User login
23rd World Congress of Dermatology
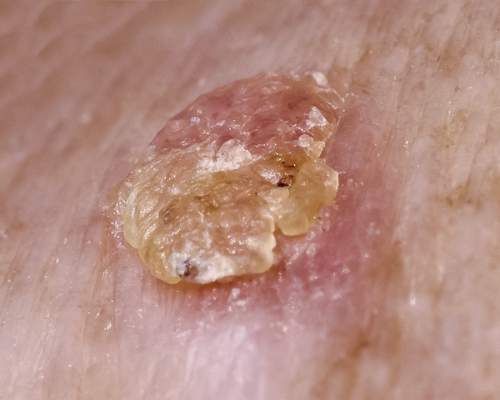
Ingenol mebutate for AKs gets thumbs-up from patients
VANCOUVER – Field therapy for actinic keratoses using topical ingenol mebutate resulted in improved patient-reported outcomes in an observational study, Dr. Thomas L. Diepgen reported at the World Congress of Dermatology.
Topical ingenol mebutate won regulatory approval for the field treatment of actinic keratoses on the strength of four randomized, double-blind placebo-controlled clinical trials, but patients and their physicians need to know how the drug performs in clinical practice. The answer is, quite well, said Dr. Diepgen of the University of Heidelberg (Germany), who presented the results of the observational study emphasizing patient-reported outcomes in 826 patients whose actinic keratoses (AKs) were treated with ingenol mebutate (Picato) in 292 German dermatologists’ offices.
Unlike in randomized clinical trials, where strict eligibility criteria often result in a skewed population of participants, this observational study provided a representative snapshot of German patients seeking AK therapy. Their mean age was 73 years, with a mean 6.2-year duration of AKs and a median baseline of 5 lesions. Eighty percent of patients had previously undergone other types of therapy for the AKs, and 34% of them had a history of nonmelanoma skin cancer.
Participants completed the Skindex-16 quality of life questionnaire at their baseline office visit, and again 8 weeks later. The Skindex-16 doesn’t ask disease-specific questions, but this 16-item questionnaire was employed in the earlier pivotal randomized trials (N. Engl. J. Med. 2012;366:1010-19), and investigators felt they should utilize the same instrument, said Dr. Diepgen.
Scores on the Skindex-16 improved significantly from a mean baseline of 24.3 out of a possible 96 points to 12.1 after 8 weeks.
Similarly, when patients were asked to rate their skin roughness, wrinkling, and/or blotchiness on a 0-3 scale, their mean scores fell from 1.46 at baseline to 0.69 at follow-up. Ninety-eight percent of patients reported no new skin anomalies such as hypopigmentation in the treatment area.
Session cochair Dr. Marc Bourcier of the University of Sherbrooke (Que.) observed that this study underscores that the timing of quality of life assessment makes an enormous difference. Had the assessment taken place at day 4, for example, when ingenol mebutate–induced skin irritation would have been prominent, the results would have been very different. Dr. Diepgen agreed, noting that he and his coinvestigators wanted to evaluate patients’ response to the long-lasting results of the treatment, rather than to the transient experience of the therapy.
The study was sponsored by Leo Pharma. Dr. Diepgen reported having received research grants and speakers fees, and/or serving on advisory boards for Leo and more than a dozen other pharmaceutical companies.
VANCOUVER – Field therapy for actinic keratoses using topical ingenol mebutate resulted in improved patient-reported outcomes in an observational study, Dr. Thomas L. Diepgen reported at the World Congress of Dermatology.
Topical ingenol mebutate won regulatory approval for the field treatment of actinic keratoses on the strength of four randomized, double-blind placebo-controlled clinical trials, but patients and their physicians need to know how the drug performs in clinical practice. The answer is, quite well, said Dr. Diepgen of the University of Heidelberg (Germany), who presented the results of the observational study emphasizing patient-reported outcomes in 826 patients whose actinic keratoses (AKs) were treated with ingenol mebutate (Picato) in 292 German dermatologists’ offices.
Unlike in randomized clinical trials, where strict eligibility criteria often result in a skewed population of participants, this observational study provided a representative snapshot of German patients seeking AK therapy. Their mean age was 73 years, with a mean 6.2-year duration of AKs and a median baseline of 5 lesions. Eighty percent of patients had previously undergone other types of therapy for the AKs, and 34% of them had a history of nonmelanoma skin cancer.
Participants completed the Skindex-16 quality of life questionnaire at their baseline office visit, and again 8 weeks later. The Skindex-16 doesn’t ask disease-specific questions, but this 16-item questionnaire was employed in the earlier pivotal randomized trials (N. Engl. J. Med. 2012;366:1010-19), and investigators felt they should utilize the same instrument, said Dr. Diepgen.
Scores on the Skindex-16 improved significantly from a mean baseline of 24.3 out of a possible 96 points to 12.1 after 8 weeks.
Similarly, when patients were asked to rate their skin roughness, wrinkling, and/or blotchiness on a 0-3 scale, their mean scores fell from 1.46 at baseline to 0.69 at follow-up. Ninety-eight percent of patients reported no new skin anomalies such as hypopigmentation in the treatment area.
Session cochair Dr. Marc Bourcier of the University of Sherbrooke (Que.) observed that this study underscores that the timing of quality of life assessment makes an enormous difference. Had the assessment taken place at day 4, for example, when ingenol mebutate–induced skin irritation would have been prominent, the results would have been very different. Dr. Diepgen agreed, noting that he and his coinvestigators wanted to evaluate patients’ response to the long-lasting results of the treatment, rather than to the transient experience of the therapy.
The study was sponsored by Leo Pharma. Dr. Diepgen reported having received research grants and speakers fees, and/or serving on advisory boards for Leo and more than a dozen other pharmaceutical companies.
VANCOUVER – Field therapy for actinic keratoses using topical ingenol mebutate resulted in improved patient-reported outcomes in an observational study, Dr. Thomas L. Diepgen reported at the World Congress of Dermatology.
Topical ingenol mebutate won regulatory approval for the field treatment of actinic keratoses on the strength of four randomized, double-blind placebo-controlled clinical trials, but patients and their physicians need to know how the drug performs in clinical practice. The answer is, quite well, said Dr. Diepgen of the University of Heidelberg (Germany), who presented the results of the observational study emphasizing patient-reported outcomes in 826 patients whose actinic keratoses (AKs) were treated with ingenol mebutate (Picato) in 292 German dermatologists’ offices.
Unlike in randomized clinical trials, where strict eligibility criteria often result in a skewed population of participants, this observational study provided a representative snapshot of German patients seeking AK therapy. Their mean age was 73 years, with a mean 6.2-year duration of AKs and a median baseline of 5 lesions. Eighty percent of patients had previously undergone other types of therapy for the AKs, and 34% of them had a history of nonmelanoma skin cancer.
Participants completed the Skindex-16 quality of life questionnaire at their baseline office visit, and again 8 weeks later. The Skindex-16 doesn’t ask disease-specific questions, but this 16-item questionnaire was employed in the earlier pivotal randomized trials (N. Engl. J. Med. 2012;366:1010-19), and investigators felt they should utilize the same instrument, said Dr. Diepgen.
Scores on the Skindex-16 improved significantly from a mean baseline of 24.3 out of a possible 96 points to 12.1 after 8 weeks.
Similarly, when patients were asked to rate their skin roughness, wrinkling, and/or blotchiness on a 0-3 scale, their mean scores fell from 1.46 at baseline to 0.69 at follow-up. Ninety-eight percent of patients reported no new skin anomalies such as hypopigmentation in the treatment area.
Session cochair Dr. Marc Bourcier of the University of Sherbrooke (Que.) observed that this study underscores that the timing of quality of life assessment makes an enormous difference. Had the assessment taken place at day 4, for example, when ingenol mebutate–induced skin irritation would have been prominent, the results would have been very different. Dr. Diepgen agreed, noting that he and his coinvestigators wanted to evaluate patients’ response to the long-lasting results of the treatment, rather than to the transient experience of the therapy.
The study was sponsored by Leo Pharma. Dr. Diepgen reported having received research grants and speakers fees, and/or serving on advisory boards for Leo and more than a dozen other pharmaceutical companies.
AT WCD 2015
Key clinical point: Field therapy for actinic keratoses using topical ingenol mebutate resulted in improved patient-reported outcomes.
Major finding: Mean scores on the Skindex-16, which reflects the quality of life impact of a patient’s skin disease, improved significantly from 24.3 pretreatment to 12.1 after 8 weeks.
Data source: A prospective observational study of patient-reported outcomes of ingenol mebutate therapy for actinic keratoses in 826 patients treated in 292 German dermatologists’ offices.
Disclosures: The study was sponsored by Leo Pharma, which markets ingenol mebutate. The presenter reported having received research grants and speakers’ fees, and/or serving on advisory boards for Leo and more than a dozen other pharmaceutical companies.
Simulated daylight PDT advantageous for AKs
VANCOUVER – Indoor simulated daylight photodynamic therapy for actinic keratoses sidesteps the major shortcoming of natural daylight PDT by providing a standardized, dermatologist-controlled light dose that’s not dependent upon the vagaries of weather, season, or outdoor temperature, Dr. Uwe Reinhold reported at the World Congress of Dermatology.
Daylight PDT, in which the photosensitizing agent is activated by natural light, is an increasingly popular concept that originated in Scandinavia but is starting to catch on in the United States. Daylight PDT is less expensive and far less painful than traditional PDT, in which the photosensitizer is activated by a pulsed dye laser or an intense pulsed light device. But on a rainy day or a cold, short, winter day, it can be a problem getting sufficient daylight outdoors to reliably activate the PDT, noted Dr. Reinhold of the Dermatology Center Bonn (Germany) Friedensplatz.
Dr. Reinhold and his colleagues solved that problem by installing a special lamp system on the ceiling of a treatment room in the office. The system enables a dermatologist to simultaneously treat several patients, who receive their 2-hour light dose while seated comfortably in the treatment room reading a book or resting.
Dr. Reinhold presented a retrospective study of 32 patients who underwent simulated daylight PDT (SDL-PDT) in his office. At baseline, the patients had a mean of 5.3 AKs on the scalp and/or face. At follow-up 12 weeks after their second and final SDL-PDT session, they averaged 0.4 AKs. Ninety-three percent of all AKs were cleared, and three-quarters of the patients were completely AK-free.
Traditional PDT is so painful that compliance becomes an issue, Dr. Reinhold noted. In contrast, SDL-PDT, like daylight PDT, is almost pain free. Pain assessment on a 0-10 visual analog scale conducted during the first SDL-PDT session showed mean scores of 0.1, 0.3, and 0.6 at 30, 60, and 90 minutes after illumination began. None of the patients required an analgesic, according to the dermatologist.
The procedure begins with curettage of hyperkeratotic lesions, followed by application of aminolevulinic acid (ALA) gel under occlusion for 30 minutes. Dr. Reinhold uses BF-200 (Ameluz), an ALA manufactured by Biofrontera, a German company, which is popular in Europe but not marketed in the United States. The gel contains 78 mg of ALA per gram. After the 30-minute incubation, the photosensitizer is removed and the special lights are switched on for 2 hours. Protective eye goggles aren’t needed. All patients receive a second treatment session 1 week later.
The lights Dr. Reinhold uses are Indoorlux, marketed by Swiss Red AG. One pair of lights is needed per patient. At a distance of 110-150 cm from the light source, the system produces 15,000-25,000 Lux. The lamps mimic the green and red components of daylight. The combined effective light dose at the wavelengths important in activating protoporphyrin IX so that it can destroy precancerous cells – green/yellow at 570-590 nm and orange/red at 620-640 nm – is 14.3-24.2 J/cm2, depending upon the distance from the light source. That’s comfortably above the 9.4-10.8 J/cm2 other investigators have determined is required for effective natural daylight PDT.
In the United States, however, as in Europe, SDL-PDT is currently an off-label therapy for AK treatment, he noted.
Dr. Reinhold reported serving as a consultant to Biofrontera and receiving speaking fees from the company.
VANCOUVER – Indoor simulated daylight photodynamic therapy for actinic keratoses sidesteps the major shortcoming of natural daylight PDT by providing a standardized, dermatologist-controlled light dose that’s not dependent upon the vagaries of weather, season, or outdoor temperature, Dr. Uwe Reinhold reported at the World Congress of Dermatology.
Daylight PDT, in which the photosensitizing agent is activated by natural light, is an increasingly popular concept that originated in Scandinavia but is starting to catch on in the United States. Daylight PDT is less expensive and far less painful than traditional PDT, in which the photosensitizer is activated by a pulsed dye laser or an intense pulsed light device. But on a rainy day or a cold, short, winter day, it can be a problem getting sufficient daylight outdoors to reliably activate the PDT, noted Dr. Reinhold of the Dermatology Center Bonn (Germany) Friedensplatz.
Dr. Reinhold and his colleagues solved that problem by installing a special lamp system on the ceiling of a treatment room in the office. The system enables a dermatologist to simultaneously treat several patients, who receive their 2-hour light dose while seated comfortably in the treatment room reading a book or resting.
Dr. Reinhold presented a retrospective study of 32 patients who underwent simulated daylight PDT (SDL-PDT) in his office. At baseline, the patients had a mean of 5.3 AKs on the scalp and/or face. At follow-up 12 weeks after their second and final SDL-PDT session, they averaged 0.4 AKs. Ninety-three percent of all AKs were cleared, and three-quarters of the patients were completely AK-free.
Traditional PDT is so painful that compliance becomes an issue, Dr. Reinhold noted. In contrast, SDL-PDT, like daylight PDT, is almost pain free. Pain assessment on a 0-10 visual analog scale conducted during the first SDL-PDT session showed mean scores of 0.1, 0.3, and 0.6 at 30, 60, and 90 minutes after illumination began. None of the patients required an analgesic, according to the dermatologist.
The procedure begins with curettage of hyperkeratotic lesions, followed by application of aminolevulinic acid (ALA) gel under occlusion for 30 minutes. Dr. Reinhold uses BF-200 (Ameluz), an ALA manufactured by Biofrontera, a German company, which is popular in Europe but not marketed in the United States. The gel contains 78 mg of ALA per gram. After the 30-minute incubation, the photosensitizer is removed and the special lights are switched on for 2 hours. Protective eye goggles aren’t needed. All patients receive a second treatment session 1 week later.
The lights Dr. Reinhold uses are Indoorlux, marketed by Swiss Red AG. One pair of lights is needed per patient. At a distance of 110-150 cm from the light source, the system produces 15,000-25,000 Lux. The lamps mimic the green and red components of daylight. The combined effective light dose at the wavelengths important in activating protoporphyrin IX so that it can destroy precancerous cells – green/yellow at 570-590 nm and orange/red at 620-640 nm – is 14.3-24.2 J/cm2, depending upon the distance from the light source. That’s comfortably above the 9.4-10.8 J/cm2 other investigators have determined is required for effective natural daylight PDT.
In the United States, however, as in Europe, SDL-PDT is currently an off-label therapy for AK treatment, he noted.
Dr. Reinhold reported serving as a consultant to Biofrontera and receiving speaking fees from the company.
VANCOUVER – Indoor simulated daylight photodynamic therapy for actinic keratoses sidesteps the major shortcoming of natural daylight PDT by providing a standardized, dermatologist-controlled light dose that’s not dependent upon the vagaries of weather, season, or outdoor temperature, Dr. Uwe Reinhold reported at the World Congress of Dermatology.
Daylight PDT, in which the photosensitizing agent is activated by natural light, is an increasingly popular concept that originated in Scandinavia but is starting to catch on in the United States. Daylight PDT is less expensive and far less painful than traditional PDT, in which the photosensitizer is activated by a pulsed dye laser or an intense pulsed light device. But on a rainy day or a cold, short, winter day, it can be a problem getting sufficient daylight outdoors to reliably activate the PDT, noted Dr. Reinhold of the Dermatology Center Bonn (Germany) Friedensplatz.
Dr. Reinhold and his colleagues solved that problem by installing a special lamp system on the ceiling of a treatment room in the office. The system enables a dermatologist to simultaneously treat several patients, who receive their 2-hour light dose while seated comfortably in the treatment room reading a book or resting.
Dr. Reinhold presented a retrospective study of 32 patients who underwent simulated daylight PDT (SDL-PDT) in his office. At baseline, the patients had a mean of 5.3 AKs on the scalp and/or face. At follow-up 12 weeks after their second and final SDL-PDT session, they averaged 0.4 AKs. Ninety-three percent of all AKs were cleared, and three-quarters of the patients were completely AK-free.
Traditional PDT is so painful that compliance becomes an issue, Dr. Reinhold noted. In contrast, SDL-PDT, like daylight PDT, is almost pain free. Pain assessment on a 0-10 visual analog scale conducted during the first SDL-PDT session showed mean scores of 0.1, 0.3, and 0.6 at 30, 60, and 90 minutes after illumination began. None of the patients required an analgesic, according to the dermatologist.
The procedure begins with curettage of hyperkeratotic lesions, followed by application of aminolevulinic acid (ALA) gel under occlusion for 30 minutes. Dr. Reinhold uses BF-200 (Ameluz), an ALA manufactured by Biofrontera, a German company, which is popular in Europe but not marketed in the United States. The gel contains 78 mg of ALA per gram. After the 30-minute incubation, the photosensitizer is removed and the special lights are switched on for 2 hours. Protective eye goggles aren’t needed. All patients receive a second treatment session 1 week later.
The lights Dr. Reinhold uses are Indoorlux, marketed by Swiss Red AG. One pair of lights is needed per patient. At a distance of 110-150 cm from the light source, the system produces 15,000-25,000 Lux. The lamps mimic the green and red components of daylight. The combined effective light dose at the wavelengths important in activating protoporphyrin IX so that it can destroy precancerous cells – green/yellow at 570-590 nm and orange/red at 620-640 nm – is 14.3-24.2 J/cm2, depending upon the distance from the light source. That’s comfortably above the 9.4-10.8 J/cm2 other investigators have determined is required for effective natural daylight PDT.
In the United States, however, as in Europe, SDL-PDT is currently an off-label therapy for AK treatment, he noted.
Dr. Reinhold reported serving as a consultant to Biofrontera and receiving speaking fees from the company.
AT WCD 2015
Key clinical point: A field containing multiple actinic keratoses can be treated virtually painlessly using lamps that simulate daylight to activate photodynamic therapy.
Major finding: 3 months after simulated daylight PDT, the mean number of AKs in treated patients was reduced from 5.3 at baseline to 0.4.
Data source: This was a retrospective study including 32 patients whose actinic keratoses was treated using simulated daylight PDT.
Disclosures: The study was supported by Biofrontera. The presenter reported serving as a consultant to and receiving speaking fees from the company.
WCD: Ustekinumab succeeds as switch agent in psoriasis
VANCOUVER, B.C. – When adults with moderate to severe psoriasis fail one anti-TNF agent, most will still respond to another anti-TNF or ustekinumab, according to data from an investigation at two University of Toronto hospitals.
Researchers reviewed the outcomes of 155 patients who were switched to a second biologic after failing at least 12 weeks of treatment with their first, which was usually an anti–tumor necrosis factor (TNF) agent. Ustekinumab was often plan B. Of the 65 patients switched to it after failing initial anti-TNF therapy, 47 (72%) had a significant response, defined as at least a 75% improvement in the Psoriasis Area & Severity Index score (PASI 75) after 12 weeks of treatment. Ten more patients (15%) achieved PASI 50.
Meanwhile, of the 82 switched to a second anti-TNF, 48 (59%) reached PASI 75 and more achieved PASI 50 after 12 weeks.
“Clinicians switch biologics all the time, but there are no clear guidelines” on how to do it, “so it was worth looking into,” investigator Whan Kim, a medical student at McMaster University in Hamilton, Ont., said at the World Congress of Dermatology.
“This is one of the largest studies to date that determines the effectiveness of real-life switching of biologic therapies in patients with moderate to severe psoriasis. This study suggests switching to ustekinumab would induce the highest response rate – 72% was surprising,” he said. “Failing a biologic doesn’t preclude responding to another one. Don’t give up,” Mr. Kim added.
The team did find, however, that patients with two previously failed biologics were about half as likely to respond to a third biologic, compared with those who had failed just one (odds ratio, 0.474; 95% confidence interval, 0.23-0.96; P = 0.04).
The patients averaged 50 years of age and had psoriasis for approximately 17 years. Half had psoriatic arthritis, and the study population included slightly more women than men. Of the 155 patients, 93 failed initial treatment with etanercept. Seven of 12 (58%) who switched to infliximab, 24 of 43 (56%) who switched to adalimumab, and 27 of 38 (71%) who switched to ustekinumab achieved PASI 75.
Twenty-three failed initial infliximab. Three of four (75%) who switched to etanercept, four of seven (57%) who switched to adalimumab, and nine of 12 (75%) who switched to ustekinumab had a significant response.
Thirty-one patients failed initial adalimumab. Seven of 11 (64%) who switched to etanercept, 3 of 5 (60%) who switched to infliximab, and 11 of 15 (73%) who switched to ustekinumab had a significant response.
Of eight patients who failed initial ustekinumab, three (38%) switched to etanercept and had a significant response after 12 weeks.
There was no outside funding for the work, and Mr. Kim had no disclosures. The senior investigator is a speaker, consultant, and investigator for AbbVie, Amgen, and Janssen.
VANCOUVER, B.C. – When adults with moderate to severe psoriasis fail one anti-TNF agent, most will still respond to another anti-TNF or ustekinumab, according to data from an investigation at two University of Toronto hospitals.
Researchers reviewed the outcomes of 155 patients who were switched to a second biologic after failing at least 12 weeks of treatment with their first, which was usually an anti–tumor necrosis factor (TNF) agent. Ustekinumab was often plan B. Of the 65 patients switched to it after failing initial anti-TNF therapy, 47 (72%) had a significant response, defined as at least a 75% improvement in the Psoriasis Area & Severity Index score (PASI 75) after 12 weeks of treatment. Ten more patients (15%) achieved PASI 50.
Meanwhile, of the 82 switched to a second anti-TNF, 48 (59%) reached PASI 75 and more achieved PASI 50 after 12 weeks.
“Clinicians switch biologics all the time, but there are no clear guidelines” on how to do it, “so it was worth looking into,” investigator Whan Kim, a medical student at McMaster University in Hamilton, Ont., said at the World Congress of Dermatology.
“This is one of the largest studies to date that determines the effectiveness of real-life switching of biologic therapies in patients with moderate to severe psoriasis. This study suggests switching to ustekinumab would induce the highest response rate – 72% was surprising,” he said. “Failing a biologic doesn’t preclude responding to another one. Don’t give up,” Mr. Kim added.
The team did find, however, that patients with two previously failed biologics were about half as likely to respond to a third biologic, compared with those who had failed just one (odds ratio, 0.474; 95% confidence interval, 0.23-0.96; P = 0.04).
The patients averaged 50 years of age and had psoriasis for approximately 17 years. Half had psoriatic arthritis, and the study population included slightly more women than men. Of the 155 patients, 93 failed initial treatment with etanercept. Seven of 12 (58%) who switched to infliximab, 24 of 43 (56%) who switched to adalimumab, and 27 of 38 (71%) who switched to ustekinumab achieved PASI 75.
Twenty-three failed initial infliximab. Three of four (75%) who switched to etanercept, four of seven (57%) who switched to adalimumab, and nine of 12 (75%) who switched to ustekinumab had a significant response.
Thirty-one patients failed initial adalimumab. Seven of 11 (64%) who switched to etanercept, 3 of 5 (60%) who switched to infliximab, and 11 of 15 (73%) who switched to ustekinumab had a significant response.
Of eight patients who failed initial ustekinumab, three (38%) switched to etanercept and had a significant response after 12 weeks.
There was no outside funding for the work, and Mr. Kim had no disclosures. The senior investigator is a speaker, consultant, and investigator for AbbVie, Amgen, and Janssen.
VANCOUVER, B.C. – When adults with moderate to severe psoriasis fail one anti-TNF agent, most will still respond to another anti-TNF or ustekinumab, according to data from an investigation at two University of Toronto hospitals.
Researchers reviewed the outcomes of 155 patients who were switched to a second biologic after failing at least 12 weeks of treatment with their first, which was usually an anti–tumor necrosis factor (TNF) agent. Ustekinumab was often plan B. Of the 65 patients switched to it after failing initial anti-TNF therapy, 47 (72%) had a significant response, defined as at least a 75% improvement in the Psoriasis Area & Severity Index score (PASI 75) after 12 weeks of treatment. Ten more patients (15%) achieved PASI 50.
Meanwhile, of the 82 switched to a second anti-TNF, 48 (59%) reached PASI 75 and more achieved PASI 50 after 12 weeks.
“Clinicians switch biologics all the time, but there are no clear guidelines” on how to do it, “so it was worth looking into,” investigator Whan Kim, a medical student at McMaster University in Hamilton, Ont., said at the World Congress of Dermatology.
“This is one of the largest studies to date that determines the effectiveness of real-life switching of biologic therapies in patients with moderate to severe psoriasis. This study suggests switching to ustekinumab would induce the highest response rate – 72% was surprising,” he said. “Failing a biologic doesn’t preclude responding to another one. Don’t give up,” Mr. Kim added.
The team did find, however, that patients with two previously failed biologics were about half as likely to respond to a third biologic, compared with those who had failed just one (odds ratio, 0.474; 95% confidence interval, 0.23-0.96; P = 0.04).
The patients averaged 50 years of age and had psoriasis for approximately 17 years. Half had psoriatic arthritis, and the study population included slightly more women than men. Of the 155 patients, 93 failed initial treatment with etanercept. Seven of 12 (58%) who switched to infliximab, 24 of 43 (56%) who switched to adalimumab, and 27 of 38 (71%) who switched to ustekinumab achieved PASI 75.
Twenty-three failed initial infliximab. Three of four (75%) who switched to etanercept, four of seven (57%) who switched to adalimumab, and nine of 12 (75%) who switched to ustekinumab had a significant response.
Thirty-one patients failed initial adalimumab. Seven of 11 (64%) who switched to etanercept, 3 of 5 (60%) who switched to infliximab, and 11 of 15 (73%) who switched to ustekinumab had a significant response.
Of eight patients who failed initial ustekinumab, three (38%) switched to etanercept and had a significant response after 12 weeks.
There was no outside funding for the work, and Mr. Kim had no disclosures. The senior investigator is a speaker, consultant, and investigator for AbbVie, Amgen, and Janssen.
AT WCD 2015
Key clinical point: Consider ustekinumab if psoriasis patients fail their first anti-TNF agent.
Major finding: Of 65 patients switched to ustekinumab after failing initial anti-TNF therapy, 47 (72%) achieved PASI 75 within 12 weeks.
Data source: Chart review of 155 psoriasis patients.
Disclosures: There was no outside funding for the work. The senior investigator is a speaker, consultant, and investigator for Abbvie, Amgen, and Janssen.
WCD: Cut Simple Carbs to Clear Acne
VANCOUVER, B.C. – Mounting evidence suggests that consuming a low glycemic index diet can substantially improve acne, according to Dr. Hyuck Hoon Kwon.
The approach has held up in several small-scale randomized clinical trials, earning it a grade of 1B last year from the American Academy of Dermatology, noted Dr. Kwon of Seoul National University in South Korea.
“Dermatologists can recommend dietary modification to patients, and can advise them to avoid foods that they believe worsen their acne,” Dr. Kwon said at the World Congress of Dermatology. He said he has seen clinically meaningful reductions in acne lesions as soon as 4 weeks after patients cut their intake of refined carbohydrates and other high glycemic index (GI) foods, although results can take up to 12 weeks, and more studies of time to effect are needed.
Scientists and clinicians have long debated the role of diet in the pathogenesis of acne, and until recently, there were no randomized, controlled trials or meta-analyses of the topic. But observational studies have repeatedly documented “astonishingly” low rates of acne in cultures with “traditional” diets that are lower in refined carbohydrates and fat than typical Western fare, said Dr. Kwon.
In one study of 1,285 individuals in Korea, those who did not have acne reported consuming significantly higher amounts of fish and yellow, leafy green, and cruciferous vegetables, while those with acne ate significantly more instant noodles, processed cheeses, and “junk” foods, he noted (Eur. J. Dermatol. 2010;20:768-72).
In another trial, Dr. Kwon and colleagues randomized 32 individuals with mild to moderate acne to either a low-GI diet that emphasized beans, barley, vegetables, fish, and whole-grain breads, or to a high-GI control diet (Acta Derm. Venereol. 2012;92:241-6). The low-GI group ate more protein to replace calories lost from cutting carbohydrates. At 10 weeks, the groups had similar mean calorie intakes and body mass indices, but the low-GI group had reduced its dietary glycemic load from baseline and had significantly fewer inflammatory and noninflammatory acne lesions compared with baseline and with the high-GI group, Dr. Kwon said. The low-GI group also had significant decreases in total average area of sebaceous glands, and decreased expression of sterol response element-binding protein-1 (SREBP-1), which stimulates lipogenesis in sebocytes.
Most recently, scientists have explored the molecular mechanisms linking diet to acne, Dr. Kwon noted. High GI diets trigger chronic hyperinsulinemia, which impairs the ability of FoxO1 transcription factor to mediate androgen receptor signaling, oxidative stress, lipogenesis, and sebaceous gland homeostasis. Importantly, FoxO1 inhibition also is associated with activation of mTORC1, which promotes lipid and protein synthesis, sebaceous gland hyperplasia, and sebaceous lipogenesis, he said.
Dr. Kwon reported having no relevant conflicts of interest.
VANCOUVER, B.C. – Mounting evidence suggests that consuming a low glycemic index diet can substantially improve acne, according to Dr. Hyuck Hoon Kwon.
The approach has held up in several small-scale randomized clinical trials, earning it a grade of 1B last year from the American Academy of Dermatology, noted Dr. Kwon of Seoul National University in South Korea.
“Dermatologists can recommend dietary modification to patients, and can advise them to avoid foods that they believe worsen their acne,” Dr. Kwon said at the World Congress of Dermatology. He said he has seen clinically meaningful reductions in acne lesions as soon as 4 weeks after patients cut their intake of refined carbohydrates and other high glycemic index (GI) foods, although results can take up to 12 weeks, and more studies of time to effect are needed.
Scientists and clinicians have long debated the role of diet in the pathogenesis of acne, and until recently, there were no randomized, controlled trials or meta-analyses of the topic. But observational studies have repeatedly documented “astonishingly” low rates of acne in cultures with “traditional” diets that are lower in refined carbohydrates and fat than typical Western fare, said Dr. Kwon.
In one study of 1,285 individuals in Korea, those who did not have acne reported consuming significantly higher amounts of fish and yellow, leafy green, and cruciferous vegetables, while those with acne ate significantly more instant noodles, processed cheeses, and “junk” foods, he noted (Eur. J. Dermatol. 2010;20:768-72).
In another trial, Dr. Kwon and colleagues randomized 32 individuals with mild to moderate acne to either a low-GI diet that emphasized beans, barley, vegetables, fish, and whole-grain breads, or to a high-GI control diet (Acta Derm. Venereol. 2012;92:241-6). The low-GI group ate more protein to replace calories lost from cutting carbohydrates. At 10 weeks, the groups had similar mean calorie intakes and body mass indices, but the low-GI group had reduced its dietary glycemic load from baseline and had significantly fewer inflammatory and noninflammatory acne lesions compared with baseline and with the high-GI group, Dr. Kwon said. The low-GI group also had significant decreases in total average area of sebaceous glands, and decreased expression of sterol response element-binding protein-1 (SREBP-1), which stimulates lipogenesis in sebocytes.
Most recently, scientists have explored the molecular mechanisms linking diet to acne, Dr. Kwon noted. High GI diets trigger chronic hyperinsulinemia, which impairs the ability of FoxO1 transcription factor to mediate androgen receptor signaling, oxidative stress, lipogenesis, and sebaceous gland homeostasis. Importantly, FoxO1 inhibition also is associated with activation of mTORC1, which promotes lipid and protein synthesis, sebaceous gland hyperplasia, and sebaceous lipogenesis, he said.
Dr. Kwon reported having no relevant conflicts of interest.
VANCOUVER, B.C. – Mounting evidence suggests that consuming a low glycemic index diet can substantially improve acne, according to Dr. Hyuck Hoon Kwon.
The approach has held up in several small-scale randomized clinical trials, earning it a grade of 1B last year from the American Academy of Dermatology, noted Dr. Kwon of Seoul National University in South Korea.
“Dermatologists can recommend dietary modification to patients, and can advise them to avoid foods that they believe worsen their acne,” Dr. Kwon said at the World Congress of Dermatology. He said he has seen clinically meaningful reductions in acne lesions as soon as 4 weeks after patients cut their intake of refined carbohydrates and other high glycemic index (GI) foods, although results can take up to 12 weeks, and more studies of time to effect are needed.
Scientists and clinicians have long debated the role of diet in the pathogenesis of acne, and until recently, there were no randomized, controlled trials or meta-analyses of the topic. But observational studies have repeatedly documented “astonishingly” low rates of acne in cultures with “traditional” diets that are lower in refined carbohydrates and fat than typical Western fare, said Dr. Kwon.
In one study of 1,285 individuals in Korea, those who did not have acne reported consuming significantly higher amounts of fish and yellow, leafy green, and cruciferous vegetables, while those with acne ate significantly more instant noodles, processed cheeses, and “junk” foods, he noted (Eur. J. Dermatol. 2010;20:768-72).
In another trial, Dr. Kwon and colleagues randomized 32 individuals with mild to moderate acne to either a low-GI diet that emphasized beans, barley, vegetables, fish, and whole-grain breads, or to a high-GI control diet (Acta Derm. Venereol. 2012;92:241-6). The low-GI group ate more protein to replace calories lost from cutting carbohydrates. At 10 weeks, the groups had similar mean calorie intakes and body mass indices, but the low-GI group had reduced its dietary glycemic load from baseline and had significantly fewer inflammatory and noninflammatory acne lesions compared with baseline and with the high-GI group, Dr. Kwon said. The low-GI group also had significant decreases in total average area of sebaceous glands, and decreased expression of sterol response element-binding protein-1 (SREBP-1), which stimulates lipogenesis in sebocytes.
Most recently, scientists have explored the molecular mechanisms linking diet to acne, Dr. Kwon noted. High GI diets trigger chronic hyperinsulinemia, which impairs the ability of FoxO1 transcription factor to mediate androgen receptor signaling, oxidative stress, lipogenesis, and sebaceous gland homeostasis. Importantly, FoxO1 inhibition also is associated with activation of mTORC1, which promotes lipid and protein synthesis, sebaceous gland hyperplasia, and sebaceous lipogenesis, he said.
Dr. Kwon reported having no relevant conflicts of interest.
AT WCD 2015
WCD: Cut simple carbs to clear acne
VANCOUVER, B.C. – Mounting evidence suggests that consuming a low glycemic index diet can substantially improve acne, according to Dr. Hyuck Hoon Kwon.
The approach has held up in several small-scale randomized clinical trials, earning it a grade of 1B last year from the American Academy of Dermatology, noted Dr. Kwon of Seoul National University in South Korea.
“Dermatologists can recommend dietary modification to patients, and can advise them to avoid foods that they believe worsen their acne,” Dr. Kwon said at the World Congress of Dermatology. He said he has seen clinically meaningful reductions in acne lesions as soon as 4 weeks after patients cut their intake of refined carbohydrates and other high glycemic index (GI) foods, although results can take up to 12 weeks, and more studies of time to effect are needed.
Scientists and clinicians have long debated the role of diet in the pathogenesis of acne, and until recently, there were no randomized, controlled trials or meta-analyses of the topic. But observational studies have repeatedly documented “astonishingly” low rates of acne in cultures with “traditional” diets that are lower in refined carbohydrates and fat than typical Western fare, said Dr. Kwon.
In one study of 1,285 individuals in Korea, those who did not have acne reported consuming significantly higher amounts of fish and yellow, leafy green, and cruciferous vegetables, while those with acne ate significantly more instant noodles, processed cheeses, and “junk” foods, he noted (Eur. J. Dermatol. 2010;20:768-72).
In another trial, Dr. Kwon and colleagues randomized 32 individuals with mild to moderate acne to either a low-GI diet that emphasized beans, barley, vegetables, fish, and whole-grain breads, or to a high-GI control diet (Acta Derm. Venereol. 2012;92:241-6). The low-GI group ate more protein to replace calories lost from cutting carbohydrates. At 10 weeks, the groups had similar mean calorie intakes and body mass indices, but the low-GI group had reduced its dietary glycemic load from baseline and had significantly fewer inflammatory and noninflammatory acne lesions compared with baseline and with the high-GI group, Dr. Kwon said. The low-GI group also had significant decreases in total average area of sebaceous glands, and decreased expression of sterol response element-binding protein-1 (SREBP-1), which stimulates lipogenesis in sebocytes.
Most recently, scientists have explored the molecular mechanisms linking diet to acne, Dr. Kwon noted. High GI diets trigger chronic hyperinsulinemia, which impairs the ability of FoxO1 transcription factor to mediate androgen receptor signaling, oxidative stress, lipogenesis, and sebaceous gland homeostasis. Importantly, FoxO1 inhibition also is associated with activation of mTORC1, which promotes lipid and protein synthesis, sebaceous gland hyperplasia, and sebaceous lipogenesis, he said.
Dr. Kwon reported having no relevant conflicts of interest.
VANCOUVER, B.C. – Mounting evidence suggests that consuming a low glycemic index diet can substantially improve acne, according to Dr. Hyuck Hoon Kwon.
The approach has held up in several small-scale randomized clinical trials, earning it a grade of 1B last year from the American Academy of Dermatology, noted Dr. Kwon of Seoul National University in South Korea.
“Dermatologists can recommend dietary modification to patients, and can advise them to avoid foods that they believe worsen their acne,” Dr. Kwon said at the World Congress of Dermatology. He said he has seen clinically meaningful reductions in acne lesions as soon as 4 weeks after patients cut their intake of refined carbohydrates and other high glycemic index (GI) foods, although results can take up to 12 weeks, and more studies of time to effect are needed.
Scientists and clinicians have long debated the role of diet in the pathogenesis of acne, and until recently, there were no randomized, controlled trials or meta-analyses of the topic. But observational studies have repeatedly documented “astonishingly” low rates of acne in cultures with “traditional” diets that are lower in refined carbohydrates and fat than typical Western fare, said Dr. Kwon.
In one study of 1,285 individuals in Korea, those who did not have acne reported consuming significantly higher amounts of fish and yellow, leafy green, and cruciferous vegetables, while those with acne ate significantly more instant noodles, processed cheeses, and “junk” foods, he noted (Eur. J. Dermatol. 2010;20:768-72).
In another trial, Dr. Kwon and colleagues randomized 32 individuals with mild to moderate acne to either a low-GI diet that emphasized beans, barley, vegetables, fish, and whole-grain breads, or to a high-GI control diet (Acta Derm. Venereol. 2012;92:241-6). The low-GI group ate more protein to replace calories lost from cutting carbohydrates. At 10 weeks, the groups had similar mean calorie intakes and body mass indices, but the low-GI group had reduced its dietary glycemic load from baseline and had significantly fewer inflammatory and noninflammatory acne lesions compared with baseline and with the high-GI group, Dr. Kwon said. The low-GI group also had significant decreases in total average area of sebaceous glands, and decreased expression of sterol response element-binding protein-1 (SREBP-1), which stimulates lipogenesis in sebocytes.
Most recently, scientists have explored the molecular mechanisms linking diet to acne, Dr. Kwon noted. High GI diets trigger chronic hyperinsulinemia, which impairs the ability of FoxO1 transcription factor to mediate androgen receptor signaling, oxidative stress, lipogenesis, and sebaceous gland homeostasis. Importantly, FoxO1 inhibition also is associated with activation of mTORC1, which promotes lipid and protein synthesis, sebaceous gland hyperplasia, and sebaceous lipogenesis, he said.
Dr. Kwon reported having no relevant conflicts of interest.
VANCOUVER, B.C. – Mounting evidence suggests that consuming a low glycemic index diet can substantially improve acne, according to Dr. Hyuck Hoon Kwon.
The approach has held up in several small-scale randomized clinical trials, earning it a grade of 1B last year from the American Academy of Dermatology, noted Dr. Kwon of Seoul National University in South Korea.
“Dermatologists can recommend dietary modification to patients, and can advise them to avoid foods that they believe worsen their acne,” Dr. Kwon said at the World Congress of Dermatology. He said he has seen clinically meaningful reductions in acne lesions as soon as 4 weeks after patients cut their intake of refined carbohydrates and other high glycemic index (GI) foods, although results can take up to 12 weeks, and more studies of time to effect are needed.
Scientists and clinicians have long debated the role of diet in the pathogenesis of acne, and until recently, there were no randomized, controlled trials or meta-analyses of the topic. But observational studies have repeatedly documented “astonishingly” low rates of acne in cultures with “traditional” diets that are lower in refined carbohydrates and fat than typical Western fare, said Dr. Kwon.
In one study of 1,285 individuals in Korea, those who did not have acne reported consuming significantly higher amounts of fish and yellow, leafy green, and cruciferous vegetables, while those with acne ate significantly more instant noodles, processed cheeses, and “junk” foods, he noted (Eur. J. Dermatol. 2010;20:768-72).
In another trial, Dr. Kwon and colleagues randomized 32 individuals with mild to moderate acne to either a low-GI diet that emphasized beans, barley, vegetables, fish, and whole-grain breads, or to a high-GI control diet (Acta Derm. Venereol. 2012;92:241-6). The low-GI group ate more protein to replace calories lost from cutting carbohydrates. At 10 weeks, the groups had similar mean calorie intakes and body mass indices, but the low-GI group had reduced its dietary glycemic load from baseline and had significantly fewer inflammatory and noninflammatory acne lesions compared with baseline and with the high-GI group, Dr. Kwon said. The low-GI group also had significant decreases in total average area of sebaceous glands, and decreased expression of sterol response element-binding protein-1 (SREBP-1), which stimulates lipogenesis in sebocytes.
Most recently, scientists have explored the molecular mechanisms linking diet to acne, Dr. Kwon noted. High GI diets trigger chronic hyperinsulinemia, which impairs the ability of FoxO1 transcription factor to mediate androgen receptor signaling, oxidative stress, lipogenesis, and sebaceous gland homeostasis. Importantly, FoxO1 inhibition also is associated with activation of mTORC1, which promotes lipid and protein synthesis, sebaceous gland hyperplasia, and sebaceous lipogenesis, he said.
Dr. Kwon reported having no relevant conflicts of interest.
AT WCD 2015
WCD: Tofacitinib’s benefits for psoriasis persist for 2 years
VANCOUVER – Oral tofacitinib for adults with moderate to severe plaque psoriasis demonstrated sustained efficacy and a “generally manageable” safety profile throughout 2 years of follow-up in a large, multicenter ongoing long-term extension study, Dr. Matthias Augustin reported at the World Congress of Dermatology.
One month after enrolling in the phase III, open-label trial (known as OPT Extend), and going on the investigational oral Janus kinase inhibitor at 10 mg b.i.d., 56.2% of the 2,847 participants showed a PASI 75 response – that is, at least a 75% reduction from their baseline Psoriasis Area and Severity Index score. After 2 years in the OPT Extend study, 64.5% of the 1,912 patients remaining in the study were PASI 75 responders.
Similarly, 56.3% of patients went from moderate or severe disease at baseline to “clear” or “almost clear” by Physician Global Assessment (PGA) at 1 month, and 53.9% of participants had a PGA score of 0 or 1 at 24 months, added Dr. Augustin, professor of dermatology at the University of Hamburg-Eppendorf and director of the German Center for Health Services Research in Dermatology and Nursing.
Psoriasis patients who had participated in any of four earlier phase III or two phase II randomized, controlled trials of tofacitinib were eligible to participate in OPT Extend. Participants were placed on tofacitinib at 10 mg b.i.d. for the first 2 months of the open-label study; then investigators adjusted their dose individually – either 5 or 10 mg b.i.d. – at each follow-up visit based on efficacy and side effects. For purposes of OPT Extend, patients’ baseline PASI and PGA scores were defined as the values recorded on the day they were randomized in the earlier round of trials.
Through 2 years of follow-up in this interim analysis of OPT Extend, less than 10% of subjects discontinued tofacitinib because of adverse events. The adverse events were the same as the ones that arose in the earlier, briefer randomized trials, the longest of which lasted 1 year. No signs of cumulative organ toxicity were evident during the additional follow-up.
Slightly more than 4% of all adverse events were categorized as severe, while 62% were mild. The most frequent adverse events were nasopharyngitis in 16% of patients, increased creatinine phosphokinase in 10%, and upper respiratory tract infections in 7%. Serious infections requiring systemic antibiotics or hospitalization occurred in 1.8% of patients on tofacitinib for 2 years, the most common of which were pneumonia, herpes zoster, and urinary tract infection. Herpes zoster occurred in 3.5% of participants, with most cases being mild or moderate. Malignancies other than nonmelanoma skin cancers occurred in 1.2% of subjects.
In terms of laboratory findings of interest, patients’ LDL/HDL ratio remained stable throughout 24 months on tofacitinib. Nor were there any clinically meaningful changes in average levels of other laboratory parameters, including hemoglobin, creatinine phosphokinase, lymphocytes, and neutrophils. A lymphocyte count below 500/mm3 occurred in 0.4% of patients at some point; however, it was unrelated to duration of exposure to tofacitinib and wasn’t linked to an increased infection rate.
Dr. Augustin said an unmet need exists for effective and nontoxic oral medications for moderate to severe psoriasis, because many patients would prefer not to take an injectable biologic. Tofacitinib is aimed at filling that need, and serving as an easier, less toxic initial therapy. However, it is essential that an optimal oral agent have a favorable safety profile because psoriasis is a lifelong disease with an average lifetime duration in excess of 40 years in adults and more than 60 years in affected children, he said.
Pfizer, which is developing the drug, has filed for marketing approval for tofacitinib for treatment of adults with moderate to severe chronic plaque psoriasis who are candidates for systemic therapy or phototherapy. The Food and Drug Administration has announced its intent to issue a ruling on the application in October 2015.
The OPT Expect study is funded by Pfizer. Dr. Augustin is an adviser to and/or a recipient of research grants from Pfizer and more than a dozen other medical companies.
VANCOUVER – Oral tofacitinib for adults with moderate to severe plaque psoriasis demonstrated sustained efficacy and a “generally manageable” safety profile throughout 2 years of follow-up in a large, multicenter ongoing long-term extension study, Dr. Matthias Augustin reported at the World Congress of Dermatology.
One month after enrolling in the phase III, open-label trial (known as OPT Extend), and going on the investigational oral Janus kinase inhibitor at 10 mg b.i.d., 56.2% of the 2,847 participants showed a PASI 75 response – that is, at least a 75% reduction from their baseline Psoriasis Area and Severity Index score. After 2 years in the OPT Extend study, 64.5% of the 1,912 patients remaining in the study were PASI 75 responders.
Similarly, 56.3% of patients went from moderate or severe disease at baseline to “clear” or “almost clear” by Physician Global Assessment (PGA) at 1 month, and 53.9% of participants had a PGA score of 0 or 1 at 24 months, added Dr. Augustin, professor of dermatology at the University of Hamburg-Eppendorf and director of the German Center for Health Services Research in Dermatology and Nursing.
Psoriasis patients who had participated in any of four earlier phase III or two phase II randomized, controlled trials of tofacitinib were eligible to participate in OPT Extend. Participants were placed on tofacitinib at 10 mg b.i.d. for the first 2 months of the open-label study; then investigators adjusted their dose individually – either 5 or 10 mg b.i.d. – at each follow-up visit based on efficacy and side effects. For purposes of OPT Extend, patients’ baseline PASI and PGA scores were defined as the values recorded on the day they were randomized in the earlier round of trials.
Through 2 years of follow-up in this interim analysis of OPT Extend, less than 10% of subjects discontinued tofacitinib because of adverse events. The adverse events were the same as the ones that arose in the earlier, briefer randomized trials, the longest of which lasted 1 year. No signs of cumulative organ toxicity were evident during the additional follow-up.
Slightly more than 4% of all adverse events were categorized as severe, while 62% were mild. The most frequent adverse events were nasopharyngitis in 16% of patients, increased creatinine phosphokinase in 10%, and upper respiratory tract infections in 7%. Serious infections requiring systemic antibiotics or hospitalization occurred in 1.8% of patients on tofacitinib for 2 years, the most common of which were pneumonia, herpes zoster, and urinary tract infection. Herpes zoster occurred in 3.5% of participants, with most cases being mild or moderate. Malignancies other than nonmelanoma skin cancers occurred in 1.2% of subjects.
In terms of laboratory findings of interest, patients’ LDL/HDL ratio remained stable throughout 24 months on tofacitinib. Nor were there any clinically meaningful changes in average levels of other laboratory parameters, including hemoglobin, creatinine phosphokinase, lymphocytes, and neutrophils. A lymphocyte count below 500/mm3 occurred in 0.4% of patients at some point; however, it was unrelated to duration of exposure to tofacitinib and wasn’t linked to an increased infection rate.
Dr. Augustin said an unmet need exists for effective and nontoxic oral medications for moderate to severe psoriasis, because many patients would prefer not to take an injectable biologic. Tofacitinib is aimed at filling that need, and serving as an easier, less toxic initial therapy. However, it is essential that an optimal oral agent have a favorable safety profile because psoriasis is a lifelong disease with an average lifetime duration in excess of 40 years in adults and more than 60 years in affected children, he said.
Pfizer, which is developing the drug, has filed for marketing approval for tofacitinib for treatment of adults with moderate to severe chronic plaque psoriasis who are candidates for systemic therapy or phototherapy. The Food and Drug Administration has announced its intent to issue a ruling on the application in October 2015.
The OPT Expect study is funded by Pfizer. Dr. Augustin is an adviser to and/or a recipient of research grants from Pfizer and more than a dozen other medical companies.
VANCOUVER – Oral tofacitinib for adults with moderate to severe plaque psoriasis demonstrated sustained efficacy and a “generally manageable” safety profile throughout 2 years of follow-up in a large, multicenter ongoing long-term extension study, Dr. Matthias Augustin reported at the World Congress of Dermatology.
One month after enrolling in the phase III, open-label trial (known as OPT Extend), and going on the investigational oral Janus kinase inhibitor at 10 mg b.i.d., 56.2% of the 2,847 participants showed a PASI 75 response – that is, at least a 75% reduction from their baseline Psoriasis Area and Severity Index score. After 2 years in the OPT Extend study, 64.5% of the 1,912 patients remaining in the study were PASI 75 responders.
Similarly, 56.3% of patients went from moderate or severe disease at baseline to “clear” or “almost clear” by Physician Global Assessment (PGA) at 1 month, and 53.9% of participants had a PGA score of 0 or 1 at 24 months, added Dr. Augustin, professor of dermatology at the University of Hamburg-Eppendorf and director of the German Center for Health Services Research in Dermatology and Nursing.
Psoriasis patients who had participated in any of four earlier phase III or two phase II randomized, controlled trials of tofacitinib were eligible to participate in OPT Extend. Participants were placed on tofacitinib at 10 mg b.i.d. for the first 2 months of the open-label study; then investigators adjusted their dose individually – either 5 or 10 mg b.i.d. – at each follow-up visit based on efficacy and side effects. For purposes of OPT Extend, patients’ baseline PASI and PGA scores were defined as the values recorded on the day they were randomized in the earlier round of trials.
Through 2 years of follow-up in this interim analysis of OPT Extend, less than 10% of subjects discontinued tofacitinib because of adverse events. The adverse events were the same as the ones that arose in the earlier, briefer randomized trials, the longest of which lasted 1 year. No signs of cumulative organ toxicity were evident during the additional follow-up.
Slightly more than 4% of all adverse events were categorized as severe, while 62% were mild. The most frequent adverse events were nasopharyngitis in 16% of patients, increased creatinine phosphokinase in 10%, and upper respiratory tract infections in 7%. Serious infections requiring systemic antibiotics or hospitalization occurred in 1.8% of patients on tofacitinib for 2 years, the most common of which were pneumonia, herpes zoster, and urinary tract infection. Herpes zoster occurred in 3.5% of participants, with most cases being mild or moderate. Malignancies other than nonmelanoma skin cancers occurred in 1.2% of subjects.
In terms of laboratory findings of interest, patients’ LDL/HDL ratio remained stable throughout 24 months on tofacitinib. Nor were there any clinically meaningful changes in average levels of other laboratory parameters, including hemoglobin, creatinine phosphokinase, lymphocytes, and neutrophils. A lymphocyte count below 500/mm3 occurred in 0.4% of patients at some point; however, it was unrelated to duration of exposure to tofacitinib and wasn’t linked to an increased infection rate.
Dr. Augustin said an unmet need exists for effective and nontoxic oral medications for moderate to severe psoriasis, because many patients would prefer not to take an injectable biologic. Tofacitinib is aimed at filling that need, and serving as an easier, less toxic initial therapy. However, it is essential that an optimal oral agent have a favorable safety profile because psoriasis is a lifelong disease with an average lifetime duration in excess of 40 years in adults and more than 60 years in affected children, he said.
Pfizer, which is developing the drug, has filed for marketing approval for tofacitinib for treatment of adults with moderate to severe chronic plaque psoriasis who are candidates for systemic therapy or phototherapy. The Food and Drug Administration has announced its intent to issue a ruling on the application in October 2015.
The OPT Expect study is funded by Pfizer. Dr. Augustin is an adviser to and/or a recipient of research grants from Pfizer and more than a dozen other medical companies.
AT WCD 2015
Key clinical point: Oral tofacitinib showed sustained efficacy for moderate to severe plaque psoriasis at 24 months in a large ongoing phase III study.
Major finding: At month 1 of the OPT Extend study, 56.2% of patients on tofacitinib had a PASI 75 response; at month 24, the PASI 75 rate was 64.5%.
Data source: The OPT Extend study is an ongoing, phase III multicenter open-label study of long-term therapy with oral tofacitinib at 5 or 10 mg b.i.d. in 2,847 patients with moderate to severe psoriasis.
Disclosures: The study is funded by Pfizer. The presenter is an adviser to and recipient of research grants from that company as well as others.
Expert advises on filling the forehead and temples
VANCOUVER – Fillers can greatly improve the age-related effects of atrophy of subcutaneous fat of the central forehead and temples, where volume loss can create an unhealthy or even skeletal appearance, according to Dr. Tatjana Pavicic.
However, dermatologists in Western countries often neglect to use fillers in the forehead and temples, said Dr. Pavicic, whose private practice in Munich specializes in cosmetic surgery and aesthetic procedures. They can learn from their colleagues in eastern Asia, where a “high, gently curved, round, or even slightly protruding forehead” is traditionally a sign of wealth, and the forehead and temples are among the most popular sites for fillers, she noted in a presentation at the World Congress of Dermatology.
Restoring volume to the central forehead and temples can shave years off patients’ appearance, but clinicians should be mindful of anatomy and use proper tools and techniques when filling these areas, Dr. Pavicic emphasized. Otherwise patients can suffer serious adverse effects, including tissue necrosis or even permanent blindness as a result of filler entering the vasculature, she said. Likewise, because the bone at the temples is thin, clinicians must be especially careful not to penetrate it. Dr. Pavicic noted that there has been at least one case in Europe of intracranial injection of filler associated with treatment of the temples.
Dr. Pavicic offered several tips for optimizing cosmetic results and keeping patients safe when filling the temples and forehead. For white patients, the cosmetic goal is to neutralize age-related concavities, while for Asian patients, it is to restore lost convexity, particularly of the forehead, she noted.
When treating the forehead, Dr. Pavicic said she places filler above the frontal bone at the subfascial level. For deep injections, the needle should be “really on the bone,” she noted. Inject small boluses of filler instead of a single large bolus, and aspirate back before injecting filler to ensure that needles are not intravascular, she said.
If using the blunt cannula technique, the cannula must be stiff enough to achieve good control – Dr. Pavicic recommends using a 22-gauge – and it should be placed into the medial and central fat pads of the forehead.
For superficial filler injections of the temples, Dr. Pavicic introduces the cannula at the zygomatic arch instead of trying to clean behind the hairline. She also recommended warning patients that the superficial veins in their temples might be more prominent for about a week after treatment. “Otherwise, they will call you in shock,” she said.
Regardless of the filler technique or the site in question, always use a high-quality product that exhibits good moldability, Dr. Pavicic concluded.
She reported having advisory or consulting relationships with Merz Pharmaceuticals, Dermaceutica, Eucerin, Galderma, and Ulthera, and receiving research support from Bayer and Ipsen.
VANCOUVER – Fillers can greatly improve the age-related effects of atrophy of subcutaneous fat of the central forehead and temples, where volume loss can create an unhealthy or even skeletal appearance, according to Dr. Tatjana Pavicic.
However, dermatologists in Western countries often neglect to use fillers in the forehead and temples, said Dr. Pavicic, whose private practice in Munich specializes in cosmetic surgery and aesthetic procedures. They can learn from their colleagues in eastern Asia, where a “high, gently curved, round, or even slightly protruding forehead” is traditionally a sign of wealth, and the forehead and temples are among the most popular sites for fillers, she noted in a presentation at the World Congress of Dermatology.
Restoring volume to the central forehead and temples can shave years off patients’ appearance, but clinicians should be mindful of anatomy and use proper tools and techniques when filling these areas, Dr. Pavicic emphasized. Otherwise patients can suffer serious adverse effects, including tissue necrosis or even permanent blindness as a result of filler entering the vasculature, she said. Likewise, because the bone at the temples is thin, clinicians must be especially careful not to penetrate it. Dr. Pavicic noted that there has been at least one case in Europe of intracranial injection of filler associated with treatment of the temples.
Dr. Pavicic offered several tips for optimizing cosmetic results and keeping patients safe when filling the temples and forehead. For white patients, the cosmetic goal is to neutralize age-related concavities, while for Asian patients, it is to restore lost convexity, particularly of the forehead, she noted.
When treating the forehead, Dr. Pavicic said she places filler above the frontal bone at the subfascial level. For deep injections, the needle should be “really on the bone,” she noted. Inject small boluses of filler instead of a single large bolus, and aspirate back before injecting filler to ensure that needles are not intravascular, she said.
If using the blunt cannula technique, the cannula must be stiff enough to achieve good control – Dr. Pavicic recommends using a 22-gauge – and it should be placed into the medial and central fat pads of the forehead.
For superficial filler injections of the temples, Dr. Pavicic introduces the cannula at the zygomatic arch instead of trying to clean behind the hairline. She also recommended warning patients that the superficial veins in their temples might be more prominent for about a week after treatment. “Otherwise, they will call you in shock,” she said.
Regardless of the filler technique or the site in question, always use a high-quality product that exhibits good moldability, Dr. Pavicic concluded.
She reported having advisory or consulting relationships with Merz Pharmaceuticals, Dermaceutica, Eucerin, Galderma, and Ulthera, and receiving research support from Bayer and Ipsen.
VANCOUVER – Fillers can greatly improve the age-related effects of atrophy of subcutaneous fat of the central forehead and temples, where volume loss can create an unhealthy or even skeletal appearance, according to Dr. Tatjana Pavicic.
However, dermatologists in Western countries often neglect to use fillers in the forehead and temples, said Dr. Pavicic, whose private practice in Munich specializes in cosmetic surgery and aesthetic procedures. They can learn from their colleagues in eastern Asia, where a “high, gently curved, round, or even slightly protruding forehead” is traditionally a sign of wealth, and the forehead and temples are among the most popular sites for fillers, she noted in a presentation at the World Congress of Dermatology.
Restoring volume to the central forehead and temples can shave years off patients’ appearance, but clinicians should be mindful of anatomy and use proper tools and techniques when filling these areas, Dr. Pavicic emphasized. Otherwise patients can suffer serious adverse effects, including tissue necrosis or even permanent blindness as a result of filler entering the vasculature, she said. Likewise, because the bone at the temples is thin, clinicians must be especially careful not to penetrate it. Dr. Pavicic noted that there has been at least one case in Europe of intracranial injection of filler associated with treatment of the temples.
Dr. Pavicic offered several tips for optimizing cosmetic results and keeping patients safe when filling the temples and forehead. For white patients, the cosmetic goal is to neutralize age-related concavities, while for Asian patients, it is to restore lost convexity, particularly of the forehead, she noted.
When treating the forehead, Dr. Pavicic said she places filler above the frontal bone at the subfascial level. For deep injections, the needle should be “really on the bone,” she noted. Inject small boluses of filler instead of a single large bolus, and aspirate back before injecting filler to ensure that needles are not intravascular, she said.
If using the blunt cannula technique, the cannula must be stiff enough to achieve good control – Dr. Pavicic recommends using a 22-gauge – and it should be placed into the medial and central fat pads of the forehead.
For superficial filler injections of the temples, Dr. Pavicic introduces the cannula at the zygomatic arch instead of trying to clean behind the hairline. She also recommended warning patients that the superficial veins in their temples might be more prominent for about a week after treatment. “Otherwise, they will call you in shock,” she said.
Regardless of the filler technique or the site in question, always use a high-quality product that exhibits good moldability, Dr. Pavicic concluded.
She reported having advisory or consulting relationships with Merz Pharmaceuticals, Dermaceutica, Eucerin, Galderma, and Ulthera, and receiving research support from Bayer and Ipsen.
AT WDC 2015