User login
Can Vitamin D Supplements Help With Hypertension?
Q) One of my patients came in and said he had read that vitamin D supplementation will help with hypertension. Now he wants to quit his blood pressure meds and use vitamin D instead. Do you have any background on this?
Vitamin D is critical for utilization of calcium, a vital nutrient for multiple metabolic and cellular processes; deficiency is associated with worsening of autoimmune disorders, osteoporosis, and certain cardiovascular conditions, among others.7 An association between vitamin D level and blood pressure has been recognized for some time, but the pathophysiology is not well understood.
A literature review of studies from 1988 to 2013 found contradictory results regarding vitamin D deficiency and concurrent elevated blood pressure (systolic and/or diastolic), as well as the impact on blood pressure with restoration of vitamin D levels. The findings were limited by several factors, including differences in study design, variables evaluated, and type of vitamin D compound used. The results suggested a link between the renin-angiotensin-aldosterone system, fibroblast growth factor 23/klotho axis, and vitamin D level.8
A study of 158 subjects (98 with newly diagnosed essential hypertension, 60 with normal blood pressure) found significantly lower 25(OH)D3 serum levels in hypertensive patients. Furthermore, the 25(OH)D3 level was significantly correlated with both systolic (r = –0.33) and diastolic blood pressure (r = –0.26). Using multiple regression analysis, after adjustment for age, smoking status, and BMI, the impact of 25(OH)D3 level accounted for 10% of the variation in systolic blood pressure.9
In a mendelian randomization study of 108,173 subjects from 35 studies, an inverse association between vitamin D level and systolic blood pressure (P = .0003) was found. A reduced risk for essential hypertension with increased vitamin D level (P = .0003) was also noted. However, no association was found between increasing vitamin D level and a reduction in diastolic blood pressure
(P = .37).10
With the ever-increasing access to health information from sources such as “Doctor Google,” it can be difficult for a non–health care professional to separate hype from evidence-based recommendations. While current evidence suggests optimal vitamin D levels may be beneficial for improving blood pressure control and may be a useful adjunctive therapy, there is no evidence to support discontinuing antihypertensive therapy and replacing it with vitamin D therapy.
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants, South Charleston, West Virginia
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
Q) One of my patients came in and said he had read that vitamin D supplementation will help with hypertension. Now he wants to quit his blood pressure meds and use vitamin D instead. Do you have any background on this?
Vitamin D is critical for utilization of calcium, a vital nutrient for multiple metabolic and cellular processes; deficiency is associated with worsening of autoimmune disorders, osteoporosis, and certain cardiovascular conditions, among others.7 An association between vitamin D level and blood pressure has been recognized for some time, but the pathophysiology is not well understood.
A literature review of studies from 1988 to 2013 found contradictory results regarding vitamin D deficiency and concurrent elevated blood pressure (systolic and/or diastolic), as well as the impact on blood pressure with restoration of vitamin D levels. The findings were limited by several factors, including differences in study design, variables evaluated, and type of vitamin D compound used. The results suggested a link between the renin-angiotensin-aldosterone system, fibroblast growth factor 23/klotho axis, and vitamin D level.8
A study of 158 subjects (98 with newly diagnosed essential hypertension, 60 with normal blood pressure) found significantly lower 25(OH)D3 serum levels in hypertensive patients. Furthermore, the 25(OH)D3 level was significantly correlated with both systolic (r = –0.33) and diastolic blood pressure (r = –0.26). Using multiple regression analysis, after adjustment for age, smoking status, and BMI, the impact of 25(OH)D3 level accounted for 10% of the variation in systolic blood pressure.9
In a mendelian randomization study of 108,173 subjects from 35 studies, an inverse association between vitamin D level and systolic blood pressure (P = .0003) was found. A reduced risk for essential hypertension with increased vitamin D level (P = .0003) was also noted. However, no association was found between increasing vitamin D level and a reduction in diastolic blood pressure
(P = .37).10
With the ever-increasing access to health information from sources such as “Doctor Google,” it can be difficult for a non–health care professional to separate hype from evidence-based recommendations. While current evidence suggests optimal vitamin D levels may be beneficial for improving blood pressure control and may be a useful adjunctive therapy, there is no evidence to support discontinuing antihypertensive therapy and replacing it with vitamin D therapy.
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants, South Charleston, West Virginia
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
Q) One of my patients came in and said he had read that vitamin D supplementation will help with hypertension. Now he wants to quit his blood pressure meds and use vitamin D instead. Do you have any background on this?
Vitamin D is critical for utilization of calcium, a vital nutrient for multiple metabolic and cellular processes; deficiency is associated with worsening of autoimmune disorders, osteoporosis, and certain cardiovascular conditions, among others.7 An association between vitamin D level and blood pressure has been recognized for some time, but the pathophysiology is not well understood.
A literature review of studies from 1988 to 2013 found contradictory results regarding vitamin D deficiency and concurrent elevated blood pressure (systolic and/or diastolic), as well as the impact on blood pressure with restoration of vitamin D levels. The findings were limited by several factors, including differences in study design, variables evaluated, and type of vitamin D compound used. The results suggested a link between the renin-angiotensin-aldosterone system, fibroblast growth factor 23/klotho axis, and vitamin D level.8
A study of 158 subjects (98 with newly diagnosed essential hypertension, 60 with normal blood pressure) found significantly lower 25(OH)D3 serum levels in hypertensive patients. Furthermore, the 25(OH)D3 level was significantly correlated with both systolic (r = –0.33) and diastolic blood pressure (r = –0.26). Using multiple regression analysis, after adjustment for age, smoking status, and BMI, the impact of 25(OH)D3 level accounted for 10% of the variation in systolic blood pressure.9
In a mendelian randomization study of 108,173 subjects from 35 studies, an inverse association between vitamin D level and systolic blood pressure (P = .0003) was found. A reduced risk for essential hypertension with increased vitamin D level (P = .0003) was also noted. However, no association was found between increasing vitamin D level and a reduction in diastolic blood pressure
(P = .37).10
With the ever-increasing access to health information from sources such as “Doctor Google,” it can be difficult for a non–health care professional to separate hype from evidence-based recommendations. While current evidence suggests optimal vitamin D levels may be beneficial for improving blood pressure control and may be a useful adjunctive therapy, there is no evidence to support discontinuing antihypertensive therapy and replacing it with vitamin D therapy.
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants, South Charleston, West Virginia
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
Megestrol Acetate for CKD and Dialysis Patients
Q) Some of my CKD patients are malnourished; in fact, some of those on dialysis do not eat well and have low albumin levels. Previously in this column, it was stated that higher albumin levels (> 4 g/dL) confer survival benefits to dialysis patients. Should I consider prescribing megestrol acetate to improve appetite? If I do prescribe it, what dose is safe for CKD and dialysis patients?
Malnutrition affects one-third of dialysis patients,1 and malnutrition-inflammation complex syndrome (MICS) is common in those with stage 5 CKD. Albumin is used as an indicator of MICS in dialysis patients; however, since other factors (stress, infection, inflammation, comorbidities) affect nutritional status,2 serum albumin alone may not be sufficient to assess it.
In fact, a recent consensus statement on malnutrition from the Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition excluded serum albumin as a diagnostic characteristic; the criteria included percentage of energy requirement, percentage of weight loss and time frame, loss of body fat and muscle mass, presence of edema, and reduced grip strength.3 These may be better measures of malnutrition in dialysis patients and could be used as criteria for determining when to prescribe an appetite stimulant, such as megestrol acetate.
In recent years, megestrol acetate (an antineoplastic drug) has been used to improve appetite, weight, albumin levels, and MICS in patients receiving maintenance dialysis.1,4-6 Rammohan et al found significant increases in weight, BMI, body fat, triceps skinfold thickness, protein/energy intake, and serum albumin in 10 dialysis patients who took megestrol acetate (400 mg/d) for 16 weeks.4
Continue for megestrol acetate's effects >>
In a 20-week randomized, double-blind, placebo-controlled trial, Yeh et al found significant increases in weight, body fat, and fat-free mass in elderly hemodialysis patients receiving megestrol acetate (800 mg/d). The treatment group also demonstrated greater improvement in ability to exercise.5
Monfared and colleagues looked specifically at megestrol acetate’s effect on serum albumin levels in dialysis patients.1 Using a much lower dose (40 mg bid for two months), they found a significant increase in serum albumin in the treatment group. Although an increase in appetite was noted, the researchers did not observe any significant change in total weight following treatment.1
In a letter to the editor of the Journal of Renal Nutrition, Golebiewska et al reported their use of megestrol acetate in maintenance hemodialysis and peritoneal dialysis patients.6 Hypoalbuminemic patients were given megestrol acetate (160 mg/d). Significant increases in weight, BMI, subjective global assessment scores (a measure of nutritional status based on clinical indices such as weight, appetite, muscle, and fat mass), and serum albumin levels were seen. Only 12 of the 32 patients completed the study; the others dropped out due to adverse effects, including high intradialytic weight gain (the amount of fluid gained between dialysis sessions), dyspnea, diarrhea, and nausea.6
Currently, there is no consensus in the literature regarding the most effective dosage of megestrol acetate. Furthermore, evidence is lacking as to whether megestrol acetate–induced increases in appetite, oral intake, weight, and serum albumin level bestow any survival benefit or affect outcomes in dialysis patients.4 However, the increased sense of well-being a patient experiences when appetite returns and weight is restored may be worth the effort.
Luanne DiGuglielmo, MS, RD, CSR
DaVita Summit Renal Center
Mountainside, New Jersey
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
Q) Some of my CKD patients are malnourished; in fact, some of those on dialysis do not eat well and have low albumin levels. Previously in this column, it was stated that higher albumin levels (> 4 g/dL) confer survival benefits to dialysis patients. Should I consider prescribing megestrol acetate to improve appetite? If I do prescribe it, what dose is safe for CKD and dialysis patients?
Malnutrition affects one-third of dialysis patients,1 and malnutrition-inflammation complex syndrome (MICS) is common in those with stage 5 CKD. Albumin is used as an indicator of MICS in dialysis patients; however, since other factors (stress, infection, inflammation, comorbidities) affect nutritional status,2 serum albumin alone may not be sufficient to assess it.
In fact, a recent consensus statement on malnutrition from the Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition excluded serum albumin as a diagnostic characteristic; the criteria included percentage of energy requirement, percentage of weight loss and time frame, loss of body fat and muscle mass, presence of edema, and reduced grip strength.3 These may be better measures of malnutrition in dialysis patients and could be used as criteria for determining when to prescribe an appetite stimulant, such as megestrol acetate.
In recent years, megestrol acetate (an antineoplastic drug) has been used to improve appetite, weight, albumin levels, and MICS in patients receiving maintenance dialysis.1,4-6 Rammohan et al found significant increases in weight, BMI, body fat, triceps skinfold thickness, protein/energy intake, and serum albumin in 10 dialysis patients who took megestrol acetate (400 mg/d) for 16 weeks.4
Continue for megestrol acetate's effects >>
In a 20-week randomized, double-blind, placebo-controlled trial, Yeh et al found significant increases in weight, body fat, and fat-free mass in elderly hemodialysis patients receiving megestrol acetate (800 mg/d). The treatment group also demonstrated greater improvement in ability to exercise.5
Monfared and colleagues looked specifically at megestrol acetate’s effect on serum albumin levels in dialysis patients.1 Using a much lower dose (40 mg bid for two months), they found a significant increase in serum albumin in the treatment group. Although an increase in appetite was noted, the researchers did not observe any significant change in total weight following treatment.1
In a letter to the editor of the Journal of Renal Nutrition, Golebiewska et al reported their use of megestrol acetate in maintenance hemodialysis and peritoneal dialysis patients.6 Hypoalbuminemic patients were given megestrol acetate (160 mg/d). Significant increases in weight, BMI, subjective global assessment scores (a measure of nutritional status based on clinical indices such as weight, appetite, muscle, and fat mass), and serum albumin levels were seen. Only 12 of the 32 patients completed the study; the others dropped out due to adverse effects, including high intradialytic weight gain (the amount of fluid gained between dialysis sessions), dyspnea, diarrhea, and nausea.6
Currently, there is no consensus in the literature regarding the most effective dosage of megestrol acetate. Furthermore, evidence is lacking as to whether megestrol acetate–induced increases in appetite, oral intake, weight, and serum albumin level bestow any survival benefit or affect outcomes in dialysis patients.4 However, the increased sense of well-being a patient experiences when appetite returns and weight is restored may be worth the effort.
Luanne DiGuglielmo, MS, RD, CSR
DaVita Summit Renal Center
Mountainside, New Jersey
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
Q) Some of my CKD patients are malnourished; in fact, some of those on dialysis do not eat well and have low albumin levels. Previously in this column, it was stated that higher albumin levels (> 4 g/dL) confer survival benefits to dialysis patients. Should I consider prescribing megestrol acetate to improve appetite? If I do prescribe it, what dose is safe for CKD and dialysis patients?
Malnutrition affects one-third of dialysis patients,1 and malnutrition-inflammation complex syndrome (MICS) is common in those with stage 5 CKD. Albumin is used as an indicator of MICS in dialysis patients; however, since other factors (stress, infection, inflammation, comorbidities) affect nutritional status,2 serum albumin alone may not be sufficient to assess it.
In fact, a recent consensus statement on malnutrition from the Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition excluded serum albumin as a diagnostic characteristic; the criteria included percentage of energy requirement, percentage of weight loss and time frame, loss of body fat and muscle mass, presence of edema, and reduced grip strength.3 These may be better measures of malnutrition in dialysis patients and could be used as criteria for determining when to prescribe an appetite stimulant, such as megestrol acetate.
In recent years, megestrol acetate (an antineoplastic drug) has been used to improve appetite, weight, albumin levels, and MICS in patients receiving maintenance dialysis.1,4-6 Rammohan et al found significant increases in weight, BMI, body fat, triceps skinfold thickness, protein/energy intake, and serum albumin in 10 dialysis patients who took megestrol acetate (400 mg/d) for 16 weeks.4
Continue for megestrol acetate's effects >>
In a 20-week randomized, double-blind, placebo-controlled trial, Yeh et al found significant increases in weight, body fat, and fat-free mass in elderly hemodialysis patients receiving megestrol acetate (800 mg/d). The treatment group also demonstrated greater improvement in ability to exercise.5
Monfared and colleagues looked specifically at megestrol acetate’s effect on serum albumin levels in dialysis patients.1 Using a much lower dose (40 mg bid for two months), they found a significant increase in serum albumin in the treatment group. Although an increase in appetite was noted, the researchers did not observe any significant change in total weight following treatment.1
In a letter to the editor of the Journal of Renal Nutrition, Golebiewska et al reported their use of megestrol acetate in maintenance hemodialysis and peritoneal dialysis patients.6 Hypoalbuminemic patients were given megestrol acetate (160 mg/d). Significant increases in weight, BMI, subjective global assessment scores (a measure of nutritional status based on clinical indices such as weight, appetite, muscle, and fat mass), and serum albumin levels were seen. Only 12 of the 32 patients completed the study; the others dropped out due to adverse effects, including high intradialytic weight gain (the amount of fluid gained between dialysis sessions), dyspnea, diarrhea, and nausea.6
Currently, there is no consensus in the literature regarding the most effective dosage of megestrol acetate. Furthermore, evidence is lacking as to whether megestrol acetate–induced increases in appetite, oral intake, weight, and serum albumin level bestow any survival benefit or affect outcomes in dialysis patients.4 However, the increased sense of well-being a patient experiences when appetite returns and weight is restored may be worth the effort.
Luanne DiGuglielmo, MS, RD, CSR
DaVita Summit Renal Center
Mountainside, New Jersey
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
Committed to Showing Results at the VA
Carolyn M. Clancy, MD, was named Interim Under Secretary for Health for the VA on July 2, 2014, just as the wait time crisis seemed to be spinning out of control. Her appointment and the confirmation of Secretary Robert A. McDonald less than a month later proved essential to calming the furor but were admittedly just the first steps in a long-term process to increase veterans’ access to care and develop better systems and procedures across the agency.
Six months after her appointment, Federal Practitioner talked with Dr. Clancy about the pace of change and the role of health care providers in improving care for veterans. In the interview, Dr. Clancy clearly noted that many VA facilities already represent the best of U.S. health care and that the path forward requires sharing of best practices. Many other facilities, of course, will have to change, but Dr. Clancy insisted it is “an incredible opportunity” for the VA to learn as a system. Perhaps most heartening to VA practitioners, Dr. Clancy also recognized that “you can’t provide veteran-centered care without employees who are inspired to do their very, very best.”
To be sure, any successful change in VA procedures and culture will require buy-in not only from across the agency, but also from veterans and Congress. Dr. Clancy has already received 2 votes of confidence: The Paralyzed Veterans of America and the Vietnam Veterans of America jointly called on President Obama to make Dr. Clancy’s appointment permanent. The White House and congressional leaders, however, have yet to schedule hearings or comment publicly.
Below is an edited and condensed version of the interview. To hear the complete interview, including an in-depth discussion of the Blueprint for Excellence, visit http://www.fedprac.com/multimedia/multimedia-library.html.
Taking Measure of VA Strengths
Interim Under Secretary for Health Carolyn M. Clancy, MD. I came to this system in August of 2013 after more than 20 years at HHS, all working for an agency that had the lead charge for funding research to improve quality and safety in health care; and I had spent the last 10 years prior to coming here as the director of that agency. I came to VHA because I thought this system was unique among all systems, public and private, in this country and had the strongest foundation in place to deliver 21st century health care. And at least as important—probably more so—was the sense of mission among all of the employees I met. These were people I’ve known in academia, people I met on the interviews, people I’ve intersected with for a number of years in the research community. You can’t replicate it, and you can’t buy it; and I figured the combination of a strong foundation and mission meant that this was one of the best systems to work for.
I still think that. Some of our best facilities could compete head to head with any facilities in the private sector. There is no question about that. We have some systems, facilities, and clinics that are struggling as well, which is also very typical of the private sector.
What we have is an incredible opportunity, first, because we have a fabulous mission. We have highly committed and dedicated employees. We have an incredible opportunity to actually learn as a system. There has been a lot of discussion at a number of levels about how health care in the new century needs to be a learning health care system. We actually have the capability of delivering on that promise. So I’m very, very excited.
VA Clinical Staff and Recruitment
Dr. Clancy. I have often observed that change can be scary, but it’s also incredibly liberating. Some of our most dedicated employees, I know, can be frustrated, because they feel like they’re doing their part; but they aren’t always sure that the members of the team are as dedicated as they are or are going to catch the ball. And there’s no question that you don’t get to high-quality care without a good team. In other words, superb health care and exceptional veteran experience is a team sport by definition.
So I think it will actually help the vast majority of our frontline clinicians. It’ll be much, much easier for them to deliver the kind of care they want to deliver every single day but sometimes feel like they get stuck in workarounds.
As you have probably read and have heard me say, one of the biggest challenges of our crisis—now quite open to everyone—is how we had limited availability and limited capacity to meet the needs of the veterans we had the privilege of serving. So we are on a very, very big recruitment drive for all kinds of clinicians. And in addition to the incredible mission, we have taken some steps to make salaries a bit more competitive with the private sector. I want to underline a bit. You wouldn’t be coming to VA because you wanted to become wealthy, but we recognize that people have to pay student loans and so forth. And speaking of student loans, we have a variety of programs to help people pay down their educational debt.
And all of these things actually help, but the opportunity to deliver care that is really focused squarely on the needs of the individual veteran. That, I think, is what people will ultimately find far more exciting than any anxiety about change. …
The answer to the question about who are we recruiting is: yes. We’re recruiting all of those people [physicians and midlevel providers]. We often speak about the health care market in this country as if there were one health care market. And actually, U.S. health care, of which VA is very much a part, particularly now with the new law, is very much a series of local and regional markets. So to some extent, the ratios and the types of people that we’re going to need will depend on the specific community; but we’re looking for people in all of those areas.
Changing VA Culture
Dr. Clancy. There are a number of things that impact culture. Some of it is about stories. And I have to say that every day I get to be inspired by real-life stories of veterans and... their caregivers. Some of those caregivers are their family members or close friends: people who love them. And many of them are people who work for us in the Veterans Health Administration, people who just go the extra mile because it’s the right thing to do. Nobody said they had to do it. We don’t have a policy or a directive for it. To them, it’s as natural as gravity. …
Secretary McDonald often uses this diagram, an inverted pyramid that I love, where he starts off by having a regular pyramid; and he said, “This is how we think of a lot of organizations with the Secretary sitting right up here at the pinnacle, and veterans and everybody else are kind of down on the lowest tier.” And he said, “It’s exactly wrong. How I think about it is—” So, he flips it. We have people who provide direct care to veterans. We have people who help those people, and then we have people who help the people who are helping the people provide the care to veterans. And so that means that customer service is everybody’s job. It means that helping people on the front lines who are our colleagues—and we’re all in this together—make sure that they can deliver the care that veterans need. That is everybody’s job.
And I actually think it’s going to be a very, very easy sell. Reinforcing all of this is being transparent about data and how we’re doing. So we’re now starting to look internally at how our facilities compare with local counterparts in their particular community, and I think you will be seeing that become more public in the near future. We just have to make it a little more visually compelling.
This is how we learn. It isn’t to say, “Gee, look, you didn’t do as well as other facilities.” It’s to say, “Huh, you know, this facility actually has improved dramatically. Why don’t we go learn what they did?” which I think is consistent with [Federal Practitioner’s] focus on best practices. This is the big, big challenge and the opportunity for health care in general.
Blueprint for Excellence
In the wake of the wait time crisis, Carolyn M. Clancy, MD, has been tasked with implementing the Blueprint for Excellence. Its intent, according to the VA, is to “frame a set of activities that simultaneously address improving the performance of VHA health care now, developing a positive service culture, transitioning from ‘sick care’ to ‘health care’ in the broadest sense, and developing agile business systems and management processes that are efficient, transparent and accountable.”
All the changes at the VA will align with 10 strategies for sustained excellence, which focus on improving performance, promoting a positive culture of service, advancing health care innovation, and increasing operational effectiveness and accountability. The strategies include:
- Operate a health care network that anticipates and meets the unique needs of enrolled veterans, in general, and the service-disabled and most vulnerable veterans, in particular.
- Deliver high-quality, veteran-centered care that compares favorably to the best of the private sector in measured outcomes, value, efficiency, and patient experience.
- Leverage information technologies, analytics, and models of health care delivery to optimize individual and population health outcomes.
- Grow an organizational culture, rooted in VA’s core values and mission, that prioritizes the veteran first; engaging and inspiring employees to their highest possible level of performance and conduct.
- Foster an environment of continuous learning, responsible risk taking, and personal accountability.
- Advance health care that is personalized, proactive, and patient-driven and engages and inspires veterans to their highest possible level of health and well-being.
- Lead the nation in research and treatment of military service-related conditions.
- Become a model integrated health services network through innovative academic, intergovernmental, and community relationships, information exchange, and public-private partnerships.
- Operate and communicate with integrity, transparency, and accountability that earns and maintains the trust of veterans, stewards of the system (Congress, Veterans Service Organizations), and the public.
- Modernize management processes in human resources, procurement, payment, capital infrastructure, and information technology to operate with benchmark agility and efficiency.
To listen to Dr. Clancy’s in-depth discussion of the Blueprint for Excellence, visit http://www.fedprac.com/multimedia/multimedia-library/article/carolyn-clancy-on-implementing-the-blueprint-for-excellence-at-the-va/f7313e00ff18fcbcf4fcaead862c285a/ocregister.html.
Measuring Success or Failure
Dr. Clancy. We will be measuring this in a lot of different ways. First is that VA is, I believe, unique among federal departments in having a very deep all-employee survey. We also participate in the broad Federal Employee Viewpoint Survey. ... In addition to that, we field our own survey internally and take that very, very seriously.
So literally, as the electrons are rolling in, we have a National Center for Organization and Development, which is sharing their results with me and looking at [the data] across the entire system by network, by facility. How are we doing? Where are there challenges? Where are there opportunities? Who’s doing incredibly well that we might learn something about how they’re doing that in order to help those facilities that are having more challenges? That is a very, very current source of information.
And the reason it’s so important is you can’t provide veteran-centered care without employees who are inspired to do their very, very best. And people who are inspired to do their very best, by definition, are not terribly unhappy. So that’s a very, very important source.
And I’ll also say that in health care, in general, as well as here, we’re seeing very important correlations between responses to employee surveys and such indicators as avoidable patient harms, rates of hospital-associated infections or health care-associated infections, and so forth. So we know that the two are very highly correlated.
The other reason that the survey is incredibly important is that most service industries have known for a long time that the best source of innovation are the people who are providing the service and care every single day. So again, that gets back to people feeling motivated and empowered and inspired. …
Time Frame for Change
Dr. Clancy. I think that people are seeing changes already. Now I’m just judging from my own e-mails and other things that we get; and we touch base regularly with veterans service organizations, with many, many stakeholders and take that input very, very seriously, because they are incredible partners in helping us identify and solve problems, because what I really worry about are veterans who are encountering difficulties, who may be fearful or hesitant in some fashion to bring that to our attention.
So I think, in a qualitative sense, we are seeing some positive signals but also recognizing that the scale of changes we’re talking about will take some time. But we will continue to see qualitative differences and, I think, real, tangible differences in the care that veterans get as the months proceed from here. I remain very optimistic about the future of this system and the size of the opportunity that we have.
Our laserlike focus for this coming year is access and exceptional veteran experience. You know, navigating health care can be pretty challenging. I know this because I’m from a very, very large extended family; and nobody else is in health or medicine, so I get regular reports. And whether you’re enrolled in our system or get your care elsewhere, it is not always easy.
But we have the incredible privilege of serving people who essentially wrote a blank check on our behalf when they agreed to serve the country. And so access is not something that, for the most part, is monitored closely in the private sector. It is certainly not publicly reported. We are making a very, very strong commitment, not just to improving, but to being able to show people the results.
Carolyn M. Clancy, MD, was named Interim Under Secretary for Health for the VA on July 2, 2014, just as the wait time crisis seemed to be spinning out of control. Her appointment and the confirmation of Secretary Robert A. McDonald less than a month later proved essential to calming the furor but were admittedly just the first steps in a long-term process to increase veterans’ access to care and develop better systems and procedures across the agency.
Six months after her appointment, Federal Practitioner talked with Dr. Clancy about the pace of change and the role of health care providers in improving care for veterans. In the interview, Dr. Clancy clearly noted that many VA facilities already represent the best of U.S. health care and that the path forward requires sharing of best practices. Many other facilities, of course, will have to change, but Dr. Clancy insisted it is “an incredible opportunity” for the VA to learn as a system. Perhaps most heartening to VA practitioners, Dr. Clancy also recognized that “you can’t provide veteran-centered care without employees who are inspired to do their very, very best.”
To be sure, any successful change in VA procedures and culture will require buy-in not only from across the agency, but also from veterans and Congress. Dr. Clancy has already received 2 votes of confidence: The Paralyzed Veterans of America and the Vietnam Veterans of America jointly called on President Obama to make Dr. Clancy’s appointment permanent. The White House and congressional leaders, however, have yet to schedule hearings or comment publicly.
Below is an edited and condensed version of the interview. To hear the complete interview, including an in-depth discussion of the Blueprint for Excellence, visit http://www.fedprac.com/multimedia/multimedia-library.html.
Taking Measure of VA Strengths
Interim Under Secretary for Health Carolyn M. Clancy, MD. I came to this system in August of 2013 after more than 20 years at HHS, all working for an agency that had the lead charge for funding research to improve quality and safety in health care; and I had spent the last 10 years prior to coming here as the director of that agency. I came to VHA because I thought this system was unique among all systems, public and private, in this country and had the strongest foundation in place to deliver 21st century health care. And at least as important—probably more so—was the sense of mission among all of the employees I met. These were people I’ve known in academia, people I met on the interviews, people I’ve intersected with for a number of years in the research community. You can’t replicate it, and you can’t buy it; and I figured the combination of a strong foundation and mission meant that this was one of the best systems to work for.
I still think that. Some of our best facilities could compete head to head with any facilities in the private sector. There is no question about that. We have some systems, facilities, and clinics that are struggling as well, which is also very typical of the private sector.
What we have is an incredible opportunity, first, because we have a fabulous mission. We have highly committed and dedicated employees. We have an incredible opportunity to actually learn as a system. There has been a lot of discussion at a number of levels about how health care in the new century needs to be a learning health care system. We actually have the capability of delivering on that promise. So I’m very, very excited.
VA Clinical Staff and Recruitment
Dr. Clancy. I have often observed that change can be scary, but it’s also incredibly liberating. Some of our most dedicated employees, I know, can be frustrated, because they feel like they’re doing their part; but they aren’t always sure that the members of the team are as dedicated as they are or are going to catch the ball. And there’s no question that you don’t get to high-quality care without a good team. In other words, superb health care and exceptional veteran experience is a team sport by definition.
So I think it will actually help the vast majority of our frontline clinicians. It’ll be much, much easier for them to deliver the kind of care they want to deliver every single day but sometimes feel like they get stuck in workarounds.
As you have probably read and have heard me say, one of the biggest challenges of our crisis—now quite open to everyone—is how we had limited availability and limited capacity to meet the needs of the veterans we had the privilege of serving. So we are on a very, very big recruitment drive for all kinds of clinicians. And in addition to the incredible mission, we have taken some steps to make salaries a bit more competitive with the private sector. I want to underline a bit. You wouldn’t be coming to VA because you wanted to become wealthy, but we recognize that people have to pay student loans and so forth. And speaking of student loans, we have a variety of programs to help people pay down their educational debt.
And all of these things actually help, but the opportunity to deliver care that is really focused squarely on the needs of the individual veteran. That, I think, is what people will ultimately find far more exciting than any anxiety about change. …
The answer to the question about who are we recruiting is: yes. We’re recruiting all of those people [physicians and midlevel providers]. We often speak about the health care market in this country as if there were one health care market. And actually, U.S. health care, of which VA is very much a part, particularly now with the new law, is very much a series of local and regional markets. So to some extent, the ratios and the types of people that we’re going to need will depend on the specific community; but we’re looking for people in all of those areas.
Changing VA Culture
Dr. Clancy. There are a number of things that impact culture. Some of it is about stories. And I have to say that every day I get to be inspired by real-life stories of veterans and... their caregivers. Some of those caregivers are their family members or close friends: people who love them. And many of them are people who work for us in the Veterans Health Administration, people who just go the extra mile because it’s the right thing to do. Nobody said they had to do it. We don’t have a policy or a directive for it. To them, it’s as natural as gravity. …
Secretary McDonald often uses this diagram, an inverted pyramid that I love, where he starts off by having a regular pyramid; and he said, “This is how we think of a lot of organizations with the Secretary sitting right up here at the pinnacle, and veterans and everybody else are kind of down on the lowest tier.” And he said, “It’s exactly wrong. How I think about it is—” So, he flips it. We have people who provide direct care to veterans. We have people who help those people, and then we have people who help the people who are helping the people provide the care to veterans. And so that means that customer service is everybody’s job. It means that helping people on the front lines who are our colleagues—and we’re all in this together—make sure that they can deliver the care that veterans need. That is everybody’s job.
And I actually think it’s going to be a very, very easy sell. Reinforcing all of this is being transparent about data and how we’re doing. So we’re now starting to look internally at how our facilities compare with local counterparts in their particular community, and I think you will be seeing that become more public in the near future. We just have to make it a little more visually compelling.
This is how we learn. It isn’t to say, “Gee, look, you didn’t do as well as other facilities.” It’s to say, “Huh, you know, this facility actually has improved dramatically. Why don’t we go learn what they did?” which I think is consistent with [Federal Practitioner’s] focus on best practices. This is the big, big challenge and the opportunity for health care in general.
Blueprint for Excellence
In the wake of the wait time crisis, Carolyn M. Clancy, MD, has been tasked with implementing the Blueprint for Excellence. Its intent, according to the VA, is to “frame a set of activities that simultaneously address improving the performance of VHA health care now, developing a positive service culture, transitioning from ‘sick care’ to ‘health care’ in the broadest sense, and developing agile business systems and management processes that are efficient, transparent and accountable.”
All the changes at the VA will align with 10 strategies for sustained excellence, which focus on improving performance, promoting a positive culture of service, advancing health care innovation, and increasing operational effectiveness and accountability. The strategies include:
- Operate a health care network that anticipates and meets the unique needs of enrolled veterans, in general, and the service-disabled and most vulnerable veterans, in particular.
- Deliver high-quality, veteran-centered care that compares favorably to the best of the private sector in measured outcomes, value, efficiency, and patient experience.
- Leverage information technologies, analytics, and models of health care delivery to optimize individual and population health outcomes.
- Grow an organizational culture, rooted in VA’s core values and mission, that prioritizes the veteran first; engaging and inspiring employees to their highest possible level of performance and conduct.
- Foster an environment of continuous learning, responsible risk taking, and personal accountability.
- Advance health care that is personalized, proactive, and patient-driven and engages and inspires veterans to their highest possible level of health and well-being.
- Lead the nation in research and treatment of military service-related conditions.
- Become a model integrated health services network through innovative academic, intergovernmental, and community relationships, information exchange, and public-private partnerships.
- Operate and communicate with integrity, transparency, and accountability that earns and maintains the trust of veterans, stewards of the system (Congress, Veterans Service Organizations), and the public.
- Modernize management processes in human resources, procurement, payment, capital infrastructure, and information technology to operate with benchmark agility and efficiency.
To listen to Dr. Clancy’s in-depth discussion of the Blueprint for Excellence, visit http://www.fedprac.com/multimedia/multimedia-library/article/carolyn-clancy-on-implementing-the-blueprint-for-excellence-at-the-va/f7313e00ff18fcbcf4fcaead862c285a/ocregister.html.
Measuring Success or Failure
Dr. Clancy. We will be measuring this in a lot of different ways. First is that VA is, I believe, unique among federal departments in having a very deep all-employee survey. We also participate in the broad Federal Employee Viewpoint Survey. ... In addition to that, we field our own survey internally and take that very, very seriously.
So literally, as the electrons are rolling in, we have a National Center for Organization and Development, which is sharing their results with me and looking at [the data] across the entire system by network, by facility. How are we doing? Where are there challenges? Where are there opportunities? Who’s doing incredibly well that we might learn something about how they’re doing that in order to help those facilities that are having more challenges? That is a very, very current source of information.
And the reason it’s so important is you can’t provide veteran-centered care without employees who are inspired to do their very, very best. And people who are inspired to do their very best, by definition, are not terribly unhappy. So that’s a very, very important source.
And I’ll also say that in health care, in general, as well as here, we’re seeing very important correlations between responses to employee surveys and such indicators as avoidable patient harms, rates of hospital-associated infections or health care-associated infections, and so forth. So we know that the two are very highly correlated.
The other reason that the survey is incredibly important is that most service industries have known for a long time that the best source of innovation are the people who are providing the service and care every single day. So again, that gets back to people feeling motivated and empowered and inspired. …
Time Frame for Change
Dr. Clancy. I think that people are seeing changes already. Now I’m just judging from my own e-mails and other things that we get; and we touch base regularly with veterans service organizations, with many, many stakeholders and take that input very, very seriously, because they are incredible partners in helping us identify and solve problems, because what I really worry about are veterans who are encountering difficulties, who may be fearful or hesitant in some fashion to bring that to our attention.
So I think, in a qualitative sense, we are seeing some positive signals but also recognizing that the scale of changes we’re talking about will take some time. But we will continue to see qualitative differences and, I think, real, tangible differences in the care that veterans get as the months proceed from here. I remain very optimistic about the future of this system and the size of the opportunity that we have.
Our laserlike focus for this coming year is access and exceptional veteran experience. You know, navigating health care can be pretty challenging. I know this because I’m from a very, very large extended family; and nobody else is in health or medicine, so I get regular reports. And whether you’re enrolled in our system or get your care elsewhere, it is not always easy.
But we have the incredible privilege of serving people who essentially wrote a blank check on our behalf when they agreed to serve the country. And so access is not something that, for the most part, is monitored closely in the private sector. It is certainly not publicly reported. We are making a very, very strong commitment, not just to improving, but to being able to show people the results.
Carolyn M. Clancy, MD, was named Interim Under Secretary for Health for the VA on July 2, 2014, just as the wait time crisis seemed to be spinning out of control. Her appointment and the confirmation of Secretary Robert A. McDonald less than a month later proved essential to calming the furor but were admittedly just the first steps in a long-term process to increase veterans’ access to care and develop better systems and procedures across the agency.
Six months after her appointment, Federal Practitioner talked with Dr. Clancy about the pace of change and the role of health care providers in improving care for veterans. In the interview, Dr. Clancy clearly noted that many VA facilities already represent the best of U.S. health care and that the path forward requires sharing of best practices. Many other facilities, of course, will have to change, but Dr. Clancy insisted it is “an incredible opportunity” for the VA to learn as a system. Perhaps most heartening to VA practitioners, Dr. Clancy also recognized that “you can’t provide veteran-centered care without employees who are inspired to do their very, very best.”
To be sure, any successful change in VA procedures and culture will require buy-in not only from across the agency, but also from veterans and Congress. Dr. Clancy has already received 2 votes of confidence: The Paralyzed Veterans of America and the Vietnam Veterans of America jointly called on President Obama to make Dr. Clancy’s appointment permanent. The White House and congressional leaders, however, have yet to schedule hearings or comment publicly.
Below is an edited and condensed version of the interview. To hear the complete interview, including an in-depth discussion of the Blueprint for Excellence, visit http://www.fedprac.com/multimedia/multimedia-library.html.
Taking Measure of VA Strengths
Interim Under Secretary for Health Carolyn M. Clancy, MD. I came to this system in August of 2013 after more than 20 years at HHS, all working for an agency that had the lead charge for funding research to improve quality and safety in health care; and I had spent the last 10 years prior to coming here as the director of that agency. I came to VHA because I thought this system was unique among all systems, public and private, in this country and had the strongest foundation in place to deliver 21st century health care. And at least as important—probably more so—was the sense of mission among all of the employees I met. These were people I’ve known in academia, people I met on the interviews, people I’ve intersected with for a number of years in the research community. You can’t replicate it, and you can’t buy it; and I figured the combination of a strong foundation and mission meant that this was one of the best systems to work for.
I still think that. Some of our best facilities could compete head to head with any facilities in the private sector. There is no question about that. We have some systems, facilities, and clinics that are struggling as well, which is also very typical of the private sector.
What we have is an incredible opportunity, first, because we have a fabulous mission. We have highly committed and dedicated employees. We have an incredible opportunity to actually learn as a system. There has been a lot of discussion at a number of levels about how health care in the new century needs to be a learning health care system. We actually have the capability of delivering on that promise. So I’m very, very excited.
VA Clinical Staff and Recruitment
Dr. Clancy. I have often observed that change can be scary, but it’s also incredibly liberating. Some of our most dedicated employees, I know, can be frustrated, because they feel like they’re doing their part; but they aren’t always sure that the members of the team are as dedicated as they are or are going to catch the ball. And there’s no question that you don’t get to high-quality care without a good team. In other words, superb health care and exceptional veteran experience is a team sport by definition.
So I think it will actually help the vast majority of our frontline clinicians. It’ll be much, much easier for them to deliver the kind of care they want to deliver every single day but sometimes feel like they get stuck in workarounds.
As you have probably read and have heard me say, one of the biggest challenges of our crisis—now quite open to everyone—is how we had limited availability and limited capacity to meet the needs of the veterans we had the privilege of serving. So we are on a very, very big recruitment drive for all kinds of clinicians. And in addition to the incredible mission, we have taken some steps to make salaries a bit more competitive with the private sector. I want to underline a bit. You wouldn’t be coming to VA because you wanted to become wealthy, but we recognize that people have to pay student loans and so forth. And speaking of student loans, we have a variety of programs to help people pay down their educational debt.
And all of these things actually help, but the opportunity to deliver care that is really focused squarely on the needs of the individual veteran. That, I think, is what people will ultimately find far more exciting than any anxiety about change. …
The answer to the question about who are we recruiting is: yes. We’re recruiting all of those people [physicians and midlevel providers]. We often speak about the health care market in this country as if there were one health care market. And actually, U.S. health care, of which VA is very much a part, particularly now with the new law, is very much a series of local and regional markets. So to some extent, the ratios and the types of people that we’re going to need will depend on the specific community; but we’re looking for people in all of those areas.
Changing VA Culture
Dr. Clancy. There are a number of things that impact culture. Some of it is about stories. And I have to say that every day I get to be inspired by real-life stories of veterans and... their caregivers. Some of those caregivers are their family members or close friends: people who love them. And many of them are people who work for us in the Veterans Health Administration, people who just go the extra mile because it’s the right thing to do. Nobody said they had to do it. We don’t have a policy or a directive for it. To them, it’s as natural as gravity. …
Secretary McDonald often uses this diagram, an inverted pyramid that I love, where he starts off by having a regular pyramid; and he said, “This is how we think of a lot of organizations with the Secretary sitting right up here at the pinnacle, and veterans and everybody else are kind of down on the lowest tier.” And he said, “It’s exactly wrong. How I think about it is—” So, he flips it. We have people who provide direct care to veterans. We have people who help those people, and then we have people who help the people who are helping the people provide the care to veterans. And so that means that customer service is everybody’s job. It means that helping people on the front lines who are our colleagues—and we’re all in this together—make sure that they can deliver the care that veterans need. That is everybody’s job.
And I actually think it’s going to be a very, very easy sell. Reinforcing all of this is being transparent about data and how we’re doing. So we’re now starting to look internally at how our facilities compare with local counterparts in their particular community, and I think you will be seeing that become more public in the near future. We just have to make it a little more visually compelling.
This is how we learn. It isn’t to say, “Gee, look, you didn’t do as well as other facilities.” It’s to say, “Huh, you know, this facility actually has improved dramatically. Why don’t we go learn what they did?” which I think is consistent with [Federal Practitioner’s] focus on best practices. This is the big, big challenge and the opportunity for health care in general.
Blueprint for Excellence
In the wake of the wait time crisis, Carolyn M. Clancy, MD, has been tasked with implementing the Blueprint for Excellence. Its intent, according to the VA, is to “frame a set of activities that simultaneously address improving the performance of VHA health care now, developing a positive service culture, transitioning from ‘sick care’ to ‘health care’ in the broadest sense, and developing agile business systems and management processes that are efficient, transparent and accountable.”
All the changes at the VA will align with 10 strategies for sustained excellence, which focus on improving performance, promoting a positive culture of service, advancing health care innovation, and increasing operational effectiveness and accountability. The strategies include:
- Operate a health care network that anticipates and meets the unique needs of enrolled veterans, in general, and the service-disabled and most vulnerable veterans, in particular.
- Deliver high-quality, veteran-centered care that compares favorably to the best of the private sector in measured outcomes, value, efficiency, and patient experience.
- Leverage information technologies, analytics, and models of health care delivery to optimize individual and population health outcomes.
- Grow an organizational culture, rooted in VA’s core values and mission, that prioritizes the veteran first; engaging and inspiring employees to their highest possible level of performance and conduct.
- Foster an environment of continuous learning, responsible risk taking, and personal accountability.
- Advance health care that is personalized, proactive, and patient-driven and engages and inspires veterans to their highest possible level of health and well-being.
- Lead the nation in research and treatment of military service-related conditions.
- Become a model integrated health services network through innovative academic, intergovernmental, and community relationships, information exchange, and public-private partnerships.
- Operate and communicate with integrity, transparency, and accountability that earns and maintains the trust of veterans, stewards of the system (Congress, Veterans Service Organizations), and the public.
- Modernize management processes in human resources, procurement, payment, capital infrastructure, and information technology to operate with benchmark agility and efficiency.
To listen to Dr. Clancy’s in-depth discussion of the Blueprint for Excellence, visit http://www.fedprac.com/multimedia/multimedia-library/article/carolyn-clancy-on-implementing-the-blueprint-for-excellence-at-the-va/f7313e00ff18fcbcf4fcaead862c285a/ocregister.html.
Measuring Success or Failure
Dr. Clancy. We will be measuring this in a lot of different ways. First is that VA is, I believe, unique among federal departments in having a very deep all-employee survey. We also participate in the broad Federal Employee Viewpoint Survey. ... In addition to that, we field our own survey internally and take that very, very seriously.
So literally, as the electrons are rolling in, we have a National Center for Organization and Development, which is sharing their results with me and looking at [the data] across the entire system by network, by facility. How are we doing? Where are there challenges? Where are there opportunities? Who’s doing incredibly well that we might learn something about how they’re doing that in order to help those facilities that are having more challenges? That is a very, very current source of information.
And the reason it’s so important is you can’t provide veteran-centered care without employees who are inspired to do their very, very best. And people who are inspired to do their very best, by definition, are not terribly unhappy. So that’s a very, very important source.
And I’ll also say that in health care, in general, as well as here, we’re seeing very important correlations between responses to employee surveys and such indicators as avoidable patient harms, rates of hospital-associated infections or health care-associated infections, and so forth. So we know that the two are very highly correlated.
The other reason that the survey is incredibly important is that most service industries have known for a long time that the best source of innovation are the people who are providing the service and care every single day. So again, that gets back to people feeling motivated and empowered and inspired. …
Time Frame for Change
Dr. Clancy. I think that people are seeing changes already. Now I’m just judging from my own e-mails and other things that we get; and we touch base regularly with veterans service organizations, with many, many stakeholders and take that input very, very seriously, because they are incredible partners in helping us identify and solve problems, because what I really worry about are veterans who are encountering difficulties, who may be fearful or hesitant in some fashion to bring that to our attention.
So I think, in a qualitative sense, we are seeing some positive signals but also recognizing that the scale of changes we’re talking about will take some time. But we will continue to see qualitative differences and, I think, real, tangible differences in the care that veterans get as the months proceed from here. I remain very optimistic about the future of this system and the size of the opportunity that we have.
Our laserlike focus for this coming year is access and exceptional veteran experience. You know, navigating health care can be pretty challenging. I know this because I’m from a very, very large extended family; and nobody else is in health or medicine, so I get regular reports. And whether you’re enrolled in our system or get your care elsewhere, it is not always easy.
But we have the incredible privilege of serving people who essentially wrote a blank check on our behalf when they agreed to serve the country. And so access is not something that, for the most part, is monitored closely in the private sector. It is certainly not publicly reported. We are making a very, very strong commitment, not just to improving, but to being able to show people the results.
Pituitary Incidentaloma
Brian, 46, is referred to endocrinology for evaluation of a pituitary “mass.” The mass was an incidental finding of head and neck CT performed three months ago, when Brian went to an emergency department following a motor vehicle accident. He has fully recovered from the accident and feels well. He describes himself as a “completely healthy” person who has no chronic medical conditions and takes neither prescription nor OTC medications.
Brian denies significant headache, visual disturbance, change in appetite, unexplained weight change, skin rash (wide purple striae) or color changes (hyperpigmentation), polyuria or polydipsia, dizziness, syncopal episodes, low libido, erectile dysfunction, joint pain, and changes in ring or shoe size. He does not wear a hat or cap and is unaware of head size changes. He has not experienced changes in his facial features or trouble with chewing.
He is a happily married engineer with two healthy children and reports that he feels well except for this “brain tumor” finding that has been a shock to him and his family. There is no family history of pituitary adenoma or multiple endocrine neoplasia syndrome.
His vital signs, all within normal ranges, include a blood pressure of 103/65 mm Hg. His height is 6 ft and his weight, 180 lb. His BMI is 24.4.
HOW COMMON IS PITUITARY INCIDENTALOMA?
A pituitary incidentaloma is a lesion in the pituitary gland that was not previously suspected and was found through an imaging study ordered for other reasons. Pituitary incidentaloma is surprisingly common, with an average prevalence of 10.6% (as estimated from combined autopsy data), although it has been found in up to 20% of patients undergoing CT and 38% undergoing MRI.1,2 Most are microadenomas (< 1 cm in size).1
Continue for recommendations from the Endocrine Society >>
SHOULD AN ASYMPTOMATIC PATIENT BE EVALUATED FURTHER?
Endocrine Society guidelines2 recommend that all patients with pituitary incidentaloma, with or without symptoms, should undergo a complete history and physical examination and laboratory evaluation to exclude hypersecretion and hyposecretion of pituitary hormones.
The “classic” presentation of pituitary hormone hypersecretion—in the form of prolactinoma, adrenocorticotropic hormone (ACTH) excess (Cushing disease), growth hormone (GH) excess (gigantism/acromegaly), and TSH excess (secondary hyperthyroidism)—may be readily detectable on history and physical examination. Subtle cases, so-called subclinical disease, however, may exhibit little or no signs and symptoms initially but can be detrimental to the patient’s health if left untreated. For example, the estimated time from onset to diagnosis of acromegaly is approximately seven to 10 years—a delay that can significantly impact the patient’s morbidity and mortality.3
Prolactinoma can be more clinically apparent in premenopausal females due to irregular menstrual cycles (oligomenorrhea/amenorrhea). However, galactorrhea, or “milky” nipple discharge, occurs in only about 50% of women with prolactinoma and is extremely rare in men. Furthermore, the clinical presentation of prolactinoma in men is vague and related to hypogonadism, resulting from increased prolactin levels. Since men are essentially asymptomatic, these tumors can grow extensively (macroadenoma) and cause “mass effect,” such as headaches and visual impairment.
Therefore, without laboratory testing, abnormal pituitary function may go unrecognized.
WHAT LABS SHOULD I ORDER FOR THIS PATIENT?
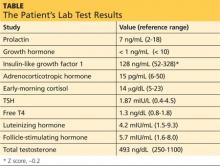
Guidelines suggest an initial screening panel of prolactin, GH, insulin-like growth factor 1 (IGF-1), ACTH, early-morning cortisol, TSH, free T4, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone.2
Note the use of “suggest” rather than “recommend.” Even among guideline task force members, there were differences in opinion as to whether certain tests (eg, TSH, LH, and FSH) should be included in initial screening. Those tests can be ordered at the clinician’s discretion, according to the level of suspicion, or can be added later if necessary.
Brian’s sample for laboratory testing is drawn at 7:50 am. Results can be found in the table (previous page).
Next page: Caveats and concerns >>
ARE THERE ANY CAVEATS TO THE INTERPRETATION OF LAB VALUES?
It is important to note that in postmenopausal women who are not taking hormone replacement therapy, LH and FSH will be elevated and estradiol may provide an additional clue in the detection of abnormal function. Conversely, low LH and FSH in postmenopausal women should raise a flag for hypopituitarism.
Another caveat is that GH secretion is pulsatile and serum levels are undetectable between pulses. Therefore, low/undetectable GH does not necessarily suggest deficiency. The GH measurement would only be helpful if it is significantly elevated (suggestive of hypersecretion—gigantism/acromegaly). Otherwise, GH has little value as a screening test.
Instead, IGF-1, which is secreted from the liver in response to GH secretion, has a longer half-life and serves as a better screening tool. IGF-1 has age- and sex-adjusted reference ranges, which are often reported by the lab or given as a Z score.
WHAT IS THE PREFERRED IMAGING STUDY FOR THE PITUITARY GLAND?
The best choice is MRI of the pituitary gland (not the whole brain) with gadolinium. If the incidentaloma was initially diagnosed by a CT, additional testing with MRI should be performed, unless contraindicated.2
Brian is referred for MRI with gadolinium. The radiologist’s report describes a 5 x 4 x 4–mm pituitary microadenoma without sellar extension or involvement of the optic chiasm.
AT WHAT POINT SHOULD OPTIC CHIASM BE A CONCERN?
Since the pituitary gland is located directly beneath the optic chiasm, any compressive effect of growth against the optic nerve(s) can cause visual impairment. This includes bitemporal hemianopsia (loss of peripheral vision) or ophthalmoplegia (abnormal movement of the ocular muscle). Since clinical signs and symptoms can be subtle or absent, all patients with evidence of a pituitary lesion abutting or compressing the optic chiasm should have a formal visual field exam.2
Continue for surgical intervention >>
WHO REQUIRES SURGICAL INTERVENTION?
Patients with mass effect (headache, increased intracranial pressure, compromised optic chiasm) and those with hyperfunctioning nonprolactin adenomas, some (but not all) macroprolactinomas, or pituitary apoplexy should be referred for surgery.2 Almost all cases involve macroadenomas rather than microadenomas.
The preferred treatment for GH-secreting tumors and ACTH-secreting tumors is surgery. However, prolactinoma can be well controlled with pharmacologic agents (dopamine agonists) in most cases. For prolactinomas refractory to these medications, surgical resection is recommended. (Detailed treatment approaches are available elsewhere; those for hyperprolactinoma can be found on the Clinician Reviews website: http://bit.ly/1HOb9Jf.)
Pituitary apoplexy, a life-threatening emergency that requires prompt surgical decompression, is an infarction of the gland due to abrupt cessation of the blood supply, caused by either pituitary artery hemorrhage or sudden hypovolemia. Increased blood supply is needed due to the extra tissue and volume of the pituitary mass; this may stress the pituitary arteries, which are not equipped for this increased flow, causing them to rupture. Hemorrhage anywhere else in the body can lead to hypovolemia and decrease the blood supply to the pituitary gland. A classic example would be postpartum hemorrhage causing pituitary infarct, called Sheehan syndrome.
Due to increased estrogen levels, the pituitary gland doubles in size during pregnancy.4 A preexisting mass may further develop and compress the optic chiasm. Therefore, women of childbearing age should be engaged in discussion of the potential risks and benefits of decompression surgery before actively pursuing pregnancy—especially if the lesion is close to the optic chiasm.
Surgery can also be considered for patients with significant growth in adenoma size during monitoring, loss of endocrinologic function due to mass effect on other pituitary cells, or unremitting headache.2
Next: How should patients be monitored?
HOW SHOULD PATIENTS BE MONITORED?
Those who do not meet criteria for surgery can be closely monitored with periodic testing. Imaging can be repeated six months after the first scan for macroadenoma and in one year for microadenoma. If there is no change in the size of the mass, imaging can be done yearly for macroadenoma and for microadenoma, every one to two years for three years and then gradually less often thereafter.2
Unless the lesion is abutting the optic chiasm (seen via imaging) or the patient reports symptoms, visual field testing does not need to be repeated.
Lab testing should be repeated six months after initial testing for macroadenoma and yearly thereafter. No further testing is suggested for nonsecretory microadenoma, unless clinically indicated.2
If there are any changes in status—noted clinically or via imaging—more frequent testing is suggested.
Brian is reassured that pituitary adenoma is not an uncommon finding and that his adenoma is relatively small in size and nonsecretory. Repeat pituitary MRI in one year is recommended.
CONCLUSION
Most pituitary incidentalomas have no consequences to a patient’s health. However, patients often become highly anxious about the “brain tumor” they were told they have. Appropriate patient education and thorough evaluation can reassure patients and alleviate their concerns.
REFERENCES
1. Molitch ME. Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinol Metab Clin North Am. 2008;37(1):151-171.
2. Freda PU, Beckers AM, Katznelson L, et al. Pituitary incidentaloma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(4):894-904.
3. Katznelson L, Atkinson JL, Cook DM, et al. American Association of Clinical Endocrinologists Medical Guidelines For Clinical Practice For The Diagnosis And Treatment Of Acromegaly–2011 Update. Endocr Pract. 2011;17(suppl 4).
4. Jameson JL. Harrison’s Endocrinology. 2nd ed. China: McGraw-Hill; 2010:16-49.
Brian, 46, is referred to endocrinology for evaluation of a pituitary “mass.” The mass was an incidental finding of head and neck CT performed three months ago, when Brian went to an emergency department following a motor vehicle accident. He has fully recovered from the accident and feels well. He describes himself as a “completely healthy” person who has no chronic medical conditions and takes neither prescription nor OTC medications.
Brian denies significant headache, visual disturbance, change in appetite, unexplained weight change, skin rash (wide purple striae) or color changes (hyperpigmentation), polyuria or polydipsia, dizziness, syncopal episodes, low libido, erectile dysfunction, joint pain, and changes in ring or shoe size. He does not wear a hat or cap and is unaware of head size changes. He has not experienced changes in his facial features or trouble with chewing.
He is a happily married engineer with two healthy children and reports that he feels well except for this “brain tumor” finding that has been a shock to him and his family. There is no family history of pituitary adenoma or multiple endocrine neoplasia syndrome.
His vital signs, all within normal ranges, include a blood pressure of 103/65 mm Hg. His height is 6 ft and his weight, 180 lb. His BMI is 24.4.
HOW COMMON IS PITUITARY INCIDENTALOMA?
A pituitary incidentaloma is a lesion in the pituitary gland that was not previously suspected and was found through an imaging study ordered for other reasons. Pituitary incidentaloma is surprisingly common, with an average prevalence of 10.6% (as estimated from combined autopsy data), although it has been found in up to 20% of patients undergoing CT and 38% undergoing MRI.1,2 Most are microadenomas (< 1 cm in size).1
Continue for recommendations from the Endocrine Society >>
SHOULD AN ASYMPTOMATIC PATIENT BE EVALUATED FURTHER?
Endocrine Society guidelines2 recommend that all patients with pituitary incidentaloma, with or without symptoms, should undergo a complete history and physical examination and laboratory evaluation to exclude hypersecretion and hyposecretion of pituitary hormones.
The “classic” presentation of pituitary hormone hypersecretion—in the form of prolactinoma, adrenocorticotropic hormone (ACTH) excess (Cushing disease), growth hormone (GH) excess (gigantism/acromegaly), and TSH excess (secondary hyperthyroidism)—may be readily detectable on history and physical examination. Subtle cases, so-called subclinical disease, however, may exhibit little or no signs and symptoms initially but can be detrimental to the patient’s health if left untreated. For example, the estimated time from onset to diagnosis of acromegaly is approximately seven to 10 years—a delay that can significantly impact the patient’s morbidity and mortality.3
Prolactinoma can be more clinically apparent in premenopausal females due to irregular menstrual cycles (oligomenorrhea/amenorrhea). However, galactorrhea, or “milky” nipple discharge, occurs in only about 50% of women with prolactinoma and is extremely rare in men. Furthermore, the clinical presentation of prolactinoma in men is vague and related to hypogonadism, resulting from increased prolactin levels. Since men are essentially asymptomatic, these tumors can grow extensively (macroadenoma) and cause “mass effect,” such as headaches and visual impairment.
Therefore, without laboratory testing, abnormal pituitary function may go unrecognized.
WHAT LABS SHOULD I ORDER FOR THIS PATIENT?
Guidelines suggest an initial screening panel of prolactin, GH, insulin-like growth factor 1 (IGF-1), ACTH, early-morning cortisol, TSH, free T4, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone.2
Note the use of “suggest” rather than “recommend.” Even among guideline task force members, there were differences in opinion as to whether certain tests (eg, TSH, LH, and FSH) should be included in initial screening. Those tests can be ordered at the clinician’s discretion, according to the level of suspicion, or can be added later if necessary.
Brian’s sample for laboratory testing is drawn at 7:50 am. Results can be found in the table (previous page).
Next page: Caveats and concerns >>
ARE THERE ANY CAVEATS TO THE INTERPRETATION OF LAB VALUES?
It is important to note that in postmenopausal women who are not taking hormone replacement therapy, LH and FSH will be elevated and estradiol may provide an additional clue in the detection of abnormal function. Conversely, low LH and FSH in postmenopausal women should raise a flag for hypopituitarism.
Another caveat is that GH secretion is pulsatile and serum levels are undetectable between pulses. Therefore, low/undetectable GH does not necessarily suggest deficiency. The GH measurement would only be helpful if it is significantly elevated (suggestive of hypersecretion—gigantism/acromegaly). Otherwise, GH has little value as a screening test.
Instead, IGF-1, which is secreted from the liver in response to GH secretion, has a longer half-life and serves as a better screening tool. IGF-1 has age- and sex-adjusted reference ranges, which are often reported by the lab or given as a Z score.
WHAT IS THE PREFERRED IMAGING STUDY FOR THE PITUITARY GLAND?
The best choice is MRI of the pituitary gland (not the whole brain) with gadolinium. If the incidentaloma was initially diagnosed by a CT, additional testing with MRI should be performed, unless contraindicated.2
Brian is referred for MRI with gadolinium. The radiologist’s report describes a 5 x 4 x 4–mm pituitary microadenoma without sellar extension or involvement of the optic chiasm.
AT WHAT POINT SHOULD OPTIC CHIASM BE A CONCERN?
Since the pituitary gland is located directly beneath the optic chiasm, any compressive effect of growth against the optic nerve(s) can cause visual impairment. This includes bitemporal hemianopsia (loss of peripheral vision) or ophthalmoplegia (abnormal movement of the ocular muscle). Since clinical signs and symptoms can be subtle or absent, all patients with evidence of a pituitary lesion abutting or compressing the optic chiasm should have a formal visual field exam.2
Continue for surgical intervention >>
WHO REQUIRES SURGICAL INTERVENTION?
Patients with mass effect (headache, increased intracranial pressure, compromised optic chiasm) and those with hyperfunctioning nonprolactin adenomas, some (but not all) macroprolactinomas, or pituitary apoplexy should be referred for surgery.2 Almost all cases involve macroadenomas rather than microadenomas.
The preferred treatment for GH-secreting tumors and ACTH-secreting tumors is surgery. However, prolactinoma can be well controlled with pharmacologic agents (dopamine agonists) in most cases. For prolactinomas refractory to these medications, surgical resection is recommended. (Detailed treatment approaches are available elsewhere; those for hyperprolactinoma can be found on the Clinician Reviews website: http://bit.ly/1HOb9Jf.)
Pituitary apoplexy, a life-threatening emergency that requires prompt surgical decompression, is an infarction of the gland due to abrupt cessation of the blood supply, caused by either pituitary artery hemorrhage or sudden hypovolemia. Increased blood supply is needed due to the extra tissue and volume of the pituitary mass; this may stress the pituitary arteries, which are not equipped for this increased flow, causing them to rupture. Hemorrhage anywhere else in the body can lead to hypovolemia and decrease the blood supply to the pituitary gland. A classic example would be postpartum hemorrhage causing pituitary infarct, called Sheehan syndrome.
Due to increased estrogen levels, the pituitary gland doubles in size during pregnancy.4 A preexisting mass may further develop and compress the optic chiasm. Therefore, women of childbearing age should be engaged in discussion of the potential risks and benefits of decompression surgery before actively pursuing pregnancy—especially if the lesion is close to the optic chiasm.
Surgery can also be considered for patients with significant growth in adenoma size during monitoring, loss of endocrinologic function due to mass effect on other pituitary cells, or unremitting headache.2
Next: How should patients be monitored?
HOW SHOULD PATIENTS BE MONITORED?
Those who do not meet criteria for surgery can be closely monitored with periodic testing. Imaging can be repeated six months after the first scan for macroadenoma and in one year for microadenoma. If there is no change in the size of the mass, imaging can be done yearly for macroadenoma and for microadenoma, every one to two years for three years and then gradually less often thereafter.2
Unless the lesion is abutting the optic chiasm (seen via imaging) or the patient reports symptoms, visual field testing does not need to be repeated.
Lab testing should be repeated six months after initial testing for macroadenoma and yearly thereafter. No further testing is suggested for nonsecretory microadenoma, unless clinically indicated.2
If there are any changes in status—noted clinically or via imaging—more frequent testing is suggested.
Brian is reassured that pituitary adenoma is not an uncommon finding and that his adenoma is relatively small in size and nonsecretory. Repeat pituitary MRI in one year is recommended.
CONCLUSION
Most pituitary incidentalomas have no consequences to a patient’s health. However, patients often become highly anxious about the “brain tumor” they were told they have. Appropriate patient education and thorough evaluation can reassure patients and alleviate their concerns.
REFERENCES
1. Molitch ME. Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinol Metab Clin North Am. 2008;37(1):151-171.
2. Freda PU, Beckers AM, Katznelson L, et al. Pituitary incidentaloma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(4):894-904.
3. Katznelson L, Atkinson JL, Cook DM, et al. American Association of Clinical Endocrinologists Medical Guidelines For Clinical Practice For The Diagnosis And Treatment Of Acromegaly–2011 Update. Endocr Pract. 2011;17(suppl 4).
4. Jameson JL. Harrison’s Endocrinology. 2nd ed. China: McGraw-Hill; 2010:16-49.
Brian, 46, is referred to endocrinology for evaluation of a pituitary “mass.” The mass was an incidental finding of head and neck CT performed three months ago, when Brian went to an emergency department following a motor vehicle accident. He has fully recovered from the accident and feels well. He describes himself as a “completely healthy” person who has no chronic medical conditions and takes neither prescription nor OTC medications.
Brian denies significant headache, visual disturbance, change in appetite, unexplained weight change, skin rash (wide purple striae) or color changes (hyperpigmentation), polyuria or polydipsia, dizziness, syncopal episodes, low libido, erectile dysfunction, joint pain, and changes in ring or shoe size. He does not wear a hat or cap and is unaware of head size changes. He has not experienced changes in his facial features or trouble with chewing.
He is a happily married engineer with two healthy children and reports that he feels well except for this “brain tumor” finding that has been a shock to him and his family. There is no family history of pituitary adenoma or multiple endocrine neoplasia syndrome.
His vital signs, all within normal ranges, include a blood pressure of 103/65 mm Hg. His height is 6 ft and his weight, 180 lb. His BMI is 24.4.
HOW COMMON IS PITUITARY INCIDENTALOMA?
A pituitary incidentaloma is a lesion in the pituitary gland that was not previously suspected and was found through an imaging study ordered for other reasons. Pituitary incidentaloma is surprisingly common, with an average prevalence of 10.6% (as estimated from combined autopsy data), although it has been found in up to 20% of patients undergoing CT and 38% undergoing MRI.1,2 Most are microadenomas (< 1 cm in size).1
Continue for recommendations from the Endocrine Society >>
SHOULD AN ASYMPTOMATIC PATIENT BE EVALUATED FURTHER?
Endocrine Society guidelines2 recommend that all patients with pituitary incidentaloma, with or without symptoms, should undergo a complete history and physical examination and laboratory evaluation to exclude hypersecretion and hyposecretion of pituitary hormones.
The “classic” presentation of pituitary hormone hypersecretion—in the form of prolactinoma, adrenocorticotropic hormone (ACTH) excess (Cushing disease), growth hormone (GH) excess (gigantism/acromegaly), and TSH excess (secondary hyperthyroidism)—may be readily detectable on history and physical examination. Subtle cases, so-called subclinical disease, however, may exhibit little or no signs and symptoms initially but can be detrimental to the patient’s health if left untreated. For example, the estimated time from onset to diagnosis of acromegaly is approximately seven to 10 years—a delay that can significantly impact the patient’s morbidity and mortality.3
Prolactinoma can be more clinically apparent in premenopausal females due to irregular menstrual cycles (oligomenorrhea/amenorrhea). However, galactorrhea, or “milky” nipple discharge, occurs in only about 50% of women with prolactinoma and is extremely rare in men. Furthermore, the clinical presentation of prolactinoma in men is vague and related to hypogonadism, resulting from increased prolactin levels. Since men are essentially asymptomatic, these tumors can grow extensively (macroadenoma) and cause “mass effect,” such as headaches and visual impairment.
Therefore, without laboratory testing, abnormal pituitary function may go unrecognized.
WHAT LABS SHOULD I ORDER FOR THIS PATIENT?
Guidelines suggest an initial screening panel of prolactin, GH, insulin-like growth factor 1 (IGF-1), ACTH, early-morning cortisol, TSH, free T4, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone.2
Note the use of “suggest” rather than “recommend.” Even among guideline task force members, there were differences in opinion as to whether certain tests (eg, TSH, LH, and FSH) should be included in initial screening. Those tests can be ordered at the clinician’s discretion, according to the level of suspicion, or can be added later if necessary.
Brian’s sample for laboratory testing is drawn at 7:50 am. Results can be found in the table (previous page).
Next page: Caveats and concerns >>
ARE THERE ANY CAVEATS TO THE INTERPRETATION OF LAB VALUES?
It is important to note that in postmenopausal women who are not taking hormone replacement therapy, LH and FSH will be elevated and estradiol may provide an additional clue in the detection of abnormal function. Conversely, low LH and FSH in postmenopausal women should raise a flag for hypopituitarism.
Another caveat is that GH secretion is pulsatile and serum levels are undetectable between pulses. Therefore, low/undetectable GH does not necessarily suggest deficiency. The GH measurement would only be helpful if it is significantly elevated (suggestive of hypersecretion—gigantism/acromegaly). Otherwise, GH has little value as a screening test.
Instead, IGF-1, which is secreted from the liver in response to GH secretion, has a longer half-life and serves as a better screening tool. IGF-1 has age- and sex-adjusted reference ranges, which are often reported by the lab or given as a Z score.
WHAT IS THE PREFERRED IMAGING STUDY FOR THE PITUITARY GLAND?
The best choice is MRI of the pituitary gland (not the whole brain) with gadolinium. If the incidentaloma was initially diagnosed by a CT, additional testing with MRI should be performed, unless contraindicated.2
Brian is referred for MRI with gadolinium. The radiologist’s report describes a 5 x 4 x 4–mm pituitary microadenoma without sellar extension or involvement of the optic chiasm.
AT WHAT POINT SHOULD OPTIC CHIASM BE A CONCERN?
Since the pituitary gland is located directly beneath the optic chiasm, any compressive effect of growth against the optic nerve(s) can cause visual impairment. This includes bitemporal hemianopsia (loss of peripheral vision) or ophthalmoplegia (abnormal movement of the ocular muscle). Since clinical signs and symptoms can be subtle or absent, all patients with evidence of a pituitary lesion abutting or compressing the optic chiasm should have a formal visual field exam.2
Continue for surgical intervention >>
WHO REQUIRES SURGICAL INTERVENTION?
Patients with mass effect (headache, increased intracranial pressure, compromised optic chiasm) and those with hyperfunctioning nonprolactin adenomas, some (but not all) macroprolactinomas, or pituitary apoplexy should be referred for surgery.2 Almost all cases involve macroadenomas rather than microadenomas.
The preferred treatment for GH-secreting tumors and ACTH-secreting tumors is surgery. However, prolactinoma can be well controlled with pharmacologic agents (dopamine agonists) in most cases. For prolactinomas refractory to these medications, surgical resection is recommended. (Detailed treatment approaches are available elsewhere; those for hyperprolactinoma can be found on the Clinician Reviews website: http://bit.ly/1HOb9Jf.)
Pituitary apoplexy, a life-threatening emergency that requires prompt surgical decompression, is an infarction of the gland due to abrupt cessation of the blood supply, caused by either pituitary artery hemorrhage or sudden hypovolemia. Increased blood supply is needed due to the extra tissue and volume of the pituitary mass; this may stress the pituitary arteries, which are not equipped for this increased flow, causing them to rupture. Hemorrhage anywhere else in the body can lead to hypovolemia and decrease the blood supply to the pituitary gland. A classic example would be postpartum hemorrhage causing pituitary infarct, called Sheehan syndrome.
Due to increased estrogen levels, the pituitary gland doubles in size during pregnancy.4 A preexisting mass may further develop and compress the optic chiasm. Therefore, women of childbearing age should be engaged in discussion of the potential risks and benefits of decompression surgery before actively pursuing pregnancy—especially if the lesion is close to the optic chiasm.
Surgery can also be considered for patients with significant growth in adenoma size during monitoring, loss of endocrinologic function due to mass effect on other pituitary cells, or unremitting headache.2
Next: How should patients be monitored?
HOW SHOULD PATIENTS BE MONITORED?
Those who do not meet criteria for surgery can be closely monitored with periodic testing. Imaging can be repeated six months after the first scan for macroadenoma and in one year for microadenoma. If there is no change in the size of the mass, imaging can be done yearly for macroadenoma and for microadenoma, every one to two years for three years and then gradually less often thereafter.2
Unless the lesion is abutting the optic chiasm (seen via imaging) or the patient reports symptoms, visual field testing does not need to be repeated.
Lab testing should be repeated six months after initial testing for macroadenoma and yearly thereafter. No further testing is suggested for nonsecretory microadenoma, unless clinically indicated.2
If there are any changes in status—noted clinically or via imaging—more frequent testing is suggested.
Brian is reassured that pituitary adenoma is not an uncommon finding and that his adenoma is relatively small in size and nonsecretory. Repeat pituitary MRI in one year is recommended.
CONCLUSION
Most pituitary incidentalomas have no consequences to a patient’s health. However, patients often become highly anxious about the “brain tumor” they were told they have. Appropriate patient education and thorough evaluation can reassure patients and alleviate their concerns.
REFERENCES
1. Molitch ME. Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinol Metab Clin North Am. 2008;37(1):151-171.
2. Freda PU, Beckers AM, Katznelson L, et al. Pituitary incidentaloma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(4):894-904.
3. Katznelson L, Atkinson JL, Cook DM, et al. American Association of Clinical Endocrinologists Medical Guidelines For Clinical Practice For The Diagnosis And Treatment Of Acromegaly–2011 Update. Endocr Pract. 2011;17(suppl 4).
4. Jameson JL. Harrison’s Endocrinology. 2nd ed. China: McGraw-Hill; 2010:16-49.
Preparing the Military Health System for the 21st Century
Assistant Secretary of Defense for Health Affairs Dr. Jonathan Woodson sits atop a massively complex health care system. With an annual budget of > $50 billion and 133,000 military and civilian health care providers, allied health professionals, and health administrators spread around the globe, the Military Health System (MHS) is exceedingly complex. Keeping the system running is challenging enough, but Woodson is focused on transforming it into a more nimble, efficient, proactive and cost-effective health care system.
Federal Practitioner recently talked with Dr. Woodson about the challenges of transforming the MHS. We also discussed the Defense Health Agency and how global health threats like Ebola fit in to the MHS mission. The following is condensed and edited, but the complete interview can be heard here: Jonathan Woodson on Military Health Readiness.
The Military Health System Mission
Assistant Secretary of Defense for Health Affairs Jonathan Woodson. The Department of Defense has a unique mission. So yes, we do deliver health care; but we deliver health care on a global basis. And in fact, we are the ones who are asked anytime there is a crisis to set up health care systems in austere places as a key enabler to those who would go in harm’s way and defend our nation.
That is a sober undertaking, and we undertake it with the full understanding that in today’s world, the American leadership and the American public expect us to not only deliver the standard of care, but really go above the standard of care and advance care anywhere in the world....If you look at the experience of the last 12 plus years of war, and you look at what has been accomplished in terms of combat casualty care, we have advanced that strategy for care.
At the beginning of the war, it was clear that we were operating from a traditional platform but a number of experts recognized that what we needed to develop was a system that could drive change based upon data. The Joint Trauma System was born, which analyzed the outcomes of just about every case that was treated. But more importantly, [it] mined the data and rapidly changed the strategies for care as we found optimal ways of delivering care.
This included everything from our strategy for evacuation and the development of critical care air medical transplant units that provided prompt evacuation from the battlefield and echelons of care so those service members would receive very sophisticated advanced care, to strategies for employing new drugs, new techniques, new training strategies for medics and bringing critical care skills closer to the point of injury. Across a wide swath of strategies for delivering care, the system has been constantly improved.
And now we see from the data that despite the fact that we’re taking care of more severely injured individuals, the fatality rates have dropped. An individual who makes it to a role 3 facility—whatever the injury severity—has a 98% chance of surviving.
This has culminated most recently in terms of this transfer of knowledge with us signing a partnership agreement with the American College of Surgeons, which will allow us to further interact with the civilian communities in terms of trying to define optimal ways for caring for injured patients. This will be good not only for us, but it will be good for the civilian communities as well.
Research and Sharing Advances
Dr. Woodson. We have outlined 6 strategic lines of effort to help modernize the military health system, and they include modernizing our management with an enterprise focus, defining our 21st century capabilities that are necessary to make us better, stronger, and more relevant for the future. [We are also] looking at the medical force structure, particularly since today we have to ensure that we understand and employ subspecialists in the right way.
In addition, we are looking at defining and investing in strategic partners. Our strategic partners are like the American College of Surgeons but represent a wide range of potential academic and research institutions that can collaborate with us to ensure that we achieve results in our research portfolio, particularly against the priorities that are very important to military medicine.
The other areas that we need to concentrate on is reforming TRICARE and defining our requirements and competencies in global health engagement. The issue is investing in and defining our strategic partners, which is what I think is going to make us extraordinarily strong, because realistically we need to approach this as the whole-of-society investment in our national defense. Our strategic partners, of course, include our other federal partners, such as the Veterans Administration.
Continuity of Care
Dr. Woodson. We are committed to serving the needs of servicemen and women who might be injured or become ill as a result of their service for decades to come. That is, we understand that they may, in fact, require care for decades. And as a result, we, of course, have several ways of ensuring that they do receive that care. We have a defined sort of insurance benefit called TRICARE; a vehicle that allows separating servicemen and women who qualify to receive care in the civilian sector.
But beyond that, we have strengthened our partnership and our collaboration with the Veterans Administration to break down barriers so that we can transition servicemen and women more effectively and easily. [For] things like transferring critical medical information, we’ve developed an integrative mental health strategy so that we have common evidence-based strategies for mental health care.
We’ve recently concluded an agreement to reform the way the 2 departments reimburse each other so that the whole issue of billing doesn’t become an encumbrance to delivery of care. We’ve agreed to a common credentialing system so that our providers can more easily serve in either system, which leads to more effective, efficient care and use of our human resources.
Across many lines—the ones I’ve mentioned, and many others—we are ensuring that we can care for the servicemen and women who might become ill or injured and require care going on into the decades to ensure that they have high-quality lives and they’re kept healthy.
The Global Response to Ebola
Dr. Woodson. We have subject matter experts that have worked in infectious diseases for some time. You know, the United States Army Institute of Infectious Diseases is a well-recognized, longstanding organization that has helped produce vaccines and strategies to care for infectious diseases and has contributed very substantially to the biosecurity not only of this nation, but of the world.
We’ve got really the indomitable spirit of the average serviceman and woman who, when given a complex job, know how to meet the mission. And so we have superb leaders, and we have become a key enabler.
When the U.S. shows up, then other countries rally. It provides a platform in which other countries can now commit to the effort. And that’s probably as important as anything else, because this needs to be a worldwide effort to stem this epidemic that is occurring in West Africa.
The Defense Health Agency
The Defense Health Agency has as its mission to put in effect common clinical and business processes to achieve those economies of scale and allow us to use our dollars and other resources more wisely. The Defense Health Agency was charged with initially standing up 10 shared services to include facilities planning, medical logistics, health information technology, managing the TRICARE health plan, pharmacy programs, the Public Health System, acquisitions, budget and resources, management systems, medical education and training, and medical research and development.
As such, we’ve achieved great results over the first year, saving about $248 million. But more importantly, we set the foundation for 21st century systems that will allow us to manage the Military Health System much more effectively, such as establishing a foundation for a common cost accounting system and the development of an enterprise performance management system.
The Military Health System added what we call the Quadruple Aim, which is providing better outcomes, better patient care experience, managing costs, and meeting our readiness mission. We have also established a solid strategic plan and framework in which to ensure that we will meet that end. We’ve had tremendous progress over the first year.
I just want to remind you and everyone of what a heavy lift this was. This is a major reorganization where thousands of people have been reassigned and reorganized to produce a more effective management system. This was no easy lift, but it has been tremendously successful to date.
You don’t necessarily flip the switch and everything is mature and working optimally overnight. The intent was to ensure that it was fully operational and capable by 1 October 2015. What we have seen to date is that it’s ahead of schedule, and that’s good news.
It represents major transformational change. Many people have to be moved. We had to build a new leadership team that, in fact, was heavily invested and contributed to by the uniformed services, the Army, Navy, and Air Force. So they’re deeply invested in the leadership and the governance of the Defense Health Agency.
The Military’s Health Care Challenges
Dr. Woodson. Our central guiding principle is the Quadruple Aim. And at the center, as I mentioned before, is this issue of readiness; and readiness is about ensuring that we have a healthy force to do the nation’s bidding in terms of defense. Not only do we have a healthy force, but we keep them healthy. So we commit to looking at environmental concerns wherever they are deployed. Of course, we provide force protection measures such as vaccines and medicines to prevent infectious diseases, such as malaria if they’re working in a part of the world where that’s endemic.
This is all part of the responsibilities of the military health system. But also, the second part of the readiness responsibility is ensuring that we have a ready medical force. A group of superbly trained providers from the embedded combat medic up to the super subspecialist neurosurgeon, nurses of all specialties and varieties, and other allied health professionals that can create a robust Military Health System and provide above the standard of care anywhere in the world where our sailors, marines, airmen, and soldiers may be operating.
We also have to deal with the issue of chronic problems, health problems that afflict our society. We have started initiatives to address obesity and fitness across a broad spectrum. As a strategy for the military health system, we’re moving from the system of health care, which is just providing treatment after an established disease has occurred, to one of health, which is looking at the whole paradigm of wellness and preventing disease from occurring. It is about reaching into that white space where people learn, work, and play to ensure that they can make healthy choices....We’re deeply invested in the health of the beneficiaries that we serve across a broad spectrum, and we’re deeply invested in the issue of prevention, not only the treatment of disease.
The issue that I would want everyone to understand is that health care and health care delivery in the 21st century is very complex. It’s about not only the actual technology, advances in medical science, but it’s also about addressing where medical science hits human systems and how do you make the system work so that you achieve the best outcomes? And in that mix are the issues of cost and ensuring that you have the ability to deliver that care wherever it’s needed. We’ve mapped out with the leadership of the Military Health System, the surgeons general, and all of their leaders a pathway forward that, in fact, will ensure that the Military Health System will be strong, better, and relevant going into the 21st century and will continue to be a key enabler for the national security, national defense, and the national military strategies.
As a designated Combat Support Agency, the Defense Health Agency is also responsible for meeting the medical needs of the combatant commanders. Central to this role is to ensure our service members are medically ready to perform their mission, and our military medical personnel are ready to perform their mission—“Medically Ready Force…Ready Medical Force.”
Assistant Secretary of Defense for Health Affairs Dr. Jonathan Woodson sits atop a massively complex health care system. With an annual budget of > $50 billion and 133,000 military and civilian health care providers, allied health professionals, and health administrators spread around the globe, the Military Health System (MHS) is exceedingly complex. Keeping the system running is challenging enough, but Woodson is focused on transforming it into a more nimble, efficient, proactive and cost-effective health care system.
Federal Practitioner recently talked with Dr. Woodson about the challenges of transforming the MHS. We also discussed the Defense Health Agency and how global health threats like Ebola fit in to the MHS mission. The following is condensed and edited, but the complete interview can be heard here: Jonathan Woodson on Military Health Readiness.
The Military Health System Mission
Assistant Secretary of Defense for Health Affairs Jonathan Woodson. The Department of Defense has a unique mission. So yes, we do deliver health care; but we deliver health care on a global basis. And in fact, we are the ones who are asked anytime there is a crisis to set up health care systems in austere places as a key enabler to those who would go in harm’s way and defend our nation.
That is a sober undertaking, and we undertake it with the full understanding that in today’s world, the American leadership and the American public expect us to not only deliver the standard of care, but really go above the standard of care and advance care anywhere in the world....If you look at the experience of the last 12 plus years of war, and you look at what has been accomplished in terms of combat casualty care, we have advanced that strategy for care.
At the beginning of the war, it was clear that we were operating from a traditional platform but a number of experts recognized that what we needed to develop was a system that could drive change based upon data. The Joint Trauma System was born, which analyzed the outcomes of just about every case that was treated. But more importantly, [it] mined the data and rapidly changed the strategies for care as we found optimal ways of delivering care.
This included everything from our strategy for evacuation and the development of critical care air medical transplant units that provided prompt evacuation from the battlefield and echelons of care so those service members would receive very sophisticated advanced care, to strategies for employing new drugs, new techniques, new training strategies for medics and bringing critical care skills closer to the point of injury. Across a wide swath of strategies for delivering care, the system has been constantly improved.
And now we see from the data that despite the fact that we’re taking care of more severely injured individuals, the fatality rates have dropped. An individual who makes it to a role 3 facility—whatever the injury severity—has a 98% chance of surviving.
This has culminated most recently in terms of this transfer of knowledge with us signing a partnership agreement with the American College of Surgeons, which will allow us to further interact with the civilian communities in terms of trying to define optimal ways for caring for injured patients. This will be good not only for us, but it will be good for the civilian communities as well.
Research and Sharing Advances
Dr. Woodson. We have outlined 6 strategic lines of effort to help modernize the military health system, and they include modernizing our management with an enterprise focus, defining our 21st century capabilities that are necessary to make us better, stronger, and more relevant for the future. [We are also] looking at the medical force structure, particularly since today we have to ensure that we understand and employ subspecialists in the right way.
In addition, we are looking at defining and investing in strategic partners. Our strategic partners are like the American College of Surgeons but represent a wide range of potential academic and research institutions that can collaborate with us to ensure that we achieve results in our research portfolio, particularly against the priorities that are very important to military medicine.
The other areas that we need to concentrate on is reforming TRICARE and defining our requirements and competencies in global health engagement. The issue is investing in and defining our strategic partners, which is what I think is going to make us extraordinarily strong, because realistically we need to approach this as the whole-of-society investment in our national defense. Our strategic partners, of course, include our other federal partners, such as the Veterans Administration.
Continuity of Care
Dr. Woodson. We are committed to serving the needs of servicemen and women who might be injured or become ill as a result of their service for decades to come. That is, we understand that they may, in fact, require care for decades. And as a result, we, of course, have several ways of ensuring that they do receive that care. We have a defined sort of insurance benefit called TRICARE; a vehicle that allows separating servicemen and women who qualify to receive care in the civilian sector.
But beyond that, we have strengthened our partnership and our collaboration with the Veterans Administration to break down barriers so that we can transition servicemen and women more effectively and easily. [For] things like transferring critical medical information, we’ve developed an integrative mental health strategy so that we have common evidence-based strategies for mental health care.
We’ve recently concluded an agreement to reform the way the 2 departments reimburse each other so that the whole issue of billing doesn’t become an encumbrance to delivery of care. We’ve agreed to a common credentialing system so that our providers can more easily serve in either system, which leads to more effective, efficient care and use of our human resources.
Across many lines—the ones I’ve mentioned, and many others—we are ensuring that we can care for the servicemen and women who might become ill or injured and require care going on into the decades to ensure that they have high-quality lives and they’re kept healthy.
The Global Response to Ebola
Dr. Woodson. We have subject matter experts that have worked in infectious diseases for some time. You know, the United States Army Institute of Infectious Diseases is a well-recognized, longstanding organization that has helped produce vaccines and strategies to care for infectious diseases and has contributed very substantially to the biosecurity not only of this nation, but of the world.
We’ve got really the indomitable spirit of the average serviceman and woman who, when given a complex job, know how to meet the mission. And so we have superb leaders, and we have become a key enabler.
When the U.S. shows up, then other countries rally. It provides a platform in which other countries can now commit to the effort. And that’s probably as important as anything else, because this needs to be a worldwide effort to stem this epidemic that is occurring in West Africa.
The Defense Health Agency
The Defense Health Agency has as its mission to put in effect common clinical and business processes to achieve those economies of scale and allow us to use our dollars and other resources more wisely. The Defense Health Agency was charged with initially standing up 10 shared services to include facilities planning, medical logistics, health information technology, managing the TRICARE health plan, pharmacy programs, the Public Health System, acquisitions, budget and resources, management systems, medical education and training, and medical research and development.
As such, we’ve achieved great results over the first year, saving about $248 million. But more importantly, we set the foundation for 21st century systems that will allow us to manage the Military Health System much more effectively, such as establishing a foundation for a common cost accounting system and the development of an enterprise performance management system.
The Military Health System added what we call the Quadruple Aim, which is providing better outcomes, better patient care experience, managing costs, and meeting our readiness mission. We have also established a solid strategic plan and framework in which to ensure that we will meet that end. We’ve had tremendous progress over the first year.
I just want to remind you and everyone of what a heavy lift this was. This is a major reorganization where thousands of people have been reassigned and reorganized to produce a more effective management system. This was no easy lift, but it has been tremendously successful to date.
You don’t necessarily flip the switch and everything is mature and working optimally overnight. The intent was to ensure that it was fully operational and capable by 1 October 2015. What we have seen to date is that it’s ahead of schedule, and that’s good news.
It represents major transformational change. Many people have to be moved. We had to build a new leadership team that, in fact, was heavily invested and contributed to by the uniformed services, the Army, Navy, and Air Force. So they’re deeply invested in the leadership and the governance of the Defense Health Agency.
The Military’s Health Care Challenges
Dr. Woodson. Our central guiding principle is the Quadruple Aim. And at the center, as I mentioned before, is this issue of readiness; and readiness is about ensuring that we have a healthy force to do the nation’s bidding in terms of defense. Not only do we have a healthy force, but we keep them healthy. So we commit to looking at environmental concerns wherever they are deployed. Of course, we provide force protection measures such as vaccines and medicines to prevent infectious diseases, such as malaria if they’re working in a part of the world where that’s endemic.
This is all part of the responsibilities of the military health system. But also, the second part of the readiness responsibility is ensuring that we have a ready medical force. A group of superbly trained providers from the embedded combat medic up to the super subspecialist neurosurgeon, nurses of all specialties and varieties, and other allied health professionals that can create a robust Military Health System and provide above the standard of care anywhere in the world where our sailors, marines, airmen, and soldiers may be operating.
We also have to deal with the issue of chronic problems, health problems that afflict our society. We have started initiatives to address obesity and fitness across a broad spectrum. As a strategy for the military health system, we’re moving from the system of health care, which is just providing treatment after an established disease has occurred, to one of health, which is looking at the whole paradigm of wellness and preventing disease from occurring. It is about reaching into that white space where people learn, work, and play to ensure that they can make healthy choices....We’re deeply invested in the health of the beneficiaries that we serve across a broad spectrum, and we’re deeply invested in the issue of prevention, not only the treatment of disease.
The issue that I would want everyone to understand is that health care and health care delivery in the 21st century is very complex. It’s about not only the actual technology, advances in medical science, but it’s also about addressing where medical science hits human systems and how do you make the system work so that you achieve the best outcomes? And in that mix are the issues of cost and ensuring that you have the ability to deliver that care wherever it’s needed. We’ve mapped out with the leadership of the Military Health System, the surgeons general, and all of their leaders a pathway forward that, in fact, will ensure that the Military Health System will be strong, better, and relevant going into the 21st century and will continue to be a key enabler for the national security, national defense, and the national military strategies.
As a designated Combat Support Agency, the Defense Health Agency is also responsible for meeting the medical needs of the combatant commanders. Central to this role is to ensure our service members are medically ready to perform their mission, and our military medical personnel are ready to perform their mission—“Medically Ready Force…Ready Medical Force.”
Assistant Secretary of Defense for Health Affairs Dr. Jonathan Woodson sits atop a massively complex health care system. With an annual budget of > $50 billion and 133,000 military and civilian health care providers, allied health professionals, and health administrators spread around the globe, the Military Health System (MHS) is exceedingly complex. Keeping the system running is challenging enough, but Woodson is focused on transforming it into a more nimble, efficient, proactive and cost-effective health care system.
Federal Practitioner recently talked with Dr. Woodson about the challenges of transforming the MHS. We also discussed the Defense Health Agency and how global health threats like Ebola fit in to the MHS mission. The following is condensed and edited, but the complete interview can be heard here: Jonathan Woodson on Military Health Readiness.
The Military Health System Mission
Assistant Secretary of Defense for Health Affairs Jonathan Woodson. The Department of Defense has a unique mission. So yes, we do deliver health care; but we deliver health care on a global basis. And in fact, we are the ones who are asked anytime there is a crisis to set up health care systems in austere places as a key enabler to those who would go in harm’s way and defend our nation.
That is a sober undertaking, and we undertake it with the full understanding that in today’s world, the American leadership and the American public expect us to not only deliver the standard of care, but really go above the standard of care and advance care anywhere in the world....If you look at the experience of the last 12 plus years of war, and you look at what has been accomplished in terms of combat casualty care, we have advanced that strategy for care.
At the beginning of the war, it was clear that we were operating from a traditional platform but a number of experts recognized that what we needed to develop was a system that could drive change based upon data. The Joint Trauma System was born, which analyzed the outcomes of just about every case that was treated. But more importantly, [it] mined the data and rapidly changed the strategies for care as we found optimal ways of delivering care.
This included everything from our strategy for evacuation and the development of critical care air medical transplant units that provided prompt evacuation from the battlefield and echelons of care so those service members would receive very sophisticated advanced care, to strategies for employing new drugs, new techniques, new training strategies for medics and bringing critical care skills closer to the point of injury. Across a wide swath of strategies for delivering care, the system has been constantly improved.
And now we see from the data that despite the fact that we’re taking care of more severely injured individuals, the fatality rates have dropped. An individual who makes it to a role 3 facility—whatever the injury severity—has a 98% chance of surviving.
This has culminated most recently in terms of this transfer of knowledge with us signing a partnership agreement with the American College of Surgeons, which will allow us to further interact with the civilian communities in terms of trying to define optimal ways for caring for injured patients. This will be good not only for us, but it will be good for the civilian communities as well.
Research and Sharing Advances
Dr. Woodson. We have outlined 6 strategic lines of effort to help modernize the military health system, and they include modernizing our management with an enterprise focus, defining our 21st century capabilities that are necessary to make us better, stronger, and more relevant for the future. [We are also] looking at the medical force structure, particularly since today we have to ensure that we understand and employ subspecialists in the right way.
In addition, we are looking at defining and investing in strategic partners. Our strategic partners are like the American College of Surgeons but represent a wide range of potential academic and research institutions that can collaborate with us to ensure that we achieve results in our research portfolio, particularly against the priorities that are very important to military medicine.
The other areas that we need to concentrate on is reforming TRICARE and defining our requirements and competencies in global health engagement. The issue is investing in and defining our strategic partners, which is what I think is going to make us extraordinarily strong, because realistically we need to approach this as the whole-of-society investment in our national defense. Our strategic partners, of course, include our other federal partners, such as the Veterans Administration.
Continuity of Care
Dr. Woodson. We are committed to serving the needs of servicemen and women who might be injured or become ill as a result of their service for decades to come. That is, we understand that they may, in fact, require care for decades. And as a result, we, of course, have several ways of ensuring that they do receive that care. We have a defined sort of insurance benefit called TRICARE; a vehicle that allows separating servicemen and women who qualify to receive care in the civilian sector.
But beyond that, we have strengthened our partnership and our collaboration with the Veterans Administration to break down barriers so that we can transition servicemen and women more effectively and easily. [For] things like transferring critical medical information, we’ve developed an integrative mental health strategy so that we have common evidence-based strategies for mental health care.
We’ve recently concluded an agreement to reform the way the 2 departments reimburse each other so that the whole issue of billing doesn’t become an encumbrance to delivery of care. We’ve agreed to a common credentialing system so that our providers can more easily serve in either system, which leads to more effective, efficient care and use of our human resources.
Across many lines—the ones I’ve mentioned, and many others—we are ensuring that we can care for the servicemen and women who might become ill or injured and require care going on into the decades to ensure that they have high-quality lives and they’re kept healthy.
The Global Response to Ebola
Dr. Woodson. We have subject matter experts that have worked in infectious diseases for some time. You know, the United States Army Institute of Infectious Diseases is a well-recognized, longstanding organization that has helped produce vaccines and strategies to care for infectious diseases and has contributed very substantially to the biosecurity not only of this nation, but of the world.
We’ve got really the indomitable spirit of the average serviceman and woman who, when given a complex job, know how to meet the mission. And so we have superb leaders, and we have become a key enabler.
When the U.S. shows up, then other countries rally. It provides a platform in which other countries can now commit to the effort. And that’s probably as important as anything else, because this needs to be a worldwide effort to stem this epidemic that is occurring in West Africa.
The Defense Health Agency
The Defense Health Agency has as its mission to put in effect common clinical and business processes to achieve those economies of scale and allow us to use our dollars and other resources more wisely. The Defense Health Agency was charged with initially standing up 10 shared services to include facilities planning, medical logistics, health information technology, managing the TRICARE health plan, pharmacy programs, the Public Health System, acquisitions, budget and resources, management systems, medical education and training, and medical research and development.
As such, we’ve achieved great results over the first year, saving about $248 million. But more importantly, we set the foundation for 21st century systems that will allow us to manage the Military Health System much more effectively, such as establishing a foundation for a common cost accounting system and the development of an enterprise performance management system.
The Military Health System added what we call the Quadruple Aim, which is providing better outcomes, better patient care experience, managing costs, and meeting our readiness mission. We have also established a solid strategic plan and framework in which to ensure that we will meet that end. We’ve had tremendous progress over the first year.
I just want to remind you and everyone of what a heavy lift this was. This is a major reorganization where thousands of people have been reassigned and reorganized to produce a more effective management system. This was no easy lift, but it has been tremendously successful to date.
You don’t necessarily flip the switch and everything is mature and working optimally overnight. The intent was to ensure that it was fully operational and capable by 1 October 2015. What we have seen to date is that it’s ahead of schedule, and that’s good news.
It represents major transformational change. Many people have to be moved. We had to build a new leadership team that, in fact, was heavily invested and contributed to by the uniformed services, the Army, Navy, and Air Force. So they’re deeply invested in the leadership and the governance of the Defense Health Agency.
The Military’s Health Care Challenges
Dr. Woodson. Our central guiding principle is the Quadruple Aim. And at the center, as I mentioned before, is this issue of readiness; and readiness is about ensuring that we have a healthy force to do the nation’s bidding in terms of defense. Not only do we have a healthy force, but we keep them healthy. So we commit to looking at environmental concerns wherever they are deployed. Of course, we provide force protection measures such as vaccines and medicines to prevent infectious diseases, such as malaria if they’re working in a part of the world where that’s endemic.
This is all part of the responsibilities of the military health system. But also, the second part of the readiness responsibility is ensuring that we have a ready medical force. A group of superbly trained providers from the embedded combat medic up to the super subspecialist neurosurgeon, nurses of all specialties and varieties, and other allied health professionals that can create a robust Military Health System and provide above the standard of care anywhere in the world where our sailors, marines, airmen, and soldiers may be operating.
We also have to deal with the issue of chronic problems, health problems that afflict our society. We have started initiatives to address obesity and fitness across a broad spectrum. As a strategy for the military health system, we’re moving from the system of health care, which is just providing treatment after an established disease has occurred, to one of health, which is looking at the whole paradigm of wellness and preventing disease from occurring. It is about reaching into that white space where people learn, work, and play to ensure that they can make healthy choices....We’re deeply invested in the health of the beneficiaries that we serve across a broad spectrum, and we’re deeply invested in the issue of prevention, not only the treatment of disease.
The issue that I would want everyone to understand is that health care and health care delivery in the 21st century is very complex. It’s about not only the actual technology, advances in medical science, but it’s also about addressing where medical science hits human systems and how do you make the system work so that you achieve the best outcomes? And in that mix are the issues of cost and ensuring that you have the ability to deliver that care wherever it’s needed. We’ve mapped out with the leadership of the Military Health System, the surgeons general, and all of their leaders a pathway forward that, in fact, will ensure that the Military Health System will be strong, better, and relevant going into the 21st century and will continue to be a key enabler for the national security, national defense, and the national military strategies.
As a designated Combat Support Agency, the Defense Health Agency is also responsible for meeting the medical needs of the combatant commanders. Central to this role is to ensure our service members are medically ready to perform their mission, and our military medical personnel are ready to perform their mission—“Medically Ready Force…Ready Medical Force.”
Early Intervention Could Lead to Reduction in CKD Cases
Q) When I see a patient for an annual physical or gynecologic exam (or even just to give a flu shot), I try to encourage healthy living. Are there any CKD statistics I can use to “encourage” my hypertensive or overweight patients to follow a better plan of care?
According to a recent study by McMahon et al, risk factors for chronic kidney disease (CKD)—including hypertension, dyslipidemia, and diabetes—may be present up to 30 years prior to diagnosis of CKD.1 Since these risk factors are modifiable, the researchers concluded that early intervention could lead to a reduction in new CKD cases.1
Using data from the Framingham Offspring Study, the researchers identified 441 patients with incident CKD and then matched them with a control group of 882 patients who did not develop CKD during the 30-year study period. Subjects who eventually developed CKD were more likely than their counterparts to have hypertension (odds ratio [OR], 1.76), obesity (OR, 1.71), and elevated triglyceride levels (OR, 1.43) 30 years prior to CKD diagnosis. Having diabetes nearly tripled a patient’s likelihood of developing CKD within 20 years (OR, 2.90).1
Early identification of these risk factors and treatment of affected patients is imperative to help prevent kidney disease. Regular screening of young and middle-aged adult patients, as well as early intervention when risk factors are identified, should slow not only the progression of these detrimental conditions but also the development of CKD.
Joanne Hindlet, ACNP, CNN-NP
Houston Nephrology Group
REFERENCES
1. McMahon GM, Preis SR, Hwang S-J, Fox CS. Mid-adulthood risk factor profiles for CKD. J Am Soc Nephrol. 2014 Jun 26; [Epub ahead of print].
2. Byham-Gray L, Stover J, Wiesen K. A Clinical Guide to Nutrition Care in Kidney Disease. 2nd ed. The Academy of Nutrition and Dietetics; 2013.
3. Crews DC. Chronic kidney disease and access to healthful foods. ASN Kidney News. 2014;6(5):11.
4. Moe SM. Phosphate additives in food: you are what you eat—but shouldn’t you know that? ASN Kidney News. 2014;6(5):8.
5. Narva A, Norton J. Medical nutrition therapy for CKD. ASN Kidney News. 2014;6(5):7.
Q) When I see a patient for an annual physical or gynecologic exam (or even just to give a flu shot), I try to encourage healthy living. Are there any CKD statistics I can use to “encourage” my hypertensive or overweight patients to follow a better plan of care?
According to a recent study by McMahon et al, risk factors for chronic kidney disease (CKD)—including hypertension, dyslipidemia, and diabetes—may be present up to 30 years prior to diagnosis of CKD.1 Since these risk factors are modifiable, the researchers concluded that early intervention could lead to a reduction in new CKD cases.1
Using data from the Framingham Offspring Study, the researchers identified 441 patients with incident CKD and then matched them with a control group of 882 patients who did not develop CKD during the 30-year study period. Subjects who eventually developed CKD were more likely than their counterparts to have hypertension (odds ratio [OR], 1.76), obesity (OR, 1.71), and elevated triglyceride levels (OR, 1.43) 30 years prior to CKD diagnosis. Having diabetes nearly tripled a patient’s likelihood of developing CKD within 20 years (OR, 2.90).1
Early identification of these risk factors and treatment of affected patients is imperative to help prevent kidney disease. Regular screening of young and middle-aged adult patients, as well as early intervention when risk factors are identified, should slow not only the progression of these detrimental conditions but also the development of CKD.
Joanne Hindlet, ACNP, CNN-NP
Houston Nephrology Group
REFERENCES
1. McMahon GM, Preis SR, Hwang S-J, Fox CS. Mid-adulthood risk factor profiles for CKD. J Am Soc Nephrol. 2014 Jun 26; [Epub ahead of print].
2. Byham-Gray L, Stover J, Wiesen K. A Clinical Guide to Nutrition Care in Kidney Disease. 2nd ed. The Academy of Nutrition and Dietetics; 2013.
3. Crews DC. Chronic kidney disease and access to healthful foods. ASN Kidney News. 2014;6(5):11.
4. Moe SM. Phosphate additives in food: you are what you eat—but shouldn’t you know that? ASN Kidney News. 2014;6(5):8.
5. Narva A, Norton J. Medical nutrition therapy for CKD. ASN Kidney News. 2014;6(5):7.
Q) When I see a patient for an annual physical or gynecologic exam (or even just to give a flu shot), I try to encourage healthy living. Are there any CKD statistics I can use to “encourage” my hypertensive or overweight patients to follow a better plan of care?
According to a recent study by McMahon et al, risk factors for chronic kidney disease (CKD)—including hypertension, dyslipidemia, and diabetes—may be present up to 30 years prior to diagnosis of CKD.1 Since these risk factors are modifiable, the researchers concluded that early intervention could lead to a reduction in new CKD cases.1
Using data from the Framingham Offspring Study, the researchers identified 441 patients with incident CKD and then matched them with a control group of 882 patients who did not develop CKD during the 30-year study period. Subjects who eventually developed CKD were more likely than their counterparts to have hypertension (odds ratio [OR], 1.76), obesity (OR, 1.71), and elevated triglyceride levels (OR, 1.43) 30 years prior to CKD diagnosis. Having diabetes nearly tripled a patient’s likelihood of developing CKD within 20 years (OR, 2.90).1
Early identification of these risk factors and treatment of affected patients is imperative to help prevent kidney disease. Regular screening of young and middle-aged adult patients, as well as early intervention when risk factors are identified, should slow not only the progression of these detrimental conditions but also the development of CKD.
Joanne Hindlet, ACNP, CNN-NP
Houston Nephrology Group
REFERENCES
1. McMahon GM, Preis SR, Hwang S-J, Fox CS. Mid-adulthood risk factor profiles for CKD. J Am Soc Nephrol. 2014 Jun 26; [Epub ahead of print].
2. Byham-Gray L, Stover J, Wiesen K. A Clinical Guide to Nutrition Care in Kidney Disease. 2nd ed. The Academy of Nutrition and Dietetics; 2013.
3. Crews DC. Chronic kidney disease and access to healthful foods. ASN Kidney News. 2014;6(5):11.
4. Moe SM. Phosphate additives in food: you are what you eat—but shouldn’t you know that? ASN Kidney News. 2014;6(5):8.
5. Narva A, Norton J. Medical nutrition therapy for CKD. ASN Kidney News. 2014;6(5):7.
CKD: Risk Before, Diet After
Q) In my admitting orders for a CKD patient, I wrote for a “renal diet.” However, the nephrology practitioners changed it to a DASH diet. What is the difference? Why would they not want a “renal diet”?
The answer to this question is: It depends—on the patient, his/her comorbidities, and the need for dialysis treatments (and if so, what type he/she is receiving). Renal diet is a general term used to refer to medical nutrition therapy (MNT) given to a patient with CKD. Each of the five stages of CKD has its own specific MNT requirements.
The MNT for CKD patients often involves modification of the following nutrients: protein, sodium, potassium, phosphorus, and sometimes fluid. The complexity of this therapy often confuses health care professionals when a CKD patient is admitted to the hospital. Let’s examine each nutrient modification to understand optimal nutrition for CKD patients.
Protein. As kidneys fail to excrete urea, protein metabolism is compromised. Thus, in CKD stages 1 and 2, the general recommendations for protein intake are 0.8 to 1.4 g/kg/d. As a patient progresses into CKD stages 3 and 4, these recommendations decrease to 0.6 to 0.8 g/kg/d. In addition, the Kidney Disease Outcomes Quality Initiative (KDOQI) and the Academy of Nutrition and Dietetics recommend that at least 50% of that protein intake be of high biological value (eg, foods of animal origin, soy proteins, dairy, legumes, and nuts and nut butters).2
Why the wide range in protein intake? Needs vary depending on the patient’s comorbidities and nutritional status. Patients with greater need (eg, documented malnutrition, infections, or wounds) will require more protein than those without documented catabolic stress. Additionally, protein needs are based on weight, making it crucial to obtain an accurate weight. When managing a very overweight or underweight patient, an appropriate standard body weight must be calculated. This assessment should be done by a registered dietitian (RD).
Also, renal replacement therapies, once introduced, sharply increase protein needs. Hemodialysis (HD) can account for free amino acid losses of 5 to 20 g per treatment. Peritoneal dialysis (PD) can result in albumin losses of 5 to 15 g/d.2 As a result, protein needs in HD and PD patients are about 1.2 g/kg/d of standard body weight. It has been reported that 30% to 50% of patients are not consuming these amounts, placing them at risk for malnutrition and a higher incidence of morbidity and mortality.2
Sodium. In CKD stages 1 to 4, dietary sodium intake should be less than 2,400 mg/d. The Dietary Approaches to Stop Hypertension (DASH) diet and a Mediterranean diet have been associated with reduced risk for decline in glomerular filtration rate (GFR) and better blood pressure control.3 Both of these diets can be employed, especially in the beginning stages of CKD. But as CKD progresses and urine output declines, recommendations for sodium intake for both HD and PD patients decrease to 2,000 mg/d. In an anuric patient, 2,000 mg/d is the maximum.2
Potassium. As kidney function declines, potassium retention occurs. In CKD stages 1 to 4, potassium restriction is not employed unless the serum level rises above normal.2 The addition of an ACE inhibitor or an angiotensin II receptor blocker to the medication regimen necessitates close monitoring of potassium levels. Potassium allowance for HD varies according to the patient’s urine output and can range from 2 to 4 g/d. PD patients generally can tolerate 3 to 4 g/d of potassium without becoming hyperkalemic, as potassium is well cleared with PD.2
Continue for further examinations >>
Phosphorus. Mineral bone abnormalities begin early in the course of CKD and lead to high-turnover bone disease, adynamic bone disease, fractures, and soft-tissue calcification. Careful monitoring of calcium, intact parathyroid hormone, and phosphorus levels is required throughout all stages of CKD, with hyperphosphatemia of particular concern.
In CKD stages 1 and 2, dietary phosphorus should be limited to maintain a normal serum level.2 As CKD progresses and phosphorus retention increases, 800 to 1,000 mg/d or 10 to 12 mg of phosphorus per gram of protein should be prescribed.
Even with the limitation of dietary phosphorus, phosphate-binding medications may be required to control serum phosphorus in later CKD stages and in HD and PD patients.2 Limiting dietary phosphorus can be difficult for patients because of inorganic phosphate salt additives widely found in canned and processed foods; they are also added to dark colas and to meats and poultry to act as preservatives and improve flavor and texture. Phosphorus additives are 100% bioavailable and therefore more readily absorbed than organic phosphorus.4
Fluid. Lastly, CKD patients need to think about their fluid intake. HD patients with a urine output of > 1,000 mL/24-h period will be allowed up to 2,000 mL/d of fluid. (A 12-oz canned drink is 355 mL.) Those with less than 1,000 mL of urine output will be allowed 1,000 to 1,500 mL/d, with anuric patients capped at 1,000 mL/d. PD patients are allowed 1,000 to 3,000 mL/d depending on urine output and overall status.2 Patients should also be reminded that foods such as soup and gelatin are counted in their fluid allowance.
The complexities of the “renal diet” make patient education by an RD critical. However, a recent article suggested that MNT for CKD patients is underutilized, with limited referrals and lack of education for primary care providers and RDs cited as reasons.5 This is mystifying considering that Medicare will pay for RD services for CKD patients.
The National Kidney Disease Education Program, in association with the Academy of Nutrition and Dietetics, has developed free professional and patient education materials to address this need; they are available at http://nkdep.nih.gov/.
Luanne DiGuglielmo, MS, RD, CSR
DaVita Summit Renal Center
Mountainside, New Jersey
REFERENCES
1. McMahon GM, Preis SR, Hwang S-J, Fox CS. Mid-adulthood risk factor profiles for CKD. J Am Soc Nephrol. 2014 Jun 26; [Epub ahead of print].
2. Byham-Gray L, Stover J, Wiesen K. A Clinical Guide to Nutrition Care in Kidney Disease. 2nd ed. The Academy of Nutrition and Dietetics; 2013.
3. Crews DC. Chronic kidney disease and access to healthful foods. ASN Kidney News. 2014;6(5):11.
4. Moe SM. Phosphate additives in food: you are what you eat—but shouldn’t you know that? ASN Kidney News. 2014;6(5):8.
5. Narva A, Norton J. Medical nutrition therapy for CKD. ASN Kidney News. 2014;6(5):7.
Q) In my admitting orders for a CKD patient, I wrote for a “renal diet.” However, the nephrology practitioners changed it to a DASH diet. What is the difference? Why would they not want a “renal diet”?
The answer to this question is: It depends—on the patient, his/her comorbidities, and the need for dialysis treatments (and if so, what type he/she is receiving). Renal diet is a general term used to refer to medical nutrition therapy (MNT) given to a patient with CKD. Each of the five stages of CKD has its own specific MNT requirements.
The MNT for CKD patients often involves modification of the following nutrients: protein, sodium, potassium, phosphorus, and sometimes fluid. The complexity of this therapy often confuses health care professionals when a CKD patient is admitted to the hospital. Let’s examine each nutrient modification to understand optimal nutrition for CKD patients.
Protein. As kidneys fail to excrete urea, protein metabolism is compromised. Thus, in CKD stages 1 and 2, the general recommendations for protein intake are 0.8 to 1.4 g/kg/d. As a patient progresses into CKD stages 3 and 4, these recommendations decrease to 0.6 to 0.8 g/kg/d. In addition, the Kidney Disease Outcomes Quality Initiative (KDOQI) and the Academy of Nutrition and Dietetics recommend that at least 50% of that protein intake be of high biological value (eg, foods of animal origin, soy proteins, dairy, legumes, and nuts and nut butters).2
Why the wide range in protein intake? Needs vary depending on the patient’s comorbidities and nutritional status. Patients with greater need (eg, documented malnutrition, infections, or wounds) will require more protein than those without documented catabolic stress. Additionally, protein needs are based on weight, making it crucial to obtain an accurate weight. When managing a very overweight or underweight patient, an appropriate standard body weight must be calculated. This assessment should be done by a registered dietitian (RD).
Also, renal replacement therapies, once introduced, sharply increase protein needs. Hemodialysis (HD) can account for free amino acid losses of 5 to 20 g per treatment. Peritoneal dialysis (PD) can result in albumin losses of 5 to 15 g/d.2 As a result, protein needs in HD and PD patients are about 1.2 g/kg/d of standard body weight. It has been reported that 30% to 50% of patients are not consuming these amounts, placing them at risk for malnutrition and a higher incidence of morbidity and mortality.2
Sodium. In CKD stages 1 to 4, dietary sodium intake should be less than 2,400 mg/d. The Dietary Approaches to Stop Hypertension (DASH) diet and a Mediterranean diet have been associated with reduced risk for decline in glomerular filtration rate (GFR) and better blood pressure control.3 Both of these diets can be employed, especially in the beginning stages of CKD. But as CKD progresses and urine output declines, recommendations for sodium intake for both HD and PD patients decrease to 2,000 mg/d. In an anuric patient, 2,000 mg/d is the maximum.2
Potassium. As kidney function declines, potassium retention occurs. In CKD stages 1 to 4, potassium restriction is not employed unless the serum level rises above normal.2 The addition of an ACE inhibitor or an angiotensin II receptor blocker to the medication regimen necessitates close monitoring of potassium levels. Potassium allowance for HD varies according to the patient’s urine output and can range from 2 to 4 g/d. PD patients generally can tolerate 3 to 4 g/d of potassium without becoming hyperkalemic, as potassium is well cleared with PD.2
Continue for further examinations >>
Phosphorus. Mineral bone abnormalities begin early in the course of CKD and lead to high-turnover bone disease, adynamic bone disease, fractures, and soft-tissue calcification. Careful monitoring of calcium, intact parathyroid hormone, and phosphorus levels is required throughout all stages of CKD, with hyperphosphatemia of particular concern.
In CKD stages 1 and 2, dietary phosphorus should be limited to maintain a normal serum level.2 As CKD progresses and phosphorus retention increases, 800 to 1,000 mg/d or 10 to 12 mg of phosphorus per gram of protein should be prescribed.
Even with the limitation of dietary phosphorus, phosphate-binding medications may be required to control serum phosphorus in later CKD stages and in HD and PD patients.2 Limiting dietary phosphorus can be difficult for patients because of inorganic phosphate salt additives widely found in canned and processed foods; they are also added to dark colas and to meats and poultry to act as preservatives and improve flavor and texture. Phosphorus additives are 100% bioavailable and therefore more readily absorbed than organic phosphorus.4
Fluid. Lastly, CKD patients need to think about their fluid intake. HD patients with a urine output of > 1,000 mL/24-h period will be allowed up to 2,000 mL/d of fluid. (A 12-oz canned drink is 355 mL.) Those with less than 1,000 mL of urine output will be allowed 1,000 to 1,500 mL/d, with anuric patients capped at 1,000 mL/d. PD patients are allowed 1,000 to 3,000 mL/d depending on urine output and overall status.2 Patients should also be reminded that foods such as soup and gelatin are counted in their fluid allowance.
The complexities of the “renal diet” make patient education by an RD critical. However, a recent article suggested that MNT for CKD patients is underutilized, with limited referrals and lack of education for primary care providers and RDs cited as reasons.5 This is mystifying considering that Medicare will pay for RD services for CKD patients.
The National Kidney Disease Education Program, in association with the Academy of Nutrition and Dietetics, has developed free professional and patient education materials to address this need; they are available at http://nkdep.nih.gov/.
Luanne DiGuglielmo, MS, RD, CSR
DaVita Summit Renal Center
Mountainside, New Jersey
REFERENCES
1. McMahon GM, Preis SR, Hwang S-J, Fox CS. Mid-adulthood risk factor profiles for CKD. J Am Soc Nephrol. 2014 Jun 26; [Epub ahead of print].
2. Byham-Gray L, Stover J, Wiesen K. A Clinical Guide to Nutrition Care in Kidney Disease. 2nd ed. The Academy of Nutrition and Dietetics; 2013.
3. Crews DC. Chronic kidney disease and access to healthful foods. ASN Kidney News. 2014;6(5):11.
4. Moe SM. Phosphate additives in food: you are what you eat—but shouldn’t you know that? ASN Kidney News. 2014;6(5):8.
5. Narva A, Norton J. Medical nutrition therapy for CKD. ASN Kidney News. 2014;6(5):7.
Q) In my admitting orders for a CKD patient, I wrote for a “renal diet.” However, the nephrology practitioners changed it to a DASH diet. What is the difference? Why would they not want a “renal diet”?
The answer to this question is: It depends—on the patient, his/her comorbidities, and the need for dialysis treatments (and if so, what type he/she is receiving). Renal diet is a general term used to refer to medical nutrition therapy (MNT) given to a patient with CKD. Each of the five stages of CKD has its own specific MNT requirements.
The MNT for CKD patients often involves modification of the following nutrients: protein, sodium, potassium, phosphorus, and sometimes fluid. The complexity of this therapy often confuses health care professionals when a CKD patient is admitted to the hospital. Let’s examine each nutrient modification to understand optimal nutrition for CKD patients.
Protein. As kidneys fail to excrete urea, protein metabolism is compromised. Thus, in CKD stages 1 and 2, the general recommendations for protein intake are 0.8 to 1.4 g/kg/d. As a patient progresses into CKD stages 3 and 4, these recommendations decrease to 0.6 to 0.8 g/kg/d. In addition, the Kidney Disease Outcomes Quality Initiative (KDOQI) and the Academy of Nutrition and Dietetics recommend that at least 50% of that protein intake be of high biological value (eg, foods of animal origin, soy proteins, dairy, legumes, and nuts and nut butters).2
Why the wide range in protein intake? Needs vary depending on the patient’s comorbidities and nutritional status. Patients with greater need (eg, documented malnutrition, infections, or wounds) will require more protein than those without documented catabolic stress. Additionally, protein needs are based on weight, making it crucial to obtain an accurate weight. When managing a very overweight or underweight patient, an appropriate standard body weight must be calculated. This assessment should be done by a registered dietitian (RD).
Also, renal replacement therapies, once introduced, sharply increase protein needs. Hemodialysis (HD) can account for free amino acid losses of 5 to 20 g per treatment. Peritoneal dialysis (PD) can result in albumin losses of 5 to 15 g/d.2 As a result, protein needs in HD and PD patients are about 1.2 g/kg/d of standard body weight. It has been reported that 30% to 50% of patients are not consuming these amounts, placing them at risk for malnutrition and a higher incidence of morbidity and mortality.2
Sodium. In CKD stages 1 to 4, dietary sodium intake should be less than 2,400 mg/d. The Dietary Approaches to Stop Hypertension (DASH) diet and a Mediterranean diet have been associated with reduced risk for decline in glomerular filtration rate (GFR) and better blood pressure control.3 Both of these diets can be employed, especially in the beginning stages of CKD. But as CKD progresses and urine output declines, recommendations for sodium intake for both HD and PD patients decrease to 2,000 mg/d. In an anuric patient, 2,000 mg/d is the maximum.2
Potassium. As kidney function declines, potassium retention occurs. In CKD stages 1 to 4, potassium restriction is not employed unless the serum level rises above normal.2 The addition of an ACE inhibitor or an angiotensin II receptor blocker to the medication regimen necessitates close monitoring of potassium levels. Potassium allowance for HD varies according to the patient’s urine output and can range from 2 to 4 g/d. PD patients generally can tolerate 3 to 4 g/d of potassium without becoming hyperkalemic, as potassium is well cleared with PD.2
Continue for further examinations >>
Phosphorus. Mineral bone abnormalities begin early in the course of CKD and lead to high-turnover bone disease, adynamic bone disease, fractures, and soft-tissue calcification. Careful monitoring of calcium, intact parathyroid hormone, and phosphorus levels is required throughout all stages of CKD, with hyperphosphatemia of particular concern.
In CKD stages 1 and 2, dietary phosphorus should be limited to maintain a normal serum level.2 As CKD progresses and phosphorus retention increases, 800 to 1,000 mg/d or 10 to 12 mg of phosphorus per gram of protein should be prescribed.
Even with the limitation of dietary phosphorus, phosphate-binding medications may be required to control serum phosphorus in later CKD stages and in HD and PD patients.2 Limiting dietary phosphorus can be difficult for patients because of inorganic phosphate salt additives widely found in canned and processed foods; they are also added to dark colas and to meats and poultry to act as preservatives and improve flavor and texture. Phosphorus additives are 100% bioavailable and therefore more readily absorbed than organic phosphorus.4
Fluid. Lastly, CKD patients need to think about their fluid intake. HD patients with a urine output of > 1,000 mL/24-h period will be allowed up to 2,000 mL/d of fluid. (A 12-oz canned drink is 355 mL.) Those with less than 1,000 mL of urine output will be allowed 1,000 to 1,500 mL/d, with anuric patients capped at 1,000 mL/d. PD patients are allowed 1,000 to 3,000 mL/d depending on urine output and overall status.2 Patients should also be reminded that foods such as soup and gelatin are counted in their fluid allowance.
The complexities of the “renal diet” make patient education by an RD critical. However, a recent article suggested that MNT for CKD patients is underutilized, with limited referrals and lack of education for primary care providers and RDs cited as reasons.5 This is mystifying considering that Medicare will pay for RD services for CKD patients.
The National Kidney Disease Education Program, in association with the Academy of Nutrition and Dietetics, has developed free professional and patient education materials to address this need; they are available at http://nkdep.nih.gov/.
Luanne DiGuglielmo, MS, RD, CSR
DaVita Summit Renal Center
Mountainside, New Jersey
REFERENCES
1. McMahon GM, Preis SR, Hwang S-J, Fox CS. Mid-adulthood risk factor profiles for CKD. J Am Soc Nephrol. 2014 Jun 26; [Epub ahead of print].
2. Byham-Gray L, Stover J, Wiesen K. A Clinical Guide to Nutrition Care in Kidney Disease. 2nd ed. The Academy of Nutrition and Dietetics; 2013.
3. Crews DC. Chronic kidney disease and access to healthful foods. ASN Kidney News. 2014;6(5):11.
4. Moe SM. Phosphate additives in food: you are what you eat—but shouldn’t you know that? ASN Kidney News. 2014;6(5):8.
5. Narva A, Norton J. Medical nutrition therapy for CKD. ASN Kidney News. 2014;6(5):7.
Noninsulinoma Pancreatogenous Hypoglycemia Syndrome Following Gastric Bypass Surgery
A 28-year-old white woman, KR, presents to primary care with episodic diaphoresis and weakness that occur one to two hours after meals. There is no history of syncope or seizures. The hypoglycemic symptoms abate with intake of oral glucose and do not occur when the patient fasts.
KR underwent Roux-en-Y gastric bypass surgery 12 months ago. At the time, her body weight was 250 lbs and her height, 62 in (BMI, 46). She has lost 60 lbs since surgery (current BMI, 35). KR has no comorbid medical conditions. She denies use of insulin injection or oral hypoglycemic medication, as well as alcohol consumption. There is no history of diarrhea or abdominal pain. Her only medication is a daily multivitamin.
Physical exam reveals a blood pressure of 126/80 mm Hg; pulse, 82 beats/min; respiratory rate, 16 breaths/min; and O2 saturation, 98%. Heart rate is regular with no murmur. Lungs are clear to auscultation. Abdominal and neurologic exams are unremarkable; musculoskeletal strength and orthostatic vital signs are normal.
The patient is instructed to test her blood sugar with a glucometer and return to the clinic in two weeks. Fingerstick monitoring reveals that her serum glucose level drops into the 40 to 50 mg/dL range approximately one to two hours after meals containing > 45 g of carbohydrate. Her fasting serum glucose readings are in the 80 to 95 mg/dL range.
The patient is presumptively diagnosed with dumping syndrome and receives nutritional counseling; she is instructed to reduce intake of simple carbohydrates and increase the protein content of meals. Despite these dietary modifications, the episodes of hypoglycemia persist.
The patient is then referred to endocrinology. Fasting labwork reveals a serum glucose level of 85 mg/dL; normal adrenocorticotropic hormone (ACTH) and cortisol levels; C-peptide level, 2.46 ng/mL (reference range, 0.80–4.00 ng/mL); and insulin level, 6.4 mIU/mL (reference range 2.6–24.9 mIU/mL). A 75-g two-hour oral glucose tolerance test (OGTT) reveals peak serum glucose of 180 mg/dL at 30 minutes followed by a nadir serum glucose of 48 mg/dL at 110 minutes, accompanied by hypoglycemic symptoms. The insulin and C-peptide levels are elevated during the entire two-hour test. The serum cortisol level is 22 mg/dL when the glucose level is 48 mg/dL. CT of the abdomen, previously ordered by the patient’s primary care provider, was unremarkable.
Since there is no laboratory evidence of fasting hypoglycemia and no pancreatic abnormalities are seen on imaging studies, the possibility of insulinoma is excluded from the differential diagnosis. Adrenal insufficiency is excluded based on the normal ACTH and cortisol levels. The possibility of noninsulinoma pancreatogenous hypoglycemia syndrome is considered.
The patient is prescribed verapamil ER 100 mg/d and notes significant reduction in the frequency of hypoglycemic episodes and symptoms. She is scheduled for follow-up in four weeks to assess for any changes in the frequency or severity of her hypoglycemic episodes.
BACKGROUND
Postprandial hypoglycemia is a rare but potentially serious complication of bariatric surgery procedures that divert nutrients into the small bowel.1,2 The Bariatric Outcomes Longitudinal Database revealed a 0.1% incidence of hypoglycemia in patients who underwent Roux-en-Y gastric bypass surgery.3
The most common cause of hypoglycemia following gastric bypass surgery is dumping syndrome, which involves rapid emptying of gastric contents with reactive hypoglycemia due to increased postprandial insulin release. In dumping syndrome, hypoglycemic symptoms—flushing, diaphoresis, weakness, and dizziness—typically occur within two to three hours after meals; patients do not experience the more severe symptoms of neuroglycopenia (eg, cognitive impairment, seizures, and loss of consciousness).4 The symptoms of dumping syndrome typically improve with reduced intake of simple carbohydrates and increased protein consumption.1
Other causes of postprandial hypoglycemia include insulinoma and noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS). Although both diagnoses are rare, they should be considered if no improvement in hypoglycemic symptoms occurs after dietary modification.1
Insulinoma is the most common cause of persistent hyperinsulinemic hypoglycemia. It is defined by Whipple’s triad: symptomatic hypoglycemia during fasting, a serum glucose level > 50 mg/dL at the time of symptom onset, and relief of symptoms after administration of glucose.5
NIPHS is less common than insulinoma. It is characterized by postprandial hypoglycemia due to increased insulin secretion resulting from pancreatic b-cell hyperplasia. Hypoglycemia does not typically occur during a 72-hour fast. In addition, pancreatic imaging studies yield normal results in cases of NIPHS. The selective arterial calcium stimulation test is positive in NIPHS.5 NIPHS is definitively diagnosed by histopathologic examination of the pancreas, which reveals nesidioblastosis.6
Nesidioblastosis involves pathologic b-cell overgrowth in the pancreas that results in excess insulin secretion.4 Nesidioblastosis is characterized by pancreatic b-cell hypertrophy, islet hyperplasia, and increased b-cell mass.2
Nesidioblastosis is the leading cause of hyperinsulinemia in newborns and infants (annual incidence, 1 in 50,000 births) but is quite rare in adults, occurring in 0.5% to 7.0% of all those with hyperinsulinism.7,8 Islet cell hypertrophy—characteristic of nesidioblastosis—is seen in both adults and children, whereas genetic mutations are present only in infants.7
Although rare in adults, nesidioblastosis is more common in the setting of gastric bypass than in the general population.7 As of 2011, there have been 40 cases of nesidioblastosis in adults who received gastric bypass.2 With the rapid increase in the number of these surgeries performed each year, nesidioblastosis should be considered in the differential diagnosis for patients who experience hypoglycemia following the procedure.2,7
Continue for hormonal mechanisms >>
HORMONAL MECHANISMS
There are multiple theories regarding the etiology of b-cell hyperplasia following bariatric surgery. The specific causes for NIPHS after gastric bypass remain under investigation.2
The most common theory is that b-cell hyperplasia may occur as a result of the surgical procedure itself and not due to obesity. The rapid delivery of food to the distal ileum after gastric bypass surgery may result in elevated production of incretin hormones (eg, GLP-1 and GIP), which increase b-cell proliferation, insulin secretion, and insulin sensitivity.7
Roux-en-Y gastric bypass also impairs ghrelin secretion. Ghrelin normally acts to suppress insulin secretion and directly opposes the action of insulin. Reduced levels of ghrelin may increase the likelihood of hypoglycemia. Other hormones that may contribute to the metabolic effects of bariatric surgery include peptide YY, oxyntomodulin, and others as yet unidentified.5,6
CLINICAL MANIFESTATIONS
NIPHS is characterized by moderate to severe postprandial hypoglycemia. Symptoms include confusion, diaphoresis, tremulousness, anxiety, weakness, blurred vision, and disorientation, as well as more severe neuroglycopenic symptoms, such as cognitive impairment, seizures, and loss of consciousness.5
These symptoms do not typically manifest until several months after gastric bypass surgery. (By contrast, symptoms experienced with dumping syndrome typically manifest shortly after the procedure.) Of note, hypoglycemic symptoms of NIPHS do not typically improve after dietary modifications aimed at reducing carbohydrate intake.2
DIAGNOSIS
Diagnosis of NIPHS is based on hypoglycemic/neuroglycopenic signs and symptoms without fasting hypoglycemia; endogenous hyperinsulinemia in the presence of hypoglycemia; negative localization studies for insulinoma (using triple-phase spiral CT); and positive selective arterial calcium stimulation test.4,6
If fasting hypoglycemia is reported or suspected, the patient should be evaluated for insulinoma using a 72-hour fast. During it, glucose, insulin, C-peptide, and pro-insulin levels should be tested every six hours; results will be normal in patients with NIPHS.5
The use of OGTT is controversial, as patients can experience variable degrees of postprandial hyperinsulinism and symptomatic hypoglycemia during the test. There are no guidelines on whether to perform OGTT in the work-up for NIPHS. In research protocols, it is common to perform a five-hour OGTT; subjects consume a mixed meal containing 50 g of carbohydrates, then their glucose, insulin, and C-peptide levels are tested every 30 to 60 minutes (or sooner if hypoglycemic symptoms occur).
Elevated insulin and C-peptide levels in the setting of hypoglycemia are characteristic findings in patients with NIPHS.5,9 In the setting of hypoglycemia, a cortisol level > 20 mg/dL is considered an appropriate adrenal response and excludes adrenal insufficiency. Triple-phase CT of the abdomen should be performed to rule out insulinoma if strongly suspected and if work-up for NIPHS is negative.5
The selective arterial calcium stimulation test is employed to confirm the diagnosis of NIPHS and to guide the extent of pancreatic resection, in an effort to minimize postoperative complications of insulin-dependent diabetes and exocrine insufficiency. In this procedure, the splenic, gastroduodenal, superior mesenteric, and hepatic arteries that supply the pancreas are selectively injected with calcium gluconate. After injection of calcium, the insulin level is measured within each artery.4,5,7 The selective arterial calcium stimulation test can also be used to localize an insulinoma. NIPHS is distinguished from insulinoma by a diffuse increase in insulin secreted from multiple segments of the arteries that supply the pancreas, following calcium stimulation.4,5,7
Continue for treatment >>
TREATMENT
There is no consensus on treatment of NIPHS in postbariatric surgery patients, and no “gold standard” exists. Pharmacologic treatment is recommended prior to surgical intervention in patients who present with symptomatic hypoglycemia without loss of consciousness or seizures.1
Pharmacologic treatments include calcium channel blockers (eg, verapamil or nifedipine), the b-cell inhibitor diazoxide, the secretory inhibitor octreotide, and a-glucosidase inhibitors.1 In one hospital group, patients were initially treated with verapamil ER 100 mg/d.5 If patients did not respond to this therapy or developed adverse effects, diazoxide was added (starting dose, 25 mg tid, titrated to 75 mg tid).5 If this combination did not produce results, octreotide (dose ranging from 25 mg/d to 50 mg tid, subcutaneously) was added. Acarbose can also be added, with the typical starting dose of 50 mg tid.1
Distal or subtotal pancreatectomy to debulk the hypertrophic islets is the most common surgical method used in patients with severe hypoglycemia that is refractory to medical management.2,5 The extent of pancreatic resection is guided by calcium angiography and typically ranges from 80% to 95%.7 Smaller pancreatic resection is associated with higher risk for persistent postoperative hypoglycemia.5 Complications associated with pancreatectomy include insulin-dependent diabetes and exocrine insufficiency.5
It is not uncommon for patients to experience recurrent symptoms after subtotal pancreatectomy, but the symptoms are typically easier to manage pharmacologically than they were pre-operatively. Occasionally, a second surgery with 95% to complete pancreatectomy is employed if recurrent hypoglycemia develops that is refractory to medical management.5
Reversal of Roux-en-Y bypass surgery has been described as an attempted treatment method in several case reports of patients with NIPHS. In at least one patient, hyperinsulinemic hypoglycemia persisted after Roux-en-Y gastric bypass reversal.2 Adjustable gastric band placement was recently reported to reverse hypoglycemic symptoms and maintain weight loss, due to restricted gastric emptying.2 Conversion of Roux-en-Y gastric banding to gastric sleeve may also be employed to restore normal gastrointestinal continuity and resolve hypoglycemia, though limited data is available regarding the efficacy of this procedure.2
Close monitoring is necessary in patients treated with pharmacologic therapy to ensure that symptoms are well controlled and that surgery is not necessary.1
SUMMARY AND CONCLUSION
Symptomatic hypoglycemia is a potential complication associated with gastric bypass surgery and is most commonly caused by dumping syndrome. It is important to consider other causes of postprandial hypoglycemia, such as insulinoma and NIPHS, in patients who continue to experience hypoglycemia despite making dietary modifications.1,4
NIPHS is a rare and poorly understood complication of gastric bypass surgery involving pathologic b-cell overgrowth, leading to hyperinsulinemia and potentially severe hypoglycemia.6 Some patients may present with complete relief of symptoms with pharmacologic treatment, while others will need surgical treatment with subtotal pancreatectomy.1
The findings of increased levels of GLP-1 hormone in patients who have received gastric bypass surgery and the fact that only a very small subset of gastric bypass patients develop NIPHS with histologic features of nesidioblastosis are subjects for further research. Further understanding of the hormonal factors involved in the pathogenesis of NIPHS and adult-onset nesidioblastosis following gastric bypass surgery could lead to novel drug development to treat diabetes.6
REFERENCES
1. Moreira RO, Moreira RBM, Machado NAM, et al. Post-prandial hypoglycemia after bariatric surgery: pharmacological treatment with verapamil and acarbose. Obes Surg. 2008;18:1618-1621.
2. Cui Y, Elahi D, Andersen D. Advances in the etiology and management of hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass. J Gastrointest Surg. 2011;15:1879-1888.
3. Sarwar H, Chapman III WH, Pender JR, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg. 2014; 24(7):1120-1124.
4. Service GJ, Thompson GB, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249-254.
5. Mathavan VK, Arregui M, Davis C, et al. Management of postgastric bypass noninsulinoma pancreatogenous hypoglycemia. Surg Endosc. 2010;24:2547-2555.
6. Cummings D. Gastric bypass and nesidioblastosis—too much of a good thing for islets? N Engl J Med. 2005;353(3):300-302.
7. Clancy TE, Moore FD, Zinner MJ. Post-gastric bypass hyperinsulinism with nesidioblastosis: subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J Gastrointest Surg. 2006;10(8):1116-1119.
8. Kaczirek K, Niederle B. Nesidioblastosis: an old term and a new understanding. World J Surg. 2004;28:1227-1230.
9. Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6): 2008-2017.
A 28-year-old white woman, KR, presents to primary care with episodic diaphoresis and weakness that occur one to two hours after meals. There is no history of syncope or seizures. The hypoglycemic symptoms abate with intake of oral glucose and do not occur when the patient fasts.
KR underwent Roux-en-Y gastric bypass surgery 12 months ago. At the time, her body weight was 250 lbs and her height, 62 in (BMI, 46). She has lost 60 lbs since surgery (current BMI, 35). KR has no comorbid medical conditions. She denies use of insulin injection or oral hypoglycemic medication, as well as alcohol consumption. There is no history of diarrhea or abdominal pain. Her only medication is a daily multivitamin.
Physical exam reveals a blood pressure of 126/80 mm Hg; pulse, 82 beats/min; respiratory rate, 16 breaths/min; and O2 saturation, 98%. Heart rate is regular with no murmur. Lungs are clear to auscultation. Abdominal and neurologic exams are unremarkable; musculoskeletal strength and orthostatic vital signs are normal.
The patient is instructed to test her blood sugar with a glucometer and return to the clinic in two weeks. Fingerstick monitoring reveals that her serum glucose level drops into the 40 to 50 mg/dL range approximately one to two hours after meals containing > 45 g of carbohydrate. Her fasting serum glucose readings are in the 80 to 95 mg/dL range.
The patient is presumptively diagnosed with dumping syndrome and receives nutritional counseling; she is instructed to reduce intake of simple carbohydrates and increase the protein content of meals. Despite these dietary modifications, the episodes of hypoglycemia persist.
The patient is then referred to endocrinology. Fasting labwork reveals a serum glucose level of 85 mg/dL; normal adrenocorticotropic hormone (ACTH) and cortisol levels; C-peptide level, 2.46 ng/mL (reference range, 0.80–4.00 ng/mL); and insulin level, 6.4 mIU/mL (reference range 2.6–24.9 mIU/mL). A 75-g two-hour oral glucose tolerance test (OGTT) reveals peak serum glucose of 180 mg/dL at 30 minutes followed by a nadir serum glucose of 48 mg/dL at 110 minutes, accompanied by hypoglycemic symptoms. The insulin and C-peptide levels are elevated during the entire two-hour test. The serum cortisol level is 22 mg/dL when the glucose level is 48 mg/dL. CT of the abdomen, previously ordered by the patient’s primary care provider, was unremarkable.
Since there is no laboratory evidence of fasting hypoglycemia and no pancreatic abnormalities are seen on imaging studies, the possibility of insulinoma is excluded from the differential diagnosis. Adrenal insufficiency is excluded based on the normal ACTH and cortisol levels. The possibility of noninsulinoma pancreatogenous hypoglycemia syndrome is considered.
The patient is prescribed verapamil ER 100 mg/d and notes significant reduction in the frequency of hypoglycemic episodes and symptoms. She is scheduled for follow-up in four weeks to assess for any changes in the frequency or severity of her hypoglycemic episodes.
BACKGROUND
Postprandial hypoglycemia is a rare but potentially serious complication of bariatric surgery procedures that divert nutrients into the small bowel.1,2 The Bariatric Outcomes Longitudinal Database revealed a 0.1% incidence of hypoglycemia in patients who underwent Roux-en-Y gastric bypass surgery.3
The most common cause of hypoglycemia following gastric bypass surgery is dumping syndrome, which involves rapid emptying of gastric contents with reactive hypoglycemia due to increased postprandial insulin release. In dumping syndrome, hypoglycemic symptoms—flushing, diaphoresis, weakness, and dizziness—typically occur within two to three hours after meals; patients do not experience the more severe symptoms of neuroglycopenia (eg, cognitive impairment, seizures, and loss of consciousness).4 The symptoms of dumping syndrome typically improve with reduced intake of simple carbohydrates and increased protein consumption.1
Other causes of postprandial hypoglycemia include insulinoma and noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS). Although both diagnoses are rare, they should be considered if no improvement in hypoglycemic symptoms occurs after dietary modification.1
Insulinoma is the most common cause of persistent hyperinsulinemic hypoglycemia. It is defined by Whipple’s triad: symptomatic hypoglycemia during fasting, a serum glucose level > 50 mg/dL at the time of symptom onset, and relief of symptoms after administration of glucose.5
NIPHS is less common than insulinoma. It is characterized by postprandial hypoglycemia due to increased insulin secretion resulting from pancreatic b-cell hyperplasia. Hypoglycemia does not typically occur during a 72-hour fast. In addition, pancreatic imaging studies yield normal results in cases of NIPHS. The selective arterial calcium stimulation test is positive in NIPHS.5 NIPHS is definitively diagnosed by histopathologic examination of the pancreas, which reveals nesidioblastosis.6
Nesidioblastosis involves pathologic b-cell overgrowth in the pancreas that results in excess insulin secretion.4 Nesidioblastosis is characterized by pancreatic b-cell hypertrophy, islet hyperplasia, and increased b-cell mass.2
Nesidioblastosis is the leading cause of hyperinsulinemia in newborns and infants (annual incidence, 1 in 50,000 births) but is quite rare in adults, occurring in 0.5% to 7.0% of all those with hyperinsulinism.7,8 Islet cell hypertrophy—characteristic of nesidioblastosis—is seen in both adults and children, whereas genetic mutations are present only in infants.7
Although rare in adults, nesidioblastosis is more common in the setting of gastric bypass than in the general population.7 As of 2011, there have been 40 cases of nesidioblastosis in adults who received gastric bypass.2 With the rapid increase in the number of these surgeries performed each year, nesidioblastosis should be considered in the differential diagnosis for patients who experience hypoglycemia following the procedure.2,7
Continue for hormonal mechanisms >>
HORMONAL MECHANISMS
There are multiple theories regarding the etiology of b-cell hyperplasia following bariatric surgery. The specific causes for NIPHS after gastric bypass remain under investigation.2
The most common theory is that b-cell hyperplasia may occur as a result of the surgical procedure itself and not due to obesity. The rapid delivery of food to the distal ileum after gastric bypass surgery may result in elevated production of incretin hormones (eg, GLP-1 and GIP), which increase b-cell proliferation, insulin secretion, and insulin sensitivity.7
Roux-en-Y gastric bypass also impairs ghrelin secretion. Ghrelin normally acts to suppress insulin secretion and directly opposes the action of insulin. Reduced levels of ghrelin may increase the likelihood of hypoglycemia. Other hormones that may contribute to the metabolic effects of bariatric surgery include peptide YY, oxyntomodulin, and others as yet unidentified.5,6
CLINICAL MANIFESTATIONS
NIPHS is characterized by moderate to severe postprandial hypoglycemia. Symptoms include confusion, diaphoresis, tremulousness, anxiety, weakness, blurred vision, and disorientation, as well as more severe neuroglycopenic symptoms, such as cognitive impairment, seizures, and loss of consciousness.5
These symptoms do not typically manifest until several months after gastric bypass surgery. (By contrast, symptoms experienced with dumping syndrome typically manifest shortly after the procedure.) Of note, hypoglycemic symptoms of NIPHS do not typically improve after dietary modifications aimed at reducing carbohydrate intake.2
DIAGNOSIS
Diagnosis of NIPHS is based on hypoglycemic/neuroglycopenic signs and symptoms without fasting hypoglycemia; endogenous hyperinsulinemia in the presence of hypoglycemia; negative localization studies for insulinoma (using triple-phase spiral CT); and positive selective arterial calcium stimulation test.4,6
If fasting hypoglycemia is reported or suspected, the patient should be evaluated for insulinoma using a 72-hour fast. During it, glucose, insulin, C-peptide, and pro-insulin levels should be tested every six hours; results will be normal in patients with NIPHS.5
The use of OGTT is controversial, as patients can experience variable degrees of postprandial hyperinsulinism and symptomatic hypoglycemia during the test. There are no guidelines on whether to perform OGTT in the work-up for NIPHS. In research protocols, it is common to perform a five-hour OGTT; subjects consume a mixed meal containing 50 g of carbohydrates, then their glucose, insulin, and C-peptide levels are tested every 30 to 60 minutes (or sooner if hypoglycemic symptoms occur).
Elevated insulin and C-peptide levels in the setting of hypoglycemia are characteristic findings in patients with NIPHS.5,9 In the setting of hypoglycemia, a cortisol level > 20 mg/dL is considered an appropriate adrenal response and excludes adrenal insufficiency. Triple-phase CT of the abdomen should be performed to rule out insulinoma if strongly suspected and if work-up for NIPHS is negative.5
The selective arterial calcium stimulation test is employed to confirm the diagnosis of NIPHS and to guide the extent of pancreatic resection, in an effort to minimize postoperative complications of insulin-dependent diabetes and exocrine insufficiency. In this procedure, the splenic, gastroduodenal, superior mesenteric, and hepatic arteries that supply the pancreas are selectively injected with calcium gluconate. After injection of calcium, the insulin level is measured within each artery.4,5,7 The selective arterial calcium stimulation test can also be used to localize an insulinoma. NIPHS is distinguished from insulinoma by a diffuse increase in insulin secreted from multiple segments of the arteries that supply the pancreas, following calcium stimulation.4,5,7
Continue for treatment >>
TREATMENT
There is no consensus on treatment of NIPHS in postbariatric surgery patients, and no “gold standard” exists. Pharmacologic treatment is recommended prior to surgical intervention in patients who present with symptomatic hypoglycemia without loss of consciousness or seizures.1
Pharmacologic treatments include calcium channel blockers (eg, verapamil or nifedipine), the b-cell inhibitor diazoxide, the secretory inhibitor octreotide, and a-glucosidase inhibitors.1 In one hospital group, patients were initially treated with verapamil ER 100 mg/d.5 If patients did not respond to this therapy or developed adverse effects, diazoxide was added (starting dose, 25 mg tid, titrated to 75 mg tid).5 If this combination did not produce results, octreotide (dose ranging from 25 mg/d to 50 mg tid, subcutaneously) was added. Acarbose can also be added, with the typical starting dose of 50 mg tid.1
Distal or subtotal pancreatectomy to debulk the hypertrophic islets is the most common surgical method used in patients with severe hypoglycemia that is refractory to medical management.2,5 The extent of pancreatic resection is guided by calcium angiography and typically ranges from 80% to 95%.7 Smaller pancreatic resection is associated with higher risk for persistent postoperative hypoglycemia.5 Complications associated with pancreatectomy include insulin-dependent diabetes and exocrine insufficiency.5
It is not uncommon for patients to experience recurrent symptoms after subtotal pancreatectomy, but the symptoms are typically easier to manage pharmacologically than they were pre-operatively. Occasionally, a second surgery with 95% to complete pancreatectomy is employed if recurrent hypoglycemia develops that is refractory to medical management.5
Reversal of Roux-en-Y bypass surgery has been described as an attempted treatment method in several case reports of patients with NIPHS. In at least one patient, hyperinsulinemic hypoglycemia persisted after Roux-en-Y gastric bypass reversal.2 Adjustable gastric band placement was recently reported to reverse hypoglycemic symptoms and maintain weight loss, due to restricted gastric emptying.2 Conversion of Roux-en-Y gastric banding to gastric sleeve may also be employed to restore normal gastrointestinal continuity and resolve hypoglycemia, though limited data is available regarding the efficacy of this procedure.2
Close monitoring is necessary in patients treated with pharmacologic therapy to ensure that symptoms are well controlled and that surgery is not necessary.1
SUMMARY AND CONCLUSION
Symptomatic hypoglycemia is a potential complication associated with gastric bypass surgery and is most commonly caused by dumping syndrome. It is important to consider other causes of postprandial hypoglycemia, such as insulinoma and NIPHS, in patients who continue to experience hypoglycemia despite making dietary modifications.1,4
NIPHS is a rare and poorly understood complication of gastric bypass surgery involving pathologic b-cell overgrowth, leading to hyperinsulinemia and potentially severe hypoglycemia.6 Some patients may present with complete relief of symptoms with pharmacologic treatment, while others will need surgical treatment with subtotal pancreatectomy.1
The findings of increased levels of GLP-1 hormone in patients who have received gastric bypass surgery and the fact that only a very small subset of gastric bypass patients develop NIPHS with histologic features of nesidioblastosis are subjects for further research. Further understanding of the hormonal factors involved in the pathogenesis of NIPHS and adult-onset nesidioblastosis following gastric bypass surgery could lead to novel drug development to treat diabetes.6
REFERENCES
1. Moreira RO, Moreira RBM, Machado NAM, et al. Post-prandial hypoglycemia after bariatric surgery: pharmacological treatment with verapamil and acarbose. Obes Surg. 2008;18:1618-1621.
2. Cui Y, Elahi D, Andersen D. Advances in the etiology and management of hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass. J Gastrointest Surg. 2011;15:1879-1888.
3. Sarwar H, Chapman III WH, Pender JR, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg. 2014; 24(7):1120-1124.
4. Service GJ, Thompson GB, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249-254.
5. Mathavan VK, Arregui M, Davis C, et al. Management of postgastric bypass noninsulinoma pancreatogenous hypoglycemia. Surg Endosc. 2010;24:2547-2555.
6. Cummings D. Gastric bypass and nesidioblastosis—too much of a good thing for islets? N Engl J Med. 2005;353(3):300-302.
7. Clancy TE, Moore FD, Zinner MJ. Post-gastric bypass hyperinsulinism with nesidioblastosis: subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J Gastrointest Surg. 2006;10(8):1116-1119.
8. Kaczirek K, Niederle B. Nesidioblastosis: an old term and a new understanding. World J Surg. 2004;28:1227-1230.
9. Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6): 2008-2017.
A 28-year-old white woman, KR, presents to primary care with episodic diaphoresis and weakness that occur one to two hours after meals. There is no history of syncope or seizures. The hypoglycemic symptoms abate with intake of oral glucose and do not occur when the patient fasts.
KR underwent Roux-en-Y gastric bypass surgery 12 months ago. At the time, her body weight was 250 lbs and her height, 62 in (BMI, 46). She has lost 60 lbs since surgery (current BMI, 35). KR has no comorbid medical conditions. She denies use of insulin injection or oral hypoglycemic medication, as well as alcohol consumption. There is no history of diarrhea or abdominal pain. Her only medication is a daily multivitamin.
Physical exam reveals a blood pressure of 126/80 mm Hg; pulse, 82 beats/min; respiratory rate, 16 breaths/min; and O2 saturation, 98%. Heart rate is regular with no murmur. Lungs are clear to auscultation. Abdominal and neurologic exams are unremarkable; musculoskeletal strength and orthostatic vital signs are normal.
The patient is instructed to test her blood sugar with a glucometer and return to the clinic in two weeks. Fingerstick monitoring reveals that her serum glucose level drops into the 40 to 50 mg/dL range approximately one to two hours after meals containing > 45 g of carbohydrate. Her fasting serum glucose readings are in the 80 to 95 mg/dL range.
The patient is presumptively diagnosed with dumping syndrome and receives nutritional counseling; she is instructed to reduce intake of simple carbohydrates and increase the protein content of meals. Despite these dietary modifications, the episodes of hypoglycemia persist.
The patient is then referred to endocrinology. Fasting labwork reveals a serum glucose level of 85 mg/dL; normal adrenocorticotropic hormone (ACTH) and cortisol levels; C-peptide level, 2.46 ng/mL (reference range, 0.80–4.00 ng/mL); and insulin level, 6.4 mIU/mL (reference range 2.6–24.9 mIU/mL). A 75-g two-hour oral glucose tolerance test (OGTT) reveals peak serum glucose of 180 mg/dL at 30 minutes followed by a nadir serum glucose of 48 mg/dL at 110 minutes, accompanied by hypoglycemic symptoms. The insulin and C-peptide levels are elevated during the entire two-hour test. The serum cortisol level is 22 mg/dL when the glucose level is 48 mg/dL. CT of the abdomen, previously ordered by the patient’s primary care provider, was unremarkable.
Since there is no laboratory evidence of fasting hypoglycemia and no pancreatic abnormalities are seen on imaging studies, the possibility of insulinoma is excluded from the differential diagnosis. Adrenal insufficiency is excluded based on the normal ACTH and cortisol levels. The possibility of noninsulinoma pancreatogenous hypoglycemia syndrome is considered.
The patient is prescribed verapamil ER 100 mg/d and notes significant reduction in the frequency of hypoglycemic episodes and symptoms. She is scheduled for follow-up in four weeks to assess for any changes in the frequency or severity of her hypoglycemic episodes.
BACKGROUND
Postprandial hypoglycemia is a rare but potentially serious complication of bariatric surgery procedures that divert nutrients into the small bowel.1,2 The Bariatric Outcomes Longitudinal Database revealed a 0.1% incidence of hypoglycemia in patients who underwent Roux-en-Y gastric bypass surgery.3
The most common cause of hypoglycemia following gastric bypass surgery is dumping syndrome, which involves rapid emptying of gastric contents with reactive hypoglycemia due to increased postprandial insulin release. In dumping syndrome, hypoglycemic symptoms—flushing, diaphoresis, weakness, and dizziness—typically occur within two to three hours after meals; patients do not experience the more severe symptoms of neuroglycopenia (eg, cognitive impairment, seizures, and loss of consciousness).4 The symptoms of dumping syndrome typically improve with reduced intake of simple carbohydrates and increased protein consumption.1
Other causes of postprandial hypoglycemia include insulinoma and noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS). Although both diagnoses are rare, they should be considered if no improvement in hypoglycemic symptoms occurs after dietary modification.1
Insulinoma is the most common cause of persistent hyperinsulinemic hypoglycemia. It is defined by Whipple’s triad: symptomatic hypoglycemia during fasting, a serum glucose level > 50 mg/dL at the time of symptom onset, and relief of symptoms after administration of glucose.5
NIPHS is less common than insulinoma. It is characterized by postprandial hypoglycemia due to increased insulin secretion resulting from pancreatic b-cell hyperplasia. Hypoglycemia does not typically occur during a 72-hour fast. In addition, pancreatic imaging studies yield normal results in cases of NIPHS. The selective arterial calcium stimulation test is positive in NIPHS.5 NIPHS is definitively diagnosed by histopathologic examination of the pancreas, which reveals nesidioblastosis.6
Nesidioblastosis involves pathologic b-cell overgrowth in the pancreas that results in excess insulin secretion.4 Nesidioblastosis is characterized by pancreatic b-cell hypertrophy, islet hyperplasia, and increased b-cell mass.2
Nesidioblastosis is the leading cause of hyperinsulinemia in newborns and infants (annual incidence, 1 in 50,000 births) but is quite rare in adults, occurring in 0.5% to 7.0% of all those with hyperinsulinism.7,8 Islet cell hypertrophy—characteristic of nesidioblastosis—is seen in both adults and children, whereas genetic mutations are present only in infants.7
Although rare in adults, nesidioblastosis is more common in the setting of gastric bypass than in the general population.7 As of 2011, there have been 40 cases of nesidioblastosis in adults who received gastric bypass.2 With the rapid increase in the number of these surgeries performed each year, nesidioblastosis should be considered in the differential diagnosis for patients who experience hypoglycemia following the procedure.2,7
Continue for hormonal mechanisms >>
HORMONAL MECHANISMS
There are multiple theories regarding the etiology of b-cell hyperplasia following bariatric surgery. The specific causes for NIPHS after gastric bypass remain under investigation.2
The most common theory is that b-cell hyperplasia may occur as a result of the surgical procedure itself and not due to obesity. The rapid delivery of food to the distal ileum after gastric bypass surgery may result in elevated production of incretin hormones (eg, GLP-1 and GIP), which increase b-cell proliferation, insulin secretion, and insulin sensitivity.7
Roux-en-Y gastric bypass also impairs ghrelin secretion. Ghrelin normally acts to suppress insulin secretion and directly opposes the action of insulin. Reduced levels of ghrelin may increase the likelihood of hypoglycemia. Other hormones that may contribute to the metabolic effects of bariatric surgery include peptide YY, oxyntomodulin, and others as yet unidentified.5,6
CLINICAL MANIFESTATIONS
NIPHS is characterized by moderate to severe postprandial hypoglycemia. Symptoms include confusion, diaphoresis, tremulousness, anxiety, weakness, blurred vision, and disorientation, as well as more severe neuroglycopenic symptoms, such as cognitive impairment, seizures, and loss of consciousness.5
These symptoms do not typically manifest until several months after gastric bypass surgery. (By contrast, symptoms experienced with dumping syndrome typically manifest shortly after the procedure.) Of note, hypoglycemic symptoms of NIPHS do not typically improve after dietary modifications aimed at reducing carbohydrate intake.2
DIAGNOSIS
Diagnosis of NIPHS is based on hypoglycemic/neuroglycopenic signs and symptoms without fasting hypoglycemia; endogenous hyperinsulinemia in the presence of hypoglycemia; negative localization studies for insulinoma (using triple-phase spiral CT); and positive selective arterial calcium stimulation test.4,6
If fasting hypoglycemia is reported or suspected, the patient should be evaluated for insulinoma using a 72-hour fast. During it, glucose, insulin, C-peptide, and pro-insulin levels should be tested every six hours; results will be normal in patients with NIPHS.5
The use of OGTT is controversial, as patients can experience variable degrees of postprandial hyperinsulinism and symptomatic hypoglycemia during the test. There are no guidelines on whether to perform OGTT in the work-up for NIPHS. In research protocols, it is common to perform a five-hour OGTT; subjects consume a mixed meal containing 50 g of carbohydrates, then their glucose, insulin, and C-peptide levels are tested every 30 to 60 minutes (or sooner if hypoglycemic symptoms occur).
Elevated insulin and C-peptide levels in the setting of hypoglycemia are characteristic findings in patients with NIPHS.5,9 In the setting of hypoglycemia, a cortisol level > 20 mg/dL is considered an appropriate adrenal response and excludes adrenal insufficiency. Triple-phase CT of the abdomen should be performed to rule out insulinoma if strongly suspected and if work-up for NIPHS is negative.5
The selective arterial calcium stimulation test is employed to confirm the diagnosis of NIPHS and to guide the extent of pancreatic resection, in an effort to minimize postoperative complications of insulin-dependent diabetes and exocrine insufficiency. In this procedure, the splenic, gastroduodenal, superior mesenteric, and hepatic arteries that supply the pancreas are selectively injected with calcium gluconate. After injection of calcium, the insulin level is measured within each artery.4,5,7 The selective arterial calcium stimulation test can also be used to localize an insulinoma. NIPHS is distinguished from insulinoma by a diffuse increase in insulin secreted from multiple segments of the arteries that supply the pancreas, following calcium stimulation.4,5,7
Continue for treatment >>
TREATMENT
There is no consensus on treatment of NIPHS in postbariatric surgery patients, and no “gold standard” exists. Pharmacologic treatment is recommended prior to surgical intervention in patients who present with symptomatic hypoglycemia without loss of consciousness or seizures.1
Pharmacologic treatments include calcium channel blockers (eg, verapamil or nifedipine), the b-cell inhibitor diazoxide, the secretory inhibitor octreotide, and a-glucosidase inhibitors.1 In one hospital group, patients were initially treated with verapamil ER 100 mg/d.5 If patients did not respond to this therapy or developed adverse effects, diazoxide was added (starting dose, 25 mg tid, titrated to 75 mg tid).5 If this combination did not produce results, octreotide (dose ranging from 25 mg/d to 50 mg tid, subcutaneously) was added. Acarbose can also be added, with the typical starting dose of 50 mg tid.1
Distal or subtotal pancreatectomy to debulk the hypertrophic islets is the most common surgical method used in patients with severe hypoglycemia that is refractory to medical management.2,5 The extent of pancreatic resection is guided by calcium angiography and typically ranges from 80% to 95%.7 Smaller pancreatic resection is associated with higher risk for persistent postoperative hypoglycemia.5 Complications associated with pancreatectomy include insulin-dependent diabetes and exocrine insufficiency.5
It is not uncommon for patients to experience recurrent symptoms after subtotal pancreatectomy, but the symptoms are typically easier to manage pharmacologically than they were pre-operatively. Occasionally, a second surgery with 95% to complete pancreatectomy is employed if recurrent hypoglycemia develops that is refractory to medical management.5
Reversal of Roux-en-Y bypass surgery has been described as an attempted treatment method in several case reports of patients with NIPHS. In at least one patient, hyperinsulinemic hypoglycemia persisted after Roux-en-Y gastric bypass reversal.2 Adjustable gastric band placement was recently reported to reverse hypoglycemic symptoms and maintain weight loss, due to restricted gastric emptying.2 Conversion of Roux-en-Y gastric banding to gastric sleeve may also be employed to restore normal gastrointestinal continuity and resolve hypoglycemia, though limited data is available regarding the efficacy of this procedure.2
Close monitoring is necessary in patients treated with pharmacologic therapy to ensure that symptoms are well controlled and that surgery is not necessary.1
SUMMARY AND CONCLUSION
Symptomatic hypoglycemia is a potential complication associated with gastric bypass surgery and is most commonly caused by dumping syndrome. It is important to consider other causes of postprandial hypoglycemia, such as insulinoma and NIPHS, in patients who continue to experience hypoglycemia despite making dietary modifications.1,4
NIPHS is a rare and poorly understood complication of gastric bypass surgery involving pathologic b-cell overgrowth, leading to hyperinsulinemia and potentially severe hypoglycemia.6 Some patients may present with complete relief of symptoms with pharmacologic treatment, while others will need surgical treatment with subtotal pancreatectomy.1
The findings of increased levels of GLP-1 hormone in patients who have received gastric bypass surgery and the fact that only a very small subset of gastric bypass patients develop NIPHS with histologic features of nesidioblastosis are subjects for further research. Further understanding of the hormonal factors involved in the pathogenesis of NIPHS and adult-onset nesidioblastosis following gastric bypass surgery could lead to novel drug development to treat diabetes.6
REFERENCES
1. Moreira RO, Moreira RBM, Machado NAM, et al. Post-prandial hypoglycemia after bariatric surgery: pharmacological treatment with verapamil and acarbose. Obes Surg. 2008;18:1618-1621.
2. Cui Y, Elahi D, Andersen D. Advances in the etiology and management of hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass. J Gastrointest Surg. 2011;15:1879-1888.
3. Sarwar H, Chapman III WH, Pender JR, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg. 2014; 24(7):1120-1124.
4. Service GJ, Thompson GB, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249-254.
5. Mathavan VK, Arregui M, Davis C, et al. Management of postgastric bypass noninsulinoma pancreatogenous hypoglycemia. Surg Endosc. 2010;24:2547-2555.
6. Cummings D. Gastric bypass and nesidioblastosis—too much of a good thing for islets? N Engl J Med. 2005;353(3):300-302.
7. Clancy TE, Moore FD, Zinner MJ. Post-gastric bypass hyperinsulinism with nesidioblastosis: subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J Gastrointest Surg. 2006;10(8):1116-1119.
8. Kaczirek K, Niederle B. Nesidioblastosis: an old term and a new understanding. World J Surg. 2004;28:1227-1230.
9. Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6): 2008-2017.
Acting Surgeon General Confident in the Battle Against Tobacco, Ebola, and Preventable Diseases
Not many health care leaders can transition smoothly from discussing the importance of walking 30 minutes per day to the need to send PHS officers to help control the Ebola epidemic in West Africa. The Surgeon General has to. As the most prominent public health official, the Surgeon General must offer a reassuring voice on health care issues big and small. With over 26 years at the PHS, Rear Admiral (RADM) Boris D. Lushniak, MD, MPH, is well equipped to handle the challenging role.
A year after assuming the role and just before delivering a plenary address at the 2014 AMSUS meeting, RADM Lushniak agreed to a wide-ranging conversation with Federal Practitioner. The following is condensed and edited, but the complete interview can be found at http://www.fedprac.com.
The 50th anniversary of the Surgeon General’s report on smoking
RADM Boris D. Lushniak, Acting Surgeon General. Go back to January 1964 and realize what a different world we lived in back then. In fact, that report, which came out after a year and a half of scientific deliberations, of looking at facts, of searching through the literature, came up with a very important conclusion. That conclusion, simply put was: Smoking is bad for you.
Now it really was a landmark report from that perspective, but when we look back 50 years, what did it prove? It proved that cigarette smoking was directly associated with only 1 cancer at that point, specifically lung cancer in men. The report had a very simple but beautiful conclusion. It said that cigarette smoking is a health hazard of significant importance in the U.S. to warrant appropriate remedial action....
A half-century later, the social norms of our society have changed. We don’t have ashtrays all around. We don’t smoke on airplanes anymore. We oftentimes can’t smoke in bars and restaurants and establishments like that. We’ve moved from 43% of our population that smoked in 1964 to 18% currently.
We’ve had 32 Surgeons’ General reports since that first one....We brought up the issue of secondhand smoke 25 years ago. We talked about the successes and failures over these years, but 50 years later smoking remains a major public health problem in this country....
When we look to the future, what’s the goal? Well we really want to get to a zero point. We reannounced with the 50th anniversary report, which was released in January 2014, that this is an endgame strategy. At some point we have to realize that it’s not good enough to get down to 18% because of the health impact. Cigarettes and tobacco use in this country bring no good; no good to the individual, no good to the individual who has to deal with secondhand smoke, and no good for the future of our nation. So we’re really talking about an endgame strategy....
Our 50th anniversary report wasn’t just looking backward....It contains current data that now show us that we’re up to 13 different cancers caused by tobacco use. We know the impact on the whole human body. In essence, it affects almost every single system of the human body now. Brand-new diseases, formerly not associated with smoking, are still being discovered.
Most recently, we’ve seen diabetes and colon and rectal cancers as some of those diseases. We’re talking about blindness associated with smoking. We’re talking about diseases such as erectile dysfunction, which are associated with smoking. This product has brought nothing but grief and sorrow into our society and continues to do so.
Now it’s not only an impact on the United States for the Office of the Surgeon General’s to speak, but also in essence we know that internationally people look at the Surgeon General reports that come out of this office as that stellar scientific information that then can be translated worldwide.
Not only do we see leadership of public health here within the United States, but we also see leadership on an international level by profiling some of the major public health issues.
The PHS response to Ebola in the U.S. and Africa
RADM Lushniak. As many of the readers may know, the Surgeon General is the commander of the U.S. Public Health Service Commissioned Corps. We have 6,800 public health professionals. These are officers working in 11 different categories working across the government, to protect, promote, and advance the health and safety of the nation.
[In October] I was in Anniston, Alabama, seeing about 70 of my officers being trained for deployment to Liberia. And in fact, in the next weeks, we will have a full team in Liberia who will be serving in the Monrovia Medical Unit and providing health care to both Liberian as well as foreign health care workers. I want to get that message out, because this battle against Ebola is occurring here in the U.S. and being done very well by the CDC and the NIH and elements of the Commissioned Corps who are working with the CDC.
At the same time, we know that the real success of eliminating Ebola and stopping the epidemic lies in Western Africa. Dr. Frieden has said that. We’re confident that there will not be an Ebola outbreak on U.S. soil; however, we need to be able to stop this outbreak. Therefore, I’m very proud of my officers who are heading off to Western Africa.
The role of the PHS Commissioned Corps
RADM Lushniak. Most of my officers are dedicated to who? To serving the underserved and vulnerable populations. Many of my clinical officers are assigned, for example, to the Indian Health Service and are providing care to that important population of our nation. They’re assigned to the Federal Bureau of Prisons and, therefore, working with the Department of Justice in getting health care to, again, a vulnerable and underserved population. They’re working at the NIH in a clinical perspective. They’re treating the Coast Guard as the main medical and dental and environment health officers. So I have officers scattered all around, and in essence, they see everything that any other practitioner sees in this country.
The emphasis certainly from the Office of the Surgeon General has been on prevention. It’s prevention of preventable diseases; many of them are chronic diseases. And certainly, my officers not only are out there treating those individual patients, but at the same time are implementing and taking to task on the importance of prevention as a general theme. We make sure that the word of the Surgeon General’s office gets spread to local communities through our practitioners.
Raising the Commissioned Corps profile
RADM Lushniak. We need to get the word out. Part of our issue, I’ll be honest with you, is that oftentimes people don’t even know the U.S. Public Health Service Commissioned Corps exists. Therefore, even when my officers are part of a Centers for Disease Control and Prevention response, they’re embedded with other facets of CDC.
What I want to proudly say is that right now this Monrovia Medical Unit will be run by U.S. Public Health Service Commissioned Corps. This is the only entity of the U.S. government that will actually have direct patient care responsibilities in Western Africa. That being said, we’re also proud that this year is the 125th anniversary of the Commissioned Corps as a uniformed service in this country. So 125 years ago an act was passed by Congress to be able to establish this uniformed service.
Finally, I’d like to say that no other nation has a uniformed service like this. I keep saying that I love my sister services. I love the Army, the Navy, the Air Force, the Marines, the Coast Guard; but many other nations have similar type entities.
The reality of the situation is that no other nation on this planet has a uniformed service purely dedicated to public health. We are an unarmed service, and we are part of the Department of Health and Human Services, but we are just as proud to be officers. We are just as proud to be serving our nation in uniform on a slightly different mission but one that has, again, a noble cause associated with it.
Reaching the top of the PHS
RADM Lushniak. I’m honored and humbled to be in this position at this stage of my career. I came in 26, almost 27 years ago into the United States Public Health Service as a young lieutenant. My goal at that time was to be an Epidemic Intelligence Service Officer at the Centers for Disease Control and Prevention. That’s how I started my career, doing what’s deemed to be shoe leather epidemiology, going out there and getting my hands dirty and being able to try to make this nation a better place and to protect the public’s health.
It’s been a great ride from the CDC to the FDA, and then ultimately, to the Office of the Secretary here within the Office of the Surgeon General as the Deputy and now as the Acting Surgeon General. The message is everyone should, first of all, acknowledge the fact that we have an incredible mission to undertake. The mission of the Commissioned Corps of the PHS is to protect, promote, and advance the health and safety of our nation. And I dare say although we captured that as our mission, that mission is translatable to almost every federal practitioner that is out there.
The burden of that is apparent—to protect, promote, and advance the health and safety of our nation. And yet it’s a bold and noble mission, one that is achievable. We’ve had incredible successes. We still have a lot of work ahead of us.
So first and foremost, I tell my young officers and I tell everyone who may be exposed to this conversation is the sense that do your job and do it well. That’s really the prime thing I’m asking my officers to do: Be dedicated to the mission and realize that incredible things are still achievable.
The National Prevention Strategy
The goal is for us to have a healthy population at every stage of life. And so 20 federal partners...came up with a National Prevention Strategy, which is a focus of priming our nation towards prevention and wellness. It’s based on 4 strategic aims, which includes the importance of healthy and safe communities. It also entails the idea of clinical community preventive services. It talks about the empowerment of people, which is a key component of change in this nation, and the elimination of health disparities throughout the nation. It focuses on the really important preventable diseases. And among them, include the elimination of tobacco use, the importance of our really looking at alcohol and substance use in general. It’s looking at the concept of active living, the importance that we move our bodies, and the importance of healthy eating.
Office of the Surgeon General initiatives
RADM Lushniak. First and foremost, the smoking issue still continues, and there will be more on tobacco use and smoking from us. We won’t give up that fight until we’re zeroed out.
In addition, recently we released a call to action on skin cancer prevention. That’s, I think, an important issue as well because we have over 5 million people in the United States each and every year who are treated for skin cancers. We have over 60,000 people who are diagnosed with the most deadly form of skin cancer, melanoma, and 9,000 people, that’s 1 person every hour, dying of melanoma. It has an incredible impact on our country, and it is, again, one of those preventable diseases. So we look at the idea of getting the message out that we, in the Office of Surgeon General, want people to live an active lifestyle. That’s an important part of the National Prevention Strategy.
I want people to be outdoors, I want them to be runners and walkers and enjoying nature; but at the same time, I need to get the message out that we need to be wary of ultraviolet radiation from sunlight, that we can protect ourselves, seek shade when possible, put on a big hat that produces shade on your face and neck and ears. Wear glasses, put on protective clothing, and then use sunscreens, broad-spectrum sunscreens of a UV protective factor of at least 15. That’s one of the initiatives that we recently released.
In the future, where we’re priming, we’re really getting back into the fitness mode. One of the things that we’re working on, and it really simplifies, I think, what has become too complex a message—the idea of how do we have a healthy and fit nation?...
I want people to start walking 30 minutes a day, 5 days a week. Do you realize just by that simple act of walking how good our nation could do in the future? How healthy we can be as a people. So we’re really looking at an emphasis on walking and walkable communities, because not every community is walkable at this stage.
Speaking at the AMSUS Continuing Education Meeting in Washington, D.C.
RADM Lushniak. AMSUS has always provided an excellent forum for the United States Public Health Service Commissioned Corps, of which I am the commander, to be able to share our information with other federal practitioners, with other parties within the federal family that are interested in health care, in public health, in contact with patients on the clinical side and the scientific side....
I’ve been a member over many years, and I’ve been a regular attendee at the meetings. It allows us to cross-fertilize, to have that ability to sit down with our sister services, to be able to sit down with nonuniformed professionals who serve in the federal system under the flag of health care or under the flag of medical care or under the big flag of science, medical science.
Not many health care leaders can transition smoothly from discussing the importance of walking 30 minutes per day to the need to send PHS officers to help control the Ebola epidemic in West Africa. The Surgeon General has to. As the most prominent public health official, the Surgeon General must offer a reassuring voice on health care issues big and small. With over 26 years at the PHS, Rear Admiral (RADM) Boris D. Lushniak, MD, MPH, is well equipped to handle the challenging role.
A year after assuming the role and just before delivering a plenary address at the 2014 AMSUS meeting, RADM Lushniak agreed to a wide-ranging conversation with Federal Practitioner. The following is condensed and edited, but the complete interview can be found at http://www.fedprac.com.
The 50th anniversary of the Surgeon General’s report on smoking
RADM Boris D. Lushniak, Acting Surgeon General. Go back to January 1964 and realize what a different world we lived in back then. In fact, that report, which came out after a year and a half of scientific deliberations, of looking at facts, of searching through the literature, came up with a very important conclusion. That conclusion, simply put was: Smoking is bad for you.
Now it really was a landmark report from that perspective, but when we look back 50 years, what did it prove? It proved that cigarette smoking was directly associated with only 1 cancer at that point, specifically lung cancer in men. The report had a very simple but beautiful conclusion. It said that cigarette smoking is a health hazard of significant importance in the U.S. to warrant appropriate remedial action....
A half-century later, the social norms of our society have changed. We don’t have ashtrays all around. We don’t smoke on airplanes anymore. We oftentimes can’t smoke in bars and restaurants and establishments like that. We’ve moved from 43% of our population that smoked in 1964 to 18% currently.
We’ve had 32 Surgeons’ General reports since that first one....We brought up the issue of secondhand smoke 25 years ago. We talked about the successes and failures over these years, but 50 years later smoking remains a major public health problem in this country....
When we look to the future, what’s the goal? Well we really want to get to a zero point. We reannounced with the 50th anniversary report, which was released in January 2014, that this is an endgame strategy. At some point we have to realize that it’s not good enough to get down to 18% because of the health impact. Cigarettes and tobacco use in this country bring no good; no good to the individual, no good to the individual who has to deal with secondhand smoke, and no good for the future of our nation. So we’re really talking about an endgame strategy....
Our 50th anniversary report wasn’t just looking backward....It contains current data that now show us that we’re up to 13 different cancers caused by tobacco use. We know the impact on the whole human body. In essence, it affects almost every single system of the human body now. Brand-new diseases, formerly not associated with smoking, are still being discovered.
Most recently, we’ve seen diabetes and colon and rectal cancers as some of those diseases. We’re talking about blindness associated with smoking. We’re talking about diseases such as erectile dysfunction, which are associated with smoking. This product has brought nothing but grief and sorrow into our society and continues to do so.
Now it’s not only an impact on the United States for the Office of the Surgeon General’s to speak, but also in essence we know that internationally people look at the Surgeon General reports that come out of this office as that stellar scientific information that then can be translated worldwide.
Not only do we see leadership of public health here within the United States, but we also see leadership on an international level by profiling some of the major public health issues.
The PHS response to Ebola in the U.S. and Africa
RADM Lushniak. As many of the readers may know, the Surgeon General is the commander of the U.S. Public Health Service Commissioned Corps. We have 6,800 public health professionals. These are officers working in 11 different categories working across the government, to protect, promote, and advance the health and safety of the nation.
[In October] I was in Anniston, Alabama, seeing about 70 of my officers being trained for deployment to Liberia. And in fact, in the next weeks, we will have a full team in Liberia who will be serving in the Monrovia Medical Unit and providing health care to both Liberian as well as foreign health care workers. I want to get that message out, because this battle against Ebola is occurring here in the U.S. and being done very well by the CDC and the NIH and elements of the Commissioned Corps who are working with the CDC.
At the same time, we know that the real success of eliminating Ebola and stopping the epidemic lies in Western Africa. Dr. Frieden has said that. We’re confident that there will not be an Ebola outbreak on U.S. soil; however, we need to be able to stop this outbreak. Therefore, I’m very proud of my officers who are heading off to Western Africa.
The role of the PHS Commissioned Corps
RADM Lushniak. Most of my officers are dedicated to who? To serving the underserved and vulnerable populations. Many of my clinical officers are assigned, for example, to the Indian Health Service and are providing care to that important population of our nation. They’re assigned to the Federal Bureau of Prisons and, therefore, working with the Department of Justice in getting health care to, again, a vulnerable and underserved population. They’re working at the NIH in a clinical perspective. They’re treating the Coast Guard as the main medical and dental and environment health officers. So I have officers scattered all around, and in essence, they see everything that any other practitioner sees in this country.
The emphasis certainly from the Office of the Surgeon General has been on prevention. It’s prevention of preventable diseases; many of them are chronic diseases. And certainly, my officers not only are out there treating those individual patients, but at the same time are implementing and taking to task on the importance of prevention as a general theme. We make sure that the word of the Surgeon General’s office gets spread to local communities through our practitioners.
Raising the Commissioned Corps profile
RADM Lushniak. We need to get the word out. Part of our issue, I’ll be honest with you, is that oftentimes people don’t even know the U.S. Public Health Service Commissioned Corps exists. Therefore, even when my officers are part of a Centers for Disease Control and Prevention response, they’re embedded with other facets of CDC.
What I want to proudly say is that right now this Monrovia Medical Unit will be run by U.S. Public Health Service Commissioned Corps. This is the only entity of the U.S. government that will actually have direct patient care responsibilities in Western Africa. That being said, we’re also proud that this year is the 125th anniversary of the Commissioned Corps as a uniformed service in this country. So 125 years ago an act was passed by Congress to be able to establish this uniformed service.
Finally, I’d like to say that no other nation has a uniformed service like this. I keep saying that I love my sister services. I love the Army, the Navy, the Air Force, the Marines, the Coast Guard; but many other nations have similar type entities.
The reality of the situation is that no other nation on this planet has a uniformed service purely dedicated to public health. We are an unarmed service, and we are part of the Department of Health and Human Services, but we are just as proud to be officers. We are just as proud to be serving our nation in uniform on a slightly different mission but one that has, again, a noble cause associated with it.
Reaching the top of the PHS
RADM Lushniak. I’m honored and humbled to be in this position at this stage of my career. I came in 26, almost 27 years ago into the United States Public Health Service as a young lieutenant. My goal at that time was to be an Epidemic Intelligence Service Officer at the Centers for Disease Control and Prevention. That’s how I started my career, doing what’s deemed to be shoe leather epidemiology, going out there and getting my hands dirty and being able to try to make this nation a better place and to protect the public’s health.
It’s been a great ride from the CDC to the FDA, and then ultimately, to the Office of the Secretary here within the Office of the Surgeon General as the Deputy and now as the Acting Surgeon General. The message is everyone should, first of all, acknowledge the fact that we have an incredible mission to undertake. The mission of the Commissioned Corps of the PHS is to protect, promote, and advance the health and safety of our nation. And I dare say although we captured that as our mission, that mission is translatable to almost every federal practitioner that is out there.
The burden of that is apparent—to protect, promote, and advance the health and safety of our nation. And yet it’s a bold and noble mission, one that is achievable. We’ve had incredible successes. We still have a lot of work ahead of us.
So first and foremost, I tell my young officers and I tell everyone who may be exposed to this conversation is the sense that do your job and do it well. That’s really the prime thing I’m asking my officers to do: Be dedicated to the mission and realize that incredible things are still achievable.
The National Prevention Strategy
The goal is for us to have a healthy population at every stage of life. And so 20 federal partners...came up with a National Prevention Strategy, which is a focus of priming our nation towards prevention and wellness. It’s based on 4 strategic aims, which includes the importance of healthy and safe communities. It also entails the idea of clinical community preventive services. It talks about the empowerment of people, which is a key component of change in this nation, and the elimination of health disparities throughout the nation. It focuses on the really important preventable diseases. And among them, include the elimination of tobacco use, the importance of our really looking at alcohol and substance use in general. It’s looking at the concept of active living, the importance that we move our bodies, and the importance of healthy eating.
Office of the Surgeon General initiatives
RADM Lushniak. First and foremost, the smoking issue still continues, and there will be more on tobacco use and smoking from us. We won’t give up that fight until we’re zeroed out.
In addition, recently we released a call to action on skin cancer prevention. That’s, I think, an important issue as well because we have over 5 million people in the United States each and every year who are treated for skin cancers. We have over 60,000 people who are diagnosed with the most deadly form of skin cancer, melanoma, and 9,000 people, that’s 1 person every hour, dying of melanoma. It has an incredible impact on our country, and it is, again, one of those preventable diseases. So we look at the idea of getting the message out that we, in the Office of Surgeon General, want people to live an active lifestyle. That’s an important part of the National Prevention Strategy.
I want people to be outdoors, I want them to be runners and walkers and enjoying nature; but at the same time, I need to get the message out that we need to be wary of ultraviolet radiation from sunlight, that we can protect ourselves, seek shade when possible, put on a big hat that produces shade on your face and neck and ears. Wear glasses, put on protective clothing, and then use sunscreens, broad-spectrum sunscreens of a UV protective factor of at least 15. That’s one of the initiatives that we recently released.
In the future, where we’re priming, we’re really getting back into the fitness mode. One of the things that we’re working on, and it really simplifies, I think, what has become too complex a message—the idea of how do we have a healthy and fit nation?...
I want people to start walking 30 minutes a day, 5 days a week. Do you realize just by that simple act of walking how good our nation could do in the future? How healthy we can be as a people. So we’re really looking at an emphasis on walking and walkable communities, because not every community is walkable at this stage.
Speaking at the AMSUS Continuing Education Meeting in Washington, D.C.
RADM Lushniak. AMSUS has always provided an excellent forum for the United States Public Health Service Commissioned Corps, of which I am the commander, to be able to share our information with other federal practitioners, with other parties within the federal family that are interested in health care, in public health, in contact with patients on the clinical side and the scientific side....
I’ve been a member over many years, and I’ve been a regular attendee at the meetings. It allows us to cross-fertilize, to have that ability to sit down with our sister services, to be able to sit down with nonuniformed professionals who serve in the federal system under the flag of health care or under the flag of medical care or under the big flag of science, medical science.
Not many health care leaders can transition smoothly from discussing the importance of walking 30 minutes per day to the need to send PHS officers to help control the Ebola epidemic in West Africa. The Surgeon General has to. As the most prominent public health official, the Surgeon General must offer a reassuring voice on health care issues big and small. With over 26 years at the PHS, Rear Admiral (RADM) Boris D. Lushniak, MD, MPH, is well equipped to handle the challenging role.
A year after assuming the role and just before delivering a plenary address at the 2014 AMSUS meeting, RADM Lushniak agreed to a wide-ranging conversation with Federal Practitioner. The following is condensed and edited, but the complete interview can be found at http://www.fedprac.com.
The 50th anniversary of the Surgeon General’s report on smoking
RADM Boris D. Lushniak, Acting Surgeon General. Go back to January 1964 and realize what a different world we lived in back then. In fact, that report, which came out after a year and a half of scientific deliberations, of looking at facts, of searching through the literature, came up with a very important conclusion. That conclusion, simply put was: Smoking is bad for you.
Now it really was a landmark report from that perspective, but when we look back 50 years, what did it prove? It proved that cigarette smoking was directly associated with only 1 cancer at that point, specifically lung cancer in men. The report had a very simple but beautiful conclusion. It said that cigarette smoking is a health hazard of significant importance in the U.S. to warrant appropriate remedial action....
A half-century later, the social norms of our society have changed. We don’t have ashtrays all around. We don’t smoke on airplanes anymore. We oftentimes can’t smoke in bars and restaurants and establishments like that. We’ve moved from 43% of our population that smoked in 1964 to 18% currently.
We’ve had 32 Surgeons’ General reports since that first one....We brought up the issue of secondhand smoke 25 years ago. We talked about the successes and failures over these years, but 50 years later smoking remains a major public health problem in this country....
When we look to the future, what’s the goal? Well we really want to get to a zero point. We reannounced with the 50th anniversary report, which was released in January 2014, that this is an endgame strategy. At some point we have to realize that it’s not good enough to get down to 18% because of the health impact. Cigarettes and tobacco use in this country bring no good; no good to the individual, no good to the individual who has to deal with secondhand smoke, and no good for the future of our nation. So we’re really talking about an endgame strategy....
Our 50th anniversary report wasn’t just looking backward....It contains current data that now show us that we’re up to 13 different cancers caused by tobacco use. We know the impact on the whole human body. In essence, it affects almost every single system of the human body now. Brand-new diseases, formerly not associated with smoking, are still being discovered.
Most recently, we’ve seen diabetes and colon and rectal cancers as some of those diseases. We’re talking about blindness associated with smoking. We’re talking about diseases such as erectile dysfunction, which are associated with smoking. This product has brought nothing but grief and sorrow into our society and continues to do so.
Now it’s not only an impact on the United States for the Office of the Surgeon General’s to speak, but also in essence we know that internationally people look at the Surgeon General reports that come out of this office as that stellar scientific information that then can be translated worldwide.
Not only do we see leadership of public health here within the United States, but we also see leadership on an international level by profiling some of the major public health issues.
The PHS response to Ebola in the U.S. and Africa
RADM Lushniak. As many of the readers may know, the Surgeon General is the commander of the U.S. Public Health Service Commissioned Corps. We have 6,800 public health professionals. These are officers working in 11 different categories working across the government, to protect, promote, and advance the health and safety of the nation.
[In October] I was in Anniston, Alabama, seeing about 70 of my officers being trained for deployment to Liberia. And in fact, in the next weeks, we will have a full team in Liberia who will be serving in the Monrovia Medical Unit and providing health care to both Liberian as well as foreign health care workers. I want to get that message out, because this battle against Ebola is occurring here in the U.S. and being done very well by the CDC and the NIH and elements of the Commissioned Corps who are working with the CDC.
At the same time, we know that the real success of eliminating Ebola and stopping the epidemic lies in Western Africa. Dr. Frieden has said that. We’re confident that there will not be an Ebola outbreak on U.S. soil; however, we need to be able to stop this outbreak. Therefore, I’m very proud of my officers who are heading off to Western Africa.
The role of the PHS Commissioned Corps
RADM Lushniak. Most of my officers are dedicated to who? To serving the underserved and vulnerable populations. Many of my clinical officers are assigned, for example, to the Indian Health Service and are providing care to that important population of our nation. They’re assigned to the Federal Bureau of Prisons and, therefore, working with the Department of Justice in getting health care to, again, a vulnerable and underserved population. They’re working at the NIH in a clinical perspective. They’re treating the Coast Guard as the main medical and dental and environment health officers. So I have officers scattered all around, and in essence, they see everything that any other practitioner sees in this country.
The emphasis certainly from the Office of the Surgeon General has been on prevention. It’s prevention of preventable diseases; many of them are chronic diseases. And certainly, my officers not only are out there treating those individual patients, but at the same time are implementing and taking to task on the importance of prevention as a general theme. We make sure that the word of the Surgeon General’s office gets spread to local communities through our practitioners.
Raising the Commissioned Corps profile
RADM Lushniak. We need to get the word out. Part of our issue, I’ll be honest with you, is that oftentimes people don’t even know the U.S. Public Health Service Commissioned Corps exists. Therefore, even when my officers are part of a Centers for Disease Control and Prevention response, they’re embedded with other facets of CDC.
What I want to proudly say is that right now this Monrovia Medical Unit will be run by U.S. Public Health Service Commissioned Corps. This is the only entity of the U.S. government that will actually have direct patient care responsibilities in Western Africa. That being said, we’re also proud that this year is the 125th anniversary of the Commissioned Corps as a uniformed service in this country. So 125 years ago an act was passed by Congress to be able to establish this uniformed service.
Finally, I’d like to say that no other nation has a uniformed service like this. I keep saying that I love my sister services. I love the Army, the Navy, the Air Force, the Marines, the Coast Guard; but many other nations have similar type entities.
The reality of the situation is that no other nation on this planet has a uniformed service purely dedicated to public health. We are an unarmed service, and we are part of the Department of Health and Human Services, but we are just as proud to be officers. We are just as proud to be serving our nation in uniform on a slightly different mission but one that has, again, a noble cause associated with it.
Reaching the top of the PHS
RADM Lushniak. I’m honored and humbled to be in this position at this stage of my career. I came in 26, almost 27 years ago into the United States Public Health Service as a young lieutenant. My goal at that time was to be an Epidemic Intelligence Service Officer at the Centers for Disease Control and Prevention. That’s how I started my career, doing what’s deemed to be shoe leather epidemiology, going out there and getting my hands dirty and being able to try to make this nation a better place and to protect the public’s health.
It’s been a great ride from the CDC to the FDA, and then ultimately, to the Office of the Secretary here within the Office of the Surgeon General as the Deputy and now as the Acting Surgeon General. The message is everyone should, first of all, acknowledge the fact that we have an incredible mission to undertake. The mission of the Commissioned Corps of the PHS is to protect, promote, and advance the health and safety of our nation. And I dare say although we captured that as our mission, that mission is translatable to almost every federal practitioner that is out there.
The burden of that is apparent—to protect, promote, and advance the health and safety of our nation. And yet it’s a bold and noble mission, one that is achievable. We’ve had incredible successes. We still have a lot of work ahead of us.
So first and foremost, I tell my young officers and I tell everyone who may be exposed to this conversation is the sense that do your job and do it well. That’s really the prime thing I’m asking my officers to do: Be dedicated to the mission and realize that incredible things are still achievable.
The National Prevention Strategy
The goal is for us to have a healthy population at every stage of life. And so 20 federal partners...came up with a National Prevention Strategy, which is a focus of priming our nation towards prevention and wellness. It’s based on 4 strategic aims, which includes the importance of healthy and safe communities. It also entails the idea of clinical community preventive services. It talks about the empowerment of people, which is a key component of change in this nation, and the elimination of health disparities throughout the nation. It focuses on the really important preventable diseases. And among them, include the elimination of tobacco use, the importance of our really looking at alcohol and substance use in general. It’s looking at the concept of active living, the importance that we move our bodies, and the importance of healthy eating.
Office of the Surgeon General initiatives
RADM Lushniak. First and foremost, the smoking issue still continues, and there will be more on tobacco use and smoking from us. We won’t give up that fight until we’re zeroed out.
In addition, recently we released a call to action on skin cancer prevention. That’s, I think, an important issue as well because we have over 5 million people in the United States each and every year who are treated for skin cancers. We have over 60,000 people who are diagnosed with the most deadly form of skin cancer, melanoma, and 9,000 people, that’s 1 person every hour, dying of melanoma. It has an incredible impact on our country, and it is, again, one of those preventable diseases. So we look at the idea of getting the message out that we, in the Office of Surgeon General, want people to live an active lifestyle. That’s an important part of the National Prevention Strategy.
I want people to be outdoors, I want them to be runners and walkers and enjoying nature; but at the same time, I need to get the message out that we need to be wary of ultraviolet radiation from sunlight, that we can protect ourselves, seek shade when possible, put on a big hat that produces shade on your face and neck and ears. Wear glasses, put on protective clothing, and then use sunscreens, broad-spectrum sunscreens of a UV protective factor of at least 15. That’s one of the initiatives that we recently released.
In the future, where we’re priming, we’re really getting back into the fitness mode. One of the things that we’re working on, and it really simplifies, I think, what has become too complex a message—the idea of how do we have a healthy and fit nation?...
I want people to start walking 30 minutes a day, 5 days a week. Do you realize just by that simple act of walking how good our nation could do in the future? How healthy we can be as a people. So we’re really looking at an emphasis on walking and walkable communities, because not every community is walkable at this stage.
Speaking at the AMSUS Continuing Education Meeting in Washington, D.C.
RADM Lushniak. AMSUS has always provided an excellent forum for the United States Public Health Service Commissioned Corps, of which I am the commander, to be able to share our information with other federal practitioners, with other parties within the federal family that are interested in health care, in public health, in contact with patients on the clinical side and the scientific side....
I’ve been a member over many years, and I’ve been a regular attendee at the meetings. It allows us to cross-fertilize, to have that ability to sit down with our sister services, to be able to sit down with nonuniformed professionals who serve in the federal system under the flag of health care or under the flag of medical care or under the big flag of science, medical science.
Genetics of Renal Disease: APOL1 Variations
Q) I have heard about a gene that causes high blood pressure. Did I hear that right? Is testing for this gene available now?
African-Americans have a higher risk for chronic kidney disease (CKD), including end-stage renal disease (ESRD; defined as kidney failure requiring dialysis or transplant), than any other racial or ethnic group in the United States.1 Previously, this has been attributed to poorly controlled hypertension and diabetes, as well as socioeconomic factors such as limited access to health care.
Research now shows that autosomal recessive genetic variations on chromosome 22q, the gene that encodes apolipoprotein-1 (APOL1; an HDL protein), promote hypertension. This subsequently increases the risk for and progression of CKD in black patients (who have up to 29x higher risk than white patients without this genetic variation).2
The APOL1 gene has two alleles. Having at least one of them provides resistance to Trypanosoma brucei, the cause of “sleeping sickness” transmitted by the tsetse fly, but increases risk for CKD and ESRD (see Table 1).2,3 Black patients descending from the southern and western portions of Africa are most likely to have two alleles, putting them at the highest risk for hypertension and associated CKD.
Foster et al reported that black patients with two altered alleles had a 31% higher risk for CKD and ESRD, compared with individuals with hypertension-induced nephrosclerosis who had zero to one altered alleles.4 Nondiabetic black patients with CKD who have two altered alleles are at highest risk for focal segmental glomerulosclerosis, HIV nephropathy, and CKD attributable to hypertension.2 The African-American Study of Kidney Disease and Hypertension found that black patients with hypertension controlled by ACE inhibitors had slower progression of CKD, regardless of allele variation.5 Currently, there is no treatment for this genetic alteration.4
One could posit that black patients undergoing renal transplant would have a higher risk for renal failure in the transplanted kidney due to APOL1-related hypertension, compared to nonblack renal transplant recipients. Additionally, a donor kidney with an altered APOL1 gene may have a higher risk for failure.6
Genotyping for APOL1 (CPT code: 81479) is available in select laboratories at a cost of approximately $400.7 For a family that has a member affected by kidney failure at a young age, knowing whether the APOL1 gene is carried in the family would allow early aggressive hypertension management to help prevent a lifetime of severe CKD.
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personalizedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Disease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Q) I have heard about a gene that causes high blood pressure. Did I hear that right? Is testing for this gene available now?
African-Americans have a higher risk for chronic kidney disease (CKD), including end-stage renal disease (ESRD; defined as kidney failure requiring dialysis or transplant), than any other racial or ethnic group in the United States.1 Previously, this has been attributed to poorly controlled hypertension and diabetes, as well as socioeconomic factors such as limited access to health care.
Research now shows that autosomal recessive genetic variations on chromosome 22q, the gene that encodes apolipoprotein-1 (APOL1; an HDL protein), promote hypertension. This subsequently increases the risk for and progression of CKD in black patients (who have up to 29x higher risk than white patients without this genetic variation).2
The APOL1 gene has two alleles. Having at least one of them provides resistance to Trypanosoma brucei, the cause of “sleeping sickness” transmitted by the tsetse fly, but increases risk for CKD and ESRD (see Table 1).2,3 Black patients descending from the southern and western portions of Africa are most likely to have two alleles, putting them at the highest risk for hypertension and associated CKD.
Foster et al reported that black patients with two altered alleles had a 31% higher risk for CKD and ESRD, compared with individuals with hypertension-induced nephrosclerosis who had zero to one altered alleles.4 Nondiabetic black patients with CKD who have two altered alleles are at highest risk for focal segmental glomerulosclerosis, HIV nephropathy, and CKD attributable to hypertension.2 The African-American Study of Kidney Disease and Hypertension found that black patients with hypertension controlled by ACE inhibitors had slower progression of CKD, regardless of allele variation.5 Currently, there is no treatment for this genetic alteration.4
One could posit that black patients undergoing renal transplant would have a higher risk for renal failure in the transplanted kidney due to APOL1-related hypertension, compared to nonblack renal transplant recipients. Additionally, a donor kidney with an altered APOL1 gene may have a higher risk for failure.6
Genotyping for APOL1 (CPT code: 81479) is available in select laboratories at a cost of approximately $400.7 For a family that has a member affected by kidney failure at a young age, knowing whether the APOL1 gene is carried in the family would allow early aggressive hypertension management to help prevent a lifetime of severe CKD.
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personalizedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Disease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Q) I have heard about a gene that causes high blood pressure. Did I hear that right? Is testing for this gene available now?
African-Americans have a higher risk for chronic kidney disease (CKD), including end-stage renal disease (ESRD; defined as kidney failure requiring dialysis or transplant), than any other racial or ethnic group in the United States.1 Previously, this has been attributed to poorly controlled hypertension and diabetes, as well as socioeconomic factors such as limited access to health care.
Research now shows that autosomal recessive genetic variations on chromosome 22q, the gene that encodes apolipoprotein-1 (APOL1; an HDL protein), promote hypertension. This subsequently increases the risk for and progression of CKD in black patients (who have up to 29x higher risk than white patients without this genetic variation).2
The APOL1 gene has two alleles. Having at least one of them provides resistance to Trypanosoma brucei, the cause of “sleeping sickness” transmitted by the tsetse fly, but increases risk for CKD and ESRD (see Table 1).2,3 Black patients descending from the southern and western portions of Africa are most likely to have two alleles, putting them at the highest risk for hypertension and associated CKD.
Foster et al reported that black patients with two altered alleles had a 31% higher risk for CKD and ESRD, compared with individuals with hypertension-induced nephrosclerosis who had zero to one altered alleles.4 Nondiabetic black patients with CKD who have two altered alleles are at highest risk for focal segmental glomerulosclerosis, HIV nephropathy, and CKD attributable to hypertension.2 The African-American Study of Kidney Disease and Hypertension found that black patients with hypertension controlled by ACE inhibitors had slower progression of CKD, regardless of allele variation.5 Currently, there is no treatment for this genetic alteration.4
One could posit that black patients undergoing renal transplant would have a higher risk for renal failure in the transplanted kidney due to APOL1-related hypertension, compared to nonblack renal transplant recipients. Additionally, a donor kidney with an altered APOL1 gene may have a higher risk for failure.6
Genotyping for APOL1 (CPT code: 81479) is available in select laboratories at a cost of approximately $400.7 For a family that has a member affected by kidney failure at a young age, knowing whether the APOL1 gene is carried in the family would allow early aggressive hypertension management to help prevent a lifetime of severe CKD.
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personalizedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Disease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.