User login
AGA publishes CPU for AI in colon polyp diagnosis and management
The American Gastroenterological Association has published a Clinical Practice Update (CPU) on artificial intelligence (AI) for diagnosing and managing colorectal polyps.
The CPU, authored by Jason Samarasena, MD, of UCI Health, Orange, Calif., and colleagues, draws on recent studies and clinical experience to discuss ways that AI is already reshaping colonoscopy, and what opportunities may lie ahead.
“As with any emerging technology, there are important questions and challenges that need to be addressed to ensure that AI tools are introduced safely and effectively into clinical endoscopic practice, ”they wrote in Gastroenterology.
With advances in processing speed and deep-learning technology, AI “computer vision” can now analyze live video of a colonoscopy in progress, enabling computer-aided detection (CADe) and computer-aided diagnosis (CADx), which the panelists described as the two most important developments in the area.
CADe
“In the last several years, numerous prospective, multicenter studies have found that real-time use of AI CADe tools during colonoscopy leads to improvements in adenoma detection and other related performance metrics,” Dr. Samarasena and colleagues wrote.
CADe has yielded mixed success in real-world practice, however, with some studies reporting worse detection metrics after implementing the new technology. Dr. Samarasena and colleagues offered a variety of possible explanations for these findings, including a “ceiling effect” among highly adept endoscopists, reduced operator vigilance caused by false confidence in the technology, and potential confounding inherent to unblinded trials.
CADe may also increase health care costs and burden, they suggested, as the technology tends to catch small benign polyps, prompting unnecessary resections and shortened colonoscopy surveillance intervals.
CADx
The above, unintended consequences of CADe may be counteracted by CADx, which uses computer vision to predict which lesions have benign histology, enabling “resect-and discard” or “diagnose-and-leave” strategies.
Such approaches could significantly reduce rates of polypectomy and/or histopathology, saving an estimated $33 million–150 million per year, according to the update.
Results of real-time CADx clinical trials have been “encouraging,” Dr. Samarasena and colleagues wrote, noting that emerging technology–compatible white-light endoscopy can achieve a negative predictive value of almost 98% for lesions less than 5 mm in diameter, potentially reducing polypectomy rate by almost half.
“Increasing endoscopist confidence in optical diagnosis may be an important step toward broader implementation of leave in situ and resect-and-discard strategies, but successful implementation will also require CADx tools that seamlessly integrate the endoscopic work flow, without the need for image enhancement or magnification,” the panelists wrote.
Reimbursement models may also need to be reworked, they suggested, as many GI practices depend on a steady stream of revenue from pathology services.
Computer-aided quality assessment systems
Beyond optical detection and diagnosis, AI tools are also being developed to improve colonoscopy technique.
Investigators are studying quality assessment systems that use AI offer feedback on a range of endoscopist skills, including colonic-fold evaluation, level of mucosal exposure, and withdrawal time, the latter of which is visualized by a “speedometer” that “paints” the mucosa with “a graphical representation of the colon.”
“In the future, these types of AI-based systems may support trainees and lower-performing endoscopists to reduce exposure errors and, more broadly, may empower physician practices and hospital systems with more nuanced and actionable data on an array of factors that contribute to colonoscopy quality,” the panelists wrote.
Looking ahead
Dr. Samarasena and colleagues concluded by suggesting that the AI tools in usage and development are just the beginning of a wave of technology that will revolutionize how colonoscopies are performed.
“Eventually, we predict an AI suite of tools for colonoscopy will seem indispensable, as a powerful adjunct to support safe and efficient clinical practice,” they wrote. “As technological innovation progresses, we can expect that the future for AI in endoscopy will be a hybrid model, where the unique capabilities of physicians and our AI tools will be seamlessly intertwined to optimize patient care.”
This CPU was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed relationships with Olympus, Neptune Medical, Conmed, and others.
The American Gastroenterological Association has published a Clinical Practice Update (CPU) on artificial intelligence (AI) for diagnosing and managing colorectal polyps.
The CPU, authored by Jason Samarasena, MD, of UCI Health, Orange, Calif., and colleagues, draws on recent studies and clinical experience to discuss ways that AI is already reshaping colonoscopy, and what opportunities may lie ahead.
“As with any emerging technology, there are important questions and challenges that need to be addressed to ensure that AI tools are introduced safely and effectively into clinical endoscopic practice, ”they wrote in Gastroenterology.
With advances in processing speed and deep-learning technology, AI “computer vision” can now analyze live video of a colonoscopy in progress, enabling computer-aided detection (CADe) and computer-aided diagnosis (CADx), which the panelists described as the two most important developments in the area.
CADe
“In the last several years, numerous prospective, multicenter studies have found that real-time use of AI CADe tools during colonoscopy leads to improvements in adenoma detection and other related performance metrics,” Dr. Samarasena and colleagues wrote.
CADe has yielded mixed success in real-world practice, however, with some studies reporting worse detection metrics after implementing the new technology. Dr. Samarasena and colleagues offered a variety of possible explanations for these findings, including a “ceiling effect” among highly adept endoscopists, reduced operator vigilance caused by false confidence in the technology, and potential confounding inherent to unblinded trials.
CADe may also increase health care costs and burden, they suggested, as the technology tends to catch small benign polyps, prompting unnecessary resections and shortened colonoscopy surveillance intervals.
CADx
The above, unintended consequences of CADe may be counteracted by CADx, which uses computer vision to predict which lesions have benign histology, enabling “resect-and discard” or “diagnose-and-leave” strategies.
Such approaches could significantly reduce rates of polypectomy and/or histopathology, saving an estimated $33 million–150 million per year, according to the update.
Results of real-time CADx clinical trials have been “encouraging,” Dr. Samarasena and colleagues wrote, noting that emerging technology–compatible white-light endoscopy can achieve a negative predictive value of almost 98% for lesions less than 5 mm in diameter, potentially reducing polypectomy rate by almost half.
“Increasing endoscopist confidence in optical diagnosis may be an important step toward broader implementation of leave in situ and resect-and-discard strategies, but successful implementation will also require CADx tools that seamlessly integrate the endoscopic work flow, without the need for image enhancement or magnification,” the panelists wrote.
Reimbursement models may also need to be reworked, they suggested, as many GI practices depend on a steady stream of revenue from pathology services.
Computer-aided quality assessment systems
Beyond optical detection and diagnosis, AI tools are also being developed to improve colonoscopy technique.
Investigators are studying quality assessment systems that use AI offer feedback on a range of endoscopist skills, including colonic-fold evaluation, level of mucosal exposure, and withdrawal time, the latter of which is visualized by a “speedometer” that “paints” the mucosa with “a graphical representation of the colon.”
“In the future, these types of AI-based systems may support trainees and lower-performing endoscopists to reduce exposure errors and, more broadly, may empower physician practices and hospital systems with more nuanced and actionable data on an array of factors that contribute to colonoscopy quality,” the panelists wrote.
Looking ahead
Dr. Samarasena and colleagues concluded by suggesting that the AI tools in usage and development are just the beginning of a wave of technology that will revolutionize how colonoscopies are performed.
“Eventually, we predict an AI suite of tools for colonoscopy will seem indispensable, as a powerful adjunct to support safe and efficient clinical practice,” they wrote. “As technological innovation progresses, we can expect that the future for AI in endoscopy will be a hybrid model, where the unique capabilities of physicians and our AI tools will be seamlessly intertwined to optimize patient care.”
This CPU was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed relationships with Olympus, Neptune Medical, Conmed, and others.
The American Gastroenterological Association has published a Clinical Practice Update (CPU) on artificial intelligence (AI) for diagnosing and managing colorectal polyps.
The CPU, authored by Jason Samarasena, MD, of UCI Health, Orange, Calif., and colleagues, draws on recent studies and clinical experience to discuss ways that AI is already reshaping colonoscopy, and what opportunities may lie ahead.
“As with any emerging technology, there are important questions and challenges that need to be addressed to ensure that AI tools are introduced safely and effectively into clinical endoscopic practice, ”they wrote in Gastroenterology.
With advances in processing speed and deep-learning technology, AI “computer vision” can now analyze live video of a colonoscopy in progress, enabling computer-aided detection (CADe) and computer-aided diagnosis (CADx), which the panelists described as the two most important developments in the area.
CADe
“In the last several years, numerous prospective, multicenter studies have found that real-time use of AI CADe tools during colonoscopy leads to improvements in adenoma detection and other related performance metrics,” Dr. Samarasena and colleagues wrote.
CADe has yielded mixed success in real-world practice, however, with some studies reporting worse detection metrics after implementing the new technology. Dr. Samarasena and colleagues offered a variety of possible explanations for these findings, including a “ceiling effect” among highly adept endoscopists, reduced operator vigilance caused by false confidence in the technology, and potential confounding inherent to unblinded trials.
CADe may also increase health care costs and burden, they suggested, as the technology tends to catch small benign polyps, prompting unnecessary resections and shortened colonoscopy surveillance intervals.
CADx
The above, unintended consequences of CADe may be counteracted by CADx, which uses computer vision to predict which lesions have benign histology, enabling “resect-and discard” or “diagnose-and-leave” strategies.
Such approaches could significantly reduce rates of polypectomy and/or histopathology, saving an estimated $33 million–150 million per year, according to the update.
Results of real-time CADx clinical trials have been “encouraging,” Dr. Samarasena and colleagues wrote, noting that emerging technology–compatible white-light endoscopy can achieve a negative predictive value of almost 98% for lesions less than 5 mm in diameter, potentially reducing polypectomy rate by almost half.
“Increasing endoscopist confidence in optical diagnosis may be an important step toward broader implementation of leave in situ and resect-and-discard strategies, but successful implementation will also require CADx tools that seamlessly integrate the endoscopic work flow, without the need for image enhancement or magnification,” the panelists wrote.
Reimbursement models may also need to be reworked, they suggested, as many GI practices depend on a steady stream of revenue from pathology services.
Computer-aided quality assessment systems
Beyond optical detection and diagnosis, AI tools are also being developed to improve colonoscopy technique.
Investigators are studying quality assessment systems that use AI offer feedback on a range of endoscopist skills, including colonic-fold evaluation, level of mucosal exposure, and withdrawal time, the latter of which is visualized by a “speedometer” that “paints” the mucosa with “a graphical representation of the colon.”
“In the future, these types of AI-based systems may support trainees and lower-performing endoscopists to reduce exposure errors and, more broadly, may empower physician practices and hospital systems with more nuanced and actionable data on an array of factors that contribute to colonoscopy quality,” the panelists wrote.
Looking ahead
Dr. Samarasena and colleagues concluded by suggesting that the AI tools in usage and development are just the beginning of a wave of technology that will revolutionize how colonoscopies are performed.
“Eventually, we predict an AI suite of tools for colonoscopy will seem indispensable, as a powerful adjunct to support safe and efficient clinical practice,” they wrote. “As technological innovation progresses, we can expect that the future for AI in endoscopy will be a hybrid model, where the unique capabilities of physicians and our AI tools will be seamlessly intertwined to optimize patient care.”
This CPU was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed relationships with Olympus, Neptune Medical, Conmed, and others.
FROM GASTROENTEROLOGY
Experts offer guidance on GLP-1 receptor agonists prior to endoscopy
to support the success of endoscopic procedures, according to a new Clinical Practice Update from the American Gastroenterological Association.
Use of glucagon-like peptide 1 (GLP-1) receptor agonists (GLP-1 RAs) has been associated with delayed gastric emptying, which raises a clinical concern about performing endoscopic procedures, especially upper endoscopies in patients using these medications, wrote Jana G. Al Hashash, MD, MSc, of the Mayo Clinic, Jacksonville, Fla., and colleagues.
The Clinical Practice Update (CPU), published in Clinical Gastroenterology and Hepatology, reviews the evidence and provides expert advice for clinicians on the evolving landscape of patients taking GLP-1 receptor agonists prior to endoscopic procedures. The CPU reflects on the most recent literature and the experience of the authors, all experts in bariatric medicine and/or endoscopy.
The American Society of Anesthesiologists (ASA) issued guidance that reflects concerns for the risk of aspiration in sedated patients because of delayed gastric motility from the use of GLP-1 RAs. The ASA advises patients on daily doses of GLP-1 RAs to refrain from taking the medications on the day of a procedure; those on weekly dosing should hold the drugs for a week prior to surgery.
However, the ASA suggestions do not differentiate based on the indication for the drug or for the type of procedure, and questions remain as to whether these changes are necessary and/or effective, the CPU authors said. The ASA’s guidance is based mainly on expert opinion, as not enough published evidence on this topic exists for a robust review and formal guideline, they added.
Recently, a multisociety statement from the AGA, AASLD, ACG, ASGE, and NASPGHAN noted that widespread implementation of the ASA guidance could be associated with unintended harms to patients.
Therefore, the AGA CPU suggests an individualized approach to managing patients on GLP-1 RAs in a pre-endoscopic setting.
For patients on GLP-1 RAs for diabetes management, discontinuing prior to endoscopic may not be worth the potential risk. Also, consider not only the dose and frequency of the GLP-1 RAs but also other comorbidities, medications, and potential gastrointestinal side effects.
“If patients taking GLP-1 RAs solely for weight loss can be identified beforehand, a dose of the medication could be withheld prior to endoscopy with likely little harm, though this should not be considered mandatory or evidence-based,” the CPU authors wrote.
However, withholding a single dose of medication may not be enough for an individual’s gastric motility to return to normal, the authors emphasized.
Additionally, the ASA’s suggestions for holding GLP-1 RAs add complexity to periprocedural medication management, which may strain resources and delay care.
The AGA CPU offers the following guidance for patients on GLP-1 RAs prior to endoscopy:
In general, patients using GLP-1 RAs who have followed the standard perioperative procedures, usually an 8-hour solid-food fast and 2-hour liquid fast, and who do not have symptoms such as ongoing nausea, vomiting, or abdominal distension should proceed with upper and/or lower endoscopy.
For symptomatic patients who may experience negative clinical consequences of endoscopy if delayed, consider rapid-sequence intubation, but the authors acknowledge that this option may not be possible in most ambulatory or office-based endoscopy settings.
Finally, consider placing patients on a liquid diet the day before a sedated procedure instead of stopping GLP-1 RAs; this strategy is “more consistent with the holistic approach to preprocedural management of other similar condi-tions,” the authors said.
The current CPU endorses the multi-society statement that puts patient safety first and encourages AGA members to follow best practices when performing endoscopies on patients who are using GLP-1 RAs, in the absence of actionable data, the authors concluded.
The Clinical Practice Update received no outside funding. Lead author Dr. Al Hashash had no financial conflicts to disclose.
to support the success of endoscopic procedures, according to a new Clinical Practice Update from the American Gastroenterological Association.
Use of glucagon-like peptide 1 (GLP-1) receptor agonists (GLP-1 RAs) has been associated with delayed gastric emptying, which raises a clinical concern about performing endoscopic procedures, especially upper endoscopies in patients using these medications, wrote Jana G. Al Hashash, MD, MSc, of the Mayo Clinic, Jacksonville, Fla., and colleagues.
The Clinical Practice Update (CPU), published in Clinical Gastroenterology and Hepatology, reviews the evidence and provides expert advice for clinicians on the evolving landscape of patients taking GLP-1 receptor agonists prior to endoscopic procedures. The CPU reflects on the most recent literature and the experience of the authors, all experts in bariatric medicine and/or endoscopy.
The American Society of Anesthesiologists (ASA) issued guidance that reflects concerns for the risk of aspiration in sedated patients because of delayed gastric motility from the use of GLP-1 RAs. The ASA advises patients on daily doses of GLP-1 RAs to refrain from taking the medications on the day of a procedure; those on weekly dosing should hold the drugs for a week prior to surgery.
However, the ASA suggestions do not differentiate based on the indication for the drug or for the type of procedure, and questions remain as to whether these changes are necessary and/or effective, the CPU authors said. The ASA’s guidance is based mainly on expert opinion, as not enough published evidence on this topic exists for a robust review and formal guideline, they added.
Recently, a multisociety statement from the AGA, AASLD, ACG, ASGE, and NASPGHAN noted that widespread implementation of the ASA guidance could be associated with unintended harms to patients.
Therefore, the AGA CPU suggests an individualized approach to managing patients on GLP-1 RAs in a pre-endoscopic setting.
For patients on GLP-1 RAs for diabetes management, discontinuing prior to endoscopic may not be worth the potential risk. Also, consider not only the dose and frequency of the GLP-1 RAs but also other comorbidities, medications, and potential gastrointestinal side effects.
“If patients taking GLP-1 RAs solely for weight loss can be identified beforehand, a dose of the medication could be withheld prior to endoscopy with likely little harm, though this should not be considered mandatory or evidence-based,” the CPU authors wrote.
However, withholding a single dose of medication may not be enough for an individual’s gastric motility to return to normal, the authors emphasized.
Additionally, the ASA’s suggestions for holding GLP-1 RAs add complexity to periprocedural medication management, which may strain resources and delay care.
The AGA CPU offers the following guidance for patients on GLP-1 RAs prior to endoscopy:
In general, patients using GLP-1 RAs who have followed the standard perioperative procedures, usually an 8-hour solid-food fast and 2-hour liquid fast, and who do not have symptoms such as ongoing nausea, vomiting, or abdominal distension should proceed with upper and/or lower endoscopy.
For symptomatic patients who may experience negative clinical consequences of endoscopy if delayed, consider rapid-sequence intubation, but the authors acknowledge that this option may not be possible in most ambulatory or office-based endoscopy settings.
Finally, consider placing patients on a liquid diet the day before a sedated procedure instead of stopping GLP-1 RAs; this strategy is “more consistent with the holistic approach to preprocedural management of other similar condi-tions,” the authors said.
The current CPU endorses the multi-society statement that puts patient safety first and encourages AGA members to follow best practices when performing endoscopies on patients who are using GLP-1 RAs, in the absence of actionable data, the authors concluded.
The Clinical Practice Update received no outside funding. Lead author Dr. Al Hashash had no financial conflicts to disclose.
to support the success of endoscopic procedures, according to a new Clinical Practice Update from the American Gastroenterological Association.
Use of glucagon-like peptide 1 (GLP-1) receptor agonists (GLP-1 RAs) has been associated with delayed gastric emptying, which raises a clinical concern about performing endoscopic procedures, especially upper endoscopies in patients using these medications, wrote Jana G. Al Hashash, MD, MSc, of the Mayo Clinic, Jacksonville, Fla., and colleagues.
The Clinical Practice Update (CPU), published in Clinical Gastroenterology and Hepatology, reviews the evidence and provides expert advice for clinicians on the evolving landscape of patients taking GLP-1 receptor agonists prior to endoscopic procedures. The CPU reflects on the most recent literature and the experience of the authors, all experts in bariatric medicine and/or endoscopy.
The American Society of Anesthesiologists (ASA) issued guidance that reflects concerns for the risk of aspiration in sedated patients because of delayed gastric motility from the use of GLP-1 RAs. The ASA advises patients on daily doses of GLP-1 RAs to refrain from taking the medications on the day of a procedure; those on weekly dosing should hold the drugs for a week prior to surgery.
However, the ASA suggestions do not differentiate based on the indication for the drug or for the type of procedure, and questions remain as to whether these changes are necessary and/or effective, the CPU authors said. The ASA’s guidance is based mainly on expert opinion, as not enough published evidence on this topic exists for a robust review and formal guideline, they added.
Recently, a multisociety statement from the AGA, AASLD, ACG, ASGE, and NASPGHAN noted that widespread implementation of the ASA guidance could be associated with unintended harms to patients.
Therefore, the AGA CPU suggests an individualized approach to managing patients on GLP-1 RAs in a pre-endoscopic setting.
For patients on GLP-1 RAs for diabetes management, discontinuing prior to endoscopic may not be worth the potential risk. Also, consider not only the dose and frequency of the GLP-1 RAs but also other comorbidities, medications, and potential gastrointestinal side effects.
“If patients taking GLP-1 RAs solely for weight loss can be identified beforehand, a dose of the medication could be withheld prior to endoscopy with likely little harm, though this should not be considered mandatory or evidence-based,” the CPU authors wrote.
However, withholding a single dose of medication may not be enough for an individual’s gastric motility to return to normal, the authors emphasized.
Additionally, the ASA’s suggestions for holding GLP-1 RAs add complexity to periprocedural medication management, which may strain resources and delay care.
The AGA CPU offers the following guidance for patients on GLP-1 RAs prior to endoscopy:
In general, patients using GLP-1 RAs who have followed the standard perioperative procedures, usually an 8-hour solid-food fast and 2-hour liquid fast, and who do not have symptoms such as ongoing nausea, vomiting, or abdominal distension should proceed with upper and/or lower endoscopy.
For symptomatic patients who may experience negative clinical consequences of endoscopy if delayed, consider rapid-sequence intubation, but the authors acknowledge that this option may not be possible in most ambulatory or office-based endoscopy settings.
Finally, consider placing patients on a liquid diet the day before a sedated procedure instead of stopping GLP-1 RAs; this strategy is “more consistent with the holistic approach to preprocedural management of other similar condi-tions,” the authors said.
The current CPU endorses the multi-society statement that puts patient safety first and encourages AGA members to follow best practices when performing endoscopies on patients who are using GLP-1 RAs, in the absence of actionable data, the authors concluded.
The Clinical Practice Update received no outside funding. Lead author Dr. Al Hashash had no financial conflicts to disclose.
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Cold snare polypectomy underused despite recommendations
, shows new research presented this week in Vancouver at the annual meeting of the American College of Gastroenterology.
Polypectomy is a key part of colorectal cancer prevention, but endoscopists’ choice of polypectomy is a major factor in quality, and the characteristics of polypectomies in clinical practice are highly variable, said Seth D. Crockett, MD, of Oregon Health & Science University, Portland, in a presentation at the meeting.
Cold snare polypectomy is preferred for the removal of polyps less than 1 cm because of a high complete resection rate and a strong safety profile, compared to forceps and hot snares, which tend to be associated with high incomplete resection rates, inadequate histopathologic specimens, and/or complication rates. The adherence of endoscopists to the recommendations was not known until now, Dr. Crockett said.
This was a cross-sectional study of 1,589,499 colonoscopies that were conducted between 2019 and 2022 in patients (aged 40-80 years) who underwent a screening or surveillance colonoscopy in which at least one small polyp of less than 1 cm was removed. The final analysis included 3,082 endoscopists. Colonoscopies in which larger polyps were detected, or there was a confirmed case of cancer, were not included.
The mean endoscopist cold snare polypectomy rate (CSPR) was 51.2%, which was “lower than expected based on current guideline recommendations,” Dr. Crockett said.
Higher cold snare polypectomy rates were more common among specialists with training in gastroenterology, and more common among those who practiced in the Midwest (69%), as compared with practitioners in the Northeast who, at 40%, had the lowest rate. Colonoscopy volume, adenoma detection rate (ADR), serrated polyp detection rate (SDR), and cecal intubation rate (CIR), were all associated with a higher CSPR.
CSPR was more than 30% higher for endoscopists with an adenoma detection rate (ADR) of greater than 35%, compared with those with an ADR of less than 25% (58% vs. 27%, respectively; P < .0001). Lower usage rates among endoscopists with low ADRs could compound the problem of interval cancer if polyps are missed, Dr. Crockett said. Endoscopist serrated polyp detection rates of 7% of higher, cecal intubation rates of 95% or higher, and mean withdrawal times greater than 9 minutes were significantly associated with higher CSPR (P < .0001 for all).
The findings suggest a correlation between higher cold snare usage and improved quality metrics, such as adenoma detection rate and cecal intubation rate, said Jonathan A. Leighton, MD, of the Mayo Clinic, Scottsdale, Ariz., in an interview.
“I would agree with the authors that much of the focus on colonoscopy quality has been directed toward polyp detection, and little on the quality of polyp resection, which can be difficult to measure,” he said. “Their results suggest that cold snare polypectomy for removal of small polyps is currently underutilized, but as with any polypectomy, it is important that all of the dysplastic tissue is removed using good technique.”
The results were strengthened by the large sample size and high fidelity of measurements of polyp size, polypectomy tools, and quality measures. But more research is needed to determine the impact of polypectomy technique on outcomes of colonoscopy efficacy and safety. In terms of limitations, small polyps carry a relatively low risk of recurrence, and the associations between an endoscopist’s polypectomy practice and polyp recurrence, interval cancer, and adverse events were not examined, Dr. Crockett said.
The study was supported by a grant from the ACG. Dr. Crockett disclosed relationships with Carelon, Exact Sciences, Freenome, and Guardant.
, shows new research presented this week in Vancouver at the annual meeting of the American College of Gastroenterology.
Polypectomy is a key part of colorectal cancer prevention, but endoscopists’ choice of polypectomy is a major factor in quality, and the characteristics of polypectomies in clinical practice are highly variable, said Seth D. Crockett, MD, of Oregon Health & Science University, Portland, in a presentation at the meeting.
Cold snare polypectomy is preferred for the removal of polyps less than 1 cm because of a high complete resection rate and a strong safety profile, compared to forceps and hot snares, which tend to be associated with high incomplete resection rates, inadequate histopathologic specimens, and/or complication rates. The adherence of endoscopists to the recommendations was not known until now, Dr. Crockett said.
This was a cross-sectional study of 1,589,499 colonoscopies that were conducted between 2019 and 2022 in patients (aged 40-80 years) who underwent a screening or surveillance colonoscopy in which at least one small polyp of less than 1 cm was removed. The final analysis included 3,082 endoscopists. Colonoscopies in which larger polyps were detected, or there was a confirmed case of cancer, were not included.
The mean endoscopist cold snare polypectomy rate (CSPR) was 51.2%, which was “lower than expected based on current guideline recommendations,” Dr. Crockett said.
Higher cold snare polypectomy rates were more common among specialists with training in gastroenterology, and more common among those who practiced in the Midwest (69%), as compared with practitioners in the Northeast who, at 40%, had the lowest rate. Colonoscopy volume, adenoma detection rate (ADR), serrated polyp detection rate (SDR), and cecal intubation rate (CIR), were all associated with a higher CSPR.
CSPR was more than 30% higher for endoscopists with an adenoma detection rate (ADR) of greater than 35%, compared with those with an ADR of less than 25% (58% vs. 27%, respectively; P < .0001). Lower usage rates among endoscopists with low ADRs could compound the problem of interval cancer if polyps are missed, Dr. Crockett said. Endoscopist serrated polyp detection rates of 7% of higher, cecal intubation rates of 95% or higher, and mean withdrawal times greater than 9 minutes were significantly associated with higher CSPR (P < .0001 for all).
The findings suggest a correlation between higher cold snare usage and improved quality metrics, such as adenoma detection rate and cecal intubation rate, said Jonathan A. Leighton, MD, of the Mayo Clinic, Scottsdale, Ariz., in an interview.
“I would agree with the authors that much of the focus on colonoscopy quality has been directed toward polyp detection, and little on the quality of polyp resection, which can be difficult to measure,” he said. “Their results suggest that cold snare polypectomy for removal of small polyps is currently underutilized, but as with any polypectomy, it is important that all of the dysplastic tissue is removed using good technique.”
The results were strengthened by the large sample size and high fidelity of measurements of polyp size, polypectomy tools, and quality measures. But more research is needed to determine the impact of polypectomy technique on outcomes of colonoscopy efficacy and safety. In terms of limitations, small polyps carry a relatively low risk of recurrence, and the associations between an endoscopist’s polypectomy practice and polyp recurrence, interval cancer, and adverse events were not examined, Dr. Crockett said.
The study was supported by a grant from the ACG. Dr. Crockett disclosed relationships with Carelon, Exact Sciences, Freenome, and Guardant.
, shows new research presented this week in Vancouver at the annual meeting of the American College of Gastroenterology.
Polypectomy is a key part of colorectal cancer prevention, but endoscopists’ choice of polypectomy is a major factor in quality, and the characteristics of polypectomies in clinical practice are highly variable, said Seth D. Crockett, MD, of Oregon Health & Science University, Portland, in a presentation at the meeting.
Cold snare polypectomy is preferred for the removal of polyps less than 1 cm because of a high complete resection rate and a strong safety profile, compared to forceps and hot snares, which tend to be associated with high incomplete resection rates, inadequate histopathologic specimens, and/or complication rates. The adherence of endoscopists to the recommendations was not known until now, Dr. Crockett said.
This was a cross-sectional study of 1,589,499 colonoscopies that were conducted between 2019 and 2022 in patients (aged 40-80 years) who underwent a screening or surveillance colonoscopy in which at least one small polyp of less than 1 cm was removed. The final analysis included 3,082 endoscopists. Colonoscopies in which larger polyps were detected, or there was a confirmed case of cancer, were not included.
The mean endoscopist cold snare polypectomy rate (CSPR) was 51.2%, which was “lower than expected based on current guideline recommendations,” Dr. Crockett said.
Higher cold snare polypectomy rates were more common among specialists with training in gastroenterology, and more common among those who practiced in the Midwest (69%), as compared with practitioners in the Northeast who, at 40%, had the lowest rate. Colonoscopy volume, adenoma detection rate (ADR), serrated polyp detection rate (SDR), and cecal intubation rate (CIR), were all associated with a higher CSPR.
CSPR was more than 30% higher for endoscopists with an adenoma detection rate (ADR) of greater than 35%, compared with those with an ADR of less than 25% (58% vs. 27%, respectively; P < .0001). Lower usage rates among endoscopists with low ADRs could compound the problem of interval cancer if polyps are missed, Dr. Crockett said. Endoscopist serrated polyp detection rates of 7% of higher, cecal intubation rates of 95% or higher, and mean withdrawal times greater than 9 minutes were significantly associated with higher CSPR (P < .0001 for all).
The findings suggest a correlation between higher cold snare usage and improved quality metrics, such as adenoma detection rate and cecal intubation rate, said Jonathan A. Leighton, MD, of the Mayo Clinic, Scottsdale, Ariz., in an interview.
“I would agree with the authors that much of the focus on colonoscopy quality has been directed toward polyp detection, and little on the quality of polyp resection, which can be difficult to measure,” he said. “Their results suggest that cold snare polypectomy for removal of small polyps is currently underutilized, but as with any polypectomy, it is important that all of the dysplastic tissue is removed using good technique.”
The results were strengthened by the large sample size and high fidelity of measurements of polyp size, polypectomy tools, and quality measures. But more research is needed to determine the impact of polypectomy technique on outcomes of colonoscopy efficacy and safety. In terms of limitations, small polyps carry a relatively low risk of recurrence, and the associations between an endoscopist’s polypectomy practice and polyp recurrence, interval cancer, and adverse events were not examined, Dr. Crockett said.
The study was supported by a grant from the ACG. Dr. Crockett disclosed relationships with Carelon, Exact Sciences, Freenome, and Guardant.
FROM ACG 2023
Endoscopic remission doubled with risankizumab vs. ustekinumab in Crohn’s disease
AT UEG WEEK 2023
COPENHAGEN – (CD) who have failed one or more anti–tumor necrosis factor (anti-TNF) therapies, according to the results of the phase 3 SEQUENCE trial.
Secondary endpoints – presented for the first time at the United European Gastroenterology Week 2023 – also showed superiority of risankizumab (Skyrizi, AbbVie), an interleulin-23 inhibitor, over ustekinumab (Stelara), an IL-12 and IL-23 inhibitor, for clinical remission at week 48 (60.8% vs. 40.8%) and a statistically significant endoscopic response also favoring risankizumab at weeks 24 and 48.
“With endoscopic remission we see that with a single agent we have doubled the endoscopic remission rate by moving from 16% to 31% with risankizumab [at week 48],” said Laurent Peyrin-Biroulet, MD, PhD, a gastroenterologist specializing in inflammatory bowel disease at Nancy University Hospital, France. “Superiority for sure was met.”
“This sort of thing happens once in your career,” noted Dr. Peyrin-Biroulet, who presented the results of the study at the meeting. “It’s totally amazing that everything you see here was in favor of risankizumab.
“Already we see the efficacy signal in the proportion of premature discontinuations at 2% vs. 13% due to lack of efficacy [in risankizumab and ustekinumab, respectively],” he said. “This is due to drug failure.”
Risankizumab is an IL-23 inhibitor that selectively blocks the cytokine IL-23, thought to be linked to a number of chronic immune-mediated diseases, by binding to its p19 subunit. It is the first IL-23 inhibitor to receive approval from the U.S. Food and Drug Administration in June 2022 for moderately to severely active CD based on data from the ADVANCE, MOTIVATE, and FORTIFY trials.
Risankizumab and ustekinumab head-to-head
The phase 3, open-label, multicenter, randomized, clinical trial evaluated risankizumab vs. ustekinumab through week 48 in patients with moderately to severely active CD.
Participants were required to have a CD Activity Index (CDAI) score of 220 to 450 at baseline, a Simple Endoscopic Score for Crohn’s Disease (SES-CD) of 6 or more for ileocolonic or colonic disease (and of 4 or more for isolated ileal disease), excluding the presence of a narrowing component, plus an average daily stool frequency of four or more and/or average daily abdominal pain score of 2 or more. They were also required to have previously failed one or more anti-TNF therapies.
Randomization was stratified by the number of anti-TNF therapies failed (one or more than one), and steroid use at baseline; steroids were then tapered from week 2. Two primary endpoints comprised clinical remission at week 24 (defined as CDAI < 150, noninferiority margin within 10% of risankizumab vs ustekinumab in 50% of participants), and also endoscopic remission (SES-CD of 4 or less, and at least a 2-point reduction vs. baseline and no subscore greater than 1 in any individual component) at week 48 demonstrating superiority of risankizumab vs ustekinumab.
Secondary endpoints included clinical remission at week 48, endoscopic response at weeks 48 and 24, steroid-free endoscopic remission at week 48, and steroid-free clinical remission at week 48 (all tested for superiority of risankizumab vs ustekinumab).
Intravenous risankizumab at 600 mg was given at weeks 0, 4 , and 8 followed by subcutaneous risankizumab at a 360-mg maintenance dose every 8 weeks through week 48 (n = 255). Participants who completed the week-48 visit continued on subcutaneous risankizumab for up to an additional 220 weeks. Ustekinumab was given as a weight-based, intravenous induction dose at week 0 followed by a 90-mg subcutaneous dose every 8 weeks, starting at week 8 through week 48 (n = 265). Participants received open-label drug administration but efficacy assessment was blinded.
Superiority of risankizumab
Both primary endpoints were met. For clinical remission at week 24, in half of the patients enrolled, rates were 58.6% (75/128) for risankizumab and 39.5% (54/137) for ustekinumab, for a difference of 18.4% [95% confidence interval, 6.6-30.3], meaning that noninferiority was met within the predefined margin of 10%. The second primary endpoint of endoscopic remission at week 48 showed rates of 31.8% (81/255) for risankizumab and 16.2% (43/265) for ustekinumab (P < .0001 for superiority).
Risankizumab was found to be superior to ustekinumab for all secondary endpoints (all with P < .0001). Steroid-free endoscopic remission at week 48 showed a 16% difference, and steroid-free clinical remission at week 48 showed a 20% difference – both in favor of risankizumab.
In addition, more participants on risankizumab completed the study (89.4%) than those on ustekinumab (74.0%), Dr. Peyrin-Biroulet reported.
Adverse event rates (events per 100 person-years) were comparable between the two drugs at 341.2 for risankizumab and 282.7 for ustekinumab. For risankizumab, no new safety risks were observed, and those recorded were consistent with the known safety profile. Serious adverse events occurred in 10% of risankizumab-treated patients, and 17% of ustekinumab-treated patients.
“We know the safety of IL-23 inhibitors is good,” said Dr. Peyrin-Biroulet. “If we look at all adverse events there was no difference across arms, and in terms of serious adverse events, it was in favor of risankizumab because a CD flare is considered a serious adverse event.”
Session comoderator, Alessandro Armuzzi, MD, head of the Inflammatory Bowel Disease Center at the IRCCS Humanitas Research Hospital in Milan, commented on the findings. “The results look in favor of risankizumab – all the endpoints were met, not only the co-endpoints but also the secondary endpoints too,” he said.
These results, showing a preference for risankizumab, have value in helping clinicians with the sequence of therapies when patients with Crohn’s disease have failed one or more TNF inhibitor, said Dr. Armuzzi.
No funding for this study was disclosed. Dr. Peyrin-Biroulet has disclosed receiving fees from Galapagos, AbbVie, Janssen, Genentech, Alimentiv, Ferring, Tillots, Celltrion, Takeda, Pfizer, Index, Sandoz, Celgene, Biogen, SamsungBioepis, Inotrem, Allergan, MSD, Roche, Arena, Gilead, Amgen, BMS, Vifor, Norgine, Mylan, Lilly, Fresenius Kabi, OSEImmunotherapeutics, Enthera, Theravance, Pandion, Gossamer, Viatris, ThermoFisher, ONOPharma, Mopac, Cytoki, Morphic, Prometheus, and Applied MolecularTransport. Dr. Armuzzi disclosed consulting/advisory board fees from AbbVie, Amgen, Arena, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, Lionhealth, MSD, Nestlé, Pfizer, Protagonist Therapeutics, Roche, Samsung Bioepis, Sandoz, Takeda, and Tillots Pharma; speaker’s fees from AbbVie, Amgen, Arena, Biogen, Bristol-Myers Squibb, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, Lionhealth, MSD, Novartis, Pfizer, Roche, Samsung Bioepis, Sandoz, and Takeda; and research grants from MSD, Takeda, Pfizer, and Biogen.
A version of this article first appeared on Medscape.com.
AT UEG WEEK 2023
COPENHAGEN – (CD) who have failed one or more anti–tumor necrosis factor (anti-TNF) therapies, according to the results of the phase 3 SEQUENCE trial.
Secondary endpoints – presented for the first time at the United European Gastroenterology Week 2023 – also showed superiority of risankizumab (Skyrizi, AbbVie), an interleulin-23 inhibitor, over ustekinumab (Stelara), an IL-12 and IL-23 inhibitor, for clinical remission at week 48 (60.8% vs. 40.8%) and a statistically significant endoscopic response also favoring risankizumab at weeks 24 and 48.
“With endoscopic remission we see that with a single agent we have doubled the endoscopic remission rate by moving from 16% to 31% with risankizumab [at week 48],” said Laurent Peyrin-Biroulet, MD, PhD, a gastroenterologist specializing in inflammatory bowel disease at Nancy University Hospital, France. “Superiority for sure was met.”
“This sort of thing happens once in your career,” noted Dr. Peyrin-Biroulet, who presented the results of the study at the meeting. “It’s totally amazing that everything you see here was in favor of risankizumab.
“Already we see the efficacy signal in the proportion of premature discontinuations at 2% vs. 13% due to lack of efficacy [in risankizumab and ustekinumab, respectively],” he said. “This is due to drug failure.”
Risankizumab is an IL-23 inhibitor that selectively blocks the cytokine IL-23, thought to be linked to a number of chronic immune-mediated diseases, by binding to its p19 subunit. It is the first IL-23 inhibitor to receive approval from the U.S. Food and Drug Administration in June 2022 for moderately to severely active CD based on data from the ADVANCE, MOTIVATE, and FORTIFY trials.
Risankizumab and ustekinumab head-to-head
The phase 3, open-label, multicenter, randomized, clinical trial evaluated risankizumab vs. ustekinumab through week 48 in patients with moderately to severely active CD.
Participants were required to have a CD Activity Index (CDAI) score of 220 to 450 at baseline, a Simple Endoscopic Score for Crohn’s Disease (SES-CD) of 6 or more for ileocolonic or colonic disease (and of 4 or more for isolated ileal disease), excluding the presence of a narrowing component, plus an average daily stool frequency of four or more and/or average daily abdominal pain score of 2 or more. They were also required to have previously failed one or more anti-TNF therapies.
Randomization was stratified by the number of anti-TNF therapies failed (one or more than one), and steroid use at baseline; steroids were then tapered from week 2. Two primary endpoints comprised clinical remission at week 24 (defined as CDAI < 150, noninferiority margin within 10% of risankizumab vs ustekinumab in 50% of participants), and also endoscopic remission (SES-CD of 4 or less, and at least a 2-point reduction vs. baseline and no subscore greater than 1 in any individual component) at week 48 demonstrating superiority of risankizumab vs ustekinumab.
Secondary endpoints included clinical remission at week 48, endoscopic response at weeks 48 and 24, steroid-free endoscopic remission at week 48, and steroid-free clinical remission at week 48 (all tested for superiority of risankizumab vs ustekinumab).
Intravenous risankizumab at 600 mg was given at weeks 0, 4 , and 8 followed by subcutaneous risankizumab at a 360-mg maintenance dose every 8 weeks through week 48 (n = 255). Participants who completed the week-48 visit continued on subcutaneous risankizumab for up to an additional 220 weeks. Ustekinumab was given as a weight-based, intravenous induction dose at week 0 followed by a 90-mg subcutaneous dose every 8 weeks, starting at week 8 through week 48 (n = 265). Participants received open-label drug administration but efficacy assessment was blinded.
Superiority of risankizumab
Both primary endpoints were met. For clinical remission at week 24, in half of the patients enrolled, rates were 58.6% (75/128) for risankizumab and 39.5% (54/137) for ustekinumab, for a difference of 18.4% [95% confidence interval, 6.6-30.3], meaning that noninferiority was met within the predefined margin of 10%. The second primary endpoint of endoscopic remission at week 48 showed rates of 31.8% (81/255) for risankizumab and 16.2% (43/265) for ustekinumab (P < .0001 for superiority).
Risankizumab was found to be superior to ustekinumab for all secondary endpoints (all with P < .0001). Steroid-free endoscopic remission at week 48 showed a 16% difference, and steroid-free clinical remission at week 48 showed a 20% difference – both in favor of risankizumab.
In addition, more participants on risankizumab completed the study (89.4%) than those on ustekinumab (74.0%), Dr. Peyrin-Biroulet reported.
Adverse event rates (events per 100 person-years) were comparable between the two drugs at 341.2 for risankizumab and 282.7 for ustekinumab. For risankizumab, no new safety risks were observed, and those recorded were consistent with the known safety profile. Serious adverse events occurred in 10% of risankizumab-treated patients, and 17% of ustekinumab-treated patients.
“We know the safety of IL-23 inhibitors is good,” said Dr. Peyrin-Biroulet. “If we look at all adverse events there was no difference across arms, and in terms of serious adverse events, it was in favor of risankizumab because a CD flare is considered a serious adverse event.”
Session comoderator, Alessandro Armuzzi, MD, head of the Inflammatory Bowel Disease Center at the IRCCS Humanitas Research Hospital in Milan, commented on the findings. “The results look in favor of risankizumab – all the endpoints were met, not only the co-endpoints but also the secondary endpoints too,” he said.
These results, showing a preference for risankizumab, have value in helping clinicians with the sequence of therapies when patients with Crohn’s disease have failed one or more TNF inhibitor, said Dr. Armuzzi.
No funding for this study was disclosed. Dr. Peyrin-Biroulet has disclosed receiving fees from Galapagos, AbbVie, Janssen, Genentech, Alimentiv, Ferring, Tillots, Celltrion, Takeda, Pfizer, Index, Sandoz, Celgene, Biogen, SamsungBioepis, Inotrem, Allergan, MSD, Roche, Arena, Gilead, Amgen, BMS, Vifor, Norgine, Mylan, Lilly, Fresenius Kabi, OSEImmunotherapeutics, Enthera, Theravance, Pandion, Gossamer, Viatris, ThermoFisher, ONOPharma, Mopac, Cytoki, Morphic, Prometheus, and Applied MolecularTransport. Dr. Armuzzi disclosed consulting/advisory board fees from AbbVie, Amgen, Arena, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, Lionhealth, MSD, Nestlé, Pfizer, Protagonist Therapeutics, Roche, Samsung Bioepis, Sandoz, Takeda, and Tillots Pharma; speaker’s fees from AbbVie, Amgen, Arena, Biogen, Bristol-Myers Squibb, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, Lionhealth, MSD, Novartis, Pfizer, Roche, Samsung Bioepis, Sandoz, and Takeda; and research grants from MSD, Takeda, Pfizer, and Biogen.
A version of this article first appeared on Medscape.com.
AT UEG WEEK 2023
COPENHAGEN – (CD) who have failed one or more anti–tumor necrosis factor (anti-TNF) therapies, according to the results of the phase 3 SEQUENCE trial.
Secondary endpoints – presented for the first time at the United European Gastroenterology Week 2023 – also showed superiority of risankizumab (Skyrizi, AbbVie), an interleulin-23 inhibitor, over ustekinumab (Stelara), an IL-12 and IL-23 inhibitor, for clinical remission at week 48 (60.8% vs. 40.8%) and a statistically significant endoscopic response also favoring risankizumab at weeks 24 and 48.
“With endoscopic remission we see that with a single agent we have doubled the endoscopic remission rate by moving from 16% to 31% with risankizumab [at week 48],” said Laurent Peyrin-Biroulet, MD, PhD, a gastroenterologist specializing in inflammatory bowel disease at Nancy University Hospital, France. “Superiority for sure was met.”
“This sort of thing happens once in your career,” noted Dr. Peyrin-Biroulet, who presented the results of the study at the meeting. “It’s totally amazing that everything you see here was in favor of risankizumab.
“Already we see the efficacy signal in the proportion of premature discontinuations at 2% vs. 13% due to lack of efficacy [in risankizumab and ustekinumab, respectively],” he said. “This is due to drug failure.”
Risankizumab is an IL-23 inhibitor that selectively blocks the cytokine IL-23, thought to be linked to a number of chronic immune-mediated diseases, by binding to its p19 subunit. It is the first IL-23 inhibitor to receive approval from the U.S. Food and Drug Administration in June 2022 for moderately to severely active CD based on data from the ADVANCE, MOTIVATE, and FORTIFY trials.
Risankizumab and ustekinumab head-to-head
The phase 3, open-label, multicenter, randomized, clinical trial evaluated risankizumab vs. ustekinumab through week 48 in patients with moderately to severely active CD.
Participants were required to have a CD Activity Index (CDAI) score of 220 to 450 at baseline, a Simple Endoscopic Score for Crohn’s Disease (SES-CD) of 6 or more for ileocolonic or colonic disease (and of 4 or more for isolated ileal disease), excluding the presence of a narrowing component, plus an average daily stool frequency of four or more and/or average daily abdominal pain score of 2 or more. They were also required to have previously failed one or more anti-TNF therapies.
Randomization was stratified by the number of anti-TNF therapies failed (one or more than one), and steroid use at baseline; steroids were then tapered from week 2. Two primary endpoints comprised clinical remission at week 24 (defined as CDAI < 150, noninferiority margin within 10% of risankizumab vs ustekinumab in 50% of participants), and also endoscopic remission (SES-CD of 4 or less, and at least a 2-point reduction vs. baseline and no subscore greater than 1 in any individual component) at week 48 demonstrating superiority of risankizumab vs ustekinumab.
Secondary endpoints included clinical remission at week 48, endoscopic response at weeks 48 and 24, steroid-free endoscopic remission at week 48, and steroid-free clinical remission at week 48 (all tested for superiority of risankizumab vs ustekinumab).
Intravenous risankizumab at 600 mg was given at weeks 0, 4 , and 8 followed by subcutaneous risankizumab at a 360-mg maintenance dose every 8 weeks through week 48 (n = 255). Participants who completed the week-48 visit continued on subcutaneous risankizumab for up to an additional 220 weeks. Ustekinumab was given as a weight-based, intravenous induction dose at week 0 followed by a 90-mg subcutaneous dose every 8 weeks, starting at week 8 through week 48 (n = 265). Participants received open-label drug administration but efficacy assessment was blinded.
Superiority of risankizumab
Both primary endpoints were met. For clinical remission at week 24, in half of the patients enrolled, rates were 58.6% (75/128) for risankizumab and 39.5% (54/137) for ustekinumab, for a difference of 18.4% [95% confidence interval, 6.6-30.3], meaning that noninferiority was met within the predefined margin of 10%. The second primary endpoint of endoscopic remission at week 48 showed rates of 31.8% (81/255) for risankizumab and 16.2% (43/265) for ustekinumab (P < .0001 for superiority).
Risankizumab was found to be superior to ustekinumab for all secondary endpoints (all with P < .0001). Steroid-free endoscopic remission at week 48 showed a 16% difference, and steroid-free clinical remission at week 48 showed a 20% difference – both in favor of risankizumab.
In addition, more participants on risankizumab completed the study (89.4%) than those on ustekinumab (74.0%), Dr. Peyrin-Biroulet reported.
Adverse event rates (events per 100 person-years) were comparable between the two drugs at 341.2 for risankizumab and 282.7 for ustekinumab. For risankizumab, no new safety risks were observed, and those recorded were consistent with the known safety profile. Serious adverse events occurred in 10% of risankizumab-treated patients, and 17% of ustekinumab-treated patients.
“We know the safety of IL-23 inhibitors is good,” said Dr. Peyrin-Biroulet. “If we look at all adverse events there was no difference across arms, and in terms of serious adverse events, it was in favor of risankizumab because a CD flare is considered a serious adverse event.”
Session comoderator, Alessandro Armuzzi, MD, head of the Inflammatory Bowel Disease Center at the IRCCS Humanitas Research Hospital in Milan, commented on the findings. “The results look in favor of risankizumab – all the endpoints were met, not only the co-endpoints but also the secondary endpoints too,” he said.
These results, showing a preference for risankizumab, have value in helping clinicians with the sequence of therapies when patients with Crohn’s disease have failed one or more TNF inhibitor, said Dr. Armuzzi.
No funding for this study was disclosed. Dr. Peyrin-Biroulet has disclosed receiving fees from Galapagos, AbbVie, Janssen, Genentech, Alimentiv, Ferring, Tillots, Celltrion, Takeda, Pfizer, Index, Sandoz, Celgene, Biogen, SamsungBioepis, Inotrem, Allergan, MSD, Roche, Arena, Gilead, Amgen, BMS, Vifor, Norgine, Mylan, Lilly, Fresenius Kabi, OSEImmunotherapeutics, Enthera, Theravance, Pandion, Gossamer, Viatris, ThermoFisher, ONOPharma, Mopac, Cytoki, Morphic, Prometheus, and Applied MolecularTransport. Dr. Armuzzi disclosed consulting/advisory board fees from AbbVie, Amgen, Arena, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, Lionhealth, MSD, Nestlé, Pfizer, Protagonist Therapeutics, Roche, Samsung Bioepis, Sandoz, Takeda, and Tillots Pharma; speaker’s fees from AbbVie, Amgen, Arena, Biogen, Bristol-Myers Squibb, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, Lionhealth, MSD, Novartis, Pfizer, Roche, Samsung Bioepis, Sandoz, and Takeda; and research grants from MSD, Takeda, Pfizer, and Biogen.
A version of this article first appeared on Medscape.com.
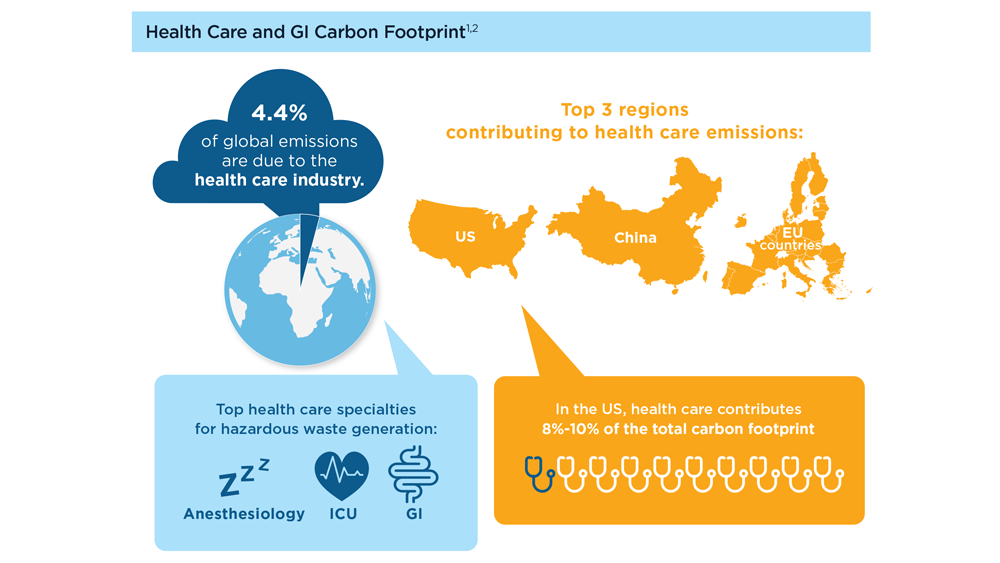
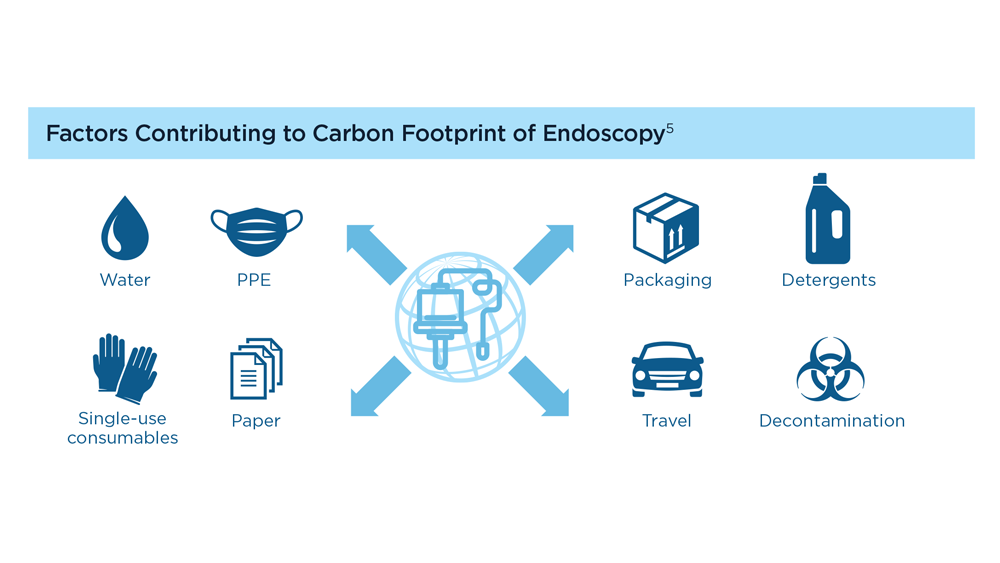
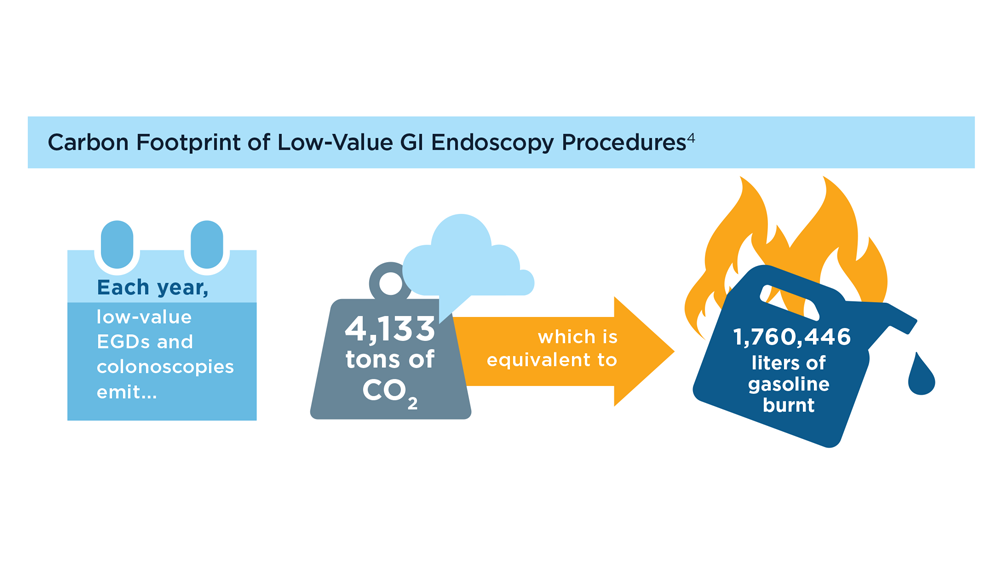
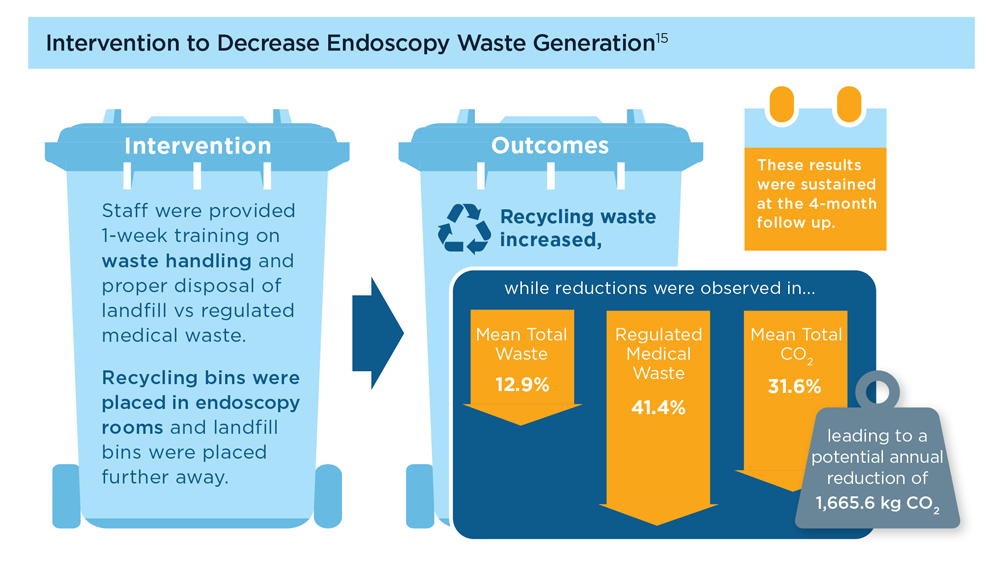
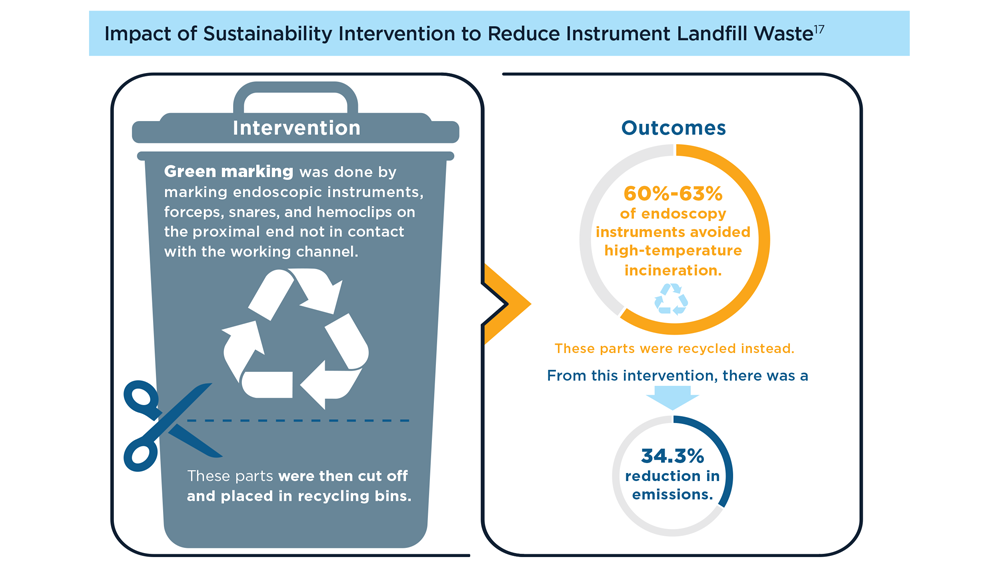
Gastroenterology and Climate Change: Assessing and Mitigating Impacts
- Karliner J et al. Eur J Public Health. 2020;30(suppl 5):v311. doi:10.1093/eurpub/ckaa165.843
- Vaccari M et al. Waste Manag Res. 2018;36(1):39-47. doi:10.1177/0734242X17739968
- Peery AF et al. Gastroenterology. 2019;156(1):254-272.e11. doi:10.1053/j.gastro.2018.08.063
- Sorge A et al. Endoscopy. 2023;55(suppl 2):S72-S73. https://www.esge.com/assets/downloads/pdfs/guidelines/ESGE_Days_2023.pdf
- Maurice JB et al. Lancet Gastroenterol Hepatol. 2020;5(7):636-638. doi:10.1016/S2468-1253(20)30157-6
- Gayam S. Am J Gastroenterol. 2020;115(12):1931-1932. doi:10.14309/ajg.0000000000001005
- Siau K et al. Tech Innov Gastrointest Endosc. 2021;23(4):344-352. doi:10.1016/j.tige.2021.06.005
- Namburar S et al. Gut. 2022;71(7):1326-1331. doi:10.1136/gutjnl-2021-324729
- Haddock R et al. Am J Gastroenterol. 2022;117(3):394-400. doi:10.14309/ajg.0000000000001604
- Donnelly MC et al. J Hepatol. 2022;76(5):995-1000. doi:10.1016/j.jhep.2022.02.01
- Leddin D, Macrae F. J Clin Gastroenterol. 2020;54(5):393-397. doi:10.1097/MCG.0000000000001336
- Pohl H et al. Hepatology. 2022;76(6):1836-1844. doi:10.1002/hep.32810
- Rodríguez de Santiago E et al. Endoscopy. 2022;54(8):797-826. doi:10.1055/a-1859-3726
- Sebastian S et al. Gut. 2023;72(1):12-26. doi:10.1136/gutjnl-2022-328460
- Cunha Neves JA et al. Gut. 2023;72(2):306-313. doi:10.1136/gutjnl-2022-327005
- Kaplan S et al. Issue Brief (Commonw Fund). 2012;29:1-14. PMID:23214181
- López-Muñoz P et al. Gut. 2023;gutjnl-2023-329544. doi:10.1136/gutjnl-2023-329544
- Karliner J et al. Eur J Public Health. 2020;30(suppl 5):v311. doi:10.1093/eurpub/ckaa165.843
- Vaccari M et al. Waste Manag Res. 2018;36(1):39-47. doi:10.1177/0734242X17739968
- Peery AF et al. Gastroenterology. 2019;156(1):254-272.e11. doi:10.1053/j.gastro.2018.08.063
- Sorge A et al. Endoscopy. 2023;55(suppl 2):S72-S73. https://www.esge.com/assets/downloads/pdfs/guidelines/ESGE_Days_2023.pdf
- Maurice JB et al. Lancet Gastroenterol Hepatol. 2020;5(7):636-638. doi:10.1016/S2468-1253(20)30157-6
- Gayam S. Am J Gastroenterol. 2020;115(12):1931-1932. doi:10.14309/ajg.0000000000001005
- Siau K et al. Tech Innov Gastrointest Endosc. 2021;23(4):344-352. doi:10.1016/j.tige.2021.06.005
- Namburar S et al. Gut. 2022;71(7):1326-1331. doi:10.1136/gutjnl-2021-324729
- Haddock R et al. Am J Gastroenterol. 2022;117(3):394-400. doi:10.14309/ajg.0000000000001604
- Donnelly MC et al. J Hepatol. 2022;76(5):995-1000. doi:10.1016/j.jhep.2022.02.01
- Leddin D, Macrae F. J Clin Gastroenterol. 2020;54(5):393-397. doi:10.1097/MCG.0000000000001336
- Pohl H et al. Hepatology. 2022;76(6):1836-1844. doi:10.1002/hep.32810
- Rodríguez de Santiago E et al. Endoscopy. 2022;54(8):797-826. doi:10.1055/a-1859-3726
- Sebastian S et al. Gut. 2023;72(1):12-26. doi:10.1136/gutjnl-2022-328460
- Cunha Neves JA et al. Gut. 2023;72(2):306-313. doi:10.1136/gutjnl-2022-327005
- Kaplan S et al. Issue Brief (Commonw Fund). 2012;29:1-14. PMID:23214181
- López-Muñoz P et al. Gut. 2023;gutjnl-2023-329544. doi:10.1136/gutjnl-2023-329544
- Karliner J et al. Eur J Public Health. 2020;30(suppl 5):v311. doi:10.1093/eurpub/ckaa165.843
- Vaccari M et al. Waste Manag Res. 2018;36(1):39-47. doi:10.1177/0734242X17739968
- Peery AF et al. Gastroenterology. 2019;156(1):254-272.e11. doi:10.1053/j.gastro.2018.08.063
- Sorge A et al. Endoscopy. 2023;55(suppl 2):S72-S73. https://www.esge.com/assets/downloads/pdfs/guidelines/ESGE_Days_2023.pdf
- Maurice JB et al. Lancet Gastroenterol Hepatol. 2020;5(7):636-638. doi:10.1016/S2468-1253(20)30157-6
- Gayam S. Am J Gastroenterol. 2020;115(12):1931-1932. doi:10.14309/ajg.0000000000001005
- Siau K et al. Tech Innov Gastrointest Endosc. 2021;23(4):344-352. doi:10.1016/j.tige.2021.06.005
- Namburar S et al. Gut. 2022;71(7):1326-1331. doi:10.1136/gutjnl-2021-324729
- Haddock R et al. Am J Gastroenterol. 2022;117(3):394-400. doi:10.14309/ajg.0000000000001604
- Donnelly MC et al. J Hepatol. 2022;76(5):995-1000. doi:10.1016/j.jhep.2022.02.01
- Leddin D, Macrae F. J Clin Gastroenterol. 2020;54(5):393-397. doi:10.1097/MCG.0000000000001336
- Pohl H et al. Hepatology. 2022;76(6):1836-1844. doi:10.1002/hep.32810
- Rodríguez de Santiago E et al. Endoscopy. 2022;54(8):797-826. doi:10.1055/a-1859-3726
- Sebastian S et al. Gut. 2023;72(1):12-26. doi:10.1136/gutjnl-2022-328460
- Cunha Neves JA et al. Gut. 2023;72(2):306-313. doi:10.1136/gutjnl-2022-327005
- Kaplan S et al. Issue Brief (Commonw Fund). 2012;29:1-14. PMID:23214181
- López-Muñoz P et al. Gut. 2023;gutjnl-2023-329544. doi:10.1136/gutjnl-2023-329544
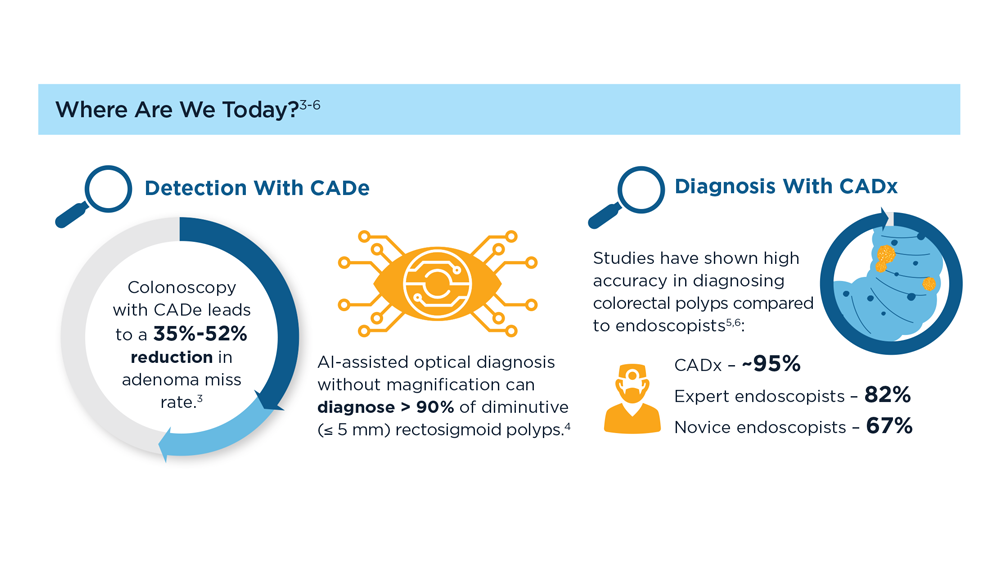
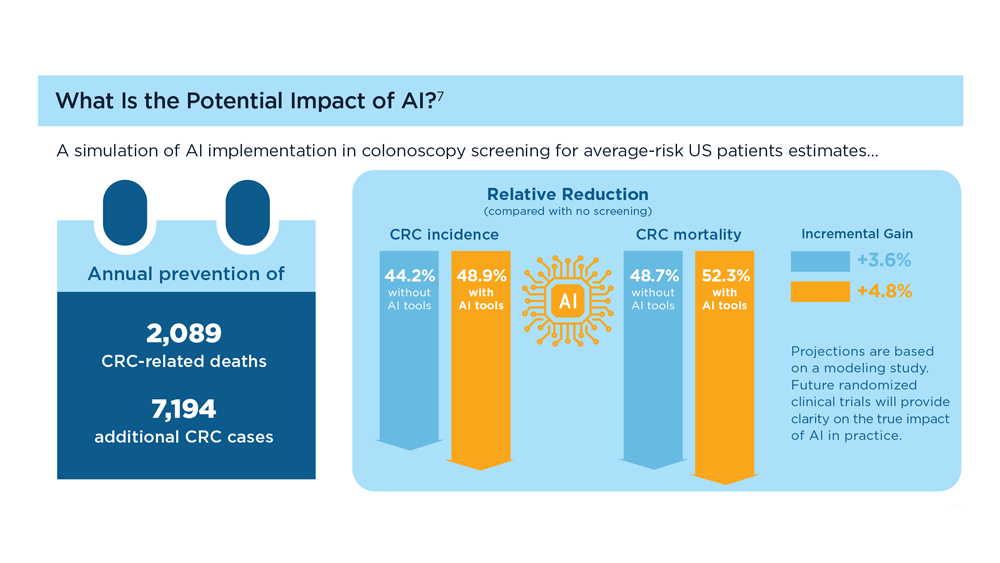
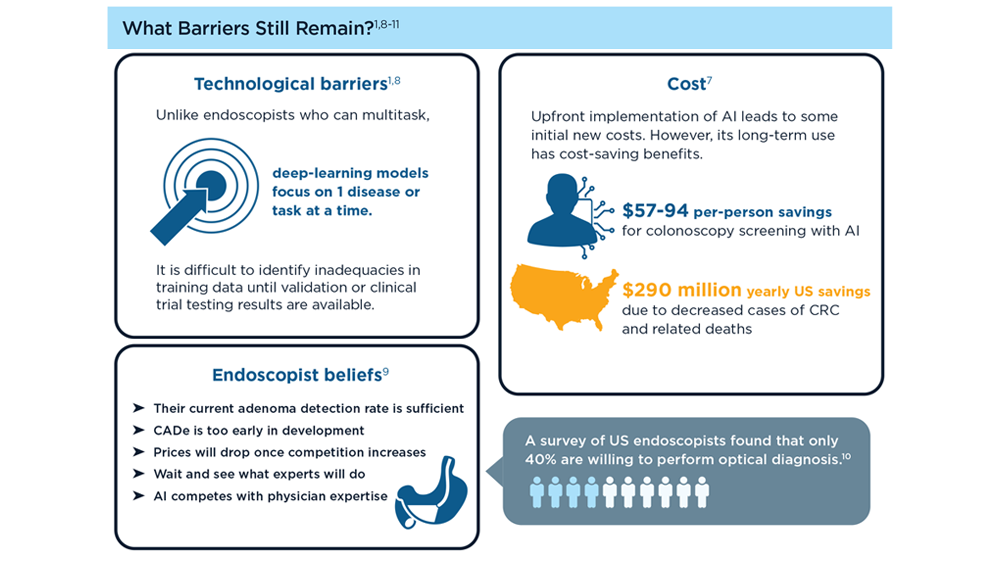
Harnessing the Power of AI to Enhance Endoscopy: Promises and Pitfalls
- Jin Z et al. BioMed Eng OnLine. 2022;21(1):12. doi:10.1186/s12938-022-00979-
- Buendgens L, Cifci D, Ghaffari Laleh N, et al. Sci Rep. 2022;12(1):4829. doi:10.1038/s41598-022-08773-1
- Uche-Anya EN, Berzin TM. Artificial intelligence applications in colonoscopy. GI & Hepatology News. January 24, 2023. https://www.mdedge.com/gihepnews/article/260769/mixed-topics/artificial-intelligence-applications-colonoscopy
- Rondonotti E et al. Endoscopy. 2023;55(1):14-22. doi:10.1055/a-1852-0330
- Antonelli G et al. Ann Gastroenterol. 2023;36(2):114-122. doi:10.20524/aog.2023.0781
- van der Zander QEW et al. Endoscopy. 2021;53(12):1219-1226. doi:10.1055/a-1343-159
- Areia PM et al. Lancet Digital Health. 2022;4(6):e436-e444. doi:10.1016/S2589-7500(22)00042-5
- Sumiyama K et al. Dig Endosc. 2021;33(2):218-230. doi:10.1111/den.13837
- Berzin TM et al. Gastrointest Endosc. 2020;92(4):951-959. doi:10.1016/j.gie.2020.06.035
- Mori Y et al. Dig Endosc. 2023;35(4):422-429. doi:10.1111/den.14531
- Uche-Anya E et al. Gut. 2022;71(9):1909-1915. doi:10.1136/gutjnl-2021-326271
- Moor M et al. Nature. 2023;616(7956):259-265. 10.1038/s41586-023-05881-4
- Kather JN et al. NPJ Digit Med. 2022;5(1):90. doi:10.1038/s41746-022-00634-5
- Jin Z et al. BioMed Eng OnLine. 2022;21(1):12. doi:10.1186/s12938-022-00979-
- Buendgens L, Cifci D, Ghaffari Laleh N, et al. Sci Rep. 2022;12(1):4829. doi:10.1038/s41598-022-08773-1
- Uche-Anya EN, Berzin TM. Artificial intelligence applications in colonoscopy. GI & Hepatology News. January 24, 2023. https://www.mdedge.com/gihepnews/article/260769/mixed-topics/artificial-intelligence-applications-colonoscopy
- Rondonotti E et al. Endoscopy. 2023;55(1):14-22. doi:10.1055/a-1852-0330
- Antonelli G et al. Ann Gastroenterol. 2023;36(2):114-122. doi:10.20524/aog.2023.0781
- van der Zander QEW et al. Endoscopy. 2021;53(12):1219-1226. doi:10.1055/a-1343-159
- Areia PM et al. Lancet Digital Health. 2022;4(6):e436-e444. doi:10.1016/S2589-7500(22)00042-5
- Sumiyama K et al. Dig Endosc. 2021;33(2):218-230. doi:10.1111/den.13837
- Berzin TM et al. Gastrointest Endosc. 2020;92(4):951-959. doi:10.1016/j.gie.2020.06.035
- Mori Y et al. Dig Endosc. 2023;35(4):422-429. doi:10.1111/den.14531
- Uche-Anya E et al. Gut. 2022;71(9):1909-1915. doi:10.1136/gutjnl-2021-326271
- Moor M et al. Nature. 2023;616(7956):259-265. 10.1038/s41586-023-05881-4
- Kather JN et al. NPJ Digit Med. 2022;5(1):90. doi:10.1038/s41746-022-00634-5
- Jin Z et al. BioMed Eng OnLine. 2022;21(1):12. doi:10.1186/s12938-022-00979-
- Buendgens L, Cifci D, Ghaffari Laleh N, et al. Sci Rep. 2022;12(1):4829. doi:10.1038/s41598-022-08773-1
- Uche-Anya EN, Berzin TM. Artificial intelligence applications in colonoscopy. GI & Hepatology News. January 24, 2023. https://www.mdedge.com/gihepnews/article/260769/mixed-topics/artificial-intelligence-applications-colonoscopy
- Rondonotti E et al. Endoscopy. 2023;55(1):14-22. doi:10.1055/a-1852-0330
- Antonelli G et al. Ann Gastroenterol. 2023;36(2):114-122. doi:10.20524/aog.2023.0781
- van der Zander QEW et al. Endoscopy. 2021;53(12):1219-1226. doi:10.1055/a-1343-159
- Areia PM et al. Lancet Digital Health. 2022;4(6):e436-e444. doi:10.1016/S2589-7500(22)00042-5
- Sumiyama K et al. Dig Endosc. 2021;33(2):218-230. doi:10.1111/den.13837
- Berzin TM et al. Gastrointest Endosc. 2020;92(4):951-959. doi:10.1016/j.gie.2020.06.035
- Mori Y et al. Dig Endosc. 2023;35(4):422-429. doi:10.1111/den.14531
- Uche-Anya E et al. Gut. 2022;71(9):1909-1915. doi:10.1136/gutjnl-2021-326271
- Moor M et al. Nature. 2023;616(7956):259-265. 10.1038/s41586-023-05881-4
- Kather JN et al. NPJ Digit Med. 2022;5(1):90. doi:10.1038/s41746-022-00634-5
Gastroenterology Data Trends 2023
In this issue:
- Gastroenterology and Climate Change: Assessing and Mitigating Impacts
Swapna Gayam, MD, FACG - MASLD/MASH and Weight Loss
Arpan Mohanty, MD, MSc - Digital Tools in the Management of IBS/Functional GI Disorders
Eric D. Shah, MD, MBA, FACG - Long COVID and the Gastrointestinal System: Emerging Evidence
Daniel E. Freedberg, MD, MS, and Lin Chang, MD, AGAF - Germline Genetic Testing in CRC: Implications for Familial and Population-Based Testing
Fay Kastrinos, MD, MPH - Evolution of Targeted Therapies for C difficile
Sahil Khanna, MBBS, MS, FACG, AGAF - Harnessing the Power of AI to Enhance Endoscopy: Promises and Pitfalls
Eugenia Uche-Anya, MD, MPH - The Evolving Role of Surgery for IBD
Julie K.M. Thacker, MD, FACS, FASCRS
In this issue:
- Gastroenterology and Climate Change: Assessing and Mitigating Impacts
Swapna Gayam, MD, FACG - MASLD/MASH and Weight Loss
Arpan Mohanty, MD, MSc - Digital Tools in the Management of IBS/Functional GI Disorders
Eric D. Shah, MD, MBA, FACG - Long COVID and the Gastrointestinal System: Emerging Evidence
Daniel E. Freedberg, MD, MS, and Lin Chang, MD, AGAF - Germline Genetic Testing in CRC: Implications for Familial and Population-Based Testing
Fay Kastrinos, MD, MPH - Evolution of Targeted Therapies for C difficile
Sahil Khanna, MBBS, MS, FACG, AGAF - Harnessing the Power of AI to Enhance Endoscopy: Promises and Pitfalls
Eugenia Uche-Anya, MD, MPH - The Evolving Role of Surgery for IBD
Julie K.M. Thacker, MD, FACS, FASCRS
In this issue:
- Gastroenterology and Climate Change: Assessing and Mitigating Impacts
Swapna Gayam, MD, FACG - MASLD/MASH and Weight Loss
Arpan Mohanty, MD, MSc - Digital Tools in the Management of IBS/Functional GI Disorders
Eric D. Shah, MD, MBA, FACG - Long COVID and the Gastrointestinal System: Emerging Evidence
Daniel E. Freedberg, MD, MS, and Lin Chang, MD, AGAF - Germline Genetic Testing in CRC: Implications for Familial and Population-Based Testing
Fay Kastrinos, MD, MPH - Evolution of Targeted Therapies for C difficile
Sahil Khanna, MBBS, MS, FACG, AGAF - Harnessing the Power of AI to Enhance Endoscopy: Promises and Pitfalls
Eugenia Uche-Anya, MD, MPH - The Evolving Role of Surgery for IBD
Julie K.M. Thacker, MD, FACS, FASCRS
More data needed on stopping GLP-1 use prior to endoscopy
In a new statement, five professional gastroenterology organizations caution that there are currently no data to support stopping glucagonlike peptide 1 (GLP-1) receptor agonists prior to elective endoscopy
The medications, which include semaglutide (Ozempic, Wegovy), tirzepatide (Mounjaro), and liraglutide (Saxenda), among others, are used for the treatment of diabetes or for weight loss and may be associated with delayed gastric emptying.
Patients taking GLP-1 receptor agonists for diabetes management “need to be cautious about withholding these medications because doing so can adversely impact blood glucose control,” said Octavia Pickett-Blakely, MD, a gastroenterologist with University of Pennsylvania in Philadelphia and spokesperson for the American Gastroenterological Association (AGA). “In patients undergoing endoscopic procedures, poorly controlled blood glucose could raise the risk of complications.”
In a commentary on Medscape, David Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, urges clinicians to learn about the topic and inform patients when prescribing GLP-1 receptor agonists.
“These are new and changing issues. In our world as gastroenterologists, we should be considering – very strongly – mitigating strategies to protect the patients on this wonderful class of therapy,” he says. “Sometimes these drugs can have significant side effects that we need to at least be aware of. Nothing is perfect, but let us be better informed.”
“We really don’t know what the risks are yet. With endoscopy, they could be significant, but perhaps they’re not,” Jonathan Leighton, MD, a gastroenterologist with Mayo Clinic Arizona in Phoenix and president-elect of the American College of Gastroenterology (ACG), told this news organization. “There are a lot of factors that go into this, and we just want to proceed cautiously and carefully until we know more.”
The ACG, AGA, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the North American Society for Pediatric Gastroenterology, Hepatology & Nutrition released the statement on Aug. 11.
It was issued in response to recent guidance on the preoperative management of adults and children on GLP-1 receptor agonists put forth by the American Society of Anesthesiologists.
In a separate statement, the AGA stated that there is little, or no data on complications from aspiration.
“While there is anecdotal experience that increased gastroparesis risk may be dose dependent or related to whether it is being used for diabetes control versus weight loss, we also acknowledge that there is little, or no data related to the relative risk of complications from aspiration. As a result, the impact associated with stopping these therapies prior to undergoing upper GI endoscopy (EGD) or other moderate to deep sedated procedures is unknown at this time.
“As clinical gastroenterologists and hepatologists, we are very familiar with safety issues regarding the performance of endoscopy in our patients suffering from gastroparesis as well as unexplained nausea, vomiting, and epigastric pain, particularly in emergency situations. As patient safety will always be paramount, and in the absence of actionable data, we encourage our members to exercise best practices when performing endoscopy on these patients who are taking GLP-1 receptor agonists. More data are needed to understand if and when these medications should be held prior to elective endoscopy. Given the need for further data regarding the emerging use of these novel compounds, we encourage our anesthesiology, endocrinology, and industry partners to work collaboratively with our members to develop the necessary evidence to appropriately inform medication adjustments prior to elective endoscopy.”
ASA recommendations
The ASA Task Force on Preoperative Fasting reviewed the available literature on GLP-1 receptor agonists and associated gastrointestinal adverse effects, including the consequences of delayed gastric emptying.
The task force acknowledges that the evidence to provide guidance for preoperative management of these drugs to prevent regurgitation and pulmonary aspiration of gastric contents is “sparse, limited only to several case reports.”
Nevertheless, given the concerns of GLP-1 receptor agonist–induced delayed gastric emptying and associated high risk for regurgitation and aspiration of gastric contents, the task force made these recommendations for elective procedures.
The day before the procedure
For patients on daily dosing, consider holding GLP-1 agonists on the day of the procedure/surgery. For patients on weekly dosing, consider holding GLP-1 agonists a week prior to the procedure/surgery.
This suggestion is irrespective of the indication (type 2 diabetes or weight loss), dose, or the type of procedure/surgery.
If GLP-1 agonists prescribed for diabetes are held for longer than the dosing schedule, consider consulting an endocrinologist for bridging the antidiabetic therapy to avoid hyperglycemia.
The day of the procedure
If GI symptoms such as severe nausea/vomiting/retching, abdominal bloating, or abdominal pain are present, consider delaying the elective procedure and discuss the concerns of potential risk of regurgitation and pulmonary aspiration of gastric contents with the proceduralist/surgeon and the patient.
If the patient has no GI symptoms and the GLP-1 agonists have been held as advised, proceed as usual.
If the patient has no GI symptoms but the GLP-1 agonists were not held as advised, proceed with “full stomach” precautions or consider evaluating gastric volume by ultrasound, if possible and if proficient with the technique. If the stomach is empty, proceed as usual. If the stomach is full or if gastric ultrasound is inconclusive or not possible, consider delaying the procedure or treat the patient as “full stomach” and manage accordingly. Discuss the concerns of potential risk of regurgitation and pulmonary aspiration of gastric contents with the proceduralist/surgeon and the patient.
There is no evidence to suggest the optimal duration of fasting for patients on GLP-1 agonists. Therefore, until we have adequate evidence, we suggest following the current ASA fasting guidelines.
For patients on GLP-1 receptor agonists who need urgent or emergent procedures, the ASA advises proceeding and treating the patient as “full stomach” and managing accordingly.
Dr. Leighton has financial relationships with Olympus and Pfizer. Dr. Pickett-Blakely has no relevant disclosures. Dr. Johnson is an adviser to ISOTHRIVE and Johnson & Johnson.
This story was adapted for GI&Hepatology News from Medscape.
In a new statement, five professional gastroenterology organizations caution that there are currently no data to support stopping glucagonlike peptide 1 (GLP-1) receptor agonists prior to elective endoscopy
The medications, which include semaglutide (Ozempic, Wegovy), tirzepatide (Mounjaro), and liraglutide (Saxenda), among others, are used for the treatment of diabetes or for weight loss and may be associated with delayed gastric emptying.
Patients taking GLP-1 receptor agonists for diabetes management “need to be cautious about withholding these medications because doing so can adversely impact blood glucose control,” said Octavia Pickett-Blakely, MD, a gastroenterologist with University of Pennsylvania in Philadelphia and spokesperson for the American Gastroenterological Association (AGA). “In patients undergoing endoscopic procedures, poorly controlled blood glucose could raise the risk of complications.”
In a commentary on Medscape, David Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, urges clinicians to learn about the topic and inform patients when prescribing GLP-1 receptor agonists.
“These are new and changing issues. In our world as gastroenterologists, we should be considering – very strongly – mitigating strategies to protect the patients on this wonderful class of therapy,” he says. “Sometimes these drugs can have significant side effects that we need to at least be aware of. Nothing is perfect, but let us be better informed.”
“We really don’t know what the risks are yet. With endoscopy, they could be significant, but perhaps they’re not,” Jonathan Leighton, MD, a gastroenterologist with Mayo Clinic Arizona in Phoenix and president-elect of the American College of Gastroenterology (ACG), told this news organization. “There are a lot of factors that go into this, and we just want to proceed cautiously and carefully until we know more.”
The ACG, AGA, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the North American Society for Pediatric Gastroenterology, Hepatology & Nutrition released the statement on Aug. 11.
It was issued in response to recent guidance on the preoperative management of adults and children on GLP-1 receptor agonists put forth by the American Society of Anesthesiologists.
In a separate statement, the AGA stated that there is little, or no data on complications from aspiration.
“While there is anecdotal experience that increased gastroparesis risk may be dose dependent or related to whether it is being used for diabetes control versus weight loss, we also acknowledge that there is little, or no data related to the relative risk of complications from aspiration. As a result, the impact associated with stopping these therapies prior to undergoing upper GI endoscopy (EGD) or other moderate to deep sedated procedures is unknown at this time.
“As clinical gastroenterologists and hepatologists, we are very familiar with safety issues regarding the performance of endoscopy in our patients suffering from gastroparesis as well as unexplained nausea, vomiting, and epigastric pain, particularly in emergency situations. As patient safety will always be paramount, and in the absence of actionable data, we encourage our members to exercise best practices when performing endoscopy on these patients who are taking GLP-1 receptor agonists. More data are needed to understand if and when these medications should be held prior to elective endoscopy. Given the need for further data regarding the emerging use of these novel compounds, we encourage our anesthesiology, endocrinology, and industry partners to work collaboratively with our members to develop the necessary evidence to appropriately inform medication adjustments prior to elective endoscopy.”
ASA recommendations
The ASA Task Force on Preoperative Fasting reviewed the available literature on GLP-1 receptor agonists and associated gastrointestinal adverse effects, including the consequences of delayed gastric emptying.
The task force acknowledges that the evidence to provide guidance for preoperative management of these drugs to prevent regurgitation and pulmonary aspiration of gastric contents is “sparse, limited only to several case reports.”
Nevertheless, given the concerns of GLP-1 receptor agonist–induced delayed gastric emptying and associated high risk for regurgitation and aspiration of gastric contents, the task force made these recommendations for elective procedures.
The day before the procedure
For patients on daily dosing, consider holding GLP-1 agonists on the day of the procedure/surgery. For patients on weekly dosing, consider holding GLP-1 agonists a week prior to the procedure/surgery.
This suggestion is irrespective of the indication (type 2 diabetes or weight loss), dose, or the type of procedure/surgery.
If GLP-1 agonists prescribed for diabetes are held for longer than the dosing schedule, consider consulting an endocrinologist for bridging the antidiabetic therapy to avoid hyperglycemia.
The day of the procedure
If GI symptoms such as severe nausea/vomiting/retching, abdominal bloating, or abdominal pain are present, consider delaying the elective procedure and discuss the concerns of potential risk of regurgitation and pulmonary aspiration of gastric contents with the proceduralist/surgeon and the patient.
If the patient has no GI symptoms and the GLP-1 agonists have been held as advised, proceed as usual.
If the patient has no GI symptoms but the GLP-1 agonists were not held as advised, proceed with “full stomach” precautions or consider evaluating gastric volume by ultrasound, if possible and if proficient with the technique. If the stomach is empty, proceed as usual. If the stomach is full or if gastric ultrasound is inconclusive or not possible, consider delaying the procedure or treat the patient as “full stomach” and manage accordingly. Discuss the concerns of potential risk of regurgitation and pulmonary aspiration of gastric contents with the proceduralist/surgeon and the patient.
There is no evidence to suggest the optimal duration of fasting for patients on GLP-1 agonists. Therefore, until we have adequate evidence, we suggest following the current ASA fasting guidelines.
For patients on GLP-1 receptor agonists who need urgent or emergent procedures, the ASA advises proceeding and treating the patient as “full stomach” and managing accordingly.
Dr. Leighton has financial relationships with Olympus and Pfizer. Dr. Pickett-Blakely has no relevant disclosures. Dr. Johnson is an adviser to ISOTHRIVE and Johnson & Johnson.
This story was adapted for GI&Hepatology News from Medscape.
In a new statement, five professional gastroenterology organizations caution that there are currently no data to support stopping glucagonlike peptide 1 (GLP-1) receptor agonists prior to elective endoscopy
The medications, which include semaglutide (Ozempic, Wegovy), tirzepatide (Mounjaro), and liraglutide (Saxenda), among others, are used for the treatment of diabetes or for weight loss and may be associated with delayed gastric emptying.
Patients taking GLP-1 receptor agonists for diabetes management “need to be cautious about withholding these medications because doing so can adversely impact blood glucose control,” said Octavia Pickett-Blakely, MD, a gastroenterologist with University of Pennsylvania in Philadelphia and spokesperson for the American Gastroenterological Association (AGA). “In patients undergoing endoscopic procedures, poorly controlled blood glucose could raise the risk of complications.”
In a commentary on Medscape, David Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, urges clinicians to learn about the topic and inform patients when prescribing GLP-1 receptor agonists.
“These are new and changing issues. In our world as gastroenterologists, we should be considering – very strongly – mitigating strategies to protect the patients on this wonderful class of therapy,” he says. “Sometimes these drugs can have significant side effects that we need to at least be aware of. Nothing is perfect, but let us be better informed.”
“We really don’t know what the risks are yet. With endoscopy, they could be significant, but perhaps they’re not,” Jonathan Leighton, MD, a gastroenterologist with Mayo Clinic Arizona in Phoenix and president-elect of the American College of Gastroenterology (ACG), told this news organization. “There are a lot of factors that go into this, and we just want to proceed cautiously and carefully until we know more.”
The ACG, AGA, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the North American Society for Pediatric Gastroenterology, Hepatology & Nutrition released the statement on Aug. 11.
It was issued in response to recent guidance on the preoperative management of adults and children on GLP-1 receptor agonists put forth by the American Society of Anesthesiologists.
In a separate statement, the AGA stated that there is little, or no data on complications from aspiration.
“While there is anecdotal experience that increased gastroparesis risk may be dose dependent or related to whether it is being used for diabetes control versus weight loss, we also acknowledge that there is little, or no data related to the relative risk of complications from aspiration. As a result, the impact associated with stopping these therapies prior to undergoing upper GI endoscopy (EGD) or other moderate to deep sedated procedures is unknown at this time.
“As clinical gastroenterologists and hepatologists, we are very familiar with safety issues regarding the performance of endoscopy in our patients suffering from gastroparesis as well as unexplained nausea, vomiting, and epigastric pain, particularly in emergency situations. As patient safety will always be paramount, and in the absence of actionable data, we encourage our members to exercise best practices when performing endoscopy on these patients who are taking GLP-1 receptor agonists. More data are needed to understand if and when these medications should be held prior to elective endoscopy. Given the need for further data regarding the emerging use of these novel compounds, we encourage our anesthesiology, endocrinology, and industry partners to work collaboratively with our members to develop the necessary evidence to appropriately inform medication adjustments prior to elective endoscopy.”
ASA recommendations
The ASA Task Force on Preoperative Fasting reviewed the available literature on GLP-1 receptor agonists and associated gastrointestinal adverse effects, including the consequences of delayed gastric emptying.
The task force acknowledges that the evidence to provide guidance for preoperative management of these drugs to prevent regurgitation and pulmonary aspiration of gastric contents is “sparse, limited only to several case reports.”
Nevertheless, given the concerns of GLP-1 receptor agonist–induced delayed gastric emptying and associated high risk for regurgitation and aspiration of gastric contents, the task force made these recommendations for elective procedures.
The day before the procedure
For patients on daily dosing, consider holding GLP-1 agonists on the day of the procedure/surgery. For patients on weekly dosing, consider holding GLP-1 agonists a week prior to the procedure/surgery.
This suggestion is irrespective of the indication (type 2 diabetes or weight loss), dose, or the type of procedure/surgery.
If GLP-1 agonists prescribed for diabetes are held for longer than the dosing schedule, consider consulting an endocrinologist for bridging the antidiabetic therapy to avoid hyperglycemia.
The day of the procedure
If GI symptoms such as severe nausea/vomiting/retching, abdominal bloating, or abdominal pain are present, consider delaying the elective procedure and discuss the concerns of potential risk of regurgitation and pulmonary aspiration of gastric contents with the proceduralist/surgeon and the patient.
If the patient has no GI symptoms and the GLP-1 agonists have been held as advised, proceed as usual.
If the patient has no GI symptoms but the GLP-1 agonists were not held as advised, proceed with “full stomach” precautions or consider evaluating gastric volume by ultrasound, if possible and if proficient with the technique. If the stomach is empty, proceed as usual. If the stomach is full or if gastric ultrasound is inconclusive or not possible, consider delaying the procedure or treat the patient as “full stomach” and manage accordingly. Discuss the concerns of potential risk of regurgitation and pulmonary aspiration of gastric contents with the proceduralist/surgeon and the patient.
There is no evidence to suggest the optimal duration of fasting for patients on GLP-1 agonists. Therefore, until we have adequate evidence, we suggest following the current ASA fasting guidelines.
For patients on GLP-1 receptor agonists who need urgent or emergent procedures, the ASA advises proceeding and treating the patient as “full stomach” and managing accordingly.
Dr. Leighton has financial relationships with Olympus and Pfizer. Dr. Pickett-Blakely has no relevant disclosures. Dr. Johnson is an adviser to ISOTHRIVE and Johnson & Johnson.
This story was adapted for GI&Hepatology News from Medscape.
Developing training pathways in advanced endoscopic resection and third-space endoscopy in the U.S.
As a gastroenterology and hepatology fellow, choosing a career path was a daunting prospect. Despite the additional specialization, there seemed to be endless career options to consider. Did I want to join an academic, private, or hybrid practice? Should I subspecialize within the field? Was it important to incorporate research or teaching into my practice? What about opportunities to take on administrative or leadership roles?
Fellowship training at a large academic research institution provided me the opportunity to work with expert faculty in inflammatory bowel disease, esophageal disease, motility and functional gastrointestinal disease, pancreaticobiliary disease, and hepatology. I enjoyed seeing patients in each of these subspecialty clinics. But, by the end of my second year of GI fellowship, I still wasn’t sure what I wanted to do professionally.
A career in academic general gastroenterology seemed to be a good fit for my personality and goals. Rather than focusing on research, I chose to position myself as a clinician educator. I knew that having a subspecialty area of expertise would help improve my clinical practice and make me a more attractive candidate to academic centers. To help narrow my choice, I looked at the clinical enterprise at our institution and assessed where the unmet clinical needs were most acute. Simultaneously, I identified potential mentors to support and guide me through the transition from fellow to independent practitioner. I decided to focus on acquiring the skills to care for patients with anorectal diseases and lower-GI motility disorders, as this area met both of my criteria – excellent mentorship and an unmet clinical need. Under the guidance of Dr. Yolanda Scarlett, I spent my 3rd year in clinic learning to interpret anorectal manometry tests, defecograms, and sitz marker studies and treating patients with refractory constipation, fecal incontinence, and anal fissures.
With a plan to develop an expertise in anorectal diseases and low-GI motility disorders, I also wanted to focus on improving my endoscopic skills to graduate as well rounded a clinician as possible. To achieve this goal, I sought out a separate endoscopy mentor, Dr. Ian Grimm, the director of endoscopy at the University of North Carolina at Chapel Hill. Dr. Grimm, a classically trained advanced endoscopist performing endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP), had a burgeoning interest in endoscopic mucosal resection (EMR) and had just returned from a few months in Japan learning to perform endoscopic submucosal dissection (ESD) and peroral endoscopic myotomy (POEM).
When I began working with Dr. Grimm, I had not even heard the term third-space endoscopy and knew nothing about ESD or POEM. I spent as much time as possible watching and assisting Dr. Grimm with complex endoscopic mucosal resection (EMR) during the first few months of my 3rd year. Soon after my exposure to advanced endoscopic resection, it was clear that I wanted to learn and incorporate this into my clinical practice. I watched Dr. Grimm perform the first POEM at UNC in the fall of 2016 and by that time I was hooked on learning third-space endoscopy. I observed and assisted with as many EMR, ESD, and POEM cases as I could that year. In addition to the hands-on and cognitive training with Dr. Grimm, I attended national meetings and workshops focused on learning third-space endoscopy. In the spring of my 3rd year I was honored to be the first fellow to complete the Olympus master class in ESD – a 2-day hands-on training course sponsored by Olympus. By the end of that year, I was performing complex EMR with minimal assistance and had completed multiple ESDs and POEMs with cognitive supervision only.
After fellowship, I joined the UNC faculty as a general gastroenterologist with expertise in anorectal disease and lower-GI motility disorders. While I was comfortable performing complex EMR, I still needed additional training and supervision before I felt ready to independently perform ESD or POEM. With the gracious support and encouragement of our division chief, I continued third-space endoscopy training with Dr. Grimm during dedicated protected time 2 days each month. Over the ensuing 4 years, I transitioned to fully independent practice performing all types of advanced EMR and third-space endoscopy including complex EMR, ESD, endoscopic full-thickness resection (EFTR), submucosal tunnel endoscopic resection (STER), esophageal POEM, gastric POEM, and Zenker’s POEM.
As one of the first gastroenterologists in the United States to perform third-space endoscopy without any formal training in advanced pancreaticobiliary endoscopy, I believe learning advanced endoscopic resection and third-space endoscopy is best achieved through a training pathway separate from the conventional advanced endoscopy fellowship focused on teaching EUS and ERCP. Although there are transferable skills learned from EUS and ERCP to the techniques used in third-space endoscopy, there is nothing inherent to performing EUS or ERCP that enables one to learn how to perform an ESD or a POEM.
There is a robust training pathway to teach advanced pancreaticobiliary endoscopy, but no formal training pathway exists to teach third-space endoscopy in the United States. Historically, a small number of interested and motivated advanced pancreaticobiliary endoscopists sought out opportunities to learn third-space endoscopy after completion of their advanced endoscopy fellowship, in some cases many years after graduation. For these early adopters in the United States, the only training opportunities required travel to Japan or another Eastern country with arrangements made to observe and participate in third-space endoscopy cases with experts there. With increased recognition of the benefits of ESD and POEM over the past 5-10 years in the United States, there has been greater adoption of third-space endoscopy and with it, more training opportunities. Still, there are very few institutions with formalized training programs in advanced endoscopic resection and third-space endoscopy in the United States to date.
Proof that this model works
In Eastern countries such as Japan, training endoscopists to perform ESD and POEM has been successfully achieved through an apprenticeship model whereby an expert in third-space endoscopy closely supervises a trainee who gains greater autonomy with increasing experience and skill over time. My personal experience is proof that this model works. But, adopting such a model more widely in the United States may prove difficult. We lack a sufficient number of experienced third-space endoscopy operators and, given the challenges to appropriate reimbursement for third-space endoscopy in the United States, there is understandable resistance to accepting the prolonged training period necessary for technical mastery of this skill.
In part, a long training period is needed because of a relative paucity of appropriate target lesions for ESD and the rarity of achalasia in the United States. While there is consensus among experts regarding the benefits of ESD for resection of early gastric cancer (EGC), relatively few EGCs are found in the United States and indications for ESD outside resection of EGC are less well defined with less clear benefits over more widely performed piecemeal EMR. Despite these challenges, it is critical that we continue to develop dedicated training pathways to teach advanced endoscopic resection and third-space endoscopy in the United States. My practice has evolved considerably since completion of fellowship nearly 6 years ago, and I now focus almost exclusively on advanced endoscopic resection and third-space endoscopy. Recently, Dr. Grimm and I began an advanced endoscopic resection elective for the general GI fellows at UNC and we are excited to welcome our first advanced endoscopic resection and third-space endoscopy fellow to UNC this July.
While there are many possible avenues to expertise in advanced endoscopic resection, few will likely follow the same path that I have taken. Trainees who are interested in pursuing this subspecialty should seek out supportive mentors in a setting where there is already a robust case volume of esophageal motility disorders and endoscopic resections. Success requires the persistent motivation to seek out diverse opportunities for self-study, exposure to experts, data on developments in the field, and hands-on exposure to as many ex-vivo and in-vivo cases as possible.
Dr. Kroch is assistant professor of medicine in the division of gastroenterology and hepatology at the University of North Carolina at Chapel Hill. He disclosed having no conflicts of interest.
As a gastroenterology and hepatology fellow, choosing a career path was a daunting prospect. Despite the additional specialization, there seemed to be endless career options to consider. Did I want to join an academic, private, or hybrid practice? Should I subspecialize within the field? Was it important to incorporate research or teaching into my practice? What about opportunities to take on administrative or leadership roles?
Fellowship training at a large academic research institution provided me the opportunity to work with expert faculty in inflammatory bowel disease, esophageal disease, motility and functional gastrointestinal disease, pancreaticobiliary disease, and hepatology. I enjoyed seeing patients in each of these subspecialty clinics. But, by the end of my second year of GI fellowship, I still wasn’t sure what I wanted to do professionally.
A career in academic general gastroenterology seemed to be a good fit for my personality and goals. Rather than focusing on research, I chose to position myself as a clinician educator. I knew that having a subspecialty area of expertise would help improve my clinical practice and make me a more attractive candidate to academic centers. To help narrow my choice, I looked at the clinical enterprise at our institution and assessed where the unmet clinical needs were most acute. Simultaneously, I identified potential mentors to support and guide me through the transition from fellow to independent practitioner. I decided to focus on acquiring the skills to care for patients with anorectal diseases and lower-GI motility disorders, as this area met both of my criteria – excellent mentorship and an unmet clinical need. Under the guidance of Dr. Yolanda Scarlett, I spent my 3rd year in clinic learning to interpret anorectal manometry tests, defecograms, and sitz marker studies and treating patients with refractory constipation, fecal incontinence, and anal fissures.
With a plan to develop an expertise in anorectal diseases and low-GI motility disorders, I also wanted to focus on improving my endoscopic skills to graduate as well rounded a clinician as possible. To achieve this goal, I sought out a separate endoscopy mentor, Dr. Ian Grimm, the director of endoscopy at the University of North Carolina at Chapel Hill. Dr. Grimm, a classically trained advanced endoscopist performing endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP), had a burgeoning interest in endoscopic mucosal resection (EMR) and had just returned from a few months in Japan learning to perform endoscopic submucosal dissection (ESD) and peroral endoscopic myotomy (POEM).
When I began working with Dr. Grimm, I had not even heard the term third-space endoscopy and knew nothing about ESD or POEM. I spent as much time as possible watching and assisting Dr. Grimm with complex endoscopic mucosal resection (EMR) during the first few months of my 3rd year. Soon after my exposure to advanced endoscopic resection, it was clear that I wanted to learn and incorporate this into my clinical practice. I watched Dr. Grimm perform the first POEM at UNC in the fall of 2016 and by that time I was hooked on learning third-space endoscopy. I observed and assisted with as many EMR, ESD, and POEM cases as I could that year. In addition to the hands-on and cognitive training with Dr. Grimm, I attended national meetings and workshops focused on learning third-space endoscopy. In the spring of my 3rd year I was honored to be the first fellow to complete the Olympus master class in ESD – a 2-day hands-on training course sponsored by Olympus. By the end of that year, I was performing complex EMR with minimal assistance and had completed multiple ESDs and POEMs with cognitive supervision only.
After fellowship, I joined the UNC faculty as a general gastroenterologist with expertise in anorectal disease and lower-GI motility disorders. While I was comfortable performing complex EMR, I still needed additional training and supervision before I felt ready to independently perform ESD or POEM. With the gracious support and encouragement of our division chief, I continued third-space endoscopy training with Dr. Grimm during dedicated protected time 2 days each month. Over the ensuing 4 years, I transitioned to fully independent practice performing all types of advanced EMR and third-space endoscopy including complex EMR, ESD, endoscopic full-thickness resection (EFTR), submucosal tunnel endoscopic resection (STER), esophageal POEM, gastric POEM, and Zenker’s POEM.
As one of the first gastroenterologists in the United States to perform third-space endoscopy without any formal training in advanced pancreaticobiliary endoscopy, I believe learning advanced endoscopic resection and third-space endoscopy is best achieved through a training pathway separate from the conventional advanced endoscopy fellowship focused on teaching EUS and ERCP. Although there are transferable skills learned from EUS and ERCP to the techniques used in third-space endoscopy, there is nothing inherent to performing EUS or ERCP that enables one to learn how to perform an ESD or a POEM.
There is a robust training pathway to teach advanced pancreaticobiliary endoscopy, but no formal training pathway exists to teach third-space endoscopy in the United States. Historically, a small number of interested and motivated advanced pancreaticobiliary endoscopists sought out opportunities to learn third-space endoscopy after completion of their advanced endoscopy fellowship, in some cases many years after graduation. For these early adopters in the United States, the only training opportunities required travel to Japan or another Eastern country with arrangements made to observe and participate in third-space endoscopy cases with experts there. With increased recognition of the benefits of ESD and POEM over the past 5-10 years in the United States, there has been greater adoption of third-space endoscopy and with it, more training opportunities. Still, there are very few institutions with formalized training programs in advanced endoscopic resection and third-space endoscopy in the United States to date.
Proof that this model works
In Eastern countries such as Japan, training endoscopists to perform ESD and POEM has been successfully achieved through an apprenticeship model whereby an expert in third-space endoscopy closely supervises a trainee who gains greater autonomy with increasing experience and skill over time. My personal experience is proof that this model works. But, adopting such a model more widely in the United States may prove difficult. We lack a sufficient number of experienced third-space endoscopy operators and, given the challenges to appropriate reimbursement for third-space endoscopy in the United States, there is understandable resistance to accepting the prolonged training period necessary for technical mastery of this skill.
In part, a long training period is needed because of a relative paucity of appropriate target lesions for ESD and the rarity of achalasia in the United States. While there is consensus among experts regarding the benefits of ESD for resection of early gastric cancer (EGC), relatively few EGCs are found in the United States and indications for ESD outside resection of EGC are less well defined with less clear benefits over more widely performed piecemeal EMR. Despite these challenges, it is critical that we continue to develop dedicated training pathways to teach advanced endoscopic resection and third-space endoscopy in the United States. My practice has evolved considerably since completion of fellowship nearly 6 years ago, and I now focus almost exclusively on advanced endoscopic resection and third-space endoscopy. Recently, Dr. Grimm and I began an advanced endoscopic resection elective for the general GI fellows at UNC and we are excited to welcome our first advanced endoscopic resection and third-space endoscopy fellow to UNC this July.
While there are many possible avenues to expertise in advanced endoscopic resection, few will likely follow the same path that I have taken. Trainees who are interested in pursuing this subspecialty should seek out supportive mentors in a setting where there is already a robust case volume of esophageal motility disorders and endoscopic resections. Success requires the persistent motivation to seek out diverse opportunities for self-study, exposure to experts, data on developments in the field, and hands-on exposure to as many ex-vivo and in-vivo cases as possible.
Dr. Kroch is assistant professor of medicine in the division of gastroenterology and hepatology at the University of North Carolina at Chapel Hill. He disclosed having no conflicts of interest.
As a gastroenterology and hepatology fellow, choosing a career path was a daunting prospect. Despite the additional specialization, there seemed to be endless career options to consider. Did I want to join an academic, private, or hybrid practice? Should I subspecialize within the field? Was it important to incorporate research or teaching into my practice? What about opportunities to take on administrative or leadership roles?
Fellowship training at a large academic research institution provided me the opportunity to work with expert faculty in inflammatory bowel disease, esophageal disease, motility and functional gastrointestinal disease, pancreaticobiliary disease, and hepatology. I enjoyed seeing patients in each of these subspecialty clinics. But, by the end of my second year of GI fellowship, I still wasn’t sure what I wanted to do professionally.
A career in academic general gastroenterology seemed to be a good fit for my personality and goals. Rather than focusing on research, I chose to position myself as a clinician educator. I knew that having a subspecialty area of expertise would help improve my clinical practice and make me a more attractive candidate to academic centers. To help narrow my choice, I looked at the clinical enterprise at our institution and assessed where the unmet clinical needs were most acute. Simultaneously, I identified potential mentors to support and guide me through the transition from fellow to independent practitioner. I decided to focus on acquiring the skills to care for patients with anorectal diseases and lower-GI motility disorders, as this area met both of my criteria – excellent mentorship and an unmet clinical need. Under the guidance of Dr. Yolanda Scarlett, I spent my 3rd year in clinic learning to interpret anorectal manometry tests, defecograms, and sitz marker studies and treating patients with refractory constipation, fecal incontinence, and anal fissures.
With a plan to develop an expertise in anorectal diseases and low-GI motility disorders, I also wanted to focus on improving my endoscopic skills to graduate as well rounded a clinician as possible. To achieve this goal, I sought out a separate endoscopy mentor, Dr. Ian Grimm, the director of endoscopy at the University of North Carolina at Chapel Hill. Dr. Grimm, a classically trained advanced endoscopist performing endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP), had a burgeoning interest in endoscopic mucosal resection (EMR) and had just returned from a few months in Japan learning to perform endoscopic submucosal dissection (ESD) and peroral endoscopic myotomy (POEM).
When I began working with Dr. Grimm, I had not even heard the term third-space endoscopy and knew nothing about ESD or POEM. I spent as much time as possible watching and assisting Dr. Grimm with complex endoscopic mucosal resection (EMR) during the first few months of my 3rd year. Soon after my exposure to advanced endoscopic resection, it was clear that I wanted to learn and incorporate this into my clinical practice. I watched Dr. Grimm perform the first POEM at UNC in the fall of 2016 and by that time I was hooked on learning third-space endoscopy. I observed and assisted with as many EMR, ESD, and POEM cases as I could that year. In addition to the hands-on and cognitive training with Dr. Grimm, I attended national meetings and workshops focused on learning third-space endoscopy. In the spring of my 3rd year I was honored to be the first fellow to complete the Olympus master class in ESD – a 2-day hands-on training course sponsored by Olympus. By the end of that year, I was performing complex EMR with minimal assistance and had completed multiple ESDs and POEMs with cognitive supervision only.
After fellowship, I joined the UNC faculty as a general gastroenterologist with expertise in anorectal disease and lower-GI motility disorders. While I was comfortable performing complex EMR, I still needed additional training and supervision before I felt ready to independently perform ESD or POEM. With the gracious support and encouragement of our division chief, I continued third-space endoscopy training with Dr. Grimm during dedicated protected time 2 days each month. Over the ensuing 4 years, I transitioned to fully independent practice performing all types of advanced EMR and third-space endoscopy including complex EMR, ESD, endoscopic full-thickness resection (EFTR), submucosal tunnel endoscopic resection (STER), esophageal POEM, gastric POEM, and Zenker’s POEM.
As one of the first gastroenterologists in the United States to perform third-space endoscopy without any formal training in advanced pancreaticobiliary endoscopy, I believe learning advanced endoscopic resection and third-space endoscopy is best achieved through a training pathway separate from the conventional advanced endoscopy fellowship focused on teaching EUS and ERCP. Although there are transferable skills learned from EUS and ERCP to the techniques used in third-space endoscopy, there is nothing inherent to performing EUS or ERCP that enables one to learn how to perform an ESD or a POEM.
There is a robust training pathway to teach advanced pancreaticobiliary endoscopy, but no formal training pathway exists to teach third-space endoscopy in the United States. Historically, a small number of interested and motivated advanced pancreaticobiliary endoscopists sought out opportunities to learn third-space endoscopy after completion of their advanced endoscopy fellowship, in some cases many years after graduation. For these early adopters in the United States, the only training opportunities required travel to Japan or another Eastern country with arrangements made to observe and participate in third-space endoscopy cases with experts there. With increased recognition of the benefits of ESD and POEM over the past 5-10 years in the United States, there has been greater adoption of third-space endoscopy and with it, more training opportunities. Still, there are very few institutions with formalized training programs in advanced endoscopic resection and third-space endoscopy in the United States to date.
Proof that this model works
In Eastern countries such as Japan, training endoscopists to perform ESD and POEM has been successfully achieved through an apprenticeship model whereby an expert in third-space endoscopy closely supervises a trainee who gains greater autonomy with increasing experience and skill over time. My personal experience is proof that this model works. But, adopting such a model more widely in the United States may prove difficult. We lack a sufficient number of experienced third-space endoscopy operators and, given the challenges to appropriate reimbursement for third-space endoscopy in the United States, there is understandable resistance to accepting the prolonged training period necessary for technical mastery of this skill.
In part, a long training period is needed because of a relative paucity of appropriate target lesions for ESD and the rarity of achalasia in the United States. While there is consensus among experts regarding the benefits of ESD for resection of early gastric cancer (EGC), relatively few EGCs are found in the United States and indications for ESD outside resection of EGC are less well defined with less clear benefits over more widely performed piecemeal EMR. Despite these challenges, it is critical that we continue to develop dedicated training pathways to teach advanced endoscopic resection and third-space endoscopy in the United States. My practice has evolved considerably since completion of fellowship nearly 6 years ago, and I now focus almost exclusively on advanced endoscopic resection and third-space endoscopy. Recently, Dr. Grimm and I began an advanced endoscopic resection elective for the general GI fellows at UNC and we are excited to welcome our first advanced endoscopic resection and third-space endoscopy fellow to UNC this July.
While there are many possible avenues to expertise in advanced endoscopic resection, few will likely follow the same path that I have taken. Trainees who are interested in pursuing this subspecialty should seek out supportive mentors in a setting where there is already a robust case volume of esophageal motility disorders and endoscopic resections. Success requires the persistent motivation to seek out diverse opportunities for self-study, exposure to experts, data on developments in the field, and hands-on exposure to as many ex-vivo and in-vivo cases as possible.
Dr. Kroch is assistant professor of medicine in the division of gastroenterology and hepatology at the University of North Carolina at Chapel Hill. He disclosed having no conflicts of interest.
Advances in endohepatology
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2
While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5
Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2

While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5

Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2

While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5

Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.