobin decrease during ICU stay, length of stay in the ICU and hospital, mortality in the ICU and hospital, and specimen tubes with insufficient volume for testing.
In a primary analysis of 21,201 patients, which excluded 6210 patients admitted during the early COVID-19 pandemic, there was no significant difference between tube-volume groups in RBC units per patient per ICU stay (relative risk [RR], 0.91). However, there was an absolute reduction of 7.24 RBC units per 100 patients per ICU stay in the small-volume group.
In addition, in a prespecified secondary analysis of 27,411 patients, RBC units per patient per ICU stay significantly decreased (RR, 0.88) after the switch to small-volume tubes, and there was an absolute reduction of 9.84 RBC units per 100 patients per ICU stay.
Overall, the median decrease in transfusion-adjusted hemoglobin wasn’t significantly different in the primary analysis but was lower in the secondary analysis. The frequency of specimens with insufficient volume for testing was low (≤0.03%) before and after the transition to small-volume tubes.
About 36,000 units of blood were given to ICU patients during the study period. The use of small-volume tubes may have saved about 1500 RBC units, the authors estimated.
“This could be an important way to help preserve the supply of blood products for patients who need them, including those undergoing cancer treatment, surgery, trauma, or other medical illnesses,” Siegal said. “The other great aspect is that this was implemented by people on the ground in the ICUs, and it’s still in use in most of those hospitals today.”
The investigators noted the need to study the switch in other patient populations, such as non-ICU hospitalized patients or outpatient settings. For instance, ICU patients often have central venous or arterial catheters for blood draws, but small-volume tubes can be used with venipuncture and could lead to additional benefits there as well.
Implementing Change
Commenting on the findings for this article, Lisa Hicks, MD, a hematologist at St. Michael’s Hospital and associate professor of medicine at the University of Toronto, said, “Routinely collecting smaller volumes of blood for diagnostic testing appears to be feasible and does not cause problems with inadequate sampling. Whether this strategy decreases transfusion is more complicated.” Hicks did not participate in the study.
“At the end of the day, we still don’t know with certainty whether reduced-volume blood collection tubes decrease transfusion burden in ICU patients — it’s possible that there are so many other factors driving down hemoglobin in this population that the impact of blood collection volume is modest to negligible,” she said. “On the other hand, it’s also possible that there is an important impact that was masked by the relatively short ICU stays in the included population.”
Hicks has researched ways to reduce unnecessary diagnostic phlebotomy in ICUs. She and colleagues found that targeting clinicians’ test ordering behavior can decrease blood draws and RBC transfusions.
“What we now know, thanks to Siegal et al, is that we don’t need to collect nearly as much blood from our ICU patients as we do, raising the question of which strategy should really be standard,” she said. “My vote goes for more blood in the patient and less in the bin.”
The study was funded by a peer-reviewed grant from the Academic Health Sciences Centers AFP Innovation Fund/Hamilton Academic Health Sciences Organization and the Hamilton Health Sciences Research Institute through the Population Health Research Institute. Siegal, who is supported by a Tier 2 Canada Research Chair in Anticoagulant Management of Cardiovascular Disease, reported honoraria for presentations paid indirectly to her institution from BMS-Pfizer, AstraZeneca, Servier, and Roche outside of the submitted work. Hicks reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
according to a new study. The change does not appear to impair biospecimen sufficiency for lab analysis.
In addition, by reducing blood transfusion during ICU admission by about 10 units per 100 patients, the change may enable hospitals and health systems to sustain blood product supply during ongoing worldwide shortages.
“It doesn’t take long working in a hospital or being a patient or family member to realize how much blood we take to do lab work. As a result, patients may develop anemia and low RBC counts, which can be associated with worse health outcomes,” lead author Deborah Siegal, MD, a hematologist at the Ottawa Hospital and associate professor of medicine at the University of Ottawa, said in an interview.
“Unfortunately, the majority of the blood we take is discarded as waste,” she said. “Here’s an opportunity to move the needle on reducing anemia in hospitalized patients, where the benefit also doesn’t come at a cost.”
The study was published online in JAMA.
Reducing Blood Loss
Among ICU patients with critical illness, there is a high prevalence of anemia, Siegal noted. More than 90% of these patients have some degree of anemia after a 3-day stay. Typically, RBC transfusions are given to correct the low blood counts, and as many as 40% of ICU patients receive at least one RBC transfusion. Anemia and RBC transfusion are each associated with adverse outcomes, including higher mortality and longer ICU and hospital stays.
Although anemia in critically ill ICU patients can have several causes, blood sampling can be substantial because of the need to draw multiple tubes several times per day. During 8 days in an ICU, the amount of blood drawn equals about 1 unit of whole blood, the authors noted, and ICU patients often struggle to increase RBC production and compensate for blood loss.
Even then, only 10% of the blood collected is required for lab testing; the remaining 90% is often discarded as waste, the authors noted. Small-volume tubes (1.8 to 3.5 mL), which are designed to draw about 50% less than standard-volume tubes (4 to 6 mL) by using less vacuum strength, are of the same size and cost as standard-volume tubes, and the collection technique is the same. They are produced by the same manufacturers and are compatible with existing lab equipment.
Siegal and colleagues conducted a stepped-wedge cluster randomized trial to test the switch to small-volume tubes in 25 adult medical-surgical ICUs in Canada between February 2019 and January 2021. They analyzed data from more than 27,000 patients admitted to the ICU for 48 hours or longer. ICUs were randomly assigned to switch from standard-volume tubes to small-volume tubes for lab testing. The research team primarily assessed RBC transfusion in units per patient per ICU stay, as well as hemoglobin decrease during ICU stay, length of stay in the ICU and hospital, mortality in the ICU and hospital, and specimen tubes with insufficient volume for testing.
In a primary analysis of 21,201 patients, which excluded 6210 patients admitted during the early COVID-19 pandemic, there was no significant difference between tube-volume groups in RBC units per patient per ICU stay (relative risk [RR], 0.91). However, there was an absolute reduction of 7.24 RBC units per 100 patients per ICU stay in the small-volume group.
In addition, in a prespecified secondary analysis of 27,411 patients, RBC units per patient per ICU stay significantly decreased (RR, 0.88) after the switch to small-volume tubes, and there was an absolute reduction of 9.84 RBC units per 100 patients per ICU stay.
Overall, the median decrease in transfusion-adjusted hemoglobin wasn’t significantly different in the primary analysis but was lower in the secondary analysis. The frequency of specimens with insufficient volume for testing was low (≤0.03%) before and after the transition to small-volume tubes.
About 36,000 units of blood were given to ICU patients during the study period. The use of small-volume tubes may have saved about 1500 RBC units, the authors estimated.
“This could be an important way to help preserve the supply of blood products for patients who need them, including those undergoing cancer treatment, surgery, trauma, or other medical illnesses,” Siegal said. “The other great aspect is that this was implemented by people on the ground in the ICUs, and it’s still in use in most of those hospitals today.”
The investigators noted the need to study the switch in other patient populations, such as non-ICU hospitalized patients or outpatient settings. For instance, ICU patients often have central venous or arterial catheters for blood draws, but small-volume tubes can be used with venipuncture and could lead to additional benefits there as well.
Implementing Change
Commenting on the findings for this article, Lisa Hicks, MD, a hematologist at St. Michael’s Hospital and associate professor of medicine at the University of Toronto, said, “Routinely collecting smaller volumes of blood for diagnostic testing appears to be feasible and does not cause problems with inadequate sampling. Whether this strategy decreases transfusion is more complicated.” Hicks did not participate in the study.
“At the end of the day, we still don’t know with certainty whether reduced-volume blood collection tubes decrease transfusion burden in ICU patients — it’s possible that there are so many other factors driving down hemoglobin in this population that the impact of blood collection volume is modest to negligible,” she said. “On the other hand, it’s also possible that there is an important impact that was masked by the relatively short ICU stays in the included population.”
Hicks has researched ways to reduce unnecessary diagnostic phlebotomy in ICUs. She and colleagues found that targeting clinicians’ test ordering behavior can decrease blood draws and RBC transfusions.
“What we now know, thanks to Siegal et al, is that we don’t need to collect nearly as much blood from our ICU patients as we do, raising the question of which strategy should really be standard,” she said. “My vote goes for more blood in the patient and less in the bin.”
The study was funded by a peer-reviewed grant from the Academic Health Sciences Centers AFP Innovation Fund/Hamilton Academic Health Sciences Organization and the Hamilton Health Sciences Research Institute through the Population Health Research Institute. Siegal, who is supported by a Tier 2 Canada Research Chair in Anticoagulant Management of Cardiovascular Disease, reported honoraria for presentations paid indirectly to her institution from BMS-Pfizer, AstraZeneca, Servier, and Roche outside of the submitted work. Hicks reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
according to a new study. The change does not appear to impair biospecimen sufficiency for lab analysis.
In addition, by reducing blood transfusion during ICU admission by about 10 units per 100 patients, the change may enable hospitals and health systems to sustain blood product supply during ongoing worldwide shortages.
“It doesn’t take long working in a hospital or being a patient or family member to realize how much blood we take to do lab work. As a result, patients may develop anemia and low RBC counts, which can be associated with worse health outcomes,” lead author Deborah Siegal, MD, a hematologist at the Ottawa Hospital and associate professor of medicine at the University of Ottawa, said in an interview.
“Unfortunately, the majority of the blood we take is discarded as waste,” she said. “Here’s an opportunity to move the needle on reducing anemia in hospitalized patients, where the benefit also doesn’t come at a cost.”
The study was published online in JAMA.
Reducing Blood Loss
Among ICU patients with critical illness, there is a high prevalence of anemia, Siegal noted. More than 90% of these patients have some degree of anemia after a 3-day stay. Typically, RBC transfusions are given to correct the low blood counts, and as many as 40% of ICU patients receive at least one RBC transfusion. Anemia and RBC transfusion are each associated with adverse outcomes, including higher mortality and longer ICU and hospital stays.
Although anemia in critically ill ICU patients can have several causes, blood sampling can be substantial because of the need to draw multiple tubes several times per day. During 8 days in an ICU, the amount of blood drawn equals about 1 unit of whole blood, the authors noted, and ICU patients often struggle to increase RBC production and compensate for blood loss.
Even then, only 10% of the blood collected is required for lab testing; the remaining 90% is often discarded as waste, the authors noted. Small-volume tubes (1.8 to 3.5 mL), which are designed to draw about 50% less than standard-volume tubes (4 to 6 mL) by using less vacuum strength, are of the same size and cost as standard-volume tubes, and the collection technique is the same. They are produced by the same manufacturers and are compatible with existing lab equipment.
Siegal and colleagues conducted a stepped-wedge cluster randomized trial to test the switch to small-volume tubes in 25 adult medical-surgical ICUs in Canada between February 2019 and January 2021. They analyzed data from more than 27,000 patients admitted to the ICU for 48 hours or longer. ICUs were randomly assigned to switch from standard-volume tubes to small-volume tubes for lab testing. The research team primarily assessed RBC transfusion in units per patient per ICU stay, as well as hemoglobin decrease during ICU stay, length of stay in the ICU and hospital, mortality in the ICU and hospital, and specimen tubes with insufficient volume for testing.
In a primary analysis of 21,201 patients, which excluded 6210 patients admitted during the early COVID-19 pandemic, there was no significant difference between tube-volume groups in RBC units per patient per ICU stay (relative risk [RR], 0.91). However, there was an absolute reduction of 7.24 RBC units per 100 patients per ICU stay in the small-volume group.
In addition, in a prespecified secondary analysis of 27,411 patients, RBC units per patient per ICU stay significantly decreased (RR, 0.88) after the switch to small-volume tubes, and there was an absolute reduction of 9.84 RBC units per 100 patients per ICU stay.
Overall, the median decrease in transfusion-adjusted hemoglobin wasn’t significantly different in the primary analysis but was lower in the secondary analysis. The frequency of specimens with insufficient volume for testing was low (≤0.03%) before and after the transition to small-volume tubes.
About 36,000 units of blood were given to ICU patients during the study period. The use of small-volume tubes may have saved about 1500 RBC units, the authors estimated.
“This could be an important way to help preserve the supply of blood products for patients who need them, including those undergoing cancer treatment, surgery, trauma, or other medical illnesses,” Siegal said. “The other great aspect is that this was implemented by people on the ground in the ICUs, and it’s still in use in most of those hospitals today.”
The investigators noted the need to study the switch in other patient populations, such as non-ICU hospitalized patients or outpatient settings. For instance, ICU patients often have central venous or arterial catheters for blood draws, but small-volume tubes can be used with venipuncture and could lead to additional benefits there as well.
Implementing Change
Commenting on the findings for this article, Lisa Hicks, MD, a hematologist at St. Michael’s Hospital and associate professor of medicine at the University of Toronto, said, “Routinely collecting smaller volumes of blood for diagnostic testing appears to be feasible and does not cause problems with inadequate sampling. Whether this strategy decreases transfusion is more complicated.” Hicks did not participate in the study.
“At the end of the day, we still don’t know with certainty whether reduced-volume blood collection tubes decrease transfusion burden in ICU patients — it’s possible that there are so many other factors driving down hemoglobin in this population that the impact of blood collection volume is modest to negligible,” she said. “On the other hand, it’s also possible that there is an important impact that was masked by the relatively short ICU stays in the included population.”
Hicks has researched ways to reduce unnecessary diagnostic phlebotomy in ICUs. She and colleagues found that targeting clinicians’ test ordering behavior can decrease blood draws and RBC transfusions.
“What we now know, thanks to Siegal et al, is that we don’t need to collect nearly as much blood from our ICU patients as we do, raising the question of which strategy should really be standard,” she said. “My vote goes for more blood in the patient and less in the bin.”
The study was funded by a peer-reviewed grant from the Academic Health Sciences Centers AFP Innovation Fund/Hamilton Academic Health Sciences Organization and the Hamilton Health Sciences Research Institute through the Population Health Research Institute. Siegal, who is supported by a Tier 2 Canada Research Chair in Anticoagulant Management of Cardiovascular Disease, reported honoraria for presentations paid indirectly to her institution from BMS-Pfizer, AstraZeneca, Servier, and Roche outside of the submitted work. Hicks reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM JAMA
In MI with anemia, results may favor liberal transfusion: MINT
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
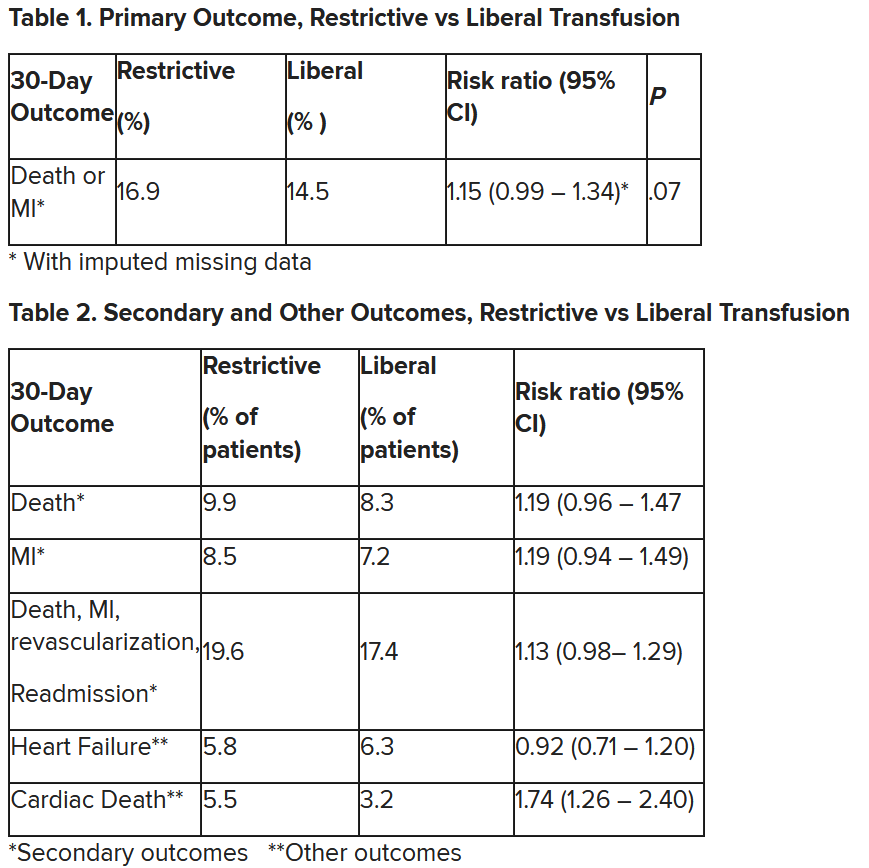
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHA 2023
Early cryoprecipitate fails to improve trauma hemorrhage outcomes
TOPLINE:
(MHP).
METHODOLOGY:
- CRYOSTAT-2 was an interventional, randomized, open-label, parallel-group controlled, international, multicenter study.
- A total of 1,604 patients were enrolled from 25 major trauma centers in the United Kingdom (n = 1,555) and 1 in the United States (n = 49) between August 2017 and November 2021.
- A total of 805 patients were randomly assigned to receive the standard MHP (standard care), and 799 were randomly assigned to receive an additional three pools of cryoprecipitate.
- The primary outcome was all-cause mortality at 28 days.
TAKEAWAY:
- Addition of early cryoprecipitate versus standard care did not improve all-cause 28-day mortality in the intent-to-treat population (25.3% vs. 26.1%; P = .74).
- In patient subgroup with penetrating trauma, 28-day mortality was significantly higher in the cryoprecipitate group than in the standard care group (16.2% vs. 10.0%; odds ratio, 1.74; P = .006).
- Massive transfusion (RBC ≥ 10 U) was similar between the cryoprecipitate and standard care groups.
IN PRACTICE:
According to the authors, it is possible that certain patients may have benefited from cryoprecipitate, but they did not receive it promptly or in adequate doses to restore functional fibrinogen levels. Despite the study’s goal of early cryoprecipitate administration, the median time to the first transfusion exceeded 1 hour after the patient’s arrival, which highlights the logistical challenges of preparing and delivering a frozen blood component from a distant blood laboratory to the patient.
SOURCE:
The study, with first author Ross Davenport, PhD, of Queen Mary University of London and colleagues, was published in JAMA).
LIMITATIONS:
There was variability of timing of cryoprecipitate administration and an overlap with patients in the standard care group receiving the intervention as part of their usual MHP treatment.
DISCLOSURES:
The study was funded by the U.K. National Institute for Health and Care Research: Health Technology Assessment and Barts Charity, U.K.
A version of this article first appeared on Medscape.com.
TOPLINE:
(MHP).
METHODOLOGY:
- CRYOSTAT-2 was an interventional, randomized, open-label, parallel-group controlled, international, multicenter study.
- A total of 1,604 patients were enrolled from 25 major trauma centers in the United Kingdom (n = 1,555) and 1 in the United States (n = 49) between August 2017 and November 2021.
- A total of 805 patients were randomly assigned to receive the standard MHP (standard care), and 799 were randomly assigned to receive an additional three pools of cryoprecipitate.
- The primary outcome was all-cause mortality at 28 days.
TAKEAWAY:
- Addition of early cryoprecipitate versus standard care did not improve all-cause 28-day mortality in the intent-to-treat population (25.3% vs. 26.1%; P = .74).
- In patient subgroup with penetrating trauma, 28-day mortality was significantly higher in the cryoprecipitate group than in the standard care group (16.2% vs. 10.0%; odds ratio, 1.74; P = .006).
- Massive transfusion (RBC ≥ 10 U) was similar between the cryoprecipitate and standard care groups.
IN PRACTICE:
According to the authors, it is possible that certain patients may have benefited from cryoprecipitate, but they did not receive it promptly or in adequate doses to restore functional fibrinogen levels. Despite the study’s goal of early cryoprecipitate administration, the median time to the first transfusion exceeded 1 hour after the patient’s arrival, which highlights the logistical challenges of preparing and delivering a frozen blood component from a distant blood laboratory to the patient.
SOURCE:
The study, with first author Ross Davenport, PhD, of Queen Mary University of London and colleagues, was published in JAMA).
LIMITATIONS:
There was variability of timing of cryoprecipitate administration and an overlap with patients in the standard care group receiving the intervention as part of their usual MHP treatment.
DISCLOSURES:
The study was funded by the U.K. National Institute for Health and Care Research: Health Technology Assessment and Barts Charity, U.K.
A version of this article first appeared on Medscape.com.
TOPLINE:
(MHP).
METHODOLOGY:
- CRYOSTAT-2 was an interventional, randomized, open-label, parallel-group controlled, international, multicenter study.
- A total of 1,604 patients were enrolled from 25 major trauma centers in the United Kingdom (n = 1,555) and 1 in the United States (n = 49) between August 2017 and November 2021.
- A total of 805 patients were randomly assigned to receive the standard MHP (standard care), and 799 were randomly assigned to receive an additional three pools of cryoprecipitate.
- The primary outcome was all-cause mortality at 28 days.
TAKEAWAY:
- Addition of early cryoprecipitate versus standard care did not improve all-cause 28-day mortality in the intent-to-treat population (25.3% vs. 26.1%; P = .74).
- In patient subgroup with penetrating trauma, 28-day mortality was significantly higher in the cryoprecipitate group than in the standard care group (16.2% vs. 10.0%; odds ratio, 1.74; P = .006).
- Massive transfusion (RBC ≥ 10 U) was similar between the cryoprecipitate and standard care groups.
IN PRACTICE:
According to the authors, it is possible that certain patients may have benefited from cryoprecipitate, but they did not receive it promptly or in adequate doses to restore functional fibrinogen levels. Despite the study’s goal of early cryoprecipitate administration, the median time to the first transfusion exceeded 1 hour after the patient’s arrival, which highlights the logistical challenges of preparing and delivering a frozen blood component from a distant blood laboratory to the patient.
SOURCE:
The study, with first author Ross Davenport, PhD, of Queen Mary University of London and colleagues, was published in JAMA).
LIMITATIONS:
There was variability of timing of cryoprecipitate administration and an overlap with patients in the standard care group receiving the intervention as part of their usual MHP treatment.
DISCLOSURES:
The study was funded by the U.K. National Institute for Health and Care Research: Health Technology Assessment and Barts Charity, U.K.
A version of this article first appeared on Medscape.com.
Using JAK inhibitors for myelofibrosis
“We are thankfully starting to be blessed with more options than we’ve ever had,” he said, but “in the front-line proliferative setting, ruxolitinib has remained the standard of care.” It’s “well established in higher-risk patients and very much an option for very symptomatic lower-risk patients.”
Dr. Hunter helped his colleagues navigate the evolving field of JAK inhibition for myelofibrosis in a presentation titled “Choosing and Properly Using a JAK Inhibitor in Myelofibrosis,”at the Society of Hematologic Oncology annual meeting.
Ruxolitinib was the first JAK inhibitor for myelofibrosis on the U.S. market, approved in 2011. Two more have followed, fedratinib in 2019 and pacritinib in 2022.
A fourth JAK inhibitor for myelofibrosis, momelotinib, is under Food and Drug Administration review with a decision expected shortly.
JAK inhibitors disrupt a key pathogenic pathway in myelofibrosis and are a mainstay of treatment, but Dr. Hunter noted that they should not replace allogeneic transplants in patients who are candidates because transplants remain “the best way to achieve long term survival, especially in higher risk patients.”
He noted that not every patient needs a JAK inhibitor, especially “lower-risk, more asymptomatic patients who are predominantly manifesting with cytopenias. [They] are less likely to benefit.”
Dr. Hunter said that although ruxolitinib remains a treatment of choice, fedratinib “is certainly an option” with comparable rates of symptom control and splenomegaly reduction. Also, while ruxolitinib is dosed according to platelet levels, fedratinib allows for full dosing down to a platelet count of 50 x 109/L.
“But there’s more GI toxicity than with ruxolitinib, especially in the first couple of months,” he said, as well as a black box warning of Wernicke’s encephalopathy. “I generally put all my [fedratinib] patients on thiamine repletion as a precaution.”
One of the most challenging aspects of using JAK inhibitors for myelofibrosis is their tendency to cause cytopenia, particularly anemia and thrombocytopenia, which, ironically, are also hallmarks of myelofibrosis itself.
Although there’s an alternative low-dose ruxolitinib regimen that can be effective in anemic settings, the approval of pacritinib and most likely momelotinib is particularly helpful for cytopenic patients, “a population which historically has been very hard to treat with our prior agents,” Dr. Hunter said.
Pacritinib is approved specifically for patients with platelet counts below 50 x 109/L; momelotinib also included lower platelet counts in several studies. Both agents indirectly boost erythropoiesis with subsequent amelioration of anemia.
“Momelotinib is an important emerging agent for these more anemic patients,” with a spleen response comparable to ruxolitinib and significantly higher rates of transfusion independence, but with lower rates of symptom control, Dr. Hunter said.
Pacritinib “really helps extend the benefit of JAK inhibitors to a group of thrombocytopenic patients who have been hard to treat with ruxolitinib,” with the added potential of improving anemia, although, like fedratinib, it has more GI toxicity, he said.
There are multiple add-on options for JAK inhibitor patients with anemia, including luspatercept, an erythropoiesis-stimulating agent approved for anemia in patients with myelodysplastic syndromes; promising results were reported recently for myelofibrosis.
Fedratinib, pacritinib, and momelotinib all have activity in the second line after ruxolitinib failure, Dr. Hunter noted, but he cautioned that ruxolitinib must be tapered over a few weeks, not stopped abruptly, to avoid withdrawal symptoms. Some clinicians overlap JAK inhibitors a day or two to avoid issues.
“Clinical trials should still be considered in many of these settings,” he said, adding that emerging agents are under development, including multiple combination therapies, often with JAK inhibitors as the background.
No disclosure information was reported.
“We are thankfully starting to be blessed with more options than we’ve ever had,” he said, but “in the front-line proliferative setting, ruxolitinib has remained the standard of care.” It’s “well established in higher-risk patients and very much an option for very symptomatic lower-risk patients.”
Dr. Hunter helped his colleagues navigate the evolving field of JAK inhibition for myelofibrosis in a presentation titled “Choosing and Properly Using a JAK Inhibitor in Myelofibrosis,”at the Society of Hematologic Oncology annual meeting.
Ruxolitinib was the first JAK inhibitor for myelofibrosis on the U.S. market, approved in 2011. Two more have followed, fedratinib in 2019 and pacritinib in 2022.
A fourth JAK inhibitor for myelofibrosis, momelotinib, is under Food and Drug Administration review with a decision expected shortly.
JAK inhibitors disrupt a key pathogenic pathway in myelofibrosis and are a mainstay of treatment, but Dr. Hunter noted that they should not replace allogeneic transplants in patients who are candidates because transplants remain “the best way to achieve long term survival, especially in higher risk patients.”
He noted that not every patient needs a JAK inhibitor, especially “lower-risk, more asymptomatic patients who are predominantly manifesting with cytopenias. [They] are less likely to benefit.”
Dr. Hunter said that although ruxolitinib remains a treatment of choice, fedratinib “is certainly an option” with comparable rates of symptom control and splenomegaly reduction. Also, while ruxolitinib is dosed according to platelet levels, fedratinib allows for full dosing down to a platelet count of 50 x 109/L.
“But there’s more GI toxicity than with ruxolitinib, especially in the first couple of months,” he said, as well as a black box warning of Wernicke’s encephalopathy. “I generally put all my [fedratinib] patients on thiamine repletion as a precaution.”
One of the most challenging aspects of using JAK inhibitors for myelofibrosis is their tendency to cause cytopenia, particularly anemia and thrombocytopenia, which, ironically, are also hallmarks of myelofibrosis itself.
Although there’s an alternative low-dose ruxolitinib regimen that can be effective in anemic settings, the approval of pacritinib and most likely momelotinib is particularly helpful for cytopenic patients, “a population which historically has been very hard to treat with our prior agents,” Dr. Hunter said.
Pacritinib is approved specifically for patients with platelet counts below 50 x 109/L; momelotinib also included lower platelet counts in several studies. Both agents indirectly boost erythropoiesis with subsequent amelioration of anemia.
“Momelotinib is an important emerging agent for these more anemic patients,” with a spleen response comparable to ruxolitinib and significantly higher rates of transfusion independence, but with lower rates of symptom control, Dr. Hunter said.
Pacritinib “really helps extend the benefit of JAK inhibitors to a group of thrombocytopenic patients who have been hard to treat with ruxolitinib,” with the added potential of improving anemia, although, like fedratinib, it has more GI toxicity, he said.
There are multiple add-on options for JAK inhibitor patients with anemia, including luspatercept, an erythropoiesis-stimulating agent approved for anemia in patients with myelodysplastic syndromes; promising results were reported recently for myelofibrosis.
Fedratinib, pacritinib, and momelotinib all have activity in the second line after ruxolitinib failure, Dr. Hunter noted, but he cautioned that ruxolitinib must be tapered over a few weeks, not stopped abruptly, to avoid withdrawal symptoms. Some clinicians overlap JAK inhibitors a day or two to avoid issues.
“Clinical trials should still be considered in many of these settings,” he said, adding that emerging agents are under development, including multiple combination therapies, often with JAK inhibitors as the background.
No disclosure information was reported.
“We are thankfully starting to be blessed with more options than we’ve ever had,” he said, but “in the front-line proliferative setting, ruxolitinib has remained the standard of care.” It’s “well established in higher-risk patients and very much an option for very symptomatic lower-risk patients.”
Dr. Hunter helped his colleagues navigate the evolving field of JAK inhibition for myelofibrosis in a presentation titled “Choosing and Properly Using a JAK Inhibitor in Myelofibrosis,”at the Society of Hematologic Oncology annual meeting.
Ruxolitinib was the first JAK inhibitor for myelofibrosis on the U.S. market, approved in 2011. Two more have followed, fedratinib in 2019 and pacritinib in 2022.
A fourth JAK inhibitor for myelofibrosis, momelotinib, is under Food and Drug Administration review with a decision expected shortly.
JAK inhibitors disrupt a key pathogenic pathway in myelofibrosis and are a mainstay of treatment, but Dr. Hunter noted that they should not replace allogeneic transplants in patients who are candidates because transplants remain “the best way to achieve long term survival, especially in higher risk patients.”
He noted that not every patient needs a JAK inhibitor, especially “lower-risk, more asymptomatic patients who are predominantly manifesting with cytopenias. [They] are less likely to benefit.”
Dr. Hunter said that although ruxolitinib remains a treatment of choice, fedratinib “is certainly an option” with comparable rates of symptom control and splenomegaly reduction. Also, while ruxolitinib is dosed according to platelet levels, fedratinib allows for full dosing down to a platelet count of 50 x 109/L.
“But there’s more GI toxicity than with ruxolitinib, especially in the first couple of months,” he said, as well as a black box warning of Wernicke’s encephalopathy. “I generally put all my [fedratinib] patients on thiamine repletion as a precaution.”
One of the most challenging aspects of using JAK inhibitors for myelofibrosis is their tendency to cause cytopenia, particularly anemia and thrombocytopenia, which, ironically, are also hallmarks of myelofibrosis itself.
Although there’s an alternative low-dose ruxolitinib regimen that can be effective in anemic settings, the approval of pacritinib and most likely momelotinib is particularly helpful for cytopenic patients, “a population which historically has been very hard to treat with our prior agents,” Dr. Hunter said.
Pacritinib is approved specifically for patients with platelet counts below 50 x 109/L; momelotinib also included lower platelet counts in several studies. Both agents indirectly boost erythropoiesis with subsequent amelioration of anemia.
“Momelotinib is an important emerging agent for these more anemic patients,” with a spleen response comparable to ruxolitinib and significantly higher rates of transfusion independence, but with lower rates of symptom control, Dr. Hunter said.
Pacritinib “really helps extend the benefit of JAK inhibitors to a group of thrombocytopenic patients who have been hard to treat with ruxolitinib,” with the added potential of improving anemia, although, like fedratinib, it has more GI toxicity, he said.
There are multiple add-on options for JAK inhibitor patients with anemia, including luspatercept, an erythropoiesis-stimulating agent approved for anemia in patients with myelodysplastic syndromes; promising results were reported recently for myelofibrosis.
Fedratinib, pacritinib, and momelotinib all have activity in the second line after ruxolitinib failure, Dr. Hunter noted, but he cautioned that ruxolitinib must be tapered over a few weeks, not stopped abruptly, to avoid withdrawal symptoms. Some clinicians overlap JAK inhibitors a day or two to avoid issues.
“Clinical trials should still be considered in many of these settings,” he said, adding that emerging agents are under development, including multiple combination therapies, often with JAK inhibitors as the background.
No disclosure information was reported.
FROM SOHO 2023
Guide explains nonsurgical management of major hemorrhage
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Study: Formula-fed extreme preemies need more iron
NEW ORLEANS –
“We were surprised that, despite actually receiving more iron in total each day on average, the formula-fed infants were significantly more iron deficient than breast-fed babies. This is the opposite of what one would expect,” study lead author Grace Power, a medical student at Dalhousie University, Halifax, N.S., said in an interview. She presented the results at the annual meeting of the American Society of Hematology.
According to Ms. Power, there’s limited research into how breastfeeding and formula feeding affect iron levels in preterm infants – especially those born extremely early, between 23 and 30 weeks’ gestation.
“This kind of research is important because preterm infants are highly susceptible to iron deficiency for a number of reasons,” she said. “Iron deficiency early in life is associated with developmental and behavioral problems later on in life. That association still stands, even if the iron deficiency is corrected, so prevention is key in this population. Knowing more about how feeding type affects iron status can help us learn about ways to prevent iron deficiency in these infants in the future.”
For the study, researchers retrospectively analyzed data about all preterm infants (< 31 weeks gestation) in Nova Scotia from 2005 to 2018. Of the 392 infants in this group (55.75% male; average age, about 5 months), 285 were fed with iron-rich formula (mean intake, 1.66 mg/kg per day), and 107 were fully or partially breast fed. The two groups were similar in terms of traits such as mean birth weight and gestational age.
The formula-fed infants were more likely to develop iron deficiency (ID, 36.8%) than the breast-fed infants (20.6%; P = .002). “Mean gestational age and birth weight were both lower in the ID group. The ID group also had a higher percentage of infants born less than 1,100 g (P = .01). More babies in the ID group received at least one blood transfusion,” the researchers reported. “ID infants had a higher daily formula intake, daily iron intake from formula, and total daily iron intake combined from formula and supplements.”
Why is there such a gap between formula-fed infants and breast-fed infants? The researchers speculated that infants absorb less iron from formula versus breast milk, possibly because of the presence of lactoferrin in breast milk.
The researchers also wondered whether physicians may pull back on iron supplementation in infants who undergo blood transfusions out of fear of the risk of iron overload, which Ms. Power said can cause infection and poor growth. By doing so, they may inadvertently deprive the babies of their need for iron.
“We don’t want clinicians to assume an infant doesn’t need iron supplementation just because they’ve received a blood transfusion,” she said.
As for an overall message from the research, Ms. Power said clinicians “should be aware that formula feeding can put infants at risk for iron deficiency and consider this when making decisions about supplementation.” And she noted that guidelines from the American Academy of Pediatrics and Canadian Pediatric Society don’t highlight the importance of iron supplementation in formula-fed, very preterm infants.
In an interview, University of Michigan pediatrician Michael K. Georgieff, MD, who has studied iron supplementation, said the study’s primary findings are surprising, although it makes sense that infants with lower gestational age and birth weight would suffer from more ID. Blood transfusion can indeed raise iron levels, but it’s important to consider that these infants may already have low levels of iron.
Dr. Georgieff advised colleagues to understand the potential for various nutritional deficiencies in preterm infants well beyond the first few weeks. When the babies are handed off to other clinicians such as pediatricians, they should undergo nutritional screening at 6 months, not at a year.
Dalhousie University funded the study. The study authors and Dr. Georgieff have no disclosures.
NEW ORLEANS –
“We were surprised that, despite actually receiving more iron in total each day on average, the formula-fed infants were significantly more iron deficient than breast-fed babies. This is the opposite of what one would expect,” study lead author Grace Power, a medical student at Dalhousie University, Halifax, N.S., said in an interview. She presented the results at the annual meeting of the American Society of Hematology.
According to Ms. Power, there’s limited research into how breastfeeding and formula feeding affect iron levels in preterm infants – especially those born extremely early, between 23 and 30 weeks’ gestation.
“This kind of research is important because preterm infants are highly susceptible to iron deficiency for a number of reasons,” she said. “Iron deficiency early in life is associated with developmental and behavioral problems later on in life. That association still stands, even if the iron deficiency is corrected, so prevention is key in this population. Knowing more about how feeding type affects iron status can help us learn about ways to prevent iron deficiency in these infants in the future.”
For the study, researchers retrospectively analyzed data about all preterm infants (< 31 weeks gestation) in Nova Scotia from 2005 to 2018. Of the 392 infants in this group (55.75% male; average age, about 5 months), 285 were fed with iron-rich formula (mean intake, 1.66 mg/kg per day), and 107 were fully or partially breast fed. The two groups were similar in terms of traits such as mean birth weight and gestational age.
The formula-fed infants were more likely to develop iron deficiency (ID, 36.8%) than the breast-fed infants (20.6%; P = .002). “Mean gestational age and birth weight were both lower in the ID group. The ID group also had a higher percentage of infants born less than 1,100 g (P = .01). More babies in the ID group received at least one blood transfusion,” the researchers reported. “ID infants had a higher daily formula intake, daily iron intake from formula, and total daily iron intake combined from formula and supplements.”
Why is there such a gap between formula-fed infants and breast-fed infants? The researchers speculated that infants absorb less iron from formula versus breast milk, possibly because of the presence of lactoferrin in breast milk.
The researchers also wondered whether physicians may pull back on iron supplementation in infants who undergo blood transfusions out of fear of the risk of iron overload, which Ms. Power said can cause infection and poor growth. By doing so, they may inadvertently deprive the babies of their need for iron.
“We don’t want clinicians to assume an infant doesn’t need iron supplementation just because they’ve received a blood transfusion,” she said.
As for an overall message from the research, Ms. Power said clinicians “should be aware that formula feeding can put infants at risk for iron deficiency and consider this when making decisions about supplementation.” And she noted that guidelines from the American Academy of Pediatrics and Canadian Pediatric Society don’t highlight the importance of iron supplementation in formula-fed, very preterm infants.
In an interview, University of Michigan pediatrician Michael K. Georgieff, MD, who has studied iron supplementation, said the study’s primary findings are surprising, although it makes sense that infants with lower gestational age and birth weight would suffer from more ID. Blood transfusion can indeed raise iron levels, but it’s important to consider that these infants may already have low levels of iron.
Dr. Georgieff advised colleagues to understand the potential for various nutritional deficiencies in preterm infants well beyond the first few weeks. When the babies are handed off to other clinicians such as pediatricians, they should undergo nutritional screening at 6 months, not at a year.
Dalhousie University funded the study. The study authors and Dr. Georgieff have no disclosures.
NEW ORLEANS –
“We were surprised that, despite actually receiving more iron in total each day on average, the formula-fed infants were significantly more iron deficient than breast-fed babies. This is the opposite of what one would expect,” study lead author Grace Power, a medical student at Dalhousie University, Halifax, N.S., said in an interview. She presented the results at the annual meeting of the American Society of Hematology.
According to Ms. Power, there’s limited research into how breastfeeding and formula feeding affect iron levels in preterm infants – especially those born extremely early, between 23 and 30 weeks’ gestation.
“This kind of research is important because preterm infants are highly susceptible to iron deficiency for a number of reasons,” she said. “Iron deficiency early in life is associated with developmental and behavioral problems later on in life. That association still stands, even if the iron deficiency is corrected, so prevention is key in this population. Knowing more about how feeding type affects iron status can help us learn about ways to prevent iron deficiency in these infants in the future.”
For the study, researchers retrospectively analyzed data about all preterm infants (< 31 weeks gestation) in Nova Scotia from 2005 to 2018. Of the 392 infants in this group (55.75% male; average age, about 5 months), 285 were fed with iron-rich formula (mean intake, 1.66 mg/kg per day), and 107 were fully or partially breast fed. The two groups were similar in terms of traits such as mean birth weight and gestational age.
The formula-fed infants were more likely to develop iron deficiency (ID, 36.8%) than the breast-fed infants (20.6%; P = .002). “Mean gestational age and birth weight were both lower in the ID group. The ID group also had a higher percentage of infants born less than 1,100 g (P = .01). More babies in the ID group received at least one blood transfusion,” the researchers reported. “ID infants had a higher daily formula intake, daily iron intake from formula, and total daily iron intake combined from formula and supplements.”
Why is there such a gap between formula-fed infants and breast-fed infants? The researchers speculated that infants absorb less iron from formula versus breast milk, possibly because of the presence of lactoferrin in breast milk.
The researchers also wondered whether physicians may pull back on iron supplementation in infants who undergo blood transfusions out of fear of the risk of iron overload, which Ms. Power said can cause infection and poor growth. By doing so, they may inadvertently deprive the babies of their need for iron.
“We don’t want clinicians to assume an infant doesn’t need iron supplementation just because they’ve received a blood transfusion,” she said.
As for an overall message from the research, Ms. Power said clinicians “should be aware that formula feeding can put infants at risk for iron deficiency and consider this when making decisions about supplementation.” And she noted that guidelines from the American Academy of Pediatrics and Canadian Pediatric Society don’t highlight the importance of iron supplementation in formula-fed, very preterm infants.
In an interview, University of Michigan pediatrician Michael K. Georgieff, MD, who has studied iron supplementation, said the study’s primary findings are surprising, although it makes sense that infants with lower gestational age and birth weight would suffer from more ID. Blood transfusion can indeed raise iron levels, but it’s important to consider that these infants may already have low levels of iron.
Dr. Georgieff advised colleagues to understand the potential for various nutritional deficiencies in preterm infants well beyond the first few weeks. When the babies are handed off to other clinicians such as pediatricians, they should undergo nutritional screening at 6 months, not at a year.
Dalhousie University funded the study. The study authors and Dr. Georgieff have no disclosures.
AT ASH 2022
Alloantibody registry would save lives, money
NEW ORLEANS – Save lives; save money. What’s not to love? That’s the claim made for a proposed nationwide data bank on alloantibodies, which develop in response to foreign red blood cells in individuals who undergo repeated blood transfusions. They can occur after pregnancy or transplants, as well as in patients with sickle cell disease.
“The findings from our model are pretty definitive,” said George Goshua, MD, MSc, of Yale University, New Haven, Conn. “Despite very conservative assumptions, our results still show a huge financial benefit to having a system in place to serve as a preventive net that catches patients before they have to go through a delayed hemolytic transfusion reaction (DHTR).”
Dr. Goshua presented the study at the American Society of Hematology annual meeting. The proposed registry would significantly reduce the risk that transfusion-dependent patients, and others who require occasional transfusions, would develop complications requiring hospitalization, he said.
A similar registry has been up and running in the Netherlands for 15 years, he said at a press briefing.
Briefing moderator Catherine Bollard, MD, of the Center for Cancer and Immunology at Children’s National Research Institute in Washington, asked Dr. Goshua why such an exchange hasn’t been started in the United States already.
“I will say first that our European colleagues are far ahead in terms of preventative care,” he replied.
“On top of that, there’s a unique environment in the United States – and this dates back about 15 years now – where we are almost allergic to putting costs on benefits, that is, attaching a cost value to a benefit that a population can gain,” Dr. Goshua said. “So in this context, there hasn’t been an analysis that shows that this [exchange] actually makes sense, but I think it’s one of those analyses kind of showing people that the sky is blue but proving it quantitatively.”
Dr. Bollard said that the potential beneficial impact of such an exchange “is huge,” but it would “require upfront expenditure to actually realize these massive gains you will get down the road for these patients.”
Would be cost-effective
Although hospitals and transfusion centers check donated blood against an individual patient’s alloantibody profile, that information is usually kept in localized records and is not typically shared across health systems nationwide.
It’s different in the Netherlands, where the Transfusion Register of Irregular Antibodies and Cross-match Problems (TRIX) was launched in 2007. Under this system, transfusion laboratories register the presence of irregular red blood cell alloantibodies for their patients and can consult the database for information that is relevant for pretransfusion testing.
To see whether such a system, if implemented in the United States, would satisfy even the most parsimonious administrator or insurer, Dr. Goshua and colleagues created a computer simulation.
They estimated age- and gender-adjusted quality-adjusted life years (QALYs) for patients living with sickle cell disease, who typically require frequent transfusions and are thus especially at risk for developing alloantibodies and immune reactions from repeat exposures to the blood of others.
The model included age- and gender-adjusted costs based on 10 years of claims data, with the assumption that equal numbers of male and females would be in the sample.
The model estimated that by reducing DHTR incidence and DHTR-specific mortality in 20% to 44% of alloimmunized patients (a very conservative estimate, according to Dr. Goshua), the existence of a U.S. exchange would result in a gain of between 7,140 and 15,710 QALYs.
Assuming a willingness to pay up to $100,000 per QALY, a commonly used threshold in economic analyses in the United States, the exchange (vs. no exchange) would be preferred in 100% of 10,000 different iterations of a cost-effectiveness acceptability curve, Dr. Goshua said.
Even if the lifetime operational costs of such an exchange exceeded $600 million, it would still be cost-effective, and the net monetary benefit to the U.S. economy would be $0.7 billion, the authors found.
And although the model was limited to patients with sickle cell anemia, many other alloimmunized patients would be likely to benefit from such an exchange, including women with a prior pregnancy, and patients with autoimmunity, myelodysplastic syndrome, or beta-thalassemia, Dr. Goshua said.
The study was supported by the American Society of Hematology, the Yale School of Medicine, and Yale Center. Dr. Goshua and Dr. Bollard reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Save lives; save money. What’s not to love? That’s the claim made for a proposed nationwide data bank on alloantibodies, which develop in response to foreign red blood cells in individuals who undergo repeated blood transfusions. They can occur after pregnancy or transplants, as well as in patients with sickle cell disease.
“The findings from our model are pretty definitive,” said George Goshua, MD, MSc, of Yale University, New Haven, Conn. “Despite very conservative assumptions, our results still show a huge financial benefit to having a system in place to serve as a preventive net that catches patients before they have to go through a delayed hemolytic transfusion reaction (DHTR).”
Dr. Goshua presented the study at the American Society of Hematology annual meeting. The proposed registry would significantly reduce the risk that transfusion-dependent patients, and others who require occasional transfusions, would develop complications requiring hospitalization, he said.
A similar registry has been up and running in the Netherlands for 15 years, he said at a press briefing.
Briefing moderator Catherine Bollard, MD, of the Center for Cancer and Immunology at Children’s National Research Institute in Washington, asked Dr. Goshua why such an exchange hasn’t been started in the United States already.
“I will say first that our European colleagues are far ahead in terms of preventative care,” he replied.
“On top of that, there’s a unique environment in the United States – and this dates back about 15 years now – where we are almost allergic to putting costs on benefits, that is, attaching a cost value to a benefit that a population can gain,” Dr. Goshua said. “So in this context, there hasn’t been an analysis that shows that this [exchange] actually makes sense, but I think it’s one of those analyses kind of showing people that the sky is blue but proving it quantitatively.”
Dr. Bollard said that the potential beneficial impact of such an exchange “is huge,” but it would “require upfront expenditure to actually realize these massive gains you will get down the road for these patients.”
Would be cost-effective
Although hospitals and transfusion centers check donated blood against an individual patient’s alloantibody profile, that information is usually kept in localized records and is not typically shared across health systems nationwide.
It’s different in the Netherlands, where the Transfusion Register of Irregular Antibodies and Cross-match Problems (TRIX) was launched in 2007. Under this system, transfusion laboratories register the presence of irregular red blood cell alloantibodies for their patients and can consult the database for information that is relevant for pretransfusion testing.
To see whether such a system, if implemented in the United States, would satisfy even the most parsimonious administrator or insurer, Dr. Goshua and colleagues created a computer simulation.
They estimated age- and gender-adjusted quality-adjusted life years (QALYs) for patients living with sickle cell disease, who typically require frequent transfusions and are thus especially at risk for developing alloantibodies and immune reactions from repeat exposures to the blood of others.
The model included age- and gender-adjusted costs based on 10 years of claims data, with the assumption that equal numbers of male and females would be in the sample.
The model estimated that by reducing DHTR incidence and DHTR-specific mortality in 20% to 44% of alloimmunized patients (a very conservative estimate, according to Dr. Goshua), the existence of a U.S. exchange would result in a gain of between 7,140 and 15,710 QALYs.
Assuming a willingness to pay up to $100,000 per QALY, a commonly used threshold in economic analyses in the United States, the exchange (vs. no exchange) would be preferred in 100% of 10,000 different iterations of a cost-effectiveness acceptability curve, Dr. Goshua said.
Even if the lifetime operational costs of such an exchange exceeded $600 million, it would still be cost-effective, and the net monetary benefit to the U.S. economy would be $0.7 billion, the authors found.
And although the model was limited to patients with sickle cell anemia, many other alloimmunized patients would be likely to benefit from such an exchange, including women with a prior pregnancy, and patients with autoimmunity, myelodysplastic syndrome, or beta-thalassemia, Dr. Goshua said.
The study was supported by the American Society of Hematology, the Yale School of Medicine, and Yale Center. Dr. Goshua and Dr. Bollard reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Save lives; save money. What’s not to love? That’s the claim made for a proposed nationwide data bank on alloantibodies, which develop in response to foreign red blood cells in individuals who undergo repeated blood transfusions. They can occur after pregnancy or transplants, as well as in patients with sickle cell disease.
“The findings from our model are pretty definitive,” said George Goshua, MD, MSc, of Yale University, New Haven, Conn. “Despite very conservative assumptions, our results still show a huge financial benefit to having a system in place to serve as a preventive net that catches patients before they have to go through a delayed hemolytic transfusion reaction (DHTR).”
Dr. Goshua presented the study at the American Society of Hematology annual meeting. The proposed registry would significantly reduce the risk that transfusion-dependent patients, and others who require occasional transfusions, would develop complications requiring hospitalization, he said.
A similar registry has been up and running in the Netherlands for 15 years, he said at a press briefing.
Briefing moderator Catherine Bollard, MD, of the Center for Cancer and Immunology at Children’s National Research Institute in Washington, asked Dr. Goshua why such an exchange hasn’t been started in the United States already.
“I will say first that our European colleagues are far ahead in terms of preventative care,” he replied.
“On top of that, there’s a unique environment in the United States – and this dates back about 15 years now – where we are almost allergic to putting costs on benefits, that is, attaching a cost value to a benefit that a population can gain,” Dr. Goshua said. “So in this context, there hasn’t been an analysis that shows that this [exchange] actually makes sense, but I think it’s one of those analyses kind of showing people that the sky is blue but proving it quantitatively.”
Dr. Bollard said that the potential beneficial impact of such an exchange “is huge,” but it would “require upfront expenditure to actually realize these massive gains you will get down the road for these patients.”
Would be cost-effective
Although hospitals and transfusion centers check donated blood against an individual patient’s alloantibody profile, that information is usually kept in localized records and is not typically shared across health systems nationwide.
It’s different in the Netherlands, where the Transfusion Register of Irregular Antibodies and Cross-match Problems (TRIX) was launched in 2007. Under this system, transfusion laboratories register the presence of irregular red blood cell alloantibodies for their patients and can consult the database for information that is relevant for pretransfusion testing.
To see whether such a system, if implemented in the United States, would satisfy even the most parsimonious administrator or insurer, Dr. Goshua and colleagues created a computer simulation.
They estimated age- and gender-adjusted quality-adjusted life years (QALYs) for patients living with sickle cell disease, who typically require frequent transfusions and are thus especially at risk for developing alloantibodies and immune reactions from repeat exposures to the blood of others.
The model included age- and gender-adjusted costs based on 10 years of claims data, with the assumption that equal numbers of male and females would be in the sample.
The model estimated that by reducing DHTR incidence and DHTR-specific mortality in 20% to 44% of alloimmunized patients (a very conservative estimate, according to Dr. Goshua), the existence of a U.S. exchange would result in a gain of between 7,140 and 15,710 QALYs.
Assuming a willingness to pay up to $100,000 per QALY, a commonly used threshold in economic analyses in the United States, the exchange (vs. no exchange) would be preferred in 100% of 10,000 different iterations of a cost-effectiveness acceptability curve, Dr. Goshua said.
Even if the lifetime operational costs of such an exchange exceeded $600 million, it would still be cost-effective, and the net monetary benefit to the U.S. economy would be $0.7 billion, the authors found.
And although the model was limited to patients with sickle cell anemia, many other alloimmunized patients would be likely to benefit from such an exchange, including women with a prior pregnancy, and patients with autoimmunity, myelodysplastic syndrome, or beta-thalassemia, Dr. Goshua said.
The study was supported by the American Society of Hematology, the Yale School of Medicine, and Yale Center. Dr. Goshua and Dr. Bollard reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ASH 2022
Beta-thalassemia: Benefits of gene therapy outweigh costs
Surveyed at 3 years, patients also reported ongoing benefits from treatment, including positive impacts on employment, school attendance, and physical activity, according to a second report.
The findings address a major question about betibeglogene autotemcel: Its durability. The therapy is priced at over $2 million per treatment, based on the premise that it will benefit patients in the long-term, in part by offsetting the cost of ongoing transfusions. Therefore, proof of long-standing benefit is important.
The Food and Drug Administration approved betibeglogene autotemcel in August 2022 for children and adults with transfusion dependent beta-thalassemia, a condition that causes patients to have absent or reduced levels of hemoglobin due to mutations in the beta-globin gene. Patients typically require transfusions every 2-5 weeks.
The treatment inserts functional copies of the mutated gene into the patients’ hematopoietic stem cells via a replication-defective lentivirus. The cells are then transfused back into the patient.
As of August 2021, 63 patients had undergone treatment and been followed for a median of 41.4 months. So far, durability looks solid.
“We now have up to 8 years efficacy and safety follow-up” with beti-cel. “Patients experience durable transfusion independence,” said Mark Walters, MD, a pediatric hematologist/oncologist at the University of California, San Francisco, who presented the long-term efficacy data at the meeting.
Overall, 89.5% of patients (34/38) in phase 3 testing achieved transfusion independence, meaning that they had hemoglobin levels of at least 9 g/dL without transfusions for a year or more.
The response rate was an improvement over phase 1/2 testing, in which 68% of subjects (15/22) became transfusion free. Improvements in the manufacturing process led to better outcomes in phase 3, Dr. Walters said.
As for quality of life (QoL), improvement “continues through 3 years following treatment,” said Franco Locatelli, MD, a pediatric hematologist/oncologist at Catholic University of the Sacred Heart, Rome, who led the QoL study.
When patients who achieved transfusion independence were surveyed 3 years after treatment, 93% of adults were employed or able to seek employment, up from 67% before treatment. School absences were down among children, almost half of subjects no longer needed symptom management, and 81% reported improvements in physical activity.
There were also improvements on various quality of life scales, including in physical functioning and mental health.
Patient age and underlying thalassemia genotype had no impact on the likelihood of transfusion independence. Those who achieved it also had reductions in markers of ineffective erythropoiesis and iron overload.
On multivariate analysis, the greatest predictor of transfusion independence was having at least 62% of cells transduced prior to reintroduction to the patient.
As for adverse events, seven subjects (11%) developed severe veno-occlusive liver disease that resolved with supportive care. Mucositis and febrile neutropenia are also a concern and related to the busulfan conditioning regimen.
No malignancies, insertional oncogenesis, or lentivirus replication have been observed.
The studies were funded by beti-cel maker Bluebird Bio, and many of the investigators are employees. Others reported ties to Bluebird and a range of other companies. Among his industry ties, Dr. Locatelli is a speaker for Bluebird. Dr. Walters also had industry relationships, but didn’t report any ties to Bluebird.
Surveyed at 3 years, patients also reported ongoing benefits from treatment, including positive impacts on employment, school attendance, and physical activity, according to a second report.
The findings address a major question about betibeglogene autotemcel: Its durability. The therapy is priced at over $2 million per treatment, based on the premise that it will benefit patients in the long-term, in part by offsetting the cost of ongoing transfusions. Therefore, proof of long-standing benefit is important.
The Food and Drug Administration approved betibeglogene autotemcel in August 2022 for children and adults with transfusion dependent beta-thalassemia, a condition that causes patients to have absent or reduced levels of hemoglobin due to mutations in the beta-globin gene. Patients typically require transfusions every 2-5 weeks.
The treatment inserts functional copies of the mutated gene into the patients’ hematopoietic stem cells via a replication-defective lentivirus. The cells are then transfused back into the patient.
As of August 2021, 63 patients had undergone treatment and been followed for a median of 41.4 months. So far, durability looks solid.
“We now have up to 8 years efficacy and safety follow-up” with beti-cel. “Patients experience durable transfusion independence,” said Mark Walters, MD, a pediatric hematologist/oncologist at the University of California, San Francisco, who presented the long-term efficacy data at the meeting.
Overall, 89.5% of patients (34/38) in phase 3 testing achieved transfusion independence, meaning that they had hemoglobin levels of at least 9 g/dL without transfusions for a year or more.
The response rate was an improvement over phase 1/2 testing, in which 68% of subjects (15/22) became transfusion free. Improvements in the manufacturing process led to better outcomes in phase 3, Dr. Walters said.
As for quality of life (QoL), improvement “continues through 3 years following treatment,” said Franco Locatelli, MD, a pediatric hematologist/oncologist at Catholic University of the Sacred Heart, Rome, who led the QoL study.
When patients who achieved transfusion independence were surveyed 3 years after treatment, 93% of adults were employed or able to seek employment, up from 67% before treatment. School absences were down among children, almost half of subjects no longer needed symptom management, and 81% reported improvements in physical activity.
There were also improvements on various quality of life scales, including in physical functioning and mental health.
Patient age and underlying thalassemia genotype had no impact on the likelihood of transfusion independence. Those who achieved it also had reductions in markers of ineffective erythropoiesis and iron overload.
On multivariate analysis, the greatest predictor of transfusion independence was having at least 62% of cells transduced prior to reintroduction to the patient.
As for adverse events, seven subjects (11%) developed severe veno-occlusive liver disease that resolved with supportive care. Mucositis and febrile neutropenia are also a concern and related to the busulfan conditioning regimen.
No malignancies, insertional oncogenesis, or lentivirus replication have been observed.
The studies were funded by beti-cel maker Bluebird Bio, and many of the investigators are employees. Others reported ties to Bluebird and a range of other companies. Among his industry ties, Dr. Locatelli is a speaker for Bluebird. Dr. Walters also had industry relationships, but didn’t report any ties to Bluebird.
Surveyed at 3 years, patients also reported ongoing benefits from treatment, including positive impacts on employment, school attendance, and physical activity, according to a second report.
The findings address a major question about betibeglogene autotemcel: Its durability. The therapy is priced at over $2 million per treatment, based on the premise that it will benefit patients in the long-term, in part by offsetting the cost of ongoing transfusions. Therefore, proof of long-standing benefit is important.
The Food and Drug Administration approved betibeglogene autotemcel in August 2022 for children and adults with transfusion dependent beta-thalassemia, a condition that causes patients to have absent or reduced levels of hemoglobin due to mutations in the beta-globin gene. Patients typically require transfusions every 2-5 weeks.
The treatment inserts functional copies of the mutated gene into the patients’ hematopoietic stem cells via a replication-defective lentivirus. The cells are then transfused back into the patient.
As of August 2021, 63 patients had undergone treatment and been followed for a median of 41.4 months. So far, durability looks solid.
“We now have up to 8 years efficacy and safety follow-up” with beti-cel. “Patients experience durable transfusion independence,” said Mark Walters, MD, a pediatric hematologist/oncologist at the University of California, San Francisco, who presented the long-term efficacy data at the meeting.
Overall, 89.5% of patients (34/38) in phase 3 testing achieved transfusion independence, meaning that they had hemoglobin levels of at least 9 g/dL without transfusions for a year or more.
The response rate was an improvement over phase 1/2 testing, in which 68% of subjects (15/22) became transfusion free. Improvements in the manufacturing process led to better outcomes in phase 3, Dr. Walters said.
As for quality of life (QoL), improvement “continues through 3 years following treatment,” said Franco Locatelli, MD, a pediatric hematologist/oncologist at Catholic University of the Sacred Heart, Rome, who led the QoL study.
When patients who achieved transfusion independence were surveyed 3 years after treatment, 93% of adults were employed or able to seek employment, up from 67% before treatment. School absences were down among children, almost half of subjects no longer needed symptom management, and 81% reported improvements in physical activity.
There were also improvements on various quality of life scales, including in physical functioning and mental health.
Patient age and underlying thalassemia genotype had no impact on the likelihood of transfusion independence. Those who achieved it also had reductions in markers of ineffective erythropoiesis and iron overload.
On multivariate analysis, the greatest predictor of transfusion independence was having at least 62% of cells transduced prior to reintroduction to the patient.
As for adverse events, seven subjects (11%) developed severe veno-occlusive liver disease that resolved with supportive care. Mucositis and febrile neutropenia are also a concern and related to the busulfan conditioning regimen.
No malignancies, insertional oncogenesis, or lentivirus replication have been observed.
The studies were funded by beti-cel maker Bluebird Bio, and many of the investigators are employees. Others reported ties to Bluebird and a range of other companies. Among his industry ties, Dr. Locatelli is a speaker for Bluebird. Dr. Walters also had industry relationships, but didn’t report any ties to Bluebird.
FROM ASH 2022
Postpartum hemorrhage rates and risk factors rising
The rate of postpartum hemorrhage for hospital deliveries in the United States increased significantly over a 20-year period, according to data from more than 76 million delivery hospitalizations from the National Inpatient Sample.
Postpartum hemorrhage remains the leading cause of maternal morbidity and mortality worldwide, and many clinical and patient-level risk factors appear to be on the rise, wrote Chiara M. Corbetta-Rastelli, MD, of the University of California, San Francisco, and colleagues.
Although practice changes have been introduced to reduce postpartum hemorrhage, recent trends in postpartum hemorrhage risk and outcomes in the context of such changes as hemorrhage safety bundles have not been examined, they said.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from hospitalizations for females aged 15-54 years for deliveries between 2000 and 2019 using the National Inpatient Sample. They used a regression analysis to estimate average annual percentage changes (AAPC). Their objectives were to characterize trends and also to assess the association between risk factors and the occurrence of postpartum hemorrhage and related interventions. Demographics, clinical factors, and hospital characteristics were mainly similar between the group of patients with postpartum hemorrhage and those with no postpartum hemorrhage.
Approximately 3% (2.3 million) of 76.7 million hospitalizations for delivery were complicated by postpartum hemorrhage during the study period, and the annual rate increased from 2.7% to 4.3%.
Overall, 21.4% of individuals with delivery hospitalizations complicated by postpartum hemorrhage had one postpartum risk factor, and 1.4% had two or more risk factors. The number of individuals with at least one risk factor for postpartum hemorrhage increased significantly, from 18.6% to 26.9%, during the study period, with an annual percentage change of 1.9%.
Compared with deliveries in individuals without risk factors, individuals with one risk factor had slightly higher odds of postpartum hemorrhage (odds ratio, 1.14), but those with two or more risk factors were more than twice as likely to experience postpartum hemorrhage as those with no risk factors (OR, 2.31).
The researchers also examined the association of specific risk factors and interventions related to hemorrhage, notably blood transfusion and peripartum hysterectomy. Blood transfusions in individuals with postpartum hemorrhage increased from 5.4% to 16.7% between 2000 and 2011, (AAPC, 10.2%) then decreased from 16.7% to 12.6% from 2011 to 2019 (AAPC, –3.9%).
Peripartum hysterectomy in the study population increased from 1.4% to 2.4% from 2000 to 2009 (AAPC 5.0%), remained steady from 2009 to 2016, and then decreased from 2.1% to 0.9% from 2016 to 2019 (AAPC –27%).
Other risk factors associated with postpartum hemorrhage itself and with blood transfusion and hysterectomy in the setting of postpartum hemorrhage included prior cesarean delivery with placenta previa or accreta, placenta previa without prior cesarean delivery, and antepartum hemorrhage or placental abruption, the researchers noted.
“In addition to placental abnormalities, risk factors such as preeclampsia with severe features, polyhydramnios, and uterine leiomyomas demonstrated the highest rates of increase in our data,” they wrote in their discussion. These trends may lead to continuing increases in postpartum hemorrhage risk, which was not fully explained by the increase in risk factors seen in the current study, the researchers said.
The study findings were limited by several factors, including the use of billing codes that could lead to misclassification of diagnoses, as well as possible differences in the definition and coding for postpartum hemorrhage among hospitals, the researchers noted. Other limitations were the exclusion of cases of readmission for postpartum hemorrhage and lack of clinical details involving use of medications or nonoperative interventions, they said.
Notably, the study finding of stable to decreasing peripartum hysterectomy rates in hospitalized patients with postpartum hemorrhage conflicts with another recent study showing an increase in peripartum hysterectomy from 2009 to 2020, but this difference may reflect changes in billing, indications for hysterectomy, or study modeling, they said.
The current study was strengthened by the use of a large database to analyze population trends, a contemporary study period, and the inclusion of meaningful outcomes such as peripartum hysterectomy, the researchers wrote.
The shift in blood transfusion and peripartum hysterectomy may reflect the implementation of protocols to promote early intervention and identification of postpartum hemorrhage, they concluded.
Interventions can have an effect
“Hemorrhage remains a leading cause of maternal mortality in the United States and blood transfusion is the most common severe maternal morbidity,” Catherine M. Albright, MD, MS, associate professor of maternal-fetal medicine at the University of Washington, Seattle, said in an interview. “It is important to understand the current state, especially given that many hospitals have implemented policies and procedures to better identify and treat postpartum hemorrhage,” she said.
Dr. Albright said, “I was pleased to see that they did not just look at a diagnosis of postpartum hemorrhage but rather also looked at complications arising from postpartum hemorrhage, such as blood transfusion or hysterectomy.”
Postpartum hemorrhage is often a clinical diagnosis that uses estimated blood loss, a notoriously inaccurate measure, said Dr. Albright. “Additionally, the definitions of postpartum hemorrhage, as well as the ICD codes, changed during the time period of the study,” she noted. “These factors all could lead to both underreporting and overreporting of the true incidence of postpartum hemorrhage. Blood transfusion and hysterectomy are more objective outcomes and demonstrate true morbidity,” she said.
“Most of the risk factors that are listed in the article are not modifiable during that pregnancy,” said Dr. Albright. For example, a history of a prior cesarean or having a twin pregnancy is not something that can be changed, she said. “Many of the other risk factors or associated clinical factors, such as obesity, chronic hypertension, and pregestational diabetes, are modifiable, but before pregnancy. Universal and easy access to primary medical care prior to and between pregnancies may help to mitigate some of these factors,” she noted.
Looking ahead, “It would be helpful to ensure that these types of data are available at the state and hospital level; this will allow for local evaluation of programs that are in place to reduce postpartum hemorrhage risk and improve identification and treatment,” Dr. Albright said.
The study received no outside funding. Dr. Corbetta-Rastelli and Dr. Albright had no financial conflicts to disclose.
The rate of postpartum hemorrhage for hospital deliveries in the United States increased significantly over a 20-year period, according to data from more than 76 million delivery hospitalizations from the National Inpatient Sample.
Postpartum hemorrhage remains the leading cause of maternal morbidity and mortality worldwide, and many clinical and patient-level risk factors appear to be on the rise, wrote Chiara M. Corbetta-Rastelli, MD, of the University of California, San Francisco, and colleagues.
Although practice changes have been introduced to reduce postpartum hemorrhage, recent trends in postpartum hemorrhage risk and outcomes in the context of such changes as hemorrhage safety bundles have not been examined, they said.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from hospitalizations for females aged 15-54 years for deliveries between 2000 and 2019 using the National Inpatient Sample. They used a regression analysis to estimate average annual percentage changes (AAPC). Their objectives were to characterize trends and also to assess the association between risk factors and the occurrence of postpartum hemorrhage and related interventions. Demographics, clinical factors, and hospital characteristics were mainly similar between the group of patients with postpartum hemorrhage and those with no postpartum hemorrhage.
Approximately 3% (2.3 million) of 76.7 million hospitalizations for delivery were complicated by postpartum hemorrhage during the study period, and the annual rate increased from 2.7% to 4.3%.
Overall, 21.4% of individuals with delivery hospitalizations complicated by postpartum hemorrhage had one postpartum risk factor, and 1.4% had two or more risk factors. The number of individuals with at least one risk factor for postpartum hemorrhage increased significantly, from 18.6% to 26.9%, during the study period, with an annual percentage change of 1.9%.
Compared with deliveries in individuals without risk factors, individuals with one risk factor had slightly higher odds of postpartum hemorrhage (odds ratio, 1.14), but those with two or more risk factors were more than twice as likely to experience postpartum hemorrhage as those with no risk factors (OR, 2.31).
The researchers also examined the association of specific risk factors and interventions related to hemorrhage, notably blood transfusion and peripartum hysterectomy. Blood transfusions in individuals with postpartum hemorrhage increased from 5.4% to 16.7% between 2000 and 2011, (AAPC, 10.2%) then decreased from 16.7% to 12.6% from 2011 to 2019 (AAPC, –3.9%).
Peripartum hysterectomy in the study population increased from 1.4% to 2.4% from 2000 to 2009 (AAPC 5.0%), remained steady from 2009 to 2016, and then decreased from 2.1% to 0.9% from 2016 to 2019 (AAPC –27%).
Other risk factors associated with postpartum hemorrhage itself and with blood transfusion and hysterectomy in the setting of postpartum hemorrhage included prior cesarean delivery with placenta previa or accreta, placenta previa without prior cesarean delivery, and antepartum hemorrhage or placental abruption, the researchers noted.
“In addition to placental abnormalities, risk factors such as preeclampsia with severe features, polyhydramnios, and uterine leiomyomas demonstrated the highest rates of increase in our data,” they wrote in their discussion. These trends may lead to continuing increases in postpartum hemorrhage risk, which was not fully explained by the increase in risk factors seen in the current study, the researchers said.
The study findings were limited by several factors, including the use of billing codes that could lead to misclassification of diagnoses, as well as possible differences in the definition and coding for postpartum hemorrhage among hospitals, the researchers noted. Other limitations were the exclusion of cases of readmission for postpartum hemorrhage and lack of clinical details involving use of medications or nonoperative interventions, they said.
Notably, the study finding of stable to decreasing peripartum hysterectomy rates in hospitalized patients with postpartum hemorrhage conflicts with another recent study showing an increase in peripartum hysterectomy from 2009 to 2020, but this difference may reflect changes in billing, indications for hysterectomy, or study modeling, they said.
The current study was strengthened by the use of a large database to analyze population trends, a contemporary study period, and the inclusion of meaningful outcomes such as peripartum hysterectomy, the researchers wrote.
The shift in blood transfusion and peripartum hysterectomy may reflect the implementation of protocols to promote early intervention and identification of postpartum hemorrhage, they concluded.
Interventions can have an effect
“Hemorrhage remains a leading cause of maternal mortality in the United States and blood transfusion is the most common severe maternal morbidity,” Catherine M. Albright, MD, MS, associate professor of maternal-fetal medicine at the University of Washington, Seattle, said in an interview. “It is important to understand the current state, especially given that many hospitals have implemented policies and procedures to better identify and treat postpartum hemorrhage,” she said.
Dr. Albright said, “I was pleased to see that they did not just look at a diagnosis of postpartum hemorrhage but rather also looked at complications arising from postpartum hemorrhage, such as blood transfusion or hysterectomy.”
Postpartum hemorrhage is often a clinical diagnosis that uses estimated blood loss, a notoriously inaccurate measure, said Dr. Albright. “Additionally, the definitions of postpartum hemorrhage, as well as the ICD codes, changed during the time period of the study,” she noted. “These factors all could lead to both underreporting and overreporting of the true incidence of postpartum hemorrhage. Blood transfusion and hysterectomy are more objective outcomes and demonstrate true morbidity,” she said.
“Most of the risk factors that are listed in the article are not modifiable during that pregnancy,” said Dr. Albright. For example, a history of a prior cesarean or having a twin pregnancy is not something that can be changed, she said. “Many of the other risk factors or associated clinical factors, such as obesity, chronic hypertension, and pregestational diabetes, are modifiable, but before pregnancy. Universal and easy access to primary medical care prior to and between pregnancies may help to mitigate some of these factors,” she noted.
Looking ahead, “It would be helpful to ensure that these types of data are available at the state and hospital level; this will allow for local evaluation of programs that are in place to reduce postpartum hemorrhage risk and improve identification and treatment,” Dr. Albright said.
The study received no outside funding. Dr. Corbetta-Rastelli and Dr. Albright had no financial conflicts to disclose.
The rate of postpartum hemorrhage for hospital deliveries in the United States increased significantly over a 20-year period, according to data from more than 76 million delivery hospitalizations from the National Inpatient Sample.
Postpartum hemorrhage remains the leading cause of maternal morbidity and mortality worldwide, and many clinical and patient-level risk factors appear to be on the rise, wrote Chiara M. Corbetta-Rastelli, MD, of the University of California, San Francisco, and colleagues.
Although practice changes have been introduced to reduce postpartum hemorrhage, recent trends in postpartum hemorrhage risk and outcomes in the context of such changes as hemorrhage safety bundles have not been examined, they said.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from hospitalizations for females aged 15-54 years for deliveries between 2000 and 2019 using the National Inpatient Sample. They used a regression analysis to estimate average annual percentage changes (AAPC). Their objectives were to characterize trends and also to assess the association between risk factors and the occurrence of postpartum hemorrhage and related interventions. Demographics, clinical factors, and hospital characteristics were mainly similar between the group of patients with postpartum hemorrhage and those with no postpartum hemorrhage.
Approximately 3% (2.3 million) of 76.7 million hospitalizations for delivery were complicated by postpartum hemorrhage during the study period, and the annual rate increased from 2.7% to 4.3%.
Overall, 21.4% of individuals with delivery hospitalizations complicated by postpartum hemorrhage had one postpartum risk factor, and 1.4% had two or more risk factors. The number of individuals with at least one risk factor for postpartum hemorrhage increased significantly, from 18.6% to 26.9%, during the study period, with an annual percentage change of 1.9%.
Compared with deliveries in individuals without risk factors, individuals with one risk factor had slightly higher odds of postpartum hemorrhage (odds ratio, 1.14), but those with two or more risk factors were more than twice as likely to experience postpartum hemorrhage as those with no risk factors (OR, 2.31).
The researchers also examined the association of specific risk factors and interventions related to hemorrhage, notably blood transfusion and peripartum hysterectomy. Blood transfusions in individuals with postpartum hemorrhage increased from 5.4% to 16.7% between 2000 and 2011, (AAPC, 10.2%) then decreased from 16.7% to 12.6% from 2011 to 2019 (AAPC, –3.9%).
Peripartum hysterectomy in the study population increased from 1.4% to 2.4% from 2000 to 2009 (AAPC 5.0%), remained steady from 2009 to 2016, and then decreased from 2.1% to 0.9% from 2016 to 2019 (AAPC –27%).
Other risk factors associated with postpartum hemorrhage itself and with blood transfusion and hysterectomy in the setting of postpartum hemorrhage included prior cesarean delivery with placenta previa or accreta, placenta previa without prior cesarean delivery, and antepartum hemorrhage or placental abruption, the researchers noted.
“In addition to placental abnormalities, risk factors such as preeclampsia with severe features, polyhydramnios, and uterine leiomyomas demonstrated the highest rates of increase in our data,” they wrote in their discussion. These trends may lead to continuing increases in postpartum hemorrhage risk, which was not fully explained by the increase in risk factors seen in the current study, the researchers said.
The study findings were limited by several factors, including the use of billing codes that could lead to misclassification of diagnoses, as well as possible differences in the definition and coding for postpartum hemorrhage among hospitals, the researchers noted. Other limitations were the exclusion of cases of readmission for postpartum hemorrhage and lack of clinical details involving use of medications or nonoperative interventions, they said.
Notably, the study finding of stable to decreasing peripartum hysterectomy rates in hospitalized patients with postpartum hemorrhage conflicts with another recent study showing an increase in peripartum hysterectomy from 2009 to 2020, but this difference may reflect changes in billing, indications for hysterectomy, or study modeling, they said.
The current study was strengthened by the use of a large database to analyze population trends, a contemporary study period, and the inclusion of meaningful outcomes such as peripartum hysterectomy, the researchers wrote.
The shift in blood transfusion and peripartum hysterectomy may reflect the implementation of protocols to promote early intervention and identification of postpartum hemorrhage, they concluded.
Interventions can have an effect
“Hemorrhage remains a leading cause of maternal mortality in the United States and blood transfusion is the most common severe maternal morbidity,” Catherine M. Albright, MD, MS, associate professor of maternal-fetal medicine at the University of Washington, Seattle, said in an interview. “It is important to understand the current state, especially given that many hospitals have implemented policies and procedures to better identify and treat postpartum hemorrhage,” she said.
Dr. Albright said, “I was pleased to see that they did not just look at a diagnosis of postpartum hemorrhage but rather also looked at complications arising from postpartum hemorrhage, such as blood transfusion or hysterectomy.”
Postpartum hemorrhage is often a clinical diagnosis that uses estimated blood loss, a notoriously inaccurate measure, said Dr. Albright. “Additionally, the definitions of postpartum hemorrhage, as well as the ICD codes, changed during the time period of the study,” she noted. “These factors all could lead to both underreporting and overreporting of the true incidence of postpartum hemorrhage. Blood transfusion and hysterectomy are more objective outcomes and demonstrate true morbidity,” she said.
“Most of the risk factors that are listed in the article are not modifiable during that pregnancy,” said Dr. Albright. For example, a history of a prior cesarean or having a twin pregnancy is not something that can be changed, she said. “Many of the other risk factors or associated clinical factors, such as obesity, chronic hypertension, and pregestational diabetes, are modifiable, but before pregnancy. Universal and easy access to primary medical care prior to and between pregnancies may help to mitigate some of these factors,” she noted.
Looking ahead, “It would be helpful to ensure that these types of data are available at the state and hospital level; this will allow for local evaluation of programs that are in place to reduce postpartum hemorrhage risk and improve identification and treatment,” Dr. Albright said.
The study received no outside funding. Dr. Corbetta-Rastelli and Dr. Albright had no financial conflicts to disclose.
FROM OBSTETRICS & GYNECOLOGY
FDA approves first gene therapy, betibeglogene autotemcel (Zynteglo), for beta-thalassemia
Betibeglogene autotemcel, a one-time gene therapy, represents a potential cure in which functional copies of the mutated gene are inserted into patients’ hematopoietic stem cells via a replication-defective lentivirus.
“Today’s approval is an important advance in the treatment of beta-thalassemia, particularly in individuals who require ongoing red blood cell transfusions,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in an FDA press release. “Given the potential health complications associated with this serious disease, this action highlights the FDA’s continued commitment to supporting development of innovative therapies for patients who have limited treatment options.”
The approval was based on phase 3 trials, in which 89% of 41 patients aged 4-34 years who received the therapy maintained normal or near-normal hemoglobin levels and didn’t need transfusions for at least a year. The patients were as young as age 4, maker Bluebird Bio said in a press release.
FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee unanimously recommended approval in June. The gene therapy had been approved in Europe, where it carried a price tag of about $1.8 million, but Bluebird pulled it from the market in 2021 because of problems with reimbursement.
“The decision to discontinue operations in Europe resulted from prolonged negotiations with European payers and challenges to achieving appropriate value recognition and market access,” the company said in a Securities and Exchange Commission filing.
The projected price in the United States is even higher: $2.1 million.
But the Institute for Clinical and Economic Review, an influential Boston-based nonprofit organization that specializes in medical cost-effectiveness analyses, concluded in June that, “given the high annual costs of standard care ... this new treatment meets commonly accepted value thresholds at an anticipated price of $2.1 million,” particularly with Bluebird’s proposal to pay back 80% of the cost if patients need a transfusion within 5 years.
The company is planning an October 2022 launch and estimates the U.S. market for betibeglogene autotemcel to be about 1,500 patients.
Adverse events in studies were “infrequent and consisted primarily of nonserious infusion-related reactions,” such as abdominal pain, hot flush, dyspnea, tachycardia, noncardiac chest pain, and cytopenias, including thrombocytopenia, leukopenia, and neutropenia. One case of thrombocytopenia was considered serious but resolved, according to the company.
Most of the serious adverse events were related to hematopoietic stem cell collection and the busulfan conditioning regimen. Insertional oncogenesis and/or cancer have been reported with Bluebird’s other gene therapy products, but no cases have been associated with betibeglogene autotemcel.
A version of this article first appeared on Medscape.com.
Betibeglogene autotemcel, a one-time gene therapy, represents a potential cure in which functional copies of the mutated gene are inserted into patients’ hematopoietic stem cells via a replication-defective lentivirus.
“Today’s approval is an important advance in the treatment of beta-thalassemia, particularly in individuals who require ongoing red blood cell transfusions,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in an FDA press release. “Given the potential health complications associated with this serious disease, this action highlights the FDA’s continued commitment to supporting development of innovative therapies for patients who have limited treatment options.”
The approval was based on phase 3 trials, in which 89% of 41 patients aged 4-34 years who received the therapy maintained normal or near-normal hemoglobin levels and didn’t need transfusions for at least a year. The patients were as young as age 4, maker Bluebird Bio said in a press release.
FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee unanimously recommended approval in June. The gene therapy had been approved in Europe, where it carried a price tag of about $1.8 million, but Bluebird pulled it from the market in 2021 because of problems with reimbursement.
“The decision to discontinue operations in Europe resulted from prolonged negotiations with European payers and challenges to achieving appropriate value recognition and market access,” the company said in a Securities and Exchange Commission filing.
The projected price in the United States is even higher: $2.1 million.
But the Institute for Clinical and Economic Review, an influential Boston-based nonprofit organization that specializes in medical cost-effectiveness analyses, concluded in June that, “given the high annual costs of standard care ... this new treatment meets commonly accepted value thresholds at an anticipated price of $2.1 million,” particularly with Bluebird’s proposal to pay back 80% of the cost if patients need a transfusion within 5 years.
The company is planning an October 2022 launch and estimates the U.S. market for betibeglogene autotemcel to be about 1,500 patients.
Adverse events in studies were “infrequent and consisted primarily of nonserious infusion-related reactions,” such as abdominal pain, hot flush, dyspnea, tachycardia, noncardiac chest pain, and cytopenias, including thrombocytopenia, leukopenia, and neutropenia. One case of thrombocytopenia was considered serious but resolved, according to the company.
Most of the serious adverse events were related to hematopoietic stem cell collection and the busulfan conditioning regimen. Insertional oncogenesis and/or cancer have been reported with Bluebird’s other gene therapy products, but no cases have been associated with betibeglogene autotemcel.
A version of this article first appeared on Medscape.com.
Betibeglogene autotemcel, a one-time gene therapy, represents a potential cure in which functional copies of the mutated gene are inserted into patients’ hematopoietic stem cells via a replication-defective lentivirus.
“Today’s approval is an important advance in the treatment of beta-thalassemia, particularly in individuals who require ongoing red blood cell transfusions,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in an FDA press release. “Given the potential health complications associated with this serious disease, this action highlights the FDA’s continued commitment to supporting development of innovative therapies for patients who have limited treatment options.”
The approval was based on phase 3 trials, in which 89% of 41 patients aged 4-34 years who received the therapy maintained normal or near-normal hemoglobin levels and didn’t need transfusions for at least a year. The patients were as young as age 4, maker Bluebird Bio said in a press release.
FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee unanimously recommended approval in June. The gene therapy had been approved in Europe, where it carried a price tag of about $1.8 million, but Bluebird pulled it from the market in 2021 because of problems with reimbursement.
“The decision to discontinue operations in Europe resulted from prolonged negotiations with European payers and challenges to achieving appropriate value recognition and market access,” the company said in a Securities and Exchange Commission filing.
The projected price in the United States is even higher: $2.1 million.
But the Institute for Clinical and Economic Review, an influential Boston-based nonprofit organization that specializes in medical cost-effectiveness analyses, concluded in June that, “given the high annual costs of standard care ... this new treatment meets commonly accepted value thresholds at an anticipated price of $2.1 million,” particularly with Bluebird’s proposal to pay back 80% of the cost if patients need a transfusion within 5 years.
The company is planning an October 2022 launch and estimates the U.S. market for betibeglogene autotemcel to be about 1,500 patients.
Adverse events in studies were “infrequent and consisted primarily of nonserious infusion-related reactions,” such as abdominal pain, hot flush, dyspnea, tachycardia, noncardiac chest pain, and cytopenias, including thrombocytopenia, leukopenia, and neutropenia. One case of thrombocytopenia was considered serious but resolved, according to the company.
Most of the serious adverse events were related to hematopoietic stem cell collection and the busulfan conditioning regimen. Insertional oncogenesis and/or cancer have been reported with Bluebird’s other gene therapy products, but no cases have been associated with betibeglogene autotemcel.
A version of this article first appeared on Medscape.com.