User login
Dynamic ultrasonography: An idea whose time has come
Ultrasonography truly has revolutionized the practice of obstetrics and gynecology. Initially, transabdominal ultrasonography was mainly a tool of the obstetrician. Early linear array, real-time equipment had barely enough resolution to perform very limited assessments, such as measure biparietal diameter and identify vertex versus breech presentation, and anterior versus posterior placenta location. The introduction of transvaginal probes, which employ higher frequency and provide closer proximity to structures, yielded a degree of image magnification that was dubbed sonomicroscopy.1 In other words, we are seeing things with our naked eye that we could not see if we could hold them in our hand at arm’s length and squint at them. An example of this is the cardiac activity clearly visible in a 3-mm embryo at 45 days from the last menstrual period. One would not appreciate this without the low power magnification of the vaginal probe.
The concept of dynamic imaging
As early as 1990, I realized that there is a difference between an ultrasound “examination” performed because of referral for imaging, which generated a report back to the referring health care provider, and “examining” one’s own patient with ultrasonography at the time of bimanual exam. I coined the phrase “the ultrasound-enhanced bimanual exam,” and I believed it should become a routine part of gynecologic care. I put forth this thesis in an article entitled, “Incorporating endovaginal ultrasonography into the overall gynecologic examination.”2 The idea is based on thinking: What exactly are we are trying to discern from a bimanual exam?
Clinicians perform the bimanual exam thousands of times. The bimanual examination consists of 2 components, an objective portion and a subjective portion. The objective component attempts to discern information that is totally objective, such as, Is the ovary enlarged? If so, is it cystic or solid? Is this uterus normal in shape and contour? If so, does it feel like leiomyomas or is it globularly enlarged as with adenomyosis? The subjective component of the bimanual examination attempts to determine whether or not tenderness is present or if there is normal mobility of the pelvic organs.
The objective component can be replaced by an image in very little time if the examiner has the equipment and the knowledge and skill. The subjective component, however, depends on the experience and often the nuance of the examiner. That was my original thought process. I wanted, and still want, the examining clinician to use imaging as part of the overall exam. But now, I want the imager to use examination as part of the overall imaging. (VIDEOS 1A and 1B.) This is the concept of dynamic imaging. It involves the liberal use of the abdominal hand as well as an in-and-out motion of the vaginal probe to ascertain aspects of the examination that in the past I deemed “subjective.” Mainly, this involves the aspects of mobility and/or tenderness.
Continue to: Guidelines concerning pelvic ultrasound do not consider dynamic imaging...
Guidelines concerning pelvic ultrasound do not consider dynamic imaging
Until now, most imagers take a myriad of pictures, mostly still snapshots, to illustrate anatomy. Most imaging physicians then look at a series of such pictures and may never even hold the transducer. This is increasingly true in instances of remote teleradiology. Even for the minority of imagers who utilize video clips (VIDEOS 2A–2C), these are still representations of anatomy .
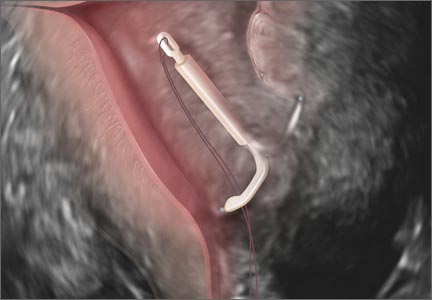
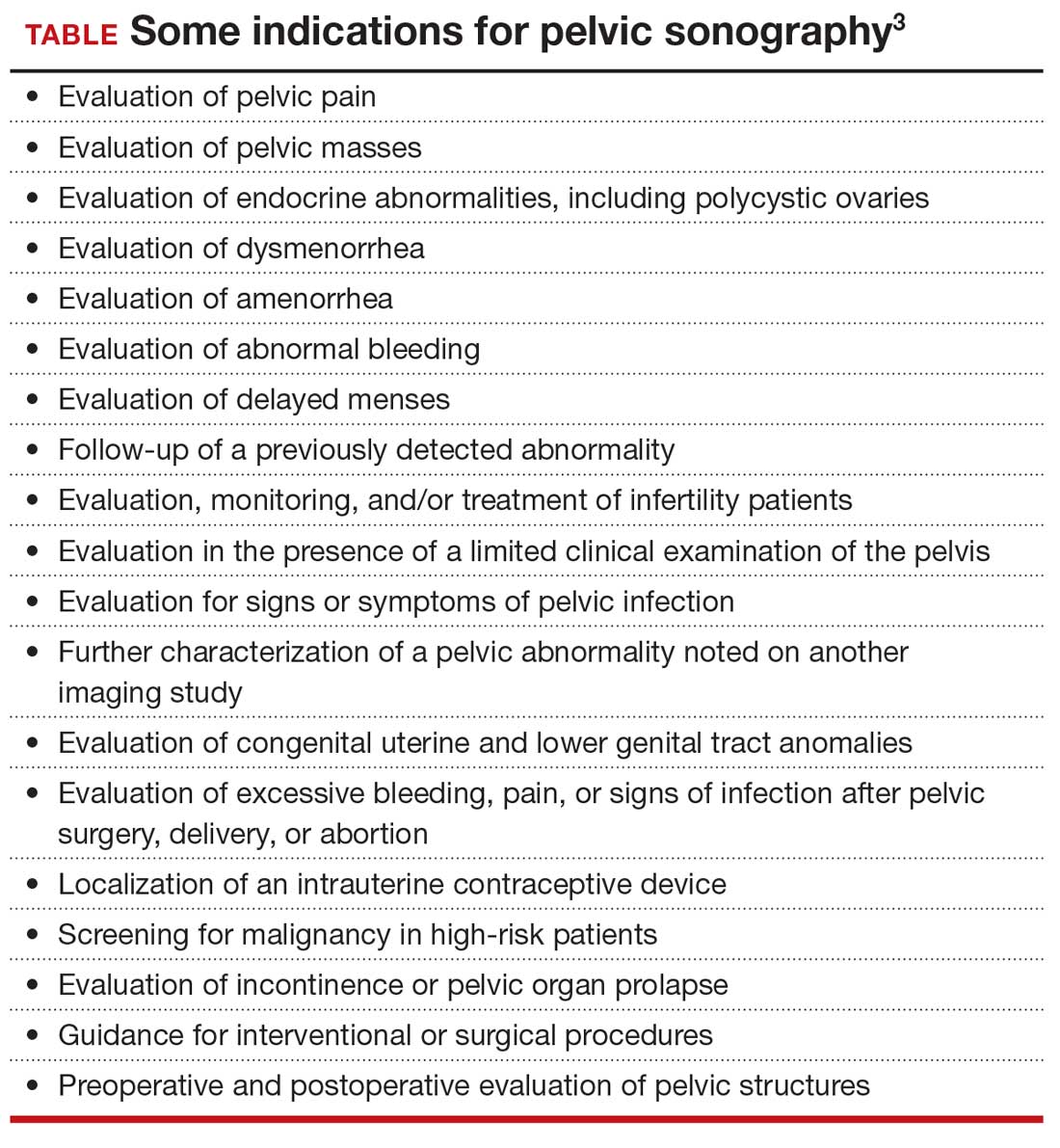
One need look no further than the guidelines that underpin the expectation of those who scan the female pelvis. The American Institute of Ultrasound in Medicine (AIUM) published a practice parameter for the performance of ultrasonography of the female pelvis, developed in collaboration with the American College of Radiology, American College of Obstetricians and Gynecologists, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound. 3 Nowhere does this document mention anything other than what images to obtain, where to look, and how to measure. Nowhere is there any mention of dynamic imaging—the concept of using one’s other hand on the abdomen, eliciting pain with the vaginal probe, checking for mobility, asking the patient to bear down. The document lists indications for pelvic sonography that include but are not limited to 19 different indications, such as pelvic pain, evaluation of dysmenorrhea, evaluation for signs or symptoms of pelvic infection, and evaluation of incontinence or pelvic organ prolapse (TABLE). 3
Dynamic ultrasonography can aid in the diagnosis of certain conditions
Specifically, what can dynamic ultrasonography add to anatomic imaging? The main considerations are pain, adhesions, endometriosis, and pelvic organ prolapse.
Pelvic pain or tenderness
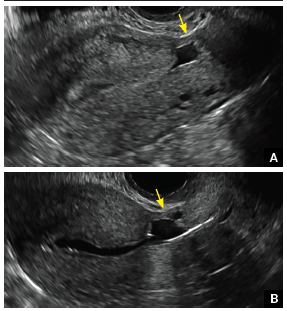
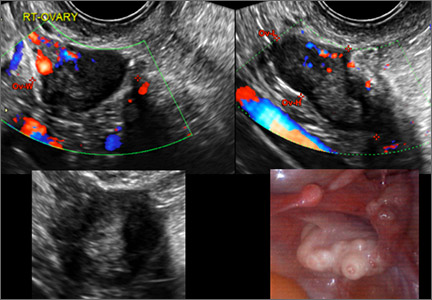
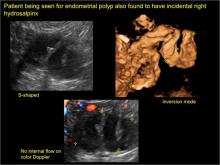
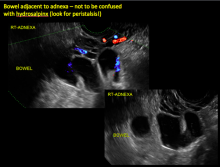
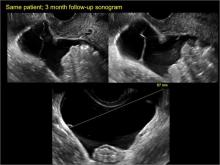
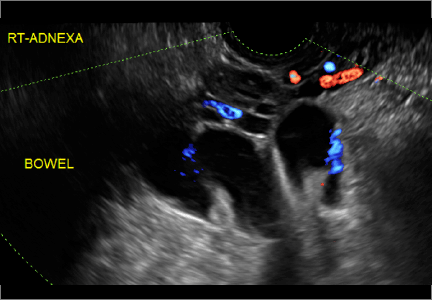
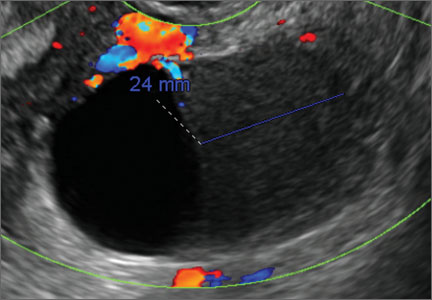
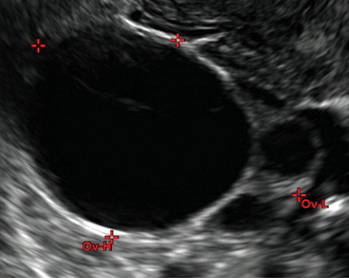
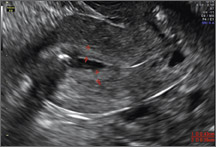
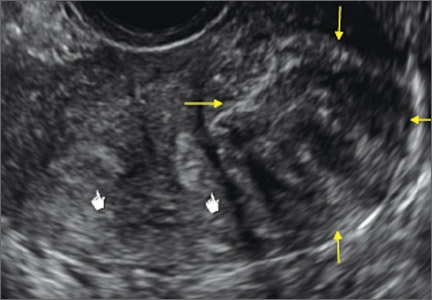
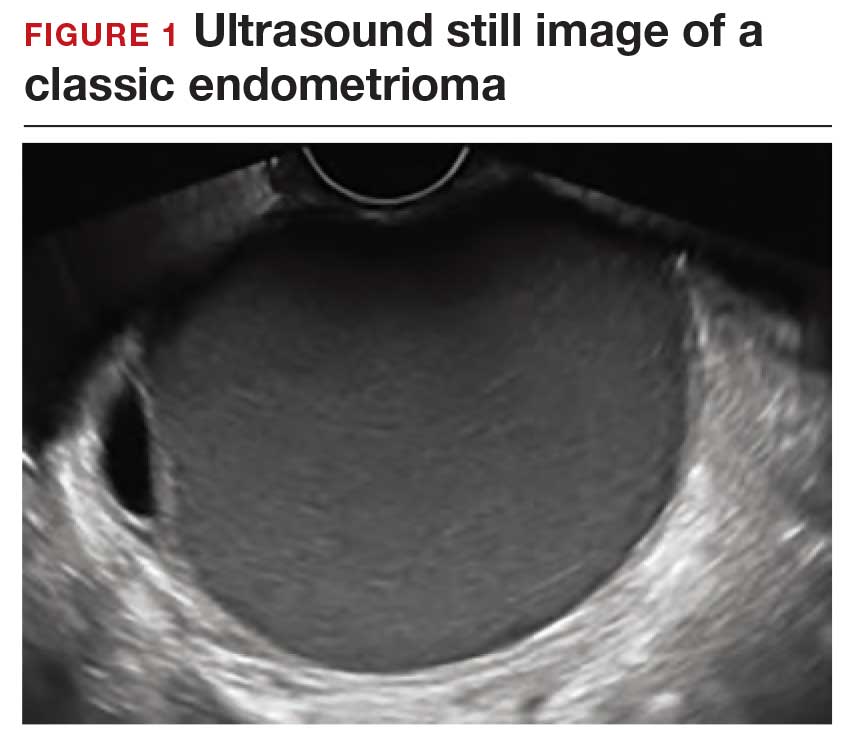
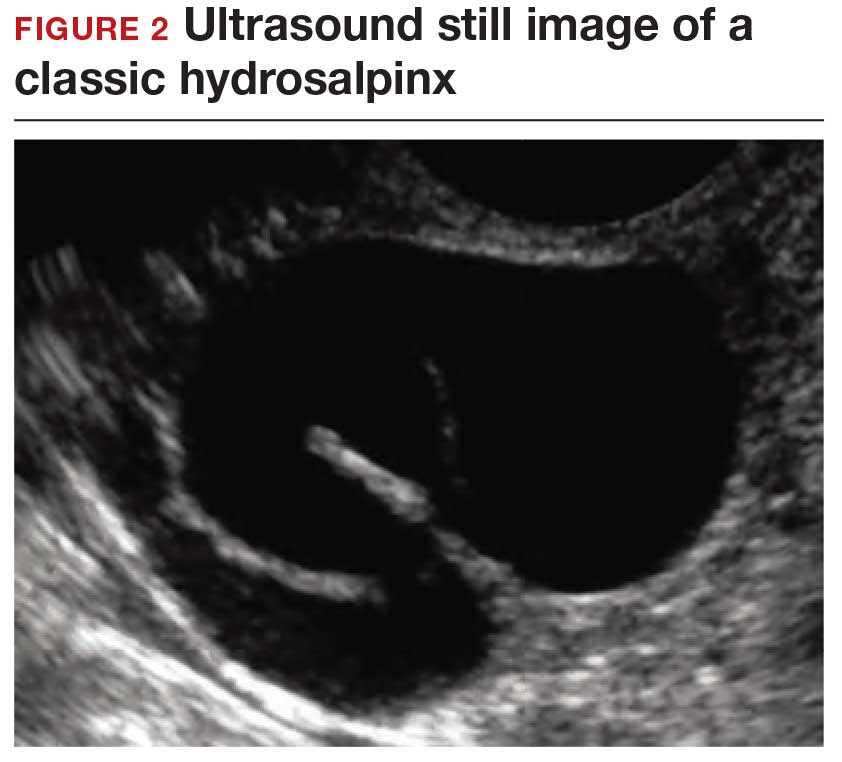
How can you evaluate a patient’s pelvic pain with an anatomic image? Perhaps pain can be corroborated if there is a classic ovarian endometrioma (FIGURE 1) (VIDEOS 3A, 3B) or classic hydrosalpinx (FIGURE 2) (VIDEOS 4A–4C). But can we evaluate pelvic pain with only an anatomic image? No, absolutely not. Evaluating pain requires dynamic assessment. As described above, in a dynamic ultrasound assessment, liberal use of the abdominal hand and the tip of the vaginal probe can elicit where the patient’s pain exists and whether the pain can be recreated.
Adhesions
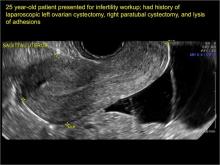
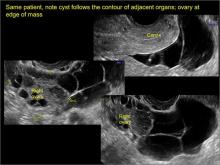
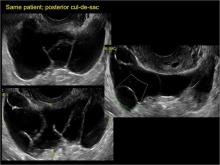
Pelvic adhesions can be a significant source of pelvic pain and, also, sometimes infertility. The adhesions themselves may not be visible on anatomic imaging. This is where the concept of the sliding organ sign is paramount, a concept first described by Dr. Ilan Timor-Tritsch in his book Transvaginal Sonography . 4 He stated, “Diagnosis of pelvic adhesions becomes possible by the ‘sliding organ sign.’ The transducer tip is pointed at the uterus, ovaries or any pelvic finding, and a gentle push-pull movement of several centimeters is started. If no adhesions are present, the organs will move freely in the pelvis. This displacement of organs is perceived on the screen as a sliding movement.” 4 Thus, if structures are in fact adherent, they will move in tandem with each other as evidenced by this dynamic assessment. If they are not adherent, they will move slightly but independently of each other ( VIDEOS 5A–5G ).
Continue to: Endometriosis...
Endometriosis
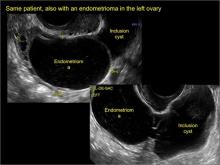
Dynamic ultrasonography can be a significant part of a nonlaparoscopic, presumptive diagnosis of endometriosis when there is no obvious ovarian endometrioma.5 The evidence for this comes from a classic paper by Okaro and colleagues, “The use of ultrasound‐based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy?”6 In that study, 120 consecutive women with chronic pelvic pain scheduled for laparoscopy underwent vaginal ultrasonography. Hard markers were defined as structural abnormalities, such as classic endometriomas or hydrosalpinges.
These markers demonstrated a 100% correlation (24 of 24 women) with laparoscopic findings, as one might have suspected. In addition, soft markers (VIDEOS 6A–6C) were defined as reduced ovarian mobility, site-specific pelvic tenderness, and the presence of loculated peritoneal fluid in the pelvis. These were predictive of pelvic pathology in 73% of these women (37 of 51).6
Thus, women who have soft markers on dynamic scanning but no obvious anatomic abnormalities can be treated with a high degree of sensitivity without the need for laparoscopic intervention.
Pelvic organ prolapse and incontinence
With the vaginal probe in place, and even a small amount of urine in the bladder, the patient can be asked to bear down (Valsalva maneuver), and cystocele (VIDEO 7) and/or hypermobility of the urethra (VIDEO 8) is easily discerned with dynamic ultrasonography. This information is not available on static anatomic imaging.
A tool that enhances patient care
Dynamic ultrasonography is an important and emerging topic in gynecologic imaging. Static images and even cine clips will yield only anatomic information. Increasingly, whoever holds the transducer—whether it be the gynecologist, radiologist, or sonographer—needs to examine the patient with the probe and include liberal use of the abdominal hand as well. Incorporating this concept will enhance the overall diagnostic input of ultrasound scanning, not just imaging, into better and more accurate patient care. ●
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in VIDEO 2A showing what appears to be a solid adnexal mass
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
VIDEO 3A Video clip of a classic endometrioma
VIDEO 3B Classic endometrioma showing no Doppler flow internally
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation
- Goldstein SR. Pregnancy I: Embryo. In: Endovaginal Ultrasound. 2nd ed. Wiley-Liss; 1991:58.
- Goldstein SR. Incorporating endovaginal ultrasonography into the overall gynecologic examination. Am J Obstet Gynecol. 1990;162:625-632.
- AIUM practice parameter for the performance of an ultrasound examination of the female pelvis. J Ultrasound Med. 2020;39:E17-E23.
- Timor-Tritsch IE, Rottem S, Elgali S. How transvaginal sonography is done. In: Timor-Tritsch IE, Rottem S, eds. Transvaginal Sonography. Elsevier Science Publishing Company, Inc; 1988:24.
- Taylor HS, Adamson GD, Diamond MP, et al. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int J Gynaecol Obstet. 2018;142:131-142.
- Okaro E, Condous G, Khalid A, et al. The use of ultrasound‐ based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy? BJOG. 2006;113:251-256.

Ultrasonography truly has revolutionized the practice of obstetrics and gynecology. Initially, transabdominal ultrasonography was mainly a tool of the obstetrician. Early linear array, real-time equipment had barely enough resolution to perform very limited assessments, such as measure biparietal diameter and identify vertex versus breech presentation, and anterior versus posterior placenta location. The introduction of transvaginal probes, which employ higher frequency and provide closer proximity to structures, yielded a degree of image magnification that was dubbed sonomicroscopy.1 In other words, we are seeing things with our naked eye that we could not see if we could hold them in our hand at arm’s length and squint at them. An example of this is the cardiac activity clearly visible in a 3-mm embryo at 45 days from the last menstrual period. One would not appreciate this without the low power magnification of the vaginal probe.
The concept of dynamic imaging
As early as 1990, I realized that there is a difference between an ultrasound “examination” performed because of referral for imaging, which generated a report back to the referring health care provider, and “examining” one’s own patient with ultrasonography at the time of bimanual exam. I coined the phrase “the ultrasound-enhanced bimanual exam,” and I believed it should become a routine part of gynecologic care. I put forth this thesis in an article entitled, “Incorporating endovaginal ultrasonography into the overall gynecologic examination.”2 The idea is based on thinking: What exactly are we are trying to discern from a bimanual exam?
Clinicians perform the bimanual exam thousands of times. The bimanual examination consists of 2 components, an objective portion and a subjective portion. The objective component attempts to discern information that is totally objective, such as, Is the ovary enlarged? If so, is it cystic or solid? Is this uterus normal in shape and contour? If so, does it feel like leiomyomas or is it globularly enlarged as with adenomyosis? The subjective component of the bimanual examination attempts to determine whether or not tenderness is present or if there is normal mobility of the pelvic organs.
The objective component can be replaced by an image in very little time if the examiner has the equipment and the knowledge and skill. The subjective component, however, depends on the experience and often the nuance of the examiner. That was my original thought process. I wanted, and still want, the examining clinician to use imaging as part of the overall exam. But now, I want the imager to use examination as part of the overall imaging. (VIDEOS 1A and 1B.) This is the concept of dynamic imaging. It involves the liberal use of the abdominal hand as well as an in-and-out motion of the vaginal probe to ascertain aspects of the examination that in the past I deemed “subjective.” Mainly, this involves the aspects of mobility and/or tenderness.
Continue to: Guidelines concerning pelvic ultrasound do not consider dynamic imaging...
Guidelines concerning pelvic ultrasound do not consider dynamic imaging
Until now, most imagers take a myriad of pictures, mostly still snapshots, to illustrate anatomy. Most imaging physicians then look at a series of such pictures and may never even hold the transducer. This is increasingly true in instances of remote teleradiology. Even for the minority of imagers who utilize video clips (VIDEOS 2A–2C), these are still representations of anatomy .
One need look no further than the guidelines that underpin the expectation of those who scan the female pelvis. The American Institute of Ultrasound in Medicine (AIUM) published a practice parameter for the performance of ultrasonography of the female pelvis, developed in collaboration with the American College of Radiology, American College of Obstetricians and Gynecologists, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound. 3 Nowhere does this document mention anything other than what images to obtain, where to look, and how to measure. Nowhere is there any mention of dynamic imaging—the concept of using one’s other hand on the abdomen, eliciting pain with the vaginal probe, checking for mobility, asking the patient to bear down. The document lists indications for pelvic sonography that include but are not limited to 19 different indications, such as pelvic pain, evaluation of dysmenorrhea, evaluation for signs or symptoms of pelvic infection, and evaluation of incontinence or pelvic organ prolapse (TABLE). 3

Dynamic ultrasonography can aid in the diagnosis of certain conditions
Specifically, what can dynamic ultrasonography add to anatomic imaging? The main considerations are pain, adhesions, endometriosis, and pelvic organ prolapse.
Pelvic pain or tenderness
How can you evaluate a patient’s pelvic pain with an anatomic image? Perhaps pain can be corroborated if there is a classic ovarian endometrioma (FIGURE 1) (VIDEOS 3A, 3B) or classic hydrosalpinx (FIGURE 2) (VIDEOS 4A–4C). But can we evaluate pelvic pain with only an anatomic image? No, absolutely not. Evaluating pain requires dynamic assessment. As described above, in a dynamic ultrasound assessment, liberal use of the abdominal hand and the tip of the vaginal probe can elicit where the patient’s pain exists and whether the pain can be recreated.


Adhesions
Pelvic adhesions can be a significant source of pelvic pain and, also, sometimes infertility. The adhesions themselves may not be visible on anatomic imaging. This is where the concept of the sliding organ sign is paramount, a concept first described by Dr. Ilan Timor-Tritsch in his book Transvaginal Sonography . 4 He stated, “Diagnosis of pelvic adhesions becomes possible by the ‘sliding organ sign.’ The transducer tip is pointed at the uterus, ovaries or any pelvic finding, and a gentle push-pull movement of several centimeters is started. If no adhesions are present, the organs will move freely in the pelvis. This displacement of organs is perceived on the screen as a sliding movement.” 4 Thus, if structures are in fact adherent, they will move in tandem with each other as evidenced by this dynamic assessment. If they are not adherent, they will move slightly but independently of each other ( VIDEOS 5A–5G ).
Continue to: Endometriosis...
Endometriosis
Dynamic ultrasonography can be a significant part of a nonlaparoscopic, presumptive diagnosis of endometriosis when there is no obvious ovarian endometrioma.5 The evidence for this comes from a classic paper by Okaro and colleagues, “The use of ultrasound‐based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy?”6 In that study, 120 consecutive women with chronic pelvic pain scheduled for laparoscopy underwent vaginal ultrasonography. Hard markers were defined as structural abnormalities, such as classic endometriomas or hydrosalpinges.
These markers demonstrated a 100% correlation (24 of 24 women) with laparoscopic findings, as one might have suspected. In addition, soft markers (VIDEOS 6A–6C) were defined as reduced ovarian mobility, site-specific pelvic tenderness, and the presence of loculated peritoneal fluid in the pelvis. These were predictive of pelvic pathology in 73% of these women (37 of 51).6
Thus, women who have soft markers on dynamic scanning but no obvious anatomic abnormalities can be treated with a high degree of sensitivity without the need for laparoscopic intervention.
Pelvic organ prolapse and incontinence
With the vaginal probe in place, and even a small amount of urine in the bladder, the patient can be asked to bear down (Valsalva maneuver), and cystocele (VIDEO 7) and/or hypermobility of the urethra (VIDEO 8) is easily discerned with dynamic ultrasonography. This information is not available on static anatomic imaging.
A tool that enhances patient care
Dynamic ultrasonography is an important and emerging topic in gynecologic imaging. Static images and even cine clips will yield only anatomic information. Increasingly, whoever holds the transducer—whether it be the gynecologist, radiologist, or sonographer—needs to examine the patient with the probe and include liberal use of the abdominal hand as well. Incorporating this concept will enhance the overall diagnostic input of ultrasound scanning, not just imaging, into better and more accurate patient care. ●
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in VIDEO 2A showing what appears to be a solid adnexal mass
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
VIDEO 3A Video clip of a classic endometrioma
VIDEO 3B Classic endometrioma showing no Doppler flow internally
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation

Ultrasonography truly has revolutionized the practice of obstetrics and gynecology. Initially, transabdominal ultrasonography was mainly a tool of the obstetrician. Early linear array, real-time equipment had barely enough resolution to perform very limited assessments, such as measure biparietal diameter and identify vertex versus breech presentation, and anterior versus posterior placenta location. The introduction of transvaginal probes, which employ higher frequency and provide closer proximity to structures, yielded a degree of image magnification that was dubbed sonomicroscopy.1 In other words, we are seeing things with our naked eye that we could not see if we could hold them in our hand at arm’s length and squint at them. An example of this is the cardiac activity clearly visible in a 3-mm embryo at 45 days from the last menstrual period. One would not appreciate this without the low power magnification of the vaginal probe.
The concept of dynamic imaging
As early as 1990, I realized that there is a difference between an ultrasound “examination” performed because of referral for imaging, which generated a report back to the referring health care provider, and “examining” one’s own patient with ultrasonography at the time of bimanual exam. I coined the phrase “the ultrasound-enhanced bimanual exam,” and I believed it should become a routine part of gynecologic care. I put forth this thesis in an article entitled, “Incorporating endovaginal ultrasonography into the overall gynecologic examination.”2 The idea is based on thinking: What exactly are we are trying to discern from a bimanual exam?
Clinicians perform the bimanual exam thousands of times. The bimanual examination consists of 2 components, an objective portion and a subjective portion. The objective component attempts to discern information that is totally objective, such as, Is the ovary enlarged? If so, is it cystic or solid? Is this uterus normal in shape and contour? If so, does it feel like leiomyomas or is it globularly enlarged as with adenomyosis? The subjective component of the bimanual examination attempts to determine whether or not tenderness is present or if there is normal mobility of the pelvic organs.
The objective component can be replaced by an image in very little time if the examiner has the equipment and the knowledge and skill. The subjective component, however, depends on the experience and often the nuance of the examiner. That was my original thought process. I wanted, and still want, the examining clinician to use imaging as part of the overall exam. But now, I want the imager to use examination as part of the overall imaging. (VIDEOS 1A and 1B.) This is the concept of dynamic imaging. It involves the liberal use of the abdominal hand as well as an in-and-out motion of the vaginal probe to ascertain aspects of the examination that in the past I deemed “subjective.” Mainly, this involves the aspects of mobility and/or tenderness.
Continue to: Guidelines concerning pelvic ultrasound do not consider dynamic imaging...
Guidelines concerning pelvic ultrasound do not consider dynamic imaging
Until now, most imagers take a myriad of pictures, mostly still snapshots, to illustrate anatomy. Most imaging physicians then look at a series of such pictures and may never even hold the transducer. This is increasingly true in instances of remote teleradiology. Even for the minority of imagers who utilize video clips (VIDEOS 2A–2C), these are still representations of anatomy .
One need look no further than the guidelines that underpin the expectation of those who scan the female pelvis. The American Institute of Ultrasound in Medicine (AIUM) published a practice parameter for the performance of ultrasonography of the female pelvis, developed in collaboration with the American College of Radiology, American College of Obstetricians and Gynecologists, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound. 3 Nowhere does this document mention anything other than what images to obtain, where to look, and how to measure. Nowhere is there any mention of dynamic imaging—the concept of using one’s other hand on the abdomen, eliciting pain with the vaginal probe, checking for mobility, asking the patient to bear down. The document lists indications for pelvic sonography that include but are not limited to 19 different indications, such as pelvic pain, evaluation of dysmenorrhea, evaluation for signs or symptoms of pelvic infection, and evaluation of incontinence or pelvic organ prolapse (TABLE). 3

Dynamic ultrasonography can aid in the diagnosis of certain conditions
Specifically, what can dynamic ultrasonography add to anatomic imaging? The main considerations are pain, adhesions, endometriosis, and pelvic organ prolapse.
Pelvic pain or tenderness
How can you evaluate a patient’s pelvic pain with an anatomic image? Perhaps pain can be corroborated if there is a classic ovarian endometrioma (FIGURE 1) (VIDEOS 3A, 3B) or classic hydrosalpinx (FIGURE 2) (VIDEOS 4A–4C). But can we evaluate pelvic pain with only an anatomic image? No, absolutely not. Evaluating pain requires dynamic assessment. As described above, in a dynamic ultrasound assessment, liberal use of the abdominal hand and the tip of the vaginal probe can elicit where the patient’s pain exists and whether the pain can be recreated.


Adhesions
Pelvic adhesions can be a significant source of pelvic pain and, also, sometimes infertility. The adhesions themselves may not be visible on anatomic imaging. This is where the concept of the sliding organ sign is paramount, a concept first described by Dr. Ilan Timor-Tritsch in his book Transvaginal Sonography . 4 He stated, “Diagnosis of pelvic adhesions becomes possible by the ‘sliding organ sign.’ The transducer tip is pointed at the uterus, ovaries or any pelvic finding, and a gentle push-pull movement of several centimeters is started. If no adhesions are present, the organs will move freely in the pelvis. This displacement of organs is perceived on the screen as a sliding movement.” 4 Thus, if structures are in fact adherent, they will move in tandem with each other as evidenced by this dynamic assessment. If they are not adherent, they will move slightly but independently of each other ( VIDEOS 5A–5G ).
Continue to: Endometriosis...
Endometriosis
Dynamic ultrasonography can be a significant part of a nonlaparoscopic, presumptive diagnosis of endometriosis when there is no obvious ovarian endometrioma.5 The evidence for this comes from a classic paper by Okaro and colleagues, “The use of ultrasound‐based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy?”6 In that study, 120 consecutive women with chronic pelvic pain scheduled for laparoscopy underwent vaginal ultrasonography. Hard markers were defined as structural abnormalities, such as classic endometriomas or hydrosalpinges.
These markers demonstrated a 100% correlation (24 of 24 women) with laparoscopic findings, as one might have suspected. In addition, soft markers (VIDEOS 6A–6C) were defined as reduced ovarian mobility, site-specific pelvic tenderness, and the presence of loculated peritoneal fluid in the pelvis. These were predictive of pelvic pathology in 73% of these women (37 of 51).6
Thus, women who have soft markers on dynamic scanning but no obvious anatomic abnormalities can be treated with a high degree of sensitivity without the need for laparoscopic intervention.
Pelvic organ prolapse and incontinence
With the vaginal probe in place, and even a small amount of urine in the bladder, the patient can be asked to bear down (Valsalva maneuver), and cystocele (VIDEO 7) and/or hypermobility of the urethra (VIDEO 8) is easily discerned with dynamic ultrasonography. This information is not available on static anatomic imaging.
A tool that enhances patient care
Dynamic ultrasonography is an important and emerging topic in gynecologic imaging. Static images and even cine clips will yield only anatomic information. Increasingly, whoever holds the transducer—whether it be the gynecologist, radiologist, or sonographer—needs to examine the patient with the probe and include liberal use of the abdominal hand as well. Incorporating this concept will enhance the overall diagnostic input of ultrasound scanning, not just imaging, into better and more accurate patient care. ●
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in VIDEO 2A showing what appears to be a solid adnexal mass
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
VIDEO 3A Video clip of a classic endometrioma
VIDEO 3B Classic endometrioma showing no Doppler flow internally
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation
- Goldstein SR. Pregnancy I: Embryo. In: Endovaginal Ultrasound. 2nd ed. Wiley-Liss; 1991:58.
- Goldstein SR. Incorporating endovaginal ultrasonography into the overall gynecologic examination. Am J Obstet Gynecol. 1990;162:625-632.
- AIUM practice parameter for the performance of an ultrasound examination of the female pelvis. J Ultrasound Med. 2020;39:E17-E23.
- Timor-Tritsch IE, Rottem S, Elgali S. How transvaginal sonography is done. In: Timor-Tritsch IE, Rottem S, eds. Transvaginal Sonography. Elsevier Science Publishing Company, Inc; 1988:24.
- Taylor HS, Adamson GD, Diamond MP, et al. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int J Gynaecol Obstet. 2018;142:131-142.
- Okaro E, Condous G, Khalid A, et al. The use of ultrasound‐ based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy? BJOG. 2006;113:251-256.
- Goldstein SR. Pregnancy I: Embryo. In: Endovaginal Ultrasound. 2nd ed. Wiley-Liss; 1991:58.
- Goldstein SR. Incorporating endovaginal ultrasonography into the overall gynecologic examination. Am J Obstet Gynecol. 1990;162:625-632.
- AIUM practice parameter for the performance of an ultrasound examination of the female pelvis. J Ultrasound Med. 2020;39:E17-E23.
- Timor-Tritsch IE, Rottem S, Elgali S. How transvaginal sonography is done. In: Timor-Tritsch IE, Rottem S, eds. Transvaginal Sonography. Elsevier Science Publishing Company, Inc; 1988:24.
- Taylor HS, Adamson GD, Diamond MP, et al. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int J Gynaecol Obstet. 2018;142:131-142.
- Okaro E, Condous G, Khalid A, et al. The use of ultrasound‐ based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy? BJOG. 2006;113:251-256.
How gynecologic procedures and pharmacologic treatments can affect the uterus
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
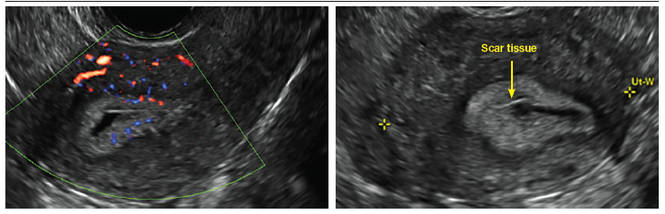
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
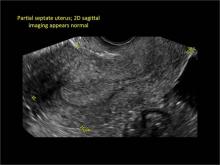
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
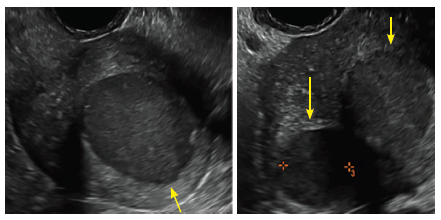
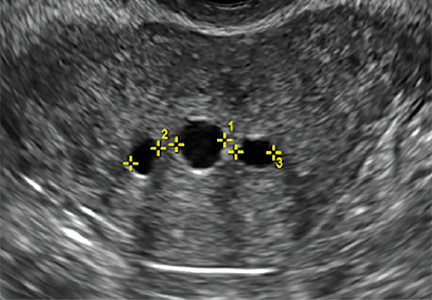
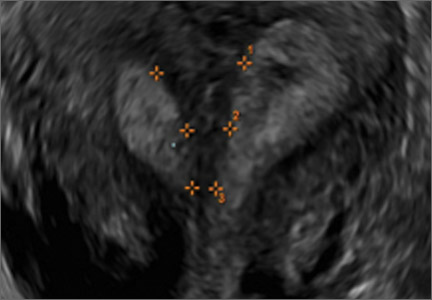
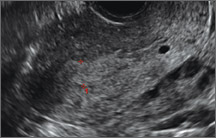
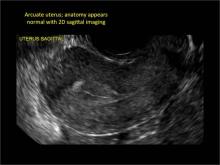
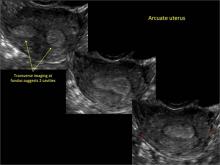
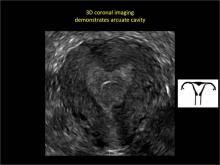
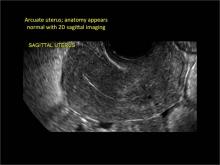
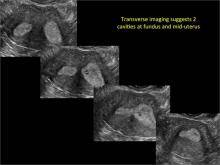
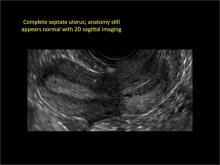
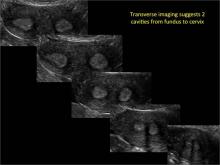
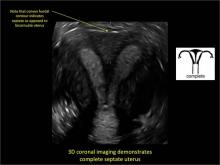
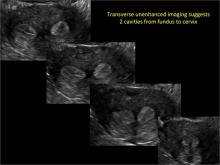
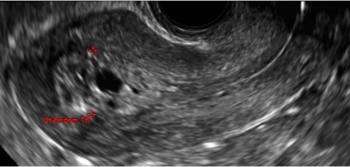
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
| FIGURE 1 Asherman syndrome | ||||
|
Postablation endometrial destruction
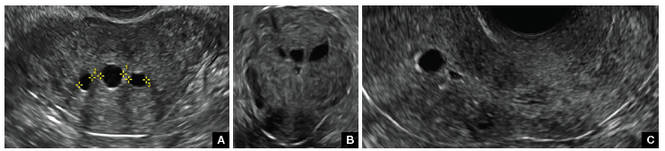
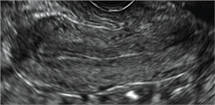
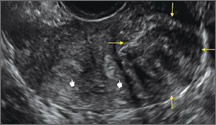
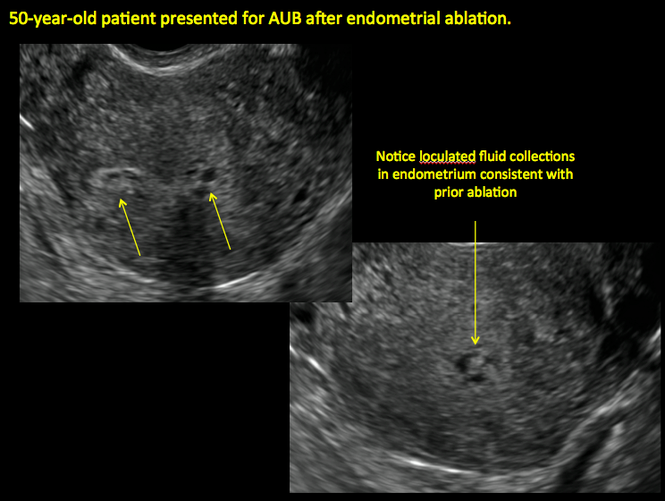
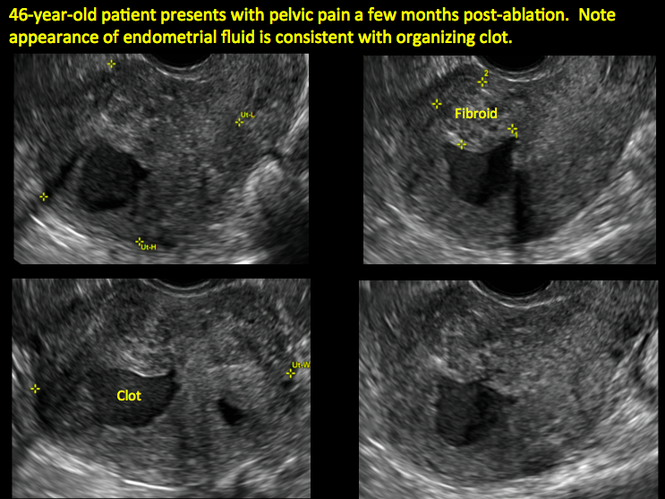
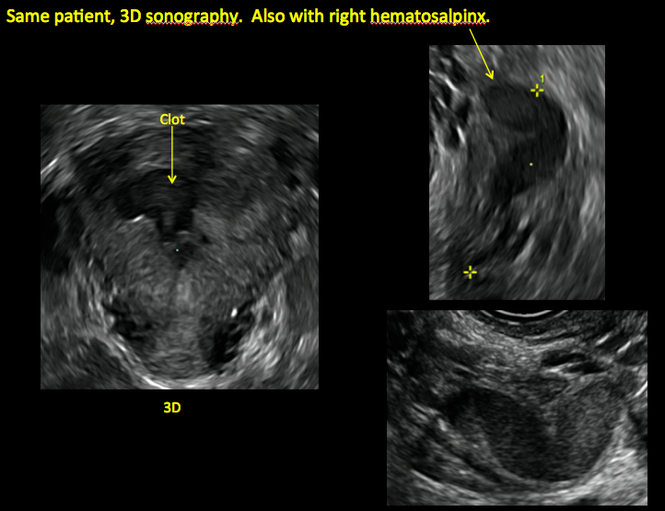
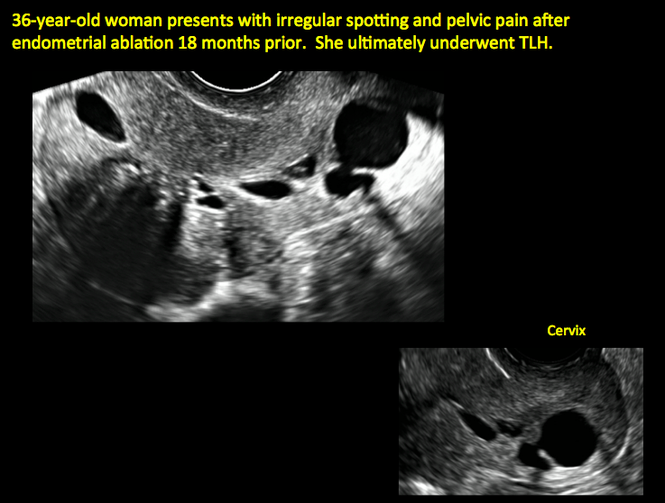
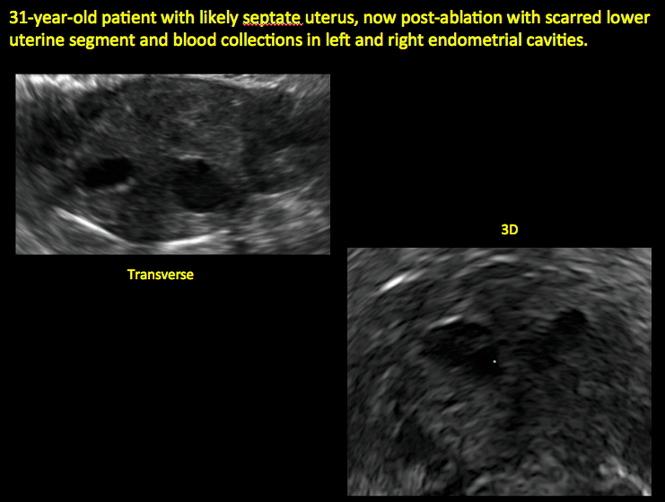
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
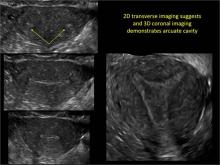
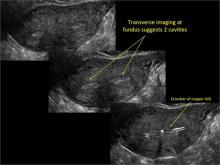
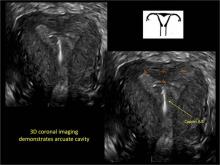
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
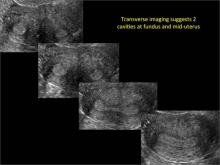
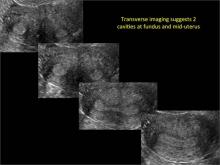
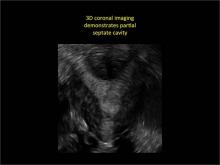
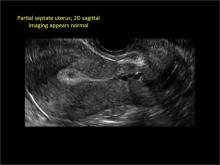
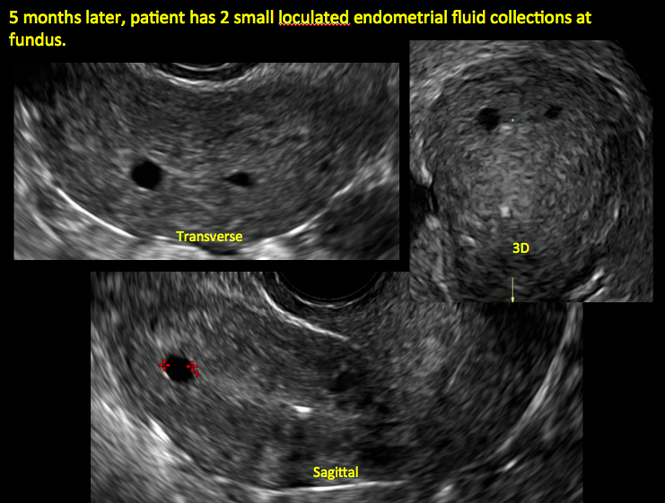
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
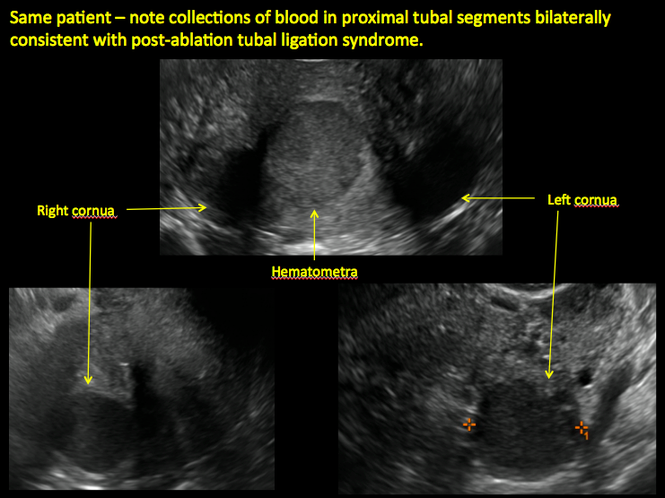
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
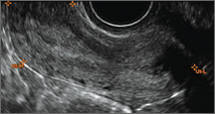
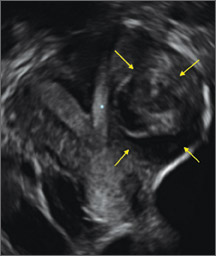
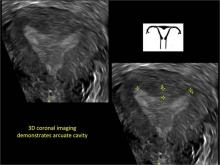
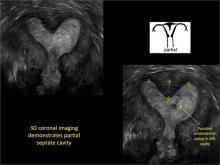
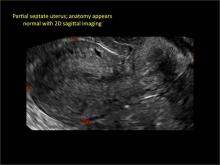
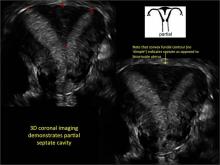
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
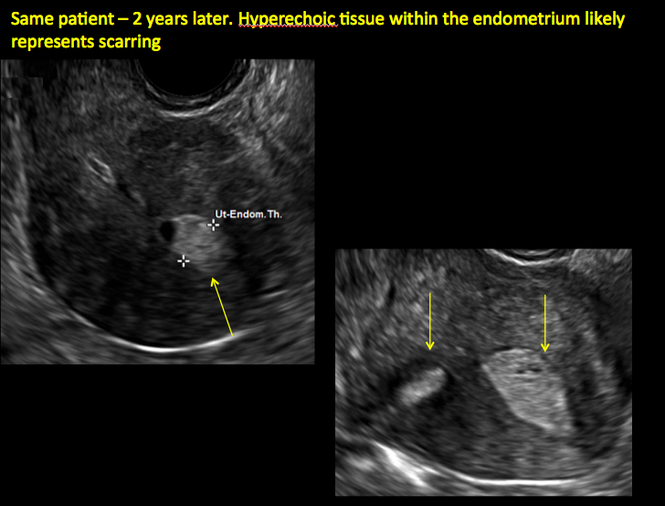
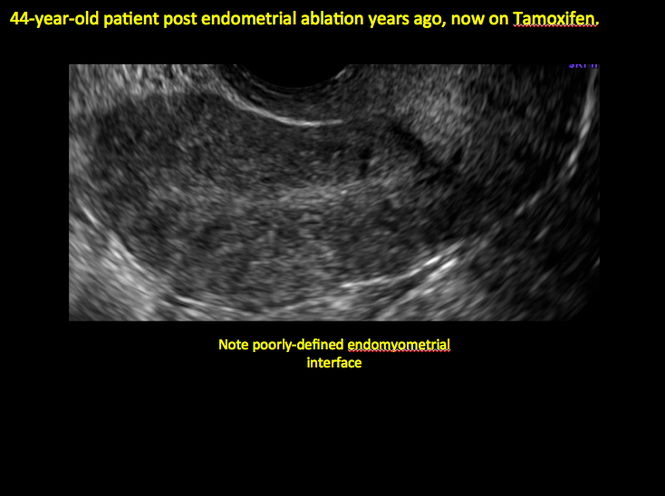
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
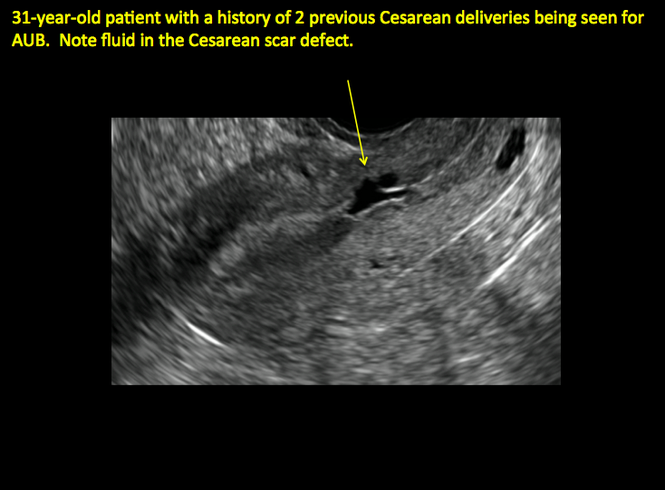
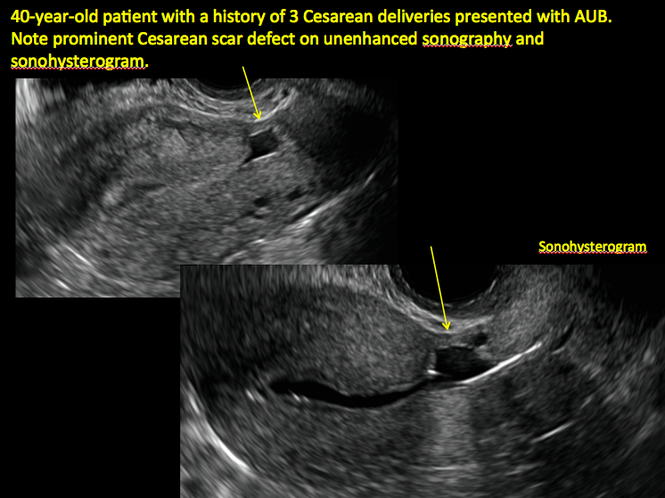
Cesarean scar defect on imaging
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||
 |  | 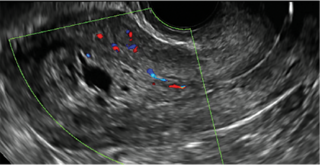 | ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
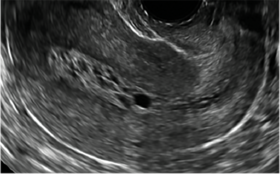
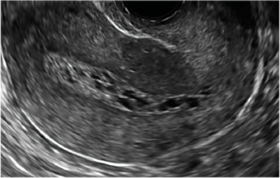
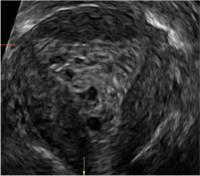
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||
 |  |  | ||
ADDITIONAL CASES - Postablation

ADDITIONAL CASES - Cesarean scar defect
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
| FIGURE 1 Asherman syndrome | ||||
|
Postablation endometrial destruction
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
Cesarean scar defect on imaging
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||
 |  |  | ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||
 |  |  | ||
ADDITIONAL CASES - Postablation










ADDITIONAL CASES - Cesarean scar defect


Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
| FIGURE 1 Asherman syndrome | ||||
|
Postablation endometrial destruction
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
Cesarean scar defect on imaging
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||
 |  |  | ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||
 |  |  | ||
ADDITIONAL CASES - Postablation










ADDITIONAL CASES - Cesarean scar defect


Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
In this Article
- Foreword by Steven R. Goldstein, MD
- Uterine changes postablation
- Endometrial changes with tamoxifen use
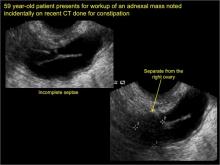
Imaging the suspected ovarian malignancy: 14 cases
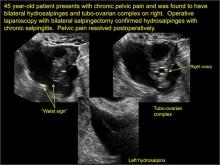
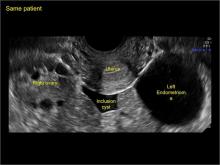
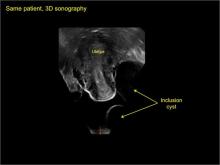
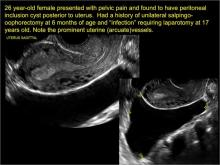
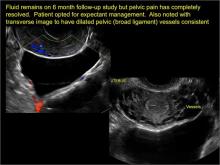
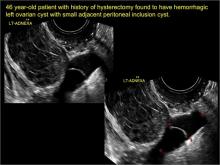
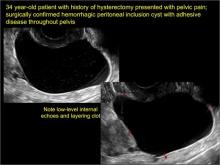
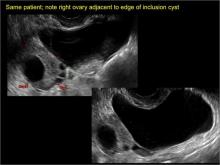
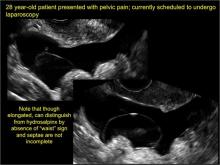
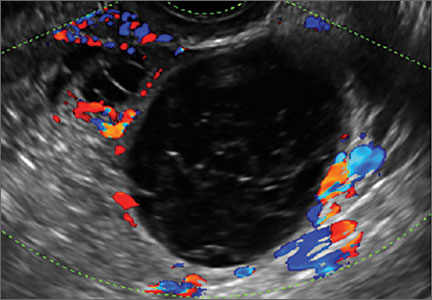
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts given its ability to characterize such cysts with high resolution and accuracy. Most cystic adnexal masses have characteristic findings that can guide counseling and management decisions. For instance, mature cystic teratomas have hyperechoic lines/dots and acoustic shadowing; hydrosalpinx are tubular or s shaped and show a “waist sign.”
In parts 1 through 3 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
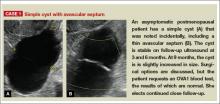
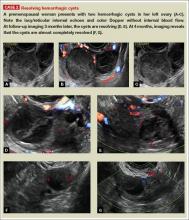
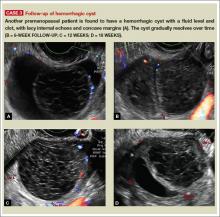
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma)
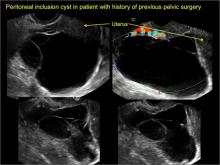
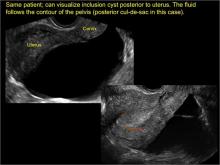
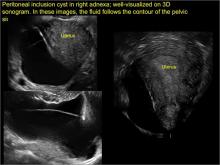
- hydrosalpinx and pelvic inclusion cysts (Part 3: “Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts)
In this conclusion to the series, we detail imaging for ovarian neoplasias (including cystadenoma and cystadenocarcinoma).
OVARIAN NEOPLASIA
A woman’s lifetime risk of undergoing surgery for suspected ovarian malignancy is 5% to 10% in the United States, and only about 13% to 21% of those undergoing surgery will actually be diagnosed with ovarian cancer.1 Therefore, the goal of diagnostic evaluation is to exclude malignancy.
Diagnostic evaluation includes:
- imaging
- lab work
- history
- physical findings.
The preferred imaging modality for a pelvic mass in asymptomatic premenopausal and postmenopausal women is transvaginal ultrasonography according to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin, which was reaffirmed in 2013.1 “No alternative imaging modality has demonstrated sufficient superiority to transvaginal ultrasonography to justify its routine use.”1
Transvaginal ultrasonography with color Doppler interrogation has demonstrated a sensitivity of 0.86% and a specificity of 0.91% for discriminating between malignant and benign ovarian masses.
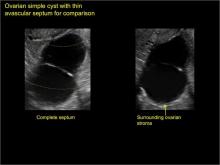
Sonographic features that are worrisome for malignancy include:
- Multiple thin septations (if indeterminate, the mass may possibly be benign)
- Thick (> 3 mm), irregular septations
- Focal areas of wall thickening (> 3 mm)
- Mural nodules or papillary projections
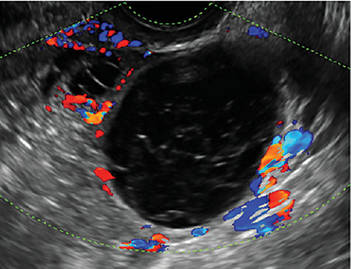
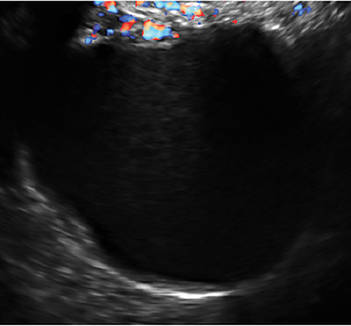
- Levine and colleagues note that a cyst with a mural nodule with internal blood flow on color Doppler has the highest likelihood of being malignant2
- Moderate or large amount of ascitic fluid in pelvis (in conjunction with ovarian mass showing the above characteristics)
Various morphology indices have been developed that combine these criteria with ovarian mass volume to determine the preoperative predictive value for malignancy.
In the images that follow, we present 14 cases that demonstrate cystadenoma, low malignant potential tumors, and ovarian neoplasia.
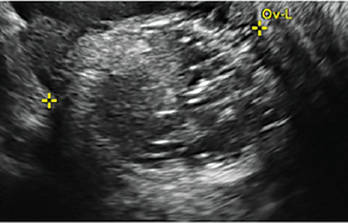
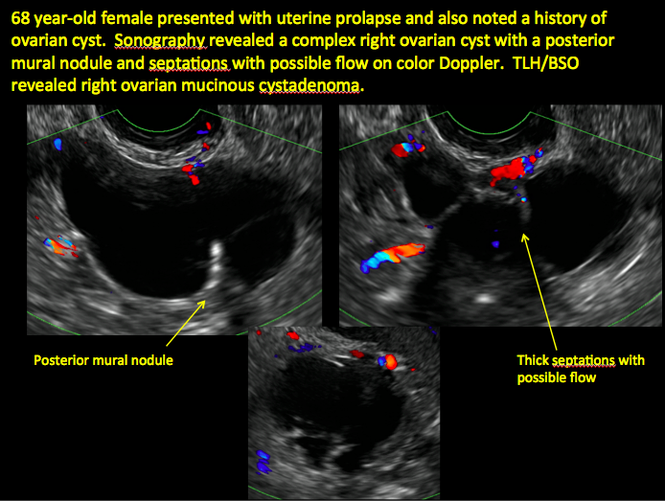
CASE 1. Right ovarian mucinous cystadenoma in 68-year-old woman with uterine prolapse and history of ovarian cyst
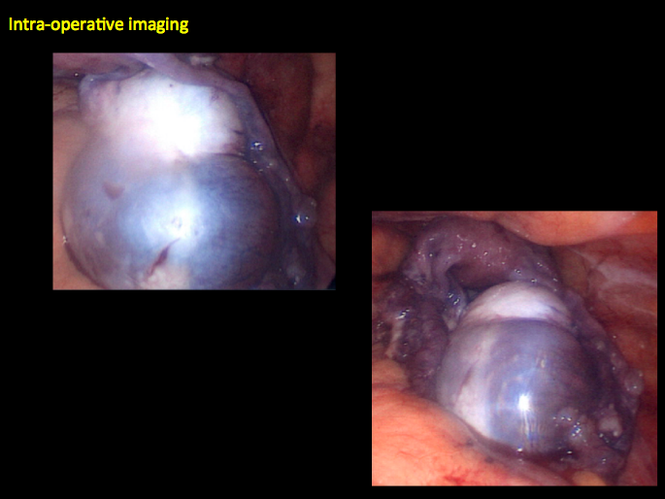
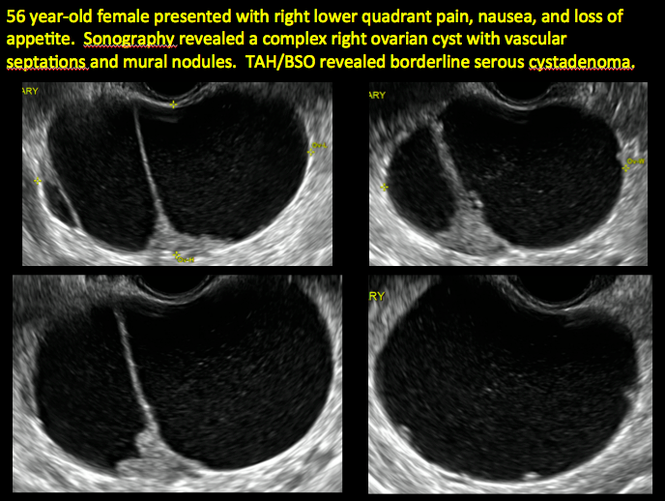
CASE 2. Borderline serous cystadenoma in 56-year-old woman with right lower quadrant pain, nausea, and loss of appetite

CASE 3. Mucinous cystadenoma in 38-year-old woman undergoing sonography for spontaneous abortion

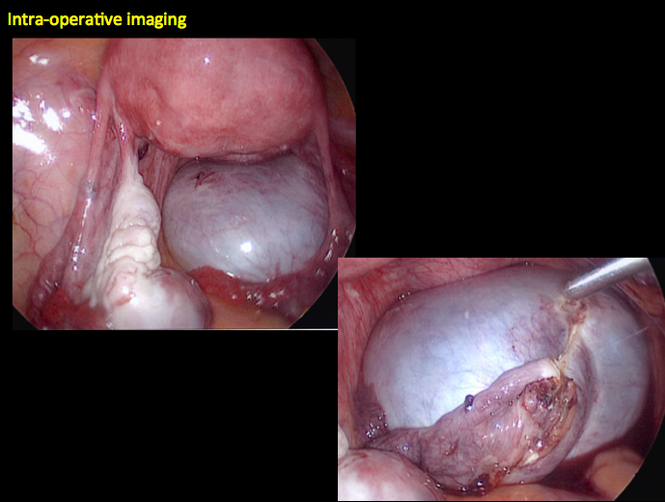
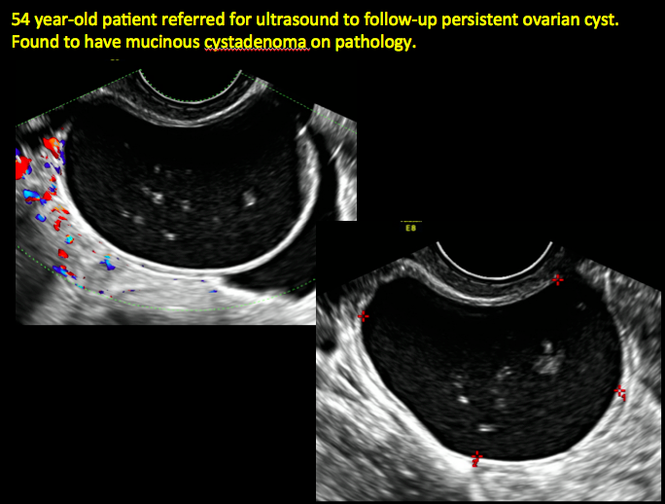
CASE 4. Mucinous cystadenoma in 54-year-old woman undergoing follow-up ultrasound for persistent ovarian cyst
CASE 7. Mature cystic teratoma in 31-year-old woman with progressively heavier bleeding and pelvic pain

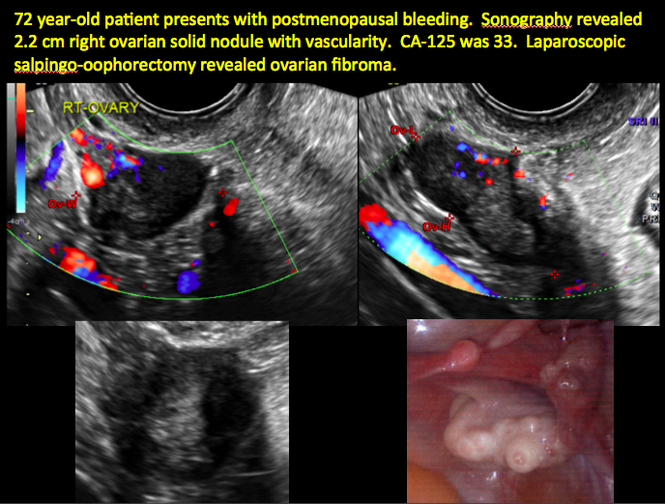
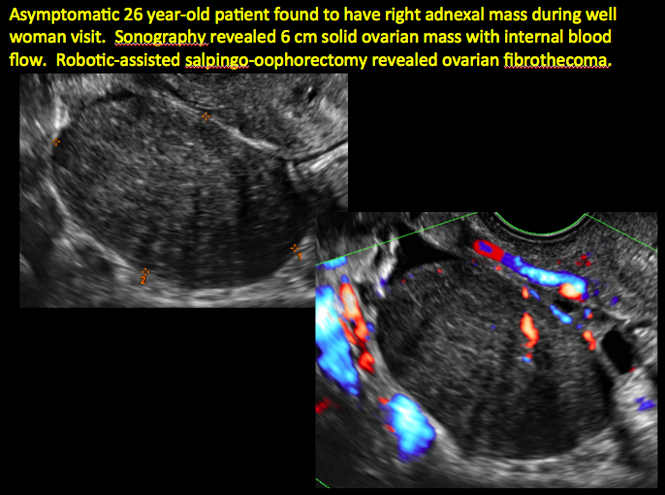
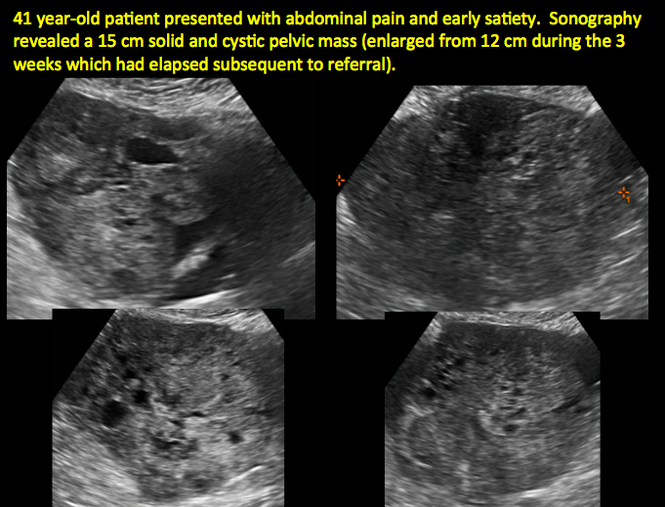
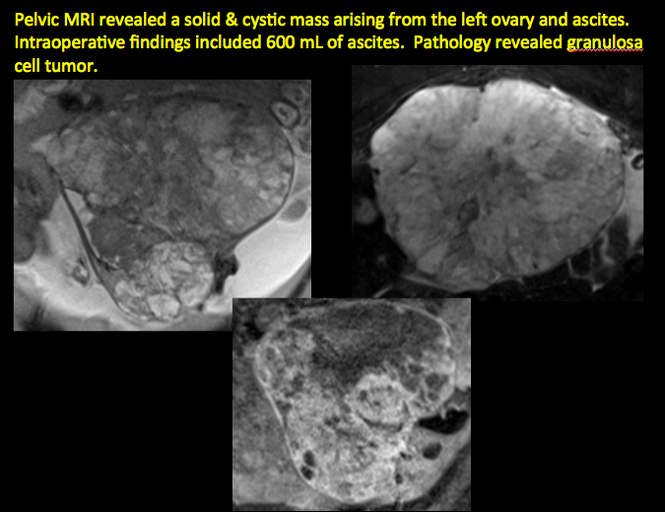
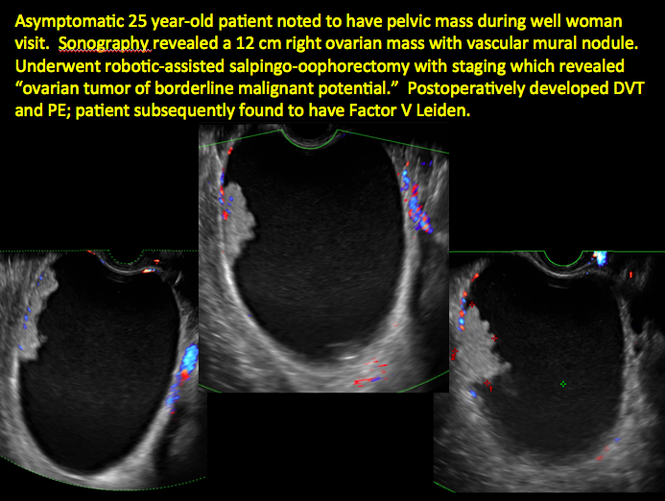
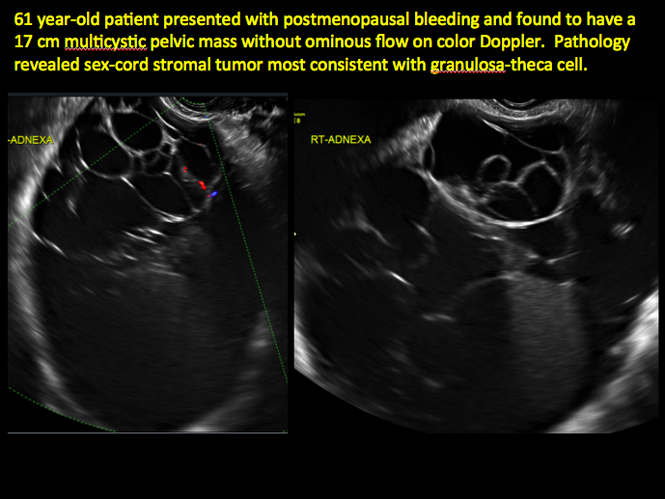
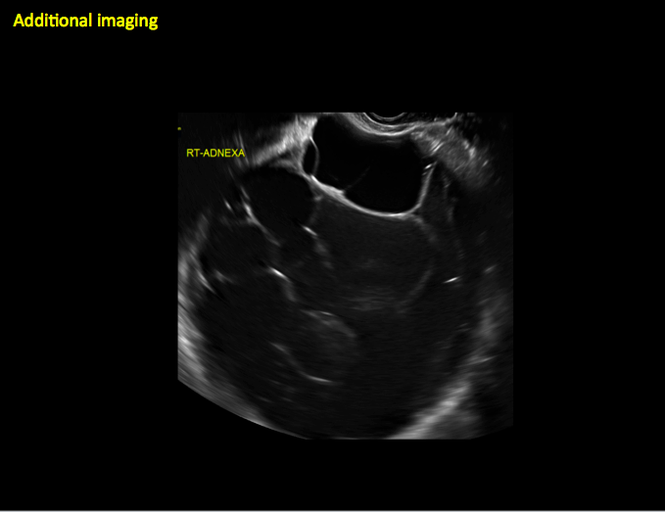
CASE 11. Sex-cord stromal tumor in 61-year-old woman with postmenopausal bleeding
CASE 12. Juvenile granulosa cell tumor in 19-year-old patient with secondary amenorrhea
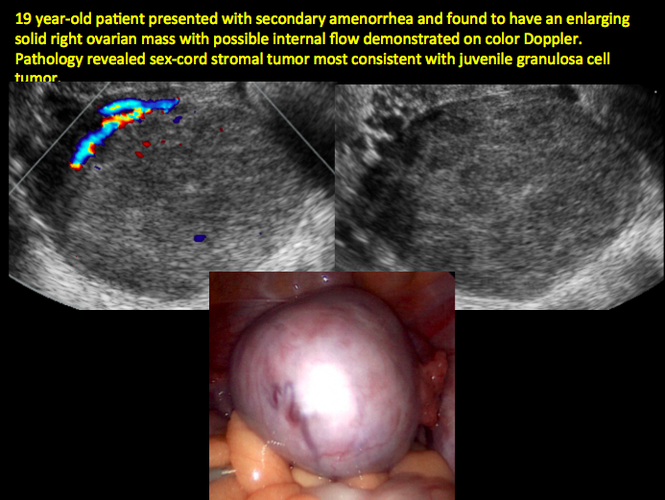
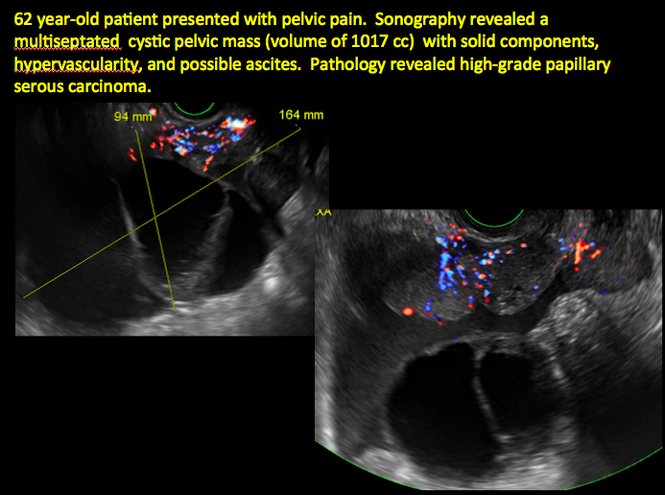
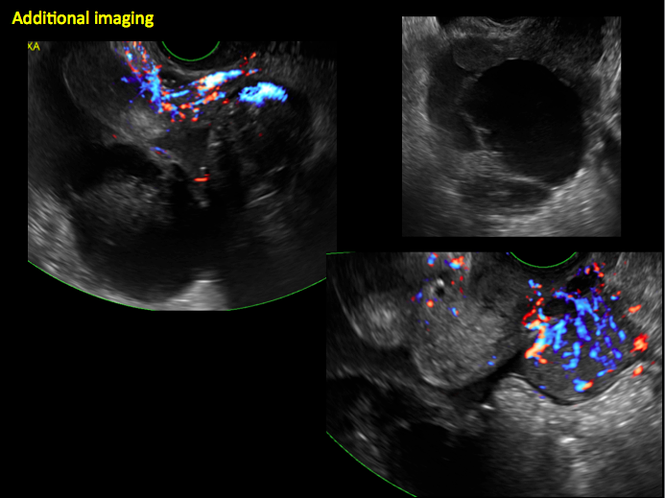
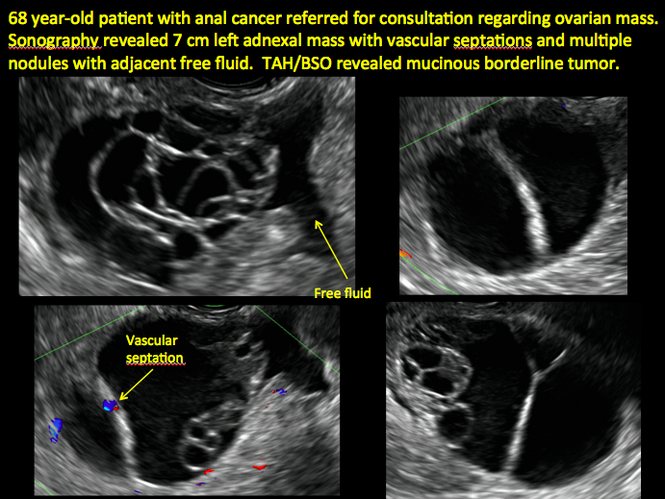
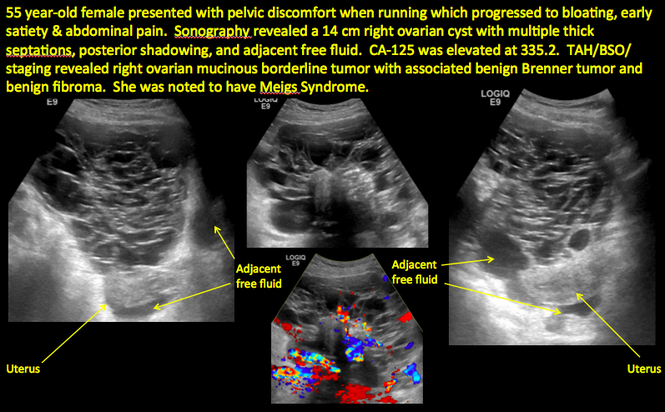
CASE 14. Mucinous borderline tumor in 55-year-old woman with pelvic discomfort
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts given its ability to characterize such cysts with high resolution and accuracy. Most cystic adnexal masses have characteristic findings that can guide counseling and management decisions. For instance, mature cystic teratomas have hyperechoic lines/dots and acoustic shadowing; hydrosalpinx are tubular or s shaped and show a “waist sign.”
In parts 1 through 3 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma)
- hydrosalpinx and pelvic inclusion cysts (Part 3: “Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts)
In this conclusion to the series, we detail imaging for ovarian neoplasias (including cystadenoma and cystadenocarcinoma).
OVARIAN NEOPLASIA
A woman’s lifetime risk of undergoing surgery for suspected ovarian malignancy is 5% to 10% in the United States, and only about 13% to 21% of those undergoing surgery will actually be diagnosed with ovarian cancer.1 Therefore, the goal of diagnostic evaluation is to exclude malignancy.
Diagnostic evaluation includes:
- imaging
- lab work
- history
- physical findings.
The preferred imaging modality for a pelvic mass in asymptomatic premenopausal and postmenopausal women is transvaginal ultrasonography according to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin, which was reaffirmed in 2013.1 “No alternative imaging modality has demonstrated sufficient superiority to transvaginal ultrasonography to justify its routine use.”1
Transvaginal ultrasonography with color Doppler interrogation has demonstrated a sensitivity of 0.86% and a specificity of 0.91% for discriminating between malignant and benign ovarian masses.
Sonographic features that are worrisome for malignancy include:
- Multiple thin septations (if indeterminate, the mass may possibly be benign)
- Thick (> 3 mm), irregular septations
- Focal areas of wall thickening (> 3 mm)
- Mural nodules or papillary projections
- Levine and colleagues note that a cyst with a mural nodule with internal blood flow on color Doppler has the highest likelihood of being malignant2
- Moderate or large amount of ascitic fluid in pelvis (in conjunction with ovarian mass showing the above characteristics)
Various morphology indices have been developed that combine these criteria with ovarian mass volume to determine the preoperative predictive value for malignancy.
In the images that follow, we present 14 cases that demonstrate cystadenoma, low malignant potential tumors, and ovarian neoplasia.
CASE 1. Right ovarian mucinous cystadenoma in 68-year-old woman with uterine prolapse and history of ovarian cyst


CASE 2. Borderline serous cystadenoma in 56-year-old woman with right lower quadrant pain, nausea, and loss of appetite


CASE 3. Mucinous cystadenoma in 38-year-old woman undergoing sonography for spontaneous abortion


CASE 4. Mucinous cystadenoma in 54-year-old woman undergoing follow-up ultrasound for persistent ovarian cyst



CASE 7. Mature cystic teratoma in 31-year-old woman with progressively heavier bleeding and pelvic pain





CASE 11. Sex-cord stromal tumor in 61-year-old woman with postmenopausal bleeding


CASE 12. Juvenile granulosa cell tumor in 19-year-old patient with secondary amenorrhea



CASE 14. Mucinous borderline tumor in 55-year-old woman with pelvic discomfort

Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts given its ability to characterize such cysts with high resolution and accuracy. Most cystic adnexal masses have characteristic findings that can guide counseling and management decisions. For instance, mature cystic teratomas have hyperechoic lines/dots and acoustic shadowing; hydrosalpinx are tubular or s shaped and show a “waist sign.”
In parts 1 through 3 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma)
- hydrosalpinx and pelvic inclusion cysts (Part 3: “Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts)
In this conclusion to the series, we detail imaging for ovarian neoplasias (including cystadenoma and cystadenocarcinoma).
OVARIAN NEOPLASIA
A woman’s lifetime risk of undergoing surgery for suspected ovarian malignancy is 5% to 10% in the United States, and only about 13% to 21% of those undergoing surgery will actually be diagnosed with ovarian cancer.1 Therefore, the goal of diagnostic evaluation is to exclude malignancy.
Diagnostic evaluation includes:
- imaging
- lab work
- history
- physical findings.
The preferred imaging modality for a pelvic mass in asymptomatic premenopausal and postmenopausal women is transvaginal ultrasonography according to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin, which was reaffirmed in 2013.1 “No alternative imaging modality has demonstrated sufficient superiority to transvaginal ultrasonography to justify its routine use.”1
Transvaginal ultrasonography with color Doppler interrogation has demonstrated a sensitivity of 0.86% and a specificity of 0.91% for discriminating between malignant and benign ovarian masses.
Sonographic features that are worrisome for malignancy include:
- Multiple thin septations (if indeterminate, the mass may possibly be benign)
- Thick (> 3 mm), irregular septations
- Focal areas of wall thickening (> 3 mm)
- Mural nodules or papillary projections
- Levine and colleagues note that a cyst with a mural nodule with internal blood flow on color Doppler has the highest likelihood of being malignant2
- Moderate or large amount of ascitic fluid in pelvis (in conjunction with ovarian mass showing the above characteristics)
Various morphology indices have been developed that combine these criteria with ovarian mass volume to determine the preoperative predictive value for malignancy.
In the images that follow, we present 14 cases that demonstrate cystadenoma, low malignant potential tumors, and ovarian neoplasia.
CASE 1. Right ovarian mucinous cystadenoma in 68-year-old woman with uterine prolapse and history of ovarian cyst


CASE 2. Borderline serous cystadenoma in 56-year-old woman with right lower quadrant pain, nausea, and loss of appetite


CASE 3. Mucinous cystadenoma in 38-year-old woman undergoing sonography for spontaneous abortion


CASE 4. Mucinous cystadenoma in 54-year-old woman undergoing follow-up ultrasound for persistent ovarian cyst



CASE 7. Mature cystic teratoma in 31-year-old woman with progressively heavier bleeding and pelvic pain





CASE 11. Sex-cord stromal tumor in 61-year-old woman with postmenopausal bleeding


CASE 12. Juvenile granulosa cell tumor in 19-year-old patient with secondary amenorrhea



CASE 14. Mucinous borderline tumor in 55-year-old woman with pelvic discomfort

“Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts
Ultrasonography is the preferred imaging method to evaluate most adnexal cysts. Most types of pelvic cyst pathology have characteristic findings that, when identified, can guide counseling and management decisions. For instance, simple cysts have thin walls, are uniformly hypoechoic, and show no blood flow on color Doppler. Endometriomas, on the other hand, demonstrate diffuse, low-level internal echoes on ultrasonography.
In parts 1 and 2 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- and mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma).
In this part 3, we detail imaging for hydrosalpinx and pelvic inclusion cysts. In part 4 we will consider cystadenomas and ovarian neoplasias.
hydrosalpinx
These cysts are caused by fimbrial obstruction and result in tubal distention with serous fluid. A hydrosalpinx may occur following an episode of salpingitis or pelvic surgery.
Sonographic features diagnostic for hydrosalpinx include a tubular or S-shaped cystic mass separate from the ovary, with:
- “beads on a string” or “cogwheel” appearance (small round nodules less than 3 mm in size that represent endosalpingeal folds when viewed in cross section)
- “waist sign” (indentations on opposite sides)
- incomplete septations, which result from segments of distended tube folding over/adhering to other tubal segments
Levine and colleagues noted that 3-dimensional imaging may be helpful when the diagnosis is uncertain.1
When a mass is noted that has features classic for hydrosalpinx, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommends1:
- no further imaging is necessary to establish the diagnosis
- frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 1 through 6 below (slides of image collections), we present 5 cases, including one of a 45-year-old patient presenting with chronic pelvic pain who was found to have bilateral hydrosalginges and right-sided tubo-ovarian complex.
pelvic inclusion cysts
Pelvic/peritoneal inclusion cysts, or peritoneal pseudocysts, are typically associated with factors that increase the risk for pelvic adhesive disease (including endometriosis, pelvic inflammatory disease, or prior pelvic surgery).
Classic sonographic features of pelvic inclusion cysts are:
- cystic mass, usually with septations/loculations
- the mass follows the contour of adjacent organs
- ovary at edge of the mass or sometimes suspended within it
- with or without flow in septation on color Doppler
When a mass is noted that has features classic for a peritoneal inclusion cyst, the US Society of Radiologists in Ultrasound recommends that1:
- no further imaging is necessary to establish the diagnosis (although further imaging may be needed if the diagnosis is uncertain)
- the frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 7 through 22 below (slides of image collections), we present several cases that demonstrate pelvic inclusion cysts on imaging. One case involves a 25-year-old patient presenting for 2- and 3-dimensional pelvic imaging due to infertility. She had a history of laparoscopic left ovarian cystectomy, right paratubal cystectomy, and lysis of adhesions. She was found to have a pelvic inclusion cyst and an endometrioma in the left ovary.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Figure 12
Figure 13
Figure 14
Figure 15
Figure 16
Figure 17
Figure 18
Figure 19
Figure 20
Figure 21
Figure 22
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Ultrasound Q. 2010;26(3):121−131.
Ultrasonography is the preferred imaging method to evaluate most adnexal cysts. Most types of pelvic cyst pathology have characteristic findings that, when identified, can guide counseling and management decisions. For instance, simple cysts have thin walls, are uniformly hypoechoic, and show no blood flow on color Doppler. Endometriomas, on the other hand, demonstrate diffuse, low-level internal echoes on ultrasonography.
In parts 1 and 2 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- and mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma).
In this part 3, we detail imaging for hydrosalpinx and pelvic inclusion cysts. In part 4 we will consider cystadenomas and ovarian neoplasias.
hydrosalpinx
These cysts are caused by fimbrial obstruction and result in tubal distention with serous fluid. A hydrosalpinx may occur following an episode of salpingitis or pelvic surgery.
Sonographic features diagnostic for hydrosalpinx include a tubular or S-shaped cystic mass separate from the ovary, with:
- “beads on a string” or “cogwheel” appearance (small round nodules less than 3 mm in size that represent endosalpingeal folds when viewed in cross section)
- “waist sign” (indentations on opposite sides)
- incomplete septations, which result from segments of distended tube folding over/adhering to other tubal segments
Levine and colleagues noted that 3-dimensional imaging may be helpful when the diagnosis is uncertain.1
When a mass is noted that has features classic for hydrosalpinx, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommends1:
- no further imaging is necessary to establish the diagnosis
- frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 1 through 6 below (slides of image collections), we present 5 cases, including one of a 45-year-old patient presenting with chronic pelvic pain who was found to have bilateral hydrosalginges and right-sided tubo-ovarian complex.
pelvic inclusion cysts
Pelvic/peritoneal inclusion cysts, or peritoneal pseudocysts, are typically associated with factors that increase the risk for pelvic adhesive disease (including endometriosis, pelvic inflammatory disease, or prior pelvic surgery).
Classic sonographic features of pelvic inclusion cysts are:
- cystic mass, usually with septations/loculations
- the mass follows the contour of adjacent organs
- ovary at edge of the mass or sometimes suspended within it
- with or without flow in septation on color Doppler
When a mass is noted that has features classic for a peritoneal inclusion cyst, the US Society of Radiologists in Ultrasound recommends that1:
- no further imaging is necessary to establish the diagnosis (although further imaging may be needed if the diagnosis is uncertain)
- the frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 7 through 22 below (slides of image collections), we present several cases that demonstrate pelvic inclusion cysts on imaging. One case involves a 25-year-old patient presenting for 2- and 3-dimensional pelvic imaging due to infertility. She had a history of laparoscopic left ovarian cystectomy, right paratubal cystectomy, and lysis of adhesions. She was found to have a pelvic inclusion cyst and an endometrioma in the left ovary.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Figure 12
Figure 13
Figure 14
Figure 15
Figure 16
Figure 17
Figure 18
Figure 19
Figure 20
Figure 21
Figure 22
Ultrasonography is the preferred imaging method to evaluate most adnexal cysts. Most types of pelvic cyst pathology have characteristic findings that, when identified, can guide counseling and management decisions. For instance, simple cysts have thin walls, are uniformly hypoechoic, and show no blood flow on color Doppler. Endometriomas, on the other hand, demonstrate diffuse, low-level internal echoes on ultrasonography.
In parts 1 and 2 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- and mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma).
In this part 3, we detail imaging for hydrosalpinx and pelvic inclusion cysts. In part 4 we will consider cystadenomas and ovarian neoplasias.
hydrosalpinx
These cysts are caused by fimbrial obstruction and result in tubal distention with serous fluid. A hydrosalpinx may occur following an episode of salpingitis or pelvic surgery.
Sonographic features diagnostic for hydrosalpinx include a tubular or S-shaped cystic mass separate from the ovary, with:
- “beads on a string” or “cogwheel” appearance (small round nodules less than 3 mm in size that represent endosalpingeal folds when viewed in cross section)
- “waist sign” (indentations on opposite sides)
- incomplete septations, which result from segments of distended tube folding over/adhering to other tubal segments
Levine and colleagues noted that 3-dimensional imaging may be helpful when the diagnosis is uncertain.1
When a mass is noted that has features classic for hydrosalpinx, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommends1:
- no further imaging is necessary to establish the diagnosis
- frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 1 through 6 below (slides of image collections), we present 5 cases, including one of a 45-year-old patient presenting with chronic pelvic pain who was found to have bilateral hydrosalginges and right-sided tubo-ovarian complex.
pelvic inclusion cysts
Pelvic/peritoneal inclusion cysts, or peritoneal pseudocysts, are typically associated with factors that increase the risk for pelvic adhesive disease (including endometriosis, pelvic inflammatory disease, or prior pelvic surgery).
Classic sonographic features of pelvic inclusion cysts are:
- cystic mass, usually with septations/loculations
- the mass follows the contour of adjacent organs
- ovary at edge of the mass or sometimes suspended within it
- with or without flow in septation on color Doppler
When a mass is noted that has features classic for a peritoneal inclusion cyst, the US Society of Radiologists in Ultrasound recommends that1:
- no further imaging is necessary to establish the diagnosis (although further imaging may be needed if the diagnosis is uncertain)
- the frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 7 through 22 below (slides of image collections), we present several cases that demonstrate pelvic inclusion cysts on imaging. One case involves a 25-year-old patient presenting for 2- and 3-dimensional pelvic imaging due to infertility. She had a history of laparoscopic left ovarian cystectomy, right paratubal cystectomy, and lysis of adhesions. She was found to have a pelvic inclusion cyst and an endometrioma in the left ovary.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Figure 12
Figure 13
Figure 14
Figure 15
Figure 16
Figure 17
Figure 18
Figure 19
Figure 20
Figure 21
Figure 22
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Ultrasound Q. 2010;26(3):121−131.
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Ultrasound Q. 2010;26(3):121−131.
Imaging the endometrioma and mature cystic teratoma
The preferred imaging method to evaluate the majority of adnexal cysts is ultrasonography, which can help characterize the cyst type. Common benign adnexal cyst types include simple, hemorrhagic, endometrioma, and mature teratoma (dermoid cyst). In this part 2 of a 4-part series on cystic adnexal pathology, we focus on imaging signs for, and follow-up of, endometriomas and mature teratomas.
Endometriomas
Endometriomas are common, typically benign, cysts that produce homogenous, low-level internal echoes and a “ground glass” appearance on ultrasonography. No internal flow is apparent on color Doppler. The presence of tiny echogenic wall foci can distinguish an endometrioma from a hemorrhagic cyst.
Rarely, endometriomas may undergo malignant transformation. Usually this occurs with cysts greater than 9 cm and in patients aged 45 years or older. A malignancy often exhibits rapid growth or the development of a solid nodule with flow on color Doppler.
Management
Although surgery remains the first-line management for women with symptomatic or enlarging endometriomas, there appears to be a role for sonographic observation, with continuous progestational treatment, in women with small (< 5 cm) asymptomatic endometriomas.
The Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommended1:
- Short-interval follow-up (6 to 12 weeks) in reproductive-aged women to ensure acute hemorrhagic cysts are not mistaken for endometriomas
- If not removed surgically, sonographic follow-up is recommended, with frequency of follow up based on patient age and symptoms and cyst size and characteristics.
In FIGURES 1 through 11 (slides of image collections), we present several cases, including one of a 25-year-old patient presenting with pelvic pain and dyspareunia who was later found to have bilateral endometriomas.
Mature teratomas
Mature cystic teratomas display several telltale signs on imaging, including:
- hyperechoic lines/dots (“dermoid mesh”) corresponding to hair/skeletal components
- “Rokitanski nodule” – a peripherally placed mass of sebum, bones, and hair
- posterior acoustic shadowing
- cystic or floating spherical structures
- no internal flow on color Doppler
Rarely, dermoid cysts may undergo malignant transformation. Usually this occurs in cysts greater than 10 cm and in patients aged 50 years or older. Internal flow on color Doppler, branching, or invasion into adjacent structures can indicate malignancy.
Management
The traditional treatment for dermoid cysts is surgical. However, given the ability for accurate diagnosis with vaginal ultrasonography, there appears to be a role for sonographic observation in asymptomatic women with small dermoids.2
If the cyst is not surgically removed, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommended initial sonographic follow up at no more than 6 months to 1 year to ensure no change in size or internal architecture.1
In FIGURES 12 through 24 below (slides of image collections), we offer imaging from the case presentation and follow-up of a 19-year-old patient with pelvic pain who has a history of ovarian cystectomy for dermoid cyst, as well as 6 additional case illustrations.
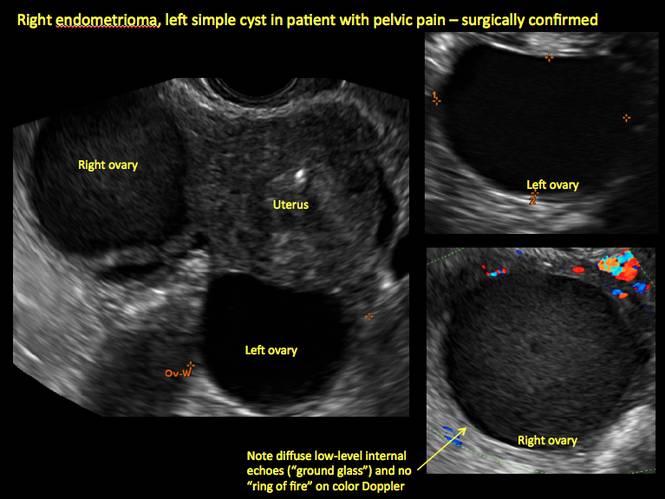
Figure 1
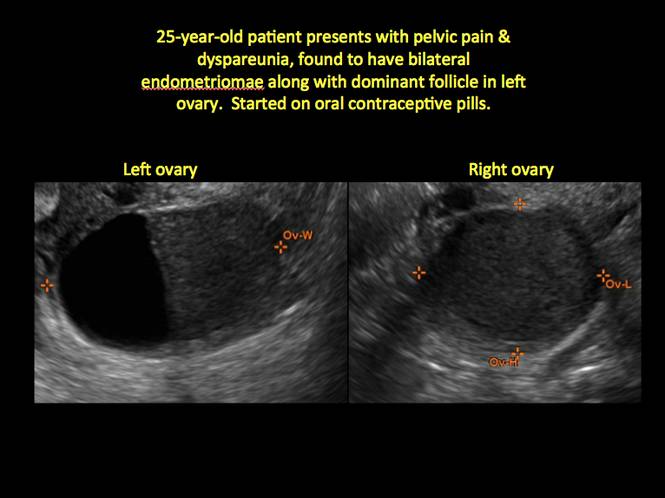
Figure 2
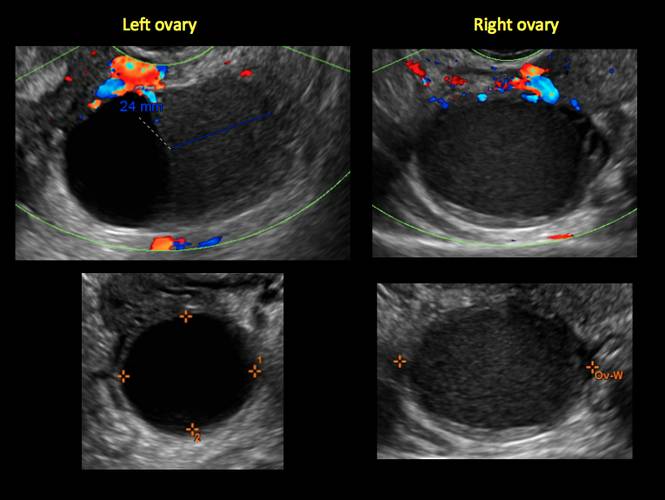
Figure 3
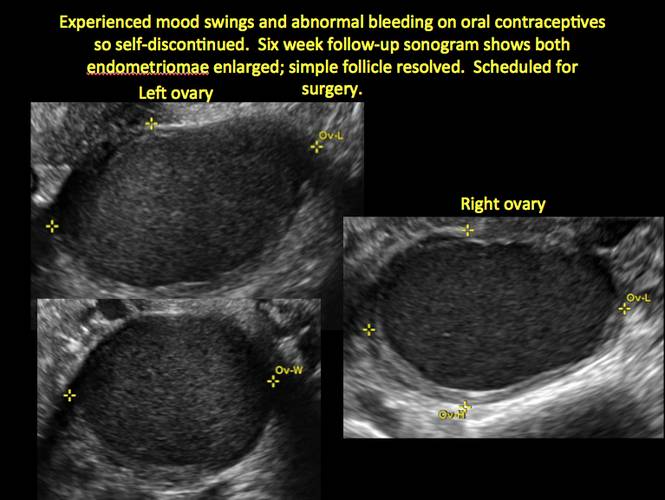
Figure 4
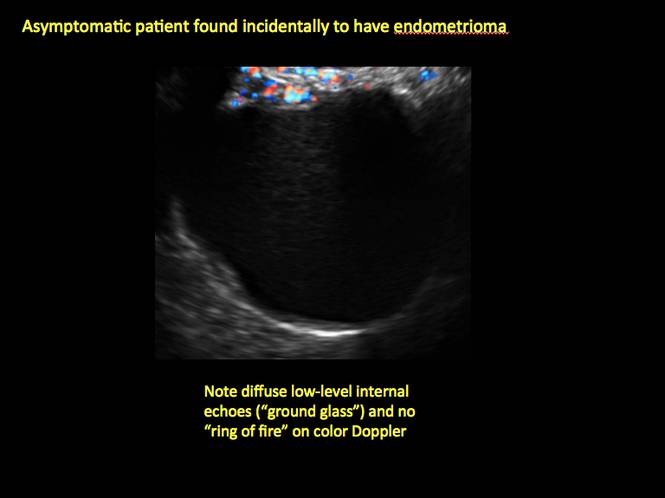
Figure 5
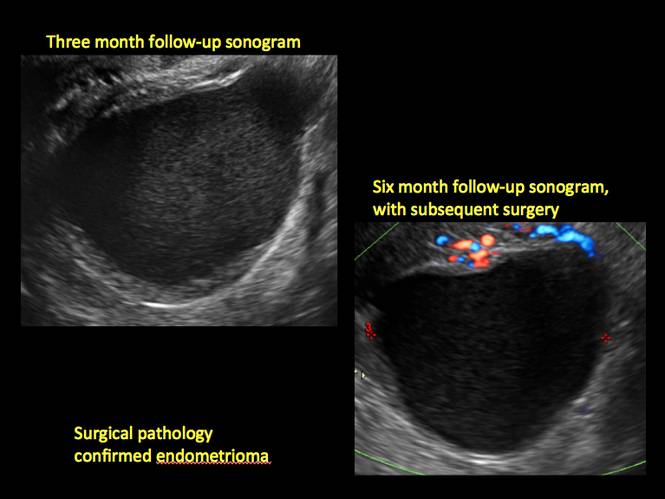
Figure 6
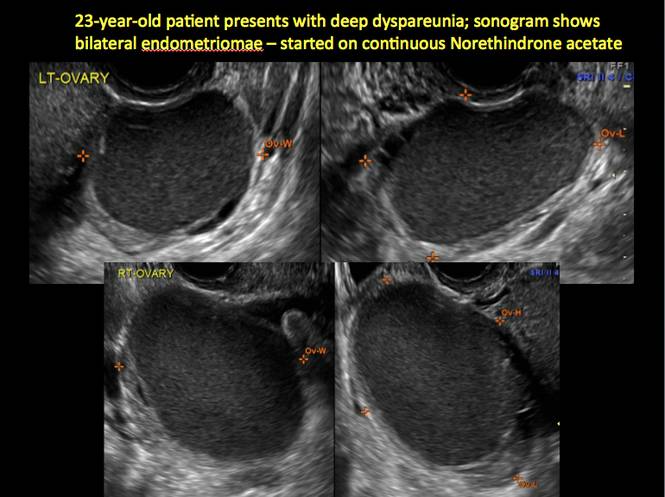
Figure 7
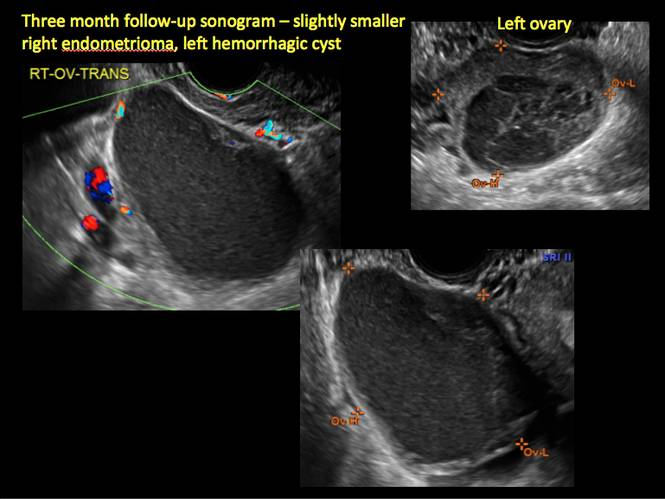
Figure 8
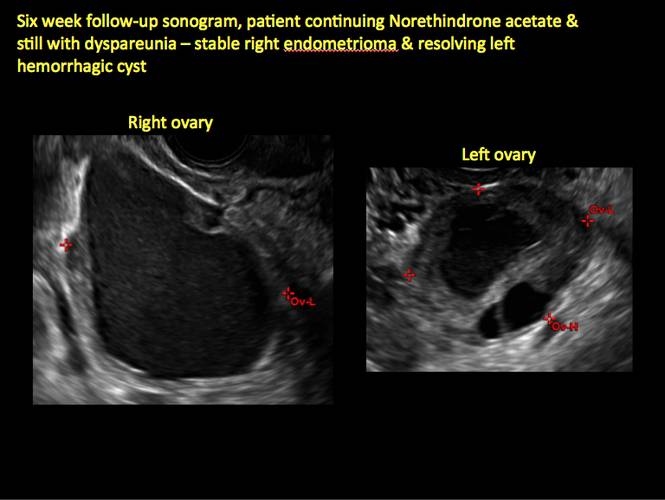
Figure 9
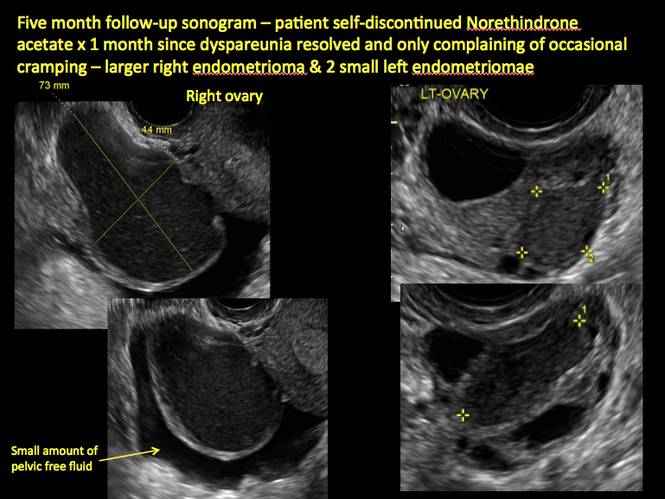
Figure 10
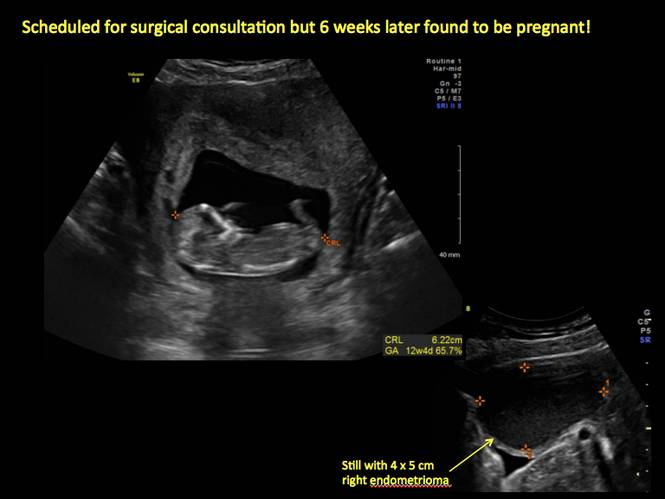
Figure 11
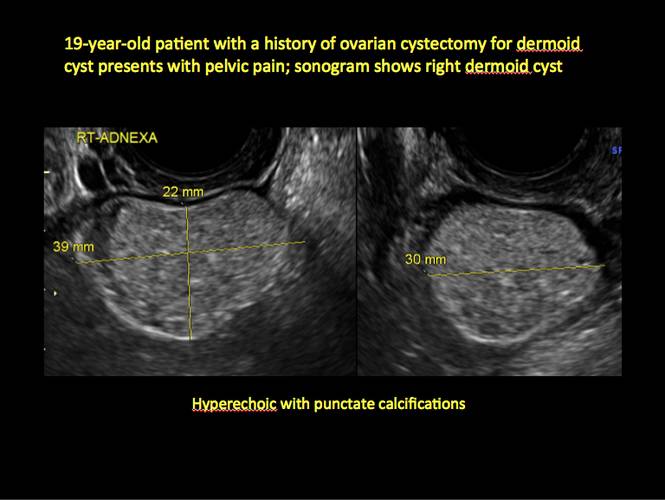
Figure 12
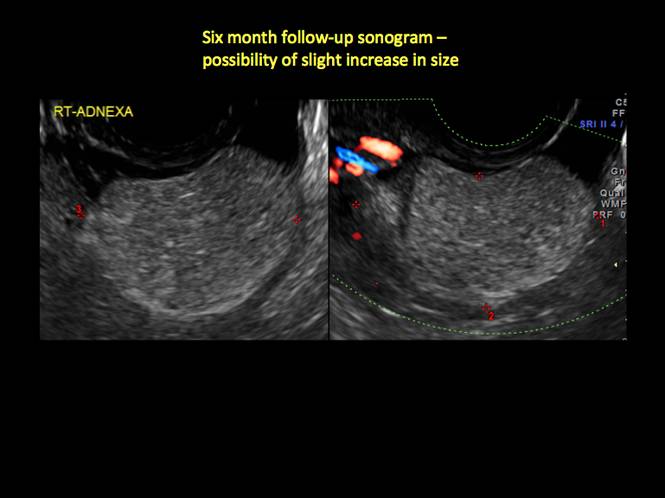
Figure 13
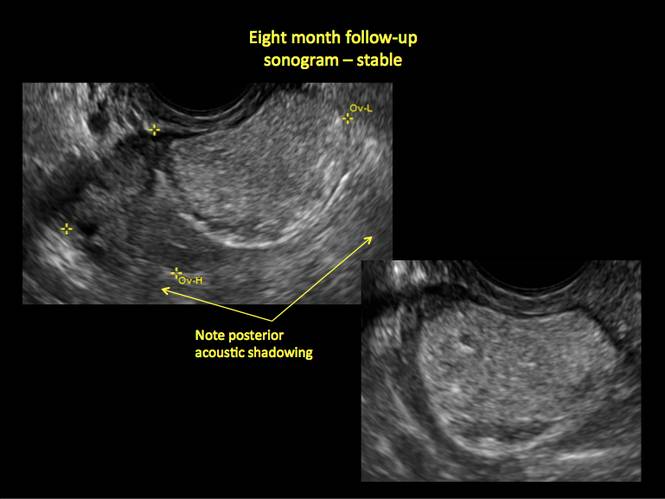
Figure 14
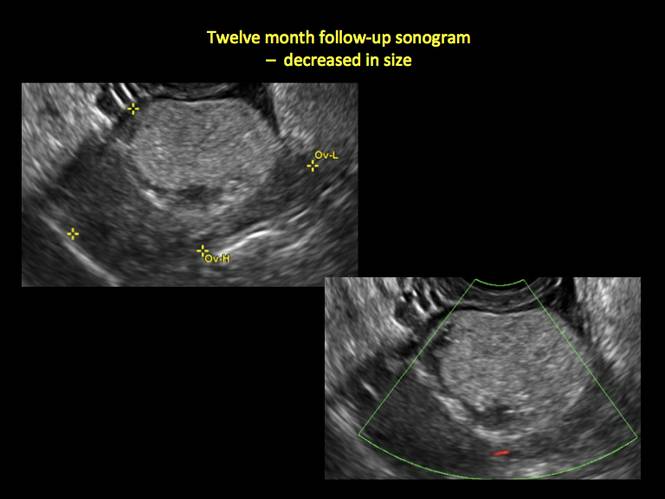
Figure 15
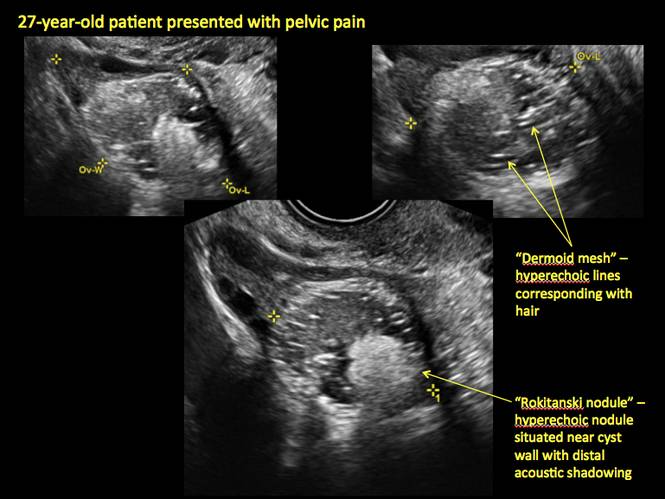
Figure 16
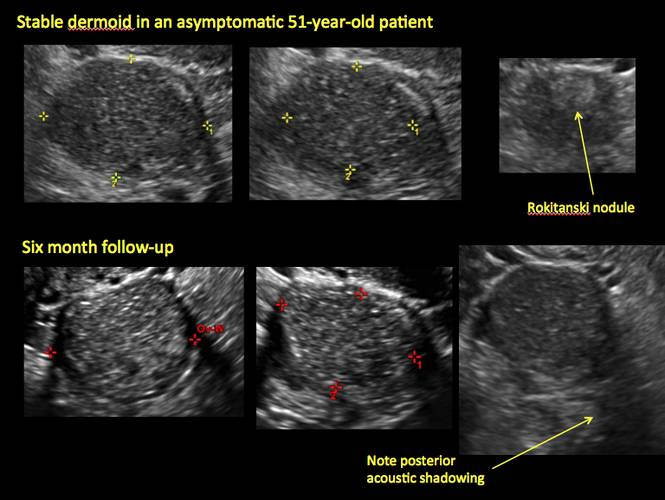
Figure 17
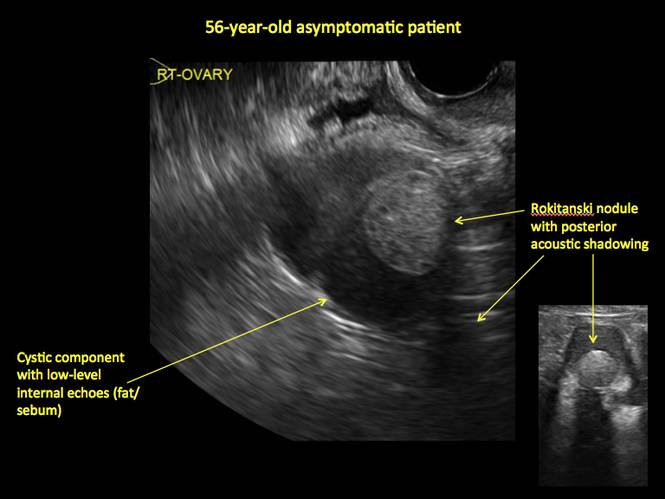
Figure 18
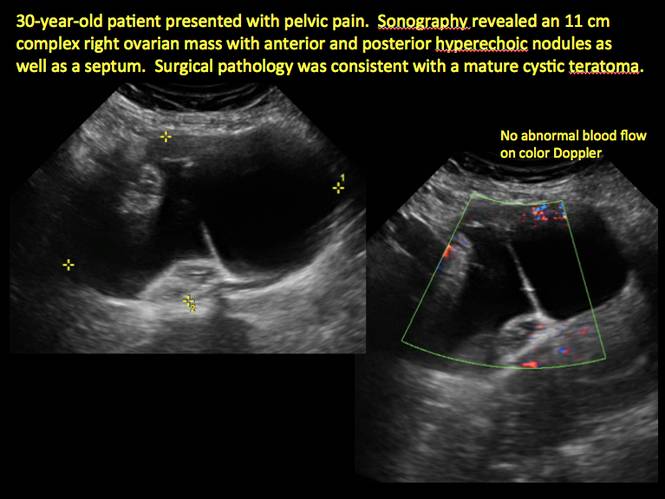
Figure 19
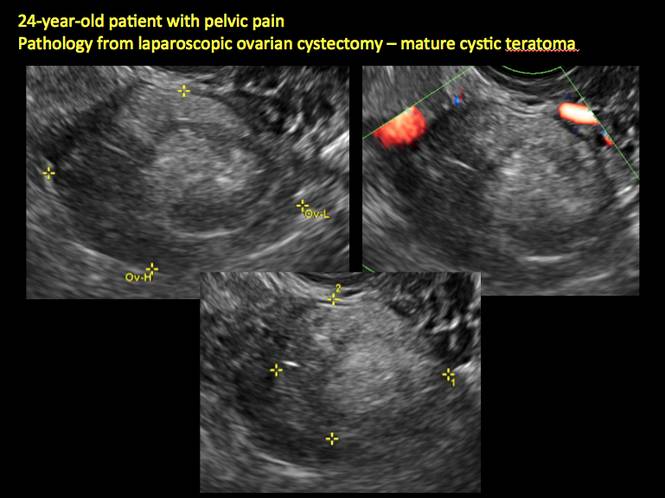
Figure 20
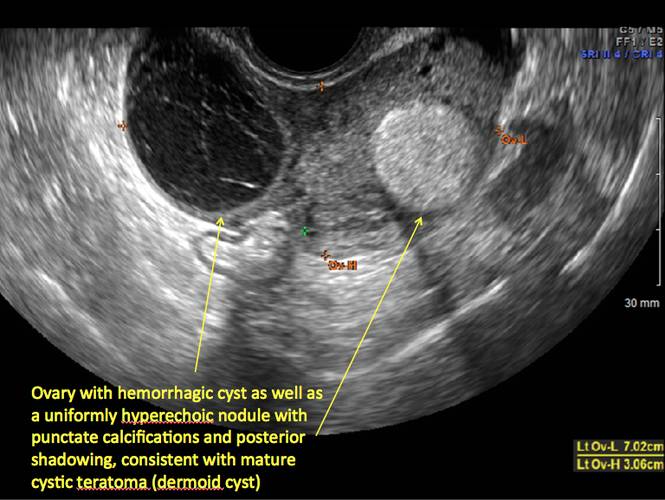
Figure 21
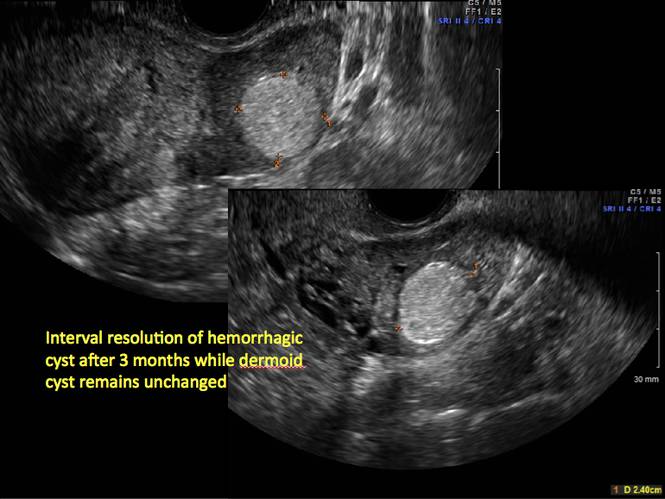
Figure 22
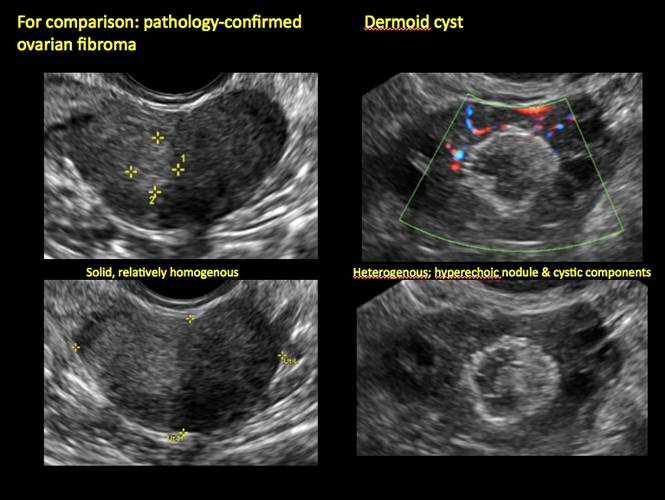
Figure 23
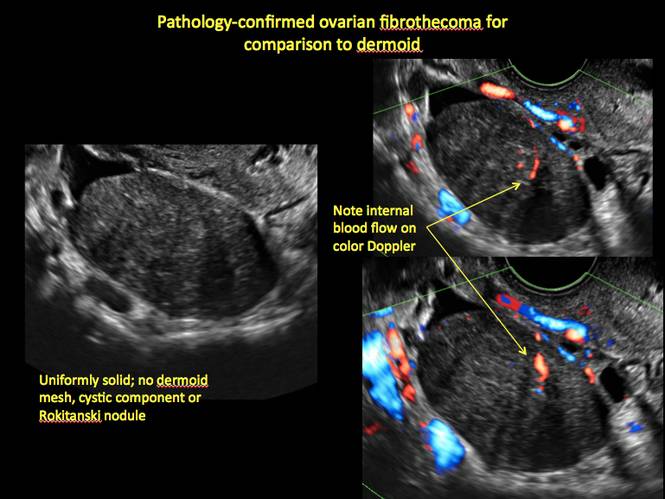
Figure 24
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943–954.
2. Hoo WL, Yazbek WL, Holland T, Mavrelos D, Tong EN, Jurkovic D. Expectant management of ultrasonically diagnosed ovarian dermoid cysts: is it possible to predict outcome? Ultrasound Obstet Gynecol. 2010;36(2): 235–240.
The preferred imaging method to evaluate the majority of adnexal cysts is ultrasonography, which can help characterize the cyst type. Common benign adnexal cyst types include simple, hemorrhagic, endometrioma, and mature teratoma (dermoid cyst). In this part 2 of a 4-part series on cystic adnexal pathology, we focus on imaging signs for, and follow-up of, endometriomas and mature teratomas.
Endometriomas
Endometriomas are common, typically benign, cysts that produce homogenous, low-level internal echoes and a “ground glass” appearance on ultrasonography. No internal flow is apparent on color Doppler. The presence of tiny echogenic wall foci can distinguish an endometrioma from a hemorrhagic cyst.
Rarely, endometriomas may undergo malignant transformation. Usually this occurs with cysts greater than 9 cm and in patients aged 45 years or older. A malignancy often exhibits rapid growth or the development of a solid nodule with flow on color Doppler.
Management
Although surgery remains the first-line management for women with symptomatic or enlarging endometriomas, there appears to be a role for sonographic observation, with continuous progestational treatment, in women with small (< 5 cm) asymptomatic endometriomas.
The Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommended1:
- Short-interval follow-up (6 to 12 weeks) in reproductive-aged women to ensure acute hemorrhagic cysts are not mistaken for endometriomas
- If not removed surgically, sonographic follow-up is recommended, with frequency of follow up based on patient age and symptoms and cyst size and characteristics.
In FIGURES 1 through 11 (slides of image collections), we present several cases, including one of a 25-year-old patient presenting with pelvic pain and dyspareunia who was later found to have bilateral endometriomas.
Mature teratomas
Mature cystic teratomas display several telltale signs on imaging, including:
- hyperechoic lines/dots (“dermoid mesh”) corresponding to hair/skeletal components
- “Rokitanski nodule” – a peripherally placed mass of sebum, bones, and hair
- posterior acoustic shadowing
- cystic or floating spherical structures
- no internal flow on color Doppler
Rarely, dermoid cysts may undergo malignant transformation. Usually this occurs in cysts greater than 10 cm and in patients aged 50 years or older. Internal flow on color Doppler, branching, or invasion into adjacent structures can indicate malignancy.
Management
The traditional treatment for dermoid cysts is surgical. However, given the ability for accurate diagnosis with vaginal ultrasonography, there appears to be a role for sonographic observation in asymptomatic women with small dermoids.2
If the cyst is not surgically removed, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommended initial sonographic follow up at no more than 6 months to 1 year to ensure no change in size or internal architecture.1
In FIGURES 12 through 24 below (slides of image collections), we offer imaging from the case presentation and follow-up of a 19-year-old patient with pelvic pain who has a history of ovarian cystectomy for dermoid cyst, as well as 6 additional case illustrations.

Figure 1

Figure 2

Figure 3

Figure 4

Figure 5

Figure 6

Figure 7

Figure 8

Figure 9

Figure 10

Figure 11

Figure 12

Figure 13

Figure 14

Figure 15

Figure 16

Figure 17

Figure 18

Figure 19

Figure 20

Figure 21

Figure 22

Figure 23

Figure 24
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The preferred imaging method to evaluate the majority of adnexal cysts is ultrasonography, which can help characterize the cyst type. Common benign adnexal cyst types include simple, hemorrhagic, endometrioma, and mature teratoma (dermoid cyst). In this part 2 of a 4-part series on cystic adnexal pathology, we focus on imaging signs for, and follow-up of, endometriomas and mature teratomas.
Endometriomas
Endometriomas are common, typically benign, cysts that produce homogenous, low-level internal echoes and a “ground glass” appearance on ultrasonography. No internal flow is apparent on color Doppler. The presence of tiny echogenic wall foci can distinguish an endometrioma from a hemorrhagic cyst.
Rarely, endometriomas may undergo malignant transformation. Usually this occurs with cysts greater than 9 cm and in patients aged 45 years or older. A malignancy often exhibits rapid growth or the development of a solid nodule with flow on color Doppler.
Management
Although surgery remains the first-line management for women with symptomatic or enlarging endometriomas, there appears to be a role for sonographic observation, with continuous progestational treatment, in women with small (< 5 cm) asymptomatic endometriomas.
The Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommended1:
- Short-interval follow-up (6 to 12 weeks) in reproductive-aged women to ensure acute hemorrhagic cysts are not mistaken for endometriomas
- If not removed surgically, sonographic follow-up is recommended, with frequency of follow up based on patient age and symptoms and cyst size and characteristics.
In FIGURES 1 through 11 (slides of image collections), we present several cases, including one of a 25-year-old patient presenting with pelvic pain and dyspareunia who was later found to have bilateral endometriomas.
Mature teratomas
Mature cystic teratomas display several telltale signs on imaging, including:
- hyperechoic lines/dots (“dermoid mesh”) corresponding to hair/skeletal components
- “Rokitanski nodule” – a peripherally placed mass of sebum, bones, and hair
- posterior acoustic shadowing
- cystic or floating spherical structures
- no internal flow on color Doppler
Rarely, dermoid cysts may undergo malignant transformation. Usually this occurs in cysts greater than 10 cm and in patients aged 50 years or older. Internal flow on color Doppler, branching, or invasion into adjacent structures can indicate malignancy.
Management
The traditional treatment for dermoid cysts is surgical. However, given the ability for accurate diagnosis with vaginal ultrasonography, there appears to be a role for sonographic observation in asymptomatic women with small dermoids.2
If the cyst is not surgically removed, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommended initial sonographic follow up at no more than 6 months to 1 year to ensure no change in size or internal architecture.1
In FIGURES 12 through 24 below (slides of image collections), we offer imaging from the case presentation and follow-up of a 19-year-old patient with pelvic pain who has a history of ovarian cystectomy for dermoid cyst, as well as 6 additional case illustrations.

Figure 1

Figure 2

Figure 3

Figure 4

Figure 5

Figure 6

Figure 7

Figure 8

Figure 9

Figure 10

Figure 11

Figure 12

Figure 13

Figure 14

Figure 15

Figure 16

Figure 17

Figure 18

Figure 19

Figure 20

Figure 21

Figure 22

Figure 23

Figure 24
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943–954.
2. Hoo WL, Yazbek WL, Holland T, Mavrelos D, Tong EN, Jurkovic D. Expectant management of ultrasonically diagnosed ovarian dermoid cysts: is it possible to predict outcome? Ultrasound Obstet Gynecol. 2010;36(2): 235–240.
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943–954.
2. Hoo WL, Yazbek WL, Holland T, Mavrelos D, Tong EN, Jurkovic D. Expectant management of ultrasonically diagnosed ovarian dermoid cysts: is it possible to predict outcome? Ultrasound Obstet Gynecol. 2010;36(2): 235–240.
Telltale sonographic features of simple and hemorrhagic cysts
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts, given its ability to accurately characterize their various aspects:
- Simple cysts are uniformly hypoechoic, with thin walls and no blood flow on color Doppler (FIGURE 1).
- Hemorrhagic cysts produce lacy/reticular echoes and clot with concave margins
(FIGURE 2). - Mature cystic teratomas produce hyperechoic lines and dots, sometimes known as “dermoid mesh,” acoustic shadowing, and a hyperechoic nodule (FIGURE 3).
- Endometriomas produce diffuse, low-level internal echoes and a “ground glass” appearance (FIGURE 4).
In the first of this 4-part series on the sonographic features of cystic adnexal pathology, we focus on simple and hemorrhagic cysts. In the following parts we will highlight:
- mature cystic teratomas and endometriomas (Part 2)
- hydrosalpinx and pelvic inclusion cysts (Part 3)
- cystadenoma and ovarian neoplasia (Part 4).
An earlier installment of this series entitled “Hemorrhagic ovarian cysts: one entity with many appearances” (May 2014) also focused on cystic pathology.
Figure 1: Simple cyst
A simple cyst in a 32-year-old patient. Figure 2: Hemorrhagic cyst
Note the lacy/reticular internal echoes and lack of internal blood flow on color Doppler. Figure 3: Cystic teratoma
This cyst exhibits the “dermoid mesh” and hyperechoic lines that correspond with hair. Figure 4: Endometrioma
Note diffuse low-level internal echoes (“ground glass”) and no “ring of fire” on color Doppler. |
Characteristics of simple cysts
A simple cyst typically is round or oval, anechoic, and has smooth, thin walls. It contains no solid component or septation (with rare exceptions), and no internal flow is visible on color Doppler imaging.
Levine and colleagues observed that simple adnexal cysts as large as 10 cm carry a risk of malignancy of less than 1%, regardless of the age of the patient. In its 2010 Consensus Conference Statement,1 the Society of Radiologists in Ultrasound recommended the following management strategies for women with simple cysts:
Reproductive-aged women
- Cyst <3 cm: No action necessary; the cyst is a normal physiologic finding and should be referred to as a follicle.
- 3–5 cm: No follow-up necessary; the cyst is almost certainly benign.
- 5–7 cm: Yearly imaging; the cyst is highly likely to be benign.
- >7 cm: Additional imaging is recommended.
Postmenopausal women
- <1 cm: No follow-up necessary; the cyst is almost certainly benign.
- 1–7 cm: Yearly imaging; the cyst is likely to be benign.
- >7 cm: Additional imaging is recommended.
Characteristics of hemorrhagic cysts
These cysts can be quite variable in appearance. Among their sonographic features:
- reticular (lacy, cobweb, or fishnet) internal echoes, due to fibrin strands
- solid-appearing areas with concave margins
- on color Doppler, there may be circumferential peripheral flow (“ring of fire”) and no internal flow.
In its 2010 Consensus Conference Statement, the Society of Radiologists in Ultrasound recommended the following management strategies1:
Premenopausal women
- ≤5 cm: No follow-up imaging unless the diagnosis is uncertain.
- >5 cm: Short-interval follow-up ultrasound (6–12 weeks).
Recently menopausal women
- Any size: Follow-up ultrasound in 6–12 weeks to ensure resolution.
Later postmenopausal women
- Any size: Consider surgical removal, as the cyst may be neoplastic.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943–954.
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts, given its ability to accurately characterize their various aspects:
- Simple cysts are uniformly hypoechoic, with thin walls and no blood flow on color Doppler (FIGURE 1).
- Hemorrhagic cysts produce lacy/reticular echoes and clot with concave margins
(FIGURE 2). - Mature cystic teratomas produce hyperechoic lines and dots, sometimes known as “dermoid mesh,” acoustic shadowing, and a hyperechoic nodule (FIGURE 3).
- Endometriomas produce diffuse, low-level internal echoes and a “ground glass” appearance (FIGURE 4).
In the first of this 4-part series on the sonographic features of cystic adnexal pathology, we focus on simple and hemorrhagic cysts. In the following parts we will highlight:
- mature cystic teratomas and endometriomas (Part 2)
- hydrosalpinx and pelvic inclusion cysts (Part 3)
- cystadenoma and ovarian neoplasia (Part 4).
An earlier installment of this series entitled “Hemorrhagic ovarian cysts: one entity with many appearances” (May 2014) also focused on cystic pathology.
Figure 1: Simple cyst
A simple cyst in a 32-year-old patient. Figure 2: Hemorrhagic cyst
Note the lacy/reticular internal echoes and lack of internal blood flow on color Doppler. Figure 3: Cystic teratoma
This cyst exhibits the “dermoid mesh” and hyperechoic lines that correspond with hair. Figure 4: Endometrioma
Note diffuse low-level internal echoes (“ground glass”) and no “ring of fire” on color Doppler. |
Characteristics of simple cysts
A simple cyst typically is round or oval, anechoic, and has smooth, thin walls. It contains no solid component or septation (with rare exceptions), and no internal flow is visible on color Doppler imaging.
Levine and colleagues observed that simple adnexal cysts as large as 10 cm carry a risk of malignancy of less than 1%, regardless of the age of the patient. In its 2010 Consensus Conference Statement,1 the Society of Radiologists in Ultrasound recommended the following management strategies for women with simple cysts:
Reproductive-aged women
- Cyst <3 cm: No action necessary; the cyst is a normal physiologic finding and should be referred to as a follicle.
- 3–5 cm: No follow-up necessary; the cyst is almost certainly benign.
- 5–7 cm: Yearly imaging; the cyst is highly likely to be benign.
- >7 cm: Additional imaging is recommended.
Postmenopausal women
- <1 cm: No follow-up necessary; the cyst is almost certainly benign.
- 1–7 cm: Yearly imaging; the cyst is likely to be benign.
- >7 cm: Additional imaging is recommended.
Characteristics of hemorrhagic cysts
These cysts can be quite variable in appearance. Among their sonographic features:
- reticular (lacy, cobweb, or fishnet) internal echoes, due to fibrin strands
- solid-appearing areas with concave margins
- on color Doppler, there may be circumferential peripheral flow (“ring of fire”) and no internal flow.
In its 2010 Consensus Conference Statement, the Society of Radiologists in Ultrasound recommended the following management strategies1:
Premenopausal women
- ≤5 cm: No follow-up imaging unless the diagnosis is uncertain.
- >5 cm: Short-interval follow-up ultrasound (6–12 weeks).
Recently menopausal women
- Any size: Follow-up ultrasound in 6–12 weeks to ensure resolution.
Later postmenopausal women
- Any size: Consider surgical removal, as the cyst may be neoplastic.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts, given its ability to accurately characterize their various aspects:
- Simple cysts are uniformly hypoechoic, with thin walls and no blood flow on color Doppler (FIGURE 1).
- Hemorrhagic cysts produce lacy/reticular echoes and clot with concave margins
(FIGURE 2). - Mature cystic teratomas produce hyperechoic lines and dots, sometimes known as “dermoid mesh,” acoustic shadowing, and a hyperechoic nodule (FIGURE 3).
- Endometriomas produce diffuse, low-level internal echoes and a “ground glass” appearance (FIGURE 4).
In the first of this 4-part series on the sonographic features of cystic adnexal pathology, we focus on simple and hemorrhagic cysts. In the following parts we will highlight:
- mature cystic teratomas and endometriomas (Part 2)
- hydrosalpinx and pelvic inclusion cysts (Part 3)
- cystadenoma and ovarian neoplasia (Part 4).
An earlier installment of this series entitled “Hemorrhagic ovarian cysts: one entity with many appearances” (May 2014) also focused on cystic pathology.
Figure 1: Simple cyst
A simple cyst in a 32-year-old patient. Figure 2: Hemorrhagic cyst
Note the lacy/reticular internal echoes and lack of internal blood flow on color Doppler. Figure 3: Cystic teratoma
This cyst exhibits the “dermoid mesh” and hyperechoic lines that correspond with hair. Figure 4: Endometrioma
Note diffuse low-level internal echoes (“ground glass”) and no “ring of fire” on color Doppler. |
Characteristics of simple cysts
A simple cyst typically is round or oval, anechoic, and has smooth, thin walls. It contains no solid component or septation (with rare exceptions), and no internal flow is visible on color Doppler imaging.
Levine and colleagues observed that simple adnexal cysts as large as 10 cm carry a risk of malignancy of less than 1%, regardless of the age of the patient. In its 2010 Consensus Conference Statement,1 the Society of Radiologists in Ultrasound recommended the following management strategies for women with simple cysts:
Reproductive-aged women
- Cyst <3 cm: No action necessary; the cyst is a normal physiologic finding and should be referred to as a follicle.
- 3–5 cm: No follow-up necessary; the cyst is almost certainly benign.
- 5–7 cm: Yearly imaging; the cyst is highly likely to be benign.
- >7 cm: Additional imaging is recommended.
Postmenopausal women
- <1 cm: No follow-up necessary; the cyst is almost certainly benign.
- 1–7 cm: Yearly imaging; the cyst is likely to be benign.
- >7 cm: Additional imaging is recommended.
Characteristics of hemorrhagic cysts
These cysts can be quite variable in appearance. Among their sonographic features:
- reticular (lacy, cobweb, or fishnet) internal echoes, due to fibrin strands
- solid-appearing areas with concave margins
- on color Doppler, there may be circumferential peripheral flow (“ring of fire”) and no internal flow.
In its 2010 Consensus Conference Statement, the Society of Radiologists in Ultrasound recommended the following management strategies1:
Premenopausal women
- ≤5 cm: No follow-up imaging unless the diagnosis is uncertain.
- >5 cm: Short-interval follow-up ultrasound (6–12 weeks).
Recently menopausal women
- Any size: Follow-up ultrasound in 6–12 weeks to ensure resolution.
Later postmenopausal women
- Any size: Consider surgical removal, as the cyst may be neoplastic.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943–954.
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943–954.
Congenital uterine anomalies: A resource of diagnostic images, Part 2
As detailed in Part 1 of this installment on uterine anomalies, a uterus that has developed abnormally can appear to be normal on 2D sonography and on unenhanced sonohysterography (Figure). Without the application of 3D coronal ultrasonography, accurate identification of the fundal contour, and ultimately the type and classification of the uterine anomaly, is not possible.1-3 Fortunately, the lowered cost (compared with magnetic resonance imaging) and the noninvasive nature of this more detailed imaging modality makes its use convenient to both the physician and the patient.
In part 1 of this 2-part installment of our imaging series, we discussed the frequency with which uterine anomalies occur and their types and classifications, as well as offered an imaging library showing the normal endometrial cavity, arcuate uterus, incomplete (partial) uterine septum, and complete uterine septum. Here, we provide two cases demonstrating 3D sonography of the unicornuate, bicornuate, didelphic, and DES-exposed uterus.
A. | B. 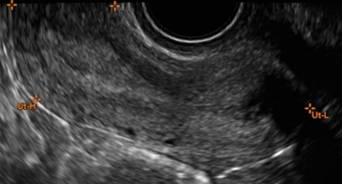 |
C. 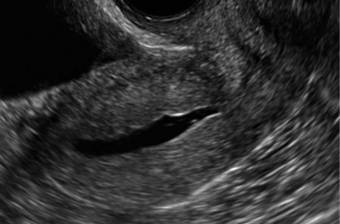 | D. 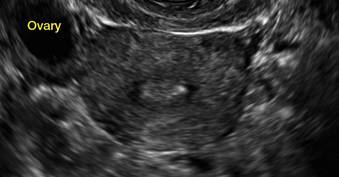 |
E.  | F. 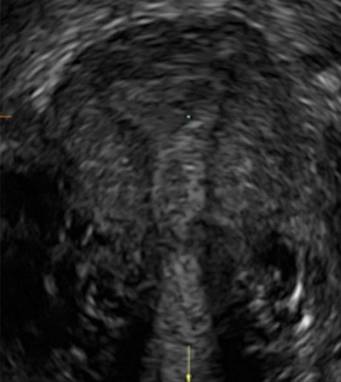 |
G. 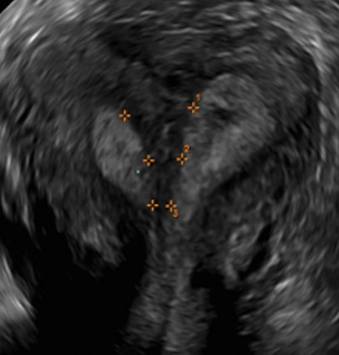
In sagittal view, a uterus with a congenital anomaly can appear normal. Sagittal views of a normal uterus (A) and didelphic uterus (B) and sonohysterogram of a unicornuate uterus (C). Transverse views of a normal (D) and didelphic uterus (E). 3D coronal views of a normal (F) and didelphic uterus (G).
Case 1: Unicornuate uterus
Transverse view of Mirena IUD in right horn and noncommunicating rudimentary left horn.
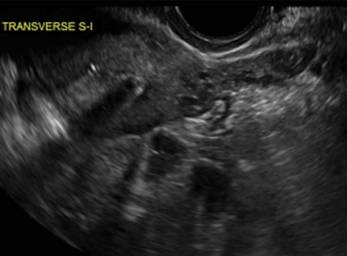
Case 2: Bicornuate uterus, with concave contour
A patient reporting pelvic pain is examined by 2D sonography, which reveals a bicornuate uterus (A). Note the concave fundal contour (arrow), indicating bicornuate uterus, both horns communicating. 3D imaging (B) revealing fundal “dimple” (concave contour, >1 cm), which is indicative of bicornuate uterus. Complete separation of cavities (C).
A. 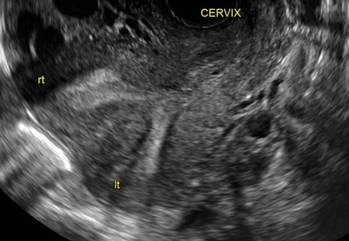
B. 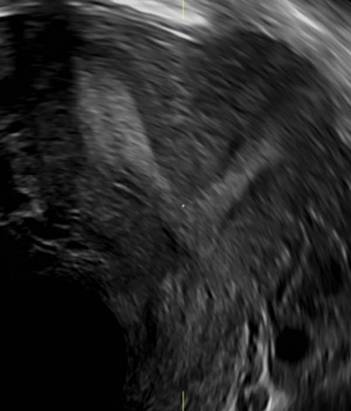
C. 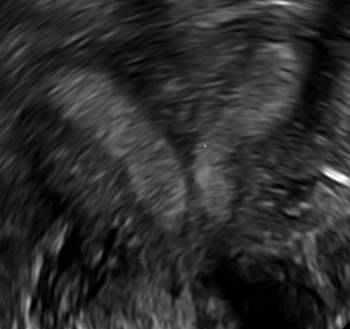
Case 3: Didelphic uterus
A patient presenting with primary infertility is found to have a didelphic uterus on 2D and 3D imaging. Note complete separation of uterine cavities on transverse, 2D views (A and B). The left horn sagittal, 2D view shows a normal appearing uterus (C). 3D imaging (D).
A. 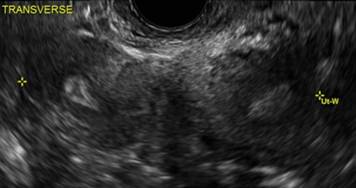
B. 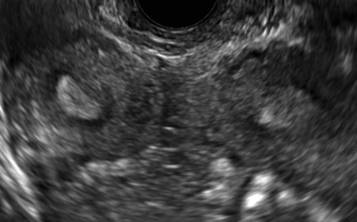
C. 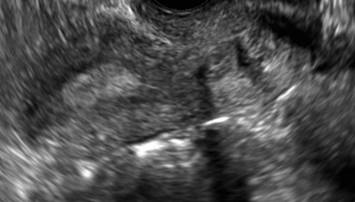
D. 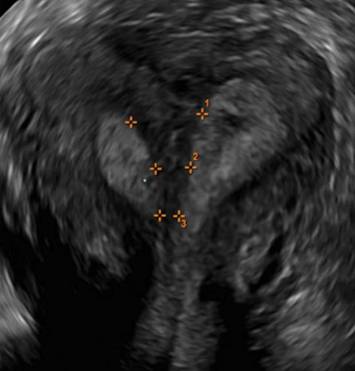
Additional images
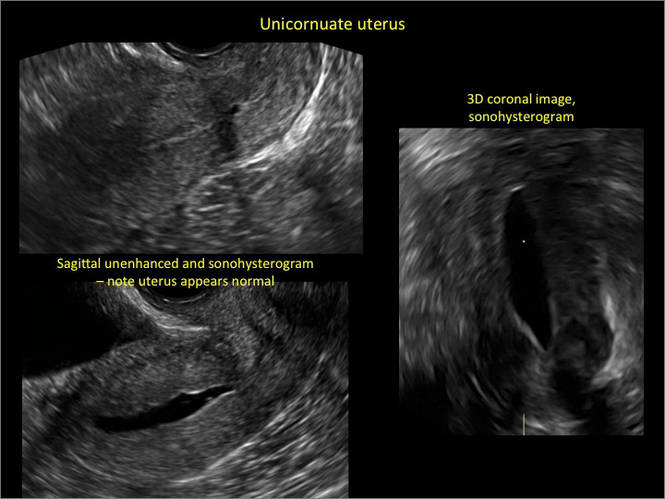
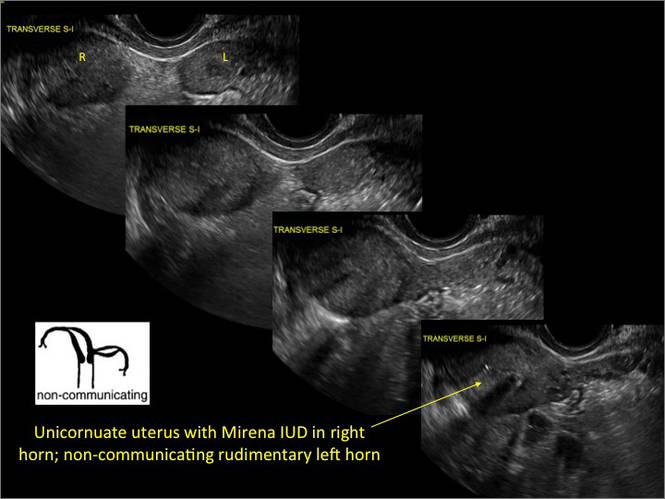
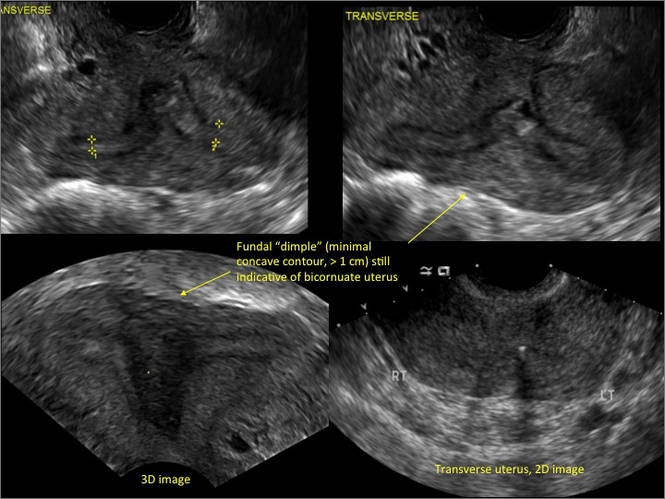
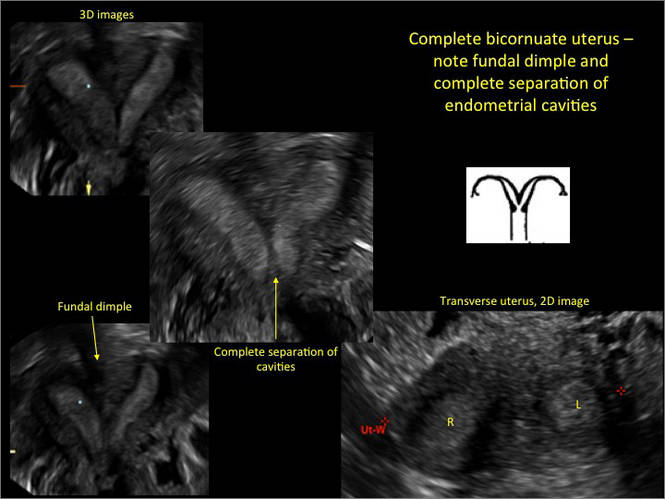
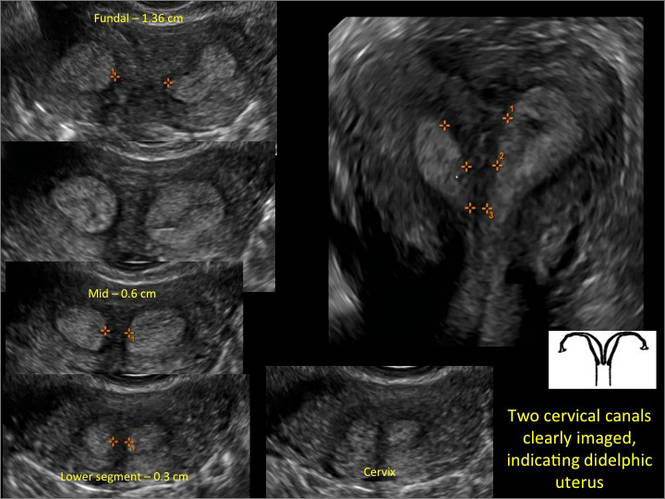

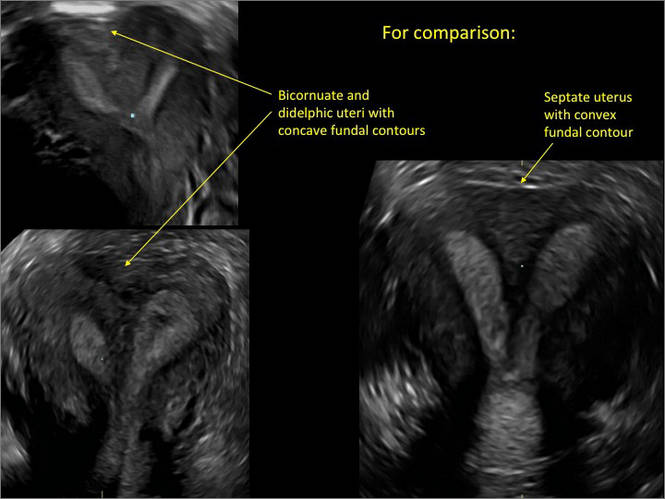
- Deutch T, Bocca S, Oehninger S, et al. Magnetic resonance imaging versus three-dimensional transvaginal ultrasound for the diagnosis of müllerian anomalies [abstract]. Fertil Steril 2006; 86(suppl):S308.15.
- Wu MH, Hsu CC, Huang KE. Detection of congenital müllerian duct anomalies using three-dimensional ultrasound. J Clin Ultrasound 1997; 25:487–492.
- Deutch TD, Abuhamad AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of müllerian duct anomalies: a review of the literature. J Ultrasound Med 2008; 27:413–423.
As detailed in Part 1 of this installment on uterine anomalies, a uterus that has developed abnormally can appear to be normal on 2D sonography and on unenhanced sonohysterography (Figure). Without the application of 3D coronal ultrasonography, accurate identification of the fundal contour, and ultimately the type and classification of the uterine anomaly, is not possible.1-3 Fortunately, the lowered cost (compared with magnetic resonance imaging) and the noninvasive nature of this more detailed imaging modality makes its use convenient to both the physician and the patient.
In part 1 of this 2-part installment of our imaging series, we discussed the frequency with which uterine anomalies occur and their types and classifications, as well as offered an imaging library showing the normal endometrial cavity, arcuate uterus, incomplete (partial) uterine septum, and complete uterine septum. Here, we provide two cases demonstrating 3D sonography of the unicornuate, bicornuate, didelphic, and DES-exposed uterus.
A. | B.  |
C.  | D.  |
E.  | F.  |
G. 
In sagittal view, a uterus with a congenital anomaly can appear normal. Sagittal views of a normal uterus (A) and didelphic uterus (B) and sonohysterogram of a unicornuate uterus (C). Transverse views of a normal (D) and didelphic uterus (E). 3D coronal views of a normal (F) and didelphic uterus (G).
Case 1: Unicornuate uterus
Transverse view of Mirena IUD in right horn and noncommunicating rudimentary left horn.

Case 2: Bicornuate uterus, with concave contour
A patient reporting pelvic pain is examined by 2D sonography, which reveals a bicornuate uterus (A). Note the concave fundal contour (arrow), indicating bicornuate uterus, both horns communicating. 3D imaging (B) revealing fundal “dimple” (concave contour, >1 cm), which is indicative of bicornuate uterus. Complete separation of cavities (C).
A. 
B. 
C. 
Case 3: Didelphic uterus
A patient presenting with primary infertility is found to have a didelphic uterus on 2D and 3D imaging. Note complete separation of uterine cavities on transverse, 2D views (A and B). The left horn sagittal, 2D view shows a normal appearing uterus (C). 3D imaging (D).
A. 
B. 
C. 
D. 
Additional images













As detailed in Part 1 of this installment on uterine anomalies, a uterus that has developed abnormally can appear to be normal on 2D sonography and on unenhanced sonohysterography (Figure). Without the application of 3D coronal ultrasonography, accurate identification of the fundal contour, and ultimately the type and classification of the uterine anomaly, is not possible.1-3 Fortunately, the lowered cost (compared with magnetic resonance imaging) and the noninvasive nature of this more detailed imaging modality makes its use convenient to both the physician and the patient.
In part 1 of this 2-part installment of our imaging series, we discussed the frequency with which uterine anomalies occur and their types and classifications, as well as offered an imaging library showing the normal endometrial cavity, arcuate uterus, incomplete (partial) uterine septum, and complete uterine septum. Here, we provide two cases demonstrating 3D sonography of the unicornuate, bicornuate, didelphic, and DES-exposed uterus.
A. | B.  |
C.  | D.  |
E.  | F.  |
G. 
In sagittal view, a uterus with a congenital anomaly can appear normal. Sagittal views of a normal uterus (A) and didelphic uterus (B) and sonohysterogram of a unicornuate uterus (C). Transverse views of a normal (D) and didelphic uterus (E). 3D coronal views of a normal (F) and didelphic uterus (G).
Case 1: Unicornuate uterus
Transverse view of Mirena IUD in right horn and noncommunicating rudimentary left horn.

Case 2: Bicornuate uterus, with concave contour
A patient reporting pelvic pain is examined by 2D sonography, which reveals a bicornuate uterus (A). Note the concave fundal contour (arrow), indicating bicornuate uterus, both horns communicating. 3D imaging (B) revealing fundal “dimple” (concave contour, >1 cm), which is indicative of bicornuate uterus. Complete separation of cavities (C).
A. 
B. 
C. 
Case 3: Didelphic uterus
A patient presenting with primary infertility is found to have a didelphic uterus on 2D and 3D imaging. Note complete separation of uterine cavities on transverse, 2D views (A and B). The left horn sagittal, 2D view shows a normal appearing uterus (C). 3D imaging (D).
A. 
B. 
C. 
D. 
Additional images













- Deutch T, Bocca S, Oehninger S, et al. Magnetic resonance imaging versus three-dimensional transvaginal ultrasound for the diagnosis of müllerian anomalies [abstract]. Fertil Steril 2006; 86(suppl):S308.15.
- Wu MH, Hsu CC, Huang KE. Detection of congenital müllerian duct anomalies using three-dimensional ultrasound. J Clin Ultrasound 1997; 25:487–492.
- Deutch TD, Abuhamad AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of müllerian duct anomalies: a review of the literature. J Ultrasound Med 2008; 27:413–423.
- Deutch T, Bocca S, Oehninger S, et al. Magnetic resonance imaging versus three-dimensional transvaginal ultrasound for the diagnosis of müllerian anomalies [abstract]. Fertil Steril 2006; 86(suppl):S308.15.
- Wu MH, Hsu CC, Huang KE. Detection of congenital müllerian duct anomalies using three-dimensional ultrasound. J Clin Ultrasound 1997; 25:487–492.
- Deutch TD, Abuhamad AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of müllerian duct anomalies: a review of the literature. J Ultrasound Med 2008; 27:413–423.
Congenital uterine anomalies: A resource of diagnostic images, Part 1
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of discussing the various uterine malformations as well as characterizing their appearance on 3D transvaginal ultrasound.
Unfortunately, many women are still subjected to the cost, inconvenience, and time involvement of magnetic resonance imaging (MRI) in cases of suspected uterine malformations. The exquisite visualization of 3D transvaginal ultrasound, so nicely depicted in this installment of Images in GYN Ultrasound, allow the observer to see the endometrial contours in the same plane as the serosal surface. This view is not available in traditional 2D ultrasound images. Thus, it is akin to doing laparoscopy and hysteroscopy simultaneously in order to arrive at the proper diagnosis. Although not mandatory, when such 3D ultrasound is performed late in the cycle, the thickened endometrium acts as a nice sonic backdrop to better delineate these structures. Alternatively, 3D saline infusion sonohysterography can be performed.
As more and more ultrasound equipment becomes available with 3D capability as a standard feature, clinicians who do perform ultrasonography will find that obtaining this “z-plane” is relatively simple and extremely informative, and can and should be done in cases of suspected uterine malformations in lieu of ordering MRI.
Congenital uterine anomalies: A resource of diagnostic images, Part 1
Michelle L. Stalnaker Ozcan, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Uterine malformations make up a diverse group of congenital anomalies that can result from various alterations in the normal development of the Müllerian ducts, including underdevelopment of one or both Müllerian ducts, disorders in Müllerian duct fusion, and alterations in septum reabsorption. How common are such anomalies, how are they classified, and what is the best approach for optimal visualization? Here, we explore these questions and offer an atlas of diagnostic images as an ongoing reference for your practice. Many of the images we offer will be found only online at obgmanagement.com.
How common are congenital uterine anomalies?
The reported prevalence of uterine malformations varies among publications due to heterogeneous population samples, differences in diagnostic techniques, and variations in nomenclature. In general, they are estimated to occur in 0.4% (0.1% to 3.0%) of the population at large, 4% of infertile women, and between 3% and 38% of women with repetitive spontaneous miscarriage.1
Classical classification
A classification of the Müllerian anomalies was introduced in 1980 and, with few modifications, was adopted by the American Fertility Society (currently, ASRM). The Society identified seven basic groups according to Müllerian development and their relationship to fertility: agenesis and hypoplasias, unicornuate uteri (unilateral hypoplasia), didelphys uteri (complete nonfusion), bicornuate uteri (incomplete fusion), septate uteri (nonreabsorption of septum), arcuate uteri (almost complete reabsorption of septum), and anomalies related to fetal DES exposure.2
Anomalies also can be categorized in terms of progression along the developmental continuum, taking into account that many cases result from partial failure of fusion and reabsorption: agenesis (Types I and II), lack of fusion (Types III and IV), lack of reabsorption(Types V and VI), and lack of posterior development (Type VII) (FIGURE 1).3
| FIGURE 1. Classification of müllerian anomalies |
|---|
 |
| Source: The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988. 49(6):944-955. |
3D ultrasonography offers accurate, cost-efficient diagnosis
Using only 2D imaging, neither an unenhanced sonogram nor a sonohysterogram can provide definitive information regarding the possibility of a uterine anomaly. The fundal contour cannot be evaluated with 2D imaging; likewise, details regarding the configuration of the uterine cavity (or cavities) may not be appreciated with the use of 2D imaging (FIGURE 2).
Figure 2: Normal appearance, but abnormal uteri
In sagittal view, a uterus with a congenital anomaly can appear normal. 2D sagittal views of a normal uterus (top), a didelphic uterus (middle), and a sonohysterogram of a septate uterus (bottom). |
To fully evaluate the uterine fundal contour and determine the type of uterine anomaly, it previously was necessary to obtain magnetic resonance imaging (MRI) or perform laparoscopy. Today, however, 3D coronal ultrasonography (US) can allow for accurate evaluation of fundal contour and diagnosis of uterine anomalies with lower cost and greater patient convenience. Several studies have confirmed the high accuracy of 3D US compared with MRI and surgical findings in the diagnosis of uterine anomalies (with 3D US showing 98% to 100% sensitivity and specificity).4-6
Case: Partial septate uterus
|
ADDITIONAL IMAGES
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Bermejo C, Martinez Ten P, Cantarero R, et al. Three-dimensional ultrasound in the diagnosis of Müllerian duct anomalies and concordance with magnetic resonance imaging. Ultrasound Obstet Gynecol. 2010;35(5):593–601.
- The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49(6):944–955.
- Acien P, Acien M. Updated classification of malformations. Hum Reprod. 2010;25(suppl 1):i81–i82.
- Deutch T, Bocca S, Oehninger S, Stadtmauer L, Abuhamad AZ. Magnetic resonance imaging versus three-dimensional transvaginal ultrasound for the diagnosis of Müllerian anomalies [abstract P-465]. Fertil Steril. 2006;86(suppl):S308.
- Wu MH, Hsu CC, Huang KE. Detection of congenital Müllerian duct anomalies using three-dimensional ultrasound. J Clin Ultrasound. 1997;25(9):487–492.
- Deutch TD, Abuhamad AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of Müllerian duct anomalies. J Ultrasound Med. 2008;27(3):413–423.
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of discussing the various uterine malformations as well as characterizing their appearance on 3D transvaginal ultrasound.
Unfortunately, many women are still subjected to the cost, inconvenience, and time involvement of magnetic resonance imaging (MRI) in cases of suspected uterine malformations. The exquisite visualization of 3D transvaginal ultrasound, so nicely depicted in this installment of Images in GYN Ultrasound, allow the observer to see the endometrial contours in the same plane as the serosal surface. This view is not available in traditional 2D ultrasound images. Thus, it is akin to doing laparoscopy and hysteroscopy simultaneously in order to arrive at the proper diagnosis. Although not mandatory, when such 3D ultrasound is performed late in the cycle, the thickened endometrium acts as a nice sonic backdrop to better delineate these structures. Alternatively, 3D saline infusion sonohysterography can be performed.
As more and more ultrasound equipment becomes available with 3D capability as a standard feature, clinicians who do perform ultrasonography will find that obtaining this “z-plane” is relatively simple and extremely informative, and can and should be done in cases of suspected uterine malformations in lieu of ordering MRI.
Congenital uterine anomalies: A resource of diagnostic images, Part 1
Michelle L. Stalnaker Ozcan, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Uterine malformations make up a diverse group of congenital anomalies that can result from various alterations in the normal development of the Müllerian ducts, including underdevelopment of one or both Müllerian ducts, disorders in Müllerian duct fusion, and alterations in septum reabsorption. How common are such anomalies, how are they classified, and what is the best approach for optimal visualization? Here, we explore these questions and offer an atlas of diagnostic images as an ongoing reference for your practice. Many of the images we offer will be found only online at obgmanagement.com.
How common are congenital uterine anomalies?
The reported prevalence of uterine malformations varies among publications due to heterogeneous population samples, differences in diagnostic techniques, and variations in nomenclature. In general, they are estimated to occur in 0.4% (0.1% to 3.0%) of the population at large, 4% of infertile women, and between 3% and 38% of women with repetitive spontaneous miscarriage.1
Classical classification
A classification of the Müllerian anomalies was introduced in 1980 and, with few modifications, was adopted by the American Fertility Society (currently, ASRM). The Society identified seven basic groups according to Müllerian development and their relationship to fertility: agenesis and hypoplasias, unicornuate uteri (unilateral hypoplasia), didelphys uteri (complete nonfusion), bicornuate uteri (incomplete fusion), septate uteri (nonreabsorption of septum), arcuate uteri (almost complete reabsorption of septum), and anomalies related to fetal DES exposure.2
Anomalies also can be categorized in terms of progression along the developmental continuum, taking into account that many cases result from partial failure of fusion and reabsorption: agenesis (Types I and II), lack of fusion (Types III and IV), lack of reabsorption(Types V and VI), and lack of posterior development (Type VII) (FIGURE 1).3
| FIGURE 1. Classification of müllerian anomalies |
|---|
 |
| Source: The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988. 49(6):944-955. |
3D ultrasonography offers accurate, cost-efficient diagnosis
Using only 2D imaging, neither an unenhanced sonogram nor a sonohysterogram can provide definitive information regarding the possibility of a uterine anomaly. The fundal contour cannot be evaluated with 2D imaging; likewise, details regarding the configuration of the uterine cavity (or cavities) may not be appreciated with the use of 2D imaging (FIGURE 2).
Figure 2: Normal appearance, but abnormal uteri
In sagittal view, a uterus with a congenital anomaly can appear normal. 2D sagittal views of a normal uterus (top), a didelphic uterus (middle), and a sonohysterogram of a septate uterus (bottom). |
To fully evaluate the uterine fundal contour and determine the type of uterine anomaly, it previously was necessary to obtain magnetic resonance imaging (MRI) or perform laparoscopy. Today, however, 3D coronal ultrasonography (US) can allow for accurate evaluation of fundal contour and diagnosis of uterine anomalies with lower cost and greater patient convenience. Several studies have confirmed the high accuracy of 3D US compared with MRI and surgical findings in the diagnosis of uterine anomalies (with 3D US showing 98% to 100% sensitivity and specificity).4-6
Case: Partial septate uterus
|
ADDITIONAL IMAGES
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of discussing the various uterine malformations as well as characterizing their appearance on 3D transvaginal ultrasound.
Unfortunately, many women are still subjected to the cost, inconvenience, and time involvement of magnetic resonance imaging (MRI) in cases of suspected uterine malformations. The exquisite visualization of 3D transvaginal ultrasound, so nicely depicted in this installment of Images in GYN Ultrasound, allow the observer to see the endometrial contours in the same plane as the serosal surface. This view is not available in traditional 2D ultrasound images. Thus, it is akin to doing laparoscopy and hysteroscopy simultaneously in order to arrive at the proper diagnosis. Although not mandatory, when such 3D ultrasound is performed late in the cycle, the thickened endometrium acts as a nice sonic backdrop to better delineate these structures. Alternatively, 3D saline infusion sonohysterography can be performed.
As more and more ultrasound equipment becomes available with 3D capability as a standard feature, clinicians who do perform ultrasonography will find that obtaining this “z-plane” is relatively simple and extremely informative, and can and should be done in cases of suspected uterine malformations in lieu of ordering MRI.
Congenital uterine anomalies: A resource of diagnostic images, Part 1
Michelle L. Stalnaker Ozcan, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Uterine malformations make up a diverse group of congenital anomalies that can result from various alterations in the normal development of the Müllerian ducts, including underdevelopment of one or both Müllerian ducts, disorders in Müllerian duct fusion, and alterations in septum reabsorption. How common are such anomalies, how are they classified, and what is the best approach for optimal visualization? Here, we explore these questions and offer an atlas of diagnostic images as an ongoing reference for your practice. Many of the images we offer will be found only online at obgmanagement.com.
How common are congenital uterine anomalies?
The reported prevalence of uterine malformations varies among publications due to heterogeneous population samples, differences in diagnostic techniques, and variations in nomenclature. In general, they are estimated to occur in 0.4% (0.1% to 3.0%) of the population at large, 4% of infertile women, and between 3% and 38% of women with repetitive spontaneous miscarriage.1
Classical classification
A classification of the Müllerian anomalies was introduced in 1980 and, with few modifications, was adopted by the American Fertility Society (currently, ASRM). The Society identified seven basic groups according to Müllerian development and their relationship to fertility: agenesis and hypoplasias, unicornuate uteri (unilateral hypoplasia), didelphys uteri (complete nonfusion), bicornuate uteri (incomplete fusion), septate uteri (nonreabsorption of septum), arcuate uteri (almost complete reabsorption of septum), and anomalies related to fetal DES exposure.2
Anomalies also can be categorized in terms of progression along the developmental continuum, taking into account that many cases result from partial failure of fusion and reabsorption: agenesis (Types I and II), lack of fusion (Types III and IV), lack of reabsorption(Types V and VI), and lack of posterior development (Type VII) (FIGURE 1).3
| FIGURE 1. Classification of müllerian anomalies |
|---|
 |
| Source: The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988. 49(6):944-955. |
3D ultrasonography offers accurate, cost-efficient diagnosis
Using only 2D imaging, neither an unenhanced sonogram nor a sonohysterogram can provide definitive information regarding the possibility of a uterine anomaly. The fundal contour cannot be evaluated with 2D imaging; likewise, details regarding the configuration of the uterine cavity (or cavities) may not be appreciated with the use of 2D imaging (FIGURE 2).
Figure 2: Normal appearance, but abnormal uteri
In sagittal view, a uterus with a congenital anomaly can appear normal. 2D sagittal views of a normal uterus (top), a didelphic uterus (middle), and a sonohysterogram of a septate uterus (bottom). |
To fully evaluate the uterine fundal contour and determine the type of uterine anomaly, it previously was necessary to obtain magnetic resonance imaging (MRI) or perform laparoscopy. Today, however, 3D coronal ultrasonography (US) can allow for accurate evaluation of fundal contour and diagnosis of uterine anomalies with lower cost and greater patient convenience. Several studies have confirmed the high accuracy of 3D US compared with MRI and surgical findings in the diagnosis of uterine anomalies (with 3D US showing 98% to 100% sensitivity and specificity).4-6
Case: Partial septate uterus
|
ADDITIONAL IMAGES
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Bermejo C, Martinez Ten P, Cantarero R, et al. Three-dimensional ultrasound in the diagnosis of Müllerian duct anomalies and concordance with magnetic resonance imaging. Ultrasound Obstet Gynecol. 2010;35(5):593–601.
- The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49(6):944–955.
- Acien P, Acien M. Updated classification of malformations. Hum Reprod. 2010;25(suppl 1):i81–i82.
- Deutch T, Bocca S, Oehninger S, Stadtmauer L, Abuhamad AZ. Magnetic resonance imaging versus three-dimensional transvaginal ultrasound for the diagnosis of Müllerian anomalies [abstract P-465]. Fertil Steril. 2006;86(suppl):S308.
- Wu MH, Hsu CC, Huang KE. Detection of congenital Müllerian duct anomalies using three-dimensional ultrasound. J Clin Ultrasound. 1997;25(9):487–492.
- Deutch TD, Abuhamad AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of Müllerian duct anomalies. J Ultrasound Med. 2008;27(3):413–423.
- Bermejo C, Martinez Ten P, Cantarero R, et al. Three-dimensional ultrasound in the diagnosis of Müllerian duct anomalies and concordance with magnetic resonance imaging. Ultrasound Obstet Gynecol. 2010;35(5):593–601.
- The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49(6):944–955.
- Acien P, Acien M. Updated classification of malformations. Hum Reprod. 2010;25(suppl 1):i81–i82.
- Deutch T, Bocca S, Oehninger S, Stadtmauer L, Abuhamad AZ. Magnetic resonance imaging versus three-dimensional transvaginal ultrasound for the diagnosis of Müllerian anomalies [abstract P-465]. Fertil Steril. 2006;86(suppl):S308.
- Wu MH, Hsu CC, Huang KE. Detection of congenital Müllerian duct anomalies using three-dimensional ultrasound. J Clin Ultrasound. 1997;25(9):487–492.
- Deutch TD, Abuhamad AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of Müllerian duct anomalies. J Ultrasound Med. 2008;27(3):413–423.
How to identify and localize IUDs on ultrasound
Although an ultrasound is not required after uncomplicated placement of an intrauterine device (IUD) or during routine management of women who are doing well with an IUD, it is invaluable in the evaluation of patients who present with pain or other symptoms suggestive of IUD malpositioning.
In this article, we outline the sonographic features of the IUDs available today in the United States and describe the basics of localization by ultrasound.
Related articles: STOP relying on 2D ultrasound for IUD localization. Steven R. Goldstein, MD, and Chrystie Fujimoto, MD (August 2014)
Update on Contraception. Melissa J. Chen, MD, MPH, and Mitchell D. Creinin, MD (August 2014)
Ultrasound features of IUDsWhen positioned normally, an IUD is centrally located within the endometrial cavity, with the crossbar positioned in the fundal area.1 Copper and progestin-releasing IUDs can be identified easily on ultrasound if one is familiar with their basic sonographic features:
- Copper IUD: The central stem is uniformly echogenic due to its copper coils (FIGURE 1)
- Levonorgestrel-releasing intrauterine system (LNG-IUS): The LNG-IUS consists of a plastic sleeve that contains the progestin and surrounds a central stem. This configuration causes acoustic shadowing and has a characteristic “laminated” sonographic appearance with parallel lines (FIGURE 2). The Mirena IUD has echogenic arms due to barium sulfate, as well as an echogenic distal tip, with acoustic shadowing from the stem. Skyla is similar except for a highly echogenic silver ring on the stem approximately 3 to 4 mm inferior to the crossbar. On occasion, the echogenic strings of Mirena and Skyla can be mistaken for the device.
Three-dimensional ultrasound is useful in imaging of an IUD. If a patient’s IUD cannot be visualized by ultrasound, plain radiography of the kidney, ureter, and bladder may be helpful. If an IUD is not apparent on plain film, consider that it may have been expelled.
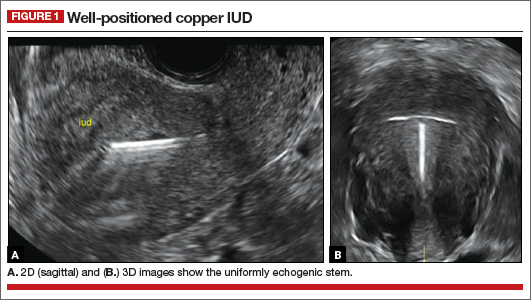
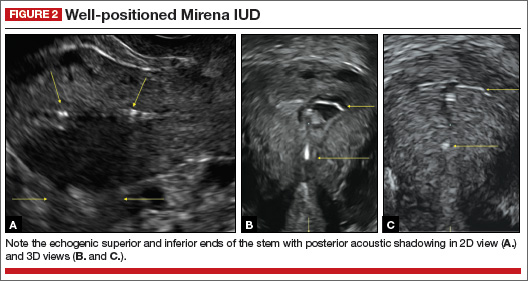
Potential malpositioningA malpositioned IUD may be partially expelled, rotated, embedded in the myometrium, or perforating the uterine serosa.
Related article: Malpositioned IUDs: When you should intervene (and when you should not). Kari Braaten, MD, MPH, and Alisa B. Goldberg, MD, MPH (August 2012)
In a retrospective case-control study that compared 182 women with sonographicallyidentified malpositioned IUDs with 182 women with properly positioned IUDs, Braaten and colleagues found that suspected adenomyosis was associated with malpositioning (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08–8.52), but a history of vaginal delivery was protective (OR, 0.53; 95% CI, 0.32–0.87).2 A distorted uterine cavity also increases the risk of malpositioning.3
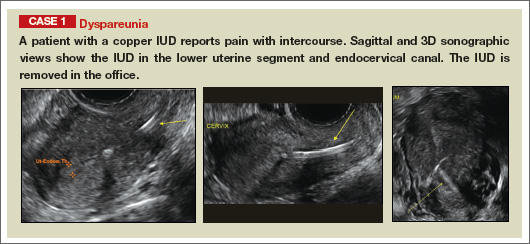
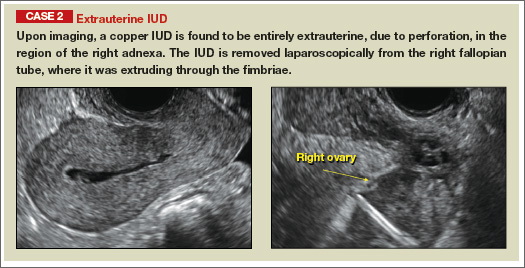
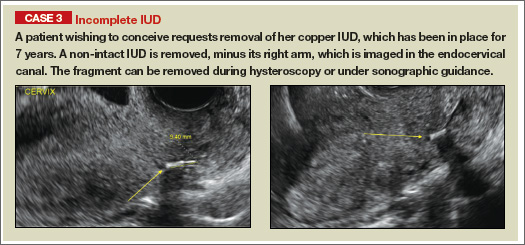 | |
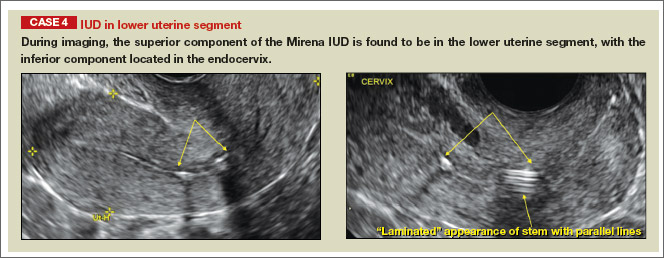 | |
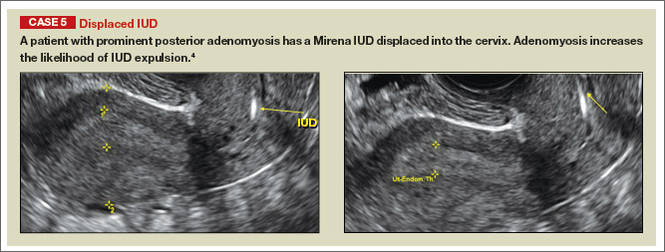 | |
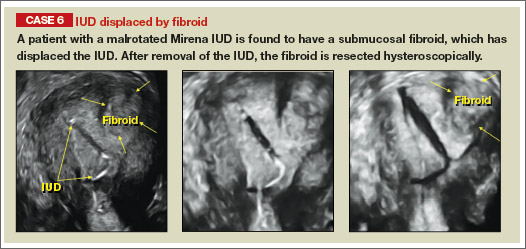 | |
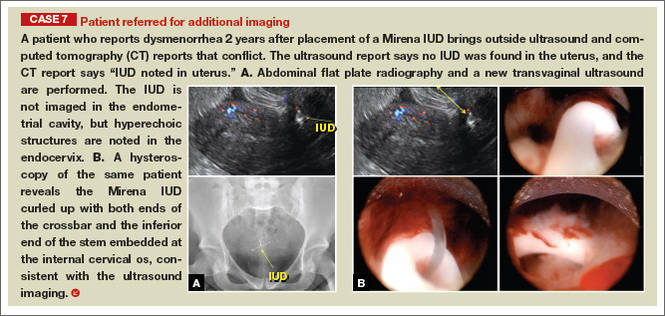 |
Although no uterine perforations were reported in a review of the LNG-IUS, expulsions were reported and may be more common among women who use the IUD for heavy menstrual bleeding.4
Additional images
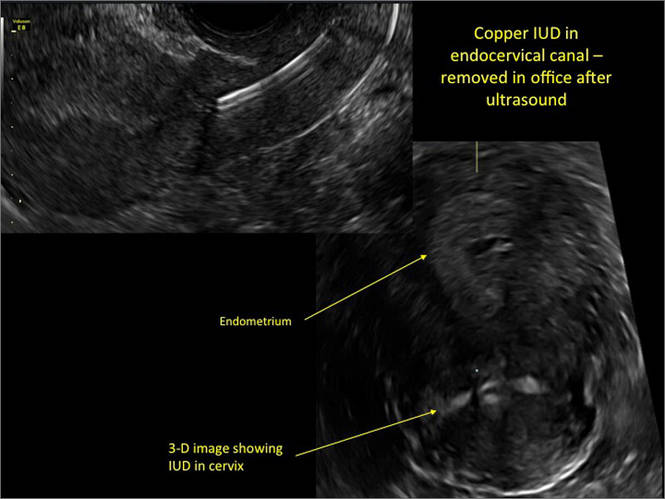
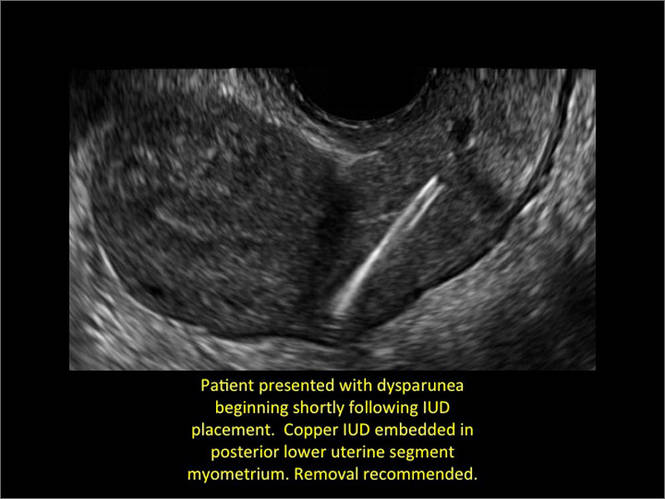
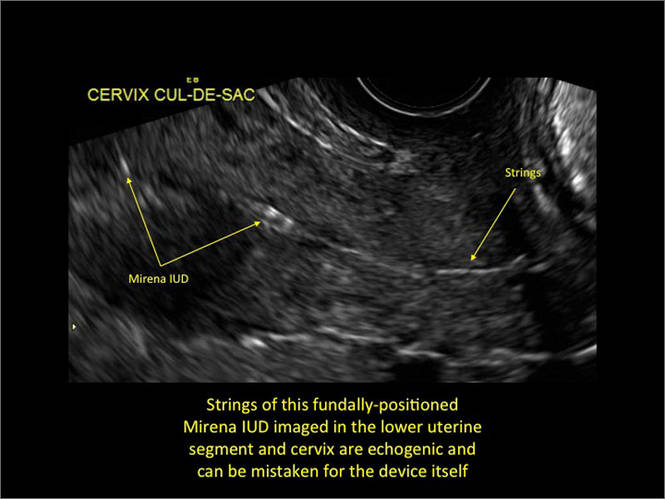
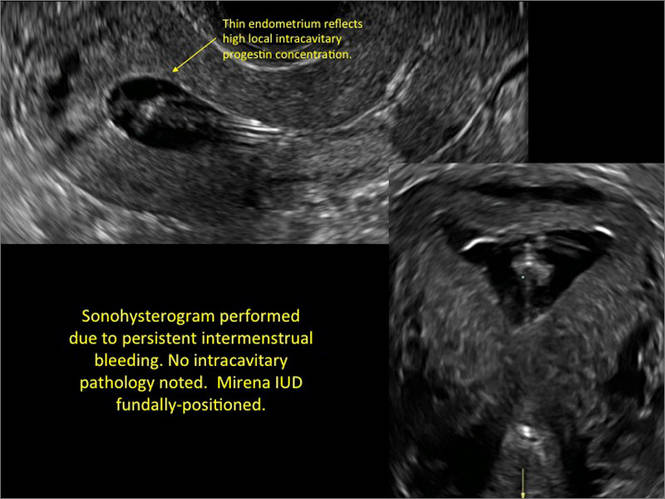
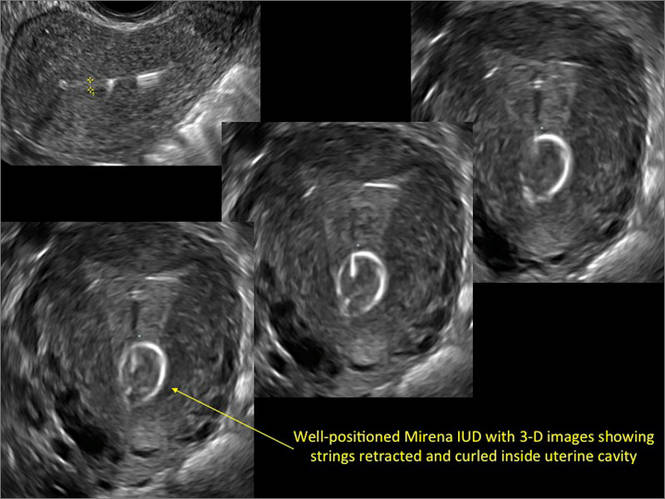
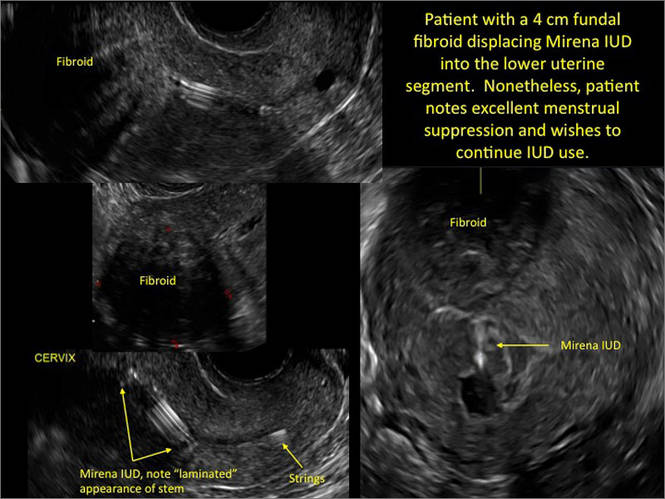
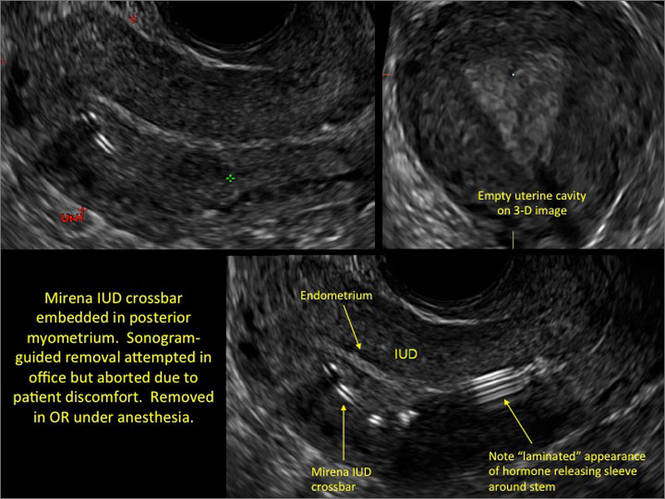
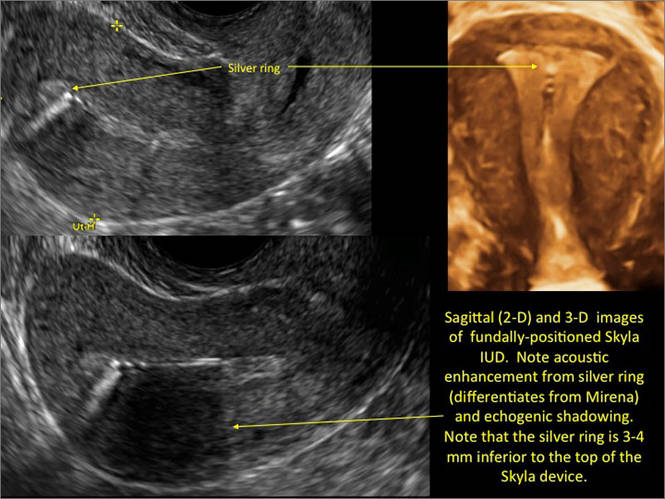
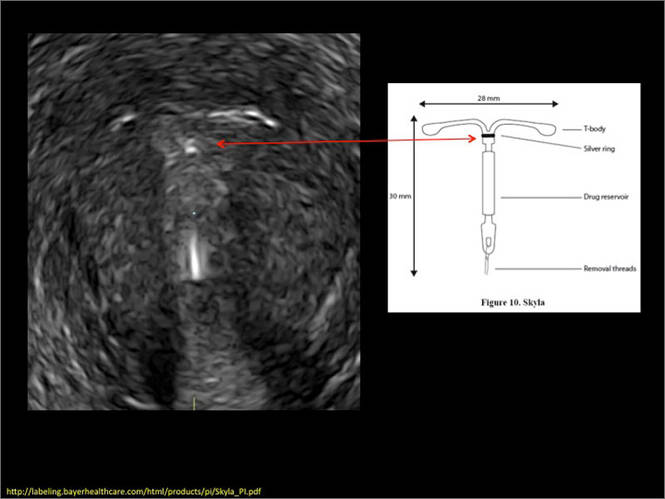
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Peri N, Graha D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26(10):1389–1401.
2. Braaten KP, Benson CB, Maurer R, Goldberg AB. Malpositioned intrauterine contraceptive devices: Risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118(5):1014–1020.
3. Braaten KP, Goldberg AB. Malpositioned IUDs: When you should intervene and when you should not. OBG Manag. 2012;24(8):39–46.
4. Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72(2):193–215.
Although an ultrasound is not required after uncomplicated placement of an intrauterine device (IUD) or during routine management of women who are doing well with an IUD, it is invaluable in the evaluation of patients who present with pain or other symptoms suggestive of IUD malpositioning.
In this article, we outline the sonographic features of the IUDs available today in the United States and describe the basics of localization by ultrasound.
Related articles: STOP relying on 2D ultrasound for IUD localization. Steven R. Goldstein, MD, and Chrystie Fujimoto, MD (August 2014)
Update on Contraception. Melissa J. Chen, MD, MPH, and Mitchell D. Creinin, MD (August 2014)
Ultrasound features of IUDsWhen positioned normally, an IUD is centrally located within the endometrial cavity, with the crossbar positioned in the fundal area.1 Copper and progestin-releasing IUDs can be identified easily on ultrasound if one is familiar with their basic sonographic features:
- Copper IUD: The central stem is uniformly echogenic due to its copper coils (FIGURE 1)
- Levonorgestrel-releasing intrauterine system (LNG-IUS): The LNG-IUS consists of a plastic sleeve that contains the progestin and surrounds a central stem. This configuration causes acoustic shadowing and has a characteristic “laminated” sonographic appearance with parallel lines (FIGURE 2). The Mirena IUD has echogenic arms due to barium sulfate, as well as an echogenic distal tip, with acoustic shadowing from the stem. Skyla is similar except for a highly echogenic silver ring on the stem approximately 3 to 4 mm inferior to the crossbar. On occasion, the echogenic strings of Mirena and Skyla can be mistaken for the device.
Three-dimensional ultrasound is useful in imaging of an IUD. If a patient’s IUD cannot be visualized by ultrasound, plain radiography of the kidney, ureter, and bladder may be helpful. If an IUD is not apparent on plain film, consider that it may have been expelled.


Potential malpositioningA malpositioned IUD may be partially expelled, rotated, embedded in the myometrium, or perforating the uterine serosa.
Related article: Malpositioned IUDs: When you should intervene (and when you should not). Kari Braaten, MD, MPH, and Alisa B. Goldberg, MD, MPH (August 2012)
In a retrospective case-control study that compared 182 women with sonographicallyidentified malpositioned IUDs with 182 women with properly positioned IUDs, Braaten and colleagues found that suspected adenomyosis was associated with malpositioning (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08–8.52), but a history of vaginal delivery was protective (OR, 0.53; 95% CI, 0.32–0.87).2 A distorted uterine cavity also increases the risk of malpositioning.3


 | |
 | |
 | |
 | |
 |
Although no uterine perforations were reported in a review of the LNG-IUS, expulsions were reported and may be more common among women who use the IUD for heavy menstrual bleeding.4
Additional images









WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
Although an ultrasound is not required after uncomplicated placement of an intrauterine device (IUD) or during routine management of women who are doing well with an IUD, it is invaluable in the evaluation of patients who present with pain or other symptoms suggestive of IUD malpositioning.
In this article, we outline the sonographic features of the IUDs available today in the United States and describe the basics of localization by ultrasound.
Related articles: STOP relying on 2D ultrasound for IUD localization. Steven R. Goldstein, MD, and Chrystie Fujimoto, MD (August 2014)
Update on Contraception. Melissa J. Chen, MD, MPH, and Mitchell D. Creinin, MD (August 2014)
Ultrasound features of IUDsWhen positioned normally, an IUD is centrally located within the endometrial cavity, with the crossbar positioned in the fundal area.1 Copper and progestin-releasing IUDs can be identified easily on ultrasound if one is familiar with their basic sonographic features:
- Copper IUD: The central stem is uniformly echogenic due to its copper coils (FIGURE 1)
- Levonorgestrel-releasing intrauterine system (LNG-IUS): The LNG-IUS consists of a plastic sleeve that contains the progestin and surrounds a central stem. This configuration causes acoustic shadowing and has a characteristic “laminated” sonographic appearance with parallel lines (FIGURE 2). The Mirena IUD has echogenic arms due to barium sulfate, as well as an echogenic distal tip, with acoustic shadowing from the stem. Skyla is similar except for a highly echogenic silver ring on the stem approximately 3 to 4 mm inferior to the crossbar. On occasion, the echogenic strings of Mirena and Skyla can be mistaken for the device.
Three-dimensional ultrasound is useful in imaging of an IUD. If a patient’s IUD cannot be visualized by ultrasound, plain radiography of the kidney, ureter, and bladder may be helpful. If an IUD is not apparent on plain film, consider that it may have been expelled.


Potential malpositioningA malpositioned IUD may be partially expelled, rotated, embedded in the myometrium, or perforating the uterine serosa.
Related article: Malpositioned IUDs: When you should intervene (and when you should not). Kari Braaten, MD, MPH, and Alisa B. Goldberg, MD, MPH (August 2012)
In a retrospective case-control study that compared 182 women with sonographicallyidentified malpositioned IUDs with 182 women with properly positioned IUDs, Braaten and colleagues found that suspected adenomyosis was associated with malpositioning (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08–8.52), but a history of vaginal delivery was protective (OR, 0.53; 95% CI, 0.32–0.87).2 A distorted uterine cavity also increases the risk of malpositioning.3


 | |
 | |
 | |
 | |
 |
Although no uterine perforations were reported in a review of the LNG-IUS, expulsions were reported and may be more common among women who use the IUD for heavy menstrual bleeding.4
Additional images









WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Peri N, Graha D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26(10):1389–1401.
2. Braaten KP, Benson CB, Maurer R, Goldberg AB. Malpositioned intrauterine contraceptive devices: Risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118(5):1014–1020.
3. Braaten KP, Goldberg AB. Malpositioned IUDs: When you should intervene and when you should not. OBG Manag. 2012;24(8):39–46.
4. Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72(2):193–215.
1. Peri N, Graha D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26(10):1389–1401.
2. Braaten KP, Benson CB, Maurer R, Goldberg AB. Malpositioned intrauterine contraceptive devices: Risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118(5):1014–1020.
3. Braaten KP, Goldberg AB. Malpositioned IUDs: When you should intervene and when you should not. OBG Manag. 2012;24(8):39–46.
4. Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72(2):193–215.