User login
Approach to dysphagia
Introduction
Dysphagia is the sensation of difficulty swallowing food or liquid in the acute or chronic setting. The prevalence of dysphagia ranges based on the type and etiology but may impact up to one in six adults.1,2 Dysphagia can cause a significant impact on a patient’s health and overall quality of life. A recent study found that only 50% of symptomatic adults seek medical care despite modifying their eating habits by either eating slowly or changing to softer foods or liquids.1 The most common, serious complications of dysphagia include aspiration pneumonia, malnutrition, and dehydration.3 According to the Agency for Healthcare Research and Quality, dysphagia may be responsible for up to 60,000 deaths annually.3
The diagnosis of esophageal dysphagia can be challenging. An initial, thorough history is essential to delineate between oropharyngeal and esophageal dysphagia and guide subsequent diagnostic testing. In recent years, there have been a number of advances in the approach to diagnosing dysphagia, including novel diagnostic modalities. The goal of this review article is to discuss the current approach to esophageal dysphagia and future direction to allow for timely diagnosis and management.
History
The diagnosis of dysphagia begins with a thorough history. Questions about the timing, onset, progression, localization of symptoms, and types of food that are difficult to swallow are essential in differentiating oropharyngeal and esophageal dysphagia.3,4 Further history taking must include medication and allergy review, smoking history, and review of prior radiation or surgical therapies to the head and neck.
Briefly, oropharyngeal dysphagia is difficulty initiating a swallow or passing food from the mouth or throat and can be caused by structural or functional etiologies.5 Clinical presentations include a sensation of food stuck in the back of the throat, coughing or choking while eating, or drooling. Structural causes include head and neck cancer, Zenker diverticulum, Killian Jamieson diverticula, prolonged intubation, or changes secondary to prior surgery or radiation.3 Functional causes may include neurologic, rheumatologic, or muscular disorders.6
Esophageal dysphagia refers to difficulty transporting food or liquid down the esophagus and can be caused by structural, inflammatory, or functional disorders.5 Patients typically localize symptoms of heartburn, regurgitation, nausea, vomiting, cough, or chest pain along the sternum or epigastric region. Alarm signs concerning for malignancy include unintentional weight loss, fevers, or night sweats.3,7 Aside from symptoms, medication review is essential, as dysphagia is a common side effect of antipsychotics, anticholinergics, antimuscarinics, narcotics, and immunosuppressant drugs.8 Larger pills such as NSAIDs, antibiotics, bisphosphonates, potassium supplements, and methylxanthines can cause drug-induced esophagitis, which can initially present as dysphagia.8 Inflammatory causes can be elucidated by obtaining a history about allergies, tobacco use, and recent infections such as thrush or pneumonia. Patients with a history of recurrent pneumonias may be silently aspirating, a complication of dysphagia.3 Once esophageal dysphagia is clinically suspected based on history, workup can begin.
Differentiating etiologies of esophageal dysphagia
The next step in diagnosing esophageal dysphagia is differentiating between structural, inflammatory, or dysmotility etiology (Figure 1).
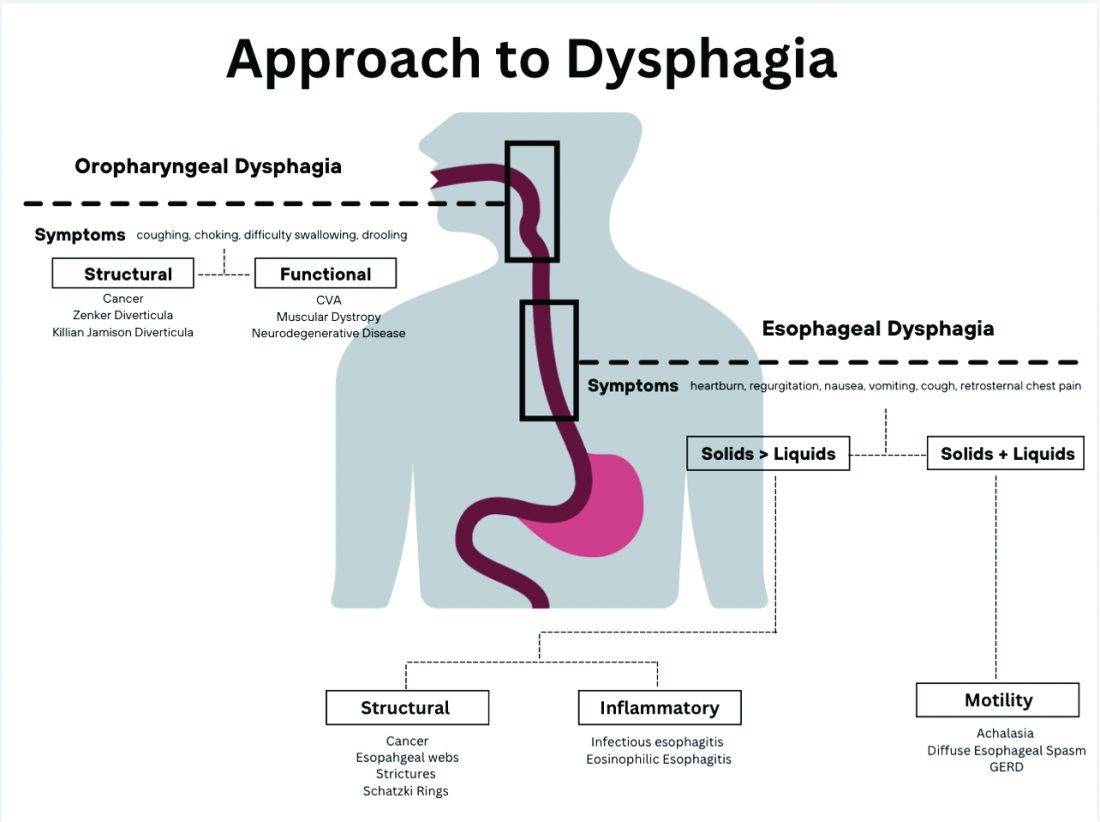
Patients with a structural cause typically have difficulty swallowing solids but are able to swallow liquids unless the disease progresses. Symptoms can rapidly worsen and lead to odynophagia, weight loss, and vomiting. In comparison, patients with motility disorders typically have difficulty swallowing both solids and liquids initially, and symptoms can be constant or intermittent.5
Prior to diagnostic studies, a 4-week trial of a proton pump inhibitor (PPI) is appropriate for patients with reflux symptoms who are younger than 50 with no alarm features concerning for malignancy.7,9 If symptoms persist after a PPI trial, then an upper endoscopy (EGD) is indicated. An EGD allows for visualization of structural etiologies, obtaining biopsies to rule out inflammatory etiologies, and the option to therapeutically treat reduced luminal diameter with dilatation.10 The most common structural and inflammatory etiologies noted on EGD include strictures, webs, carcinomas, Schatzki rings, and gastroesophageal reflux or eosinophilic esophagitis.4
If upper endoscopy is normal and clinical suspicion for an obstructive cause remains high, barium esophagram can be utilized as an adjunctive study. Previously, barium esophagram was the initial test to distinguish between structural and motility disorders. The benefits of endoscopy over barium esophagram as the first diagnostic study include higher diagnostic yield, higher sensitivity and specificity, and lower costs.7 However, barium studies may be more sensitive for lower esophageal rings or extrinsic esophageal compression.3
Evaluation of esophageal motility disorder
If a structural or inflammatory etiology of dysphagia is not identified, investigation for an esophageal motility disorder (EMD) is warranted. Examples of motility disorders include achalasia, ineffective esophageal motility, hypercontractility, spasticity, or esophagogastric junction outflow obstruction (EGJOO).10,11 High-resolution esophageal manometry (HRM) remains the gold standard in diagnosis of EMD.12 An HRM catheter utilizes 36 sensors placed two centimeters apart and is placed in the esophagus to evaluate pressure and peristalsis between the upper and lower esophageal sphincters.13 In 2009, the Chicago Classification System was developed to provide a diagnostic algorithm that categorizes EMD based on HRM testing, with the most recent version (4.0) being published in 2020.12,14 Motility diagnoses are divided into two general classifications of disorders of body peristalsis and disorders of EGJ outflow. The most recent updates also include changes in swallow protocols, patient positioning, targeted symptoms, addition of impedance sensors, and consideration of supplemental testing when HRM is inconclusive based on the clinical context.12 There are some limitations of HRM to highlight. One of the main diagnostic values used with HRM is the integrated relaxation pressure (IRP). Despite standardization, IRP measurements vary based on the recorder and patient position. A minority of patients with achalasia may have IRP that does not approach the accepted cutoff and, therefore, the EGJ is not accurately assessed on HRM.15,16 In addition, some swallow protocols have lower sensitivity and specificity for certain motility disorders, and the test can result as inconclusive.14 In these scenarios, supplemental testing with timed barium esophagram or functional luminal imaging probe (EndoFLIP) is indicated.10,11
Over the past decade, EndoFLIP has emerged as a novel diagnostic tool in evaluating EMD. EndoFLIP is usually completed during an upper endoscopy and utilizes impedance planimetry to measure cross-sectional area and esophageal distensibility and evaluate contractile patterns.16 During the procedure, a small catheter with an inflatable balloon is inserted into the esophagus with the distal end in the stomach, traversing the esophagogastric junction (EGJ). The pressure transducer has electrodes every centimeter to allow for a three-dimensional construction of the esophagus and EGJ.17 EndoFLIP has been shown to accurately measure pyloric diameter, pressure, and distensibility at certain balloon volumes.18 In addition, FLIP is being used to further identify aspects of esophageal dysmotility in patients with eosinophilic esophagitis, thought primarily to be an inflammatory disorder.19 However, limitations include minimal accessibility of EndoFLIP within clinical practice and a specific computer program needed to generate the topographic plots.20
When used in conjunction with HRM, EndoFLIP provides complementary data that can be used to better detect major motility disorders.15,20,21 Each study adds unique information about the different physiologic events comprising the esophageal response to distention. Overall, the benefits of EndoFLIP include expediting workup during index endoscopy, patient comfort with sedation, and real-time diagnostic data that supplement results obtained during HRM.10,16,20,2223
Of note, if the diagnostic evaluation for structural, inflammatory, and motility disorders are unrevealing, investigating for atypical reflux symptoms can be pursued for patients with persistent dysphagia. Studies investigating pH, or acidity in the esophagus, in relation to symptoms, can be conducted wirelessly via a capsule fixed to the mucosa or with a nasal catheter.3
Normal workup – hypervigilance
In a subset of patients, all diagnostic testing for structural, inflammatory, or motility disorders is normal. These patients are classified as having a functional esophageal disorder. Despite normal testing, patients still have significant symptoms including epigastric pain, chest pain, globus sensation, or difficulty swallowing. It is theorized that a degree of visceral hypersensitivity between the brain-gut axis contributes to ongoing symptoms.24 Studies for effective treatments are ongoing but typically include cognitive-behavioral therapy, brain-gut behavioral therapy, swallow therapy antidepressants, or short courses of proton pump inhibitors.9
Conclusion
In this review article, we discussed the diagnostic approach for esophageal dysphagia. Initial assessment requires a thorough history, differentiation between oropharyngeal and esophageal dysphagia, and determination of who warrants an upper endoscopy. Upper endoscopy may reveal structural or inflammatory causes of dysphagia, including strictures, masses, or esophagitis, to name a few. If a structural or inflammatory cause is ruled out, this warrants investigation for esophageal motility disorders. The current gold standard for diagnosing EMD is manometry, and supplemental studies, including EndoFLIP, barium esophagram, and pH studies, may provide complimentary data. If workup for dysphagia is normal, evaluation for esophageal hypervigilance causing increased sensitivity to normal or mild sensations may be warranted. In conclusion, the diagnosis of dysphagia is challenging and requires investigation with a systematic approach to ensure timely diagnosis and treatment
Dr. Ronnie and Dr. Bloomberg are in the department of internal medicine at Loyola University Chicago, Maywood, Ill. Dr. Venu is in the division of gastroenterology at Loyola. He is on the speakers bureau at Medtronic.
References
1. Adkins C et al. Clin Gastroenterol Hepatol. 2020;18(9):1970-9.e2.
2. Bhattacharyya N. Otolaryngol Head Neck Surg. 2014;151(5):765-9.
3. McCarty EB and Chao TN. Med Clin North Am. 2021;105(5):939-54.
4. Thiyagalingam S et al. Mayo Clin Proc. 2021;96(2):488-97.
5. Malagelada JR et al. J Clin Gastroenterol. 2015;49(5):370-8.
6. Rommel, N and Hamdy S. Nat Rev Gastroenterol Hepatol. 2016;13(1):49-59.
7. Liu LWC et al. J Can Assoc Gastroenterol. 2018;1(1):5-19.
8. Schwemmle C et al. HNO. 2015;63(7):504-10.
9. Moayyedi P et al. Am J Gastroenterol. 2017;112(7):988-1013.
10. Triggs J and Pandolfino J. F1000Res. 2019 Aug 29. doi: 10.12688/f1000research.18900.1.
11. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14058.
12. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14053.
13. Fox M et al. Neurogastroenterol Motil. 2004;16(5):533-42.
14. Sweis R and Fox M. Curr Gastroenterol Rep. 2020;22(10):49.
15. Carlson DA et al. Gastroenterology. 2015;149(7):1742-51.
16. Donnan EN and Pandolfino JE. Gastroenterol Clin North Am. 2020;49(3):427-35.
17. Carlson DA. Curr Opin Gastroenterol. 2016;32(4):310-8.
18. Zheng T et al. Neurogastroenterol Motil. 2022;34(10):e14386.
19. Carlson DA et al. Clin Gastroenterol Hepatol. 2022;20(8):1719-28.e3.
20. Carlson DA et al. Am J Gastroenterol. 2016;111(12):1726-35.
21. Carlson DA et al. Neurogastroenterol Motil. 2021;33(10):e14116.
22. Carlson DA et al. Gastrointest Endosc. 2019;90(6):915-923.e1.
23. Fox MR et al. Neurogastroenterol Motil. 2021;33(4):e14120.
24. Aziz Q et al. Gastroenterology. 2016 Feb 15. doi: 10.1053/j.gastro.2016.02.012.
Introduction
Dysphagia is the sensation of difficulty swallowing food or liquid in the acute or chronic setting. The prevalence of dysphagia ranges based on the type and etiology but may impact up to one in six adults.1,2 Dysphagia can cause a significant impact on a patient’s health and overall quality of life. A recent study found that only 50% of symptomatic adults seek medical care despite modifying their eating habits by either eating slowly or changing to softer foods or liquids.1 The most common, serious complications of dysphagia include aspiration pneumonia, malnutrition, and dehydration.3 According to the Agency for Healthcare Research and Quality, dysphagia may be responsible for up to 60,000 deaths annually.3
The diagnosis of esophageal dysphagia can be challenging. An initial, thorough history is essential to delineate between oropharyngeal and esophageal dysphagia and guide subsequent diagnostic testing. In recent years, there have been a number of advances in the approach to diagnosing dysphagia, including novel diagnostic modalities. The goal of this review article is to discuss the current approach to esophageal dysphagia and future direction to allow for timely diagnosis and management.
History
The diagnosis of dysphagia begins with a thorough history. Questions about the timing, onset, progression, localization of symptoms, and types of food that are difficult to swallow are essential in differentiating oropharyngeal and esophageal dysphagia.3,4 Further history taking must include medication and allergy review, smoking history, and review of prior radiation or surgical therapies to the head and neck.
Briefly, oropharyngeal dysphagia is difficulty initiating a swallow or passing food from the mouth or throat and can be caused by structural or functional etiologies.5 Clinical presentations include a sensation of food stuck in the back of the throat, coughing or choking while eating, or drooling. Structural causes include head and neck cancer, Zenker diverticulum, Killian Jamieson diverticula, prolonged intubation, or changes secondary to prior surgery or radiation.3 Functional causes may include neurologic, rheumatologic, or muscular disorders.6
Esophageal dysphagia refers to difficulty transporting food or liquid down the esophagus and can be caused by structural, inflammatory, or functional disorders.5 Patients typically localize symptoms of heartburn, regurgitation, nausea, vomiting, cough, or chest pain along the sternum or epigastric region. Alarm signs concerning for malignancy include unintentional weight loss, fevers, or night sweats.3,7 Aside from symptoms, medication review is essential, as dysphagia is a common side effect of antipsychotics, anticholinergics, antimuscarinics, narcotics, and immunosuppressant drugs.8 Larger pills such as NSAIDs, antibiotics, bisphosphonates, potassium supplements, and methylxanthines can cause drug-induced esophagitis, which can initially present as dysphagia.8 Inflammatory causes can be elucidated by obtaining a history about allergies, tobacco use, and recent infections such as thrush or pneumonia. Patients with a history of recurrent pneumonias may be silently aspirating, a complication of dysphagia.3 Once esophageal dysphagia is clinically suspected based on history, workup can begin.
Differentiating etiologies of esophageal dysphagia
The next step in diagnosing esophageal dysphagia is differentiating between structural, inflammatory, or dysmotility etiology (Figure 1).

Patients with a structural cause typically have difficulty swallowing solids but are able to swallow liquids unless the disease progresses. Symptoms can rapidly worsen and lead to odynophagia, weight loss, and vomiting. In comparison, patients with motility disorders typically have difficulty swallowing both solids and liquids initially, and symptoms can be constant or intermittent.5
Prior to diagnostic studies, a 4-week trial of a proton pump inhibitor (PPI) is appropriate for patients with reflux symptoms who are younger than 50 with no alarm features concerning for malignancy.7,9 If symptoms persist after a PPI trial, then an upper endoscopy (EGD) is indicated. An EGD allows for visualization of structural etiologies, obtaining biopsies to rule out inflammatory etiologies, and the option to therapeutically treat reduced luminal diameter with dilatation.10 The most common structural and inflammatory etiologies noted on EGD include strictures, webs, carcinomas, Schatzki rings, and gastroesophageal reflux or eosinophilic esophagitis.4
If upper endoscopy is normal and clinical suspicion for an obstructive cause remains high, barium esophagram can be utilized as an adjunctive study. Previously, barium esophagram was the initial test to distinguish between structural and motility disorders. The benefits of endoscopy over barium esophagram as the first diagnostic study include higher diagnostic yield, higher sensitivity and specificity, and lower costs.7 However, barium studies may be more sensitive for lower esophageal rings or extrinsic esophageal compression.3
Evaluation of esophageal motility disorder
If a structural or inflammatory etiology of dysphagia is not identified, investigation for an esophageal motility disorder (EMD) is warranted. Examples of motility disorders include achalasia, ineffective esophageal motility, hypercontractility, spasticity, or esophagogastric junction outflow obstruction (EGJOO).10,11 High-resolution esophageal manometry (HRM) remains the gold standard in diagnosis of EMD.12 An HRM catheter utilizes 36 sensors placed two centimeters apart and is placed in the esophagus to evaluate pressure and peristalsis between the upper and lower esophageal sphincters.13 In 2009, the Chicago Classification System was developed to provide a diagnostic algorithm that categorizes EMD based on HRM testing, with the most recent version (4.0) being published in 2020.12,14 Motility diagnoses are divided into two general classifications of disorders of body peristalsis and disorders of EGJ outflow. The most recent updates also include changes in swallow protocols, patient positioning, targeted symptoms, addition of impedance sensors, and consideration of supplemental testing when HRM is inconclusive based on the clinical context.12 There are some limitations of HRM to highlight. One of the main diagnostic values used with HRM is the integrated relaxation pressure (IRP). Despite standardization, IRP measurements vary based on the recorder and patient position. A minority of patients with achalasia may have IRP that does not approach the accepted cutoff and, therefore, the EGJ is not accurately assessed on HRM.15,16 In addition, some swallow protocols have lower sensitivity and specificity for certain motility disorders, and the test can result as inconclusive.14 In these scenarios, supplemental testing with timed barium esophagram or functional luminal imaging probe (EndoFLIP) is indicated.10,11
Over the past decade, EndoFLIP has emerged as a novel diagnostic tool in evaluating EMD. EndoFLIP is usually completed during an upper endoscopy and utilizes impedance planimetry to measure cross-sectional area and esophageal distensibility and evaluate contractile patterns.16 During the procedure, a small catheter with an inflatable balloon is inserted into the esophagus with the distal end in the stomach, traversing the esophagogastric junction (EGJ). The pressure transducer has electrodes every centimeter to allow for a three-dimensional construction of the esophagus and EGJ.17 EndoFLIP has been shown to accurately measure pyloric diameter, pressure, and distensibility at certain balloon volumes.18 In addition, FLIP is being used to further identify aspects of esophageal dysmotility in patients with eosinophilic esophagitis, thought primarily to be an inflammatory disorder.19 However, limitations include minimal accessibility of EndoFLIP within clinical practice and a specific computer program needed to generate the topographic plots.20
When used in conjunction with HRM, EndoFLIP provides complementary data that can be used to better detect major motility disorders.15,20,21 Each study adds unique information about the different physiologic events comprising the esophageal response to distention. Overall, the benefits of EndoFLIP include expediting workup during index endoscopy, patient comfort with sedation, and real-time diagnostic data that supplement results obtained during HRM.10,16,20,2223
Of note, if the diagnostic evaluation for structural, inflammatory, and motility disorders are unrevealing, investigating for atypical reflux symptoms can be pursued for patients with persistent dysphagia. Studies investigating pH, or acidity in the esophagus, in relation to symptoms, can be conducted wirelessly via a capsule fixed to the mucosa or with a nasal catheter.3
Normal workup – hypervigilance
In a subset of patients, all diagnostic testing for structural, inflammatory, or motility disorders is normal. These patients are classified as having a functional esophageal disorder. Despite normal testing, patients still have significant symptoms including epigastric pain, chest pain, globus sensation, or difficulty swallowing. It is theorized that a degree of visceral hypersensitivity between the brain-gut axis contributes to ongoing symptoms.24 Studies for effective treatments are ongoing but typically include cognitive-behavioral therapy, brain-gut behavioral therapy, swallow therapy antidepressants, or short courses of proton pump inhibitors.9
Conclusion
In this review article, we discussed the diagnostic approach for esophageal dysphagia. Initial assessment requires a thorough history, differentiation between oropharyngeal and esophageal dysphagia, and determination of who warrants an upper endoscopy. Upper endoscopy may reveal structural or inflammatory causes of dysphagia, including strictures, masses, or esophagitis, to name a few. If a structural or inflammatory cause is ruled out, this warrants investigation for esophageal motility disorders. The current gold standard for diagnosing EMD is manometry, and supplemental studies, including EndoFLIP, barium esophagram, and pH studies, may provide complimentary data. If workup for dysphagia is normal, evaluation for esophageal hypervigilance causing increased sensitivity to normal or mild sensations may be warranted. In conclusion, the diagnosis of dysphagia is challenging and requires investigation with a systematic approach to ensure timely diagnosis and treatment
Dr. Ronnie and Dr. Bloomberg are in the department of internal medicine at Loyola University Chicago, Maywood, Ill. Dr. Venu is in the division of gastroenterology at Loyola. He is on the speakers bureau at Medtronic.
References
1. Adkins C et al. Clin Gastroenterol Hepatol. 2020;18(9):1970-9.e2.
2. Bhattacharyya N. Otolaryngol Head Neck Surg. 2014;151(5):765-9.
3. McCarty EB and Chao TN. Med Clin North Am. 2021;105(5):939-54.
4. Thiyagalingam S et al. Mayo Clin Proc. 2021;96(2):488-97.
5. Malagelada JR et al. J Clin Gastroenterol. 2015;49(5):370-8.
6. Rommel, N and Hamdy S. Nat Rev Gastroenterol Hepatol. 2016;13(1):49-59.
7. Liu LWC et al. J Can Assoc Gastroenterol. 2018;1(1):5-19.
8. Schwemmle C et al. HNO. 2015;63(7):504-10.
9. Moayyedi P et al. Am J Gastroenterol. 2017;112(7):988-1013.
10. Triggs J and Pandolfino J. F1000Res. 2019 Aug 29. doi: 10.12688/f1000research.18900.1.
11. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14058.
12. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14053.
13. Fox M et al. Neurogastroenterol Motil. 2004;16(5):533-42.
14. Sweis R and Fox M. Curr Gastroenterol Rep. 2020;22(10):49.
15. Carlson DA et al. Gastroenterology. 2015;149(7):1742-51.
16. Donnan EN and Pandolfino JE. Gastroenterol Clin North Am. 2020;49(3):427-35.
17. Carlson DA. Curr Opin Gastroenterol. 2016;32(4):310-8.
18. Zheng T et al. Neurogastroenterol Motil. 2022;34(10):e14386.
19. Carlson DA et al. Clin Gastroenterol Hepatol. 2022;20(8):1719-28.e3.
20. Carlson DA et al. Am J Gastroenterol. 2016;111(12):1726-35.
21. Carlson DA et al. Neurogastroenterol Motil. 2021;33(10):e14116.
22. Carlson DA et al. Gastrointest Endosc. 2019;90(6):915-923.e1.
23. Fox MR et al. Neurogastroenterol Motil. 2021;33(4):e14120.
24. Aziz Q et al. Gastroenterology. 2016 Feb 15. doi: 10.1053/j.gastro.2016.02.012.
Introduction
Dysphagia is the sensation of difficulty swallowing food or liquid in the acute or chronic setting. The prevalence of dysphagia ranges based on the type and etiology but may impact up to one in six adults.1,2 Dysphagia can cause a significant impact on a patient’s health and overall quality of life. A recent study found that only 50% of symptomatic adults seek medical care despite modifying their eating habits by either eating slowly or changing to softer foods or liquids.1 The most common, serious complications of dysphagia include aspiration pneumonia, malnutrition, and dehydration.3 According to the Agency for Healthcare Research and Quality, dysphagia may be responsible for up to 60,000 deaths annually.3
The diagnosis of esophageal dysphagia can be challenging. An initial, thorough history is essential to delineate between oropharyngeal and esophageal dysphagia and guide subsequent diagnostic testing. In recent years, there have been a number of advances in the approach to diagnosing dysphagia, including novel diagnostic modalities. The goal of this review article is to discuss the current approach to esophageal dysphagia and future direction to allow for timely diagnosis and management.
History
The diagnosis of dysphagia begins with a thorough history. Questions about the timing, onset, progression, localization of symptoms, and types of food that are difficult to swallow are essential in differentiating oropharyngeal and esophageal dysphagia.3,4 Further history taking must include medication and allergy review, smoking history, and review of prior radiation or surgical therapies to the head and neck.
Briefly, oropharyngeal dysphagia is difficulty initiating a swallow or passing food from the mouth or throat and can be caused by structural or functional etiologies.5 Clinical presentations include a sensation of food stuck in the back of the throat, coughing or choking while eating, or drooling. Structural causes include head and neck cancer, Zenker diverticulum, Killian Jamieson diverticula, prolonged intubation, or changes secondary to prior surgery or radiation.3 Functional causes may include neurologic, rheumatologic, or muscular disorders.6
Esophageal dysphagia refers to difficulty transporting food or liquid down the esophagus and can be caused by structural, inflammatory, or functional disorders.5 Patients typically localize symptoms of heartburn, regurgitation, nausea, vomiting, cough, or chest pain along the sternum or epigastric region. Alarm signs concerning for malignancy include unintentional weight loss, fevers, or night sweats.3,7 Aside from symptoms, medication review is essential, as dysphagia is a common side effect of antipsychotics, anticholinergics, antimuscarinics, narcotics, and immunosuppressant drugs.8 Larger pills such as NSAIDs, antibiotics, bisphosphonates, potassium supplements, and methylxanthines can cause drug-induced esophagitis, which can initially present as dysphagia.8 Inflammatory causes can be elucidated by obtaining a history about allergies, tobacco use, and recent infections such as thrush or pneumonia. Patients with a history of recurrent pneumonias may be silently aspirating, a complication of dysphagia.3 Once esophageal dysphagia is clinically suspected based on history, workup can begin.
Differentiating etiologies of esophageal dysphagia
The next step in diagnosing esophageal dysphagia is differentiating between structural, inflammatory, or dysmotility etiology (Figure 1).

Patients with a structural cause typically have difficulty swallowing solids but are able to swallow liquids unless the disease progresses. Symptoms can rapidly worsen and lead to odynophagia, weight loss, and vomiting. In comparison, patients with motility disorders typically have difficulty swallowing both solids and liquids initially, and symptoms can be constant or intermittent.5
Prior to diagnostic studies, a 4-week trial of a proton pump inhibitor (PPI) is appropriate for patients with reflux symptoms who are younger than 50 with no alarm features concerning for malignancy.7,9 If symptoms persist after a PPI trial, then an upper endoscopy (EGD) is indicated. An EGD allows for visualization of structural etiologies, obtaining biopsies to rule out inflammatory etiologies, and the option to therapeutically treat reduced luminal diameter with dilatation.10 The most common structural and inflammatory etiologies noted on EGD include strictures, webs, carcinomas, Schatzki rings, and gastroesophageal reflux or eosinophilic esophagitis.4
If upper endoscopy is normal and clinical suspicion for an obstructive cause remains high, barium esophagram can be utilized as an adjunctive study. Previously, barium esophagram was the initial test to distinguish between structural and motility disorders. The benefits of endoscopy over barium esophagram as the first diagnostic study include higher diagnostic yield, higher sensitivity and specificity, and lower costs.7 However, barium studies may be more sensitive for lower esophageal rings or extrinsic esophageal compression.3
Evaluation of esophageal motility disorder
If a structural or inflammatory etiology of dysphagia is not identified, investigation for an esophageal motility disorder (EMD) is warranted. Examples of motility disorders include achalasia, ineffective esophageal motility, hypercontractility, spasticity, or esophagogastric junction outflow obstruction (EGJOO).10,11 High-resolution esophageal manometry (HRM) remains the gold standard in diagnosis of EMD.12 An HRM catheter utilizes 36 sensors placed two centimeters apart and is placed in the esophagus to evaluate pressure and peristalsis between the upper and lower esophageal sphincters.13 In 2009, the Chicago Classification System was developed to provide a diagnostic algorithm that categorizes EMD based on HRM testing, with the most recent version (4.0) being published in 2020.12,14 Motility diagnoses are divided into two general classifications of disorders of body peristalsis and disorders of EGJ outflow. The most recent updates also include changes in swallow protocols, patient positioning, targeted symptoms, addition of impedance sensors, and consideration of supplemental testing when HRM is inconclusive based on the clinical context.12 There are some limitations of HRM to highlight. One of the main diagnostic values used with HRM is the integrated relaxation pressure (IRP). Despite standardization, IRP measurements vary based on the recorder and patient position. A minority of patients with achalasia may have IRP that does not approach the accepted cutoff and, therefore, the EGJ is not accurately assessed on HRM.15,16 In addition, some swallow protocols have lower sensitivity and specificity for certain motility disorders, and the test can result as inconclusive.14 In these scenarios, supplemental testing with timed barium esophagram or functional luminal imaging probe (EndoFLIP) is indicated.10,11
Over the past decade, EndoFLIP has emerged as a novel diagnostic tool in evaluating EMD. EndoFLIP is usually completed during an upper endoscopy and utilizes impedance planimetry to measure cross-sectional area and esophageal distensibility and evaluate contractile patterns.16 During the procedure, a small catheter with an inflatable balloon is inserted into the esophagus with the distal end in the stomach, traversing the esophagogastric junction (EGJ). The pressure transducer has electrodes every centimeter to allow for a three-dimensional construction of the esophagus and EGJ.17 EndoFLIP has been shown to accurately measure pyloric diameter, pressure, and distensibility at certain balloon volumes.18 In addition, FLIP is being used to further identify aspects of esophageal dysmotility in patients with eosinophilic esophagitis, thought primarily to be an inflammatory disorder.19 However, limitations include minimal accessibility of EndoFLIP within clinical practice and a specific computer program needed to generate the topographic plots.20
When used in conjunction with HRM, EndoFLIP provides complementary data that can be used to better detect major motility disorders.15,20,21 Each study adds unique information about the different physiologic events comprising the esophageal response to distention. Overall, the benefits of EndoFLIP include expediting workup during index endoscopy, patient comfort with sedation, and real-time diagnostic data that supplement results obtained during HRM.10,16,20,2223
Of note, if the diagnostic evaluation for structural, inflammatory, and motility disorders are unrevealing, investigating for atypical reflux symptoms can be pursued for patients with persistent dysphagia. Studies investigating pH, or acidity in the esophagus, in relation to symptoms, can be conducted wirelessly via a capsule fixed to the mucosa or with a nasal catheter.3
Normal workup – hypervigilance
In a subset of patients, all diagnostic testing for structural, inflammatory, or motility disorders is normal. These patients are classified as having a functional esophageal disorder. Despite normal testing, patients still have significant symptoms including epigastric pain, chest pain, globus sensation, or difficulty swallowing. It is theorized that a degree of visceral hypersensitivity between the brain-gut axis contributes to ongoing symptoms.24 Studies for effective treatments are ongoing but typically include cognitive-behavioral therapy, brain-gut behavioral therapy, swallow therapy antidepressants, or short courses of proton pump inhibitors.9
Conclusion
In this review article, we discussed the diagnostic approach for esophageal dysphagia. Initial assessment requires a thorough history, differentiation between oropharyngeal and esophageal dysphagia, and determination of who warrants an upper endoscopy. Upper endoscopy may reveal structural or inflammatory causes of dysphagia, including strictures, masses, or esophagitis, to name a few. If a structural or inflammatory cause is ruled out, this warrants investigation for esophageal motility disorders. The current gold standard for diagnosing EMD is manometry, and supplemental studies, including EndoFLIP, barium esophagram, and pH studies, may provide complimentary data. If workup for dysphagia is normal, evaluation for esophageal hypervigilance causing increased sensitivity to normal or mild sensations may be warranted. In conclusion, the diagnosis of dysphagia is challenging and requires investigation with a systematic approach to ensure timely diagnosis and treatment
Dr. Ronnie and Dr. Bloomberg are in the department of internal medicine at Loyola University Chicago, Maywood, Ill. Dr. Venu is in the division of gastroenterology at Loyola. He is on the speakers bureau at Medtronic.
References
1. Adkins C et al. Clin Gastroenterol Hepatol. 2020;18(9):1970-9.e2.
2. Bhattacharyya N. Otolaryngol Head Neck Surg. 2014;151(5):765-9.
3. McCarty EB and Chao TN. Med Clin North Am. 2021;105(5):939-54.
4. Thiyagalingam S et al. Mayo Clin Proc. 2021;96(2):488-97.
5. Malagelada JR et al. J Clin Gastroenterol. 2015;49(5):370-8.
6. Rommel, N and Hamdy S. Nat Rev Gastroenterol Hepatol. 2016;13(1):49-59.
7. Liu LWC et al. J Can Assoc Gastroenterol. 2018;1(1):5-19.
8. Schwemmle C et al. HNO. 2015;63(7):504-10.
9. Moayyedi P et al. Am J Gastroenterol. 2017;112(7):988-1013.
10. Triggs J and Pandolfino J. F1000Res. 2019 Aug 29. doi: 10.12688/f1000research.18900.1.
11. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14058.
12. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14053.
13. Fox M et al. Neurogastroenterol Motil. 2004;16(5):533-42.
14. Sweis R and Fox M. Curr Gastroenterol Rep. 2020;22(10):49.
15. Carlson DA et al. Gastroenterology. 2015;149(7):1742-51.
16. Donnan EN and Pandolfino JE. Gastroenterol Clin North Am. 2020;49(3):427-35.
17. Carlson DA. Curr Opin Gastroenterol. 2016;32(4):310-8.
18. Zheng T et al. Neurogastroenterol Motil. 2022;34(10):e14386.
19. Carlson DA et al. Clin Gastroenterol Hepatol. 2022;20(8):1719-28.e3.
20. Carlson DA et al. Am J Gastroenterol. 2016;111(12):1726-35.
21. Carlson DA et al. Neurogastroenterol Motil. 2021;33(10):e14116.
22. Carlson DA et al. Gastrointest Endosc. 2019;90(6):915-923.e1.
23. Fox MR et al. Neurogastroenterol Motil. 2021;33(4):e14120.
24. Aziz Q et al. Gastroenterology. 2016 Feb 15. doi: 10.1053/j.gastro.2016.02.012.



