User login
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
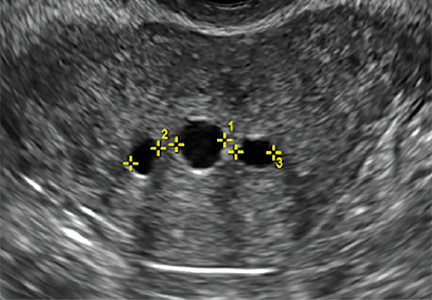
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
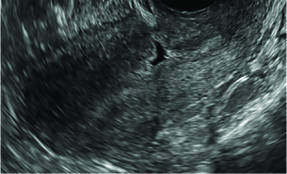
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
| FIGURE 1 Asherman syndrome | ||||
|
Postablation endometrial destruction
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
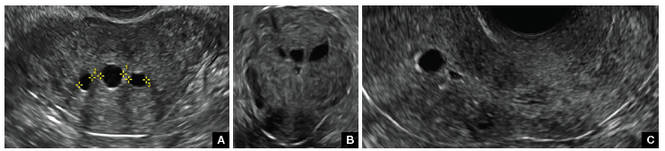
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
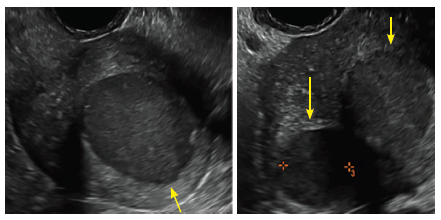
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
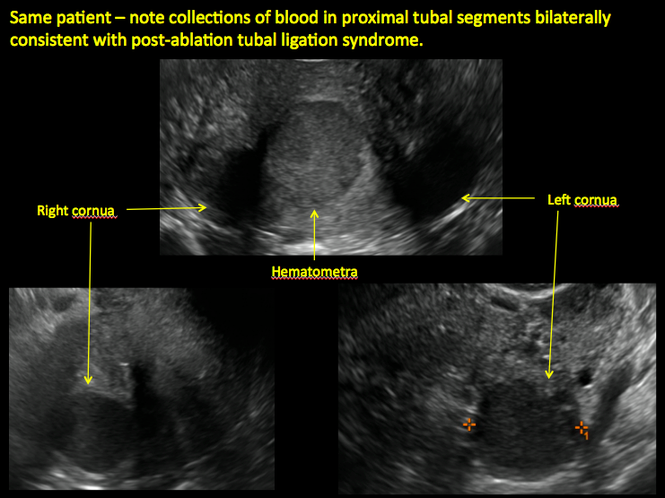
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
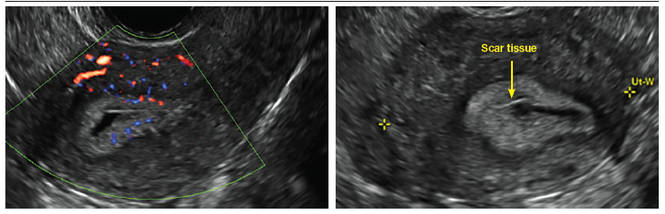
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
Cesarean scar defect on imaging
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
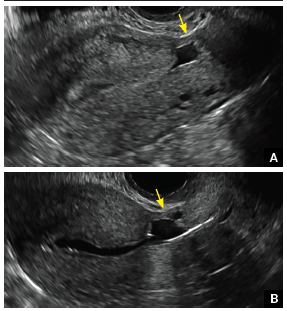
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||
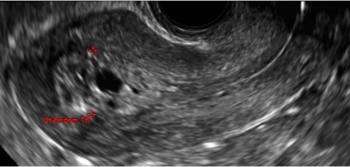 |  | 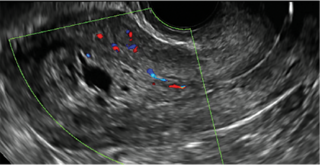 | ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||
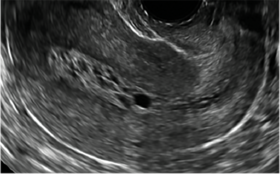 | 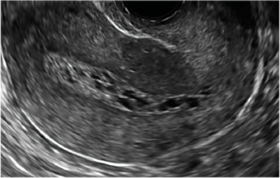 | 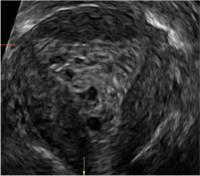 | ||
ADDITIONAL CASES - Postablation
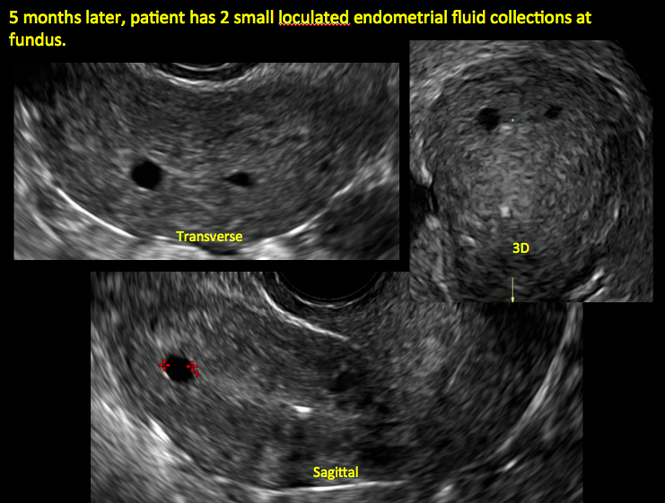
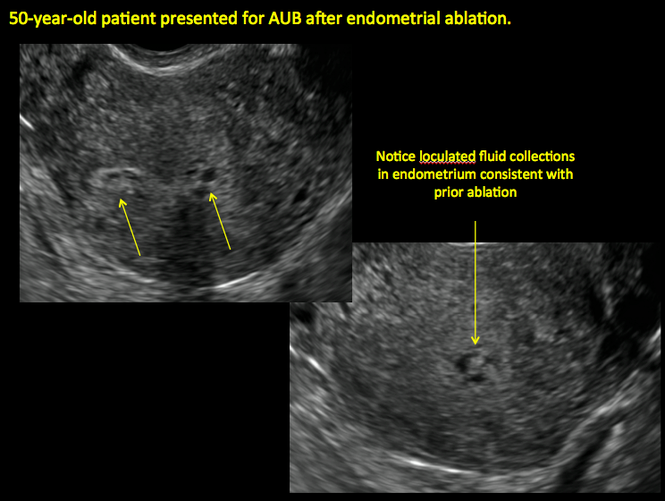
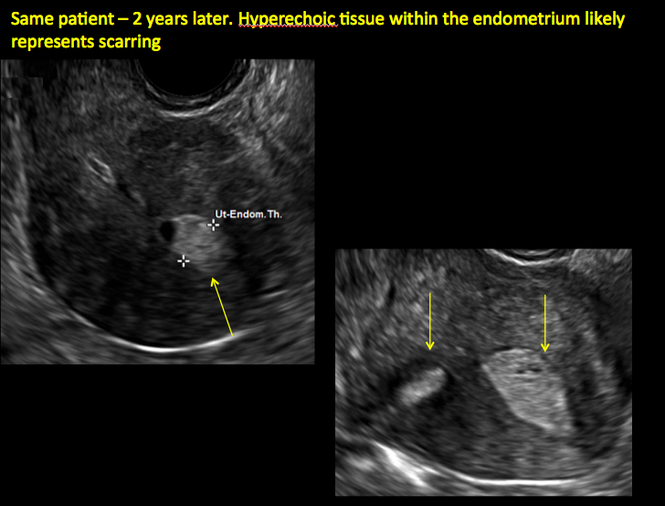
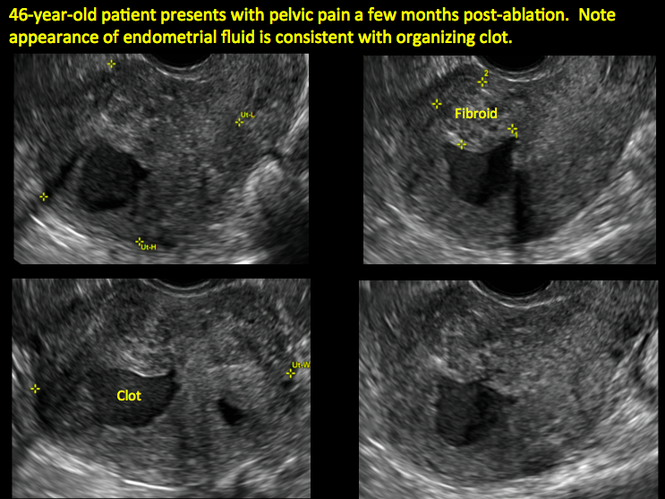
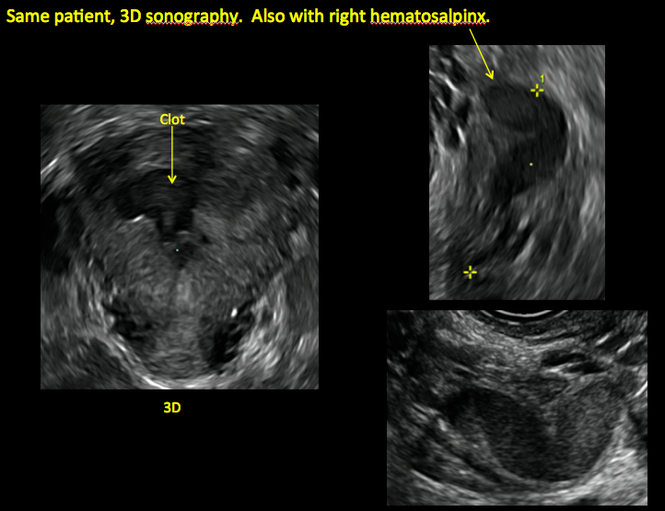
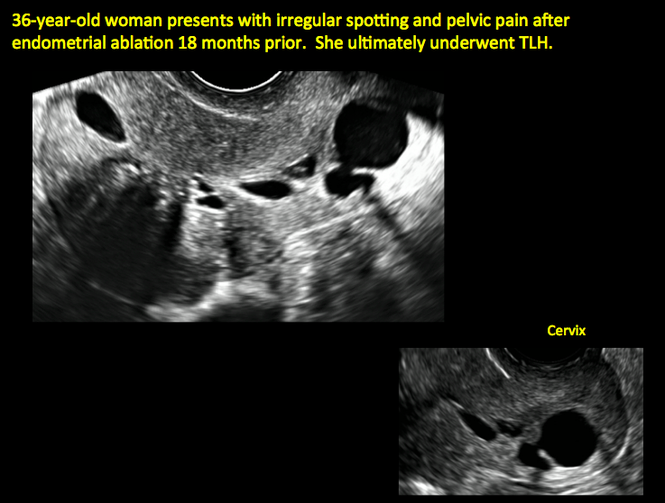

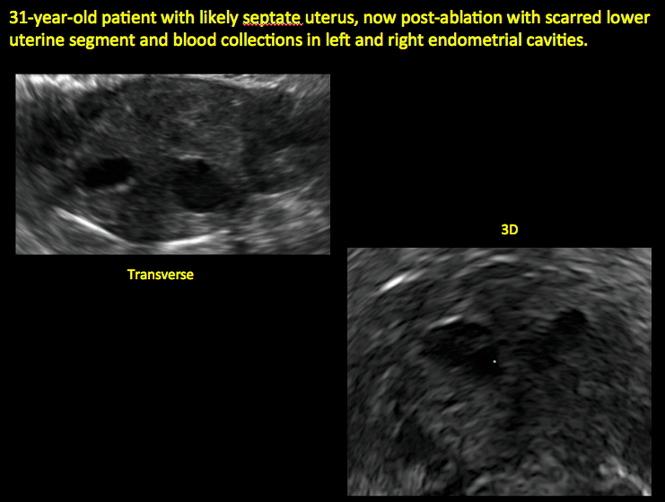
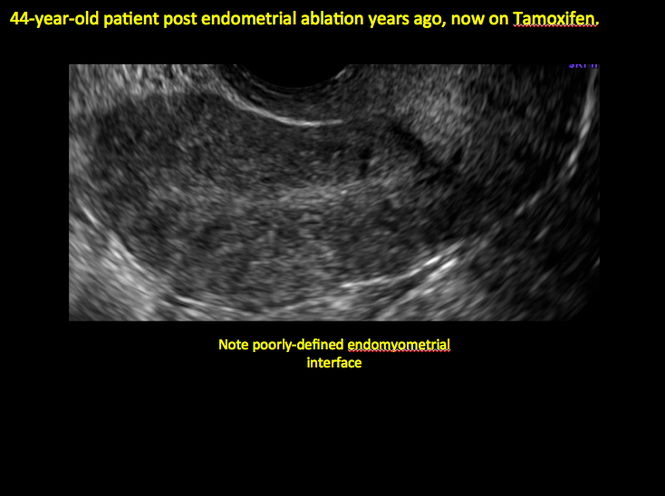
ADDITIONAL CASES - Cesarean scar defect
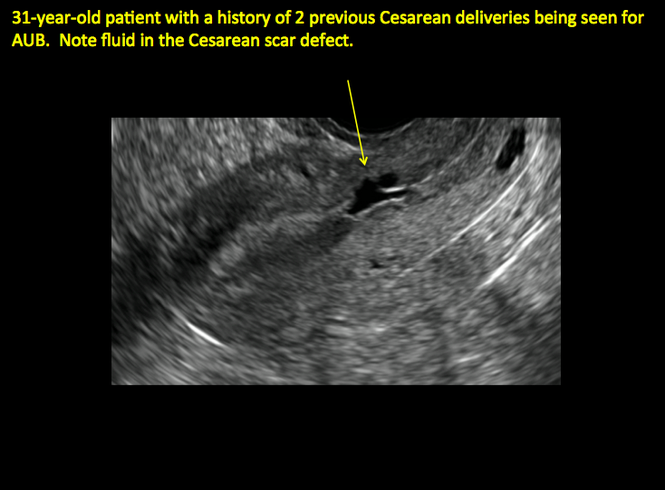
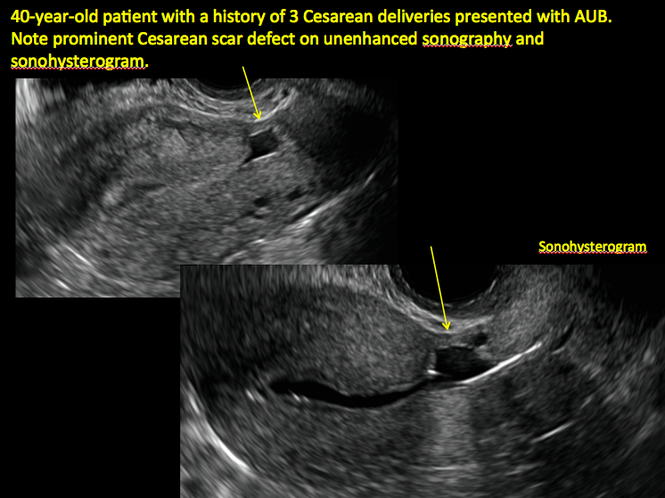
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
| FIGURE 1 Asherman syndrome | ||||
|
Postablation endometrial destruction
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
Cesarean scar defect on imaging
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||
 |  |  | ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||
 |  |  | ||
ADDITIONAL CASES - Postablation










ADDITIONAL CASES - Cesarean scar defect


Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
| FIGURE 1 Asherman syndrome | ||||
|
Postablation endometrial destruction
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
Cesarean scar defect on imaging
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||
 |  |  | ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||
 |  |  | ||
ADDITIONAL CASES - Postablation










ADDITIONAL CASES - Cesarean scar defect


Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
In this Article
- Foreword by Steven R. Goldstein, MD
- Uterine changes postablation
- Endometrial changes with tamoxifen use