User login
Vonoprazan beats PPIs in H. pylori eradication
LAS VEGAS – In the treatment of Helicobacter pylori infection, combination therapies using the oral potassium-competitive acid blocker vonoprazan were superior to standard proton pump inhibitor (PPI)–based triple therapy, producing higher eradication rates, according to combined data from a U.S. and a European phase 3 randomized, controlled trial.
Vonoprazan has been submitted to the Food and Drug Administration for approval with a Fast Track designation in combination with amoxicillin and clarithromycin (triple therapy) or amoxicillin alone (dual therapy) for treating H. pylori infection. It has already been approved in Japan for the treatment of gastric and duodenal ulcers, reflux esophagitis, secondary prevention of low-dose aspirin– or nonsteroidal anti-inflammatory drug–induced gastric mucosal damage, and for first and second-line H. pylori eradication therapy.
Study details
The study included 1,046 treatment-naive patients who had dyspepsia, a recent or new diagnosis of a nonbleeding peptic ulcer, a history of a peptic ulcer, or long-term stable use of an NSAID. Patients were randomized to PPI-based triple therapy (lansoprazole, amoxicillin, clarithromycin), vonoprazan triple therapy (plus amoxicillin, clarithromycin), or vonoprazan dual therapy (amoxicillin). The treatment period was 14 days, followed by 13C urea breath test (UBT) 4 weeks after treatment.
The researchers conducted several analyses, including: Modified intention-to-treat analyses, which included all enrollees; per protocol analyses, which included patients who took at least 75% of each study medication and underwent 13C UBT in the expected time frame; and a safety population of all patients who took at least one study drug.
Among patients with H. pylori strains that were not resistant to clarithromycin, the PPI-based triple-therapy group had an eradication rate of 78.8%, compared with 84.7% in the vonoprazan triple-therapy group (P < .0001), and 78.5% in the vonoprazan dual-therapy group (P = .0037). In the per protocol analysis, PPI-based triple therapy eradicated H. pylori 82.1% of the time, compared with 90.4% in the vonoprazan triple-therapy group (P < .0001) and 81.2% in the vonoprazan dual-therapy group (P = .0077). Both vonoprazan treatment groups were noninferior to PPI-based triple therapy.
A prespecified exploratory analysis found that vonoprazan triple therapy outperformed PPI-based triple therapy in the modified intention-to-treat population (P = .0408) and the per protocol population (P = .0059).
Among patients with clarithromycin-resistant strains of H. pylori, in the modified intention-to-treat population, 31.9% achieved eradication with PPI triple therapy, compared with 65.8% in the vonoprazan triple-therapy group, and 69.6% in the vonoprazan dual-therapy group. In the per protocol population, the numbers were 29.0% versus 67.2% and 79.5%, respectively (P < .0001 for both versus PPI triple therapy).
Among all patients, in the modified intention-to-treat population, 68.5% achieved eradication with PPI triple therapy, 80.8% with vonoprazan triple therapy (P =. 0001), and 77.2% with vonoprazan dual therapy (P = .0063)*. In the per protocol population, the numbers were 70.0%, 85.7% (P < .0001), and 81.1% (P = .0013), respectively.
Safety outcomes were similar among the three groups, with treatment-emergent adverse events occurring in 34.5% of the PPI triple-therapy group (1.2% discontinued), 34.1% of the vonoprazan triple-therapy group (2.3% discontinued), and 29.9% in the vonoprazan dual-therapy group (0.9% discontinued).
Fighting against resistance
The efficacy of PPI-based clarithromycin-based triple therapy has fallen below 80% in the United States and Europe over the past few decades, largely because of antibiotic resistance, said William Chey, MD, during a presentation of the results at the annual meeting of the American College of Gastroenterology. Dr. Chey is a professor of medicine and director of the GI physiology laboratory at Michigan Medicine.
Vonoprazan is more stable in acid than are PPIs, and produces greater and more durable acid reduction, according to Dr. Chey. That’s important for two reasons: One is that some antibiotics are acid-labile, and so may have their efficacy directly impacted in a more acidic environment. The other factor is that most antibiotics work better on bacteria that are actively replicating, and H. pylori reproduces better in a more neutral environment. “So, you increase the replication, you increase the bioavailability of the antibiotics. And therefore, hopefully, that underlies why we see it working better in the patients with [antibiotic] resistance,” Dr. Chey said in an interview.
It remains to be seen whether or not the drug will receive FDA approval, but he pointed to other regimens like bismuth quadruple therapy and rifabutin-based triple therapy that are already available. “If I had the choice, I would never use a PPI-based triple therapy again. People should not be doing that,” said Dr. Chey.
“More successful H. pylori eradication regimens are certainly needed, and these results are particularly relevant and interesting given the increasing failure of initial treatment regimens,” said Kimberly Harer, MD, who moderated the session. She noted that the secondary analysis of patients with clarithromycin-resistant infections was particularly relevant. “The superiority analysis indicating vonoprazan triple therapy resulted in increased H. pylori eradication compared to lanzoprazole triple therapy was especially interesting,” said Dr. Harer, who is a clinical lecturer at University of Michigan Health, Ann Arbor.
One downside to the study is that it didn’t compare vonoprazan combinations to quadruple therapy of a PPI, bismuth, tetracycline, and a nitroimidazole, said Joseph Jennings, MD, who was asked to comment on the study. Other treatment approaches include sequential antibiotics and other combinations. Dr. Jennings also highlighted the findings that the vonoprazan regimens were superior against clarithromycin-resistant strains. “The more different regimens we can add to the armamentarium, the better chance we have because the resistant patterns fluctuate all throughout the world,” said Dr. Jennings, who is an assistant professor of medicine at Georgetown University and director of the center for GI bleeding at MedStar Georgetown University Hospital, both in Washington.
He also pointed out that physicians can face a conundrum when patients fail multiple lines of therapy and have testing done that shows high levels of resistance. Some have allergies that prevent them from turning to other antibiotics. “That’s a market where lots of doctors struggle. Something like this would be a nice add-on,” said Dr. Jennings.
The study was funded by Phathom Pharmaceuticals.** Dr. Chey has consulted and/or received research support from Abbvie, Alfasigma, Allakos, Alnylam, Bayer, Bioamerica, Cosmo, Intrinsic Medicine, Ironwood, Modify Health, My GI Health, My Nutrition Health, Nestle, Phathom Pharmaceuticals, QOL Medical, Redhill, Salix/Valeant, Takeda, Urovant, and Vibrant. Dr. Harer and Dr. Jennings have no relevant financial disclosures.
*Correction, 10/29/21: An earlier version of this article misstated the percentage of patients in the modified intention-to-treat population who achieved eradication with vonoprazan triple therapy.
**Correction, 10/29/21: An earlier version of this article misstated the name of Phathom Pharmaceuticals.
LAS VEGAS – In the treatment of Helicobacter pylori infection, combination therapies using the oral potassium-competitive acid blocker vonoprazan were superior to standard proton pump inhibitor (PPI)–based triple therapy, producing higher eradication rates, according to combined data from a U.S. and a European phase 3 randomized, controlled trial.
Vonoprazan has been submitted to the Food and Drug Administration for approval with a Fast Track designation in combination with amoxicillin and clarithromycin (triple therapy) or amoxicillin alone (dual therapy) for treating H. pylori infection. It has already been approved in Japan for the treatment of gastric and duodenal ulcers, reflux esophagitis, secondary prevention of low-dose aspirin– or nonsteroidal anti-inflammatory drug–induced gastric mucosal damage, and for first and second-line H. pylori eradication therapy.
Study details
The study included 1,046 treatment-naive patients who had dyspepsia, a recent or new diagnosis of a nonbleeding peptic ulcer, a history of a peptic ulcer, or long-term stable use of an NSAID. Patients were randomized to PPI-based triple therapy (lansoprazole, amoxicillin, clarithromycin), vonoprazan triple therapy (plus amoxicillin, clarithromycin), or vonoprazan dual therapy (amoxicillin). The treatment period was 14 days, followed by 13C urea breath test (UBT) 4 weeks after treatment.
The researchers conducted several analyses, including: Modified intention-to-treat analyses, which included all enrollees; per protocol analyses, which included patients who took at least 75% of each study medication and underwent 13C UBT in the expected time frame; and a safety population of all patients who took at least one study drug.
Among patients with H. pylori strains that were not resistant to clarithromycin, the PPI-based triple-therapy group had an eradication rate of 78.8%, compared with 84.7% in the vonoprazan triple-therapy group (P < .0001), and 78.5% in the vonoprazan dual-therapy group (P = .0037). In the per protocol analysis, PPI-based triple therapy eradicated H. pylori 82.1% of the time, compared with 90.4% in the vonoprazan triple-therapy group (P < .0001) and 81.2% in the vonoprazan dual-therapy group (P = .0077). Both vonoprazan treatment groups were noninferior to PPI-based triple therapy.
A prespecified exploratory analysis found that vonoprazan triple therapy outperformed PPI-based triple therapy in the modified intention-to-treat population (P = .0408) and the per protocol population (P = .0059).
Among patients with clarithromycin-resistant strains of H. pylori, in the modified intention-to-treat population, 31.9% achieved eradication with PPI triple therapy, compared with 65.8% in the vonoprazan triple-therapy group, and 69.6% in the vonoprazan dual-therapy group. In the per protocol population, the numbers were 29.0% versus 67.2% and 79.5%, respectively (P < .0001 for both versus PPI triple therapy).
Among all patients, in the modified intention-to-treat population, 68.5% achieved eradication with PPI triple therapy, 80.8% with vonoprazan triple therapy (P =. 0001), and 77.2% with vonoprazan dual therapy (P = .0063)*. In the per protocol population, the numbers were 70.0%, 85.7% (P < .0001), and 81.1% (P = .0013), respectively.
Safety outcomes were similar among the three groups, with treatment-emergent adverse events occurring in 34.5% of the PPI triple-therapy group (1.2% discontinued), 34.1% of the vonoprazan triple-therapy group (2.3% discontinued), and 29.9% in the vonoprazan dual-therapy group (0.9% discontinued).
Fighting against resistance
The efficacy of PPI-based clarithromycin-based triple therapy has fallen below 80% in the United States and Europe over the past few decades, largely because of antibiotic resistance, said William Chey, MD, during a presentation of the results at the annual meeting of the American College of Gastroenterology. Dr. Chey is a professor of medicine and director of the GI physiology laboratory at Michigan Medicine.
Vonoprazan is more stable in acid than are PPIs, and produces greater and more durable acid reduction, according to Dr. Chey. That’s important for two reasons: One is that some antibiotics are acid-labile, and so may have their efficacy directly impacted in a more acidic environment. The other factor is that most antibiotics work better on bacteria that are actively replicating, and H. pylori reproduces better in a more neutral environment. “So, you increase the replication, you increase the bioavailability of the antibiotics. And therefore, hopefully, that underlies why we see it working better in the patients with [antibiotic] resistance,” Dr. Chey said in an interview.
It remains to be seen whether or not the drug will receive FDA approval, but he pointed to other regimens like bismuth quadruple therapy and rifabutin-based triple therapy that are already available. “If I had the choice, I would never use a PPI-based triple therapy again. People should not be doing that,” said Dr. Chey.
“More successful H. pylori eradication regimens are certainly needed, and these results are particularly relevant and interesting given the increasing failure of initial treatment regimens,” said Kimberly Harer, MD, who moderated the session. She noted that the secondary analysis of patients with clarithromycin-resistant infections was particularly relevant. “The superiority analysis indicating vonoprazan triple therapy resulted in increased H. pylori eradication compared to lanzoprazole triple therapy was especially interesting,” said Dr. Harer, who is a clinical lecturer at University of Michigan Health, Ann Arbor.
One downside to the study is that it didn’t compare vonoprazan combinations to quadruple therapy of a PPI, bismuth, tetracycline, and a nitroimidazole, said Joseph Jennings, MD, who was asked to comment on the study. Other treatment approaches include sequential antibiotics and other combinations. Dr. Jennings also highlighted the findings that the vonoprazan regimens were superior against clarithromycin-resistant strains. “The more different regimens we can add to the armamentarium, the better chance we have because the resistant patterns fluctuate all throughout the world,” said Dr. Jennings, who is an assistant professor of medicine at Georgetown University and director of the center for GI bleeding at MedStar Georgetown University Hospital, both in Washington.
He also pointed out that physicians can face a conundrum when patients fail multiple lines of therapy and have testing done that shows high levels of resistance. Some have allergies that prevent them from turning to other antibiotics. “That’s a market where lots of doctors struggle. Something like this would be a nice add-on,” said Dr. Jennings.
The study was funded by Phathom Pharmaceuticals.** Dr. Chey has consulted and/or received research support from Abbvie, Alfasigma, Allakos, Alnylam, Bayer, Bioamerica, Cosmo, Intrinsic Medicine, Ironwood, Modify Health, My GI Health, My Nutrition Health, Nestle, Phathom Pharmaceuticals, QOL Medical, Redhill, Salix/Valeant, Takeda, Urovant, and Vibrant. Dr. Harer and Dr. Jennings have no relevant financial disclosures.
*Correction, 10/29/21: An earlier version of this article misstated the percentage of patients in the modified intention-to-treat population who achieved eradication with vonoprazan triple therapy.
**Correction, 10/29/21: An earlier version of this article misstated the name of Phathom Pharmaceuticals.
LAS VEGAS – In the treatment of Helicobacter pylori infection, combination therapies using the oral potassium-competitive acid blocker vonoprazan were superior to standard proton pump inhibitor (PPI)–based triple therapy, producing higher eradication rates, according to combined data from a U.S. and a European phase 3 randomized, controlled trial.
Vonoprazan has been submitted to the Food and Drug Administration for approval with a Fast Track designation in combination with amoxicillin and clarithromycin (triple therapy) or amoxicillin alone (dual therapy) for treating H. pylori infection. It has already been approved in Japan for the treatment of gastric and duodenal ulcers, reflux esophagitis, secondary prevention of low-dose aspirin– or nonsteroidal anti-inflammatory drug–induced gastric mucosal damage, and for first and second-line H. pylori eradication therapy.
Study details
The study included 1,046 treatment-naive patients who had dyspepsia, a recent or new diagnosis of a nonbleeding peptic ulcer, a history of a peptic ulcer, or long-term stable use of an NSAID. Patients were randomized to PPI-based triple therapy (lansoprazole, amoxicillin, clarithromycin), vonoprazan triple therapy (plus amoxicillin, clarithromycin), or vonoprazan dual therapy (amoxicillin). The treatment period was 14 days, followed by 13C urea breath test (UBT) 4 weeks after treatment.
The researchers conducted several analyses, including: Modified intention-to-treat analyses, which included all enrollees; per protocol analyses, which included patients who took at least 75% of each study medication and underwent 13C UBT in the expected time frame; and a safety population of all patients who took at least one study drug.
Among patients with H. pylori strains that were not resistant to clarithromycin, the PPI-based triple-therapy group had an eradication rate of 78.8%, compared with 84.7% in the vonoprazan triple-therapy group (P < .0001), and 78.5% in the vonoprazan dual-therapy group (P = .0037). In the per protocol analysis, PPI-based triple therapy eradicated H. pylori 82.1% of the time, compared with 90.4% in the vonoprazan triple-therapy group (P < .0001) and 81.2% in the vonoprazan dual-therapy group (P = .0077). Both vonoprazan treatment groups were noninferior to PPI-based triple therapy.
A prespecified exploratory analysis found that vonoprazan triple therapy outperformed PPI-based triple therapy in the modified intention-to-treat population (P = .0408) and the per protocol population (P = .0059).
Among patients with clarithromycin-resistant strains of H. pylori, in the modified intention-to-treat population, 31.9% achieved eradication with PPI triple therapy, compared with 65.8% in the vonoprazan triple-therapy group, and 69.6% in the vonoprazan dual-therapy group. In the per protocol population, the numbers were 29.0% versus 67.2% and 79.5%, respectively (P < .0001 for both versus PPI triple therapy).
Among all patients, in the modified intention-to-treat population, 68.5% achieved eradication with PPI triple therapy, 80.8% with vonoprazan triple therapy (P =. 0001), and 77.2% with vonoprazan dual therapy (P = .0063)*. In the per protocol population, the numbers were 70.0%, 85.7% (P < .0001), and 81.1% (P = .0013), respectively.
Safety outcomes were similar among the three groups, with treatment-emergent adverse events occurring in 34.5% of the PPI triple-therapy group (1.2% discontinued), 34.1% of the vonoprazan triple-therapy group (2.3% discontinued), and 29.9% in the vonoprazan dual-therapy group (0.9% discontinued).
Fighting against resistance
The efficacy of PPI-based clarithromycin-based triple therapy has fallen below 80% in the United States and Europe over the past few decades, largely because of antibiotic resistance, said William Chey, MD, during a presentation of the results at the annual meeting of the American College of Gastroenterology. Dr. Chey is a professor of medicine and director of the GI physiology laboratory at Michigan Medicine.
Vonoprazan is more stable in acid than are PPIs, and produces greater and more durable acid reduction, according to Dr. Chey. That’s important for two reasons: One is that some antibiotics are acid-labile, and so may have their efficacy directly impacted in a more acidic environment. The other factor is that most antibiotics work better on bacteria that are actively replicating, and H. pylori reproduces better in a more neutral environment. “So, you increase the replication, you increase the bioavailability of the antibiotics. And therefore, hopefully, that underlies why we see it working better in the patients with [antibiotic] resistance,” Dr. Chey said in an interview.
It remains to be seen whether or not the drug will receive FDA approval, but he pointed to other regimens like bismuth quadruple therapy and rifabutin-based triple therapy that are already available. “If I had the choice, I would never use a PPI-based triple therapy again. People should not be doing that,” said Dr. Chey.
“More successful H. pylori eradication regimens are certainly needed, and these results are particularly relevant and interesting given the increasing failure of initial treatment regimens,” said Kimberly Harer, MD, who moderated the session. She noted that the secondary analysis of patients with clarithromycin-resistant infections was particularly relevant. “The superiority analysis indicating vonoprazan triple therapy resulted in increased H. pylori eradication compared to lanzoprazole triple therapy was especially interesting,” said Dr. Harer, who is a clinical lecturer at University of Michigan Health, Ann Arbor.
One downside to the study is that it didn’t compare vonoprazan combinations to quadruple therapy of a PPI, bismuth, tetracycline, and a nitroimidazole, said Joseph Jennings, MD, who was asked to comment on the study. Other treatment approaches include sequential antibiotics and other combinations. Dr. Jennings also highlighted the findings that the vonoprazan regimens were superior against clarithromycin-resistant strains. “The more different regimens we can add to the armamentarium, the better chance we have because the resistant patterns fluctuate all throughout the world,” said Dr. Jennings, who is an assistant professor of medicine at Georgetown University and director of the center for GI bleeding at MedStar Georgetown University Hospital, both in Washington.
He also pointed out that physicians can face a conundrum when patients fail multiple lines of therapy and have testing done that shows high levels of resistance. Some have allergies that prevent them from turning to other antibiotics. “That’s a market where lots of doctors struggle. Something like this would be a nice add-on,” said Dr. Jennings.
The study was funded by Phathom Pharmaceuticals.** Dr. Chey has consulted and/or received research support from Abbvie, Alfasigma, Allakos, Alnylam, Bayer, Bioamerica, Cosmo, Intrinsic Medicine, Ironwood, Modify Health, My GI Health, My Nutrition Health, Nestle, Phathom Pharmaceuticals, QOL Medical, Redhill, Salix/Valeant, Takeda, Urovant, and Vibrant. Dr. Harer and Dr. Jennings have no relevant financial disclosures.
*Correction, 10/29/21: An earlier version of this article misstated the percentage of patients in the modified intention-to-treat population who achieved eradication with vonoprazan triple therapy.
**Correction, 10/29/21: An earlier version of this article misstated the name of Phathom Pharmaceuticals.
AT ACG 2021
Balloon-enhanced colonoscopy finds more adenomas
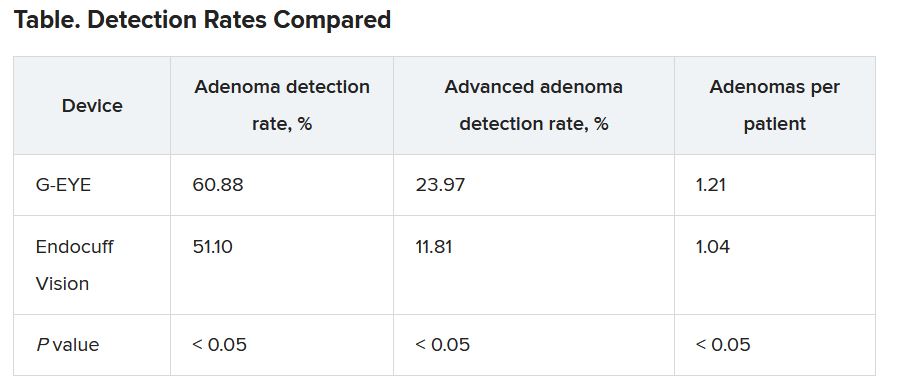
LAS VEGAS – The G-EYE colonoscope facilitates detection of adenomas more so than does the Endocuff Vision, researchers say.
In the first head-to-head comparison of two mechanical enhancement colonoscopy devices, “the G-EYE demonstrated a meaningful increase in adenoma detection rate [ADR] over Endocuff, particularly for advanced adenomas,” said Seth Gross, MD, a professor of medicine at New York University.
Previous studies have shown that mechanical enhancements are more effective than optical enhancements, Dr. Gross said. “To take it a step further, when you look at mechanical devices, especially these two, in past studies, the G-EYE has been sort of the leader in adenoma detection,” he told this news organization.
But until now, no studies had compared them head to head, said Dr. Gross, who presented the finding here at the American College of Gastroenterology (ACG) 2021 Annual Scientific Meeting.
The two devices work differently. The Endocuff Vision fits onto the colonoscope tip. During withdrawal, it expands radially, and its arms flatten the folds within the colon. The G-EYE balloon is deflated at insertion, then is inflated at the cecum, smoothing the colon wall while centering the colonoscopic view.
To compare the two, Dr. Gross and colleagues randomly assigned 363 patients to undergo colonoscopy with G-EYE and 364 patients to undergo colonoscopy with Endocuff Vision. The two groups were similar in demographics.
Withdrawal times were >6 minutes in both groups. The researchers detected adenomas in a higher percentage of patients with the G-EYE than with the Endocuff Vision. The same was true for advanced adenomas.
When using the G-EYE, the researchers also found more adenomas per patient, more sessile serrated adenomas per patient, more large adenomas per patient, and more right colon adenomas per patient.
The benchmark for ADR is only 25%, Dr. Gross said, suggesting that both devices are a worthwhile improvement over standard colonoscopes. “It supports the past literature that a mechanical enhancement is something that should be considered during colonoscopy,” he said.
Costs differ as well. The G-EYE requires a permanent modification to the bending rubber of the colonoscope, so the cost is up front. The Endocuff Vision utilizes a single-use cap that is placed on the tip, so costs are spread over time.
The G-EYE gained U.S. Food and Drug Administration clearance in May 2020. In 2016, the Endocuff (an earlier version of the Endocuff Vision) became the first mechanical device the use of which the FDA acknowledged improved ADRs.
Dr. Gross said that it would be interesting to see whether the mechanical devices and artificial intelligence enhancements could complement each other so as to yield even higher detection rates.
Session moderator Brooks Cash, MD, a professor of medicine at the University of Texas Health Science Center, Houston, said the difference in detection rates made an impressive case for the G-EYE.
“I wouldn’t say I’m convinced,” Dr. Cash said in an interview. “I’d like to see more data. But I think that the plurality of the evidence that they presented and the size of the study were certainly compelling.”
He added that he’d like to see evidence that adding the balloon to a colonoscope doesn’t complicate the cleaning of the device.
Dr. Gross has a financial relationship with Olympus, the maker of the Endocuff Vision. Dr. Cash reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LAS VEGAS – The G-EYE colonoscope facilitates detection of adenomas more so than does the Endocuff Vision, researchers say.
In the first head-to-head comparison of two mechanical enhancement colonoscopy devices, “the G-EYE demonstrated a meaningful increase in adenoma detection rate [ADR] over Endocuff, particularly for advanced adenomas,” said Seth Gross, MD, a professor of medicine at New York University.
Previous studies have shown that mechanical enhancements are more effective than optical enhancements, Dr. Gross said. “To take it a step further, when you look at mechanical devices, especially these two, in past studies, the G-EYE has been sort of the leader in adenoma detection,” he told this news organization.
But until now, no studies had compared them head to head, said Dr. Gross, who presented the finding here at the American College of Gastroenterology (ACG) 2021 Annual Scientific Meeting.
The two devices work differently. The Endocuff Vision fits onto the colonoscope tip. During withdrawal, it expands radially, and its arms flatten the folds within the colon. The G-EYE balloon is deflated at insertion, then is inflated at the cecum, smoothing the colon wall while centering the colonoscopic view.
To compare the two, Dr. Gross and colleagues randomly assigned 363 patients to undergo colonoscopy with G-EYE and 364 patients to undergo colonoscopy with Endocuff Vision. The two groups were similar in demographics.
Withdrawal times were >6 minutes in both groups. The researchers detected adenomas in a higher percentage of patients with the G-EYE than with the Endocuff Vision. The same was true for advanced adenomas.
When using the G-EYE, the researchers also found more adenomas per patient, more sessile serrated adenomas per patient, more large adenomas per patient, and more right colon adenomas per patient.
The benchmark for ADR is only 25%, Dr. Gross said, suggesting that both devices are a worthwhile improvement over standard colonoscopes. “It supports the past literature that a mechanical enhancement is something that should be considered during colonoscopy,” he said.
Costs differ as well. The G-EYE requires a permanent modification to the bending rubber of the colonoscope, so the cost is up front. The Endocuff Vision utilizes a single-use cap that is placed on the tip, so costs are spread over time.
The G-EYE gained U.S. Food and Drug Administration clearance in May 2020. In 2016, the Endocuff (an earlier version of the Endocuff Vision) became the first mechanical device the use of which the FDA acknowledged improved ADRs.
Dr. Gross said that it would be interesting to see whether the mechanical devices and artificial intelligence enhancements could complement each other so as to yield even higher detection rates.
Session moderator Brooks Cash, MD, a professor of medicine at the University of Texas Health Science Center, Houston, said the difference in detection rates made an impressive case for the G-EYE.
“I wouldn’t say I’m convinced,” Dr. Cash said in an interview. “I’d like to see more data. But I think that the plurality of the evidence that they presented and the size of the study were certainly compelling.”
He added that he’d like to see evidence that adding the balloon to a colonoscope doesn’t complicate the cleaning of the device.
Dr. Gross has a financial relationship with Olympus, the maker of the Endocuff Vision. Dr. Cash reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LAS VEGAS – The G-EYE colonoscope facilitates detection of adenomas more so than does the Endocuff Vision, researchers say.
In the first head-to-head comparison of two mechanical enhancement colonoscopy devices, “the G-EYE demonstrated a meaningful increase in adenoma detection rate [ADR] over Endocuff, particularly for advanced adenomas,” said Seth Gross, MD, a professor of medicine at New York University.
Previous studies have shown that mechanical enhancements are more effective than optical enhancements, Dr. Gross said. “To take it a step further, when you look at mechanical devices, especially these two, in past studies, the G-EYE has been sort of the leader in adenoma detection,” he told this news organization.
But until now, no studies had compared them head to head, said Dr. Gross, who presented the finding here at the American College of Gastroenterology (ACG) 2021 Annual Scientific Meeting.
The two devices work differently. The Endocuff Vision fits onto the colonoscope tip. During withdrawal, it expands radially, and its arms flatten the folds within the colon. The G-EYE balloon is deflated at insertion, then is inflated at the cecum, smoothing the colon wall while centering the colonoscopic view.
To compare the two, Dr. Gross and colleagues randomly assigned 363 patients to undergo colonoscopy with G-EYE and 364 patients to undergo colonoscopy with Endocuff Vision. The two groups were similar in demographics.
Withdrawal times were >6 minutes in both groups. The researchers detected adenomas in a higher percentage of patients with the G-EYE than with the Endocuff Vision. The same was true for advanced adenomas.
When using the G-EYE, the researchers also found more adenomas per patient, more sessile serrated adenomas per patient, more large adenomas per patient, and more right colon adenomas per patient.
The benchmark for ADR is only 25%, Dr. Gross said, suggesting that both devices are a worthwhile improvement over standard colonoscopes. “It supports the past literature that a mechanical enhancement is something that should be considered during colonoscopy,” he said.
Costs differ as well. The G-EYE requires a permanent modification to the bending rubber of the colonoscope, so the cost is up front. The Endocuff Vision utilizes a single-use cap that is placed on the tip, so costs are spread over time.
The G-EYE gained U.S. Food and Drug Administration clearance in May 2020. In 2016, the Endocuff (an earlier version of the Endocuff Vision) became the first mechanical device the use of which the FDA acknowledged improved ADRs.
Dr. Gross said that it would be interesting to see whether the mechanical devices and artificial intelligence enhancements could complement each other so as to yield even higher detection rates.
Session moderator Brooks Cash, MD, a professor of medicine at the University of Texas Health Science Center, Houston, said the difference in detection rates made an impressive case for the G-EYE.
“I wouldn’t say I’m convinced,” Dr. Cash said in an interview. “I’d like to see more data. But I think that the plurality of the evidence that they presented and the size of the study were certainly compelling.”
He added that he’d like to see evidence that adding the balloon to a colonoscope doesn’t complicate the cleaning of the device.
Dr. Gross has a financial relationship with Olympus, the maker of the Endocuff Vision. Dr. Cash reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACG 2021
After POEM, FLIP matches HRM for measuring patient response
LAS VEGAS – Functional lumen imaging probe (FLIP) was equivalent to high-resolution manometry (HRM) in predicting clinical response by Eckardt score 6 months or more after per oral endoscopic myotomy (POEM) for achalasia or esophagogastric junction (EGJ) outlet obstruction (EGJOO).
Measures for clinical response following lower esophageal sphincter myotomy procedures include Eckardt Score, timed barium esophagram, HRM, and FLIP. However, since FLIP is a relatively new technique, there are few clinical data comparing its efficacy versus HRM in patients who have a positive response to POEM measured by the Eckardt score, according to John DeWitt, MD, who presented the research at the annual meeting of the American College of Gastroenterology.
FLIP can be performed during a follow-up endoscopy while a patient is sedated, while HRM requires the patient to be awake. Some patients find the procedure intolerable, and Dr. DeWitt estimates that 10%-20% of patients don’t return for follow-up assessments because of the discomfort.
“[FLIP] is a relatively new technology, the role of which is still being discovered. We have a lot more information on the diagnosis side of things. The role in follow-up, particularly after myotomy, is really not defined well. This is the first study to my knowledge that has evaluated manometry and FLIP head-to-head to compare patient-reported outcomes,” said Dr. DeWitt in an interview. He is a professor of medicine and the director of endoscopic ultrasound at Indiana University Medical Center, in Indianapolis.
Going head-to-head
The researchers conducted a retrospective, single-center study of 265 consecutive patients who underwent POEM for achalasia or EGJOO from 2016 through 2020. A clinical response was defined as an Eckardt score ≤3, EGJ distensibility index (EGJ-DI) higher than 2.8 mm2/mm Hg, maximum integrated relaxation pressure (IRP) <15 mm Hg, or a maximum EGJ diameter greater than 14 mm at any balloon distension.
In all, 126 patients returned for follow-up and completed an upper endoscopy with FLIP, HRM, and Eckardt scores within a 6-12 month period after the POEM procedure.
With respect to HRM, an IRP measurement <15 mm Hg predicted post-POEM Eckardt score with a sensitivity of 86.7% (95% confidence interval, 79.3-92.2) and a specificity of 33.3% (95% CI, 4.3-77.7), with an area under the curve of 0.60 (95% CI, 0.39-0.81). A maximum EJG diameter ≥ 14 mm had a sensitivity of 77.5% (95% CI, 69.0-84.6) and a specificity of 33.3% (95% CI, 4.3-77.7), with an AUC of 0.55 (95% CI, 0.34-0.76).
The performance was similar with FLIP: EGJ-DI > 2.8 mm2/mm Hg at any balloon setting had a sensitivity of 95.0% (95% CI, 89.4-98.1) and a specificity% of 0.0, and an AUC of 0.53 (95% CI, 0.51-0.55). A similar measurement at 40 mL or 50 mL distension had a sensitivity of 93.3% (95% CI, 87.3-97.1) and a specificity of 16.7% (95% CI, 0.4-64.1), with an AUC of 0.55 (95% CI, 0.39-0.72). Receiver operator characteristic analysis showed no significant difference between ability of FLIP and HRM to predict a normal Eckardt score.
If the study is repeated in other patient populations, Dr. DeWitt hopes that it could eliminate manometry altogether in a large majority of patients. “That would be potentially a game changer for bringing patients back to see how well they’re doing,” said Dr. DeWitt.
Not all patients who undergo POEM would be good candidates for FLIP, said Dr. DeWitt. The study was limited to patients with hypertension in the lower esophageal sphincter. Other disorders such as diffuse esophageal spasm, jackhammer esophagus, and type III achalasia would not likely be candidates for FLIP. “Those patients are going to probably still need manometry because if the esophageal body abnormalities are still present, then repeat testing might need to be performed,” said Dr. DeWitt. Still, he estimated about 80% of patients could be eligible for FLIP instead.
Impact on patients
“I think it’s interesting new data,” said Patrick Young, MD, who comoderated the session where the research was presented. He noted that the treatment of achalasia is evolving away from surgery, and the techniques to measure response are evolving along with it. “As we progress in that technology and using that procedure, we need to understand better how to follow those people up. I think adding this new device may help us to understand who’s going to respond well, and who’s not going to respond well. This is an early investigation, so I think we’ll need to do trials, but I think this is a good first step,” said Dr. Young, who is a professor of medicine at the Uniformed Services University of the Health Sciences, Bethesda, Md.
Comoderator Mohammad Yaghoobi, MD, also praised the study, but noted that the cost of FLIP could be a concern. “We want to have a reasonable ratio of the cost versus the effectiveness,” said Dr. Yaghoobi, who is an associate professor of medicine at McMaster University in Hamilton, Ont.
Dr. DeWitt, Dr. Young, and Dr. Yaghoobi had no relevant disclosures.
LAS VEGAS – Functional lumen imaging probe (FLIP) was equivalent to high-resolution manometry (HRM) in predicting clinical response by Eckardt score 6 months or more after per oral endoscopic myotomy (POEM) for achalasia or esophagogastric junction (EGJ) outlet obstruction (EGJOO).
Measures for clinical response following lower esophageal sphincter myotomy procedures include Eckardt Score, timed barium esophagram, HRM, and FLIP. However, since FLIP is a relatively new technique, there are few clinical data comparing its efficacy versus HRM in patients who have a positive response to POEM measured by the Eckardt score, according to John DeWitt, MD, who presented the research at the annual meeting of the American College of Gastroenterology.
FLIP can be performed during a follow-up endoscopy while a patient is sedated, while HRM requires the patient to be awake. Some patients find the procedure intolerable, and Dr. DeWitt estimates that 10%-20% of patients don’t return for follow-up assessments because of the discomfort.
“[FLIP] is a relatively new technology, the role of which is still being discovered. We have a lot more information on the diagnosis side of things. The role in follow-up, particularly after myotomy, is really not defined well. This is the first study to my knowledge that has evaluated manometry and FLIP head-to-head to compare patient-reported outcomes,” said Dr. DeWitt in an interview. He is a professor of medicine and the director of endoscopic ultrasound at Indiana University Medical Center, in Indianapolis.
Going head-to-head
The researchers conducted a retrospective, single-center study of 265 consecutive patients who underwent POEM for achalasia or EGJOO from 2016 through 2020. A clinical response was defined as an Eckardt score ≤3, EGJ distensibility index (EGJ-DI) higher than 2.8 mm2/mm Hg, maximum integrated relaxation pressure (IRP) <15 mm Hg, or a maximum EGJ diameter greater than 14 mm at any balloon distension.
In all, 126 patients returned for follow-up and completed an upper endoscopy with FLIP, HRM, and Eckardt scores within a 6-12 month period after the POEM procedure.
With respect to HRM, an IRP measurement <15 mm Hg predicted post-POEM Eckardt score with a sensitivity of 86.7% (95% confidence interval, 79.3-92.2) and a specificity of 33.3% (95% CI, 4.3-77.7), with an area under the curve of 0.60 (95% CI, 0.39-0.81). A maximum EJG diameter ≥ 14 mm had a sensitivity of 77.5% (95% CI, 69.0-84.6) and a specificity of 33.3% (95% CI, 4.3-77.7), with an AUC of 0.55 (95% CI, 0.34-0.76).
The performance was similar with FLIP: EGJ-DI > 2.8 mm2/mm Hg at any balloon setting had a sensitivity of 95.0% (95% CI, 89.4-98.1) and a specificity% of 0.0, and an AUC of 0.53 (95% CI, 0.51-0.55). A similar measurement at 40 mL or 50 mL distension had a sensitivity of 93.3% (95% CI, 87.3-97.1) and a specificity of 16.7% (95% CI, 0.4-64.1), with an AUC of 0.55 (95% CI, 0.39-0.72). Receiver operator characteristic analysis showed no significant difference between ability of FLIP and HRM to predict a normal Eckardt score.
If the study is repeated in other patient populations, Dr. DeWitt hopes that it could eliminate manometry altogether in a large majority of patients. “That would be potentially a game changer for bringing patients back to see how well they’re doing,” said Dr. DeWitt.
Not all patients who undergo POEM would be good candidates for FLIP, said Dr. DeWitt. The study was limited to patients with hypertension in the lower esophageal sphincter. Other disorders such as diffuse esophageal spasm, jackhammer esophagus, and type III achalasia would not likely be candidates for FLIP. “Those patients are going to probably still need manometry because if the esophageal body abnormalities are still present, then repeat testing might need to be performed,” said Dr. DeWitt. Still, he estimated about 80% of patients could be eligible for FLIP instead.
Impact on patients
“I think it’s interesting new data,” said Patrick Young, MD, who comoderated the session where the research was presented. He noted that the treatment of achalasia is evolving away from surgery, and the techniques to measure response are evolving along with it. “As we progress in that technology and using that procedure, we need to understand better how to follow those people up. I think adding this new device may help us to understand who’s going to respond well, and who’s not going to respond well. This is an early investigation, so I think we’ll need to do trials, but I think this is a good first step,” said Dr. Young, who is a professor of medicine at the Uniformed Services University of the Health Sciences, Bethesda, Md.
Comoderator Mohammad Yaghoobi, MD, also praised the study, but noted that the cost of FLIP could be a concern. “We want to have a reasonable ratio of the cost versus the effectiveness,” said Dr. Yaghoobi, who is an associate professor of medicine at McMaster University in Hamilton, Ont.
Dr. DeWitt, Dr. Young, and Dr. Yaghoobi had no relevant disclosures.
LAS VEGAS – Functional lumen imaging probe (FLIP) was equivalent to high-resolution manometry (HRM) in predicting clinical response by Eckardt score 6 months or more after per oral endoscopic myotomy (POEM) for achalasia or esophagogastric junction (EGJ) outlet obstruction (EGJOO).
Measures for clinical response following lower esophageal sphincter myotomy procedures include Eckardt Score, timed barium esophagram, HRM, and FLIP. However, since FLIP is a relatively new technique, there are few clinical data comparing its efficacy versus HRM in patients who have a positive response to POEM measured by the Eckardt score, according to John DeWitt, MD, who presented the research at the annual meeting of the American College of Gastroenterology.
FLIP can be performed during a follow-up endoscopy while a patient is sedated, while HRM requires the patient to be awake. Some patients find the procedure intolerable, and Dr. DeWitt estimates that 10%-20% of patients don’t return for follow-up assessments because of the discomfort.
“[FLIP] is a relatively new technology, the role of which is still being discovered. We have a lot more information on the diagnosis side of things. The role in follow-up, particularly after myotomy, is really not defined well. This is the first study to my knowledge that has evaluated manometry and FLIP head-to-head to compare patient-reported outcomes,” said Dr. DeWitt in an interview. He is a professor of medicine and the director of endoscopic ultrasound at Indiana University Medical Center, in Indianapolis.
Going head-to-head
The researchers conducted a retrospective, single-center study of 265 consecutive patients who underwent POEM for achalasia or EGJOO from 2016 through 2020. A clinical response was defined as an Eckardt score ≤3, EGJ distensibility index (EGJ-DI) higher than 2.8 mm2/mm Hg, maximum integrated relaxation pressure (IRP) <15 mm Hg, or a maximum EGJ diameter greater than 14 mm at any balloon distension.
In all, 126 patients returned for follow-up and completed an upper endoscopy with FLIP, HRM, and Eckardt scores within a 6-12 month period after the POEM procedure.
With respect to HRM, an IRP measurement <15 mm Hg predicted post-POEM Eckardt score with a sensitivity of 86.7% (95% confidence interval, 79.3-92.2) and a specificity of 33.3% (95% CI, 4.3-77.7), with an area under the curve of 0.60 (95% CI, 0.39-0.81). A maximum EJG diameter ≥ 14 mm had a sensitivity of 77.5% (95% CI, 69.0-84.6) and a specificity of 33.3% (95% CI, 4.3-77.7), with an AUC of 0.55 (95% CI, 0.34-0.76).
The performance was similar with FLIP: EGJ-DI > 2.8 mm2/mm Hg at any balloon setting had a sensitivity of 95.0% (95% CI, 89.4-98.1) and a specificity% of 0.0, and an AUC of 0.53 (95% CI, 0.51-0.55). A similar measurement at 40 mL or 50 mL distension had a sensitivity of 93.3% (95% CI, 87.3-97.1) and a specificity of 16.7% (95% CI, 0.4-64.1), with an AUC of 0.55 (95% CI, 0.39-0.72). Receiver operator characteristic analysis showed no significant difference between ability of FLIP and HRM to predict a normal Eckardt score.
If the study is repeated in other patient populations, Dr. DeWitt hopes that it could eliminate manometry altogether in a large majority of patients. “That would be potentially a game changer for bringing patients back to see how well they’re doing,” said Dr. DeWitt.
Not all patients who undergo POEM would be good candidates for FLIP, said Dr. DeWitt. The study was limited to patients with hypertension in the lower esophageal sphincter. Other disorders such as diffuse esophageal spasm, jackhammer esophagus, and type III achalasia would not likely be candidates for FLIP. “Those patients are going to probably still need manometry because if the esophageal body abnormalities are still present, then repeat testing might need to be performed,” said Dr. DeWitt. Still, he estimated about 80% of patients could be eligible for FLIP instead.
Impact on patients
“I think it’s interesting new data,” said Patrick Young, MD, who comoderated the session where the research was presented. He noted that the treatment of achalasia is evolving away from surgery, and the techniques to measure response are evolving along with it. “As we progress in that technology and using that procedure, we need to understand better how to follow those people up. I think adding this new device may help us to understand who’s going to respond well, and who’s not going to respond well. This is an early investigation, so I think we’ll need to do trials, but I think this is a good first step,” said Dr. Young, who is a professor of medicine at the Uniformed Services University of the Health Sciences, Bethesda, Md.
Comoderator Mohammad Yaghoobi, MD, also praised the study, but noted that the cost of FLIP could be a concern. “We want to have a reasonable ratio of the cost versus the effectiveness,” said Dr. Yaghoobi, who is an associate professor of medicine at McMaster University in Hamilton, Ont.
Dr. DeWitt, Dr. Young, and Dr. Yaghoobi had no relevant disclosures.
AT ACG 2021
Dupilumab shows long-term efficacy in EoE
LAS VEGAS –Data from the 28-week extension of the Liberty EoE TREET phase 3 clinical trial showed that the anti–interleukin-4/IL-13 antibody dupilumab led to long-term improvement in eosinophil count, histology, and patient-reported symptoms of eosinophilic esophagitis (EoE) out to 28 weeks. Dupilumab is Food and Drug Administration approved for the treatment of atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyposis.
Many patients don’t respond to the standard therapies of proton pump inhibitors, steroids, or diet. Some evidence suggests that EoE might be driven by type 2 inflammation, and dupilumab’s effect on the shared receptor of IL-4 and IL-13 directly counters that pathway.
“The current treatments are [proton pump inhibitors], steroids, or diet – a good proportion of patients don’t respond to them. And they’re also not targeted,” Evan Dellon, MD, professor of medicine and epidemiology at the University of North Carolina at Chapel Hill, said in an interview. Dr. Dellon presented the research at the annual meeting of the American College of Gastroenterology.
“The bottom line is that people who responded up front to dupilumab maintain that response to a year, and the people on placebo gained a similar response as the people who were treated. It looked good. It was histologic, symptomatic, and endoscopic outcomes,” said Dr. Dellon.
Many of the patients in the new study were steroid refractory, making it a difficult population to treat, according to Dr. Dellon. “You can’t compare to the steroid-treated patients, but the 6-month data showed about a 60% response rate histologically, which is right up there with where steroids and diet are for easier to treat patients. So the fact that it’s a harder to treat cohort is pretty impressive from that standpoint,” said Dr. Dellon.
Data from the first 24 weeks was previously reported at UEG Week 2020 and showed that dupilumab outperformed placebo in EoE patients aged 12 years and older, with dupilumab producing better outcomes in peak esophageal intraepithelial eosinophil count and change in Dysphagia Symptom Questionnaire (DSQ) Score at 24 weeks.
At ACG 2021, Dr. Dellon reported on 52-week results, where all patients from both treated and placebo groups received dupilumab after the initial 24-week phase. Dupilumab reduced dysphagia symptoms as measured by the absolute change in DSQ score at 24 weeks (–21.9 vs. –9.6; P < .001). At 52 weeks, the dupilumab group showed a change of –23.4 from the start of the study, and the placebo-to-dupilumab group had a DSQ score change of –21.7. Dupilumab also led to a greater percentage reduction in DSQ score by 24 weeks (69.2% versus 31.7%; P < .001); at 52 weeks, the dupilumab group had a 75.9% reduction and the placebo-to-dupilumab group had a 65.9% reduction (no significant difference).
The dupilumab group had a greater proportion of patients who achieved peak esophageal eosinophil count of 6 eosinophils or less per high power field at 24 weeks (59.5% vs. 5.1%); at 52 weeks, 55.9% had achieved this measure, versus 60.0% of the placebo-to-dupilumab group. At 24 weeks, the dupilumab group had a 71.2% reduction in peak eosinophil count from baseline versus –3.0% in placebo (P < .001). At week 52, the reductions were 88.6% and 83.8%, respectively.
Histology features were improved with dupilumab. At week 24, the absolute change in histology scoring system mean grade score (histologic severity) from initial baseline was greater in the dupilumab group (least squares mean, –0.761 vs. –0.001; P < .001). The improvement continued at week 52 (LS mean, –0.87) and occurred in the placebo-to-dupilumab group (LS mean, –0.87). The dupilumab group had a greater absolute change in mean stage score at 24 weeks (histologic extent, LS mean, –0.753 vs. –0.012; P < .001) and 52 weeks (LS mean, –0.89), while the placebo-to-dupilumab group achieved a similar change at 52 weeks (LS mean, –0.87).
Endoscopic features improved in the dupilumab group as measured by endoscopic reference score at 24 weeks (LS mean, –3.2 versus –0.3; P <.001) and at 52 weeks (LS mean, –4.1). The placebo-to-dupilumab group had a similar outcome at 52 weeks (LS mean, –3.9).
Dupilumab was well tolerated, with the only significant difference in treatment-emergent adverse events being injection-site reactions and injection-site erythema.
“I thought the data was really impressive and compelling,” said Amy Oxentenko, MD, chair of medicine at the Mayo Clinic in Phoenix, who comoderated the session. “It’d be nice to have something like this that is a targeted therapy that clearly shows improvement in not only some of the symptoms and histology, but also having an impact possibly on that fibrotic piece, which I think is really the area of morbidity in these patients long term.”
If approved, dupilumab could improve compliance among patients, who sometimes struggle with taking topical steroids properly, said comoderator David Hass, MD, who is an associate clinical professor at Yale University, New Haven, Conn. He also agreed that the potential for remodeling would be a significant benefit over steroids.
One concern with dupilumab would be any potential for immune suppression. “It’s always something to think about,” Dr. Hass said.
LIBERTY EoE TREET was funded by Sanofi and Regeneron. Dr. Dellon has consulted and received research support from numerous pharmaceutical companies. Dr. Oxentenko and Dr. Hass have no relevant financial disclosures.
This article was updated Nov. 4, 2021.
LAS VEGAS –Data from the 28-week extension of the Liberty EoE TREET phase 3 clinical trial showed that the anti–interleukin-4/IL-13 antibody dupilumab led to long-term improvement in eosinophil count, histology, and patient-reported symptoms of eosinophilic esophagitis (EoE) out to 28 weeks. Dupilumab is Food and Drug Administration approved for the treatment of atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyposis.
Many patients don’t respond to the standard therapies of proton pump inhibitors, steroids, or diet. Some evidence suggests that EoE might be driven by type 2 inflammation, and dupilumab’s effect on the shared receptor of IL-4 and IL-13 directly counters that pathway.
“The current treatments are [proton pump inhibitors], steroids, or diet – a good proportion of patients don’t respond to them. And they’re also not targeted,” Evan Dellon, MD, professor of medicine and epidemiology at the University of North Carolina at Chapel Hill, said in an interview. Dr. Dellon presented the research at the annual meeting of the American College of Gastroenterology.
“The bottom line is that people who responded up front to dupilumab maintain that response to a year, and the people on placebo gained a similar response as the people who were treated. It looked good. It was histologic, symptomatic, and endoscopic outcomes,” said Dr. Dellon.
Many of the patients in the new study were steroid refractory, making it a difficult population to treat, according to Dr. Dellon. “You can’t compare to the steroid-treated patients, but the 6-month data showed about a 60% response rate histologically, which is right up there with where steroids and diet are for easier to treat patients. So the fact that it’s a harder to treat cohort is pretty impressive from that standpoint,” said Dr. Dellon.
Data from the first 24 weeks was previously reported at UEG Week 2020 and showed that dupilumab outperformed placebo in EoE patients aged 12 years and older, with dupilumab producing better outcomes in peak esophageal intraepithelial eosinophil count and change in Dysphagia Symptom Questionnaire (DSQ) Score at 24 weeks.
At ACG 2021, Dr. Dellon reported on 52-week results, where all patients from both treated and placebo groups received dupilumab after the initial 24-week phase. Dupilumab reduced dysphagia symptoms as measured by the absolute change in DSQ score at 24 weeks (–21.9 vs. –9.6; P < .001). At 52 weeks, the dupilumab group showed a change of –23.4 from the start of the study, and the placebo-to-dupilumab group had a DSQ score change of –21.7. Dupilumab also led to a greater percentage reduction in DSQ score by 24 weeks (69.2% versus 31.7%; P < .001); at 52 weeks, the dupilumab group had a 75.9% reduction and the placebo-to-dupilumab group had a 65.9% reduction (no significant difference).
The dupilumab group had a greater proportion of patients who achieved peak esophageal eosinophil count of 6 eosinophils or less per high power field at 24 weeks (59.5% vs. 5.1%); at 52 weeks, 55.9% had achieved this measure, versus 60.0% of the placebo-to-dupilumab group. At 24 weeks, the dupilumab group had a 71.2% reduction in peak eosinophil count from baseline versus –3.0% in placebo (P < .001). At week 52, the reductions were 88.6% and 83.8%, respectively.
Histology features were improved with dupilumab. At week 24, the absolute change in histology scoring system mean grade score (histologic severity) from initial baseline was greater in the dupilumab group (least squares mean, –0.761 vs. –0.001; P < .001). The improvement continued at week 52 (LS mean, –0.87) and occurred in the placebo-to-dupilumab group (LS mean, –0.87). The dupilumab group had a greater absolute change in mean stage score at 24 weeks (histologic extent, LS mean, –0.753 vs. –0.012; P < .001) and 52 weeks (LS mean, –0.89), while the placebo-to-dupilumab group achieved a similar change at 52 weeks (LS mean, –0.87).
Endoscopic features improved in the dupilumab group as measured by endoscopic reference score at 24 weeks (LS mean, –3.2 versus –0.3; P <.001) and at 52 weeks (LS mean, –4.1). The placebo-to-dupilumab group had a similar outcome at 52 weeks (LS mean, –3.9).
Dupilumab was well tolerated, with the only significant difference in treatment-emergent adverse events being injection-site reactions and injection-site erythema.
“I thought the data was really impressive and compelling,” said Amy Oxentenko, MD, chair of medicine at the Mayo Clinic in Phoenix, who comoderated the session. “It’d be nice to have something like this that is a targeted therapy that clearly shows improvement in not only some of the symptoms and histology, but also having an impact possibly on that fibrotic piece, which I think is really the area of morbidity in these patients long term.”
If approved, dupilumab could improve compliance among patients, who sometimes struggle with taking topical steroids properly, said comoderator David Hass, MD, who is an associate clinical professor at Yale University, New Haven, Conn. He also agreed that the potential for remodeling would be a significant benefit over steroids.
One concern with dupilumab would be any potential for immune suppression. “It’s always something to think about,” Dr. Hass said.
LIBERTY EoE TREET was funded by Sanofi and Regeneron. Dr. Dellon has consulted and received research support from numerous pharmaceutical companies. Dr. Oxentenko and Dr. Hass have no relevant financial disclosures.
This article was updated Nov. 4, 2021.
LAS VEGAS –Data from the 28-week extension of the Liberty EoE TREET phase 3 clinical trial showed that the anti–interleukin-4/IL-13 antibody dupilumab led to long-term improvement in eosinophil count, histology, and patient-reported symptoms of eosinophilic esophagitis (EoE) out to 28 weeks. Dupilumab is Food and Drug Administration approved for the treatment of atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyposis.
Many patients don’t respond to the standard therapies of proton pump inhibitors, steroids, or diet. Some evidence suggests that EoE might be driven by type 2 inflammation, and dupilumab’s effect on the shared receptor of IL-4 and IL-13 directly counters that pathway.
“The current treatments are [proton pump inhibitors], steroids, or diet – a good proportion of patients don’t respond to them. And they’re also not targeted,” Evan Dellon, MD, professor of medicine and epidemiology at the University of North Carolina at Chapel Hill, said in an interview. Dr. Dellon presented the research at the annual meeting of the American College of Gastroenterology.
“The bottom line is that people who responded up front to dupilumab maintain that response to a year, and the people on placebo gained a similar response as the people who were treated. It looked good. It was histologic, symptomatic, and endoscopic outcomes,” said Dr. Dellon.
Many of the patients in the new study were steroid refractory, making it a difficult population to treat, according to Dr. Dellon. “You can’t compare to the steroid-treated patients, but the 6-month data showed about a 60% response rate histologically, which is right up there with where steroids and diet are for easier to treat patients. So the fact that it’s a harder to treat cohort is pretty impressive from that standpoint,” said Dr. Dellon.
Data from the first 24 weeks was previously reported at UEG Week 2020 and showed that dupilumab outperformed placebo in EoE patients aged 12 years and older, with dupilumab producing better outcomes in peak esophageal intraepithelial eosinophil count and change in Dysphagia Symptom Questionnaire (DSQ) Score at 24 weeks.
At ACG 2021, Dr. Dellon reported on 52-week results, where all patients from both treated and placebo groups received dupilumab after the initial 24-week phase. Dupilumab reduced dysphagia symptoms as measured by the absolute change in DSQ score at 24 weeks (–21.9 vs. –9.6; P < .001). At 52 weeks, the dupilumab group showed a change of –23.4 from the start of the study, and the placebo-to-dupilumab group had a DSQ score change of –21.7. Dupilumab also led to a greater percentage reduction in DSQ score by 24 weeks (69.2% versus 31.7%; P < .001); at 52 weeks, the dupilumab group had a 75.9% reduction and the placebo-to-dupilumab group had a 65.9% reduction (no significant difference).
The dupilumab group had a greater proportion of patients who achieved peak esophageal eosinophil count of 6 eosinophils or less per high power field at 24 weeks (59.5% vs. 5.1%); at 52 weeks, 55.9% had achieved this measure, versus 60.0% of the placebo-to-dupilumab group. At 24 weeks, the dupilumab group had a 71.2% reduction in peak eosinophil count from baseline versus –3.0% in placebo (P < .001). At week 52, the reductions were 88.6% and 83.8%, respectively.
Histology features were improved with dupilumab. At week 24, the absolute change in histology scoring system mean grade score (histologic severity) from initial baseline was greater in the dupilumab group (least squares mean, –0.761 vs. –0.001; P < .001). The improvement continued at week 52 (LS mean, –0.87) and occurred in the placebo-to-dupilumab group (LS mean, –0.87). The dupilumab group had a greater absolute change in mean stage score at 24 weeks (histologic extent, LS mean, –0.753 vs. –0.012; P < .001) and 52 weeks (LS mean, –0.89), while the placebo-to-dupilumab group achieved a similar change at 52 weeks (LS mean, –0.87).
Endoscopic features improved in the dupilumab group as measured by endoscopic reference score at 24 weeks (LS mean, –3.2 versus –0.3; P <.001) and at 52 weeks (LS mean, –4.1). The placebo-to-dupilumab group had a similar outcome at 52 weeks (LS mean, –3.9).
Dupilumab was well tolerated, with the only significant difference in treatment-emergent adverse events being injection-site reactions and injection-site erythema.
“I thought the data was really impressive and compelling,” said Amy Oxentenko, MD, chair of medicine at the Mayo Clinic in Phoenix, who comoderated the session. “It’d be nice to have something like this that is a targeted therapy that clearly shows improvement in not only some of the symptoms and histology, but also having an impact possibly on that fibrotic piece, which I think is really the area of morbidity in these patients long term.”
If approved, dupilumab could improve compliance among patients, who sometimes struggle with taking topical steroids properly, said comoderator David Hass, MD, who is an associate clinical professor at Yale University, New Haven, Conn. He also agreed that the potential for remodeling would be a significant benefit over steroids.
One concern with dupilumab would be any potential for immune suppression. “It’s always something to think about,” Dr. Hass said.
LIBERTY EoE TREET was funded by Sanofi and Regeneron. Dr. Dellon has consulted and received research support from numerous pharmaceutical companies. Dr. Oxentenko and Dr. Hass have no relevant financial disclosures.
This article was updated Nov. 4, 2021.
AT AGC 2021
Tracking adenomas per colonoscopy shows promise as quality measure
The number of adenomas per colonoscopy (APC) is inversely correlated with postcolonoscopy colorectal cancer (PCCRC), which supports use of APC as a new quality control measure, according to investigators.
Data from 138 endoscopists showed that patients screened by physicians with higher APCs had significantly lower rates of PCCRC, and an APC of 0.6 offered more protection than either an APC of 0.4 or an adenoma detection rate (ADR) of 25%, reported lead author Joseph C. Anderson, MD, of White River Junction VA Medical Center, Hanover, N.H., and colleagues.
“Unfortunately, APC has never been validated as a quality measure by demonstrating a reduction in PCCRC in exams performed by endoscopists with higher rates,” Dr. Anderson said at the annual meeting of the American College of Gastroenterology.
To this end, Dr. Anderson and colleagues reviewed data from the New Hampshire Colonoscopy Registry (NHCR), including 9,023 screening colonoscopies with a follow-up event 6-60 months after the initial exam. Procedures were conducted by 138 endoscopists in New Hampshire, Vermont, Massachusetts, and Maine.
Three quality measures were analyzed for associations with PCCRC: an APC of 0.4, an APC of 0.6, and an ADR of 25%. Hazard ratios were calculated for all PCCRCs, as well as PCCRCs diagnosed at first follow-up event. Rates were reported for two time periods: 6-36 months and 6-60 months.
From 6 to 60 months, 82 cases of PCCRC were diagnosed, among which 50 were diagnosed between 6 and 36 months.
For both periods, all three quality measures were significantly associated with reductions in PCCRC. The higher APC of 0.6, however, offered greater protection, reducing all PCCRCs by 71% and 61% in the shorter and longer period, respectively. In comparison, the lower APC of 0.4 reduced rates by 63% and 53%, while the ADR benchmark reduced rates by 62% and 42%.
These trends were maintained for PCCRCs diagnosed at first follow-up event. An APC of 0.6 was associated with respective reductions of 79% and 65% for the shorter and longer period, compared with 64% and 57% for the lower APC, and 67% and 49% for ADR.
Additional analysis clarified the relationship between APC level and likelihood of developing PCCRC. In terms of absolute risk, patients screened by an endoscopist with an APC greater than 0.6 had a 0.5% chance of developing PCCRC from 6 to 36 months, compared with 0.7% for an APC of 0.4-0.6, and 2.1% for an APC of less than 0.4 (P = .0001). This pattern held through 60 months, during which time an APC greater than 0.6 was associated with an absolute risk of PCCRC of 0.4%, compared with 0.7% for an APC of 0.4-0.6, and 1.6% for an APC less than 0.4 (P = .0001).
“Our novel data support the use of APC as a quality measure by demonstrating a reduction in PCCRC risk in exams performed by endoscopists with higher APCs,” Dr. Anderson concluded, noting that an APC of 0.6 appeared to offer more protection than an APC of 0.4. “I feel that ... APC as a quality measure, now that we’ve validated it, may be accepted because of its ability to differentiate endoscopists on their adenoma detection skills.”
According to Lawrence Hookey, MD, of Queen’s University, Kingston, Ont., “It’s an important study that will probably contribute to where we’re going forward.”
Dr. Lawrence, chair of the division and medical director of the endoscopy units at Kingston General and Hotel Dieu hospitals, said that APC may overcome the main concern with ADR – that endoscopists who find one adenoma may not be motivated to seek out as many as possible.
“The problem with ADR, in general, is that if you find one polyp, and if ADR is the stat you’re living by, then you don’t need to find any other polyps, and that obviously doesn’t do that patient a favor, necessarily,” Dr. Hookey said in an interview. “It does bring them back sooner for surveillance, but it doesn’t help remove the rest of the polyps that they have. And not that someone is going to find one polyp and turn off the light and pull the scope out, but you may not be looking as hard.”
APC mitigates this issue, he explained, because it determines “whether or not you’re truly clearing things out and getting rid of as many [polyps] as possible.”
Dr. Hookey said that APC is “probably the best” quality control measure on the horizon, and he suggested that more work is needed to determine the optimal benchmark figure, which should ideally be investigated through larger studies.
“I just want to see it in bigger groups,” he said.
The investigators and Dr. Hookey reported no conflicts of interest.
The number of adenomas per colonoscopy (APC) is inversely correlated with postcolonoscopy colorectal cancer (PCCRC), which supports use of APC as a new quality control measure, according to investigators.
Data from 138 endoscopists showed that patients screened by physicians with higher APCs had significantly lower rates of PCCRC, and an APC of 0.6 offered more protection than either an APC of 0.4 or an adenoma detection rate (ADR) of 25%, reported lead author Joseph C. Anderson, MD, of White River Junction VA Medical Center, Hanover, N.H., and colleagues.
“Unfortunately, APC has never been validated as a quality measure by demonstrating a reduction in PCCRC in exams performed by endoscopists with higher rates,” Dr. Anderson said at the annual meeting of the American College of Gastroenterology.
To this end, Dr. Anderson and colleagues reviewed data from the New Hampshire Colonoscopy Registry (NHCR), including 9,023 screening colonoscopies with a follow-up event 6-60 months after the initial exam. Procedures were conducted by 138 endoscopists in New Hampshire, Vermont, Massachusetts, and Maine.
Three quality measures were analyzed for associations with PCCRC: an APC of 0.4, an APC of 0.6, and an ADR of 25%. Hazard ratios were calculated for all PCCRCs, as well as PCCRCs diagnosed at first follow-up event. Rates were reported for two time periods: 6-36 months and 6-60 months.
From 6 to 60 months, 82 cases of PCCRC were diagnosed, among which 50 were diagnosed between 6 and 36 months.
For both periods, all three quality measures were significantly associated with reductions in PCCRC. The higher APC of 0.6, however, offered greater protection, reducing all PCCRCs by 71% and 61% in the shorter and longer period, respectively. In comparison, the lower APC of 0.4 reduced rates by 63% and 53%, while the ADR benchmark reduced rates by 62% and 42%.
These trends were maintained for PCCRCs diagnosed at first follow-up event. An APC of 0.6 was associated with respective reductions of 79% and 65% for the shorter and longer period, compared with 64% and 57% for the lower APC, and 67% and 49% for ADR.
Additional analysis clarified the relationship between APC level and likelihood of developing PCCRC. In terms of absolute risk, patients screened by an endoscopist with an APC greater than 0.6 had a 0.5% chance of developing PCCRC from 6 to 36 months, compared with 0.7% for an APC of 0.4-0.6, and 2.1% for an APC of less than 0.4 (P = .0001). This pattern held through 60 months, during which time an APC greater than 0.6 was associated with an absolute risk of PCCRC of 0.4%, compared with 0.7% for an APC of 0.4-0.6, and 1.6% for an APC less than 0.4 (P = .0001).
“Our novel data support the use of APC as a quality measure by demonstrating a reduction in PCCRC risk in exams performed by endoscopists with higher APCs,” Dr. Anderson concluded, noting that an APC of 0.6 appeared to offer more protection than an APC of 0.4. “I feel that ... APC as a quality measure, now that we’ve validated it, may be accepted because of its ability to differentiate endoscopists on their adenoma detection skills.”
According to Lawrence Hookey, MD, of Queen’s University, Kingston, Ont., “It’s an important study that will probably contribute to where we’re going forward.”
Dr. Lawrence, chair of the division and medical director of the endoscopy units at Kingston General and Hotel Dieu hospitals, said that APC may overcome the main concern with ADR – that endoscopists who find one adenoma may not be motivated to seek out as many as possible.
“The problem with ADR, in general, is that if you find one polyp, and if ADR is the stat you’re living by, then you don’t need to find any other polyps, and that obviously doesn’t do that patient a favor, necessarily,” Dr. Hookey said in an interview. “It does bring them back sooner for surveillance, but it doesn’t help remove the rest of the polyps that they have. And not that someone is going to find one polyp and turn off the light and pull the scope out, but you may not be looking as hard.”
APC mitigates this issue, he explained, because it determines “whether or not you’re truly clearing things out and getting rid of as many [polyps] as possible.”
Dr. Hookey said that APC is “probably the best” quality control measure on the horizon, and he suggested that more work is needed to determine the optimal benchmark figure, which should ideally be investigated through larger studies.
“I just want to see it in bigger groups,” he said.
The investigators and Dr. Hookey reported no conflicts of interest.
The number of adenomas per colonoscopy (APC) is inversely correlated with postcolonoscopy colorectal cancer (PCCRC), which supports use of APC as a new quality control measure, according to investigators.
Data from 138 endoscopists showed that patients screened by physicians with higher APCs had significantly lower rates of PCCRC, and an APC of 0.6 offered more protection than either an APC of 0.4 or an adenoma detection rate (ADR) of 25%, reported lead author Joseph C. Anderson, MD, of White River Junction VA Medical Center, Hanover, N.H., and colleagues.
“Unfortunately, APC has never been validated as a quality measure by demonstrating a reduction in PCCRC in exams performed by endoscopists with higher rates,” Dr. Anderson said at the annual meeting of the American College of Gastroenterology.
To this end, Dr. Anderson and colleagues reviewed data from the New Hampshire Colonoscopy Registry (NHCR), including 9,023 screening colonoscopies with a follow-up event 6-60 months after the initial exam. Procedures were conducted by 138 endoscopists in New Hampshire, Vermont, Massachusetts, and Maine.
Three quality measures were analyzed for associations with PCCRC: an APC of 0.4, an APC of 0.6, and an ADR of 25%. Hazard ratios were calculated for all PCCRCs, as well as PCCRCs diagnosed at first follow-up event. Rates were reported for two time periods: 6-36 months and 6-60 months.
From 6 to 60 months, 82 cases of PCCRC were diagnosed, among which 50 were diagnosed between 6 and 36 months.
For both periods, all three quality measures were significantly associated with reductions in PCCRC. The higher APC of 0.6, however, offered greater protection, reducing all PCCRCs by 71% and 61% in the shorter and longer period, respectively. In comparison, the lower APC of 0.4 reduced rates by 63% and 53%, while the ADR benchmark reduced rates by 62% and 42%.
These trends were maintained for PCCRCs diagnosed at first follow-up event. An APC of 0.6 was associated with respective reductions of 79% and 65% for the shorter and longer period, compared with 64% and 57% for the lower APC, and 67% and 49% for ADR.
Additional analysis clarified the relationship between APC level and likelihood of developing PCCRC. In terms of absolute risk, patients screened by an endoscopist with an APC greater than 0.6 had a 0.5% chance of developing PCCRC from 6 to 36 months, compared with 0.7% for an APC of 0.4-0.6, and 2.1% for an APC of less than 0.4 (P = .0001). This pattern held through 60 months, during which time an APC greater than 0.6 was associated with an absolute risk of PCCRC of 0.4%, compared with 0.7% for an APC of 0.4-0.6, and 1.6% for an APC less than 0.4 (P = .0001).
“Our novel data support the use of APC as a quality measure by demonstrating a reduction in PCCRC risk in exams performed by endoscopists with higher APCs,” Dr. Anderson concluded, noting that an APC of 0.6 appeared to offer more protection than an APC of 0.4. “I feel that ... APC as a quality measure, now that we’ve validated it, may be accepted because of its ability to differentiate endoscopists on their adenoma detection skills.”
According to Lawrence Hookey, MD, of Queen’s University, Kingston, Ont., “It’s an important study that will probably contribute to where we’re going forward.”
Dr. Lawrence, chair of the division and medical director of the endoscopy units at Kingston General and Hotel Dieu hospitals, said that APC may overcome the main concern with ADR – that endoscopists who find one adenoma may not be motivated to seek out as many as possible.
“The problem with ADR, in general, is that if you find one polyp, and if ADR is the stat you’re living by, then you don’t need to find any other polyps, and that obviously doesn’t do that patient a favor, necessarily,” Dr. Hookey said in an interview. “It does bring them back sooner for surveillance, but it doesn’t help remove the rest of the polyps that they have. And not that someone is going to find one polyp and turn off the light and pull the scope out, but you may not be looking as hard.”
APC mitigates this issue, he explained, because it determines “whether or not you’re truly clearing things out and getting rid of as many [polyps] as possible.”
Dr. Hookey said that APC is “probably the best” quality control measure on the horizon, and he suggested that more work is needed to determine the optimal benchmark figure, which should ideally be investigated through larger studies.
“I just want to see it in bigger groups,” he said.
The investigators and Dr. Hookey reported no conflicts of interest.
FROM ACG 2021
Immune response detected in most IBD patients after COVID vaccines
Most patients with inflammatory bowel disease (IBD) develop a humoral immune response after completing an mRNA SARS-CoV-2 vaccine series, according to data from almost 800 patients.
Anti–receptor binding domain IgG antibodies specific to SARS-CoV-2 were detectable in 95% of patients, with “generally similar” results across vaccine type, age group, and medication class, apart from corticosteroid users, who had an 86% antibody detection rate, reported lead author Kimberly N. Weaver, MD, of the University of North Carolina at Chapel Hill, and colleagues.
“Patients with IBD on immunosuppressive medications have the potential for attenuated response to the SARS-CoV-2 vaccination,” Dr. Weaver said at the annual meeting of the American College of Gastroenterology.
In support of this possibility, Dr. Weaver cited two recent trials from earlier in 2021: one demonstrated blunted antibody responses in IBD patients taking infliximab, while the other showed that full vaccination was less effective at preventing SARS-CoV-2 infection among patients with IBD than nonimmunosuppressed individuals.
To better characterize antibody responses after receiving an mRNA vaccination series, Dr. Weaver and colleagues launched the PREVENT-COVID trial, including the present dataset of 787 patients with IBD older than 12 years, all of whom provided serum samples 8 weeks after completing an mRNA vaccine series. Patients with positive nucleocapsid antibody (indicating prior infection), and/or those who reported prior COVID-19 infection, were excluded. Most patients were White (95%) and female (73%), with an average age of 48 years. Slightly more patients received the BNT162b2 vaccine than the mRNA-1273 vaccine (58% vs. 42%).
At 8 weeks, 752 out of 787 patients had detectable antibodies (95%). Antibody rates were highest among patients receiving vedolizumab monotherapy (n = 83; 99%) or ustekinumab monotherapy (n = 102; 99%), followed by mercaptopurine, azathioprine, or methotrexate monotherapy (n = 67; 97%); anti–tumor necrosis factor monotherapy (n = 270; 96%); mesalamine, sulfasalazine, or budesonide monotherapy or no medication (n = 143; 95%); and finally anti-TNF/immunosuppressive combination therapy (n = 75; 86%). Median and mean antibody titers were lowest for anti-TNF combination therapy and highest for vedolizumab.
Thirty-five patients taking corticosteroids had an antibody detection rate of 85.7% (95% CI, 70.6-93.7), compared with 95.9% (95% CI, 94.2-97.1) among nonsteroid users. In contrast, antibody detection rates were not significantly affected by age or vaccine type.
“Reassuringly, most IBD medications do not prevent an initial antibody response after SARS-CoV-2 vaccination, and this is unlike other classes of immune suppression such as B-cell depletion therapy,” Dr. Weaver concluded. “Additional data are forthcoming on a larger subset of participants in the PREVENT-COVID study which will allow for analysis of factors associated with humoral immune response and potential optimization of immunization strategies.” She described a dataset of about 500 IBD patients in which booster vaccines overcame poor antibody responses to the initial vaccine series.
‘The data we need’
Serre-yu Wong, MD, PhD, of Icahn School of Medicine at Mount Sinai, New York, agreed that the findings should offer some reassurance to patients with IBD and their care providers.
“At the end of the day we have really nice seroconversion rates for the IBD population,” Dr. Wong said.
In April 2021, Dr. Wong and the ICARUS-IBD Working Group published a similar report of 48 patients with IBD receiving biologic therapies, among whom the seroconversion rate was 100%.
“A lot of the early data, including ours, are on infusion medications, and that’s sort of a practical thing because those were the only patients we could get samples from, but [Dr. Weaver and colleagues] were able to get samples from patients not on medications, on oral medications, and on other injection medications that people can take at home, and these are really the data we need for all of our other IBD patients,” Dr. Wong said.
Dr. Wong highlighted that both trials showed some IBD patients generating “very, very high” titers, many of them above the threshold needed for donating convalescent plasma for COVID-19 treatment; still, exact titer levels needed to protect against SARS-CoV-2 infection remain unclear.
“This is going to require longitudinal studies,” Dr. Wong said. “We can’t answer that perfectly right now. We don’t know the magic level of antibodies. I don’t know if you need a titer of 1:100 or 1:1,000.”
Although postvaccination antibody testing is not recommended by the Centers for Disease Control and Prevention, Dr. Wong said that “many patients” check their titers anyway, leading to anxiety if antibodies are low or undetectable.
“I know that it’s very disconcerting sometimes when you don’t see an antibody response, and this is one of the hardest things to try to explain to patients,” Dr. Wong said. “[It’s necessary] to have a frank discussion about the fact that we don’t know the magic level of antibodies, and that there are also other parts of the immune system that we haven’t tested with antibodies. We haven’t tested the T-cell response, and we do know you can have a T-cell response even if you don’t have a B-cell response.”
Dr. Wong suggested that more work is needed to determine the impact of the IBD disease process on susceptibility to SARS-CoV-2 infection, and the rates of antibody responses for the various other vaccines being used around the world.
The PREVENT-COVID study was supported by the Leona M. and Harry B. Helmsley Charitable Trust. The investigators disclosed additional relationships with AbbVie, Johnson & Johnson, Genentech, and others. Dr. Wong reported no relevant conflicts of interest.
This article was updated Oct. 28, 2021.
Most patients with inflammatory bowel disease (IBD) develop a humoral immune response after completing an mRNA SARS-CoV-2 vaccine series, according to data from almost 800 patients.
Anti–receptor binding domain IgG antibodies specific to SARS-CoV-2 were detectable in 95% of patients, with “generally similar” results across vaccine type, age group, and medication class, apart from corticosteroid users, who had an 86% antibody detection rate, reported lead author Kimberly N. Weaver, MD, of the University of North Carolina at Chapel Hill, and colleagues.
“Patients with IBD on immunosuppressive medications have the potential for attenuated response to the SARS-CoV-2 vaccination,” Dr. Weaver said at the annual meeting of the American College of Gastroenterology.
In support of this possibility, Dr. Weaver cited two recent trials from earlier in 2021: one demonstrated blunted antibody responses in IBD patients taking infliximab, while the other showed that full vaccination was less effective at preventing SARS-CoV-2 infection among patients with IBD than nonimmunosuppressed individuals.
To better characterize antibody responses after receiving an mRNA vaccination series, Dr. Weaver and colleagues launched the PREVENT-COVID trial, including the present dataset of 787 patients with IBD older than 12 years, all of whom provided serum samples 8 weeks after completing an mRNA vaccine series. Patients with positive nucleocapsid antibody (indicating prior infection), and/or those who reported prior COVID-19 infection, were excluded. Most patients were White (95%) and female (73%), with an average age of 48 years. Slightly more patients received the BNT162b2 vaccine than the mRNA-1273 vaccine (58% vs. 42%).
At 8 weeks, 752 out of 787 patients had detectable antibodies (95%). Antibody rates were highest among patients receiving vedolizumab monotherapy (n = 83; 99%) or ustekinumab monotherapy (n = 102; 99%), followed by mercaptopurine, azathioprine, or methotrexate monotherapy (n = 67; 97%); anti–tumor necrosis factor monotherapy (n = 270; 96%); mesalamine, sulfasalazine, or budesonide monotherapy or no medication (n = 143; 95%); and finally anti-TNF/immunosuppressive combination therapy (n = 75; 86%). Median and mean antibody titers were lowest for anti-TNF combination therapy and highest for vedolizumab.
Thirty-five patients taking corticosteroids had an antibody detection rate of 85.7% (95% CI, 70.6-93.7), compared with 95.9% (95% CI, 94.2-97.1) among nonsteroid users. In contrast, antibody detection rates were not significantly affected by age or vaccine type.
“Reassuringly, most IBD medications do not prevent an initial antibody response after SARS-CoV-2 vaccination, and this is unlike other classes of immune suppression such as B-cell depletion therapy,” Dr. Weaver concluded. “Additional data are forthcoming on a larger subset of participants in the PREVENT-COVID study which will allow for analysis of factors associated with humoral immune response and potential optimization of immunization strategies.” She described a dataset of about 500 IBD patients in which booster vaccines overcame poor antibody responses to the initial vaccine series.
‘The data we need’
Serre-yu Wong, MD, PhD, of Icahn School of Medicine at Mount Sinai, New York, agreed that the findings should offer some reassurance to patients with IBD and their care providers.
“At the end of the day we have really nice seroconversion rates for the IBD population,” Dr. Wong said.
In April 2021, Dr. Wong and the ICARUS-IBD Working Group published a similar report of 48 patients with IBD receiving biologic therapies, among whom the seroconversion rate was 100%.
“A lot of the early data, including ours, are on infusion medications, and that’s sort of a practical thing because those were the only patients we could get samples from, but [Dr. Weaver and colleagues] were able to get samples from patients not on medications, on oral medications, and on other injection medications that people can take at home, and these are really the data we need for all of our other IBD patients,” Dr. Wong said.
Dr. Wong highlighted that both trials showed some IBD patients generating “very, very high” titers, many of them above the threshold needed for donating convalescent plasma for COVID-19 treatment; still, exact titer levels needed to protect against SARS-CoV-2 infection remain unclear.
“This is going to require longitudinal studies,” Dr. Wong said. “We can’t answer that perfectly right now. We don’t know the magic level of antibodies. I don’t know if you need a titer of 1:100 or 1:1,000.”
Although postvaccination antibody testing is not recommended by the Centers for Disease Control and Prevention, Dr. Wong said that “many patients” check their titers anyway, leading to anxiety if antibodies are low or undetectable.
“I know that it’s very disconcerting sometimes when you don’t see an antibody response, and this is one of the hardest things to try to explain to patients,” Dr. Wong said. “[It’s necessary] to have a frank discussion about the fact that we don’t know the magic level of antibodies, and that there are also other parts of the immune system that we haven’t tested with antibodies. We haven’t tested the T-cell response, and we do know you can have a T-cell response even if you don’t have a B-cell response.”
Dr. Wong suggested that more work is needed to determine the impact of the IBD disease process on susceptibility to SARS-CoV-2 infection, and the rates of antibody responses for the various other vaccines being used around the world.
The PREVENT-COVID study was supported by the Leona M. and Harry B. Helmsley Charitable Trust. The investigators disclosed additional relationships with AbbVie, Johnson & Johnson, Genentech, and others. Dr. Wong reported no relevant conflicts of interest.
This article was updated Oct. 28, 2021.
Most patients with inflammatory bowel disease (IBD) develop a humoral immune response after completing an mRNA SARS-CoV-2 vaccine series, according to data from almost 800 patients.
Anti–receptor binding domain IgG antibodies specific to SARS-CoV-2 were detectable in 95% of patients, with “generally similar” results across vaccine type, age group, and medication class, apart from corticosteroid users, who had an 86% antibody detection rate, reported lead author Kimberly N. Weaver, MD, of the University of North Carolina at Chapel Hill, and colleagues.
“Patients with IBD on immunosuppressive medications have the potential for attenuated response to the SARS-CoV-2 vaccination,” Dr. Weaver said at the annual meeting of the American College of Gastroenterology.
In support of this possibility, Dr. Weaver cited two recent trials from earlier in 2021: one demonstrated blunted antibody responses in IBD patients taking infliximab, while the other showed that full vaccination was less effective at preventing SARS-CoV-2 infection among patients with IBD than nonimmunosuppressed individuals.
To better characterize antibody responses after receiving an mRNA vaccination series, Dr. Weaver and colleagues launched the PREVENT-COVID trial, including the present dataset of 787 patients with IBD older than 12 years, all of whom provided serum samples 8 weeks after completing an mRNA vaccine series. Patients with positive nucleocapsid antibody (indicating prior infection), and/or those who reported prior COVID-19 infection, were excluded. Most patients were White (95%) and female (73%), with an average age of 48 years. Slightly more patients received the BNT162b2 vaccine than the mRNA-1273 vaccine (58% vs. 42%).
At 8 weeks, 752 out of 787 patients had detectable antibodies (95%). Antibody rates were highest among patients receiving vedolizumab monotherapy (n = 83; 99%) or ustekinumab monotherapy (n = 102; 99%), followed by mercaptopurine, azathioprine, or methotrexate monotherapy (n = 67; 97%); anti–tumor necrosis factor monotherapy (n = 270; 96%); mesalamine, sulfasalazine, or budesonide monotherapy or no medication (n = 143; 95%); and finally anti-TNF/immunosuppressive combination therapy (n = 75; 86%). Median and mean antibody titers were lowest for anti-TNF combination therapy and highest for vedolizumab.
Thirty-five patients taking corticosteroids had an antibody detection rate of 85.7% (95% CI, 70.6-93.7), compared with 95.9% (95% CI, 94.2-97.1) among nonsteroid users. In contrast, antibody detection rates were not significantly affected by age or vaccine type.
“Reassuringly, most IBD medications do not prevent an initial antibody response after SARS-CoV-2 vaccination, and this is unlike other classes of immune suppression such as B-cell depletion therapy,” Dr. Weaver concluded. “Additional data are forthcoming on a larger subset of participants in the PREVENT-COVID study which will allow for analysis of factors associated with humoral immune response and potential optimization of immunization strategies.” She described a dataset of about 500 IBD patients in which booster vaccines overcame poor antibody responses to the initial vaccine series.
‘The data we need’
Serre-yu Wong, MD, PhD, of Icahn School of Medicine at Mount Sinai, New York, agreed that the findings should offer some reassurance to patients with IBD and their care providers.
“At the end of the day we have really nice seroconversion rates for the IBD population,” Dr. Wong said.
In April 2021, Dr. Wong and the ICARUS-IBD Working Group published a similar report of 48 patients with IBD receiving biologic therapies, among whom the seroconversion rate was 100%.
“A lot of the early data, including ours, are on infusion medications, and that’s sort of a practical thing because those were the only patients we could get samples from, but [Dr. Weaver and colleagues] were able to get samples from patients not on medications, on oral medications, and on other injection medications that people can take at home, and these are really the data we need for all of our other IBD patients,” Dr. Wong said.
Dr. Wong highlighted that both trials showed some IBD patients generating “very, very high” titers, many of them above the threshold needed for donating convalescent plasma for COVID-19 treatment; still, exact titer levels needed to protect against SARS-CoV-2 infection remain unclear.
“This is going to require longitudinal studies,” Dr. Wong said. “We can’t answer that perfectly right now. We don’t know the magic level of antibodies. I don’t know if you need a titer of 1:100 or 1:1,000.”
Although postvaccination antibody testing is not recommended by the Centers for Disease Control and Prevention, Dr. Wong said that “many patients” check their titers anyway, leading to anxiety if antibodies are low or undetectable.
“I know that it’s very disconcerting sometimes when you don’t see an antibody response, and this is one of the hardest things to try to explain to patients,” Dr. Wong said. “[It’s necessary] to have a frank discussion about the fact that we don’t know the magic level of antibodies, and that there are also other parts of the immune system that we haven’t tested with antibodies. We haven’t tested the T-cell response, and we do know you can have a T-cell response even if you don’t have a B-cell response.”
Dr. Wong suggested that more work is needed to determine the impact of the IBD disease process on susceptibility to SARS-CoV-2 infection, and the rates of antibody responses for the various other vaccines being used around the world.
The PREVENT-COVID study was supported by the Leona M. and Harry B. Helmsley Charitable Trust. The investigators disclosed additional relationships with AbbVie, Johnson & Johnson, Genentech, and others. Dr. Wong reported no relevant conflicts of interest.
This article was updated Oct. 28, 2021.
AT ACG 2021
A pill for C. difficile works by increasing microbiome diversity
CP101, under development by Finch Therapeutics, proved more effective than a placebo in preventing recurrent infections for up to 24 weeks.
The CP101 capsules contain a powder of freeze-dried human stools from screened donors. They restore natural diversity that has been disrupted by antibiotics, said Jessica Allegretti, MD, MPH a gastroenterologist at Brigham and Women’s Hospital in Boston.
The treatment offers an alternative to fecal microbiota transplant, which can effectively treat antibiotic-resistant C. difficile infections but is difficult to standardize and administer – and doesn’t have full approval from the U.S. Food and Drug Administration, she added.
“I think this marks a moment in this space where we’re going to have better, safer, and more available options for patients,” she said in an interview. “It’s exciting.”
Dr. Allegretti is an author on three presentations of results from PRISM3, a phase 2 trial of CP101. They will be presented this week at the annual meeting of the American College of Gastroenterology. These results extend out to 24 weeks, whereas the 8-week results of this trial were presented a year ago at the same meeting.
Study details
The study enrolled 198 people who received antibiotics for recurrent C. difficile infections. Some patients had two or more recurrences, while others had only one recurrence but were 65 years of age or older.
“That was a unique aspect of this study, to see the effect of bringing a therapy like CP101 earlier in the treatment paradigm,” said Dr. Allegretti. “You can imagine for an older, frail, or more fragile patient that you would want to get rid of this [infection] earlier.”
After waiting 2-6 days for the antibiotics to wash out, the researchers randomly assigned 102 of these patients to take the CP101 pills orally and 96 to take placebo pills, both without bowel preparation.
The two groups were not significantly different in age, gender, comorbidities, the number of C. difficile recurrences, or the type of test used to diagnose the infection (PCR-based vs. toxin EIA-based).
After 8 weeks, 74.5% of those given the CP101 pills had not had a recurrence, compared with 61.5% of those given the placebo. The difference was just barely statistically significant (P = .0488).
Sixteen weeks later, the effect endured, with 73.5% of the CP101 group and 59.4% of the placebo group still free of recurrence. The statistical significance of the difference improved slightly (P = .0347).
Drug-related emergent adverse events were similar between the two groups: 16.3% for the CP101 group vs. 19.2% for the placebo group. These were mostly gastrointestinal symptoms, and none were serious.
Some of the patients received vancomycin as a first-line treatment for C. difficile infections, and the researchers wondered if the washout period was not sufficient to purge that antibiotic, leaving enough to interfere with the effectiveness of CP101.
Therefore, they separately analyzed 40 patients treated with fidaxomicin, which they expected to wash out more quickly. Among these patients, 81% who received CP101 were free of recurrences, at 8 weeks and 24 weeks. This compared with 42.1% of those who received the placebo, at both time points. This difference was more statistically significant (P = .0211).
Understanding how it works
To understand better how CP101 achieves its effects, the researchers collected stool samples from the patients and counted the number of different kinds organisms in each sample.
At baseline, the patients had about the same number, but after a week the diversity was greater in the patients treated with CP101, and that difference had increased at week 8. The researchers also found much less diversity of organisms in the stools of those patients who had recurrences of C. difficile infection.
The diversity of microbes in the successfully treated patients appeared to have been introduced by CP101. Dr. Allegretti and colleagues measured the number of organisms in the stool samples that came from CP101. They found that 96% of patients colonized by the CP101 organisms had avoided recurrence of the C. difficile infections, compared with 54.2% of those patients not colonized by these microbes.
“We now have some microbiome-based markers that show us as early as week 1 that the patient is going to be cured or not,” Dr. Allegretti said.
Based on these results, Finch plans to launch a phase 3 trial soon, she said.
The data on colonization is interesting because it has not been found with fecal microbiota transplants, said Purna Kashyap, MBBS, codirector of the Microbiome Program at the Mayo Clinic College of Medicine in Rochester, Minn., who was not involved in the study.
But to better interpret the data, it would be helpful to know more about how the placebo and CP101 groups compared at baseline with regard to medications, immunosuppression, and antibiotics used to treat the C. difficile infections, Dr. Kashyap said. He was struck by the lower cure rate in the portion of the placebo group treated with fidaxomicin.
“Overall, I think these are exciting observations based on the data but require careful review of the entire data to make sense of [them], which will happen when it goes through peer review,” he told this news organization in an email.
Several other standardized microbiota restoration products are under development, including at least two other capsules. In contrast to CP101, which is made up of whole stool, VE303 (Vedanta Biosciences) is a “rationally defined bacterial consortium,” and SER-109 (Seres Therapeutics) is a “consortium of highly purified Firmicutes spores.” VE303 has completed a phase 2 trial, and SER-109 has completed a phase 3 trial.
Dr. Allegretti is a consultant for Finch Therapeutics, which funded the trial. Dr. Kashyap has disclosed no relevant financial relationships.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
CP101, under development by Finch Therapeutics, proved more effective than a placebo in preventing recurrent infections for up to 24 weeks.
The CP101 capsules contain a powder of freeze-dried human stools from screened donors. They restore natural diversity that has been disrupted by antibiotics, said Jessica Allegretti, MD, MPH a gastroenterologist at Brigham and Women’s Hospital in Boston.
The treatment offers an alternative to fecal microbiota transplant, which can effectively treat antibiotic-resistant C. difficile infections but is difficult to standardize and administer – and doesn’t have full approval from the U.S. Food and Drug Administration, she added.
“I think this marks a moment in this space where we’re going to have better, safer, and more available options for patients,” she said in an interview. “It’s exciting.”
Dr. Allegretti is an author on three presentations of results from PRISM3, a phase 2 trial of CP101. They will be presented this week at the annual meeting of the American College of Gastroenterology. These results extend out to 24 weeks, whereas the 8-week results of this trial were presented a year ago at the same meeting.
Study details
The study enrolled 198 people who received antibiotics for recurrent C. difficile infections. Some patients had two or more recurrences, while others had only one recurrence but were 65 years of age or older.
“That was a unique aspect of this study, to see the effect of bringing a therapy like CP101 earlier in the treatment paradigm,” said Dr. Allegretti. “You can imagine for an older, frail, or more fragile patient that you would want to get rid of this [infection] earlier.”
After waiting 2-6 days for the antibiotics to wash out, the researchers randomly assigned 102 of these patients to take the CP101 pills orally and 96 to take placebo pills, both without bowel preparation.
The two groups were not significantly different in age, gender, comorbidities, the number of C. difficile recurrences, or the type of test used to diagnose the infection (PCR-based vs. toxin EIA-based).
After 8 weeks, 74.5% of those given the CP101 pills had not had a recurrence, compared with 61.5% of those given the placebo. The difference was just barely statistically significant (P = .0488).
Sixteen weeks later, the effect endured, with 73.5% of the CP101 group and 59.4% of the placebo group still free of recurrence. The statistical significance of the difference improved slightly (P = .0347).
Drug-related emergent adverse events were similar between the two groups: 16.3% for the CP101 group vs. 19.2% for the placebo group. These were mostly gastrointestinal symptoms, and none were serious.
Some of the patients received vancomycin as a first-line treatment for C. difficile infections, and the researchers wondered if the washout period was not sufficient to purge that antibiotic, leaving enough to interfere with the effectiveness of CP101.
Therefore, they separately analyzed 40 patients treated with fidaxomicin, which they expected to wash out more quickly. Among these patients, 81% who received CP101 were free of recurrences, at 8 weeks and 24 weeks. This compared with 42.1% of those who received the placebo, at both time points. This difference was more statistically significant (P = .0211).
Understanding how it works
To understand better how CP101 achieves its effects, the researchers collected stool samples from the patients and counted the number of different kinds organisms in each sample.
At baseline, the patients had about the same number, but after a week the diversity was greater in the patients treated with CP101, and that difference had increased at week 8. The researchers also found much less diversity of organisms in the stools of those patients who had recurrences of C. difficile infection.
The diversity of microbes in the successfully treated patients appeared to have been introduced by CP101. Dr. Allegretti and colleagues measured the number of organisms in the stool samples that came from CP101. They found that 96% of patients colonized by the CP101 organisms had avoided recurrence of the C. difficile infections, compared with 54.2% of those patients not colonized by these microbes.
“We now have some microbiome-based markers that show us as early as week 1 that the patient is going to be cured or not,” Dr. Allegretti said.
Based on these results, Finch plans to launch a phase 3 trial soon, she said.
The data on colonization is interesting because it has not been found with fecal microbiota transplants, said Purna Kashyap, MBBS, codirector of the Microbiome Program at the Mayo Clinic College of Medicine in Rochester, Minn., who was not involved in the study.
But to better interpret the data, it would be helpful to know more about how the placebo and CP101 groups compared at baseline with regard to medications, immunosuppression, and antibiotics used to treat the C. difficile infections, Dr. Kashyap said. He was struck by the lower cure rate in the portion of the placebo group treated with fidaxomicin.
“Overall, I think these are exciting observations based on the data but require careful review of the entire data to make sense of [them], which will happen when it goes through peer review,” he told this news organization in an email.
Several other standardized microbiota restoration products are under development, including at least two other capsules. In contrast to CP101, which is made up of whole stool, VE303 (Vedanta Biosciences) is a “rationally defined bacterial consortium,” and SER-109 (Seres Therapeutics) is a “consortium of highly purified Firmicutes spores.” VE303 has completed a phase 2 trial, and SER-109 has completed a phase 3 trial.
Dr. Allegretti is a consultant for Finch Therapeutics, which funded the trial. Dr. Kashyap has disclosed no relevant financial relationships.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
CP101, under development by Finch Therapeutics, proved more effective than a placebo in preventing recurrent infections for up to 24 weeks.
The CP101 capsules contain a powder of freeze-dried human stools from screened donors. They restore natural diversity that has been disrupted by antibiotics, said Jessica Allegretti, MD, MPH a gastroenterologist at Brigham and Women’s Hospital in Boston.
The treatment offers an alternative to fecal microbiota transplant, which can effectively treat antibiotic-resistant C. difficile infections but is difficult to standardize and administer – and doesn’t have full approval from the U.S. Food and Drug Administration, she added.
“I think this marks a moment in this space where we’re going to have better, safer, and more available options for patients,” she said in an interview. “It’s exciting.”
Dr. Allegretti is an author on three presentations of results from PRISM3, a phase 2 trial of CP101. They will be presented this week at the annual meeting of the American College of Gastroenterology. These results extend out to 24 weeks, whereas the 8-week results of this trial were presented a year ago at the same meeting.
Study details
The study enrolled 198 people who received antibiotics for recurrent C. difficile infections. Some patients had two or more recurrences, while others had only one recurrence but were 65 years of age or older.
“That was a unique aspect of this study, to see the effect of bringing a therapy like CP101 earlier in the treatment paradigm,” said Dr. Allegretti. “You can imagine for an older, frail, or more fragile patient that you would want to get rid of this [infection] earlier.”
After waiting 2-6 days for the antibiotics to wash out, the researchers randomly assigned 102 of these patients to take the CP101 pills orally and 96 to take placebo pills, both without bowel preparation.
The two groups were not significantly different in age, gender, comorbidities, the number of C. difficile recurrences, or the type of test used to diagnose the infection (PCR-based vs. toxin EIA-based).
After 8 weeks, 74.5% of those given the CP101 pills had not had a recurrence, compared with 61.5% of those given the placebo. The difference was just barely statistically significant (P = .0488).
Sixteen weeks later, the effect endured, with 73.5% of the CP101 group and 59.4% of the placebo group still free of recurrence. The statistical significance of the difference improved slightly (P = .0347).
Drug-related emergent adverse events were similar between the two groups: 16.3% for the CP101 group vs. 19.2% for the placebo group. These were mostly gastrointestinal symptoms, and none were serious.
Some of the patients received vancomycin as a first-line treatment for C. difficile infections, and the researchers wondered if the washout period was not sufficient to purge that antibiotic, leaving enough to interfere with the effectiveness of CP101.
Therefore, they separately analyzed 40 patients treated with fidaxomicin, which they expected to wash out more quickly. Among these patients, 81% who received CP101 were free of recurrences, at 8 weeks and 24 weeks. This compared with 42.1% of those who received the placebo, at both time points. This difference was more statistically significant (P = .0211).
Understanding how it works
To understand better how CP101 achieves its effects, the researchers collected stool samples from the patients and counted the number of different kinds organisms in each sample.
At baseline, the patients had about the same number, but after a week the diversity was greater in the patients treated with CP101, and that difference had increased at week 8. The researchers also found much less diversity of organisms in the stools of those patients who had recurrences of C. difficile infection.
The diversity of microbes in the successfully treated patients appeared to have been introduced by CP101. Dr. Allegretti and colleagues measured the number of organisms in the stool samples that came from CP101. They found that 96% of patients colonized by the CP101 organisms had avoided recurrence of the C. difficile infections, compared with 54.2% of those patients not colonized by these microbes.
“We now have some microbiome-based markers that show us as early as week 1 that the patient is going to be cured or not,” Dr. Allegretti said.
Based on these results, Finch plans to launch a phase 3 trial soon, she said.
The data on colonization is interesting because it has not been found with fecal microbiota transplants, said Purna Kashyap, MBBS, codirector of the Microbiome Program at the Mayo Clinic College of Medicine in Rochester, Minn., who was not involved in the study.
But to better interpret the data, it would be helpful to know more about how the placebo and CP101 groups compared at baseline with regard to medications, immunosuppression, and antibiotics used to treat the C. difficile infections, Dr. Kashyap said. He was struck by the lower cure rate in the portion of the placebo group treated with fidaxomicin.
“Overall, I think these are exciting observations based on the data but require careful review of the entire data to make sense of [them], which will happen when it goes through peer review,” he told this news organization in an email.
Several other standardized microbiota restoration products are under development, including at least two other capsules. In contrast to CP101, which is made up of whole stool, VE303 (Vedanta Biosciences) is a “rationally defined bacterial consortium,” and SER-109 (Seres Therapeutics) is a “consortium of highly purified Firmicutes spores.” VE303 has completed a phase 2 trial, and SER-109 has completed a phase 3 trial.
Dr. Allegretti is a consultant for Finch Therapeutics, which funded the trial. Dr. Kashyap has disclosed no relevant financial relationships.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
FROM ACG 2021
Stool samples meet gastric biopsies for H. pylori antibiotic resistance testing
Using stool samples to test for Helicobacter pylori antibiotic resistance provides highly similar results to those of gastric biopsy samples, which suggests that stool testing may be a safer, more convenient, and more cost-effective option, according to investigators.
Head-to-head testing for resistance-associated mutations using next-generation sequencing (NGS) showed 92% concordance between the two sample types, with 100% technical success among polymerase chain reaction (PCR)–positive stool samples, lead author Steven Moss, MD, of Brown University, Providence, R.I., and colleagues reported.
“H. pylori eradication rates have declined largely due to rising antimicrobial resistance worldwide,” Dr. Moss said at the annual meeting of the American College of Gastroenterology. “There is therefore a need for rapid, accurate, reliable antibiotic resistance testing.”
According to Dr. Moss, molecular resistance testing of gastric biopsies yields similar results to culture-based testing of gastric biopsies, but endoscopic sample collection remains inconvenient and relatively costly, so “it is not commonly performed in many GI practices.
“Whether reliable resistance testing by NGS is possible from stool samples remains unclear,” Dr. Moss said.
To explore this possibility, Dr. Moss and colleagues recruited 262 patients scheduled for upper endoscopy at four sites in the United States. From each patient, two gastric biopsies were taken, and within 2 weeks of the procedure, prior to starting anti–H. pylori therapy, one stool sample was collected.
For gastric biopsy samples, H. pylori positivity was confirmed by PCR, whereas positivity in stool samples was confirmed by both fecal antigen testing and PCR. After confirmation, NGS was conducted, with screening for resistance-associated mutations to six commonly used antibiotics: clarithromycin, levofloxacin, metronidazole, tetracycline, amoxicillin, and rifabutin.
Out of 262 patients, 73 tested positive for H. pylori via stool testing; however, 2 of these patients had inadequate gastric DNA for analysis, leaving 71 patients in the evaluable dataset. Within this group, samples from 50 patients (70.4%) had at least one resistance-association mutation.
Among all 71 individuals, 65 patients (91.5%) had fully concordant results between the two sample types. In four out of the six discordant cases, there was only one difference in antibiotic-associated mutations. Concordance ranged from 89% for metronidazole mutations to 100% for tetracycline, amoxicillin, and rifabutin mutations.
“It is now possible to rapidly obtain susceptibility data without endoscopy,” Dr. Moss concluded. “Using NGS to determine H. pylori antibiotic resistance using stool obviates the cost, inconvenience, and risks of endoscopy resistance profiling.”
Dr. Moss noted that the cost of the stool-based test, through study sponsor American Molecular Laboratories, is about $450, and that the company is “working with various insurance companies to try to get [the test] reimbursed.”
For cases of H. pylori infection without resistance testing results, Dr. Moss recommended first-line treatment with quadruple bismuth–based therapy; however, he noted that “most gastroenterologists, in all kinds of practice, are not measuring their eradication success rate ... so it’s really difficult to know if your best guess is really the appropriate treatment.”
According to Lukasz Kwapisz, MD, of Baylor College of Medicine, Houston, the concordance results are “encouraging,” and suggest that stool-based testing “could be much easier for the patient and the clinician” to find ways to eradicate H. pylori infection.
Dr. Kwapisz predicted that it will take additional successful studies, as well as real-world data, to convert clinicians to the new approach. He suggested that the transition may be gradual, like the adoption of fecal calprotectin testing.
“I don’t know if it’s one singular defining study that will tell you: ‘Okay, we all have to use this [stool-based resistance testing],’ ” he said. “It kind of happens over time – over a 2- or 3-year stretch, I would think, with positive results.”
The study was supported by American Molecular Labs. The investigators disclosed additional relationships with Takeda, Phathom, and Redhill. Dr. Kwapisz reported no conflicts of interest.
Using stool samples to test for Helicobacter pylori antibiotic resistance provides highly similar results to those of gastric biopsy samples, which suggests that stool testing may be a safer, more convenient, and more cost-effective option, according to investigators.
Head-to-head testing for resistance-associated mutations using next-generation sequencing (NGS) showed 92% concordance between the two sample types, with 100% technical success among polymerase chain reaction (PCR)–positive stool samples, lead author Steven Moss, MD, of Brown University, Providence, R.I., and colleagues reported.
“H. pylori eradication rates have declined largely due to rising antimicrobial resistance worldwide,” Dr. Moss said at the annual meeting of the American College of Gastroenterology. “There is therefore a need for rapid, accurate, reliable antibiotic resistance testing.”
According to Dr. Moss, molecular resistance testing of gastric biopsies yields similar results to culture-based testing of gastric biopsies, but endoscopic sample collection remains inconvenient and relatively costly, so “it is not commonly performed in many GI practices.
“Whether reliable resistance testing by NGS is possible from stool samples remains unclear,” Dr. Moss said.
To explore this possibility, Dr. Moss and colleagues recruited 262 patients scheduled for upper endoscopy at four sites in the United States. From each patient, two gastric biopsies were taken, and within 2 weeks of the procedure, prior to starting anti–H. pylori therapy, one stool sample was collected.
For gastric biopsy samples, H. pylori positivity was confirmed by PCR, whereas positivity in stool samples was confirmed by both fecal antigen testing and PCR. After confirmation, NGS was conducted, with screening for resistance-associated mutations to six commonly used antibiotics: clarithromycin, levofloxacin, metronidazole, tetracycline, amoxicillin, and rifabutin.
Out of 262 patients, 73 tested positive for H. pylori via stool testing; however, 2 of these patients had inadequate gastric DNA for analysis, leaving 71 patients in the evaluable dataset. Within this group, samples from 50 patients (70.4%) had at least one resistance-association mutation.
Among all 71 individuals, 65 patients (91.5%) had fully concordant results between the two sample types. In four out of the six discordant cases, there was only one difference in antibiotic-associated mutations. Concordance ranged from 89% for metronidazole mutations to 100% for tetracycline, amoxicillin, and rifabutin mutations.
“It is now possible to rapidly obtain susceptibility data without endoscopy,” Dr. Moss concluded. “Using NGS to determine H. pylori antibiotic resistance using stool obviates the cost, inconvenience, and risks of endoscopy resistance profiling.”
Dr. Moss noted that the cost of the stool-based test, through study sponsor American Molecular Laboratories, is about $450, and that the company is “working with various insurance companies to try to get [the test] reimbursed.”
For cases of H. pylori infection without resistance testing results, Dr. Moss recommended first-line treatment with quadruple bismuth–based therapy; however, he noted that “most gastroenterologists, in all kinds of practice, are not measuring their eradication success rate ... so it’s really difficult to know if your best guess is really the appropriate treatment.”
According to Lukasz Kwapisz, MD, of Baylor College of Medicine, Houston, the concordance results are “encouraging,” and suggest that stool-based testing “could be much easier for the patient and the clinician” to find ways to eradicate H. pylori infection.
Dr. Kwapisz predicted that it will take additional successful studies, as well as real-world data, to convert clinicians to the new approach. He suggested that the transition may be gradual, like the adoption of fecal calprotectin testing.
“I don’t know if it’s one singular defining study that will tell you: ‘Okay, we all have to use this [stool-based resistance testing],’ ” he said. “It kind of happens over time – over a 2- or 3-year stretch, I would think, with positive results.”
The study was supported by American Molecular Labs. The investigators disclosed additional relationships with Takeda, Phathom, and Redhill. Dr. Kwapisz reported no conflicts of interest.
Using stool samples to test for Helicobacter pylori antibiotic resistance provides highly similar results to those of gastric biopsy samples, which suggests that stool testing may be a safer, more convenient, and more cost-effective option, according to investigators.
Head-to-head testing for resistance-associated mutations using next-generation sequencing (NGS) showed 92% concordance between the two sample types, with 100% technical success among polymerase chain reaction (PCR)–positive stool samples, lead author Steven Moss, MD, of Brown University, Providence, R.I., and colleagues reported.
“H. pylori eradication rates have declined largely due to rising antimicrobial resistance worldwide,” Dr. Moss said at the annual meeting of the American College of Gastroenterology. “There is therefore a need for rapid, accurate, reliable antibiotic resistance testing.”
According to Dr. Moss, molecular resistance testing of gastric biopsies yields similar results to culture-based testing of gastric biopsies, but endoscopic sample collection remains inconvenient and relatively costly, so “it is not commonly performed in many GI practices.
“Whether reliable resistance testing by NGS is possible from stool samples remains unclear,” Dr. Moss said.
To explore this possibility, Dr. Moss and colleagues recruited 262 patients scheduled for upper endoscopy at four sites in the United States. From each patient, two gastric biopsies were taken, and within 2 weeks of the procedure, prior to starting anti–H. pylori therapy, one stool sample was collected.
For gastric biopsy samples, H. pylori positivity was confirmed by PCR, whereas positivity in stool samples was confirmed by both fecal antigen testing and PCR. After confirmation, NGS was conducted, with screening for resistance-associated mutations to six commonly used antibiotics: clarithromycin, levofloxacin, metronidazole, tetracycline, amoxicillin, and rifabutin.
Out of 262 patients, 73 tested positive for H. pylori via stool testing; however, 2 of these patients had inadequate gastric DNA for analysis, leaving 71 patients in the evaluable dataset. Within this group, samples from 50 patients (70.4%) had at least one resistance-association mutation.
Among all 71 individuals, 65 patients (91.5%) had fully concordant results between the two sample types. In four out of the six discordant cases, there was only one difference in antibiotic-associated mutations. Concordance ranged from 89% for metronidazole mutations to 100% for tetracycline, amoxicillin, and rifabutin mutations.
“It is now possible to rapidly obtain susceptibility data without endoscopy,” Dr. Moss concluded. “Using NGS to determine H. pylori antibiotic resistance using stool obviates the cost, inconvenience, and risks of endoscopy resistance profiling.”
Dr. Moss noted that the cost of the stool-based test, through study sponsor American Molecular Laboratories, is about $450, and that the company is “working with various insurance companies to try to get [the test] reimbursed.”
For cases of H. pylori infection without resistance testing results, Dr. Moss recommended first-line treatment with quadruple bismuth–based therapy; however, he noted that “most gastroenterologists, in all kinds of practice, are not measuring their eradication success rate ... so it’s really difficult to know if your best guess is really the appropriate treatment.”
According to Lukasz Kwapisz, MD, of Baylor College of Medicine, Houston, the concordance results are “encouraging,” and suggest that stool-based testing “could be much easier for the patient and the clinician” to find ways to eradicate H. pylori infection.
Dr. Kwapisz predicted that it will take additional successful studies, as well as real-world data, to convert clinicians to the new approach. He suggested that the transition may be gradual, like the adoption of fecal calprotectin testing.
“I don’t know if it’s one singular defining study that will tell you: ‘Okay, we all have to use this [stool-based resistance testing],’ ” he said. “It kind of happens over time – over a 2- or 3-year stretch, I would think, with positive results.”
The study was supported by American Molecular Labs. The investigators disclosed additional relationships with Takeda, Phathom, and Redhill. Dr. Kwapisz reported no conflicts of interest.
FROM ACG 2021
A pill for C. difficile works by increasing microbiome diversity
CP101, under development by Finch Therapeutics, proved more effective than a placebo in preventing recurrent infections for up to 24 weeks.
The CP101 capsules contain a powder of freeze-dried human stools from screened donors. They restore natural diversity that has been disrupted by antibiotics, said Jessica Allegretti, MD, MPH a gastroenterologist at Brigham and Women’s Hospital in Boston.
The treatment offers an alternative to fecal microbiota transplant, which can effectively treat antibiotic-resistant C. difficile infections but is difficult to standardize and administer – and doesn’t have full approval from the U.S. Food and Drug Administration, she added.
“I think this marks a moment in this space where we’re going to have better, safer, and more available options for patients,” she said in an interview. “It’s exciting.”
Dr. Allegretti is an author on three presentations of results from PRISM3, a phase 2 trial of CP101. They will be presented this week at the annual meeting of the American College of Gastroenterology. These results extend out to 24 weeks, whereas the 8-week results of this trial were presented a year ago at the same meeting.
Study details
The study enrolled 198 people who received antibiotics for recurrent C. difficile infections. Some patients had two or more recurrences, while others had only one recurrence but were 65 years of age or older.
“That was a unique aspect of this study, to see the effect of bringing a therapy like CP101 earlier in the treatment paradigm,” said Dr. Allegretti. “You can imagine for an older, frail, or more fragile patient that you would want to get rid of this [infection] earlier.”
After waiting 2-6 days for the antibiotics to wash out, the researchers randomly assigned 102 of these patients to take the CP101 pills orally and 96 to take placebo pills, both without bowel preparation.
The two groups were not significantly different in age, gender, comorbidities, the number of C. difficile recurrences, or the type of test used to diagnose the infection (PCR-based vs. toxin EIA-based).
After 8 weeks, 74.5% of those given the CP101 pills had not had a recurrence, compared with 61.5% of those given the placebo. The difference was just barely statistically significant (P = .0488).
Sixteen weeks later, the effect endured, with 73.5% of the CP101 group and 59.4% of the placebo group still free of recurrence. The statistical significance of the difference improved slightly (P = .0347).
Drug-related emergent adverse events were similar between the two groups: 16.3% for the CP101 group vs. 19.2% for the placebo group. These were mostly gastrointestinal symptoms, and none were serious.
Some of the patients received vancomycin as a first-line treatment for C. difficile infections, and the researchers wondered if the washout period was not sufficient to purge that antibiotic, leaving enough to interfere with the effectiveness of CP101.
Therefore, they separately analyzed 40 patients treated with fidaxomicin, which they expected to wash out more quickly. Among these patients, 81% who received CP101 were free of recurrences, at 8 weeks and 24 weeks. This compared with 42.1% of those who received the placebo, at both time points. This difference was more statistically significant (P = .0211).
Understanding how it works
To understand better how CP101 achieves its effects, the researchers collected stool samples from the patients and counted the number of different kinds organisms in each sample.
At baseline, the patients had about the same number, but after a week the diversity was greater in the patients treated with CP101, and that difference had increased at week 8. The researchers also found much less diversity of organisms in the stools of those patients who had recurrences of C. difficile infection.
The diversity of microbes in the successfully treated patients appeared to have been introduced by CP101. Dr. Allegretti and colleagues measured the number of organisms in the stool samples that came from CP101. They found that 96% of patients colonized by the CP101 organisms had avoided recurrence of the C. difficile infections, compared with 54.2% of those patients not colonized by these microbes.
“We now have some microbiome-based markers that show us as early as week 1 that the patient is going to be cured or not,” Dr. Allegretti said.
Based on these results, Finch plans to launch a phase 3 trial soon, she said.
The data on colonization is interesting because it has not been found with fecal microbiota transplants, said Purna Kashyap, MBBS, codirector of the Microbiome Program at the Mayo Clinic College of Medicine in Rochester, Minn., who was not involved in the study.
But to better interpret the data, it would be helpful to know more about how the placebo and CP101 groups compared at baseline with regard to medications, immunosuppression, and antibiotics used to treat the C. difficile infections, Dr. Kashyap said. He was struck by the lower cure rate in the portion of the placebo group treated with fidaxomicin.
“Overall, I think these are exciting observations based on the data but require careful review of the entire data to make sense of [them], which will happen when it goes through peer review,” he told this news organization in an email.
Several other standardized microbiota restoration products are under development, including at least two other capsules. In contrast to CP101, which is made up of whole stool, VE303 (Vedanta Biosciences) is a “rationally defined bacterial consortium,” and SER-109 (Seres Therapeutics) is a “consortium of highly purified Firmicutes spores.” VE303 has completed a phase 2 trial, and SER-109 has completed a phase 3 trial.
Dr. Allegretti is a consultant for Finch Therapeutics, which funded the trial. Dr. Kashyap has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CP101, under development by Finch Therapeutics, proved more effective than a placebo in preventing recurrent infections for up to 24 weeks.
The CP101 capsules contain a powder of freeze-dried human stools from screened donors. They restore natural diversity that has been disrupted by antibiotics, said Jessica Allegretti, MD, MPH a gastroenterologist at Brigham and Women’s Hospital in Boston.
The treatment offers an alternative to fecal microbiota transplant, which can effectively treat antibiotic-resistant C. difficile infections but is difficult to standardize and administer – and doesn’t have full approval from the U.S. Food and Drug Administration, she added.
“I think this marks a moment in this space where we’re going to have better, safer, and more available options for patients,” she said in an interview. “It’s exciting.”
Dr. Allegretti is an author on three presentations of results from PRISM3, a phase 2 trial of CP101. They will be presented this week at the annual meeting of the American College of Gastroenterology. These results extend out to 24 weeks, whereas the 8-week results of this trial were presented a year ago at the same meeting.
Study details
The study enrolled 198 people who received antibiotics for recurrent C. difficile infections. Some patients had two or more recurrences, while others had only one recurrence but were 65 years of age or older.
“That was a unique aspect of this study, to see the effect of bringing a therapy like CP101 earlier in the treatment paradigm,” said Dr. Allegretti. “You can imagine for an older, frail, or more fragile patient that you would want to get rid of this [infection] earlier.”
After waiting 2-6 days for the antibiotics to wash out, the researchers randomly assigned 102 of these patients to take the CP101 pills orally and 96 to take placebo pills, both without bowel preparation.
The two groups were not significantly different in age, gender, comorbidities, the number of C. difficile recurrences, or the type of test used to diagnose the infection (PCR-based vs. toxin EIA-based).
After 8 weeks, 74.5% of those given the CP101 pills had not had a recurrence, compared with 61.5% of those given the placebo. The difference was just barely statistically significant (P = .0488).
Sixteen weeks later, the effect endured, with 73.5% of the CP101 group and 59.4% of the placebo group still free of recurrence. The statistical significance of the difference improved slightly (P = .0347).
Drug-related emergent adverse events were similar between the two groups: 16.3% for the CP101 group vs. 19.2% for the placebo group. These were mostly gastrointestinal symptoms, and none were serious.
Some of the patients received vancomycin as a first-line treatment for C. difficile infections, and the researchers wondered if the washout period was not sufficient to purge that antibiotic, leaving enough to interfere with the effectiveness of CP101.
Therefore, they separately analyzed 40 patients treated with fidaxomicin, which they expected to wash out more quickly. Among these patients, 81% who received CP101 were free of recurrences, at 8 weeks and 24 weeks. This compared with 42.1% of those who received the placebo, at both time points. This difference was more statistically significant (P = .0211).
Understanding how it works
To understand better how CP101 achieves its effects, the researchers collected stool samples from the patients and counted the number of different kinds organisms in each sample.
At baseline, the patients had about the same number, but after a week the diversity was greater in the patients treated with CP101, and that difference had increased at week 8. The researchers also found much less diversity of organisms in the stools of those patients who had recurrences of C. difficile infection.
The diversity of microbes in the successfully treated patients appeared to have been introduced by CP101. Dr. Allegretti and colleagues measured the number of organisms in the stool samples that came from CP101. They found that 96% of patients colonized by the CP101 organisms had avoided recurrence of the C. difficile infections, compared with 54.2% of those patients not colonized by these microbes.
“We now have some microbiome-based markers that show us as early as week 1 that the patient is going to be cured or not,” Dr. Allegretti said.
Based on these results, Finch plans to launch a phase 3 trial soon, she said.
The data on colonization is interesting because it has not been found with fecal microbiota transplants, said Purna Kashyap, MBBS, codirector of the Microbiome Program at the Mayo Clinic College of Medicine in Rochester, Minn., who was not involved in the study.
But to better interpret the data, it would be helpful to know more about how the placebo and CP101 groups compared at baseline with regard to medications, immunosuppression, and antibiotics used to treat the C. difficile infections, Dr. Kashyap said. He was struck by the lower cure rate in the portion of the placebo group treated with fidaxomicin.
“Overall, I think these are exciting observations based on the data but require careful review of the entire data to make sense of [them], which will happen when it goes through peer review,” he told this news organization in an email.
Several other standardized microbiota restoration products are under development, including at least two other capsules. In contrast to CP101, which is made up of whole stool, VE303 (Vedanta Biosciences) is a “rationally defined bacterial consortium,” and SER-109 (Seres Therapeutics) is a “consortium of highly purified Firmicutes spores.” VE303 has completed a phase 2 trial, and SER-109 has completed a phase 3 trial.
Dr. Allegretti is a consultant for Finch Therapeutics, which funded the trial. Dr. Kashyap has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CP101, under development by Finch Therapeutics, proved more effective than a placebo in preventing recurrent infections for up to 24 weeks.
The CP101 capsules contain a powder of freeze-dried human stools from screened donors. They restore natural diversity that has been disrupted by antibiotics, said Jessica Allegretti, MD, MPH a gastroenterologist at Brigham and Women’s Hospital in Boston.
The treatment offers an alternative to fecal microbiota transplant, which can effectively treat antibiotic-resistant C. difficile infections but is difficult to standardize and administer – and doesn’t have full approval from the U.S. Food and Drug Administration, she added.
“I think this marks a moment in this space where we’re going to have better, safer, and more available options for patients,” she said in an interview. “It’s exciting.”
Dr. Allegretti is an author on three presentations of results from PRISM3, a phase 2 trial of CP101. They will be presented this week at the annual meeting of the American College of Gastroenterology. These results extend out to 24 weeks, whereas the 8-week results of this trial were presented a year ago at the same meeting.
Study details
The study enrolled 198 people who received antibiotics for recurrent C. difficile infections. Some patients had two or more recurrences, while others had only one recurrence but were 65 years of age or older.
“That was a unique aspect of this study, to see the effect of bringing a therapy like CP101 earlier in the treatment paradigm,” said Dr. Allegretti. “You can imagine for an older, frail, or more fragile patient that you would want to get rid of this [infection] earlier.”
After waiting 2-6 days for the antibiotics to wash out, the researchers randomly assigned 102 of these patients to take the CP101 pills orally and 96 to take placebo pills, both without bowel preparation.
The two groups were not significantly different in age, gender, comorbidities, the number of C. difficile recurrences, or the type of test used to diagnose the infection (PCR-based vs. toxin EIA-based).
After 8 weeks, 74.5% of those given the CP101 pills had not had a recurrence, compared with 61.5% of those given the placebo. The difference was just barely statistically significant (P = .0488).
Sixteen weeks later, the effect endured, with 73.5% of the CP101 group and 59.4% of the placebo group still free of recurrence. The statistical significance of the difference improved slightly (P = .0347).
Drug-related emergent adverse events were similar between the two groups: 16.3% for the CP101 group vs. 19.2% for the placebo group. These were mostly gastrointestinal symptoms, and none were serious.
Some of the patients received vancomycin as a first-line treatment for C. difficile infections, and the researchers wondered if the washout period was not sufficient to purge that antibiotic, leaving enough to interfere with the effectiveness of CP101.
Therefore, they separately analyzed 40 patients treated with fidaxomicin, which they expected to wash out more quickly. Among these patients, 81% who received CP101 were free of recurrences, at 8 weeks and 24 weeks. This compared with 42.1% of those who received the placebo, at both time points. This difference was more statistically significant (P = .0211).
Understanding how it works
To understand better how CP101 achieves its effects, the researchers collected stool samples from the patients and counted the number of different kinds organisms in each sample.
At baseline, the patients had about the same number, but after a week the diversity was greater in the patients treated with CP101, and that difference had increased at week 8. The researchers also found much less diversity of organisms in the stools of those patients who had recurrences of C. difficile infection.
The diversity of microbes in the successfully treated patients appeared to have been introduced by CP101. Dr. Allegretti and colleagues measured the number of organisms in the stool samples that came from CP101. They found that 96% of patients colonized by the CP101 organisms had avoided recurrence of the C. difficile infections, compared with 54.2% of those patients not colonized by these microbes.
“We now have some microbiome-based markers that show us as early as week 1 that the patient is going to be cured or not,” Dr. Allegretti said.
Based on these results, Finch plans to launch a phase 3 trial soon, she said.
The data on colonization is interesting because it has not been found with fecal microbiota transplants, said Purna Kashyap, MBBS, codirector of the Microbiome Program at the Mayo Clinic College of Medicine in Rochester, Minn., who was not involved in the study.
But to better interpret the data, it would be helpful to know more about how the placebo and CP101 groups compared at baseline with regard to medications, immunosuppression, and antibiotics used to treat the C. difficile infections, Dr. Kashyap said. He was struck by the lower cure rate in the portion of the placebo group treated with fidaxomicin.
“Overall, I think these are exciting observations based on the data but require careful review of the entire data to make sense of [them], which will happen when it goes through peer review,” he told this news organization in an email.
Several other standardized microbiota restoration products are under development, including at least two other capsules. In contrast to CP101, which is made up of whole stool, VE303 (Vedanta Biosciences) is a “rationally defined bacterial consortium,” and SER-109 (Seres Therapeutics) is a “consortium of highly purified Firmicutes spores.” VE303 has completed a phase 2 trial, and SER-109 has completed a phase 3 trial.
Dr. Allegretti is a consultant for Finch Therapeutics, which funded the trial. Dr. Kashyap has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACG 2021