User login
‘Diagnosis creep’: Are some AFib patients overtreated?
without those treatments having been validated in those particular groups.
This concern has been highlighted recently in the atrial fibrillation (AF) field, with the recent change in the definition of hypertension in the United States at lower levels of blood pressure causing a lot more patients to become eligible for oral anticoagulation at an earlier stage in their AF course.
U.S. researchers analyzed data from 316,388 patients with AF from the National Cardiovascular Data Registry Practice Innovation and Clinical Excellence outpatient quality improvement registry, and found that at 36 months’ follow-up, 83.5% of patients met the new 130/80 mm Hg definition of hypertension, while only 53.3% met the previous 140/90 mm Hg definition.
The diagnosis of hypertension gives 1 point in the CHA2DS2-VASc score, which is used to determine risk in AF patients, those with scores of 2 or more being eligible for oral anticoagulation.
The researchers report that in patients with an index CHA2DS2-VASc score of 1 (before the hypertension diagnosis), at 36 months, 83% fulfilled the 130/80 mm Hg definition of hypertension while the 140/90 mm Hg definition was met by only 50%, giving a large increase in the number of patients who could qualify for oral anticoagulation therapy.
“While the definition of hypertension has changed in response to landmark clinical trials, CHA2DS2-VASc was validated using an older hypertension definition, with limited ambulatory blood pressure monitoring and higher blood pressure goals for treatment,” the authors state.
“Now, patients with AF will meet the CHA2DS2-VASc threshold for oral anticoagulation earlier in their disease course. However, it is not known if patients with scores of 1 or 2 using the new hypertension definition have sufficient stroke risk to offset the bleeding risk of oral anticoagulation and will receive net clinical benefit,” they point out.
This study was published online as a research letter in JAMA Network Open.
Senior author of the report, Mintu Turakhia, MD, Stanford (Calif.) University/iRhythm Technologies Inc., said AF is a good example of how “diagnosis creep” may lead to patients receiving inappropriate treatment.
“Risk scores derived when risk variables were described in one way are starting to be applied based on a diagnosis made in a totally different way,” he said in an interview. “Diagnosis creep is a problem everywhere in medicine. The goal of this study was to quantify what this means for the new definition of hypertension in the context of risk scoring AF patients for anticoagulation treatment. We are calling attention to this issue so clinicians are aware of possible implications.”
Dr. Turakhia explained that the CHA2DS2-VASc score was formulated based on claims data so there was a record of hypertension on the clinical encounter. That hypertension diagnosis would have been based on the old definition of 140/90 mm Hg.
“But now we apply a label of hypertension in the office every time someone has a measurement of elevated blood pressure – treated or untreated – and the blood pressure threshold for a hypertension diagnosis has changed to 130/80 mm Hg,” he said. “We are asking what this means for risk stratification scores such as CHA2DS2-VASc, and how do we quantify what that means for anticoagulation eligibility?”
He said that while identifying hypertension at lower blood pressures may be beneficial with regard to starting antihypertensive treatment earlier with a consequent reduction in cardiovascular outcomes, when this also affects risk scores that determine treatment for other conditions, as is the case for AF, the case is not so clear.
Dr. Turakhia pointed out that with AF, there are additional factors causing diagnosis creep, including earlier detection of AF and identification of shorter episodes due to the use of higher sensitivity tools to detect abnormal rhythms.
“What about the patient who has been identified as having AF based on just a few seconds found on monitoring and who is aged 65 (so just over the age threshold for 1 point on the CHA2DS2-VASc score)?” he asked. “Now we’re going to throw in hypertension with a blood pressure measurement just over 130/80 mm Hg, and they will be eligible for anticoagulation.”
Dr. Turakhia noted that in addition to earlier classification of hypertension, other conditions contributing to the CHA2DS2-VASc score are also being detected earlier, including diabetes and reduced ejection fractions that are considered heart failure.
“I worry about the sum of the parts. We don’t know if the risk score performs equally well when we’re using these different thresholds. We have to be careful that we are not exposing patients to the bleeding risks of anticoagulation unnecessarily. There is a clear issue here,” he said.
What should clinicians do?
In a comment, Gregory Lip, MD, chair of cardiovascular medicine at the University of Liverpool, England, who helped develop the CHA2DS2-VASc score, said clinicians needed to think more broadly when considering hypertension as a risk factor for the score.
He points out that if a patient had a history of hypertension but is now controlled to below 130/80 mm Hg, they would still be considered to be at risk per the CHA2DS2-VASc score.
And for patients without a history of hypertension, and who have a current blood pressure measurement of around 130/80 mm Hg, Dr. Lip advises that it would be premature to diagnose hypertension immediately.
“Hypertension is not a yes/no diagnosis. If you look at the relationship between blood pressure and risk of stroke, it is like a continual dose-response. It doesn’t mean that at 129/79 there is no stroke risk but that at 130/80 there is a stroke risk. It’s not like that,” he said.
“I wouldn’t make a diagnosis on a one-off blood pressure measurement. I would want to monitor that patient and get them to do home measurements,” he commented. “If someone constantly has levels around that 130/80 mm Hg, I don’t necessarily rush in with a definite diagnosis of hypertension and start drug treatment. I would look at lifestyle first. And in such patients, I wouldn’t give them the 1 point for hypertension on the CHA2DS2-VASc score.”
Dr. Lip points out that a hypertension diagnosis is not just about blood pressure numbers. “We have to assess the patients much more completely before giving them a diagnosis and consider factors such as whether there is evidence of hypertension-related end-organ damage, and if lifestyle issues have been addressed.”
Are new risk scores needed?
Dr. Turakhia agreed that clinicians need to look at the bigger picture, but he also suggested that new risk scores may need to be developed.
“All of us in the medical community need to think about whether we should be recalibrating risk prediction with more contemporary evidence – based on our ability to detect disease now,” he commented.
“This could even be a different risk score altogether, possibly incorporating a wider range of parameters or perhaps incorporating machine learning. That’s really the question we need to be asking ourselves,” Dr. Turakhia added.
Dr. Lip noted that there are many stroke risk factors and only those that are most common and have been well validated go into clinical risk scores such as CHA2DS2-VASc.
“These risks scores are by design simplifications, and only have modest predictive value for identifying patients at high risk of stroke. You can always improve on clinical risk scores by adding in other variables,” he said. “There are some risk scores in AF with 26 variables. But the practical application of these more complex scores can be difficult in clinical practice. These risks scores are meant to be simple so that they can be used by busy clinicians in the outpatient clinic or on a ward round. It is not easy to input 26 different variables.”
He also noted that many guidelines are now veering away from categorizing patients at high, medium, or low risk of stroke, which he refers to as “artificial” classifications. “There is now more of a default position that patients should receive stroke prevention normally with a DOAC [direct oral anticoagulant] unless they are low risk.”
Dr. Turakhia agreed that it is imperative to look at the bigger picture when identifying AF patients for anticoagulation. “We have to be careful not to take things at face value. It is more important than ever to use clinical judgment to avoid overtreatment in borderline situations,” he concluded.
This study was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry. Dr. Turakhia reported employment from iRhythm Technologies; equity from AliveCor, Connect America, Evidently, and Forward; grants from U.S. Food and Drug Administration, American Heart Association, Bayer, Sanofi, Gilead, and Bristol Myers Squibb; and personal fees from Pfizer and JAMA Cardiology (prior associate editor) outside the submitted work. Dr. Lip has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
without those treatments having been validated in those particular groups.
This concern has been highlighted recently in the atrial fibrillation (AF) field, with the recent change in the definition of hypertension in the United States at lower levels of blood pressure causing a lot more patients to become eligible for oral anticoagulation at an earlier stage in their AF course.
U.S. researchers analyzed data from 316,388 patients with AF from the National Cardiovascular Data Registry Practice Innovation and Clinical Excellence outpatient quality improvement registry, and found that at 36 months’ follow-up, 83.5% of patients met the new 130/80 mm Hg definition of hypertension, while only 53.3% met the previous 140/90 mm Hg definition.
The diagnosis of hypertension gives 1 point in the CHA2DS2-VASc score, which is used to determine risk in AF patients, those with scores of 2 or more being eligible for oral anticoagulation.
The researchers report that in patients with an index CHA2DS2-VASc score of 1 (before the hypertension diagnosis), at 36 months, 83% fulfilled the 130/80 mm Hg definition of hypertension while the 140/90 mm Hg definition was met by only 50%, giving a large increase in the number of patients who could qualify for oral anticoagulation therapy.
“While the definition of hypertension has changed in response to landmark clinical trials, CHA2DS2-VASc was validated using an older hypertension definition, with limited ambulatory blood pressure monitoring and higher blood pressure goals for treatment,” the authors state.
“Now, patients with AF will meet the CHA2DS2-VASc threshold for oral anticoagulation earlier in their disease course. However, it is not known if patients with scores of 1 or 2 using the new hypertension definition have sufficient stroke risk to offset the bleeding risk of oral anticoagulation and will receive net clinical benefit,” they point out.
This study was published online as a research letter in JAMA Network Open.
Senior author of the report, Mintu Turakhia, MD, Stanford (Calif.) University/iRhythm Technologies Inc., said AF is a good example of how “diagnosis creep” may lead to patients receiving inappropriate treatment.
“Risk scores derived when risk variables were described in one way are starting to be applied based on a diagnosis made in a totally different way,” he said in an interview. “Diagnosis creep is a problem everywhere in medicine. The goal of this study was to quantify what this means for the new definition of hypertension in the context of risk scoring AF patients for anticoagulation treatment. We are calling attention to this issue so clinicians are aware of possible implications.”
Dr. Turakhia explained that the CHA2DS2-VASc score was formulated based on claims data so there was a record of hypertension on the clinical encounter. That hypertension diagnosis would have been based on the old definition of 140/90 mm Hg.
“But now we apply a label of hypertension in the office every time someone has a measurement of elevated blood pressure – treated or untreated – and the blood pressure threshold for a hypertension diagnosis has changed to 130/80 mm Hg,” he said. “We are asking what this means for risk stratification scores such as CHA2DS2-VASc, and how do we quantify what that means for anticoagulation eligibility?”
He said that while identifying hypertension at lower blood pressures may be beneficial with regard to starting antihypertensive treatment earlier with a consequent reduction in cardiovascular outcomes, when this also affects risk scores that determine treatment for other conditions, as is the case for AF, the case is not so clear.
Dr. Turakhia pointed out that with AF, there are additional factors causing diagnosis creep, including earlier detection of AF and identification of shorter episodes due to the use of higher sensitivity tools to detect abnormal rhythms.
“What about the patient who has been identified as having AF based on just a few seconds found on monitoring and who is aged 65 (so just over the age threshold for 1 point on the CHA2DS2-VASc score)?” he asked. “Now we’re going to throw in hypertension with a blood pressure measurement just over 130/80 mm Hg, and they will be eligible for anticoagulation.”
Dr. Turakhia noted that in addition to earlier classification of hypertension, other conditions contributing to the CHA2DS2-VASc score are also being detected earlier, including diabetes and reduced ejection fractions that are considered heart failure.
“I worry about the sum of the parts. We don’t know if the risk score performs equally well when we’re using these different thresholds. We have to be careful that we are not exposing patients to the bleeding risks of anticoagulation unnecessarily. There is a clear issue here,” he said.
What should clinicians do?
In a comment, Gregory Lip, MD, chair of cardiovascular medicine at the University of Liverpool, England, who helped develop the CHA2DS2-VASc score, said clinicians needed to think more broadly when considering hypertension as a risk factor for the score.
He points out that if a patient had a history of hypertension but is now controlled to below 130/80 mm Hg, they would still be considered to be at risk per the CHA2DS2-VASc score.
And for patients without a history of hypertension, and who have a current blood pressure measurement of around 130/80 mm Hg, Dr. Lip advises that it would be premature to diagnose hypertension immediately.
“Hypertension is not a yes/no diagnosis. If you look at the relationship between blood pressure and risk of stroke, it is like a continual dose-response. It doesn’t mean that at 129/79 there is no stroke risk but that at 130/80 there is a stroke risk. It’s not like that,” he said.
“I wouldn’t make a diagnosis on a one-off blood pressure measurement. I would want to monitor that patient and get them to do home measurements,” he commented. “If someone constantly has levels around that 130/80 mm Hg, I don’t necessarily rush in with a definite diagnosis of hypertension and start drug treatment. I would look at lifestyle first. And in such patients, I wouldn’t give them the 1 point for hypertension on the CHA2DS2-VASc score.”
Dr. Lip points out that a hypertension diagnosis is not just about blood pressure numbers. “We have to assess the patients much more completely before giving them a diagnosis and consider factors such as whether there is evidence of hypertension-related end-organ damage, and if lifestyle issues have been addressed.”
Are new risk scores needed?
Dr. Turakhia agreed that clinicians need to look at the bigger picture, but he also suggested that new risk scores may need to be developed.
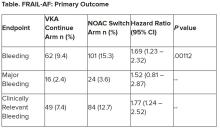
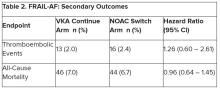
“All of us in the medical community need to think about whether we should be recalibrating risk prediction with more contemporary evidence – based on our ability to detect disease now,” he commented.
“This could even be a different risk score altogether, possibly incorporating a wider range of parameters or perhaps incorporating machine learning. That’s really the question we need to be asking ourselves,” Dr. Turakhia added.
Dr. Lip noted that there are many stroke risk factors and only those that are most common and have been well validated go into clinical risk scores such as CHA2DS2-VASc.
“These risks scores are by design simplifications, and only have modest predictive value for identifying patients at high risk of stroke. You can always improve on clinical risk scores by adding in other variables,” he said. “There are some risk scores in AF with 26 variables. But the practical application of these more complex scores can be difficult in clinical practice. These risks scores are meant to be simple so that they can be used by busy clinicians in the outpatient clinic or on a ward round. It is not easy to input 26 different variables.”
He also noted that many guidelines are now veering away from categorizing patients at high, medium, or low risk of stroke, which he refers to as “artificial” classifications. “There is now more of a default position that patients should receive stroke prevention normally with a DOAC [direct oral anticoagulant] unless they are low risk.”
Dr. Turakhia agreed that it is imperative to look at the bigger picture when identifying AF patients for anticoagulation. “We have to be careful not to take things at face value. It is more important than ever to use clinical judgment to avoid overtreatment in borderline situations,” he concluded.
This study was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry. Dr. Turakhia reported employment from iRhythm Technologies; equity from AliveCor, Connect America, Evidently, and Forward; grants from U.S. Food and Drug Administration, American Heart Association, Bayer, Sanofi, Gilead, and Bristol Myers Squibb; and personal fees from Pfizer and JAMA Cardiology (prior associate editor) outside the submitted work. Dr. Lip has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
without those treatments having been validated in those particular groups.
This concern has been highlighted recently in the atrial fibrillation (AF) field, with the recent change in the definition of hypertension in the United States at lower levels of blood pressure causing a lot more patients to become eligible for oral anticoagulation at an earlier stage in their AF course.
U.S. researchers analyzed data from 316,388 patients with AF from the National Cardiovascular Data Registry Practice Innovation and Clinical Excellence outpatient quality improvement registry, and found that at 36 months’ follow-up, 83.5% of patients met the new 130/80 mm Hg definition of hypertension, while only 53.3% met the previous 140/90 mm Hg definition.
The diagnosis of hypertension gives 1 point in the CHA2DS2-VASc score, which is used to determine risk in AF patients, those with scores of 2 or more being eligible for oral anticoagulation.
The researchers report that in patients with an index CHA2DS2-VASc score of 1 (before the hypertension diagnosis), at 36 months, 83% fulfilled the 130/80 mm Hg definition of hypertension while the 140/90 mm Hg definition was met by only 50%, giving a large increase in the number of patients who could qualify for oral anticoagulation therapy.
“While the definition of hypertension has changed in response to landmark clinical trials, CHA2DS2-VASc was validated using an older hypertension definition, with limited ambulatory blood pressure monitoring and higher blood pressure goals for treatment,” the authors state.
“Now, patients with AF will meet the CHA2DS2-VASc threshold for oral anticoagulation earlier in their disease course. However, it is not known if patients with scores of 1 or 2 using the new hypertension definition have sufficient stroke risk to offset the bleeding risk of oral anticoagulation and will receive net clinical benefit,” they point out.
This study was published online as a research letter in JAMA Network Open.
Senior author of the report, Mintu Turakhia, MD, Stanford (Calif.) University/iRhythm Technologies Inc., said AF is a good example of how “diagnosis creep” may lead to patients receiving inappropriate treatment.
“Risk scores derived when risk variables were described in one way are starting to be applied based on a diagnosis made in a totally different way,” he said in an interview. “Diagnosis creep is a problem everywhere in medicine. The goal of this study was to quantify what this means for the new definition of hypertension in the context of risk scoring AF patients for anticoagulation treatment. We are calling attention to this issue so clinicians are aware of possible implications.”
Dr. Turakhia explained that the CHA2DS2-VASc score was formulated based on claims data so there was a record of hypertension on the clinical encounter. That hypertension diagnosis would have been based on the old definition of 140/90 mm Hg.
“But now we apply a label of hypertension in the office every time someone has a measurement of elevated blood pressure – treated or untreated – and the blood pressure threshold for a hypertension diagnosis has changed to 130/80 mm Hg,” he said. “We are asking what this means for risk stratification scores such as CHA2DS2-VASc, and how do we quantify what that means for anticoagulation eligibility?”
He said that while identifying hypertension at lower blood pressures may be beneficial with regard to starting antihypertensive treatment earlier with a consequent reduction in cardiovascular outcomes, when this also affects risk scores that determine treatment for other conditions, as is the case for AF, the case is not so clear.
Dr. Turakhia pointed out that with AF, there are additional factors causing diagnosis creep, including earlier detection of AF and identification of shorter episodes due to the use of higher sensitivity tools to detect abnormal rhythms.
“What about the patient who has been identified as having AF based on just a few seconds found on monitoring and who is aged 65 (so just over the age threshold for 1 point on the CHA2DS2-VASc score)?” he asked. “Now we’re going to throw in hypertension with a blood pressure measurement just over 130/80 mm Hg, and they will be eligible for anticoagulation.”
Dr. Turakhia noted that in addition to earlier classification of hypertension, other conditions contributing to the CHA2DS2-VASc score are also being detected earlier, including diabetes and reduced ejection fractions that are considered heart failure.
“I worry about the sum of the parts. We don’t know if the risk score performs equally well when we’re using these different thresholds. We have to be careful that we are not exposing patients to the bleeding risks of anticoagulation unnecessarily. There is a clear issue here,” he said.
What should clinicians do?
In a comment, Gregory Lip, MD, chair of cardiovascular medicine at the University of Liverpool, England, who helped develop the CHA2DS2-VASc score, said clinicians needed to think more broadly when considering hypertension as a risk factor for the score.
He points out that if a patient had a history of hypertension but is now controlled to below 130/80 mm Hg, they would still be considered to be at risk per the CHA2DS2-VASc score.
And for patients without a history of hypertension, and who have a current blood pressure measurement of around 130/80 mm Hg, Dr. Lip advises that it would be premature to diagnose hypertension immediately.
“Hypertension is not a yes/no diagnosis. If you look at the relationship between blood pressure and risk of stroke, it is like a continual dose-response. It doesn’t mean that at 129/79 there is no stroke risk but that at 130/80 there is a stroke risk. It’s not like that,” he said.
“I wouldn’t make a diagnosis on a one-off blood pressure measurement. I would want to monitor that patient and get them to do home measurements,” he commented. “If someone constantly has levels around that 130/80 mm Hg, I don’t necessarily rush in with a definite diagnosis of hypertension and start drug treatment. I would look at lifestyle first. And in such patients, I wouldn’t give them the 1 point for hypertension on the CHA2DS2-VASc score.”
Dr. Lip points out that a hypertension diagnosis is not just about blood pressure numbers. “We have to assess the patients much more completely before giving them a diagnosis and consider factors such as whether there is evidence of hypertension-related end-organ damage, and if lifestyle issues have been addressed.”
Are new risk scores needed?
Dr. Turakhia agreed that clinicians need to look at the bigger picture, but he also suggested that new risk scores may need to be developed.
“All of us in the medical community need to think about whether we should be recalibrating risk prediction with more contemporary evidence – based on our ability to detect disease now,” he commented.
“This could even be a different risk score altogether, possibly incorporating a wider range of parameters or perhaps incorporating machine learning. That’s really the question we need to be asking ourselves,” Dr. Turakhia added.
Dr. Lip noted that there are many stroke risk factors and only those that are most common and have been well validated go into clinical risk scores such as CHA2DS2-VASc.
“These risks scores are by design simplifications, and only have modest predictive value for identifying patients at high risk of stroke. You can always improve on clinical risk scores by adding in other variables,” he said. “There are some risk scores in AF with 26 variables. But the practical application of these more complex scores can be difficult in clinical practice. These risks scores are meant to be simple so that they can be used by busy clinicians in the outpatient clinic or on a ward round. It is not easy to input 26 different variables.”
He also noted that many guidelines are now veering away from categorizing patients at high, medium, or low risk of stroke, which he refers to as “artificial” classifications. “There is now more of a default position that patients should receive stroke prevention normally with a DOAC [direct oral anticoagulant] unless they are low risk.”
Dr. Turakhia agreed that it is imperative to look at the bigger picture when identifying AF patients for anticoagulation. “We have to be careful not to take things at face value. It is more important than ever to use clinical judgment to avoid overtreatment in borderline situations,” he concluded.
This study was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry. Dr. Turakhia reported employment from iRhythm Technologies; equity from AliveCor, Connect America, Evidently, and Forward; grants from U.S. Food and Drug Administration, American Heart Association, Bayer, Sanofi, Gilead, and Bristol Myers Squibb; and personal fees from Pfizer and JAMA Cardiology (prior associate editor) outside the submitted work. Dr. Lip has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
EMA warns that omega-3-acid ethyl esters may cause AFib
In its September meeting, the Should atrial fibrillation develop, intake of the medication must be stopped permanently.
Omega-3-acid ethyl esters are used to treat hypertriglyceridemia if lifestyle changes, particularly those related to nutrition, have not been sufficient to lower the blood triglyceride level. Hypertriglyceridemia is a risk factor for coronary heart disease.
During a Periodic Safety Update Single Assessment Procedure, the EMA safety committee analyzed systematic overviews and meta-analyses of randomized, controlled clinical studies. Experts found a dose-dependent increase in the risk for atrial fibrillation in patients with cardiovascular diseases or cardiovascular risk factors who were being treated with omega-3-acid ethyl esters, compared with those treated with placebo. The observed risk was at its highest at a dose of 4 g/d.
The PRAC will recommend an update to the Summary of Product Characteristics for preparations that contain omega-3-acid ethyl esters. The aim is to inform physicians, pharmacists, and patients of the risk for atrial fibrillation. A notification will be sent to health care professionals soon to inform them of further details.
This article was translated from the Medscape German Edition. A version appeared on Medscape.com.
In its September meeting, the Should atrial fibrillation develop, intake of the medication must be stopped permanently.
Omega-3-acid ethyl esters are used to treat hypertriglyceridemia if lifestyle changes, particularly those related to nutrition, have not been sufficient to lower the blood triglyceride level. Hypertriglyceridemia is a risk factor for coronary heart disease.
During a Periodic Safety Update Single Assessment Procedure, the EMA safety committee analyzed systematic overviews and meta-analyses of randomized, controlled clinical studies. Experts found a dose-dependent increase in the risk for atrial fibrillation in patients with cardiovascular diseases or cardiovascular risk factors who were being treated with omega-3-acid ethyl esters, compared with those treated with placebo. The observed risk was at its highest at a dose of 4 g/d.
The PRAC will recommend an update to the Summary of Product Characteristics for preparations that contain omega-3-acid ethyl esters. The aim is to inform physicians, pharmacists, and patients of the risk for atrial fibrillation. A notification will be sent to health care professionals soon to inform them of further details.
This article was translated from the Medscape German Edition. A version appeared on Medscape.com.
In its September meeting, the Should atrial fibrillation develop, intake of the medication must be stopped permanently.
Omega-3-acid ethyl esters are used to treat hypertriglyceridemia if lifestyle changes, particularly those related to nutrition, have not been sufficient to lower the blood triglyceride level. Hypertriglyceridemia is a risk factor for coronary heart disease.
During a Periodic Safety Update Single Assessment Procedure, the EMA safety committee analyzed systematic overviews and meta-analyses of randomized, controlled clinical studies. Experts found a dose-dependent increase in the risk for atrial fibrillation in patients with cardiovascular diseases or cardiovascular risk factors who were being treated with omega-3-acid ethyl esters, compared with those treated with placebo. The observed risk was at its highest at a dose of 4 g/d.
The PRAC will recommend an update to the Summary of Product Characteristics for preparations that contain omega-3-acid ethyl esters. The aim is to inform physicians, pharmacists, and patients of the risk for atrial fibrillation. A notification will be sent to health care professionals soon to inform them of further details.
This article was translated from the Medscape German Edition. A version appeared on Medscape.com.
Decoding AFib recurrence: PCPs’ role in personalized care
One in three patients who experience their first bout of atrial fibrillation (AFib) during hospitalization can expect to experience a recurrence of the arrhythmia within the year, new research shows.
The findings, reported in Annals of Internal Medicine, suggest these patients may be good candidates for oral anticoagulants to reduce their risk for stroke.
“Atrial fibrillation is very common in patients for the very first time in their life when they’re sick and in the hospital,” said William F. McIntyre, MD, PhD, a cardiologist at McMaster University, Hamilton, Ont., who led the study. These new insights into AFib management suggest there is a need for primary care physicians to be on the lookout for potential recurrence.
AFib is strongly linked to stroke, and patients at greater risk for stroke may be prescribed oral anticoagulants. Although the arrhythmia can be reversed before the patient is discharged from the hospital, risk for recurrence was unclear, Dr. McIntyre said.
“We wanted to know if the patient was in atrial fibrillation because of the physiologic stress that they were under, or if they just have the disease called atrial fibrillation, which should usually be followed lifelong by a specialist,” Dr. McIntyre said.
Dr. McIntyre and colleagues followed 139 patients (mean age, 71 years) at three medical centers in Ontario who experienced new-onset AFib during their hospital stay, along with an equal number of patients who had no history of AFib and who served as controls. The research team used a Holter monitor to record study participants’ heart rhythm for 14 days to detect incident AFib at 1 and 6 months after discharge. They also followed up with periodic phone calls for up to 12 months. Among the study participants, half were admitted for noncardiac surgeries, and the other half were admitted for medical illnesses, including infections and pneumonia. Participants with a prior history of AFib were excluded from the analysis.
The primary outcome of the study was an episode of AFib that lasted at least 30 seconds on the monitor or one detected during routine care at the 12-month mark.
Patients who experienced AFib for the first time in the hospital had roughly a 33% risk for recurrence within a year, nearly sevenfold higher than their age- and sex-matched counterparts who had not had an arrhythmia during their hospital stay (3%; confidence interval, 0%-6.4%).
“This study has important implications for management of patients who have a first presentation of AFib that is concurrent with a reversible physiologic stressor,” the authors wrote. “An AFib recurrence risk of 33.1% at 1 year is neither low enough to conclude that transient new-onset AFib in the setting of another illness is benign nor high enough that all such transient new-onset AFib can be assumed to be paroxysmal AFib. Instead, these results call for risk stratification and follow-up in these patients.”
The researchers reported that among people with recurrent AFib in the study, the median total time in arrhythmia was 9 hours. “This far exceeds the cutoff of 6 minutes that was established as being associated with stroke using simulated AFib screening in patients with implanted continuous monitors,” they wrote. “These results suggest that the patients in our study who had AFib detected in follow-up are similar to contemporary patients with AFib for whom evidence-based therapies, including oral anticoagulation, are warranted.”
Dr. McIntyre and colleagues were able to track outcomes and treatments for the patients in the study. In the group with recurrent AFib, 1 had a stroke, 2 experienced systemic embolism, 3 had a heart failure event, 6 experienced bleeding, and 11 died. In the other group, there was one case of stroke, one of heart failure, four cases involving bleeding, and seven deaths. “The proportion of participants with new-onset AFib during their initial hospitalization who were taking oral anticoagulants was 47.1% at 6 months and 49.2% at 12 months. This included 73% of participants with AFib detected during follow-up and 39% who did not have AFib detected during follow-up,” they wrote.
The uncertain nature of AFib recurrence complicates predictions about patients’ posthospitalization experiences within the following year. “We cannot just say: ‘Hey, this is just a reversible illness, and now we can forget about it,’ ” Dr. McIntyre said. “Nor is the risk of recurrence so strong in the other direction that you can give patients a lifelong diagnosis of atrial fibrillation.”
Role for primary care
Without that certainty, physicians cannot refer everyone who experiences new-onset AFib to a cardiologist for long-term care. The variability in recurrence rates necessitates a more nuanced and personalized approach. Here, primary care physicians step in, offering tailored care based on their established, long-term patient relationships, Dr. McIntyre said.
The study participants already have chronic health conditions that bring them into regular contact with their family physician. This gives primary care physicians a golden opportunity to be on lookout and to recommend care from a cardiologist at the appropriate time if it becomes necessary, he said.
“I have certainly seen cases of recurrent atrial fibrillation in patients who had an episode while hospitalized, and consistent with this study, this is a common clinical occurrence,” said Deepak L. Bhatt, MD, MPH, director of Mount Sinai Heart, New York. Primary care physicians must remain vigilant and avoid the temptation to attribute AFib solely to illness or surgery
“Ideally, we would have randomized clinical trial data to guide the decision about whether to use prophylactic anticoagulation,” said Dr. Bhatt, who added that a cardiology consultation may also be appropriate.
Dr. McIntyre reported no relevant financial relationships. Dr. Bhatt reported numerous relationships with industry.
A version of this article appeared on Medscape.com.
One in three patients who experience their first bout of atrial fibrillation (AFib) during hospitalization can expect to experience a recurrence of the arrhythmia within the year, new research shows.
The findings, reported in Annals of Internal Medicine, suggest these patients may be good candidates for oral anticoagulants to reduce their risk for stroke.
“Atrial fibrillation is very common in patients for the very first time in their life when they’re sick and in the hospital,” said William F. McIntyre, MD, PhD, a cardiologist at McMaster University, Hamilton, Ont., who led the study. These new insights into AFib management suggest there is a need for primary care physicians to be on the lookout for potential recurrence.
AFib is strongly linked to stroke, and patients at greater risk for stroke may be prescribed oral anticoagulants. Although the arrhythmia can be reversed before the patient is discharged from the hospital, risk for recurrence was unclear, Dr. McIntyre said.
“We wanted to know if the patient was in atrial fibrillation because of the physiologic stress that they were under, or if they just have the disease called atrial fibrillation, which should usually be followed lifelong by a specialist,” Dr. McIntyre said.
Dr. McIntyre and colleagues followed 139 patients (mean age, 71 years) at three medical centers in Ontario who experienced new-onset AFib during their hospital stay, along with an equal number of patients who had no history of AFib and who served as controls. The research team used a Holter monitor to record study participants’ heart rhythm for 14 days to detect incident AFib at 1 and 6 months after discharge. They also followed up with periodic phone calls for up to 12 months. Among the study participants, half were admitted for noncardiac surgeries, and the other half were admitted for medical illnesses, including infections and pneumonia. Participants with a prior history of AFib were excluded from the analysis.
The primary outcome of the study was an episode of AFib that lasted at least 30 seconds on the monitor or one detected during routine care at the 12-month mark.
Patients who experienced AFib for the first time in the hospital had roughly a 33% risk for recurrence within a year, nearly sevenfold higher than their age- and sex-matched counterparts who had not had an arrhythmia during their hospital stay (3%; confidence interval, 0%-6.4%).
“This study has important implications for management of patients who have a first presentation of AFib that is concurrent with a reversible physiologic stressor,” the authors wrote. “An AFib recurrence risk of 33.1% at 1 year is neither low enough to conclude that transient new-onset AFib in the setting of another illness is benign nor high enough that all such transient new-onset AFib can be assumed to be paroxysmal AFib. Instead, these results call for risk stratification and follow-up in these patients.”
The researchers reported that among people with recurrent AFib in the study, the median total time in arrhythmia was 9 hours. “This far exceeds the cutoff of 6 minutes that was established as being associated with stroke using simulated AFib screening in patients with implanted continuous monitors,” they wrote. “These results suggest that the patients in our study who had AFib detected in follow-up are similar to contemporary patients with AFib for whom evidence-based therapies, including oral anticoagulation, are warranted.”
Dr. McIntyre and colleagues were able to track outcomes and treatments for the patients in the study. In the group with recurrent AFib, 1 had a stroke, 2 experienced systemic embolism, 3 had a heart failure event, 6 experienced bleeding, and 11 died. In the other group, there was one case of stroke, one of heart failure, four cases involving bleeding, and seven deaths. “The proportion of participants with new-onset AFib during their initial hospitalization who were taking oral anticoagulants was 47.1% at 6 months and 49.2% at 12 months. This included 73% of participants with AFib detected during follow-up and 39% who did not have AFib detected during follow-up,” they wrote.
The uncertain nature of AFib recurrence complicates predictions about patients’ posthospitalization experiences within the following year. “We cannot just say: ‘Hey, this is just a reversible illness, and now we can forget about it,’ ” Dr. McIntyre said. “Nor is the risk of recurrence so strong in the other direction that you can give patients a lifelong diagnosis of atrial fibrillation.”
Role for primary care
Without that certainty, physicians cannot refer everyone who experiences new-onset AFib to a cardiologist for long-term care. The variability in recurrence rates necessitates a more nuanced and personalized approach. Here, primary care physicians step in, offering tailored care based on their established, long-term patient relationships, Dr. McIntyre said.
The study participants already have chronic health conditions that bring them into regular contact with their family physician. This gives primary care physicians a golden opportunity to be on lookout and to recommend care from a cardiologist at the appropriate time if it becomes necessary, he said.
“I have certainly seen cases of recurrent atrial fibrillation in patients who had an episode while hospitalized, and consistent with this study, this is a common clinical occurrence,” said Deepak L. Bhatt, MD, MPH, director of Mount Sinai Heart, New York. Primary care physicians must remain vigilant and avoid the temptation to attribute AFib solely to illness or surgery
“Ideally, we would have randomized clinical trial data to guide the decision about whether to use prophylactic anticoagulation,” said Dr. Bhatt, who added that a cardiology consultation may also be appropriate.
Dr. McIntyre reported no relevant financial relationships. Dr. Bhatt reported numerous relationships with industry.
A version of this article appeared on Medscape.com.
One in three patients who experience their first bout of atrial fibrillation (AFib) during hospitalization can expect to experience a recurrence of the arrhythmia within the year, new research shows.
The findings, reported in Annals of Internal Medicine, suggest these patients may be good candidates for oral anticoagulants to reduce their risk for stroke.
“Atrial fibrillation is very common in patients for the very first time in their life when they’re sick and in the hospital,” said William F. McIntyre, MD, PhD, a cardiologist at McMaster University, Hamilton, Ont., who led the study. These new insights into AFib management suggest there is a need for primary care physicians to be on the lookout for potential recurrence.
AFib is strongly linked to stroke, and patients at greater risk for stroke may be prescribed oral anticoagulants. Although the arrhythmia can be reversed before the patient is discharged from the hospital, risk for recurrence was unclear, Dr. McIntyre said.
“We wanted to know if the patient was in atrial fibrillation because of the physiologic stress that they were under, or if they just have the disease called atrial fibrillation, which should usually be followed lifelong by a specialist,” Dr. McIntyre said.
Dr. McIntyre and colleagues followed 139 patients (mean age, 71 years) at three medical centers in Ontario who experienced new-onset AFib during their hospital stay, along with an equal number of patients who had no history of AFib and who served as controls. The research team used a Holter monitor to record study participants’ heart rhythm for 14 days to detect incident AFib at 1 and 6 months after discharge. They also followed up with periodic phone calls for up to 12 months. Among the study participants, half were admitted for noncardiac surgeries, and the other half were admitted for medical illnesses, including infections and pneumonia. Participants with a prior history of AFib were excluded from the analysis.
The primary outcome of the study was an episode of AFib that lasted at least 30 seconds on the monitor or one detected during routine care at the 12-month mark.
Patients who experienced AFib for the first time in the hospital had roughly a 33% risk for recurrence within a year, nearly sevenfold higher than their age- and sex-matched counterparts who had not had an arrhythmia during their hospital stay (3%; confidence interval, 0%-6.4%).
“This study has important implications for management of patients who have a first presentation of AFib that is concurrent with a reversible physiologic stressor,” the authors wrote. “An AFib recurrence risk of 33.1% at 1 year is neither low enough to conclude that transient new-onset AFib in the setting of another illness is benign nor high enough that all such transient new-onset AFib can be assumed to be paroxysmal AFib. Instead, these results call for risk stratification and follow-up in these patients.”
The researchers reported that among people with recurrent AFib in the study, the median total time in arrhythmia was 9 hours. “This far exceeds the cutoff of 6 minutes that was established as being associated with stroke using simulated AFib screening in patients with implanted continuous monitors,” they wrote. “These results suggest that the patients in our study who had AFib detected in follow-up are similar to contemporary patients with AFib for whom evidence-based therapies, including oral anticoagulation, are warranted.”
Dr. McIntyre and colleagues were able to track outcomes and treatments for the patients in the study. In the group with recurrent AFib, 1 had a stroke, 2 experienced systemic embolism, 3 had a heart failure event, 6 experienced bleeding, and 11 died. In the other group, there was one case of stroke, one of heart failure, four cases involving bleeding, and seven deaths. “The proportion of participants with new-onset AFib during their initial hospitalization who were taking oral anticoagulants was 47.1% at 6 months and 49.2% at 12 months. This included 73% of participants with AFib detected during follow-up and 39% who did not have AFib detected during follow-up,” they wrote.
The uncertain nature of AFib recurrence complicates predictions about patients’ posthospitalization experiences within the following year. “We cannot just say: ‘Hey, this is just a reversible illness, and now we can forget about it,’ ” Dr. McIntyre said. “Nor is the risk of recurrence so strong in the other direction that you can give patients a lifelong diagnosis of atrial fibrillation.”
Role for primary care
Without that certainty, physicians cannot refer everyone who experiences new-onset AFib to a cardiologist for long-term care. The variability in recurrence rates necessitates a more nuanced and personalized approach. Here, primary care physicians step in, offering tailored care based on their established, long-term patient relationships, Dr. McIntyre said.
The study participants already have chronic health conditions that bring them into regular contact with their family physician. This gives primary care physicians a golden opportunity to be on lookout and to recommend care from a cardiologist at the appropriate time if it becomes necessary, he said.
“I have certainly seen cases of recurrent atrial fibrillation in patients who had an episode while hospitalized, and consistent with this study, this is a common clinical occurrence,” said Deepak L. Bhatt, MD, MPH, director of Mount Sinai Heart, New York. Primary care physicians must remain vigilant and avoid the temptation to attribute AFib solely to illness or surgery
“Ideally, we would have randomized clinical trial data to guide the decision about whether to use prophylactic anticoagulation,” said Dr. Bhatt, who added that a cardiology consultation may also be appropriate.
Dr. McIntyre reported no relevant financial relationships. Dr. Bhatt reported numerous relationships with industry.
A version of this article appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Anxiety, depression ease after AFib ablation: Clinical or placebo effect?
who had initially tested high for such psychological distress.
The finding, said the researchers, may point to an overlooked potential benefit of ablation that can be discussed with patients considering whether to have the procedure.
Importantly, the 100 adults with symptomatic paroxysmal or persistent AFib in the randomized trial weren’t blinded to treatment assignment, which was either ablation or continued medical therapy.
That leaves open the possibility that psychological distress improved in the ablation group not from any unique effect of ablation itself but because patients expected to benefit from the procedure.
The investigators acknowledged that their trial, called REMEDIAL, can’t rule out a placebo effect as part of the observed benefit. Indeed, studies suggest that there is a substantial placebo component of AFib ablation – which, notably, is usually done to make patients feel better.
But the current findings are more consistent with the conventional view that patients feel better primarily because ablation reduces the AFib causing their symptoms, the group said.
Psychological stress in the study started to fall early after the procedure and continued to decline consistently over the next 6 months (P = .006) and 12 months (P = .005), not a typical pattern for placebo, they wrote.
Moreover, the mental health benefits “correlated very strongly” with less recurrent AFib, reduced AFib burden, and withdrawal of beta-blockers and antiarrhythmic agents, outcomes that might be expected from ablation, said Jonathan M. Kalman, MBBS, PhD.
“Of course, I cannot say there is no placebo effect from having had the procedure, and maybe that something to consider,” but it’s probably not the main driver of benefit, he said in an interview. The relationship between successful AFib ablation “and improvements in physical and now mental health is overwhelming.”
Dr. Kalman, who is affiliated with Royal Melbourne Hospital, is senior author on the study, published in JAMA.
The findings add to “strong, reproducible evidence that ablation is the best way to tackle rhythm control in [AFib] populations” regardless of age, mental health status, or AFib burden, said Auroa Badin, MD, who wasn’t involved in REMEDIAL but has studied the psychological effects of arrhythmia ablation.
For example, there is “very good evidence” from CABANA and other trials that AFib ablation “considerably improves quality of life,” Dr. Badin, of OhioHealth Heart & Vascular Physicians, Columbus, said in an interview. The current study “just emphasizes that there’s also a psychological effect.”
Some of that response could be a placebo or even a nocebo effect. Most of the patients assigned to the medical arm had already been on medications that failed at rhythm control. And their management in the trial, he said, “even if you optimize it, was still drug therapy.”
Patients in the control group, therefore, could have been “disappointed” at the prospect of continued ineffective therapy in a way that influenced their outcomes. “That is another confounding factor,” Dr. Badin said.
But if the psychological results of ablation in the trial were predominantly a placebo effect, early differences in psychological test scores would not have persisted for long, certainly not for a year, he observed. Moreover, the ablation group had better test scores at 12 months than at 6 months, “indicating a likelihood of improvement over time.”
Differences between the groups would probably have been less pronounced if the control group had received a sham procedure, Dr. Badin proposed. That would potentially differentiate ablation’s clinical and placebo contributions to the outcomes.
Still, he said, any observed placebo effect in a sham-controlled trial would probably have been limited. “I think it still would have been a positive trial. It may not show the same difference, but I don’t think you would have a neutral trial just by doing a sham.”
REMEDIAL has “good data,” and its conclusions about ablation’s potential psychological benefits are “reasonable” and worth bringing up when discussing the procedure with patients, Dr. Badin said.
Indeed, psychological distress is “important and often overlooked” in patients with AFib, Dr. Kalman observed. “The dominant indication for atrial fibrillation ablation is symptomatic impact on quality of life. We should think about that broadly, about not just the physical symptoms but the impact on their mental health.”
The trial was conducted at two centers in Australia. It enrolled patients, one-third of whom were women, who were on medical management for AFib. Patients receiving treatment for severe depression were excluded. The included patients were randomly assigned to undergo catheter ablation or to continue on closely managed rhythm-control medication, with cardioversion as indicated.
Psychological distress was measured at baseline and throughout follow-up by a battery of self-administered, validated questionnaires. Baseline test scores for the two groups were similar.
Recurrence and burden of AFib were tracked primarily by daily KardiaMobile (AliveCor) ECG monitoring. A few patients were followed using already implanted cardiac rhythm devices or by 24-hour Holter monitor every 3 months, Dr. Kalman said.
Composite scores on the Hospital Anxiety and Depression Scale (HADS) at 12 months, the primary endpoint, were 7.6 and 11.8 (P = .005) for the ablation and medical groups, respectively. They were 8.2 and 11.9 (P = .006), respectively, at 6 months.
The prevalence of severe psychological distress, defined as a HADS score greater than 15, was lower in the ablation group at 6 months (14.2% vs. 34%; P = .02) and 12 months (10.2% vs. 31.9%; P = .01).
Scores on the Beck Depression Inventory–II questionnaire were also consistently and significantly better for the ablation group at 6 and at 12 months (P = .01 for both).
Monitoring picked up AFib in 47% of the ablation group and 96% of the control group (P < .001) over 12 months. Their median AFib burdens were 0% (interquartile range, 0%-3.2%) and 15.5% (IQR, 1%-46%), respectively (P < .001).
Antiarrhythmic drug use fell from a baseline of 90% to 53% 3 months after ablation and 30% at 12 months (P = .003). Use of these drugs in the control group was 89% at baseline and remained essentially the same, 85%, at 12 months.
AFib symptom severity scores were significantly lower after ablation, compared with medical management at 3, 6, and 12 months.
The observed effect of ablation on psychological stress “clearly speaks in favor of effective rhythm control, and moreover catheter ablation” and is a “novel argument” in support of catheter ablation for AFib, Julia Lurz, MD, Heart Center Leipzig (Germany) at University Leipzig, and Karl-Heinz Ladwig, MD, PhD, Technical University Munich (Germany), wrote in an editorial accompanying publication of REMEDIAL.
But the findings also “raise the question of why rhythm control was so ineffective in the medical treatment group,” they wrote.
They agreed that the randomization process itself may have had its own psychological effects. “Potential disappointment” in the medical group and “high expectations” among patients who received ablation “could have fueled the success of catheter ablation” with respect to mental health endpoints.
Dr. Kalman reported receiving grants from the National Health and Medical Research Council of Australia, Medtronic, Mooney, and Biosense Webster. Dr. Badin, Dr. Lurz, and Dr. Ladwig reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
who had initially tested high for such psychological distress.
The finding, said the researchers, may point to an overlooked potential benefit of ablation that can be discussed with patients considering whether to have the procedure.
Importantly, the 100 adults with symptomatic paroxysmal or persistent AFib in the randomized trial weren’t blinded to treatment assignment, which was either ablation or continued medical therapy.
That leaves open the possibility that psychological distress improved in the ablation group not from any unique effect of ablation itself but because patients expected to benefit from the procedure.
The investigators acknowledged that their trial, called REMEDIAL, can’t rule out a placebo effect as part of the observed benefit. Indeed, studies suggest that there is a substantial placebo component of AFib ablation – which, notably, is usually done to make patients feel better.
But the current findings are more consistent with the conventional view that patients feel better primarily because ablation reduces the AFib causing their symptoms, the group said.
Psychological stress in the study started to fall early after the procedure and continued to decline consistently over the next 6 months (P = .006) and 12 months (P = .005), not a typical pattern for placebo, they wrote.
Moreover, the mental health benefits “correlated very strongly” with less recurrent AFib, reduced AFib burden, and withdrawal of beta-blockers and antiarrhythmic agents, outcomes that might be expected from ablation, said Jonathan M. Kalman, MBBS, PhD.
“Of course, I cannot say there is no placebo effect from having had the procedure, and maybe that something to consider,” but it’s probably not the main driver of benefit, he said in an interview. The relationship between successful AFib ablation “and improvements in physical and now mental health is overwhelming.”
Dr. Kalman, who is affiliated with Royal Melbourne Hospital, is senior author on the study, published in JAMA.
The findings add to “strong, reproducible evidence that ablation is the best way to tackle rhythm control in [AFib] populations” regardless of age, mental health status, or AFib burden, said Auroa Badin, MD, who wasn’t involved in REMEDIAL but has studied the psychological effects of arrhythmia ablation.
For example, there is “very good evidence” from CABANA and other trials that AFib ablation “considerably improves quality of life,” Dr. Badin, of OhioHealth Heart & Vascular Physicians, Columbus, said in an interview. The current study “just emphasizes that there’s also a psychological effect.”
Some of that response could be a placebo or even a nocebo effect. Most of the patients assigned to the medical arm had already been on medications that failed at rhythm control. And their management in the trial, he said, “even if you optimize it, was still drug therapy.”
Patients in the control group, therefore, could have been “disappointed” at the prospect of continued ineffective therapy in a way that influenced their outcomes. “That is another confounding factor,” Dr. Badin said.
But if the psychological results of ablation in the trial were predominantly a placebo effect, early differences in psychological test scores would not have persisted for long, certainly not for a year, he observed. Moreover, the ablation group had better test scores at 12 months than at 6 months, “indicating a likelihood of improvement over time.”
Differences between the groups would probably have been less pronounced if the control group had received a sham procedure, Dr. Badin proposed. That would potentially differentiate ablation’s clinical and placebo contributions to the outcomes.
Still, he said, any observed placebo effect in a sham-controlled trial would probably have been limited. “I think it still would have been a positive trial. It may not show the same difference, but I don’t think you would have a neutral trial just by doing a sham.”
REMEDIAL has “good data,” and its conclusions about ablation’s potential psychological benefits are “reasonable” and worth bringing up when discussing the procedure with patients, Dr. Badin said.
Indeed, psychological distress is “important and often overlooked” in patients with AFib, Dr. Kalman observed. “The dominant indication for atrial fibrillation ablation is symptomatic impact on quality of life. We should think about that broadly, about not just the physical symptoms but the impact on their mental health.”
The trial was conducted at two centers in Australia. It enrolled patients, one-third of whom were women, who were on medical management for AFib. Patients receiving treatment for severe depression were excluded. The included patients were randomly assigned to undergo catheter ablation or to continue on closely managed rhythm-control medication, with cardioversion as indicated.
Psychological distress was measured at baseline and throughout follow-up by a battery of self-administered, validated questionnaires. Baseline test scores for the two groups were similar.
Recurrence and burden of AFib were tracked primarily by daily KardiaMobile (AliveCor) ECG monitoring. A few patients were followed using already implanted cardiac rhythm devices or by 24-hour Holter monitor every 3 months, Dr. Kalman said.
Composite scores on the Hospital Anxiety and Depression Scale (HADS) at 12 months, the primary endpoint, were 7.6 and 11.8 (P = .005) for the ablation and medical groups, respectively. They were 8.2 and 11.9 (P = .006), respectively, at 6 months.
The prevalence of severe psychological distress, defined as a HADS score greater than 15, was lower in the ablation group at 6 months (14.2% vs. 34%; P = .02) and 12 months (10.2% vs. 31.9%; P = .01).
Scores on the Beck Depression Inventory–II questionnaire were also consistently and significantly better for the ablation group at 6 and at 12 months (P = .01 for both).
Monitoring picked up AFib in 47% of the ablation group and 96% of the control group (P < .001) over 12 months. Their median AFib burdens were 0% (interquartile range, 0%-3.2%) and 15.5% (IQR, 1%-46%), respectively (P < .001).
Antiarrhythmic drug use fell from a baseline of 90% to 53% 3 months after ablation and 30% at 12 months (P = .003). Use of these drugs in the control group was 89% at baseline and remained essentially the same, 85%, at 12 months.
AFib symptom severity scores were significantly lower after ablation, compared with medical management at 3, 6, and 12 months.
The observed effect of ablation on psychological stress “clearly speaks in favor of effective rhythm control, and moreover catheter ablation” and is a “novel argument” in support of catheter ablation for AFib, Julia Lurz, MD, Heart Center Leipzig (Germany) at University Leipzig, and Karl-Heinz Ladwig, MD, PhD, Technical University Munich (Germany), wrote in an editorial accompanying publication of REMEDIAL.
But the findings also “raise the question of why rhythm control was so ineffective in the medical treatment group,” they wrote.
They agreed that the randomization process itself may have had its own psychological effects. “Potential disappointment” in the medical group and “high expectations” among patients who received ablation “could have fueled the success of catheter ablation” with respect to mental health endpoints.
Dr. Kalman reported receiving grants from the National Health and Medical Research Council of Australia, Medtronic, Mooney, and Biosense Webster. Dr. Badin, Dr. Lurz, and Dr. Ladwig reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
who had initially tested high for such psychological distress.
The finding, said the researchers, may point to an overlooked potential benefit of ablation that can be discussed with patients considering whether to have the procedure.
Importantly, the 100 adults with symptomatic paroxysmal or persistent AFib in the randomized trial weren’t blinded to treatment assignment, which was either ablation or continued medical therapy.
That leaves open the possibility that psychological distress improved in the ablation group not from any unique effect of ablation itself but because patients expected to benefit from the procedure.
The investigators acknowledged that their trial, called REMEDIAL, can’t rule out a placebo effect as part of the observed benefit. Indeed, studies suggest that there is a substantial placebo component of AFib ablation – which, notably, is usually done to make patients feel better.
But the current findings are more consistent with the conventional view that patients feel better primarily because ablation reduces the AFib causing their symptoms, the group said.
Psychological stress in the study started to fall early after the procedure and continued to decline consistently over the next 6 months (P = .006) and 12 months (P = .005), not a typical pattern for placebo, they wrote.
Moreover, the mental health benefits “correlated very strongly” with less recurrent AFib, reduced AFib burden, and withdrawal of beta-blockers and antiarrhythmic agents, outcomes that might be expected from ablation, said Jonathan M. Kalman, MBBS, PhD.
“Of course, I cannot say there is no placebo effect from having had the procedure, and maybe that something to consider,” but it’s probably not the main driver of benefit, he said in an interview. The relationship between successful AFib ablation “and improvements in physical and now mental health is overwhelming.”
Dr. Kalman, who is affiliated with Royal Melbourne Hospital, is senior author on the study, published in JAMA.
The findings add to “strong, reproducible evidence that ablation is the best way to tackle rhythm control in [AFib] populations” regardless of age, mental health status, or AFib burden, said Auroa Badin, MD, who wasn’t involved in REMEDIAL but has studied the psychological effects of arrhythmia ablation.
For example, there is “very good evidence” from CABANA and other trials that AFib ablation “considerably improves quality of life,” Dr. Badin, of OhioHealth Heart & Vascular Physicians, Columbus, said in an interview. The current study “just emphasizes that there’s also a psychological effect.”
Some of that response could be a placebo or even a nocebo effect. Most of the patients assigned to the medical arm had already been on medications that failed at rhythm control. And their management in the trial, he said, “even if you optimize it, was still drug therapy.”
Patients in the control group, therefore, could have been “disappointed” at the prospect of continued ineffective therapy in a way that influenced their outcomes. “That is another confounding factor,” Dr. Badin said.
But if the psychological results of ablation in the trial were predominantly a placebo effect, early differences in psychological test scores would not have persisted for long, certainly not for a year, he observed. Moreover, the ablation group had better test scores at 12 months than at 6 months, “indicating a likelihood of improvement over time.”
Differences between the groups would probably have been less pronounced if the control group had received a sham procedure, Dr. Badin proposed. That would potentially differentiate ablation’s clinical and placebo contributions to the outcomes.
Still, he said, any observed placebo effect in a sham-controlled trial would probably have been limited. “I think it still would have been a positive trial. It may not show the same difference, but I don’t think you would have a neutral trial just by doing a sham.”
REMEDIAL has “good data,” and its conclusions about ablation’s potential psychological benefits are “reasonable” and worth bringing up when discussing the procedure with patients, Dr. Badin said.
Indeed, psychological distress is “important and often overlooked” in patients with AFib, Dr. Kalman observed. “The dominant indication for atrial fibrillation ablation is symptomatic impact on quality of life. We should think about that broadly, about not just the physical symptoms but the impact on their mental health.”
The trial was conducted at two centers in Australia. It enrolled patients, one-third of whom were women, who were on medical management for AFib. Patients receiving treatment for severe depression were excluded. The included patients were randomly assigned to undergo catheter ablation or to continue on closely managed rhythm-control medication, with cardioversion as indicated.
Psychological distress was measured at baseline and throughout follow-up by a battery of self-administered, validated questionnaires. Baseline test scores for the two groups were similar.
Recurrence and burden of AFib were tracked primarily by daily KardiaMobile (AliveCor) ECG monitoring. A few patients were followed using already implanted cardiac rhythm devices or by 24-hour Holter monitor every 3 months, Dr. Kalman said.
Composite scores on the Hospital Anxiety and Depression Scale (HADS) at 12 months, the primary endpoint, were 7.6 and 11.8 (P = .005) for the ablation and medical groups, respectively. They were 8.2 and 11.9 (P = .006), respectively, at 6 months.
The prevalence of severe psychological distress, defined as a HADS score greater than 15, was lower in the ablation group at 6 months (14.2% vs. 34%; P = .02) and 12 months (10.2% vs. 31.9%; P = .01).
Scores on the Beck Depression Inventory–II questionnaire were also consistently and significantly better for the ablation group at 6 and at 12 months (P = .01 for both).
Monitoring picked up AFib in 47% of the ablation group and 96% of the control group (P < .001) over 12 months. Their median AFib burdens were 0% (interquartile range, 0%-3.2%) and 15.5% (IQR, 1%-46%), respectively (P < .001).
Antiarrhythmic drug use fell from a baseline of 90% to 53% 3 months after ablation and 30% at 12 months (P = .003). Use of these drugs in the control group was 89% at baseline and remained essentially the same, 85%, at 12 months.
AFib symptom severity scores were significantly lower after ablation, compared with medical management at 3, 6, and 12 months.
The observed effect of ablation on psychological stress “clearly speaks in favor of effective rhythm control, and moreover catheter ablation” and is a “novel argument” in support of catheter ablation for AFib, Julia Lurz, MD, Heart Center Leipzig (Germany) at University Leipzig, and Karl-Heinz Ladwig, MD, PhD, Technical University Munich (Germany), wrote in an editorial accompanying publication of REMEDIAL.
But the findings also “raise the question of why rhythm control was so ineffective in the medical treatment group,” they wrote.
They agreed that the randomization process itself may have had its own psychological effects. “Potential disappointment” in the medical group and “high expectations” among patients who received ablation “could have fueled the success of catheter ablation” with respect to mental health endpoints.
Dr. Kalman reported receiving grants from the National Health and Medical Research Council of Australia, Medtronic, Mooney, and Biosense Webster. Dr. Badin, Dr. Lurz, and Dr. Ladwig reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM JAMA
Trial halted for bleeding reduction with abelacimab vs. rivaroxaban in AFib
– major and clinically relevant nonmajor bleeding – in patients taking abelacimab versus those on rivaroxaban.
The announcement of topline results of the AZALEA-TIMI 71 trial was made by Anthos Therapeutics, the company developing abelacimab.
“The AZALEA-TIMI 71 study is the largest and longest head-to-head study of a Factor XI inhibitor to provide definitive evidence of a highly significant reduction in bleeding as compared to the standard-of-care anticoagulant,” Marc Sabatine, MD, chair of cardiovascular medicine at Brigham and Women’s Hospital and chair of the TIMI study group, both in Boston, stated in the Anthos press release.
“With a median of 21 months of follow-up, spanning more than 2,000 patient years, AZALEA-TIMI 71 represents a landmark study confirming the promise of Factor XI inhibition as causing substantially less bleeding than a current standard-of-care,” Dr. Sabatine added.
Abelacimab is a novel, highly selective, fully human monoclonal antibody with dual inhibitory activity against factor XI and its active form, factor XIa. At the 150-mg dose given subcutaneously once monthly, the drug maintains around 98% inhibition of factor XI, in line with the benign bleeding profile of patients with genetic factor XI deficiency, the company notes.
The AZALEA-TIMI 71 study is an event-driven, randomized study comparing two blinded doses of abelacimab (90 mg or 150 mg given by subcutaneous injection once-monthly) with rivaroxaban 20 mg daily in 1,287 patients with AFib who are at moderate to high risk for stroke. Full results of the study will be presented at an upcoming scientific congress.
Patients in the rivaroxaban arm can transition to abelacimab in an extension study.
In a previous proof-of-concept study published in The New England Journal of Medicine, a single IV dose of abelacimab achieved a large reduction in venous thromboembolism versus enoxaparin in patients undergoing knee surgery.
A phase 3 trial in AFib patients is now planned. The LILAC-TIMI 76 study is an event-driven, randomized trial to evaluate the efficacy and safety of abelacimab relative to placebo on the rate of ischemic stroke or systemic embolism in AFib patients who have been deemed to be unsuitable for currently available anticoagulation therapy. Patients will be randomized to receive abelacimab 150 mg subcutaneously or matching placebo once monthly. The researchers aim to enroll approximately 1,900 patients from North America, Europe, Latin America, the Middle East, and Asia.
Dan Bloomfield, MD, chief medical officer of Anthos Therapeutics, said that, “Abelacimab embodies its promise as a hemostasis-sparing anticoagulant and represents a paradigm shift in the prevention of stroke and other thrombotic conditions.”
It is estimated that 12.1 million people in the United States will have AFib by 2030, but 40%-60% of patients with AFib are not prescribed anticoagulants today, one of the main reasons being concerns about bleeding, the company notes.
“Abelacimab has the potential to provide a game-changing treatment option for all those patients who live with the daily fear of bleeding while taking current anticoagulants,” said Leslie Lake, president of the National Blood Clot Alliance.
Abelacimab has been granted a fast-track designation by the U.S. Food and Drug Administration for the prevention of stroke and systemic embolism in patients with atrial fibrillation.
Several other Factor XI inhibitors are in development and have also shown promising results in terms of a more benign bleeding profile than current standard-of-care anticoagulants.
A version of this article first appeared on Medscape.com.
– major and clinically relevant nonmajor bleeding – in patients taking abelacimab versus those on rivaroxaban.
The announcement of topline results of the AZALEA-TIMI 71 trial was made by Anthos Therapeutics, the company developing abelacimab.
“The AZALEA-TIMI 71 study is the largest and longest head-to-head study of a Factor XI inhibitor to provide definitive evidence of a highly significant reduction in bleeding as compared to the standard-of-care anticoagulant,” Marc Sabatine, MD, chair of cardiovascular medicine at Brigham and Women’s Hospital and chair of the TIMI study group, both in Boston, stated in the Anthos press release.
“With a median of 21 months of follow-up, spanning more than 2,000 patient years, AZALEA-TIMI 71 represents a landmark study confirming the promise of Factor XI inhibition as causing substantially less bleeding than a current standard-of-care,” Dr. Sabatine added.
Abelacimab is a novel, highly selective, fully human monoclonal antibody with dual inhibitory activity against factor XI and its active form, factor XIa. At the 150-mg dose given subcutaneously once monthly, the drug maintains around 98% inhibition of factor XI, in line with the benign bleeding profile of patients with genetic factor XI deficiency, the company notes.
The AZALEA-TIMI 71 study is an event-driven, randomized study comparing two blinded doses of abelacimab (90 mg or 150 mg given by subcutaneous injection once-monthly) with rivaroxaban 20 mg daily in 1,287 patients with AFib who are at moderate to high risk for stroke. Full results of the study will be presented at an upcoming scientific congress.
Patients in the rivaroxaban arm can transition to abelacimab in an extension study.
In a previous proof-of-concept study published in The New England Journal of Medicine, a single IV dose of abelacimab achieved a large reduction in venous thromboembolism versus enoxaparin in patients undergoing knee surgery.
A phase 3 trial in AFib patients is now planned. The LILAC-TIMI 76 study is an event-driven, randomized trial to evaluate the efficacy and safety of abelacimab relative to placebo on the rate of ischemic stroke or systemic embolism in AFib patients who have been deemed to be unsuitable for currently available anticoagulation therapy. Patients will be randomized to receive abelacimab 150 mg subcutaneously or matching placebo once monthly. The researchers aim to enroll approximately 1,900 patients from North America, Europe, Latin America, the Middle East, and Asia.
Dan Bloomfield, MD, chief medical officer of Anthos Therapeutics, said that, “Abelacimab embodies its promise as a hemostasis-sparing anticoagulant and represents a paradigm shift in the prevention of stroke and other thrombotic conditions.”
It is estimated that 12.1 million people in the United States will have AFib by 2030, but 40%-60% of patients with AFib are not prescribed anticoagulants today, one of the main reasons being concerns about bleeding, the company notes.
“Abelacimab has the potential to provide a game-changing treatment option for all those patients who live with the daily fear of bleeding while taking current anticoagulants,” said Leslie Lake, president of the National Blood Clot Alliance.
Abelacimab has been granted a fast-track designation by the U.S. Food and Drug Administration for the prevention of stroke and systemic embolism in patients with atrial fibrillation.
Several other Factor XI inhibitors are in development and have also shown promising results in terms of a more benign bleeding profile than current standard-of-care anticoagulants.
A version of this article first appeared on Medscape.com.
– major and clinically relevant nonmajor bleeding – in patients taking abelacimab versus those on rivaroxaban.
The announcement of topline results of the AZALEA-TIMI 71 trial was made by Anthos Therapeutics, the company developing abelacimab.
“The AZALEA-TIMI 71 study is the largest and longest head-to-head study of a Factor XI inhibitor to provide definitive evidence of a highly significant reduction in bleeding as compared to the standard-of-care anticoagulant,” Marc Sabatine, MD, chair of cardiovascular medicine at Brigham and Women’s Hospital and chair of the TIMI study group, both in Boston, stated in the Anthos press release.
“With a median of 21 months of follow-up, spanning more than 2,000 patient years, AZALEA-TIMI 71 represents a landmark study confirming the promise of Factor XI inhibition as causing substantially less bleeding than a current standard-of-care,” Dr. Sabatine added.
Abelacimab is a novel, highly selective, fully human monoclonal antibody with dual inhibitory activity against factor XI and its active form, factor XIa. At the 150-mg dose given subcutaneously once monthly, the drug maintains around 98% inhibition of factor XI, in line with the benign bleeding profile of patients with genetic factor XI deficiency, the company notes.
The AZALEA-TIMI 71 study is an event-driven, randomized study comparing two blinded doses of abelacimab (90 mg or 150 mg given by subcutaneous injection once-monthly) with rivaroxaban 20 mg daily in 1,287 patients with AFib who are at moderate to high risk for stroke. Full results of the study will be presented at an upcoming scientific congress.
Patients in the rivaroxaban arm can transition to abelacimab in an extension study.
In a previous proof-of-concept study published in The New England Journal of Medicine, a single IV dose of abelacimab achieved a large reduction in venous thromboembolism versus enoxaparin in patients undergoing knee surgery.
A phase 3 trial in AFib patients is now planned. The LILAC-TIMI 76 study is an event-driven, randomized trial to evaluate the efficacy and safety of abelacimab relative to placebo on the rate of ischemic stroke or systemic embolism in AFib patients who have been deemed to be unsuitable for currently available anticoagulation therapy. Patients will be randomized to receive abelacimab 150 mg subcutaneously or matching placebo once monthly. The researchers aim to enroll approximately 1,900 patients from North America, Europe, Latin America, the Middle East, and Asia.
Dan Bloomfield, MD, chief medical officer of Anthos Therapeutics, said that, “Abelacimab embodies its promise as a hemostasis-sparing anticoagulant and represents a paradigm shift in the prevention of stroke and other thrombotic conditions.”
It is estimated that 12.1 million people in the United States will have AFib by 2030, but 40%-60% of patients with AFib are not prescribed anticoagulants today, one of the main reasons being concerns about bleeding, the company notes.
“Abelacimab has the potential to provide a game-changing treatment option for all those patients who live with the daily fear of bleeding while taking current anticoagulants,” said Leslie Lake, president of the National Blood Clot Alliance.
Abelacimab has been granted a fast-track designation by the U.S. Food and Drug Administration for the prevention of stroke and systemic embolism in patients with atrial fibrillation.
Several other Factor XI inhibitors are in development and have also shown promising results in terms of a more benign bleeding profile than current standard-of-care anticoagulants.
A version of this article first appeared on Medscape.com.
Stress, insomnia tied to increased AFib risk for older women
TOPLINE:
Eight psychosocial factors, grouped into two distinct clusters, are significantly associated with risk for atrial fibrillation in postmenopausal women, with insomnia and stressful life events (SLEs) being the most strongly associated with AFib, a large new study has found.
METHODOLOGY:
- In addition to traditional risk factors such as obesity, advanced age, ethnicity, smoking, alcohol, hypertension, diabetes, coronary artery disease, heart failure, and emotional and psychological distress may also affect AFib.
- The study included 83,736 postmenopausal women in the Women’s Health Initiative (mean age, 63.9 years; 88.1% White) who did not have AFib at baseline.
- From questionnaires, researchers collected information on psychosocial stressors and used hierarchical cluster analysis to identify patterns of psychosocial predictors.
- They separated these clusters into quartiles, identified associations between psychosocial exposure variables, and adjusted for traditional risk factors.
- Over an average follow-up of 10.5 years, 23,954 participants (28.6%) developed incident AFib.
TAKEAWAY:
- The analysis generated two clusters of distinct psychosocial variables that were significantly associated with AFib: the Stress Cluster, including SLEs, depressive symptoms, and insomnia; and the Strain Cluster, including three personality traits: optimism, cynical hostility, and emotional expressiveness; and two social measures: social support, and social strain.
- Those in the highest quartiles of both the Stress Cluster and the Strain Cluster had greater rates of AFib, compared with those in the lowest quartiles.
- In a final model, the association between SLEs (hazard ratio, 1.02; 95% confidence interval, 1.01-1.04) and insomnia (HR, 1.04; 95% CI, 1.03-1.06) were most strongly linked to increased incidence of AFib, and a sensitivity analysis using snoring as a surrogate marker for sleep apnea didn’t change this outcome, supporting the independent effect of insomnia on AFib.
- In subgroup analyses, the Stress Cluster had a stronger association with AFib incidence in younger (50-69 years) versus older women (70-79 years), and in non-Hispanic White and Asian women versus Hispanic and non-Hispanic Black women.
IN PRACTICE:
The results support the hypothesis that psychosocial predictors account for additional risk for AFib “above and beyond” traditional risk factors, the authors wrote. Identifying and addressing sex-specific, modifiable risk factors, including insomnia, “may help reduce the burden of AF[ib] in aging women.”
SOURCE:
The study was conducted by Susan X. Zhao, MD, division of cardiology, department of medicine, Santa Clara Valley Medical Center, San Jose, Calif., and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
The psychometric questionnaires were administered only at study entry, but psychosocial variables may change over time. Data on sleep apnea and other sleep disorders, which may confound the relationship between insomnia and AFib, were not available, and although the study included a sensitivity analysis controlling for snoring, this is an imperfect surrogate for sleep apnea. Generalizability to other demographic, racial, and ethnic groups is limited.
DISCLOSURES:
The Women’s Health Initiative program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Eight psychosocial factors, grouped into two distinct clusters, are significantly associated with risk for atrial fibrillation in postmenopausal women, with insomnia and stressful life events (SLEs) being the most strongly associated with AFib, a large new study has found.
METHODOLOGY:
- In addition to traditional risk factors such as obesity, advanced age, ethnicity, smoking, alcohol, hypertension, diabetes, coronary artery disease, heart failure, and emotional and psychological distress may also affect AFib.
- The study included 83,736 postmenopausal women in the Women’s Health Initiative (mean age, 63.9 years; 88.1% White) who did not have AFib at baseline.
- From questionnaires, researchers collected information on psychosocial stressors and used hierarchical cluster analysis to identify patterns of psychosocial predictors.
- They separated these clusters into quartiles, identified associations between psychosocial exposure variables, and adjusted for traditional risk factors.
- Over an average follow-up of 10.5 years, 23,954 participants (28.6%) developed incident AFib.
TAKEAWAY:
- The analysis generated two clusters of distinct psychosocial variables that were significantly associated with AFib: the Stress Cluster, including SLEs, depressive symptoms, and insomnia; and the Strain Cluster, including three personality traits: optimism, cynical hostility, and emotional expressiveness; and two social measures: social support, and social strain.
- Those in the highest quartiles of both the Stress Cluster and the Strain Cluster had greater rates of AFib, compared with those in the lowest quartiles.
- In a final model, the association between SLEs (hazard ratio, 1.02; 95% confidence interval, 1.01-1.04) and insomnia (HR, 1.04; 95% CI, 1.03-1.06) were most strongly linked to increased incidence of AFib, and a sensitivity analysis using snoring as a surrogate marker for sleep apnea didn’t change this outcome, supporting the independent effect of insomnia on AFib.
- In subgroup analyses, the Stress Cluster had a stronger association with AFib incidence in younger (50-69 years) versus older women (70-79 years), and in non-Hispanic White and Asian women versus Hispanic and non-Hispanic Black women.
IN PRACTICE:
The results support the hypothesis that psychosocial predictors account for additional risk for AFib “above and beyond” traditional risk factors, the authors wrote. Identifying and addressing sex-specific, modifiable risk factors, including insomnia, “may help reduce the burden of AF[ib] in aging women.”
SOURCE:
The study was conducted by Susan X. Zhao, MD, division of cardiology, department of medicine, Santa Clara Valley Medical Center, San Jose, Calif., and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
The psychometric questionnaires were administered only at study entry, but psychosocial variables may change over time. Data on sleep apnea and other sleep disorders, which may confound the relationship between insomnia and AFib, were not available, and although the study included a sensitivity analysis controlling for snoring, this is an imperfect surrogate for sleep apnea. Generalizability to other demographic, racial, and ethnic groups is limited.
DISCLOSURES:
The Women’s Health Initiative program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Eight psychosocial factors, grouped into two distinct clusters, are significantly associated with risk for atrial fibrillation in postmenopausal women, with insomnia and stressful life events (SLEs) being the most strongly associated with AFib, a large new study has found.
METHODOLOGY:
- In addition to traditional risk factors such as obesity, advanced age, ethnicity, smoking, alcohol, hypertension, diabetes, coronary artery disease, heart failure, and emotional and psychological distress may also affect AFib.
- The study included 83,736 postmenopausal women in the Women’s Health Initiative (mean age, 63.9 years; 88.1% White) who did not have AFib at baseline.
- From questionnaires, researchers collected information on psychosocial stressors and used hierarchical cluster analysis to identify patterns of psychosocial predictors.
- They separated these clusters into quartiles, identified associations between psychosocial exposure variables, and adjusted for traditional risk factors.
- Over an average follow-up of 10.5 years, 23,954 participants (28.6%) developed incident AFib.
TAKEAWAY:
- The analysis generated two clusters of distinct psychosocial variables that were significantly associated with AFib: the Stress Cluster, including SLEs, depressive symptoms, and insomnia; and the Strain Cluster, including three personality traits: optimism, cynical hostility, and emotional expressiveness; and two social measures: social support, and social strain.
- Those in the highest quartiles of both the Stress Cluster and the Strain Cluster had greater rates of AFib, compared with those in the lowest quartiles.
- In a final model, the association between SLEs (hazard ratio, 1.02; 95% confidence interval, 1.01-1.04) and insomnia (HR, 1.04; 95% CI, 1.03-1.06) were most strongly linked to increased incidence of AFib, and a sensitivity analysis using snoring as a surrogate marker for sleep apnea didn’t change this outcome, supporting the independent effect of insomnia on AFib.
- In subgroup analyses, the Stress Cluster had a stronger association with AFib incidence in younger (50-69 years) versus older women (70-79 years), and in non-Hispanic White and Asian women versus Hispanic and non-Hispanic Black women.
IN PRACTICE:
The results support the hypothesis that psychosocial predictors account for additional risk for AFib “above and beyond” traditional risk factors, the authors wrote. Identifying and addressing sex-specific, modifiable risk factors, including insomnia, “may help reduce the burden of AF[ib] in aging women.”
SOURCE:
The study was conducted by Susan X. Zhao, MD, division of cardiology, department of medicine, Santa Clara Valley Medical Center, San Jose, Calif., and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
The psychometric questionnaires were administered only at study entry, but psychosocial variables may change over time. Data on sleep apnea and other sleep disorders, which may confound the relationship between insomnia and AFib, were not available, and although the study included a sensitivity analysis controlling for snoring, this is an imperfect surrogate for sleep apnea. Generalizability to other demographic, racial, and ethnic groups is limited.
DISCLOSURES:
The Women’s Health Initiative program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
Steady VKA therapy beats switch to NOAC in frail AFib patients: FRAIL-AF
Switching frail patients with atrial fibrillation (AFib) from anticoagulation therapy with vitamin K antagonists (VKAs) to a novel oral anticoagulant (NOAC) resulted in more bleeding without any reduction in thromboembolic complications or all-cause mortality, randomized trial results show.
The study, FRAIL-AF, is the first randomized NOAC trial to exclusively include frail older patients, said lead author Linda P.T. Joosten, MD, Julius Center for Health Sciences and Primary Care in Utrecht, the Netherlands, and these unexpected findings provide evidence that goes beyond what is currently available.
“Data from the FRAIL-AF trial showed that switching from a VKA to a NOAC should not be considered without a clear indication in frail older patients with AF[ib], as switching to a NOAC leads to 69% more bleeding,” she concluded, without any benefit on secondary clinical endpoints, including thromboembolic events and all-cause mortality.
“The results turned out different than we expected,” Dr. Joosten said. “The hypothesis of this superiority trial was that switching from VKA therapy to a NOAC would result in less bleeding. However, we observed the opposite. After the interim analysis, the data and safety monitoring board advised to stop inclusion because switching from a VKA to a NOAC was clearly contraindicated with a hazard ratio of 1.69 and a highly significant P value of .001.”
Results of FRAIL-AF were presented at the annual congress of the European Society of Cardiology and published online in the journal Circulation.
Session moderator Renate B. Schnabel, MD, interventional cardiologist with University Heart & Vascular Center Hamburg (Germany), congratulated the researchers on these “astonishing” data.
“The thing I want to emphasize here is that, in the absence of randomized controlled trial data, we should be very cautious in extrapolating data from the landmark trials to populations not enrolled in those, and to rely on observational data only,” Dr. Schnabel told Dr. Joosten. “We need randomized controlled trials that sometimes give astonishing results.”
Frailty a clinical syndrome
Frailty is “a lot more than just aging, multiple comorbidities and polypharmacy,” Dr. Joosten explained. “It’s really a clinical syndrome, with people with a high biological vulnerability, dependency on significant others, and a reduced capacity to resist stressors, all leading to a reduced homeostatic reserve.”
Frailty is common in the community, with a prevalence of about 12%, she noted, “and even more important, AF[ib] in frail older people is very common, with a prevalence of 18%. And “without any doubt, we have to adequately anticoagulate frail AF[ib] patients, as they have a high stroke risk, with an incidence of 12.4% per year,” Dr. Joosten noted, compared with 3.9% per year among nonfrail AFib patients.
NOACs are preferred over VKAs in nonfrail AFib patients, after four major trials, RE-LY with dabigatran, ROCKET-AF with rivaroxaban, ARISTOTLE with apixaban, and ENGAGE-AF with edoxaban, showed that NOAC treatment resulted in less major bleeding while stroke risk was comparable with treatment with warfarin, she noted.
The 2023 European Heart Rhythm Association consensus document on management of arrhythmias in frailty syndrome concludes that the advantages of NOACs relative to VKAs are “likely consistent” in frail and nonfrail AFib patients, but the level of evidence is low.
So it’s unknown if NOACs are preferred over VKAs in frail AFib patients, “and it’s even more questionable whether patients on VKAs should switch to NOAC therapy,” Dr. Joosten said.
This new trial aimed to answer the question of whether switching frail AFib patients currently managed on a VKA to a NOAC would reduce bleeding. FRAIL-AF was a pragmatic, multicenter, open-label, randomized, controlled superiority trial.
Older AFib patients were deemed frail if they were aged 75 years or older and had a score of 3 or more on the validated Groningen Frailty Indicator (GFI). Patients with a glomerular filtration rate of less than 30 mL/min per 1.73 m2 or with valvular AFib were excluded.
Eligible patients were then assigned randomly to switch from their international normalized ratio (INR)–guided VKA treatment with either 1 mg acenocoumarol or 3 mg phenprocoumon, to a NOAC, or to continue VKA treatment. They were followed for 12 months for the primary outcome – major bleeding or clinically relevant nonmajor bleeding complication, whichever came first – accounting for death as a competing risk.
A total of 1,330 patients were randomly assigned between January 2018 and June 2022. Their mean age was 83 years, and they had a median GFI of 4. After randomization, 6 patients in the switch-to-NOAC arm, and 1 in the continue-VKA arm were found to have exclusion criteria, so in the end, 662 patients were switched from a VKA to NOAC, while 661 continued on VKA therapy. The choice of NOAC was made by the treating physician.
Major bleeding was defined as a fatal bleeding; bleeding in a critical area or organ; bleeding leading to transfusion; and/or bleeding leading to a fall in hemoglobin level of 2 g/dL (1.24 mmol/L) or more. Nonmajor bleeding was bleeding not considered major but requiring face-to-face consultation, hospitalization or increased level of care, or medical intervention.
After a prespecified futility analysis planned after 163 primary outcome events, the trial was halted when it was seen that there were 101 primary outcome events in the switch arm compared to 62 in the continue arm, Dr. Joosten said. The difference appeared to be driven by clinically relevant nonmajor bleeding.
Secondary outcomes of thromboembolic events and all-cause mortality were similar between the groups.
Completely different patients
Discussant at the meeting for the presentation was Isabelle C. Van Gelder, MD, University Medical Centre Groningen (the Netherlands). She said the results are important and relevant because it “provides data on an important gap of knowledge in our AF[ib] guidelines, and a note for all the cardiologists – this study was not done in the hospital. This trial was done in general practitioner practices, so that’s important to consider.”
Comparing FRAIL-AF patients with those of the four previous NOAC trials, “you see that enormous difference in age,” with an average age of 83 years versus 70-73 years in those trials. “These are completely different patients than have been included previously,” she said.
That GFI score of 4 or more includes patients on four or more different types of medication, as well as memory complaints, an inability to walk around the house, and problems with vision or hearing.
The finding of a 69% increase in bleeding with NOACs in FRAIL-AF was “completely unexpected, and I think that we as cardiologists and as NOAC believers did not expect it at all, but it is as clear as it is.” The curves don’t diverge immediately, but rather after 3 months or thereafter, “so it has nothing to do with the switching process. So why did it occur?”
The Netherlands has dedicated thrombosis services that might improve time in therapeutic range for VKA patients, but there is no real difference in TTRs in FRAIL-AF versus the other NOAC trials, Dr. Van Gelder noted.
The most likely suspect in her view is frailty itself, in particular the tendency for patients to be on a high number of medications. A previous study showed, for example, that polypharmacy could be used as a proxy for the effect of frailty on bleeding risk; patients on 10 or more medications had a higher risk for bleeding on treatment with rivaroxaban versus those on 4 or fewer medications.
“Therefore, in my view, why was there such a high risk of bleeding? It’s because these are other patients than we are normally used to treat, we as cardiologists,” although general practitioners see these patients all the time. “It’s all about frailty.”
NOACs are still relatively new drugs, with possible unknown interactions, she added. Because of their frailty and polypharmacy, these patients may benefit from INR control, Dr. Van Gelder speculated. “Therefore, I agree with them that we should be careful; if such old, frail patients survive on VKA, do not change medications and do not switch!”
The study was supported by the Dutch government with additional and unrestricted educational grants from Boehringer Ingelheim, BMS-Pfizer, Bayer, and Daiichi Sankyo. Dr. Joosten reported no relevant financial relationships. Dr. Van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Switching frail patients with atrial fibrillation (AFib) from anticoagulation therapy with vitamin K antagonists (VKAs) to a novel oral anticoagulant (NOAC) resulted in more bleeding without any reduction in thromboembolic complications or all-cause mortality, randomized trial results show.
The study, FRAIL-AF, is the first randomized NOAC trial to exclusively include frail older patients, said lead author Linda P.T. Joosten, MD, Julius Center for Health Sciences and Primary Care in Utrecht, the Netherlands, and these unexpected findings provide evidence that goes beyond what is currently available.
“Data from the FRAIL-AF trial showed that switching from a VKA to a NOAC should not be considered without a clear indication in frail older patients with AF[ib], as switching to a NOAC leads to 69% more bleeding,” she concluded, without any benefit on secondary clinical endpoints, including thromboembolic events and all-cause mortality.
“The results turned out different than we expected,” Dr. Joosten said. “The hypothesis of this superiority trial was that switching from VKA therapy to a NOAC would result in less bleeding. However, we observed the opposite. After the interim analysis, the data and safety monitoring board advised to stop inclusion because switching from a VKA to a NOAC was clearly contraindicated with a hazard ratio of 1.69 and a highly significant P value of .001.”
Results of FRAIL-AF were presented at the annual congress of the European Society of Cardiology and published online in the journal Circulation.
Session moderator Renate B. Schnabel, MD, interventional cardiologist with University Heart & Vascular Center Hamburg (Germany), congratulated the researchers on these “astonishing” data.
“The thing I want to emphasize here is that, in the absence of randomized controlled trial data, we should be very cautious in extrapolating data from the landmark trials to populations not enrolled in those, and to rely on observational data only,” Dr. Schnabel told Dr. Joosten. “We need randomized controlled trials that sometimes give astonishing results.”
Frailty a clinical syndrome
Frailty is “a lot more than just aging, multiple comorbidities and polypharmacy,” Dr. Joosten explained. “It’s really a clinical syndrome, with people with a high biological vulnerability, dependency on significant others, and a reduced capacity to resist stressors, all leading to a reduced homeostatic reserve.”
Frailty is common in the community, with a prevalence of about 12%, she noted, “and even more important, AF[ib] in frail older people is very common, with a prevalence of 18%. And “without any doubt, we have to adequately anticoagulate frail AF[ib] patients, as they have a high stroke risk, with an incidence of 12.4% per year,” Dr. Joosten noted, compared with 3.9% per year among nonfrail AFib patients.
NOACs are preferred over VKAs in nonfrail AFib patients, after four major trials, RE-LY with dabigatran, ROCKET-AF with rivaroxaban, ARISTOTLE with apixaban, and ENGAGE-AF with edoxaban, showed that NOAC treatment resulted in less major bleeding while stroke risk was comparable with treatment with warfarin, she noted.
The 2023 European Heart Rhythm Association consensus document on management of arrhythmias in frailty syndrome concludes that the advantages of NOACs relative to VKAs are “likely consistent” in frail and nonfrail AFib patients, but the level of evidence is low.
So it’s unknown if NOACs are preferred over VKAs in frail AFib patients, “and it’s even more questionable whether patients on VKAs should switch to NOAC therapy,” Dr. Joosten said.
This new trial aimed to answer the question of whether switching frail AFib patients currently managed on a VKA to a NOAC would reduce bleeding. FRAIL-AF was a pragmatic, multicenter, open-label, randomized, controlled superiority trial.
Older AFib patients were deemed frail if they were aged 75 years or older and had a score of 3 or more on the validated Groningen Frailty Indicator (GFI). Patients with a glomerular filtration rate of less than 30 mL/min per 1.73 m2 or with valvular AFib were excluded.
Eligible patients were then assigned randomly to switch from their international normalized ratio (INR)–guided VKA treatment with either 1 mg acenocoumarol or 3 mg phenprocoumon, to a NOAC, or to continue VKA treatment. They were followed for 12 months for the primary outcome – major bleeding or clinically relevant nonmajor bleeding complication, whichever came first – accounting for death as a competing risk.
A total of 1,330 patients were randomly assigned between January 2018 and June 2022. Their mean age was 83 years, and they had a median GFI of 4. After randomization, 6 patients in the switch-to-NOAC arm, and 1 in the continue-VKA arm were found to have exclusion criteria, so in the end, 662 patients were switched from a VKA to NOAC, while 661 continued on VKA therapy. The choice of NOAC was made by the treating physician.
Major bleeding was defined as a fatal bleeding; bleeding in a critical area or organ; bleeding leading to transfusion; and/or bleeding leading to a fall in hemoglobin level of 2 g/dL (1.24 mmol/L) or more. Nonmajor bleeding was bleeding not considered major but requiring face-to-face consultation, hospitalization or increased level of care, or medical intervention.
After a prespecified futility analysis planned after 163 primary outcome events, the trial was halted when it was seen that there were 101 primary outcome events in the switch arm compared to 62 in the continue arm, Dr. Joosten said. The difference appeared to be driven by clinically relevant nonmajor bleeding.
Secondary outcomes of thromboembolic events and all-cause mortality were similar between the groups.
Completely different patients
Discussant at the meeting for the presentation was Isabelle C. Van Gelder, MD, University Medical Centre Groningen (the Netherlands). She said the results are important and relevant because it “provides data on an important gap of knowledge in our AF[ib] guidelines, and a note for all the cardiologists – this study was not done in the hospital. This trial was done in general practitioner practices, so that’s important to consider.”
Comparing FRAIL-AF patients with those of the four previous NOAC trials, “you see that enormous difference in age,” with an average age of 83 years versus 70-73 years in those trials. “These are completely different patients than have been included previously,” she said.
That GFI score of 4 or more includes patients on four or more different types of medication, as well as memory complaints, an inability to walk around the house, and problems with vision or hearing.
The finding of a 69% increase in bleeding with NOACs in FRAIL-AF was “completely unexpected, and I think that we as cardiologists and as NOAC believers did not expect it at all, but it is as clear as it is.” The curves don’t diverge immediately, but rather after 3 months or thereafter, “so it has nothing to do with the switching process. So why did it occur?”
The Netherlands has dedicated thrombosis services that might improve time in therapeutic range for VKA patients, but there is no real difference in TTRs in FRAIL-AF versus the other NOAC trials, Dr. Van Gelder noted.
The most likely suspect in her view is frailty itself, in particular the tendency for patients to be on a high number of medications. A previous study showed, for example, that polypharmacy could be used as a proxy for the effect of frailty on bleeding risk; patients on 10 or more medications had a higher risk for bleeding on treatment with rivaroxaban versus those on 4 or fewer medications.
“Therefore, in my view, why was there such a high risk of bleeding? It’s because these are other patients than we are normally used to treat, we as cardiologists,” although general practitioners see these patients all the time. “It’s all about frailty.”
NOACs are still relatively new drugs, with possible unknown interactions, she added. Because of their frailty and polypharmacy, these patients may benefit from INR control, Dr. Van Gelder speculated. “Therefore, I agree with them that we should be careful; if such old, frail patients survive on VKA, do not change medications and do not switch!”
The study was supported by the Dutch government with additional and unrestricted educational grants from Boehringer Ingelheim, BMS-Pfizer, Bayer, and Daiichi Sankyo. Dr. Joosten reported no relevant financial relationships. Dr. Van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Switching frail patients with atrial fibrillation (AFib) from anticoagulation therapy with vitamin K antagonists (VKAs) to a novel oral anticoagulant (NOAC) resulted in more bleeding without any reduction in thromboembolic complications or all-cause mortality, randomized trial results show.
The study, FRAIL-AF, is the first randomized NOAC trial to exclusively include frail older patients, said lead author Linda P.T. Joosten, MD, Julius Center for Health Sciences and Primary Care in Utrecht, the Netherlands, and these unexpected findings provide evidence that goes beyond what is currently available.
“Data from the FRAIL-AF trial showed that switching from a VKA to a NOAC should not be considered without a clear indication in frail older patients with AF[ib], as switching to a NOAC leads to 69% more bleeding,” she concluded, without any benefit on secondary clinical endpoints, including thromboembolic events and all-cause mortality.
“The results turned out different than we expected,” Dr. Joosten said. “The hypothesis of this superiority trial was that switching from VKA therapy to a NOAC would result in less bleeding. However, we observed the opposite. After the interim analysis, the data and safety monitoring board advised to stop inclusion because switching from a VKA to a NOAC was clearly contraindicated with a hazard ratio of 1.69 and a highly significant P value of .001.”
Results of FRAIL-AF were presented at the annual congress of the European Society of Cardiology and published online in the journal Circulation.
Session moderator Renate B. Schnabel, MD, interventional cardiologist with University Heart & Vascular Center Hamburg (Germany), congratulated the researchers on these “astonishing” data.
“The thing I want to emphasize here is that, in the absence of randomized controlled trial data, we should be very cautious in extrapolating data from the landmark trials to populations not enrolled in those, and to rely on observational data only,” Dr. Schnabel told Dr. Joosten. “We need randomized controlled trials that sometimes give astonishing results.”
Frailty a clinical syndrome
Frailty is “a lot more than just aging, multiple comorbidities and polypharmacy,” Dr. Joosten explained. “It’s really a clinical syndrome, with people with a high biological vulnerability, dependency on significant others, and a reduced capacity to resist stressors, all leading to a reduced homeostatic reserve.”
Frailty is common in the community, with a prevalence of about 12%, she noted, “and even more important, AF[ib] in frail older people is very common, with a prevalence of 18%. And “without any doubt, we have to adequately anticoagulate frail AF[ib] patients, as they have a high stroke risk, with an incidence of 12.4% per year,” Dr. Joosten noted, compared with 3.9% per year among nonfrail AFib patients.
NOACs are preferred over VKAs in nonfrail AFib patients, after four major trials, RE-LY with dabigatran, ROCKET-AF with rivaroxaban, ARISTOTLE with apixaban, and ENGAGE-AF with edoxaban, showed that NOAC treatment resulted in less major bleeding while stroke risk was comparable with treatment with warfarin, she noted.
The 2023 European Heart Rhythm Association consensus document on management of arrhythmias in frailty syndrome concludes that the advantages of NOACs relative to VKAs are “likely consistent” in frail and nonfrail AFib patients, but the level of evidence is low.
So it’s unknown if NOACs are preferred over VKAs in frail AFib patients, “and it’s even more questionable whether patients on VKAs should switch to NOAC therapy,” Dr. Joosten said.
This new trial aimed to answer the question of whether switching frail AFib patients currently managed on a VKA to a NOAC would reduce bleeding. FRAIL-AF was a pragmatic, multicenter, open-label, randomized, controlled superiority trial.
Older AFib patients were deemed frail if they were aged 75 years or older and had a score of 3 or more on the validated Groningen Frailty Indicator (GFI). Patients with a glomerular filtration rate of less than 30 mL/min per 1.73 m2 or with valvular AFib were excluded.
Eligible patients were then assigned randomly to switch from their international normalized ratio (INR)–guided VKA treatment with either 1 mg acenocoumarol or 3 mg phenprocoumon, to a NOAC, or to continue VKA treatment. They were followed for 12 months for the primary outcome – major bleeding or clinically relevant nonmajor bleeding complication, whichever came first – accounting for death as a competing risk.
A total of 1,330 patients were randomly assigned between January 2018 and June 2022. Their mean age was 83 years, and they had a median GFI of 4. After randomization, 6 patients in the switch-to-NOAC arm, and 1 in the continue-VKA arm were found to have exclusion criteria, so in the end, 662 patients were switched from a VKA to NOAC, while 661 continued on VKA therapy. The choice of NOAC was made by the treating physician.
Major bleeding was defined as a fatal bleeding; bleeding in a critical area or organ; bleeding leading to transfusion; and/or bleeding leading to a fall in hemoglobin level of 2 g/dL (1.24 mmol/L) or more. Nonmajor bleeding was bleeding not considered major but requiring face-to-face consultation, hospitalization or increased level of care, or medical intervention.
After a prespecified futility analysis planned after 163 primary outcome events, the trial was halted when it was seen that there were 101 primary outcome events in the switch arm compared to 62 in the continue arm, Dr. Joosten said. The difference appeared to be driven by clinically relevant nonmajor bleeding.
Secondary outcomes of thromboembolic events and all-cause mortality were similar between the groups.
Completely different patients
Discussant at the meeting for the presentation was Isabelle C. Van Gelder, MD, University Medical Centre Groningen (the Netherlands). She said the results are important and relevant because it “provides data on an important gap of knowledge in our AF[ib] guidelines, and a note for all the cardiologists – this study was not done in the hospital. This trial was done in general practitioner practices, so that’s important to consider.”
Comparing FRAIL-AF patients with those of the four previous NOAC trials, “you see that enormous difference in age,” with an average age of 83 years versus 70-73 years in those trials. “These are completely different patients than have been included previously,” she said.
That GFI score of 4 or more includes patients on four or more different types of medication, as well as memory complaints, an inability to walk around the house, and problems with vision or hearing.
The finding of a 69% increase in bleeding with NOACs in FRAIL-AF was “completely unexpected, and I think that we as cardiologists and as NOAC believers did not expect it at all, but it is as clear as it is.” The curves don’t diverge immediately, but rather after 3 months or thereafter, “so it has nothing to do with the switching process. So why did it occur?”
The Netherlands has dedicated thrombosis services that might improve time in therapeutic range for VKA patients, but there is no real difference in TTRs in FRAIL-AF versus the other NOAC trials, Dr. Van Gelder noted.
The most likely suspect in her view is frailty itself, in particular the tendency for patients to be on a high number of medications. A previous study showed, for example, that polypharmacy could be used as a proxy for the effect of frailty on bleeding risk; patients on 10 or more medications had a higher risk for bleeding on treatment with rivaroxaban versus those on 4 or fewer medications.
“Therefore, in my view, why was there such a high risk of bleeding? It’s because these are other patients than we are normally used to treat, we as cardiologists,” although general practitioners see these patients all the time. “It’s all about frailty.”
NOACs are still relatively new drugs, with possible unknown interactions, she added. Because of their frailty and polypharmacy, these patients may benefit from INR control, Dr. Van Gelder speculated. “Therefore, I agree with them that we should be careful; if such old, frail patients survive on VKA, do not change medications and do not switch!”
The study was supported by the Dutch government with additional and unrestricted educational grants from Boehringer Ingelheim, BMS-Pfizer, Bayer, and Daiichi Sankyo. Dr. Joosten reported no relevant financial relationships. Dr. Van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE ESC CONGRESS 2023
Is AFib ablation the fifth pillar in heart failure care? CASTLE-HTx
Recorded Aug. 28, 2023. This transcript has been edited for clarity.
John M. Mandrola, MD: I’m here at the European Society of Cardiology meeting, and I’m very excited to have two colleagues whom I met at the Western Atrial Fibrillation Symposium (Western AFib) and who presented the CASTLE-HTx study. This is Christian Sohns and Philipp Sommer, and the CASTLE-HTx study is very exciting.
Before I get into that, I really want to introduce the concept of atrial fibrillation in heart failure. I like to say that there are two big populations of patients with atrial fibrillation, and the vast majority can be treated slowly with reassurance and education. There is a group of patients who have heart failure who, when they develop atrial fibrillation, can degenerate rapidly. The CASTLE-HTx study looked at catheter ablation versus medical therapy in patients with advanced heart failure.
Christian, why don’t you tell us the top-line results and what you found.
CASTLE-HTx key findings
Christian Sohns, MD, PhD: Thanks, first of all, for mentioning this special cohort of patients in end-stage heart failure, which is very important. The endpoint of the study was a composite of death from any cause or left ventricular assist device (LVAD) implantation and heart transplantation. These are very hard, strong clinical endpoints, not the rate of rehospitalization or something like that.
Catheter ablation was superior to medical therapy alone in terms of this composite endpoint. That was driven by cardiovascular death and all-cause mortality, which highlights the fact that you should always consider atrial fibrillation ablation in the end-stage heart failure cohort. The findings were driven by the fact that we saw left ventricular reverse remodeling and the reduction of atrial fibrillation in these patients.
Dr. Mandrola: Tell me about how it came about. It was conducted at your center. Who were these patients?
Philipp Sommer, MD: As one of the biggest centers for heart transplantations all over Europe, with roughly 100 transplants per year, we had many patients being referred to our center with the questions of whether those patients are eligible for a heart transplantation. Not all of the patients in our study were listed for a transplant, but all of them were admitted in that end-stage heart failure status to evaluate their eligibility for transplant.
If we look at the baseline data of those patients, they had an ejection fraction of 29%. They had a 6-minute walk test as a functional capacity parameter of around 300 m. Approximately two thirds of them were New York Heart Association class III and IV, which is significantly worse than what we saw in the previous studies dealing with heart failure patients.
I think overall, if you also look at NT-proBNP levels, this is a really sick patient population where some people might doubt if they should admit and refer those patients for an ablation procedure. Therefore, it’s really interesting and fascinating to see the results.
Dr. Mandrola: I did read in the manuscript, and I heard from you, that these were recruited as outpatients. So they were stable outpatients who were referred to the center for consideration of an LVAD or transplant?
Dr. Sohns: The definition of stability is very difficult in these patients because they have hospital stays, they have a history of drug therapy, and they have a history of interventions also behind them – not atrial fibrillation ablation, but others. I think these patients are referred because the referring physicians are done with the case. They can no longer offer any option to the patients other than surgical treatment, assist device, pump implantation, or transplantation.
If you look at the guidelines, they do not comment on atrial fibrillation ablation in this cohort of patients. Also, they have different recommendations between the American societies and the European societies regarding what is end-stage heart failure and how to treat these patients. Therefore, it was a big benefit of CASTLE-HTx that we randomized a cohort of patients with advanced end-stage heart failure.
How can AFib ablation have such big, early effects?
Dr. Mandrola: These are very clinically significant findings, with large effect sizes and very early separation of the Kaplan-Meier curves. How do you explain how dramatic an effect that is, and how early of an effect?
Dr. Sommer: That’s one of the key questions at the end of the day. I think our job basically was to provide the data and to ensure that the data are clean and that it’s all perfectly done. The interpretation of these data is really kind of difficult, although we do not have the 100% perfect and obvious explanation why the curves separated so early. Our view on that is that we are talking about a pretty fragile patient population, so little differences like having a tachyarrhythmia of 110 day in, day out or being in sinus rhythm of 60 can make a huge difference. That’s obviously pretty early.
The one that remains in tachyarrhythmia will deteriorate and will require an LVAD after a couple of months, and the one that you may keep in sinus rhythm, even with reduced atrial fibrillation burden – not zero, but reduced atrial fibrillation burden – and improved LV function, all of a sudden this patient will still remain on a low level of being stable, but he or she will remain stable and will not require any surgical interventions for the next 1.5-2 years. If we can manage to do this, just postponing the natural cause of the disease, I think that is a great benefit for the patient.
Dr. Mandrola: One of the things that comes up in our center is that I look at some of these patients and think, there’s no way I can put this patient under general anesthetic and do all of this. Your ablation procedure wasn’t that extensive, was it?
Dr. Sohns: On the one hand, no. On the other hand, yes. You need to take into consideration that it has been performed by experienced physicians with experience in heart failure treatment and atrial fibrillation in heart transplantation centers, though it›s not sure that we can transfer these results one-to-one to all other centers in the world.
It is very clear that we have almost no major complications in these patients. We were able to do these ablation procedures without general anesthesia. We have 60% of patients who had pulmonary vein isolation only and 40% of patients who have PVI and additional therapy. We have a procedure duration of almost 90 minutes during radiofrequency ablation.
We have different categories. When you talk about the different patient cohorts, we also see different stages of myocardial tissue damage, which will be part of another publication for sure. It is, in part, surprising how normal some of the atria were despite having a volume of 180 mL, but they had no fibrosis. That was very interesting.
Dr. Mandrola: How did the persistent vs paroxysmal atrial fibrillation sort out? Were these mostly patients with persistent atrial fibrillation?
Dr. Sommer: Two-thirds were persistent. It would be expected in this patient population that you would not find so many paroxysmal cases. I think it›s very important what Christian was just mentioning that when we discussed the trial design, we were anticipating problems with the sedation, for example. With the follow-up of those procedures, would they decompensate because of the fluid that you have to deliver during such a procedure.
We were quite surprised at the end of the day that the procedures were quite straightforward. Fortunately, we had no major complications. I think there were four complications in the 100 ablated patients. I think we were really positive about how the procedures turned out.
I should mention that one of the exclusion criteria was a left atrial diameter of about 60 mm. The huge ones may be very diseased, and maybe the hopeless ones were excluded from the study. Below 60 mm, we did the ablation.
Rhythm control
Dr. Mandrola: One of my colleagues, who is even more skeptical than me, wanted me to ask you, why wouldn’t you take a patient with persistent atrial fibrillation who had heart failure and just cardiovert and use amiodarone and try and maintain sinus rhythm that way?
Dr. Sohns: It is important to mention that 50% of the patients have already had amiodarone before they were randomized and enrolled for the trial. It might bring you a couple of minutes or a couple of hours [of relief], but the patients would get recurrence.
It was very interesting also, and this is in line with the data from Jason Andrade, who demonstrated that we were able to reduce the percentage of patients with persistent atrial fibrillation to paroxysmal. We did a down-staging of the underlying disease. This is not possible with cardioversion or drugs, for example.
Dr. Sommer: What I really like about that question and that comment is the idea that rhythm control in this subset of patients obviously has a role and an importance. It may be a cardioversion initially, giving amiodarone if they didn’t have that before, and you can keep the patient in sinus rhythm with this therapy, I think we’re reaching the same goal.
I think the critical point to get into the mind of physicians who treat heart failure is that sinus rhythm is beneficial, however you get there. Ablation, of course, as in other studies, is the most powerful tool to get there. Cardioversion can be a really good thing to do; you just have to think about it and consider it.
Dr. Mandrola: I do want to say to everybody that there is a tension sometimes between the heart failure community and the electrophysiology community. I think the ideal situation is that we work together, because I think that we can help with the maintenance of sinus rhythm. The control group mortality at 1 year was 20%, and I’ve heard people say that that’s not advanced heart failure. Advanced heart failure patients have much higher mortality than that. My colleague who is a heart failure specialist was criticizing a selection bias in picking the best patients. How would you answer that?
Dr. Sohns: There are data available from Eurotransplant, for example, that the waiting list mortality is 18%, so I think we are almost in line with this 20% mortality in this conservative group. You cannot generalize it. All these patients have different histories. We have 60% dilated cardiomyopathy and 40% ischemic cardiomyopathy. I think it is a very representative group in contrast to your friend who suggests that it is not.
Dr. Sommer: What I like about the discussion is that some approach us to say that the mortality in the control group is much too high – like, what are you doing with those patients that you create so many endpoints? Then others say that it’s not high enough because that is not end-stage heart failure. Come on! We have a patient cohort that is very well described and very well characterized.
If the label is end-stage heart failure, advanced heart failure, or whatever, they are sicker than the patients that we had in earlier trials. The patients that we treated were mostly excluded from all other trials. We opened the door. We found a clear result. I think everyone can see whatever you like to see.
Dr. Mandrola: What would your take-home message be after having done this trial design, the trial was conducted in your single center, and you come up with these amazing results? What would your message be to the whole community?
Dr. Sohns: Taking into consideration how severely sick these patients are, I can just repeat it: They are one step away from death, more or less, or from surgical intervention that can prolong their life. You should also consider that there are options like atrial fibrillation ablation that can buy time, postpone the natural course, or even in some patients replace the destination therapy. Therefore, in my opinion the next guidelines should recommend that every patient should carefully be checked for sinus rhythm before bringing these patients into the environment of transplantation.
Dr. Sommer: My interpretation is that we have to try to bring into physicians’ minds that besides a well-established and well-documented effect of drug therapy with the fabulous four, we may now have the fabulous five, including an ablation option for patients with atrial fibrillation.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. Dr. Sohns is deputy director of the Heart and Diabetes Center NRW, Ruhr University Bochum, Bad Oeynhausen, Germany. Dr. Sommer is professor of cardiology at the Heart and Diabetes Center NRW. Dr. Mandrola reported no conflicts of interest. Dr. Sohns reported receiving research funding from Else Kröner–Fresenius–Stiftung. Dr. Sommer reported consulting with Abbott, Biosense Webster, Boston Scientific, and Medtronic USA.
A version of this article first appeared on Medscape.com.
Recorded Aug. 28, 2023. This transcript has been edited for clarity.
John M. Mandrola, MD: I’m here at the European Society of Cardiology meeting, and I’m very excited to have two colleagues whom I met at the Western Atrial Fibrillation Symposium (Western AFib) and who presented the CASTLE-HTx study. This is Christian Sohns and Philipp Sommer, and the CASTLE-HTx study is very exciting.
Before I get into that, I really want to introduce the concept of atrial fibrillation in heart failure. I like to say that there are two big populations of patients with atrial fibrillation, and the vast majority can be treated slowly with reassurance and education. There is a group of patients who have heart failure who, when they develop atrial fibrillation, can degenerate rapidly. The CASTLE-HTx study looked at catheter ablation versus medical therapy in patients with advanced heart failure.
Christian, why don’t you tell us the top-line results and what you found.
CASTLE-HTx key findings
Christian Sohns, MD, PhD: Thanks, first of all, for mentioning this special cohort of patients in end-stage heart failure, which is very important. The endpoint of the study was a composite of death from any cause or left ventricular assist device (LVAD) implantation and heart transplantation. These are very hard, strong clinical endpoints, not the rate of rehospitalization or something like that.
Catheter ablation was superior to medical therapy alone in terms of this composite endpoint. That was driven by cardiovascular death and all-cause mortality, which highlights the fact that you should always consider atrial fibrillation ablation in the end-stage heart failure cohort. The findings were driven by the fact that we saw left ventricular reverse remodeling and the reduction of atrial fibrillation in these patients.
Dr. Mandrola: Tell me about how it came about. It was conducted at your center. Who were these patients?
Philipp Sommer, MD: As one of the biggest centers for heart transplantations all over Europe, with roughly 100 transplants per year, we had many patients being referred to our center with the questions of whether those patients are eligible for a heart transplantation. Not all of the patients in our study were listed for a transplant, but all of them were admitted in that end-stage heart failure status to evaluate their eligibility for transplant.
If we look at the baseline data of those patients, they had an ejection fraction of 29%. They had a 6-minute walk test as a functional capacity parameter of around 300 m. Approximately two thirds of them were New York Heart Association class III and IV, which is significantly worse than what we saw in the previous studies dealing with heart failure patients.
I think overall, if you also look at NT-proBNP levels, this is a really sick patient population where some people might doubt if they should admit and refer those patients for an ablation procedure. Therefore, it’s really interesting and fascinating to see the results.
Dr. Mandrola: I did read in the manuscript, and I heard from you, that these were recruited as outpatients. So they were stable outpatients who were referred to the center for consideration of an LVAD or transplant?
Dr. Sohns: The definition of stability is very difficult in these patients because they have hospital stays, they have a history of drug therapy, and they have a history of interventions also behind them – not atrial fibrillation ablation, but others. I think these patients are referred because the referring physicians are done with the case. They can no longer offer any option to the patients other than surgical treatment, assist device, pump implantation, or transplantation.
If you look at the guidelines, they do not comment on atrial fibrillation ablation in this cohort of patients. Also, they have different recommendations between the American societies and the European societies regarding what is end-stage heart failure and how to treat these patients. Therefore, it was a big benefit of CASTLE-HTx that we randomized a cohort of patients with advanced end-stage heart failure.
How can AFib ablation have such big, early effects?
Dr. Mandrola: These are very clinically significant findings, with large effect sizes and very early separation of the Kaplan-Meier curves. How do you explain how dramatic an effect that is, and how early of an effect?
Dr. Sommer: That’s one of the key questions at the end of the day. I think our job basically was to provide the data and to ensure that the data are clean and that it’s all perfectly done. The interpretation of these data is really kind of difficult, although we do not have the 100% perfect and obvious explanation why the curves separated so early. Our view on that is that we are talking about a pretty fragile patient population, so little differences like having a tachyarrhythmia of 110 day in, day out or being in sinus rhythm of 60 can make a huge difference. That’s obviously pretty early.
The one that remains in tachyarrhythmia will deteriorate and will require an LVAD after a couple of months, and the one that you may keep in sinus rhythm, even with reduced atrial fibrillation burden – not zero, but reduced atrial fibrillation burden – and improved LV function, all of a sudden this patient will still remain on a low level of being stable, but he or she will remain stable and will not require any surgical interventions for the next 1.5-2 years. If we can manage to do this, just postponing the natural cause of the disease, I think that is a great benefit for the patient.
Dr. Mandrola: One of the things that comes up in our center is that I look at some of these patients and think, there’s no way I can put this patient under general anesthetic and do all of this. Your ablation procedure wasn’t that extensive, was it?
Dr. Sohns: On the one hand, no. On the other hand, yes. You need to take into consideration that it has been performed by experienced physicians with experience in heart failure treatment and atrial fibrillation in heart transplantation centers, though it›s not sure that we can transfer these results one-to-one to all other centers in the world.
It is very clear that we have almost no major complications in these patients. We were able to do these ablation procedures without general anesthesia. We have 60% of patients who had pulmonary vein isolation only and 40% of patients who have PVI and additional therapy. We have a procedure duration of almost 90 minutes during radiofrequency ablation.
We have different categories. When you talk about the different patient cohorts, we also see different stages of myocardial tissue damage, which will be part of another publication for sure. It is, in part, surprising how normal some of the atria were despite having a volume of 180 mL, but they had no fibrosis. That was very interesting.
Dr. Mandrola: How did the persistent vs paroxysmal atrial fibrillation sort out? Were these mostly patients with persistent atrial fibrillation?
Dr. Sommer: Two-thirds were persistent. It would be expected in this patient population that you would not find so many paroxysmal cases. I think it›s very important what Christian was just mentioning that when we discussed the trial design, we were anticipating problems with the sedation, for example. With the follow-up of those procedures, would they decompensate because of the fluid that you have to deliver during such a procedure.
We were quite surprised at the end of the day that the procedures were quite straightforward. Fortunately, we had no major complications. I think there were four complications in the 100 ablated patients. I think we were really positive about how the procedures turned out.
I should mention that one of the exclusion criteria was a left atrial diameter of about 60 mm. The huge ones may be very diseased, and maybe the hopeless ones were excluded from the study. Below 60 mm, we did the ablation.
Rhythm control
Dr. Mandrola: One of my colleagues, who is even more skeptical than me, wanted me to ask you, why wouldn’t you take a patient with persistent atrial fibrillation who had heart failure and just cardiovert and use amiodarone and try and maintain sinus rhythm that way?
Dr. Sohns: It is important to mention that 50% of the patients have already had amiodarone before they were randomized and enrolled for the trial. It might bring you a couple of minutes or a couple of hours [of relief], but the patients would get recurrence.
It was very interesting also, and this is in line with the data from Jason Andrade, who demonstrated that we were able to reduce the percentage of patients with persistent atrial fibrillation to paroxysmal. We did a down-staging of the underlying disease. This is not possible with cardioversion or drugs, for example.
Dr. Sommer: What I really like about that question and that comment is the idea that rhythm control in this subset of patients obviously has a role and an importance. It may be a cardioversion initially, giving amiodarone if they didn’t have that before, and you can keep the patient in sinus rhythm with this therapy, I think we’re reaching the same goal.
I think the critical point to get into the mind of physicians who treat heart failure is that sinus rhythm is beneficial, however you get there. Ablation, of course, as in other studies, is the most powerful tool to get there. Cardioversion can be a really good thing to do; you just have to think about it and consider it.
Dr. Mandrola: I do want to say to everybody that there is a tension sometimes between the heart failure community and the electrophysiology community. I think the ideal situation is that we work together, because I think that we can help with the maintenance of sinus rhythm. The control group mortality at 1 year was 20%, and I’ve heard people say that that’s not advanced heart failure. Advanced heart failure patients have much higher mortality than that. My colleague who is a heart failure specialist was criticizing a selection bias in picking the best patients. How would you answer that?
Dr. Sohns: There are data available from Eurotransplant, for example, that the waiting list mortality is 18%, so I think we are almost in line with this 20% mortality in this conservative group. You cannot generalize it. All these patients have different histories. We have 60% dilated cardiomyopathy and 40% ischemic cardiomyopathy. I think it is a very representative group in contrast to your friend who suggests that it is not.
Dr. Sommer: What I like about the discussion is that some approach us to say that the mortality in the control group is much too high – like, what are you doing with those patients that you create so many endpoints? Then others say that it’s not high enough because that is not end-stage heart failure. Come on! We have a patient cohort that is very well described and very well characterized.
If the label is end-stage heart failure, advanced heart failure, or whatever, they are sicker than the patients that we had in earlier trials. The patients that we treated were mostly excluded from all other trials. We opened the door. We found a clear result. I think everyone can see whatever you like to see.
Dr. Mandrola: What would your take-home message be after having done this trial design, the trial was conducted in your single center, and you come up with these amazing results? What would your message be to the whole community?
Dr. Sohns: Taking into consideration how severely sick these patients are, I can just repeat it: They are one step away from death, more or less, or from surgical intervention that can prolong their life. You should also consider that there are options like atrial fibrillation ablation that can buy time, postpone the natural course, or even in some patients replace the destination therapy. Therefore, in my opinion the next guidelines should recommend that every patient should carefully be checked for sinus rhythm before bringing these patients into the environment of transplantation.
Dr. Sommer: My interpretation is that we have to try to bring into physicians’ minds that besides a well-established and well-documented effect of drug therapy with the fabulous four, we may now have the fabulous five, including an ablation option for patients with atrial fibrillation.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. Dr. Sohns is deputy director of the Heart and Diabetes Center NRW, Ruhr University Bochum, Bad Oeynhausen, Germany. Dr. Sommer is professor of cardiology at the Heart and Diabetes Center NRW. Dr. Mandrola reported no conflicts of interest. Dr. Sohns reported receiving research funding from Else Kröner–Fresenius–Stiftung. Dr. Sommer reported consulting with Abbott, Biosense Webster, Boston Scientific, and Medtronic USA.
A version of this article first appeared on Medscape.com.
Recorded Aug. 28, 2023. This transcript has been edited for clarity.
John M. Mandrola, MD: I’m here at the European Society of Cardiology meeting, and I’m very excited to have two colleagues whom I met at the Western Atrial Fibrillation Symposium (Western AFib) and who presented the CASTLE-HTx study. This is Christian Sohns and Philipp Sommer, and the CASTLE-HTx study is very exciting.
Before I get into that, I really want to introduce the concept of atrial fibrillation in heart failure. I like to say that there are two big populations of patients with atrial fibrillation, and the vast majority can be treated slowly with reassurance and education. There is a group of patients who have heart failure who, when they develop atrial fibrillation, can degenerate rapidly. The CASTLE-HTx study looked at catheter ablation versus medical therapy in patients with advanced heart failure.
Christian, why don’t you tell us the top-line results and what you found.
CASTLE-HTx key findings
Christian Sohns, MD, PhD: Thanks, first of all, for mentioning this special cohort of patients in end-stage heart failure, which is very important. The endpoint of the study was a composite of death from any cause or left ventricular assist device (LVAD) implantation and heart transplantation. These are very hard, strong clinical endpoints, not the rate of rehospitalization or something like that.
Catheter ablation was superior to medical therapy alone in terms of this composite endpoint. That was driven by cardiovascular death and all-cause mortality, which highlights the fact that you should always consider atrial fibrillation ablation in the end-stage heart failure cohort. The findings were driven by the fact that we saw left ventricular reverse remodeling and the reduction of atrial fibrillation in these patients.
Dr. Mandrola: Tell me about how it came about. It was conducted at your center. Who were these patients?
Philipp Sommer, MD: As one of the biggest centers for heart transplantations all over Europe, with roughly 100 transplants per year, we had many patients being referred to our center with the questions of whether those patients are eligible for a heart transplantation. Not all of the patients in our study were listed for a transplant, but all of them were admitted in that end-stage heart failure status to evaluate their eligibility for transplant.
If we look at the baseline data of those patients, they had an ejection fraction of 29%. They had a 6-minute walk test as a functional capacity parameter of around 300 m. Approximately two thirds of them were New York Heart Association class III and IV, which is significantly worse than what we saw in the previous studies dealing with heart failure patients.
I think overall, if you also look at NT-proBNP levels, this is a really sick patient population where some people might doubt if they should admit and refer those patients for an ablation procedure. Therefore, it’s really interesting and fascinating to see the results.
Dr. Mandrola: I did read in the manuscript, and I heard from you, that these were recruited as outpatients. So they were stable outpatients who were referred to the center for consideration of an LVAD or transplant?
Dr. Sohns: The definition of stability is very difficult in these patients because they have hospital stays, they have a history of drug therapy, and they have a history of interventions also behind them – not atrial fibrillation ablation, but others. I think these patients are referred because the referring physicians are done with the case. They can no longer offer any option to the patients other than surgical treatment, assist device, pump implantation, or transplantation.
If you look at the guidelines, they do not comment on atrial fibrillation ablation in this cohort of patients. Also, they have different recommendations between the American societies and the European societies regarding what is end-stage heart failure and how to treat these patients. Therefore, it was a big benefit of CASTLE-HTx that we randomized a cohort of patients with advanced end-stage heart failure.
How can AFib ablation have such big, early effects?
Dr. Mandrola: These are very clinically significant findings, with large effect sizes and very early separation of the Kaplan-Meier curves. How do you explain how dramatic an effect that is, and how early of an effect?
Dr. Sommer: That’s one of the key questions at the end of the day. I think our job basically was to provide the data and to ensure that the data are clean and that it’s all perfectly done. The interpretation of these data is really kind of difficult, although we do not have the 100% perfect and obvious explanation why the curves separated so early. Our view on that is that we are talking about a pretty fragile patient population, so little differences like having a tachyarrhythmia of 110 day in, day out or being in sinus rhythm of 60 can make a huge difference. That’s obviously pretty early.
The one that remains in tachyarrhythmia will deteriorate and will require an LVAD after a couple of months, and the one that you may keep in sinus rhythm, even with reduced atrial fibrillation burden – not zero, but reduced atrial fibrillation burden – and improved LV function, all of a sudden this patient will still remain on a low level of being stable, but he or she will remain stable and will not require any surgical interventions for the next 1.5-2 years. If we can manage to do this, just postponing the natural cause of the disease, I think that is a great benefit for the patient.
Dr. Mandrola: One of the things that comes up in our center is that I look at some of these patients and think, there’s no way I can put this patient under general anesthetic and do all of this. Your ablation procedure wasn’t that extensive, was it?
Dr. Sohns: On the one hand, no. On the other hand, yes. You need to take into consideration that it has been performed by experienced physicians with experience in heart failure treatment and atrial fibrillation in heart transplantation centers, though it›s not sure that we can transfer these results one-to-one to all other centers in the world.
It is very clear that we have almost no major complications in these patients. We were able to do these ablation procedures without general anesthesia. We have 60% of patients who had pulmonary vein isolation only and 40% of patients who have PVI and additional therapy. We have a procedure duration of almost 90 minutes during radiofrequency ablation.
We have different categories. When you talk about the different patient cohorts, we also see different stages of myocardial tissue damage, which will be part of another publication for sure. It is, in part, surprising how normal some of the atria were despite having a volume of 180 mL, but they had no fibrosis. That was very interesting.
Dr. Mandrola: How did the persistent vs paroxysmal atrial fibrillation sort out? Were these mostly patients with persistent atrial fibrillation?
Dr. Sommer: Two-thirds were persistent. It would be expected in this patient population that you would not find so many paroxysmal cases. I think it›s very important what Christian was just mentioning that when we discussed the trial design, we were anticipating problems with the sedation, for example. With the follow-up of those procedures, would they decompensate because of the fluid that you have to deliver during such a procedure.
We were quite surprised at the end of the day that the procedures were quite straightforward. Fortunately, we had no major complications. I think there were four complications in the 100 ablated patients. I think we were really positive about how the procedures turned out.
I should mention that one of the exclusion criteria was a left atrial diameter of about 60 mm. The huge ones may be very diseased, and maybe the hopeless ones were excluded from the study. Below 60 mm, we did the ablation.
Rhythm control
Dr. Mandrola: One of my colleagues, who is even more skeptical than me, wanted me to ask you, why wouldn’t you take a patient with persistent atrial fibrillation who had heart failure and just cardiovert and use amiodarone and try and maintain sinus rhythm that way?
Dr. Sohns: It is important to mention that 50% of the patients have already had amiodarone before they were randomized and enrolled for the trial. It might bring you a couple of minutes or a couple of hours [of relief], but the patients would get recurrence.
It was very interesting also, and this is in line with the data from Jason Andrade, who demonstrated that we were able to reduce the percentage of patients with persistent atrial fibrillation to paroxysmal. We did a down-staging of the underlying disease. This is not possible with cardioversion or drugs, for example.
Dr. Sommer: What I really like about that question and that comment is the idea that rhythm control in this subset of patients obviously has a role and an importance. It may be a cardioversion initially, giving amiodarone if they didn’t have that before, and you can keep the patient in sinus rhythm with this therapy, I think we’re reaching the same goal.
I think the critical point to get into the mind of physicians who treat heart failure is that sinus rhythm is beneficial, however you get there. Ablation, of course, as in other studies, is the most powerful tool to get there. Cardioversion can be a really good thing to do; you just have to think about it and consider it.
Dr. Mandrola: I do want to say to everybody that there is a tension sometimes between the heart failure community and the electrophysiology community. I think the ideal situation is that we work together, because I think that we can help with the maintenance of sinus rhythm. The control group mortality at 1 year was 20%, and I’ve heard people say that that’s not advanced heart failure. Advanced heart failure patients have much higher mortality than that. My colleague who is a heart failure specialist was criticizing a selection bias in picking the best patients. How would you answer that?
Dr. Sohns: There are data available from Eurotransplant, for example, that the waiting list mortality is 18%, so I think we are almost in line with this 20% mortality in this conservative group. You cannot generalize it. All these patients have different histories. We have 60% dilated cardiomyopathy and 40% ischemic cardiomyopathy. I think it is a very representative group in contrast to your friend who suggests that it is not.
Dr. Sommer: What I like about the discussion is that some approach us to say that the mortality in the control group is much too high – like, what are you doing with those patients that you create so many endpoints? Then others say that it’s not high enough because that is not end-stage heart failure. Come on! We have a patient cohort that is very well described and very well characterized.
If the label is end-stage heart failure, advanced heart failure, or whatever, they are sicker than the patients that we had in earlier trials. The patients that we treated were mostly excluded from all other trials. We opened the door. We found a clear result. I think everyone can see whatever you like to see.
Dr. Mandrola: What would your take-home message be after having done this trial design, the trial was conducted in your single center, and you come up with these amazing results? What would your message be to the whole community?
Dr. Sohns: Taking into consideration how severely sick these patients are, I can just repeat it: They are one step away from death, more or less, or from surgical intervention that can prolong their life. You should also consider that there are options like atrial fibrillation ablation that can buy time, postpone the natural course, or even in some patients replace the destination therapy. Therefore, in my opinion the next guidelines should recommend that every patient should carefully be checked for sinus rhythm before bringing these patients into the environment of transplantation.
Dr. Sommer: My interpretation is that we have to try to bring into physicians’ minds that besides a well-established and well-documented effect of drug therapy with the fabulous four, we may now have the fabulous five, including an ablation option for patients with atrial fibrillation.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. Dr. Sohns is deputy director of the Heart and Diabetes Center NRW, Ruhr University Bochum, Bad Oeynhausen, Germany. Dr. Sommer is professor of cardiology at the Heart and Diabetes Center NRW. Dr. Mandrola reported no conflicts of interest. Dr. Sohns reported receiving research funding from Else Kröner–Fresenius–Stiftung. Dr. Sommer reported consulting with Abbott, Biosense Webster, Boston Scientific, and Medtronic USA.
A version of this article first appeared on Medscape.com.
How do you prescribe exercise in primary prevention?
To avoid cardiovascular disease, the American Heart Association (AHA) recommends performing at least 150 minutes of moderate-intensity aerobic activity every week, 75 minutes of intense aerobic activity every week, or a combination of both, preferably spread out throughout the week. But how knowledgeable are physicians when it comes to prescribing exercise, and how should patients be assessed so that appropriate physical activity can be recommended?
In a presentation titled, “Patient Evaluation and Exercise Prescription in Primary Prevention,”
“Exercise has cardioprotective, emotional, antiarrhythmic, and antithrombotic benefits, and it reduces stress,” she explained.
She also noted that the risk regarding cardiopulmonary and musculoskeletal components must be evaluated, because exercise can itself trigger coronary events, and the last thing intended when prescribing exercise is to cause complications. “We must recommend exercise progressively. We can’t suggest a high-intensity regimen to a patient if they haven’t had any preconditioning where collateral circulation could be developed and lung and cardiac capacity could be improved.”
Dr. Sánchez went on to say that, according to the AHA, patients should be classified as follows: those who exercise and those who don’t, those with a history of cardiovascular, metabolic, or renal disease, and those with symptomatic and asymptomatic diseases, in order to consider the parameters when recommending exercise.
“If the patient has symptoms and is doing light physical activity, like walking, they can keep doing this exercise and don’t need further assessments. But if they have a symptomatic disease and are not exercising, they need to be evaluated after exercise has been prescribed, and not just clinically, either. Some sort of diagnostic method should be considered. Also, for patients who are physically active and who desire to increase the intensity of their exercise, the recommendation is to perform a detailed clinical examination and, if necessary, perform additional imaging studies.”
Warning signs
- Dizziness.
- Orthopnea.
- Abnormal heart rate.
- Edema in the lower extremities.
- Chest pain, especially when occurring with exercise.
- Intermittent claudication.
- Heart murmurs.
- Dyspnea.
- Reduced output.
- Fatigue.
Calibrating exercise parameters
The parameters of frequency (number of sessions per week), intensity (perceived exertion measured by heart rate reached), time, and type (aerobic exercise vs. strength training) should be considered when forming an appropriate prescription for exercise, explained Dr. Sánchez.
“The big problem is that most physicians don’t know how to prescribe it properly. And beyond knowing how, the important thing is that, when we’re with the patient during the consultation, we ought to be doing more than just establishing a routine. We need to be motivators and we need to be identifying obstacles and the patient’s interest in exercise, because it’s clear that incorporating physical activity into our daily lives helps improve the quality and length of life,” the specialist added.
The recommendations are straightforward: for individuals aged 18-64 years, 150 minutes of moderate-intensity activity per week, whether aerobic, strength training, or mixed, should be prescribed. “We need to encourage moving more and sitting less, and recommend comprehensive programs that include coordination, balance, and muscle strengthening. If a sedentary lifestyle is a risk factor, we need to encourage patients to start performing physical activity for 1-2 minutes every hour, because any exercise must be gradual and progressive to avoid complications,” she noted.
Evaluate, then recommend
The specialist emphasized the importance of making personalized prescriptions, exercising caution, and performing adequate assessments to know which exercise routine to recommend. “The patient should also be involved in their self-care and must have an adequate diet and hydration, and we need to remind them that they shouldn’t be exercising if they have an infection, due to the risk of myocarditis and sudden death,” she added.
Rafaelina Concepción, MD, cardiologist from the Dominican Republic and vice president of the Inter-American Society of Cardiology for Central America and the Caribbean, agreed with the importance of assessing risk and risk factors for patients who request an exercise routine. “For example, in patients with prediabetes, it has been shown that exercising can slow the progression to diabetes. The essential thing is to use stratification and know what kind of exercise to recommend, whether aerobic, strength training, or a combination of the two, to improve functional capacity without reaching the threshold heart rate while reducing the risk of other comorbidities like hypertension, obesity, and high lipids, and achieving lifestyle changes.”
Carlos Franco, MD, a cardiologist in El Salvador, emphasized that there is no such thing as zero risk when evaluating a patient. “Of course, there’s a difference between an athlete and someone who isn’t physically active, but we need to profile all patients correctly, evaluate risk factors in detail, not overlook subclinical cardiovascular disease, and check whether they need stress testing or additional imaging to assess cardiac functional capacity. Also, exercise must be prescribed gradually, and the patient’s nutritional status must be assessed.”
Dr. Franco ended by explaining that physicians must understand how to prescribe the basics of exercise and make small interventions of reasonable intensity, provide practical advice, and, to the extent possible, rely on specialists such as physiatrists, sports specialists, and physical therapists.
This article was translated from the Medscape Spanish Edition. A version of this article appeared on Medscape.com.
To avoid cardiovascular disease, the American Heart Association (AHA) recommends performing at least 150 minutes of moderate-intensity aerobic activity every week, 75 minutes of intense aerobic activity every week, or a combination of both, preferably spread out throughout the week. But how knowledgeable are physicians when it comes to prescribing exercise, and how should patients be assessed so that appropriate physical activity can be recommended?
In a presentation titled, “Patient Evaluation and Exercise Prescription in Primary Prevention,”
“Exercise has cardioprotective, emotional, antiarrhythmic, and antithrombotic benefits, and it reduces stress,” she explained.
She also noted that the risk regarding cardiopulmonary and musculoskeletal components must be evaluated, because exercise can itself trigger coronary events, and the last thing intended when prescribing exercise is to cause complications. “We must recommend exercise progressively. We can’t suggest a high-intensity regimen to a patient if they haven’t had any preconditioning where collateral circulation could be developed and lung and cardiac capacity could be improved.”
Dr. Sánchez went on to say that, according to the AHA, patients should be classified as follows: those who exercise and those who don’t, those with a history of cardiovascular, metabolic, or renal disease, and those with symptomatic and asymptomatic diseases, in order to consider the parameters when recommending exercise.
“If the patient has symptoms and is doing light physical activity, like walking, they can keep doing this exercise and don’t need further assessments. But if they have a symptomatic disease and are not exercising, they need to be evaluated after exercise has been prescribed, and not just clinically, either. Some sort of diagnostic method should be considered. Also, for patients who are physically active and who desire to increase the intensity of their exercise, the recommendation is to perform a detailed clinical examination and, if necessary, perform additional imaging studies.”
Warning signs
- Dizziness.
- Orthopnea.
- Abnormal heart rate.
- Edema in the lower extremities.
- Chest pain, especially when occurring with exercise.
- Intermittent claudication.
- Heart murmurs.
- Dyspnea.
- Reduced output.
- Fatigue.
Calibrating exercise parameters
The parameters of frequency (number of sessions per week), intensity (perceived exertion measured by heart rate reached), time, and type (aerobic exercise vs. strength training) should be considered when forming an appropriate prescription for exercise, explained Dr. Sánchez.
“The big problem is that most physicians don’t know how to prescribe it properly. And beyond knowing how, the important thing is that, when we’re with the patient during the consultation, we ought to be doing more than just establishing a routine. We need to be motivators and we need to be identifying obstacles and the patient’s interest in exercise, because it’s clear that incorporating physical activity into our daily lives helps improve the quality and length of life,” the specialist added.
The recommendations are straightforward: for individuals aged 18-64 years, 150 minutes of moderate-intensity activity per week, whether aerobic, strength training, or mixed, should be prescribed. “We need to encourage moving more and sitting less, and recommend comprehensive programs that include coordination, balance, and muscle strengthening. If a sedentary lifestyle is a risk factor, we need to encourage patients to start performing physical activity for 1-2 minutes every hour, because any exercise must be gradual and progressive to avoid complications,” she noted.
Evaluate, then recommend
The specialist emphasized the importance of making personalized prescriptions, exercising caution, and performing adequate assessments to know which exercise routine to recommend. “The patient should also be involved in their self-care and must have an adequate diet and hydration, and we need to remind them that they shouldn’t be exercising if they have an infection, due to the risk of myocarditis and sudden death,” she added.
Rafaelina Concepción, MD, cardiologist from the Dominican Republic and vice president of the Inter-American Society of Cardiology for Central America and the Caribbean, agreed with the importance of assessing risk and risk factors for patients who request an exercise routine. “For example, in patients with prediabetes, it has been shown that exercising can slow the progression to diabetes. The essential thing is to use stratification and know what kind of exercise to recommend, whether aerobic, strength training, or a combination of the two, to improve functional capacity without reaching the threshold heart rate while reducing the risk of other comorbidities like hypertension, obesity, and high lipids, and achieving lifestyle changes.”
Carlos Franco, MD, a cardiologist in El Salvador, emphasized that there is no such thing as zero risk when evaluating a patient. “Of course, there’s a difference between an athlete and someone who isn’t physically active, but we need to profile all patients correctly, evaluate risk factors in detail, not overlook subclinical cardiovascular disease, and check whether they need stress testing or additional imaging to assess cardiac functional capacity. Also, exercise must be prescribed gradually, and the patient’s nutritional status must be assessed.”
Dr. Franco ended by explaining that physicians must understand how to prescribe the basics of exercise and make small interventions of reasonable intensity, provide practical advice, and, to the extent possible, rely on specialists such as physiatrists, sports specialists, and physical therapists.
This article was translated from the Medscape Spanish Edition. A version of this article appeared on Medscape.com.
To avoid cardiovascular disease, the American Heart Association (AHA) recommends performing at least 150 minutes of moderate-intensity aerobic activity every week, 75 minutes of intense aerobic activity every week, or a combination of both, preferably spread out throughout the week. But how knowledgeable are physicians when it comes to prescribing exercise, and how should patients be assessed so that appropriate physical activity can be recommended?
In a presentation titled, “Patient Evaluation and Exercise Prescription in Primary Prevention,”
“Exercise has cardioprotective, emotional, antiarrhythmic, and antithrombotic benefits, and it reduces stress,” she explained.
She also noted that the risk regarding cardiopulmonary and musculoskeletal components must be evaluated, because exercise can itself trigger coronary events, and the last thing intended when prescribing exercise is to cause complications. “We must recommend exercise progressively. We can’t suggest a high-intensity regimen to a patient if they haven’t had any preconditioning where collateral circulation could be developed and lung and cardiac capacity could be improved.”
Dr. Sánchez went on to say that, according to the AHA, patients should be classified as follows: those who exercise and those who don’t, those with a history of cardiovascular, metabolic, or renal disease, and those with symptomatic and asymptomatic diseases, in order to consider the parameters when recommending exercise.
“If the patient has symptoms and is doing light physical activity, like walking, they can keep doing this exercise and don’t need further assessments. But if they have a symptomatic disease and are not exercising, they need to be evaluated after exercise has been prescribed, and not just clinically, either. Some sort of diagnostic method should be considered. Also, for patients who are physically active and who desire to increase the intensity of their exercise, the recommendation is to perform a detailed clinical examination and, if necessary, perform additional imaging studies.”
Warning signs
- Dizziness.
- Orthopnea.
- Abnormal heart rate.
- Edema in the lower extremities.
- Chest pain, especially when occurring with exercise.
- Intermittent claudication.
- Heart murmurs.
- Dyspnea.
- Reduced output.
- Fatigue.
Calibrating exercise parameters
The parameters of frequency (number of sessions per week), intensity (perceived exertion measured by heart rate reached), time, and type (aerobic exercise vs. strength training) should be considered when forming an appropriate prescription for exercise, explained Dr. Sánchez.
“The big problem is that most physicians don’t know how to prescribe it properly. And beyond knowing how, the important thing is that, when we’re with the patient during the consultation, we ought to be doing more than just establishing a routine. We need to be motivators and we need to be identifying obstacles and the patient’s interest in exercise, because it’s clear that incorporating physical activity into our daily lives helps improve the quality and length of life,” the specialist added.
The recommendations are straightforward: for individuals aged 18-64 years, 150 minutes of moderate-intensity activity per week, whether aerobic, strength training, or mixed, should be prescribed. “We need to encourage moving more and sitting less, and recommend comprehensive programs that include coordination, balance, and muscle strengthening. If a sedentary lifestyle is a risk factor, we need to encourage patients to start performing physical activity for 1-2 minutes every hour, because any exercise must be gradual and progressive to avoid complications,” she noted.
Evaluate, then recommend
The specialist emphasized the importance of making personalized prescriptions, exercising caution, and performing adequate assessments to know which exercise routine to recommend. “The patient should also be involved in their self-care and must have an adequate diet and hydration, and we need to remind them that they shouldn’t be exercising if they have an infection, due to the risk of myocarditis and sudden death,” she added.
Rafaelina Concepción, MD, cardiologist from the Dominican Republic and vice president of the Inter-American Society of Cardiology for Central America and the Caribbean, agreed with the importance of assessing risk and risk factors for patients who request an exercise routine. “For example, in patients with prediabetes, it has been shown that exercising can slow the progression to diabetes. The essential thing is to use stratification and know what kind of exercise to recommend, whether aerobic, strength training, or a combination of the two, to improve functional capacity without reaching the threshold heart rate while reducing the risk of other comorbidities like hypertension, obesity, and high lipids, and achieving lifestyle changes.”
Carlos Franco, MD, a cardiologist in El Salvador, emphasized that there is no such thing as zero risk when evaluating a patient. “Of course, there’s a difference between an athlete and someone who isn’t physically active, but we need to profile all patients correctly, evaluate risk factors in detail, not overlook subclinical cardiovascular disease, and check whether they need stress testing or additional imaging to assess cardiac functional capacity. Also, exercise must be prescribed gradually, and the patient’s nutritional status must be assessed.”
Dr. Franco ended by explaining that physicians must understand how to prescribe the basics of exercise and make small interventions of reasonable intensity, provide practical advice, and, to the extent possible, rely on specialists such as physiatrists, sports specialists, and physical therapists.
This article was translated from the Medscape Spanish Edition. A version of this article appeared on Medscape.com.
Minimizing atrial pacing no benefit in sinus node disease: DANPACE II
suggest results of a trial that randomly assigned patients with SND who had received their first pacemaker implant to one of two pacing programs.
Over 2 years of follow-up with remote monitoring, there was no difference in the primary endpoint of time to first device-detected episode of AF lasting more than 6 minutes, reported Max Brix Kronborg, MD, PhD, department of cardiology, Aarhus University Hospital, Denmark.
The study, DANPACE II, excluded patients with permanent or persistent AF or persistent bradycardia prior to or at the time of enrollment.
The findings were presented at annual congress of the European Society of Cardiology and were published online simultaneously in the European Heart Journal.
The 539 participants in the trial were randomly assigned in a 1:1 ratio to a pacing program of 60 beats/minute with rate-adaptive pacing (DDR-60) or 40 beats/minute without rate-adaptive pacing (DDD-40). All patients were equipped with remote monitoring and were followed for 2 years. Tracings were adjudicated for atrial high-rate episodes by experienced device specialists, Dr. Kronborg said.
No difference seen in primary outcome
When graphed, curves for the primary outcome in the two groups were essentially superimposable. For the secondary outcomes of AF lasting more than 6 hours and AF lasting more than 24 hours, there was a modest but progressive separation in the lines favoring the DDR-60 group for both. However, the P value did not approach significance in the first of these endpoints (P = .35) and remained only a trend (P = .08) in the second.
There were no substantial differences in results when patients were stratified by age (> 73 years vs. younger), gender (women represented 50% of patients), PR interval (> 150 milliseconds vs. less), or history of AF prior to study entry; the latter group represented approximately 40% of the trial participants.
There was a between-group difference in the primary composite safety endpoint of syncope and presyncope. By 2 years, 13% of those in the DDR-60 group had experienced one of these safety events, vs. 22% (P = .01) of the DDD-40 group.
The study was not designed to determine a cause for these episodes, but Dr. Kronborg reported that bradycardia was suspected in the majority of cases.
Crossovers more common on minimal pacing
Crossovers were permitted, and 26% of patients did so at some point in the trial. Of these, about one-third were switched to the opposite arm in response to syncope. Almost all of the others crossed over because of chronotropic incompetence. The greater crossover rate in the DDD-40 group (23% vs. 3%; P < .001) was highly significant.
Quality of life was measured with the SF36 tool, and physical function was evaluated with the 6-minute walk distance test (6MWD). Results on these measures did not differ significantly between groups. For 6MWD, the mean gain from baseline was 8 m in both groups.
The results of this study are important because they challenge what has been a widely held perception among electrophysiologists, according to Cecilia Linde, MD, PhD, a professor of cardiology at the Karolinska Institute, Stockholm.
“I think many of us involved in pacing thought for many years that minimizing pacing would be beneficial, and this clearly shows it is not,” said Dr. Linde, who was the moderator of the scientific session in which these results were presented.
Results appear definitive
The ESC-invited discussant, Jose L. Merino, MD, PhD, director of arrhythmia and electrophysiology research, La Paz University Hospital, Madrid, concurred. He said these results are convincing.
On the basis of these findings, which not only failed to show a benefit but showed in the experimental arm a higher incidence of syncope and chronotropic incompetence, Dr. Merino concluded, “Programming intended to minimize atrial pacing should not be used as routine in unselected patients with SND.”
A trend for protection from DDR-60 over DDD-40 from the longest episodes of AF caught Dr. Merino’s attention, leading him to question whether the optimal rate of pacing might be even higher than 60 beats/minute in SND, but he said that is a separate issue. DANPACE was not powered to examine the effect in long duration episodes.
Ultimately, while Dr. Merino characterized the increased risk of syncope with minimized pacing as “an important finding” in regard to dissuading clinicians to pursue this strategy, he said that the underlying question of the DANPACE trial remains unanswered.
Pacing remains “a treatment of choice” in SND, but further investigation is needed “about the optimal pacing rate to minimize AF and syncope” in this population, he said.
Dr. Kronborg reports a financial relationship with Abbott. Dr. Linde reports financial relationships with Cardio 3, Medtronic, St. Jude, and Vifor. Dr. Merino reports financial relationships with Abbott, Medtronic, and Microport.
A version of this article first appeared on Medscape.com.
suggest results of a trial that randomly assigned patients with SND who had received their first pacemaker implant to one of two pacing programs.
Over 2 years of follow-up with remote monitoring, there was no difference in the primary endpoint of time to first device-detected episode of AF lasting more than 6 minutes, reported Max Brix Kronborg, MD, PhD, department of cardiology, Aarhus University Hospital, Denmark.
The study, DANPACE II, excluded patients with permanent or persistent AF or persistent bradycardia prior to or at the time of enrollment.
The findings were presented at annual congress of the European Society of Cardiology and were published online simultaneously in the European Heart Journal.
The 539 participants in the trial were randomly assigned in a 1:1 ratio to a pacing program of 60 beats/minute with rate-adaptive pacing (DDR-60) or 40 beats/minute without rate-adaptive pacing (DDD-40). All patients were equipped with remote monitoring and were followed for 2 years. Tracings were adjudicated for atrial high-rate episodes by experienced device specialists, Dr. Kronborg said.
No difference seen in primary outcome
When graphed, curves for the primary outcome in the two groups were essentially superimposable. For the secondary outcomes of AF lasting more than 6 hours and AF lasting more than 24 hours, there was a modest but progressive separation in the lines favoring the DDR-60 group for both. However, the P value did not approach significance in the first of these endpoints (P = .35) and remained only a trend (P = .08) in the second.
There were no substantial differences in results when patients were stratified by age (> 73 years vs. younger), gender (women represented 50% of patients), PR interval (> 150 milliseconds vs. less), or history of AF prior to study entry; the latter group represented approximately 40% of the trial participants.
There was a between-group difference in the primary composite safety endpoint of syncope and presyncope. By 2 years, 13% of those in the DDR-60 group had experienced one of these safety events, vs. 22% (P = .01) of the DDD-40 group.
The study was not designed to determine a cause for these episodes, but Dr. Kronborg reported that bradycardia was suspected in the majority of cases.
Crossovers more common on minimal pacing
Crossovers were permitted, and 26% of patients did so at some point in the trial. Of these, about one-third were switched to the opposite arm in response to syncope. Almost all of the others crossed over because of chronotropic incompetence. The greater crossover rate in the DDD-40 group (23% vs. 3%; P < .001) was highly significant.
Quality of life was measured with the SF36 tool, and physical function was evaluated with the 6-minute walk distance test (6MWD). Results on these measures did not differ significantly between groups. For 6MWD, the mean gain from baseline was 8 m in both groups.
The results of this study are important because they challenge what has been a widely held perception among electrophysiologists, according to Cecilia Linde, MD, PhD, a professor of cardiology at the Karolinska Institute, Stockholm.
“I think many of us involved in pacing thought for many years that minimizing pacing would be beneficial, and this clearly shows it is not,” said Dr. Linde, who was the moderator of the scientific session in which these results were presented.
Results appear definitive
The ESC-invited discussant, Jose L. Merino, MD, PhD, director of arrhythmia and electrophysiology research, La Paz University Hospital, Madrid, concurred. He said these results are convincing.
On the basis of these findings, which not only failed to show a benefit but showed in the experimental arm a higher incidence of syncope and chronotropic incompetence, Dr. Merino concluded, “Programming intended to minimize atrial pacing should not be used as routine in unselected patients with SND.”
A trend for protection from DDR-60 over DDD-40 from the longest episodes of AF caught Dr. Merino’s attention, leading him to question whether the optimal rate of pacing might be even higher than 60 beats/minute in SND, but he said that is a separate issue. DANPACE was not powered to examine the effect in long duration episodes.
Ultimately, while Dr. Merino characterized the increased risk of syncope with minimized pacing as “an important finding” in regard to dissuading clinicians to pursue this strategy, he said that the underlying question of the DANPACE trial remains unanswered.
Pacing remains “a treatment of choice” in SND, but further investigation is needed “about the optimal pacing rate to minimize AF and syncope” in this population, he said.
Dr. Kronborg reports a financial relationship with Abbott. Dr. Linde reports financial relationships with Cardio 3, Medtronic, St. Jude, and Vifor. Dr. Merino reports financial relationships with Abbott, Medtronic, and Microport.
A version of this article first appeared on Medscape.com.
suggest results of a trial that randomly assigned patients with SND who had received their first pacemaker implant to one of two pacing programs.
Over 2 years of follow-up with remote monitoring, there was no difference in the primary endpoint of time to first device-detected episode of AF lasting more than 6 minutes, reported Max Brix Kronborg, MD, PhD, department of cardiology, Aarhus University Hospital, Denmark.
The study, DANPACE II, excluded patients with permanent or persistent AF or persistent bradycardia prior to or at the time of enrollment.
The findings were presented at annual congress of the European Society of Cardiology and were published online simultaneously in the European Heart Journal.
The 539 participants in the trial were randomly assigned in a 1:1 ratio to a pacing program of 60 beats/minute with rate-adaptive pacing (DDR-60) or 40 beats/minute without rate-adaptive pacing (DDD-40). All patients were equipped with remote monitoring and were followed for 2 years. Tracings were adjudicated for atrial high-rate episodes by experienced device specialists, Dr. Kronborg said.
No difference seen in primary outcome
When graphed, curves for the primary outcome in the two groups were essentially superimposable. For the secondary outcomes of AF lasting more than 6 hours and AF lasting more than 24 hours, there was a modest but progressive separation in the lines favoring the DDR-60 group for both. However, the P value did not approach significance in the first of these endpoints (P = .35) and remained only a trend (P = .08) in the second.
There were no substantial differences in results when patients were stratified by age (> 73 years vs. younger), gender (women represented 50% of patients), PR interval (> 150 milliseconds vs. less), or history of AF prior to study entry; the latter group represented approximately 40% of the trial participants.
There was a between-group difference in the primary composite safety endpoint of syncope and presyncope. By 2 years, 13% of those in the DDR-60 group had experienced one of these safety events, vs. 22% (P = .01) of the DDD-40 group.
The study was not designed to determine a cause for these episodes, but Dr. Kronborg reported that bradycardia was suspected in the majority of cases.
Crossovers more common on minimal pacing
Crossovers were permitted, and 26% of patients did so at some point in the trial. Of these, about one-third were switched to the opposite arm in response to syncope. Almost all of the others crossed over because of chronotropic incompetence. The greater crossover rate in the DDD-40 group (23% vs. 3%; P < .001) was highly significant.
Quality of life was measured with the SF36 tool, and physical function was evaluated with the 6-minute walk distance test (6MWD). Results on these measures did not differ significantly between groups. For 6MWD, the mean gain from baseline was 8 m in both groups.
The results of this study are important because they challenge what has been a widely held perception among electrophysiologists, according to Cecilia Linde, MD, PhD, a professor of cardiology at the Karolinska Institute, Stockholm.
“I think many of us involved in pacing thought for many years that minimizing pacing would be beneficial, and this clearly shows it is not,” said Dr. Linde, who was the moderator of the scientific session in which these results were presented.
Results appear definitive
The ESC-invited discussant, Jose L. Merino, MD, PhD, director of arrhythmia and electrophysiology research, La Paz University Hospital, Madrid, concurred. He said these results are convincing.
On the basis of these findings, which not only failed to show a benefit but showed in the experimental arm a higher incidence of syncope and chronotropic incompetence, Dr. Merino concluded, “Programming intended to minimize atrial pacing should not be used as routine in unselected patients with SND.”
A trend for protection from DDR-60 over DDD-40 from the longest episodes of AF caught Dr. Merino’s attention, leading him to question whether the optimal rate of pacing might be even higher than 60 beats/minute in SND, but he said that is a separate issue. DANPACE was not powered to examine the effect in long duration episodes.
Ultimately, while Dr. Merino characterized the increased risk of syncope with minimized pacing as “an important finding” in regard to dissuading clinicians to pursue this strategy, he said that the underlying question of the DANPACE trial remains unanswered.
Pacing remains “a treatment of choice” in SND, but further investigation is needed “about the optimal pacing rate to minimize AF and syncope” in this population, he said.
Dr. Kronborg reports a financial relationship with Abbott. Dr. Linde reports financial relationships with Cardio 3, Medtronic, St. Jude, and Vifor. Dr. Merino reports financial relationships with Abbott, Medtronic, and Microport.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2023