User login
Product Update
REVIEW
By James Greenberg, MD
Chief of Gynecology
Associate Professor, Harvard Medical School
Boston, Massachusetts
Guardenia: A “really great solution” for contained tissue extraction
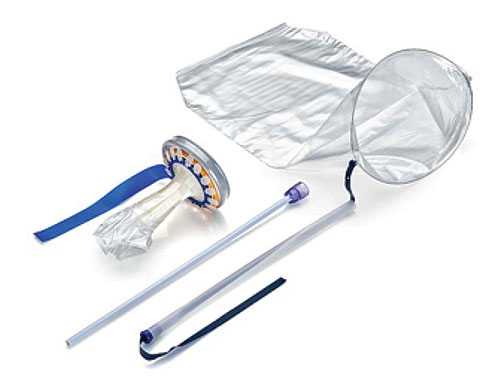
The Guardenia Contained Extraction System, developed by Advanced Surgical Concepts (Wicklow, Ireland), offers a comprehensive approach to contained tissue extraction.
Background. Contained tissue extraction has been an integral part of laparoscopic procedures from at least the late 1980s and early 1990s. Bags of one sort or another are routinely used to remove from the abdomen all sorts of human tissues, including but not limited to ovaries, ectopic pregnancies, gallbladders, kidneys, spleens, and the list goes on. However, after the April 17, 2014, FDA Safety Communication discouraging the use of power morcellators with myomectomy or hysterectomy, the need for more robust contained tissue extraction systems has been ongoing, and there has yet to be a really good solution despite some innovative attempts.
Design/Functionality. Advanced Surgical Concepts’ Guardenia System is the latest attempt to provide a “really great solution” for surgeons to address my 3 “musts” for any system to gain traction in this niche.
- Must #1. An easy method for getting the device into the abdomen.
- Must #2. An easy method for getting the tissue into the bag.
- Must #3. An easy method for getting the tissue out of the body while still containing the cells within the system.
In my opinion based on my usage, Guardenia does a pretty good job addressing all my “musts.”
The Guardenia System is a sterile, single-use device with 3 main components—an introducer with a plunger that will fit through any standard 12-mm trocar, a polyurethane film bag supported by a nitinol ring, and an opening ring with expandable semi-rigid polyethylene “Guard Petals.” When I used it in the operating room to manually morcellate a 10-cm myoma through the umbilicus, I thought it was pretty spot on. Getting the bag into the abdomen is 100% intuitive but even has a nifty up-arrow built onto the tip to make sure the bag is opened in the proper direction. The bag material and the nitinol ring, in combination with the 17.5-cm opening ring, make the process of getting the specimen into the bag and exteriorized easier than any other system I have ever used. And, the opening ring with the Guard Petals yields a very large retraction area for the incision size while providing excellent protection to the surrounding tissues. Overall, Guardenia worked better than anything else I have previously used.
Innovation. Guardenia does not really introduce any fundamentally novel ideas, but it does combine a lot of standard technologies into a product whose sum is much larger than its parts. I would like to see it combined with an occlusive top piece to allow the retractor to be used for single-port laparoscopy as well, but that would just be the cherry on top.
Summary. I have been working on inventing a really good contained tissue extraction system for a long time, and I am a bit chagrined to see someone else outsmart me (low bar), but Advanced Surgical Concepts did, and I really like Guardenia. For pathology that is appropriate for contained morcellation, this device is definitely worth a try, and I suspect many surgeons will switch to it from whatever they are currently using.
FOR MORE INFORMATION, VISIT https://advancedsurgical.ie/guardenia-contained-extraction-system/
The views of the author are personal opinions and do not necessarily represent the views of
REVIEW
By James Greenberg, MD
Chief of Gynecology
Associate Professor, Harvard Medical School
Boston, Massachusetts
Guardenia: A “really great solution” for contained tissue extraction
The Guardenia Contained Extraction System, developed by Advanced Surgical Concepts (Wicklow, Ireland), offers a comprehensive approach to contained tissue extraction.
Background. Contained tissue extraction has been an integral part of laparoscopic procedures from at least the late 1980s and early 1990s. Bags of one sort or another are routinely used to remove from the abdomen all sorts of human tissues, including but not limited to ovaries, ectopic pregnancies, gallbladders, kidneys, spleens, and the list goes on. However, after the April 17, 2014, FDA Safety Communication discouraging the use of power morcellators with myomectomy or hysterectomy, the need for more robust contained tissue extraction systems has been ongoing, and there has yet to be a really good solution despite some innovative attempts.
Design/Functionality. Advanced Surgical Concepts’ Guardenia System is the latest attempt to provide a “really great solution” for surgeons to address my 3 “musts” for any system to gain traction in this niche.
- Must #1. An easy method for getting the device into the abdomen.
- Must #2. An easy method for getting the tissue into the bag.
- Must #3. An easy method for getting the tissue out of the body while still containing the cells within the system.
In my opinion based on my usage, Guardenia does a pretty good job addressing all my “musts.”
The Guardenia System is a sterile, single-use device with 3 main components—an introducer with a plunger that will fit through any standard 12-mm trocar, a polyurethane film bag supported by a nitinol ring, and an opening ring with expandable semi-rigid polyethylene “Guard Petals.” When I used it in the operating room to manually morcellate a 10-cm myoma through the umbilicus, I thought it was pretty spot on. Getting the bag into the abdomen is 100% intuitive but even has a nifty up-arrow built onto the tip to make sure the bag is opened in the proper direction. The bag material and the nitinol ring, in combination with the 17.5-cm opening ring, make the process of getting the specimen into the bag and exteriorized easier than any other system I have ever used. And, the opening ring with the Guard Petals yields a very large retraction area for the incision size while providing excellent protection to the surrounding tissues. Overall, Guardenia worked better than anything else I have previously used.
Innovation. Guardenia does not really introduce any fundamentally novel ideas, but it does combine a lot of standard technologies into a product whose sum is much larger than its parts. I would like to see it combined with an occlusive top piece to allow the retractor to be used for single-port laparoscopy as well, but that would just be the cherry on top.
Summary. I have been working on inventing a really good contained tissue extraction system for a long time, and I am a bit chagrined to see someone else outsmart me (low bar), but Advanced Surgical Concepts did, and I really like Guardenia. For pathology that is appropriate for contained morcellation, this device is definitely worth a try, and I suspect many surgeons will switch to it from whatever they are currently using.
FOR MORE INFORMATION, VISIT https://advancedsurgical.ie/guardenia-contained-extraction-system/
The views of the author are personal opinions and do not necessarily represent the views of
REVIEW
By James Greenberg, MD
Chief of Gynecology
Associate Professor, Harvard Medical School
Boston, Massachusetts
Guardenia: A “really great solution” for contained tissue extraction
The Guardenia Contained Extraction System, developed by Advanced Surgical Concepts (Wicklow, Ireland), offers a comprehensive approach to contained tissue extraction.
Background. Contained tissue extraction has been an integral part of laparoscopic procedures from at least the late 1980s and early 1990s. Bags of one sort or another are routinely used to remove from the abdomen all sorts of human tissues, including but not limited to ovaries, ectopic pregnancies, gallbladders, kidneys, spleens, and the list goes on. However, after the April 17, 2014, FDA Safety Communication discouraging the use of power morcellators with myomectomy or hysterectomy, the need for more robust contained tissue extraction systems has been ongoing, and there has yet to be a really good solution despite some innovative attempts.
Design/Functionality. Advanced Surgical Concepts’ Guardenia System is the latest attempt to provide a “really great solution” for surgeons to address my 3 “musts” for any system to gain traction in this niche.
- Must #1. An easy method for getting the device into the abdomen.
- Must #2. An easy method for getting the tissue into the bag.
- Must #3. An easy method for getting the tissue out of the body while still containing the cells within the system.
In my opinion based on my usage, Guardenia does a pretty good job addressing all my “musts.”
The Guardenia System is a sterile, single-use device with 3 main components—an introducer with a plunger that will fit through any standard 12-mm trocar, a polyurethane film bag supported by a nitinol ring, and an opening ring with expandable semi-rigid polyethylene “Guard Petals.” When I used it in the operating room to manually morcellate a 10-cm myoma through the umbilicus, I thought it was pretty spot on. Getting the bag into the abdomen is 100% intuitive but even has a nifty up-arrow built onto the tip to make sure the bag is opened in the proper direction. The bag material and the nitinol ring, in combination with the 17.5-cm opening ring, make the process of getting the specimen into the bag and exteriorized easier than any other system I have ever used. And, the opening ring with the Guard Petals yields a very large retraction area for the incision size while providing excellent protection to the surrounding tissues. Overall, Guardenia worked better than anything else I have previously used.
Innovation. Guardenia does not really introduce any fundamentally novel ideas, but it does combine a lot of standard technologies into a product whose sum is much larger than its parts. I would like to see it combined with an occlusive top piece to allow the retractor to be used for single-port laparoscopy as well, but that would just be the cherry on top.
Summary. I have been working on inventing a really good contained tissue extraction system for a long time, and I am a bit chagrined to see someone else outsmart me (low bar), but Advanced Surgical Concepts did, and I really like Guardenia. For pathology that is appropriate for contained morcellation, this device is definitely worth a try, and I suspect many surgeons will switch to it from whatever they are currently using.
FOR MORE INFORMATION, VISIT https://advancedsurgical.ie/guardenia-contained-extraction-system/
The views of the author are personal opinions and do not necessarily represent the views of
Product updates and reviews
Product Update
Newly available single-use vaginal speculum
Ceek Women’s Health introduces the Nella single-use vaginal speculum for use during gynecologic examinations and procedures. Designed “by women for women, along with trusted clinicians to enhance patient comfort,” according to Ceek’s press release, the Nella speculum has a quiet operating mechanism, an LED light, and sidewall retractors. Its narrow shape allows for patient comfort and cervical visualization and because it is single use, it eliminates possibilities of cross contamination, according to the manufacturer. In addition, Ceek says it is an ergonomic tool, made from premium material, and is available in one size.
For more information, visit https://www.nellaspec.com

Product Update
Newly available single-use vaginal speculum
Ceek Women’s Health introduces the Nella single-use vaginal speculum for use during gynecologic examinations and procedures. Designed “by women for women, along with trusted clinicians to enhance patient comfort,” according to Ceek’s press release, the Nella speculum has a quiet operating mechanism, an LED light, and sidewall retractors. Its narrow shape allows for patient comfort and cervical visualization and because it is single use, it eliminates possibilities of cross contamination, according to the manufacturer. In addition, Ceek says it is an ergonomic tool, made from premium material, and is available in one size.
For more information, visit https://www.nellaspec.com

Product Update
Newly available single-use vaginal speculum
Ceek Women’s Health introduces the Nella single-use vaginal speculum for use during gynecologic examinations and procedures. Designed “by women for women, along with trusted clinicians to enhance patient comfort,” according to Ceek’s press release, the Nella speculum has a quiet operating mechanism, an LED light, and sidewall retractors. Its narrow shape allows for patient comfort and cervical visualization and because it is single use, it eliminates possibilities of cross contamination, according to the manufacturer. In addition, Ceek says it is an ergonomic tool, made from premium material, and is available in one size.
For more information, visit https://www.nellaspec.com

Product updates and reviews
REVIEW
Butterfly iQ+: Offering day-to-day portable ultrasound tech
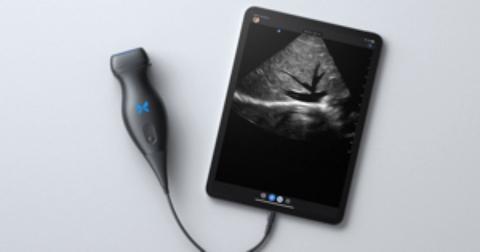
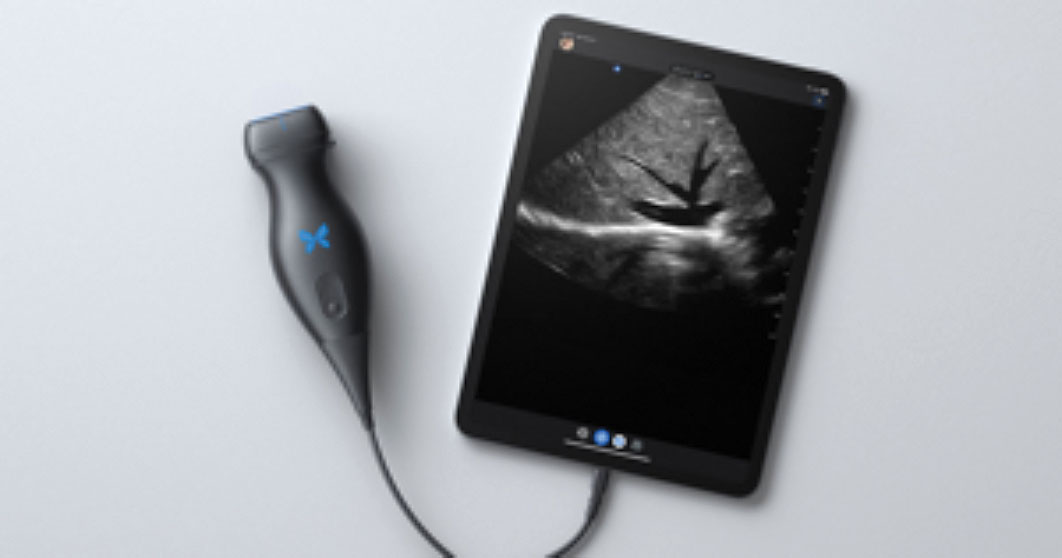
The Butterfly iQ+ app with an ultrasound probe and cable is available from Butterfly Network, Inc, in Guilford, Connecticut.
Background. It could be reasonably argued that ultrasonography has surpassed the speculum as the single most important tool in ObGyn. From its origins in 1949 with the pioneering work of George Ludwig using A-mode (amplitude-mode) ultrasound and the first publication of its use in pregnancy using B-mode (brightness-mode) ultrasound in Lancet in 1958 by Donald and colleagues, this technology has become so ingrained into ObGyn that it is often frustrating to practice comfortably without it. Thus, today, the biggest question facing most practitioners is not whether or not to have an ultrasound in their practice but which one to have.
Given the wide range of quality, functionality, and price within the ultrasound device space, choosing the right technology can feel as daunting as choosing the perfect restaurant in New York City. That said, when looking for entry-level ultrasound technology to address the day-to-day basic needs of your average ObGyn, the Butterfly iQ+ may be an easy choice.
Design/Functionality. The Butterfly iQ+ app does not come with a screen. Rather, the device is compatible with both iOS and Android systems and readily connects to a vast array of easily purchased devices, with either lightening or USB-C ports. In our office, we use an iPad mini. The probe is lightweight (309 g) and contains a rechargeable 2600 mAh lithium ion battery, so that its power source is independent of the device to which it is attached. The probe is a 2D array with 9000 micro-machined sensors. It allows for imaging using M-mode, B-mode, Color Doppler, Power Doppler, and Pulsed Wave Doppler. (I don’t know what the last two are or what they are used for, but they sound important.) It has a scan depth range of 1 cm to 30 cm. The downloadable Butterfly iQ+ app that has the software that makes the probe functional has more tools, controls, and presets than anyone could ever need. But that’s not all. The App has data encrypted HIPAA/HITECH-compliant Cloud-based connectivity that offers unlimited image storage, access to reports, and embedded CPT codes should billing capabilities be needed.
The true beauty of the Butterfly iQ+ is that the image quality is awesome and it is really easy to use. The software is mostly intuitive and takes only a minimal effort to learn. The device holds its charge more than adequately for a day in the office and the recharging process is fast and easy. When it comes to the device’s design and functionality–as a Capricorn–I am still looking for its flaws.
Innovation. The real innovations of the Butterfly iQ+ are its “ultrasound-on-a-chip”™ technology and its incorporation of a rechargeable battery into the probe. This combination allows for crystal clear imaging in a cordless, portable device. While most other similar technologies waste their time, technology, space, and cost on the screen, the Butterfly iQ+ punted on that challenge and put all their efforts into the probe and the software. It was a great choice.
Summary. In our office, the Butterfly iQ+ has changed the way we practice. Our trusty fetal dopplers are mostly gone, having been replaced by the Butterfly iQ+. At almost every prenatal visit, patients can now see their baby rather than just hear the heartbeat (and they can hear it too if they want by using the M-mode functionality on the device). Patients love it, and so do the doctors. Instead of just hearing heart beats, fetal position and quick fluid checks are now routine, so we think our care is actually a little better than it was. The Butterfly iQ+ is also great for confirming IUD locations after placement or when the strings are not visible. All-in-all, I love this product. Who doesn’t love butterflies?!
For more information, visit https://www.butterflynetwork.com
The views of the author are personal opinions and do not necessarily represent the views of OBG
References
- Kaproth-Joslin KA, Nicola R, Dogra VS. The History of US: from bats and boats to the bedside and beyond: RSNA centennial article. Radiographics. 2015;35:960-970.
- Donald I, MacVicar J, Brown TG. Investigation of abdominal masses by pulsed ultrasound. Lancet. 1958;1:1188-1195.
REVIEW
Butterfly iQ+: Offering day-to-day portable ultrasound tech
The Butterfly iQ+ app with an ultrasound probe and cable is available from Butterfly Network, Inc, in Guilford, Connecticut.
Background. It could be reasonably argued that ultrasonography has surpassed the speculum as the single most important tool in ObGyn. From its origins in 1949 with the pioneering work of George Ludwig using A-mode (amplitude-mode) ultrasound and the first publication of its use in pregnancy using B-mode (brightness-mode) ultrasound in Lancet in 1958 by Donald and colleagues, this technology has become so ingrained into ObGyn that it is often frustrating to practice comfortably without it. Thus, today, the biggest question facing most practitioners is not whether or not to have an ultrasound in their practice but which one to have.
Given the wide range of quality, functionality, and price within the ultrasound device space, choosing the right technology can feel as daunting as choosing the perfect restaurant in New York City. That said, when looking for entry-level ultrasound technology to address the day-to-day basic needs of your average ObGyn, the Butterfly iQ+ may be an easy choice.
Design/Functionality. The Butterfly iQ+ app does not come with a screen. Rather, the device is compatible with both iOS and Android systems and readily connects to a vast array of easily purchased devices, with either lightening or USB-C ports. In our office, we use an iPad mini. The probe is lightweight (309 g) and contains a rechargeable 2600 mAh lithium ion battery, so that its power source is independent of the device to which it is attached. The probe is a 2D array with 9000 micro-machined sensors. It allows for imaging using M-mode, B-mode, Color Doppler, Power Doppler, and Pulsed Wave Doppler. (I don’t know what the last two are or what they are used for, but they sound important.) It has a scan depth range of 1 cm to 30 cm. The downloadable Butterfly iQ+ app that has the software that makes the probe functional has more tools, controls, and presets than anyone could ever need. But that’s not all. The App has data encrypted HIPAA/HITECH-compliant Cloud-based connectivity that offers unlimited image storage, access to reports, and embedded CPT codes should billing capabilities be needed.
The true beauty of the Butterfly iQ+ is that the image quality is awesome and it is really easy to use. The software is mostly intuitive and takes only a minimal effort to learn. The device holds its charge more than adequately for a day in the office and the recharging process is fast and easy. When it comes to the device’s design and functionality–as a Capricorn–I am still looking for its flaws.
Innovation. The real innovations of the Butterfly iQ+ are its “ultrasound-on-a-chip”™ technology and its incorporation of a rechargeable battery into the probe. This combination allows for crystal clear imaging in a cordless, portable device. While most other similar technologies waste their time, technology, space, and cost on the screen, the Butterfly iQ+ punted on that challenge and put all their efforts into the probe and the software. It was a great choice.
Summary. In our office, the Butterfly iQ+ has changed the way we practice. Our trusty fetal dopplers are mostly gone, having been replaced by the Butterfly iQ+. At almost every prenatal visit, patients can now see their baby rather than just hear the heartbeat (and they can hear it too if they want by using the M-mode functionality on the device). Patients love it, and so do the doctors. Instead of just hearing heart beats, fetal position and quick fluid checks are now routine, so we think our care is actually a little better than it was. The Butterfly iQ+ is also great for confirming IUD locations after placement or when the strings are not visible. All-in-all, I love this product. Who doesn’t love butterflies?!
For more information, visit https://www.butterflynetwork.com
The views of the author are personal opinions and do not necessarily represent the views of OBG
References
- Kaproth-Joslin KA, Nicola R, Dogra VS. The History of US: from bats and boats to the bedside and beyond: RSNA centennial article. Radiographics. 2015;35:960-970.
- Donald I, MacVicar J, Brown TG. Investigation of abdominal masses by pulsed ultrasound. Lancet. 1958;1:1188-1195.
REVIEW
Butterfly iQ+: Offering day-to-day portable ultrasound tech
The Butterfly iQ+ app with an ultrasound probe and cable is available from Butterfly Network, Inc, in Guilford, Connecticut.
Background. It could be reasonably argued that ultrasonography has surpassed the speculum as the single most important tool in ObGyn. From its origins in 1949 with the pioneering work of George Ludwig using A-mode (amplitude-mode) ultrasound and the first publication of its use in pregnancy using B-mode (brightness-mode) ultrasound in Lancet in 1958 by Donald and colleagues, this technology has become so ingrained into ObGyn that it is often frustrating to practice comfortably without it. Thus, today, the biggest question facing most practitioners is not whether or not to have an ultrasound in their practice but which one to have.
Given the wide range of quality, functionality, and price within the ultrasound device space, choosing the right technology can feel as daunting as choosing the perfect restaurant in New York City. That said, when looking for entry-level ultrasound technology to address the day-to-day basic needs of your average ObGyn, the Butterfly iQ+ may be an easy choice.
Design/Functionality. The Butterfly iQ+ app does not come with a screen. Rather, the device is compatible with both iOS and Android systems and readily connects to a vast array of easily purchased devices, with either lightening or USB-C ports. In our office, we use an iPad mini. The probe is lightweight (309 g) and contains a rechargeable 2600 mAh lithium ion battery, so that its power source is independent of the device to which it is attached. The probe is a 2D array with 9000 micro-machined sensors. It allows for imaging using M-mode, B-mode, Color Doppler, Power Doppler, and Pulsed Wave Doppler. (I don’t know what the last two are or what they are used for, but they sound important.) It has a scan depth range of 1 cm to 30 cm. The downloadable Butterfly iQ+ app that has the software that makes the probe functional has more tools, controls, and presets than anyone could ever need. But that’s not all. The App has data encrypted HIPAA/HITECH-compliant Cloud-based connectivity that offers unlimited image storage, access to reports, and embedded CPT codes should billing capabilities be needed.
The true beauty of the Butterfly iQ+ is that the image quality is awesome and it is really easy to use. The software is mostly intuitive and takes only a minimal effort to learn. The device holds its charge more than adequately for a day in the office and the recharging process is fast and easy. When it comes to the device’s design and functionality–as a Capricorn–I am still looking for its flaws.
Innovation. The real innovations of the Butterfly iQ+ are its “ultrasound-on-a-chip”™ technology and its incorporation of a rechargeable battery into the probe. This combination allows for crystal clear imaging in a cordless, portable device. While most other similar technologies waste their time, technology, space, and cost on the screen, the Butterfly iQ+ punted on that challenge and put all their efforts into the probe and the software. It was a great choice.
Summary. In our office, the Butterfly iQ+ has changed the way we practice. Our trusty fetal dopplers are mostly gone, having been replaced by the Butterfly iQ+. At almost every prenatal visit, patients can now see their baby rather than just hear the heartbeat (and they can hear it too if they want by using the M-mode functionality on the device). Patients love it, and so do the doctors. Instead of just hearing heart beats, fetal position and quick fluid checks are now routine, so we think our care is actually a little better than it was. The Butterfly iQ+ is also great for confirming IUD locations after placement or when the strings are not visible. All-in-all, I love this product. Who doesn’t love butterflies?!
For more information, visit https://www.butterflynetwork.com
The views of the author are personal opinions and do not necessarily represent the views of OBG
References
- Kaproth-Joslin KA, Nicola R, Dogra VS. The History of US: from bats and boats to the bedside and beyond: RSNA centennial article. Radiographics. 2015;35:960-970.
- Donald I, MacVicar J, Brown TG. Investigation of abdominal masses by pulsed ultrasound. Lancet. 1958;1:1188-1195.
Product updates and reviews
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort
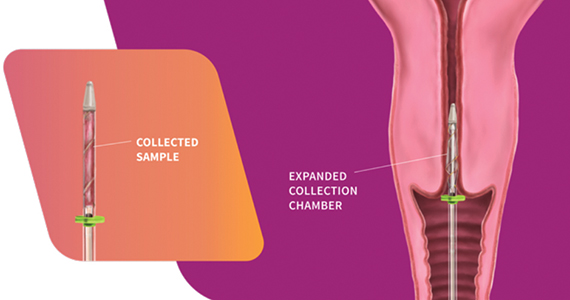
The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort

The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort

The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.
Product updates and reviews
HEGENBERGER RETRACTOR: IS IT HELPFUL FOR PERINEAL REPAIR?
The Hegenberger Retractor, manufactured by Hegenberger Medical (Abingdon, United Kingdom) is available for purchase in the United States through Rocket Medical. A video that I find particularly useful for explaining its use is available here: https://www.youtube.com /watch?v=p-jilXgXZLY
Background. About 85% of women having a vaginal birth experience some form of perineal trauma, and 60% to 70% receive stitches for those spontaneous tears or intentional incisions. As such, repairing perineal lacerations is a requisite skill for all obstetricians and midwives, and every provider has developed exposure techniques to perform their suturing with the goals of good tissue re-approximation, efficiency, minimized patient discomfort, reduced blood loss, and safety from needle sticks. For several millennia, the most commonly used tissue retractor for these repairs has been one’s own fingers, or those of a colleague. While cost-effective and readily available, fingers do have drawbacks as a vaginal retractor. First, their use as a retractor precludes their use for other tasks. Second, their frequent need to be inserted and replaced (see drawback #1) can be uncomfortable for patients. Third, their limited surface area is often insufficient to appropriately provide adequate tissue retraction for optimal surgical site visualization. Finally, they get tired and typically do not appreciate being stuck with needles. Given all this, it is surprising that so many centuries have passed with so little innovation for this ubiquitous procedure. Fortunately, Danish midwife Malene Hegenberger thought now was a good time to change the status quo.
Design/Functionality. The Hegenberger Retractor is brilliant in its simplicity. Its unique molded plastic design is smooth, ergonomic, nonconductive, and packaged as a single-use sterile device. Amazingly, it has a near-perfect pliability balance, making it simultaneously easy to compress for insertion while providing enough retraction tension for good visualization once it has been reexpanded. The subtle ridges on the compression points are just enough to allow for a good grip, and the notches on the sides are a convenient addition for holding extra suture if needed. The device has been cleared by the US Food and Drug Administration (FDA) as a Class 1 device and is approved for sale in the United States. In my experience with its use, I thought it was easy to place and provided excellent exposure for the repairs I was doing. In fact, I thought it provided as good if not better exposure than what I would expect from a Gelpi retractor without any of the trauma the Gelpi adds with its pointed ends. Smile emoji!
Innovation. In the early 1800s, French midwifery pioneer Marie Boivin introduced a novel pelvimeter and a revolutionary 2-part speculum to the technology of the day. Why it took more than 200 years for the ideas of another cutting-edge midwife to breach the walls of the obstetric technological establishment remains a mystery, but fortunately it has been done. While seemingly obvious, the Hegenberger Retractor is the culmination of years of work and 88 prototypes. It looks simple, but the secret to its functionality is the precision with which each dimension and every curve was designed. The device has been cleared by the FDA as a Class 1 device and is approved for sale in the United States.
Summary. There are a lot of reasons to like the Hegenberger Retractor. I like it for its simplicity; I like it for its functionality; I like it for its ability to fill a real need. On the downside, I do not like that it is a single-use plastic device, and I am not happy about adding cost to obstetric care. Most of all, I hate that I did not invent it.
Is the Hegenberger Retractor going to be needed to repair every obstetric laceration? No. Will it provide perfect exposure to repair every obstetric laceration? Of course not. But it is an incredibly clever device that will be very helpful in many situations, and I suspect it will soon become a mainstay on most maternity units as it gains recognition.
FOR MORE INFORMATION, VISIT www.rocketmedical.com
- McCandlish R, Bowler U, van Asten H, et al. A randomised controlled trial of care of the perineum during second stage of normal labour. Br J Obstet Gynaecol. 1998;105:1262-1272.
- Ferry G. Marie Boivin: from midwife to gynaecologist. Lancet. 2019;393:2192-2193. doi: 10.1016/S0140-6736(19)31188-2.
HEGENBERGER RETRACTOR: IS IT HELPFUL FOR PERINEAL REPAIR?
The Hegenberger Retractor, manufactured by Hegenberger Medical (Abingdon, United Kingdom) is available for purchase in the United States through Rocket Medical. A video that I find particularly useful for explaining its use is available here: https://www.youtube.com /watch?v=p-jilXgXZLY
Background. About 85% of women having a vaginal birth experience some form of perineal trauma, and 60% to 70% receive stitches for those spontaneous tears or intentional incisions. As such, repairing perineal lacerations is a requisite skill for all obstetricians and midwives, and every provider has developed exposure techniques to perform their suturing with the goals of good tissue re-approximation, efficiency, minimized patient discomfort, reduced blood loss, and safety from needle sticks. For several millennia, the most commonly used tissue retractor for these repairs has been one’s own fingers, or those of a colleague. While cost-effective and readily available, fingers do have drawbacks as a vaginal retractor. First, their use as a retractor precludes their use for other tasks. Second, their frequent need to be inserted and replaced (see drawback #1) can be uncomfortable for patients. Third, their limited surface area is often insufficient to appropriately provide adequate tissue retraction for optimal surgical site visualization. Finally, they get tired and typically do not appreciate being stuck with needles. Given all this, it is surprising that so many centuries have passed with so little innovation for this ubiquitous procedure. Fortunately, Danish midwife Malene Hegenberger thought now was a good time to change the status quo.
Design/Functionality. The Hegenberger Retractor is brilliant in its simplicity. Its unique molded plastic design is smooth, ergonomic, nonconductive, and packaged as a single-use sterile device. Amazingly, it has a near-perfect pliability balance, making it simultaneously easy to compress for insertion while providing enough retraction tension for good visualization once it has been reexpanded. The subtle ridges on the compression points are just enough to allow for a good grip, and the notches on the sides are a convenient addition for holding extra suture if needed. The device has been cleared by the US Food and Drug Administration (FDA) as a Class 1 device and is approved for sale in the United States. In my experience with its use, I thought it was easy to place and provided excellent exposure for the repairs I was doing. In fact, I thought it provided as good if not better exposure than what I would expect from a Gelpi retractor without any of the trauma the Gelpi adds with its pointed ends. Smile emoji!
Innovation. In the early 1800s, French midwifery pioneer Marie Boivin introduced a novel pelvimeter and a revolutionary 2-part speculum to the technology of the day. Why it took more than 200 years for the ideas of another cutting-edge midwife to breach the walls of the obstetric technological establishment remains a mystery, but fortunately it has been done. While seemingly obvious, the Hegenberger Retractor is the culmination of years of work and 88 prototypes. It looks simple, but the secret to its functionality is the precision with which each dimension and every curve was designed. The device has been cleared by the FDA as a Class 1 device and is approved for sale in the United States.
Summary. There are a lot of reasons to like the Hegenberger Retractor. I like it for its simplicity; I like it for its functionality; I like it for its ability to fill a real need. On the downside, I do not like that it is a single-use plastic device, and I am not happy about adding cost to obstetric care. Most of all, I hate that I did not invent it.
Is the Hegenberger Retractor going to be needed to repair every obstetric laceration? No. Will it provide perfect exposure to repair every obstetric laceration? Of course not. But it is an incredibly clever device that will be very helpful in many situations, and I suspect it will soon become a mainstay on most maternity units as it gains recognition.
FOR MORE INFORMATION, VISIT www.rocketmedical.com
HEGENBERGER RETRACTOR: IS IT HELPFUL FOR PERINEAL REPAIR?
The Hegenberger Retractor, manufactured by Hegenberger Medical (Abingdon, United Kingdom) is available for purchase in the United States through Rocket Medical. A video that I find particularly useful for explaining its use is available here: https://www.youtube.com /watch?v=p-jilXgXZLY
Background. About 85% of women having a vaginal birth experience some form of perineal trauma, and 60% to 70% receive stitches for those spontaneous tears or intentional incisions. As such, repairing perineal lacerations is a requisite skill for all obstetricians and midwives, and every provider has developed exposure techniques to perform their suturing with the goals of good tissue re-approximation, efficiency, minimized patient discomfort, reduced blood loss, and safety from needle sticks. For several millennia, the most commonly used tissue retractor for these repairs has been one’s own fingers, or those of a colleague. While cost-effective and readily available, fingers do have drawbacks as a vaginal retractor. First, their use as a retractor precludes their use for other tasks. Second, their frequent need to be inserted and replaced (see drawback #1) can be uncomfortable for patients. Third, their limited surface area is often insufficient to appropriately provide adequate tissue retraction for optimal surgical site visualization. Finally, they get tired and typically do not appreciate being stuck with needles. Given all this, it is surprising that so many centuries have passed with so little innovation for this ubiquitous procedure. Fortunately, Danish midwife Malene Hegenberger thought now was a good time to change the status quo.
Design/Functionality. The Hegenberger Retractor is brilliant in its simplicity. Its unique molded plastic design is smooth, ergonomic, nonconductive, and packaged as a single-use sterile device. Amazingly, it has a near-perfect pliability balance, making it simultaneously easy to compress for insertion while providing enough retraction tension for good visualization once it has been reexpanded. The subtle ridges on the compression points are just enough to allow for a good grip, and the notches on the sides are a convenient addition for holding extra suture if needed. The device has been cleared by the US Food and Drug Administration (FDA) as a Class 1 device and is approved for sale in the United States. In my experience with its use, I thought it was easy to place and provided excellent exposure for the repairs I was doing. In fact, I thought it provided as good if not better exposure than what I would expect from a Gelpi retractor without any of the trauma the Gelpi adds with its pointed ends. Smile emoji!
Innovation. In the early 1800s, French midwifery pioneer Marie Boivin introduced a novel pelvimeter and a revolutionary 2-part speculum to the technology of the day. Why it took more than 200 years for the ideas of another cutting-edge midwife to breach the walls of the obstetric technological establishment remains a mystery, but fortunately it has been done. While seemingly obvious, the Hegenberger Retractor is the culmination of years of work and 88 prototypes. It looks simple, but the secret to its functionality is the precision with which each dimension and every curve was designed. The device has been cleared by the FDA as a Class 1 device and is approved for sale in the United States.
Summary. There are a lot of reasons to like the Hegenberger Retractor. I like it for its simplicity; I like it for its functionality; I like it for its ability to fill a real need. On the downside, I do not like that it is a single-use plastic device, and I am not happy about adding cost to obstetric care. Most of all, I hate that I did not invent it.
Is the Hegenberger Retractor going to be needed to repair every obstetric laceration? No. Will it provide perfect exposure to repair every obstetric laceration? Of course not. But it is an incredibly clever device that will be very helpful in many situations, and I suspect it will soon become a mainstay on most maternity units as it gains recognition.
FOR MORE INFORMATION, VISIT www.rocketmedical.com
- McCandlish R, Bowler U, van Asten H, et al. A randomised controlled trial of care of the perineum during second stage of normal labour. Br J Obstet Gynaecol. 1998;105:1262-1272.
- Ferry G. Marie Boivin: from midwife to gynaecologist. Lancet. 2019;393:2192-2193. doi: 10.1016/S0140-6736(19)31188-2.
- McCandlish R, Bowler U, van Asten H, et al. A randomised controlled trial of care of the perineum during second stage of normal labour. Br J Obstet Gynaecol. 1998;105:1262-1272.
- Ferry G. Marie Boivin: from midwife to gynaecologist. Lancet. 2019;393:2192-2193. doi: 10.1016/S0140-6736(19)31188-2.
Product update: HPV assay
BD ONCLARITYTM HPV ASSAY
FOR MORE INFORMATION, VISIT: https://womens-health-solutions.bd.com/
BD ONCLARITYTM HPV ASSAY
FOR MORE INFORMATION, VISIT: https://womens-health-solutions.bd.com/
BD ONCLARITYTM HPV ASSAY
FOR MORE INFORMATION, VISIT: https://womens-health-solutions.bd.com/