User login
Expanding Treatment Options
Q) One of my diabetic patients read about finerenone in The New York Times. Apparently, it’s the “newest cure for albuminuria”! Is this just hype, or do the trials on this medication really show progress against kidney disease? Should I buy stock in the company?
Albuminuria (> 500 mg/d) associated with diabetic nephropathy and other glomerular diseases increases patient risk for chronic kidney disease (CKD) and its progression to end-stage renal disease (ESRD). Reduction of albuminuria has been shown to slow the progression of CKD.
Renin-angiotensin-aldosterone system (RAAS) blockers, such as ACE inhibitors or angiotensin receptor blockers, are considered firstline therapy to reduce albuminuria. Additional treatment modalities include diuretics, nondihydropyridine calcium channel blockers, ß-blockers, and aldosterone antagonist therapy. Limiting dietary sodium helps control blood pressure, thus slowing disease progression. In addition, some studies show that limiting phosphorus and protein (for the latter, intake of no more than 0.7 g/kg ideal body weight per day) may slow the progression of CKD. Unfortunately, despite these interventions, patients may still advance to ESRD.1
The aldosterone and steroidal mineralocorticoid receptor antagonists (MRA) spironolactone and eplerenone have been found to reduce albuminuria when used in conjunction with RAAS blockade. However, patients using this combination are up to eight times more likely to experience hyperkalemia—a serious, potentially life-threatening adverse condition—than those not using an MRA.2 The presence of hyperkalemia requires discontinuation of the RAAS blocker and the MRA, at least temporarily.
Finerenone, a nonsteroidal MRA with “greater receptor selectivity than spironolactone and better receptor affinity than eplerenone in vitro,” is in phase III trials for the treatment of systolic and diastolic dysfunction and reduction of morbidity and mortality associated with heart failure.2 One study has already demonstrated that finerenone (5 to 10 mg/d) is at least as effective as spironolactone (25 mg/d) for heart failure patients.3
The Mineralocorticoid Receptor Antagonist Tolerability Study-Diabetic Nephropathy (ARTS-DN) found that finerenone at 10 to 20 mg/d was superior to spironolactone and eplerenone, partly due to the decreased incidence of hyperkalemia. However, it should be noted that the lower incidence of hyperkalemia may be attributable to the fact that 66% of the study participants had an estimated glomerular filtration rate (eGFR) greater than 60 mL/min and that potential participants with a serum potassium level of more than 4.8 mEq/L were not included in the study.2
Additional research is needed to confirm superiority of finerenone over spironolactone and eplerenone, in conjunction with RAAS blockers, in the treatment of albuminuria and hyperkalemia. Including subjects with lower eGFR (such as patients with stage IV CKD who are at higher risk for hyperkalemia) would give a better indication of finerenone’s efficacy. In the meantime, it’s probably too soon to corner the market on this stock! —SEB
Susan E. Brown, MS, ARNP, ACNP-BC, CCRN
Great River Nephrology, West Burlington, Iowa
References
1. Parikh SV, Haddad NJ, Hebert LA. Retarding progression of kidney disease. In: Johnson RJ, Feehally J, Floege J, eds. Comprehensive Clinical Nephrology. 5th ed. Philadelphia, PA: Saunders; 2015:931-940.
2. Bakris GL, Agarwal R, Chan JC, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884-894.
3. Kolkhof P, Delbeck M, Kretschmer A, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist, protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69-78.
Q) One of my diabetic patients read about finerenone in The New York Times. Apparently, it’s the “newest cure for albuminuria”! Is this just hype, or do the trials on this medication really show progress against kidney disease? Should I buy stock in the company?
Albuminuria (> 500 mg/d) associated with diabetic nephropathy and other glomerular diseases increases patient risk for chronic kidney disease (CKD) and its progression to end-stage renal disease (ESRD). Reduction of albuminuria has been shown to slow the progression of CKD.
Renin-angiotensin-aldosterone system (RAAS) blockers, such as ACE inhibitors or angiotensin receptor blockers, are considered firstline therapy to reduce albuminuria. Additional treatment modalities include diuretics, nondihydropyridine calcium channel blockers, ß-blockers, and aldosterone antagonist therapy. Limiting dietary sodium helps control blood pressure, thus slowing disease progression. In addition, some studies show that limiting phosphorus and protein (for the latter, intake of no more than 0.7 g/kg ideal body weight per day) may slow the progression of CKD. Unfortunately, despite these interventions, patients may still advance to ESRD.1
The aldosterone and steroidal mineralocorticoid receptor antagonists (MRA) spironolactone and eplerenone have been found to reduce albuminuria when used in conjunction with RAAS blockade. However, patients using this combination are up to eight times more likely to experience hyperkalemia—a serious, potentially life-threatening adverse condition—than those not using an MRA.2 The presence of hyperkalemia requires discontinuation of the RAAS blocker and the MRA, at least temporarily.
Finerenone, a nonsteroidal MRA with “greater receptor selectivity than spironolactone and better receptor affinity than eplerenone in vitro,” is in phase III trials for the treatment of systolic and diastolic dysfunction and reduction of morbidity and mortality associated with heart failure.2 One study has already demonstrated that finerenone (5 to 10 mg/d) is at least as effective as spironolactone (25 mg/d) for heart failure patients.3
The Mineralocorticoid Receptor Antagonist Tolerability Study-Diabetic Nephropathy (ARTS-DN) found that finerenone at 10 to 20 mg/d was superior to spironolactone and eplerenone, partly due to the decreased incidence of hyperkalemia. However, it should be noted that the lower incidence of hyperkalemia may be attributable to the fact that 66% of the study participants had an estimated glomerular filtration rate (eGFR) greater than 60 mL/min and that potential participants with a serum potassium level of more than 4.8 mEq/L were not included in the study.2
Additional research is needed to confirm superiority of finerenone over spironolactone and eplerenone, in conjunction with RAAS blockers, in the treatment of albuminuria and hyperkalemia. Including subjects with lower eGFR (such as patients with stage IV CKD who are at higher risk for hyperkalemia) would give a better indication of finerenone’s efficacy. In the meantime, it’s probably too soon to corner the market on this stock! —SEB
Susan E. Brown, MS, ARNP, ACNP-BC, CCRN
Great River Nephrology, West Burlington, Iowa
References
1. Parikh SV, Haddad NJ, Hebert LA. Retarding progression of kidney disease. In: Johnson RJ, Feehally J, Floege J, eds. Comprehensive Clinical Nephrology. 5th ed. Philadelphia, PA: Saunders; 2015:931-940.
2. Bakris GL, Agarwal R, Chan JC, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884-894.
3. Kolkhof P, Delbeck M, Kretschmer A, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist, protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69-78.
Q) One of my diabetic patients read about finerenone in The New York Times. Apparently, it’s the “newest cure for albuminuria”! Is this just hype, or do the trials on this medication really show progress against kidney disease? Should I buy stock in the company?
Albuminuria (> 500 mg/d) associated with diabetic nephropathy and other glomerular diseases increases patient risk for chronic kidney disease (CKD) and its progression to end-stage renal disease (ESRD). Reduction of albuminuria has been shown to slow the progression of CKD.
Renin-angiotensin-aldosterone system (RAAS) blockers, such as ACE inhibitors or angiotensin receptor blockers, are considered firstline therapy to reduce albuminuria. Additional treatment modalities include diuretics, nondihydropyridine calcium channel blockers, ß-blockers, and aldosterone antagonist therapy. Limiting dietary sodium helps control blood pressure, thus slowing disease progression. In addition, some studies show that limiting phosphorus and protein (for the latter, intake of no more than 0.7 g/kg ideal body weight per day) may slow the progression of CKD. Unfortunately, despite these interventions, patients may still advance to ESRD.1
The aldosterone and steroidal mineralocorticoid receptor antagonists (MRA) spironolactone and eplerenone have been found to reduce albuminuria when used in conjunction with RAAS blockade. However, patients using this combination are up to eight times more likely to experience hyperkalemia—a serious, potentially life-threatening adverse condition—than those not using an MRA.2 The presence of hyperkalemia requires discontinuation of the RAAS blocker and the MRA, at least temporarily.
Finerenone, a nonsteroidal MRA with “greater receptor selectivity than spironolactone and better receptor affinity than eplerenone in vitro,” is in phase III trials for the treatment of systolic and diastolic dysfunction and reduction of morbidity and mortality associated with heart failure.2 One study has already demonstrated that finerenone (5 to 10 mg/d) is at least as effective as spironolactone (25 mg/d) for heart failure patients.3
The Mineralocorticoid Receptor Antagonist Tolerability Study-Diabetic Nephropathy (ARTS-DN) found that finerenone at 10 to 20 mg/d was superior to spironolactone and eplerenone, partly due to the decreased incidence of hyperkalemia. However, it should be noted that the lower incidence of hyperkalemia may be attributable to the fact that 66% of the study participants had an estimated glomerular filtration rate (eGFR) greater than 60 mL/min and that potential participants with a serum potassium level of more than 4.8 mEq/L were not included in the study.2
Additional research is needed to confirm superiority of finerenone over spironolactone and eplerenone, in conjunction with RAAS blockers, in the treatment of albuminuria and hyperkalemia. Including subjects with lower eGFR (such as patients with stage IV CKD who are at higher risk for hyperkalemia) would give a better indication of finerenone’s efficacy. In the meantime, it’s probably too soon to corner the market on this stock! —SEB
Susan E. Brown, MS, ARNP, ACNP-BC, CCRN
Great River Nephrology, West Burlington, Iowa
References
1. Parikh SV, Haddad NJ, Hebert LA. Retarding progression of kidney disease. In: Johnson RJ, Feehally J, Floege J, eds. Comprehensive Clinical Nephrology. 5th ed. Philadelphia, PA: Saunders; 2015:931-940.
2. Bakris GL, Agarwal R, Chan JC, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884-894.
3. Kolkhof P, Delbeck M, Kretschmer A, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist, protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69-78.
Genetics of Renal Disease: APOL1 Variations
Q) I have heard about a gene that causes high blood pressure. Did I hear that right? Is testing for this gene available now?
African-Americans have a higher risk for chronic kidney disease (CKD), including end-stage renal disease (ESRD; defined as kidney failure requiring dialysis or transplant), than any other racial or ethnic group in the United States.1 Previously, this has been attributed to poorly controlled hypertension and diabetes, as well as socioeconomic factors such as limited access to health care.
Research now shows that autosomal recessive genetic variations on chromosome 22q, the gene that encodes apolipoprotein-1 (APOL1; an HDL protein), promote hypertension. This subsequently increases the risk for and progression of CKD in black patients (who have up to 29x higher risk than white patients without this genetic variation).2
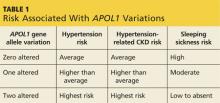
The APOL1 gene has two alleles. Having at least one of them provides resistance to Trypanosoma brucei, the cause of “sleeping sickness” transmitted by the tsetse fly, but increases risk for CKD and ESRD (see Table 1).2,3 Black patients descending from the southern and western portions of Africa are most likely to have two alleles, putting them at the highest risk for hypertension and associated CKD.
Foster et al reported that black patients with two altered alleles had a 31% higher risk for CKD and ESRD, compared with individuals with hypertension-induced nephrosclerosis who had zero to one altered alleles.4 Nondiabetic black patients with CKD who have two altered alleles are at highest risk for focal segmental glomerulosclerosis, HIV nephropathy, and CKD attributable to hypertension.2 The African-American Study of Kidney Disease and Hypertension found that black patients with hypertension controlled by ACE inhibitors had slower progression of CKD, regardless of allele variation.5 Currently, there is no treatment for this genetic alteration.4
One could posit that black patients undergoing renal transplant would have a higher risk for renal failure in the transplanted kidney due to APOL1-related hypertension, compared to nonblack renal transplant recipients. Additionally, a donor kidney with an altered APOL1 gene may have a higher risk for failure.6
Genotyping for APOL1 (CPT code: 81479) is available in select laboratories at a cost of approximately $400.7 For a family that has a member affected by kidney failure at a young age, knowing whether the APOL1 gene is carried in the family would allow early aggressive hypertension management to help prevent a lifetime of severe CKD.
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personalizedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Disease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Q) I have heard about a gene that causes high blood pressure. Did I hear that right? Is testing for this gene available now?
African-Americans have a higher risk for chronic kidney disease (CKD), including end-stage renal disease (ESRD; defined as kidney failure requiring dialysis or transplant), than any other racial or ethnic group in the United States.1 Previously, this has been attributed to poorly controlled hypertension and diabetes, as well as socioeconomic factors such as limited access to health care.
Research now shows that autosomal recessive genetic variations on chromosome 22q, the gene that encodes apolipoprotein-1 (APOL1; an HDL protein), promote hypertension. This subsequently increases the risk for and progression of CKD in black patients (who have up to 29x higher risk than white patients without this genetic variation).2
The APOL1 gene has two alleles. Having at least one of them provides resistance to Trypanosoma brucei, the cause of “sleeping sickness” transmitted by the tsetse fly, but increases risk for CKD and ESRD (see Table 1).2,3 Black patients descending from the southern and western portions of Africa are most likely to have two alleles, putting them at the highest risk for hypertension and associated CKD.
Foster et al reported that black patients with two altered alleles had a 31% higher risk for CKD and ESRD, compared with individuals with hypertension-induced nephrosclerosis who had zero to one altered alleles.4 Nondiabetic black patients with CKD who have two altered alleles are at highest risk for focal segmental glomerulosclerosis, HIV nephropathy, and CKD attributable to hypertension.2 The African-American Study of Kidney Disease and Hypertension found that black patients with hypertension controlled by ACE inhibitors had slower progression of CKD, regardless of allele variation.5 Currently, there is no treatment for this genetic alteration.4
One could posit that black patients undergoing renal transplant would have a higher risk for renal failure in the transplanted kidney due to APOL1-related hypertension, compared to nonblack renal transplant recipients. Additionally, a donor kidney with an altered APOL1 gene may have a higher risk for failure.6
Genotyping for APOL1 (CPT code: 81479) is available in select laboratories at a cost of approximately $400.7 For a family that has a member affected by kidney failure at a young age, knowing whether the APOL1 gene is carried in the family would allow early aggressive hypertension management to help prevent a lifetime of severe CKD.
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personalizedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Disease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Q) I have heard about a gene that causes high blood pressure. Did I hear that right? Is testing for this gene available now?
African-Americans have a higher risk for chronic kidney disease (CKD), including end-stage renal disease (ESRD; defined as kidney failure requiring dialysis or transplant), than any other racial or ethnic group in the United States.1 Previously, this has been attributed to poorly controlled hypertension and diabetes, as well as socioeconomic factors such as limited access to health care.
Research now shows that autosomal recessive genetic variations on chromosome 22q, the gene that encodes apolipoprotein-1 (APOL1; an HDL protein), promote hypertension. This subsequently increases the risk for and progression of CKD in black patients (who have up to 29x higher risk than white patients without this genetic variation).2
The APOL1 gene has two alleles. Having at least one of them provides resistance to Trypanosoma brucei, the cause of “sleeping sickness” transmitted by the tsetse fly, but increases risk for CKD and ESRD (see Table 1).2,3 Black patients descending from the southern and western portions of Africa are most likely to have two alleles, putting them at the highest risk for hypertension and associated CKD.
Foster et al reported that black patients with two altered alleles had a 31% higher risk for CKD and ESRD, compared with individuals with hypertension-induced nephrosclerosis who had zero to one altered alleles.4 Nondiabetic black patients with CKD who have two altered alleles are at highest risk for focal segmental glomerulosclerosis, HIV nephropathy, and CKD attributable to hypertension.2 The African-American Study of Kidney Disease and Hypertension found that black patients with hypertension controlled by ACE inhibitors had slower progression of CKD, regardless of allele variation.5 Currently, there is no treatment for this genetic alteration.4
One could posit that black patients undergoing renal transplant would have a higher risk for renal failure in the transplanted kidney due to APOL1-related hypertension, compared to nonblack renal transplant recipients. Additionally, a donor kidney with an altered APOL1 gene may have a higher risk for failure.6
Genotyping for APOL1 (CPT code: 81479) is available in select laboratories at a cost of approximately $400.7 For a family that has a member affected by kidney failure at a young age, knowing whether the APOL1 gene is carried in the family would allow early aggressive hypertension management to help prevent a lifetime of severe CKD.
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personalizedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Disease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
The Risk and Treatment for Wilms Tumors
Q) In school, they always emphasized the abdominal exam to rule out Wilms tumors. Are Wilms tumors still with us? Has treatment and evaluation changed?
Wilms tumor is a renal cancer found most commonly in children younger than 9 and represents approximately 7% of all malignancies in children.8,9 It can occur in one or both kidneys, with earlier diagnosis noted with bilateral involvement. Risk is highest among non-Hispanic white persons and African-Americans and lowest among Asians.8
Wilms tumor develops due to a genetic mutation in the WT1 gene located on the 11p13 chromosome. Defects are also noted on the 11p15 chromosome and the p53 tumor suppressor gene.10 Urbach et al recently identified a relationship between the LIN28 gene and Wilms tumor.11 Tumors develop when embryonic renal cells that should cease growing at the time of birth continue to grow in the postnatal period. Wilms tumor can be familial or sporadic. It can also be associated with various congenital anomalies manifested within various syndromes (see Table 2), as well as isolated genitourinary abnormalities, especially in boys.10
Most children present with a palpable, smooth, firm, generally painless mass in the abdomen; those who have bilateral renal involvement usually present earlier than those with unilateral involvement. Palpation of the abdomen during examination, if vigorous, can result in rupture of the renal capsule and tumor spillage. Additional symptoms include hematuria, fever, and hypertension. Referral to pediatric oncology is imperative.12
Definitive diagnosis is made by histologic evaluation following biopsy or surgical excision.13 Other possible diagnostic tests include but are not limited to abdominal ultrasound or CT; chest CT (to rule out metastatic lung disease); urinalysis (to evaluate for hematuria and proteinuria); liver function studies (to evaluate for hepatic involvement); and laboratory studies to measure coagulation, serum calcium, blood urea nitrogen, creatinine, and complete blood count.
Histologic examination for staging (I-V) occurs following surgical excision of the tumor. There are two staging systems available: the National Wilms Tumor Study, based on postoperative tumor evaluation, and the International Society of Pediatric Oncology, based on postchemotherapy evaluation.13
Treatment options include surgical excision (including complete nephrectomy of the affected kidney), chemotherapy based on tumor staging, and internal and/or external radiation therapy.13
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personal izedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Dis ease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Q) In school, they always emphasized the abdominal exam to rule out Wilms tumors. Are Wilms tumors still with us? Has treatment and evaluation changed?
Wilms tumor is a renal cancer found most commonly in children younger than 9 and represents approximately 7% of all malignancies in children.8,9 It can occur in one or both kidneys, with earlier diagnosis noted with bilateral involvement. Risk is highest among non-Hispanic white persons and African-Americans and lowest among Asians.8
Wilms tumor develops due to a genetic mutation in the WT1 gene located on the 11p13 chromosome. Defects are also noted on the 11p15 chromosome and the p53 tumor suppressor gene.10 Urbach et al recently identified a relationship between the LIN28 gene and Wilms tumor.11 Tumors develop when embryonic renal cells that should cease growing at the time of birth continue to grow in the postnatal period. Wilms tumor can be familial or sporadic. It can also be associated with various congenital anomalies manifested within various syndromes (see Table 2), as well as isolated genitourinary abnormalities, especially in boys.10
Most children present with a palpable, smooth, firm, generally painless mass in the abdomen; those who have bilateral renal involvement usually present earlier than those with unilateral involvement. Palpation of the abdomen during examination, if vigorous, can result in rupture of the renal capsule and tumor spillage. Additional symptoms include hematuria, fever, and hypertension. Referral to pediatric oncology is imperative.12
Definitive diagnosis is made by histologic evaluation following biopsy or surgical excision.13 Other possible diagnostic tests include but are not limited to abdominal ultrasound or CT; chest CT (to rule out metastatic lung disease); urinalysis (to evaluate for hematuria and proteinuria); liver function studies (to evaluate for hepatic involvement); and laboratory studies to measure coagulation, serum calcium, blood urea nitrogen, creatinine, and complete blood count.
Histologic examination for staging (I-V) occurs following surgical excision of the tumor. There are two staging systems available: the National Wilms Tumor Study, based on postoperative tumor evaluation, and the International Society of Pediatric Oncology, based on postchemotherapy evaluation.13
Treatment options include surgical excision (including complete nephrectomy of the affected kidney), chemotherapy based on tumor staging, and internal and/or external radiation therapy.13
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personal izedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Dis ease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Q) In school, they always emphasized the abdominal exam to rule out Wilms tumors. Are Wilms tumors still with us? Has treatment and evaluation changed?
Wilms tumor is a renal cancer found most commonly in children younger than 9 and represents approximately 7% of all malignancies in children.8,9 It can occur in one or both kidneys, with earlier diagnosis noted with bilateral involvement. Risk is highest among non-Hispanic white persons and African-Americans and lowest among Asians.8
Wilms tumor develops due to a genetic mutation in the WT1 gene located on the 11p13 chromosome. Defects are also noted on the 11p15 chromosome and the p53 tumor suppressor gene.10 Urbach et al recently identified a relationship between the LIN28 gene and Wilms tumor.11 Tumors develop when embryonic renal cells that should cease growing at the time of birth continue to grow in the postnatal period. Wilms tumor can be familial or sporadic. It can also be associated with various congenital anomalies manifested within various syndromes (see Table 2), as well as isolated genitourinary abnormalities, especially in boys.10
Most children present with a palpable, smooth, firm, generally painless mass in the abdomen; those who have bilateral renal involvement usually present earlier than those with unilateral involvement. Palpation of the abdomen during examination, if vigorous, can result in rupture of the renal capsule and tumor spillage. Additional symptoms include hematuria, fever, and hypertension. Referral to pediatric oncology is imperative.12
Definitive diagnosis is made by histologic evaluation following biopsy or surgical excision.13 Other possible diagnostic tests include but are not limited to abdominal ultrasound or CT; chest CT (to rule out metastatic lung disease); urinalysis (to evaluate for hematuria and proteinuria); liver function studies (to evaluate for hepatic involvement); and laboratory studies to measure coagulation, serum calcium, blood urea nitrogen, creatinine, and complete blood count.
Histologic examination for staging (I-V) occurs following surgical excision of the tumor. There are two staging systems available: the National Wilms Tumor Study, based on postoperative tumor evaluation, and the International Society of Pediatric Oncology, based on postchemotherapy evaluation.13
Treatment options include surgical excision (including complete nephrectomy of the affected kidney), chemotherapy based on tumor staging, and internal and/or external radiation therapy.13
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personal izedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Dis ease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.