User login
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2
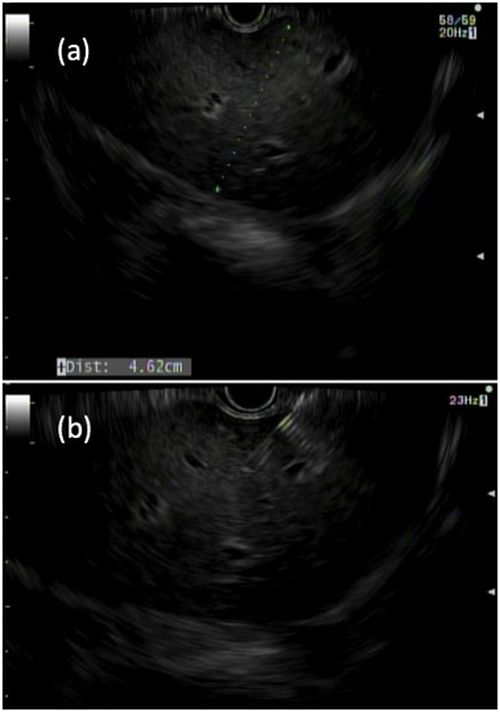
While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5
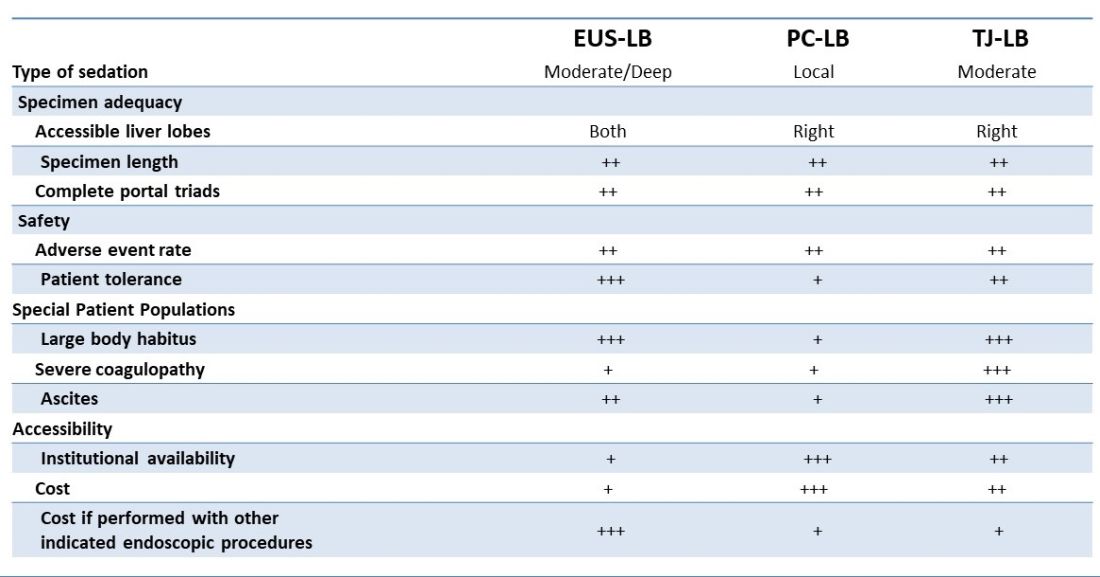
Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2

While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5

Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2

While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5

Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.

