User login
EUS-guided RF ablation doubles survival for unresectable pancreatic cancer
CHARLOTTE, N.C. – In a small proof-of-concept study, patients with small unresectable pancreatic cancers treated with endoscopic ultrasound–guided radiofrequency ablation (EUS-RFA) had a more than twofold improvement in overall survival compared with historical controls with a similar disease history, investigators in Thailand found.
In a weighted analysis, median weighted overall survival – the primary outcome – was 14 months among 11 patients who underwent EUS-RFA, compared with 6.1 months for 35 matched controls, translating into a hazard ratio for death with EUS-RFA of 0.38 (P = .016), reported Chawin Lopimpisuth, MD, from King Chulalongkorn Memorial Hospital in Bangkok, Thailand.
Median weighted progression-free survival (PFS) was longer among cases than controls, but did not differ significantly, at 6.1 months and 3.9 months, respectively.
“In patients with unresectable pancreatic ductal adenocarcinomas that are less than 4 cm, EUS-RFA alone or combined with chemotherapy resulted in significantly improved overall survival and tended to improve progression-free survival with minimal adverse events,” Dr. Lopimpisuth reported at the annual meeting of the American College of Gastroenterology.
Small but unresectable tumors
Endoscopically guided radiofrequency ablation of pancreatic ductal tumors has been shown to be both feasible and safe in previous studies, he said, prompting his group to explore whether EUS-RFA could help to control the primary tumor and improve survival outcomes.
They enrolled 11 patients with primary pancreatic ductal adenocarcinoma tumors less than 4 cm in diameter that were unresectable due to blood vessel involvement or distant metastasis, and used propensity-score matching to pair them with a total of 35 controls. Controls were matched by tumor size, staging, age-adjusted Charlson Comorbidity Index, chemotherapy regimen received, and interactions between CCI, regimen, and staging.
The results were weighted to assure that covariate distribution among patients treated with chemotherapy only equaled that of patients who underwent EUS-RFA.
Patients underwent EUS-RFA with a 19-gauge needle, with 50 watts of energy delivered with an impedance of 100 ohms. Those patients deemed able to tolerate chemotherapy received that as well.
After a minimum of 1 year of follow-up, the median weighted survival, as noted before, was 14 months for patients who received EUS-RFA, compared with 6.1 months for controls.
Adjusted survival probabilities at 6 and 12 months were 73% and 64%, respectively, for patients in the EUS-RFA group, compared with 69% and 17% for controls. Adjusted PFS rates at 6 and 12 months were 55% and 36% in the EUS-RFA group, compared with 28% and 4% in the control group.
The only adverse event of significance was mild abdominal pain, reported by 8.3% of total EUS-RFA procedures.
Promising but preliminary
In an interview with this news organization, ACG President Samir A. Shah, MD, from Brown University and Miriam Hospital in Providence, R.I., who was not involved in the study, commented that “we have limited options with these patients, so it’s really exciting to see an initial trend toward efficacy, and their survival improvement was significant by several months.”
Dr. Shah was a moderator of the presidential symposium where the data were presented.
Comoderator Brooks D. Cash, MD, from the University of Texas Health Science Center at Houston, said that the advantage of EUS-RFA is that it’s only minimally invasive and appears to offer a significant survival advantage for patients with few effective treatment options.
He cautioned, however, that “it’s a small study and needs to be replicated in a larger venue and different sites as well, but I think it looks very promising.”
The investigators did not report a funding source for the study. Dr. Lopimpisuth, Dr. Shah, and Dr. Cash all reported having no relevant financial relationships to disclose.
CHARLOTTE, N.C. – In a small proof-of-concept study, patients with small unresectable pancreatic cancers treated with endoscopic ultrasound–guided radiofrequency ablation (EUS-RFA) had a more than twofold improvement in overall survival compared with historical controls with a similar disease history, investigators in Thailand found.
In a weighted analysis, median weighted overall survival – the primary outcome – was 14 months among 11 patients who underwent EUS-RFA, compared with 6.1 months for 35 matched controls, translating into a hazard ratio for death with EUS-RFA of 0.38 (P = .016), reported Chawin Lopimpisuth, MD, from King Chulalongkorn Memorial Hospital in Bangkok, Thailand.
Median weighted progression-free survival (PFS) was longer among cases than controls, but did not differ significantly, at 6.1 months and 3.9 months, respectively.
“In patients with unresectable pancreatic ductal adenocarcinomas that are less than 4 cm, EUS-RFA alone or combined with chemotherapy resulted in significantly improved overall survival and tended to improve progression-free survival with minimal adverse events,” Dr. Lopimpisuth reported at the annual meeting of the American College of Gastroenterology.
Small but unresectable tumors
Endoscopically guided radiofrequency ablation of pancreatic ductal tumors has been shown to be both feasible and safe in previous studies, he said, prompting his group to explore whether EUS-RFA could help to control the primary tumor and improve survival outcomes.
They enrolled 11 patients with primary pancreatic ductal adenocarcinoma tumors less than 4 cm in diameter that were unresectable due to blood vessel involvement or distant metastasis, and used propensity-score matching to pair them with a total of 35 controls. Controls were matched by tumor size, staging, age-adjusted Charlson Comorbidity Index, chemotherapy regimen received, and interactions between CCI, regimen, and staging.
The results were weighted to assure that covariate distribution among patients treated with chemotherapy only equaled that of patients who underwent EUS-RFA.
Patients underwent EUS-RFA with a 19-gauge needle, with 50 watts of energy delivered with an impedance of 100 ohms. Those patients deemed able to tolerate chemotherapy received that as well.
After a minimum of 1 year of follow-up, the median weighted survival, as noted before, was 14 months for patients who received EUS-RFA, compared with 6.1 months for controls.
Adjusted survival probabilities at 6 and 12 months were 73% and 64%, respectively, for patients in the EUS-RFA group, compared with 69% and 17% for controls. Adjusted PFS rates at 6 and 12 months were 55% and 36% in the EUS-RFA group, compared with 28% and 4% in the control group.
The only adverse event of significance was mild abdominal pain, reported by 8.3% of total EUS-RFA procedures.
Promising but preliminary
In an interview with this news organization, ACG President Samir A. Shah, MD, from Brown University and Miriam Hospital in Providence, R.I., who was not involved in the study, commented that “we have limited options with these patients, so it’s really exciting to see an initial trend toward efficacy, and their survival improvement was significant by several months.”
Dr. Shah was a moderator of the presidential symposium where the data were presented.
Comoderator Brooks D. Cash, MD, from the University of Texas Health Science Center at Houston, said that the advantage of EUS-RFA is that it’s only minimally invasive and appears to offer a significant survival advantage for patients with few effective treatment options.
He cautioned, however, that “it’s a small study and needs to be replicated in a larger venue and different sites as well, but I think it looks very promising.”
The investigators did not report a funding source for the study. Dr. Lopimpisuth, Dr. Shah, and Dr. Cash all reported having no relevant financial relationships to disclose.
CHARLOTTE, N.C. – In a small proof-of-concept study, patients with small unresectable pancreatic cancers treated with endoscopic ultrasound–guided radiofrequency ablation (EUS-RFA) had a more than twofold improvement in overall survival compared with historical controls with a similar disease history, investigators in Thailand found.
In a weighted analysis, median weighted overall survival – the primary outcome – was 14 months among 11 patients who underwent EUS-RFA, compared with 6.1 months for 35 matched controls, translating into a hazard ratio for death with EUS-RFA of 0.38 (P = .016), reported Chawin Lopimpisuth, MD, from King Chulalongkorn Memorial Hospital in Bangkok, Thailand.
Median weighted progression-free survival (PFS) was longer among cases than controls, but did not differ significantly, at 6.1 months and 3.9 months, respectively.
“In patients with unresectable pancreatic ductal adenocarcinomas that are less than 4 cm, EUS-RFA alone or combined with chemotherapy resulted in significantly improved overall survival and tended to improve progression-free survival with minimal adverse events,” Dr. Lopimpisuth reported at the annual meeting of the American College of Gastroenterology.
Small but unresectable tumors
Endoscopically guided radiofrequency ablation of pancreatic ductal tumors has been shown to be both feasible and safe in previous studies, he said, prompting his group to explore whether EUS-RFA could help to control the primary tumor and improve survival outcomes.
They enrolled 11 patients with primary pancreatic ductal adenocarcinoma tumors less than 4 cm in diameter that were unresectable due to blood vessel involvement or distant metastasis, and used propensity-score matching to pair them with a total of 35 controls. Controls were matched by tumor size, staging, age-adjusted Charlson Comorbidity Index, chemotherapy regimen received, and interactions between CCI, regimen, and staging.
The results were weighted to assure that covariate distribution among patients treated with chemotherapy only equaled that of patients who underwent EUS-RFA.
Patients underwent EUS-RFA with a 19-gauge needle, with 50 watts of energy delivered with an impedance of 100 ohms. Those patients deemed able to tolerate chemotherapy received that as well.
After a minimum of 1 year of follow-up, the median weighted survival, as noted before, was 14 months for patients who received EUS-RFA, compared with 6.1 months for controls.
Adjusted survival probabilities at 6 and 12 months were 73% and 64%, respectively, for patients in the EUS-RFA group, compared with 69% and 17% for controls. Adjusted PFS rates at 6 and 12 months were 55% and 36% in the EUS-RFA group, compared with 28% and 4% in the control group.
The only adverse event of significance was mild abdominal pain, reported by 8.3% of total EUS-RFA procedures.
Promising but preliminary
In an interview with this news organization, ACG President Samir A. Shah, MD, from Brown University and Miriam Hospital in Providence, R.I., who was not involved in the study, commented that “we have limited options with these patients, so it’s really exciting to see an initial trend toward efficacy, and their survival improvement was significant by several months.”
Dr. Shah was a moderator of the presidential symposium where the data were presented.
Comoderator Brooks D. Cash, MD, from the University of Texas Health Science Center at Houston, said that the advantage of EUS-RFA is that it’s only minimally invasive and appears to offer a significant survival advantage for patients with few effective treatment options.
He cautioned, however, that “it’s a small study and needs to be replicated in a larger venue and different sites as well, but I think it looks very promising.”
The investigators did not report a funding source for the study. Dr. Lopimpisuth, Dr. Shah, and Dr. Cash all reported having no relevant financial relationships to disclose.
AT ACG 2022
Real-world evidence seen for metal stents in biliary strictures
A real-world analysis in the United Kingdom found that a fully covered metal stent is safe and effective at controlling anastomotic strictures (AS) following liver transplants.
Biliary AS occurs in an estimated 5%-32% of patients following a liver transplant. Generally, these have been managed by insertion of side-by-side plastic stents to remodel the stricture, but this often required multiple procedures to resolve the problem. More recently, transpapillary fully covered self-expanding metallic stents (FCSEMSs) have been introduced and they appear to perform equivalently to their plastic counterparts while requiring fewer procedures.
The new study “is yet another large experience demonstrating that use of fully covered metal stents for treating anastomotic biliary strictures is highly effective and also cost-effective because you really decrease the number of ERCPs [endoscopic retrograde cholangiopancreatographies] that are required to treat an anastomotic stricture,” said Vladimir Kushnir, MD, who was asked to comment on the study, which was published in Therapeutic Advances in Gastroenterology.
The researchers analyzed retrospective data from 162 consecutive patients who underwent ERCP with intraductal self-expanding metal stent (IDSEMS) insertion at nine tertiary centers. The procedures employed the Kaffes (Taewoong Niti-S) biliary covered stent, which is not available in the United States. Unlike conventional FCSEMSs, the device does not have to traverse the papilla. It is also shorter and includes an antimigration waist and removal wires that may reduce the risk of silent migration. Small case series suggested efficacy in the treatment of post–liver transplant AS.
There were 176 episodes of stent insertion among the 162 included patients; 62% of patients were male, and the median age at transplant was 54 years. Etiologies included hepatocellular carcinoma (22%), alcohol-related liver disease (18%), and nonalcoholic fatty liver disease (12%). The median time to development of a stricture was 24.9 weeks. Among all patients, 35% had previously received stents; 75% of those were plastic stents.
Overall, 10% of patients experienced stricture recurrence at a median interval of 19 weeks following stent removal. Median stent emplacement was 15 weeks, and 81% of patients had a resolution of their strictures.
Dr. Kushnir, from Washington University in St. Louis, highlighted the differences between the stent used in the study and those currently available in the United States. “This type of stent is a self-expanding metal stent that’s covered, but what’s different about it is that it’s designed to go completely within the bile duct, whereas a traditional fully covered metal stent traverses the major duodenal papilla.”
Despite those differences, he believes that the study can inform current practice in the United States. “In situations where you’re faced with a question of whether or not you leave multiple plastic stents in, or you put a full metal stent in that’s going to be fully within the bile duct, I think this data does provide some reassurance. If you’re using one of the traditional stents that we have in the United States and putting it fully within the bile duct, you do need to be prepared to have a little bit of a harder time removing the stent when the time comes for the removal procedure, which could require cholangioscopy. But this does provide some evidence to back up the practice of using fully covered metal stents fully within the bile duct to remediate anastomotic strictures that may be just a little too high up to treat traditionally with a stent that remains transpapillary,” said Dr. Kushnir.
The study also suggests an avenue for further research. “What’s also interesting about this study is that they only left the stents in for 3 months. In most clinical trials, where we’ve used fully covered metal stents for treating anastomotic biliary strictures, you leave the stent in from anywhere from 6 to 12 months. So with only 3 months dwell time they were able to get pretty impressive results, at least in the short term, in a retrospective study, so it does raise the question of should we be evaluating shorter dwell times for stents in treating anastomotic strictures when we’re using a fully covered metal stent that’s a larger diameter?” said Dr. Kushnir.
The authors noted some limitations, such as the retrospective design, small sample size, and lack of control group. They also noted that the multicenter design may have introduced heterogeneity in patient management and follow-up.
“In conclusion, IDSEMS appear to be safe and highly efficacious in the management of [post–liver transplant] AS,” concluded the authors. “Long-term outcomes appear good with low rates of AS recurrence.”
The authors declare no conflicts of interest. Dr. Kushnir is a consultant for ConMed and Boston Scientific.
A real-world analysis in the United Kingdom found that a fully covered metal stent is safe and effective at controlling anastomotic strictures (AS) following liver transplants.
Biliary AS occurs in an estimated 5%-32% of patients following a liver transplant. Generally, these have been managed by insertion of side-by-side plastic stents to remodel the stricture, but this often required multiple procedures to resolve the problem. More recently, transpapillary fully covered self-expanding metallic stents (FCSEMSs) have been introduced and they appear to perform equivalently to their plastic counterparts while requiring fewer procedures.
The new study “is yet another large experience demonstrating that use of fully covered metal stents for treating anastomotic biliary strictures is highly effective and also cost-effective because you really decrease the number of ERCPs [endoscopic retrograde cholangiopancreatographies] that are required to treat an anastomotic stricture,” said Vladimir Kushnir, MD, who was asked to comment on the study, which was published in Therapeutic Advances in Gastroenterology.
The researchers analyzed retrospective data from 162 consecutive patients who underwent ERCP with intraductal self-expanding metal stent (IDSEMS) insertion at nine tertiary centers. The procedures employed the Kaffes (Taewoong Niti-S) biliary covered stent, which is not available in the United States. Unlike conventional FCSEMSs, the device does not have to traverse the papilla. It is also shorter and includes an antimigration waist and removal wires that may reduce the risk of silent migration. Small case series suggested efficacy in the treatment of post–liver transplant AS.
There were 176 episodes of stent insertion among the 162 included patients; 62% of patients were male, and the median age at transplant was 54 years. Etiologies included hepatocellular carcinoma (22%), alcohol-related liver disease (18%), and nonalcoholic fatty liver disease (12%). The median time to development of a stricture was 24.9 weeks. Among all patients, 35% had previously received stents; 75% of those were plastic stents.
Overall, 10% of patients experienced stricture recurrence at a median interval of 19 weeks following stent removal. Median stent emplacement was 15 weeks, and 81% of patients had a resolution of their strictures.
Dr. Kushnir, from Washington University in St. Louis, highlighted the differences between the stent used in the study and those currently available in the United States. “This type of stent is a self-expanding metal stent that’s covered, but what’s different about it is that it’s designed to go completely within the bile duct, whereas a traditional fully covered metal stent traverses the major duodenal papilla.”
Despite those differences, he believes that the study can inform current practice in the United States. “In situations where you’re faced with a question of whether or not you leave multiple plastic stents in, or you put a full metal stent in that’s going to be fully within the bile duct, I think this data does provide some reassurance. If you’re using one of the traditional stents that we have in the United States and putting it fully within the bile duct, you do need to be prepared to have a little bit of a harder time removing the stent when the time comes for the removal procedure, which could require cholangioscopy. But this does provide some evidence to back up the practice of using fully covered metal stents fully within the bile duct to remediate anastomotic strictures that may be just a little too high up to treat traditionally with a stent that remains transpapillary,” said Dr. Kushnir.
The study also suggests an avenue for further research. “What’s also interesting about this study is that they only left the stents in for 3 months. In most clinical trials, where we’ve used fully covered metal stents for treating anastomotic biliary strictures, you leave the stent in from anywhere from 6 to 12 months. So with only 3 months dwell time they were able to get pretty impressive results, at least in the short term, in a retrospective study, so it does raise the question of should we be evaluating shorter dwell times for stents in treating anastomotic strictures when we’re using a fully covered metal stent that’s a larger diameter?” said Dr. Kushnir.
The authors noted some limitations, such as the retrospective design, small sample size, and lack of control group. They also noted that the multicenter design may have introduced heterogeneity in patient management and follow-up.
“In conclusion, IDSEMS appear to be safe and highly efficacious in the management of [post–liver transplant] AS,” concluded the authors. “Long-term outcomes appear good with low rates of AS recurrence.”
The authors declare no conflicts of interest. Dr. Kushnir is a consultant for ConMed and Boston Scientific.
A real-world analysis in the United Kingdom found that a fully covered metal stent is safe and effective at controlling anastomotic strictures (AS) following liver transplants.
Biliary AS occurs in an estimated 5%-32% of patients following a liver transplant. Generally, these have been managed by insertion of side-by-side plastic stents to remodel the stricture, but this often required multiple procedures to resolve the problem. More recently, transpapillary fully covered self-expanding metallic stents (FCSEMSs) have been introduced and they appear to perform equivalently to their plastic counterparts while requiring fewer procedures.
The new study “is yet another large experience demonstrating that use of fully covered metal stents for treating anastomotic biliary strictures is highly effective and also cost-effective because you really decrease the number of ERCPs [endoscopic retrograde cholangiopancreatographies] that are required to treat an anastomotic stricture,” said Vladimir Kushnir, MD, who was asked to comment on the study, which was published in Therapeutic Advances in Gastroenterology.
The researchers analyzed retrospective data from 162 consecutive patients who underwent ERCP with intraductal self-expanding metal stent (IDSEMS) insertion at nine tertiary centers. The procedures employed the Kaffes (Taewoong Niti-S) biliary covered stent, which is not available in the United States. Unlike conventional FCSEMSs, the device does not have to traverse the papilla. It is also shorter and includes an antimigration waist and removal wires that may reduce the risk of silent migration. Small case series suggested efficacy in the treatment of post–liver transplant AS.
There were 176 episodes of stent insertion among the 162 included patients; 62% of patients were male, and the median age at transplant was 54 years. Etiologies included hepatocellular carcinoma (22%), alcohol-related liver disease (18%), and nonalcoholic fatty liver disease (12%). The median time to development of a stricture was 24.9 weeks. Among all patients, 35% had previously received stents; 75% of those were plastic stents.
Overall, 10% of patients experienced stricture recurrence at a median interval of 19 weeks following stent removal. Median stent emplacement was 15 weeks, and 81% of patients had a resolution of their strictures.
Dr. Kushnir, from Washington University in St. Louis, highlighted the differences between the stent used in the study and those currently available in the United States. “This type of stent is a self-expanding metal stent that’s covered, but what’s different about it is that it’s designed to go completely within the bile duct, whereas a traditional fully covered metal stent traverses the major duodenal papilla.”
Despite those differences, he believes that the study can inform current practice in the United States. “In situations where you’re faced with a question of whether or not you leave multiple plastic stents in, or you put a full metal stent in that’s going to be fully within the bile duct, I think this data does provide some reassurance. If you’re using one of the traditional stents that we have in the United States and putting it fully within the bile duct, you do need to be prepared to have a little bit of a harder time removing the stent when the time comes for the removal procedure, which could require cholangioscopy. But this does provide some evidence to back up the practice of using fully covered metal stents fully within the bile duct to remediate anastomotic strictures that may be just a little too high up to treat traditionally with a stent that remains transpapillary,” said Dr. Kushnir.
The study also suggests an avenue for further research. “What’s also interesting about this study is that they only left the stents in for 3 months. In most clinical trials, where we’ve used fully covered metal stents for treating anastomotic biliary strictures, you leave the stent in from anywhere from 6 to 12 months. So with only 3 months dwell time they were able to get pretty impressive results, at least in the short term, in a retrospective study, so it does raise the question of should we be evaluating shorter dwell times for stents in treating anastomotic strictures when we’re using a fully covered metal stent that’s a larger diameter?” said Dr. Kushnir.
The authors noted some limitations, such as the retrospective design, small sample size, and lack of control group. They also noted that the multicenter design may have introduced heterogeneity in patient management and follow-up.
“In conclusion, IDSEMS appear to be safe and highly efficacious in the management of [post–liver transplant] AS,” concluded the authors. “Long-term outcomes appear good with low rates of AS recurrence.”
The authors declare no conflicts of interest. Dr. Kushnir is a consultant for ConMed and Boston Scientific.
FROM THERAPEUTIC ADVANCES IN GASTROENTEROLOGY
Water exchange boosts colonoscopy training experience
A new study finds that colonoscopy trainees had a better experience with and performed better when using water exchange (WE) than when using air insufflation. The new study was published in the Journal of Clinical Gastroenterology.
According to study author Felix W. Leung, MD, from the Veterans Affairs Greater Los Angeles Healthcare System in North Hills, Calif., and the University of California, Los Angeles, WE is less painful than air insufflation and increases cecal intubation rate because it reduces loop formation. He added that it also increases polyp and adenoma detection rates.
Although WE has compared favorably with air insufflation for ADR and pain, there is little evidence regarding how trainees view WE versus air insufflation. Dr. Leung pointed out that the issue could be particularly important among millennial trainees, who may have a different learning style than previous generations. He also noted that previous studies of WE versus air insufflation among trainees measured the perspective of trainers, and did not include the trainees’ opinions of the learning process or trainee outcomes like polyp detection rate.
Seeking to fill this knowledge gap, Dr. Leung conducted a prospective observational study at a Veterans Administration Hospital. Trainees conducted unsedated colonoscopies using WE, as well as WE and air insufflation colonoscopies in alternating order in sedated patients. A total of 83 air insufflation and 119 WE colonoscopies were performed. Trainees rated their experiences on a 1- to 5-point scale, with 1 being “strongly agree” and 5 “strongly disagree” to two statements: “My colonoscopy experience was better than expected” then “I was confident with my technical skills using this method.”
On average, trainees using WE reported a better than expected experience when using WE, compared with air insufflation (2.02 vs. 2.43; P = .0087), but no significant difference in the ensuing confidence in their technical skills (2.76 vs. 2.85; P = .48). There was a longer insertion time for WE (40 minutes vs. 30 minutes; P = .0008). WE was associated with a significantly higher adjusted cecal intubation rate (99% vs. 89%; P = .0031) and a significantly higher polyp detection rate (54% vs. 32%; P = .0447). Overall insertion time was longer with WE than air insufflation (40 minutes vs. 30 minutes; P = .0008), but withdrawal times were similar (22 minutes vs. 20 minutes; P = .3369).
The reduction in pain associated with WE can potentially improve training, in which cases procedures are typically performed on patients under moderate sedation, according to John Allen, MD, who was asked to comment on the study.
He also said that WE can sometimes do a better job than air of opening the lumen. It can help clean the colon surface, and even improve visibility. “Viewing the mucosa under water is like having a lens that helps view the surface and enhance polyp detection,” said Dr. Allen, who is a retired clinical professor of medicine at the University of Michigan, Ann Arbor.
Dr. Allen noted that either air sufflation or WE can be used to overcome the inexperience of the trainee, and that there shouldn’t be much difference between the two methods for sedated colonoscopies. The time of exam is similar, and WE does not require use of carbon dioxide or other gases, which avoids extra costs. “A highly skilled colonoscopist can perform exams using any of the available media. That said, WE is proving to be helpful no matter what your skill level. The only disadvantage I can see is that many trainers do not know how WE works and are unused to this process, although it is easy to learn,” said Dr. Allen.
The study is limited by the fact that it was conducted at a single institution in a nonblinded, nonrandomized population.
Dr. Leung declared there are no conflicts of interest to disclose. Dr. Allen has no relevant financial disclosures.
A new study finds that colonoscopy trainees had a better experience with and performed better when using water exchange (WE) than when using air insufflation. The new study was published in the Journal of Clinical Gastroenterology.
According to study author Felix W. Leung, MD, from the Veterans Affairs Greater Los Angeles Healthcare System in North Hills, Calif., and the University of California, Los Angeles, WE is less painful than air insufflation and increases cecal intubation rate because it reduces loop formation. He added that it also increases polyp and adenoma detection rates.
Although WE has compared favorably with air insufflation for ADR and pain, there is little evidence regarding how trainees view WE versus air insufflation. Dr. Leung pointed out that the issue could be particularly important among millennial trainees, who may have a different learning style than previous generations. He also noted that previous studies of WE versus air insufflation among trainees measured the perspective of trainers, and did not include the trainees’ opinions of the learning process or trainee outcomes like polyp detection rate.
Seeking to fill this knowledge gap, Dr. Leung conducted a prospective observational study at a Veterans Administration Hospital. Trainees conducted unsedated colonoscopies using WE, as well as WE and air insufflation colonoscopies in alternating order in sedated patients. A total of 83 air insufflation and 119 WE colonoscopies were performed. Trainees rated their experiences on a 1- to 5-point scale, with 1 being “strongly agree” and 5 “strongly disagree” to two statements: “My colonoscopy experience was better than expected” then “I was confident with my technical skills using this method.”
On average, trainees using WE reported a better than expected experience when using WE, compared with air insufflation (2.02 vs. 2.43; P = .0087), but no significant difference in the ensuing confidence in their technical skills (2.76 vs. 2.85; P = .48). There was a longer insertion time for WE (40 minutes vs. 30 minutes; P = .0008). WE was associated with a significantly higher adjusted cecal intubation rate (99% vs. 89%; P = .0031) and a significantly higher polyp detection rate (54% vs. 32%; P = .0447). Overall insertion time was longer with WE than air insufflation (40 minutes vs. 30 minutes; P = .0008), but withdrawal times were similar (22 minutes vs. 20 minutes; P = .3369).
The reduction in pain associated with WE can potentially improve training, in which cases procedures are typically performed on patients under moderate sedation, according to John Allen, MD, who was asked to comment on the study.
He also said that WE can sometimes do a better job than air of opening the lumen. It can help clean the colon surface, and even improve visibility. “Viewing the mucosa under water is like having a lens that helps view the surface and enhance polyp detection,” said Dr. Allen, who is a retired clinical professor of medicine at the University of Michigan, Ann Arbor.
Dr. Allen noted that either air sufflation or WE can be used to overcome the inexperience of the trainee, and that there shouldn’t be much difference between the two methods for sedated colonoscopies. The time of exam is similar, and WE does not require use of carbon dioxide or other gases, which avoids extra costs. “A highly skilled colonoscopist can perform exams using any of the available media. That said, WE is proving to be helpful no matter what your skill level. The only disadvantage I can see is that many trainers do not know how WE works and are unused to this process, although it is easy to learn,” said Dr. Allen.
The study is limited by the fact that it was conducted at a single institution in a nonblinded, nonrandomized population.
Dr. Leung declared there are no conflicts of interest to disclose. Dr. Allen has no relevant financial disclosures.
A new study finds that colonoscopy trainees had a better experience with and performed better when using water exchange (WE) than when using air insufflation. The new study was published in the Journal of Clinical Gastroenterology.
According to study author Felix W. Leung, MD, from the Veterans Affairs Greater Los Angeles Healthcare System in North Hills, Calif., and the University of California, Los Angeles, WE is less painful than air insufflation and increases cecal intubation rate because it reduces loop formation. He added that it also increases polyp and adenoma detection rates.
Although WE has compared favorably with air insufflation for ADR and pain, there is little evidence regarding how trainees view WE versus air insufflation. Dr. Leung pointed out that the issue could be particularly important among millennial trainees, who may have a different learning style than previous generations. He also noted that previous studies of WE versus air insufflation among trainees measured the perspective of trainers, and did not include the trainees’ opinions of the learning process or trainee outcomes like polyp detection rate.
Seeking to fill this knowledge gap, Dr. Leung conducted a prospective observational study at a Veterans Administration Hospital. Trainees conducted unsedated colonoscopies using WE, as well as WE and air insufflation colonoscopies in alternating order in sedated patients. A total of 83 air insufflation and 119 WE colonoscopies were performed. Trainees rated their experiences on a 1- to 5-point scale, with 1 being “strongly agree” and 5 “strongly disagree” to two statements: “My colonoscopy experience was better than expected” then “I was confident with my technical skills using this method.”
On average, trainees using WE reported a better than expected experience when using WE, compared with air insufflation (2.02 vs. 2.43; P = .0087), but no significant difference in the ensuing confidence in their technical skills (2.76 vs. 2.85; P = .48). There was a longer insertion time for WE (40 minutes vs. 30 minutes; P = .0008). WE was associated with a significantly higher adjusted cecal intubation rate (99% vs. 89%; P = .0031) and a significantly higher polyp detection rate (54% vs. 32%; P = .0447). Overall insertion time was longer with WE than air insufflation (40 minutes vs. 30 minutes; P = .0008), but withdrawal times were similar (22 minutes vs. 20 minutes; P = .3369).
The reduction in pain associated with WE can potentially improve training, in which cases procedures are typically performed on patients under moderate sedation, according to John Allen, MD, who was asked to comment on the study.
He also said that WE can sometimes do a better job than air of opening the lumen. It can help clean the colon surface, and even improve visibility. “Viewing the mucosa under water is like having a lens that helps view the surface and enhance polyp detection,” said Dr. Allen, who is a retired clinical professor of medicine at the University of Michigan, Ann Arbor.
Dr. Allen noted that either air sufflation or WE can be used to overcome the inexperience of the trainee, and that there shouldn’t be much difference between the two methods for sedated colonoscopies. The time of exam is similar, and WE does not require use of carbon dioxide or other gases, which avoids extra costs. “A highly skilled colonoscopist can perform exams using any of the available media. That said, WE is proving to be helpful no matter what your skill level. The only disadvantage I can see is that many trainers do not know how WE works and are unused to this process, although it is easy to learn,” said Dr. Allen.
The study is limited by the fact that it was conducted at a single institution in a nonblinded, nonrandomized population.
Dr. Leung declared there are no conflicts of interest to disclose. Dr. Allen has no relevant financial disclosures.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Colonoscopy in FIT-based screening demands higher ADR
Adenoma detection rate (ADR) targets for endoscopists performing colonoscopy after a positive fecal immunochemical test (FIT) should be markedly higher compared with ADR targets used in primary colonoscopy, researchers report.
Data from the Netherlands FIT-based screening program show that the ADR is “linearly and inversely” associated with interim colorectal cancer (CRC) occurrence, first author Pieter H.A. Wisse, MD, with Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization.
“Endoscopists should strive to obtain ADRs as high as possible” in FIT-positive screenees, Wisse said.
The study was published online in Annals of Internal Medicine.
Small differences, huge consequences
The ADR is a key quality indicator for endoscopists performing colonoscopies for CRC because it reflects their ability to detect lesions and is inversely associated with the risk of interval postcolonoscopy CRC (PCCRC).
Adults with a positive FIT result have a high prevalence of adenomas, leading to high ADRs for endoscopists performing colonoscopies in this setting. However, data on optimal ADRs of endoscopists performing colonoscopies in FIT-based screening are scarce.
To investigate, Dr. Wisse and colleagues evaluated the association between the ADR and interval PCCRC in patients undergoing colonoscopy after a positive FIT result. The analysis included 362 accredited and audited endoscopists who performed 116,360 colonoscopies.
During a median follow-up of 52 months, 209 interval PCCRCs were identified.
The quality of the colonoscopic examinations in FIT-positive screenees was high; endoscopists’ ADRs ranged between 40% and 82%, with a median ADR of 67%.
A higher ADR was strongly associated with lower incidence of interval PCCRC, with an adjusted hazard ratio of 0.95 (95% confidence interval, 0.92-0.97) per 1% increase in ADR.
For endoscopists with an ADR of 60%, the cumulative incidence of interval PCCRC was nearly two times as high as that of endoscopists with an ADR of 70%. The risk was even higher for endoscopists with ADRs less than 60%.
For every 1,000 FIT-positive colonoscopies, the expected number of patients diagnosed with interval PCCRC in 5 years was roughly 2 for endoscopists with an ADR of 70%, compared with almost 3.5 for ADRs of 60% and more than 4.5 for ADRs of 55%.
The authors note that the relatively short duration of follow-up (median, 52 months) could be considered a study limitation.
Quality metrics needed
“These seemingly small ADR differences are deceptive – if an endoscopist increases their ADR by just 10%, their patients’ associated decrease in relative interval cancer risk is a remarkable 40% to 50%,” Douglas Corley, MD, PhD, MPH, from Kaiser Permanente, Oakland, Calif., points out in an accompanying editorial.
Dr. Wisse and colleagues add that FIT-based colonoscopy has now surpassed primary colonoscopy as the most commonly used primary CRC-screening method.
They say there is a need to determine specific ADR targets for FIT-positive screenees to assure quality of colonoscopies and optimize the effect of screening programs by reducing interval PCCRC risk.
For primary colonoscopy, most professional societies recommend an ADR of at least 25% as an indicator of adequate performance. The new study suggests that FIT-positive colonoscopy “demands a markedly higher ADR target than primary colonoscopy,” the authors write.
Dr. Corley said this study provides “an excellent framework for evaluating nine concepts regarding effective quality metrics and how these can illustrate pathways for meaningful metrics for the care of other cancers and disorders.”
Quality metrics must be trustworthy, important, strategic, relevant, actionable, simple, gaming-resistant, time-stamped, and owned, he explained.
Questions concerning goals, plans for implementation of interventions, and the application of goals while maintaining simplicity must be considered in metric development, Dr. Corley said.
The study had no funding.
A version of this article first appeared on Medscape.com.
Adenoma detection rate (ADR) targets for endoscopists performing colonoscopy after a positive fecal immunochemical test (FIT) should be markedly higher compared with ADR targets used in primary colonoscopy, researchers report.
Data from the Netherlands FIT-based screening program show that the ADR is “linearly and inversely” associated with interim colorectal cancer (CRC) occurrence, first author Pieter H.A. Wisse, MD, with Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization.
“Endoscopists should strive to obtain ADRs as high as possible” in FIT-positive screenees, Wisse said.
The study was published online in Annals of Internal Medicine.
Small differences, huge consequences
The ADR is a key quality indicator for endoscopists performing colonoscopies for CRC because it reflects their ability to detect lesions and is inversely associated with the risk of interval postcolonoscopy CRC (PCCRC).
Adults with a positive FIT result have a high prevalence of adenomas, leading to high ADRs for endoscopists performing colonoscopies in this setting. However, data on optimal ADRs of endoscopists performing colonoscopies in FIT-based screening are scarce.
To investigate, Dr. Wisse and colleagues evaluated the association between the ADR and interval PCCRC in patients undergoing colonoscopy after a positive FIT result. The analysis included 362 accredited and audited endoscopists who performed 116,360 colonoscopies.
During a median follow-up of 52 months, 209 interval PCCRCs were identified.
The quality of the colonoscopic examinations in FIT-positive screenees was high; endoscopists’ ADRs ranged between 40% and 82%, with a median ADR of 67%.
A higher ADR was strongly associated with lower incidence of interval PCCRC, with an adjusted hazard ratio of 0.95 (95% confidence interval, 0.92-0.97) per 1% increase in ADR.
For endoscopists with an ADR of 60%, the cumulative incidence of interval PCCRC was nearly two times as high as that of endoscopists with an ADR of 70%. The risk was even higher for endoscopists with ADRs less than 60%.
For every 1,000 FIT-positive colonoscopies, the expected number of patients diagnosed with interval PCCRC in 5 years was roughly 2 for endoscopists with an ADR of 70%, compared with almost 3.5 for ADRs of 60% and more than 4.5 for ADRs of 55%.
The authors note that the relatively short duration of follow-up (median, 52 months) could be considered a study limitation.
Quality metrics needed
“These seemingly small ADR differences are deceptive – if an endoscopist increases their ADR by just 10%, their patients’ associated decrease in relative interval cancer risk is a remarkable 40% to 50%,” Douglas Corley, MD, PhD, MPH, from Kaiser Permanente, Oakland, Calif., points out in an accompanying editorial.
Dr. Wisse and colleagues add that FIT-based colonoscopy has now surpassed primary colonoscopy as the most commonly used primary CRC-screening method.
They say there is a need to determine specific ADR targets for FIT-positive screenees to assure quality of colonoscopies and optimize the effect of screening programs by reducing interval PCCRC risk.
For primary colonoscopy, most professional societies recommend an ADR of at least 25% as an indicator of adequate performance. The new study suggests that FIT-positive colonoscopy “demands a markedly higher ADR target than primary colonoscopy,” the authors write.
Dr. Corley said this study provides “an excellent framework for evaluating nine concepts regarding effective quality metrics and how these can illustrate pathways for meaningful metrics for the care of other cancers and disorders.”
Quality metrics must be trustworthy, important, strategic, relevant, actionable, simple, gaming-resistant, time-stamped, and owned, he explained.
Questions concerning goals, plans for implementation of interventions, and the application of goals while maintaining simplicity must be considered in metric development, Dr. Corley said.
The study had no funding.
A version of this article first appeared on Medscape.com.
Adenoma detection rate (ADR) targets for endoscopists performing colonoscopy after a positive fecal immunochemical test (FIT) should be markedly higher compared with ADR targets used in primary colonoscopy, researchers report.
Data from the Netherlands FIT-based screening program show that the ADR is “linearly and inversely” associated with interim colorectal cancer (CRC) occurrence, first author Pieter H.A. Wisse, MD, with Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization.
“Endoscopists should strive to obtain ADRs as high as possible” in FIT-positive screenees, Wisse said.
The study was published online in Annals of Internal Medicine.
Small differences, huge consequences
The ADR is a key quality indicator for endoscopists performing colonoscopies for CRC because it reflects their ability to detect lesions and is inversely associated with the risk of interval postcolonoscopy CRC (PCCRC).
Adults with a positive FIT result have a high prevalence of adenomas, leading to high ADRs for endoscopists performing colonoscopies in this setting. However, data on optimal ADRs of endoscopists performing colonoscopies in FIT-based screening are scarce.
To investigate, Dr. Wisse and colleagues evaluated the association between the ADR and interval PCCRC in patients undergoing colonoscopy after a positive FIT result. The analysis included 362 accredited and audited endoscopists who performed 116,360 colonoscopies.
During a median follow-up of 52 months, 209 interval PCCRCs were identified.
The quality of the colonoscopic examinations in FIT-positive screenees was high; endoscopists’ ADRs ranged between 40% and 82%, with a median ADR of 67%.
A higher ADR was strongly associated with lower incidence of interval PCCRC, with an adjusted hazard ratio of 0.95 (95% confidence interval, 0.92-0.97) per 1% increase in ADR.
For endoscopists with an ADR of 60%, the cumulative incidence of interval PCCRC was nearly two times as high as that of endoscopists with an ADR of 70%. The risk was even higher for endoscopists with ADRs less than 60%.
For every 1,000 FIT-positive colonoscopies, the expected number of patients diagnosed with interval PCCRC in 5 years was roughly 2 for endoscopists with an ADR of 70%, compared with almost 3.5 for ADRs of 60% and more than 4.5 for ADRs of 55%.
The authors note that the relatively short duration of follow-up (median, 52 months) could be considered a study limitation.
Quality metrics needed
“These seemingly small ADR differences are deceptive – if an endoscopist increases their ADR by just 10%, their patients’ associated decrease in relative interval cancer risk is a remarkable 40% to 50%,” Douglas Corley, MD, PhD, MPH, from Kaiser Permanente, Oakland, Calif., points out in an accompanying editorial.
Dr. Wisse and colleagues add that FIT-based colonoscopy has now surpassed primary colonoscopy as the most commonly used primary CRC-screening method.
They say there is a need to determine specific ADR targets for FIT-positive screenees to assure quality of colonoscopies and optimize the effect of screening programs by reducing interval PCCRC risk.
For primary colonoscopy, most professional societies recommend an ADR of at least 25% as an indicator of adequate performance. The new study suggests that FIT-positive colonoscopy “demands a markedly higher ADR target than primary colonoscopy,” the authors write.
Dr. Corley said this study provides “an excellent framework for evaluating nine concepts regarding effective quality metrics and how these can illustrate pathways for meaningful metrics for the care of other cancers and disorders.”
Quality metrics must be trustworthy, important, strategic, relevant, actionable, simple, gaming-resistant, time-stamped, and owned, he explained.
Questions concerning goals, plans for implementation of interventions, and the application of goals while maintaining simplicity must be considered in metric development, Dr. Corley said.
The study had no funding.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
AGA Clinical Practice Update: Expert review of management of subepithelial lesions
The proper management of subepithelial lesions (SELs) depends on the size, histopathology, malignant potential, and presence of symptoms, according to a new American Gastroenterological Association clinical practice update published in Clinical Gastroenterology and Hepatology.
“SELs are found in 1 in every 300 endoscopies, and two-thirds of these lesions are located in the stomach,” explained Kaveh Sharzehi, MD, an associate professor of medicine in the division of gastroenterology and hepatology at Oregon Health & Science University, Portland, and colleagues. “They represent a heterogeneous group of lesions including nonneoplastic lesions such as ectopic pancreatic tissue and neoplastic lesions. The neoplastic SELs can vary from lesions with no malignant potential such as lipomas to those with malignant potential such as gastrointestinal stromal tumors (GISTs). The majority of SELs are small and found incidentally.”
The authors developed 10 clinical practice advice statements on the diagnosis and management of subepithelial lesions based on a review of the published literature and expert opinion.
First, standard mucosal biopsies often don’t reach deep enough to obtain a pathologic diagnosis for SELs because the lesions have normal overlying mucosa. Forceps bite-on-bite/deep-well biopsies or tunnel biopsies may help to establish a pathologic diagnosis.
Used as an adjunct to standard endoscopy, endoscopic ultrasound (EUS) has become the primary method for determining diagnostic and prognostic characteristics of SELs – such as the layer of origin, echogenicity, and presence of blood vessels within the lesion. It can also help with tissue acquisition.
For SELs arising from the submucosa, EUS-guided fine-needle aspiration and fine-needle biopsy have evolved as widely used methods for obtaining tissue. For SELs arising from muscularis propria, fine-needle aspiration and fine-needle biopsy should be used to determine whether the lesion is a GIST or leiomyoma. Using structural assessment and staining will allow for the differentiation of mesenchymal tumors and assessment of their malignant potential.
To remove SELs, multiple endoscopic resection techniques may be appropriate, depending on the layer of origin, size, and location, with the goal of complete, en bloc resection with no disruption to the wall or capsule of the lesion. These techniques should be limited to endoscopists skilled in advanced tissue resection.
SELs without malignant potential, such as lipoma or pancreatic rest, don’t need further evaluation or surveillance.
SELs that are ulcerated, bleeding, or causing symptoms should be considered for resection.
Other lesions are managed with resection or surveillance based on pathology. For example, leiomyomas, which are benign and most often found in the esophagus, generally don’t require surveillance or resection. On the other hand, all GISTs have some malignant potential, and management varies by size, location, and presence of symptoms. GISTs larger than 2 cm, should be considered for resection. Some GISTs between 2 cm and 4 cm without high-risk features can be removed by using advanced endoscopic resection techniques.
The determination for resection in all cases should include a multidisciplinary approach, with confirmation of a low mitotic index and lack of metastatic disease on cross-sectional imaging.
“The ultimate goal of endoscopic resection is to have a complete resection,” the authors wrote. “Determining the layer of involvement by EUS is critical in planning resection techniques.”
The authors reported no grant support or funding sources for this report. One author serves as a consultant for Boston Scientific, Fujifilm, Intuitive Surgical, Medtronic, and Olympus. The remaining authors disclosed no conflicts.
The proper management of subepithelial lesions (SELs) depends on the size, histopathology, malignant potential, and presence of symptoms, according to a new American Gastroenterological Association clinical practice update published in Clinical Gastroenterology and Hepatology.
“SELs are found in 1 in every 300 endoscopies, and two-thirds of these lesions are located in the stomach,” explained Kaveh Sharzehi, MD, an associate professor of medicine in the division of gastroenterology and hepatology at Oregon Health & Science University, Portland, and colleagues. “They represent a heterogeneous group of lesions including nonneoplastic lesions such as ectopic pancreatic tissue and neoplastic lesions. The neoplastic SELs can vary from lesions with no malignant potential such as lipomas to those with malignant potential such as gastrointestinal stromal tumors (GISTs). The majority of SELs are small and found incidentally.”
The authors developed 10 clinical practice advice statements on the diagnosis and management of subepithelial lesions based on a review of the published literature and expert opinion.
First, standard mucosal biopsies often don’t reach deep enough to obtain a pathologic diagnosis for SELs because the lesions have normal overlying mucosa. Forceps bite-on-bite/deep-well biopsies or tunnel biopsies may help to establish a pathologic diagnosis.
Used as an adjunct to standard endoscopy, endoscopic ultrasound (EUS) has become the primary method for determining diagnostic and prognostic characteristics of SELs – such as the layer of origin, echogenicity, and presence of blood vessels within the lesion. It can also help with tissue acquisition.
For SELs arising from the submucosa, EUS-guided fine-needle aspiration and fine-needle biopsy have evolved as widely used methods for obtaining tissue. For SELs arising from muscularis propria, fine-needle aspiration and fine-needle biopsy should be used to determine whether the lesion is a GIST or leiomyoma. Using structural assessment and staining will allow for the differentiation of mesenchymal tumors and assessment of their malignant potential.
To remove SELs, multiple endoscopic resection techniques may be appropriate, depending on the layer of origin, size, and location, with the goal of complete, en bloc resection with no disruption to the wall or capsule of the lesion. These techniques should be limited to endoscopists skilled in advanced tissue resection.
SELs without malignant potential, such as lipoma or pancreatic rest, don’t need further evaluation or surveillance.
SELs that are ulcerated, bleeding, or causing symptoms should be considered for resection.
Other lesions are managed with resection or surveillance based on pathology. For example, leiomyomas, which are benign and most often found in the esophagus, generally don’t require surveillance or resection. On the other hand, all GISTs have some malignant potential, and management varies by size, location, and presence of symptoms. GISTs larger than 2 cm, should be considered for resection. Some GISTs between 2 cm and 4 cm without high-risk features can be removed by using advanced endoscopic resection techniques.
The determination for resection in all cases should include a multidisciplinary approach, with confirmation of a low mitotic index and lack of metastatic disease on cross-sectional imaging.
“The ultimate goal of endoscopic resection is to have a complete resection,” the authors wrote. “Determining the layer of involvement by EUS is critical in planning resection techniques.”
The authors reported no grant support or funding sources for this report. One author serves as a consultant for Boston Scientific, Fujifilm, Intuitive Surgical, Medtronic, and Olympus. The remaining authors disclosed no conflicts.
The proper management of subepithelial lesions (SELs) depends on the size, histopathology, malignant potential, and presence of symptoms, according to a new American Gastroenterological Association clinical practice update published in Clinical Gastroenterology and Hepatology.
“SELs are found in 1 in every 300 endoscopies, and two-thirds of these lesions are located in the stomach,” explained Kaveh Sharzehi, MD, an associate professor of medicine in the division of gastroenterology and hepatology at Oregon Health & Science University, Portland, and colleagues. “They represent a heterogeneous group of lesions including nonneoplastic lesions such as ectopic pancreatic tissue and neoplastic lesions. The neoplastic SELs can vary from lesions with no malignant potential such as lipomas to those with malignant potential such as gastrointestinal stromal tumors (GISTs). The majority of SELs are small and found incidentally.”
The authors developed 10 clinical practice advice statements on the diagnosis and management of subepithelial lesions based on a review of the published literature and expert opinion.
First, standard mucosal biopsies often don’t reach deep enough to obtain a pathologic diagnosis for SELs because the lesions have normal overlying mucosa. Forceps bite-on-bite/deep-well biopsies or tunnel biopsies may help to establish a pathologic diagnosis.
Used as an adjunct to standard endoscopy, endoscopic ultrasound (EUS) has become the primary method for determining diagnostic and prognostic characteristics of SELs – such as the layer of origin, echogenicity, and presence of blood vessels within the lesion. It can also help with tissue acquisition.
For SELs arising from the submucosa, EUS-guided fine-needle aspiration and fine-needle biopsy have evolved as widely used methods for obtaining tissue. For SELs arising from muscularis propria, fine-needle aspiration and fine-needle biopsy should be used to determine whether the lesion is a GIST or leiomyoma. Using structural assessment and staining will allow for the differentiation of mesenchymal tumors and assessment of their malignant potential.
To remove SELs, multiple endoscopic resection techniques may be appropriate, depending on the layer of origin, size, and location, with the goal of complete, en bloc resection with no disruption to the wall or capsule of the lesion. These techniques should be limited to endoscopists skilled in advanced tissue resection.
SELs without malignant potential, such as lipoma or pancreatic rest, don’t need further evaluation or surveillance.
SELs that are ulcerated, bleeding, or causing symptoms should be considered for resection.
Other lesions are managed with resection or surveillance based on pathology. For example, leiomyomas, which are benign and most often found in the esophagus, generally don’t require surveillance or resection. On the other hand, all GISTs have some malignant potential, and management varies by size, location, and presence of symptoms. GISTs larger than 2 cm, should be considered for resection. Some GISTs between 2 cm and 4 cm without high-risk features can be removed by using advanced endoscopic resection techniques.
The determination for resection in all cases should include a multidisciplinary approach, with confirmation of a low mitotic index and lack of metastatic disease on cross-sectional imaging.
“The ultimate goal of endoscopic resection is to have a complete resection,” the authors wrote. “Determining the layer of involvement by EUS is critical in planning resection techniques.”
The authors reported no grant support or funding sources for this report. One author serves as a consultant for Boston Scientific, Fujifilm, Intuitive Surgical, Medtronic, and Olympus. The remaining authors disclosed no conflicts.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Then and now: Endoscopy
In the second issue of GI & Hepatology News in February 2007, an article reviewed the disruptive forces to colonoscopy including CT colonography and the colon capsule. The article stated that “colonoscopy is still the preferred method, but the emerging technology could catch up in 3-5 years.”
While this prediction did not come to pass, the field of endoscopy has evolved in remarkable ways over the last 15 years. From the development of high-definition endoscopes to the transformation of interventional endoscopy to include “third space” procedures, previously unimaginable techniques have now become commonplace. This transformation has changed the nature and training of our field and, even more importantly, dramatically improved the care of our patients.
Just as notably, the regulatory and practice environment for endoscopy has also changed in the last 15 years, albeit at a slower pace. In January of 2007, as the first issue of GI & Hepatology News came out, Medicare announced that it would cover all screening procedures without a copay but left a loophole that charged patients if their screening colonoscopy became therapeutic. That loophole was finally fixed this year as GI & Hepatology News celebrates its 15-year anniversary.
If the past 15 years are any indication, endoscopy practice will continue to change at a humbling pace over the next 15 years. I look forward to seeing those changes unfold through the pages of GI & Hepatology News.
Dr. Gellad is associate professor of medicine and associate vice chair of ambulatory services at Duke University Medical Center, Durham, N.C. He is also a staff physician with the Durham VA Health Care system. He disclosed ties with Merck, Novo Nordisk, and Higgs Boson Health.
In the second issue of GI & Hepatology News in February 2007, an article reviewed the disruptive forces to colonoscopy including CT colonography and the colon capsule. The article stated that “colonoscopy is still the preferred method, but the emerging technology could catch up in 3-5 years.”
While this prediction did not come to pass, the field of endoscopy has evolved in remarkable ways over the last 15 years. From the development of high-definition endoscopes to the transformation of interventional endoscopy to include “third space” procedures, previously unimaginable techniques have now become commonplace. This transformation has changed the nature and training of our field and, even more importantly, dramatically improved the care of our patients.
Just as notably, the regulatory and practice environment for endoscopy has also changed in the last 15 years, albeit at a slower pace. In January of 2007, as the first issue of GI & Hepatology News came out, Medicare announced that it would cover all screening procedures without a copay but left a loophole that charged patients if their screening colonoscopy became therapeutic. That loophole was finally fixed this year as GI & Hepatology News celebrates its 15-year anniversary.
If the past 15 years are any indication, endoscopy practice will continue to change at a humbling pace over the next 15 years. I look forward to seeing those changes unfold through the pages of GI & Hepatology News.
Dr. Gellad is associate professor of medicine and associate vice chair of ambulatory services at Duke University Medical Center, Durham, N.C. He is also a staff physician with the Durham VA Health Care system. He disclosed ties with Merck, Novo Nordisk, and Higgs Boson Health.
In the second issue of GI & Hepatology News in February 2007, an article reviewed the disruptive forces to colonoscopy including CT colonography and the colon capsule. The article stated that “colonoscopy is still the preferred method, but the emerging technology could catch up in 3-5 years.”
While this prediction did not come to pass, the field of endoscopy has evolved in remarkable ways over the last 15 years. From the development of high-definition endoscopes to the transformation of interventional endoscopy to include “third space” procedures, previously unimaginable techniques have now become commonplace. This transformation has changed the nature and training of our field and, even more importantly, dramatically improved the care of our patients.
Just as notably, the regulatory and practice environment for endoscopy has also changed in the last 15 years, albeit at a slower pace. In January of 2007, as the first issue of GI & Hepatology News came out, Medicare announced that it would cover all screening procedures without a copay but left a loophole that charged patients if their screening colonoscopy became therapeutic. That loophole was finally fixed this year as GI & Hepatology News celebrates its 15-year anniversary.
If the past 15 years are any indication, endoscopy practice will continue to change at a humbling pace over the next 15 years. I look forward to seeing those changes unfold through the pages of GI & Hepatology News.
Dr. Gellad is associate professor of medicine and associate vice chair of ambulatory services at Duke University Medical Center, Durham, N.C. He is also a staff physician with the Durham VA Health Care system. He disclosed ties with Merck, Novo Nordisk, and Higgs Boson Health.
Lean and clean: Minimally invasive endoscopic and pharmacologic approaches to obesity
Obesity currently affects more than 40% of the U.S. population. It is the second-leading preventable cause of mortality behind smoking with an estimated 300,000 deaths per year.1,2 Weight loss can reduce the risk of metabolic comorbidities such as diabetes, heart disease, and stroke. However, 5%-10% total body weight loss (TBWL) is required for risk reduction.3 Sustained weight loss involves dietary alterations and physical activity, although it is difficult to maintain long term with lifestyle changes alone. Less than 10% of Americans with a BMI greater than 30 kg/m2 will achieve 5% TBWL each year, and nearly 80% of people will regain the weight within 5 years, a phenomenon known as “weight cycling.”4,5 Not only can these weight fluctuations make future weight-loss efforts more difficult, but they can also negatively impact cardiometabolic health in the long term.5 Thus, additional therapies are typically needed in conjunction with lifestyle interventions to treat obesity.
Current guidelines recommend bariatric surgery for patients unable to achieve or maintain weight loss through lifestyle changes.6 Surgeries like Roux-en-Y gastric bypass and sleeve gastrectomy lead to improvements in morbidity and mortality from metabolic diseases but are often only approved for select patients with a BMI of at least 40 or at least 35 with obesity-related comorbidities.7 These restrictions exclude patients at lower BMIs who may have early metabolic disease. Furthermore, only a small proportion of eligible patients are referred or willing to undergo surgery because of access issues, socioeconomic barriers, and concerns about adverse events.8,9 Endoscopic bariatric therapy and antiobesity medications (AOMs) have blossomed because of the need for other less-invasive options to stimulate weight loss.
Minimally invasive and noninvasive therapies in obesity
Endoscopic bariatric and metabolic therapies
Endoscopic bariatric and metabolic therapies (EBMTs) are used for the treatment of obesity in patients with a BMI of 30 kg/m2, a cohort that may be ineligible for bariatric surgery.10,11 EBMTs involve three categories: space-occupying devices (intragastric balloons [IGBs], transpyloric shuttle [TPS]), aspiration therapy, and gastric remodeling (endoscopic sleeve gastroplasty [ESG]).21,13 Presently, TPS and aspiration therapy are not commercially available in the United States. There are three types of IGB approved by the Food and Drug Administration, and Apollo ESGTM recently received de novo marketing authorization for the treatment of obesity. TBWL with EBMTs is promising at 12 months post procedure. Ranges include 7%-12% TBWL for IGBs and 15%-19% for ESG, with low rates of serious adverse events (AEs).13-18 Weight loss often reaches or exceeds the 10% TBWL needed to improve or completely reverse metabolic complications.
Obesity pharmacotherapy
Multiple professional societies support the use of obesity pharmacotherapy as an effective adjunct to lifestyle interventions.19 AOMs are classified as peripherally-acting to prevent nutrition absorption (e.g. orlistat), centrally acting to suppress appetite and/or cravings (e.g., phentermine/topiramate or naltrexone/bupropion), or incretin mimetics such as glucagonlike peptide–1 agonists (e.g., liraglutide, semaglutide).20 With the exception of orlistat, most agents have some effects on the hypothalamus to suppress appetite.21 Obesity medications tend to lead to a minimum weight loss of 3-10 kg after 12 months of treatment, and newer medications have even greater efficacy.22 Despite these results, discontinuation rates of the popular GLP-1 agonists can be as high as 47.7% and 70.1% at 12 and 24 months, respectively, because of the high cost of medications, gastrointestinal side effects, and poor tolerance.23,24
An ongoing challenge for patients is maintaining weight loss following cessation of pharmacotherapy when weight loss goals have been achieved. In this context, the combination of obesity pharmacotherapy and EBMTs can be utilized for long-term weight loss and weight maintenance given the chronic, relapsing, and complex nature of obesity.25
Advantages of less-invasive therapies in obesity management
The advantages of both pharmacologic and endoscopic weight-loss therapies are numerous. Pharmacotherapies are noninvasive, and their multiple mechanisms allow for combined use to synergistically promote weight reduction.26,27 Medications can be used in both the short- and long-term management of obesity, allowing for flexibility in use for patients pending fluctuations in weight. Furthermore, medications can improve markers of cardiovascular health including total cholesterol, LDL cholesterol, blood pressure, and glycemic control.28
As minimally invasive therapies, EBMTs have less morbidity and mortality, compared with bariatric surgeries.29 The most common side effects of IGBs or ESG include abdominal pain, nausea, and worsening of acid reflux symptoms, which can be medically managed unlike some of the AEs associated with surgery, such as bowel obstruction, anastomotic dehiscence, fistulization, and postoperative infections.30 Long-term AEs from surgery also include malabsorption, nutritional deficiencies, cholelithiasis, and anastomotic stenosis.31 Even with improvement in surgical techniques, the rate of perioperative and postoperative mortality in Roux-en-Y gastric bypass is estimated to be 0.4% and 0.7%, respectively, compared with only 0.08% with IGBs.30,32
In addition, EBMTs are also more cost effective than surgery, as they are often same-day outpatient procedures, leading to decreased length of stay (LOS) for patients. In ongoing research conducted by Sharaiha and colleagues, it was found that patients undergoing ESG had an average LOS of only 0.13 days, compared with 3.09 days for laparoscopic sleeve gastrectomy and 1.68 for laparoscopic gastric banding. The cost for ESG was approximately $12,000, compared with $15,000-$22,000 for laparoscopic bariatric surgeries.33 With their availability to patients with lower BMIs and their less-invasive nature, EBMTs and pharmacotherapy can be utilized on the spectrum of obesity care as bridge therapies both before and after surgery.
Our clinical approach
In 2015, the first Veterans Affairs hospital-based endoscopic bariatric program was established at the VA New York Harbor Healthcare System utilizing IGBs and weight loss pharmacotherapy in conjunction with the VA MOVE! Program to treat obesity and metabolic comorbidities in veterans. Since then, EBMTs have expanded to include ESG and novel medications. Our treatment algorithm accounts for the chronic nature of obesity, the risk of weight regain after any intervention, and the need for longitudinal patient care.
Patients undergo work-up by a multidisciplinary team (MD team) with a nutritionist, psychologist, primary care physician, gastroenterologist, and endocrinologist to determine the optimal treatment plan (Fig. 1).29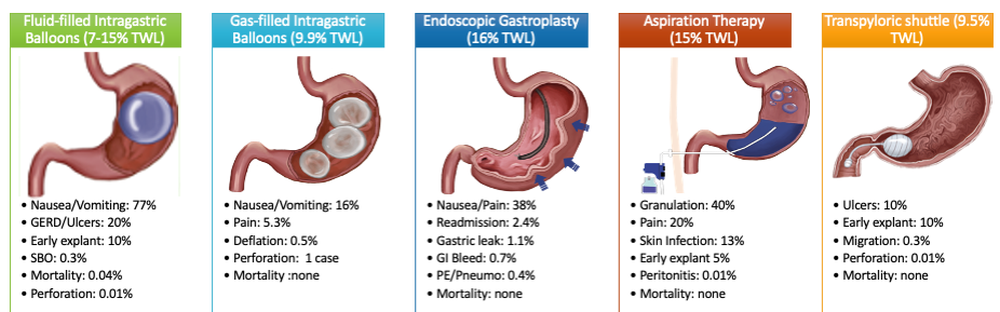
Patients are required to attend multiple information sessions, where all weight-loss methods are presented, including surgery, bariatric endoscopy, and pharmacotherapy. Other specialists also help manage comorbid conditions. Prior to selecting an initial intervention, patients undergo intensive lifestyle and behavioral therapy (Fig. 2 and 3). Depending on the selected therapy, initial treatment lasts between 3 and 12 months with ongoing support from the MD team.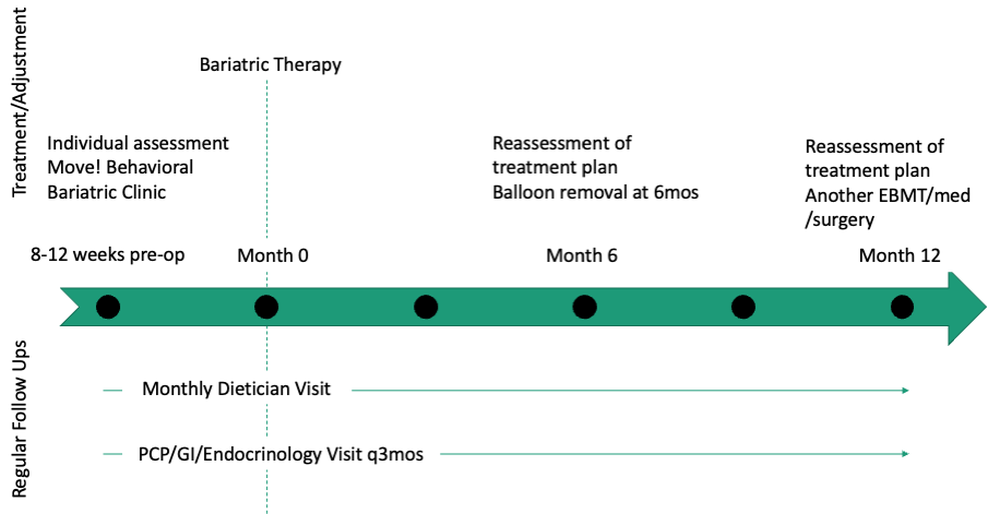
If patients do not achieve their targeted weight loss after initial treatment, a new strategy is selected. This includes a different EBMT such as ESG, alternate pharmacotherapy, or surgery until the weight and health goals of the patient are achieved and sustained (Fig. 3). From the start, patients are informed that our program is a long-term intervention and that active participation in the MOVE! Program, as well as follow-up with the MD team are keys to success. EBMTs and medications are presented as effective tools that only work to enhance the effects of lifestyle changes.
Our multidisciplinary approach provides flexibility for patients to trial different options depending on their progress. Research on long-term outcomes with weight loss and metabolic parameters is ongoing, though early results are promising. Thus far, we have observed that patients undergoing a combination therapy of EBMTs and AOMs have greater weight loss than patients on a single therapeutic approach with either EBMT or AOMs alone.34 Racial and socioeconomic disparities in referrals to bariatric surgery are yet another barrier for patients to access weight reduction and improvement in cardiovascular health.35 EBMTs and pharmacotherapy are no longer just on the horizon; they are here as accessible, effective, and long-term treatments for all patients with obesity. More expansive insurance coverage is needed for EBMTs and AOMs in order to prevent progression of obesity-related comorbidities, reduce high costs, and ensure more equitable access to these effective therapies.
Dr. Young and Dr. Zenger are resident physicians in the department of internal medicine at New York University. Dr. Holzwanger is an advanced endoscopy fellow in the division of gastroenterology at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston. Dr. Popov is director of bariatric endoscopy at VA New York Harbor Healthcare System, and assistant professor of medicine at New York University. Dr. Popov reported relationships with Obalon, Microtech, and Spatz, but the remaining authors reported no competing interests.
References
1. Ward ZJ et al. N Engl J Med. 2019;381(25):2440-50.
2. Stein CJ and Colditz GA. J Clin Endocrinol Metab. 2004;89(6):2522-5.
3. Ryan DH and Yockey SR. Curr Obes Rep. 2017;6(2):187-94.
4. Fildes A et al. Am J Public Health. 2015;105(9):e54-9.
5. Rhee E-J. J Obes Metab Syndr. 2017;26(4):237-42.
6. American College of Cardiology/American Heart Association Task Force on Practice Guidelines OEP. Obesity (Silver Spring). 2014;22 Suppl 2:S5-39.
7. Adams TD et al. N Engl J Med. 2018;378(1):93-6.
8. Wharton S et al. Clin Obes. 2016;6(2):154-60.
9. Iuzzolino E and Kim Y. Obes Res Clin Pract. 2020;14(4):310-20.
10. Goyal D, Watson RR. Endoscopic Bariatric Therapies. Curr Gastroenterol Rep. 2016;18(6):26.
11. Ali MR et al. Surg Obes Relat Dis. 2016;12(3):462-467.
12. Turkeltaub JA, Edmundowicz SA. Curr Treat Options Gastroenterol. 2019;17(2):187-201.
13. Reja D et al. Transl Gastroenterol Hepatol. 2022;7:21.
14. Force ABET et al. Gastrointest Endosc. 2015;82(3):425-38e5.
15. Thompson CC et al. Am J Gastroenterol. 2017;112(3):447-57.
16. Nystrom M et al. Obes Surg. 2018;28(7):1860-8.
17. Abu Dayyeh BK et al. Surg Obes Relat Dis. 2019;15(8):1423-4.
18. Sharaiha RZ et al. Clin Gastroenterol Hepatol. 2017;15(4):504-10.
19. Apovian CM et al. J Clin Endocrinol Metab. 2015;100(2):342-62.
20. Son JW and Kim S. Diabetes Metab J. 2020;44(6):802-18.
21. Holst JJ. Int J Obes (Lond). Int J Obes (Lond). 2013;37(9):1161-8.
22. Joo JK and Lee KS. J Menopausal Med. 2014;20(3):90-6.
23. Weiss T et al. Patient Prefer Adherence. 2020;14:2337-45.
24. Sikirica MV et al. Diabetes Metab Syndr Obes. 2017;10:403-12.
25. Kahan S et al. Tech Innov Gastrointest Endosc. 2020;22(3):154-8.
26. Bhat SP and Sharma A. Curr Drug Targets. 2017;18(8):983-93.
27. Pendse J et al. Obesity (Silver Spring). 2021;29(2):308-16.
28. Rucker D et al. BMJ. 2007;335(7631):1194-9.
29. Jirapinyo P and Thompson CC. Clin Gastroenterol Hepatol. 2017;15(5):619-30.
30. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;81(5):1073-86.
31. Schulman AR and Thompson CC. Am J Gastroenterol. 2017;112(11):1640-55.
32. Ma IT and Madura JA, 2nd. Gastroenterol Hepatol (NY). 2015;11(8):526-35.
33. Sharaiha RZ. Endoscopic sleeve gastroplasty as a nonsurgical weight loss alternative. Digestive Disease Week, oral presentation. 2017.
34. Young S et al. Long-term efficacy of a multidisciplinary minimally invasive approach to weight management compared to single endoscopic therapy: A cohort study. P0865. American College of Gastroenterology Meeting, Abstract P0865. 2021.
35. Johnson-Mann C et al. Surg Obes Relat Dis. 2019;15(4):615-20.
Obesity currently affects more than 40% of the U.S. population. It is the second-leading preventable cause of mortality behind smoking with an estimated 300,000 deaths per year.1,2 Weight loss can reduce the risk of metabolic comorbidities such as diabetes, heart disease, and stroke. However, 5%-10% total body weight loss (TBWL) is required for risk reduction.3 Sustained weight loss involves dietary alterations and physical activity, although it is difficult to maintain long term with lifestyle changes alone. Less than 10% of Americans with a BMI greater than 30 kg/m2 will achieve 5% TBWL each year, and nearly 80% of people will regain the weight within 5 years, a phenomenon known as “weight cycling.”4,5 Not only can these weight fluctuations make future weight-loss efforts more difficult, but they can also negatively impact cardiometabolic health in the long term.5 Thus, additional therapies are typically needed in conjunction with lifestyle interventions to treat obesity.
Current guidelines recommend bariatric surgery for patients unable to achieve or maintain weight loss through lifestyle changes.6 Surgeries like Roux-en-Y gastric bypass and sleeve gastrectomy lead to improvements in morbidity and mortality from metabolic diseases but are often only approved for select patients with a BMI of at least 40 or at least 35 with obesity-related comorbidities.7 These restrictions exclude patients at lower BMIs who may have early metabolic disease. Furthermore, only a small proportion of eligible patients are referred or willing to undergo surgery because of access issues, socioeconomic barriers, and concerns about adverse events.8,9 Endoscopic bariatric therapy and antiobesity medications (AOMs) have blossomed because of the need for other less-invasive options to stimulate weight loss.
Minimally invasive and noninvasive therapies in obesity
Endoscopic bariatric and metabolic therapies
Endoscopic bariatric and metabolic therapies (EBMTs) are used for the treatment of obesity in patients with a BMI of 30 kg/m2, a cohort that may be ineligible for bariatric surgery.10,11 EBMTs involve three categories: space-occupying devices (intragastric balloons [IGBs], transpyloric shuttle [TPS]), aspiration therapy, and gastric remodeling (endoscopic sleeve gastroplasty [ESG]).21,13 Presently, TPS and aspiration therapy are not commercially available in the United States. There are three types of IGB approved by the Food and Drug Administration, and Apollo ESGTM recently received de novo marketing authorization for the treatment of obesity. TBWL with EBMTs is promising at 12 months post procedure. Ranges include 7%-12% TBWL for IGBs and 15%-19% for ESG, with low rates of serious adverse events (AEs).13-18 Weight loss often reaches or exceeds the 10% TBWL needed to improve or completely reverse metabolic complications.
Obesity pharmacotherapy
Multiple professional societies support the use of obesity pharmacotherapy as an effective adjunct to lifestyle interventions.19 AOMs are classified as peripherally-acting to prevent nutrition absorption (e.g. orlistat), centrally acting to suppress appetite and/or cravings (e.g., phentermine/topiramate or naltrexone/bupropion), or incretin mimetics such as glucagonlike peptide–1 agonists (e.g., liraglutide, semaglutide).20 With the exception of orlistat, most agents have some effects on the hypothalamus to suppress appetite.21 Obesity medications tend to lead to a minimum weight loss of 3-10 kg after 12 months of treatment, and newer medications have even greater efficacy.22 Despite these results, discontinuation rates of the popular GLP-1 agonists can be as high as 47.7% and 70.1% at 12 and 24 months, respectively, because of the high cost of medications, gastrointestinal side effects, and poor tolerance.23,24
An ongoing challenge for patients is maintaining weight loss following cessation of pharmacotherapy when weight loss goals have been achieved. In this context, the combination of obesity pharmacotherapy and EBMTs can be utilized for long-term weight loss and weight maintenance given the chronic, relapsing, and complex nature of obesity.25
Advantages of less-invasive therapies in obesity management
The advantages of both pharmacologic and endoscopic weight-loss therapies are numerous. Pharmacotherapies are noninvasive, and their multiple mechanisms allow for combined use to synergistically promote weight reduction.26,27 Medications can be used in both the short- and long-term management of obesity, allowing for flexibility in use for patients pending fluctuations in weight. Furthermore, medications can improve markers of cardiovascular health including total cholesterol, LDL cholesterol, blood pressure, and glycemic control.28
As minimally invasive therapies, EBMTs have less morbidity and mortality, compared with bariatric surgeries.29 The most common side effects of IGBs or ESG include abdominal pain, nausea, and worsening of acid reflux symptoms, which can be medically managed unlike some of the AEs associated with surgery, such as bowel obstruction, anastomotic dehiscence, fistulization, and postoperative infections.30 Long-term AEs from surgery also include malabsorption, nutritional deficiencies, cholelithiasis, and anastomotic stenosis.31 Even with improvement in surgical techniques, the rate of perioperative and postoperative mortality in Roux-en-Y gastric bypass is estimated to be 0.4% and 0.7%, respectively, compared with only 0.08% with IGBs.30,32
In addition, EBMTs are also more cost effective than surgery, as they are often same-day outpatient procedures, leading to decreased length of stay (LOS) for patients. In ongoing research conducted by Sharaiha and colleagues, it was found that patients undergoing ESG had an average LOS of only 0.13 days, compared with 3.09 days for laparoscopic sleeve gastrectomy and 1.68 for laparoscopic gastric banding. The cost for ESG was approximately $12,000, compared with $15,000-$22,000 for laparoscopic bariatric surgeries.33 With their availability to patients with lower BMIs and their less-invasive nature, EBMTs and pharmacotherapy can be utilized on the spectrum of obesity care as bridge therapies both before and after surgery.
Our clinical approach
In 2015, the first Veterans Affairs hospital-based endoscopic bariatric program was established at the VA New York Harbor Healthcare System utilizing IGBs and weight loss pharmacotherapy in conjunction with the VA MOVE! Program to treat obesity and metabolic comorbidities in veterans. Since then, EBMTs have expanded to include ESG and novel medications. Our treatment algorithm accounts for the chronic nature of obesity, the risk of weight regain after any intervention, and the need for longitudinal patient care.
Patients undergo work-up by a multidisciplinary team (MD team) with a nutritionist, psychologist, primary care physician, gastroenterologist, and endocrinologist to determine the optimal treatment plan (Fig. 1).29
Patients are required to attend multiple information sessions, where all weight-loss methods are presented, including surgery, bariatric endoscopy, and pharmacotherapy. Other specialists also help manage comorbid conditions. Prior to selecting an initial intervention, patients undergo intensive lifestyle and behavioral therapy (Fig. 2 and 3). Depending on the selected therapy, initial treatment lasts between 3 and 12 months with ongoing support from the MD team.
If patients do not achieve their targeted weight loss after initial treatment, a new strategy is selected. This includes a different EBMT such as ESG, alternate pharmacotherapy, or surgery until the weight and health goals of the patient are achieved and sustained (Fig. 3). From the start, patients are informed that our program is a long-term intervention and that active participation in the MOVE! Program, as well as follow-up with the MD team are keys to success. EBMTs and medications are presented as effective tools that only work to enhance the effects of lifestyle changes.
Our multidisciplinary approach provides flexibility for patients to trial different options depending on their progress. Research on long-term outcomes with weight loss and metabolic parameters is ongoing, though early results are promising. Thus far, we have observed that patients undergoing a combination therapy of EBMTs and AOMs have greater weight loss than patients on a single therapeutic approach with either EBMT or AOMs alone.34 Racial and socioeconomic disparities in referrals to bariatric surgery are yet another barrier for patients to access weight reduction and improvement in cardiovascular health.35 EBMTs and pharmacotherapy are no longer just on the horizon; they are here as accessible, effective, and long-term treatments for all patients with obesity. More expansive insurance coverage is needed for EBMTs and AOMs in order to prevent progression of obesity-related comorbidities, reduce high costs, and ensure more equitable access to these effective therapies.
Dr. Young and Dr. Zenger are resident physicians in the department of internal medicine at New York University. Dr. Holzwanger is an advanced endoscopy fellow in the division of gastroenterology at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston. Dr. Popov is director of bariatric endoscopy at VA New York Harbor Healthcare System, and assistant professor of medicine at New York University. Dr. Popov reported relationships with Obalon, Microtech, and Spatz, but the remaining authors reported no competing interests.
References
1. Ward ZJ et al. N Engl J Med. 2019;381(25):2440-50.
2. Stein CJ and Colditz GA. J Clin Endocrinol Metab. 2004;89(6):2522-5.
3. Ryan DH and Yockey SR. Curr Obes Rep. 2017;6(2):187-94.
4. Fildes A et al. Am J Public Health. 2015;105(9):e54-9.
5. Rhee E-J. J Obes Metab Syndr. 2017;26(4):237-42.
6. American College of Cardiology/American Heart Association Task Force on Practice Guidelines OEP. Obesity (Silver Spring). 2014;22 Suppl 2:S5-39.
7. Adams TD et al. N Engl J Med. 2018;378(1):93-6.
8. Wharton S et al. Clin Obes. 2016;6(2):154-60.
9. Iuzzolino E and Kim Y. Obes Res Clin Pract. 2020;14(4):310-20.
10. Goyal D, Watson RR. Endoscopic Bariatric Therapies. Curr Gastroenterol Rep. 2016;18(6):26.
11. Ali MR et al. Surg Obes Relat Dis. 2016;12(3):462-467.
12. Turkeltaub JA, Edmundowicz SA. Curr Treat Options Gastroenterol. 2019;17(2):187-201.
13. Reja D et al. Transl Gastroenterol Hepatol. 2022;7:21.
14. Force ABET et al. Gastrointest Endosc. 2015;82(3):425-38e5.
15. Thompson CC et al. Am J Gastroenterol. 2017;112(3):447-57.
16. Nystrom M et al. Obes Surg. 2018;28(7):1860-8.
17. Abu Dayyeh BK et al. Surg Obes Relat Dis. 2019;15(8):1423-4.
18. Sharaiha RZ et al. Clin Gastroenterol Hepatol. 2017;15(4):504-10.
19. Apovian CM et al. J Clin Endocrinol Metab. 2015;100(2):342-62.
20. Son JW and Kim S. Diabetes Metab J. 2020;44(6):802-18.
21. Holst JJ. Int J Obes (Lond). Int J Obes (Lond). 2013;37(9):1161-8.
22. Joo JK and Lee KS. J Menopausal Med. 2014;20(3):90-6.
23. Weiss T et al. Patient Prefer Adherence. 2020;14:2337-45.
24. Sikirica MV et al. Diabetes Metab Syndr Obes. 2017;10:403-12.
25. Kahan S et al. Tech Innov Gastrointest Endosc. 2020;22(3):154-8.
26. Bhat SP and Sharma A. Curr Drug Targets. 2017;18(8):983-93.
27. Pendse J et al. Obesity (Silver Spring). 2021;29(2):308-16.
28. Rucker D et al. BMJ. 2007;335(7631):1194-9.
29. Jirapinyo P and Thompson CC. Clin Gastroenterol Hepatol. 2017;15(5):619-30.
30. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;81(5):1073-86.
31. Schulman AR and Thompson CC. Am J Gastroenterol. 2017;112(11):1640-55.
32. Ma IT and Madura JA, 2nd. Gastroenterol Hepatol (NY). 2015;11(8):526-35.
33. Sharaiha RZ. Endoscopic sleeve gastroplasty as a nonsurgical weight loss alternative. Digestive Disease Week, oral presentation. 2017.
34. Young S et al. Long-term efficacy of a multidisciplinary minimally invasive approach to weight management compared to single endoscopic therapy: A cohort study. P0865. American College of Gastroenterology Meeting, Abstract P0865. 2021.
35. Johnson-Mann C et al. Surg Obes Relat Dis. 2019;15(4):615-20.
Obesity currently affects more than 40% of the U.S. population. It is the second-leading preventable cause of mortality behind smoking with an estimated 300,000 deaths per year.1,2 Weight loss can reduce the risk of metabolic comorbidities such as diabetes, heart disease, and stroke. However, 5%-10% total body weight loss (TBWL) is required for risk reduction.3 Sustained weight loss involves dietary alterations and physical activity, although it is difficult to maintain long term with lifestyle changes alone. Less than 10% of Americans with a BMI greater than 30 kg/m2 will achieve 5% TBWL each year, and nearly 80% of people will regain the weight within 5 years, a phenomenon known as “weight cycling.”4,5 Not only can these weight fluctuations make future weight-loss efforts more difficult, but they can also negatively impact cardiometabolic health in the long term.5 Thus, additional therapies are typically needed in conjunction with lifestyle interventions to treat obesity.
Current guidelines recommend bariatric surgery for patients unable to achieve or maintain weight loss through lifestyle changes.6 Surgeries like Roux-en-Y gastric bypass and sleeve gastrectomy lead to improvements in morbidity and mortality from metabolic diseases but are often only approved for select patients with a BMI of at least 40 or at least 35 with obesity-related comorbidities.7 These restrictions exclude patients at lower BMIs who may have early metabolic disease. Furthermore, only a small proportion of eligible patients are referred or willing to undergo surgery because of access issues, socioeconomic barriers, and concerns about adverse events.8,9 Endoscopic bariatric therapy and antiobesity medications (AOMs) have blossomed because of the need for other less-invasive options to stimulate weight loss.
Minimally invasive and noninvasive therapies in obesity
Endoscopic bariatric and metabolic therapies
Endoscopic bariatric and metabolic therapies (EBMTs) are used for the treatment of obesity in patients with a BMI of 30 kg/m2, a cohort that may be ineligible for bariatric surgery.10,11 EBMTs involve three categories: space-occupying devices (intragastric balloons [IGBs], transpyloric shuttle [TPS]), aspiration therapy, and gastric remodeling (endoscopic sleeve gastroplasty [ESG]).21,13 Presently, TPS and aspiration therapy are not commercially available in the United States. There are three types of IGB approved by the Food and Drug Administration, and Apollo ESGTM recently received de novo marketing authorization for the treatment of obesity. TBWL with EBMTs is promising at 12 months post procedure. Ranges include 7%-12% TBWL for IGBs and 15%-19% for ESG, with low rates of serious adverse events (AEs).13-18 Weight loss often reaches or exceeds the 10% TBWL needed to improve or completely reverse metabolic complications.
Obesity pharmacotherapy
Multiple professional societies support the use of obesity pharmacotherapy as an effective adjunct to lifestyle interventions.19 AOMs are classified as peripherally-acting to prevent nutrition absorption (e.g. orlistat), centrally acting to suppress appetite and/or cravings (e.g., phentermine/topiramate or naltrexone/bupropion), or incretin mimetics such as glucagonlike peptide–1 agonists (e.g., liraglutide, semaglutide).20 With the exception of orlistat, most agents have some effects on the hypothalamus to suppress appetite.21 Obesity medications tend to lead to a minimum weight loss of 3-10 kg after 12 months of treatment, and newer medications have even greater efficacy.22 Despite these results, discontinuation rates of the popular GLP-1 agonists can be as high as 47.7% and 70.1% at 12 and 24 months, respectively, because of the high cost of medications, gastrointestinal side effects, and poor tolerance.23,24
An ongoing challenge for patients is maintaining weight loss following cessation of pharmacotherapy when weight loss goals have been achieved. In this context, the combination of obesity pharmacotherapy and EBMTs can be utilized for long-term weight loss and weight maintenance given the chronic, relapsing, and complex nature of obesity.25
Advantages of less-invasive therapies in obesity management
The advantages of both pharmacologic and endoscopic weight-loss therapies are numerous. Pharmacotherapies are noninvasive, and their multiple mechanisms allow for combined use to synergistically promote weight reduction.26,27 Medications can be used in both the short- and long-term management of obesity, allowing for flexibility in use for patients pending fluctuations in weight. Furthermore, medications can improve markers of cardiovascular health including total cholesterol, LDL cholesterol, blood pressure, and glycemic control.28
As minimally invasive therapies, EBMTs have less morbidity and mortality, compared with bariatric surgeries.29 The most common side effects of IGBs or ESG include abdominal pain, nausea, and worsening of acid reflux symptoms, which can be medically managed unlike some of the AEs associated with surgery, such as bowel obstruction, anastomotic dehiscence, fistulization, and postoperative infections.30 Long-term AEs from surgery also include malabsorption, nutritional deficiencies, cholelithiasis, and anastomotic stenosis.31 Even with improvement in surgical techniques, the rate of perioperative and postoperative mortality in Roux-en-Y gastric bypass is estimated to be 0.4% and 0.7%, respectively, compared with only 0.08% with IGBs.30,32
In addition, EBMTs are also more cost effective than surgery, as they are often same-day outpatient procedures, leading to decreased length of stay (LOS) for patients. In ongoing research conducted by Sharaiha and colleagues, it was found that patients undergoing ESG had an average LOS of only 0.13 days, compared with 3.09 days for laparoscopic sleeve gastrectomy and 1.68 for laparoscopic gastric banding. The cost for ESG was approximately $12,000, compared with $15,000-$22,000 for laparoscopic bariatric surgeries.33 With their availability to patients with lower BMIs and their less-invasive nature, EBMTs and pharmacotherapy can be utilized on the spectrum of obesity care as bridge therapies both before and after surgery.
Our clinical approach
In 2015, the first Veterans Affairs hospital-based endoscopic bariatric program was established at the VA New York Harbor Healthcare System utilizing IGBs and weight loss pharmacotherapy in conjunction with the VA MOVE! Program to treat obesity and metabolic comorbidities in veterans. Since then, EBMTs have expanded to include ESG and novel medications. Our treatment algorithm accounts for the chronic nature of obesity, the risk of weight regain after any intervention, and the need for longitudinal patient care.
Patients undergo work-up by a multidisciplinary team (MD team) with a nutritionist, psychologist, primary care physician, gastroenterologist, and endocrinologist to determine the optimal treatment plan (Fig. 1).29
Patients are required to attend multiple information sessions, where all weight-loss methods are presented, including surgery, bariatric endoscopy, and pharmacotherapy. Other specialists also help manage comorbid conditions. Prior to selecting an initial intervention, patients undergo intensive lifestyle and behavioral therapy (Fig. 2 and 3). Depending on the selected therapy, initial treatment lasts between 3 and 12 months with ongoing support from the MD team.
If patients do not achieve their targeted weight loss after initial treatment, a new strategy is selected. This includes a different EBMT such as ESG, alternate pharmacotherapy, or surgery until the weight and health goals of the patient are achieved and sustained (Fig. 3). From the start, patients are informed that our program is a long-term intervention and that active participation in the MOVE! Program, as well as follow-up with the MD team are keys to success. EBMTs and medications are presented as effective tools that only work to enhance the effects of lifestyle changes.
Our multidisciplinary approach provides flexibility for patients to trial different options depending on their progress. Research on long-term outcomes with weight loss and metabolic parameters is ongoing, though early results are promising. Thus far, we have observed that patients undergoing a combination therapy of EBMTs and AOMs have greater weight loss than patients on a single therapeutic approach with either EBMT or AOMs alone.34 Racial and socioeconomic disparities in referrals to bariatric surgery are yet another barrier for patients to access weight reduction and improvement in cardiovascular health.35 EBMTs and pharmacotherapy are no longer just on the horizon; they are here as accessible, effective, and long-term treatments for all patients with obesity. More expansive insurance coverage is needed for EBMTs and AOMs in order to prevent progression of obesity-related comorbidities, reduce high costs, and ensure more equitable access to these effective therapies.
Dr. Young and Dr. Zenger are resident physicians in the department of internal medicine at New York University. Dr. Holzwanger is an advanced endoscopy fellow in the division of gastroenterology at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston. Dr. Popov is director of bariatric endoscopy at VA New York Harbor Healthcare System, and assistant professor of medicine at New York University. Dr. Popov reported relationships with Obalon, Microtech, and Spatz, but the remaining authors reported no competing interests.
References
1. Ward ZJ et al. N Engl J Med. 2019;381(25):2440-50.
2. Stein CJ and Colditz GA. J Clin Endocrinol Metab. 2004;89(6):2522-5.
3. Ryan DH and Yockey SR. Curr Obes Rep. 2017;6(2):187-94.
4. Fildes A et al. Am J Public Health. 2015;105(9):e54-9.
5. Rhee E-J. J Obes Metab Syndr. 2017;26(4):237-42.
6. American College of Cardiology/American Heart Association Task Force on Practice Guidelines OEP. Obesity (Silver Spring). 2014;22 Suppl 2:S5-39.
7. Adams TD et al. N Engl J Med. 2018;378(1):93-6.
8. Wharton S et al. Clin Obes. 2016;6(2):154-60.
9. Iuzzolino E and Kim Y. Obes Res Clin Pract. 2020;14(4):310-20.
10. Goyal D, Watson RR. Endoscopic Bariatric Therapies. Curr Gastroenterol Rep. 2016;18(6):26.
11. Ali MR et al. Surg Obes Relat Dis. 2016;12(3):462-467.
12. Turkeltaub JA, Edmundowicz SA. Curr Treat Options Gastroenterol. 2019;17(2):187-201.
13. Reja D et al. Transl Gastroenterol Hepatol. 2022;7:21.
14. Force ABET et al. Gastrointest Endosc. 2015;82(3):425-38e5.
15. Thompson CC et al. Am J Gastroenterol. 2017;112(3):447-57.
16. Nystrom M et al. Obes Surg. 2018;28(7):1860-8.
17. Abu Dayyeh BK et al. Surg Obes Relat Dis. 2019;15(8):1423-4.
18. Sharaiha RZ et al. Clin Gastroenterol Hepatol. 2017;15(4):504-10.
19. Apovian CM et al. J Clin Endocrinol Metab. 2015;100(2):342-62.
20. Son JW and Kim S. Diabetes Metab J. 2020;44(6):802-18.
21. Holst JJ. Int J Obes (Lond). Int J Obes (Lond). 2013;37(9):1161-8.
22. Joo JK and Lee KS. J Menopausal Med. 2014;20(3):90-6.
23. Weiss T et al. Patient Prefer Adherence. 2020;14:2337-45.
24. Sikirica MV et al. Diabetes Metab Syndr Obes. 2017;10:403-12.
25. Kahan S et al. Tech Innov Gastrointest Endosc. 2020;22(3):154-8.
26. Bhat SP and Sharma A. Curr Drug Targets. 2017;18(8):983-93.
27. Pendse J et al. Obesity (Silver Spring). 2021;29(2):308-16.
28. Rucker D et al. BMJ. 2007;335(7631):1194-9.
29. Jirapinyo P and Thompson CC. Clin Gastroenterol Hepatol. 2017;15(5):619-30.
30. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;81(5):1073-86.
31. Schulman AR and Thompson CC. Am J Gastroenterol. 2017;112(11):1640-55.
32. Ma IT and Madura JA, 2nd. Gastroenterol Hepatol (NY). 2015;11(8):526-35.
33. Sharaiha RZ. Endoscopic sleeve gastroplasty as a nonsurgical weight loss alternative. Digestive Disease Week, oral presentation. 2017.
34. Young S et al. Long-term efficacy of a multidisciplinary minimally invasive approach to weight management compared to single endoscopic therapy: A cohort study. P0865. American College of Gastroenterology Meeting, Abstract P0865. 2021.
35. Johnson-Mann C et al. Surg Obes Relat Dis. 2019;15(4):615-20.
Endoscopy experts review training, assessment evidence
Endoscopic training is increasingly complex as benchmarks for quality evolve and tools and procedures advance with innovation.
A team of experts, led by Matthew J. Whitson, MD, with Hofstra University/Northwell Health in Hempstead, N.Y., aimed to simplify challenges for educators and clinical endoscopists with a review of tools and techniques for education, as well as assessment methods.
Their review was published in the Techniques and Innovations in Gastrointestinal Endoscopy.
Giving feedback
Key steps to effective feedback include first talking about the goals for the endoscopy session, then careful observation, but minimal feedback during the endoscopy. Most of the feedback should come after the endoscopy, the authors wrote.
A paper by Walsh et al. demonstrated that with beginning endoscopists, giving feedback afterward led to long-term skill development as compared with short-term benefits of frequent feedback in the middle of procedures.
Feedback should be constructive and specific with emphasis on goals for the next procedure. It should be delivered in a respectful, nonthreatening way for greatest effectiveness.
Mastery learning
In this model, each trainee must achieve competence in specific skills to progress to the next level.
“For example, the trainee must master retroflexion in the stomach prior to attempting clip hemostasis in the stomach,” the authors wrote.
Repetitively practicing the skill is coupled with direct feedback.
Mastery learning is often paired with simulation so trainees can practice in a safe space before working with patients.
Cognitive load theory
Knowing the challenges of learners can help educators with instruction techniques. An important concept is cognitive load theory (CLT). CLT is focused on the working memory of a learner and the harm that an overload of information can have on learning. A learner’s working memory can process only a few pieces of information at any given time, the theory states.
One mitigation strategy by educators may be to assign a trainee a smaller piece of a specific task appropriate to the trainee’s skill level.
“For example, an early trainee endoscopist may be able to inject epinephrine for a bleeding vessel, but not be ready to perform effective BiCap cautery,” the authors suggested.
Different learning styles
Learning styles include visual, aural, reading/writing, and kinesthetic styles (when learners need to touch or manipulate to learn a skill).
“A study of surgical trainees demonstrated that kinesthetic learning was the most preferred unimodal learning style of those entering the field,” the authors wrote.
Dr. Whitson and coauthors gave examples of working with trainees with different learning styles.
A trainee who learns visually, they say, might need to learn about “loop reduction” by looking at images of alpha or beta loops or using ScopeGuide during endoscopy. A kinesthetic learner may need to feel a successful loop reduction with hands on the endoscope during simulation to understand the skill better.
“There is some suggestion that the millennial generation – the demographic of the current gastroenterology fellows – may have higher preferences for kinesthetic learning,” the authors wrote.
Role of simulation
The Accreditation Council for Graduate Medical Education, which oversees Gastroenterology Fellowship training, mandates simulation in gastroenterology education but does not specify endoscopic simulation. The American Board of Surgeons, however, does require their trainees to complete the flexible endoscopic curriculum, which is simulation-based.
Simulator use appears particularly helpful early in training. One study demonstrated that colonoscopy simulation has benefit in the first 30 colonoscopies in depth of insertion, independent completion, and ability to identify landmarks.
However, another study showed simulation after 50 colonoscopies has shown no benefit, the authors wrote. Finding uses for simulators beyond diagnostics will be important for justifying buying more of them for medical centers given the high cost.
Procedural volume
Dr. Whitson and colleagues wrote that using sheer volume of procedures as a measure of competency is falling out of favor and there is recognition in the field that competency will come at different times and at different volumes for individual trainees.
A study assessing competency in esophagogastroduodenoscopy (EGD), for example, demonstrated that, while most trainees will achieve independent intubation rates of the second part of the duodenum by 150 procedures, it will take between 200 and 250 for the average fellow to reach competency of all motor skills for a standard EGD, and 300 to become efficient (Gastrointest Endosc. 2019;90(4):613-20).
Assessment of skills has evolved from numbers of procedures to competency-based assessments to the development of direct observation tools.
Coaching for the practicing endoscopist
Most studies on coaching have focused on trainees, but coaching can be used with experienced endoscopists as well.
One study investigated use of direct verbal coaching to train experienced practitioners in water immersion colonoscopy “which resulted in shorter cecal intubation times, improved [adenoma detection rate], and less use of sedation during procedures,” the authors noted. Another study currently underway in the United Kingdom uses electronic feedback coupled with education and training to change behaviors to improve polyp detection performance in colonoscopy.
The authors noted that using one of these tools or strategies does not preclude using another.
“[I]n fact educators likely will recognize the utility of incorporating multiple of these techniques in the same endoscopy session with a trainee,” the authors wrote.
One author holds stock in Boston Scientific. The remaining authors disclose no conflicts.
Whitson, Williams, and Shah eloquently and thoroughly explore the principles central to successful training and review the latest understanding of best practices to apply them. Beyond fellows, this will have ongoing relevance to practicing endoscopists as they must learn new skills and in turn apply them in teaching others.
The authors rightly emphasize the concern for cognitive overload. In practice, a maximum of one or two take-home lessons per mentored training session is a good rule of thumb; the converse should be equally emphasized, that every single proctored training examination ought to be mined for at least one relevant lesson, be it technical, cognitive, or another nontechnical pearl related to teamwork, professionalism, and so on.
Much simulator investigation to date, including my own, has focused on technical skills and performance outcomes – more work is needed especially in web, simulator, and even AI-based tools to teach cognitive skills for recognizing abnormalities, identifying them, and making real-time evidence-based decisions. The value of simulator-based teaching of endoscopic nontechnical skills, practice in troubleshooting common mishaps, and teaching by counter example of what not to do are other areas of great promise. There is not yet a prescribed, evidence-based guideline regarding which simulation devices should be used, at what stages of fellowship, and how often; however, the principles described in this paper provide the road map for how simulation ought to be integrated into endoscopic teaching.
Jonathan Cohen, MD is a clinical professor of medicine at New York University. He is the Editor of “Successful Training in Gastrointestinal Endoscopy,” 2nd ed. (Hoboken, N.J.: Wiley-Blackwell, 2022) and an investigator in ex vivo and computer endoscopy simulators. He is a consultant for Olympus America and Micro-Tech Endoscopy, receives royalties from Wouters-Kluwer and Wiley, and holds stock in GI Windows, Virtual Health Partners, ROMtech, and MD Medical Navigators.
Whitson, Williams, and Shah eloquently and thoroughly explore the principles central to successful training and review the latest understanding of best practices to apply them. Beyond fellows, this will have ongoing relevance to practicing endoscopists as they must learn new skills and in turn apply them in teaching others.
The authors rightly emphasize the concern for cognitive overload. In practice, a maximum of one or two take-home lessons per mentored training session is a good rule of thumb; the converse should be equally emphasized, that every single proctored training examination ought to be mined for at least one relevant lesson, be it technical, cognitive, or another nontechnical pearl related to teamwork, professionalism, and so on.
Much simulator investigation to date, including my own, has focused on technical skills and performance outcomes – more work is needed especially in web, simulator, and even AI-based tools to teach cognitive skills for recognizing abnormalities, identifying them, and making real-time evidence-based decisions. The value of simulator-based teaching of endoscopic nontechnical skills, practice in troubleshooting common mishaps, and teaching by counter example of what not to do are other areas of great promise. There is not yet a prescribed, evidence-based guideline regarding which simulation devices should be used, at what stages of fellowship, and how often; however, the principles described in this paper provide the road map for how simulation ought to be integrated into endoscopic teaching.
Jonathan Cohen, MD is a clinical professor of medicine at New York University. He is the Editor of “Successful Training in Gastrointestinal Endoscopy,” 2nd ed. (Hoboken, N.J.: Wiley-Blackwell, 2022) and an investigator in ex vivo and computer endoscopy simulators. He is a consultant for Olympus America and Micro-Tech Endoscopy, receives royalties from Wouters-Kluwer and Wiley, and holds stock in GI Windows, Virtual Health Partners, ROMtech, and MD Medical Navigators.
Whitson, Williams, and Shah eloquently and thoroughly explore the principles central to successful training and review the latest understanding of best practices to apply them. Beyond fellows, this will have ongoing relevance to practicing endoscopists as they must learn new skills and in turn apply them in teaching others.
The authors rightly emphasize the concern for cognitive overload. In practice, a maximum of one or two take-home lessons per mentored training session is a good rule of thumb; the converse should be equally emphasized, that every single proctored training examination ought to be mined for at least one relevant lesson, be it technical, cognitive, or another nontechnical pearl related to teamwork, professionalism, and so on.
Much simulator investigation to date, including my own, has focused on technical skills and performance outcomes – more work is needed especially in web, simulator, and even AI-based tools to teach cognitive skills for recognizing abnormalities, identifying them, and making real-time evidence-based decisions. The value of simulator-based teaching of endoscopic nontechnical skills, practice in troubleshooting common mishaps, and teaching by counter example of what not to do are other areas of great promise. There is not yet a prescribed, evidence-based guideline regarding which simulation devices should be used, at what stages of fellowship, and how often; however, the principles described in this paper provide the road map for how simulation ought to be integrated into endoscopic teaching.
Jonathan Cohen, MD is a clinical professor of medicine at New York University. He is the Editor of “Successful Training in Gastrointestinal Endoscopy,” 2nd ed. (Hoboken, N.J.: Wiley-Blackwell, 2022) and an investigator in ex vivo and computer endoscopy simulators. He is a consultant for Olympus America and Micro-Tech Endoscopy, receives royalties from Wouters-Kluwer and Wiley, and holds stock in GI Windows, Virtual Health Partners, ROMtech, and MD Medical Navigators.
Endoscopic training is increasingly complex as benchmarks for quality evolve and tools and procedures advance with innovation.
A team of experts, led by Matthew J. Whitson, MD, with Hofstra University/Northwell Health in Hempstead, N.Y., aimed to simplify challenges for educators and clinical endoscopists with a review of tools and techniques for education, as well as assessment methods.
Their review was published in the Techniques and Innovations in Gastrointestinal Endoscopy.
Giving feedback
Key steps to effective feedback include first talking about the goals for the endoscopy session, then careful observation, but minimal feedback during the endoscopy. Most of the feedback should come after the endoscopy, the authors wrote.
A paper by Walsh et al. demonstrated that with beginning endoscopists, giving feedback afterward led to long-term skill development as compared with short-term benefits of frequent feedback in the middle of procedures.
Feedback should be constructive and specific with emphasis on goals for the next procedure. It should be delivered in a respectful, nonthreatening way for greatest effectiveness.
Mastery learning
In this model, each trainee must achieve competence in specific skills to progress to the next level.
“For example, the trainee must master retroflexion in the stomach prior to attempting clip hemostasis in the stomach,” the authors wrote.
Repetitively practicing the skill is coupled with direct feedback.
Mastery learning is often paired with simulation so trainees can practice in a safe space before working with patients.
Cognitive load theory
Knowing the challenges of learners can help educators with instruction techniques. An important concept is cognitive load theory (CLT). CLT is focused on the working memory of a learner and the harm that an overload of information can have on learning. A learner’s working memory can process only a few pieces of information at any given time, the theory states.
One mitigation strategy by educators may be to assign a trainee a smaller piece of a specific task appropriate to the trainee’s skill level.
“For example, an early trainee endoscopist may be able to inject epinephrine for a bleeding vessel, but not be ready to perform effective BiCap cautery,” the authors suggested.
Different learning styles
Learning styles include visual, aural, reading/writing, and kinesthetic styles (when learners need to touch or manipulate to learn a skill).
“A study of surgical trainees demonstrated that kinesthetic learning was the most preferred unimodal learning style of those entering the field,” the authors wrote.
Dr. Whitson and coauthors gave examples of working with trainees with different learning styles.
A trainee who learns visually, they say, might need to learn about “loop reduction” by looking at images of alpha or beta loops or using ScopeGuide during endoscopy. A kinesthetic learner may need to feel a successful loop reduction with hands on the endoscope during simulation to understand the skill better.
“There is some suggestion that the millennial generation – the demographic of the current gastroenterology fellows – may have higher preferences for kinesthetic learning,” the authors wrote.
Role of simulation
The Accreditation Council for Graduate Medical Education, which oversees Gastroenterology Fellowship training, mandates simulation in gastroenterology education but does not specify endoscopic simulation. The American Board of Surgeons, however, does require their trainees to complete the flexible endoscopic curriculum, which is simulation-based.
Simulator use appears particularly helpful early in training. One study demonstrated that colonoscopy simulation has benefit in the first 30 colonoscopies in depth of insertion, independent completion, and ability to identify landmarks.
However, another study showed simulation after 50 colonoscopies has shown no benefit, the authors wrote. Finding uses for simulators beyond diagnostics will be important for justifying buying more of them for medical centers given the high cost.
Procedural volume
Dr. Whitson and colleagues wrote that using sheer volume of procedures as a measure of competency is falling out of favor and there is recognition in the field that competency will come at different times and at different volumes for individual trainees.
A study assessing competency in esophagogastroduodenoscopy (EGD), for example, demonstrated that, while most trainees will achieve independent intubation rates of the second part of the duodenum by 150 procedures, it will take between 200 and 250 for the average fellow to reach competency of all motor skills for a standard EGD, and 300 to become efficient (Gastrointest Endosc. 2019;90(4):613-20).
Assessment of skills has evolved from numbers of procedures to competency-based assessments to the development of direct observation tools.
Coaching for the practicing endoscopist
Most studies on coaching have focused on trainees, but coaching can be used with experienced endoscopists as well.
One study investigated use of direct verbal coaching to train experienced practitioners in water immersion colonoscopy “which resulted in shorter cecal intubation times, improved [adenoma detection rate], and less use of sedation during procedures,” the authors noted. Another study currently underway in the United Kingdom uses electronic feedback coupled with education and training to change behaviors to improve polyp detection performance in colonoscopy.
The authors noted that using one of these tools or strategies does not preclude using another.
“[I]n fact educators likely will recognize the utility of incorporating multiple of these techniques in the same endoscopy session with a trainee,” the authors wrote.
One author holds stock in Boston Scientific. The remaining authors disclose no conflicts.
Endoscopic training is increasingly complex as benchmarks for quality evolve and tools and procedures advance with innovation.
A team of experts, led by Matthew J. Whitson, MD, with Hofstra University/Northwell Health in Hempstead, N.Y., aimed to simplify challenges for educators and clinical endoscopists with a review of tools and techniques for education, as well as assessment methods.
Their review was published in the Techniques and Innovations in Gastrointestinal Endoscopy.
Giving feedback
Key steps to effective feedback include first talking about the goals for the endoscopy session, then careful observation, but minimal feedback during the endoscopy. Most of the feedback should come after the endoscopy, the authors wrote.
A paper by Walsh et al. demonstrated that with beginning endoscopists, giving feedback afterward led to long-term skill development as compared with short-term benefits of frequent feedback in the middle of procedures.
Feedback should be constructive and specific with emphasis on goals for the next procedure. It should be delivered in a respectful, nonthreatening way for greatest effectiveness.
Mastery learning
In this model, each trainee must achieve competence in specific skills to progress to the next level.
“For example, the trainee must master retroflexion in the stomach prior to attempting clip hemostasis in the stomach,” the authors wrote.
Repetitively practicing the skill is coupled with direct feedback.
Mastery learning is often paired with simulation so trainees can practice in a safe space before working with patients.
Cognitive load theory
Knowing the challenges of learners can help educators with instruction techniques. An important concept is cognitive load theory (CLT). CLT is focused on the working memory of a learner and the harm that an overload of information can have on learning. A learner’s working memory can process only a few pieces of information at any given time, the theory states.
One mitigation strategy by educators may be to assign a trainee a smaller piece of a specific task appropriate to the trainee’s skill level.
“For example, an early trainee endoscopist may be able to inject epinephrine for a bleeding vessel, but not be ready to perform effective BiCap cautery,” the authors suggested.
Different learning styles
Learning styles include visual, aural, reading/writing, and kinesthetic styles (when learners need to touch or manipulate to learn a skill).
“A study of surgical trainees demonstrated that kinesthetic learning was the most preferred unimodal learning style of those entering the field,” the authors wrote.
Dr. Whitson and coauthors gave examples of working with trainees with different learning styles.
A trainee who learns visually, they say, might need to learn about “loop reduction” by looking at images of alpha or beta loops or using ScopeGuide during endoscopy. A kinesthetic learner may need to feel a successful loop reduction with hands on the endoscope during simulation to understand the skill better.
“There is some suggestion that the millennial generation – the demographic of the current gastroenterology fellows – may have higher preferences for kinesthetic learning,” the authors wrote.
Role of simulation
The Accreditation Council for Graduate Medical Education, which oversees Gastroenterology Fellowship training, mandates simulation in gastroenterology education but does not specify endoscopic simulation. The American Board of Surgeons, however, does require their trainees to complete the flexible endoscopic curriculum, which is simulation-based.
Simulator use appears particularly helpful early in training. One study demonstrated that colonoscopy simulation has benefit in the first 30 colonoscopies in depth of insertion, independent completion, and ability to identify landmarks.
However, another study showed simulation after 50 colonoscopies has shown no benefit, the authors wrote. Finding uses for simulators beyond diagnostics will be important for justifying buying more of them for medical centers given the high cost.
Procedural volume
Dr. Whitson and colleagues wrote that using sheer volume of procedures as a measure of competency is falling out of favor and there is recognition in the field that competency will come at different times and at different volumes for individual trainees.
A study assessing competency in esophagogastroduodenoscopy (EGD), for example, demonstrated that, while most trainees will achieve independent intubation rates of the second part of the duodenum by 150 procedures, it will take between 200 and 250 for the average fellow to reach competency of all motor skills for a standard EGD, and 300 to become efficient (Gastrointest Endosc. 2019;90(4):613-20).
Assessment of skills has evolved from numbers of procedures to competency-based assessments to the development of direct observation tools.
Coaching for the practicing endoscopist
Most studies on coaching have focused on trainees, but coaching can be used with experienced endoscopists as well.
One study investigated use of direct verbal coaching to train experienced practitioners in water immersion colonoscopy “which resulted in shorter cecal intubation times, improved [adenoma detection rate], and less use of sedation during procedures,” the authors noted. Another study currently underway in the United Kingdom uses electronic feedback coupled with education and training to change behaviors to improve polyp detection performance in colonoscopy.
The authors noted that using one of these tools or strategies does not preclude using another.
“[I]n fact educators likely will recognize the utility of incorporating multiple of these techniques in the same endoscopy session with a trainee,” the authors wrote.
One author holds stock in Boston Scientific. The remaining authors disclose no conflicts.
FROM TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
EUS-guided gallbladder drainage for acute cholecystitis
Percutaneous transhepatic gallbladder drainage (PT-GBD) is the most common, nonoperative method for gallbladder decompression in patients unfit for cholecystectomy. However, drain-related complications (20%-75%), including tube changes, dyscosmesis, discomfort, and recurrent cholecystitis (up to 15%), limit its long-term use. Endoscopic transpapillary gallbladder drainage (ET-GBD) and now, endoscopic ultrasound–guided gallbladder drainage (EUS-GBD), have emerged as options.
ET-GBD is performed at endoscopic retrograde cholangiopancreatography (ERCP) by cannulating the cystic duct, allowing placement of a pigtail plastic stent into the gallbladder. However, obstructing pathology (stone, stricture, metal stent or mass) may result in lower technical and clinical success when compared with EUS-GBD (84% vs. 98% and 91% vs. 97%, respectively). Furthermore, it does not allow for treatment of gallstones, and may require stent exchanges.
EUS-GBD involves placing a stent from the duodenum/stomach into the gallbladder under EUS guidance. Initial use of pigtail plastic stents and biliary self-expandable metal stents were not ideal, because of their risk of leakage, longer length (contralateral wall injury, occlusions), and migration (lack of flanges). Lumen-apposing metal stents (LAMS) overcame these limitations because of their short length and large flanges, and their large diameters (up to 20 mm) aid passage of gallstones or cholecystoscopy. Several case series and comparative trials have been published on EUS-GBD including a randomized prospective trial of EUS-GBD vs. PT-GBD demonstrating its superiority. Adverse events are uncommon and include misdeployments, bleeding, perforation, bile leaks, occlusion (commonly with food, prompting some endoscopists to place pigtails stents through the LAMS and avoiding the stomach as a target), and migration.
EUS-GBD should be avoided in patients who have a perforated gallbladder, have large volume ascites, or are too sick to tolerate anesthesia. Although there are patients who have subsequently undergone cholecystectomy post EUS-GBD, a discussion with one’s surgeon must be had prior to choosing this approach over ET-GBD.
In conclusion, determining the ideal method for endoscopic GBD in high-surgical-risk patients requires consideration of comorbidities, anatomy (GB position, cystic duct characteristics), presence of ascites, future surgical candidacy, and local expertise. ET-GBD should be prioritized for patients requiring ERCP for alternative reasons, large volume ascites, and as a bridge to cholecystectomy. Conversely, EUS-GBD is preferred with indwelling metal biliary stents covering the cystic duct and/or high-volume cholelithiasis. LAMS can be left long term; however, in patients willing to undergo an additional procedure, exchanging the LAMS for plastic stents can be undertaken at 4-6 weeks. Ultimately, more randomized and prospective data are needed to compare ET- and EUS-GBD outcomes, including a formal cost analysis.
Dr. Irani is with Virginia Mason Medical Center, Seattle. He reports being a consultant for Boston Scientific and Gore, as well as remittance to his clinic. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
Percutaneous transhepatic gallbladder drainage (PT-GBD) is the most common, nonoperative method for gallbladder decompression in patients unfit for cholecystectomy. However, drain-related complications (20%-75%), including tube changes, dyscosmesis, discomfort, and recurrent cholecystitis (up to 15%), limit its long-term use. Endoscopic transpapillary gallbladder drainage (ET-GBD) and now, endoscopic ultrasound–guided gallbladder drainage (EUS-GBD), have emerged as options.
ET-GBD is performed at endoscopic retrograde cholangiopancreatography (ERCP) by cannulating the cystic duct, allowing placement of a pigtail plastic stent into the gallbladder. However, obstructing pathology (stone, stricture, metal stent or mass) may result in lower technical and clinical success when compared with EUS-GBD (84% vs. 98% and 91% vs. 97%, respectively). Furthermore, it does not allow for treatment of gallstones, and may require stent exchanges.
EUS-GBD involves placing a stent from the duodenum/stomach into the gallbladder under EUS guidance. Initial use of pigtail plastic stents and biliary self-expandable metal stents were not ideal, because of their risk of leakage, longer length (contralateral wall injury, occlusions), and migration (lack of flanges). Lumen-apposing metal stents (LAMS) overcame these limitations because of their short length and large flanges, and their large diameters (up to 20 mm) aid passage of gallstones or cholecystoscopy. Several case series and comparative trials have been published on EUS-GBD including a randomized prospective trial of EUS-GBD vs. PT-GBD demonstrating its superiority. Adverse events are uncommon and include misdeployments, bleeding, perforation, bile leaks, occlusion (commonly with food, prompting some endoscopists to place pigtails stents through the LAMS and avoiding the stomach as a target), and migration.
EUS-GBD should be avoided in patients who have a perforated gallbladder, have large volume ascites, or are too sick to tolerate anesthesia. Although there are patients who have subsequently undergone cholecystectomy post EUS-GBD, a discussion with one’s surgeon must be had prior to choosing this approach over ET-GBD.
In conclusion, determining the ideal method for endoscopic GBD in high-surgical-risk patients requires consideration of comorbidities, anatomy (GB position, cystic duct characteristics), presence of ascites, future surgical candidacy, and local expertise. ET-GBD should be prioritized for patients requiring ERCP for alternative reasons, large volume ascites, and as a bridge to cholecystectomy. Conversely, EUS-GBD is preferred with indwelling metal biliary stents covering the cystic duct and/or high-volume cholelithiasis. LAMS can be left long term; however, in patients willing to undergo an additional procedure, exchanging the LAMS for plastic stents can be undertaken at 4-6 weeks. Ultimately, more randomized and prospective data are needed to compare ET- and EUS-GBD outcomes, including a formal cost analysis.
Dr. Irani is with Virginia Mason Medical Center, Seattle. He reports being a consultant for Boston Scientific and Gore, as well as remittance to his clinic. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
Percutaneous transhepatic gallbladder drainage (PT-GBD) is the most common, nonoperative method for gallbladder decompression in patients unfit for cholecystectomy. However, drain-related complications (20%-75%), including tube changes, dyscosmesis, discomfort, and recurrent cholecystitis (up to 15%), limit its long-term use. Endoscopic transpapillary gallbladder drainage (ET-GBD) and now, endoscopic ultrasound–guided gallbladder drainage (EUS-GBD), have emerged as options.
ET-GBD is performed at endoscopic retrograde cholangiopancreatography (ERCP) by cannulating the cystic duct, allowing placement of a pigtail plastic stent into the gallbladder. However, obstructing pathology (stone, stricture, metal stent or mass) may result in lower technical and clinical success when compared with EUS-GBD (84% vs. 98% and 91% vs. 97%, respectively). Furthermore, it does not allow for treatment of gallstones, and may require stent exchanges.
EUS-GBD involves placing a stent from the duodenum/stomach into the gallbladder under EUS guidance. Initial use of pigtail plastic stents and biliary self-expandable metal stents were not ideal, because of their risk of leakage, longer length (contralateral wall injury, occlusions), and migration (lack of flanges). Lumen-apposing metal stents (LAMS) overcame these limitations because of their short length and large flanges, and their large diameters (up to 20 mm) aid passage of gallstones or cholecystoscopy. Several case series and comparative trials have been published on EUS-GBD including a randomized prospective trial of EUS-GBD vs. PT-GBD demonstrating its superiority. Adverse events are uncommon and include misdeployments, bleeding, perforation, bile leaks, occlusion (commonly with food, prompting some endoscopists to place pigtails stents through the LAMS and avoiding the stomach as a target), and migration.
EUS-GBD should be avoided in patients who have a perforated gallbladder, have large volume ascites, or are too sick to tolerate anesthesia. Although there are patients who have subsequently undergone cholecystectomy post EUS-GBD, a discussion with one’s surgeon must be had prior to choosing this approach over ET-GBD.
In conclusion, determining the ideal method for endoscopic GBD in high-surgical-risk patients requires consideration of comorbidities, anatomy (GB position, cystic duct characteristics), presence of ascites, future surgical candidacy, and local expertise. ET-GBD should be prioritized for patients requiring ERCP for alternative reasons, large volume ascites, and as a bridge to cholecystectomy. Conversely, EUS-GBD is preferred with indwelling metal biliary stents covering the cystic duct and/or high-volume cholelithiasis. LAMS can be left long term; however, in patients willing to undergo an additional procedure, exchanging the LAMS for plastic stents can be undertaken at 4-6 weeks. Ultimately, more randomized and prospective data are needed to compare ET- and EUS-GBD outcomes, including a formal cost analysis.
Dr. Irani is with Virginia Mason Medical Center, Seattle. He reports being a consultant for Boston Scientific and Gore, as well as remittance to his clinic. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
AT DDW 2022
New model could boost BE screening, early diagnosis
A new model that predicts Barrett’s esophagus (BE) risk relies on data that can be sourced from the electronic health record.
The tool was developed and validated by researchers seeking to improve early diagnosis of esophageal adenocarcinoma (EAC) through early detection of BE. Currently, 5-year survival from EAC is just 20%, and fewer than 30% of patients have curative options at diagnosis because of late-stage disease.
Several clinical guidelines recommend screening for BE in high-risk individuals. The trouble is that adherence is low: A meta-analysis of more than 33,000 patients found that 57% of newly diagnosed EAC patients had a simultaneous, first-time diagnosis of BE, which suggests missed screening opportunities. These patients also had higher probability of late-stage disease and worse mortality outcomes than patients who had a previous BE diagnosis. Low adherence to screening guidelines can be attributed to difficulties in implementation, as well as unsatisfactory outcomes in real-world settings. In a previous study, the researchers examined the efficacy of existing guidelines in diagnosing BE in a primary care population, and found all of these screening guidelines had a low area under the receiver operating curve ranging from 0.50 to 0.60.
The researchers sought to address these challenges in the current study published in Clinical Gastroenterology and Hepatology using a model development cohort of 274 patients with BE and 1,350 patients without BE. The patients were seen at Houston Veterans Affairs clinics between 2008 and 2012, and were between ages 40 and 80 years. The researchers included common risk factors identified among existing guidelines. The final model, Houston-BEST, incorporated sex, age, race/ethnicity, smoking status, body mass index, symptoms of gastroesophageal reflux disease (heartburn or reflux at least 1 day/week), and first-degree relative history of esophageal cancer.
The validation cohorts included patients from primary care clinics at the Houston VA who were undergoing screening colonoscopy (44 with BE, 469 without BE), as well as patients at the University of Michigan, Ann Arbor, or Ann Arbor VA clinics who presented for first esophagogastroduodenoscopy (71 with BE, 916 without BE).
In the development cohort, the researchers set an a priori threshold of predicted probability that corresponded to sensitivity of 90%; the threshold predicted probability of BE was 9.3% (AUROC, 0.69; 95% confidence interval, 0.66-0.72). The specificity was 39.9% (95% CI, 37.2%-42.5%). In the Houston area validation cohort, the model had a sensitivity of 84.1% (AUROC, 0.68; 95% CI, 0.60-0.76). The number needed to treat to detect a single BE case was 11.
Among the University of Michigan/Ann Arbor validation cohort, the model had an AUROC of 0.70 (95% CI, 0.64-0.76), but it had a sensitivity of 0%. The researchers also tested the ability of the model to discriminate early neoplasia from no BE, and found an AUROC of 0.72 (95% CI, 0.67-0.77).
The researchers tested other models based on existing guidelines in the Houston area cohort and found that their model performed at the high end of the range when compared with those other models (AUROCs, 0.65-0.70 vs. 0.58-0.70). “While the predictive performance of Houston-BEST model is modest, it has much better discriminative ability compared to current societal clinical practice guidelines. However, the model may need to be further refined for lower risk (nonveteran) populations,” the authors wrote.
The strength of the model is that it relies on data found in the EHR. The researchers suggest that future studies should look employing the model alongside e-Trigger tools that can mine electronic clinical data to identify patients at risk for a missed diagnosis.
The authors reported no personal or financial conflicts of interest.
A new model that predicts Barrett’s esophagus (BE) risk relies on data that can be sourced from the electronic health record.
The tool was developed and validated by researchers seeking to improve early diagnosis of esophageal adenocarcinoma (EAC) through early detection of BE. Currently, 5-year survival from EAC is just 20%, and fewer than 30% of patients have curative options at diagnosis because of late-stage disease.
Several clinical guidelines recommend screening for BE in high-risk individuals. The trouble is that adherence is low: A meta-analysis of more than 33,000 patients found that 57% of newly diagnosed EAC patients had a simultaneous, first-time diagnosis of BE, which suggests missed screening opportunities. These patients also had higher probability of late-stage disease and worse mortality outcomes than patients who had a previous BE diagnosis. Low adherence to screening guidelines can be attributed to difficulties in implementation, as well as unsatisfactory outcomes in real-world settings. In a previous study, the researchers examined the efficacy of existing guidelines in diagnosing BE in a primary care population, and found all of these screening guidelines had a low area under the receiver operating curve ranging from 0.50 to 0.60.
The researchers sought to address these challenges in the current study published in Clinical Gastroenterology and Hepatology using a model development cohort of 274 patients with BE and 1,350 patients without BE. The patients were seen at Houston Veterans Affairs clinics between 2008 and 2012, and were between ages 40 and 80 years. The researchers included common risk factors identified among existing guidelines. The final model, Houston-BEST, incorporated sex, age, race/ethnicity, smoking status, body mass index, symptoms of gastroesophageal reflux disease (heartburn or reflux at least 1 day/week), and first-degree relative history of esophageal cancer.
The validation cohorts included patients from primary care clinics at the Houston VA who were undergoing screening colonoscopy (44 with BE, 469 without BE), as well as patients at the University of Michigan, Ann Arbor, or Ann Arbor VA clinics who presented for first esophagogastroduodenoscopy (71 with BE, 916 without BE).
In the development cohort, the researchers set an a priori threshold of predicted probability that corresponded to sensitivity of 90%; the threshold predicted probability of BE was 9.3% (AUROC, 0.69; 95% confidence interval, 0.66-0.72). The specificity was 39.9% (95% CI, 37.2%-42.5%). In the Houston area validation cohort, the model had a sensitivity of 84.1% (AUROC, 0.68; 95% CI, 0.60-0.76). The number needed to treat to detect a single BE case was 11.
Among the University of Michigan/Ann Arbor validation cohort, the model had an AUROC of 0.70 (95% CI, 0.64-0.76), but it had a sensitivity of 0%. The researchers also tested the ability of the model to discriminate early neoplasia from no BE, and found an AUROC of 0.72 (95% CI, 0.67-0.77).
The researchers tested other models based on existing guidelines in the Houston area cohort and found that their model performed at the high end of the range when compared with those other models (AUROCs, 0.65-0.70 vs. 0.58-0.70). “While the predictive performance of Houston-BEST model is modest, it has much better discriminative ability compared to current societal clinical practice guidelines. However, the model may need to be further refined for lower risk (nonveteran) populations,” the authors wrote.
The strength of the model is that it relies on data found in the EHR. The researchers suggest that future studies should look employing the model alongside e-Trigger tools that can mine electronic clinical data to identify patients at risk for a missed diagnosis.
The authors reported no personal or financial conflicts of interest.
A new model that predicts Barrett’s esophagus (BE) risk relies on data that can be sourced from the electronic health record.
The tool was developed and validated by researchers seeking to improve early diagnosis of esophageal adenocarcinoma (EAC) through early detection of BE. Currently, 5-year survival from EAC is just 20%, and fewer than 30% of patients have curative options at diagnosis because of late-stage disease.
Several clinical guidelines recommend screening for BE in high-risk individuals. The trouble is that adherence is low: A meta-analysis of more than 33,000 patients found that 57% of newly diagnosed EAC patients had a simultaneous, first-time diagnosis of BE, which suggests missed screening opportunities. These patients also had higher probability of late-stage disease and worse mortality outcomes than patients who had a previous BE diagnosis. Low adherence to screening guidelines can be attributed to difficulties in implementation, as well as unsatisfactory outcomes in real-world settings. In a previous study, the researchers examined the efficacy of existing guidelines in diagnosing BE in a primary care population, and found all of these screening guidelines had a low area under the receiver operating curve ranging from 0.50 to 0.60.
The researchers sought to address these challenges in the current study published in Clinical Gastroenterology and Hepatology using a model development cohort of 274 patients with BE and 1,350 patients without BE. The patients were seen at Houston Veterans Affairs clinics between 2008 and 2012, and were between ages 40 and 80 years. The researchers included common risk factors identified among existing guidelines. The final model, Houston-BEST, incorporated sex, age, race/ethnicity, smoking status, body mass index, symptoms of gastroesophageal reflux disease (heartburn or reflux at least 1 day/week), and first-degree relative history of esophageal cancer.
The validation cohorts included patients from primary care clinics at the Houston VA who were undergoing screening colonoscopy (44 with BE, 469 without BE), as well as patients at the University of Michigan, Ann Arbor, or Ann Arbor VA clinics who presented for first esophagogastroduodenoscopy (71 with BE, 916 without BE).
In the development cohort, the researchers set an a priori threshold of predicted probability that corresponded to sensitivity of 90%; the threshold predicted probability of BE was 9.3% (AUROC, 0.69; 95% confidence interval, 0.66-0.72). The specificity was 39.9% (95% CI, 37.2%-42.5%). In the Houston area validation cohort, the model had a sensitivity of 84.1% (AUROC, 0.68; 95% CI, 0.60-0.76). The number needed to treat to detect a single BE case was 11.
Among the University of Michigan/Ann Arbor validation cohort, the model had an AUROC of 0.70 (95% CI, 0.64-0.76), but it had a sensitivity of 0%. The researchers also tested the ability of the model to discriminate early neoplasia from no BE, and found an AUROC of 0.72 (95% CI, 0.67-0.77).
The researchers tested other models based on existing guidelines in the Houston area cohort and found that their model performed at the high end of the range when compared with those other models (AUROCs, 0.65-0.70 vs. 0.58-0.70). “While the predictive performance of Houston-BEST model is modest, it has much better discriminative ability compared to current societal clinical practice guidelines. However, the model may need to be further refined for lower risk (nonveteran) populations,” the authors wrote.
The strength of the model is that it relies on data found in the EHR. The researchers suggest that future studies should look employing the model alongside e-Trigger tools that can mine electronic clinical data to identify patients at risk for a missed diagnosis.
The authors reported no personal or financial conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY











