User login
Developing training pathways in advanced endoscopic resection and third-space endoscopy in the U.S.
As a gastroenterology and hepatology fellow, choosing a career path was a daunting prospect. Despite the additional specialization, there seemed to be endless career options to consider. Did I want to join an academic, private, or hybrid practice? Should I subspecialize within the field? Was it important to incorporate research or teaching into my practice? What about opportunities to take on administrative or leadership roles?
Fellowship training at a large academic research institution provided me the opportunity to work with expert faculty in inflammatory bowel disease, esophageal disease, motility and functional gastrointestinal disease, pancreaticobiliary disease, and hepatology. I enjoyed seeing patients in each of these subspecialty clinics. But, by the end of my second year of GI fellowship, I still wasn’t sure what I wanted to do professionally.
A career in academic general gastroenterology seemed to be a good fit for my personality and goals. Rather than focusing on research, I chose to position myself as a clinician educator. I knew that having a subspecialty area of expertise would help improve my clinical practice and make me a more attractive candidate to academic centers. To help narrow my choice, I looked at the clinical enterprise at our institution and assessed where the unmet clinical needs were most acute. Simultaneously, I identified potential mentors to support and guide me through the transition from fellow to independent practitioner. I decided to focus on acquiring the skills to care for patients with anorectal diseases and lower-GI motility disorders, as this area met both of my criteria – excellent mentorship and an unmet clinical need. Under the guidance of Dr. Yolanda Scarlett, I spent my 3rd year in clinic learning to interpret anorectal manometry tests, defecograms, and sitz marker studies and treating patients with refractory constipation, fecal incontinence, and anal fissures.
With a plan to develop an expertise in anorectal diseases and low-GI motility disorders, I also wanted to focus on improving my endoscopic skills to graduate as well rounded a clinician as possible. To achieve this goal, I sought out a separate endoscopy mentor, Dr. Ian Grimm, the director of endoscopy at the University of North Carolina at Chapel Hill. Dr. Grimm, a classically trained advanced endoscopist performing endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP), had a burgeoning interest in endoscopic mucosal resection (EMR) and had just returned from a few months in Japan learning to perform endoscopic submucosal dissection (ESD) and peroral endoscopic myotomy (POEM).
When I began working with Dr. Grimm, I had not even heard the term third-space endoscopy and knew nothing about ESD or POEM. I spent as much time as possible watching and assisting Dr. Grimm with complex endoscopic mucosal resection (EMR) during the first few months of my 3rd year. Soon after my exposure to advanced endoscopic resection, it was clear that I wanted to learn and incorporate this into my clinical practice. I watched Dr. Grimm perform the first POEM at UNC in the fall of 2016 and by that time I was hooked on learning third-space endoscopy. I observed and assisted with as many EMR, ESD, and POEM cases as I could that year. In addition to the hands-on and cognitive training with Dr. Grimm, I attended national meetings and workshops focused on learning third-space endoscopy. In the spring of my 3rd year I was honored to be the first fellow to complete the Olympus master class in ESD – a 2-day hands-on training course sponsored by Olympus. By the end of that year, I was performing complex EMR with minimal assistance and had completed multiple ESDs and POEMs with cognitive supervision only.
After fellowship, I joined the UNC faculty as a general gastroenterologist with expertise in anorectal disease and lower-GI motility disorders. While I was comfortable performing complex EMR, I still needed additional training and supervision before I felt ready to independently perform ESD or POEM. With the gracious support and encouragement of our division chief, I continued third-space endoscopy training with Dr. Grimm during dedicated protected time 2 days each month. Over the ensuing 4 years, I transitioned to fully independent practice performing all types of advanced EMR and third-space endoscopy including complex EMR, ESD, endoscopic full-thickness resection (EFTR), submucosal tunnel endoscopic resection (STER), esophageal POEM, gastric POEM, and Zenker’s POEM.
As one of the first gastroenterologists in the United States to perform third-space endoscopy without any formal training in advanced pancreaticobiliary endoscopy, I believe learning advanced endoscopic resection and third-space endoscopy is best achieved through a training pathway separate from the conventional advanced endoscopy fellowship focused on teaching EUS and ERCP. Although there are transferable skills learned from EUS and ERCP to the techniques used in third-space endoscopy, there is nothing inherent to performing EUS or ERCP that enables one to learn how to perform an ESD or a POEM.
There is a robust training pathway to teach advanced pancreaticobiliary endoscopy, but no formal training pathway exists to teach third-space endoscopy in the United States. Historically, a small number of interested and motivated advanced pancreaticobiliary endoscopists sought out opportunities to learn third-space endoscopy after completion of their advanced endoscopy fellowship, in some cases many years after graduation. For these early adopters in the United States, the only training opportunities required travel to Japan or another Eastern country with arrangements made to observe and participate in third-space endoscopy cases with experts there. With increased recognition of the benefits of ESD and POEM over the past 5-10 years in the United States, there has been greater adoption of third-space endoscopy and with it, more training opportunities. Still, there are very few institutions with formalized training programs in advanced endoscopic resection and third-space endoscopy in the United States to date.
Proof that this model works
In Eastern countries such as Japan, training endoscopists to perform ESD and POEM has been successfully achieved through an apprenticeship model whereby an expert in third-space endoscopy closely supervises a trainee who gains greater autonomy with increasing experience and skill over time. My personal experience is proof that this model works. But, adopting such a model more widely in the United States may prove difficult. We lack a sufficient number of experienced third-space endoscopy operators and, given the challenges to appropriate reimbursement for third-space endoscopy in the United States, there is understandable resistance to accepting the prolonged training period necessary for technical mastery of this skill.
In part, a long training period is needed because of a relative paucity of appropriate target lesions for ESD and the rarity of achalasia in the United States. While there is consensus among experts regarding the benefits of ESD for resection of early gastric cancer (EGC), relatively few EGCs are found in the United States and indications for ESD outside resection of EGC are less well defined with less clear benefits over more widely performed piecemeal EMR. Despite these challenges, it is critical that we continue to develop dedicated training pathways to teach advanced endoscopic resection and third-space endoscopy in the United States. My practice has evolved considerably since completion of fellowship nearly 6 years ago, and I now focus almost exclusively on advanced endoscopic resection and third-space endoscopy. Recently, Dr. Grimm and I began an advanced endoscopic resection elective for the general GI fellows at UNC and we are excited to welcome our first advanced endoscopic resection and third-space endoscopy fellow to UNC this July.
While there are many possible avenues to expertise in advanced endoscopic resection, few will likely follow the same path that I have taken. Trainees who are interested in pursuing this subspecialty should seek out supportive mentors in a setting where there is already a robust case volume of esophageal motility disorders and endoscopic resections. Success requires the persistent motivation to seek out diverse opportunities for self-study, exposure to experts, data on developments in the field, and hands-on exposure to as many ex-vivo and in-vivo cases as possible.
Dr. Kroch is assistant professor of medicine in the division of gastroenterology and hepatology at the University of North Carolina at Chapel Hill. He disclosed having no conflicts of interest.
As a gastroenterology and hepatology fellow, choosing a career path was a daunting prospect. Despite the additional specialization, there seemed to be endless career options to consider. Did I want to join an academic, private, or hybrid practice? Should I subspecialize within the field? Was it important to incorporate research or teaching into my practice? What about opportunities to take on administrative or leadership roles?
Fellowship training at a large academic research institution provided me the opportunity to work with expert faculty in inflammatory bowel disease, esophageal disease, motility and functional gastrointestinal disease, pancreaticobiliary disease, and hepatology. I enjoyed seeing patients in each of these subspecialty clinics. But, by the end of my second year of GI fellowship, I still wasn’t sure what I wanted to do professionally.
A career in academic general gastroenterology seemed to be a good fit for my personality and goals. Rather than focusing on research, I chose to position myself as a clinician educator. I knew that having a subspecialty area of expertise would help improve my clinical practice and make me a more attractive candidate to academic centers. To help narrow my choice, I looked at the clinical enterprise at our institution and assessed where the unmet clinical needs were most acute. Simultaneously, I identified potential mentors to support and guide me through the transition from fellow to independent practitioner. I decided to focus on acquiring the skills to care for patients with anorectal diseases and lower-GI motility disorders, as this area met both of my criteria – excellent mentorship and an unmet clinical need. Under the guidance of Dr. Yolanda Scarlett, I spent my 3rd year in clinic learning to interpret anorectal manometry tests, defecograms, and sitz marker studies and treating patients with refractory constipation, fecal incontinence, and anal fissures.
With a plan to develop an expertise in anorectal diseases and low-GI motility disorders, I also wanted to focus on improving my endoscopic skills to graduate as well rounded a clinician as possible. To achieve this goal, I sought out a separate endoscopy mentor, Dr. Ian Grimm, the director of endoscopy at the University of North Carolina at Chapel Hill. Dr. Grimm, a classically trained advanced endoscopist performing endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP), had a burgeoning interest in endoscopic mucosal resection (EMR) and had just returned from a few months in Japan learning to perform endoscopic submucosal dissection (ESD) and peroral endoscopic myotomy (POEM).
When I began working with Dr. Grimm, I had not even heard the term third-space endoscopy and knew nothing about ESD or POEM. I spent as much time as possible watching and assisting Dr. Grimm with complex endoscopic mucosal resection (EMR) during the first few months of my 3rd year. Soon after my exposure to advanced endoscopic resection, it was clear that I wanted to learn and incorporate this into my clinical practice. I watched Dr. Grimm perform the first POEM at UNC in the fall of 2016 and by that time I was hooked on learning third-space endoscopy. I observed and assisted with as many EMR, ESD, and POEM cases as I could that year. In addition to the hands-on and cognitive training with Dr. Grimm, I attended national meetings and workshops focused on learning third-space endoscopy. In the spring of my 3rd year I was honored to be the first fellow to complete the Olympus master class in ESD – a 2-day hands-on training course sponsored by Olympus. By the end of that year, I was performing complex EMR with minimal assistance and had completed multiple ESDs and POEMs with cognitive supervision only.
After fellowship, I joined the UNC faculty as a general gastroenterologist with expertise in anorectal disease and lower-GI motility disorders. While I was comfortable performing complex EMR, I still needed additional training and supervision before I felt ready to independently perform ESD or POEM. With the gracious support and encouragement of our division chief, I continued third-space endoscopy training with Dr. Grimm during dedicated protected time 2 days each month. Over the ensuing 4 years, I transitioned to fully independent practice performing all types of advanced EMR and third-space endoscopy including complex EMR, ESD, endoscopic full-thickness resection (EFTR), submucosal tunnel endoscopic resection (STER), esophageal POEM, gastric POEM, and Zenker’s POEM.
As one of the first gastroenterologists in the United States to perform third-space endoscopy without any formal training in advanced pancreaticobiliary endoscopy, I believe learning advanced endoscopic resection and third-space endoscopy is best achieved through a training pathway separate from the conventional advanced endoscopy fellowship focused on teaching EUS and ERCP. Although there are transferable skills learned from EUS and ERCP to the techniques used in third-space endoscopy, there is nothing inherent to performing EUS or ERCP that enables one to learn how to perform an ESD or a POEM.
There is a robust training pathway to teach advanced pancreaticobiliary endoscopy, but no formal training pathway exists to teach third-space endoscopy in the United States. Historically, a small number of interested and motivated advanced pancreaticobiliary endoscopists sought out opportunities to learn third-space endoscopy after completion of their advanced endoscopy fellowship, in some cases many years after graduation. For these early adopters in the United States, the only training opportunities required travel to Japan or another Eastern country with arrangements made to observe and participate in third-space endoscopy cases with experts there. With increased recognition of the benefits of ESD and POEM over the past 5-10 years in the United States, there has been greater adoption of third-space endoscopy and with it, more training opportunities. Still, there are very few institutions with formalized training programs in advanced endoscopic resection and third-space endoscopy in the United States to date.
Proof that this model works
In Eastern countries such as Japan, training endoscopists to perform ESD and POEM has been successfully achieved through an apprenticeship model whereby an expert in third-space endoscopy closely supervises a trainee who gains greater autonomy with increasing experience and skill over time. My personal experience is proof that this model works. But, adopting such a model more widely in the United States may prove difficult. We lack a sufficient number of experienced third-space endoscopy operators and, given the challenges to appropriate reimbursement for third-space endoscopy in the United States, there is understandable resistance to accepting the prolonged training period necessary for technical mastery of this skill.
In part, a long training period is needed because of a relative paucity of appropriate target lesions for ESD and the rarity of achalasia in the United States. While there is consensus among experts regarding the benefits of ESD for resection of early gastric cancer (EGC), relatively few EGCs are found in the United States and indications for ESD outside resection of EGC are less well defined with less clear benefits over more widely performed piecemeal EMR. Despite these challenges, it is critical that we continue to develop dedicated training pathways to teach advanced endoscopic resection and third-space endoscopy in the United States. My practice has evolved considerably since completion of fellowship nearly 6 years ago, and I now focus almost exclusively on advanced endoscopic resection and third-space endoscopy. Recently, Dr. Grimm and I began an advanced endoscopic resection elective for the general GI fellows at UNC and we are excited to welcome our first advanced endoscopic resection and third-space endoscopy fellow to UNC this July.
While there are many possible avenues to expertise in advanced endoscopic resection, few will likely follow the same path that I have taken. Trainees who are interested in pursuing this subspecialty should seek out supportive mentors in a setting where there is already a robust case volume of esophageal motility disorders and endoscopic resections. Success requires the persistent motivation to seek out diverse opportunities for self-study, exposure to experts, data on developments in the field, and hands-on exposure to as many ex-vivo and in-vivo cases as possible.
Dr. Kroch is assistant professor of medicine in the division of gastroenterology and hepatology at the University of North Carolina at Chapel Hill. He disclosed having no conflicts of interest.
As a gastroenterology and hepatology fellow, choosing a career path was a daunting prospect. Despite the additional specialization, there seemed to be endless career options to consider. Did I want to join an academic, private, or hybrid practice? Should I subspecialize within the field? Was it important to incorporate research or teaching into my practice? What about opportunities to take on administrative or leadership roles?
Fellowship training at a large academic research institution provided me the opportunity to work with expert faculty in inflammatory bowel disease, esophageal disease, motility and functional gastrointestinal disease, pancreaticobiliary disease, and hepatology. I enjoyed seeing patients in each of these subspecialty clinics. But, by the end of my second year of GI fellowship, I still wasn’t sure what I wanted to do professionally.
A career in academic general gastroenterology seemed to be a good fit for my personality and goals. Rather than focusing on research, I chose to position myself as a clinician educator. I knew that having a subspecialty area of expertise would help improve my clinical practice and make me a more attractive candidate to academic centers. To help narrow my choice, I looked at the clinical enterprise at our institution and assessed where the unmet clinical needs were most acute. Simultaneously, I identified potential mentors to support and guide me through the transition from fellow to independent practitioner. I decided to focus on acquiring the skills to care for patients with anorectal diseases and lower-GI motility disorders, as this area met both of my criteria – excellent mentorship and an unmet clinical need. Under the guidance of Dr. Yolanda Scarlett, I spent my 3rd year in clinic learning to interpret anorectal manometry tests, defecograms, and sitz marker studies and treating patients with refractory constipation, fecal incontinence, and anal fissures.
With a plan to develop an expertise in anorectal diseases and low-GI motility disorders, I also wanted to focus on improving my endoscopic skills to graduate as well rounded a clinician as possible. To achieve this goal, I sought out a separate endoscopy mentor, Dr. Ian Grimm, the director of endoscopy at the University of North Carolina at Chapel Hill. Dr. Grimm, a classically trained advanced endoscopist performing endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP), had a burgeoning interest in endoscopic mucosal resection (EMR) and had just returned from a few months in Japan learning to perform endoscopic submucosal dissection (ESD) and peroral endoscopic myotomy (POEM).
When I began working with Dr. Grimm, I had not even heard the term third-space endoscopy and knew nothing about ESD or POEM. I spent as much time as possible watching and assisting Dr. Grimm with complex endoscopic mucosal resection (EMR) during the first few months of my 3rd year. Soon after my exposure to advanced endoscopic resection, it was clear that I wanted to learn and incorporate this into my clinical practice. I watched Dr. Grimm perform the first POEM at UNC in the fall of 2016 and by that time I was hooked on learning third-space endoscopy. I observed and assisted with as many EMR, ESD, and POEM cases as I could that year. In addition to the hands-on and cognitive training with Dr. Grimm, I attended national meetings and workshops focused on learning third-space endoscopy. In the spring of my 3rd year I was honored to be the first fellow to complete the Olympus master class in ESD – a 2-day hands-on training course sponsored by Olympus. By the end of that year, I was performing complex EMR with minimal assistance and had completed multiple ESDs and POEMs with cognitive supervision only.
After fellowship, I joined the UNC faculty as a general gastroenterologist with expertise in anorectal disease and lower-GI motility disorders. While I was comfortable performing complex EMR, I still needed additional training and supervision before I felt ready to independently perform ESD or POEM. With the gracious support and encouragement of our division chief, I continued third-space endoscopy training with Dr. Grimm during dedicated protected time 2 days each month. Over the ensuing 4 years, I transitioned to fully independent practice performing all types of advanced EMR and third-space endoscopy including complex EMR, ESD, endoscopic full-thickness resection (EFTR), submucosal tunnel endoscopic resection (STER), esophageal POEM, gastric POEM, and Zenker’s POEM.
As one of the first gastroenterologists in the United States to perform third-space endoscopy without any formal training in advanced pancreaticobiliary endoscopy, I believe learning advanced endoscopic resection and third-space endoscopy is best achieved through a training pathway separate from the conventional advanced endoscopy fellowship focused on teaching EUS and ERCP. Although there are transferable skills learned from EUS and ERCP to the techniques used in third-space endoscopy, there is nothing inherent to performing EUS or ERCP that enables one to learn how to perform an ESD or a POEM.
There is a robust training pathway to teach advanced pancreaticobiliary endoscopy, but no formal training pathway exists to teach third-space endoscopy in the United States. Historically, a small number of interested and motivated advanced pancreaticobiliary endoscopists sought out opportunities to learn third-space endoscopy after completion of their advanced endoscopy fellowship, in some cases many years after graduation. For these early adopters in the United States, the only training opportunities required travel to Japan or another Eastern country with arrangements made to observe and participate in third-space endoscopy cases with experts there. With increased recognition of the benefits of ESD and POEM over the past 5-10 years in the United States, there has been greater adoption of third-space endoscopy and with it, more training opportunities. Still, there are very few institutions with formalized training programs in advanced endoscopic resection and third-space endoscopy in the United States to date.
Proof that this model works
In Eastern countries such as Japan, training endoscopists to perform ESD and POEM has been successfully achieved through an apprenticeship model whereby an expert in third-space endoscopy closely supervises a trainee who gains greater autonomy with increasing experience and skill over time. My personal experience is proof that this model works. But, adopting such a model more widely in the United States may prove difficult. We lack a sufficient number of experienced third-space endoscopy operators and, given the challenges to appropriate reimbursement for third-space endoscopy in the United States, there is understandable resistance to accepting the prolonged training period necessary for technical mastery of this skill.
In part, a long training period is needed because of a relative paucity of appropriate target lesions for ESD and the rarity of achalasia in the United States. While there is consensus among experts regarding the benefits of ESD for resection of early gastric cancer (EGC), relatively few EGCs are found in the United States and indications for ESD outside resection of EGC are less well defined with less clear benefits over more widely performed piecemeal EMR. Despite these challenges, it is critical that we continue to develop dedicated training pathways to teach advanced endoscopic resection and third-space endoscopy in the United States. My practice has evolved considerably since completion of fellowship nearly 6 years ago, and I now focus almost exclusively on advanced endoscopic resection and third-space endoscopy. Recently, Dr. Grimm and I began an advanced endoscopic resection elective for the general GI fellows at UNC and we are excited to welcome our first advanced endoscopic resection and third-space endoscopy fellow to UNC this July.
While there are many possible avenues to expertise in advanced endoscopic resection, few will likely follow the same path that I have taken. Trainees who are interested in pursuing this subspecialty should seek out supportive mentors in a setting where there is already a robust case volume of esophageal motility disorders and endoscopic resections. Success requires the persistent motivation to seek out diverse opportunities for self-study, exposure to experts, data on developments in the field, and hands-on exposure to as many ex-vivo and in-vivo cases as possible.
Dr. Kroch is assistant professor of medicine in the division of gastroenterology and hepatology at the University of North Carolina at Chapel Hill. He disclosed having no conflicts of interest.
Advances in endohepatology
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2
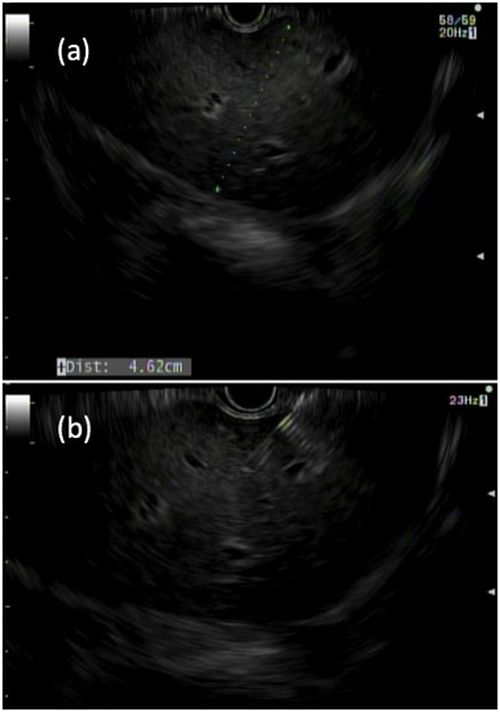
While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5
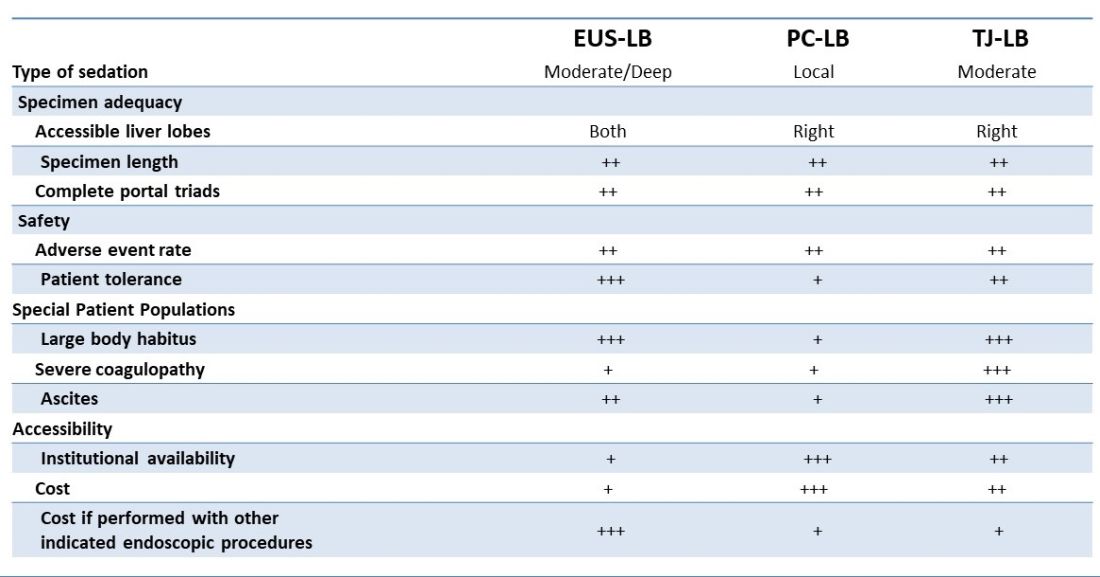
Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2

While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5

Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2

While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5

Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.
I selected a GI career path aligned with my goals
In this video, Dr. David Ramsay of Digestive Health Specialists in Winston Salem, N.C., discusses the different career paths available to fellows and early-career physicians, and why he chose to become a private practice gastroenterologist. Dr. Ramsay shares his insights into different private practice models and what physicians should consider when beginning their careers, as well as what questions to ask when trying to determine if an organization will be a good fit for their future career plans. He has no financial conflicts relative to the topics in this video.

In this video, Dr. David Ramsay of Digestive Health Specialists in Winston Salem, N.C., discusses the different career paths available to fellows and early-career physicians, and why he chose to become a private practice gastroenterologist. Dr. Ramsay shares his insights into different private practice models and what physicians should consider when beginning their careers, as well as what questions to ask when trying to determine if an organization will be a good fit for their future career plans. He has no financial conflicts relative to the topics in this video.

In this video, Dr. David Ramsay of Digestive Health Specialists in Winston Salem, N.C., discusses the different career paths available to fellows and early-career physicians, and why he chose to become a private practice gastroenterologist. Dr. Ramsay shares his insights into different private practice models and what physicians should consider when beginning their careers, as well as what questions to ask when trying to determine if an organization will be a good fit for their future career plans. He has no financial conflicts relative to the topics in this video.

Increase in message volume begs the question: ‘Should we be compensated for our time?’
The American Gastroenterological Association and other gastrointestinal-specific organizations have excellent resources available to members that focus on optimizing reimbursement in your clinical and endoscopic practice.
During the COVID-19 pandemic and public health emergency (PHE), many previously noncovered services were now covered under rules of the Centers for Medicare & Medicaid Services. During the pandemic, patient portal messages increased by 157%, meaning more work for health care teams, negatively impacting physician satisfaction, and increasing burnout.1 Medical burnout has been associated with increased time spent on electronic health records, with some subspeciality gastroenterology (GI) groups having a high EHR burden, according to a recently published article in the American Journal of Gastroenterology.2
This topic is a timely discussion as several large health systems have implemented processes to bill for non–face-to-face services (termed “asynchronous care”), some of which have not been well received in the lay media. It is important to note that despite these implementations, studies have shown only 1% of all incoming portal messages would meet criteria to be submitted for reimbursement. This impact might be slightly higher in chronic care management practices.
Providers and practices have several options when considering billing for non–face-to-face encounters, which we outline in Table 1.3
The focus of this article will be to review the more common non–face-to-face evaluation and management services, such as telephone E/M (patient phone call) and e-visits (patient portal messages) as these have recently generated the most interest and discussion amongst health care providers.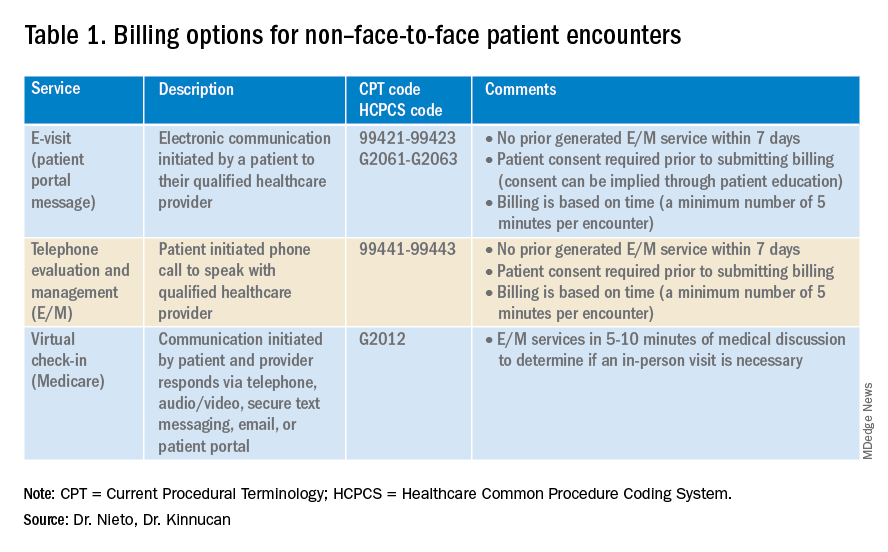
Telemedicine after COVID-19 pandemic
During the beginning of the pandemic, a web-based survey study found that almost all providers in GI practices implemented some form of telemedicine to continue to provide care for patients, compared to 32% prior to the pandemic.4,5 The high demand and essential requirement for telehealth evaluation facilitated its reimbursement, eliminating the primary barrier to previous use.6
One of the new covered benefits by CMS was asynchronous telehealth care.7 The PHE ended in May 2023, and since then a qualified health care provider (QHCP) does not have the full flexibility to deliver telemedicine services across state lines. The U.S. Department of Health and Human Services has considered some telehealth policy changes after the COVID-19 PHE and many of those will be extended, at least through 2024.8 As during the pandemic, where the U.S. national payer network (CMS, state Medicaid, and private payers) and state health agencies assisted to ensure patients get the care they need by authorizing providers to be compensated for non–face-to-face services, we believe this service will continue to be part of our clinical practice.
We recommend you stay informed about local and federal laws, regulations, and alternatives for reimbursement as they may be modified at the beginning of a new calendar year. Remember, you can always talk with your revenue cycle team to clarify any query.
Telephone evaluation and management services
The patient requests to speak with you.
Telephone evaluation and management services became more widely used after the pandemic and were recognized by CMS as a covered medical service under PHE. As outlined in Table 1, there are associated codes with this service and it can only apply to an established patient in your practice. The cumulative time spent over a 7-day period without generating an immediate follow-up visit could qualify for this CPT code. However, for a patient with a high-complexity diagnosis and/or decisions being made about care, it might be better to consider a virtual office visit as this would value the complex care at a higher level than the time spent during the telephone E/M encounter.
A common question comes up: Can my nurse or support team bill for telephone care? No, only QHCP can, which means physicians and advanced practice providers can bill for this E/M service, and it does not include time spent by other members of clinical staff in patient care. However, there are CPT codes for chronic care management, which is not covered in this article.
Virtual evaluation and management services
You respond to a patient-initiated portal message.
Patient portal messages increased exponentially during the pandemic with 2.5 more minutes spent per message, resulting in more EHR work by practitioners, compared with prior to the pandemic. One study showed an immediate postpandemic increase in EHR patient-initiated messages with no return to prepandemic baseline.1
Although studies evaluating postpandemic telemedicine services are needed, we believe that this trend will continue, and for this reason, it is important to create sustainable workflows to continue to provide this patient driven avenue of care.9
E-visits are asynchronous patient or guardian portal messages that require a minimum of 5 minutes to provide medical decision-making without prior E/M services in the last 7 days. To obtain reimbursement for this service, it cannot be initiated by the provider, and patient consent must be obtained. Documentation should include this information and the time spent in the encounter. The associated CPT codes with this e-service are outlined in Table 1.
A common question is, “Are there additional codes I should use if a portal message E/M visit lasts more than 30 minutes?” No. If an e-visit lasts more than 30 minutes, the QHCP should bill the CPT code 99423. However, we would advise that, if this care requires more than 30 minutes, then either virtual or face-to-face E/M be considered for the optimal reimbursement for provider time spent. Another common question is around consent for services, and we advise providers to review this requirement with their compliance colleagues as each institution has different policies.
Virtual check-in
Medicare also covers brief communication technology–based services also known as virtual check-ins, where patients can communicate with their provider after having established care. During this brief conversation that can be via telephone, audio/video, secure text messaging, email, or patient portal, providers will determine if an in-person visit is necessary. CMS has designed G codes for these virtual check-ins that are from the Healthcare Common Procedure Coding System (HCPCS). Two codes are available for this E/M service: G2012, which is outlined in Table 1, and G2010, which covers the evaluation of images and/or recorded videos. In order to be reimbursed for a G2010 code, providers need at least a 5-minute response to make a clinical determination or give the patient a medical impression.
Patient satisfaction, physician well-being and quality of care outcomes
Large health care systems like Kaiser Permanente implemented secure message patient-physician communication (the patient portal) even before the pandemic, showing promising results in 2010 with reduction in office visits, improvement in measurable quality outcomes, and high level of patient satisfaction.10 Post pandemic, several large health care centers opted to announce the billing implementation for patient-initiated portal messages.11 A focus was placed on educating their patients about when a message will and will not be billed. Using this type of strategy can help to improve patient awareness about potential billing without affecting patient satisfaction and care outcomes. Studies have shown the EHR has contributed to physician burnout and some physicians reducing their clinical time or leaving medicine; a reduction in messaging might have a positive impact on physician well-being.
The challenge is that medical billing is not routinely included as a curriculum topic in many residency and fellowship programs; however, trainees are part of E/M services and have limited knowledge of billing processes. Unfortunately, at this time, trainees cannot submit for reimbursement for asynchronous care as described above. We hope that this brief article will help junior gastroenterologists optimize their outpatient billing practices.
Dr. Nieto is an internal medicine chief resident with WellStar Cobb Medical Center, Austell, Ga. Dr. Kinnucan is a gastroenterologist with Mayo Clinic, Jacksonville, Fla. The authors have no conflicts of interest to disclose for this article. The authors certify that no financial and grant support has been received for this article.
References
1. Holmgren AJ et al. J Am Med Inform Assoc. 2021 Dec 9. doi: 10.1093/jamia/ocab268.
2. Bali AS et al. Am J Gastroenterol. 2023 Apr 24. doi: 10.14309/ajg.0000000000002254.
3. AAFP. Family Physician. “Coding Scenario: Coding for Virtual-Digital Visits”
4. Keihanian T. et al. Telehealth Utilization in Gastroenterology Clinics Amid the COVID-19 Pandemic: Impact on Clinical Practice and Gastroenterology Training. Gastroenterology. 2020 Jun 20. doi: 10.1053/j.gastro.2020.06.040.
5. Lewin S et al. J Crohns Colitis. 2020 Oct 21. doi: 10.1093/ecco-jcc/jjaa140.
6. Perisetti A and H Goyal. Dig Dis Sci. 2021 Mar 3. doi: 10.1007/s10620-021-06874-x.
7. Telehealth.HHS.gov. Medicaid and Medicare billing for asynchronous telehealth. Updated: 2022 May 4.
8. Telehealth.HHS.gov. Telehealth policy changes after the COVID-19 public health emergency. Last updated: 2023 Jan 23.
9. Fox B and Sizemore JO. Telehealth: Fad or the future. Epic Health Research Network. 2020 Aug 18.
10. Baer D. Patient-physician e-mail communication: the kaiser permanente experience. J Oncol Pract. 2011 Jul. doi: 10.1200/JOP.2011.000323.
11. Myclevelandclinic.org. MyChart Messaging.
12. Sinsky CA et al. J Gen Intern Med. 2022 Aug 29. doi: 10.1007/s11606-022-07766-0.
The American Gastroenterological Association and other gastrointestinal-specific organizations have excellent resources available to members that focus on optimizing reimbursement in your clinical and endoscopic practice.
During the COVID-19 pandemic and public health emergency (PHE), many previously noncovered services were now covered under rules of the Centers for Medicare & Medicaid Services. During the pandemic, patient portal messages increased by 157%, meaning more work for health care teams, negatively impacting physician satisfaction, and increasing burnout.1 Medical burnout has been associated with increased time spent on electronic health records, with some subspeciality gastroenterology (GI) groups having a high EHR burden, according to a recently published article in the American Journal of Gastroenterology.2
This topic is a timely discussion as several large health systems have implemented processes to bill for non–face-to-face services (termed “asynchronous care”), some of which have not been well received in the lay media. It is important to note that despite these implementations, studies have shown only 1% of all incoming portal messages would meet criteria to be submitted for reimbursement. This impact might be slightly higher in chronic care management practices.
Providers and practices have several options when considering billing for non–face-to-face encounters, which we outline in Table 1.3
The focus of this article will be to review the more common non–face-to-face evaluation and management services, such as telephone E/M (patient phone call) and e-visits (patient portal messages) as these have recently generated the most interest and discussion amongst health care providers.
Telemedicine after COVID-19 pandemic
During the beginning of the pandemic, a web-based survey study found that almost all providers in GI practices implemented some form of telemedicine to continue to provide care for patients, compared to 32% prior to the pandemic.4,5 The high demand and essential requirement for telehealth evaluation facilitated its reimbursement, eliminating the primary barrier to previous use.6
One of the new covered benefits by CMS was asynchronous telehealth care.7 The PHE ended in May 2023, and since then a qualified health care provider (QHCP) does not have the full flexibility to deliver telemedicine services across state lines. The U.S. Department of Health and Human Services has considered some telehealth policy changes after the COVID-19 PHE and many of those will be extended, at least through 2024.8 As during the pandemic, where the U.S. national payer network (CMS, state Medicaid, and private payers) and state health agencies assisted to ensure patients get the care they need by authorizing providers to be compensated for non–face-to-face services, we believe this service will continue to be part of our clinical practice.
We recommend you stay informed about local and federal laws, regulations, and alternatives for reimbursement as they may be modified at the beginning of a new calendar year. Remember, you can always talk with your revenue cycle team to clarify any query.
Telephone evaluation and management services
The patient requests to speak with you.
Telephone evaluation and management services became more widely used after the pandemic and were recognized by CMS as a covered medical service under PHE. As outlined in Table 1, there are associated codes with this service and it can only apply to an established patient in your practice. The cumulative time spent over a 7-day period without generating an immediate follow-up visit could qualify for this CPT code. However, for a patient with a high-complexity diagnosis and/or decisions being made about care, it might be better to consider a virtual office visit as this would value the complex care at a higher level than the time spent during the telephone E/M encounter.
A common question comes up: Can my nurse or support team bill for telephone care? No, only QHCP can, which means physicians and advanced practice providers can bill for this E/M service, and it does not include time spent by other members of clinical staff in patient care. However, there are CPT codes for chronic care management, which is not covered in this article.
Virtual evaluation and management services
You respond to a patient-initiated portal message.
Patient portal messages increased exponentially during the pandemic with 2.5 more minutes spent per message, resulting in more EHR work by practitioners, compared with prior to the pandemic. One study showed an immediate postpandemic increase in EHR patient-initiated messages with no return to prepandemic baseline.1
Although studies evaluating postpandemic telemedicine services are needed, we believe that this trend will continue, and for this reason, it is important to create sustainable workflows to continue to provide this patient driven avenue of care.9
E-visits are asynchronous patient or guardian portal messages that require a minimum of 5 minutes to provide medical decision-making without prior E/M services in the last 7 days. To obtain reimbursement for this service, it cannot be initiated by the provider, and patient consent must be obtained. Documentation should include this information and the time spent in the encounter. The associated CPT codes with this e-service are outlined in Table 1.
A common question is, “Are there additional codes I should use if a portal message E/M visit lasts more than 30 minutes?” No. If an e-visit lasts more than 30 minutes, the QHCP should bill the CPT code 99423. However, we would advise that, if this care requires more than 30 minutes, then either virtual or face-to-face E/M be considered for the optimal reimbursement for provider time spent. Another common question is around consent for services, and we advise providers to review this requirement with their compliance colleagues as each institution has different policies.
Virtual check-in
Medicare also covers brief communication technology–based services also known as virtual check-ins, where patients can communicate with their provider after having established care. During this brief conversation that can be via telephone, audio/video, secure text messaging, email, or patient portal, providers will determine if an in-person visit is necessary. CMS has designed G codes for these virtual check-ins that are from the Healthcare Common Procedure Coding System (HCPCS). Two codes are available for this E/M service: G2012, which is outlined in Table 1, and G2010, which covers the evaluation of images and/or recorded videos. In order to be reimbursed for a G2010 code, providers need at least a 5-minute response to make a clinical determination or give the patient a medical impression.
Patient satisfaction, physician well-being and quality of care outcomes
Large health care systems like Kaiser Permanente implemented secure message patient-physician communication (the patient portal) even before the pandemic, showing promising results in 2010 with reduction in office visits, improvement in measurable quality outcomes, and high level of patient satisfaction.10 Post pandemic, several large health care centers opted to announce the billing implementation for patient-initiated portal messages.11 A focus was placed on educating their patients about when a message will and will not be billed. Using this type of strategy can help to improve patient awareness about potential billing without affecting patient satisfaction and care outcomes. Studies have shown the EHR has contributed to physician burnout and some physicians reducing their clinical time or leaving medicine; a reduction in messaging might have a positive impact on physician well-being.
The challenge is that medical billing is not routinely included as a curriculum topic in many residency and fellowship programs; however, trainees are part of E/M services and have limited knowledge of billing processes. Unfortunately, at this time, trainees cannot submit for reimbursement for asynchronous care as described above. We hope that this brief article will help junior gastroenterologists optimize their outpatient billing practices.
Dr. Nieto is an internal medicine chief resident with WellStar Cobb Medical Center, Austell, Ga. Dr. Kinnucan is a gastroenterologist with Mayo Clinic, Jacksonville, Fla. The authors have no conflicts of interest to disclose for this article. The authors certify that no financial and grant support has been received for this article.
References
1. Holmgren AJ et al. J Am Med Inform Assoc. 2021 Dec 9. doi: 10.1093/jamia/ocab268.
2. Bali AS et al. Am J Gastroenterol. 2023 Apr 24. doi: 10.14309/ajg.0000000000002254.
3. AAFP. Family Physician. “Coding Scenario: Coding for Virtual-Digital Visits”
4. Keihanian T. et al. Telehealth Utilization in Gastroenterology Clinics Amid the COVID-19 Pandemic: Impact on Clinical Practice and Gastroenterology Training. Gastroenterology. 2020 Jun 20. doi: 10.1053/j.gastro.2020.06.040.
5. Lewin S et al. J Crohns Colitis. 2020 Oct 21. doi: 10.1093/ecco-jcc/jjaa140.
6. Perisetti A and H Goyal. Dig Dis Sci. 2021 Mar 3. doi: 10.1007/s10620-021-06874-x.
7. Telehealth.HHS.gov. Medicaid and Medicare billing for asynchronous telehealth. Updated: 2022 May 4.
8. Telehealth.HHS.gov. Telehealth policy changes after the COVID-19 public health emergency. Last updated: 2023 Jan 23.
9. Fox B and Sizemore JO. Telehealth: Fad or the future. Epic Health Research Network. 2020 Aug 18.
10. Baer D. Patient-physician e-mail communication: the kaiser permanente experience. J Oncol Pract. 2011 Jul. doi: 10.1200/JOP.2011.000323.
11. Myclevelandclinic.org. MyChart Messaging.
12. Sinsky CA et al. J Gen Intern Med. 2022 Aug 29. doi: 10.1007/s11606-022-07766-0.
The American Gastroenterological Association and other gastrointestinal-specific organizations have excellent resources available to members that focus on optimizing reimbursement in your clinical and endoscopic practice.
During the COVID-19 pandemic and public health emergency (PHE), many previously noncovered services were now covered under rules of the Centers for Medicare & Medicaid Services. During the pandemic, patient portal messages increased by 157%, meaning more work for health care teams, negatively impacting physician satisfaction, and increasing burnout.1 Medical burnout has been associated with increased time spent on electronic health records, with some subspeciality gastroenterology (GI) groups having a high EHR burden, according to a recently published article in the American Journal of Gastroenterology.2
This topic is a timely discussion as several large health systems have implemented processes to bill for non–face-to-face services (termed “asynchronous care”), some of which have not been well received in the lay media. It is important to note that despite these implementations, studies have shown only 1% of all incoming portal messages would meet criteria to be submitted for reimbursement. This impact might be slightly higher in chronic care management practices.
Providers and practices have several options when considering billing for non–face-to-face encounters, which we outline in Table 1.3
The focus of this article will be to review the more common non–face-to-face evaluation and management services, such as telephone E/M (patient phone call) and e-visits (patient portal messages) as these have recently generated the most interest and discussion amongst health care providers.
Telemedicine after COVID-19 pandemic
During the beginning of the pandemic, a web-based survey study found that almost all providers in GI practices implemented some form of telemedicine to continue to provide care for patients, compared to 32% prior to the pandemic.4,5 The high demand and essential requirement for telehealth evaluation facilitated its reimbursement, eliminating the primary barrier to previous use.6
One of the new covered benefits by CMS was asynchronous telehealth care.7 The PHE ended in May 2023, and since then a qualified health care provider (QHCP) does not have the full flexibility to deliver telemedicine services across state lines. The U.S. Department of Health and Human Services has considered some telehealth policy changes after the COVID-19 PHE and many of those will be extended, at least through 2024.8 As during the pandemic, where the U.S. national payer network (CMS, state Medicaid, and private payers) and state health agencies assisted to ensure patients get the care they need by authorizing providers to be compensated for non–face-to-face services, we believe this service will continue to be part of our clinical practice.
We recommend you stay informed about local and federal laws, regulations, and alternatives for reimbursement as they may be modified at the beginning of a new calendar year. Remember, you can always talk with your revenue cycle team to clarify any query.
Telephone evaluation and management services
The patient requests to speak with you.
Telephone evaluation and management services became more widely used after the pandemic and were recognized by CMS as a covered medical service under PHE. As outlined in Table 1, there are associated codes with this service and it can only apply to an established patient in your practice. The cumulative time spent over a 7-day period without generating an immediate follow-up visit could qualify for this CPT code. However, for a patient with a high-complexity diagnosis and/or decisions being made about care, it might be better to consider a virtual office visit as this would value the complex care at a higher level than the time spent during the telephone E/M encounter.
A common question comes up: Can my nurse or support team bill for telephone care? No, only QHCP can, which means physicians and advanced practice providers can bill for this E/M service, and it does not include time spent by other members of clinical staff in patient care. However, there are CPT codes for chronic care management, which is not covered in this article.
Virtual evaluation and management services
You respond to a patient-initiated portal message.
Patient portal messages increased exponentially during the pandemic with 2.5 more minutes spent per message, resulting in more EHR work by practitioners, compared with prior to the pandemic. One study showed an immediate postpandemic increase in EHR patient-initiated messages with no return to prepandemic baseline.1
Although studies evaluating postpandemic telemedicine services are needed, we believe that this trend will continue, and for this reason, it is important to create sustainable workflows to continue to provide this patient driven avenue of care.9
E-visits are asynchronous patient or guardian portal messages that require a minimum of 5 minutes to provide medical decision-making without prior E/M services in the last 7 days. To obtain reimbursement for this service, it cannot be initiated by the provider, and patient consent must be obtained. Documentation should include this information and the time spent in the encounter. The associated CPT codes with this e-service are outlined in Table 1.
A common question is, “Are there additional codes I should use if a portal message E/M visit lasts more than 30 minutes?” No. If an e-visit lasts more than 30 minutes, the QHCP should bill the CPT code 99423. However, we would advise that, if this care requires more than 30 minutes, then either virtual or face-to-face E/M be considered for the optimal reimbursement for provider time spent. Another common question is around consent for services, and we advise providers to review this requirement with their compliance colleagues as each institution has different policies.
Virtual check-in
Medicare also covers brief communication technology–based services also known as virtual check-ins, where patients can communicate with their provider after having established care. During this brief conversation that can be via telephone, audio/video, secure text messaging, email, or patient portal, providers will determine if an in-person visit is necessary. CMS has designed G codes for these virtual check-ins that are from the Healthcare Common Procedure Coding System (HCPCS). Two codes are available for this E/M service: G2012, which is outlined in Table 1, and G2010, which covers the evaluation of images and/or recorded videos. In order to be reimbursed for a G2010 code, providers need at least a 5-minute response to make a clinical determination or give the patient a medical impression.
Patient satisfaction, physician well-being and quality of care outcomes
Large health care systems like Kaiser Permanente implemented secure message patient-physician communication (the patient portal) even before the pandemic, showing promising results in 2010 with reduction in office visits, improvement in measurable quality outcomes, and high level of patient satisfaction.10 Post pandemic, several large health care centers opted to announce the billing implementation for patient-initiated portal messages.11 A focus was placed on educating their patients about when a message will and will not be billed. Using this type of strategy can help to improve patient awareness about potential billing without affecting patient satisfaction and care outcomes. Studies have shown the EHR has contributed to physician burnout and some physicians reducing their clinical time or leaving medicine; a reduction in messaging might have a positive impact on physician well-being.
The challenge is that medical billing is not routinely included as a curriculum topic in many residency and fellowship programs; however, trainees are part of E/M services and have limited knowledge of billing processes. Unfortunately, at this time, trainees cannot submit for reimbursement for asynchronous care as described above. We hope that this brief article will help junior gastroenterologists optimize their outpatient billing practices.
Dr. Nieto is an internal medicine chief resident with WellStar Cobb Medical Center, Austell, Ga. Dr. Kinnucan is a gastroenterologist with Mayo Clinic, Jacksonville, Fla. The authors have no conflicts of interest to disclose for this article. The authors certify that no financial and grant support has been received for this article.
References
1. Holmgren AJ et al. J Am Med Inform Assoc. 2021 Dec 9. doi: 10.1093/jamia/ocab268.
2. Bali AS et al. Am J Gastroenterol. 2023 Apr 24. doi: 10.14309/ajg.0000000000002254.
3. AAFP. Family Physician. “Coding Scenario: Coding for Virtual-Digital Visits”
4. Keihanian T. et al. Telehealth Utilization in Gastroenterology Clinics Amid the COVID-19 Pandemic: Impact on Clinical Practice and Gastroenterology Training. Gastroenterology. 2020 Jun 20. doi: 10.1053/j.gastro.2020.06.040.
5. Lewin S et al. J Crohns Colitis. 2020 Oct 21. doi: 10.1093/ecco-jcc/jjaa140.
6. Perisetti A and H Goyal. Dig Dis Sci. 2021 Mar 3. doi: 10.1007/s10620-021-06874-x.
7. Telehealth.HHS.gov. Medicaid and Medicare billing for asynchronous telehealth. Updated: 2022 May 4.
8. Telehealth.HHS.gov. Telehealth policy changes after the COVID-19 public health emergency. Last updated: 2023 Jan 23.
9. Fox B and Sizemore JO. Telehealth: Fad or the future. Epic Health Research Network. 2020 Aug 18.
10. Baer D. Patient-physician e-mail communication: the kaiser permanente experience. J Oncol Pract. 2011 Jul. doi: 10.1200/JOP.2011.000323.
11. Myclevelandclinic.org. MyChart Messaging.
12. Sinsky CA et al. J Gen Intern Med. 2022 Aug 29. doi: 10.1007/s11606-022-07766-0.
May 2023 - ICYMI
Gastroenterology
January 2023
Yardeni D et al. Current Best Practice in Hepatitis B Management and Understanding Long-term Prospects for Cure. Gastroenterology. 2023 Jan;164(1):42-60.e6. doi: 10.1053/j.gastro.2022.10.008. Epub 2022 Oct 12. PMID: 36243037; PMCID: PMC9772068.
Laine L et al. Vonoprazan Versus Lansoprazole for Healing and Maintenance of Healing of Erosive Esophagitis: A Randomized Trial. Gastroenterology. 2023 Jan;164(1):61-71. doi: 10.1053/j.gastro.2022.09.041. Epub 2022 Oct 10. PMID: 36228734.
February 2023
Ufere NN et al. Promoting Prognostic Understanding and Health Equity for Patients With Advanced Liver Disease: Using “Best Case/Worst Case.” Gastroenterology. 2023 Feb;164(2):171-6. doi: 10.1053/j.gastro.2022.12.005. PMID: 36702571.
March 2023
Heath JK et al. Training Generations of Clinician Educators: Applying the Novel Clinician Educator Milestones to Faculty Development. Gastroenterology. 2023 Mar;164(3):325-8.e1. doi: 10.1053/j.gastro.2022.12.003. Epub 2022 Dec 9. PMID: 36509156.
Singh S et al. AGA Clinical Guidelines Committee. Electronic address: clinicalpractice@gastro.org. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Ulcerative Colitis. Gastroenterology. 2023 Mar;164(3):344-72. doi: 10.1053/j.gastro.2022.12.007. PMID: 36822736.
Clinical Gastroenterology and Hepatology
January 2023
Speicher LL and Francis D. Improving Employee Experience: Reducing Burnout, Decreasing Turnover and Building Well-being. Clin Gastroenterol Hepatol. 2023 Jan;21(1):11-4. doi: 10.1016/j.cgh.2022.09.020. Epub 2022 Sep 22. PMID: 36155248; PMCID: PMC9547273.
Penagini R et al. Rapid Drink Challenge During High-resolution Manometry for Evaluation of Esophageal Emptying in Treated Achalasia. Clin Gastroenterol Hepatol. 2023 Jan;21(1):55-63. doi: 10.1016/j.cgh.2022.02.047. Epub 2022 Feb 28. PMID: 35240328.
February 2023
Zaki TA et al. Racial and Ethnic Disparities in Early-Onset Colorectal Cancer Survival. Clin Gastroenterol Hepatol. 2023 Feb;21(2):497-506.e3. doi: 10.1016/j.cgh.2022.05.035. Epub 2022 Jun 16. PMID: 35716905; PMCID: PMC9835097.
Brenner DM et al. Rare, Overlooked, or Underappreciated Causes of Recurrent Abdominal Pain: A Primer for Gastroenterologists. Clin Gastroenterol Hepatol. 2023 Feb;21(2):264-79. doi: 10.1016/j.cgh.2022.09.022. Epub 2022 Sep 27. PMID: 36180010.
March 2023
Hanna M et al. Emerging Tests for Noninvasive Colorectal Cancer Screening. Clin Gastroenterol Hepatol. 2023 Mar;21(3):604-16. doi: 10.1016/j.cgh.2022.12.008. Epub 2022 Dec 17. PMID: 36539002; PMCID: PMC9974876.
Ormsby EL et al. Association of Standardized Radiology Reporting and Management of Abdominal CT and MRI With Diagnosis of Pancreatic Cancer. Clin Gastroenterol Hepatol. 2023 Mar;21(3):644-52.e2. doi: 10.1016/j.cgh.2022.03.047. Epub 2022 Apr 15. PMID: 35436626.
Techniques and Innovations in Gastrointestinal Endoscopy
Mohapatra S et al. (Accepted/In press). Outcomes of Endoscopic Resection for Colorectal Polyps with High-Grade Dysplasia or Intramucosal Cancer. Tech Innov Gastrointest Endosc. 2023 Jan 22. doi: 10.1016/j.tige.2023.01.003.
Holzwanger EA et al. Improving Dysplasia Detection in Barrett’s Esophagus. Techniques and Innovations in Gastrointestinal Endoscopy. Tech Innov Gastrointest Endosc. 2023;25(2):157-66. doi: 10.1016/j.tige.2023.01.002.
Gastroenterology
January 2023
Yardeni D et al. Current Best Practice in Hepatitis B Management and Understanding Long-term Prospects for Cure. Gastroenterology. 2023 Jan;164(1):42-60.e6. doi: 10.1053/j.gastro.2022.10.008. Epub 2022 Oct 12. PMID: 36243037; PMCID: PMC9772068.
Laine L et al. Vonoprazan Versus Lansoprazole for Healing and Maintenance of Healing of Erosive Esophagitis: A Randomized Trial. Gastroenterology. 2023 Jan;164(1):61-71. doi: 10.1053/j.gastro.2022.09.041. Epub 2022 Oct 10. PMID: 36228734.
February 2023
Ufere NN et al. Promoting Prognostic Understanding and Health Equity for Patients With Advanced Liver Disease: Using “Best Case/Worst Case.” Gastroenterology. 2023 Feb;164(2):171-6. doi: 10.1053/j.gastro.2022.12.005. PMID: 36702571.
March 2023
Heath JK et al. Training Generations of Clinician Educators: Applying the Novel Clinician Educator Milestones to Faculty Development. Gastroenterology. 2023 Mar;164(3):325-8.e1. doi: 10.1053/j.gastro.2022.12.003. Epub 2022 Dec 9. PMID: 36509156.
Singh S et al. AGA Clinical Guidelines Committee. Electronic address: clinicalpractice@gastro.org. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Ulcerative Colitis. Gastroenterology. 2023 Mar;164(3):344-72. doi: 10.1053/j.gastro.2022.12.007. PMID: 36822736.
Clinical Gastroenterology and Hepatology
January 2023
Speicher LL and Francis D. Improving Employee Experience: Reducing Burnout, Decreasing Turnover and Building Well-being. Clin Gastroenterol Hepatol. 2023 Jan;21(1):11-4. doi: 10.1016/j.cgh.2022.09.020. Epub 2022 Sep 22. PMID: 36155248; PMCID: PMC9547273.
Penagini R et al. Rapid Drink Challenge During High-resolution Manometry for Evaluation of Esophageal Emptying in Treated Achalasia. Clin Gastroenterol Hepatol. 2023 Jan;21(1):55-63. doi: 10.1016/j.cgh.2022.02.047. Epub 2022 Feb 28. PMID: 35240328.
February 2023
Zaki TA et al. Racial and Ethnic Disparities in Early-Onset Colorectal Cancer Survival. Clin Gastroenterol Hepatol. 2023 Feb;21(2):497-506.e3. doi: 10.1016/j.cgh.2022.05.035. Epub 2022 Jun 16. PMID: 35716905; PMCID: PMC9835097.
Brenner DM et al. Rare, Overlooked, or Underappreciated Causes of Recurrent Abdominal Pain: A Primer for Gastroenterologists. Clin Gastroenterol Hepatol. 2023 Feb;21(2):264-79. doi: 10.1016/j.cgh.2022.09.022. Epub 2022 Sep 27. PMID: 36180010.
March 2023
Hanna M et al. Emerging Tests for Noninvasive Colorectal Cancer Screening. Clin Gastroenterol Hepatol. 2023 Mar;21(3):604-16. doi: 10.1016/j.cgh.2022.12.008. Epub 2022 Dec 17. PMID: 36539002; PMCID: PMC9974876.
Ormsby EL et al. Association of Standardized Radiology Reporting and Management of Abdominal CT and MRI With Diagnosis of Pancreatic Cancer. Clin Gastroenterol Hepatol. 2023 Mar;21(3):644-52.e2. doi: 10.1016/j.cgh.2022.03.047. Epub 2022 Apr 15. PMID: 35436626.
Techniques and Innovations in Gastrointestinal Endoscopy
Mohapatra S et al. (Accepted/In press). Outcomes of Endoscopic Resection for Colorectal Polyps with High-Grade Dysplasia or Intramucosal Cancer. Tech Innov Gastrointest Endosc. 2023 Jan 22. doi: 10.1016/j.tige.2023.01.003.
Holzwanger EA et al. Improving Dysplasia Detection in Barrett’s Esophagus. Techniques and Innovations in Gastrointestinal Endoscopy. Tech Innov Gastrointest Endosc. 2023;25(2):157-66. doi: 10.1016/j.tige.2023.01.002.
Gastroenterology
January 2023
Yardeni D et al. Current Best Practice in Hepatitis B Management and Understanding Long-term Prospects for Cure. Gastroenterology. 2023 Jan;164(1):42-60.e6. doi: 10.1053/j.gastro.2022.10.008. Epub 2022 Oct 12. PMID: 36243037; PMCID: PMC9772068.
Laine L et al. Vonoprazan Versus Lansoprazole for Healing and Maintenance of Healing of Erosive Esophagitis: A Randomized Trial. Gastroenterology. 2023 Jan;164(1):61-71. doi: 10.1053/j.gastro.2022.09.041. Epub 2022 Oct 10. PMID: 36228734.
February 2023
Ufere NN et al. Promoting Prognostic Understanding and Health Equity for Patients With Advanced Liver Disease: Using “Best Case/Worst Case.” Gastroenterology. 2023 Feb;164(2):171-6. doi: 10.1053/j.gastro.2022.12.005. PMID: 36702571.
March 2023
Heath JK et al. Training Generations of Clinician Educators: Applying the Novel Clinician Educator Milestones to Faculty Development. Gastroenterology. 2023 Mar;164(3):325-8.e1. doi: 10.1053/j.gastro.2022.12.003. Epub 2022 Dec 9. PMID: 36509156.
Singh S et al. AGA Clinical Guidelines Committee. Electronic address: clinicalpractice@gastro.org. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Ulcerative Colitis. Gastroenterology. 2023 Mar;164(3):344-72. doi: 10.1053/j.gastro.2022.12.007. PMID: 36822736.
Clinical Gastroenterology and Hepatology
January 2023
Speicher LL and Francis D. Improving Employee Experience: Reducing Burnout, Decreasing Turnover and Building Well-being. Clin Gastroenterol Hepatol. 2023 Jan;21(1):11-4. doi: 10.1016/j.cgh.2022.09.020. Epub 2022 Sep 22. PMID: 36155248; PMCID: PMC9547273.
Penagini R et al. Rapid Drink Challenge During High-resolution Manometry for Evaluation of Esophageal Emptying in Treated Achalasia. Clin Gastroenterol Hepatol. 2023 Jan;21(1):55-63. doi: 10.1016/j.cgh.2022.02.047. Epub 2022 Feb 28. PMID: 35240328.
February 2023
Zaki TA et al. Racial and Ethnic Disparities in Early-Onset Colorectal Cancer Survival. Clin Gastroenterol Hepatol. 2023 Feb;21(2):497-506.e3. doi: 10.1016/j.cgh.2022.05.035. Epub 2022 Jun 16. PMID: 35716905; PMCID: PMC9835097.
Brenner DM et al. Rare, Overlooked, or Underappreciated Causes of Recurrent Abdominal Pain: A Primer for Gastroenterologists. Clin Gastroenterol Hepatol. 2023 Feb;21(2):264-79. doi: 10.1016/j.cgh.2022.09.022. Epub 2022 Sep 27. PMID: 36180010.
March 2023
Hanna M et al. Emerging Tests for Noninvasive Colorectal Cancer Screening. Clin Gastroenterol Hepatol. 2023 Mar;21(3):604-16. doi: 10.1016/j.cgh.2022.12.008. Epub 2022 Dec 17. PMID: 36539002; PMCID: PMC9974876.
Ormsby EL et al. Association of Standardized Radiology Reporting and Management of Abdominal CT and MRI With Diagnosis of Pancreatic Cancer. Clin Gastroenterol Hepatol. 2023 Mar;21(3):644-52.e2. doi: 10.1016/j.cgh.2022.03.047. Epub 2022 Apr 15. PMID: 35436626.
Techniques and Innovations in Gastrointestinal Endoscopy
Mohapatra S et al. (Accepted/In press). Outcomes of Endoscopic Resection for Colorectal Polyps with High-Grade Dysplasia or Intramucosal Cancer. Tech Innov Gastrointest Endosc. 2023 Jan 22. doi: 10.1016/j.tige.2023.01.003.
Holzwanger EA et al. Improving Dysplasia Detection in Barrett’s Esophagus. Techniques and Innovations in Gastrointestinal Endoscopy. Tech Innov Gastrointest Endosc. 2023;25(2):157-66. doi: 10.1016/j.tige.2023.01.002.
Approach to dysphagia
Introduction
Dysphagia is the sensation of difficulty swallowing food or liquid in the acute or chronic setting. The prevalence of dysphagia ranges based on the type and etiology but may impact up to one in six adults.1,2 Dysphagia can cause a significant impact on a patient’s health and overall quality of life. A recent study found that only 50% of symptomatic adults seek medical care despite modifying their eating habits by either eating slowly or changing to softer foods or liquids.1 The most common, serious complications of dysphagia include aspiration pneumonia, malnutrition, and dehydration.3 According to the Agency for Healthcare Research and Quality, dysphagia may be responsible for up to 60,000 deaths annually.3
The diagnosis of esophageal dysphagia can be challenging. An initial, thorough history is essential to delineate between oropharyngeal and esophageal dysphagia and guide subsequent diagnostic testing. In recent years, there have been a number of advances in the approach to diagnosing dysphagia, including novel diagnostic modalities. The goal of this review article is to discuss the current approach to esophageal dysphagia and future direction to allow for timely diagnosis and management.
History
The diagnosis of dysphagia begins with a thorough history. Questions about the timing, onset, progression, localization of symptoms, and types of food that are difficult to swallow are essential in differentiating oropharyngeal and esophageal dysphagia.3,4 Further history taking must include medication and allergy review, smoking history, and review of prior radiation or surgical therapies to the head and neck.
Briefly, oropharyngeal dysphagia is difficulty initiating a swallow or passing food from the mouth or throat and can be caused by structural or functional etiologies.5 Clinical presentations include a sensation of food stuck in the back of the throat, coughing or choking while eating, or drooling. Structural causes include head and neck cancer, Zenker diverticulum, Killian Jamieson diverticula, prolonged intubation, or changes secondary to prior surgery or radiation.3 Functional causes may include neurologic, rheumatologic, or muscular disorders.6
Esophageal dysphagia refers to difficulty transporting food or liquid down the esophagus and can be caused by structural, inflammatory, or functional disorders.5 Patients typically localize symptoms of heartburn, regurgitation, nausea, vomiting, cough, or chest pain along the sternum or epigastric region. Alarm signs concerning for malignancy include unintentional weight loss, fevers, or night sweats.3,7 Aside from symptoms, medication review is essential, as dysphagia is a common side effect of antipsychotics, anticholinergics, antimuscarinics, narcotics, and immunosuppressant drugs.8 Larger pills such as NSAIDs, antibiotics, bisphosphonates, potassium supplements, and methylxanthines can cause drug-induced esophagitis, which can initially present as dysphagia.8 Inflammatory causes can be elucidated by obtaining a history about allergies, tobacco use, and recent infections such as thrush or pneumonia. Patients with a history of recurrent pneumonias may be silently aspirating, a complication of dysphagia.3 Once esophageal dysphagia is clinically suspected based on history, workup can begin.
Differentiating etiologies of esophageal dysphagia
The next step in diagnosing esophageal dysphagia is differentiating between structural, inflammatory, or dysmotility etiology (Figure 1).
Patients with a structural cause typically have difficulty swallowing solids but are able to swallow liquids unless the disease progresses. Symptoms can rapidly worsen and lead to odynophagia, weight loss, and vomiting. In comparison, patients with motility disorders typically have difficulty swallowing both solids and liquids initially, and symptoms can be constant or intermittent.5
Prior to diagnostic studies, a 4-week trial of a proton pump inhibitor (PPI) is appropriate for patients with reflux symptoms who are younger than 50 with no alarm features concerning for malignancy.7,9 If symptoms persist after a PPI trial, then an upper endoscopy (EGD) is indicated. An EGD allows for visualization of structural etiologies, obtaining biopsies to rule out inflammatory etiologies, and the option to therapeutically treat reduced luminal diameter with dilatation.10 The most common structural and inflammatory etiologies noted on EGD include strictures, webs, carcinomas, Schatzki rings, and gastroesophageal reflux or eosinophilic esophagitis.4
If upper endoscopy is normal and clinical suspicion for an obstructive cause remains high, barium esophagram can be utilized as an adjunctive study. Previously, barium esophagram was the initial test to distinguish between structural and motility disorders. The benefits of endoscopy over barium esophagram as the first diagnostic study include higher diagnostic yield, higher sensitivity and specificity, and lower costs.7 However, barium studies may be more sensitive for lower esophageal rings or extrinsic esophageal compression.3
Evaluation of esophageal motility disorder
If a structural or inflammatory etiology of dysphagia is not identified, investigation for an esophageal motility disorder (EMD) is warranted. Examples of motility disorders include achalasia, ineffective esophageal motility, hypercontractility, spasticity, or esophagogastric junction outflow obstruction (EGJOO).10,11 High-resolution esophageal manometry (HRM) remains the gold standard in diagnosis of EMD.12 An HRM catheter utilizes 36 sensors placed two centimeters apart and is placed in the esophagus to evaluate pressure and peristalsis between the upper and lower esophageal sphincters.13 In 2009, the Chicago Classification System was developed to provide a diagnostic algorithm that categorizes EMD based on HRM testing, with the most recent version (4.0) being published in 2020.12,14 Motility diagnoses are divided into two general classifications of disorders of body peristalsis and disorders of EGJ outflow. The most recent updates also include changes in swallow protocols, patient positioning, targeted symptoms, addition of impedance sensors, and consideration of supplemental testing when HRM is inconclusive based on the clinical context.12 There are some limitations of HRM to highlight. One of the main diagnostic values used with HRM is the integrated relaxation pressure (IRP). Despite standardization, IRP measurements vary based on the recorder and patient position. A minority of patients with achalasia may have IRP that does not approach the accepted cutoff and, therefore, the EGJ is not accurately assessed on HRM.15,16 In addition, some swallow protocols have lower sensitivity and specificity for certain motility disorders, and the test can result as inconclusive.14 In these scenarios, supplemental testing with timed barium esophagram or functional luminal imaging probe (EndoFLIP) is indicated.10,11
Over the past decade, EndoFLIP has emerged as a novel diagnostic tool in evaluating EMD. EndoFLIP is usually completed during an upper endoscopy and utilizes impedance planimetry to measure cross-sectional area and esophageal distensibility and evaluate contractile patterns.16 During the procedure, a small catheter with an inflatable balloon is inserted into the esophagus with the distal end in the stomach, traversing the esophagogastric junction (EGJ). The pressure transducer has electrodes every centimeter to allow for a three-dimensional construction of the esophagus and EGJ.17 EndoFLIP has been shown to accurately measure pyloric diameter, pressure, and distensibility at certain balloon volumes.18 In addition, FLIP is being used to further identify aspects of esophageal dysmotility in patients with eosinophilic esophagitis, thought primarily to be an inflammatory disorder.19 However, limitations include minimal accessibility of EndoFLIP within clinical practice and a specific computer program needed to generate the topographic plots.20
When used in conjunction with HRM, EndoFLIP provides complementary data that can be used to better detect major motility disorders.15,20,21 Each study adds unique information about the different physiologic events comprising the esophageal response to distention. Overall, the benefits of EndoFLIP include expediting workup during index endoscopy, patient comfort with sedation, and real-time diagnostic data that supplement results obtained during HRM.10,16,20,2223
Of note, if the diagnostic evaluation for structural, inflammatory, and motility disorders are unrevealing, investigating for atypical reflux symptoms can be pursued for patients with persistent dysphagia. Studies investigating pH, or acidity in the esophagus, in relation to symptoms, can be conducted wirelessly via a capsule fixed to the mucosa or with a nasal catheter.3
Normal workup – hypervigilance
In a subset of patients, all diagnostic testing for structural, inflammatory, or motility disorders is normal. These patients are classified as having a functional esophageal disorder. Despite normal testing, patients still have significant symptoms including epigastric pain, chest pain, globus sensation, or difficulty swallowing. It is theorized that a degree of visceral hypersensitivity between the brain-gut axis contributes to ongoing symptoms.24 Studies for effective treatments are ongoing but typically include cognitive-behavioral therapy, brain-gut behavioral therapy, swallow therapy antidepressants, or short courses of proton pump inhibitors.9
Conclusion
In this review article, we discussed the diagnostic approach for esophageal dysphagia. Initial assessment requires a thorough history, differentiation between oropharyngeal and esophageal dysphagia, and determination of who warrants an upper endoscopy. Upper endoscopy may reveal structural or inflammatory causes of dysphagia, including strictures, masses, or esophagitis, to name a few. If a structural or inflammatory cause is ruled out, this warrants investigation for esophageal motility disorders. The current gold standard for diagnosing EMD is manometry, and supplemental studies, including EndoFLIP, barium esophagram, and pH studies, may provide complimentary data. If workup for dysphagia is normal, evaluation for esophageal hypervigilance causing increased sensitivity to normal or mild sensations may be warranted. In conclusion, the diagnosis of dysphagia is challenging and requires investigation with a systematic approach to ensure timely diagnosis and treatment
Dr. Ronnie and Dr. Bloomberg are in the department of internal medicine at Loyola University Chicago, Maywood, Ill. Dr. Venu is in the division of gastroenterology at Loyola. He is on the speakers bureau at Medtronic.
References
1. Adkins C et al. Clin Gastroenterol Hepatol. 2020;18(9):1970-9.e2.
2. Bhattacharyya N. Otolaryngol Head Neck Surg. 2014;151(5):765-9.
3. McCarty EB and Chao TN. Med Clin North Am. 2021;105(5):939-54.
4. Thiyagalingam S et al. Mayo Clin Proc. 2021;96(2):488-97.
5. Malagelada JR et al. J Clin Gastroenterol. 2015;49(5):370-8.
6. Rommel, N and Hamdy S. Nat Rev Gastroenterol Hepatol. 2016;13(1):49-59.
7. Liu LWC et al. J Can Assoc Gastroenterol. 2018;1(1):5-19.
8. Schwemmle C et al. HNO. 2015;63(7):504-10.
9. Moayyedi P et al. Am J Gastroenterol. 2017;112(7):988-1013.
10. Triggs J and Pandolfino J. F1000Res. 2019 Aug 29. doi: 10.12688/f1000research.18900.1.
11. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14058.
12. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14053.
13. Fox M et al. Neurogastroenterol Motil. 2004;16(5):533-42.
14. Sweis R and Fox M. Curr Gastroenterol Rep. 2020;22(10):49.
15. Carlson DA et al. Gastroenterology. 2015;149(7):1742-51.
16. Donnan EN and Pandolfino JE. Gastroenterol Clin North Am. 2020;49(3):427-35.
17. Carlson DA. Curr Opin Gastroenterol. 2016;32(4):310-8.
18. Zheng T et al. Neurogastroenterol Motil. 2022;34(10):e14386.
19. Carlson DA et al. Clin Gastroenterol Hepatol. 2022;20(8):1719-28.e3.
20. Carlson DA et al. Am J Gastroenterol. 2016;111(12):1726-35.
21. Carlson DA et al. Neurogastroenterol Motil. 2021;33(10):e14116.
22. Carlson DA et al. Gastrointest Endosc. 2019;90(6):915-923.e1.
23. Fox MR et al. Neurogastroenterol Motil. 2021;33(4):e14120.
24. Aziz Q et al. Gastroenterology. 2016 Feb 15. doi: 10.1053/j.gastro.2016.02.012.
Introduction
Dysphagia is the sensation of difficulty swallowing food or liquid in the acute or chronic setting. The prevalence of dysphagia ranges based on the type and etiology but may impact up to one in six adults.1,2 Dysphagia can cause a significant impact on a patient’s health and overall quality of life. A recent study found that only 50% of symptomatic adults seek medical care despite modifying their eating habits by either eating slowly or changing to softer foods or liquids.1 The most common, serious complications of dysphagia include aspiration pneumonia, malnutrition, and dehydration.3 According to the Agency for Healthcare Research and Quality, dysphagia may be responsible for up to 60,000 deaths annually.3
The diagnosis of esophageal dysphagia can be challenging. An initial, thorough history is essential to delineate between oropharyngeal and esophageal dysphagia and guide subsequent diagnostic testing. In recent years, there have been a number of advances in the approach to diagnosing dysphagia, including novel diagnostic modalities. The goal of this review article is to discuss the current approach to esophageal dysphagia and future direction to allow for timely diagnosis and management.
History
The diagnosis of dysphagia begins with a thorough history. Questions about the timing, onset, progression, localization of symptoms, and types of food that are difficult to swallow are essential in differentiating oropharyngeal and esophageal dysphagia.3,4 Further history taking must include medication and allergy review, smoking history, and review of prior radiation or surgical therapies to the head and neck.
Briefly, oropharyngeal dysphagia is difficulty initiating a swallow or passing food from the mouth or throat and can be caused by structural or functional etiologies.5 Clinical presentations include a sensation of food stuck in the back of the throat, coughing or choking while eating, or drooling. Structural causes include head and neck cancer, Zenker diverticulum, Killian Jamieson diverticula, prolonged intubation, or changes secondary to prior surgery or radiation.3 Functional causes may include neurologic, rheumatologic, or muscular disorders.6
Esophageal dysphagia refers to difficulty transporting food or liquid down the esophagus and can be caused by structural, inflammatory, or functional disorders.5 Patients typically localize symptoms of heartburn, regurgitation, nausea, vomiting, cough, or chest pain along the sternum or epigastric region. Alarm signs concerning for malignancy include unintentional weight loss, fevers, or night sweats.3,7 Aside from symptoms, medication review is essential, as dysphagia is a common side effect of antipsychotics, anticholinergics, antimuscarinics, narcotics, and immunosuppressant drugs.8 Larger pills such as NSAIDs, antibiotics, bisphosphonates, potassium supplements, and methylxanthines can cause drug-induced esophagitis, which can initially present as dysphagia.8 Inflammatory causes can be elucidated by obtaining a history about allergies, tobacco use, and recent infections such as thrush or pneumonia. Patients with a history of recurrent pneumonias may be silently aspirating, a complication of dysphagia.3 Once esophageal dysphagia is clinically suspected based on history, workup can begin.
Differentiating etiologies of esophageal dysphagia
The next step in diagnosing esophageal dysphagia is differentiating between structural, inflammatory, or dysmotility etiology (Figure 1).

Patients with a structural cause typically have difficulty swallowing solids but are able to swallow liquids unless the disease progresses. Symptoms can rapidly worsen and lead to odynophagia, weight loss, and vomiting. In comparison, patients with motility disorders typically have difficulty swallowing both solids and liquids initially, and symptoms can be constant or intermittent.5
Prior to diagnostic studies, a 4-week trial of a proton pump inhibitor (PPI) is appropriate for patients with reflux symptoms who are younger than 50 with no alarm features concerning for malignancy.7,9 If symptoms persist after a PPI trial, then an upper endoscopy (EGD) is indicated. An EGD allows for visualization of structural etiologies, obtaining biopsies to rule out inflammatory etiologies, and the option to therapeutically treat reduced luminal diameter with dilatation.10 The most common structural and inflammatory etiologies noted on EGD include strictures, webs, carcinomas, Schatzki rings, and gastroesophageal reflux or eosinophilic esophagitis.4
If upper endoscopy is normal and clinical suspicion for an obstructive cause remains high, barium esophagram can be utilized as an adjunctive study. Previously, barium esophagram was the initial test to distinguish between structural and motility disorders. The benefits of endoscopy over barium esophagram as the first diagnostic study include higher diagnostic yield, higher sensitivity and specificity, and lower costs.7 However, barium studies may be more sensitive for lower esophageal rings or extrinsic esophageal compression.3
Evaluation of esophageal motility disorder
If a structural or inflammatory etiology of dysphagia is not identified, investigation for an esophageal motility disorder (EMD) is warranted. Examples of motility disorders include achalasia, ineffective esophageal motility, hypercontractility, spasticity, or esophagogastric junction outflow obstruction (EGJOO).10,11 High-resolution esophageal manometry (HRM) remains the gold standard in diagnosis of EMD.12 An HRM catheter utilizes 36 sensors placed two centimeters apart and is placed in the esophagus to evaluate pressure and peristalsis between the upper and lower esophageal sphincters.13 In 2009, the Chicago Classification System was developed to provide a diagnostic algorithm that categorizes EMD based on HRM testing, with the most recent version (4.0) being published in 2020.12,14 Motility diagnoses are divided into two general classifications of disorders of body peristalsis and disorders of EGJ outflow. The most recent updates also include changes in swallow protocols, patient positioning, targeted symptoms, addition of impedance sensors, and consideration of supplemental testing when HRM is inconclusive based on the clinical context.12 There are some limitations of HRM to highlight. One of the main diagnostic values used with HRM is the integrated relaxation pressure (IRP). Despite standardization, IRP measurements vary based on the recorder and patient position. A minority of patients with achalasia may have IRP that does not approach the accepted cutoff and, therefore, the EGJ is not accurately assessed on HRM.15,16 In addition, some swallow protocols have lower sensitivity and specificity for certain motility disorders, and the test can result as inconclusive.14 In these scenarios, supplemental testing with timed barium esophagram or functional luminal imaging probe (EndoFLIP) is indicated.10,11
Over the past decade, EndoFLIP has emerged as a novel diagnostic tool in evaluating EMD. EndoFLIP is usually completed during an upper endoscopy and utilizes impedance planimetry to measure cross-sectional area and esophageal distensibility and evaluate contractile patterns.16 During the procedure, a small catheter with an inflatable balloon is inserted into the esophagus with the distal end in the stomach, traversing the esophagogastric junction (EGJ). The pressure transducer has electrodes every centimeter to allow for a three-dimensional construction of the esophagus and EGJ.17 EndoFLIP has been shown to accurately measure pyloric diameter, pressure, and distensibility at certain balloon volumes.18 In addition, FLIP is being used to further identify aspects of esophageal dysmotility in patients with eosinophilic esophagitis, thought primarily to be an inflammatory disorder.19 However, limitations include minimal accessibility of EndoFLIP within clinical practice and a specific computer program needed to generate the topographic plots.20
When used in conjunction with HRM, EndoFLIP provides complementary data that can be used to better detect major motility disorders.15,20,21 Each study adds unique information about the different physiologic events comprising the esophageal response to distention. Overall, the benefits of EndoFLIP include expediting workup during index endoscopy, patient comfort with sedation, and real-time diagnostic data that supplement results obtained during HRM.10,16,20,2223
Of note, if the diagnostic evaluation for structural, inflammatory, and motility disorders are unrevealing, investigating for atypical reflux symptoms can be pursued for patients with persistent dysphagia. Studies investigating pH, or acidity in the esophagus, in relation to symptoms, can be conducted wirelessly via a capsule fixed to the mucosa or with a nasal catheter.3
Normal workup – hypervigilance
In a subset of patients, all diagnostic testing for structural, inflammatory, or motility disorders is normal. These patients are classified as having a functional esophageal disorder. Despite normal testing, patients still have significant symptoms including epigastric pain, chest pain, globus sensation, or difficulty swallowing. It is theorized that a degree of visceral hypersensitivity between the brain-gut axis contributes to ongoing symptoms.24 Studies for effective treatments are ongoing but typically include cognitive-behavioral therapy, brain-gut behavioral therapy, swallow therapy antidepressants, or short courses of proton pump inhibitors.9
Conclusion
In this review article, we discussed the diagnostic approach for esophageal dysphagia. Initial assessment requires a thorough history, differentiation between oropharyngeal and esophageal dysphagia, and determination of who warrants an upper endoscopy. Upper endoscopy may reveal structural or inflammatory causes of dysphagia, including strictures, masses, or esophagitis, to name a few. If a structural or inflammatory cause is ruled out, this warrants investigation for esophageal motility disorders. The current gold standard for diagnosing EMD is manometry, and supplemental studies, including EndoFLIP, barium esophagram, and pH studies, may provide complimentary data. If workup for dysphagia is normal, evaluation for esophageal hypervigilance causing increased sensitivity to normal or mild sensations may be warranted. In conclusion, the diagnosis of dysphagia is challenging and requires investigation with a systematic approach to ensure timely diagnosis and treatment
Dr. Ronnie and Dr. Bloomberg are in the department of internal medicine at Loyola University Chicago, Maywood, Ill. Dr. Venu is in the division of gastroenterology at Loyola. He is on the speakers bureau at Medtronic.
References
1. Adkins C et al. Clin Gastroenterol Hepatol. 2020;18(9):1970-9.e2.
2. Bhattacharyya N. Otolaryngol Head Neck Surg. 2014;151(5):765-9.
3. McCarty EB and Chao TN. Med Clin North Am. 2021;105(5):939-54.
4. Thiyagalingam S et al. Mayo Clin Proc. 2021;96(2):488-97.
5. Malagelada JR et al. J Clin Gastroenterol. 2015;49(5):370-8.
6. Rommel, N and Hamdy S. Nat Rev Gastroenterol Hepatol. 2016;13(1):49-59.
7. Liu LWC et al. J Can Assoc Gastroenterol. 2018;1(1):5-19.
8. Schwemmle C et al. HNO. 2015;63(7):504-10.
9. Moayyedi P et al. Am J Gastroenterol. 2017;112(7):988-1013.
10. Triggs J and Pandolfino J. F1000Res. 2019 Aug 29. doi: 10.12688/f1000research.18900.1.
11. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14058.
12. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14053.
13. Fox M et al. Neurogastroenterol Motil. 2004;16(5):533-42.
14. Sweis R and Fox M. Curr Gastroenterol Rep. 2020;22(10):49.
15. Carlson DA et al. Gastroenterology. 2015;149(7):1742-51.
16. Donnan EN and Pandolfino JE. Gastroenterol Clin North Am. 2020;49(3):427-35.
17. Carlson DA. Curr Opin Gastroenterol. 2016;32(4):310-8.
18. Zheng T et al. Neurogastroenterol Motil. 2022;34(10):e14386.
19. Carlson DA et al. Clin Gastroenterol Hepatol. 2022;20(8):1719-28.e3.
20. Carlson DA et al. Am J Gastroenterol. 2016;111(12):1726-35.
21. Carlson DA et al. Neurogastroenterol Motil. 2021;33(10):e14116.
22. Carlson DA et al. Gastrointest Endosc. 2019;90(6):915-923.e1.
23. Fox MR et al. Neurogastroenterol Motil. 2021;33(4):e14120.
24. Aziz Q et al. Gastroenterology. 2016 Feb 15. doi: 10.1053/j.gastro.2016.02.012.
Introduction
Dysphagia is the sensation of difficulty swallowing food or liquid in the acute or chronic setting. The prevalence of dysphagia ranges based on the type and etiology but may impact up to one in six adults.1,2 Dysphagia can cause a significant impact on a patient’s health and overall quality of life. A recent study found that only 50% of symptomatic adults seek medical care despite modifying their eating habits by either eating slowly or changing to softer foods or liquids.1 The most common, serious complications of dysphagia include aspiration pneumonia, malnutrition, and dehydration.3 According to the Agency for Healthcare Research and Quality, dysphagia may be responsible for up to 60,000 deaths annually.3
The diagnosis of esophageal dysphagia can be challenging. An initial, thorough history is essential to delineate between oropharyngeal and esophageal dysphagia and guide subsequent diagnostic testing. In recent years, there have been a number of advances in the approach to diagnosing dysphagia, including novel diagnostic modalities. The goal of this review article is to discuss the current approach to esophageal dysphagia and future direction to allow for timely diagnosis and management.
History
The diagnosis of dysphagia begins with a thorough history. Questions about the timing, onset, progression, localization of symptoms, and types of food that are difficult to swallow are essential in differentiating oropharyngeal and esophageal dysphagia.3,4 Further history taking must include medication and allergy review, smoking history, and review of prior radiation or surgical therapies to the head and neck.
Briefly, oropharyngeal dysphagia is difficulty initiating a swallow or passing food from the mouth or throat and can be caused by structural or functional etiologies.5 Clinical presentations include a sensation of food stuck in the back of the throat, coughing or choking while eating, or drooling. Structural causes include head and neck cancer, Zenker diverticulum, Killian Jamieson diverticula, prolonged intubation, or changes secondary to prior surgery or radiation.3 Functional causes may include neurologic, rheumatologic, or muscular disorders.6
Esophageal dysphagia refers to difficulty transporting food or liquid down the esophagus and can be caused by structural, inflammatory, or functional disorders.5 Patients typically localize symptoms of heartburn, regurgitation, nausea, vomiting, cough, or chest pain along the sternum or epigastric region. Alarm signs concerning for malignancy include unintentional weight loss, fevers, or night sweats.3,7 Aside from symptoms, medication review is essential, as dysphagia is a common side effect of antipsychotics, anticholinergics, antimuscarinics, narcotics, and immunosuppressant drugs.8 Larger pills such as NSAIDs, antibiotics, bisphosphonates, potassium supplements, and methylxanthines can cause drug-induced esophagitis, which can initially present as dysphagia.8 Inflammatory causes can be elucidated by obtaining a history about allergies, tobacco use, and recent infections such as thrush or pneumonia. Patients with a history of recurrent pneumonias may be silently aspirating, a complication of dysphagia.3 Once esophageal dysphagia is clinically suspected based on history, workup can begin.
Differentiating etiologies of esophageal dysphagia
The next step in diagnosing esophageal dysphagia is differentiating between structural, inflammatory, or dysmotility etiology (Figure 1).

Patients with a structural cause typically have difficulty swallowing solids but are able to swallow liquids unless the disease progresses. Symptoms can rapidly worsen and lead to odynophagia, weight loss, and vomiting. In comparison, patients with motility disorders typically have difficulty swallowing both solids and liquids initially, and symptoms can be constant or intermittent.5
Prior to diagnostic studies, a 4-week trial of a proton pump inhibitor (PPI) is appropriate for patients with reflux symptoms who are younger than 50 with no alarm features concerning for malignancy.7,9 If symptoms persist after a PPI trial, then an upper endoscopy (EGD) is indicated. An EGD allows for visualization of structural etiologies, obtaining biopsies to rule out inflammatory etiologies, and the option to therapeutically treat reduced luminal diameter with dilatation.10 The most common structural and inflammatory etiologies noted on EGD include strictures, webs, carcinomas, Schatzki rings, and gastroesophageal reflux or eosinophilic esophagitis.4
If upper endoscopy is normal and clinical suspicion for an obstructive cause remains high, barium esophagram can be utilized as an adjunctive study. Previously, barium esophagram was the initial test to distinguish between structural and motility disorders. The benefits of endoscopy over barium esophagram as the first diagnostic study include higher diagnostic yield, higher sensitivity and specificity, and lower costs.7 However, barium studies may be more sensitive for lower esophageal rings or extrinsic esophageal compression.3
Evaluation of esophageal motility disorder
If a structural or inflammatory etiology of dysphagia is not identified, investigation for an esophageal motility disorder (EMD) is warranted. Examples of motility disorders include achalasia, ineffective esophageal motility, hypercontractility, spasticity, or esophagogastric junction outflow obstruction (EGJOO).10,11 High-resolution esophageal manometry (HRM) remains the gold standard in diagnosis of EMD.12 An HRM catheter utilizes 36 sensors placed two centimeters apart and is placed in the esophagus to evaluate pressure and peristalsis between the upper and lower esophageal sphincters.13 In 2009, the Chicago Classification System was developed to provide a diagnostic algorithm that categorizes EMD based on HRM testing, with the most recent version (4.0) being published in 2020.12,14 Motility diagnoses are divided into two general classifications of disorders of body peristalsis and disorders of EGJ outflow. The most recent updates also include changes in swallow protocols, patient positioning, targeted symptoms, addition of impedance sensors, and consideration of supplemental testing when HRM is inconclusive based on the clinical context.12 There are some limitations of HRM to highlight. One of the main diagnostic values used with HRM is the integrated relaxation pressure (IRP). Despite standardization, IRP measurements vary based on the recorder and patient position. A minority of patients with achalasia may have IRP that does not approach the accepted cutoff and, therefore, the EGJ is not accurately assessed on HRM.15,16 In addition, some swallow protocols have lower sensitivity and specificity for certain motility disorders, and the test can result as inconclusive.14 In these scenarios, supplemental testing with timed barium esophagram or functional luminal imaging probe (EndoFLIP) is indicated.10,11
Over the past decade, EndoFLIP has emerged as a novel diagnostic tool in evaluating EMD. EndoFLIP is usually completed during an upper endoscopy and utilizes impedance planimetry to measure cross-sectional area and esophageal distensibility and evaluate contractile patterns.16 During the procedure, a small catheter with an inflatable balloon is inserted into the esophagus with the distal end in the stomach, traversing the esophagogastric junction (EGJ). The pressure transducer has electrodes every centimeter to allow for a three-dimensional construction of the esophagus and EGJ.17 EndoFLIP has been shown to accurately measure pyloric diameter, pressure, and distensibility at certain balloon volumes.18 In addition, FLIP is being used to further identify aspects of esophageal dysmotility in patients with eosinophilic esophagitis, thought primarily to be an inflammatory disorder.19 However, limitations include minimal accessibility of EndoFLIP within clinical practice and a specific computer program needed to generate the topographic plots.20
When used in conjunction with HRM, EndoFLIP provides complementary data that can be used to better detect major motility disorders.15,20,21 Each study adds unique information about the different physiologic events comprising the esophageal response to distention. Overall, the benefits of EndoFLIP include expediting workup during index endoscopy, patient comfort with sedation, and real-time diagnostic data that supplement results obtained during HRM.10,16,20,2223
Of note, if the diagnostic evaluation for structural, inflammatory, and motility disorders are unrevealing, investigating for atypical reflux symptoms can be pursued for patients with persistent dysphagia. Studies investigating pH, or acidity in the esophagus, in relation to symptoms, can be conducted wirelessly via a capsule fixed to the mucosa or with a nasal catheter.3
Normal workup – hypervigilance
In a subset of patients, all diagnostic testing for structural, inflammatory, or motility disorders is normal. These patients are classified as having a functional esophageal disorder. Despite normal testing, patients still have significant symptoms including epigastric pain, chest pain, globus sensation, or difficulty swallowing. It is theorized that a degree of visceral hypersensitivity between the brain-gut axis contributes to ongoing symptoms.24 Studies for effective treatments are ongoing but typically include cognitive-behavioral therapy, brain-gut behavioral therapy, swallow therapy antidepressants, or short courses of proton pump inhibitors.9
Conclusion
In this review article, we discussed the diagnostic approach for esophageal dysphagia. Initial assessment requires a thorough history, differentiation between oropharyngeal and esophageal dysphagia, and determination of who warrants an upper endoscopy. Upper endoscopy may reveal structural or inflammatory causes of dysphagia, including strictures, masses, or esophagitis, to name a few. If a structural or inflammatory cause is ruled out, this warrants investigation for esophageal motility disorders. The current gold standard for diagnosing EMD is manometry, and supplemental studies, including EndoFLIP, barium esophagram, and pH studies, may provide complimentary data. If workup for dysphagia is normal, evaluation for esophageal hypervigilance causing increased sensitivity to normal or mild sensations may be warranted. In conclusion, the diagnosis of dysphagia is challenging and requires investigation with a systematic approach to ensure timely diagnosis and treatment
Dr. Ronnie and Dr. Bloomberg are in the department of internal medicine at Loyola University Chicago, Maywood, Ill. Dr. Venu is in the division of gastroenterology at Loyola. He is on the speakers bureau at Medtronic.
References
1. Adkins C et al. Clin Gastroenterol Hepatol. 2020;18(9):1970-9.e2.
2. Bhattacharyya N. Otolaryngol Head Neck Surg. 2014;151(5):765-9.
3. McCarty EB and Chao TN. Med Clin North Am. 2021;105(5):939-54.
4. Thiyagalingam S et al. Mayo Clin Proc. 2021;96(2):488-97.
5. Malagelada JR et al. J Clin Gastroenterol. 2015;49(5):370-8.
6. Rommel, N and Hamdy S. Nat Rev Gastroenterol Hepatol. 2016;13(1):49-59.
7. Liu LWC et al. J Can Assoc Gastroenterol. 2018;1(1):5-19.
8. Schwemmle C et al. HNO. 2015;63(7):504-10.
9. Moayyedi P et al. Am J Gastroenterol. 2017;112(7):988-1013.
10. Triggs J and Pandolfino J. F1000Res. 2019 Aug 29. doi: 10.12688/f1000research.18900.1.
11. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14058.
12. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14053.
13. Fox M et al. Neurogastroenterol Motil. 2004;16(5):533-42.
14. Sweis R and Fox M. Curr Gastroenterol Rep. 2020;22(10):49.
15. Carlson DA et al. Gastroenterology. 2015;149(7):1742-51.
16. Donnan EN and Pandolfino JE. Gastroenterol Clin North Am. 2020;49(3):427-35.
17. Carlson DA. Curr Opin Gastroenterol. 2016;32(4):310-8.
18. Zheng T et al. Neurogastroenterol Motil. 2022;34(10):e14386.
19. Carlson DA et al. Clin Gastroenterol Hepatol. 2022;20(8):1719-28.e3.
20. Carlson DA et al. Am J Gastroenterol. 2016;111(12):1726-35.
21. Carlson DA et al. Neurogastroenterol Motil. 2021;33(10):e14116.
22. Carlson DA et al. Gastrointest Endosc. 2019;90(6):915-923.e1.
23. Fox MR et al. Neurogastroenterol Motil. 2021;33(4):e14120.
24. Aziz Q et al. Gastroenterology. 2016 Feb 15. doi: 10.1053/j.gastro.2016.02.012.
Spring reflections
Dear friends,
I celebrate my achievements (both personal and work related), try not to be too hard on myself with unaccomplished tasks, and plan goals for the upcoming year. Most importantly, it’s a time to be grateful for both opportunities and challenges. Thank you for your engagement with The New Gastroenterologist, and as you go through this issue, I hope you can find time for some spring reflections as well!
In this issue’s In Focus, Dr. Tanisha Ronnie, Dr. Lauren Bloomberg, and Dr. Mukund Venu break down the approach to a patient with dysphagia, a common and difficult encounter in GI practice. They emphasize the importance of a good clinical history as well as understanding the role of diagnostic testing. In our Short Clinical Review section, Dr. Noa Krugliak Cleveland and Dr. David Rubin review the rising role of intestinal ultrasound in inflammatory bowel disease, how to be trained, and how to incorporate it in clinical practice.
As early-career gastroenterologists, Dr. Samad Soudagar and Dr. Mohammad Bilal were tasked with establishing an advanced endoscopy practice, which may be overwhelming for many. They synthesized their experiences into 10 practical tips to build a successful practice. Our Post-fellowship Pathways article highlights Dr. Katie Hutchins’s journey from private practice to academic medicine; she provides insights into the life-changing decision and what she learned about herself to make that pivot.
In our Finance section, Dr. Kelly Hathorn and Dr. David Creighton reflect on navigating as new parents while both working full time in medicine; their article weighs the pros and cons of various childcare options in the post–COVID pandemic world.
In an additional contribution this issue, gastroenterology and hepatology fellowship program leaders at the University of Florida, Gainesville, describe their experience with virtual recruitment, including feedback from their candidates, especially as we enter another cycle of GI Match.
If you are interested in contributing or have ideas for future TNG topics, please contact me (jtrieu23@gmail.com), or Jillian Schweitzer (jschweitzer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are without appreciating where we were: The first formalized gastroenterology fellowship curriculum was a joint publication by four major GI and hepatology societies in 1996 – just 27 years ago!
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Advanced Endoscopy Fellow
Division of gastroenterology & hepatology
University of North Carolina at Chapel Hill
Dear friends,
I celebrate my achievements (both personal and work related), try not to be too hard on myself with unaccomplished tasks, and plan goals for the upcoming year. Most importantly, it’s a time to be grateful for both opportunities and challenges. Thank you for your engagement with The New Gastroenterologist, and as you go through this issue, I hope you can find time for some spring reflections as well!
In this issue’s In Focus, Dr. Tanisha Ronnie, Dr. Lauren Bloomberg, and Dr. Mukund Venu break down the approach to a patient with dysphagia, a common and difficult encounter in GI practice. They emphasize the importance of a good clinical history as well as understanding the role of diagnostic testing. In our Short Clinical Review section, Dr. Noa Krugliak Cleveland and Dr. David Rubin review the rising role of intestinal ultrasound in inflammatory bowel disease, how to be trained, and how to incorporate it in clinical practice.
As early-career gastroenterologists, Dr. Samad Soudagar and Dr. Mohammad Bilal were tasked with establishing an advanced endoscopy practice, which may be overwhelming for many. They synthesized their experiences into 10 practical tips to build a successful practice. Our Post-fellowship Pathways article highlights Dr. Katie Hutchins’s journey from private practice to academic medicine; she provides insights into the life-changing decision and what she learned about herself to make that pivot.
In our Finance section, Dr. Kelly Hathorn and Dr. David Creighton reflect on navigating as new parents while both working full time in medicine; their article weighs the pros and cons of various childcare options in the post–COVID pandemic world.
In an additional contribution this issue, gastroenterology and hepatology fellowship program leaders at the University of Florida, Gainesville, describe their experience with virtual recruitment, including feedback from their candidates, especially as we enter another cycle of GI Match.
If you are interested in contributing or have ideas for future TNG topics, please contact me (jtrieu23@gmail.com), or Jillian Schweitzer (jschweitzer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are without appreciating where we were: The first formalized gastroenterology fellowship curriculum was a joint publication by four major GI and hepatology societies in 1996 – just 27 years ago!
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Advanced Endoscopy Fellow
Division of gastroenterology & hepatology
University of North Carolina at Chapel Hill
Dear friends,
I celebrate my achievements (both personal and work related), try not to be too hard on myself with unaccomplished tasks, and plan goals for the upcoming year. Most importantly, it’s a time to be grateful for both opportunities and challenges. Thank you for your engagement with The New Gastroenterologist, and as you go through this issue, I hope you can find time for some spring reflections as well!
In this issue’s In Focus, Dr. Tanisha Ronnie, Dr. Lauren Bloomberg, and Dr. Mukund Venu break down the approach to a patient with dysphagia, a common and difficult encounter in GI practice. They emphasize the importance of a good clinical history as well as understanding the role of diagnostic testing. In our Short Clinical Review section, Dr. Noa Krugliak Cleveland and Dr. David Rubin review the rising role of intestinal ultrasound in inflammatory bowel disease, how to be trained, and how to incorporate it in clinical practice.
As early-career gastroenterologists, Dr. Samad Soudagar and Dr. Mohammad Bilal were tasked with establishing an advanced endoscopy practice, which may be overwhelming for many. They synthesized their experiences into 10 practical tips to build a successful practice. Our Post-fellowship Pathways article highlights Dr. Katie Hutchins’s journey from private practice to academic medicine; she provides insights into the life-changing decision and what she learned about herself to make that pivot.
In our Finance section, Dr. Kelly Hathorn and Dr. David Creighton reflect on navigating as new parents while both working full time in medicine; their article weighs the pros and cons of various childcare options in the post–COVID pandemic world.
In an additional contribution this issue, gastroenterology and hepatology fellowship program leaders at the University of Florida, Gainesville, describe their experience with virtual recruitment, including feedback from their candidates, especially as we enter another cycle of GI Match.
If you are interested in contributing or have ideas for future TNG topics, please contact me (jtrieu23@gmail.com), or Jillian Schweitzer (jschweitzer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are without appreciating where we were: The first formalized gastroenterology fellowship curriculum was a joint publication by four major GI and hepatology societies in 1996 – just 27 years ago!
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Advanced Endoscopy Fellow
Division of gastroenterology & hepatology
University of North Carolina at Chapel Hill
Why my independent GI practice started a GI fellowship program
In this video Naresh Gunaratnam, MD, discusses the gastroenterology fellowship program that Huron Gastroenterology developed with Trinity Health in Ann Arbor, Mich. Dr. Gunaratnam helped create the program because he and his colleagues felt that traditional fellowship programs don’t always provide information or guidance about non-academic career pathways in gastroenterology. Hear from Dr. Gunaratnam how the fellowship program at Huron Gastroenterology is training fellows to become excellent clinicians who care for patients in the community setting. He has no financial conflicts relative to the topics in this video.

In this video Naresh Gunaratnam, MD, discusses the gastroenterology fellowship program that Huron Gastroenterology developed with Trinity Health in Ann Arbor, Mich. Dr. Gunaratnam helped create the program because he and his colleagues felt that traditional fellowship programs don’t always provide information or guidance about non-academic career pathways in gastroenterology. Hear from Dr. Gunaratnam how the fellowship program at Huron Gastroenterology is training fellows to become excellent clinicians who care for patients in the community setting. He has no financial conflicts relative to the topics in this video.

In this video Naresh Gunaratnam, MD, discusses the gastroenterology fellowship program that Huron Gastroenterology developed with Trinity Health in Ann Arbor, Mich. Dr. Gunaratnam helped create the program because he and his colleagues felt that traditional fellowship programs don’t always provide information or guidance about non-academic career pathways in gastroenterology. Hear from Dr. Gunaratnam how the fellowship program at Huron Gastroenterology is training fellows to become excellent clinicians who care for patients in the community setting. He has no financial conflicts relative to the topics in this video.

AGA News – May 2023
Season 2 of Small Talk, Big Topics is here!
AGA’s podcast for trainees and early career GIs, Small Talk, Big Topics, is back for season two. To kick off the new season, hosts Drs. Matthew Whitson, Nina Nandy, and CS Tse sit down with AGA President Dr. John Carethers in a two-part special to chat about his career and how his involvement with AGA has impacted him.
In episode one, Drs. Whitson, Nandy and Tse take a deep dive with Dr. Carethers to reflect on how he first got involved with AGA, his experience with different committees, and how those roles paved the way to leadership positions.
Now, as president, he says, “I am having so much fun. AGA has been with me for my entire GI career. It’s really the voice of the science and practice of gastroenterology.”
In episode two, Dr. Carethers examines the career advice he’s received, how it shaped his leadership style and provides guidance to early career GIs.
“What’s important about some of these higher-level [decisions] is to set a vision. You can’t be a leader if you have no followers, and people have to believe in something, that they’re moving toward something.”
Listen to more of Dr. Carethers’ insight in the first two episodes of Small Talk, Big Topics wherever you listen to podcasts and subscribe to stay up to date on new episodes.
Maximize your first day at DDW® 2023
Held during the first day of Digestive Disease Week®, this year’s AGA Postgraduate Course will be held live on Saturday, May 6, from 8:30 a.m. to 5 p.m. CT. This year’s theme – Advances in Gastroenterology: News You Can Use – will help you cut through the noise surrounding best practices for GI physicians.
Pricing is the same for both in-person and virtual attendees, giving you the flexibility to experience the course in-person or from the comfort of your home. All registrants will have on-demand access to the course for three months and the opportunity to earn up to 17.5 total credits when you complete all on-demand content.
What’s new this year?
General session format
Presentations will be given in an engaging format that will feel less didactic and more akin to a discussion among faculty, or a conversation with the experts! It’s also an exciting opportunity to mix junior and senior lecturers on the same platform.
Recent clinical practices
Session panelists will work together to select the key papers in their topic areas for discussion. Only the newest — within one year — and most important papers, clinical guidelines and pathways in the field will be selected.
Register to attend DDW and the Postgraduate Course today.
And the winner of this year’s Shark Tank is …
The 13th annual AGA Tech Summit took place in San Francisco, Calif., recently, bringing together GI entrepreneurs, clinicians, medical technology companies, venture capitalists, and regulatory agencies working to improve patient care in the field. A highlight of the event is the annual Shark Tank competition, where forward-thinking companies showcase and pitch their innovations to a panel of expert judges.
Congratulations to this year’s winner – Endiatx!
From devices providing rapid cancer detection to technology that makes endoscopy safer, the five companies selected for the 2023 AGA Shark Tank represented a glimpse of the future of GI patient care.
While each team offered a creative solution to modern-day GI challenges, only one could be declared the winner. Congratulations to our 2023 winner, Endiatx! Endiatx will represent AGA in the upcoming Shark Tank competition at DDW®.
Endiatx has developed a vitamin-sized intrabody robot
PillBot is a miniature robotic capsule endoscopy. Shipped to a patient’s home or picked up from a pharmacy, the standard size capsule is swallowed and then controlled by an external joystick-like device or a phone app by a physician in a physically separate location. Using real-time video transmissions visible to both operator and patient, the capsule navigates the entire stomach in a few minutes without anesthesia and ultimately is excreted outside the body without the need for recapture.
Future GI physician innovators
This year the AGA Center for GI Innovation and Technology (CGIT) welcomed 22 first-year to advanced endoscopy fellows to the AGA Innovation Fellows Program. The program provides a unique opportunity for the fellows to learn from GI clinicians, innovators, entrepreneurs, and medical technology executives on how new technologies are developed and brought to market. 
The fellows received an exclusive behind-the-scenes tour of Medtronic’s R&D facility in Santa Clara, Calif., and got to experience hands-on demonstrations of GI GeniusTM, PillCamTM, EndoflipTM, NexpowderTM, BravoTM, BarrxTM and ProdiGITM technologies. The group was also hosted by Boston Scientific Corporation, Castle Biosciences and PENTAX Medical at a dinner that included an innovators panel discussion. The program will continue throughout the year with monthly educational sessions moderated by members of the AGA CGIT committee.
- Mohd Amer Alsamman, MD, Georgetown University
- Mohammad Arfeen, MD, Franciscan Health Olympia Fields
- Alexis Bayudan, MD, University of California, San Francisco
- Aileen Bui, MD, University of Southern California
- Divya Chalikonda, MD, Thomas Jefferson University Hospital
- Alec Faggen, MD, University of California, San Francisco
- Sweta Ghosh, PhD, University of Louisville School of Medicine
- Hemant Goyal, MD, University of Texas Houston
- Averill Guo, MD, Brown University
- Omar Jamil, MD, University of Chicago
- Christina Kratschmer, MD, Washington University in St. Louis
- Thi Khuc, MD, University of Maryland School of Medicine
- Anand Kumar, MD, Northwell Health – Lenox Hill Hospital
- Xing Li, MD, Massachusetts General Hospital
- Alana Persaud, MD, SUNY Downstate Medical Center
- Itegbemie Obaitan, MD, Indiana University School of Medicine
- Chethan Ramprasad, MD, University of Pennsylvania
- Abhishek Satishchandran, MD, University of Michigan
- Kevin Shah, MD, Emory University School of Medicine
- Shifa Umar, MD, University of Chicago
- Kornpong Vantanasiri, MD, Mayo Clinic Rochester
- Shaleen Vasavada, MD, Baylor College of Medicine
Highlights from social media
See what else attendees shared with #AGATech on Twitter.
The 2023 AGA Tech Summit was made possible by support from Castle Biosciences and Medtronic (Diamond Sponsors), AI Medical Services, Boston Scientific, Exact Sciences Corporation, FUJIFILM Medical Systems and Olympus Corporation (Gold Sponsors), Cook Medical Inc., and STERIS Endoscopy (Silver Sponsors), and Apollo Endosurgery and EvoEndo (Bronze Sponsors).
AGA takes CRC month to Capitol Hill
Participating in Colorectal Cancer Awareness Month in Washington, D.C., means one thing – taking the fight to save lives from CRC to Capitol Hill and advocating for increased access to screening and research to improve outcomes.

In March, AGA joined the national advocacy organization Fight Colorectal Cancer (Fight CRC) and partners in the colorectal cancer community for events in our nation’s capital. The goal was to destigmatize talking about gut health and CRC and to collaboratively develop solutions that will improve and increase access to CRC screening.
Fight CRC working lunch
Former AGA president Dr. David Lieberman and fellow AGA member and FORWARD graduate Dr. Fola May served as facilitators for the coalition of public and private leaders assembled by Fight CRC. The group is working to develop an action plan to further equitable CRC screening and lower the number of lives impacted by CRC. Among the participants were insurers, industry, federal agencies, healthcare providers, retail businesses, and patients.
White House Cancer Moonshot colorectal cancer forum
In partnership with President Biden’s reignited Cancer Moonshot initiative, we joined Fight CRC and other advocacy and industry leaders in the colorectal cancer community for the Cancer Moonshot Colorectal Cancer Forum, hosted by the White House.
Dr. May participated as a panelist during the forum and discussed how we should address disparities in CRC. “Research dollars are essential in [combating CRC inequity]. We do not know how to effectively deliver care and preventive services to these populations unless we do deep dives into these particular settings to understand how to best deliver that care. This is not a “pick a model and apply broadly” approach. We need to go to the people, and we need to go to the people with the methods that work for that particular setting, and that’s going to be different in every community.”
In addition to Dr. Lieberman, who attended on behalf of AGA, fellow AGA members Drs. Austin Chiang, Swati Patel and AGA FORWARD Scholar Rachel Issaka were in attendance. We are appreciative of the opportunity to be included in these important discussions with the Administration and partners in the CRC community as we work together to reduce the burden of CRC and save lives.
Fight CRC United in Blue rally on the National Mall
It’s become an annual tradition for us to join Fight CRC’s United in Blue rally and blue flag installation on the National Mall, and this year was no different. We joined industry and patient advocacy groups in the CRC community to raise our voices about the need for screening, research, and advocacy to improve colon cancer outcomes.
The rally included inspiring calls to action and CRC testimonials from individuals who have been personally impacted by the disease, including Rep. Donald Payne Jr. (D-NJ), who lost his father to CRC and who personally underwent screening, which led to the discovery of 13 polyps.
Dr. Manish Singla from Capital Digestive Care spoke on behalf of AGA and provided encouragement and a reminder for patients and providers.
“What I keep hearing here is patients feel like they’re not being heard – so we’re listening. We’re trying and we’re here to fight the disease with you all. Everyone here knows somebody who is due for a colonoscopy and isn’t getting it, so use your persuasion – talk about it, convince, cajole, shame – use whatever you need so that everyone gets the screenings they need,” Dr. Singla said.
Our work is just beginning: Let’s work together to encourage screenings for colorectal cancer and save lives. Join us as we remind everyone that 45 is the new 50.
Season 2 of Small Talk, Big Topics is here!
AGA’s podcast for trainees and early career GIs, Small Talk, Big Topics, is back for season two. To kick off the new season, hosts Drs. Matthew Whitson, Nina Nandy, and CS Tse sit down with AGA President Dr. John Carethers in a two-part special to chat about his career and how his involvement with AGA has impacted him.
In episode one, Drs. Whitson, Nandy and Tse take a deep dive with Dr. Carethers to reflect on how he first got involved with AGA, his experience with different committees, and how those roles paved the way to leadership positions.
Now, as president, he says, “I am having so much fun. AGA has been with me for my entire GI career. It’s really the voice of the science and practice of gastroenterology.”
In episode two, Dr. Carethers examines the career advice he’s received, how it shaped his leadership style and provides guidance to early career GIs.
“What’s important about some of these higher-level [decisions] is to set a vision. You can’t be a leader if you have no followers, and people have to believe in something, that they’re moving toward something.”
Listen to more of Dr. Carethers’ insight in the first two episodes of Small Talk, Big Topics wherever you listen to podcasts and subscribe to stay up to date on new episodes.
Maximize your first day at DDW® 2023
Held during the first day of Digestive Disease Week®, this year’s AGA Postgraduate Course will be held live on Saturday, May 6, from 8:30 a.m. to 5 p.m. CT. This year’s theme – Advances in Gastroenterology: News You Can Use – will help you cut through the noise surrounding best practices for GI physicians.
Pricing is the same for both in-person and virtual attendees, giving you the flexibility to experience the course in-person or from the comfort of your home. All registrants will have on-demand access to the course for three months and the opportunity to earn up to 17.5 total credits when you complete all on-demand content.
What’s new this year?
General session format
Presentations will be given in an engaging format that will feel less didactic and more akin to a discussion among faculty, or a conversation with the experts! It’s also an exciting opportunity to mix junior and senior lecturers on the same platform.
Recent clinical practices
Session panelists will work together to select the key papers in their topic areas for discussion. Only the newest — within one year — and most important papers, clinical guidelines and pathways in the field will be selected.
Register to attend DDW and the Postgraduate Course today.
And the winner of this year’s Shark Tank is …
The 13th annual AGA Tech Summit took place in San Francisco, Calif., recently, bringing together GI entrepreneurs, clinicians, medical technology companies, venture capitalists, and regulatory agencies working to improve patient care in the field. A highlight of the event is the annual Shark Tank competition, where forward-thinking companies showcase and pitch their innovations to a panel of expert judges.
Congratulations to this year’s winner – Endiatx!
From devices providing rapid cancer detection to technology that makes endoscopy safer, the five companies selected for the 2023 AGA Shark Tank represented a glimpse of the future of GI patient care.
While each team offered a creative solution to modern-day GI challenges, only one could be declared the winner. Congratulations to our 2023 winner, Endiatx! Endiatx will represent AGA in the upcoming Shark Tank competition at DDW®.
Endiatx has developed a vitamin-sized intrabody robot
PillBot is a miniature robotic capsule endoscopy. Shipped to a patient’s home or picked up from a pharmacy, the standard size capsule is swallowed and then controlled by an external joystick-like device or a phone app by a physician in a physically separate location. Using real-time video transmissions visible to both operator and patient, the capsule navigates the entire stomach in a few minutes without anesthesia and ultimately is excreted outside the body without the need for recapture.
Future GI physician innovators
This year the AGA Center for GI Innovation and Technology (CGIT) welcomed 22 first-year to advanced endoscopy fellows to the AGA Innovation Fellows Program. The program provides a unique opportunity for the fellows to learn from GI clinicians, innovators, entrepreneurs, and medical technology executives on how new technologies are developed and brought to market. 
The fellows received an exclusive behind-the-scenes tour of Medtronic’s R&D facility in Santa Clara, Calif., and got to experience hands-on demonstrations of GI GeniusTM, PillCamTM, EndoflipTM, NexpowderTM, BravoTM, BarrxTM and ProdiGITM technologies. The group was also hosted by Boston Scientific Corporation, Castle Biosciences and PENTAX Medical at a dinner that included an innovators panel discussion. The program will continue throughout the year with monthly educational sessions moderated by members of the AGA CGIT committee.
- Mohd Amer Alsamman, MD, Georgetown University
- Mohammad Arfeen, MD, Franciscan Health Olympia Fields
- Alexis Bayudan, MD, University of California, San Francisco
- Aileen Bui, MD, University of Southern California
- Divya Chalikonda, MD, Thomas Jefferson University Hospital
- Alec Faggen, MD, University of California, San Francisco
- Sweta Ghosh, PhD, University of Louisville School of Medicine
- Hemant Goyal, MD, University of Texas Houston
- Averill Guo, MD, Brown University
- Omar Jamil, MD, University of Chicago
- Christina Kratschmer, MD, Washington University in St. Louis
- Thi Khuc, MD, University of Maryland School of Medicine
- Anand Kumar, MD, Northwell Health – Lenox Hill Hospital
- Xing Li, MD, Massachusetts General Hospital
- Alana Persaud, MD, SUNY Downstate Medical Center
- Itegbemie Obaitan, MD, Indiana University School of Medicine
- Chethan Ramprasad, MD, University of Pennsylvania
- Abhishek Satishchandran, MD, University of Michigan
- Kevin Shah, MD, Emory University School of Medicine
- Shifa Umar, MD, University of Chicago
- Kornpong Vantanasiri, MD, Mayo Clinic Rochester
- Shaleen Vasavada, MD, Baylor College of Medicine
Highlights from social media
See what else attendees shared with #AGATech on Twitter.
The 2023 AGA Tech Summit was made possible by support from Castle Biosciences and Medtronic (Diamond Sponsors), AI Medical Services, Boston Scientific, Exact Sciences Corporation, FUJIFILM Medical Systems and Olympus Corporation (Gold Sponsors), Cook Medical Inc., and STERIS Endoscopy (Silver Sponsors), and Apollo Endosurgery and EvoEndo (Bronze Sponsors).
AGA takes CRC month to Capitol Hill
Participating in Colorectal Cancer Awareness Month in Washington, D.C., means one thing – taking the fight to save lives from CRC to Capitol Hill and advocating for increased access to screening and research to improve outcomes.

In March, AGA joined the national advocacy organization Fight Colorectal Cancer (Fight CRC) and partners in the colorectal cancer community for events in our nation’s capital. The goal was to destigmatize talking about gut health and CRC and to collaboratively develop solutions that will improve and increase access to CRC screening.
Fight CRC working lunch
Former AGA president Dr. David Lieberman and fellow AGA member and FORWARD graduate Dr. Fola May served as facilitators for the coalition of public and private leaders assembled by Fight CRC. The group is working to develop an action plan to further equitable CRC screening and lower the number of lives impacted by CRC. Among the participants were insurers, industry, federal agencies, healthcare providers, retail businesses, and patients.
White House Cancer Moonshot colorectal cancer forum
In partnership with President Biden’s reignited Cancer Moonshot initiative, we joined Fight CRC and other advocacy and industry leaders in the colorectal cancer community for the Cancer Moonshot Colorectal Cancer Forum, hosted by the White House.
Dr. May participated as a panelist during the forum and discussed how we should address disparities in CRC. “Research dollars are essential in [combating CRC inequity]. We do not know how to effectively deliver care and preventive services to these populations unless we do deep dives into these particular settings to understand how to best deliver that care. This is not a “pick a model and apply broadly” approach. We need to go to the people, and we need to go to the people with the methods that work for that particular setting, and that’s going to be different in every community.”
In addition to Dr. Lieberman, who attended on behalf of AGA, fellow AGA members Drs. Austin Chiang, Swati Patel and AGA FORWARD Scholar Rachel Issaka were in attendance. We are appreciative of the opportunity to be included in these important discussions with the Administration and partners in the CRC community as we work together to reduce the burden of CRC and save lives.
Fight CRC United in Blue rally on the National Mall
It’s become an annual tradition for us to join Fight CRC’s United in Blue rally and blue flag installation on the National Mall, and this year was no different. We joined industry and patient advocacy groups in the CRC community to raise our voices about the need for screening, research, and advocacy to improve colon cancer outcomes.
The rally included inspiring calls to action and CRC testimonials from individuals who have been personally impacted by the disease, including Rep. Donald Payne Jr. (D-NJ), who lost his father to CRC and who personally underwent screening, which led to the discovery of 13 polyps.
Dr. Manish Singla from Capital Digestive Care spoke on behalf of AGA and provided encouragement and a reminder for patients and providers.
“What I keep hearing here is patients feel like they’re not being heard – so we’re listening. We’re trying and we’re here to fight the disease with you all. Everyone here knows somebody who is due for a colonoscopy and isn’t getting it, so use your persuasion – talk about it, convince, cajole, shame – use whatever you need so that everyone gets the screenings they need,” Dr. Singla said.
Our work is just beginning: Let’s work together to encourage screenings for colorectal cancer and save lives. Join us as we remind everyone that 45 is the new 50.
Season 2 of Small Talk, Big Topics is here!
AGA’s podcast for trainees and early career GIs, Small Talk, Big Topics, is back for season two. To kick off the new season, hosts Drs. Matthew Whitson, Nina Nandy, and CS Tse sit down with AGA President Dr. John Carethers in a two-part special to chat about his career and how his involvement with AGA has impacted him.
In episode one, Drs. Whitson, Nandy and Tse take a deep dive with Dr. Carethers to reflect on how he first got involved with AGA, his experience with different committees, and how those roles paved the way to leadership positions.
Now, as president, he says, “I am having so much fun. AGA has been with me for my entire GI career. It’s really the voice of the science and practice of gastroenterology.”
In episode two, Dr. Carethers examines the career advice he’s received, how it shaped his leadership style and provides guidance to early career GIs.
“What’s important about some of these higher-level [decisions] is to set a vision. You can’t be a leader if you have no followers, and people have to believe in something, that they’re moving toward something.”
Listen to more of Dr. Carethers’ insight in the first two episodes of Small Talk, Big Topics wherever you listen to podcasts and subscribe to stay up to date on new episodes.
Maximize your first day at DDW® 2023
Held during the first day of Digestive Disease Week®, this year’s AGA Postgraduate Course will be held live on Saturday, May 6, from 8:30 a.m. to 5 p.m. CT. This year’s theme – Advances in Gastroenterology: News You Can Use – will help you cut through the noise surrounding best practices for GI physicians.
Pricing is the same for both in-person and virtual attendees, giving you the flexibility to experience the course in-person or from the comfort of your home. All registrants will have on-demand access to the course for three months and the opportunity to earn up to 17.5 total credits when you complete all on-demand content.
What’s new this year?
General session format
Presentations will be given in an engaging format that will feel less didactic and more akin to a discussion among faculty, or a conversation with the experts! It’s also an exciting opportunity to mix junior and senior lecturers on the same platform.
Recent clinical practices
Session panelists will work together to select the key papers in their topic areas for discussion. Only the newest — within one year — and most important papers, clinical guidelines and pathways in the field will be selected.
Register to attend DDW and the Postgraduate Course today.
And the winner of this year’s Shark Tank is …
The 13th annual AGA Tech Summit took place in San Francisco, Calif., recently, bringing together GI entrepreneurs, clinicians, medical technology companies, venture capitalists, and regulatory agencies working to improve patient care in the field. A highlight of the event is the annual Shark Tank competition, where forward-thinking companies showcase and pitch their innovations to a panel of expert judges.
Congratulations to this year’s winner – Endiatx!
From devices providing rapid cancer detection to technology that makes endoscopy safer, the five companies selected for the 2023 AGA Shark Tank represented a glimpse of the future of GI patient care.
While each team offered a creative solution to modern-day GI challenges, only one could be declared the winner. Congratulations to our 2023 winner, Endiatx! Endiatx will represent AGA in the upcoming Shark Tank competition at DDW®.
Endiatx has developed a vitamin-sized intrabody robot
PillBot is a miniature robotic capsule endoscopy. Shipped to a patient’s home or picked up from a pharmacy, the standard size capsule is swallowed and then controlled by an external joystick-like device or a phone app by a physician in a physically separate location. Using real-time video transmissions visible to both operator and patient, the capsule navigates the entire stomach in a few minutes without anesthesia and ultimately is excreted outside the body without the need for recapture.
Future GI physician innovators
This year the AGA Center for GI Innovation and Technology (CGIT) welcomed 22 first-year to advanced endoscopy fellows to the AGA Innovation Fellows Program. The program provides a unique opportunity for the fellows to learn from GI clinicians, innovators, entrepreneurs, and medical technology executives on how new technologies are developed and brought to market. 
The fellows received an exclusive behind-the-scenes tour of Medtronic’s R&D facility in Santa Clara, Calif., and got to experience hands-on demonstrations of GI GeniusTM, PillCamTM, EndoflipTM, NexpowderTM, BravoTM, BarrxTM and ProdiGITM technologies. The group was also hosted by Boston Scientific Corporation, Castle Biosciences and PENTAX Medical at a dinner that included an innovators panel discussion. The program will continue throughout the year with monthly educational sessions moderated by members of the AGA CGIT committee.
- Mohd Amer Alsamman, MD, Georgetown University
- Mohammad Arfeen, MD, Franciscan Health Olympia Fields
- Alexis Bayudan, MD, University of California, San Francisco
- Aileen Bui, MD, University of Southern California
- Divya Chalikonda, MD, Thomas Jefferson University Hospital
- Alec Faggen, MD, University of California, San Francisco
- Sweta Ghosh, PhD, University of Louisville School of Medicine
- Hemant Goyal, MD, University of Texas Houston
- Averill Guo, MD, Brown University
- Omar Jamil, MD, University of Chicago
- Christina Kratschmer, MD, Washington University in St. Louis
- Thi Khuc, MD, University of Maryland School of Medicine
- Anand Kumar, MD, Northwell Health – Lenox Hill Hospital
- Xing Li, MD, Massachusetts General Hospital
- Alana Persaud, MD, SUNY Downstate Medical Center
- Itegbemie Obaitan, MD, Indiana University School of Medicine
- Chethan Ramprasad, MD, University of Pennsylvania
- Abhishek Satishchandran, MD, University of Michigan
- Kevin Shah, MD, Emory University School of Medicine
- Shifa Umar, MD, University of Chicago
- Kornpong Vantanasiri, MD, Mayo Clinic Rochester
- Shaleen Vasavada, MD, Baylor College of Medicine
Highlights from social media
See what else attendees shared with #AGATech on Twitter.
The 2023 AGA Tech Summit was made possible by support from Castle Biosciences and Medtronic (Diamond Sponsors), AI Medical Services, Boston Scientific, Exact Sciences Corporation, FUJIFILM Medical Systems and Olympus Corporation (Gold Sponsors), Cook Medical Inc., and STERIS Endoscopy (Silver Sponsors), and Apollo Endosurgery and EvoEndo (Bronze Sponsors).
AGA takes CRC month to Capitol Hill
Participating in Colorectal Cancer Awareness Month in Washington, D.C., means one thing – taking the fight to save lives from CRC to Capitol Hill and advocating for increased access to screening and research to improve outcomes.

In March, AGA joined the national advocacy organization Fight Colorectal Cancer (Fight CRC) and partners in the colorectal cancer community for events in our nation’s capital. The goal was to destigmatize talking about gut health and CRC and to collaboratively develop solutions that will improve and increase access to CRC screening.
Fight CRC working lunch
Former AGA president Dr. David Lieberman and fellow AGA member and FORWARD graduate Dr. Fola May served as facilitators for the coalition of public and private leaders assembled by Fight CRC. The group is working to develop an action plan to further equitable CRC screening and lower the number of lives impacted by CRC. Among the participants were insurers, industry, federal agencies, healthcare providers, retail businesses, and patients.
White House Cancer Moonshot colorectal cancer forum
In partnership with President Biden’s reignited Cancer Moonshot initiative, we joined Fight CRC and other advocacy and industry leaders in the colorectal cancer community for the Cancer Moonshot Colorectal Cancer Forum, hosted by the White House.
Dr. May participated as a panelist during the forum and discussed how we should address disparities in CRC. “Research dollars are essential in [combating CRC inequity]. We do not know how to effectively deliver care and preventive services to these populations unless we do deep dives into these particular settings to understand how to best deliver that care. This is not a “pick a model and apply broadly” approach. We need to go to the people, and we need to go to the people with the methods that work for that particular setting, and that’s going to be different in every community.”
In addition to Dr. Lieberman, who attended on behalf of AGA, fellow AGA members Drs. Austin Chiang, Swati Patel and AGA FORWARD Scholar Rachel Issaka were in attendance. We are appreciative of the opportunity to be included in these important discussions with the Administration and partners in the CRC community as we work together to reduce the burden of CRC and save lives.
Fight CRC United in Blue rally on the National Mall
It’s become an annual tradition for us to join Fight CRC’s United in Blue rally and blue flag installation on the National Mall, and this year was no different. We joined industry and patient advocacy groups in the CRC community to raise our voices about the need for screening, research, and advocacy to improve colon cancer outcomes.
The rally included inspiring calls to action and CRC testimonials from individuals who have been personally impacted by the disease, including Rep. Donald Payne Jr. (D-NJ), who lost his father to CRC and who personally underwent screening, which led to the discovery of 13 polyps.
Dr. Manish Singla from Capital Digestive Care spoke on behalf of AGA and provided encouragement and a reminder for patients and providers.
“What I keep hearing here is patients feel like they’re not being heard – so we’re listening. We’re trying and we’re here to fight the disease with you all. Everyone here knows somebody who is due for a colonoscopy and isn’t getting it, so use your persuasion – talk about it, convince, cajole, shame – use whatever you need so that everyone gets the screenings they need,” Dr. Singla said.
Our work is just beginning: Let’s work together to encourage screenings for colorectal cancer and save lives. Join us as we remind everyone that 45 is the new 50.
Establishing an advanced endoscopy practice: Tips for trainees and early faculty
Establishing an advanced endoscopy practice can appear challenging and overwhelming. It is often the culmination of more than a decade of education and training for advanced endoscopists and is usually their first foray into employment. all while creating a rewarding opportunity to provide a population with necessary services, which, more than likely, were not previously being offered at your institution or in your region.
Tip 1: Understand the current landscape
When joining a hospital-employed or private practice, it is important for the advanced endoscopist to gauge the current landscape of the job, beginning with gaining an understanding of the current services provided by your gastroenterology colleagues. This includes knowing the types of advanced endoscopy services previously provided, especially if you have partners or colleagues who perform these procedures, and their prior referral patterns, either within or outside their respective group. Also, it is important to understand the services that are provided locally at other institutions. This will allow you to develop a niche of the types of services you can provide that are not available in the current practice set-up.
Tip 2: Connect with peers, interspecialty collaborators, and referring physicians
It is important that you connect with your GI colleagues once you start a new job. This can differ in ease depending on the size of your group. For example, in a small group, it may be easier to familiarize yourself with your colleagues through regular interactions. If you are a part of a larger practice, however, it is necessary to be more proactive and set up introductory meetings/sessions. These interactions provide a great opportunity to share your goals and start building a relationship.
Efforts also should be made to reach out to primary care, hematology/oncology, surgical/radiation oncology, general surgery, and interventional radiology physicians, as these are the specialists with whom an advanced endoscopist typically has the most interaction. The relationship with these colleagues is bidirectional, as the majority of our patients need multidisciplinary decision-making and care. For example, the first time you speak to the colorectal surgeon at your institution should not be in the middle of a complication. The purpose of these introductions should not be solely to inform them of the services you are offering but to start developing a relationship in a true sense, because eventually those relationships will transform into excellent patient care.
Tip 3: Communication
Communication is a key principle in building a practice. Referring physicians often entrust you with managing a part of their patient’s medical problems. Patient/procedure outcomes should be relayed promptly to referring physicians, as this not only helps build the trust of the referring physician, but also enhances the patient’s trust in the health system, knowing that all physicians are communicating with the common goal of improving the patient’s disease course.
Communication with the referring physician is important not only after a procedure but also before it. Know that a consult is an “ask for help.” For example, even if you are not the correct specialist for a referral (for example, an inflammatory bowel disease patient was sent to an advanced endoscopist), it is good practice to take ownership of the patient and forward that person to the appropriate colleague.
Tip 4: Build a local reputation
Building upon this, it is also important to connect with other GI groups in the community, regardless of whether they have their own affiliated advanced endoscopists. This helps determine the advanced endoscopy services being offered regionally, which will further allow an understanding of the unmet needs of the region. In addition, building a relationship with local advanced endoscopists in the region can help establish a collaborative relationship going forward, rather than a contentious/competitive dynamic.
Tip 5: Advance your skills
As advanced endoscopy fellows are aware, completing an advanced endoscopy fellowship allows for building a strong foundation of skills, which will continue to refine and grow as you advance in your career.
Depending on your skill-set and training, the first year should focus on developing and establishing “your style” (since the training is tailored to follow the practice patterns of your mentors). The first few months are good to focus on refining endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, endoscopic mucosal resection, and luminal stenting techniques. As you start to build a reputation of being “safe, thoughtful, and skilled” and depending on your interests and goals, continued engagement in the advanced endoscopy community to understand new technologies/procedures is helpful. It is important to remember that new skills and procedures can be introduced in your practice, but this should be done in a timely and patient manner. You should appropriately educate and train yourself for such procedures through educational conferences/courses, shadowing and routine engagement with mentors, and collaboration with industry partners.
Tip 6: Team building
From a procedural standpoint, certain staff members should be recognized to be part of or lead an “advanced endoscopy team,” with a goal of dedicated exposure to a high volume of complex procedures. This builds camaraderie and trust within the team of advanced endoscopy nurses and technicians going forward, which is crucial to introducing and building a high-complexity procedural service. This is also an excellent opportunity to partner with our industry colleagues to ensure that they can train your team on the use of novel devices.
Tip 7: Offering new services to your patients
Advanced endoscopy is a rapidly evolving specialty, and new procedures, technology, and devices are allowing us to provide minimally invasive options to our patients. It is important that prior to introducing new services and programs, your hospital/practice administration should be informed about any such plans. Also, all potential collaborating services (surgery, interventional radiology, etc.) should be part of the decision-making to ensure patients receive the best possible multidisciplinary care.
Tip 8: Mentorship and peer-mentorship
Establishing a network of regional and national advanced endoscopy colleagues and mentors is critical. This may be harder to develop in community-based and private practice, where one may feel that they are on an “island.” Engagement with national organizations, use of social media, and other avenues are excellent ways to build this network. Advanced endoscopic procedures also are associated with higher rates of adverse events, so having a peer-support group to provide emotional and moral support when these adverse events occur also is important. Such a network also includes those collaborating specialties to which you would refer (surgical oncology, thoracic surgery, etc.). Being involved in local tumor boards and “gut clubs” is another way of remaining engaged and not feeling isolated.
Tip 9: Have fun
Advanced endoscopy can be busy, as well as physically and mentally exhausting. It is important to maintain a good work-life balance. In addition, planning scheduled retreats or social events with your advanced endoscopy team (nurses, technicians, schedulers, colleagues) is important not only to show appreciation, but also to help build camaraderie and develop relationships.
Tip 10: Remember your ‘why’
Often times, there can be stressors associated with building a practice and increasing your volume, therefore, it is always important to remember why you became a medical professional and advanced endoscopist. This will get you through the days where you had a complication or when things didn’t go as planned.
Conclusion
Lastly, it is important to keep revisiting your skill sets and practice and evaluate what is working well and what can be improved. To all the advanced endoscopists starting their careers: Be patient and have a positive attitude! The leaders in our field did not become so overnight, and an advanced endoscopy–based career resembles a marathon rather than a sprint. Mistakes during procedures and practice building can be made, but how you grow and learn from those mistakes is what determines how likely you are to succeed going forward. Respect and acknowledge your staff, your collaborating physicians, and mentors. It takes time and effort to develop an advanced endoscopy practice. Being proud of your achievements and promoting procedural and patient care advances that you have made are beneficial and encouraged. We are fortunate to be in an ever-evolving specialty, and it is an exciting time to be practicing advanced endoscopy. Good luck!
Dr. Soudagar is a gastroenterologist at Northwestern Medical Group, Lake Forest, Ill. Dr. Bilal, assistant professor of medicine at the University of Minnesota, Minneapolis, is an advanced endoscopist and gastroenterologist at Minneapolis VA Medical Center. The authors have no conflicts of interest.
Establishing an advanced endoscopy practice can appear challenging and overwhelming. It is often the culmination of more than a decade of education and training for advanced endoscopists and is usually their first foray into employment. all while creating a rewarding opportunity to provide a population with necessary services, which, more than likely, were not previously being offered at your institution or in your region.
Tip 1: Understand the current landscape
When joining a hospital-employed or private practice, it is important for the advanced endoscopist to gauge the current landscape of the job, beginning with gaining an understanding of the current services provided by your gastroenterology colleagues. This includes knowing the types of advanced endoscopy services previously provided, especially if you have partners or colleagues who perform these procedures, and their prior referral patterns, either within or outside their respective group. Also, it is important to understand the services that are provided locally at other institutions. This will allow you to develop a niche of the types of services you can provide that are not available in the current practice set-up.
Tip 2: Connect with peers, interspecialty collaborators, and referring physicians
It is important that you connect with your GI colleagues once you start a new job. This can differ in ease depending on the size of your group. For example, in a small group, it may be easier to familiarize yourself with your colleagues through regular interactions. If you are a part of a larger practice, however, it is necessary to be more proactive and set up introductory meetings/sessions. These interactions provide a great opportunity to share your goals and start building a relationship.
Efforts also should be made to reach out to primary care, hematology/oncology, surgical/radiation oncology, general surgery, and interventional radiology physicians, as these are the specialists with whom an advanced endoscopist typically has the most interaction. The relationship with these colleagues is bidirectional, as the majority of our patients need multidisciplinary decision-making and care. For example, the first time you speak to the colorectal surgeon at your institution should not be in the middle of a complication. The purpose of these introductions should not be solely to inform them of the services you are offering but to start developing a relationship in a true sense, because eventually those relationships will transform into excellent patient care.
Tip 3: Communication
Communication is a key principle in building a practice. Referring physicians often entrust you with managing a part of their patient’s medical problems. Patient/procedure outcomes should be relayed promptly to referring physicians, as this not only helps build the trust of the referring physician, but also enhances the patient’s trust in the health system, knowing that all physicians are communicating with the common goal of improving the patient’s disease course.
Communication with the referring physician is important not only after a procedure but also before it. Know that a consult is an “ask for help.” For example, even if you are not the correct specialist for a referral (for example, an inflammatory bowel disease patient was sent to an advanced endoscopist), it is good practice to take ownership of the patient and forward that person to the appropriate colleague.
Tip 4: Build a local reputation
Building upon this, it is also important to connect with other GI groups in the community, regardless of whether they have their own affiliated advanced endoscopists. This helps determine the advanced endoscopy services being offered regionally, which will further allow an understanding of the unmet needs of the region. In addition, building a relationship with local advanced endoscopists in the region can help establish a collaborative relationship going forward, rather than a contentious/competitive dynamic.
Tip 5: Advance your skills
As advanced endoscopy fellows are aware, completing an advanced endoscopy fellowship allows for building a strong foundation of skills, which will continue to refine and grow as you advance in your career.
Depending on your skill-set and training, the first year should focus on developing and establishing “your style” (since the training is tailored to follow the practice patterns of your mentors). The first few months are good to focus on refining endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, endoscopic mucosal resection, and luminal stenting techniques. As you start to build a reputation of being “safe, thoughtful, and skilled” and depending on your interests and goals, continued engagement in the advanced endoscopy community to understand new technologies/procedures is helpful. It is important to remember that new skills and procedures can be introduced in your practice, but this should be done in a timely and patient manner. You should appropriately educate and train yourself for such procedures through educational conferences/courses, shadowing and routine engagement with mentors, and collaboration with industry partners.
Tip 6: Team building
From a procedural standpoint, certain staff members should be recognized to be part of or lead an “advanced endoscopy team,” with a goal of dedicated exposure to a high volume of complex procedures. This builds camaraderie and trust within the team of advanced endoscopy nurses and technicians going forward, which is crucial to introducing and building a high-complexity procedural service. This is also an excellent opportunity to partner with our industry colleagues to ensure that they can train your team on the use of novel devices.
Tip 7: Offering new services to your patients
Advanced endoscopy is a rapidly evolving specialty, and new procedures, technology, and devices are allowing us to provide minimally invasive options to our patients. It is important that prior to introducing new services and programs, your hospital/practice administration should be informed about any such plans. Also, all potential collaborating services (surgery, interventional radiology, etc.) should be part of the decision-making to ensure patients receive the best possible multidisciplinary care.
Tip 8: Mentorship and peer-mentorship
Establishing a network of regional and national advanced endoscopy colleagues and mentors is critical. This may be harder to develop in community-based and private practice, where one may feel that they are on an “island.” Engagement with national organizations, use of social media, and other avenues are excellent ways to build this network. Advanced endoscopic procedures also are associated with higher rates of adverse events, so having a peer-support group to provide emotional and moral support when these adverse events occur also is important. Such a network also includes those collaborating specialties to which you would refer (surgical oncology, thoracic surgery, etc.). Being involved in local tumor boards and “gut clubs” is another way of remaining engaged and not feeling isolated.
Tip 9: Have fun
Advanced endoscopy can be busy, as well as physically and mentally exhausting. It is important to maintain a good work-life balance. In addition, planning scheduled retreats or social events with your advanced endoscopy team (nurses, technicians, schedulers, colleagues) is important not only to show appreciation, but also to help build camaraderie and develop relationships.
Tip 10: Remember your ‘why’
Often times, there can be stressors associated with building a practice and increasing your volume, therefore, it is always important to remember why you became a medical professional and advanced endoscopist. This will get you through the days where you had a complication or when things didn’t go as planned.
Conclusion
Lastly, it is important to keep revisiting your skill sets and practice and evaluate what is working well and what can be improved. To all the advanced endoscopists starting their careers: Be patient and have a positive attitude! The leaders in our field did not become so overnight, and an advanced endoscopy–based career resembles a marathon rather than a sprint. Mistakes during procedures and practice building can be made, but how you grow and learn from those mistakes is what determines how likely you are to succeed going forward. Respect and acknowledge your staff, your collaborating physicians, and mentors. It takes time and effort to develop an advanced endoscopy practice. Being proud of your achievements and promoting procedural and patient care advances that you have made are beneficial and encouraged. We are fortunate to be in an ever-evolving specialty, and it is an exciting time to be practicing advanced endoscopy. Good luck!
Dr. Soudagar is a gastroenterologist at Northwestern Medical Group, Lake Forest, Ill. Dr. Bilal, assistant professor of medicine at the University of Minnesota, Minneapolis, is an advanced endoscopist and gastroenterologist at Minneapolis VA Medical Center. The authors have no conflicts of interest.
Establishing an advanced endoscopy practice can appear challenging and overwhelming. It is often the culmination of more than a decade of education and training for advanced endoscopists and is usually their first foray into employment. all while creating a rewarding opportunity to provide a population with necessary services, which, more than likely, were not previously being offered at your institution or in your region.
Tip 1: Understand the current landscape
When joining a hospital-employed or private practice, it is important for the advanced endoscopist to gauge the current landscape of the job, beginning with gaining an understanding of the current services provided by your gastroenterology colleagues. This includes knowing the types of advanced endoscopy services previously provided, especially if you have partners or colleagues who perform these procedures, and their prior referral patterns, either within or outside their respective group. Also, it is important to understand the services that are provided locally at other institutions. This will allow you to develop a niche of the types of services you can provide that are not available in the current practice set-up.
Tip 2: Connect with peers, interspecialty collaborators, and referring physicians
It is important that you connect with your GI colleagues once you start a new job. This can differ in ease depending on the size of your group. For example, in a small group, it may be easier to familiarize yourself with your colleagues through regular interactions. If you are a part of a larger practice, however, it is necessary to be more proactive and set up introductory meetings/sessions. These interactions provide a great opportunity to share your goals and start building a relationship.
Efforts also should be made to reach out to primary care, hematology/oncology, surgical/radiation oncology, general surgery, and interventional radiology physicians, as these are the specialists with whom an advanced endoscopist typically has the most interaction. The relationship with these colleagues is bidirectional, as the majority of our patients need multidisciplinary decision-making and care. For example, the first time you speak to the colorectal surgeon at your institution should not be in the middle of a complication. The purpose of these introductions should not be solely to inform them of the services you are offering but to start developing a relationship in a true sense, because eventually those relationships will transform into excellent patient care.
Tip 3: Communication
Communication is a key principle in building a practice. Referring physicians often entrust you with managing a part of their patient’s medical problems. Patient/procedure outcomes should be relayed promptly to referring physicians, as this not only helps build the trust of the referring physician, but also enhances the patient’s trust in the health system, knowing that all physicians are communicating with the common goal of improving the patient’s disease course.
Communication with the referring physician is important not only after a procedure but also before it. Know that a consult is an “ask for help.” For example, even if you are not the correct specialist for a referral (for example, an inflammatory bowel disease patient was sent to an advanced endoscopist), it is good practice to take ownership of the patient and forward that person to the appropriate colleague.
Tip 4: Build a local reputation
Building upon this, it is also important to connect with other GI groups in the community, regardless of whether they have their own affiliated advanced endoscopists. This helps determine the advanced endoscopy services being offered regionally, which will further allow an understanding of the unmet needs of the region. In addition, building a relationship with local advanced endoscopists in the region can help establish a collaborative relationship going forward, rather than a contentious/competitive dynamic.
Tip 5: Advance your skills
As advanced endoscopy fellows are aware, completing an advanced endoscopy fellowship allows for building a strong foundation of skills, which will continue to refine and grow as you advance in your career.
Depending on your skill-set and training, the first year should focus on developing and establishing “your style” (since the training is tailored to follow the practice patterns of your mentors). The first few months are good to focus on refining endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, endoscopic mucosal resection, and luminal stenting techniques. As you start to build a reputation of being “safe, thoughtful, and skilled” and depending on your interests and goals, continued engagement in the advanced endoscopy community to understand new technologies/procedures is helpful. It is important to remember that new skills and procedures can be introduced in your practice, but this should be done in a timely and patient manner. You should appropriately educate and train yourself for such procedures through educational conferences/courses, shadowing and routine engagement with mentors, and collaboration with industry partners.
Tip 6: Team building
From a procedural standpoint, certain staff members should be recognized to be part of or lead an “advanced endoscopy team,” with a goal of dedicated exposure to a high volume of complex procedures. This builds camaraderie and trust within the team of advanced endoscopy nurses and technicians going forward, which is crucial to introducing and building a high-complexity procedural service. This is also an excellent opportunity to partner with our industry colleagues to ensure that they can train your team on the use of novel devices.
Tip 7: Offering new services to your patients
Advanced endoscopy is a rapidly evolving specialty, and new procedures, technology, and devices are allowing us to provide minimally invasive options to our patients. It is important that prior to introducing new services and programs, your hospital/practice administration should be informed about any such plans. Also, all potential collaborating services (surgery, interventional radiology, etc.) should be part of the decision-making to ensure patients receive the best possible multidisciplinary care.
Tip 8: Mentorship and peer-mentorship
Establishing a network of regional and national advanced endoscopy colleagues and mentors is critical. This may be harder to develop in community-based and private practice, where one may feel that they are on an “island.” Engagement with national organizations, use of social media, and other avenues are excellent ways to build this network. Advanced endoscopic procedures also are associated with higher rates of adverse events, so having a peer-support group to provide emotional and moral support when these adverse events occur also is important. Such a network also includes those collaborating specialties to which you would refer (surgical oncology, thoracic surgery, etc.). Being involved in local tumor boards and “gut clubs” is another way of remaining engaged and not feeling isolated.
Tip 9: Have fun
Advanced endoscopy can be busy, as well as physically and mentally exhausting. It is important to maintain a good work-life balance. In addition, planning scheduled retreats or social events with your advanced endoscopy team (nurses, technicians, schedulers, colleagues) is important not only to show appreciation, but also to help build camaraderie and develop relationships.
Tip 10: Remember your ‘why’
Often times, there can be stressors associated with building a practice and increasing your volume, therefore, it is always important to remember why you became a medical professional and advanced endoscopist. This will get you through the days where you had a complication or when things didn’t go as planned.
Conclusion
Lastly, it is important to keep revisiting your skill sets and practice and evaluate what is working well and what can be improved. To all the advanced endoscopists starting their careers: Be patient and have a positive attitude! The leaders in our field did not become so overnight, and an advanced endoscopy–based career resembles a marathon rather than a sprint. Mistakes during procedures and practice building can be made, but how you grow and learn from those mistakes is what determines how likely you are to succeed going forward. Respect and acknowledge your staff, your collaborating physicians, and mentors. It takes time and effort to develop an advanced endoscopy practice. Being proud of your achievements and promoting procedural and patient care advances that you have made are beneficial and encouraged. We are fortunate to be in an ever-evolving specialty, and it is an exciting time to be practicing advanced endoscopy. Good luck!
Dr. Soudagar is a gastroenterologist at Northwestern Medical Group, Lake Forest, Ill. Dr. Bilal, assistant professor of medicine at the University of Minnesota, Minneapolis, is an advanced endoscopist and gastroenterologist at Minneapolis VA Medical Center. The authors have no conflicts of interest.
















