User login
European Society of Cardiology (ESC): Annual Congress
VIDEO: Less may be best when ablating persistent AF
BARCELONA – Current guidelines recommend more extensive ablation procedures beyond pulmonary vein isolation for persistent atrial fibrillation. At the annual congress of the European Society of Cardiology, Dr. Atul Verma explains in this video interview that the fresh results of STAR-AF 2, the largest-ever randomized trial of popular ablation strategies in persistent AF, turn the conventional wisdom on its head and suggest that less ablation may be more.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BARCELONA – Current guidelines recommend more extensive ablation procedures beyond pulmonary vein isolation for persistent atrial fibrillation. At the annual congress of the European Society of Cardiology, Dr. Atul Verma explains in this video interview that the fresh results of STAR-AF 2, the largest-ever randomized trial of popular ablation strategies in persistent AF, turn the conventional wisdom on its head and suggest that less ablation may be more.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BARCELONA – Current guidelines recommend more extensive ablation procedures beyond pulmonary vein isolation for persistent atrial fibrillation. At the annual congress of the European Society of Cardiology, Dr. Atul Verma explains in this video interview that the fresh results of STAR-AF 2, the largest-ever randomized trial of popular ablation strategies in persistent AF, turn the conventional wisdom on its head and suggest that less ablation may be more.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ESC CONGRESS 2014
Prehospital administration of ticagrelor confers no benefit over in-hospital
Prehospital administration of ticagrelor did not improve either short- or long-term outcomes for patients with ST-elevation myocardial infarction over the standard in-hospital administration of the drug.
Patients who received ticagrelor in the field had virtually identical rates of 70% or greater post–percutaneous coronary intervention resolution and post-PCI cardiovascular events as those who got the drug after hospital admission, Dr. Gilles Montalescot and his colleagues reported at the online at the annual congress of the European Society of Cardiology. The results were simultaneously published online (N. Engl. J. Med. 2014 Sept. 1; [doi:10.1056/NEJMoa1407024]).
The phase IV ATLANTIC (Administration of Ticagrelor in the Cath Lab or in the Ambulance for New ST Elevation Myocardial Infarction to Open the Coronary artery) trial, conducted in 112 PCI centers among 13 countries, randomized 1,862 patients with ongoing STEMI of less than 6 hours’ duration to either a 180-mg loading dose of ticagrelor before hospital transfer, or to the same protocol in the PCI lab. All patients then received an ongoing regimen of 90 mg twice daily for 30 days, with the recommendation that this be continued for 12 months. Some patients also received glycoprotein IIB/IIIa inhibitors in the ambulance, but this was delivered at the physicians’ discretion.
In addition to STEMI resolution, primary endpoint included the proportion of patients who didn’t meet TIMI flow grade 3 in the infarcted artery before PCI. Secondary endpoints were a composite measure of death, MI, stent thrombosis, or urgent revascularization at 30 days; thrombotic bailout with glycoprotein inhibition, TIMI flow grade 3 at the end of the procedure, and complete STEMI resolution at by 60 minutes after PCI, reported Dr. Montalescot, director of cardiac care at the Pitié-Salpêtrière Hospital in Paris, and his coauthors.
Safety endpoints included major bleeding after PCI.
The patients were a mean of 61 years old. About 19% had a body mass index of 30 kg/m2 or higher. For 75%, the first point of care was in the ambulance. About 30% had glycoprotein inhibition before PCI.
The rates of a STEMI resolution of at least 70% were almost identical in the prehospital and in-hospital groups (86.8% and 87.6%, respectively), as was the absence of TIMI flow grade 3 in the infarcted artery by the time of PCI (82.6% and 83.1%). Likewise, a similar proportion of patients met either one or both secondary endpoints (49.6% and 52.8%).
While there was no significant between-group difference in the composite endpoint, stent thrombosis at 24 hours after PCI was lower in the prehospital group than in the in-hospital group (0% and 8%, respectively), and also at 30 days afterward (0.2% and 1.2%).
In all, 49 patients died, 30 in the prehospital group and 19 in the in-hospital group – not a significant difference (3.3% vs. 2%). The most common causes were cardiogenic shock, cardiac arrest, mechanical complications, and heart failure.
Major bleeding rates within the first 48 hours were low and similar (1.8% vs.1.6%); 30-day rates were identical (1.2% vs. 1.2%).
"Our study shows that there is no downside to earlier administration of ticagrelor, and it reduces the risk of post-procedure stent thrombosis. ... It is also a more practical time point for administration of the drug than in the catheterisation laboratory, where considerable staff and technical demands already exist," Dr. Montalescot said in a statement.
AstraZeneca sponsored the study. Dr. Montalescot reported receiving grants from the company and other pharmaceutical companies. He also reported consulting fees from 25 different pharmaceutical companies and study groups, including the ATLANTIC study.
The coauthors also reported numerous financial relationships with pharmaceutical companies, including AstraZeneca.
On Twitter @alz_gal
Prehospital administration of ticagrelor did not improve either short- or long-term outcomes for patients with ST-elevation myocardial infarction over the standard in-hospital administration of the drug.
Patients who received ticagrelor in the field had virtually identical rates of 70% or greater post–percutaneous coronary intervention resolution and post-PCI cardiovascular events as those who got the drug after hospital admission, Dr. Gilles Montalescot and his colleagues reported at the online at the annual congress of the European Society of Cardiology. The results were simultaneously published online (N. Engl. J. Med. 2014 Sept. 1; [doi:10.1056/NEJMoa1407024]).
The phase IV ATLANTIC (Administration of Ticagrelor in the Cath Lab or in the Ambulance for New ST Elevation Myocardial Infarction to Open the Coronary artery) trial, conducted in 112 PCI centers among 13 countries, randomized 1,862 patients with ongoing STEMI of less than 6 hours’ duration to either a 180-mg loading dose of ticagrelor before hospital transfer, or to the same protocol in the PCI lab. All patients then received an ongoing regimen of 90 mg twice daily for 30 days, with the recommendation that this be continued for 12 months. Some patients also received glycoprotein IIB/IIIa inhibitors in the ambulance, but this was delivered at the physicians’ discretion.
In addition to STEMI resolution, primary endpoint included the proportion of patients who didn’t meet TIMI flow grade 3 in the infarcted artery before PCI. Secondary endpoints were a composite measure of death, MI, stent thrombosis, or urgent revascularization at 30 days; thrombotic bailout with glycoprotein inhibition, TIMI flow grade 3 at the end of the procedure, and complete STEMI resolution at by 60 minutes after PCI, reported Dr. Montalescot, director of cardiac care at the Pitié-Salpêtrière Hospital in Paris, and his coauthors.
Safety endpoints included major bleeding after PCI.
The patients were a mean of 61 years old. About 19% had a body mass index of 30 kg/m2 or higher. For 75%, the first point of care was in the ambulance. About 30% had glycoprotein inhibition before PCI.
The rates of a STEMI resolution of at least 70% were almost identical in the prehospital and in-hospital groups (86.8% and 87.6%, respectively), as was the absence of TIMI flow grade 3 in the infarcted artery by the time of PCI (82.6% and 83.1%). Likewise, a similar proportion of patients met either one or both secondary endpoints (49.6% and 52.8%).
While there was no significant between-group difference in the composite endpoint, stent thrombosis at 24 hours after PCI was lower in the prehospital group than in the in-hospital group (0% and 8%, respectively), and also at 30 days afterward (0.2% and 1.2%).
In all, 49 patients died, 30 in the prehospital group and 19 in the in-hospital group – not a significant difference (3.3% vs. 2%). The most common causes were cardiogenic shock, cardiac arrest, mechanical complications, and heart failure.
Major bleeding rates within the first 48 hours were low and similar (1.8% vs.1.6%); 30-day rates were identical (1.2% vs. 1.2%).
"Our study shows that there is no downside to earlier administration of ticagrelor, and it reduces the risk of post-procedure stent thrombosis. ... It is also a more practical time point for administration of the drug than in the catheterisation laboratory, where considerable staff and technical demands already exist," Dr. Montalescot said in a statement.
AstraZeneca sponsored the study. Dr. Montalescot reported receiving grants from the company and other pharmaceutical companies. He also reported consulting fees from 25 different pharmaceutical companies and study groups, including the ATLANTIC study.
The coauthors also reported numerous financial relationships with pharmaceutical companies, including AstraZeneca.
On Twitter @alz_gal
Prehospital administration of ticagrelor did not improve either short- or long-term outcomes for patients with ST-elevation myocardial infarction over the standard in-hospital administration of the drug.
Patients who received ticagrelor in the field had virtually identical rates of 70% or greater post–percutaneous coronary intervention resolution and post-PCI cardiovascular events as those who got the drug after hospital admission, Dr. Gilles Montalescot and his colleagues reported at the online at the annual congress of the European Society of Cardiology. The results were simultaneously published online (N. Engl. J. Med. 2014 Sept. 1; [doi:10.1056/NEJMoa1407024]).
The phase IV ATLANTIC (Administration of Ticagrelor in the Cath Lab or in the Ambulance for New ST Elevation Myocardial Infarction to Open the Coronary artery) trial, conducted in 112 PCI centers among 13 countries, randomized 1,862 patients with ongoing STEMI of less than 6 hours’ duration to either a 180-mg loading dose of ticagrelor before hospital transfer, or to the same protocol in the PCI lab. All patients then received an ongoing regimen of 90 mg twice daily for 30 days, with the recommendation that this be continued for 12 months. Some patients also received glycoprotein IIB/IIIa inhibitors in the ambulance, but this was delivered at the physicians’ discretion.
In addition to STEMI resolution, primary endpoint included the proportion of patients who didn’t meet TIMI flow grade 3 in the infarcted artery before PCI. Secondary endpoints were a composite measure of death, MI, stent thrombosis, or urgent revascularization at 30 days; thrombotic bailout with glycoprotein inhibition, TIMI flow grade 3 at the end of the procedure, and complete STEMI resolution at by 60 minutes after PCI, reported Dr. Montalescot, director of cardiac care at the Pitié-Salpêtrière Hospital in Paris, and his coauthors.
Safety endpoints included major bleeding after PCI.
The patients were a mean of 61 years old. About 19% had a body mass index of 30 kg/m2 or higher. For 75%, the first point of care was in the ambulance. About 30% had glycoprotein inhibition before PCI.
The rates of a STEMI resolution of at least 70% were almost identical in the prehospital and in-hospital groups (86.8% and 87.6%, respectively), as was the absence of TIMI flow grade 3 in the infarcted artery by the time of PCI (82.6% and 83.1%). Likewise, a similar proportion of patients met either one or both secondary endpoints (49.6% and 52.8%).
While there was no significant between-group difference in the composite endpoint, stent thrombosis at 24 hours after PCI was lower in the prehospital group than in the in-hospital group (0% and 8%, respectively), and also at 30 days afterward (0.2% and 1.2%).
In all, 49 patients died, 30 in the prehospital group and 19 in the in-hospital group – not a significant difference (3.3% vs. 2%). The most common causes were cardiogenic shock, cardiac arrest, mechanical complications, and heart failure.
Major bleeding rates within the first 48 hours were low and similar (1.8% vs.1.6%); 30-day rates were identical (1.2% vs. 1.2%).
"Our study shows that there is no downside to earlier administration of ticagrelor, and it reduces the risk of post-procedure stent thrombosis. ... It is also a more practical time point for administration of the drug than in the catheterisation laboratory, where considerable staff and technical demands already exist," Dr. Montalescot said in a statement.
AstraZeneca sponsored the study. Dr. Montalescot reported receiving grants from the company and other pharmaceutical companies. He also reported consulting fees from 25 different pharmaceutical companies and study groups, including the ATLANTIC study.
The coauthors also reported numerous financial relationships with pharmaceutical companies, including AstraZeneca.
On Twitter @alz_gal
FROM THE ESC CONGRESS 2014
Key clinical point: Prehospital administration of ticagrelor did not improve PCI outcomes above the rates achieved with in-hospital administration.
Major finding: The rate of STEMI resolution was virtually identical in those who received the drug at transfer vs. those who received in the hospital (86.8% and 87.6%, respectively).
Data source: ATLANTIC, a randomized, placebo-controlled study in 1,862 patients.
Disclosures: AstraZeneca sponsored the study. Dr. Montalescot reported receiving grants from the company and other pharmaceutical companies. He also reported consulting fees from 25 different pharmaceutical companies and study groups, including the ATLANTIC study. The coauthors also reported numerous financial relationships with pharmaceutical companies, including AstraZeneca.
Beta-blockers no help for heart failure with atrial fib
Beta-blocker therapy doesn’t reduce all-cause mortality, cardiovascular mortality, cardiovascular hospitalization, or stroke in patients with heart failure who also have atrial fibrillation, Dipak Kotecha, Ph.D., reported at the annual congress of the European Society of Cardiology in Barcelona.
In a meta-analysis that used the "arduous" process of analyzing individual-patient data from all the high-quality randomized controlled trials available, Dr. Kotecha and his associates found no evidence that beta-blockers prevent adverse clinical events in this patient population. "Beta-blockers should no longer be regarded as standard therapy to improve prognosis" in patients with concomitant heart failure (HF) and atrial fibrillation (AF), he said.
In contrast, the drugs are effective and are strongly recommended for patients with HF who are in sinus rhythm, Dr. Kotecha said in a paper presented at the meeting and simultaneously published online Sept. 2 (Lancet 2014 [doi:10.1016/S0140-6736(14)61373-8]).
Heart failure and atrial fibrillation are common disorders, and both are becoming more prevalent. They often coexist, and an estimated 14%-50% of patients who have symptomatic HF also have AF.
At present, both European and American guidelines advise the use of beta-blockers for symptomatic heart failure without regard to AF status, based on trials that predominantly enrolled patients in sinus rhythm. There have been concerns about the drugs’ efficacy in certain subgroups of patients, including those with AF. "Patients with AF are often prescribed beta-blockers for both prognostic benefit in HF and heart-rate control, although there is little and underpowered evidence for efficacy in terms of clinical outcomes," he noted.
Dr. Kotecha and his associates in the Beta-Blockers in Heart Failure Collaborative Group – a multinational organization "formed to provide definitive answers to open questions about HF and beta-blocker therapy" and to provide clinicians with clear guidance – reviewed data from 18,254 study participants who were followed for a mean of 1.5 years, which allowed a robust and adequately powered analysis of this issue. They included "only unconfounded head-to-head trials with recruitment of more than 300 patients and a planned follow-up of more than 6 months."
A total of 3,066 (17%) of the study participants had AF as well as heart failure, and 633 of them died during follow-up. "A consistent benefit of beta-blockers versus placebo was noted for all death or hospital admission outcomes in patients with sinus rhythm, but the differences were not significant in patients with AF," said Dr. Kotecha of the University of Birmingham (England) Centre for Cardiovascular Sciences.
"The substantial benefit identified in patients with sinus rhythm should not be extrapolated to patients with AF," he said.
It is important to note that beta-blockers did appear to be safe, with no increase in mortality or adverse events, compared with placebo. "This should reassure clinicians, particularly for patients with another indication for beta-blockers, such as MI or the need for rate control of rapid AF with ongoing symptoms," he added.
This study was funded by Menarini Farmaceutica Internazionale, and GlaxoSmithKline provided data extraction support. Neither pharmaceutical group had a role in data analysis or manuscript preparation. Dr. Kotecha is supported by the U.K. National Institute for Health Research and reported receiving grants and honoraria from Menarini. His associates reported ties to numerous device and pharmaceutical sources.
Beta-blocker therapy doesn’t reduce all-cause mortality, cardiovascular mortality, cardiovascular hospitalization, or stroke in patients with heart failure who also have atrial fibrillation, Dipak Kotecha, Ph.D., reported at the annual congress of the European Society of Cardiology in Barcelona.
In a meta-analysis that used the "arduous" process of analyzing individual-patient data from all the high-quality randomized controlled trials available, Dr. Kotecha and his associates found no evidence that beta-blockers prevent adverse clinical events in this patient population. "Beta-blockers should no longer be regarded as standard therapy to improve prognosis" in patients with concomitant heart failure (HF) and atrial fibrillation (AF), he said.
In contrast, the drugs are effective and are strongly recommended for patients with HF who are in sinus rhythm, Dr. Kotecha said in a paper presented at the meeting and simultaneously published online Sept. 2 (Lancet 2014 [doi:10.1016/S0140-6736(14)61373-8]).
Heart failure and atrial fibrillation are common disorders, and both are becoming more prevalent. They often coexist, and an estimated 14%-50% of patients who have symptomatic HF also have AF.
At present, both European and American guidelines advise the use of beta-blockers for symptomatic heart failure without regard to AF status, based on trials that predominantly enrolled patients in sinus rhythm. There have been concerns about the drugs’ efficacy in certain subgroups of patients, including those with AF. "Patients with AF are often prescribed beta-blockers for both prognostic benefit in HF and heart-rate control, although there is little and underpowered evidence for efficacy in terms of clinical outcomes," he noted.
Dr. Kotecha and his associates in the Beta-Blockers in Heart Failure Collaborative Group – a multinational organization "formed to provide definitive answers to open questions about HF and beta-blocker therapy" and to provide clinicians with clear guidance – reviewed data from 18,254 study participants who were followed for a mean of 1.5 years, which allowed a robust and adequately powered analysis of this issue. They included "only unconfounded head-to-head trials with recruitment of more than 300 patients and a planned follow-up of more than 6 months."
A total of 3,066 (17%) of the study participants had AF as well as heart failure, and 633 of them died during follow-up. "A consistent benefit of beta-blockers versus placebo was noted for all death or hospital admission outcomes in patients with sinus rhythm, but the differences were not significant in patients with AF," said Dr. Kotecha of the University of Birmingham (England) Centre for Cardiovascular Sciences.
"The substantial benefit identified in patients with sinus rhythm should not be extrapolated to patients with AF," he said.
It is important to note that beta-blockers did appear to be safe, with no increase in mortality or adverse events, compared with placebo. "This should reassure clinicians, particularly for patients with another indication for beta-blockers, such as MI or the need for rate control of rapid AF with ongoing symptoms," he added.
This study was funded by Menarini Farmaceutica Internazionale, and GlaxoSmithKline provided data extraction support. Neither pharmaceutical group had a role in data analysis or manuscript preparation. Dr. Kotecha is supported by the U.K. National Institute for Health Research and reported receiving grants and honoraria from Menarini. His associates reported ties to numerous device and pharmaceutical sources.
Beta-blocker therapy doesn’t reduce all-cause mortality, cardiovascular mortality, cardiovascular hospitalization, or stroke in patients with heart failure who also have atrial fibrillation, Dipak Kotecha, Ph.D., reported at the annual congress of the European Society of Cardiology in Barcelona.
In a meta-analysis that used the "arduous" process of analyzing individual-patient data from all the high-quality randomized controlled trials available, Dr. Kotecha and his associates found no evidence that beta-blockers prevent adverse clinical events in this patient population. "Beta-blockers should no longer be regarded as standard therapy to improve prognosis" in patients with concomitant heart failure (HF) and atrial fibrillation (AF), he said.
In contrast, the drugs are effective and are strongly recommended for patients with HF who are in sinus rhythm, Dr. Kotecha said in a paper presented at the meeting and simultaneously published online Sept. 2 (Lancet 2014 [doi:10.1016/S0140-6736(14)61373-8]).
Heart failure and atrial fibrillation are common disorders, and both are becoming more prevalent. They often coexist, and an estimated 14%-50% of patients who have symptomatic HF also have AF.
At present, both European and American guidelines advise the use of beta-blockers for symptomatic heart failure without regard to AF status, based on trials that predominantly enrolled patients in sinus rhythm. There have been concerns about the drugs’ efficacy in certain subgroups of patients, including those with AF. "Patients with AF are often prescribed beta-blockers for both prognostic benefit in HF and heart-rate control, although there is little and underpowered evidence for efficacy in terms of clinical outcomes," he noted.
Dr. Kotecha and his associates in the Beta-Blockers in Heart Failure Collaborative Group – a multinational organization "formed to provide definitive answers to open questions about HF and beta-blocker therapy" and to provide clinicians with clear guidance – reviewed data from 18,254 study participants who were followed for a mean of 1.5 years, which allowed a robust and adequately powered analysis of this issue. They included "only unconfounded head-to-head trials with recruitment of more than 300 patients and a planned follow-up of more than 6 months."
A total of 3,066 (17%) of the study participants had AF as well as heart failure, and 633 of them died during follow-up. "A consistent benefit of beta-blockers versus placebo was noted for all death or hospital admission outcomes in patients with sinus rhythm, but the differences were not significant in patients with AF," said Dr. Kotecha of the University of Birmingham (England) Centre for Cardiovascular Sciences.
"The substantial benefit identified in patients with sinus rhythm should not be extrapolated to patients with AF," he said.
It is important to note that beta-blockers did appear to be safe, with no increase in mortality or adverse events, compared with placebo. "This should reassure clinicians, particularly for patients with another indication for beta-blockers, such as MI or the need for rate control of rapid AF with ongoing symptoms," he added.
This study was funded by Menarini Farmaceutica Internazionale, and GlaxoSmithKline provided data extraction support. Neither pharmaceutical group had a role in data analysis or manuscript preparation. Dr. Kotecha is supported by the U.K. National Institute for Health Research and reported receiving grants and honoraria from Menarini. His associates reported ties to numerous device and pharmaceutical sources.
FROM THE ESC CONGRESS 2014
Key clinical point: The benefit of beta-blockers in patients with HF and sinus rhythm cannot be extended to those with HF and atrial fibrillation.
Major finding: A consistent benefit of beta-blockers versus placebo was noted for all-cause mortality, CV mortality, CV hospitalization, and stroke in patients with sinus rhythm, but the differences were not significant in patients with AF.
Data source: A metaanalysis of all large high-quality randomized controlled trials assessing mortality and CV outcomes for 18,254 adults with HF, including 3,066 with concomitant AF, who received either beta-blockers or placebo and were followed for a mean of 1.5 years.
Disclosures: This study was funded by Menarini Farmaceutica Internazionale, and GlaxoSmithKline provided data extraction support. Neither pharmaceutical group had a role in data analysis or manuscript preparation. Dr. Kotecha is supported by the U.K. National Institute for Health Research and reported receiving grants and honoraria from Menarini. His associates reported ties to numerous device and pharmaceutical sources.
Prednisolone, immunotherapy ineffective for most tuberculous pericarditis
Neither standard anti-inflammatory therapy using prednisolone nor an experimental immunotherapy using Mycobacterium indicus pranii injections reduced the composite outcome of death, cardiac tamponade requiring pericardiocentesis, or constrictive pericarditis in an international clinical trial of adults with tuberculous pericarditis, a study showed.
However, prednisolone was beneficial for one component of this composite outcome – lowering the rate of constrictive pericarditis – compared with placebo, Dr. Bongani M. Mayosi reported at the annual congress of the European Society of Cardiology in Barcelona. His report was simultaneously presented at the meeting and published online Sept. 2 (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1407380]).
Glucocorticoids are thought to attenuate the inflammatory response in patients with tuberculous pericarditis, and are recommended as adjunctive therapy in current American and World Health Organization treatment guidelines. But the studies on which these recommendations are based had "very small" numbers of events and patients, and the treatment effect was minimal, leading European expert groups to advise against using the drugs in this patient population. In addition, glucocorticoids may raise the risk of cancer in patients who are coinfected with HIV, which is common in the regions of sub-Saharan Africa and Asia where tuberculous pericarditis is most frequent, said Dr. Mayosi of the department of medicine, Old Groote Schuur Hospital, Cape Town, South Africa.
The 1,400 participants in the Investigation of the Management of Pericarditis (IMPI) trial had pericardial effusion confirmed by echocardiography and evidence of definite or probable tuberculous pericarditis; two-thirds also had concomitant HIV infection. They were treated at 19 hospitals across 8 countries in Africa during a 5-year period. All received background antimicrobial therapy for tuberculosis, and all patients with HIV received antiretrovirals according to WHO guidelines.
The participants first were randomly assigned to receive prednisolone (706 patients) or placebo (694 patients) in tapering doses for 6 weeks. In the second phase of the study, 1,250 of these participants were then randomly assigned to receive five intradermal injections of either heat-killed M. indicus pranii or placebo at intervals over the course of 3 months. This nonpathogenic, rapidly growing mycobacterium species has been shown to suppress inflammation in patients with leprosy.
The primary efficacy outcome was a composite of death, cardiac tamponade requiring pericardiocentesis, or constrictive pericarditis. After a median follow-up of 636 days, there were 14.3 such events per 100 patient-years in the prednisolone group and 14.8 in the placebo group, a nonsignificant difference.
When each component of this composite outcome was considered individually, prednisolone did not improve the death rate or the rate of cardiac tamponade, compared with placebo, but did reduce the rate of constrictive pericarditis (4.4%, vs 7.8% with placebo), and thus the rate of hospitalization (20.7%, vs 25.2% with placebo). "This finding is important because pericardiectomy, the definitive treatment for chronic pericardial constriction, is associated with high perioperative mortality and morbidity, and cardiac surgery is not widely available in Africa," Dr. Mayosi said.
Prednisolone also raised the rate of opportunistic infections, chiefly candidiasis, compared with placebo. And it markedly increased the rate of cancer, primarily Kaposi’s sarcoma, in patients coinfected with HIV (1.05 cases per 100 person-years, vs. 0.32 cases with placebo).
In the M. indicus pranii comparison, the active treatment was no different from placebo with regard to the composite outcome or any secondary outcomes, and that portion of the trial was halted early for futility. Like prednisolone, this experimental agent raised the rate of cancer in HIV-positive patients (0.92 cases per 100 person-years, vs. 0.24 cases with placebo) – an adverse event that has not been reported previously with M. indicus pranii.
In addition, significantly more patients who received M. indicus pranii (41.4%) than placebo (2.9%) developed injection-site reactions. Fifteen percent of patients given the active injections developed abscesses at the injection site, compared with only 1% of those given placebo injections.
The IMPI trial was supported by the Canadian Institutes of Health Research, the Canadian Network and Centre for Trials Internationally, the Population Health Research Institute, the South African Medical Research Council, the Lily and Ernst Hausmann Research Trust, and Cadila Pharma (India). Cadila donated the study drugs but had no role in the design or conduct of the study or in data analysis. The authors had no relevant financial conflicts of interest to disclose.
These findings clearly suggest that adjunctive glucocorticoids should not be used routinely in patients with tuberculous pericarditis, which may surprise many clinicians. But because they prevent constrictive pericarditis and reduce hospitalizations, the drugs may be appropriate for patients at highest risk for complications, such as those with large effusions, high levels of inflammatory cells or markers in the pericardial fluid, and early signs of constriction.
The use of glucocorticoids should be curtailed in patients coinfected with HIV unless the risk of constrictive pericarditis is high, since these drugs increase the risk of cancer in this patient population.
Dr. Richard E. Chaisson and Dr. Wendy S. Post of Johns Hopkins University, Baltimore, made these remarks in an editorial accompanying Dr. Mayosi’s report (N. Engl. J. Med. 2014 Sept. 2 [doi:10.1056/NEJMe1409356]. Dr. Chaisson reported receiving support from Merck.
These findings clearly suggest that adjunctive glucocorticoids should not be used routinely in patients with tuberculous pericarditis, which may surprise many clinicians. But because they prevent constrictive pericarditis and reduce hospitalizations, the drugs may be appropriate for patients at highest risk for complications, such as those with large effusions, high levels of inflammatory cells or markers in the pericardial fluid, and early signs of constriction.
The use of glucocorticoids should be curtailed in patients coinfected with HIV unless the risk of constrictive pericarditis is high, since these drugs increase the risk of cancer in this patient population.
Dr. Richard E. Chaisson and Dr. Wendy S. Post of Johns Hopkins University, Baltimore, made these remarks in an editorial accompanying Dr. Mayosi’s report (N. Engl. J. Med. 2014 Sept. 2 [doi:10.1056/NEJMe1409356]. Dr. Chaisson reported receiving support from Merck.
These findings clearly suggest that adjunctive glucocorticoids should not be used routinely in patients with tuberculous pericarditis, which may surprise many clinicians. But because they prevent constrictive pericarditis and reduce hospitalizations, the drugs may be appropriate for patients at highest risk for complications, such as those with large effusions, high levels of inflammatory cells or markers in the pericardial fluid, and early signs of constriction.
The use of glucocorticoids should be curtailed in patients coinfected with HIV unless the risk of constrictive pericarditis is high, since these drugs increase the risk of cancer in this patient population.
Dr. Richard E. Chaisson and Dr. Wendy S. Post of Johns Hopkins University, Baltimore, made these remarks in an editorial accompanying Dr. Mayosi’s report (N. Engl. J. Med. 2014 Sept. 2 [doi:10.1056/NEJMe1409356]. Dr. Chaisson reported receiving support from Merck.
Neither standard anti-inflammatory therapy using prednisolone nor an experimental immunotherapy using Mycobacterium indicus pranii injections reduced the composite outcome of death, cardiac tamponade requiring pericardiocentesis, or constrictive pericarditis in an international clinical trial of adults with tuberculous pericarditis, a study showed.
However, prednisolone was beneficial for one component of this composite outcome – lowering the rate of constrictive pericarditis – compared with placebo, Dr. Bongani M. Mayosi reported at the annual congress of the European Society of Cardiology in Barcelona. His report was simultaneously presented at the meeting and published online Sept. 2 (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1407380]).
Glucocorticoids are thought to attenuate the inflammatory response in patients with tuberculous pericarditis, and are recommended as adjunctive therapy in current American and World Health Organization treatment guidelines. But the studies on which these recommendations are based had "very small" numbers of events and patients, and the treatment effect was minimal, leading European expert groups to advise against using the drugs in this patient population. In addition, glucocorticoids may raise the risk of cancer in patients who are coinfected with HIV, which is common in the regions of sub-Saharan Africa and Asia where tuberculous pericarditis is most frequent, said Dr. Mayosi of the department of medicine, Old Groote Schuur Hospital, Cape Town, South Africa.
The 1,400 participants in the Investigation of the Management of Pericarditis (IMPI) trial had pericardial effusion confirmed by echocardiography and evidence of definite or probable tuberculous pericarditis; two-thirds also had concomitant HIV infection. They were treated at 19 hospitals across 8 countries in Africa during a 5-year period. All received background antimicrobial therapy for tuberculosis, and all patients with HIV received antiretrovirals according to WHO guidelines.
The participants first were randomly assigned to receive prednisolone (706 patients) or placebo (694 patients) in tapering doses for 6 weeks. In the second phase of the study, 1,250 of these participants were then randomly assigned to receive five intradermal injections of either heat-killed M. indicus pranii or placebo at intervals over the course of 3 months. This nonpathogenic, rapidly growing mycobacterium species has been shown to suppress inflammation in patients with leprosy.
The primary efficacy outcome was a composite of death, cardiac tamponade requiring pericardiocentesis, or constrictive pericarditis. After a median follow-up of 636 days, there were 14.3 such events per 100 patient-years in the prednisolone group and 14.8 in the placebo group, a nonsignificant difference.
When each component of this composite outcome was considered individually, prednisolone did not improve the death rate or the rate of cardiac tamponade, compared with placebo, but did reduce the rate of constrictive pericarditis (4.4%, vs 7.8% with placebo), and thus the rate of hospitalization (20.7%, vs 25.2% with placebo). "This finding is important because pericardiectomy, the definitive treatment for chronic pericardial constriction, is associated with high perioperative mortality and morbidity, and cardiac surgery is not widely available in Africa," Dr. Mayosi said.
Prednisolone also raised the rate of opportunistic infections, chiefly candidiasis, compared with placebo. And it markedly increased the rate of cancer, primarily Kaposi’s sarcoma, in patients coinfected with HIV (1.05 cases per 100 person-years, vs. 0.32 cases with placebo).
In the M. indicus pranii comparison, the active treatment was no different from placebo with regard to the composite outcome or any secondary outcomes, and that portion of the trial was halted early for futility. Like prednisolone, this experimental agent raised the rate of cancer in HIV-positive patients (0.92 cases per 100 person-years, vs. 0.24 cases with placebo) – an adverse event that has not been reported previously with M. indicus pranii.
In addition, significantly more patients who received M. indicus pranii (41.4%) than placebo (2.9%) developed injection-site reactions. Fifteen percent of patients given the active injections developed abscesses at the injection site, compared with only 1% of those given placebo injections.
The IMPI trial was supported by the Canadian Institutes of Health Research, the Canadian Network and Centre for Trials Internationally, the Population Health Research Institute, the South African Medical Research Council, the Lily and Ernst Hausmann Research Trust, and Cadila Pharma (India). Cadila donated the study drugs but had no role in the design or conduct of the study or in data analysis. The authors had no relevant financial conflicts of interest to disclose.
Neither standard anti-inflammatory therapy using prednisolone nor an experimental immunotherapy using Mycobacterium indicus pranii injections reduced the composite outcome of death, cardiac tamponade requiring pericardiocentesis, or constrictive pericarditis in an international clinical trial of adults with tuberculous pericarditis, a study showed.
However, prednisolone was beneficial for one component of this composite outcome – lowering the rate of constrictive pericarditis – compared with placebo, Dr. Bongani M. Mayosi reported at the annual congress of the European Society of Cardiology in Barcelona. His report was simultaneously presented at the meeting and published online Sept. 2 (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1407380]).
Glucocorticoids are thought to attenuate the inflammatory response in patients with tuberculous pericarditis, and are recommended as adjunctive therapy in current American and World Health Organization treatment guidelines. But the studies on which these recommendations are based had "very small" numbers of events and patients, and the treatment effect was minimal, leading European expert groups to advise against using the drugs in this patient population. In addition, glucocorticoids may raise the risk of cancer in patients who are coinfected with HIV, which is common in the regions of sub-Saharan Africa and Asia where tuberculous pericarditis is most frequent, said Dr. Mayosi of the department of medicine, Old Groote Schuur Hospital, Cape Town, South Africa.
The 1,400 participants in the Investigation of the Management of Pericarditis (IMPI) trial had pericardial effusion confirmed by echocardiography and evidence of definite or probable tuberculous pericarditis; two-thirds also had concomitant HIV infection. They were treated at 19 hospitals across 8 countries in Africa during a 5-year period. All received background antimicrobial therapy for tuberculosis, and all patients with HIV received antiretrovirals according to WHO guidelines.
The participants first were randomly assigned to receive prednisolone (706 patients) or placebo (694 patients) in tapering doses for 6 weeks. In the second phase of the study, 1,250 of these participants were then randomly assigned to receive five intradermal injections of either heat-killed M. indicus pranii or placebo at intervals over the course of 3 months. This nonpathogenic, rapidly growing mycobacterium species has been shown to suppress inflammation in patients with leprosy.
The primary efficacy outcome was a composite of death, cardiac tamponade requiring pericardiocentesis, or constrictive pericarditis. After a median follow-up of 636 days, there were 14.3 such events per 100 patient-years in the prednisolone group and 14.8 in the placebo group, a nonsignificant difference.
When each component of this composite outcome was considered individually, prednisolone did not improve the death rate or the rate of cardiac tamponade, compared with placebo, but did reduce the rate of constrictive pericarditis (4.4%, vs 7.8% with placebo), and thus the rate of hospitalization (20.7%, vs 25.2% with placebo). "This finding is important because pericardiectomy, the definitive treatment for chronic pericardial constriction, is associated with high perioperative mortality and morbidity, and cardiac surgery is not widely available in Africa," Dr. Mayosi said.
Prednisolone also raised the rate of opportunistic infections, chiefly candidiasis, compared with placebo. And it markedly increased the rate of cancer, primarily Kaposi’s sarcoma, in patients coinfected with HIV (1.05 cases per 100 person-years, vs. 0.32 cases with placebo).
In the M. indicus pranii comparison, the active treatment was no different from placebo with regard to the composite outcome or any secondary outcomes, and that portion of the trial was halted early for futility. Like prednisolone, this experimental agent raised the rate of cancer in HIV-positive patients (0.92 cases per 100 person-years, vs. 0.24 cases with placebo) – an adverse event that has not been reported previously with M. indicus pranii.
In addition, significantly more patients who received M. indicus pranii (41.4%) than placebo (2.9%) developed injection-site reactions. Fifteen percent of patients given the active injections developed abscesses at the injection site, compared with only 1% of those given placebo injections.
The IMPI trial was supported by the Canadian Institutes of Health Research, the Canadian Network and Centre for Trials Internationally, the Population Health Research Institute, the South African Medical Research Council, the Lily and Ernst Hausmann Research Trust, and Cadila Pharma (India). Cadila donated the study drugs but had no role in the design or conduct of the study or in data analysis. The authors had no relevant financial conflicts of interest to disclose.
FROM THE ESC CONGRESS 2014
Key clinical point: Although treatment with either prednisolone or M. indicus pranii did not improve overall outcomes in patients with tuberculosis pericarditis, prednisone did reduce the risk of developing constrictive pericarditis and hospitalizations.
Major finding: After a median follow-up of 636 days, the primary efficacy outcome – a composite of death, cardiac tamponade requiring pericardiocentesis, or constrictive pericarditis – occurred in 14.3 cases per 100 patient-years in the prednisolone group, compared with 14.8 in the placebo group.
Data source: IMPI, a randomized controlled trial in 1,400 adults with presumed tuberculous pericarditis who were treated at 19 hospitals in 8 African countries with 6 weeks of either prednisolone or placebo, followed by 3 months of M. indicus pranii or placebo injections.
Disclosures: The IMPI trial was supported by the Canadian Institutes of Health Research, the Canadian Network and Centre for Trials Internationally, the Population Health Research Institute, the South African Medical Research Council, the Lily and Ernst Hausmann Research Trust, and Cadila Pharma (India). Cadila donated the study drugs but had no role in the design or conduct of the study or in data analysis. The authors had no relevant financial conflicts of interest to disclose.
VIDEO: Repositionable TAVR valve holds promise
BARCELONA – The transcatheter aortic valve replacement technology is changing rapidly, especially in Europe where the regulatory process is different and more types of valves are on the market.
One of the current focus areas in the development of TAVR devices is making retrievable and repositionable valves to improve the implantation process and patient outcomes. Late last year, Lotus Valve System reported favorable results for its valve in the REPRISE II study.
At the annual congress of the European Society of Cardiology, Dr. Stylianos A. Pyxaras presented another study showing that the repositionable Direct Flow Medical valve in elderly high-risk patients with severe aortic stenosis compared well in safety and efficacy with the results of the DISCOVER trial.
Dr. Deepak L. Bhatt, executive director of Interventional Cardiovascular Programs and professor of medicine at Harvard Medical School in Boston, shared his opinion about the findings and the future implications on practice. He was not involved in any of the mentioned studies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Twitter: @naseemmiller
Lotus Valve System, REPRISE II, ESC
BARCELONA – The transcatheter aortic valve replacement technology is changing rapidly, especially in Europe where the regulatory process is different and more types of valves are on the market.
One of the current focus areas in the development of TAVR devices is making retrievable and repositionable valves to improve the implantation process and patient outcomes. Late last year, Lotus Valve System reported favorable results for its valve in the REPRISE II study.
At the annual congress of the European Society of Cardiology, Dr. Stylianos A. Pyxaras presented another study showing that the repositionable Direct Flow Medical valve in elderly high-risk patients with severe aortic stenosis compared well in safety and efficacy with the results of the DISCOVER trial.
Dr. Deepak L. Bhatt, executive director of Interventional Cardiovascular Programs and professor of medicine at Harvard Medical School in Boston, shared his opinion about the findings and the future implications on practice. He was not involved in any of the mentioned studies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Twitter: @naseemmiller
BARCELONA – The transcatheter aortic valve replacement technology is changing rapidly, especially in Europe where the regulatory process is different and more types of valves are on the market.
One of the current focus areas in the development of TAVR devices is making retrievable and repositionable valves to improve the implantation process and patient outcomes. Late last year, Lotus Valve System reported favorable results for its valve in the REPRISE II study.
At the annual congress of the European Society of Cardiology, Dr. Stylianos A. Pyxaras presented another study showing that the repositionable Direct Flow Medical valve in elderly high-risk patients with severe aortic stenosis compared well in safety and efficacy with the results of the DISCOVER trial.
Dr. Deepak L. Bhatt, executive director of Interventional Cardiovascular Programs and professor of medicine at Harvard Medical School in Boston, shared his opinion about the findings and the future implications on practice. He was not involved in any of the mentioned studies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Twitter: @naseemmiller
Lotus Valve System, REPRISE II, ESC
Lotus Valve System, REPRISE II, ESC
AT THE ESC CONGRESS 2014
VIDEO: Alirocumab study showing sharp reduction in MACE promising but not definitive
CHICAGO – Discussant Dr. Robert M. Califf called the results of the ODYSSEY LONG TERM trial presented by Dr. Jennifer G. Robinson at the annual congress of the European Society of Cardiology "alluring" and "fantastic," with a reported 54% reduction in major adverse cardiovascular events in high-risk, statin-treated patients on add-on alirocumab. But neither he nor Dr. Robinson consider these results to be the final word on the subject.
Here’s what Dr. Robinson had to say about the investigational PCSK9 inhibitor’s performance in the study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Discussant Dr. Robert M. Califf called the results of the ODYSSEY LONG TERM trial presented by Dr. Jennifer G. Robinson at the annual congress of the European Society of Cardiology "alluring" and "fantastic," with a reported 54% reduction in major adverse cardiovascular events in high-risk, statin-treated patients on add-on alirocumab. But neither he nor Dr. Robinson consider these results to be the final word on the subject.
Here’s what Dr. Robinson had to say about the investigational PCSK9 inhibitor’s performance in the study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Discussant Dr. Robert M. Califf called the results of the ODYSSEY LONG TERM trial presented by Dr. Jennifer G. Robinson at the annual congress of the European Society of Cardiology "alluring" and "fantastic," with a reported 54% reduction in major adverse cardiovascular events in high-risk, statin-treated patients on add-on alirocumab. But neither he nor Dr. Robinson consider these results to be the final word on the subject.
Here’s what Dr. Robinson had to say about the investigational PCSK9 inhibitor’s performance in the study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ESC CONGRESS 2014
Fractional flow reserve-guided PCI improves outcomes in stable heart disease
Fractional flow reserve-guided percutaneous coronary intervention plus best medical therapy improved outcomes when compared with medical therapy alone in patients with stable coronary artery disease in the Fractional Flow Reserve versus Angiography for Multivessel Evaluation 2 (FAME 2) trial.
A composite outcome of death from any cause, nonfatal myocardial infarction, or urgent revascularization within 2 years occurred in 8.1% of 447 patients who had at least one stenosis with a fractional flow reserve (FFR) of 0.80 and were randomized to undergo percutaneous coronary intervention (PCI) performed on the basis of the FFR, compared with 19.5% of 441 such patients who received medical therapy alone.
The difference was driven mainly by a 77% reduction in the need for urgent revascularization in interventional group as compared with the medical therapy group (4.0% vs. 16.3%, hazard ratio, 0.23). The overall rates of death and myocardial infarction did not differ significantly between the groups, Dr. Bernard D. Bruyne of the Cardiovascular Center Aalst, Belgium, and his colleagues reported at the annual congress of the European Society of Cardiology.
The findings of the open-label, randomized, multicenter trial were simultaneously published online Sept. 1 (N. Engl. J. Med. 2014 Sept. 1[doi:10.1056/NEJMoa1408758]).
The improved outcomes in the PCI group were the result of outcomes that occurred between 8 days and 2 years after randomization; in the first 7 days, more primary end-point events occurred in the PCI group than in the medical therapy group (2.2% vs. 0.9%, hazard ratio 2.49).
The rate of the primary endpoint was 9.0% among 332 additional patients who had an FFR of more than 0.80 in all stenoses, were enrolled into a registry, and received medical therapy alone, the researchers said.
The original FAME trial studied the procedure in patients who had already been selected for PCI. Compared with patients whose PCI was guided by angiography alone, those whose PCI was guided by FFR had significantly reduced rates of the composite end point of death, nonfatal myocardial infarction, and repeat revascularization at 1 year (N. Engl. J. Med. 2009;360:213-24).
FAME 2 evaluated use of FFR for improving the benefits of initial stenting as an alternative to noninvasive medical therapy. The trial was halted after a median of 7 months’ follow-up, when data safety and monitoring board found a highly statistically significant reduction in hospital readmission and urgent revascularization in the patients who received FFR-based stenting compared with those who received optimal medical therapy alone.
FAME 2 was supported by St. Jude Medical. Dr. De Bruyne reported that his institution receives grant support and consulting fees on his behalf from St. Jude Medical. Detailed disclosure information for several other authors is available with the full text of the article at www.NEJM.org.
The most impressive finding in the FAME 2 trial was a sustained reduced rate of urgent revascularization among patients undergoing early PCI.
Though the trigger in 60% of patients was based solely on clinical features and was potentially influenced by knowledge of the coronary anatomy, there was nonetheless a lower incidence of revascularization triggered by myocardial infarction or electrocardiographic changes in the PCI group than in the medical therapy group (3.4% versus 7.0%). This finding is consistent with recent evidence challenging the long-held view that acute coronary syndromes occur mainly at sites of noncritical stenoses.
It is plausible that stenting of vulnerable, hemodynamically significant lesions could prevent future coronary events.
The FAME 2 results show that early FFR-guided PCI in patients with stable coronary disease sustainably reduced the need for urgent revascularization. Given the continued improvement in the safety of stents and the procedures to implant them, PCI may eventually be shown to also have a favorable effect on other hard end points.
Dr. Jeffrey J. Rade is with the University of Massachusetts, Worcester. He made these comments in an accompanying editorial (N. Engl. J. Med. 2014 Sept. 1[doi:10.1056/NEJMe1410336]). He reported having no disclosures.
The most impressive finding in the FAME 2 trial was a sustained reduced rate of urgent revascularization among patients undergoing early PCI.
Though the trigger in 60% of patients was based solely on clinical features and was potentially influenced by knowledge of the coronary anatomy, there was nonetheless a lower incidence of revascularization triggered by myocardial infarction or electrocardiographic changes in the PCI group than in the medical therapy group (3.4% versus 7.0%). This finding is consistent with recent evidence challenging the long-held view that acute coronary syndromes occur mainly at sites of noncritical stenoses.
It is plausible that stenting of vulnerable, hemodynamically significant lesions could prevent future coronary events.
The FAME 2 results show that early FFR-guided PCI in patients with stable coronary disease sustainably reduced the need for urgent revascularization. Given the continued improvement in the safety of stents and the procedures to implant them, PCI may eventually be shown to also have a favorable effect on other hard end points.
Dr. Jeffrey J. Rade is with the University of Massachusetts, Worcester. He made these comments in an accompanying editorial (N. Engl. J. Med. 2014 Sept. 1[doi:10.1056/NEJMe1410336]). He reported having no disclosures.
The most impressive finding in the FAME 2 trial was a sustained reduced rate of urgent revascularization among patients undergoing early PCI.
Though the trigger in 60% of patients was based solely on clinical features and was potentially influenced by knowledge of the coronary anatomy, there was nonetheless a lower incidence of revascularization triggered by myocardial infarction or electrocardiographic changes in the PCI group than in the medical therapy group (3.4% versus 7.0%). This finding is consistent with recent evidence challenging the long-held view that acute coronary syndromes occur mainly at sites of noncritical stenoses.
It is plausible that stenting of vulnerable, hemodynamically significant lesions could prevent future coronary events.
The FAME 2 results show that early FFR-guided PCI in patients with stable coronary disease sustainably reduced the need for urgent revascularization. Given the continued improvement in the safety of stents and the procedures to implant them, PCI may eventually be shown to also have a favorable effect on other hard end points.
Dr. Jeffrey J. Rade is with the University of Massachusetts, Worcester. He made these comments in an accompanying editorial (N. Engl. J. Med. 2014 Sept. 1[doi:10.1056/NEJMe1410336]). He reported having no disclosures.
Fractional flow reserve-guided percutaneous coronary intervention plus best medical therapy improved outcomes when compared with medical therapy alone in patients with stable coronary artery disease in the Fractional Flow Reserve versus Angiography for Multivessel Evaluation 2 (FAME 2) trial.
A composite outcome of death from any cause, nonfatal myocardial infarction, or urgent revascularization within 2 years occurred in 8.1% of 447 patients who had at least one stenosis with a fractional flow reserve (FFR) of 0.80 and were randomized to undergo percutaneous coronary intervention (PCI) performed on the basis of the FFR, compared with 19.5% of 441 such patients who received medical therapy alone.
The difference was driven mainly by a 77% reduction in the need for urgent revascularization in interventional group as compared with the medical therapy group (4.0% vs. 16.3%, hazard ratio, 0.23). The overall rates of death and myocardial infarction did not differ significantly between the groups, Dr. Bernard D. Bruyne of the Cardiovascular Center Aalst, Belgium, and his colleagues reported at the annual congress of the European Society of Cardiology.
The findings of the open-label, randomized, multicenter trial were simultaneously published online Sept. 1 (N. Engl. J. Med. 2014 Sept. 1[doi:10.1056/NEJMoa1408758]).
The improved outcomes in the PCI group were the result of outcomes that occurred between 8 days and 2 years after randomization; in the first 7 days, more primary end-point events occurred in the PCI group than in the medical therapy group (2.2% vs. 0.9%, hazard ratio 2.49).
The rate of the primary endpoint was 9.0% among 332 additional patients who had an FFR of more than 0.80 in all stenoses, were enrolled into a registry, and received medical therapy alone, the researchers said.
The original FAME trial studied the procedure in patients who had already been selected for PCI. Compared with patients whose PCI was guided by angiography alone, those whose PCI was guided by FFR had significantly reduced rates of the composite end point of death, nonfatal myocardial infarction, and repeat revascularization at 1 year (N. Engl. J. Med. 2009;360:213-24).
FAME 2 evaluated use of FFR for improving the benefits of initial stenting as an alternative to noninvasive medical therapy. The trial was halted after a median of 7 months’ follow-up, when data safety and monitoring board found a highly statistically significant reduction in hospital readmission and urgent revascularization in the patients who received FFR-based stenting compared with those who received optimal medical therapy alone.
FAME 2 was supported by St. Jude Medical. Dr. De Bruyne reported that his institution receives grant support and consulting fees on his behalf from St. Jude Medical. Detailed disclosure information for several other authors is available with the full text of the article at www.NEJM.org.
Fractional flow reserve-guided percutaneous coronary intervention plus best medical therapy improved outcomes when compared with medical therapy alone in patients with stable coronary artery disease in the Fractional Flow Reserve versus Angiography for Multivessel Evaluation 2 (FAME 2) trial.
A composite outcome of death from any cause, nonfatal myocardial infarction, or urgent revascularization within 2 years occurred in 8.1% of 447 patients who had at least one stenosis with a fractional flow reserve (FFR) of 0.80 and were randomized to undergo percutaneous coronary intervention (PCI) performed on the basis of the FFR, compared with 19.5% of 441 such patients who received medical therapy alone.
The difference was driven mainly by a 77% reduction in the need for urgent revascularization in interventional group as compared with the medical therapy group (4.0% vs. 16.3%, hazard ratio, 0.23). The overall rates of death and myocardial infarction did not differ significantly between the groups, Dr. Bernard D. Bruyne of the Cardiovascular Center Aalst, Belgium, and his colleagues reported at the annual congress of the European Society of Cardiology.
The findings of the open-label, randomized, multicenter trial were simultaneously published online Sept. 1 (N. Engl. J. Med. 2014 Sept. 1[doi:10.1056/NEJMoa1408758]).
The improved outcomes in the PCI group were the result of outcomes that occurred between 8 days and 2 years after randomization; in the first 7 days, more primary end-point events occurred in the PCI group than in the medical therapy group (2.2% vs. 0.9%, hazard ratio 2.49).
The rate of the primary endpoint was 9.0% among 332 additional patients who had an FFR of more than 0.80 in all stenoses, were enrolled into a registry, and received medical therapy alone, the researchers said.
The original FAME trial studied the procedure in patients who had already been selected for PCI. Compared with patients whose PCI was guided by angiography alone, those whose PCI was guided by FFR had significantly reduced rates of the composite end point of death, nonfatal myocardial infarction, and repeat revascularization at 1 year (N. Engl. J. Med. 2009;360:213-24).
FAME 2 evaluated use of FFR for improving the benefits of initial stenting as an alternative to noninvasive medical therapy. The trial was halted after a median of 7 months’ follow-up, when data safety and monitoring board found a highly statistically significant reduction in hospital readmission and urgent revascularization in the patients who received FFR-based stenting compared with those who received optimal medical therapy alone.
FAME 2 was supported by St. Jude Medical. Dr. De Bruyne reported that his institution receives grant support and consulting fees on his behalf from St. Jude Medical. Detailed disclosure information for several other authors is available with the full text of the article at www.NEJM.org.
FROM THE ESC CONGRESS 2014
Key clinical point: Stenting cut the need for urgent revascularization in patients who had early PCI.
Major finding: The composite outcome occurred in 8.1% vs. 19.5% of intervention and medical therapy patients, respectively.
Data source: The open-label, randomized, multicenter FAME 2 study in 888 patients.
Disclosures: FAME 2 was supported by St. Jude Medical. Dr. De Bruyne reported that his institution receives grant support and consulting fees on his behalf from St. Jude Medical. Detailed disclosure information for several other authors is available with the full text of the article at www.NEJM.org.
Ultrathin, biodegradable stent proves noninferior to durable polymer stent
An ultrathin, sirolimus-eluting biodegradable stent was noninferior to a durable everolimus-eluting stent for a primary endpoint of target lesion failure, according to the results of a 12-month randomized, single-blind trial presented at the annual congress of the European Society of Cardiology.
The noninferiority BIOSCIENCE trial in 2,119 patients – 1063 given the sirolimus-eluting, biodegradable stent, and 1,056 given the durable everolimus-eluting stent – recorded a 6.5% failure rate with sirolimus-eluting stent and 6.6% failure rate with the everolimus-eluting stent, and found no significant differences in the rates of definite stent thrombosis.
However, the investigators did note slightly better outcomes among patients with ST-segment elevation myocardial infarction given the biodegradable, stent compared with those given the durable polymer stent, according to a paper simultaneously published online September 1 in The Lancet [http://dx.doi.org/10.1016/ S0140-6736(14)61038-2].
"Our findings document excellent clinical outcomes for both stent types in a patient population with minimal exclusion criteria – a substantial proportion of whom presented with acute coronary syndromes and complex lesion characteristics," wrote Dr. Thomas Pilgrim, from University Hospital, Bern, Switzerland, and colleagues.
Biodegradable polymer stents have the potential to abolish the late adverse outcomes from durable polymer, such as late stent thrombosis and neoatherosclerosis, which may result from permanent contact between fluoropolymers and the vessel wall, an accompanying editorial noted.
"The large body of accumulated scientific evidence, including the important results from BIOSCIENCE, should trigger research efforts and economic resources to focus on functional restoration of the stented coronary artery, for example, with fully bioresorbable vascular scaffolds," wrote the editorial’s authors Dr. Julinda Mehilli from the Munich (Germany) University Clinic, and Dr. Steffen Massberg of the Munich Heart Alliance.
The study was partly funded by a grant from the Swiss National Science Foundation, and some authors declared travel expenses, grants, lecture fees and other funding from a range of companies including the stent’s manufacturer Biotronik. Dr Mehilli declared speaker’s fees from Abbott Vascular, Biotronik, and Lilly/Daiichi Sankyo.
An ultrathin, sirolimus-eluting biodegradable stent was noninferior to a durable everolimus-eluting stent for a primary endpoint of target lesion failure, according to the results of a 12-month randomized, single-blind trial presented at the annual congress of the European Society of Cardiology.
The noninferiority BIOSCIENCE trial in 2,119 patients – 1063 given the sirolimus-eluting, biodegradable stent, and 1,056 given the durable everolimus-eluting stent – recorded a 6.5% failure rate with sirolimus-eluting stent and 6.6% failure rate with the everolimus-eluting stent, and found no significant differences in the rates of definite stent thrombosis.
However, the investigators did note slightly better outcomes among patients with ST-segment elevation myocardial infarction given the biodegradable, stent compared with those given the durable polymer stent, according to a paper simultaneously published online September 1 in The Lancet [http://dx.doi.org/10.1016/ S0140-6736(14)61038-2].
"Our findings document excellent clinical outcomes for both stent types in a patient population with minimal exclusion criteria – a substantial proportion of whom presented with acute coronary syndromes and complex lesion characteristics," wrote Dr. Thomas Pilgrim, from University Hospital, Bern, Switzerland, and colleagues.
Biodegradable polymer stents have the potential to abolish the late adverse outcomes from durable polymer, such as late stent thrombosis and neoatherosclerosis, which may result from permanent contact between fluoropolymers and the vessel wall, an accompanying editorial noted.
"The large body of accumulated scientific evidence, including the important results from BIOSCIENCE, should trigger research efforts and economic resources to focus on functional restoration of the stented coronary artery, for example, with fully bioresorbable vascular scaffolds," wrote the editorial’s authors Dr. Julinda Mehilli from the Munich (Germany) University Clinic, and Dr. Steffen Massberg of the Munich Heart Alliance.
The study was partly funded by a grant from the Swiss National Science Foundation, and some authors declared travel expenses, grants, lecture fees and other funding from a range of companies including the stent’s manufacturer Biotronik. Dr Mehilli declared speaker’s fees from Abbott Vascular, Biotronik, and Lilly/Daiichi Sankyo.
An ultrathin, sirolimus-eluting biodegradable stent was noninferior to a durable everolimus-eluting stent for a primary endpoint of target lesion failure, according to the results of a 12-month randomized, single-blind trial presented at the annual congress of the European Society of Cardiology.
The noninferiority BIOSCIENCE trial in 2,119 patients – 1063 given the sirolimus-eluting, biodegradable stent, and 1,056 given the durable everolimus-eluting stent – recorded a 6.5% failure rate with sirolimus-eluting stent and 6.6% failure rate with the everolimus-eluting stent, and found no significant differences in the rates of definite stent thrombosis.
However, the investigators did note slightly better outcomes among patients with ST-segment elevation myocardial infarction given the biodegradable, stent compared with those given the durable polymer stent, according to a paper simultaneously published online September 1 in The Lancet [http://dx.doi.org/10.1016/ S0140-6736(14)61038-2].
"Our findings document excellent clinical outcomes for both stent types in a patient population with minimal exclusion criteria – a substantial proportion of whom presented with acute coronary syndromes and complex lesion characteristics," wrote Dr. Thomas Pilgrim, from University Hospital, Bern, Switzerland, and colleagues.
Biodegradable polymer stents have the potential to abolish the late adverse outcomes from durable polymer, such as late stent thrombosis and neoatherosclerosis, which may result from permanent contact between fluoropolymers and the vessel wall, an accompanying editorial noted.
"The large body of accumulated scientific evidence, including the important results from BIOSCIENCE, should trigger research efforts and economic resources to focus on functional restoration of the stented coronary artery, for example, with fully bioresorbable vascular scaffolds," wrote the editorial’s authors Dr. Julinda Mehilli from the Munich (Germany) University Clinic, and Dr. Steffen Massberg of the Munich Heart Alliance.
The study was partly funded by a grant from the Swiss National Science Foundation, and some authors declared travel expenses, grants, lecture fees and other funding from a range of companies including the stent’s manufacturer Biotronik. Dr Mehilli declared speaker’s fees from Abbott Vascular, Biotronik, and Lilly/Daiichi Sankyo.
FROM THE ESC CONGRESS 2014
Key clinical point: An ultrathin sirolimus-eluting stent made from biodegradable polymer was noninferior to a durable polymer everolimus-eluting stent.
Major finding: The primary endpoint of target-lesion failure was 6.5% with the biodegradable stent and 6.6% with the durable stent.
Data source: BIOSCIENCE, a randomized, single-blind, noninferiority trial in 2,119 patients.
Disclosures: BIOSCIENCE was partly funded by a grant from the Swiss National Science Foundation, and some authors declared travel expenses, grants, lecture fees and other funding from a range of companies including the stent’s manufacturer Biotronik.
Alirocumab sharply reduced major cardiovascular events
BARCELONA – When added to maximally tolerated statin therapy, the investigational PCSK9 inhibitor alirocumab resulted in a further 54% reduction in major cardiovascular events among high-cardiovascular-risk patients, based on a post-hoc analysis of a large randomized controlled Phase-3 trial.
The ODYSSEY LONG TERM trial is the largest and longest study of a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor to report results to date, with roughly 1,900 patient-years of double-blind exposure to alirocumab. And although the ongoing trial is primarily a safety study, it is also now the first PCSK9 trial to provide what everyone watching the development of this novel drug class has been eagerly awaiting: clinical outcomes data, albeit in this case from a post-hoc secondary analysis.
“This is the first trial with any of the PCSK9 inhibitors to suggest that there will be a further significant reduction in cardiovascular events when added on to maximized statin therapy,” Dr. Jennifer G. Robinson said in presenting interim results of ODYSSEY LONG TERM at the annual congress of the European Society of Cardiology.
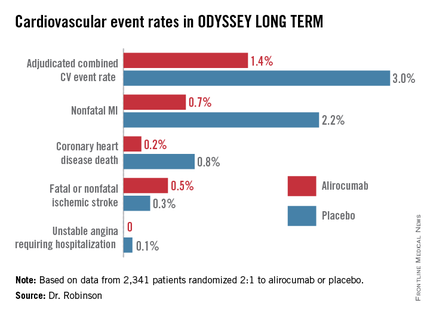
“We’re on the right track in terms of trying to achieve further reduction in cardiovascular events through additional lipid lowering. But this is not the definitive evidence. We need the prospective outcomes trials to validate this data and also to establish the long-term safety of these drugs when added to the statins,” cautioned Dr. Robinson, professor of epidemiology and of medicine and director of the prevention intervention center at the University of Iowa, Iowa City.
Nonetheless, on the basis of the dramatic LDL-lowering and reassuring evidence of safety shown in ODYSSEY LONG TERM and the other double-blind phase III trials presented at the congress, Sanofi and Regeneron announced plans to file for U.S. and European Union marketing approval of alirocumab before the end of the year. The proposed indication will be for LDL lowering, which regulatory agencies have accepted as a surrogate endpoint for prevention of clinical events.
Meanwhile, the definitive ODYSSEY OUTCOMES trial is underway in 18,000 patients with acute coronary syndromes, with prospective evaluation of CV outcomes as its primary endpoint. The composite endpoint employed in the big OUTCOMES trial is identical to that used in the ODYSSEY LONG TERM post hoc analysis.
ODYSSEY LONG TERM includes 2,341 patients at high CV risk and an LDL level greater than 70 mg/dL despite maximally tolerated statin therapy. The patients fall into two categories: those with heterozygous familial hypercholesterolemia and others at very high risk because of known coronary heart disease. Participants were randomized 2:1 to 150 mg of alirocumab by self-administered subcutaneous injection at home every 2 weeks or placebo in addition to their statin.
In the interim post-hoc efficacy analysis at 65 weeks, the combined rate of cardiac death, nonfatal MI, stroke, and unstable angina requiring hospitalization was 1.4% in the alirocumab arm compared to 3.0% in placebo-treated controls, for a highly significant 54% relative risk reduction (see graphic).
At 24 weeks, the alirocumab group showed a mean 62% reduction in LDL compared to placebo, a difference that remained constant at 52 weeks. The average LDL level at 52 weeks in the alirocumab group was 53 mg/dL, down from 123 mg/dL on active treatment at baseline; 79% of alirocumab-treated patients achieved an LDL below 70 mg/dL.
The incidence and types of adverse events in the alirocumab arm were essentially the same as with placebo, with no signal of problems in any domains, including neurocognitive function or allergic reactions.
ODYSSEY FH I and FH II
In a separate presentation, Dr. Michel Farnier reported on the alirocumab experience in 735 patients with heterozygous familial hypercholesterolemia in two phase III trials known as ODYSSEY FH I and FH II. At baseline, all were above their LDL goal despite maximally tolerated statin therapy, in two-thirds of cases with add-on ezetimibe. Participants were randomized 2:1 to add-on alirocumab at 75 mg every 2 weeks or to placebo.
The alirocumab-treated patients had 58% and 51% reductions in LDL, compared to actively treated controls at 24 weeks in the FH I and FH II trials. Of the alirocumab-treated patients, 72% and 81% achieved their prespecified LDL goal at 24 weeks, compared with 2% and 11% of controls.
“We have never before seen these kinds of percentages of patients with familial hypercholesterolemia reaching these LDL levels,” commented Dr. Farnier of Point Medical in Dijon, France.
ODYSSEY COMBO II
At the same hot-line clinical trials session, Dr. Christopher P. Cannon reported that alirocumab markedly outperformed ezetimibe as add-on therapy in the 720-patient, phase III, double-blind ODYSSEY COMBO II trial.
In this study, patients at very high CV risk who were unable to reach their desired goal of an LDL below 70 mg/dL despite maximum tolerated statin doses were randomized 2:1 to alirocumab at 75 mg once every 2 weeks or oral ezetimibe (Zetia) at its approved dose of 10 mg/day as an active comparator. Each participant also received placebo therapy.
By week 24, patients on alirocumab plus high-dose statin averaged a 51% reduction in LDL compared to baseline, versus a 21% reduction with ezetimibe plus statin. These effects were maintained at 1 year, with no evidence of tolerance.
Of patients on alirocumab, 77% achieved an LDL goal of less than 70 mg/dL at week 24, compared with 45% on ezetimibe. In addition, 60% of the alirocumab group had an LDL below 50 mg/dL, as did 15% on ezetimibe.
The study design called for patients in the alirocumab group who still had an LDL above 70 mg/dL at week 12 to be uptitrated from 75 mg to 150 mg every 2 weeks. But only 20% of patients needed to do so, according to Dr. Cannon, professor of medicine at Harvard University, Boston.
Will high-risk patient adhere long-term to treatment by self-injection? Dr. Cannon thinks so. He noted that 85% of patients in ODYSSEY COMBO II remained adherent to the biweekly self-injection protocol through 1 year.
“That has been a very pleasant surprise,” the cardiologist said. “The notion of injections for cholesterol management is foreign. It was a big surprise to us that patients really did it.”
‘Great news’ from alirocumab
The alirocumab results in the heterozygous familial hypercholesterolemia trials are “great news for patients with this disease,” discussant Dr. Robert M. Califf said. He noted that recent estimates put the prevalence of heterozygous familial hypercholesterolemia at roughly 1 in 250 persons in the general population, making the genetic disorder considerably more common than most physicians realize.
“These are people who have a terrible disease where a massive reduction in LDL, it seems to me, is clearly worthwhile,” commented Dr. Califf, professor of medicine and vice chancellor for clinical and translational research at Duke University in Durham, N.C.
As for Dr. Robinson’s ODYSSEY LONG TERM data showing a 54% reduction in major CV events, he said “It’s alluring. It looks great. It looks fantastic. And it sets up the large events trial. The only caution out of all of this from my perspective is that any study with less than 100 events is something that should be regarded with great interest but is not definitive.”
If approved, alirocumab could see widespread use, especially if the ODYSSEY OUTCOMES results prove positive. After all, heterozygous familial hypercholesterolemia is now recognized to be one of the most common of all inherited diseases. And Dr. Cannon said that in clinical trials in post-acute coronary syndrome patients placed on high-dose statins, it’s common for only about 40% to achieve an LDL level below 70 mg/dL. In clinical practice, the rate is probably even lower.*
“Down the line I think this level of LDL lowering is needed very badly. To get high-risk patients who have high cholesterol despite maximally dosed statins or statin-intolerance down to an LDL level of 50 mg/dL would be a pretty good thing. This will be applicable to millions of patients,” he predicted.*
Dr. Robinson said that at the American Heart Association meeting this November she and her ODYSSEY LONG TERM coinvestigators plan to present subgroup analyses looking at alirocumab efficacy and safety in patients with achieved LDL levels below 25 and even 15 mg/dL.
Dr. Robinson, Dr. Cannon, and Dr. Farnier reported receiving research grants and consultants’ fees from Sanofi and Regeneron as well as other pharmaceutical companies. Dr. Califf reported having no financial conflicts.
bjancin@frontlinemedcom.com
*CORRECTION: An earlier version of this story misattributed these statements.
BARCELONA – When added to maximally tolerated statin therapy, the investigational PCSK9 inhibitor alirocumab resulted in a further 54% reduction in major cardiovascular events among high-cardiovascular-risk patients, based on a post-hoc analysis of a large randomized controlled Phase-3 trial.
The ODYSSEY LONG TERM trial is the largest and longest study of a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor to report results to date, with roughly 1,900 patient-years of double-blind exposure to alirocumab. And although the ongoing trial is primarily a safety study, it is also now the first PCSK9 trial to provide what everyone watching the development of this novel drug class has been eagerly awaiting: clinical outcomes data, albeit in this case from a post-hoc secondary analysis.
“This is the first trial with any of the PCSK9 inhibitors to suggest that there will be a further significant reduction in cardiovascular events when added on to maximized statin therapy,” Dr. Jennifer G. Robinson said in presenting interim results of ODYSSEY LONG TERM at the annual congress of the European Society of Cardiology.

“We’re on the right track in terms of trying to achieve further reduction in cardiovascular events through additional lipid lowering. But this is not the definitive evidence. We need the prospective outcomes trials to validate this data and also to establish the long-term safety of these drugs when added to the statins,” cautioned Dr. Robinson, professor of epidemiology and of medicine and director of the prevention intervention center at the University of Iowa, Iowa City.
Nonetheless, on the basis of the dramatic LDL-lowering and reassuring evidence of safety shown in ODYSSEY LONG TERM and the other double-blind phase III trials presented at the congress, Sanofi and Regeneron announced plans to file for U.S. and European Union marketing approval of alirocumab before the end of the year. The proposed indication will be for LDL lowering, which regulatory agencies have accepted as a surrogate endpoint for prevention of clinical events.
Meanwhile, the definitive ODYSSEY OUTCOMES trial is underway in 18,000 patients with acute coronary syndromes, with prospective evaluation of CV outcomes as its primary endpoint. The composite endpoint employed in the big OUTCOMES trial is identical to that used in the ODYSSEY LONG TERM post hoc analysis.
ODYSSEY LONG TERM includes 2,341 patients at high CV risk and an LDL level greater than 70 mg/dL despite maximally tolerated statin therapy. The patients fall into two categories: those with heterozygous familial hypercholesterolemia and others at very high risk because of known coronary heart disease. Participants were randomized 2:1 to 150 mg of alirocumab by self-administered subcutaneous injection at home every 2 weeks or placebo in addition to their statin.
In the interim post-hoc efficacy analysis at 65 weeks, the combined rate of cardiac death, nonfatal MI, stroke, and unstable angina requiring hospitalization was 1.4% in the alirocumab arm compared to 3.0% in placebo-treated controls, for a highly significant 54% relative risk reduction (see graphic).
At 24 weeks, the alirocumab group showed a mean 62% reduction in LDL compared to placebo, a difference that remained constant at 52 weeks. The average LDL level at 52 weeks in the alirocumab group was 53 mg/dL, down from 123 mg/dL on active treatment at baseline; 79% of alirocumab-treated patients achieved an LDL below 70 mg/dL.
The incidence and types of adverse events in the alirocumab arm were essentially the same as with placebo, with no signal of problems in any domains, including neurocognitive function or allergic reactions.
ODYSSEY FH I and FH II
In a separate presentation, Dr. Michel Farnier reported on the alirocumab experience in 735 patients with heterozygous familial hypercholesterolemia in two phase III trials known as ODYSSEY FH I and FH II. At baseline, all were above their LDL goal despite maximally tolerated statin therapy, in two-thirds of cases with add-on ezetimibe. Participants were randomized 2:1 to add-on alirocumab at 75 mg every 2 weeks or to placebo.
The alirocumab-treated patients had 58% and 51% reductions in LDL, compared to actively treated controls at 24 weeks in the FH I and FH II trials. Of the alirocumab-treated patients, 72% and 81% achieved their prespecified LDL goal at 24 weeks, compared with 2% and 11% of controls.
“We have never before seen these kinds of percentages of patients with familial hypercholesterolemia reaching these LDL levels,” commented Dr. Farnier of Point Medical in Dijon, France.
ODYSSEY COMBO II
At the same hot-line clinical trials session, Dr. Christopher P. Cannon reported that alirocumab markedly outperformed ezetimibe as add-on therapy in the 720-patient, phase III, double-blind ODYSSEY COMBO II trial.
In this study, patients at very high CV risk who were unable to reach their desired goal of an LDL below 70 mg/dL despite maximum tolerated statin doses were randomized 2:1 to alirocumab at 75 mg once every 2 weeks or oral ezetimibe (Zetia) at its approved dose of 10 mg/day as an active comparator. Each participant also received placebo therapy.
By week 24, patients on alirocumab plus high-dose statin averaged a 51% reduction in LDL compared to baseline, versus a 21% reduction with ezetimibe plus statin. These effects were maintained at 1 year, with no evidence of tolerance.
Of patients on alirocumab, 77% achieved an LDL goal of less than 70 mg/dL at week 24, compared with 45% on ezetimibe. In addition, 60% of the alirocumab group had an LDL below 50 mg/dL, as did 15% on ezetimibe.
The study design called for patients in the alirocumab group who still had an LDL above 70 mg/dL at week 12 to be uptitrated from 75 mg to 150 mg every 2 weeks. But only 20% of patients needed to do so, according to Dr. Cannon, professor of medicine at Harvard University, Boston.
Will high-risk patient adhere long-term to treatment by self-injection? Dr. Cannon thinks so. He noted that 85% of patients in ODYSSEY COMBO II remained adherent to the biweekly self-injection protocol through 1 year.
“That has been a very pleasant surprise,” the cardiologist said. “The notion of injections for cholesterol management is foreign. It was a big surprise to us that patients really did it.”
‘Great news’ from alirocumab
The alirocumab results in the heterozygous familial hypercholesterolemia trials are “great news for patients with this disease,” discussant Dr. Robert M. Califf said. He noted that recent estimates put the prevalence of heterozygous familial hypercholesterolemia at roughly 1 in 250 persons in the general population, making the genetic disorder considerably more common than most physicians realize.
“These are people who have a terrible disease where a massive reduction in LDL, it seems to me, is clearly worthwhile,” commented Dr. Califf, professor of medicine and vice chancellor for clinical and translational research at Duke University in Durham, N.C.
As for Dr. Robinson’s ODYSSEY LONG TERM data showing a 54% reduction in major CV events, he said “It’s alluring. It looks great. It looks fantastic. And it sets up the large events trial. The only caution out of all of this from my perspective is that any study with less than 100 events is something that should be regarded with great interest but is not definitive.”
If approved, alirocumab could see widespread use, especially if the ODYSSEY OUTCOMES results prove positive. After all, heterozygous familial hypercholesterolemia is now recognized to be one of the most common of all inherited diseases. And Dr. Cannon said that in clinical trials in post-acute coronary syndrome patients placed on high-dose statins, it’s common for only about 40% to achieve an LDL level below 70 mg/dL. In clinical practice, the rate is probably even lower.*
“Down the line I think this level of LDL lowering is needed very badly. To get high-risk patients who have high cholesterol despite maximally dosed statins or statin-intolerance down to an LDL level of 50 mg/dL would be a pretty good thing. This will be applicable to millions of patients,” he predicted.*
Dr. Robinson said that at the American Heart Association meeting this November she and her ODYSSEY LONG TERM coinvestigators plan to present subgroup analyses looking at alirocumab efficacy and safety in patients with achieved LDL levels below 25 and even 15 mg/dL.
Dr. Robinson, Dr. Cannon, and Dr. Farnier reported receiving research grants and consultants’ fees from Sanofi and Regeneron as well as other pharmaceutical companies. Dr. Califf reported having no financial conflicts.
bjancin@frontlinemedcom.com
*CORRECTION: An earlier version of this story misattributed these statements.
BARCELONA – When added to maximally tolerated statin therapy, the investigational PCSK9 inhibitor alirocumab resulted in a further 54% reduction in major cardiovascular events among high-cardiovascular-risk patients, based on a post-hoc analysis of a large randomized controlled Phase-3 trial.
The ODYSSEY LONG TERM trial is the largest and longest study of a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor to report results to date, with roughly 1,900 patient-years of double-blind exposure to alirocumab. And although the ongoing trial is primarily a safety study, it is also now the first PCSK9 trial to provide what everyone watching the development of this novel drug class has been eagerly awaiting: clinical outcomes data, albeit in this case from a post-hoc secondary analysis.
“This is the first trial with any of the PCSK9 inhibitors to suggest that there will be a further significant reduction in cardiovascular events when added on to maximized statin therapy,” Dr. Jennifer G. Robinson said in presenting interim results of ODYSSEY LONG TERM at the annual congress of the European Society of Cardiology.

“We’re on the right track in terms of trying to achieve further reduction in cardiovascular events through additional lipid lowering. But this is not the definitive evidence. We need the prospective outcomes trials to validate this data and also to establish the long-term safety of these drugs when added to the statins,” cautioned Dr. Robinson, professor of epidemiology and of medicine and director of the prevention intervention center at the University of Iowa, Iowa City.
Nonetheless, on the basis of the dramatic LDL-lowering and reassuring evidence of safety shown in ODYSSEY LONG TERM and the other double-blind phase III trials presented at the congress, Sanofi and Regeneron announced plans to file for U.S. and European Union marketing approval of alirocumab before the end of the year. The proposed indication will be for LDL lowering, which regulatory agencies have accepted as a surrogate endpoint for prevention of clinical events.
Meanwhile, the definitive ODYSSEY OUTCOMES trial is underway in 18,000 patients with acute coronary syndromes, with prospective evaluation of CV outcomes as its primary endpoint. The composite endpoint employed in the big OUTCOMES trial is identical to that used in the ODYSSEY LONG TERM post hoc analysis.
ODYSSEY LONG TERM includes 2,341 patients at high CV risk and an LDL level greater than 70 mg/dL despite maximally tolerated statin therapy. The patients fall into two categories: those with heterozygous familial hypercholesterolemia and others at very high risk because of known coronary heart disease. Participants were randomized 2:1 to 150 mg of alirocumab by self-administered subcutaneous injection at home every 2 weeks or placebo in addition to their statin.
In the interim post-hoc efficacy analysis at 65 weeks, the combined rate of cardiac death, nonfatal MI, stroke, and unstable angina requiring hospitalization was 1.4% in the alirocumab arm compared to 3.0% in placebo-treated controls, for a highly significant 54% relative risk reduction (see graphic).
At 24 weeks, the alirocumab group showed a mean 62% reduction in LDL compared to placebo, a difference that remained constant at 52 weeks. The average LDL level at 52 weeks in the alirocumab group was 53 mg/dL, down from 123 mg/dL on active treatment at baseline; 79% of alirocumab-treated patients achieved an LDL below 70 mg/dL.
The incidence and types of adverse events in the alirocumab arm were essentially the same as with placebo, with no signal of problems in any domains, including neurocognitive function or allergic reactions.
ODYSSEY FH I and FH II
In a separate presentation, Dr. Michel Farnier reported on the alirocumab experience in 735 patients with heterozygous familial hypercholesterolemia in two phase III trials known as ODYSSEY FH I and FH II. At baseline, all were above their LDL goal despite maximally tolerated statin therapy, in two-thirds of cases with add-on ezetimibe. Participants were randomized 2:1 to add-on alirocumab at 75 mg every 2 weeks or to placebo.
The alirocumab-treated patients had 58% and 51% reductions in LDL, compared to actively treated controls at 24 weeks in the FH I and FH II trials. Of the alirocumab-treated patients, 72% and 81% achieved their prespecified LDL goal at 24 weeks, compared with 2% and 11% of controls.
“We have never before seen these kinds of percentages of patients with familial hypercholesterolemia reaching these LDL levels,” commented Dr. Farnier of Point Medical in Dijon, France.
ODYSSEY COMBO II
At the same hot-line clinical trials session, Dr. Christopher P. Cannon reported that alirocumab markedly outperformed ezetimibe as add-on therapy in the 720-patient, phase III, double-blind ODYSSEY COMBO II trial.
In this study, patients at very high CV risk who were unable to reach their desired goal of an LDL below 70 mg/dL despite maximum tolerated statin doses were randomized 2:1 to alirocumab at 75 mg once every 2 weeks or oral ezetimibe (Zetia) at its approved dose of 10 mg/day as an active comparator. Each participant also received placebo therapy.
By week 24, patients on alirocumab plus high-dose statin averaged a 51% reduction in LDL compared to baseline, versus a 21% reduction with ezetimibe plus statin. These effects were maintained at 1 year, with no evidence of tolerance.
Of patients on alirocumab, 77% achieved an LDL goal of less than 70 mg/dL at week 24, compared with 45% on ezetimibe. In addition, 60% of the alirocumab group had an LDL below 50 mg/dL, as did 15% on ezetimibe.
The study design called for patients in the alirocumab group who still had an LDL above 70 mg/dL at week 12 to be uptitrated from 75 mg to 150 mg every 2 weeks. But only 20% of patients needed to do so, according to Dr. Cannon, professor of medicine at Harvard University, Boston.
Will high-risk patient adhere long-term to treatment by self-injection? Dr. Cannon thinks so. He noted that 85% of patients in ODYSSEY COMBO II remained adherent to the biweekly self-injection protocol through 1 year.
“That has been a very pleasant surprise,” the cardiologist said. “The notion of injections for cholesterol management is foreign. It was a big surprise to us that patients really did it.”
‘Great news’ from alirocumab
The alirocumab results in the heterozygous familial hypercholesterolemia trials are “great news for patients with this disease,” discussant Dr. Robert M. Califf said. He noted that recent estimates put the prevalence of heterozygous familial hypercholesterolemia at roughly 1 in 250 persons in the general population, making the genetic disorder considerably more common than most physicians realize.
“These are people who have a terrible disease where a massive reduction in LDL, it seems to me, is clearly worthwhile,” commented Dr. Califf, professor of medicine and vice chancellor for clinical and translational research at Duke University in Durham, N.C.
As for Dr. Robinson’s ODYSSEY LONG TERM data showing a 54% reduction in major CV events, he said “It’s alluring. It looks great. It looks fantastic. And it sets up the large events trial. The only caution out of all of this from my perspective is that any study with less than 100 events is something that should be regarded with great interest but is not definitive.”
If approved, alirocumab could see widespread use, especially if the ODYSSEY OUTCOMES results prove positive. After all, heterozygous familial hypercholesterolemia is now recognized to be one of the most common of all inherited diseases. And Dr. Cannon said that in clinical trials in post-acute coronary syndrome patients placed on high-dose statins, it’s common for only about 40% to achieve an LDL level below 70 mg/dL. In clinical practice, the rate is probably even lower.*
“Down the line I think this level of LDL lowering is needed very badly. To get high-risk patients who have high cholesterol despite maximally dosed statins or statin-intolerance down to an LDL level of 50 mg/dL would be a pretty good thing. This will be applicable to millions of patients,” he predicted.*
Dr. Robinson said that at the American Heart Association meeting this November she and her ODYSSEY LONG TERM coinvestigators plan to present subgroup analyses looking at alirocumab efficacy and safety in patients with achieved LDL levels below 25 and even 15 mg/dL.
Dr. Robinson, Dr. Cannon, and Dr. Farnier reported receiving research grants and consultants’ fees from Sanofi and Regeneron as well as other pharmaceutical companies. Dr. Califf reported having no financial conflicts.
bjancin@frontlinemedcom.com
*CORRECTION: An earlier version of this story misattributed these statements.
AT THE ESC CONGRESS 2014
Key clinical point: The lower-is-better approach to LDL reduction gets a big boost from a study that showed adding alirocumab to maximally tolerated statin therapy was associated with 54% fewer major adverse cardiovascular events compared to statin plus placebo in high-cardiovascular-risk patients.
Major finding: The incidence of the composite cardiovascular event endpoint at 65 weeks was 1.4% in those on alirocumab plus maximally tolerated statin therapy and 3.0% in those on placebo plus statin.
Data source: This was an interim post hoc analysis from the Phase-3, double-blind, randomized, prospective ODYSSEY LONG TERM trial of more than 2,300 randomized patients.
Disclosures: The ODYSSEY clinical trials program is sponsored by Sanofi and Regeneron. The study presenters reported receiving research grants and consultants’ fees from those and other companies.
LCZ696 surpasses enalapril for heart failure
BARCELONA – A new, dual-agent formulation for treating heart failure patients with reduced ejection fraction showed a dramatic benefit for cutting the rate of cardiovascular death and hospitalizations in an international, controlled, pivotal trial with more than 8,000 patients.
The superiority of the new compound, which combines the novel neprilysin-inhibitor drug sacubitril with the established angiotensin receptor (ARB) blocking drug valsartan, was so strong that it immediately loomed as the new cornerstone-drug of choice for heart-failure patients with reduced ejection fraction pending regulatory approvals, said Dr. Milton Packer at the annual congress of the European Society of Cardiology.
Treatment with the new combination agent, currently known as LCZ696, doubled the cardiovascular-death benefit of the ACE inhibitor enalapril, while simultaneously edging out enalapril for safety and tolerability. Neprilysin is an endopeptidase that degrades several endogenous vasoactive peptides. Inhibiting this degradation process by treatment with LCZ696 boosts levels of these peptides and counters the neurohormonal overactivation that characterizes heart failure and leads to vasoconstriction, sodium retention, and maladaptive remodeling.
"This robust finding provides strong evidence supporting use of LCZ696 as the treatment of choice for heart failure instead of an ACE inhibitor or ARB" alone, said Dr. Packer, professor and chairman of clinical sciences at UT Southwestern Medical Center in Dallas. "The intent of this trial was to provide persuasive evidence that LCZ696 should replace current use of ACE inhibitors and ARBs as the cornerstone of heart failure treatment," said Dr. Packer, coprincipal investigator of the study.
Especially striking to several heart failure experts who heard the study results was the range of clinically important benefits seen when the new combination substituted for enalapril.
"This is the first study in a long time where we saw not only a reduction in the combined endpoint of cardiovascular death and hospitalization, but also significant effect on each of these two endpoints individually as well as on all-cause mortality," commented Dr. Mariell Jessup, professor of medicine at the University of Pennsylvania in Philadelphia. The new trial "was really trying to upset the foundation of heart failure treatment that we’ve used for the last 2 decades, and it succeeded. We were hoping for a positive outcome; what we got was remarkable. It very convincingly showed that this drug has incredible promise," she said in an interview.
"This will be an important change," commented Dr. John G.F. Cleland, professor of cardiology at the University of Hull, U.K. "The science and medicine will make it hard not to switch everyone with low ejection fraction heart failure [and other features that mirror the study’s enrollment criteria] from an ACE inhibitor to this the moment it becomes available," he said in an interview. "This is now the drug of choice for any heart failure patient with persistently elevated natriuretic peptide and left ventricular systolic dysfunction."
The Prospective Comparison of ARNI [angiotensin receptor–neprilysin inhibitor] with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial randomized 8,399 patients with heart failure and a left ventricular ejection fraction of 40% of less to treatment with either 200 mg b.i.d. of LCZ696 or 10 mg b.i.d. enalapril plus other standard heart failure medications, including a beta-blocker (in 93%), a diuretic (in 80%) and a mineralocorticoid antagonist such as spironolactone (in about 55%). About 70% of enrolled patients had New York Heart Association class II heart failure, and another 24% had class III heart failure.
After a median treatment duration of 27 months, the primary combined endpoint of cardiovascular death or first hospitalization for heart failure occurred in 22% of the patients treated with LCZ696 and in 27% of those treated with enalapril, a 20% relative risk reduction that was statistically significant, and which showed a number needed to treat of 21 to prevent one of these primary endpoints. LCZ696 treatment also linked with a significant reduction in all-cause death, and a statistically significant and clinically meaningful improvement in patient quality of life as measured using the Kansas City Cardiomyopathy Questionnaire after patients had been on treatment for 8 months. Online publication of the full results occurred concurrent with Dr. Packer’s report at the meeting (N.Engl. J. Med 2014;DOI: 10.1056/NEJMoa1409077).
While Dr. Packer highlighted the positive impact of LCZ696 on quality of life, he also stressed that the primary impact of treatment with LCZ696 is not to make patients feel better. "The major benefit of this drug is to change the natural course and rate of progression of heart failure. It should be used in patients with mild heart failure to reduce progression and death," he said.
"By switching patients from an ACE inhibitor to LCZ696 you can prolong patient survival. It is exciting to have a new drug" that can confer as many benefits as LCZ696, Dr. Jessup said.
PARADIGM-HF was sponsored by Novartis. Dr. Packer has been a consultant to Novartis as well as several other companies. Dr. Jessup had no disclosures. Dr. Cleland has received research support and honoraria from Sorin, St. Jude, and Medtronic.
Twitter: @mitchelzoler
PARADIGM-HF is the first contemporary trial to test the concept of substituting a new agent for an established heart-failure drug rather than adding a new compound to already established treatments. It was a large trial that enrolled patients with an elevated level of natriuretic peptide, used very relevant primary and secondary outcomes, and had a modest discontinuation rate during 27 months of treatment. The result showed a significant, 20% relative risk reduction in patients who were well treated for heart failure based on current guidelines, and the outcomes showed consistent results across all prespecified subgroups studied.
The investigational drug also showed good safety for renal function and potassium levels. Although patients who received LCZ696 had significantly more hypotensive episodes than patients in the enalapril comparator group, the investigational arm had no significant excess of treatment discontinuations. Patients who received LCZ696 also had a small increase in episodes of angioedema, but the difference did no reach statistical significance.
The trial used a clever run-in design that screened out patients who were intolerant of either enalapril or the study drug, a step that excluded 12% of the initial patient group.
The magnitude of the treatment effect and the number needed to treat suggests that LCZ696 will be a cost-effective treatment for the types of patients studied. Future studies should explore the efficacy of this drug combination in patients with heart failure with preserved ejection fraction, and in patients with acute decompensated heart failure, and a study should also compare LCZ696 with the safety and efficacy of a direct renin inhibitor such as aliskiren.
Michel Komajda, M.D., is professor of cardiology at University Pierre and Marie Curie in Paris, and is past president of the European Society of Cardiology. He has been a consultant to or speaker on behalf of Novartis and several other companies. He made these comments as the designated discussant for PARADIGM-HF.
PARADIGM-HF is the first contemporary trial to test the concept of substituting a new agent for an established heart-failure drug rather than adding a new compound to already established treatments. It was a large trial that enrolled patients with an elevated level of natriuretic peptide, used very relevant primary and secondary outcomes, and had a modest discontinuation rate during 27 months of treatment. The result showed a significant, 20% relative risk reduction in patients who were well treated for heart failure based on current guidelines, and the outcomes showed consistent results across all prespecified subgroups studied.
The investigational drug also showed good safety for renal function and potassium levels. Although patients who received LCZ696 had significantly more hypotensive episodes than patients in the enalapril comparator group, the investigational arm had no significant excess of treatment discontinuations. Patients who received LCZ696 also had a small increase in episodes of angioedema, but the difference did no reach statistical significance.
The trial used a clever run-in design that screened out patients who were intolerant of either enalapril or the study drug, a step that excluded 12% of the initial patient group.
The magnitude of the treatment effect and the number needed to treat suggests that LCZ696 will be a cost-effective treatment for the types of patients studied. Future studies should explore the efficacy of this drug combination in patients with heart failure with preserved ejection fraction, and in patients with acute decompensated heart failure, and a study should also compare LCZ696 with the safety and efficacy of a direct renin inhibitor such as aliskiren.
Michel Komajda, M.D., is professor of cardiology at University Pierre and Marie Curie in Paris, and is past president of the European Society of Cardiology. He has been a consultant to or speaker on behalf of Novartis and several other companies. He made these comments as the designated discussant for PARADIGM-HF.
PARADIGM-HF is the first contemporary trial to test the concept of substituting a new agent for an established heart-failure drug rather than adding a new compound to already established treatments. It was a large trial that enrolled patients with an elevated level of natriuretic peptide, used very relevant primary and secondary outcomes, and had a modest discontinuation rate during 27 months of treatment. The result showed a significant, 20% relative risk reduction in patients who were well treated for heart failure based on current guidelines, and the outcomes showed consistent results across all prespecified subgroups studied.
The investigational drug also showed good safety for renal function and potassium levels. Although patients who received LCZ696 had significantly more hypotensive episodes than patients in the enalapril comparator group, the investigational arm had no significant excess of treatment discontinuations. Patients who received LCZ696 also had a small increase in episodes of angioedema, but the difference did no reach statistical significance.
The trial used a clever run-in design that screened out patients who were intolerant of either enalapril or the study drug, a step that excluded 12% of the initial patient group.
The magnitude of the treatment effect and the number needed to treat suggests that LCZ696 will be a cost-effective treatment for the types of patients studied. Future studies should explore the efficacy of this drug combination in patients with heart failure with preserved ejection fraction, and in patients with acute decompensated heart failure, and a study should also compare LCZ696 with the safety and efficacy of a direct renin inhibitor such as aliskiren.
Michel Komajda, M.D., is professor of cardiology at University Pierre and Marie Curie in Paris, and is past president of the European Society of Cardiology. He has been a consultant to or speaker on behalf of Novartis and several other companies. He made these comments as the designated discussant for PARADIGM-HF.
BARCELONA – A new, dual-agent formulation for treating heart failure patients with reduced ejection fraction showed a dramatic benefit for cutting the rate of cardiovascular death and hospitalizations in an international, controlled, pivotal trial with more than 8,000 patients.
The superiority of the new compound, which combines the novel neprilysin-inhibitor drug sacubitril with the established angiotensin receptor (ARB) blocking drug valsartan, was so strong that it immediately loomed as the new cornerstone-drug of choice for heart-failure patients with reduced ejection fraction pending regulatory approvals, said Dr. Milton Packer at the annual congress of the European Society of Cardiology.
Treatment with the new combination agent, currently known as LCZ696, doubled the cardiovascular-death benefit of the ACE inhibitor enalapril, while simultaneously edging out enalapril for safety and tolerability. Neprilysin is an endopeptidase that degrades several endogenous vasoactive peptides. Inhibiting this degradation process by treatment with LCZ696 boosts levels of these peptides and counters the neurohormonal overactivation that characterizes heart failure and leads to vasoconstriction, sodium retention, and maladaptive remodeling.
"This robust finding provides strong evidence supporting use of LCZ696 as the treatment of choice for heart failure instead of an ACE inhibitor or ARB" alone, said Dr. Packer, professor and chairman of clinical sciences at UT Southwestern Medical Center in Dallas. "The intent of this trial was to provide persuasive evidence that LCZ696 should replace current use of ACE inhibitors and ARBs as the cornerstone of heart failure treatment," said Dr. Packer, coprincipal investigator of the study.
Especially striking to several heart failure experts who heard the study results was the range of clinically important benefits seen when the new combination substituted for enalapril.
"This is the first study in a long time where we saw not only a reduction in the combined endpoint of cardiovascular death and hospitalization, but also significant effect on each of these two endpoints individually as well as on all-cause mortality," commented Dr. Mariell Jessup, professor of medicine at the University of Pennsylvania in Philadelphia. The new trial "was really trying to upset the foundation of heart failure treatment that we’ve used for the last 2 decades, and it succeeded. We were hoping for a positive outcome; what we got was remarkable. It very convincingly showed that this drug has incredible promise," she said in an interview.
"This will be an important change," commented Dr. John G.F. Cleland, professor of cardiology at the University of Hull, U.K. "The science and medicine will make it hard not to switch everyone with low ejection fraction heart failure [and other features that mirror the study’s enrollment criteria] from an ACE inhibitor to this the moment it becomes available," he said in an interview. "This is now the drug of choice for any heart failure patient with persistently elevated natriuretic peptide and left ventricular systolic dysfunction."
The Prospective Comparison of ARNI [angiotensin receptor–neprilysin inhibitor] with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial randomized 8,399 patients with heart failure and a left ventricular ejection fraction of 40% of less to treatment with either 200 mg b.i.d. of LCZ696 or 10 mg b.i.d. enalapril plus other standard heart failure medications, including a beta-blocker (in 93%), a diuretic (in 80%) and a mineralocorticoid antagonist such as spironolactone (in about 55%). About 70% of enrolled patients had New York Heart Association class II heart failure, and another 24% had class III heart failure.
After a median treatment duration of 27 months, the primary combined endpoint of cardiovascular death or first hospitalization for heart failure occurred in 22% of the patients treated with LCZ696 and in 27% of those treated with enalapril, a 20% relative risk reduction that was statistically significant, and which showed a number needed to treat of 21 to prevent one of these primary endpoints. LCZ696 treatment also linked with a significant reduction in all-cause death, and a statistically significant and clinically meaningful improvement in patient quality of life as measured using the Kansas City Cardiomyopathy Questionnaire after patients had been on treatment for 8 months. Online publication of the full results occurred concurrent with Dr. Packer’s report at the meeting (N.Engl. J. Med 2014;DOI: 10.1056/NEJMoa1409077).
While Dr. Packer highlighted the positive impact of LCZ696 on quality of life, he also stressed that the primary impact of treatment with LCZ696 is not to make patients feel better. "The major benefit of this drug is to change the natural course and rate of progression of heart failure. It should be used in patients with mild heart failure to reduce progression and death," he said.
"By switching patients from an ACE inhibitor to LCZ696 you can prolong patient survival. It is exciting to have a new drug" that can confer as many benefits as LCZ696, Dr. Jessup said.
PARADIGM-HF was sponsored by Novartis. Dr. Packer has been a consultant to Novartis as well as several other companies. Dr. Jessup had no disclosures. Dr. Cleland has received research support and honoraria from Sorin, St. Jude, and Medtronic.
Twitter: @mitchelzoler
BARCELONA – A new, dual-agent formulation for treating heart failure patients with reduced ejection fraction showed a dramatic benefit for cutting the rate of cardiovascular death and hospitalizations in an international, controlled, pivotal trial with more than 8,000 patients.
The superiority of the new compound, which combines the novel neprilysin-inhibitor drug sacubitril with the established angiotensin receptor (ARB) blocking drug valsartan, was so strong that it immediately loomed as the new cornerstone-drug of choice for heart-failure patients with reduced ejection fraction pending regulatory approvals, said Dr. Milton Packer at the annual congress of the European Society of Cardiology.
Treatment with the new combination agent, currently known as LCZ696, doubled the cardiovascular-death benefit of the ACE inhibitor enalapril, while simultaneously edging out enalapril for safety and tolerability. Neprilysin is an endopeptidase that degrades several endogenous vasoactive peptides. Inhibiting this degradation process by treatment with LCZ696 boosts levels of these peptides and counters the neurohormonal overactivation that characterizes heart failure and leads to vasoconstriction, sodium retention, and maladaptive remodeling.
"This robust finding provides strong evidence supporting use of LCZ696 as the treatment of choice for heart failure instead of an ACE inhibitor or ARB" alone, said Dr. Packer, professor and chairman of clinical sciences at UT Southwestern Medical Center in Dallas. "The intent of this trial was to provide persuasive evidence that LCZ696 should replace current use of ACE inhibitors and ARBs as the cornerstone of heart failure treatment," said Dr. Packer, coprincipal investigator of the study.
Especially striking to several heart failure experts who heard the study results was the range of clinically important benefits seen when the new combination substituted for enalapril.
"This is the first study in a long time where we saw not only a reduction in the combined endpoint of cardiovascular death and hospitalization, but also significant effect on each of these two endpoints individually as well as on all-cause mortality," commented Dr. Mariell Jessup, professor of medicine at the University of Pennsylvania in Philadelphia. The new trial "was really trying to upset the foundation of heart failure treatment that we’ve used for the last 2 decades, and it succeeded. We were hoping for a positive outcome; what we got was remarkable. It very convincingly showed that this drug has incredible promise," she said in an interview.
"This will be an important change," commented Dr. John G.F. Cleland, professor of cardiology at the University of Hull, U.K. "The science and medicine will make it hard not to switch everyone with low ejection fraction heart failure [and other features that mirror the study’s enrollment criteria] from an ACE inhibitor to this the moment it becomes available," he said in an interview. "This is now the drug of choice for any heart failure patient with persistently elevated natriuretic peptide and left ventricular systolic dysfunction."
The Prospective Comparison of ARNI [angiotensin receptor–neprilysin inhibitor] with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial randomized 8,399 patients with heart failure and a left ventricular ejection fraction of 40% of less to treatment with either 200 mg b.i.d. of LCZ696 or 10 mg b.i.d. enalapril plus other standard heart failure medications, including a beta-blocker (in 93%), a diuretic (in 80%) and a mineralocorticoid antagonist such as spironolactone (in about 55%). About 70% of enrolled patients had New York Heart Association class II heart failure, and another 24% had class III heart failure.
After a median treatment duration of 27 months, the primary combined endpoint of cardiovascular death or first hospitalization for heart failure occurred in 22% of the patients treated with LCZ696 and in 27% of those treated with enalapril, a 20% relative risk reduction that was statistically significant, and which showed a number needed to treat of 21 to prevent one of these primary endpoints. LCZ696 treatment also linked with a significant reduction in all-cause death, and a statistically significant and clinically meaningful improvement in patient quality of life as measured using the Kansas City Cardiomyopathy Questionnaire after patients had been on treatment for 8 months. Online publication of the full results occurred concurrent with Dr. Packer’s report at the meeting (N.Engl. J. Med 2014;DOI: 10.1056/NEJMoa1409077).
While Dr. Packer highlighted the positive impact of LCZ696 on quality of life, he also stressed that the primary impact of treatment with LCZ696 is not to make patients feel better. "The major benefit of this drug is to change the natural course and rate of progression of heart failure. It should be used in patients with mild heart failure to reduce progression and death," he said.
"By switching patients from an ACE inhibitor to LCZ696 you can prolong patient survival. It is exciting to have a new drug" that can confer as many benefits as LCZ696, Dr. Jessup said.
PARADIGM-HF was sponsored by Novartis. Dr. Packer has been a consultant to Novartis as well as several other companies. Dr. Jessup had no disclosures. Dr. Cleland has received research support and honoraria from Sorin, St. Jude, and Medtronic.
Twitter: @mitchelzoler
AT THE ESC CONGRESS 2014
Key clinical point: Substituting LCZ696 for enalapril in heart failure patients was linked with significant improvements in survival, heart failure hospitalization, and quality of life.
Major finding: LCZ696 was associated with a 20% relative risk reduction in cardiovascular death and heart-failure hospitalization compared with enalapril in heart failure patients.
Data source: PARADIGM-HF, which assessed 8,399 patients randomized at 1,043 centers in 47 countries.
Disclosures: PARADIGM-HF was sponsored by Novartis. Dr. Packer has been a consultant to Novartis as well as several other companies. Dr. Jessup had no disclosures. Dr. Cleland has received research support and honoraria from Sorin, St. Jude, and Medtronic.








