User login
Fine-needle aspiration alternative allows closer look at pancreatic cystic lesions
Endoscopic ultrasound (EUS)–guided through-the-needle biopsies (TTNBs) of pancreatic cystic lesions are sufficient for accurate molecular analysis, which offers a superior alternative to cyst fluid obtained via fine-needle aspiration, based on a prospective study.
For highest diagnostic clarity, next-generation sequencing (NGS) of TTNBs can be paired with histology, lead author Charlotte Vestrup Rift, MD, PhD, of Copenhagen University Hospital, and colleagues reported.
“The diagnostic algorithm for the management of [pancreatic cystic lesions] includes endoscopic ultrasound examination with aspiration of cyst fluid for cytology,” the investigators wrote in Gastrointestinal Endoscopy. “However, the reported sensitivity of cytology is low [at 54%]. A new microforceps, introduced through a 19-gauge needle, has proven useful for procurement of [TTNBs] that represent both the epithelial and stromal component of the cyst wall. TTNBs have a high sensitivity of 86% for the diagnosis of mucinous cysts.”
Dr. Rift and colleagues evaluated the impact of introducing NGS to the diagnostic process. They noted that concomitant mutations in GNAS and KRAS are diagnostic for intraductal papillary mucinous neoplasms (IPMNs), while other mutations have been linked with progression to cancer.
The study involved 101 patients with pancreatic cystic lesions larger than 15 mm in diameter, mean age of 68 years, among whom 91 had residual TTNBs available after microscopic analysis. These samples underwent a 51-gene NGS panel that included the “most prevalent hot-spot mutations.” Diagnoses were sorted into four categories: neoplastic cyst, mucinous cyst, IPMN, or serous cystic neoplasm.
The primary endpoint was diagnostic yield, both for molecular analysis of TTNBs and for molecular analysis plus histopathology of TTNBs. Sensitivity and specificity of NGS were also determined using histopathology as the gold standard.
Relying on NGS alone, diagnostic yields were 44.5% and 27.7% for detecting a mucinous cyst and determining type of cyst, respectively. These yields rose to 73.3% and 70.3%, respectively, when NGS was used with microscopic evaluation. Continuing with this combined approach, sensitivity and specificity were 83.7% and 81.8%, respectively, for the diagnosis of a mucinous cyst. Sensitivity and specificity were higher still, at 87.2% and 84.6%, respectively, for identifying IPMNs.
The adverse-event rate was 9.9%, with a risk of postprocedure acute pancreatitis of 8.9 % and procedure-associated intracystic bleeding of 3%, according to the authors.
Limitations of the study include the relatively small sample size and the single-center design.
“TTNB-NGS is not sufficient as a stand-alone diagnostic tool as of yet but has a high diagnostic yield when combined with microscopic evaluation and subtyping by immunohistochemistry,” the investigators concluded. “The advantage of EUS-TTNB over EUS–[fine-needle aspiration] is the ability to perform detailed cyst subtyping and the high technical success rate of the procedure. ... However, the procedure comes with a risk of adverse events and thus should be offered to patients where the value of an exact diagnosis outweighs the risks.”
“Molecular subtyping is emerging as a useful clinical test for diagnosing pancreatic cysts,” said Margaret Geraldine Keane, MBBS, MSc, of Johns Hopkins Medicine, Baltimore, although she noted that NGS remains expensive and sporadically available, “which limits its clinical utility and incorporation into diagnostic algorithms for pancreatic cysts. In the future, as the cost of sequencing reduces, and availability improves, this may change.”
For now, Dr. Keane advised physicians to reserve molecular subtyping for cases in which “accurate cyst subtyping will change management ... or when other tests have not provided a clear diagnosis.”
She said the present study is valuable because better diagnostic tests are badly needed for patients with pancreatic cysts, considering the high rate of surgical overtreatment.
“Having more diagnostic tests, such as those described in this publication [to be used on their own or in combination] to decide which patients need surgery, is important,” Dr. Keane said who was not involved in the study.
Better diagnostic tests could also improve outcomes for patients with pancreatic cancer, she said, noting a 5-year survival rate of 10%.
“This outcome is in large part attributable to the late stage at which the majority of patients are diagnosed,” Dr. Keane said. “If patients can be diagnosed earlier, survival dramatically improves. Improvements in diagnostic tests for premalignant pancreatic cystic lesions are therefore vital.”
The study was supported by Rigshospitalets Research Foundation, The Novo Nordisk Foundation, The Danish Cancer Society, and others, although they did not have a role in conducting the study or preparing the manuscript. One investigator disclosed a relationship with MediGlobe. The other investigators reported no conflicts of interest. Dr. Keane disclosed no conflicts of interest.
Endoscopic ultrasound (EUS)–guided through-the-needle biopsies (TTNBs) of pancreatic cystic lesions are sufficient for accurate molecular analysis, which offers a superior alternative to cyst fluid obtained via fine-needle aspiration, based on a prospective study.
For highest diagnostic clarity, next-generation sequencing (NGS) of TTNBs can be paired with histology, lead author Charlotte Vestrup Rift, MD, PhD, of Copenhagen University Hospital, and colleagues reported.
“The diagnostic algorithm for the management of [pancreatic cystic lesions] includes endoscopic ultrasound examination with aspiration of cyst fluid for cytology,” the investigators wrote in Gastrointestinal Endoscopy. “However, the reported sensitivity of cytology is low [at 54%]. A new microforceps, introduced through a 19-gauge needle, has proven useful for procurement of [TTNBs] that represent both the epithelial and stromal component of the cyst wall. TTNBs have a high sensitivity of 86% for the diagnosis of mucinous cysts.”
Dr. Rift and colleagues evaluated the impact of introducing NGS to the diagnostic process. They noted that concomitant mutations in GNAS and KRAS are diagnostic for intraductal papillary mucinous neoplasms (IPMNs), while other mutations have been linked with progression to cancer.
The study involved 101 patients with pancreatic cystic lesions larger than 15 mm in diameter, mean age of 68 years, among whom 91 had residual TTNBs available after microscopic analysis. These samples underwent a 51-gene NGS panel that included the “most prevalent hot-spot mutations.” Diagnoses were sorted into four categories: neoplastic cyst, mucinous cyst, IPMN, or serous cystic neoplasm.
The primary endpoint was diagnostic yield, both for molecular analysis of TTNBs and for molecular analysis plus histopathology of TTNBs. Sensitivity and specificity of NGS were also determined using histopathology as the gold standard.
Relying on NGS alone, diagnostic yields were 44.5% and 27.7% for detecting a mucinous cyst and determining type of cyst, respectively. These yields rose to 73.3% and 70.3%, respectively, when NGS was used with microscopic evaluation. Continuing with this combined approach, sensitivity and specificity were 83.7% and 81.8%, respectively, for the diagnosis of a mucinous cyst. Sensitivity and specificity were higher still, at 87.2% and 84.6%, respectively, for identifying IPMNs.
The adverse-event rate was 9.9%, with a risk of postprocedure acute pancreatitis of 8.9 % and procedure-associated intracystic bleeding of 3%, according to the authors.
Limitations of the study include the relatively small sample size and the single-center design.
“TTNB-NGS is not sufficient as a stand-alone diagnostic tool as of yet but has a high diagnostic yield when combined with microscopic evaluation and subtyping by immunohistochemistry,” the investigators concluded. “The advantage of EUS-TTNB over EUS–[fine-needle aspiration] is the ability to perform detailed cyst subtyping and the high technical success rate of the procedure. ... However, the procedure comes with a risk of adverse events and thus should be offered to patients where the value of an exact diagnosis outweighs the risks.”
“Molecular subtyping is emerging as a useful clinical test for diagnosing pancreatic cysts,” said Margaret Geraldine Keane, MBBS, MSc, of Johns Hopkins Medicine, Baltimore, although she noted that NGS remains expensive and sporadically available, “which limits its clinical utility and incorporation into diagnostic algorithms for pancreatic cysts. In the future, as the cost of sequencing reduces, and availability improves, this may change.”
For now, Dr. Keane advised physicians to reserve molecular subtyping for cases in which “accurate cyst subtyping will change management ... or when other tests have not provided a clear diagnosis.”
She said the present study is valuable because better diagnostic tests are badly needed for patients with pancreatic cysts, considering the high rate of surgical overtreatment.
“Having more diagnostic tests, such as those described in this publication [to be used on their own or in combination] to decide which patients need surgery, is important,” Dr. Keane said who was not involved in the study.
Better diagnostic tests could also improve outcomes for patients with pancreatic cancer, she said, noting a 5-year survival rate of 10%.
“This outcome is in large part attributable to the late stage at which the majority of patients are diagnosed,” Dr. Keane said. “If patients can be diagnosed earlier, survival dramatically improves. Improvements in diagnostic tests for premalignant pancreatic cystic lesions are therefore vital.”
The study was supported by Rigshospitalets Research Foundation, The Novo Nordisk Foundation, The Danish Cancer Society, and others, although they did not have a role in conducting the study or preparing the manuscript. One investigator disclosed a relationship with MediGlobe. The other investigators reported no conflicts of interest. Dr. Keane disclosed no conflicts of interest.
Endoscopic ultrasound (EUS)–guided through-the-needle biopsies (TTNBs) of pancreatic cystic lesions are sufficient for accurate molecular analysis, which offers a superior alternative to cyst fluid obtained via fine-needle aspiration, based on a prospective study.
For highest diagnostic clarity, next-generation sequencing (NGS) of TTNBs can be paired with histology, lead author Charlotte Vestrup Rift, MD, PhD, of Copenhagen University Hospital, and colleagues reported.
“The diagnostic algorithm for the management of [pancreatic cystic lesions] includes endoscopic ultrasound examination with aspiration of cyst fluid for cytology,” the investigators wrote in Gastrointestinal Endoscopy. “However, the reported sensitivity of cytology is low [at 54%]. A new microforceps, introduced through a 19-gauge needle, has proven useful for procurement of [TTNBs] that represent both the epithelial and stromal component of the cyst wall. TTNBs have a high sensitivity of 86% for the diagnosis of mucinous cysts.”
Dr. Rift and colleagues evaluated the impact of introducing NGS to the diagnostic process. They noted that concomitant mutations in GNAS and KRAS are diagnostic for intraductal papillary mucinous neoplasms (IPMNs), while other mutations have been linked with progression to cancer.
The study involved 101 patients with pancreatic cystic lesions larger than 15 mm in diameter, mean age of 68 years, among whom 91 had residual TTNBs available after microscopic analysis. These samples underwent a 51-gene NGS panel that included the “most prevalent hot-spot mutations.” Diagnoses were sorted into four categories: neoplastic cyst, mucinous cyst, IPMN, or serous cystic neoplasm.
The primary endpoint was diagnostic yield, both for molecular analysis of TTNBs and for molecular analysis plus histopathology of TTNBs. Sensitivity and specificity of NGS were also determined using histopathology as the gold standard.
Relying on NGS alone, diagnostic yields were 44.5% and 27.7% for detecting a mucinous cyst and determining type of cyst, respectively. These yields rose to 73.3% and 70.3%, respectively, when NGS was used with microscopic evaluation. Continuing with this combined approach, sensitivity and specificity were 83.7% and 81.8%, respectively, for the diagnosis of a mucinous cyst. Sensitivity and specificity were higher still, at 87.2% and 84.6%, respectively, for identifying IPMNs.
The adverse-event rate was 9.9%, with a risk of postprocedure acute pancreatitis of 8.9 % and procedure-associated intracystic bleeding of 3%, according to the authors.
Limitations of the study include the relatively small sample size and the single-center design.
“TTNB-NGS is not sufficient as a stand-alone diagnostic tool as of yet but has a high diagnostic yield when combined with microscopic evaluation and subtyping by immunohistochemistry,” the investigators concluded. “The advantage of EUS-TTNB over EUS–[fine-needle aspiration] is the ability to perform detailed cyst subtyping and the high technical success rate of the procedure. ... However, the procedure comes with a risk of adverse events and thus should be offered to patients where the value of an exact diagnosis outweighs the risks.”
“Molecular subtyping is emerging as a useful clinical test for diagnosing pancreatic cysts,” said Margaret Geraldine Keane, MBBS, MSc, of Johns Hopkins Medicine, Baltimore, although she noted that NGS remains expensive and sporadically available, “which limits its clinical utility and incorporation into diagnostic algorithms for pancreatic cysts. In the future, as the cost of sequencing reduces, and availability improves, this may change.”
For now, Dr. Keane advised physicians to reserve molecular subtyping for cases in which “accurate cyst subtyping will change management ... or when other tests have not provided a clear diagnosis.”
She said the present study is valuable because better diagnostic tests are badly needed for patients with pancreatic cysts, considering the high rate of surgical overtreatment.
“Having more diagnostic tests, such as those described in this publication [to be used on their own or in combination] to decide which patients need surgery, is important,” Dr. Keane said who was not involved in the study.
Better diagnostic tests could also improve outcomes for patients with pancreatic cancer, she said, noting a 5-year survival rate of 10%.
“This outcome is in large part attributable to the late stage at which the majority of patients are diagnosed,” Dr. Keane said. “If patients can be diagnosed earlier, survival dramatically improves. Improvements in diagnostic tests for premalignant pancreatic cystic lesions are therefore vital.”
The study was supported by Rigshospitalets Research Foundation, The Novo Nordisk Foundation, The Danish Cancer Society, and others, although they did not have a role in conducting the study or preparing the manuscript. One investigator disclosed a relationship with MediGlobe. The other investigators reported no conflicts of interest. Dr. Keane disclosed no conflicts of interest.
FROM GASTROINTESTINAL ENDOSCOPY
MERIT: Endoscopic sleeve gastroplasty shows ‘very impressive’ outcomes in randomized clinical trial
In a randomized, controlled trial, endoscopic sleeve gastroplasty (ESG) combined with lifestyle modifications was safe and effective for weight loss among individuals with class I and class II obesity, compared with lifestyle modifications alone.
“Lifestyle modifications and pharmacological therapy have several limitations, and the use of bariatric surgery is hampered by its invasive nature and patient perceptions,” the study authors wrote. ESG is a minimally invasive, reversible, organ-sparing bariatric procedure that might be able to fill those care gaps, they explained.
Previous retrospective studies have suggested that ESG is effective, and a meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%). However, according to the authors of the current study, known as MERIT and published in the Lancet, there have been no randomized clinical trials investigating ESG's efficacy to date.
“[This is] the kind of study that we have been looking forward to. The outcomes were very impressive,” said Danny Issa, MD, who was asked to comment on the study. He is a clinical assistant professor of medicine at the University of California, Los Angeles. meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%).
Understanding the study and its results
Between December 2017 and June 2019, the researchers randomized 209 participants to ESG plus lifestyle modification or lifestyle modification only, which served as the control. The mean age was 47.3 in the ESG group (88% female) and 45.7 in the control group (84% female). The mean body mass index (BMI) was 35.5 kg/m2 in the ESG group and 35.7 among controls.
After 1 year, the intervention group had a mean percentage of excess weight loss (EWL) of 49.2% , compared with 3.2% for the control group (P < .0001). The mean percentage of total body weight lost was 13.6% in the ESG group and 0.8% in the control group (P < .0001). After adjustment for age, sex, type 2 diabetes, hypertension, and baseline BMI, the ESG group had a mean difference of excess weight loss of 44.7% (95% CI, 37.5%-51.9%) and a mean difference of total weight loss of 12.6% (95% CI, 10.7%-14.5%), compared with the control group at 52 weeks. At 52 weeks, 77% of the ESG group had at least a 25% excess weight loss, which was the secondary endpoint, compared with 12% of the control group (P < .0001).
Overall, 80% of the ESG group had an improvement in at least one metabolic comorbidity, while 12% experienced a worsening. Among the control group, 45% had an improvement and 50% worsened. Among 27 patients in the treatment group with diabetes, 93% experienced an improvement in hemoglobin A1c levels, compared with 15% of patients with diabetes in the control group. Similarly among patients with hypertension, 60% in the intervention group had an improvement, compared with 40% of controls. Of those with metabolic syndrome, 83% improved after undergoing surgery, compared with 35% of controls.
At 2 years, 68% of the ESG group who achieved a 25% EWL continued to have at least 25% EWL; 2% in the treatment group had a serious ESG-related adverse event, but there was no mortality or need for intensive care or follow-up surgery.
Aiming for level I evidence
“The results are very encouraging, so I think it’s good news for the field of bariatric endoscopy. I think it’s going to provide more confidence to patients and physicians, and for new trainees who are interested in this field, I think it’s going to inspire them,” said Shailendra Singh, MD, who was asked to comment on the study. Dr. Singh is an associate professor of medicine and director of bariatric medicine at West Virginia University, Morgantown.
The study could also improve insurance coverage of the procedure, said Dr. Singh. “I think this study will help us reach out to the payers and give them the data behind this because they always look for level I evidence. ESG is a relatively new endoscopic procedure; I think this is a step forward in that direction,” he said.
The study underlines the applicability of the procedure to patients who don’t want more invasive surgery, or who can’t tolerate some of the higher efficacy medications that are increasingly available.
It is also just one of various options for obesity treatment, which are increasingly being used in combination, according to Avlin Imaeda, MD. “Just like we see in hypertension, where you progressively add more and more medications, I think we’re going to see obesity treatment go that way too. I see this as adding choice for patients and adding to this potentially multimodal approach,” said Dr. Imaeda, an associate professor of medicine at Yale University, New Haven, Conn., who was not involved in the study.
The study authors report various financial relationships, including some with Apollo Endosurgery, which funded this study. Dr. Issa and Dr. Imaeda have no relevant financial disclosures. Dr. Singh is a consultant for Apollo Endosurgery.
This article was updated Aug. 18, 2022.
In a randomized, controlled trial, endoscopic sleeve gastroplasty (ESG) combined with lifestyle modifications was safe and effective for weight loss among individuals with class I and class II obesity, compared with lifestyle modifications alone.
“Lifestyle modifications and pharmacological therapy have several limitations, and the use of bariatric surgery is hampered by its invasive nature and patient perceptions,” the study authors wrote. ESG is a minimally invasive, reversible, organ-sparing bariatric procedure that might be able to fill those care gaps, they explained.
Previous retrospective studies have suggested that ESG is effective, and a meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%). However, according to the authors of the current study, known as MERIT and published in the Lancet, there have been no randomized clinical trials investigating ESG's efficacy to date.
“[This is] the kind of study that we have been looking forward to. The outcomes were very impressive,” said Danny Issa, MD, who was asked to comment on the study. He is a clinical assistant professor of medicine at the University of California, Los Angeles. meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%).
Understanding the study and its results
Between December 2017 and June 2019, the researchers randomized 209 participants to ESG plus lifestyle modification or lifestyle modification only, which served as the control. The mean age was 47.3 in the ESG group (88% female) and 45.7 in the control group (84% female). The mean body mass index (BMI) was 35.5 kg/m2 in the ESG group and 35.7 among controls.
After 1 year, the intervention group had a mean percentage of excess weight loss (EWL) of 49.2% , compared with 3.2% for the control group (P < .0001). The mean percentage of total body weight lost was 13.6% in the ESG group and 0.8% in the control group (P < .0001). After adjustment for age, sex, type 2 diabetes, hypertension, and baseline BMI, the ESG group had a mean difference of excess weight loss of 44.7% (95% CI, 37.5%-51.9%) and a mean difference of total weight loss of 12.6% (95% CI, 10.7%-14.5%), compared with the control group at 52 weeks. At 52 weeks, 77% of the ESG group had at least a 25% excess weight loss, which was the secondary endpoint, compared with 12% of the control group (P < .0001).
Overall, 80% of the ESG group had an improvement in at least one metabolic comorbidity, while 12% experienced a worsening. Among the control group, 45% had an improvement and 50% worsened. Among 27 patients in the treatment group with diabetes, 93% experienced an improvement in hemoglobin A1c levels, compared with 15% of patients with diabetes in the control group. Similarly among patients with hypertension, 60% in the intervention group had an improvement, compared with 40% of controls. Of those with metabolic syndrome, 83% improved after undergoing surgery, compared with 35% of controls.
At 2 years, 68% of the ESG group who achieved a 25% EWL continued to have at least 25% EWL; 2% in the treatment group had a serious ESG-related adverse event, but there was no mortality or need for intensive care or follow-up surgery.
Aiming for level I evidence
“The results are very encouraging, so I think it’s good news for the field of bariatric endoscopy. I think it’s going to provide more confidence to patients and physicians, and for new trainees who are interested in this field, I think it’s going to inspire them,” said Shailendra Singh, MD, who was asked to comment on the study. Dr. Singh is an associate professor of medicine and director of bariatric medicine at West Virginia University, Morgantown.
The study could also improve insurance coverage of the procedure, said Dr. Singh. “I think this study will help us reach out to the payers and give them the data behind this because they always look for level I evidence. ESG is a relatively new endoscopic procedure; I think this is a step forward in that direction,” he said.
The study underlines the applicability of the procedure to patients who don’t want more invasive surgery, or who can’t tolerate some of the higher efficacy medications that are increasingly available.
It is also just one of various options for obesity treatment, which are increasingly being used in combination, according to Avlin Imaeda, MD. “Just like we see in hypertension, where you progressively add more and more medications, I think we’re going to see obesity treatment go that way too. I see this as adding choice for patients and adding to this potentially multimodal approach,” said Dr. Imaeda, an associate professor of medicine at Yale University, New Haven, Conn., who was not involved in the study.
The study authors report various financial relationships, including some with Apollo Endosurgery, which funded this study. Dr. Issa and Dr. Imaeda have no relevant financial disclosures. Dr. Singh is a consultant for Apollo Endosurgery.
This article was updated Aug. 18, 2022.
In a randomized, controlled trial, endoscopic sleeve gastroplasty (ESG) combined with lifestyle modifications was safe and effective for weight loss among individuals with class I and class II obesity, compared with lifestyle modifications alone.
“Lifestyle modifications and pharmacological therapy have several limitations, and the use of bariatric surgery is hampered by its invasive nature and patient perceptions,” the study authors wrote. ESG is a minimally invasive, reversible, organ-sparing bariatric procedure that might be able to fill those care gaps, they explained.
Previous retrospective studies have suggested that ESG is effective, and a meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%). However, according to the authors of the current study, known as MERIT and published in the Lancet, there have been no randomized clinical trials investigating ESG's efficacy to date.
“[This is] the kind of study that we have been looking forward to. The outcomes were very impressive,” said Danny Issa, MD, who was asked to comment on the study. He is a clinical assistant professor of medicine at the University of California, Los Angeles. meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%).
Understanding the study and its results
Between December 2017 and June 2019, the researchers randomized 209 participants to ESG plus lifestyle modification or lifestyle modification only, which served as the control. The mean age was 47.3 in the ESG group (88% female) and 45.7 in the control group (84% female). The mean body mass index (BMI) was 35.5 kg/m2 in the ESG group and 35.7 among controls.
After 1 year, the intervention group had a mean percentage of excess weight loss (EWL) of 49.2% , compared with 3.2% for the control group (P < .0001). The mean percentage of total body weight lost was 13.6% in the ESG group and 0.8% in the control group (P < .0001). After adjustment for age, sex, type 2 diabetes, hypertension, and baseline BMI, the ESG group had a mean difference of excess weight loss of 44.7% (95% CI, 37.5%-51.9%) and a mean difference of total weight loss of 12.6% (95% CI, 10.7%-14.5%), compared with the control group at 52 weeks. At 52 weeks, 77% of the ESG group had at least a 25% excess weight loss, which was the secondary endpoint, compared with 12% of the control group (P < .0001).
Overall, 80% of the ESG group had an improvement in at least one metabolic comorbidity, while 12% experienced a worsening. Among the control group, 45% had an improvement and 50% worsened. Among 27 patients in the treatment group with diabetes, 93% experienced an improvement in hemoglobin A1c levels, compared with 15% of patients with diabetes in the control group. Similarly among patients with hypertension, 60% in the intervention group had an improvement, compared with 40% of controls. Of those with metabolic syndrome, 83% improved after undergoing surgery, compared with 35% of controls.
At 2 years, 68% of the ESG group who achieved a 25% EWL continued to have at least 25% EWL; 2% in the treatment group had a serious ESG-related adverse event, but there was no mortality or need for intensive care or follow-up surgery.
Aiming for level I evidence
“The results are very encouraging, so I think it’s good news for the field of bariatric endoscopy. I think it’s going to provide more confidence to patients and physicians, and for new trainees who are interested in this field, I think it’s going to inspire them,” said Shailendra Singh, MD, who was asked to comment on the study. Dr. Singh is an associate professor of medicine and director of bariatric medicine at West Virginia University, Morgantown.
The study could also improve insurance coverage of the procedure, said Dr. Singh. “I think this study will help us reach out to the payers and give them the data behind this because they always look for level I evidence. ESG is a relatively new endoscopic procedure; I think this is a step forward in that direction,” he said.
The study underlines the applicability of the procedure to patients who don’t want more invasive surgery, or who can’t tolerate some of the higher efficacy medications that are increasingly available.
It is also just one of various options for obesity treatment, which are increasingly being used in combination, according to Avlin Imaeda, MD. “Just like we see in hypertension, where you progressively add more and more medications, I think we’re going to see obesity treatment go that way too. I see this as adding choice for patients and adding to this potentially multimodal approach,” said Dr. Imaeda, an associate professor of medicine at Yale University, New Haven, Conn., who was not involved in the study.
The study authors report various financial relationships, including some with Apollo Endosurgery, which funded this study. Dr. Issa and Dr. Imaeda have no relevant financial disclosures. Dr. Singh is a consultant for Apollo Endosurgery.
This article was updated Aug. 18, 2022.
FROM THE LANCET
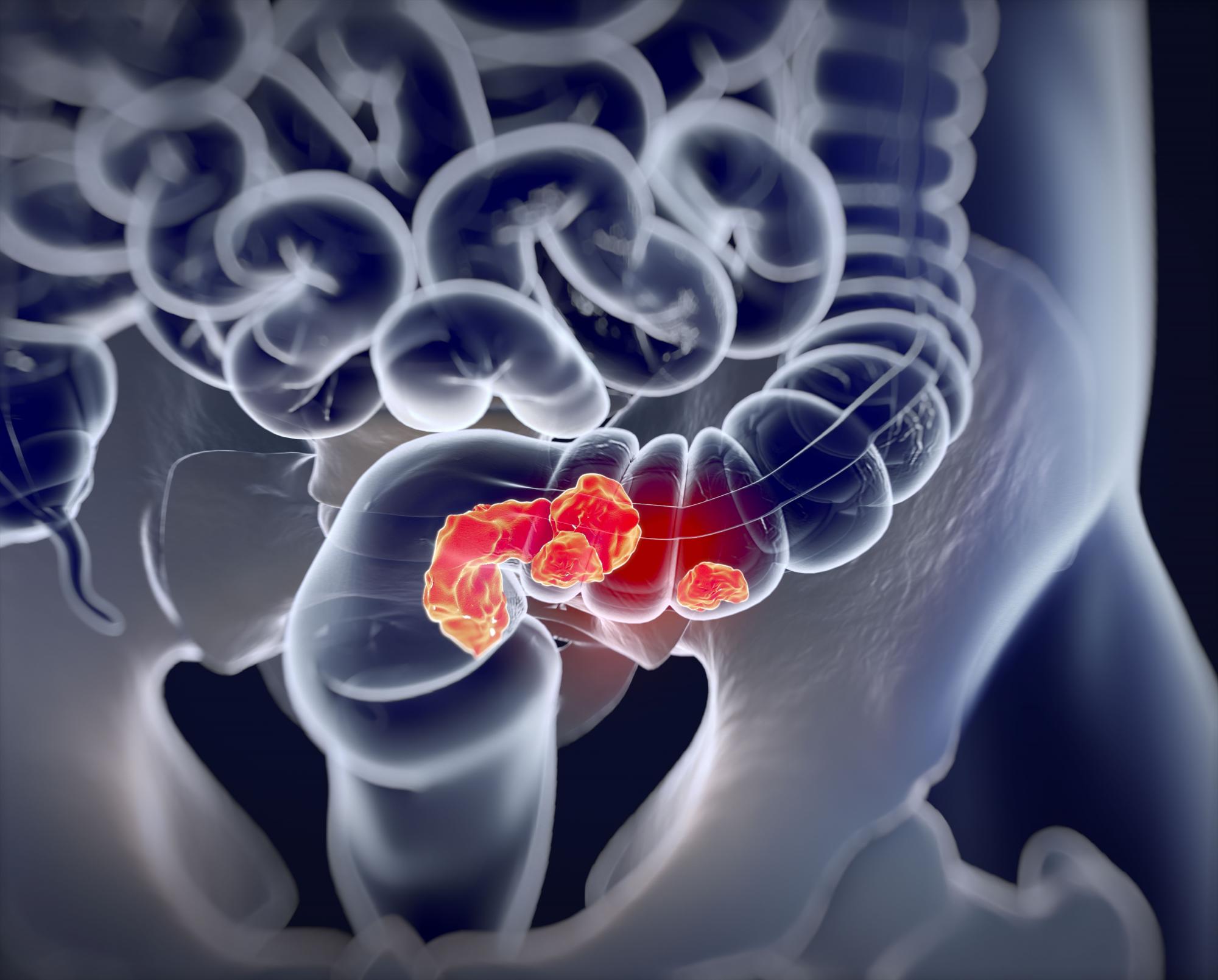
Higher ADR continues to show ‘strong, consistent’ link with lower interval CRC
Higher adenoma detection rates (ADR) during colonoscopies were associated with lower rates of interim colorectal cancer (CRC), and the relationship held true along a broad range of ADR values, according to a retrospective study.
The new study, published online in JAMA, examined ADRs and rates of interim colorectal cancer among patients in California and Washington State between 2011 and 2017. The authors found a 3% reduction in risk for each additional 1% value of ADR. The reduction in risk held true even at high ADRs.
“It basically reaffirms what we’ve believed for the longest time, and other research work has documented – that interim cancers are higher in association with lower adenoma detection rates. The higher you can get that adenoma detection rate, the more we’re going to be able to lower the [rate of] cancers that develop within 3 years of a colonoscopy,” said Lawrence Kosinski, MD, who was asked to comment on the study.
The study included 735,396 patients with a median age of 61.4 years. Among these patients, 852,624 negative colonoscopies were performed by 383 eligible physicians. Participating physicians had to perform at least 25 screening colonoscopies and 100 total colonoscopies per year. After 2.4 million person-years of follow-up, the researchers observed 619 postcolonoscopy colorectal cancers and 36 related deaths over a median follow-up of 3.25 years.
There was an association between each 1% increase in ADR and a reduced probability of postcolonoscopy CRC (hazard ratio [HR], 0.97; 95% confidence interval [CI], 0.96-0.98) and mortality from postcolonoscopy CRC (HR, 0.95; 95% CI, 0.92-0.99).
The median ADR was 28.3%. There was an association between ADR above the median versus below the median and a reduced risk of postcolonoscopy CRC with 1.79 cases versus 3.10 cases per 10,000 person-years, respectively (absolute difference in 7-year risk, –12.2 per 10,000 negative colonoscopies; HR, 0.61; 95% CI, 0.52-0.73). There was a similar reduction in risk of postcolonoscopy CRC-related mortality (0.05 versus 0.22 per 10,000 person-years; absolute difference in 7-year risk, –1.2 per 10,000 negative colonoscopies; HR, 0.26; 95% CI, 0.11-0.65).
These findings may be limited in generalizability to physicians with lower procedure volumes or to populations with different adenoma prevalence.
“Given the strong, consistent associations of higher adenoma detection rates with colonoscopy effectiveness for reducing colorectal cancer incidence and mortality, the current results support more research to identify reliable and readily adoptable methods for increasing adenoma detection rates among physicians with lower values across diverse settings,” the researchers wrote.
The improvement over a broad range of ADRs, along with other recent findings, suggests that there may need to be updates to the use of ADRs as a quality metric, according to an accompanying editorial by Douglas K. Rex, MD, of the division of gastroenterology/hepatology at Indiana University, Indianapolis. For example, it’s possible that ADRs could be measured by averaging values from screening, diagnostic, and surveillance colonoscopy. The editorialist suggested that, if improvements in interim cancer rates continue as ADRs approach 50%, the current view of ADRs, as a minimally acceptable standard, may require reconsideration. Instead, it may be appropriate to continue with a minimum threshold, but add a much higher, aspirational target. Dr. Rex also suggested that highly-variable detection of sessile serrated lesions could be excluded from ADRs in order to reduce variability.
Factors to consider
The study is useful, but it doesn’t address the disparity in adenoma detection that exists between individual doctors, according to Dr. Kosinski, founder and chief medical officer of SonarMD and previously director of a large gastroenterology clinic. “Even if you look at doctors who do a minimum of 250 screening colonoscopies in a year, there’s still variability. There was even a study published in 2014 showing ADRs anywhere from 7.4% to 52.5%. The bell curve is broad,” he said.
As patients age, they have a higher frequency of polyps appearing on the right side of the colon, and those polyps are flatter and more easily missed than polyps on the left side. “The variation in ADR is higher on the right side of the colon than it is on the left. Doctors have to really do a very good job of examining that right side of the colon so that they don’t miss the flat polyps,” said Dr. Kosinski.
To improve ADRs, Dr. Kosinski emphasized the need to take the required time out to complete a procedure, despite the tight schedules often faced by ambulatory centers. “It’s the time you take coming out of the colon that’s critical. You owe it to the patient,” he said.
And if a patient hasn’t prepped well enough, it’s better to send the patient home without the procedure than to conduct a poor-quality screening. “If you can’t see the mucosal surface, you can’t tell the patient that they have a negative colonoscopy. If you have to do more cleaning during the procedure, then do more cleaning during the procedure. If you have to cancel the procedure and bring the patient back, it’s better to do that than it is to do an incomplete colonoscopy,” said Dr. Kosinski.
He also stressed the need to make sure that the patient is properly sedated and comfortable “so that you can do the job you’re supposed to do,” he said.
Some authors disclosed relationships with Amgen and the National Cancer Institute. Dr. Rex disclosed relationships with Olympus, Boston Scientific, Aries, and others, all outside the submitted work.
Higher adenoma detection rates (ADR) during colonoscopies were associated with lower rates of interim colorectal cancer (CRC), and the relationship held true along a broad range of ADR values, according to a retrospective study.
The new study, published online in JAMA, examined ADRs and rates of interim colorectal cancer among patients in California and Washington State between 2011 and 2017. The authors found a 3% reduction in risk for each additional 1% value of ADR. The reduction in risk held true even at high ADRs.
“It basically reaffirms what we’ve believed for the longest time, and other research work has documented – that interim cancers are higher in association with lower adenoma detection rates. The higher you can get that adenoma detection rate, the more we’re going to be able to lower the [rate of] cancers that develop within 3 years of a colonoscopy,” said Lawrence Kosinski, MD, who was asked to comment on the study.
The study included 735,396 patients with a median age of 61.4 years. Among these patients, 852,624 negative colonoscopies were performed by 383 eligible physicians. Participating physicians had to perform at least 25 screening colonoscopies and 100 total colonoscopies per year. After 2.4 million person-years of follow-up, the researchers observed 619 postcolonoscopy colorectal cancers and 36 related deaths over a median follow-up of 3.25 years.
There was an association between each 1% increase in ADR and a reduced probability of postcolonoscopy CRC (hazard ratio [HR], 0.97; 95% confidence interval [CI], 0.96-0.98) and mortality from postcolonoscopy CRC (HR, 0.95; 95% CI, 0.92-0.99).
The median ADR was 28.3%. There was an association between ADR above the median versus below the median and a reduced risk of postcolonoscopy CRC with 1.79 cases versus 3.10 cases per 10,000 person-years, respectively (absolute difference in 7-year risk, –12.2 per 10,000 negative colonoscopies; HR, 0.61; 95% CI, 0.52-0.73). There was a similar reduction in risk of postcolonoscopy CRC-related mortality (0.05 versus 0.22 per 10,000 person-years; absolute difference in 7-year risk, –1.2 per 10,000 negative colonoscopies; HR, 0.26; 95% CI, 0.11-0.65).
These findings may be limited in generalizability to physicians with lower procedure volumes or to populations with different adenoma prevalence.
“Given the strong, consistent associations of higher adenoma detection rates with colonoscopy effectiveness for reducing colorectal cancer incidence and mortality, the current results support more research to identify reliable and readily adoptable methods for increasing adenoma detection rates among physicians with lower values across diverse settings,” the researchers wrote.
The improvement over a broad range of ADRs, along with other recent findings, suggests that there may need to be updates to the use of ADRs as a quality metric, according to an accompanying editorial by Douglas K. Rex, MD, of the division of gastroenterology/hepatology at Indiana University, Indianapolis. For example, it’s possible that ADRs could be measured by averaging values from screening, diagnostic, and surveillance colonoscopy. The editorialist suggested that, if improvements in interim cancer rates continue as ADRs approach 50%, the current view of ADRs, as a minimally acceptable standard, may require reconsideration. Instead, it may be appropriate to continue with a minimum threshold, but add a much higher, aspirational target. Dr. Rex also suggested that highly-variable detection of sessile serrated lesions could be excluded from ADRs in order to reduce variability.
Factors to consider
The study is useful, but it doesn’t address the disparity in adenoma detection that exists between individual doctors, according to Dr. Kosinski, founder and chief medical officer of SonarMD and previously director of a large gastroenterology clinic. “Even if you look at doctors who do a minimum of 250 screening colonoscopies in a year, there’s still variability. There was even a study published in 2014 showing ADRs anywhere from 7.4% to 52.5%. The bell curve is broad,” he said.
As patients age, they have a higher frequency of polyps appearing on the right side of the colon, and those polyps are flatter and more easily missed than polyps on the left side. “The variation in ADR is higher on the right side of the colon than it is on the left. Doctors have to really do a very good job of examining that right side of the colon so that they don’t miss the flat polyps,” said Dr. Kosinski.
To improve ADRs, Dr. Kosinski emphasized the need to take the required time out to complete a procedure, despite the tight schedules often faced by ambulatory centers. “It’s the time you take coming out of the colon that’s critical. You owe it to the patient,” he said.
And if a patient hasn’t prepped well enough, it’s better to send the patient home without the procedure than to conduct a poor-quality screening. “If you can’t see the mucosal surface, you can’t tell the patient that they have a negative colonoscopy. If you have to do more cleaning during the procedure, then do more cleaning during the procedure. If you have to cancel the procedure and bring the patient back, it’s better to do that than it is to do an incomplete colonoscopy,” said Dr. Kosinski.
He also stressed the need to make sure that the patient is properly sedated and comfortable “so that you can do the job you’re supposed to do,” he said.
Some authors disclosed relationships with Amgen and the National Cancer Institute. Dr. Rex disclosed relationships with Olympus, Boston Scientific, Aries, and others, all outside the submitted work.
Higher adenoma detection rates (ADR) during colonoscopies were associated with lower rates of interim colorectal cancer (CRC), and the relationship held true along a broad range of ADR values, according to a retrospective study.
The new study, published online in JAMA, examined ADRs and rates of interim colorectal cancer among patients in California and Washington State between 2011 and 2017. The authors found a 3% reduction in risk for each additional 1% value of ADR. The reduction in risk held true even at high ADRs.
“It basically reaffirms what we’ve believed for the longest time, and other research work has documented – that interim cancers are higher in association with lower adenoma detection rates. The higher you can get that adenoma detection rate, the more we’re going to be able to lower the [rate of] cancers that develop within 3 years of a colonoscopy,” said Lawrence Kosinski, MD, who was asked to comment on the study.
The study included 735,396 patients with a median age of 61.4 years. Among these patients, 852,624 negative colonoscopies were performed by 383 eligible physicians. Participating physicians had to perform at least 25 screening colonoscopies and 100 total colonoscopies per year. After 2.4 million person-years of follow-up, the researchers observed 619 postcolonoscopy colorectal cancers and 36 related deaths over a median follow-up of 3.25 years.
There was an association between each 1% increase in ADR and a reduced probability of postcolonoscopy CRC (hazard ratio [HR], 0.97; 95% confidence interval [CI], 0.96-0.98) and mortality from postcolonoscopy CRC (HR, 0.95; 95% CI, 0.92-0.99).
The median ADR was 28.3%. There was an association between ADR above the median versus below the median and a reduced risk of postcolonoscopy CRC with 1.79 cases versus 3.10 cases per 10,000 person-years, respectively (absolute difference in 7-year risk, –12.2 per 10,000 negative colonoscopies; HR, 0.61; 95% CI, 0.52-0.73). There was a similar reduction in risk of postcolonoscopy CRC-related mortality (0.05 versus 0.22 per 10,000 person-years; absolute difference in 7-year risk, –1.2 per 10,000 negative colonoscopies; HR, 0.26; 95% CI, 0.11-0.65).
These findings may be limited in generalizability to physicians with lower procedure volumes or to populations with different adenoma prevalence.
“Given the strong, consistent associations of higher adenoma detection rates with colonoscopy effectiveness for reducing colorectal cancer incidence and mortality, the current results support more research to identify reliable and readily adoptable methods for increasing adenoma detection rates among physicians with lower values across diverse settings,” the researchers wrote.
The improvement over a broad range of ADRs, along with other recent findings, suggests that there may need to be updates to the use of ADRs as a quality metric, according to an accompanying editorial by Douglas K. Rex, MD, of the division of gastroenterology/hepatology at Indiana University, Indianapolis. For example, it’s possible that ADRs could be measured by averaging values from screening, diagnostic, and surveillance colonoscopy. The editorialist suggested that, if improvements in interim cancer rates continue as ADRs approach 50%, the current view of ADRs, as a minimally acceptable standard, may require reconsideration. Instead, it may be appropriate to continue with a minimum threshold, but add a much higher, aspirational target. Dr. Rex also suggested that highly-variable detection of sessile serrated lesions could be excluded from ADRs in order to reduce variability.
Factors to consider
The study is useful, but it doesn’t address the disparity in adenoma detection that exists between individual doctors, according to Dr. Kosinski, founder and chief medical officer of SonarMD and previously director of a large gastroenterology clinic. “Even if you look at doctors who do a minimum of 250 screening colonoscopies in a year, there’s still variability. There was even a study published in 2014 showing ADRs anywhere from 7.4% to 52.5%. The bell curve is broad,” he said.
As patients age, they have a higher frequency of polyps appearing on the right side of the colon, and those polyps are flatter and more easily missed than polyps on the left side. “The variation in ADR is higher on the right side of the colon than it is on the left. Doctors have to really do a very good job of examining that right side of the colon so that they don’t miss the flat polyps,” said Dr. Kosinski.
To improve ADRs, Dr. Kosinski emphasized the need to take the required time out to complete a procedure, despite the tight schedules often faced by ambulatory centers. “It’s the time you take coming out of the colon that’s critical. You owe it to the patient,” he said.
And if a patient hasn’t prepped well enough, it’s better to send the patient home without the procedure than to conduct a poor-quality screening. “If you can’t see the mucosal surface, you can’t tell the patient that they have a negative colonoscopy. If you have to do more cleaning during the procedure, then do more cleaning during the procedure. If you have to cancel the procedure and bring the patient back, it’s better to do that than it is to do an incomplete colonoscopy,” said Dr. Kosinski.
He also stressed the need to make sure that the patient is properly sedated and comfortable “so that you can do the job you’re supposed to do,” he said.
Some authors disclosed relationships with Amgen and the National Cancer Institute. Dr. Rex disclosed relationships with Olympus, Boston Scientific, Aries, and others, all outside the submitted work.
FROM JAMA
Smartphone tool helps gauge bowel prep quality before colonoscopy
An artificial intelligence (AI) tool that runs on a smartphone can help patients scheduled for a colonoscopy evaluate independently how well they do with bowel cleansing and may be an alternative approach for evaluating bowel preparation quality before the colonoscopy, especially in the COVID-19 era.
The AI tool is a “manpower-saving” option that reduces the need for nurses to evaluate the quality of bowel preparation, say Wei Gong, MD, Southern Medical University, Shenzhen, China, and colleagues.
Having the tool on a patient’s smartphone means caregivers and nurses would not be required to assess the adequacy of bowel cleansing for patients, which, in turn, would reduce person-to-person contact and the spread of infectious diseases, they add.
The study was published online in the American Journal of Gastroenterology.
Better than do-it-yourself evaluation?
The study was conducted at two hospitals in China among consecutive patients prepping for colonoscopy. All participants received standard bowel preparation instructions and were given a leaflet with general guidelines on bowel preparation.
The leaflet included photos representing bowel preparation quality and informed patients that their stool should eventually be a yellowish clear liquid; if any cloudiness (including turbid liquid, particles, or small amounts of feces) is observed in the liquid stool, the bowel preparation is not complete.
All patients were prescribed standard polyethylene glycol electrolyte solution for bowel cleansing 4-6 hours before the colonoscopy.
After consuming the solution, all patients scanned a QR (quick response) code with a smartphone for randomization into an experimental group using the AI-convolutional neural network (AI-CNN) model or a control group using self-evaluation.
The system gave instructions for using the application, taking photos of their feces, and uploading the images.
After uploading the images, the 730 patients in the AI-CNN group automatically received a “pass” or “not pass” alert, which indicated whether their bowel preparation was adequate or not.
The 704 patients in the control group evaluated the adequacy of bowel preparation on their own according to the leaflet instructions after uploading their images.
Colonoscopists and nurses were blinded to the bowel evaluation method that each patient used.
According to the investigators, evaluation results (“pass” or “not pass”) in terms of adequacy of bowel preparation as represented by Boston Bowel Preparation Scale (BBPS) scores were consistent between the two methods (AI-CNN or self-evaluation).
Overall, there were no significant differences in the two methods in terms of mean BBPS scores, polyp detection rate, or adenoma detection rate.
In subgroup analysis, however, the mean BBPS score of patients with “pass” results was significantly higher in the AI-CNN group than in the self-evaluation control group.
This suggests that the AI-CNN model may further improve the quality of bowel preparation in patients exhibiting adequate bowel preparation, the researchers say.
The results also suggest improved bowel preparation quality of the right colon under the aid of the AI-CNN model, which may be crucial for the prevention of interval colorectal cancer.
The study did not investigate the user acceptability of the AI-CNN model.
“To improve the model and broaden its application in routine practice, evaluating its convenience, accessibility, aspects that cause users difficulty, and user satisfaction is crucial,” the study team concludes.
The study was supported by the Xiamen Medical Health Science and Technology Project and the Xiamen Chang Gung Hospital Science Project. The authors have declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An artificial intelligence (AI) tool that runs on a smartphone can help patients scheduled for a colonoscopy evaluate independently how well they do with bowel cleansing and may be an alternative approach for evaluating bowel preparation quality before the colonoscopy, especially in the COVID-19 era.
The AI tool is a “manpower-saving” option that reduces the need for nurses to evaluate the quality of bowel preparation, say Wei Gong, MD, Southern Medical University, Shenzhen, China, and colleagues.
Having the tool on a patient’s smartphone means caregivers and nurses would not be required to assess the adequacy of bowel cleansing for patients, which, in turn, would reduce person-to-person contact and the spread of infectious diseases, they add.
The study was published online in the American Journal of Gastroenterology.
Better than do-it-yourself evaluation?
The study was conducted at two hospitals in China among consecutive patients prepping for colonoscopy. All participants received standard bowel preparation instructions and were given a leaflet with general guidelines on bowel preparation.
The leaflet included photos representing bowel preparation quality and informed patients that their stool should eventually be a yellowish clear liquid; if any cloudiness (including turbid liquid, particles, or small amounts of feces) is observed in the liquid stool, the bowel preparation is not complete.
All patients were prescribed standard polyethylene glycol electrolyte solution for bowel cleansing 4-6 hours before the colonoscopy.
After consuming the solution, all patients scanned a QR (quick response) code with a smartphone for randomization into an experimental group using the AI-convolutional neural network (AI-CNN) model or a control group using self-evaluation.
The system gave instructions for using the application, taking photos of their feces, and uploading the images.
After uploading the images, the 730 patients in the AI-CNN group automatically received a “pass” or “not pass” alert, which indicated whether their bowel preparation was adequate or not.
The 704 patients in the control group evaluated the adequacy of bowel preparation on their own according to the leaflet instructions after uploading their images.
Colonoscopists and nurses were blinded to the bowel evaluation method that each patient used.
According to the investigators, evaluation results (“pass” or “not pass”) in terms of adequacy of bowel preparation as represented by Boston Bowel Preparation Scale (BBPS) scores were consistent between the two methods (AI-CNN or self-evaluation).
Overall, there were no significant differences in the two methods in terms of mean BBPS scores, polyp detection rate, or adenoma detection rate.
In subgroup analysis, however, the mean BBPS score of patients with “pass” results was significantly higher in the AI-CNN group than in the self-evaluation control group.
This suggests that the AI-CNN model may further improve the quality of bowel preparation in patients exhibiting adequate bowel preparation, the researchers say.
The results also suggest improved bowel preparation quality of the right colon under the aid of the AI-CNN model, which may be crucial for the prevention of interval colorectal cancer.
The study did not investigate the user acceptability of the AI-CNN model.
“To improve the model and broaden its application in routine practice, evaluating its convenience, accessibility, aspects that cause users difficulty, and user satisfaction is crucial,” the study team concludes.
The study was supported by the Xiamen Medical Health Science and Technology Project and the Xiamen Chang Gung Hospital Science Project. The authors have declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An artificial intelligence (AI) tool that runs on a smartphone can help patients scheduled for a colonoscopy evaluate independently how well they do with bowel cleansing and may be an alternative approach for evaluating bowel preparation quality before the colonoscopy, especially in the COVID-19 era.
The AI tool is a “manpower-saving” option that reduces the need for nurses to evaluate the quality of bowel preparation, say Wei Gong, MD, Southern Medical University, Shenzhen, China, and colleagues.
Having the tool on a patient’s smartphone means caregivers and nurses would not be required to assess the adequacy of bowel cleansing for patients, which, in turn, would reduce person-to-person contact and the spread of infectious diseases, they add.
The study was published online in the American Journal of Gastroenterology.
Better than do-it-yourself evaluation?
The study was conducted at two hospitals in China among consecutive patients prepping for colonoscopy. All participants received standard bowel preparation instructions and were given a leaflet with general guidelines on bowel preparation.
The leaflet included photos representing bowel preparation quality and informed patients that their stool should eventually be a yellowish clear liquid; if any cloudiness (including turbid liquid, particles, or small amounts of feces) is observed in the liquid stool, the bowel preparation is not complete.
All patients were prescribed standard polyethylene glycol electrolyte solution for bowel cleansing 4-6 hours before the colonoscopy.
After consuming the solution, all patients scanned a QR (quick response) code with a smartphone for randomization into an experimental group using the AI-convolutional neural network (AI-CNN) model or a control group using self-evaluation.
The system gave instructions for using the application, taking photos of their feces, and uploading the images.
After uploading the images, the 730 patients in the AI-CNN group automatically received a “pass” or “not pass” alert, which indicated whether their bowel preparation was adequate or not.
The 704 patients in the control group evaluated the adequacy of bowel preparation on their own according to the leaflet instructions after uploading their images.
Colonoscopists and nurses were blinded to the bowel evaluation method that each patient used.
According to the investigators, evaluation results (“pass” or “not pass”) in terms of adequacy of bowel preparation as represented by Boston Bowel Preparation Scale (BBPS) scores were consistent between the two methods (AI-CNN or self-evaluation).
Overall, there were no significant differences in the two methods in terms of mean BBPS scores, polyp detection rate, or adenoma detection rate.
In subgroup analysis, however, the mean BBPS score of patients with “pass” results was significantly higher in the AI-CNN group than in the self-evaluation control group.
This suggests that the AI-CNN model may further improve the quality of bowel preparation in patients exhibiting adequate bowel preparation, the researchers say.
The results also suggest improved bowel preparation quality of the right colon under the aid of the AI-CNN model, which may be crucial for the prevention of interval colorectal cancer.
The study did not investigate the user acceptability of the AI-CNN model.
“To improve the model and broaden its application in routine practice, evaluating its convenience, accessibility, aspects that cause users difficulty, and user satisfaction is crucial,” the study team concludes.
The study was supported by the Xiamen Medical Health Science and Technology Project and the Xiamen Chang Gung Hospital Science Project. The authors have declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Barrett’s esophagus: AGA screening update ‘goes above and beyond’
A new clinical practice update from the American Gastroenterological Association offers practical advice around surveillance and use of new screening technologies for Barrett’s esophagus.
The AGA clinical practice update, published in Clinical Gastroenterology and Hepatology comes from the AGA’s Center for GI Innovation and Technology. It offers 15 best practice advice statements based on expert review of existing literature combined with discussion and expert opinion. The aim is “to provide an update on advances and innovation” but not to replace current guidelines.
“Guidelines operate on rigorous methodology which requires the use of [Grading of Recommendations, Assessment, Development and Evaluation] methodology and a higher level of evidence. In gastroenterology especially, innovation is moving quickly and there’s no way for patients to reap their benefits if clinical practice was dictated by guidelines alone. That said, we do need documents that support and drive innovation in clinical practice,” corresponding author Srinadh Komanduri, MD, professor of medicine and surgery in the division of gastroenterology and hepatology at Northwestern University, Chicago, told this news publication.
Asked to comment, Vivek Kaul, MD, the Segal-Watson Professor of Medicine in the Center for Advanced Therapeutic Endoscopy in the division of gastroenterology and hepatology at the University of Rochester (N.Y.) Medical Center, said that the document is “an important attempt to not only present the available scientific literature in a very concise and understandable manner, but it goes above and beyond that in terms of diving into some novel paradigms and technologies and procedures that are either emerging or will be emerging in the near future.”
Improving detection by dropping GERD requirement
The first of the 15 statements may also be the most paradigm shifting: The panel suggests screening via standard upper endoscopy of people with at least three risk factors for Barrett’s esophagus and esophageal adenocarcinoma, including those who are male, are non-Hispanic White, are aged above 50 years, and have a history of smoking, chronic gastroesophageal reflux disease (GERD), obesity, or a family history of Barrett’s esophagus or esophageal adenocarcinoma.
This represents a departure from all current guidelines, which stipulate GERD as a necessary prerequisite for screening. But the reason is simple, according to the authors: A majority of patients diagnosed with esophageal cancer never experience classic GERD symptoms.
“There is growing evidence in high-level publications over the last couple of years that reflux is not the ideal predictor, based on odds, for development of Barrett’s esophagus. So the consensus among the experts was that we need to remove GERD as an absolute prerequisite or we’re never going to make progress. In order to make an impact on the rise of esophageal adenocarcinoma we have to increase the denominator of patients we are seeing,” Dr. Komanduri explained.
While it might be difficult to screen every White male over 50 years of age, the data do suggest screening those who also have obesity and/or are current smokers. “That’s a perfect subset you might want to start with. There are permutations that have greater value that don’t occupy unnecessary resource utilization. Most critical are the family history of esophageal cancer or Barrett’s esophagus,” he noted.
Dr. Kaul said that a one-time Barrett’s esophagus screening of all White males over 50 years old “is not unreasonable, especially given the rising rates of esophageal cancer.”
However, he also noted, “The feasibility, preferred screening modality, incremental costs, and yield of this new strategy will need to be studied further. Access to GI endoscopy in the postpandemic world is already a concern and will need to be factored into execution of this [advice statement] and will likely impact adoption in some way.”
For his part, Dr. Komanduri said that more investigation will be needed to validate which patients most benefit from screening and that the AGA is planning educational programs for clinicians about interpreting this new paradigm.
New technology could make screening easier and cheaper
The availability of nonendoscopic cell collection devices, including the swallowable Cytosponge (Medtronic), EsoCheck (Lucid), and EsoCap (Capnostics) could help make screening for Barrett’s esophagus easier and more cost effective. They are designed for in-office use and don’t require sedation. Each one is currently in various stages of development and clinical trials. As of now they’re approved in the United States only for cell collection but not for Barrett’s esophagus screening, but their use is endorsed by some guidelines. The Cytosponge in particular is widely available and has been used extensively in the United Kingdom.
Dr. Kaul commented, “While there is a need for nonendoscopic screening devices, the ideal patient population and practice setting for administration of these devices has not been clearly defined. Also, who will be delivering these tests: Primary care or gastroenterology providers? These devices ... represent a major step forward and a novel paradigm for Barrett’s esophagus screening, and the only platform that non-GI providers could use.”
Virtual chromoendoscopy: A must have in 2022
A third best practice advice statement shouldn’t be controversial because it’s in other guidelines already, but data show clinicians aren’t always doing it: Performing screening and surveillance endoscopic examinations using virtual chromoendoscopy in addition to high-definition white light endoscopy, with adequate time spent inspecting the Barrett’s segment. The majority of data supporting this is for narrow-band imaging only.
“The blue light lets you pick up early mucosal and vascular changes which might represent dysplastic lesions. It’s not a question of should. It’s a medicolegal slam dunk; you must do it. It’s been a guideline recommendation in the last few years, and it’s just a switch on the scope. It doesn’t require separate equipment, yet people are often still skipping it,” Dr. Komanduri said.
Indeed, Dr. Kaul concurred, “The importance of a high quality, meticulous endoscopic examination for screening and surveillance in Barrett’s esophagus cannot be overemphasized.”
‘Finally pushing the needle in the right direction’
The overall goals, Dr. Komanduri said, are “increasing the denominator, using less invasive screening, but finding more patients. If we find more patients we’ll need to stratify their risk. We hope that all these things eventually tie together in a nice story, all with the aim of preventing an invasive cancer that can’t be treated.”
He believes the new update “is a pivotal document in this field that’s going to be a paradigm changer. A lot of aspects need further validation. It’s by no means the end. But I think we’re finally pushing the needle in the right direction as things move forward with innovation.”
Dr. Kaul agrees. “It’s highlighting the principles that may become established paradigms in the future.”
Dr. Komanduri and the other authors of the update reported relationships, including consulting and research support, with companies like Boston Scientific, Medtronic, Virgo Video Solutions, and Castle Biosciences. Dr. Kaul serves as a consultant and advisory board member for CDx Diagnostics, an advisory board member for Castle Biosciences, and an investigator for Lucid Diagnostics.
A new clinical practice update from the American Gastroenterological Association offers practical advice around surveillance and use of new screening technologies for Barrett’s esophagus.
The AGA clinical practice update, published in Clinical Gastroenterology and Hepatology comes from the AGA’s Center for GI Innovation and Technology. It offers 15 best practice advice statements based on expert review of existing literature combined with discussion and expert opinion. The aim is “to provide an update on advances and innovation” but not to replace current guidelines.
“Guidelines operate on rigorous methodology which requires the use of [Grading of Recommendations, Assessment, Development and Evaluation] methodology and a higher level of evidence. In gastroenterology especially, innovation is moving quickly and there’s no way for patients to reap their benefits if clinical practice was dictated by guidelines alone. That said, we do need documents that support and drive innovation in clinical practice,” corresponding author Srinadh Komanduri, MD, professor of medicine and surgery in the division of gastroenterology and hepatology at Northwestern University, Chicago, told this news publication.
Asked to comment, Vivek Kaul, MD, the Segal-Watson Professor of Medicine in the Center for Advanced Therapeutic Endoscopy in the division of gastroenterology and hepatology at the University of Rochester (N.Y.) Medical Center, said that the document is “an important attempt to not only present the available scientific literature in a very concise and understandable manner, but it goes above and beyond that in terms of diving into some novel paradigms and technologies and procedures that are either emerging or will be emerging in the near future.”
Improving detection by dropping GERD requirement
The first of the 15 statements may also be the most paradigm shifting: The panel suggests screening via standard upper endoscopy of people with at least three risk factors for Barrett’s esophagus and esophageal adenocarcinoma, including those who are male, are non-Hispanic White, are aged above 50 years, and have a history of smoking, chronic gastroesophageal reflux disease (GERD), obesity, or a family history of Barrett’s esophagus or esophageal adenocarcinoma.
This represents a departure from all current guidelines, which stipulate GERD as a necessary prerequisite for screening. But the reason is simple, according to the authors: A majority of patients diagnosed with esophageal cancer never experience classic GERD symptoms.
“There is growing evidence in high-level publications over the last couple of years that reflux is not the ideal predictor, based on odds, for development of Barrett’s esophagus. So the consensus among the experts was that we need to remove GERD as an absolute prerequisite or we’re never going to make progress. In order to make an impact on the rise of esophageal adenocarcinoma we have to increase the denominator of patients we are seeing,” Dr. Komanduri explained.
While it might be difficult to screen every White male over 50 years of age, the data do suggest screening those who also have obesity and/or are current smokers. “That’s a perfect subset you might want to start with. There are permutations that have greater value that don’t occupy unnecessary resource utilization. Most critical are the family history of esophageal cancer or Barrett’s esophagus,” he noted.
Dr. Kaul said that a one-time Barrett’s esophagus screening of all White males over 50 years old “is not unreasonable, especially given the rising rates of esophageal cancer.”
However, he also noted, “The feasibility, preferred screening modality, incremental costs, and yield of this new strategy will need to be studied further. Access to GI endoscopy in the postpandemic world is already a concern and will need to be factored into execution of this [advice statement] and will likely impact adoption in some way.”
For his part, Dr. Komanduri said that more investigation will be needed to validate which patients most benefit from screening and that the AGA is planning educational programs for clinicians about interpreting this new paradigm.
New technology could make screening easier and cheaper
The availability of nonendoscopic cell collection devices, including the swallowable Cytosponge (Medtronic), EsoCheck (Lucid), and EsoCap (Capnostics) could help make screening for Barrett’s esophagus easier and more cost effective. They are designed for in-office use and don’t require sedation. Each one is currently in various stages of development and clinical trials. As of now they’re approved in the United States only for cell collection but not for Barrett’s esophagus screening, but their use is endorsed by some guidelines. The Cytosponge in particular is widely available and has been used extensively in the United Kingdom.
Dr. Kaul commented, “While there is a need for nonendoscopic screening devices, the ideal patient population and practice setting for administration of these devices has not been clearly defined. Also, who will be delivering these tests: Primary care or gastroenterology providers? These devices ... represent a major step forward and a novel paradigm for Barrett’s esophagus screening, and the only platform that non-GI providers could use.”
Virtual chromoendoscopy: A must have in 2022
A third best practice advice statement shouldn’t be controversial because it’s in other guidelines already, but data show clinicians aren’t always doing it: Performing screening and surveillance endoscopic examinations using virtual chromoendoscopy in addition to high-definition white light endoscopy, with adequate time spent inspecting the Barrett’s segment. The majority of data supporting this is for narrow-band imaging only.
“The blue light lets you pick up early mucosal and vascular changes which might represent dysplastic lesions. It’s not a question of should. It’s a medicolegal slam dunk; you must do it. It’s been a guideline recommendation in the last few years, and it’s just a switch on the scope. It doesn’t require separate equipment, yet people are often still skipping it,” Dr. Komanduri said.
Indeed, Dr. Kaul concurred, “The importance of a high quality, meticulous endoscopic examination for screening and surveillance in Barrett’s esophagus cannot be overemphasized.”
‘Finally pushing the needle in the right direction’
The overall goals, Dr. Komanduri said, are “increasing the denominator, using less invasive screening, but finding more patients. If we find more patients we’ll need to stratify their risk. We hope that all these things eventually tie together in a nice story, all with the aim of preventing an invasive cancer that can’t be treated.”
He believes the new update “is a pivotal document in this field that’s going to be a paradigm changer. A lot of aspects need further validation. It’s by no means the end. But I think we’re finally pushing the needle in the right direction as things move forward with innovation.”
Dr. Kaul agrees. “It’s highlighting the principles that may become established paradigms in the future.”
Dr. Komanduri and the other authors of the update reported relationships, including consulting and research support, with companies like Boston Scientific, Medtronic, Virgo Video Solutions, and Castle Biosciences. Dr. Kaul serves as a consultant and advisory board member for CDx Diagnostics, an advisory board member for Castle Biosciences, and an investigator for Lucid Diagnostics.
A new clinical practice update from the American Gastroenterological Association offers practical advice around surveillance and use of new screening technologies for Barrett’s esophagus.
The AGA clinical practice update, published in Clinical Gastroenterology and Hepatology comes from the AGA’s Center for GI Innovation and Technology. It offers 15 best practice advice statements based on expert review of existing literature combined with discussion and expert opinion. The aim is “to provide an update on advances and innovation” but not to replace current guidelines.
“Guidelines operate on rigorous methodology which requires the use of [Grading of Recommendations, Assessment, Development and Evaluation] methodology and a higher level of evidence. In gastroenterology especially, innovation is moving quickly and there’s no way for patients to reap their benefits if clinical practice was dictated by guidelines alone. That said, we do need documents that support and drive innovation in clinical practice,” corresponding author Srinadh Komanduri, MD, professor of medicine and surgery in the division of gastroenterology and hepatology at Northwestern University, Chicago, told this news publication.
Asked to comment, Vivek Kaul, MD, the Segal-Watson Professor of Medicine in the Center for Advanced Therapeutic Endoscopy in the division of gastroenterology and hepatology at the University of Rochester (N.Y.) Medical Center, said that the document is “an important attempt to not only present the available scientific literature in a very concise and understandable manner, but it goes above and beyond that in terms of diving into some novel paradigms and technologies and procedures that are either emerging or will be emerging in the near future.”
Improving detection by dropping GERD requirement
The first of the 15 statements may also be the most paradigm shifting: The panel suggests screening via standard upper endoscopy of people with at least three risk factors for Barrett’s esophagus and esophageal adenocarcinoma, including those who are male, are non-Hispanic White, are aged above 50 years, and have a history of smoking, chronic gastroesophageal reflux disease (GERD), obesity, or a family history of Barrett’s esophagus or esophageal adenocarcinoma.
This represents a departure from all current guidelines, which stipulate GERD as a necessary prerequisite for screening. But the reason is simple, according to the authors: A majority of patients diagnosed with esophageal cancer never experience classic GERD symptoms.
“There is growing evidence in high-level publications over the last couple of years that reflux is not the ideal predictor, based on odds, for development of Barrett’s esophagus. So the consensus among the experts was that we need to remove GERD as an absolute prerequisite or we’re never going to make progress. In order to make an impact on the rise of esophageal adenocarcinoma we have to increase the denominator of patients we are seeing,” Dr. Komanduri explained.
While it might be difficult to screen every White male over 50 years of age, the data do suggest screening those who also have obesity and/or are current smokers. “That’s a perfect subset you might want to start with. There are permutations that have greater value that don’t occupy unnecessary resource utilization. Most critical are the family history of esophageal cancer or Barrett’s esophagus,” he noted.
Dr. Kaul said that a one-time Barrett’s esophagus screening of all White males over 50 years old “is not unreasonable, especially given the rising rates of esophageal cancer.”
However, he also noted, “The feasibility, preferred screening modality, incremental costs, and yield of this new strategy will need to be studied further. Access to GI endoscopy in the postpandemic world is already a concern and will need to be factored into execution of this [advice statement] and will likely impact adoption in some way.”
For his part, Dr. Komanduri said that more investigation will be needed to validate which patients most benefit from screening and that the AGA is planning educational programs for clinicians about interpreting this new paradigm.
New technology could make screening easier and cheaper
The availability of nonendoscopic cell collection devices, including the swallowable Cytosponge (Medtronic), EsoCheck (Lucid), and EsoCap (Capnostics) could help make screening for Barrett’s esophagus easier and more cost effective. They are designed for in-office use and don’t require sedation. Each one is currently in various stages of development and clinical trials. As of now they’re approved in the United States only for cell collection but not for Barrett’s esophagus screening, but their use is endorsed by some guidelines. The Cytosponge in particular is widely available and has been used extensively in the United Kingdom.
Dr. Kaul commented, “While there is a need for nonendoscopic screening devices, the ideal patient population and practice setting for administration of these devices has not been clearly defined. Also, who will be delivering these tests: Primary care or gastroenterology providers? These devices ... represent a major step forward and a novel paradigm for Barrett’s esophagus screening, and the only platform that non-GI providers could use.”
Virtual chromoendoscopy: A must have in 2022
A third best practice advice statement shouldn’t be controversial because it’s in other guidelines already, but data show clinicians aren’t always doing it: Performing screening and surveillance endoscopic examinations using virtual chromoendoscopy in addition to high-definition white light endoscopy, with adequate time spent inspecting the Barrett’s segment. The majority of data supporting this is for narrow-band imaging only.
“The blue light lets you pick up early mucosal and vascular changes which might represent dysplastic lesions. It’s not a question of should. It’s a medicolegal slam dunk; you must do it. It’s been a guideline recommendation in the last few years, and it’s just a switch on the scope. It doesn’t require separate equipment, yet people are often still skipping it,” Dr. Komanduri said.
Indeed, Dr. Kaul concurred, “The importance of a high quality, meticulous endoscopic examination for screening and surveillance in Barrett’s esophagus cannot be overemphasized.”
‘Finally pushing the needle in the right direction’
The overall goals, Dr. Komanduri said, are “increasing the denominator, using less invasive screening, but finding more patients. If we find more patients we’ll need to stratify their risk. We hope that all these things eventually tie together in a nice story, all with the aim of preventing an invasive cancer that can’t be treated.”
He believes the new update “is a pivotal document in this field that’s going to be a paradigm changer. A lot of aspects need further validation. It’s by no means the end. But I think we’re finally pushing the needle in the right direction as things move forward with innovation.”
Dr. Kaul agrees. “It’s highlighting the principles that may become established paradigms in the future.”
Dr. Komanduri and the other authors of the update reported relationships, including consulting and research support, with companies like Boston Scientific, Medtronic, Virgo Video Solutions, and Castle Biosciences. Dr. Kaul serves as a consultant and advisory board member for CDx Diagnostics, an advisory board member for Castle Biosciences, and an investigator for Lucid Diagnostics.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Pre-endoscopy COVID-19 testing may not be needed
Pre-endoscopy viral testing may not be necessary to prevent coronavirus transmission from patients to endoscopy staff members, according to a new study published in Gut.
Instead, using personal protective equipment (PPE) and ensuring up-to-date COVID-19 vaccination among the medical team was found to be enough to substantially reduce the risk of spreading SARS-CoV-2, wrote Alexander Hann, Dr.med., gastroenterologist at University Hospital W
“We suggest that pre-selection of patients using respective questionnaires, vaccination, and particularly PPE appears to be sufficient for the prevention of SARS-CoV-2 transmission in GI endoscopy,” they wrote.
Dr. Hann and colleagues analyzed 15,750 endoscopies performed by 29 staff members during the period between May 2020 and December 2021. The researchers looked at three test approaches: No testing (4,543 patients), rapid antigen testing (682 patients), and real-time PCR testing (10,465 patients). In addition, 60 endoscopies were performed in patients with known COVID-19. Overall, no staff members became infected with SARS-CoV-2 during the study period. In all three scenarios, staff used PPE, and the vaccination rate of the team was 97%.
University Hospital W
All patients were interviewed before admission for COVID-19 symptoms, close contact with infected people, and recent travel to high-risk countries. Moreover, some endoscopies were performed even if a patient had positive markers for COVID-19.
The clinical team wore recommended PPE, including a high-filter FFP2 mask, one pair of gloves, protective eyewear, and disposable gowns. For patients with known COVID-19, staff wore two pairs of gloves, a disposable hairnet, and a water-resistant disposable gown. In addition, endoscopies were performed in negative pressure intervention rooms.
Among the 29 staff members involved, 16 physicians and 13 assistants worked in the endoscopy unit for at least 2 days per week for at least 6 months. The hospital’s internal policy required medical staff to undergo PCR testing if a rapid antigen test was positive or symptoms developed. Staff were vaccinated with two doses of the Pfizer-BioNTech vaccine in January and February 2021. A single booster dose of the Pfizer or Moderna vaccine was administered in November and December 2021.
The clinical team was not tested routinely, so asymptomatic infections may have existed. Moreover, the relatively low COVID-19 incidence in the local area might have influenced the risk of transmission. “However, even at the end of 2021, when the incidence was increasing, we did not see any higher risk of transmission,” the researchers explained.
“An important limitation of our study relates to the new variant Omicron that was dominant in our local area after the analyzed time frame.” Additional studies may be needed to understand the risk of transmission with the latest Omicron variants, and given the additional costs and implications on routine activity, current testing guidelines may need to be reconsidered.
“Although our data were not part of a randomized prospective study, we were able to demonstrate on a fairly high number of patients that PPE measures in addition to a short interview for assessment of a patient’s individual risks appear to be highly effective to control transmission of SARS-CoV-2 during an endoscopy. ... Pre-procedural RT-PCR testing or RA testing did not show any additional benefit,” Dr. Hann and colleagues concluded.
The authors reported no conflicts of interest.
Pre-endoscopy viral testing may not be necessary to prevent coronavirus transmission from patients to endoscopy staff members, according to a new study published in Gut.
Instead, using personal protective equipment (PPE) and ensuring up-to-date COVID-19 vaccination among the medical team was found to be enough to substantially reduce the risk of spreading SARS-CoV-2, wrote Alexander Hann, Dr.med., gastroenterologist at University Hospital W
“We suggest that pre-selection of patients using respective questionnaires, vaccination, and particularly PPE appears to be sufficient for the prevention of SARS-CoV-2 transmission in GI endoscopy,” they wrote.
Dr. Hann and colleagues analyzed 15,750 endoscopies performed by 29 staff members during the period between May 2020 and December 2021. The researchers looked at three test approaches: No testing (4,543 patients), rapid antigen testing (682 patients), and real-time PCR testing (10,465 patients). In addition, 60 endoscopies were performed in patients with known COVID-19. Overall, no staff members became infected with SARS-CoV-2 during the study period. In all three scenarios, staff used PPE, and the vaccination rate of the team was 97%.
University Hospital W
All patients were interviewed before admission for COVID-19 symptoms, close contact with infected people, and recent travel to high-risk countries. Moreover, some endoscopies were performed even if a patient had positive markers for COVID-19.
The clinical team wore recommended PPE, including a high-filter FFP2 mask, one pair of gloves, protective eyewear, and disposable gowns. For patients with known COVID-19, staff wore two pairs of gloves, a disposable hairnet, and a water-resistant disposable gown. In addition, endoscopies were performed in negative pressure intervention rooms.
Among the 29 staff members involved, 16 physicians and 13 assistants worked in the endoscopy unit for at least 2 days per week for at least 6 months. The hospital’s internal policy required medical staff to undergo PCR testing if a rapid antigen test was positive or symptoms developed. Staff were vaccinated with two doses of the Pfizer-BioNTech vaccine in January and February 2021. A single booster dose of the Pfizer or Moderna vaccine was administered in November and December 2021.
The clinical team was not tested routinely, so asymptomatic infections may have existed. Moreover, the relatively low COVID-19 incidence in the local area might have influenced the risk of transmission. “However, even at the end of 2021, when the incidence was increasing, we did not see any higher risk of transmission,” the researchers explained.
“An important limitation of our study relates to the new variant Omicron that was dominant in our local area after the analyzed time frame.” Additional studies may be needed to understand the risk of transmission with the latest Omicron variants, and given the additional costs and implications on routine activity, current testing guidelines may need to be reconsidered.
“Although our data were not part of a randomized prospective study, we were able to demonstrate on a fairly high number of patients that PPE measures in addition to a short interview for assessment of a patient’s individual risks appear to be highly effective to control transmission of SARS-CoV-2 during an endoscopy. ... Pre-procedural RT-PCR testing or RA testing did not show any additional benefit,” Dr. Hann and colleagues concluded.
The authors reported no conflicts of interest.
Pre-endoscopy viral testing may not be necessary to prevent coronavirus transmission from patients to endoscopy staff members, according to a new study published in Gut.
Instead, using personal protective equipment (PPE) and ensuring up-to-date COVID-19 vaccination among the medical team was found to be enough to substantially reduce the risk of spreading SARS-CoV-2, wrote Alexander Hann, Dr.med., gastroenterologist at University Hospital W
“We suggest that pre-selection of patients using respective questionnaires, vaccination, and particularly PPE appears to be sufficient for the prevention of SARS-CoV-2 transmission in GI endoscopy,” they wrote.
Dr. Hann and colleagues analyzed 15,750 endoscopies performed by 29 staff members during the period between May 2020 and December 2021. The researchers looked at three test approaches: No testing (4,543 patients), rapid antigen testing (682 patients), and real-time PCR testing (10,465 patients). In addition, 60 endoscopies were performed in patients with known COVID-19. Overall, no staff members became infected with SARS-CoV-2 during the study period. In all three scenarios, staff used PPE, and the vaccination rate of the team was 97%.
University Hospital W
All patients were interviewed before admission for COVID-19 symptoms, close contact with infected people, and recent travel to high-risk countries. Moreover, some endoscopies were performed even if a patient had positive markers for COVID-19.
The clinical team wore recommended PPE, including a high-filter FFP2 mask, one pair of gloves, protective eyewear, and disposable gowns. For patients with known COVID-19, staff wore two pairs of gloves, a disposable hairnet, and a water-resistant disposable gown. In addition, endoscopies were performed in negative pressure intervention rooms.
Among the 29 staff members involved, 16 physicians and 13 assistants worked in the endoscopy unit for at least 2 days per week for at least 6 months. The hospital’s internal policy required medical staff to undergo PCR testing if a rapid antigen test was positive or symptoms developed. Staff were vaccinated with two doses of the Pfizer-BioNTech vaccine in January and February 2021. A single booster dose of the Pfizer or Moderna vaccine was administered in November and December 2021.
The clinical team was not tested routinely, so asymptomatic infections may have existed. Moreover, the relatively low COVID-19 incidence in the local area might have influenced the risk of transmission. “However, even at the end of 2021, when the incidence was increasing, we did not see any higher risk of transmission,” the researchers explained.
“An important limitation of our study relates to the new variant Omicron that was dominant in our local area after the analyzed time frame.” Additional studies may be needed to understand the risk of transmission with the latest Omicron variants, and given the additional costs and implications on routine activity, current testing guidelines may need to be reconsidered.
“Although our data were not part of a randomized prospective study, we were able to demonstrate on a fairly high number of patients that PPE measures in addition to a short interview for assessment of a patient’s individual risks appear to be highly effective to control transmission of SARS-CoV-2 during an endoscopy. ... Pre-procedural RT-PCR testing or RA testing did not show any additional benefit,” Dr. Hann and colleagues concluded.
The authors reported no conflicts of interest.
FROM GUT
Will ESD replace EMR for large colorectal polyps?
Dear colleagues,
Resection of polyps is the bread and butter of endoscopy. Advances in technology have enabled us to tackle larger and more complex lesions throughout the gastrointestinal tract, especially through endoscopic mucosal resection (EMR). Endoscopic submucosal dissection (ESD) is another technique that offers much promise for complex colorectal polyps and is being rapidly adopted in the West. But do its benefits outweigh the costs in time, money and additional training needed for successful ESD? How can we justify higher recurrence rates with EMR when ESD is available? Will reimbursement continue to favor EMR? In this issue of Perspectives, Dr. Alexis Bayudan and Dr. Craig A. Munroe make the case for adopting ESD, while Dr. Sumeet Tewani highlights all the benefits of EMR. I invite you to a great debate and look forward to hearing your own thoughts on Twitter @AGA_GIHN and by email at ginews@gastro.org.
Gyanprakash A. Ketwaroo, MD, MSc, is assistant professor of medicine at Baylor College of Medicine, Houston. He is an associate editor for GI & Hepatology News.
The future standard of care
BY ALEXIS BAYUDAN, MD, AND CRAIG A. MUNROE, MD
Endoscopic submucosal dissection (ESD) is a minimally invasive, organ-sparing, flexible endoscopic technique used to treat advanced neoplasia of the digestive tract, with the goal of en bloc resection for accurate histologic assessment. ESD was introduced over 25 years ago in Japan by a small group of innovative endoscopists.1 After its initial adoption and success with removing gastric lesions, ESD later evolved as a technique used for complete resection of lesions throughout the gastrointestinal tract.
The intent of ESD is to achieve clear pathologic evaluation of deep and lateral margins, which is generally lost when piecemeal EMR (pEMR) is performed on lesions larger than 2 cm. With growing global experience, the evidence is clear that ESD is advantageous when compared to pEMR in the resection of large colorectal lesions en bloc, leading to improved curative resection rates and less local recurrence.
From our own experience, and from the results of many studies, we know that although procedure time in ESD can be longer, the rate of complete resection is far superior. ESD was previously cited as having a 10% risk of perforation in the 1990s and early 2000s, but current rates are closer to 4.5%, as noted by Nimii et al., with nearly complete successful treatment with endoscopic closure.1 In a 2021 meta-analysis reviewing a total of 21 studies, Lim et. al demonstrate that, although there is an increased risk of perforation with ESD compared to EMR (risk ratio, 7.597; 95% confidence interval, 4.281-13.479; P < .001), there is no significant difference in bleeding risk between the two techniques (RR, 7.597; 95% CI, 4.281-13.479; P < .001).2
Since its inception, many refinements of the ESD technique have occurred through technology, and better understanding of anatomy and disease states. These include, but are not limited to, improvements in hemostatic and closure techniques, electrosurgical equipment, resection and traction devices, the use of carbon dioxide, the ability to perform full-thickness endoscopic surgery, and submucosal lifting.1 The realm of endoscopic innovation is moving at a rapid pace within commercial and noncommercial entities, and advancements in ESD devices will allow for further improvements in procedure times and decreased procedural complications. Conversely, there have been few advancements in EMR technique in decades.
Further developments in ESD will continue to democratize this intervention, so that it can be practiced in all medical centers, not just expert centers. However, for ESD to become standard of care in the Western world, it will require more exposure and training. ESD has rapidly spread throughout Japan because of the master-mentor relationship that fosters safe learning, in addition to an abundance of highly skilled EMR-experienced physicians who went on to acquire their skills under the supervision of ESD experts. Current methods of teaching ESD, such as using pig models to practice specific steps of the procedure, can be implemented in Western gastroenterology training programs and through GI and surgical society training programs to learn safe operation in the third space. Mentorship and proctorship are also mandatory. The incorporation of ESD into standard practice over pEMR is very akin to laparoscopic cholecystectomy revolutionizing gallbladder surgery, even though open cholecystectomy was known to be effective.
A major limitation in the adoption of ESD in the West is reimbursement. Despite mounting evidence of the superiority of ESD in well-trained hands, and the additional training needed to safely perform these procedures, there had not been a pathway forward for payment for the increased requirements needed to perform these procedures safely.3 This leads to more endoscopists performing pEMR in the West which is anti-innovative. In October 2021, the Centers for Medicare and Medicaid Services expanded the reimbursement for ESD (Healthcare Common Procedure Coding System C9779). The availability of billing codes paves the way for increasing patient access to these therapies. Hopefully, additional codes will follow.
With the mounting evidence demonstrating ESD is superior to pEMR in terms of curative resection and recurrence rates, we think it is time for ESD to be incorporated widely into Western practice. ESD is still evolving and improving; ESD will become both safer and more effective. ESD has revolutionized endoscopic resection, and we are just beginning to see the possibilities and value of these techniques.
Dr. Baydan is a second-year fellow, and Dr. Munroe is an associate professor, both at the University of California, San Francisco. They have no relevant conflicts of interest.
References
1. Ferreira J et al. Clin Colon Rectal Surg. 2015 Sep; 28(3):146-151.
2. Lim X et al. World J Gastroenterol. 2021 Jul 7;27(25):3925-39.
3. Iqbal S et al. World J Gastrointest Endosc. 2020 Jan 16; 12(1):49-52.
More investment than payoff
Most large colorectal polyps are best managed by endoscopic mucosal resection (EMR) and do not require endoscopic submucosal dissection (ESD). EMR can provide complete, safe, and effective removal, preventing colorectal cancer while avoiding the risks of surgery or ESD. EMR has several advantages over ESD. It is minimally invasive, low cost, well tolerated, and allows excellent histopathologic examination. It is performed during colonoscopy in an outpatient endoscopy lab or ambulatory surgery center. There are several techniques that can be performed safely and efficiently using accessories that are readily available. It is easier to learn and perform, with lower risks and fewer resources. Endoscopists can effectively integrate EMR into a busy practice, without making significant additional investments.
EMR of large adenomas has improved morbidity, mortality, and cost compared to surgery.1-3 I first carefully inspect the lesion to plan the approach and exclude submucosal invasion, which should be referred for ESD or surgery instead. This includes understanding the size, location, morphology, and surface characteristics, using high-definition and narrow-band imaging or Fujinon intelligent chromoendoscopy. Conventional EMR utilizes submucosal injection to lift the polyp away from the underlying muscle layer before hot snare resection. Injection needles and snares of various shapes, sizes, and stiffness are available in most endoscopy labs. The goal is en bloc resection to achieve potential cure with complete histological assessment and low rate of recurrence. This can be achieved for lesions up to 2 cm in size, although larger lesions require piecemeal resection, which limits accurate histopathology and carries a recurrence rate up to 25%.1 Thermal ablation of the resection margins with argon plasma coagulation or snare-tip soft coagulation can reduce the rate of recurrence. Additionally, most recurrences are identified during early surveillance within 6 months and managed endoscopically. The rates of adverse events, including bleeding (6%-15%), perforation (1%-2%), and postpolypectomy syndrome (< 1%) remain at acceptable low levels.1,4
For many polyps, saline injection is safe, effective, and inexpensive, but it dissipates rapidly with limited duration of effect. Alternative agents can improve the lift, at additional cost.4 I prefer adding dye, such as methylene blue, to differentiate the submucosa from the muscularis, demarcate the lesion margins, and allow easier inspection of the defect. Dilute epinephrine can also be added to reduce intraprocedural bleeding and maintain a clean resection field. I reserve this for the duodenum, but it can be an important adjunct for some colorectal polyps. Submucosal injection also allows assessment for a “nonlifting sign,” which raises suspicion for invasive carcinoma but can also occur with benign submucosal fibrosis from previous biopsy, partial resection, or adjacent tattoo. In these cases, effective management can still be achieved using EMR in combination with avulsion and thermal ablation techniques.
Alternative techniques include cold EMR and underwater EMR.1,4 These are gaining popularity because of their excellent safety profile and favorable outcomes. Cold EMR involves submucosal injection followed by cold-snare resection, eliminating the use of cautery and its associated risks. Cold EMR is very safe and effective for small polyps, and we use this for progressively larger polyps given the low complication rate. Despite the need for piecemeal resection of polyps larger than 10 mm, local recurrence rates are comparable to conventional EMR. Sessile serrated polyps are especially ideal for piecemeal cold EMR. Meanwhile, underwater EMR eliminates the need for submucosal injection by utilizing water immersion, which elevates the mucosal lesion away from the muscularis layer. Either hot or cold snare resection can be performed. Benefits include reduced procedure time and cost, and relatively low complication and recurrence rates, compared with conventional EMR. I find this to be a nice option for laterally spreading polyps, with potentially higher rates of en bloc resection.1,4
ESD involves similar techniques but includes careful dissection of the submucosal layer beneath the lesion. In addition to the tools for EMR, a specialized electrosurgical knife is necessary, as well as dedicated training and mentorship that can be difficult to accommodate for an active endoscopist in practice. The primary advantage of ESD is higher en bloc resection rates for larger and potentially deeper lesions, with accurate histologic assessment and staging, and very low recurrence rates.1,4,5 However, ESD is more complex, technically challenging, and time and resource intensive, with higher risk of complications. Intraprocedural bleeding is common and requires immediate management. Additional risks include 2% risk of delayed bleeding and 5% risk of perforation.1,5 ESD involves an operating room, longer procedure times, and higher cost including surgical, anesthesia, and nursing costs. Some of this may be balanced by reduced frequency of surveillance and therapeutic procedures. While both EMR and ESD carry significant cost savings, compared with surgery, ESD is additionally disadvantaged by lack of reimbursement.
Regardless of the technique, EMR is easier to learn and perform than ESD, uses a limited number of devices that are readily available, and carries lower cost-burden. EMR is successful for most colorectal polyps, with the primary disadvantage being piecemeal resection of larger polyps. The rates of adverse events are lower, and appropriate surveillance is essential to ensuring complete resection and eliminating recurrence. Japanese and European guidelines endorse ESD for lesions that have a high likelihood of cancer invading the submucosa and for lesions that cannot be removed by EMR because of submucosal fibrosis. Ultimately, patients need to be treated individually with the most appropriate technique.
Dr. Tewani of Rockford Gastroenterology Associates is clinical assistant professor of medicine at the University of Illinois, Rockford. He has no relevant conflicts of interest to disclose.
References
1. Rashid MU et al. Surg Oncol. 2022 Mar 18;101742.
2. Law R et al. Gastrointest Endosc. 2016 Jun;83(6):1248-57.
3. Backes Y et al. BMC Gastroenterol. 2016 May 26;16(1):56.
4. Thiruvengadam SS et al. Gastroenterol Hepatol. 2022 Mar;18(3):133-44.
5. Wang J et al. World J Gastroenterol. 2014 Jul 7;20(25):8282-7l.
Dear colleagues,
Resection of polyps is the bread and butter of endoscopy. Advances in technology have enabled us to tackle larger and more complex lesions throughout the gastrointestinal tract, especially through endoscopic mucosal resection (EMR). Endoscopic submucosal dissection (ESD) is another technique that offers much promise for complex colorectal polyps and is being rapidly adopted in the West. But do its benefits outweigh the costs in time, money and additional training needed for successful ESD? How can we justify higher recurrence rates with EMR when ESD is available? Will reimbursement continue to favor EMR? In this issue of Perspectives, Dr. Alexis Bayudan and Dr. Craig A. Munroe make the case for adopting ESD, while Dr. Sumeet Tewani highlights all the benefits of EMR. I invite you to a great debate and look forward to hearing your own thoughts on Twitter @AGA_GIHN and by email at ginews@gastro.org.
Gyanprakash A. Ketwaroo, MD, MSc, is assistant professor of medicine at Baylor College of Medicine, Houston. He is an associate editor for GI & Hepatology News.
The future standard of care
BY ALEXIS BAYUDAN, MD, AND CRAIG A. MUNROE, MD
Endoscopic submucosal dissection (ESD) is a minimally invasive, organ-sparing, flexible endoscopic technique used to treat advanced neoplasia of the digestive tract, with the goal of en bloc resection for accurate histologic assessment. ESD was introduced over 25 years ago in Japan by a small group of innovative endoscopists.1 After its initial adoption and success with removing gastric lesions, ESD later evolved as a technique used for complete resection of lesions throughout the gastrointestinal tract.
The intent of ESD is to achieve clear pathologic evaluation of deep and lateral margins, which is generally lost when piecemeal EMR (pEMR) is performed on lesions larger than 2 cm. With growing global experience, the evidence is clear that ESD is advantageous when compared to pEMR in the resection of large colorectal lesions en bloc, leading to improved curative resection rates and less local recurrence.
From our own experience, and from the results of many studies, we know that although procedure time in ESD can be longer, the rate of complete resection is far superior. ESD was previously cited as having a 10% risk of perforation in the 1990s and early 2000s, but current rates are closer to 4.5%, as noted by Nimii et al., with nearly complete successful treatment with endoscopic closure.1 In a 2021 meta-analysis reviewing a total of 21 studies, Lim et. al demonstrate that, although there is an increased risk of perforation with ESD compared to EMR (risk ratio, 7.597; 95% confidence interval, 4.281-13.479; P < .001), there is no significant difference in bleeding risk between the two techniques (RR, 7.597; 95% CI, 4.281-13.479; P < .001).2
Since its inception, many refinements of the ESD technique have occurred through technology, and better understanding of anatomy and disease states. These include, but are not limited to, improvements in hemostatic and closure techniques, electrosurgical equipment, resection and traction devices, the use of carbon dioxide, the ability to perform full-thickness endoscopic surgery, and submucosal lifting.1 The realm of endoscopic innovation is moving at a rapid pace within commercial and noncommercial entities, and advancements in ESD devices will allow for further improvements in procedure times and decreased procedural complications. Conversely, there have been few advancements in EMR technique in decades.
Further developments in ESD will continue to democratize this intervention, so that it can be practiced in all medical centers, not just expert centers. However, for ESD to become standard of care in the Western world, it will require more exposure and training. ESD has rapidly spread throughout Japan because of the master-mentor relationship that fosters safe learning, in addition to an abundance of highly skilled EMR-experienced physicians who went on to acquire their skills under the supervision of ESD experts. Current methods of teaching ESD, such as using pig models to practice specific steps of the procedure, can be implemented in Western gastroenterology training programs and through GI and surgical society training programs to learn safe operation in the third space. Mentorship and proctorship are also mandatory. The incorporation of ESD into standard practice over pEMR is very akin to laparoscopic cholecystectomy revolutionizing gallbladder surgery, even though open cholecystectomy was known to be effective.
A major limitation in the adoption of ESD in the West is reimbursement. Despite mounting evidence of the superiority of ESD in well-trained hands, and the additional training needed to safely perform these procedures, there had not been a pathway forward for payment for the increased requirements needed to perform these procedures safely.3 This leads to more endoscopists performing pEMR in the West which is anti-innovative. In October 2021, the Centers for Medicare and Medicaid Services expanded the reimbursement for ESD (Healthcare Common Procedure Coding System C9779). The availability of billing codes paves the way for increasing patient access to these therapies. Hopefully, additional codes will follow.
With the mounting evidence demonstrating ESD is superior to pEMR in terms of curative resection and recurrence rates, we think it is time for ESD to be incorporated widely into Western practice. ESD is still evolving and improving; ESD will become both safer and more effective. ESD has revolutionized endoscopic resection, and we are just beginning to see the possibilities and value of these techniques.
Dr. Baydan is a second-year fellow, and Dr. Munroe is an associate professor, both at the University of California, San Francisco. They have no relevant conflicts of interest.
References
1. Ferreira J et al. Clin Colon Rectal Surg. 2015 Sep; 28(3):146-151.
2. Lim X et al. World J Gastroenterol. 2021 Jul 7;27(25):3925-39.
3. Iqbal S et al. World J Gastrointest Endosc. 2020 Jan 16; 12(1):49-52.
More investment than payoff
Most large colorectal polyps are best managed by endoscopic mucosal resection (EMR) and do not require endoscopic submucosal dissection (ESD). EMR can provide complete, safe, and effective removal, preventing colorectal cancer while avoiding the risks of surgery or ESD. EMR has several advantages over ESD. It is minimally invasive, low cost, well tolerated, and allows excellent histopathologic examination. It is performed during colonoscopy in an outpatient endoscopy lab or ambulatory surgery center. There are several techniques that can be performed safely and efficiently using accessories that are readily available. It is easier to learn and perform, with lower risks and fewer resources. Endoscopists can effectively integrate EMR into a busy practice, without making significant additional investments.
EMR of large adenomas has improved morbidity, mortality, and cost compared to surgery.1-3 I first carefully inspect the lesion to plan the approach and exclude submucosal invasion, which should be referred for ESD or surgery instead. This includes understanding the size, location, morphology, and surface characteristics, using high-definition and narrow-band imaging or Fujinon intelligent chromoendoscopy. Conventional EMR utilizes submucosal injection to lift the polyp away from the underlying muscle layer before hot snare resection. Injection needles and snares of various shapes, sizes, and stiffness are available in most endoscopy labs. The goal is en bloc resection to achieve potential cure with complete histological assessment and low rate of recurrence. This can be achieved for lesions up to 2 cm in size, although larger lesions require piecemeal resection, which limits accurate histopathology and carries a recurrence rate up to 25%.1 Thermal ablation of the resection margins with argon plasma coagulation or snare-tip soft coagulation can reduce the rate of recurrence. Additionally, most recurrences are identified during early surveillance within 6 months and managed endoscopically. The rates of adverse events, including bleeding (6%-15%), perforation (1%-2%), and postpolypectomy syndrome (< 1%) remain at acceptable low levels.1,4
For many polyps, saline injection is safe, effective, and inexpensive, but it dissipates rapidly with limited duration of effect. Alternative agents can improve the lift, at additional cost.4 I prefer adding dye, such as methylene blue, to differentiate the submucosa from the muscularis, demarcate the lesion margins, and allow easier inspection of the defect. Dilute epinephrine can also be added to reduce intraprocedural bleeding and maintain a clean resection field. I reserve this for the duodenum, but it can be an important adjunct for some colorectal polyps. Submucosal injection also allows assessment for a “nonlifting sign,” which raises suspicion for invasive carcinoma but can also occur with benign submucosal fibrosis from previous biopsy, partial resection, or adjacent tattoo. In these cases, effective management can still be achieved using EMR in combination with avulsion and thermal ablation techniques.
Alternative techniques include cold EMR and underwater EMR.1,4 These are gaining popularity because of their excellent safety profile and favorable outcomes. Cold EMR involves submucosal injection followed by cold-snare resection, eliminating the use of cautery and its associated risks. Cold EMR is very safe and effective for small polyps, and we use this for progressively larger polyps given the low complication rate. Despite the need for piecemeal resection of polyps larger than 10 mm, local recurrence rates are comparable to conventional EMR. Sessile serrated polyps are especially ideal for piecemeal cold EMR. Meanwhile, underwater EMR eliminates the need for submucosal injection by utilizing water immersion, which elevates the mucosal lesion away from the muscularis layer. Either hot or cold snare resection can be performed. Benefits include reduced procedure time and cost, and relatively low complication and recurrence rates, compared with conventional EMR. I find this to be a nice option for laterally spreading polyps, with potentially higher rates of en bloc resection.1,4
ESD involves similar techniques but includes careful dissection of the submucosal layer beneath the lesion. In addition to the tools for EMR, a specialized electrosurgical knife is necessary, as well as dedicated training and mentorship that can be difficult to accommodate for an active endoscopist in practice. The primary advantage of ESD is higher en bloc resection rates for larger and potentially deeper lesions, with accurate histologic assessment and staging, and very low recurrence rates.1,4,5 However, ESD is more complex, technically challenging, and time and resource intensive, with higher risk of complications. Intraprocedural bleeding is common and requires immediate management. Additional risks include 2% risk of delayed bleeding and 5% risk of perforation.1,5 ESD involves an operating room, longer procedure times, and higher cost including surgical, anesthesia, and nursing costs. Some of this may be balanced by reduced frequency of surveillance and therapeutic procedures. While both EMR and ESD carry significant cost savings, compared with surgery, ESD is additionally disadvantaged by lack of reimbursement.
Regardless of the technique, EMR is easier to learn and perform than ESD, uses a limited number of devices that are readily available, and carries lower cost-burden. EMR is successful for most colorectal polyps, with the primary disadvantage being piecemeal resection of larger polyps. The rates of adverse events are lower, and appropriate surveillance is essential to ensuring complete resection and eliminating recurrence. Japanese and European guidelines endorse ESD for lesions that have a high likelihood of cancer invading the submucosa and for lesions that cannot be removed by EMR because of submucosal fibrosis. Ultimately, patients need to be treated individually with the most appropriate technique.
Dr. Tewani of Rockford Gastroenterology Associates is clinical assistant professor of medicine at the University of Illinois, Rockford. He has no relevant conflicts of interest to disclose.
References
1. Rashid MU et al. Surg Oncol. 2022 Mar 18;101742.
2. Law R et al. Gastrointest Endosc. 2016 Jun;83(6):1248-57.
3. Backes Y et al. BMC Gastroenterol. 2016 May 26;16(1):56.
4. Thiruvengadam SS et al. Gastroenterol Hepatol. 2022 Mar;18(3):133-44.
5. Wang J et al. World J Gastroenterol. 2014 Jul 7;20(25):8282-7l.
Dear colleagues,
Resection of polyps is the bread and butter of endoscopy. Advances in technology have enabled us to tackle larger and more complex lesions throughout the gastrointestinal tract, especially through endoscopic mucosal resection (EMR). Endoscopic submucosal dissection (ESD) is another technique that offers much promise for complex colorectal polyps and is being rapidly adopted in the West. But do its benefits outweigh the costs in time, money and additional training needed for successful ESD? How can we justify higher recurrence rates with EMR when ESD is available? Will reimbursement continue to favor EMR? In this issue of Perspectives, Dr. Alexis Bayudan and Dr. Craig A. Munroe make the case for adopting ESD, while Dr. Sumeet Tewani highlights all the benefits of EMR. I invite you to a great debate and look forward to hearing your own thoughts on Twitter @AGA_GIHN and by email at ginews@gastro.org.
Gyanprakash A. Ketwaroo, MD, MSc, is assistant professor of medicine at Baylor College of Medicine, Houston. He is an associate editor for GI & Hepatology News.
The future standard of care
BY ALEXIS BAYUDAN, MD, AND CRAIG A. MUNROE, MD
Endoscopic submucosal dissection (ESD) is a minimally invasive, organ-sparing, flexible endoscopic technique used to treat advanced neoplasia of the digestive tract, with the goal of en bloc resection for accurate histologic assessment. ESD was introduced over 25 years ago in Japan by a small group of innovative endoscopists.1 After its initial adoption and success with removing gastric lesions, ESD later evolved as a technique used for complete resection of lesions throughout the gastrointestinal tract.
The intent of ESD is to achieve clear pathologic evaluation of deep and lateral margins, which is generally lost when piecemeal EMR (pEMR) is performed on lesions larger than 2 cm. With growing global experience, the evidence is clear that ESD is advantageous when compared to pEMR in the resection of large colorectal lesions en bloc, leading to improved curative resection rates and less local recurrence.
From our own experience, and from the results of many studies, we know that although procedure time in ESD can be longer, the rate of complete resection is far superior. ESD was previously cited as having a 10% risk of perforation in the 1990s and early 2000s, but current rates are closer to 4.5%, as noted by Nimii et al., with nearly complete successful treatment with endoscopic closure.1 In a 2021 meta-analysis reviewing a total of 21 studies, Lim et. al demonstrate that, although there is an increased risk of perforation with ESD compared to EMR (risk ratio, 7.597; 95% confidence interval, 4.281-13.479; P < .001), there is no significant difference in bleeding risk between the two techniques (RR, 7.597; 95% CI, 4.281-13.479; P < .001).2
Since its inception, many refinements of the ESD technique have occurred through technology, and better understanding of anatomy and disease states. These include, but are not limited to, improvements in hemostatic and closure techniques, electrosurgical equipment, resection and traction devices, the use of carbon dioxide, the ability to perform full-thickness endoscopic surgery, and submucosal lifting.1 The realm of endoscopic innovation is moving at a rapid pace within commercial and noncommercial entities, and advancements in ESD devices will allow for further improvements in procedure times and decreased procedural complications. Conversely, there have been few advancements in EMR technique in decades.
Further developments in ESD will continue to democratize this intervention, so that it can be practiced in all medical centers, not just expert centers. However, for ESD to become standard of care in the Western world, it will require more exposure and training. ESD has rapidly spread throughout Japan because of the master-mentor relationship that fosters safe learning, in addition to an abundance of highly skilled EMR-experienced physicians who went on to acquire their skills under the supervision of ESD experts. Current methods of teaching ESD, such as using pig models to practice specific steps of the procedure, can be implemented in Western gastroenterology training programs and through GI and surgical society training programs to learn safe operation in the third space. Mentorship and proctorship are also mandatory. The incorporation of ESD into standard practice over pEMR is very akin to laparoscopic cholecystectomy revolutionizing gallbladder surgery, even though open cholecystectomy was known to be effective.
A major limitation in the adoption of ESD in the West is reimbursement. Despite mounting evidence of the superiority of ESD in well-trained hands, and the additional training needed to safely perform these procedures, there had not been a pathway forward for payment for the increased requirements needed to perform these procedures safely.3 This leads to more endoscopists performing pEMR in the West which is anti-innovative. In October 2021, the Centers for Medicare and Medicaid Services expanded the reimbursement for ESD (Healthcare Common Procedure Coding System C9779). The availability of billing codes paves the way for increasing patient access to these therapies. Hopefully, additional codes will follow.
With the mounting evidence demonstrating ESD is superior to pEMR in terms of curative resection and recurrence rates, we think it is time for ESD to be incorporated widely into Western practice. ESD is still evolving and improving; ESD will become both safer and more effective. ESD has revolutionized endoscopic resection, and we are just beginning to see the possibilities and value of these techniques.
Dr. Baydan is a second-year fellow, and Dr. Munroe is an associate professor, both at the University of California, San Francisco. They have no relevant conflicts of interest.
References
1. Ferreira J et al. Clin Colon Rectal Surg. 2015 Sep; 28(3):146-151.
2. Lim X et al. World J Gastroenterol. 2021 Jul 7;27(25):3925-39.
3. Iqbal S et al. World J Gastrointest Endosc. 2020 Jan 16; 12(1):49-52.
More investment than payoff
Most large colorectal polyps are best managed by endoscopic mucosal resection (EMR) and do not require endoscopic submucosal dissection (ESD). EMR can provide complete, safe, and effective removal, preventing colorectal cancer while avoiding the risks of surgery or ESD. EMR has several advantages over ESD. It is minimally invasive, low cost, well tolerated, and allows excellent histopathologic examination. It is performed during colonoscopy in an outpatient endoscopy lab or ambulatory surgery center. There are several techniques that can be performed safely and efficiently using accessories that are readily available. It is easier to learn and perform, with lower risks and fewer resources. Endoscopists can effectively integrate EMR into a busy practice, without making significant additional investments.
EMR of large adenomas has improved morbidity, mortality, and cost compared to surgery.1-3 I first carefully inspect the lesion to plan the approach and exclude submucosal invasion, which should be referred for ESD or surgery instead. This includes understanding the size, location, morphology, and surface characteristics, using high-definition and narrow-band imaging or Fujinon intelligent chromoendoscopy. Conventional EMR utilizes submucosal injection to lift the polyp away from the underlying muscle layer before hot snare resection. Injection needles and snares of various shapes, sizes, and stiffness are available in most endoscopy labs. The goal is en bloc resection to achieve potential cure with complete histological assessment and low rate of recurrence. This can be achieved for lesions up to 2 cm in size, although larger lesions require piecemeal resection, which limits accurate histopathology and carries a recurrence rate up to 25%.1 Thermal ablation of the resection margins with argon plasma coagulation or snare-tip soft coagulation can reduce the rate of recurrence. Additionally, most recurrences are identified during early surveillance within 6 months and managed endoscopically. The rates of adverse events, including bleeding (6%-15%), perforation (1%-2%), and postpolypectomy syndrome (< 1%) remain at acceptable low levels.1,4
For many polyps, saline injection is safe, effective, and inexpensive, but it dissipates rapidly with limited duration of effect. Alternative agents can improve the lift, at additional cost.4 I prefer adding dye, such as methylene blue, to differentiate the submucosa from the muscularis, demarcate the lesion margins, and allow easier inspection of the defect. Dilute epinephrine can also be added to reduce intraprocedural bleeding and maintain a clean resection field. I reserve this for the duodenum, but it can be an important adjunct for some colorectal polyps. Submucosal injection also allows assessment for a “nonlifting sign,” which raises suspicion for invasive carcinoma but can also occur with benign submucosal fibrosis from previous biopsy, partial resection, or adjacent tattoo. In these cases, effective management can still be achieved using EMR in combination with avulsion and thermal ablation techniques.
Alternative techniques include cold EMR and underwater EMR.1,4 These are gaining popularity because of their excellent safety profile and favorable outcomes. Cold EMR involves submucosal injection followed by cold-snare resection, eliminating the use of cautery and its associated risks. Cold EMR is very safe and effective for small polyps, and we use this for progressively larger polyps given the low complication rate. Despite the need for piecemeal resection of polyps larger than 10 mm, local recurrence rates are comparable to conventional EMR. Sessile serrated polyps are especially ideal for piecemeal cold EMR. Meanwhile, underwater EMR eliminates the need for submucosal injection by utilizing water immersion, which elevates the mucosal lesion away from the muscularis layer. Either hot or cold snare resection can be performed. Benefits include reduced procedure time and cost, and relatively low complication and recurrence rates, compared with conventional EMR. I find this to be a nice option for laterally spreading polyps, with potentially higher rates of en bloc resection.1,4
ESD involves similar techniques but includes careful dissection of the submucosal layer beneath the lesion. In addition to the tools for EMR, a specialized electrosurgical knife is necessary, as well as dedicated training and mentorship that can be difficult to accommodate for an active endoscopist in practice. The primary advantage of ESD is higher en bloc resection rates for larger and potentially deeper lesions, with accurate histologic assessment and staging, and very low recurrence rates.1,4,5 However, ESD is more complex, technically challenging, and time and resource intensive, with higher risk of complications. Intraprocedural bleeding is common and requires immediate management. Additional risks include 2% risk of delayed bleeding and 5% risk of perforation.1,5 ESD involves an operating room, longer procedure times, and higher cost including surgical, anesthesia, and nursing costs. Some of this may be balanced by reduced frequency of surveillance and therapeutic procedures. While both EMR and ESD carry significant cost savings, compared with surgery, ESD is additionally disadvantaged by lack of reimbursement.
Regardless of the technique, EMR is easier to learn and perform than ESD, uses a limited number of devices that are readily available, and carries lower cost-burden. EMR is successful for most colorectal polyps, with the primary disadvantage being piecemeal resection of larger polyps. The rates of adverse events are lower, and appropriate surveillance is essential to ensuring complete resection and eliminating recurrence. Japanese and European guidelines endorse ESD for lesions that have a high likelihood of cancer invading the submucosa and for lesions that cannot be removed by EMR because of submucosal fibrosis. Ultimately, patients need to be treated individually with the most appropriate technique.
Dr. Tewani of Rockford Gastroenterology Associates is clinical assistant professor of medicine at the University of Illinois, Rockford. He has no relevant conflicts of interest to disclose.
References
1. Rashid MU et al. Surg Oncol. 2022 Mar 18;101742.
2. Law R et al. Gastrointest Endosc. 2016 Jun;83(6):1248-57.
3. Backes Y et al. BMC Gastroenterol. 2016 May 26;16(1):56.
4. Thiruvengadam SS et al. Gastroenterol Hepatol. 2022 Mar;18(3):133-44.
5. Wang J et al. World J Gastroenterol. 2014 Jul 7;20(25):8282-7l.
Common endoscopic procedure needs quality improvement
One of the most common procedures in gastroenterology – esophagogastroduodenoscopy (EGD) – needs to consistently meet quality measures, but data on interventions to improve them is lacking, according to a recent review.
Researchers, led by Fateh Bazerbachi, MD, with CentraCare, Interventional Endoscopy Program, at St. Cloud (Minn.) Hospital performed a systematic review of the literature to identify which interventions and measures have improved the performance of EGD quality indicators previously identified by the American Society for Gastrointestinal Endoscopy. They also looked for demonstrations of improving compliance with the prioritized indicators. The review appeared in Gastrointestinal Endoscopy.
The authors pointed out that more than 6.1 million EGDs are performed every year in the United States. Although gastroenterologists perform most of them, other providers also perform them, including primary care physicians, surgeons, and sometimes advanced practice providers. Therefore, establishing well-defined quality measures is critical for consistent outcomes.
Daniel C. Buckles, MD, associate professor of gastroenterology, hepatology & motility at the University of Kansas Medical Center in Kansas City, who was not part of the review, said high-quality EGDs are critical for many reasons, including avoiding overuse when results of the procedure are not likely to change a patient’s treatment but add risk to the patient and increase costs to the health care system.
“Lack of training to recognize important GI pathology seen on an EGD and lack of standardized reporting of GI abnormalities using validated classification systems can lead to suboptimal treatment and follow-up for patients,” he noted.
Testing provider adherence to guidelines years after publication helps providers understand what works and can improve outcomes, Dr. Buckles said.
Dr. Buckles said that one of the highlights of the review was that researchers were able to confidently say that use of standardized checklists and frequent auditing had value in improving preprocedural and postprocedural quality indicators.
“The authors also concluded that focused educational interventions might improve endoscopists’ abilities to adhere to standardized Barrett’s esophagus examinations,” he added. “Unfortunately, the authors were not able to find much evidence for interventions that would improve intraprocedural EGD quality indicators.”
The authors pointed to a prospective study that evaluated whether an audit intervention helped in 10,000 consecutive EGDs. They found the audits “improved EGD report quality, such as justification for incompleteness or accurate lesion/segment description, regardless of the endoscopist’s experience documenting the report (specialist vs. trainee).” When audits were used in other studies to evaluate endoscopy overall performance (including EGD and colonoscopy) results showed similar improvement in important endpoints, the authors wrote. Additionally, “use of dictation templates has been demonstrated to improve the completeness of endoscopy report.”
A study led by the European Network for the Investigation of Gastrointestinal Mucosal Alterations found inconsistent compliance with EGD biopsy–sampling guidelines in patients with signs of gastric pathology, even in academic centers. The authors of that study recommended dedicated educational programs to raise awareness of which scenarios warrant gastric sampling during EGD.
The authors of the current study also acknowledge that many sound practices that likely improve quality, for instance time-outs or checklists for administering antibiotics during percutaneous feeding tube placement, are unlikely to be formally studied.
“Nevertheless, such practices should be encouraged and monitored,” they wrote.
“This document synthesizes practices and interventions that may allow for a high-quality upper endoscopy,” they concluded. “Furthermore, the scarcity of strong data to support interventions that can improve important quality indicators in upper-GI endoscopy should be seen as an opportunity.”
Several authors disclosed relationships with commercial interests, such as Boston Scientific, Salix, and Janssen. Dr. Buckles reports no relevant financial relationships.
One of the most common procedures in gastroenterology – esophagogastroduodenoscopy (EGD) – needs to consistently meet quality measures, but data on interventions to improve them is lacking, according to a recent review.
Researchers, led by Fateh Bazerbachi, MD, with CentraCare, Interventional Endoscopy Program, at St. Cloud (Minn.) Hospital performed a systematic review of the literature to identify which interventions and measures have improved the performance of EGD quality indicators previously identified by the American Society for Gastrointestinal Endoscopy. They also looked for demonstrations of improving compliance with the prioritized indicators. The review appeared in Gastrointestinal Endoscopy.
The authors pointed out that more than 6.1 million EGDs are performed every year in the United States. Although gastroenterologists perform most of them, other providers also perform them, including primary care physicians, surgeons, and sometimes advanced practice providers. Therefore, establishing well-defined quality measures is critical for consistent outcomes.
Daniel C. Buckles, MD, associate professor of gastroenterology, hepatology & motility at the University of Kansas Medical Center in Kansas City, who was not part of the review, said high-quality EGDs are critical for many reasons, including avoiding overuse when results of the procedure are not likely to change a patient’s treatment but add risk to the patient and increase costs to the health care system.
“Lack of training to recognize important GI pathology seen on an EGD and lack of standardized reporting of GI abnormalities using validated classification systems can lead to suboptimal treatment and follow-up for patients,” he noted.
Testing provider adherence to guidelines years after publication helps providers understand what works and can improve outcomes, Dr. Buckles said.
Dr. Buckles said that one of the highlights of the review was that researchers were able to confidently say that use of standardized checklists and frequent auditing had value in improving preprocedural and postprocedural quality indicators.
“The authors also concluded that focused educational interventions might improve endoscopists’ abilities to adhere to standardized Barrett’s esophagus examinations,” he added. “Unfortunately, the authors were not able to find much evidence for interventions that would improve intraprocedural EGD quality indicators.”
The authors pointed to a prospective study that evaluated whether an audit intervention helped in 10,000 consecutive EGDs. They found the audits “improved EGD report quality, such as justification for incompleteness or accurate lesion/segment description, regardless of the endoscopist’s experience documenting the report (specialist vs. trainee).” When audits were used in other studies to evaluate endoscopy overall performance (including EGD and colonoscopy) results showed similar improvement in important endpoints, the authors wrote. Additionally, “use of dictation templates has been demonstrated to improve the completeness of endoscopy report.”
A study led by the European Network for the Investigation of Gastrointestinal Mucosal Alterations found inconsistent compliance with EGD biopsy–sampling guidelines in patients with signs of gastric pathology, even in academic centers. The authors of that study recommended dedicated educational programs to raise awareness of which scenarios warrant gastric sampling during EGD.
The authors of the current study also acknowledge that many sound practices that likely improve quality, for instance time-outs or checklists for administering antibiotics during percutaneous feeding tube placement, are unlikely to be formally studied.
“Nevertheless, such practices should be encouraged and monitored,” they wrote.
“This document synthesizes practices and interventions that may allow for a high-quality upper endoscopy,” they concluded. “Furthermore, the scarcity of strong data to support interventions that can improve important quality indicators in upper-GI endoscopy should be seen as an opportunity.”
Several authors disclosed relationships with commercial interests, such as Boston Scientific, Salix, and Janssen. Dr. Buckles reports no relevant financial relationships.
One of the most common procedures in gastroenterology – esophagogastroduodenoscopy (EGD) – needs to consistently meet quality measures, but data on interventions to improve them is lacking, according to a recent review.
Researchers, led by Fateh Bazerbachi, MD, with CentraCare, Interventional Endoscopy Program, at St. Cloud (Minn.) Hospital performed a systematic review of the literature to identify which interventions and measures have improved the performance of EGD quality indicators previously identified by the American Society for Gastrointestinal Endoscopy. They also looked for demonstrations of improving compliance with the prioritized indicators. The review appeared in Gastrointestinal Endoscopy.
The authors pointed out that more than 6.1 million EGDs are performed every year in the United States. Although gastroenterologists perform most of them, other providers also perform them, including primary care physicians, surgeons, and sometimes advanced practice providers. Therefore, establishing well-defined quality measures is critical for consistent outcomes.
Daniel C. Buckles, MD, associate professor of gastroenterology, hepatology & motility at the University of Kansas Medical Center in Kansas City, who was not part of the review, said high-quality EGDs are critical for many reasons, including avoiding overuse when results of the procedure are not likely to change a patient’s treatment but add risk to the patient and increase costs to the health care system.
“Lack of training to recognize important GI pathology seen on an EGD and lack of standardized reporting of GI abnormalities using validated classification systems can lead to suboptimal treatment and follow-up for patients,” he noted.
Testing provider adherence to guidelines years after publication helps providers understand what works and can improve outcomes, Dr. Buckles said.
Dr. Buckles said that one of the highlights of the review was that researchers were able to confidently say that use of standardized checklists and frequent auditing had value in improving preprocedural and postprocedural quality indicators.
“The authors also concluded that focused educational interventions might improve endoscopists’ abilities to adhere to standardized Barrett’s esophagus examinations,” he added. “Unfortunately, the authors were not able to find much evidence for interventions that would improve intraprocedural EGD quality indicators.”
The authors pointed to a prospective study that evaluated whether an audit intervention helped in 10,000 consecutive EGDs. They found the audits “improved EGD report quality, such as justification for incompleteness or accurate lesion/segment description, regardless of the endoscopist’s experience documenting the report (specialist vs. trainee).” When audits were used in other studies to evaluate endoscopy overall performance (including EGD and colonoscopy) results showed similar improvement in important endpoints, the authors wrote. Additionally, “use of dictation templates has been demonstrated to improve the completeness of endoscopy report.”
A study led by the European Network for the Investigation of Gastrointestinal Mucosal Alterations found inconsistent compliance with EGD biopsy–sampling guidelines in patients with signs of gastric pathology, even in academic centers. The authors of that study recommended dedicated educational programs to raise awareness of which scenarios warrant gastric sampling during EGD.
The authors of the current study also acknowledge that many sound practices that likely improve quality, for instance time-outs or checklists for administering antibiotics during percutaneous feeding tube placement, are unlikely to be formally studied.
“Nevertheless, such practices should be encouraged and monitored,” they wrote.
“This document synthesizes practices and interventions that may allow for a high-quality upper endoscopy,” they concluded. “Furthermore, the scarcity of strong data to support interventions that can improve important quality indicators in upper-GI endoscopy should be seen as an opportunity.”
Several authors disclosed relationships with commercial interests, such as Boston Scientific, Salix, and Janssen. Dr. Buckles reports no relevant financial relationships.
FROM GASTROINTESTINAL ENDOSCOPY
Repeat endoscopy for deliberate foreign body ingestions
A 35-year-old female with a complex psychiatric history and polysubstance use presents to the emergency department following ingestion of three sewing needles. The patient has a long history of multiple suicide attempts and foreign-body ingestions requiring repeated endoscopy. Prior ingestions include, but are not limited to, razor blades, screws, toothbrushes, batteries, plastic cutlery, and shower curtain rings. The patient has had over 50 upper endoscopies within the past year in addition to a laryngoscopy and bronchoscopy for retrieval of foreign bodies. Despite intensive inpatient psychiatric treatment and outpatient behavioral therapy, the patient continues to present with recurrent ingestions, creating frustration among multiple health care providers. Are gastroenterologists obligated to perform repeated endoscopies for recurrent foreign-body ingestions? Is there a point at which it would be medically and ethically appropriate to defer endoscopy in this clinical scenario?
Deliberate foreign-body ingestion (DFBI) is a psychological disorder in which patients swallow nonnutritive objects. The disorder is commonly seen in young female patients with psychiatric disorders.1 It is also associated with substance abuse, intellectual disabilities, and malingering (such as external motivation to avoid jail). Of those with psychiatric disorders, repeat ingestions are primarily seen in patients with borderline personality disorder (BPD) or part of a syndrome of self-mutilation or attention-seeking behavior.2 Patients with BPD are thought to have atrophic changes in the brain causing neurocognitive dysfunction accounting for such behaviors.1 Self-injurious behavior is also associated with a history of abandonment and childhood abuse.3 Studies show that 85% of patients evaluated for DFBI have a prior psychiatric diagnosis and 84% of these patients have a history of prior ingestions.4
In this case, the patient’s needles were successfully removed endoscopically. The psychiatry service adjusted her medication regimen and conducted a prolonged behavioral therapy session focused on coping strategies and impulse control. The following morning, the patient managed to overpower her 24-hour 1:1 sitter to ingest a pen. Endoscopy was performed again, with successful removal of the pen.
Although intentional ingestions occur in a small subset of patients, DFBI utilizes significant hospital and fiscal resources. The startling economic impact of caring for these patients was demonstrated in a cost analysis at a large academic center in Rhode Island. It found 33 patients with repeated ingestions accounted for over 300 endoscopies in an 8-year period culminating in a total hospitalization cost of 2 million dollars per year.5 Another study estimated the average cost of a patient with DFBI per hospital visit to be $6,616 in the United States with an average length of stay of 5.6 days.6 The cost burden is largely caused by the repetitive nature of the clinical presentation and involvement of multiple disciplines, including emergency medicine, gastroenterology, anesthesia, psychiatry, social work, security services, and in some cases, otolaryngology, pulmonology, and surgery.
In addition to endoscopy, an inpatient admission for DFBI centers around preventing repeated ingestions. This entails constant observation by security or a sitter, limiting access to objects through restraints or room modifications, and psychiatry consultation for management of the underlying psychiatric disorder. Studies show this management approach rarely succeeds in preventing recurrent ingestions.6 Interestingly, data also shows inpatient psychiatric admission is not beneficial in preventing recurrent DFBI and can paradoxically increase the frequency of swallowing behavior in some patients.6 This patient failed multiple inpatient treatment programs and was noncompliant with outpatient therapies. Given the costly burden to the health care system and propensity of repeated behavior, should this patient continue to receive endoscopies? Would it ever be justifiable to forgo endoscopic retrieval?
One of the fundamental principles of medical ethics is beneficence, supporting the notion that all providers should act in the best interest of the patient. Adults may make poor or self-destructive choices, but that does not preclude our moral obligation to treat them. Patients with substance abuse disorders may repeatedly use emergency room services for acute intoxication and overdose treatment. An emergency department physician would not withhold Narcan from a patient simply because of the frequency of repeated overdoses. A similar rationale could be applied to patients with DFBI – they should undergo endoscopy if they are accepting of the risks/benefits of repeated procedures. Given that this patient’s repeated ingestions are suicide attempts, it could be argued that not removing the object would make a clinician complicit with a patient’s suicide attempt or intent of self-harm.
From an alternative vantage point, patients with repeated DFBI have an increased risk of complications with repeated endoscopy, especially when performed emergently. Patients may have an increased risk of aspiration because of insufficient preoperative fasting, and attempted removal of ingested needles and other sharp objects carries a high risk of penetrating trauma, bleeding, and perforation. The patient’s swallowing history predicts a high likelihood of repeat ingestion which, over time, makes subsequent endoscopies seem futile. Endoscopic treatment does not address the underlying problem and only serves as a temporary fix to bridge the patient to their next ingestion. Furthermore, the utilization of resources is substantial – namely, the repeated emergency use of anesthesia and operating room and endoscopy staff, as well as the psychiatry, surgical, internal medicine, and gastroenterology services. Inevitably, treatment of a patient such as this diverts limited health care resources away from other patients who may have equally or more pressing medical needs.
Despite the seemingly futile nature of these procedures and strain on resources, it would be difficult from a medicolegal perspective to justify withholding endoscopy. In 1986, the Emergency Medical Treatment and Labor Act was enacted that requires anyone presenting to an emergency department to be stabilized and treated.7 In this particular patient case, an ethics consultation was obtained and recommended that the patient continue to undergo endoscopy. However, the team also suggested that a multidisciplinary meeting with ethics, the primary and procedural teams, and the hospital’s medicolegal department be held to further elucidate a plan for future admissions and to decide if or when it may be appropriate to withhold invasive procedures. This case was presented at our weekly gastroenterology grand rounds, and procedural guidelines were reviewed. Given the size and nature of most of the objects the patient ingests, we reviewed that it would be safe in the majority of scenarios to wait until the morning for removal if called overnight – providing some relief to those on call while minimizing utilization of emergency anesthesia resources as well as operating room and endoscopy staff.
Caring for these patients is challenging as providers may feel frustrated and angry after repeated admissions. The patient may sense the low morale from providers and feel judged for their actions. It is theorized that this leads to repeated ingestions as a defense mechanism and a means of acting out.1 Additionally, friction can develop between teams as there is a common perception that psychiatry is not “doing enough” to treat the psychiatric disorder to prevent recurrences.8
In conclusion, DFBIs occur in a small number of patients with psychiatric disorders, but account for a large utilization of health care recourses. Gastroenterologists have an ethical and legal obligation to provide treatment including repeat endoscopies as long as the therapeutic benefit of the procedure outweighs risks. A multidisciplinary approach with individualized care plans can help prevent recurrent hospitalizations and procedures which may, in turn, improve outcomes and reduce health care costs.1 Until the patient and clinicians can successfully mitigate the psychiatric and social factors perpetuating repeated ingestions, gastroenterologists will continue to provide endoscopic management. Individual cases should be discussed with the hospital’s ethics and medicolegal teams for further guidance on deferring endoscopic treatment in cases of medically refractory psychological disease.
Dr. Sims is a gastroenterology fellow in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. Dr. Rao is assistant professor in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. They had no conflicts of interest to disclose.
References
1. Bangash F et al. Cureus. 2021 Feb;13(2):e13179. doi: 10.7759/cureus.13179
2. Palese C et al. Gastroenterol Hepatol (N Y). 2012 July;8(7):485-6
3. Gitlin GF et al. Psychosomatics, 2007 March;48(2):162-6. doi: 10.1176/appi.psy.48.2.162
4. Palta R et al. Gastrointest Endosc. 2009 March;69(3):426-33. doi: 10.1016/j.gie.2008.05.072
5. Huang BL et al. Clin Gastroenterol Hepatol. 2010 Nov;8(11):941-6. doi: 10.1016/j.cgh.2010.07.013
6. Poynter BA et al. Gen Hosp Psychiatry. 2011 Sep-Oct;33(5):518-24. doi: 10.1016/j.genhosppsych.2011.06.011
7. American College of Emergency Physicians, EMTALA Fact Sheet. https://www.acep.org/life-as-a-physician/ethics--legal/emtala/emtala-fact-sheet/
8. Grzenda A. Carlat Hosp Psych Report. 2021 Jan;1(1 ):5-9
A 35-year-old female with a complex psychiatric history and polysubstance use presents to the emergency department following ingestion of three sewing needles. The patient has a long history of multiple suicide attempts and foreign-body ingestions requiring repeated endoscopy. Prior ingestions include, but are not limited to, razor blades, screws, toothbrushes, batteries, plastic cutlery, and shower curtain rings. The patient has had over 50 upper endoscopies within the past year in addition to a laryngoscopy and bronchoscopy for retrieval of foreign bodies. Despite intensive inpatient psychiatric treatment and outpatient behavioral therapy, the patient continues to present with recurrent ingestions, creating frustration among multiple health care providers. Are gastroenterologists obligated to perform repeated endoscopies for recurrent foreign-body ingestions? Is there a point at which it would be medically and ethically appropriate to defer endoscopy in this clinical scenario?
Deliberate foreign-body ingestion (DFBI) is a psychological disorder in which patients swallow nonnutritive objects. The disorder is commonly seen in young female patients with psychiatric disorders.1 It is also associated with substance abuse, intellectual disabilities, and malingering (such as external motivation to avoid jail). Of those with psychiatric disorders, repeat ingestions are primarily seen in patients with borderline personality disorder (BPD) or part of a syndrome of self-mutilation or attention-seeking behavior.2 Patients with BPD are thought to have atrophic changes in the brain causing neurocognitive dysfunction accounting for such behaviors.1 Self-injurious behavior is also associated with a history of abandonment and childhood abuse.3 Studies show that 85% of patients evaluated for DFBI have a prior psychiatric diagnosis and 84% of these patients have a history of prior ingestions.4
In this case, the patient’s needles were successfully removed endoscopically. The psychiatry service adjusted her medication regimen and conducted a prolonged behavioral therapy session focused on coping strategies and impulse control. The following morning, the patient managed to overpower her 24-hour 1:1 sitter to ingest a pen. Endoscopy was performed again, with successful removal of the pen.
Although intentional ingestions occur in a small subset of patients, DFBI utilizes significant hospital and fiscal resources. The startling economic impact of caring for these patients was demonstrated in a cost analysis at a large academic center in Rhode Island. It found 33 patients with repeated ingestions accounted for over 300 endoscopies in an 8-year period culminating in a total hospitalization cost of 2 million dollars per year.5 Another study estimated the average cost of a patient with DFBI per hospital visit to be $6,616 in the United States with an average length of stay of 5.6 days.6 The cost burden is largely caused by the repetitive nature of the clinical presentation and involvement of multiple disciplines, including emergency medicine, gastroenterology, anesthesia, psychiatry, social work, security services, and in some cases, otolaryngology, pulmonology, and surgery.
In addition to endoscopy, an inpatient admission for DFBI centers around preventing repeated ingestions. This entails constant observation by security or a sitter, limiting access to objects through restraints or room modifications, and psychiatry consultation for management of the underlying psychiatric disorder. Studies show this management approach rarely succeeds in preventing recurrent ingestions.6 Interestingly, data also shows inpatient psychiatric admission is not beneficial in preventing recurrent DFBI and can paradoxically increase the frequency of swallowing behavior in some patients.6 This patient failed multiple inpatient treatment programs and was noncompliant with outpatient therapies. Given the costly burden to the health care system and propensity of repeated behavior, should this patient continue to receive endoscopies? Would it ever be justifiable to forgo endoscopic retrieval?
One of the fundamental principles of medical ethics is beneficence, supporting the notion that all providers should act in the best interest of the patient. Adults may make poor or self-destructive choices, but that does not preclude our moral obligation to treat them. Patients with substance abuse disorders may repeatedly use emergency room services for acute intoxication and overdose treatment. An emergency department physician would not withhold Narcan from a patient simply because of the frequency of repeated overdoses. A similar rationale could be applied to patients with DFBI – they should undergo endoscopy if they are accepting of the risks/benefits of repeated procedures. Given that this patient’s repeated ingestions are suicide attempts, it could be argued that not removing the object would make a clinician complicit with a patient’s suicide attempt or intent of self-harm.
From an alternative vantage point, patients with repeated DFBI have an increased risk of complications with repeated endoscopy, especially when performed emergently. Patients may have an increased risk of aspiration because of insufficient preoperative fasting, and attempted removal of ingested needles and other sharp objects carries a high risk of penetrating trauma, bleeding, and perforation. The patient’s swallowing history predicts a high likelihood of repeat ingestion which, over time, makes subsequent endoscopies seem futile. Endoscopic treatment does not address the underlying problem and only serves as a temporary fix to bridge the patient to their next ingestion. Furthermore, the utilization of resources is substantial – namely, the repeated emergency use of anesthesia and operating room and endoscopy staff, as well as the psychiatry, surgical, internal medicine, and gastroenterology services. Inevitably, treatment of a patient such as this diverts limited health care resources away from other patients who may have equally or more pressing medical needs.
Despite the seemingly futile nature of these procedures and strain on resources, it would be difficult from a medicolegal perspective to justify withholding endoscopy. In 1986, the Emergency Medical Treatment and Labor Act was enacted that requires anyone presenting to an emergency department to be stabilized and treated.7 In this particular patient case, an ethics consultation was obtained and recommended that the patient continue to undergo endoscopy. However, the team also suggested that a multidisciplinary meeting with ethics, the primary and procedural teams, and the hospital’s medicolegal department be held to further elucidate a plan for future admissions and to decide if or when it may be appropriate to withhold invasive procedures. This case was presented at our weekly gastroenterology grand rounds, and procedural guidelines were reviewed. Given the size and nature of most of the objects the patient ingests, we reviewed that it would be safe in the majority of scenarios to wait until the morning for removal if called overnight – providing some relief to those on call while minimizing utilization of emergency anesthesia resources as well as operating room and endoscopy staff.
Caring for these patients is challenging as providers may feel frustrated and angry after repeated admissions. The patient may sense the low morale from providers and feel judged for their actions. It is theorized that this leads to repeated ingestions as a defense mechanism and a means of acting out.1 Additionally, friction can develop between teams as there is a common perception that psychiatry is not “doing enough” to treat the psychiatric disorder to prevent recurrences.8
In conclusion, DFBIs occur in a small number of patients with psychiatric disorders, but account for a large utilization of health care recourses. Gastroenterologists have an ethical and legal obligation to provide treatment including repeat endoscopies as long as the therapeutic benefit of the procedure outweighs risks. A multidisciplinary approach with individualized care plans can help prevent recurrent hospitalizations and procedures which may, in turn, improve outcomes and reduce health care costs.1 Until the patient and clinicians can successfully mitigate the psychiatric and social factors perpetuating repeated ingestions, gastroenterologists will continue to provide endoscopic management. Individual cases should be discussed with the hospital’s ethics and medicolegal teams for further guidance on deferring endoscopic treatment in cases of medically refractory psychological disease.
Dr. Sims is a gastroenterology fellow in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. Dr. Rao is assistant professor in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. They had no conflicts of interest to disclose.
References
1. Bangash F et al. Cureus. 2021 Feb;13(2):e13179. doi: 10.7759/cureus.13179
2. Palese C et al. Gastroenterol Hepatol (N Y). 2012 July;8(7):485-6
3. Gitlin GF et al. Psychosomatics, 2007 March;48(2):162-6. doi: 10.1176/appi.psy.48.2.162
4. Palta R et al. Gastrointest Endosc. 2009 March;69(3):426-33. doi: 10.1016/j.gie.2008.05.072
5. Huang BL et al. Clin Gastroenterol Hepatol. 2010 Nov;8(11):941-6. doi: 10.1016/j.cgh.2010.07.013
6. Poynter BA et al. Gen Hosp Psychiatry. 2011 Sep-Oct;33(5):518-24. doi: 10.1016/j.genhosppsych.2011.06.011
7. American College of Emergency Physicians, EMTALA Fact Sheet. https://www.acep.org/life-as-a-physician/ethics--legal/emtala/emtala-fact-sheet/
8. Grzenda A. Carlat Hosp Psych Report. 2021 Jan;1(1 ):5-9
A 35-year-old female with a complex psychiatric history and polysubstance use presents to the emergency department following ingestion of three sewing needles. The patient has a long history of multiple suicide attempts and foreign-body ingestions requiring repeated endoscopy. Prior ingestions include, but are not limited to, razor blades, screws, toothbrushes, batteries, plastic cutlery, and shower curtain rings. The patient has had over 50 upper endoscopies within the past year in addition to a laryngoscopy and bronchoscopy for retrieval of foreign bodies. Despite intensive inpatient psychiatric treatment and outpatient behavioral therapy, the patient continues to present with recurrent ingestions, creating frustration among multiple health care providers. Are gastroenterologists obligated to perform repeated endoscopies for recurrent foreign-body ingestions? Is there a point at which it would be medically and ethically appropriate to defer endoscopy in this clinical scenario?
Deliberate foreign-body ingestion (DFBI) is a psychological disorder in which patients swallow nonnutritive objects. The disorder is commonly seen in young female patients with psychiatric disorders.1 It is also associated with substance abuse, intellectual disabilities, and malingering (such as external motivation to avoid jail). Of those with psychiatric disorders, repeat ingestions are primarily seen in patients with borderline personality disorder (BPD) or part of a syndrome of self-mutilation or attention-seeking behavior.2 Patients with BPD are thought to have atrophic changes in the brain causing neurocognitive dysfunction accounting for such behaviors.1 Self-injurious behavior is also associated with a history of abandonment and childhood abuse.3 Studies show that 85% of patients evaluated for DFBI have a prior psychiatric diagnosis and 84% of these patients have a history of prior ingestions.4
In this case, the patient’s needles were successfully removed endoscopically. The psychiatry service adjusted her medication regimen and conducted a prolonged behavioral therapy session focused on coping strategies and impulse control. The following morning, the patient managed to overpower her 24-hour 1:1 sitter to ingest a pen. Endoscopy was performed again, with successful removal of the pen.
Although intentional ingestions occur in a small subset of patients, DFBI utilizes significant hospital and fiscal resources. The startling economic impact of caring for these patients was demonstrated in a cost analysis at a large academic center in Rhode Island. It found 33 patients with repeated ingestions accounted for over 300 endoscopies in an 8-year period culminating in a total hospitalization cost of 2 million dollars per year.5 Another study estimated the average cost of a patient with DFBI per hospital visit to be $6,616 in the United States with an average length of stay of 5.6 days.6 The cost burden is largely caused by the repetitive nature of the clinical presentation and involvement of multiple disciplines, including emergency medicine, gastroenterology, anesthesia, psychiatry, social work, security services, and in some cases, otolaryngology, pulmonology, and surgery.
In addition to endoscopy, an inpatient admission for DFBI centers around preventing repeated ingestions. This entails constant observation by security or a sitter, limiting access to objects through restraints or room modifications, and psychiatry consultation for management of the underlying psychiatric disorder. Studies show this management approach rarely succeeds in preventing recurrent ingestions.6 Interestingly, data also shows inpatient psychiatric admission is not beneficial in preventing recurrent DFBI and can paradoxically increase the frequency of swallowing behavior in some patients.6 This patient failed multiple inpatient treatment programs and was noncompliant with outpatient therapies. Given the costly burden to the health care system and propensity of repeated behavior, should this patient continue to receive endoscopies? Would it ever be justifiable to forgo endoscopic retrieval?
One of the fundamental principles of medical ethics is beneficence, supporting the notion that all providers should act in the best interest of the patient. Adults may make poor or self-destructive choices, but that does not preclude our moral obligation to treat them. Patients with substance abuse disorders may repeatedly use emergency room services for acute intoxication and overdose treatment. An emergency department physician would not withhold Narcan from a patient simply because of the frequency of repeated overdoses. A similar rationale could be applied to patients with DFBI – they should undergo endoscopy if they are accepting of the risks/benefits of repeated procedures. Given that this patient’s repeated ingestions are suicide attempts, it could be argued that not removing the object would make a clinician complicit with a patient’s suicide attempt or intent of self-harm.
From an alternative vantage point, patients with repeated DFBI have an increased risk of complications with repeated endoscopy, especially when performed emergently. Patients may have an increased risk of aspiration because of insufficient preoperative fasting, and attempted removal of ingested needles and other sharp objects carries a high risk of penetrating trauma, bleeding, and perforation. The patient’s swallowing history predicts a high likelihood of repeat ingestion which, over time, makes subsequent endoscopies seem futile. Endoscopic treatment does not address the underlying problem and only serves as a temporary fix to bridge the patient to their next ingestion. Furthermore, the utilization of resources is substantial – namely, the repeated emergency use of anesthesia and operating room and endoscopy staff, as well as the psychiatry, surgical, internal medicine, and gastroenterology services. Inevitably, treatment of a patient such as this diverts limited health care resources away from other patients who may have equally or more pressing medical needs.
Despite the seemingly futile nature of these procedures and strain on resources, it would be difficult from a medicolegal perspective to justify withholding endoscopy. In 1986, the Emergency Medical Treatment and Labor Act was enacted that requires anyone presenting to an emergency department to be stabilized and treated.7 In this particular patient case, an ethics consultation was obtained and recommended that the patient continue to undergo endoscopy. However, the team also suggested that a multidisciplinary meeting with ethics, the primary and procedural teams, and the hospital’s medicolegal department be held to further elucidate a plan for future admissions and to decide if or when it may be appropriate to withhold invasive procedures. This case was presented at our weekly gastroenterology grand rounds, and procedural guidelines were reviewed. Given the size and nature of most of the objects the patient ingests, we reviewed that it would be safe in the majority of scenarios to wait until the morning for removal if called overnight – providing some relief to those on call while minimizing utilization of emergency anesthesia resources as well as operating room and endoscopy staff.
Caring for these patients is challenging as providers may feel frustrated and angry after repeated admissions. The patient may sense the low morale from providers and feel judged for their actions. It is theorized that this leads to repeated ingestions as a defense mechanism and a means of acting out.1 Additionally, friction can develop between teams as there is a common perception that psychiatry is not “doing enough” to treat the psychiatric disorder to prevent recurrences.8
In conclusion, DFBIs occur in a small number of patients with psychiatric disorders, but account for a large utilization of health care recourses. Gastroenterologists have an ethical and legal obligation to provide treatment including repeat endoscopies as long as the therapeutic benefit of the procedure outweighs risks. A multidisciplinary approach with individualized care plans can help prevent recurrent hospitalizations and procedures which may, in turn, improve outcomes and reduce health care costs.1 Until the patient and clinicians can successfully mitigate the psychiatric and social factors perpetuating repeated ingestions, gastroenterologists will continue to provide endoscopic management. Individual cases should be discussed with the hospital’s ethics and medicolegal teams for further guidance on deferring endoscopic treatment in cases of medically refractory psychological disease.
Dr. Sims is a gastroenterology fellow in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. Dr. Rao is assistant professor in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. They had no conflicts of interest to disclose.
References
1. Bangash F et al. Cureus. 2021 Feb;13(2):e13179. doi: 10.7759/cureus.13179
2. Palese C et al. Gastroenterol Hepatol (N Y). 2012 July;8(7):485-6
3. Gitlin GF et al. Psychosomatics, 2007 March;48(2):162-6. doi: 10.1176/appi.psy.48.2.162
4. Palta R et al. Gastrointest Endosc. 2009 March;69(3):426-33. doi: 10.1016/j.gie.2008.05.072
5. Huang BL et al. Clin Gastroenterol Hepatol. 2010 Nov;8(11):941-6. doi: 10.1016/j.cgh.2010.07.013
6. Poynter BA et al. Gen Hosp Psychiatry. 2011 Sep-Oct;33(5):518-24. doi: 10.1016/j.genhosppsych.2011.06.011
7. American College of Emergency Physicians, EMTALA Fact Sheet. https://www.acep.org/life-as-a-physician/ethics--legal/emtala/emtala-fact-sheet/
8. Grzenda A. Carlat Hosp Psych Report. 2021 Jan;1(1 ):5-9
AI-based CADe outperforms high-definition white light in colonoscopy
An artificial intelligence (AI)–based computer-aided polyp detection (CADe) system missed fewer adenomas, polyps, and sessile serrated lesions and identified more adenomas per colonoscopy than a high-definition white light (HDWL) colonoscopy, according to findings from a randomized study.
While adenoma detection by colonoscopy is associated with a reduced risk of interval colon cancer, detection rates of adenomas vary among physicians. AI approaches, such as machine learning and deep learning, may improve adenoma detection rates during colonoscopy and thus potentially improve outcomes for patients, suggested study authors led by Jeremy R. Glissen Brown, MD, of the Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, who reported their trial findings in Clinical Gastroenterology and Hepatology.
The investigators explained that, although AI approaches may offer benefits in adenoma detection, there have been no prospective data for U.S. populations on the efficacy of an AI-based CADe system for improving adenoma detection rates (ADRs) and reducing adenoma miss rates (AMRs). To overcome this research gap, the investigators performed a prospective, multicenter, single-blind randomized tandem colonoscopy study which assessed a deep learning–based CADe system in 232 patients.
Individuals who presented to the four included U.S. medical centers for either colorectal cancer screening or surveillance were randomly assigned to the CADe system colonoscopy first (n = 116) or HDWL colonoscopy first (n = 116). This was immediately followed by the other procedure, in tandem fashion, performed by the same endoscopist. AMR was the primary outcome of interest, while secondary outcomes were adenomas per colonoscopy (APC) and the miss rate of sessile serrated lesions (SSL).
The researchers excluded 9 patients, which resulted in a total patient population of 223 patients. Approximately 45.3% of the cohort was female, 67.7% were White, and 21% were Black. Most patients (60%) were indicated for primary colorectal cancer screening.
Compared with the HDWL-first group, the AMR was significantly lower in the CADe-first group (31.25% vs. 20.12%, respectively; P = .0247). The researchers commented that, although the CADe system resulted in a statistically significantly lower AMR, the rate still reflects missed adenomas.
Additionally, the CADe-first group had a lower SSL miss rate, compared with the HDWL-first group (7.14% vs. 42.11%, respectively; P = .0482). The researchers noted that their study is one of the first research studies to show that a computer-assisted polyp detection system can reduce the SSL miss rate. The first-pass APC was also significantly higher in the CADe-first group (1.19 vs. 0.90; P = .0323). No statistically significant difference was observed between the groups in regard to the first-pass ADR (50.44% for the CADe-first group vs. 43.64 % for the HDWL-first group; P = .3091).
A multivariate logistic regression analysis identified three significant factors predictive of missed polyps: use of HDWL first vs. the computer-assisted detection system first (odds ratio, 1.8830; P = .0214), age 65 years or younger (OR, 1.7390; P = .0451), and right colon vs. other location (OR, 1.7865; P = .0436).
According to the researchers, the study was not powered to identify differences in ADR, thereby limiting the interpretation of this analysis. In addition, the investigators noted that the tandem colonoscopy study design is limited in its generalizability to real-world clinical settings. Also, given that endoscopists were not blinded to group assignments while performing each withdrawal, the researchers commented that “it is possible that endoscopist performance was influenced by being observed or that endoscopists who participated for the length of the study became over-reliant on” the CADe system during withdrawal, resulting in an underestimate or overestimation of the system’s performance.
The authors concluded that their findings suggest that an AI-based CADe system with colonoscopy “has the potential to decrease interprovider variability in colonoscopy quality by reducing AMR, even in experienced providers.”
This was an investigator-initiated study, with research software and study funding provided by Wision AI. The investigators reported relationships with Wision AI, as well as Olympus, Fujifilm, and Medtronic.
Several randomized trials testing artificial intelligence (AI)–assisted colonoscopy showed improvement in adenoma detection. This study adds to the growing body of evidence that computer-aided detection (CADe) systems for adenoma augment adenoma detection rates, even among highly skilled endoscopists whose baseline ADRs are much higher than the currently recommended threshold for quality colonoscopy (25%).
This study also highlights the usefulness of CADe in aiding detection of sessile serrated lesions (SSL). Recognition of SSL appears to be challenging for trainees and the most likely type of missed large adenomas overall.
AI-based systems will enhance but will not replace the highly skilled operator. As this study pointed out, despite the superior ADR, adenomas were still missed by CADe. The main reason for this was that the missed polyps were not brought into the visual field by the operator. A combination of a CADe program and a distal attachment mucosa exposure device in the hands of an experienced endoscopists might bring the best results.
Monika Fischer, MD, is an associate professor of medicine at Indiana University, Indianapolis. She reported no relevant conflicts of interest.
Several randomized trials testing artificial intelligence (AI)–assisted colonoscopy showed improvement in adenoma detection. This study adds to the growing body of evidence that computer-aided detection (CADe) systems for adenoma augment adenoma detection rates, even among highly skilled endoscopists whose baseline ADRs are much higher than the currently recommended threshold for quality colonoscopy (25%).
This study also highlights the usefulness of CADe in aiding detection of sessile serrated lesions (SSL). Recognition of SSL appears to be challenging for trainees and the most likely type of missed large adenomas overall.
AI-based systems will enhance but will not replace the highly skilled operator. As this study pointed out, despite the superior ADR, adenomas were still missed by CADe. The main reason for this was that the missed polyps were not brought into the visual field by the operator. A combination of a CADe program and a distal attachment mucosa exposure device in the hands of an experienced endoscopists might bring the best results.
Monika Fischer, MD, is an associate professor of medicine at Indiana University, Indianapolis. She reported no relevant conflicts of interest.
Several randomized trials testing artificial intelligence (AI)–assisted colonoscopy showed improvement in adenoma detection. This study adds to the growing body of evidence that computer-aided detection (CADe) systems for adenoma augment adenoma detection rates, even among highly skilled endoscopists whose baseline ADRs are much higher than the currently recommended threshold for quality colonoscopy (25%).
This study also highlights the usefulness of CADe in aiding detection of sessile serrated lesions (SSL). Recognition of SSL appears to be challenging for trainees and the most likely type of missed large adenomas overall.
AI-based systems will enhance but will not replace the highly skilled operator. As this study pointed out, despite the superior ADR, adenomas were still missed by CADe. The main reason for this was that the missed polyps were not brought into the visual field by the operator. A combination of a CADe program and a distal attachment mucosa exposure device in the hands of an experienced endoscopists might bring the best results.
Monika Fischer, MD, is an associate professor of medicine at Indiana University, Indianapolis. She reported no relevant conflicts of interest.
An artificial intelligence (AI)–based computer-aided polyp detection (CADe) system missed fewer adenomas, polyps, and sessile serrated lesions and identified more adenomas per colonoscopy than a high-definition white light (HDWL) colonoscopy, according to findings from a randomized study.
While adenoma detection by colonoscopy is associated with a reduced risk of interval colon cancer, detection rates of adenomas vary among physicians. AI approaches, such as machine learning and deep learning, may improve adenoma detection rates during colonoscopy and thus potentially improve outcomes for patients, suggested study authors led by Jeremy R. Glissen Brown, MD, of the Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, who reported their trial findings in Clinical Gastroenterology and Hepatology.
The investigators explained that, although AI approaches may offer benefits in adenoma detection, there have been no prospective data for U.S. populations on the efficacy of an AI-based CADe system for improving adenoma detection rates (ADRs) and reducing adenoma miss rates (AMRs). To overcome this research gap, the investigators performed a prospective, multicenter, single-blind randomized tandem colonoscopy study which assessed a deep learning–based CADe system in 232 patients.
Individuals who presented to the four included U.S. medical centers for either colorectal cancer screening or surveillance were randomly assigned to the CADe system colonoscopy first (n = 116) or HDWL colonoscopy first (n = 116). This was immediately followed by the other procedure, in tandem fashion, performed by the same endoscopist. AMR was the primary outcome of interest, while secondary outcomes were adenomas per colonoscopy (APC) and the miss rate of sessile serrated lesions (SSL).
The researchers excluded 9 patients, which resulted in a total patient population of 223 patients. Approximately 45.3% of the cohort was female, 67.7% were White, and 21% were Black. Most patients (60%) were indicated for primary colorectal cancer screening.
Compared with the HDWL-first group, the AMR was significantly lower in the CADe-first group (31.25% vs. 20.12%, respectively; P = .0247). The researchers commented that, although the CADe system resulted in a statistically significantly lower AMR, the rate still reflects missed adenomas.
Additionally, the CADe-first group had a lower SSL miss rate, compared with the HDWL-first group (7.14% vs. 42.11%, respectively; P = .0482). The researchers noted that their study is one of the first research studies to show that a computer-assisted polyp detection system can reduce the SSL miss rate. The first-pass APC was also significantly higher in the CADe-first group (1.19 vs. 0.90; P = .0323). No statistically significant difference was observed between the groups in regard to the first-pass ADR (50.44% for the CADe-first group vs. 43.64 % for the HDWL-first group; P = .3091).
A multivariate logistic regression analysis identified three significant factors predictive of missed polyps: use of HDWL first vs. the computer-assisted detection system first (odds ratio, 1.8830; P = .0214), age 65 years or younger (OR, 1.7390; P = .0451), and right colon vs. other location (OR, 1.7865; P = .0436).
According to the researchers, the study was not powered to identify differences in ADR, thereby limiting the interpretation of this analysis. In addition, the investigators noted that the tandem colonoscopy study design is limited in its generalizability to real-world clinical settings. Also, given that endoscopists were not blinded to group assignments while performing each withdrawal, the researchers commented that “it is possible that endoscopist performance was influenced by being observed or that endoscopists who participated for the length of the study became over-reliant on” the CADe system during withdrawal, resulting in an underestimate or overestimation of the system’s performance.
The authors concluded that their findings suggest that an AI-based CADe system with colonoscopy “has the potential to decrease interprovider variability in colonoscopy quality by reducing AMR, even in experienced providers.”
This was an investigator-initiated study, with research software and study funding provided by Wision AI. The investigators reported relationships with Wision AI, as well as Olympus, Fujifilm, and Medtronic.
An artificial intelligence (AI)–based computer-aided polyp detection (CADe) system missed fewer adenomas, polyps, and sessile serrated lesions and identified more adenomas per colonoscopy than a high-definition white light (HDWL) colonoscopy, according to findings from a randomized study.
While adenoma detection by colonoscopy is associated with a reduced risk of interval colon cancer, detection rates of adenomas vary among physicians. AI approaches, such as machine learning and deep learning, may improve adenoma detection rates during colonoscopy and thus potentially improve outcomes for patients, suggested study authors led by Jeremy R. Glissen Brown, MD, of the Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, who reported their trial findings in Clinical Gastroenterology and Hepatology.
The investigators explained that, although AI approaches may offer benefits in adenoma detection, there have been no prospective data for U.S. populations on the efficacy of an AI-based CADe system for improving adenoma detection rates (ADRs) and reducing adenoma miss rates (AMRs). To overcome this research gap, the investigators performed a prospective, multicenter, single-blind randomized tandem colonoscopy study which assessed a deep learning–based CADe system in 232 patients.
Individuals who presented to the four included U.S. medical centers for either colorectal cancer screening or surveillance were randomly assigned to the CADe system colonoscopy first (n = 116) or HDWL colonoscopy first (n = 116). This was immediately followed by the other procedure, in tandem fashion, performed by the same endoscopist. AMR was the primary outcome of interest, while secondary outcomes were adenomas per colonoscopy (APC) and the miss rate of sessile serrated lesions (SSL).
The researchers excluded 9 patients, which resulted in a total patient population of 223 patients. Approximately 45.3% of the cohort was female, 67.7% were White, and 21% were Black. Most patients (60%) were indicated for primary colorectal cancer screening.
Compared with the HDWL-first group, the AMR was significantly lower in the CADe-first group (31.25% vs. 20.12%, respectively; P = .0247). The researchers commented that, although the CADe system resulted in a statistically significantly lower AMR, the rate still reflects missed adenomas.
Additionally, the CADe-first group had a lower SSL miss rate, compared with the HDWL-first group (7.14% vs. 42.11%, respectively; P = .0482). The researchers noted that their study is one of the first research studies to show that a computer-assisted polyp detection system can reduce the SSL miss rate. The first-pass APC was also significantly higher in the CADe-first group (1.19 vs. 0.90; P = .0323). No statistically significant difference was observed between the groups in regard to the first-pass ADR (50.44% for the CADe-first group vs. 43.64 % for the HDWL-first group; P = .3091).
A multivariate logistic regression analysis identified three significant factors predictive of missed polyps: use of HDWL first vs. the computer-assisted detection system first (odds ratio, 1.8830; P = .0214), age 65 years or younger (OR, 1.7390; P = .0451), and right colon vs. other location (OR, 1.7865; P = .0436).
According to the researchers, the study was not powered to identify differences in ADR, thereby limiting the interpretation of this analysis. In addition, the investigators noted that the tandem colonoscopy study design is limited in its generalizability to real-world clinical settings. Also, given that endoscopists were not blinded to group assignments while performing each withdrawal, the researchers commented that “it is possible that endoscopist performance was influenced by being observed or that endoscopists who participated for the length of the study became over-reliant on” the CADe system during withdrawal, resulting in an underestimate or overestimation of the system’s performance.
The authors concluded that their findings suggest that an AI-based CADe system with colonoscopy “has the potential to decrease interprovider variability in colonoscopy quality by reducing AMR, even in experienced providers.”
This was an investigator-initiated study, with research software and study funding provided by Wision AI. The investigators reported relationships with Wision AI, as well as Olympus, Fujifilm, and Medtronic.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY