User login
Postpartum hypertension
When managing our pregnant patients, we often might be tempted to view the delivery of the baby as the conclusion of prenatal care. For many women, the baby’s birth coincides with a resolution of health conditions that they may have experienced during pregnancy, including edema, gestational diabetes, and hypertensive disorders. However, the postpartum period remains a critical time in the health of the mother. Indeed, the weeks immediately following parturition often are colloquially referred to as the fourth trimester, further emphasizing the importance of appropriate patient management and care during this time.
One of the key health conditions we must monitor in the immediate postpartum period is hypertension. According to a 2018 report compiling data from nine of the Centers for Disease Control and Prevention’s Maternal Mortality Review Committees, hypertensive disorders accounted for approximately 9.3% of pregnancy-related maternal deaths within 42 days after delivery (http://reviewtoaction.org/Report_from_Nine_MMRCs). Although women who have hypertensive disorders during pregnancy are at risk for complications after giving birth, women without gestational hypertension, preeclampsia, or eclampsia can experience these conditions post partum at a rate between 0.3% and 27.5% (Am J Obstet Gynecol 2012 Jun;206[6]:470-5). Therefore, we cannot assume that a patient with an uncomplicated pregnancy is completely “in the clear” after delivery.
Despite these somewhat grim statistics, With vigilant monitoring and strong communication with our patients, ob.gyns. can reduce the risks of these complications from occurring, more quickly resolve symptoms as they might arise, and significantly improve the health and well-being of new mothers in the fourth trimester.
The importance of caring for all of our patients along the continuum of pregnancy, especially as it pertains to monitoring and preventing postpartum hypertension, is the focus of the third and final installment of this Master Class series on hypertension in pregnancy authored by Dr. Baha Sibai, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
When managing our pregnant patients, we often might be tempted to view the delivery of the baby as the conclusion of prenatal care. For many women, the baby’s birth coincides with a resolution of health conditions that they may have experienced during pregnancy, including edema, gestational diabetes, and hypertensive disorders. However, the postpartum period remains a critical time in the health of the mother. Indeed, the weeks immediately following parturition often are colloquially referred to as the fourth trimester, further emphasizing the importance of appropriate patient management and care during this time.
One of the key health conditions we must monitor in the immediate postpartum period is hypertension. According to a 2018 report compiling data from nine of the Centers for Disease Control and Prevention’s Maternal Mortality Review Committees, hypertensive disorders accounted for approximately 9.3% of pregnancy-related maternal deaths within 42 days after delivery (http://reviewtoaction.org/Report_from_Nine_MMRCs). Although women who have hypertensive disorders during pregnancy are at risk for complications after giving birth, women without gestational hypertension, preeclampsia, or eclampsia can experience these conditions post partum at a rate between 0.3% and 27.5% (Am J Obstet Gynecol 2012 Jun;206[6]:470-5). Therefore, we cannot assume that a patient with an uncomplicated pregnancy is completely “in the clear” after delivery.
Despite these somewhat grim statistics, With vigilant monitoring and strong communication with our patients, ob.gyns. can reduce the risks of these complications from occurring, more quickly resolve symptoms as they might arise, and significantly improve the health and well-being of new mothers in the fourth trimester.
The importance of caring for all of our patients along the continuum of pregnancy, especially as it pertains to monitoring and preventing postpartum hypertension, is the focus of the third and final installment of this Master Class series on hypertension in pregnancy authored by Dr. Baha Sibai, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
When managing our pregnant patients, we often might be tempted to view the delivery of the baby as the conclusion of prenatal care. For many women, the baby’s birth coincides with a resolution of health conditions that they may have experienced during pregnancy, including edema, gestational diabetes, and hypertensive disorders. However, the postpartum period remains a critical time in the health of the mother. Indeed, the weeks immediately following parturition often are colloquially referred to as the fourth trimester, further emphasizing the importance of appropriate patient management and care during this time.
One of the key health conditions we must monitor in the immediate postpartum period is hypertension. According to a 2018 report compiling data from nine of the Centers for Disease Control and Prevention’s Maternal Mortality Review Committees, hypertensive disorders accounted for approximately 9.3% of pregnancy-related maternal deaths within 42 days after delivery (http://reviewtoaction.org/Report_from_Nine_MMRCs). Although women who have hypertensive disorders during pregnancy are at risk for complications after giving birth, women without gestational hypertension, preeclampsia, or eclampsia can experience these conditions post partum at a rate between 0.3% and 27.5% (Am J Obstet Gynecol 2012 Jun;206[6]:470-5). Therefore, we cannot assume that a patient with an uncomplicated pregnancy is completely “in the clear” after delivery.
Despite these somewhat grim statistics, With vigilant monitoring and strong communication with our patients, ob.gyns. can reduce the risks of these complications from occurring, more quickly resolve symptoms as they might arise, and significantly improve the health and well-being of new mothers in the fourth trimester.
The importance of caring for all of our patients along the continuum of pregnancy, especially as it pertains to monitoring and preventing postpartum hypertension, is the focus of the third and final installment of this Master Class series on hypertension in pregnancy authored by Dr. Baha Sibai, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
Recognition, evaluation, and management of postpartum hypertension
Postpartum hypertension has a host of potential causes, some of which may be benign (such as the persistence of mild gestational hypertension or mild chronic hypertension) whereas others (such as severe de novo preeclampsia-eclampsia and HELLP syndrome [a complication of pregnancy characterized by hemolysis, elevated liver enzymes, and a low platelet count]) can be life threatening.
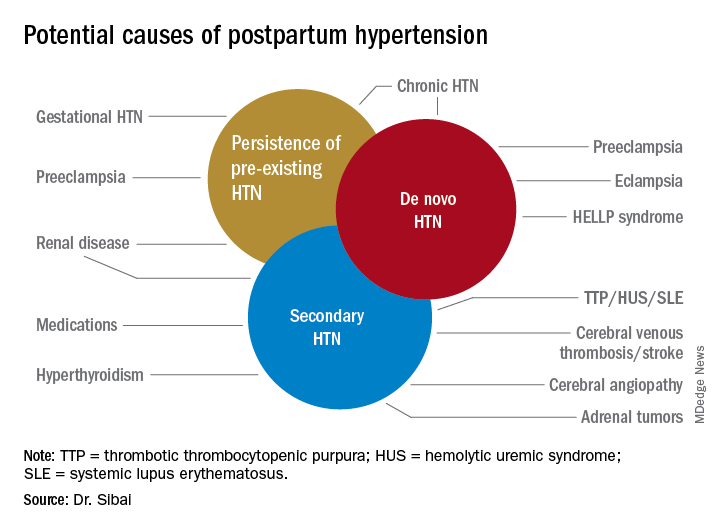
Postpartum hypertension may occur secondary to lupus, hyperthyroidism, hemolytic uremic syndrome, stroke, and other conditions, which means that we must have a high index of suspicion for secondary dangerous causes of hypertension when evaluating such women.
With monitoring, reporting, and prompt evaluation of symptoms in the postpartum period – and with patient education on signs and symptoms of severe hypertension and preeclampsia (PE) – we can expect to avoid a range of potential maternal complications, from hypertensive encephalopathy, liver hemorrhage, renal failure, and the development of eclampsia, ischemic stroke/cerebral hemorrhage, pulmonary edema, and cardiomyopathy.
Most women with gestational hypertension (GHTN) become normotensive during the first week post partum, but in women who develop PE during pregnancy, hypertension often takes longer to resolve. Some of these women may have an initial decrease in blood pressure immediately post partum followed by development of hypertension again between days 3 and 6. Therefore, This can be achieved either in-hospital, through home BP monitoring, or with in-office visits.
In addition, all women – including those who did not have hypertension during their pregnancies – should be educated about the signs and symptoms of severe hypertension or PE and instructed to report these to a medical provider in a timely fashion. Severe hypertension or PE with severe features may develop for the first time during the postpartum period either before or after hospital discharge. It is important to appreciate, moreover, that approximately 25%-40% of cases of eclampsia develop in the postpartum period with onset ranging from 2 days to 6 weeks after delivery. Moreover, almost one-third of women who develop the HELLP syndrome do so during the postpartum period.
Management of persistent hypertension
The most common causes for persistent hypertension beyond 48 hours after delivery are GHTN, PE, or chronic hypertension. Initial management will depend on history, clinical findings, presence or absence of associated symptoms, results of laboratory findings (urine protein, platelet count, liver enzymes, serum creatinine, and electrolytes), and response to prior treatment of hypertension.
Certain medications that frequently are prescribed in the postpartum period, such as ergonovine and decongestants, should be discontinued if they are being used. These agents can aggravate preexisting hypertension or result in new-onset hypertension if used in large or frequent doses. Their use also may be associated with cerebral symptoms, nausea, and vomiting.
Subsequent management includes close observation until resolution of hypertension and associated symptoms. If the patient has hypertension only with no symptoms, no proteinuria, and normal laboratory findings, BP control is the focus; antihypertensives are used if systolic BP remains persistently greater than or equal to 150 mm Hg and/or if diastolic BP persists at greater than or equal to 100 mm Hg. Intravenous boluses of either labetalol or hydralazine or oral rapid-acting nifedipine are used initially if systolic BP is greater than or equal to 160 mm Hg or diastolic BP greater than or equal to 110 mm Hg persists for at least 30 minutes. This is followed by oral medication to keep systolic BP less than 150 mg Hg and diastolic BP less than 100 mm Hg.
For patients with persistent hypertension after GHTN or PE, I recommend oral long-acting nifedipine XL (30 mg every 12 hours) or oral labetalol (200 mg every 8-12 hours). Compared with labetalol, oral nifedipine is associated with improved renal blood flow with resultant diuresis, which makes it the drug of choice in women with volume overload. In some, it is necessary to switch to a new agent such as an angiotensin-converting enzyme (ACE) inhibitor; an ACE inhibitor is the drug of choice in those with pregestational diabetes mellitus, renal disease, or cardiomyopathy. In addition, thiazide or loop diuretics may be needed in women with circulatory overload and in those with pulmonary edema. Antihypertensives such as nifedipine, labetalol, furosemide, captopril, and enalapril are compatible with breastfeeding.
If the BP remains less than 150 mm Hg (systolic) and/or less than 100 mm Hg (diastolic) for 24 hours, and there are no maternal symptoms, the patient may be discharged home with instructions for daily BP measurements (self or by a visiting nurse) and the reporting of symptoms until her next visit in 1 week. Antihypertensives then are discontinued if the BP remains below the hypertensive levels for at least 48 hours. This may take 1 or several weeks to achieve.
Women with PE with severe features should receive close monitoring of BP and of symptoms during the immediate postpartum period, as well as accurate measurements of fluid intake, urinary output, and weight gain. These women often have received large amounts of IV fluids during labor as a result of prehydration before epidural analgesia, as well as IV fluids administered during the use of oxytocin and magnesium sulfate in labor and post partum. Mobilization of extracellular fluid also leads to increased intravascular volume. As a result, women who have PE with severe features – particularly those with abnormal renal function, capillary leak, or early-onset disease – are at increased risk for pulmonary edema and exacerbation of severe hypertension.
Careful evaluation of the volume of IV fluids, oral intake, blood products, urine output, respiratory symptoms, and vital signs is advised. Patients who develop tachycardia or respiratory symptoms such as dry cough, shortness of breath, or orthopnea also should be monitored with pulse oximetry and frequent chest auscultation, as well as chest x-ray.
New-onset severe symptoms
Because severe hypertension or PE with severe features may develop for the first time during the postpartum period, postpartum women – and the medical providers and personnel who respond to patient phone calls – should be well educated about the signs and symptoms of severe hypertension or PE. These include new-onset severe headaches that do not respond to maximum doses of analgesics, persistent severe visual changes, and new-onset epigastric pain with nausea and vomiting, dyspnea, orthopnea, shortness of breath, or palpitations. These women are at increased risk for eclampsia, pulmonary edema, stroke, and thromboembolism; these women require careful evaluation and potential hospitalization.
Severe new onset of persistent headaches and/or visual symptoms. Women with hypertension in association with new-onset persistent headaches and/or visual changes should be suspected to have severe PE. Patients who have hypertension with seizure should be initially treated as having eclampsia and should receive brain imaging to rule out other etiologies. Magnesium sulfate therapy must be initiated promptly for seizure prophylaxis and/or treatment. In addition, intravenous antihypertensive medications are recommended to lower BP to the desired goal while considering an alternative cause for the cerebral symptoms.
Women presenting with hypertension in association with refractory and/or thunderclap headaches, visual disturbances, or neurologic deficits should be evaluated for possible cerebrovascular complications such as reversible cerebral vasoconstriction syndrome (RCVS), cerebral venous thrombosis, or stroke. These women will require selective diagnostic neuroimaging and consultation with neurology and/or neurosurgery. Such an evaluation may include CT scan for hemorrhage, MRI for detection of vasogenic edema and/or ischemia or infarction, cerebral angiography for diagnosis of RCVS, and cerebral venography for detection of cerebral venous thrombosis. Subsequent treatment will depend on the etiology.
Severe new-onset epigastric/right upper quadrant pain with nausea and vomiting. Women with persistent nausea, vomiting, or epigastric pain should be evaluated for HELLP syndrome because up to 30% who develop the syndrome do so post partum. The time of onset of clinical and laboratory findings ranges from 1 to 7 days post partum. Women are managed as they are before delivery, with the use of magnesium sulfate, antihypertensives, and close monitoring of vital signs and laboratory values.
In general, patients with HELLP syndrome will demonstrate an improvement in clinical and laboratory findings within 72 hours after treatment. If there is either no improvement or a deterioration in these findings, then it is important to consult with appropriate specialists for evaluation and subsequent management of possible rare syndromes such as acute fatty liver, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, or exacerbation of lupus.
Severe new-onset shortness of breath, dyspnea, orthopnea, or palpitations. Women with these symptoms in the postpartum period should be evaluated for possible pulmonary edema, pulmonary embolism, or peripartum cardiomyopathy. Women with postpartum hypertension are at risk for pulmonary edema with onset at 3-6 days after delivery. Diagnosis is confirmed by physical exam (tachycardia, tachypnea), presence of rales on lung exam, pulse oximetry (oxygen saturation less than 93%), and chest x-ray, and echocardiography to exclude other etiologies. Treatment of pulmonary edema includes oxygen supplementation, 40 mg IV furosemide, control of severe hypertension, fluid restriction, and supportive care.
Pulmonary embolism usually is confirmed by chest CT angiography and managed with therapeutic anticoagulation. Peripartum cardiomyopathy is diagnosed by echocardiography revealing left ventricular systolic dysfunction (ejection fraction less than 45%, dilated left ventricle). Treatment includes IV furosemide, use of a vasodilator, and ACE inhibitor therapy.
Remote prognosis
Recent research suggests that women who develop PE may be at increased risk for future cardiovascular disease such as heart failure, coronary artery disease, and stroke later in life. Indeed, many of the risk factors and pathophysiologic abnormalities of PE are similar to those of coronary artery disease.
The American College of Obstetricians and Gynecologists and the American Heart Association recommend that women with PE receive close observation in the postpartum period and careful evaluation in the first year after delivery to identify those who could benefit from early intervention to prevent subsequent cardiovascular disease. In general, when pregnancies are complicated by PE, there are opportunities for lifestyle and risk factor modification.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston
Postpartum hypertension has a host of potential causes, some of which may be benign (such as the persistence of mild gestational hypertension or mild chronic hypertension) whereas others (such as severe de novo preeclampsia-eclampsia and HELLP syndrome [a complication of pregnancy characterized by hemolysis, elevated liver enzymes, and a low platelet count]) can be life threatening.

Postpartum hypertension may occur secondary to lupus, hyperthyroidism, hemolytic uremic syndrome, stroke, and other conditions, which means that we must have a high index of suspicion for secondary dangerous causes of hypertension when evaluating such women.
With monitoring, reporting, and prompt evaluation of symptoms in the postpartum period – and with patient education on signs and symptoms of severe hypertension and preeclampsia (PE) – we can expect to avoid a range of potential maternal complications, from hypertensive encephalopathy, liver hemorrhage, renal failure, and the development of eclampsia, ischemic stroke/cerebral hemorrhage, pulmonary edema, and cardiomyopathy.
Most women with gestational hypertension (GHTN) become normotensive during the first week post partum, but in women who develop PE during pregnancy, hypertension often takes longer to resolve. Some of these women may have an initial decrease in blood pressure immediately post partum followed by development of hypertension again between days 3 and 6. Therefore, This can be achieved either in-hospital, through home BP monitoring, or with in-office visits.
In addition, all women – including those who did not have hypertension during their pregnancies – should be educated about the signs and symptoms of severe hypertension or PE and instructed to report these to a medical provider in a timely fashion. Severe hypertension or PE with severe features may develop for the first time during the postpartum period either before or after hospital discharge. It is important to appreciate, moreover, that approximately 25%-40% of cases of eclampsia develop in the postpartum period with onset ranging from 2 days to 6 weeks after delivery. Moreover, almost one-third of women who develop the HELLP syndrome do so during the postpartum period.
Management of persistent hypertension
The most common causes for persistent hypertension beyond 48 hours after delivery are GHTN, PE, or chronic hypertension. Initial management will depend on history, clinical findings, presence or absence of associated symptoms, results of laboratory findings (urine protein, platelet count, liver enzymes, serum creatinine, and electrolytes), and response to prior treatment of hypertension.
Certain medications that frequently are prescribed in the postpartum period, such as ergonovine and decongestants, should be discontinued if they are being used. These agents can aggravate preexisting hypertension or result in new-onset hypertension if used in large or frequent doses. Their use also may be associated with cerebral symptoms, nausea, and vomiting.
Subsequent management includes close observation until resolution of hypertension and associated symptoms. If the patient has hypertension only with no symptoms, no proteinuria, and normal laboratory findings, BP control is the focus; antihypertensives are used if systolic BP remains persistently greater than or equal to 150 mm Hg and/or if diastolic BP persists at greater than or equal to 100 mm Hg. Intravenous boluses of either labetalol or hydralazine or oral rapid-acting nifedipine are used initially if systolic BP is greater than or equal to 160 mm Hg or diastolic BP greater than or equal to 110 mm Hg persists for at least 30 minutes. This is followed by oral medication to keep systolic BP less than 150 mg Hg and diastolic BP less than 100 mm Hg.
For patients with persistent hypertension after GHTN or PE, I recommend oral long-acting nifedipine XL (30 mg every 12 hours) or oral labetalol (200 mg every 8-12 hours). Compared with labetalol, oral nifedipine is associated with improved renal blood flow with resultant diuresis, which makes it the drug of choice in women with volume overload. In some, it is necessary to switch to a new agent such as an angiotensin-converting enzyme (ACE) inhibitor; an ACE inhibitor is the drug of choice in those with pregestational diabetes mellitus, renal disease, or cardiomyopathy. In addition, thiazide or loop diuretics may be needed in women with circulatory overload and in those with pulmonary edema. Antihypertensives such as nifedipine, labetalol, furosemide, captopril, and enalapril are compatible with breastfeeding.
If the BP remains less than 150 mm Hg (systolic) and/or less than 100 mm Hg (diastolic) for 24 hours, and there are no maternal symptoms, the patient may be discharged home with instructions for daily BP measurements (self or by a visiting nurse) and the reporting of symptoms until her next visit in 1 week. Antihypertensives then are discontinued if the BP remains below the hypertensive levels for at least 48 hours. This may take 1 or several weeks to achieve.
Women with PE with severe features should receive close monitoring of BP and of symptoms during the immediate postpartum period, as well as accurate measurements of fluid intake, urinary output, and weight gain. These women often have received large amounts of IV fluids during labor as a result of prehydration before epidural analgesia, as well as IV fluids administered during the use of oxytocin and magnesium sulfate in labor and post partum. Mobilization of extracellular fluid also leads to increased intravascular volume. As a result, women who have PE with severe features – particularly those with abnormal renal function, capillary leak, or early-onset disease – are at increased risk for pulmonary edema and exacerbation of severe hypertension.
Careful evaluation of the volume of IV fluids, oral intake, blood products, urine output, respiratory symptoms, and vital signs is advised. Patients who develop tachycardia or respiratory symptoms such as dry cough, shortness of breath, or orthopnea also should be monitored with pulse oximetry and frequent chest auscultation, as well as chest x-ray.
New-onset severe symptoms
Because severe hypertension or PE with severe features may develop for the first time during the postpartum period, postpartum women – and the medical providers and personnel who respond to patient phone calls – should be well educated about the signs and symptoms of severe hypertension or PE. These include new-onset severe headaches that do not respond to maximum doses of analgesics, persistent severe visual changes, and new-onset epigastric pain with nausea and vomiting, dyspnea, orthopnea, shortness of breath, or palpitations. These women are at increased risk for eclampsia, pulmonary edema, stroke, and thromboembolism; these women require careful evaluation and potential hospitalization.
Severe new onset of persistent headaches and/or visual symptoms. Women with hypertension in association with new-onset persistent headaches and/or visual changes should be suspected to have severe PE. Patients who have hypertension with seizure should be initially treated as having eclampsia and should receive brain imaging to rule out other etiologies. Magnesium sulfate therapy must be initiated promptly for seizure prophylaxis and/or treatment. In addition, intravenous antihypertensive medications are recommended to lower BP to the desired goal while considering an alternative cause for the cerebral symptoms.
Women presenting with hypertension in association with refractory and/or thunderclap headaches, visual disturbances, or neurologic deficits should be evaluated for possible cerebrovascular complications such as reversible cerebral vasoconstriction syndrome (RCVS), cerebral venous thrombosis, or stroke. These women will require selective diagnostic neuroimaging and consultation with neurology and/or neurosurgery. Such an evaluation may include CT scan for hemorrhage, MRI for detection of vasogenic edema and/or ischemia or infarction, cerebral angiography for diagnosis of RCVS, and cerebral venography for detection of cerebral venous thrombosis. Subsequent treatment will depend on the etiology.
Severe new-onset epigastric/right upper quadrant pain with nausea and vomiting. Women with persistent nausea, vomiting, or epigastric pain should be evaluated for HELLP syndrome because up to 30% who develop the syndrome do so post partum. The time of onset of clinical and laboratory findings ranges from 1 to 7 days post partum. Women are managed as they are before delivery, with the use of magnesium sulfate, antihypertensives, and close monitoring of vital signs and laboratory values.
In general, patients with HELLP syndrome will demonstrate an improvement in clinical and laboratory findings within 72 hours after treatment. If there is either no improvement or a deterioration in these findings, then it is important to consult with appropriate specialists for evaluation and subsequent management of possible rare syndromes such as acute fatty liver, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, or exacerbation of lupus.
Severe new-onset shortness of breath, dyspnea, orthopnea, or palpitations. Women with these symptoms in the postpartum period should be evaluated for possible pulmonary edema, pulmonary embolism, or peripartum cardiomyopathy. Women with postpartum hypertension are at risk for pulmonary edema with onset at 3-6 days after delivery. Diagnosis is confirmed by physical exam (tachycardia, tachypnea), presence of rales on lung exam, pulse oximetry (oxygen saturation less than 93%), and chest x-ray, and echocardiography to exclude other etiologies. Treatment of pulmonary edema includes oxygen supplementation, 40 mg IV furosemide, control of severe hypertension, fluid restriction, and supportive care.
Pulmonary embolism usually is confirmed by chest CT angiography and managed with therapeutic anticoagulation. Peripartum cardiomyopathy is diagnosed by echocardiography revealing left ventricular systolic dysfunction (ejection fraction less than 45%, dilated left ventricle). Treatment includes IV furosemide, use of a vasodilator, and ACE inhibitor therapy.
Remote prognosis
Recent research suggests that women who develop PE may be at increased risk for future cardiovascular disease such as heart failure, coronary artery disease, and stroke later in life. Indeed, many of the risk factors and pathophysiologic abnormalities of PE are similar to those of coronary artery disease.
The American College of Obstetricians and Gynecologists and the American Heart Association recommend that women with PE receive close observation in the postpartum period and careful evaluation in the first year after delivery to identify those who could benefit from early intervention to prevent subsequent cardiovascular disease. In general, when pregnancies are complicated by PE, there are opportunities for lifestyle and risk factor modification.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston
Postpartum hypertension has a host of potential causes, some of which may be benign (such as the persistence of mild gestational hypertension or mild chronic hypertension) whereas others (such as severe de novo preeclampsia-eclampsia and HELLP syndrome [a complication of pregnancy characterized by hemolysis, elevated liver enzymes, and a low platelet count]) can be life threatening.

Postpartum hypertension may occur secondary to lupus, hyperthyroidism, hemolytic uremic syndrome, stroke, and other conditions, which means that we must have a high index of suspicion for secondary dangerous causes of hypertension when evaluating such women.
With monitoring, reporting, and prompt evaluation of symptoms in the postpartum period – and with patient education on signs and symptoms of severe hypertension and preeclampsia (PE) – we can expect to avoid a range of potential maternal complications, from hypertensive encephalopathy, liver hemorrhage, renal failure, and the development of eclampsia, ischemic stroke/cerebral hemorrhage, pulmonary edema, and cardiomyopathy.
Most women with gestational hypertension (GHTN) become normotensive during the first week post partum, but in women who develop PE during pregnancy, hypertension often takes longer to resolve. Some of these women may have an initial decrease in blood pressure immediately post partum followed by development of hypertension again between days 3 and 6. Therefore, This can be achieved either in-hospital, through home BP monitoring, or with in-office visits.
In addition, all women – including those who did not have hypertension during their pregnancies – should be educated about the signs and symptoms of severe hypertension or PE and instructed to report these to a medical provider in a timely fashion. Severe hypertension or PE with severe features may develop for the first time during the postpartum period either before or after hospital discharge. It is important to appreciate, moreover, that approximately 25%-40% of cases of eclampsia develop in the postpartum period with onset ranging from 2 days to 6 weeks after delivery. Moreover, almost one-third of women who develop the HELLP syndrome do so during the postpartum period.
Management of persistent hypertension
The most common causes for persistent hypertension beyond 48 hours after delivery are GHTN, PE, or chronic hypertension. Initial management will depend on history, clinical findings, presence or absence of associated symptoms, results of laboratory findings (urine protein, platelet count, liver enzymes, serum creatinine, and electrolytes), and response to prior treatment of hypertension.
Certain medications that frequently are prescribed in the postpartum period, such as ergonovine and decongestants, should be discontinued if they are being used. These agents can aggravate preexisting hypertension or result in new-onset hypertension if used in large or frequent doses. Their use also may be associated with cerebral symptoms, nausea, and vomiting.
Subsequent management includes close observation until resolution of hypertension and associated symptoms. If the patient has hypertension only with no symptoms, no proteinuria, and normal laboratory findings, BP control is the focus; antihypertensives are used if systolic BP remains persistently greater than or equal to 150 mm Hg and/or if diastolic BP persists at greater than or equal to 100 mm Hg. Intravenous boluses of either labetalol or hydralazine or oral rapid-acting nifedipine are used initially if systolic BP is greater than or equal to 160 mm Hg or diastolic BP greater than or equal to 110 mm Hg persists for at least 30 minutes. This is followed by oral medication to keep systolic BP less than 150 mg Hg and diastolic BP less than 100 mm Hg.
For patients with persistent hypertension after GHTN or PE, I recommend oral long-acting nifedipine XL (30 mg every 12 hours) or oral labetalol (200 mg every 8-12 hours). Compared with labetalol, oral nifedipine is associated with improved renal blood flow with resultant diuresis, which makes it the drug of choice in women with volume overload. In some, it is necessary to switch to a new agent such as an angiotensin-converting enzyme (ACE) inhibitor; an ACE inhibitor is the drug of choice in those with pregestational diabetes mellitus, renal disease, or cardiomyopathy. In addition, thiazide or loop diuretics may be needed in women with circulatory overload and in those with pulmonary edema. Antihypertensives such as nifedipine, labetalol, furosemide, captopril, and enalapril are compatible with breastfeeding.
If the BP remains less than 150 mm Hg (systolic) and/or less than 100 mm Hg (diastolic) for 24 hours, and there are no maternal symptoms, the patient may be discharged home with instructions for daily BP measurements (self or by a visiting nurse) and the reporting of symptoms until her next visit in 1 week. Antihypertensives then are discontinued if the BP remains below the hypertensive levels for at least 48 hours. This may take 1 or several weeks to achieve.
Women with PE with severe features should receive close monitoring of BP and of symptoms during the immediate postpartum period, as well as accurate measurements of fluid intake, urinary output, and weight gain. These women often have received large amounts of IV fluids during labor as a result of prehydration before epidural analgesia, as well as IV fluids administered during the use of oxytocin and magnesium sulfate in labor and post partum. Mobilization of extracellular fluid also leads to increased intravascular volume. As a result, women who have PE with severe features – particularly those with abnormal renal function, capillary leak, or early-onset disease – are at increased risk for pulmonary edema and exacerbation of severe hypertension.
Careful evaluation of the volume of IV fluids, oral intake, blood products, urine output, respiratory symptoms, and vital signs is advised. Patients who develop tachycardia or respiratory symptoms such as dry cough, shortness of breath, or orthopnea also should be monitored with pulse oximetry and frequent chest auscultation, as well as chest x-ray.
New-onset severe symptoms
Because severe hypertension or PE with severe features may develop for the first time during the postpartum period, postpartum women – and the medical providers and personnel who respond to patient phone calls – should be well educated about the signs and symptoms of severe hypertension or PE. These include new-onset severe headaches that do not respond to maximum doses of analgesics, persistent severe visual changes, and new-onset epigastric pain with nausea and vomiting, dyspnea, orthopnea, shortness of breath, or palpitations. These women are at increased risk for eclampsia, pulmonary edema, stroke, and thromboembolism; these women require careful evaluation and potential hospitalization.
Severe new onset of persistent headaches and/or visual symptoms. Women with hypertension in association with new-onset persistent headaches and/or visual changes should be suspected to have severe PE. Patients who have hypertension with seizure should be initially treated as having eclampsia and should receive brain imaging to rule out other etiologies. Magnesium sulfate therapy must be initiated promptly for seizure prophylaxis and/or treatment. In addition, intravenous antihypertensive medications are recommended to lower BP to the desired goal while considering an alternative cause for the cerebral symptoms.
Women presenting with hypertension in association with refractory and/or thunderclap headaches, visual disturbances, or neurologic deficits should be evaluated for possible cerebrovascular complications such as reversible cerebral vasoconstriction syndrome (RCVS), cerebral venous thrombosis, or stroke. These women will require selective diagnostic neuroimaging and consultation with neurology and/or neurosurgery. Such an evaluation may include CT scan for hemorrhage, MRI for detection of vasogenic edema and/or ischemia or infarction, cerebral angiography for diagnosis of RCVS, and cerebral venography for detection of cerebral venous thrombosis. Subsequent treatment will depend on the etiology.
Severe new-onset epigastric/right upper quadrant pain with nausea and vomiting. Women with persistent nausea, vomiting, or epigastric pain should be evaluated for HELLP syndrome because up to 30% who develop the syndrome do so post partum. The time of onset of clinical and laboratory findings ranges from 1 to 7 days post partum. Women are managed as they are before delivery, with the use of magnesium sulfate, antihypertensives, and close monitoring of vital signs and laboratory values.
In general, patients with HELLP syndrome will demonstrate an improvement in clinical and laboratory findings within 72 hours after treatment. If there is either no improvement or a deterioration in these findings, then it is important to consult with appropriate specialists for evaluation and subsequent management of possible rare syndromes such as acute fatty liver, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, or exacerbation of lupus.
Severe new-onset shortness of breath, dyspnea, orthopnea, or palpitations. Women with these symptoms in the postpartum period should be evaluated for possible pulmonary edema, pulmonary embolism, or peripartum cardiomyopathy. Women with postpartum hypertension are at risk for pulmonary edema with onset at 3-6 days after delivery. Diagnosis is confirmed by physical exam (tachycardia, tachypnea), presence of rales on lung exam, pulse oximetry (oxygen saturation less than 93%), and chest x-ray, and echocardiography to exclude other etiologies. Treatment of pulmonary edema includes oxygen supplementation, 40 mg IV furosemide, control of severe hypertension, fluid restriction, and supportive care.
Pulmonary embolism usually is confirmed by chest CT angiography and managed with therapeutic anticoagulation. Peripartum cardiomyopathy is diagnosed by echocardiography revealing left ventricular systolic dysfunction (ejection fraction less than 45%, dilated left ventricle). Treatment includes IV furosemide, use of a vasodilator, and ACE inhibitor therapy.
Remote prognosis
Recent research suggests that women who develop PE may be at increased risk for future cardiovascular disease such as heart failure, coronary artery disease, and stroke later in life. Indeed, many of the risk factors and pathophysiologic abnormalities of PE are similar to those of coronary artery disease.
The American College of Obstetricians and Gynecologists and the American Heart Association recommend that women with PE receive close observation in the postpartum period and careful evaluation in the first year after delivery to identify those who could benefit from early intervention to prevent subsequent cardiovascular disease. In general, when pregnancies are complicated by PE, there are opportunities for lifestyle and risk factor modification.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston
Opportunistic salpingectomy appears to reduce risk of ovarian cancer
Women at high risk of ovarian cancer secondary to genetic predisposition (BRCA gene mutation, Lynch syndrome) still are recommended to undergo bilateral salpingo-oophorectomy after completion of child bearing or by age 40-45 years depending on the specific mutation and family history. For a woman not at risk of hereditary-related ovarian cancer, opportunistic salpingectomy would appear to reduce the risk of ovarian cancer.
Unlike bilateral tubal ligation, which has a greater protective risk of endometrioid and clear-cell carcinoma of the ovary, as well. A Swedish population-based cohort study involving over a quarter of a million women undergoing benign surgery noted a statistically significant decrease in ovarian cancer risk with salpingectomy. The degree of risk reduction was greater when bilateral salpingectomy was performed.1 Moreover, a Danish case-control study of over 13,000 women with ovarian cancer demonstrated a 42% decrease in epithelial carcinoma risk following bilateral salpingectomy.2
Bilateral salpingectomy does not appear to decrease ovarian function. A study by Venturella et al. that compared 91 women undergoing bilateral salpingectomy with 95 women with mesosalpinx removal within the tubes during salpingectomy observed no significant difference in change of ovarian reserve.3 Moreover, Kotlyar et al. performed a literature review and noted similar findings.4 Finally, in another study by Venturella et al. no effects were noted 3-5 years following prophylactic bilateral salpingectomy on ovarian reserve in women undergoing total laparoscopic hysterectomy in their late reproductive years, compared with healthy women with intact uterus and adnexa.5
Introduction of opportunistic salpingectomy secondary to potential ovarian cancer reduction has seen increased adoption over the years. A U.S. study of 400,000 hysterectomies performed for benign indications from 1998 to 2011 showed an increased annual rate of bilateral salpingectomy of 8% (1998-2008) and a 24% annual increase (2008-2011).6 A retrospective study of 12,143 hysterectomies performed within a large U.S. health care system reported an increased rate of salpingectomy from 15% in 2011 to 45% in 2012 to 73% in 2014.7
Given the fact that the American College of Obstetricians and Gynecologists and the AAGL recommend vaginal hysterectomy as the approach of choice when feasible, tips and tricks on opportunistic salpingectomy form an important topic.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Rosanne M. Kho, MD. Dr. Kho’s academic and clinical work focuses on advancing vaginal and minimally invasive surgery. Dr. Kho is a strong advocate of the vaginal approach for benign hysterectomy and is recognized for her passion for bringing vaginal surgery back into the armamentarium of the gynecologic surgeon. Dr. Kho is published in the field of gynecologic surgery, having authored many peer-reviewed manuscripts and book chapters. She is currently an associate editor for the Journal of Minimally Invasive Gynecology (JMIG).
It is truly a pleasure to welcome Dr. Kho to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Natl Cancer Inst. 2015 Jan 27. doi: 10.1093/jnci/dju410.
2. Acta Obstet Gynecol Scand. 2015 Jan;94(1):86-94.
3. Fertil Steril. 2015 Nov;104(5):1332-9.
4. J Minim Invasive Gynecol. 2017 May-Jun;24(4):563-78.
5. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
6. Am J Obstet Gynecol. 2015 Nov;213(5):713.e1-13.
7. Obstet Gynecol. 2016 Aug;128(2):277-83.
Women at high risk of ovarian cancer secondary to genetic predisposition (BRCA gene mutation, Lynch syndrome) still are recommended to undergo bilateral salpingo-oophorectomy after completion of child bearing or by age 40-45 years depending on the specific mutation and family history. For a woman not at risk of hereditary-related ovarian cancer, opportunistic salpingectomy would appear to reduce the risk of ovarian cancer.
Unlike bilateral tubal ligation, which has a greater protective risk of endometrioid and clear-cell carcinoma of the ovary, as well. A Swedish population-based cohort study involving over a quarter of a million women undergoing benign surgery noted a statistically significant decrease in ovarian cancer risk with salpingectomy. The degree of risk reduction was greater when bilateral salpingectomy was performed.1 Moreover, a Danish case-control study of over 13,000 women with ovarian cancer demonstrated a 42% decrease in epithelial carcinoma risk following bilateral salpingectomy.2
Bilateral salpingectomy does not appear to decrease ovarian function. A study by Venturella et al. that compared 91 women undergoing bilateral salpingectomy with 95 women with mesosalpinx removal within the tubes during salpingectomy observed no significant difference in change of ovarian reserve.3 Moreover, Kotlyar et al. performed a literature review and noted similar findings.4 Finally, in another study by Venturella et al. no effects were noted 3-5 years following prophylactic bilateral salpingectomy on ovarian reserve in women undergoing total laparoscopic hysterectomy in their late reproductive years, compared with healthy women with intact uterus and adnexa.5
Introduction of opportunistic salpingectomy secondary to potential ovarian cancer reduction has seen increased adoption over the years. A U.S. study of 400,000 hysterectomies performed for benign indications from 1998 to 2011 showed an increased annual rate of bilateral salpingectomy of 8% (1998-2008) and a 24% annual increase (2008-2011).6 A retrospective study of 12,143 hysterectomies performed within a large U.S. health care system reported an increased rate of salpingectomy from 15% in 2011 to 45% in 2012 to 73% in 2014.7
Given the fact that the American College of Obstetricians and Gynecologists and the AAGL recommend vaginal hysterectomy as the approach of choice when feasible, tips and tricks on opportunistic salpingectomy form an important topic.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Rosanne M. Kho, MD. Dr. Kho’s academic and clinical work focuses on advancing vaginal and minimally invasive surgery. Dr. Kho is a strong advocate of the vaginal approach for benign hysterectomy and is recognized for her passion for bringing vaginal surgery back into the armamentarium of the gynecologic surgeon. Dr. Kho is published in the field of gynecologic surgery, having authored many peer-reviewed manuscripts and book chapters. She is currently an associate editor for the Journal of Minimally Invasive Gynecology (JMIG).
It is truly a pleasure to welcome Dr. Kho to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Natl Cancer Inst. 2015 Jan 27. doi: 10.1093/jnci/dju410.
2. Acta Obstet Gynecol Scand. 2015 Jan;94(1):86-94.
3. Fertil Steril. 2015 Nov;104(5):1332-9.
4. J Minim Invasive Gynecol. 2017 May-Jun;24(4):563-78.
5. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
6. Am J Obstet Gynecol. 2015 Nov;213(5):713.e1-13.
7. Obstet Gynecol. 2016 Aug;128(2):277-83.
Women at high risk of ovarian cancer secondary to genetic predisposition (BRCA gene mutation, Lynch syndrome) still are recommended to undergo bilateral salpingo-oophorectomy after completion of child bearing or by age 40-45 years depending on the specific mutation and family history. For a woman not at risk of hereditary-related ovarian cancer, opportunistic salpingectomy would appear to reduce the risk of ovarian cancer.
Unlike bilateral tubal ligation, which has a greater protective risk of endometrioid and clear-cell carcinoma of the ovary, as well. A Swedish population-based cohort study involving over a quarter of a million women undergoing benign surgery noted a statistically significant decrease in ovarian cancer risk with salpingectomy. The degree of risk reduction was greater when bilateral salpingectomy was performed.1 Moreover, a Danish case-control study of over 13,000 women with ovarian cancer demonstrated a 42% decrease in epithelial carcinoma risk following bilateral salpingectomy.2
Bilateral salpingectomy does not appear to decrease ovarian function. A study by Venturella et al. that compared 91 women undergoing bilateral salpingectomy with 95 women with mesosalpinx removal within the tubes during salpingectomy observed no significant difference in change of ovarian reserve.3 Moreover, Kotlyar et al. performed a literature review and noted similar findings.4 Finally, in another study by Venturella et al. no effects were noted 3-5 years following prophylactic bilateral salpingectomy on ovarian reserve in women undergoing total laparoscopic hysterectomy in their late reproductive years, compared with healthy women with intact uterus and adnexa.5
Introduction of opportunistic salpingectomy secondary to potential ovarian cancer reduction has seen increased adoption over the years. A U.S. study of 400,000 hysterectomies performed for benign indications from 1998 to 2011 showed an increased annual rate of bilateral salpingectomy of 8% (1998-2008) and a 24% annual increase (2008-2011).6 A retrospective study of 12,143 hysterectomies performed within a large U.S. health care system reported an increased rate of salpingectomy from 15% in 2011 to 45% in 2012 to 73% in 2014.7
Given the fact that the American College of Obstetricians and Gynecologists and the AAGL recommend vaginal hysterectomy as the approach of choice when feasible, tips and tricks on opportunistic salpingectomy form an important topic.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Rosanne M. Kho, MD. Dr. Kho’s academic and clinical work focuses on advancing vaginal and minimally invasive surgery. Dr. Kho is a strong advocate of the vaginal approach for benign hysterectomy and is recognized for her passion for bringing vaginal surgery back into the armamentarium of the gynecologic surgeon. Dr. Kho is published in the field of gynecologic surgery, having authored many peer-reviewed manuscripts and book chapters. She is currently an associate editor for the Journal of Minimally Invasive Gynecology (JMIG).
It is truly a pleasure to welcome Dr. Kho to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Natl Cancer Inst. 2015 Jan 27. doi: 10.1093/jnci/dju410.
2. Acta Obstet Gynecol Scand. 2015 Jan;94(1):86-94.
3. Fertil Steril. 2015 Nov;104(5):1332-9.
4. J Minim Invasive Gynecol. 2017 May-Jun;24(4):563-78.
5. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
6. Am J Obstet Gynecol. 2015 Nov;213(5):713.e1-13.
7. Obstet Gynecol. 2016 Aug;128(2):277-83.
Can prophylactic salpingectomies be achieved with the vaginal approach?
In the last decade, there has been a major shift in our understanding of the pathogenesis of ovarian cancers. Current literature suggests that many high-grade serous carcinomas develop from the distal aspect of the fallopian tube and that serous tubal intraepithelial carcinoma is likely the precursor. The critical role that the fallopian tubes play as the likely origin of many serous ovarian and pelvic cancers has resulted in a shift from prophylactic salpingo-oophorectomy, which may increase risk for cardiovascular disease, to prophylactic bilateral salpingectomy (PBS) at the time of hysterectomy.
It is important that this shift occur with vaginal hysterectomy (VH) and not only with other surgical approaches. It is known that PBS is performed more commonly during laparoscopic or abdominal hysterectomy, and it’s possible that the need for adnexal surgery may further contribute to the decline in the rate of VH performed in the United States. This is despite evidence that the vaginal approach is preferred for benign hysterectomy even in patients with a nonprolapsed and large fibroid uterus, obesity, or previous pelvic surgery. Current American College of Obstetricians and Gynecologists’ guidelines also state that the need to perform adnexal surgery is not a contraindication to the vaginal approach.
So that more women may attain the benefits and advantages of VH, we need more effective teaching programs for vaginal surgery in residency training programs, hospitals, and community surgical centers. Moreover, we must appreciate that PBS with VH is safe and feasible. There are multiple techniques and tools available to facilitate the successful removal of the tubes, particularly in difficult cases.
The benefit and safety of PBS
Is PBS really effective in decreasing the incidence and mortality of ovarian cancer? A proposed randomized trial in Sweden with a target accrual of 4,400 patients – the Hysterectomy and Opportunistic Salpingectromy Study (HOPPSA, NCT03045965) – will evaluate the risk of ovarian cancer over a 10- to 30-year follow-up period in patients undergoing hysterectomy through all routes. While we wait for these prospective results, an elegant decision-model analysis suggests that routine PBS during VH would eliminate one diagnosis of ovarian cancer for every 225 women undergoing hysterectomy (reducing the risk from 0.956% to 0.511%) and would prevent one death for every 450 women (reducing the risk from 0.478% to 0.256%). The analysis, which drew upon published literature, Medicare reimbursement data, and the National Surgical Quality Improvement Program database, also found that PBS with VH is a less expensive strategy than VH alone because of an increased risk of future adnexal surgery in women retaining their tubes.1

The question of whether PBS places a woman at risk for early menopause is a relevant one. A study following women for 3-5 years after surgery showed that the addition of PBS to total laparoscopic hysterectomy in women of reproductive age does not appear to modify ovarian function.2 However, a recently published retrospective study from the Swedish National Registry showed that women who underwent PBS with abdominal or laparoscopic benign hysterectomy had an increased risk of menopausal symptoms 1 year after surgery.3 Women between the ages of 45-49 years were at highest risk, suggesting increased vulnerability to possible vascular effects of PBS. A longer follow-up period may be necessary to assess younger age groups.
In a multicenter, prospective and observational trial involving 69 patients undergoing VH, PBS was feasible in 75% (a majority of whom [78%] had pelvic organ prolapse) and increased operating time by 11 minutes with no additional complications noted. The surgeons in this study, primarily urogynecologists, utilized a clamp or double-clamp technique to remove the fimbriae.4
The decision-model analysis mentioned above found that PBS would involve slightly more complications than VH alone (7.95% vs. 7.68%),1 and a systematic review that I coauthored of PBS in low-risk women found a small to no increase in operative time and no additional estimated blood loss, hospital stay, or complications for PBS.5
Tools and techniques
Vaginal PBS can be accomplished easily with traditional clamp-cut-tie technique in cases where the fallopian tubes are accessible, such as in patients with uterine prolapse. Generally, most surgeons perform a distal fimbriectomy only for risk-reduction purposes because this is where precursor lesions known as serous tubal intraepithelial cancer (STIC) reside.
To perform a fimbriectomy in cases where the distal portion of the tube is easily accessible, a Kelly clamp is placed across the mesosalpinx, and a fine tie is used for ligature. In more challenging hysterectomy cases, such as in lack of uterine prolapse, large fibroid uterus, morbid obesity, and in patients with previous tubal ligation, the fallopian tubes can be more difficult to access. In these cases, I prefer the use of the vessel-sealing device to seal and divide the mesosalpinx.
Here I describe three specific techniques that can facilitate the removal of the fallopian tubes in more challenging cases. In each technique, the entire fallopian tubes are removed – without leaving behind the proximal stump. The residual stump has the potential of developing into a hydrosalpinx that may necessitate another procedure in the future for the patient.
Separate the fallopian tube before clamping the ‘utero-ovarian ligament’ technique
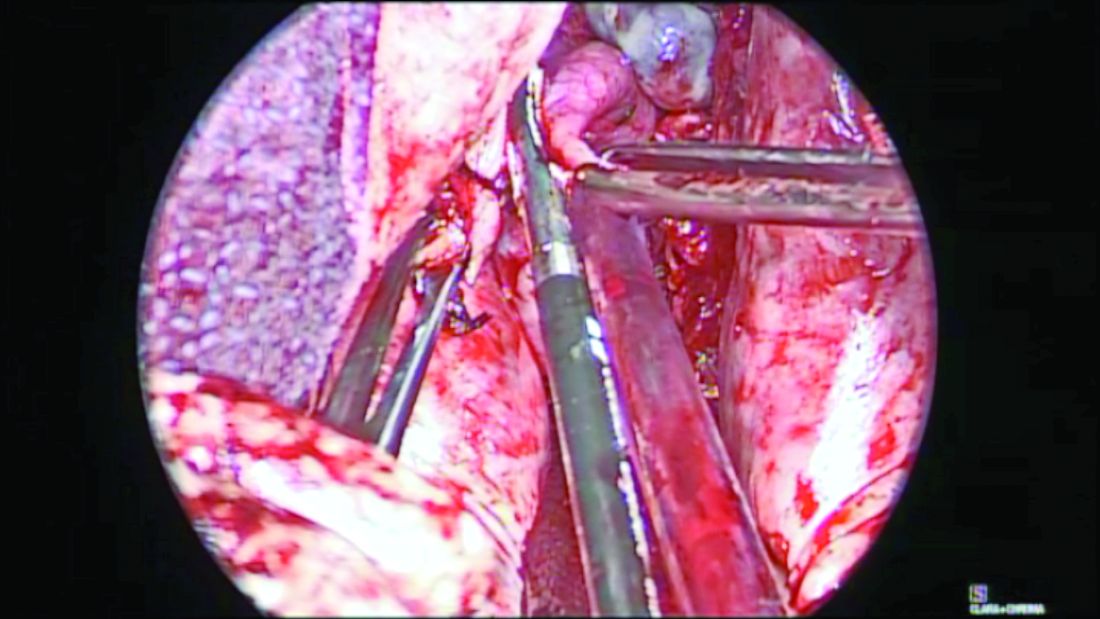
Before completion of the hysterectomy and clamping of the round ligament/fallopian tube/utero-ovarian ligament (RFUO) complex (commonly referred as the “utero-ovarian ligament”), I recommend first identifying the proximal portion of the fallopian tube. The isthmus is sealed and divided from its attachment to the uterine cornua, and a clamp is placed on the remaining round ligament/utero-ovarian ligament complex. The pedicle is then cut and tied. (Figure 1.) After removal of the uterus, the fallopian tube is ready to be grasped with an Allis clamp or Babcock forceps, and the remaining mesosalpinx is sealed and divided all the way to the distal portion/fimbriae.
Round ligament–mesosalpinx technique
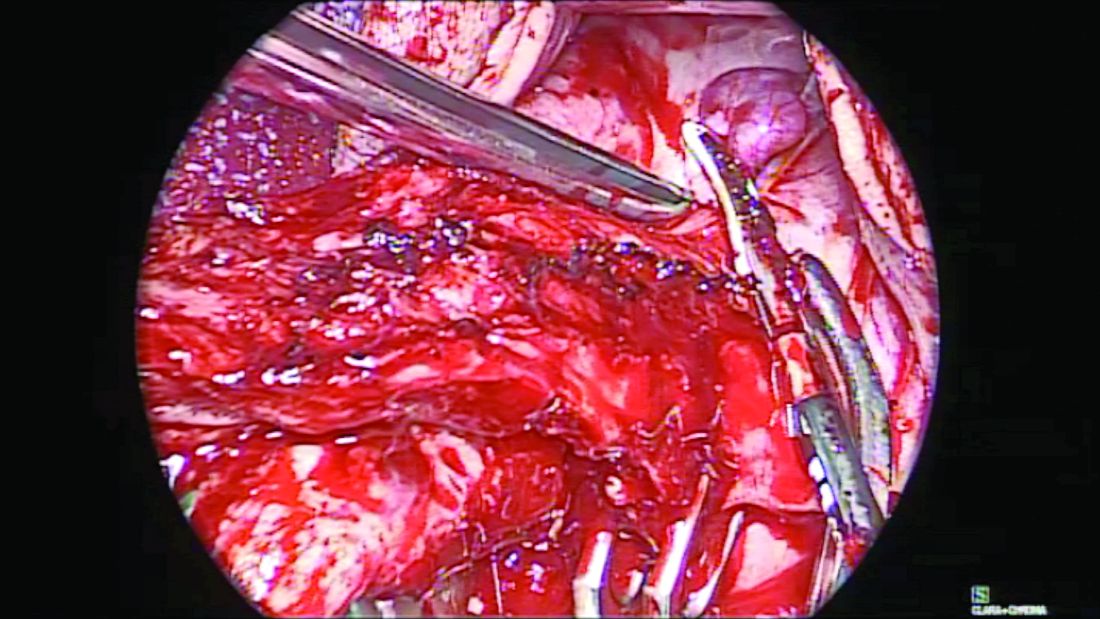
When the uterus is large or lacks prolapse, the fallopian tubes can be difficult to visualize. In such cases, I recommend the use of the round ligament–mesosalpinx technique. After completion of the hysterectomy and ligation of the RFUO complex, a long and moist vaginal pack (I prefer the 4” x 36” cotton vaginal pack by Dukal) is used to push the bowels back and expose the adnexae. The round ligament is identified within the RFUO complex and transected using a monopolar instrument. This step that separates the round ligament from the RFUO complex successfully releases the adnexae from the pelvic sidewall, making it easier to access the fallopian tubes (and the ovaries, when needed). A window is created in the mesosalpinx, and a curved clamp is placed on the ovarian vessels. Using sharp scissors, the proximal portion of the fallopian tube contained within the RFUO complex is separated, and the mesosalpinx is sealed and divided all the way to the distal end using the vessel-sealing device. (Figure 2.)
vNOTES (transvaginal Natural Orifice Translumenal Endoscopic Surgery) salpingectomy technique

When the adnexae is noted to be high in the pelvis or when it is adherent to the pelvic sidewall, I recommend the vNOTES technique. It involves insertion of a mini-gel port into the vaginal opening. (Figure 3.) A 5-mm or 10-mm scope is inserted through this port for visualization. The fallopian tube can be grasped with a laparoscopic grasper and the mesosalpinx sealed and divided using a vessel-sealing device. (Figure 4.) Often, because the bowel is already retracted up with the vaginal pack, insufflation is not necessary with this procedure.

The change in our understanding of the etiology of ovarian cancer calls for salpingectomy during hysterectomy. With such tools, devices, and techniques that facilitate the vaginal removal of the fallopian tubes, the need for prophylactic salpingectomy should not be a deterrent to pursuing a hysterectomy vaginally.
Dr. Kho is head of the section of benign gynecology at the Cleveland Clinic.
References
1. Am J Obstet Gynecol. 2017;217(5):503-4.
2. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
3. Am J Obstet Gynecol. 2019;220:85.e1-10.
4. Am J Obstet Gynecol. 2017;217:605.e1-5.
5. J Minim Invasive Gynecol. 2017 Feb;24(2):218-29.
In the last decade, there has been a major shift in our understanding of the pathogenesis of ovarian cancers. Current literature suggests that many high-grade serous carcinomas develop from the distal aspect of the fallopian tube and that serous tubal intraepithelial carcinoma is likely the precursor. The critical role that the fallopian tubes play as the likely origin of many serous ovarian and pelvic cancers has resulted in a shift from prophylactic salpingo-oophorectomy, which may increase risk for cardiovascular disease, to prophylactic bilateral salpingectomy (PBS) at the time of hysterectomy.
It is important that this shift occur with vaginal hysterectomy (VH) and not only with other surgical approaches. It is known that PBS is performed more commonly during laparoscopic or abdominal hysterectomy, and it’s possible that the need for adnexal surgery may further contribute to the decline in the rate of VH performed in the United States. This is despite evidence that the vaginal approach is preferred for benign hysterectomy even in patients with a nonprolapsed and large fibroid uterus, obesity, or previous pelvic surgery. Current American College of Obstetricians and Gynecologists’ guidelines also state that the need to perform adnexal surgery is not a contraindication to the vaginal approach.
So that more women may attain the benefits and advantages of VH, we need more effective teaching programs for vaginal surgery in residency training programs, hospitals, and community surgical centers. Moreover, we must appreciate that PBS with VH is safe and feasible. There are multiple techniques and tools available to facilitate the successful removal of the tubes, particularly in difficult cases.
The benefit and safety of PBS
Is PBS really effective in decreasing the incidence and mortality of ovarian cancer? A proposed randomized trial in Sweden with a target accrual of 4,400 patients – the Hysterectomy and Opportunistic Salpingectromy Study (HOPPSA, NCT03045965) – will evaluate the risk of ovarian cancer over a 10- to 30-year follow-up period in patients undergoing hysterectomy through all routes. While we wait for these prospective results, an elegant decision-model analysis suggests that routine PBS during VH would eliminate one diagnosis of ovarian cancer for every 225 women undergoing hysterectomy (reducing the risk from 0.956% to 0.511%) and would prevent one death for every 450 women (reducing the risk from 0.478% to 0.256%). The analysis, which drew upon published literature, Medicare reimbursement data, and the National Surgical Quality Improvement Program database, also found that PBS with VH is a less expensive strategy than VH alone because of an increased risk of future adnexal surgery in women retaining their tubes.1

The question of whether PBS places a woman at risk for early menopause is a relevant one. A study following women for 3-5 years after surgery showed that the addition of PBS to total laparoscopic hysterectomy in women of reproductive age does not appear to modify ovarian function.2 However, a recently published retrospective study from the Swedish National Registry showed that women who underwent PBS with abdominal or laparoscopic benign hysterectomy had an increased risk of menopausal symptoms 1 year after surgery.3 Women between the ages of 45-49 years were at highest risk, suggesting increased vulnerability to possible vascular effects of PBS. A longer follow-up period may be necessary to assess younger age groups.
In a multicenter, prospective and observational trial involving 69 patients undergoing VH, PBS was feasible in 75% (a majority of whom [78%] had pelvic organ prolapse) and increased operating time by 11 minutes with no additional complications noted. The surgeons in this study, primarily urogynecologists, utilized a clamp or double-clamp technique to remove the fimbriae.4
The decision-model analysis mentioned above found that PBS would involve slightly more complications than VH alone (7.95% vs. 7.68%),1 and a systematic review that I coauthored of PBS in low-risk women found a small to no increase in operative time and no additional estimated blood loss, hospital stay, or complications for PBS.5
Tools and techniques
Vaginal PBS can be accomplished easily with traditional clamp-cut-tie technique in cases where the fallopian tubes are accessible, such as in patients with uterine prolapse. Generally, most surgeons perform a distal fimbriectomy only for risk-reduction purposes because this is where precursor lesions known as serous tubal intraepithelial cancer (STIC) reside.
To perform a fimbriectomy in cases where the distal portion of the tube is easily accessible, a Kelly clamp is placed across the mesosalpinx, and a fine tie is used for ligature. In more challenging hysterectomy cases, such as in lack of uterine prolapse, large fibroid uterus, morbid obesity, and in patients with previous tubal ligation, the fallopian tubes can be more difficult to access. In these cases, I prefer the use of the vessel-sealing device to seal and divide the mesosalpinx.
Here I describe three specific techniques that can facilitate the removal of the fallopian tubes in more challenging cases. In each technique, the entire fallopian tubes are removed – without leaving behind the proximal stump. The residual stump has the potential of developing into a hydrosalpinx that may necessitate another procedure in the future for the patient.
Separate the fallopian tube before clamping the ‘utero-ovarian ligament’ technique

Before completion of the hysterectomy and clamping of the round ligament/fallopian tube/utero-ovarian ligament (RFUO) complex (commonly referred as the “utero-ovarian ligament”), I recommend first identifying the proximal portion of the fallopian tube. The isthmus is sealed and divided from its attachment to the uterine cornua, and a clamp is placed on the remaining round ligament/utero-ovarian ligament complex. The pedicle is then cut and tied. (Figure 1.) After removal of the uterus, the fallopian tube is ready to be grasped with an Allis clamp or Babcock forceps, and the remaining mesosalpinx is sealed and divided all the way to the distal portion/fimbriae.
Round ligament–mesosalpinx technique

When the uterus is large or lacks prolapse, the fallopian tubes can be difficult to visualize. In such cases, I recommend the use of the round ligament–mesosalpinx technique. After completion of the hysterectomy and ligation of the RFUO complex, a long and moist vaginal pack (I prefer the 4” x 36” cotton vaginal pack by Dukal) is used to push the bowels back and expose the adnexae. The round ligament is identified within the RFUO complex and transected using a monopolar instrument. This step that separates the round ligament from the RFUO complex successfully releases the adnexae from the pelvic sidewall, making it easier to access the fallopian tubes (and the ovaries, when needed). A window is created in the mesosalpinx, and a curved clamp is placed on the ovarian vessels. Using sharp scissors, the proximal portion of the fallopian tube contained within the RFUO complex is separated, and the mesosalpinx is sealed and divided all the way to the distal end using the vessel-sealing device. (Figure 2.)
vNOTES (transvaginal Natural Orifice Translumenal Endoscopic Surgery) salpingectomy technique

When the adnexae is noted to be high in the pelvis or when it is adherent to the pelvic sidewall, I recommend the vNOTES technique. It involves insertion of a mini-gel port into the vaginal opening. (Figure 3.) A 5-mm or 10-mm scope is inserted through this port for visualization. The fallopian tube can be grasped with a laparoscopic grasper and the mesosalpinx sealed and divided using a vessel-sealing device. (Figure 4.) Often, because the bowel is already retracted up with the vaginal pack, insufflation is not necessary with this procedure.

The change in our understanding of the etiology of ovarian cancer calls for salpingectomy during hysterectomy. With such tools, devices, and techniques that facilitate the vaginal removal of the fallopian tubes, the need for prophylactic salpingectomy should not be a deterrent to pursuing a hysterectomy vaginally.
Dr. Kho is head of the section of benign gynecology at the Cleveland Clinic.
References
1. Am J Obstet Gynecol. 2017;217(5):503-4.
2. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
3. Am J Obstet Gynecol. 2019;220:85.e1-10.
4. Am J Obstet Gynecol. 2017;217:605.e1-5.
5. J Minim Invasive Gynecol. 2017 Feb;24(2):218-29.
In the last decade, there has been a major shift in our understanding of the pathogenesis of ovarian cancers. Current literature suggests that many high-grade serous carcinomas develop from the distal aspect of the fallopian tube and that serous tubal intraepithelial carcinoma is likely the precursor. The critical role that the fallopian tubes play as the likely origin of many serous ovarian and pelvic cancers has resulted in a shift from prophylactic salpingo-oophorectomy, which may increase risk for cardiovascular disease, to prophylactic bilateral salpingectomy (PBS) at the time of hysterectomy.
It is important that this shift occur with vaginal hysterectomy (VH) and not only with other surgical approaches. It is known that PBS is performed more commonly during laparoscopic or abdominal hysterectomy, and it’s possible that the need for adnexal surgery may further contribute to the decline in the rate of VH performed in the United States. This is despite evidence that the vaginal approach is preferred for benign hysterectomy even in patients with a nonprolapsed and large fibroid uterus, obesity, or previous pelvic surgery. Current American College of Obstetricians and Gynecologists’ guidelines also state that the need to perform adnexal surgery is not a contraindication to the vaginal approach.
So that more women may attain the benefits and advantages of VH, we need more effective teaching programs for vaginal surgery in residency training programs, hospitals, and community surgical centers. Moreover, we must appreciate that PBS with VH is safe and feasible. There are multiple techniques and tools available to facilitate the successful removal of the tubes, particularly in difficult cases.
The benefit and safety of PBS
Is PBS really effective in decreasing the incidence and mortality of ovarian cancer? A proposed randomized trial in Sweden with a target accrual of 4,400 patients – the Hysterectomy and Opportunistic Salpingectromy Study (HOPPSA, NCT03045965) – will evaluate the risk of ovarian cancer over a 10- to 30-year follow-up period in patients undergoing hysterectomy through all routes. While we wait for these prospective results, an elegant decision-model analysis suggests that routine PBS during VH would eliminate one diagnosis of ovarian cancer for every 225 women undergoing hysterectomy (reducing the risk from 0.956% to 0.511%) and would prevent one death for every 450 women (reducing the risk from 0.478% to 0.256%). The analysis, which drew upon published literature, Medicare reimbursement data, and the National Surgical Quality Improvement Program database, also found that PBS with VH is a less expensive strategy than VH alone because of an increased risk of future adnexal surgery in women retaining their tubes.1

The question of whether PBS places a woman at risk for early menopause is a relevant one. A study following women for 3-5 years after surgery showed that the addition of PBS to total laparoscopic hysterectomy in women of reproductive age does not appear to modify ovarian function.2 However, a recently published retrospective study from the Swedish National Registry showed that women who underwent PBS with abdominal or laparoscopic benign hysterectomy had an increased risk of menopausal symptoms 1 year after surgery.3 Women between the ages of 45-49 years were at highest risk, suggesting increased vulnerability to possible vascular effects of PBS. A longer follow-up period may be necessary to assess younger age groups.
In a multicenter, prospective and observational trial involving 69 patients undergoing VH, PBS was feasible in 75% (a majority of whom [78%] had pelvic organ prolapse) and increased operating time by 11 minutes with no additional complications noted. The surgeons in this study, primarily urogynecologists, utilized a clamp or double-clamp technique to remove the fimbriae.4
The decision-model analysis mentioned above found that PBS would involve slightly more complications than VH alone (7.95% vs. 7.68%),1 and a systematic review that I coauthored of PBS in low-risk women found a small to no increase in operative time and no additional estimated blood loss, hospital stay, or complications for PBS.5
Tools and techniques
Vaginal PBS can be accomplished easily with traditional clamp-cut-tie technique in cases where the fallopian tubes are accessible, such as in patients with uterine prolapse. Generally, most surgeons perform a distal fimbriectomy only for risk-reduction purposes because this is where precursor lesions known as serous tubal intraepithelial cancer (STIC) reside.
To perform a fimbriectomy in cases where the distal portion of the tube is easily accessible, a Kelly clamp is placed across the mesosalpinx, and a fine tie is used for ligature. In more challenging hysterectomy cases, such as in lack of uterine prolapse, large fibroid uterus, morbid obesity, and in patients with previous tubal ligation, the fallopian tubes can be more difficult to access. In these cases, I prefer the use of the vessel-sealing device to seal and divide the mesosalpinx.
Here I describe three specific techniques that can facilitate the removal of the fallopian tubes in more challenging cases. In each technique, the entire fallopian tubes are removed – without leaving behind the proximal stump. The residual stump has the potential of developing into a hydrosalpinx that may necessitate another procedure in the future for the patient.
Separate the fallopian tube before clamping the ‘utero-ovarian ligament’ technique

Before completion of the hysterectomy and clamping of the round ligament/fallopian tube/utero-ovarian ligament (RFUO) complex (commonly referred as the “utero-ovarian ligament”), I recommend first identifying the proximal portion of the fallopian tube. The isthmus is sealed and divided from its attachment to the uterine cornua, and a clamp is placed on the remaining round ligament/utero-ovarian ligament complex. The pedicle is then cut and tied. (Figure 1.) After removal of the uterus, the fallopian tube is ready to be grasped with an Allis clamp or Babcock forceps, and the remaining mesosalpinx is sealed and divided all the way to the distal portion/fimbriae.
Round ligament–mesosalpinx technique

When the uterus is large or lacks prolapse, the fallopian tubes can be difficult to visualize. In such cases, I recommend the use of the round ligament–mesosalpinx technique. After completion of the hysterectomy and ligation of the RFUO complex, a long and moist vaginal pack (I prefer the 4” x 36” cotton vaginal pack by Dukal) is used to push the bowels back and expose the adnexae. The round ligament is identified within the RFUO complex and transected using a monopolar instrument. This step that separates the round ligament from the RFUO complex successfully releases the adnexae from the pelvic sidewall, making it easier to access the fallopian tubes (and the ovaries, when needed). A window is created in the mesosalpinx, and a curved clamp is placed on the ovarian vessels. Using sharp scissors, the proximal portion of the fallopian tube contained within the RFUO complex is separated, and the mesosalpinx is sealed and divided all the way to the distal end using the vessel-sealing device. (Figure 2.)
vNOTES (transvaginal Natural Orifice Translumenal Endoscopic Surgery) salpingectomy technique

When the adnexae is noted to be high in the pelvis or when it is adherent to the pelvic sidewall, I recommend the vNOTES technique. It involves insertion of a mini-gel port into the vaginal opening. (Figure 3.) A 5-mm or 10-mm scope is inserted through this port for visualization. The fallopian tube can be grasped with a laparoscopic grasper and the mesosalpinx sealed and divided using a vessel-sealing device. (Figure 4.) Often, because the bowel is already retracted up with the vaginal pack, insufflation is not necessary with this procedure.

The change in our understanding of the etiology of ovarian cancer calls for salpingectomy during hysterectomy. With such tools, devices, and techniques that facilitate the vaginal removal of the fallopian tubes, the need for prophylactic salpingectomy should not be a deterrent to pursuing a hysterectomy vaginally.
Dr. Kho is head of the section of benign gynecology at the Cleveland Clinic.
References
1. Am J Obstet Gynecol. 2017;217(5):503-4.
2. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
3. Am J Obstet Gynecol. 2019;220:85.e1-10.
4. Am J Obstet Gynecol. 2017;217:605.e1-5.
5. J Minim Invasive Gynecol. 2017 Feb;24(2):218-29.
Management of hypertensive disorders in pregnancy
In the last installment of the Master Class, I addressed the importance of clarity in the classification of hypertensive disorders in pregnancy, and proposed several key diagnostic definitions. Here, I address the management of “mild” gestational hypertension (GHTN) and preeclampsia without severe features, which I believe should be managed similarly. I also address the management of preeclampsia with severe features, and I share an algorithm that I have developed and fine-tuned over the years to control acute severe hypertension with the use of intravenous labetalol, intravenous hydralazine, or oral nifedipine.
Management of “mild” gestational hypertension/Preeclampsia without severe features
Mild gestational hypertension in and of itself has little effect on maternal or perinatal morbidity and mortality when it develops at or beyond 37 weeks’ gestation. However, approximately 40% of patients diagnosed with preterm GHTN will subsequently develop preeclampsia or progress to severe GHTN. In addition, these pregnancies may result in fetal growth restriction and placental abruption.
Antihypertensive drugs should not be used during ambulatory management of women with GHTN. Patients who receive antihypertensive therapy, including those diagnosed with severe GHTN, should be hospitalized and initially treated as having preeclampsia with or without severe features. Subsequent management will depend on initial response to therapy, blood pressure values after treatment, gestational age, and laboratory findings.
Preeclampsia without severe features is usually managed as in those with GHTN. (See related figure.) 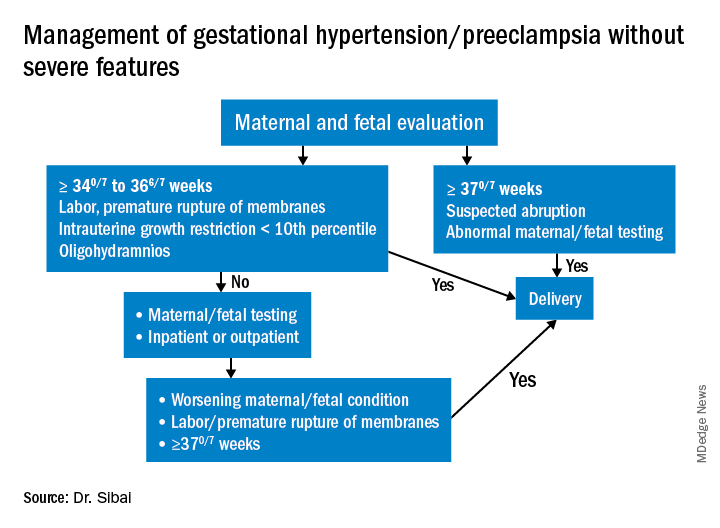
Close surveillance is warranted, as either type may progress to fulminant disease. Maternal surveillance should include blood pressure measurements twice per week, and CBC, liver enzymes, and serum creatinine measurements once every week. Patients also should be instructed to immediately report any of these symptoms: Persistent severe headaches; right upper quadrant or epigastric pain, nausea, and vomiting; scotomata, blurred vision, photophobia, or double vision; shortness of breath or orthopnea; altered mental changes; decreased fetal movement; rupture of membranes; vaginal bleeding; or regular uterine contractions.
Fetal evaluation for patients with GHTN/preeclampsia includes ultrasound at the time of diagnosis for evaluation of fetal growth and amniotic fluid value (deepest vertical pocket, or DVP) as well as fetal movement count and non-stress testing (NST). Subsequently, NST and DVP need to be checked twice per week. A decision for delivery will depend on gestational age, fetal status, and development of severe disease.
Management of preeclampsia with severe features
Any patient who has preeclampsia with severe features should be admitted and initially observed in a labor and delivery unit. (See related figure.) 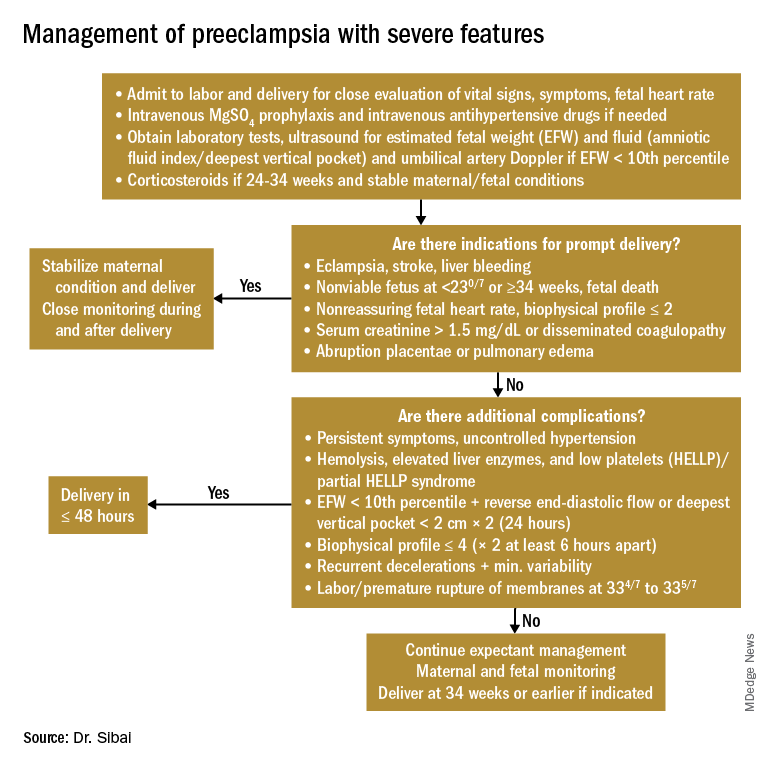
Initial workup should include assessment for fetal well-being, monitoring of maternal blood pressure and symptomatology, and laboratory evaluation. Laboratory assessment should include hematocrit, platelet count, serum creatinine, and aspartate aminotransferase (AST). An ultrasound for fetal growth and amniotic fluid index/DVP also should be obtained. Candidates for expectant management should be carefully selected, counseled regarding its risks and benefits, and managed only at tertiary care hospitals.
Fetal well-being should be assessed on a daily basis by NST and on a weekly basis with amniotic fluid/DVP determination. An ultrasound for fetal growth should be performed every 2-3 weeks. Maternal laboratory evaluation should be done daily or every other day. If the patient maintains a stable maternal and fetal course, she may be expectantly managed until 34 weeks. Worsening maternal or fetal status warrants delivery, regardless of gestational age.
Maternal blood pressure (BP) control is essential with expectant management or during delivery. Medications can be given orally or intravenously, as necessary, to maintain a systolic BP of 140-150 mm Hg and a diastolic BP of 90-100 mm Hg. The most commonly used intravenous medications for this purpose are labetalol and hydralazine. Other medications can include oral rapid-acting nifedipine. Subsequent management can include oral medications such as labetalol and long-acting nifedipine. Care should be taken not to drop the blood pressure too rapidly to avoid reduced renal and placental perfusion.
A trial of labor is indicated in patients with severe preeclampsia if gestational age is greater than 30 weeks and/or if cervical Bishop Score is greater than or equal to 6. However, an appropriate time frame should be established regarding achievement of active labor.
Patients should be closely monitored for at least 24 hours post partum. Post partum eclampsia occurs in 30% of patients; thus, women who are receiving magnesium sulfate should continue it for 24 hours after delivery. In addition, women with preeclampsia who are receiving magnesium sulfate are at risk for postpartum hemorrhage due to uterine atony and should be managed accordingly.
Some patients with severe preeclampsia also are at risk for pulmonary edema and exacerbation of severe hypertension 3-5 days post partum. Therefore, all patients should receive frequent monitoring of intake and output.
Control of acute severe hypertension antepartum, in labor, or post partum
Uncontrolled severe hypertension for several hours may be associated with stroke and pulmonary edema. Therefore, several guidelines recommend initiation of antihypertensive medications for acute lowering of maternal blood pressure within 30-60 minutes. Several antihypertensive agents are available for the control of sustained severe hypertension before, during, and after delivery. It is important to be familiar with the maternal and fetal side effects, as well as mode of action of each agent, to select the best one. Antihypertensive agents can exert an effect by decreasing cardiac output, peripheral vascular resistance, and central blood pressure, or by inhibiting angiotensin production. Indications for therapy and commonly used drugs in pregnancy are listed in the accompanying table.
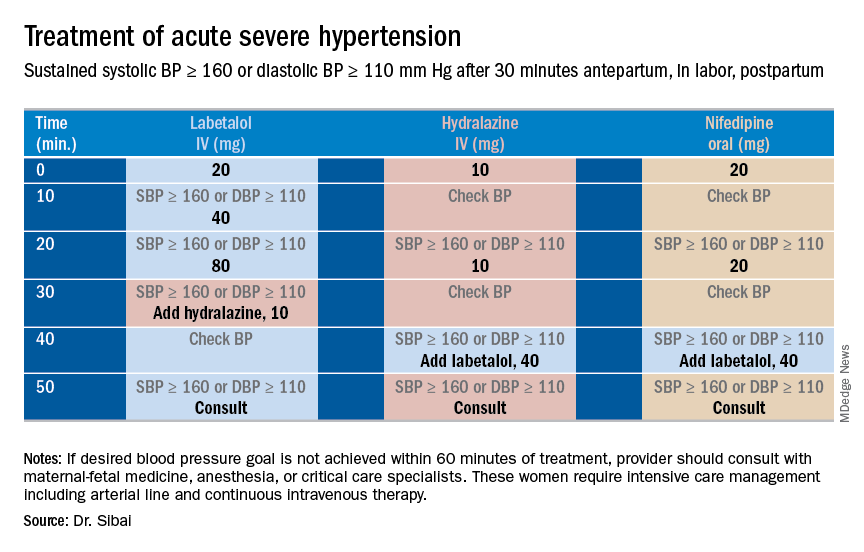
Several trials have compared the efficacy and side effects of intravenous bolus injections of hydralazine to either IV labetalol or oral rapid-acting nifedipine as well as oral nifedipine to IV labetalol. The results of these studies suggest that any of these three medications can be used to treat severe hypertension in pregnancy as long as the physician is familiar with the doses to be used, the expected onset of action, and potential side effects.
Because both hydralazine and nifedipine are associated with tachycardia, it is recommended that these agents not be used in patients with a heart rate above 105-110 beats per minute (bpm). It also is important to be attentive to patients with generalized swelling and/or hemoconcentration (hematocrit great than or equal to 40%), as these patients usually have marked reduction in plasma volume and can develop an excessive hypotensive response, with secondary reduction in tissue perfusion and uteroplacental blood flow, when treated with a combination of rapid-acting vasodilators (hydralazine or nifedipine). Such patients may require a bolus infusion of 250-500 mL of isotonic saline prior to the administration of vasodilators. In these patients, labetalol may be the appropriate drug to use.
Labetalol should be avoided in patients with bradycardia (heart rate less than 60 bpm), in those with moderate to severe asthma, and in those with heart failure. In these patients, either hydralazine or nifedipine is the drug of choice. If an intravenous access is not available or difficult to obtain, oral nifedipine should be the drug of choice. In addition, because nifedipine is associated with improved renal blood flow with resultant increase in urine output, it is the drug of choice for treatment in those with decreased urine output, and for treatment of severe hypertension in the postpartum period.
In a third and final installment, I will elaborate on the postpartum management of women who have experienced hypertension with or without associated symptoms. Recently, postpartum hypertension has become a major cause of hospital readmission, as well as severe maternal morbidity and mortality.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
In the last installment of the Master Class, I addressed the importance of clarity in the classification of hypertensive disorders in pregnancy, and proposed several key diagnostic definitions. Here, I address the management of “mild” gestational hypertension (GHTN) and preeclampsia without severe features, which I believe should be managed similarly. I also address the management of preeclampsia with severe features, and I share an algorithm that I have developed and fine-tuned over the years to control acute severe hypertension with the use of intravenous labetalol, intravenous hydralazine, or oral nifedipine.
Management of “mild” gestational hypertension/Preeclampsia without severe features
Mild gestational hypertension in and of itself has little effect on maternal or perinatal morbidity and mortality when it develops at or beyond 37 weeks’ gestation. However, approximately 40% of patients diagnosed with preterm GHTN will subsequently develop preeclampsia or progress to severe GHTN. In addition, these pregnancies may result in fetal growth restriction and placental abruption.
Antihypertensive drugs should not be used during ambulatory management of women with GHTN. Patients who receive antihypertensive therapy, including those diagnosed with severe GHTN, should be hospitalized and initially treated as having preeclampsia with or without severe features. Subsequent management will depend on initial response to therapy, blood pressure values after treatment, gestational age, and laboratory findings.
Preeclampsia without severe features is usually managed as in those with GHTN. (See related figure.) 
Close surveillance is warranted, as either type may progress to fulminant disease. Maternal surveillance should include blood pressure measurements twice per week, and CBC, liver enzymes, and serum creatinine measurements once every week. Patients also should be instructed to immediately report any of these symptoms: Persistent severe headaches; right upper quadrant or epigastric pain, nausea, and vomiting; scotomata, blurred vision, photophobia, or double vision; shortness of breath or orthopnea; altered mental changes; decreased fetal movement; rupture of membranes; vaginal bleeding; or regular uterine contractions.
Fetal evaluation for patients with GHTN/preeclampsia includes ultrasound at the time of diagnosis for evaluation of fetal growth and amniotic fluid value (deepest vertical pocket, or DVP) as well as fetal movement count and non-stress testing (NST). Subsequently, NST and DVP need to be checked twice per week. A decision for delivery will depend on gestational age, fetal status, and development of severe disease.
Management of preeclampsia with severe features
Any patient who has preeclampsia with severe features should be admitted and initially observed in a labor and delivery unit. (See related figure.) 
Initial workup should include assessment for fetal well-being, monitoring of maternal blood pressure and symptomatology, and laboratory evaluation. Laboratory assessment should include hematocrit, platelet count, serum creatinine, and aspartate aminotransferase (AST). An ultrasound for fetal growth and amniotic fluid index/DVP also should be obtained. Candidates for expectant management should be carefully selected, counseled regarding its risks and benefits, and managed only at tertiary care hospitals.
Fetal well-being should be assessed on a daily basis by NST and on a weekly basis with amniotic fluid/DVP determination. An ultrasound for fetal growth should be performed every 2-3 weeks. Maternal laboratory evaluation should be done daily or every other day. If the patient maintains a stable maternal and fetal course, she may be expectantly managed until 34 weeks. Worsening maternal or fetal status warrants delivery, regardless of gestational age.
Maternal blood pressure (BP) control is essential with expectant management or during delivery. Medications can be given orally or intravenously, as necessary, to maintain a systolic BP of 140-150 mm Hg and a diastolic BP of 90-100 mm Hg. The most commonly used intravenous medications for this purpose are labetalol and hydralazine. Other medications can include oral rapid-acting nifedipine. Subsequent management can include oral medications such as labetalol and long-acting nifedipine. Care should be taken not to drop the blood pressure too rapidly to avoid reduced renal and placental perfusion.
A trial of labor is indicated in patients with severe preeclampsia if gestational age is greater than 30 weeks and/or if cervical Bishop Score is greater than or equal to 6. However, an appropriate time frame should be established regarding achievement of active labor.
Patients should be closely monitored for at least 24 hours post partum. Post partum eclampsia occurs in 30% of patients; thus, women who are receiving magnesium sulfate should continue it for 24 hours after delivery. In addition, women with preeclampsia who are receiving magnesium sulfate are at risk for postpartum hemorrhage due to uterine atony and should be managed accordingly.
Some patients with severe preeclampsia also are at risk for pulmonary edema and exacerbation of severe hypertension 3-5 days post partum. Therefore, all patients should receive frequent monitoring of intake and output.
Control of acute severe hypertension antepartum, in labor, or post partum
Uncontrolled severe hypertension for several hours may be associated with stroke and pulmonary edema. Therefore, several guidelines recommend initiation of antihypertensive medications for acute lowering of maternal blood pressure within 30-60 minutes. Several antihypertensive agents are available for the control of sustained severe hypertension before, during, and after delivery. It is important to be familiar with the maternal and fetal side effects, as well as mode of action of each agent, to select the best one. Antihypertensive agents can exert an effect by decreasing cardiac output, peripheral vascular resistance, and central blood pressure, or by inhibiting angiotensin production. Indications for therapy and commonly used drugs in pregnancy are listed in the accompanying table.

Several trials have compared the efficacy and side effects of intravenous bolus injections of hydralazine to either IV labetalol or oral rapid-acting nifedipine as well as oral nifedipine to IV labetalol. The results of these studies suggest that any of these three medications can be used to treat severe hypertension in pregnancy as long as the physician is familiar with the doses to be used, the expected onset of action, and potential side effects.
Because both hydralazine and nifedipine are associated with tachycardia, it is recommended that these agents not be used in patients with a heart rate above 105-110 beats per minute (bpm). It also is important to be attentive to patients with generalized swelling and/or hemoconcentration (hematocrit great than or equal to 40%), as these patients usually have marked reduction in plasma volume and can develop an excessive hypotensive response, with secondary reduction in tissue perfusion and uteroplacental blood flow, when treated with a combination of rapid-acting vasodilators (hydralazine or nifedipine). Such patients may require a bolus infusion of 250-500 mL of isotonic saline prior to the administration of vasodilators. In these patients, labetalol may be the appropriate drug to use.
Labetalol should be avoided in patients with bradycardia (heart rate less than 60 bpm), in those with moderate to severe asthma, and in those with heart failure. In these patients, either hydralazine or nifedipine is the drug of choice. If an intravenous access is not available or difficult to obtain, oral nifedipine should be the drug of choice. In addition, because nifedipine is associated with improved renal blood flow with resultant increase in urine output, it is the drug of choice for treatment in those with decreased urine output, and for treatment of severe hypertension in the postpartum period.
In a third and final installment, I will elaborate on the postpartum management of women who have experienced hypertension with or without associated symptoms. Recently, postpartum hypertension has become a major cause of hospital readmission, as well as severe maternal morbidity and mortality.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
In the last installment of the Master Class, I addressed the importance of clarity in the classification of hypertensive disorders in pregnancy, and proposed several key diagnostic definitions. Here, I address the management of “mild” gestational hypertension (GHTN) and preeclampsia without severe features, which I believe should be managed similarly. I also address the management of preeclampsia with severe features, and I share an algorithm that I have developed and fine-tuned over the years to control acute severe hypertension with the use of intravenous labetalol, intravenous hydralazine, or oral nifedipine.
Management of “mild” gestational hypertension/Preeclampsia without severe features
Mild gestational hypertension in and of itself has little effect on maternal or perinatal morbidity and mortality when it develops at or beyond 37 weeks’ gestation. However, approximately 40% of patients diagnosed with preterm GHTN will subsequently develop preeclampsia or progress to severe GHTN. In addition, these pregnancies may result in fetal growth restriction and placental abruption.
Antihypertensive drugs should not be used during ambulatory management of women with GHTN. Patients who receive antihypertensive therapy, including those diagnosed with severe GHTN, should be hospitalized and initially treated as having preeclampsia with or without severe features. Subsequent management will depend on initial response to therapy, blood pressure values after treatment, gestational age, and laboratory findings.
Preeclampsia without severe features is usually managed as in those with GHTN. (See related figure.) 
Close surveillance is warranted, as either type may progress to fulminant disease. Maternal surveillance should include blood pressure measurements twice per week, and CBC, liver enzymes, and serum creatinine measurements once every week. Patients also should be instructed to immediately report any of these symptoms: Persistent severe headaches; right upper quadrant or epigastric pain, nausea, and vomiting; scotomata, blurred vision, photophobia, or double vision; shortness of breath or orthopnea; altered mental changes; decreased fetal movement; rupture of membranes; vaginal bleeding; or regular uterine contractions.
Fetal evaluation for patients with GHTN/preeclampsia includes ultrasound at the time of diagnosis for evaluation of fetal growth and amniotic fluid value (deepest vertical pocket, or DVP) as well as fetal movement count and non-stress testing (NST). Subsequently, NST and DVP need to be checked twice per week. A decision for delivery will depend on gestational age, fetal status, and development of severe disease.
Management of preeclampsia with severe features
Any patient who has preeclampsia with severe features should be admitted and initially observed in a labor and delivery unit. (See related figure.) 
Initial workup should include assessment for fetal well-being, monitoring of maternal blood pressure and symptomatology, and laboratory evaluation. Laboratory assessment should include hematocrit, platelet count, serum creatinine, and aspartate aminotransferase (AST). An ultrasound for fetal growth and amniotic fluid index/DVP also should be obtained. Candidates for expectant management should be carefully selected, counseled regarding its risks and benefits, and managed only at tertiary care hospitals.
Fetal well-being should be assessed on a daily basis by NST and on a weekly basis with amniotic fluid/DVP determination. An ultrasound for fetal growth should be performed every 2-3 weeks. Maternal laboratory evaluation should be done daily or every other day. If the patient maintains a stable maternal and fetal course, she may be expectantly managed until 34 weeks. Worsening maternal or fetal status warrants delivery, regardless of gestational age.
Maternal blood pressure (BP) control is essential with expectant management or during delivery. Medications can be given orally or intravenously, as necessary, to maintain a systolic BP of 140-150 mm Hg and a diastolic BP of 90-100 mm Hg. The most commonly used intravenous medications for this purpose are labetalol and hydralazine. Other medications can include oral rapid-acting nifedipine. Subsequent management can include oral medications such as labetalol and long-acting nifedipine. Care should be taken not to drop the blood pressure too rapidly to avoid reduced renal and placental perfusion.
A trial of labor is indicated in patients with severe preeclampsia if gestational age is greater than 30 weeks and/or if cervical Bishop Score is greater than or equal to 6. However, an appropriate time frame should be established regarding achievement of active labor.
Patients should be closely monitored for at least 24 hours post partum. Post partum eclampsia occurs in 30% of patients; thus, women who are receiving magnesium sulfate should continue it for 24 hours after delivery. In addition, women with preeclampsia who are receiving magnesium sulfate are at risk for postpartum hemorrhage due to uterine atony and should be managed accordingly.
Some patients with severe preeclampsia also are at risk for pulmonary edema and exacerbation of severe hypertension 3-5 days post partum. Therefore, all patients should receive frequent monitoring of intake and output.
Control of acute severe hypertension antepartum, in labor, or post partum
Uncontrolled severe hypertension for several hours may be associated with stroke and pulmonary edema. Therefore, several guidelines recommend initiation of antihypertensive medications for acute lowering of maternal blood pressure within 30-60 minutes. Several antihypertensive agents are available for the control of sustained severe hypertension before, during, and after delivery. It is important to be familiar with the maternal and fetal side effects, as well as mode of action of each agent, to select the best one. Antihypertensive agents can exert an effect by decreasing cardiac output, peripheral vascular resistance, and central blood pressure, or by inhibiting angiotensin production. Indications for therapy and commonly used drugs in pregnancy are listed in the accompanying table.

Several trials have compared the efficacy and side effects of intravenous bolus injections of hydralazine to either IV labetalol or oral rapid-acting nifedipine as well as oral nifedipine to IV labetalol. The results of these studies suggest that any of these three medications can be used to treat severe hypertension in pregnancy as long as the physician is familiar with the doses to be used, the expected onset of action, and potential side effects.
Because both hydralazine and nifedipine are associated with tachycardia, it is recommended that these agents not be used in patients with a heart rate above 105-110 beats per minute (bpm). It also is important to be attentive to patients with generalized swelling and/or hemoconcentration (hematocrit great than or equal to 40%), as these patients usually have marked reduction in plasma volume and can develop an excessive hypotensive response, with secondary reduction in tissue perfusion and uteroplacental blood flow, when treated with a combination of rapid-acting vasodilators (hydralazine or nifedipine). Such patients may require a bolus infusion of 250-500 mL of isotonic saline prior to the administration of vasodilators. In these patients, labetalol may be the appropriate drug to use.
Labetalol should be avoided in patients with bradycardia (heart rate less than 60 bpm), in those with moderate to severe asthma, and in those with heart failure. In these patients, either hydralazine or nifedipine is the drug of choice. If an intravenous access is not available or difficult to obtain, oral nifedipine should be the drug of choice. In addition, because nifedipine is associated with improved renal blood flow with resultant increase in urine output, it is the drug of choice for treatment in those with decreased urine output, and for treatment of severe hypertension in the postpartum period.
In a third and final installment, I will elaborate on the postpartum management of women who have experienced hypertension with or without associated symptoms. Recently, postpartum hypertension has become a major cause of hospital readmission, as well as severe maternal morbidity and mortality.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Treating preeclampsia
Preeclampsia is such a complicated and insidious disease – and one with such serious implications for the fetus, the infant at birth, and the mother – that we decided to run a three-part series on its diagnosis and management. The complication can have an acute onset in many patients, and this acute onset may rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for obstetricians to be able to affect the health and well-being of both the mother and fetus.
I have invited Dr. Baha M. Sibai, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to deliver this series. Our first installment addressed diagnostic criteria and attempted to clarify confusion that may have been introduced with the 2013 publication of the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy. It is important that the diagnostic criteria are well established and understood because the management of patients is very much based on accurate placement within these diagnostic criteria.
This second installment of our series focuses on the application of appropriate therapeutic measures for various diagnostic groups. Dr. Sibai has spent decades studying hypertensive disorders in pregnancy and developing practical clinical strategies for management. It is our hope that the guidance and algorithms presented here will be useful for improving patient care and outcomes of this serious obstetrical syndrome. A third installment on postpartum management will come later.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
Preeclampsia is such a complicated and insidious disease – and one with such serious implications for the fetus, the infant at birth, and the mother – that we decided to run a three-part series on its diagnosis and management. The complication can have an acute onset in many patients, and this acute onset may rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for obstetricians to be able to affect the health and well-being of both the mother and fetus.
I have invited Dr. Baha M. Sibai, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to deliver this series. Our first installment addressed diagnostic criteria and attempted to clarify confusion that may have been introduced with the 2013 publication of the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy. It is important that the diagnostic criteria are well established and understood because the management of patients is very much based on accurate placement within these diagnostic criteria.
This second installment of our series focuses on the application of appropriate therapeutic measures for various diagnostic groups. Dr. Sibai has spent decades studying hypertensive disorders in pregnancy and developing practical clinical strategies for management. It is our hope that the guidance and algorithms presented here will be useful for improving patient care and outcomes of this serious obstetrical syndrome. A third installment on postpartum management will come later.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
Preeclampsia is such a complicated and insidious disease – and one with such serious implications for the fetus, the infant at birth, and the mother – that we decided to run a three-part series on its diagnosis and management. The complication can have an acute onset in many patients, and this acute onset may rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for obstetricians to be able to affect the health and well-being of both the mother and fetus.
I have invited Dr. Baha M. Sibai, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to deliver this series. Our first installment addressed diagnostic criteria and attempted to clarify confusion that may have been introduced with the 2013 publication of the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy. It is important that the diagnostic criteria are well established and understood because the management of patients is very much based on accurate placement within these diagnostic criteria.
This second installment of our series focuses on the application of appropriate therapeutic measures for various diagnostic groups. Dr. Sibai has spent decades studying hypertensive disorders in pregnancy and developing practical clinical strategies for management. It is our hope that the guidance and algorithms presented here will be useful for improving patient care and outcomes of this serious obstetrical syndrome. A third installment on postpartum management will come later.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
Understanding hypertensive disorders in pregnancy
Preeclampsia is one of the most significant medical complications in pregnancy because of the acute onset it can have in so many affected patients. This acute onset may then rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth.
It is an obstetrical syndrome with serious implications for the fetus, the infant at birth, and the mother, and it is one whose incidence has been increasing. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for the health and well-being of both the mother and fetus.
A new classification system introduced in 2013 by the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy has added further complexity to an already complicated disease. On one hand, attempting to precisely achieve a diagnosis with such an imprecise and insidious disease seems ill advised. On the other hand, it is important to achieve some level of clarity with respect to diagnosis and management. In doing so, we must lean toward overdiagnosis and maintain a low threshold for treatment and intervention in the interest of the mother and infant.
I have engaged Baha M. Sibai, MD, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to introduce a practical approach for interpreting and utilizing the ACOG report. This installment is the first of a two-part series in which we hope to provide practical clinical strategies for this complex disease.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@MDedge.com.
Preeclampsia is one of the most significant medical complications in pregnancy because of the acute onset it can have in so many affected patients. This acute onset may then rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth.
It is an obstetrical syndrome with serious implications for the fetus, the infant at birth, and the mother, and it is one whose incidence has been increasing. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for the health and well-being of both the mother and fetus.
A new classification system introduced in 2013 by the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy has added further complexity to an already complicated disease. On one hand, attempting to precisely achieve a diagnosis with such an imprecise and insidious disease seems ill advised. On the other hand, it is important to achieve some level of clarity with respect to diagnosis and management. In doing so, we must lean toward overdiagnosis and maintain a low threshold for treatment and intervention in the interest of the mother and infant.
I have engaged Baha M. Sibai, MD, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to introduce a practical approach for interpreting and utilizing the ACOG report. This installment is the first of a two-part series in which we hope to provide practical clinical strategies for this complex disease.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@MDedge.com.
Preeclampsia is one of the most significant medical complications in pregnancy because of the acute onset it can have in so many affected patients. This acute onset may then rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth.
It is an obstetrical syndrome with serious implications for the fetus, the infant at birth, and the mother, and it is one whose incidence has been increasing. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for the health and well-being of both the mother and fetus.
A new classification system introduced in 2013 by the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy has added further complexity to an already complicated disease. On one hand, attempting to precisely achieve a diagnosis with such an imprecise and insidious disease seems ill advised. On the other hand, it is important to achieve some level of clarity with respect to diagnosis and management. In doing so, we must lean toward overdiagnosis and maintain a low threshold for treatment and intervention in the interest of the mother and infant.
I have engaged Baha M. Sibai, MD, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to introduce a practical approach for interpreting and utilizing the ACOG report. This installment is the first of a two-part series in which we hope to provide practical clinical strategies for this complex disease.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@MDedge.com.
Clarifying the categories of hypertensive disorders in pregnancy
Prenatal care always has been in part about identifying women with medical complications including preeclampsia. We have long measured blood pressure, checked the urine for high levels of protein, and monitored weight gain. We still do.
However, over the years, the diagnostic criteria for preeclampsia have evolved, first with the exclusion of edema and more recently with the exclusion of proteinuria as a necessary element of the diagnosis. The American College of Obstetricians and Gynecologists’ Task Force Report, Hypertension in Pregnancy, published in 2013, concluded that while preeclampsia may still be defined by the occurrence of hypertension with proteinuria, it also may be diagnosed when hypertension occurs in association with other multisystemic signs indicative of disease severity. The change came based on evidence that some women develop eclampsia, HELLP syndrome, and other serious complications in the absence of proteinuria.
The 2013 document also attempted to review and clarify various issues relating to the classifications, diagnosis, prediction and prevention, and management of hypertension during pregnancy, including the postpartum period. In many respects, it was successful in doing so. However, there is still much confusion regarding the diagnosis of certain categories of hypertensive disorders – particularly preeclampsia with severe features and superimposed preeclampsia with or without severe features.
While it is difficult to establish precise definitions given the often insidious nature of preeclampsia, it still is important to achieve a higher level of clarity with respect to these categories. Overdiagnosis may be preferable. However, improper classification also may influence management decisions that could prove detrimental to the fetus.
Severe gestational hypertension
ACOG’s 2013 Report on Hypertension in Pregnancy classifies hypertensive disorders of pregnancy into these categories: Gestational hypertension (GHTN), preeclampsia, preeclampsia with severe features (this includes HELLP), chronic hypertension (CHTN), superimposed preeclampsia with or without severe features, and eclampsia.
Some of the definitions and diagnostic criteria are clear. For instance, GHTN is defined as the new onset of hypertension after 20 weeks’ gestation in the absence of proteinuria or systemic findings such as thrombocytopenia or impaired liver function. CHTN is defined as hypertension that predates conception or is detected before 20 weeks’ gestation. In both cases there should be elevated blood pressure on two occasions at least 4 hours apart.
A major omission is the lack of a definition for severe GHTN. Removal of this previously well-understood classification category combined with unclear statements regarding preeclampsia with or without severe features has made it difficult for physicians to know in some cases of severe hypertension only what diagnosis a woman should receive and how she should be managed.
I recommend that we maintain the category of severe GHTN, and that it be defined as a systolic blood pressure (BP) greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions at least 4 hours apart when antihypertensive medications have not been initiated. There should be no proteinuria or severe features such as thrombocytopenia or impaired liver function.
The physician may elect in these cases to administer antihypertensive medication and observe the patient in the hospital. An individualized decision can then be made regarding how the patient should be managed, including whether she should be admitted and whether the pregnancy should continue beyond 34 weeks. Blood pressure, gestational age at diagnosis, the presence or absence of symptoms, and laboratory tests all should be taken into consideration.
Preeclampsia with or without severe features
We need to clarify and simplify how we think about GHTN and preeclampsia with or without severe features.
Most cases of preeclampsia will involve new-onset proteinuria, with proteinuria being defined as greater than or equal to 300 mg/day or a protein-creatinine ratio of greater than or equal to 0.3 mg/dL. In cases in which a dipstick test must be used, proteinuria is suggested by a urine protein reading of 1+. (It is important to note that dipstick readings should be taken on two separate occasions.) According to the report, preeclampsia also may be established by the presence of GHTN in association with any one of a list of features that are generally referred to as “severe features.”
Various boxes and textual descriptions in the report offer a sometimes confusing picture, however, of the terms preeclampsia and preeclampsia with severe features and their differences. For clarification, I recommend that we define preeclampsia with severe features as GHTN (mild or severe) in association with any one of the severe features.
Severe features of preeclampsia
- Platelet count less than 100,000/microliter.
- Elevated hepatic transaminases greater than two times the upper limit of normal for specific laboratory adult reference ranges.
- Severe persistent right upper quadrant abdominal pain or epigastric pain unresponsive to analgesics and unexplained by other etiology.
- Serum creatinine greater than 1.1 mg/dL.
- Pulmonary edema.
- Persistent cerebral disturbances such as severe persistent new-onset headaches unresponsive to nonnarcotic analgesics, altered mental status or other neurologic deficits.
- Visual disturbances such as blurred vision, scotomata, photophobia, or loss of vision.
I also suggest that we think of “mild” GHTN (systolic BP of 140-159 mm Hg or diastolic BP 90-109 mm Hg) and preeclampsia without severe features as one in the same, and that we manage them similarly. The presence or absence of proteinuria is currently the only difference diagnostically. The only difference with respect to management – aside from a weekly urine protein check in the case of GHTN – is the frequency of nonstress testing (NST) and amniotic fluid index (AFI) measurement (currently once a week for GHTN and twice a week for preeclampsia).
Given that unnecessary time and energy may be spent differentiating the two when management is essentially the same, I suggest that preeclampsia be diagnosed in any patient with GHTN with or without proteinuria. All patients can then be managed with blood pressure checks twice a week; symptoms and kick count daily; NST and AFI twice a week; estimated fetal weight by ultrasound every third week; lab tests (CBC, liver enzymes, and creatinine) once a week, and delivery at 37 weeks.
Superimposed preeclampsia with or without severe features
As the report states, the recognition of preeclampsia superimposed on chronic hypertension is “perhaps the greatest challenge” in the diagnosis and management of hypertensive disorders in pregnancy. Overdiagnosis “may be preferable,” the report says, given the high risk of adverse pregnancy outcomes with superimposed preeclampsia. On the other hand, it says, a “more stratified approach based on severity and predictors of adverse outcome may be useful” in avoiding unnecessary preterm births.
Ultimately, the task force proposed that we utilize the two categories of “superimposed preeclampsia” and “superimposed preeclampsia with severe features,” and in doing so, it noted that there “often is ambiguity in the diagnosis of superimposed preeclampsia and that the clinical spectrum of disease is broad.” Indeed, the diagnosis of superimposed preeclampsia as presented in the report remains vague and open to interpretation. In my institution, it has created significant confusion.
The report states that superimposed preeclampsia is likely when any of the following are present: 1) a sudden increase in blood pressure that was previously well controlled or escalation of antihypertensive medications to control blood pressure, or 2) new onset of proteinuria or a sudden increase in proteinuria in a woman with known proteinuria before or early in pregnancy.
It is not clear, however, what is considered a sudden increase in blood pressure, and it is concerning that any escalation of medication could potentially prompt this diagnosis. Is an increase in systolic blood pressure from 140 mm Hg to 150 mm Hg or an increase in diastolic blood pressure from 90 mm Hg to 100 mm Hg between two prenatal visits considered a “sudden increase”? Does an increase in methyldopa dosage from 250 mg daily to 500 mg daily to keep blood pressure within the range of mild hypertension mean that the patient should be diagnosed with superimposed preeclampsia? Hypertension is likely to increase and require an escalation of antihypertensive medications as patients with chronic hypertension progress through their pregnancies.
Similarly, a “sudden increase in proteinuria” – or “sudden, substantial, and sustained increases in protein excretion,” as written elsewhere in the report with respect to superimposed preeclampsia – also is undefined. What exactly does this mean? That we lack clinically meaningful parameters and clear descriptions of acceptable criteria/scenarios for observation rather than intervention is troubling, particularly because some of these women may have preexisting renal disease with expected increases and fluctuations in protein excretion during advanced gestation.
We must be cautious about making a diagnosis of superimposed preeclampsia based on changes in blood pressure or urinary protein alone, lest we have unnecessary hospitalizations and interventions. I recommend that the diagnosis of superimposed preeclampsia be made based on either the new onset of proteinuria in association with mild hypertension after 20 weeks or on elevation in blood pressure to severe ranges (systolic BP greater than or equal to160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of maximum doses of one antihypertensive drug.
Regarding superimposed preeclampsia with severe features, I recommend that in the case of blood pressure elevation, it be diagnosed only after maximal doses of two medications have been used. Specifically, I recommend that superimposed preeclampsia with severe features be defined as either CHTN or superimposed preeclampsia in association with either systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions despite use of maximum doses of labetalol (2,400 mg/day) plus long-acting nifedipine (120 mg/day), or with any of the other severe features.
In a second installment of the Master Class, I will elaborate on the treatment of severe GHTN and address the management of preeclampsia with severe features as well as postpartum management of hypertension during pregnancy.
Suggested diagnostic definitions
- Preeclampsia with severe features: GHTN in association with severe features.
- Superimposed preeclampsia: CHTN with either the new onset of proteinuria in association with mild hypertension after 20 weeks, or an elevation in blood pressure to severe ranges (systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of the maximal dose of one antihypertensive drug.
- Superimposed preeclampsia with severe features: CHTN or superimposed preeclampsia with severe features or with a rise in blood pressure to severe ranges despite the maximal doses of two antihypertensive drugs (e.g. 2,400 mg/day labetalol plus 120 mg/day long-acting nifedipine).
Note: These definitions reflect adaptations and clarifications of ACOG’s 2013 Task Force Report on Hypertension in Pregnancy.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Prenatal care always has been in part about identifying women with medical complications including preeclampsia. We have long measured blood pressure, checked the urine for high levels of protein, and monitored weight gain. We still do.
However, over the years, the diagnostic criteria for preeclampsia have evolved, first with the exclusion of edema and more recently with the exclusion of proteinuria as a necessary element of the diagnosis. The American College of Obstetricians and Gynecologists’ Task Force Report, Hypertension in Pregnancy, published in 2013, concluded that while preeclampsia may still be defined by the occurrence of hypertension with proteinuria, it also may be diagnosed when hypertension occurs in association with other multisystemic signs indicative of disease severity. The change came based on evidence that some women develop eclampsia, HELLP syndrome, and other serious complications in the absence of proteinuria.
The 2013 document also attempted to review and clarify various issues relating to the classifications, diagnosis, prediction and prevention, and management of hypertension during pregnancy, including the postpartum period. In many respects, it was successful in doing so. However, there is still much confusion regarding the diagnosis of certain categories of hypertensive disorders – particularly preeclampsia with severe features and superimposed preeclampsia with or without severe features.
While it is difficult to establish precise definitions given the often insidious nature of preeclampsia, it still is important to achieve a higher level of clarity with respect to these categories. Overdiagnosis may be preferable. However, improper classification also may influence management decisions that could prove detrimental to the fetus.
Severe gestational hypertension
ACOG’s 2013 Report on Hypertension in Pregnancy classifies hypertensive disorders of pregnancy into these categories: Gestational hypertension (GHTN), preeclampsia, preeclampsia with severe features (this includes HELLP), chronic hypertension (CHTN), superimposed preeclampsia with or without severe features, and eclampsia.
Some of the definitions and diagnostic criteria are clear. For instance, GHTN is defined as the new onset of hypertension after 20 weeks’ gestation in the absence of proteinuria or systemic findings such as thrombocytopenia or impaired liver function. CHTN is defined as hypertension that predates conception or is detected before 20 weeks’ gestation. In both cases there should be elevated blood pressure on two occasions at least 4 hours apart.
A major omission is the lack of a definition for severe GHTN. Removal of this previously well-understood classification category combined with unclear statements regarding preeclampsia with or without severe features has made it difficult for physicians to know in some cases of severe hypertension only what diagnosis a woman should receive and how she should be managed.
I recommend that we maintain the category of severe GHTN, and that it be defined as a systolic blood pressure (BP) greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions at least 4 hours apart when antihypertensive medications have not been initiated. There should be no proteinuria or severe features such as thrombocytopenia or impaired liver function.
The physician may elect in these cases to administer antihypertensive medication and observe the patient in the hospital. An individualized decision can then be made regarding how the patient should be managed, including whether she should be admitted and whether the pregnancy should continue beyond 34 weeks. Blood pressure, gestational age at diagnosis, the presence or absence of symptoms, and laboratory tests all should be taken into consideration.
Preeclampsia with or without severe features
We need to clarify and simplify how we think about GHTN and preeclampsia with or without severe features.
Most cases of preeclampsia will involve new-onset proteinuria, with proteinuria being defined as greater than or equal to 300 mg/day or a protein-creatinine ratio of greater than or equal to 0.3 mg/dL. In cases in which a dipstick test must be used, proteinuria is suggested by a urine protein reading of 1+. (It is important to note that dipstick readings should be taken on two separate occasions.) According to the report, preeclampsia also may be established by the presence of GHTN in association with any one of a list of features that are generally referred to as “severe features.”
Various boxes and textual descriptions in the report offer a sometimes confusing picture, however, of the terms preeclampsia and preeclampsia with severe features and their differences. For clarification, I recommend that we define preeclampsia with severe features as GHTN (mild or severe) in association with any one of the severe features.
Severe features of preeclampsia
- Platelet count less than 100,000/microliter.
- Elevated hepatic transaminases greater than two times the upper limit of normal for specific laboratory adult reference ranges.
- Severe persistent right upper quadrant abdominal pain or epigastric pain unresponsive to analgesics and unexplained by other etiology.
- Serum creatinine greater than 1.1 mg/dL.
- Pulmonary edema.
- Persistent cerebral disturbances such as severe persistent new-onset headaches unresponsive to nonnarcotic analgesics, altered mental status or other neurologic deficits.
- Visual disturbances such as blurred vision, scotomata, photophobia, or loss of vision.
I also suggest that we think of “mild” GHTN (systolic BP of 140-159 mm Hg or diastolic BP 90-109 mm Hg) and preeclampsia without severe features as one in the same, and that we manage them similarly. The presence or absence of proteinuria is currently the only difference diagnostically. The only difference with respect to management – aside from a weekly urine protein check in the case of GHTN – is the frequency of nonstress testing (NST) and amniotic fluid index (AFI) measurement (currently once a week for GHTN and twice a week for preeclampsia).
Given that unnecessary time and energy may be spent differentiating the two when management is essentially the same, I suggest that preeclampsia be diagnosed in any patient with GHTN with or without proteinuria. All patients can then be managed with blood pressure checks twice a week; symptoms and kick count daily; NST and AFI twice a week; estimated fetal weight by ultrasound every third week; lab tests (CBC, liver enzymes, and creatinine) once a week, and delivery at 37 weeks.
Superimposed preeclampsia with or without severe features
As the report states, the recognition of preeclampsia superimposed on chronic hypertension is “perhaps the greatest challenge” in the diagnosis and management of hypertensive disorders in pregnancy. Overdiagnosis “may be preferable,” the report says, given the high risk of adverse pregnancy outcomes with superimposed preeclampsia. On the other hand, it says, a “more stratified approach based on severity and predictors of adverse outcome may be useful” in avoiding unnecessary preterm births.
Ultimately, the task force proposed that we utilize the two categories of “superimposed preeclampsia” and “superimposed preeclampsia with severe features,” and in doing so, it noted that there “often is ambiguity in the diagnosis of superimposed preeclampsia and that the clinical spectrum of disease is broad.” Indeed, the diagnosis of superimposed preeclampsia as presented in the report remains vague and open to interpretation. In my institution, it has created significant confusion.
The report states that superimposed preeclampsia is likely when any of the following are present: 1) a sudden increase in blood pressure that was previously well controlled or escalation of antihypertensive medications to control blood pressure, or 2) new onset of proteinuria or a sudden increase in proteinuria in a woman with known proteinuria before or early in pregnancy.
It is not clear, however, what is considered a sudden increase in blood pressure, and it is concerning that any escalation of medication could potentially prompt this diagnosis. Is an increase in systolic blood pressure from 140 mm Hg to 150 mm Hg or an increase in diastolic blood pressure from 90 mm Hg to 100 mm Hg between two prenatal visits considered a “sudden increase”? Does an increase in methyldopa dosage from 250 mg daily to 500 mg daily to keep blood pressure within the range of mild hypertension mean that the patient should be diagnosed with superimposed preeclampsia? Hypertension is likely to increase and require an escalation of antihypertensive medications as patients with chronic hypertension progress through their pregnancies.
Similarly, a “sudden increase in proteinuria” – or “sudden, substantial, and sustained increases in protein excretion,” as written elsewhere in the report with respect to superimposed preeclampsia – also is undefined. What exactly does this mean? That we lack clinically meaningful parameters and clear descriptions of acceptable criteria/scenarios for observation rather than intervention is troubling, particularly because some of these women may have preexisting renal disease with expected increases and fluctuations in protein excretion during advanced gestation.
We must be cautious about making a diagnosis of superimposed preeclampsia based on changes in blood pressure or urinary protein alone, lest we have unnecessary hospitalizations and interventions. I recommend that the diagnosis of superimposed preeclampsia be made based on either the new onset of proteinuria in association with mild hypertension after 20 weeks or on elevation in blood pressure to severe ranges (systolic BP greater than or equal to160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of maximum doses of one antihypertensive drug.
Regarding superimposed preeclampsia with severe features, I recommend that in the case of blood pressure elevation, it be diagnosed only after maximal doses of two medications have been used. Specifically, I recommend that superimposed preeclampsia with severe features be defined as either CHTN or superimposed preeclampsia in association with either systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions despite use of maximum doses of labetalol (2,400 mg/day) plus long-acting nifedipine (120 mg/day), or with any of the other severe features.
In a second installment of the Master Class, I will elaborate on the treatment of severe GHTN and address the management of preeclampsia with severe features as well as postpartum management of hypertension during pregnancy.
Suggested diagnostic definitions
- Preeclampsia with severe features: GHTN in association with severe features.
- Superimposed preeclampsia: CHTN with either the new onset of proteinuria in association with mild hypertension after 20 weeks, or an elevation in blood pressure to severe ranges (systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of the maximal dose of one antihypertensive drug.
- Superimposed preeclampsia with severe features: CHTN or superimposed preeclampsia with severe features or with a rise in blood pressure to severe ranges despite the maximal doses of two antihypertensive drugs (e.g. 2,400 mg/day labetalol plus 120 mg/day long-acting nifedipine).
Note: These definitions reflect adaptations and clarifications of ACOG’s 2013 Task Force Report on Hypertension in Pregnancy.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Prenatal care always has been in part about identifying women with medical complications including preeclampsia. We have long measured blood pressure, checked the urine for high levels of protein, and monitored weight gain. We still do.
However, over the years, the diagnostic criteria for preeclampsia have evolved, first with the exclusion of edema and more recently with the exclusion of proteinuria as a necessary element of the diagnosis. The American College of Obstetricians and Gynecologists’ Task Force Report, Hypertension in Pregnancy, published in 2013, concluded that while preeclampsia may still be defined by the occurrence of hypertension with proteinuria, it also may be diagnosed when hypertension occurs in association with other multisystemic signs indicative of disease severity. The change came based on evidence that some women develop eclampsia, HELLP syndrome, and other serious complications in the absence of proteinuria.
The 2013 document also attempted to review and clarify various issues relating to the classifications, diagnosis, prediction and prevention, and management of hypertension during pregnancy, including the postpartum period. In many respects, it was successful in doing so. However, there is still much confusion regarding the diagnosis of certain categories of hypertensive disorders – particularly preeclampsia with severe features and superimposed preeclampsia with or without severe features.
While it is difficult to establish precise definitions given the often insidious nature of preeclampsia, it still is important to achieve a higher level of clarity with respect to these categories. Overdiagnosis may be preferable. However, improper classification also may influence management decisions that could prove detrimental to the fetus.
Severe gestational hypertension
ACOG’s 2013 Report on Hypertension in Pregnancy classifies hypertensive disorders of pregnancy into these categories: Gestational hypertension (GHTN), preeclampsia, preeclampsia with severe features (this includes HELLP), chronic hypertension (CHTN), superimposed preeclampsia with or without severe features, and eclampsia.
Some of the definitions and diagnostic criteria are clear. For instance, GHTN is defined as the new onset of hypertension after 20 weeks’ gestation in the absence of proteinuria or systemic findings such as thrombocytopenia or impaired liver function. CHTN is defined as hypertension that predates conception or is detected before 20 weeks’ gestation. In both cases there should be elevated blood pressure on two occasions at least 4 hours apart.
A major omission is the lack of a definition for severe GHTN. Removal of this previously well-understood classification category combined with unclear statements regarding preeclampsia with or without severe features has made it difficult for physicians to know in some cases of severe hypertension only what diagnosis a woman should receive and how she should be managed.
I recommend that we maintain the category of severe GHTN, and that it be defined as a systolic blood pressure (BP) greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions at least 4 hours apart when antihypertensive medications have not been initiated. There should be no proteinuria or severe features such as thrombocytopenia or impaired liver function.
The physician may elect in these cases to administer antihypertensive medication and observe the patient in the hospital. An individualized decision can then be made regarding how the patient should be managed, including whether she should be admitted and whether the pregnancy should continue beyond 34 weeks. Blood pressure, gestational age at diagnosis, the presence or absence of symptoms, and laboratory tests all should be taken into consideration.
Preeclampsia with or without severe features
We need to clarify and simplify how we think about GHTN and preeclampsia with or without severe features.
Most cases of preeclampsia will involve new-onset proteinuria, with proteinuria being defined as greater than or equal to 300 mg/day or a protein-creatinine ratio of greater than or equal to 0.3 mg/dL. In cases in which a dipstick test must be used, proteinuria is suggested by a urine protein reading of 1+. (It is important to note that dipstick readings should be taken on two separate occasions.) According to the report, preeclampsia also may be established by the presence of GHTN in association with any one of a list of features that are generally referred to as “severe features.”
Various boxes and textual descriptions in the report offer a sometimes confusing picture, however, of the terms preeclampsia and preeclampsia with severe features and their differences. For clarification, I recommend that we define preeclampsia with severe features as GHTN (mild or severe) in association with any one of the severe features.
Severe features of preeclampsia
- Platelet count less than 100,000/microliter.
- Elevated hepatic transaminases greater than two times the upper limit of normal for specific laboratory adult reference ranges.
- Severe persistent right upper quadrant abdominal pain or epigastric pain unresponsive to analgesics and unexplained by other etiology.
- Serum creatinine greater than 1.1 mg/dL.
- Pulmonary edema.
- Persistent cerebral disturbances such as severe persistent new-onset headaches unresponsive to nonnarcotic analgesics, altered mental status or other neurologic deficits.
- Visual disturbances such as blurred vision, scotomata, photophobia, or loss of vision.
I also suggest that we think of “mild” GHTN (systolic BP of 140-159 mm Hg or diastolic BP 90-109 mm Hg) and preeclampsia without severe features as one in the same, and that we manage them similarly. The presence or absence of proteinuria is currently the only difference diagnostically. The only difference with respect to management – aside from a weekly urine protein check in the case of GHTN – is the frequency of nonstress testing (NST) and amniotic fluid index (AFI) measurement (currently once a week for GHTN and twice a week for preeclampsia).
Given that unnecessary time and energy may be spent differentiating the two when management is essentially the same, I suggest that preeclampsia be diagnosed in any patient with GHTN with or without proteinuria. All patients can then be managed with blood pressure checks twice a week; symptoms and kick count daily; NST and AFI twice a week; estimated fetal weight by ultrasound every third week; lab tests (CBC, liver enzymes, and creatinine) once a week, and delivery at 37 weeks.
Superimposed preeclampsia with or without severe features
As the report states, the recognition of preeclampsia superimposed on chronic hypertension is “perhaps the greatest challenge” in the diagnosis and management of hypertensive disorders in pregnancy. Overdiagnosis “may be preferable,” the report says, given the high risk of adverse pregnancy outcomes with superimposed preeclampsia. On the other hand, it says, a “more stratified approach based on severity and predictors of adverse outcome may be useful” in avoiding unnecessary preterm births.
Ultimately, the task force proposed that we utilize the two categories of “superimposed preeclampsia” and “superimposed preeclampsia with severe features,” and in doing so, it noted that there “often is ambiguity in the diagnosis of superimposed preeclampsia and that the clinical spectrum of disease is broad.” Indeed, the diagnosis of superimposed preeclampsia as presented in the report remains vague and open to interpretation. In my institution, it has created significant confusion.
The report states that superimposed preeclampsia is likely when any of the following are present: 1) a sudden increase in blood pressure that was previously well controlled or escalation of antihypertensive medications to control blood pressure, or 2) new onset of proteinuria or a sudden increase in proteinuria in a woman with known proteinuria before or early in pregnancy.
It is not clear, however, what is considered a sudden increase in blood pressure, and it is concerning that any escalation of medication could potentially prompt this diagnosis. Is an increase in systolic blood pressure from 140 mm Hg to 150 mm Hg or an increase in diastolic blood pressure from 90 mm Hg to 100 mm Hg between two prenatal visits considered a “sudden increase”? Does an increase in methyldopa dosage from 250 mg daily to 500 mg daily to keep blood pressure within the range of mild hypertension mean that the patient should be diagnosed with superimposed preeclampsia? Hypertension is likely to increase and require an escalation of antihypertensive medications as patients with chronic hypertension progress through their pregnancies.
Similarly, a “sudden increase in proteinuria” – or “sudden, substantial, and sustained increases in protein excretion,” as written elsewhere in the report with respect to superimposed preeclampsia – also is undefined. What exactly does this mean? That we lack clinically meaningful parameters and clear descriptions of acceptable criteria/scenarios for observation rather than intervention is troubling, particularly because some of these women may have preexisting renal disease with expected increases and fluctuations in protein excretion during advanced gestation.
We must be cautious about making a diagnosis of superimposed preeclampsia based on changes in blood pressure or urinary protein alone, lest we have unnecessary hospitalizations and interventions. I recommend that the diagnosis of superimposed preeclampsia be made based on either the new onset of proteinuria in association with mild hypertension after 20 weeks or on elevation in blood pressure to severe ranges (systolic BP greater than or equal to160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of maximum doses of one antihypertensive drug.
Regarding superimposed preeclampsia with severe features, I recommend that in the case of blood pressure elevation, it be diagnosed only after maximal doses of two medications have been used. Specifically, I recommend that superimposed preeclampsia with severe features be defined as either CHTN or superimposed preeclampsia in association with either systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions despite use of maximum doses of labetalol (2,400 mg/day) plus long-acting nifedipine (120 mg/day), or with any of the other severe features.
In a second installment of the Master Class, I will elaborate on the treatment of severe GHTN and address the management of preeclampsia with severe features as well as postpartum management of hypertension during pregnancy.
Suggested diagnostic definitions
- Preeclampsia with severe features: GHTN in association with severe features.
- Superimposed preeclampsia: CHTN with either the new onset of proteinuria in association with mild hypertension after 20 weeks, or an elevation in blood pressure to severe ranges (systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of the maximal dose of one antihypertensive drug.
- Superimposed preeclampsia with severe features: CHTN or superimposed preeclampsia with severe features or with a rise in blood pressure to severe ranges despite the maximal doses of two antihypertensive drugs (e.g. 2,400 mg/day labetalol plus 120 mg/day long-acting nifedipine).
Note: These definitions reflect adaptations and clarifications of ACOG’s 2013 Task Force Report on Hypertension in Pregnancy.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Minilaparoscopy is the next step in minimally invasive surgery
Minimally invasive surgeons have been intrigued for more than 2 decades by the clinical aspects and benefits of minilaparoscopy. Miniature instruments (2-3.5 mm) were introduced starting in the late 1980s, and through the 1990s minilaparoscopic procedures were performed across multiple specialties. However, the instrumentation available at the time had limited durability and functionality (for example, a lack of electrosurgical capability), and clinical experience and resulting data were sparse. The minilaparoscopic approach failed to gain momentum and was never widely adopted.
In the past 5-10 years, with new innovations in technology and improved instrumentation, minilaparoscopy is undergoing a renaissance in surgical circles. Medical device companies have developed numerous electrosurgical and other advanced energy options as well as a variety of needle holders, graspers, and other instruments – all with diameters of 3.5 mm or less and with significantly more durability than the earlier generation of mini-instruments. While surgeons oftentimes still use larger telescopes for better visualization, 2- to 3.5-mm telescopes are available in various lengths and angles, and optic quality is continually improving.
The minilaparoscopic approach is more similar to conventional laparoscopy than laparoendoscopic single-site surgery, which has not met early expectations. It is a more logical next step in the evolution of minimally invasive surgery and its goals of further reducing surgical trauma and improving cosmesis. I am performing hysterectomies in which I place two 5-mm nonbladed trocars through incisions inside the umbilicus and a minilaparoscopic percutaneous cannula below the bikini line; it is a “hybrid” procedure, in essence, that incorporates the use of mini-instrumentation.
In addition to diagnostic laparoscopy, I also use minilaparoscopy for some of my patients who need ovarian cystectomy, oophorectomy, appendectomy, treatment of early-stage endometriosis or adhesiolysis. Throughout the world and across multiple specialties, it is being adopted for a wide range of adult and pediatric procedures, from abdominopelvic adhesions and inguinal hernia repair to cholecystectomy, and even to enhance diagnosis in the ED or ICU.1
The importance of surgical scars
The resurgence of interest in minilaparoscopy has been driven largely by its clinical advantages. From a clinical standpoint, less intrusion through the abdominal wall with the use of smaller instruments and fewer insertion points generally means less surgical trauma, and less analgesic medication and postoperative pain, for instance, as well as fewer vascular injuries and a more minimal risk of adhesions. Scar cosmesis also has been viewed as an advantage, just as it was when the abdominal hysterectomy was being replaced by laparoscopic hysterectomy starting in 1989. Still, for me, the clinical aspects have long been at the forefront.
My interest in providing my patients the very best cosmetic results changed after we surveyed patients who were scheduled for a hysterectomy in my practice over the span of 1 year. All patients seen during that time (from November 2012 to November 2013) were asked to complete a questionnaire on their knowledge of hysterectomy incisional scars, their perceptions, and their desires. Almost all of the 200 women who completed the survey – 93% – indicated that cosmetic issues such as scars are important to them (“slightly,” “moderately,” “quite,” or “extremely” important), and of these, 24% chose “extremely important.”
Asked how they feel about the appearance of their scars from prior abdominal surgery, 58% indicated the appearance bothered them to some extent, and 11% said they were “extremely” bothered. Almost all of the 200 patients – 92% – said they would be interested in a surgery that would leave no scars, and 45% said they were “extremely” interested.2
The findings juxtaposed the clinical benefits of more minimally invasive surgery – what had been foremost on my mind – with patients’ attention to and concern about scars. The study demonstrated that patient preferences are just as compelling, if not more, than what the surgeon wants. It showed, moreover, how important it is to discuss hysterectomy incision options – and patient preferences regarding incision location, size, and number – prior to surgery.
When asked about their familiarity with the locations of skin incisions in different hysterectomy procedures (abdominal, vaginal, laparoscopic, robotic, and mini), between 25% and 56% indicated they were not at all familiar with them. Familiarity was greatest with incisions in traditional laparoscopic hysterectomy. Yet patients want to have that knowledge: Almost all of the survey participants – 93% – indicated it is important to discuss the location, number, and size of incisions prior to surgery, and 59% said it is “extremely” important.
Patients also were asked to rank a short list of incision locations (above or below the belly button, and above or below the bikini line) from the least desirable to most desirable, and the results suggest just how different personal preferences can be. The most-desirable incision location was below the bikini line for 68% of patients, followed by above the belly button for 16%. The least-desirable location was above the belly button for 69%, followed by below the bikini line for 15%. Asked whether it is cosmetically superior for one’s incisions to be low (below the bikini line), 86% said they agreed.
Other research has similarly shown that cosmesis is important for women undergoing gynecologic surgery. For instance, women in another single-practice study were more likely to prefer single-site and traditional laparoscopic incisions over robotic ones when they were shown photos of an abdomen marked up with the incision lengths and locations typical for each of these three approaches.3 And notably, there has been research looking at the psychological impact of incisional scars specifically in patients who are morbidly obese.
While we may not be accustomed to discussing incisions and scars, it behooves us as surgeons to consider initiating a conversation about incisions with all our patients – regardless of their body mass index and prior surgical history – during the preoperative evaluation.
My hysterectomy approach
I have utilized one of the most recent developments in minilaparoscopy instrumentation – the MiniLap percutaneous surgical system (Teleflex) – to develop a mini technique for hysterectomy I’ve trademarked as the Cosmetic Hysterectomy. The percutaneous system has an outer diameter of 2.3 mm, integrated needle tips that facilitate insertion without a trocar, and a selection of integrated graspers (e.g., a miniature clutch or alligator) that open up to 12.5 mm and can be advanced and retracted through the cannula. The graspers can be locked onto the tissue, and the system itself can be stabilized extracorporeally so that it can be hands free.
For the hysterectomy, I make two 5-mm vertical incisions within the umbilicus – one for a nonbladed 5-mm trocar at 12 o’clock and the other for a second nonbladed 5-mm trocar at 6 o’clock, penetrating the fascia. The trocars house a 30-degree extra-long laparoscope with camera attached, and an advanced bipolar electrosurgery device.
The minilaparoscopic cannula is inserted in the lower-abdominal area through a single 1-mm stab incision, and one or two instruments can be placed as needed. Tissues can be removed vaginally once dissection is completed, and the vaginal cuff can be closed laparoscopically or vaginally. The edges of the minilaparoscopic cannula are approximated together and held with surgical glue or a sterile skin-closure strip. There is no need to close the fascia.4
The percutaneous system opens new windows for minimally invasive surgery. It can be moved and used in several locations throughout a surgical procedure such that we can achieve more patient-specific “incisional mapping,” as I’m now calling it, rather than uniformly utilizing standard trocar placement sites.
Even without use of this particular innovation, the use of smaller instruments is proving both feasible and advantageous. A study that randomized 75 women scheduled for a hysterectomy to traditional laparoscopy (with a 5- to 10-mm port size) or minilaparoscopy (with a 3-mm port size) found no statistically significant differences in blood loss, hemoglobin drop, pain scores, or analgesic use. The authors concluded that the smaller port sizes did not affect the ability to perform the procedure. Moreover, they noted, the minilaparoscopy group had consistently smaller scars and better cosmesis.5
Another retrospective study of perioperative outcomes with standard laparoscopic, minilaparoscopic, and laparoendoscopic single-site hysterectomy found that postoperative pain control and the need for analgesic medication was significantly less with minilaparoscopy and laparoendoscopic single-site (LESS) hysterectomy, compared with traditional laparoscopy. Pain and medication in patients undergoing minilaparoscopy was reduced by more than 50%, compared with the traditional laparoscopy group, which suggests less operative trauma.6
In my practice, postoperative analgesia is simply intranasal ketorolac tromethamine (Sprix) and/or long-acting tramadol (Conzip); opioids have been eliminated in all minilaparoscopic procedures. We have had no complications, including no trocar-site bleeding, nerve entrapments, trocar-site herniations, or infections. Not every patient is a candidate for consideration of a minilaparoscopic hysterectomy, of course. The patient who has extensive adhesions from multiple previous surgeries or a large uterus with fibroids, for instance, should be treated with traditional laparoscopy regardless of her concerns regarding cosmesis.
No two surgeons are alike; each has his/her own ideas, skill sets, and approaches. Minilaparoscopy may not be for everyone, but given the number of durable miniature instruments now available, it’s an approach to consider integrating into a variety of gynecologic procedures.
For a right salpingo-oophorectomy, for instance, a 3-mm trocar placed at 12 o’clock through the umbilicus can accommodate a 3-mm scope with a high-definition camera, and an 11-mm trocar placed at 6 o’clock can house an energy device. In the right and left lower quadrants, two additional 3-mm trocars can be placed – one to accommodate a grasping instrument and the other to house the scope after the fallopian tube has been transected. A specimen bag can be passed through the 11-mm trocar in the umbilicus for removal of the ovary and tube. With the umbilicus hiding the largest of scars, the procedure is less invasive with better cosmetic results.
Dr. McCarus disclosed that he is a consultant for Ethicon.
References
1. Surg Technol Int. 2015 Nov;27:19-30.
2. Surg Technol Int. 2014 Nov;25:150-6.
3. J Minim Invasive Gynecol. 2011 Sep-Oct;18(5):640-3.
4. Surg Technol Int 2013 Sep;23:129-32.
5. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
6. Surg Endosc. 2012 Dec;26(12):3592-6.
Minimally invasive surgeons have been intrigued for more than 2 decades by the clinical aspects and benefits of minilaparoscopy. Miniature instruments (2-3.5 mm) were introduced starting in the late 1980s, and through the 1990s minilaparoscopic procedures were performed across multiple specialties. However, the instrumentation available at the time had limited durability and functionality (for example, a lack of electrosurgical capability), and clinical experience and resulting data were sparse. The minilaparoscopic approach failed to gain momentum and was never widely adopted.
In the past 5-10 years, with new innovations in technology and improved instrumentation, minilaparoscopy is undergoing a renaissance in surgical circles. Medical device companies have developed numerous electrosurgical and other advanced energy options as well as a variety of needle holders, graspers, and other instruments – all with diameters of 3.5 mm or less and with significantly more durability than the earlier generation of mini-instruments. While surgeons oftentimes still use larger telescopes for better visualization, 2- to 3.5-mm telescopes are available in various lengths and angles, and optic quality is continually improving.
The minilaparoscopic approach is more similar to conventional laparoscopy than laparoendoscopic single-site surgery, which has not met early expectations. It is a more logical next step in the evolution of minimally invasive surgery and its goals of further reducing surgical trauma and improving cosmesis. I am performing hysterectomies in which I place two 5-mm nonbladed trocars through incisions inside the umbilicus and a minilaparoscopic percutaneous cannula below the bikini line; it is a “hybrid” procedure, in essence, that incorporates the use of mini-instrumentation.
In addition to diagnostic laparoscopy, I also use minilaparoscopy for some of my patients who need ovarian cystectomy, oophorectomy, appendectomy, treatment of early-stage endometriosis or adhesiolysis. Throughout the world and across multiple specialties, it is being adopted for a wide range of adult and pediatric procedures, from abdominopelvic adhesions and inguinal hernia repair to cholecystectomy, and even to enhance diagnosis in the ED or ICU.1
The importance of surgical scars
The resurgence of interest in minilaparoscopy has been driven largely by its clinical advantages. From a clinical standpoint, less intrusion through the abdominal wall with the use of smaller instruments and fewer insertion points generally means less surgical trauma, and less analgesic medication and postoperative pain, for instance, as well as fewer vascular injuries and a more minimal risk of adhesions. Scar cosmesis also has been viewed as an advantage, just as it was when the abdominal hysterectomy was being replaced by laparoscopic hysterectomy starting in 1989. Still, for me, the clinical aspects have long been at the forefront.
My interest in providing my patients the very best cosmetic results changed after we surveyed patients who were scheduled for a hysterectomy in my practice over the span of 1 year. All patients seen during that time (from November 2012 to November 2013) were asked to complete a questionnaire on their knowledge of hysterectomy incisional scars, their perceptions, and their desires. Almost all of the 200 women who completed the survey – 93% – indicated that cosmetic issues such as scars are important to them (“slightly,” “moderately,” “quite,” or “extremely” important), and of these, 24% chose “extremely important.”
Asked how they feel about the appearance of their scars from prior abdominal surgery, 58% indicated the appearance bothered them to some extent, and 11% said they were “extremely” bothered. Almost all of the 200 patients – 92% – said they would be interested in a surgery that would leave no scars, and 45% said they were “extremely” interested.2
The findings juxtaposed the clinical benefits of more minimally invasive surgery – what had been foremost on my mind – with patients’ attention to and concern about scars. The study demonstrated that patient preferences are just as compelling, if not more, than what the surgeon wants. It showed, moreover, how important it is to discuss hysterectomy incision options – and patient preferences regarding incision location, size, and number – prior to surgery.
When asked about their familiarity with the locations of skin incisions in different hysterectomy procedures (abdominal, vaginal, laparoscopic, robotic, and mini), between 25% and 56% indicated they were not at all familiar with them. Familiarity was greatest with incisions in traditional laparoscopic hysterectomy. Yet patients want to have that knowledge: Almost all of the survey participants – 93% – indicated it is important to discuss the location, number, and size of incisions prior to surgery, and 59% said it is “extremely” important.
Patients also were asked to rank a short list of incision locations (above or below the belly button, and above or below the bikini line) from the least desirable to most desirable, and the results suggest just how different personal preferences can be. The most-desirable incision location was below the bikini line for 68% of patients, followed by above the belly button for 16%. The least-desirable location was above the belly button for 69%, followed by below the bikini line for 15%. Asked whether it is cosmetically superior for one’s incisions to be low (below the bikini line), 86% said they agreed.
Other research has similarly shown that cosmesis is important for women undergoing gynecologic surgery. For instance, women in another single-practice study were more likely to prefer single-site and traditional laparoscopic incisions over robotic ones when they were shown photos of an abdomen marked up with the incision lengths and locations typical for each of these three approaches.3 And notably, there has been research looking at the psychological impact of incisional scars specifically in patients who are morbidly obese.
While we may not be accustomed to discussing incisions and scars, it behooves us as surgeons to consider initiating a conversation about incisions with all our patients – regardless of their body mass index and prior surgical history – during the preoperative evaluation.
My hysterectomy approach
I have utilized one of the most recent developments in minilaparoscopy instrumentation – the MiniLap percutaneous surgical system (Teleflex) – to develop a mini technique for hysterectomy I’ve trademarked as the Cosmetic Hysterectomy. The percutaneous system has an outer diameter of 2.3 mm, integrated needle tips that facilitate insertion without a trocar, and a selection of integrated graspers (e.g., a miniature clutch or alligator) that open up to 12.5 mm and can be advanced and retracted through the cannula. The graspers can be locked onto the tissue, and the system itself can be stabilized extracorporeally so that it can be hands free.
For the hysterectomy, I make two 5-mm vertical incisions within the umbilicus – one for a nonbladed 5-mm trocar at 12 o’clock and the other for a second nonbladed 5-mm trocar at 6 o’clock, penetrating the fascia. The trocars house a 30-degree extra-long laparoscope with camera attached, and an advanced bipolar electrosurgery device.
The minilaparoscopic cannula is inserted in the lower-abdominal area through a single 1-mm stab incision, and one or two instruments can be placed as needed. Tissues can be removed vaginally once dissection is completed, and the vaginal cuff can be closed laparoscopically or vaginally. The edges of the minilaparoscopic cannula are approximated together and held with surgical glue or a sterile skin-closure strip. There is no need to close the fascia.4
The percutaneous system opens new windows for minimally invasive surgery. It can be moved and used in several locations throughout a surgical procedure such that we can achieve more patient-specific “incisional mapping,” as I’m now calling it, rather than uniformly utilizing standard trocar placement sites.
Even without use of this particular innovation, the use of smaller instruments is proving both feasible and advantageous. A study that randomized 75 women scheduled for a hysterectomy to traditional laparoscopy (with a 5- to 10-mm port size) or minilaparoscopy (with a 3-mm port size) found no statistically significant differences in blood loss, hemoglobin drop, pain scores, or analgesic use. The authors concluded that the smaller port sizes did not affect the ability to perform the procedure. Moreover, they noted, the minilaparoscopy group had consistently smaller scars and better cosmesis.5
Another retrospective study of perioperative outcomes with standard laparoscopic, minilaparoscopic, and laparoendoscopic single-site hysterectomy found that postoperative pain control and the need for analgesic medication was significantly less with minilaparoscopy and laparoendoscopic single-site (LESS) hysterectomy, compared with traditional laparoscopy. Pain and medication in patients undergoing minilaparoscopy was reduced by more than 50%, compared with the traditional laparoscopy group, which suggests less operative trauma.6
In my practice, postoperative analgesia is simply intranasal ketorolac tromethamine (Sprix) and/or long-acting tramadol (Conzip); opioids have been eliminated in all minilaparoscopic procedures. We have had no complications, including no trocar-site bleeding, nerve entrapments, trocar-site herniations, or infections. Not every patient is a candidate for consideration of a minilaparoscopic hysterectomy, of course. The patient who has extensive adhesions from multiple previous surgeries or a large uterus with fibroids, for instance, should be treated with traditional laparoscopy regardless of her concerns regarding cosmesis.
No two surgeons are alike; each has his/her own ideas, skill sets, and approaches. Minilaparoscopy may not be for everyone, but given the number of durable miniature instruments now available, it’s an approach to consider integrating into a variety of gynecologic procedures.
For a right salpingo-oophorectomy, for instance, a 3-mm trocar placed at 12 o’clock through the umbilicus can accommodate a 3-mm scope with a high-definition camera, and an 11-mm trocar placed at 6 o’clock can house an energy device. In the right and left lower quadrants, two additional 3-mm trocars can be placed – one to accommodate a grasping instrument and the other to house the scope after the fallopian tube has been transected. A specimen bag can be passed through the 11-mm trocar in the umbilicus for removal of the ovary and tube. With the umbilicus hiding the largest of scars, the procedure is less invasive with better cosmetic results.
Dr. McCarus disclosed that he is a consultant for Ethicon.
References
1. Surg Technol Int. 2015 Nov;27:19-30.
2. Surg Technol Int. 2014 Nov;25:150-6.
3. J Minim Invasive Gynecol. 2011 Sep-Oct;18(5):640-3.
4. Surg Technol Int 2013 Sep;23:129-32.
5. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
6. Surg Endosc. 2012 Dec;26(12):3592-6.
Minimally invasive surgeons have been intrigued for more than 2 decades by the clinical aspects and benefits of minilaparoscopy. Miniature instruments (2-3.5 mm) were introduced starting in the late 1980s, and through the 1990s minilaparoscopic procedures were performed across multiple specialties. However, the instrumentation available at the time had limited durability and functionality (for example, a lack of electrosurgical capability), and clinical experience and resulting data were sparse. The minilaparoscopic approach failed to gain momentum and was never widely adopted.
In the past 5-10 years, with new innovations in technology and improved instrumentation, minilaparoscopy is undergoing a renaissance in surgical circles. Medical device companies have developed numerous electrosurgical and other advanced energy options as well as a variety of needle holders, graspers, and other instruments – all with diameters of 3.5 mm or less and with significantly more durability than the earlier generation of mini-instruments. While surgeons oftentimes still use larger telescopes for better visualization, 2- to 3.5-mm telescopes are available in various lengths and angles, and optic quality is continually improving.
The minilaparoscopic approach is more similar to conventional laparoscopy than laparoendoscopic single-site surgery, which has not met early expectations. It is a more logical next step in the evolution of minimally invasive surgery and its goals of further reducing surgical trauma and improving cosmesis. I am performing hysterectomies in which I place two 5-mm nonbladed trocars through incisions inside the umbilicus and a minilaparoscopic percutaneous cannula below the bikini line; it is a “hybrid” procedure, in essence, that incorporates the use of mini-instrumentation.
In addition to diagnostic laparoscopy, I also use minilaparoscopy for some of my patients who need ovarian cystectomy, oophorectomy, appendectomy, treatment of early-stage endometriosis or adhesiolysis. Throughout the world and across multiple specialties, it is being adopted for a wide range of adult and pediatric procedures, from abdominopelvic adhesions and inguinal hernia repair to cholecystectomy, and even to enhance diagnosis in the ED or ICU.1
The importance of surgical scars
The resurgence of interest in minilaparoscopy has been driven largely by its clinical advantages. From a clinical standpoint, less intrusion through the abdominal wall with the use of smaller instruments and fewer insertion points generally means less surgical trauma, and less analgesic medication and postoperative pain, for instance, as well as fewer vascular injuries and a more minimal risk of adhesions. Scar cosmesis also has been viewed as an advantage, just as it was when the abdominal hysterectomy was being replaced by laparoscopic hysterectomy starting in 1989. Still, for me, the clinical aspects have long been at the forefront.
My interest in providing my patients the very best cosmetic results changed after we surveyed patients who were scheduled for a hysterectomy in my practice over the span of 1 year. All patients seen during that time (from November 2012 to November 2013) were asked to complete a questionnaire on their knowledge of hysterectomy incisional scars, their perceptions, and their desires. Almost all of the 200 women who completed the survey – 93% – indicated that cosmetic issues such as scars are important to them (“slightly,” “moderately,” “quite,” or “extremely” important), and of these, 24% chose “extremely important.”
Asked how they feel about the appearance of their scars from prior abdominal surgery, 58% indicated the appearance bothered them to some extent, and 11% said they were “extremely” bothered. Almost all of the 200 patients – 92% – said they would be interested in a surgery that would leave no scars, and 45% said they were “extremely” interested.2
The findings juxtaposed the clinical benefits of more minimally invasive surgery – what had been foremost on my mind – with patients’ attention to and concern about scars. The study demonstrated that patient preferences are just as compelling, if not more, than what the surgeon wants. It showed, moreover, how important it is to discuss hysterectomy incision options – and patient preferences regarding incision location, size, and number – prior to surgery.
When asked about their familiarity with the locations of skin incisions in different hysterectomy procedures (abdominal, vaginal, laparoscopic, robotic, and mini), between 25% and 56% indicated they were not at all familiar with them. Familiarity was greatest with incisions in traditional laparoscopic hysterectomy. Yet patients want to have that knowledge: Almost all of the survey participants – 93% – indicated it is important to discuss the location, number, and size of incisions prior to surgery, and 59% said it is “extremely” important.
Patients also were asked to rank a short list of incision locations (above or below the belly button, and above or below the bikini line) from the least desirable to most desirable, and the results suggest just how different personal preferences can be. The most-desirable incision location was below the bikini line for 68% of patients, followed by above the belly button for 16%. The least-desirable location was above the belly button for 69%, followed by below the bikini line for 15%. Asked whether it is cosmetically superior for one’s incisions to be low (below the bikini line), 86% said they agreed.
Other research has similarly shown that cosmesis is important for women undergoing gynecologic surgery. For instance, women in another single-practice study were more likely to prefer single-site and traditional laparoscopic incisions over robotic ones when they were shown photos of an abdomen marked up with the incision lengths and locations typical for each of these three approaches.3 And notably, there has been research looking at the psychological impact of incisional scars specifically in patients who are morbidly obese.
While we may not be accustomed to discussing incisions and scars, it behooves us as surgeons to consider initiating a conversation about incisions with all our patients – regardless of their body mass index and prior surgical history – during the preoperative evaluation.
My hysterectomy approach
I have utilized one of the most recent developments in minilaparoscopy instrumentation – the MiniLap percutaneous surgical system (Teleflex) – to develop a mini technique for hysterectomy I’ve trademarked as the Cosmetic Hysterectomy. The percutaneous system has an outer diameter of 2.3 mm, integrated needle tips that facilitate insertion without a trocar, and a selection of integrated graspers (e.g., a miniature clutch or alligator) that open up to 12.5 mm and can be advanced and retracted through the cannula. The graspers can be locked onto the tissue, and the system itself can be stabilized extracorporeally so that it can be hands free.
For the hysterectomy, I make two 5-mm vertical incisions within the umbilicus – one for a nonbladed 5-mm trocar at 12 o’clock and the other for a second nonbladed 5-mm trocar at 6 o’clock, penetrating the fascia. The trocars house a 30-degree extra-long laparoscope with camera attached, and an advanced bipolar electrosurgery device.
The minilaparoscopic cannula is inserted in the lower-abdominal area through a single 1-mm stab incision, and one or two instruments can be placed as needed. Tissues can be removed vaginally once dissection is completed, and the vaginal cuff can be closed laparoscopically or vaginally. The edges of the minilaparoscopic cannula are approximated together and held with surgical glue or a sterile skin-closure strip. There is no need to close the fascia.4
The percutaneous system opens new windows for minimally invasive surgery. It can be moved and used in several locations throughout a surgical procedure such that we can achieve more patient-specific “incisional mapping,” as I’m now calling it, rather than uniformly utilizing standard trocar placement sites.
Even without use of this particular innovation, the use of smaller instruments is proving both feasible and advantageous. A study that randomized 75 women scheduled for a hysterectomy to traditional laparoscopy (with a 5- to 10-mm port size) or minilaparoscopy (with a 3-mm port size) found no statistically significant differences in blood loss, hemoglobin drop, pain scores, or analgesic use. The authors concluded that the smaller port sizes did not affect the ability to perform the procedure. Moreover, they noted, the minilaparoscopy group had consistently smaller scars and better cosmesis.5
Another retrospective study of perioperative outcomes with standard laparoscopic, minilaparoscopic, and laparoendoscopic single-site hysterectomy found that postoperative pain control and the need for analgesic medication was significantly less with minilaparoscopy and laparoendoscopic single-site (LESS) hysterectomy, compared with traditional laparoscopy. Pain and medication in patients undergoing minilaparoscopy was reduced by more than 50%, compared with the traditional laparoscopy group, which suggests less operative trauma.6
In my practice, postoperative analgesia is simply intranasal ketorolac tromethamine (Sprix) and/or long-acting tramadol (Conzip); opioids have been eliminated in all minilaparoscopic procedures. We have had no complications, including no trocar-site bleeding, nerve entrapments, trocar-site herniations, or infections. Not every patient is a candidate for consideration of a minilaparoscopic hysterectomy, of course. The patient who has extensive adhesions from multiple previous surgeries or a large uterus with fibroids, for instance, should be treated with traditional laparoscopy regardless of her concerns regarding cosmesis.
No two surgeons are alike; each has his/her own ideas, skill sets, and approaches. Minilaparoscopy may not be for everyone, but given the number of durable miniature instruments now available, it’s an approach to consider integrating into a variety of gynecologic procedures.
For a right salpingo-oophorectomy, for instance, a 3-mm trocar placed at 12 o’clock through the umbilicus can accommodate a 3-mm scope with a high-definition camera, and an 11-mm trocar placed at 6 o’clock can house an energy device. In the right and left lower quadrants, two additional 3-mm trocars can be placed – one to accommodate a grasping instrument and the other to house the scope after the fallopian tube has been transected. A specimen bag can be passed through the 11-mm trocar in the umbilicus for removal of the ovary and tube. With the umbilicus hiding the largest of scars, the procedure is less invasive with better cosmetic results.
Dr. McCarus disclosed that he is a consultant for Ethicon.
References
1. Surg Technol Int. 2015 Nov;27:19-30.
2. Surg Technol Int. 2014 Nov;25:150-6.
3. J Minim Invasive Gynecol. 2011 Sep-Oct;18(5):640-3.
4. Surg Technol Int 2013 Sep;23:129-32.
5. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
6. Surg Endosc. 2012 Dec;26(12):3592-6.
Minilaparoscopy is a relevant surgical technique
With the wax and wane in the popularity of single-port surgery and with the advent of improved instrumentation, minilaparoscopy would appear to be the next long-lasting surgical technique to enhance postsurgical cosmetic appearance. For this reason, it is surprising that the use of minilaparoscopy has not been acknowledged and evaluated as a viable option more often in general surgery and urology. This, despite the fact that the use of this technique in hysterectomy was described nearly 20 years ago.1
Our minimally invasive gynecologic surgery (MIGS) team has utilized minilaparoscopy for diagnostic laparoscopy, lysis of adhesions, treatment of stage I, II, and occasionally stage III endometriosis, ovarian cystectomy, ureterolysis, presacral neurectomy, and total laparoscopic hysterectomy – as has our guest author Steven McCarus, MD. When performing hysterectomy via minilaparoscopy, our team closes the vaginal cuff laparoscopically, placing the suture transvaginally.
By removing the fibroid via a colpotomy incision, the Italian MIGS surgeon Fabio Ghezzi, MD, is able to perform myomectomy and hysterectomy routinely via minilaparoscopy.2 Articles have been published regarding the feasibility of performing minilaparoscopic surgery for both the treatment of benign adnexal mases3 and endometriosis.4
Dr. McCarus presents compelling evidence regarding the cosmetic advantage of minilaparoscopy, but the reported impact on pain has been variable: As Alyssa Small Layne et al. states, “Some studies associate minilaparoscopy with decreased pain, whereas others did not find a difference.”5 In part, this is attributable to the fact that no matter what technique is performed, the pathology must be excised. However, it is my belief that with improvements in instrumentation – as noted by Dr. McCarus and our collected added experience – the postoperative pain profile for the patient undergoing minilaparoscopy will change dramatically.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Dr. McCarus, who is the chief of gynecological surgery at Florida Hospital Celebration Health, Celebration. With over 25 years of experience, Dr. McCarus is nationally known as a leader in the practice of minimally invasive gynecologic surgery.
It is a pleasure to welcome Dr. McCarus to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Am Assoc Gynecol Laparosc. 1999 Feb;6(1):97-100.
2. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
3. J Clin Med Res. 2017 Jul;9(7):613-7.
4. Gynecol Minim Invasive Ther. 2013 Aug;2(3):85-8.
5. Curr Opin Obstet Gynecol. 2016 Aug;28(4):255-60.
With the wax and wane in the popularity of single-port surgery and with the advent of improved instrumentation, minilaparoscopy would appear to be the next long-lasting surgical technique to enhance postsurgical cosmetic appearance. For this reason, it is surprising that the use of minilaparoscopy has not been acknowledged and evaluated as a viable option more often in general surgery and urology. This, despite the fact that the use of this technique in hysterectomy was described nearly 20 years ago.1
Our minimally invasive gynecologic surgery (MIGS) team has utilized minilaparoscopy for diagnostic laparoscopy, lysis of adhesions, treatment of stage I, II, and occasionally stage III endometriosis, ovarian cystectomy, ureterolysis, presacral neurectomy, and total laparoscopic hysterectomy – as has our guest author Steven McCarus, MD. When performing hysterectomy via minilaparoscopy, our team closes the vaginal cuff laparoscopically, placing the suture transvaginally.
By removing the fibroid via a colpotomy incision, the Italian MIGS surgeon Fabio Ghezzi, MD, is able to perform myomectomy and hysterectomy routinely via minilaparoscopy.2 Articles have been published regarding the feasibility of performing minilaparoscopic surgery for both the treatment of benign adnexal mases3 and endometriosis.4
Dr. McCarus presents compelling evidence regarding the cosmetic advantage of minilaparoscopy, but the reported impact on pain has been variable: As Alyssa Small Layne et al. states, “Some studies associate minilaparoscopy with decreased pain, whereas others did not find a difference.”5 In part, this is attributable to the fact that no matter what technique is performed, the pathology must be excised. However, it is my belief that with improvements in instrumentation – as noted by Dr. McCarus and our collected added experience – the postoperative pain profile for the patient undergoing minilaparoscopy will change dramatically.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Dr. McCarus, who is the chief of gynecological surgery at Florida Hospital Celebration Health, Celebration. With over 25 years of experience, Dr. McCarus is nationally known as a leader in the practice of minimally invasive gynecologic surgery.
It is a pleasure to welcome Dr. McCarus to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Am Assoc Gynecol Laparosc. 1999 Feb;6(1):97-100.
2. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
3. J Clin Med Res. 2017 Jul;9(7):613-7.
4. Gynecol Minim Invasive Ther. 2013 Aug;2(3):85-8.
5. Curr Opin Obstet Gynecol. 2016 Aug;28(4):255-60.
With the wax and wane in the popularity of single-port surgery and with the advent of improved instrumentation, minilaparoscopy would appear to be the next long-lasting surgical technique to enhance postsurgical cosmetic appearance. For this reason, it is surprising that the use of minilaparoscopy has not been acknowledged and evaluated as a viable option more often in general surgery and urology. This, despite the fact that the use of this technique in hysterectomy was described nearly 20 years ago.1
Our minimally invasive gynecologic surgery (MIGS) team has utilized minilaparoscopy for diagnostic laparoscopy, lysis of adhesions, treatment of stage I, II, and occasionally stage III endometriosis, ovarian cystectomy, ureterolysis, presacral neurectomy, and total laparoscopic hysterectomy – as has our guest author Steven McCarus, MD. When performing hysterectomy via minilaparoscopy, our team closes the vaginal cuff laparoscopically, placing the suture transvaginally.
By removing the fibroid via a colpotomy incision, the Italian MIGS surgeon Fabio Ghezzi, MD, is able to perform myomectomy and hysterectomy routinely via minilaparoscopy.2 Articles have been published regarding the feasibility of performing minilaparoscopic surgery for both the treatment of benign adnexal mases3 and endometriosis.4
Dr. McCarus presents compelling evidence regarding the cosmetic advantage of minilaparoscopy, but the reported impact on pain has been variable: As Alyssa Small Layne et al. states, “Some studies associate minilaparoscopy with decreased pain, whereas others did not find a difference.”5 In part, this is attributable to the fact that no matter what technique is performed, the pathology must be excised. However, it is my belief that with improvements in instrumentation – as noted by Dr. McCarus and our collected added experience – the postoperative pain profile for the patient undergoing minilaparoscopy will change dramatically.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Dr. McCarus, who is the chief of gynecological surgery at Florida Hospital Celebration Health, Celebration. With over 25 years of experience, Dr. McCarus is nationally known as a leader in the practice of minimally invasive gynecologic surgery.
It is a pleasure to welcome Dr. McCarus to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Am Assoc Gynecol Laparosc. 1999 Feb;6(1):97-100.
2. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
3. J Clin Med Res. 2017 Jul;9(7):613-7.
4. Gynecol Minim Invasive Ther. 2013 Aug;2(3):85-8.
5. Curr Opin Obstet Gynecol. 2016 Aug;28(4):255-60.