User login
2015 South Beach Symposium
OTC phototherapy devices can enhance outcomes
MIAMI BEACH – Over-the-counter phototherapy devices for anti-aging and acne treatment can improve office-based procedure results and enhance clinical practice, according to Dr. Z. Paul Lorenc.
Light-emitting diode (LED) devices now available for home use, such as the illuMask (La Lumiere) anti-aging and anti-acne masks, are safe and effective and can be used along with office-based treatments to speed healing and boost results, Dr. Lorenc said at the South Beach Symposium.
The illuMask anti-aging mask uses a combination of 630 nm wavelength red light and 855 nm wavelength infrared light, and the two work synergistically to improve signs of aging, such as wrinkles, photoaging, and hyperpigmentation.
Dr. Lorenc, chief medical and scientific officer for La Lumiere, worked on the pivotal trials for the devices, and said that in a study of 30 patients aged 14 -40 years, 15 minutes of daily mask use was associated with 82% and 94% improvement in 17 clinical skin attributes at weeks 4 and 8, respectively. Improvement in 20 sham-treated subjects was 24% at both 4 and 8 weeks.
Subject self-assessment showed significant improvement in 93% and 100% of 15 clinical attributes at weeks 4 and 8, respectively, in the treated subjects, compared with 33% and 40% in the sham-treated subjects, he said.
The illuMask anti-acne mask uses 630 nm red light and 440 nm blue light, which also work synergistically.
In addition, 15 minutes of daily use was associated with a 71% average decrease in the number of noninflammatory lesions and an 83% decrease in the number of inflammatory lesions at 8 weeks. The median decrease in the 5-point Acne Severity Assessment Scale score was 2 points – from 4 (moderate) to 2 (almost clear).
Dr. Lorenc, who practices plastic surgery in New York, said he does not treat acne very often, but he does encounter patients with adult acne who benefit from the acne mask.
He said he uses the anti-aging mask often.
“I’m using it on every single resurfacing patient,” he said, explaining that red light is anti-inflammatory in nature, and increases production of collagen and elastin.
Patients undergoing partial or total resurfacing, those undergoing other procedures such as microneedling, and even those undergoing a face-lift, can benefit from use of the anti-aging mask beginning the day after the procedure, he said, adding that it decreases inflammatory processes, and can provide some added anti-aging effects that may not be addressed by the initial procedures (dyschromia in face-lift patients, for example).
The masks are simple to use, don’t take much time, can be used with existing treatment regimens, are appropriate for all Fitzpatrick skin types, and are associated with a high compliance rate – even among teens, Dr. Lorenc said.
While some physicians have expressed concern that the OTC availability of phototherapy devices could hurt clinical practice, his experience has been quite the opposite.
The boost that the masks give both to the recovery time and efficacy of office-based procedures makes for happier patients who come back – and send their friends, he said.
“I have incorporated [the masks] into my practice, and what it has done is the opposite of what you would think intuitively; it has expanded my practice – both surgical and nonsurgical, and we think that this is a very good adjunct therapy to both acne and anti-aging treatment,” he said.
Dr. Lorenc had no additional disclosures.
MIAMI BEACH – Over-the-counter phototherapy devices for anti-aging and acne treatment can improve office-based procedure results and enhance clinical practice, according to Dr. Z. Paul Lorenc.
Light-emitting diode (LED) devices now available for home use, such as the illuMask (La Lumiere) anti-aging and anti-acne masks, are safe and effective and can be used along with office-based treatments to speed healing and boost results, Dr. Lorenc said at the South Beach Symposium.
The illuMask anti-aging mask uses a combination of 630 nm wavelength red light and 855 nm wavelength infrared light, and the two work synergistically to improve signs of aging, such as wrinkles, photoaging, and hyperpigmentation.
Dr. Lorenc, chief medical and scientific officer for La Lumiere, worked on the pivotal trials for the devices, and said that in a study of 30 patients aged 14 -40 years, 15 minutes of daily mask use was associated with 82% and 94% improvement in 17 clinical skin attributes at weeks 4 and 8, respectively. Improvement in 20 sham-treated subjects was 24% at both 4 and 8 weeks.
Subject self-assessment showed significant improvement in 93% and 100% of 15 clinical attributes at weeks 4 and 8, respectively, in the treated subjects, compared with 33% and 40% in the sham-treated subjects, he said.
The illuMask anti-acne mask uses 630 nm red light and 440 nm blue light, which also work synergistically.
In addition, 15 minutes of daily use was associated with a 71% average decrease in the number of noninflammatory lesions and an 83% decrease in the number of inflammatory lesions at 8 weeks. The median decrease in the 5-point Acne Severity Assessment Scale score was 2 points – from 4 (moderate) to 2 (almost clear).
Dr. Lorenc, who practices plastic surgery in New York, said he does not treat acne very often, but he does encounter patients with adult acne who benefit from the acne mask.
He said he uses the anti-aging mask often.
“I’m using it on every single resurfacing patient,” he said, explaining that red light is anti-inflammatory in nature, and increases production of collagen and elastin.
Patients undergoing partial or total resurfacing, those undergoing other procedures such as microneedling, and even those undergoing a face-lift, can benefit from use of the anti-aging mask beginning the day after the procedure, he said, adding that it decreases inflammatory processes, and can provide some added anti-aging effects that may not be addressed by the initial procedures (dyschromia in face-lift patients, for example).
The masks are simple to use, don’t take much time, can be used with existing treatment regimens, are appropriate for all Fitzpatrick skin types, and are associated with a high compliance rate – even among teens, Dr. Lorenc said.
While some physicians have expressed concern that the OTC availability of phototherapy devices could hurt clinical practice, his experience has been quite the opposite.
The boost that the masks give both to the recovery time and efficacy of office-based procedures makes for happier patients who come back – and send their friends, he said.
“I have incorporated [the masks] into my practice, and what it has done is the opposite of what you would think intuitively; it has expanded my practice – both surgical and nonsurgical, and we think that this is a very good adjunct therapy to both acne and anti-aging treatment,” he said.
Dr. Lorenc had no additional disclosures.
MIAMI BEACH – Over-the-counter phototherapy devices for anti-aging and acne treatment can improve office-based procedure results and enhance clinical practice, according to Dr. Z. Paul Lorenc.
Light-emitting diode (LED) devices now available for home use, such as the illuMask (La Lumiere) anti-aging and anti-acne masks, are safe and effective and can be used along with office-based treatments to speed healing and boost results, Dr. Lorenc said at the South Beach Symposium.
The illuMask anti-aging mask uses a combination of 630 nm wavelength red light and 855 nm wavelength infrared light, and the two work synergistically to improve signs of aging, such as wrinkles, photoaging, and hyperpigmentation.
Dr. Lorenc, chief medical and scientific officer for La Lumiere, worked on the pivotal trials for the devices, and said that in a study of 30 patients aged 14 -40 years, 15 minutes of daily mask use was associated with 82% and 94% improvement in 17 clinical skin attributes at weeks 4 and 8, respectively. Improvement in 20 sham-treated subjects was 24% at both 4 and 8 weeks.
Subject self-assessment showed significant improvement in 93% and 100% of 15 clinical attributes at weeks 4 and 8, respectively, in the treated subjects, compared with 33% and 40% in the sham-treated subjects, he said.
The illuMask anti-acne mask uses 630 nm red light and 440 nm blue light, which also work synergistically.
In addition, 15 minutes of daily use was associated with a 71% average decrease in the number of noninflammatory lesions and an 83% decrease in the number of inflammatory lesions at 8 weeks. The median decrease in the 5-point Acne Severity Assessment Scale score was 2 points – from 4 (moderate) to 2 (almost clear).
Dr. Lorenc, who practices plastic surgery in New York, said he does not treat acne very often, but he does encounter patients with adult acne who benefit from the acne mask.
He said he uses the anti-aging mask often.
“I’m using it on every single resurfacing patient,” he said, explaining that red light is anti-inflammatory in nature, and increases production of collagen and elastin.
Patients undergoing partial or total resurfacing, those undergoing other procedures such as microneedling, and even those undergoing a face-lift, can benefit from use of the anti-aging mask beginning the day after the procedure, he said, adding that it decreases inflammatory processes, and can provide some added anti-aging effects that may not be addressed by the initial procedures (dyschromia in face-lift patients, for example).
The masks are simple to use, don’t take much time, can be used with existing treatment regimens, are appropriate for all Fitzpatrick skin types, and are associated with a high compliance rate – even among teens, Dr. Lorenc said.
While some physicians have expressed concern that the OTC availability of phototherapy devices could hurt clinical practice, his experience has been quite the opposite.
The boost that the masks give both to the recovery time and efficacy of office-based procedures makes for happier patients who come back – and send their friends, he said.
“I have incorporated [the masks] into my practice, and what it has done is the opposite of what you would think intuitively; it has expanded my practice – both surgical and nonsurgical, and we think that this is a very good adjunct therapy to both acne and anti-aging treatment,” he said.
Dr. Lorenc had no additional disclosures.
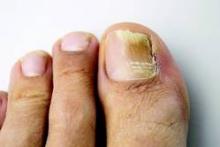
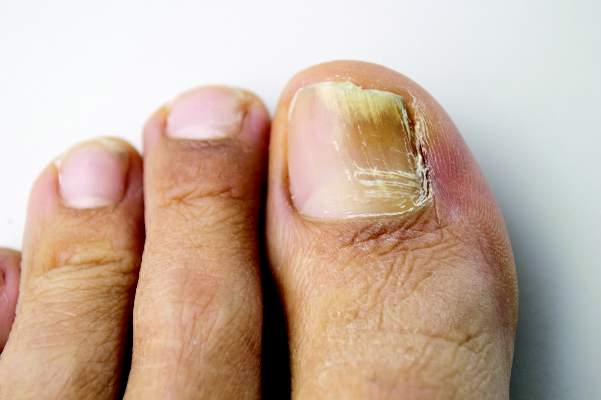
Combine topicals, orals for onychomycosis
MIAMI BEACH – Two new topical solutions approved in 2014 for the treatment of distal subungual onychomycosis don’t eliminate the need for oral treatment, but they do represent improvement in the options available to patients, according to Dr. Boni E. Elewski.
Oral treatments, including terbinafine, itraconazole, and fluconazole are still of value – either alone or in combination with the new solutions or other agents – for this type of onychomycosis, which is “essentially a nail bed dermatophytosis,” she said at the South Beach Symposium.
Terbinafine has been used for 2 decades, and is probably the most commonly used treatment for onychomycosis. It is approved as a once-daily pill given for 90 days, and reportedly has a cure rate just under 40%, she said.
Itraconazole is approved as a 200-mg daily dose for 12 weeks, although most physicians use pulse dosing, prescribing 400 mg daily for a week, then for 1 week each month.
“I like this drug for my nondermatophyte patients … if you have something that you don’t think is a dermatophyte, or if they’ve failed terbinafine, this is an excellent option,” she said.
Fluconazole, her “personal favorite,” is used off label for onychomycosis, but has been shown to have good cure rates with once-weekly treatment, said Dr. Elewski of the University of Alabama at Birmingham.
“I’ll confess, I like this drug because I feel comfortable with it,” she said adding that she tells her patients to think about “Fungal Fridays” or “Toesdays” as a way to remember to take their weekly treatments.
The cure rates are quite good, she said, noting that because the nails grow so slowly, the pace of the treatment matches patient expectations. They don’t finish treatment and still have a “cruddy-looking” nail, she explained.
If any of these oral drugs are used, laboratory monitoring and periodic assessments are necessary, so treatment is a bit complicated. Adverse events remain a concern – particularly drug-drug interactions, drug eruption, cardiac issues (with itraconazole), and loss of taste (with terbinafine).
“So we do have to worry about some of these conditions, which is why having other treatments is so nice,” Dr. Elewski said.
Another reason it’s good to have more options is that no matter which drug you choose, it won’t cure everyone, she noted. Sometimes that’s because the condition is severe; patients with a dermatophyte abscess, those with very thick nails, and those with complete nail involvement associated with a nondermatophyte mold, for example, will have a poorer prognosis, regardless of which oral treatment given. Also, the condition is often complicated by concomitant disorders such as psoriasis. About 5% of patients with psoriasis have nail-only disease, and about a third of them also have onychomycosis. Others are misdiagnosed as having onychomycosis.
Alternatives that can be used alone or in combination with the oral therapies, include the two new topical solutions: efinaconazole and tavaborole, Dr. Elewski said.
Solutions are good, because you can apply them on, under, and around the nail. Both of the drugs have demonstrated effective penetration of the nail plate, allowing penetration to the nail bed where the infection exists, she noted.
The mycological cure rate with efinaconazole yields outcomes comparable to those with oral drugs.
“I think [the mycological cure] is actually the most important endpoint. Because when you want to get rid of the fungus, what do you do? You want to kill the fungus,” said Dr. Elewski, adding that mycological cure is the first sign a patient will go on to experience complete cure.
The complete cure rate is lower, but that appears to be a time-related factor. The nail takes a long time to grow, so the complete cure rate will lag behind the mycological cure rate, Dr. Elewski explained.
The other topical solution – tavaborole – is a new molecule that contains boron. Mycological cure rates in studies of the drug were in the mid-30% range, and it appears able to be used with nail polish without issues to the polish or the outcomes, she said.
In Dr. Elewski’s experience, these topicals work better than expected in clinical practice, based on the clinical trials. One patient who wasn’t eligible for the clinical trials because of a dermatophytosis, for example, was nearly clear within 5 months, and is now totally cured, she said.
“So these treatments are effective as monotherapy, but could be used as an adjunct with systemic therapy, and perhaps also in nondermatophyte cases of onychomycosis,” Dr. Elewski noted. “I think it would be a better option to pick one of these topical drugs than putting someone on a prolonged course of itraconazole if at all possible.” The safety issue would be more favorable with the topical antifungal solution, she said.
A treatment that should never be used is ketoconazole, she noted, explaining that although the drug was never approved specifically for onychomycosis, it was often prescribed for the treatment of tinea versicolor. Because of safety concerns, the FDA removed its indication for all cutaneous fungal infections in 2013.
Dr. Elewski is a consultant for Valeant Pharmaceuticals and a contracted researcher for Anacor Pharmaceuticals.
MIAMI BEACH – Two new topical solutions approved in 2014 for the treatment of distal subungual onychomycosis don’t eliminate the need for oral treatment, but they do represent improvement in the options available to patients, according to Dr. Boni E. Elewski.
Oral treatments, including terbinafine, itraconazole, and fluconazole are still of value – either alone or in combination with the new solutions or other agents – for this type of onychomycosis, which is “essentially a nail bed dermatophytosis,” she said at the South Beach Symposium.
Terbinafine has been used for 2 decades, and is probably the most commonly used treatment for onychomycosis. It is approved as a once-daily pill given for 90 days, and reportedly has a cure rate just under 40%, she said.
Itraconazole is approved as a 200-mg daily dose for 12 weeks, although most physicians use pulse dosing, prescribing 400 mg daily for a week, then for 1 week each month.
“I like this drug for my nondermatophyte patients … if you have something that you don’t think is a dermatophyte, or if they’ve failed terbinafine, this is an excellent option,” she said.
Fluconazole, her “personal favorite,” is used off label for onychomycosis, but has been shown to have good cure rates with once-weekly treatment, said Dr. Elewski of the University of Alabama at Birmingham.
“I’ll confess, I like this drug because I feel comfortable with it,” she said adding that she tells her patients to think about “Fungal Fridays” or “Toesdays” as a way to remember to take their weekly treatments.
The cure rates are quite good, she said, noting that because the nails grow so slowly, the pace of the treatment matches patient expectations. They don’t finish treatment and still have a “cruddy-looking” nail, she explained.
If any of these oral drugs are used, laboratory monitoring and periodic assessments are necessary, so treatment is a bit complicated. Adverse events remain a concern – particularly drug-drug interactions, drug eruption, cardiac issues (with itraconazole), and loss of taste (with terbinafine).
“So we do have to worry about some of these conditions, which is why having other treatments is so nice,” Dr. Elewski said.
Another reason it’s good to have more options is that no matter which drug you choose, it won’t cure everyone, she noted. Sometimes that’s because the condition is severe; patients with a dermatophyte abscess, those with very thick nails, and those with complete nail involvement associated with a nondermatophyte mold, for example, will have a poorer prognosis, regardless of which oral treatment given. Also, the condition is often complicated by concomitant disorders such as psoriasis. About 5% of patients with psoriasis have nail-only disease, and about a third of them also have onychomycosis. Others are misdiagnosed as having onychomycosis.
Alternatives that can be used alone or in combination with the oral therapies, include the two new topical solutions: efinaconazole and tavaborole, Dr. Elewski said.
Solutions are good, because you can apply them on, under, and around the nail. Both of the drugs have demonstrated effective penetration of the nail plate, allowing penetration to the nail bed where the infection exists, she noted.
The mycological cure rate with efinaconazole yields outcomes comparable to those with oral drugs.
“I think [the mycological cure] is actually the most important endpoint. Because when you want to get rid of the fungus, what do you do? You want to kill the fungus,” said Dr. Elewski, adding that mycological cure is the first sign a patient will go on to experience complete cure.
The complete cure rate is lower, but that appears to be a time-related factor. The nail takes a long time to grow, so the complete cure rate will lag behind the mycological cure rate, Dr. Elewski explained.
The other topical solution – tavaborole – is a new molecule that contains boron. Mycological cure rates in studies of the drug were in the mid-30% range, and it appears able to be used with nail polish without issues to the polish or the outcomes, she said.
In Dr. Elewski’s experience, these topicals work better than expected in clinical practice, based on the clinical trials. One patient who wasn’t eligible for the clinical trials because of a dermatophytosis, for example, was nearly clear within 5 months, and is now totally cured, she said.
“So these treatments are effective as monotherapy, but could be used as an adjunct with systemic therapy, and perhaps also in nondermatophyte cases of onychomycosis,” Dr. Elewski noted. “I think it would be a better option to pick one of these topical drugs than putting someone on a prolonged course of itraconazole if at all possible.” The safety issue would be more favorable with the topical antifungal solution, she said.
A treatment that should never be used is ketoconazole, she noted, explaining that although the drug was never approved specifically for onychomycosis, it was often prescribed for the treatment of tinea versicolor. Because of safety concerns, the FDA removed its indication for all cutaneous fungal infections in 2013.
Dr. Elewski is a consultant for Valeant Pharmaceuticals and a contracted researcher for Anacor Pharmaceuticals.
MIAMI BEACH – Two new topical solutions approved in 2014 for the treatment of distal subungual onychomycosis don’t eliminate the need for oral treatment, but they do represent improvement in the options available to patients, according to Dr. Boni E. Elewski.
Oral treatments, including terbinafine, itraconazole, and fluconazole are still of value – either alone or in combination with the new solutions or other agents – for this type of onychomycosis, which is “essentially a nail bed dermatophytosis,” she said at the South Beach Symposium.
Terbinafine has been used for 2 decades, and is probably the most commonly used treatment for onychomycosis. It is approved as a once-daily pill given for 90 days, and reportedly has a cure rate just under 40%, she said.
Itraconazole is approved as a 200-mg daily dose for 12 weeks, although most physicians use pulse dosing, prescribing 400 mg daily for a week, then for 1 week each month.
“I like this drug for my nondermatophyte patients … if you have something that you don’t think is a dermatophyte, or if they’ve failed terbinafine, this is an excellent option,” she said.
Fluconazole, her “personal favorite,” is used off label for onychomycosis, but has been shown to have good cure rates with once-weekly treatment, said Dr. Elewski of the University of Alabama at Birmingham.
“I’ll confess, I like this drug because I feel comfortable with it,” she said adding that she tells her patients to think about “Fungal Fridays” or “Toesdays” as a way to remember to take their weekly treatments.
The cure rates are quite good, she said, noting that because the nails grow so slowly, the pace of the treatment matches patient expectations. They don’t finish treatment and still have a “cruddy-looking” nail, she explained.
If any of these oral drugs are used, laboratory monitoring and periodic assessments are necessary, so treatment is a bit complicated. Adverse events remain a concern – particularly drug-drug interactions, drug eruption, cardiac issues (with itraconazole), and loss of taste (with terbinafine).
“So we do have to worry about some of these conditions, which is why having other treatments is so nice,” Dr. Elewski said.
Another reason it’s good to have more options is that no matter which drug you choose, it won’t cure everyone, she noted. Sometimes that’s because the condition is severe; patients with a dermatophyte abscess, those with very thick nails, and those with complete nail involvement associated with a nondermatophyte mold, for example, will have a poorer prognosis, regardless of which oral treatment given. Also, the condition is often complicated by concomitant disorders such as psoriasis. About 5% of patients with psoriasis have nail-only disease, and about a third of them also have onychomycosis. Others are misdiagnosed as having onychomycosis.
Alternatives that can be used alone or in combination with the oral therapies, include the two new topical solutions: efinaconazole and tavaborole, Dr. Elewski said.
Solutions are good, because you can apply them on, under, and around the nail. Both of the drugs have demonstrated effective penetration of the nail plate, allowing penetration to the nail bed where the infection exists, she noted.
The mycological cure rate with efinaconazole yields outcomes comparable to those with oral drugs.
“I think [the mycological cure] is actually the most important endpoint. Because when you want to get rid of the fungus, what do you do? You want to kill the fungus,” said Dr. Elewski, adding that mycological cure is the first sign a patient will go on to experience complete cure.
The complete cure rate is lower, but that appears to be a time-related factor. The nail takes a long time to grow, so the complete cure rate will lag behind the mycological cure rate, Dr. Elewski explained.
The other topical solution – tavaborole – is a new molecule that contains boron. Mycological cure rates in studies of the drug were in the mid-30% range, and it appears able to be used with nail polish without issues to the polish or the outcomes, she said.
In Dr. Elewski’s experience, these topicals work better than expected in clinical practice, based on the clinical trials. One patient who wasn’t eligible for the clinical trials because of a dermatophytosis, for example, was nearly clear within 5 months, and is now totally cured, she said.
“So these treatments are effective as monotherapy, but could be used as an adjunct with systemic therapy, and perhaps also in nondermatophyte cases of onychomycosis,” Dr. Elewski noted. “I think it would be a better option to pick one of these topical drugs than putting someone on a prolonged course of itraconazole if at all possible.” The safety issue would be more favorable with the topical antifungal solution, she said.
A treatment that should never be used is ketoconazole, she noted, explaining that although the drug was never approved specifically for onychomycosis, it was often prescribed for the treatment of tinea versicolor. Because of safety concerns, the FDA removed its indication for all cutaneous fungal infections in 2013.
Dr. Elewski is a consultant for Valeant Pharmaceuticals and a contracted researcher for Anacor Pharmaceuticals.
AT THE SOUTH BEACH SYMPOSIUM
Plant-based supplement safely protects against UV radiation
MIAMI BEACH – An oral Polypodium leucotomos extract supplement was safe and effective for sun protection in a randomized, double-blind, placebo-controlled study of 40 participants.
The plant-based supplement, marketed as Heliocare by Ferndale Healthcare, was associated with a significant reduction in the risk of sunburn and the risk of erythema after ultraviolet B exposure, and with a significant increase in sun tolerability, according to study coauthor Dr. Brian Berman, who presented the findings at the South Beach Symposium.
After 2 months of treatment, 8 of 20 participants in the placebo group experienced at least one episode of sunburn, compared with 2 of 20 in the treatment group. At day 28 of treatment, 1 vs. 8 participants in the placebo and treatment groups, respectively, experienced an increased minimal erythema dose (MED), and 3 vs. 10 experienced decreased ultraviolet-induced erythema intensity (J. Clin. Aesthet. Dermatol. 2015;8:19-23).
Study participants were adults aged 18-65 years with Fitzpatrick skin types I-IV. They took 240-mg Heliocare capsules twice daily for 60 days and were assessed by physical examination. Vital signs were measured and clinical laboratory parameters, including hematology, comprehensive metabolic panel, and prothrombin time-partial thromboplastin time, were assessed at baseline and at the end of treatment. Twelve participants in each group also underwent MED testing. The treatment and placebo groups were similar with respect to the prestudy number of sunburns and the number of hours of sun exposure both before and during the study, said Dr. Berman of the University of Miami.
No safety issues associated with treatment were detected. Four participants in the treatment group reported transient mild fatigue, bloating, and headache, and one in the placebo group reported headache.
Polypodium leucotomos is a South American species of fern. Extracts from the fern have been used for at least 4 decades for photoprotection and for treatment of various skin disorders. The current findings suggest that the supplement is a safe and effective means for reducing the damaging effects of ultraviolet radiation, he said.
As with any dietary supplement, Polypodium leucotomos extract is not approved by the Food and Drug Administration to diagnose, treat, cure, or prevent any disease.
Dr. Berman and his colleagues concluded that, based on the excellent safety profile, additional studies assessing higher doses may be warranted.
Dr. Berman is a consultant/speaker for Ferndale Pharmaceuticals and has relationships with several other pharmaceutical companies.
MIAMI BEACH – An oral Polypodium leucotomos extract supplement was safe and effective for sun protection in a randomized, double-blind, placebo-controlled study of 40 participants.
The plant-based supplement, marketed as Heliocare by Ferndale Healthcare, was associated with a significant reduction in the risk of sunburn and the risk of erythema after ultraviolet B exposure, and with a significant increase in sun tolerability, according to study coauthor Dr. Brian Berman, who presented the findings at the South Beach Symposium.
After 2 months of treatment, 8 of 20 participants in the placebo group experienced at least one episode of sunburn, compared with 2 of 20 in the treatment group. At day 28 of treatment, 1 vs. 8 participants in the placebo and treatment groups, respectively, experienced an increased minimal erythema dose (MED), and 3 vs. 10 experienced decreased ultraviolet-induced erythema intensity (J. Clin. Aesthet. Dermatol. 2015;8:19-23).
Study participants were adults aged 18-65 years with Fitzpatrick skin types I-IV. They took 240-mg Heliocare capsules twice daily for 60 days and were assessed by physical examination. Vital signs were measured and clinical laboratory parameters, including hematology, comprehensive metabolic panel, and prothrombin time-partial thromboplastin time, were assessed at baseline and at the end of treatment. Twelve participants in each group also underwent MED testing. The treatment and placebo groups were similar with respect to the prestudy number of sunburns and the number of hours of sun exposure both before and during the study, said Dr. Berman of the University of Miami.
No safety issues associated with treatment were detected. Four participants in the treatment group reported transient mild fatigue, bloating, and headache, and one in the placebo group reported headache.
Polypodium leucotomos is a South American species of fern. Extracts from the fern have been used for at least 4 decades for photoprotection and for treatment of various skin disorders. The current findings suggest that the supplement is a safe and effective means for reducing the damaging effects of ultraviolet radiation, he said.
As with any dietary supplement, Polypodium leucotomos extract is not approved by the Food and Drug Administration to diagnose, treat, cure, or prevent any disease.
Dr. Berman and his colleagues concluded that, based on the excellent safety profile, additional studies assessing higher doses may be warranted.
Dr. Berman is a consultant/speaker for Ferndale Pharmaceuticals and has relationships with several other pharmaceutical companies.
MIAMI BEACH – An oral Polypodium leucotomos extract supplement was safe and effective for sun protection in a randomized, double-blind, placebo-controlled study of 40 participants.
The plant-based supplement, marketed as Heliocare by Ferndale Healthcare, was associated with a significant reduction in the risk of sunburn and the risk of erythema after ultraviolet B exposure, and with a significant increase in sun tolerability, according to study coauthor Dr. Brian Berman, who presented the findings at the South Beach Symposium.
After 2 months of treatment, 8 of 20 participants in the placebo group experienced at least one episode of sunburn, compared with 2 of 20 in the treatment group. At day 28 of treatment, 1 vs. 8 participants in the placebo and treatment groups, respectively, experienced an increased minimal erythema dose (MED), and 3 vs. 10 experienced decreased ultraviolet-induced erythema intensity (J. Clin. Aesthet. Dermatol. 2015;8:19-23).
Study participants were adults aged 18-65 years with Fitzpatrick skin types I-IV. They took 240-mg Heliocare capsules twice daily for 60 days and were assessed by physical examination. Vital signs were measured and clinical laboratory parameters, including hematology, comprehensive metabolic panel, and prothrombin time-partial thromboplastin time, were assessed at baseline and at the end of treatment. Twelve participants in each group also underwent MED testing. The treatment and placebo groups were similar with respect to the prestudy number of sunburns and the number of hours of sun exposure both before and during the study, said Dr. Berman of the University of Miami.
No safety issues associated with treatment were detected. Four participants in the treatment group reported transient mild fatigue, bloating, and headache, and one in the placebo group reported headache.
Polypodium leucotomos is a South American species of fern. Extracts from the fern have been used for at least 4 decades for photoprotection and for treatment of various skin disorders. The current findings suggest that the supplement is a safe and effective means for reducing the damaging effects of ultraviolet radiation, he said.
As with any dietary supplement, Polypodium leucotomos extract is not approved by the Food and Drug Administration to diagnose, treat, cure, or prevent any disease.
Dr. Berman and his colleagues concluded that, based on the excellent safety profile, additional studies assessing higher doses may be warranted.
Dr. Berman is a consultant/speaker for Ferndale Pharmaceuticals and has relationships with several other pharmaceutical companies.
AT THE SOUTH BEACH SYMPOSIUM
Key clinical point: An oral Polypodium leucotomos supplement provides safe and effective photoprotection.
Major finding:. At 2 months, eight placebo-group and two treatment-group participants experienced at least one episode of sunburn.
Data source: A randomized, double-blind, placebo-controlled study of 40 subjects.
Disclosures: Dr. Berman is a consultant/speaker for Ferndale Pharmaceuticals and has relationships with several other pharmaceutical companies.
KOH solution, AK treatment both improve genital warts
MIAMI BEACH – Two novel therapies have potential for the treatment of genital warts, according to Dr. Theodore Rosen.
The first of these “way-off-label” treatments involves application of 5% potassium hydroxide (KOH) solution daily for 12 weeks, said Dr. Rosen, professor of dermatology at Baylor College of Medicine in Houston, Tex.
In a randomized, open-label study of 60 patients conducted in Turkey, researchers compared the KOH treatment with a 10% salicylic acid and 0.5% 5-fluorouracil compound available outside of the United States. The compound used in the study is similar to the WartPEEL (MedCara Pharmaceuticals) product available in the United States, Dr. Rosen said at the South Beach Symposium.
Both treatments were associated with a significant decrease in the number of lesions, and the outcomes were similar in both groups at 12 weeks (mean decrease from 17.03 to 3.73 lesions with KOH and from 16.13 to 3.10 with the 5-FU product). The investigators reported that excellent clearance was achieved by 70% and 76.7% of patients in the KOH and 5-FU groups, respectively, and marked improvement was seen in 13.3% and 20% of patients in the groups, respectively (Int. J. Dermatol. 2014; 53:1145-50).
No difference was seen between the groups in the rate of relapse at 16 weeks, and no serious adverse events were reported.
“This is a dirt-cheap way to treat genital warts,” Dr. Rosen said, adding that clinicians can simply give patients 5% KOH in a small bottle and instruct them to apply it once daily.
A second potential – but “not-so-dirt-cheap” – treatment worth noting is ingenol mebutate, based on findings from another small study, Dr. Rosen said.
Ingenol mebutate (Picato), which is approved for the treatment of actinic keratosis, was shown in 10 patients with human papillomovirus-6–positive genital warts to provide complete clearance with a single application.
The treatment was compared with vehicle in each patient – ingenol mebutate was applied to one affected area, and vehicle was applied to another affected area. The areas with active treatment were completely clear within 3-7 days, and the areas where vehicle was applied were not clear. No recurrence was noted at 3 months in the areas treated with ingenol mebutate.
Not surprisingly, the treatment was associated with mild to moderate burning, but it is a “very, very interesting, very short treatment,” Dr. Rosen said.
Dr. Rosen reported having no relevant disclosures.
MIAMI BEACH – Two novel therapies have potential for the treatment of genital warts, according to Dr. Theodore Rosen.
The first of these “way-off-label” treatments involves application of 5% potassium hydroxide (KOH) solution daily for 12 weeks, said Dr. Rosen, professor of dermatology at Baylor College of Medicine in Houston, Tex.
In a randomized, open-label study of 60 patients conducted in Turkey, researchers compared the KOH treatment with a 10% salicylic acid and 0.5% 5-fluorouracil compound available outside of the United States. The compound used in the study is similar to the WartPEEL (MedCara Pharmaceuticals) product available in the United States, Dr. Rosen said at the South Beach Symposium.
Both treatments were associated with a significant decrease in the number of lesions, and the outcomes were similar in both groups at 12 weeks (mean decrease from 17.03 to 3.73 lesions with KOH and from 16.13 to 3.10 with the 5-FU product). The investigators reported that excellent clearance was achieved by 70% and 76.7% of patients in the KOH and 5-FU groups, respectively, and marked improvement was seen in 13.3% and 20% of patients in the groups, respectively (Int. J. Dermatol. 2014; 53:1145-50).
No difference was seen between the groups in the rate of relapse at 16 weeks, and no serious adverse events were reported.
“This is a dirt-cheap way to treat genital warts,” Dr. Rosen said, adding that clinicians can simply give patients 5% KOH in a small bottle and instruct them to apply it once daily.
A second potential – but “not-so-dirt-cheap” – treatment worth noting is ingenol mebutate, based on findings from another small study, Dr. Rosen said.
Ingenol mebutate (Picato), which is approved for the treatment of actinic keratosis, was shown in 10 patients with human papillomovirus-6–positive genital warts to provide complete clearance with a single application.
The treatment was compared with vehicle in each patient – ingenol mebutate was applied to one affected area, and vehicle was applied to another affected area. The areas with active treatment were completely clear within 3-7 days, and the areas where vehicle was applied were not clear. No recurrence was noted at 3 months in the areas treated with ingenol mebutate.
Not surprisingly, the treatment was associated with mild to moderate burning, but it is a “very, very interesting, very short treatment,” Dr. Rosen said.
Dr. Rosen reported having no relevant disclosures.
MIAMI BEACH – Two novel therapies have potential for the treatment of genital warts, according to Dr. Theodore Rosen.
The first of these “way-off-label” treatments involves application of 5% potassium hydroxide (KOH) solution daily for 12 weeks, said Dr. Rosen, professor of dermatology at Baylor College of Medicine in Houston, Tex.
In a randomized, open-label study of 60 patients conducted in Turkey, researchers compared the KOH treatment with a 10% salicylic acid and 0.5% 5-fluorouracil compound available outside of the United States. The compound used in the study is similar to the WartPEEL (MedCara Pharmaceuticals) product available in the United States, Dr. Rosen said at the South Beach Symposium.
Both treatments were associated with a significant decrease in the number of lesions, and the outcomes were similar in both groups at 12 weeks (mean decrease from 17.03 to 3.73 lesions with KOH and from 16.13 to 3.10 with the 5-FU product). The investigators reported that excellent clearance was achieved by 70% and 76.7% of patients in the KOH and 5-FU groups, respectively, and marked improvement was seen in 13.3% and 20% of patients in the groups, respectively (Int. J. Dermatol. 2014; 53:1145-50).
No difference was seen between the groups in the rate of relapse at 16 weeks, and no serious adverse events were reported.
“This is a dirt-cheap way to treat genital warts,” Dr. Rosen said, adding that clinicians can simply give patients 5% KOH in a small bottle and instruct them to apply it once daily.
A second potential – but “not-so-dirt-cheap” – treatment worth noting is ingenol mebutate, based on findings from another small study, Dr. Rosen said.
Ingenol mebutate (Picato), which is approved for the treatment of actinic keratosis, was shown in 10 patients with human papillomovirus-6–positive genital warts to provide complete clearance with a single application.
The treatment was compared with vehicle in each patient – ingenol mebutate was applied to one affected area, and vehicle was applied to another affected area. The areas with active treatment were completely clear within 3-7 days, and the areas where vehicle was applied were not clear. No recurrence was noted at 3 months in the areas treated with ingenol mebutate.
Not surprisingly, the treatment was associated with mild to moderate burning, but it is a “very, very interesting, very short treatment,” Dr. Rosen said.
Dr. Rosen reported having no relevant disclosures.
EXPERT ANALYSIS FROM THE SOUTH BEACH SYMPOSIUM
Household contamination may promote recurrent MRSA skin infections
MIAMI BEACH – Patients with recurrent methicillin-resistant Staphylococcus aureus skin infections should be advised to address their environment, according to Dr. Theodore Rosen.
Toilets, telephones, hairbrushes, and bathroom sink and door handles are particular culprits in harboring MRSA, Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston, said at the South Beach Symposium.
That was the finding of a recent prospective longitudinal study looking at households in Los Angeles and Chicago with at least one index case of S. aureus skin infection, he said.
Samantha J. Eells of the Los Angeles Biomedical Research Center at Harbor-UCLA Medical Center, Torrance, Calif., and her colleagues enrolled 346 households of adults and children with such infections. Household fomites were surveyed for contamination at baseline and 3 months, and all isolates underwent genetic typing.
Nearly half (49%) of households were contaminated with S. aureus at baseline, and 51% of the 304 that completed follow-up were contaminated at 3 months. Of those with a USA300 MRSA body infection isolate, 58% and 63% had environmental contamination with an indistinguishable MRSA strain at baseline and at 3 months, respectively, the investigators found (Infect. Control. Hosp. Epidemiol. 2014;35:1373-82).
Numerous environmental locations were contaminated up to 3 months after the index case, but among the most common locations with MRSA contamination, with 7%-8% affected among the study households, were toilets, landline phones, index subjects’ hairbrushes, and bathroom sink and door handles. Other areas with contamination detected were children’s toys, television remote controls, kitchen counters, and kitchen sink handles.
“We found that an index subject’s infection isolate frequently persisted for at least 3 months, suggesting that it potentially serves as a reservoir that places all household members at risk for future S. aureus infections. While multiple S. aureus strain types were recovered from some households, the index subject’s infection strain persisted in more than 50% of households after 3 months,” the investigators wrote.
The findings, in the context of the high prevalence of body colonization found in the community-based population, suggests that successful outpatient decolonization may require not only eradication of nasal, skin, and oropharyngeal colonization, but also of environmental contamination, they said.
So not only can patients get MRSA from family members, sex partners, dogs and cats, and raw food sold in grocery stores, as shown in prior studies, they can also get it from the household environment, Dr. Rosen said.
“If you’re doing everything else [to eradicate colonization], you need to make sure someone who is having recurring MRSA outbreaks basically cleans up their environment,” he said.
Dr. Rosen reported having no disclosures.
MIAMI BEACH – Patients with recurrent methicillin-resistant Staphylococcus aureus skin infections should be advised to address their environment, according to Dr. Theodore Rosen.
Toilets, telephones, hairbrushes, and bathroom sink and door handles are particular culprits in harboring MRSA, Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston, said at the South Beach Symposium.
That was the finding of a recent prospective longitudinal study looking at households in Los Angeles and Chicago with at least one index case of S. aureus skin infection, he said.
Samantha J. Eells of the Los Angeles Biomedical Research Center at Harbor-UCLA Medical Center, Torrance, Calif., and her colleagues enrolled 346 households of adults and children with such infections. Household fomites were surveyed for contamination at baseline and 3 months, and all isolates underwent genetic typing.
Nearly half (49%) of households were contaminated with S. aureus at baseline, and 51% of the 304 that completed follow-up were contaminated at 3 months. Of those with a USA300 MRSA body infection isolate, 58% and 63% had environmental contamination with an indistinguishable MRSA strain at baseline and at 3 months, respectively, the investigators found (Infect. Control. Hosp. Epidemiol. 2014;35:1373-82).
Numerous environmental locations were contaminated up to 3 months after the index case, but among the most common locations with MRSA contamination, with 7%-8% affected among the study households, were toilets, landline phones, index subjects’ hairbrushes, and bathroom sink and door handles. Other areas with contamination detected were children’s toys, television remote controls, kitchen counters, and kitchen sink handles.
“We found that an index subject’s infection isolate frequently persisted for at least 3 months, suggesting that it potentially serves as a reservoir that places all household members at risk for future S. aureus infections. While multiple S. aureus strain types were recovered from some households, the index subject’s infection strain persisted in more than 50% of households after 3 months,” the investigators wrote.
The findings, in the context of the high prevalence of body colonization found in the community-based population, suggests that successful outpatient decolonization may require not only eradication of nasal, skin, and oropharyngeal colonization, but also of environmental contamination, they said.
So not only can patients get MRSA from family members, sex partners, dogs and cats, and raw food sold in grocery stores, as shown in prior studies, they can also get it from the household environment, Dr. Rosen said.
“If you’re doing everything else [to eradicate colonization], you need to make sure someone who is having recurring MRSA outbreaks basically cleans up their environment,” he said.
Dr. Rosen reported having no disclosures.
MIAMI BEACH – Patients with recurrent methicillin-resistant Staphylococcus aureus skin infections should be advised to address their environment, according to Dr. Theodore Rosen.
Toilets, telephones, hairbrushes, and bathroom sink and door handles are particular culprits in harboring MRSA, Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston, said at the South Beach Symposium.
That was the finding of a recent prospective longitudinal study looking at households in Los Angeles and Chicago with at least one index case of S. aureus skin infection, he said.
Samantha J. Eells of the Los Angeles Biomedical Research Center at Harbor-UCLA Medical Center, Torrance, Calif., and her colleagues enrolled 346 households of adults and children with such infections. Household fomites were surveyed for contamination at baseline and 3 months, and all isolates underwent genetic typing.
Nearly half (49%) of households were contaminated with S. aureus at baseline, and 51% of the 304 that completed follow-up were contaminated at 3 months. Of those with a USA300 MRSA body infection isolate, 58% and 63% had environmental contamination with an indistinguishable MRSA strain at baseline and at 3 months, respectively, the investigators found (Infect. Control. Hosp. Epidemiol. 2014;35:1373-82).
Numerous environmental locations were contaminated up to 3 months after the index case, but among the most common locations with MRSA contamination, with 7%-8% affected among the study households, were toilets, landline phones, index subjects’ hairbrushes, and bathroom sink and door handles. Other areas with contamination detected were children’s toys, television remote controls, kitchen counters, and kitchen sink handles.
“We found that an index subject’s infection isolate frequently persisted for at least 3 months, suggesting that it potentially serves as a reservoir that places all household members at risk for future S. aureus infections. While multiple S. aureus strain types were recovered from some households, the index subject’s infection strain persisted in more than 50% of households after 3 months,” the investigators wrote.
The findings, in the context of the high prevalence of body colonization found in the community-based population, suggests that successful outpatient decolonization may require not only eradication of nasal, skin, and oropharyngeal colonization, but also of environmental contamination, they said.
So not only can patients get MRSA from family members, sex partners, dogs and cats, and raw food sold in grocery stores, as shown in prior studies, they can also get it from the household environment, Dr. Rosen said.
“If you’re doing everything else [to eradicate colonization], you need to make sure someone who is having recurring MRSA outbreaks basically cleans up their environment,” he said.
Dr. Rosen reported having no disclosures.
Newer antifungals shorten tinea pedis treatment duration, promote adherence
MIAMI BEACH – Two new antifungal agents on the market – luliconazole and naftifine – each have something unique to offer when it comes to treating tinea pedis, according to Dr. Boni E. Elewski.
Luliconazole is an azole drug, meaning it is broad spectrum and kills dermatophytes, yeast, and molds. Also, like all azoles, it has some antibacterial activity, she said at the South Beach Symposium.
Naftifine is an allylamine drug, and is mainly an antidermatophyte agent – albeit a “very, very potent antidermatophyte” – with no antibacterial activity, she said.
Both are approved for once-daily use for 2 weeks, and that’s good because the short treatment duration improves adherence to the regimen, especially compared with other drugs that require 4-6 weeks of treatment to eradicate the problem, noted Dr. Elewski, professor of dermatology and director of clinical trials research at the University of Alabama at Birmingham.
Both drugs also stay in the skin and continue working after treatment stops.
Making the choice regarding which drug or class of drugs to use depends on the patient’s symptoms.
“First of all, tinea pedis may not be obvious. People don’t often tell you, ‘This is what I have – it’s tinea pedis,’ ” she said.
Keep in mind that tinea pedis and onychomycosis are related. If you have a patient who you think has onychomycosis, look at the bottom of their foot, she advised.
“If they don’t have tinea pedis, they probably don’t have onychomycosis unless they’ve had tinea pedis recently and got rid of it,” she said.
Also, look for collarettes of scale, which may be very subtle and may look like “tiny little circular pieces of scale on the medial or lateral foot.”
“If you are not sure, just keep looking harder because you might see it,” Dr. Elewski said.
Interdigital tinea pedis will be a little more obvious, with scaling and crusting between the toes, as well as maceration and oozing in many cases.
When the toe web is oozing, you’re likely dealing with intertrigo, she said.
In such cases, an azole cream is the better treatment choice, because azoles will kill Candida, bacteria, and dermatophytes that are there, she said.
“So when I have a moist macerated space, I like an azole. If you have a dry scaly process – with or without the collarettes – you’re probably better with an allylamine, particularly if you use a keratolytic with it, something that has urea or a lactic acid,” she said.
Dr. Elewski is a consultant for Valeant Pharmaceuticals International and a contracted researcher for Anacor Pharmaceuticals.
MIAMI BEACH – Two new antifungal agents on the market – luliconazole and naftifine – each have something unique to offer when it comes to treating tinea pedis, according to Dr. Boni E. Elewski.
Luliconazole is an azole drug, meaning it is broad spectrum and kills dermatophytes, yeast, and molds. Also, like all azoles, it has some antibacterial activity, she said at the South Beach Symposium.
Naftifine is an allylamine drug, and is mainly an antidermatophyte agent – albeit a “very, very potent antidermatophyte” – with no antibacterial activity, she said.
Both are approved for once-daily use for 2 weeks, and that’s good because the short treatment duration improves adherence to the regimen, especially compared with other drugs that require 4-6 weeks of treatment to eradicate the problem, noted Dr. Elewski, professor of dermatology and director of clinical trials research at the University of Alabama at Birmingham.
Both drugs also stay in the skin and continue working after treatment stops.
Making the choice regarding which drug or class of drugs to use depends on the patient’s symptoms.
“First of all, tinea pedis may not be obvious. People don’t often tell you, ‘This is what I have – it’s tinea pedis,’ ” she said.
Keep in mind that tinea pedis and onychomycosis are related. If you have a patient who you think has onychomycosis, look at the bottom of their foot, she advised.
“If they don’t have tinea pedis, they probably don’t have onychomycosis unless they’ve had tinea pedis recently and got rid of it,” she said.
Also, look for collarettes of scale, which may be very subtle and may look like “tiny little circular pieces of scale on the medial or lateral foot.”
“If you are not sure, just keep looking harder because you might see it,” Dr. Elewski said.
Interdigital tinea pedis will be a little more obvious, with scaling and crusting between the toes, as well as maceration and oozing in many cases.
When the toe web is oozing, you’re likely dealing with intertrigo, she said.
In such cases, an azole cream is the better treatment choice, because azoles will kill Candida, bacteria, and dermatophytes that are there, she said.
“So when I have a moist macerated space, I like an azole. If you have a dry scaly process – with or without the collarettes – you’re probably better with an allylamine, particularly if you use a keratolytic with it, something that has urea or a lactic acid,” she said.
Dr. Elewski is a consultant for Valeant Pharmaceuticals International and a contracted researcher for Anacor Pharmaceuticals.
MIAMI BEACH – Two new antifungal agents on the market – luliconazole and naftifine – each have something unique to offer when it comes to treating tinea pedis, according to Dr. Boni E. Elewski.
Luliconazole is an azole drug, meaning it is broad spectrum and kills dermatophytes, yeast, and molds. Also, like all azoles, it has some antibacterial activity, she said at the South Beach Symposium.
Naftifine is an allylamine drug, and is mainly an antidermatophyte agent – albeit a “very, very potent antidermatophyte” – with no antibacterial activity, she said.
Both are approved for once-daily use for 2 weeks, and that’s good because the short treatment duration improves adherence to the regimen, especially compared with other drugs that require 4-6 weeks of treatment to eradicate the problem, noted Dr. Elewski, professor of dermatology and director of clinical trials research at the University of Alabama at Birmingham.
Both drugs also stay in the skin and continue working after treatment stops.
Making the choice regarding which drug or class of drugs to use depends on the patient’s symptoms.
“First of all, tinea pedis may not be obvious. People don’t often tell you, ‘This is what I have – it’s tinea pedis,’ ” she said.
Keep in mind that tinea pedis and onychomycosis are related. If you have a patient who you think has onychomycosis, look at the bottom of their foot, she advised.
“If they don’t have tinea pedis, they probably don’t have onychomycosis unless they’ve had tinea pedis recently and got rid of it,” she said.
Also, look for collarettes of scale, which may be very subtle and may look like “tiny little circular pieces of scale on the medial or lateral foot.”
“If you are not sure, just keep looking harder because you might see it,” Dr. Elewski said.
Interdigital tinea pedis will be a little more obvious, with scaling and crusting between the toes, as well as maceration and oozing in many cases.
When the toe web is oozing, you’re likely dealing with intertrigo, she said.
In such cases, an azole cream is the better treatment choice, because azoles will kill Candida, bacteria, and dermatophytes that are there, she said.
“So when I have a moist macerated space, I like an azole. If you have a dry scaly process – with or without the collarettes – you’re probably better with an allylamine, particularly if you use a keratolytic with it, something that has urea or a lactic acid,” she said.
Dr. Elewski is a consultant for Valeant Pharmaceuticals International and a contracted researcher for Anacor Pharmaceuticals.
AT THE SOUTH BEACH SYMPOSIUM
Novel ‘soft anticholinergic’ shows promise for axillary hyperhidrosis
MIAMI BEACH – A promising new treatment for axillary hyperhidrosis will soon be evaluated in a clinical trial.
The novel molecular compound – a “soft anticholinergic” – was safe and effective as a topical treatment in a recent pilot study, Dr. Brian Berman reported in a session on innovations in clinical therapeutics at the South Beach Symposium.
Currently known as BBI-4000, the product is locally active but quickly metabolized if absorbed, which reduces the risk of treatment-related adverse events, said Dr. Berman of the University of Miami.
No comparable first-line topical prescription treatment exists for axillary hyperhidrosis, he noted.
In the pilot study – the results of which were reported in September by the manufacturer, Brickell Biotech – 18 subjects were randomized to receive once-daily treatment with either a high topical concentration, a low topical concentration, or topical vehicle for 14 days. Of the 12 patients receiving active treatment, 75% achieved at least a 50% sweat reduction, compared with 33% of the 6 patients in the vehicle group.
Further, 67% of those receiving active treatment, compared with 33% in the vehicle group, achieved at least a two-point improvement in their Hyperhidrosis Disease Severity Scale score, indicating substantial improvement, Dr. Berman said.
No treatment-related adverse events were reported, and none of the patients discontinued treatment because of adverse events.
Based on these findings, Brickell Biotech has initiated a randomized, double-blind, vehicle-controlled phase IIb clinical trial with plans to enroll 180 patients with primary axillary hyperhidrosis. The trial will assess the safety and efficacy of three different concentrations of BBI-4000, compared with vehicle.
Dr. Berman is a consultant, researcher, and/or speaker for Beiersdorf, DUSA Pharmaceuticals, Ferndale Pharmaceuticals, Galderma Laboratories, Genentech, Halscion, LEO Pharmaceuticals, Onset Therapeutics, Sensus Healthcare, Tigercat Pharma, and TopMD Skin Care. He also owns stock in TopMD Skin Care.
MIAMI BEACH – A promising new treatment for axillary hyperhidrosis will soon be evaluated in a clinical trial.
The novel molecular compound – a “soft anticholinergic” – was safe and effective as a topical treatment in a recent pilot study, Dr. Brian Berman reported in a session on innovations in clinical therapeutics at the South Beach Symposium.
Currently known as BBI-4000, the product is locally active but quickly metabolized if absorbed, which reduces the risk of treatment-related adverse events, said Dr. Berman of the University of Miami.
No comparable first-line topical prescription treatment exists for axillary hyperhidrosis, he noted.
In the pilot study – the results of which were reported in September by the manufacturer, Brickell Biotech – 18 subjects were randomized to receive once-daily treatment with either a high topical concentration, a low topical concentration, or topical vehicle for 14 days. Of the 12 patients receiving active treatment, 75% achieved at least a 50% sweat reduction, compared with 33% of the 6 patients in the vehicle group.
Further, 67% of those receiving active treatment, compared with 33% in the vehicle group, achieved at least a two-point improvement in their Hyperhidrosis Disease Severity Scale score, indicating substantial improvement, Dr. Berman said.
No treatment-related adverse events were reported, and none of the patients discontinued treatment because of adverse events.
Based on these findings, Brickell Biotech has initiated a randomized, double-blind, vehicle-controlled phase IIb clinical trial with plans to enroll 180 patients with primary axillary hyperhidrosis. The trial will assess the safety and efficacy of three different concentrations of BBI-4000, compared with vehicle.
Dr. Berman is a consultant, researcher, and/or speaker for Beiersdorf, DUSA Pharmaceuticals, Ferndale Pharmaceuticals, Galderma Laboratories, Genentech, Halscion, LEO Pharmaceuticals, Onset Therapeutics, Sensus Healthcare, Tigercat Pharma, and TopMD Skin Care. He also owns stock in TopMD Skin Care.
MIAMI BEACH – A promising new treatment for axillary hyperhidrosis will soon be evaluated in a clinical trial.
The novel molecular compound – a “soft anticholinergic” – was safe and effective as a topical treatment in a recent pilot study, Dr. Brian Berman reported in a session on innovations in clinical therapeutics at the South Beach Symposium.
Currently known as BBI-4000, the product is locally active but quickly metabolized if absorbed, which reduces the risk of treatment-related adverse events, said Dr. Berman of the University of Miami.
No comparable first-line topical prescription treatment exists for axillary hyperhidrosis, he noted.
In the pilot study – the results of which were reported in September by the manufacturer, Brickell Biotech – 18 subjects were randomized to receive once-daily treatment with either a high topical concentration, a low topical concentration, or topical vehicle for 14 days. Of the 12 patients receiving active treatment, 75% achieved at least a 50% sweat reduction, compared with 33% of the 6 patients in the vehicle group.
Further, 67% of those receiving active treatment, compared with 33% in the vehicle group, achieved at least a two-point improvement in their Hyperhidrosis Disease Severity Scale score, indicating substantial improvement, Dr. Berman said.
No treatment-related adverse events were reported, and none of the patients discontinued treatment because of adverse events.
Based on these findings, Brickell Biotech has initiated a randomized, double-blind, vehicle-controlled phase IIb clinical trial with plans to enroll 180 patients with primary axillary hyperhidrosis. The trial will assess the safety and efficacy of three different concentrations of BBI-4000, compared with vehicle.
Dr. Berman is a consultant, researcher, and/or speaker for Beiersdorf, DUSA Pharmaceuticals, Ferndale Pharmaceuticals, Galderma Laboratories, Genentech, Halscion, LEO Pharmaceuticals, Onset Therapeutics, Sensus Healthcare, Tigercat Pharma, and TopMD Skin Care. He also owns stock in TopMD Skin Care.
AT THE SOUTH BEACH SYMPOSIUM
Hyaluronic acid wrinkle filler also appears useful for midface augmentation
MIAMI BEACH – A large gel particle hyaluronic acid filler with lidocaine (LGP-HAL) was safe and effective for midface augmentation and correction of midface contour deficiencies in a randomized, multicenter, pivotal study.
The product (Perlane, Galderma Laboratories), which is currently approved for moderate to severe facial folds and wrinkles, provided visible, clinically meaningful, aesthetic results for at least 12 months based on assessment by a blinded evaluator, Dr. David E. Bank reported in a poster at the South Beach Symposium.
At 8 weeks after initial treatment, 89% of 150 treated subjects experienced at least a one-point improvement in their combined right and left side Medicis Midface Volume Scale (MMVS) score, compared with 16% of 50 patients who were not treated.
Further, the treated subjects were assessed as having greater midface fullness than the nontreated subjects at every measurement during the first 12 months after treatment, according to Dr. Bank of New York–Presbyterian Hospital/Columbia University Medical Center, New York.
Study participants were men and women aged 18-65 years (mean of 53 years) with MMVS scores of two, three, or four on the validated one- to four-point scale, with one indicating a fairly full midface and four indicating substantial loss of fullness in the midface area. At the initial treatment, the 150 treatment group subjects were injected with a mean volume of 4.21 mL. All but one received subcutaneous injections, and 115 received supraperiosteal injections.
They were evaluated at weeks 2 (when an optional touch-up was allowed) and 4, and months 2, 4, 6, 8, 10, and 12 following the initial treatment.
The treatment group subjects were treated again at 12 months, along with those not treated initially. Another touch-up was allowed at a 2-week assessment, and additional assessments were conducted at weeks 3 and 4.
Treatment was well tolerated. More than 80% of events reported after treatment were mild in severity, and the adverse events that occurred in more than 5% of subjects included implant site hematoma, hemorrhage, pain, swelling, and headache.
One subject had two cases of implant site inflammation that were deemed related by the investigators, and one experienced a serious adverse event (implant site hematoma) deemed related to the procedure as opposed to the device.
No additional risk was seen in those who were treated twice, Dr. Bank noted.
“Facial shape plays an important role in facial attractiveness,” he wrote, adding that full cheeks, in particular, play an important role.
Aging, however, is associated with increased tissue laxity, soft-tissue descent and deflation, and loss of bone, which all contribute to deficiencies in the midface.
Hyaluronic fillers have been used successfully to correct such deficiencies, and the current findings suggest that LGP-HAL also can be used safely to enhance midface aesthetics, he concluded.
This study was funded by Galderma Laboratories.
MIAMI BEACH – A large gel particle hyaluronic acid filler with lidocaine (LGP-HAL) was safe and effective for midface augmentation and correction of midface contour deficiencies in a randomized, multicenter, pivotal study.
The product (Perlane, Galderma Laboratories), which is currently approved for moderate to severe facial folds and wrinkles, provided visible, clinically meaningful, aesthetic results for at least 12 months based on assessment by a blinded evaluator, Dr. David E. Bank reported in a poster at the South Beach Symposium.
At 8 weeks after initial treatment, 89% of 150 treated subjects experienced at least a one-point improvement in their combined right and left side Medicis Midface Volume Scale (MMVS) score, compared with 16% of 50 patients who were not treated.
Further, the treated subjects were assessed as having greater midface fullness than the nontreated subjects at every measurement during the first 12 months after treatment, according to Dr. Bank of New York–Presbyterian Hospital/Columbia University Medical Center, New York.
Study participants were men and women aged 18-65 years (mean of 53 years) with MMVS scores of two, three, or four on the validated one- to four-point scale, with one indicating a fairly full midface and four indicating substantial loss of fullness in the midface area. At the initial treatment, the 150 treatment group subjects were injected with a mean volume of 4.21 mL. All but one received subcutaneous injections, and 115 received supraperiosteal injections.
They were evaluated at weeks 2 (when an optional touch-up was allowed) and 4, and months 2, 4, 6, 8, 10, and 12 following the initial treatment.
The treatment group subjects were treated again at 12 months, along with those not treated initially. Another touch-up was allowed at a 2-week assessment, and additional assessments were conducted at weeks 3 and 4.
Treatment was well tolerated. More than 80% of events reported after treatment were mild in severity, and the adverse events that occurred in more than 5% of subjects included implant site hematoma, hemorrhage, pain, swelling, and headache.
One subject had two cases of implant site inflammation that were deemed related by the investigators, and one experienced a serious adverse event (implant site hematoma) deemed related to the procedure as opposed to the device.
No additional risk was seen in those who were treated twice, Dr. Bank noted.
“Facial shape plays an important role in facial attractiveness,” he wrote, adding that full cheeks, in particular, play an important role.
Aging, however, is associated with increased tissue laxity, soft-tissue descent and deflation, and loss of bone, which all contribute to deficiencies in the midface.
Hyaluronic fillers have been used successfully to correct such deficiencies, and the current findings suggest that LGP-HAL also can be used safely to enhance midface aesthetics, he concluded.
This study was funded by Galderma Laboratories.
MIAMI BEACH – A large gel particle hyaluronic acid filler with lidocaine (LGP-HAL) was safe and effective for midface augmentation and correction of midface contour deficiencies in a randomized, multicenter, pivotal study.
The product (Perlane, Galderma Laboratories), which is currently approved for moderate to severe facial folds and wrinkles, provided visible, clinically meaningful, aesthetic results for at least 12 months based on assessment by a blinded evaluator, Dr. David E. Bank reported in a poster at the South Beach Symposium.
At 8 weeks after initial treatment, 89% of 150 treated subjects experienced at least a one-point improvement in their combined right and left side Medicis Midface Volume Scale (MMVS) score, compared with 16% of 50 patients who were not treated.
Further, the treated subjects were assessed as having greater midface fullness than the nontreated subjects at every measurement during the first 12 months after treatment, according to Dr. Bank of New York–Presbyterian Hospital/Columbia University Medical Center, New York.
Study participants were men and women aged 18-65 years (mean of 53 years) with MMVS scores of two, three, or four on the validated one- to four-point scale, with one indicating a fairly full midface and four indicating substantial loss of fullness in the midface area. At the initial treatment, the 150 treatment group subjects were injected with a mean volume of 4.21 mL. All but one received subcutaneous injections, and 115 received supraperiosteal injections.
They were evaluated at weeks 2 (when an optional touch-up was allowed) and 4, and months 2, 4, 6, 8, 10, and 12 following the initial treatment.
The treatment group subjects were treated again at 12 months, along with those not treated initially. Another touch-up was allowed at a 2-week assessment, and additional assessments were conducted at weeks 3 and 4.
Treatment was well tolerated. More than 80% of events reported after treatment were mild in severity, and the adverse events that occurred in more than 5% of subjects included implant site hematoma, hemorrhage, pain, swelling, and headache.
One subject had two cases of implant site inflammation that were deemed related by the investigators, and one experienced a serious adverse event (implant site hematoma) deemed related to the procedure as opposed to the device.
No additional risk was seen in those who were treated twice, Dr. Bank noted.
“Facial shape plays an important role in facial attractiveness,” he wrote, adding that full cheeks, in particular, play an important role.
Aging, however, is associated with increased tissue laxity, soft-tissue descent and deflation, and loss of bone, which all contribute to deficiencies in the midface.
Hyaluronic fillers have been used successfully to correct such deficiencies, and the current findings suggest that LGP-HAL also can be used safely to enhance midface aesthetics, he concluded.
This study was funded by Galderma Laboratories.
Key clinical point: LGP-HAL (Perlane) is safe and effective for midface augmentation.
Major finding: At 8 weeks, 89% of treated subjects vs. 16% of controls experienced at least a one-point improvement in MMVS score.
Data source: A randomized, multicenter, pivotal study of 200 subjects.
Disclosures This study was funded by Galderma Laboratories.
Acne scar type determines treatment approach
MIAMI BEACH – Do you cringe when a patient comes in wanting treatment for acne scars? Fear not; such patients are usually very motivated, and effective treatment options exist, according to Dr. Gary Monheit.
It’s true that you can’t perfect their skin, that there is a need to deal with the psychology of what their scarring has meant for them, and that it may take a significant amount of time to temper expectations, he said at the South Beach Symposium.
“But once you win them over, they can be some of the most grateful patients you have. … Don’t turn them away,” he said.
There’s not a lot of training available for treating acne scars, but using existing skill, good judgment, and a combination of tools and techniques can lead to excellent results, said Dr. Monheit, who is in private practice in Birmingham, Ala.
Leveling patient expectations comes down to educating patients about the different kinds of scars and the fact that each type requires different tools.
For example, fillers can be useful – but only for distensible scars. If the scar rises up when the skin is stretched, that’s a distensible scar that will respond to a filler, he explained.
Conversely, if injectable fillers are used in scars with bands that hold them down in deeper tissue, the filler will migrate, surround the scar, and exaggerate its appearance.
A classification system described by Dr. Greg Goodman (Int. J. Dermatol. 2011;50:1179-94]) is useful for determining the best treatment approach based on scar type, he said.
Dr. Goodman described level 1 scars as flat with red, white, or brown marks. Level 2 scars are mild scars visible primarily at close distance, such as while looking in the mirror. Level 3 scars are visible at a conversational distance, but are distensible. Level 4 scars are more severe and are nondistensible.
Peels, cosmeceuticals, and light resurfacing techniques such as the V-beam laser or intense pulsed light, are best for mild scars like level 1 scars, as they typically are erythematous and involve color change, Dr. Monheit said.
For hyperpigmented level 1 scars, consider using topical treatments such as salicylic acid or bleaching agents. For hypopigmented scars, there is a need to blend skin color.
The main thing with these types of scars is to address the color and texture, he said.
Level 2 scars are atrophic, and involve textural and color change. Other resurfacing techniques, including deeper peels, dermabrasion, and laser resurfacing such as with a fractional laser or an ablative laser are particularly useful for these, he said, noting that the treatments can be combined as necessary to achieve the desired results.
Dermarolling also can be helpful for deeper scars.
“As you get into the dermis and deeper, you are breaking collagen bonds and letting skin rise to the surface, and you can also use [dermarolling] as a conduit for whatever you are doing,” he explained.
Needling is another approach that is relatively safe, inexpensive, and involves minimal down time. However, the treatment can be bloody and painful with the depths typically needed for treating scars, he noted.
Moderate scars that are distensible, like level 3 scars, generally need lifting. Fillers are good for these types of scars, and often there is very little more that needs to be done.
Nondistensible scars that are more severe typically require a combination approach. One of the best tools for these types of scars is subcision to loosen them, which creates a pocket that then can be either allowed to fill with blood to raise the scar up or treated with an injectable filler. This is followed by resurfacing to smooth the surface of the skin.
Dr. Monheit described one patient in whom he subcised the nondistensible scars and injected a very small amount – just enough for a surface lift – of Radiesse filler. Significant improvement was seen after two treatment sessions, and then resurfacing was performed.
In another patient with nondistensible scars, he excised a little bit of skin at the scar and performed what amounted to a collagen graft by “chopping it into little pieces – making little pellets – and essentially stuffing those pellets into the pockets with a tiny little needle, giving it lift and stability.” This was followed by resurfacing.
“It really does give a nice result,” he said, noting that subcision may cause some red bumps at first, but these will settle down and the scar will smooth out.
A different approach is needed for other types of scars, such as ice pick scars.
One approach is to punch out the scar surgically and either float the scar up or put a little punch graft in, he said.
Another approach that he uses for ice pick scars is to lightly apply 90% trichloroacetic acid to the base of the scar with a toothpick. This causes an inflammatory reaction that destroys the scar and raises it up, making it distensible and resurfaceable, he said.
Ice pick scars, as well as boxcar scars, divot scars, and hypertrophic scars, need to be refined during the treatment process.
Also keep in mind that patients lose volume as they age, which can cause acne scars to become more prominent. Sometimes a patient will come in with old scars that never bothered them before, and suddenly the scars are much more visible.
“If you volume-fill the face, you’ll improve the scars,” he said, describing one 48-year-old woman who was treated successfully with three sessions of Sculptra, topped off with a little bit of Radiesse.
Dr. Monheit has consulted or performed contracted research for Allergan, Contura, Dermik Laboratories, Galderma Laboratories, Ipsen/Medicis, Kythera Biopharmaceuticals, MELA Sciences, Merz Pharmaceuticals, MyoScience, and Revance Therapeutics.
MIAMI BEACH – Do you cringe when a patient comes in wanting treatment for acne scars? Fear not; such patients are usually very motivated, and effective treatment options exist, according to Dr. Gary Monheit.
It’s true that you can’t perfect their skin, that there is a need to deal with the psychology of what their scarring has meant for them, and that it may take a significant amount of time to temper expectations, he said at the South Beach Symposium.
“But once you win them over, they can be some of the most grateful patients you have. … Don’t turn them away,” he said.
There’s not a lot of training available for treating acne scars, but using existing skill, good judgment, and a combination of tools and techniques can lead to excellent results, said Dr. Monheit, who is in private practice in Birmingham, Ala.
Leveling patient expectations comes down to educating patients about the different kinds of scars and the fact that each type requires different tools.
For example, fillers can be useful – but only for distensible scars. If the scar rises up when the skin is stretched, that’s a distensible scar that will respond to a filler, he explained.
Conversely, if injectable fillers are used in scars with bands that hold them down in deeper tissue, the filler will migrate, surround the scar, and exaggerate its appearance.
A classification system described by Dr. Greg Goodman (Int. J. Dermatol. 2011;50:1179-94]) is useful for determining the best treatment approach based on scar type, he said.
Dr. Goodman described level 1 scars as flat with red, white, or brown marks. Level 2 scars are mild scars visible primarily at close distance, such as while looking in the mirror. Level 3 scars are visible at a conversational distance, but are distensible. Level 4 scars are more severe and are nondistensible.
Peels, cosmeceuticals, and light resurfacing techniques such as the V-beam laser or intense pulsed light, are best for mild scars like level 1 scars, as they typically are erythematous and involve color change, Dr. Monheit said.
For hyperpigmented level 1 scars, consider using topical treatments such as salicylic acid or bleaching agents. For hypopigmented scars, there is a need to blend skin color.
The main thing with these types of scars is to address the color and texture, he said.
Level 2 scars are atrophic, and involve textural and color change. Other resurfacing techniques, including deeper peels, dermabrasion, and laser resurfacing such as with a fractional laser or an ablative laser are particularly useful for these, he said, noting that the treatments can be combined as necessary to achieve the desired results.
Dermarolling also can be helpful for deeper scars.
“As you get into the dermis and deeper, you are breaking collagen bonds and letting skin rise to the surface, and you can also use [dermarolling] as a conduit for whatever you are doing,” he explained.
Needling is another approach that is relatively safe, inexpensive, and involves minimal down time. However, the treatment can be bloody and painful with the depths typically needed for treating scars, he noted.
Moderate scars that are distensible, like level 3 scars, generally need lifting. Fillers are good for these types of scars, and often there is very little more that needs to be done.
Nondistensible scars that are more severe typically require a combination approach. One of the best tools for these types of scars is subcision to loosen them, which creates a pocket that then can be either allowed to fill with blood to raise the scar up or treated with an injectable filler. This is followed by resurfacing to smooth the surface of the skin.
Dr. Monheit described one patient in whom he subcised the nondistensible scars and injected a very small amount – just enough for a surface lift – of Radiesse filler. Significant improvement was seen after two treatment sessions, and then resurfacing was performed.
In another patient with nondistensible scars, he excised a little bit of skin at the scar and performed what amounted to a collagen graft by “chopping it into little pieces – making little pellets – and essentially stuffing those pellets into the pockets with a tiny little needle, giving it lift and stability.” This was followed by resurfacing.
“It really does give a nice result,” he said, noting that subcision may cause some red bumps at first, but these will settle down and the scar will smooth out.
A different approach is needed for other types of scars, such as ice pick scars.
One approach is to punch out the scar surgically and either float the scar up or put a little punch graft in, he said.
Another approach that he uses for ice pick scars is to lightly apply 90% trichloroacetic acid to the base of the scar with a toothpick. This causes an inflammatory reaction that destroys the scar and raises it up, making it distensible and resurfaceable, he said.
Ice pick scars, as well as boxcar scars, divot scars, and hypertrophic scars, need to be refined during the treatment process.
Also keep in mind that patients lose volume as they age, which can cause acne scars to become more prominent. Sometimes a patient will come in with old scars that never bothered them before, and suddenly the scars are much more visible.
“If you volume-fill the face, you’ll improve the scars,” he said, describing one 48-year-old woman who was treated successfully with three sessions of Sculptra, topped off with a little bit of Radiesse.
Dr. Monheit has consulted or performed contracted research for Allergan, Contura, Dermik Laboratories, Galderma Laboratories, Ipsen/Medicis, Kythera Biopharmaceuticals, MELA Sciences, Merz Pharmaceuticals, MyoScience, and Revance Therapeutics.
MIAMI BEACH – Do you cringe when a patient comes in wanting treatment for acne scars? Fear not; such patients are usually very motivated, and effective treatment options exist, according to Dr. Gary Monheit.
It’s true that you can’t perfect their skin, that there is a need to deal with the psychology of what their scarring has meant for them, and that it may take a significant amount of time to temper expectations, he said at the South Beach Symposium.
“But once you win them over, they can be some of the most grateful patients you have. … Don’t turn them away,” he said.
There’s not a lot of training available for treating acne scars, but using existing skill, good judgment, and a combination of tools and techniques can lead to excellent results, said Dr. Monheit, who is in private practice in Birmingham, Ala.
Leveling patient expectations comes down to educating patients about the different kinds of scars and the fact that each type requires different tools.
For example, fillers can be useful – but only for distensible scars. If the scar rises up when the skin is stretched, that’s a distensible scar that will respond to a filler, he explained.
Conversely, if injectable fillers are used in scars with bands that hold them down in deeper tissue, the filler will migrate, surround the scar, and exaggerate its appearance.
A classification system described by Dr. Greg Goodman (Int. J. Dermatol. 2011;50:1179-94]) is useful for determining the best treatment approach based on scar type, he said.
Dr. Goodman described level 1 scars as flat with red, white, or brown marks. Level 2 scars are mild scars visible primarily at close distance, such as while looking in the mirror. Level 3 scars are visible at a conversational distance, but are distensible. Level 4 scars are more severe and are nondistensible.
Peels, cosmeceuticals, and light resurfacing techniques such as the V-beam laser or intense pulsed light, are best for mild scars like level 1 scars, as they typically are erythematous and involve color change, Dr. Monheit said.
For hyperpigmented level 1 scars, consider using topical treatments such as salicylic acid or bleaching agents. For hypopigmented scars, there is a need to blend skin color.
The main thing with these types of scars is to address the color and texture, he said.
Level 2 scars are atrophic, and involve textural and color change. Other resurfacing techniques, including deeper peels, dermabrasion, and laser resurfacing such as with a fractional laser or an ablative laser are particularly useful for these, he said, noting that the treatments can be combined as necessary to achieve the desired results.
Dermarolling also can be helpful for deeper scars.
“As you get into the dermis and deeper, you are breaking collagen bonds and letting skin rise to the surface, and you can also use [dermarolling] as a conduit for whatever you are doing,” he explained.
Needling is another approach that is relatively safe, inexpensive, and involves minimal down time. However, the treatment can be bloody and painful with the depths typically needed for treating scars, he noted.
Moderate scars that are distensible, like level 3 scars, generally need lifting. Fillers are good for these types of scars, and often there is very little more that needs to be done.
Nondistensible scars that are more severe typically require a combination approach. One of the best tools for these types of scars is subcision to loosen them, which creates a pocket that then can be either allowed to fill with blood to raise the scar up or treated with an injectable filler. This is followed by resurfacing to smooth the surface of the skin.
Dr. Monheit described one patient in whom he subcised the nondistensible scars and injected a very small amount – just enough for a surface lift – of Radiesse filler. Significant improvement was seen after two treatment sessions, and then resurfacing was performed.
In another patient with nondistensible scars, he excised a little bit of skin at the scar and performed what amounted to a collagen graft by “chopping it into little pieces – making little pellets – and essentially stuffing those pellets into the pockets with a tiny little needle, giving it lift and stability.” This was followed by resurfacing.
“It really does give a nice result,” he said, noting that subcision may cause some red bumps at first, but these will settle down and the scar will smooth out.
A different approach is needed for other types of scars, such as ice pick scars.
One approach is to punch out the scar surgically and either float the scar up or put a little punch graft in, he said.
Another approach that he uses for ice pick scars is to lightly apply 90% trichloroacetic acid to the base of the scar with a toothpick. This causes an inflammatory reaction that destroys the scar and raises it up, making it distensible and resurfaceable, he said.
Ice pick scars, as well as boxcar scars, divot scars, and hypertrophic scars, need to be refined during the treatment process.
Also keep in mind that patients lose volume as they age, which can cause acne scars to become more prominent. Sometimes a patient will come in with old scars that never bothered them before, and suddenly the scars are much more visible.
“If you volume-fill the face, you’ll improve the scars,” he said, describing one 48-year-old woman who was treated successfully with three sessions of Sculptra, topped off with a little bit of Radiesse.
Dr. Monheit has consulted or performed contracted research for Allergan, Contura, Dermik Laboratories, Galderma Laboratories, Ipsen/Medicis, Kythera Biopharmaceuticals, MELA Sciences, Merz Pharmaceuticals, MyoScience, and Revance Therapeutics.
New Psoriasis Drugs Offer Treatment Advantages
MIAMI BEACH – Two of the newest treatments available for psoriasis – apremilast and secukinumab – are true “game-changers,” according to Dr. David M. Pariser.
Apremilast (Otezla), a recently approved oral phosphodiesterase 4 inhibitor, will be a particularly attractive treatment option for many dermatologists and patients, he said at the South Beach Symposium.
Apremilast has a very limited effect on the immune system, and it’s an oral therapy and thus requires no needles. It is very safe – with “strikingly few” serious adverse events – and no laboratory monitoring is required, he explained.
Efficacy results with apremilast are modest. In the phase III ESTEEM trial, for example, 33% of patients achieved at least 75% improvement (PASI-75), compared with 5% of patients who received placebo, said Dr. Pariser of Eastern Virginia Medical School, Norfolk, and an investigator for the trial.
Further, the drug can be used for almost any patient and type of psoriasis; it is an option for those who want systemic therapy, but who don’t want to go on a biologic or methotrexate, and its use is not precluded by a history of cancer or infections, as is the case with biologics, he added.
Apremilast also will be attractive for dermatologists who do not currently prescribe systemic therapy for psoriasis, or who don’t use aggressive systemic therapy for psoriasis, he said.
“If a prescriber feels safety is more important than efficacy, this might be a good choice,” he said.
Continue for information on secukinumab >>
Secukinumab (Cosentyx), on the other hand, is a “big gun,” Dr. Pariser said of the biologic, which was approved in January 2015 for the treatment of adults with moderate to severe plaque psoriasis.
“It’s the biggest gun we’ve got now … and it really has a safety profile similar to existing biologics so far,” he said.
The fully human monoclonal antibody inhibits interleukin-17A and is administered by subcutaneous injection. Its safety and efficacy were demonstrated in numerous of studies involving about 4,500 patients. Treatment was associated with significant improvement, compared with placebo, said Dr. Pariser, who also was an investigator on secukinumab trials.
Of note, while the PASI-75 findings for secukinumab are “a nice number but not dramatically higher than things we have had in the past,” the PASI-90 and PASI-100 scores are remarkable, he said.
At the highest dose studied (300 mg given at weeks 1, 2, 3, 4, and 8, and monthly thereafter), PASI-90 was achieved by 59% of patients in one study, and PASI-100 was achieved in 28%.
“That’s significant. We haven’t had that before, and that is really, really, really nice,” he said.
Further, the primary efficacy endpoint of the study was outcome at 12 weeks, but patients continued to improve at least until 16 weeks.
PASI-100 – no psoriasis whatsoever – was 40% at 16 weeks, he said.
In another phase III trial, secukinumab was again shown to be superior to placebo, but it also compared favorably with etanercept, he noted.
Safety was reasonable in the secukinumab trials. No deaths occurred, but there were more serious adverse events and discontinuations in the active treatment group. Nasopharyngitis was the most common serious adverse events, and it occurred in all groups. Upper respiratory tract infections appeared to be more common in the secukinumab patients, he noted.
An important consideration with secukinumab, however, is the need for continuous treatment, he said.
“The bottom line, really, is that patients should stay on it. They will lose effectiveness if they go off of it or take it on an as-needed basis,” he said.
Dr. Pariser is a consultant and/or researcher for Amgen, AbbVie, Celgene, Eli Lilly, Janssen Pharmaceuticals, Merck, and Pfizer.
MIAMI BEACH – Two of the newest treatments available for psoriasis – apremilast and secukinumab – are true “game-changers,” according to Dr. David M. Pariser.
Apremilast (Otezla), a recently approved oral phosphodiesterase 4 inhibitor, will be a particularly attractive treatment option for many dermatologists and patients, he said at the South Beach Symposium.
Apremilast has a very limited effect on the immune system, and it’s an oral therapy and thus requires no needles. It is very safe – with “strikingly few” serious adverse events – and no laboratory monitoring is required, he explained.
Efficacy results with apremilast are modest. In the phase III ESTEEM trial, for example, 33% of patients achieved at least 75% improvement (PASI-75), compared with 5% of patients who received placebo, said Dr. Pariser of Eastern Virginia Medical School, Norfolk, and an investigator for the trial.
Further, the drug can be used for almost any patient and type of psoriasis; it is an option for those who want systemic therapy, but who don’t want to go on a biologic or methotrexate, and its use is not precluded by a history of cancer or infections, as is the case with biologics, he added.
Apremilast also will be attractive for dermatologists who do not currently prescribe systemic therapy for psoriasis, or who don’t use aggressive systemic therapy for psoriasis, he said.
“If a prescriber feels safety is more important than efficacy, this might be a good choice,” he said.
Continue for information on secukinumab >>
Secukinumab (Cosentyx), on the other hand, is a “big gun,” Dr. Pariser said of the biologic, which was approved in January 2015 for the treatment of adults with moderate to severe plaque psoriasis.
“It’s the biggest gun we’ve got now … and it really has a safety profile similar to existing biologics so far,” he said.
The fully human monoclonal antibody inhibits interleukin-17A and is administered by subcutaneous injection. Its safety and efficacy were demonstrated in numerous of studies involving about 4,500 patients. Treatment was associated with significant improvement, compared with placebo, said Dr. Pariser, who also was an investigator on secukinumab trials.
Of note, while the PASI-75 findings for secukinumab are “a nice number but not dramatically higher than things we have had in the past,” the PASI-90 and PASI-100 scores are remarkable, he said.
At the highest dose studied (300 mg given at weeks 1, 2, 3, 4, and 8, and monthly thereafter), PASI-90 was achieved by 59% of patients in one study, and PASI-100 was achieved in 28%.
“That’s significant. We haven’t had that before, and that is really, really, really nice,” he said.
Further, the primary efficacy endpoint of the study was outcome at 12 weeks, but patients continued to improve at least until 16 weeks.
PASI-100 – no psoriasis whatsoever – was 40% at 16 weeks, he said.
In another phase III trial, secukinumab was again shown to be superior to placebo, but it also compared favorably with etanercept, he noted.
Safety was reasonable in the secukinumab trials. No deaths occurred, but there were more serious adverse events and discontinuations in the active treatment group. Nasopharyngitis was the most common serious adverse events, and it occurred in all groups. Upper respiratory tract infections appeared to be more common in the secukinumab patients, he noted.
An important consideration with secukinumab, however, is the need for continuous treatment, he said.
“The bottom line, really, is that patients should stay on it. They will lose effectiveness if they go off of it or take it on an as-needed basis,” he said.
Dr. Pariser is a consultant and/or researcher for Amgen, AbbVie, Celgene, Eli Lilly, Janssen Pharmaceuticals, Merck, and Pfizer.
MIAMI BEACH – Two of the newest treatments available for psoriasis – apremilast and secukinumab – are true “game-changers,” according to Dr. David M. Pariser.
Apremilast (Otezla), a recently approved oral phosphodiesterase 4 inhibitor, will be a particularly attractive treatment option for many dermatologists and patients, he said at the South Beach Symposium.
Apremilast has a very limited effect on the immune system, and it’s an oral therapy and thus requires no needles. It is very safe – with “strikingly few” serious adverse events – and no laboratory monitoring is required, he explained.
Efficacy results with apremilast are modest. In the phase III ESTEEM trial, for example, 33% of patients achieved at least 75% improvement (PASI-75), compared with 5% of patients who received placebo, said Dr. Pariser of Eastern Virginia Medical School, Norfolk, and an investigator for the trial.
Further, the drug can be used for almost any patient and type of psoriasis; it is an option for those who want systemic therapy, but who don’t want to go on a biologic or methotrexate, and its use is not precluded by a history of cancer or infections, as is the case with biologics, he added.
Apremilast also will be attractive for dermatologists who do not currently prescribe systemic therapy for psoriasis, or who don’t use aggressive systemic therapy for psoriasis, he said.
“If a prescriber feels safety is more important than efficacy, this might be a good choice,” he said.
Continue for information on secukinumab >>
Secukinumab (Cosentyx), on the other hand, is a “big gun,” Dr. Pariser said of the biologic, which was approved in January 2015 for the treatment of adults with moderate to severe plaque psoriasis.
“It’s the biggest gun we’ve got now … and it really has a safety profile similar to existing biologics so far,” he said.
The fully human monoclonal antibody inhibits interleukin-17A and is administered by subcutaneous injection. Its safety and efficacy were demonstrated in numerous of studies involving about 4,500 patients. Treatment was associated with significant improvement, compared with placebo, said Dr. Pariser, who also was an investigator on secukinumab trials.
Of note, while the PASI-75 findings for secukinumab are “a nice number but not dramatically higher than things we have had in the past,” the PASI-90 and PASI-100 scores are remarkable, he said.
At the highest dose studied (300 mg given at weeks 1, 2, 3, 4, and 8, and monthly thereafter), PASI-90 was achieved by 59% of patients in one study, and PASI-100 was achieved in 28%.
“That’s significant. We haven’t had that before, and that is really, really, really nice,” he said.
Further, the primary efficacy endpoint of the study was outcome at 12 weeks, but patients continued to improve at least until 16 weeks.
PASI-100 – no psoriasis whatsoever – was 40% at 16 weeks, he said.
In another phase III trial, secukinumab was again shown to be superior to placebo, but it also compared favorably with etanercept, he noted.
Safety was reasonable in the secukinumab trials. No deaths occurred, but there were more serious adverse events and discontinuations in the active treatment group. Nasopharyngitis was the most common serious adverse events, and it occurred in all groups. Upper respiratory tract infections appeared to be more common in the secukinumab patients, he noted.
An important consideration with secukinumab, however, is the need for continuous treatment, he said.
“The bottom line, really, is that patients should stay on it. They will lose effectiveness if they go off of it or take it on an as-needed basis,” he said.
Dr. Pariser is a consultant and/or researcher for Amgen, AbbVie, Celgene, Eli Lilly, Janssen Pharmaceuticals, Merck, and Pfizer.
EXPERT ANALYSIS FROM THE ANNUAL SOUTH BEACH SYMPOSIUM