User login
Composite Scale Better Gauges Mucosal Injury in Celiac Disease
, according to a study in Clinical Gastroenterology and Hepatology.
The new morphometric duodenal biopsy mucosal scale joins together villous height-to-crypt depth ratio (Vh:Cd) and intraepithelial lymphocytes (IEL) — each key CeD histological measures of the small intestine — in a scale called VCIEL.
The authors believe the VCIEL will enable a broader and more accurate measurement of mucosal health in CeD. It will be particularly useful for population analysis in clinical trials and could improve the powering of trial design. “Use of VCIEL may lead to better outcome measures for potential new therapeutic treatments benefiting patients,” wrote Jocelyn A. Silvester, MD, PhD, a pediatrician at Boston Children’s Hospital and an assistant professor at Harvard Medical School, and colleagues.
This chronic enteropathy affects about 1% of the world’s population and requires a lifelong adherence to a gluten-free diet, the authors noted.
The authors pointed to weaknesses in the current quantitative and qualitative ways of measuring gluten-induced mucosal injury on biopsy for CeD. “Morphometry measures the injury continuum for architecture and inflammation, but these are used as separate outcomes,” they wrote. “The original Marsh-Oberhuber [M-O] classifications are rather contrived approaches to assess a biologic continuum, forcing the injury in categorical groups of unclear clinical relevance and where clinically significant changes may occur within one single category.”
Moreover, the quantitation of inflammation relies on binary assessment as normal or increased, which results in histology that is unscorable by M-O if villous atrophy persists without increased IELs, they added.
The Study
In the absence of a broadly accepted single measure of mucosal injury in CeD, the group assessed whether the composite metric could improve statistical precision for assessing histology.
Enter VCIEL, which combines the Vh:Cd and IEL for individual patients with equal weighting by converting each scale to a fraction of their standard deviation and summing the results.
The researchers applied the VCIEL formula in a reanalysis of four clinical gluten-challenge trials and compared the results for Vh:Cd and IEL separately with those for VCIEL for clinical significance (effect size) and statistical significance.
In reanalysis of the ALV003-1021 trial, for example, the researchers observed an effect size and P value (analysis of covariance) of 1.37 and .038 for a delta (difference) value of Vh:Cd 1.17 and .005 for IEL and 1.86 and .004 for VCIEL.
For the similar gluten-challenge IMGX003-NCCIH-1721 trial, the corresponding delta results were .76 and .057 for Vh:Cd, .98 and .018 for IEL, and 1.14 and .007 for VCIEL. Comparable improvements with VCIEL over individual Vh:Cd and IEL were observed for other studies, including a nontherapeutic gluten challenge study.
In NCT03409796 trial data, the computation of VCIEL values showed an improved statistical significance relative to the component values of Vh:Cd and IEL by the within-group paired 2-tailed t test P values from baseline to day 15, particularly at a 10-g gluten challenge dose: Vh:Cd, IEL, VCIEL = .0050, .0031, and .0014, respectively.
Little correlation emerged between baseline values and changes with intervention for Vh:Cd and IEL on an individual patient basis.
The greater accuracy and statistical precision of the VCIEL scale are presumably due to averaging over some of the measurement uncertainty in individual patient and timepoint Vh:Cd and IEL values and creating a composite of different histologic properties, the authors noted.
This study was funded by ImmunogenX, Inc. First author Jack A. Syage is a cofounder and shareholder in ImmunogenX Inc. Dr. Silvester has served on an advisory board for Takeda Pharmaceuticals and has received research funding from Biomedal S.L., Cour Pharmaceuticals, and Glutenostics LLC. Several coauthors disclosed various financial ties to multiple private-sector pharmaceutical and biomedical companies, including ImmunogenX.
, according to a study in Clinical Gastroenterology and Hepatology.
The new morphometric duodenal biopsy mucosal scale joins together villous height-to-crypt depth ratio (Vh:Cd) and intraepithelial lymphocytes (IEL) — each key CeD histological measures of the small intestine — in a scale called VCIEL.
The authors believe the VCIEL will enable a broader and more accurate measurement of mucosal health in CeD. It will be particularly useful for population analysis in clinical trials and could improve the powering of trial design. “Use of VCIEL may lead to better outcome measures for potential new therapeutic treatments benefiting patients,” wrote Jocelyn A. Silvester, MD, PhD, a pediatrician at Boston Children’s Hospital and an assistant professor at Harvard Medical School, and colleagues.
This chronic enteropathy affects about 1% of the world’s population and requires a lifelong adherence to a gluten-free diet, the authors noted.
The authors pointed to weaknesses in the current quantitative and qualitative ways of measuring gluten-induced mucosal injury on biopsy for CeD. “Morphometry measures the injury continuum for architecture and inflammation, but these are used as separate outcomes,” they wrote. “The original Marsh-Oberhuber [M-O] classifications are rather contrived approaches to assess a biologic continuum, forcing the injury in categorical groups of unclear clinical relevance and where clinically significant changes may occur within one single category.”
Moreover, the quantitation of inflammation relies on binary assessment as normal or increased, which results in histology that is unscorable by M-O if villous atrophy persists without increased IELs, they added.
The Study
In the absence of a broadly accepted single measure of mucosal injury in CeD, the group assessed whether the composite metric could improve statistical precision for assessing histology.
Enter VCIEL, which combines the Vh:Cd and IEL for individual patients with equal weighting by converting each scale to a fraction of their standard deviation and summing the results.
The researchers applied the VCIEL formula in a reanalysis of four clinical gluten-challenge trials and compared the results for Vh:Cd and IEL separately with those for VCIEL for clinical significance (effect size) and statistical significance.
In reanalysis of the ALV003-1021 trial, for example, the researchers observed an effect size and P value (analysis of covariance) of 1.37 and .038 for a delta (difference) value of Vh:Cd 1.17 and .005 for IEL and 1.86 and .004 for VCIEL.
For the similar gluten-challenge IMGX003-NCCIH-1721 trial, the corresponding delta results were .76 and .057 for Vh:Cd, .98 and .018 for IEL, and 1.14 and .007 for VCIEL. Comparable improvements with VCIEL over individual Vh:Cd and IEL were observed for other studies, including a nontherapeutic gluten challenge study.
In NCT03409796 trial data, the computation of VCIEL values showed an improved statistical significance relative to the component values of Vh:Cd and IEL by the within-group paired 2-tailed t test P values from baseline to day 15, particularly at a 10-g gluten challenge dose: Vh:Cd, IEL, VCIEL = .0050, .0031, and .0014, respectively.
Little correlation emerged between baseline values and changes with intervention for Vh:Cd and IEL on an individual patient basis.
The greater accuracy and statistical precision of the VCIEL scale are presumably due to averaging over some of the measurement uncertainty in individual patient and timepoint Vh:Cd and IEL values and creating a composite of different histologic properties, the authors noted.
This study was funded by ImmunogenX, Inc. First author Jack A. Syage is a cofounder and shareholder in ImmunogenX Inc. Dr. Silvester has served on an advisory board for Takeda Pharmaceuticals and has received research funding from Biomedal S.L., Cour Pharmaceuticals, and Glutenostics LLC. Several coauthors disclosed various financial ties to multiple private-sector pharmaceutical and biomedical companies, including ImmunogenX.
, according to a study in Clinical Gastroenterology and Hepatology.
The new morphometric duodenal biopsy mucosal scale joins together villous height-to-crypt depth ratio (Vh:Cd) and intraepithelial lymphocytes (IEL) — each key CeD histological measures of the small intestine — in a scale called VCIEL.
The authors believe the VCIEL will enable a broader and more accurate measurement of mucosal health in CeD. It will be particularly useful for population analysis in clinical trials and could improve the powering of trial design. “Use of VCIEL may lead to better outcome measures for potential new therapeutic treatments benefiting patients,” wrote Jocelyn A. Silvester, MD, PhD, a pediatrician at Boston Children’s Hospital and an assistant professor at Harvard Medical School, and colleagues.
This chronic enteropathy affects about 1% of the world’s population and requires a lifelong adherence to a gluten-free diet, the authors noted.
The authors pointed to weaknesses in the current quantitative and qualitative ways of measuring gluten-induced mucosal injury on biopsy for CeD. “Morphometry measures the injury continuum for architecture and inflammation, but these are used as separate outcomes,” they wrote. “The original Marsh-Oberhuber [M-O] classifications are rather contrived approaches to assess a biologic continuum, forcing the injury in categorical groups of unclear clinical relevance and where clinically significant changes may occur within one single category.”
Moreover, the quantitation of inflammation relies on binary assessment as normal or increased, which results in histology that is unscorable by M-O if villous atrophy persists without increased IELs, they added.
The Study
In the absence of a broadly accepted single measure of mucosal injury in CeD, the group assessed whether the composite metric could improve statistical precision for assessing histology.
Enter VCIEL, which combines the Vh:Cd and IEL for individual patients with equal weighting by converting each scale to a fraction of their standard deviation and summing the results.
The researchers applied the VCIEL formula in a reanalysis of four clinical gluten-challenge trials and compared the results for Vh:Cd and IEL separately with those for VCIEL for clinical significance (effect size) and statistical significance.
In reanalysis of the ALV003-1021 trial, for example, the researchers observed an effect size and P value (analysis of covariance) of 1.37 and .038 for a delta (difference) value of Vh:Cd 1.17 and .005 for IEL and 1.86 and .004 for VCIEL.
For the similar gluten-challenge IMGX003-NCCIH-1721 trial, the corresponding delta results were .76 and .057 for Vh:Cd, .98 and .018 for IEL, and 1.14 and .007 for VCIEL. Comparable improvements with VCIEL over individual Vh:Cd and IEL were observed for other studies, including a nontherapeutic gluten challenge study.
In NCT03409796 trial data, the computation of VCIEL values showed an improved statistical significance relative to the component values of Vh:Cd and IEL by the within-group paired 2-tailed t test P values from baseline to day 15, particularly at a 10-g gluten challenge dose: Vh:Cd, IEL, VCIEL = .0050, .0031, and .0014, respectively.
Little correlation emerged between baseline values and changes with intervention for Vh:Cd and IEL on an individual patient basis.
The greater accuracy and statistical precision of the VCIEL scale are presumably due to averaging over some of the measurement uncertainty in individual patient and timepoint Vh:Cd and IEL values and creating a composite of different histologic properties, the authors noted.
This study was funded by ImmunogenX, Inc. First author Jack A. Syage is a cofounder and shareholder in ImmunogenX Inc. Dr. Silvester has served on an advisory board for Takeda Pharmaceuticals and has received research funding from Biomedal S.L., Cour Pharmaceuticals, and Glutenostics LLC. Several coauthors disclosed various financial ties to multiple private-sector pharmaceutical and biomedical companies, including ImmunogenX.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Check out our new Crohn’s disease clinician toolkit!
Have you ever wished you could access all of our Crohn’s disease resources in one place? We’ve compiled our Crohn’s disease clinical guidance, continuing education resources, patient education, and FAQs into one convenient toolkit.
Toolkit includes clinical guidance on:
- Role of biomarkers for the management of Crohn’s disease
- Medical management of moderate to severe luminal and perianal fistulizing Crohn’s disease
- Diet and nutritional therapies in patients with IBD
Check it out at www.gastro.org/toolkit.
Have you ever wished you could access all of our Crohn’s disease resources in one place? We’ve compiled our Crohn’s disease clinical guidance, continuing education resources, patient education, and FAQs into one convenient toolkit.
Toolkit includes clinical guidance on:
- Role of biomarkers for the management of Crohn’s disease
- Medical management of moderate to severe luminal and perianal fistulizing Crohn’s disease
- Diet and nutritional therapies in patients with IBD
Check it out at www.gastro.org/toolkit.
Have you ever wished you could access all of our Crohn’s disease resources in one place? We’ve compiled our Crohn’s disease clinical guidance, continuing education resources, patient education, and FAQs into one convenient toolkit.
Toolkit includes clinical guidance on:
- Role of biomarkers for the management of Crohn’s disease
- Medical management of moderate to severe luminal and perianal fistulizing Crohn’s disease
- Diet and nutritional therapies in patients with IBD
Check it out at www.gastro.org/toolkit.
High-Quality Diet in Early Life May Ward Off Later IBD
, prospective pooled data from two Scandinavian birth cohorts suggested.
It appears important to feed children a quality diet at a very young age, in particular one rich in vegetables and fish, since by age three, only dietary fish intake had any impact on IBD risk.
Although high intakes of these two food categories in very early life correlated with lower IBD risk, exposure to sugar-sweetened beverages (SSBs) was associated with an increased risk. “While non-causal explanations for our results cannot be ruled out, these novel findings are consistent with the hypothesis that early-life diet, possibly mediated through changes in the gut microbiome, may affect the risk of developing IBD,” wrote lead author Annie Guo, a PhD candidate in the Department of Pediatrics, University of Gothenburg, Sweden, and colleagues. The report was published in Gut.
“This is a population-based study investigating the risk for IBD, rather than the specific effect of diet,” Ms. Guo said in an interview. “Therefore, the results are not enough on their own to be translated into individual advice that can be applicable in the clinic. However, the study supports current dietary guidelines for small children, that is, the intake of sugar should be limited and a higher intake of fish and vegetables is beneficial for overall health.”
Two-Cohort Study
The investigators prospectively recorded food-group information on children (just under half were female) from the All Babies in Southeast Sweden and The Norwegian Mother, Father and Child Cohort Study to assess the diet quality using a Healthy Eating Index and intake frequency. Parents answered questions about their offspring’s diet at ages 12-18 months and 30-36 months. Quality of diet was measured by intake of meat, fish, fruit, vegetables, dairy, sweets, snacks, and drinks.
The Swedish cohort included 21,700 children born between October 1997 and October 1999, while the Norwegian analysis included 114,500 children, 95,200 mothers, and 75,200 fathers recruited from across Norway from 1999 to 2008. In 1,304,433 person-years of follow-up, the researchers tracked 81,280 participants from birth to childhood and adolescence, with median follow-ups in the two cohorts ranging from 1 year of age to 21.3 years (Sweden) and to 15.2 years of age (Norway). Of these children, 307 were diagnosed with IBD: Crohn’s disease (CD; n = 131); ulcerative colitis (UC; n = 97); and IBD unclassified (n = 79).
Adjusting for parental IBD history, sex, origin, education, and maternal comorbidities, the study found:
- Compared with low-quality diet, both medium- and high-quality diets at 1 year were associated with a roughly 25% reduced risk for IBD (pooled adjusted hazard ratio [aHR], 0.75 [95% CI, 0.58-0.98] and 0.75 [0.56-1.0], respectively).
- The pooled aHR per increase of category was 0.86 (95% CI, 0.74-0.99). The pooled aHR for IBD in 1-year-olds with high vs low fish intake was 0.70 (95% CI, 0.49-1.0), and this diet showed an association with a reduced risk for UC (pooled aHR, 0.46; 95% CI, 0.21-0.99). Higher vegetable intake at 1 year was also associated with a risk reduction in IBD (HR, 0.72; 95% CI, 0.55-0.95). It has been hypothesized that intake of vegetables and vegetable fibers may have programming effects on the immune system.
- AutoWith 72% of children reportedly consuming SSBs at age 1, pooled aHRs showed that some vs no intake of SSBs was associated with an increased risk for later IBD (pooled aHR, 1.42; 95% CI, 1.05-1.90).
- There were no obvious associations between overall IBD or CD/UC risk and meat, dairy, fruit, grains, potatoes, and foods high in sugar and/or fat. Diet at age 3 years was not associated with incident IBD (pooled aHR, 1.02; 95% CI, 0.76-1.37), suggesting that the risk impact of diet is greatest on very young and vulnerable microbiomes.
Ms. Guo noted that a Swedish national survey among 4-year-olds found a mean SSB consumption of 187 g/d with a mean frequency of once daily. The most desired changes in food habits are a lower intake of soft drinks, sweets, crisps, cakes, and biscuits and an increase in the intake of fruits and vegetables. A similar Norwegian survey among 2-year-olds showed that SSBs were consumed by 36% of all children with a mean intake of 40 g/d.
The exact mechanism by which sugar affects the intestinal microbiota is not established. “However, what we do know is that an excessive intake of sugar can disrupt the balance of the gut microbiome,” Ms. Guo said. “And if the child has a high intake of foods with high in sugar, that also increases the chances that the child’s overall diet has a lower intake of other foods that contribute to a diverse microbiome such as fruits and vegetables.”
An ‘Elegant’ Study
In an accompanying editorial, gastroenterologist Ashwin N. Ananthakrishnan, MBBS, MPH, AGAF, of Mass General Brigham and the Mass General Research Institute, Boston, cautioned that accurately measuring food intake in very young children is difficult, and dietary questionnaires in this study did not address food additives and emulsifiers common in commercial baby food, which may play a role in the pathogenesis of IBD.
Another study limitation is that the dietary questionnaire used has not been qualitatively or quantitatively validated against other more conventional methods, said Dr. Ananthakrishnan, who was not involved in the research.
Nevertheless, he called the study “elegant” and expanding of the data on the importance of this period in IBD development. “Although in the present study there was no association between diet at 3 years and development of IBD (in contrast to the association observed for dietary intake at 1 year), other prospective cohorts of adult-onset IBD have demonstrated an inverse association between vegetable or fish intake and reduced risk for CD while sugar-sweetened beverages have been linked to a higher risk for IBD.”
As to the question of recommending early preventive diet for IBD, “thus far, data on the impact of diet very early in childhood, outside of breastfeeding, on the risk for IBD has been lacking,” Dr. Ananthakrishnan said in an interview. “This important study highlights that diet as early as 1 year can modify subsequent risk for IBD. This raises the intriguing possibility of whether early changes in diet could be used, particularly in those at higher risk, to reduce or even prevent future development of IBD. Of course, more works needs to be done to define modifiability of diet as a risk factor, but this is an important supportive data.”
In his editorial, Dr. Ananthakrishnan stated that despite the absence of gold-standard interventional data demonstrating a benefit of dietary interventions, “in my opinion, it may still be reasonable to suggest such interventions to motivate individuals who incorporate several of the dietary patterns associated with lower risk for IBD from this and other studies. This includes ensuring adequate dietary fiber, particularly from fruits and vegetables, intake of fish, minimizing sugar-sweetened beverages and preferring fresh over processed and ultra-processed foods and snacks.” According to the study authors, their novel findings support further research on the role of childhood diet in the prevention of IBD.
The All Babies in Southeast Sweden Study is supported by Barndiabetesfonden (Swedish Child Diabetes Foundation), the Swedish Council for Working Life and Social Research, the Swedish Research Council, the Medical Research Council of Southeast Sweden, the JDRF Wallenberg Foundation, ALF and LFoU grants from Region Östergötland and Linköping University, and the Joanna Cocozza Foundation.
The Norwegian Mother, Father and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research.
Ms. Guo received grants from the Swedish Society for Medical Research and the Henning and Johan Throne-Holst Foundation to conduct this study. Co-author Karl Mårild has received funding from the Swedish Society for Medical Research, the Swedish Research Council, and ALF, Sweden’s medical research and education co-ordinating body. The authors declared no competing interests. Dr. Ananthakrishnan is supported by the National Institutes of Health, the Leona M. and Harry B. Helmsley Charitable Trust, and the Chleck Family Foundation. He has served on the scientific advisory board for Geneoscopy.
, prospective pooled data from two Scandinavian birth cohorts suggested.
It appears important to feed children a quality diet at a very young age, in particular one rich in vegetables and fish, since by age three, only dietary fish intake had any impact on IBD risk.
Although high intakes of these two food categories in very early life correlated with lower IBD risk, exposure to sugar-sweetened beverages (SSBs) was associated with an increased risk. “While non-causal explanations for our results cannot be ruled out, these novel findings are consistent with the hypothesis that early-life diet, possibly mediated through changes in the gut microbiome, may affect the risk of developing IBD,” wrote lead author Annie Guo, a PhD candidate in the Department of Pediatrics, University of Gothenburg, Sweden, and colleagues. The report was published in Gut.
“This is a population-based study investigating the risk for IBD, rather than the specific effect of diet,” Ms. Guo said in an interview. “Therefore, the results are not enough on their own to be translated into individual advice that can be applicable in the clinic. However, the study supports current dietary guidelines for small children, that is, the intake of sugar should be limited and a higher intake of fish and vegetables is beneficial for overall health.”
Two-Cohort Study
The investigators prospectively recorded food-group information on children (just under half were female) from the All Babies in Southeast Sweden and The Norwegian Mother, Father and Child Cohort Study to assess the diet quality using a Healthy Eating Index and intake frequency. Parents answered questions about their offspring’s diet at ages 12-18 months and 30-36 months. Quality of diet was measured by intake of meat, fish, fruit, vegetables, dairy, sweets, snacks, and drinks.
The Swedish cohort included 21,700 children born between October 1997 and October 1999, while the Norwegian analysis included 114,500 children, 95,200 mothers, and 75,200 fathers recruited from across Norway from 1999 to 2008. In 1,304,433 person-years of follow-up, the researchers tracked 81,280 participants from birth to childhood and adolescence, with median follow-ups in the two cohorts ranging from 1 year of age to 21.3 years (Sweden) and to 15.2 years of age (Norway). Of these children, 307 were diagnosed with IBD: Crohn’s disease (CD; n = 131); ulcerative colitis (UC; n = 97); and IBD unclassified (n = 79).
Adjusting for parental IBD history, sex, origin, education, and maternal comorbidities, the study found:
- Compared with low-quality diet, both medium- and high-quality diets at 1 year were associated with a roughly 25% reduced risk for IBD (pooled adjusted hazard ratio [aHR], 0.75 [95% CI, 0.58-0.98] and 0.75 [0.56-1.0], respectively).
- The pooled aHR per increase of category was 0.86 (95% CI, 0.74-0.99). The pooled aHR for IBD in 1-year-olds with high vs low fish intake was 0.70 (95% CI, 0.49-1.0), and this diet showed an association with a reduced risk for UC (pooled aHR, 0.46; 95% CI, 0.21-0.99). Higher vegetable intake at 1 year was also associated with a risk reduction in IBD (HR, 0.72; 95% CI, 0.55-0.95). It has been hypothesized that intake of vegetables and vegetable fibers may have programming effects on the immune system.
- AutoWith 72% of children reportedly consuming SSBs at age 1, pooled aHRs showed that some vs no intake of SSBs was associated with an increased risk for later IBD (pooled aHR, 1.42; 95% CI, 1.05-1.90).
- There were no obvious associations between overall IBD or CD/UC risk and meat, dairy, fruit, grains, potatoes, and foods high in sugar and/or fat. Diet at age 3 years was not associated with incident IBD (pooled aHR, 1.02; 95% CI, 0.76-1.37), suggesting that the risk impact of diet is greatest on very young and vulnerable microbiomes.
Ms. Guo noted that a Swedish national survey among 4-year-olds found a mean SSB consumption of 187 g/d with a mean frequency of once daily. The most desired changes in food habits are a lower intake of soft drinks, sweets, crisps, cakes, and biscuits and an increase in the intake of fruits and vegetables. A similar Norwegian survey among 2-year-olds showed that SSBs were consumed by 36% of all children with a mean intake of 40 g/d.
The exact mechanism by which sugar affects the intestinal microbiota is not established. “However, what we do know is that an excessive intake of sugar can disrupt the balance of the gut microbiome,” Ms. Guo said. “And if the child has a high intake of foods with high in sugar, that also increases the chances that the child’s overall diet has a lower intake of other foods that contribute to a diverse microbiome such as fruits and vegetables.”
An ‘Elegant’ Study
In an accompanying editorial, gastroenterologist Ashwin N. Ananthakrishnan, MBBS, MPH, AGAF, of Mass General Brigham and the Mass General Research Institute, Boston, cautioned that accurately measuring food intake in very young children is difficult, and dietary questionnaires in this study did not address food additives and emulsifiers common in commercial baby food, which may play a role in the pathogenesis of IBD.
Another study limitation is that the dietary questionnaire used has not been qualitatively or quantitatively validated against other more conventional methods, said Dr. Ananthakrishnan, who was not involved in the research.
Nevertheless, he called the study “elegant” and expanding of the data on the importance of this period in IBD development. “Although in the present study there was no association between diet at 3 years and development of IBD (in contrast to the association observed for dietary intake at 1 year), other prospective cohorts of adult-onset IBD have demonstrated an inverse association between vegetable or fish intake and reduced risk for CD while sugar-sweetened beverages have been linked to a higher risk for IBD.”
As to the question of recommending early preventive diet for IBD, “thus far, data on the impact of diet very early in childhood, outside of breastfeeding, on the risk for IBD has been lacking,” Dr. Ananthakrishnan said in an interview. “This important study highlights that diet as early as 1 year can modify subsequent risk for IBD. This raises the intriguing possibility of whether early changes in diet could be used, particularly in those at higher risk, to reduce or even prevent future development of IBD. Of course, more works needs to be done to define modifiability of diet as a risk factor, but this is an important supportive data.”
In his editorial, Dr. Ananthakrishnan stated that despite the absence of gold-standard interventional data demonstrating a benefit of dietary interventions, “in my opinion, it may still be reasonable to suggest such interventions to motivate individuals who incorporate several of the dietary patterns associated with lower risk for IBD from this and other studies. This includes ensuring adequate dietary fiber, particularly from fruits and vegetables, intake of fish, minimizing sugar-sweetened beverages and preferring fresh over processed and ultra-processed foods and snacks.” According to the study authors, their novel findings support further research on the role of childhood diet in the prevention of IBD.
The All Babies in Southeast Sweden Study is supported by Barndiabetesfonden (Swedish Child Diabetes Foundation), the Swedish Council for Working Life and Social Research, the Swedish Research Council, the Medical Research Council of Southeast Sweden, the JDRF Wallenberg Foundation, ALF and LFoU grants from Region Östergötland and Linköping University, and the Joanna Cocozza Foundation.
The Norwegian Mother, Father and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research.
Ms. Guo received grants from the Swedish Society for Medical Research and the Henning and Johan Throne-Holst Foundation to conduct this study. Co-author Karl Mårild has received funding from the Swedish Society for Medical Research, the Swedish Research Council, and ALF, Sweden’s medical research and education co-ordinating body. The authors declared no competing interests. Dr. Ananthakrishnan is supported by the National Institutes of Health, the Leona M. and Harry B. Helmsley Charitable Trust, and the Chleck Family Foundation. He has served on the scientific advisory board for Geneoscopy.
, prospective pooled data from two Scandinavian birth cohorts suggested.
It appears important to feed children a quality diet at a very young age, in particular one rich in vegetables and fish, since by age three, only dietary fish intake had any impact on IBD risk.
Although high intakes of these two food categories in very early life correlated with lower IBD risk, exposure to sugar-sweetened beverages (SSBs) was associated with an increased risk. “While non-causal explanations for our results cannot be ruled out, these novel findings are consistent with the hypothesis that early-life diet, possibly mediated through changes in the gut microbiome, may affect the risk of developing IBD,” wrote lead author Annie Guo, a PhD candidate in the Department of Pediatrics, University of Gothenburg, Sweden, and colleagues. The report was published in Gut.
“This is a population-based study investigating the risk for IBD, rather than the specific effect of diet,” Ms. Guo said in an interview. “Therefore, the results are not enough on their own to be translated into individual advice that can be applicable in the clinic. However, the study supports current dietary guidelines for small children, that is, the intake of sugar should be limited and a higher intake of fish and vegetables is beneficial for overall health.”
Two-Cohort Study
The investigators prospectively recorded food-group information on children (just under half were female) from the All Babies in Southeast Sweden and The Norwegian Mother, Father and Child Cohort Study to assess the diet quality using a Healthy Eating Index and intake frequency. Parents answered questions about their offspring’s diet at ages 12-18 months and 30-36 months. Quality of diet was measured by intake of meat, fish, fruit, vegetables, dairy, sweets, snacks, and drinks.
The Swedish cohort included 21,700 children born between October 1997 and October 1999, while the Norwegian analysis included 114,500 children, 95,200 mothers, and 75,200 fathers recruited from across Norway from 1999 to 2008. In 1,304,433 person-years of follow-up, the researchers tracked 81,280 participants from birth to childhood and adolescence, with median follow-ups in the two cohorts ranging from 1 year of age to 21.3 years (Sweden) and to 15.2 years of age (Norway). Of these children, 307 were diagnosed with IBD: Crohn’s disease (CD; n = 131); ulcerative colitis (UC; n = 97); and IBD unclassified (n = 79).
Adjusting for parental IBD history, sex, origin, education, and maternal comorbidities, the study found:
- Compared with low-quality diet, both medium- and high-quality diets at 1 year were associated with a roughly 25% reduced risk for IBD (pooled adjusted hazard ratio [aHR], 0.75 [95% CI, 0.58-0.98] and 0.75 [0.56-1.0], respectively).
- The pooled aHR per increase of category was 0.86 (95% CI, 0.74-0.99). The pooled aHR for IBD in 1-year-olds with high vs low fish intake was 0.70 (95% CI, 0.49-1.0), and this diet showed an association with a reduced risk for UC (pooled aHR, 0.46; 95% CI, 0.21-0.99). Higher vegetable intake at 1 year was also associated with a risk reduction in IBD (HR, 0.72; 95% CI, 0.55-0.95). It has been hypothesized that intake of vegetables and vegetable fibers may have programming effects on the immune system.
- AutoWith 72% of children reportedly consuming SSBs at age 1, pooled aHRs showed that some vs no intake of SSBs was associated with an increased risk for later IBD (pooled aHR, 1.42; 95% CI, 1.05-1.90).
- There were no obvious associations between overall IBD or CD/UC risk and meat, dairy, fruit, grains, potatoes, and foods high in sugar and/or fat. Diet at age 3 years was not associated with incident IBD (pooled aHR, 1.02; 95% CI, 0.76-1.37), suggesting that the risk impact of diet is greatest on very young and vulnerable microbiomes.
Ms. Guo noted that a Swedish national survey among 4-year-olds found a mean SSB consumption of 187 g/d with a mean frequency of once daily. The most desired changes in food habits are a lower intake of soft drinks, sweets, crisps, cakes, and biscuits and an increase in the intake of fruits and vegetables. A similar Norwegian survey among 2-year-olds showed that SSBs were consumed by 36% of all children with a mean intake of 40 g/d.
The exact mechanism by which sugar affects the intestinal microbiota is not established. “However, what we do know is that an excessive intake of sugar can disrupt the balance of the gut microbiome,” Ms. Guo said. “And if the child has a high intake of foods with high in sugar, that also increases the chances that the child’s overall diet has a lower intake of other foods that contribute to a diverse microbiome such as fruits and vegetables.”
An ‘Elegant’ Study
In an accompanying editorial, gastroenterologist Ashwin N. Ananthakrishnan, MBBS, MPH, AGAF, of Mass General Brigham and the Mass General Research Institute, Boston, cautioned that accurately measuring food intake in very young children is difficult, and dietary questionnaires in this study did not address food additives and emulsifiers common in commercial baby food, which may play a role in the pathogenesis of IBD.
Another study limitation is that the dietary questionnaire used has not been qualitatively or quantitatively validated against other more conventional methods, said Dr. Ananthakrishnan, who was not involved in the research.
Nevertheless, he called the study “elegant” and expanding of the data on the importance of this period in IBD development. “Although in the present study there was no association between diet at 3 years and development of IBD (in contrast to the association observed for dietary intake at 1 year), other prospective cohorts of adult-onset IBD have demonstrated an inverse association between vegetable or fish intake and reduced risk for CD while sugar-sweetened beverages have been linked to a higher risk for IBD.”
As to the question of recommending early preventive diet for IBD, “thus far, data on the impact of diet very early in childhood, outside of breastfeeding, on the risk for IBD has been lacking,” Dr. Ananthakrishnan said in an interview. “This important study highlights that diet as early as 1 year can modify subsequent risk for IBD. This raises the intriguing possibility of whether early changes in diet could be used, particularly in those at higher risk, to reduce or even prevent future development of IBD. Of course, more works needs to be done to define modifiability of diet as a risk factor, but this is an important supportive data.”
In his editorial, Dr. Ananthakrishnan stated that despite the absence of gold-standard interventional data demonstrating a benefit of dietary interventions, “in my opinion, it may still be reasonable to suggest such interventions to motivate individuals who incorporate several of the dietary patterns associated with lower risk for IBD from this and other studies. This includes ensuring adequate dietary fiber, particularly from fruits and vegetables, intake of fish, minimizing sugar-sweetened beverages and preferring fresh over processed and ultra-processed foods and snacks.” According to the study authors, their novel findings support further research on the role of childhood diet in the prevention of IBD.
The All Babies in Southeast Sweden Study is supported by Barndiabetesfonden (Swedish Child Diabetes Foundation), the Swedish Council for Working Life and Social Research, the Swedish Research Council, the Medical Research Council of Southeast Sweden, the JDRF Wallenberg Foundation, ALF and LFoU grants from Region Östergötland and Linköping University, and the Joanna Cocozza Foundation.
The Norwegian Mother, Father and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research.
Ms. Guo received grants from the Swedish Society for Medical Research and the Henning and Johan Throne-Holst Foundation to conduct this study. Co-author Karl Mårild has received funding from the Swedish Society for Medical Research, the Swedish Research Council, and ALF, Sweden’s medical research and education co-ordinating body. The authors declared no competing interests. Dr. Ananthakrishnan is supported by the National Institutes of Health, the Leona M. and Harry B. Helmsley Charitable Trust, and the Chleck Family Foundation. He has served on the scientific advisory board for Geneoscopy.
FROM GUT
May 2024 – ICYMI
Gastroenterology
January 2024
Hirano I, et al; ASCENT WORKING GROUP. Ascending to New Heights for Novel Therapeutics for Eosinophilic Esophagitis. Gastroenterology. 2024 Jan;166(1):1-10. doi: 10.1053/j.gastro.2023.09.004. Epub 2023 Sep 9. PMID: 37690772; PMCID: PMC10872872.
Åkerström JH, et al. Antireflux Surgery Versus Antireflux Medication and Risk of Esophageal Adenocarcinoma in Patients With Barrett’s Esophagus. Gastroenterology. 2024 Jan;166(1):132-138.e3. doi: 10.1053/j.gastro.2023.08.050. Epub 2023 Sep 9. PMID: 37690771.
Barnes EL, et al; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on the Management of Pouchitis and Inflammatory Pouch Disorders. Gastroenterology. 2024 Jan;166(1):59-85. doi: 10.1053/j.gastro.2023.10.015. PMID: 38128971.
February 2024
Yoo HW, et al. Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study. Gastroenterology. 2024 Feb;166(2):313-322.e3. doi: 10.1053/j.gastro.2023.10.013. Epub 2023 Oct 18. PMID: 37863270.
Yang J, et al. High Soluble Fiber Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites in Mice. Gastroenterology. 2024 Feb;166(2):323-337.e7. doi: 10.1053/j.gastro.2023.10.012. Epub 2023 Oct 18. PMID: 37858797.
Young E, et al. Texture and Color Enhancement Imaging Improves Colonic Adenoma Detection: A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Feb;166(2):338-340.e3. doi: 10.1053/j.gastro.2023.10.008. Epub 2023 Oct 14. PMID: 37839498.
Clinical Gastroenterology and Hepatology
January 2024
Overbeek KA, et al; Dutch Familial Pancreatic Cancer Surveillance Study work group. Intraductal Papillary Mucinous Neoplasms in High-Risk Individuals: Incidence, Growth Rate, and Malignancy Risk. Clin Gastroenterol Hepatol. 2024 Jan;22(1):62-71.e7. doi: 10.1016/j.cgh.2023.03.035. Epub 2023 Apr 7. PMID: 37031711.
Reddy CA, et al. Achalasia is Strongly Associated With Eosinophilic Esophagitis and Other Allergic Disorders. Clin Gastroenterol Hepatol. 2024 Jan;22(1):34-41.e2. doi: 10.1016/j.cgh.2023.06.013. Epub 2023 Jun 28. PMID: 37391057; PMCID: PMC10753026.
Thiruvengadam NR, et al. The Clinical Impact and Cost-Effectiveness of Surveillance of Incidentally Detected Gastric Intestinal Metaplasia: A Microsimulation Analysis. Clin Gastroenterol Hepatol. 2024 Jan;22(1):51-61. doi: 10.1016/j.cgh.2023.05.028. Epub 2023 Jun 9. Erratum in: Clin Gastroenterol Hepatol. 2024 Jan 19;: PMID: 37302442.
February 2024
Goodoory VC, et al. Systematic Review and Meta-analysis: Efficacy of Mesalamine in Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):243-251.e5. doi: 10.1016/j.cgh.2023.02.014. Epub 2023 Feb 27. PMID: 36858143.
Brenner DM, et al. Development and Current State of Digital Therapeutics for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):222-234. doi: 10.1016/j.cgh.2023.09.013. Epub 2023 Sep 22. PMID: 37743035.
Techniques and Innovations in Gastrointestinal Endoscopy
January 2024
Ramirez PR, et al. Gaps and Improvement Opportunities in Post-Colonoscopy Communication. Tech Innov Gastrointest Endosc. 2024 Jan;26(1):90-92. doi: 10.1016/j.tige.2023.10.001. Epub 2023 Oct 22.
Gonzaga ER, et al. Gastric Peroral Endoscopic Myotomy (G-POEM) for the Management of Gastroparesis. Tech Innov Gastrointest Endosc. 2024 Jan; 26(1): 46-55. doi: 10.1016/j.tige.2023.09.002. Epub 2023 Oct 13.
Wang D, et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2024 Jan;26(1): 30-37. doi: 10.1016/j.tige.2023.10.003. Epub 2023 Nov 8.
Gastro Hep Advances
January 2024
Adeniran E, et al. Intense and Sustained Alcohol Consumption Associated With Acute Pancreatitis Warrants Early Intervention. Gastro Hep Advances. 2024 Jan;3(1):61-63. doi: 10.1016/j.gastha.2023.08.017. Epub 2023 Sep 2.
Alkhouri N, et al. A Novel Prescription Digital Therapeutic Option for the Treatment of Metabolic Dysfunction-Associated Steatotic Liver Disease. Gastro Hep Advances. 2024 Jan;3(1): 9-16. doi: 10.1016/j.gastha.2023.08.019. Epub 2023 Oct 1.
Gastroenterology
January 2024
Hirano I, et al; ASCENT WORKING GROUP. Ascending to New Heights for Novel Therapeutics for Eosinophilic Esophagitis. Gastroenterology. 2024 Jan;166(1):1-10. doi: 10.1053/j.gastro.2023.09.004. Epub 2023 Sep 9. PMID: 37690772; PMCID: PMC10872872.
Åkerström JH, et al. Antireflux Surgery Versus Antireflux Medication and Risk of Esophageal Adenocarcinoma in Patients With Barrett’s Esophagus. Gastroenterology. 2024 Jan;166(1):132-138.e3. doi: 10.1053/j.gastro.2023.08.050. Epub 2023 Sep 9. PMID: 37690771.
Barnes EL, et al; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on the Management of Pouchitis and Inflammatory Pouch Disorders. Gastroenterology. 2024 Jan;166(1):59-85. doi: 10.1053/j.gastro.2023.10.015. PMID: 38128971.
February 2024
Yoo HW, et al. Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study. Gastroenterology. 2024 Feb;166(2):313-322.e3. doi: 10.1053/j.gastro.2023.10.013. Epub 2023 Oct 18. PMID: 37863270.
Yang J, et al. High Soluble Fiber Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites in Mice. Gastroenterology. 2024 Feb;166(2):323-337.e7. doi: 10.1053/j.gastro.2023.10.012. Epub 2023 Oct 18. PMID: 37858797.
Young E, et al. Texture and Color Enhancement Imaging Improves Colonic Adenoma Detection: A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Feb;166(2):338-340.e3. doi: 10.1053/j.gastro.2023.10.008. Epub 2023 Oct 14. PMID: 37839498.
Clinical Gastroenterology and Hepatology
January 2024
Overbeek KA, et al; Dutch Familial Pancreatic Cancer Surveillance Study work group. Intraductal Papillary Mucinous Neoplasms in High-Risk Individuals: Incidence, Growth Rate, and Malignancy Risk. Clin Gastroenterol Hepatol. 2024 Jan;22(1):62-71.e7. doi: 10.1016/j.cgh.2023.03.035. Epub 2023 Apr 7. PMID: 37031711.
Reddy CA, et al. Achalasia is Strongly Associated With Eosinophilic Esophagitis and Other Allergic Disorders. Clin Gastroenterol Hepatol. 2024 Jan;22(1):34-41.e2. doi: 10.1016/j.cgh.2023.06.013. Epub 2023 Jun 28. PMID: 37391057; PMCID: PMC10753026.
Thiruvengadam NR, et al. The Clinical Impact and Cost-Effectiveness of Surveillance of Incidentally Detected Gastric Intestinal Metaplasia: A Microsimulation Analysis. Clin Gastroenterol Hepatol. 2024 Jan;22(1):51-61. doi: 10.1016/j.cgh.2023.05.028. Epub 2023 Jun 9. Erratum in: Clin Gastroenterol Hepatol. 2024 Jan 19;: PMID: 37302442.
February 2024
Goodoory VC, et al. Systematic Review and Meta-analysis: Efficacy of Mesalamine in Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):243-251.e5. doi: 10.1016/j.cgh.2023.02.014. Epub 2023 Feb 27. PMID: 36858143.
Brenner DM, et al. Development and Current State of Digital Therapeutics for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):222-234. doi: 10.1016/j.cgh.2023.09.013. Epub 2023 Sep 22. PMID: 37743035.
Techniques and Innovations in Gastrointestinal Endoscopy
January 2024
Ramirez PR, et al. Gaps and Improvement Opportunities in Post-Colonoscopy Communication. Tech Innov Gastrointest Endosc. 2024 Jan;26(1):90-92. doi: 10.1016/j.tige.2023.10.001. Epub 2023 Oct 22.
Gonzaga ER, et al. Gastric Peroral Endoscopic Myotomy (G-POEM) for the Management of Gastroparesis. Tech Innov Gastrointest Endosc. 2024 Jan; 26(1): 46-55. doi: 10.1016/j.tige.2023.09.002. Epub 2023 Oct 13.
Wang D, et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2024 Jan;26(1): 30-37. doi: 10.1016/j.tige.2023.10.003. Epub 2023 Nov 8.
Gastro Hep Advances
January 2024
Adeniran E, et al. Intense and Sustained Alcohol Consumption Associated With Acute Pancreatitis Warrants Early Intervention. Gastro Hep Advances. 2024 Jan;3(1):61-63. doi: 10.1016/j.gastha.2023.08.017. Epub 2023 Sep 2.
Alkhouri N, et al. A Novel Prescription Digital Therapeutic Option for the Treatment of Metabolic Dysfunction-Associated Steatotic Liver Disease. Gastro Hep Advances. 2024 Jan;3(1): 9-16. doi: 10.1016/j.gastha.2023.08.019. Epub 2023 Oct 1.
Gastroenterology
January 2024
Hirano I, et al; ASCENT WORKING GROUP. Ascending to New Heights for Novel Therapeutics for Eosinophilic Esophagitis. Gastroenterology. 2024 Jan;166(1):1-10. doi: 10.1053/j.gastro.2023.09.004. Epub 2023 Sep 9. PMID: 37690772; PMCID: PMC10872872.
Åkerström JH, et al. Antireflux Surgery Versus Antireflux Medication and Risk of Esophageal Adenocarcinoma in Patients With Barrett’s Esophagus. Gastroenterology. 2024 Jan;166(1):132-138.e3. doi: 10.1053/j.gastro.2023.08.050. Epub 2023 Sep 9. PMID: 37690771.
Barnes EL, et al; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on the Management of Pouchitis and Inflammatory Pouch Disorders. Gastroenterology. 2024 Jan;166(1):59-85. doi: 10.1053/j.gastro.2023.10.015. PMID: 38128971.
February 2024
Yoo HW, et al. Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study. Gastroenterology. 2024 Feb;166(2):313-322.e3. doi: 10.1053/j.gastro.2023.10.013. Epub 2023 Oct 18. PMID: 37863270.
Yang J, et al. High Soluble Fiber Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites in Mice. Gastroenterology. 2024 Feb;166(2):323-337.e7. doi: 10.1053/j.gastro.2023.10.012. Epub 2023 Oct 18. PMID: 37858797.
Young E, et al. Texture and Color Enhancement Imaging Improves Colonic Adenoma Detection: A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Feb;166(2):338-340.e3. doi: 10.1053/j.gastro.2023.10.008. Epub 2023 Oct 14. PMID: 37839498.
Clinical Gastroenterology and Hepatology
January 2024
Overbeek KA, et al; Dutch Familial Pancreatic Cancer Surveillance Study work group. Intraductal Papillary Mucinous Neoplasms in High-Risk Individuals: Incidence, Growth Rate, and Malignancy Risk. Clin Gastroenterol Hepatol. 2024 Jan;22(1):62-71.e7. doi: 10.1016/j.cgh.2023.03.035. Epub 2023 Apr 7. PMID: 37031711.
Reddy CA, et al. Achalasia is Strongly Associated With Eosinophilic Esophagitis and Other Allergic Disorders. Clin Gastroenterol Hepatol. 2024 Jan;22(1):34-41.e2. doi: 10.1016/j.cgh.2023.06.013. Epub 2023 Jun 28. PMID: 37391057; PMCID: PMC10753026.
Thiruvengadam NR, et al. The Clinical Impact and Cost-Effectiveness of Surveillance of Incidentally Detected Gastric Intestinal Metaplasia: A Microsimulation Analysis. Clin Gastroenterol Hepatol. 2024 Jan;22(1):51-61. doi: 10.1016/j.cgh.2023.05.028. Epub 2023 Jun 9. Erratum in: Clin Gastroenterol Hepatol. 2024 Jan 19;: PMID: 37302442.
February 2024
Goodoory VC, et al. Systematic Review and Meta-analysis: Efficacy of Mesalamine in Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):243-251.e5. doi: 10.1016/j.cgh.2023.02.014. Epub 2023 Feb 27. PMID: 36858143.
Brenner DM, et al. Development and Current State of Digital Therapeutics for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):222-234. doi: 10.1016/j.cgh.2023.09.013. Epub 2023 Sep 22. PMID: 37743035.
Techniques and Innovations in Gastrointestinal Endoscopy
January 2024
Ramirez PR, et al. Gaps and Improvement Opportunities in Post-Colonoscopy Communication. Tech Innov Gastrointest Endosc. 2024 Jan;26(1):90-92. doi: 10.1016/j.tige.2023.10.001. Epub 2023 Oct 22.
Gonzaga ER, et al. Gastric Peroral Endoscopic Myotomy (G-POEM) for the Management of Gastroparesis. Tech Innov Gastrointest Endosc. 2024 Jan; 26(1): 46-55. doi: 10.1016/j.tige.2023.09.002. Epub 2023 Oct 13.
Wang D, et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2024 Jan;26(1): 30-37. doi: 10.1016/j.tige.2023.10.003. Epub 2023 Nov 8.
Gastro Hep Advances
January 2024
Adeniran E, et al. Intense and Sustained Alcohol Consumption Associated With Acute Pancreatitis Warrants Early Intervention. Gastro Hep Advances. 2024 Jan;3(1):61-63. doi: 10.1016/j.gastha.2023.08.017. Epub 2023 Sep 2.
Alkhouri N, et al. A Novel Prescription Digital Therapeutic Option for the Treatment of Metabolic Dysfunction-Associated Steatotic Liver Disease. Gastro Hep Advances. 2024 Jan;3(1): 9-16. doi: 10.1016/j.gastha.2023.08.019. Epub 2023 Oct 1.
A Simplified Approach to Pelvic Floor Dysfunction
Pelvic floor dysfunction (PFD) represents a spectrum of symptoms involving sensory and emptying abnormalities of the bowel and bladder and pelvic organ prolapse. The pelvic floor refers to a group of muscles that spans the pelvic outlet, providing support to the pelvic organs and coordinating constrictor mechanisms to control urination and defecation. Symptoms reported by patients experiencing PFD include involuntary loss of stool or urine, incomplete emptying of the bowel and bladder, a sensation of fullness, bulging in the vagina, and sexual dysfunction.1
As such, symptoms related to PFD are very common concerns raised by patients to their gastroenterologists. Data from the National Health and Nutrition Examination Survey show that 23.7% of women over the age of 20 had at least one symptom of PFD.2 Unfortunately, patients experiencing pelvic floor dysfunction often are hesitant to seek care because of embarrassment or perception that limited treatment options exist for their symptoms.
Pelvic Floor Anatomy
Regions of the pelvis are often referred to by anatomic compartment: anterior (bladder and urethra), middle (vagina and uterus or prostate), and posterior (colon, rectum, and anal canal). Supporting these compartments is the levator ani, a muscle group that is used synonymously with the term “pelvic diaphragm.”
Continence of stool is provided by the anal sphincter muscles and the puborectalis muscle, which wraps around the posterior aspect of the anorectal canal. Damage to the musculature or sensory perception to this area may result in fecal incontinence. Defecation is a coordinated process during which the abdominal and rectal muscles contract, while the anal sphincter muscles and puborectalis simultaneously relax. A disturbance in neuromuscular coordination (dyssynergic defecation) or structural pathology such as pelvic organ prolapse may lead to obstructed defecation.
PFD is thought to be a result of one or more insults to the pelvic floor such as chronic straining, childbirth, iatrogenic injury, or systemic disease such as diabetes.3
Evaluation of PFD Symptoms
Patients presenting with suspected PFD necessitate a comprehensive interdisciplinary assessment. In addition to obtaining a medical, surgical, and obstetric history, details about symptoms and lifestyle should include toileting habits, diet, and physical activity. The Pelvic Floor Distress Inventory (PFDI-20) is a commonly used tool that can be employed in the clinical setting.4
A pelvic exam can reveal pelvic organ prolapse and other mucosal pathology. The Pelvic Organ Prolapse Quantification System (POP-Q) is a widely used classification system for describing pelvic organ prolapse.5 Protrusion of the rectal wall into the vagina is referred to as a rectocele, while prolapse of small bowel into the upper posterior wall of the vagina is called an enterocele. While the finding of a rectocele on exam is common in parous women and may not cause any symptoms, a larger rectocele may cause a sensation of incomplete evacuation of stool.
A digital rectal exam (DRE) should be performed to assess pelvic floor function and help identify structural abnormalities.
Initial Management
A stepwise approach to the management of PFD can allow many patients to be effectively treated without the need for surgical intervention. For patients reporting liquid stool consistency, the evaluation should pivot toward the workup and management of diarrhea, which can easily overwhelm continence mechanisms and cause fecal incontinence. Fiber supplementation to normalize stool consistency is considered first-line therapy for patients presenting with both fecal incontinence and obstructed defecation. Other tools for fecal incontinence include avoiding foods that trigger diarrhea and use of loperamide.6 For patients with obstructed defecation, a trial of laxatives can be followed by a prescription agent if needed, such as a secretagogue or prokinetic.7
Vaginal splinting is a technique that can be used in patients with rectocele, whereby a finger is inserted into the vagina and pressure is applied on the posterior vaginal wall toward the rectum. Reducing the rectocele can facilitate emptying stool from the rectum and prevent leakage of retained stool.8 Similarly, use of rectal irrigation enemas can also help clear retained stool.
Pelvic floor physical therapists examine the strength, coordination, and tone of the pelvic floor muscles. When hypertonic musculature is present, manual interventions may be performed including trigger point release, myofascial release, and dry needling.9 When hypotonic musculature or dyssynergia is present, strengthening and neuromuscular re-education are recommended. Biofeedback can be administered via surface electromyography and/or balloon training to improve rectal sensitivity. Proper defecation techniques, including positioning, breathing, and behavioral modifications, improve clinical outcomes.
Diagnostic Testing
For patients who do not improve with conservative management, further testing is recommended to characterize the underlying pathology. Typically, anorectal manometry (ARM) is performed in conjunction with the balloon expulsion test and imaging. Each modality has its strengths and limitations (see Table 1).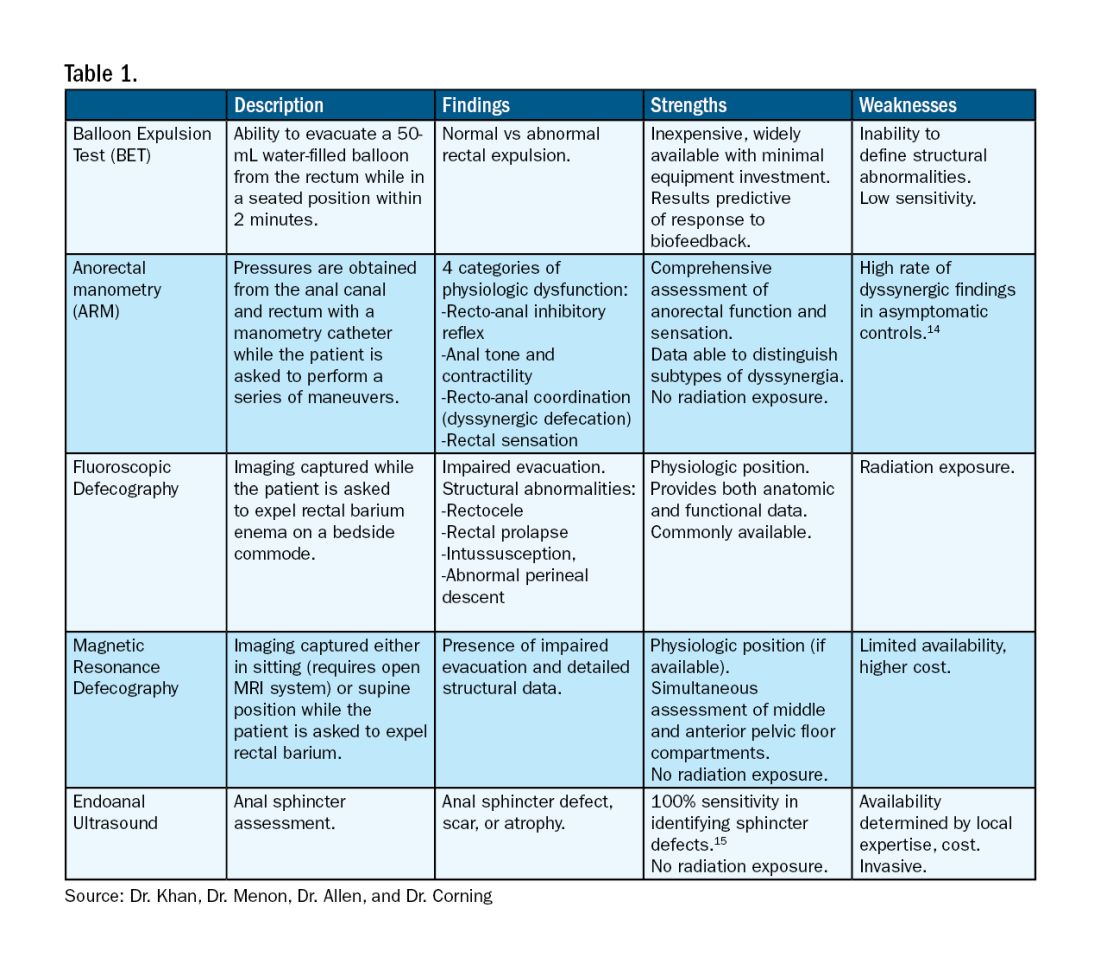
ARM allows for the assessment of rectal sensation and recto-anal pressures and coordination.10
Dynamic imaging, by barium defecography under fluoroscopy or MRI, captures anatomy at rest and with simulated defecation to identify pelvic organ prolapse, compartmental defects, and organ mobility.11 Endoanal ultrasonography is considered in patients experiencing fecal incontinence to evaluate the integrity of the anal sphincter muscles.
Minimally Invasive Procedures and Surgical Options for PFD
Functional abnormalities such as dyssynergia often coexist with structural abnormalities. Because structural abnormalities are commonly found in asymptomatic patients, noninvasive functional therapy, such as pelvic floor physical therapy and anorectal biofeedback, are preferred prior to surgical repair of a structural finding. For patients with fecal incontinence, sacral nerve stimulation (SNS) has emerged as a preferred therapy due to demonstrated efficacy in symptom improvement.12 Sphincteroplasty is reserved for those with acute sphincter injury or failure of SNS.
In patients with findings of intussusception, prolapse, or rectocele that have not responded to conservative therapy, referral for surgical repair may be considered. While the specific surgical approach will depend on many factors, the goal is typically excision and/or suspension of rectal tissue and reinforcement of the rectovaginal septum.
It is critical that we are equipped with the available knowledge and tools to provide these patients with optimal care.
Dr. Khan, Dr. Menon, Dr. Allen, and Dr. Corning are based at the University of Texas Medical Branch in Galveston, Texas. They report no conflicts of interest.
References
1. Grimes WR and Stratton M. Pelvic floor dysfunction. 2023 Jun 26. In: StatPearls [Internet]. Treasure Island (Fla.): StatPearls Publishing; 2024 Jan. PMID: 32644672.
2. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008 Sep 17. doi: 10.1001/jama.300.11.1311.
3. Lawrence JM et al. Pelvic floor disorders, diabetes, and obesity in women: Findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care. 2007 Oct. doi: 10.2337/dc07-0262.
4. Barber MD et al. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol. 2005 Jul. doi: 10.1016/j.ajog.2004.12.025.
5. Persu C et al. Pelvic Organ Prolapse Quantification System (POP-Q) — A new era in pelvic prolapse staging. J Med Life. 2011 Jan-Mar. PMID: 21505577.
6. Wald A et al. ACG Clinical Guidelines: Management of benign anorectal disorders. Am J Gastroenterol. 2021 Oct 1. doi: 10.14309/ajg.0000000000001507.
7. Bharucha AE and Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
8. Menees S and Chey WD. Fecal incontinence: Pathogenesis, diagnosis, and updated treatment strategies. Gastroenterol Clin North Am. 2022 Mar. doi: 10.1016/j.gtc.2021.10.005.
9. Wallace SL et al. Pelvic floor physical therapy in the treatment of pelvic floor dysfunction in women. Curr Opin Obstet Gynecol. 2019 Dec. doi: 10.1097/GCO.0000000000000584.
10. Carrington EV et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil. 2020 Jan. doi: 10.1111/nmo.13679.
11. El Sayed RF et al. Magnetic resonance imaging of pelvic floor dysfunction — Joint recommendations of the ESUR and ESGAR Pelvic Floor Working Group. Eur Radiol. 2017 May. doi: 10.1007/s00330-016-4471-7.
12. Thaha MA et al. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015 Aug 24. doi: 10.1002/14651858.CD004464.pub3.
13. Chiarioni G et al. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005 Jul. doi: 10.1053/j.gastro.2005.05.015.
14. Grossi U et al. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2016 Mar. doi: 10.1136/gutjnl-2014-308835.
15. Albuquerque A. Endoanal ultrasonography in fecal incontinence: Current and future perspectives. World J Gastrointest Endosc. 2015 Jun 10. doi: 10.4253/wjge.v7.i6.575.
Pelvic floor dysfunction (PFD) represents a spectrum of symptoms involving sensory and emptying abnormalities of the bowel and bladder and pelvic organ prolapse. The pelvic floor refers to a group of muscles that spans the pelvic outlet, providing support to the pelvic organs and coordinating constrictor mechanisms to control urination and defecation. Symptoms reported by patients experiencing PFD include involuntary loss of stool or urine, incomplete emptying of the bowel and bladder, a sensation of fullness, bulging in the vagina, and sexual dysfunction.1
As such, symptoms related to PFD are very common concerns raised by patients to their gastroenterologists. Data from the National Health and Nutrition Examination Survey show that 23.7% of women over the age of 20 had at least one symptom of PFD.2 Unfortunately, patients experiencing pelvic floor dysfunction often are hesitant to seek care because of embarrassment or perception that limited treatment options exist for their symptoms.
Pelvic Floor Anatomy
Regions of the pelvis are often referred to by anatomic compartment: anterior (bladder and urethra), middle (vagina and uterus or prostate), and posterior (colon, rectum, and anal canal). Supporting these compartments is the levator ani, a muscle group that is used synonymously with the term “pelvic diaphragm.”
Continence of stool is provided by the anal sphincter muscles and the puborectalis muscle, which wraps around the posterior aspect of the anorectal canal. Damage to the musculature or sensory perception to this area may result in fecal incontinence. Defecation is a coordinated process during which the abdominal and rectal muscles contract, while the anal sphincter muscles and puborectalis simultaneously relax. A disturbance in neuromuscular coordination (dyssynergic defecation) or structural pathology such as pelvic organ prolapse may lead to obstructed defecation.
PFD is thought to be a result of one or more insults to the pelvic floor such as chronic straining, childbirth, iatrogenic injury, or systemic disease such as diabetes.3
Evaluation of PFD Symptoms
Patients presenting with suspected PFD necessitate a comprehensive interdisciplinary assessment. In addition to obtaining a medical, surgical, and obstetric history, details about symptoms and lifestyle should include toileting habits, diet, and physical activity. The Pelvic Floor Distress Inventory (PFDI-20) is a commonly used tool that can be employed in the clinical setting.4
A pelvic exam can reveal pelvic organ prolapse and other mucosal pathology. The Pelvic Organ Prolapse Quantification System (POP-Q) is a widely used classification system for describing pelvic organ prolapse.5 Protrusion of the rectal wall into the vagina is referred to as a rectocele, while prolapse of small bowel into the upper posterior wall of the vagina is called an enterocele. While the finding of a rectocele on exam is common in parous women and may not cause any symptoms, a larger rectocele may cause a sensation of incomplete evacuation of stool.
A digital rectal exam (DRE) should be performed to assess pelvic floor function and help identify structural abnormalities.
Initial Management
A stepwise approach to the management of PFD can allow many patients to be effectively treated without the need for surgical intervention. For patients reporting liquid stool consistency, the evaluation should pivot toward the workup and management of diarrhea, which can easily overwhelm continence mechanisms and cause fecal incontinence. Fiber supplementation to normalize stool consistency is considered first-line therapy for patients presenting with both fecal incontinence and obstructed defecation. Other tools for fecal incontinence include avoiding foods that trigger diarrhea and use of loperamide.6 For patients with obstructed defecation, a trial of laxatives can be followed by a prescription agent if needed, such as a secretagogue or prokinetic.7
Vaginal splinting is a technique that can be used in patients with rectocele, whereby a finger is inserted into the vagina and pressure is applied on the posterior vaginal wall toward the rectum. Reducing the rectocele can facilitate emptying stool from the rectum and prevent leakage of retained stool.8 Similarly, use of rectal irrigation enemas can also help clear retained stool.
Pelvic floor physical therapists examine the strength, coordination, and tone of the pelvic floor muscles. When hypertonic musculature is present, manual interventions may be performed including trigger point release, myofascial release, and dry needling.9 When hypotonic musculature or dyssynergia is present, strengthening and neuromuscular re-education are recommended. Biofeedback can be administered via surface electromyography and/or balloon training to improve rectal sensitivity. Proper defecation techniques, including positioning, breathing, and behavioral modifications, improve clinical outcomes.
Diagnostic Testing
For patients who do not improve with conservative management, further testing is recommended to characterize the underlying pathology. Typically, anorectal manometry (ARM) is performed in conjunction with the balloon expulsion test and imaging. Each modality has its strengths and limitations (see Table 1).
ARM allows for the assessment of rectal sensation and recto-anal pressures and coordination.10
Dynamic imaging, by barium defecography under fluoroscopy or MRI, captures anatomy at rest and with simulated defecation to identify pelvic organ prolapse, compartmental defects, and organ mobility.11 Endoanal ultrasonography is considered in patients experiencing fecal incontinence to evaluate the integrity of the anal sphincter muscles.
Minimally Invasive Procedures and Surgical Options for PFD
Functional abnormalities such as dyssynergia often coexist with structural abnormalities. Because structural abnormalities are commonly found in asymptomatic patients, noninvasive functional therapy, such as pelvic floor physical therapy and anorectal biofeedback, are preferred prior to surgical repair of a structural finding. For patients with fecal incontinence, sacral nerve stimulation (SNS) has emerged as a preferred therapy due to demonstrated efficacy in symptom improvement.12 Sphincteroplasty is reserved for those with acute sphincter injury or failure of SNS.
In patients with findings of intussusception, prolapse, or rectocele that have not responded to conservative therapy, referral for surgical repair may be considered. While the specific surgical approach will depend on many factors, the goal is typically excision and/or suspension of rectal tissue and reinforcement of the rectovaginal septum.
It is critical that we are equipped with the available knowledge and tools to provide these patients with optimal care.
Dr. Khan, Dr. Menon, Dr. Allen, and Dr. Corning are based at the University of Texas Medical Branch in Galveston, Texas. They report no conflicts of interest.
References
1. Grimes WR and Stratton M. Pelvic floor dysfunction. 2023 Jun 26. In: StatPearls [Internet]. Treasure Island (Fla.): StatPearls Publishing; 2024 Jan. PMID: 32644672.
2. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008 Sep 17. doi: 10.1001/jama.300.11.1311.
3. Lawrence JM et al. Pelvic floor disorders, diabetes, and obesity in women: Findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care. 2007 Oct. doi: 10.2337/dc07-0262.
4. Barber MD et al. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol. 2005 Jul. doi: 10.1016/j.ajog.2004.12.025.
5. Persu C et al. Pelvic Organ Prolapse Quantification System (POP-Q) — A new era in pelvic prolapse staging. J Med Life. 2011 Jan-Mar. PMID: 21505577.
6. Wald A et al. ACG Clinical Guidelines: Management of benign anorectal disorders. Am J Gastroenterol. 2021 Oct 1. doi: 10.14309/ajg.0000000000001507.
7. Bharucha AE and Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
8. Menees S and Chey WD. Fecal incontinence: Pathogenesis, diagnosis, and updated treatment strategies. Gastroenterol Clin North Am. 2022 Mar. doi: 10.1016/j.gtc.2021.10.005.
9. Wallace SL et al. Pelvic floor physical therapy in the treatment of pelvic floor dysfunction in women. Curr Opin Obstet Gynecol. 2019 Dec. doi: 10.1097/GCO.0000000000000584.
10. Carrington EV et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil. 2020 Jan. doi: 10.1111/nmo.13679.
11. El Sayed RF et al. Magnetic resonance imaging of pelvic floor dysfunction — Joint recommendations of the ESUR and ESGAR Pelvic Floor Working Group. Eur Radiol. 2017 May. doi: 10.1007/s00330-016-4471-7.
12. Thaha MA et al. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015 Aug 24. doi: 10.1002/14651858.CD004464.pub3.
13. Chiarioni G et al. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005 Jul. doi: 10.1053/j.gastro.2005.05.015.
14. Grossi U et al. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2016 Mar. doi: 10.1136/gutjnl-2014-308835.
15. Albuquerque A. Endoanal ultrasonography in fecal incontinence: Current and future perspectives. World J Gastrointest Endosc. 2015 Jun 10. doi: 10.4253/wjge.v7.i6.575.
Pelvic floor dysfunction (PFD) represents a spectrum of symptoms involving sensory and emptying abnormalities of the bowel and bladder and pelvic organ prolapse. The pelvic floor refers to a group of muscles that spans the pelvic outlet, providing support to the pelvic organs and coordinating constrictor mechanisms to control urination and defecation. Symptoms reported by patients experiencing PFD include involuntary loss of stool or urine, incomplete emptying of the bowel and bladder, a sensation of fullness, bulging in the vagina, and sexual dysfunction.1
As such, symptoms related to PFD are very common concerns raised by patients to their gastroenterologists. Data from the National Health and Nutrition Examination Survey show that 23.7% of women over the age of 20 had at least one symptom of PFD.2 Unfortunately, patients experiencing pelvic floor dysfunction often are hesitant to seek care because of embarrassment or perception that limited treatment options exist for their symptoms.
Pelvic Floor Anatomy
Regions of the pelvis are often referred to by anatomic compartment: anterior (bladder and urethra), middle (vagina and uterus or prostate), and posterior (colon, rectum, and anal canal). Supporting these compartments is the levator ani, a muscle group that is used synonymously with the term “pelvic diaphragm.”
Continence of stool is provided by the anal sphincter muscles and the puborectalis muscle, which wraps around the posterior aspect of the anorectal canal. Damage to the musculature or sensory perception to this area may result in fecal incontinence. Defecation is a coordinated process during which the abdominal and rectal muscles contract, while the anal sphincter muscles and puborectalis simultaneously relax. A disturbance in neuromuscular coordination (dyssynergic defecation) or structural pathology such as pelvic organ prolapse may lead to obstructed defecation.
PFD is thought to be a result of one or more insults to the pelvic floor such as chronic straining, childbirth, iatrogenic injury, or systemic disease such as diabetes.3
Evaluation of PFD Symptoms
Patients presenting with suspected PFD necessitate a comprehensive interdisciplinary assessment. In addition to obtaining a medical, surgical, and obstetric history, details about symptoms and lifestyle should include toileting habits, diet, and physical activity. The Pelvic Floor Distress Inventory (PFDI-20) is a commonly used tool that can be employed in the clinical setting.4
A pelvic exam can reveal pelvic organ prolapse and other mucosal pathology. The Pelvic Organ Prolapse Quantification System (POP-Q) is a widely used classification system for describing pelvic organ prolapse.5 Protrusion of the rectal wall into the vagina is referred to as a rectocele, while prolapse of small bowel into the upper posterior wall of the vagina is called an enterocele. While the finding of a rectocele on exam is common in parous women and may not cause any symptoms, a larger rectocele may cause a sensation of incomplete evacuation of stool.
A digital rectal exam (DRE) should be performed to assess pelvic floor function and help identify structural abnormalities.
Initial Management
A stepwise approach to the management of PFD can allow many patients to be effectively treated without the need for surgical intervention. For patients reporting liquid stool consistency, the evaluation should pivot toward the workup and management of diarrhea, which can easily overwhelm continence mechanisms and cause fecal incontinence. Fiber supplementation to normalize stool consistency is considered first-line therapy for patients presenting with both fecal incontinence and obstructed defecation. Other tools for fecal incontinence include avoiding foods that trigger diarrhea and use of loperamide.6 For patients with obstructed defecation, a trial of laxatives can be followed by a prescription agent if needed, such as a secretagogue or prokinetic.7
Vaginal splinting is a technique that can be used in patients with rectocele, whereby a finger is inserted into the vagina and pressure is applied on the posterior vaginal wall toward the rectum. Reducing the rectocele can facilitate emptying stool from the rectum and prevent leakage of retained stool.8 Similarly, use of rectal irrigation enemas can also help clear retained stool.
Pelvic floor physical therapists examine the strength, coordination, and tone of the pelvic floor muscles. When hypertonic musculature is present, manual interventions may be performed including trigger point release, myofascial release, and dry needling.9 When hypotonic musculature or dyssynergia is present, strengthening and neuromuscular re-education are recommended. Biofeedback can be administered via surface electromyography and/or balloon training to improve rectal sensitivity. Proper defecation techniques, including positioning, breathing, and behavioral modifications, improve clinical outcomes.
Diagnostic Testing
For patients who do not improve with conservative management, further testing is recommended to characterize the underlying pathology. Typically, anorectal manometry (ARM) is performed in conjunction with the balloon expulsion test and imaging. Each modality has its strengths and limitations (see Table 1).
ARM allows for the assessment of rectal sensation and recto-anal pressures and coordination.10
Dynamic imaging, by barium defecography under fluoroscopy or MRI, captures anatomy at rest and with simulated defecation to identify pelvic organ prolapse, compartmental defects, and organ mobility.11 Endoanal ultrasonography is considered in patients experiencing fecal incontinence to evaluate the integrity of the anal sphincter muscles.
Minimally Invasive Procedures and Surgical Options for PFD
Functional abnormalities such as dyssynergia often coexist with structural abnormalities. Because structural abnormalities are commonly found in asymptomatic patients, noninvasive functional therapy, such as pelvic floor physical therapy and anorectal biofeedback, are preferred prior to surgical repair of a structural finding. For patients with fecal incontinence, sacral nerve stimulation (SNS) has emerged as a preferred therapy due to demonstrated efficacy in symptom improvement.12 Sphincteroplasty is reserved for those with acute sphincter injury or failure of SNS.
In patients with findings of intussusception, prolapse, or rectocele that have not responded to conservative therapy, referral for surgical repair may be considered. While the specific surgical approach will depend on many factors, the goal is typically excision and/or suspension of rectal tissue and reinforcement of the rectovaginal septum.
It is critical that we are equipped with the available knowledge and tools to provide these patients with optimal care.
Dr. Khan, Dr. Menon, Dr. Allen, and Dr. Corning are based at the University of Texas Medical Branch in Galveston, Texas. They report no conflicts of interest.
References
1. Grimes WR and Stratton M. Pelvic floor dysfunction. 2023 Jun 26. In: StatPearls [Internet]. Treasure Island (Fla.): StatPearls Publishing; 2024 Jan. PMID: 32644672.
2. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008 Sep 17. doi: 10.1001/jama.300.11.1311.
3. Lawrence JM et al. Pelvic floor disorders, diabetes, and obesity in women: Findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care. 2007 Oct. doi: 10.2337/dc07-0262.
4. Barber MD et al. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol. 2005 Jul. doi: 10.1016/j.ajog.2004.12.025.
5. Persu C et al. Pelvic Organ Prolapse Quantification System (POP-Q) — A new era in pelvic prolapse staging. J Med Life. 2011 Jan-Mar. PMID: 21505577.
6. Wald A et al. ACG Clinical Guidelines: Management of benign anorectal disorders. Am J Gastroenterol. 2021 Oct 1. doi: 10.14309/ajg.0000000000001507.
7. Bharucha AE and Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
8. Menees S and Chey WD. Fecal incontinence: Pathogenesis, diagnosis, and updated treatment strategies. Gastroenterol Clin North Am. 2022 Mar. doi: 10.1016/j.gtc.2021.10.005.
9. Wallace SL et al. Pelvic floor physical therapy in the treatment of pelvic floor dysfunction in women. Curr Opin Obstet Gynecol. 2019 Dec. doi: 10.1097/GCO.0000000000000584.
10. Carrington EV et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil. 2020 Jan. doi: 10.1111/nmo.13679.
11. El Sayed RF et al. Magnetic resonance imaging of pelvic floor dysfunction — Joint recommendations of the ESUR and ESGAR Pelvic Floor Working Group. Eur Radiol. 2017 May. doi: 10.1007/s00330-016-4471-7.
12. Thaha MA et al. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015 Aug 24. doi: 10.1002/14651858.CD004464.pub3.
13. Chiarioni G et al. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005 Jul. doi: 10.1053/j.gastro.2005.05.015.
14. Grossi U et al. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2016 Mar. doi: 10.1136/gutjnl-2014-308835.
15. Albuquerque A. Endoanal ultrasonography in fecal incontinence: Current and future perspectives. World J Gastrointest Endosc. 2015 Jun 10. doi: 10.4253/wjge.v7.i6.575.
GI Doc Aims to Lift Barriers to CRC Screening for Black Patients
In gastroenterology, a good bedside manner is a vital attribute. Visiting with an anxious patient before a colonoscopy, Adjoa Anyane-Yeboa, MD, MPH, knew what to say to calm him down.
“I could tell he was really nervous about the procedure, even though he wasn’t letting on,” said Dr. Anyane-Yeboa, a gastroenterologist with Massachusetts General Hospital in Boston. She put him at ease by cracking jokes and making him smile during the consent process. After it was over, he thanked her for making him feel more comfortable.
“I will have it done again, and I’ll come back to you next time,” said the patient.
GI doctors perform colonoscopies all day, every day, “so we sometimes forget how nervous people are. But it’s nice to be able to connect with people and put them at ease,” she said.
Interacting with patients gives her joy. Addressing health disparities is her long-term goal. Dr. Anyane-Yeboa’s research has focused on the barriers to colorectal cancer screening in the Black population, as well as disparities in inflammatory bowel disease (IBD).
“I think there’s a lot that still needs to be done around colorectal cancer screening,” she said.
In an interview, she talks more in depth about her research and her ongoing work to increase public knowledge and awareness about colorectal cancer screening.
Q: Why did you choose GI?
Dr. Anyane-Yeboa: When I got to residency, GI was the rotation that was the most fun. I was the most excited to read about it, the most excited to go to work the next day.
I remember people saying, “You should look at the people who are in the field and look at their personalities, and then think about which personalities match you best.” In residency I considered hematology, cardiology, and GI. The cardiologists were so serious, so intense, talking about research methods all the time. Whereas, the GI folks were joking, laughing, making fart jokes. I felt like these were my people, lighthearted and easy-going. And I genuinely enjoyed going to work every day and learning about the disorders of the GI tract. I still do to this day.
Q: Let’s discuss your research with IBD in Black populations and colorectal cancer screening.
Dr. Anyane-Yeboa: My two main areas of work are in IBD and minority populations, predominantly Black populations, and in colorectal cancer screening in minority populations, and again, mostly in historically marginalized populations.
With colon cancer, we know that there are disparities with incidence in mortality. Black individuals have had the second highest incidence in mortality from colorectal cancer. For me, being a Black female physician and seeing people who look like me, time and time again, being diagnosed with colorectal cancer and dying is really what drives me, because in GI, colon cancer screening is our bread and butter.
Some of the work that I’m doing now around colorectal cancer is in predominantly Black community health centers, working on increasing colorectal cancer screening rates in this population, and figuring out what the barriers are to screening and how we can address them, and what are some strategies that will work in a health center setting to get people screened.
Q: One study of yours surveyed unscreened Black individuals age ≥ 45 and found age-specific barriers to CRC screening in this population, as well as a lack of targeted messaging to incentivize screening.
Dr. Anyane-Yeboa: That mixed method study was done in partnership with the National Colorectal Cancer Roundtable and American Cancer Society.
In that study, we found that the most common barrier to screening was self-procrastination or delay of screening, meaning, “I’m going to get screened, just not right now.” It’s not a priority. What was unique about this is we looked at it from age breakdown, so 45-49, 50-54, 55-plus. With the younger 45-49 group, we don’t know as much about how to get them screened. We also saw that healthcare providers weren’t starting conversations about screening with these younger newly eligible patients.
We also described effective messages to get people screened in that paper as well.
Q: What changes would you like to see going forward with screening? What still needs to happen?
Dr. Anyane-Yeboa: In some of the other work that I’ve done, particularly with the health centers and younger populations interviewed in focus groups, I’m seeing that those who are younger don’t really know much about colorectal cancer screening. Those who do know about it have seen commercials about popular stool-based testing brands, and that’s how they’ve learned about screening.
What I would like to see is ways to increase the knowledge and awareness about colorectal cancer screening and colorectal cancer on a broad scale, on a more national, public-facing scale. Because I’m realizing that if they’re healthy young folks who aren’t going to the physician, who don’t have a primary care provider, then they might not even really hear about colorectal cancer screening. We need ways to educate the general public so individuals can advocate for themselves around screening.
I also want to see more providers discussing screening with all patients, starting from those 45-49, and younger if they have a family history. Providers should screen every single patient that they see. We know that every single person should be screened at 45 and older, and not all providers, surprisingly, are discussing it with their patients.
Q: When you’re not being a GI, how do you spend your free weekend afternoons?
Dr. Anyane-Yeboa: Saturday morning is my favorite time of the week. I’m either catching up on my TV shows, or I might be on a walk with my dog, particularly in the afternoon. I live near an arboretum, so I usually walk through there on the weekend afternoons. I also might be trying out a new restaurant with my friends. I love traveling, so I might also be sightseeing in another country.
Lightning Round
Texting or talking?
Texting
Favorite junk food?
Cookies
Cat or dog person?
Both; love cats, have a dog
If you weren’t a gastroenterologist, what would you be?
Fashion boutique owner
Best place you’ve traveled to?
Morocco
How many cups of coffee do you drink per day?
Two
Favorite ice cream?
Don’t eat ice cream, only cookies
Favorite sport?
Tennis
Optimist or pessimist?
Optimist (glass half full)
In gastroenterology, a good bedside manner is a vital attribute. Visiting with an anxious patient before a colonoscopy, Adjoa Anyane-Yeboa, MD, MPH, knew what to say to calm him down.
“I could tell he was really nervous about the procedure, even though he wasn’t letting on,” said Dr. Anyane-Yeboa, a gastroenterologist with Massachusetts General Hospital in Boston. She put him at ease by cracking jokes and making him smile during the consent process. After it was over, he thanked her for making him feel more comfortable.
“I will have it done again, and I’ll come back to you next time,” said the patient.
GI doctors perform colonoscopies all day, every day, “so we sometimes forget how nervous people are. But it’s nice to be able to connect with people and put them at ease,” she said.
Interacting with patients gives her joy. Addressing health disparities is her long-term goal. Dr. Anyane-Yeboa’s research has focused on the barriers to colorectal cancer screening in the Black population, as well as disparities in inflammatory bowel disease (IBD).
“I think there’s a lot that still needs to be done around colorectal cancer screening,” she said.
In an interview, she talks more in depth about her research and her ongoing work to increase public knowledge and awareness about colorectal cancer screening.
Q: Why did you choose GI?
Dr. Anyane-Yeboa: When I got to residency, GI was the rotation that was the most fun. I was the most excited to read about it, the most excited to go to work the next day.
I remember people saying, “You should look at the people who are in the field and look at their personalities, and then think about which personalities match you best.” In residency I considered hematology, cardiology, and GI. The cardiologists were so serious, so intense, talking about research methods all the time. Whereas, the GI folks were joking, laughing, making fart jokes. I felt like these were my people, lighthearted and easy-going. And I genuinely enjoyed going to work every day and learning about the disorders of the GI tract. I still do to this day.
Q: Let’s discuss your research with IBD in Black populations and colorectal cancer screening.
Dr. Anyane-Yeboa: My two main areas of work are in IBD and minority populations, predominantly Black populations, and in colorectal cancer screening in minority populations, and again, mostly in historically marginalized populations.
With colon cancer, we know that there are disparities with incidence in mortality. Black individuals have had the second highest incidence in mortality from colorectal cancer. For me, being a Black female physician and seeing people who look like me, time and time again, being diagnosed with colorectal cancer and dying is really what drives me, because in GI, colon cancer screening is our bread and butter.
Some of the work that I’m doing now around colorectal cancer is in predominantly Black community health centers, working on increasing colorectal cancer screening rates in this population, and figuring out what the barriers are to screening and how we can address them, and what are some strategies that will work in a health center setting to get people screened.
Q: One study of yours surveyed unscreened Black individuals age ≥ 45 and found age-specific barriers to CRC screening in this population, as well as a lack of targeted messaging to incentivize screening.
Dr. Anyane-Yeboa: That mixed method study was done in partnership with the National Colorectal Cancer Roundtable and American Cancer Society.
In that study, we found that the most common barrier to screening was self-procrastination or delay of screening, meaning, “I’m going to get screened, just not right now.” It’s not a priority. What was unique about this is we looked at it from age breakdown, so 45-49, 50-54, 55-plus. With the younger 45-49 group, we don’t know as much about how to get them screened. We also saw that healthcare providers weren’t starting conversations about screening with these younger newly eligible patients.
We also described effective messages to get people screened in that paper as well.
Q: What changes would you like to see going forward with screening? What still needs to happen?
Dr. Anyane-Yeboa: In some of the other work that I’ve done, particularly with the health centers and younger populations interviewed in focus groups, I’m seeing that those who are younger don’t really know much about colorectal cancer screening. Those who do know about it have seen commercials about popular stool-based testing brands, and that’s how they’ve learned about screening.
What I would like to see is ways to increase the knowledge and awareness about colorectal cancer screening and colorectal cancer on a broad scale, on a more national, public-facing scale. Because I’m realizing that if they’re healthy young folks who aren’t going to the physician, who don’t have a primary care provider, then they might not even really hear about colorectal cancer screening. We need ways to educate the general public so individuals can advocate for themselves around screening.
I also want to see more providers discussing screening with all patients, starting from those 45-49, and younger if they have a family history. Providers should screen every single patient that they see. We know that every single person should be screened at 45 and older, and not all providers, surprisingly, are discussing it with their patients.
Q: When you’re not being a GI, how do you spend your free weekend afternoons?
Dr. Anyane-Yeboa: Saturday morning is my favorite time of the week. I’m either catching up on my TV shows, or I might be on a walk with my dog, particularly in the afternoon. I live near an arboretum, so I usually walk through there on the weekend afternoons. I also might be trying out a new restaurant with my friends. I love traveling, so I might also be sightseeing in another country.
Lightning Round
Texting or talking?
Texting
Favorite junk food?
Cookies
Cat or dog person?
Both; love cats, have a dog
If you weren’t a gastroenterologist, what would you be?
Fashion boutique owner
Best place you’ve traveled to?
Morocco
How many cups of coffee do you drink per day?
Two
Favorite ice cream?
Don’t eat ice cream, only cookies
Favorite sport?
Tennis
Optimist or pessimist?
Optimist (glass half full)
In gastroenterology, a good bedside manner is a vital attribute. Visiting with an anxious patient before a colonoscopy, Adjoa Anyane-Yeboa, MD, MPH, knew what to say to calm him down.
“I could tell he was really nervous about the procedure, even though he wasn’t letting on,” said Dr. Anyane-Yeboa, a gastroenterologist with Massachusetts General Hospital in Boston. She put him at ease by cracking jokes and making him smile during the consent process. After it was over, he thanked her for making him feel more comfortable.
“I will have it done again, and I’ll come back to you next time,” said the patient.
GI doctors perform colonoscopies all day, every day, “so we sometimes forget how nervous people are. But it’s nice to be able to connect with people and put them at ease,” she said.
Interacting with patients gives her joy. Addressing health disparities is her long-term goal. Dr. Anyane-Yeboa’s research has focused on the barriers to colorectal cancer screening in the Black population, as well as disparities in inflammatory bowel disease (IBD).
“I think there’s a lot that still needs to be done around colorectal cancer screening,” she said.
In an interview, she talks more in depth about her research and her ongoing work to increase public knowledge and awareness about colorectal cancer screening.
Q: Why did you choose GI?
Dr. Anyane-Yeboa: When I got to residency, GI was the rotation that was the most fun. I was the most excited to read about it, the most excited to go to work the next day.
I remember people saying, “You should look at the people who are in the field and look at their personalities, and then think about which personalities match you best.” In residency I considered hematology, cardiology, and GI. The cardiologists were so serious, so intense, talking about research methods all the time. Whereas, the GI folks were joking, laughing, making fart jokes. I felt like these were my people, lighthearted and easy-going. And I genuinely enjoyed going to work every day and learning about the disorders of the GI tract. I still do to this day.
Q: Let’s discuss your research with IBD in Black populations and colorectal cancer screening.
Dr. Anyane-Yeboa: My two main areas of work are in IBD and minority populations, predominantly Black populations, and in colorectal cancer screening in minority populations, and again, mostly in historically marginalized populations.
With colon cancer, we know that there are disparities with incidence in mortality. Black individuals have had the second highest incidence in mortality from colorectal cancer. For me, being a Black female physician and seeing people who look like me, time and time again, being diagnosed with colorectal cancer and dying is really what drives me, because in GI, colon cancer screening is our bread and butter.
Some of the work that I’m doing now around colorectal cancer is in predominantly Black community health centers, working on increasing colorectal cancer screening rates in this population, and figuring out what the barriers are to screening and how we can address them, and what are some strategies that will work in a health center setting to get people screened.
Q: One study of yours surveyed unscreened Black individuals age ≥ 45 and found age-specific barriers to CRC screening in this population, as well as a lack of targeted messaging to incentivize screening.
Dr. Anyane-Yeboa: That mixed method study was done in partnership with the National Colorectal Cancer Roundtable and American Cancer Society.
In that study, we found that the most common barrier to screening was self-procrastination or delay of screening, meaning, “I’m going to get screened, just not right now.” It’s not a priority. What was unique about this is we looked at it from age breakdown, so 45-49, 50-54, 55-plus. With the younger 45-49 group, we don’t know as much about how to get them screened. We also saw that healthcare providers weren’t starting conversations about screening with these younger newly eligible patients.
We also described effective messages to get people screened in that paper as well.
Q: What changes would you like to see going forward with screening? What still needs to happen?
Dr. Anyane-Yeboa: In some of the other work that I’ve done, particularly with the health centers and younger populations interviewed in focus groups, I’m seeing that those who are younger don’t really know much about colorectal cancer screening. Those who do know about it have seen commercials about popular stool-based testing brands, and that’s how they’ve learned about screening.
What I would like to see is ways to increase the knowledge and awareness about colorectal cancer screening and colorectal cancer on a broad scale, on a more national, public-facing scale. Because I’m realizing that if they’re healthy young folks who aren’t going to the physician, who don’t have a primary care provider, then they might not even really hear about colorectal cancer screening. We need ways to educate the general public so individuals can advocate for themselves around screening.
I also want to see more providers discussing screening with all patients, starting from those 45-49, and younger if they have a family history. Providers should screen every single patient that they see. We know that every single person should be screened at 45 and older, and not all providers, surprisingly, are discussing it with their patients.
Q: When you’re not being a GI, how do you spend your free weekend afternoons?
Dr. Anyane-Yeboa: Saturday morning is my favorite time of the week. I’m either catching up on my TV shows, or I might be on a walk with my dog, particularly in the afternoon. I live near an arboretum, so I usually walk through there on the weekend afternoons. I also might be trying out a new restaurant with my friends. I love traveling, so I might also be sightseeing in another country.
Lightning Round
Texting or talking?
Texting
Favorite junk food?
Cookies
Cat or dog person?
Both; love cats, have a dog
If you weren’t a gastroenterologist, what would you be?
Fashion boutique owner
Best place you’ve traveled to?
Morocco
How many cups of coffee do you drink per day?
Two
Favorite ice cream?
Don’t eat ice cream, only cookies
Favorite sport?
Tennis
Optimist or pessimist?
Optimist (glass half full)
Are Direct-to-Consumer Microbiome Tests Clinically Useful?
Companies selling gut microbiome tests directly to consumers offer up a variety of claims to promote their products.
“We analyze the trillions of microbes in your gut microflora and craft a unique formula for your unique gut needs,” one says. “Get actionable dietary, supplement, and lifestyle recommendations from our microbiome experts based on your results, tailored to mom and baby’s biomarkers. ... Any family member like dads or siblings are welcome too,” says another.
The companies assert that they can improve gut health by offering individuals personalized treatments based on their gut microbiome test results. The trouble is, no provider, company, or technology can reliably do that yet.
Clinical Implications, Not Applications
The microbiome is the “constellation of microorganisms that call the human body home,” including many strains of bacteria, fungi, and viruses. That constellation comprises some 39 trillion cells.
Although knowledge is increasing on the oral, cutaneous, and vaginal microbiomes, the gut microbiome is arguably the most studied. However, while research is increasingly demonstrating that the gut microbiome has clinical implications, much work needs to be done before reliable applications based on that research are available.
But , Erik C. von Rosenvinge, MD, AGAF, a professor at the University of Maryland School of Medicine and chief of gastroenterology at the VA Maryland Health Care System, Baltimore, said in an interview.
“If you go to their websites, even if it’s not stated overtly, these companies at least give the impression that they’re providing actionable, useful information,” he said. “The sites recommend microbiome testing, and often supplements, probiotics, or other products that they sell. And consumers are told they need to be tested again once they start taking any of these products to see if they’re receiving any benefit.”
Dr. von Rosenvinge and colleagues authored a recent article in Science arguing that DTC microbiome tests “lack analytical and clinical validity” — and yet regulation of the industry has been “generally ignored.” They identified 31 companies globally, 17 of which are based in the United States, claiming to have products and/or services aimed at changing the intestinal microbiome.
Unreliable, Unregulated
The lack of reliability has been shown by experts who have tested the tests.
“People have taken the same stool sample, sent it to multiple companies, and gotten different results back,” Dr. von Rosenvinge said. “People also have taken a stool sample and sent it to the same company under two different names and received two different results. If the test is unreliable at its foundational level, it’s hard to use it in any clinical way.”
Test users’ methods and the companies’ procedures can affect the results, Dina Kao, MD, a professor at the University of Alberta, Edmonton, Alberta, Canada, said in an interview.
“So many biases can be introduced at every single step of the way, starting from how the stool sample was collected and how it’s preserved or not being preserved, because that can introduce a lot of noise that would change the analyses. Which primer they’re using to amplify the signals and which bioinformatic pipeline they use are also important,” said Dr. Kao, who presented at the recent Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association (AGA) and the European Society of Neurogastroenterology and Motility (ESNM).
Different investigators and companies use different technologies, so it’s very difficult to compare them and to create a standard, said Mahmoud Ghannoum, PhD, a professor in the dermatology and pathology departments at Case Western Reserve University School of Medicine and director of the Center for Medical Mycology at University Hospitals in Cleveland.
The complexity of the gut microbiome makes test standardization more difficult than it is when just one organism is involved, Dr. Ghannoum, who chaired the antifungal subcommittee at the Clinical and Laboratory Standards Institute, said in an interview.
“Even though many researchers are focusing on bacteria, we also have fungi and viruses. We need standardization of methods for testing these organisms if we want to have regulations,” said Dr. Ghannoum, a cofounder of BIOHM, a microbiome company that offers nondiagnostic tests and markets a variety of probiotics, prebiotics, and immunity supplements. BIOHM is one of the 31 companies identified by Dr. von Rosenvinge and colleagues, as noted above.
Dr. Ghannoum believes that taking a systematic approach could facilitate standardization and, ultimately, regulation of the DTC microbiome testing products. He and his colleagues described such an approach by outlining the stages for designing probiotics capable of modulating the microbiome in chronic diseases, using Crohn’s disease as a model. Their strategy involved the following steps:
- Using primary microbiome data to identify, by abundance, the microorganisms underlying dysbiosis.
- Gaining insight into the interactions among the identified pathogens.
- Conducting a correlation analysis to identify potential lead probiotic strains that antagonize these pathogens and discovering metabolites that can interrupt their interactions.
- Creating a prototype formulation for testing.
- Validating the efficacy of the candidate formulation via preclinical in vitro and in vivo testing.
- Conducting clinical testing.
Dr. Ghannoum recommends that companies use a similar process “to provide evidence that what they are doing will be helpful, not only for them but also for the reputation of the whole industry.”
Potential Pitfalls
Whether test results from commercial companies are positioned as wellness aids or diagnostic tools, providing advice based on the results “is where the danger can really come in,” Dr. Kao said. “There is still so much we don’t know about which microbial signatures are associated with each condition.”
“Even when we have a solution, like the Crohn’s exclusion diet, a physician doesn’t know enough of the nuances to give advice to a patient,” she said. “That really should be done under the guidance of an expert dietitian. And if a company is selling probiotics, I personally feel that’s not ethical. I’m pretty sure there’s always going to be some kind of conflict of interest.”
Supplements and probiotics are generally safe, but negative consequences can occur, Dr. von Rosenvinge noted.
“We occasionally see people who end up with liver problems as a result of certain supplements, and rarely, probiotics have been associated with infections from those organisms, usually in those with a compromised immune system,” he said.
Other risks include people taking supplements or probiotics when they actually have a medically treatable condition or delays in diagnosis of a potentially serious underlying condition, such as colon cancer, he said. Some patients may stop taking their traditional medication in favor of taking supplements or may experience a drug-supplement interaction if they take both.
What to Tell Patients
“Doctors should be advising against this testing for their patients,” gastroenterologist Colleen R. Kelly, MD, AGAF, Brigham and Women’s Hospital, Boston, said in an interview. “I explain to patients that these tests are not validated and are clinically meaningless data and not worth the money. There is a reason they are not covered by insurance.
“Recommendations to purchase probiotics or supplements manufactured by the testing company to ‘restore a balanced or healthy microbiome’ clearly seem like a scam,” she added. “I believe some of these companies are capitalizing on patients who are desperate for answers to explain chronic symptoms, such as bloating in irritable bowel syndrome.”
Dr. von Rosenvinge said that the message to patients “is that the science isn’t there yet to support using the results of these tests in a meaningful way. We believe the microbiome is very important in health and disease, but the tests themselves in their current state are not as reliable and reproducible as we would like.”
When patients come in with test results, the first question a clinician should ask is what led them to seek out this type of information in the first place, Dr. von Rosenvinge said.
“Our patient focus groups suggested that many have not gotten clear, satisfactory answers from traditional medicine,” he said. “We don’t have a single test that says, yes, you have irritable bowel syndrome, or no, you don’t. We might suggest things that are helpful for some people and are less helpful for others.”
Dr. Kelly said she worries that “there are snake oil salesmen and cons out there who will gladly take your money. These may be smart people, capable of doing very high-level testing, and even producing very detailed and accurate results, but that doesn’t mean we know what to do with them.”
She hopes to see a microbiome-based diagnostic test in the future, particularly if the ability to therapeutically manipulate the gut microbiome in various diseases becomes a reality.
Educate Clinicians, Companies
More education is needed on the subject, so we can become “microbial clinicians,” Dr. Kao said.
“The microbiome never came up when I was going through my medical education,” she said. But we, and the next generation of physicians, “need to at least be able to understand the basics.
“Hopefully, one day, we will be in a position where we can have meaningful interpretations of the test results and make some kind of meaningful dietary interventions,” Dr. Kao added.
As for clinicians who are currently ordering these tests and products directly from the DTC companies, Dr. Kao said, “I roll my eyes.”
Dr. Ghannoum reiterated that companies offering microbiome tests and products also need to be educated and encouraged to use systematic approaches to product development and interpretation.
“Companies should be open to calls from clinicians and be ready to explain findings on a report, as well as the basis for any recommendations,” he said.
Dr. von Rosenvinge, Dr. Kao, and Dr. Kelly had no relevant conflicts of interest. Dr. Ghannoum is a cofounder of BIOHM.
A version of this article appeared on Medscape.com.
Companies selling gut microbiome tests directly to consumers offer up a variety of claims to promote their products.
“We analyze the trillions of microbes in your gut microflora and craft a unique formula for your unique gut needs,” one says. “Get actionable dietary, supplement, and lifestyle recommendations from our microbiome experts based on your results, tailored to mom and baby’s biomarkers. ... Any family member like dads or siblings are welcome too,” says another.
The companies assert that they can improve gut health by offering individuals personalized treatments based on their gut microbiome test results. The trouble is, no provider, company, or technology can reliably do that yet.
Clinical Implications, Not Applications
The microbiome is the “constellation of microorganisms that call the human body home,” including many strains of bacteria, fungi, and viruses. That constellation comprises some 39 trillion cells.
Although knowledge is increasing on the oral, cutaneous, and vaginal microbiomes, the gut microbiome is arguably the most studied. However, while research is increasingly demonstrating that the gut microbiome has clinical implications, much work needs to be done before reliable applications based on that research are available.
But , Erik C. von Rosenvinge, MD, AGAF, a professor at the University of Maryland School of Medicine and chief of gastroenterology at the VA Maryland Health Care System, Baltimore, said in an interview.
“If you go to their websites, even if it’s not stated overtly, these companies at least give the impression that they’re providing actionable, useful information,” he said. “The sites recommend microbiome testing, and often supplements, probiotics, or other products that they sell. And consumers are told they need to be tested again once they start taking any of these products to see if they’re receiving any benefit.”
Dr. von Rosenvinge and colleagues authored a recent article in Science arguing that DTC microbiome tests “lack analytical and clinical validity” — and yet regulation of the industry has been “generally ignored.” They identified 31 companies globally, 17 of which are based in the United States, claiming to have products and/or services aimed at changing the intestinal microbiome.
Unreliable, Unregulated
The lack of reliability has been shown by experts who have tested the tests.
“People have taken the same stool sample, sent it to multiple companies, and gotten different results back,” Dr. von Rosenvinge said. “People also have taken a stool sample and sent it to the same company under two different names and received two different results. If the test is unreliable at its foundational level, it’s hard to use it in any clinical way.”
Test users’ methods and the companies’ procedures can affect the results, Dina Kao, MD, a professor at the University of Alberta, Edmonton, Alberta, Canada, said in an interview.
“So many biases can be introduced at every single step of the way, starting from how the stool sample was collected and how it’s preserved or not being preserved, because that can introduce a lot of noise that would change the analyses. Which primer they’re using to amplify the signals and which bioinformatic pipeline they use are also important,” said Dr. Kao, who presented at the recent Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association (AGA) and the European Society of Neurogastroenterology and Motility (ESNM).
Different investigators and companies use different technologies, so it’s very difficult to compare them and to create a standard, said Mahmoud Ghannoum, PhD, a professor in the dermatology and pathology departments at Case Western Reserve University School of Medicine and director of the Center for Medical Mycology at University Hospitals in Cleveland.
The complexity of the gut microbiome makes test standardization more difficult than it is when just one organism is involved, Dr. Ghannoum, who chaired the antifungal subcommittee at the Clinical and Laboratory Standards Institute, said in an interview.
“Even though many researchers are focusing on bacteria, we also have fungi and viruses. We need standardization of methods for testing these organisms if we want to have regulations,” said Dr. Ghannoum, a cofounder of BIOHM, a microbiome company that offers nondiagnostic tests and markets a variety of probiotics, prebiotics, and immunity supplements. BIOHM is one of the 31 companies identified by Dr. von Rosenvinge and colleagues, as noted above.
Dr. Ghannoum believes that taking a systematic approach could facilitate standardization and, ultimately, regulation of the DTC microbiome testing products. He and his colleagues described such an approach by outlining the stages for designing probiotics capable of modulating the microbiome in chronic diseases, using Crohn’s disease as a model. Their strategy involved the following steps:
- Using primary microbiome data to identify, by abundance, the microorganisms underlying dysbiosis.
- Gaining insight into the interactions among the identified pathogens.
- Conducting a correlation analysis to identify potential lead probiotic strains that antagonize these pathogens and discovering metabolites that can interrupt their interactions.
- Creating a prototype formulation for testing.
- Validating the efficacy of the candidate formulation via preclinical in vitro and in vivo testing.
- Conducting clinical testing.
Dr. Ghannoum recommends that companies use a similar process “to provide evidence that what they are doing will be helpful, not only for them but also for the reputation of the whole industry.”
Potential Pitfalls
Whether test results from commercial companies are positioned as wellness aids or diagnostic tools, providing advice based on the results “is where the danger can really come in,” Dr. Kao said. “There is still so much we don’t know about which microbial signatures are associated with each condition.”
“Even when we have a solution, like the Crohn’s exclusion diet, a physician doesn’t know enough of the nuances to give advice to a patient,” she said. “That really should be done under the guidance of an expert dietitian. And if a company is selling probiotics, I personally feel that’s not ethical. I’m pretty sure there’s always going to be some kind of conflict of interest.”
Supplements and probiotics are generally safe, but negative consequences can occur, Dr. von Rosenvinge noted.
“We occasionally see people who end up with liver problems as a result of certain supplements, and rarely, probiotics have been associated with infections from those organisms, usually in those with a compromised immune system,” he said.
Other risks include people taking supplements or probiotics when they actually have a medically treatable condition or delays in diagnosis of a potentially serious underlying condition, such as colon cancer, he said. Some patients may stop taking their traditional medication in favor of taking supplements or may experience a drug-supplement interaction if they take both.
What to Tell Patients
“Doctors should be advising against this testing for their patients,” gastroenterologist Colleen R. Kelly, MD, AGAF, Brigham and Women’s Hospital, Boston, said in an interview. “I explain to patients that these tests are not validated and are clinically meaningless data and not worth the money. There is a reason they are not covered by insurance.
“Recommendations to purchase probiotics or supplements manufactured by the testing company to ‘restore a balanced or healthy microbiome’ clearly seem like a scam,” she added. “I believe some of these companies are capitalizing on patients who are desperate for answers to explain chronic symptoms, such as bloating in irritable bowel syndrome.”
Dr. von Rosenvinge said that the message to patients “is that the science isn’t there yet to support using the results of these tests in a meaningful way. We believe the microbiome is very important in health and disease, but the tests themselves in their current state are not as reliable and reproducible as we would like.”
When patients come in with test results, the first question a clinician should ask is what led them to seek out this type of information in the first place, Dr. von Rosenvinge said.
“Our patient focus groups suggested that many have not gotten clear, satisfactory answers from traditional medicine,” he said. “We don’t have a single test that says, yes, you have irritable bowel syndrome, or no, you don’t. We might suggest things that are helpful for some people and are less helpful for others.”
Dr. Kelly said she worries that “there are snake oil salesmen and cons out there who will gladly take your money. These may be smart people, capable of doing very high-level testing, and even producing very detailed and accurate results, but that doesn’t mean we know what to do with them.”
She hopes to see a microbiome-based diagnostic test in the future, particularly if the ability to therapeutically manipulate the gut microbiome in various diseases becomes a reality.
Educate Clinicians, Companies
More education is needed on the subject, so we can become “microbial clinicians,” Dr. Kao said.
“The microbiome never came up when I was going through my medical education,” she said. But we, and the next generation of physicians, “need to at least be able to understand the basics.
“Hopefully, one day, we will be in a position where we can have meaningful interpretations of the test results and make some kind of meaningful dietary interventions,” Dr. Kao added.
As for clinicians who are currently ordering these tests and products directly from the DTC companies, Dr. Kao said, “I roll my eyes.”
Dr. Ghannoum reiterated that companies offering microbiome tests and products also need to be educated and encouraged to use systematic approaches to product development and interpretation.
“Companies should be open to calls from clinicians and be ready to explain findings on a report, as well as the basis for any recommendations,” he said.
Dr. von Rosenvinge, Dr. Kao, and Dr. Kelly had no relevant conflicts of interest. Dr. Ghannoum is a cofounder of BIOHM.
A version of this article appeared on Medscape.com.
Companies selling gut microbiome tests directly to consumers offer up a variety of claims to promote their products.
“We analyze the trillions of microbes in your gut microflora and craft a unique formula for your unique gut needs,” one says. “Get actionable dietary, supplement, and lifestyle recommendations from our microbiome experts based on your results, tailored to mom and baby’s biomarkers. ... Any family member like dads or siblings are welcome too,” says another.
The companies assert that they can improve gut health by offering individuals personalized treatments based on their gut microbiome test results. The trouble is, no provider, company, or technology can reliably do that yet.
Clinical Implications, Not Applications
The microbiome is the “constellation of microorganisms that call the human body home,” including many strains of bacteria, fungi, and viruses. That constellation comprises some 39 trillion cells.
Although knowledge is increasing on the oral, cutaneous, and vaginal microbiomes, the gut microbiome is arguably the most studied. However, while research is increasingly demonstrating that the gut microbiome has clinical implications, much work needs to be done before reliable applications based on that research are available.
But , Erik C. von Rosenvinge, MD, AGAF, a professor at the University of Maryland School of Medicine and chief of gastroenterology at the VA Maryland Health Care System, Baltimore, said in an interview.
“If you go to their websites, even if it’s not stated overtly, these companies at least give the impression that they’re providing actionable, useful information,” he said. “The sites recommend microbiome testing, and often supplements, probiotics, or other products that they sell. And consumers are told they need to be tested again once they start taking any of these products to see if they’re receiving any benefit.”
Dr. von Rosenvinge and colleagues authored a recent article in Science arguing that DTC microbiome tests “lack analytical and clinical validity” — and yet regulation of the industry has been “generally ignored.” They identified 31 companies globally, 17 of which are based in the United States, claiming to have products and/or services aimed at changing the intestinal microbiome.
Unreliable, Unregulated
The lack of reliability has been shown by experts who have tested the tests.
“People have taken the same stool sample, sent it to multiple companies, and gotten different results back,” Dr. von Rosenvinge said. “People also have taken a stool sample and sent it to the same company under two different names and received two different results. If the test is unreliable at its foundational level, it’s hard to use it in any clinical way.”
Test users’ methods and the companies’ procedures can affect the results, Dina Kao, MD, a professor at the University of Alberta, Edmonton, Alberta, Canada, said in an interview.
“So many biases can be introduced at every single step of the way, starting from how the stool sample was collected and how it’s preserved or not being preserved, because that can introduce a lot of noise that would change the analyses. Which primer they’re using to amplify the signals and which bioinformatic pipeline they use are also important,” said Dr. Kao, who presented at the recent Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association (AGA) and the European Society of Neurogastroenterology and Motility (ESNM).
Different investigators and companies use different technologies, so it’s very difficult to compare them and to create a standard, said Mahmoud Ghannoum, PhD, a professor in the dermatology and pathology departments at Case Western Reserve University School of Medicine and director of the Center for Medical Mycology at University Hospitals in Cleveland.
The complexity of the gut microbiome makes test standardization more difficult than it is when just one organism is involved, Dr. Ghannoum, who chaired the antifungal subcommittee at the Clinical and Laboratory Standards Institute, said in an interview.
“Even though many researchers are focusing on bacteria, we also have fungi and viruses. We need standardization of methods for testing these organisms if we want to have regulations,” said Dr. Ghannoum, a cofounder of BIOHM, a microbiome company that offers nondiagnostic tests and markets a variety of probiotics, prebiotics, and immunity supplements. BIOHM is one of the 31 companies identified by Dr. von Rosenvinge and colleagues, as noted above.
Dr. Ghannoum believes that taking a systematic approach could facilitate standardization and, ultimately, regulation of the DTC microbiome testing products. He and his colleagues described such an approach by outlining the stages for designing probiotics capable of modulating the microbiome in chronic diseases, using Crohn’s disease as a model. Their strategy involved the following steps:
- Using primary microbiome data to identify, by abundance, the microorganisms underlying dysbiosis.
- Gaining insight into the interactions among the identified pathogens.
- Conducting a correlation analysis to identify potential lead probiotic strains that antagonize these pathogens and discovering metabolites that can interrupt their interactions.
- Creating a prototype formulation for testing.
- Validating the efficacy of the candidate formulation via preclinical in vitro and in vivo testing.
- Conducting clinical testing.
Dr. Ghannoum recommends that companies use a similar process “to provide evidence that what they are doing will be helpful, not only for them but also for the reputation of the whole industry.”
Potential Pitfalls
Whether test results from commercial companies are positioned as wellness aids or diagnostic tools, providing advice based on the results “is where the danger can really come in,” Dr. Kao said. “There is still so much we don’t know about which microbial signatures are associated with each condition.”
“Even when we have a solution, like the Crohn’s exclusion diet, a physician doesn’t know enough of the nuances to give advice to a patient,” she said. “That really should be done under the guidance of an expert dietitian. And if a company is selling probiotics, I personally feel that’s not ethical. I’m pretty sure there’s always going to be some kind of conflict of interest.”
Supplements and probiotics are generally safe, but negative consequences can occur, Dr. von Rosenvinge noted.
“We occasionally see people who end up with liver problems as a result of certain supplements, and rarely, probiotics have been associated with infections from those organisms, usually in those with a compromised immune system,” he said.
Other risks include people taking supplements or probiotics when they actually have a medically treatable condition or delays in diagnosis of a potentially serious underlying condition, such as colon cancer, he said. Some patients may stop taking their traditional medication in favor of taking supplements or may experience a drug-supplement interaction if they take both.
What to Tell Patients
“Doctors should be advising against this testing for their patients,” gastroenterologist Colleen R. Kelly, MD, AGAF, Brigham and Women’s Hospital, Boston, said in an interview. “I explain to patients that these tests are not validated and are clinically meaningless data and not worth the money. There is a reason they are not covered by insurance.
“Recommendations to purchase probiotics or supplements manufactured by the testing company to ‘restore a balanced or healthy microbiome’ clearly seem like a scam,” she added. “I believe some of these companies are capitalizing on patients who are desperate for answers to explain chronic symptoms, such as bloating in irritable bowel syndrome.”
Dr. von Rosenvinge said that the message to patients “is that the science isn’t there yet to support using the results of these tests in a meaningful way. We believe the microbiome is very important in health and disease, but the tests themselves in their current state are not as reliable and reproducible as we would like.”
When patients come in with test results, the first question a clinician should ask is what led them to seek out this type of information in the first place, Dr. von Rosenvinge said.
“Our patient focus groups suggested that many have not gotten clear, satisfactory answers from traditional medicine,” he said. “We don’t have a single test that says, yes, you have irritable bowel syndrome, or no, you don’t. We might suggest things that are helpful for some people and are less helpful for others.”
Dr. Kelly said she worries that “there are snake oil salesmen and cons out there who will gladly take your money. These may be smart people, capable of doing very high-level testing, and even producing very detailed and accurate results, but that doesn’t mean we know what to do with them.”
She hopes to see a microbiome-based diagnostic test in the future, particularly if the ability to therapeutically manipulate the gut microbiome in various diseases becomes a reality.
Educate Clinicians, Companies
More education is needed on the subject, so we can become “microbial clinicians,” Dr. Kao said.
“The microbiome never came up when I was going through my medical education,” she said. But we, and the next generation of physicians, “need to at least be able to understand the basics.
“Hopefully, one day, we will be in a position where we can have meaningful interpretations of the test results and make some kind of meaningful dietary interventions,” Dr. Kao added.
As for clinicians who are currently ordering these tests and products directly from the DTC companies, Dr. Kao said, “I roll my eyes.”
Dr. Ghannoum reiterated that companies offering microbiome tests and products also need to be educated and encouraged to use systematic approaches to product development and interpretation.
“Companies should be open to calls from clinicians and be ready to explain findings on a report, as well as the basis for any recommendations,” he said.
Dr. von Rosenvinge, Dr. Kao, and Dr. Kelly had no relevant conflicts of interest. Dr. Ghannoum is a cofounder of BIOHM.
A version of this article appeared on Medscape.com.
FDA OKs Subcutaneous Vedolizumab for Crohn’s Maintenance Therapy
The move follows the FDA’s approval last year of subcutaneous vedolizumab for maintenance treatment of adults with moderately to severely active ulcerative colitis (UC).
The humanized immunoglobulin G1 monoclonal antibody is available as a single-dose prefilled pen (Entyvio Pen).
The FDA first approved the IV formulation of the biologic in 2014 for patients with moderate to severe UC and CD who cannot tolerate other therapies or in whom such therapies have failed.
The approval of subcutaneous vedolizumab for maintenance treatment of CD is based on the phase 3, randomized, double-blind, placebo-controlled VISIBLE 2 trial.
The trial enrolled 409 adult patients with moderately to severely active CD who had clinical response at week 6 following two doses of open-label IV vedolizumab at weeks 0 and 2.
At week 6, they were randomly allocated in a 2:1 ratio to receive vedolizumab 108 mg administered by subcutaneous injection or placebo every 2 weeks. The primary endpoint was clinical remission at week 52, which was defined as a total Crohn’s Disease Activity Index score ≤ 150.
The results showed that significantly more patients receiving subcutaneous vedolizumab than placebo achieved long-term clinical remission (48% vs 34%; P < .01), the company said in a news release.
The safety profile of subcutaneous vedolizumab is generally consistent with the known safety profile of IV vedolizumab, with the addition of injection-site reactions (including injection-site erythema, rash, pruritus, swelling, bruising, hematoma, pain, urticaria, and edema).
“Crohn’s disease is a complex and usually progressive disease for which an appropriate management plan is critical. My primary goal as a clinician is always to get patients to achieve remission,” Timothy Ritter, MD, senior medical director, GI Alliance Research, and assistant professor of medicine, Burnett School of Medicine at TCU, Fort Worth, Texas, said in the news release.
“In VISIBLE 2, about half of patients treated with Entyvio SC achieved long-term clinical remission. The data from VISIBLE 2 reaffirm the well-established efficacy profile of Entyvio, regardless of route of administration,” Dr. Ritter added.
A version of this article appeared on Medscape.com.
The move follows the FDA’s approval last year of subcutaneous vedolizumab for maintenance treatment of adults with moderately to severely active ulcerative colitis (UC).
The humanized immunoglobulin G1 monoclonal antibody is available as a single-dose prefilled pen (Entyvio Pen).
The FDA first approved the IV formulation of the biologic in 2014 for patients with moderate to severe UC and CD who cannot tolerate other therapies or in whom such therapies have failed.
The approval of subcutaneous vedolizumab for maintenance treatment of CD is based on the phase 3, randomized, double-blind, placebo-controlled VISIBLE 2 trial.
The trial enrolled 409 adult patients with moderately to severely active CD who had clinical response at week 6 following two doses of open-label IV vedolizumab at weeks 0 and 2.
At week 6, they were randomly allocated in a 2:1 ratio to receive vedolizumab 108 mg administered by subcutaneous injection or placebo every 2 weeks. The primary endpoint was clinical remission at week 52, which was defined as a total Crohn’s Disease Activity Index score ≤ 150.
The results showed that significantly more patients receiving subcutaneous vedolizumab than placebo achieved long-term clinical remission (48% vs 34%; P < .01), the company said in a news release.
The safety profile of subcutaneous vedolizumab is generally consistent with the known safety profile of IV vedolizumab, with the addition of injection-site reactions (including injection-site erythema, rash, pruritus, swelling, bruising, hematoma, pain, urticaria, and edema).
“Crohn’s disease is a complex and usually progressive disease for which an appropriate management plan is critical. My primary goal as a clinician is always to get patients to achieve remission,” Timothy Ritter, MD, senior medical director, GI Alliance Research, and assistant professor of medicine, Burnett School of Medicine at TCU, Fort Worth, Texas, said in the news release.
“In VISIBLE 2, about half of patients treated with Entyvio SC achieved long-term clinical remission. The data from VISIBLE 2 reaffirm the well-established efficacy profile of Entyvio, regardless of route of administration,” Dr. Ritter added.
A version of this article appeared on Medscape.com.
The move follows the FDA’s approval last year of subcutaneous vedolizumab for maintenance treatment of adults with moderately to severely active ulcerative colitis (UC).
The humanized immunoglobulin G1 monoclonal antibody is available as a single-dose prefilled pen (Entyvio Pen).
The FDA first approved the IV formulation of the biologic in 2014 for patients with moderate to severe UC and CD who cannot tolerate other therapies or in whom such therapies have failed.
The approval of subcutaneous vedolizumab for maintenance treatment of CD is based on the phase 3, randomized, double-blind, placebo-controlled VISIBLE 2 trial.
The trial enrolled 409 adult patients with moderately to severely active CD who had clinical response at week 6 following two doses of open-label IV vedolizumab at weeks 0 and 2.
At week 6, they were randomly allocated in a 2:1 ratio to receive vedolizumab 108 mg administered by subcutaneous injection or placebo every 2 weeks. The primary endpoint was clinical remission at week 52, which was defined as a total Crohn’s Disease Activity Index score ≤ 150.
The results showed that significantly more patients receiving subcutaneous vedolizumab than placebo achieved long-term clinical remission (48% vs 34%; P < .01), the company said in a news release.
The safety profile of subcutaneous vedolizumab is generally consistent with the known safety profile of IV vedolizumab, with the addition of injection-site reactions (including injection-site erythema, rash, pruritus, swelling, bruising, hematoma, pain, urticaria, and edema).
“Crohn’s disease is a complex and usually progressive disease for which an appropriate management plan is critical. My primary goal as a clinician is always to get patients to achieve remission,” Timothy Ritter, MD, senior medical director, GI Alliance Research, and assistant professor of medicine, Burnett School of Medicine at TCU, Fort Worth, Texas, said in the news release.
“In VISIBLE 2, about half of patients treated with Entyvio SC achieved long-term clinical remission. The data from VISIBLE 2 reaffirm the well-established efficacy profile of Entyvio, regardless of route of administration,” Dr. Ritter added.
A version of this article appeared on Medscape.com.
Which Probiotics Are Effective in Irritable Bowel Syndrome?
PARIS — Irritable bowel syndrome (IBS) is a common brain-gut axis disorder, and patients are often dissatisfied with conventional treatments.
The role of the microbiota in IBS is now well established, and patients frequently take probiotics on their own initiative or on the advice of a physician or pharmacist. However, not all probiotics have equal efficacy, so which ones should be recommended?
Jean-Marc Sabaté, MD, PhD, a gastroenterologist at Avicenne Hospital in Bobigny, France, shared insights about probiotics at the Francophone Days of Hepatology, Gastroenterology, and Digestive Oncology.
IBS, according to the Rome IV symptom-based classification, is a “disorder of brain-gut axis interactions” with a prevalence of about 4% in the adult population. In France, during an average care pathway of about 8 years, patients try an average of five therapeutic strategies (and as many as 11), including antispasmodics (85%), diets (78%), and probiotics. In addition, 66.4% of patients had either taken or were taking probiotics at the time of a recent survey.
While the 2022 recommendations from the American College of Gastroenterology on the diagnosis and management of IBS do not support the use of probiotics for overall symptom relief — a recommendation for which they cite a low level of evidence — “there is nevertheless a rationale for prescribing probiotics in IBS due to the significant role of the microbiota (or dysbiosis) in this condition,” said Dr. Sabaté.
Microbiota in IBS
Evidence indicating that antibiotics exacerbate IBS symptoms and revealing chronic bacterial overgrowth in the small intestine of patients with IBS supports the role of the microbiota. Studies using a molecular approach (16s rRNA) have settled the debate, confirming differences in the intestinal flora between patients with IBS and healthy subjects. Data also indicate differences in flora between patient subtypes, such as an increased Firmicutes to Bacteroidetes ratio. However, one subgroup, which can represent as much as a third of patients, seems to harbor a “normal” microbiota.
Nonetheless, the microbiota plays a significant role in IBS. A Swedish study highlighted the influence of bacterial enterotypes on transit type associated with IBS and symptom severity, independent of diet composition or medication use.
This dysbiosis could play a significant role as it interacts with other mechanisms involved in IBS, including changes in intestinal motility related to diet (related to fermentable carbohydrates, for example). Moreover, the microbiota seems to induce a low level of immune activation in patients with IBS, leading to microinflammation and increased intestinal permeability, especially after an infection.
Furthermore, alterations in the regulation of bile acid deconjugation by the microbiota partly explain the frequency and consistency of stools in diarrhea-predominant IBS patients.
In addition, colonic gas production is higher in these patients. Those complaining of flatulence have poor tolerance to intestinal gases after a flatulent meal, associated with microbiota instability.
Data regarding the interaction between the microbiota and central mechanisms mainly come from animal studies. In rodents, microbiota constituents seem to affect brain development, function, and morphology. Emotional and physical traumas during childhood appear to be risk factors. Moreover, even brief exposure to broad-spectrum antibiotics in neonates could cause subsequent visceral hypersensitivity.
Lastly, the role of the microbiota in changes in medullary pain control after visceral stimulation (eg, rectal distension) has still not been demonstrated in humans.
Recent Guideline
In its February 2023 Global Guideline “Probiotics and Prebiotics” for IBS, the World Gastroenterology Organization looked at the level of evidence for probiotics.
Three strains, as well as a combination of several strains, were supported by level 2 evidence, meaning at least two randomized studies with converging results. These are Bifidobacterium bifidum MIMBb75, which improves overall symptoms and quality of life; Lactobacillus plantarum 299v (DSM 9843), which acts on the severity of abdominal pain and bloating; and B infantis 35624 (new name: B longum 35624), which improves the overall assessment of IBS symptoms, as does the multistrain product containing L rhamnosus GG, L rhamnosus LC705, Propionibacterium freudenreichii ssp shermanii JS DSM 7067, and B animalis ssp lactis B012 DSM 15954.
Efficacy and Availability
Probiotics belonging to the category of dietary supplements or medical devices are not required to provide evidence for a mechanism of action or even efficacy to be marketed. Thus, for most probiotics sold, there are no human or even animal studies available.
Dr. Sabaté proposed a choice of probiotics based on the literature and the presence of at least one randomized placebo-controlled trial conducted in patients with IBS showing positive results.
“,” he emphasized. The parameters that can be improved include symptom severity, quality of life, abdominal pain, and bloating.
Effective Probiotics
B longum 35624, which was developed with researchers from University College Cork in Ireland, is probably the most studied in animals and humans. Research has encompassed the mechanistic, clinical, and safety aspects of the probiotic. It has shown good results on the IBS-Symptom Severity Score (SSS), quality of life, abdominal pain, bowel disturbances, and bloating. The treatment duration in studies is 4-8 weeks.
L plantarum 299v (DSM 9843) affects the frequency of abdominal pain and pain score. The treatment duration in studies is 4 weeks.
The multistrain product that includes L plantarum CECT 7484/L plantarum CECT 7485/ Pediococcus acidilactici CECT 7483 allows for an improvement in quality of life and anxiety related to digestive symptoms. No positive effect has been described on digestive symptoms, especially diarrhea. The treatment duration is 6 weeks.
B bifidum MIMBb75 (both normal and heat-inactivated forms) is beneficial for pain, the composite IBS-SSS score, and quality of life. The treatment duration is 4-8 weeks.
“Except for the multistrain combination, which is more suited to patients with diarrhea-predominant IBS, the other three probiotics can be prescribed regardless of the IBS subtype,” said Dr. Sabaté. “Treatment durations are typically 4 weeks, but it is possible to continue up to 8 weeks, which is the maximum duration of these studies. In practice, there are no tolerance issues with probiotics prescribed for IBS based on the literature. These should be tested under the conditions and for the duration of the published studies and should only be continued if there is individual benefit on symptoms or quality of life.”
Note that microbiota analyses conducted for individual purposes are of no help in choosing probiotics.
Mechanisms of Action
In a murine model, but not in humans, some strains, especially L acidophilus NCFM, have shown an antinociceptive effect by inducing opioid and cannabinoid receptors.
Only in animals to date, L farciminis and B lactis CNCM I-2494 have shown prevention of induced hypersensitivity (ie, inhibition of the cytoskeleton contraction of colon epithelial cells and subsequent opening of tight junctions).
B infantis 35624 has an anti-inflammatory action by modifying the IL-10 and IL-12 cytokine ratio in animals and humans. It has an immunomodulatory action by increasing dendritic cells in the mucosa and decreasing Th1 and Th7 helper T cells.
B infantis 35624 and L farciminis are two strains that decrease visceral sensitivity in mice.
Escherichia coli Nissle 1917 acts on lipopeptide production with an antinociceptive effect, as observed in mice, by decreasing visceral sensitivity through calcium nociceptor flux blockade (action on GABA type B receptor).
Acting on dysbiosis by modifying fecal microbiota during probiotic intake is possible but depends on the probiotics, like B infantis 35624. In humans, B longum NCC 3001 could modify brain activations.
Dr. Sabaté disclosed financial relationships with Mayoly Spindler, Kyowa Kirin, Tillotts, Servier, Norgine, Biocodex, Merck, Viatris, Abivax, and Inventiva.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
PARIS — Irritable bowel syndrome (IBS) is a common brain-gut axis disorder, and patients are often dissatisfied with conventional treatments.
The role of the microbiota in IBS is now well established, and patients frequently take probiotics on their own initiative or on the advice of a physician or pharmacist. However, not all probiotics have equal efficacy, so which ones should be recommended?
Jean-Marc Sabaté, MD, PhD, a gastroenterologist at Avicenne Hospital in Bobigny, France, shared insights about probiotics at the Francophone Days of Hepatology, Gastroenterology, and Digestive Oncology.
IBS, according to the Rome IV symptom-based classification, is a “disorder of brain-gut axis interactions” with a prevalence of about 4% in the adult population. In France, during an average care pathway of about 8 years, patients try an average of five therapeutic strategies (and as many as 11), including antispasmodics (85%), diets (78%), and probiotics. In addition, 66.4% of patients had either taken or were taking probiotics at the time of a recent survey.
While the 2022 recommendations from the American College of Gastroenterology on the diagnosis and management of IBS do not support the use of probiotics for overall symptom relief — a recommendation for which they cite a low level of evidence — “there is nevertheless a rationale for prescribing probiotics in IBS due to the significant role of the microbiota (or dysbiosis) in this condition,” said Dr. Sabaté.
Microbiota in IBS
Evidence indicating that antibiotics exacerbate IBS symptoms and revealing chronic bacterial overgrowth in the small intestine of patients with IBS supports the role of the microbiota. Studies using a molecular approach (16s rRNA) have settled the debate, confirming differences in the intestinal flora between patients with IBS and healthy subjects. Data also indicate differences in flora between patient subtypes, such as an increased Firmicutes to Bacteroidetes ratio. However, one subgroup, which can represent as much as a third of patients, seems to harbor a “normal” microbiota.
Nonetheless, the microbiota plays a significant role in IBS. A Swedish study highlighted the influence of bacterial enterotypes on transit type associated with IBS and symptom severity, independent of diet composition or medication use.
This dysbiosis could play a significant role as it interacts with other mechanisms involved in IBS, including changes in intestinal motility related to diet (related to fermentable carbohydrates, for example). Moreover, the microbiota seems to induce a low level of immune activation in patients with IBS, leading to microinflammation and increased intestinal permeability, especially after an infection.
Furthermore, alterations in the regulation of bile acid deconjugation by the microbiota partly explain the frequency and consistency of stools in diarrhea-predominant IBS patients.
In addition, colonic gas production is higher in these patients. Those complaining of flatulence have poor tolerance to intestinal gases after a flatulent meal, associated with microbiota instability.
Data regarding the interaction between the microbiota and central mechanisms mainly come from animal studies. In rodents, microbiota constituents seem to affect brain development, function, and morphology. Emotional and physical traumas during childhood appear to be risk factors. Moreover, even brief exposure to broad-spectrum antibiotics in neonates could cause subsequent visceral hypersensitivity.
Lastly, the role of the microbiota in changes in medullary pain control after visceral stimulation (eg, rectal distension) has still not been demonstrated in humans.
Recent Guideline
In its February 2023 Global Guideline “Probiotics and Prebiotics” for IBS, the World Gastroenterology Organization looked at the level of evidence for probiotics.
Three strains, as well as a combination of several strains, were supported by level 2 evidence, meaning at least two randomized studies with converging results. These are Bifidobacterium bifidum MIMBb75, which improves overall symptoms and quality of life; Lactobacillus plantarum 299v (DSM 9843), which acts on the severity of abdominal pain and bloating; and B infantis 35624 (new name: B longum 35624), which improves the overall assessment of IBS symptoms, as does the multistrain product containing L rhamnosus GG, L rhamnosus LC705, Propionibacterium freudenreichii ssp shermanii JS DSM 7067, and B animalis ssp lactis B012 DSM 15954.
Efficacy and Availability
Probiotics belonging to the category of dietary supplements or medical devices are not required to provide evidence for a mechanism of action or even efficacy to be marketed. Thus, for most probiotics sold, there are no human or even animal studies available.
Dr. Sabaté proposed a choice of probiotics based on the literature and the presence of at least one randomized placebo-controlled trial conducted in patients with IBS showing positive results.
“,” he emphasized. The parameters that can be improved include symptom severity, quality of life, abdominal pain, and bloating.
Effective Probiotics
B longum 35624, which was developed with researchers from University College Cork in Ireland, is probably the most studied in animals and humans. Research has encompassed the mechanistic, clinical, and safety aspects of the probiotic. It has shown good results on the IBS-Symptom Severity Score (SSS), quality of life, abdominal pain, bowel disturbances, and bloating. The treatment duration in studies is 4-8 weeks.
L plantarum 299v (DSM 9843) affects the frequency of abdominal pain and pain score. The treatment duration in studies is 4 weeks.
The multistrain product that includes L plantarum CECT 7484/L plantarum CECT 7485/ Pediococcus acidilactici CECT 7483 allows for an improvement in quality of life and anxiety related to digestive symptoms. No positive effect has been described on digestive symptoms, especially diarrhea. The treatment duration is 6 weeks.
B bifidum MIMBb75 (both normal and heat-inactivated forms) is beneficial for pain, the composite IBS-SSS score, and quality of life. The treatment duration is 4-8 weeks.
“Except for the multistrain combination, which is more suited to patients with diarrhea-predominant IBS, the other three probiotics can be prescribed regardless of the IBS subtype,” said Dr. Sabaté. “Treatment durations are typically 4 weeks, but it is possible to continue up to 8 weeks, which is the maximum duration of these studies. In practice, there are no tolerance issues with probiotics prescribed for IBS based on the literature. These should be tested under the conditions and for the duration of the published studies and should only be continued if there is individual benefit on symptoms or quality of life.”
Note that microbiota analyses conducted for individual purposes are of no help in choosing probiotics.
Mechanisms of Action
In a murine model, but not in humans, some strains, especially L acidophilus NCFM, have shown an antinociceptive effect by inducing opioid and cannabinoid receptors.
Only in animals to date, L farciminis and B lactis CNCM I-2494 have shown prevention of induced hypersensitivity (ie, inhibition of the cytoskeleton contraction of colon epithelial cells and subsequent opening of tight junctions).
B infantis 35624 has an anti-inflammatory action by modifying the IL-10 and IL-12 cytokine ratio in animals and humans. It has an immunomodulatory action by increasing dendritic cells in the mucosa and decreasing Th1 and Th7 helper T cells.
B infantis 35624 and L farciminis are two strains that decrease visceral sensitivity in mice.
Escherichia coli Nissle 1917 acts on lipopeptide production with an antinociceptive effect, as observed in mice, by decreasing visceral sensitivity through calcium nociceptor flux blockade (action on GABA type B receptor).
Acting on dysbiosis by modifying fecal microbiota during probiotic intake is possible but depends on the probiotics, like B infantis 35624. In humans, B longum NCC 3001 could modify brain activations.
Dr. Sabaté disclosed financial relationships with Mayoly Spindler, Kyowa Kirin, Tillotts, Servier, Norgine, Biocodex, Merck, Viatris, Abivax, and Inventiva.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
PARIS — Irritable bowel syndrome (IBS) is a common brain-gut axis disorder, and patients are often dissatisfied with conventional treatments.
The role of the microbiota in IBS is now well established, and patients frequently take probiotics on their own initiative or on the advice of a physician or pharmacist. However, not all probiotics have equal efficacy, so which ones should be recommended?
Jean-Marc Sabaté, MD, PhD, a gastroenterologist at Avicenne Hospital in Bobigny, France, shared insights about probiotics at the Francophone Days of Hepatology, Gastroenterology, and Digestive Oncology.
IBS, according to the Rome IV symptom-based classification, is a “disorder of brain-gut axis interactions” with a prevalence of about 4% in the adult population. In France, during an average care pathway of about 8 years, patients try an average of five therapeutic strategies (and as many as 11), including antispasmodics (85%), diets (78%), and probiotics. In addition, 66.4% of patients had either taken or were taking probiotics at the time of a recent survey.
While the 2022 recommendations from the American College of Gastroenterology on the diagnosis and management of IBS do not support the use of probiotics for overall symptom relief — a recommendation for which they cite a low level of evidence — “there is nevertheless a rationale for prescribing probiotics in IBS due to the significant role of the microbiota (or dysbiosis) in this condition,” said Dr. Sabaté.
Microbiota in IBS
Evidence indicating that antibiotics exacerbate IBS symptoms and revealing chronic bacterial overgrowth in the small intestine of patients with IBS supports the role of the microbiota. Studies using a molecular approach (16s rRNA) have settled the debate, confirming differences in the intestinal flora between patients with IBS and healthy subjects. Data also indicate differences in flora between patient subtypes, such as an increased Firmicutes to Bacteroidetes ratio. However, one subgroup, which can represent as much as a third of patients, seems to harbor a “normal” microbiota.
Nonetheless, the microbiota plays a significant role in IBS. A Swedish study highlighted the influence of bacterial enterotypes on transit type associated with IBS and symptom severity, independent of diet composition or medication use.
This dysbiosis could play a significant role as it interacts with other mechanisms involved in IBS, including changes in intestinal motility related to diet (related to fermentable carbohydrates, for example). Moreover, the microbiota seems to induce a low level of immune activation in patients with IBS, leading to microinflammation and increased intestinal permeability, especially after an infection.
Furthermore, alterations in the regulation of bile acid deconjugation by the microbiota partly explain the frequency and consistency of stools in diarrhea-predominant IBS patients.
In addition, colonic gas production is higher in these patients. Those complaining of flatulence have poor tolerance to intestinal gases after a flatulent meal, associated with microbiota instability.
Data regarding the interaction between the microbiota and central mechanisms mainly come from animal studies. In rodents, microbiota constituents seem to affect brain development, function, and morphology. Emotional and physical traumas during childhood appear to be risk factors. Moreover, even brief exposure to broad-spectrum antibiotics in neonates could cause subsequent visceral hypersensitivity.
Lastly, the role of the microbiota in changes in medullary pain control after visceral stimulation (eg, rectal distension) has still not been demonstrated in humans.
Recent Guideline
In its February 2023 Global Guideline “Probiotics and Prebiotics” for IBS, the World Gastroenterology Organization looked at the level of evidence for probiotics.
Three strains, as well as a combination of several strains, were supported by level 2 evidence, meaning at least two randomized studies with converging results. These are Bifidobacterium bifidum MIMBb75, which improves overall symptoms and quality of life; Lactobacillus plantarum 299v (DSM 9843), which acts on the severity of abdominal pain and bloating; and B infantis 35624 (new name: B longum 35624), which improves the overall assessment of IBS symptoms, as does the multistrain product containing L rhamnosus GG, L rhamnosus LC705, Propionibacterium freudenreichii ssp shermanii JS DSM 7067, and B animalis ssp lactis B012 DSM 15954.
Efficacy and Availability
Probiotics belonging to the category of dietary supplements or medical devices are not required to provide evidence for a mechanism of action or even efficacy to be marketed. Thus, for most probiotics sold, there are no human or even animal studies available.
Dr. Sabaté proposed a choice of probiotics based on the literature and the presence of at least one randomized placebo-controlled trial conducted in patients with IBS showing positive results.
“,” he emphasized. The parameters that can be improved include symptom severity, quality of life, abdominal pain, and bloating.
Effective Probiotics
B longum 35624, which was developed with researchers from University College Cork in Ireland, is probably the most studied in animals and humans. Research has encompassed the mechanistic, clinical, and safety aspects of the probiotic. It has shown good results on the IBS-Symptom Severity Score (SSS), quality of life, abdominal pain, bowel disturbances, and bloating. The treatment duration in studies is 4-8 weeks.
L plantarum 299v (DSM 9843) affects the frequency of abdominal pain and pain score. The treatment duration in studies is 4 weeks.
The multistrain product that includes L plantarum CECT 7484/L plantarum CECT 7485/ Pediococcus acidilactici CECT 7483 allows for an improvement in quality of life and anxiety related to digestive symptoms. No positive effect has been described on digestive symptoms, especially diarrhea. The treatment duration is 6 weeks.
B bifidum MIMBb75 (both normal and heat-inactivated forms) is beneficial for pain, the composite IBS-SSS score, and quality of life. The treatment duration is 4-8 weeks.
“Except for the multistrain combination, which is more suited to patients with diarrhea-predominant IBS, the other three probiotics can be prescribed regardless of the IBS subtype,” said Dr. Sabaté. “Treatment durations are typically 4 weeks, but it is possible to continue up to 8 weeks, which is the maximum duration of these studies. In practice, there are no tolerance issues with probiotics prescribed for IBS based on the literature. These should be tested under the conditions and for the duration of the published studies and should only be continued if there is individual benefit on symptoms or quality of life.”
Note that microbiota analyses conducted for individual purposes are of no help in choosing probiotics.
Mechanisms of Action
In a murine model, but not in humans, some strains, especially L acidophilus NCFM, have shown an antinociceptive effect by inducing opioid and cannabinoid receptors.
Only in animals to date, L farciminis and B lactis CNCM I-2494 have shown prevention of induced hypersensitivity (ie, inhibition of the cytoskeleton contraction of colon epithelial cells and subsequent opening of tight junctions).
B infantis 35624 has an anti-inflammatory action by modifying the IL-10 and IL-12 cytokine ratio in animals and humans. It has an immunomodulatory action by increasing dendritic cells in the mucosa and decreasing Th1 and Th7 helper T cells.
B infantis 35624 and L farciminis are two strains that decrease visceral sensitivity in mice.
Escherichia coli Nissle 1917 acts on lipopeptide production with an antinociceptive effect, as observed in mice, by decreasing visceral sensitivity through calcium nociceptor flux blockade (action on GABA type B receptor).
Acting on dysbiosis by modifying fecal microbiota during probiotic intake is possible but depends on the probiotics, like B infantis 35624. In humans, B longum NCC 3001 could modify brain activations.
Dr. Sabaté disclosed financial relationships with Mayoly Spindler, Kyowa Kirin, Tillotts, Servier, Norgine, Biocodex, Merck, Viatris, Abivax, and Inventiva.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
IBD: Histologic Inflammation Linked With Lower Female Fertility
, according to a Swedish nationwide cohort study.
Reduced fertility was linked with histologic inflammation even in the absence of clinical disease activity, highlighting the importance of achieving deep remission in women planning pregnancy, reported lead author Karl Mårild, MD, PhD, of Sahlgrenska Academy, Gothenburg, Sweden, and colleagues.
“Reduced female fertility (ie, number of live births) is believed to be primarily confined to women with clinically active IBD, especially in Crohn’s disease (CD), where symptoms may inhibit sexual activity, and inflammation may affect the fallopian tubes and ovaries,” the investigators wrote in Gastroenterology. “Despite the increasing appreciation of histologic activity in IBD, its association with female fertility has not been clarified, including whether histologic activity in the absence of clinical disease activity impairs fertility.”
Dr. Mårild and colleagues aimed to address this knowledge gap by analyzing fertility rates and histologic inflammation or IBD activity in two cohorts of women with IBD aged 15-44 years. The first group included approximately 21,000 women with and without histologic inflammation from 1990 to 2016. The second group included approximately 25,000 women with or without IBD clinical activity from 2006 to 2020. In each group, the relationship between fertility and IBD was compared with fertility in matched general population comparator individuals.
This approach showed that clinical IBD activity was associated with an adjusted fertility rate ratio (aFRR) of 0.76 (95% CI, 0.72-0.79), which equates to one fewer child per six women with 10 years of clinical activity. Impacts on fertility were similar for UC (aFRR, 0.75) and CD (aFRR, 0.76).
“Fertility rates were notably reduced during periods of clinical IBD activity and, contrary to a generally accepted belief, equally reduced in clinically active UC and CD,” the investigators wrote. “Besides inflammation, clinically active IBD may reduce fertility through psychological mechanisms (eg, depression), dyspareunia (especially in perianal CD), bowel pain, urgency, and other symptoms that hinder sexual activity.”
Compared with histologic remission, histologic inflammation was also associated with reduced fertility (aFRR, 0.90). This means that in periods of histologic inflammation, 6.35 live births occurred per 100 person-years of follow-up, compared with 7.09 lives births for periods of histologic remission. This amounts to one fewer child per 14 women with 10 years of histologic inflammation.
Finally, the study revealed that, in women with clinically quiescent IBD, those with histologic inflammation had significantly reduced fertility, compared with those in histologic remission (aFRR, 0.85). This association persisted after controlling for contraceptive use.
“Even if histologic inflammation was associated with an overall modest fertility reduction … its impact on the individual might be substantial, with potential ramifications beyond reproductive health, given that reduced female fertility is linked to poor quality of life and mental health,” Dr. Mårild and colleagues wrote. “At a societal level, involuntary childlessness causes high and increasing costs, highlighting the need to focus on preventable causes of reduced fertility.”
The investigators suggested that inflammation may be driving infertility by reducing ovulation and fertilization, or by reducing endometrial receptivity, which increases risk of pregnancy loss.
“This is the first study, to our knowledge, to show reduced fertility during histologic inflammation in IBD compared to histologic remission,” the investigators wrote. “Our findings suggest that achieving histologic remission may improve the fertility of women with IBD, even in the absence of clinically defined disease activity.”
The investigators disclosed relationships with AbbVie, Pfizer, Janssen, and others.
The importance of controlling inflammation to ensure a healthy pregnancy cannot be overstated. With regard to fertility, the literature has emphasized that surgery has been the major risk factor for decreasing fertility in both ulcerative colitis and Crohn’s disease. Disease activity has been more influential on Crohn’s disease versus ulcerative colitis. Other factors such as voluntary childlessness, premature ovarian failure, and malnutrition can also play a role. There have been data to show that anti–tumor necrosis factor use increases the chances of successful implantation for women with sub-fertility who do not have concomitant IBD, perhaps by decreasing inflammation in the pelvis.
Sunanda Kane, MD, MSPH, AGAF, is based in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. She reports serving as a consultant to Boehringer Ingelheim, Bristol Myers Squibb, Fresenius Kabi, Gilead, Janssen, and Takeda. She is also Section Editor for IBD for UptoDate.
The importance of controlling inflammation to ensure a healthy pregnancy cannot be overstated. With regard to fertility, the literature has emphasized that surgery has been the major risk factor for decreasing fertility in both ulcerative colitis and Crohn’s disease. Disease activity has been more influential on Crohn’s disease versus ulcerative colitis. Other factors such as voluntary childlessness, premature ovarian failure, and malnutrition can also play a role. There have been data to show that anti–tumor necrosis factor use increases the chances of successful implantation for women with sub-fertility who do not have concomitant IBD, perhaps by decreasing inflammation in the pelvis.
Sunanda Kane, MD, MSPH, AGAF, is based in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. She reports serving as a consultant to Boehringer Ingelheim, Bristol Myers Squibb, Fresenius Kabi, Gilead, Janssen, and Takeda. She is also Section Editor for IBD for UptoDate.
The importance of controlling inflammation to ensure a healthy pregnancy cannot be overstated. With regard to fertility, the literature has emphasized that surgery has been the major risk factor for decreasing fertility in both ulcerative colitis and Crohn’s disease. Disease activity has been more influential on Crohn’s disease versus ulcerative colitis. Other factors such as voluntary childlessness, premature ovarian failure, and malnutrition can also play a role. There have been data to show that anti–tumor necrosis factor use increases the chances of successful implantation for women with sub-fertility who do not have concomitant IBD, perhaps by decreasing inflammation in the pelvis.
Sunanda Kane, MD, MSPH, AGAF, is based in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. She reports serving as a consultant to Boehringer Ingelheim, Bristol Myers Squibb, Fresenius Kabi, Gilead, Janssen, and Takeda. She is also Section Editor for IBD for UptoDate.
, according to a Swedish nationwide cohort study.
Reduced fertility was linked with histologic inflammation even in the absence of clinical disease activity, highlighting the importance of achieving deep remission in women planning pregnancy, reported lead author Karl Mårild, MD, PhD, of Sahlgrenska Academy, Gothenburg, Sweden, and colleagues.
“Reduced female fertility (ie, number of live births) is believed to be primarily confined to women with clinically active IBD, especially in Crohn’s disease (CD), where symptoms may inhibit sexual activity, and inflammation may affect the fallopian tubes and ovaries,” the investigators wrote in Gastroenterology. “Despite the increasing appreciation of histologic activity in IBD, its association with female fertility has not been clarified, including whether histologic activity in the absence of clinical disease activity impairs fertility.”
Dr. Mårild and colleagues aimed to address this knowledge gap by analyzing fertility rates and histologic inflammation or IBD activity in two cohorts of women with IBD aged 15-44 years. The first group included approximately 21,000 women with and without histologic inflammation from 1990 to 2016. The second group included approximately 25,000 women with or without IBD clinical activity from 2006 to 2020. In each group, the relationship between fertility and IBD was compared with fertility in matched general population comparator individuals.
This approach showed that clinical IBD activity was associated with an adjusted fertility rate ratio (aFRR) of 0.76 (95% CI, 0.72-0.79), which equates to one fewer child per six women with 10 years of clinical activity. Impacts on fertility were similar for UC (aFRR, 0.75) and CD (aFRR, 0.76).
“Fertility rates were notably reduced during periods of clinical IBD activity and, contrary to a generally accepted belief, equally reduced in clinically active UC and CD,” the investigators wrote. “Besides inflammation, clinically active IBD may reduce fertility through psychological mechanisms (eg, depression), dyspareunia (especially in perianal CD), bowel pain, urgency, and other symptoms that hinder sexual activity.”
Compared with histologic remission, histologic inflammation was also associated with reduced fertility (aFRR, 0.90). This means that in periods of histologic inflammation, 6.35 live births occurred per 100 person-years of follow-up, compared with 7.09 lives births for periods of histologic remission. This amounts to one fewer child per 14 women with 10 years of histologic inflammation.
Finally, the study revealed that, in women with clinically quiescent IBD, those with histologic inflammation had significantly reduced fertility, compared with those in histologic remission (aFRR, 0.85). This association persisted after controlling for contraceptive use.
“Even if histologic inflammation was associated with an overall modest fertility reduction … its impact on the individual might be substantial, with potential ramifications beyond reproductive health, given that reduced female fertility is linked to poor quality of life and mental health,” Dr. Mårild and colleagues wrote. “At a societal level, involuntary childlessness causes high and increasing costs, highlighting the need to focus on preventable causes of reduced fertility.”
The investigators suggested that inflammation may be driving infertility by reducing ovulation and fertilization, or by reducing endometrial receptivity, which increases risk of pregnancy loss.
“This is the first study, to our knowledge, to show reduced fertility during histologic inflammation in IBD compared to histologic remission,” the investigators wrote. “Our findings suggest that achieving histologic remission may improve the fertility of women with IBD, even in the absence of clinically defined disease activity.”
The investigators disclosed relationships with AbbVie, Pfizer, Janssen, and others.
, according to a Swedish nationwide cohort study.
Reduced fertility was linked with histologic inflammation even in the absence of clinical disease activity, highlighting the importance of achieving deep remission in women planning pregnancy, reported lead author Karl Mårild, MD, PhD, of Sahlgrenska Academy, Gothenburg, Sweden, and colleagues.
“Reduced female fertility (ie, number of live births) is believed to be primarily confined to women with clinically active IBD, especially in Crohn’s disease (CD), where symptoms may inhibit sexual activity, and inflammation may affect the fallopian tubes and ovaries,” the investigators wrote in Gastroenterology. “Despite the increasing appreciation of histologic activity in IBD, its association with female fertility has not been clarified, including whether histologic activity in the absence of clinical disease activity impairs fertility.”
Dr. Mårild and colleagues aimed to address this knowledge gap by analyzing fertility rates and histologic inflammation or IBD activity in two cohorts of women with IBD aged 15-44 years. The first group included approximately 21,000 women with and without histologic inflammation from 1990 to 2016. The second group included approximately 25,000 women with or without IBD clinical activity from 2006 to 2020. In each group, the relationship between fertility and IBD was compared with fertility in matched general population comparator individuals.
This approach showed that clinical IBD activity was associated with an adjusted fertility rate ratio (aFRR) of 0.76 (95% CI, 0.72-0.79), which equates to one fewer child per six women with 10 years of clinical activity. Impacts on fertility were similar for UC (aFRR, 0.75) and CD (aFRR, 0.76).
“Fertility rates were notably reduced during periods of clinical IBD activity and, contrary to a generally accepted belief, equally reduced in clinically active UC and CD,” the investigators wrote. “Besides inflammation, clinically active IBD may reduce fertility through psychological mechanisms (eg, depression), dyspareunia (especially in perianal CD), bowel pain, urgency, and other symptoms that hinder sexual activity.”
Compared with histologic remission, histologic inflammation was also associated with reduced fertility (aFRR, 0.90). This means that in periods of histologic inflammation, 6.35 live births occurred per 100 person-years of follow-up, compared with 7.09 lives births for periods of histologic remission. This amounts to one fewer child per 14 women with 10 years of histologic inflammation.
Finally, the study revealed that, in women with clinically quiescent IBD, those with histologic inflammation had significantly reduced fertility, compared with those in histologic remission (aFRR, 0.85). This association persisted after controlling for contraceptive use.
“Even if histologic inflammation was associated with an overall modest fertility reduction … its impact on the individual might be substantial, with potential ramifications beyond reproductive health, given that reduced female fertility is linked to poor quality of life and mental health,” Dr. Mårild and colleagues wrote. “At a societal level, involuntary childlessness causes high and increasing costs, highlighting the need to focus on preventable causes of reduced fertility.”
The investigators suggested that inflammation may be driving infertility by reducing ovulation and fertilization, or by reducing endometrial receptivity, which increases risk of pregnancy loss.
“This is the first study, to our knowledge, to show reduced fertility during histologic inflammation in IBD compared to histologic remission,” the investigators wrote. “Our findings suggest that achieving histologic remission may improve the fertility of women with IBD, even in the absence of clinically defined disease activity.”
The investigators disclosed relationships with AbbVie, Pfizer, Janssen, and others.
FROM GASTROENTEROLOGY


















