User login
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort
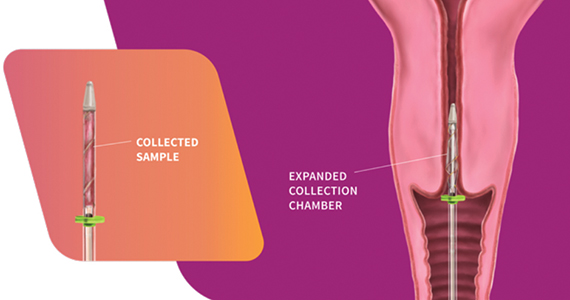
The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort

The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort

The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.