User login
Congenital uterine anomalies: A resource of diagnostic images, Part 1
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of discussing the various uterine malformations as well as characterizing their appearance on 3D transvaginal ultrasound.
Unfortunately, many women are still subjected to the cost, inconvenience, and time involvement of magnetic resonance imaging (MRI) in cases of suspected uterine malformations. The exquisite visualization of 3D transvaginal ultrasound, so nicely depicted in this installment of Images in GYN Ultrasound, allow the observer to see the endometrial contours in the same plane as the serosal surface. This view is not available in traditional 2D ultrasound images. Thus, it is akin to doing laparoscopy and hysteroscopy simultaneously in order to arrive at the proper diagnosis. Although not mandatory, when such 3D ultrasound is performed late in the cycle, the thickened endometrium acts as a nice sonic backdrop to better delineate these structures. Alternatively, 3D saline infusion sonohysterography can be performed.
As more and more ultrasound equipment becomes available with 3D capability as a standard feature, clinicians who do perform ultrasonography will find that obtaining this “z-plane” is relatively simple and extremely informative, and can and should be done in cases of suspected uterine malformations in lieu of ordering MRI.
Congenital uterine anomalies: A resource of diagnostic images, Part 1
Michelle L. Stalnaker Ozcan, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Uterine malformations make up a diverse group of congenital anomalies that can result from various alterations in the normal development of the Müllerian ducts, including underdevelopment of one or both Müllerian ducts, disorders in Müllerian duct fusion, and alterations in septum reabsorption. How common are such anomalies, how are they classified, and what is the best approach for optimal visualization? Here, we explore these questions and offer an atlas of diagnostic images as an ongoing reference for your practice. Many of the images we offer will be found only online at obgmanagement.com.
How common are congenital uterine anomalies?
The reported prevalence of uterine malformations varies among publications due to heterogeneous population samples, differences in diagnostic techniques, and variations in nomenclature. In general, they are estimated to occur in 0.4% (0.1% to 3.0%) of the population at large, 4% of infertile women, and between 3% and 38% of women with repetitive spontaneous miscarriage.1
Classical classification
A classification of the Müllerian anomalies was introduced in 1980 and, with few modifications, was adopted by the American Fertility Society (currently, ASRM). The Society identified seven basic groups according to Müllerian development and their relationship to fertility: agenesis and hypoplasias, unicornuate uteri (unilateral hypoplasia), didelphys uteri (complete nonfusion), bicornuate uteri (incomplete fusion), septate uteri (nonreabsorption of septum), arcuate uteri (almost complete reabsorption of septum), and anomalies related to fetal DES exposure.2
Anomalies also can be categorized in terms of progression along the developmental continuum, taking into account that many cases result from partial failure of fusion and reabsorption: agenesis (Types I and II), lack of fusion (Types III and IV), lack of reabsorption(Types V and VI), and lack of posterior development (Type VII) (FIGURE 1).3
| FIGURE 1. Classification of müllerian anomalies |
|---|
 |
| Source: The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988. 49(6):944-955. |
3D ultrasonography offers accurate, cost-efficient diagnosis
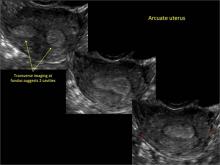
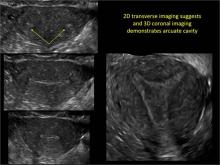
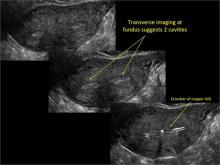
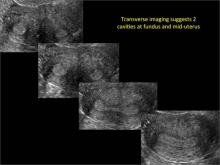
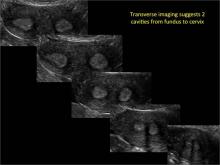
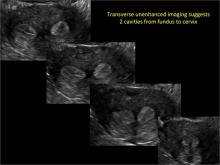
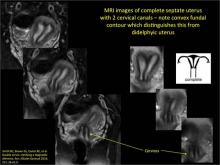
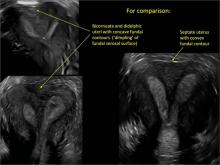
Using only 2D imaging, neither an unenhanced sonogram nor a sonohysterogram can provide definitive information regarding the possibility of a uterine anomaly. The fundal contour cannot be evaluated with 2D imaging; likewise, details regarding the configuration of the uterine cavity (or cavities) may not be appreciated with the use of 2D imaging (FIGURE 2).
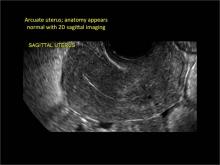
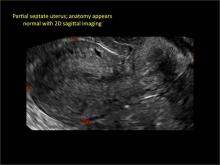
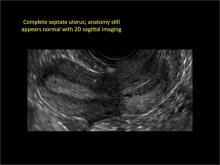
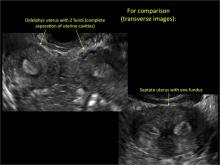
Figure 2: Normal appearance, but abnormal uteri
In sagittal view, a uterus with a congenital anomaly can appear normal. 2D sagittal views of a normal uterus (top), a didelphic uterus (middle), and a sonohysterogram of a septate uterus (bottom). |
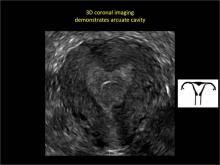
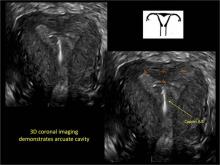
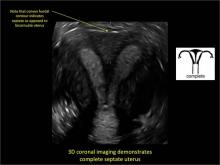
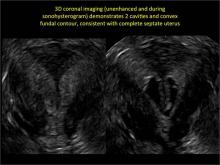
To fully evaluate the uterine fundal contour and determine the type of uterine anomaly, it previously was necessary to obtain magnetic resonance imaging (MRI) or perform laparoscopy. Today, however, 3D coronal ultrasonography (US) can allow for accurate evaluation of fundal contour and diagnosis of uterine anomalies with lower cost and greater patient convenience. Several studies have confirmed the high accuracy of 3D US compared with MRI and surgical findings in the diagnosis of uterine anomalies (with 3D US showing 98% to 100% sensitivity and specificity).4-6
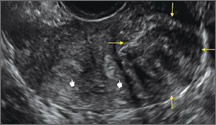
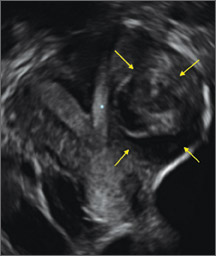
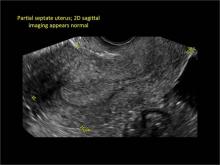
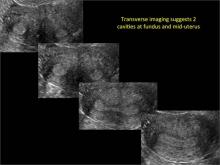
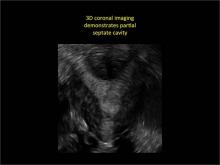
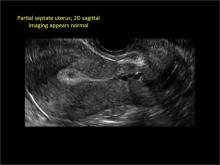
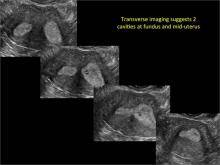
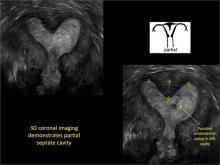
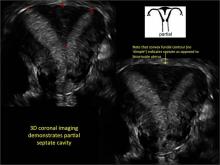
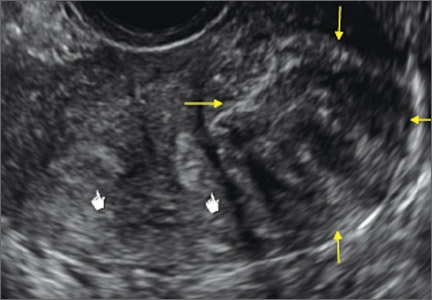
Case: Partial septate uterus
|
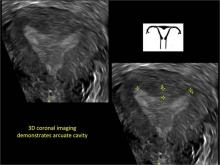
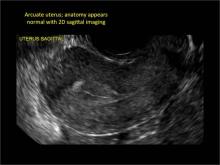
ADDITIONAL IMAGES
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Bermejo C, Martinez Ten P, Cantarero R, et al. Three-dimensional ultrasound in the diagnosis of Müllerian duct anomalies and concordance with magnetic resonance imaging. Ultrasound Obstet Gynecol. 2010;35(5):593–601.
- The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49(6):944–955.
- Acien P, Acien M. Updated classification of malformations. Hum Reprod. 2010;25(suppl 1):i81–i82.
- Deutch T, Bocca S, Oehninger S, Stadtmauer L, Abuhamad AZ. Magnetic resonance imaging versus three-dimensional transvaginal ultrasound for the diagnosis of Müllerian anomalies [abstract P-465]. Fertil Steril. 2006;86(suppl):S308.
- Wu MH, Hsu CC, Huang KE. Detection of congenital Müllerian duct anomalies using three-dimensional ultrasound. J Clin Ultrasound. 1997;25(9):487–492.
- Deutch TD, Abuhamad AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of Müllerian duct anomalies. J Ultrasound Med. 2008;27(3):413–423.
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of discussing the various uterine malformations as well as characterizing their appearance on 3D transvaginal ultrasound.
Unfortunately, many women are still subjected to the cost, inconvenience, and time involvement of magnetic resonance imaging (MRI) in cases of suspected uterine malformations. The exquisite visualization of 3D transvaginal ultrasound, so nicely depicted in this installment of Images in GYN Ultrasound, allow the observer to see the endometrial contours in the same plane as the serosal surface. This view is not available in traditional 2D ultrasound images. Thus, it is akin to doing laparoscopy and hysteroscopy simultaneously in order to arrive at the proper diagnosis. Although not mandatory, when such 3D ultrasound is performed late in the cycle, the thickened endometrium acts as a nice sonic backdrop to better delineate these structures. Alternatively, 3D saline infusion sonohysterography can be performed.
As more and more ultrasound equipment becomes available with 3D capability as a standard feature, clinicians who do perform ultrasonography will find that obtaining this “z-plane” is relatively simple and extremely informative, and can and should be done in cases of suspected uterine malformations in lieu of ordering MRI.
Congenital uterine anomalies: A resource of diagnostic images, Part 1
Michelle L. Stalnaker Ozcan, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Uterine malformations make up a diverse group of congenital anomalies that can result from various alterations in the normal development of the Müllerian ducts, including underdevelopment of one or both Müllerian ducts, disorders in Müllerian duct fusion, and alterations in septum reabsorption. How common are such anomalies, how are they classified, and what is the best approach for optimal visualization? Here, we explore these questions and offer an atlas of diagnostic images as an ongoing reference for your practice. Many of the images we offer will be found only online at obgmanagement.com.
How common are congenital uterine anomalies?
The reported prevalence of uterine malformations varies among publications due to heterogeneous population samples, differences in diagnostic techniques, and variations in nomenclature. In general, they are estimated to occur in 0.4% (0.1% to 3.0%) of the population at large, 4% of infertile women, and between 3% and 38% of women with repetitive spontaneous miscarriage.1
Classical classification
A classification of the Müllerian anomalies was introduced in 1980 and, with few modifications, was adopted by the American Fertility Society (currently, ASRM). The Society identified seven basic groups according to Müllerian development and their relationship to fertility: agenesis and hypoplasias, unicornuate uteri (unilateral hypoplasia), didelphys uteri (complete nonfusion), bicornuate uteri (incomplete fusion), septate uteri (nonreabsorption of septum), arcuate uteri (almost complete reabsorption of septum), and anomalies related to fetal DES exposure.2
Anomalies also can be categorized in terms of progression along the developmental continuum, taking into account that many cases result from partial failure of fusion and reabsorption: agenesis (Types I and II), lack of fusion (Types III and IV), lack of reabsorption(Types V and VI), and lack of posterior development (Type VII) (FIGURE 1).3
| FIGURE 1. Classification of müllerian anomalies |
|---|
 |
| Source: The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988. 49(6):944-955. |
3D ultrasonography offers accurate, cost-efficient diagnosis
Using only 2D imaging, neither an unenhanced sonogram nor a sonohysterogram can provide definitive information regarding the possibility of a uterine anomaly. The fundal contour cannot be evaluated with 2D imaging; likewise, details regarding the configuration of the uterine cavity (or cavities) may not be appreciated with the use of 2D imaging (FIGURE 2).
Figure 2: Normal appearance, but abnormal uteri
In sagittal view, a uterus with a congenital anomaly can appear normal. 2D sagittal views of a normal uterus (top), a didelphic uterus (middle), and a sonohysterogram of a septate uterus (bottom). |
To fully evaluate the uterine fundal contour and determine the type of uterine anomaly, it previously was necessary to obtain magnetic resonance imaging (MRI) or perform laparoscopy. Today, however, 3D coronal ultrasonography (US) can allow for accurate evaluation of fundal contour and diagnosis of uterine anomalies with lower cost and greater patient convenience. Several studies have confirmed the high accuracy of 3D US compared with MRI and surgical findings in the diagnosis of uterine anomalies (with 3D US showing 98% to 100% sensitivity and specificity).4-6
Case: Partial septate uterus
|
ADDITIONAL IMAGES
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of discussing the various uterine malformations as well as characterizing their appearance on 3D transvaginal ultrasound.
Unfortunately, many women are still subjected to the cost, inconvenience, and time involvement of magnetic resonance imaging (MRI) in cases of suspected uterine malformations. The exquisite visualization of 3D transvaginal ultrasound, so nicely depicted in this installment of Images in GYN Ultrasound, allow the observer to see the endometrial contours in the same plane as the serosal surface. This view is not available in traditional 2D ultrasound images. Thus, it is akin to doing laparoscopy and hysteroscopy simultaneously in order to arrive at the proper diagnosis. Although not mandatory, when such 3D ultrasound is performed late in the cycle, the thickened endometrium acts as a nice sonic backdrop to better delineate these structures. Alternatively, 3D saline infusion sonohysterography can be performed.
As more and more ultrasound equipment becomes available with 3D capability as a standard feature, clinicians who do perform ultrasonography will find that obtaining this “z-plane” is relatively simple and extremely informative, and can and should be done in cases of suspected uterine malformations in lieu of ordering MRI.
Congenital uterine anomalies: A resource of diagnostic images, Part 1
Michelle L. Stalnaker Ozcan, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Uterine malformations make up a diverse group of congenital anomalies that can result from various alterations in the normal development of the Müllerian ducts, including underdevelopment of one or both Müllerian ducts, disorders in Müllerian duct fusion, and alterations in septum reabsorption. How common are such anomalies, how are they classified, and what is the best approach for optimal visualization? Here, we explore these questions and offer an atlas of diagnostic images as an ongoing reference for your practice. Many of the images we offer will be found only online at obgmanagement.com.
How common are congenital uterine anomalies?
The reported prevalence of uterine malformations varies among publications due to heterogeneous population samples, differences in diagnostic techniques, and variations in nomenclature. In general, they are estimated to occur in 0.4% (0.1% to 3.0%) of the population at large, 4% of infertile women, and between 3% and 38% of women with repetitive spontaneous miscarriage.1
Classical classification
A classification of the Müllerian anomalies was introduced in 1980 and, with few modifications, was adopted by the American Fertility Society (currently, ASRM). The Society identified seven basic groups according to Müllerian development and their relationship to fertility: agenesis and hypoplasias, unicornuate uteri (unilateral hypoplasia), didelphys uteri (complete nonfusion), bicornuate uteri (incomplete fusion), septate uteri (nonreabsorption of septum), arcuate uteri (almost complete reabsorption of septum), and anomalies related to fetal DES exposure.2
Anomalies also can be categorized in terms of progression along the developmental continuum, taking into account that many cases result from partial failure of fusion and reabsorption: agenesis (Types I and II), lack of fusion (Types III and IV), lack of reabsorption(Types V and VI), and lack of posterior development (Type VII) (FIGURE 1).3
| FIGURE 1. Classification of müllerian anomalies |
|---|
 |
| Source: The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988. 49(6):944-955. |
3D ultrasonography offers accurate, cost-efficient diagnosis
Using only 2D imaging, neither an unenhanced sonogram nor a sonohysterogram can provide definitive information regarding the possibility of a uterine anomaly. The fundal contour cannot be evaluated with 2D imaging; likewise, details regarding the configuration of the uterine cavity (or cavities) may not be appreciated with the use of 2D imaging (FIGURE 2).
Figure 2: Normal appearance, but abnormal uteri
In sagittal view, a uterus with a congenital anomaly can appear normal. 2D sagittal views of a normal uterus (top), a didelphic uterus (middle), and a sonohysterogram of a septate uterus (bottom). |
To fully evaluate the uterine fundal contour and determine the type of uterine anomaly, it previously was necessary to obtain magnetic resonance imaging (MRI) or perform laparoscopy. Today, however, 3D coronal ultrasonography (US) can allow for accurate evaluation of fundal contour and diagnosis of uterine anomalies with lower cost and greater patient convenience. Several studies have confirmed the high accuracy of 3D US compared with MRI and surgical findings in the diagnosis of uterine anomalies (with 3D US showing 98% to 100% sensitivity and specificity).4-6
Case: Partial septate uterus
|
ADDITIONAL IMAGES
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Bermejo C, Martinez Ten P, Cantarero R, et al. Three-dimensional ultrasound in the diagnosis of Müllerian duct anomalies and concordance with magnetic resonance imaging. Ultrasound Obstet Gynecol. 2010;35(5):593–601.
- The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49(6):944–955.
- Acien P, Acien M. Updated classification of malformations. Hum Reprod. 2010;25(suppl 1):i81–i82.
- Deutch T, Bocca S, Oehninger S, Stadtmauer L, Abuhamad AZ. Magnetic resonance imaging versus three-dimensional transvaginal ultrasound for the diagnosis of Müllerian anomalies [abstract P-465]. Fertil Steril. 2006;86(suppl):S308.
- Wu MH, Hsu CC, Huang KE. Detection of congenital Müllerian duct anomalies using three-dimensional ultrasound. J Clin Ultrasound. 1997;25(9):487–492.
- Deutch TD, Abuhamad AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of Müllerian duct anomalies. J Ultrasound Med. 2008;27(3):413–423.
- Bermejo C, Martinez Ten P, Cantarero R, et al. Three-dimensional ultrasound in the diagnosis of Müllerian duct anomalies and concordance with magnetic resonance imaging. Ultrasound Obstet Gynecol. 2010;35(5):593–601.
- The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49(6):944–955.
- Acien P, Acien M. Updated classification of malformations. Hum Reprod. 2010;25(suppl 1):i81–i82.
- Deutch T, Bocca S, Oehninger S, Stadtmauer L, Abuhamad AZ. Magnetic resonance imaging versus three-dimensional transvaginal ultrasound for the diagnosis of Müllerian anomalies [abstract P-465]. Fertil Steril. 2006;86(suppl):S308.
- Wu MH, Hsu CC, Huang KE. Detection of congenital Müllerian duct anomalies using three-dimensional ultrasound. J Clin Ultrasound. 1997;25(9):487–492.
- Deutch TD, Abuhamad AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of Müllerian duct anomalies. J Ultrasound Med. 2008;27(3):413–423.
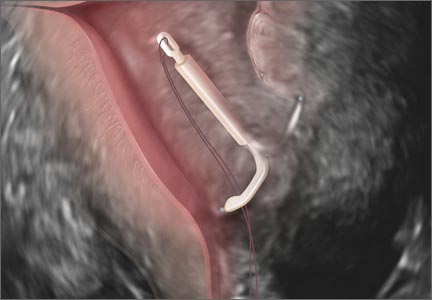
How to identify and localize IUDs on ultrasound
Although an ultrasound is not required after uncomplicated placement of an intrauterine device (IUD) or during routine management of women who are doing well with an IUD, it is invaluable in the evaluation of patients who present with pain or other symptoms suggestive of IUD malpositioning.
In this article, we outline the sonographic features of the IUDs available today in the United States and describe the basics of localization by ultrasound.
Related articles: STOP relying on 2D ultrasound for IUD localization. Steven R. Goldstein, MD, and Chrystie Fujimoto, MD (August 2014)
Update on Contraception. Melissa J. Chen, MD, MPH, and Mitchell D. Creinin, MD (August 2014)
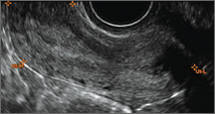
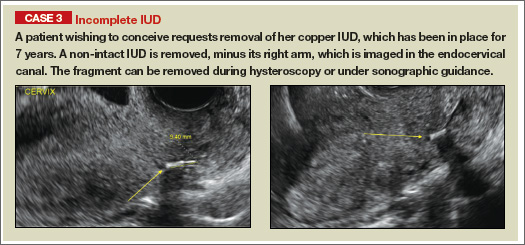
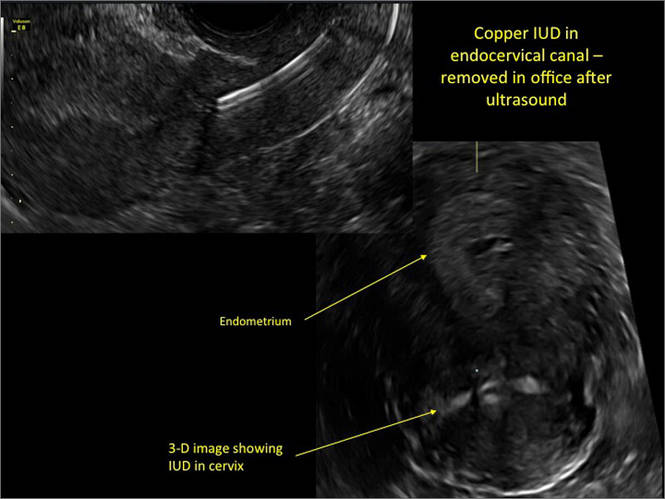
Ultrasound features of IUDsWhen positioned normally, an IUD is centrally located within the endometrial cavity, with the crossbar positioned in the fundal area.1 Copper and progestin-releasing IUDs can be identified easily on ultrasound if one is familiar with their basic sonographic features:
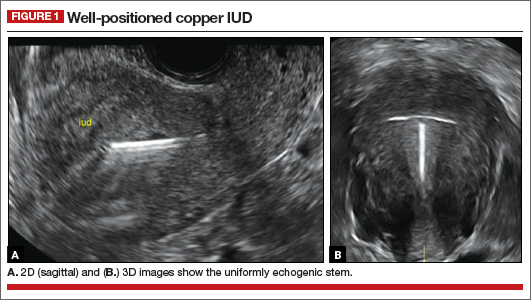
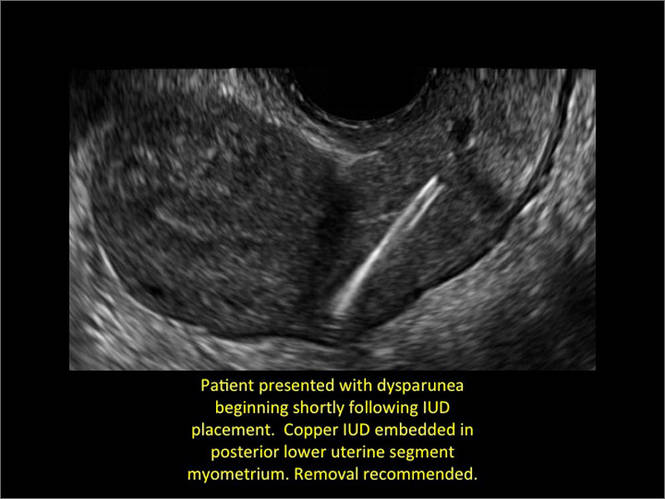
- Copper IUD: The central stem is uniformly echogenic due to its copper coils (FIGURE 1)
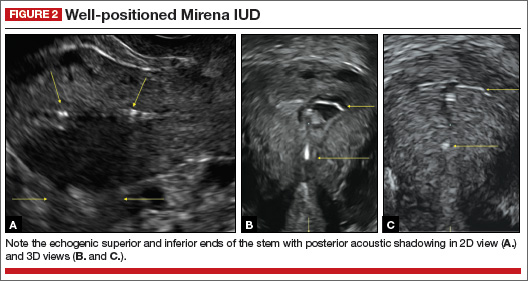
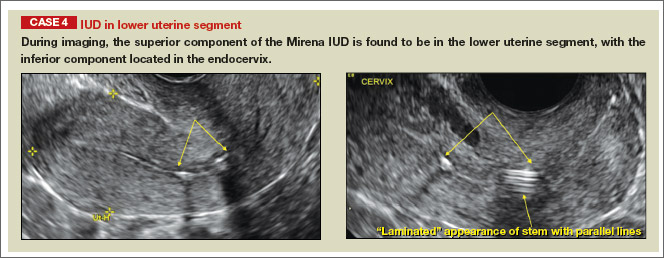
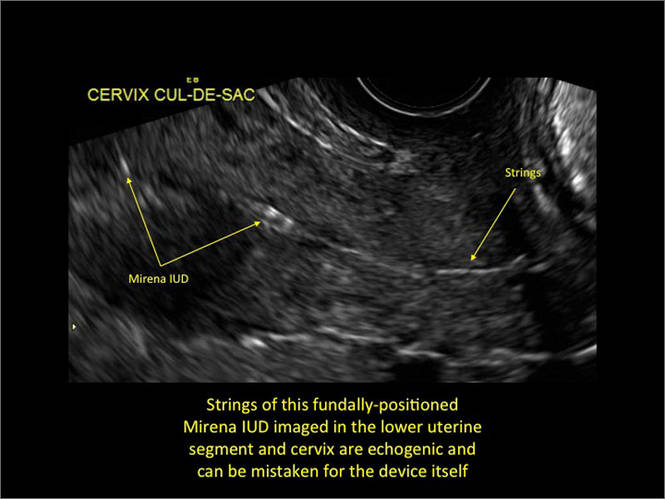
- Levonorgestrel-releasing intrauterine system (LNG-IUS): The LNG-IUS consists of a plastic sleeve that contains the progestin and surrounds a central stem. This configuration causes acoustic shadowing and has a characteristic “laminated” sonographic appearance with parallel lines (FIGURE 2). The Mirena IUD has echogenic arms due to barium sulfate, as well as an echogenic distal tip, with acoustic shadowing from the stem. Skyla is similar except for a highly echogenic silver ring on the stem approximately 3 to 4 mm inferior to the crossbar. On occasion, the echogenic strings of Mirena and Skyla can be mistaken for the device.
Three-dimensional ultrasound is useful in imaging of an IUD. If a patient’s IUD cannot be visualized by ultrasound, plain radiography of the kidney, ureter, and bladder may be helpful. If an IUD is not apparent on plain film, consider that it may have been expelled.
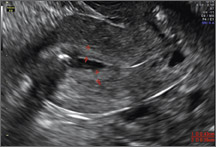
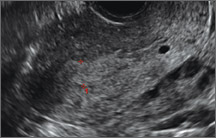
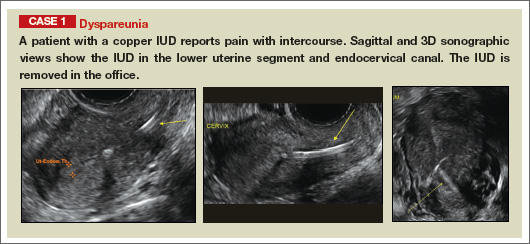
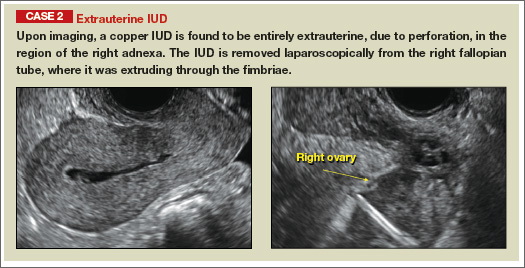
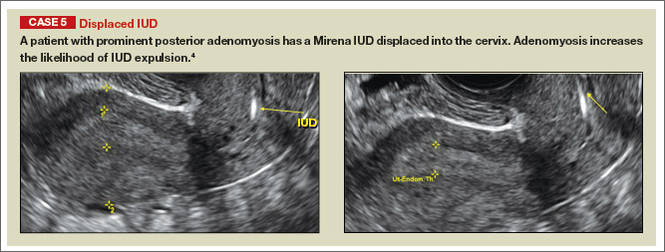
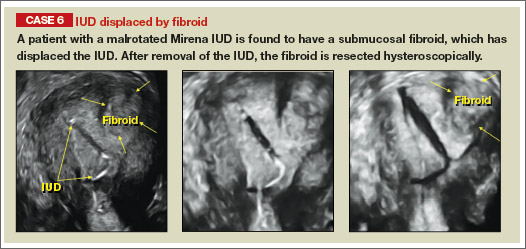
Potential malpositioningA malpositioned IUD may be partially expelled, rotated, embedded in the myometrium, or perforating the uterine serosa.
Related article: Malpositioned IUDs: When you should intervene (and when you should not). Kari Braaten, MD, MPH, and Alisa B. Goldberg, MD, MPH (August 2012)
In a retrospective case-control study that compared 182 women with sonographicallyidentified malpositioned IUDs with 182 women with properly positioned IUDs, Braaten and colleagues found that suspected adenomyosis was associated with malpositioning (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08–8.52), but a history of vaginal delivery was protective (OR, 0.53; 95% CI, 0.32–0.87).2 A distorted uterine cavity also increases the risk of malpositioning.3
 | |
 | |
 | |
 | |
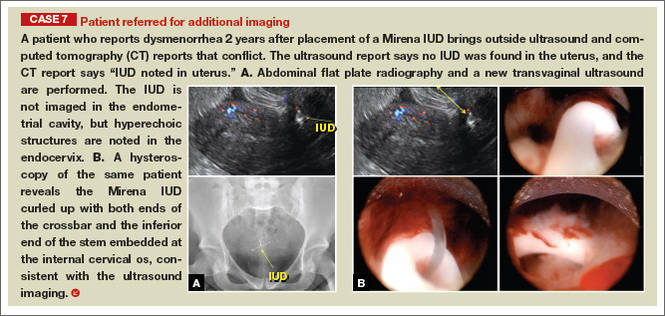 |
Although no uterine perforations were reported in a review of the LNG-IUS, expulsions were reported and may be more common among women who use the IUD for heavy menstrual bleeding.4
Additional images
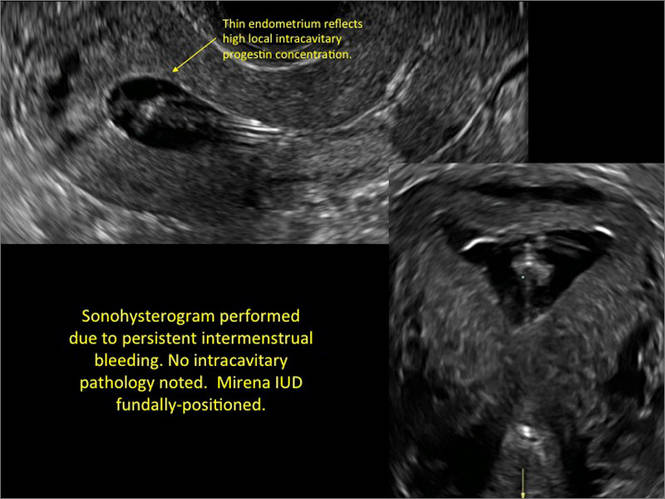
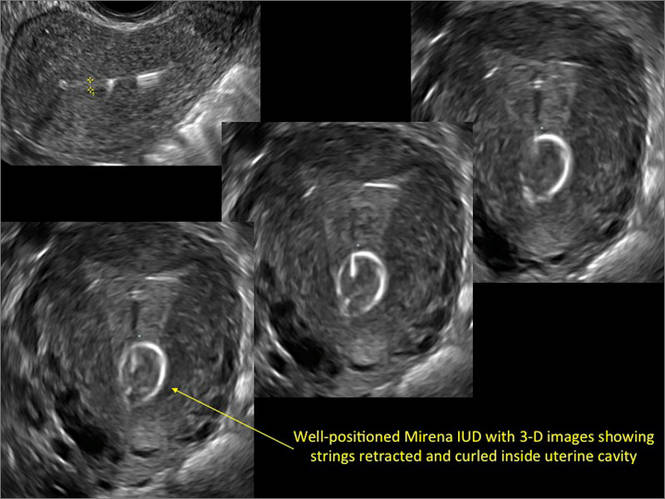
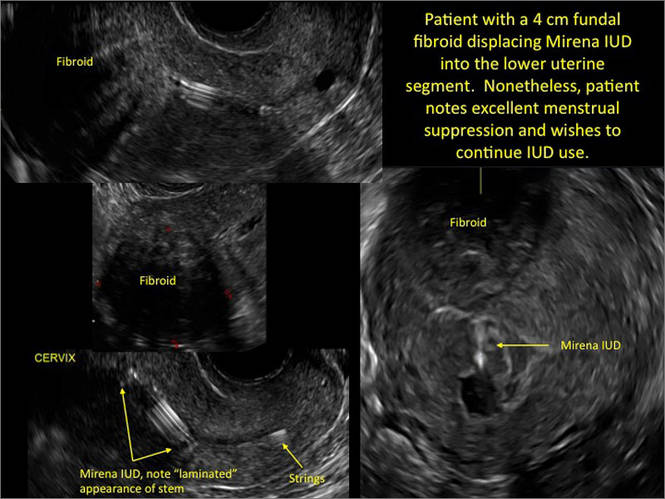
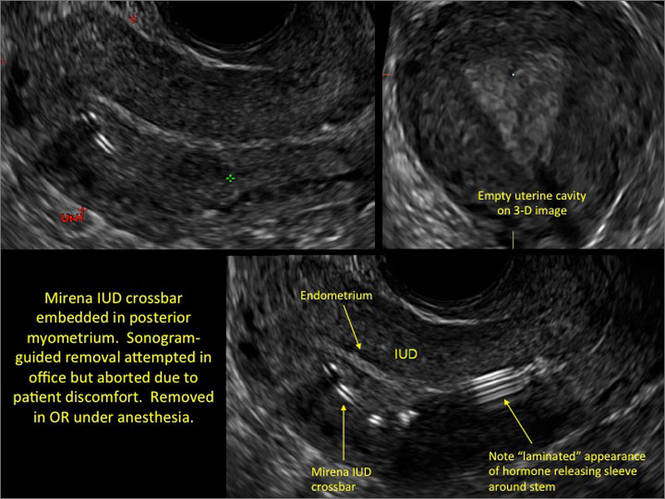
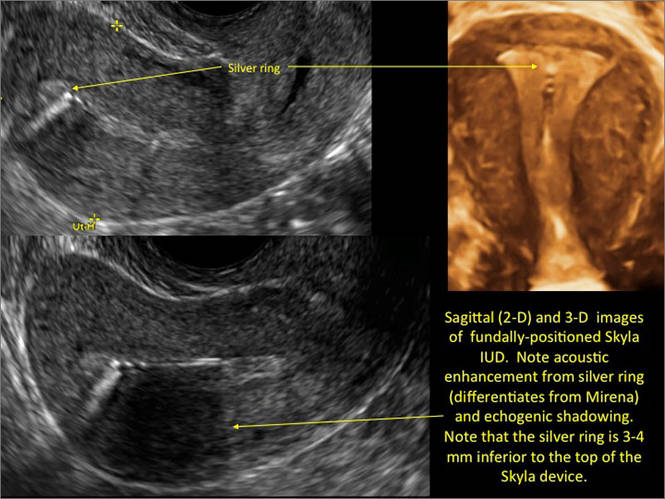
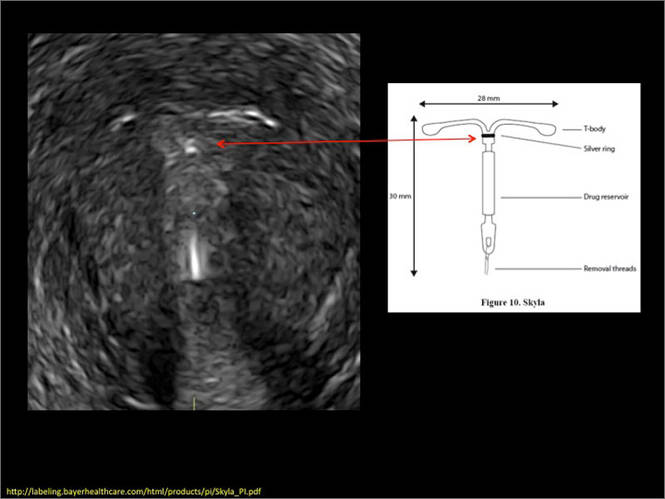
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Peri N, Graha D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26(10):1389–1401.
2. Braaten KP, Benson CB, Maurer R, Goldberg AB. Malpositioned intrauterine contraceptive devices: Risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118(5):1014–1020.
3. Braaten KP, Goldberg AB. Malpositioned IUDs: When you should intervene and when you should not. OBG Manag. 2012;24(8):39–46.
4. Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72(2):193–215.
Although an ultrasound is not required after uncomplicated placement of an intrauterine device (IUD) or during routine management of women who are doing well with an IUD, it is invaluable in the evaluation of patients who present with pain or other symptoms suggestive of IUD malpositioning.
In this article, we outline the sonographic features of the IUDs available today in the United States and describe the basics of localization by ultrasound.
Related articles: STOP relying on 2D ultrasound for IUD localization. Steven R. Goldstein, MD, and Chrystie Fujimoto, MD (August 2014)
Update on Contraception. Melissa J. Chen, MD, MPH, and Mitchell D. Creinin, MD (August 2014)
Ultrasound features of IUDsWhen positioned normally, an IUD is centrally located within the endometrial cavity, with the crossbar positioned in the fundal area.1 Copper and progestin-releasing IUDs can be identified easily on ultrasound if one is familiar with their basic sonographic features:
- Copper IUD: The central stem is uniformly echogenic due to its copper coils (FIGURE 1)
- Levonorgestrel-releasing intrauterine system (LNG-IUS): The LNG-IUS consists of a plastic sleeve that contains the progestin and surrounds a central stem. This configuration causes acoustic shadowing and has a characteristic “laminated” sonographic appearance with parallel lines (FIGURE 2). The Mirena IUD has echogenic arms due to barium sulfate, as well as an echogenic distal tip, with acoustic shadowing from the stem. Skyla is similar except for a highly echogenic silver ring on the stem approximately 3 to 4 mm inferior to the crossbar. On occasion, the echogenic strings of Mirena and Skyla can be mistaken for the device.
Three-dimensional ultrasound is useful in imaging of an IUD. If a patient’s IUD cannot be visualized by ultrasound, plain radiography of the kidney, ureter, and bladder may be helpful. If an IUD is not apparent on plain film, consider that it may have been expelled.


Potential malpositioningA malpositioned IUD may be partially expelled, rotated, embedded in the myometrium, or perforating the uterine serosa.
Related article: Malpositioned IUDs: When you should intervene (and when you should not). Kari Braaten, MD, MPH, and Alisa B. Goldberg, MD, MPH (August 2012)
In a retrospective case-control study that compared 182 women with sonographicallyidentified malpositioned IUDs with 182 women with properly positioned IUDs, Braaten and colleagues found that suspected adenomyosis was associated with malpositioning (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08–8.52), but a history of vaginal delivery was protective (OR, 0.53; 95% CI, 0.32–0.87).2 A distorted uterine cavity also increases the risk of malpositioning.3


 | |
 | |
 | |
 | |
 |
Although no uterine perforations were reported in a review of the LNG-IUS, expulsions were reported and may be more common among women who use the IUD for heavy menstrual bleeding.4
Additional images









WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
Although an ultrasound is not required after uncomplicated placement of an intrauterine device (IUD) or during routine management of women who are doing well with an IUD, it is invaluable in the evaluation of patients who present with pain or other symptoms suggestive of IUD malpositioning.
In this article, we outline the sonographic features of the IUDs available today in the United States and describe the basics of localization by ultrasound.
Related articles: STOP relying on 2D ultrasound for IUD localization. Steven R. Goldstein, MD, and Chrystie Fujimoto, MD (August 2014)
Update on Contraception. Melissa J. Chen, MD, MPH, and Mitchell D. Creinin, MD (August 2014)
Ultrasound features of IUDsWhen positioned normally, an IUD is centrally located within the endometrial cavity, with the crossbar positioned in the fundal area.1 Copper and progestin-releasing IUDs can be identified easily on ultrasound if one is familiar with their basic sonographic features:
- Copper IUD: The central stem is uniformly echogenic due to its copper coils (FIGURE 1)
- Levonorgestrel-releasing intrauterine system (LNG-IUS): The LNG-IUS consists of a plastic sleeve that contains the progestin and surrounds a central stem. This configuration causes acoustic shadowing and has a characteristic “laminated” sonographic appearance with parallel lines (FIGURE 2). The Mirena IUD has echogenic arms due to barium sulfate, as well as an echogenic distal tip, with acoustic shadowing from the stem. Skyla is similar except for a highly echogenic silver ring on the stem approximately 3 to 4 mm inferior to the crossbar. On occasion, the echogenic strings of Mirena and Skyla can be mistaken for the device.
Three-dimensional ultrasound is useful in imaging of an IUD. If a patient’s IUD cannot be visualized by ultrasound, plain radiography of the kidney, ureter, and bladder may be helpful. If an IUD is not apparent on plain film, consider that it may have been expelled.


Potential malpositioningA malpositioned IUD may be partially expelled, rotated, embedded in the myometrium, or perforating the uterine serosa.
Related article: Malpositioned IUDs: When you should intervene (and when you should not). Kari Braaten, MD, MPH, and Alisa B. Goldberg, MD, MPH (August 2012)
In a retrospective case-control study that compared 182 women with sonographicallyidentified malpositioned IUDs with 182 women with properly positioned IUDs, Braaten and colleagues found that suspected adenomyosis was associated with malpositioning (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08–8.52), but a history of vaginal delivery was protective (OR, 0.53; 95% CI, 0.32–0.87).2 A distorted uterine cavity also increases the risk of malpositioning.3


 | |
 | |
 | |
 | |
 |
Although no uterine perforations were reported in a review of the LNG-IUS, expulsions were reported and may be more common among women who use the IUD for heavy menstrual bleeding.4
Additional images









WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Peri N, Graha D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26(10):1389–1401.
2. Braaten KP, Benson CB, Maurer R, Goldberg AB. Malpositioned intrauterine contraceptive devices: Risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118(5):1014–1020.
3. Braaten KP, Goldberg AB. Malpositioned IUDs: When you should intervene and when you should not. OBG Manag. 2012;24(8):39–46.
4. Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72(2):193–215.
1. Peri N, Graha D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26(10):1389–1401.
2. Braaten KP, Benson CB, Maurer R, Goldberg AB. Malpositioned intrauterine contraceptive devices: Risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118(5):1014–1020.
3. Braaten KP, Goldberg AB. Malpositioned IUDs: When you should intervene and when you should not. OBG Manag. 2012;24(8):39–46.
4. Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72(2):193–215.
Uterine adenomyosis: Noninvasive diagnosis
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s installment of Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of describing what adenomyosis will look like on transvaginal ultrasound
In my first book, entitled Endovaginal Ultrasound,1 I coined the phrase “sonomicoscopy.” I maintain that we are seeing things with transvaginal ultrasound that you could not see with your naked eye if you could hold the structure at arms length and squint at it.
Adenomyosis is defined as endometrial glands and stroma embedded within the myometrium. Literature has shown that if you do three sections on a routine hysterectomy specimen the incidence of adenomyosis is 31%; with six sections the incidence is 61%! In other words, it is a very prevalent occurrence.
There is no question that adenomyosis CAN be a source of uterine enlargement, pain, and bleeding. But it is such a prevalent finding that the real question is: What percent of women, especially parous women, will have sonographic evidence of adenomyosis but be totally asymptomatic? Such women represent the denominator while the symptomatic ones represent the numerator. I worry about labeling asymptomatic patients with this entity—when they become perimenopausal and oligo-ovulatory, and may have irregular bleeding—their symptoms can be judged to be FROM adenomyosis and surgical correction is offered.
An important part of successful ultrasound use is being sure that we redefine what is “normal” as we examine patients with this “low power microscope.” So, while transvaginal ultrasound CAN identify glands and stroma within the myometrium, we must be careful not to automatically label this finding as a “disease.”
Reference
1. Goldstein SR. Endovaginal Ultrasound. 2nd ed. John Wiley & Sons, Inc: Hoboken, NJ; January 1991.
Uterine adenomyosis: Noninvasive diagnosis
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
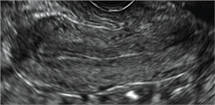
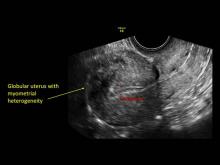
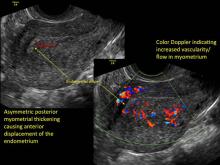
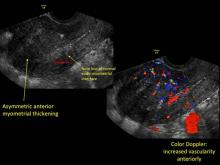
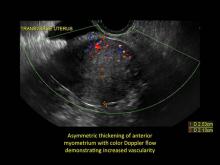
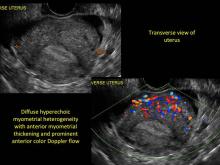
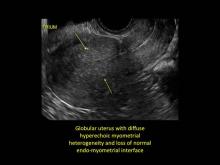
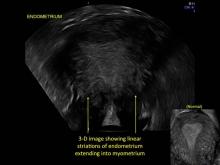
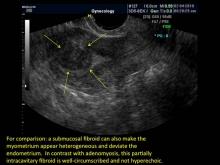
Uterine adenomyosis is a pathologic condition in which endometrial glands and stroma are present in the uterine myometrium. Uterine adenomyosis is common, and may coexist with leiomyomata or endometriosis. When present, it may cause dysmenorrhea and heavy menses.
Until recently, the best way to establish a diagnosis of uterine adenomyosis was through histologic examination of a hysterectomy specimen. However, transvaginal ultrasound and pelvic magnetic resonance imaging have been shown to be accurate for noninvasive diagnosis.
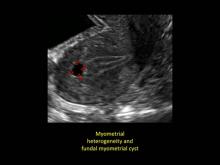
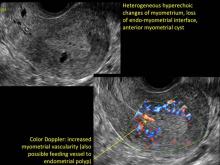
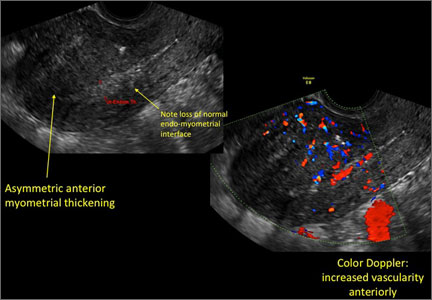
Signs on imaging include:
- Globular/bulky uterus
- Asymmetric thickening of myometrium
- Loss of clarity of endo-myometrial interface
- Diffuse heterogenous myometrial echogenicity
- Myometrial cysts
| Click to enlarge image |
1. Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstetricia et Gynecologica. 2010;89(11):1374-1384.
2. Meredith SM, Sanchez-Ramos L, Kaunitz AM. Diagnostic accuracy of transvaginal sonography for the diagnosis of adenomyosis: systematic review and meta-analysis. Am J Obstet Gynecol. 2009;201(1):107.e1-e6.
3. Munro MG. Update: abnormal uterine bleeding. OBG Manag. 2014;26(3):27-32.
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s installment of Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of describing what adenomyosis will look like on transvaginal ultrasound
In my first book, entitled Endovaginal Ultrasound,1 I coined the phrase “sonomicoscopy.” I maintain that we are seeing things with transvaginal ultrasound that you could not see with your naked eye if you could hold the structure at arms length and squint at it.
Adenomyosis is defined as endometrial glands and stroma embedded within the myometrium. Literature has shown that if you do three sections on a routine hysterectomy specimen the incidence of adenomyosis is 31%; with six sections the incidence is 61%! In other words, it is a very prevalent occurrence.
There is no question that adenomyosis CAN be a source of uterine enlargement, pain, and bleeding. But it is such a prevalent finding that the real question is: What percent of women, especially parous women, will have sonographic evidence of adenomyosis but be totally asymptomatic? Such women represent the denominator while the symptomatic ones represent the numerator. I worry about labeling asymptomatic patients with this entity—when they become perimenopausal and oligo-ovulatory, and may have irregular bleeding—their symptoms can be judged to be FROM adenomyosis and surgical correction is offered.
An important part of successful ultrasound use is being sure that we redefine what is “normal” as we examine patients with this “low power microscope.” So, while transvaginal ultrasound CAN identify glands and stroma within the myometrium, we must be careful not to automatically label this finding as a “disease.”
Reference
1. Goldstein SR. Endovaginal Ultrasound. 2nd ed. John Wiley & Sons, Inc: Hoboken, NJ; January 1991.
Uterine adenomyosis: Noninvasive diagnosis
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Uterine adenomyosis is a pathologic condition in which endometrial glands and stroma are present in the uterine myometrium. Uterine adenomyosis is common, and may coexist with leiomyomata or endometriosis. When present, it may cause dysmenorrhea and heavy menses.
Until recently, the best way to establish a diagnosis of uterine adenomyosis was through histologic examination of a hysterectomy specimen. However, transvaginal ultrasound and pelvic magnetic resonance imaging have been shown to be accurate for noninvasive diagnosis.
Signs on imaging include:
- Globular/bulky uterus
- Asymmetric thickening of myometrium
- Loss of clarity of endo-myometrial interface
- Diffuse heterogenous myometrial echogenicity
- Myometrial cysts
| Click to enlarge image |
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s installment of Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of describing what adenomyosis will look like on transvaginal ultrasound
In my first book, entitled Endovaginal Ultrasound,1 I coined the phrase “sonomicoscopy.” I maintain that we are seeing things with transvaginal ultrasound that you could not see with your naked eye if you could hold the structure at arms length and squint at it.
Adenomyosis is defined as endometrial glands and stroma embedded within the myometrium. Literature has shown that if you do three sections on a routine hysterectomy specimen the incidence of adenomyosis is 31%; with six sections the incidence is 61%! In other words, it is a very prevalent occurrence.
There is no question that adenomyosis CAN be a source of uterine enlargement, pain, and bleeding. But it is such a prevalent finding that the real question is: What percent of women, especially parous women, will have sonographic evidence of adenomyosis but be totally asymptomatic? Such women represent the denominator while the symptomatic ones represent the numerator. I worry about labeling asymptomatic patients with this entity—when they become perimenopausal and oligo-ovulatory, and may have irregular bleeding—their symptoms can be judged to be FROM adenomyosis and surgical correction is offered.
An important part of successful ultrasound use is being sure that we redefine what is “normal” as we examine patients with this “low power microscope.” So, while transvaginal ultrasound CAN identify glands and stroma within the myometrium, we must be careful not to automatically label this finding as a “disease.”
Reference
1. Goldstein SR. Endovaginal Ultrasound. 2nd ed. John Wiley & Sons, Inc: Hoboken, NJ; January 1991.
Uterine adenomyosis: Noninvasive diagnosis
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Uterine adenomyosis is a pathologic condition in which endometrial glands and stroma are present in the uterine myometrium. Uterine adenomyosis is common, and may coexist with leiomyomata or endometriosis. When present, it may cause dysmenorrhea and heavy menses.
Until recently, the best way to establish a diagnosis of uterine adenomyosis was through histologic examination of a hysterectomy specimen. However, transvaginal ultrasound and pelvic magnetic resonance imaging have been shown to be accurate for noninvasive diagnosis.
Signs on imaging include:
- Globular/bulky uterus
- Asymmetric thickening of myometrium
- Loss of clarity of endo-myometrial interface
- Diffuse heterogenous myometrial echogenicity
- Myometrial cysts
| Click to enlarge image |
1. Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstetricia et Gynecologica. 2010;89(11):1374-1384.
2. Meredith SM, Sanchez-Ramos L, Kaunitz AM. Diagnostic accuracy of transvaginal sonography for the diagnosis of adenomyosis: systematic review and meta-analysis. Am J Obstet Gynecol. 2009;201(1):107.e1-e6.
3. Munro MG. Update: abnormal uterine bleeding. OBG Manag. 2014;26(3):27-32.
1. Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstetricia et Gynecologica. 2010;89(11):1374-1384.
2. Meredith SM, Sanchez-Ramos L, Kaunitz AM. Diagnostic accuracy of transvaginal sonography for the diagnosis of adenomyosis: systematic review and meta-analysis. Am J Obstet Gynecol. 2009;201(1):107.e1-e6.
3. Munro MG. Update: abnormal uterine bleeding. OBG Manag. 2014;26(3):27-32.