User login
At-home monitoring device can predict Crohn’s disease flares
CHICAGO – , including an inability to signal a change in disease activity without laboratory testing or before symptoms arise.
A new device developed at Massachusetts Institute of Technology could change all that.
Using data collected via a passive at-home monitoring device (Emerald sensor, Emerald Innovations Inc.), researchers found that increases in breathing rate, more awakenings at night, and slower walking speed accurately predicted that a person’s Crohn’s disease activity was about to flare, according to a study presented May 7 at Digestive Disease Week® (DDW) 2023.
In some cases, the prediction of a flare came up to 25 days sooner than via traditional measures.
“In order to provide optimal care, providers need to monitor patients closely with regard to accurate active disease.” said Joshua Korzenik, MD, a gastroenterologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School in Boston. “The problem is the clinical symptoms are not accurate.”
Tracking flares with technology
Traditionally, measuring flares in Crohn’s disease activity depends on imaging, colonoscopy, and/or laboratory measures of calprotectin or other biomarkers. These approaches can be costly, can involve delays, and can carry risks, Dr. Korzenik said.
“They are also a single snapshot in time,” he added.
To determine how well a noninvasive device could perform, investigators enrolled 120 people with 105 continuing in the study long enough to be evaluable; 44 people whose Crohn’s disease was in remission, 35 with active Crohn’s disease, as well as 26 healthy controls. Among those with Crohn’s disease, 83% were on biologic therapy.
The groups were matched for age and gender, with a mean age of 47 years and mean disease duration of 13 years.
People with certain medical conditions were excluded, as was anyone who owned a large dog that sometimes slept in bed with them because that might throw off the readings.
The participants put the device – which resembles a closed laptop or a large Wi-Fi router – in their homes and were monitored for a mean 306 days. Participants wore an ankle bracelet the first 2 weeks of the study so the device could learn to distinguish them from others in the home.
The device sent out radio waves with frequencies like Wi-Fi for researchers to measure the factors that may be associated with flares, such as sleep quality and cycles, breathing rate, and gait speed.
Traditional clinical measures based on blood and stool samples, along with patient-reported outcome surveys, were taken to compare with the accuracy of the device.
Data from the device were collected and transmitted securely to a cloud database without any interaction from the participant. Data included information on more than 25,000 nights of sleep, 200,000 hours of breathing signals, and 400,000 measurements of walking speed.
Sleep quality and cycles were straightforward, as was breathing rate. But gait speed was a little more complicated to measure. To illustrate, Dr. Korzenik showed the layout of an example apartment with data on how someone moved around. To distill the data, the researchers focused on one path in the home, relatively straight and not obstructed by furniture, and limited the measurements to a certain amount of time. People who spent more time at home during the COVID-19 pandemic did not skew the results, according to Dr. Korzenik, who added that it wasn’t total time walking around but a snippet in time.
A variety of sleep, breathing, and mobility metrics extracted by the device were integrated to assess disease activity. Investigators noted that during flares, sleep quality decreased, and more nocturnal awakenings occurred. They also found that gait speed slowed, and respiratory rate increased with flares.
When the investigators looked at sleep as a function of disease activity in the patient-reported surveys, they found a significant difference between people in remission and those with active disease. For example, people with active disease had a greater number of awakenings at night (P = .0016), less REM sleep at night (P = .0000), and less time in deep sleep (P = .000) compared with those in remission.
The technology “can identify flares with a predictive value that approaches fecal calprotectin,” Dr. Korzenik said.
Machine learning was used to look at severity of disease vs. fecal calprotectin values and “showed the data could be used as a marker of disease,” he added.
Use of a remote monitor, the comparison of validated vs. conventional data, and the large dataset were among the strengths of the study. The single-center design and exclusion of people with some comorbidities are potential limitations.
Further studies are warranted to confirm these findings and guide optimal care of people with Crohn’s disease, the investigators noted.
Earlier detection, earlier intervention
“The study is really important,” said session comoderator Raymond K. Cross Jr., MD, professor of medicine and director of the IBD program at the University of Maryland, Baltimore.
Monitoring devices like this “could be very useful,” Dr. Cross said. “It is not invasive, unless you consider a device in your house invasive. But, to me, I don’t think a box in my bedroom would be unnerving to me.”
Dr. Cross shared a couple of caveats. “The one devil in the details is always going to be cost,” he said. Also, it’s unclear who will read and interpret all the data generated by the device among “providers who are already overwhelmed with the volume of information.”
Moving forward, a device like this could offer multiple uses, Dr. Cross noted. If the device can detect relapses earlier, physicians could intervene sooner, he said. Also, the device could potentially flag people who are not taking their medications as recommended, or it could be used as a guide to optimize treatment response.
Whether data from the device could indicate when it’s appropriate to reduce the frequency or dose of medication or even when to withdraw therapy would be “really aspirational,” Dr. Cross added.
The study was funded by The Leona M. and Harry B. Helmsley Charitable Trust. Dr. Korzenik and Dr. Cross report no relevant financial relationships.
DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
A version of this article first appeared on Medscape.com.
CHICAGO – , including an inability to signal a change in disease activity without laboratory testing or before symptoms arise.
A new device developed at Massachusetts Institute of Technology could change all that.
Using data collected via a passive at-home monitoring device (Emerald sensor, Emerald Innovations Inc.), researchers found that increases in breathing rate, more awakenings at night, and slower walking speed accurately predicted that a person’s Crohn’s disease activity was about to flare, according to a study presented May 7 at Digestive Disease Week® (DDW) 2023.
In some cases, the prediction of a flare came up to 25 days sooner than via traditional measures.
“In order to provide optimal care, providers need to monitor patients closely with regard to accurate active disease.” said Joshua Korzenik, MD, a gastroenterologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School in Boston. “The problem is the clinical symptoms are not accurate.”
Tracking flares with technology
Traditionally, measuring flares in Crohn’s disease activity depends on imaging, colonoscopy, and/or laboratory measures of calprotectin or other biomarkers. These approaches can be costly, can involve delays, and can carry risks, Dr. Korzenik said.
“They are also a single snapshot in time,” he added.
To determine how well a noninvasive device could perform, investigators enrolled 120 people with 105 continuing in the study long enough to be evaluable; 44 people whose Crohn’s disease was in remission, 35 with active Crohn’s disease, as well as 26 healthy controls. Among those with Crohn’s disease, 83% were on biologic therapy.
The groups were matched for age and gender, with a mean age of 47 years and mean disease duration of 13 years.
People with certain medical conditions were excluded, as was anyone who owned a large dog that sometimes slept in bed with them because that might throw off the readings.
The participants put the device – which resembles a closed laptop or a large Wi-Fi router – in their homes and were monitored for a mean 306 days. Participants wore an ankle bracelet the first 2 weeks of the study so the device could learn to distinguish them from others in the home.
The device sent out radio waves with frequencies like Wi-Fi for researchers to measure the factors that may be associated with flares, such as sleep quality and cycles, breathing rate, and gait speed.
Traditional clinical measures based on blood and stool samples, along with patient-reported outcome surveys, were taken to compare with the accuracy of the device.
Data from the device were collected and transmitted securely to a cloud database without any interaction from the participant. Data included information on more than 25,000 nights of sleep, 200,000 hours of breathing signals, and 400,000 measurements of walking speed.
Sleep quality and cycles were straightforward, as was breathing rate. But gait speed was a little more complicated to measure. To illustrate, Dr. Korzenik showed the layout of an example apartment with data on how someone moved around. To distill the data, the researchers focused on one path in the home, relatively straight and not obstructed by furniture, and limited the measurements to a certain amount of time. People who spent more time at home during the COVID-19 pandemic did not skew the results, according to Dr. Korzenik, who added that it wasn’t total time walking around but a snippet in time.
A variety of sleep, breathing, and mobility metrics extracted by the device were integrated to assess disease activity. Investigators noted that during flares, sleep quality decreased, and more nocturnal awakenings occurred. They also found that gait speed slowed, and respiratory rate increased with flares.
When the investigators looked at sleep as a function of disease activity in the patient-reported surveys, they found a significant difference between people in remission and those with active disease. For example, people with active disease had a greater number of awakenings at night (P = .0016), less REM sleep at night (P = .0000), and less time in deep sleep (P = .000) compared with those in remission.
The technology “can identify flares with a predictive value that approaches fecal calprotectin,” Dr. Korzenik said.
Machine learning was used to look at severity of disease vs. fecal calprotectin values and “showed the data could be used as a marker of disease,” he added.
Use of a remote monitor, the comparison of validated vs. conventional data, and the large dataset were among the strengths of the study. The single-center design and exclusion of people with some comorbidities are potential limitations.
Further studies are warranted to confirm these findings and guide optimal care of people with Crohn’s disease, the investigators noted.
Earlier detection, earlier intervention
“The study is really important,” said session comoderator Raymond K. Cross Jr., MD, professor of medicine and director of the IBD program at the University of Maryland, Baltimore.
Monitoring devices like this “could be very useful,” Dr. Cross said. “It is not invasive, unless you consider a device in your house invasive. But, to me, I don’t think a box in my bedroom would be unnerving to me.”
Dr. Cross shared a couple of caveats. “The one devil in the details is always going to be cost,” he said. Also, it’s unclear who will read and interpret all the data generated by the device among “providers who are already overwhelmed with the volume of information.”
Moving forward, a device like this could offer multiple uses, Dr. Cross noted. If the device can detect relapses earlier, physicians could intervene sooner, he said. Also, the device could potentially flag people who are not taking their medications as recommended, or it could be used as a guide to optimize treatment response.
Whether data from the device could indicate when it’s appropriate to reduce the frequency or dose of medication or even when to withdraw therapy would be “really aspirational,” Dr. Cross added.
The study was funded by The Leona M. and Harry B. Helmsley Charitable Trust. Dr. Korzenik and Dr. Cross report no relevant financial relationships.
DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
A version of this article first appeared on Medscape.com.
CHICAGO – , including an inability to signal a change in disease activity without laboratory testing or before symptoms arise.
A new device developed at Massachusetts Institute of Technology could change all that.
Using data collected via a passive at-home monitoring device (Emerald sensor, Emerald Innovations Inc.), researchers found that increases in breathing rate, more awakenings at night, and slower walking speed accurately predicted that a person’s Crohn’s disease activity was about to flare, according to a study presented May 7 at Digestive Disease Week® (DDW) 2023.
In some cases, the prediction of a flare came up to 25 days sooner than via traditional measures.
“In order to provide optimal care, providers need to monitor patients closely with regard to accurate active disease.” said Joshua Korzenik, MD, a gastroenterologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School in Boston. “The problem is the clinical symptoms are not accurate.”
Tracking flares with technology
Traditionally, measuring flares in Crohn’s disease activity depends on imaging, colonoscopy, and/or laboratory measures of calprotectin or other biomarkers. These approaches can be costly, can involve delays, and can carry risks, Dr. Korzenik said.
“They are also a single snapshot in time,” he added.
To determine how well a noninvasive device could perform, investigators enrolled 120 people with 105 continuing in the study long enough to be evaluable; 44 people whose Crohn’s disease was in remission, 35 with active Crohn’s disease, as well as 26 healthy controls. Among those with Crohn’s disease, 83% were on biologic therapy.
The groups were matched for age and gender, with a mean age of 47 years and mean disease duration of 13 years.
People with certain medical conditions were excluded, as was anyone who owned a large dog that sometimes slept in bed with them because that might throw off the readings.
The participants put the device – which resembles a closed laptop or a large Wi-Fi router – in their homes and were monitored for a mean 306 days. Participants wore an ankle bracelet the first 2 weeks of the study so the device could learn to distinguish them from others in the home.
The device sent out radio waves with frequencies like Wi-Fi for researchers to measure the factors that may be associated with flares, such as sleep quality and cycles, breathing rate, and gait speed.
Traditional clinical measures based on blood and stool samples, along with patient-reported outcome surveys, were taken to compare with the accuracy of the device.
Data from the device were collected and transmitted securely to a cloud database without any interaction from the participant. Data included information on more than 25,000 nights of sleep, 200,000 hours of breathing signals, and 400,000 measurements of walking speed.
Sleep quality and cycles were straightforward, as was breathing rate. But gait speed was a little more complicated to measure. To illustrate, Dr. Korzenik showed the layout of an example apartment with data on how someone moved around. To distill the data, the researchers focused on one path in the home, relatively straight and not obstructed by furniture, and limited the measurements to a certain amount of time. People who spent more time at home during the COVID-19 pandemic did not skew the results, according to Dr. Korzenik, who added that it wasn’t total time walking around but a snippet in time.
A variety of sleep, breathing, and mobility metrics extracted by the device were integrated to assess disease activity. Investigators noted that during flares, sleep quality decreased, and more nocturnal awakenings occurred. They also found that gait speed slowed, and respiratory rate increased with flares.
When the investigators looked at sleep as a function of disease activity in the patient-reported surveys, they found a significant difference between people in remission and those with active disease. For example, people with active disease had a greater number of awakenings at night (P = .0016), less REM sleep at night (P = .0000), and less time in deep sleep (P = .000) compared with those in remission.
The technology “can identify flares with a predictive value that approaches fecal calprotectin,” Dr. Korzenik said.
Machine learning was used to look at severity of disease vs. fecal calprotectin values and “showed the data could be used as a marker of disease,” he added.
Use of a remote monitor, the comparison of validated vs. conventional data, and the large dataset were among the strengths of the study. The single-center design and exclusion of people with some comorbidities are potential limitations.
Further studies are warranted to confirm these findings and guide optimal care of people with Crohn’s disease, the investigators noted.
Earlier detection, earlier intervention
“The study is really important,” said session comoderator Raymond K. Cross Jr., MD, professor of medicine and director of the IBD program at the University of Maryland, Baltimore.
Monitoring devices like this “could be very useful,” Dr. Cross said. “It is not invasive, unless you consider a device in your house invasive. But, to me, I don’t think a box in my bedroom would be unnerving to me.”
Dr. Cross shared a couple of caveats. “The one devil in the details is always going to be cost,” he said. Also, it’s unclear who will read and interpret all the data generated by the device among “providers who are already overwhelmed with the volume of information.”
Moving forward, a device like this could offer multiple uses, Dr. Cross noted. If the device can detect relapses earlier, physicians could intervene sooner, he said. Also, the device could potentially flag people who are not taking their medications as recommended, or it could be used as a guide to optimize treatment response.
Whether data from the device could indicate when it’s appropriate to reduce the frequency or dose of medication or even when to withdraw therapy would be “really aspirational,” Dr. Cross added.
The study was funded by The Leona M. and Harry B. Helmsley Charitable Trust. Dr. Korzenik and Dr. Cross report no relevant financial relationships.
DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
A version of this article first appeared on Medscape.com.
AT DDW 2023
Fewer discontinuations with infliximab vs. vedolizumab for UC maintenance therapy
CHICAGO – an updated meta-analysis of randomized clinical trials reveals.
At 1 year, 2% of people taking the anti–tumor necrosis factor (TNF) agent infliximab discontinued for lack of efficacy, compared with 24% of patients taking vedolizumab (Entyvio), an integrin receptor antagonist.
The safety profile of each agent is also important.
“We know that vedolizumab has less safety risks than an anti-TNF agent, but we also have the gut feeling that the anti-TNF agents are more efficacious,” lead author Marc Ferrante, MD, said in an interview.
“Of course, I don’t think we can really say that vedolizumab is the safest and infliximab is not safe, but there is some difference,” added Dr. Ferrante, a professor in the department of gastroenterology and hepatology, University Hospitals Leuven (Belgium).
The study was presented as a poster at the annual Digestive Disease Week (DDW).
The researchers conducted a pooled analysis of six randomized controlled trials from the past 10 years. They analyzed the NOR-SWITCH IV Q8W, the NCT02883452 SC Q2W, and LIBERTY-UC SC Q2W studies for infliximab, and the VISIBLE 1 SC Q2W, GEMINI 1 IV Q4W, and VARSITY IV Q8W trials for vedolizumab.
Their work expands on a meta-analysis by Dr. Ferrante and colleagues presented at DDW 2022. They added the 1-year results from the phase 3 LIBERTY-UC study to increase the number of participants taking infliximab or an infliximab biosimilar.
“Luckily, the results are very similar,” Dr. Ferrante said, and noted that they support previous findings that discontinuation of infliximab was lower than that of vedolizumab.
Most of the patients in the infliximab group were taking an infliximab biosimilar, whereas the vedolizumab group received the originator. Dr. Ferrante noted that the economic considerations involved in deciding between a biosimilar and an originator were not part of the research but that “there will be a difference in costs.”
Same mechanism, different route
The novel finding from the study includes the subcutaneous form of infliximab, which is not yet available in the United States, noted Joshua M. Steinberg, MD, director of inflammatory bowel disease at Gastroenterology of the Rockies, Denver, when asked to comment on the study. Currently, intravenously administered infliximab and vedolizumab are available in the United States.
A better comparator in the future would be looking at subcutaneous forms of both agents, especially “with the impending launch of subcutaneous vedolizumab in the United States,” said Dr. Steinberg, who is also a clinical instructor of medicine at the University of Colorado at Denver, Aurora.
He added that it’s reassuring overall that with a newer mode of administration but same mechanism of action, it is still feasible and durable for at least 1 year.
“The general consensus is that in terms of our biologics, vedolizumab is the safest because of its targeted mechanism of action. But sometimes the ‘safest choice’ isn’t the best choice,” Dr. Steinberg said. “I think in the right patient, the most effective treatment is going to be the one that works the best, and that’s not going to be universal.”
The study was sponsored by Celltrion, which makes an infliximab biosimilar. Dr. Ferrante receives honoraria as a consultant and speaker for Celltrion. Dr. Steinberg reported no relevant financial relationships.
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and The Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
CHICAGO – an updated meta-analysis of randomized clinical trials reveals.
At 1 year, 2% of people taking the anti–tumor necrosis factor (TNF) agent infliximab discontinued for lack of efficacy, compared with 24% of patients taking vedolizumab (Entyvio), an integrin receptor antagonist.
The safety profile of each agent is also important.
“We know that vedolizumab has less safety risks than an anti-TNF agent, but we also have the gut feeling that the anti-TNF agents are more efficacious,” lead author Marc Ferrante, MD, said in an interview.
“Of course, I don’t think we can really say that vedolizumab is the safest and infliximab is not safe, but there is some difference,” added Dr. Ferrante, a professor in the department of gastroenterology and hepatology, University Hospitals Leuven (Belgium).
The study was presented as a poster at the annual Digestive Disease Week (DDW).
The researchers conducted a pooled analysis of six randomized controlled trials from the past 10 years. They analyzed the NOR-SWITCH IV Q8W, the NCT02883452 SC Q2W, and LIBERTY-UC SC Q2W studies for infliximab, and the VISIBLE 1 SC Q2W, GEMINI 1 IV Q4W, and VARSITY IV Q8W trials for vedolizumab.
Their work expands on a meta-analysis by Dr. Ferrante and colleagues presented at DDW 2022. They added the 1-year results from the phase 3 LIBERTY-UC study to increase the number of participants taking infliximab or an infliximab biosimilar.
“Luckily, the results are very similar,” Dr. Ferrante said, and noted that they support previous findings that discontinuation of infliximab was lower than that of vedolizumab.
Most of the patients in the infliximab group were taking an infliximab biosimilar, whereas the vedolizumab group received the originator. Dr. Ferrante noted that the economic considerations involved in deciding between a biosimilar and an originator were not part of the research but that “there will be a difference in costs.”
Same mechanism, different route
The novel finding from the study includes the subcutaneous form of infliximab, which is not yet available in the United States, noted Joshua M. Steinberg, MD, director of inflammatory bowel disease at Gastroenterology of the Rockies, Denver, when asked to comment on the study. Currently, intravenously administered infliximab and vedolizumab are available in the United States.
A better comparator in the future would be looking at subcutaneous forms of both agents, especially “with the impending launch of subcutaneous vedolizumab in the United States,” said Dr. Steinberg, who is also a clinical instructor of medicine at the University of Colorado at Denver, Aurora.
He added that it’s reassuring overall that with a newer mode of administration but same mechanism of action, it is still feasible and durable for at least 1 year.
“The general consensus is that in terms of our biologics, vedolizumab is the safest because of its targeted mechanism of action. But sometimes the ‘safest choice’ isn’t the best choice,” Dr. Steinberg said. “I think in the right patient, the most effective treatment is going to be the one that works the best, and that’s not going to be universal.”
The study was sponsored by Celltrion, which makes an infliximab biosimilar. Dr. Ferrante receives honoraria as a consultant and speaker for Celltrion. Dr. Steinberg reported no relevant financial relationships.
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and The Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
CHICAGO – an updated meta-analysis of randomized clinical trials reveals.
At 1 year, 2% of people taking the anti–tumor necrosis factor (TNF) agent infliximab discontinued for lack of efficacy, compared with 24% of patients taking vedolizumab (Entyvio), an integrin receptor antagonist.
The safety profile of each agent is also important.
“We know that vedolizumab has less safety risks than an anti-TNF agent, but we also have the gut feeling that the anti-TNF agents are more efficacious,” lead author Marc Ferrante, MD, said in an interview.
“Of course, I don’t think we can really say that vedolizumab is the safest and infliximab is not safe, but there is some difference,” added Dr. Ferrante, a professor in the department of gastroenterology and hepatology, University Hospitals Leuven (Belgium).
The study was presented as a poster at the annual Digestive Disease Week (DDW).
The researchers conducted a pooled analysis of six randomized controlled trials from the past 10 years. They analyzed the NOR-SWITCH IV Q8W, the NCT02883452 SC Q2W, and LIBERTY-UC SC Q2W studies for infliximab, and the VISIBLE 1 SC Q2W, GEMINI 1 IV Q4W, and VARSITY IV Q8W trials for vedolizumab.
Their work expands on a meta-analysis by Dr. Ferrante and colleagues presented at DDW 2022. They added the 1-year results from the phase 3 LIBERTY-UC study to increase the number of participants taking infliximab or an infliximab biosimilar.
“Luckily, the results are very similar,” Dr. Ferrante said, and noted that they support previous findings that discontinuation of infliximab was lower than that of vedolizumab.
Most of the patients in the infliximab group were taking an infliximab biosimilar, whereas the vedolizumab group received the originator. Dr. Ferrante noted that the economic considerations involved in deciding between a biosimilar and an originator were not part of the research but that “there will be a difference in costs.”
Same mechanism, different route
The novel finding from the study includes the subcutaneous form of infliximab, which is not yet available in the United States, noted Joshua M. Steinberg, MD, director of inflammatory bowel disease at Gastroenterology of the Rockies, Denver, when asked to comment on the study. Currently, intravenously administered infliximab and vedolizumab are available in the United States.
A better comparator in the future would be looking at subcutaneous forms of both agents, especially “with the impending launch of subcutaneous vedolizumab in the United States,” said Dr. Steinberg, who is also a clinical instructor of medicine at the University of Colorado at Denver, Aurora.
He added that it’s reassuring overall that with a newer mode of administration but same mechanism of action, it is still feasible and durable for at least 1 year.
“The general consensus is that in terms of our biologics, vedolizumab is the safest because of its targeted mechanism of action. But sometimes the ‘safest choice’ isn’t the best choice,” Dr. Steinberg said. “I think in the right patient, the most effective treatment is going to be the one that works the best, and that’s not going to be universal.”
The study was sponsored by Celltrion, which makes an infliximab biosimilar. Dr. Ferrante receives honoraria as a consultant and speaker for Celltrion. Dr. Steinberg reported no relevant financial relationships.
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and The Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
AT DDW 2023
Endoscopic sleeve gastroplasty plus obesity drugs add up to more weight loss
CHICAGO – Antiobesity medications and endoscopic sleeve gastroplasty (ESG) are popular strategies for weight loss on their own. Now researchers are looking at what happens when you combine them.
In a study presented at the annual Digestive Disease Week® (DDW), they found
Starting medication within 6 months of ESG was more ideal than other timing intervals. Initiating medical therapy more than 6 months before ESG was associated with less weight loss.
In the single-center, retrospective study, 224 patients were enrolled, of whom 34% were on monotherapy (ESG alone), 31% had combination therapy (medication prescribed within 6 months prior to or after ESG), and 35% had sequential therapy (medication more than 6 months prior to or after ESG).
Most patients were female, ranging from 74% to 95% of each group, and baseline BMI ranged from a mean 37.5 kg/m2 to 40.1 kg/m2.
The medications involved in the study were phentermine, phentermine/topiramate extended release (Qsymia), orlistat (Xenical, Alli), bupropion/naltrexone ER (Contrave), or the glucagonlike peptide–1 receptor agonist (GLP-1RA) liraglutide (Saxenda, Victoza) or semaglutide (Ozempic, Wegovy, Rybelsus). Of the patients who underwent combination therapy, 30% were prescribed a regimen that included a GLP-1RA. Of the patients who underwent sequential therapy, 81% were prescribed a medication first and 19% underwent ESG first.
At 1 year, the greatest total weight loss was a mean 23.7% with the combination of ESG and a GLP-1RA. Total weight loss was 18% with ESG plus a non–GLP-1RA medication. ESG alone led to 17.3%. Sequential therapy that began with ESG yielded 14.7% total weight loss, whereas sequential therapy that began with medication first resulted in 12% weight loss.
It’s possible that gastroplasty performed second was less impressive because the medications were very effective, and there was not as much weight to lose, said Pichamol Jirapinyo, MD, MPH, a bariatric endoscopist at Brigham and Women’s Hospital, Boston, and lead author of the study.
Researchers stopped medication therapy if people did not experience at least 5% total weight loss after 3 months on a maintenance dose.
Waiting for weight loss to start to plateau after gastroplasty might be an ideal time to add weight loss medication, said Dr. Jirapinyo. “Usually when I see them at 3 months, I plot how fast their weight loss has been. If it’s been going down [steadily], we do not offer an antiobesity medication until I see them again at 6 months.”
The serious adverse event (SAE) rate associated with ESG was similar among the three cohorts: 2.6% with monotherapy group, 1.4% with combination therapy, and 1.3% with sequential therapy. SAEs associated with antiobesity medication occurred in 1.3% of the sequential therapy group and was not reported in either of the other two groups.
“I certainly think combination therapy should be more effective than just gastroplasty alone and is probably better,” said Gregory L. Austin, MD, session comoderator and a gastroenterologist at the UCHealth Digestive Health Center, Denver.
“Whether you start immediately or wait 3 months afterwards is a question that still needs to be answered,” he added.
Dr. Austin agreed that taking an antiobesity medicine more than 6 months before gastroplasty might be associated with enough weight loss to make the gastroplasty look less effective.
He also noted that the study “doesn’t really address the question of whether you should offer gastroplasty to somebody who’s been on [medication] for more than 6 months because you probably still should if they haven’t achieved an appropriate weight loss that’s associated with reduced comorbidity risk going forward.”
Different study, similar result
In a second study, also presented at DDW 2023, investigators looked at timing of liraglutide for weight loss in a randomized controlled trial. They found that administration of GLP-1RA right after transoral outlet reduction endoscopy (TORe) in people with a history of Roux-en-Y gastric bypass extended weight loss longer than a placebo injection. This strategy was also favorable versus waiting to give liraglutide 1 year later.
The researchers randomly assigned 51 people to get weekly subcutaneous liraglutide injections following TORe for 12 months, then placebo injections for 12 months. They assigned 58 patients to receive weekly placebo injections following TORe for 12 months, then liraglutide injections for 12 months.
At 12 months following the procedure, total body weight loss (TBWL) among participants receiving liraglutide was about 22%, compared with about 14% among patients receiving placebo. At 24 months following the procedure (12 months after crossover), TBWL among patients in the liraglutide-first group was almost 35%, compared with about 24% in the placebo-first/liraglutide-second group.
There was a durable effect associated with liraglutide even after switching to placebo, said Ali Lahooti, lead study author and second-year medical student at Weill Cornell Medicine, New York.
“There did seem to be a better benefit of starting on it for the first year and then stopping it,” Dr. Austin noted.
These two studies come at a time when the debate over the timing of different obesity interventions continues. Some experts believe weight loss medications can help with the rebound in weight that some people experience months after bariatric surgery, for example.
‘Wave of the future’
The study by Dr. Jirapinyo and colleagues is “really exciting and interesting,” said Linda S. Lee, MD, medical director of endoscopy, Brigham and Women’s Hospital, Boston, when asked to comment.
Medication begun within 6 months of the endoscopic procedure “led to superior outcomes, compared to just endoscopy alone,” Dr. Lee said. “I think that’s really the wave of the future as far as treating patients with obesity issues. We clearly know that diet and exercise alone for most people is not good enough. Of course, we have surgery, but we also realize that with surgery sometimes the weight starts to creep back up over time.”
Dr. Lee noted that the study was limited because it was retrospective. Ideally, it would be good if future, prospective research randomly assigns people to endoscopy alone or endoscopy plus medication.
Dr. Lee also noted there is a limited number of bariatric endoscopists. By the time people with obesity get to a specialist, they’ve likely tried diet and exercise and “probably have seen all the commercials for these different medications. I think the reality is that most people will ask their primary care physicians about antiobesity medication.
“From my point of view, as long as the medicine is safe and not harming them, then let’s do both of them together,” Dr. Lee added.
Dr. Lee also mentioned another study (Abstract Mo1898) presented at DDW 2023 that showed total weight loss with endoscopic sleeve gastroplasty was durable over 10 years. Follow-up was with only seven patients, however.
Larger numbers are needed to confirm the finding, but it’s “exciting,” she said.
Dr. Jirapinyo receives grant/research support from Apollo Endosurgery, Fractyl, and USGI Medical, and is a consultant for ERBE, GI Dynamics, and Spatz Medical. Dr. Lahooti, Dr. Austin, and Dr. Lee reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
CHICAGO – Antiobesity medications and endoscopic sleeve gastroplasty (ESG) are popular strategies for weight loss on their own. Now researchers are looking at what happens when you combine them.
In a study presented at the annual Digestive Disease Week® (DDW), they found
Starting medication within 6 months of ESG was more ideal than other timing intervals. Initiating medical therapy more than 6 months before ESG was associated with less weight loss.
In the single-center, retrospective study, 224 patients were enrolled, of whom 34% were on monotherapy (ESG alone), 31% had combination therapy (medication prescribed within 6 months prior to or after ESG), and 35% had sequential therapy (medication more than 6 months prior to or after ESG).
Most patients were female, ranging from 74% to 95% of each group, and baseline BMI ranged from a mean 37.5 kg/m2 to 40.1 kg/m2.
The medications involved in the study were phentermine, phentermine/topiramate extended release (Qsymia), orlistat (Xenical, Alli), bupropion/naltrexone ER (Contrave), or the glucagonlike peptide–1 receptor agonist (GLP-1RA) liraglutide (Saxenda, Victoza) or semaglutide (Ozempic, Wegovy, Rybelsus). Of the patients who underwent combination therapy, 30% were prescribed a regimen that included a GLP-1RA. Of the patients who underwent sequential therapy, 81% were prescribed a medication first and 19% underwent ESG first.
At 1 year, the greatest total weight loss was a mean 23.7% with the combination of ESG and a GLP-1RA. Total weight loss was 18% with ESG plus a non–GLP-1RA medication. ESG alone led to 17.3%. Sequential therapy that began with ESG yielded 14.7% total weight loss, whereas sequential therapy that began with medication first resulted in 12% weight loss.
It’s possible that gastroplasty performed second was less impressive because the medications were very effective, and there was not as much weight to lose, said Pichamol Jirapinyo, MD, MPH, a bariatric endoscopist at Brigham and Women’s Hospital, Boston, and lead author of the study.
Researchers stopped medication therapy if people did not experience at least 5% total weight loss after 3 months on a maintenance dose.
Waiting for weight loss to start to plateau after gastroplasty might be an ideal time to add weight loss medication, said Dr. Jirapinyo. “Usually when I see them at 3 months, I plot how fast their weight loss has been. If it’s been going down [steadily], we do not offer an antiobesity medication until I see them again at 6 months.”
The serious adverse event (SAE) rate associated with ESG was similar among the three cohorts: 2.6% with monotherapy group, 1.4% with combination therapy, and 1.3% with sequential therapy. SAEs associated with antiobesity medication occurred in 1.3% of the sequential therapy group and was not reported in either of the other two groups.
“I certainly think combination therapy should be more effective than just gastroplasty alone and is probably better,” said Gregory L. Austin, MD, session comoderator and a gastroenterologist at the UCHealth Digestive Health Center, Denver.
“Whether you start immediately or wait 3 months afterwards is a question that still needs to be answered,” he added.
Dr. Austin agreed that taking an antiobesity medicine more than 6 months before gastroplasty might be associated with enough weight loss to make the gastroplasty look less effective.
He also noted that the study “doesn’t really address the question of whether you should offer gastroplasty to somebody who’s been on [medication] for more than 6 months because you probably still should if they haven’t achieved an appropriate weight loss that’s associated with reduced comorbidity risk going forward.”
Different study, similar result
In a second study, also presented at DDW 2023, investigators looked at timing of liraglutide for weight loss in a randomized controlled trial. They found that administration of GLP-1RA right after transoral outlet reduction endoscopy (TORe) in people with a history of Roux-en-Y gastric bypass extended weight loss longer than a placebo injection. This strategy was also favorable versus waiting to give liraglutide 1 year later.
The researchers randomly assigned 51 people to get weekly subcutaneous liraglutide injections following TORe for 12 months, then placebo injections for 12 months. They assigned 58 patients to receive weekly placebo injections following TORe for 12 months, then liraglutide injections for 12 months.
At 12 months following the procedure, total body weight loss (TBWL) among participants receiving liraglutide was about 22%, compared with about 14% among patients receiving placebo. At 24 months following the procedure (12 months after crossover), TBWL among patients in the liraglutide-first group was almost 35%, compared with about 24% in the placebo-first/liraglutide-second group.
There was a durable effect associated with liraglutide even after switching to placebo, said Ali Lahooti, lead study author and second-year medical student at Weill Cornell Medicine, New York.
“There did seem to be a better benefit of starting on it for the first year and then stopping it,” Dr. Austin noted.
These two studies come at a time when the debate over the timing of different obesity interventions continues. Some experts believe weight loss medications can help with the rebound in weight that some people experience months after bariatric surgery, for example.
‘Wave of the future’
The study by Dr. Jirapinyo and colleagues is “really exciting and interesting,” said Linda S. Lee, MD, medical director of endoscopy, Brigham and Women’s Hospital, Boston, when asked to comment.
Medication begun within 6 months of the endoscopic procedure “led to superior outcomes, compared to just endoscopy alone,” Dr. Lee said. “I think that’s really the wave of the future as far as treating patients with obesity issues. We clearly know that diet and exercise alone for most people is not good enough. Of course, we have surgery, but we also realize that with surgery sometimes the weight starts to creep back up over time.”
Dr. Lee noted that the study was limited because it was retrospective. Ideally, it would be good if future, prospective research randomly assigns people to endoscopy alone or endoscopy plus medication.
Dr. Lee also noted there is a limited number of bariatric endoscopists. By the time people with obesity get to a specialist, they’ve likely tried diet and exercise and “probably have seen all the commercials for these different medications. I think the reality is that most people will ask their primary care physicians about antiobesity medication.
“From my point of view, as long as the medicine is safe and not harming them, then let’s do both of them together,” Dr. Lee added.
Dr. Lee also mentioned another study (Abstract Mo1898) presented at DDW 2023 that showed total weight loss with endoscopic sleeve gastroplasty was durable over 10 years. Follow-up was with only seven patients, however.
Larger numbers are needed to confirm the finding, but it’s “exciting,” she said.
Dr. Jirapinyo receives grant/research support from Apollo Endosurgery, Fractyl, and USGI Medical, and is a consultant for ERBE, GI Dynamics, and Spatz Medical. Dr. Lahooti, Dr. Austin, and Dr. Lee reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
CHICAGO – Antiobesity medications and endoscopic sleeve gastroplasty (ESG) are popular strategies for weight loss on their own. Now researchers are looking at what happens when you combine them.
In a study presented at the annual Digestive Disease Week® (DDW), they found
Starting medication within 6 months of ESG was more ideal than other timing intervals. Initiating medical therapy more than 6 months before ESG was associated with less weight loss.
In the single-center, retrospective study, 224 patients were enrolled, of whom 34% were on monotherapy (ESG alone), 31% had combination therapy (medication prescribed within 6 months prior to or after ESG), and 35% had sequential therapy (medication more than 6 months prior to or after ESG).
Most patients were female, ranging from 74% to 95% of each group, and baseline BMI ranged from a mean 37.5 kg/m2 to 40.1 kg/m2.
The medications involved in the study were phentermine, phentermine/topiramate extended release (Qsymia), orlistat (Xenical, Alli), bupropion/naltrexone ER (Contrave), or the glucagonlike peptide–1 receptor agonist (GLP-1RA) liraglutide (Saxenda, Victoza) or semaglutide (Ozempic, Wegovy, Rybelsus). Of the patients who underwent combination therapy, 30% were prescribed a regimen that included a GLP-1RA. Of the patients who underwent sequential therapy, 81% were prescribed a medication first and 19% underwent ESG first.
At 1 year, the greatest total weight loss was a mean 23.7% with the combination of ESG and a GLP-1RA. Total weight loss was 18% with ESG plus a non–GLP-1RA medication. ESG alone led to 17.3%. Sequential therapy that began with ESG yielded 14.7% total weight loss, whereas sequential therapy that began with medication first resulted in 12% weight loss.
It’s possible that gastroplasty performed second was less impressive because the medications were very effective, and there was not as much weight to lose, said Pichamol Jirapinyo, MD, MPH, a bariatric endoscopist at Brigham and Women’s Hospital, Boston, and lead author of the study.
Researchers stopped medication therapy if people did not experience at least 5% total weight loss after 3 months on a maintenance dose.
Waiting for weight loss to start to plateau after gastroplasty might be an ideal time to add weight loss medication, said Dr. Jirapinyo. “Usually when I see them at 3 months, I plot how fast their weight loss has been. If it’s been going down [steadily], we do not offer an antiobesity medication until I see them again at 6 months.”
The serious adverse event (SAE) rate associated with ESG was similar among the three cohorts: 2.6% with monotherapy group, 1.4% with combination therapy, and 1.3% with sequential therapy. SAEs associated with antiobesity medication occurred in 1.3% of the sequential therapy group and was not reported in either of the other two groups.
“I certainly think combination therapy should be more effective than just gastroplasty alone and is probably better,” said Gregory L. Austin, MD, session comoderator and a gastroenterologist at the UCHealth Digestive Health Center, Denver.
“Whether you start immediately or wait 3 months afterwards is a question that still needs to be answered,” he added.
Dr. Austin agreed that taking an antiobesity medicine more than 6 months before gastroplasty might be associated with enough weight loss to make the gastroplasty look less effective.
He also noted that the study “doesn’t really address the question of whether you should offer gastroplasty to somebody who’s been on [medication] for more than 6 months because you probably still should if they haven’t achieved an appropriate weight loss that’s associated with reduced comorbidity risk going forward.”
Different study, similar result
In a second study, also presented at DDW 2023, investigators looked at timing of liraglutide for weight loss in a randomized controlled trial. They found that administration of GLP-1RA right after transoral outlet reduction endoscopy (TORe) in people with a history of Roux-en-Y gastric bypass extended weight loss longer than a placebo injection. This strategy was also favorable versus waiting to give liraglutide 1 year later.
The researchers randomly assigned 51 people to get weekly subcutaneous liraglutide injections following TORe for 12 months, then placebo injections for 12 months. They assigned 58 patients to receive weekly placebo injections following TORe for 12 months, then liraglutide injections for 12 months.
At 12 months following the procedure, total body weight loss (TBWL) among participants receiving liraglutide was about 22%, compared with about 14% among patients receiving placebo. At 24 months following the procedure (12 months after crossover), TBWL among patients in the liraglutide-first group was almost 35%, compared with about 24% in the placebo-first/liraglutide-second group.
There was a durable effect associated with liraglutide even after switching to placebo, said Ali Lahooti, lead study author and second-year medical student at Weill Cornell Medicine, New York.
“There did seem to be a better benefit of starting on it for the first year and then stopping it,” Dr. Austin noted.
These two studies come at a time when the debate over the timing of different obesity interventions continues. Some experts believe weight loss medications can help with the rebound in weight that some people experience months after bariatric surgery, for example.
‘Wave of the future’
The study by Dr. Jirapinyo and colleagues is “really exciting and interesting,” said Linda S. Lee, MD, medical director of endoscopy, Brigham and Women’s Hospital, Boston, when asked to comment.
Medication begun within 6 months of the endoscopic procedure “led to superior outcomes, compared to just endoscopy alone,” Dr. Lee said. “I think that’s really the wave of the future as far as treating patients with obesity issues. We clearly know that diet and exercise alone for most people is not good enough. Of course, we have surgery, but we also realize that with surgery sometimes the weight starts to creep back up over time.”
Dr. Lee noted that the study was limited because it was retrospective. Ideally, it would be good if future, prospective research randomly assigns people to endoscopy alone or endoscopy plus medication.
Dr. Lee also noted there is a limited number of bariatric endoscopists. By the time people with obesity get to a specialist, they’ve likely tried diet and exercise and “probably have seen all the commercials for these different medications. I think the reality is that most people will ask their primary care physicians about antiobesity medication.
“From my point of view, as long as the medicine is safe and not harming them, then let’s do both of them together,” Dr. Lee added.
Dr. Lee also mentioned another study (Abstract Mo1898) presented at DDW 2023 that showed total weight loss with endoscopic sleeve gastroplasty was durable over 10 years. Follow-up was with only seven patients, however.
Larger numbers are needed to confirm the finding, but it’s “exciting,” she said.
Dr. Jirapinyo receives grant/research support from Apollo Endosurgery, Fractyl, and USGI Medical, and is a consultant for ERBE, GI Dynamics, and Spatz Medical. Dr. Lahooti, Dr. Austin, and Dr. Lee reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
AT DDW 2023
A nod to the future of AGA
CHICAGO – It’s been 125 years since the founding of the American Gastroenterological Association (AGA). It’s gone from a small organization in which gastroenterology wasn’t even a known medical specialty, to an organization that grants millions of dollars in research funding each year.
He spoke with optimism about gastroenterology’s future during his presidential address on May 8 at the annual Digestive Disease Week® (DDW) meeting in Chicago.
“I congratulate the AGA on its quasquicentennial, or 125th anniversary,” said Dr. Carethers, who is distinguished professor of medicine and vice chancellor for health sciences at the University of California, San Diego.
The AGA was founded in 1897 by Detroit-based physician Charles Aaron, MD. His passion was gastroenterology, but at that point, it wasn’t an established medical discipline. Dr. Aaron, Max Einhorn, MD, and 8 other colleagues formed the American Gastroenterological Association. Today, with nearly 16,000 members, the organization has become a driving force in improving the care of patients with gastrointestinal conditions.
Among AGA’s accomplishments since its founding: In 1940, the American Board of Internal Medicine certified gastroenterology as a subspecialty. Three years later, the first issue of Gastroenterology, the AGA’s flagship journal, was published. And, in 1971, the very first Digestive Disease Week® meeting took place.
In terms of medical advances that have been made since those early years, the list is vast: From the description of ileitis in 1932 by Burril B. Crohn, MD, in 1932 to the discovery of the hepatitis B surface antigen in 1965 and the more recent discovery of germline mutations in DNA mismatch repair genes as a cause of Lynch syndrome.
Dr. Carethers outlined goals for the future, including building a leadership team that is “reflective of our practice here in the United States,” Dr. Carethers said. Creating a culturally and gender diverse leadership team will only strengthen the organization and the practice of gastroenterology. The AGA’s first female president, Sarah Jordan, MD, was named in 1942, and since then, the AGA has been led by women and men from different ethnic backgrounds, including himself, who is AGA’s first president of African American heritage.
The AGA has committed to a number of diversity and equity objectives, including the AGA Equity Project, an initiative launched in 2020 whose goal is to achieve equity and eradicate disparities in digestive diseases with a focus on justice and equity, research and funding, workforce and leadership, recognition of the achievements of people of color, unconscious bias, and engagement with early career members.
“I am not only excited about the diversity and equity objectives within our specialty ... but also the innovation and things to come for our specialty,” Dr. Carethers said.
Securing funding for early-stage innovations in medicine can be difficult across medical disciplines, including gastroenterology. So, last year, the AGA, with Varia Ventures, launched the GI Opportunity Fund 1 to support early-stage GI-based companies. The goal is to raise $25 million for the initial fund. Through the AGA’s Center for GI Innovation and Technology and the AGA Tech Summit, early-stage companies may have new funding opportunities.
And, through the AGA Research Foundation, the organization will continue to support clinical research. Last year, $2.6 million in grants were awarded to investigators.
Dr. Carethers is a board director at Avantor, a life sciences supply company.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
CHICAGO – It’s been 125 years since the founding of the American Gastroenterological Association (AGA). It’s gone from a small organization in which gastroenterology wasn’t even a known medical specialty, to an organization that grants millions of dollars in research funding each year.
He spoke with optimism about gastroenterology’s future during his presidential address on May 8 at the annual Digestive Disease Week® (DDW) meeting in Chicago.
“I congratulate the AGA on its quasquicentennial, or 125th anniversary,” said Dr. Carethers, who is distinguished professor of medicine and vice chancellor for health sciences at the University of California, San Diego.
The AGA was founded in 1897 by Detroit-based physician Charles Aaron, MD. His passion was gastroenterology, but at that point, it wasn’t an established medical discipline. Dr. Aaron, Max Einhorn, MD, and 8 other colleagues formed the American Gastroenterological Association. Today, with nearly 16,000 members, the organization has become a driving force in improving the care of patients with gastrointestinal conditions.
Among AGA’s accomplishments since its founding: In 1940, the American Board of Internal Medicine certified gastroenterology as a subspecialty. Three years later, the first issue of Gastroenterology, the AGA’s flagship journal, was published. And, in 1971, the very first Digestive Disease Week® meeting took place.
In terms of medical advances that have been made since those early years, the list is vast: From the description of ileitis in 1932 by Burril B. Crohn, MD, in 1932 to the discovery of the hepatitis B surface antigen in 1965 and the more recent discovery of germline mutations in DNA mismatch repair genes as a cause of Lynch syndrome.
Dr. Carethers outlined goals for the future, including building a leadership team that is “reflective of our practice here in the United States,” Dr. Carethers said. Creating a culturally and gender diverse leadership team will only strengthen the organization and the practice of gastroenterology. The AGA’s first female president, Sarah Jordan, MD, was named in 1942, and since then, the AGA has been led by women and men from different ethnic backgrounds, including himself, who is AGA’s first president of African American heritage.
The AGA has committed to a number of diversity and equity objectives, including the AGA Equity Project, an initiative launched in 2020 whose goal is to achieve equity and eradicate disparities in digestive diseases with a focus on justice and equity, research and funding, workforce and leadership, recognition of the achievements of people of color, unconscious bias, and engagement with early career members.
“I am not only excited about the diversity and equity objectives within our specialty ... but also the innovation and things to come for our specialty,” Dr. Carethers said.
Securing funding for early-stage innovations in medicine can be difficult across medical disciplines, including gastroenterology. So, last year, the AGA, with Varia Ventures, launched the GI Opportunity Fund 1 to support early-stage GI-based companies. The goal is to raise $25 million for the initial fund. Through the AGA’s Center for GI Innovation and Technology and the AGA Tech Summit, early-stage companies may have new funding opportunities.
And, through the AGA Research Foundation, the organization will continue to support clinical research. Last year, $2.6 million in grants were awarded to investigators.
Dr. Carethers is a board director at Avantor, a life sciences supply company.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
CHICAGO – It’s been 125 years since the founding of the American Gastroenterological Association (AGA). It’s gone from a small organization in which gastroenterology wasn’t even a known medical specialty, to an organization that grants millions of dollars in research funding each year.
He spoke with optimism about gastroenterology’s future during his presidential address on May 8 at the annual Digestive Disease Week® (DDW) meeting in Chicago.
“I congratulate the AGA on its quasquicentennial, or 125th anniversary,” said Dr. Carethers, who is distinguished professor of medicine and vice chancellor for health sciences at the University of California, San Diego.
The AGA was founded in 1897 by Detroit-based physician Charles Aaron, MD. His passion was gastroenterology, but at that point, it wasn’t an established medical discipline. Dr. Aaron, Max Einhorn, MD, and 8 other colleagues formed the American Gastroenterological Association. Today, with nearly 16,000 members, the organization has become a driving force in improving the care of patients with gastrointestinal conditions.
Among AGA’s accomplishments since its founding: In 1940, the American Board of Internal Medicine certified gastroenterology as a subspecialty. Three years later, the first issue of Gastroenterology, the AGA’s flagship journal, was published. And, in 1971, the very first Digestive Disease Week® meeting took place.
In terms of medical advances that have been made since those early years, the list is vast: From the description of ileitis in 1932 by Burril B. Crohn, MD, in 1932 to the discovery of the hepatitis B surface antigen in 1965 and the more recent discovery of germline mutations in DNA mismatch repair genes as a cause of Lynch syndrome.
Dr. Carethers outlined goals for the future, including building a leadership team that is “reflective of our practice here in the United States,” Dr. Carethers said. Creating a culturally and gender diverse leadership team will only strengthen the organization and the practice of gastroenterology. The AGA’s first female president, Sarah Jordan, MD, was named in 1942, and since then, the AGA has been led by women and men from different ethnic backgrounds, including himself, who is AGA’s first president of African American heritage.
The AGA has committed to a number of diversity and equity objectives, including the AGA Equity Project, an initiative launched in 2020 whose goal is to achieve equity and eradicate disparities in digestive diseases with a focus on justice and equity, research and funding, workforce and leadership, recognition of the achievements of people of color, unconscious bias, and engagement with early career members.
“I am not only excited about the diversity and equity objectives within our specialty ... but also the innovation and things to come for our specialty,” Dr. Carethers said.
Securing funding for early-stage innovations in medicine can be difficult across medical disciplines, including gastroenterology. So, last year, the AGA, with Varia Ventures, launched the GI Opportunity Fund 1 to support early-stage GI-based companies. The goal is to raise $25 million for the initial fund. Through the AGA’s Center for GI Innovation and Technology and the AGA Tech Summit, early-stage companies may have new funding opportunities.
And, through the AGA Research Foundation, the organization will continue to support clinical research. Last year, $2.6 million in grants were awarded to investigators.
Dr. Carethers is a board director at Avantor, a life sciences supply company.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
AT DDW 2023
Study of environmental impact of GI endoscopy finds room for improvement
CHICAGO – according to Madhav Desai, MD, MPH, assistant professor of medicine at the University of Minnesota, Minneapolis. About 20% of the waste, most of which went to landfills, was potentially recyclable, he said in a presentation given at the annual Digestive Disease Week® meeting.
Gastrointestinal endoscopies are critical for the screening, diagnosis, and treatment of a variety of gastrointestinal conditions. But like other medical procedures, endoscopies are a source of environmental waste, including plastic, sharps, personal protective equipment (PPE), and cleaning supplies, and also energy waste.
“This all goes back to the damage that mankind is inflicting on the environment in general, with the health care sector as one of the top contributors to plastic waste generation, landfills and water wastage,” Dr. Desai said. “Endoscopies, with their numerous benefits, substantially increase waste generation through landfill waste and liquid consumption and waste through the cleaning of endoscopes. We have a responsibility to look into this topic.”
To prospectively assess total waste generation from their institution, Dr. Desai, who was with the Kansas City (Mo.) Veterans Administration Medical Center, when the research was conducted, collected data on the items used in 450 consecutive procedures from May to June 2022. The data included procedure type, accessory use, intravenous tubing, numbers of biopsy jars, linens, PPE, and more, beginning at the point of patient entry to the endoscopy unit until discharge. They also collected data on waste generation related to reprocessing after each procedure and daily energy use (including endoscopy equipment, lights, and computers). With an eye toward finding opportunities to improve and maximize waste recycling, they stratified waste into the three categories of biohazardous, nonbiohazardous, or potentially recyclable.
“We found that the total waste generated during the time period was 1,398.6 kg, with more than half of it, 61.6%, going directly to landfill,” Dr. Desai said in an interview. “That’s an amount that an average family in the U.S. would use for 2 months. That’s a huge amount.”
Most waste consists of sharps
Exactly one-third was biohazard waste and 5.1% was sharps, they found. A single procedure, on average, sent 2.19 kg of waste to landfill. Extrapolated to 1 year, the waste total amounts to 9,189 kg (equivalent to just over 10 U.S. tons) and per 100 procedures to 219 kg (about 483 pounds).
They estimated 20% of the landfill waste was potentially recyclable (such as plastic CO2 tubing, O2 connector, syringes, etc.), which could reduce the total landfill burden by 8.6 kg per day or 2,580 kg per year (or 61 kg per 100 procedures). Reprocessing endoscopes generated 194 gallons of liquid waste (735.26 kg) per day or 1,385 gallons per 100 procedures.
Turning to energy consumption, Dr. Desai reported that daily use in the endoscopy unit was 277.1 kW-hours (equivalent to 8.2 gallons of gasoline), adding up to about 1,980 kW per 100 procedures. “That 100-procedure amount is the equivalent of the energy used for an average fuel efficiency car to travel 1,200 miles, the distance from Seattle to San Diego,” he said.
“One next step,” Dr. Desai said, “is getting help from GI societies to come together and have endoscopy units track their own performance. You need benchmarks so that you can determine how good an endoscopist you are with respect to waste.”
He commented further:“We all owe it to the environment. And, we have all witnessed what Mother Nature can do to you.”
Working on the potentially recyclable materials that account for 20% of the total waste would be a simple initial step to reduce waste going to landfills, Dr. Desai and colleagues concluded in the meeting abstract. “These data could serve as an actionable model for health systems to reduce total waste generation and move toward environmentally sustainable endoscopy units,” they wrote.
The authors reported no disclosures.
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and The Society for Surgery of the Alimentary Tract.
CHICAGO – according to Madhav Desai, MD, MPH, assistant professor of medicine at the University of Minnesota, Minneapolis. About 20% of the waste, most of which went to landfills, was potentially recyclable, he said in a presentation given at the annual Digestive Disease Week® meeting.
Gastrointestinal endoscopies are critical for the screening, diagnosis, and treatment of a variety of gastrointestinal conditions. But like other medical procedures, endoscopies are a source of environmental waste, including plastic, sharps, personal protective equipment (PPE), and cleaning supplies, and also energy waste.
“This all goes back to the damage that mankind is inflicting on the environment in general, with the health care sector as one of the top contributors to plastic waste generation, landfills and water wastage,” Dr. Desai said. “Endoscopies, with their numerous benefits, substantially increase waste generation through landfill waste and liquid consumption and waste through the cleaning of endoscopes. We have a responsibility to look into this topic.”
To prospectively assess total waste generation from their institution, Dr. Desai, who was with the Kansas City (Mo.) Veterans Administration Medical Center, when the research was conducted, collected data on the items used in 450 consecutive procedures from May to June 2022. The data included procedure type, accessory use, intravenous tubing, numbers of biopsy jars, linens, PPE, and more, beginning at the point of patient entry to the endoscopy unit until discharge. They also collected data on waste generation related to reprocessing after each procedure and daily energy use (including endoscopy equipment, lights, and computers). With an eye toward finding opportunities to improve and maximize waste recycling, they stratified waste into the three categories of biohazardous, nonbiohazardous, or potentially recyclable.
“We found that the total waste generated during the time period was 1,398.6 kg, with more than half of it, 61.6%, going directly to landfill,” Dr. Desai said in an interview. “That’s an amount that an average family in the U.S. would use for 2 months. That’s a huge amount.”
Most waste consists of sharps
Exactly one-third was biohazard waste and 5.1% was sharps, they found. A single procedure, on average, sent 2.19 kg of waste to landfill. Extrapolated to 1 year, the waste total amounts to 9,189 kg (equivalent to just over 10 U.S. tons) and per 100 procedures to 219 kg (about 483 pounds).
They estimated 20% of the landfill waste was potentially recyclable (such as plastic CO2 tubing, O2 connector, syringes, etc.), which could reduce the total landfill burden by 8.6 kg per day or 2,580 kg per year (or 61 kg per 100 procedures). Reprocessing endoscopes generated 194 gallons of liquid waste (735.26 kg) per day or 1,385 gallons per 100 procedures.
Turning to energy consumption, Dr. Desai reported that daily use in the endoscopy unit was 277.1 kW-hours (equivalent to 8.2 gallons of gasoline), adding up to about 1,980 kW per 100 procedures. “That 100-procedure amount is the equivalent of the energy used for an average fuel efficiency car to travel 1,200 miles, the distance from Seattle to San Diego,” he said.
“One next step,” Dr. Desai said, “is getting help from GI societies to come together and have endoscopy units track their own performance. You need benchmarks so that you can determine how good an endoscopist you are with respect to waste.”
He commented further:“We all owe it to the environment. And, we have all witnessed what Mother Nature can do to you.”
Working on the potentially recyclable materials that account for 20% of the total waste would be a simple initial step to reduce waste going to landfills, Dr. Desai and colleagues concluded in the meeting abstract. “These data could serve as an actionable model for health systems to reduce total waste generation and move toward environmentally sustainable endoscopy units,” they wrote.
The authors reported no disclosures.
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and The Society for Surgery of the Alimentary Tract.
CHICAGO – according to Madhav Desai, MD, MPH, assistant professor of medicine at the University of Minnesota, Minneapolis. About 20% of the waste, most of which went to landfills, was potentially recyclable, he said in a presentation given at the annual Digestive Disease Week® meeting.
Gastrointestinal endoscopies are critical for the screening, diagnosis, and treatment of a variety of gastrointestinal conditions. But like other medical procedures, endoscopies are a source of environmental waste, including plastic, sharps, personal protective equipment (PPE), and cleaning supplies, and also energy waste.
“This all goes back to the damage that mankind is inflicting on the environment in general, with the health care sector as one of the top contributors to plastic waste generation, landfills and water wastage,” Dr. Desai said. “Endoscopies, with their numerous benefits, substantially increase waste generation through landfill waste and liquid consumption and waste through the cleaning of endoscopes. We have a responsibility to look into this topic.”
To prospectively assess total waste generation from their institution, Dr. Desai, who was with the Kansas City (Mo.) Veterans Administration Medical Center, when the research was conducted, collected data on the items used in 450 consecutive procedures from May to June 2022. The data included procedure type, accessory use, intravenous tubing, numbers of biopsy jars, linens, PPE, and more, beginning at the point of patient entry to the endoscopy unit until discharge. They also collected data on waste generation related to reprocessing after each procedure and daily energy use (including endoscopy equipment, lights, and computers). With an eye toward finding opportunities to improve and maximize waste recycling, they stratified waste into the three categories of biohazardous, nonbiohazardous, or potentially recyclable.
“We found that the total waste generated during the time period was 1,398.6 kg, with more than half of it, 61.6%, going directly to landfill,” Dr. Desai said in an interview. “That’s an amount that an average family in the U.S. would use for 2 months. That’s a huge amount.”
Most waste consists of sharps
Exactly one-third was biohazard waste and 5.1% was sharps, they found. A single procedure, on average, sent 2.19 kg of waste to landfill. Extrapolated to 1 year, the waste total amounts to 9,189 kg (equivalent to just over 10 U.S. tons) and per 100 procedures to 219 kg (about 483 pounds).
They estimated 20% of the landfill waste was potentially recyclable (such as plastic CO2 tubing, O2 connector, syringes, etc.), which could reduce the total landfill burden by 8.6 kg per day or 2,580 kg per year (or 61 kg per 100 procedures). Reprocessing endoscopes generated 194 gallons of liquid waste (735.26 kg) per day or 1,385 gallons per 100 procedures.
Turning to energy consumption, Dr. Desai reported that daily use in the endoscopy unit was 277.1 kW-hours (equivalent to 8.2 gallons of gasoline), adding up to about 1,980 kW per 100 procedures. “That 100-procedure amount is the equivalent of the energy used for an average fuel efficiency car to travel 1,200 miles, the distance from Seattle to San Diego,” he said.
“One next step,” Dr. Desai said, “is getting help from GI societies to come together and have endoscopy units track their own performance. You need benchmarks so that you can determine how good an endoscopist you are with respect to waste.”
He commented further:“We all owe it to the environment. And, we have all witnessed what Mother Nature can do to you.”
Working on the potentially recyclable materials that account for 20% of the total waste would be a simple initial step to reduce waste going to landfills, Dr. Desai and colleagues concluded in the meeting abstract. “These data could serve as an actionable model for health systems to reduce total waste generation and move toward environmentally sustainable endoscopy units,” they wrote.
The authors reported no disclosures.
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and The Society for Surgery of the Alimentary Tract.
AT DDW 2023
CRC screening rates are higher in Medicaid expansion states
CHICAGO – presented on May 6 in Chicago at the annual Digestive Disease Week®.
Researchers from the University of California, Los Angeles, reported that states with expanded Medicaid coverage had significantly higher rates of colorectal cancer (CRC) screening than states where officials refused federal support for Medicaid expansion.
Led by Megan R. McLeod, MD, an internal medicine resident at the University of California, Los Angeles, researchers compared CRC screening rates in states that did not adopt Medicaid expansion in 2021 with screening rates in states that invested Medicaid expansion into 1,284 Federally Qualified Health Centers, which are nonprofit health centers or clinics that serve medically underserved areas and populations. In this study, 76% of these centers were in states that accepted Medicaid expansion. The median colorectal cancer screening rate was 42.1% in Medicaid expansion states, compared with 36.5% in nonexpansion states
“The impact of being uninsured on CRC screening participation was profound in nonexpansion states,” said Dr. McLeod, who will be a UCLA gastroenterology fellow this year.
The study adds to a growing body of evidence that shows Medicaid expansion, which increases access to health care services to previously uninsured or underinsured patients, can improve health outcomes and may reduce racial and economic disparities.
For example, a 2019 study based on electronic health record data presented at the annual meeting of the American Society of Clinical Oncology showed that, after Medicaid expansion, racial differences in timely cancer treatment effectively disappeared. Before Medicaid expansion, Black patients were 4.8% less likely than White patients to receive timely cancer treatment, which is defined as treatment starting within 30 days of the diagnosis of an advanced or metastatic solid tumor. After Medicaid expansion, however, the difference between the racial groups dwindled to 0.8% and was no longer statistically significant.
Researchers at Weill Cornell Medical Center in New York reported in 2020 at the virtual annual meeting of the American Association for the Study of Liver Diseases that, 1 year after Medicaid expansion began on Jan. 1, 2014, the rate of liver-related mortality began to decline in 18 states with expanded coverage, whereas the rate of liver-related deaths continued to climb in 14 states that did not expand Medicaid.
The U.S. Health Resources and Services Administration funds Federally Qualified Health Centers (FQHC) that serve nearly 29 million patients throughout the country, including a large proportion whose care is covered by Medicaid. Among patients cared for in these centers, one in three have incomes below the federal poverty line, and one in five are uninsured.
Screening rates compared
Dr. McLeod and colleagues sought to determine whether Medicaid expansion would have an effect on CRC screening rates at these centers. The final analysis included 6,940,879 patients (between 50 and 74 years), of whom 1.7% were unhoused and 17.6% were uninsured.
Medicaid expansion status appeared to have a direct impact on whether screenings were even offered to patients. Centers in rural areas and those with a high proportion of uninsured patients were found to have significantly higher odds for doing fewer CRC screenings. In Medicaid expansion states, CRC screening rates were significantly lower for patients who were male, Black, Hispanic, had low income, were unhoused, or were uninsured.
In a Q&A that followed the presentation, Steven Itzkowitz, MD, director of the GI fellowship program at the Icahn School of Medicine at Mount Sinai, New York, suggested the type of CRC test patients are offered is directly related to Medicaid expansion status.
“In New York, before Cologuard (a colon and rectal cancer screening test) was covered by Medicaid, it wasn’t used very much, but once it got paid for by Medicaid, rates went up,” he said.
The study was internally supported. Dr. McLeod reported no conflicts of interest. Dr. Itzkowitz has been a consultant for Exact Sciences, the maker of Cologuard.
DDW is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
CHICAGO – presented on May 6 in Chicago at the annual Digestive Disease Week®.
Researchers from the University of California, Los Angeles, reported that states with expanded Medicaid coverage had significantly higher rates of colorectal cancer (CRC) screening than states where officials refused federal support for Medicaid expansion.
Led by Megan R. McLeod, MD, an internal medicine resident at the University of California, Los Angeles, researchers compared CRC screening rates in states that did not adopt Medicaid expansion in 2021 with screening rates in states that invested Medicaid expansion into 1,284 Federally Qualified Health Centers, which are nonprofit health centers or clinics that serve medically underserved areas and populations. In this study, 76% of these centers were in states that accepted Medicaid expansion. The median colorectal cancer screening rate was 42.1% in Medicaid expansion states, compared with 36.5% in nonexpansion states
“The impact of being uninsured on CRC screening participation was profound in nonexpansion states,” said Dr. McLeod, who will be a UCLA gastroenterology fellow this year.
The study adds to a growing body of evidence that shows Medicaid expansion, which increases access to health care services to previously uninsured or underinsured patients, can improve health outcomes and may reduce racial and economic disparities.
For example, a 2019 study based on electronic health record data presented at the annual meeting of the American Society of Clinical Oncology showed that, after Medicaid expansion, racial differences in timely cancer treatment effectively disappeared. Before Medicaid expansion, Black patients were 4.8% less likely than White patients to receive timely cancer treatment, which is defined as treatment starting within 30 days of the diagnosis of an advanced or metastatic solid tumor. After Medicaid expansion, however, the difference between the racial groups dwindled to 0.8% and was no longer statistically significant.
Researchers at Weill Cornell Medical Center in New York reported in 2020 at the virtual annual meeting of the American Association for the Study of Liver Diseases that, 1 year after Medicaid expansion began on Jan. 1, 2014, the rate of liver-related mortality began to decline in 18 states with expanded coverage, whereas the rate of liver-related deaths continued to climb in 14 states that did not expand Medicaid.
The U.S. Health Resources and Services Administration funds Federally Qualified Health Centers (FQHC) that serve nearly 29 million patients throughout the country, including a large proportion whose care is covered by Medicaid. Among patients cared for in these centers, one in three have incomes below the federal poverty line, and one in five are uninsured.
Screening rates compared
Dr. McLeod and colleagues sought to determine whether Medicaid expansion would have an effect on CRC screening rates at these centers. The final analysis included 6,940,879 patients (between 50 and 74 years), of whom 1.7% were unhoused and 17.6% were uninsured.
Medicaid expansion status appeared to have a direct impact on whether screenings were even offered to patients. Centers in rural areas and those with a high proportion of uninsured patients were found to have significantly higher odds for doing fewer CRC screenings. In Medicaid expansion states, CRC screening rates were significantly lower for patients who were male, Black, Hispanic, had low income, were unhoused, or were uninsured.
In a Q&A that followed the presentation, Steven Itzkowitz, MD, director of the GI fellowship program at the Icahn School of Medicine at Mount Sinai, New York, suggested the type of CRC test patients are offered is directly related to Medicaid expansion status.
“In New York, before Cologuard (a colon and rectal cancer screening test) was covered by Medicaid, it wasn’t used very much, but once it got paid for by Medicaid, rates went up,” he said.
The study was internally supported. Dr. McLeod reported no conflicts of interest. Dr. Itzkowitz has been a consultant for Exact Sciences, the maker of Cologuard.
DDW is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
CHICAGO – presented on May 6 in Chicago at the annual Digestive Disease Week®.
Researchers from the University of California, Los Angeles, reported that states with expanded Medicaid coverage had significantly higher rates of colorectal cancer (CRC) screening than states where officials refused federal support for Medicaid expansion.
Led by Megan R. McLeod, MD, an internal medicine resident at the University of California, Los Angeles, researchers compared CRC screening rates in states that did not adopt Medicaid expansion in 2021 with screening rates in states that invested Medicaid expansion into 1,284 Federally Qualified Health Centers, which are nonprofit health centers or clinics that serve medically underserved areas and populations. In this study, 76% of these centers were in states that accepted Medicaid expansion. The median colorectal cancer screening rate was 42.1% in Medicaid expansion states, compared with 36.5% in nonexpansion states
“The impact of being uninsured on CRC screening participation was profound in nonexpansion states,” said Dr. McLeod, who will be a UCLA gastroenterology fellow this year.
The study adds to a growing body of evidence that shows Medicaid expansion, which increases access to health care services to previously uninsured or underinsured patients, can improve health outcomes and may reduce racial and economic disparities.
For example, a 2019 study based on electronic health record data presented at the annual meeting of the American Society of Clinical Oncology showed that, after Medicaid expansion, racial differences in timely cancer treatment effectively disappeared. Before Medicaid expansion, Black patients were 4.8% less likely than White patients to receive timely cancer treatment, which is defined as treatment starting within 30 days of the diagnosis of an advanced or metastatic solid tumor. After Medicaid expansion, however, the difference between the racial groups dwindled to 0.8% and was no longer statistically significant.
Researchers at Weill Cornell Medical Center in New York reported in 2020 at the virtual annual meeting of the American Association for the Study of Liver Diseases that, 1 year after Medicaid expansion began on Jan. 1, 2014, the rate of liver-related mortality began to decline in 18 states with expanded coverage, whereas the rate of liver-related deaths continued to climb in 14 states that did not expand Medicaid.
The U.S. Health Resources and Services Administration funds Federally Qualified Health Centers (FQHC) that serve nearly 29 million patients throughout the country, including a large proportion whose care is covered by Medicaid. Among patients cared for in these centers, one in three have incomes below the federal poverty line, and one in five are uninsured.
Screening rates compared
Dr. McLeod and colleagues sought to determine whether Medicaid expansion would have an effect on CRC screening rates at these centers. The final analysis included 6,940,879 patients (between 50 and 74 years), of whom 1.7% were unhoused and 17.6% were uninsured.
Medicaid expansion status appeared to have a direct impact on whether screenings were even offered to patients. Centers in rural areas and those with a high proportion of uninsured patients were found to have significantly higher odds for doing fewer CRC screenings. In Medicaid expansion states, CRC screening rates were significantly lower for patients who were male, Black, Hispanic, had low income, were unhoused, or were uninsured.
In a Q&A that followed the presentation, Steven Itzkowitz, MD, director of the GI fellowship program at the Icahn School of Medicine at Mount Sinai, New York, suggested the type of CRC test patients are offered is directly related to Medicaid expansion status.
“In New York, before Cologuard (a colon and rectal cancer screening test) was covered by Medicaid, it wasn’t used very much, but once it got paid for by Medicaid, rates went up,” he said.
The study was internally supported. Dr. McLeod reported no conflicts of interest. Dr. Itzkowitz has been a consultant for Exact Sciences, the maker of Cologuard.
DDW is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
AT DDW 2023
Bariatric surgery cuts risk for obesity-related cancers in half: Study
For years evidence has pointed to multiple health benefits associated with bariatric surgery, including improvements in diabetes, sleep apnea, and blood pressure. Now researchers are adding cutting cancer risk by more than half to the list.
compared with 8.9% of their peers who did not undergo such surgery.
“We did see a difference in breast cancer, colon cancer, liver cancer, and ovarian cancer incidence. ... with patients in the bariatric surgery group having lower incidence of these four types of cancers when compared to the nonsurgical control group,” said Vibhu Chittajallu, MD, lead author and a gastroenterology fellow at Case Western Reserve University and University Hospitals in Cleveland.
The obesity epidemic is “one of the most serious health challenges in the United States today,” Dr. Chittajallu added at an April 27 media briefing during which select research was previewed for the annual Digestive Disease Week®. Obesity has been associated with multiple serious illnesses, including type 2 diabetes, heart disease, and cancer.
Obesity is also common. The Centers for Disease Control and Prevention reports that nearly 42% of American adults have obesity, and rates continue to rise.
Dr. Chittajallu and colleagues used billing codes in a national database to identify 55,789 patients with obesity who underwent bariatric surgery (sleeve gastrectomy, gastric bypass, or gastric band procedures) and a control group of the same size who did not have surgery.
Investigators controlled for risk factors that contribute to cancer development, including smoking history, alcohol use, heart disease, and hormone therapies.
Key findings
In 10 years of follow-up, 2,206 patients who underwent bariatric surgery developed an obesity-associated cancer, compared with 4,960 patients who did not have bariatric surgery.
The bariatric surgery group had lower numbers of new cases for six types of cancers (Table 1).
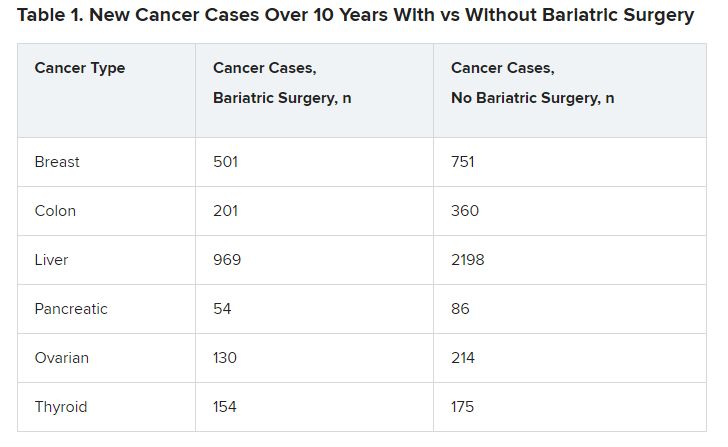
The differences were significant in four cancer types associated with obesity: breast cancer (P = .001), colon cancer (P < .01), liver cancer (P < .01), and ovarian cancer (P = .002).
The incidence of several other cancers, including renal carcinoma, and rectal and endometrial cancers, was not significantly different between the groups.
The mechanisms underlying excess cancer cases in patients with obesity are not completely understood, Dr. Chittajallu said. Bariatric surgery has been shown to decrease excess inflammation, elevate insulin, and moderate hormone levels.
‘Fascinating’ study but questions remain
The study is “fascinating,” said Loren Laine, MD, moderator of the media briefing. “Obesity is clearly associated with a number of different cancers, and that’s very important. So, it makes logical sense that if you lose weight, you will reduce that risk.”
Although investigators controlled for several known cancer risk factors, there are some they couldn’t control for because they were not included in the database, and there could be unknowns that also affected the results, noted Dr. Laine, who is professor of medicine (digestive diseases) and chief of digestive health at Yale University in New Haven, Conn.
“You have to be circumspect when you look at retrospective observational studies,” he added.
It would be helpful to know when most cancers developed over the 10 years, Dr. Laine said. Dr. Chittajallu responded that the research team did not include cancers that developed in the first year after bariatric surgery to minimize incidental findings, but he did not provide a timeline for the cancers that developed.
Another unanswered question, Dr. Laine said, is whether a dose-response relationship exists. If future research shows that the more weight a person loses, the more likely they are to have a reduction in cancer risk, “that would be fascinating,” he said. Also, it would be interesting to know if endoscopic interventions and weight-loss medications decrease cancer risks in people with obesity.
More research is needed to understand how bariatric surgery affects cancer risk, Dr. Chittajallu said. “But the significant findings from this study suggest it’s an exciting avenue for further study.”
DDW 2023 will be held May 6-9 in Chicago and virtually.
The study was independently supported. Dr. Chittajallu and Dr. Laine have reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
For years evidence has pointed to multiple health benefits associated with bariatric surgery, including improvements in diabetes, sleep apnea, and blood pressure. Now researchers are adding cutting cancer risk by more than half to the list.
compared with 8.9% of their peers who did not undergo such surgery.
“We did see a difference in breast cancer, colon cancer, liver cancer, and ovarian cancer incidence. ... with patients in the bariatric surgery group having lower incidence of these four types of cancers when compared to the nonsurgical control group,” said Vibhu Chittajallu, MD, lead author and a gastroenterology fellow at Case Western Reserve University and University Hospitals in Cleveland.
The obesity epidemic is “one of the most serious health challenges in the United States today,” Dr. Chittajallu added at an April 27 media briefing during which select research was previewed for the annual Digestive Disease Week®. Obesity has been associated with multiple serious illnesses, including type 2 diabetes, heart disease, and cancer.
Obesity is also common. The Centers for Disease Control and Prevention reports that nearly 42% of American adults have obesity, and rates continue to rise.
Dr. Chittajallu and colleagues used billing codes in a national database to identify 55,789 patients with obesity who underwent bariatric surgery (sleeve gastrectomy, gastric bypass, or gastric band procedures) and a control group of the same size who did not have surgery.
Investigators controlled for risk factors that contribute to cancer development, including smoking history, alcohol use, heart disease, and hormone therapies.
Key findings
In 10 years of follow-up, 2,206 patients who underwent bariatric surgery developed an obesity-associated cancer, compared with 4,960 patients who did not have bariatric surgery.
The bariatric surgery group had lower numbers of new cases for six types of cancers (Table 1).

The differences were significant in four cancer types associated with obesity: breast cancer (P = .001), colon cancer (P < .01), liver cancer (P < .01), and ovarian cancer (P = .002).
The incidence of several other cancers, including renal carcinoma, and rectal and endometrial cancers, was not significantly different between the groups.
The mechanisms underlying excess cancer cases in patients with obesity are not completely understood, Dr. Chittajallu said. Bariatric surgery has been shown to decrease excess inflammation, elevate insulin, and moderate hormone levels.
‘Fascinating’ study but questions remain
The study is “fascinating,” said Loren Laine, MD, moderator of the media briefing. “Obesity is clearly associated with a number of different cancers, and that’s very important. So, it makes logical sense that if you lose weight, you will reduce that risk.”
Although investigators controlled for several known cancer risk factors, there are some they couldn’t control for because they were not included in the database, and there could be unknowns that also affected the results, noted Dr. Laine, who is professor of medicine (digestive diseases) and chief of digestive health at Yale University in New Haven, Conn.
“You have to be circumspect when you look at retrospective observational studies,” he added.
It would be helpful to know when most cancers developed over the 10 years, Dr. Laine said. Dr. Chittajallu responded that the research team did not include cancers that developed in the first year after bariatric surgery to minimize incidental findings, but he did not provide a timeline for the cancers that developed.
Another unanswered question, Dr. Laine said, is whether a dose-response relationship exists. If future research shows that the more weight a person loses, the more likely they are to have a reduction in cancer risk, “that would be fascinating,” he said. Also, it would be interesting to know if endoscopic interventions and weight-loss medications decrease cancer risks in people with obesity.
More research is needed to understand how bariatric surgery affects cancer risk, Dr. Chittajallu said. “But the significant findings from this study suggest it’s an exciting avenue for further study.”
DDW 2023 will be held May 6-9 in Chicago and virtually.
The study was independently supported. Dr. Chittajallu and Dr. Laine have reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
For years evidence has pointed to multiple health benefits associated with bariatric surgery, including improvements in diabetes, sleep apnea, and blood pressure. Now researchers are adding cutting cancer risk by more than half to the list.
compared with 8.9% of their peers who did not undergo such surgery.
“We did see a difference in breast cancer, colon cancer, liver cancer, and ovarian cancer incidence. ... with patients in the bariatric surgery group having lower incidence of these four types of cancers when compared to the nonsurgical control group,” said Vibhu Chittajallu, MD, lead author and a gastroenterology fellow at Case Western Reserve University and University Hospitals in Cleveland.
The obesity epidemic is “one of the most serious health challenges in the United States today,” Dr. Chittajallu added at an April 27 media briefing during which select research was previewed for the annual Digestive Disease Week®. Obesity has been associated with multiple serious illnesses, including type 2 diabetes, heart disease, and cancer.
Obesity is also common. The Centers for Disease Control and Prevention reports that nearly 42% of American adults have obesity, and rates continue to rise.
Dr. Chittajallu and colleagues used billing codes in a national database to identify 55,789 patients with obesity who underwent bariatric surgery (sleeve gastrectomy, gastric bypass, or gastric band procedures) and a control group of the same size who did not have surgery.
Investigators controlled for risk factors that contribute to cancer development, including smoking history, alcohol use, heart disease, and hormone therapies.
Key findings
In 10 years of follow-up, 2,206 patients who underwent bariatric surgery developed an obesity-associated cancer, compared with 4,960 patients who did not have bariatric surgery.
The bariatric surgery group had lower numbers of new cases for six types of cancers (Table 1).

The differences were significant in four cancer types associated with obesity: breast cancer (P = .001), colon cancer (P < .01), liver cancer (P < .01), and ovarian cancer (P = .002).
The incidence of several other cancers, including renal carcinoma, and rectal and endometrial cancers, was not significantly different between the groups.
The mechanisms underlying excess cancer cases in patients with obesity are not completely understood, Dr. Chittajallu said. Bariatric surgery has been shown to decrease excess inflammation, elevate insulin, and moderate hormone levels.
‘Fascinating’ study but questions remain
The study is “fascinating,” said Loren Laine, MD, moderator of the media briefing. “Obesity is clearly associated with a number of different cancers, and that’s very important. So, it makes logical sense that if you lose weight, you will reduce that risk.”
Although investigators controlled for several known cancer risk factors, there are some they couldn’t control for because they were not included in the database, and there could be unknowns that also affected the results, noted Dr. Laine, who is professor of medicine (digestive diseases) and chief of digestive health at Yale University in New Haven, Conn.
“You have to be circumspect when you look at retrospective observational studies,” he added.
It would be helpful to know when most cancers developed over the 10 years, Dr. Laine said. Dr. Chittajallu responded that the research team did not include cancers that developed in the first year after bariatric surgery to minimize incidental findings, but he did not provide a timeline for the cancers that developed.
Another unanswered question, Dr. Laine said, is whether a dose-response relationship exists. If future research shows that the more weight a person loses, the more likely they are to have a reduction in cancer risk, “that would be fascinating,” he said. Also, it would be interesting to know if endoscopic interventions and weight-loss medications decrease cancer risks in people with obesity.
More research is needed to understand how bariatric surgery affects cancer risk, Dr. Chittajallu said. “But the significant findings from this study suggest it’s an exciting avenue for further study.”
DDW 2023 will be held May 6-9 in Chicago and virtually.
The study was independently supported. Dr. Chittajallu and Dr. Laine have reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
AT DDW 2023
Can an endoscopic procedure treat type 2 diabetes?
Called recellularization via electroporation therapy (ReCET), the technology, manufactured by Endogenex, uses a specialized catheter to deliver alternating electric pulses to the duodenum to induce cellular regeneration. This process is thought to improve insulin sensitivity, in part, by altering gut hormones and nutritional sensing, principal investigator Jacques Bergman, MD, PhD, said in a press briefing held in conjunction with the annual Digestive Disease Week® (DDW), where he will present the data on May 9.
In the first-in-human study of ReCET, 12 of 14 patients were able to come off insulin for up to a year following the procedure when combined with the use of the glucagonlike peptide–1 agonist semaglutide.
“This might be a game changer in the management of type 2 diabetes because a single outpatient endoscopic intervention was suggested to have a pretty long therapeutic effect, which is compliance-free, as opposed to drug therapy that relies on patients taking the drugs on a daily basis,” said Dr. Bergman, professor of gastrointestinal endoscopy at Amsterdam University Medical Center.
Moreover, he added, “this technique is disease-modifying, so it goes to the root cause of type 2 diabetes and tackles the insulin resistance, as opposed to drug therapy, which at best, is disease-controlling, and the effect is immediately gone if you stop the medication.”
ReCET is similar to another product, Fractyl’s Revita DMR, for which Dr. Bergman was involved in a randomized clinical trial. He said in an interview that the two technologies differ in that the Revita uses heat with submucosal lifting to avoid deeper heat penetration, whereas ReCET is nonthermal. He is also involved in a second randomized trial of the Revita.
Is semaglutide muddying the findings?
Asked to comment about the current study with ReCET, Ali Aminian, MD, professor of surgery and director of the Bariatric and Metabolic Institute at the Cleveland Clinic, said that the treatment effect is certainly plausible.
“The observation that hyperglycemia rapidly and substantially improves after bariatric surgery has prompted innovators to search for novel endoscopic procedures targeting the GI tract to improve diabetes and metabolic disease. Over the years, we learned that in addition to its role in digestion and absorption, the GI tract is actually a large endocrine organ which contributes to development of diabetes and metabolic disease.”
However, Dr. Aminian said that, “while these preliminary findings on a very small number of patients with a very short follow-up time are interesting,” he faulted the study design for including semaglutide. “When patients are treated with a combination of therapies, it will be hard to understand the true effect of each therapy,” and particularly, “when we add a strong diabetes medication like semaglutide.”
Dr. Bergman said semaglutide was used to “boost the insulin-resistant effect of the endoscopic treatment,” and that a planned double-blind, randomized trial will “show how much semaglutide actually contributed to the effect.” The ultimate goal, he noted, is to eliminate the need for all medications.
Moreover, when people with type 2 diabetes add semaglutide to insulin treatment, only about 20% typically are able to quit taking the insulin, in contrast to the 86% seen in this study, lead author Celine Busch, MBBS, a PhD candidate in gastroenterology at Amsterdam University, said in a DDW statement.
Dr. Aminian said, “I’m looking forward to better quality data ... from studies with a stronger design to prove safety, efficacy, and durability of this endoscopic intervention in patients with diabetes.”
But, he also cautioned, “in the past few years, other endoscopic procedures targeting the duodenum were introduced with exciting initial findings based on a small series [with a] short-term follow-up time. However, their safety, efficacy, and durability were not proven in subsequent studies.”
All patients stopped insulin, most for a year
The single-arm, single-center study involved 14 patients with type 2 diabetes taking basal but not premeal insulin. All underwent the 1-hour outpatient ReCET procedure, which involved placing a catheter into the first part of the small bowel and delivering electrical pulses to the duodenum.
Patients adhered to a calorie-controlled liquid diet for 2 weeks, after which they were initiated on semaglutide. All 14 patients were able to come off insulin for 3 months while maintaining glycemic control, and 12 were able to come off insulin for 12 months. They also experienced a 50% reduction in liver fat.
Dr. Bergman said a randomized, double-blind study using a sham procedure for controls is expected to start in about 2 months. “But for now, we are very encouraged by the potential for controlling type 2 diabetes with a single endoscopic treatment.”
Dr. Bergman has reported serving on the advisory board for Endogenex. Dr. Aminian has reported receiving research support and honorarium from Medtronic and Ethicon.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
Called recellularization via electroporation therapy (ReCET), the technology, manufactured by Endogenex, uses a specialized catheter to deliver alternating electric pulses to the duodenum to induce cellular regeneration. This process is thought to improve insulin sensitivity, in part, by altering gut hormones and nutritional sensing, principal investigator Jacques Bergman, MD, PhD, said in a press briefing held in conjunction with the annual Digestive Disease Week® (DDW), where he will present the data on May 9.
In the first-in-human study of ReCET, 12 of 14 patients were able to come off insulin for up to a year following the procedure when combined with the use of the glucagonlike peptide–1 agonist semaglutide.
“This might be a game changer in the management of type 2 diabetes because a single outpatient endoscopic intervention was suggested to have a pretty long therapeutic effect, which is compliance-free, as opposed to drug therapy that relies on patients taking the drugs on a daily basis,” said Dr. Bergman, professor of gastrointestinal endoscopy at Amsterdam University Medical Center.
Moreover, he added, “this technique is disease-modifying, so it goes to the root cause of type 2 diabetes and tackles the insulin resistance, as opposed to drug therapy, which at best, is disease-controlling, and the effect is immediately gone if you stop the medication.”
ReCET is similar to another product, Fractyl’s Revita DMR, for which Dr. Bergman was involved in a randomized clinical trial. He said in an interview that the two technologies differ in that the Revita uses heat with submucosal lifting to avoid deeper heat penetration, whereas ReCET is nonthermal. He is also involved in a second randomized trial of the Revita.
Is semaglutide muddying the findings?
Asked to comment about the current study with ReCET, Ali Aminian, MD, professor of surgery and director of the Bariatric and Metabolic Institute at the Cleveland Clinic, said that the treatment effect is certainly plausible.
“The observation that hyperglycemia rapidly and substantially improves after bariatric surgery has prompted innovators to search for novel endoscopic procedures targeting the GI tract to improve diabetes and metabolic disease. Over the years, we learned that in addition to its role in digestion and absorption, the GI tract is actually a large endocrine organ which contributes to development of diabetes and metabolic disease.”
However, Dr. Aminian said that, “while these preliminary findings on a very small number of patients with a very short follow-up time are interesting,” he faulted the study design for including semaglutide. “When patients are treated with a combination of therapies, it will be hard to understand the true effect of each therapy,” and particularly, “when we add a strong diabetes medication like semaglutide.”
Dr. Bergman said semaglutide was used to “boost the insulin-resistant effect of the endoscopic treatment,” and that a planned double-blind, randomized trial will “show how much semaglutide actually contributed to the effect.” The ultimate goal, he noted, is to eliminate the need for all medications.
Moreover, when people with type 2 diabetes add semaglutide to insulin treatment, only about 20% typically are able to quit taking the insulin, in contrast to the 86% seen in this study, lead author Celine Busch, MBBS, a PhD candidate in gastroenterology at Amsterdam University, said in a DDW statement.
Dr. Aminian said, “I’m looking forward to better quality data ... from studies with a stronger design to prove safety, efficacy, and durability of this endoscopic intervention in patients with diabetes.”
But, he also cautioned, “in the past few years, other endoscopic procedures targeting the duodenum were introduced with exciting initial findings based on a small series [with a] short-term follow-up time. However, their safety, efficacy, and durability were not proven in subsequent studies.”
All patients stopped insulin, most for a year
The single-arm, single-center study involved 14 patients with type 2 diabetes taking basal but not premeal insulin. All underwent the 1-hour outpatient ReCET procedure, which involved placing a catheter into the first part of the small bowel and delivering electrical pulses to the duodenum.
Patients adhered to a calorie-controlled liquid diet for 2 weeks, after which they were initiated on semaglutide. All 14 patients were able to come off insulin for 3 months while maintaining glycemic control, and 12 were able to come off insulin for 12 months. They also experienced a 50% reduction in liver fat.
Dr. Bergman said a randomized, double-blind study using a sham procedure for controls is expected to start in about 2 months. “But for now, we are very encouraged by the potential for controlling type 2 diabetes with a single endoscopic treatment.”
Dr. Bergman has reported serving on the advisory board for Endogenex. Dr. Aminian has reported receiving research support and honorarium from Medtronic and Ethicon.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
Called recellularization via electroporation therapy (ReCET), the technology, manufactured by Endogenex, uses a specialized catheter to deliver alternating electric pulses to the duodenum to induce cellular regeneration. This process is thought to improve insulin sensitivity, in part, by altering gut hormones and nutritional sensing, principal investigator Jacques Bergman, MD, PhD, said in a press briefing held in conjunction with the annual Digestive Disease Week® (DDW), where he will present the data on May 9.
In the first-in-human study of ReCET, 12 of 14 patients were able to come off insulin for up to a year following the procedure when combined with the use of the glucagonlike peptide–1 agonist semaglutide.
“This might be a game changer in the management of type 2 diabetes because a single outpatient endoscopic intervention was suggested to have a pretty long therapeutic effect, which is compliance-free, as opposed to drug therapy that relies on patients taking the drugs on a daily basis,” said Dr. Bergman, professor of gastrointestinal endoscopy at Amsterdam University Medical Center.
Moreover, he added, “this technique is disease-modifying, so it goes to the root cause of type 2 diabetes and tackles the insulin resistance, as opposed to drug therapy, which at best, is disease-controlling, and the effect is immediately gone if you stop the medication.”
ReCET is similar to another product, Fractyl’s Revita DMR, for which Dr. Bergman was involved in a randomized clinical trial. He said in an interview that the two technologies differ in that the Revita uses heat with submucosal lifting to avoid deeper heat penetration, whereas ReCET is nonthermal. He is also involved in a second randomized trial of the Revita.
Is semaglutide muddying the findings?
Asked to comment about the current study with ReCET, Ali Aminian, MD, professor of surgery and director of the Bariatric and Metabolic Institute at the Cleveland Clinic, said that the treatment effect is certainly plausible.
“The observation that hyperglycemia rapidly and substantially improves after bariatric surgery has prompted innovators to search for novel endoscopic procedures targeting the GI tract to improve diabetes and metabolic disease. Over the years, we learned that in addition to its role in digestion and absorption, the GI tract is actually a large endocrine organ which contributes to development of diabetes and metabolic disease.”
However, Dr. Aminian said that, “while these preliminary findings on a very small number of patients with a very short follow-up time are interesting,” he faulted the study design for including semaglutide. “When patients are treated with a combination of therapies, it will be hard to understand the true effect of each therapy,” and particularly, “when we add a strong diabetes medication like semaglutide.”
Dr. Bergman said semaglutide was used to “boost the insulin-resistant effect of the endoscopic treatment,” and that a planned double-blind, randomized trial will “show how much semaglutide actually contributed to the effect.” The ultimate goal, he noted, is to eliminate the need for all medications.
Moreover, when people with type 2 diabetes add semaglutide to insulin treatment, only about 20% typically are able to quit taking the insulin, in contrast to the 86% seen in this study, lead author Celine Busch, MBBS, a PhD candidate in gastroenterology at Amsterdam University, said in a DDW statement.
Dr. Aminian said, “I’m looking forward to better quality data ... from studies with a stronger design to prove safety, efficacy, and durability of this endoscopic intervention in patients with diabetes.”
But, he also cautioned, “in the past few years, other endoscopic procedures targeting the duodenum were introduced with exciting initial findings based on a small series [with a] short-term follow-up time. However, their safety, efficacy, and durability were not proven in subsequent studies.”
All patients stopped insulin, most for a year
The single-arm, single-center study involved 14 patients with type 2 diabetes taking basal but not premeal insulin. All underwent the 1-hour outpatient ReCET procedure, which involved placing a catheter into the first part of the small bowel and delivering electrical pulses to the duodenum.
Patients adhered to a calorie-controlled liquid diet for 2 weeks, after which they were initiated on semaglutide. All 14 patients were able to come off insulin for 3 months while maintaining glycemic control, and 12 were able to come off insulin for 12 months. They also experienced a 50% reduction in liver fat.
Dr. Bergman said a randomized, double-blind study using a sham procedure for controls is expected to start in about 2 months. “But for now, we are very encouraged by the potential for controlling type 2 diabetes with a single endoscopic treatment.”
Dr. Bergman has reported serving on the advisory board for Endogenex. Dr. Aminian has reported receiving research support and honorarium from Medtronic and Ethicon.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
FROM DDW 2023








