User login
Game-changing results in fluid resuscitation for acute pancreatitis
Early, aggressive fluid resuscitation in acute pancreatitis led to a higher incidence of fluid overload without improving clinical outcomes in the landmark WATERFALL trial.
Early aggressive hydration is widely recommended for the management of acute pancreatitis, but evidence for this practice is limited.
“The WATERFALL trial demonstrates that aggressive fluid resuscitation in acute pancreatitis is not safe, it is not associated with improved outcomes, and it should be abandoned,” Enrique de-Madaria, MD, PhD, with Hospital General Universitario Dr. Balmis, Alicante, Spain, told this news organization.
The trial settles a “new and clear reference for fluid resuscitation in this frequent disease: lactated Ringer’s solution 1.5 mL/kg per hour (preceded by a 10 mL/kg bolus over 2 hours only in case of hypovolemia),” added Dr. de-Madaria, president of the Spanish Association of Gastroenterology.
“This moderate fluid resuscitation strategy is associated with a much lower frequency of fluid overload and a trend toward improved outcomes. For such reasons, it should be considered as a new standard of care in the early management of acute pancreatitis,” Dr. de-Madaria said.
The WATERFALL trial results were published in the New England Journal of Medicine.
The results are “stunning and, given the carefully crafted trial methods, irrefutable,” Timothy Gardner, MD, with the section of gastroenterology and hepatology, Dartmouth–Hitchcock Medical Center, Lebanon, N.H., wrote in a linked editorial.
Trial details
The trial was conducted at 18 centers across India, Italy, Mexico, and Spain. Patients who presented with acute pancreatitis were randomly allocated to aggressive or moderate resuscitation with lactated Ringer’s solution.
Aggressive fluid resuscitation consisted of a bolus of 20 mL/kg of body weight, followed by 3 mL/kg per hour. Moderate fluid resuscitation consisted of a bolus of 10 mL/kg in patients with hypovolemia or no bolus in patients with normovolemia, followed by 1.5 mL/kg per hour in all patients in this group.
Patients were assessed at 12, 24, 48, and 72 hours, and fluid resuscitation was adjusted according to clinical status.
A total of 249 patients were included in the interim analysis – 122 in the aggressive-resuscitation group and 127 in the moderate-resuscitation group.
The data and safety monitoring board terminated the trial at the first interim safety analysis as a result of the development of fluid overload in 20.5% of the patients in the aggressive-resuscitation group versus 6.3% of those in the moderate-resuscitation group (adjusted relative risk, 2.85; 95% confidence interval, 1.36-5.94; P = .004).
“An increased risk of fluid overload was detected in the overall population of patients and also in subgroups of patients without systemic inflammatory response syndrome at baseline, patients with SIRS at baseline (thus, with a higher risk of development of severe pancreatitis), and patients with hypovolemia,” the investigators reported.
This clear signal of harm was coupled with no significant difference in the incidence of moderately severe or severe pancreatitis (22.1% in the aggressive-resuscitation group and 17.3% in the moderate-resuscitation group; aRR, 1.30; 95% CI, 0.78-2.18; P = .32).
Patients in the aggressive-resuscitation group spent a median of 6 days in the hospital, compared with 5 days for patients in the moderate-resuscitation group.
“These findings do not support current management guidelines, which recommend early aggressive resuscitation for the treatment of acute pancreatitis,” the study team wrote.
‘Landmark’ trial
This is a “landmark” trial and “so clinically relevant because of its choice of real world-appropriate aggressive-resuscitation and moderate-resuscitation treatment groups, its use of pancreatitis severity as the main clinical outcome, and its reliance on the carefully defined variable of fluid overload as the main safety outcome,” Dr. Gardner wrote in his editorial.
“Unlike in most other randomized, controlled trials of fluid resuscitation in acute pancreatitis, patients with varying baseline pancreatitis severity were included, and changes in the rate of resuscitation were determined on the basis of a dynamic assessment of hemodynamic testing, imaging, and clinical factors,” he added.
Dr. Gardner said the WATERFALL trial results lead to several conclusions.
First, the need to focus on a steady rate of initial resuscitation – no more than 1.5 mL/kg of body weight per hour. Clinicians should administer a bolus of 10 mL/kg only if there are signs of initial hypovolemia.
Second, that careful clinical and hemodynamic monitoring are essential during the first 72 hours after admission to make sure that patients remain euvolemic and to avoid fluid overload.
Third, that diuresis in patients with fluid overload in the first 72 hours is most likely beneficial and certainly not detrimental to important clinical outcomes.
Dr. Gardner said the trial also highlights the need to focus research efforts on evaluating other pharmacologic therapies instead of crystalloid fluids.
“Performing randomized controlled trials in acute pancreatitis is notoriously difficult, and the limited human and financial resources that are available for appropriately powered trials in this field post WATERFALL are much better spent on comparative-effectiveness and placebo-controlled trials evaluating new therapeutic agents,” Dr. Gardner said.
“Now that we have gone over the WATERFALL, it is time to look downstream at new targets to treat this challenging disease,” he concluded.
Support for the trial was provided by Instituto de Salud Carlos III, the Spanish Association of Gastroenterology, and ISABIAL (Instituto de Investigación Sanitaria y Biomédica de Alicante).
A version of this article first appeared on Medscape.com.
Early, aggressive fluid resuscitation in acute pancreatitis led to a higher incidence of fluid overload without improving clinical outcomes in the landmark WATERFALL trial.
Early aggressive hydration is widely recommended for the management of acute pancreatitis, but evidence for this practice is limited.
“The WATERFALL trial demonstrates that aggressive fluid resuscitation in acute pancreatitis is not safe, it is not associated with improved outcomes, and it should be abandoned,” Enrique de-Madaria, MD, PhD, with Hospital General Universitario Dr. Balmis, Alicante, Spain, told this news organization.
The trial settles a “new and clear reference for fluid resuscitation in this frequent disease: lactated Ringer’s solution 1.5 mL/kg per hour (preceded by a 10 mL/kg bolus over 2 hours only in case of hypovolemia),” added Dr. de-Madaria, president of the Spanish Association of Gastroenterology.
“This moderate fluid resuscitation strategy is associated with a much lower frequency of fluid overload and a trend toward improved outcomes. For such reasons, it should be considered as a new standard of care in the early management of acute pancreatitis,” Dr. de-Madaria said.
The WATERFALL trial results were published in the New England Journal of Medicine.
The results are “stunning and, given the carefully crafted trial methods, irrefutable,” Timothy Gardner, MD, with the section of gastroenterology and hepatology, Dartmouth–Hitchcock Medical Center, Lebanon, N.H., wrote in a linked editorial.
Trial details
The trial was conducted at 18 centers across India, Italy, Mexico, and Spain. Patients who presented with acute pancreatitis were randomly allocated to aggressive or moderate resuscitation with lactated Ringer’s solution.
Aggressive fluid resuscitation consisted of a bolus of 20 mL/kg of body weight, followed by 3 mL/kg per hour. Moderate fluid resuscitation consisted of a bolus of 10 mL/kg in patients with hypovolemia or no bolus in patients with normovolemia, followed by 1.5 mL/kg per hour in all patients in this group.
Patients were assessed at 12, 24, 48, and 72 hours, and fluid resuscitation was adjusted according to clinical status.
A total of 249 patients were included in the interim analysis – 122 in the aggressive-resuscitation group and 127 in the moderate-resuscitation group.
The data and safety monitoring board terminated the trial at the first interim safety analysis as a result of the development of fluid overload in 20.5% of the patients in the aggressive-resuscitation group versus 6.3% of those in the moderate-resuscitation group (adjusted relative risk, 2.85; 95% confidence interval, 1.36-5.94; P = .004).
“An increased risk of fluid overload was detected in the overall population of patients and also in subgroups of patients without systemic inflammatory response syndrome at baseline, patients with SIRS at baseline (thus, with a higher risk of development of severe pancreatitis), and patients with hypovolemia,” the investigators reported.
This clear signal of harm was coupled with no significant difference in the incidence of moderately severe or severe pancreatitis (22.1% in the aggressive-resuscitation group and 17.3% in the moderate-resuscitation group; aRR, 1.30; 95% CI, 0.78-2.18; P = .32).
Patients in the aggressive-resuscitation group spent a median of 6 days in the hospital, compared with 5 days for patients in the moderate-resuscitation group.
“These findings do not support current management guidelines, which recommend early aggressive resuscitation for the treatment of acute pancreatitis,” the study team wrote.
‘Landmark’ trial
This is a “landmark” trial and “so clinically relevant because of its choice of real world-appropriate aggressive-resuscitation and moderate-resuscitation treatment groups, its use of pancreatitis severity as the main clinical outcome, and its reliance on the carefully defined variable of fluid overload as the main safety outcome,” Dr. Gardner wrote in his editorial.
“Unlike in most other randomized, controlled trials of fluid resuscitation in acute pancreatitis, patients with varying baseline pancreatitis severity were included, and changes in the rate of resuscitation were determined on the basis of a dynamic assessment of hemodynamic testing, imaging, and clinical factors,” he added.
Dr. Gardner said the WATERFALL trial results lead to several conclusions.
First, the need to focus on a steady rate of initial resuscitation – no more than 1.5 mL/kg of body weight per hour. Clinicians should administer a bolus of 10 mL/kg only if there are signs of initial hypovolemia.
Second, that careful clinical and hemodynamic monitoring are essential during the first 72 hours after admission to make sure that patients remain euvolemic and to avoid fluid overload.
Third, that diuresis in patients with fluid overload in the first 72 hours is most likely beneficial and certainly not detrimental to important clinical outcomes.
Dr. Gardner said the trial also highlights the need to focus research efforts on evaluating other pharmacologic therapies instead of crystalloid fluids.
“Performing randomized controlled trials in acute pancreatitis is notoriously difficult, and the limited human and financial resources that are available for appropriately powered trials in this field post WATERFALL are much better spent on comparative-effectiveness and placebo-controlled trials evaluating new therapeutic agents,” Dr. Gardner said.
“Now that we have gone over the WATERFALL, it is time to look downstream at new targets to treat this challenging disease,” he concluded.
Support for the trial was provided by Instituto de Salud Carlos III, the Spanish Association of Gastroenterology, and ISABIAL (Instituto de Investigación Sanitaria y Biomédica de Alicante).
A version of this article first appeared on Medscape.com.
Early, aggressive fluid resuscitation in acute pancreatitis led to a higher incidence of fluid overload without improving clinical outcomes in the landmark WATERFALL trial.
Early aggressive hydration is widely recommended for the management of acute pancreatitis, but evidence for this practice is limited.
“The WATERFALL trial demonstrates that aggressive fluid resuscitation in acute pancreatitis is not safe, it is not associated with improved outcomes, and it should be abandoned,” Enrique de-Madaria, MD, PhD, with Hospital General Universitario Dr. Balmis, Alicante, Spain, told this news organization.
The trial settles a “new and clear reference for fluid resuscitation in this frequent disease: lactated Ringer’s solution 1.5 mL/kg per hour (preceded by a 10 mL/kg bolus over 2 hours only in case of hypovolemia),” added Dr. de-Madaria, president of the Spanish Association of Gastroenterology.
“This moderate fluid resuscitation strategy is associated with a much lower frequency of fluid overload and a trend toward improved outcomes. For such reasons, it should be considered as a new standard of care in the early management of acute pancreatitis,” Dr. de-Madaria said.
The WATERFALL trial results were published in the New England Journal of Medicine.
The results are “stunning and, given the carefully crafted trial methods, irrefutable,” Timothy Gardner, MD, with the section of gastroenterology and hepatology, Dartmouth–Hitchcock Medical Center, Lebanon, N.H., wrote in a linked editorial.
Trial details
The trial was conducted at 18 centers across India, Italy, Mexico, and Spain. Patients who presented with acute pancreatitis were randomly allocated to aggressive or moderate resuscitation with lactated Ringer’s solution.
Aggressive fluid resuscitation consisted of a bolus of 20 mL/kg of body weight, followed by 3 mL/kg per hour. Moderate fluid resuscitation consisted of a bolus of 10 mL/kg in patients with hypovolemia or no bolus in patients with normovolemia, followed by 1.5 mL/kg per hour in all patients in this group.
Patients were assessed at 12, 24, 48, and 72 hours, and fluid resuscitation was adjusted according to clinical status.
A total of 249 patients were included in the interim analysis – 122 in the aggressive-resuscitation group and 127 in the moderate-resuscitation group.
The data and safety monitoring board terminated the trial at the first interim safety analysis as a result of the development of fluid overload in 20.5% of the patients in the aggressive-resuscitation group versus 6.3% of those in the moderate-resuscitation group (adjusted relative risk, 2.85; 95% confidence interval, 1.36-5.94; P = .004).
“An increased risk of fluid overload was detected in the overall population of patients and also in subgroups of patients without systemic inflammatory response syndrome at baseline, patients with SIRS at baseline (thus, with a higher risk of development of severe pancreatitis), and patients with hypovolemia,” the investigators reported.
This clear signal of harm was coupled with no significant difference in the incidence of moderately severe or severe pancreatitis (22.1% in the aggressive-resuscitation group and 17.3% in the moderate-resuscitation group; aRR, 1.30; 95% CI, 0.78-2.18; P = .32).
Patients in the aggressive-resuscitation group spent a median of 6 days in the hospital, compared with 5 days for patients in the moderate-resuscitation group.
“These findings do not support current management guidelines, which recommend early aggressive resuscitation for the treatment of acute pancreatitis,” the study team wrote.
‘Landmark’ trial
This is a “landmark” trial and “so clinically relevant because of its choice of real world-appropriate aggressive-resuscitation and moderate-resuscitation treatment groups, its use of pancreatitis severity as the main clinical outcome, and its reliance on the carefully defined variable of fluid overload as the main safety outcome,” Dr. Gardner wrote in his editorial.
“Unlike in most other randomized, controlled trials of fluid resuscitation in acute pancreatitis, patients with varying baseline pancreatitis severity were included, and changes in the rate of resuscitation were determined on the basis of a dynamic assessment of hemodynamic testing, imaging, and clinical factors,” he added.
Dr. Gardner said the WATERFALL trial results lead to several conclusions.
First, the need to focus on a steady rate of initial resuscitation – no more than 1.5 mL/kg of body weight per hour. Clinicians should administer a bolus of 10 mL/kg only if there are signs of initial hypovolemia.
Second, that careful clinical and hemodynamic monitoring are essential during the first 72 hours after admission to make sure that patients remain euvolemic and to avoid fluid overload.
Third, that diuresis in patients with fluid overload in the first 72 hours is most likely beneficial and certainly not detrimental to important clinical outcomes.
Dr. Gardner said the trial also highlights the need to focus research efforts on evaluating other pharmacologic therapies instead of crystalloid fluids.
“Performing randomized controlled trials in acute pancreatitis is notoriously difficult, and the limited human and financial resources that are available for appropriately powered trials in this field post WATERFALL are much better spent on comparative-effectiveness and placebo-controlled trials evaluating new therapeutic agents,” Dr. Gardner said.
“Now that we have gone over the WATERFALL, it is time to look downstream at new targets to treat this challenging disease,” he concluded.
Support for the trial was provided by Instituto de Salud Carlos III, the Spanish Association of Gastroenterology, and ISABIAL (Instituto de Investigación Sanitaria y Biomédica de Alicante).
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
AGA Clinical Practice Update: Expert review on endoscopic management for recurrent acute and chronic pancreatitis
Endoscopy plays an integral role in the evaluation and management of patients with recurrent acute pancreatitis and chronic pancreatitis, according to a new American Gastroenterological Association clinical practice update published in Gastroenterology.
Acute pancreatitis remains the leading cause of inpatient care among gastrointestinal conditions, with about 10%-30% of patients developing recurrent acute pancreatitis, wrote co–first authors Daniel Strand, MD, from the University of Virginia Health System, Charlottesville, and Ryan J. Law, MD, from the Mayo Clinic, Rochester, Minn., and colleagues. About 35% of patients with recurrent acute pancreatitis will progress to chronic pancreatitis. Both conditions are associated with significant morbidity and mortality.
“Interventions aimed to better evaluate, mitigate the progression of, and treat symptoms related to [acute pancreatitis] and [chronic pancreatitis] are critical to improve patients’ quality of life and other long-term outcomes,” the authors of the expert review wrote.
The authors reviewed randomized controlled trials, observational studies, systematic reviews and meta-analyses, and expert consensus in the field to develop eight clinical practice advice statements.
First, when the initial evaluation reveals no clear explanation for acute or recurrent pancreatitis, endoscopic ultrasound is the preferred diagnostic test. The authors noted that, although there isn’t a concretely defined optimal timing for EUS defined, most experts advise a short delay of 2-6 weeks after resolution of acute pancreatitis. MRI with contrast and cholangiopancreatography can be a reasonable complementary or alternative test, based on local expertise and availability.
Second, the role of ERCP remains controversial for reducing the frequency of acute pancreatitis episodes in patients with pancreas divisum, the most common congenital pancreatic anomaly, the authors wrote. However, minor papilla endotherapy may be useful, particularly for those with objective signs of outflow obstruction, such as a dilated dorsal pancreatic duct or santorinicele. However, there is no role for ERCP in treating pain alone in patients with pancreas divisum.
Third, ERCP remains even more controversial for reducing the frequency of pancreatitis episodes in patients with unexplained recurrent acute pancreatitis and standard pancreatic ductal anatomy, according to the authors. It should only be considered after a comprehensive discussion of the uncertain benefits and potentially severe procedure-related adverse events. When used, ERCP with biliary sphincterotomy alone may be preferable to dual sphincterotomy.
Fourth, for long-term treatment of patients with painful obstructive chronic pancreatitis, surgical intervention should be considered over endoscopic therapy, the study authors wrote. Pain is the most common symptom and important driver of impaired quality of life in patients with chronic pancreatitis, among whom a subset will be affected by intraductal hypertension from an obstructed pancreatic duct. The authors noted that endoscopic intervention remains a reasonable alternative to surgery for suboptimal operative candidates or patients who want a less-invasive approach, as long as they are clearly informed that the best practice advice primarily favors surgery.
Fifth, when using ERCP for pancreatic duct stones, small main pancreatic duct stones of 5 mm or less can be treated with pancreatography and conventional stone extraction maneuvers. For larger stones, however, extracorporeal shockwave lithotripsy or pancreatoscopy with intraductal lithotripsy can be considered, although the former is not widely available in the United States and the success rates for the latter vary.
Sixth, when using ERCP for pancreatic duct strictures, prolonged stent therapy for 6-12 months is effective for treating symptoms and remodeling main pancreatic duct strictures. The preferred approach is to place and sequentially add multiple plastic stents in parallel, or up-sizing. Emerging evidence suggests that fully covered self-expanding metal stents may be useful in this case, but additional research is needed. For example, one study suggested that patients treated with these self-expanding stents required fewer ERCPs, but their adverse event rate was significantly higher (39% vs. 14%).
Seventh, ERCP with stent insertion is the preferred treatment for benign biliary stricture caused by chronic pancreatitis. Fully covered self-expanding metal stents are favored over placing multiple plastic stents when feasible, given the similar efficacy but significantly lower need for stent exchange procedures during the treatment course.
Eighth, celiac plexus block shouldn’t be routinely performed for the management of pain caused by chronic pancreatitis. Celiac plexus block could be considered in certain patients on a case-by-case basis if they have debilitating pain that hasn’t responded to other therapeutic measures. However, this should only be considered after a discussion about the unclear outcomes and its procedural risks.
“Given the current lack of evidence, additional well-designed prospective comparative studies are needed to support a more unified diagnostic and therapeutic pathway for the treatment of these complex cases,” the authors concluded.
The authors reported no grant support or funding sources for this report. Several authors disclosed financial relationships with companies such as Olympus America, Medtronic, and Microtech.
Endoscopy plays an integral role in the evaluation and management of patients with recurrent acute pancreatitis and chronic pancreatitis, according to a new American Gastroenterological Association clinical practice update published in Gastroenterology.
Acute pancreatitis remains the leading cause of inpatient care among gastrointestinal conditions, with about 10%-30% of patients developing recurrent acute pancreatitis, wrote co–first authors Daniel Strand, MD, from the University of Virginia Health System, Charlottesville, and Ryan J. Law, MD, from the Mayo Clinic, Rochester, Minn., and colleagues. About 35% of patients with recurrent acute pancreatitis will progress to chronic pancreatitis. Both conditions are associated with significant morbidity and mortality.
“Interventions aimed to better evaluate, mitigate the progression of, and treat symptoms related to [acute pancreatitis] and [chronic pancreatitis] are critical to improve patients’ quality of life and other long-term outcomes,” the authors of the expert review wrote.
The authors reviewed randomized controlled trials, observational studies, systematic reviews and meta-analyses, and expert consensus in the field to develop eight clinical practice advice statements.
First, when the initial evaluation reveals no clear explanation for acute or recurrent pancreatitis, endoscopic ultrasound is the preferred diagnostic test. The authors noted that, although there isn’t a concretely defined optimal timing for EUS defined, most experts advise a short delay of 2-6 weeks after resolution of acute pancreatitis. MRI with contrast and cholangiopancreatography can be a reasonable complementary or alternative test, based on local expertise and availability.
Second, the role of ERCP remains controversial for reducing the frequency of acute pancreatitis episodes in patients with pancreas divisum, the most common congenital pancreatic anomaly, the authors wrote. However, minor papilla endotherapy may be useful, particularly for those with objective signs of outflow obstruction, such as a dilated dorsal pancreatic duct or santorinicele. However, there is no role for ERCP in treating pain alone in patients with pancreas divisum.
Third, ERCP remains even more controversial for reducing the frequency of pancreatitis episodes in patients with unexplained recurrent acute pancreatitis and standard pancreatic ductal anatomy, according to the authors. It should only be considered after a comprehensive discussion of the uncertain benefits and potentially severe procedure-related adverse events. When used, ERCP with biliary sphincterotomy alone may be preferable to dual sphincterotomy.
Fourth, for long-term treatment of patients with painful obstructive chronic pancreatitis, surgical intervention should be considered over endoscopic therapy, the study authors wrote. Pain is the most common symptom and important driver of impaired quality of life in patients with chronic pancreatitis, among whom a subset will be affected by intraductal hypertension from an obstructed pancreatic duct. The authors noted that endoscopic intervention remains a reasonable alternative to surgery for suboptimal operative candidates or patients who want a less-invasive approach, as long as they are clearly informed that the best practice advice primarily favors surgery.
Fifth, when using ERCP for pancreatic duct stones, small main pancreatic duct stones of 5 mm or less can be treated with pancreatography and conventional stone extraction maneuvers. For larger stones, however, extracorporeal shockwave lithotripsy or pancreatoscopy with intraductal lithotripsy can be considered, although the former is not widely available in the United States and the success rates for the latter vary.
Sixth, when using ERCP for pancreatic duct strictures, prolonged stent therapy for 6-12 months is effective for treating symptoms and remodeling main pancreatic duct strictures. The preferred approach is to place and sequentially add multiple plastic stents in parallel, or up-sizing. Emerging evidence suggests that fully covered self-expanding metal stents may be useful in this case, but additional research is needed. For example, one study suggested that patients treated with these self-expanding stents required fewer ERCPs, but their adverse event rate was significantly higher (39% vs. 14%).
Seventh, ERCP with stent insertion is the preferred treatment for benign biliary stricture caused by chronic pancreatitis. Fully covered self-expanding metal stents are favored over placing multiple plastic stents when feasible, given the similar efficacy but significantly lower need for stent exchange procedures during the treatment course.
Eighth, celiac plexus block shouldn’t be routinely performed for the management of pain caused by chronic pancreatitis. Celiac plexus block could be considered in certain patients on a case-by-case basis if they have debilitating pain that hasn’t responded to other therapeutic measures. However, this should only be considered after a discussion about the unclear outcomes and its procedural risks.
“Given the current lack of evidence, additional well-designed prospective comparative studies are needed to support a more unified diagnostic and therapeutic pathway for the treatment of these complex cases,” the authors concluded.
The authors reported no grant support or funding sources for this report. Several authors disclosed financial relationships with companies such as Olympus America, Medtronic, and Microtech.
Endoscopy plays an integral role in the evaluation and management of patients with recurrent acute pancreatitis and chronic pancreatitis, according to a new American Gastroenterological Association clinical practice update published in Gastroenterology.
Acute pancreatitis remains the leading cause of inpatient care among gastrointestinal conditions, with about 10%-30% of patients developing recurrent acute pancreatitis, wrote co–first authors Daniel Strand, MD, from the University of Virginia Health System, Charlottesville, and Ryan J. Law, MD, from the Mayo Clinic, Rochester, Minn., and colleagues. About 35% of patients with recurrent acute pancreatitis will progress to chronic pancreatitis. Both conditions are associated with significant morbidity and mortality.
“Interventions aimed to better evaluate, mitigate the progression of, and treat symptoms related to [acute pancreatitis] and [chronic pancreatitis] are critical to improve patients’ quality of life and other long-term outcomes,” the authors of the expert review wrote.
The authors reviewed randomized controlled trials, observational studies, systematic reviews and meta-analyses, and expert consensus in the field to develop eight clinical practice advice statements.
First, when the initial evaluation reveals no clear explanation for acute or recurrent pancreatitis, endoscopic ultrasound is the preferred diagnostic test. The authors noted that, although there isn’t a concretely defined optimal timing for EUS defined, most experts advise a short delay of 2-6 weeks after resolution of acute pancreatitis. MRI with contrast and cholangiopancreatography can be a reasonable complementary or alternative test, based on local expertise and availability.
Second, the role of ERCP remains controversial for reducing the frequency of acute pancreatitis episodes in patients with pancreas divisum, the most common congenital pancreatic anomaly, the authors wrote. However, minor papilla endotherapy may be useful, particularly for those with objective signs of outflow obstruction, such as a dilated dorsal pancreatic duct or santorinicele. However, there is no role for ERCP in treating pain alone in patients with pancreas divisum.
Third, ERCP remains even more controversial for reducing the frequency of pancreatitis episodes in patients with unexplained recurrent acute pancreatitis and standard pancreatic ductal anatomy, according to the authors. It should only be considered after a comprehensive discussion of the uncertain benefits and potentially severe procedure-related adverse events. When used, ERCP with biliary sphincterotomy alone may be preferable to dual sphincterotomy.
Fourth, for long-term treatment of patients with painful obstructive chronic pancreatitis, surgical intervention should be considered over endoscopic therapy, the study authors wrote. Pain is the most common symptom and important driver of impaired quality of life in patients with chronic pancreatitis, among whom a subset will be affected by intraductal hypertension from an obstructed pancreatic duct. The authors noted that endoscopic intervention remains a reasonable alternative to surgery for suboptimal operative candidates or patients who want a less-invasive approach, as long as they are clearly informed that the best practice advice primarily favors surgery.
Fifth, when using ERCP for pancreatic duct stones, small main pancreatic duct stones of 5 mm or less can be treated with pancreatography and conventional stone extraction maneuvers. For larger stones, however, extracorporeal shockwave lithotripsy or pancreatoscopy with intraductal lithotripsy can be considered, although the former is not widely available in the United States and the success rates for the latter vary.
Sixth, when using ERCP for pancreatic duct strictures, prolonged stent therapy for 6-12 months is effective for treating symptoms and remodeling main pancreatic duct strictures. The preferred approach is to place and sequentially add multiple plastic stents in parallel, or up-sizing. Emerging evidence suggests that fully covered self-expanding metal stents may be useful in this case, but additional research is needed. For example, one study suggested that patients treated with these self-expanding stents required fewer ERCPs, but their adverse event rate was significantly higher (39% vs. 14%).
Seventh, ERCP with stent insertion is the preferred treatment for benign biliary stricture caused by chronic pancreatitis. Fully covered self-expanding metal stents are favored over placing multiple plastic stents when feasible, given the similar efficacy but significantly lower need for stent exchange procedures during the treatment course.
Eighth, celiac plexus block shouldn’t be routinely performed for the management of pain caused by chronic pancreatitis. Celiac plexus block could be considered in certain patients on a case-by-case basis if they have debilitating pain that hasn’t responded to other therapeutic measures. However, this should only be considered after a discussion about the unclear outcomes and its procedural risks.
“Given the current lack of evidence, additional well-designed prospective comparative studies are needed to support a more unified diagnostic and therapeutic pathway for the treatment of these complex cases,” the authors concluded.
The authors reported no grant support or funding sources for this report. Several authors disclosed financial relationships with companies such as Olympus America, Medtronic, and Microtech.
FROM GASTROENTEROLOGY
Fine-needle aspiration alternative allows closer look at pancreatic cystic lesions
Endoscopic ultrasound (EUS)–guided through-the-needle biopsies (TTNBs) of pancreatic cystic lesions are sufficient for accurate molecular analysis, which offers a superior alternative to cyst fluid obtained via fine-needle aspiration, based on a prospective study.
For highest diagnostic clarity, next-generation sequencing (NGS) of TTNBs can be paired with histology, lead author Charlotte Vestrup Rift, MD, PhD, of Copenhagen University Hospital, and colleagues reported.
“The diagnostic algorithm for the management of [pancreatic cystic lesions] includes endoscopic ultrasound examination with aspiration of cyst fluid for cytology,” the investigators wrote in Gastrointestinal Endoscopy. “However, the reported sensitivity of cytology is low [at 54%]. A new microforceps, introduced through a 19-gauge needle, has proven useful for procurement of [TTNBs] that represent both the epithelial and stromal component of the cyst wall. TTNBs have a high sensitivity of 86% for the diagnosis of mucinous cysts.”
Dr. Rift and colleagues evaluated the impact of introducing NGS to the diagnostic process. They noted that concomitant mutations in GNAS and KRAS are diagnostic for intraductal papillary mucinous neoplasms (IPMNs), while other mutations have been linked with progression to cancer.
The study involved 101 patients with pancreatic cystic lesions larger than 15 mm in diameter, mean age of 68 years, among whom 91 had residual TTNBs available after microscopic analysis. These samples underwent a 51-gene NGS panel that included the “most prevalent hot-spot mutations.” Diagnoses were sorted into four categories: neoplastic cyst, mucinous cyst, IPMN, or serous cystic neoplasm.
The primary endpoint was diagnostic yield, both for molecular analysis of TTNBs and for molecular analysis plus histopathology of TTNBs. Sensitivity and specificity of NGS were also determined using histopathology as the gold standard.
Relying on NGS alone, diagnostic yields were 44.5% and 27.7% for detecting a mucinous cyst and determining type of cyst, respectively. These yields rose to 73.3% and 70.3%, respectively, when NGS was used with microscopic evaluation. Continuing with this combined approach, sensitivity and specificity were 83.7% and 81.8%, respectively, for the diagnosis of a mucinous cyst. Sensitivity and specificity were higher still, at 87.2% and 84.6%, respectively, for identifying IPMNs.
The adverse-event rate was 9.9%, with a risk of postprocedure acute pancreatitis of 8.9 % and procedure-associated intracystic bleeding of 3%, according to the authors.
Limitations of the study include the relatively small sample size and the single-center design.
“TTNB-NGS is not sufficient as a stand-alone diagnostic tool as of yet but has a high diagnostic yield when combined with microscopic evaluation and subtyping by immunohistochemistry,” the investigators concluded. “The advantage of EUS-TTNB over EUS–[fine-needle aspiration] is the ability to perform detailed cyst subtyping and the high technical success rate of the procedure. ... However, the procedure comes with a risk of adverse events and thus should be offered to patients where the value of an exact diagnosis outweighs the risks.”
“Molecular subtyping is emerging as a useful clinical test for diagnosing pancreatic cysts,” said Margaret Geraldine Keane, MBBS, MSc, of Johns Hopkins Medicine, Baltimore, although she noted that NGS remains expensive and sporadically available, “which limits its clinical utility and incorporation into diagnostic algorithms for pancreatic cysts. In the future, as the cost of sequencing reduces, and availability improves, this may change.”
For now, Dr. Keane advised physicians to reserve molecular subtyping for cases in which “accurate cyst subtyping will change management ... or when other tests have not provided a clear diagnosis.”
She said the present study is valuable because better diagnostic tests are badly needed for patients with pancreatic cysts, considering the high rate of surgical overtreatment.
“Having more diagnostic tests, such as those described in this publication [to be used on their own or in combination] to decide which patients need surgery, is important,” Dr. Keane said who was not involved in the study.
Better diagnostic tests could also improve outcomes for patients with pancreatic cancer, she said, noting a 5-year survival rate of 10%.
“This outcome is in large part attributable to the late stage at which the majority of patients are diagnosed,” Dr. Keane said. “If patients can be diagnosed earlier, survival dramatically improves. Improvements in diagnostic tests for premalignant pancreatic cystic lesions are therefore vital.”
The study was supported by Rigshospitalets Research Foundation, The Novo Nordisk Foundation, The Danish Cancer Society, and others, although they did not have a role in conducting the study or preparing the manuscript. One investigator disclosed a relationship with MediGlobe. The other investigators reported no conflicts of interest. Dr. Keane disclosed no conflicts of interest.
Endoscopic ultrasound (EUS)–guided through-the-needle biopsies (TTNBs) of pancreatic cystic lesions are sufficient for accurate molecular analysis, which offers a superior alternative to cyst fluid obtained via fine-needle aspiration, based on a prospective study.
For highest diagnostic clarity, next-generation sequencing (NGS) of TTNBs can be paired with histology, lead author Charlotte Vestrup Rift, MD, PhD, of Copenhagen University Hospital, and colleagues reported.
“The diagnostic algorithm for the management of [pancreatic cystic lesions] includes endoscopic ultrasound examination with aspiration of cyst fluid for cytology,” the investigators wrote in Gastrointestinal Endoscopy. “However, the reported sensitivity of cytology is low [at 54%]. A new microforceps, introduced through a 19-gauge needle, has proven useful for procurement of [TTNBs] that represent both the epithelial and stromal component of the cyst wall. TTNBs have a high sensitivity of 86% for the diagnosis of mucinous cysts.”
Dr. Rift and colleagues evaluated the impact of introducing NGS to the diagnostic process. They noted that concomitant mutations in GNAS and KRAS are diagnostic for intraductal papillary mucinous neoplasms (IPMNs), while other mutations have been linked with progression to cancer.
The study involved 101 patients with pancreatic cystic lesions larger than 15 mm in diameter, mean age of 68 years, among whom 91 had residual TTNBs available after microscopic analysis. These samples underwent a 51-gene NGS panel that included the “most prevalent hot-spot mutations.” Diagnoses were sorted into four categories: neoplastic cyst, mucinous cyst, IPMN, or serous cystic neoplasm.
The primary endpoint was diagnostic yield, both for molecular analysis of TTNBs and for molecular analysis plus histopathology of TTNBs. Sensitivity and specificity of NGS were also determined using histopathology as the gold standard.
Relying on NGS alone, diagnostic yields were 44.5% and 27.7% for detecting a mucinous cyst and determining type of cyst, respectively. These yields rose to 73.3% and 70.3%, respectively, when NGS was used with microscopic evaluation. Continuing with this combined approach, sensitivity and specificity were 83.7% and 81.8%, respectively, for the diagnosis of a mucinous cyst. Sensitivity and specificity were higher still, at 87.2% and 84.6%, respectively, for identifying IPMNs.
The adverse-event rate was 9.9%, with a risk of postprocedure acute pancreatitis of 8.9 % and procedure-associated intracystic bleeding of 3%, according to the authors.
Limitations of the study include the relatively small sample size and the single-center design.
“TTNB-NGS is not sufficient as a stand-alone diagnostic tool as of yet but has a high diagnostic yield when combined with microscopic evaluation and subtyping by immunohistochemistry,” the investigators concluded. “The advantage of EUS-TTNB over EUS–[fine-needle aspiration] is the ability to perform detailed cyst subtyping and the high technical success rate of the procedure. ... However, the procedure comes with a risk of adverse events and thus should be offered to patients where the value of an exact diagnosis outweighs the risks.”
“Molecular subtyping is emerging as a useful clinical test for diagnosing pancreatic cysts,” said Margaret Geraldine Keane, MBBS, MSc, of Johns Hopkins Medicine, Baltimore, although she noted that NGS remains expensive and sporadically available, “which limits its clinical utility and incorporation into diagnostic algorithms for pancreatic cysts. In the future, as the cost of sequencing reduces, and availability improves, this may change.”
For now, Dr. Keane advised physicians to reserve molecular subtyping for cases in which “accurate cyst subtyping will change management ... or when other tests have not provided a clear diagnosis.”
She said the present study is valuable because better diagnostic tests are badly needed for patients with pancreatic cysts, considering the high rate of surgical overtreatment.
“Having more diagnostic tests, such as those described in this publication [to be used on their own or in combination] to decide which patients need surgery, is important,” Dr. Keane said who was not involved in the study.
Better diagnostic tests could also improve outcomes for patients with pancreatic cancer, she said, noting a 5-year survival rate of 10%.
“This outcome is in large part attributable to the late stage at which the majority of patients are diagnosed,” Dr. Keane said. “If patients can be diagnosed earlier, survival dramatically improves. Improvements in diagnostic tests for premalignant pancreatic cystic lesions are therefore vital.”
The study was supported by Rigshospitalets Research Foundation, The Novo Nordisk Foundation, The Danish Cancer Society, and others, although they did not have a role in conducting the study or preparing the manuscript. One investigator disclosed a relationship with MediGlobe. The other investigators reported no conflicts of interest. Dr. Keane disclosed no conflicts of interest.
Endoscopic ultrasound (EUS)–guided through-the-needle biopsies (TTNBs) of pancreatic cystic lesions are sufficient for accurate molecular analysis, which offers a superior alternative to cyst fluid obtained via fine-needle aspiration, based on a prospective study.
For highest diagnostic clarity, next-generation sequencing (NGS) of TTNBs can be paired with histology, lead author Charlotte Vestrup Rift, MD, PhD, of Copenhagen University Hospital, and colleagues reported.
“The diagnostic algorithm for the management of [pancreatic cystic lesions] includes endoscopic ultrasound examination with aspiration of cyst fluid for cytology,” the investigators wrote in Gastrointestinal Endoscopy. “However, the reported sensitivity of cytology is low [at 54%]. A new microforceps, introduced through a 19-gauge needle, has proven useful for procurement of [TTNBs] that represent both the epithelial and stromal component of the cyst wall. TTNBs have a high sensitivity of 86% for the diagnosis of mucinous cysts.”
Dr. Rift and colleagues evaluated the impact of introducing NGS to the diagnostic process. They noted that concomitant mutations in GNAS and KRAS are diagnostic for intraductal papillary mucinous neoplasms (IPMNs), while other mutations have been linked with progression to cancer.
The study involved 101 patients with pancreatic cystic lesions larger than 15 mm in diameter, mean age of 68 years, among whom 91 had residual TTNBs available after microscopic analysis. These samples underwent a 51-gene NGS panel that included the “most prevalent hot-spot mutations.” Diagnoses were sorted into four categories: neoplastic cyst, mucinous cyst, IPMN, or serous cystic neoplasm.
The primary endpoint was diagnostic yield, both for molecular analysis of TTNBs and for molecular analysis plus histopathology of TTNBs. Sensitivity and specificity of NGS were also determined using histopathology as the gold standard.
Relying on NGS alone, diagnostic yields were 44.5% and 27.7% for detecting a mucinous cyst and determining type of cyst, respectively. These yields rose to 73.3% and 70.3%, respectively, when NGS was used with microscopic evaluation. Continuing with this combined approach, sensitivity and specificity were 83.7% and 81.8%, respectively, for the diagnosis of a mucinous cyst. Sensitivity and specificity were higher still, at 87.2% and 84.6%, respectively, for identifying IPMNs.
The adverse-event rate was 9.9%, with a risk of postprocedure acute pancreatitis of 8.9 % and procedure-associated intracystic bleeding of 3%, according to the authors.
Limitations of the study include the relatively small sample size and the single-center design.
“TTNB-NGS is not sufficient as a stand-alone diagnostic tool as of yet but has a high diagnostic yield when combined with microscopic evaluation and subtyping by immunohistochemistry,” the investigators concluded. “The advantage of EUS-TTNB over EUS–[fine-needle aspiration] is the ability to perform detailed cyst subtyping and the high technical success rate of the procedure. ... However, the procedure comes with a risk of adverse events and thus should be offered to patients where the value of an exact diagnosis outweighs the risks.”
“Molecular subtyping is emerging as a useful clinical test for diagnosing pancreatic cysts,” said Margaret Geraldine Keane, MBBS, MSc, of Johns Hopkins Medicine, Baltimore, although she noted that NGS remains expensive and sporadically available, “which limits its clinical utility and incorporation into diagnostic algorithms for pancreatic cysts. In the future, as the cost of sequencing reduces, and availability improves, this may change.”
For now, Dr. Keane advised physicians to reserve molecular subtyping for cases in which “accurate cyst subtyping will change management ... or when other tests have not provided a clear diagnosis.”
She said the present study is valuable because better diagnostic tests are badly needed for patients with pancreatic cysts, considering the high rate of surgical overtreatment.
“Having more diagnostic tests, such as those described in this publication [to be used on their own or in combination] to decide which patients need surgery, is important,” Dr. Keane said who was not involved in the study.
Better diagnostic tests could also improve outcomes for patients with pancreatic cancer, she said, noting a 5-year survival rate of 10%.
“This outcome is in large part attributable to the late stage at which the majority of patients are diagnosed,” Dr. Keane said. “If patients can be diagnosed earlier, survival dramatically improves. Improvements in diagnostic tests for premalignant pancreatic cystic lesions are therefore vital.”
The study was supported by Rigshospitalets Research Foundation, The Novo Nordisk Foundation, The Danish Cancer Society, and others, although they did not have a role in conducting the study or preparing the manuscript. One investigator disclosed a relationship with MediGlobe. The other investigators reported no conflicts of interest. Dr. Keane disclosed no conflicts of interest.
FROM GASTROINTESTINAL ENDOSCOPY
Acute pancreatitis: Procalcitonin algorithm safely reduces antibiotic overuse
A procalcitonin-based algorithm could safely reduce unnecessary usage of antibiotics in patients with acute pancreatitis, based on results of a randomized controlled trial.
Physicians should consider incorporating the decision-making process into their daily practice, suggested lead author Ajith K. Siriwardena, MD, of Manchester (England) University and colleagues, who also recommended that the algorithm be added to future guidelines.
“Overuse of antibiotics and the resultant emergence of multidrug resistant microorganisms is a potent threat to the welfare of humanity in the 21st century,” the investigators wrote in The Lancet Gastroenterology & Hepatology.
Antibiotic overuse is common in cases of acute pancreatitis, they noted, because clinical features are typically insufficient to distinguish between inflammation and infection. While measuring procalcitonin can help can detect infection, “indiscriminate measurement” of the biomarker is not cost effective, according to the investigators, leading previous reviews and analyses to conclude that further research is needed before widespread usage can be recommended.
Dr. Siriwardena and colleagues aimed to meet this need by conducting a randomized controlled trial involving 260 patients hospitalized for acute pancreatitis at Manchester Royal Infirmary. Patients were randomized in a near 1:1 ratio. Both the intervention group (n = 132) and the control group (n = 128) received guideline-based care; however, in addition to standard of care, procalcitonin was measured in the intervention group at days 0, 4, and 7 then weekly. Among these patients, antibiotics were stopped or not started when procalcitonin was below 1.0 ng/mL, but antibiotics were started or continued when procalcitonin was 1.0 ng/mL or more.
The primary outcome was presence or absence of antibiotic use during hospital stay. A range of secondary outcomes were also reported, included all-cause mortality, days of antibiotic use, rates of infection, and endoscopic, radiological, or surgical intervention.
Significantly fewer patients in the procalcitonin group received antibiotics during their stay, compared with the usual-care group (45% vs. 63%), which translated to an adjusted risk difference of –15.6% (P = .0071). Patients in the procalcitonin group who did receive antibiotics received about 1 day less of antibiotic treatment.
Despite the reduced antibiotic usage, length of hospital stay was similar between groups, as were rates of clinical infection, hospital-acquired infection, death, and adverse events, which suggests that the algorithm safely reduced antibiotic usage without negatively impacting clinical outcomes, according to investigators.
“Procalcitonin-based algorithms to guide antibiotic use should be considered in the care of this group of patients and be incorporated into future guidelines on the management of acute pancreatitis,” the investigators concluded.
Aaron Sasson, MD, director of the pancreatic cancer center and codirector of the gastrointestinal oncology team at Stony Brook (N.Y.) Medicine, said the study is noteworthy because it addresses an important topic with a large prospective randomized trial; however, he pointed out some limitations.
“There are several issues with this trial,” Dr. Sasson said in a written comment. “First, it included a large percentage of patients with mild acute pancreatitis, a group of patients for whom the use of antibiotics is not controversial. Secondly, the rate of infected pancreatic necrosis was 5% in both arms of the study, indicating the lack of severity of the cohort of patients.”
Dr. Sasson said that the algorithm “could be useful” to differentiate between inflammation and infection in patients with acute pancreatitis, “but only as an adjunct with other clinical parameters.”
He suggested that the algorithm would offer more utility if it could distinguish between pancreatic necrosis and infected pancreatic necrosis. “Unfortunately, this trial did not answer this question,” he said, noting that a similar trial involving “only patients with severe pancreatitis” would be needed.
The investigators and Dr. Sasson disclosed no competing interests.
A procalcitonin-based algorithm could safely reduce unnecessary usage of antibiotics in patients with acute pancreatitis, based on results of a randomized controlled trial.
Physicians should consider incorporating the decision-making process into their daily practice, suggested lead author Ajith K. Siriwardena, MD, of Manchester (England) University and colleagues, who also recommended that the algorithm be added to future guidelines.
“Overuse of antibiotics and the resultant emergence of multidrug resistant microorganisms is a potent threat to the welfare of humanity in the 21st century,” the investigators wrote in The Lancet Gastroenterology & Hepatology.
Antibiotic overuse is common in cases of acute pancreatitis, they noted, because clinical features are typically insufficient to distinguish between inflammation and infection. While measuring procalcitonin can help can detect infection, “indiscriminate measurement” of the biomarker is not cost effective, according to the investigators, leading previous reviews and analyses to conclude that further research is needed before widespread usage can be recommended.
Dr. Siriwardena and colleagues aimed to meet this need by conducting a randomized controlled trial involving 260 patients hospitalized for acute pancreatitis at Manchester Royal Infirmary. Patients were randomized in a near 1:1 ratio. Both the intervention group (n = 132) and the control group (n = 128) received guideline-based care; however, in addition to standard of care, procalcitonin was measured in the intervention group at days 0, 4, and 7 then weekly. Among these patients, antibiotics were stopped or not started when procalcitonin was below 1.0 ng/mL, but antibiotics were started or continued when procalcitonin was 1.0 ng/mL or more.
The primary outcome was presence or absence of antibiotic use during hospital stay. A range of secondary outcomes were also reported, included all-cause mortality, days of antibiotic use, rates of infection, and endoscopic, radiological, or surgical intervention.
Significantly fewer patients in the procalcitonin group received antibiotics during their stay, compared with the usual-care group (45% vs. 63%), which translated to an adjusted risk difference of –15.6% (P = .0071). Patients in the procalcitonin group who did receive antibiotics received about 1 day less of antibiotic treatment.
Despite the reduced antibiotic usage, length of hospital stay was similar between groups, as were rates of clinical infection, hospital-acquired infection, death, and adverse events, which suggests that the algorithm safely reduced antibiotic usage without negatively impacting clinical outcomes, according to investigators.
“Procalcitonin-based algorithms to guide antibiotic use should be considered in the care of this group of patients and be incorporated into future guidelines on the management of acute pancreatitis,” the investigators concluded.
Aaron Sasson, MD, director of the pancreatic cancer center and codirector of the gastrointestinal oncology team at Stony Brook (N.Y.) Medicine, said the study is noteworthy because it addresses an important topic with a large prospective randomized trial; however, he pointed out some limitations.
“There are several issues with this trial,” Dr. Sasson said in a written comment. “First, it included a large percentage of patients with mild acute pancreatitis, a group of patients for whom the use of antibiotics is not controversial. Secondly, the rate of infected pancreatic necrosis was 5% in both arms of the study, indicating the lack of severity of the cohort of patients.”
Dr. Sasson said that the algorithm “could be useful” to differentiate between inflammation and infection in patients with acute pancreatitis, “but only as an adjunct with other clinical parameters.”
He suggested that the algorithm would offer more utility if it could distinguish between pancreatic necrosis and infected pancreatic necrosis. “Unfortunately, this trial did not answer this question,” he said, noting that a similar trial involving “only patients with severe pancreatitis” would be needed.
The investigators and Dr. Sasson disclosed no competing interests.
A procalcitonin-based algorithm could safely reduce unnecessary usage of antibiotics in patients with acute pancreatitis, based on results of a randomized controlled trial.
Physicians should consider incorporating the decision-making process into their daily practice, suggested lead author Ajith K. Siriwardena, MD, of Manchester (England) University and colleagues, who also recommended that the algorithm be added to future guidelines.
“Overuse of antibiotics and the resultant emergence of multidrug resistant microorganisms is a potent threat to the welfare of humanity in the 21st century,” the investigators wrote in The Lancet Gastroenterology & Hepatology.
Antibiotic overuse is common in cases of acute pancreatitis, they noted, because clinical features are typically insufficient to distinguish between inflammation and infection. While measuring procalcitonin can help can detect infection, “indiscriminate measurement” of the biomarker is not cost effective, according to the investigators, leading previous reviews and analyses to conclude that further research is needed before widespread usage can be recommended.
Dr. Siriwardena and colleagues aimed to meet this need by conducting a randomized controlled trial involving 260 patients hospitalized for acute pancreatitis at Manchester Royal Infirmary. Patients were randomized in a near 1:1 ratio. Both the intervention group (n = 132) and the control group (n = 128) received guideline-based care; however, in addition to standard of care, procalcitonin was measured in the intervention group at days 0, 4, and 7 then weekly. Among these patients, antibiotics were stopped or not started when procalcitonin was below 1.0 ng/mL, but antibiotics were started or continued when procalcitonin was 1.0 ng/mL or more.
The primary outcome was presence or absence of antibiotic use during hospital stay. A range of secondary outcomes were also reported, included all-cause mortality, days of antibiotic use, rates of infection, and endoscopic, radiological, or surgical intervention.
Significantly fewer patients in the procalcitonin group received antibiotics during their stay, compared with the usual-care group (45% vs. 63%), which translated to an adjusted risk difference of –15.6% (P = .0071). Patients in the procalcitonin group who did receive antibiotics received about 1 day less of antibiotic treatment.
Despite the reduced antibiotic usage, length of hospital stay was similar between groups, as were rates of clinical infection, hospital-acquired infection, death, and adverse events, which suggests that the algorithm safely reduced antibiotic usage without negatively impacting clinical outcomes, according to investigators.
“Procalcitonin-based algorithms to guide antibiotic use should be considered in the care of this group of patients and be incorporated into future guidelines on the management of acute pancreatitis,” the investigators concluded.
Aaron Sasson, MD, director of the pancreatic cancer center and codirector of the gastrointestinal oncology team at Stony Brook (N.Y.) Medicine, said the study is noteworthy because it addresses an important topic with a large prospective randomized trial; however, he pointed out some limitations.
“There are several issues with this trial,” Dr. Sasson said in a written comment. “First, it included a large percentage of patients with mild acute pancreatitis, a group of patients for whom the use of antibiotics is not controversial. Secondly, the rate of infected pancreatic necrosis was 5% in both arms of the study, indicating the lack of severity of the cohort of patients.”
Dr. Sasson said that the algorithm “could be useful” to differentiate between inflammation and infection in patients with acute pancreatitis, “but only as an adjunct with other clinical parameters.”
He suggested that the algorithm would offer more utility if it could distinguish between pancreatic necrosis and infected pancreatic necrosis. “Unfortunately, this trial did not answer this question,” he said, noting that a similar trial involving “only patients with severe pancreatitis” would be needed.
The investigators and Dr. Sasson disclosed no competing interests.
FROM THE LANCET GASTROENTEROLOGY & HEPATOLOGY
Index cholecystectomy reduces readmissions after acute cholangitis
SAN DIEGO – Patients with acute cholangitis are twice as likely to be readmitted within 30 days if they don’t get a cholecystectomy in the same hospital admission for which they get biliary decompression, researchers say.
The readmissions result mostly from sepsis and recurrence of the acute cholangitis, said Ahmad Khan, MD, MS, a gastroenterology fellow at Case Western Reserve University in Cleveland, at Digestive Diseases Week® (DDW) 2022. “These added readmissions can cause a significant burden in terms of costs and extra days of hospitalization in these patients.”
Acute cholangitis in patients without bile duct stents is most often caused by biliary calculi, benign biliary stricture, or malignancy. A gastrointestinal emergency, it requires treatment with biliary decompression followed by cholecystectomy, but the cholecystectomy is considered an elective procedure.
Surgeons may delay it if the patient is very sick, or simply for scheduling reasons, Dr. Khan said. “There are some areas where the surgeons may be too busy,” he said. Or if the patient first presents at the end of the week, some surgeons will send the patient home so they don’t have to operate on the weekend, he said.
To understand the consequences of these decisions, Dr. Khan and his colleagues analyzed data from 2016 to 2018 from the National Readmission Database of the U.S. Agency for Healthcare Research and Quality.
They found that 11% of patients who went home before returning for a cholecystectomy had to be readmitted versus only 5.5% of those who got a cholecystectomy during the same (index) admission as their biliary decompression.
Patients who got cholecystectomies during their index admissions were slightly younger and healthier: Their mean age was 67.29 years and 20.59% had three or more comorbidities at index admission versus 70.77 years of age and 39.80% with three or more comorbidities at index admission for those who got their cholecystectomies later.
The researchers did not find any significant differences in the hospitals’ characteristics, such as being urban or academic, between the two groups.
Mortality was higher for those who received their cholecystectomy after returning home, but they spent less time in the hospital at lower total cost. The differences in outcomes between the index admission and readmission were all statistically significant (P < .01).
This observational study could not determine cause and effect, but it justifies a prospective trial that could more definitely determine which approach results in better outcomes, Dr. Khan said.
That patients are less likely to need readmission if they return home without a gall bladder after treatment for acute cholangitis “makes sense,” said session comoderator Richard Sterling, MD, MSc, chief of hepatology at Virginia Commonwealth University in Richmond.
“Should you do it immediately or can you wait a day or 2? They didn’t really address when during that admission, so we still don’t know the optimal sequence of events.”
If a patient has so many comorbidities that the surgeon and anesthesiologist don’t think the patient could survive a cholecystectomy, then the surgeon might do a cholecystostomy instead, he said.
Dr. Khan said he hopes to delve deeper into the data to determine what factors might have influenced the surgeons’ decisions to delay the cholecystectomy. “I want to see, of the patients who did not get same-admission cholecystectomies, how many had diabetes, how many had coronary artery disease, how many were on blood thinners, and things like that.”
Neither Dr. Khan nor Dr. Sterling reported any relevant financial interests.
SAN DIEGO – Patients with acute cholangitis are twice as likely to be readmitted within 30 days if they don’t get a cholecystectomy in the same hospital admission for which they get biliary decompression, researchers say.
The readmissions result mostly from sepsis and recurrence of the acute cholangitis, said Ahmad Khan, MD, MS, a gastroenterology fellow at Case Western Reserve University in Cleveland, at Digestive Diseases Week® (DDW) 2022. “These added readmissions can cause a significant burden in terms of costs and extra days of hospitalization in these patients.”
Acute cholangitis in patients without bile duct stents is most often caused by biliary calculi, benign biliary stricture, or malignancy. A gastrointestinal emergency, it requires treatment with biliary decompression followed by cholecystectomy, but the cholecystectomy is considered an elective procedure.
Surgeons may delay it if the patient is very sick, or simply for scheduling reasons, Dr. Khan said. “There are some areas where the surgeons may be too busy,” he said. Or if the patient first presents at the end of the week, some surgeons will send the patient home so they don’t have to operate on the weekend, he said.
To understand the consequences of these decisions, Dr. Khan and his colleagues analyzed data from 2016 to 2018 from the National Readmission Database of the U.S. Agency for Healthcare Research and Quality.
They found that 11% of patients who went home before returning for a cholecystectomy had to be readmitted versus only 5.5% of those who got a cholecystectomy during the same (index) admission as their biliary decompression.
Patients who got cholecystectomies during their index admissions were slightly younger and healthier: Their mean age was 67.29 years and 20.59% had three or more comorbidities at index admission versus 70.77 years of age and 39.80% with three or more comorbidities at index admission for those who got their cholecystectomies later.
The researchers did not find any significant differences in the hospitals’ characteristics, such as being urban or academic, between the two groups.
Mortality was higher for those who received their cholecystectomy after returning home, but they spent less time in the hospital at lower total cost. The differences in outcomes between the index admission and readmission were all statistically significant (P < .01).
This observational study could not determine cause and effect, but it justifies a prospective trial that could more definitely determine which approach results in better outcomes, Dr. Khan said.
That patients are less likely to need readmission if they return home without a gall bladder after treatment for acute cholangitis “makes sense,” said session comoderator Richard Sterling, MD, MSc, chief of hepatology at Virginia Commonwealth University in Richmond.
“Should you do it immediately or can you wait a day or 2? They didn’t really address when during that admission, so we still don’t know the optimal sequence of events.”
If a patient has so many comorbidities that the surgeon and anesthesiologist don’t think the patient could survive a cholecystectomy, then the surgeon might do a cholecystostomy instead, he said.
Dr. Khan said he hopes to delve deeper into the data to determine what factors might have influenced the surgeons’ decisions to delay the cholecystectomy. “I want to see, of the patients who did not get same-admission cholecystectomies, how many had diabetes, how many had coronary artery disease, how many were on blood thinners, and things like that.”
Neither Dr. Khan nor Dr. Sterling reported any relevant financial interests.
SAN DIEGO – Patients with acute cholangitis are twice as likely to be readmitted within 30 days if they don’t get a cholecystectomy in the same hospital admission for which they get biliary decompression, researchers say.
The readmissions result mostly from sepsis and recurrence of the acute cholangitis, said Ahmad Khan, MD, MS, a gastroenterology fellow at Case Western Reserve University in Cleveland, at Digestive Diseases Week® (DDW) 2022. “These added readmissions can cause a significant burden in terms of costs and extra days of hospitalization in these patients.”
Acute cholangitis in patients without bile duct stents is most often caused by biliary calculi, benign biliary stricture, or malignancy. A gastrointestinal emergency, it requires treatment with biliary decompression followed by cholecystectomy, but the cholecystectomy is considered an elective procedure.
Surgeons may delay it if the patient is very sick, or simply for scheduling reasons, Dr. Khan said. “There are some areas where the surgeons may be too busy,” he said. Or if the patient first presents at the end of the week, some surgeons will send the patient home so they don’t have to operate on the weekend, he said.
To understand the consequences of these decisions, Dr. Khan and his colleagues analyzed data from 2016 to 2018 from the National Readmission Database of the U.S. Agency for Healthcare Research and Quality.
They found that 11% of patients who went home before returning for a cholecystectomy had to be readmitted versus only 5.5% of those who got a cholecystectomy during the same (index) admission as their biliary decompression.
Patients who got cholecystectomies during their index admissions were slightly younger and healthier: Their mean age was 67.29 years and 20.59% had three or more comorbidities at index admission versus 70.77 years of age and 39.80% with three or more comorbidities at index admission for those who got their cholecystectomies later.
The researchers did not find any significant differences in the hospitals’ characteristics, such as being urban or academic, between the two groups.
Mortality was higher for those who received their cholecystectomy after returning home, but they spent less time in the hospital at lower total cost. The differences in outcomes between the index admission and readmission were all statistically significant (P < .01).
This observational study could not determine cause and effect, but it justifies a prospective trial that could more definitely determine which approach results in better outcomes, Dr. Khan said.
That patients are less likely to need readmission if they return home without a gall bladder after treatment for acute cholangitis “makes sense,” said session comoderator Richard Sterling, MD, MSc, chief of hepatology at Virginia Commonwealth University in Richmond.
“Should you do it immediately or can you wait a day or 2? They didn’t really address when during that admission, so we still don’t know the optimal sequence of events.”
If a patient has so many comorbidities that the surgeon and anesthesiologist don’t think the patient could survive a cholecystectomy, then the surgeon might do a cholecystostomy instead, he said.
Dr. Khan said he hopes to delve deeper into the data to determine what factors might have influenced the surgeons’ decisions to delay the cholecystectomy. “I want to see, of the patients who did not get same-admission cholecystectomies, how many had diabetes, how many had coronary artery disease, how many were on blood thinners, and things like that.”
Neither Dr. Khan nor Dr. Sterling reported any relevant financial interests.
AT DDW 2022
Obesity interactions complex in acute pancreatitis
Obesity, in combination with other risk factors, is associated with increased morbidity and mortality in acute pancreatitis (AP); however, body mass index (BMI) alone is not a successful predictor of disease severity, new research shows.
“As there was no agreement or consistency between BMI and AP severity, it can be concluded that AP severity cannot be predicted successfully by examining BMI only,” reported the authors in research published recently in Pancreatology.
The course of acute pancreatitis is typically mild in the majority (80%-85%) of cases; however, in severe cases, permanent organ failure can occur, with much worse outcomes and mortality rates of up to 35%.
Research has previously shown not only a link between obesity and acute pancreatitis but also an increased risk for complications and in-hospital mortality in obese patients with severe cases of acute pancreatitis – though a wide range of factors and comorbidities may complicate the association.
To more closely evaluate the course and outcomes of acute pancreatitis based on BMI classification, study authors led by Ali Tuzun Ince, MD, of the department of internal medicine, Gastroenterology Clinic of Bezmialem Vakif University, Istanbul, analyzed retrospective data from 2010 to 2020 on 1,334 adult patients (720 female, 614 male) who were diagnosed with acute pancreatitis per the Revised Atlanta Classification (RAC) criteria.
The patients were stratified based on their BMI as normal weight, overweight, or obese and whether they had mild, moderate, or severe (with permanent organ failure) acute pancreatitis.
In terms of acute pancreatitis severity, based on RAC criteria, 57.1% of patients had mild disease, 20.4% had moderate disease, and 22.5% had severe disease.
The overall mortality rate was 9.9% (n = 132); half of these patients were obese, and 87% had severe acute pancreatitis.
The overall rate of complications was 42.9%, including 20.8% in the normal weight group, 40.6% in the overweight group, and 38.6% in the obese group.
Patients in the overweight and obese groups also had higher mortality rates (3.7% and 4.9%, respectively), interventional procedures (36% and 39%, respectively), and length of hospital stay (11.6% and 9.8%, respectively), compared with the normal-weight group.
Other factors that were significantly associated with an increased mortality risk, in addition to obesity (P = .046), included old age (P = .000), male sex (P = .05), alcohol use (P = .014), low hematocrit (P = .044), high C-reactive protein (P = .024), moderate to severe and severe acute pancreatitis (P = .02 and P < .001, respectively), and any complications (P < .001).
Risk factors associated with increased admission to the ICU differed from those for mortality, and included female gender (P = .024), smoking (P = .021), hypertriglyceridemia (P = .047), idiopathic etiology (P = .023), and moderate to severe and severe acute pancreatitis (P < .001).
Of note, there were no significant associations between BMI and either the RAC score or Balthazar CT severity index (Balthazar CTSI) groups.
Specifically, among patients considered to have severe acute pancreatitis per Balthazar CTSI, 6.3% were of normal weight, 5% were overweight, and 7.1% were obese.
“In addition, since agreement and consistency between BMI and Balthazar score cannot be determined, the Balthazar score cannot be estimated from BMI,” the authors reported.
While the prediction of prognosis in acute pancreatitis is gaining interest, the findings underscore the role of combined factors, they added.
“Although many scoring systems are currently in use attempt to estimate the severity [in acute pancreatitis], none is 100% accurate yet,” the authors noted. “Each risk factor exacerbates the course of disease. Therefore, it would be better to consider the combined effects of risk factors.”
That being said, the findings show “mortality is increased significantly by the combined presence of risk factors such as male sex, OB [obesity], alcohol, MSAP [moderate to severe acute pancreatitis] and SAP [severe acute pancreatitis], all kinds of complications, old age, low Hct, and high CRP,” they wrote.
Obesity’s complex interactions
Commenting on the study, Vijay P. Singh, MD, a professor of medicine in the division of gastroenterology and hepatology at the Mayo Clinic in Scottsdale, Ariz., agreed that the complexities risk factors, particularly with obesity, can be tricky to detangle.
“Broadly, the study confirms several previous reports from different parts of the world that obesity was associated with increased mortality in acute pancreatitis,” he said in an interview.
“However, obesity had two complex interactions, the first that obesity is also associated with increased diabetes, and hypertriglyceridemia, which may themselves be risk factors for severity,” he explained.
“The second one is that intermediary severity markers [e.g., Balthazar score on imaging] did not correlate with the BMI categories.”
Dr. Singh noted that is “likely because therapies like IV fluids that may get more intense in predicted severe disease alter the natural course of pancreatitis.”
The findings are a reminder that “BMI is only a number that attempts to quantify fat,” Dr. Singh said, noting that BMI doesn’t address either the location of fat, such as being in close proximity to the pancreas, or fat composition, such as the proportion of unsaturated fat.
“When the unsaturated fat proportion is higher, the pancreatitis is worse, even at smaller total fat amounts [for example, at a lower BMI],” he said. “Taking these aspects into account may help in risk assessment.”
The authors and Dr. Singh had no disclosures to report.
Obesity, in combination with other risk factors, is associated with increased morbidity and mortality in acute pancreatitis (AP); however, body mass index (BMI) alone is not a successful predictor of disease severity, new research shows.
“As there was no agreement or consistency between BMI and AP severity, it can be concluded that AP severity cannot be predicted successfully by examining BMI only,” reported the authors in research published recently in Pancreatology.
The course of acute pancreatitis is typically mild in the majority (80%-85%) of cases; however, in severe cases, permanent organ failure can occur, with much worse outcomes and mortality rates of up to 35%.
Research has previously shown not only a link between obesity and acute pancreatitis but also an increased risk for complications and in-hospital mortality in obese patients with severe cases of acute pancreatitis – though a wide range of factors and comorbidities may complicate the association.
To more closely evaluate the course and outcomes of acute pancreatitis based on BMI classification, study authors led by Ali Tuzun Ince, MD, of the department of internal medicine, Gastroenterology Clinic of Bezmialem Vakif University, Istanbul, analyzed retrospective data from 2010 to 2020 on 1,334 adult patients (720 female, 614 male) who were diagnosed with acute pancreatitis per the Revised Atlanta Classification (RAC) criteria.
The patients were stratified based on their BMI as normal weight, overweight, or obese and whether they had mild, moderate, or severe (with permanent organ failure) acute pancreatitis.
In terms of acute pancreatitis severity, based on RAC criteria, 57.1% of patients had mild disease, 20.4% had moderate disease, and 22.5% had severe disease.
The overall mortality rate was 9.9% (n = 132); half of these patients were obese, and 87% had severe acute pancreatitis.
The overall rate of complications was 42.9%, including 20.8% in the normal weight group, 40.6% in the overweight group, and 38.6% in the obese group.
Patients in the overweight and obese groups also had higher mortality rates (3.7% and 4.9%, respectively), interventional procedures (36% and 39%, respectively), and length of hospital stay (11.6% and 9.8%, respectively), compared with the normal-weight group.
Other factors that were significantly associated with an increased mortality risk, in addition to obesity (P = .046), included old age (P = .000), male sex (P = .05), alcohol use (P = .014), low hematocrit (P = .044), high C-reactive protein (P = .024), moderate to severe and severe acute pancreatitis (P = .02 and P < .001, respectively), and any complications (P < .001).
Risk factors associated with increased admission to the ICU differed from those for mortality, and included female gender (P = .024), smoking (P = .021), hypertriglyceridemia (P = .047), idiopathic etiology (P = .023), and moderate to severe and severe acute pancreatitis (P < .001).
Of note, there were no significant associations between BMI and either the RAC score or Balthazar CT severity index (Balthazar CTSI) groups.
Specifically, among patients considered to have severe acute pancreatitis per Balthazar CTSI, 6.3% were of normal weight, 5% were overweight, and 7.1% were obese.
“In addition, since agreement and consistency between BMI and Balthazar score cannot be determined, the Balthazar score cannot be estimated from BMI,” the authors reported.
While the prediction of prognosis in acute pancreatitis is gaining interest, the findings underscore the role of combined factors, they added.
“Although many scoring systems are currently in use attempt to estimate the severity [in acute pancreatitis], none is 100% accurate yet,” the authors noted. “Each risk factor exacerbates the course of disease. Therefore, it would be better to consider the combined effects of risk factors.”
That being said, the findings show “mortality is increased significantly by the combined presence of risk factors such as male sex, OB [obesity], alcohol, MSAP [moderate to severe acute pancreatitis] and SAP [severe acute pancreatitis], all kinds of complications, old age, low Hct, and high CRP,” they wrote.
Obesity’s complex interactions
Commenting on the study, Vijay P. Singh, MD, a professor of medicine in the division of gastroenterology and hepatology at the Mayo Clinic in Scottsdale, Ariz., agreed that the complexities risk factors, particularly with obesity, can be tricky to detangle.
“Broadly, the study confirms several previous reports from different parts of the world that obesity was associated with increased mortality in acute pancreatitis,” he said in an interview.
“However, obesity had two complex interactions, the first that obesity is also associated with increased diabetes, and hypertriglyceridemia, which may themselves be risk factors for severity,” he explained.
“The second one is that intermediary severity markers [e.g., Balthazar score on imaging] did not correlate with the BMI categories.”
Dr. Singh noted that is “likely because therapies like IV fluids that may get more intense in predicted severe disease alter the natural course of pancreatitis.”
The findings are a reminder that “BMI is only a number that attempts to quantify fat,” Dr. Singh said, noting that BMI doesn’t address either the location of fat, such as being in close proximity to the pancreas, or fat composition, such as the proportion of unsaturated fat.
“When the unsaturated fat proportion is higher, the pancreatitis is worse, even at smaller total fat amounts [for example, at a lower BMI],” he said. “Taking these aspects into account may help in risk assessment.”
The authors and Dr. Singh had no disclosures to report.
Obesity, in combination with other risk factors, is associated with increased morbidity and mortality in acute pancreatitis (AP); however, body mass index (BMI) alone is not a successful predictor of disease severity, new research shows.
“As there was no agreement or consistency between BMI and AP severity, it can be concluded that AP severity cannot be predicted successfully by examining BMI only,” reported the authors in research published recently in Pancreatology.
The course of acute pancreatitis is typically mild in the majority (80%-85%) of cases; however, in severe cases, permanent organ failure can occur, with much worse outcomes and mortality rates of up to 35%.
Research has previously shown not only a link between obesity and acute pancreatitis but also an increased risk for complications and in-hospital mortality in obese patients with severe cases of acute pancreatitis – though a wide range of factors and comorbidities may complicate the association.
To more closely evaluate the course and outcomes of acute pancreatitis based on BMI classification, study authors led by Ali Tuzun Ince, MD, of the department of internal medicine, Gastroenterology Clinic of Bezmialem Vakif University, Istanbul, analyzed retrospective data from 2010 to 2020 on 1,334 adult patients (720 female, 614 male) who were diagnosed with acute pancreatitis per the Revised Atlanta Classification (RAC) criteria.
The patients were stratified based on their BMI as normal weight, overweight, or obese and whether they had mild, moderate, or severe (with permanent organ failure) acute pancreatitis.
In terms of acute pancreatitis severity, based on RAC criteria, 57.1% of patients had mild disease, 20.4% had moderate disease, and 22.5% had severe disease.
The overall mortality rate was 9.9% (n = 132); half of these patients were obese, and 87% had severe acute pancreatitis.
The overall rate of complications was 42.9%, including 20.8% in the normal weight group, 40.6% in the overweight group, and 38.6% in the obese group.
Patients in the overweight and obese groups also had higher mortality rates (3.7% and 4.9%, respectively), interventional procedures (36% and 39%, respectively), and length of hospital stay (11.6% and 9.8%, respectively), compared with the normal-weight group.
Other factors that were significantly associated with an increased mortality risk, in addition to obesity (P = .046), included old age (P = .000), male sex (P = .05), alcohol use (P = .014), low hematocrit (P = .044), high C-reactive protein (P = .024), moderate to severe and severe acute pancreatitis (P = .02 and P < .001, respectively), and any complications (P < .001).
Risk factors associated with increased admission to the ICU differed from those for mortality, and included female gender (P = .024), smoking (P = .021), hypertriglyceridemia (P = .047), idiopathic etiology (P = .023), and moderate to severe and severe acute pancreatitis (P < .001).
Of note, there were no significant associations between BMI and either the RAC score or Balthazar CT severity index (Balthazar CTSI) groups.
Specifically, among patients considered to have severe acute pancreatitis per Balthazar CTSI, 6.3% were of normal weight, 5% were overweight, and 7.1% were obese.
“In addition, since agreement and consistency between BMI and Balthazar score cannot be determined, the Balthazar score cannot be estimated from BMI,” the authors reported.
While the prediction of prognosis in acute pancreatitis is gaining interest, the findings underscore the role of combined factors, they added.
“Although many scoring systems are currently in use attempt to estimate the severity [in acute pancreatitis], none is 100% accurate yet,” the authors noted. “Each risk factor exacerbates the course of disease. Therefore, it would be better to consider the combined effects of risk factors.”
That being said, the findings show “mortality is increased significantly by the combined presence of risk factors such as male sex, OB [obesity], alcohol, MSAP [moderate to severe acute pancreatitis] and SAP [severe acute pancreatitis], all kinds of complications, old age, low Hct, and high CRP,” they wrote.
Obesity’s complex interactions
Commenting on the study, Vijay P. Singh, MD, a professor of medicine in the division of gastroenterology and hepatology at the Mayo Clinic in Scottsdale, Ariz., agreed that the complexities risk factors, particularly with obesity, can be tricky to detangle.
“Broadly, the study confirms several previous reports from different parts of the world that obesity was associated with increased mortality in acute pancreatitis,” he said in an interview.
“However, obesity had two complex interactions, the first that obesity is also associated with increased diabetes, and hypertriglyceridemia, which may themselves be risk factors for severity,” he explained.
“The second one is that intermediary severity markers [e.g., Balthazar score on imaging] did not correlate with the BMI categories.”
Dr. Singh noted that is “likely because therapies like IV fluids that may get more intense in predicted severe disease alter the natural course of pancreatitis.”
The findings are a reminder that “BMI is only a number that attempts to quantify fat,” Dr. Singh said, noting that BMI doesn’t address either the location of fat, such as being in close proximity to the pancreas, or fat composition, such as the proportion of unsaturated fat.
“When the unsaturated fat proportion is higher, the pancreatitis is worse, even at smaller total fat amounts [for example, at a lower BMI],” he said. “Taking these aspects into account may help in risk assessment.”
The authors and Dr. Singh had no disclosures to report.
FROM PANCREATOLOGY
In diabetes, fast-growing pancreatic cysts may be a red flag
LAS VEGAS – New results from a single center, retrospective analysis suggest that individuals with diabetes and pancreatic cysts have larger cyst sizes at diagnosis, and a faster subsequent cyst growth rate. Smoking was independently associated with faster growth rate.
Most pancreatic cancer patients were previously diagnosed with hyperglycemia and diabetes, and pancreatic cancer can cause diabetes. “This sort of dual causality raises questions as to whether or not hyperglycemia, or the new diagnosis of diabetes itself, could be a harbinger of cancer or precancer. And should these patients be more closely monitored?” David Robbins, MD, said in an interview.
Dr. Robbins, associate professor of medicine and program director in gastroenterology in the Northwell Health System, New York, presented the study at the annual meeting of the American College of Gastroenterology.
Faster growth rates of pancreatic cysts in the presence of diabetes are important because they represent a potential mark for cyst aggressiveness. “So the question really is, in the setting of diabetes, are there factors perhaps circulating in the bloodstream, or other intrinsic factors, that make these cysts more dangerous and require a different surveillance approach than someone who doesn’t have diabetes? We have (surveillance) guidelines that address the average population, but they don’t really hone in on what do you do with (individuals with diabetes),” Dr. Robbins said during the presentation.
The study could have implications for screening, said session moderator Dayna Early, MD, professor of medicine at Washington University and director of endoscopy at Barnes Jewish Hospital, both in St. Louis. “I think this is important information to guide us to look more closely at patients with diabetes who do have pancreatic cysts,” she said in an interview.
The study included 177 adults with pancreatic cysts or abnormal imaging results between 2013 and 2020. Sixty-five percent were female, and the mean age was 65.4 years; 64% were White, 10% were Black, and 8.5% were Asian. Among the participants, 24.8% were smokers and 32.2% had type 2 diabetes.
Patients with diabetes had larger cyst sizes (2.23 cm versus 2.76 cm), as well as a higher annual cyst growth rate (1.90 cm versus 1.30 cm). Cyst size and growth rate were similar between patients with controlled and uncontrolled diabetes. Smoking was associated with a larger cyst size overall (2.2 cm versus 1.81 cm), and were larger still among patients with diabetes who smoked (2.35 cm).
Seventy-one patients went on to have pathologic confirmation by endoscopic ultrasound-guided fine needle aspiration. “In the diabetic group, two developed adenocarcinoma, six of the nondiabetics developed adenocarcinoma, and there was no difference in CEA or serum CA 19-9,” Dr. Robbins said during his presentation.
Of 28 patients diagnosed with pancreatic cancer, 13 had type 2 diabetes.
Defining danger
There remains uncertainty about what cyst growth rate is most dangerous. Some guidelines recommend that individuals with new-onset or worsening diabetes and intraductal papillary mucinous neoplasm or mucinous cystic neoplasm cysts, or cysts alone that are growing faster than 3 mm per year, may be at significantly increased risk of pancreatic cancer. These guidelines recommend that they be screened with short-interval magnetic resonance imaging or endoscopic ultrasound (EUS) fine needle aspiration. However, this recommendation is conditional and is backed by a very low level of evidence.
Other reports have shown varying risks at different growth rates. “It’s not really clear at this point. And that’s why I think, while our study is small and exploratory, this is a particular area that is relatively easy to evaluate. We have huge databases of pancreatic cyst evolution, and we know that 30 million Americans have diabetes. So, the next obvious study is to do a more systematic look at that, and work towards refining and making sense of these divergent guidelines, all of which are saying the same thing but using different threshold numbers,” said Dr. Robbins.
The next step is do larger, multicenter studies in the context of other risk factors such as family history and smoking, but the current finding represents an opportunity to catch at least some pancreatic cancers earlier, according to Dr. Robbins. He suggested that individuals with diabetes who are diagnosed with a pancreatic cyst should be referred to a gastroenterologist or another specialist to track cyst growth. “That is going to miss a lot of folks who didn’t get imaging for whatever reason (and so don’t have a cyst identified), but it is an early opportunity, and it’s better than what we’re doing now.”
During the talk, Dr. Robbins said, “Given the ease, availability and low cost of diabetes screening in the general clinic population, we encourage the inclusion of HbA1c and fasting glucose in algorithms for pancreatic cyst surveillance.”
Dr. Early found the suggestion intriguing, but wasn’t ready to lend full support. “I think looking at the suggestion of possibly monitoring hemoglobin A1c levels was novel. I don’t know that we’ll necessarily adopt that as standard practice, but that’s something I think that could be looked at in the future as a way to help risk stratify whether patients need to be surveyed more frequently,” she said.
Dr. Robbins and Dr. Early have no relevant financial disclosures.
LAS VEGAS – New results from a single center, retrospective analysis suggest that individuals with diabetes and pancreatic cysts have larger cyst sizes at diagnosis, and a faster subsequent cyst growth rate. Smoking was independently associated with faster growth rate.
Most pancreatic cancer patients were previously diagnosed with hyperglycemia and diabetes, and pancreatic cancer can cause diabetes. “This sort of dual causality raises questions as to whether or not hyperglycemia, or the new diagnosis of diabetes itself, could be a harbinger of cancer or precancer. And should these patients be more closely monitored?” David Robbins, MD, said in an interview.
Dr. Robbins, associate professor of medicine and program director in gastroenterology in the Northwell Health System, New York, presented the study at the annual meeting of the American College of Gastroenterology.
Faster growth rates of pancreatic cysts in the presence of diabetes are important because they represent a potential mark for cyst aggressiveness. “So the question really is, in the setting of diabetes, are there factors perhaps circulating in the bloodstream, or other intrinsic factors, that make these cysts more dangerous and require a different surveillance approach than someone who doesn’t have diabetes? We have (surveillance) guidelines that address the average population, but they don’t really hone in on what do you do with (individuals with diabetes),” Dr. Robbins said during the presentation.
The study could have implications for screening, said session moderator Dayna Early, MD, professor of medicine at Washington University and director of endoscopy at Barnes Jewish Hospital, both in St. Louis. “I think this is important information to guide us to look more closely at patients with diabetes who do have pancreatic cysts,” she said in an interview.
The study included 177 adults with pancreatic cysts or abnormal imaging results between 2013 and 2020. Sixty-five percent were female, and the mean age was 65.4 years; 64% were White, 10% were Black, and 8.5% were Asian. Among the participants, 24.8% were smokers and 32.2% had type 2 diabetes.
Patients with diabetes had larger cyst sizes (2.23 cm versus 2.76 cm), as well as a higher annual cyst growth rate (1.90 cm versus 1.30 cm). Cyst size and growth rate were similar between patients with controlled and uncontrolled diabetes. Smoking was associated with a larger cyst size overall (2.2 cm versus 1.81 cm), and were larger still among patients with diabetes who smoked (2.35 cm).
Seventy-one patients went on to have pathologic confirmation by endoscopic ultrasound-guided fine needle aspiration. “In the diabetic group, two developed adenocarcinoma, six of the nondiabetics developed adenocarcinoma, and there was no difference in CEA or serum CA 19-9,” Dr. Robbins said during his presentation.
Of 28 patients diagnosed with pancreatic cancer, 13 had type 2 diabetes.
Defining danger
There remains uncertainty about what cyst growth rate is most dangerous. Some guidelines recommend that individuals with new-onset or worsening diabetes and intraductal papillary mucinous neoplasm or mucinous cystic neoplasm cysts, or cysts alone that are growing faster than 3 mm per year, may be at significantly increased risk of pancreatic cancer. These guidelines recommend that they be screened with short-interval magnetic resonance imaging or endoscopic ultrasound (EUS) fine needle aspiration. However, this recommendation is conditional and is backed by a very low level of evidence.
Other reports have shown varying risks at different growth rates. “It’s not really clear at this point. And that’s why I think, while our study is small and exploratory, this is a particular area that is relatively easy to evaluate. We have huge databases of pancreatic cyst evolution, and we know that 30 million Americans have diabetes. So, the next obvious study is to do a more systematic look at that, and work towards refining and making sense of these divergent guidelines, all of which are saying the same thing but using different threshold numbers,” said Dr. Robbins.
The next step is do larger, multicenter studies in the context of other risk factors such as family history and smoking, but the current finding represents an opportunity to catch at least some pancreatic cancers earlier, according to Dr. Robbins. He suggested that individuals with diabetes who are diagnosed with a pancreatic cyst should be referred to a gastroenterologist or another specialist to track cyst growth. “That is going to miss a lot of folks who didn’t get imaging for whatever reason (and so don’t have a cyst identified), but it is an early opportunity, and it’s better than what we’re doing now.”
During the talk, Dr. Robbins said, “Given the ease, availability and low cost of diabetes screening in the general clinic population, we encourage the inclusion of HbA1c and fasting glucose in algorithms for pancreatic cyst surveillance.”
Dr. Early found the suggestion intriguing, but wasn’t ready to lend full support. “I think looking at the suggestion of possibly monitoring hemoglobin A1c levels was novel. I don’t know that we’ll necessarily adopt that as standard practice, but that’s something I think that could be looked at in the future as a way to help risk stratify whether patients need to be surveyed more frequently,” she said.
Dr. Robbins and Dr. Early have no relevant financial disclosures.
LAS VEGAS – New results from a single center, retrospective analysis suggest that individuals with diabetes and pancreatic cysts have larger cyst sizes at diagnosis, and a faster subsequent cyst growth rate. Smoking was independently associated with faster growth rate.
Most pancreatic cancer patients were previously diagnosed with hyperglycemia and diabetes, and pancreatic cancer can cause diabetes. “This sort of dual causality raises questions as to whether or not hyperglycemia, or the new diagnosis of diabetes itself, could be a harbinger of cancer or precancer. And should these patients be more closely monitored?” David Robbins, MD, said in an interview.
Dr. Robbins, associate professor of medicine and program director in gastroenterology in the Northwell Health System, New York, presented the study at the annual meeting of the American College of Gastroenterology.
Faster growth rates of pancreatic cysts in the presence of diabetes are important because they represent a potential mark for cyst aggressiveness. “So the question really is, in the setting of diabetes, are there factors perhaps circulating in the bloodstream, or other intrinsic factors, that make these cysts more dangerous and require a different surveillance approach than someone who doesn’t have diabetes? We have (surveillance) guidelines that address the average population, but they don’t really hone in on what do you do with (individuals with diabetes),” Dr. Robbins said during the presentation.
The study could have implications for screening, said session moderator Dayna Early, MD, professor of medicine at Washington University and director of endoscopy at Barnes Jewish Hospital, both in St. Louis. “I think this is important information to guide us to look more closely at patients with diabetes who do have pancreatic cysts,” she said in an interview.
The study included 177 adults with pancreatic cysts or abnormal imaging results between 2013 and 2020. Sixty-five percent were female, and the mean age was 65.4 years; 64% were White, 10% were Black, and 8.5% were Asian. Among the participants, 24.8% were smokers and 32.2% had type 2 diabetes.
Patients with diabetes had larger cyst sizes (2.23 cm versus 2.76 cm), as well as a higher annual cyst growth rate (1.90 cm versus 1.30 cm). Cyst size and growth rate were similar between patients with controlled and uncontrolled diabetes. Smoking was associated with a larger cyst size overall (2.2 cm versus 1.81 cm), and were larger still among patients with diabetes who smoked (2.35 cm).
Seventy-one patients went on to have pathologic confirmation by endoscopic ultrasound-guided fine needle aspiration. “In the diabetic group, two developed adenocarcinoma, six of the nondiabetics developed adenocarcinoma, and there was no difference in CEA or serum CA 19-9,” Dr. Robbins said during his presentation.
Of 28 patients diagnosed with pancreatic cancer, 13 had type 2 diabetes.
Defining danger
There remains uncertainty about what cyst growth rate is most dangerous. Some guidelines recommend that individuals with new-onset or worsening diabetes and intraductal papillary mucinous neoplasm or mucinous cystic neoplasm cysts, or cysts alone that are growing faster than 3 mm per year, may be at significantly increased risk of pancreatic cancer. These guidelines recommend that they be screened with short-interval magnetic resonance imaging or endoscopic ultrasound (EUS) fine needle aspiration. However, this recommendation is conditional and is backed by a very low level of evidence.
Other reports have shown varying risks at different growth rates. “It’s not really clear at this point. And that’s why I think, while our study is small and exploratory, this is a particular area that is relatively easy to evaluate. We have huge databases of pancreatic cyst evolution, and we know that 30 million Americans have diabetes. So, the next obvious study is to do a more systematic look at that, and work towards refining and making sense of these divergent guidelines, all of which are saying the same thing but using different threshold numbers,” said Dr. Robbins.
The next step is do larger, multicenter studies in the context of other risk factors such as family history and smoking, but the current finding represents an opportunity to catch at least some pancreatic cancers earlier, according to Dr. Robbins. He suggested that individuals with diabetes who are diagnosed with a pancreatic cyst should be referred to a gastroenterologist or another specialist to track cyst growth. “That is going to miss a lot of folks who didn’t get imaging for whatever reason (and so don’t have a cyst identified), but it is an early opportunity, and it’s better than what we’re doing now.”
During the talk, Dr. Robbins said, “Given the ease, availability and low cost of diabetes screening in the general clinic population, we encourage the inclusion of HbA1c and fasting glucose in algorithms for pancreatic cyst surveillance.”
Dr. Early found the suggestion intriguing, but wasn’t ready to lend full support. “I think looking at the suggestion of possibly monitoring hemoglobin A1c levels was novel. I don’t know that we’ll necessarily adopt that as standard practice, but that’s something I think that could be looked at in the future as a way to help risk stratify whether patients need to be surveyed more frequently,” she said.
Dr. Robbins and Dr. Early have no relevant financial disclosures.
AT ACG 2021
Developing a career in medical pancreatology: An emerging postfellowship career path
Although described by the Greek physician Herophilos around 300 B.C., it was not until the 19th century that enzymes began to be isolated from pancreatic secretions and their digestive action described, and not until early in the 20th century that Banting, Macleod, and Best received the Nobel prize for purifying insulin from the pancreata of dogs. For centuries in between, the pancreas was considered to be just a ‘beautiful piece of flesh’ (kallikreas), the main role of which was to protect the blood vessels in the abdomen and to serve as a cushion to the stomach.1 Certainly, the pancreas has come a long way since then but, like most other organs in the body, is oft ignored until it develops issues.
Like many other disorders in gastroenterology, pancreatic disorders were historically approached as mechanical or “plumbing” issues. As modern technology and innovation percolated through the world of endoscopy, a wide array of state-of-the-art tools were devised. Availability of newer “toys” and development of newer techniques also means that an ever-increasing curriculum has been squeezed into a generally single year of therapeutic endoscopy training, such that trainees can no longer limit themselves to learning only endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) or intervening on pancreatic disease alone. Modern, subspecialized approaches to disease and economic considerations often dictate that the therapeutic endoscopist of today must perform a wide range of procedures besides ERCP and EUS, such as advanced resection using endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), per-oral endoscopic myotomy (POEM), endoscopic bariatric procedures, and newer techniques and acronyms that continue to evolve on a regular basis. This leaves the therapeutic endoscopist with little time for outpatient management of many patients that don’t need interventional procedures but are often very complex and need ongoing, long-term follow-up. In addition, any clinic slots available for interventional endoscopists may be utilized by patients coming in to discuss complex procedures or for postprocedure follow-up. Endoscopic management is not the definitive treatment for most pancreatic disorders. In fact, as our knowledge of pancreatic disease has continued to evolve, endoscopic intervention is now required in a minority of cases.
Role of the medical pancreatologist
Patient Care
As part of a comprehensive, multidisciplinary team that also includes an interventional gastroenterologist, pancreatic surgeon, transplant surgeon (in centers offering islet autotransplantation with total pancreatectomy), radiology, endocrinology, and GI pathologist, the medical pancreatologist helps lead the care of patients with pancreatic disorders, such as pancreatic cysts, acute and chronic pancreatitis (especially in cases where there is no role for active endoscopic intervention), autoimmune pancreatitis, indeterminate pancreatic masses, as well as screens high-risk patients for pancreatic cancer in conjunction with a genetic counselor. The medical pancreatologist often also serves as a bridge between various members of a large multidisciplinary team that, formally in the form of conferences or informally, discusses the management of complex patients, with each member available to help the other based on the patient’s most immediate clinical need at that time. A schematic showing how the medical pancreatologist collaborates with the therapeutic endoscopist is provided in Figure 1.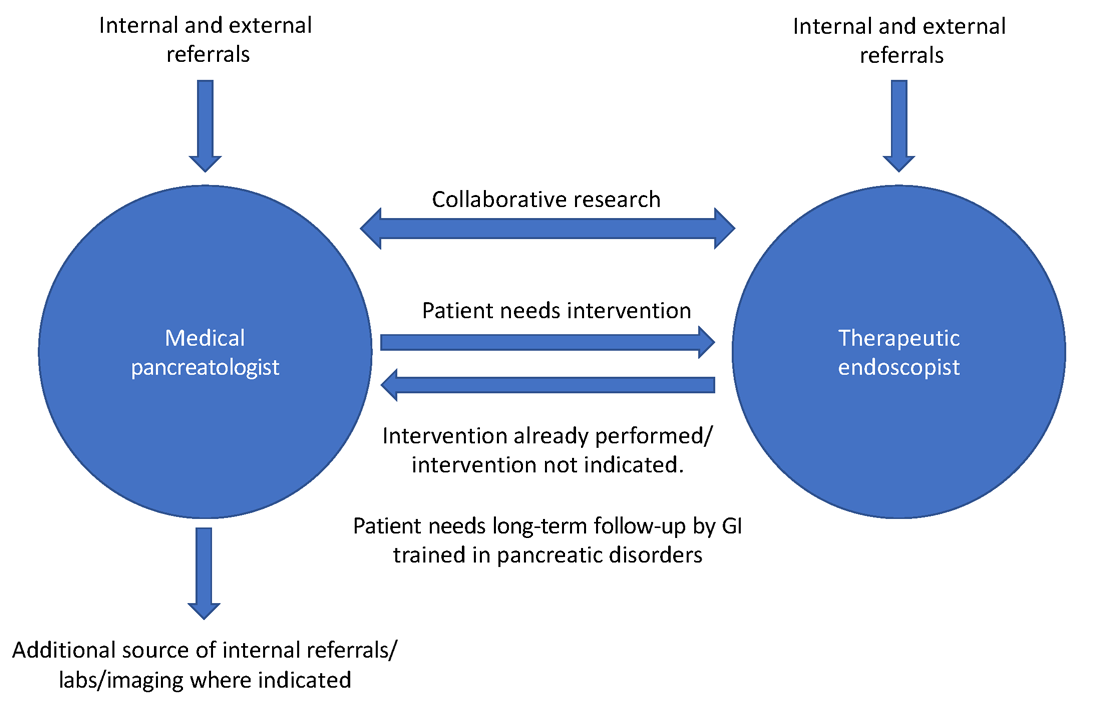
Uzma Siddiqui, MD, director for the Center for Endoscopic Research and Technology (CERT) at the University of Chicago said, “The management of pancreatic diseases is often challenging. Surgeons and endoscopists can offer some treatments that focus on one aspect or symptom, but the medical pancreatologist brings focus to the patient as a whole and helps organize care. It is only with everyone’s combined efforts and the added perspective of the medical pancreatologist that we can provide the best care for our shared patients.”
David Xin, MD, MPH, a medical pancreatologist at Brigham and Women’s Hospital, Boston, added, “I am often asked what it means to be a medical pancreatologist. What do I do if not EUS and ERCP? I provide longitudinal care, coordinate multidisciplinary management, assess nutritional status, optimize quality of life, and manage pain. But perhaps most importantly, I make myself available for patients who seek understanding and sympathy regarding their complex disease. I became a medical pancreatologist because my mentors during training helped me recognize how rewarding this career would be.”
Insights from other medical pancreatologists and therapeutic endoscopists are provided in Figure 2.

Education
Having a dedicated medical pancreatology clinic has the potential to add a unique element to the training of gastroenterology fellows. In my own experience, besides fellows interested in medical pancreatology, even those interested in therapeutic endoscopy find it useful to rotate through the pancreas clinic and follow patients after or leading to their procedures, becoming comfortable with noninterventional pain management of patients with pancreatic disorders and risk stratification of pancreatic cystic lesions, and learning about the management of rare disorders such as autoimmune pancreatitis. Most importantly, this allows trainees to identify cases where endoscopic intervention may not offer definitive treatment for complex conditions such as pancreatic pain. Trainee-centered organizations such as the Collaborative Alliance for Pancreatic Education and Research (CAPER) enable trainees and young investigators to network with other physicians who are passionate about the pancreas and establish early research collaborations for current and future research endeavors that will help advance this field.
Research
Having a trained medical pancreatologist adds the possibility of adding a unique angle to ongoing research within a gastroenterology division, especially in collaboration with others. For example, during my fellowship training I was able to focus on histological changes in pancreatic islets of patients with pancreatic cancer that develop diabetes, compared with those that do not, in collaboration with a pathologist who focused on studying islet pathology and under the guidance of my mentor, Dr. Suresh Chari, a medical pancreatologist.2 I was also part of other studies within the GI division with other medical pancreatologists, such as Dr. Santhi Vege and Dr. Shounak Majumder, who have continued to serve as career and research mentors.3 Collaborative, multicenter studies on pancreatic disease are also conducted by CAPER, the organization mentioned above. A list of potential collaborations for the fellow interested
in medical pancreatology is provided in Figure 3.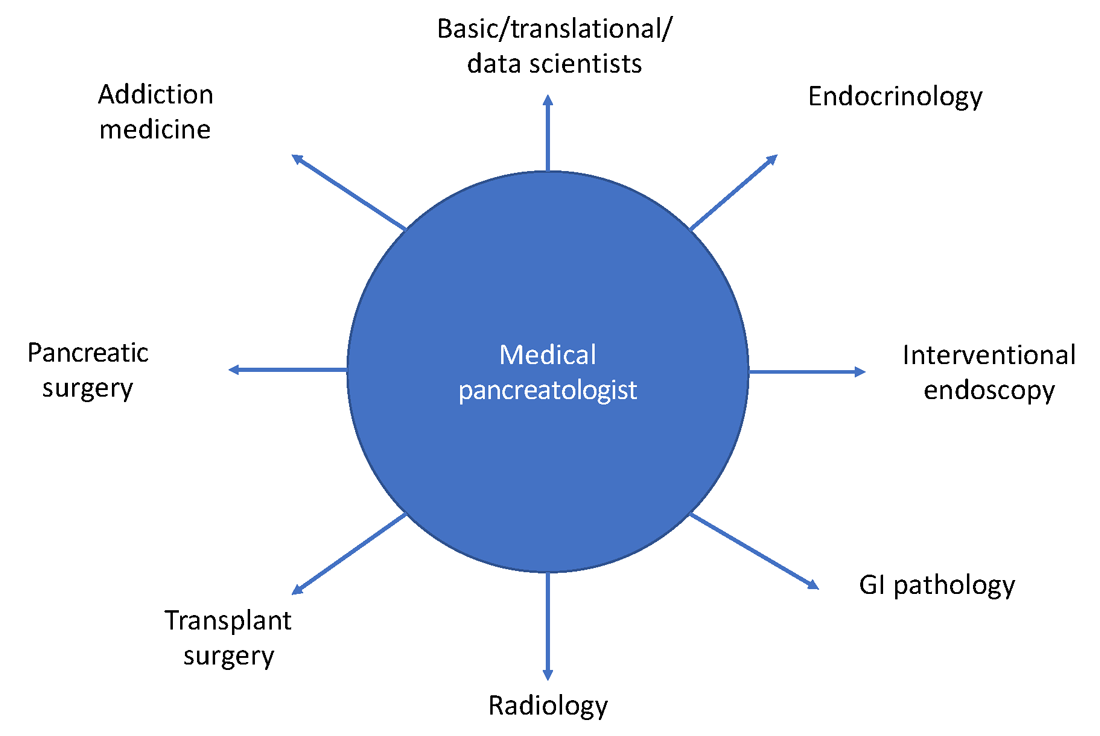
Marketing considerations for the gastroenterology division
Having a medical pancreatologist in the team is not only attractive for referring physicians within an institution but is often a great asset from a marketing standpoint, especially for tertiary care academic centers and large community practices with a broad referral base. Given that there are a limited number of medical pancreatologists in the country, having one as part of the faculty can certainly provide a competitive edge to that center within the area, especially with an ever-increasing preference of patients for hyperspecialized care.
How to develop a career in medical pancreatology
Gastroenterology fellows often start their fellowships “undifferentiated” and try to get exposed to a wide variety of GI pathology, either through general GI clinics or as part of subspecialized clinics, as they attempt to decide how they want their careers to look down the line. Similar to other subspecialities, if a trainee has already decided to pursue medical pancreatology (as happened in my case), they should strongly consider ranking programs with available opportunities for research/clinic in medical pancreatology and ideally undergo an additional year of training. Fellows who decide during the course of their fellowship that they want to pursue a career in medical pancreatology should consider applying for a 4th year in the subject to not only obtain further training in the field but to also conduct research in the area and become more “marketable” as a person that could start a medical pancreatology program at their future academic or community position. Trainees interested in medical pancreatology should try to focus their time on long-term, clinical management of patients with pancreatic disorders, engaging a multidisciplinary team composed of interventional endoscopists, pancreatic surgeons, transplant surgeons (if total pancreatectomy and islet autotransplantation is available), radiology, addiction medicine (if available), endocrinology, and pathology. The list of places that offer a 4th year in medical pancreatology is increasing every year, and as of the writing of this article there are six programs that have this opportunity, which include:
- Mayo Clinic, Rochester, Minn.
- Beth Israel Deaconess Medical Center, Boston
- Brigham and Women’s Hospital, Boston
- Johns Hopkins Hospital, Baltimore
- University of Pittsburgh Medical Center, Pittsburgh, Penn.
The CAPER website is also a great resource for education as well as for identifying potential medical pancreatology programs.
In summary, medical pancreatology is an evolving and rapidly growing career path for gastroenterology fellows interested in providing care to patients with pancreatic disease in close collaboration with multiple other subspecialties, especially therapeutic endoscopy and pancreatic surgery. The field is also ripe for fellows interested in clinical, translational, and basic science research related to pancreatic disorders.
Dr. Nagpal is assistant professor of medicine, director, pancreas clinic, University of Chicago. He had no conflicts to disclose.
References
1. Feldman M et al. “Sleisenger and Fordtran’s Gastrointestinal and Liver Disease,” 11th ed. (Philadelphia: Elsevier, 2021).
2. Nagpal SJS et al. Pancreatology. 2020 Jul;20(5):929-35.
3. Nagpal SJS et al. Pancreatology. 2019 Mar;19(2):290-5.
Although described by the Greek physician Herophilos around 300 B.C., it was not until the 19th century that enzymes began to be isolated from pancreatic secretions and their digestive action described, and not until early in the 20th century that Banting, Macleod, and Best received the Nobel prize for purifying insulin from the pancreata of dogs. For centuries in between, the pancreas was considered to be just a ‘beautiful piece of flesh’ (kallikreas), the main role of which was to protect the blood vessels in the abdomen and to serve as a cushion to the stomach.1 Certainly, the pancreas has come a long way since then but, like most other organs in the body, is oft ignored until it develops issues.
Like many other disorders in gastroenterology, pancreatic disorders were historically approached as mechanical or “plumbing” issues. As modern technology and innovation percolated through the world of endoscopy, a wide array of state-of-the-art tools were devised. Availability of newer “toys” and development of newer techniques also means that an ever-increasing curriculum has been squeezed into a generally single year of therapeutic endoscopy training, such that trainees can no longer limit themselves to learning only endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) or intervening on pancreatic disease alone. Modern, subspecialized approaches to disease and economic considerations often dictate that the therapeutic endoscopist of today must perform a wide range of procedures besides ERCP and EUS, such as advanced resection using endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), per-oral endoscopic myotomy (POEM), endoscopic bariatric procedures, and newer techniques and acronyms that continue to evolve on a regular basis. This leaves the therapeutic endoscopist with little time for outpatient management of many patients that don’t need interventional procedures but are often very complex and need ongoing, long-term follow-up. In addition, any clinic slots available for interventional endoscopists may be utilized by patients coming in to discuss complex procedures or for postprocedure follow-up. Endoscopic management is not the definitive treatment for most pancreatic disorders. In fact, as our knowledge of pancreatic disease has continued to evolve, endoscopic intervention is now required in a minority of cases.
Role of the medical pancreatologist
Patient Care
As part of a comprehensive, multidisciplinary team that also includes an interventional gastroenterologist, pancreatic surgeon, transplant surgeon (in centers offering islet autotransplantation with total pancreatectomy), radiology, endocrinology, and GI pathologist, the medical pancreatologist helps lead the care of patients with pancreatic disorders, such as pancreatic cysts, acute and chronic pancreatitis (especially in cases where there is no role for active endoscopic intervention), autoimmune pancreatitis, indeterminate pancreatic masses, as well as screens high-risk patients for pancreatic cancer in conjunction with a genetic counselor. The medical pancreatologist often also serves as a bridge between various members of a large multidisciplinary team that, formally in the form of conferences or informally, discusses the management of complex patients, with each member available to help the other based on the patient’s most immediate clinical need at that time. A schematic showing how the medical pancreatologist collaborates with the therapeutic endoscopist is provided in Figure 1.
Uzma Siddiqui, MD, director for the Center for Endoscopic Research and Technology (CERT) at the University of Chicago said, “The management of pancreatic diseases is often challenging. Surgeons and endoscopists can offer some treatments that focus on one aspect or symptom, but the medical pancreatologist brings focus to the patient as a whole and helps organize care. It is only with everyone’s combined efforts and the added perspective of the medical pancreatologist that we can provide the best care for our shared patients.”
David Xin, MD, MPH, a medical pancreatologist at Brigham and Women’s Hospital, Boston, added, “I am often asked what it means to be a medical pancreatologist. What do I do if not EUS and ERCP? I provide longitudinal care, coordinate multidisciplinary management, assess nutritional status, optimize quality of life, and manage pain. But perhaps most importantly, I make myself available for patients who seek understanding and sympathy regarding their complex disease. I became a medical pancreatologist because my mentors during training helped me recognize how rewarding this career would be.”
Insights from other medical pancreatologists and therapeutic endoscopists are provided in Figure 2.

Education
Having a dedicated medical pancreatology clinic has the potential to add a unique element to the training of gastroenterology fellows. In my own experience, besides fellows interested in medical pancreatology, even those interested in therapeutic endoscopy find it useful to rotate through the pancreas clinic and follow patients after or leading to their procedures, becoming comfortable with noninterventional pain management of patients with pancreatic disorders and risk stratification of pancreatic cystic lesions, and learning about the management of rare disorders such as autoimmune pancreatitis. Most importantly, this allows trainees to identify cases where endoscopic intervention may not offer definitive treatment for complex conditions such as pancreatic pain. Trainee-centered organizations such as the Collaborative Alliance for Pancreatic Education and Research (CAPER) enable trainees and young investigators to network with other physicians who are passionate about the pancreas and establish early research collaborations for current and future research endeavors that will help advance this field.
Research
Having a trained medical pancreatologist adds the possibility of adding a unique angle to ongoing research within a gastroenterology division, especially in collaboration with others. For example, during my fellowship training I was able to focus on histological changes in pancreatic islets of patients with pancreatic cancer that develop diabetes, compared with those that do not, in collaboration with a pathologist who focused on studying islet pathology and under the guidance of my mentor, Dr. Suresh Chari, a medical pancreatologist.2 I was also part of other studies within the GI division with other medical pancreatologists, such as Dr. Santhi Vege and Dr. Shounak Majumder, who have continued to serve as career and research mentors.3 Collaborative, multicenter studies on pancreatic disease are also conducted by CAPER, the organization mentioned above. A list of potential collaborations for the fellow interested
in medical pancreatology is provided in Figure 3.
Marketing considerations for the gastroenterology division
Having a medical pancreatologist in the team is not only attractive for referring physicians within an institution but is often a great asset from a marketing standpoint, especially for tertiary care academic centers and large community practices with a broad referral base. Given that there are a limited number of medical pancreatologists in the country, having one as part of the faculty can certainly provide a competitive edge to that center within the area, especially with an ever-increasing preference of patients for hyperspecialized care.
How to develop a career in medical pancreatology
Gastroenterology fellows often start their fellowships “undifferentiated” and try to get exposed to a wide variety of GI pathology, either through general GI clinics or as part of subspecialized clinics, as they attempt to decide how they want their careers to look down the line. Similar to other subspecialities, if a trainee has already decided to pursue medical pancreatology (as happened in my case), they should strongly consider ranking programs with available opportunities for research/clinic in medical pancreatology and ideally undergo an additional year of training. Fellows who decide during the course of their fellowship that they want to pursue a career in medical pancreatology should consider applying for a 4th year in the subject to not only obtain further training in the field but to also conduct research in the area and become more “marketable” as a person that could start a medical pancreatology program at their future academic or community position. Trainees interested in medical pancreatology should try to focus their time on long-term, clinical management of patients with pancreatic disorders, engaging a multidisciplinary team composed of interventional endoscopists, pancreatic surgeons, transplant surgeons (if total pancreatectomy and islet autotransplantation is available), radiology, addiction medicine (if available), endocrinology, and pathology. The list of places that offer a 4th year in medical pancreatology is increasing every year, and as of the writing of this article there are six programs that have this opportunity, which include:
- Mayo Clinic, Rochester, Minn.
- Beth Israel Deaconess Medical Center, Boston
- Brigham and Women’s Hospital, Boston
- Johns Hopkins Hospital, Baltimore
- University of Pittsburgh Medical Center, Pittsburgh, Penn.
The CAPER website is also a great resource for education as well as for identifying potential medical pancreatology programs.
In summary, medical pancreatology is an evolving and rapidly growing career path for gastroenterology fellows interested in providing care to patients with pancreatic disease in close collaboration with multiple other subspecialties, especially therapeutic endoscopy and pancreatic surgery. The field is also ripe for fellows interested in clinical, translational, and basic science research related to pancreatic disorders.
Dr. Nagpal is assistant professor of medicine, director, pancreas clinic, University of Chicago. He had no conflicts to disclose.
References
1. Feldman M et al. “Sleisenger and Fordtran’s Gastrointestinal and Liver Disease,” 11th ed. (Philadelphia: Elsevier, 2021).
2. Nagpal SJS et al. Pancreatology. 2020 Jul;20(5):929-35.
3. Nagpal SJS et al. Pancreatology. 2019 Mar;19(2):290-5.
Although described by the Greek physician Herophilos around 300 B.C., it was not until the 19th century that enzymes began to be isolated from pancreatic secretions and their digestive action described, and not until early in the 20th century that Banting, Macleod, and Best received the Nobel prize for purifying insulin from the pancreata of dogs. For centuries in between, the pancreas was considered to be just a ‘beautiful piece of flesh’ (kallikreas), the main role of which was to protect the blood vessels in the abdomen and to serve as a cushion to the stomach.1 Certainly, the pancreas has come a long way since then but, like most other organs in the body, is oft ignored until it develops issues.
Like many other disorders in gastroenterology, pancreatic disorders were historically approached as mechanical or “plumbing” issues. As modern technology and innovation percolated through the world of endoscopy, a wide array of state-of-the-art tools were devised. Availability of newer “toys” and development of newer techniques also means that an ever-increasing curriculum has been squeezed into a generally single year of therapeutic endoscopy training, such that trainees can no longer limit themselves to learning only endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) or intervening on pancreatic disease alone. Modern, subspecialized approaches to disease and economic considerations often dictate that the therapeutic endoscopist of today must perform a wide range of procedures besides ERCP and EUS, such as advanced resection using endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), per-oral endoscopic myotomy (POEM), endoscopic bariatric procedures, and newer techniques and acronyms that continue to evolve on a regular basis. This leaves the therapeutic endoscopist with little time for outpatient management of many patients that don’t need interventional procedures but are often very complex and need ongoing, long-term follow-up. In addition, any clinic slots available for interventional endoscopists may be utilized by patients coming in to discuss complex procedures or for postprocedure follow-up. Endoscopic management is not the definitive treatment for most pancreatic disorders. In fact, as our knowledge of pancreatic disease has continued to evolve, endoscopic intervention is now required in a minority of cases.
Role of the medical pancreatologist
Patient Care
As part of a comprehensive, multidisciplinary team that also includes an interventional gastroenterologist, pancreatic surgeon, transplant surgeon (in centers offering islet autotransplantation with total pancreatectomy), radiology, endocrinology, and GI pathologist, the medical pancreatologist helps lead the care of patients with pancreatic disorders, such as pancreatic cysts, acute and chronic pancreatitis (especially in cases where there is no role for active endoscopic intervention), autoimmune pancreatitis, indeterminate pancreatic masses, as well as screens high-risk patients for pancreatic cancer in conjunction with a genetic counselor. The medical pancreatologist often also serves as a bridge between various members of a large multidisciplinary team that, formally in the form of conferences or informally, discusses the management of complex patients, with each member available to help the other based on the patient’s most immediate clinical need at that time. A schematic showing how the medical pancreatologist collaborates with the therapeutic endoscopist is provided in Figure 1.
Uzma Siddiqui, MD, director for the Center for Endoscopic Research and Technology (CERT) at the University of Chicago said, “The management of pancreatic diseases is often challenging. Surgeons and endoscopists can offer some treatments that focus on one aspect or symptom, but the medical pancreatologist brings focus to the patient as a whole and helps organize care. It is only with everyone’s combined efforts and the added perspective of the medical pancreatologist that we can provide the best care for our shared patients.”
David Xin, MD, MPH, a medical pancreatologist at Brigham and Women’s Hospital, Boston, added, “I am often asked what it means to be a medical pancreatologist. What do I do if not EUS and ERCP? I provide longitudinal care, coordinate multidisciplinary management, assess nutritional status, optimize quality of life, and manage pain. But perhaps most importantly, I make myself available for patients who seek understanding and sympathy regarding their complex disease. I became a medical pancreatologist because my mentors during training helped me recognize how rewarding this career would be.”
Insights from other medical pancreatologists and therapeutic endoscopists are provided in Figure 2.

Education
Having a dedicated medical pancreatology clinic has the potential to add a unique element to the training of gastroenterology fellows. In my own experience, besides fellows interested in medical pancreatology, even those interested in therapeutic endoscopy find it useful to rotate through the pancreas clinic and follow patients after or leading to their procedures, becoming comfortable with noninterventional pain management of patients with pancreatic disorders and risk stratification of pancreatic cystic lesions, and learning about the management of rare disorders such as autoimmune pancreatitis. Most importantly, this allows trainees to identify cases where endoscopic intervention may not offer definitive treatment for complex conditions such as pancreatic pain. Trainee-centered organizations such as the Collaborative Alliance for Pancreatic Education and Research (CAPER) enable trainees and young investigators to network with other physicians who are passionate about the pancreas and establish early research collaborations for current and future research endeavors that will help advance this field.
Research
Having a trained medical pancreatologist adds the possibility of adding a unique angle to ongoing research within a gastroenterology division, especially in collaboration with others. For example, during my fellowship training I was able to focus on histological changes in pancreatic islets of patients with pancreatic cancer that develop diabetes, compared with those that do not, in collaboration with a pathologist who focused on studying islet pathology and under the guidance of my mentor, Dr. Suresh Chari, a medical pancreatologist.2 I was also part of other studies within the GI division with other medical pancreatologists, such as Dr. Santhi Vege and Dr. Shounak Majumder, who have continued to serve as career and research mentors.3 Collaborative, multicenter studies on pancreatic disease are also conducted by CAPER, the organization mentioned above. A list of potential collaborations for the fellow interested
in medical pancreatology is provided in Figure 3.
Marketing considerations for the gastroenterology division
Having a medical pancreatologist in the team is not only attractive for referring physicians within an institution but is often a great asset from a marketing standpoint, especially for tertiary care academic centers and large community practices with a broad referral base. Given that there are a limited number of medical pancreatologists in the country, having one as part of the faculty can certainly provide a competitive edge to that center within the area, especially with an ever-increasing preference of patients for hyperspecialized care.
How to develop a career in medical pancreatology
Gastroenterology fellows often start their fellowships “undifferentiated” and try to get exposed to a wide variety of GI pathology, either through general GI clinics or as part of subspecialized clinics, as they attempt to decide how they want their careers to look down the line. Similar to other subspecialities, if a trainee has already decided to pursue medical pancreatology (as happened in my case), they should strongly consider ranking programs with available opportunities for research/clinic in medical pancreatology and ideally undergo an additional year of training. Fellows who decide during the course of their fellowship that they want to pursue a career in medical pancreatology should consider applying for a 4th year in the subject to not only obtain further training in the field but to also conduct research in the area and become more “marketable” as a person that could start a medical pancreatology program at their future academic or community position. Trainees interested in medical pancreatology should try to focus their time on long-term, clinical management of patients with pancreatic disorders, engaging a multidisciplinary team composed of interventional endoscopists, pancreatic surgeons, transplant surgeons (if total pancreatectomy and islet autotransplantation is available), radiology, addiction medicine (if available), endocrinology, and pathology. The list of places that offer a 4th year in medical pancreatology is increasing every year, and as of the writing of this article there are six programs that have this opportunity, which include:
- Mayo Clinic, Rochester, Minn.
- Beth Israel Deaconess Medical Center, Boston
- Brigham and Women’s Hospital, Boston
- Johns Hopkins Hospital, Baltimore
- University of Pittsburgh Medical Center, Pittsburgh, Penn.
The CAPER website is also a great resource for education as well as for identifying potential medical pancreatology programs.
In summary, medical pancreatology is an evolving and rapidly growing career path for gastroenterology fellows interested in providing care to patients with pancreatic disease in close collaboration with multiple other subspecialties, especially therapeutic endoscopy and pancreatic surgery. The field is also ripe for fellows interested in clinical, translational, and basic science research related to pancreatic disorders.
Dr. Nagpal is assistant professor of medicine, director, pancreas clinic, University of Chicago. He had no conflicts to disclose.
References
1. Feldman M et al. “Sleisenger and Fordtran’s Gastrointestinal and Liver Disease,” 11th ed. (Philadelphia: Elsevier, 2021).
2. Nagpal SJS et al. Pancreatology. 2020 Jul;20(5):929-35.
3. Nagpal SJS et al. Pancreatology. 2019 Mar;19(2):290-5.
The risk factors behind infected pancreatic necrosis’ deadly toll
Patients with infected pancreatic necrosis (IPN) are more likely to experience organ failure and mortality, which makes identifying them as quickly as possible especially crucial. A new study aimed to make this task a bit easier by categorizing the main risk factors for IPN in a cohort of patients with severe acute pancreatitis, which included extensive spread of necrotic collections, preceding bacteremia, and preceding open abdomen treatment, as well as postinterventional pancreatitis.
In their study, published in the Journal of Gastrointestinal Surgery, Henrik L. Husu, MD, of the University of Helsinki, and colleagues noted the inherent challenges of rendering a preoperative diagnosis of IPN.
“Fever and increasing inflammation markers may indicate suspicion of IPN, but these are very common in patients with severe acute pancreatitis treated in the ICU,” and more knowledge of specific IPN risk factors is needed to improve clinical decision-making, they said.
Dr. Husu and colleagues identified 163 adults with acute pancreatitis admitted to the ICU at a single center between 2010 and 2018, approximately 68% of whom had alcoholic necrotizing pancreatitis. Pneumonia, bacteremia, and IPN occurred at an average of 4, 16, and 23 days, respectively, after ICU admission.
Forty-seven patients (28.8%) developed IPN within 90 days of ICU admission, all patients had a least one persistent organ failure, and 60% had multiple organ failure within 24 hours of ICU admission.
In a multivariate regression analysis, independent risk factors for IPN included postoperative or postendoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (odds ratio,13.5) and widespread necrotic collections (OR, 5.7 for unilateral paracolic or retromesenteric; OR, 21.8 for bilateral paracolic or unilateral paracolic and retromesenteric). Other risk factors were preceding bacteremia (OR, 4.8) and preceding open abdomen treatment for abdominal compartment syndrome (OR, 3.6).
After 90 days, 29 patients had died, including 7 with IPN and 22 without IPN. In addition, patients with IPN had longer overall hospital stays and ICU stays, higher rates of ICU readmission, and greater use of open necrosectomy, the researchers noted.
The study findings were limited by several factors, including the retrospective design, lack of controls, potential differences in treatment protocols, and the survival bias that prevented direct comparison of mortality in patients with and without IPN, the researchers noted. “This study cannot provide a reliable estimate of the difference in mortality attributable to IPN itself.”
However, the researchers noted that “the strength of the present study was to include only patients with persistent organ failure and admission to ICU in the early disease course,” and results indicate a significant morbid outcome associated with IPN. “In attempting to decrease the rate of IPN, efforts to identify and treat incipient organ failure with subsequent low threshold for admission to ICU becomes essential,” they emphasized.
More data may prompt greater intervention
“IPN portends a poor prognosis, and can be challenging to both diagnose and treat,” Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, said in an interview. “Identifying risk factors for development of IPN may facilitate earlier therapy that could modify the natural history of this disease.”
Dr. Ketwaroo said he was not surprised by the study findings. “This was a small single-center, retrospective study, where infection could only be ascertained among those who received interventions, and the findings should thus be interpreted within these limitations. Overall, however, I was not surprised. More extensive necrosis and opportunities for infectious seeding of necrosis such as interventions (ERCP) and bacteremia would be expected risk factors. I was surprised by the use of prophylactic antibiotics, as well as the high rate of open necrosectomy, though this should not affect the main findings of risk factors for infection.
“The studies highlight that a significant portion of patients with severe acute pancreatitis with necrosis will develop infection,” said Dr. Ketwaroo. “Being aware of the risk factors for infection, as identified in this study, can add to our clinical judgment in suspecting infection and opting for debridement. Especially with advancements in endoscopic necrosectomy, gastroenterologists may be more inclined to intervene when suspecting IPN. The next steps for research are to validate risk factors in larger, prospective studies.”
The study was supported by governmental competitive funds for medical research, a research grant from the Medical Society of Finland, and a research grant from Perkléns Foundation. The researchers had no financial conflicts to disclose. Dr. Ketwaroo had no financial conflicts to disclose but is a member of the GI & Hepatology News editorial advisory board.
Patients with infected pancreatic necrosis (IPN) are more likely to experience organ failure and mortality, which makes identifying them as quickly as possible especially crucial. A new study aimed to make this task a bit easier by categorizing the main risk factors for IPN in a cohort of patients with severe acute pancreatitis, which included extensive spread of necrotic collections, preceding bacteremia, and preceding open abdomen treatment, as well as postinterventional pancreatitis.
In their study, published in the Journal of Gastrointestinal Surgery, Henrik L. Husu, MD, of the University of Helsinki, and colleagues noted the inherent challenges of rendering a preoperative diagnosis of IPN.
“Fever and increasing inflammation markers may indicate suspicion of IPN, but these are very common in patients with severe acute pancreatitis treated in the ICU,” and more knowledge of specific IPN risk factors is needed to improve clinical decision-making, they said.
Dr. Husu and colleagues identified 163 adults with acute pancreatitis admitted to the ICU at a single center between 2010 and 2018, approximately 68% of whom had alcoholic necrotizing pancreatitis. Pneumonia, bacteremia, and IPN occurred at an average of 4, 16, and 23 days, respectively, after ICU admission.
Forty-seven patients (28.8%) developed IPN within 90 days of ICU admission, all patients had a least one persistent organ failure, and 60% had multiple organ failure within 24 hours of ICU admission.
In a multivariate regression analysis, independent risk factors for IPN included postoperative or postendoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (odds ratio,13.5) and widespread necrotic collections (OR, 5.7 for unilateral paracolic or retromesenteric; OR, 21.8 for bilateral paracolic or unilateral paracolic and retromesenteric). Other risk factors were preceding bacteremia (OR, 4.8) and preceding open abdomen treatment for abdominal compartment syndrome (OR, 3.6).
After 90 days, 29 patients had died, including 7 with IPN and 22 without IPN. In addition, patients with IPN had longer overall hospital stays and ICU stays, higher rates of ICU readmission, and greater use of open necrosectomy, the researchers noted.
The study findings were limited by several factors, including the retrospective design, lack of controls, potential differences in treatment protocols, and the survival bias that prevented direct comparison of mortality in patients with and without IPN, the researchers noted. “This study cannot provide a reliable estimate of the difference in mortality attributable to IPN itself.”
However, the researchers noted that “the strength of the present study was to include only patients with persistent organ failure and admission to ICU in the early disease course,” and results indicate a significant morbid outcome associated with IPN. “In attempting to decrease the rate of IPN, efforts to identify and treat incipient organ failure with subsequent low threshold for admission to ICU becomes essential,” they emphasized.
More data may prompt greater intervention
“IPN portends a poor prognosis, and can be challenging to both diagnose and treat,” Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, said in an interview. “Identifying risk factors for development of IPN may facilitate earlier therapy that could modify the natural history of this disease.”
Dr. Ketwaroo said he was not surprised by the study findings. “This was a small single-center, retrospective study, where infection could only be ascertained among those who received interventions, and the findings should thus be interpreted within these limitations. Overall, however, I was not surprised. More extensive necrosis and opportunities for infectious seeding of necrosis such as interventions (ERCP) and bacteremia would be expected risk factors. I was surprised by the use of prophylactic antibiotics, as well as the high rate of open necrosectomy, though this should not affect the main findings of risk factors for infection.
“The studies highlight that a significant portion of patients with severe acute pancreatitis with necrosis will develop infection,” said Dr. Ketwaroo. “Being aware of the risk factors for infection, as identified in this study, can add to our clinical judgment in suspecting infection and opting for debridement. Especially with advancements in endoscopic necrosectomy, gastroenterologists may be more inclined to intervene when suspecting IPN. The next steps for research are to validate risk factors in larger, prospective studies.”
The study was supported by governmental competitive funds for medical research, a research grant from the Medical Society of Finland, and a research grant from Perkléns Foundation. The researchers had no financial conflicts to disclose. Dr. Ketwaroo had no financial conflicts to disclose but is a member of the GI & Hepatology News editorial advisory board.
Patients with infected pancreatic necrosis (IPN) are more likely to experience organ failure and mortality, which makes identifying them as quickly as possible especially crucial. A new study aimed to make this task a bit easier by categorizing the main risk factors for IPN in a cohort of patients with severe acute pancreatitis, which included extensive spread of necrotic collections, preceding bacteremia, and preceding open abdomen treatment, as well as postinterventional pancreatitis.
In their study, published in the Journal of Gastrointestinal Surgery, Henrik L. Husu, MD, of the University of Helsinki, and colleagues noted the inherent challenges of rendering a preoperative diagnosis of IPN.
“Fever and increasing inflammation markers may indicate suspicion of IPN, but these are very common in patients with severe acute pancreatitis treated in the ICU,” and more knowledge of specific IPN risk factors is needed to improve clinical decision-making, they said.
Dr. Husu and colleagues identified 163 adults with acute pancreatitis admitted to the ICU at a single center between 2010 and 2018, approximately 68% of whom had alcoholic necrotizing pancreatitis. Pneumonia, bacteremia, and IPN occurred at an average of 4, 16, and 23 days, respectively, after ICU admission.
Forty-seven patients (28.8%) developed IPN within 90 days of ICU admission, all patients had a least one persistent organ failure, and 60% had multiple organ failure within 24 hours of ICU admission.
In a multivariate regression analysis, independent risk factors for IPN included postoperative or postendoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (odds ratio,13.5) and widespread necrotic collections (OR, 5.7 for unilateral paracolic or retromesenteric; OR, 21.8 for bilateral paracolic or unilateral paracolic and retromesenteric). Other risk factors were preceding bacteremia (OR, 4.8) and preceding open abdomen treatment for abdominal compartment syndrome (OR, 3.6).
After 90 days, 29 patients had died, including 7 with IPN and 22 without IPN. In addition, patients with IPN had longer overall hospital stays and ICU stays, higher rates of ICU readmission, and greater use of open necrosectomy, the researchers noted.
The study findings were limited by several factors, including the retrospective design, lack of controls, potential differences in treatment protocols, and the survival bias that prevented direct comparison of mortality in patients with and without IPN, the researchers noted. “This study cannot provide a reliable estimate of the difference in mortality attributable to IPN itself.”
However, the researchers noted that “the strength of the present study was to include only patients with persistent organ failure and admission to ICU in the early disease course,” and results indicate a significant morbid outcome associated with IPN. “In attempting to decrease the rate of IPN, efforts to identify and treat incipient organ failure with subsequent low threshold for admission to ICU becomes essential,” they emphasized.
More data may prompt greater intervention
“IPN portends a poor prognosis, and can be challenging to both diagnose and treat,” Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, said in an interview. “Identifying risk factors for development of IPN may facilitate earlier therapy that could modify the natural history of this disease.”
Dr. Ketwaroo said he was not surprised by the study findings. “This was a small single-center, retrospective study, where infection could only be ascertained among those who received interventions, and the findings should thus be interpreted within these limitations. Overall, however, I was not surprised. More extensive necrosis and opportunities for infectious seeding of necrosis such as interventions (ERCP) and bacteremia would be expected risk factors. I was surprised by the use of prophylactic antibiotics, as well as the high rate of open necrosectomy, though this should not affect the main findings of risk factors for infection.
“The studies highlight that a significant portion of patients with severe acute pancreatitis with necrosis will develop infection,” said Dr. Ketwaroo. “Being aware of the risk factors for infection, as identified in this study, can add to our clinical judgment in suspecting infection and opting for debridement. Especially with advancements in endoscopic necrosectomy, gastroenterologists may be more inclined to intervene when suspecting IPN. The next steps for research are to validate risk factors in larger, prospective studies.”
The study was supported by governmental competitive funds for medical research, a research grant from the Medical Society of Finland, and a research grant from Perkléns Foundation. The researchers had no financial conflicts to disclose. Dr. Ketwaroo had no financial conflicts to disclose but is a member of the GI & Hepatology News editorial advisory board.
FROM THE JOURNAL OF GASTROINTESTINAL SURGERY
AGA Shark Tank 2021: A simple design survives
William of Ockham would have been proud because, at this year’s American Gastroenterological Association’s Shark Tank pitch competition, one product clearly demonstrated Ockham’s razor – that sometimes the simplest solution is best – and came away as the winner at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Out of five innovative products, ranging from an educational app to a high-tech anorectal sensor, all aimed at improving outcomes in patients with gastrointestinal disorders, the winner was ... drumroll please ...
A needle.
That’s it. A needle. But not like any other needle.
Winner: Toufic Kachaamy, MD, FASGE, AGAF – An EUS-guided access needle
This EUS-guided access needle, invented by Dr. Kachaamy, enterprise clinical leader at Cancer Treatment Centers of America, Phoenix, is a simple device that overcomes a longstanding challenge presented by endoscopic retrograde cholangiopancreatography (ERCP): biliary access.
Many “ERCPs are considered difficult, and sometimes fail, depending on the center and the endoscopist,” Dr. Kachaamy said during a virtual presentation. “Most failures are due to failed initial access to the bile duct.”
Indeed, one study cited a failure rate in ductal cannulation of 5%-15% even among experienced hands.
Failure can have several consequences, Dr. Kachaamy noted, including increased complications, higher cost, delayed care, longer hospitalization, and greater likelihood of patient transfer.
He went on to explain why biliary access can be so challenging and how this EUS-guided access needle helps address these issues.
“[The] two main limitations [during endoscopic ultrasound–guided biliary access] are directing the wire into the narrowed areas and the wire shearing as we are manipulating the wire to get it to where we want it,” Dr. Kachaamy said. “[This EUS-guided access needle] is a 19-22 gauge, rotatable needle with a smooth, side exit for the wire to allow wire manipulation and direction without shearing.”
Dr. Kachaamy highlighted the simple design, which will keep the production cost below $300 per unit, and suggested that failed ERCPs are just the first potential indication of many. Future uses may include gallbladder access, peri-GI collection, gastrojejunostomy, and others.
In an interview, Dr. Kachaamy reacted to the win, which follows 2 years of collaborative development with Cancer Treatment Centers of America.
“For people who are innovators, there’s nothing that feels more rewarding than their ideas being recognized as adding something to the field and potentially helping people and patients,” Dr. Kachaamy said. “So [this is] very, very, very exciting. Very rewarding. Pride would probably be the best way I’d describe it.”
Dr. Kachaamy anticipates that this EUS-guided access needle will be commercially available within 1-2 years, pending regulatory approval. In the meantime, he and his colleagues are seeking a strategic partner.
A shark speaks
V. Raman Muthusamy, MD, AGAF, immediate past chair of the AGA Center for GI Innovation and Technology and director of endoscopy at UCLA Health System, moderated the Shark Tank session, calling it “the highlight” of the AGA Tech Summit.
Dr. Muthusamy and four other “sharks,” including a gastroenterologist, venture capitalist, regulatory device reviewer, and entrepreneur, scored the pitches using three equally weighted categories: the quality of the pitch, the level of innovation and impact on the field, and the quality of the business plan and overall feasibility.
“We saw a full spectrum [of innovations],” Dr. Muthusamy said. “I think it was an enjoyable session.”
Behind closed doors, the sharks narrowed the field to two top contenders. Ultimately, however, there could be only one winner: Dr. Kachaamy. Their decision aligned with a “Fan Favorite” audience poll.
“A lot of [Dr. Kachaamy’s win] had to do with the potential applications and commonality of the problem,” Dr. Muthusamy said in an interview. He highlighted how the EUS-guided access needle allows for an immediate response to ERCP failure without the need for a second procedure.
Dr. Muthusamy also noted that several product designs previously failed to achieve what the EUS-guided access needle has the potential to do.
“I think the feeling was that this seemed to be a way that may address some of the limitations and challenges that we’ve had with earlier [attempts at solving this problem],” Dr. Muthusamy said.
For innovators who didn’t make the cut this year, or those with products still in development, Dr. Muthusamy suggested applying next year.
“We encourage our colleagues and members of the AGA to continue to apply to this program,” Dr. Muthusamy said.
Other fish in the sea
Four other innovators entered the AGA Shark Tank this year. Here are snippets of their pitches:
Hans Gregersen, MD, PhD, MPH – Fecobionics
“Fecobionics is a simulated electronic stool with the consistency and shape of normal stool,” Dr. Gregersen said.
The balloon device, which contains multiple sensors, provides “real-time, quantitative, and mechanistic insights by simulating defecation.”
“It ... is inserted into the rectum,” Dr. Gregersen said. “It measures multiple pressures; it has gyroscopes that measure orientation; we can compute the bending of the device; and we can calculate the shape of the device.”
According to Dr. Gregersen, Fecobionics has “diagnostic potential for patients with fecal incontinence and for subtyping patients with constipation.” He highlighted fewer false-positives than current technology, alongside greater efficiency and lower cost.
Dr. Gregersen is a research professor at California Medical Innovations Institute, San Diego.
Mary J. Pattison, RN – Trans-Abdominal Gastric Surgical System (TAGSS)
TAGSS is a trans-abdominal gastric access device that “represents a novel and exciting means to address multiple gastrointestinal conditions that are without a standardized approach,” Ms. Pattison said. “Placed as simply as a [percutaneous endoscopic gastrostomy tube], TAGSS offers disruptive technology to address [gastroesophageal reflux disease], fundoplication, achalasia, gastroparesis, gastric tumors, and even obesity in a safe, efficient, and cost effective manner. TAGSS offers the first true hybrid approach for endoscopic/laparoscopic collaboration.”
Ms. Pattison is a nurse clinician and endoscopy assistant at WestGlen GI Consultants, Weston, Mo.
Pankaj Rajvanshi, MD, FAASLD – Healthswim App
“At this time, most patient education is provided by Dr. Google,” Dr. Rajvanshi said, “and we want to change that. We have built a platform which allows you, the physician, to create custom, curated, credible content that can be delivered seamlessly to your patients on an ongoing basis.”
Through the Healthswim app, patients subscribe to their providers, allowing access physician-approved content. Subscribers also receive provider updates through their social media feeds.
Dr. Rajvanshi is a gastroenterologist at Swedish Medical Center, Seattle.
Ali S. Karakurum, MD, FACP, FACG – A Device for Removal of Esophageal Food Impactions
“I would like to propose a device which consists of a clear overtube, a collapsible plastic cylindrical basket secured to the distal end of the overtube ... and a snare wire attached to the distal end of the basket which is controlled by the snare handle externally,” Dr. Karakurum said. “The device is ... gradually advanced over the scope for the basket to encompass the food bolus under direct visualization. Once the food bolus is within the basket, the wire loop at the end of the basket is closed via the external handle, securing the food bolus in the basket for safe removal.”
Dr. Karakurum is a gastroenterologist at Advanced Gastroenterology & Endoscopy, Port Jefferson, N.Y.
This article was updated 5/14/21.
William of Ockham would have been proud because, at this year’s American Gastroenterological Association’s Shark Tank pitch competition, one product clearly demonstrated Ockham’s razor – that sometimes the simplest solution is best – and came away as the winner at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Out of five innovative products, ranging from an educational app to a high-tech anorectal sensor, all aimed at improving outcomes in patients with gastrointestinal disorders, the winner was ... drumroll please ...
A needle.
That’s it. A needle. But not like any other needle.
Winner: Toufic Kachaamy, MD, FASGE, AGAF – An EUS-guided access needle
This EUS-guided access needle, invented by Dr. Kachaamy, enterprise clinical leader at Cancer Treatment Centers of America, Phoenix, is a simple device that overcomes a longstanding challenge presented by endoscopic retrograde cholangiopancreatography (ERCP): biliary access.
Many “ERCPs are considered difficult, and sometimes fail, depending on the center and the endoscopist,” Dr. Kachaamy said during a virtual presentation. “Most failures are due to failed initial access to the bile duct.”
Indeed, one study cited a failure rate in ductal cannulation of 5%-15% even among experienced hands.
Failure can have several consequences, Dr. Kachaamy noted, including increased complications, higher cost, delayed care, longer hospitalization, and greater likelihood of patient transfer.
He went on to explain why biliary access can be so challenging and how this EUS-guided access needle helps address these issues.
“[The] two main limitations [during endoscopic ultrasound–guided biliary access] are directing the wire into the narrowed areas and the wire shearing as we are manipulating the wire to get it to where we want it,” Dr. Kachaamy said. “[This EUS-guided access needle] is a 19-22 gauge, rotatable needle with a smooth, side exit for the wire to allow wire manipulation and direction without shearing.”
Dr. Kachaamy highlighted the simple design, which will keep the production cost below $300 per unit, and suggested that failed ERCPs are just the first potential indication of many. Future uses may include gallbladder access, peri-GI collection, gastrojejunostomy, and others.
In an interview, Dr. Kachaamy reacted to the win, which follows 2 years of collaborative development with Cancer Treatment Centers of America.
“For people who are innovators, there’s nothing that feels more rewarding than their ideas being recognized as adding something to the field and potentially helping people and patients,” Dr. Kachaamy said. “So [this is] very, very, very exciting. Very rewarding. Pride would probably be the best way I’d describe it.”
Dr. Kachaamy anticipates that this EUS-guided access needle will be commercially available within 1-2 years, pending regulatory approval. In the meantime, he and his colleagues are seeking a strategic partner.
A shark speaks
V. Raman Muthusamy, MD, AGAF, immediate past chair of the AGA Center for GI Innovation and Technology and director of endoscopy at UCLA Health System, moderated the Shark Tank session, calling it “the highlight” of the AGA Tech Summit.
Dr. Muthusamy and four other “sharks,” including a gastroenterologist, venture capitalist, regulatory device reviewer, and entrepreneur, scored the pitches using three equally weighted categories: the quality of the pitch, the level of innovation and impact on the field, and the quality of the business plan and overall feasibility.
“We saw a full spectrum [of innovations],” Dr. Muthusamy said. “I think it was an enjoyable session.”
Behind closed doors, the sharks narrowed the field to two top contenders. Ultimately, however, there could be only one winner: Dr. Kachaamy. Their decision aligned with a “Fan Favorite” audience poll.
“A lot of [Dr. Kachaamy’s win] had to do with the potential applications and commonality of the problem,” Dr. Muthusamy said in an interview. He highlighted how the EUS-guided access needle allows for an immediate response to ERCP failure without the need for a second procedure.
Dr. Muthusamy also noted that several product designs previously failed to achieve what the EUS-guided access needle has the potential to do.
“I think the feeling was that this seemed to be a way that may address some of the limitations and challenges that we’ve had with earlier [attempts at solving this problem],” Dr. Muthusamy said.
For innovators who didn’t make the cut this year, or those with products still in development, Dr. Muthusamy suggested applying next year.
“We encourage our colleagues and members of the AGA to continue to apply to this program,” Dr. Muthusamy said.
Other fish in the sea
Four other innovators entered the AGA Shark Tank this year. Here are snippets of their pitches:
Hans Gregersen, MD, PhD, MPH – Fecobionics
“Fecobionics is a simulated electronic stool with the consistency and shape of normal stool,” Dr. Gregersen said.
The balloon device, which contains multiple sensors, provides “real-time, quantitative, and mechanistic insights by simulating defecation.”
“It ... is inserted into the rectum,” Dr. Gregersen said. “It measures multiple pressures; it has gyroscopes that measure orientation; we can compute the bending of the device; and we can calculate the shape of the device.”
According to Dr. Gregersen, Fecobionics has “diagnostic potential for patients with fecal incontinence and for subtyping patients with constipation.” He highlighted fewer false-positives than current technology, alongside greater efficiency and lower cost.
Dr. Gregersen is a research professor at California Medical Innovations Institute, San Diego.
Mary J. Pattison, RN – Trans-Abdominal Gastric Surgical System (TAGSS)
TAGSS is a trans-abdominal gastric access device that “represents a novel and exciting means to address multiple gastrointestinal conditions that are without a standardized approach,” Ms. Pattison said. “Placed as simply as a [percutaneous endoscopic gastrostomy tube], TAGSS offers disruptive technology to address [gastroesophageal reflux disease], fundoplication, achalasia, gastroparesis, gastric tumors, and even obesity in a safe, efficient, and cost effective manner. TAGSS offers the first true hybrid approach for endoscopic/laparoscopic collaboration.”
Ms. Pattison is a nurse clinician and endoscopy assistant at WestGlen GI Consultants, Weston, Mo.
Pankaj Rajvanshi, MD, FAASLD – Healthswim App
“At this time, most patient education is provided by Dr. Google,” Dr. Rajvanshi said, “and we want to change that. We have built a platform which allows you, the physician, to create custom, curated, credible content that can be delivered seamlessly to your patients on an ongoing basis.”
Through the Healthswim app, patients subscribe to their providers, allowing access physician-approved content. Subscribers also receive provider updates through their social media feeds.
Dr. Rajvanshi is a gastroenterologist at Swedish Medical Center, Seattle.
Ali S. Karakurum, MD, FACP, FACG – A Device for Removal of Esophageal Food Impactions
“I would like to propose a device which consists of a clear overtube, a collapsible plastic cylindrical basket secured to the distal end of the overtube ... and a snare wire attached to the distal end of the basket which is controlled by the snare handle externally,” Dr. Karakurum said. “The device is ... gradually advanced over the scope for the basket to encompass the food bolus under direct visualization. Once the food bolus is within the basket, the wire loop at the end of the basket is closed via the external handle, securing the food bolus in the basket for safe removal.”
Dr. Karakurum is a gastroenterologist at Advanced Gastroenterology & Endoscopy, Port Jefferson, N.Y.
This article was updated 5/14/21.
William of Ockham would have been proud because, at this year’s American Gastroenterological Association’s Shark Tank pitch competition, one product clearly demonstrated Ockham’s razor – that sometimes the simplest solution is best – and came away as the winner at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Out of five innovative products, ranging from an educational app to a high-tech anorectal sensor, all aimed at improving outcomes in patients with gastrointestinal disorders, the winner was ... drumroll please ...
A needle.
That’s it. A needle. But not like any other needle.
Winner: Toufic Kachaamy, MD, FASGE, AGAF – An EUS-guided access needle
This EUS-guided access needle, invented by Dr. Kachaamy, enterprise clinical leader at Cancer Treatment Centers of America, Phoenix, is a simple device that overcomes a longstanding challenge presented by endoscopic retrograde cholangiopancreatography (ERCP): biliary access.
Many “ERCPs are considered difficult, and sometimes fail, depending on the center and the endoscopist,” Dr. Kachaamy said during a virtual presentation. “Most failures are due to failed initial access to the bile duct.”
Indeed, one study cited a failure rate in ductal cannulation of 5%-15% even among experienced hands.
Failure can have several consequences, Dr. Kachaamy noted, including increased complications, higher cost, delayed care, longer hospitalization, and greater likelihood of patient transfer.
He went on to explain why biliary access can be so challenging and how this EUS-guided access needle helps address these issues.
“[The] two main limitations [during endoscopic ultrasound–guided biliary access] are directing the wire into the narrowed areas and the wire shearing as we are manipulating the wire to get it to where we want it,” Dr. Kachaamy said. “[This EUS-guided access needle] is a 19-22 gauge, rotatable needle with a smooth, side exit for the wire to allow wire manipulation and direction without shearing.”
Dr. Kachaamy highlighted the simple design, which will keep the production cost below $300 per unit, and suggested that failed ERCPs are just the first potential indication of many. Future uses may include gallbladder access, peri-GI collection, gastrojejunostomy, and others.
In an interview, Dr. Kachaamy reacted to the win, which follows 2 years of collaborative development with Cancer Treatment Centers of America.
“For people who are innovators, there’s nothing that feels more rewarding than their ideas being recognized as adding something to the field and potentially helping people and patients,” Dr. Kachaamy said. “So [this is] very, very, very exciting. Very rewarding. Pride would probably be the best way I’d describe it.”
Dr. Kachaamy anticipates that this EUS-guided access needle will be commercially available within 1-2 years, pending regulatory approval. In the meantime, he and his colleagues are seeking a strategic partner.
A shark speaks
V. Raman Muthusamy, MD, AGAF, immediate past chair of the AGA Center for GI Innovation and Technology and director of endoscopy at UCLA Health System, moderated the Shark Tank session, calling it “the highlight” of the AGA Tech Summit.
Dr. Muthusamy and four other “sharks,” including a gastroenterologist, venture capitalist, regulatory device reviewer, and entrepreneur, scored the pitches using three equally weighted categories: the quality of the pitch, the level of innovation and impact on the field, and the quality of the business plan and overall feasibility.
“We saw a full spectrum [of innovations],” Dr. Muthusamy said. “I think it was an enjoyable session.”
Behind closed doors, the sharks narrowed the field to two top contenders. Ultimately, however, there could be only one winner: Dr. Kachaamy. Their decision aligned with a “Fan Favorite” audience poll.
“A lot of [Dr. Kachaamy’s win] had to do with the potential applications and commonality of the problem,” Dr. Muthusamy said in an interview. He highlighted how the EUS-guided access needle allows for an immediate response to ERCP failure without the need for a second procedure.
Dr. Muthusamy also noted that several product designs previously failed to achieve what the EUS-guided access needle has the potential to do.
“I think the feeling was that this seemed to be a way that may address some of the limitations and challenges that we’ve had with earlier [attempts at solving this problem],” Dr. Muthusamy said.
For innovators who didn’t make the cut this year, or those with products still in development, Dr. Muthusamy suggested applying next year.
“We encourage our colleagues and members of the AGA to continue to apply to this program,” Dr. Muthusamy said.
Other fish in the sea
Four other innovators entered the AGA Shark Tank this year. Here are snippets of their pitches:
Hans Gregersen, MD, PhD, MPH – Fecobionics
“Fecobionics is a simulated electronic stool with the consistency and shape of normal stool,” Dr. Gregersen said.
The balloon device, which contains multiple sensors, provides “real-time, quantitative, and mechanistic insights by simulating defecation.”
“It ... is inserted into the rectum,” Dr. Gregersen said. “It measures multiple pressures; it has gyroscopes that measure orientation; we can compute the bending of the device; and we can calculate the shape of the device.”
According to Dr. Gregersen, Fecobionics has “diagnostic potential for patients with fecal incontinence and for subtyping patients with constipation.” He highlighted fewer false-positives than current technology, alongside greater efficiency and lower cost.
Dr. Gregersen is a research professor at California Medical Innovations Institute, San Diego.
Mary J. Pattison, RN – Trans-Abdominal Gastric Surgical System (TAGSS)
TAGSS is a trans-abdominal gastric access device that “represents a novel and exciting means to address multiple gastrointestinal conditions that are without a standardized approach,” Ms. Pattison said. “Placed as simply as a [percutaneous endoscopic gastrostomy tube], TAGSS offers disruptive technology to address [gastroesophageal reflux disease], fundoplication, achalasia, gastroparesis, gastric tumors, and even obesity in a safe, efficient, and cost effective manner. TAGSS offers the first true hybrid approach for endoscopic/laparoscopic collaboration.”
Ms. Pattison is a nurse clinician and endoscopy assistant at WestGlen GI Consultants, Weston, Mo.
Pankaj Rajvanshi, MD, FAASLD – Healthswim App
“At this time, most patient education is provided by Dr. Google,” Dr. Rajvanshi said, “and we want to change that. We have built a platform which allows you, the physician, to create custom, curated, credible content that can be delivered seamlessly to your patients on an ongoing basis.”
Through the Healthswim app, patients subscribe to their providers, allowing access physician-approved content. Subscribers also receive provider updates through their social media feeds.
Dr. Rajvanshi is a gastroenterologist at Swedish Medical Center, Seattle.
Ali S. Karakurum, MD, FACP, FACG – A Device for Removal of Esophageal Food Impactions
“I would like to propose a device which consists of a clear overtube, a collapsible plastic cylindrical basket secured to the distal end of the overtube ... and a snare wire attached to the distal end of the basket which is controlled by the snare handle externally,” Dr. Karakurum said. “The device is ... gradually advanced over the scope for the basket to encompass the food bolus under direct visualization. Once the food bolus is within the basket, the wire loop at the end of the basket is closed via the external handle, securing the food bolus in the basket for safe removal.”
Dr. Karakurum is a gastroenterologist at Advanced Gastroenterology & Endoscopy, Port Jefferson, N.Y.
This article was updated 5/14/21.
FROM THE 2021 AGA TECH SUMMIT MEETING










